Abstract
Background
A panic attack is a discrete period of fear or anxiety that has a rapid onset and reaches a peak within 10 minutes. The main symptoms involve bodily systems, such as racing heart, chest pain, sweating, shaking, dizziness, flushing, churning stomach, faintness and breathlessness. Other recognised panic attack symptoms involve fearful cognitions, such as the fear of collapse, going mad or dying, and derealisation (the sensation that the world is unreal). Panic disorder is common in the general population with a prevalence of 1% to 4%. The treatment of panic disorder includes psychological and pharmacological interventions, including antidepressants and benzodiazepines.
Objectives
To compare, via network meta‐analysis, individual drugs (antidepressants and benzodiazepines) or placebo in terms of efficacy and acceptability in the acute treatment of panic disorder, with or without agoraphobia.
To rank individual active drugs for panic disorder (antidepressants, benzodiazepines and placebo) according to their effectiveness and acceptability.
To rank drug classes for panic disorder (selective serotonin reuptake inhibitors (SSRIs), serotonin‐norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), mono‐amine oxidase inhibitors (MAOIs) and benzodiazepines (BDZs) and placebo) according to their effectiveness and acceptability.
To explore heterogeneity and inconsistency between direct and indirect evidence in a network meta‐analysis.
Search methods
We searched the Cochrane Common Mental Disorders Specialised Register, CENTRAL, CDSR, MEDLINE, Ovid Embase and PsycINFO to 26 May 2022.
Selection criteria
We included randomised controlled trials (RCTs) of people aged 18 years or older of either sex and any ethnicity with clinically diagnosed panic disorder, with or without agoraphobia. We included trials that compared the effectiveness of antidepressants and benzodiazepines with each other or with a placebo.
Data collection and analysis
Two authors independently screened titles/abstracts and full texts, extracted data and assessed risk of bias. We analysed dichotomous data and continuous data as risk ratios (RRs), mean differences (MD) or standardised mean differences (SMD): response to treatment (i.e. substantial improvement from baseline as defined by the original investigators: dichotomous outcome), total number of dropouts due to any reason (as a proxy measure of treatment acceptability: dichotomous outcome), remission (i.e. satisfactory end state as defined by global judgement of the original investigators: dichotomous outcome), panic symptom scales and global judgement (continuous outcome), frequency of panic attacks (as recorded, for example, by a panic diary; continuous outcome), agoraphobia (dichotomous outcome). We assessed the certainty of evidence using threshold analyses.
Main results
Overall, we included 70 trials in this review. Sample sizes ranged between 5 and 445 participants in each arm, and the total sample size per study ranged from 10 to 1168. Thirty‐five studies included sample sizes of over 100 participants.
There is evidence from 48 RCTs (N = 10,118) that most medications are more effective in the response outcome than placebo. In particular, diazepam, alprazolam, clonazepam, paroxetine, venlafaxine, clomipramine, fluoxetine and adinazolam showed the strongest effect, with diazepam, alprazolam and clonazepam ranking as the most effective. We found heterogeneity in most of the comparisons, but our threshold analyses suggest that this is unlikely to impact the findings of the network meta‐analysis.
Results from 64 RCTs (N = 12,310) suggest that most medications are associated with either a reduced or similar risk of dropouts to placebo. Alprazolam and diazepam were associated with a lower dropout rate compared to placebo and were ranked as the most tolerated of all the medications examined.
Thirty‐two RCTs (N = 8569) were included in the remission outcome. Most medications were more effective than placebo, namely desipramine, fluoxetine, clonazepam, diazepam, fluvoxamine, imipramine, venlafaxine and paroxetine, and their effects were clinically meaningful. Amongst these medications, desipramine and alprazolam were ranked highest.
Thirty‐five RCTs (N = 8826) are included in the continuous outcome reduction in panic scale scores. Brofaromine, clonazepam and reboxetine had the strongest reductions in panic symptoms compared to placebo, but results were based on either one trial or very small trials.
Forty‐one RCTs (N = 7853) are included in the frequency of panic attack outcome. Only clonazepam and alprazolam showed a strong reduction in the frequency of panic attacks compared to placebo, and were ranked highest.
Twenty‐six RCTs (N = 7044) provided data for agoraphobia. The strongest reductions in agoraphobia symptoms were found for citalopram, reboxetine, escitalopram, clomipramine and diazepam, compared to placebo.
For the pooled intervention classes, we examined the two primary outcomes (response and dropout). The classes of medication were: SSRIs, SNRIs, TCAs, MAOIs and BDZs.
For the response outcome, all classes of medications examined were more effective than placebo. TCAs as a class ranked as the most effective, followed by BDZs and MAOIs. SSRIs as a class ranked fifth on average, while SNRIs were ranked lowest. When we compared classes of medication with each other for the response outcome, we found no difference between classes. Comparisons between MAOIs and TCAs and between BDZs and TCAs also suggested no differences between these medications, but the results were imprecise.
For the dropout outcome, BDZs were the only class associated with a lower dropout compared to placebo and were ranked first in terms of tolerability. The other classes did not show any difference in dropouts compared to placebo. In terms of ranking, TCAs are on average second to BDZs, followed by SNRIs, then by SSRIs and lastly by MAOIs. BDZs were associated with lower dropout rates compared to SSRIs, SNRIs and TCAs.
The quality of the studies comparing antidepressants with placebo was moderate, while the quality of the studies comparing BDZs with placebo and antidepressants was low.
Authors' conclusions
In terms of efficacy, SSRIs, SNRIs (venlafaxine), TCAs, MAOIs and BDZs may be effective, with little difference between classes. However, it is important to note that the reliability of these findings may be limited due to the overall low quality of the studies, with all having unclear or high risk of bias across multiple domains. Within classes, some differences emerged. For example, amongst the SSRIs paroxetine and fluoxetine seem to have stronger evidence of efficacy than sertraline. Benzodiazepines appear to have a small but significant advantage in terms of tolerability (incidence of dropouts) over other classes.
Keywords: Adult; Humans; Alprazolam; Alprazolam/therapeutic use; Antidepressive Agents; Antidepressive Agents/therapeutic use; Antidepressive Agents, Tricyclic; Antidepressive Agents, Tricyclic/therapeutic use; Benzodiazepines; Benzodiazepines/therapeutic use; Clomipramine; Clomipramine/therapeutic use; Clonazepam; Clonazepam/therapeutic use; Desipramine; Desipramine/therapeutic use; Diazepam; Diazepam/therapeutic use; Fluoxetine; Fluoxetine/therapeutic use; Network Meta-Analysis; Panic Disorder; Panic Disorder/complications; Panic Disorder/drug therapy; Paroxetine; Paroxetine/therapeutic use; Reboxetine; Reboxetine/therapeutic use; Selective Serotonin Reuptake Inhibitors; Selective Serotonin Reuptake Inhibitors/therapeutic use; Serotonin and Noradrenaline Reuptake Inhibitors; Serotonin and Noradrenaline Reuptake Inhibitors/therapeutic use; Venlafaxine Hydrochloride; Venlafaxine Hydrochloride/therapeutic use
Plain language summary
Pharmacological treatments in panic disorder in adults: a network meta‐analysis
Why is this review important?
People with panic disorder are profoundly impacted by this condition, often experiencing challenges with work, education and social or family life. We wanted to evaluate which medications, if any, are the most effective and safe. In particular, we aimed to assess whether the network meta‐analysis findings were valid enough to identify the best medications, in order to improve care. These analyses have also generated suggestions for future research to reduce key uncertainties in the evidence.
Who will be interested in this research?
The research in this Cochrane Review will interest:
‐ people who decide policy, and influence decisions about the prescription of medications for panic disorder;
‐ people who prescribe these medicines to people with panic disorder;
‐ people with panic disorder;
‐ those who support and care for them.
What did we want to find out?
We wanted to find out how well antidepressants, benzodiazepines and azapirones work to improve panic disorder symptoms in adults (i.e. people aged 18 years or older).
We wanted to know how these medications affect:
‐ the symptoms of panic disorder;
‐ dropout from studies, as a measure of the side effects of medication;
‐ recovery: no longer meeting the diagnostic criteria for panic disorder;
‐ response or remission: scores on a scale indicating an important reduction in panic or no longer experiencing panic;
‐ reduction in the frequency of panic attacks;
‐ reduction in agoraphobia (fear of being in situations where escape might be difficult or that help would not be available if things go wrong).
What did we do?
We searched electronic databases and study registers to find all relevant studies. We only included randomised controlled trials (a type of study in which participants are assigned to a treatment group using a random method) that compared treatment with antidepressants, benzodiazepines, azapirones and placebo in adults with a diagnosis of panic disorder, with or without agoraphobia. We only included studies in which the patients and clinicians did not know which treatment they received. We included 70 studies in our review with a total of 12,703 participants. The date of our search was 26 May 2022.
What does the evidence from the review tell us?
‐ We found that most medications may be more effective in the response outcome than placebo. In particular, diazepam, alprazolam, clonazepam, paroxetine, venlafaxine, clomipramine, fluoxetine and adinazolam showed the strongest effect. Also, most medications were either associated with a reduced or similar risk of dropouts to placebo. Alprazolam and diazepam were associated with a lower dropout rate than placebo and were ranked as the most tolerated of all the medications examined.
‐ Most medications may have been more effective than placebo in remitting the symptoms of panic disorder and their effects were clinically meaningful. In terms of the reduction in panic scale scores, brofaromine, clonazepam and reboxetine seem to have the strongest reductions in panic symptoms compared to placebo, but the results were based on either one trial or very small trials. For the frequency of panic attacks outcome, only clonazepam and alprazolam showed a strong reduction in the frequency of attacks compared to placebo. The strongest reductions in agoraphobia symptoms were found for citalopram, reboxetine, escitalopram, clomipramine and diazepam, compared to placebo.
‐ If we consider the classes of medications together (selective serotonin reuptake inhibitors (SSRIs), serotonin‐norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), mono‐amine oxidase inhibitors (MAOIs) and benzodiazepines), all classes of medications examined were more effective than placebo. TCAs as a class ranked as the most effective, followed by benzodiazepines and MAOIs. SSRIs as a class ranked fifth on average, while SNRIs were ranked as the lowest.
‐ If classes of medication are compared with each other for the response outcome, no difference is found between classes. For the dropout outcome, benzodiazepines were the only class associated with a lower dropout than placebo, and they were ranked as first in terms of tolerability. The other classes did not show any difference in dropouts compared to placebo.
‐ It is important to note that, while the quality of the studies comparing antidepressants with placebo was acceptable, the quality of the studies comparing benzodiazepines with placebo and antidepressants was low. This may limit the certainty of our results.
‐ Our review has limitations as it is based on short‐term studies.
What should happen next?
‐ Almost all the studies examined in this network meta‐analysis were of short duration. For benzodiazepines, there has been considerable debate about whether they can be used in the long term given their propensity for abuse and the possible risk of tolerance. More research on their long‐term effects (i.e. longer than eight weeks and maybe up to one year) is needed.
‐ It will be important to systematically assess the efficacy of medications compared to talking therapies, perhaps in a network meta‐analysis. Data for depression seem to show that psychotherapies can lead to a more sustained effect. The same may apply to anxiety disorders in general and panic disorder in particular and this needs to be investigated.
Summary of findings
Summary of findings 1. Summary of findings: response at end of treatment.
|
Population: people with panic disorder diagnosis Settings: inpatient, outpatient and primary care Intervention: antidepressants (such as sertraline) or benzodiazepines (such as diazepam) Comparison: placebo, alternative antidepressant or benzodiazepine | ||||
| Anticipated absolute effects (95% CrI)* | ||||
|
48 RCTs, 10,118 participants |
Assumed comparator risk per 1000 |
Corresponding intervention risk per 1000 (95% CrI) |
Relative effect (NMA): RR (95% CrI) |
Threshold analysis |
| Diazepam vs placebo | 617 | 401 (173 to 592) | 0.65 (0.28 to 0.96) |
No concerns |
| Alprazolam vs placebo | 617 | 419 (241 to 568) | 0.68 (0.39 to 0.92) |
No concerns |
| Clonazepam vs placebo | 617 | 438 (253 to 592) | 0.71 (0.41 to 0.96) |
No concerns |
| Escitalopram vs placebo | 617 | 481 (259 to 635) | 0.78 (0.42 to 1.03) |
No concerns |
| Fluoxetine vs placebo | 617 | 481 (259 to 617) | 0.78 (0.42 to 1.00) |
No concerns |
| Adinazolam vs placebo | 617 | 506 (308 to 617) | 0.82 (0.50 to 1.00) | No concerns |
| Imipramine vs placebo | 617 | 506 (247 to 672) | 0.82 (0.40 to 1.09) | No concerns |
| Paroxetine vs placebo | 617 | 524 (395 to 598) | 0.85 (0.64 to 0.97) |
No concerns |
| Venlafaxine vs placebo | 617 | 518 (370 to 598) | 0.84 (0.60 to 0.97) |
No concerns |
| Clomipramine vs placebo | 617 | 524 (352 to 611) | 0.85 (0.57 to 0.99) |
No concerns |
| Fluvoxamine vs placebo | 617 | 531 (327 to 648) | 0.86 (0.53 to 1.05) |
No concerns |
| Citalopram vs placebo | 617 | 537 (352 to 629) | 0.87 (0.57 to 1.02 |
No concerns |
| Sertraline vs placebo | 617 | 549 (413 to 629) | 0.89 (0.67 to 1.02) |
No concerns |
| Desipramine vs placebo | 617 | 580 (265 to 845) |
0.94 (0.43 to 1.37) |
No concerns |
| Buspirone vs placebo | 617 | 703 (296 to 1271) |
1.14 (0.48 to 2.06) |
No concerns |
| Ritanserin vs placebo | 617 | 734 (6 to 1666) | 1.19 (0.01 to 2.70) |
No concerns |
| Etizolam vs placebo | 617 | 358 (19 to 882) | 0.58 (0.03 to 1.43) |
Findings sensitive to imprecision1 |
| Reboxetine vs placebo | 617 | 475 (148 to 734) | 0.77 (0.24 to 1.19) |
Findings sensitive to imprecision1 |
| Moclobemide vs fluoxetine | 185 | 213 (52 to 771) | 1.15 (0.28 to 4.17) |
No concerns |
| Citalopram vs fluoxetine | 185 | 281 (159 to 1097) | 1.52 (0.86 to 5.93) |
No concerns |
| Desipramine vs fluoxetine | 185 | 216 (83 to 783) | 1.17 (0.45 to 4.23) |
No concerns |
| Paroxetine vs sertraline | 556 | 506 (322 to 645) | 0.91 (0.58 to 1.16) |
No concerns |
| Paroxetine vs venlafaxine | 330 | 333 (277 to 416) | 1.01 (0.84 to 1.26) |
No concerns |
| Imipramine vs fluvoxamine | 379 | 326 (163 to 462) |
0.86 (0.43 to 1.22) |
No concerns |
| Ritanserin vs fluvoxamine | 379 | 595 (243 to 1762) | 1.57 (0.64 to 4.65) |
No concerns |
| Paroxetine vs clomipramine | 314 | 323 (232 to 506) | 1.03 (0.74 to 1.61) |
No concerns |
| Moclobemide vs clomipramine | 314 | 298 (60 to 612) | 0.95 (0.19 to 1.95) |
No concerns |
| Citalopram vs clomipramine | 314 | 374 (279 to 647) | 1.19 (0.89 to 2.06) |
No concerns |
| Alprazolam vs imipramine | 550 | 424 (215 to 671) | 0.77 (0.39 to 1.22) |
No concerns |
| Alprazolam vs paroxetine | 351 | 291 (176 to 393) | 0.83 (0.50 to 1.12) |
No concerns |
| Escitalopram vs citalopram | 484 | 499 (257 to 886) | 1.03 (0.53 to 1.83) |
No concerns |
| Diazepam vs alprazolam | 294 | 315 (153 to 585) | 1.07 (0.52 to 1.99) |
No concerns |
| Buspirone vs alprazolam | 294 | 547 (326 to 1558) | 1.86 (1.11 to 5.30) |
No concerns |
195% CrI crosses invariant range.
*The corresponding risk (and its 95% credible interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CrI). In comparisons with placebo, estimates of assumed risk were based on the mean risk of non‐response in the placebo group. In head‐to‐head comparisons, estimates of assumed risk were based on the median risk of non‐response in the comparator group as there were fewer trials.
CrI: credible interval; NMA: network meta‐analysis; RR: risk ratio; RCT: randomised controlled trial
Summary of findings 2. Summary of findings: dropout at end of treatment.
|
Population: people with panic disorder diagnosis Settings: inpatient, outpatient and primary care Intervention: antidepressants (such as sertraline) or benzodiazepines (such as diazepam) Comparison: placebo, alternative antidepressant or benzodiazepine | ||||
| Anticipated absolute effects (95% CrI)* | ||||
|
64 RCTs; 12,310 participants |
Assumed comparator risk per 1000 | Corresponding intervention risk per 1000 (95% CrI) |
Relative effect: RR (95% CrI) |
Threshold analysis |
| Fluvoxamine vs placebo | 340 | 398 (289 to 564) | 1.17 (0.85 to 1.66) | No concerns |
| Paroxetine vs placebo | 340 | 364 (313 to 364) | 1.07 (0.92 to 1.07) |
No concerns |
| Imipramine vs placebo | 340 | 289 (214 to 381) | 0.85 (0.63 to 1.12) |
No concerns |
| Venlafaxine vs placebo | 340 | 337 (272 to 411) | 0.99 (0.80 to 1.21) |
No concerns |
| Clomipramine vs placebo | 340 | 330 (252 to 422) | 0.97 (0.74 to 1.24) |
No concerns |
| Sertraline vs placebo | 340 | 343 (275 to 445) | 1.01 (0.81 to 1.31) |
No concerns |
| Escitalopram vs placebo | 340 | 231.2 (129 to 367) | 0.68 (0.38 to 1.08) |
No concerns |
| Citalopram vs placebo | 340 | 299.2 (211 to 408) | 0.88 (0.62 to 1.20) |
No concerns |
| Desipramine vs placebo | 340 | 214.2 (48 to 578) | 0.63 (0.14 to 1.70) |
Incoherence1 |
| Fluoxetine vs placebo | 340 | 384.2 (204 to 646) | 1.13 (0.60 to 1.90) |
Incoherence2 |
| Reboxetine vs placebo | 340 | 136 (44 to 398) | 0.40 (0.13 to 1.17) |
No concerns |
| Clonazepam vs placebo | 340 | 319.6 (251 to 384) | 0.94 (0.74 to 1.13) |
No concerns |
| Adinazolam vs placebo | 340 | 404.6 (296 to 575) | 1.19 (0.87 to 1.69) |
No concerns |
| Alprazolam vs placebo | 340 | 156.4 (112 to 224) | 0.46 (0.33 to 0.66) |
No concerns |
| Etizolam vs placebo | 340 | 125.8 (3 to 847) | 0.37 (0.01 to 2.49) |
Imprecision3 |
| Buspirone vs placebo | 340 | 622.2 (398 to 1136) | 1.83 (1.17 to 3.34) |
No concerns |
| Diazepam vs placebo | 340 | 170 (78 to 309) | 0.50 (0.23 to 0.91) |
No concerns |
| Imipramine vs fluoxetine | 50 | 38 (20 to 72) | 0.75 (0.40 to 1.44) |
No concerns |
| Citalopram vs fluoxetine | 50 | 39 (21 to 77) | 0.78 (0.42 to 1.53) |
No concerns |
| Desipramine vs fluoxetine | 50 | 28 (7 to 80) | 0.56 (0.13 to 1.59) |
No concerns |
| Mirtazapine vs fluoxetine | 50 | 35 (5 to 107) | 0.70 (0.09 to 2.13) |
No concerns |
| Brofaromine vs fluvoxamine | 194 | 204 (103 to 371) | 1.05 (0.53 to 1.91) |
No concerns |
| Imipramine vs fluvoxamine | 194 | 142 (93 to 202) | 0.73 (0.48 to 1.04) |
No concerns |
| Paroxetine vs sertraline | 265 | 281 (220 to 356) | 1.06 (0.83 to 1.34) |
No concerns |
| Brofaromine vs clomipramine | 255 | 324 (179 to 561) | 1.27 (0.70 to 2.20) |
No concerns |
| Adinazolam vs clomipramine | 255 | 316 (232 to 446) | 1.24 (0.91 to 1.75) |
No concerns |
| Moclobemide vs clomipramine | 255 | 286 (151 to 497) | 1.12 (0.59 to 1.95) |
No concerns |
| Imipramine vs clomipramine | 255 | 224 (156 to 316) | 0.88 (0.61 to 1.24) |
No concerns |
| Citalopram vs clomipramine | 255 | 235 (158 to 329) | 0.92 (0.62 to 1.29) |
No concerns |
| Paroxetine vs clomipramine | 255 | 283 (217 to 378) | 1.11 (0.85 to 1.48) |
No concerns |
| Buspirone vs imipramine | 302 | 649 (395 to 1295) | 2.15 (1.31 to 4.29) |
No concerns |
| Alprazolam vs imipramine | 302 | 166 (118 to 226) | 0.55 (0.39 to 0.75) |
No concerns |
| Diazepam vs alprazolam | 167 | 177 (87 to 328) | 1.06 (0.52 to 1.97) |
No concerns |
| Buspirone vs alprazolam | 167 | 660 (338 to 1411) | 3.96 (2.03 to 8.47) |
No concerns |
| Alprazolam vs clonazepam | 77 | 40 (25 to 61) | 0.52 (0.33 to 0.79) |
No concerns |
| Paroxetine vs venlafaxine | 257 | 278 (224 to 355) | 1.08 (0.87 to 1.38) |
No concerns |
| Escitalopram vs citalopram | 231 | 180 (104 to 285) | 0.78 (0.45 to 1.23) |
No concerns |
1Direct estimates but not indirect estimates crossed equivalence range. 2Indirect estimates but not direct estimates crossed equivalence range. 395% CrI crossed equivalence.
*The corresponding risk (and its 95% CrI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CrI). In comparisons with placebo, estimates of assumed risk were based on the mean risk of dropout in the placebo group. In head‐to‐head comparisons, estimates of assumed risk were based on the median risk of dropout in the comparator group as there were fewer trials.
CrI: credible interval; RR: risk ratio; RCT: randomised controlled trial
Background
Description of the condition
A panic attack is a discrete period of fear or anxiety that has a rapid onset and reaches a peak within 10 minutes (APA 2013a). The main symptoms involve bodily systems, such as racing heart, chest pain, sweating, shaking, dizziness, flushing, churning stomach, faintness and breathlessness. Other recognised panic attack symptoms involve fearful cognitions, such as the fear of collapse, going mad or dying, and derealisation (sensation that the world is unreal) (APA 2013a).
Panic disorder first entered diagnostic classification systems in 1980 with the publication of the Diagnostic and Statistical Manual of Mental Disorders ‐ 3rd edition (DSM‐III), following observations that patients with panic attacks responded to treatment with imipramine, which is a tricyclic antidepressant (TCA) (Klein 1964). To diagnose panic disorder, further conditions must be met relating to the frequency of attacks, the need for some attacks to come on ‘out of the blue’ rather than in a predictable, externally triggered situation, and exclusions where attacks are attributable solely to medical causes or panic‐inducing substances, notably caffeine. DSM‐IV also requires that at least one attack has been followed by: a) persistent concern about having additional attacks; b) worry about the implications of the attack or its consequences; or c) a significant change in behaviour related to the attacks (APA 1994). The core features of panic attacks remained unchanged in DSM‐5 (APA 2013a), but in DSM‐5 panic disorder and agoraphobia are no longer linked and are now coded as two diagnoses (APA 2013b).
Panic disorder is common in the general population; it occurs in 1% to 4% of people (lifetime prevalence) (Bijl 1998; Eaton 1994; Kessler 2012). In primary care settings, panic has been reported to have a prevalence of around 10% (King 2008). This is because common mental disorders are more often dealt with in primary care (King 2008). Women and previously married people have consistently elevated odds of panic (Kessler 2006). There seems to be some weak association between unemployment and retirement and the likelihood of suffering from panic disorder (Kessler 2006). Its cause is not fully understood and there are probably several reasons why panic occurs. Biological theories incorporate the faulty triggering of an inbuilt anxiety response, possibly a suffocation alarm. Evidence for this comes from biological challenge tests (lactate and carbon dioxide trigger panic in those with the disorder) and from animal experiments and neuroimaging studies in humans that show activation of fear circuits in the brain, such as that involving a part of the brain called periaqueductal grey matter (Gorman 2000).
About one‐quarter of people with panic disorder also have agoraphobia (Kessler 2006). Agoraphobia is defined as anxiety about being in places or situations from which escape might be difficult or embarrassing, or in which help may not be available in the event of having a panic attack (APA 2013a). The presence of agoraphobia is associated with increased severity and worse outcome (Kessler 2006). There are several risk factors that predict the development of agoraphobia in people with panic disorder: female gender, more intense dizziness during a panic attack, cognitive factors, dependent personality traits and social anxiety disorder (Starcevic 2009).
Panic disorder, with or without agoraphobia, co‐occurs very frequently with other psychiatric disorders, such as drug dependence, major depression, bipolar I disorder, social phobia, specific phobia and generalised anxiety disorder (Grant 2006). It is estimated that generalised anxiety disorder co‐occurs in 68% of people with panic disorder, whilst 24% to 88% of people with panic disorder have major depression (Starcevic 2009).
Description of the intervention
This review is focused on antidepressants and benzodiazepines, two pharmacological interventions. The treatment of panic disorder includes psychological and pharmacological interventions, often used in combination (Furukawa 2007; Watanabe 2009). The main pharmacological treatments used in panic disorder are antidepressants and benzodiazepines (BDZs). Azapirones, gabapentinoids, anticonvulsants, beta‐blockers and inositol have also been studied but are not a focus of this review.
Historically, pharmacological interventions for panic disorder have been based on the use of older antidepressants, such as mono‐amine oxidase inhibitors (MAOIs) and tricyclic antidepressants (TCAs) (Bruce 2003). MAOIs and TCAs are, however, burdened by severe adverse effects, such as dietary restrictions (to avoid hypertensive crisis) for MAOIs, and anticholinergic (e.g. memory problems and confusion) and arrhythmogenic (heart rhythm problems) effects, and overall poor tolerability for TCAs (Wade 1999). Benzodiazepines (BDZs), particularly high‐potency ones, have been used as a safer alternative in panic disorder (Stein 2010), although they may work less effectively in the long term (NICE 2011). Recent guidelines, for example APA 2009, NICE 2011, BAP 2014 and Katzman 2014, consider newer antidepressants, such as the selective serotonin reuptake inhibitors (SSRIs) and the serotonin noradrenaline reuptake inhibitor venlafaxine, as first‐line treatment for panic disorder, due in part to their more favourable adverse effect profile over the older antidepressant groups (MAOIs and TCAs). A meta‐analysis comparing SSRIs and TCAs in panic disorder showed that SSRIs are as effective as TCAs, and are better tolerated (Bakker 2002), although other studies showed a possible overestimation of the efficacy of SSRIs over older antidepressants in panic disorder (Anderson 2000; Otto 2001).
BDZs have a higher incidence of dependence and withdrawal reaction when compared to antidepressants (Wade 1999), and they may not be effective in treating panic disorder that occurs together with depression (Ballenger 1998). In spite of these caveats, it appears that BDZs continue to be widely prescribed for the treatment of panic disorder (Bruce 2003).
How the intervention might work
Antidepressant drugs augment the function of the monoamines serotonin and noradrenaline. Serotonergic antidepressants (SSRIs) promote the transmission of the neurotransmitter serotonin across brain synapses. They most notably do it in the part of the brain called the dorsal raphe nucleus (Briley 1993). They prevent reuptake of serotonin into nerve terminals by inhibiting serotonin transporters, thus allowing more serotonin to be available for neurotransmission. In panic disorder, imaging studies have revealed reduced expression of the 5H1A serotonin receptor (Nash 2008), which has an inhibitory function, so the increased serotonin throughput may in part serve to overcome this deficit of inhibition. Noradrenergic antidepressants can similarly increase transmission of the catecholamine noradrenaline. Some antidepressants, such as the serotonin‐norepinephrine reuptake inhibitor (SNRI) drugs (e.g. venlafaxine, duloxetine) and TCAs, can enhance both serotonin and noradrenaline transmission by inhibiting both transporters.
BDZs moderate the gamma‐aminobutyric acid (GABA) neurotransmitter system, which is the brain’s main inhibitory neurotransmitter. They activate the GABA‐A BDZ receptor. This receptor complex contains a chloride channel, opened by agonists, which ultimately reduces anxiety and creates sedation. The BDZ binding site communicates only indirectly with the channel, meaning that BDZs are safer than their predecessors, the barbiturates. It is known through imaging studies that the inhibitory GABA system is deficient in panic disorder (Cameron 2007; Malizia 1998), thus the ability of BDZs to activate the GABA‐A BDZ receptor can counteract this. It is likely that both monoamine‐based systems and GABA‐based systems converge, allowing both antidepressants and BDZs to have efficacy in panic disorder despite their differing actions on neurotransmitter systems. One possibility is via serotonergic neurones that modulate GABA input to the part of the brain called periaqueductal grey matter.
Why it is important to do this review
People with panic disorder are profoundly impacted by this condition often experiencing challenges engaging with work, education and social or family life. These challenges not only impact people with panic disorder but also have substantial social and economic costs (Batelaan 2007). A recent German study found that 60% of societal costs associated with panic disorder were due to productivity losses and absences from work (Brettschneider 2019). Therefore further information on the safety and effectiveness of pharmacological interventions has the potential to benefit both people with panic disorder and society.
Pharmacological treatments are widely used in clinical practice to treat panic disorder. To our knowledge, the last meta‐analysis specifically focused on benzodiazepines for panic disorder was published in 1991 (Wilkinson 1991), and the last two meta‐analyses focusing on antidepressants for this condition were published more than 10 years ago and seven years ago (Bakker 2002 and Andrisano 2013, respectively). Standard pair‐wise meta‐analyses of psychopharmacological interventions in panic disorder have been published within Cochrane (Bighelli 2016; Bighelli 2018; Breilmann 2019; Imai 2014). Other reviews have been published on combined psychotherapy and pharmacotherapy in panic disorder (Furukawa 2007; Watanabe 2009). However, given the complexity of the condition, it is very important to carry out a comprehensive and comparative evaluation of the main pharmacological treatment options within the framework of a network meta‐analysis (NMA). NMAs produce estimates of the relative effects between any pair of interventions in the network, and usually yield more precise estimates than a single direct or indirect estimate (Higgins 2019).
We wanted to evaluate which treatments, if any, are the most effective and safe. In particular, we aimed to assess whether the NMA findings are of sufficient validity to help patients, mental health professionals and policymakers identify the best pharmacological treatments for panic disorder, in order to improve clinical practice and patient care. These analyses also generate suggestions for future research to reduce key uncertainties in the evidence base.
Objectives
To assess the effects of individual active drugs (antidepressants and benzodiazepines) and placebo in terms of efficacy and acceptability for the acute treatment of panic disorder, with or without agoraphobia.
To rank individual active drugs (antidepressants, benzodiazepines and placebo) according to their effectiveness and acceptability for panic disorder, with or without agoraphobia.
To rank drug classes (SSRIs, SNRIs, TCAs, MAOIs and BDZs and placebo) according to their effectiveness and acceptability, for panic disorder, with or without agoraphobia.
To explore heterogeneity and inconsistency between direct and indirect evidence for individual active drugs and placebo in the network meta‐analyses, for panic disorder, with or without agoraphobia.
Methods
Criteria for considering studies for this review
Types of studies
We included double‐blind randomised controlled trials (RCTs) of the included drugs (see Types of interventions), either compared to one another or to placebo, in the acute treatment of panic disorder. We excluded trials in which drugs were used as an augmentation strategy for any other psychotropic drugs. For trials that had a cross‐over design, we only considered results from the first randomisation period. Cluster‐randomised trials were included only if intracluster correlation coefficients were reported. If a study was reported as double‐blind, we included it; any risk of bias associated with implementing this procedure informed our risk of bias assessment.
We excluded the following:
Relapse prevention trials.
Studies in patients with a diagnosis of panic disorder where the effects of treatments were measured after panic attacks had been induced (for example with CO₂ inhalations or lactate infusions).
Studies concurrently administering psychosocial therapies targeted at panic disorder.
Studies comparing psychosocial interventions.
Quasi‐randomised trials.
Types of participants
The fundamental assumption underpinning a network meta‐analysis is that of consistency/transitivity (Caldwell 2005; Cipriani 2013). We assumed that any patient who met the inclusion criteria below was, in principle, equally likely to have been randomised to any of the eligible interventions examined in this review, that is, that they are 'jointly randomisable' (Salanti 2012).
Participant characteristics
People aged 18 or older, of either sex, with a primary diagnosis of panic disorder, with or without agoraphobia.
Diagnosis
Diagnosis was according to any of the following criteria: DSM‐III‐R; DSM‐IV or the International Classification of Diseases, 10th edition (ICD‐10); DSM‐5. We did not include studies using operationalised criteria before DSM‐III because their conceptualisation of panic disorder is substantively different.
Comorbidities
When the study eligibility focused on agoraphobia rather than panic disorder, and was operationally diagnosed according to the above‐named criteria, and when we could safely assume that at least some of the patients experienced panic disorder as defined by the above criteria, we included the study. Considering that over 95% of patients with agoraphobia seen clinically also suffer from panic disorder (Goisman 1995), we planned to investigate the effect of their inclusion in a subgroup analysis. However, this subgroup analysis was not possible as all studies included people with agoraphobia.
We excluded trials in which all participants had a concurrent primary diagnosis of any psychiatric disorder other than panic disorder or agoraphobia when the focus was not the treatment of panic disorder. We excluded trials in which participants had a serious concomitant medical illness.
Setting
Inpatient, outpatient and primary care.
Subset data
We did not include trials that provided data on a relevant subset of their participants (e.g. a study that included a subset of participants meeting the criteria for panic disorder).
Types of interventions
We only included studies where medications were used at a therapeutic dosage. We defined therapeutic doses as doses that are indicated for panic disorder by any of the North American, European or Japanese regulatory agencies. Where such were not available, we followed the same dose ranges as for major depression (for antidepressants) and generalised anxiety disorder (for benzodiazepines).
Antidepressants
TCAs and related antidepressants: amitriptyline, clomipramine, desipramine, dosulepin/dothiepin, doxepin, imipramine, lofepramine, protriptyline, maprotiline, nortriptyline, trimipramine, amitriptylinoxide, butriptyline, cianopramine, demexiptilline, dibenzepin, dimetacrine, fluotracen, iprindole, imipraminoxide, melitracen, metapramine, nitroxazepine, noxiptiline, opipramol, pipofezine, propizepine, quinupramine.
Selective serotonin reuptake inhibitors: citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, femoxetine, indalpine, zimelidine.
Monoamine‐oxidase inhibitors: isocarboxazid, moclobemide, phenelzine, tranylcypromine, brofaromine, triRimaᵀᴹ, befloxatone, benmoxin, caroxazone, cimoxatone, clorgyline, deprenyl, iproclozide, mebanazine, minaprine, nialamide, octamoxin, pheniprazine, phenoxypropazine, pirlindole, pivhydrazine, safrazine, selegiline, toloxatone.
Serotonin‐noradrenaline reuptake inhibitors: desvenlafaxine, duloxetine, levomilnacipran, milnacipran, venlafaxine.
Noradrenergic and specific serotonergic antidepressants: mirtazapine, setiptiline.
Noradrenergic and dopaminergic reuptake inhibitors: bupropion, cilobamin, diclofensine, nomifensine.
Noradrenergic reuptake inhibitors: reboxetine, viloxazine.
Others: agomelatine, amineptine, trazodone, nefazodone, mianserin, vortioxetine and non‐conventional herbal products (e.g. Hypericum), viqualine, tianeptine, etoperidone, medifoxamine, pizotifen, benacytine, ritanserin, tedatioxetine, thozalinone.
Benzodiazepines (BDZs)
Alprazolam, bretazenil, bromazepam, chlordiazepoxide, cinolazepam, clonazepam, cloxazolam, clorazepate, delorazepam, diazepam, estazolam, etizolam, fludiazepam, flunitrazepam, flurazepam, flutoprazepam, halazepam, ketazolam, loprazolam, lorazepam, lormatezepam, medazepam, nimatazepam, nitrazepam, nodazepam, oxazepam, phenazepam, pinazepam, prazepam, premazepam, quazepam, temazepam, tetrazepam, triazolam and any other drug belonging to the BDZ class.
Placebo
Placebo can be active (i.e. mimicking side effects) or inactive (completely inert). We included studies using active and inactive placebo. This could be a potential source of heterogeneity or inconsistency (or both).
Types of outcome measures
We included studies that met the above inclusion criteria regardless of whether they reported on the following primary and secondary outcomes, which were pre‐defined at the protocol stage (Guaiana 2020). We chose continuous and dichotomous data as they provide complementary data.
Primary outcomes
Response to treatment (i.e. substantial improvement from baseline as defined by the original investigators). We used the following definitions of response: “much or very much improved” according to the Clinical Global Impression Change Scale; more than 40% reduction in the Panic Disorder Severity Scale score; or more than 50% reduction in the Fear Questionnaire Agoraphobia Subscale. When multiple measures were used, we gave preference to the most global measure.
Total number of dropouts due to any reason (as a proxy measure of treatment acceptability).
Secondary outcomes
Remission (i.e. satisfactory end state as defined by global judgement of the original investigators). Examples of this outcome included “panic free” and “no or minimal symptoms” according to the Clinical Global Impression Severity Scale. When multiple measures were used, we gave preference to the most global measure.
Panic symptom rating scales and global clinical judgement on a continuous scale. Examples include the Panic Disorder Severity Scale total score (0 to 28), Clinical Global Impression Severity Scale (1 to 7) and Clinical Global Impression Change Scale (1 to 7).
Frequency of panic attacks per unit of time (ex. days, weeks, months..., as recorded, for example, by a panic diary).
Agoraphobia symptom (as measured, for example, by the Fear Questionnaire, Mobility Inventory or behavioural avoidance test).
When more than one scale was available in the paper, we gave preference in the following order:
Panic Disorder Severity Scale (PDSS) > Panic and Agoraphobia Scale (PAS) > Anxiety Sensitivity Index‐Revised (ASI‐R) > Anxiety Sensitivity Index (ASI) > Anxiety Control Questionnaire (ACQ) > Body Sensations Questionnaire (BSQ) > other scales specific for panic disorder.
Clinical Global Impression ‐ Severity (CGI‐S) > Clinical Global Impression ‐ Improvement (CGI‐I) > Global Assessment Scale (GAS) > Global Assessment of Functioning (GAF) > other global scales.
Fear Questionnaire ‐ Agoraphobia subscale (FQ‐ag) > Fear Questionnaire ‐ Global (FQ‐global) > Mobile Inventory for Agoraphobia‐Avoidance‐Alone (MI‐AAL) > MI‐Avoidance‐Accompanied (MI‐AAC) > other scales specific for agoraphobia only.
Panic frequency > panic severity > other scales specific for panic attacks only.
Once the scale was chosen, if both self‐ and observer‐rated assessments were available, we gave preference to the latter. The actual measure entered into the meta‐analysis is indicated at the top of the listings in Characteristics of included studies.
Timing of outcome assessment
All outcomes were short term: we defined this as acute‐phase treatment, which normally lasted two to six months. When studies reported more response rates at different time points within two to six months, we gave preference to the time point closest to three months (i.e. 12 weeks).
Hierarchy of outcome measures
When several possible outcome measures were reported for the same outcome, we used the primary outcome according to the original study.
Search methods for identification of studies
Trials that included at least two of the interventions were eligible for inclusion in the review. We searched for all possible comparisons formed by the interventions of interest, as defined above.
Electronic searches
We searched the following databases using relevant subject headings (controlled vocabulary) and search syntax appropriate to each resource (all years to 26 May 2022).
Cochrane Common Mental Disorders Specialised Register (CCMDCTR) (all available years) (Appendix 1);
Cochrane Central Register of Controlled Trials (CENTRAL 2022, Issue 5) in the Cochrane Library;
Ovid MEDLINE databases (2014 to 26 May 2022) (Appendix 2);
Ovid Embase (2014 to May Week 2 2022);
Ovid PsycINFO (2014 to May Week 2 2022).
We searched the trial registers ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch) via CCMDCTR and CENTRAL in the Cochrane Library.
We applied date restrictions to the searches of MEDLINE, Embase, PsycINFO and CENTRAL for the following reason: Cochrane Common Mental Disorders relocated to the University of York in 2016 and the group's Specialised Register (which previously included RCTs from these databases) fell out of date at this time. We conducted the additional searches to account for this period from 2014 onwards.
We applied no further restrictions on date, language or publication status to the searches.
Searching other resources
Two review authors independently checked the reference lists of all included studies, non‐Cochrane systematic reviews and major textbooks of affective disorders (written in English), for published reports and citations of unpublished research. We also conducted a citation search via the Web of Science (included studies only) to identify additional works. We also contacted experts in the field.
Data collection and analysis
For details about who performed the original systematic review tasks among the authors please see Bighelli 2016, Bighelli 2018 and Breilmann 2019.
Selection of studies
At least two review authors independently screened the titles and abstracts of all studies we identified as a result of the search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved the full‐text study reports/publications and two review authors independently screened them and identified studies for inclusion; they also identified and recorded reasons for exclusion of the ineligible studies.
The two review authors resolved any disagreement through discussion or, when required, through consultation with a third member of the review team. We identified and excluded duplicate records and collated multiple reports related to the same study so that each study rather than each report is the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and a Characteristics of excluded studies table (Moher 2009).
Data extraction and management
We used a data collection form, piloted on at least one study in the review, to extract study characteristics and outcome data. Two authors from the review team extracted study characteristics and outcome data from the included studies.
From each included study we extracted data on the following study, intervention and population characteristics that may act as possible effect modifiers.
Methods: study design, randomisation (individual or cluster), total duration of study, number of study centres and location, study setting, withdrawals and date of study.
Participants: number, setting, sex, diagnostic criteria, presence or absence of medical and psychiatric co‐morbidities, presence or absence of elderly participants, percentage of patients with agoraphobia, percentage of patients with baseline depression, inclusion criteria and exclusion criteria.
Interventions: medication dose, medication dose range, use of rescue medication.
Outcomes: primary and secondary outcomes specified and collected, and time points reported. Where possible, we extracted data at the arm level, not summary effects.
Notes: sponsorship/funding for trial, and notable conflicts of interest of trial authors.
We compiled a table of important trial and patient characteristics and visually inspected the similarity of factors we considered likely to modify treatment effect.
We noted in the Characteristics of included studies table if outcome data were not reported in a usable way. We resolved disagreements through consensus or by involving a third person. One review author transferred data into Review Manager 5 (Review Manager 2014), WinBUGS or OpenBUGS software. We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports. A second review author spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
To assess risk of bias in RCTs, we used the Cochrane risk of bias tool (Higgins 2011).
Two review authors independently assessed the risk of bias for each included study. We resolved any disagreements by discussion or by involving another author.
For each trial, we assessed the following domains:
sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessors;
incomplete outcome data;
selective reporting.
We judged each domain as being at a low, high or unclear risk of bias. We also extracted relevant text that underpinned our judgement and presented this in the risk of bias tables.
We decided to include sponsorship bias because of the high number of sponsored studies. Scientific literature on depression shows some level of sponsorship bias (Cristea 2017), which may be applicable to anxiety.
Measures of treatment effect
Dichotomous data
For binary outcomes, we estimated the risk ratio (RR) and its 95% confidence interval (CI) using a random‐effects model. It has been shown that a random‐effects model has good generalisability (Furukawa 2002), and that a RR is more intuitive than an odds ratio (OR) (Boissel 1999). Furthermore, ORs tend to be interpreted as RRs by clinicians (Deeks 2000). This may lead to an overestimation of the impression of the effect (Higgins 2019).
Continuous data
(1) Summary statistics
Different studies used varied panic rating scales, therefore we used the standardised mean difference (SMD) to pool continuous data. We interpreted the magnitude of SMDs using standard rules of thumb (Cohen 1992). If all included studies used the same instrument, we used the mean difference (MD).
(2) Endpoint versus change data
Trials report results with a combination of endpoint means and change from baseline means of assessment rating scales. We preferred to use endpoint data, which typically cannot have negative values and are easier to interpret from a clinical point of view. If endpoint data were unavailable, we extracted the change from baseline data in separate analyses. If we used the MD, we pooled results from change from baseline and endpoint data in the same analysis.
Considering that clinical trials for panic disorder are usually small, and that data distribution is difficult to assess for studies with small samples, in this review we gave priority to the use and analysis of dichotomous variables both for efficacy and acceptability. Where outcome data or standard deviations (SDs) were not recorded, we asked authors to supply the data. When only the standard error (SE) or t‐statistics or P values were reported, we calculated SDs according to Altman 1996. In the absence of data from the authors, we calculated the mean value of known SDs from the group of included studies according to Furukawa 2006. We checked that the original SDs were properly distributed, so that the imputed SD represented the average.
Relative treatment rankings
We estimated the mean rank (and their 95% CrIs) for all treatments.
Unit of analysis issues
Cluster‐randomised trials
In cluster‐randomised trials, groups of individuals rather than individuals are randomised to different interventions. If we identified cluster placebo‐controlled randomised trials, we appropriately analysed these data by taking into account intraclass correlation coefficients to adjust for cluster effects. Where trialists had not adjusted for the effects of clustering, we attempted to do this by obtaining an intracluster correlation coefficient and then following the guidance given in chapter 16.3.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019).
Cross‐over trials
Cross‐over trials are trials in which all participants receive both the control and intervention treatment but in a different order. The major problem is a carry‐over effect from the first phase to the second phase of the study, especially if the condition of interest is unstable (Elbourne 2002). As this is the case with panic disorder, we included randomised cross‐over studies but only used data up to the point of the first cross‐over.
Studies with multiple treatment groups
Multi‐arm studies where the same medication at different doses is compared remained intact with no adjustments to the numerator or denominator of the shared intervention group. We accounted for the correlation between the effect sizes from multi‐arm studies using the approach suggested in Higgins 1996 and Dias 2013a.
Dose‐ranging studies
We also included dose‐ranging studies, where different doses of the same medication are compared to each other, and pooled the different dose arms, considering them to be one as long as they were within the standard range (see above).
Dealing with missing data
We tried to contact the study authors for all relevant missing data.
(1) Dichotomous outcomes
We calculated response, or remission on treatment, using an intention‐to‐treat analysis (ITT). We followed the principle 'once randomised, always analysed'. Where participants left the study before the intended endpoint, we assumed that they would have experienced the negative outcome. When dichotomous outcomes were not reported but the baseline mean and SD on a panic disorder scale were reported, we calculated the number of responding or remitted participants according to a validated imputation method (Furukawa 2005). We analysed the validity of the above approach by sensitivity analysis. If necessary, we contacted the authors of studies to obtain data or clarification (or both).
(2) Continuous outcomes
Concerning continuous data, the CochraneHandbook for Systematic Reviews of Interventions recommends avoiding imputation of continuous data and suggests using the data as presented by the original authors (Higgins 2019). Where ITT data were available, we preferred ITT to 'per‐protocol analysis'. If necessary, we contacted the authors of studies to obtain data or clarification (or both).
(3) Skewed or qualitative data
Where available we presented skewed and qualitative data descriptively.
We considered several strategies for skewed data. If papers reported a mean and SD and there was also an absolute minimum possible value for the outcome, we divided the mean by the SD. If this was less than 2, then we concluded that there was some indication of skewness. If it was less than 1 (that is the SD was bigger than the mean) then there was almost certainly skewness. If papers had not reported the skewness and simply reported means, SDs and sample sizes, we used these numbers. Because there is a possibility that these data may not have been properly analysed, and can also be misleading, we conducted analyses with and without these studies. If the data have been log‐transformed for analysis, and the geometric means are reported, skewness will be reduced. This is the recommended method of analysis of skewed data (Higgins 2019). If papers used non‐parametric tests and described averages using medians, they could not be formally pooled in the analysis. We followed the recommendation in the CochraneHandbook for Systematic Reviews of Interventions that results of these studies be reported in a table in our review, along with all other papers (Higgins 2019). This means that the data will not be lost from the review and the results can be considered when drawing conclusions, even if they cannot be formally pooled in the analyses.
(4) Missing statistics
When only P or SE values were reported, we calculated SDs (Altman 1996). In the absence of supplementary data after requests to the study authors, we calculated the SDs according to a validated imputation method (Furukawa 2006). We examined the validity of these imputations in the sensitivity analyses.
Assessment of heterogeneity
We assumed a homogeneous between‐study variability across studies (Lu 2004). We based the statistical assessment of heterogeneity in the entire network on the magnitude of the heterogeneity standard deviation parameter, Tau², estimated from the model and the 95% prediction interval for the relative treatment effects.
Inconsistency can be considered an additional layer of heterogeneity, which can occur in networks of evidence. It can occur when there is a discrepancy between a direct and indirect estimate of treatment effect. We conducted node‐splitting analyses to identify in greater detail inconsistencies in the network (van Valkenhoef 2016). We conducted these analyses on the two primary outcomes: response to treatment and total dropouts for any reason.
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results. These are described in Section 10 of the CochraneHandbook for Systematic Reviews of Interventions (Higgins 2019). We examined small‐study effects in the network, including publication bias, through network meta‐regression (Chaimani 2012); see the Sensitivity analysis section below for further details.
Assessment of transitivity across treatment comparisons
Transitivity characterises a network of interventions when the distributions of potential effect modifiers (as described above) are balanced across all pair‐wise comparisons. Transitivity can be interpreted as the extension of the clinical and methodological heterogeneity across the network of different comparisons, and is necessary to ensure a valid network meta‐analysis. We evaluated transitivity in this review as follows:
We assessed whether the included interventions were similar when they were evaluated in RCTs with different designs; for example, whether antidepressants were administered in the same way in studies comparing antidepressants to placebo and in those comparing antidepressants to benzodiazepines.
We compared the distribution of the potential effect modifiers across the different pair‐wise comparisons.
Data synthesis
We conducted random‐effects network meta‐analyses (NMAs) comparing three or more interventions across a network of studies. NMAs combine together both direct (interventions compared in trials) and indirect evidence (interventions not compared directly in trials but part of the network) (Higgins 2019). We conducted all NMAs in a Bayesian framework, and took into account the correlations induced by multi‐arm trials, using WinBUGS 1.4.3 (Winbugs 2012) or OpenBUGS (Lunn 2009). We used standard non‐informative priors based on published WinBUGS code (Dias 2013a).
We initially considered three possible models:
A class (lumped) model (i.e. antidepressants (ADs) and benzodiazepines (BDZs) were compared with each other and with placebo).
An individual treatment model (i.e. all ADs and BDZs listed in the 'Types of interventions' section were compared with each other and with placebo).
A hierarchical model (class‐effects) where we included both class and treatments.
We concluded that it was feasible to conduct individual‐effects and class‐effects models. We initially compared goodness of fit statistics of these models. We measured goodness of fit of the model to the data by the posterior mean of the residual deviance. This is defined as the difference between the deviance for the fitted model and the deviance for the saturated model, where deviance measures the fit of the model to the data points using the likelihood function. We assessed convergence using two chains and based on the Brooks‐Gelman‐Rubin diagnostic tool in WinBUGS.
Where neither individual‐effects nor class‐effects models fitted the data adequately, we explored potential sources of heterogeneity, inconsistency and risk of bias.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses are often exploratory in nature and should be interpreted cautiously: firstly, because they often involve multiple analyses leading to false‐positive results, and secondly because these analyses lack power and are more likely to result in false‐negative results. Therefore, we explored heterogeneity using the following covariates in the network meta‐analyses for the two primary outcomes.
People with panic disorder without agoraphobia versus people with panic disorder and agoraphobia.
Date: we included the publication year as a continuous variable, centred on the mean date. An earlier review noted evidence of attrition bias in earlier studies of benzodiazepines (Breilmann 2019). Design and statistical analyses of clinical trials have changed over time; we therefore assessed if this was a source of heterogeneity.
Placebo response: related to the earlier point, Breilmann 2019 found that trials of benzodiazepines may underestimate placebo response rates. In addition, the onset of action differed between interventions (e.g. SSRIs, TCAs, benzodiazepines) included in the network. Therefore, this may be a source of heterogeneity in placebo response that may impact on the network. We included placebo response as a random effect, allowing response rates to differ by intervention.
Sensitivity analysis
The following sensitivity analyses were planned a priori. We examined whether the results changed and checked for the robustness of the observed findings using the following.
Excluding trials with imputed response rate.
Excluding studies using an ad hoc outcome scale versus studies using a validated scale, such as the Panic Disorder Severity Scale (PDSS), Clinical Global Impression Severity Scale, and Clinical Global Impression Change Scale (for response and remission outcomes only).
-
Conducting bias‐adjustment models for the two primary outcomes (Dias 2013b). The following models were fitted:
Bias adjustment: an initial exploration of the data suggested there may be differences between small and large studies. To estimate the influence of small‐study effects on the network meta‐analyses, we examined the association between effect estimates and their variance (small studies usually have larger variances). We also investigated the impact of high risk of bias for each of the domains of the Cochrane risk of bias tool. Analyses were conducted on the primary outcomes using WinBUGS. We assessed the magnitude of the bias parameter along with its 95% credible intervals (CrIs). The impact on relative effects estimates and between‐trial standard deviation were also examined.
Bias arising from missing data: as we have noted above, trial analyses of missing data may have resulted in bias. Therefore, we aimed to estimate the magnitude of 'informative missing parameters' and assess the impact of adjusting for these effects in the network meta‐analyses. We proposed to conduct sensitivity analyses for the two primary outcomes. However, data were not reported in sufficient detail to enable us to conduct these sensitivity analyses.
Summary of findings and assessment of the certainty of the evidence
We created summary of findings tables for the primary outcomes: response and total number of dropouts.
Currently, two methods for evaluating confidence in the results of an NMA have been recommended in the CochraneHandbook for Systematic Reviews of Interventions: CINeMA (CINeMA 2017; Nikolakopoulou 2019) and GRADE working group approaches (Puhan 2014). However, only frequentist NMA estimates are compatible with CINeMA software. The complexity of our analyses required modelling to be conducted in a Bayesian framework. Therefore, we were unable to use the CINeMA approach in our review. There are also potential limitations with the Puhan 2014 approach, noted in a recent paper (Phillippo 2019). Since confidence ratings are based on individual pairwise comparisons, rather than the network as a whole, applying this method could have potentially generated logically incoherent judgements in some contexts.
We therefore used threshold analyses to explore the impact of potential biases and evaluate the confidence in our NMA estimates (Phillippo 2018; Phillippo 2019). We conducted threshold analyses at the contrast level (Phillippo 2019). We judged a clinically important effect to consist of OR = 0.67 or OR = 1.50 compared with placebo for both primary outcomes. Some concerns about imprecision were indicated by a 95% CrI exceeding 0.67 or 1.50, depending on effect direction. Major concerns about imprecision were indicated by a 95% CrI exceeding both 0.67 and 1.50. We estimated invariant intervals where any changes (at the contrast level) within this threshold would not impact our conclusions on the precision of our NMA estimates.
To assess the impact of risk of bias, we conducted meta‐regression analyses to examine whether each of the domains of the risk of bias tool were associated with the outcome.
To assess the impact of heterogeneity, we compared whether findings based on 95% CrIs led to different conclusions than analyses based on 95% prediction intervals (PIs), which capture heterogeneity not taken into account by CrIs. That is, we examined when the 95% CrI was within the invariant interval and the 95% PI extended beyond the invariant interval.
In terms of incoherence, where inconsistency between direct and indirect evidence was identified in our analyses, we assessed the extent to which the conclusions were likely to be robust to these data issues.
Similarly, if indirectness was identified we assessed the likely impact on our conclusions based on the estimated invariant intervals. However, indirectness was not identified in any analyses.
We formally checked the presence of publication bias with visual inspection of funnel plots in the head‐to‐head published Cochrane Review (Bighelli 2018).
Results
Description of studies
Results of the search
Searching and selection of studies has been done in the previous Cochrane head‐to‐head comparison reviews on antidepressants and benzodiazepines in panic disorder (Bighelli 2016), antidepressants versus placebo in panic disorder (Bighelli 2018) and benzodiazepines versus placebo in panic disorder (Breilmann 2019). This NMA includes all the studies selected in those reviews. We carried out two new searches on 1 February 2021 and 26 May 2022. No new studies in addition to the ones already included in the previous Cochrane head‐to‐head comparison reviews were found after the two new searches.
The number of records identified by the searches was 3677 and 3199 remained after de‐duplication. We excluded 3013 references after assessment of titles and abstracts. We retrieved 186 full‐text articles for full inspection. Of these, 116 studies were excluded. Finally, 70 trials, including 12,703 participants, were included in the review. See Figure 1 for a PRISMA flow diagram (Moher 2009) depicting the study selection process.
1.
Included studies
We included 70 trials in this review (see Characteristics of included studies and Figure 1).
Sample sizes
The sample sizes ranged between 5 and 445 participants in each arm. Total sample size per study ranged from 10 to 1168. Thirty‐five studies had sample sizes of over 100 participants.
Setting
A total of 29 trials enrolled only outpatients, three trials enrolled only inpatients, and both inpatients and outpatients were enrolled in three trials. For the remaining 35 trials, the setting was unclear.
Thirty‐three trials were conducted in the USA, four in the Netherlands, two in Italy, four in Canada, three in Brazil, two in China, two in the UK, four in Japan and one in Finland; 13 trials were multinational and two did not provide information about the country.
Participants
The proportion of women ranged from 40% to 90%. The mean age of participants ranged from 32 to 46 years.
Interventions
Fifty‐two trials included two arms, while the remaining studies had three arms. Eight trials included a comparison between antidepressants and benzodiazepines, 15 between individual antidepressants and two trials between individual benzodiazepines. Fifty‐five trials had a placebo arm.
Duration of the intervention
Intervention duration ranged from 4 to 24 weeks.
Outcomes
Fifty trials reported data on response rates, while the number of dropouts for any reason was reported in 64 trials. Thirty‐six trials reported on remission rates, 37 trials reported data on panic symptoms, 40 on frequency of panic attacks and 25 on agoraphobia outcomes.
Excluded studies
There are 116 excluded studies. The most common reason for exclusion was that participants did not meet our inclusion criteria for panic disorder (51 studies). The next most common reason for exclusion was not meeting our study design criteria (31 studies), then comparator not meeting our inclusion criteria (13 studies). Intervention inclusion criteria were not met in 15 studies, one study was conducted in a population that did not meet our inclusion criteria and, finally, five studies did not provide sufficient data to be included in our review (see Characteristics of excluded studies and Figure 1).
Risk of bias in included studies
For details of the risk of bias judgements for each study, see the Characteristics of included studies table. Graphical representations of the overall risk of bias in the included studies are presented in Figure 2 and Figure 3.
2.

3.

Allocation
Allocation concealment and random sequence generation were rarely reported in sufficient detail. For random sequence generation, we rated only four studies at low risk of bias. We rated all other studies at unclear risk of bias. For allocation concealment, we rated only five studies at low risk of bias. We rated all other studies at an unclear risk of bias.
Blinding
We judged 26 studies at low risk of bias for blinding of participants and personnel. We judged two studies to be at high risk of bias. All the other studies had unclear risk of bias.
We judged 14 studies at low risk of bias for blinding of outcome assessment and one study at high risk of bias. We judged all the other studies to be at unclear risk of bias.
Incomplete outcome data
We judged 17 studies to be at low risk of bias for incomplete outcome data. We judged 24 studies to be at high risk of bias and all the other studies to be at unclear risk of bias.
Selective reporting
We judged 27 studies to be at low risk of bias for selective reporting. We judged 24 studies to be at high risk of bias. We judged all the other studies to be at unclear risk of bias.
Other potential sources of bias
We judged eight studies to be at low risk of bias. We judged 35 studies to be at high risk of bias. All the other studies were at unclear risk of bias. The most common reason for studies to be at high risk of bias was potential or actual sponsorship bias.
Effects of interventions
A. Primary outcomes
Response
Model selection
Figure 4 presents a network plot for each individual treatment compared with placebo and other interventions. Nodes and width of the edges are weighted by sample size. Forty‐eight RCTs and 10,118 participants are included in the main NMA. Results from Figure 4 are commented on below.
4.
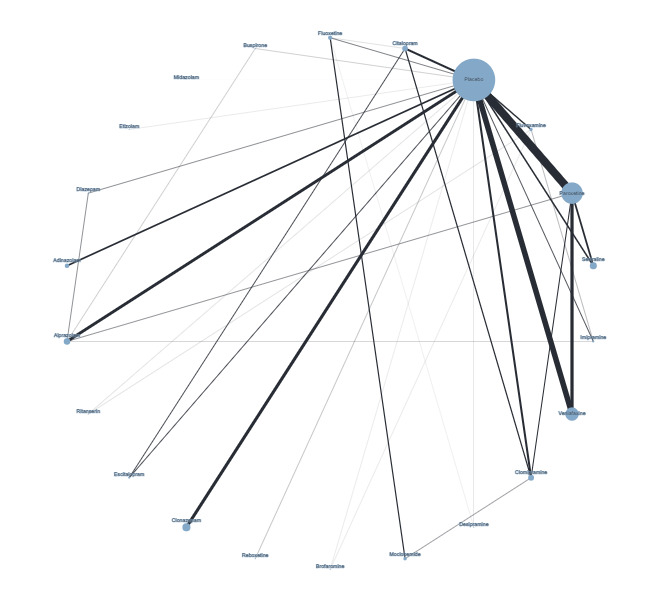
Network plot for response outcome (node size and edge width weighted by sample size)
Table 3 summarises the model selection process. We began by fitting the two models proposed in our protocol: an individual‐effects model of antidepressants and benzodiazepines and a class‐effects model that included individual medications but also allowed clustering between treatments from the class. Neither model fitted the data well, therefore we assessed goodness of fit for individual‐effects models with a covariate for publication date, adjustment for baseline risk and bias adjustment for small studies models.
1. Model selection for non‐response outcome.
| Model | Deviance information criterion | Total residual deviance |
| Individual‐effects model | 669.12 | Mean = 123.7, data points = 107 |
| Class‐effects model | 678.58 | Mean = 136.0, data points = 107 |
| Individual‐effects model adjusting for small‐study effects (variance) | 664.55 | Mean = 108.6, data points = 107 |
| Individual‐effects model adjusting for baseline risk | 673.2 | Mean = 112.4, data points = 107 |
| Individual‐effects model adjusting for risk of bias in attrition and selective reporting | 679.317 | Mean = 120.7, data points = 107 |
| Individual‐effects model adjusting for publication date | 689.96 | Mean = 126.3, data points = 107 |
| Individual‐effects model adjusting for use of validated measures | 679.91 | Mean = 124.0 data points = 107 |
The model that included a covariate for publication date did not substantially improve goodness of fit. However, models adjusting for baseline risk or small‐study effects fitted the data better than either the individual‐effects or class‐effects models. However, the bias adjustment model had a lower between‐study standard deviation (SD 0.28, 95% CrI 0.05 to 0.50) than the baseline risk model (SD 0.54, 95% CrI 0.35 to 0.78). The bias adjustment model (mean = 108.6) also had a lower total residual deviance than the baseline risk model (mean = 112.4); we therefore selected this model for our main results.
We ran models for an initial 50,000 iterations and confirmed that the model had reached convergence. We discarded the initial 50,000 iterations and ran the model for 100,000 further iterations.
Assessment of transitivity: node‐splitting analyses and inspection of residual deviances
Consistent with the protocol, to aid model selection we first explored the potential for inconsistency (transitivity) between direct and indirect evidence using node‐splitting analyses.
There was evidence of inconsistency for the brofaromine–fluvoxamine‐placebo loop (brofaromine versus placebo, P = 0.001; fluvoxamine versus placebo, P = 0.008; brofaromine versus fluvoxamine, P = 0.001). For further details, please see Appendix 3. This is consistent with the residual deviances in the standard NMA model, which also suggested that these trials were outliers. In addition, threshold analyses found that the NMA findings were sensitive to imprecision in the comparison between brofaromine and fluvoxamine (see Figure 5).
5.
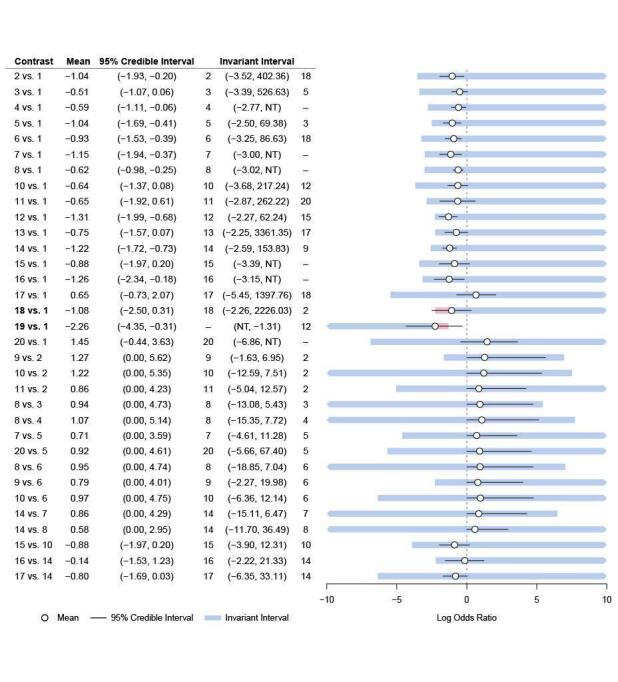
Forest plot for threshold analysis on response
Medications: 1 = placebo, 2 = fluoxetine, 3 = sertraline, 4 = venlafaxine, 5 = fluvoxamine, 6 = clomipramine, 7 = imipramine, 8 = paroxetine, 9 = moclobemide, 10 = citalopram, 11 = desipramine, 12 = clonazepam, 13 = adinazolam, 14 = alprazolam, 15 = escitalopram, 16 = diazepam, 17 = buspirone, 18 = reboxetine, 19 = etizolam, 20 = ritanserin
Given these issues with the brofaromine‐fluvoxamine‐placebo evidence loop, we excluded these studies (Van Vliet 1993; Van Vliet 1996) from the main analysis. In addition, we also identified problematic residual deviances for another study (Schweizer 1992), with only five participants and 100% events in one arm; we therefore also excluded this study from the main analyses.
Of course, it is never possible to affirm the transitivity assumption with certainty. However, the above measures have helped to explore transitivity and to minimise the potential for violation of this assumption.
Meta‐regression analyses
The main purpose of the meta‐regression analyses was to identify potential prognostic factors associated with treatment effect, which may contribute to the risk of intransitivity. We planned to assess the impact of three covariates in meta‐regression analyses (presence of agoraphobia, publication date and placebo response rate). It was not possible to assess the impact of agoraphobia as all studies included participants with this condition (see Table 4). In addition, we planned to adjust for small‐study effects in a sensitivity analysis, but due to poor fit for the models proposed in the protocol this model became our main analyses.
2. Meta‐regression analyses for response outcome.
| Model | Covariates |
Median covariate estimate (95% CrI) |
Between‐study SD1 (95% CrI) |
| Small‐study effects | Variance in individual study (continuous) | ‐1.20 (‐2.59 to 0.46) |
0.28 (0.05 to 0.50) |
| Baseline risk | Baseline risk (continuous) | ‐0.79 (‐1.02 to ‐0.40) |
0.52 (0.32 to 0.75) |
| Risk of bias | Attrition bias (low risk of bias vs unclear or high risk of bias) | ‐0.01 (‐0.57 to 0.48) |
0.54 (0.29 to 0.85) |
| Outcome reporting bias (low risk of bias vs unclear or high risk of bias) | 0.02 (‐0.47 to 0.57) |
||
| Publication date | Publication date (continuous) | ‐0.03 (‐0.06 to 0.04) |
0.45 (0.23 to 0.74) |
| Validated outcome | Validated measure of response (yes vs no) | ‐0.36 (‐0.84 to 0.14) |
0.46 (0.24 to 0.75) |
1Between‐study SD in individual‐effects model without covariates = 0.50 (95% CrI 0.28 to 0.79).
CrI: credible interval; SD: standard deviation
There may be a strong association between the variance in individual studies and response, but the credible intervals were wide (beta ‐1.20, 95% CrI ‐2.59 to 0.46). The bias estimate also suggested that there is likely some variation in effect due to small study bias (Kappa 1.41, 95% CrI 0.15 to 2.98). However, there was a lot of variability in estimating this parameter.
There was a strong association between effect estimates and placebo response rates with a tight CrI (beta ‐0.79, 95% CrI ‐1.02 to ‐0.40). However, the heterogeneity estimate was a little higher than for the no covariate model (SD 0.57, 95% CrI 0.39 to 0.81).
Publication date was not associated with effect estimates (beta ‐0.03, 95% CrI ‐0.06 to 0.04) and had a limited impact on heterogeneity (no covariate model SD 0.50, 95% CrI 0.28 to 0.79; covariate model SD 0.45, 95% CrI 0.23 to 0.74)
Sensitivity analyses
We also identified several methodological factors that may contribute to intransitivity. These are explored below.
1) Excluding trials with imputed response rates
Excluding four trials with imputed response rates did not impact on goodness of fit. For example, the individual‐effects model had a very high total residual deviance (mean 117, from 98 data points), indicating a poor fit with the data. Excluding these studies also did not reduce heterogeneity (SD 0.57, 95% CrI 0.33 to 0.90).
2) Excluding studies using an ad hoc outcome scale versus studies using a validated panic scale
Most trials did not use a validated panic scale, therefore we excluded 30 trials in the sensitivity analyses, leaving only 21 included studies. Total residual deviance remained high (mean 53.46, from 48 data points) and heterogeneity slightly increased (SD 0.58, 95% CrI 0.12 to 1.25).
3) Bias adjustment model (missing data)
Bias adjustment models were not possible as insufficient data were reported in individual trials. Most studies either conducted last observation carried forward (LOCF) analyses or did not report the method of incomplete outcome data management.
See Table 1 for more information.
Main results
Although we focus on the findings of the model adjusting for small‐study effects, comparisons between medications and placebo for the model adjusting for baseline risk are also provided in Table 5.
3. Summary results comparing interventions with placebo for non‐response (sorted by mean rank, equivalence range and invariant range).
| Intervention | RR (95% CrI): small‐study effects | OR (95% CrI): small‐study effects | Mean rank (95% CrI): small‐study effects | RR (95% CrI): baseline risk | Mean rank (95% CrI): baseline risk | No. trials | Sample size |
| 95% CrI does not cross equivalence range or invariant range | |||||||
| Diazepam | 0.65 (0.28 to 0.96) |
0.33 (0.14 to 0.82) |
3 (1 to 15) |
0.67 (0.24 to 0.99) |
7 (1 to 17) |
1 | 160 |
| Alprazolam | 0.68 (0.39 to 0.92) |
0.37 (0.23 to 0.61) |
4 (1 to 11) |
0.60 (0.31 to 0.89) |
5 (2 to 11) |
7 | 895 |
| Clonazepam | 0.71 (0.41 to 0.94) |
0.40 (0.24 to 0.71) |
5 (1 to 14) |
0.63 (0.30 to 0.91) |
6 (2 to 13) |
5 | 938 |
| Paroxetine | 0.85 (0.64 to 0.97) |
0.60 (0.45 to 0.82) |
11 (6 to 16) |
0.75 (0.46 to 0.95 |
10 (4 to 16) |
8 | 1635 |
| Venlafaxine | 0.84 (0.60 to 0.97) |
0.58 (0.41 to 0.84) |
11 (4 to 17) |
0.71 (0.34 to 0.97) |
8 (2 to 17) |
4 | 1693 |
| Clomipramine | 0.85 (0.57 to 0.99) |
0.60 (0.37 to 0.96) |
11 (4 to 17) |
0.72 (0.40 to 0.94) |
9 (3 to 15) |
4 | 468 |
| Fluoxetine | 0.78 (0.42 to 1.00) |
0.50 (0.24 to 0.99) |
8 (2 to 17) |
0.57 (0.14 to 0.97) |
4 (1 to 16) |
1 | 180 |
| Adinazolam | 0.82 (0.50 to 1.00) |
0.54 (0.29 to 0.99) |
9 (2 to 17) |
0.72 (0.33 to 0.98) |
9 (2 to 17) |
2 | 517 |
| 95% CrI crosses equivalence range but not invariant range | |||||||
| Escitalopram | 0.78 (0.40 to 1.03) |
0.48 (0.21 to 1.11) |
8 (1 to 18) |
0.93 (0.41 to 1.32) |
16 (3 to 19) |
1 | 254 |
| Imipramine | 0.82 (0.40 to 1.09) |
0.54 (0.20 to 1.38) |
9 (2 to 18) |
0.83 (0.41 to 1.07) |
13 (3 to 18) |
2 | 147 |
| Fluvoxamine | 0.86 (0.53 to 1.05) |
0.62 (0.32 to 1.20) |
12 (3 to 18) |
0.71 (0.34 to 0.97) |
10 (3 to 16) |
5 | 450 |
| Citalopram | 0.87 (0.57 to 1.02) |
0.62 (0.37 to 1.09) |
12 (3 to 18) |
0.89 (0.54 to 1.07) |
15 (7 to 18) |
2 | 628 |
| Sertraline | 0.89 (0.67 to 1.02) |
0.67 (0.43 to 1.07) |
13 (6 to 18) |
0.84 (0.58 to 0.99) |
13 (6 to 17) |
3 | 470 |
| 95% CrI crosses equivalence range in both directions but not invariant range | |||||||
| Desipramine | 0.94 (0.43 to 1.37) |
0.82 (0.22 to 3.01) |
15 (2 to 20) |
0.69 (0.22 to 1.05) |
7 (1 to 18) |
1 | 56 |
| Buspirone | 1.14 (0.48 to 2.06) |
2.40 (0.32 to 14.3) |
19 (2 to 20) |
1.13 (0.76 to 1.88) |
19 (12 to 20) |
1 | 67 |
| Ritanserin | 1.19 (0.01 to 2.70) |
10. 43 (0.04 to 2807) |
20 (1 to 20) |
1.18 (0.46 to 2.23) |
20 (3 to 20) |
1 | 39 |
| 95% CrI crosses equivalence and/or invariant ranges | |||||||
| Etizolam | 0.58 (0.03 to 1.43) |
0.29 (0.01 to 5.69) |
2 (1 to 20) |
0.37 (0.05 to 0.92) |
1 (1 to 15) |
1 | 30 |
| Reboxetine* | 0.77 (0.24 to 1.19) |
0.46 (0.10 to 1.86) |
7 (1 to 19) |
0.85 (0.32 to 1.20) |
13 (2 to 19) |
1 | 82 |
*Does not cross invariant range in baseline risk model.
CrI: credible interval; OR: odds ratio; RR: risk ratio
Most medications were more effective than placebo. The following medications were effective and 95% CrIs did not cross the equivalence range:
diazepam (RR 0.65, 95% CrI 0.28 to 0.96; mean rank 3, 95% CrI 1 to 15);
alprazolam (RR 0.68, 95% CrI 0.39 to 0.92; mean rank 4, 95% CrI 1 to 11);
clonazepam (RR 0.71, 95% CrI 0.41 to 0.94; mean rank 6, 95% CrI 2 to 13);
paroxetine (RR 0.85, 95% CrI 0.64 to 0.97; mean rank 11, 95% CrI 6 to 16);
venlafaxine (RR 0.84, 95% CrI 0.60 to 0.97; mean rank 11, 95% CrI 4 to 17).
The following medications were more effective than placebo but 95% CrIs crossed the equivalence range:
escitalopram (RR 0.78, 95% CrI 0.39 to 1.03; mean rank 8, 95% CrI 1 to 18);
fluoxetine (RR 0.78, 95% CrI 0.43 to 1.00; mean rank 8, 95% CrI 2 to 17);
adinazolam (RR 0.82, 95% CrI 0.49 to 1.00; mean rank 9, 95% CrI 2 to 17);
imipramine (RR 0.82, 95% CrI 0.40 to 1.09; mean rank 9, 95% CrI 2 to 18);
clomipramine (RR 0.85, 95% CrI 0.57 to 0.99; mean rank 11, 95% CrI 4 to 17);
fluvoxamine (RR 0.86, 95% CrI 0.53 to 1.05; mean rank 12, 95% CrI 3 to 18);
citalopram (RR 0.87, 95% CrI 0.57 to 1.02; mean rank 12, 95% CrI 3 to 18);
sertraline (RR 0.89, 95% CrI 0.66 to 1.02; mean rank 13, 95% CrI 6 to 18).
For three medications, 95% CrIs crossed the equivalence range in both directions but not the invariant range:
desipramine (RR 0.94, 95% CrI 0.43 to 1.37; mean rank 15, 95% CrI 2 to 20);
buspirone (RR 1.14, 95% CrI 0.48 to 2.06; mean rank 19, 95% CrI 2 to 20);
ritanserin (RR 1.19, 95% CrI 0.01 to 2.70; mean rank 20, 95% CrI 1 to 20).
For two medications, 95% CrIs crossed both the equivalence range and the invariant range:
etizolam (RR 0.58, 95% CrI 0.03 to 1.43; mean rank 2, 95% CrI 1 to 20);
reboxetine (RR 0.77, 95% CrI 0.24 to 1.19; mean rank 7, 95% CrI 1 to 19).
Threshold analysis
Risk of bias: meta‐regression analyses did not find an association between effect estimates and domains (attrition bias and outcome reporting bias) judged to potentially be at risk of bias.
Imprecision: imprecision of findings was of potential concern; 95% CrIs crossed our a priori determined equivalence range for most comparisons (e.g. escitalopram versus placebo, fluoxetine versus placebo, adinazolam versus placebo). There were particular concerns about imprecision for three comparisons (desipramine versus placebo, buspirone versus placebo, ritanserin versus placebo) since the 95% CrIs crossed the equivalence range in both directions.
However, threshold analyses suggested that the NMA findings were robust to imprecision for most comparisons. Although the findings were sensitive to imprecision for the following comparisons (see Figure 5), this imprecision is unlikely to impact on effect estimates for other medications in the network as they were both based on one small RCT and not directly compared with other medications:
etizolam versus placebo: OR 0.28 (95% CrI 0.01 to 5.69); RR 0.58 (95% CrI 0.03 to 1.43);
reboxetine versus placebo: OR 0.46 (95% CrI 0.10 to 1.86); RR 0.77 (95% CrI 0.24 to 1.19).
Heterogeneity: for the following comparisons the prediction interval (PI), but not the credible interval, crossed our a priori equivalence range, suggesting a potential concern about heterogeneity:
fluoxetine versus placebo (OR 0.50, 95% PI 0.19 to 1.22);
sertraline versus placebo (OR 0.60, 95% PI 0.24 to 1.51);
venlafaxine versus placebo (OR 0.58, 95% PI 0.29 to 1.20);
fluvoxamine versus placebo (OR 0.61, 95% PI 0.26 to 1.52);
clomipramine versus placebo (OR 0.61, 95% PI 0.28 to 1.28);
imipramine versus placebo (OR 0.53, 95% PI 0.17 to 1.65);
paroxetine versus placebo (OR 0.59, 95% PI 0.31 to 1.22);
adinazolam versus placebo (OR 0.54, 95% PI 0.23 to 1.26);
sertraline versus paroxetine (OR 0.90, 95% PI 0.36 to 2.26);
paroxetine versus alprazolam (OR 0.61, 95% PI 0.23 to 1.55).
However, the threshold analysis indicated that heterogeneity was unlikely to impact on the NMA findings. Prediction intervals remained within the invariant intervals for all three comparisons (see Figure 5).
Indirectness: we identified several factors that may impact on the directness of the evidence:
In some studies, the placebo arm had more dropouts than the active treatment arm.
Different methodology is used in newer studies compared to older studies.
Some studies have used validated measures, while some others have used clinician judgement.
Some medications, for example etizolam, are not widely used in practice.
Incoherence (transitivity): node‐splitting analyses found evidence of incoherence for the brofaromine‐fluvoxamine‐placebo loop. However, since studies within this evidence loop were excluded from the main analyses there were no longer concerns about potential incoherence.
Small‐study effects: we found evidence of small‐study bias. The base‐case models proposed in our protocol all fitted the data poorly. However, analyses adjusted for the magnitude of the variance of individual studies substantially improved model fit. This suggests that findings from studies with larger sample sizes (and smaller variances) may have differed from smaller studies (and larger variances). However, it is unclear whether the NMA findings were impacted by any residual bias.
Dropout
Figure 6 presents a network plot for each individual treatment compared with placebo and other interventions. Nodes were weighted by the number of studies and width of the edges was weighted by the inverse of the variance. Sixty‐four RCTs, including 12,310 participants, were included in the NMA. Results from Figure 6 are commented on below.
6.
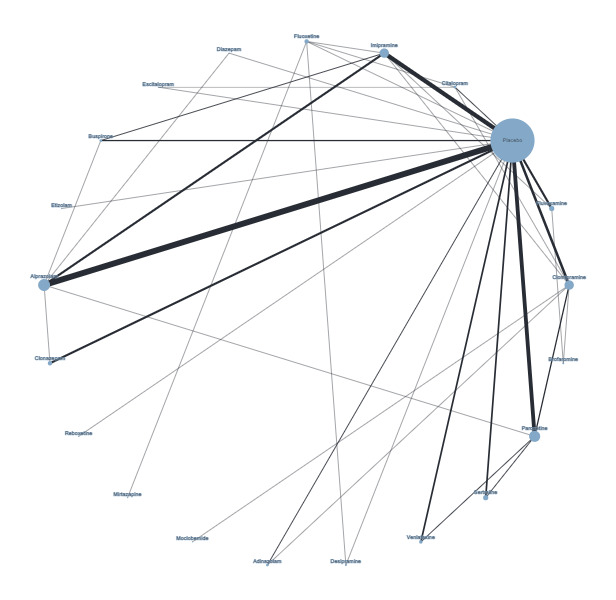
Network plot for dropout (node size and edge width weighted by number of studies)
We fitted two models: one that included individual antidepressants and benzodiazepines and a class‐effects model that included medications but also allowed clustering between treatments from the class. Neither model fitted the data well, for example total residual deviance was much higher than the number of data points (see Table 6). Therefore, we selected the model with adjustment for small‐study effects for the main results.
4. Model selection for dropout outcome.
| Model | DIC | Total residual deviance |
SD (95% CrI) |
| Individual‐effects model | 844.64 | Mean = 172.2, data points = 146 | 0.24 (0.04 to 0.46) |
| Class‐effects model | 840.91 | Mean = 169.6, data points = 146 | 0.27 (0.06 to 0.48) |
| Adjustment for small studies | 831.46 | Mean = 149.1, data points = 146 | 0.18 (0.01 to 0.37) |
CrI: credible interval; DIC: deviation information criterion; SD: standard deviation
We ran models for an initial 50,000 iterations and confirmed that the model had reached convergence. We discarded the initial 50,000 iterations and ran the model for 100,000 further iterations.
Node‐splitting analyses: assessment of transitivity
Consistent with the protocol, to aid model selection we first explored the potential for inconsistency between direct and indirect evidence using node‐splitting analyses. There were a number of inconsistencies identified between direct and indirect evidence: fluoxetine versus placebo (P = 0.03), sertraline versus placebo (P = 0.04), sertraline versus paroxetine (P = 0.04), fluvoxamine versus imipramine (P = 0.03), desipramine versus placebo (P = 0.03), desipramine versus fluoxetine (P = 0.03), clonazepam versus alprazolam (P = 0.03), sertraline versus paroxetine (P = 0.04), fluvoxamine versus imipramine (P = 0.05). In addition, the difference between clomipramine versus paroxetine (P = 0.06) was borderline statistically significant. For further details, please see Appendix 4.
Meta‐regression analyses
The main purpose of the meta‐regression analyses was to identify potential prognostic factors associated with treatment effect that may contribute to the risk of intransitivity. We planned to assess the impact of three covariates in meta‐regression analyses (presence of agoraphobia, publication date and placebo response rate). It was not possible to assess the impact of agoraphobia as all studies included participants with this condition (see Table 4). In addition, we planned to adjust for small‐study effects in a sensitivity analysis, but due to poor fit for the models proposed in the protocol, this model became our main analyses.
Meta‐regression analyses were only possible for the association between effect estimates and the size of variance in individual studies. There was a strong association between the variance for included trials and the effects (beta ‐1.07, 95% CrI ‐1.77 to ‐0.38; kappa = 0.71, 95% CrI 0.09 to 1.55). There was a more precise estimate of small‐study bias for dropouts, with both lower and upper credible interval suggesting substantial bias. When comparing the smallest to the largest study the exaggeration of effect is estimated to be 3.207 on a logOR scale and 24.705 on an OR scale. As above, this is potentially an overestimate of the likely bias as reflected by relatively wide credible intervals. However, even at the lower credible interval there is a strong suggestion of bias (exaggeration can vary between 3.61 and 184.69 on an OR scale based on credible intervals).
It was not possible to conclude anything regarding the association between the other covariates and effect estimates. All the meta‐regression models crashed for this outcome. The likely causes of these problems were a number of studies with zero events as well as other studies with a very low number of events. Therefore, we fitted models with a continuity correction (adding 0.5 to all cells in the 2 x 2 table in studies with no events in either intervention or control). We also fitted models with varying priors for the heterogeneity parameter (a minimally informative prior with large variance compared with an informative prior for mental health studies). It was not possible to run either of these models. We then excluded studies from the analyses where there were zero events either in the intervention or control group. However, there still remained a number of studies with a small number of events and this model also failed to run.
See Table 2 for more information.
Main results
Most medications were either associated with a reduced or similar proportion of participants who dropped out, compared to placebo (see Table 7).
5. Summary results comparing interventions with placebo for dropout (sorted by mean rank, equivalence range and invariant range).
| Intervention | RR (95% CrI) | OR (95% CrI) | Mean rank (95% CrI) | No. trials | Sample size: participants |
| 95% CrI does not cross equivalence range or invariant interval | |||||
| Alprazolam | 0.46 (0.33 to 0.66) |
0.37 (0.28 to 0.50) |
3 (1 to 6) |
14 | 1979 |
| Diazepam | 0.50 (0.23 to 0.91) |
0.39 (0.17 to 0.87) |
4 (1 to 9) |
1 | 160 |
| Venlafaxine | 0.99 (0.80 to 1.21) |
0.98 (0.73 to 1.33) |
12 (7 to 18) |
4 | 1693 |
| Sertraline | 1.01 (0.81 to 1.31) |
1.01 (0.71 to 1.44) |
13 (7 to 19) |
4 | 647 |
| Paroxetine | 1.07 (0.92 to 1.07) |
1.11 (0.89 to 1.39) |
15 (10 to 19) |
8 | 2524 |
| Buspirone | 1.83 (1.17 to 3.34) |
3.36 (1.25 to 9.10) |
21 (18 to 21) |
3 | 170 |
| 95% CrI crosses equivalence range but not invariant interval | |||||
| Reboxetine | 0.40 (0.13 to 1.17) |
0.40 (0.13 to 1.17) |
4 (1 to 15) |
1 | 82 |
| Escitalopram | 0.68 (0.38 to 1.08) |
0.59 (0.30 to 1.12) |
6 (2 to 15) |
1 | 254 |
| Imipramine | 0.85 (0.63 to 1.12) |
0.78 (0.55 to 1.18) |
8 (5 to 16) |
9 | 1207 |
| Citalopram | 0.88 (0.62 to 1.20) |
0.83 (0.53 to 1.31) |
9 (5 to 17) |
2 | 628 |
| Clonazepam | 0.94 (0.74 to 1.13) |
0.88 (0.55 to 1.36) |
10 (5 to 18) |
5 | 959 |
| Clomipramine | 0.97 (0.74 to 1.24) |
0.94 (0.67 to 1.38) |
11 (6 to 17) |
7 | 720 |
| Fluvoxamine | 1.17 (0.85 to 1.66) |
1.28 (0.75 to 2.10) |
17 (8 to 20) |
4 | 467 |
| Adinazolam | 1.19 (0.87 to 1.69) |
1.35 (0.82 to 2.20) |
17 (9 to 20) |
2 | 517 |
| 95% CrI crosses equivalence range in both directions but not invariant interval | |||||
| Desipramine | 0.63 (0.14 to 1.70) |
0.54 (0.11 to 2.61) |
5 (1 to 20) |
1 | 56 |
| Fluoxetine | 1.13 (0.60 to 1.90) |
1.24 (0.51 to 2.87) |
16 (5 to 20) |
1 | 180 |
| 95% CrI crosses equivalence range in both directions and invariant interval | |||||
| Etizolam | 0.37 (0.01 to 2.49) |
0.28 (0.01 to 8.29) |
2 (1 to 21) |
1 | 30 |
CrI: credible interval; OR: odds ratio; RR: risk ratio
There was a reduction in dropout rate compared with placebo for the following medications and 95% CrIs did not cross the equivalence range or invariant range:
alprazolam (RR 0.46, 95% CrI 0.33 to 0.65; mean rank 3, 95% CrI 1 to 6);
diazepam (RR 0.50, 95% CrI 0.23 to 0.91; mean rank 3, 95% CrI 1 to 9).
There was no difference in dropout rate compared with placebo for the following medications and 95% CrIs did not cross the equivalence range or invariant range:
venlafaxine (RR 0.99, 95% CrI 0.80 to 1.21; mean rank 12, 95% CrI 6 to 18);
sertraline (RR 1.00, 95% CrI 0.80 to 1.30; mean rank 13, 95% CrI 7 to 18);
paroxetine (RR 1.07, 95% CrI 0.92 to 1.07; mean rank 15, 95% CrI 10 to 19).
For one medication, there was an increased dropout rate compared with placebo that did not cross the equivalence range:
buspirone (RR 1.83, 95% CrI 1.14 to 3.34; mean rank 21, 95% CrI 18 to 21).
Several medications had a reduced dropout rate compared with placebo, and 95% CrIs crossed the equivalence range but not the invariant range:
reboxetine (RR 0.40, 95% CrI 0.13 to 1.17; mean rank 3, 95% CrI 1 to 15);
escitalopram (RR 0.68, 95% CrI 0.38 to 1.08; mean rank 6, 95% CrI 2 to 15);
imipramine (RR 0.85, 95% CrI 0.63 to 1.12; mean rank 8, 95% CrI 4 to 15);
citalopram (RR 0.88, 95% CrI 0.62 to 1.20; mean rank 9, 95% CrI 5 to 17).
There was no difference in dropout rate compared with placebo for the following medications, however 95% CrIs crossed the equivalence range but not the invariant range:
clonazepam (RR 0.92, 95% CrI 0.63 to 1.22; mean rank 10, 95% CrI 5 to 18);
clomipramine (RR 0.96, 95% CrI 0.74 to 1.24; mean rank 11, 95% CrI 6 to 17);
fluvoxamine (RR 1.16, 95% CrI 0.85 to 1.63; mean rank 17, 95% CrI 9 to 20);
adinazolam (RR 1.19, 95% CrI 0.87 to 1.68; mean rank 17, 95% CrI 9 to 20).
For two medications compared with placebo, 95% CrIs were wide and crossed the equivalence range in both directions but not the invariant range:
desipramine (RR 0.63, 95% CrI 0.14 to 1.73; mean rank 5, 95% CrI 1 to 20);
fluoxetine (RR 1.13, 95% CrI 0.62 to 1.94; mean rank 16, 95% CrI 5 to 20).
For one medication compared with placebo, the 95% CrI crossed the equivalence range in both directions and also crossed the invariant range:
etizolam (RR 0.39, 95% CrI 0.01 to 2.69; mean rank 2, 95% CrI 1 to 21).
Threshold analysis
Risk of bias: we were unable to assess the association between effect estimates and the impact of risk of bias. Therefore, it is unclear the extent to which our findings are sensitive to domains (attrition bias and outcome reporting bias) where a substantial proportion of studies were rated at a high or unclear risk of bias.
Imprecision: NMA findings were not sensitive to imprecision for any comparisons, with the exception of etizolam versus placebo, a small trial of 30 participants. It is unlikely that this imprecision substantially impacted on the effect estimates for other medications in the network.
Heterogeneity: there was evidence of substantial heterogeneity. For the following comparisons 95% prediction intervals extended beyond the equivalence range in both directions, whilst 95% credible intervals were within the equivalence range, potentially indicating major concerns with heterogeneity:
sertraline versus placebo (OR 1.00, 95% PI 0.59 to 1.78);
venlafaxine versus placebo (OR 0.98, 95% PI 0.58 to 1.68).
However, the threshold analysis indicated this heterogeneity was unlikely to impact on the NMA findings (Figure 7). For both comparisons 95% PIs remained within the invariant intervals.
7.
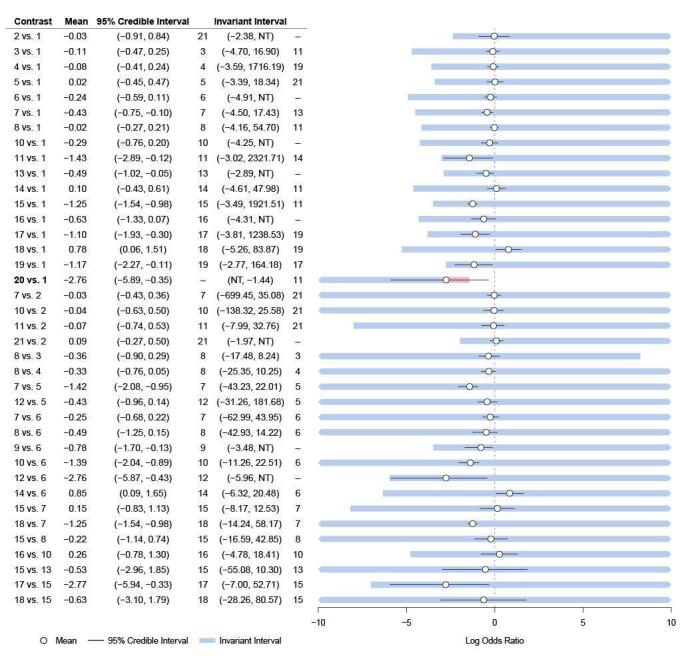
Forest plot of dropout threshold analysis
Medications: 1 = placebo, 2 = fluoxetine, 3 = sertraline, 4 = venlafaxine, 5 = fluvoxamine, 6 = clomipramine, 7 = imipramine, 8 = paroxetine, 9 = moclobemide, 10 = citalopram, 11 = desipramine, 12 = clonazepam, 13 = adinazolam, 14 = alprazolam, 15 = escitalopram, 16 = diazepam, 17 = buspirone, 18 = reboxetine, 19 = etizolam, 20 = ritanserin
For several medications, 95% PIs extended beyond the equivalence range in one direction in comparison with 95% CrIs:
clomipramine versus placebo (OR 0.94, 95% PI 0.56 to 1.72);
paroxetine versus placebo (OR 1.11, 95% PI 0.68 to 1.81);
clonazepam versus placebo (OR 0.88, 95% PI 0.46 to 1.61);
alprazolam versus placebo (OR 0.36, 95% PI 0.22 to 0.64);
paroxetine versus sertraline (OR 1.10, 95% PI 0.62 to 1.88);
paroxetine versus venlafaxine (OR 1.13, 95% PI 0.65 to 1.94);
imipramine versus clomipramine (OR 0.83, 95% PI 0.45 to 1.63);
paroxetine versus clomipramine (OR 1.18, 95% PI 0.64 to 2.03);
citalopram versus clomipramine (OR 0.88, 95% PI 0.45 to 1.67);
escitalopram versus citalopram (OR 0.70, 95% PI 0.32 to 1.53).
However, the threshold analysis indicated that this heterogeneity was unlikely to impact on the NMA findings. For all these comparisons 95% PIs remained within the invariant intervals.
Incoherence (transitivity): node‐splitting analyses identified incoherence between direct and indirect evidence for several comparisons. However, the NMA findings were sensitive to incoherence for two comparisons:
Desipramine versus placebo: direct estimates crossed the invariant threshold (log OR ‐2.30, 95% CrI ‐4.40 to ‐0.77) but not indirect estimates (log OR 1.30, 95% CrI ‐1.60 to 4.90) or network estimates (log OR ‐1.5, 95% CrI ‐2.90 to ‐0.23). This incoherence may reflect the instability of estimates in an evidence loop based on two small trials: desipramine versus placebo (N = 56) and desipramine versus fluoxetine (N = 22).
Fluoxetine versus placebo: indirect estimates crossed the invariant threshold (log OR ‐1.60, 95% CrI ‐3.60 to 0.08) but not direct estimates (log OR 0.48, 95% CrI ‐0.38 to 1.40) or network estimates (log OR 0.04, 95% CrI ‐0.72 to 0.81). This incoherence may partly be explained by small‐study effects, since the fluoxetine versus placebo estimate was based on a larger trial (N = 180), whereas head‐to‐head trials between fluoxetine and other medications were mainly based on small trials: versus imipramine (N = 18), citalopram (N = 42), desipramine (N = 22), mirtazapine (N = 30). Therefore, the bias‐adjusted analyses may have reduced the impact of the incoherence in these estimates.
Reporting bias/small‐study effects: we found evidence of small‐study bias, therefore our main findings are based on a model adjusting for this potential bias. The base‐case models proposed in our protocol all fitted the data poorly. However, analyses adjusted for the magnitude of the variance of individual studies led to an acceptable model fit. This suggests that findings from studies with larger sample sizes (and smaller variances) may have differed from smaller studies (and larger variances). However, it is unclear whether the NMA findings were impacted by any residual bias.
B. Secondary outcomes
Remission
Figure 8 presents a network plot for each individual treatment compared with placebo and other interventions. Nodes were weighted by the number of studies and the width of the edges was weighted by the inverse of the variance. Thirty‐two RCTs, including 8569 participants, are included in the NMA.
8.
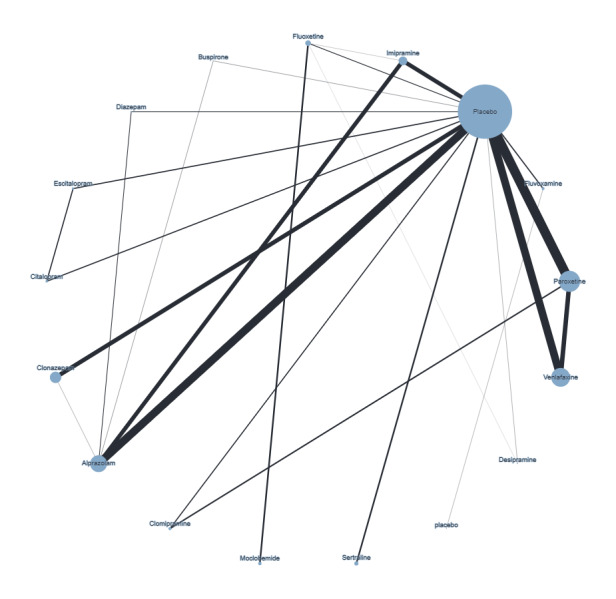
Network plot remission (node size and edge width weighted by sample size)
We began by fitting both individual‐effects and class‐effects models but neither fitted the data well (see Table 8). Given the limited differences between the individual‐level and class‐level models, we focused on the individual‐level model. High deviances (> 2) were observed for five, mostly small, studies (Black 1993; GSK 1994/04; Klosko 1990; Nair 1996; Pohl 1989b). Removing these studies improved the fit of the model. In addition, the between‐study standard deviation was 0.22 (95% CrI 0.02 to 0.42). Since the removal of outliers led to an acceptable fit, we selected this model for further analyses.
6. Model selection for remission outcome.
| Model | Deviance information criterion | Total residual deviance |
| Individual‐effects model | 560.47 | Mean = 95.00, from 88 data points |
| Class‐effects model | 554.86 | Mean = 96.34, from 88 data points |
| Individual‐effects model ‐ outliers removed | 472.19 | Mean = 72.53, from 71 data points |
Table 9 summarises the NMA findings on remission. Most medications were more effective than placebo:
7. Summary results comparing interventions with placebo for remission (sorted by mean rank and equivalence range).
| Comparator |
RR (95% CrI) |
OR (95% CrI | Mean rank (95% CrI) | No. of trials | No. of participants |
| 95% CrI does not cross the equivalence range | |||||
| Desipramine | 0.66 (0.29 to 0.97) |
0.31 (0.11 to 0.89) |
2 (1 to 13) |
1 | 52 |
| Alprazolam | 0.65 (0.44 to 0.84) |
0.31 (0.23 to 0.40) |
2 (1 to 5) |
9 | 1732 |
| Fluoxetine | 0.76 (0.46 to 0.96) |
0.43 (0.22 to 0.84) |
5 (1 to 13) |
1 | 180 |
| Clonazepam | 0.76 (0.53 to 0.92) |
0.43 (0.28 to 0.64) |
5 (1 to 11) |
4 | 940 |
| Diazepam | 0.74 (0.43 to 0.96) |
0.41 (0.20 to 0.82) |
5 (1 to 13) |
1 | 160 |
| Fluvoxamine | 0.77 (0.50 to 0.95) |
0.44 (0.25 to 0.77) |
6 (1 to 12) |
3 | 311 |
| Imipramine | 0.79 (0.57 to 0.94) |
0.48 (0.31 to 0.71) |
7 (2 to 12) |
3 | 904 |
| Venlafaxine | 0.87 (0.70 to 0.96) |
0.61 (0.45 to 0.83) |
10 (5 to 13) |
4 | 1693 |
| Paroxetine | 0.88 (0.71 to 0.97) |
0.62 (0.47 to 0.82) |
10 (6 to 13) |
5 | 2065 |
| 95% CrI crosses equivalence range | |||||
| Sertraline | 0.86 (0.68 to 1.01) |
0.58 (0.33 to 1.02) |
9 (3 to 15) |
2 | 353 |
| Escitalopram | 0.92 (0.65 to 1.09) |
0.73 (0.36 to 1.45) |
12 (3 to 16) |
1 | 254 |
| 95% CrI crosses equivalence range in both directions | |||||
| Citalopram | 0.97 (0.73 to 1.15) |
0.89 (0.44 to 1.79) |
13 (6 to 16) |
1 | 251 |
| Buspirone | 0.99 (0.65 to 1.24) |
0.95 (0.35 to 2.64) |
14 (3 to 16) |
1 | 67 |
| Clomipramine | 1.01 (0.83 to 1.16) |
1.02 (0.58 to 1.81) |
15 (9 to 16) |
1 | 244 |
CrI: credible interval; OR: odds ratio; RR: risk ratio
desipramine (RR 0.66, 95% CrI 0.29 to 0.97; mean rank 2, 95% CrI 1 to 13);
alprazolam (RR 0.65, 95% CrI 0.44 to 0.84; mean rank 2, 95% CrI 1 to 5);
fluoxetine (RR 0.77, 95% CrI 0.46 to 0.96; mean rank 5, 95% CrI 1 to 13);
clonazepam (RR 0.76, 95% CrI 0.53 to 0.92; mean rank 5, 95% CrI 1 to 11);
diazepam (RR 0.74, 95% CrI 0.43 to 0.96; mean rank 5, 95% CrI 1 to 13);
fluvoxamine (RR 0.77, 95% CrI 0.50 to 0.95; mean rank 6, 95% CrI 1 to 12);
imipramine (RR 0.79, 95% CrI 0.57 to 0.94; mean rank 7, 95% CrI 2 to 12);
venlafaxine (RR 0.87, 95% CrI 0.70 to 0.96; mean rank 10, 95% CrI 5 to 13);
paroxetine (RR 0.88, 95% CrI 0.71 to 0.97; mean rank 10, 95% CrI 6 to 13).
Two medications probably were more effective than placebo, but the 95% CrIs crossed our equivalence range:
sertraline (RR 0.86, 95% CrI 0.68 to 1.01; mean rank 9, 95% CrI 3 to 15);
escitalopram (RR 0.92, 95% CrI 0.65 to 1.09; mean rank 12, 95% CrI 3 to 16).
Three medications may be no different from placebo, but there was considerable uncertainty about these estimates as the 95% CrIs crossed our equivalence range in both directions:
citalopram (RR 0.97, 95% CrI 0.73 to 1.15; mean rank 13, 95% CrI 6 to 16);
buspirone (RR 0.99, 95% CrI 0.65 to 1.24; mean rank 14, 95% CrI 3 to 16);
clomipramine (RR 1.01, 95% CrI 0.83 to 1.16; mean rank 15, 95% CrI 9 to 16).
Sensitivity analysis
Most trials did not use a validated panic scale, therefore this sensitivity analysis, excluding unvalidated scales, included only 15 trials and 12 medications:
clonazepam RR 0.20 (95% CrI 0.01 to 0.88);
alprazolam RR 0.56 (95% CrI 0.24 to 0.89);
imipramine RR 0.78 (95% CrI 0.45 to 1.04);
fluoxetine RR 0.80 (95% CrI 0.46 to 1.04);
sertraline RR 0.86 (95% CrI 0.75 to 1.09);
paroxetine RR 0.86 (95% CrI 0.61 to 1.03);
venlafaxine RR 0.87 (95% CrI 0.68 to 1.00);
escitalopram RR 0.92 (95% CrI 0.58 to 1.14);
fluvoxamine RR 0.93 (95% CrI 0.71 to 1.09);
buspirone RR 0.94 (95% CrI 0.52 to 1.21);
citalopram RR 0.98 (95% CrI 0.0.65 to 1.18).
Effect estimates for the remaining treatments did not differ substantially from the main analyses for most treatments. There were two exceptions: in the main analyses there were four trials of clonazepam and only one small trial (N = 24) in the sensitivity analyses (Valenca 2000), which had a very high placebo non‐response rate (90%) that may have led to an overestimate of the effectiveness of clonazepam. In addition, fluvoxamine was less effective compared to placebo in the sensitivity analysis compared with the main analyses. One trial was excluded from the sensitivity analysis (Hoehn‐Saric 1993); this was a relatively small trial (N = 50) and had a very high placebo non‐response rate (84%), which may have led to an overestimate of the effectiveness of fluvoxamine in the main analyses.
Panic scales
Figure 9 presents a network plot for each individual treatment compared with placebo and other interventions for change from baseline. Figure 10 presents a network plot for endpoint scores. Nodes and widths of edges were weighted by sample size. Thirty‐five RCTs, including 8826 participants, were included in the NMAs.
9.
Network plot intervention combinations for panic scales (change from baseline)
10.
Network plot of intervention comparisons included in network meta‐analysis of panic scales (endpoint)
Studies reported change from baseline and/or endpoint scores, therefore we analysed these data separately. We fitted two models: one that included individual antidepressants and benzodiazepines and a class‐effects model that included medications but also allowed clustering between treatments from the class. Both models fitted the data well, therefore we selected the simpler individual‐effects model for both change from baseline (between‐study standard deviation 0.63, 95% CrI 0.33 to 1.30) and endpoint data (between‐study standard deviation 0.46, 95% CrI 0.29 to 0.82) (see Table 10). Below we summarise the NMA endpoint data but for more details and change from baseline data, see Table 11.
8. Model selection for panic scales outcome.
| Model | Deviance information criterion | Total residual deviance |
| Endpoint | ||
| Individual‐effects | 22.17 | Mean = 40.54, from 41 data points |
| Class‐effects | 22.31 | Mean = 42.16, from 41 data points |
| Change from baseline | ||
| Individual‐effects | 49.27 | Mean = 37.89, from 37 data points |
| Class‐effects | 51.37 | Mean = 41.11, from 37 data points |
9. Summary results comparing interventions with placebo for mean score on panic scales.
| Comparator | Endpoint | Change from baseline | No. trials | No. participants | ||
|
SMD (95% CrI) |
Mean rank (95% CrI) |
SMD (95% CrI) |
Mean rank (95% CrI) | |||
| Brofaromine | ‐3.78 (‐5.02 to ‐2.55) |
1 (1 to 2) |
‐ | ‐ | 1 | 29 |
| Clonazepam | ‐2.36 (‐3.27 to ‐1.45) |
2 (1 to 3) |
‐1.23 (‐2.63 to 0.17) |
3 (1 to 13) |
3 | 101 |
| Reboxetine | ‐1.03 (‐2.13 to 0.08) |
3 (2 to 10) |
‐ | ‐ | 1 | 82 |
| Clomipramine | ‐0.68 (‐1.38 to 0.03) |
5 (3 to 9) |
‐1.96 (‐3.27 to ‐0.81) |
1 (1 to 6) |
2 | 210 |
| Alprazolam | ‐0.48 (‐1.19 to 0.24) |
6 (3 to 11) |
‐0.86 (‐1.62 to ‐0.11) |
6 (2 to 11) |
7 | 1255 |
| Imipramine | ‐0.28 (‐1.03 to 0.47) |
7 (3 to 12) |
‐0.57 (‐1.60 to 0.46) |
8 (2 to 14) |
5 | 1032 |
| Paroxetine | ‐0.22 (‐0.69 to 0.25) |
8 (5 to 11) |
‐0.94 (‐1.97 to ‐0.01) |
5 (2 to 12) |
5 | 1968 |
| Fluvoxamine | ‐0.17 (‐0.79 to 0.45) |
8 (4 to 12) |
‐ | ‐ | 3 | 338 |
| Venlafaxine | 0.30 (‐0.39 to 0.99) |
12 (7 to 12) |
‐0.59 (‐1.60 to 0.40) |
8 (2 to 14) |
4 | 1693 |
| Adinazolam | ‐0.18 (‐1.00 to 0.63) |
8 (4 to 12) |
‐0.17 (‐1.66 to 1.30) |
11 (2 to 14) |
2 | 517 |
| Diazepam | ‐ | ‐ | ‐0.80 (‐2.15 to 0.53) |
6 (1 to 14) |
1 | 160 |
| Fluoxetine | ‐ | ‐ | ‐0.61 (‐2.08 to 0.87) |
8 (1 to 14) |
1 | 180 |
| Escitalopram | ‐ | ‐ | ‐0.40 (‐1.87 to 1.08) |
10 (2 to 14) |
1 | 254 |
| Citalopram | ‐ | ‐ | ‐0.30 (‐1.77 to 1.17) |
11 (2 to 14) |
1 | 251 |
| Sertraline | ‐ | ‐ | ‐0.78 (‐1.90 to 0.27) |
7 (2 to 13) |
1 | 176 |
| Desipramine | ‐ | ‐ | ‐0.63 (‐2.18 to 0.92) |
8 (1 to 14) |
1 | 56 |
CrI: credible interval; SMD: standardised mean difference
Compared with placebo there was a large reduction in panic symptoms for the following interventions, however they were all based on either one trial or a few small trials:
brofaromine SMD ‐3.78 (95% CrI ‐5.02 to ‐2.55), mean rank 1 (95% CrI 1 to 2);
clonazepam SMD ‐2.36 (95% CrI ‐3.27 to ‐1.45), mean rank 2 (95% CrI 1 to 3);
reboxetine SMD ‐1.03 (95% CrI ‐2.13 to 0.08), mean rank 3 (95% CrI 2 to 10).
Compared with placebo there were medium‐to‐large imprecise reductions in panic symptoms for these interventions:
clomipramine SMD ‐0.68 (95% CrI ‐1.38 to 0.03), mean rank 5 (95% CrI 3 to 9);
alprazolam SMD ‐0.48 (95% CrI ‐1.19 to 0.24), mean rank 6 (95% CrI 3 to 11).
Compared with placebo there were small reductions in panic symptoms for these interventions:
imipramine SMD ‐0.28 (95% CrI ‐1.03 to 0.47), mean rank 7 (95% CrI 3 to 12);
fluvoxamine SMD ‐0.17 (95% CrI ‐0.79 to 0.45), mean rank 8 (95% CrI 5 to 11);
paroxetine SMD ‐0.22 (95% CrI ‐0.69 to 0.25), mean rank 8 (95% CrI 5 to 11);
adinazolam SMD ‐0.18 (95% CrI ‐1.00 to 0.63), mean rank 8 (95% CrI 4 to 12);
venlafaxine SMD 0.30 (95% CrI ‐0.39 to 0.99), mean rank 12 (95% CrI 7 to 12).
Frequency of panic attacks
Figure 11 presents a network plot for each individual treatment compared with placebo and other interventions. Nodes were weighted by the number of studies and the width of the edges was weighted by the inverse of the variance. Forty‐one RCTs, including 7853 participants, were included in the NMA.
11.
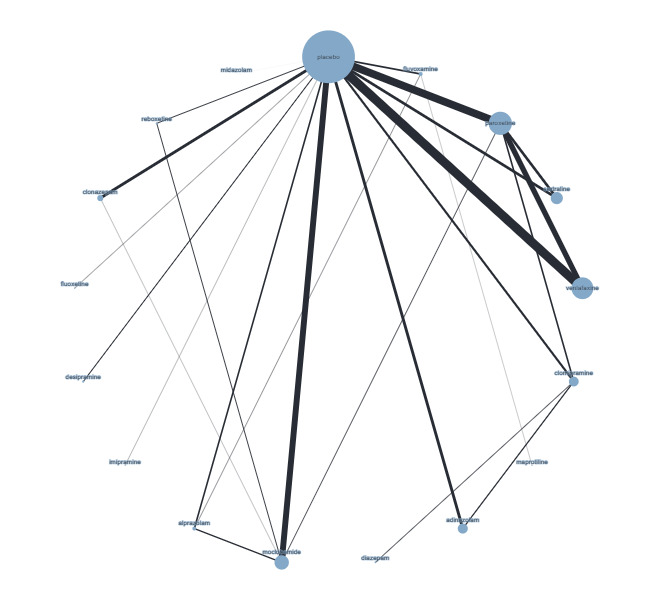
Network plot frequency of panic attacks (node size and edge width weighted by sample size)
We fitted two models: one that included individual antidepressants and benzodiazepines and a class‐effects model that included medications but also allowed clustering between treatments from the class. Both models fitted the data well; we therefore preferred the individual‐effects model as the more complex class‐effects model was not found to fit the data any better (see Table 12).
10. Model selection for frequency of panic attacks.
| Model | Deviance information criterion | Total residual deviance |
| Individual‐effects | 212.73 | Mean = 90.08, from 93 data points |
| Class‐effects | 211.56 | Mean = 90.76, from 93 data points |
| Individual‐effects, removed midazolam | 204.98 | Mean = 88.04, from 90 data points |
Examining the network plot, there was one study of midazolam compared with placebo with only five participants. Given the very large effect size, and lack of connection to other nodes in the network, we excluded this study from the individual‐effects model. This led to a much better fit compared with the main individual‐effects model, and total residual deviance remained acceptable (mean 88.04, from 90 data points). We therefore selected this sensitivity analysis for the main results. The between‐study standard deviation was 2.72 (2.06 to 3.69).
Main results
Compared with placebo, only two medications were associated with a reduction in the frequency of panic attacks that did not include zero in the 95% credible intervals (see Table 13):
11. Summary results comparing interventions with placebo for frequency of panic attacks.
| Comparator | MD (95% CrI) | Mean rank (95% CrI) | No. trials | No. participants |
| Clonazepam | ‐3.75 (‐7.64 to ‐0.01) |
3 (1 to 12) |
3 | 532 |
| Reboxetine | ‐3.54 (‐8.57 to 1.50) |
4 (1 to 14) |
1 | 82 |
| Alprazolam | ‐2.58 (‐4.79 to ‐0.43) |
6 (2 to 12) |
10 | 958 |
| Paroxetine | ‐1.97 (‐4.22 to 0.27) |
7 (2 to 13) |
6 | 1496 |
| Sertraline | ‐1.68 (‐4.81 to 1.42) |
8 (2 to 15) |
3 | 522 |
| Venlafaxine | ‐1.28 (‐3.93 to 1.37) |
9 (3 to 15) |
4 | 1693 |
| Clomipramine | ‐0.96 (‐4.06 to 2.15) |
10 (3 to 15) |
2 | 424 |
| Fluoxetine | ‐0.70 (‐6.29 to 4.89) |
10 (1 to 16) |
1 | 180 |
| Adinazolam | ‐0.33 (‐3.75 to 3.08) |
11 (3 to 16) |
2 | 517 |
| Imipramine | ‐0.71 (‐6.43 to 5.03) |
11 (1 to 16) |
6 | 319 |
| Desipramine | ‐4.60 (‐10.55 to 1.33) |
2 (1 to 14) |
1 | 56 |
| Diazepam | ‐0.66 (‐7.67 to 6.35) |
11 (1 to 16) |
1 | 160 |
| Fluvoxamine | 0.06 (‐3.46 to 3.55) |
12 (4 to 15) |
3 | 338 |
CrI: credible interval; MD: mean difference
desipramine: MD ‐4.60 (‐10.55 to 1.33), mean rank 2 (95% CrI 1 to 14);
clonazepam: MD ‐3.76 (‐7.61 to ‐0.03), mean rank 3 (95% CrI 1 to 12);
alprazolam: MD ‐2.58 (‐4.79 to ‐0.43), mean rank 6 (95% CrI 2 to 12).
Compared with placebo, several medications were associated with a reduction of at least one panic attack, but with wide 95% credible intervals:
reboxetine: MD ‐3.54 (‐8.57 to 1.50), mean rank 4 (95% CrI to 1 to 14);
paroxetine: MD ‐1.97 (95% CrI ‐4.22 to 0.27), mean rank 7 (95% CrI 2 to 13);
sertraline: MD ‐1.68 (95% CrI ‐4.81 to 1.42), mean rank 8 (95% CrI 2 to 15);
venlafaxine: MD ‐1.28 (95% CrI ‐3.93 to 1.37), mean rank 9 (95% CrI 3 to 15).
Compared with placebo, several medications were associated with either no reduction or less than one panic attack, but with wide 95% credible intervals:
clomipramine: MD ‐0.96 (95% CrI ‐4.06 to 2.15), mean rank 10 (95% CrI 3 to 15);
fluoxetine: MD ‐0.71 (‐6.30 to 4.89), mean rank 10 (95% CrI 1 to 16);
imipramine: MD ‐0.71 (‐6.43 to 5.03), mean rank 10 (95% CrI 1 to 16);
adinazolam: MD ‐0.33 (‐3.75 to 3.08), mean rank 11 (95% CrI 3 to 16);
diazepam: MD ‐0.66 (‐7.67 to 6.35), mean rank 11 (95% CrI 1 to 16);
fluvoxamine: MD 0.06 (95% CrI ‐3.46 to 3.55), mean rank 12 (95% CrI 4 to 15).
Agoraphobia
Figure 12 (change from baseline) and Figure 13 (endpoint) presents a network plot for each individual treatment compared with placebo and other interventions. Nodes were weighted by the number of studies and the width of the edges was weighted by the inverse of the variance. Twenty‐six RCTs, including 7044 participants, were included in the NMAs.
12.
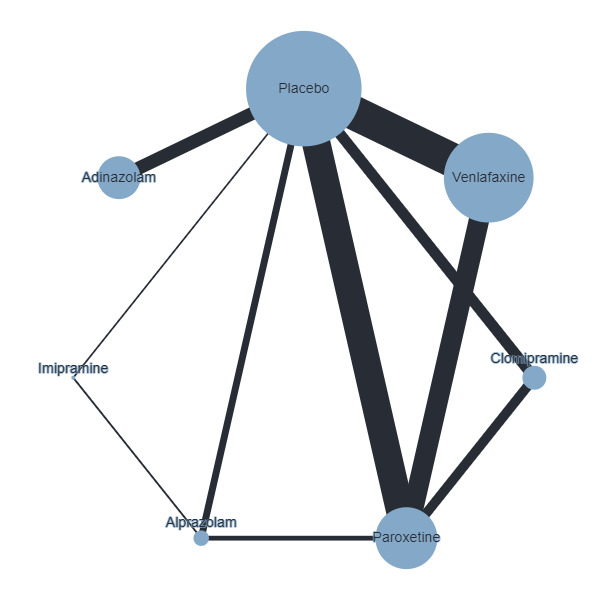
Network plot intervention combinations for agoraphobia (change from baseline)
13.
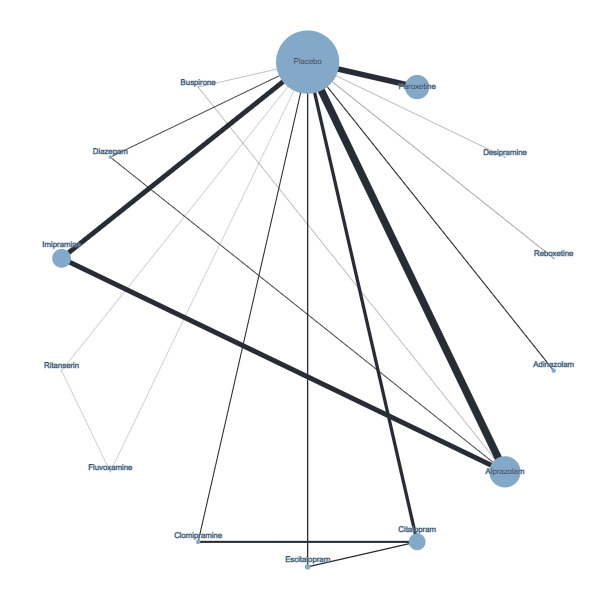
Network plot of intervention combinations for agoraphobia symptoms (endpoint)
We removed two small studies (Van Vliet 1993; Van Vliet 1996) with high deviances (> 3) that were clear outliers and represented a risk to transitivity assumption.
Individual‐effects and class‐effects models both fitted the data well for both endpoint and change from baseline; we therefore selected the individual‐effects model for both datasets (see Table 14 for further details). The results below are for endpoint data (for further details and change from baseline data, see Table 15).
12. Model selection for agoraphobia symptoms.
| Model | Deviance information criterion | Total residual deviance |
| Individual‐effects, outliers removed (endpoint) | 92.93 | Mean = 37.34, from 38 data points |
| Class‐effects, outliers removed (endpoint) | 91.01 | Mean = 37.1, from 38 data points |
| Individual‐effects (change from baseline) | 50.56 | Mean = 18.36, from 18 data points |
13. Summary results comparing interventions with placebo for mean score on agoraphobia symptoms scales.
| Endpoint | Change from baseline | |||||
| Comparator |
SMD (95% CrI) |
Mean rank (95% CrI) |
SMD (95% CrI) |
Mean rank (95% CrI) | No. trials | No. participants |
| Citalopram | ‐0.87 (‐1.32 to ‐0.40) |
2 (1 to 10) |
‐ | ‐ | 2 | 628 |
| Reboxetine | ‐0.86 (‐1.62 to ‐0.11) |
2 (1 to 10) |
‐ | ‐ | 1 | 82 |
| Escitalopram | ‐0.78 (‐1.40 to ‐0.16) |
3 (1 to 10) |
‐ | ‐ | 1 | 254 |
| Clomipramine | ‐0.60 (‐1.18 to ‐0.01) |
5 (1 to 11) |
‐0.54 (‐0.95 to ‐0.17) |
2 (1 to 5) |
3 | 468 |
| Diazepam | ‐0.52 (‐1.14 to 0.08) |
6 (1 to 12) |
‐ | ‐ | 1 | 160 |
| Fluvoxamine | ‐0.50 (‐1.42 to 0.41) |
6 (1 to 13) |
‐ | ‐ | 1 | 39 |
| Alprazolam | ‐0.46 (‐0.75 to ‐0.20) |
6 (3 to 10) |
‐0.44 (‐0.74 to ‐0.11) |
3 (1 to 6) |
10 | 1951 |
| Desipramine | ‐0.41 (‐1.22 to 0.39) |
7 (1 to 14) |
‐ | ‐ | 1 | 56 |
| Paroxetine | ‐0.30 (‐0.76 to 0.16) |
8 (3 to 13) |
‐0.48 (‐0.71 to ‐0.19) |
3 (1 to 5) |
5 | 1891 |
| Imipramine | ‐0.22 (‐0.59 to 0.16) |
9 (5 to 13) |
‐0.46 (‐1.22 to 0.29) |
3 (1 to 7) |
4 | 944 |
| Buspirone | ‐0.03 (‐0.77 to 0.70) |
11 (3 to 14) |
‐ | ‐ | 1 | 67 |
| Adinazolam | 0.10 (‐0.57 to 0.76) |
13 (8 to 16) |
‐0.07 (‐0.56 to 0.43) |
6 (2 to 7) |
2 | 517 |
| Ritanserin | 0.22 (‐0.63 to 1.08) |
13 (5 to 14) |
‐ | ‐ | 1 | 39 |
CrI: credible interval; SMD: standardised mean difference
Main results
Compared with placebo, there were several medications associated with medium‐to‐large reductions in agoraphobia symptoms:
citalopram SMD ‐0.87 (95% CrI ‐1.32 to ‐0.41), mean rank 2 (95% CrI 1 to 7);
reboxetine SMD ‐0.86 (95% CrI ‐1.62 to ‐0.11), mean rank 2 (95% CrI 1 to 10);
escitalopram SMD ‐0.78 (95% CrI ‐1.40 to ‐0.16), mean rank 3 (95% CrI 1 to 10);
clomipramine SMD ‐0.60 (95% CrI ‐1.18 to ‐0.01), mean rank 5 (95% CrI 1 to 11);
diazepam SMD ‐0.52 (95% CrI ‐1.14 to 0.08), mean rank 6 (95% CrI 1 to 12).
Compared with placebo, there were several medications associated with a small‐to‐medium reduction in agoraphobia symptoms, but the 95% CrIs were mainly imprecise:
fluvoxamine SMD ‐0.50 (95% CrI ‐1.42 to 0.41), mean rank 6 (95% CrI 1 to 13);
alprazolam SMD ‐0.46 (95% CrI ‐0.75 to ‐0.20), mean rank 6 (95% CrI 3 to 10);
desipramine SMD ‐0.41 (95% CrI ‐1.22 to 0.39), mean rank 7 (95% CrI 1 to 14);
paroxetine SMD ‐0.30 (95% CrI ‐0.76 to 0.16), mean rank 8 (95% CrI 3 to 13);
imipramine SMD ‐0.22 (95% CrI ‐0.59 to 0.16), mean rank 9 (95% CrI 5 to 13).
There were three medications, in comparison with placebo, associated either with negligible change or a small increase in agoraphobia symptoms. However, in each case the 95% credible intervals for these estimates were very wide:
buspirone SMD ‐0.03 (95% CrI ‐0.77 to 0.70), mean rank 11 (95% CrI 3 to 14);
adinazolam SMD 0.10 (95% CrI ‐0.57 to 0.76), mean rank 13 (95% CrI 5 to 14);
ritanserin SMD 0.22 (95% CrI ‐0.63 to 1.08), mean rank 13 (95% CrI 5 to 14).
2. Pooled intervention classes
Data for individual interventions were insufficiently precise to compare across active interventions. Therefore, we also conducted analyses on pooled intervention classes (SSRIs, SNRIs, TCAs, MAOIs, benzodiazepines), comparing the effectiveness of these intervention classes with placebo and one another. We limited our analyses to the primary outcomes of response and dropout.
Response
Figure 14 illustrates the network of comparisons included in the NMA. The bias adjustment model best fitted the data, therefore we based our estimates on this model (see Table 16). The between‐study standard deviation was 0.25 (95% CrI 0.04 to 0.44). All intervention classes were effective compared with placebo (see Table 17):
14.
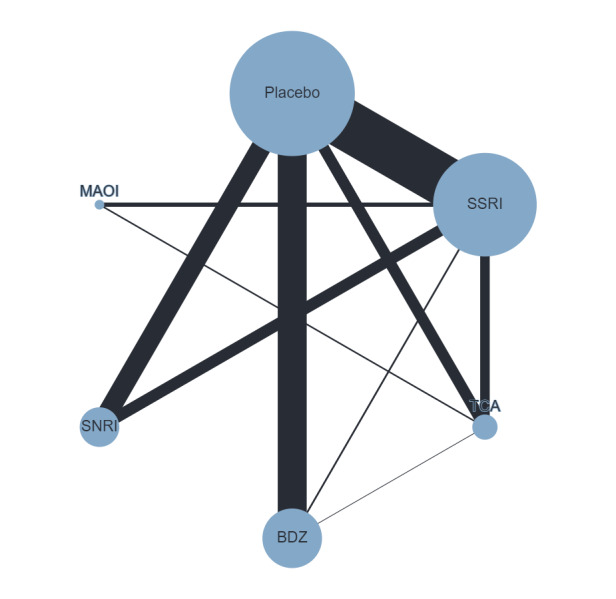
Network plot comparing medication classes for response (node size and edge width weighted by sample size)
14. Model selection for pooled intervention classes (response).
| Model | Total residual deviance | DIC |
| Pooled classes | Mean = 117.7, from 100 data points | 624.4 |
| Pooled classes (small‐study effects) | Mean = 100.1, from 100 data points | 612.2 |
| Pooled classes (baseline risk) | Mean = 107.7, from 100 data points | 619.9 |
DIC: deviation information criterion
15. Pooled intervention classes versus placebo and other pooled intervention classes for response (adjusted for small‐study effects).
| Intervention | RR (95% CrI) | OR (95% CrI) | Mean rank (95% CrI) | No. studies | Sample size |
| SSRIs versus placebo | 0.82 (0.61 to 0.96) |
0.54 (0.43 to 0.68) |
SSRIs: 5 (3 to 6) |
20 | 4306 |
| SNRIs versus placebo | 0.86 (0.67 to 0.97) |
0.61 (0.46 to 0.83) |
SNRIs: 6 (3 to 6) |
4 | 1693 |
| TCAs versus placebo | 0.74 (0.47 to 0.94) |
0.43 (0.29 to 0.64) |
TCAs: 2 (1 to 6) |
9 | 957 |
| MAOIs versus placebo |
0.76 (0.49 to 0.95) |
0.45 (0.31 to 0.68) |
MAOIs: 3 (1 to 6) |
‐ | ‐ |
| BDZs versus placebo | 0.76 (0.51 to 0.94) |
0.46 (0.33 to 0.64) |
BDZs: 3 (1 to 6) |
15 | 2471 |
| SNRIs versus SSRIs | 1.04 (0.93 to 1.27) |
1.13 (0.83 to 1.55) |
‐ | 2 | 991 |
| TCAs versus SSRIs | 0.92 (0.70 to 1.06) |
0.79 (0.54 to 1.15) |
‐ | 4 | 572 |
| MAOIs versus SSRIs | 0.93 (0.72 to 1.09) |
0.83 (0.57 to 1.23) |
‐ | 1 | 366 |
| BDZs versus SSRIs | 0.94 (0.74 to 1.08) |
0.84 (0.59 to 1.19) |
‐ | 1 | 154 |
| TCAs versus SNRIs | 0.87 (0.61 to 1.03) |
0.70 (0.44 to 1.10) |
‐ | ‐ | ‐ |
| MAOIs versus SNRIs | 0.89 (0.64 to 1.06) |
0.73 (0.47 to 1.17) |
‐ | ‐ | ‐ |
| BDZs versus SNRIs | 0.90 (0.66 to 1.04) |
0.74 (0.49 to 1.13) |
‐ | ‐ | ‐ |
| MAOIs versus TCAs | 1.02 (0.80 to 1.33) |
1.05 (0.66 to 1.70) |
‐ | 1 | 135 |
| BDZs versus TCAs | 1.02 (0.81 to 1.34) |
1.06 (0.67 to 1.70) |
‐ | 1 | 61 |
| BDZs versus MAOIs | 1.01 (0.78 to 1.30) |
1.02 (0.62 to 1.62) |
‐ | ‐ | ‐ |
BDZ: benzodiazepine; CrI: credible interval; MAOI: mono‐amine oxidase inhibitor; OR: odds ratio; RR: risk ratio; SNRI: serotonin‐norepinephrine reuptake inhibitor; SSRI: selective serotonin reuptake inhibitor; TCA: tricyclic antidepressant
SSRIs: RR 0.83 (95% CrI 0.63 to 0.96), mean rank 5 (95% CrI 2 to 6);
SNRIs: RR 0.85 (95% CrI 0.63 to 0.97), mean rank 5 (95% CrI 1 to 6);
TCAs: RR 0.82 (95% CrI 0.57 to 0.96), mean rank 4 (95% CrI 1 to 6);
MAOIs: RR 0.79 (95% CrI 0.52 to 0.96), mean rank 3 (95% CrI 1 to 6);
BDZs: RR 0.78 (95% CrI 0.52 to 0.95), mean rank 3 (95% CrI 1 to 6).
There was no difference between the following classes, with all 95% CrIs remaining within the equivalence range:
SNRIs versus SSRIs: RR 1.01 (95% CrI 0.86 to 1.21);
TCAs versus SSRIs: RR 0.98 (95% CrI 0.81 to 1.13).
There was no difference between the following classes, although the 95% CrIs crossed the equivalence range:
TCAs versus SNRIs: RR 0.97 (95% CrI 0.75 to 1.16);
MAOIs versus SSRIs: RR 0.95 (95% CrI 0.74 to 1.10);
BDZs versus SSRIs: RR 0.94 (95% CrI 0.74 to 1.08);
MAOIs versus SNRIs: RR 0.94 (95% CrI 0.69 to 1.14);
BDZs versus SNRIs: RR 0.94 (95% CrI 0.69 to 1.11);
MAOIs versus TCAs: RR 0.97 (95% CrI 0.75 to 1.19);
BDZs versus TCAs: RR 0.96 (95% CrI 0.75 to 1.17).
There was also no difference between the following classes, but the 95% CrI crossed both sides of the equivalence range:
BDZs versus MAOIs: RR 1.00 (95% CrI 0.77 to 1.27).
Dropout
Figure 15 illustrates the network of comparisons included in the NMA for dropout. The bias adjustment model best fitted the data, therefore we based our estimates on this model (see Table 18). The between‐study standard deviation was 0.38 (95% CrI 0.22 to 0.58).
15.
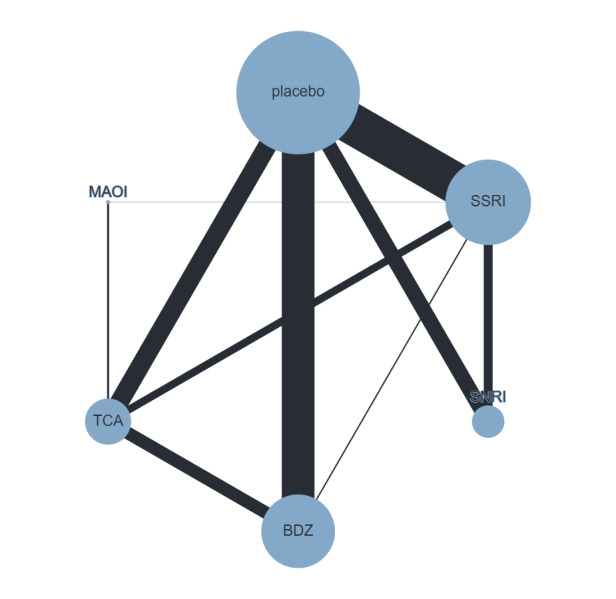
Network diagram comparing medication classes with placebo and one another for dropout (node size and edge width weighted by sample size)
16. Model selection pooled intervention classes (dropout).
| Model | Total residual deviance | DIC |
| Pooled classes | Mean = 137.2, from 128 data points | 756.0 |
| Pooled classes (small‐study effects) | Mean = 129, from 128 data points | 752.5 |
| Pooled classes (baseline risk) | Mean = 135.2, from 128 data points | 750.4 |
DIC: deviation information criterion
Benzodiazepines (BDZs) were the only treatment where dropout was less likely than with placebo (see Table 19):
17. Pooled intervention classes versus placebo and other pooled intervention classes for dropout (adjusted for small study effects).
| Intervention | RR (95% CrI) | OR (95% CrI) | Mean rank (95% CrI) | No. studies | Sample size |
| SSRIs versus placebo | 1.01 (0.85 to 1.22) |
1.02 (0.79 to 1.33) |
SSRIs: 5 (2 to 7) |
24 | 7260 |
| SNRIs versus placebo | 0.97 (0.73 to 1.33) |
0.96 (0.62 to 1.48) |
SNRIs: 4 (2 to 7) |
4 | 2020 |
| TCAs versus placebo | 0.89 (0.67 to 1.14) |
0.83 (0.58 to 1.22) |
TCAs: 3 (2 to 6) |
13 | 2642 |
| MAOIs versus placebo |
1.06 (0.58 to 1.80) |
1.11 (0.49 to 2.65) |
MAOIs: 6 (1 to 7) |
‐ | ‐ |
| BDZs versus placebo | 0.63 (0.45 to 0.83) |
0.52 (0.37 to 0.72) |
BDZs: 1 (1 to 2) |
19 | 4085 |
| SNRIs versus SSRIs | 0.96 (0.71 to 1.33) |
0.94 (0.59 to 1.48) |
‐ | 2 | 1316 |
| TCAs versus SSRIs | 0.88 (0.66 to 1.12) |
0.82 (0.58 to 1.18) |
‐ | 3 | 133 |
| MAOIs versus SSRIs | 1.05 (0.58 to 1.76) |
1.08 (0.48 to 2.57) |
‐ | 1 | 30 |
| BDZs versus SSRIs | 0.62 (0.44 to 0.83) |
0.51 (0.35 to 0.73) |
‐ | 2 | 452 |
| TCAs versus SNRIs | 0.91 (0.61 to 1.31) |
0.87 (0.52 to 1.51) |
‐ | ‐ | ‐ |
| MAOIs versus SNRIs | 1.10 (0.56 to 1.93) |
1.16 (0.47 to 3.01) |
‐ | ‐ | ‐ |
| BDZs versus SNRIs | 0.65 (0.40 to 0.95) |
0.55 (0.32 to 0.92) |
‐ | ‐ | ‐ |
| MAOIs versus TCAs | 1.20 (0.69 to 1.97) |
1.33 (0.62 to 2.89) |
‐ | 2 | 228 |
| BDZs versus TCAs | 0.72 (0.50 to 0.94) |
0.63 (0.41 to 0.92) |
‐ | 5 | 1749 |
| BDZs versus MAOIs | 0.60 (0.32 to 1.07) |
0.47 (0.19 to 1.08) |
‐ | ‐ | ‐ |
BDZ: benzodiazepine; CrI: credible interval; MAOI: mono‐amine oxidase inhibitor; OR: odds ratio; RR: risk ratio; SNRI: serotonin‐norepinephrine reuptake inhibitor; SSRI: selective serotonin reuptake inhibitor; TCA: tricyclic antidepressant
BDZs: RR 0.63 (0.45 to 0.83), mean rank 1 (95% CrI 1 to 2);
SSRIs: RR 1.01 (0.85 to 1.22), mean rank 5 (95% CrI 2 to 7);
SNRIs: RR 0.97 (0.73 to 1.33), mean rank 4 (95% CrI 2 to 7);
TCAs: RR 0.89 (0.67 to 1.14), mean rank 3 (95% CrI 2 to 6);
MAOIs: RR 1.06 (0.58 to 1.80), mean rank 6 (95% CrI 1 to 7).
BDZs were associated with a reduced risk of dropout compared with the following treatment classes:
SSRIs: RR 0.51 (0.35 to 0.73);
SNRIs: RR 0.55 (0.32 to 0.92);
TCAs: RR 0.63 (0.41 to 0.92).
There were differences in the risk of dropout (although the 95% CrIs crossed the equivalence range) for the following treatment classes:
BDZs versus MAOIs: RR 0.60 (0.32 to 1.07) ‐ favours BDZs;
TCAs versus SSRIs: RR 0.88 (0.66 to 1.12) ‐ favours TCAs.
There was no difference in the risk of dropout (although the 95% CrI cross the equivalence range) for the following treatment classes:
SNRIs versus SSRIs: RR 0.96 (0.71 to 1.33).
It was unclear whether there was a difference in the risk of dropout for the following treatments (95% CrIs crossed the equivalence range in both directions):
MAOIs versus SSRIs: RR 1.05 (0.58 to 1.76);
TCAs versus SNRIs: RR 0.91 (0.61 to 1.31);
MAOIs versus SNRIs: RR 1.10 (0.56 to 1.93);
MAOIs versus TCAs: RR 1.20 (0.69 to 1.97).
Discussion
Summary of main results
1. Individual interventions analysis
There was evidence from 48 RCTs (N = 10,118) that most medications may be more effective in the response outcome than placebo. In particular, diazepam, alprazolam, clonazepam, paroxetine, venlafaxine, clomipramine, fluoxetine and adinazolam showed the strongest effect, with diazepam, alprazolam and clonazepam ranking as the most effective. Escitalopram, imipramine, fluvoxamine, citalopram and sertraline are more effective than placebo, but the results are imprecise, given the wider 95% CrIs. Desipramine, buspirone, ritanserin, etizolam and reboxetine do not seem to be more effective than placebo, but the 95% CrIs were very wide. Heterogeneity was found for most comparisons, but our threshold analyses suggest that this is unlikely to impact the NMA findings. Out of the included trials, only 21 used a validated panic scale. The sensitivity analysis conducted on the studies using a validated panic scale showed a slight increase in heterogeneity. In terms of ranking, diazepam, alprazolam and clonazepam ranked as most effective, followed by fluoxetine and adinazolam. Paroxetine, venlafaxine and clomipramine ranked the lowest.
Results from 64 RCTs (N = 12,310) suggest that most medications were either associated with a reduced or similar risk of dropouts to placebo. Alprazolam and diazepam were associated with a lower dropout rate compared to placebo and were ranked as the most tolerated of all the medications examined. No difference in dropout rate was found for venlafaxine, sertraline and paroxetine compared with placebo. Buspirone was associated with a higher rate of dropouts and was ranked as the least tolerated medication. While reboxetine, escitalopram, imipramine and citalopram showed a reduction in dropout rates compared to placebo, the effects are imprecise due to the wide 95% CrI. Similarly, clonazepam, clomipramine, fluvoxamine, adinazolam, desipramine, fluoxetine and etizolam did not show any difference in dropout rates compared to placebo, but the effects are imprecise due to the wide 95% CrI. The dropout outcome showed evidence of substantial heterogeneity. Also, we identified incoherence in the desipramine versus placebo and in the fluoxetine versus placebo comparisons, mostly due to the effect of small studies.
Thirty‐two RCTs (N = 8569) were included in the remission outcome. Most medications seemed to be more effective than placebo, namely desipramine, fluoxetine, clonazepam, diazepam, fluvoxamine, imipramine, venlafaxine and paroxetine, and their effects were clinically meaningful. Amongst these medications, desipramine and alprazolam were ranked the highest; fluoxetine, clonazepam, diazepam, fluvoxamine and imipramine were ranked in the middle; venlafaxine and paroxetine were ranked lowest. Sertraline and escitalopram were more effective than placebo, but their effects are imprecise due to the wide 95% CrI. Citalopram, buspirone and clomipramine may not be more effective than placebo, but the 95% CrIs were very wide, indicating considerable uncertainty. Most studies did not include a validated panic scale. However, sensitivity analysis, including studies using a validated panic scale, did not differ substantially from the main analysis, except for clonazepam, the effect of which may have been overestimated by a high placebo non‐response rate, and fluvoxamine, which was less effective than placebo, compared to the main analyses.
Thirty‐five RCTs were included (N = 8826) for the continuous outcome reduction in panic scale scores. Brofaromine, clonazepam and reboxetine had the strongest reductions in panic symptoms compared to placebo, but the results were based on either one trial or very small trials. Clomipramine, imipramine and alprazolam showed evidence of a reduction in panic scale scores compared to placebo, but the reductions showed a high level of imprecision. Venlafaxine, fluvoxamine, paroxetine and adinazolam showed small reductions in panic scale scores compared to placebo. Brofaromine ranked the highest, followed by clonazepam and reboxetine, while clomipramine, imipramine and alprazolam had an intermediate ranking. However, these results are either based on small trials or are imprecise. Venlafaxine, fluoxetine, paroxetine and adinazolam showed the lowest ranking as they reduced panic symptoms to a minor extent.
Forty‐one RCTs are included (N = 7853) in the frequency of panic attack outcome. Only clonazepam and alprazolam showed a strong reduction in the frequency of panic attacks compared to placebo, and were ranked as highest. Fluoxetine, reboxetine, paroxetine, sertraline and venlafaxine tended to reduce panic attacks, but the CrIs were wide. Weak effects were found for clomipramine, adinazolam, imipramine, desipramine, diazepam and fluvoxamine. Ranking is difficult to interpret for most medications, other than clonazepam and alprazolam, due to imprecision.
Twenty‐six RCTs (N = 7044) provided data for agoraphobia. The strongest reductions in agoraphobia symptoms were found for citalopram, reboxetine, escitalopram, clomipramine and diazepam, compared to placebo. Smaller effects were observed for alprazolam, fluvoxamine, desipramine, paroxetine and imipramine compared to placebo, with imprecise results. Negligible or small effects were found for buspirone, adinazolam and ritanserin, compared to placebo, and the results were imprecise. Citalopram and reboxetine were ranked as the highest in terms of reduction in agoraphobia, while escitalopram, clomipramine and diazepam were ranked as less effective.
2. Pooled intervention classes
The two outcomes examined were the primary outcomes (response and dropout). The classes of medication examined were: SSRIs, SNRIs, TCAs, MAOIs and BDZs.
For the response outcome, all classes of medications examined (SSRIs, SNRIs, TCAs, MAOIs, BDZs) seem to be more effective than placebo. TCAs as a class ranked as the most effective, followed by BDZs and MAOIs. SSRIs as a class ranked fifth on average, while SNRIs were ranked as the lowest. However, differences in rankings do not reflect substantial differences in effectiveness between these classes. If classes of medication are compared with each other for the response outcome, no difference is found between classes. Comparisons between MAOIs and TCAs and between BDZs and TCAs also suggested no differences between these medications, but the results were imprecise.
For the dropout outcome, BDZs were the only class associated with lower dropout compared to placebo, and they were ranked as first in terms of tolerability. The other classes did not show any difference in dropouts compared to placebo. In terms of ranking, TCAs are on average second to BDZs, followed by SNRIs, then by SSRIs and lastly by MAOIs. BDZs were associated with lower dropout rates compared to SSRIs, SNRIs and TCAs. BDZs were also associated with lower dropout rates compared to MAOIs, but the results were imprecise due to the wide 95% CrIs. Similarly, TCAs were associated with lower dropout rates than SSRIs, but the results were also imprecise due to wide 95% CrIs. SSRIs were associated with the same risk of dropout as SNRIs, but the results were imprecise due to the wide 95% CrIs. It was not possible to determine whether MAOIs were associated with a higher or lower dropout compared to SSRIs, SNRIs and TCAs, due to the 95% CrIs crossing the equivalence range in both directions. For the same reason, it was also not clear whether TCAs were associated with higher or lower dropout compared to SNRIs.
Overall completeness and applicability of evidence
The patient populations of the included studies were highly selected. For example, most studies excluded patients with psychiatric co‐morbidities or patients with intake of other drugs, although panic disorder is highly comorbid with other psychiatric disorders (e.g. drug dependence, major depression, bipolar I disorder, social phobia, specific phobia and generalised anxiety disorder) (Grant 2006; Preti 2016). The analysed population is therefore probably not fully representative of patients usually seen in routine practice, and the results of this review may not automatically apply to the general population. Also, although the studies included in the review were carried out in different countries from several continents, the majority of studies were conducted in the USA and Europe and thus may be not transferable to Asia, Africa and other regions of the world. Finally, the validity of the outcome used to measure severity of panic disorder may be a further limitation. Panic disorder is a multifaceted disorder, typically characterised by panic attacks and avoidance, both of which deteriorate the afflicted person's functioning, but the two may be compensatory of each other (e.g. when one is completely agoraphobic, one may be free of panic attacks). More recent measures of panic disorder severity (e.g. the Panic Disorder Severity Scale) take into account all of these aspects, but older studies often focused on one aspect of the disorder and thus may have neglected the other aspects. Our review was only able to synthesise what was measured in the original studies.
For the antidepressant versus placebo studies, the majority of RCTs provided data for the primary outcomes specified in the protocol, allowing us to include a considerable number of studies and participants in the analyses. It was therefore possible to generate useful information on the efficacy and acceptability of antidepressants in comparison with placebo. In terms of applicability, considering the high number of studies and participants, we can argue that this population may reflect in a satisfactory way the characteristics of people with panic disorder seen in 'real‐world' settings, despite the well‐known limitations of all randomised studies that should always be acknowledged. One limitation to generalisability may also be connected with the exclusion of studies in which regular use of benzodiazepines was allowed, since this practice might be common in real‐life settings.
For the BDZs versus placebo studies, the completeness and applicability may have been limited by various factors. Some analyses were underpowered (e.g. number of participants experiencing at least one adverse effect) because only a few studies provided appropriate data for these outcomes. Moreover, we did not investigate other side effects of BDZs (cognitive impairment, risk of falls, tolerance, dependence, less optimal reaction time, risk of dangers when using instruments, etc.), which may have limited the applicability of our findings. It is important to point out the implications and consequences of longer BDZ use. A recent population‐based study reported a significant excess of hospital attendance for falls, fractures, long‐term care admission and death over a one‐year follow‐up period in continuous BDZs users relative to intermittent users after a 180‐day index period (Davies 2022). Unlike the issue of short‐term tolerability, these aspects of BDZ‐related adverse outcomes in the long term cannot be addressed in the present review using data from randomised trials with a median of only eight weeks' treatment. Nevertheless, in younger adults not at immediate risk of the adverse outcomes described above, especially those with no history of substance misuse, BDZs, with their good short‐term tolerability and rapid onset of action, may well have a useful role in the initial or short‐term management of panic disorder, when antidepressants may not be practicable after initial management of panic disorder. However, it is worth noting that the findings presented in this review may be limited by the low quality of the trials comparing BDZs to placebo and BDZs to antidepressants.
The identified studies comparing antidepressants and benzodiazepines are not sufficient to comprehensively address the objectives. The majority of studies enrolled a very small number of participants and did not provide data for all the outcomes specified in the protocol. Only short‐term data on acceptability and adverse effects of antidepressants and benzodiazepines were available. Clinically, this is a major limitation as long‐term use of benzodiazepines is controversial due to concerns about adverse psychological and physical effects, physical dependence and withdrawal. Similar concerns have been raised about long‐term exposure to antidepressants, in particular the SSRIs.
Quality of the evidence
The quality of the studies varied depending on the comparisons, and was usually low or unclear.
For the studies comparing antidepressants with placebo, the overall methodological quality of the included studies was unclear. No study showed an overall low risk of bias. The majority of studies showed mixed features, with a large prevalence of unclear risk of bias in different domains; however, this may reflect a lack of exhaustive reporting rather than clear evidence of bias. In general, confidence in the estimates of effect ranged from 'low' to 'moderate' for most of the outcomes assessed. Study findings were generally quite precise, with small confidence intervals and a high number of participants. Reasons to downgrade the quality of the evidence were primarily due to limitations in the included studies and inconsistency (heterogeneity between studies' results). In agreement with this judgement, we argue that, for the primary outcomes, treatment estimates may be considered quite robust, and further research is unlikely to change our confidence in the estimate of effect.
For the studies comparing benzodiazepines with placebo, the overall methodological quality of the included studies was poor. We rated all studies as having an unclear risk of bias in at least three domains. In addition, the majority of the studies had a high risk of bias in at least one domain, including a high risk of attrition bias and high risk of bias for blinding of participants and physicians. These potential biases are a major threat to the validity of the studies included in this review. Most studies with a high risk of attrition bias reported unequal dropout rates between the treatment groups, with higher rates in the placebo groups. Furthermore, participants in the placebo group dropped out early in trials comparing benzodiazepines and placebo. The missing data are thus clearly not completely random, resulting in a high risk of an underestimation of the placebo effect and therefore of an overestimation of the treatment effect, because in the last observation carried forward analyses participants are included with higher values in the placebo group, without taking into account that symptoms usually decline over time (e.g. due to the natural course of the disorder, regression to the mean, etc.). Furthermore, the bias may be reinforced by censorship of participant data at protocol violation in the first weeks, which was a standard procedure accepted by the regulatory authorities in the past.
For the studies comparing antidepressants with benzodiazepines, the overall methodological quality of the included studies was poor. No study showed an overall low risk of bias. The majority of studies showed mixed features, with a large prevalence of an unclear risk of bias in different domains, which seems to reflect a lack of exhaustive reporting rather than clear evidence of bias. In general, the confidence in the estimate of effect appeared to range from 'very low' to 'moderate' for most of the outcomes assessed. This judgement is primarily due to limitations in the included studies (high dropout rates), imprecision (wide confidence intervals) and inconsistency (heterogeneity between study results). In accordance with that, any estimate of effect should be considered very uncertain, and further research is very likely to change the estimate of effect and thus the degree of confidence in its applicability in routine clinical practice.
Potential biases in the review process
Several potential biases have been identified in the review process.
The search and selection of the studies was done in the previous Cochrane head‐to‐head comparisons on panic disorder (Bighelli 2016; Bighelli 2018; Breilmann 2019), as this NMA includes all the studies selected in those reviews and no new studies.
Several possible limitations of this review should be highlighted for all the studies. Some limitations are intrinsically related to the actual process of retrieving, collecting, selecting and extracting data. In order to reduce the potential bias of this complex process, two review authors independently worked on each of these steps. It has been highlighted that two independent extractors are overall more reliable than extraction performed by a single author followed by verification by a second author. We applied the same process for the risk of bias assessment. Furthermore, disagreements were always discussed with a third author. Another relevant problem concerns the 'systematic' nature of the search. We chose to include only randomised studies as they provide the strongest level of evidence available. In this type of review there is some risk of publication bias, which means that negative studies may not have been published. Although the search was thorough, it is possible that we may not have identified some unpublished studies, considering that there are no shared procedures to perform this kind of search. It is expected that the analysis of published literature only would lead to overestimation of the efficacy of a given intervention. Finally, it is important to bear in mind that some included studies were funded by the pharmaceutical industry, and this may again introduce an overestimation of the efficacy of interventions.
For the antidepressant versus placebo comparisons, we formally checked the presence of publication bias with visual inspection of funnel plots in the head‐to‐head published Cochrane Review (Bighelli 2018). Regarding the primary outcome, 'Failure to respond', a visual inspection of the funnel plot suggested that some studies with a low number of participants favouring placebo against TCAs may be missing, and this may have led to an overestimation of the efficacy of TCAs compared to placebo. For the primary outcome, 'Total dropouts', a visual investigation of the funnel plot suggested that some small studies favouring placebo against SSRIs might be missing, and this might have led to an overestimation of the acceptability of SSRIs.
For the benzodiazepines versus placebo comparisons, we formally checked the presence of publication bias with visual inspection of funnel plots in the head‐to‐head published Cochrane Review (Breilmann 2019). The funnel plots are indicative of the presence of publication bias, and we identified only one unpublished trial for inclusion in the review. Most of the included studies were published more than 15 years ago, and the availability of information on the licensing procedures of these drugs is very limited. Considering that for some individual benzodiazepines only one study was included, we think that it is rather likely that there are some other unpublished trials.
For the antidepressants versus placebo comparisons, the impact of unpublished literature on the results of this review is uncertain, however it is expected that an analysis of only published literature would lead to overestimation of the efficacy of a given intervention. We did not check this formally with a funnel plot analysis in the Cochrane head‐to‐head review (Bighelli 2016), as fewer than 10 studies contributed to any analyses, thus making the funnel plot methodology less informative.
Agreements and disagreements with other studies or reviews
This NMA review is based on the Cochrane head‐to‐head comparisons on panic disorder published in previous years (Bighelli 2016; Bighelli 2018; Breilmann 2019). The Cochrane head‐to‐head review on antidepressants versus placebo in panic disorder showed that antidepressants as a group are more effective than placebo, although the evidence was of low quality (Bighelli 2018). Antidepressants as a class were less tolerated than placebo. The Cochrane head‐to‐head review on BDZs versus placebo showed that BDZs as a group are more effective than placebo, although the evidence was of low quality (Breilmann 2019). BDZs as a class were more tolerated than placebo. The Cochrane head‐to‐head review on antidepressants versus BDZs showed that antidepressants as a class were not more or less effective than BDZs (Bighelli 2016). Remission rates showed a benefit for BDZs compared to antidepressants, but the effect was very small and close to no difference. In terms of tolerability, the review found evidence suggesting a benefit for benzodiazepines compared to antidepressants when looking at the number of dropouts due to any cause. The methodology in this NMA allowed us to rank treatments, which was an advantage compared to the Cochrane head‐to‐head comparisons. In line with the previous Cochrane head‐to‐head meta‐analyses, antidepressants and BDZs seem effective compared to placebo. However, we found that some BDZs ranked higher, if compared to placebo, in terms of efficacy and tolerability, except for remission where desipramine ranked as high as alprazolam. For class comparisons, the head‐to‐head NMA comparing antidepressants and BDZs did not show any difference (Bighelli 2016). Our NMA, instead, ranked TCAs as the class with the strongest effect, compared to placebo. However, in line with the Bighelli 2016 review, our NMA found no difference between classes of medications for response outcomes. For tolerability outcomes, in line with Bighelli 2016, BDZs were ranked as the most tolerated class of medications for panic disorder.
Two other NMAs have been published on panic disorder (Chawla 2022; Du 2021). To our knowledge, these are the only other NMAs published on the topic.
Du 2021 is based on 42 trials comparing antidepressants and BDZs (a lower number compared to our NMA), all published, and also included single‐blind trials, unlike our review. This review did not adjust for small‐study bias. Du and colleagues concluded that escitalopram and venlafaxine, as well as BDZs, are effective choices for panic disorder. The findings of Du and colleagues are similar to this review, although they compared BDZs as a class with other individual antidepressants. We also found that most antidepressants and BDZs were more effective than placebo, and also found no substantial differences between these medication classes.
Chawla 2022 is based on 87 trials, a higher number of trials compared to our NMA. This is likely explained by Chawla and colleagues' broader inclusion criteria. Our review only included monotherapy of antidepressants and BDZs whereas Chawla and colleagues included monotherapy and combination therapy, and included further medication classes (azapirones, beta‐blockers). A further difference was that our review only included double‐blind trials, while Chawla and colleagues also included single‐blind trials. However, it should be noted that reporting of blinding was unclear in many studies, so the impact of this inclusion criterion on the results is unclear. Apart from the studies that Chawla included but were ineligible for our review as per our eligibility criteria, there was one unpublished study that we had missed that they had included (Pfizer 2008). By contrast, some studies seemed to be eligible as per their study protocol but were not included in their review. Whereas our current review adjusted for small‐study bias, the review by Chawla and colleagues did not. Given the large number of small studies included in this NMA, and the potential risk of bias identified, this is an important advantage of our current review. Chawla and colleagues conclude that SSRIs provide high rates of remission, with sertraline and escitalopram associated with a higher remission and low risk of adverse events. However, the authors pointed out that the studies had moderate to very low‐certainty levels of evidence, mostly as a result of within‐study bias, inconsistency and imprecision of the findings reported. They did not recommend BDZs as first‐line treatments due to the potential risk of adverse events. They found that BDZs were more effective than SSRI and SNRIs, whereas our review did not find substantial differences between these medication classes. This difference in findings is probably accounted for by the bias adjustment used in our analyses; in our unadjusted analyses we found similar effects to Chawla and colleagues. We feel that the methodology used in our review, such as limiting to double‐blind studies and adjusting for small‐study effects, makes our findings more robust.
Authors' conclusions
Implications for practice.
This Cochrane Review seems to suggest that selective serotonin reuptake inhibitors (SSRIs), serotonin‐norepinephrine reuptake inhibitors (SNRIs) (venlafaxine), tricyclic antidepressants (TCAs), mono‐amine oxidase inhibitors (MAOIs) and benzodiazepines (BDZs) may be effective, with little difference between classes in terms of efficacy. However, it is important to note that the reliability of these findings may be limited due to the overall low quality of the studies, with all trials rated unclear or high across multiple domains.
Within classes, some differences emerged. For example, amongst the SSRIs paroxetine and fluoxetine seem to have stronger evidence of efficacy than sertraline. Benzodiazepines appear to have a small but significant advantage in terms of tolerability (assessed by the incidence of dropouts) over other classes over the time period of the studies (median eight weeks).
Existing guidelines (Katzman 2014; Baldwin 2014; Andrews 2018) and other systematic reviews on panic disorder (Chawla 2022; Du 2021) favour SSRIs and sometimes the SNRI venlafaxine as first‐line treatment. In light of this, our findings bring up two issues for clinicians to consider:
Amongst SSRIs, are paroxetine and fluoxetine preferable to sertraline and citalopram/escitalopram on the basis of the finding of slightly better efficacy? However, paroxetine is known to be associated with difficult withdrawal and is a strong mechanistic CYP2D6 inhibitor, while fluoxetine and its metabolite nor‐fluoxetine inhibit a range of important cytochrome (CYP) enzymes, increasing the likelihood of drug interactions. In contrast, sertraline is a substrate of multiple CYPs but a strong inhibitor of none and is not noted for problems on withdrawal, so these benefits might offset the slightly weaker evidence of efficacy.
BDZs performed well on efficacy and tolerability in the time frame examined here (4 to 24 weeks with a median of 8 weeks), but most guidelines advise that they are not used as first‐line treatment. The British Association of Psychopharmacology guidelines state that BDZs "will usually be reserved for the further treatment of patients who have not responded to at least three previous treatments" (Baldwin 2014). Thus, while the findings for BDZs were positive in terms of efficacy and tolerability in the short term, the limitation that we could not examine the consequences of longer‐term use, despite there being well‐characterised concerns, means that the evidence reported in this Cochrane Review is insufficient to override the many current treatment guidelines that suggest that BDZs may be a less desirable choice overall than SSRIs and SNRIs. Moreover, guidelines encourage prescribers and patients to keep using antidepressants as prophylaxis for periods of at least six months to two years after response (Andrews 2018; Baldwin 2014; Cleare 2015; Katzman 2014).
Finally, while some guidelines recommend other drugs such as buspirone, gabapentin and mirtazapine, there is a lack of positive randomised evidence to support these drugs in panic disorder.
Another important point to bear in mind is the relationship between pharmacological treatments and psychotherapy in panic. The evidence for depression points in the direction of the superiority of psychotherapy (alone or in combination with medications) in the long term (Furukawa 2021). It will be important not to discount the relevance of psychotherapy and its combination with pharmacotherapy in the treatment of panic disorder.
Implications for research.
Threshold analyses found that the network meta‐analysis (NMA) results were relatively robust to the impact of potential biases. Future randomised studies may not add much to our overall findings comparing all medications to placebo and comparing medication classes. However, some uncertainties remain. Comparisons of individual medications are still very imprecise. It is worth mentioning that the networks themselves might not be fully mature, adding another layer of uncertainty to the conclusions drawn from the review. In addition, few studies measured quality of life and social functioning.
An important limitation of this NMA is the fact that there have been limited studies in the past 15 years. Most clinical drug trials of panic disorder date back to the 1980s and 1990s and there are not many recent trials on panic. Research methodology may have become more refined over time. It will be highly desirable to carry out new trials on antidepressants and benzodiazepines in panic disorders, possibly comparing them to novel treatments. A search of ClinicalTrials.gov (2 March 2023) showed that there are very few ongoing clinical studies on pharmacological treatment in panic disorder.
A further limitation is that almost all the studies examined in this NMA were of short duration. This may have had some implications for the long‐term efficacy in a clinical setting of the medication examined. For the BDZs, there has been a considerable debate about whether they can be used in the long term given their propensity for abuse, possible risk of tolerance (Horowitz 2021), and the existence of withdrawal symptoms (Allison 2003). Some authors advocate against the long‐term use of BDZs in any case (Horowitz 2021). Nonetheless, other authors have been more open to the idea of using BDZs in case other treatments, such as antidepressants, fail and when the likelihood of abuse is low (Hirschtritt 2021; Silberman 2021). Experts belonging to the International Task Force on BDZs talk about a "bias" against BDZs (Silberman 2021): they say that the evidence that BDZs are likely to be abused in any case, that they create tolerance or are dangerous in overdose, does not match the beliefs many clinicians have against them (Silberman 2021). Antidepressants seem to have a lower propensity for abuse (Fluyau 2022), but are also associated with withdrawal symptoms that can be severe and possibly worse than BDZ withdrawal (Fava 2019). Their use is not devoid of problems, as they may even worsen the conditions they are supposed to treat (Fava 2020). Studies where BDZs and antidepressants are assessed in the long term (i.e. longer than a year) are needed for anxiety disorders, as the efficacy of medications in anxiety disorders is less established for longer durations. BDZs may be an alternative for people who do not respond to antidepressants and/or psychotherapy.
Another important question is: are BDZs more helpful and less risky in the long term if they are only taken intermittently (i.e. a few times per week, as needed) as rescue medications? Studies where regular versus intermittent use of BDZs in anxiety disorders is compared will be particularly useful to guide the clinician to the optimal course of treatment.
Finally, it will be important to systematically assess the efficacy of medications compared to psychotherapy, perhaps in a NMA. Data from depression seem to show that psychotherapies can lead to a more sustained effect. The same may apply to anxiety disorders in general and panic disorder in particular and needs to be investigated. Psychotherapy can be a valid first‐line alternative or add‐on treatment in panic disorder (Papola 2022), and needs to be compared to medications in future research trials.
History
Protocol first published: Issue 7, 2017
Acknowledgements
Editorial contributions
Cochrane Common Mental Disorders supported the authors in the development of this systematic review.
The following people conducted the editorial process for this article:
Sign‐off Editor (final editorial decision): Neil O'Connell, Brunel University, London, UK; Senior Managing Editor (provided editorial guidance to authors, edited the article): Joey Kwong; Managing Editor (selected peer reviewers, collated peer reviewer comments, provided editorial guidance to authors, edited the article): Marwah Anas El‐Wegoud, Central Editorial Service; Editorial Assistant (conducted editorial policy checks and supported the editorial team): Leticia Rodrigues, Central Editorial Service; Copy Editor (copy editing and production): Jenny Bellorini, Cochrane Central Production Service.
Peer reviewers (provided comments and recommended an editorial decision): Amir Garakani, MD, Department of Psychiatry and Behavioral Health, Greenwich Hospital, Greenwich, CT, Department of Psychiatry, Yale School of Medicine, New Haven, CT (clinical review); Anton JLM van Balkom, MD, PhD Department of Psychiatry Amsterdam UMC, Vrije Universiteit, Amsterdam, the Netherlands (clinical review); Rafael C Freire ‐ Department of Psychiatry and Center for Neuroscience Studies, Queen's University, Kingston, ON, Canada (clinical review); Brian Duncan (consumer review); Benjamin Rouse, Research Analyst, ECRI (methods review); Sofia Tsokani, Cochrane (methods review); Anne Littlewood, Cochrane Oral Health (search review). One additional peer reviewer provided clinical peer review but chose not to be publicly acknowledged.
Appendices
Appendix 1. Cochrane Specialised Register
The Cochrane Common Mental Disorders Group (CCMD) maintains an archived controlled trials register known as the CCMDCTR. This specialised register contains over 40,000 reference records (reports of RCTs) for anxiety disorders, depression, bipolar disorder, eating disorders, self‐harm and other mental disorders within the scope of this Group. The CCMDCTR is a partially studies‐based register with more than 50% of reference records tagged to around 12,500 individually PICO‐coded study records. Reports of studies for inclusion in the register were collated from (weekly) generic searches of key bibliographic databases to June 2016, which included: MEDLINE (1950 onwards), Embase (1974 onwards), PsycINFO (1967 onwards), quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), and review‐specific searches of additional databases. Reports of studies were also sourced from international trials registries, drug companies, the handsearching of key journals, conference proceedings and other (non‐Cochrane) systematic reviews and meta‐analyses. An example of the core MEDLINE search is displayed below.
[MeSH Headings]: eating disorders/ or anorexia nervosa/ or binge‐eating disorder/ or bulimia nervosa/ or female athlete triad syndrome/ or pica/ or hyperphagia/ or bulimia/ or self‐injurious behavior/ or self mutilation/ or suicide/ or suicidal ideation/ or suicide, attempted/ or mood disorders/ or affective disorders, psychotic/ or bipolar disorder/ or cyclothymic disorder/ or depressive disorder/ or depression, postpartum/ or depressive disorder, major/ or depressive disorder, treatment‐resistant/ or dysthymic disorder/ or seasonal affective disorder/ or neurotic disorders/ or depression/ or adjustment disorders/ or exp antidepressive agents/ or anxiety disorders/ or agoraphobia/ or neurocirculatory asthenia/ or obsessive‐compulsive disorder/ or obsessive hoarding/ or panic disorder/ or phobic disorders/ or stress disorders, traumatic/ or combat disorders/ or stress disorders, post‐traumatic/ or stress disorders, traumatic, acute/ or anxiety/ or anxiety, castration/ or koro/ or anxiety, separation/ or panic/ or exp anti‐anxiety agents/ or somatoform disorders/ or body dysmorphic disorders/ or conversion disorder/ or hypochondriasis/ or neurasthenia/ or hysteria/ or munchausen syndrome by proxy/ or munchausen syndrome/ or fatigue syndrome, chronic/ or obsessive behavior/ or compulsive behavior/ or behavior, addictive/ or impulse control disorders/ or firesetting behavior/ or gambling/ or trichotillomania/ or stress, psychological/ or burnout, professional/ or sexual dysfunctions, psychological/ or vaginismus/ or Anhedonia/ or Affective Symptoms/ or *Mental Disorders/ OR [Title/ Author Keywords]: (eating disorder* or anorexia nervosa or bulimi* or binge eat* or (self adj (injur* or mutilat*)) or suicide* or suicidal or parasuicid* or mood disorder* or affective disorder* or bipolar i or bipolar ii or (bipolar and (affective or disorder*)) or mania or manic or cyclothymic* or depression or depressive or dysthymi* or neurotic or neurosis or adjustment disorder* or antidepress* or anxiety disorder* or agoraphobia or obsess* or compulsi* or panic or phobi* or ptsd or posttrauma* or post trauma* or combat or somatoform or somati#ation or medical* unexplained or body dysmorphi* or conversion disorder or hypochondria* or neurastheni* or hysteria or munchausen or chronic fatigue* or gambling or trichotillomania or vaginismus or anhedoni* or affective symptoms or mental disorder* or mental health).tw,kf. AND [RCT filter]: (controlled clinical trial.pt. or randomised controlled trial.pt. or (randomi#ed or randomi#ation).ab,ti. or randomly.ab. or (random* adj3 (administ* or allocat* or assign* or class* or control* or determine* or divide* or distribut* or expose* or fashion or number* or place* or recruit* or subsitut* or treat*)).ab. or placebo*.ab,ti. or drug therapy.fs. or trial.ab,ti. or groups.ab. or (control* adj3 (trial* or study or studies)).ab,ti. or ((singl* or doubl* or tripl* or trebl*) adj3 (blind* or mask* or dummy*)).mp. or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or randomised controlled trial/ or pragmatic clinical trial/ or (quasi adj (experimental or random*)).ti,ab. or ((waitlist* or wait* list* or treatment as usual or TAU) adj3 (control or group)).ab.)
Records were screened for reports of RCTs within the scope of the Cochrane Common Mental Disorders Group. Secondary reports of RCTs were tagged to the appropriate study record.
The CCMDCTR‐Studies Register was searched for a suite of panic reviews on condition alone. Condition = panic Records were manually screened for drug therapy trials.
The CCMDCTR‐References Register was searched using a more sensitive set of free‐text terms to identify additional untagged/uncoded reports of RCTs. A further search was conducted to identify drug therapy trials for ‘Anxiety Disorders Not Otherwise Specified’ (ADNOS), which may include a subset of participants with panic disorder.
CCDANCTR‐Refs Search 1 (panic): #1. panic or agoraphobi* #2. (antidepress* or anti‐depress* or "anti depress*" or MAOI* or RIMA* or “monoamine oxidase inhibit*” or ((serotonin or norepinephrine or noradrenaline or neurotransmitter* or dopamin*) NEAR (uptake or reuptake or re‐uptake or "re uptake")) or SSRI* or SNRI* or NARI* or SARI* or NDRI* or TCA* or tricyclic* or tetracyclic* or pharmacotherap* or psychotropic* or "drug therapy") #3. (agomelatine or alaproclate or amoxapine or amineptine or amitriptylin* or amitriptylinoxide or atomoxetine or befloxatone or benactyzine or binospirone or brofaromine or (buproprion or amfebutamone) or butriptyline or caroxazone or cianopramine or cilobamine or cimoxatone or citalopram or (chlorimipramin* or clomipramin* or chlomipramin* or clomipramine) or clorgyline or clovoxamine or (cx157 or tyrima) or demexiptiline or deprenyl or (desipramine* or pertofrane) or desvenlafaxine or dibenzepin or diclofensine or dimetacrin* or dosulepin or dothiepin or doxepin or duloxetine or desvenlafaxine or dvs‐233 or escitalopram or etoperidone or femoxetine or fluotracen or fluoxetine or fluvoxamine or (hyperforin or hypericum or “st john*”) or imipramin* or iprindole or iproniazid* or ipsapirone or isocarboxazid* or levomilnacipran or lofepramine* or (“lu aa21004” or vortioxetine) or "lu aa24530" or (ly2216684 or edivoxetine) or maprotiline or melitracen or metapramine or mianserin or milnacipran or minaprine or mirtazapine or moclobemide or nefazodone or nialamide or nitroxazepine or nomifensine or norfenfluramine or nortriptylin* or noxiptilin* or opipramol or oxaflozane or paroxetine or phenelzine or pheniprazine or pipofezine or pirlindole or pivagabine or pizotyline or propizepine or protriptylin* or quinupramine or reboxetine or rolipram or scopolamine or selegiline or sertraline or setiptiline or teciptiline or thozalinone or tianeptin* or toloxatone or tranylcypromin* or trazodone or trimipramine or venlafaxine or viloxazine or vilazodone or viqualine or zalospirone) #4. (benzodiazepin* or BZD or abecarnil or adinazolam or alprazolam or arfendazam or bentazepam or bretazenil or bromazepam or brotizolam or camazepam or chlordiazepoxide or chlordesmethyldiazepam or cinolazepam or clobazam or clonazepam or clorazepate or chlorazepate or clotiazepam or cloxazolam or delorazepam or demoxepam or desmethyldiazepam or desoxydemoxepam or devazepide or diazepam or doxefazepam or estazolam or “ethyl loflazepate” or “cm 6912” or cm‐6912 or etizolam or fludiazepam or flunitrazepam or flurazepam or dealkylflurazepam or flutoprazepam or fosazepam or gidazepam or girisopam or halazepam or haloxazolam or ketazolam or loflazepate or loprazolam or lorazepam or lormetazepam or meclonazepam or medazepam or metaclazepam or mexazolam or midazolam or nerisopam or nimetazepam or nitrazepam or norchlordiazepoxide or norclobazam or nordazepam or norfludiazepam or norflunitrazepam or oxazepam or “wy 3498” or wy‐3498 or oxazolam or phenazepam or pinazepam or prazepam or premazepam or propazepam or quazepam or ripazepam or serazepine or sograzepide or talampanel or tarazepide or temazepam or tetrazepam or tofisopam or triazolam or (zolazepam or zaleplon or zolpidem or zopiclone or eszopiclone or z‐drugs or “z drugs”) or *pam or *lam or nonbenzo*) #5. (azapirone or alnespirone or binospirone or buspirone or enilospirone or eptapirone or gepirone or ipsapirone or revospirone or tandospirone or zalospirone or *piron* or or gabapentin* or pregabalin or mirogabalin or imagabalin) #6. (#1 and (#2 or #3 or #4 or #5)) CCDANCTR‐Refs Search 2 (ADNOS): #7. ((anxiety or anxious):ti or ADNOS) and not (agoraphobi* or panic or (social NEAR (anxi* or phobi*)) or generalised or generalized or obsessive or compulsive or OCD or PTSD or post‐trauma* or “post trauma*” or posttrauma*) #8. (#7 and (#2 or #3 or #4 or #5))
The search of the CCMDCTR was conducted at several different time points, across a suite of associated panic reviews:
Benzodiazepines versus placebo for panic disorder in adults (all years to 26 March 2014, 11 September 2015 and 29 May 2018)
Antidepressants and benzodiazepines for panic disorder in adults (all years to 11 September 2015)
Antidepressants versus placebo for panic disorder in adults (all years to May 2017)
Hence, for this review, the search in January 2021 was date‐limited (2014 onwards).
Appendix 2. Other database searches
Panic NMA search (22‐Jan‐2021)
Ovid Embase (2014 to 2021 Week 03), n = 600
Ovid MEDLINE (2014 to January 22, 2021), n = 133
Ovid PsycINFO (2014 to January Week 2 2021), n = 239
CLib:CENTRAL (2014 to Issue 1 of 12, 2021), n = 412
CCMDCTR (2014‐2016), n = 223
Total = 1607
Duplicates removed = 408
To screen, n = 1199
Database: Embase <1980 to 2021 Week 03>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 Panic/ (23677)
2 Agoraphobia/ (6204)
3 (panic or agoraphobi* or agrophobi*).mp. (30012)
4 or/1‐3 (30012)
5 exp antidepressant agent/ (428171)
6 exp serotonin uptake inhibitor/ (261582)
7 exp serotonin noradrenalin reuptake inhibitor/ (177554)
8 exp noradrenalin uptake inhibitor/ (220773)
9 (Agomelatine or Alaproclate or Amoxapine or Amineptine or Amitriptylin* or Amitriptylinoxide or Atomoxetine or Befloxatone or Benactyzine or Binospirone or Brofaromine or (Buproprion or Amfebutamone) or Butriptyline or Caroxazone or Cianopramine or Cilobamine or Cimoxatone or Citalopram or (Chlorimipramin* or Clomipramin* or Chlomipramin* or Clomipramine) or Clorgyline or Clovoxamine or (CX157 or Tyrima) or Demexiptiline or Deprenyl or (Desipramine* or Pertofrane) or Desvenlafaxine or Dibenzepin or Diclofensine or Dimetacrin* or Dosulepin or Dothiepin or Doxepin or Duloxetine or Desvenlafaxine or DVS‐233 or Escitalopram or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluvoxamine or (Hyperforin or Hypericum or St John*) or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramine* or (Lu AA21004 or Vortioxetine) or Lu AA24530 or (LY2216684 or Edivoxetine) or Maprotiline or Melitracen or Metapramine or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptylin* or Noxiptilin* or Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramine or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone).mp. (230665)
10 (antidepress* or anti depress* or MAOI* or monoamine oxidase inhibit* or ((serotonin or norepinephrine or noradrenaline or nor epinephrine or nor adrenaline or neurotransmitt* or dopamine*) and (uptake or reuptake or re‐uptake)) or noradrenerg* or antiadrenergic or anti adrenergic or SSRI* or SNRI* or TCA* or tricyclic* or tetracyclic* or heterocyclic* or psychotropic*).mp. (332300)
11 exp Benzodiazepine derivative/ (217044)
12 (benzodiazepin* or BZD or abecarnil or adinazolam or alprazolam or arfendazam or bentazepam or bretazenil or bromazepam or brotizolam or camazepam or chlordiazepoxide or chlordesmethyldiazepam or cinolazepam or clobazam or clonazepam or clorazepate or chlorazepate or clotiazepam or cloxazolam or delorazepam or demoxepam or desmethyldiazepam or desoxydemoxepam or devazepide or diazepam or doxefazepam or estazolam or ethyl loflazepate or cm 6912 or cm‐6912 or etizolam or fludiazepam or flunitrazepam or flurazepam or dealkylflurazepam or flutoprazepam or fosazepam or gidazepam or girisopam or halazepam or haloxazolam or ketazolam or loflazepate or loprazolam or lorazepam or lormetazepam or meclonazepam or medazepam or metaclazepam or mexazolam or midazolam or nerisopam or nimetazepam or nitrazepam or norchlordiazepoxide or norclobazam or nordazepam or norfludiazepam or norflunitrazepam or oxazepam or wy 3498 or wy‐3498 or oxazolam or phenazepam or pinazepam or prazepam or premazepam or propazepam or quazepam or ripazepam or serazepine or sograzepide or talampanel or tarazepide or temazepam or tetrazepam or tofisopam or triazolam or zolazepam or zaleplon or zolpidem or zopiclone or eszopiclone or z‐drugs or z drugs).mp. (240315)
13 (azapirone or alnespirone or binospirone or buspirone or enilospirone or eptapirone or gepirone or ipsapirone or revospirone or tandospirone or zalospirone).mp. (10678)
14 (placebo* or dummy or sugar pill*).mp. (458097)
15 or/5‐14 (1174248)
16 major clinical study/ (3666712)
17 Randomized controlled trial/ (637112)
18 Controlled clinical study/ (465662)
19 double blind procedure/ (177381)
20 randomization/ (89576)
21 (RCT or randomi#ed).ti,ab,kw. (952346)
22 ((at random or random*) adj2 (allocat* or assign* or divide* or division or number)).ti,ab,kw. (289159)
23 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab,kw. (233577)
24 or/16‐23 (4754094)
25 ((animal or nonhuman) not (human and (animal or nonhuman))).de. (5727997)
26 24 not 25 (4613841)
27 4 and 15 and 26 (2703)
28 elsevier.cr. (25284730)
29 27 and 28 (2625)
30 (2014* or 2015* or 2016* or 2017* or 2018* or 2019* or 2020* or 2021*).yr,dc,dd. (11982917)
31 29 and 30 (685)
32 (random$ adj sampl$ adj7 ("cross section$" or questionnaire$1 or survey$ or database$1)).ti,ab. not (comparative study/ or controlled study/ or randomi?ed controlled.ti,ab. or randomly assigned.ti,ab.) (8318)
33 Cross‐sectional study/ not (randomized controlled trial/ or controlled clinical study/ or controlled study/ or randomi?ed controlled.ti,ab. or control group$1.ti,ab.) (258041)
34 (((case adj control$) and random$) not randomi?ed controlled).ti,ab. (18055)
35 (Systematic review not (trial or study)).ti. (163799)
36 (review.ab. and review.pt.) not trial.ti. (856407)
37 or/32‐36 (1233786)
38 31 not 37 (600)
***************************
Database: Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Daily <1946 to January 22, 2021>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 (panic or agoraphobi*).mp. (17630)
2 exp Antidepressive Agents/ (150189)
3 exp Neurotransmitter Uptake Inhibitors/ (147746)
4 exp Monoamine Oxidase Inhibitors/ (21782)
5 (antidepress* or anti depress* or MAOI* or monoamine oxidase inhibit* or ((serotonin or norepinephrine or noradrenaline or nor epinephrine or nor adrenaline or neurotransmitt* or dopamine*) and (uptake or reuptake or re‐uptake)) or noradrenerg* or antiadrenergic or anti adrenergic or SSRI* or SNRI* or TCA* or tricyclic* or tetracyclic* or heterocyclic* or psychotropic*).mp. (254597)
6 (Agomelatine or Alaproclate or Amoxapine or Amineptine or Amitriptylin* or Amitriptylinoxide or Atomoxetine or Befloxatone or Benactyzine or Binospirone or Brofaromine or (Buproprion or Amfebutamone) or Butriptyline or Caroxazone or Cianopramine or Cilobamine or Cimoxatone or Citalopram or (Chlorimipramin* or Clomipramin* or Chlomipramin* or Clomipramine) or Clorgyline or Clovoxamine or (CX157 or Tyrima) or Demexiptiline or Deprenyl or (Desipramine* or Pertofrane) or Desvenlafaxine or Dibenzepin or Diclofensine or Dimetacrin* or Dosulepin or Dothiepin or Doxepin or Duloxetine or Desvenlafaxine or DVS‐233 or Escitalopram or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluvoxamine or (Hyperforin or Hypericum or St John*) or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramine* or (Lu AA21004 or Vortioxetine) or Lu AA24530 or (LY2216684 or Edivoxetine) or Maprotiline or Melitracen or Metapramine or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptylin* or Noxiptilin* or Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramine or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone).mp. (103692)
7 exp Benzodiazepines/ (65890)
8 (benzodiazepin* or BZD or abecarnil or adinazolam or alprazolam or arfendazam or bentazepam or bretazenil or bromazepam or brotizolam or camazepam or chlordiazepoxide or chlordesmethyldiazepam or cinolazepam or clobazam or clonazepam or clorazepate or chlorazepate or clotiazepam or cloxazolam or delorazepam or demoxepam or desmethyldiazepam or desoxydemoxepam or devazepide or diazepam or doxefazepam or estazolam or ethyl loflazepate or cm 6912 or cm‐6912 or etizolam or fludiazepam or flunitrazepam or flurazepam or dealkylflurazepam or flutoprazepam or fosazepam or gidazepam or girisopam or halazepam or haloxazolam or ketazolam or loflazepate or loprazolam or lorazepam or lormetazepam or meclonazepam or medazepam or metaclazepam or mexazolam or midazolam or nerisopam or nimetazepam or nitrazepam or norchlordiazepoxide or norclobazam or nordazepam or norfludiazepam or norflunitrazepam or oxazepam or wy 3498 or wy‐3498 or oxazolam or phenazepam or pinazepam or prazepam or premazepam or propazepam or quazepam or ripazepam or serazepine or sograzepide or talampanel or tarazepide or temazepam or tetrazepam or tofisopam or triazolam or zolazepam or zaleplon or zolpidem or zopiclone or eszopiclone or z‐drugs or z drugs).mp. (95432)
9 (azapirone or alnespirone or binospirone or buspirone or enilospirone or eptapirone or gepirone or ipsapirone or revospirone or tandospirone or zalospirone).mp. (3696)
10 (placebo* or dummy or sugar pill*).mp. (240983)
11 or/2‐10 (716304)
12 randomized controlled trial.pt. (521298)
13 randomi#ed.ti,ab,kf. (660246)
14 controlled clinical trial.pt. (94034)
15 Double‐Blind Method/ (161967)
16 clinical trials as topic.sh. (194395)
17 randomly.ab. (351007)
18 (RCT or at random or (random* adj (assign* or allocat* or divid* or division or number))).ti,ab,kf. (235272)
19 trial.ti,kf. (249466)
20 (animals not (humans and animals)).sh. (4746234)
21 or/12‐19 (1389632)
22 21 not 20 (1286054)
23 1 and 11 and 22 (1221)
24 (2014* or 2015* or 2016* or 2017* or 2018* or 2019* or 2020* or 2021*).yr,dc,ed,ez. (9248835)
25 23 and 24 (133)
***************************
Database: APA PsycInfo <1806 to January Week 2 2021>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 Panic Attack/ or Panic/ or Panic Disorder/ (9720)
2 Agoraphobia/ (2901)
3 (panic or agoraphobi*).mp. (19403)
4 adnos.ti,ab,id. (5)
5 (anxiety disorder* adj2 otherwise specified).ti,ab,id. (72)
6 or/1‐5 (19447)
7 exp Antidepressant Drugs/ (39143)
8 Neurotransmitter Uptake Inhibitors/ or exp serotonin norepinephrine reuptake inhibitors/ or exp serotonin reuptake inhibitors/ (13726)
9 exp Monoamine Oxidase Inhibitors/ (2253)
10 exp Tricyclic Antidepressant Drugs/ (6412)
11 (antidepress* or anti depress* or MAOI* or monoamine oxidase inhibit* or ((serotonin or norepinephrine or noradrenaline or nor epinephrine or nor adrenaline or neurotransmitt* or dopamine*) and (uptake or reuptake or re‐uptake)) or noradrenerg* or antiadrenergic or anti adrenergic or SSRI* or SNRI* or TCA* or tricyclic* or tetracyclic* or heterocyclic* or psychotropic*).mp. (81357)
12 (Agomelatine or Alaproclate or Amoxapine or Amineptine or Amitriptylin* or Amitriptylinoxide or Atomoxetine or Befloxatone or Benactyzine or Binospirone or Brofaromine or (Buproprion or Amfebutamone) or Butriptyline or Caroxazone or Cianopramine or Cilobamine or Cimoxatone or Citalopram or (Chlorimipramin* or Clomipramin* or Chlomipramin* or Clomipramine) or Clorgyline or Clovoxamine or (CX157 or Tyrima) or Demexiptiline or Deprenyl or (Desipramine* or Pertofrane) or Desvenlafaxine or Dibenzepin or Diclofensine or Dimetacrin* or Dosulepin or Dothiepin or Doxepin or Duloxetine or Desvenlafaxine or DVS‐233 or Escitalopram or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluvoxamine or (Hyperforin or Hypericum or St John*) or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramine* or (Lu AA21004 or Vortioxetine) or Lu AA24530 or (LY2216684 or Edivoxetine) or Maprotiline or Melitracen or Metapramine or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptylin* or Noxiptilin* or Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramine or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone).mp. (38204)
13 exp benzodiazepines/ (10824)
14 (benzodiazepin* or BZD or abecarnil or adinazolam or alprazolam or arfendazam or bentazepam or bretazenil or bromazepam or brotizolam or camazepam or chlordiazepoxide or chlordesmethyldiazepam or cinolazepam or clobazam or clonazepam or clorazepate or chlorazepate or clotiazepam or cloxazolam or delorazepam or demoxepam or desmethyldiazepam or desoxydemoxepam or devazepide or diazepam or doxefazepam or estazolam or ethyl loflazepate or cm 6912 or cm‐6912 or etizolam or fludiazepam or flunitrazepam or flurazepam or dealkylflurazepam or flutoprazepam or fosazepam or gidazepam or girisopam or halazepam or haloxazolam or ketazolam or loflazepate or loprazolam or lorazepam or lormetazepam or meclonazepam or medazepam or metaclazepam or mexazolam or midazolam or nerisopam or nimetazepam or nitrazepam or norchlordiazepoxide or norclobazam or nordazepam or norfludiazepam or norflunitrazepam or oxazepam or wy 3498 or wy‐3498 or oxazolam or phenazepam or pinazepam or prazepam or premazepam or propazepam or quazepam or ripazepam or serazepine or sograzepide or talampanel or tarazepide or temazepam or tetrazepam or tofisopam or triazolam or zolazepam or zaleplon or zolpidem or zopiclone or eszopiclone or z‐drugs or z drugs).mp. (25520)
15 (azapirone or alnespirone or binospirone or buspirone or enilospirone or eptapirone or gepirone or ipsapirone or revospirone or tandospirone or zalospirone).mp. (1905)
16 (placebo* or dummy or sugar pill*).mp. (43421)
17 or/7‐16 (149294)
18 (RCT or at random or (random* adj3 (administ* or allocat* or assign* or class* or control* or crossover or cross‐over or determine* or divide* or division or distribut* or expose* or fashion or number* or place* or recruit* or split or subsitut* or treat*))).ti,ab,id. (107013)
19 trial.ti,id. (36775)
20 randomi#ed.ti,ab,id. (88005)
21 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$ or dummy)).ti,ab,id. (26947)
22 (placebo* or dummy or sugar pill*).mp. (43421)
23 or/18‐22 (174540)
24 6 and 17 and 23 (1090)
25 (2014* or 2015* or 2016* or 2017* or 2018* or 2019* or 2020* or 2021*).yr,an. (1307557)
26 24 and 25 (101)
27 (anxiety disorder? not (agoraphobi* or panic or (social adj3 (anxi* or phobi*)) or generalised or generalized or obsessive or compulsive or OCD or PTSD or post‐trauma* or post trauma* or posttrauma*)).ti,id,hw. (15437)
28 17 and 23 and 27 (596)
29 25 and 28 (153)
30 26 or 29 (239)
***************************
Search Name:
Date Run: 24/01/2021 16:49:05
Comment:
ID Search Hits
#1 MeSH descriptor: [Panic] this term only 264
#2 MeSH descriptor: [Panic Disorder] this term only 946
#3 MeSH descriptor: [Agoraphobia] this term only 433
#4 (panic or agoraphobi*):ti,ab,kw 3120
#5 (#1 or #2 or #3 or #4) 3120
#6 MeSH descriptor: [Antidepressive Agents] explode all trees 5773
#7 MeSH descriptor: [Neurotransmitter Uptake Inhibitors] explode all trees 3466
#8 MeSH descriptor: [Monoamine Oxidase Inhibitors] explode all trees 385
#9 (antidepress* or "anti depress*" or MAOI* or "monoamine oxidase inhibit*" or ((serotonin or norepinephrine or noradrenaline or nor epinephrine or nor adrenaline or neurotransmitt* or dopamine*) near (uptake or reuptake or re‐uptake)) or noradrenerg* or antiadrenergic or anti adrenergic or SSRI* or SNRI* or TCA* or tricyclic* or tetracyclic* or heterocyclic* or psychotropic*):ti,ab,kw 26071
#10 (Agomelatine or Alaproclate or Amoxapine or Amineptine or Amitriptylin* or Amitriptylinoxide or Atomoxetine or Befloxatone or Benactyzine or Binospirone or Brofaromine or (Buproprion or Amfebutamone) or Butriptyline or Caroxazone or Cianopramine or Cilobamine or Cimoxatone or Citalopram or (Chlorimipramin* or Clomipramin* or Chlomipramin* or Clomipramine) or Clorgyline or Clovoxamine or (CX157 or Tyrima) or Demexiptiline or Deprenyl or (Desipramine* or Pertofrane) or Desvenlafaxine or Dibenzepin or Diclofensine or Dimetacrin* or Dosulepin or Dothiepin or Doxepin or Duloxetine or Desvenlafaxine or DVS‐233 or Escitalopram or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluvoxamine or (Hyperforin or Hypericum or "St John*") or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramine* or ("Lu AA21004" or Vortioxetine) or "Lu AA24530" or (LY2216684 or Edivoxetine) or Maprotiline or Melitracen or Metapramine or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptylin* or Noxiptilin* or Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramine or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone):ti,ab,kw 24856
#11 MeSH descriptor: [Benzodiazepines] explode all trees 9637
#12 (benzodiazepin* or BZD or abecarnil or adinazolam or alprazolam or arfendazam or bentazepam or bretazenil or bromazepam or brotizolam or camazepam or chlordiazepoxide or chlordesmethyldiazepam or cinolazepam or clobazam or clonazepam or clorazepate or chlorazepate or clotiazepam or cloxazolam or delorazepam or demoxepam or desmethyldiazepam or desoxydemoxepam or devazepide or diazepam or doxefazepam or estazolam or ethyl loflazepate or "cm 6912" or cm‐6912 or etizolam or fludiazepam or flunitrazepam or flurazepam or dealkylflurazepam or flutoprazepam or fosazepam or gidazepam or girisopam or halazepam or haloxazolam or ketazolam or loflazepate or loprazolam or lorazepam or lormetazepam or meclonazepam or medazepam or metaclazepam or mexazolam or midazolam or nerisopam or nimetazepam or nitrazepam or norchlordiazepoxide or norclobazam or nordazepam or norfludiazepam or norflunitrazepam or oxazepam or wy 3498 or wy‐3498 or oxazolam or phenazepam or pinazepam or prazepam or premazepam or propazepam or quazepam or ripazepam or serazepine or sograzepide or talampanel or tarazepide or temazepam or tetrazepam or tofisopam or triazolam or zolazepam or zaleplon or zolpidem or zopiclone or eszopiclone or z‐drugs or z drugs or nonbenzo*):ti,ab,kw 23211
#13 (azapirone or alnespirone or binospirone or buspirone or enilospirone or eptapirone or gepirone or ipsapirone or revospirone or tandospirone or zalospirone or gabapentin* or pregabalin or mirogabalin or imagabalin):ti,ab,kw 5116
#14 (placebo* or dummy or "sugar pill*"):ti,ab,kw 320034
#15 (#6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14) 357277
#16 #5 and #15 1560
Limit 2014‐CLib: CENTRAL, Issue 1 of 12, 2021 = 421 trials
Appendix 3. Node‐splitting for response: direct, indirect and network estimates
The table below summarises the direct, indirect and network estimates for each comparison. P values < 0.05 reflect a statistically significant difference between direct and indirect estimates. These comparisons are in bold:
| Comparison |
Direct estimate: log OR (95% CrI) |
Indirect estimate: log OR (95% CrI) |
Network estimate: log OR (95% CrI) |
P value |
| Citalopram vs placebo | ‐0.62 (‐1.50 to 0.27) |
‐0.16 (‐2.30 to 2.00) |
‐0.64 (‐1.40 to 0.10) |
0.69 |
| Desipramine vs placebo | ‐0.93 (‐2.50 to 0.60) |
0.12 (‐2.3 to 2.8) |
‐0.65 (‐1.9 to 0.64) |
0.48 |
| Brofaromine vs placebo |
‐27.00 (‐64.00 to ‐3.90) |
‐1.60 (‐3.60 to 0.29) |
‐2.40 (‐4.20 to ‐0.89) |
0.01 |
| Fluoxetine vs placebo | ‐1.10 (‐2.40 to 0.19) |
‐0.99 (‐2.30 to 0.23) |
‐1.00 (‐1.90 to ‐0.18) |
0.90 |
| Sertraline vs placebo | ‐0.36 (‐1.10 to 0.38) |
‐0.72 (‐1.70 to 0.21) |
‐0.51 (‐1.10 to 0.06) |
0.53 |
| Fluvoxamine vs placebo |
‐1.00 (‐1.70 to ‐0.37) |
‐40.00 (‐96.00 to ‐3.70) |
‐1.10 (‐1.80 to ‐0.50) |
0.008 |
| Clomipramine vs placebo | ‐0.98 (‐1.80 to ‐0.33) |
‐1.30 (‐2.70 to 0.16) |
‐0.93 (‐1.50 to ‐0.38) |
0.70 |
| Paroxetine vs placebo | ‐0.58 (‐0.96 to ‐0.16) |
‐0.49 (‐1.50 to 0.39) |
‐0.62 (‐0.98 to ‐0.25) |
0.85 |
| Citalopram vs fluoxetine | ‐0.80 (‐2.70 to 0.99) |
‐0.22 (‐1.50 to 1.00) |
‐0.39 (‐1.40 to 0.60) |
0.60 |
| Citalopram vs clomipramine | 0.06 (‐1.20 to 1.30) |
‐0.69 (‐2.00 to 0.54) |
‐0.28 (‐1.10 to 0.51) |
0.37 |
| Desipramine vs fluoxetine | ‐1.10 (‐3.60 to 1.20) |
‐0.04 (‐1.80 to 1.70) |
‐0.39 (‐1.80 to 0.98) |
0.48 |
| Brofaromine vs fluvoxamine |
0.59 (‐1.20 to 2.50) |
33.00 (2.90 to 88.00) |
1.30 (‐0.18 to 3.00) |
0.01 |
| Alprazolam vs imipramine | 0.06 (‐1.50 to 1.60) |
0.08 (‐1.10 to 1.20) |
0.06 (‐0.83 to 0.94) |
0.98 |
| Alprazolam vs paroxetine | ‐0.08 (‐1.40 to 1.20) |
0.82 (0.17 to 1.50) |
0.60 (0.01 to 1.20) |
0.21 |
| Moclobemide vs fluoxetine | ‐0.10 (‐1.20 to 1.00) |
1.10 (‐0.66 to 2.90) |
0.24 (‐0.72 to 1.20) |
0.24 |
| Paroxetine vs sertraline | 0.07 (‐0.78 to 0.91) |
‐0.29 (‐1.10 to 0.55) |
‐0.11 (‐0.69 to 0.48) |
0.53 |
| Paroxetine vs venlafaxine | ‐0.04 (‐0.91 to 0.81) |
‐0.21 (‐1.20 to 0.75) |
‐0.03 (‐0.62 to 0.56) |
0.78 |
| Imipramine vs fluvoxamine | ‐0.75 (‐2.10 to 0.58) |
0.23 (‐0.98 to 1.50) |
‐0.05 (‐0.97 to 0.89) |
0.28 |
| Paroxetine vs clomipramine | ‐0.75 (‐2.10 to 0.58) |
0.23 (‐0.98 to 1.50) |
‐0.05 (‐0.97 to 0.89) |
0.07 |
| Moclobemide vs clomipramine | 0.66 (‐0.71 to 2.00) |
‐0.58 (‐2.10 to 1.00) |
0.13 (‐0.90 to 1.2) |
0.24 |
Appendix 4. Node splitting for dropout: direct, indirect and network estimates
The table below summarises the direct, indirect and network estimates for each comparison. P values < 0.05 reflect a statistically significant difference between direct and indirect estimates. These comparisons are in bold:
| Comparison |
Direct estimate: log OR (95% CrI) |
Indirect estimate: log OR (95% CrI) |
Network estimate: log OR (95% CrI) |
P value |
| Citalopram vs placebo | ‐0.25 (‐0.62 to 0.12) |
0.080 (‐3.7 to 3.8) |
‐0.29 (‐0.63 to 0.05) |
0.84 |
| Desipramine vs placebo |
‐2.3 (‐4.40 to ‐0.77) |
1.3 (‐1.60 to 4.9) |
‐1.5 (‐2.90 to ‐0.23) |
0.03 |
| Adinazolam vs placebo | ‐0.07 (‐0.62 to 0.49) |
0.32 (‐0.25 to 0.90) |
0.12 (‐0.28 to 0.52) |
0.33 |
| Fluoxetine vs placebo |
0.48 (‐0.38 to 1.40) |
‐1.6 (‐3.6 to 0.08) |
0.041 (‐0.72 to 0.81) |
0.03 |
| Sertraline vs placebo |
0.13 (‐0.23 to 0.49) |
‐0.45 (‐0.88 to ‐0.02) |
‐0.11 (‐0.38 to 0.17) |
0.04 |
| Fluvoxamine vs placebo | ‐0.09 (‐0.49 to 0.32) |
0.13 (‐3.70 to 3.90) |
0.01 (‐0.37 to 0.40) |
0.90 |
| Clomipramine vs placebo | ‐0.33 (‐0.66 to ‐0.01) |
‐0.09 (‐0.73 to 0.55) |
‐0.26 (‐0.54 to 0.02) |
0.51 |
| Imipramine vs placebo | ‐0.54 (‐0.78 to ‐0.31) |
1.10 (‐1.7 to 4.80) |
‐0.49 (‐0.71 to ‐0.27) |
0.24 |
| Paroxetine vs placebo | ‐0.02 (‐0.19 to 0.16) |
0.24 (‐0.25 to 0.74) |
0.03 (‐0.13 to 0.19) |
0.34 |
| Citalopram vs fluoxetine | 0.01 (‐3.70 to 3.70) |
0.36 (‐0.49 to 1.20) |
0.33 (‐0.49 to 1.20) |
0.84 |
| Citalopram vs clomipramine | 0.12 (‐0.42 to 0.65) |
0.02 (‐0.59 to 0.62) |
0.03 (‐0.35 to 0.42) |
0.79 |
| Desipramine vs fluoxetine |
‐1.00 (‐4.60 to 1.70) |
2.50 (0.82 to 4.80) |
1.50 (0.13 to 3.00) |
0.03 |
| Brofaromine vs fluvoxamine | 0.01 (‐3.70 to 3.80) |
‐0.12 (‐1.10 to 0.84) |
‐0.11 (‐1.00 to 0.78) |
0.94 |
| Brofaromine vs clomipramine | ‐0.40 (‐1.20 to 0.43) |
‐0.27 (‐4.00 to 3.50) |
‐0.39 (‐1.20 to 0.40) |
0.94 |
| Alprazolam vs clonazepam |
0.98 (‐0.84 to 3.20) |
‐1.10 (‐1.50 to ‐0.66) |
‐0.88 (‐1.30 to ‐0.50) |
0.03 |
| Clomipramine vs adinazolam | ‐0.53 (‐1.00 to ‐0.03) |
‐0.13 (‐0.77 to 0.49) |
‐0.38 (‐0.77 to 0.01) |
0.34 |
| Buspirone vs alprazolam | 3.10 (1.20 to 6.40) |
1.60 (0.76 to 2.50) |
2.00 (1.30 to 2.70) |
0.19 |
| Imipramine vs alprazolam | 0.85 (0.54 to 1.20) |
0.68 (0.14 to 1.20) |
0.74 (0.48 to 0.99) |
0.60 |
| Paroxetine vs alprazolam | 0.77 (0.06 to 1.50) |
1.3 (1.10 to 1.60) |
1.3 (1.00 to 1.50) |
0.16 |
| Imipramine vs buspirone | ‐0.56 (‐1.60 to 0.43) |
‐1.90 (‐3.20 to ‐0.73) |
‐1.20 (‐1.90 to ‐0.55) |
0.10 |
| Imipramine vs fluoxetine | 0.95 (‐1.70 to 4.50) |
‐0.68 (‐1.50 to 0.14) |
‐0.53 (‐1.30 to 0.25) |
0.25 |
| Paroxetine vs sertraline |
0.43 (0.04 to 0.83) |
‐0.16 (‐0.56 to 0.24) |
0.14 (‐0.14 to 0.42) |
0.04 |
| Paroxetine vs venlafaxine | 0.12 (‐0.22 to 0.45) |
‐0.15 (‐0.53 to 0.23) |
0.11 (‐0.13 to 0.35) |
0.30 |
| Imipramine vs clomipramine | ‐1.2 (‐2.1 to ‐0.38) |
‐0.26 (‐0.77 to 0.26) |
‐0.50 (‐0.93 to ‐0.08) |
0.05 |
| Imipramine vs clomipramine | ‐0.24 (‐1.60 to 1.10) |
‐0.21 (‐0.58 to 0.15) |
‐0.23 (‐0.58 to 0.13) |
0.97 |
| Paroxetine vs clomipramine | ‐0.14 (‐0.65 to 0.38) |
0.49 (0.10 to 0.88) |
0.29 (‐0.01 to 0.59) |
0.06 |
Appendix 5. WinBUGS and OpenBUGS code
Non‐response – bias adjusted model (run in OpenBUGS)
Placebo 1
Fluoxetine 2
Sertraline 3
Venlafaxine 4
Fluvoxamine 5
Clomipramine 6
Imipramine 7
Paroxetine 8
Moclobemide 9
Citalopram 10
Desipramine 11
Clonazepam 12
Adinazolam 13
Alprazolam 14
Escitalopram 15
Diazepam 16
Buspirone 17
Reboxetine 18
Etizolam 19
Ritanserin 20
model{
for(i in 1:ns){
w[i,1] <‐ 0 # adjustment for multi‐arm trials is zero for control arm
beta[i,1] <‐ 0 # no bias term in baseline arm
V[i,1] <‐ 0 # no variance term in baseline arm
Z[i,1] <‐ 0 # no bias term in baseline arm
delta[i,1] <‐ 0 # treatment effect is zero for control arm
mu[i] ~ dnorm(0,.0001) # vague priors for all trial baselines
for (k in 1:na[i]) {
r[i,k] ~ dbin(p[i,k],n[i,k]) # binomial likelihood
logit(p[i,k]) <‐ mu[i] + delta[i,k] + beta[i, k]* V[i,k] * Z[i,k] # model for linear predictor
rhat[i,k] <‐ p[i,k] * n[i,k] # expected value of the numerators
#Deviance contribution
dev[i,k] <‐ 2 * (r[i,k] * (log(r[i,k])‐log(rhat[i,k]))
+ (n[i,k]‐r[i,k]) * (log(n[i,k]‐r[i,k]) ‐ log(n[i,k]‐rhat[i,k]))) }
#Summed residual deviance contribution for this trial
resdev[i] <‐ sum(dev[i,1:na[i]])
for (k in 2:na[i]) {
# calculate variance of log odds ratio for comparisons with arm 1
# check for zero or 100% events in arm k
aux.a[i,k] <‐ equals(r[i,k],0)+equals(r[i,k],n[i,k])
# check for zero or 100% events in arm 1
aux.b[i,k] <‐ equals(r[i,1],0)+equals(r[i,1],n[i,1])
aux[i,k] <‐ max(aux.a[i,k],aux.b[i,k]) # any zero or 100% events?
# add 0.5 if zero or 100% events
V[i,k] <‐ 1/(r[i,k]+(0.5*aux[i,k])) + 1/(r[i,1]+(0.5*aux[i,k])) + 1/(n[i,k] r[i,k]+(0.5*aux[i,k])) + 1/(n[i,1]‐r[i,1]+(0.5*aux[i,k]))
# model for bias parameter beta
beta[i,k] ~ dnorm(mb[i,k], Pkappa)
mb[i,k] <‐ A[C[i,k]]
delta[i,k] ~ dnorm(md[i,k],taud[i,k]) # trial‐specific LOR distributions
md[i,k] <‐ d[t[i,k]] ‐ d[t[i,1]] + sw[i,k] # mean of LOR distributions (with multi‐arm trial correction)
taud[i,k] <‐ tau *2*(k‐1)/k # precision of LOR distributions (with multi‐arm trial correction)
w[i,k] <‐ (delta[i,k] ‐ d[t[i,k]] + d[t[i,1]]) # adjustment for multi‐arm RCTs
sw[i,k] <‐ sum(w[i,1:k‐1])/(k‐1) # cumulative adjustment for multi‐arm trials
} }
totresdev <‐ sum(resdev[]) # Total Residual Deviance
d[1]<‐0 # treatment effect is zero for reference treatment
# vague priors for treatment effects
for (k in 2:nt){ d[k] ~ dnorm(0,.0001) }
sd ~ dunif(0,5) # vague prior for between‐trial SD
tau <‐ pow(sd,‐2) # between‐trial precision = (1/between‐trial variance)
# mean bias: assumptions
A[1] <‐ 0 # Placebo v Placebo
A[2] <‐ b # Placebo v Any Drug
A[3] <‐ 0 #Drug vs Drug
# bias model prior for variance
kappa ~ dunif(0,5)
kappa.sq <‐ pow(kappa,2)
Pkappa <‐ 1/kappa.sq
# bias model prior for mean
b~dnorm(0,.001)
#prediction intervals
delta.new[1] <‐ 0
w.new[1] <‐ 0
for (k in 2:nt){
delta.new[k] ~dnorm(m.new[k], tau.new[k])
m.new[k] <‐ d[k] + sw.new[k]
tau.new[k] <‐ tau *2*(k‐1)/k
w.new[k] <‐ delta.new[k] ‐ d[k]
sw.new[k] <‐ sum(w.new[1:k‐1])/(k‐1)
}
# pairwise ORs and LORs for all possible pair‐wise comparisons
for (c in 1:(nt‐1)) {
for (k in (c+1):nt) {
or[c,k] <‐ exp(d[k] ‐ d[c])
lor[c,k] <‐ (d[k]‐d[c])
} }
# ranking on relative scale
for (k in 1:nt) {
rk[k] <‐ rank(d[],k) # assumes events are “bad”
best[k] <‐ equals(rk[k],1) #calculate probability that treat k is best
for (h in 1:nt){ prob[h,k] <‐ equals(rk[k],h) } # calculates probability that treat k is h‐th best
}
# Provide estimates of treatment effects T[k] on the natural (probability) scale given a Mean Effect, meanA, for #'standard' treatment A, with precision (1/variance) precA
E ~ dnorm(meanE,precE)
for (k in 1:nt) { logit(T[k]) <‐ E + (d[k] ‐ d[3]) }
# Provide estimates of number needed to treat NNT[k], Risk Difference RD[k],
# and Relative Risk RR[k], for each treatment, relative to treatment 1
for (c in 1:(nt‐1)) {
for (k in (c+1):nt) {
rr[c,k] <‐(T[k]/T[c])
logrr[c,k] <‐log(T[k]/T[c])
} }
#pairwise prediction intervals: ORs and LORs
for (c in 1: (nt‐1)) {
for (k in (c+1) :nt) {
lor.new[c,k] <‐ delta.new[k] ‐ delta.new[c]
or.new[c,k] <‐ exp(lor.new[c,k])
}
}
}
list(ns=48, nt=20, meanE=0.496, precE=1.267)
t[,1] t[,2] t[,3] r[,1] n[,1] r[,2] n[,2] r[,3] n[,3] na[] C[,1] C[,2] C[,3] Z[,1] Z[,2] Z[,3] #Name
2 10 NA 4 21 7 21 NA NA 2 NA 3 NA NA 0 NA #Amore 1999 bis
1 5 NA 52 92 33 87 NA NA 2 NA 2 NA NA 1 NA #Asnis 2011
1 8 NA 20 69 54 209 NA NA 2 NA 2 NA NA 1 NA #Ballenger 1998
3 8 NA 62 112 64 113 NA NA 2 NA 3 NA NA 0 NA #Bandelow 2004
1 7 NA 19 24 45 83 NA NA 2 NA 2 NA NA 1 NA #Barlow 2000
1 8 NA 5 10 6 9 NA NA 2 NA 2 NA NA 1 NA #Bergink 2005
1 5 NA 10 18 9 21 NA NA 2 NA 2 NA NA 1 NA #Black 1993
1 4 NA 87 180 72 181 NA NA 2 NA 2 NA NA 1 NA #Bradwejn 2005
1 6 NA 14 15 2 15 NA NA 2 NA 2 NA NA 1 NA #Broocks 1998
2 11 NA 2 11 4 11 NA NA 2 NA 3 NA NA 0 NA #Bystritsky 1995
1 6 NA 42 51 63 107 NA NA 2 NA 2 NA NA 1 NA #Caillard 1999
1 3 NA 22 62 18 63 NA NA 2 NA 2 NA NA 1 NA #Koszycki 2011
9 6 NA 15 67 9 68 NA NA 2 NA 3 NA NA 0 NA #Krueger 1999
1 4 NA 81 168 71 175 NA NA 2 NA 2 NA NA 1 NA #Liebowitz 2009
1 11 NA 15 28 9 28 NA NA 2 NA 2 NA NA 1 NA #Lydiard 1993
1 2 NA 35 90 16 90 NA NA 2 NA 2 NA NA 1 NA #Michelson 2001
3 8 NA 51 157 56 164 NA NA 2 NA 3 NA NA 0 NA #Pfizer 2008
1 3 NA 73 88 62 88 NA NA 2 NA 2 NA NA 1 NA #Pollack 1998
1 5 NA 24 37 9 36 NA NA 2 NA 2 NA NA 1 NA #Sharp 1990
1 8 NA 226 421 149 413 NA NA 2 NA 2 NA NA 1 NA #Sheehan 2005
9 2 NA 44 182 48 184 NA NA 2 NA 3 NA NA 0 NA #Tiller 1999
1 3 NA 32 56 63 113 NA NA 2 NA 2 NA NA 1 NA #Tsutsui 1997
1 8 NA 57 84 43 87 NA NA 2 NA 2 NA NA 1 NA #Tsutsui 2000a
1 8 NA 21 37 44 83 NA NA 2 NA 2 NA NA 1 NA #Tsutsui 2000b
1 18 NA 31 40 23 42 NA NA 2 NA 2 NA NA 1 NA #Versiani 2002
8 6 NA 5 38 4 35 NA NA 2 NA 3 NA NA 0 NA #Zhang 2000
1 12 NA 10 17 5 10 NA NA 2 NA 2 NA NA 1 NA #Baker 2003
1 6 8 52 123 49 121 36 123 3 NA 2 2 NA 1 1 #Lecrubier 1997
1 4 8 68 162 67 330 36 161 3 NA 2 2 NA 1 1 #Pollack 2007a
1 4 8 76 163 87 334 37 166 3 NA 2 2 NA 1 1 #Pollack 2007b
1 10 15 104 125 96 126 90 129 3 NA 2 2 NA 1 1 #Stahl 2003
1 6 10 64 96 49 98 136 281 3 NA 2 2 NA 1 1 #Wade 1997
1 5 20 18 19 5 20 18 20 3 NA 2 2 NA 1 1 #Den Boer 1990
1 5 7 37 50 41 50 33 48 3 NA 2 2 NA 1 1 #Nair 1996
1 8 14 15 72 14 77 15 77 3 NA 2 2 NA 1 1 #GSK 1994/04
1 7 14 17 20 11 20 22 41 3 NA 2 2 NA 1 1 #Uhlenhuth 1989
1 12 NA 15 16 2 13 NA NA 2 NA 2 NA NA 1 NA #Beauclair 1994
1 13 NA 43 83 107 232 NA NA 2 NA 2 NA NA 1 NA #Carter 1995
1 13 NA 63 103 30 99 NA NA 2 NA 2 NA NA 1 NA #Davidson 1994
1 14 NA 13 18 9 17 NA NA 2 NA 2 NA NA 1 NA #Klosko 1990
1 12 NA 140 225 80 230 NA NA 2 NA 2 NA NA 1 NA #Moroz 1999
1 14 16 57 79 29 78 32 81 3 NA 2 2 NA 1 1 #Noyes 1996
1 12 NA 41 69 134 344 NA NA 2 NA 2 NA NA 1 NA #Rosenbaum 1997
1 12 NA 7 10 4 14 NA NA 2 NA 2 NA NA 1 NA #Valenca 2000
1 14 NA 28 70 26 139 NA NA 2 NA 2 NA NA 1 NA #Pecknold 1994
1 19 NA 10 15 3 15 NA NA 2 NA 2 NA NA 1 NA #Savoldi 1990
1 14 17 28 33 10 34 28 34 3 NA 2 2 NA 1 1 #Sheehan 1993
1 14 NA 59 108 28 109 NA NA 2 NA 2 NA NA 1 NA #Schweizer 1993
END
Drop out – bias adjusted model (run in winBUGS)
Placebo 1
Fluoxetine 2
Sertraline 3
Venlafaxine 4
Fluvoxamine 5
Clomipramine 6
Imipramine 7
Paroxetine 8
Moclobemide 9
Citalopram 10
Desipramine 11
Brofaromine 12
Clonazepam 13
Adinazolam 14
Alprazolam 15
Escitalopram 16
Diazepam 17
Buspirone 18
Reboxetine 19
Etizolam 20
Mirtazapine 21
model{
for(i in 1:ns){
w[i,1] <‐ 0 # adjustment for multi‐arm trials is zero for control arm
beta[i,1] <‐ 0 # no bias term in baseline arm
V[i,1] <‐ 0 # no variance term in baseline arm
Z[i,1] <‐ 0 # no bias term in baseline arm
delta[i,1] <‐ 0 # treatment effect is zero for control arm
mu[i] ~ dnorm(0,.0001) # vague priors for all trial baselines
for (k in 1:na[i]) {
r[i,k] ~ dbin(p[i,k],n[i,k]) # binomial likelihood
logit(p[i,k]) <‐ mu[i] + delta[i,k] + beta[i, k]* V[i,k] * Z[i,k] # model for linear predictor
rhat[i,k] <‐ p[i,k] * n[i,k] # expected value of the numerators
#Deviance contribution
dev[i,k] <‐ 2 * (r[i,k] * (log(r[i,k])‐log(rhat[i,k]))
+ (n[i,k]‐r[i,k]) * (log(n[i,k]‐r[i,k]) ‐ log(n[i,k]‐rhat[i,k]))) }
#Summed residual deviance contribution for this trial
resdev[i] <‐ sum(dev[i,1:na[i]])
for (k in 2:na[i]) {
# calculate variance of log odds ratio for comparisons with arm 1
# check for zero or 100% events in arm k
aux.a[i,k] <‐ equals(r[i,k],0)+equals(r[i,k],n[i,k])
# check for zero or 100% events in arm 1
aux.b[i,k] <‐ equals(r[i,1],0)+equals(r[i,1],n[i,1])
aux[i,k] <‐ max(aux.a[i,k],aux.b[i,k]) # any zero or 100% events?
# add 0.5 if zero or 100% events
V[i,k] <‐ 1/(r[i,k]+(0.5*aux[i,k])) + 1/(r[i,1]+(0.5*aux[i,k])) + 1/(n[i,k]‐r[i,k]+(0.5*aux[i,k])) + 1/(n[i,1]‐r[i,1]+(0.5*aux[i,k]))
# model for bias parameter beta
beta[i,k] ~ dnorm(mb[i,k], Pkappa)
mb[i,k] <‐ A[C[i,k]]
delta[i,k] ~ dnorm(md[i,k],taud[i,k]) # trial‐specific LOR distributions
md[i,k] <‐ d[t[i,k]] ‐ d[t[i,1]] + sw[i,k] # mean of LOR distributions (with multi‐arm trial correction)
taud[i,k] <‐ tau *2*(k‐1)/k # precision of LOR distributions (with multi‐arm trial correction)
w[i,k] <‐ (delta[i,k] ‐ d[t[i,k]] + d[t[i,1]]) # adjustment for multi‐arm RCTs
sw[i,k] <‐ sum(w[i,1:k‐1])/(k‐1) # cumulative adjustment for multi‐arm trials
} }
totresdev <‐ sum(resdev[]) # Total Residual Deviance
d[1]<‐0 # treatment effect is zero for reference treatment
# vague priors for treatment effects
for (k in 2:nt){ d[k] ~ dnorm(0,.0001) }
sd ~ dunif(0,5) # vague prior for between‐trial SD
tau <‐ pow(sd,‐2) # between‐trial precision = (1/between‐trial variance)
# mean bias: assumptions
A[1] <‐ 0 # Placebo v Placebo
A[2] <‐ b # Placebo v Any Drug
A[3] <‐ 0 #Drug vs Drug
# bias model prior for variance
kappa ~ dunif(0,5)
kappa.sq <‐ pow(kappa,2)
Pkappa <‐ 1/kappa.sq
# bias model prior for mean
b~dnorm(0,.001)
#prediction intervals
delta.new[1] <‐ 0
w.new[1] <‐ 0
for (k in 2:nt){
delta.new[k] ~dnorm(m.new[k], tau.new[k])
m.new[k] <‐ d[k] + sw.new[k]
tau.new[k] <‐ tau *2*(k‐1)/k
w.new[k] <‐ delta.new[k] ‐ d[k]
sw.new[k] <‐ sum(w.new[1:k‐1])/(k‐1)
}
# pairwise ORs and LORs for all possible pair‐wise comparisons
for (c in 1:(nt‐1)) {
for (k in (c+1):nt) {
or[c,k] <‐ exp(d[k] ‐ d[c])
lor[c,k] <‐ (d[k]‐d[c])
} }
# ranking on relative scale
for (k in 1:nt) {
rk[k] <‐ rank(d[],k) # assumes events are “bad”
best[k] <‐ equals(rk[k],1) #calculate probability that treat k is best
for (h in 1:nt){ prob[h,k] <‐ equals(rk[k],h) } # calculates probability that treat k is h‐th best
}
# Provide estimates of treatment effects T[k] on the natural (probability) scale given a Mean Effect, meanA, for #'standard' treatment A, with precision (1/variance) precA
E ~ dnorm(meanE,precE)
for (k in 1:nt) { logit(T[k]) <‐ E + (d[k] ‐ d[3]) }
for (c in 1:(nt‐1)) {
for (k in (c+1):nt) {
rr[c,k] <‐(T[k]/T[c])
logrr[c,k] <‐log(T[k]/T[c])
} }
#pairwise prediction intervals: ORs and LORs
for (c in 1: (nt‐1)) {
for (k in (c+1) :nt) {
lor.new[c,k] <‐ delta.new[k] ‐ delta.new[c]
or.new[c,k] <‐ exp(lor.new[c,k])
}
}
}
list(ns=64, nt=21, meanE=‐0.76, precE=2.303)
t[,1] t[,2] t[,3] r[,1] n[,1] r[,2] n[,2] r[,3] n[,3] na[] C[,2] C[,3] Z[,2] Z[,3] #Name
2 7 NA 1 19 2 19 NA NA 2 3 NA 0 NA #Amore 1999
2 10 NA 1 21 1 21 NA NA 2 3 NA 0 NA #Amore 1999 bis
1 5 NA 29 95 29 93 NA NA 2 2 NA 1 NA #Asnis 2011
6 12 NA 22 46 27 47 NA NA 2 3 NA 0 NA #Bakish 1993
1 8 NA 23 69 67 209 NA NA 2 2 NA 1 NA #Ballenger 1998
3 8 NA 31 112 37 113 NA NA 2 3 NA 0 NA #Bandelow 2004
1 7 NA 10 24 32 83 NA NA 2 2 NA 1 NA #Barlow 2000
1 8 NA 3 10 2 9 NA NA 2 2 NA 1 NA #Bergink 2005
1 5 NA 7 25 4 25 NA NA 2 2 NA 1 NA #Black 1993
1 4 NA 45 180 51 181 NA NA 2 2 NA 1 NA #Bradwejn 2005
1 6 NA 4 15 0 15 NA NA 2 2 NA 1 NA #Broocks 1998
2 11 NA 1 11 2 11 NA NA 2 3 NA 0 NA #Bystritsky 1995
1 6 NA 25 57 37 123 NA NA 2 2 NA 1 NA #Caillard 1999
6 8 NA 4 35 1 38 NA NA 2 3 NA 0 NA #GSK 29060 525
1 5 NA 7 25 6 25 NA NA 2 2 NA 1 NA #Hoehn‐Saric 1993
6 14 NA 36 149 58 166 NA NA 2 3 NA 0 NA #Holland 1999
1 3 NA 19 62 16 63 NA NA 2 2 NA 1 NA #Koszycki 2011
6 9 NA 15 68 17 67 NA NA 2 3 NA 0 NA #Krueger 1999
1 4 NA 43 168 55 175 NA NA 2 2 NA 1 NA #Liebowitz 2009
1 3 NA 14 45 49 132 NA NA 2 2 NA 1 NA #Londborg 1998
1 11 NA 11 28 2 28 NA NA 2 2 NA 1 NA #Lydiard 1993
1 2 NA 10 90 15 90 NA NA 2 2 NA 1 NA #Michelson 2001
3 8 NA 25 157 42 164 NA NA 2 3 NA 0 NA #Pfizer 2008
1 7 18 3 22 4 20 5 18 3 2 2 1 1 #Pohl 1989
1 3 NA 15 88 17 88 NA NA 2 2 NA 1 NA #Pollack 1998
2 21 NA 3 15 2 15 NA NA 2 3 NA 0 NA #Ribeiro 2001
1 5 NA 9 37 7 36 NA NA 2 2 NA 1 NA #Sharp 1990
1 8 NA 117 445 133 444 NA NA 2 2 NA 1 NA #Sheehan 2005
1 3 NA 23 56 54 113 NA NA 2 2 NA 1 NA #Tsutsui 1997
1 8 NA 34 84 41 87 NA NA 2 2 NA 1 NA #Tsutsui 2000a
1 8 NA 18 37 38 83 NA NA 2 2 NA 1 NA #Tsutsui 2000b
5 12 NA 1 15 1 15 NA NA 2 3 NA 0 NA #Van Vliet 1996
1 19 NA 21 40 11 42 NA NA 2 2 NA 1 NA #Versiani 2002
6 8 NA 4 35 1 38 NA NA 2 3 NA 0 NA #Zhang 2000
1 6 NA 12 15 10 16 NA NA 2 2 NA 1 NA #Johnston 1995
1 6 7 2 20 7 20 6 20 3 2 2 1 1 #Gentil 1993
1 6 8 44 123 33 121 36 123 3 2 2 1 1 #Lecrubier 1997
1 4 8 42 162 53 330 35 161 3 2 2 1 1 #Pollack 2007a
1 4 8 42 163 67 334 30 166 3 2 2 1 1 #Pollack 2007b
1 10 16 47 125 38 126 31 129 3 2 2 1 1 #Stahl 2003
Non‐remission (run in winBUGS)
Placebo 1
Fluoxetine 2
Sertraline 3
Venlafaxine 4
Fluvoxamine 5
Clomipramine 6
Imipramine 7
Paroxetine 8
Moclobemide 9
Citalopram 10
Desipramine 11
Clonazepam 12
Alprazolam 13
Escitalopram 14
Diazepam 15
Buspirone 16
model{
for(i in 1:ns){
w[i,1] <‐ 0 # adjustment for multi‐arm trials is zero for control arm
delta[i,1] <‐ 0 # treatment effect is zero for control arm
mu[i] ~ dnorm(0,.0001) # vague priors for all trial baselines
for (k in 1:na[i]) {
r[i,k] ~ dbin(p[i,k],n[i,k]) # binomial likelihood
logit(p[i,k]) <‐ mu[i] + delta[i,k] # model for linear predictor
rhat[i,k] <‐ p[i,k] * n[i,k] # expected value of the numerators
#Deviance contribution
dev[i,k] <‐ 2 * (r[i,k] * (log(r[i,k])‐log(rhat[i,k]))
+ (n[i,k]‐r[i,k]) * (log(n[i,k]‐r[i,k]) ‐ log(n[i,k]‐rhat[i,k]))) }
#Summed residual deviance contribution for this trial
resdev[i] <‐ sum(dev[i,1:na[i]])
for (k in 2:na[i]) {
delta[i,k] ~ dnorm(md[i,k],taud[i,k]) # trial‐specific LOR distributions
md[i,k] <‐ d[t[i,k]] ‐ d[t[i,1]] + sw[i,k] # mean of LOR distributions (with multi‐arm trial correction)
taud[i,k] <‐ tau *2*(k‐1)/k # precision of LOR distributions (with multi‐arm trial correction)
w[i,k] <‐ (delta[i,k] ‐ d[t[i,k]] + d[t[i,1]]) # adjustment for multi‐arm RCTs
sw[i,k] <‐ sum(w[i,1:k‐1])/(k‐1) # cumulative adjustment for multi‐arm trials
} }
totresdev <‐ sum(resdev[]) # Total Residual Deviance
d[1]<‐0 # treatment effect is zero for reference treatment
# vague priors for treatment effects
for (k in 2:nt){ d[k] ~ dnorm(0,.0001) }
sd ~ dunif(0,5) # vague prior for between‐trial SD
tau <‐ pow(sd,‐2) # between‐trial precision = (1/between‐trial variance)
# pairwise ORs and LORs for all possible pair‐wise comparisons
for (c in 1:(nt‐1)) {
for (k in (c+1):nt) {
or[c,k] <‐ exp(d[k] ‐ d[c])
lor[c,k] <‐ (d[k]‐d[c])
} }
# ranking on relative scale
for (k in 1:nt) {
rk[k] <‐ rank(d[],k) # assumes events are “bad”
best[k] <‐ equals(rk[k],1) #calculate probability that treat k is best
for (h in 1:nt){ prob[h,k] <‐ equals(rk[k],h) } # calculates probability that treat k is h‐th best
}
# Provide estimates of treatment effects T[k] on the natural (probability) scale given a Mean Effect, meanA, for #'standard' treatment A, with precision (1/variance) precA
A ~ dnorm(meanA,precA)
for (k in 1:nt) { logit(T[k]) <‐ A + (d[k] ‐ d[3]) }
# Provide estimates of number needed to treat NNT[k], Risk Difference RD[k],
# and Relative Risk RR[k], for each treatment, relative to treatment 1
for (k in 2:nt) {
NNT[k] <‐ 1/(T[1]‐ T[k]) # assumes events are “bad”
RD[k] <‐ T[k] ‐ T[1]
RR[k] <‐ T[k]/T[1]
}
}
list(ns=32, nt=16, meanA=0.5624, precA=3.7355)
t[,1] t[,2] t[,3] r[,1] n[,1] r[,2] n[,2] r[,3] n[,3] na[] #trial
2 7 NA 5 19 6 19 NA NA 2 #Amore 1999
1 5 NA 53 95 33 93 NA NA 2 #Asnis 2011
1 8 NA 40 69 112 209 NA NA 2 #Ballenger 1998
1 4 NA 92 180 93 181 NA NA 2 #Bradwejn 2005
2 11 NA 4 11 5 11 NA NA 2 #Bystritsky 1995
1 5 NA 21 25 14 25 NA NA 2 #Hoehn‐Saric 1993
1 4 NA 126 168 117 175 NA NA 2 #Liebowitz 2009
1 3 NA 27 45 57 132 NA NA 2 #Londborg 1998
1 11 NA 15 28 6 28 NA NA 2 #Lydiard 1993
1 2 NA 65 90 52 90 NA NA 2 #Michelson 2001
1 3 NA 47 88 38 88 NA NA 2 #Pollack 1998
1 5 NA 20 37 16 36 NA NA 2 #Sharp 1990
1 8 NA 209 445 164 444 NA NA 2 #Sheehan 2005
2 9 NA 80 184 85 182 NA NA 2 #Tiller 1999
END
Frequency of Panic attacks
Placebo 1
Fluvoxamine 2
Paroxetine 3
Sertraline 4
Venlafaxine 5
Clomipramine 6
Maprotiline 7
Adinazolam 8
Moclobemide 9
Alprazolam 10
Imipramine 11
Desipramine 12
Fluoxetine 13
Reboxetine 14
Clonazepam 15
Diazepam 16
# Normal likelihood, identity link
# Random effects model for multi‐arm trials
model{ # *** PROGRAM STARTS
for(i in 1:ns){ # LOOP THROUGH STUDIES
w[i,1] <‐ 0 # adjustment for multi‐arm trials is zero for control arm
delta[i,1] <‐ 0 # treatment effect is zero for control arm
mu[i] ~ dnorm(0,.0001) # vague priors for all trial baselines
for (k in 1:na[i]) { # LOOP THROUGH ARMS
var[i,k] <‐ pow(se[i,k],2) # calculate variances
prec[i,k] <‐ 1/var[i,k] # set precisions
y[i,k] ~ dnorm(theta[i,k],prec[i,k]) # normal likelihood
theta[i,k] <‐ mu[i] + delta[i,k] # model for linear predictor
dev[i,k] <‐ (y[i,k]‐theta[i,k])*(y[i,k]‐theta[i,k])*prec[i,k] #Deviance contribution
}
resdev[i] <‐ sum(dev[i,1:na[i]]) # summed residual deviance contribution for this trial
for (k in 2:na[i]) { # LOOP THROUGH ARMS
delta[i,k] ~ dnorm(md[i,k],taud[i,k]) # trial‐specific LOR distributions
md[i,k] <‐ d[t[i,k]] ‐ d[t[i,1]] + sw[i,k] # mean of treat effects distributions (with multi‐arm trial correction)
taud[i,k] <‐ tau *2*(k‐1)/k # precision of treat effects distributions (with multi‐arm trial correction)
w[i,k] <‐ (delta[i,k] ‐ d[t[i,k]] + d[t[i,1]]) # adjustment for multi‐arm RCTs
sw[i,k] <‐ sum(w[i,1:k‐1])/(k‐1) # cumulative adjustment for multi‐arm trials
}
}
totresdev <‐ sum(resdev[]) #Total Residual Deviance
d[1]<‐0 # treatment effect is zero for reference treatment
for (k in 2:nt){ d[k] ~ dnorm(0,.0001) } # vague priors for treatment effects
sd ~ dunif(0,5) # vague prior for between‐trial SD.
tau <‐ pow(sd,‐2) # between‐trial precision = (1/between‐trial variance)
# ranking on relative scale
for (k in 1:nt) {
rk[k] <‐ rank(d[],k) # assumes events are “bad”
best[k] <‐ equals(rk[k],1) #calculate probability that treat k is best
for (h in 1:nt){ prob[h,k] <‐ equals(rk[k],h) } # calculates probability that treat k is h‐th best
}
} # *** PROGRAM ENDS
list(ns=40, nt=16)
t[,1] t[,2] t[,3] y[,1] y[,2] y[,3] se[,1] se[,2] se[,3] na[] #Study#
1 2 NA 2.1 1.2 NA 0.43788027 0.246585883 NA 2 #Asnis 2011
1 3 NA 9.8 6.37 NA 2.118791014 0.87086851 NA 2 #Ballenger 1998
4 3 NA ‐1.82 ‐2.13 NA 1.174833708 1.189814663 NA 2 #Bandelow 2004
1 5 NA ‐3.7 ‐5 NA 0.105697795 0.10830801 NA 2 #Bradwejn 2005
1 6 NA 1.1 0.55 NA 0.266053216 0.112141433 NA 2 #Caillard 1999
7 2 NA 5 1.6 NA 0.40824829 0.402492236 NA 2 #Den Boer 1988
3 6 NA 0.38 0.16 NA 1.310002864 0.450001084 NA 2 #GSK 29060 525
1 2 NA 1.9 0.8 NA 4.735258411 1.42128463 NA 2 #Hoehn‐Saric 1993
8 6 NA 3.1 1.5 NA 0.624500541 0.499522781 NA 2 #Holland 1999
16 6 NA 3.7 3.4 NA 1.099524999 1.103537094 NA 2 #Krueger 1999
9 10 NA 4.7 2.4 NA 2.863295573 1.407124728 NA 2 #Lepola 1990
1 5 NA ‐1.56 ‐1.82 NA 0.110397746 0.110397746 NA 2 #Liebowitz 2009
1 4 NA 8.8 2.17 NA 3.028681456 0.535075975 NA 2 #Londborg 1998
1 11 NA 1.6 0.9 NA 0.62364138 0.491353815 NA 2 #Lydiard 1993
1 12 NA ‐2.2 ‐2.9 NA 0.337309617 0.337309617 NA 2 #Michelson 2001
4 3 NA ‐4.07 ‐4.59 NA 0.543062184 0.667292095 NA 2 #Pfizer 2008
1 10 NA 14 13 NA 0.63107412 0.436033256 NA 2 #Pohl 1989
1 4 NA 1.31 0.74 NA 2.141601196 0.646483859 NA 2 #Pollack 1998
1 4 NA 4.47 4.39 NA 0.828571429 1.170574176 NA 2 #Tsutsui 1997
1 3 NA 0 0 NA 1.688749537 1.78991886 NA 2 #Tsutsui 2000a
1 13 NA 5.8 1.2 NA 1.103105664 0.279478278 NA 2 #Versiani 2002
1 6 3 ‐8.5 ‐8.7 ‐12.2 1.258881491 1.227755341 1.475052479 3 #Lecrubier 1997
1 5 3 ‐5.1 ‐6.9 ‐7.6 0.109337903 0.077068521 1.342750957 3 #Pollack 2007a
1 5 3 ‐4.8 ‐6.25 ‐6 0.109687785 0.078070178 1.30038217 3 #Pollack 2007b
1 2 10 4.6 5.8 2.5 0.431760375 0.297372212 0.300891532 3 #Nair 1996
1 3 9 ‐8.6 ‐10.1 ‐11.3 1.499996438 1.29499611 1.900413489 3 #GSK 1994/04
1 10 9 2.8 1.3 0.9 0.654073773 0.38340579 0.312358076 3 #Schweizer 1993b
1 10 9 0 0.13 0 0.01 0.116666667 0.003535534 3 #Sheikh 1999
1 10 9 ‐3.3 ‐5.9 ‐7.5 2.437314095 1.00623059 1.510518675 3 #Taylor 1990
1 10 9 20.05 8.85 4.12 0.661876121 1.030827338 0.877696542 3 #Uhlenhuth 1989
1 15 NA 10.8 2.4 NA 1.725 0.610170216 NA 2 #Beauclair 1994
1 8 NA ‐1.23 ‐2.3 NA 0.45 0.285436723 NA 2 #Carter 1995
1 8 NA 1.8 1.3 NA 0.299540101 0.299501269 NA 2 #Davidson 1994
1 9 NA 0.56 0.51 NA 0.256284643 0.2025 NA 2 #Klosko 1990
1 9 NA 5.36 1.93 NA 1.086244573 0.540315633 NA 2 #Lydiard 1992
1 15 NA 2.2 1.5 NA 0.000686803 0.000677285 NA 2 #Moroz 1999
1 9 14 4.9 1.8 1.4 0.956324716 0.837885005 0.288888889 3 #Noyes 1996
1 9 NA 2.7 1.35 NA 0.590442933 0.246822979 NA 2 #Pecknold 1994
1 9 NA ‐0.5 ‐2.7 NA 0.59426608 0.415861968 NA 2 #Schweizer 1993
1 15 9 ‐2 ‐5.6 ‐5.3 5.820379557 2.431840076 2.653613888 3 #Tesar 1991
END
Panic scales, endpoint (run in WinBUGS)
1 Placebo
2 Fluvoxamine
3 Paroxetine
4 Imipramine
5 Venlafaxine
6 Clomipramine
7 Adinazolam
8 Brofaromine
9 Reboxetine
10 Alprazolam
11 Clonazepam
12 Moclobemide
# Normal likelihood, identity link: SMD with arm‐based means
# Random effects model for multi‐arm trials
model{ # *** PROGRAM STARTS
for(i in 1:ns){ # LOOP THROUGH STUDIES
w[i,1] <‐ 0 # adjustment for multi‐arm trials is zero for control arm
delta[i,1] <‐ 0 # treatment effect is zero for control arm
mu[i] ~ dnorm(0,.0001) # vague priors for all trial baselines
for (k in 1:na[i]){
var[i,k] <‐ pow(se[i,k],2) # calcultate variances
prec[i,k] <‐ 1/var[i,k] # set precisions
y[i,k] ~ dnorm(phi[i,k], prec[i,k]) # normal likelihood
phi[i,k] <‐ theta[i,k] * Pooled.sd[i] # theta is SMD
theta[i,k] <‐ mu[i] + delta[i,k] # model for linear predictor
#Deviance contribution
dev[i,k] <‐ (y[i,k]‐phi[i,k])*(y[i,k]‐phi[i,k])/var[i,k]
}
# summed residual deviance contribution for this trial
resdev[i] <‐ sum(dev[i,1:na[i]])
for (k in 2:na[i]){ # LOOP THROUGH ARMS
# trial‐specific RE distributions
delta[i,k] ~ dnorm(md[i,k], taud[i,k])
md[i,k] <‐ d[t[i,k]] ‐ d[t[i,1]] + sw[i,k]
# precision of RE distributions (with multi‐arm trial correction)
taud[i,k] <‐ tau *2*(k‐1)/k
#adjustment, multi‐arm RCTs
w[i,k] <‐ delta[i,k] ‐ d[t[i,k]] + d[t[i,1]]
# cumulative adjustment for multi‐arm trials
sw[i,k] <‐sum(w[i,1:k‐1])/(k‐1)
}
}
totresdev <‐ sum(resdev[]) #Total Residual Deviance
d[1]<‐0 # treatment effect is zero for control arm
# vague priors for treatment effects
for (k in 2:nt){ d[k] ~ dnorm(0,.0001) }
sd ~ dunif(0,10) # vague prior for for between‐trial SD
tau <‐ pow(sd,‐2) # between‐trial precision = (1/between‐trial variance)
# pairwise differences
for (c in 1:(nt‐1)) { for (k in (c+1):nt) { diff[c,k] <‐ d[k] ‐ d[c] } }
# rank treatments
for (k in 1:nt) {
rk[k] <‐ rank(d[],k)
best[k] <‐ equals(rk[k],1) # Smallest is best (i.e. rank 1)
# prob treat k is h‐th best, prob[1,k]=best[k]
for (h in 1:nt) { prob[h,k] <‐ equals(rk[k],h) }
}
}
} # *** PROGRAM ENDS
DATA
#nt=no. treatments, ns=no. studies;
list(nt=12,ns=19)
t[,1] t[,2] t[,3] y[,1] y[,2] y[,3] se[,1] se[,2] se[,3] Pooled.sd[] na[] #Study#
1 2 NA 0.9 0.5 NA 0.093831486 0.085769003 NA 0.852878145 2 #Asnis 2011
1 3 NA 3 2.86 NA 0.156501609 0.090614595 NA 1.307543332 2 #Ballenger 1998
1 4 NA 0.9 1.05 NA 0.183711731 0.08451848 NA 0.800284473 2 #Barlow 2000
1 2 NA 14.8 8.1 NA 3.488393454 4.997189686 NA 19.59859344 2 #Black 1993
1 5 NA 1.27 2.71 NA 0.097982627 0.099611746 NA 1.265132574 2 #Bradwejn 2005
1 6 NA 3.9 2.85 NA 0.210042013 0.145010473 NA 1.5 2 #Caillard 1999
7 6 NA 2.7 1.7 NA 0.160695825 0.103479296 NA 1.407631354 2 #Holland 1999
12 6 NA 3.1 2.9 NA 0.200003597 0.199994876 NA 1.643206626 2 #Krueger 1999
1 3 NA 3.21 2.83 NA 0.059946532 0.059540208 NA 1.22013713 2 #Sheehan 2005
1 8 NA 12.8 6.6 NA 0.347439614 0.490577891 NA 1.638766474 2 #Van Vliet 1993
1 9 NA 3.8 2.5 NA 0.206021205 0.207142724 NA 1.265078372 2 #Versiani 2002
3 6 NA 3.32 3.1 NA 0.212074694 0.176013196 NA 1.159412077 2 #Zhang 2000
1 11 NA 6.6 2.5 NA 0.45 0.332820118 NA 1.562049935 2 #Beauclair 1994
1 7 NA 3.6 3.2 NA 0.100503586 0.099498744 NA 1.005411856 2 #Davidson 1994
1 11 NA 3.5 1.5 NA 0.4 0.221880078 NA 0.979795897 2 #Valenca 2000
1 10 NA 3.6 2.75 NA 0.169722463 0.099238105 NA 1.25886192 2 #Pecknold 1994
1 5 3 9.2 5.44 6.2 0.626498204 0.668302213 0.964339324 10.98180467 3 #Pollack 2007a
1 2 4 3.3 3.4 2.6 0.218797487 0.192148199 0.231455025 1.415371448 3 #Nair 1996
1 3 10 2.3 2 1.9 0.100399203 0.099484975 0.200140362 1.203177189 3 #GSK 1994/04
END
Panic scales, change from baseline (run in WinBUGS)
1 Placebo
2 Paroxetine
3 Sertraline
4 Imipramine
5 Venlafaxine
6 Clomipramine
7 Fluoxetine
8 Desipramine
9 Adinazolam
10 Citalopram
11 Escitalopram
12 Alprazolam
13 Clonazepam
14 Diazepam
# Normal likelihood, identity link: SMD with arm‐based means
# Random effects model for multi‐arm trials
model{ # *** PROGRAM STARTS
for(i in 1:ns){ # LOOP THROUGH STUDIES
w[i,1] <‐ 0 # adjustment for multi‐arm trials is zero for control arm
delta[i,1] <‐ 0 # treatment effect is zero for control arm
mu[i] ~ dnorm(0,.0001) # vague priors for all trial baselines
for (k in 1:na[i]){
var[i,k] <‐ pow(se[i,k],2) # calcultate variances
prec[i,k] <‐ 1/var[i,k] # set precisions
y[i,k] ~ dnorm(phi[i,k], prec[i,k]) # normal likelihood
phi[i,k] <‐ theta[i,k] * Pooled.sd[i] # theta is SMD
theta[i,k] <‐ mu[i] + delta[i,k] # model for linear predictor
#Deviance contribution
dev[i,k] <‐ (y[i,k]‐phi[i,k])*(y[i,k]‐phi[i,k])/var[i,k]
}
# summed residual deviance contribution for this trial
resdev[i] <‐ sum(dev[i,1:na[i]])
for (k in 2:na[i]){ # LOOP THROUGH ARMS
# trial‐specific RE distributions
delta[i,k] ~ dnorm(md[i,k], taud[i,k])
md[i,k] <‐ d[t[i,k]] ‐ d[t[i,1]] + sw[i,k]
# precision of RE distributions (with multi‐arm trial correction)
taud[i,k] <‐ tau *2*(k‐1)/k
#adjustment, multi‐arm RCTs
w[i,k] <‐ delta[i,k] ‐ d[t[i,k]] + d[t[i,1]]
# cumulative adjustment for multi‐arm trials
sw[i,k] <‐sum(w[i,1:k‐1])/(k‐1)
}
}
totresdev <‐ sum(resdev[]) #Total Residual Deviance
d[1]<‐0 # treatment effect is zero for control arm
# vague priors for treatment effects
for (k in 2:nt){ d[k] ~ dnorm(0,.0001) }
sd ~ dunif(0,10) # vague prior for for between‐trial SD
tau <‐ pow(sd,‐2) # between‐trial precision = (1/between‐trial variance)
# pairwise differences
for (c in 1:(nt‐1)) { for (k in (c+1):nt) { diff[c,k] <‐ d[k] ‐ d[c] } }
# rank treatments
for (k in 1:nt) {
rk[k] <‐ rank(d[],k)
best[k] <‐ equals(rk[k],1) # Smallest is best (i.e. rank 1)
# prob treat k is h‐th best, prob[1,k]=best[k]
for (h in 1:nt) { prob[h,k] <‐ equals(rk[k],h) }
}
}
} # *** PROGRAM ENDS
DATA
#nt=no. treatments, ns=no. studies;
list(nt=14,ns=16)
t[,1] t[,2] t[,3] y[,1] y[,2] y[,3] se[,1] se[,2] se[,3] Pooled.sd[] na[] #Study#
1 6 NA ‐0.3 ‐3.1 NA 0.25819889 0.180739223 NA 0.863133825 2 #Broocks 1998
1 5 NA ‐7.5 ‐9.28 NA 0.449889616 0.459763915 NA 5.476326989 2 #Liebowitz 2009
1 8 NA ‐7.2 ‐8.4 NA 0.396862697 0.321269802 NA 1.910497317 2 #Lydiard 1993
1 7 NA ‐7.6 ‐11.5 NA 0.664078309 0.68516016 NA 6.400781202 2 #Michelson 2001
1 4 NA ‐4.7 ‐5.8 NA 0.558585877 0.56348913 NA 2.572984648 2 #Pohl 1989
1 3 NA ‐0.64 ‐0.88 NA 0.077192103 0.073554247 NA 0.705072866 2 #Pollack 1998
1 9 NA ‐0.85 ‐1.04 NA 0.127777778 0.070046772 NA 1.08409517 2 #Carter 1995
1 12 NA ‐1 ‐2.1 NA 0.14596009 0.128719181 NA 1.348320715 2 #Schweizer 1993
3 2 NA ‐13.5 ‐12.7 NA 1.210692425 1.261431006 NA 12.93972252 2 #Bandelow 2004
2 6 NA ‐3.32 ‐3.1 NA 1.289660298 0.978848896 NA 6.942802028 2 #GSK 29060 525
3 2 NA ‐17.4 ‐17 NA 0.757802459 0.707223138 NA 8.25991828 2 #Pfizer 2008
1 5 2 ‐6.8 ‐9.61 ‐9.51 0.28102491 0.304979184 0.283719746 4.583858606 3 #Pollack 2007b
1 10 11 ‐1.2 ‐1.5 ‐1.6 0.070243936 0.099215674 0.100175845 0.987761683 3 #Stahl 2003
1 4 12 ‐0.9 ‐1.9 ‐2.3 0.223606798 0.156524758 0.400083325 1.360828412 3 #Taylor 1990
1 13 12 ‐0.9 ‐2.4 ‐1.8 0.298481003 0.254950976 0.265361389 1.312346571 3 #Tesar 1991
1 12 14 ‐6.1 ‐7.8 ‐7.6 0.22501758 0.169841555 0.188888889 1.738676445 3 #Noyes 1996
END
Agoraphobia, end of treatment (run in WinBUGS)
1 Placebo
2 Paroxetine
3 Desipramine
4 Reboxetine
5 Citalopram
6 Escitalopram
7 Clomipramine
8 Fluvoxamine
9 Ritanserin
10 Imipramine
11 Alprazolam
12 Adinazolam
13 Diazepam
14 Buspirone
# Normal likelihood, identity link: SMD with arm‐based means
# Random effects model for multi‐arm trials
model{ # *** PROGRAM STARTS
for(i in 1:ns){ # LOOP THROUGH STUDIES
w[i,1] <‐ 0 # adjustment for multi‐arm trials is zero for control arm
delta[i,1] <‐ 0 # treatment effect is zero for control arm
mu[i] ~ dnorm(0,.0001) # vague priors for all trial baselines
for (k in 1:na[i]){
var[i,k] <‐ pow(se[i,k],2) # calcultate variances
prec[i,k] <‐ 1/var[i,k] # set precisions
y[i,k] ~ dnorm(phi[i,k], prec[i,k]) # normal likelihood
phi[i,k] <‐ theta[i,k] * Pooled.sd[i] # theta is SMD
theta[i,k] <‐ mu[i] + delta[i,k] # model for linear predictor
#Deviance contribution
dev[i,k] <‐ (y[i,k]‐phi[i,k])*(y[i,k]‐phi[i,k])/var[i,k]
}
# summed residual deviance contribution for this trial
resdev[i] <‐ sum(dev[i,1:na[i]])
for (k in 2:na[i]){ # LOOP THROUGH ARMS
# trial‐specific RE distributions
delta[i,k] ~ dnorm(md[i,k], taud[i,k])
md[i,k] <‐ d[t[i,k]] ‐ d[t[i,1]] + sw[i,k]
# precision of RE distributions (with multi‐arm trial correction)
taud[i,k] <‐ tau *2*(k‐1)/k
#adjustment, multi‐arm RCTs
w[i,k] <‐ delta[i,k] ‐ d[t[i,k]] + d[t[i,1]]
# cumulative adjustment for multi‐arm trials
sw[i,k] <‐sum(w[i,1:k‐1])/(k‐1)
}
}
totresdev <‐ sum(resdev[]) #Total Residual Deviance
d[1]<‐0 # treatment effect is zero for control arm
# vague priors for treatment effects
for (k in 2:nt){ d[k] ~ dnorm(0,.0001) }
sd ~ dunif(0,10) # vague prior for for between‐trial SD
tau <‐ pow(sd,‐2) # between‐trial precision = (1/between‐trial variance)
# pairwise differences
for (c in 1:(nt‐1)) { for (k in (c+1):nt) { diff[c,k] <‐ d[k] ‐ d[c] } }
# rank treatments
for (k in 1:nt) {
rk[k] <‐ rank(d[],k)
best[k] <‐ equals(rk[k],1) # Smallest is best (i.e. rank 1)
# prob treat k is h‐th best, prob[1,k]=best[k]
for (h in 1:nt) { prob[h,k] <‐ equals(rk[k],h) }
}
}
} # *** PROGRAM ENDS
DATA
#nt=no. treatments, ns=no. studies;
list(nt=14,ns=15)
t[,1] t[,2] t[,3] y[,1] y[,2] y[,3] se[,1] se[,2] se[,3] Pooled.sd[] na[] #Study#
1 2 NA 4.8 4.09 NA 0.38523473 0.225498915 NA 3.245320377 2 #Ballenger 1998
1 3 NA 4 2.8 NA 0.56694671 0.529150262 NA 2.901723626 2 #Lydiard 1993
1 2 NA 31 24.2 NA 0.949904626 0.98 NA 18.6045613 2 #Sheehan 2005
1 4 NA 5.2 3.2 NA 0.486664263 0.207142724 NA 2.311832577 2 #Versiani 2002
1 5 6 21.1 17.2 16.1 0.635941766 0.800339772 0.899793754 8.56053517 3 #Stahl 2003
1 7 5 34 27 22.78 0.306186218 0.282842712 0.689015234 9.057498202 3 #Wade 1997
1 8 9 28.84 23.75 31.1 2.473101611 2.569242106 1.996808704 10.1808113 3 #Den Boer 1990
1 10 11 3.2 2.6 2.6 0.151716521 0.141602086 0.157785846 2.964898818 3 #CNCPS 1992
1 10 11 1.83 1.68 1.47 0.141421356 0.133279156 0.088775453 0.680104716 3 #Schweizer 1993b
1 10 11 4.9 5 3.66 0.670820393 0.626099034 1.138506724 5.494917966 3 #Uhlenhuth 1989
1 12 NA 4.7 4.9 NA 0.200002137 0.200002525 NA 2.010396454 2 #Davidson 1994
1 11 13 5.2 3.6 3.5 0.382529886 0.32836034 0.333333333 3.087825103 3 #Noyes 1996
1 11 NA 5.3 3.9 NA 0.300002381 0.247671167 NA 2.790035842 2 #Pecknold 1994
1 11 14 27.2 16.8 25.5 3.435363756 3.063766745 3.899219543 17.88476183 3 #Sheehan 1993
1 11 NA 14.63 8.89 NA 1.36 1.209712776 NA 5.282290997 2 #Munjack 1989
END
Agoraphobia, change in baseline (run in WinBUGS)
1=Placebo
2=Venlafaxine
3=Clomipramine
4=Paroxetine
5=Alprazolam
6=Imipramine
7=Adinazolam
# Normal likelihood, identity link: SMD with arm‐based means
# Random effects model for multi‐arm trials
model{ # *** PROGRAM STARTS
for(i in 1:ns){ # LOOP THROUGH STUDIES
w[i,1] <‐ 0 # adjustment for multi‐arm trials is zero for control arm
delta[i,1] <‐ 0 # treatment effect is zero for control arm
mu[i] ~ dnorm(0,.0001) # vague priors for all trial baselines
for (k in 1:na[i]){
var[i,k] <‐ pow(se[i,k],2) # calcultate variances
prec[i,k] <‐ 1/var[i,k] # set precisions
y[i,k] ~ dnorm(phi[i,k], prec[i,k]) # normal likelihood
phi[i,k] <‐ theta[i,k] * Pooled.sd[i] # theta is SMD
theta[i,k] <‐ mu[i] + delta[i,k] # model for linear predictor
#Deviance contribution
dev[i,k] <‐ (y[i,k]‐phi[i,k])*(y[i,k]‐phi[i,k])/var[i,k]
}
# summed residual deviance contribution for this trial
resdev[i] <‐ sum(dev[i,1:na[i]])
for (k in 2:na[i]){ # LOOP THROUGH ARMS
# trial‐specific RE distributions
delta[i,k] ~ dnorm(md[i,k], taud[i,k])
md[i,k] <‐ d[t[i,k]] ‐ d[t[i,1]] + sw[i,k]
# precision of RE distributions (with multi‐arm trial correction)
taud[i,k] <‐ tau *2*(k‐1)/k
#adjustment, multi‐arm RCTs
w[i,k] <‐ delta[i,k] ‐ d[t[i,k]] + d[t[i,1]]
# cumulative adjustment for multi‐arm trials
sw[i,k] <‐sum(w[i,1:k‐1])/(k‐1)
}
}
totresdev <‐ sum(resdev[]) #Total Residual Deviance
d[1]<‐0 # treatment effect is zero for control arm
# vague priors for treatment effects
for (k in 2:nt){ d[k] ~ dnorm(0,.0001) }
sd ~ dunif(0,10) # vague prior for for between‐trial SD
tau <‐ pow(sd,‐2) # between‐trial precision = (1/between‐trial variance)
# pairwise differences
for (c in 1:(nt‐1)) { for (k in (c+1):nt) { diff[c,k] <‐ d[k] ‐ d[c] } }
# rank treatments
for (k in 1:nt) {
rk[k] <‐ rank(d[],k)
best[k] <‐ equals(rk[k],1) # Smallest is best (i.e. rank 1)
# prob treat k is h‐th best, prob[1,k]=best[k]
for (h in 1:nt) { prob[h,k] <‐ equals(rk[k],h) }
}
}
} # *** PROGRAM ENDS
DATA
#nt=no. treatments, ns=no. studies;
list(nt=7, ns=9)
t[,1] t[,2] t[,3] y[,1] y[,2] y[,3] se[,1] se[,2] se[,3] Pooled.sd[] na[] #Study#
1 2 NA ‐14.83 ‐21.06 NA 1.637158543 1.666520327 NA 21.15183355 2 #Bradwejn 2005
1 3 NA ‐8.2 ‐30.3 NA 5.939773488 5.990214242 NA 21.8100321 2 #Broocks 1998
1 2 NA ‐14.99 ‐21.56 NA 1.70995633 1.709813779 NA 21.15057636 2 #Liebowitz 2009
1 3 4 ‐1.4 ‐2.7 ‐2.8 0.230143654 0.264575131 0.29970746 2.831821872 3 #Lecrubier 1997
1 2 4 ‐16.38 ‐25.1 ‐25.55 0.224179415 1.182285788 0.240373674 15.00388961 3 #Pollack 2007b
1 4 5 ‐0.9 ‐1.1 ‐1.3 0.20010414 0.198680835 0.199585778 1.498866044 3 #GSK 1994/04
1 6 5 ‐3.2 ‐4.9 ‐4.3 0.894427191 1.498165545 0.816496581 4.881262004 3 #Taylor 1990
1 7 NA ‐2.63 ‐2.95 NA 0.522222222 0.312589636 NA 4.714796447 2 #Carter 1995
1 5 NA ‐2.1 ‐3.7 NA 0.29192018 0.287142787 NA 2.853041141 2 #Schweizer 1993
END
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Amore 1999.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐IV panic disorder with or without agoraphobia Method of diagnosis: not stated Age, mean (SD) years: fluoxetine 37.0 (7.1); imipramine 37.2 (8.2) Sex: for fluoxetine, 57.89% women, 42.11% men; for imipramine, 36.84% women, 63.16% men Location: Italy; setting unclear Co‐morbidities: patients with a history of psychosis, current major depression, organic brain syndromes or significant neurological disorders, seizures, clinically relevant cardiovascular, hepatic, renal or haematological diseases were excluded Rescue medication: oxazepam (up to a maximum daily dose of 30 mg) permitted during the first 4 weeks of double‐blind treatment |
|
| Interventions | Participants were randomly assigned to either: 1. Fluoxetine arm (n = 19) Duration: 24 weeks of active treatment (acute and continuation phase), 6 months maintenance phase for responders Treatment protocol: flexible dosage; range = 10 mg to 50 mg, mean 20 mg/day (SD 10) (responder group) 2. Imipramine arm (n = 19) Duration: 24 weeks of active treatment (acute and continuation phase), 6 months maintenance phase for responders Treatment protocol: flexible dosage; range = 2 mg to 250 mg, mean 150 mg/day (SD 25) (responder group) |
|
| Outcomes |
Time points for assessment: baseline and weekly for 16 weeks, every 2 weeks between week 17 and 24, later monthly Outcomes:
|
|
| Notes |
Date of study: not stated Funding source: not stated Declarations of interest among the primary researchers: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "they were randomly assigned to fluoxetine or imipramine treatment". No further details. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double blind". No further details. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double blind". No further details. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided. |
| Selective reporting (reporting bias) | High risk | Data on the scales CGI, PASS and HRSD not reported at endpoint. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
Amore 1999 bis.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐IV panic disorder with or without agoraphobia Method of diagnosis: not stated Age: for fluoxetine, mean = 37.2 (SD 7.0); for citalopram, mean = 36.7 (SD 7.4) Sex: for fluoxetine, 57.1% women, 42.9% men; for imipramine, 61.9% women, 38.1% men Location: Italy; setting unclear Co‐morbidities: patients with a history of psychosis, current major depression, organic brain syndromes or significant neurological disorders, seizures, clinically relevant cardiovascular, hepatic, renal or haematological diseases, alcohol or drugs abuse were excluded Rescue medication: oxazepam (up to a maximum daily dose of 30 mg) permitted |
|
| Interventions | Participants were randomly assigned to either: 1. Fluoxetine arm (n = 21) Duration: 24 weeks of active treatment (acute and continuation phase), 6 months maintenance phase for responders Treatment protocol: flexible dosage; range = 10 mg to 50 mg, mean = 20 mg/day (SD 10) 2. Citalopram arm (n = 21) Duration: 24 weeks of active treatment (acute and continuation phase), 6 months maintenance phase for responders Treatment protocol: flexible dosage; range = 20 mg to 60 mg, mean = 40 mg/day (SD 10) |
|
| Outcomes |
Time points for assessment: baseline and weekly for 16 weeks, every 2 weeks between week 17 and 24, later monthly Outcomes:
|
|
| Notes |
Date of study: not stated Funding source: not stated Declarations of interest among the primary researchers: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "they were randomly assigned". No further details. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double blind". No further details. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double blind". No further details. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided. |
| Selective reporting (reporting bias) | High risk | Data on the scales CGI, PASS and HAMA not reported at endpoint. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
Asnis 2001.
| Study characteristics | ||
| Methods | Study design: 8 weeks, multi‐centre, double‐blind, placebo‐controlled outpatient clinical trial, parallel groups, individual randomisation | |
| Participants |
Diagnosis: DSM‐III‐R panic disorder with or without agoraphobia Method of diagnosis: not specified Age (years): fluvoxamine arm mean age (years) 34.2 (SD 10.2, range 19 to 65), placebo arm mean age (years) 36.7 (SD 9.8, range 20 to 63) Sex: 64 men, 115 women Location: outpatients, 4 centres throughout the USA Co‐morbidities: excluded Rescue medication: discouraged, but allowed for night‐time sedation (lorazepam 1 mg to 2 mg or chloral hydrate 1 mg to 2 mg) |
|
| Interventions | Participants were randomly assigned to either: 1. Fluvoxamine arm (randomised n = 93) Duration: 8 weeks Treatment protocol: flexible dosage; range = 100 to 300 mg/day, mean 4.2 cps/day (SD 1.4) 2. Placebo arm (randomised n = 95) Duration: 8 weeks Treatment protocol: flexible dosage, mean 5.1 cps/day (SD 1.2) |
|
| Outcomes |
Time points for assessment: at baseline and weekly until week 8 Outcomes
|
|
| Notes |
Date of study: not specified Funding source: unclear Declarations of interest among the primary researchers: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is described as "randomized", however the sequence generation process is not discussed. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study is defined as "double‐blind". Quote: "Treatment was started with a daily dosage of one capsule (50 mg fluvoxamine or matching placebo) [...]" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts: fluvoxamine group 29/93 (31.2%), placebo group 29/95 (30.5%). There are high dropout rates in each arm. Reasons for leaving the study early are relatively balanced between the 2 groups (see table 1). Quote: "Conclusions were based on the last observation carried forward (LOCF) to the end of the study analyses for the intention to treat population (all patients randomized to double‐blind treatment who provided some on‐drug efficacy data)". However, the tables do not report the number of analysed participants. |
| Selective reporting (reporting bias) | High risk | The primary outcome is clearly reported in the methods, quote: "The primary efficacy measurement, the DPAI, was designed to identify panic attacks". However, the DPAI scores are not reported in the text and tables. All other measurements are reported. |
| Other bias | High risk | Quote: "The authors thank Drs. R.I.H. and A.M. who were at Solvay Duphar for their help in providing statistical assistance and a thorough review of the manuscript". A risk of sponsorship bias cannot be excluded. |
Baker 2003.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐IV panic disorder Method of diagnosis: Structured Clinical Interview for DSM‐IV (modified version) Age: clonazepam: mean = 47.3 (SE 2.76); placebo: mean = 44.4 (SE 1.87) Sex: clonazepam: female 30%; placebo: female 56% Location: Canada (Toronto Western Hospital) Comorbidities: not stated Rescue medication: none |
|
| Interventions | Participants were randomly assigned to either: 1. Clonazepam (n = 10) Duration: 4 weeks Treatment protocol: not stated 2. Placebo (n = 17) Duration: 4 weeks Treatment protocol: not stated |
|
| Outcomes |
Time points for assessment: baseline, end of trial Primary outcomes:
|
|
| Notes |
Date of study: not stated Funding source: supported by a grant from the Heart and Stroke Foundation of Ontario Declarations of interest among the primary researchers: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided on blinding. Quote: "27 patients (...) were randomised in a double‐blinded fashion to 4 weeks of treatment with clonazepam or placebo" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind; no further information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided. |
| Selective reporting (reporting bias) | Unclear risk | The efficacy data of rating scales are not reported. There are data on sleep measures only. |
| Other bias | Low risk | No evidence of other bias was found. |
Bakish 1993.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III‐R panic disorder with or without agoraphobia Method of diagnosis: not stated Age: not stated Sex: not stated Location: Canada; setting: outpatients Co‐morbidities: not stated Rescue medication: chloral hydrate, up to 1 g at night |
|
| Interventions | Participants were randomly assigned to either: 1) Brofaromine arm (n = 47) Duration: 8 weeks Treatment protocol: flexible dosage; range = 50 mg to 150 mg, mean and SD not provided 2) Clomipramine arm (n = 46) Duration: 8 weeks Treatment protocol: flexible dosage; range = 25 mg to 75 mg, mean and SD not provided |
|
| Outcomes |
Time points for assessment: baseline, every 2 weeks Outcomes:
|
|
| Notes |
Date of study: not stated Funding source: not stated Declarations of interest among the primary researchers: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised". No further details. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double blind". No further details. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double blind". No further details. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided. |
| Selective reporting (reporting bias) | High risk | Data on the scales HAMD, CRIDS, CRGCS, PRIDS, PRAS, PRCGS, DPI not reported at endpoint; data on the scales HAMA and Mark Matthews Phobia Scale are reported only in graphs; number of patients evaluated not specified. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
Ballenger 1998.
| Study characteristics | ||
| Methods | Study design: 10 weeks, double‐blind, randomised (cluster‐randomisation), placebo‐controlled, parallel‐design, multi‐centre clinical trial | |
| Participants |
Diagnosis: DSM‐III‐R criteria for panic disorder, with or without agoraphobia Method of diagnosis: not specified Age (years): placebo arm mean age 37.3 (SD 10.4), paroxetine 10 mg arm mean age 36.1 (SD 9.1), paroxetine 20 mg arm mean age 35.9 (SD 10.1) and paroxetine 40 mg mean age 36.3 (SD 10.8) Sex: 95 men, 183 women Location: outpatients Co‐morbidities: excluded Rescue medication: not allowed |
|
| Interventions | Participants were randomly assigned to either: 1. Paroxetine 10 mg arm (randomised n = 67) Duration: 10 weeks Treatment protocol: fixed dosage 10 mg/day 2. Paroxetine 20 mg arm (randomised n = 70) Duration: 10 weeks Treatment protocol: fixed dosage 20 mg/day 3. Paroxetine 40 mg arm (randomised n = 72) Duration: 10 weeks Treatment protocol: fixed dosage 40 mg/day 4. Placebo arm (randomised n = 69) Duration: 10 weeks Treatment protocol: fixed dosage |
|
| Outcomes |
Time points for assessment: at baseline, week 4 and week 10 Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: sponsored by the drug company marketing the drug Declarations of interest among the primary researchers: apparently connected with the drug company marketing the drug |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is described as "randomized", however the sequence generation process is not discussed. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study is described as "double‐blind", however procedures for ensuring the blindness of participants and who administered the intervention are not discussed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts: paroxetine 10 mg group 22/67 (32.8%), paroxetine 20 mg group 23/70 (32.8%), paroxetine 40 mg group 22/72 (30.5%), placebo group 23/69 (33.3%). The dropout rate is high in every arm and reasons for leaving the study are apparently balanced between groups as reported in table 2 in the paper. Quote: "Results for the intent‐to‐treat population were determined on the basis of the data sets for both completer analysis (observed cases) and endpoint analysis (last observation carried forward)". Outcome measures reported are consistent with an ITT analysis (as reported in table 3 in the paper). |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes are clearly pre‐specified in the protocol of the study and in the "measurements" paragraph of the paper. All relevant data are clearly reported in tables. |
| Other bias | Unclear risk | A "disclosure of interest" paragraph is not reported. |
Bandelow 2004.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐IV and ICD‐10 diagnosis of panic disorder with or without agoraphobia Method of diagnosis: not stated Age: for sertraline, mean = 39.6 (SD 11.7); for paroxetine, mean = 38.1 (SD 11.7) Sex: for sertraline, 60% women, 40% men; for paroxetine, 66% women, 34% men Location: 5 centres in Denmark, 22 centres in Germany, 2 centres in the Netherlands, 2 centres in Switzerland, 2 centres in Turkey; setting: outpatients Co‐morbidities: patients with clinically significant and unstable medical illness, bipolar disorder, schizophrenic disorder, delusional disorder, epilepsy, major depressive disorder (MDD), obsessive‐compulsive disorder (OCD), social phobia, history of alcoholism or drug abuse were excluded Rescue medication: chloral hydrate, zolpidem or zopiclone allowed if necessary to treat severe insomnia, less than 3 times per week |
|
| Interventions | Participants were randomly assigned to either: 1. Sertraline arm (n = 112) Duration: 12 weeks Treatment protocol: flexible dosage; range = 25 mg to 150 mg, mean = 84.5 (SD 39.1) 2. Paroxetine arm (n = 113) Duration: 12 weeks Treatment protocol: flexible dosage; range = 10 mg to 60 mg, mean = 48.1 (SD 11.2) |
|
| Outcomes |
Time points for assessment: baseline, week 1, 2, 4, 6, 8, 12 and 15 Outcomes:
|
|
| Notes |
Date of study: data were collected from January 2000 to June 2001 Funding source: Funded by Pfizer Inc, New York Declarations of interest among the primary researchers: Dr Bandelow has received grant/research support from GlaxoSmithKline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "they were randomly assigned". No further details. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double blind". No further details. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double blind". No further details. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "[...] a secondary analysis was performed on the ITT population, which consisted of all patients who were randomly assigned to study drug and for whom at least one post baseline PAS assessment was available" |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | High risk | Sponsored by Pfizer; the role of the funder in planning, conducting and writing the study is not discussed. |
Barlow 2000.
| Study characteristics | ||
| Methods | Study design: 12 weeks and then 6 months, multi‐centre, randomised, double‐blind, placebo‐controlled clinical trial, parallel groups, cluster‐randomisation | |
| Participants |
Diagnosis: panic disorder with or without mild agoraphobia Method of diagnosis: ADIS‐R (Anxiety Disorder Interview Schedule‐Revised, diagnosis confirmed 2 weeks prior to first treatment visit) Age (years): mean 36.1 (SD 10.7) Sex: 62.5% women Location: not specified Co‐morbidities: patients with depression were not excluded, unless suicidal Rescue medication: allowed up to 20 doses of benzodiazepines (or 10 alprazolam equivalent) |
|
| Interventions | Participants were randomly assigned to either: 1. imipramine arm (randomised n = 83) Duration: 12 weeks Treatment protocol: flexible dosage; "the dose was titrated 10 mg every other day until 50 mg per day and then was flexible, with efforts to reach 100 mg by the end of week 3 and 200 by week 5" 2. CBT alone arm (randomised n = 77) Duration: 12 weeks Treatment protocol: unclear 3. CBT plus imipramine arm (randomised n = 65) Duration: 12 weeks Treatment protocol: flexible dosage; range = 10 to 60 mg/day 4. CBT plus placebo arm (randomised n = 63) Duration: 12 weeks Treatment protocol: flexible dosage 5. placebo arm (randomised n = 24) Duration: 12 weeks Treatment protocol: flexible dosage |
|
| Outcomes |
Time points for assessment: at baseline, at week 12 and then at month 4, 5 and 6 Outcomes:
|
|
| Notes |
Date of study: May 1991 to April 1998 Funding source: the study was mostly funded by public financial support. Sponsorship bias is unlikely to have occurred. Declarations of interest among the primary researchers: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is described as randomised, however details on the random sequence generation are not discussed. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Trained independent evaluators were employed (see "Assessment" paragraph). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Trained independent evaluators were employed. |
| Selective reporting (reporting bias) | Low risk | Primary endpoints are divided into continuous outcome measures (average item score for the PDSS) and categorical outcome measures (responders based on CGI). All relevant data are reported in tables. |
| Other bias | Unclear risk | Study authors received various financial support from pharmaceutical agencies. Quote: "Imipramine and matching placebo were provided by Teva Pharmaceuticals USA". The study was mostly funded by public financial support. |
Beauclair 1994.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III panic disorder or agoraphobia with panic attacks Method of diagnosis: semi‐structured interview Age: range: 21 to 49 years (median: 34) Sex: M = 12; F = 17 Location: Canada; setting: outpatients Comorbidities: none Rescue medication: none |
|
| Interventions | Participants were randomly assigned to either: 1. Clonazepam (n = 13) Duration: 4 weeks Treatment protocol: flexible dosage; range = 1 to 5 mg (the actual maximum daily dose was 3.5 mg) 2. Placebo (n = 16) Duration: 4 weeks Treatment protocol: flexible |
|
| Outcomes |
Time points for assessment: days 0, 7, 14, 21, 28 Primary outcomes:
Secondary outcome:
|
|
| Notes |
Date of study: June 1987 to June 1988 Funding source: not stated Declarations of interest among the primary researchers: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated to 4 weeks of treatment". No information on random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients were randomly allocated to 4 weeks of treatment under double‐blind conditions". "Medications were administered in tablets of identical appearance under double‐blind conditions" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Concentration of clonazepam in plasma were measured under double‐blind conditions with placebo controls" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There is an imbalance in the number of participants completing 4 weeks of treatment (clonazepam = 12/13 (92.3%), placebo = 8/16 (50%)). Quote: "The proportion of patients treated with placebo who terminated the study prematurely (50%) was significantly higher than that in the clonazepam‐treated group (15.4%)". "Two types of analysis were carried out on the data: an ITT on the 29 patients who entered the trial and an efficacy analysis on the subgroup of 20 patients who completed the full 4 weeks of treatment" |
| Selective reporting (reporting bias) | Low risk | The clinical measures declared in the methods are reported in the results. |
| Other bias | Unclear risk | Quote: "The authors thank Hoffman ‐ La Roche for assistance in carrying out this study" |
Bergink 2005.
| Study characteristics | ||
| Methods | Study design: 9 weeks, randomised (individual randomisation), double‐blind, parallel, placebo‐controlled clinical trial, parallel groups | |
| Participants |
Diagnosis: panic disorder with and without agoraphobia according to the DSM‐IV criteria Method of diagnosis: PDSS, CGI and number of panic attacks per week Age (years): the mean age was 41 for the metabotropic glutamate (LY354740), 44 for paroxetine and 45 for placebo Sex: 18 men and 27 women Location: University Medical Centre (UMC) in Utrecht, the Netherlands Co‐morbidities: excluded Rescue medication: not permitted |
|
| Interventions | Participants were randomly assigned to either: 1. LY354740 arm (randomised n = 18) Duration: 9 weeks Treatment protocol: flexible dosage; range = 100 to 200 mg/day 2. Paroxetine arm (randomised n = 9) Duration: 9 weeks Treatment protocol: flexible dosage; range = 10 to 60 mg/day 3. Placebo arm (randomised n = 0) Duration: 9 weeks Treatment protocol: flexible dosage |
|
| Outcomes |
Time points for assessment: at baseline and then at week 3, 6, 9 Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: unclear Declarations of interest among the primary researchers: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "eligible patients were assigned in a 1:1:1:1 ratio to one of the following four treatment groups: LY354740 100 mg/day, LY354740 200 mg/day, paroxetine, placebo". No further information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study is described as "double blind" but no further details are given. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number and the reasons for dropouts are specified. Data analysis was performed on the intention‐to‐treat population using the LOCF. |
| Selective reporting (reporting bias) | Low risk | The primary and secondary outcome data are shown in a table. |
| Other bias | Unclear risk | It is unclear whether the study authors received a grant for the study. |
Black 1993.
| Study characteristics | ||
| Methods | Study design: 8 weeks, double‐blind, placebo‐controlled trial, parallel groups, individual randomisation | |
| Participants |
Diagnosis: DSM‐III‐R criteria for panic disorder with or without agoraphobia Method of diagnosis: SCID Age (years): fluvoxamine arm mean age 35.1 (SD 10.4), CBT arm mean age 38.7 (SD 12.4) and placebo arm mean age 37.0 (SD 9.9) Sex: 22 men, 53 women Location: outpatient setting, multi‐centre, USA Co‐morbidities: patients with a diagnosis of major depression were also included, medical co‐morbidities were excluded Rescue medication: not allowed |
|
| Interventions | Participants were randomly assigned to either: 1. Fluvoxamine arm (randomised n = 25) Duration: 8 weeks Treatment protocol: flexible dosage; range = up to 300 mg per day, mean 230 mg (4.6 cps)/day 2. CBT arm (randomised n = 25) Duration: 8 weeks Treatment protocol: psychotherapy sessions 3. Placebo arm (randomised n = 25) Duration: 8 weeks Treatment protocol: flexible dosage, 5.5 cps/day |
|
| Outcomes |
Time points for assessment: at baseline and then at week 4 and 8 Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: financed by a drug company Declarations of interest among the primary researchers: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to the drug study (n=50) or to the cognitive therapy (n=25) [...]". The sequence generation process is not described. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Investigators and subjects remained "blind" to this assignment (ie, fluvoxamine vs placebo)". However, procedures for ensuring the concealment of allocation are not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Medications [...] were administered in a double‐blind fashion". However, procedures for ensuring the blinding are not discussed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Assessments were made by the project coordinator (JG) or a psychiatrist (DWB or RW)". No further information provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout rate: fluvoxamine group 4/25 (16%), placebo group 7/25 (28%). The rate of dropouts in the placebo group was higher than in the fluvoxamine group, and reasons for leaving the study early are unbalanced, particularly considering dropouts for ineffectiveness. In the "statistical analysis" paragraph both "completer analysis" and ITT analysis with a "last observation carried forward" approach are mentioned, however it is not clear which one was employed for data reported in tables, since the number of analysed participants is not reported. |
| Selective reporting (reporting bias) | High risk | Primary and secondary outcomes are not clearly pre‐specified in the text. Data from all the rating scales are clearly reported in graphs, with the exception of the frequency of panic attacks. |
| Other bias | High risk | Quote: "The study was sponsored in part through a grant from Reid‐Rowell Pharmaceuticals Inc, Atlanta, Ga". The role of the funder in planning, conducting and writing the study is not discussed. |
Bradweijn 2005.
| Study characteristics | ||
| Methods | Study design: 10 weeks, flexible dose, double‐blind, randomised (individual randomisation), parallel groups, placebo‐controlled study | |
| Participants |
Diagnosis: DSM‐IV panic disorder with or without agoraphobia Method of diagnosis: DSM‐IV and modified Mini International Neuropsychiatric Interview Age (years): 38.9 (SD 12.4) for the venlafaxine ER arm and 38.8 (SD 12.1) for the placebo arm Sex: venlafaxine arm, 61 men and 99 women; placebo arm, 69 men, 99 women Location: outpatient setting, 50 sites in Canada, Europe and South Africa Co‐morbidities: excluded Rescue medication: not allowed |
|
| Interventions | Participants were randomly assigned to either: 1. Venlafaxine ER arm (randomised n = 181) Duration: 10 weeks Treatment protocol: flexible dosage; range = 75 to 225 mg/day, mean = 162.9 mg/day (SD 60.6) at week 10 2. Placebo arm (randomised n = 180) Duration: 10 weeks Treatment protocol: flexible dosage; range = 1 to 3 capsules |
|
| Outcomes |
Timepoints for assessment: at baseline and then at 2, 3, 4, 6, 8 and 10 weeks Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: the study was funded by the company marketing the drug Declarations of interest among the primary researchers: the primary researcher received funding from drug companies for the study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is described as "randomised". No further information about the random sequence generation is provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study is described as double‐blind but it is unclear whether the investigators were 'blind'. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study is described as double‐blind but it is unclear whether the investigators were 'blind'. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The dropout rate is over 25% and it is reported in the flow chart of the study. The study authors used ITT analysis. |
| Selective reporting (reporting bias) | Low risk | The results are clearly reported in the tables and in the text. |
| Other bias | High risk | The study was funded by the company marketing the drug. The primary researcher received funding from drug companies for the study. |
Broocks 1998.
| Study characteristics | ||
| Methods | Study design: 10 weeks, placebo‐controlled study, parallel groups, individual randomisation | |
| Participants |
Diagnosis: DSM‐III‐R and ICD‐10 criteria diagnosis of panic disorder and agoraphobia Method of diagnosis: SCID for DSM‐III‐R Age (years): 18 to 50; exercise arm mean age 31.8 (SD 9.5), clomipramine arm mean age 33.9 (SD 9.2) and placebo arm mean age 34.8 (SD 6.8) Sex: 23 men, 23 women Location: outpatient setting, Germany Co‐morbidities: excluded Rescue medication: promethazine 25 mg to 50 mg |
|
| Interventions | Participants were randomly assigned to either: 1. Clomipramine arm (randomised n = 15) Duration: 10 weeks Treatment protocol: fixed dosage; range = 37.5 to 112.5 mg/day 2. Aerobic exercise ‐ running arm (randomised n = 16) Duration: 10 weeks Treatment protocol: running schedule 3. Placebo arm (randomised n = 15) Duration: 10 weeks Treatment protocol: fixed dosage |
|
| Outcomes |
Time points for assessment: at baseline and then at 10 weeks Outcomes:
|
|
| Notes |
Date of study: unclear Funding source: grant from a car factory Declarations of interest among the primary researchers: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The sequence generation process is not described. Moreover, the randomisation procedure was divided into 2 steps, quote: "At baseline, patients were randomly assigned to the clomipramine/placebo group (n = 30) or the exercise group (n = 6). The study therapists (A.B., G.P., and A.G.) were not blind to this assignment. Patients in the drug group were further randomly assigned to receive either clomipramine (n = 15) or placebo (n = 15). The assignment was done by the hospital pharmacist; investigators and subjects remained blind to this assignment". This may have altered the balance between the 3 arms, which are, however, described as comparable. |
| Allocation concealment (selection bias) | Unclear risk | Selection bias is likely to have occurred due to the lack of description of the sequence generation process and the division of the randomisation procedure. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts: exercise group 5/16 (31.2%); clomipramine group 0/15 (0%); placebo group 4/15 (26.7%). Dropout rates are high for 2 groups, with reasons for leaving the study apparently balanced. An ITT analysis was performed and data were imputed with a LOCF approach. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes are clearly reported in tables. |
| Other bias | Low risk | Supported by a grant from a car factory, so it is unlikely that a sponsorship bias might have occurred. |
Bystritsky 1994.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III‐R panic disorder with or without agoraphobia Method of diagnosis: not stated Age: average age of 37 years, no between‐group differences Sex: 12 males and 9 females, no between‐group differences Location: USA; setting unclear Co‐morbidities: lack of significant drug or alcohol history or significant medical illness; patients that had an additional diagnosis of major depression (MD) or generalised anxiety disorder (GAD) were allowed to participate only if they presented a predominant picture of panic disorder and if panic symptoms preceded the onset of the current episode of MD or GAD Rescue medication: not stated |
|
| Interventions | Participants were randomly assigned to either: 1. Desipramine arm (n = 11) Duration: 10 weeks Treatment protocol: flexible dosage; range = 10 mg to 300 mg, mean = 110 (SD 49) 2. Fluoxetine arm (n = 11) Duration: 10 weeks Treatment protocol: flexible dosage; range = 2.5 mg to 60 mg, mean = 19 (SD 10) |
|
| Outcomes |
Time points for assessment: weekly Outcomes:
|
|
| Notes |
Date of study: not stated Funding source: this research has been supported in part by NIMH grant MH 45342‐02 and by an NPI Opportunity Grant Declarations of interest among the primary researchers: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "they were assigned randomly". No further details. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "both patients and investigators were blind to the assignment"; "patients were administered identical capsules labeled A, B or C: Capsules A, containing 2,5 mg of fluoxetine or 10 mg of desipramine were administered for one week [...], capsules B (containing) 25 mg of desipramine or 5 mg of fluoxetine, (capsules) C (containing) 50 mg of desipramine or 10 mg of fluoxetine". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "both patients and investigators were blind to the assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Unclear risk | Quote: "this research has been supported in part by NIMH grant MH 45342‐02 and by an NPI Opportunity Grant". |
Caillard 1999.
| Study characteristics | ||
| Methods | Study design: 8 weeks, multi‐centre, randomised (individual randomisation), parallel groups, double‐blind, 3 arms, placebo‐controlled trial | |
| Participants |
Diagnosis: DSM‐III‐R criteria for panic disorder with or without agoraphobia Method of diagnosis: participants had to fulfil the DSM‐III‐R criteria for panic disorder, with a minimum score of 20 on the HAMA, and a minimum of 5 points for the 2 first items (anxious mood and tension), after the 1‐week, single‐blind period Age (years): clomipramine low‐dose arm mean age 38 (SD 10), clomipramine high‐dose arm mean age 35.5 (SD 11) and placebo arm mean age 37 (SD 10) Sex: 64 men, 94 women Location: outpatient setting, multi‐centre (15 sites in France) Co‐morbidities: excluded Rescue medication: not allowed |
|
| Interventions | Participants were randomly assigned to either: 1. Clomipramine low‐dose arm (randomised n = 61) Duration: 8 weeks Treatment protocol: fixed dosage; 60 mg/day 2. Clomipramine high‐dose arm (randomised n = 62) Duration: 8 weeks Treatment protocol: fixed dosage; 150 mg/day 3. Placebo arm (randomised n = 57) Duration: 8 weeks Treatment protocol: fixed dosage |
|
| Outcomes |
Time points for assessment: at baseline and weekly Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: the sponsor is the drug company marketing clomipramine Declarations of interest among the primary researchers: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is described as randomised, however details on the sequence generation process are not provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts: clomipramine "low dose" group 15/61 (25%); clomipramine "high dose" group 22/62 (37%); placebo 25/57 (45%). Dropout rates are high (more than 20%), and unbalanced between groups both in number and in terms of reasons for leaving the study early. The intention‐to‐treat analysis included all 180 randomised participants and was applied only to categorical data. Instead, only participants who strictly observed the protocol were included in the explanatory analysis. However, according to Table 2, not all randomised participants were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Quote: "The aim of this study was to investigate the dose‐response relationship for clomipramine in patients with panic disorder [...]". However, the primary outcome measure and time point employed are not clearly reported. All relevant data are reported in the text and tables. |
| Other bias | High risk | Quote: "This study was supported in part by the NOVARTIS Company and by the French University Antidepressant Group". The role of the funder in planning, conducting and writing the study is not discussed. |
Carter 1995.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III‐R panic disorder with agoraphobia Method of diagnosis: Structured Clinical Interview for DSM‐III‐R, Upjohn version (SCID‐UP‐R); medical questionnaire; physical examination; laboratory test Age: not stated Sex: not stated Location: USA (10 study sites) Comorbidities: none Rescue medication: none |
|
| Interventions | Participants were randomly assigned to either: 1. Adinazolam SR 30 mg (n = 79) Duration: 4 weeks Treatment protocol: fixed dose: 30 mg 2. Adinazolam SR 60 mg (n = 81) Duration: 4 weeks Treatment protocol: fixed dose: 60 mg 3. Adinazolam SR 90 mg (n = 72) Duration: 4 weeks Treatment protocol: fixed dose: 90 mg 4. Placebo (n = 83) Duration: 4 weeks |
|
| Outcomes |
Time points for assessment: baseline, weeks 1, 2, 4 Primary outcomes:
Secondary outcome:
|
|
| Notes |
Date of study: not stated Funding source: supported by grants from the Upjohn Company Declarations of interest among the primary researchers: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients (...) were randomly assigned to receive one of three doses of adinazolam or placebo". No information on random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study is double‐blind. Quote: "Medication was dispensed in blister packs with morning and evening doses cells, with contained a fixed number of identical tablets (containing either 15 mg of adinazolam or placebo)". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind; no further information. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only modified ITT (1 post‐baseline assessment) data available; number of randomised participants is unclear. |
| Selective reporting (reporting bias) | High risk | Side effects data published only selectively (discontinuation symptoms). |
| Other bias | Unclear risk | Supported by grants from the Upjohn Company. |
CNCPS 1992.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III‐R panic disorder with limited or extensive phobic avoidance (panic attacks with agoraphobia) Method of diagnosis: "patients were evaluated by Structured Clinical Interview for DSM‐III Diagnosis, Upjohn (SCID‐UP)" Age: mean = 34 (SD not provided) Sex: 62% female, 38% male Location: 12 centres in the USA, Spain, Denmark, Germany, England, Italy, Brazil, Mexico, France, Colombia, Austria, Sweden, Canada, Belgium; setting: inpatients and outpatients Co‐morbidities: patients with psychotic disorders, dementia, bipolar disorder, alcoholism or drug abuse within the last 6 months or significant medical problems were excluded. Patients with current major depression were excluded unless the depression was judged to be secondary to the anxiety disorder and did not have melancholic or psychotic features. Rescue medication: Quote "patients taking CNS drugs, including benzodiazepines, were excluded from the study. During the washout period, blood was drawn for benzodiazepines screening". |
|
| Interventions |
Participants were randomly assigned to either: 1. Imipramine arm (n = 391) Duration: 8 weeks Treatment protocol: flexible dosage; range = 25 mg to 250 mg, mean = 155 mg (SD not provided) 2. Alprazolam arm (n = 386) Duration: 8 weeks Treatment protocol: flexible dosage; range = 1 mg to 10 mg, mean = 5.7 (SD not provided) 3. Placebo arm (n = 391) Duration: 8 weeks |
|
| Outcomes |
Time points for assessment: baseline, weekly, endpoint Outcomes:
|
|
| Notes |
Date of study: data collection: 1984 to 1987 Funding source: sponsored by Upjohn Company, Kalamazoo, Michigan Declarations of interest among the primary researchers: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned"; "alprazolam, imipramine or placebo were assigned in 12 randomization blocks of the basic three cell random‐assignment, parallel treatment‐design. [...] At each center patients were blindly and randomly assigned to alprazolam, imipramine or placebo treatment, based on a table of random numbers [...]. Patients removed from the protocol before three weeks had to be replaced; after three weeks, non‐completers were not replaced." No further information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote "double‐blind design". No further details. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote "double‐blind design". No further details. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "of 1168 patients randomized, 1122 met criteria for ITT". No further information provided. |
| Selective reporting (reporting bias) | High risk | In the primary publication, data on the Panic Attack scale are not reported; data on the Physician's Global Improvement scale are only partially reported, and without the number of patients evaluated; data on other continuous outcomes (HAMA, HRSD) are reported without the number of patients evaluated. Other data are partially reported in a secondary publication of this study. |
| Other bias | High risk | Sponsored by Upjohn Company, Kalamazoo, Michigan; the role of the funder in planning, conducting and writing the study is not discussed. |
Davidson 1994.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III‐R panic disorder with agoraphobia Method of diagnosis: Structured Clinical Interview for DSM‐III‐R, Upjohn version (SCID‐UP‐R) Age: adinazolam: mean = 36.1 (SD 10.8); placebo: mean = 35.5 (SD 8.9) Sex: adinazolam: M = 34%, F = 66%; placebo: M = 33%, F = 67% Location: USA (at 4 centres: University of California, Duke University Medical Center, University of Missouri‐Columbia, University of Wisconsin) Comorbidities: controlled physical illness Rescue medication: none |
|
| Interventions | Participants were randomly assigned to either: 1. Adinazolam SR (n = 99) Duration: 4 weeks Treatment protocol: flexible dosage; mean = 84.1 (SD 28.6); range = 3.5 to 7.5 capsules 2. Placebo (n = 103) Duration: 4 weeks Treatment protocol: flexible; mean = 92.3 (SD 27.3 mg equivalents); range = 4 to 8 tablets |
|
| Outcomes |
Time points for assessment: baseline, weeks 1, 2, 4 Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Date of study: not stated Funding source: not stated Declarations of interest among the primary researchers: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This was a parallel, double‐blind, flexible dose, 4 week efficacy and safety study with patients randomised to receive either adinazolam or matching placebo tablets". "Randomised assignment to treatment groups determined that equal numbers of patients received both treatment possibilities". No information on random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "This was a parallel, double‐blind, flexible dose, 4 week efficacy and safety study". “Medication was packed in individual bottles”. No information on blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind; no further information. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were dropouts in each group (drug 1 = 12 out of 99, placebo = 15 out of 103), but there was no imbalance between the 2 groups. Quote: "No statistical difference was found in the dropouts rates between the two treatment groups". |
| Selective reporting (reporting bias) | High risk | Side effects only reported if group difference was statistically significant; SDs only reported as P value. |
| Other bias | Unclear risk | Bristol‐Myers Squibb Pharmaceutical Research Institute was involved in this study. Analysis at baseline for centres, treatment and centre by treatment effects in baseline severity scores according to centre for situational panic attack frequency, unexpected panic attack duration, main phobia severity and overall phobia severity |
Den Boer 1988.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III panic disorder without phobic avoidance or panic disorder with severe phobic avoidance behaviour Method of diagnosis: not stated Age: for maprotiline, mean = 35.0 (SD 7.4); for fluvoxamine, mean = 37.3 (SD 10.6) Sex: for maprotiline, 4 males and 20 females; for fluvoxamine, 5 males and 15 females Location: the Netherlands; setting: outpatients Co‐morbidities: patients with major affective disorders, schizophrenia, other psychotic disorders or significant medical problems were excluded Rescue medication: not stated |
|
| Interventions |
Participants were randomly assigned to either: 1. Maprotiline arm ("24 patients were included in the maprotiline group") Duration: 6 weeks Treatment protocol: flexible dosage; range = 50 mg to 150 mg, mean and SD not provided 2. Fluvoxamine arm ("20 patients were included in the fluvoxamine group") Duration: 6 weeks Treatment protocol: flexible dosage; range = 50 mg to 150 mg, mean and SD not provided |
|
| Outcomes |
Time points for assessment: baseline and weekly Outcomes:
|
|
| Notes |
Date of study: not stated Funding source: not stated Declarations of interest among the primary researchers: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "they were randomly allocated". No further details. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote "double‐blind treatment". No further details. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote "double‐blind treatment". No further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Number of patients randomised per group not reported (number of total randomised patients = 47); only number of patients evaluated per group was available, respectively 24 in maprotiline and 20 in fluvoxamine group. |
| Selective reporting (reporting bias) | High risk | Continuous outcome data are reported only in graphs. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
Den Boer 1990.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III‐R Method of diagnosis: not stated Age: for fluvoxamine mean = 37, for ritanserin mean = 35, for placebo mean = 37 Sex: the female to male ratio was almost 3 to 1 in all groups Location: the Netherlands; setting: outpatients Co‐morbidities: patients with a primary diagnosis other than panic disorder were excluded Rescue medication: none |
|
| Interventions |
Participants were randomly assigned to either: 1. Fluvoxamine arm Duration: 8 weeks Treatment protocol: fixed dosage = 150 mg 2. Ritanserin arm Duration: 8 weeks Treatment protocol: fixed dosage = 20 mg 3. Placebo arm Duration: 8 weeks Total number of randomised patients = 60. The number of patients randomised for each arm is not provided. |
|
| Outcomes |
Time points for assessment: baseline, weekly Outcomes:
|
|
| Notes |
Date of study: not stated Funding source: not stated Declarations of interest among the primary researchers: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised". No further information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double blind". No further information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double blind". No further information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided about the management of incomplete outcome data. |
| Selective reporting (reporting bias) | High risk | Data are reported in graphs (HAMA, FQ); other data are only partially reported. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
Gentil 1993.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III‐R panic disorder with or without agoraphobia Method of diagnosis: semi‐structured interview Age: for imipramine, mean = 36.35 (SEM 2.12); for clomipramine, mean = 34.1 (SEM 1.89) Sex: for imipramine, 70% women, 30% men; for clomipramine, 50% women, 50% men Location: Brazil; setting: outpatients Co‐morbidities: patients with other medical condition, drug abuse, OCD, primary major depression or psychoses were excluded; major depression without melancholia, secondary to panic disorder, could still be included Rescue medication: not stated |
|
| Interventions |
Participants were randomly assigned to either: 1. Imipramine arm (n = 20) Duration: 8 weeks Treatment protocol: flexible dosage; range = 25 mg to 200 mg, mean = 113.8 (SD 9.5) 2. Clomipramine arm (n = 20) Duration: 8 weeks Treatment protocol: flexible dosage; range = 10 mg to 80 mg, mean = 50 (SD 4.2) (3) placebo arm (propantheline) (n = 20) Duration: 8 weeks Treatment protocol: flexible dosage; mean = 85.5 (SD 5.7) |
|
| Outcomes |
Time points for assessment: baseline, week 2, 4, 6 and 8 Outcomes:
|
|
| Notes |
Date of study: not stated Funding source: grants from FAPESP and FINEP, donations from Rhodia SA, Metalurgica Matarazzo, Itautec, Soft Consultoria an Industrias Bardella SPA, Fundacao Zerbini and Fundacao Faculdade de Medicina Declarations of interest among the primary researchers: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "they were randomly allocated". Dropouts before completing the fourth week of treatment were replaced (therefore we considered only data before replacing: number of dropouts at fourth week). No further information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote "double‐blind treatment"; "Capsules were in the hospital pharmacy with tablets of the commercially available TCAs or propanteline (placebo) and filled up with lactose. The dose range of propanteline was selected to give mild to moderate peripheral anticholinergic effects". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote "double‐blind treatment"; "Capsules were in the hospital pharmacy with tablets of the commercially available TCAs or propanteline (placebo) and filled up with lactose. The dose range of propanteline was selected to give mild to moderate peripheral anticholinergic effects". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 15 patients left the trial before completing the first 4 weeks of treatment and were replaced. No information provided on incomplete outcome data management. |
| Selective reporting (reporting bias) | High risk | Data on the scales HAMD and BDI are not reported at endpoint. Data on the scales CGI and Sheehan are reported only in graphs; the number of patients evaluated is not specified. |
| Other bias | Low risk | Quote: "this study was not supported by the manufacturers of the drugs tested". |
GSK 1994/04.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III‐R panic disorder Method of diagnosis: Structured Clinical Interview for DSM‐III‐R Age: paroxetine: mean = 39.1 (SD 11.1); alprazolam: mean = 39.5 (SD 12.5); placebo: mean = 39.0 (SD 11.8) Sex: paroxetine: M = 28; alprazolam: M = 29; placebo: M = 23 Location: 16 centres in the USA Comorbidities: major depression (if secondary) Rescue medication: none |
|
| Interventions | Participants were randomly assigned to either: 1. Paroxetine (n = 77) Duration: 10 weeks Treatment protocol: flexible dosage; range: 10 to 60 mg/day 2. Alprazolam (n = 77) Duration: 10 weeks Treatment protocol: flexible dosage; range: 1 to 6 mg/day 3. Placebo (n = 72) Duration: 10 weeks Treatment protocol: flexible |
|
| Outcomes |
Time points for assessment: not stated Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Date of study: November 1992 to April 1994 Funding source: GlaxoSmithKline Declarations of interest among the primary researchers: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind; no further information. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind; no further information. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing outcome data: drug 1 = 29 out of 77, drug 2 = 17 out of 77, placebo = 22 out of 72. LOCF data available. |
| Selective reporting (reporting bias) | Unclear risk | Only short study synopsis available. |
| Other bias | Low risk | No evidence of other bias was found. |
GSK 29060 525.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: panic disorder; no further details provided Method of diagnosis: not stated Age: for paroxetine, mean = 37.12 (SD 9.92); for clomipramine, mean = 40.13 (SD 11.34) Sex: for paroxetine, 14 women, 23 men, 1 unknown; for clomipramine, 17 women, 14 men Location: China; setting unclear Co‐morbidities: patients with current major depression were excluded. No other co‐morbidities mentioned Rescue medication: not stated |
|
| Interventions |
Participants were randomly assigned to either: 1. Paroxetine arm (n = 38) Duration: 10 weeks Treatment protocol: flexible dosage; range = 10 mg to 50 mg, mean and SD not provided 2. Clomipramine arm (n = 35) Duration: 10 weeks Treatment protocol: flexible dosage; range = 50 mg to 100 mg, mean and SD not provided |
|
| Outcomes |
Time points for assessment: baseline, endpoint (10 weeks) Outcomes:
|
|
| Notes |
Date of study: September 1998 to September 1999 Funding source: GSK Declarations of interest among the primary researchers: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized". No further information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double‐blind". No further details. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double‐blind". No further details. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "ITT population consisted of all subjects who received treatment and have one post treatment evaluation". No further information provided. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | High risk | Sponsored by GSK; the role of the funder in planning, conducting and writing the study is not discussed. |
Hoehn‐Saric 1993.
| Study characteristics | ||
| Methods | Study design: 8 weeks, double‐blind, placebo‐controlled outpatient clinical trial, parallel groups, individual randomisation | |
| Participants |
Diagnosis: DSM‐III‐R panic disorder with or without agoraphobia Method of diagnosis: SCID Age (years): mean age 38.0 (SD 9.6) Sex: 16 men, 20 women Location: outpatient department at Johns Hopkins Hospital (Baltimore, Maryland, USA) Co‐morbidities: excluded Rescue medication: not allowed |
|
| Interventions | Participants were randomly assigned to either: 1. Fluvoxamine arm (randomised n = 25) Duration: 8 weeks Treatment protocol: flexible dosage; range = 100 to 300 mg/day, mean 206.8 mg/day 2. Placebo arm (randomised n = 25) Duration: 8 weeks Treatment protocol: flexible dosage, mean = 5.6 cps/day |
|
| Outcomes |
Time points for assessment: at baseline and then weekly until week 8 Outcomes:
|
|
| Notes |
Date of study: not stated Funding source: cps of fluvoxamine or placebo were provided by the drug company marketing the drug Declarations of interest among the primary researchers: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The sequence generation procedure is not discussed. 50 patients were randomised (25 in each group), however only those who were still eligible after the single‐blind phase took the medication. This procedure may have affected the effect of randomisation. The balance between the 2 arms is not discussed or reported in graphs. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts: fluvoxamine group 6/25 (24%); placebo group 7/25 (28%), which are high dropout rates. However, 25 is the number originally allocated to each arm (see above, selection bias). Among the original 50 participants, some (not clear how many) were excluded after a single‐blind phase. 37 participants completed the study, however only those who had complete sets of data (36 participants) were analysed, which seems to be consistent with a 'per protocol' analysis. |
| Selective reporting (reporting bias) | High risk | Quote: " [...] we predicted that treatment with fluvoxamine would be more effective than placebo in reducing the frequency and severity of panic attacks". However, it is not clear which exactly is the primary outcome and how it was assessed. Mean scores and SDs are clearly reported for the baseline assessment (figure 1), but only graphically reported for weekly assessments. |
| Other bias | High risk | Cps of fluvoxamine or placebo were provided by Solvay Co. The role of the funder in planning and conducting the study is not discussed. |
Holland 1999.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III‐R panic disorder with or without agoraphobia Method of diagnosis: not stated Age: for adinazolam, mean = 36.5; for clomipramine, mean = 35.8 (SD not provided) Sex: for adinazolam, 36% male; for clomipramine, 38% male Location: UK; setting unclear Co‐morbidities: patients with psychiatric co‐morbidities were excluded Rescue medication: not stated |
|
| Interventions |
Participants were randomly assigned to either: 1. Adinazolam arm (n = 166) Duration: 24 weeks Treatment protocol: flexible dosage; range = 30 mg to 90 mg, mean and SD not provided 2. Clomipramine arm (n = 149) Duration: 24 weeks Treatment protocol: flexible dosage; range = 50 mg to 150 mg, mean and SD not provided |
|
| Outcomes |
Time points for assessment: weeks 1, 2, 4, 8, 12, 16, 20 and 24 Outcomes:
|
|
| Notes |
Date of study: not stated Funding source: not stated Declarations of interest among the primary researchers: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized". No further information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double‐blind". No further details. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double‐blind". No further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | LOCF data are reported, but without specifying the number of patients evaluated. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes were reported, but without specifying the number of patients evaluated. |
| Other bias | Unclear risk | Authors' affiliations refer to pharmaceutical companies. |
Johnston 1995.
| Study characteristics | ||
| Methods | Study design: 28 weeks, placebo‐controlled, double‐blind clinical trial, parallel groups, individual randomisation | |
| Participants |
Diagnosis: DSM‐III agoraphobia Method of diagnosis: unclear Age (years): 18 to 70, mean = 37 (SD 10) Sex: women Location: unclear Co‐morbidities: excluded Rescue medication: unclear |
|
| Interventions | Participants were randomly assigned to either: 1. Clomipramine arm (randomised n = 16) Duration: 28 weeks Treatment protocol: flexible/fixed dosage; range = 25 to 300 mg/day, mean = 68.3 mg/day (SD 39.7) 2. Clomipramine + CBT arm (randomised n = 17) Duration: 28 weeks Treatment protocol: flexible dosage; range = 25 to 300 mg/day, mean = 133.3 mg/day (SD 58.7) 3. Placebo arm (randomised n = 16) Duration: 28 weeks Treatment protocol: flexible/fixed dosage; range = 25 to 300 mg/day, mean = 154.41 mg/day (SD 51.7) 4. Placebo + CBT (randomised n = 15) Duration: 28 weeks Treatment protocol: flexible/fixed dosage; range = 25 to 300 mg/day, mean = 139.3 mg/day (SD 73.7) |
|
| Outcomes |
Time points for assessment: at baseline, week 1, 2, 3, 4 and then at 4‐weekly intervals thereafter for a total of 28 weeks Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: the drug was supplied by the drug company that produces it and by Health and Welfare Canada Declarations of interest among the primary researchers: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about the sequence generation is provided. Quote: "random sequential assignment of patients to each of the four groups was carried out". |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both the participants and the personnel administering the drug are described as blinded. Quote: "the study was double blind for medication status with the principal investigator, therapists and subjects being unaware of whether placebo or clomipramine was being administered to individuals" and "study medications were supplied in coded vials with sealed keys to be consulted in emergency". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The assessors are described as blinded. Quote: "the study was double blind for medication status with the principal investigator, therapists and subjects being unaware of whether placebo or clomipramine was being administered to individuals". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number of dropouts is reported and it seems that there were some significant differences between dropouts and participants. Quote: "mean scores on 45 of the 48 outcome and demographic measures were higher for the drop‐out group than for those who completed the clinical trial". |
| Selective reporting (reporting bias) | Unclear risk | Data are only graphically reported (in box and whisker plot) so their interpretation is not easy. The only table reported does not specify the differences between clomipramine and placebo. |
| Other bias | High risk | The study was supported by the drug company marketing clomipramine. |
Klosko 1990.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III‐R panic disorder with agoraphobia Method of diagnosis: Anxiety Disorder Interview Schedule‐Revised Age: mean = 37 (SD 11.04) Sex: M = 26%, F = 74% Location: not stated (USA) Comorbidities: major depression (if secondary) Rescue medication: none |
|
| Interventions | Participants were randomly assigned to either: 1. Alprazolam (n = 17) Duration: 15 weeks Treatment protocol: flexible dosage; mean = 4.60 (SD 1.82) 2. Panic control treatment (PCT) (behaviour therapy treatment group) (n = 18) Duration: 15 weeks Treatment protocol: 15 individual sessions of an integrated CBT in weekly meetings 3. Waiting list (n = 16) Duration: 15 weeks Treatment protocol: no treatment 4. Placebo arm (n = 18) Duration: 15 weeks Treatment protocol: flexible dosage; mean = 5.08 (SD 2.65) |
|
| Outcomes |
Time points for assessment: clinical assessment before and after treatment; self‐monitoring measures throughout treatment Primary outcomes:
|
|
| Notes |
Date of study: not stated Funding source: this research was supported in part by a grant from the National Institute of Mental Health (MH‐36800) and the Upjohn Company Declarations of interest among the primary researchers: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Medication was supplied by the Upjohn Company in matching 1‐mg tablets, packaged in matching bottles containing sufficient medication for 1 week, and was administered double‐blind". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the ADIS‐r administrators were blind to group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Out of 69 initial subjects, 57 subjects completed the study and 12 subjects dropped out. A higher rate of drop out was observed in the placebo group compared with the other three groups. A chi‐square analysis on these dropout frequencies was significant. Separate chi‐squares on each pair of groups showed significant differences in between the placebo and alprazolam groups, the placebo and PCT groups and the placebo and waiting‐list groups. Those who dropped from the study where compared with study completers on major pre‐treatment variables. Since all the placebo subjects dropped from the study before completion of 3 weeks of treatment, endpoint analysis were not conducted. (...) Since the placebo group had a disproportionate number of dropouts, it is reasonable to argue that analysis of end state functioning that includes only study completers represents a distortion of results. Given the reasons and the rapidity with which most subjects dropped from the study, it is likely that, at time of study withdrawal, dropouts maintained their pretreatment low end state functioning status." |
| Selective reporting (reporting bias) | High risk | Numerous outcomes, e.g. side effects, are not reported. |
| Other bias | Unclear risk | This research was supported in part by a grant from the National Institute of Mental Health (MH‐36800) and the Upjohn Company. |
Koszycki 2011.
| Study characteristics | ||
| Methods | Study design: 12 weeks randomised (individual randomisation), parallel groups, double‐blind, placebo‐controlled, multi‐centre clinical trial. The "acute phase" lasted 12 weeks. Participants who showed adequate response were eligible to enter a 12‐week extension treatment. | |
| Participants |
Diagnosis: DSM‐IV criteria for panic disorder with or without agoraphobia Method of diagnosis: psychiatric interview and a Structured Clinical Interview for DSM‐IV (SCID) Age (years): sertraline arm mean age 36.40 (SD 10.0), placebo arm mean age 35.24 (SD 9.9), sertraline + SCBT arm mean age 36.22 (SD 10.9), placebo + SCBT arm mean age 36.80 (SD 12.2) Sex: 90 men, 161 women Location: outpatient, 15 academic health centres in Canada Co‐morbidities: "co‐morbid depression, generalized anxiety disorder, social phobia, somatization disorder and specific phobia were allowed as long as these conditions were secondary to and not clinically more prominent than the PD with or without agoraphobia" Rescue medication: oxazepam up to 60 mg/week allowed. It was used at least once by 55.9% of the participants and the weekly mean dose range was 24.8 mg/week (SD 30.9) to 33.7 mg/week (SD 18) |
|
| Interventions | Participants were randomly assigned to either: 1. Sertraline arm (randomised n = 63) Duration: 12 weeks Treatment protocol: flexible dosage; range = 25 to 200 mg/day, mean = 116.1 mg/day (SD 59.6) 2. Sertraline + SCBT arm (randomised n = 61) Duration: 12 weeks Treatment protocol: flexible dosage; range = 25 to 200 mg/day, mean = 95.8 mg/day (SD 57.6) 3. Placebo + SCBT arm (randomised n = 65) Duration: 12 weeks Treatment protocol: flexible dosage; mean = 138.3 mg/day (SD 59.5) 4. Placebo arm (randomised n = 62) Duration: 12 weeks Treatment protocol: flexible dosage, mean = 138.3 mg/day (SD 59.5) |
|
| Outcomes |
Time points for assessment: at baseline at week 1, 2, 3, 4, 6, 8, 10 and 12 Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: the study was supported by the drug company marketing sertraline Declarations of interest among the primary researchers: one of the primary researchers declared a conflict of interest with several drug companies |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly allocated to one of four groups by a computer generated randomization code [...]". |
| Allocation concealment (selection bias) | Low risk | Quote: "Investigators at each site were provided with a sealed envelope that contained the identification of the study drug being administered to the patient. In a medical emergency, the investigator was authorized to break the code for that subject only". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Placebo and sertraline were provided as matching capsules and administered double‐blind". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Outcome assessments were made by investigators who were blind to allocation of the drug and who were not told whether the patient was assigned to SCBT. Patients were instructed not to divulge their SCBT assignment to the investigators". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts: placebo arm (30.6%); sertraline arm (25.4%). Dropout rates are high. Reasons for leaving the study early are apparently balanced between groups, with the exception of adverse effects (9 in placebo arms versus 5 in antidepressant arm). An ITT was performed. Quote: "The mixed model methodology, as opposed to conventional repeated‐measures ANOVA, allows all available observations on each patient to be used without having to use an imputation procedure such as last‐observation carried forward". Only those who had no post‐baseline assessment were excluded from the ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Data are poorly reported. |
| Other bias | High risk | The study was supported by the drug company marketing sertraline; the role of the funder in planning, conducting and writing the study is not discussed. |
Krueger 1999.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III‐R panic disorder with or without agoraphobia Method of diagnosis: SCID Axis I, Roche edition Age: for moclobemide, mean = 35.0 (SD 8.9); for clomipramine, mean = 36.0 (SD 9.5) Sex: for moclobemide, 41.8% males, 58.2 females; for clomipramine, 39.7% males, 60.3% females Location: Norway, Sweden, the Netherlands; setting unclear Co‐morbidities: none, except of generalised anxiety disorders and social phobia of less than moderate severity Rescue medication: chloral hydrate as an occasional night‐time hypnotic |
|
| Interventions |
Participants were randomly assigned to either: 1. Moclobemide arm (n = 67) Duration: 8 weeks Treatment protocol: fixed‐flexible dosage, range = 300 to 600 mg, mean and SD not provided 2. Clomipramine arm (n = 68) Duration: 8 weeks Treatment protocol: fixed‐flexible dosage, range = 100 mg to 200 mg, mean and SD not provided |
|
| Outcomes |
Time points for assessment: week 1, 2, 4 and 8 Outcomes:
|
|
| Notes |
Date of study: not stated Funding source: Hoffmann ‐ La Roche Declarations of interest among the primary researchers: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized". No further information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double‐blind". No further details. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double‐blind". No further details. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "it was estimated that the ITT population with two‐sided significance level of 0.05 and a power of at least 0.8 had to be at least 66 patients in each treatment group"; "the ITT population comprised 135 patients who had received treatment and at least one assessment after baseline". |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | High risk | Sponsored by Hoffmann‐La Roche; the role of the funder in planning, conducting and writing the study is not discussed. |
Lecrubier 1997.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III‐R panic disorder with or without agoraphobia Method of diagnosis: not stated Age: for paroxetine, mean = 34.7 (SD 9.3); for clomipramine, mean = 35.1 (SD 9.2) Sex: for paroxetine, 53 males, 70 females; for clomipramine, 46 males, 75 females Location: 39 centres in Belgium, Denmark, France, Hungary, Ireland, Israel, Italy, the Netherlands, Norway, Spain, Switzerland, UK, Yugoslavia; setting: outpatients Co‐morbidities: none Rescue medication: chloral hydrate for night‐time sedation allowed |
|
| Interventions |
Participants were randomly assigned to either: 1. Paroxetine arm (n = 123) Duration: 12 weeks Treatment protocol: flexible dosage, range = 10 mg to 60 mg, mean and SD not provided 2. Clomipramine arm (n = 122) Duration: 12 weeks Treatment protocol: flexible dosage, range = 10 mg to 150 mg, mean and SD not provided 3. Placebo arm (n = 123) Duration: 12 weeks |
|
| Outcomes |
Time points for assessment: weeks 3, 6, 9, 12 Outcomes:
|
|
| Notes |
Date of study: October 1991 to November 1993 Funding source: sponsored by GSK Declarations of interest among the primary researchers: Department of Clinical Research, Development and Medical Affairs, SmithKline Beecham Pharmaceuticals |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized". No further information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double‐blind". No further details. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double‐blind". No further details. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "the primary and secondary efficacy analysis were performed on the ITT population, which included all subjects who were randomized, who received their randomized treatment and for whom at least one assessment was available after active treatment. Safety assessment were performed on the ITT population. Dropouts rates were around 30% in both treatment arms." |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | High risk | Sponsored by GSK; the role of the funder in planning, conducting and writing the study is not discussed. |
Lepola 1990.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III panic disorder with or without agoraphobia Method of diagnosis: not stated Age: mean = 37.4 (SD not provided) Sex: not stated Location: Finland; setting: inpatients Co‐morbidities: patients with psychiatric co‐morbidities were excluded; medical co‐morbidities are not mentioned; 6 patients suspected cases of epilepsy Rescue medication: "the patients did not receive any other treatment during the trial period" |
|
| Interventions |
Participants were randomly assigned to either: 1. Alprazolam arm (n = 27) Duration: 9 weeks Treatment protocol: flexible dosage, range = 1.5 mg to 8 mg, mean = 4.9 (SD not provided) 2. Imipramine arm (n = 28) Duration: 9 weeks Treatment protocol: flexible dosage, range = 30 mg to 225 mg, mean = 130 (SD not provided) |
|
| Outcomes |
Time points for assessment: baseline, 3 weeks, 9 weeks Outcomes:
|
|
| Notes |
Date of study: not stated Funding source: not stated Declarations of interest among the primary researchers: none (but authors' affiliations refer to pharmaceutical companies) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized". No further information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double‐blind". No further details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double‐blind". No further details |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were reported. |
| Other bias | Unclear risk | Authors' affiliations refer to pharmaceutical companies. |
Liebowitz 2009.
| Study characteristics | ||
| Methods | Study design: 10 weeks, randomised (individual), parallel groups, double‐blind, placebo‐controlled, multi‐centre clinical trial | |
| Participants |
Diagnosis: DSM‐IV criteria for panic disorder with or without agoraphobia Method of diagnosis: not specified Age (years): venlafaxine ER arm mean age 36 (SD 12.4) and placebo arm mean age 36.7 (SD 12.0) Sex: 107 men, 203 women Location: outpatient setting, in 56 sites (7 in Canada and 49 in the USA) Co‐morbidities: people with a secondary major depression or GAD were eligible. Any other clinically significant Axis I or Axis II disorders, or HAM‐D score ≥ 18 at baseline were excluded Rescue medication: unclear |
|
| Interventions | Participants were randomly assigned to either: 1. Venlafaxine ER arm (randomised n = 175) Duration: 10 weeks Treatment protocol: flexible dosage; range = 37.5 to 225 mg/day 2. Placebo arm (randomised n = 168) Duration: 10 weeks Treatment protocol: flexible dosage |
|
| Outcomes |
Time points for assessment: at baseline and then at week 1, 2, 3, 4, 6, 8 and 10 Outcomes:
|
|
| Notes |
Date of study: the study was conducted from April 2001 to December 2002 Funding source: the drug company marketing the drug is likely to have sponsored the study Declarations of interest among the primary researchers: declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is described as randomised, however the process of sequence generation is not clearly reported. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study is described as double‐blind, however the methods for ensuring blindness of both participants and who administered the intervention are not discussed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rates: venlafaxine arm 55/175 (31.4%); placebo arm 43/168 (25.6%). Dropout rates are high in both arms and reasons for leaving the study early are apparently balanced, according to Figure 1. Quote: "The primary analysis population for efficacy variables was the intent‐to‐treat (ITT) population". However, as reported in Figure 1, the ITT population does not match with participants randomly assigned at baseline. Quote: "Patients in the ITT population were those who had a baseline PAAS evaluation and at least 1 double‐blind, on‐therapy evaluation of the primary efficacy variable during visits 3 to 10 and within 3 days of stopping the study medication before taper". This is consistent with an 'as treated' analysis. In the ITT population, imputations were performed with a LOCF approach. |
| Selective reporting (reporting bias) | Low risk | The primary outcome measure is defined as "the percentage of patients free of full‐symptom panic attacks as measured with the Panic and Anticipatory Anxiety Scale (PAAS)", however the precise time point of interest is not clearly specified. All relevant data are clearly reported in the text and tables. |
| Other bias | High risk | Quote: "This clinical trial and analysis were sponsored by Wyeth Research, Collegeville, Pa". No other details on the role of funder in planning and conducting the study are provided. |
Londborg 1998.
| Study characteristics | ||
| Methods | Study design: multisite, double‐blind, parallel and fixed‐dose design, randomised (individual randomisation) controlled trial | |
| Participants |
Diagnosis: DSM‐III‐R diagnosis of panic disorder with or without agoraphobia Method of diagnosis: SCID (Structured Clinical interview for DSM‐III‐R) Age (years): 18.9 to 74.5 (the average age of participants was 38.8 years) Sex: 53% men, 47% women Location: outpatient setting, 7 sites in the USA (6 in western USA and 1 in West Virginia) Co‐morbidities: participants with a secondary diagnosis of an affective disorder, anxiety states including generalised anxiety disorder, social or simple phobia, obsessive‐compulsive disorder or post‐traumatic stress disorder or personality disorder were permitted to participate Rescue medication: choral hydrate for sleep |
|
| Interventions | Participants were randomly assigned to either: 1. Sertraline 50 mg arm (randomised n = 43) Duration: 12 weeks Treatment protocol: fixed dosage 50 mg/day 2. Sertraline 100 mg arm (randomised n = 44) Duration: 12 weeks Treatment protocol: fixed dosage 100 mg/day 3. Sertraline 200 mg arm (randomised n = 45) Duration: 12 weeks Treatment protocol: fixed dosage 200 mg/day 4. Placebo arm (randomised n = 45) Duration: 12 weeks Treatment protocol: fixed dosage, number of tablets not specified |
|
| Outcomes |
Time points for assessment: at the end of weeks 1, 2, 3, 4, 6, 8, 10 and 12 Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: drug company marketing sertraline Declarations of interest among the primary researchers: RW is a Senior Associate Medical Director at the drug company marketing sertraline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly assigned by site, with a blocking factor of four". No further information provided. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "the subjects were randomly assigned by site". No further details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "study medication was taken with the evening meal as a single dose of two capsules contained in a blister pack". No further information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The dropout rate is high (> 20%). Quote: "of the 177 safety‐evaluable subjects, 63 (36%) withdrew from the study, 28 due to adverse experiences and 12 because of insufficient clinical response [...] The difference among the groups was not statistically significant when subjects in the placebo group were compared with pooled subjects taking sertraline (31% and 37%)". The investigators used the LOCF. Quote: "parallel analyses of efficacy parameters were performed both for end‐point with last observation carried forward". |
| Selective reporting (reporting bias) | Low risk | The data related to the primary outcomes are reported in the text, tables and graphs. |
| Other bias | High risk | The study was funded by the drug company marketing sertraline. RW is a Senior Associate Medical Director at the drug company marketing sertraline. |
Lydiard 1992.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III panic disorder or agoraphobia with panic attacks Method of diagnosis: Structured Clinical Interview for DSM‐III, Upjohn version Age: placebo: mean = 36.3 (SD 8.1); alprazolam 2 mg: mean = 39.1 (SD 9.5); alprazolam 6 mg: mean = 36.2 (SD 9.1) Sex: unclear Location: USA Comorbidities: major depression only if depressive symptoms were secondary to their panic symptoms; panic symptoms dominated the clinical picture; the symptoms of the panic disorder preceded the affective disorder chronologically Rescue medication: none |
|
| Interventions | Participants were randomly assigned to either: 1. Alprazolam 2 mg (n = 30) Duration: 6 weeks Treatment protocol: fixed = 2 mg 2. Alprazolam 6 mg (n = 31) Duration: 6 weeks Treatment protocol: fixed = 6 mg 3. Placebo arm (n = 33) Duration: 6 weeks Treatment protocol: fixed |
|
| Outcomes |
Time points for assessment: baseline, week 1, 2, 3, 4, 6 Primary outcomes:
Secondary outcome:
|
|
| Notes |
Date of study: not stated Funding source: not stated Declarations of interest among the primary researchers: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This was a double‐blind study. Quote: "Identically appearing capsules containing alprazolam 1 mg or placebo were packaged for each study week". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind; no further information. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Unequal dropout rates. Quote: "Differential drop‐out rates were noted across treatment groups, with 45% of placebo treated patients, 76.7% alprazolam 2 mg and 48.4% of the alprazoalm 6 mg completing the study". |
| Selective reporting (reporting bias) | High risk | Numerical data of the clinical outcome measures described in the methods are not reported in the results. Only graphs for a few outcome measures are presented. There are different reasons for missing data across groups. |
| Other bias | Low risk | No evidence of other bias was found. |
Lydiard 1993.
| Study characteristics | ||
| Methods | Study design: 12‐week, placebo‐controlled, parallel groups, individual randomisation, double‐blind study | |
| Participants |
Diagnosis: DSM‐III‐R diagnosis of panic disorder with or without agoraphobia Method of diagnosis: structured interview for DSM‐III‐R Age (years): desipramine arm mean age = 38.1 (SD 6.9), placebo arm mean age = 35.1 (SD 1.3) Sex: sex distribution between the 2 arms is unclear Location: primary care setting, South Carolina (USA) Co‐morbidities: excluded Rescue medication: apparently not permitted, but this is not explicit |
|
| Interventions | Participants were randomly assigned to either: 1. Desipramine arm (randomised n = 28) Duration: 12 weeks Treatment protocol: flexible dosage; range = 50 to 200 mg/day, mean = 177 mg (SD 81) 2. Placebo arm (randomised n = 28) Duration: 12 weeks Treatment protocol: flexible dosage; range = 50 to 200 mg/day, mean = 242 mg/day (SD 54) |
|
| Outcomes |
Time points for assessment: at baseline, 8 and 12 weeks Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: unclear Declarations of interest among the primary researchers: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is described as "randomised", but no information about the random sequence generation is provided. Quote: "the patients were randomly assigned to either DMI or placebo". |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as "double blind", no further information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as "double blind", no further information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The dropout rate in the desipramine group is around 7%, while the dropout rate in the placebo group is 39%, so it is high. Investigators used a data imputation technique. Quote: "we calculated the 12‐week outcome for all patients completing at least 8 weeks' treatment by bringing the last observed value forward, expressing these as 12‐week outcome". |
| Selective reporting (reporting bias) | Low risk | All the outcomes are reported in a table in a clear way. |
| Other bias | Unclear risk | It is unclear whether the study was funded by a drug company marketing desipramine or not. No declaration of interest is mentioned. |
Michelson 2001.
| Study characteristics | ||
| Methods | Study design: 12 weeks, randomised (individual randomisation), parallel groups, double‐blind, placebo‐controlled | |
| Participants |
Diagnosis: DSM‐IV criteria for panic disorder with or without agoraphobia Method of diagnosis: SCID Age (years): mean age in fluoxetine arm 36.5 (SD 10.3), mean age in placebo arm 34.8 (SD 9.8) Sex: in fluoxetine arm, 48% (n = 43) men, 52% (n = 47) women; in placebo arm, 41% (n = 37) men, 59% (n = 53) women (overall number: 80 men and 100 women) Location: outpatients, psychiatric clinics, 9 sites in Europe Co‐morbidities: excluded Rescue medication: unclear |
|
| Interventions | Participants were randomly assigned to either: 1. Fluoxetine arm (randomised n = 90) Duration: 12 weeks Treatment protocol: flexible dosage; range = 20 to 60 mg/day, mean = 29.8 mg/day (SD is not specified) 2. Placebo arm (randomised n = 90) Duration: 12 weeks Treatment protocol: flexible dosage (the number of tablets is not specified) |
|
| Outcomes |
Time points for assessment: at baseline, 6, 12 weeks (endpoint) Outcomes:
|
|
| Notes |
Date of study: not reported in the primary publication Funding source: unclear Declarations of interest among the primary researchers: some authors are employees of the company marketing the drug, others are paid consultants |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is reported as randomised, but no information is provided about the random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described just as quote: "double blind trial". No further information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The dropout rate is reported in the text. Quote: "among randomised patients, the number of patients reaching the final visit after 12 weeks of fluoxetine or placebo therapy was similar for both groups (fluoxetine n = 75, 83.3%); placebo n = 80 (88.8%). The total number of discontinuations due to adverse effects was similar for both groups (fluoxetine n = 5, 5.5%), (placebo n = 3, 3.3%)... other reasons for discontinuation included lack of efficacy (fluoxetine n = 5, 5.5%), (placebo n = 3, 3.3%)... patients lost to follow up... patient decision... and protocol requirement..." Despite the dropouts the groups still seem comparable. Data imputation was performed (ITT analysis). |
| Selective reporting (reporting bias) | Low risk | The data of all the outcome measures are clearly reported in tables as mean scores and mean changes from baseline. Standard deviations are specified. |
| Other bias | High risk | Sponsorship bias: some study authors are employees of the company marketing the drug, others are paid consultants. |
Moroz 1999.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III‐R panic disorder with or without agoraphobia Method of diagnosis: Structured Clinical Interview for DSM‐III‐R and a psychiatric interview Age: clonazepam: mean = 36.7 (SD 11.3); placebo: mean = 36.8 (SD 11.4) Sex: clonazepam: F = 141, M = 81; placebo: F = 140, M = 76 Location: not stated (USA); setting: outpatients Comorbidities: psychiatric (major depression, social phobia, obsessive‐compulsive disorder, generalised anxiety disorder) were excluded Rescue medication: none |
|
| Interventions | Participants were randomly assigned to either: 1. Clonazepam (n = 230) Duration: 6 weeks Treatment protocol: flexible dosage; mean = 2.3 mg/day; range = 0.5 mg to 4 mg (daily dose) 2. Placebo (n = 225) Duration: 6 weeks Treatment protocol: flexible dosage; mean = 3.0 mg/day |
|
| Outcomes |
Time points for assessment: assessment week 1, 2 and 6. For CGI‐S, PGI‐C, WSDS and monitoring of adverse events: weeks 0, 1, 2, 3, 6. HAMA, HAMD: at screening visit and week 6 Primary outcomes:
Secondary outcome:
|
|
| Notes |
Date of study: not stated Funding source: sponsored by Hoffmann‐La Roche Inc., Nutley, NJ Declarations of interest among the primary researchers: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. Quote: "The study medications were clonazepam and identical‐looking placebo tablets". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind; no further information. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There is an imbalance in missing outcome data between the groups (drug 1 = 44 out of 230, placebo = 65 out of 225), with different reasons for missing data across groups. Furthermore, the total number of dropouts in each group is not fully transparent. |
| Selective reporting (reporting bias) | High risk | The outcomes of interest in the review are reported incompletely (no mean, SD) so that they cannot be entered into a meta‐analysis. |
| Other bias | High risk | Quote: "The demographic and baseline disease characteristics of the clonazepam and placebo ITT groups were similar". Sponsored by Hoffmann‐La Roche Inc., Nutley, NJ. |
Munjack 1989.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III panic disorder or agoraphobia with panic attacks Method of diagnosis: not stated Age: mean = 31 (range = 18 to 62) Sex: M = 17; F = 38 Location: California, USA (psychiatric outpatients clinic) Comorbidities: not stated Rescue medication: none |
|
| Interventions | Participants were randomly assigned to either: 1. Alprazolam (n = 20) Duration: 5 weeks Treatment protocol: flexible dosage; range = 1.5 mg to 6 mg, mean = 3.62 (7.24 capsules, SD 4.09) 2. Placebo (n = 21) Duration: 5 weeks Treatment protocol: flexible; mean = 9.90 capsules (SD 3.74) |
|
| Outcomes |
Time points for assessment: weekly Primary outcomes:
Secondary outcome:
|
|
| Notes |
Date of study: not stated Funding source: not stated Declarations of interest among the primary researchers: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Patients were randomly and blindly assigned to one of 3 treatment groups. All of the visually identical capsules contained either (...) were administered three times a day". Additional analysis of the success of blinding showed that physicians were able to distinguish between alprazolam and placebo regardless of the blinding procedure (Munjack 1989b). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind; outcome assessments were conducted by physicians and independent "assessors"; results are reported separately, no further information available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There is an imbalance in missing outcome data between the groups (drug 1 = 0, placebo = 5). Quote: "A chi‐square analysis indicated a significant difference in the dropout rates among the 3 treatment groups and specifically between alprazolam and placebo". Observed case analysis only. |
| Selective reporting (reporting bias) | High risk | Not all the efficacy outcome measures described in the methods are reported in the results section (Sheehan). No baseline data are presented. No data on side effects |
| Other bias | Low risk | No evidence of other bias was found. |
Nair 1996.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III‐R panic disorder with or without agoraphobia Method of diagnosis: not stated Age: for fluvoxamine, mean = 34.5; for imipramine, mean = 34.5, SD not provided Sex: for fluvoxamine 56% females 44% males; for imipramine 50% females 50% males Location: Canada; setting: outpatients Co‐morbidities: patients with a history of bipolar disorder, organic brain syndrome, schizophrenia or other psychotic disorders were excluded Rescue medication: oxazepam up to 60 mg daily or chloral hydrate up to 2000 mg daily were permitted during the first 4 weeks of treatment |
|
| Interventions |
Participants were randomly assigned to either: 1. Fluvoxamine arm (n = 50) Duration: 8 weeks Treatment protocol: flexible dosage, range = 50 mg to 300 mg, mean = 171.4 mg, SD not provided 2. Imipramine arm (n = 48) Duration: 8 weeks Treatment protocol: flexible dosage, range = 50 mg to 300 mg, mean = 164.7, SD not provided 3. Placebo arm (n = 50) Duration: 8 weeks |
|
| Outcomes |
Time points for assessment: weekly Outcomes:
|
|
| Notes |
Date of study: not stated Funding source: Orto McNeil Ltd. Declarations of interest among the primary researchers: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized". No further information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the study medication was in the form of identically appearing capsules each containing either placebo, 50 mg of fluvoxamine or 50 mg of imipramine". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the study medication was in the form of identically appearing capsules each containing either placebo, 50 mg of fluvoxamine or 50 mg of imipramine". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "two patient samples were identified for analysis and reporting purposes prior to unblinding: an all patients analysis and an ITT. The all patients sample was defined as those randomised to double blind treatment and who provided at least some drug safety and tolerance data [...] the main efficacy analysis of the study was based on the LOCF of the ITT sample". |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | High risk | Sponsored by Orto McNeil Ltd; the role of the funder in planning, conducting and writing the study is not discussed. |
Noyes 1996.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III panic disorder or agoraphobia with panic attacks Method of diagnosis: Structured Clinical Interview for DSM‐III, Upjohn Version Age: mean = 36.6 (SD 10.5) Sex: women = 157, men = 84 Location: USA, Australia; setting: outpatients Co‐morbidities: patients with major psychiatric co‐morbidities, head trauma or seizures were excluded Rescue medication: none |
|
| Interventions |
Participants were randomly assigned to either: 1. Diazepam arm (n = 81) Duration: 8 weeks Treatment protocol: flexible dosage, range = 10 mg to 100 mg, mean = 43, SD not provided 2. Alprazolam arm (n = 78) Duration: 8 weeks Treatment protocol: flexible dosage, range = 1 mg to 10 mg, mean = 4.9, SD not provided 3. Placebo arm (n = 79) Duration: 8 weeks |
|
| Outcomes |
Time points for assessment: baseline, 4 weeks, 8 weeks Outcomes:
|
|
| Notes |
Date of study: not stated Funding source: supported by a grant from the Upjohn Company Declarations of interest among the primary researchers: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized". No further information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double‐blind". No further details. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double‐blind". No further details. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "to examine differences in treatment groups over time we completed ITT analysis using logistic regression procedures. The results of analysis using the completer sample were very similar to those using the III subjects". |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | High risk | Supported by a grant from the Upjohn Company; the role of the funder in planning, conducting and writing the study is not discussed. |
Pecknold 1994.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III‐R panic disorder and extensive phobic avoidance (agoraphobia with panic attacks) or limited phobic avoidance Method of diagnosis: Structured Clinical Interview for DSM‐III‐R Age: for alprazolam CT, mean = 36.4 (SD 10.5) (range 19 to 64); for alprazolam XR, mean = 33.8 (SD 10.3) (range 24 to 65); for placebo, mean = 35.5 (SD 10.0) (range 22 to 64) Sex: for alprazolam CT, 59% female; for alprazolam XR, 63% female; for placebo, 58% female Location: USA (2 sites: Rhode Island and Los Angeles) and Canada (1 site: Montreal) Comorbidities: major depression (if depressive symptoms were secondary to panic symptoms or if panic dominated the clinical picture and panic disorder preceded the development of the affective symptoms) Rescue medication: none |
|
| Interventions | Participants were randomly assigned to either: 1. Alprazolam CT (n = 69*) Duration: 6 weeks Treatment protocol: flexible dosage; range = 2 to 7(?) mg; mean = 3.95 (SD 1.86) 2. Alprazolam XR (n = 70*) Duration: 6 weeks Treatment protocol: flexible dosage; range = 2 to 7(?) mg; mean = 4.35 (SD 2.30) 3. Placebo (n = 70*) Duration: 6 weeks Treatment protocol: flexible dosage; range = 2 to 7(?) mg; mean = 5.46 (SD 2.26) *The number of participants in the different arms is inconsistently reported. We used the number of participants in the LOCF analyses. |
|
| Outcomes |
Time points for assessment: at baseline and weekly thereafter for 6 weeks Primary outcomes:
Secondary outcome:
|
|
| Notes |
Date of study: not stated Funding source: the study was supported by the Upjohn Company Declarations of interest among the primary researchers: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No clear information on blinding. Quote: "This study was design as a double‐blind (...). Medication was dispensed weekly to patients in two bottles". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear information on blinding. Quote: "This study was design as a double‐blind (...). Medication was dispensed weekly to patients in two bottles". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only ITT population reported. There are inconsistencies in the reported N. Data censored for participants with at least 3 weeks of treatment. There is an imbalance in dropouts among the groups (drug 1 = 7, drug 2 = 12, placebo = 20). Quote: "During the first 3 weeks of the study, 4.2% of the CT alprazolam, 14.3% of the XR alprazolam and 14.7% of the placebo recipients dropped out of the study after beginning medication from the total ITT group of 209 patients". "However, there was a significantly higher dropout rate, probably because of effectiveness, in the placebo group compared with the CT or XR groups". |
| Selective reporting (reporting bias) | High risk | Quote: “When completer analysis showed no statistical significance, endpoint results were reported”. Some scales are only reported not to have shown significant differences. 1 outcome measure (WSDS) is not reported in the results. |
| Other bias | High risk | The study was supported by the Upjohn Company. |
Pfizer 2008.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: panic disorder with or without agoraphobia according to DSM‐IV Method of diagnosis: no information provided Age: range = 18 to 64 years, mean and SD not provided Sex: sertraline: female = 113, male = 44; paroxetine: female = 109, male = 53 Location: Japan; setting unclear Co‐morbidities: "patients with bipolar disorder, schizophrenia, delusional disorder, epilepsy, MDD, OCD, seasonal affective disorder or GAD were excluded; patients who concurrently have depression/depressive state, anxiety disorder and generalized anxiety disorder may be included if the primary diagnosis is identified to be panic disorder" Rescue medication: none |
|
| Interventions |
Participants were randomly assigned to either: 1. Sertraline arm (n = 157) Duration: 12 weeks Treatment protocol: flexible dosage, range = 25 mg to 100 mg 2. Paroxetine arm (n = 164) Duration: 12 weeks Treatment protocol: flexible dosage, range = 10 mg to 30 mg |
|
| Outcomes |
Time points for assessment: Outcomes:
|
|
| Notes |
Date of study: May 2008 to February 2010 Funding source: Pfizer Declarations of interest among the primary researchers: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Allocation: randomized". No further information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Masking: double‐blind (subject, investigator)". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Masking: double‐blind (subject, investigator)". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Last obsevation carried forward". No further information provided. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | High risk | Sponsored by Pfizer; the role of the funder in planning, conducting and writing the study is not discussed. |
Pohl 1989b.
| Study characteristics | ||
| Methods | Study design: 8 weeks, randomised controlled trial, individual randomisation, parallel groups | |
| Participants | Diagnosis: DSM‐III panic disorder or agoraphobia with panic attacks Method of diagnosis: not stated Age (years): for buspirone, mean = 31.1 (SD 2.1); for placebo, mean = 31.6 (SD 2.2); for imipramine, mean = 29.2 (SD 2.2) Sex: for buspirone, 44% women, 56% men; for placebo, 50% women, 50% men Location: outpatients, USA Co‐morbidities: excluded Rescue medication: none | |
| Interventions | Participants were randomly assigned to either: 1. Buspirone arm (randomised n = 18) Duration: 8 weeks Treatment protocol: flexible dosage; range = 10 mg to 60 mg, mean = 29.5 (SD 4.0) 2. Imipramine arm (randomised n = 20) Duration: 8 weeks Treatment protocol: flexible dosage; range = 50 mg to 300 mg, mean = 140 (SD 17.5) 3. Placebo arm (randomised n = 22) Duration: 8 weeks Treatment protocol: flexible |
|
| Outcomes |
Time points for assessment: weekly for the first 4 weeks, and biweekly for the last 4 weeks Outcomes:
|
|
| Notes | Date of study: not stated Funding source: not stated Declarations of interest among the primary researchers: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "All eligible patients were randomized to 8 weeks of double‐blind treatment with buspirone, imipramine or placebo following an initial 4‐7 days of single blind placebo wash‐out." No further details about randomisation are provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical capsules were used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of the assessors is not described even though the trial is described as "double blind". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High attrition rate. |
| Selective reporting (reporting bias) | High risk | The measures of primary outcome are specified in the text (in the "efficacy measures" chapter under the "methods" section), but the results are reported in graphs and not in a table or in the text as numbers. |
| Other bias | Unclear risk | No information is provided about possible sponsorship of the study. |
Pollack 1998.
| Study characteristics | ||
| Methods | Study design: 10 weeks, flexible dose, multi‐centre trial, random assignment (individual), parallel groups, placebo‐controlled | |
| Participants |
Diagnosis: DSM‐III‐R criteria for panic disorder with or without agoraphobia Method of diagnosis: SCID Age (years): mean age in sertraline arm 37.8 (SD 11.6), mean age in placebo arm 34.9 (SD 9.6) Sex: 115 women, 63 men Location: outpatient setting, 10 sites, USA and Brazil Co‐morbidities: "patients with comorbid dystimic, personality, or other anxiety disorders could be included if the panic disorder was judged to be the principal diagnosis" Rescue medication: not allowed |
|
| Interventions | Participants were randomly assigned to either: 1. Sertraline arm (randomised n = 88) Duration: 10 weeks Treatment protocol: flexible dose, range 25 to 200 mg/day, mean 118.1 mg/day (SD 62.9) 2. Placebo arm (randomised n = 88) Duration: 10 weeks Treatment protocol: flexible dose, range unknown, mean 147.5 mg/day (SD 55.5) |
|
| Outcomes |
Time points for assessment: at baseline and at weeks 1, 2, 3, 4, 6, 8 and 10 Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: supported by the company marketing the drug Declarations of interest among the primary researchers: one of the primary researchers is an employee of the company marketing the drug |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The random sequence generation is explained. Quote: "patients were randomly assigned by computer‐generated numbers to 10 weeks of double blind treatment with either sertraline or placebo". |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rate is less than 20%. They apparently imputed missing data. Quote: "patients who took at least one dose of double blind medication and completed any additional assessment were included in the analysis for safety and efficacy". |
| Selective reporting (reporting bias) | Low risk | Outcomes are clearly reported in tables. |
| Other bias | High risk | The study was financially supported by the drug company marketing the drug and one of the primary researchers was an employee of the company itself. |
Pollack 2007a.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐IV panic disorder with or without agoraphobia Method of diagnosis: Mini‐International Neuropsychiatric Interview Age: for venlafaxine 75 mg, mean = 35.8 (SD 9.97); for venlafaxine 225 mg, mean = 37.1 (SD 11.8), for paroxetine mean = 37.5 (SD 11) Sex: for venlafaxine 75 mg, females = 65%, males = 35%; for venlafaxine 225 mg, females = 68%, males = 33%; for paroxetine, females = 68%, males = 32% Location: Argentina, Mexico, Chile, Costa Rica; setting: outpatients Co‐morbidities: patients with other predominant Axis I or II disorders and important medical conditions were excluded Rescue medication: zaleplon or zolpidem permitted up to 3 times per week for the first 2 weeks of randomised treatment |
|
| Interventions |
Participants were randomly assigned to either: 1. Venlafaxine 75 mg arm (n = 163) Duration: 12 weeks Treatment protocol: fixed dosage = 75 mg/day 2. Venlafaxine 225 mg arm (n = 167) Duration: 12 weeks Treatment protocol: fixed dosage = 225 mg/day 3. Paroxetine arm (n = 161) Duration: 12 weeks Treatment protocol: fixed dosage = 40 mg/day 4. Placebo arm (n = 162) Duration: 12 weeks |
|
| Outcomes |
Time points for assessment: baseline, weeks 1, 2, 3, 4, 6, 8, 10, 12 Outcomes:
|
|
| Notes |
Date of study: not stated Funding source: Wyeth Research, Collegeville, Pennsylvania Declarations of interest among the primary researchers: members of advisory boards, and research support received by many pharmaceutical companies, including AstraZeneca, GlaxoSmithKline, Eli Lilly, Pfizer, Roche, Wyeth |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized". No further information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "... they were randomly assigned to receive venlafaxine 75 mg/day, venlafaxine 225 mg/day, paroxetine or placebo once daily in identically appearing capsules". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "... they were randomly assigned to receive venlafaxine 75 mg/day, venlafaxine 225 mg/day, paroxetine or placebo once daily in identically appearing capsules". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "statistical analysis on the primary and secondary outcome measures were performed for an ITT population of patients who had at least one post randomisation visit on therapy using LOCF values". |
| Selective reporting (reporting bias) | Unclear risk | Continuous data at endpoint are reported only in graphs. |
| Other bias | High risk | Sponsored by Wyeth; the role of the funder in planning, conducting and writing the study is not discussed. |
Pollack 2007b.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐IV panic disorder with or without agoraphobia Method of diagnosis: Mini‐International Neuropsychiatric Interview Age: for venlafaxine 75 mg, mean = 36.2 (SD 10.7); for venlafaxine 150 mg, mean = 37.7 (SD 11.5), for paroxetine mean = 37.6 (SD 10.5) Sex: for venlafaxine 75 mg, females = 66%, males = 34%; for venlafaxine 150 mg, females = 70%, males = 30%; for paroxetine, females = 64%, males = 36% Location: Europe; setting: outpatients Co‐morbidities: patients with other predominant Axis I or II disorders and important medical conditions were excluded Rescue medication: zaleplon or zolpidem permitted up to 3 times per week for the first 2 weeks of randomised treatment |
|
| Interventions |
Participants were randomly assigned to either: 1. Venlafaxine 75 mg arm (n = 166) Duration: 12 weeks Treatment protocol: fixed dosage = 75 mg/day 2. Venlafaxine 150 mg arm (n = 168) Duration: 12 weeks Treatment protocol: fixed dosage = 150 mg/day 3. Paroxetine arm (n = 166) Duration: 12 weeks Treatment protocol: fixed dosage = 40 mg/day 4. Placebo arm (n = 163) Duration: 12 weeks |
|
| Outcomes |
Time points for assessment: baseline, weeks 1, 2, 3, 4, 6, 8, 10, 12 Outcomes:
|
|
| Notes |
Date of study: not stated Funding source: sponsored by Wyeth Research Declarations of interest among the primary researchers: members of advisory boards, and research support received by many pharmaceutical companies, including AstraZeneca, GlaxoSmithKline, Eli Lilly, Pfizer, Roche, Wyeth; some authors' affiliations refer to Wyeth |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized". No further information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Study medication was provided as identical appearing capsules and was to be taken once daily with food". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Study medication was provided as identical appearing capsules and was to be taken once daily with food". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "statistical analysis on the primary and secondary outcome measures were performed for an ITT population of patients who had at least one post randomisation visit on therapy using LOCF values". No further information provided. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | High risk | Sponsored by Wyeth; the role of the funder in planning, conducting and writing the study is not discussed. |
Ribeiro 2001.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐IV panic disorder with or without agoraphobia Method of diagnosis: not stated Age: for mirtazapine, mean = 36.1 (SD 10.9); for fluoxetine, mean = 36.4 (SD 10.1) Sex: for mirtazapine, 86.7% females, for fluoxetine, 66.7% females Location: Brazil; setting: outpatients Co‐morbidities: patients with psychiatric and physical disorders were excluded Rescue medication: none |
|
| Interventions |
Participants were randomly assigned to either: 1. Mirtazapine arm (n = 15) Duration: 8 weeks Treatment protocol: flexible dosage, range = 15 mg to 30 mg, mean = 17.9 (SD 4.3) 2. Fluoxetine arm (n = 15) Duration: 8 weeks Treatment protocol: flexible dosage, range = 10 mg to 20 mg, mean = 13.1 (SD 3.2) |
|
| Outcomes |
Time points for assessment: baseline, week 1, 2, 4, 6 and 8 Outcomes:
|
|
| Notes |
Date of study: November 1998 to March 1999 Funding source: research supported by FIPE‐HCPA (Fundo de Incentivo à Pesquisa e Eventos) Declarations of interest among the primary researchers: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomised to mirtazapine or fluoxetine using a computer program which assigned 15 patients to each group". |
| Allocation concealment (selection bias) | Low risk | Quote: "a person who was not participating in the study labeled flasks containing enough medications for periods between visits" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double‐blind". No further details. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double‐blind". No further details. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "the analysis included all patients who took at least one dose of medication during the double‐blind phase and who provided any follow‐up data". No further information provided. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Unclear risk | Quote: "Organon Pharmaceutical kindly provided mirtazapine for the trial". No more information provided. |
Robinson 1989.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III panic disorder Method of diagnosis: not stated Age: for buspirone, mean = 34.4 (SD 1.8); for placebo, mean = 33.1 (SD 1.9); for imipramine, mean = 30.1 (SD 1.0) Sex: for buspirone, 64% women, 36% men; for placebo, 62% women, 38% men; for imipramine, 75% women, 25% men Location: United States of America Co‐morbidities: unclear Rescue medication: none |
|
| Interventions | Participants were randomly assigned to either: 1. Buspirone arm (n = 34) Duration: 8 weeks Treatment protocol: flexible dosage; range = not stated, mean = 43 (SD 3) 2. Placebo arm (n = 29) Duration: 8 weeks Treatment protocol: flexible 3. Imipramine arm (n = 28) Duration: 8 weeks Treatment protocol: flexible dosage; range = not stated, mean = 221 (SD 18) |
|
| Outcomes |
Time points for assessment: at 0, 2, 4, 6, 7, 8 weeks Outcomes:
|
|
| Notes |
Date of study: not stated Funding source: not stated Declarations of interest among the primary researchers: one of the authors belonged to Bristol‐Myers Company Pharmaceutical Research and Development Division. The authors were advised by employees from Bristol‐Myers Company. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical capsules were used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | This is a double‐blind trial. No other information. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High attrition rate. |
| Selective reporting (reporting bias) | Unclear risk | No information provided. |
| Other bias | High risk | All the authors were employed by the drug company marketing the drug. |
Rosenbaum 1997.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III‐R panic disorder with or without agoraphobia Method of diagnosis: SCID‐Ro (a version of the Structured Clinical Interview for DSM‐III‐R) and a psychiatry interview Age: mean = 37.3, range = 18 to 76 Sex: F = 56% Location: USA (12 sites); outpatient setting Co‐morbidities: none Rescue medication: none (protocol) |
|
| Interventions | Participants were randomly assigned to either: 1. Clonazepam 0.5 mg (n = 68) Duration: 9 weeks (+ discontinuance phase: 7 weeks) Treatment protocol: fixed dose = 0.5 mg 2. Clonazepam 1.0 mg (n = 68) Duration: 9 weeks (+ discontinuance phase: 7 weeks) Treatment protocol: fixed dose = 1.0 mg 3. Clonazepam 2.0 mg (n = 69) Duration: 9 weeks (+ discontinuance phase: 7 weeks) Treatment protocol: fixed dose = 2.0 mg 4. Clonazepam 3.0 mg (n = 67) Duration: 9 weeks (+ discontinuance phase: 7 weeks) Treatment protocol: fixed dose = 3.0 mg 5. Clonazepam 4.0 mg (n = 72) Duration: 9 weeks (+ discontinuance phase: 7 weeks) Treatment protocol: fixed dose = 4.0 mg 6. Placebo arm (n = 69) Duration: 9 weeks (+ discontinuance phase: 7 weeks) Treatment protocol: fixed |
|
| Outcomes |
Time points for assessment: at each visit (CGI‐S, mean duration of anticipatory anxiety); at each post‐baseline visit (CGI‐C); at baseline, week 9, week 16 (severity of fear associated with the main phobia) Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Date of study: October 1992 to June 1995 Funding source: this clinical trial was supported by Hoffmann‐La Roche Declarations of interest among the primary researchers: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization to treatment groups was done by means of computer‐generated codes for each centre, using the fixed‐block method with a block size of six". |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. Quote: "All study medications were taken in divided doses, half in the morning and half at bedtime, and were identical in appearance and packaging (blister cards)". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind; no further information. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only data for modified ITT reported. |
| Selective reporting (reporting bias) | High risk | Not all the data are reported completely, so some could not be entered into a meta‐analysis. |
| Other bias | High risk | This clinical trial was supported by Hoffmann‐La Roche. |
Savoldi 1990.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III panic disorder with agoraphobia Method of diagnosis: not stated Age: mean = 37.7 (SD 7.97) Sex: M = 12; F = 18 Location: not stated Comorbidities: none Rescue medication: none |
|
| Interventions | Participants were randomly assigned to either: 1. Etizolam (n = 15) Duration: 4 weeks Treatment protocol: fixed dosage = 0.50 mg 2. Placebo arm (n = 15) Duration: 4 weeks Treatment protocol: fixed |
|
| Outcomes |
Time points for assessment: baseline, week 2, 4 Primary outcomes:
Secondary outcome:
|
|
| Notes |
Date of study: not stated Funding source: not stated Declarations of interest among the primary researchers: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were allocated at random to receive twice daily doses of either etizoalm or placebo". No information on random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "32 patients were enrolled in a double‐blind study. (...) The psychometric evaluations were carried out by two independent examiners, not the trial clinician". It is not clear who was blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "32 patients were enrolled in a double‐blind study. (...) The psychometric evaluations were carried out by two independent examiners, not the trial clinician". It is not clear who was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There is an imbalance in dropouts (drug 1 = 1 out of 15, placebo = 6 out of 15). The dropouts were not included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | The frequency of panic attacks was not reported. |
| Other bias | Low risk | No evidence of other bias was found. |
Schweizer 1992.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III‐R panic disorder, either uncomplicated, with limited phobic avoidance, or with agoraphobia Method of diagnosis: not stated Age: mean = 35 Sex: 3 males, 2 females Location: not stated Comorbidities: social phobia (n = 1) Rescue medication: none (besides midazolam when necessary) |
|
| Interventions | Participants were randomly assigned to either: 1. Midazolam (n = 3 + 2) Duration: 3 + 3 weeks Treatment protocol: flexible dosage; range = 0.25 to 1 mg; mean number of doses (week 3) = 6.1 (0.44 mg/day) 2. Placebo (n = 2 + 3) Duration: 3 + 3 weeks Treatment protocol: flexible |
|
| Outcomes |
Time points for assessment: baseline, week 1, 2, 3, 4, 5, 6 Primary outcomes:
|
|
| Notes |
Date of study: not stated Funding source: not stated Declarations of interest among the primary researchers: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The study had a double‐blind design. (...) Investigators were careful not to indicate anything about the order of timing of the crossover". No further information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The study had a double‐blind design. (...) Investigators were careful not to indicate anything about the order of timing of the crossover". No further information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Individual participant data available. No evidence of selective outcome reporting. |
| Selective reporting (reporting bias) | Unclear risk | No side effects reported. |
| Other bias | Unclear risk | Very small pilot cross‐over trial. |
Schweizer 1993.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III panic disorder Method of diagnosis: Structured Clinical Interview for DSM‐III, Upjohn Version Age: mean = 33 (SD 7 ) Sex: female = 75%, male = 25% Location: USA; setting: in‐ and outpatients Co‐morbidities: none Rescue medication: Quote: "no concomitant centrally active medication therapy was permitted during the study" |
|
| Interventions |
Participants were randomly assigned to either: 1. Alprazolam arm (n = 37) Duration: 8 weeks short term, 32 weeks long term Treatment protocol: flexible dosage, range = 2 mg to 10 mg, mean = 5.4 (SD 2.1) 2. Imipramine arm (n = 34) Duration: 8 weeks short term, 32 weeks long term Treatment protocol: flexible dosage, range = 50 mg to 250 mg, mean = 152 (SD 65) 3. Placebo arm (n = 35) Duration: 8 weeks short term, 32 weeks long term |
|
| Outcomes |
Time points for assessment: weekly until week 6, week 8, monthly for 6 months Outcomes:
|
|
| Notes |
Date of study: not stated Funding source: sponsored by Upjohn Co. Declarations of interest among the primary researchers: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized". No further information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "patients were dispensed identical capsules containing either 1 mg of alprazolam or 25 mg of imipramine". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "patients were dispensed identical capsules containing either 1 mg of alprazolam or 25 mg of imipramine". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "ITT endpoint analysis, including all patients with at least one week of treatment and 'evaluable patients' or 'decreasing N' analysis, using only those patients available at each visit, were the primary set of analysis conducted. Supplementary completers analysis using only patients who completed either 8 weeks or 32 weeks of treatment were also conducted". "While the high attrition rate in the imipramine and placebo treatment groups posed a problem for the statistical analysis of the various outcome measures, attrition rates themselves constituted an important and independent outcome measures. Survival analysis was performed for on‐study treatment". |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were reported. |
| Other bias | High risk | Sponsored by Upjohn Co.; the role of the funder in planning, conducting and writing the study is not discussed. |
Sharp 1990.
| Study characteristics | ||
| Methods | Study design: randomised (individual randomisation), parallel groups, double‐blind, fixed‐dose design, 12 weeks + 6 months of follow‐up | |
| Participants |
Diagnosis: DSM‐III‐R panic disorder with or without agoraphobia Method of diagnosis: not specified Age (years): 18 to 70, 36.62 in fluvoxamine arm, 42.28 in placebo arm, 37.27 in fluvoxamine + placebo arm, 38.81 in placebo + CBT arm, 33.23 in CBT arm Sex: 115 women, 32 men Location: general practice/primary care, Scotland, UK Co‐morbidities: excluded Rescue medication: not allowed |
|
| Interventions | Participants were randomly assigned to either: 1. Fluvoxamine arm (randomised n = 36) Treatment protocol: fixed dose, range 50 to 150 mg/day, mean = 150 mg/day Duration: 12 weeks 2. Placebo arm (randomised n = 37) Treatment protocol: fixed dose; range not stated Duration: 12 weeks 3. Fluvoxamine + CBT (randomised n = 38) Treatment protocol: fixed dose; 150 mg/day Duration: 12 weeks 4. Placebo + CBT arm (randomised n = 36) Treatment protocol: fixed dose; range not stated Duration: 12 weeks 5. CBT arm (randomised n = 43) Treatment protocol: 30‐ to 60‐min sessions Duration: 12 weeks |
|
| Outcomes |
Time points for assessment: at baseline and at weeks 1, 2, 4, 6, 8, 10 and 12 Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: funded by the company marketing the drug Declarations of interest among the primary researchers: not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but no further information about random sequence generation is provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The active and placebo tablets seemed to be identical. Quote: "medication was supplied in 50 mg tablets, patients receiving placebo were given the equivalent number of tablets at each appointment, thus maintaining the double blind status". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | There was an independent assessor monitoring the data collection. Quote: "JA acted as independent monitor; data collected were monitored at monthly intervals throughout the duration of study". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout rate is around 19% in the fluvoxamine group and around 24% in the placebo group. It is not clear whether missing data were imputed. |
| Selective reporting (reporting bias) | Unclear risk | Scores of the scales used for the treatment evaluation are poorly reported. |
| Other bias | High risk | Funded by the company marketing the drug. |
Sheehan 1993.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III‐R panic disorder with extensive phobic avoidance, panic disorder with limited phobic avoidance Method of diagnosis: Structured Clinical Interview for DSM‐III (SCID‐UP) Age: alprazolam: mean = 36.4 (SD 8.8); buspirone: mean = 36.6 (SD 9.4); placebo: mean = 37.2 (SD 10.9) Sex: alprazolam: F = 76%, buspirone: F = 67%, placebo: F = 77% Location: USA Comorbidities: major depressive disorder (if secondary to panic disorder) Rescue medication: none |
|
| Interventions | Participants were randomly assigned to either: 1. Alprazolam (n = 34) Duration: 8 weeks Treatment protocol: flexible dosage; range = 1.5 mg to 10 mg, mean = 5.2 (SD 2.6) 2. Buspirone (n = 34) Duration: 8 weeks Treatment protocol: flexible dosage; range = 15 mg to 100 mg, mean = 61 (SD 26.5) 3. Placebo (n = 33) Duration: 8 weeks Treatment protocol: flexible dosage; range = 3 to 20 capsules, mean = 16.5 capsules (SD 5) |
|
| Outcomes |
Time points for assessment: baseline, weekly for 8 visits Primary outcomes:
Secondary outcome:
|
|
| Notes |
Date of study: not stated Funding source: this study was supported in part by grant 4447 from the Upjohn Company Declarations of interest among the primary researchers: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "a total of 101 patients entered the trial and were randomly assigned to the 3 treatment groups". No information on random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "a double‐blind design was used. Medication was prepared in identical‐appearing capsules containing 0.5 mg of alprazolam, 5 mg of buspirone or placebo." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind; no further information. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data censored for participants with at least 3 weeks of treatment; analyses mainly reported from observed case analysis. |
| Selective reporting (reporting bias) | High risk | Not all of the study's prespecified primary outcome measures have been reported (e.g. SCL‐90, MADRS). |
| Other bias | Unclear risk | This study was supported in part by grant 4447 from the Upjohn Company. The results are based entirely on the authors' statistical analysis and management of the data and not on any analysis by the sponsors. The article was written exclusively by the authors without any assistance or input from any pharmaceutical company. |
Sheehan 2005.
| Study characteristics | ||
| Methods | Study design: pooled analysis of 3 identical, double‐blind, placebo‐controlled, parallel‐group, individually randomised, 10‐week clinical trials | |
| Participants |
Diagnosis: DSM‐IV panic disorder with or without agoraphobia Method of diagnosis: DSM‐IV Age (years): 18 to 65, mean 37.6 (SD 10.22) in paroxetine CR group, 37.8 (SD 10.61) in placebo group Sex: 356 men, 543 women Location: USA and Canada, outpatient setting Co‐morbidities: inclusion of people with secondary Axis I disorders Rescue medication: not allowed |
|
| Interventions | Participants were randomly assigned to either: 1. Paroxetine CR arm (randomised n = 444) Duration: 10 weeks Treatment protocol: flexible dosage; range = 12.5 to 75 mg/day, mean = 50 mg/day (SD not specified) 2. Placebo arm (randomised n = 445) Duration: 10 weeks Treatment protocol: flexible dosage |
|
| Outcomes |
Time points for assessment: at baseline and at weekly and bi‐weekly intervals Outcomes:
|
|
| Notes |
Date of study: November 1996 to September 1997 Funding source: the studies were sponsored by the company marketing paroxetine CR Declarations of interest among the primary researchers: the study author declares to have financial associations with many companies that produce psychoactive pharmaceutical agents |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The studies are described as "randomised", but no information about the random sequence generation is provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The studies are described as "double blind"; no other information is provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of dropouts and the reasons for withdrawal are clearly reported. The study authors used data imputation. Quote: "Efficacy and safety analysis were carried out on the modified intention‐to‐treat (ITT) population, defined as all patients who were randomly assigned to treatment, received at least 1 dose of study medication, and had at least 1 postbaseline assessment". |
| Selective reporting (reporting bias) | Low risk | The results of the primary and secondary efficacy outcomes are reported in tables and graphs. |
| Other bias | High risk | The studies were sponsored by the company marketing paroxetine CR; the role of the funder in planning, conducting and writing the study is not discussed. |
Sheikh 1999.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III‐R panic disorder with or without agoraphobia Method of diagnosis: Structured Clinical Interview for DSM‐III‐R ‐ patient version (SCID‐P) Age: mean = 61.24 (SD 5.27), range = 55 to 73 Sex: M = 2; F = 32 Location: USA Comorbidities: none Rescue medication: none |
|
| Interventions | Participants were randomly assigned to either: 1. Alprazolam (n = 8) Duration: 8 weeks Treatment protocol: flexible dosage; range = 1 mg to 6 mg, mean = 2.87 (SD 1.66) 2. Imipramine (n = 10) Duration: 8 weeks Treatment protocol: flexible dosage; range = 10 mg to 200 mg, mean = 77.5 (SD 59.4) 3. Placebo arm (n = 7) Duration: 8 weeks Treatment protocol: flexible |
|
| Outcomes |
Time points for assessment: baseline, at each subsequent medication visit Primary outcomes:
|
|
| Notes |
Date of study: 2‐year period (1988 to 1990) Funding source: this research was supported in part by the Medical Research Service of the VAPAHCS, by grant MH‐49226 from the National Institutes of Health, US Department of Health and Human Services, and the Upjohn Company Declarations of interest among the primary researchers: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Those subjects selected for inclusion were randomised to one of three medication treatment conditions". No information on sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Medication for this double‐blinded protocol were provided by the UpJohn Company in the form of identical looking capsules". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind; no further information. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were 7 dropouts (6 in the placebo group, 0 in the alprazolam group, 1 in the imipramine group). There is an imbalance between the groups. Quote: "The small sample size prevents statistical analyses of the data". Placebo group analysed as LOCF, others as observed case. |
| Selective reporting (reporting bias) | Unclear risk | The results of the rating scales are all reported. No data on side effects, but they are not mentioned in the methods. |
| Other bias | Unclear risk | This research was supported in part by the Medical Research Service of the VAPAHCS, by grant MH‐49226 from the National Institutes of Health, US Department of Health and Human Services, and the Upjohn Company. |
Stahl 2003.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐IV panic disorder Method of diagnosis: not stated Age: for escitalopram, mean = 37.5, for citalopram mean = 37.1 Sex: for escitalopram 57.6% female, for citalopram 61.6% female Location: USA; setting: outpatients Co‐morbidities: patients with bipolar disorder, schizophrenia, obsessive‐compulsive disorder or other psychotic disorder, psychoactive substance use disorder, clinically significant abnormalities in laboratory evaluations or electrocardiographic readings were excluded Rescue medication: zolpidem |
|
| Interventions |
Participants were randomly assigned to either: 1. Escitalopram arm (n = 129) Duration: 10 weeks Treatment protocol: flexible dosage, range = 5 mg to 20 mg, mean = 10.8 (SD not provided) 2. Citalopram arm (n = 126) Duration: 10 weeks Treatment protocol: flexible dosage, range = 10 mg to 40 mg, mean = 21.3 (SD not provided) 3. Placebo arm (n = 125) Duration: 10 weeks |
|
| Outcomes |
Time points for assessment: baseline, weeks 1, 2, 4, 6, 8 and 10 Outcomes:
|
|
| Notes |
Date of study: 1999 to 2001 Funding source: sponsored by Forest Laboratories Declarations of interest among the primary researchers: one of the authors has received research support from many drug companies; other authors are employees of Forest Laboratories |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized". No further information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double blind". No further information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double blind". No further information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "The ITT set consisted of 351 patients, 125 treated with escitalopram, 112 with citalopram and 114 with placebo". Dropout rates were different between treatment groups (escitalopram = 24.2%, citalopram = 31.9%). |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | High risk | Sponsored by Forest Laboratories; the role of the funder in planning, conducting and writing the study is not discussed. |
Taylor 1990.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: panic disorder with phobic avoidance Method of diagnosis: Structured Clinical Interview for Diagnoses‐Upjohn version (SCID‐UP) Age: alprazolam: mean = 35.0, imipramine: mean = 34.1, placebo: mean = 34.9 Sex: alprazolam: male = 19%, imipramine: 30%, placebo: 31% Location: USA Comorbidities: none Rescue medication: none |
|
| Interventions | Participants were randomly assigned to either: 1. Alprazolam (n = 26) Duration: 8 weeks Treatment protocol: flexible dosage; range = 1 to 8 mg, mean = 3.7 2. Imipramine (n = 27) Duration: 8 weeks Treatment protocol: flexible dosage; range = 1 to 9 mg, mean = 4.9 3. Placebo (n = 26) Duration: 8 weeks Treatment protocol: flexible; number of pills: 2 to 10, mean = 6.8 |
|
| Outcomes |
Time points for assessment: baseline, weeks 1, 4, 8 Primary outcomes:
Secondary outcome:
|
|
| Notes |
Date of study: not stated Funding source: this research was supported in part by National Institute of Mental Health grant 40118 and by a gift from the Upjohn Company Declarations of interest among the primary researchers: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | “Double blind”, “identical capsules”. Additional analysis of the success of blinding showed that despite the blinding procedure, participants and physicians were able to distinguish between alprazolam and placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | “Double blind”; no further information available |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis only, unequal dropout rate (alprazolam: 8%, placebo: 23%). |
| Selective reporting (reporting bias) | High risk | Almost all of the efficacy outcome measures described in the methods are reported in the results, but data are incomplete (standard deviations are not always presented). Furthermore, SAFTEE‐UP event form is not reported. |
| Other bias | Unclear risk | This research was supported in part by National Institute of Mental Health grant 40118 and by a gift from the Upjohn Company. |
Tesar 1991.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III panic disorder with phobic avoidance Method of diagnosis: Structured Clinical Interview for DSM‐III‐Upjohn version (SCID‐UP) Age: alprazolam: mean = 32.8 (SD 8.9); clonazepam: mean = 30.5 (SD 6.5); placebo: mean = 30.7 (SD 9.0) Sex: alprazolam: M = 42%; clonazepam: M = 42%; placebo: M = 27 Location: USA (Clinical Psychopharmacology Unit at Massachusetts General Hospital) Comorbidities: major depression (if secondary to panic disorder) Rescue medication: none |
|
| Interventions | Participants were randomly assigned to either: 1. Alprazolam (n = 24) Duration: 6 weeks Treatment protocol: flexible dosage; range = 1 mg to 10 mg, mean = 5.39 (SD 2.89) 2. Clonazepam (n = 26) Duration: 6 weeks Treatment protocol: flexible dosage; range = 0.5 mg to 5 mg, mean = 2.5 (SD 0.94) 3. Placebo arm (n = 22) Duration: 6 weeks Treatment protocol: flexible |
|
| Outcomes |
Time points for assessment: baseline, week 3 and 6 Primary outcomes:
Secondary outcome:
|
|
| Notes |
Date of study: not stated Funding source: supported in part by a grant from the Upjohn Corporation, Kalamazoo, Michigan Declarations of interest among the primary researchers: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "72 subjects (...) were randomised to a treatment group. The study utilised a double‐blind, placebo controlled trial with random assignment and flexible dosing of study medication". No information on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The study utilised a double‐blind, placebo‐controlled trial (...)". "The study drugs were administered in identical capsules." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind; no further information. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There is an imbalance in dropouts between the groups (drug 1 = 4 out of 24, drug 2 = 2 out of 26, placebo = 14 out of 22). Quote: "Follow‐up chi‐squared analysis indicated a significantly greater proportion of patients dropping out of the placebo group than the active treatment groups. The high dropout rate in the placebo group required a more complex evaluation of treatment outcome". For this reason, both completer and endpoint analyses are provided. |
| Selective reporting (reporting bias) | Low risk | All the measures declared in the methods are reported in the results. |
| Other bias | High risk | Supported in part by a grant from the Upjohn Corporation, Kalamazoo, Michigan. |
Tiller 1999.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III‐R panic disorder Method of diagnosis: Structured Clinical Interview (SCID) Age: mean = 35 Sex: 67% female Location: not stated; setting: unclear Co‐morbidities: not stated Rescue medication: not stated; "there was not extensive co‐prescription of hypnotics, sedatives or beta‐blockers". |
|
| Interventions |
Participants were randomly assigned to either: 1. Moclobemide arm (n = 182) Duration: 8 weeks Treatment protocol: flexible dosage, range = 300 mg to 600 mg, mean = 498 (SD 68) 2. Fluoxetine arm (n = 184) Duration: 8 weeks Treatment protocol: flexible dosage, range = 10 mg to 30 mg, mean = 20.5 (SD 2.7) |
|
| Outcomes |
Time points for assessment: Outcomes:
|
|
| Notes |
Date of study: not stated Funding source: sponsored by Hoffmann‐La Roche Declarations of interest among the primary researchers: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allocated". No further information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double blind". No further information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double blind". No further information provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No information provided about the management of incomplete outcome data; number of total dropouts not reported. |
| Selective reporting (reporting bias) | Unclear risk | All relevant outcomes mentioned in the methods section were reported. |
| Other bias | High risk | Sponsored by Hoffmann‐La Roche; the role of the funder in planning, conducting and writing the study is not discussed. |
Tsutsui 1997.
| Study characteristics | ||
| Methods | Study design: 12 weeks, randomised (cluster‐randomisation), parallel design, placebo‐controlled, double‐blind | |
| Participants |
Diagnosis: DSM‐III‐R panic disorder Method of diagnosis: not specified Age (years): some participants > 65, range unclear Sex: they show the ratio of gender, however it is not for the randomised population, but for the population included in the analysis Location: inpatient, multi‐centre trial all over Japan Co‐morbidities: excluded Rescue medication: lorazepam |
|
| Interventions | Participants were randomly assigned to either: 1. Sertraline low‐dose arm (randomised n = 59) Duration: 12 weeks Treatment protocol: fixed dosage; 75 mg/day 2. Sertraline high‐dose arm (randomised n = 54) Duration: 12 weeks Treatment protocol: fixed dosage; 150 mg/day 3. Placebo arm (randomised n = 56) Duration: 12 weeks Treatment protocol: fixed dosage; number of tablets not specified |
|
| Outcomes |
Time points for assessment: baseline and at 12 weeks Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: unclear Declarations of interest among the primary researchers: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster‐randomisation. The method is not specified. |
| Allocation concealment (selection bias) | Low risk | An independent researcher randomly allocated participants. He passed identical tablets to the clinician. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both the participants and the physician were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts from analysis were over 20%; no imputation for missing data was performed. |
| Selective reporting (reporting bias) | Unclear risk | There is no protocol for this study, so we cannot judge selective reporting. |
| Other bias | Unclear risk | Researcher conflicts of interest are unclear. |
Tsutsui 2000a.
| Study characteristics | ||
| Methods | Study design: 8 weeks, randomised controlled trial (cluster‐randomisation), parallel design, double‐blind | |
| Participants |
Diagnosis: DSM‐IV panic disorder Method of diagnosis: not stated Age (years): inclusion criteria includes 65 years. They show the age range of the population included in their analysis as from 18 to 60, however this is not the age range of the population randomised. Therefore we cannot ascertain if the randomised population included 65‐year old persons = “unclear”. Sex: they show the gender ratio, however it is not for the randomised population, but for the population included in the analysis Location: in‐ and outpatient setting, all over Japan (multi‐centre trial) Co‐morbidities: excluded Rescue medication: lorazepam, zopiclone, brotizolam, lormetazepam, rilmazafone |
|
| Interventions | Participants were randomly assigned to either: 1. Paroxetine arm (randomised n = 87) Duration: 8 weeks Treatment protocol: fixed dosage 30 mg/day 2. Placebo arm (randomised n = 84) Duration: 8 weeks Treatment protocol: fixed dosage |
|
| Outcomes |
Time points for assessment: at baseline, at 8 weeks Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: the study was sponsored by the company marketing the drug Declarations of interest among the primary researchers: conflict of interest among primary researchers |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster‐randomised trial. No further details are provided about the random sequence generation. |
| Allocation concealment (selection bias) | Low risk | An independent researcher randomly allocated participants. He passed identical tablets to the clinician. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both the participants and the personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is unclear whether the researchers were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts from analysis were over 20%; no imputation for missing data was performed. |
| Selective reporting (reporting bias) | Unclear risk | There is no protocol for this study, so we cannot judge selective reporting. |
| Other bias | High risk | The study was sponsored by the company marketing the drug. Conflict of interest among the primary researchers. |
Tsutsui 2000b.
| Study characteristics | ||
| Methods | Study design: 8 weeks, randomised (cluster‐randomisation), parallel design, placebo‐controlled, double‐blind trial | |
| Participants |
Diagnosis: DSM‐IV panic disorder Method of diagnosis: not stated Age (years): range 18 to 72 Sex: distribution of gender in randomised population not reported Location: in‐ and outpatient setting, all over Japan Co‐morbidities: excluded Rescue medication: lorazepam |
|
| Interventions | Participants were randomly assigned to either: 1. Paroxetine low‐dose arm (randomised n = 38) Treatment protocol: fixed dosage 20 mg/day Duration: 8 weeks 2. Paroxetine high‐dose arm (randomised n = 45) Treatment protocol: fixed dosage 30 mg/day Duration: 8 weeks 3. Placebo arm (randomised n = 37) Treatment protocol: fixed dosage Duration: 8 weeks |
|
| Outcomes |
Time points for assessment: baseline and 8 weeks Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: the study was sponsored by the company marketing the drug Declarations of interest among the primary researchers: conflict of interest among the primary researchers |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster‐randomisation. No further details are provided about the random sequence generation. |
| Allocation concealment (selection bias) | Low risk | An independent researcher randomly allocated participants. He passed identical tablets to the clinician. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both participants and physicians were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is unclear whether the assessors were blinded or not. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts from analysis were over 20%. ITT analysis was used, but the method of imputation was not mentioned. |
| Selective reporting (reporting bias) | Unclear risk | There is no protocol for this study, so we cannot judge selective reporting. |
| Other bias | High risk | The study was sponsored by the company marketing the drug. Conflict of interest among the primary researchers. |
Uhlenhuth 1989.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III panic disorder or agoraphobia with panic attacks Method of diagnosis: SCID‐UP Age: mean = 31.54 (SD 7.12) Sex: 58% female Location: USA; setting: outpatients Co‐morbidities: patients with another primary psychiatric disorder or a physical disorder judged likely to interfere with the study were excluded Rescue medication: not stated |
|
| Interventions |
Participants were randomly assigned to either: 1. Alprazolam 2 mg arm (n = 20) Duration: 8 weeks Treatment protocol: fixed dosage 2 mg 2. Alprazolam 6 mg arm (n = 21) Duration: 8 weeks Treatment protocol: fixed dosage 6 mg 3. Imipramine arm (n = 20) Duration: 8 weeks Treatment protocol: fixed dosage 225 mg 4. Placebo arm (n = 20) Duration: 8 weeks |
|
| Outcomes |
Time points for assessment: weeks 1, 2, 3, 4, 6, 8 Outcomes:
|
|
| Notes |
Date of study: not stated Funding source: sponsored by Upjohn Company Declarations of interest among the primary researchers: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "random". No further information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All patients received two identical appearing capsules four times daily". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All patients received two identical appearing capsules four times daily". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "two sets of outcome analysis were employed; one included all 81 patients who entered treatment, and the other included only the 63 patients who completed at least 4 weeks of treatment. Both sets of analysis presented here were based on the final (last available) clinical score for each patient (endpoint analysis). Patterns of dropout by treatment were analysed by survival analysis using the actuarial life table method." |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | High risk | Sponsored by Upjohn Company; the role of the funder in planning, conducting and writing the study is not discussed. |
Valenca 2000.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐IV panic disorder with agoraphobia Method of diagnosis: Structured Clinical Interview for DSM‐IV Age: clonazepam group: mean = 37.5 (SD 6.6); placebo group: mean = 36.8 (SD 7.2) Sex: M = 10; F = 14 Location: University of Rio de Janeiro (at the Laboratory of Panic and Respiration) Comorbidities: none Rescue medication: none |
|
| Interventions | Participants were randomly assigned to either: 1. Clonazepam (n = 14) Duration: 6 weeks Treatment protocol: fixed dosage: 2 mg/day 2. Placebo arm (n = 10) Duration: 6 weeks |
|
| Outcomes |
Time points for assessment: not stated Primary outcomes: 1. Number of panic attacks: participant's diary 2. Global improvement of panic disorder: CGI 3. Anxiety: HAMA 4. Panic‐Associated Symptom Scale (PASS) (panic attacks, anticipatory anxiety, phobias) |
|
| Notes |
Date of study: not stated Funding source: not stated Declarations of interest among the primary researchers: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "All 24 subjects were randomly assigned to either treatment with clonazepam or placebo." No further details. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding is only mentioned in the study title; no further information. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding is only mentioned in the study title; no further information. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Observed case data only; missing 1 person per group. |
| Selective reporting (reporting bias) | High risk | The study protocol is not available. An efficacy outcome (PGI) reported in the results was not prespecified in the methods section. This outcome is reported incompletely (no baseline data). |
| Other bias | Unclear risk | Supported by the Brazilian Council for Scientific and Technological Development (CNPq). |
Van Vliet 1993.
| Study characteristics | ||
| Methods | Study design: 12‐week, double‐blind, placebo‐controlled, individual randomisation, parallel groups | |
| Participants |
Diagnosis: DSM‐III‐R panic disorder with or without agoraphobia Method of diagnosis: SCL‐90 Age (years): 26 to 49, mean = 32 (SD 6.4) Sex: 27 women, 3 men Location: outpatient clinic of the department of Biological Psychiatry of the University Hospital in Utrecht, Netherlands Co‐morbidities: excluded Rescue medication: oxazepam to a maximum of 30 mg daily, if required |
|
| Interventions | Participants were randomly assigned to either: 1. Brofaromine arm (randomised n = 15) Duration: 12 weeks Treatment protocol: flexible dosage; range = 50 to 150 mg/day, mean = not stated (SD not stated) 2. Placebo arm (randomised n = 14) Duration: 12 weeks Treatment protocol: flexible dosage; range = not stated, mean = not stated (SD not stated) |
|
| Outcomes |
Time points for assessment: weekly for 12 weeks (some outcomes were evaluated at the baseline and at the endpoint only) Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: none declared Declarations of interest among the primary researchers: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated to one of the two treatment groups". No further details are provided. The number of participants randomised per arm is unclear. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double blind". No further information provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The only information reported about dropouts is that 1 participant in the placebo group was withdrawn from the study at week 8 because of lack of efficacy. Other reasons for withdrawal are not discussed. Thus it is not clear whether the 2 groups are still comparable or not after the dropout. Data imputation is not clearly discussed, however apparently only completers were analysed (consistent with 'per protocol analysis'). |
| Selective reporting (reporting bias) | High risk | The measures of the primary outcome are not clearly specified and mean scores for the scales are graphically reported in figures and only partially reported in the text. |
| Other bias | Low risk | It is unlikely that sponsorship bias could have influenced the results. Quote: "The authors wish to thank Mrs M de Wol˜Ferdinandusse, director of the Dutch Foundation of Phobic Disorders, and the Laboratory of Biological Psychiatry of the University Hospital Utrecht, head Mr A Klompmakers". |
Van Vliet 1996.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III‐R panic disorder with or without agoraphobia Method of diagnosis: open interview Age: mean = 35 (SD 7.46) Sex: 26 women, 6 men Location: the Netherlands; setting: outpatients Co‐morbidities: patients with another anxiety disorder, major affective disorders or psychotic disorder, alcohol or drug abuse and medical problems were excluded Rescue medication: oxazepam maximum 30 mg daily |
|
| Interventions |
Participants were randomly assigned to either: 1. Brofaromine arm (n = 15) Duration: 12 weeks Treatment protocol: fixed dosage 150 mg 2. Fluvoxamine arm (n = 15) Duration: 12 weeks Treatment protocol: fixed dosage 150 mg |
|
| Outcomes |
Time points for assessment: weekly Outcomes:
|
|
| Notes |
Date of study: not stated Funding source: not stated Declarations of interest among the primary researchers: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly". No further information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double blind". No further information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double blind". No further information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided about management of incomplete outcome data. |
| Selective reporting (reporting bias) | High risk | Continuous outcomes are reported incompletely (number of evaluated patients is not reported), so that they cannot be entered in a meta‐analysis. Fear Questionnaire data for agoraphobia are only reported in graphs. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
Versiani 2002.
| Study characteristics | ||
| Methods | Study design: 8 weeks, multi‐centre, placebo‐controlled, randomised (individual) parallel‐group, double‐blind clinical trial | |
| Participants |
Diagnosis: DSM‐III‐R panic disorder with or without agoraphobia Method of diagnosis: not specified Age (years): mean age in reboxetine arm 36.5 (SD 10.4), mean age in placebo arm 35.1 (SD 10.9) Sex: 50 women, 25 men Location: Brazil and Italy Co‐morbidities: excluded Rescue medication: unclear |
|
| Interventions | Participants were randomly assigned to either: 1. Reboxetine arm (randomised n = 42) Duration: 8 weeks Treatment protocol: flexible dosage; range = 2 to 8 mg/day 2. Placebo arm (randomised n = 40) Duration: 8 weeks Treatment protocol: flexible dosage |
|
| Outcomes |
Time points for assessment: weekly for 8 weeks Outcomes:
|
|
| Notes |
Date of study: not specified Funding source: not specified Declarations of interest among the primary researchers: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "randomised" but no further information is given about the random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts. Quote: "a last observation carried forward analysis was conducted and included all patients who received at least 3 weeks of treatment". |
| Selective reporting (reporting bias) | Unclear risk | The outcomes are reported in the graphs and in the text. For some data they do not specify the SD. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
Wade 1997.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III‐R panic disorder Method of diagnosis: not stated Age: mean = 38 (SD not provided) Sex: 70% female, 30% male Location: not stated; setting unclear Co‐morbidities: patients with depression, organic brain damage, drug/alcohol misuse and other severe psychiatric or somatic disorders were excluded Rescue medication: treatment with oxazepam was permitted during weeks 1 and 2 (maximum dose 20 mg daily), discontinued during weeks 3 and 4, and prohibited during weeks 5 to 8 |
|
| Interventions |
Participants were randomly assigned to either: 1. Citalopram 10 mg to 15 mg arm (n = 97) Duration: 8 weeks Treatment protocol: 10 mg, with the option of increasing to 15 mg if efficacy was not seen 2. Citalopram 20 mg to 30 mg arm (n = 95) Duration: 8 weeks Treatment protocol: 20 mg, with the option of increasing to 30 mg if efficacy was not seen 3. Citalopram 40 mg to 60 mg arm (n = 89) Duration: 8 weeks Treatment protocol: 40 mg, with the option of increasing to 60 mg if efficacy was not seen 4. Clomipramine (n = 98) Duration: 8 weeks Treatment protocol: 60 mg, with the option of increasing to 90 mg if efficacy was not seen 5. Placebo (n = 96) |
|
| Outcomes |
Time points for assessment: baseline, last assessment (no further details provided) Outcomes:
|
|
| Notes |
Date of study: not stated Funding source: not stated Declarations of interest among the primary researchers: none (but authors' affiliations refer to pharmaceutical companies) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised". No further information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double blind". No further information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double blind". No further information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "the primary analysis of efficacy was based upon the relative number of responding patients for the ITT population and by use of the LOCF". |
| Selective reporting (reporting bias) | Unclear risk | All outcomes were reported; data on CAS are reported only in graphs. |
| Other bias | Unclear risk | One of the authors' affiliations refers to Lundbeck. |
Zhang 2000.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM III‐R Method of diagnosis: no available information Age: not stated Sex: not stated Location: China; setting: in‐ and outpatients Co‐morbidities: none Rescue medication: not stated |
|
| Interventions |
Participants were randomly assigned to either: 1. Paroxetine arm (n = 38) Duration: 10 weeks Treatment protocol: week 1: 20 mg, week 2: 30 mg, week 3: 40 mg, week 4 to 10: 40 mg to 50 mg; mean = 43.5 mg (SD 4.8) 2. Clomipramine arm (n = 35) Duration: 10 weeks Treatment protocol: week 1: 50 mg, week 2: 100 mg, week 3: 150 mg, week 4 to 10: 150 mg to 200 mg; mean = 159.7 mg (SD 20.1) |
|
| Outcomes |
Time points for assessment: not stated Outcomes: not stated |
|
| Notes |
Date of study: not stated Funding source: sponsored by the drug company marketing the drug Declarations of interest among the primary researchers: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided. |
| Selective reporting (reporting bias) | Unclear risk | No information provided. |
| Other bias | High risk | Sponsored by the drug company marketing the drug; the role of the funder in planning, conducting and writing the study is not discussed. |
BDI: Beck Depression Inventory CAS: Clinical Anxiety Scale CBT: cognitive behavioural therapy CGI: Clinical Global Impression CGI‐I: Clinical Global Impression Improvement Score CGI‐S: Clinical Global Impression Severity of Illness Score cps: capsules CR: controlled release CT: compressed tablet CRGCS: Clinician Rated Global Change Scale CRIDS: Clinician Rated Impairment and Disability Scale DPAI: Daily Panic Attack Inventory DPI: Daily Panic Inventory DOTES: Dosage Record and Treatment Emergent Symptom Scale DSM III/IV/5: Diagnostic and Statistical Manual of Mental Disorders (third/fourth/fifth revision) ER: extended release F: female FQ: Fear Questionnaire GAD: generalised anxiety disorder HAMA: Hamilton Rating Scale for Anxiety HAMD/HDRS: Hamilton Rating Scale for Depression ICD‐10: International Statistical Classification of Diseases and Related Health Problems, 10th revision ITT: intention‐to‐treat LOCF: last observation carried forward M: male MADRS: Montgomery‐Åsberg Depression Rating Scale mg: milligram MDD: major depressive disorder MHPG: 3‐methoxy‐4‐hydroxyphenylglycol n: number OCD: obsessive compulsive disorder PAAS: Panic and Anticipatory Anxiety Scale PASS: Panic‐Associated Symptoms Scale PDSS: Panic Disorder Severity Scale PGE: Patient Global Evaluation PGI: Patient Global Impression PGI‐P: Patient Rated Global Improvement Scale PRAS: Patient Rated Anxiety Scale PRGCS: Patient Rated Global Change Scale PRIDS: Patient Rating Impairment Disability Scale Q‐LES‐Q: Quality of Life Enjoyment and Satisfaction Questionnaire SAFTEE: Systematic Assessment for Treatment Emergent Events SAFTEE‐UP: Systematic Assessment for Treatment‐Emergent Events (Upjohn) SCBT: self‐administered cognitive behaviour therapy SCID: Structured Clinical Interview for DSM SCID‐UP: Structured Clinical Interview for DSM, update SCL‐90: Anxiety Subscale of Symptom Checklist‐90‐Revised SD: standard deviation SDS: Sheehan Disability Scale ShAS: Sheehan Anxiety Scales SE: standard error SEM: standard error of the mean SR: sustained release STAI: State‐Trait Anxiety Inventory TCAs: tricyclic antidepressants UPI: Utrecht Panic Inventory WSDS: Work and Social Disability Scale XR: extended release
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ananth 1979 | Wrong diagnosis (anxiety neurosis) |
| Bakish 1994 | Wrong study design (single case) |
| Baldini Rossi 2000 | Wrong diagnosis (participants were not primarily diagnosed with panic disorder) |
| Ballenger 1988 | Wrong diagnosis (the main diagnosis is agoraphobia, and less than 30% of participants suffer from panic disorder) |
| Balon 1991 | Wrong study design (panicogenic) |
| Balon 1993 | Wrong study design (panicogenic) |
| Barbosa 1980 | Wrong diagnosis (anxiety disorder) |
| Bernardi 1998 | Wrong comparator (comorbidity of anxiety and depression) |
| Bueno 1988 | Wrong diagnosis (anxiety disorder) |
| Bystritsky 1990 | Wrong study design (not double‐blind) |
| Charney 1986 | Wrong study design (not randomised) |
| Chen 1997 | Wrong comparator (buspirone) |
| Chen 1998 | Wrong comparator (buspirone) |
| Chen 2003 | Wrong diagnosis (anxiety) |
| Chouinard 1983 | Wrong diagnosis (psychoneurotic patients) |
| Chounaird 1982 | Wrong diagnosis (generalised anxiety and panic disorder) |
| Cohn 1984 | Wrong diagnosis (anxiety disorder) |
| Cooper 1990 | Wrong diagnosis (anxiety disorder) |
| Cooper 1991 | Wrong diagnosis (anxiety disorder) |
| Csanalosi 1977 | Wrong diagnosis (anxiety disorder) |
| Cunha 1988 | Wrong diagnosis (anxiety disorder) |
| Dager 1992 | No usable data |
| Dasberg 1974 | Wrong diagnosis (anxiety disorder) |
| Davis 1981 | Wrong study design (not a RCT) |
| De Candia 2009 | Wrong diagnosis (mild to moderate anxiety disorder) |
| de Jonghe 1989 | Wrong diagnosis (participants were diagnosed with anxiety disorders including panic disorder, but the randomisation was not stratified by diagnosis) |
| De Rosa 1980 | Wrong diagnosis (anxiety disorder) |
| Dell'Erba 2006 | Wrong study design (not randomised) |
| den Boer 1987 | Wrong diagnosis (participants were diagnosed with anxiety disorders including panic disorder, but the randomisation was not stratified by diagnosis) |
| Downing 1978 | Wrong diagnosis (anxiety disorder) |
| Downing 1979 | Wrong diagnosis (anxiety disorder) |
| Downing 1983 | Wrong diagnosis (anxiety disorder) |
| Dunner 1986 | Wrong diagnosis (participants were diagnosed with anxiety disorders including panic disorder, but the randomisation was not stratified by diagnosis) |
| Dyukova 1992 | Wrong study design (not randomised) |
| Dyukova 1993 | Wrong diagnosis (autonomic crisis) |
| Evans 1986 | Wrong study comparator (concomitant psychotherapy) |
| Fahy 1992 | Wrong study comparator (concomitant psychotherapy) |
| Fava 1989 | No usable data |
| Filip 1981 | Wrong diagnosis (anxiety disorder) |
| Franulic 1989 | Wrong study design (not randomised) |
| Furukawa 2009 | Wrong study design (review) |
| Greiss 1980 | Wrong diagnosis (anxiety disorder) |
| Grilo 1988 | Wrong intervention (combined therapy with cognitive behaviour therapy) |
| Hare 1974 | Wrong diagnosis (anxiety and depression) |
| Hofmeijer‐Sevink 2017 | Wrong intervention (D‐cycloserine enhancement) |
| Hu 2002 | Wrong comparator (psychotherapy and drug) |
| Huppert 2004 | Wrong comparator (CBT and medication) |
| Kahn 1986 | Wrong diagnosis (depressive and anxiety disorder) |
| Kaplan 2000 | Wrong study design (comparison with healthy people) |
| Keller 1993 | Wrong diagnosis (participants were not primarily diagnosed with panic disorder) |
| Kerry 1983 | Wrong diagnosis (neurotic anxiety) |
| Klein 1988 | Wrong study design (not a RCT) |
| Klerman 1990 | Wrong study design (not a RCT) |
| Knijnik 1990 | Wrong diagnosis (anxiety neurosis) |
| Laakmann 1980 | Wrong diagnosis (anxiety neurosis) |
| Lapierre 1975 | Wrong diagnosis (anxiety neurosis) |
| Lepola 1989 | Wrong study design (not randomised) |
| Lorch 1995 | Wrong intervention (concomitant psychotherapy) |
| Marks 1993 | Wrong intervention (concomitant psychotherapy) |
| Mavissakalian 1982 | Wrong study design (review) |
| Mavissakalian 2003 | Wrong study design (long‐term phase of a discontinuation/maintenance open‐label study) |
| McCurdy 1978 | Wrong diagnosis (anxiety neurosis and depressive symptomatology) |
| McEvilly 1981 | Wrong diagnosis (anxiety disorder) |
| McHugh 2007 | Wrong intervention (concomitant psychotherapy) |
| Mellman 1986 | Wrong study design (withdrawal study) |
| Miretzky 1992 | Wrong intervention (concomitant psychotherapy) |
| Mueller 1986 | Wrong diagnosis (anxiety neurosis) |
| Muncy 1981 | Wrong comparator (imipramine compared with two psychoterapeutic modalities plus no treatment; no placebo or other intervention arm) |
| Nair 1982 | Wrong diagnosis (participants were diagnosed with anxiety disorders including panic disorder, but the randomisation was not stratified by diagnosis) |
| Nanivadekar 1973 | Wrong diagnosis (anxiety neurosis) |
| Nardi 2011 | Wrong study design (not double‐blind) |
| Ogunremi 1973 | Wrong diagnosis (healthy participants) |
| Padron 1974 | Wrong comparator (no placebo group) |
| Pareek 2014 | Wrong comparison (clonazepam‐CR versus clonazepam) |
| Pasini 1972 | Wrong diagnosis (anxiety disorder) |
| Pfizer 2002 | No data available |
| Pfizer 2005 | Wrong study design (not double‐blind) |
| Piedade 1987 | Wrong diagnosis (anxious status) |
| Pohl 1989a | Wrong intervention (concomitant psychotherapy) |
| Pollack 2002 | Wrong study design (review) |
| Pollack 2003 | Wrong intervention (combined therapy with different drugs) |
| Pols 1996 | Wrong study design (induced panic attacks) |
| Porta 1974 | Wrong diagnosis (anxiety disorder) |
| Predescu 1969 | Wrong study design (not a RCT) |
| Pyke 1989 | Wrong study design (panicogenic) |
| Raffaele 2002 | Wrong study design (only one group) |
| Rapaport 2000 | Wrong intervention (concomitant psychological therapy) |
| Rifkin 1991 | Wrong study design (not a RCT) |
| Rizley 1986 | No usable data |
| Roll 2004 | Wrong intervention (concomitant psychotherapy) |
| Roy‐Byrne 2001 | Wrong comparator (paroxetine versus usual care) |
| Rynn 2003 | Wrong population (patient discontinuing benzodiazepine therapy) |
| Saiz‐Ruiz 1992 | No usable data |
| Scieghi 1986 | Wrong diagnosis (neurotic anxiety) |
| Sheehan 1980 | Wrong diagnosis (participants were not diagnosed with panic disorder) |
| Sladka 1979 | Wrong diagnosis (anxiety neurosis) |
| Sonne 1986 | Wrong diagnosis (all anxiety disorders) |
| Surman 1986 | Wrong study design (not randomised) |
| Sveback 1990 | Wrong study design (not randomised) |
| Taylor 1982 | Wrong intervention (concomitant psychological therapy) |
| Telch 1985 | Wrong intervention (concomitant psychotherapy) |
| Terra 1971 | Wrong diagnosis (anxiety disorder) |
| Tesar 1990 | Wrong study design (not a RCT) |
| Tyrer 1984 | Wrong diagnosis (generalised anxiety disorder) |
| Tyrer 1988 | Wrong diagnosis (participants with different diagnoses, and randomisation was not stratified according to diagnosis) |
| van Apeldoorn 2008 | Wrong intervention (concomitant psychotherapy) |
| Van Balkom 1996 | Wrong comparator (concomitant exposure in vivo) |
| Van Boeijen 2007 | Wrong comparator (psychotherapy) |
| Versiani 1983 | Wrong diagnosis (anxiety disorder) |
| Wiesner 1993 | Wrong intervention (benzodiazepine agonist) |
| Woods 1988 | Wrong intervention (benzodiazepine antagonist) |
| Yang 2005 | Wrong study design (not double‐blind) |
| Yang 2006 | Wrong study design (not double‐blind) |
| Yeragani 1992 | Wrong study design (panicogenic) |
| Zajecka 1996 | Wrong diagnosis (participants were not diagnosed with panic disorder) |
| Zmorski 1985 | Wrong diagnosis (anxiety disorder) |
CBT: cognitive behavioural therapy RCT: randomised controlled trial
Differences between protocol and review
While conducting our systematic review of antidepressants, benzodiazepines and azapirones to treat panic disorder, we identified inconsistency between direct and indirect evidence in the network meta‐analyses. We judged it important to explore the sources of this inconsistency. This required a substantial addition to the methods proposed in the original protocol. Therefore, we updated our protocol to outline the methods we planned to use to quantify and explore this inconsistency. For example, we had originally proposed to conduct global tests of inconsistency to guide whether to use more intensive methods (node‐splitting). However, since we were concerned about potential inconsistency, we decided to conduct node‐splitting and did not conduct global tests of inconsistency.
The protocol stated that bias‐adjustment models would be conducted as sensitivity analyses. However, given the poor fit of standard models, results from bias‐adjustment models were reported as the main analyses as they fitted the data much better.
The protocol stated that we would not include studies using DSM‐III criteria. However, since the other Cochrane pairwise meta‐analyses on which this NMA is based have included studies using DSM‐III criteria, we decided to include these studies.
In addition, we had proposed to conduct sensitivity analyses where different doses were treated as separate nodes. However, given the large number of meta‐regression analyses and sensitivity analyses conducted we chose not to do this.
We initially planned to include studies with no useable data, but since these studies could not be entered in the analysis we decided to exclude them.
Contributions of authors
GG, CB, MK, TAF and AC conceived the review. GG, DC and AC wrote the draft of the protocol. NM, SJCD and DC contributed to the formal analysis of the review. All authors critically commented on the protocol. HI, AT, AP, IB, LR, SDn and AC selected the studies, appraised their quality and extracted data. GG and NM wrote the draft of the review. All authors contributed to reviewing and editing the draft.
Sources of support
Internal sources
-
Western University, Canada
Salary and protected research time for GG
-
University of Verona, Italy
Salary for CB, IB
-
University of Bristol, UK
Salary for DC
-
University of Toronto, Canada
Salary and protected time for SJD
-
Kyoto University, Japan
Salary for TAF, HI, AT
-
University of Oxford, UK
Salary for AC
-
National Institute for Health and Care Research (NIHR), UK
Support to AC (NIHR Research Professorship ‐ grant RP‐2017‐08‐ST2‐006, NIHR Oxford Cognitive Health Clinical Research Facility, NIHR Oxford and Thames Valley Applied Research Collaboration and NIHR Oxford Health Biomedical Research Centre ‐ grant BRC‐1215‐20005)
External sources
-
None, Other
No external source of support
Declarations of interest
GG: is a Cochrane Editor. He was not involved in the editorial process for the manuscript. He is a diplomate of the Academy of Cognitive Therapy.
NM: is a Cochrane Editor. He was not involved in the editorial process for the manuscript.
CB: is a Cochrane Editor. He was not involved in the editorial process for the manuscript.
SJCD: is a Cochrane Editor. He was not involved in the editorial process for the manuscript. He is a member of the European College of Neuropsychopharmacology and co‐chair of their Anxiety Disorders Research Network. He has published opinions in medical journals relevant to the interventions in this review. He is a member of the Anxiety Disorders Research Network of the European College of Neuropsychopharmacology (ECNP) and of the British Association of Psychopharmacology (BAP).
TAF: has received lecture fees from Eli Lilly, Meiji, Mochida, MSD, Otsuka, Pfizer, Shionogi and Mitsubishi‐Tanabe, and consultancy fees from Sekisui Chemicals and Takeda Science Foundation. He has received royalties from Igaku‐Shoin, Seiwa‐Shoten and Nihon Bunka Kagaku‐sha publishers. He has received grant or research support from the Japanese Ministry of Education, Science, and Technology, the Japanese Ministry of Health, Labor and Welfare, the Japan Foundation for Neuroscience and Mental Health, Mitsubishi‐Tanabe and Mochida. He is a diplomate of the Academy of Cognitive Therapy. TAF has a patent 2018‐177688 pending.
HI: received an honorarium for a lecture from Otsuka.
SD: no conflicts of interest
DC: no conflicts of interest.
MK: no conflicts of interest.
AT: received lecture fees from Sumitomo Dainippon Pharma, Eisai, Janssen Pharmaceutical, Meiji‐Seika Pharma, Mitsubishi Tanabe Pharma, Otsuka and Takeda Pharmaceutical.
IB: is Deputy Co‐ordinating Editor of Cochrane Schizophrenia. She was not involved in the editorial process for the current review.
AP: no conflicts of interest.
AC: is supported by the National Institute for Health Research (NIHR) Oxford Cognitive Health Clinical Research Facility, by an NIHR Research Professorship (grant RP‐2017‐08‐ST2‐006), by the NIHR Oxford and Thames Valley Applied Research Collaboration and by the NIHR Oxford Health Biomedical Research Centre (grant BRC‐1215‐20005). The views expressed are those of the authors and not necessarily those of the UK National Health Service, the NIHR or the UK Department of Health. He has received research, educational and travel support from INCiPiT (Italian Network for Paediatric Trials), CARIPLO Foundation, Lundbeck and Angelini Pharma. He is the CI/PI of two trials about seltorexant in depression, sponsored by Janssen.
SDn: is a Cochrane Editor. She was not involved in the editorial process for the manuscript.
LR: no conflicts of interest.
New
References
References to studies included in this review
Amore 1999 {published data only}
- Amore M, Magnani K, Cerisoli M, Casagrande C, Ferrari G. Panic disorder. A long‐term treatment study: fluoxetine vs imipramine. Human Psychopharmacology: Clinical and Experimental 1999;14(6):429-34. [Google Scholar]
Amore 1999 bis {published data only}
- Amore M, Magnani K, Cerisoli M, Ferrari G. Short‐term and long‐term evaluation of selective serotonin reuptake inhibitors in the treatment of panic disorder: fluoxetine vs citalopram. Human Psychopharmacology: Clinical and Experimental 1999;14(6):435‐40. [Google Scholar]
Asnis 2001 {published data only}
- Asnis GM, Hameedi FA, Goddard AW, Potkin SG, Black D, Jameel M, et al. Fluvoxamine in the treatment of panic disorder: a multi-center, double-blind, placebo-controlled study in outpatients. Psychiatry Research 2001;103:1-14. [DOI] [PubMed] [Google Scholar]
Baker 2003 {published data only}
- Baker B, Khaykin Y, Devins G, Dorian P, Shapiro C, Newman D. Correlates of therapeutic response in panic disorder presenting with palpitations: heart rate variability, sleep, and placebo effect. Canadian Journal of Psychiatry/Revue Canadienne de Psychiatrie 2003;48(6):381-7. [DOI] [PubMed] [Google Scholar]
Bakish 1993 {published data only}
- Bakish D, Saxena BM, Bowen R, D'Souza J. Reversible monoamine oxidase‐A inhibitors in panic disorder. Clinical Neuropharmacology 1993;16(Suppl 2):S77-S82. [PubMed] [Google Scholar]
Ballenger 1998 {published data only}
- Ballenger JC, Wheadon DE, Steiner M, Bushnell W, Gergel IP. Double-blind, fixed-dose, placebo-controlled study ofparoxetine in the treatment of panic disorder. American Journal of Psychiatry 1998;155:36-42. [DOI] [PubMed] [Google Scholar]
Bandelow 2004 {published data only}
- Bandelow B, Behnke K, Lenoir S, Hendriks GJ, Alkin T, Goebel C, et al. Sertraline versus paroxetine in the treatment of panic disorder: an acute, double‐blind noninferiority comparison. Journal of Clinical Psychiatry 2004;65(3):405-13. [DOI] [PubMed] [Google Scholar]
Barlow 2000 {published data only}
- Barlow DH, Gorman JM, Shear MK, Woods SW. Cognitive behavioral therapy, imipramine, or their combination for panic disorder: a randomized controlled trial. JAMA 2000;283:2529-36. [DOI] [PubMed] [Google Scholar]
Beauclair 1994 {published data only}
- Beauclair L, Fontaine R, Annable L, Holobow N, Chouinard G. Clonazepam in the treatment of panic disorder: a double‐blind, placebo‐controlled trial investigating the correlation between clonazepam concentrations in plasma and clinical response. Journal of Clinical Psychopharmacology 1994;14(2):111-8. [PubMed] [Google Scholar]
Bergink 2005 {published data only}
- Bergink V, Westenberg HGM. Metabotropic glutamate II receptor agonists in panic disorder: a double-blind clinicaltrial with LY354740. International Clinical Psychopharmacology 2005;20:291-3. [DOI] [PubMed] [Google Scholar]
Black 1993 {published data only}
- Black DW, Wesner R, Bowers W, Gabel J. A comparison off luvoxamine, cognitive therapy and placebo in the treatment of panic disorder. Archives of General Psychiatry 1993;50:44-50. [DOI] [PubMed] [Google Scholar]
Bradweijn 2005 {published data only}
- Bradwejn J, Ahokas A, Stein DJ, Salinas E, Emilien G, Whitaker T. Venlafaxine extended-release capsules in panic disorder: flexible-dose, double-blind, placebo-controlled study. British Journal of Psychiatry 2005;187:352-9. [DOI] [PubMed] [Google Scholar]
Broocks 1998 {published data only}
- Bandelow B, Broocks A, Pekrun G, George A, Meyer T, Pralle L, et al. The use of the panic and agoraphobia scale (P & A) in a controlled clinical trial. Pharmacopsychiatry 2000;33:174-81. [DOI] [PubMed] [Google Scholar]
- Broocks A, Bandelow B, Pekrun G, George A, Meyer T, Bartmann U, et al. Comparison of aerobic exercise, clomipramine and placebo in the treatment of panic disorder. American Journal of Psychiatry 1998;155:603-9. [DOI] [PubMed] [Google Scholar]
- Broocks A, Stevinson C. Exercise may help treat panic disorder. Focus on Alternative and Complementary Therapies 1999;4:84-5. [Google Scholar]
Bystritsky 1994 {published data only}
- Bystritsky A, Rosen RM, Murphy KJ, Bohn P, Keys SA, Vapnik T. Double‐blind pilot trial of desipramine versus fluoxetine in panic patients. Anxiety 1994;1(6):287-90. [DOI] [PubMed] [Google Scholar]
Caillard 1999 {published data only}
- Caillard V, Rouillon F, Viel JF, Markabi S, French University Antidepressants Group. Comparative effects of low and high doses of clomipramine and placebo in panic disorder: a double-blind controlled study. Acta Psychiatrica Scandinavica 1999;99:51-8. [DOI] [PubMed] [Google Scholar]
Carter 1995 {published data only}
- Carter CS, Fawcett J, Hertzman M, Papp LA, Jones W, Patterson WM, et al. Adinazolam‐SR in panic disorder with agoraphobia: relationship of daily dose to efficacy. Journal of Clinical Psychiatry 1995;56(5):202-10. [PubMed] [Google Scholar]
CNCPS 1992 {published data only}
- Cross National Collaborative Panic Study Second Phase Investigators. Drug treatment of panic disorder: comparative efficacy of alprazolam, imipramine, and placebo. British Journal of Psychiatry 1992;160:191-202. [DOI] [PubMed] [Google Scholar]
Davidson 1994 {published data only}
- Davidson JR, Beitman B, Greist JH, Maddock RJ, Lewis CP, Sheridan AQ, et al. Adinazolam sustained‐release treatment of panic disorder: a double‐blind study. Journal of Clinical Psychopharmacology 1994;14(4):255-63. [PubMed] [Google Scholar]
Den Boer 1988 {published data only}
- Den Boer JA, Westenberg HGM. Effect of a serotonin and noradrenaline uptake inhibitor in panic disorder; a double‐blind comparative study with fluvoxamine and maprotiline. International Clinical Psychopharmacology 1988;3(1):59-74. [DOI] [PubMed] [Google Scholar]
Den Boer 1990 {published data only}
- Den Boer JA, Westenberg HGM. Serotonin function in panic disorder: a double blind placebo controlled study with fluvoxamine and ritanserin. Psychopharmacology 1990;102:85-94. [DOI] [PubMed] [Google Scholar]
Gentil 1993 {published data only}
- Gentil V, Lotufo‐Neto F, Andrade L, Cordás T, Bernik M, Ramos R, et al. Clomipramine, a better reference drug for panic/agoraphobia. Effectiveness comparison with imipramine. Journal of Psychopharmacology 1993;7(4):316-24. [DOI] [PubMed] [Google Scholar]
GSK 1994/04 {published data only}
- GlaxoSmithKline. A double‐blind, multicentered, flexible‐dose study of paroxetine, alprazolam and placebo in the treatment of panic disorder.. www.gsk‐clinicalstudyregister.com/study/29060/223 2008.
GSK 29060 525 {published data only}
- GSK. A double blind, multicenter randomized drug‐controlled study to assess the efficacy and tolerance of paroxetine compared with clomipramine in treatment of panic disorder. https://www.gsk-studyregister.com/en/trial-details/?id=29060/525.
Hoehn‐Saric 1993 {published data only}
- Hoehn-Saric R, McLeod DR, Hipsley PA. Effect of fluvoxamine on panic disorder. Journal of Clinical Psychopharmacology 1993;13:321-6. [PubMed] [Google Scholar]
Holland 1999 {published data only}
- Holland R, Musch B, Hindmarch I. Specific effects of benzodiazepines and tricyclic antidepressants in panic disorder: comparisons of clomipramine with alprazolam SR and adinazolam SR. Human Psychopharmacology Clinical Experimental 1999;14:119-24. [Google Scholar]
Johnston 1995 {published data only}
- Johnston DG, Troyer IE, Whitsett SF, Dalby T. Clomipramine treatment and behaviour therapy with agoraphobic women. Canadian Journal of Psychiatry 1995;40(4):192‐9. [PubMed] [Google Scholar]
Klosko 1990 {published data only}
- Klosko JS, Barlow DH, Tassinari R, Cerny JA. A comparison of alprazolam and behavior therapy in treatment of panic disorder. Journal of Consulting and Clinical Psychology 1990;58(1):77-84. [DOI] [PubMed] [Google Scholar]
Koszycki 2011 {published data only}
- Koszycki D, Taljaard M, Segal Z, Bradwejn J. A randomized trial of sertraline, self-administered cognitive behavior therapy, and their combination for panic disorder. Psychological Medicine 2011;41:373-83. [DOI] [PubMed] [Google Scholar]
Krueger 1999 {published data only}
- Krueger MB, Dahl AA. The efficacy and safety of moclobemide compared to clomipramine in the treatment of panic disorder. European Archives of Psychiatry and Clinical Neuroscience 1999;249(Suppl 1):S19‐S24. [DOI] [PubMed] [Google Scholar]
Lecrubier 1997 {published data only}
- Bakker A, Dyck R, Spinhoven P, Balkom AJ. Paroxetine, clomipramine, and cognitive therapy in the treatment of panic disorder. Journal of Clinical Psychiatry 1999;60:831-8. [DOI] [PubMed] [Google Scholar]
- Lecrubier Y, Bakker A, Dunbar G, Judge R and the Collaborative Panic Study Investigators. A comparison of paroxetine, clomipramine and placebo in the treatment of panic disorder. Acta Psychiatrica Scandinavica 1997;95:145-52. [DOI] [PubMed] [Google Scholar]
- Lecrubier Y, Judge R and the Collaborative Paroxetine Panic Study Investigators. Long‐term evaluation of paroxetine, clomipramine and placebo in panic disorder. Acta Psychiatrica Scandinavica 1997;95:153-60. [DOI] [PubMed] [Google Scholar]
Lepola 1990 {published data only}
- Lepola U, Heikkinen H, Rimon R, Riekkinen P. Clinical evaluation of alprazolam in patients with panic disorder; a double‐blind comparison with imipramine. Human Psychopharmacology 1990;5:159‐63. [Google Scholar]
Liebowitz 2009 {published data only}
- Liebowitz MR, Asnis G, Mangano R, Tzanis E. A double-blind, placebo-controlled, parallel-group, flexible-dose study of venlafaxine extended release capsules in adult outpatients with panic disorder. Journal of Clinical Psychiatry 2009;70:550-61. [DOI] [PubMed] [Google Scholar]
Londborg 1998 {published data only}
- Londborg PD, Wolkow R, Smith WT, DuBoff E, England D, Ferguson J, et al. Sertraline in the treatment of panic disorder. A multi‐site, double‐blind, placebo‐controlled, fixed‐dose investigation. British Journal of Psychiatry 1998;173:54‐60. [DOI] [PubMed] [Google Scholar]
Lydiard 1992 {published data only}
- Lydiard RB, Lesser IM, Ballenger JC, Rubin RT, Laraia M, DuPont R. A fixed‐dose study of alprazolam 2 mg, alprazolam 6 mg, and placebo in panic disorder. Journal of Clinical Psychopharmacology 1992;12(2):96-103. [PubMed] [Google Scholar]
Lydiard 1993 {published data only}
- Lydiard RB, Morton WA, Emmanuel NP, Zealberg JJ, Laraia MT, Stuart GW, et al. Preliminary report: placebo‐controlled, double‐blind study of the clinical and metabolic effects of desipramine in panic disorder. Psychopharmacology Bulletin 1993;29(2):183-8. [PubMed] [Google Scholar]
Michelson 2001 {published data only}
- Michelson D, Allgulander C, Dantendorfer K, Knezevic A, Maierhofer D, Micev V, et al. Efficacy of usual antidepressant dosing regimens of fluoxetine in panic disorder: randomised, placebo‐controlled trial. British Journal of Psychiatry 2001;179:514-8. [DOI] [PubMed] [Google Scholar]
Moroz 1999 {published data only}
- Moroz G, Rosenbaum JF. Efficacy, safety, and gradual discontinuation of clonazepam in panic disorder: a placebo‐controlled, multicenter study using optimized dosages. Journal of Clinical Psychiatry 1999;60(9):604-12. [DOI] [PubMed] [Google Scholar]
Munjack 1989 {published data only}
- Munjack DJ, Brown RA, McDowell D, Palmer R. Actual medication versus therapist guesses: in a blind study, how blind is blind? Journal of Clinical Psychopharmacology 1989;9(2):148-9. [DOI] [PubMed] [Google Scholar]
Nair 1996 {published data only}
- Nair NP, Bakish D, Saxena B, Amin M, Schwartz G, West TE. Comparison of fluvoxamine, imipramine, and placebo in the treatment of outpatients with panic disorder. Anxiety 1996;2(4):192-8. [DOI] [PubMed] [Google Scholar]
Noyes 1996 {published data only}
- Noyes R, Burrows GD, Reich JH, Judd FK, Garvey MJ, Norman TR, et al. Diazepam versus alprazolam for the treatment of panic disorder. Journal of Clinical Psychiatry 1996;57(8):349-55. [PubMed] [Google Scholar]
Pecknold 1994 {published data only}
- Pecknold J, Luthe L, Munjack D, Alexander P. A double‐blind, placebo‐controlled, multicenter study with alprazolam and extended‐release alprazolam in the treatment of panic disorder. Journal of Clinical Psychopharmacology 1994;14(5):314-21. [PubMed] [Google Scholar]
Pfizer 2008 {published data only}
- Pfizer. A randomized, double‐blind, multicenter study of sertraline compared with paroxetine In the treatment of panic disorder. http://clinicaltrials.gov/show/NCT00677352 (first received 14 May 2008).
Pohl 1989b {published data only}
- Pohl R, Balon R, Yeragani VK, Gershon S. Serotoninergic anxiolytics in the treatment of panic disorder: a controlled study with buspirone. Psychopathology 1989;22(Suppl 1):60‐7. [DOI] [PubMed] [Google Scholar]
Pollack 1998 {published data only}
- Pollack MH, Otto MW, Worthington JJ, Gus Manfro G, Wolkow R. Sertraline in the treatment of panic disorder: a flexible‐dose multicenter trial. Archives of General Psychiatry 1998;55:1010-6. [DOI] [PubMed] [Google Scholar]
Pollack 2007a {published data only}
- Pollack MH, Mangano R, Entsuah R, Tzanis E, Simon NM. A randomized controlled trial of venlafaxine ER and paroxetine in the treatment of outpatients with panic disorder. Psychopharmacology 2007;194:233-42. [DOI] [PubMed] [Google Scholar]
Pollack 2007b {published data only}
- Pollack MH, Lepola U, Koponen H, Simon NM, Worthington JJ, Emilien G, et al. A double-blind study of the efficacy of venlafaxine extended-release, paroxetine and placebo in the treatment of panic disorder. Depression and Anxiety 2007;24:1-14. [DOI] [PubMed] [Google Scholar]
Ribeiro 2001 {published data only}
- Ribeiro L, Busnello JV, Kauer‐Sant'Anna M, Madruga M, Quevedo J, Busnello EAD, et al. Mirtazapine versus fluoxetine in the treatment of panic disorder. Brazilian Journal of Medical and Biological Research 2001;34:1303‐7. [DOI] [PubMed] [Google Scholar]
Robinson 1989 {published data only}
- Robinson D, Shrotriya RC, Alms DR, Messina M, Andary J. Treatment of panic disorder: nonbenzodiazepine anxiolytics, including buspirone. Psychopharmacology Bulletin 1989;25(1):21‐6. [PubMed] [Google Scholar]
Rosenbaum 1997 {published data only}
- Rosenbaum JF, Moroz G, Bowden CL. Clonazepam in the treatment of panic disorder with or without agoraphobia: a dose‐response study of efficacy, safety, and discontinuance. Clonazepam Panic Disorder Dose‐Response Study Group. Journal of Clinical Psychopharmacology 1997;17(5):390-400. [DOI] [PubMed] [Google Scholar]
Savoldi 1990 {published data only}
- Savoldi F, Somenzini G, Ecari U. Etizolam versus placebo in the treatment of panic disorder with agoraphobia: a double‐blind study. Current Medical Research and Opinion 1990;12(3):185-90. [DOI] [PubMed] [Google Scholar]
Schweizer 1992 {published data only}
- Schweizer E, Clary C, Dever AI, Mandos LA. The use of low‐dose intranasal midazolam to treat panic disorder: a pilot study.. Journal of Clinical Psychiatry 1992;53(1):19-22. [PubMed] [Google Scholar]
Schweizer 1993 {published data only}
- Schweizer E, Rickels K, Weiss S, Zavodnick S. Maintenance drug treatment of panic disorder. I. Results of a prospective, placebo‐controlled comparison of alprazolam and imipramine. Archives of General Psychiatry 1993;50:51-60. [DOI] [PubMed] [Google Scholar]
Sharp 1990 {published data only}
- Sharp DM, Power KG, Simpson RJ, Swanson V, Moodie E, Anstee JA, et al. Fluvoxamine, placebo and cognitive behaviour therapy used alone and in combination in the treatment of panic disorder and agoraphobia. Journal of Anxiety Disorders 1996;10(4):219-42. [Google Scholar]
Sheehan 1993 {published data only}
- Sheehan DV, Raj AB, Harnett Sheehan K, Soto S, Knapp E. The relative efficacy of high‐dose buspirone and alprazolam in the treatment of panic disorder: a double‐blind placebo‐controlled study. Acta Psychiatrica Scandinavica 1993;88(1):1-11. [DOI] [PubMed] [Google Scholar]
Sheehan 2005 {published data only}
- Sheehan DV, Burnham DB, Iyengar MK, Perera P. Efficacy and tolerability of controlled-release paroxetine in the treatment of panic disorder. Journal of Clinical Psychiatry 2005;66:34-40. [PubMed] [Google Scholar]
Sheikh 1999 {published data only}
- Sheikh JI, Swales PJ. Treatment of panic disorder in older adults: a pilot study comparison of alprazolam, imipramine, and placebo. International Journal of Psychiatry in Medicine 1999;29(1):107‐17. [DOI] [PubMed] [Google Scholar]
Stahl 2003 {published data only}
- Stahl S, Gergel I, Li D. Escitalopram in the treatment of panic disorder: a randomized, double‐blind, placebo‐controlled trial. Journal of Clinical Psychiatry 2003;64:1322-7. [DOI] [PubMed] [Google Scholar]
Taylor 1990 {published data only}
- Taylor CB, Hayward C, King R, Ehlers A, Margraf J, Maddock R, et al. Cardiovascular and symptomatic reduction effects of alprazolam and imipramine in patients with panic disorder: results of a double‐blind, placebo‐controlled trial. Journal of Clinical Psychopharmacology 1990;10(2):112‐8. [DOI] [PubMed] [Google Scholar]
Tesar 1991 {published data only}
- Tesar GE, Rosenbaum JF, Pollack MH, Otto MW, Sachs GS, Herman JB, et al. Double‐blind, placebo‐controlled comparison of clonazepam and alprazolam for panic disorder. Journal of Clinical Psychiatry 1991;52(2):69‐76. [PubMed] [Google Scholar]
Tiller 1999 {published data only}
- Tiller JWG, Bouwer C, Behnke K. Moclobemide and fluoxetine for panic disorder. European Archives of Psychiatry and Clinical Neuroscience 1999;249(Suppl 1):S7‐S10. [DOI] [PubMed] [Google Scholar]
Tsutsui 1997 {published data only}
- Tsutsui S. Clinical evaluation of paroxetine Hcl, a selective serotonin reuptake inhibitor, in the treatment of panic disorder: late phase II double-blind, parallel group study. Japanese Pharmacology and Therapeutics 2000;28:S271-94. [Google Scholar]
Tsutsui 2000a {published data only}
- Tsutsui S. Clinical evaluation of paroxetine Hcl, a selective serotonin reuptake inhibitor, in the treatment of panic disorder: late phase II double-blind, parallel group study. Japanese Pharmacology and Therapeutics 2000;28:S271-94. [Google Scholar]
Tsutsui 2000b {published data only}
- Tsutsui S. Clinical evaluation of paroxetine Hcl, a selective serotonin reuptake inhibitor, in the treatment of panic disorder: phase III double-blind, parallel group study. Japanese Pharmacology and Therapeutics 2000;28:S295-314. [Google Scholar]
Uhlenhuth 1989 {published data only}
- Uhlenhuth EH, Matuzas W, Glass RM, Easton C. Response of panic disorder to fixed doses of alprazolam or imipramine. Journal of Affective Disorders 1989;17(3):261‐70. [DOI] [PubMed] [Google Scholar]
Valenca 2000 {published data only}
- Valenca AM, Nardi AE, Nascimento I, Mezzasalma MA, Lopes FL, Zin W. Double‐blind clonazepam vs placebo in panic disorder treatment. Arquivos de Neuro‐Psiquiatria 2000;58(4):1025-9. [DOI] [PubMed] [Google Scholar]
Van Vliet 1993 {published data only}
- Van Vliet IM, Westenberg HGM, Den Boer JA. MAO inhibitors in panic disorder: clinical effects of treatment with brofaromine. Psychopharmacology 1993;112:483-9. [DOI] [PubMed] [Google Scholar]
Van Vliet 1996 {published data only}
- Vliet IM, den Boer JA, Westenberg HGM, Slaap BR. A double‐blind comparative study of brofaromine and fluvoxamine in outpatients with panic disorder. Journal of Clinical Psychopharmacology 1996;16(4):299-306. [DOI] [PubMed] [Google Scholar]
Versiani 2002 {published data only}
- Versiani M, Cassano G, Perugi G, Benedetti A, Mastalli L, Nardi A, et al. Reboxetine, a selective norepinephrine reuptake inhibitor, is an effective and well-tolerated treatment for panic disorder. Journal of Clinical Psychiatry 2002;63:31-7. [DOI] [PubMed] [Google Scholar]
Wade 1997 {published data only}
- Wade AG, Lepola U, Koponen HJ, Pedersen V, Pedersen T. The effect of citalopram in panic disorder. British Journal of Psychiatry 1997;170:549-53. [DOI] [PubMed] [Google Scholar]
Zhang 2000 {published data only}
- Zhang HY, Zhao QP, Ma C. Paroxetine versus clomipramine for the treatment of panic disorders: a double‐blind randomised study. Chinese Mental Health Journal 2000;14(6):410-3. [Google Scholar]
References to studies excluded from this review
Ananth 1979 {published data only}
- Ananth J, Van den Steen N. Clobazam in the treatment of anxiety neurosis: a double‐blind study. Current Therapeutic Research, Clinical and Experimental 1979;26(1):119-26. [Google Scholar]
Bakish 1994 {published data only}
- Bakish D. The use of the reversible monoamine oxidase‐A inhibitor brofaromine in social phobia complicated by panic disorder with or without agoraphobia. Journal of Clinical Psychopharmacology 1994;14(1):74-5. [DOI] [PubMed] [Google Scholar]
Baldini Rossi 2000 {published data only}
- Baldini Rossi NA, Cassano PA, Dell'Osso LA, Ciapparelli AA, Bandettini di Poggio AA, Russo AA, et al. Depression comorbid with panic disorder or other anxiety disorders: a 16‐week multicentre randomised parallel‐group trial of moclobemide versus paroxetine. European Neuropsychopharmacology 2000;10(Suppl 2):S52-3. [Google Scholar]
Ballenger 1988 {published data only}
- Ballenger JC, Burrows GD, DuPont RL, Lesser IM, Noyes R, Pecknold JC. Alprazolam in panic disorder and agoraphobia: results from a multicenter trial. I. Efficacy in short‐term treatment. Archives of General Psychiatry 1988;45(5):413-22. [DOI] [PubMed] [Google Scholar]
Balon 1991 {published data only}
- Balon R, Pohl R, Yeragani VK, Ramesh C, Glitz DA. The changes of thyroid hormone during pharmacological treatment of panic disorder patients. Progress in Neuropsychopharmacology and Biological Psychiatry 1991;15(5):595-600. [DOI] [PubMed] [Google Scholar]
Balon 1993 {published data only}
- Balon R, Yeragani VK, Pohl R, Merlos B, Sherwood P. Changes in appetite and weight during the pharmacological treatment of patients with panic disorder. Canadian Journal of Psychiatry 1993;38(1):19-22. [DOI] [PubMed] [Google Scholar]
Barbosa 1980 {published data only}
- Barbosa MFS. Treatment of neurotic anxiety with clobazam: double‐blind clinical trial against placebo. Clinica Terapeutica 1980;9(4):285-8. [Google Scholar]
Bernardi 1998 {published data only}
- Bernardi F, Cairoli S, D'Aurizio C, De Rosa A, Grasso A, Sannino V, et al. Double‐blind comparative study of alprazolam (Xanax) and amitriptyline in the treatment of anxiety associated with depression [Studio in doppio cieco di confronto fra alprazolam (Xanax) e amitriptilina nel trattamento dell'ansia associata a depressione]. Minerva Psichiatrica 1988;29(4):203-10. [PubMed] [Google Scholar]
Bueno 1988 {published data only}
- Bueno JR, Laks J. Anti anxiety activity of buspirone: comparative trial with placebo and diazepam [Atividade ansiolitica da buspirona: estudo comparativo com placebo e diazepam]. Jornal Brasileiro de Psiquiatria 1988;37(2):97-9. [Google Scholar]
Bystritsky 1990 {published data only}
- Bystritsky AA, Pasnau RO. Initial reaction and subsequent response to antidepressants in panic patients. American Journal of Psychiatry 1990;147(11):1575. [DOI] [PubMed] [Google Scholar]
Charney 1986 {published data only}
- Charney DS, Woods SW, Goodman WK, Rifkin B, Kinch M, Aiken B, et al. Drug treatment of panic disorder: the comparative efficacy of imipramine, alprazolam, and trazodone. Journal of Clinical Psychiatry 1986;47(12):580-6. [PubMed] [Google Scholar]
Chen 1997 {published data only}
- Chen ZM, Hu XZ, Zhang YL, Zhang JH. Buspirone vs diazepam treatment of anxiety disorders in a double blind study. Zhongyuan Journal of Psychologic Medicine 1997;3(3):146-7. [Google Scholar]
Chen 1998 {published data only}
- Chen ZM, Hu XZ, Zhang YL, Zhang JH. Buspirone vs diazepam in treating anxiety disorders in a double‐blind study [Chinese Journal of New Drugs and Clinical Remedies]. 1998 17;2:99-100. [Google Scholar]
Chen 2003 {published data only}
- Chen Z, Guo B, Zhang J. Mianserin vs. alprazolam in treating anxiety disorder. Chinese Journal of New Drugs and Clinical Remedies 2003;22(7):405-7. [Google Scholar]
Chouinard 1983 {published data only}
- Chouinard G, Annable L, Fontaine R, Solyom L. Alprazolam in the treatment of generalized anxiety and panic disorders: a double‐blind placebo‐controlled study. Psychopharmacology Bulletin 1983;18(1):115-6. [DOI] [PubMed] [Google Scholar]
Chounaird 1982 {published data only}
- Chouinard G, Annable L, Fontaine R, Solyom L. Alprazolam in the treatment of generalized anxiety and panic disorders: a double‐blind placebo‐controlled study. Psychopharmacology 1982;77(3):229-33. [DOI] [PubMed] [Google Scholar]
Cohn 1984 {published data only}
- Cohn JB, Wilcox CS. Long‐term comparison of alprazolam, lorazepam and placebo in patients with an anxiety disorder. Pharmacotherapy 1984;4(2):93-8. [DOI] [PubMed] [Google Scholar]
Cooper 1990 {published data only}
- Cooper SJ, Kelly CB, McGilloway S, Gilliland A. Beta 2‐adrenoceptor antagonism in anxiety. European Neuropsychopharmacology 1990;1(1):75-7. [DOI] [PubMed] [Google Scholar]
Cooper 1991 {published data only}
- Cooper SJ, Gilliland A, Kelly C, McGilloway S. A comparison of beta‐2‐adrenoceptor antagonist (ICI 118,551), diazepam and placebo in the treatment of acute anxiety. Journal of Psychopharmacology 1991;5(2):155-9. [DOI] [PubMed] [Google Scholar]
Csanalosi 1977 {published data only}
- Csanalosi I, Pereira Ogan J, Case G, Werblowsky J, Rickels K. Triflubazam (ORF 8063), a new benzodiazepine in anxiety neurosis. Current Therapeutic Research, Clinical and Experimental 1977;22(1):166-71. [Google Scholar]
Cunha 1988 {published data only}
- Cunha JM, Swicker AP. Anti‐anxiety activity of cannabidiol; double‐blind, comparative trial with diazepam and placebo [Efeito ansiolítico do canabidiol; um estudo comparativo duplo‐cego com diazepam e placebo. R. Cent. Ci.c Bioméd. Univ. Fed. Uberlândia 1988;4(1):27-34. [Google Scholar]
Dager 1992 {published data only}
- Dager SR, Roy‐Byrne PP, Hendrickson H, Cowley DS, Avery DH, Hall KC, et al. Long‐term outcome of panic states during double‐blind treatment and after withdrawal of alprazolam and placebo. Annals of Clinical Psychiatry 1992;4(4):251-8. [Google Scholar]
Dasberg 1974 {published data only}
- Dasberg H. The effect of daily oral dosage of diazepam, plasma concentrations and metabolic clearance of diazepam and demethyldiazepam on various constituents of the acute clinical anxiety syndrome. Psychotherapy and Psychosomatics 1974;24(2):113-8. [DOI] [PubMed] [Google Scholar]
Davis 1981 {published data only}
- Davis JM, Nasr S, Spira N, Vogel C. Anxiety: differential diagnosis and treatment from a biologic perspective. Journal of Clinical Psychiatry 1981;42(11 Pt 2):4-14. [PubMed] [Google Scholar]
De Candia 2009 {published data only}
- De Candia MP, DiSciascio G, Durbano F, Mencacci C, Rubiera M, Aguglia E, et al. Effects of treatment with etizolam 0.5 mg BID on cognitive performance: a 3‐week, multicenter, randomized, double‐blind, placebo‐controlled, two‐treatment, three‐period, noninferiority crossover study in patients with anxiety disorder. Clinical Therapeutics 2009;31(12):2851-9. [DOI] [PubMed] [Google Scholar]
de Jonghe 1989 {published data only}
- Jonghe F, Swinkels J, Tuynman‐Qua H, Jonkers F. A comparative study of suriclone, lorazepam and placebo in anxiety disorder. Pharmacopsychiatry 1989;22(6):266-71. [DOI] [PubMed] [Google Scholar]
Dell'Erba 2006 {published data only}
- Dell'Erba GL, Nuzzo E. Effectiveness treatment for panic and agoraphobia in comparison between drug and specialized psychological treatment [Il trattamento efficace nella pratica del disturbo di panico e agorafobia in una valutazione comparativa tra psicofarmacologia e trattamento psicologico specifico]. Rivista di Psichiatria 2006;41(6):397-403. [Google Scholar]
den Boer 1987 {published data only}
- den Boer JA, Westenberg HG, Kamerbeek WD, Verhoeven WM, Kahn RS. Effect of serotonin uptake inhibitors in anxiety disorders; a double‐blind comparison of clomipramine and fluvoxamine. International Clinical Psychopharmacology 1987;2(1):21-32. [DOI] [PubMed] [Google Scholar]
De Rosa 1980 {published data only}
- De Rosa E, De Rosa G, Coppi R, Zannella F, Pepe C. Randomized double‐blind study of loxapine as compared with diazepam in therapy of patients with anxiety neuroses [Ricerca in doppio cieco randomizzata sulla loxapina in comparazione al diazepam nell'ambito di pazienti affeti da nevrosi d'ansia]. Clinica Terapeutica 1980;95(2):127-46. [PubMed] [Google Scholar]
Downing 1978 {published data only}
- Downing RW, Rickels K. Prediction of response to chlordiazepoxide and placebo in anxious outpatients: an attempt at replication. Pharmakopsychiatr Neuropsychopharmakol 1978;11(5):207-19. [DOI] [PubMed] [Google Scholar]
Downing 1979 {published data only}
- Downing RW, Rickels K, Rickels LA, Downing D. Nonspecific factors and side effect complaints. Factors affecting the incidence of drowsiness in drug and placebo treated anxious and depressed outpatients. Acta Psychiatrica Scandinavica 1979;60(5):438-48. [DOI] [PubMed] [Google Scholar]
Downing 1983 {published data only}
- Downing RW, Rickels K. Physician prognosis in relationship to drug and placebo response in anxious and depressed psychiatric outpatients. Journal of Nervous and Mental Disease 1983;171(3):182-5. [DOI] [PubMed] [Google Scholar]
Dunner 1986 {published data only}
- Dunner DL, Ishiki D, Avery DH, Wilson LG, Hyde TS. Effect of alprazolam and diazepam on anxiety and panic attacks in panic disorder: a controlled study. Journal of Clinical Psychiatry 1986;47(9):458-60. [PubMed] [Google Scholar]
Dyukova 1992 {published data only}
- Dyukova GM, Shepeleva IP, Vorob'eva OV. Treatment of negative crises (panic attacks). Neuroscience and Behavioral Physiology 1992;22(4):343-5. [DOI] [PubMed] [Google Scholar]
Dyukova 1993 {published data only}
- Dyukova GM, Shepeleva IP, Vorov'eva OV. Treatment of autonomic attacks (panic attacks). Journal of Russian and East European Psychiatry 1993;26(1):22-7. [Google Scholar]
Evans 1986 {published data only}
- Evans L, Kenardy J, Schneider P, Hoey H. Effect of a selective serotonin uptake inhibitor in agoraphobia with panic attacks. A double‐blind comparison of zimeldine, imipramine and placebo. Acta Psychiatrica Scandinavica 1986;73(1):49-53. [DOI] [PubMed] [Google Scholar]
Fahy 1992 {published data only}
- Fahy TJ, O'Rourke D, Brophy J, Schazmann W, Sciascia S. The Galway Study of Panic Disorder. I: Clomipramine and lofepramine in DSM III‐R panic disorder: a placebo controlled trial. Journal of Affective Disorders 1992;26(1):63-75. [DOI] [PubMed] [Google Scholar]
Fava 1989 {published data only}
- Fava M, Rosenbaum JF, MacLaughlin RA, Tesar GE, Pollack MH, Cohen LS, et al. Dehydroepiandrosterone‐sulfate/cortisol ratio in panic disorder. Psychiatry Research 1989;28:345-50. [DOI] [PubMed] [Google Scholar]
Filip 1981 {published data only}
- Filip V, Sladka R, Dostalova J, Haskovcova V, Jarosova M, Faltus F, et al. A double‐blind, placebo‐controlled study with tofizopam in anxiety neurosis. Agressologie 1981;22(C):27-30. [PubMed] [Google Scholar]
Franulic 1989 {published data only}
- Franulic AM, Sanchez GV, O'Ryan FG, Gladic DM, Barahona MC, Gloger SK. Clomipramine and diazepam plasma levels in panic disorder and agoraphobia. Preliminary findings [Concentraciones plasmaticas de clomipramina y diazepam en Desorden de Panico y Agoraphobia. Un estudio preliminar]. Revista Chilena de Neuro‐Psiquiatria 1989;27:101-10. [Google Scholar]
Furukawa 2009 {published data only}
- Furukawa TA, Katherine Shear M, Barlow DH, Gorman JM, Woods SW, Money R, et al. Evidence‐based guidelines for interpretation of the Panic Disorder Severity Scale. Depression and Anxiety 2009;26(10):922-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greiss 1980 {published data only}
- Greiss KC, Fogari R. Double‐blind clinical assessment of alprazolam, a new benzodiazepine derivative, in the treatment of moderate to severe anxiety. Journal of Clinical Pharmacology 1980;20(11-12):693-9. [DOI] [PubMed] [Google Scholar]
Grilo 1988 {published data only}
- Grilo CM, Money R, Barlow DH, Goddard AW, Gorman JM, Hofmann SG, et al. Pretreatment patient factors predicting attrition from a multicenter randomized controlled treatment study for panic disorder. Comprehensive Psychiatry 1998;39(6):323-32. [DOI] [PubMed] [Google Scholar]
Hare 1974 {published data only}
- Hare MK. Treatment of anxiety and depression: a comparative trial of amitriptyline (Laroxyl) and diazepam (Valium). Clinical Trials Journal 1974;11(1):39-44. [Google Scholar]
Hofmeijer‐Sevink 2017 {published data only}
- Hofmeijer‐Sevink MK, Duits P, Rijkeboer MM, Hoogendoorn AW, Megen HJ, Vulink NC, et al. No effects of d‐cycloserine enhancement in exposure with response prevention therapy in panic disorder with agoraphobia: a double‐blind, randomized controlled trial. Journal of Clinical Psychopharmacology 2017;37(5):531-9. [DOI] [PubMed] [Google Scholar]
Hu 2002 {published data only}
- Hu H, Meng HQ. A comparative study in psychotherapy and drug in treatment of anxiety disorders. Chinese Journal of Nervous and Mental Diseases 2002;28(2):85-7. [Google Scholar]
Huppert 2004 {published data only}
- Huppert JD, Schultz LT, Foa EB, Barlow DH, Davidson JR, Gorman JM, et al. Differential response to placebo among patients with social phobia, panic disorder, and obsessive‐compulsive disorder. American Journal of Psychiatry 2004;161(8):1485-7. [DOI] [PubMed] [Google Scholar]
Kahn 1986 {published data only}
- Kahn RJ, McNair DM, Lipman RS, Covi L, Rickels K, Downing R, et al. Imipramine and chlordiazepoxide in depressive and anxiety disorders. II. Efficacy in anxious outpatients. Archives of General Psychiatry 1986;43(1):79-85. [DOI] [PubMed] [Google Scholar]
Kaplan 2000 {published data only}
- Kaplan GB, Greenblatt DJ, Ehrenberg BL, Goddard JE, Harmatz JS, Shader RI. Differences in pharmacodynamics but not pharmacokinetics between subjects with panic disorder and healthy subjects after treatment with a single dose of alprazolam. Journal of Clinical Psychopharmacology 2000;20(3):338-46. [DOI] [PubMed] [Google Scholar]
Keller 1993 {published data only}
- Keller MB, Lavori PW, Goldenberg IM, Baker LA, Pollack MH, Sachs GS, et al. Influence of depression on the treatment of panic disorder with imipramine, alprazolam and placebo. Journal of Affective Disorders 1993;28(1):27-38. [DOI] [PubMed] [Google Scholar]
Kerry 1983 {published data only}
- Kerry RJ, McDermott CM. Alprazolam in the treatment of neurotic anxiety. Pharmatherapeutica 1983;3(7):451-5. [PubMed] [Google Scholar]
Klein 1988 {published data only}
- Klein DF. Nottingham study of neurotic disorder. Lancet 1988;2(8618):1915. [DOI] [PubMed] [Google Scholar]
Klerman 1990 {published data only}
- Klerman GL. Depression and panic anxiety: the effect of depressive co‐morbidity on response to drug treatment of patients with panic disorder and agoraphobia. Journal of Psychiatric Research 1990;24(Suppl 2):27-41. [DOI] [PubMed] [Google Scholar]
Knijnik 1990 {published data only}
- Knijnik L, D'Arrigo BE. Comparative study of cloxazolam and placebo in anxiety neurosis [Estudo comparativo sobre o emprego do cloxazolam e placebo em neurose de ansiedade]. Jornal Brasileiro de Psiquiatria 1990;39(4):209-12. [Google Scholar]
Laakmann 1980 {published data only}
- Laakmann G, Blaschke D, Buttermann M, Hippius H, Schewe S, Uberla K. Double blind study with the benzodiazepine derivative Ka‐2547 in outpatients with anxiety neurosis [Doppelblindstudie mit dem Benzodiazepin‐Derivat Ka‐2547 bei ambulanten Patienten mit Angstneurose]. Arzneimittelforschung 1980;30(8):1233-4. [Google Scholar]
Lapierre 1975 {published data only}
- Lapierre YD. Clinical and physiological assessment of chlorazepate, diazepam and placebo in anxious neurotics. International Journal of Clinical Pharmacology and Biopharmacy 1975;11(4):315-22. [PubMed] [Google Scholar]
Lepola 1989 {published data only}
- Lepola U, Jolkkonen J, Rimón R, Riekkinen P. Long‐term effects of alprazolam and imipramine on cerebrospinal fluid monoamine metabolites and neuropeptides in panic disorder. Neuropsychobiology 1989;2(4):182-6. [DOI] [PubMed] [Google Scholar]
Lorch 1995 {published data only}
- Lorch B, Graf‐Morgenstern M, Hain C, Sandmann J, Schlegel S, Hautzinger M, et al. Treatment of panic disorder: pharmacological versus behavioral therapy? Pharmacopsychiatry 1995;28:199. [Google Scholar]
Marks 1993 {published data only}
- Marks IM, Swinson RP, Basoglu M, Kuch K, Noshirvani H, O'Sullivan G, et al. Alprazolam and exposure alone and combined in panic disorder with agoraphobia. A controlled study in London and Toronto. British Journal of Psychiatry 1993;162:776-87. [DOI] [PubMed] [Google Scholar]
Mavissakalian 1982 {published data only}
- Mavissakalian M, Michelson L. Agoraphobia: behavioral and pharmacological treatments, preliminary outcome, and process findings. Psychopharmacology Bulletin 1982;18(4):91-103. [PubMed] [Google Scholar]
Mavissakalian 2003 {published data only}
- Mavissakalian MR. Imipramine vs. sertraline in panic disorder: 24‐week treatment completers. Annals of Clinical Psychiatry 2003;15(3):171-80. [DOI] [PubMed] [Google Scholar]
McCurdy 1978 {published data only}
- McCurdy L, Schatzberg AF. Studies with oral lorazepam in anxiety neurosis associated with depressive symptomatology. Journal of Clinical Psychiatry 1978;39(10 Pt 2):30-4. [PubMed] [Google Scholar]
McEvilly 1981 {published data only}
- McEvilly JP, Etemad B. Double‐blind comparison in parallel groups with nightly single doses of halazepam and placebo[Comparacion dobleciega en grupos paralelos con dosis unicas nocturnas de halazepam y placebo]. Investigación Médica Internacional 1981;8(2):202-8. [Google Scholar]
McHugh 2007 {published data only}
- McHugh RK, Otto MW, Barlow DH, Gorman JM, Shear MK, Woods SW. Cost‐efficacy of individual and combined treatments for panic disorder. Journal of Clinical Psychiatry 2007;68(7):1038-44. [DOI] [PubMed] [Google Scholar]
Mellman 1986 {published data only}
- Mellman TA, Uhde TW. Withdrawal syndrome with gradual tapering of alprazolam. American Journal of Psychiatry 1986;143(11):1464-6. [DOI] [PubMed] [Google Scholar]
Miretzky 1992 {published data only}
- Miretzky A, Horn R, Koehler K, Moeller HJ. Combination of alprazolam, antidepressive drugs and cognitive behavior therapy in the treatment of panic disorder. Clinical Neuropharmacology 1992;15(1 pt B):536. [Google Scholar]
Mueller 1986 {published data only}
- Mueller AA, Binz U, Wendt G, Stoll KD. Treatment outcomes of diazepam and oxprenolol with anxiety neurosis patients [Therapieerfolge von Diazepam und Oxprenolol bei Patienten mit Angstneurose]. In: Angst und Psychopharmaka: Methoden und Ergebnisse pharmakopsychologischer, pharmakopsychiatrischer und verhaltenspharmakologischer Forschung. Kohlkammer, 1986:261-70. [Google Scholar]
Muncy 1981 {published data only}
- Muncy SM. Panic: a comparison of four treatment methods. Dissertation Abstracts International 1991;51(12-B Pt 1):6115. [Google Scholar]
Nair 1982 {published data only}
- Nair NP, Singh AN, Lapierre Y, Saxena BM, Nestoros JN, Schwartz G. Ketazolam in the treatment of anxiety: a standard and placebo controlled study. Current Therapeutic Research, Clinical & Experimental 1982;31(5):679-91. [Google Scholar]
Nanivadekar 1973 {published data only}
- Nanivadekar AS, Wig NN, Khorana AB, Master RS, Kulkarni SS. A multicenter investigation of lorazepam in anxiety neurosis. Current Therapeutic Research, Clinical and Experimental 1973;15(7):500-7. [PubMed] [Google Scholar]
Nardi 2011 {published data only}
- Nardi AE, Valença AM, Freire RC, Mochcovitch MD, Amrein R, Sardinha A, et al. Psychopharmacotherapy of panic disorder: 8‐week randomized trial with clonazepam and paroxetine. Brazilian Journal of Medical and Biological Research 2011;44(4):366-73. [DOI] [PubMed] [Google Scholar]
Ogunremi 1973 {published data only}
- Ogunremi OO, Adamson L, Brezinová V, Hunter WM, Maclean AW, Oswald I, et al. Two anti‐anxiety drugs: a psychoneuroendocrine study. British Medical Journal 1973;2(5860):202-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Padron 1974 {published data only}
- Padron C. Comparative clinical evaluation of lorazepam [Essai clinique comparatif lorazepamdiazepam]. Schweizerische Rundschau fur Medizin Praxis 1974;63(16):494-6. [PubMed] [Google Scholar]
Pareek 2014 {published data only}
- Ipca Laboratories Ltd. Comparative evaluation of efficacy and safety of clonazepam‐CR and conventional clonazepam in patients with panic disorder. https://www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=3027&EncHid=&modid=&compid=%27,%273027det%27.
Pasini 1972 {published data only}
- Pasini E. Double blind study on the use of medazepam in ambulatory therapy] [Etude en doubleaveugle sur l'emploi du medazepam en therapeutique ambulatoire]. Cahiers de Médecine (Europa Medica) 1972;13(6):456-7. [PubMed] [Google Scholar]
Pfizer 2002 {published data only}
- Pfizer. A double‐blind, placebo‐controlled, parallel‐group comparison of venlafaxine extended‐release capsules and paroxetine in outpatients with panic disorder. http://clinicaltrials.gov/show/NCT00044772 (first received 6 September 2002).
Pfizer 2005 {published data only}
- Pfizer. Pilot study of venlafaxine extended release (XR) in the treatment of panic disorder (PD) in comparison to paroxetine. http://clinicaltrials.gov/show/NCT00195598 (first received 19 September 2005).
Piedade 1987 {published data only}
- Piedade RAM, Sougey EB, Almeida FJB, Knijnik L, Barros Camargo I, Del Porto JA, et al. Efficacy of cloxazolam versus placebo in the therapy of anxious status: double‐blind controlled study [Estudo da efic cia do cloxazolam versus placebo na terapia de estados ansiosos: estudo duplocego controlado]. Jornal Brasileiro de Psiquiatri 1987;36(3):189-97. [Google Scholar]
Pohl 1989a {published data only}
- Pohl R, Rickels K, Charney D. Clinical results on the use of lorazepam to treat panic attacks [Risultati clinici sull'uso del lorazepam nel disturbo da attacchi di panico]. Rivista di Psichiatri 1989;24(2):99-100. [Google Scholar]
Pollack 2002 {published data only}
- Pollack MH, Rapaport MH, Fayyad R, Otto MW, Nierenberg AA, Clary CM. Early improvement predicts endpoint remission status in sertraline and placebo treatments of panic disorder. Journal of Psychiatric Research 2002;36(4):229-36. [DOI] [PubMed] [Google Scholar]
Pollack 2003 {published data only}
- Pollack MH, Simon NM, Worthington JJ, Doyle AL, Peters P, Toshkov F, et al. Combined paroxetine and clonazepam treatment strategies compared to paroxetine monotherapy for panic disorder. Journal of Psychopharmacology 2003;17(3):276-82. [DOI] [PubMed] [Google Scholar]
Pols 1996 {published data only}
- Pols H, Verburg K, Hauzer R, Meijer J, Griez E. Alprazolam premedication and 35% carbon dioxide vulnerability in panic patients. Biological Psychiatry 1996;40(9):913-7. [DOI] [PubMed] [Google Scholar]
Porta 1974 {published data only}
- Porta V, Jann G, Delzanno GB. Comparative double‐blind clinical trial of SB 5833 and temazapam [Essai clinique compare, en double aveugle, du SB 5833 et du temazepam]. Bruxelles Medical 1974;54(11):655-8. [PubMed] [Google Scholar]
Predescu 1969 {published data only}
- Predescu V, Ciurezu T, Romila A, Piree S, Ionescu G, Roman I, et al. The "double‐blind" procedure in study of the anxiolytic effects of the preparation Wy 3498 (Oxazepam). Evaluation of anxiety states with the Hamilton scale (HS) [Procedeul "dubluorb" in studiul efectelor anxiolitice ale preparatului Wy 3498 (Oxazepam). Evaluarea starilor de anxietate la scala Hamilton (SH)]. Neurologia, Psihiatria, Neurochirurgia 1969;14(2):153-65. [PubMed] [Google Scholar]
Pyke 1989 {published data only}
- Pyke RE, Greenberg HS. Double‐blind comparison of alprazolam and adinazolam for panic and phobic disorders. Journal of Clinical Psychopharmacology 1989;9(1):15-21. [PubMed] [Google Scholar]
Raffaele 2002 {published data only}
- Raffaele R, Vecchio I, Malaguarnera M, Rampello L, Ruggieri M, Nicoletti F. Therapy of panic attacks in the elderly. Archives of Gerontology and Geriatrics. Supplement 2002;8:295-301. [DOI] [PubMed] [Google Scholar]
Rapaport 2000 {published data only}
- Rapaport MH, Gladsjo J, McKinney R, Auerbach M, Hahn T, Rabin A, et al. Alprazolam‐XR and neuropsychological function in panic disorder. International Journal of Neuropsychopharmacology 2000;3(Suppl 1):272. [Google Scholar]
Rifkin 1991 {published data only}
- Rifkin A. The sequence of improvement of the symptoms encountered in patients with panic disorder. Comprehensive Psychiatry 1991;32(6):559-60. [DOI] [PubMed] [Google Scholar]
Rizley 1986 {published data only}
- Rizley R, Kahn RJ, McNair DM, Frankenthaler LM. A comparison of alprazolam and imipramine in the treatment of agoraphobia and panic disorder. Psychopharmacology Bulletin 1986;22(1):167-72. [PubMed] [Google Scholar]
Roll 2004 {published data only}
- Roll D, Ray SE, Marcus SM, Passarelli V, Money R, Barlow DH, et al. Independent evaluator knowledge of treatment in a multicenter comparative treatment study of panic disorder. Neuropsychopharmacology 2004;29(3):612-8. [DOI] [PubMed] [Google Scholar]
Roy‐Byrne 2001 {published data only}
- Roy‐Byrne PP, Katon W, Cowley DS, Russo J. A randomized effectiveness trial of collaborative care for patients with panic disorder in primary care. Archives of General Psychiatry 2001;58(9):869-76. [DOI] [PubMed] [Google Scholar]
Rynn 2003 {published data only}
- Rynn M, Garcia‐Espana F, Greenblatt DJ, Mandos LA, Schweizer E, Rickels K. Imipramine and buspirone in patients with panic disorder who are discontinuing long‐term benzodiazepine therapy. Journal of Clinical Psychopharmacology 2003;23(5):505-8. [DOI] [PubMed] [Google Scholar]
Saiz‐Ruiz 1992 {published data only}
- Saiz‐Ruiz J, Ibanez A. Personality traits and treatment response in panic disorder. Clinical Neuropharmacology 1992;15(1 Pt B):533. [Google Scholar]
Scieghi 1986 {published data only}
- Scieghi G, Levi‐Minzi A, Greco C. The intravenous infusion use of chlordemethyldiazepam in neurotic anxiety [Psicopatologia e psicofarmacologia dell'ansia nevrotica A proposito dell'impiego del clordemetildiazepam per infusione venosa]. Rivista Sperimentale Freniatria 1986;3:531-42. [Google Scholar]
Sheehan 1980 {published data only}
- Sheehan DV, Ballenger J, Jacobsen G. Treatment of endogenous anxiety with phobic, hysterical, and hypochondriacal symptoms. Archives of General Psychiatry 1980;37(1):51-9. [DOI] [PubMed] [Google Scholar]
Sladka 1979 {published data only}
- Sladka R, Dostalova J, Haskovcova V, Jarosova M, Faltus F, Slanska J, et al. A placebo‐controlled clinical trial with tofizopam in the treatment of anxiety neurosis. Therapia Hungarica 1979;27(4):176-80. [PubMed] [Google Scholar]
Sonne 1986 {published data only}
- Sonne LM, Bruun Hansen J. Alprazolam (Tafil) and bromazepam (Lexotan) in the treatment of anxiety: a randomized, double‐blind comparison in psychiatric outpatients [Alprazolam (Tafil) og bromazepam (Lexotan) i angstbehandlingen En randomiseret, dobbeltblind sammenligning fra psykiatrisk specialpraksis]. Ugeskrift for Laeger 1986;148(23):1392-5. [PubMed] [Google Scholar]
Surman 1986 {published data only}
- Surman OS, Williams J, Sheehan DV, Strom TB, Jones KJ, Coleman J4. Immunological response to stress in agoraphobia and panic attacks. Biological Psychiatry 1986;21(8-9):768-74. [DOI] [PubMed] [Google Scholar]
Sveback 1990 {published data only}
- Svebak S, Cameron A, Levander S. Clonazepam and imipramine in the treatment of panic attacks: a double‐blind comparison of efficacy and side effects. Journal of Clinical Psychiatry 1990;51(Suppl 5):14-7. [PubMed] [Google Scholar]
Taylor 1982 {published data only}
- Taylor CB, Kenigsberg ML, Robinson JM. A controlled comparison of relaxation and diazepam in panic disorder. Journal of Clinical Psychiatry 1982;43(10):423-5. [PubMed] [Google Scholar]
Telch 1985 {published data only}
- Telch MJ, Agras WS, Taylor CB, Roth WT, Gallen CC. Combined pharmacological and behavioral treatment for agoraphobia. Behaviour Research and Therapy 1985;23(3):325-35. [DOI] [PubMed] [Google Scholar]
Terra 1971 {published data only}
- Terra SO, Bueno JR, Pires LL. Evaluation of the anti‐anxiety activity of lorazepam in ambulatory patients [Avaliacao da atividade ansiolitica dolorazepam empacientes de ambulatorio]. Jornal Brasileiro de Psiquiatria 1971;20(3):237-47. [PubMed] [Google Scholar]
Tesar 1990 {published data only}
- Tesar GE. High‐potency benzodiazepines for short‐term management of panic disorder: the US experience. Journal of Clinical Psychiatry 1990;51(5 Suppl):4-10. [PubMed] [Google Scholar]
Tyrer 1984 {published data only}
- Tyrer P, Owen R. Anxiety in primary care: is short‐term drug treatment appropriate? Journal of Psychiatric Research 1984;18(1):73-8. [DOI] [PubMed] [Google Scholar]
Tyrer 1988 {published data only}
- Tyrer P, Seivewright N, Murphy S, Ferguson B, Kingdon D, Barczak P, et al. The Nottingham study of neurotic disorder: comparison of drug and psychological treatments. Lancet 1988;2(8605):235-40. [DOI] [PubMed] [Google Scholar]
van Apeldoorn 2008 {published data only}
- Apeldoorn FJ, Hout WJ, Mersch PP, Huisman M, Slaap BR, Hale WW, et al. Is a combined therapy more effective than either CBT or SSRI alone? Results of a multicenter trial on panic disorder with or without agoraphobia. Acta Psychiatrica 2008;117(4):260-70. [DOI] [PubMed] [Google Scholar]
Van Balkom 1996 {published data only}
- Van Balkom AJ, Beurs E, Koele P, Lange A, Dyck R. Long‐term benzodiazepine use is associated with smaller treatment gain in panic disorder with agoraphobia. Journal of Nervous and Mental Disease 1996;184(2):133-5. [DOI] [PubMed] [Google Scholar]
Van Boeijen 2007 {published data only}
- Van Boeijen C, Van Oppen P, Boeke J, Visser S, Kempe P, Blankenstein N, et al. First‐line treatment of anxiety disorders: a randomized controlled trial [Angststoornissen in de eerste lijn vaak goed te behandelen: Een gerandomiseerd gecontroleerd onderzoek]. Huisarts en Wetenschap 2007;50(7):315-20. [Google Scholar]
Versiani 1983 {published data only}
- Versiani M, Bueno JR. Evaluation of the use of cloxazolam in patients with moderate anxiety [Avaliacao do emprego do cloxazolam em portadores de ansiedade moderada]. Jornal Brasileiro de Psiquiatria 1983;32(1):27-30. [Google Scholar]
Wiesner 1993 {published data only}
- Wiesner J, Grunder G, Wetzel H, Hiemke C. Bretazenil: neuroendocrinological profile of a partial benzodiazepine agonist in patients suffering from panic disorder with agoraphobia. Pharmacopsychiatry 1993;26:212. [Google Scholar]
Woods 1988 {published data only}
- Woods WS, Charney SD, Silver MJ, Krystal HJ, Heninger RG. Benzodiazepine receptor antagonist effects in panic disorder. In: 141st Annual Meeting of the American Psychiatric Association, 1988 May 7-12. Montreal, Quebec, 1988.
Yang 2005 {published data only}
- Yang H, Yu C, Gao H. The control study of mirtazapine in treating patients with panic disorder. Medical Journal of Chinese People Health 2005;17(3):133-5. [Google Scholar]
Yang 2006 {published data only}
- Yang H, Gao H, Yu C. Effect of citalopram in the treatment of panic disorder. Shandong Archives of Psychiatr 2006;19(2):186-7. [Google Scholar]
Yeragani 1992 {published data only}
- Yeragani VK, Pohl R, Balon R, Ramesh C, Weinberg P. Imipramine‐induced jitteriness and decreased serum iron levels. Neuropsychobiology 1992;25(1):8-10. [DOI] [PubMed] [Google Scholar]
Zajecka 1996 {published data only}
- Zajecka JM. The effect of nefazodone on comorbid anxiety symptoms associated with depression: experience in family practice and psychiatric outpatient settings. Journal of Clinical Psychiatry 1996;57(Suppl 2):10-4. [PubMed] [Google Scholar]
Zmorski 1985 {published data only}
- Zmorski T, Fischer Cornelssen KA. Clinical experiences with the new‐generation anxiolytic agent cloxazolam: a double‐blind study. [Klinische Erfahrungen mit dem Anxiolytikum der neuen Generation: CloxazolamEine Doppelblindstudie]. Schweizerische Rundschau fur Medizin Praxis 1985;74(27):728-34. [PubMed] [Google Scholar]
Additional references
Allison 2003
- Allison C, Pratt JA. Neuroadaptive processes in GABAergic and glutamatergic systems in benzodiazepine dependence. Pharmacology & Therapeutics 2003;98:171-95. [DOI] [PubMed] [Google Scholar]
Altman 1996
- Altman DG, Bland MJ. Detecting skewness from summary information. BMJ 1996;313(7066):1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 2000
- Anderson IM. Selective serotonin reuptake inhibitors versus tricyclic antidepressants: a meta-analysis of efficacy and tolerability. Journal of Affective Disorders 2000;58(1):19-36. [DOI] [PubMed] [Google Scholar]
Andrews 2018
- Andrews G, Bell C, Boyce P, Gale C, Lampe L, Marwat O, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the treatment of panic disorder, social anxiety disorder and generalised anxiety disorder. Australian & New Zealand Journal of Psychiatry 2018;52:1109-72. [Google Scholar]
Andrisano 2013
- Andrisano C, Chiesa A, Serretti A. Newer antidepressants and panic disorder: a meta-analysis. International Clinical Psychopharmacology 2013;28:33-45. [DOI] [PubMed] [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th edition. Washington, DC: American Psychiatric Publishing, 1994. [Google Scholar]
APA 2009
- American Psychiatric Association. American Psychiatric Association Practice Guideline for the Treatment of Panic Disorder. Washington, DC: American Psychiatric Publishing, 2009. [Google Scholar]
APA 2013a
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Washington, DC: American Psychiatric Publishing, 2013. [Google Scholar]
APA 2013b
- American Psychiatric Association. Highlights of changes from DSM-IV-TR to DSM-5. www.dsm5.org/Documents/changes%20from%20dsm-iv-tr%20to%20dsm-5.pdf (accessed prior to 10 June 2017).
Bakker 2002
- Bakker A, Balkom AJ, Spinhoven P. SSRIs vs. TCAs in the treatment of panic disorder: a meta-analysis. Acta Psychiatrica Scandinavica 2002;106:163-7. [DOI] [PubMed] [Google Scholar]
Baldwin 2014
- Baldwin DS, Anderson IM, Nutt DJ, Allgulander C, Bandelow B, den Boer JA, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. Journal of Psychopharmacology 2014;28:403-39. [DOI] [PubMed] [Google Scholar]
Ballenger 1998
- Ballenger JC, Davidson JR, Lecrubier Y, Nutt DJ, Baldwin DS, den Boer JA, et al. Consensus statement on panic disorder from the International Consensus Group on Depression and Anxiety. Journal of Clinical Psychiatry 1998;59(Suppl 8):47-54. [PubMed] [Google Scholar]
BAP 2014
- Baldwin DS, Anderson IM, Nutt DJ, Allgulander C, Bandelow B, den Boer JA, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. Journal of Psychopharmacology (Oxford, England) 2014;28(5):403-39. [DOI] [PubMed] [Google Scholar]
Batelaan 2007
- Batelaan N, Smit F, Graaf R, Balkom A, Vollebergh W, Beekman A. Economic costs of full-blown and subthreshold panic disorder. Journal of Affective Disorders 2007;104(1-3):127-36. [DOI] [PubMed] [Google Scholar]
Bighelli 2016
- Bighelli I, Trespidi C, Castellazzi M, Cipriani A, Furukawa TA, Girlanda F, et al. Antidepressants and benzodiazepines for panic disorder in adults. Cochrane Database of Systematic Reviews 2016, Issue 9. Art. No: CD011567. [DOI: 10.1002/14651858.CD011567.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bighelli 2018
- Bighelli I, Castellazzi M, Cipriani A, Girlanda F, Guaiana G, Koesters M, et al. Antidepressants versus placebo for panic disorder in adults. Cochrane Database of Systematic Reviews 2018, Issue 4. Art. No: CD010676. [DOI: 10.1002/14651858.CD010676.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bijl 1998
- Bijl RV, Ravelli A, Zessen G. Prevalence of psychiatric disorder in the general population: results of The Netherlands Mental Health Survey and Incidence Study (NEMESIS). Social Psychiatry and Psychiatric Epidemiology 1998;33:587-95. [DOI] [PubMed] [Google Scholar]
Boissel 1999
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. Comparison of the indices and their use. Therapie 1999;54:405-11. [PubMed] [Google Scholar]
Breilmann 2019
- Breilmann J, Girlanda F, Guaiana G, Barbui C, Cipriani A, Castellazzi M, et al. Benzodiazepines versus placebo for panic disorder in adults. Cochrane Database of Systematic Reviews 2019, Issue 3. Art. No: CD010677. [DOI: 10.1002/14651858.CD010677.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brettschneider 2019
- Brettschneider C, Bleiber F, Hiller TS, Konnopka A, Breitbart J, Margraf J, et al. The allocation of resources in the care for patients with panic disorder in Germany: an excess cost analysis informing policy and science. Cost effectiveness and Resource Allocation 2019;17:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Briley 1993
- Briley M, Moret C. Neurobiological mechanisms involved in antidepressant therapies. Clinical Neuropharmacology 1993;16:387-400. [DOI] [PubMed] [Google Scholar]
Bruce 2003
- Bruce SE, Vasile RG, Goisman RM, Salzman C, Spencer M, Machan JT, et al. Are benzodiazepines still the medication of choice for patients with panic disorder with or without agoraphobia? American Journal of Psychiatry 2003;160:1432-8. [DOI] [PubMed] [Google Scholar]
Caldwell 2005
- Caldwell DM, Ades AE, Higgins JPT. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005;331:897-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cameron 2007
- Cameron OG, Huang GC, Nichols T, Koeppe RA, Minoshima S, Rose D, et al. Reduced gamma-aminobutyric acid(A)-benzodiazepine binding sites in insular cortex of individuals with panic disorder. Archives of General Psychiatry 2007;64:793-800. [DOI] [PubMed] [Google Scholar]
Chaimani 2012
- Chaimani A, Salanti G. Using network meta-analysis to evaluate the existence of small-study effects in a network of interventions. Research Synthesis Methods 2012;3(2):161-76. [DOI] [PubMed] [Google Scholar]
Chawla 2022
- Chawla N, Anothaisintawee T, Charoenrungrueangchai K, Thaipisuttikul P, McKay GJ, Attia J, et al. Drug treatment for panic disorder with or without agoraphobia: systematic review and network meta-analysis of randomised controlled trials. BMJ 2022;376:e066084. [DOI: doi: 10.1136/bmj-2021-066084] [DOI] [PMC free article] [PubMed] [Google Scholar]
CINeMA 2017 [Computer program]
- CINeMA. Confidence in Network Meta-Analysis. Bern: Institute of Social and Preventive Medicine, University of Bern, 2017.
Cipriani 2013
- Cipriani A, Higgins JP, Geddes JR, Salanti G. Conceptual and technical challenges in network meta-analysis. Annals of Internal Medicine 2013;159(2):130-7. [DOI] [PubMed] [Google Scholar]
Cleare 2015
- Cleare A, Pariante CM, Young AH, Anderson IM, Christmas D, Cowen PJ, et al, Members of the Consensus Meeting. Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2008 British Association for Psychopharmacology guidelines. Journal of Psychopharmacology 2015;29:459-525. [DOI] [PubMed] [Google Scholar]
Cohen 1992
- Cohen J. A power primer. Psychological Bulletin 1992;112(1):155-9. [DOI] [PubMed] [Google Scholar]
Cristea 2017
- Cristea IA, Gentili C, Pietrini P, Cuijpers P. Sponsorship bias in the comparative efficacy of psychotherapy and pharmacotherapy for adult depression: meta-analysis. British Journal of Psychiatry 2017;210:16-23. [DOI] [PubMed] [Google Scholar]
Davies 2022
- Davies SJ, Rudoler D, Oliveira C, Huang A, Kurdyak P, Iaboni A. Comparative safety of chronic versus intermittent benzodiazepine prescribing in older adults: a population-based cohort study. Journal of Psychopharmacology 2022;36:460-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2000
- Deeks J. Issues in the selection for meta-analyses of binary data. In: Proceedings of the 8th International Cochrane Colloquium; Cape Town, South Africa, 2000 Oct 25-28. 2000.
Dias 2013a
- Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Medical Decision Making 2013;33:607-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dias 2013b
- Dias S, Sutton AJ, Welton NJ, Ades AE. Evidence synthesis for decision making 3: heterogeneity--subgroups, meta-regression, bias, and bias-adjustment. Medical Decision Making 2013;33:618-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Du 2021
- Du Y, Du B, Diao Y, Yin Z, Li J, Shu Y, et al. Comparative efficacy and acceptability of antidepressants and benzodiazepines for the treatment of panic disorder: a systematic review and network meta-analysis. Asian Journal of Psychiatry 2021;60:102664. [DOI: doi: 10.1016/j.ajp.2021.102664] [DOI] [PubMed] [Google Scholar]
Eaton 1994
- Eaton WW, Kessler RC, Wittchen H-U, Magee WJ. Panic and panic disorder in the United States. American Journal of Psychiatry 1994;151:413-20. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31:140-9. [DOI] [PubMed] [Google Scholar]
Fava 2019
- Fava GA, Cosci F. Understanding and managing withdrawal syndromes after discontinuation of antidepressant drugs. Journal of Clinical Psychiatry 2019;80(6):19com12794. [DOI] [PubMed] [Google Scholar]
Fava 2020
- Fava GA. May antidepressant drugs worsen the conditions they are supposed to treat? The clinical foundations of the oppositional model of tolerance. Therapeutic Advances in Psychopharmacology 2020;10:2045125320970325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fluyau 2022
- Fluyau D, Mitra P, Jain A, Kailasam VK, Pierre CG. Selective serotonin reuptake inhibitors in the treatment of depression, anxiety, and post-traumatic stress disorder in substance use disorders: a Bayesian meta-analysis. European Journal of Clinical Pharmacology 2022;78:931-42. [DOI] [PubMed] [Google Scholar]
Furukawa 2002
- Furukawa TA, Guyatt GH, Griffith LE. Can we individualize the 'number needed to treat'? An empirical study of summary effect measures in meta-analyses. International Journal of Epidemiology 2002;31:72-6. [DOI] [PubMed] [Google Scholar]
Furukawa 2005
- Furukawa TA, Cipriani A, Barbui C, Brambilla P, Watanabe N. Imputing response rates from means and standard deviations in meta-analysis. International Clinical Psychopharmacology 2005;20:49-52. [DOI] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. Journal of Clinical Epidemiology 2000;59:7-10. [DOI] [PubMed] [Google Scholar]
Furukawa 2007
- Furukawa TA, Watanabe N, Churchill R. Combined psychotherapy plus antidepressants for panic disorder with or without agoraphobia. Cochrane Database of Systematic Reviews 2007, Issue 1. Art. No: CD004364. [DOI: 10.1002/14651858.CD004364.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Furukawa 2021
- Furukawa TA, Shinohara K, Sahker E, Karyotaki E, Miguel C, Ciharova M, et al. Initial treatment choices to achieve sustained response in major depression: a systematic review and network meta-analysis. World Psychiatry 2021;20:387-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goisman 1995
- Goisman RM, Warshaw MG, Steketee GS, Fierman EJ, Rogers MP, Goldenberg I, et al. DSM-IV and the disappearance of agoraphobia without a history of panic disorder: new data on a controversial diagnosis. American Journal of Psychiatry 1995;152:1438-43. [DOI] [PubMed] [Google Scholar]
Gorman 2000
- Gorman JM, Kent JM, Sullivan GM, Coplan JD. Neuroanatomical hypothesis of panic disorder, revised. American Journal of Psychiatry 2000;157:493-505. [DOI] [PubMed] [Google Scholar]
Grant 2006
- Grant BF, Hasin DS, Stinson FS, Dawson DA, Goldstein RB, Smith S, et al. The epidemiology of DSM-IV panic disorder and agoraphobia in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Clinical Psychiatry 2006;67:363-74. [DOI] [PubMed] [Google Scholar]
Higgins 1996
- Higgins JPT, Whitehead A. Borrowing strength from external trials in a meta-analysis. Statistics in Medicine 1996;15:2733-49. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman D, Gotzsche P, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2019
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6. The Cochrane Collaboration, 2019. Available from www.training.cochrane.org/handbook.
Hirschtritt 2021
- Hirschtritt ME, Olfson M, Kroenke K. Balancing the risks and benefits of benzodiazepines. JAMA 2021;325:347-8. [DOI] [PubMed] [Google Scholar]
Horowitz 2021
- Horowitz MA, Wright JM, Taylor D. Risks and benefits of benzodiazepines. JAMA 2021;325:2208. [DOI] [PubMed] [Google Scholar]
Imai 2014
- Imai H, Tajika A, Chen P, Pompoli A, Guaiana G, Castellazzi M, et al. Azapirones versus placebo for panic disorder in adults. Cochrane Database of Systematic Reviews 2014, Issue 9. Art. No: CD010828. [DOI: 10.1002/14651858.CD010828.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Katzman 2014
- Katzman MA, Bleau P, Blier P, Chokka P, Kjernisted K, Van Ameringen M, Canadian Anxiety Guidelines Initiative Group on behalf of the Anxiety Disorders Association of Canada/Association Canadienne des troubles anxieux and McGill University. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry 2014;14 Suppl 1:S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kessler 2006
- Kessler RC, Chiu WT, Jin R, Ruscio AM, Shear K, Walters EE. The epidemiology of panic attacks, panic disorder, and agoraphobia in the National Comorbidity Survey Replication. Archives of General Psychiatry 2006;63:415-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kessler 2012
- Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. International Journal of Methods in Psychiatric Research 2012;21:169-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
King 2008
- King M, Nazareth I, Levy G, Walker C, Morris R, Weich S, et al. Prevalence of common mental disorders in general practice attendees across Europe. British Journal of Psychiatry 2008;192:362-7. [DOI] [PubMed] [Google Scholar]
Klein 1964
- Klein DF. Delineation of two drug-responsive anxiety syndromes. Psychopharmacologia 1964;5:397-408. [DOI] [PubMed] [Google Scholar]
Lu 2004
- Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Statistics in Medicine 2004;23:3105-24. [DOI] [PubMed] [Google Scholar]
Lunn 2009
- Lunn D J, Spiegelhalter D, Thomas A, Best N. The BUGS project: evolution, critique and future directions. Statistics in Medicine 2009;28:3049-67. [DOI] [PubMed] [Google Scholar]
Malizia 1998
- Malizia AL, Cunningham VJ, Bell CJ, Liddle PF, Jones T, Nutt DJ. Decreased brain GABA(A)-benzodiazepine receptor binding in panic disorder: preliminary results from a quantitative PET study. Archives of General Psychiatry 1998;55:715-20. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, the PRISMA group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement. PLOS Medicine 2009;6(6):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nash 2008
- Nash JR, Sargent PA, Rabiner EA, Hood SD, Argyropoulos SV, Potokar JP, et al. Serotonin 5-HT1A receptor binding in people with panic disorder: positron emission tomography study. British Journal of Psychiatry 2008;1993:229-34. [DOI] [PubMed] [Google Scholar]
NICE 2011
- National Institute for Health and Care Excellence. Generalised anxiety disorder and panic disorder (with or without agoraphobia) in adults [CG113]. London: National Institute for Health and Care Excellence, 2011. [Google Scholar]
Nikolakopoulou 2019
- Nikolakopoulou A, Higgins JPT, Papakonstantinou T, Chaimani A, Del Giovane C, Egger M, et al. Assessing confidence in the results of network meta-analysis (CINeMA). Available at www.biorxiv.org/content/10.1101/597047v1. [DOI] [PMC free article] [PubMed]
Otto 2001
- Otto MW, Tuby KS, Gould RA, McLean RY, Pollack MH. An effect-size analysis of the relative efficacy and tolerability of serotonin selective reuptake inhibitors for panic disorder. American Journal of Psychiatry 2000;158:1989-92. [DOI] [PubMed] [Google Scholar]
Papola 2022
- Papola D, Ostuzzi G, Tedeschi F, Gastaldon C, Purgato M, Del Giovane C, et al. Comparative efficacy and acceptability of psychotherapies for panic disorder with or without agoraphobia: systematic review and network meta-analysis of randomised controlled trials. British Journal of Psychiatry 2022;221:507-19. [DOI] [PubMed] [Google Scholar]
Pfizer 2008
- Pfizer. A study of sertraline compared with paroxetine in the treatment of panic disorder. https://clinicaltrials.gov/study/NCT00677352 (first received 14 May 2008).
Phillippo 2018
- Phillippo DM, Dias S, Ades AE, Didelez V, Welton NJ. Sensitivity of treatment recommendations to bias in network meta-analysis. Journal of the Royal Statistical Society. Series A, (Statistics in Society) 2018;181(3):843-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Phillippo 2019
- Phillippo DM, Dias S, Welton NJ, Caldwell DM, Taske N, Ades AE. Threshold analysis as an alternative to GRADE for assessing confidence in guideline recommendations based on network meta-analyses. Annals of Internal Medicine 2019;170(8):538-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Preti 2016
- Preti A, Vrublevska J, Veroniki AA, Huedo‐Medina TB, Fountoulakis KN. Prevalence, impact and treatment of generalised anxiety disorder in bipolar disorder: a systematic review and meta‐analysis. Evidence‐Based Mental Health 2016;19:73-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Puhan 2014
- Puhan MA, Schunemann, Murad MH, Li T, Brignardello-Peterson R, Singh JA, Kessels AG, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014;349:g5630. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Salanti 2012
- Salanti G. Indirect and mixed-treatment comparison, network or multiple-treatments meta-analysis: many names, many benefits. Research Synthesis Methods 2012;3(2):80-97. [DOI] [PubMed] [Google Scholar]
Silberman 2021
- Silberman E, Balon R, Starcevic V, Shader R, Cosci F, Fava GA, et al. Benzodiazepines: it's time to return to the evidence. British Journal of Psychiatry 2021;218:125-7. [DOI] [PubMed] [Google Scholar]
Starcevic 2009
- Starcevic V. Anxiety Disorders in Adults: A Clinical Guide. Oxford: Oxford University Press, 2009. [Google Scholar]
Stein 2010
- Stein M, Steckler T, Lightfoot JD, Hay E, Goddard AW. Pharmacologic treatment of panic disorder. Current Topics in Behavioral Neurosciences 2010;2:469-85. [DOI] [PubMed] [Google Scholar]
van Valkenhoef 2016
- Valkenhoef G, Dias S, Ades AE, Welton NJ. Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Research Synthesis and Methods 2016;7:80-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wade 1999
- Wade AG. Antidepressants in panic disorder. International Clinical Psychopharmacology 1999;14(Suppl 2):13-7. [PubMed] [Google Scholar]
Watanabe 2009
- Watanabe N, Churchill R, Furukawa TA. Combined psychotherapy plus benzodiazepines for panic disorder. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No: CD005335. [DOI: 10.1002/14651858.CD005335.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilkinson 1991
- Wilkinson G, Balestrieri M, Ruggeri M, Bellantuono C. Meta-analysis of double-blind placebo-controlled trials of antidepressants and benzodiazepines for patients with panic disorders. Psychological Medicine 1991;21:991-8. [DOI] [PubMed] [Google Scholar]
Winbugs 2012 [Computer program]
- Winbugs. MRC Medical Biostatistics Unit Cambridge, 2012. www.mrc-bsu.cam.ac.uk/bugs/.
References to other published versions of this review
Guaiana 2020
- Guaiana G, Barbui C, Meader N, Davies SJC, Furukawa TA, Imai H, et al. Pharmacological treatments in panic disorder in adults: a network meta‐analysis. Cochrane Database of Systematic Reviews 2020, Issue 10. Art. No: CD012729. [DOI: 10.1002/14651858.CD012729.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


