Abstract
Background
The worldwide prevalence of diabetic foot ulcers (DFUs) among persons with diabetes is estimated at 6.3%, with an annual incidence of 9.1 to 26.1 million persons. The early detection of asymmetrical plantar temperature elevation, followed by reduction of weight-bearing on the affected foot, may be an effective mode of prevention.
Methods
Patients with diabetes and peripheral neuropathy (DFU risk groups 2/3) were monitored for plantar abnormalities with a telemedical system consisting of sole inserts with temperature sensors and photographic documentation. An open, prospective, randomized controlled trial was performed to determine whether this system prevented DFUs. The intervention and control groups were also trained in ulcer prevention and observed in follow-up at 6-month intervals for 24 months.
Results
283 patients were recruited. In 85 137 observation days, DFUs arose in five patients in the control group (n = 143) and in no patient in the intervention group (n = 140). The primary outcome measure was the hazard ratio, which was calculated to be 0.015 (95% confidence interval [0; 19,717]; p = 0.25) after adjustment for age, sex, severity of neuropathy, and risk class. There were 239 alarms and 75 instructions to reduce weight-bearing on the foot. The subjects carried out the telemedical application on about 70% of the days of observation. Quality of life improved in both groups.
Conclusion
The tele-health system used in this trial is practical and enables the early detection of morbidity. Likely explanations for the unexpectedly low ulceration rate in this trial (and, in turn, for the lack of statistical significance) include the availability of a training program and regular follow-up examinations to patients in both arms of the trial, along with lower mobility levels due to the COVID pandemic.
Diabetic foot ulcer (DFU) is the most commonly occurring complication of diabetes. It carries a risk of amputation of the lower extremity with associated high morbidity and mortality (1, 2). Around 20% of severely infected ulcers result in amputation (3, 4). The enormous social and economic burden is reflected by the increased prevalence rates of DFU in diabetes (˜6.3%, i.e., approximately 9.1 to 26.1 million patients annually worldwide) (1, 5). Unfortunately, the standard preventive interventions lower the incidence of DFU only slightly (1, 6, 7). The lifetime incidence of DFU remains as high as 34% in patients with diabetes, and the incidence of ulcer recurrence amounts to ˜ 40% within 1 year and ˜ 60% within 3 years (1, 8). DFU remains the leading cause for non-traumatic lower extremity amputation, accounting for 85% of cases (9). There is thus an urgent need not only for strategies to identify patients at high risk, but also for the establishment of warning systems for the critical pre-ulcer status so that preventive interventions can be implemented (10).
In 80% of cases DFU is accompanied by peripheral polyneuropathy, resulting in defective sensation of pain and temperature (11, 12). DFU arises from repetitive plantar stress through shear forces or vertical loading, resulting in tissue microtrauma with local inflammation, immune cell recruitment, and enzymatic autolysis (13). One of the classical signs is calor, a locoregional temperature elevation. In randomized controlled trials (RCTs) a temperature increase preceded ulcer formation by 5 days to 5 weeks, indicating that temperature monitoring can be used for early detection (14–18). In a RCT performed in a population at high risk of DFU, the ulceration rate was 12.2% in the standard care group, but only 4.7% in the group of patients monitored by means of dermal thermometry (odds ratio 3.0 [95% confidence interval 1.0; 8.5]; p = 0.038) (16). However, the measurements were not integrated into a telehealth application. High false-alarm rates may compromise patient adherence in telehealth applications (the specificity is between 32% and 57% at thresholds of 3.20 °C and 2.22 °C, respectively) (19, 20).
In our RCT, a telehealth application was set up with twice daily home recordings of plantar temperatures by means of sensor-equipped insoles and photodocumentation of foot abnormalities using cell phones. The study evaluated the effectiveness of the established telehealth system with close exchange of health data in preventing the onset of diabetic foot syndrome and improving patient compliance and quality of life in a domestic setting.
Methods
Study design
This open-label, prospective, randomized clinical trial (German Clinical Trials Registry DRKS00013798) was designed for patients with diabetes and at risk for DFU due to the presence of intermediate to severe peripheral neuropathy. The study protocol was approved by the local ethics committee on 8 January 2018 and the complete study protocol has been published (21). Patient recruitment was carried out at the Department of Nephrology and Hypertension, Diabetes, and Endocrinology of the University Hospital, Otto-von-Guericke University, Magdeburg. Written informed consent was provided by all eligible participants before study commencement. The enrolled patients with polyneuropathy and/or prior DFU were randomly assigned to the control or the intervention arm in a 1:1 ratio, stratified by age, sex, severity of neuropathy (restriction of vibration sensation), and risk group (2 or 3) (22–24). The randomization was performed by the medical statistician using the software RITA (Statsol, Lübeck). The allocation sequence was concealed from the physicians who had recruited the patients. The study physicians and patients were then informed about the outcome of randomization and 2 weeks later, at visit 0, patients were shown how to perform regular foot care measures for prevention of foot ulcers by a qualified study physician (standardized patient training).
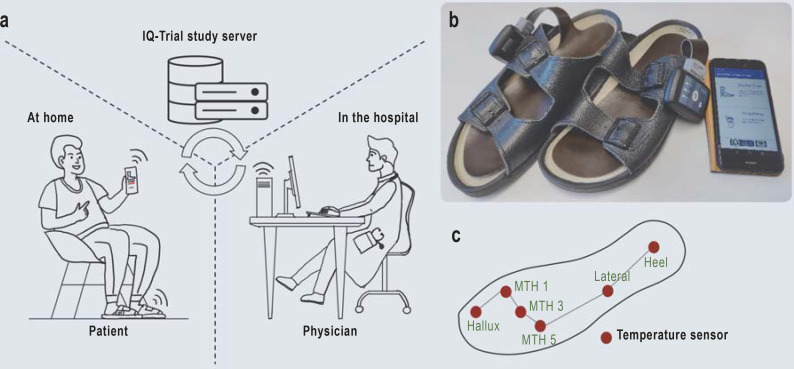
In addition to the training session, probands in the intervention group were provided with sensor-equipped insoles manufactured by Thorsis Technologies GmbH and a cellphone app programed at the Department of Nephrology and Hypertension, Diabetes, and Endocrinology that exchanged data via the IQ-Trial Study Server with the physicians in the clinic (Figure 1). Plantar foot temperature recordings were recommended twice daily at an interval of > 4 hours (each lasting ˜ 5 minutes). The preset alarm algorithm automatically notified the study physician via the dashboard when temperature asymmetries (> 1.5 °C) between corresponding sensor sites exceeded 32 hours (eFigure 1). The study participants were instructed to use the app to perform foot inspections, provide foot status reports, and upload foot photographs. The study physician decided on the basis of all available data whether or not to recommend a 5-day intervention period of foot offloading. On-site visits were arranged if temperature asymmetries persisted.
Figure 1.
Overview of the Smart Prevent Diabetic Feet telehealth system
a) Patients in the intervention group were provided with sensor-equipped insoles and a cellphone app that exchanged data with the physicians in the hospital via the IQ-Trial study server. Plantar foot temperature recordings were recommended twice daily. The preset alarm algorithm automatically notified the study physician via the dashboard when temperature asymmetries (> 1.5 °C) between corresponding sensor sites lasted > 32 hours. Patients were instructed to perform foot inspections, document the foot status, and upload foot photographs. The physician decided, based on all available data, whether or not to recommend a 5-day period of foot offloading. On-site visits were arranged when temperature asymmetries persisted.
b) Sensor-equipped insoles in sandals and start page of app.
c) Insole sensor sites.
All patients were instructed to consult their study physician immediately if the recommended daily self-inspection revealed signs of inflammation (e.g., redness, pain, and sores), foot abnormalities, or ulceration. Regular study visits were planned every 6 months. These measures represent a very high standard of care. A detailed explanation of interventions is provided in the eSupplement.
Study outcomes
The primary outcome of this study was the time to onset of the first foot ulcer (≥ Wagner grade 1), classified according to the Wagner/Armstrong criteria (25, 26) and adjusted by age (in years), sex, severity of neuropathy, and risk class (22–24).
The secondary outcomes were:
Adherence to daily temperature measurements
Frequency of temperature alarms and intervention instructions
Temperature drops correlating with limb ischemia
Number of adverse events (AEs) and severe adverse events (SAEs)
Quality of life (QOL) based on the World Health Organization-5 score
Precursors of DFU: redness, infections, or wounds on the foot
Results
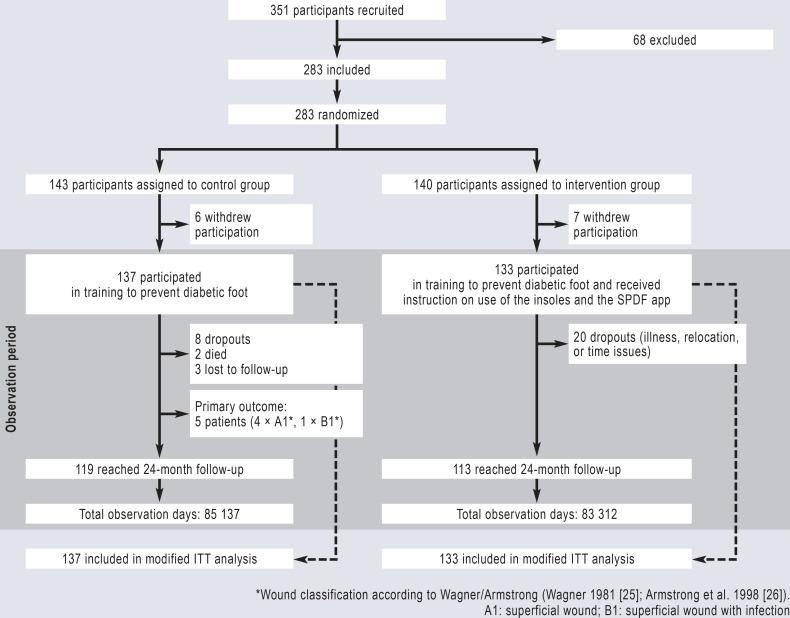
From January 2018 to February 2020, 351 patients with diabetes and peripheral polyneuropathy were recruited (Figure 2). Of these patients, 283 consented to the study protocol and were randomized to either the control or the intervention group in a ratio of 1:1. Six patients from the control group and seven from the intervention group were excluded from the intention-to-treat (ITT) analyses (“modified protocol”) because they withdrew their consent before the training session due to either the complexity of the study protocol or dissatisfaction with their group allocation (n = 13). The baseline characteristics of the patients (n = 270) were similar in the two groups (Table, eTable 1). The study closed on 20 March 2021. A total of 85 137 observation days for the control group and 83 312 observation days for the intervention group were included in the modified ITT analysis.
Figure 2.
Flow diagram of Smart Prevent Diabetic Feet study
A total of 283 patients with diabetes and peripheral polyneuropathy were enrolled in the study and randomized to the control or intervention group. Of these, 137 patients in the control group and 133 patients in the intervention group were evaluated after participating in training sessions on diabetic foot care. The patients in the intervention group additionally received the SPDF insole system for temperature measurement and instruction in use of the app. ITT, Intention to treat; SPDF, Smart Prevent Diabetic Feet
Table. The participants’ characteristics at the beginning of the study.
| Group |
Control group
(n = 137) |
Intervention group
(n = 133) |
| Age, years | 65.6 (8.6) | 65.2 (9.1) |
| Sex (male) | 93 (67.9%) | 90 (67.7%) |
| Diabetes mellitus | ||
| Type 1 | 23 (16.8%) | 25 (18.8%) |
| Type 2 | 114 (83.2%) | 108 (81.2%) |
| Duration of diabetes mellitus (in years)*1 | 16.4 (11.7) | 16.6 (11.9) |
| Participants’ height (in m) | 1.7 (0.1) | 1.7 (0.1) |
| Weight (in kg) | 93.2 (20.1) | 93.5 (20.4) |
| Body mass index (BMI), kg/m2 | 30.9 (5.8) | 30.7 (6.3) |
| WHO-5 Well-Being Index (0–25) | 17.6 (4.9) | 18.2 (4.5) |
| Neuropathy symptoms score (NSS) | ||
| Moderate (5–6) | 27 (19.7%) | 27 (20.3%) |
| Severe (7–10) | 84 (61.3%) | 80 (60.2%) |
| Neuropathy disability score (NDS) | ||
| Moderate (6–8) | 62 (45.3%) | 66 (49.6%) |
| Severe (9–10) | 13 (9.5%) | 11 (8.3%) |
| Ankle–brachial index (ABI), right foot | ||
| ≥ 1.3 | 7 (5.15%) | 9 (6.8%) |
| ≤ 0.9–20.75 | 10 (7.3%) | 10 (7.5%) |
| ≤ 0.75–20.5 | 3 (2.2%) | 2 (1.5%) |
| Ankle–brachial index (ABI), left foot | ||
| ≥1.3 | 6 (4.4%) | 7 (5.2%) |
| ≤ 0.9–0.75 | 10 (7.3%) | 7 (5.3%) |
| ≤ 0.75–0.5 | 1 (0.7%) | 4 (3.0%) |
| Classification of previous plantar foot ulcers*2 | ||
| A0, A1, A2, A3 | 4 (2.8%) | 4 (3.0%) |
| Unclassified | 5 (3.8%) | 3 (2.3%) |
| DFU risk group | ||
| Group 2 | 129 (94.2%) | 127 (95.5%) |
| Group 3 | 8 (5.8%) | 6 (4.5%) |
Primary results
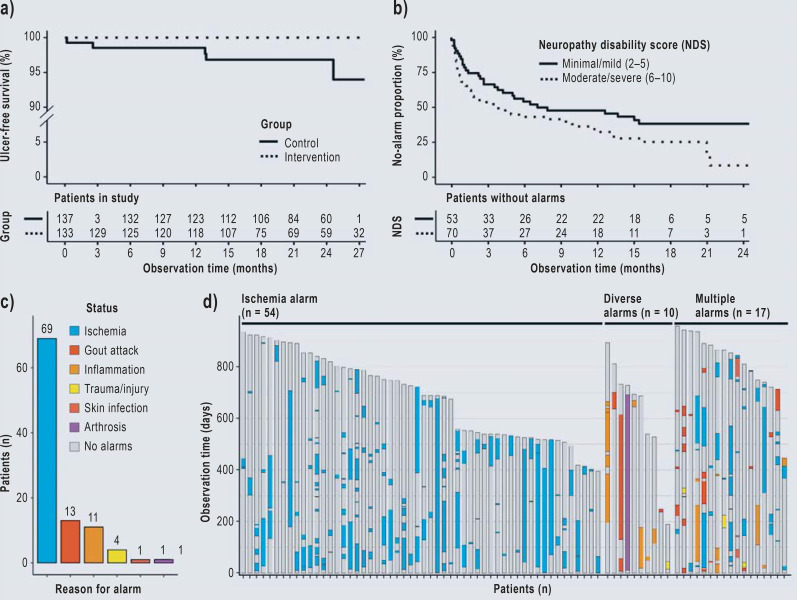
Five patients developed foot ulcers in the control group versus no patients in the intervention group (classified according to the Wagner/Armstrong criteria as four DFU grade 1 stage A and one grade 1 stage B) (25, 26). The duration of the ulceration ranged from 3 to 8 weeks and required foot offloading, prescription of orthopedic shoes, and/or hospitalization. None of the patients developed severe ulceration requiring limb amputation (eTable 2). When Cox proportional hazards regression analysis with adjustment of age, sex, risk class, and severity of neuropathy was performed, the level of significance was not reached due to the low incidence of ulcer formation (hazard ratio 0.015 [0.000; 19.717]; p = 0.25). The unadjusted Kaplan–Meier survival analyses of ulcer occurrence over time showed that participants in the intervention group had a lower ulceration rate than those in the control group (log-rank test, p = 0.021, Figure 3a).
Figure 3.
Study results and interpretation of the reasons for temperature alarms
(a) Kaplan–Meier plot of plantar foot ulcer occurrence in the modified ITT population (n = 270)
(b) Evaluation of alarms in the subgroups with minimal/mild or moderate/severe neuropathy within the intervention group (n = 123). Only events within the first 24 months of the study were included.
(c) Overview of the alarms and the underlying reasons in the intervention group from the post-hoc analysis.
(d) Alarm categories and duration for individual patients in the intervention group. Patients without alarms were not visualized (n = 52).
Secondary results
The plantar temperature recordings were “normalized” by subtraction of ambient temperature recordings from the same insole by an additional sensor integrated in the Bluetooth device. The predefined algorithm calculated asymmetries of temperatures recorded by sensors from both insoles and indicated on the dashboard when the alarm level was ≥ 3 (eFigure 1). The study physicians assessed the plausibility of these findings on the basis of all available information. In 75 cases of verified alarms, instructions on the 5-day foot offloading intervention were initiated by the physician (eFigure 2). In cases of misclassification due to technical defects (missing or faulty sensor data), absent contact of the plantar foot with the hallux sensor, or temperature decrease (possibly due to intermittent limb ischemia), the alarm levels were reset.
The overall adherence rate was defined as measurement days divided by observation days. This rate was calculated at 70% (eFigure 3). The numbers of AEs and SAEs recorded within the study did not differ between the two groups (eTable 3). The QOL of both groups increased during the study period (eTable 4). After 24 months, QOL was slightly better in patients with the telehealth app.
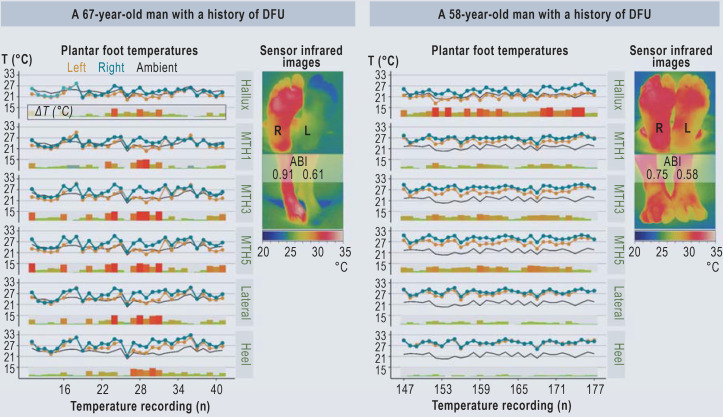
According to the post hoc analyses, patients with advanced neuropathy had shorter time-to-alarm periods than those with minor neuropathy (log-rank test, p = 0.048, Figure 3b). Overall, 239 alarms within the 83 312 observation days of the intervention group were identified post hoc by means of manual supervised classification by two physicians (Figure 3c, d). In 33 of 40 inflammation-related alarms, foot offloading was performed as instructed (eTable 5). Figure 4 presents examples of plantar foot temperature asymmetry due to intermittent ischemia.
Figure 4.
Temperature recordings and clinical findings of two patients with temperature abnormalities most likely due to transient ischemia. The temperature asymmetries correlated to clinical findings, infrared imaging, and ABI measurements. ABI, Ankle–brachial index; DFU, diabetic foot ulcer; MTH, metatarsal head; T, absolute temperature values measured by a sensor; ΔT: difference in absolute temperature between corresponding sensor sites, e.g., left MTH1 versus right MTH1.
Discussion
Telehealth technology applications may aid medical professionals in their interaction with patients and may also help patients with chronic diseases to monitor their health status (27, 28). Telehealth applications have been set up in various fields, but relate mostly to telecounseling (29). Key trends with innovations in the consumer technology market (e.g., wearable sensors with wireless monitoring functions) have the potential to enhance clinical decision making in wide-reaching fashion and to become a means of delivering patient care (30, 31). The projected shortages of medical professionals in rural areas will increase the need for innovative surveillance strategies in patients with chronic diseases, e.g., those with diabetes and the late complications of polyneuropathy. The applications will need to be user-friendly and easy to operate, and the patients will need to possess basic skills in digital media.
The first reports on the utility of plantar temperature recordings for early detection of tissue damage and incipient DFU formation were published more than 15 years ago, and several small to medium-sized clinical trials have described positive results (14–18, 32, 33). The risk of developing a DFU over a period of 18 months was reduced from 12.2% to 4.7% by temperature monitoring in a study of 225 patients with diabetes (16); however, controversy persists in relation to the reproducibility of such measurements with hand-held infrared devices, infrared cameras, or using a temperature matrix (1, 14–18, 32, 33). Our RCT was designed to integrate a telemedical temperature monitoring device in the study participants’ daily routine. Compared with a hand-held thermometer, sensor-equipped insoles are convenient and provide accurate and efficient temperature recording at six predefined positions on the foot, together with the ambient temperature (15–17). A cellphone app provided the basis for doctor–patient interactions, presentation of the temperature measurements, display of alarm levels, and the completion of short questionnaires on foot abnormalities and general well-being. Use of the conventional cellphone camera was eased by providing templates and a submenu of the app so that images could be transferred immediately to the study center. At the time of the study, each pair of insoles including electronic setup cost € 1000; the number of pairs was therefore limited. The recruited cohort can be classified as having a moderate to high risk of DFU occurrence. The actual number of ulcers in the control group was five over 85 137 observation days, while no ulcers were observed in the intervention group, which was equipped with the telehealth application and also interacted with the study physician. These numbers are both unexpectedly low, given that in similar patient cohorts ulcer formation has been observed at rates of 8.4–41.5% within 6–18 months (14–18). The low ulcer rate may be due to the extensive training sessions on foot care at study commencement, which were received very positively and encouraged adherence to preventive measures (daily foot inspection, moisturizing creams). The close monitoring of the participants every 6 months with on-site study visits and clinical examinations as well as infrared imaging may also have lowered the DFU rate. A possible alternative explanation is general physical inactivity of the study population during the corona virus pandemic (34).
The number of alarms triggered by the predefined algorithm was unexpectedly high. The caring study physician evaluated all data on a daily basis and compared the plantar temperature recordings with those of the preceding weeks and with the ambient temperature before recommending foot offloading. By this means, transient ischemia could be distinguished from asymmetric inflammation in most cases. The main cause of alarms related to transient episodes of ischemia, not to inflammatory events with asymmetric plantar and ambient temperature values. Furthermore, comparison with historical values indicated a downshift of temperatures in these patients. Individual cases with infrared imaging confirmed these findings. Thus, temperature decreases possibly indicating ischemic events are highly prevalent (35, 36), and such downshifts were observed in 52% of the participants in the intervention group.
Do the Smart Prevent Diabetic Feet study results indicate more efficient delivery of care for patients? The Cox regression analysis of our data reveals no statistically significant benefit of the telemedicine intervention. Nevertheless, the estimated effect is clearly in the direction of reduced risk for DFU. Because of the small number of cases, however, the confidence interval is very wide. An advantage of the telehealth approach is the implementation of a surveillance strategy that it allows information to be gathered without organizational obstacles (e.g., consulting hours, on-site physician visits, travel). During the study, a learning curve in patient care by telehealth was accompanied by expanding functionality of the dashboard, allowing the physicians to interact with the participants via messages, short notices, standardized questionnaires and foot photographs. Furthermore the availability of temperature recordings from the preceding days and weeks permitted the physicians to detect changes over time and swiftly to differentiate between increases and decreases in temperature. The user-centered design facilitates the integration of sensor data with foot findings from the pictorial material. In this way, medical interpretation and evaluation could be integrated into clinical assessment into workflows and clinical routines (37).
One drawback of the study is the unblinded nature of the temperature measurement for patients, physicians, and biostatisticians. In view of the uncertainties that would arise from false reporting of temperatures or the lack of intervention in the case of asymmetric temperature elevation, both mock insole recording and measurement without intervention recommendations from the study center seemed unrealistic. On the other hand, ulcer diagnosis in the presence of skin lesions is an objective finding with low likelihood of bias. The assessment and classification of ulcers was confirmed by performance of clinical examinations by two independent examiners. The time investment of the dashboard supervising physician was not recorded systematically, so no objective number can be given. It is noteworthy that functionality improved considerably and that color coding of the preset alarm levels facilitated an immediate overview of patient status. Since the number of primary events (DFU) was much lower than expected, the results should be confirmed in another study.
In summary, this study provides the first experience of telehealth interactions between physicians and patients over an extended period in persons with diabetes and polyneuropathy. The dashboard facilitated data interpretation and the diagnosis of comorbidities by virtue of the combination of digital images and temperature recordings. The adherence rates and the QOL indicated acceptance of this approach by the majority of the mostly elderly participants, which is regarded as a basic requirement for implementation (27, 38).
Acknowledgments
Acknowledgments
We would like to thank Ingeborg Bloos-Walzer, Marie Theres Sarji, Andre Pfannkuche, and Fred Samland (Thorsis GmbH) for their support in the early stages of the study. The statistical analyses were planned and performed by the study team, Mrs. Anke Lux and Prof. Dr. Siegfried Kropf of the Institute for Biometry and Medical Informatics, Otto-von-Guericke University, Magdeburg. We dedicate this study to Thorsten Szczepanski of Thorsis Technologies GmbH, who inspired us all with his enthusiasm and his commitment to improving patient care.
Funding
The European Fund for Regional Development and the Ministry of Science, Economics and Digitalization of the State of Saxony-Anhalt funded this project under the Autonomy in Old Age Program (project nos. ZS/2016/05/78615, ZS/2018/12/95325). AM was funded by the China Scholarship Council (CSC no. 201508120093).
Data sharing statement
We are open to any reasonable requests for the original data directed to the corresponding author, provided the requested data relates to the data published in the article and do not compromise any future publication or any other related issues. These data may include anonymous data from the sensor measurements and statistical analyses. The data will be available for at least 10 years from the date of publication.
Footnotes
Conflict of interest statement
PRM has filed a patent on temperature measurement for early detection of diabetic foot ulcers. Together with Thorsten Szczepanski he has founded the company Medixmind GmbH to optimize the analyses of the sensor data, which were transferred in double-pseudonymized manner to a server. The technical staff of Medixmind and of Thorsis GmbH had access to the pseudonymized data sets. Furthermore, PRM has received personal fees from Berlin-Chemie, Boehringer Ingelheim, Bristol-Myers Squibb, Lilly, Novo Nordisk, and Novartis for consultation as well as fees for presentations and memberships in advisory boards.
The remaining authors declare that no conflict of interest exists.
References
- 1.Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376:2367–2375. doi: 10.1056/NEJMra1615439. [DOI] [PubMed] [Google Scholar]
- 2.Grennan D. Diabetic foot ulcers. JAMA. 2019;321 doi: 10.1001/jama.2018.18323. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong DG, Tan T-W, Boulton AJM, Bus SA. Diabetic foot ulcers: a review. JAMA. 2023;330:62–75. doi: 10.1001/jama.2023.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ndosi M, Wright-Hughes A, Brown S, et al. Prognosis of the infected diabetic foot ulcer: a 12-month prospective observational study. Diabet Med. 2018;35:78–88. doi: 10.1111/dme.13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis. Ann Med. 2017;49:106–116. doi: 10.1080/07853890.2016.1231932. [DOI] [PubMed] [Google Scholar]
- 6.van Netten JJ, Raspovic A, Lavery LA, et al. Prevention of foot ulcers in the at-risk patient with diabetes: a systematic review. Diabetes Metab Res Rev. 2020;36(Suppl 1) doi: 10.1002/dmrr.3270. [DOI] [PubMed] [Google Scholar]
- 7.Lazzarini PA, Jarl G, Gooday C, et al. Effectiveness of offloading interventions to heal foot ulcers in persons with diabetes: a systematic review. Diabetes Metab Res Rev. 2020 36;(Suppl 1) doi: 10.1002/dmrr.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366:1719–1724. doi: 10.1016/S0140-6736(05)67698-2. [DOI] [PubMed] [Google Scholar]
- 9.Alavi A, Sibbald RG, Mayer D, et al. Diabetic foot ulcers: Part I. Pathophysiology and prevention. Journal of the American Academy of Dermatology. 2014;70:1.e1–1e18. doi: 10.1016/j.jaad.2013.06.055. [DOI] [PubMed] [Google Scholar]
- 10.Abbott CA, Chatwin KE, Foden P, et al. Innovative intelligent insole system reduces diabetic foot ulcer recurrence at plantar sites: a prospective, randomised, proof-of-concept study. Lancet Digit Health. 2019;1:e308–e318. doi: 10.1016/S2589-7500(19)30128-1. [DOI] [PubMed] [Google Scholar]
- 11.Caputo GM, Cavanagh PR, Ulbrecht JS, Gibbons GW, Karchmer AW. Assessment and management of foot disease in patients with diabetes. N Engl J Med. 1994;331:854–860. doi: 10.1056/NEJM199409293311307. [DOI] [PubMed] [Google Scholar]
- 12.Boulton AJ, Kubrusly DB, Bowker JH, et al. Impaired vibratory perception and diabetic foot ulceration. Diabet Med. 1986;3:335–337. doi: 10.1111/j.1464-5491.1986.tb00775.x. [DOI] [PubMed] [Google Scholar]
- 13.Monteiro-Soares M, Boyko EJ, Ribeiro J, Ribeiro I, Dinis-Ribeiro M. Predictive factors for diabetic foot ulceration: a systematic review. Diabetes Metab Res Rev. 2012;28:574–600. doi: 10.1002/dmrr.2319. [DOI] [PubMed] [Google Scholar]
- 14.Lavery LA, Higgins KR, Lanctot DR, et al. Home monitoring of foot skin temperatures to prevent ulceration. Diabetes Care. 2004;27:2642–2647. doi: 10.2337/diacare.27.11.2642. [DOI] [PubMed] [Google Scholar]
- 15.Lavery LA, Higgins KR, Lanctot DR, et al. Preventing diabetic foot ulcer recurrence in high-risk patients: use of temperature monitoring as a self-assessment tool. Diabetes Care. 2007;30:14–20. doi: 10.2337/dc06-1600. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong DG, Holtz-Neiderer K, Wendel C, Mohler MJ, Kimbriel HR, Lavery LA. Skin temperature monitoring reduces the risk for diabetic foot ulceration in high-risk patients. Am J Med. 2007;120:1042–1046. doi: 10.1016/j.amjmed.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 17.Bus SA, Aan de Stegge WB, van Baal JG, Busch-Westbroek TE, Nollet F, van Netten JJ. Effectiveness of at-home skin temperature monitoring in reducing the incidence of foot ulcer recurrence in people with diabetes: a multicenter randomized controlled trial (DIATEMP) BMJ Open Diabetes Res Care. 2021;9 doi: 10.1136/bmjdrc-2021-002392. e002392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skafjeld A, Iversen MM, Holme I, Ribu L, Hvaal K, Kilhovd BK. A pilot study testing the feasibility of skin temperature monitoring to reduce recurrent foot ulcers in patients with diabetes—a randomized controlled trial. BMC Endocr Disord. 2015;15 doi: 10.1186/s12902-015-0054-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Netten JJ, Prijs M, van Baal JG, Liu C, van der Heijden F, Bus SA. Diagnostic values for skin temperature assessment to detect diabetes-related foot complications. Diabetes Technol Thes. 2014;16:714–721. doi: 10.1089/dia.2014.0052. [DOI] [PubMed] [Google Scholar]
- 20.Frykberg RG, Gordon IL, Reyzelman AM, et al. Feasibility and efficacy of a smart mat technology to predict development of diabetic plantar ulcers. Diabetes Care. 2017;40:973–980. doi: 10.2337/dc16-2294. [DOI] [PubMed] [Google Scholar]
- 21.Ming A, Walter I, Alhajjar A, Leuckert M, Mertens PR. Study protocol for a randomized controlled trial to test for preventive effects of diabetic foot ulceration by telemedicine that includes sensor-equipped insoles combined with photo documentation. Trials. 2019;20 doi: 10.1186/s13063-019-3623-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peters EJ, Lavery LA International Working Group on the Diabetic Foot. effectiveness of the diabetic foot risk classification system of the International Working Group on the Diabetic Foot. Diabetes Care. 2001;24:1442–1447. doi: 10.2337/diacare.24.8.1442. [DOI] [PubMed] [Google Scholar]
- 23.Bus SA, van Netten JJ, Lavery LA, et al. IWGDF guidance on the prevention of foot ulcers in at-risk patients with diabetes. Diabetes Metab Res Rev. 2016;32(Suppl 1):16–24. doi: 10.1002/dmrr.2696. [DOI] [PubMed] [Google Scholar]
- 24.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–115. [PubMed] [Google Scholar]
- 25.Wagner FW. The dysvascular foot: a system for diagnosis and treatment. Foot Ankle. 1981;2:64–122. doi: 10.1177/107110078100200202. [DOI] [PubMed] [Google Scholar]
- 26.Armstrong DG, Lavery LA, Harkless LB. Validation of a diabetic wound classification system. The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care. 1998;21:855–859. doi: 10.2337/diacare.21.5.855. [DOI] [PubMed] [Google Scholar]
- 27.Tuckson RV, Edmunds M, Hodgkins ML. Telehealth. N Engl J Med. 2017;377:1585–1592. doi: 10.1056/NEJMsr1503323. [DOI] [PubMed] [Google Scholar]
- 28.Dinesen B, Nonnecke B, Lindeman D, et al. Personalized telehealth in the future: a global research agenda. J Med Internet Res. 2016;18 doi: 10.2196/jmir.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dorsey ER, Topol EJ. State of telehealth. N Engl J Med. 2016;375:154–161. doi: 10.1056/NEJMra1601705. [DOI] [PubMed] [Google Scholar]
- 30.Perez MV, Mahaffey KW, Hedlin H, et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. 2019;381:1909–1917. doi: 10.1056/NEJMoa1901183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bayoumy K, Gaber M, Elshafeey A, et al. Smart wearable devices in cardiovascular care: where we are and how to move forward. Nat Rev Cardiol. 2021;18:581–599. doi: 10.1038/s41569-021-00522-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Houghton VJ, Bower VM, Chant DC. Is an increase in skin temperature predictive of neuropathic foot ulceration in people with diabetes? A systematic review and meta-analysis. J Foot Ankle Res. 2013;6 doi: 10.1186/1757-1146-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ena J, Carretero-Gomez J, Arevalo-Lorido JC, Sanchez-Ardila C, Zapatero-Gaviria A, Gómez-Huelgas R. The association between elevated foot skin temperature and the incidence of diabetic foot ulcers: a meta-analysis. Int J Low Extrem Wounds. 2021;20:111–118. doi: 10.1177/1534734619897501. [DOI] [PubMed] [Google Scholar]
- 34.Ruiz-Roso MB, Knott-Torcal C, Matilla-Escalante DC, et al. COVID-19 lockdown and changes of the dietary pattern and physical activity habits in a cohort of patients with type 2 diabetes mellitus. Nutrients. 2020;12 doi: 10.3390/nu12082327. E2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howard DPJ, Banerjee A, Fairhead JF, et al. Population-based study of incidence, risk factors, outcome, and prognosis of ischemic peripheral arterial events: implications for prevention. Circulation. 2015;132:1805–1815. doi: 10.1161/CIRCULATIONAHA.115.016424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conte MS, Bradbury AW, Kolh P, et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J Vasc Surg. 2019;69 doi: 10.1016/j.jvs.2019.02.016. 3S-125S. e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agboola SO, Bates DW, Kvedar JC. Digital health and patient safety. JAMA. 2016;315:1697–1698. doi: 10.1001/jama.2016.2402. [DOI] [PubMed] [Google Scholar]
- 38.Flodgren G, Rachas A, Farmer AJ, Inzitari M, Shepperd S. Interactive telemedicine: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD002098.pub2. CD002098. [DOI] [PMC free article] [PubMed] [Google Scholar]