Abstract
BACKGROUND
Pregnant women infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are more likely to experience preterm birth and their neonates are more likely to be stillborn or admitted to a neonatal unit. The World Health Organization declared in May 2023 an end to the coronavirus disease 2019 (COVID-19) pandemic as a global health emergency. However, pregnant women are still becoming infected with SARS-CoV-2 and there is limited information available regarding the effect of SARS-CoV-2 infection in early pregnancy on pregnancy outcomes.
OBJECTIVE AND RATIONALE
We conducted this systematic review to determine the prevalence of early pregnancy loss in women with SARS-Cov-2 infection and compare the risk to pregnant women without SARS-CoV-2 infection.
SEARCH METHODS
Our systematic review is based on a prospectively registered protocol. The search of PregCov19 consortium was supplemented with an extra electronic search specifically on pregnancy loss in pregnant women infected with SARS-CoV-2 up to 10 March 2023 in PubMed, Google Scholar, and LitCovid. We included retrospective and prospective studies of pregnant women with SARS-CoV-2 infection, provided that they contained information on pregnancy losses in the first and/or second trimester. Primary outcome was miscarriage defined as a pregnancy loss before 20 weeks of gestation, however, studies that reported loss up to 22 or 24 weeks were also included. Additionally, we report on studies that defined the pregnancy loss to occur at the first and/or second trimester of pregnancy without specifying gestational age, and for second trimester miscarriage only when the study presented stillbirths and/or foetal losses separately from miscarriages. Data were stratified into first and second trimester. Secondary outcomes were ectopic pregnancy (any extra-uterine pregnancy), and termination of pregnancy. At least three researchers independently extracted the data and assessed study quality. We calculated odds ratios (OR) and risk differences (RDs) with corresponding 95% CI and pooled the data using random effects meta-analysis. To estimate risk prevalence, we performed meta-analysis on proportions. Heterogeneity was assessed by I2.
OUTCOMES
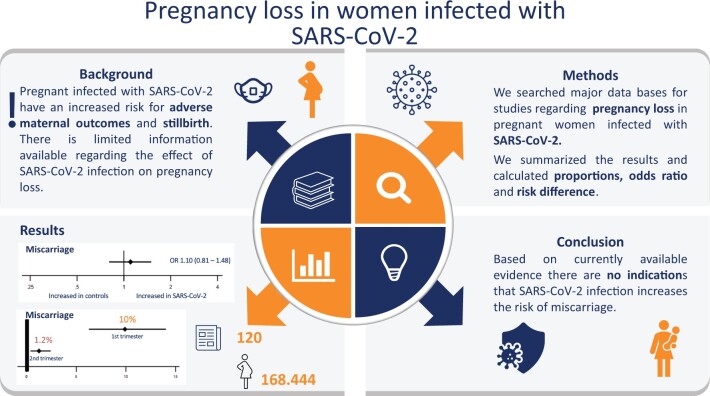
We included 120 studies comprising a total of 168 444 pregnant women with SARS-CoV-2 infection; of which 18 233 women were in their first or second trimester of pregnancy. Evidence level was considered to be of low to moderate certainty, mostly owing to selection bias. We did not find evidence of an association between SARS-CoV-2 infection and miscarriage (OR 1.10, 95% CI 0.81–1.48; I2 = 0.0%; RD 0.0012, 95% CI −0.0103 to 0.0127; I2 = 0%; 9 studies, 4439 women). Miscarriage occurred in 9.9% (95% CI 6.2–14.0%; I2 = 68%; 46 studies, 1797 women) of the women with SARS CoV-2 infection in their first trimester and in 1.2% (95% CI 0.3–2.4%; I2 = 34%; 33 studies; 3159 women) in the second trimester. The proportion of ectopic pregnancies in women with SARS-CoV-2 infection was 1.4% (95% CI 0.02–4.2%; I2 = 66%; 14 studies, 950 women). Termination of pregnancy occurred in 0.6% of the women (95% CI 0.01–1.6%; I2 = 79%; 39 studies; 1166 women).
WIDER IMPLICATIONS
Our study found no indication that SARS-CoV-2 infection in the first or second trimester increases the risk of miscarriages. To provide better risk estimates, well-designed studies are needed that include pregnant women with and without SARS-CoV-2 infection at conception and early pregnancy and consider the association of clinical manifestation and severity of SARS-CoV-2 infection with pregnancy loss, as well as potential confounding factors such as previous pregnancy loss. For clinical practice, pregnant women should still be advised to take precautions to avoid risk of SARS-CoV-2 exposure and receive SARS-CoV-2 vaccination.
Keywords: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2, COVID-19, coronavirus disease 2019, miscarriage, (early) pregnancy loss, ectopic pregnancy, abortion, spontaneous abortion, termination of pregnancy
Graphical abstract
An analysis of studies on the prevalence of early pregnancy loss in women infected with SARS-CoV-2 compared to pregnant women without SARS-CoV-2 infection showed no indications that SARS-CoV-2 infection increases risk of miscarriage. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; OR, odds ratio.
Introduction
Pregnant women infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been shown to be at increased risk for severe coronavirus disease 2019 (COVID-19), with higher rates of pneumonia and respiratory failure, compared to non-pregnant women with SARS-CoV-2 infection. In addition, SARS-CoV-2 infected pregnant women also have an increased risk for adverse pregnancy outcomes compared to pregnant women without SARS-CoV-2 infection (Allotey et al., 2020; Ahmad et al., 2022). However, most data on SARS-CoV-2 infection in pregnancy stems from surveillance or research cohorts primarily including pregnant women infected with SARS-CoV-2 late in pregnancy; there is only limited information available regarding the effect of SARS-CoV-2 infection in early pregnancy and its relation to pregnancy outcome.
The PregCOV19 consortium is an international team of experts that aim to undertake living systematic reviews involving pregnant and postnatal women at risk, suspected, and diagnosed to have SARS-CoV-2 infection, and synthesize the relevant evidence on prevalence, risk factors, mother-to-child transmission, diagnosis, and treatment of the disease. The consortium began a living systematic review and meta-analysis in April 2020 to determine the clinical manifestations of SARS-CoV-2 infection in pregnant women, identify risk factors for complications, and quantify maternal and perinatal outcomes. The review found that pregnant women with SARS-CoV-2 infection are at an increased risk to deliver a stillborn child and to deliver preterm than pregnant women without SARS-CoV-2 infection (Allotey et al., 2020). Furthermore, venous thromboembolism and disseminated intravascular coagulation (DIC) have also been noted more frequently in pregnant women with SARS-CoV-2 infection than those without infection (Al-Samkari et al., 2020; Cruz Melguizo et al., 2021). DIC is a pathological disruption of the process of haemostasis and is a leading cause for maternal mortality, often secondary to underlying maternal/foetal complications, such as placental abruption, amniotic fluid embolism or HELLP (Haemolysis, Elevated Liver enzymes and Low Platelets) syndrome (Erez et al., 2022). Placental abnormalities related to maternal and foetal malperfusion, villous fibrin deposits, foetal vasculopathy, as well as inflammatory alterations have been described with SARS-CoV-2 infection in third trimester of pregnancy, which in some studies has been associated with increased risk of stillbirth (Joshi et al., 2022; Schwartz et al., 2022). The presence of such placental abnormalities associated with SARS-CoV-2 infection in early pregnancy might also result in higher miscarriage rates (Pabinger et al., 2001; Collins et al., 2022). Few studies have evaluated the question of whether SARS-CoV-2 infection in pregnant women during the first or second trimester of pregnancy might lead to pregnancy loss.
Viral infections during pregnancy have been linked with adverse pregnancy outcomes and birth defects (Racicot and Mor, 2017). Particular viruses can infect several cellular components of the placenta, while other viruses can directly infect the foetus at specific times during gestation. This increase in adverse maternal/foetal outcomes can especially be seen during pandemics such as influenza, Ebola, and Lassa fever (Silasi et al., 2015). Moreover, a recent study found that influenza during pregnancy is associated with pregnancy loss at >13 weeks of gestation and decreased infant birthweight, and that the risk of influenza is highest in the first trimester of pregnancy (Dawood et al., 2021). Since influenza and SARS-CoV-2 share immunopathological similarities, SARS-CoV-2 infection in early pregnancy might increase the risk of pregnancy loss as well (Khorramdelazad et al., 2021).
Early pregnancy loss includes miscarriages and ectopic pregnancies (EPs). A miscarriage is generally defined as the spontaneous loss of pregnancy before 20–22 weeks of gestation, though in some countries the definition includes pregnancy loss up to 24 weeks (Prager et al., 2023). Globally, an estimated 23 million miscarriages occur every year, affecting 1 in 10 women in their lifetime (Lancet, 2021). Most miscarriages (80%) occur before 12 weeks of gestation, while late miscarriages (usually between 12 and 20–22 weeks of gestation) are less frequent, with an estimated rate of 1–2% of all pregnancies (Practice Committee of the American Society for Reproductive Medicine, 2012; Dugas and Slane, 2021). EP occurs when the embryo implants outside the uterus, usually in one of the fallopian tubes, and occurs in an estimated 2% of pregnancies. SARS-CoV-2 infection should not affect development of EP, but some studies have described an increased rate of ruptured EP during the COVID-19 pandemic, possibly linked to delayed access to medical care during the pandemic (Casadio et al., 2020; Barg et al., 2021).
Even though the World Health Organization (WHO) has declared an end to the COVID-19 pandemic as a global health emergency, people are still becoming infected with SARS-CoV-2. This systematic review specifically aims to study the prevalence of pregnancy loss in pregnant women with confirmed SARS-CoV-2 infection and whether this differs compared to pregnant women without SARS-CoV-2 infection. We hypothesize that SARS-CoV-2 infection in pregnant women increases the chance of a first or second trimester miscarriage.
Methods
Our systematic review is based on a prospectively registered protocol (PROSPERO CRD42020178076; registered 22 April 2020). For this project, a short separate protocol was developed (https://osf.io/e8dhr/).
Search strategy
We used the PregCOV19 search, as described previously (Yap et al., 2020). Subsequently, an extra electronic literature search was conducted (in duplicate by J.v.B., J.K., M.S., M.V., B.C., E.K., and/or M.v.W.) specifically addressing pregnancy loss in pregnant women with COVID-19 up to 10 March 2023 in the following medical databases: PubMed, Google Scholar, and LitCovid (Supplementary Table S1). Finally, the reference lists of relevant studies were examined to identify additional studies.
Study selection
We screened the queried articles on title and abstract for eligibility. All studies of pregnant women with confirmed or suspected SARS-CoV-2 infection were included, provided that they contained information on pregnancy loss (miscarriage and/or EP) or on termination of pregnancy (TOP). The cases were defined as pregnant women with SARS-CoV-2 infection who had a pregnancy loss preferably before 20 weeks of gestation, however, studies that reported loss up to 22 or 24 weeks were also included. Moreover, we included studies that defined pregnancy loss to occur at the first and/or second trimester of pregnancy without specifying gestational age. The control group was defined as pregnant and postpartum women without SARS-CoV-2 infection. Study groups used cohorts for sequential publications over time. To prevent multiple inclusion of the same data we selected the latest or largest study and excluded the overlapping studies. Studies were also excluded if they were non-peer reviewed papers, review articles, guidelines, and opinion pieces. When an overlap in data was expected, the study with most complete data was included.
Women were defined as having confirmed SARS-CoV-2 infection if they had confirmation through reverse transcription PCT (RT-PCR). Suspected SARS-CoV-2 infection was defined as women with a diagnosis based solely on clinical, serological, and radiological findings. We excluded studies when pregnancy loss data and/or having had SARS-CoV-2 infection was based on self-reporting.
Data extraction and study quality assessment
A structured data-extraction form was used, and data were extracted by multiple reviewers (J.v.B., J.K., M.S., M.V., B.C., E.K., M.v.W.). The data-extraction sheet of the main search from the PregCov group located in Birmingham was shared (J.Z. and S.T.), and all data extracted were cross-checked. We went back to the original studies to recheck the data (by J.v.B., E.K., M.v.W.) in case of discrepancies or missing data. The following study design characteristics were extracted: the first author’s name, setting, year of publication, country of origin, and study design. The documented patient’s characteristics were total number of patients included, number of patients included with confirmed SARS-CoV-2 infection, age of patients in years, BMI in kg/m2, and other information about the patient spectrum including demographic characteristics, type of pregnancy loss, severity of COVID-19-related disease, previous miscarriages, smoking, and the week and/or trimester of gestation the pregnancy loss/termination occurred. The extracted data are also part of an open database (https://cgf.cochrane.org/news/covid-19-coronavirus-disease-fertility-and-pregnancy).
Methodological quality of included comparative cohort studies was assessed using the Newcastle-Ottawa Scale (Wells et al., 2000) for selection, comparability, and outcome ascertainment bias. As described previously (Allotey et al., 2020; Yap et al., 2020), studies achieving four stars for selection, two for comparability, and three for ascertainment of the outcome were considered to have a low risk of bias. Studies achieving two or three stars for selection, one for comparability, and two for outcome ascertainment were considered to have moderate risk of bias, and any study achieving one star for selection or outcome ascertainment, or zero for any of the three domains, was regarded as having a high risk of bias. The quality of prevalence studies was assessed using the validated tool by Hoy et al. (2012). The following domains were considered on risk of bias for external validity: population, sampling frame, selection, and non-response bias. The following domains were assessed on risk of bias for internal validity: data collection, case definition, reliability and validity, and mode of data collection, adequate follow up and appropriate numerator and denominator. The critical appraisal of included studies was carried out by three reviewers (J.v.B., E.K., M.v.W.).
GRADE was used to determine the certainty of the evidence; because all studies were observational the certainty of the evidence was initially set at ‘low’, with the possibility to be down or upgraded.
We excluded studies from the meta-analyses that reported on 10 or less cases, and those with 100% miscarriage in the first or second trimester in view of the selection bias. We excluded studies from the comparative meta-analysis when numerator and/or denominators were unclear and when SARS-CoV-2 infection was based on self-reporting.
Outcomes and definitions
Primary outcome was miscarriage ≤20 weeks of gestation, however, studies that reported pregnancy loss up to 22 or 24 weeks were also included. Additionally, we included studies that defined the pregnancy loss to occur at the first and/or second trimester of pregnancy without specifying gestational age—this was an amendment to the review protocol. Second trimester miscarriages without a clear definition were included only when the study presented stillbirths and/or foetal losses separately from miscarriages. Miscarriage was stratified for gestational age (first trimester miscarriage up to 12 weeks of gestation and second trimester miscarriage above 12 weeks of gestation).
Secondary outcomes were EP, defined as any extra-uterine pregnancy, TOP, defined as an induced abortion, and recurrent miscarriage, defined as a spontaneous miscarriage after two previous spontaneous miscarriages. We evaluated EP for completeness as early pregnancy loss includes EP. We evaluated TOP/induced abortions to ensure induced abortions were distinguished from spontaneous abortions.
Statistical analysis
For studies comparing dichotomous outcomes in pregnant women with and without SARS-CoV-2 infection, we calculated odds ratios (OR) and risk differences (RDs) with corresponding 95% CI and pooled the data using random effects meta-analysis. To estimate the rate of miscarriage, EP, and TOP, we pooled proportions with 95% CI using DerSimonian and Laird random effects meta-analysis after transforming the data using Freeman–Tukey double arcsin transformation. Statistical heterogeneity between studies was reported as I2 statistics, I2 > 50% representing substantial heterogeneity. The impact of heterogeneity on pooled results was evaluated by calculating predictive intervals. We used the Metan and Metaprop routine in STATA for the analyses (StataCorp, 2019, Stata Statistical Software: Release 16, College Station, TX, USA: StataCorp LLC).
We aimed to describe miscarriage before 20 weeks of gestation per registered first and second trimester pregnancy and stratified for time of gestation (before and after 12 weeks of gestation). The study specific and summarized effect measures were calculated using a random effect model. When available, recurrent pregnancy loss was summarized as prevalence and OR with 95% CI, as described above.
For the prevalence estimates, we performed subgroup analyses per year of publication (2020, 2021, and 2022), per study region according to geographic World Bank regions (https://www.worldbank.org/), and on the basis of the study size (below or at least 20) to evaluate small study bias. A generalized linear mixed model (GLMM) instead of Freeman–Tukey double arcsin transformation was used as sensitivity analysis for the primary outcomes and GLMM was applied when zero counts resulted in inconsistencies in the estimates.
Patient and public involvement
There was no patient or public involvement in the design and reporting of the present review.
Results
Search results
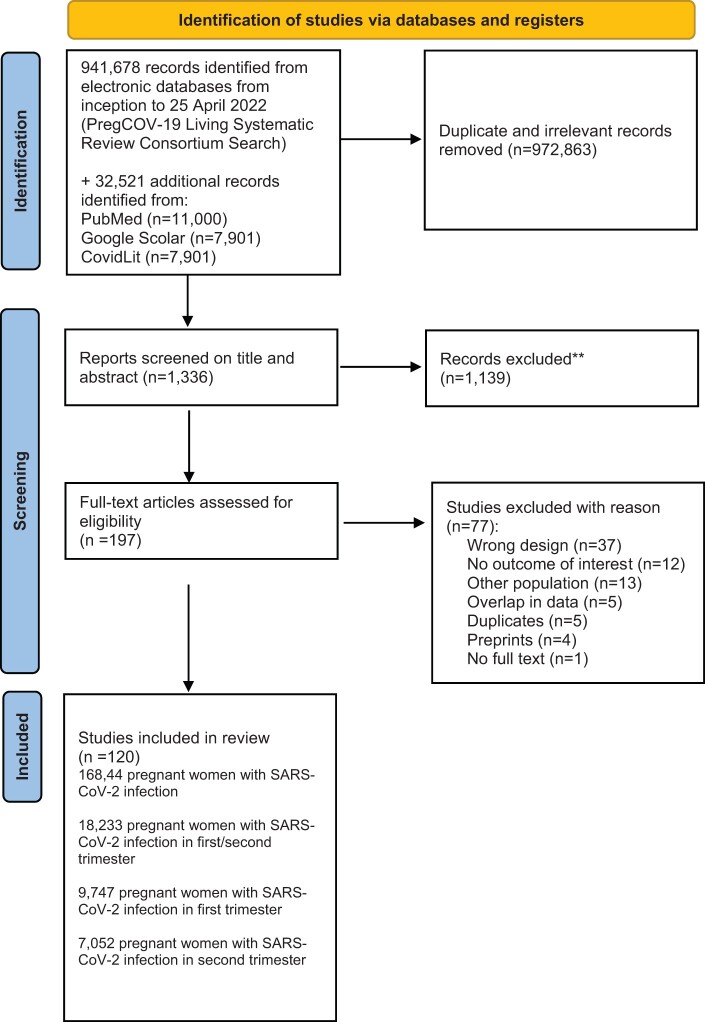
A total of 941 678 citations were identified after screening electronic databases from inception to 25 April 2022 (PregCOV-19 Living Systematic Review Consortium search). Subsequently, with the additional electronic search from inception to 10 March 2023, conducted to specifically address pregnancy loss, we identified an additional 32 521 studies. The databases we used for the extra search were PubMed, Google Scholar, and the LitCovid Database. After the removal of duplicates and irrelevant articles, 1336 studies remained for screening; 1139 studies were excluded based on screening titles and abstracts. After screening the remaining 197 full text articles, we excluded 77 studies (Fig. 1).
Figure 1.
PRISMA flowchart of selection of studies for a systematic review and meta-analysis on the risk and prevalence of pregnancy loss in pregnant women infected with SARS-CoV-2. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Finally, we included 120 studies comprising a total of 168 444 pregnant women registered with SARS-CoV-2 infection in first or second trimester of pregnancy (Adhikari et al., 2020; Ayed et al., 2020; Calderón et al., 2020; Chen et al., 2020, 2021; Curi et al., 2020; Delahoy et al., 2020; Diouf et al., 2020; Edlow et al., 2020; Emeruwa et al., 2020; Fox and Melka, 2020; Grechukhina et al., 2020; Hernández et al., 2020, 2023; Kayem et al., 2020; Lei et al., 2020; London et al., 2020; Mattar et al., 2020; Metkari and Palve, 2020; Nambiar et al., 2020; Omrani et al., 2020; Pirjani et al., 2020; Qiancheng et al., 2020; Sentilhes et al., 2020; Shah et al., 2020; Shmakov et al., 2022; Tug et al., 2020; Woodworth et al., 2020; Wu et al., 2020a; Yan et al., 2020; Crovetto et al., 2021; Damar Çakırca et al., 2021, 2022; D’Antonio et al., 2021; Deng et al., 2021; Devi et al., 2021; Donati et al., 2021; Erol et al., 2021; Fan et al., 2021; Foo et al., 2021; Gajbhiye et al., 2021; Guo et al., 2021; Hazari et al., 2021; Hcini et al., 2021; Jacoby et al., 2021; Jang et al., 2021; Kuzan et al., 2021; Lokken et al., 2021; Mahajan et al., 2021; Manasova et al., 2021; Metz et al., 2021; Mullins et al., 2021; Overtoom etal., 2022; Poon et al., 2021; Priyadharshini et al., 2021; Aabakke et al., 2021, 2023; Abedzadeh-Kalahroudi et al., 2021; Ajith et al., 2021; Akram et al., 2021; Anand et al., 2021; Arinkan et al., 2021; Basu et al., 2021; Burwick et al., 2021; Cardona-Pérez et al., 2021; Chaudhary et al., 2021; Saimin et al., 2021; Santhosh et al., 2021; Singh et al., 2021; Taghavi et al., 2021; Tanacan et al., 2021; Vizheh et al., 2021; Vouga et al., 2021; Vousden et al., 2021; Yassa et al., 2021; Yazihan et al., 2021; Ahmad et al., 2022; Al-Hajjar et al., 2022; Arakaki et al., 2022; Babic et al., 2022; Balachandren et al., 2022; Barris et al., 2022; Bhoora et al., 2022; Borges-Charepe et al., 2022; Cambou et al., 2023; Chung et al., 2022; Collins et al., 2022; Cosma et al., 2022; Daclin et al., 2022; Fallach et al., 2022; Grandone et al., 2022; Hamadneh et al., 2022; Haye et al., 2022; Khoiwal et al., 2022; Kiremitli et al., 2022; Kumari et al., 2022; Martínez-Varea et al., 2022; McCreary et al., 2022; Neelam et al., 2023; Péju et al., 2022; Qudsieh et al., 2022; Regan et al., 2022; Rozo et al., 2022; Sahin et al., 2022a,b; Santos et al., 2022; Schell et al., 2022; Sekkarie et al., 2022; Souza et al., 2022; Sunder et al., 2022; Takemoto et al., 2022; Zelini et al., 2022; Ziert et al., 2022; Göklü et al., 2023; Hughes et al., 2023; Martinez-Baladejo et al., 2023; Poisson et al., 2023; Sertel and Demir, 2023; Tavakoli et al., 2023; Youssef et al., 2023). For studies with overlapping populations, we always selected the last most updated studies. In two cases both studies were included: Woodworth et al. (2020) had details on number of women in first and second trimester pregnancy that were lacking in Neelam et al. (2023); and Aabakke et al. (2021) had details on number of EP, but for all other outcomes we included the updated study (Aabakke et al., 2023).
Of the 120 included studies, 20 studies were cohort studies with a non-infected control group (Adhikari et al., 2020; Edlow et al., 2020; Pirjani et al., 2020; Cardona-Pérez et al., 2021; Crovetto et al., 2021; Donati et al., 2021; Erol et al., 2021; Gajbhiye et al., 2021; Jacoby et al., 2021; Metz et al., 2021; Taghavi et al., 2021; Tanacan et al., 2021; Yazihan et al., 2021; Balachandren et al., 2022; Cosma et al., 2022; Daclin et al., 2022; Fallach et al., 2022; Khoiwal et al., 2022; Souza et al., 2022; Sunder et al., 2022).
The details of the selection and review process are provided in Fig. 1.
Risk of bias assessment of included studies
Supplementary Tables S2 and S3 illustrate the results of the risk of bias assessment. Overall, half of the prevalence studies were judged to be at moderate risk of bias (60/120), 39% were judged to be at low risk of bias (47/120), and 13 studies were judged to be of high risk of bias (13/120). In addition, 39% of cohort studies were judged to be at high risk of bias (7/18), 28% were judged to be at low risk of bias (5/18), and 33% were judged to be at medium risk of bias (6/18).
The following domains were considered as low risk of bias for external validity: representative of national population for relevant variables (population), representative of target population (sampling frame), some form of random selection was used to select the sample (selection bias), and more than 75% response rate in individuals with and without the outcome (non-response bias). The following domains were considered as low risk of bias for internal validity: all data were collected directly from the subjects, an acceptable case definition was used, the study instrument that measured the parameter of interest showed to have reliability and validity, the same mode of data collection was used for all subjects, the length of the shortest prevalence period of the parameter was appropriate and the paper provided appropriate numerators and denominators for the parameter of interest (Hoy et al., 2012).
Characteristics of included studies
The 120 included studies assessed 168 444 pregnant women with SARS-CoV-2 infection. Most studies were from the USA (23), Turkey (13), India (13), China (9), Iran (5), Italy (4), France (4), with five studies performed in multiple countries. The majority of the women diagnosed with SARS-CoV-2 were in their third trimester of pregnancy, with 18 233 women in their first or second trimester of pregnancy.
Most studies confirmed diagnosis on the basis of SARS-CoV-2 RT-PCR (105/120); 15 studies tested for SARS-CoV-2 using either RT-PCR or antibodies to confirm the presence of SARS-CoV-2; one study confirmed a COVID-19 disease diagnosis through reports in the official COVID-19 test surveillance system of Brazil (Takemoto et al., 2022) and one study confirmed diagnosis based on clinical signs (Curi et al., 2020).
The average age ranged from 23 to 36 years in women with SARS-CoV-2 infection and from 27 to 34 years in the control group without SARS-CoV-2 infection. One study specifically reported on an infertile population that conceived following fertility treatment (Cousins, 2020).
The average BMI ranged from 23 to 33 kg/m2 in women with SARS-CoV-2 infection. Smoking was reported in 23 studies (Delahoy et al., 2020; Grechukhina et al., 2020; Kayem et al., 2020; WAPM, 2021; Aabakke et al., 2021; Cardona-Pérez et al., 2021; Crovetto et al., 2021; D’Antonio et al., 2021; Jacoby et al., 2021; Metz et al., 2021; Overtoom etal., 2022; Vouga et al., 2021; Vousden et al., 2021; Balachandren et al., 2022; Cosma et al., 2022; Daclin et al., 2022; Göklü et al., 2023; Hamadneh et al., 2022; Martínez-Varea et al., 2022; Hughes et al., 2023; Poisson et al., 2023; Souza et al., 2022; Martinez-Baladejo et al., 2023). One study reported on smoking marijuana (Edlow et al., 2020). Moreover, previous pregnancy loss was reported in two studies (Cosma et al., 2022; Sahin et al., 2022b). Characteristics of included studies are described in Supplementary Table S4.
The definition of miscarriage differed between studies and is reported in Supplementary Table S5. Seven studies defined miscarriage as pregnancy loss up to 24 weeks (Calderón et al., 2020; Emeruwa et al., 2020; Wu et al., 2020a; Akram et al., 2021; Poisson et al., 2023; Regan et al., 2022; Sertel and Demir, 2023).
One study provided mean gestational age for pregnancy loss in the second trimester (Schell et al., 2022). Forty-six studies did not provide a definition of miscarriage (Adhikari et al., 2020; Chen et al., 2020; Curi et al., 2020; Edlow et al., 2020; Emeruwa et al., 2020; Hernández et al., 2020; Sentilhes et al., 2020; Martinez-Baladejo et al., 2023; Metkari and Palve, 2020; Omrani et al., 2020; Pirjani et al., 2020; Ajith et al., 2021; Anand et al., 2021; Abedzadeh-Kalahroudi et al., 2021; Basu et al., 2021; Cardona-Pérez et al., 2021; Chaudhary et al., 2021; Crovetto et al., 2021; Donati et al., 2021; Guo et al., 2021; Jang et al., 2021; Saimin et al., 2021; Mahajan et al., 2021; Mullins et al., 2021; Overtoom etal., 2022; Poon et al., 2021; Priyadharshini et al., 2021; Tanacan et al., 2021; Yazihan et al., 2021; Vizheh et al., 2021; Vousden et al., 2021; Ahmad et al., 2022; Arakaki et al., 2022; Babic et al., 2022; Chung et al., 2022; Cosma et al., 2022; Daclin et al., 2022; Hamadneh et al., 2022; Sahin et al., 2022a; Souza et al., 2022; Zelini et al., 2022; Qudsieh et al., 2022; Regan et al., 2022; Santos et al., 2022; Ziert et al., 2022; Youssef et al., 2023).
Miscarriage in women with SARS-CoV-2 infection versus non-infected controls
Eleven studies reported on miscarriage in the first or second trimester of pregnancy and had data on number of women in their first or second trimester of pregnancy with SARS-CoV-2 infection versus non-infected pregnant controls (Edlow et al., 2020; Crovetto et al., 2021; Donati et al., 2021; Erol et al., 2021; Taghavi et al., 2021; Tanacan et al., 2021; Yazihan et al., 2021; Balachandren et al., 2022; Cosma et al., 2022; Fallach et al., 2022; Souza et al., 2022). In the women with SARS-CoV-2 infection the average age was 30.4 (SD 3.0) years, and the average BMI was 25.3 kg/m2 (SD 4.7) versus 27.3 (SD 3.5) years and 25.0 kg/m2 (SD 5.1), respectively, in non-infected controls. Two studies were excluded from the meta-analysis: one study because presence of SARS-CoV-2 infection was based on self-reporting, which was an exclusion criterium (Balachandren et al., 2022), and the other study because miscarriage was only mentioned in the flowchart with unclear data in the control group (Donati et al., 2021).
Of the nine included studies, four studies were judged to have a low risk of bias (Crovetto et al., 2021; Tanacan et al., 2021; Yazihan et al., 2021; Fallach et al., 2022), two studies had a moderate risk of bias (Taghavi et al., 2021; Souza et al., 2022), and three a high risk of bias (Edlow et al., 2020; Erol et al., 2021; Cosma et al., 2022) (Supplementary Table S2).
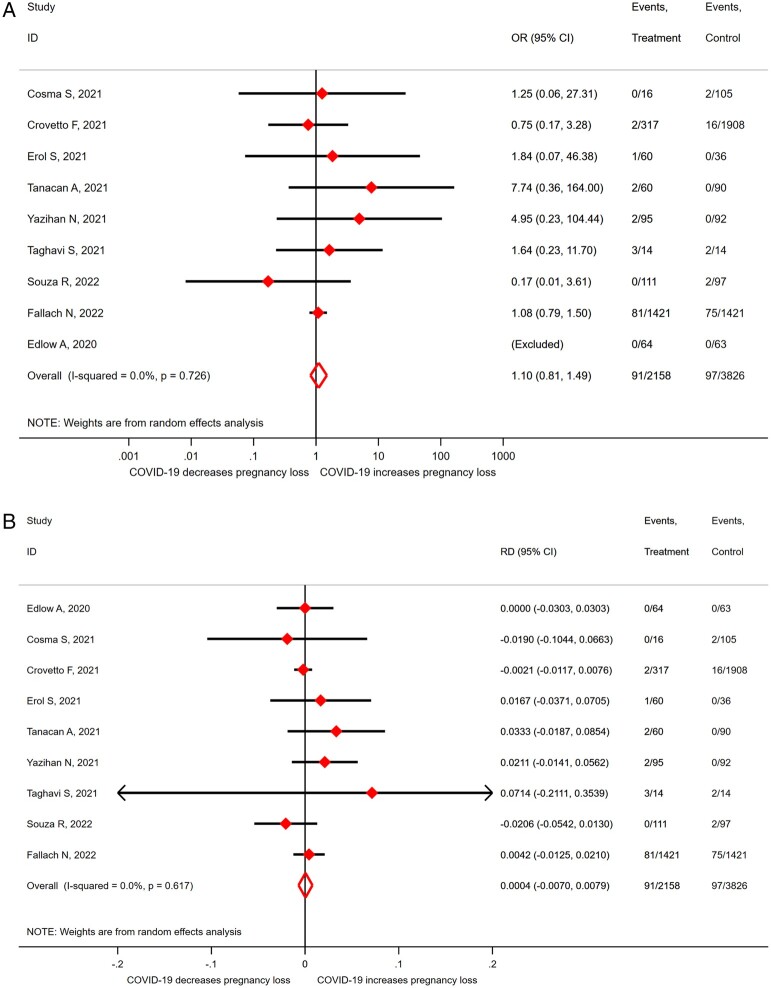
The OR for miscarriage in SARS-CoV-2 infected women versus non-infected controls was 1.10 (95% CI 0.81–1.49; I2 = 0.0%; 9 studies, 5984 women; moderate quality evidence; Fig. 2a). This corresponds to an RD of 0.0004 (95% CI −0.0070 to 0.0079; I2 = 0.0%; 9 studies, 5984 women; Fig. 2b). No stratified analyses were performed in view of the limited number of studies and lack of heterogeneity (I2 = 0). The majority of women were likely at the end of the first trimester; given a miscarriage rate of 10% in uninfected controls, the risk of a miscarriage in women with SARS-CoV-2 infection is between 9.3% and 10.8%.
Figure 2.
Odds ratio (A) and risk difference (B) for miscarriage in pregnant women with SARS-CoV-2 versus without-SARS-CoV-2 infection. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; COVID-19, coronavirus disease 2019; OR, odds ratio; RD, risk difference.
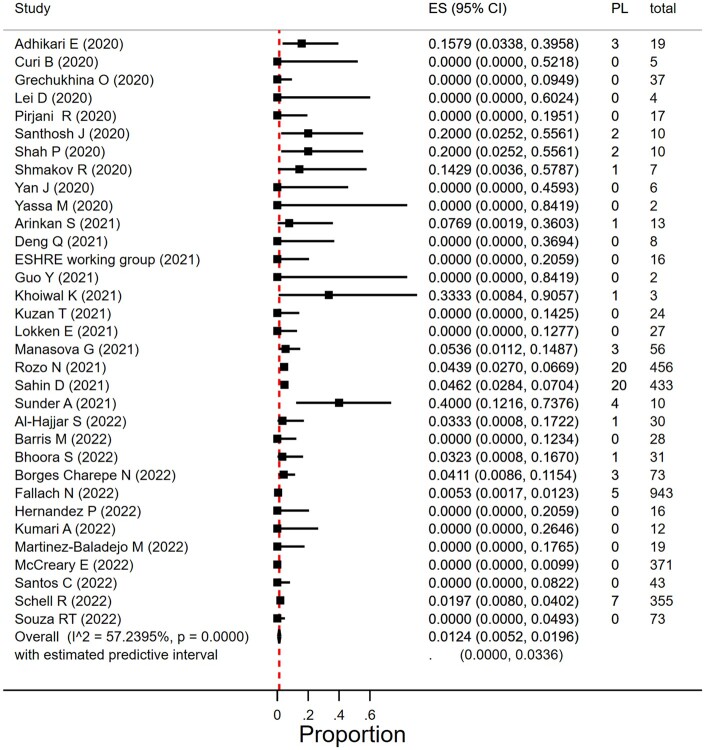
Proportion of first trimester miscarriage in women with SARS-CoV-2 infection
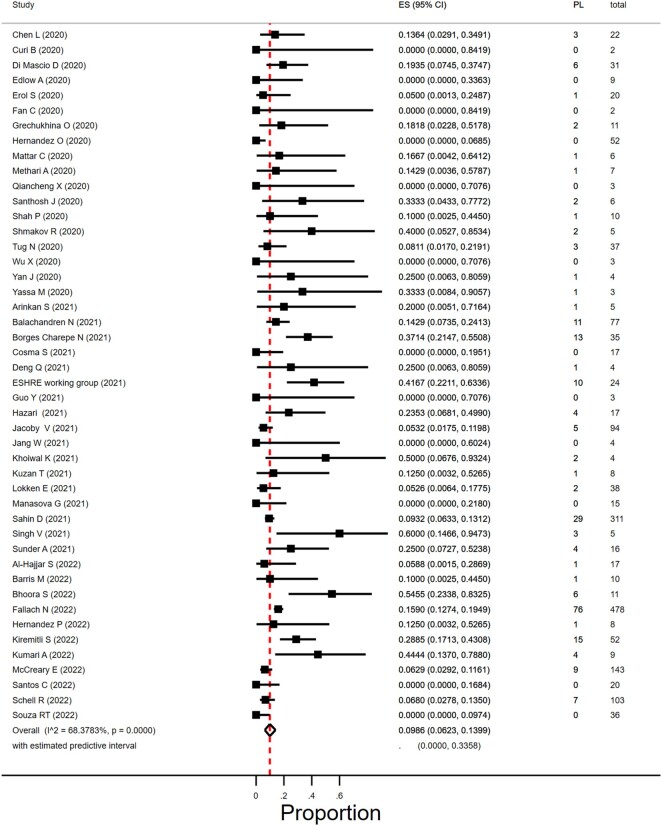
Miscarriage occurring in the first trimester was reported by 46 studies that included 1797 women diagnosed with SARS-CoV-2 infection in the first trimester (Curi et al., 2020; Edlow et al., 2020; Erol et al., 2021; Hernández et al., 2020, 2023; Grechukhina et al., 2020; Mattar et al., 2020; Metkari and Palve, 2020; Qiancheng et al., 2020; Shah et al., 2020; Tug et al., 2020; Wu et al., 2020a; Yan et al., 2020; Arinkan et al., 2021; Chen et al., 2021; Deng et al., 2021; ESHRE Working Group, 2021; Fan et al., 2021; Hazari et al., 2021; Guo et al., 2021; Jacoby et al., 2021; Jang et al., 2021; Kuzan et al., 2021; Lokken et al., 2021; Manasova et al., 2021; Santhosh et al., 2021; Singh et al., 2021; Yassa et al., 2021; WAPM, 2021; Al-Hajjar et al., 2022; Balachandren et al., 2022; Barris et al., 2022; Bhoora et al., 2022; Borges-Charepe et al., 2022; Cosma et al., 2022; Fallach et al., 2022; Khoiwal et al., 2022; Kiremitli et al., 2022; Kumari et al., 2022; McCreary et al., 2022; Sahin et al., 2022b; Santos et al., 2022; Schell et al., 2022; Shmakov et al., 2022; Souza et al., 2022; Sunder et al., 2022).
In women with SARS-CoV-2 infection in their first trimester, the miscarriage rate was 9.9% (95% CI 6.2–14.0%; I2 = 68.4%; 46 studies, 1797 women; Fig. 3). There was substantial heterogeneity in the reported miscarriage estimates. The heterogeneity could partly be explained by differences in geographical region; in five studies from South Asia with 10 or less women in their first trimester of pregnancy, the miscarriage prevalence was between 10% and 60%. Subgroup analyses according to study size and year of publication resulted in overlapping estimates. Table 1 reports on overall, sensitivity, and subgroup results.
Figure 3.
Proportion of miscarriage in pregnant women with SARS-CoV-2 in the first trimester. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ES, estimate of proportion; PL, pregnancy loss.
Table 1.
Sensitivity and subgroup analyses for miscarriage in pregnant women with SARS-CoV-2 infection.
| Study N | Prevalence (95% CI) | I2 | Subgroup heterogeneity | |
|---|---|---|---|---|
| First trimester miscarriage | 46 | 9.86% (6.23–13.99) | 68.4% | |
| Sensitivity analysis with GLMM | 10.02% (6.71–14.51) | |||
| Sensitivity analysis fixed effect | 7.85% (6.32–9.50) | |||
| Sensitivity analysis with CC (0.5) | 10.77% (7.69–13.85) | |||
| Predictive interval | 0–33.6% | |||
| By World Bank region | P = 0.0003 | |||
| North America | 8 | 6.24% (3.61–8.87)* | 0.0% | |
| East Asia and Pacific | 9 | 7.13% (0.02–19.27) | 0.0% | |
| Europe and West Asia | 13 | 13.98% (6.71–22.83) | 75.6% | |
| Middle East and North Africa | 5 | 14.70% (10.25–19.67) | 13.6% | |
| South Asia | 5 | 30.58% (11.70–52.66) | 38.4% | |
| World, other regions | 6 | 6.90% (0.00–22.83) | 85.4% | |
| By study size, no of patients | P = 0.23 | |||
| <20 | 33 | 10.93 (4.91–16.94) | 50.0% | |
| ≥20 | 13 | 9.73 (5.07–14.39) | 74.1% | |
| By publication year | P = 0.80 | |||
| 2020 | 18 | 6.86 (1.56–14.30) | 40.7% | |
| 2021 | 17 | 12.81 (6.24–20.76) | 71.8% | |
| 2022 | 11 | 11.01 (4.84–18.83) | 82.0% | |
| Second trimester miscarriage | 33 | 1.24% (0.38–2.42) | 58.8% | |
| Sensitivity analysis with GLMM | 1.16% (0.73–2.43) | |||
| Sensitivity analysis fixed effect | 0.57% (0.28–0.87) | |||
| Sensitivity analysis with CC (0.5) | 1.58% (0.73–2.43) | |||
| Predictive interval | 0–3.8% | |||
| By WB region | P = 0.24 | |||
| North America | 7 | 0.69% (0.00–1.99) | 69.9% | |
| East Asia and Pacific | 5 | 0.00% (0.00–5.22) | 0% | |
| Europe and West Asia | 8 | 1.12% (0.20–2.84) | 0% | |
| Middle East and North Africa | 4 | 8.75% (0.00–29.98) | 72.5% | |
| Latin-America and Caribbean | 4 | 0.77% (0.00–4.08) | 67.2% | |
| South Asia | 4 | 3.83% (0.00–22.17) | 54.6% | |
| World, other regions | 1 | 3.23% (0.08–16.70) | ||
| By study size, no of patients | P = 0.004 | |||
| <20 | 18 | 2.91 (0.00–8.60) | 31.3% | |
| ≥20 | 15 | 1.13 (0.18–2.60) | 78.1% | |
| By publication year | P = 0.09 | |||
| 2020 | 10 | 2.87 (0.00–10.95) | 37.0% | |
| 2021 | 11 | 1.13 (0.00–3.68) | 40.7% | |
| 2022 | 12 | 0.45 (0.00–1.96)* | 46.7% |
Unstable proportion owing to incorrect back transformation, recalculated with GLMM.
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; GLMM, generalized linear mixed models; CC, continuity correction.
Proportion of second trimester miscarriage in women with SARS-CoV-2 infection
In 33 studies, the proportion of second trimester miscarriage could be retrieved (Adhikari et al., 2020; Curi et al., 2020; Grechukhina et al., 2020; Lei et al., 2020; Pirjani et al., 2020; Shah et al., 2020; Santhosh et al., 2021; Yan et al., 2020; Arinkan et al., 2021; Deng et al., 2021; ESHRE Working Group, 2021; Guo et al., 2021; Kuzan et al., 2021; Lokken et al., 2021; Manasova et al., 2021; Sunder et al., 2022; Yassa et al., 2021; Al-Hajjar et al., 2022; Barris et al., 2022; Bhoora et al., 2022; Borges-Charepe et al., 2022; Fallach et al., 2022; Khoiwal et al., 2022; Kumari et al., 2022; McCreary et al., 2022; Rozo et al., 2022; Sahin et al., 2022b; Santos et al., 2022; Schell et al., 2022; Shmakov et al., 2022; Souza et al., 2022; Hernández et al., 2023; Martinez-Baladejo et al., 2023). Second trimester miscarriage was usually defined as loss before 20 or 22 weeks. Two studies that did not provide a definition were also included as number of women in their second trimester and number of miscarriages during second trimester were provided (Curi et al., 2020; Souza et al., 2022).
The miscarriage rate in women with SARS-CoV-2 infection during the second trimester was 1.2% (95% CI 0.5–2.0%; I2 = 57%; 33 studies; 3159 women; Fig. 4 and Table 1). There was moderate heterogeneity in the reported miscarriage estimates between the studies. The proportion of second trimester miscarriages seemed to vary by size of the included study (P = 0.004) and was 2.9% (95% CI 0.0–8.6%) in studies that included <20 women and 1.1% (95% CI 0.2–2.6%) in studies with at least 20 women (Table 1). Subgroup analyses according to year of publication and geographical region resulted in overlapping estimates (respectively, P = 0.09 and P = 0.24) (Table 1).
Figure 4.
Proportion of miscarriage in pregnant women with SARS-CoV-2 infection in the second trimester. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ES, estimate of proportion; PL, pregnancy loss.
Additional analysis to estimate the prevalence of miscarriage in women with SARS-CoV-2 infection
In 42 studies, the number of miscarriages and number of women with SARS-CoV-2 infection and number of women in either first or second trimester was reported, but miscarriage rates could not be extracted for the first and second trimester separately. Supplementary Figure S1 provides an overall average for all studies that reported on miscarriage per number of women in either first or second trimester pregnancies. For the studies with incomplete trimester information the miscarriage rate in women that became infected at any time during their first or second trimester of pregnancy was 5.7% (95% CI 3.8–79%; I2 = 92%; 42 studies, 2909 women), and in the studies with trimester information 4.2% (95% 2.6–6.2%; I2 = 77%), leading to a total average of 4.9% (95% CI 3.6–6.4%; I2 = 88%; 92 studies). When assuming an equal distribution of women over the first and second trimester, the overall expected total chance to have a miscarriage would be twice these estimates; on basis of the calculated total average (95% CI of 3.61–6.44) this would be between 7.2% and 12.9%. This is in line with the sum of our reported first and second trimester estimates for miscarriage rate of 9.9% and 1.2%, respectively.
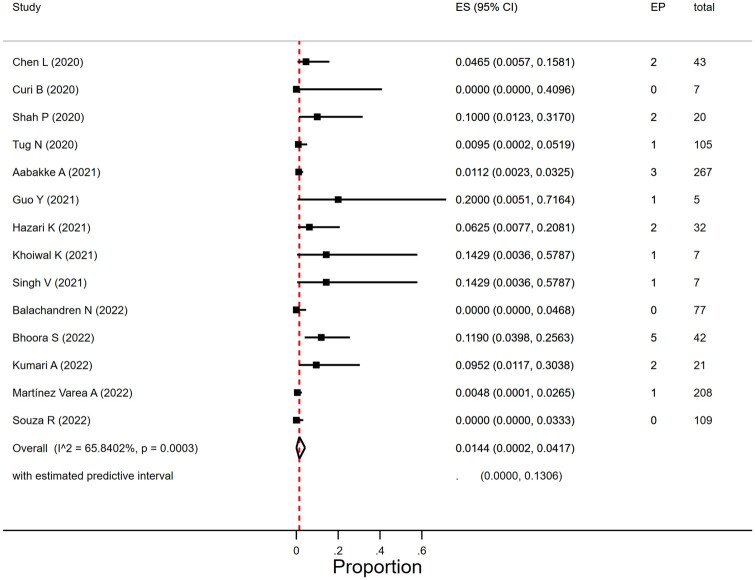
Proportion of ectopic pregnancy in women with SARS-CoV-2 infection
We found 18 studies that reported on EP (Calderón et al., 2020; Chen et al., 2020; Curi et al., 2020; Metkari and Palve, 2020; Shah et al., 2020; Tug et al., 2020; Aabakke et al., 2021; Devi et al., 2021; Gajbhiye et al., 2021; Guo et al., 2021; Hazari et al., 2021; Singh et al., 2021; Balachandren et al., 2022; Bhoora et al., 2022; Khoiwal et al., 2022; Kumari et al., 2022; Martínez-Varea et al., 2022; Souza et al., 2022) but only for 15 studies could the number of women that were included in their first or second trimester of pregnancy be retrieved (Chen et al., 2020; Curi et al., 2020; Metkari and Palve, 2020; Shah et al., 2020; Tug et al., 2020; Aabakke et al., 2021; Guo et al., 2021; Hazari et al., 2021; Singh et al., 2021; Balachandren et al., 2022; Bhoora et al., 2022; Khoiwal et al., 2022; Kumari et al., 2022; Martínez-Varea et al., 2022; Souza et al., 2022). One study was excluded in view of an unexpectedly high number of EP suggesting extreme selection bias (four cases in seven women in their first trimester) (Metkari and Palve, 2020).
The EP rate in women with SARS-CoV-2 infection was 1.4% (95% CI 0.02–4.2%; I2 = 65.8%; 14 studies, 950 women; Fig. 5). The heterogeneity was moderate to severe. In view of the limited number of studies we did not do subgroup analyses per geographical region on EP rate. The proportion of EP varied by size of study (P = 0.01) and was 1.0% (95% CI 0.0–3.4%) in 10 studies that included more than 20 women and 9.4% (95% CI 0.08–26.4) in studies with <20 women. Year of publication (2020, 2021, and 2022) did not affect EP rates (P = 0.41).
Figure 5.
Proportion of ectopic pregnancies in women with SARS-CoV-2 infection in the first or second trimester. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; EP, ectopic pregnancy; ES, estimate of proportion.
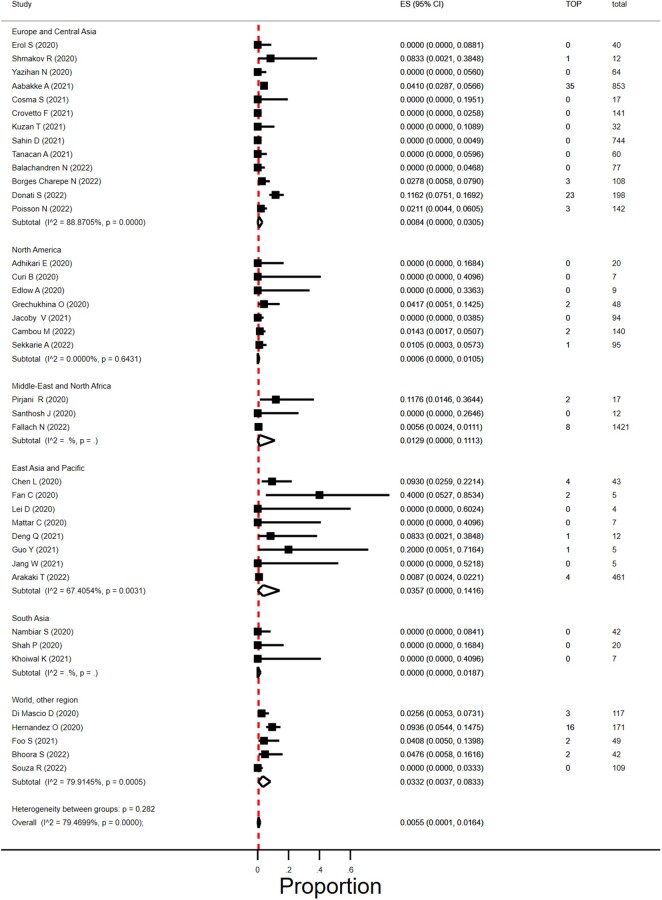
Proportion of termination of pregnancy in women with SARS-CoV-2 infection
We found 49 studies that reported on TOP (Adhikari et al., 2020; Ayed et al., 2020; Chen et al., 2020; Curi et al., 2020; Edlow et al., 2020; Grechukhina et al., 2020; Hernández et al., 2020; Lei et al., 2020; London et al., 2020; Mattar et al., 2020; Nambiar et al., 2020; Pirjani et al., 2020; Qiancheng et al., 2020; Sentilhes et al., 2020; Shah et al., 2020; Wu et al., 2020a; Anand et al., 2021; Arinkan et al., 2021; Crovetto et al., 2021; Deng et al., 2021; Devi et al., 2021; Donati et al., 2021; Erol et al., 2021; Fan et al., 2021; Foo et al., 2021; Gajbhiye et al., 2021; Guo et al., 2021; Hcini et al., 2021; Jacoby et al., 2021; Jang et al., 2021; Kuzan et al., 2021; Mahajan et al., 2021; Santhosh et al., 2021; Tanacan et al., 2021; Vouga et al., 2021; Yazihan et al., 2021; Arakaki et al., 2022; Balachandren et al., 2022; Bhoora et al., 2022; Borges-Charepe et al., 2022; Cambou et al., 2023; Cosma et al., 2022; Fallach et al., 2022; Khoiwal et al., 2022; Poisson et al., 2023; Sahin et al., 2022b; Sekkarie et al., 2022; Shmakov et al., 2022; Souza et al., 2022; Aabakke et al., 2023) and, of these, 43 studies reported on the number of women in their first and/or second trimester of pregnancy (Adhikari et al., 2020; Ayed et al., 2020; Chen et al., 2020; Curi et al., 2020; Edlow et al., 2020; Grechukhina et al., 2020; Hernández et al., 2020; Lei et al., 2020; London et al., 2020; Mattar et al., 2020; Nambiar et al., 2020; Pirjani et al., 2020; Qiancheng et al., 2020; Sentilhes et al., 2020; Shah et al., 2020; Wu et al., 2020a; Crovetto et al., 2021; Deng et al., 2021; Donati et al., 2021; Erol et al., 2021; Fan et al., 2021; Foo et al., 2021; Guo et al., 2021; Jacoby et al., 2021; Jang et al., 2021; Kuzan et al., 2021; Santhosh et al., 2021; Tanacan et al., 2021; Yazihan et al., 2021; Arinkan et al., 2021; Arakaki et al., 2022; Balachandren et al., 2022; Bhoora et al., 2022; Borges-Charepe et al., 2022; Cambou et al., 2023; Cosma et al., 2022; Fallach et al., 2022; Khoiwal et al., 2022; Sahin et al., 2022b; Sekkarie et al., 2022; Shmakov et al., 2022; Souza et al., 2022; Aabakke et al., 2023; Poisson et al., 2023). Four studies were excluded, two studies as only one woman was in her first or second trimester of pregnancy (London et al., 2020; Sentilhes et al., 2020) and two studies as all of the women registered with an infection in their first or second trimester of pregnancies were TOP pregnancies (Qiancheng et al., 2020; Wu et al., 2020a). The overall proportion of termination in SARS-CoV-2 infected pregnancies was 0.6% (95% CI 0.01–1.6%; I2 = 79%; 39 studies; 1166 women; Fig. 6). Reason for TOP was only provided in a few early studies; in one early Chinese study two women asked for TOP owing to fear of SARS-CoV-2 effect on the pregnancy (Fan et al., 2021); in two studies TOP was medically indicated (Donati et al., 2021; Fan et al., 2021).
Figure 6.
Proportion of termination of pregnancies in women with SARS-CoV-2 infection in first or second trimester in different geographical regions. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TOP, termination of pregnancy; ES, estimate of proportion.
The proportion of TOP seemed to vary by size of the included study (P = 0.005) and was 2.5% (95% CI 0.01–7.6%) in studies that included <20 women and 1.3% (95% CI 0.39–2.5%) in studies with at least 20 women. Geographical regions did not differ in TOP rates (P = 0.28), nor did year of publication (2020, 2021, and 2022) (P = 0.28).
Discussion
Findings in context
In this systematic review, we found no evidence for an association of SARS-CoV-2 infection in early pregnancy with pregnancy loss. We found that pregnant women in the first or second trimester of pregnancy with SARS-CoV-2 infection were not at increased risk for a miscarriage compared to pregnant women without the infection. On average, first trimester miscarriage occurred in 1 in 10 pregnant women (10%) with SARS-CoV-2 infection and second trimester miscarriage in 12 of 1000 pregnant women (1.2%) with SARS-CoV-2 infection. These data suggest an overall miscarriage rate of 11%. This is in line with the estimate of the studies for which miscarriage rate per first and second trimester could not be extracted separately. These miscarriage rates also correspond to what would be expected in women without SARS-CoV-2 infection.
We found EP to occur in of 1.4% in early pregnancies. This compares to the overall rate of EP of 1–2% found in the general population (Panelli et al., 2015). The average proportion of TOP in women with SARS-CoV-2 infection was 0.6%.
Prevalence estimates showed substantial heterogeneity for first trimester miscarriage and moderate heterogeneity for second trimester miscarriage. For first trimester miscarriages, the prevalence differences between geographical region were largely caused by high prevalence in some small studies. Overall, study size and year of publication did not have significant effects on the estimates. Part of the heterogeneity in the first trimester could possibly be related to inclusion or exclusion of biochemical pregnancies, i.e. pregnancies diagnosed based on hCG. This would suggest that actual early miscarriage rates may be larger than estimated. On the other hand, in hospital-based studies selection bias is feasible, as women may be more inclined to visit the hospital in case of a miscarriage than when the pregnancy is ongoing, particularly when the pandemic was at the highest. For second trimester miscarriages the prevalence differed by study size and was highest in the smaller studies, with no significant effect of geographical region or year of publication. For EP heterogeneity was moderate, while it was substantial for TOP. TOP estimates varied by sample size of the individual study. Health care provider uncertainty of the effect of SARS-CoV-2 infection on mother and child may have increased the rate of TOP early in the pandemic (Wu et al., 2020b). On the other hand, lower abortion access because of COVID-19 pandemic-related restrictions may have resulted in a decrease in TOP (Cousins, 2020).
How SARS-CoV-2 could be related to pregnancy loss
There is a known association of some viral infections with foetal malformation and pregnancy complications. Particular viruses can infect several cellular components of the placenta, such as trophoblasts, and can affect placental function, which may result in pregnancy complications such as preterm birth, miscarriage, and intrauterine growth restriction (Racicot and Mor, 2017). Also, it has been suggested that the adverse pregnancy outcomes following SARS-CoV-2 infection might be caused by an inflammatory cytokine imbalance (Vesce et al., 2022).
A review on 11 pregnant women infected with Middle East Respiratory Syndrome coronavirus (MERS-CoV) reported adverse outcomes in over 90% of the presented cases; there was no information related to placental infection (Alfaraj et al., 2019).
A study investigating the pregnancy and perinatal outcomes of pregnant women with SARS in 2002 reported that 57% of the patients had a miscarriage, while case fatality rate was 25%. Placental tissues and cord blood were negative for SARS-CoV in this study (Wong et al., 2004).
Viruses can directly infect the foetus at specific times during gestation. This can result in severe birth defects or even pregnancy loss (Racicot and Mor, 2017; Badr et al., 2020). How viruses cross the placental barrier and reach the foetus remains largely unknown. A proposed mechanism involves infection of extravillous trophoblasts and/or direct infection of maternal immune cells. Other possible routes of vertical transmission include direct infection of the syncytium or via inflammation-mediated disruption of the syncytiotrophoblast layer, thus damaging the placental barrier and allowing for transmission (Megli and Coyne, 2022). Results from a systematic review on mother-to-child transmission in SARS-CoV-2 infection confirmed vertical transmission of the virus could occur, although the absolute numbers were low and transmission was rare (Allotey et al., 2020).
Several case studies have reported on SARS-CoV-2 positive placental tissue in the second trimester using a variety of assays before 20 weeks of gestation (Baud et al., 2020; Michel et al., 2021). SARS-CoV-2-related placentitis, which is an inflammation of the placenta caused by infection with SARS-CoV-2 and is characterized by increased perivillous fibrin deposition, histiocytic intervillositis, and villous trophoblast necrosis (Mithal et al., 2022), has been associated with pregnancy loss and stillbirth (Stenton et al., 2022). On the other hand, another study found no placental differences between SARS-CoV-2 infected and non-infected women and suggested that maternal SARS-CoV-2-related respiratory failure and the resulting hypoxia is the major risk factor for pregnancy loss and stillbirth (Suhren et al., 2022). In addition, SARS-CoV-2 infection can trigger a cytokine storm, which may lead to both an inflammatory response in the foetus and to placental damage with consecutive foetal growth retardation, preterm birth, and miscarriage (Cavalcante et al., 2021).
The MaterCov study investigated the impact of SARS-CoV-2 infection on subclinical placental thrombosis and maternal thrombotic factors. They found an increased risk of developing obstetric complications in pregnant women with SARS-CoV-2, such as intrauterine growth restriction and stillbirth. However, there were no more placental pathologies identified in pregnant women infected by SARS-CoV-2 compared to pregnant women without infection (Carbonnel et al., 2022).
Given that SARS-CoV-2 infection during pregnancy is associated with more severe maternal disease than in SARS-CoV-2-infected non-pregnant women of reproductive age as well as associated with an increase in adverse perinatal outcomes such as stillbirth and preterm delivery with infection in later pregnancy, pregnant women should be advised to take precautions to avoid risk of SARS-CoV-2 exposure and receive a COVID-19 vaccine to reduce the risk of severe disease.
There is no reason to speculate that SARS-CoV-2 should be associated with abnormal fertilization and implantation outside the uterus. One study reported that SARS-CoV-2 can infect human embryos in vitro (Montano et al., 2022); it remains to be elucidated whether this finding has any implications in vivo.
Comparison with existing data
A recent European study (Balachandren et al., 2022) suggested that women who had SARS-CoV-2 infection in the first trimester had a higher risk of early miscarriage. The authors found an early miscarriage rate of 14% in the ‘presumed infected’ group (11/77 [95% CI 6–22]), 5% in the ‘uncertain’ group (15/295 [95% CI 3–8]), and 8% in the ‘presumed uninfected’ group (212/2669 [95% CI 7–9], P = 0.02). After adjusting for age, BMI, ethnicity, smoking status, gestational age at registration and the number of previous miscarriages, they found the risk of early miscarriage to be higher among women with presumed SARS-CoV-2 infection in the first trimester compared to those with no infection (relative rate 1.7, 95% CI 1.0–3.0, P = 0.06). This study used self-reported data on diagnosis of SARS-CoV-2 and pregnancy outcomes, which may have resulted in higher reporting of miscarriage in the presumed infected group.
Two systematic reviews and meta-analyses reported on the proportion of miscarriage in women with SARS-CoV-2. One review included seven cases-series and concluded higher miscarriage rates were found in infected women (Kazemi et al., 2021), the other review included 17 studies, both case-series and cohort studies, and suggested miscarriage rates were comparable to non-infected women (Cavalcante et al., 2021). A recent systematic review and meta-analysis reported, among several other outcomes, that there was no significant difference in the rates of total miscarriage between SARS-CoV-2-infected and non-infected pregnant women (Jeong and Kim, 2023). In this review, the denominator included women in their third trimester both in the infected and non-infected controls, thus not allowing a fair comparison.
A systematic review on foetal demise, including stillbirths and late miscarriages following SARS-CoV-2 infection, concluded that most cases with late miscarriages (between 14 and 22 weeks) and stillbirths presented with placental abnormalities associated with potential transplacental SARS-CoV-2 infection, which may cause placental insufficiency and foetal hypoxia. The review included mostly case studies and case series (Alcover et al., 2023).
Study strengths and limitations
The strength of this systematic review and meta-analysis is the review process that combined the extensive search of the PregCov consortium with specific searches, which enabled us to include a large population of pregnant women with and without SARS-CoV-2 infection during the first and second trimester.
Our review has several limitations. Most included studies were hospital-based studies such that selection bias towards more severe infections seems likely. On the other hand, if the prevalence of miscarriage is not clearly higher in the women with more severe COVID-19, then it is very unlikely it will be higher in infection with mild symptoms. The majority of studies did not include very early pregnancies, i.e. before 5 or 6 weeks of gestation. This implies that we cannot make a statement on the possible effect of SARS-CoV-2 infection on biochemical pregnancy. However, as the majority of biochemical pregnancies miscarry owing to chromosomal abnormalities, and as comparable viral infections, such as influenza, only increased miscarriage rates later in pregnancy, it does not seem likely that SARS-CoV-2 is associated with very early miscarriage (Dawood et al., 2021). The effect estimates have large 95% confidence boundaries and there was high heterogeneity across studies in first trimester miscarriage. This could only partly be explained by small study effect and may further be explained by differences in definition of miscarriage and differences in gestational age at which women were included in the cohorts and registries. Therefore, uncertainties remain for key outcomes that require further evidence. Furthermore, different variants of SARS-CoV-2, treatment of pregnant women with COVID-19 and access to vaccines could have affected outcomes but could not be studied as detailed data on variants, treatment and prevention in women in their first of second trimester of pregnancy was usually lacking. SARS-CoV-2 variants differed between 2020 and 2022, however, prevalence estimates for 2020, when no treatment was available yet, overlapped with prevalence estimates for 2021 and 2022. Meanwhile, a large proportion of vaccinated women could only be expected in the studies published in 2022. The overlapping estimates between 2020, 2021, and 2022 suggest there is no large impact of variants of SARS-CoV-2, treatment of pregnant women with COVID-19 and access to vaccines on pregnancy loss. Additionally, data on prior pregnancy history, such as previous pregnancy loss, were mostly not available. These could be important confounders when assessing the risk of miscarriages.
Relevance for clinical practice and research
In order to provide better risk estimates more studies are needed. These are preferably well-designed prospective studies that include pregnant women with and without SARS-CoV-2 infection at conception and early pregnancy, and consider the association of clinical manifestation and severity of COVID-19 disease with pregnancy loss, as well as potential confounding factors such as previous pregnancy loss. In SARS-CoV-infected women with a miscarriage, foetal karyotyping could be carried out to exclude a genetic factor.
Even though the WHO has declared an end to the COVID-19 pandemic as a global health emergency, women are at risk of SARS-CoV-2 infection. Pregnant women should be advised to take precautions to avoid risk of SARS-CoV-2 exposure and to be vaccinated with a COVID-19 vaccine.
Conclusion
There are still many unknowns regarding SARS-CoV-2 infection in early pregnancy. Reassuringly, based on currently available evidence, there are no indications that SARS-CoV-2 infection in early pregnancy increases the risk of miscarriages. In order to provide better risk estimates, well-designed studies are needed.
Supplementary Material
Acknowledgements
We would like to acknowledge the following members of the PregCOV-19 Living Systematic Review Consortium Elena Stallings, Magnus Yap, Shaunak Chatterjee, Tania Kew, Luke Debenham, Anna Clavé Llavall, Anushka Dixit, Dengyi Zhou, Rishab Balaji, Xiu Qiu, Mingyang Yuan, Dyuti Coomar, Siang Ing Lee, Vanessa Brizuela, Nathalie Jeanne Nicole Broutet, Edna Kara, Anna Thorson, Olufemi Taiwo Oladapo.
Contributor Information
Janneke A C van Baar, Department of Obstetrics and Gynaecology, Center for Reproductive Medicine, Amsterdam UMC, Amsterdam, The Netherlands.
Elena B Kostova, Department of Obstetrics and Gynaecology, Center for Reproductive Medicine, Amsterdam UMC, Amsterdam, The Netherlands; Cochrane Gynaecology and Fertility Satellite, Amsterdam, The Netherlands; Amsterdam Reproduction and Development Research Institute, Amsterdam, The Netherlands.
John Allotey, Birmingham Women’s and Children’s NHS Foundation Trust, Birmingham, UK; NIHR Biomedical Research Center, University Hospitals Birmingham, Birmingham, UK.
Shakila Thangaratinam, Birmingham Women’s and Children’s NHS Foundation Trust, Birmingham, UK; NIHR Biomedical Research Center, University Hospitals Birmingham, Birmingham, UK; WHO Collaborating Centre for Global Women’s Health, Institute of Metabolism and Systems Research, University of Birmingham, Birmingham, UK.
Javier R Zamora, WHO Collaborating Centre for Global Women’s Health, Institute of Metabolism and Systems Research, University of Birmingham, Birmingham, UK; Clinical Biostatistics Unit, Hospital Universitario Ramón y Cajal (IRYCIS), Madrid, Spain; CIBER Epidemiology and Public Health (CIBERESP), Madrid, Spain.
Mercedes Bonet, UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva, Switzerland.
Caron Rahn Kim, UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva, Switzerland.
Lynne M Mofenson, Elizabeth Glaser Pediatric AIDS Foundation, Washington, DC, USA.
Heinke Kunst, Blizard Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, London, UK; Barts Health NHS Trust, London, UK.
Asma Khalil, St George’s University London, London, UK.
Elisabeth van Leeuwen, Amsterdam Reproduction and Development Research Institute, Amsterdam, The Netherlands; Women and Childrens Hospital, Amsterdam UMC, Amsterdam, The Netherlands.
Julia Keijzer, Department of Obstetrics and Gynaecology, Center for Reproductive Medicine, Amsterdam UMC, Amsterdam, The Netherlands.
Marije Strikwerda, Department Vrouw & Baby, Utrecht UMC, location University of Utrecht, Utrecht, The Netherlands.
Bethany Clark, Department Vrouw & Baby, Utrecht UMC, location University of Utrecht, Utrecht, The Netherlands.
Maxime Verschuuren, Department of Obstetrics and Gynaecology, Center for Reproductive Medicine, Amsterdam UMC, Amsterdam, The Netherlands.
Arri Coomarasamy, Birmingham Women’s and Children’s NHS Foundation Trust, Birmingham, UK; NIHR Biomedical Research Center, University Hospitals Birmingham, Birmingham, UK; Tommy's Centre for Miscarriage Research, Birmingham, UK.
Mariëtte Goddijn, Department of Obstetrics and Gynaecology, Center for Reproductive Medicine, Amsterdam UMC, Amsterdam, The Netherlands; Amsterdam Reproduction and Development Research Institute, Amsterdam, The Netherlands.
Madelon van Wely, Department of Obstetrics and Gynaecology, Center for Reproductive Medicine, Amsterdam UMC, Amsterdam, The Netherlands; Cochrane Gynaecology and Fertility Satellite, Amsterdam, The Netherlands; Amsterdam Reproduction and Development Research Institute, Amsterdam, The Netherlands.
PregCOV-19 Living Systematic Review Consortium:
Elena Stallings, Magnus Yap, Shaunak Chatterjee, Tania Kew, Luke Debenham, Anna Clavé Llavall, Anushka Dixit, Dengyi Zhou, Rishab Balaji, Xiu Qiu, Mingyang Yuan, Dyuti Coomar, Siang Ing Lee, Vanessa Brizuela, Nathalie Jeanne Nicole Broutet, Edna Kara, Caron Rahn Kim, Anna Thorson, and Olufemi Taiwo Oladapo
Supplementary data
Supplementary data are available at Human Reproduction Update online.
Data availability
Data collected including data on pregnancy loss is available at https://cgf.cochrane.org/news/covid-19-coronavirus-disease-fertility-and-pregnancy. Dissemination to participants and related patient and public communities: The PregCov-19 LSR Group will disseminate the findings through dedicated websites: www.birmingham.ac.uk/research/who-collaborating-centre/pregcov/index.aspx and https://cgf.cochrane.org/news/covid-19-coronavirus-disease-fertility-and-pregnancy as well as through social media.
Authors’ roles
M.v.W. and E.K. conceptualized the study. J.A., S.T., M.B., C.K., J.Z., L.M., H.K., A.K., E.v.L., E.K., and M.v.W. are members of the PregCOV-19 Living Systematic Review Consortium. M.G. and A.C. advised on the protocol. J.A., S.T., and J.Z. shared data from this infrastructure. J.B., B.C., J.K., M.S., M.V., M.v.W., and E.K. performed literature searches, selected studies, and extracted data. M.v.W. conducted the analyses. J.Z. gave statistical advice. All authors contributed to the interpretation and writing of the manuscript and approved the final version. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. The corresponding author is the guarantor of the data (M.v.W) and affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been disclosed.
Funding
The presented work on pregnancy loss received no specific funding. However, it was facilitated by the infrastructure of the PRegCOV-19 Living Systematic Review Consortium that was partially supported by the German Federal Ministry of Health (BMG) COVID-19 Research and development support to the World Health Organization (WHO) and UNDP-UNFPA-UNICEF-WHO-World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), a co-sponsored programme executed by WHO. The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions, or policies of the institutions with which they are affiliated.
Conflict of interest
Authors declare that partial funding by WHO and HRP for the infrastructure of the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
- Aabakke AJM, Krebs L, Petersen TG, Kjeldsen FS, Corn G, Wøjdemann K, Ibsen MH, Jonsdottir F, Rønneberg E, Andersen CS. et al. SARS-CoV-2 infection in pregnancy in Denmark-characteristics and outcomes after confirmed infection in pregnancy: a nationwide, prospective, population-based cohort study. Acta Obstet Gynecol Scand 2021;100:2097–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aabakke AJM, Petersen TG, Wøjdemann K, Ibsen MH, Jonsdottir F, Rønneberg E, Andersen CS, Hammer A, Clausen TD, Milbak J. et al. Risk factors for and pregnancy outcomes after SARS-CoV-2 in pregnancy according to disease severity: a nationwide cohort study with validation of the SARS-CoV-2 diagnosis. Acta Obstet Gynecol Scand 2023;102:282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abedzadeh-Kalahroudi M, Sehat M, Vahedpour Z, Talebian P.. Maternal and neonatal outcomes of pregnant patients with COVID-19: a prospective cohort study. Int J Gynaecol Obstet 2021;153:449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari EH, Moreno W, Zofkie AC, MacDonald L, McIntire DD, Collins RRJ, Spong CY.. Pregnancy outcomes among women with and without severe acute respiratory syndrome coronavirus 2 infection. JAMA Netw Open 2020;3:e2029256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad SN, Sameen D, Dar MA, Jallu R, Shora TN, Dhingra M.. Do SARS-CoV-2-infected pregnant women have adverse pregnancy outcomes as compared to non-infected pregnant women? Int J Womens Health 2022;14:1201–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajith S, Reshmi VP, Nambiar S, Naser A, Athulya B.. Prevalence and risk factors of neonatal Covid-19 infection: a single-centre observational study. J Obstet Gynaecol India 2021;71:235–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akram EG, Mohammed SI, Khoshaba JZ.. Relation of COVID-19 infection to outcomes of pregnancy during the pandemic in Kirkuk city. Eur J Mol Clin Med 2021;7:4314–4318. [Google Scholar]
- Al-Hajjar S, Ibrahim L, Kurdi W, Tulbah M, Alnemer M, Bin Jabr M, Elsaidawi W, Binmanee A, Ali M, Bukhari H. et al. Observational cohort study of perinatal outcomes of women with COVID-19. J Infect Public Health 2022;15:1503–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Samkari H, Karp Leaf RS, Dzik WH, Carlson JCT, Fogerty AE, Waheed A, Goodarzi K, Bendapudi PK, Bornikova L, Gupta S. et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood 2020;136:489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcover N, Regiroli G, Benachi A, Vauloup-Fellous C, Vivanti AJ, De Luca D.. Systematic review and synthesis of stillbirths and late miscarriages following SARS-CoV-2 infections. Am J Obstet Gynecol 2023;229:118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaraj SH, Al-Tawfiq JA, Memish ZA.. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection during pregnancy: report of two cases & review of the literature. J Microbiol Immunol Infect 2019;52:501–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, Debenham L, Llavall AC, Dixit A, Zhou D. et al. ; For PregCOV-19 Living Systematic Review Consortium. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ 2020;370:m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand P, Yadav A, Debata P, Bachani S, Gupta N, Gera R.. Clinical profile, viral load, management and outcome of neonates born to COVID 19 positive mothers: a tertiary care centre experience from India. Eur J Pediatr 2021;180:547–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakaki T, Junichi H, Akihiko S, Tomoaki I, Isamu I, Katsuyuki K, Department of Medical Safety, the Japan Association of Obstetricians and Gynecologists (JAOG). Risk factors for severe disease and impact of severity on pregnant women with COVID-19: a case–control study based on data from a nationwide survey of maternity services in Japan. BMJ Open 2022;12:e068575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arinkan SA, Dall? Alper EC, Topcu G, Muhcu M.. Perinatal outcomes of pregnant women having SARS-CoV-2 infection. Taiwan J Obstet Gynecol 2021;60:1043–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayed A, Embaireeg A, Benawadh A, Al-Fouzan W, Hammoud M, Al-Hathal M, Alzaydai A, Ahmad A, Ayed M.. Maternal and perinatal characteristics and outcomes of pregnancies complicated with COVID-19 in Kuwait. BMC Pregnancy Childbirth 2020;20:754. doi: 10.1186/s12884-020-03461-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babic I, Alsomali F, Aljuhani S, Baeissa S, Alhabib I, AlAhmari E, Omer M, Alkhalifa K.. COVID-19 pandemic and its impact on perinatal outcomes between symptomatic and asymptomatic women. Obstet Gynecol Int 2022;2022:1756266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badr DA, Mattern J, Carlin A, Cordier AG, Maillart E, El Hachem L, El Kenz H, Andronikof M, De Bels D, Damoisel C. et al. Are clinical outcomes worse for pregnant women at ≥20 weeks' gestation infected with coronavirus disease 2019? A multicenter case-control study with propensity score matching. Am J Obstet Gynecol 2020;223:764–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandren N, Davies MC, Hall JA, Stephenson JM, David AL, Barrett G, O’Neill HC, Ploubidis GB, Yasmin E, Mavrelos D.. SARS-CoV-2 infection in the first trimester and the risk of early miscarriage: a UK population-based prospective cohort study of 3041 pregnancies conceived during the pandemic. Hum Reprod 2022;37:1126–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barg M, Rotem R, Mor P, Rottenstreich M, Khatib F, Grisaru-Granovsky S, Armon S.. Delayed presentation of ectopic pregnancy during the COVID-19 pandemic: a retrospective study of a collateral effect. Int J Gynaecol Obstet 2021;153:457–461. [DOI] [PubMed] [Google Scholar]
- Barris M, Figueras L, Zala C, Abojer L, Carro C, Silenzi V.. Cohort of pregnant and postpartum women with COVID-19. Comparative analysis between two pandemic waves. Medicina (B Aires) 2022;82:830–835. [PubMed] [Google Scholar]
- Basu JK, Chauke L, Cert M, Fetal M, Magoro T, Cert Maternal and Fetal Medicine. Clinical features and outcomes of COVID-19 infection among pregnant women in South Africa. Int J MCH AIDS 2021;10:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud D, Greub G, Favre G, Gengler C, Jaton K, Dubruc E, Pomar L.. Second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infection. JAMA 2020;323:2198–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhoora S, Zamparini J, Odell N, Murray L, Balie G, Sanyika N, Mall K, Ramdin T, Mahomed A, Chauke L.. COVID-19 in pregnant women in South Africa: a retrospective review. S Afr Med J 2022;112:911–918. [DOI] [PubMed] [Google Scholar]
- Borges-Charepe N, Queirós A, Alves MJ, Serrano F, Ferreira C, Gamito M, Smet C, Silva V, Féria B, Laranjo M. et al. One year of COVID-19 in pregnancy: a national wide collaborative study. Acta Med Port 2022;35:357–366. [DOI] [PubMed] [Google Scholar]
- Burwick RM, Yawetz S, Stephenson KE, Collier AY, Sen P, Blackburn BG, Kojic EM, Hirshberg A, Suarez JF, Sobieszczyk ME. et al. Compassionate use of remdesivir in pregnant women with severe coronavirus disease 2019. Clin Infect Dis 2021;73:e3996–e4004. doi: 10.1093/cid/ciaa1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón JM, Figueroa Flores MDR, Paniagua Coria L, Briones Garduño JC, Meneses Figueroa J, Vargas Contretas MJ, De la Cruz Ávila L, Díaz Meza S, Ramírez Chacón R, Padmanabhan S. et al. Nitazoxanide against COVID-19 in three explorative scenarios. J Infect Dev Ctries 2020;14:982–986. [DOI] [PubMed] [Google Scholar]
- Cambou MC, Liu CM, Mok T, Fajardo-Martinez V, Paiola SG, Ibarrondo FJ, Kerin T, Fuller T, Tobin NH, Garcia G. et al. Longitudinal evaluation of antibody persistence in mother-infant dyads after severe acute respiratory syndrome coronavirus 2 infection in pregnancy. J Infect Dis 2023;227:236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonnel M, Daclin C, Tourne M, Roux E, Le-Marchand M, Racowsky C, Kennel T, Farfour E, Vasse M, Ayoubi JM. et al. Impact of COVID-19 on subclinical placental thrombosis and maternal thrombotic factors. J Clin Med 2022;11:4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona-Pérez JA, Villegas-Mota I, Helguera-Repetto AC, Acevedo-Gallegos S, Rodríguez-Bosch M, Aguinaga-Ríos M, Coronado-Zarco I, León-Juárez M, Aguilar-Ayala D, Valdespino-Vázquez MY. et al. Prevalence, clinical features, and outcomes of SARS-CoV-2 infection in pregnant women with or without mild/moderate symptoms: Results from universal screening in a tertiary care center in Mexico City, Mexico. PLoS One 2021;16:e0249584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadio P, Youssef A, Arena A, Gamal N, Pilu G, Seracchioli R.. Increased rate of ruptured ectopic pregnancy in COVID-19 pandemic: analysis from the North of Italy. Ultrasound Obstet Gynecol 2020;56:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalcante MB, de Melo Bezerra Cavalcante CT, Cavalcante ANM, Sarno M, Barini R, Kwak-Kim J.. COVID-19 and miscarriage: from immunopathological mechanisms to actual clinical evidence. J Reprod Immunol 2021;148:103382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary S, Nazir S, Shahbaz F, Humayun S, Akhtar N, Humayun S.. Pregnancy outcome in COVID-19 suspected and COVID-19 confirmed women: a comparative analysis. Annals KEMU 2021;27:255–261. [Google Scholar]
- Chen L, Li Q, Zheng D, Jiang H, Wei Y, Zou L, Feng L, Xiong G, Sun G, Wang H. et al. Clinical characteristics of pregnant women with Covid-19 in Wuhan, China. N Engl J Med 2020;382:e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Bernstein P, Nair S, Romanelli E, Khoury R, Labins J, Fiorica G, Reddy S.. A review of 92 obstetric patients with COVID-19 in the Bronx, New York and their peripartum anaesthetic management. Anaesthesiol Intensive Ther 2021;53:115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y, Kim EJ, Kim HS, Park KH, Baek JH, Kim J, Lee JY, Lee CS, Lim S, Kim SW. et al. Maternal and neonatal outcomes in pregnant women with coronavirus disease 2019 in Korea. J Korean Med Sci 2022;37:e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins A, Memtsa M, Kirk E, Othman M, Abdul Kadir R.. The risk of venous thromboembolism in early pregnancy loss: review of the literature and current guidelines and the need for guidance—Communication from the SSC on Women's Health Issues for thrombosis and haemostasis. J Thromb Haemost 2022;20:767–776. [DOI] [PubMed] [Google Scholar]
- Cosma S, Carosso AR, Cusato J, Borella F, Bertero L, Bovetti M, Bevilacqua F, Mengozzi G, Mazzone R, Ghisetti V. et al. Obstetric and neonatal outcomes after SARS-CoV-2 infection in the first trimester of pregnancy: a prospective comparative study. J Obstet Gynaecol Res 2022;48:393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins S. COVID-19 has "devastating" effect on women and girls. Lancet 2020;396:301–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crovetto F, Crispi F, Llurba E, Pascal R, Larroya M, Trilla C, Camacho M, Medina C, Dobaño C, Gomez-Roig MD. et al. ; KidsCorona Pregnancy COVID-19 Group. Impact of severe acute respiratory syndrome coronavirus 2 infection on pregnancy outcomes: a population-based study. Clin Infect Dis 2021;73:1768–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz Melguizo S, de la Cruz Conty ML, Carmona Payán P, Abascal-Saiz A, Pintando Recarte P, González Rodríguez L, Cuenca Marín C, Martínez Varea A, Oreja Cuesta AB, Rodríguez PP. et al. ; on behalf of the Spanish Obstetric Emergency Group S O E G. Pregnancy outcomes and SARS-CoV-2 infection: the Spanish Obstetric Emergency Group Study. Viruses 2021;13:853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curi B, Sabre A, Benjamin I, Serventi L, Nuritdinova D.. Coronavirus infection in a high-risk obstetrical population of the South Bronx, New York. Am J Obstet Gynecol MFM 2020;2:100203–100203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Antonio F, Sen C, Di Mascio D, Galindo A, Villalain C, Herraiz I, Resul A, Ali O, Hasan E, Manuel Guerra C. et al. ; WAMP. Maternal and perinatal outcomes in high compared to low risk pregnancies complicated by severe acute respiratory syndrome coronavirus 2 infection (phase 2): the World Association of Perinatal Medicine working group on coronavirus disease 2019. Am J Obstetr Gynecol MFM 2021;3:100329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daclin C, Carbonnel M, Rossignol M, Abbou H, Trabelsi H, Cimmino A, Delmas J, Rifai AS, Coiquaud LA, Tiberon A. et al. Impact of COVID-19 infection in pregnancy and neonates: a case control study. J Gynecol Obstet Hum Reprod 2022;51:102366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damar Çakırca T, Torun A, Hamidanoğlu M, Portakal RD, Ölçen M, Çakırca G, Haksever M.. COVID-19 infection in pregnancy: a single center experience with 75 cases. Ginekol Pol 2022;93:410–415. [DOI] [PubMed] [Google Scholar]
- Dawood FS, Kittikraisak W, Patel A, Rentz Hunt D, Suntarattiwong P, Wesley MG, Thompson MG, Soto G, Mundhada S, Arriola CS. et al. Incidence of influenza during pregnancy and association with pregnancy and perinatal outcomes in three middle-income countries: a multisite prospective longitudinal cohort study. Lancet Infect Dis 2021;21:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delahoy MJ, Whitaker M, O'Halloran A, Chai SJ, Kirley PD, Alden N, Kawasaki B, Meek J, Yousey-Hindes K, Anderson EJ. et al. ; COVID-NET Surveillance Team. Characteristics and maternal and birth outcomes of hospitalized pregnant women with laboratory-confirmed COVID-19—COVID-NET, 13 States, March 1–August 22, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1347–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Q, Cao S, Wang H, Zhang Y, Chen L, Yang Z, Peng Z, Zhou Q.. Application of quantitative lung ultrasound instead of CT for monitoring COVID-19 pneumonia in pregnant women: a single-center retrospective study. BMC Pregnancy Childbirth 2021;21:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi KP, Devi AB, Rameswar M, Singh LR, Bharati SD.. Obstetric and perinatal outcomes in the first wave of COVID-19 infected pregnant women. Int J Clin Obstet Gynaecol 2021;5:96–100. [Google Scholar]
- Diouf AA, Mbaye KD, Gueye M, Thioub D, Niang N, Dekou CY, Gueye MDN, Diallo M, Mbaye M, Dieme MEF. et al. Clinical characteristics and outcomes of COVID-19 infection in nine pregnant women: a report from a sub-Saharan African country, Senegal. Pan Afr Med J 2020;35:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donati S, Corsi E, Maraschini A, Salvatore MA; ItOSS COVID-19 Working Group; ItOSS COVID-19 WORKING GROUP. The first SARS-CoV-2 wave among pregnant women in Italy: results from a prospective population-based study. Ann Ist Super Sanita 2021;57:272–285. [DOI] [PubMed] [Google Scholar]
- Dugas C, Slane VH.. Miscarriage. In: StatPearls 2021. Treasure Island (FL: ): StatPearls Publishing, 2023. https://www.ncbi.nlm.nih.gov/books/NBK532992/. [Google Scholar]
- Edlow AG, Li JZ, Collier A-RY, Atyeo C, James KE, Boatin AA, Gray KJ, Bordt EA, Shook LL, Yonker LM. et al. Assessment of maternal and neonatal SARS-CoV-2 viral load, transplacental antibody transfer, and placental pathology in pregnancies during the COVID-19 pandemic. JAMA Netw Open 2020;3:e2030455. doi: 10.1001/jamanetworkopen.2020.30455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emeruwa UN, Spiegelman J, Ona S, Kahe K, Miller RS, Fuchs KM, Aubey JJ, Booker W, D'Alton ME, Friedman AM. et al. Influence of race and ethnicity on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection rates and clinical outcomes in pregnancy. Obstetr Gynecol 2020;136:1040–1043. [DOI] [PubMed] [Google Scholar]
- Erez O, Othman M, Rabinovich A, Leron E, Gotsch F, Thachil J.. DIC in pregnancy—pathophysiology, clinical characteristics, diagnostic scores, and treatments. J Blood Med 2022;13:21–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erol SA, Tanacan A, Anuk AT, Tokalioglu EO, Biriken D, Keskin HL, Moraloglu OT, Yazihan N, Sahin D.. Evaluation of maternal serum afamin and vitamin E levels in pregnant women with COVID-19 and its association with composite adverse perinatal outcomes. J Med Virol 2021;93:2350–2358. [DOI] [PubMed] [Google Scholar]
- Fallach N, Segal Y, Agassy J, Perez G, Peretz A, Chodick G, Gazit S, Patalon T, Ben Tov A, Goldshtein I.. Pregnancy outcomes after SARS-CoV-2 infection by trimester: a large, population-based cohort study. PLoS One 2022;17:e0270893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C, Guo Y, Qu P, Wang S, Wang M, Yuan J, Li C, Gao L, Pang Y, Li Z. et al. No obviously adverse pregnancy complications and outcomes of the recovered pregnant women from COVID-19. Reprod Toxicol 2021;100:163–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo S-S, Cambou MC, Mok T, Fajardo VM, Jung KL, Fuller T, Chen W, Kerin T, Mei J, Bhattacharya D. et al. The systemic inflammatory landscape of COVID-19 in pregnancy: extensive serum proteomic profiling of mother-infant dyads with in utero SARS-CoV-2. Cell Rep Med 2021;2:100453–100453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NS, Melka S.. COVID-19 in pregnant women: case series from one large New York city obstetrical practice. Am J Perinatol 2020;37:1002–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajbhiye R, Mahajan N, Waghmare R, Zala S, Chaaithanya I, Kuppusamy P, Bhurke A, Pious M, Surve S, Modi D. et al. Clinical characteristics, outcomes, & mortality in pregnant women with COVID-19 in Maharashtra, India: results from PregCovid registry. Indian J Med Res 2021;153:629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göklü MR, Oğlak SC, Gedik Özköse Z, Tunç Ş, Bolluk G.. The course of infection with the Delta variant of COVID-19 in pregnancy: analysis of clinical, laboratory, and neonatal outcomes. J Turk Ger Gynecol Assoc 2023;24:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandone E, Vimercati A, Sorrentino F, Colaizzo D, Ostuni A, Ceci O, Capozza M, Tiscia G, De Laurenzo A, Mastroianno M. et al. Obstetric outcomes in pregnant COVID-19 women: the imbalance of von Willebrand factor and ADAMTS13 axis. BMC Pregnancy Childbirth 2022;22:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grechukhina O, Greenberg V, Lundsberg LS, Deshmukh U, Cate J, Lipkind HS, Campbell KH, Pettker CM, Kohari KS, Reddy UM.. Coronavirus disease 2019 pregnancy outcomes in a racially and ethnically diverse population. Am J Obstet Gynecol MFM 2020;2:100246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Yuan J, Wang M, Yu Y, Bian J, Fan C.. Case series of 20 pregnant women with 2019 novel coronavirus disease in Wuhan, China. J Obstet Gynaecol Res 2021;47:1344–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamadneh J, Hamadneh S, ALBashtawy M, Alkhawaldeh A, Bashtawi M, Alshloul M, Rayan A, Abdalrahim A.. Impact of COVID-19 on perinatal mental health among pregnant mothers infected with COVID-19, during the first wave of the epidemic in Jordan. Heliyon 2022;8:e12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haye MT, Cartes G, Gutiérrez J, Ahumada P, Krause B, Merialdi M, Gonzalez R.. Maternal and perinatal outcomes in pregnant women with confirmed severe and mild COVID-19 at one large maternity hospital in Chile. J Matern Fetal Neonatal Med 2022;35:5917–5922. [DOI] [PubMed] [Google Scholar]
- Hazari KS, Abdeldayem R, Paulose L, Kurien N, Almahloul Z, Mohammad H, Elgergawi TFA, Alkhanbouli M, Mahmoud K, Fazari AB. et al. Covid-19 infection in pregnant women in Dubai: a case-control study. BMC Pregnancy Childbirth 2021;21:658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hcini N, Maamri F, Picone O, Carod JF, Lambert V, Mathieu M, Carles G, Pomar L.. Maternal, fetal and neonatal outcomes of large series of SARS-CoV-2 positive pregnancies in peripartum period: a single-center prospective comparative study. Eur J Obstet Gynecol Reprod Biol 2021;257:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández BO, Honorato S M, Silva G MC, Sepúlveda-Martínez Á, Fuenzalida C J, Abarzúa C F, Von Bischhoffshausen P S, Morales M N, García P, Oyarce M MI. et al. COVID 19 y embarazo en Chile: informe preliminar del estudio multicéntrico GESTACOVID. Rev Chil Obstet Ginecol 2020;85:S75–S89. [Google Scholar]
- Hernández PV, Chen L, Zhang R, Jackups R, Nelson DM, He M.. The effects of preconception and early gestation SARS-CoV-2 infection on pregnancy outcomes and placental pathology. Ann Diagn Pathol 2023;62:152076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, Baker P, Smith E, Buchbinder R.. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol 2012;65:934–939. [DOI] [PubMed] [Google Scholar]
- Hughes BL, Sandoval GJ, Metz TD, Clifton RG, Grobman WA, Saade GR, Manuck TA, Longo M, Sowles A, Clark K. et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. First- or second-trimester SARS-CoV-2 infection and subsequent pregnancy outcomes. Am J Obstet Gynecol 2023;228:226.e221–226.e229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby VL, Murtha A, Afshar Y, Gaw SL, Asiodu I, Tolosa J, Norton ME, Boscardin WJ, Flaherman V.. Risk of pregnancy loss before 20 weeks' gestation in study participants with COVID-19. Am J Obstet Gynecol 2021;225:456–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang WK, Lee SY, Park S, Ryoo NH, Hwang I, Park JM, Bae JG.. Pregnancy outcome, antibodies, and placental pathology in SARS-CoV-2 infection during early pregnancy. Int J Environ Res Public Health 2021;18:5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong Y, Kim MA.. SARS-CoV-2 infection in pregnancy and adverse pregnancy outcomes: a systematic review and meta-analysis. Obstet Gynecol Sci 2023;66:270–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi B, Chandi A, Srinivasan R, Saini SS, Prasad GRV, Puri GD, Bhalla A, Suri V, Bagga R.. The placental pathology in Coronavirus disease 2019 infected mothers and its impact on pregnancy outcome. Placenta 2022;127:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayem G, Lecarpentier E, Deruelle P, Bretelle F, Azria E, Blanc J, Bohec C, Bornes M, Ceccaldi P-F, Chalet Y. et al. A snapshot of the Covid-19 pandemic among pregnant women in France. J Gynecol Obstet Hum Reprod 2020;49:101826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazemi SN, Hajikhani B, Didar H, Hosseini SS, Haddadi S, Khalili F, Mirsaeidi M, Nasiri MJ.. COVID-19 and cause of pregnancy loss during the pandemic: a systematic review. PLoS One 2021;16:e0255994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoiwal K, Agarwal A, Gaurav A, Kumari R, Mittal A, Sabnani S, Mundhra R, Chawla L, Bahadur A, Chaturvedi J.. Obstetric and perinatal outcomes in pregnant women with COVID-19: an interim analysis. Women Health 2022;62:12–20. [DOI] [PubMed] [Google Scholar]
- Khorramdelazad H, Kazemi MH, Najafi A, Keykhaee M, Zolfaghari Emameh R, Falak R.. Immunopathological similarities between COVID-19 and influenza: investigating the consequences of Co-infection. Microb Pathog 2021;152:104554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiremitli S, Kiremitli T, Ulug P, Kirkinci A, Kurnuc FZ, Yilmaz N, Dinc K, Yilmaz BK, Gul OI, Uzel K.. Does being infected with SARS-CoV-2 in the first-trimester increase the risk of miscarriage? An Acad Bras Cienc 2022;94:e20211283. [DOI] [PubMed] [Google Scholar]
- Kumari A, Anand S, Vidyarthi A.. Effects of COVID-19 during pregnancy on maternal and neonatal outcome: a retrospective observational study in tertiary teaching hospital, India. J Family Med Prim Care 2022;11:1820–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzan TY, Murzoğlu Altıntoprak K, Çiftçi HÖ, Kuzan BN, Yassa M, Tuğ N, Çimşit NÇ.. Clinical and radiologic characteristics of symptomatic pregnant women with COVID-19 pneumonia. J Turk Ger Gynecol Assoc 2021;22:196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancet T. Miscarriage: worldwide reform of care is needed. Lancet 2021;397:1597. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)00954-5/fulltext. [DOI] [PubMed] [Google Scholar]
- Lei D, Wang C, Li C, Fang C, Yang W, Chen B, Wei M, Xu X, Yang H, Wang S. et al. Clinical characteristics of COVID-19 in pregnancy: analysis of nine cases. Chin J Perinatal Med 2020;12:159–165. [Google Scholar]
- Lokken EM, Huebner EM, Taylor GG, Hendrickson S, Vanderhoeven J, Kachikis A, Coler B, Walker CL, Sheng JS, Al-Haddad BJS. et al. ; Washington State COVID-19 in Pregnancy Collaborative. Disease severity, pregnancy outcomes, and maternal deaths among pregnant patients with severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am J Obstet Gynecol 2021;225:77.e1–77.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London V, McLaren R Jr, Atallah F, Cepeda C, McCalla S, Fisher N, Stein JL, Haberman S, Minkoff H.. The relationship between status at presentation and outcomes among pregnant women with COVID-19. Am J Perinatol 2020;37:991–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan NN, Ansari M, Gaikwad C, Jadhav P, Tirkey D, Pophalkar MP, Bhurke AV, Modi DN, Mahale SD, Gajbhiye RK.. Impact of SARS-CoV-2 on multiple gestation pregnancy. Int J Gynaecol Obstet 2021;152:220–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manasova G, Golubenko M, Didenkul N, Radchenko Y, Gladchuk I.. CLINICAL AND EPIDEMIOLOGICAL FEATURES OF COVID-19 COURSE IN PREGNANT WOMEN. Georgian Med News 2021;320:90–96. [PubMed] [Google Scholar]
- Martinez-Baladejo MT, Graul A, Gifford T, McGorry D, Bell J, Anastasio H, Zighelboim I, Tolosa JE.. Pregnancy outcomes after administration of monoclonal antibody therapy for COVID-19. Am J Obstet Gynecol MFM 2023;5:100761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Varea A, Satorres E, Florez S, Domenech J, Desco-Blay J, Monfort-Pitarch S, Hueso M, Perales-Marín A, Diago-Almela V.. Comparison of maternal-fetal outcomes among unvaccinated and vaccinated pregnant women with COVID-19. J Pers Med 2022;12:2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattar CN, Kalimuddin S, Sadarangani SP, Tagore S, Thain S, Thoon KC, Hong EY, Kanneganti A, Ku CW, Chan GM. et al. Pregnancy outcomes in COVID-19: a prospective cohort study in Singapore. Ann Acad Med Singap 2020;49:857–869. [PubMed] [Google Scholar]
- McCreary EK, Lemon L, Megli C, Oakes A, Seymour CW; UPMC Magee Monoclonal Antibody Treatment Group. Monoclonal antibodies for treatment of SARS-CoV-2 infection during pregnancy: a cohort study. Ann Intern Med 2022;175:1707–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Practice Committee of the American Society for Reproductive Medicine. Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril 2012;98:1103–1111. [DOI] [PubMed] [Google Scholar]
- Megli CJ, Coyne CB.. Infections at the maternal-fetal interface: an overview of pathogenesis and defence. Nat Rev Microbiol 2022;20:67–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metkari AM, Palve TT.. Study of symptomatology and presentations of COVID-19 in pregnancy at a tertiary care hospital. Int J Reprod Contracept Obstet Gynecol 2020;9:4410–4414. [Google Scholar]
- Metz TD, Clifton RG, Hughes BL, Sandoval G, Saade GR, Grobman WA, Manuck TA, Miodovnik M, Sowles A, Clark K. et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Disease severity and perinatal outcomes of pregnant patients with coronavirus disease 2019 (COVID-19). Obstet Gynecol 2021;137:571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel AS, De Logiviere V, Schnuriger A, Lefebvre M, Maisonneuve E, Kayem G.. Description of a late miscarriage case at 16 weeks of gestation associated with a SARS-CoV-2 infection. J Gynecol Obstet Hum Reprod 2021;50:102064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithal LB, Otero S, Simons LM, Hultquist JF, Miller ES, Ozer EA, Shanes ED, Goldstein JA.. Low-level SARS-CoV-2 viremia coincident with COVID placentitis and stillbirth. Placenta 2022;121:79–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montano M, Victor AR, Griffin DK, Duong T, Bolduc N, Farmer A, Garg V, Hadjantonakis AK, Coates A, Barnes FL. et al. SARS-CoV-2 can infect human embryos. Sci Rep 2022;12:15451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins E, Hudak ML, Banerjee J, Getzlaff T, Townson J, Barnette K, Playle R, Perry A, Bourne T, Lees CC; PAN-COVID Investigators and the National Perinatal COVID-19 Registry Study Group. Pregnancy and neonatal outcomes of COVID‐19: coreporting of common outcomes from PAN‐COVID and AAP‐SONPM registries. Ultrasound Obstet Gynecol 2021;57:573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambiar SS, P RV, S A.. COVID-19 symptoms-does pregnancy alter the course of the disease. Int J Reprod Contracept Obstet Gynecol 2020;9:5032. [Google Scholar]
- Neelam V, Reeves EL, Woodworth KR, O'Malley Olsen E, Reynolds MR, Rende J, Wingate H, Manning SE, Romitti P, Ojo KD. et al. Pregnancy and infant outcomes by trimester of SARS-CoV-2 infection in pregnancy–SET-NET, 22 jurisdictions, January 25, 2020–December 31, 2020. Birth Defects Res 2023;115:145–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omrani AS, Almaslamani MA, Daghfal J, Alattar RA, Elgara M, Shaar SH, Ibrahim TBH, Zaqout A, Bakdach D, Akkari AM. et al. The first consecutive 5000 patients with coronavirus disease 2019 from Qatar; a nation-wide cohort study. BMC Infect Dis 2020;20:777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omrani AS, Almaslamani MA, Daghfal J, Alattar RA, Elgara M, Shaar SH, Ibrahim TBH, Zaqout A, Bakdach D, Akkari AM. et al. SARS‐CoV‐2 infection in pregnancy during the first wave of COVID‐19 in the Netherlands: a prospective nationwide population‐based cohort study (NethOSS). BJOG 2021;20:777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overtoom EM, , RosmanAN, , ZwartJJ, , VogelvangTE, , SchaapTP, , Van Den AkkerT, , Bloemenkamp KWM.. SARS-CoV-2 infection in pregnancy during the first wave of COVID-19 in the Netherlands: a prospective nationwide population-based cohort study (NethOSS). BJOG 2022;129:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabinger I, Grafenhofer H, Kaider A, Ilic A, Eichinger S, Quehenberger P, Husslein P, Mannhalter C, Lechner K.. Preeclampsia and fetal loss in women with a history of venous thromboembolism. Arterioscler Thromb Vasc Biol 2001;21:874–879. [DOI] [PubMed] [Google Scholar]
- Panelli DM, Phillips CH, Brady PC.. Incidence, diagnosis and management of tubal and nontubal ectopic pregnancies: a review. Fertil Res Pract 2015;1:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péju E, Belicard F, Silva S, Hraiech S, Painvin B, Kamel T, Thille AW, Goury A, Grimaldi D, Jung B. et al. ; COVIDPREG Study Group. Management and outcomes of pregnant women admitted to intensive care unit for severe pneumonia related to SARS-CoV-2 infection: the multicenter and international COVIDPREG study. Intensive Care Med 2022;48:1185–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirjani R, Hosseini R, Soori T, Rabiei M, Hosseini L, Abiri A, Moini A, Shizarpour A, Razani G, Sepidarkish M.. Maternal and neonatal outcomes in COVID-19 infected pregnancies: a prospective cohort study. J Travel Med 2020;27:taaa158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poisson M, Sibiude J, Mosnino E, Koual M, Landraud L, Fidouh N, Mandelbrot L, Vauloup-Fellous C, Luton D, Benachi A. et al. Impact of variants of SARS-CoV-2 on obstetrical and neonatal outcomes. J Gynecol Obstet Hum Reprod 2023;52:102566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon LC, Leung BW, Ma T, Yu FNY, Kong CW, Lo TK, So PL, Leung WC, Shu W, Cheung KW. et al. Relationship between viral load, infection-to-delivery interval and mother-to-child transfer of anti-SARS-CoV-2 antibodies. Ultrasound Obstet Gynecol 2021;57:974–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prager S, Micks ME, Dalton VK. Pregnancy loss (miscarriage): terminology, risk factors, and etiology, UpToDate 2023. https://www.uptodate.com/contents/pregnancy-loss-miscarriage-terminology-risk-factors-and-etiology (16 April 2023, date last accessed).
- Priyadharshini CB, Priya S, Selvameena M, Waseemsha S, Muthurajesh E, Shalini M.. Demographic profile of COVID-19 positive mothers & their outcome in government Rajaji hospital, Madurai, Tamilnadu—a cross sectional Study. Clin Epidemiol Glob Health 2021;12:100864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiancheng X, Jian S, Lingling P, Lei H, Xiaogan J, Weihua L, Gang Y, Shirong L, Zhen W, GuoPing X. et al. ; Sixth Batch of Anhui Medical Team Aiding Wuhan for COVID-19. Coronavirus disease 2019 in pregnancy. Int J Infect Dis 2020;95:376–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qudsieh S, Mahfouz IA, Assaf GA.. Clinical features and outcome of corona virus disease–19 infection among pregnant Jordanian women. JMJ 2022;56:345–353. [Google Scholar]
- Racicot K, Mor G.. Risks associated with viral infections during pregnancy. J Clin Invest 2017;127:1591–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan AK, Arah OA, Sullivan SG.. Performance of diagnostic coding and laboratory testing results to measure COVID-19 during pregnancy and associations with pregnancy outcomes. Paediatr Perinat Epidemiol 2022;36:508–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozo N, Valencia D, Newton SM, Avila G, Gonzalez MA, Sancken CL, Burkel VK, Ellington SR, Gilboa SM, Rao CY. et al. Severity of illness by pregnancy status among laboratory‐confirmed SARS‐CoV‐2 infections occurring in reproductive‐aged women in Colombia. Paediatr Perinat Epidemiol 2022;36:456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin D, Tanacan A, Anuk AT, Sinaci S, Besimoglu B, Oluklu D, Hendem DU, Beser DM, Yildirim M, Sakcak B. et al. Comparison of clinical features and perinatal outcomes between pre-variant and post-variant periods in pregnant women with SARS-CoV-2: analysis of 1935 cases. Arch Gynecol Obstet 2022a;306:1939–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin D, Tanacan A, Erol SA, Yucel Yetiskin FD, Besimoglu B, Ozden Tokalioglu E, Anuk AT, Turgut E, Goncu Ayhan S, Turgay B. et al. Management of pregnant women with COVID-19: a tertiary pandemic center experience on 1416 cases. J Med Virol 2022b;94:1074–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saimin J, Ridwan S, Irawaty I, Arimaswati A, Salman S, Hermawan W.. Clinical profile of pregnant women with COVID-19 hospitalized in regional referral hospital. Indones J Obstet Gynecol 2021;9:5–9. [Google Scholar]
- Santhosh J, Al Salmani M, Khamis F, Ali Al Ubaidani S, Al-Zakwani I.. Clinical characteristics of COVID-19 in pregnant women: A retrospective descriptive single-center study from a tertiary hospital in Muscat, Oman. Int J Gynaecol Obstet 2021;152:270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos CAD, Fonseca Filho GG, Alves MM, Macedo EYL, Pontes MGA, Paula AP, Barreto CTR, Zeneide FN, Nery AF, Freitas RAO. et al. Maternal and neonatal outcomes associated with mild COVID-19 infection in an obstetric cohort in Brazil. Am J Trop Med Hyg 2022;107:1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell RC, Macias DA, Garner WH, White AM, McIntire DD, Pruszynski J, Adhikari EH.. Examining the impact of trimester of diagnosis on COVID-19 disease progression in pregnancy. Am J Obstet Gynecol MFM 2022;4:100728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz DA, Avvad-Portari E, Babál P, Baldewijns M, Blomberg M, Bouachba A, Camacho J, Collardeau-Frachon S, Colson A, Dehaene I. et al. Placental tissue destruction and insufficiency from COVID-19 causes stillbirth and neonatal death from hypoxic-ischemic injury. Arch Pathol Lab Med 2022;146:660–676. [DOI] [PubMed] [Google Scholar]
- Sekkarie A, Woodruff R, Whitaker M, Kramer MR, Zapata LB, Ellington SR, Meaney-Delman DM, Pham H, Patel K, Taylor CA. et al. ; COVID-19-Associated Hospitalization Surveillance Network COVID-NET Surveillance Team. Characteristics and treatment of hospitalized pregnant women with COVID-19. Am J Obstet Gynecol MFM 2022;4:100715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentilhes L, De Marcillac F, Jouffrieau C, Kuhn P, Thuet V, Hansmann Y, Ruch Y, Fafi-Kremer S, Deruelle P.. Coronavirus disease 2019 in pregnancy was associated with maternal morbidity and preterm birth. Am J Obstet Gynecol 2020;223:914.e1–914.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sertel E, Demir M.. Evaluation of the effects of COVID-19 disease and the trimester in which the disease is diagnosed on obstetric and neonatal outcomes: a retrospective cohort study. J Obstet Gynaecol Res 2023;49:614–624. [DOI] [PubMed] [Google Scholar]
- Shah PT, Shah SR, Shah SR, Yadava PA, Patel BS, Chudasama TJ.. Fetomaternal outcome in COVID-19 infected pregnant women: a preliminary clinical study. Int J Reprod Contracept Obstet Gynecol 2020;9:3704–3711. [Google Scholar]
- Shmakov RG, Prikhodko A, Polushkina E, Shmakova E, Pyregov A, Bychenko V, Priputnevich TV, Dolgushin GO, Yarotskaya E, Pekarev O. et al. Clinical course of novel COVID-19 infection in pregnant women. J Matern Fetal Neonatal Med 2022;35:4431–4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silasi M, Cardenas I, Kwon JY, Racicot K, Aldo P, Mor G.. Viral infections during pregnancy. Am J Reprod Immunol 2015;73:199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V, Choudhary A, Datta MR, Ray A.. Maternal and neonatal outcomes of COVID-19 in pregnancy: a single-centre observational study. Cureus 2021;13:e13184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza RT, Cecatti JG, Pacagnella RC, Ribeiro-Do-Valle CC, Luz AG, Lajos GJ, Nobrega GM, Griggio TB, Charles CM, Bento SF. et al. ; REBRACO Study Group. The COVID-19 pandemic in Brazilian pregnant and postpartum women: results from the REBRACO prospective cohort study. Sci Rep 2022;12:11758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenton S, McPartland J, Shukla R, Turner K, Marton T, Hargitai B, Bamber A, Pryce J, Peres CL, Burguess N. et al. SARS-COV2 placentitis and pregnancy outcome: a multicentre experience during the alpha and early delta waves of coronavirus pandemic in England. EClinicalMedicine 2022;47:101389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhren JT, Meinardus A, Hussein K, Schaumann N.. Meta-analysis on COVID-19-pregnancy-related placental pathologies shows no specific pattern. Placenta 2022;117:72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunder A, , VargheseB, , DarwishB, , ShaikhoN, , Rashid M.. Impacts and effects of COVID-19 infection in pregnancy. Saudi Med J 2022;43:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghavi SA, Heidari S, Jahanfar S, Amirjani S, Aji-Ramkani A, Azizi-Kutenaee M, Bazarganipour F.. Obstetric, maternal, and neonatal outcomes in COVID-19 compared to healthy pregnant women in Iran: a retrospective, case-control study. Middle East Fertil Soc J 2021;26:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto MLS, Menezes MO, Andreucci CB, Knobel R, Sousa LAR, Katz L, Fonseca EB, Magalhães CG, Oliveira WK, Rezende-Filho J. et al. Maternal mortality and COVID-19. J Matern Fetal Neonatal Med 2022;35:2355–2361. [DOI] [PubMed] [Google Scholar]
- Tanacan A, Yazihan N, Erol SA, Anuk AT, Yucel Yetiskin FD, Biriken D, Ozgu-Erdinc AS, Keskin HL, Moraloglu Tekin O, Sahin D.. The impact of COVID-19 infection on the cytokine profile of pregnant women: a prospective case-control study. Cytokine 2021;140:155431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavakoli N, Chaichian S, Sadraei JS, Sarhadi S, Bidgoli SA, Rokhsat E, Anoushirvani K, Nikfar B, Mehdizadehkashi A.. Is it possible to reduce the rate of vertical transmission and improve perinatal outcomes by inclusion of remdesivir in treatment regimen of pregnant women with COVID-19? BMC Pregnancy Childbirth 2023;23:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The ESHRE COVID-19 Working Group, Ata B, Gianaroli L, Lundin K, Mcheik S, Mocanu E, Rautakallio-Hokkanen S, Tapanainen JS, Vermeulen N, Veiga A. Outcomes of SARS-CoV-2 infected pregnancies after medically assisted reproduction. Human Reproduction 2021;36:2883–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tug N, Yassa M, Köle E, Sakin Ö, Çak?r Köle M, Karateke A, Yiyit N, Yavuz E, Birol P, Budak D. et al. Pregnancy worsens the morbidity of COVID-19 and this effect becomes more prominent as pregnancy advances. Turk J Obstet Gynecol 2020;17:149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAPM (World Association of Perinatal Medicine) Working Group on COVID-19. Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection. Ultrasound Obstet Gynecol 2021;57:232–241. [DOI] [PubMed] [Google Scholar]
- Vesce F, Battisti C, Crudo M.. The inflammatory cytokine imbalance for miscarriage, pregnancy loss and COVID-19 pneumonia. Front Immunol 2022;13:861245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizheh M, Muhidin S, Aghajani F, Maleki Z, Bagheri F, Hosamirudsari H, Aleyasin A, Tehranian A.. Characteristics and outcomes of COVID-19 pneumonia in pregnancy compared with infected nonpregnant women. Int J Gynaecol Obstet 2021;153:462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vouga M, Favre G, Martinez-Perez O, Pomar L, Acebal LF, Abascal-Saiz A, Vila Hernandez MR, Hcini N, Lambert V, Carles G. et al. Maternal outcomes and risk factors for COVID-19 severity among pregnant women. Sci Rep 2021;11:13898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousden N, Bunch K, Morris E, Simpson N, Gale C, O'Brien P, Quigley M, Brocklehurst P, Kurinczuk JJ, Knight M.. The incidence, characteristics and outcomes of pregnant women hospitalized with symptomatic and asymptomatic SARS-CoV-2 infection in the UK from March to September 2020: a national cohort study using the UK Obstetric Surveillance System (UKOSS). PLoS One 2021;16:e0251123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses, 2000. Available from: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (16 April 2023,date last accessed).
- Wong SF, Chow KM, Leung TN, Ng WF, Ng TK, Shek CC, Ng PC, Lam PW, Ho LC, To WW. et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol 2004;191:292–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth KR, Olsen EO, Neelam V, Lewis EL, Galang RR, Oduyebo T, Aveni K, Yazdy MM, Harvey E, Longcore ND. et al. ; COVID-19 Pregnancy and Infant Linked Outcomes Team (PILOT). Birth and infant outcomes following laboratory-confirmed SARS-CoV-2 infection in pregnancy—SET-NET, 16 jurisdictions, March 29–October 14, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1635–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Sun R, Chen J, Xie Y, Zhang S, Wang X.. Radiological findings and clinical characteristics of pregnant women with COVID-19 pneumonia. Int J Gynaecol Obstet 2020a;150:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y-T, Li C, Zhang C-J, Huang H-F; FRCOG. Is termination of early pregnancy indicated in women with COVID-19? Eur J Obstet Gynecol Reprod Biol 2020b;251:271–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Guo J, Fan C, Juan J, Yu X, Li J, Feng L, Li C, Chen H, Qiao Y. et al. Coronavirus disease 2019 in pregnant women: a report based on 116 cases. Am J Obstet Gynecol 2020;223:111. E111–111. E114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap M, Debenham L, Kew T, Chatterjee SR, Allotey J, Stallings E, Coomar D, Lee SI, Qiu X, Yuan M. et al. ; PregCOV-19 Consortium. Clinical manifestations, prevalence, risk factors, outcomes, transmission, diagnosis and treatment of COVID-19 in pregnancy and postpartum: a living systematic review protocol. BMJ Open 2020;10:e041868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa M, Birol P, Mutlu AM, Tekin AB, Sandal K, Tug N.. Lung ultrasound can influence the clinical treatment of pregnant women with COVID-19. J Ultrasound Med 2021;40:191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazihan N, Tanacan A, Erol SA, Anuk AT, Sinaci S, Biriken D, Keskin HL, Moraloglu OT, Sahin D.. Comparison of VEGF-A values between pregnant women with COVID-19 and healthy pregnancies and its association with composite adverse outcomes. J Med Virol 2021;93:2204–2209. [DOI] [PubMed] [Google Scholar]
- Youssef AM, EL-Gelany S, Ibrahim MH, Gedawy AR.. Maternal and perinatal outcomes in pregnant women with Covid-19: a retrospective Egyptian study. Ann Neonatol J 2023;5:79–96. [Google Scholar]
- Zelini P, Perotti F, Scatigno AL, Dominoni M, Zavaglio F, Arossa A, Piccini S, Angelini M, Ghirardello S, Lilleri D. et al. Impact of SARS-CoV-2 infection during pregnancy and persistence of antibody response. New Microbiol 2022;45:181–189. [PubMed] [Google Scholar]
- Ziert Y, Abou-Dakn M, Backes C, Banz-Jansen C, Bock N, Bohlmann M, Engelbrecht C, Gruber TM, Iannaccone A, Jegen M. et al. ; COVID-19-Related Obstetric and Neonatal Outcome Study (CRONOS) Network. Maternal and neonatal outcomes of pregnancies with COVID-19 after medically assisted reproduction: results from the prospective COVID-19-Related Obstetrical and Neonatal Outcome Study. Am J Obstet Gynecol 2022;227:495.e1–495.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data collected including data on pregnancy loss is available at https://cgf.cochrane.org/news/covid-19-coronavirus-disease-fertility-and-pregnancy. Dissemination to participants and related patient and public communities: The PregCov-19 LSR Group will disseminate the findings through dedicated websites: www.birmingham.ac.uk/research/who-collaborating-centre/pregcov/index.aspx and https://cgf.cochrane.org/news/covid-19-coronavirus-disease-fertility-and-pregnancy as well as through social media.