Abstract
This review summarizes the correlation between diabetes mellitus (DM) and gastric cancer (GC) from the perspectives of epidemiology, drug use, and potential mechanisms. The association between DM and GC is inconclusive, and the positive direction of the association reported in most published meta-analyses suggests that DM may be an independent risk factor for GC. Many clinical investigations have shown that people with DM and GC who undergo gastrectomy may have better glycemic control. The potential link between DM and GC may involve the interaction of multiple common risk factors, such as obesity, hyperglycemia and hyperinsulinemia, H. pylori infection, and the use of metformin. Although in vitro and in vivo data support that H. pylori infection status and metformin can influence GC risk in DM patients, there are conflicting results. Patient survival outcomes are influenced by multiple factors, so further research is needed to identify the patients who may benefit.
1. Introduction
Diabetes mellitus (DM) is projected to affect 500 million people worldwide by 2030 [1]. Gastric cancer (GC) is a lethal tumor that affects the digestive system. It is the fifth most frequent cancer and the fourth major cause of cancer mortality in the world. There are nearly one million new GC cases and nearly 700,000 deaths worldwide per year [2]. However, despite a vast literature on the relationship between DM and cancer, the association with GC varied in different studies. Therefore, this review summarizes the correlation between DM and GC from the perspectives of epidemiology, drug use, and potential mechanisms.
2. Epidemiological Analysis of DM and GC
DM is a metabolic disorder characterized by insulin malfunction that is often accompanied by severe consequences, such as hyperglycemia, hypoinsulinemia, and insulin-like growth factor- (IGF-) related metabolic dysfunction [3]. DM increases the risk of certain cancer types, such as pancreatic cancer [4], breast cancer [5], endometrial cancer [6], and colorectal cancer [7] and increases mortality from any cancer [8]. DM is present in 8% to 18% of all cancer patients. Compared with nondiabetic patients, diabetic cancer patients have a 42% increased risk of death and a 21% increased risk of tumor recurrence [9]. Although epidemiological studies have shown that DM has a certain impact on gastrointestinal tumors [10], the association between DM and GC is still controversial.
2.1. DM Increases the Risk of GC
An analysis of 80,193 gastrointestinal cancers from five European and three Asian countries revealed that the overall prevalence of DM was 14.8% (11,866/80,193). Among them, the prevalence of DM was highest in colon and rectal cancer patients (15.5% vs. 15.3%, respectively) and 14.0% in GC patients, both of which were significantly correlated with the high incidence of DM [11]. A meta-analysis also revealed a statistically significant relationship between DM and GC incidence (RR = 1.11, 95% CI: 1.00-1.24, P = 0.045, I2 = 79.5%) [12]. The results of a large retrospective cohort study conducted in Korea that included 195,312 patients who had a more accurate diagnosis by endoscopic examination revealed that individuals with DM were at an elevated risk of developing GC (estimated adjusted HR = 1.76, 95% CI: 1.04-2.97) [13].
2.2. DM Does Not Increase the Risk of GC
A meta-analysis of 22 cohort studies and 8,559,861 participants found that DM had little or no change in the risk of GC [14]. There was no evidence of a significant difference in the RR for GC between men and women (RR = 1.10, 95% CI: 0.94-1.29, I2 = 22.9% in men; RR = 1.00, 95% CI: 0.90-1.11, I2 = 97.2% in women) [14]. Compared with normal blood glucose participants, the risk of GC was not increased among participants with prediabetes (HR = 1.07, 95% CI: 0.79-1.44), DM (HR = 0.77, 95% CI: 0.46-1.29), or any of these exposures (HR = 0.96, 95% CI: 0.73-1.27) [15]. The relationship between DM and GC is unclear based on previous epidemiological studies. A two-stage individual participant data meta-analysis including 5,592 cases of GC and 12,477 controls from 14 studies from North America, South America, Europe, and Asia did not find an association between DM and GC (pooled OR = 1.01, 95% CI: 0.94-1.07). However, the risk of gastric cardia cancer was significantly higher with T2DM (OR = 1.16, 95% CI: 1.02-1.33) [16].
2.3. Whether DM Increases the Risk of Death from GC
DM can disrupt the body's immune function and metabolic processes [17], leading to disturbances in energy balance that may impact the development and outcomes of cancer [18]. However, it is important to note that specific prognostic outcomes can vary from individual to individual, depending on factors such as DM management, treatment modalities (e.g., surgery and adjuvant chemotherapy), and antidiabetic medications such as insulin [19].
There are some studies that suggest that patients with DM may face a higher risk and lower survival rate in GC treatment [11–13]. Studies showed that high fasting blood glucose (≥126 mg/dL; RR = 1.09) increases the risk of GC [20]. The blood glucose variability in GC patients is significantly higher than that in non-GC patients, and higher blood glucose variability in patients without DM will also increase the risk of GC [21]. Preoperative metabolic syndrome, particularly hyperglycemia, predicted GC mortality in patients receiving radical gastrectomy, particularly in patients with early GC, according to a sizable cohort research by Hu et al. [22]. However, other studies have not observed this association. According to Miao et al., Zheng et al., and Dabo et al., there has not been much of a difference in the death rate or risk of getting GC in DM individuals [14–16]. In addition, Bae's meta-analysis of prospective cohort studies found no evidence linking a history of DM to an increased risk of GC [23].
Differences in study populations, exposure assessments, lengths of follow-up, and adjustment for confounders might explain the high degree of heterogeneity in the findings. In particular, the exposure assessments and duration of follow-up varied considerably across studies. Furthermore, confounding variables such as gender, age, BMI, population, race, culture, lifestyle, environment, and socioeconomic position will alter the incidence of diabetes or GC and may even raise the risk of GC among people with diabetes [24]. Significant gender and geographical disparities in the prevalence of type 2 diabetes mellitus (T2DM) and GC have emerged over the last 30 years, suggesting complicated links with race, immigration, culture, lifestyle, gene-environment interactions, socioeconomic level, and social role inequalities [25]. The influence of genetic effects, epigenetic processes, dietary variables, and lifestyle on the risk and result of T2DM and GC development differs between men and women [26]. Sex hormones influence insulin sensitivity and secretion, stomach emptying and glucose absorption, vascular function, energy metabolism, and inflammatory response in women with excess androgen or males with impaired gonadal activity [27]. GC has a significant male advantage, and greater levels of circulating dehydroepiandrosterone may be related to a decreased risk of noncardiac GC [28]. In addition, there is a link between blood levels of androgens, estrogen, and sex hormone-binding globulin in males with the chance of developing primary GC [29].
Furthermore, physiological and psychological variables contribute to gender variations in T2DM and GC risk and prognosis [30]. However, there is currently a scarcity of randomized controlled studies that show gender-specific benefits using well-designed intervention measures. Gender differences must be studied using appropriate animal models and translational research to better understand the pathophysiology and complicated interplay of hormones, genes, lifestyle, and environment in T2DM and GC patients. As a result, the effect of DM on the risk of GC or death must be explored further. Understanding the possible impact of DM on GC risk is a critical component of DM treatment.
2.4. Remission of DM after Gastrectomy
Many clinical investigations have shown that people with DM and GC who undergo gastrectomy may have better glycemic control [31, 32]. T2DM remission rates vary from 42.5% to 65.4% in patients with GC following gastrectomy (Table 1). A meta-analysis of 11 randomized controlled trials provided class 1A evidence demonstrating that patients who undergo bariatric surgery experience T2DM remission [33]. After gastrectomy, insulin resistance is shown to decrease, and fasting glucose returned to normal; however, the cause of remission is still unknown [34]. According to An et al.'s study, the length of T2DM remission was substantially associated with the degree of remission [35]. Kim et al. concluded that BMI reduction was significantly associated with remission of T2DM [36]. Total gastrectomy with RY reconstruction has a greater remission rate than other surgical procedures; however, it is unclear whether the scope of the gastrectomy or the manner of reconstruction has a role in T2DM remission. According to Wang et al., the degree of gastrectomy, rather than the method of reconstruction, was the most important factor determining T2DM remission [37]. On the other hand, Choi et al. found that RY reconstruction is crucial for T2DM remission [38]. A meta-analysis by Peng et al. suggested that only the degree of gastrectomy can affect T2DM remission [39], which may also affect overall survival. Wei et al. found that recovery from preexisting T2DM following radical gastrectomy was highly related to higher overall survival in a sample of 67 patients [40]. Although the mechanism of T2DM remission following gastrectomy is unknown, bariatric surgery may promote T2DM remission by promoting lifestyle modifications, including reduced food intake, weight loss, and intestinal malabsorption. In addition, there are a few theories that might explain why hyperglycemia improves following gastrectomy. The foregut theory states that resection of the duodenum and proximal jejunum may prevent the secretion of some signals that promote insulin resistance, but this signal is still unknown and remains less well proven in human subjects [41]. According to the hindgut hypothesis, rapid transport of unabsorbed nutrients to the distal intestine might boost intestinal hormone release [42]. In addition, ghrelin, another gut hormone that stimulates appetite and food intake, is mainly produced by gastric X/A cells, and its level is reduced after gastrectomy [43]. Changes in gut microbiota following the Billroth II or Roux-en-Y gastric bypass have also been linked to DM remission and improved metabolic control in two recent investigations; the main manifestations were reduced incidence of metabolic syndrome and T2DM and increased postoperative intestinal microbial richness and diversity [44, 45].
Table 1.
Remission of DM after gastrectomy.
| Author | Surgery | Sample size | CR | PR | Follow-up (mo) |
|---|---|---|---|---|---|
| Lee et al. [34] | RYTG, BI, BII, RYGJ | 229 | 19.70% | 37.10% | NA |
| An et al. [35] | RYTG, BI, BII | 64 | 3.10% | 54.70% | 12 |
| Kim et al. [36] | RYTG, BI, BII | 385 | 15.10% | 30.40% | 33.7 |
| Choi et al. [38] | RYTG, BI | 40 | 2.50% | 40.00% | 12 |
| Wei et al. [40] | RYTG, BII | 67 | 26.90% | 32.80% | 57.4 |
CR: complete remission; PR: partial remission; RYTG: Roux-en-Y total gastrectomy; BI: Billroth I reconstruction; BII: Billroth II reconstruction; RYGJ: subtotal gastrectomy with Roux-en-Y gastrojejunostomy reconstruction; NA: not available.
3. Potential Mechanisms between DM and GC
Although the connection between DM and GC is yet unknown, numerous biological theories have been postulated, including obesity, hyperglycemia and hyperinsulinemia, Helicobacter pylori (H. pylori) infection, and the use of certain medications (e.g., metformin) [16, 46–49] (Figure 1).
Figure 1.
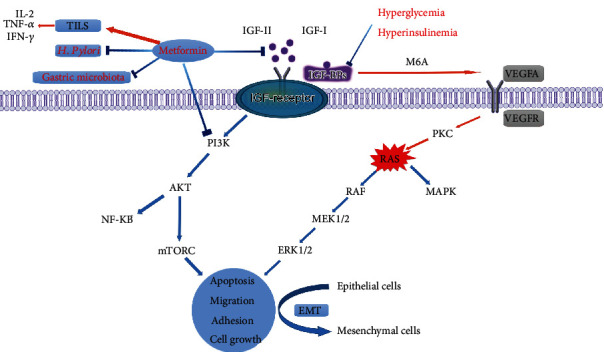
Potential mechanisms between DM and GC. Abb: IL-2: interleukin-2; TNF-α: tumor necrosis factor alpha; INF-γ: interferon-gamma; TILS: tumor-infiltrating lymphocytes; IGF: insulin-like growth factor; IGFBP: insulin-like growth factor binding protein; VEGF: vascular endothelial growth factor; EMT: epithelial-mesenchymal transition.
3.1. Influence of Obesity on DM and GC
Multiple meta-analyses suggest that obesity and unhealthy lifestyles may have deleterious effects on GC risk [50]. The prevalence of DM significantly increased from 1995 to 2014, and associations between DM and obesity are well established [51]. Obesity is associated with insulin resistance, compensatory hyperinsulinemia, metabolic syndrome, and T2DM. Overweight or obesity is linked to a higher risk of GC, and the intensity of this link is stronger as BMI rises, particularly in Asian populations [52]. Rawla and Barsouk suggested that DM patients were more likely to suffer from obesity and gastroesophageal reflux disease, leading to a significant increase in the risk of gastric cardiac cancer [53]. On the other hand, Lin et al. found no change in the incidence of stomach cardiac carcinoma attributable to DM across BMI strata [54], suggesting that other factors unrelated to obesity may be involved in the pathogenesis of gastric cardiac cancer. Further studies are needed to prove these hypotheses.
3.2. Effects of Hyperglycemia and Hyperinsulinemia on DM and GC
At the molecular level, in vitro and in vivo studies show different mechanisms for hyperglycemia and hyperinsulinemia leading to the development of GC, such as increased cell proliferation, promotion of angiogenesis, oxidative DNA damage and overstimulation of tumorigenic pathways [55]. Hyperglycemia has been linked to tumor vascularity, metastasis, and the expression of vascular endothelial growth factor in many investigations [56]. Hyperglycemia can lead to DNA damage directly or can damage through the production of reactive oxygen species (ROS). Metabolism-induced oxidative stress may promote epithelial mesenchymal transformation, leading to the accumulation of tumor genes and tumor suppressor gene mutations, promoting gastric mucosal damage and interfering with repair [13]. Furthermore, hyperglycemia can generate more energy through glycolysis and lactic acid pathways, leading to energy balance imbalance, affecting intracellular metabolism and damaging immune function, complement activation, and antioxidant systems [57]. Patients with DM may have increased susceptibility to H. pylori infection and delayed wound healing after infection due to immunosuppression caused by hyperglycemia [58].
Hyperglycemia can also significantly trigger insulin secretion, and hyperinsulinemia can overactivate insulin signaling. Furthermore, chronic hyperglycemia may also cause an increase in the formation of ROS and oxidative stress [59], both of which are thought to promote carcinogenesis and cancer development [60]. Additionally, insulin resistance in DM promotes inflammation and activates nuclear factor-κB (NF-κB), which is a light-chain enhancer of activated B cell signaling that plays a major role in GC development and progression [61]. Hyperglycemia can also provide more glucose to tumor cells, promote tumor proliferation and migration, and activate GC cells to migrate to lymph nodes [62]. In addition, abnormal fluctuations in glucose levels in DM patients also increase oxidative stress, endothelial dysfunction, and subclinical inflammation. NADPH oxidase activity in mitochondria induces superoxide production [63], and the AKT signaling pathway is inhibited by increased NF-κB and caspase-3 expression [64]. Therefore, fluctuations in fasting glucose are considered to be linked to an increased risk of GC [21, 65]. Plasma insulin levels were also positively associated with GC compared with hyperglycemia [66].
Hyperinsulinemia leads to elevated levels of insulin-like growth factor 1 (IGF-I), a potent pro-mitogen that can cause cancer and decrease apoptosis in cancer cells [67]. Hyperinsulinemia may also overstimulate tumorigenic pathways, such as IGF-II/IR-A signaling, which is thought to be a key promoter of cancer in people with diabetes or prediabetes [66, 68]. Insulin receptor (IR), IGF-I, and IGF-II are all significantly expressed in GC cells, and the IGF-I/IGF-IR pathway has been considered an important therapeutic target for GC [69]. The fact that GC cell survival is dependent on IR but not the IGF-I receptor suggests that IGF-I/IGF-II increase GC cell survival through IR [70]. Hyperinsulinemia and overexpression of IGF can activate the mitotic pathway or stimulate tumor growth by inhibiting the expression of IGF-binding proteins (IGFBPs), which play a key role in the carcinogenesis and metastasis of GC [71]. Hyperinsulinemia may also downregulate IGFBP levels, indirectly leading to elevated levels of IGF [72]. In addition, insulin is a mitogen and cell survival factor expressed in almost all cell types that activates signal transduction, stimulates cell growth, and promotes cell survival and is considered to be a potential mechanism for the association between DM and cancer [55]. Saisana et al. confirmed that high expression of the insulin receptor can be detected in metastatic GC cells and cell lines, which can stimulate PI3K/Akt signal transduction, cell proliferation, and the survival of GC cells [73]. Knockdown of the insulin receptor can inhibit tumor cell proliferation and induce programmed cell death. These results suggest that insulin and insulin receptors can synergistically promote the occurrence and development of GC. Some studies have also found that H. pylori infection can potentially disrupt the balance of gastrointestinal microbiota, consequently impacting energy metabolism and insulin sensitivity in the body. This disruption may lead to insulin resistance, where the cells become less responsive to insulin, ultimately developing hyperinsulinemia [74]. Previous studies have also confirmed that insulin use in DM patients is significantly associated with a high incidence of H. pylori eradication [75].
3.3. Effects of Biomarker on DM and GC
IGFBP, IGF-I, and numerous growth factors, including vascular endothelial growth factor (VEGF), are now known biomarker between DM and GC. IGFBP family members have been shown to have a role in tumor formation and progression, and they may be valuable prognostic indicators in a variety of malignant tumors, including ovarian cancer [76], pancreatic cancer [77], and GC [78]. Currently, there is a scarcity of thorough research on IGFBP as a biomarker for GC.
Bioinformatics investigation reveals that IGFBP expression differs among GC cell lines and tissues [79]. IGFBP-1 is a blood biomarker with good diagnostic sensitivity for upper gastrointestinal cancer. Overexpression of IGFBP-1 inhibits MMP-9-induced GC cell migration and protects against H. pylori-induced GC [80]. Although clinical studies have shown that IGFBP-3 can be used as a biomarker for the diagnosis and prognosis of esophageal gastric junction adenocarcinoma [81] and that the simultaneous decrease of IGFBP-3 and increase of IGF-I may promote tumor growth [82], the mechanism underlying the relationship between these two potential biomarkers and GC has not been established. IGFBP-5 overexpression promotes the activity of the tumor suppressor factor PKNOX2, which can limit the development of GC [83]. IGFBP-7 mRNA expression is associated with a poor outcome in GC [84].
GC patients have a systemic biochemical imbalance of several growth factors, including notably raised levels of IGF-I and VEGF in advanced GC patients [85]. According to Saisana et al.'s findings, GC cells' survival depends on insulin receptors, insulin, and IGF signaling pathways that play a prominent role in gastric adenocarcinoma [73]. Higher IGF-IR expression is linked to a shorter overall survival. Serum IGF-I levels are considerably higher in patients with H. pylori-induced GC [86]. Upregulation of IGF-IR may activate the PI3K/AKT/mTOR signaling pathway, promoting GC cell migration and invasion [87].
VEGF is a critical proangiogenic factor that has emerged as the primary target of immunotherapy for GC [88]. Furthermore, animal models have demonstrated that IGFBP-4 can increase VEGF-induced angiogenesis [89] and that the m6A binding protein METTL3 can target VEGFA via IGFBP-2, encouraging the creation of colorectal cancer vasculogenic mimicry via the PI3K/AKT/mTOR and ERK1/2 signaling pathways [90]. Because IGF-IR regulates the production of VEGF ligands in GC cells and contributes to angiogenesis and lymphangiogenesis, inhibiting the IGF-I receptor can increase the antitumor impact of bevacizumab [91].
The interaction between numerous extracellular vesicles and immune-related cytokines released by GC cells [85], which are thought to be connected with the initiation and poor prognosis of GC [92], may be related to the imbalance of these growth factors. However, the peripheral concentration of growth factors does not have significant diagnostic potential. It cannot be utilized as an independent biomarker in patients to differentiate between different forms of GC. As a result, more experimental and clinical investigations, including other indicators, are required for validation.
3.4. Effect of Helicobacter Pylori Infection on DM and GC
H. pylori, a gram-negative, active, microaerobic, and spiral-shaped bacterium, is a major known risk factor for GC, and H. pylori infection is closely associated with more than 60% of GC cases [93]. Currently, the only natural host of H. pylori is the human stomach. The World Health Organization (WHO) has classified H. pylori as a Class I carcinogen [94]. H. pylori can cause oxidative stress and DNA damage through specific toxic cytokines such as cytotoxin-associated gene A (CagA), vacuolar cytotoxin A (VacA), and outer membrane protein and eventually lead to tumor formation [95]. Mucosal integrity can be compromised by phosphorylated CagA, which controls cytoskeleton and intercellular connections and their shape and function [96]. By turning on the carcinogenic YAP pathway, CagA also promotes GC's epithelial-mesenchymal transition [97]. Both CagA and VacA may induce autophagy [98, 99], and VacA is another virulence factor that can alter host cell metabolism by inhibiting mTORC1 [100].
Recent studies have demonstrated that H. pylori infection is closely related to DM and insulin resistance [101]. The creation of biofilms, decreased bacterial diversity, drastically reduced facultative anaerobic function, and increased abundance of H. pylori and Haemophilus are only a few of the important alterations in the stomach microbiota that can result from H. pylori infection [102]. Additionally, it was discovered that H. pylori corejected strongly with Fusobacterium, Neisseria, Prevotella, Wechterella, and Roche in patients with GC [103], and the gastrointestinal microbiota of these microorganisms would play a role in the pathogenesis of DM by controlling fatty acid synthesis and energy metabolism [104]. C.H. Tseng and F.H. Tseng found that patients with DM were shown to have a greater infection rate, a poorer eradication rate, and a higher reinfection rate [48]. H. pylori infection can lead to DNA damage by increasing the production of reactive oxygen species in epithelial cells of the gastrointestinal system, resulting in gastric mucosal atrophy, intestinal metaplasia, and, ultimately, the development of GC [105]. Ikeda et al. reported a significantly increased risk of GC in DM patients with H. pylori infection with baseline HBA1c levels higher than 6.0% [106]. Results of a large cohort study by Youn et al. showed that GC was associated with first-degree relatives with GC (OR = 3.23) in the absence of H. pylori and with hyperglycemia (OR = 1.98) in the presence of H. pylori [107]. However, according to Jun et al., there is no link between blood glucose and the risk of GC in either H. pylori-positive or H. pylori-negative DM patients [108]. In prediabetes patients, no correlation has been found between H. pylori infection and the risk of GC [109]. Interestingly, GC was shown to reduce the abundance of Helicobacter [110], H. pylori infection decreased with the progression of GC, and the diagnostic effectiveness of H. pylori decreased [111]. These contradictory findings imply that the impact of H. pylori infection on the risk of GC in DM individuals should be investigated further.
3.5. Effects of Gastric Microbiota (Other than H. Pylori) on DM and GC
Although successful H. pylori eradication does not completely prevent the development of GC and only about 1% of infected individuals develop GC [112], H. pylori infection plays a critical role in the early stages of carcinogenesis by increasing inflammation and gradually degrading gastric epithelial structure and function [113]. Additionally, compared to superficial gastritis, intestinal metaplasia and GC exhibit much lower levels of microbial diversity, which is now understood to be a characteristic of inflammatory illnesses and malignancies [114]. Some Escherichia coli branched-chain proteins, Bacteroides fragilis, Clostridium nuclear, and other pathogenic bacteria may contribute to the development of colorectal cancer [115]. Compared with the microbiota in chronic gastritis, the microbiota in GC patients not only increased the function of nitrite reductase, which promoted the reduction of nitrite to nitric oxide, but also increased the function of nitrite reductase, which promoted the reduction of nitrite to nitrite [112, 116]. Therefore, in addition to H. pylori, other gastric microorganisms may also contribute to the persistent inflammation of gastric mucosa and the development of GC, including Citrobacter, Clostridium, Lactobacillus, Achromobacter, and Rhodococcus, which reside in the intestinal mucosa as commensals [117].
All of these findings suggest that the mechanism by which bacteria promote tumor growth may be connected to producing an inflammatory response and altering host immunological function [118]. The immune system is an essential regulator that promotes or inhibits tumor biological function [119], and the intestinal microbiota can drive immune system development and function [120], as well as alter intestinal function and immune system [121]. More and more research suggests that the gastrointestinal symbiotic microflora can modulate host immunity and maintain host immunological homeostasis. For example, in the GC microenvironment, the amount of BDCA2+ plasmacytoid dendritic cells is positively connected with the number of stenotrophomonas, whereas the number of Foxp3+ regulatory T cells is strongly correlated with the number of selenodont [122]. An imbalance in the microorganisms of the intestine promotes the establishment of an immunosuppressive microenvironment [123]. AMP, IgA, ROC, and phagocytosis are ways the immune system modulates the microbiota. In turn, the microbiome creates compounds that control immune system activity [124].
Although the influence of H. pylori infection on the incidence of GC in DM individuals is still debated, alterations in gastrointestinal microbiota other than H. pylori have been linked to DM, and these gastrointestinal microbiota are thought to be key players in the interaction between H. pylori infection and metabolic diseases such as DM [125]. The study discovered abnormalities in the gastrointestinal microbiota of DM. Among the commonly reported findings, the genera of A. muciniphila [126, 127], Bifidobacterium [128], Bacteroides [129], Faecalibacterium [130], F. prausnitzii [131], C. leptum [132], Oscillospiraceae [126], and Akkermansia [133] were negatively associated with T2DM, while the genera of Ruminococcus [131, 134], Dorea [135], and Blautia [126] were positively associated with T2DM. These microbiome alterations influence inflammation, glucose and lipid metabolism, insulin sensitivity, and overall energy balance [135, 136]. For example, lipopolysaccharides, as a product of gastrointestinal microbiota, can promote metabolic endotoxemia and low-grade inflammation [137], and Roseburia intestinalis, Bacteroides fragilis, Akkermansia muciniphila, Lactobacillus plantarum, and L. casei can stimulate the production of anti-inflammatory cytokines and chemokines [138]. R. intestinalis can increase T regulatory cell development, stimulate TGF-β, and suppress intestinal inflammation [139]. Bacteroides also increased gene expression in T regulatory cells [140]. L. plantarum, L. paracasei, and L. case can decrease IL-1β, monocyte chemoattractant protein-1, intercellular adhesion molecule-1, IL-8, CD36, and C-reactive protein [141]. Lactobacillus [142] and Akkermansia [143] have been found to suppress TNF-α. L. paracasei and microbial anti-inflammatory molecule from F. prausnitzii inhibit the activity of NF-κB [144]. As a metabolic product of gastrointestinal microbiota, short-chain fatty acids (SCFA) can not only directly prevent low-grade inflammation and enhance the secretion of glucagon-like peptide 1 (GLP-1) but also increase insulin sensitivity and affect cell function and insulin secretion [145].
Changes in the composition, variety, and activity of the microbiota can cause a disruption in glucose metabolism, which is a key factor in the development of T2DM [146]. Bifidobacterium lactis can both boost glycogen production and decrease the expression of gluconeogenesis-related genes in the liver, such as glucose-6-phosphatase and phosphoenolpyruvate carboxykinase [147]. It can also improve endotoxin-related inflammation and impaired intestinal barrier function, perhaps with antidiabetic benefits [148]. Lactobacillus butyrate reduces insulin resistance in the liver by raising mRNA levels of PI3K, insulin receptor substrate-2, AMPK, Akt2, and glycogen production [149]. Lactobacillus tyrosine also lowers blood sugar levels via the cholic acid-chlorine exchange pathway [150]. Furthermore, certain gastrointestinal bacteria can promote fatty acid oxidation and energy expenditure while decreasing fatty acid synthesis, improving T2DM, such as Akkermansia muciniphila, Bacteroides acidifaciens, Lactobacillus gasseri, and SCFA [136]. Moreover, the products of these microorganisms, such as butyrate, can promote fatty acid oxidation and thermogenesis by inhibiting the histone deacetylation process in the muscle, thereby increasing energy expenditure by promoting mitochondrial function in the muscle [151].
In conclusion, a diverse gastrointestinal microbiota is critical for general metabolic health. The intestinal microbiota may be a crucial regulator of host glucose metabolism and immune response. When the microbiota is out of balance, it can contribute to pathological processes such as GC and DM. However, the gastrointestinal microbiota is a complex ecosystem, and further study is needed to determine which microorganisms are responsible for the pathophysiology and molecular processes of GC and DM.
3.6. Effect of Metformin on DM and GC
Metformin, used as a first-line medicine in the treatment of DM, has a direct anticancer impact on a wide variety of tumor cells, including tumor stem cells, in both insulin-dependent and insulin-independent models [152]. It can not only promote the expression of metabolic checkpoints related to T cells and immunosuppressive cells in the tumor environment in cancer cells [153, 154] but also has systemic impacts on metabolism by interfering with gastrointestinal microbiota [155]. In vitro and in vivo model studies have shown that in digestive system cancers, metformin provides chemoprophylactic effects and direct therapeutic action [156], and it has the potential to be a chemical and radiosensitizer, increasing the sensitivity of cancer cell lines to 5-fluorouracil (5-FU) and paclitaxel [157]. Most clinical studies have demonstrated that metformin can reduce the risk of gastrointestinal cancer and improve survival rates [158]. However, there is no solid evidence showing that metformin usage increases the risk of GC [159, 160].
Metformin has been proven to protect against GC in various observational studies in recent years [161, 162] (Table 2). Cheung et al. showed that metformin can reduce GC risk (HR = 0.49, 95% CI: 0.24-0.98), which decreases further with increasing dose and duration [162]. Tseng also demonstrated that metformin reduces GC risk, especially when the cumulative duration exceeds 2 years [163]. Another meta-analysis also showed a 21% reduction in GC risk with the use of metformin (HR = 0.790; 95% CI: 0.624-1.001), especially in Asian populations [164]. Metformin was shown to minimize GC recurrence in gastrectomy patients in two retrospective investigations [165, 166]. Despite this, some observational studies in the USA [167], the Netherlands [168], and Sweden [169] did not show a lower risk of GC associated with metformin use. Whether metformin can improve the prognosis of GC in patients with DM remains controversial. In the study of Dulskas et al., although metformin was associated with a reduced risk of GC, it did not affect the survival rate of patients with DM and GC [170, 171]. The studies conducted by Baglia et al. and Chen et al. did not similarly observe the survival benefits of metformin for GC [172, 173]. In contrast, metformin improved overall survival but not cancer-specific survival. Studies conducted by Lacroix et al. [174], Seo et al. [166], and Chung et al. [175] showed that metformin can improve the survival rate of patients with T2DM and reduce GC recurrence.
Table 2.
Clinical studies of metformin for the treatment of GC.
| Author | Study design | Inclusion criteria | HR |
|---|---|---|---|
| Tseng [163] | Retrospective cohort study | DM2 + antidiabetic drugs | HR: 0.45 (0.36-0.56) |
| Lee et al. [165] | Retrospective cohort study | GC + gastrectomy | HR: 0.58 (0.37-0.93) |
| de Jong et al. [168] | Retrospective cohort study | DM2 + oral antidiabetic drugs | HR: 0.97 (0.82-1.15) |
| Zhou et al. [183] | Meta-analysis, 7 cohort studies | GC + metformin | HR: 0.76 (0.64-0.91) |
| Lacroix et al. [174] | Retrospective cohort study | GC | HR: 0.86 (0.56-1.33) |
| Zheng et al. [169] | Prospective cohort study | DM2 + antidiabetic drugs | Noncardia: HR: 0.93 (0.78-1.12). Cardia: HR: 1.49 (1.09-2.02) |
| Baglia et al. [172] | Prospective cohort study | Breast, CRC, lung, and GC patients | OS-HR: 1.11 (0.81-1.53) |
| Seo et al. [166] | Retrospective cohort study | GC + curative gastrectomy | HR: 0.45 (0.30-0.66) |
| Dulskas et al. [170] | Retrospective cohort study | DM2 + GC | SIR: 0.75 (0.66-0.86) |
| Shuai et al. [164] | Meta-analysis, 11 cohort studies | GC + metformin | HR: 0.79 (0.62-1.00) |
Most previous clinical studies have been retrospective and often limited by immortal time bias and selection bias, and the link between metformin use and GC risk has been exaggerated [176]. More clinical research is needed to validate the role of metformin in the treatment and chemoprophylaxis of GC. In particular, in in vitro and in vivo studies on metabolism and cell cycle arrest, possible therapeutic targets for metformin have been identified to enhance the anticancer effects of chemotherapy by regulating inflammation. In tumor xenograft models, metformin alone decreased tumor volume, and cisplatin, rapamycin, or both boosted the impact of each treatment alone and blocked GC cell peritoneal spread [177]. In vitro studies have shown that combining metformin with cisplatin, adriamycin, and paclitaxel may improve the unique effects of each treatment, and the combination with the three chemotherapy drugs can effectively induce the apoptosis of AGS cells [178]. However, the biological mechanism of this association remains unclear; several possible mechanisms could explain the protective effect of metformin. First, metformin directly activates AMPK and inhibits cell proliferation by inhibiting cancer-related central signaling pathways, such as the PI3K/Akt/mTOR pathway [179]. Second, metformin-induced decreases in IGF concentrations in circulating insulin may lower activation of IGF/IGF1-R signaling, resulting in reduced growth promotion and mitogenesis [180]. As a result, the anticancer effects of metformin may be attributed to its capacity to modify the metabolic milieu or to directly act on tumor cells. Third, the significant intracellular metabolic changes induced by metformin are the reduced accumulation of glycolytic intermediates and the synergistic reduction in tricarboxylic acid cycle intermediates, contributing to a reduction in gluconeogenesis [181]. The activation of AMPK promotes glucose uptake in fat and muscle, inhibiting tumor cell proliferation and migration [182]. Fourth, the protective effect of metformin may be related to the inhibition of HIF1α/PKM2 signal transduction [183]. Metformin induces the downregulation of hypoxia-inducible factor 1α and TNF-α, which can inhibit angiogenesis and improve immune surveillance by reducing tumor hypoxia [184]. Finally, studies in recent years have suggested that metformin may have a potential protective effect against H. pylori infection. After eradication of H. pylori, metformin reduced the risk of GC by 51% in DM patients [138]. On the one hand, the persistent inflammatory response brought on by H. pylori colonization is the strongest single risk factor for GC [185]. Metformin plays an anti-inflammatory role by inhibiting cell signaling pathways and reducing the production of proinflammatory factors [186], which can reduce the inflammatory response brought on by H. pylori. On the other hand, metformin can regulate the function of the immune system, including enhancing the activity of natural immune cells and regulating the immune response [187]. In addition to improving the body's resistance to H. pylori infection, metformin can also enhance the effectiveness of cancer treatments, though the molecular mechanisms underlying these effects are not fully understood [188]. Recent research has also demonstrated that metformin can not only regulate gastrointestinal microbiota in composition and function to enhance its glucose-regulating effect [189] but also promote gastric acid secretion by activating AMPK to differentiate gastric epithelial progenitor cells into acid-secreting parietal cells [190], thereby alleviating the reduction in gastric acid secretion brought on by H. pylori infection [116]. This enhances metformin's glucose-regulating effect. Metformin may have the potential to be an anti-GC medication by encouraging the differentiation of gastric epithelial progenitor cells into acid-secreting parietal cells [191], which is thought to be important in H. pylori infection and the occurrence and development of GC [192].
The changes in intracellular pathways caused by tumorigenesis and the underlying mechanism of the antitumor activities of metformin have been confirmed, revealing new therapeutic targets. However, these are not the only treatments available to reduce cancer risk. Insulin resistance, DM, the chronic diseases associated with inflammation in the microenvironment, and specific tumor-driven oncogenic pathways may interfere with the direct and indirect antitumor effects of metformin. Although epidemiological in nature, in vivo and vitro studies and clinical data support the benefit of metformin in some patients with digestive tumors, but survival outcomes are influenced by a variety of factors, such as cancer type, differentiation, staging, and treatment. Therefore, to fully understand metformin use in gastrointestinal tumors, rigorous clinical trials are needed to identify patients who may benefit from metformin.
4. Conclusions
DM is linked to an increased risk of cancer and cancer-related mortality, and the association between DM and GC is inconclusive. The positive association reported in most published meta-analyses suggests that DM may be an independent risk factor for GC, regardless of statistical significance. Potential mechanisms may include hyperinsulinemia, insulin resistance, elevated IGF-I levels, oxidative stress, chronic inflammation, and anti-insulin medication use. Activation of these signaling pathways is responsible for the development of GC in DM patients. Understanding the relationship between DM and GC may provide novel therapeutic strategies to counter the poor prognosis caused by this correlation.
Acknowledgments
This paper is supported by the Hangzhou Science and Technology Development Plan Project (20201203B56).
Data Availability
The data used to support the findings of this study are included in the article.
Conflicts of Interest
All the authors have no competing interest.
Authors' Contributions
Zhe Zhang designed the project, collected data, and contributed to the analysis. Li Wang analyzed the data and did all the experiments. Zhe Zhang drafted the manuscript. All the authors revised and corrected the manuscript.
References
- 1.van Baar A. C. G., Holleman F., Crenier L., et al. Endoscopic duodenal mucosal resurfacing for the treatment of type 2 diabetes mellitus: one year results from the first international, open-label, prospective, multicentre study. Gut . 2020;69(2):295–303. doi: 10.1136/gutjnl-2019-318349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H., Ferlay J., Siegel R. L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians . 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Simon T. G., King L. Y., Chong D. Q., et al. Diabetes, metabolic comorbidities, and risk of hepatocellular carcinoma: results from two prospective cohort studies. Hepatology . 2018;67(5):1797–1806. doi: 10.1002/hep.29660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho J., Scragg R., Pandol S. J., Goodarzi M. O., Petrov M. S. Antidiabetic medications and mortality risk in individuals with pancreatic cancer-related diabetes and postpancreatitis diabetes: a nationwide cohort study. Diabetes Care . 2019;42(9):1675–1683. doi: 10.2337/dc19-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shao S., Gill A. A., Zahm S. H., et al. Diabetes and overall survival among breast cancer patients in the US military health system. Cancer Epidemiology, Biomarkers & Prevention . 2018;27(1):50–57. doi: 10.1158/1055-9965.EPI-17-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Z., Yu Z., Meng X., et al. Long noncoding RNA GAS5 impairs the proliferation and invasion of endometrial carcinoma induced by high glucose via targeting miR-222-3p/p27. American Journal of Translational Research . 2019;11(4):2413–2421. [PMC free article] [PubMed] [Google Scholar]
- 7.Saito A., Kitayama J., Horie H., et al. Metformin changes the immune microenvironment of colorectal cancer in patients with type 2 diabetes mellitus. Cancer Science . 2020;111(11):4012–4020. doi: 10.1111/cas.14615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qi J., He P., Yao H., et al. Cancer risk among patients with type 2 diabetes: a real-world study in Shanghai, China. Journal of Diabetes . 2019;11(11):878–883. doi: 10.1111/1753-0407.12926. [DOI] [PubMed] [Google Scholar]
- 9.Chatterjee A., Kosmacek E. A., Shrishrimal S., McDonald J. T., Oberley-Deegan R. E. MnTE-2-PyP, a manganese porphyrin, reduces cytotoxicity caused by irradiation in a diabetic environment through the induction of endogenous antioxidant defenses. Redox Biology . 2020;34, article 101542 doi: 10.1016/j.redox.2020.101542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Jong R. G. P. J., Peeters P. J. H. L., Burden A. M., et al. Gastrointestinal cancer incidence in type 2 diabetes mellitus; results from a large population-based cohort study in the UK. Cancer Epidemiology . 2018;54:104–111. doi: 10.1016/j.canep.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Roderburg C., Loosen S. H., Hoyer L., Luedde T., Kostev K. Prevalence of diabetes mellitus among 80,193 gastrointestinal cancer patients in five European and three Asian countries. Journal of Cancer Research and Clinical Oncology . 2022;148(5):1057–1062. doi: 10.1007/s00432-021-03861-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian T., Zhang L. Q., Ma X. H., Zhou J. N., Shen J. Diabetes mellitus and incidence and mortality of gastric cancer: a meta-analysis. Experimental and Clinical Endocrinology & Diabetes . 2012;120(4):217–223. doi: 10.1055/s-0031-1297969. [DOI] [PubMed] [Google Scholar]
- 13.Yang H. J., Kang D., Chang Y., et al. Diabetes mellitus is associated with an increased risk of gastric cancer: a cohort study. Gastric Cancer . 2020;23(3):382–390. doi: 10.1007/s10120-019-01033-8. [DOI] [PubMed] [Google Scholar]
- 14.Miao Z. F., Xu H., Xu Y. Y., et al. Diabetes mellitus and the risk of gastric cancer: a meta-analysis of cohort studies. Oncotarget . 2017;8(27):44881–44892. doi: 10.18632/oncotarget.16487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng J., Rutegård M., Santoni G., et al. Prediabetes and diabetes in relation to risk of gastric adenocarcinoma. British Journal of Cancer . 2019;120(12):1147–1152. doi: 10.1038/s41416-019-0470-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dabo B., Pelucchi C., Rota M., et al. The association between diabetes and gastric cancer: results from the stomach cancer pooling project consortium. European Journal of Cancer Prevention . 2022;31(3):260–269. doi: 10.1097/CEJ.0000000000000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abd Ellah N. H., Ahmed E. A., Abd-Ellatief R. B., Ali M. F., Zahran A. M., Hetta H. F. Metoclopramide nanoparticles modulate immune response in a diabetic rat model: association with regulatory T cells and proinflammatory cytokines. International Journal of Nanomedicine . 2019;14:2383–2395. doi: 10.2147/IJN.S196842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.García-Jiménez C., Gutiérrez-Salmerón M., Chocarro-Calvo A., García-Martinez J. M., Castaño A., De la Vieja A. From obesity to diabetes and cancer: epidemiological links and role of therapies. British Journal of Cancer . 2016;114(7):716–722. doi: 10.1038/bjc.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu H., Chen K., Jia X., et al. Metformin use is associated with better survival of breast cancer patients with diabetes: a meta-analysis. The Oncologist . 2015;20(11):1236–1244. doi: 10.1634/theoncologist.2015-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nam S. Y., Jeong J., Lee W. K., Jeon S. W. Sex-specific effect of body mass index and fasting glucose on gastric cancer risk and all causes mortality; a cohort study of 5.17 million. International Journal of Obesity . 2022;46(9):1644–1651. doi: 10.1038/s41366-022-01161-9. [DOI] [PubMed] [Google Scholar]
- 21.Hong S. H., Noh E., Kim J., et al. Fasting plasma glucose variability and gastric cancer risk in individuals without diabetes mellitus: a nationwide population-based cohort study. Clinical and Translational Gastroenterology . 2020;11(9, article e00221) doi: 10.14309/ctg.0000000000000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu D., Peng F., Lin X., et al. Preoperative metabolic syndrome is predictive of significant gastric cancer mortality after gastrectomy: the Fujian prospective investigation of cancer (FIESTA) study. EBioMedicine . 2017;15:73–80. doi: 10.1016/j.ebiom.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bae J. M. Diabetes history and gastric cancer risk: different results by types of follow-up studies. Asian Pacific Journal of Cancer Prevention . 2022;23(5):1523–1528. doi: 10.31557/APJCP.2022.23.5.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pearson-Stuttard J., Papadimitriou N., Markozannes G., et al. Type 2 diabetes and cancer: an umbrella review of observational and Mendelian randomization studies. Cancer Epidemiology, Biomarkers & Prevention . 2021;30(6):1218–1228. doi: 10.1158/1055-9965.EPI-20-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Danaei G., Finucane M. M., Lu Y., et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet . 2011;378(9785):31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 26.Navarro G., Allard C., Xu W., Mauvais-Jarvis F. The role of androgens in metabolism, obesity, and diabetes in males and females. Obesity . 2015;23(4):713–719. doi: 10.1002/oby.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kautzky-Willer A., Harreiter J., Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocrine Reviews . 2016;37(3):278–316. doi: 10.1210/er.2015-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leal Y. A., Song M., Zabaleta J., et al. Circulating levels of sex steroid hormones and gastric cancer. Archives of Medical Research . 2021;52(6):660–664. doi: 10.1016/j.arcmed.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Zhu Z., Chen Y., Ren J., et al. Serum levels of androgens, estrogens, and sex hormone binding globulin and risk of primary gastric cancer in Chinese men: a nested case-control study. Cancer Prevention Research . 2021;14(6):659–666. doi: 10.1158/1940-6207.CAPR-20-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee S. P., Sung I. K., Kim J. H., Lee S. Y., Park H. S., Shim C. S. The effect of emotional stress and depression on the prevalence of digestive diseases. Journal of Neurogastroenterology and Motility . 2015;21(2):273–282. doi: 10.5056/jnm14116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim W. J., Kwon Y., Lee C. M., et al. Oncometabolic surgery: emergence and legitimacy for investigation. Chinese Journal of Cancer Research . 2020;32(2):252–262. doi: 10.21147/j.issn.1000-9604.2020.02.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kashihara H., Shimada M., Yoshikawa K., et al. The effect of Roux-en-Y reconstruction on type 2 diabetes in the early postoperative period. Anticancer Research . 2018;38(8):4901–4905. doi: 10.21873/anticanres.12805. [DOI] [PubMed] [Google Scholar]
- 33.Buchwald H., Buchwald J. N. Metabolic (bariatric and nonbariatric) surgery for type 2 diabetes: a personal perspective review. Diabetes Care . 2019;42(2):331–340. doi: 10.2337/dc17-2654. [DOI] [PubMed] [Google Scholar]
- 34.Lee W., Ahn S. H., Lee J. H., et al. Comparative study of diabetes mellitus resolution according to reconstruction type after gastrectomy in gastric cancer patients with diabetes mellitus. Obesity Surgery . 2012;22(8):1238–1243. doi: 10.1007/s11695-011-0580-1. [DOI] [PubMed] [Google Scholar]
- 35.An J. Y., Kim Y. M., Yun M. A., Jeon B. H., Noh S. H. Improvement of type 2 diabetes mellitus after gastric cancer surgery: short-term outcome analysis after gastrectomy. World Journal of Gastroenterology . 2013;19(48):9410–9417. doi: 10.3748/wjg.v19.i48.9410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim J. W., Cheong J. H., Hyung W. J., Choi S. H., Noh S. H. Outcome after gastrectomy in gastric cancer patients with type 2 diabetes. World Journal of Gastroenterology . 2012;18(1):49–54. doi: 10.3748/wjg.v18.i1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang K. C., Huang K. H., Lan Y. T., et al. Outcome after curative surgery for gastric cancer patients with type 2 diabetes. World Journal of Surgery . 2014;38(2):431–438. doi: 10.1007/s00268-013-2291-3. [DOI] [PubMed] [Google Scholar]
- 38.Choi Y. Y., Noh S. H., An J. Y. A randomized controlled trial of Roux-en-Y gastrojejunostomy vs. gastroduodenostomy with respect to the improvement of type 2 diabetes mellitus after distal gastrectomy in gastric cancer patients. PLoS One . 2017;12(12, article e0188904) doi: 10.1371/journal.pone.0188904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peng D., Cheng Y. X., Zhang W. Does Roux-en-Y construction really bring benefit of type 2 diabetes mellitus remission after gastrectomy in patients with gastric cancer? A systematic review and meta-analysis. Diabetes Therapy . 2020;11(12):2863–2872. doi: 10.1007/s13300-020-00934-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei Z. W., Li J. L., Wu Y., et al. Impact of pre-existing type-2 diabetes on patient outcomes after radical resection for gastric cancer: a retrospective cohort study. Digestive Diseases and Sciences . 2014;59(5):1017–1024. doi: 10.1007/s10620-013-2965-6. [DOI] [PubMed] [Google Scholar]
- 41.Jirapinyo P., Thompson A. C., Kröner P. T., Chan W. W., Thompson C. C. Metabolic effect of foregut exclusion demonstrated by the impact of gastrogastric fistula on recurrence of diabetes. Journal of the American College of Surgeons . 2018;226(3):259–266.e1. doi: 10.1016/j.jamcollsurg.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwon Y., Jung Kim H., Lo Menzo E., Park S., Szomstein S., Rosenthal R. J. A systematic review and meta-analysis of the effect of Billroth reconstruction on type 2 diabetes: a new perspective on old surgical methods. Surgery for Obesity and Related Diseases . 2015;11(6):1386–1395. doi: 10.1016/j.soard.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Papamargaritis D., le Roux C. W. Do gut hormones contribute to weight loss and glycaemic outcomes after bariatric surgery? Nutrients . 2021;13(3):p. 762. doi: 10.3390/nu13030762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin X. H., Huang K. H., Chuang W. H., et al. The long term effect of metabolic profile and microbiota status in early gastric cancer patients after subtotal gastrectomy. PLoS One . 2018;13(11, article e0206930) doi: 10.1371/journal.pone.0206930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Al Assal K., Prifti E., Belda E., et al. Gut microbiota profile of obese diabetic women submitted to Roux-en-Y gastric bypass and its association with food intake and postoperative diabetes remission. Nutrients . 2020;12(2):p. 278. doi: 10.3390/nu12020278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheung K. S., Chan E. W., Wong A. Y. S., Chen L., Wong I. C. K., Leung W. K. Long-term proton pump inhibitors and risk of gastric cancer development after treatment for helicobacter pylori: a population-based study. Gut . 2018;67(1):28–35. doi: 10.1136/gutjnl-2017-314605. [DOI] [PubMed] [Google Scholar]
- 47.Li L., Gan Y., Wu C., Qu X., Sun G., Lu Z. Coffee consumption and the risk of gastric cancer: a meta-analysis of prospective cohort studies. BMC Cancer . 2015;15(1):p. 733. doi: 10.1186/s12885-015-1758-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tseng C. H., Tseng F. H. Diabetes and gastric cancer: the potential links. World Journal of Gastroenterology . 2014;20(7):1701–1711. doi: 10.3748/wjg.v20.i7.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tseng C. H. The relationship between diabetes mellitus and gastric cancer and the potential benefits of metformin: an extensive review of the literature. Biomolecules . 2021;11(7):p. 1022. doi: 10.3390/biom11071022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han J., Jiang Y., Liu X., et al. Dietary fat intake and risk of gastric cancer: a meta-analysis of observational studies. PLoS One . 2015;10(9, article e0138580) doi: 10.1371/journal.pone.0138580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arora S., Stouffer G. A., Kucharska-Newton A. M., et al. Twenty year trends and sex differences in young adults hospitalized with acute myocardial infarction. Circulation . 2019;139(8):1047–1056. doi: 10.1161/CIRCULATIONAHA.118.037137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nussbaumer R., Meyer-Gerspach A. C., Peterli R., et al. First-phase insulin and amylin after bariatric surgery: a prospective randomized trial on patients with insulin resistance or diabetes after gastric bypass or sleeve gastrectomy. Obesity Facts . 2020;13(6):584–595. doi: 10.1159/000511928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rawla P., Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Review/Przegląd Gastroenterologiczny . 2019;14(1):26–38. doi: 10.5114/pg.2018.80001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin S. W., Freedman N. D., Hollenbeck A. R., Schatzkin A., Abnet C. C. Prospective study of self-reported diabetes and risk of upper gastrointestinal cancers. Cancer Epidemiology, Biomarkers & Prevention . 2011;20(5):954–961. doi: 10.1158/1055-9965.EPI-10-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang A. M. Y., Wellberg E. A., Kopp J. L., Johnson J. D. Hyperinsulinemia in obesity, inflammation, and cancer. Diabetes and Metabolism Journal . 2021;45(3):285–311. doi: 10.4093/dmj.2020.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoon J. M., Son K. Y., Eom C. S., Durrance D., Park S. M. Pre-existing diabetes mellitus increases the risk of gastric cancer: a meta-analysis. World Journal of Gastroenterology . 2013;19(6):936–945. doi: 10.3748/wjg.v19.i6.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang M., Muraki I., Liu K., et al. Diabetes and mortality from respiratory diseases: the Japan collaborative cohort study. Journal of Epidemiology . 2020;30(10):457–463. doi: 10.2188/jea.JE20190091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tiffon C. Histone deacetylase inhibition restores expression of hypoxia-inducible protein NDRG1 in pancreatic cancer. Pancreas . 2018;47(2):200–207. doi: 10.1097/MPA.0000000000000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cao X., Liu D., Xia Y., Cai T., He Y., Liu J. A novel polysaccharide from Lentinus edodes mycelia protects MIN6 cells against high glucose-induced damage via the MAPKs and Nrf2 pathways. Food & Nutrition Research . 2019;63 doi: 10.29219/fnr.v63.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kraft V. A. N., Bezjian C. T., Pfeiffer S., et al. GTP cyclohydrolase 1/tetrahydrobiopterin counteract ferroptosis through lipid remodeling. ACS Central Science . 2020;6(1):41–53. doi: 10.1021/acscentsci.9b01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yue W., Zhu M., Zuo L., et al. Early pattern of Epstein-Barr virus infection in gastric epithelial cells by "cell-in-cell". Virologica Sinica . 2019;34(3):253–261. doi: 10.1007/s12250-019-00097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen X., Chen Y., Li T., et al. Diabetes mellitus promoted lymph node metastasis in gastric cancer: a 15-year single-institution experience. Chinese Medical Journal . 2022;135(8):950–961. doi: 10.1097/CM9.0000000000001795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maeda M., Hayashi T., Mizuno N., Hattori Y., Kuzuya M. Intermittent high glucose implements stress-induced senescence in human vascular endothelial cells: role of superoxide production by NADPH oxidase. PLoS One . 2015;10(4, article e0123169) doi: 10.1371/journal.pone.0123169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ying C., Liu T., Ling H., et al. Glucose variability aggravates cardiac fibrosis by altering AKT signalling path. Diabetes & Vascular Disease Research . 2017;14(4):327–335. doi: 10.1177/1479164117698917. [DOI] [PubMed] [Google Scholar]
- 65.Zhang N., Wang Y., Tse G., Li G., Wu S., Liu T. Association of visit-to-visit variability in fasting plasma glucose with digestive cancer risk. Oxidative Medicine and Cellular Longevity . 2022;2022:12. doi: 10.1155/2022/4530894.4530894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gallagher E. J., LeRoith D. Hyperinsulinaemia in cancer. Nature Reviews Cancer . 2020;20(11):629–644. doi: 10.1038/s41568-020-0295-5. [DOI] [PubMed] [Google Scholar]
- 67.Stefansdottir G., Zoungas S., Chalmers J., et al. Intensive glucose control and risk of cancer in patients with type 2 diabetes. Diabetologia . 2011;54(7):1608–1614. doi: 10.1007/s00125-011-2104-x. [DOI] [PubMed] [Google Scholar]
- 68.Scalia P., Giordano A., Martini C., Williams S. J. Isoform-and paralog-switching in IR-signaling: when diabetes opens the gates to cancer. Biomolecules . 2020;10(12):p. 1617. doi: 10.3390/biom10121617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heckl S. M., Wiesener V., Behrens H. M., Ulase D., Krüger S., Röcken C. The expression of the insulin receptor in gastric cancer correlates with the HER2 status and may have putative therapeutic implications. Gastric Cancer . 2019;22(6):1130–1142. doi: 10.1007/s10120-019-00964-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu L., Zhou R., Yuan L., et al. IGF1/IGF1R/STAT3 signaling-inducible IFITM2 promotes gastric cancer growth and metastasis. Cancer Letters . 2017;393:76–85. doi: 10.1016/j.canlet.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 71.Orliaguet L., Dalmas E., Drareni K., Venteclef N., Alzaid F. Mechanisms of macrophage polarization in insulin signaling and sensitivity. Frontiers in Endocrinology . 2020;11:p. 62. doi: 10.3389/fendo.2020.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lutz S. Z., Hennenlotter J., Scharpf M. O., et al. Androgen receptor overexpression in prostate cancer in type 2 diabetes. Molecular Metabolism . 2018;8:158–166. doi: 10.1016/j.molmet.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saisana M., Griffin S. M., May F. E. B. Insulin and the insulin receptor collaborate to promote human gastric cancer. Gastric Cancer . 2022;25(1):107–123. doi: 10.1007/s10120-021-01236-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen Y. Y., Fang W. H., Wang C. C., et al. Helicobacter pylori infection increases risk of incident metabolic syndrome and diabetes: a cohort study. PLoS One . 2019;14(2, article e0208913) doi: 10.1371/journal.pone.0208913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tseng C. H. Diabetes, insulin use, and gastric Cancer. Journal of Clinical Gastroenterology . 2013;47(6):e60–e64. doi: 10.1097/MCG.0b013e31827245eb. [DOI] [PubMed] [Google Scholar]
- 76.Wu Y. H., Huang Y. F., Chang T. H., et al. COL11A1 activates cancer-associated fibroblasts by modulating TGF-β3 through the NF-κB/IGFBP2 axis in ovarian cancer cells. Oncogene . 2021;40(26):4503–4519. doi: 10.1038/s41388-021-01865-8. [DOI] [PubMed] [Google Scholar]
- 77.Masuo K., Chen R., Yogo A., et al. SNAIL2 contributes to tumorigenicity and chemotherapy resistance in pancreatic cancer by regulating IGFBP2. Cancer Science . 2021;112(12):4987–4999. doi: 10.1111/cas.15162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu Q., Jiang J., Zhang X., Zhang M., Fu Y. Comprehensive analysis of IGFBPs as biomarkers in gastric cancer. Frontiers in Oncology . 2021;11, article 723131 doi: 10.3389/fonc.2021.723131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu Y., Shen S., Yan Z., et al. Expression characteristics and their functional role of IGFBP gene family in pan-cancer. BMC Cancer . 2023;23(1):p. 371. doi: 10.1186/s12885-023-10832-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Luo C., Sun F., Zhu H., et al. Insulin-like growth factor binding protein-1 (IGFBP-1) upregulated by helicobacter pylori and is associated with gastric cancer cells migration. Pathology, Research and Practice . 2017;213(9):1029–1036. doi: 10.1016/j.prp.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 81.Ding T. Y., Peng Y. H., Hong C. Q., et al. Serum insulin-like growth factor binding protein 3 as a promising diagnostic and prognostic biomarker in esophagogastric junction adenocarcinoma. Discover Oncology . 2022;13(1):p. 128. doi: 10.1007/s12672-022-00591-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu H., Gu H., Kutbi E. H., Tan S. C., Low T. Y., Zhang C. Association of IGF-1 and IGFBP-3 levels with gastric cancer: a systematic review and meta-analysis. International Journal of Clinical Practice . 2021;75(11, article e14764) doi: 10.1111/ijcp.14764. [DOI] [PubMed] [Google Scholar]
- 83.Zhang L., Li W., Cao L., et al. PKNOX2 suppresses gastric cancer through the transcriptional activation of IGFBP5 and p53. Oncogene . 2019;38(23):4590–4604. doi: 10.1038/s41388-019-0743-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu C. T., Wu F. C., Zhuang Y. X., et al. The diagnostic value of serum insulin-like growth factor binding protein 7 in gastric cancer. PeerJ . 2023;11, article e15419 doi: 10.7717/peerj.15419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kędzierska L., Madej-Michniewicz A., Marczuk N., Dołęgowska B., Starzyńska T., Błogowski W. Clinical significance of various growth factors in patients with different gastric neoplasms. American Journal of Translational Research . 2020;12(1):118–129. [PMC free article] [PubMed] [Google Scholar]
- 86.Ghafari F., Alizadeh A. M., Agah S., Irani S., Mokhtare M. Insulin-like growth factor 1 serum levels in different stages of gastric cancer and their association with Helicobacter pylori status. Peptides . 2022;158, article 170892 doi: 10.1016/j.peptides.2022.170892. [DOI] [PubMed] [Google Scholar]
- 87.Guo C., Chu H., Gong Z., et al. HOXB13 promotes gastric cancer cell migration and invasion via IGF-1R upregulation and subsequent activation of PI3K/AKT/mTOR signaling pathway. Life Sciences . 2021;278, article 119522 doi: 10.1016/j.lfs.2021.119522. [DOI] [PubMed] [Google Scholar]
- 88.Chen Z., Li Y., Tan B., et al. Progress and current status of molecule-targeted therapy and drug resistance in gastric cancer. Drugs of Today . 2020;56(7):469–482. doi: 10.1358/dot.2020.56.7.3112071. [DOI] [PubMed] [Google Scholar]
- 89.Wo D., Chen J., Li Q., et al. IGFBP-4 enhances VEGF-induced angiogenesis in a mouse model of myocardial infarction. Journal of Cellular and Molecular Medicine . 2020;24(16):9466–9471. doi: 10.1111/jcmm.15516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu X., He H., Zhang F., et al. m6A methylated EphA2 and VEGFA through IGF2BP2/3 regulation promotes vasculogenic mimicry in colorectal cancer via PI3K/AKT and ERK1/2 signaling. Cell Death & Disease . 2022;13(5):p. 483. doi: 10.1038/s41419-022-04950-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li H., Adachi Y., Yamamoto H., et al. Insulin-like growth factor-I receptor blockade reduces tumor angiogenesis and enhances the effects of bevacizumab for a human gastric cancer cell line, MKN45. Cancer . 2011;117(14):3135–3147. doi: 10.1002/cncr.25893. [DOI] [PubMed] [Google Scholar]
- 92.Zhang H., Deng T., Liu R., et al. Exosome-delivered EGFR regulates liver microenvironment to promote gastric cancer liver metastasis. Nature Communications . 2017;8(1, article 15016) doi: 10.1038/ncomms15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Matsunaga S., Nishiumi S., Tagawa R., Yoshida M. Alterations in metabolic pathways in gastric epithelial cells infected with Helicobacter pylori. Microbial Pathogenesis . 2018;124:122–129. doi: 10.1016/j.micpath.2018.08.033. [DOI] [PubMed] [Google Scholar]
- 94.Fischbach W., Malfertheiner P. Helicobacter pylori infection. Deutsches Ärzteblatt International . 2018;115(25):429–436. doi: 10.3238/arztebl.2018.0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Saribas S., Demiryas S., Yilmaz E., et al. Association between human leukocyte antigen gene polymorphisms and multiple EPIYA-C repeats in gastrointestinal disorders. World Journal of Gastroenterology . 2020;26(32):4817–4832. doi: 10.3748/wjg.v26.i32.4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ansari S., Yamaoka Y. Helicobacter pylori virulence factor cytotoxin-associated gene a (CagA)-mediated gastric pathogenicity. International Journal of Molecular Sciences . 2020;21(19):p. 7430. doi: 10.3390/ijms21197430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li N., Feng Y., Hu Y., et al. Helicobacter pylori CagA promotes epithelial mesenchymal transition in gastric carcinogenesis via triggering oncogenic YAP pathway. Journal of Experimental & Clinical Cancer Research . 2018;37(1):p. 280. doi: 10.1186/s13046-018-0962-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tsugawa H., Mori H., Matsuzaki J., et al. CAPZA1 determines the risk of gastric carcinogenesis by inhibiting Helicobacter pylori CagA-degraded autophagy. Autophagy . 2019;15(2):242–258. doi: 10.1080/15548627.2018.1515530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhu P., Xue J., Zhang Z. J., et al. Helicobacter pylori VacA induces autophagic cell death in gastric epithelial cells via the endoplasmic reticulum stress pathway. Cell Death & Disease . 2017;8(12):p. 3207. doi: 10.1038/s41419-017-0011-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim I. J., Lee J., Oh S. J., et al. Helicobacter pylori Infection Modulates Host Cell Metabolism through VacA- Dependent Inhibition of mTORC1. Cell Host & Microbe . 2018;23(5):583–593.e8. doi: 10.1016/j.chom.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cornejo-Pareja I., Martín-Núñez G. M., Roca-Rodríguez M. M., et al. H. Pylori eradication treatment alters gut microbiota and GLP-1 secretion in humans. Journal of Clinical Medicine . 2019;8(4):p. 451. doi: 10.3390/jcm8040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhao Y., Gao X., Guo J., et al. Helicobacter pylori infection alters gastric and tongue coating microbial communities. Helicobacter . 2019;24(2, article e12567) doi: 10.1111/hel.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Guo Y., Zhang Y., Gerhard M., et al. Effect of helicobacter pylori on gastrointestinal microbiota: a population-based study in Linqu, a high-risk area of gastric cancer. Gut . 2020;69(9):1598–1607. doi: 10.1136/gutjnl-2019-319696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wu R., Zhao D., An R., et al. Linggui Zhugan formula improves glucose and lipid levels and alters gut microbiota in high-fat diet-induced diabetic mice. Frontiers in Physiology . 2019;10:p. 918. doi: 10.3389/fphys.2019.00918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Luo J., Xiang Y., Xu X., et al. High glucose-induced ROS production stimulates proliferation of pancreatic cancer via inactivating the JNK pathway. Oxidative Medicine and Cellular Longevity . 2018;2018:10. doi: 10.1155/2018/6917206.6917206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ikeda F., Doi Y., Yonemoto K., et al. Hyperglycemia Increases Risk of Gastric Cancer Posed by Helicobacter pylori Infection: A Population-Based Cohort Study. Gastroenterology . 2009;136(4):1234–1241. doi: 10.1053/j.gastro.2008.12.045. [DOI] [PubMed] [Google Scholar]
- 107.Youn Nam S., Park B. J., Nam J. H., et al. Association of currentHelicobacter pyloriinfection and metabolic factors with gastric cancer in 35,519 subjects: a cross-sectional study. United European Gastroenterology Journal . 2019;7(2):287–296. doi: 10.1177/2050640618819402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jun J. K., Gwack J., Park S. K., et al. Fasting serum glucose level and gastric cancer risk in a nested case-control study. Journal of Preventive Medicine and Public Health . 2006;39(6):493–498. [PubMed] [Google Scholar]
- 109.Gravina A. G., Zagari R. M., De Musis C., Romano L., Loguercio C., Romano M. Helicobacter pylori and extragastric diseases: a review. World Journal of Gastroenterology . 2018;24(29):3204–3221. doi: 10.3748/wjg.v24.i29.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zheng C., Chen T., Wang Y., et al. A randomised trial of probiotics to reduce severity of physiological and microbial disorders induced by partial gastrectomy for patients with gastric cancer. Journal of Cancer . 2019;10(3):568–576. doi: 10.7150/jca.29072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Biranjia-Hurdoyal S. D., Seetulsingh-Goorah S. P. Performances of four Helicobacter pylori serological detection kits using stool antigen test as gold standard. PLoS One . 2016;11(10, article e0163834) doi: 10.1371/journal.pone.0163834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang Z., Gao X., Zeng R., et al. Changes of the gastric mucosal microbiome associated with histological stages of gastric carcinogenesis. Frontiers in Microbiology . 2020;11:p. 997. doi: 10.3389/fmicb.2020.00997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Noto J. M., Zackular J. P., Varga M. G., et al. Modification of the gastric mucosal microbiota by a strain-specific helicobacter pylori oncoprotein and carcinogenic histologic phenotype. MBio . 2019;10(3, article e00955-19) doi: 10.1128/mBio.00955-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Coker O. O., Dai Z., Nie Y., et al. Mucosal microbiome dysbiosis in gastric carcinogenesis. Gut . 2018;67(6):1024–1032. doi: 10.1136/gutjnl-2017-314281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wong S. H., Yu J. Gut microbiota in colorectal cancer: mechanisms of action and clinical applications. Nature Reviews, Gastroenterology & Hepatology . 2019;16(11):690–704. doi: 10.1038/s41575-019-0209-8. [DOI] [PubMed] [Google Scholar]
- 116.Ferreira R. M., Pereira-Marques J., Pinto-Ribeiro I., et al. Gastric microbial community profiling reveals a dysbiotic cancer-associated microbiota. Gut . 2018;67(2):226–236. doi: 10.1136/gutjnl-2017-314205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dong Z., Chen B., Pan H., et al. Detection of microbial 16S rRNA gene in the serum of patients with gastric cancer. Frontiers in Oncology . 2019;9:p. 608. doi: 10.3389/fonc.2019.00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li Q., Yu H. The role of non-H. Pylori bacteria in the development of gastric cancer. American Journal of Cancer Research . 2020;10(8):2271–2281. [PMC free article] [PubMed] [Google Scholar]
- 119.Mashukov A., Shapochka D., Seleznov O., et al. Histological differentiation impacts the tumor immune microenvironment in gastric carcinoma: relation to the immune cycle. World Journal of Gastroenterology . 2021;27(31):5259–5271. doi: 10.3748/wjg.v27.i31.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang T., Zheng N., Luo Q., et al. Probiotics Lactobacillus reuteri abrogates immune checkpoint blockade-associated colitis by inhibiting group 3 innate lymphoid cells. Frontiers in Immunology . 2019;10:p. 1235. doi: 10.3389/fimmu.2019.01235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fujisaka S., Avila-Pacheco J., Soto M., et al. Diet, genetics, and the gut microbiome drive dynamic changes in plasma metabolites. Cell Reports . 2018;22(11):3072–3086. doi: 10.1016/j.celrep.2018.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ling Z., Shao L., Liu X., et al. Regulatory T cells and plasmacytoid dendritic cells within the tumor microenvironment in gastric cancer are correlated with gastric microbiota dysbiosis: a preliminary study. Frontiers in Immunology . 2019;10:p. 533. doi: 10.3389/fimmu.2019.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ling Z., Cheng Y., Yan X., et al. Alterations of the fecal microbiota in Chinese patients with multiple sclerosis. Frontiers in Immunology . 2020;11, article 590783 doi: 10.3389/fimmu.2020.590783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Noor F., Kaysen A., Wilmes P., Schneider J. G. The gut microbiota and hematopoietic stem cell transplantation: challenges and potentials. Journal of Innate Immunity . 2019;11(5):405–415. doi: 10.1159/000492943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Martin-Nuñez G. M., Cornejo-Pareja I., Clemente-Postigo M., Tinahones F. J. Gut microbiota: the missing link between helicobacter pylori infection and metabolic disorders? Frontiers in Endocrinology . 2021;12, article 639856 doi: 10.3389/fendo.2021.639856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Allin K. H., Tremaroli V., Caesar R., et al. Aberrant intestinal microbiota in individuals with prediabetes. Diabetologia . 2018;61(4):810–820. doi: 10.1007/s00125-018-4550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mitsou E. K., Detopoulou M., Kakali A., et al. Mining possible associations of faecal A. muciniphila colonisation patterns with host adiposity and cardiometabolic markers in an adult population. Beneficial Microbes . 2019;10(7):741–749. doi: 10.3920/BM2019.0033. [DOI] [PubMed] [Google Scholar]
- 128.Tabasi M., Eybpoosh S., Sadeghpour Heravi F., et al. Gut microbiota and serum biomarker analyses in obese patients diagnosed with diabetes and hypothyroid disorder. Metabolic Syndrome and Related Disorders . 2021;19(3):144–151. doi: 10.1089/met.2020.0119. [DOI] [PubMed] [Google Scholar]
- 129.Li H., Liu B., Song J., et al. Characteristics of gut microbiota in patients with hypertension and/or hyperlipidemia: a cross-sectional study on rural residents in Xinxiang County, Henan Province. Microorganisms . 2019;7(10):p. 399. doi: 10.3390/microorganisms7100399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zeng Q., Li D., He Y., et al. Discrepant gut microbiota markers for the classification of obesity-related metabolic abnormalities. Scientific Reports . 2019;9(1, article 13424) doi: 10.1038/s41598-019-49462-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Asnicar F., Berry S. E., Valdes A. M., et al. Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. Nature Medicine . 2021;27(2):321–332. doi: 10.1038/s41591-020-01183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shih C. T., Yeh Y. T., Lin C. C., Yang L. Y., Chiang C. P. Akkermansia muciniphila is negatively correlated with hemoglobin A1c in refractory diabetes. Microorganisms . 2020;8(9):p. 1360. doi: 10.3390/microorganisms8091360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wu H., Tremaroli V., Schmidt C., et al. The Gut Microbiota in Prediabetes and Diabetes: A Population-Based Cross- Sectional Study. Cell Metabolism . 2020;32(3):379–390.e3. doi: 10.1016/j.cmet.2020.06.011. [DOI] [PubMed] [Google Scholar]
- 134.Pinna N. K., Anjana R. M., Saxena S., et al. Trans-ethnic gut microbial signatures of prediabetic subjects from India and Denmark. Genome Medicine . 2021;13(1):p. 36. doi: 10.1186/s13073-021-00851-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Naderpoor N., Mousa A., Gomez-Arango L. F., Barrett H. L., Dekker Nitert M., de Courten B. Faecal microbiota are related to insulin sensitivity and secretion in overweight or obese adults. Journal of Clinical Medicine . 2019;8(4):p. 452. doi: 10.3390/jcm8040452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gurung M., Li Z., You H., et al. Role of gut microbiota in type 2 diabetes pathophysiology. eBioMedicine . 2020;51, article 102590 doi: 10.1016/j.ebiom.2019.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rorato R., Borges B. C., Uchoa E. T., Antunes-Rodrigues J., Elias C. F., Elias L. L. K. LPS-induced low-grade inflammation increases hypothalamic JNK expression and causes central insulin resistance irrespective of body weight changes. International Journal of Molecular Sciences . 2017;18(7):p. 1431. doi: 10.3390/ijms18071431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shen Z., Zhu C., Quan Y., et al. Insights into Roseburia intestinalis which alleviates experimental colitis pathology by inducing anti-inflammatory responses. Journal of Gastroenterology and Hepatology . 2018;33(10):1751–1760. doi: 10.1111/jgh.14144. [DOI] [PubMed] [Google Scholar]
- 139.Zhu C., Song K., Shen Z., et al. Roseburia intestinalis inhibits interleukin-17 excretion and promotes regulatory T cells differentiation in colitis. Molecular Medicine Reports . 2018;17(6):7567–7574. doi: 10.3892/mmr.2018.8833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hoffmann T. W., Pham H. P., Bridonneau C., et al. Microorganisms linked to inflammatory bowel disease-associated dysbiosis differentially impact host physiology in gnotobiotic mice. The ISME Journal . 2016;10(2):460–477. doi: 10.1038/ismej.2015.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu W. C., Yang M. C., Wu Y. Y., Chen P. H., Hsu C. M., Chen L. W. Lactobacillus plantarum reverse diabetes-induced Fmo3 and ICAM expression in mice through enteric dysbiosis-related c-Jun NH2-terminal kinase pathways. PLoS One . 2018;13(5, article e0196511) doi: 10.1371/journal.pone.0196511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yong C. C., Yoon Y., Yoo H. S., Oh S. Effect of lactobacillus fermentation on the anti-inflammatory potential of turmeric. Journal of Microbiology and Biotechnology . 2019;29(10):1561–1569. doi: 10.4014/jmb.1906.06032. [DOI] [PubMed] [Google Scholar]
- 143.Luo Y., Lan C., Xie K., et al. Active or autoclaved Akkermansia muciniphila relieves TNF-α-induced inflammation in intestinal epithelial cells through distinct pathways. Frontiers in Immunology . 2021;12, article 788638 doi: 10.3389/fimmu.2021.788638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Breyner N. M., Michon C., de Sousa C. S., et al. Microbial anti-inflammatory molecule (MAM) from Faecalibacterium prausnitzii shows a protective effect on DNBS and DSS-induced colitis model in mice through inhibition of NF-κB pathway. Frontiers in Microbiology . 2017;8:p. 114. doi: 10.3389/fmicb.2017.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Palmnäs-Bédard M. S. A., Costabile G., Vetrani C., et al. The human gut microbiota and glucose metabolism: a scoping review of key bacteria and the potential role of SCFAs. The American Journal of Clinical Nutrition . 2022;116(4):862–874. doi: 10.1093/ajcn/nqac217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Koh A., Bäckhed F. From association to causality: the role of the gut microbiota and its functional products on host metabolism. Molecular Cell . 2020;78(4):584–596. doi: 10.1016/j.molcel.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 147.Kim S. H., Huh C. S., Choi I. D., et al. The anti-diabetic activity of Bifidobacterium lactis HY8101 in vitro and in vivo. Journal of Applied Microbiology . 2014;117(3):834–845. doi: 10.1111/jam.12573. [DOI] [PubMed] [Google Scholar]
- 148.Hao J., Zhang Y., Wu T., et al. The antidiabetic effects of Bifidobacterium longum subsp. longum BL21 through regulating gut microbiota structure in type 2 diabetic mice. Food & Function . 2022;13(19):9947–9958. doi: 10.1039/d2fo01109c. [DOI] [PubMed] [Google Scholar]
- 149.Li X., Wang E., Yin B., et al. Effects of Lactobacillus casei CCFM419 on insulin resistance and gut microbiota in type 2 diabetic mice. Beneficial Microbes . 2017;8(3):421–432. doi: 10.3920/BM2016.0167. [DOI] [PubMed] [Google Scholar]
- 150.Zhang Y., Guo X., Guo J., et al. Lactobacillus casei reduces susceptibility to type 2 diabetes via microbiota-mediated body chloride ion influx. Scientific Reports . 2014;4:p. 5654. doi: 10.1038/srep05654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang Q., Xiao X., Zheng J., et al. Featured article: structure moderation of gut microbiota in liraglutide-treated diabetic male rats. Experimental Biology and Medicine . 2018;243(1):34–44. doi: 10.1177/1535370217743765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gong J., Kelekar G., Shen J., Shen J., Kaur S., Mita M. The expanding role of metformin in cancer: an update on antitumor mechanisms and clinical development. Targeted Oncology . 2016;11(4):447–467. doi: 10.1007/s11523-016-0423-z. [DOI] [PubMed] [Google Scholar]
- 153.Verdura S., Cuyàs E., Martin-Castillo B., Menendez J. A. Metformin as an archetype immuno-metabolic adjuvant for cancer immunotherapy. Oncoimmunology . 2019;8(10, article e1633235) doi: 10.1080/2162402X.2019.1633235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang J. J., Zhang Q. S., Li Z. Q., Zhou J. W., Du J. Metformin attenuates PD-L1 expression through activating hippo signaling pathway in colorectal cancer cells. American Journal of Translational Research . 2019;11(11):6965–6976. [PMC free article] [PubMed] [Google Scholar]
- 155.Bryrup T., Thomsen C. W., Kern T., et al. Metformin-induced changes of the gut microbiota in healthy young men: results of a non-blinded, one-armed intervention study. Diabetologia . 2019;62(6):1024–1035. doi: 10.1007/s00125-019-4848-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bridgeman S. C., Ellison G. C., Melton P. E., Newsholme P., Mamotte C. D. S. Epigenetic effects of metformin: from molecular mechanisms to clinical implications. Diabetes, Obesity & Metabolism . 2018;20(7):1553–1562. doi: 10.1111/dom.13262. [DOI] [PubMed] [Google Scholar]
- 157.Kim S. H., Kim S. C., Ku J. L. Metformin increases chemo-sensitivity via gene downregulation encoding DNA replication proteins in 5-Fu resistant colorectal cancer cells. Oncotarget . 2017;8(34):56546–56557. doi: 10.18632/oncotarget.17798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bradley M. C., Ferrara A., Achacoso N., Ehrlich S. F., Quesenberry C. P., Jr., Habel L. A. A cohort study of metformin and colorectal cancer risk among patients with diabetes mellitus. Cancer Epidemiology, Biomarkers & Prevention . 2018;27(5):525–530. doi: 10.1158/1055-9965.EPI-17-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ražuka-Ebela D., Giupponi B., Franceschi F. Helicobacter pyloriand extragastric diseases. Helicobacter . 2018;23, article e12520(Supplement 1) doi: 10.1111/hel.12520. [DOI] [PubMed] [Google Scholar]
- 160.Li P., Zhang C., Gao P., et al. Metformin use and its effect on gastric cancer in patients with type 2 diabetes: a systematic review of observational studies. Oncology Letters . 2018;15(1):1191–1199. doi: 10.3892/ol.2017.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tran T. T., Lee J., Gunathilake M., Cho H., Kim J. Influence of fasting glucose level on gastric cancer incidence in a prospective cohort study. Cancer Epidemiology, Biomarkers & Prevention . 2022;31(1):254–261. doi: 10.1158/1055-9965.EPI-21-0670. [DOI] [PubMed] [Google Scholar]
- 162.Cheung K. S., Chan E. W., Wong A. Y. S., et al. Metformin use and gastric cancer risk in diabetic patients after Helicobacter pylori eradication. Journal of the National Cancer Institute . 2019;111(5):484–489. doi: 10.1093/jnci/djy144. [DOI] [PubMed] [Google Scholar]
- 163.Tseng C. H. Metformin reduces gastric cancer risk in patients with type 2 diabetes mellitus. Aging . 2016;8(8):1636–1649. doi: 10.18632/aging.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Shuai Y., Li C., Zhou X. The effect of metformin on gastric cancer in patients with type 2 diabetes: a systematic review and meta-analysis. Clinical & Translational Oncology . 2020;22(9):1580–1590. doi: 10.1007/s12094-020-02304-y. [DOI] [PubMed] [Google Scholar]
- 165.Lee C. K., Jung M., Jung I., et al. Cumulative metformin use and its impact on survival in gastric cancer patients after gastrectomy. Annals of Surgery . 2016;263(1):96–102. doi: 10.1097/SLA.0000000000001086. [DOI] [PubMed] [Google Scholar]
- 166.Seo H. S., Jung Y. J., Kim J. H., Lee H. H., Park C. H. The effect of metformin on prognosis in patients with locally advanced gastric cancer associated with type 2 diabetes mellitus. American Journal of Clinical Oncology . 2019;42(12):909–917. doi: 10.1097/COC.0000000000000627. [DOI] [PubMed] [Google Scholar]
- 167.Murff H. J., Roumie C. L., Greevy R. A., et al. Metformin use and incidence cancer risk: evidence for a selective protective effect against liver cancer. Cancer Causes & Control . 2018;29(9):823–832. doi: 10.1007/s10552-018-1058-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.de Jong R. G., Burden A. M., de Kort S., et al. No decreased risk of gastrointestinal cancers in users of metformin in the Netherlands; a time-varying analysis of metformin exposure. Cancer Prevention Research . 2017;10(5):290–297. doi: 10.1158/1940-6207.CAPR-16-0277. [DOI] [PubMed] [Google Scholar]
- 169.Zheng J., Xie S. H., Santoni G., Lagergren J. Metformin use and risk of gastric adenocarcinoma in a Swedish population- based cohort study. British Journal of Cancer . 2019;121(10):877–882. doi: 10.1038/s41416-019-0598-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dulskas A., Patasius A., Kaceniene A., Linkeviciute-Ulinskiene D., Zabuliene L., Smailyte G. A cohort study of antihyperglycemic medication exposure and gastric cancer risk. Journal of Clinical Medicine . 2020;9(2):p. 435. doi: 10.3390/jcm9020435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dulskas A., Patasius A., Linkeviciute-Ulinskiene D., Zabuliene L., Smailyte G. A cohort study of antihyperglycemic medication exposure and survival in patients with gastric cancer. Aging . 2019;11(17):7197–7205. doi: 10.18632/aging.102245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Baglia M. L., Cui Y., Zheng T., et al. Diabetes medication use in association with survival among patients of breast, colorectal, lung, or gastric cancer. Cancer Research and Treatment . 2019;51(2):538–546. doi: 10.4143/crt.2017.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chen X., Chen Y., Li T., et al. Impact of diabetes on prognosis of gastric cancer patients performed with gastrectomy. Chinese Journal of Cancer Research . 2020;32(5):631–644. doi: 10.21147/j.issn.1000-9604.2020.05.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lacroix O., Couttenier A., Vaes E., Cardwell C. R., De Schutter H., Robert A. Impact of metformin on gastric adenocarcinoma survival: a Belgian population based study. Cancer Epidemiology . 2018;53:149–155. doi: 10.1016/j.canep.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 175.Chung W. S., Le P. H., Kuo C. J., et al. Impact of metformin use on survival in patients with gastric cancer and diabetes mellitus following gastrectomy. Cancers . 2020;12(8):p. 2013. doi: 10.3390/cancers12082013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang Y. B., Tan L. M., Luo L., et al. Immortal time bias exaggerates the effect of metformin on the risk of gastric cancer: a meta-analysis. Pharmacological Research . 2021;165, article 105425 doi: 10.1016/j.phrs.2021.105425. [DOI] [PubMed] [Google Scholar]
- 177.Yu G., Fang W., Xia T., et al. Metformin potentiates rapamycin and cisplatin in gastric cancer in mice. Oncotarget . 2015;6(14):12748–12762. doi: 10.18632/oncotarget.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wu X. Effect of metformin combined with chemotherapeutic agents on gastric cancer cell line AGS. Pakistan Journal of Pharmaceutical Sciences . 2017;30(5):1833–1836. [PubMed] [Google Scholar]
- 179.DeWaal D., Nogueira V., Terry A. R., et al. Hexokinase-2 depletion inhibits glycolysis and induces oxidative phosphorylation in hepatocellular carcinoma and sensitizes to metformin. Nature Communications . 2018;9(1):p. 446. doi: 10.1038/s41467-017-02733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nature Reviews Cancer . 2012;12(3):159–169. doi: 10.1038/nrc3215. [DOI] [PubMed] [Google Scholar]
- 181.Janzer A., German N. J., Gonzalez-Herrera K. N., Asara J. M., Haigis M. C., Struhl K. Metformin and phenformin deplete tricarboxylic acid cycle and glycolytic intermediates during cell transformation and NTPs in cancer stem cells. Proceedings of the National Academy of Sciences of the United States of America . 2014;111(29):10574–10579. doi: 10.1073/pnas.1409844111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhou G., Myers R., Li Y., et al. Role of AMP-activated protein kinase in mechanism of metformin action. The Journal of Clinical Investigation . 2001;108(8):1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhou X. L., Xue W. H., Ding X. F., et al. Association between metformin and the risk of gastric cancer in patients with type 2 diabetes mellitus: a meta-analysis of cohort studies. Oncotarget . 2017;8(33):55622–55631. doi: 10.18632/oncotarget.16973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cha J. H., Yang W. H., Xia W., et al. Metformin promotes antitumor immunity via endoplasmic-reticulum-associated degradation of PD-L1. Molecular Cell . 2018;71(4):606–620.e7. doi: 10.1016/j.molcel.2018.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chen X., Zhang J., Wang R., et al. UPLC-Q-TOF/MS-based serum and urine metabonomics study on the ameliorative effects of palmatine on helicobacter pylori-induced chronic atrophic gastritis. Frontiers in Pharmacology . 2020;11, article 586954 doi: 10.3389/fphar.2020.586954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kulkarni A. S., Gubbi S., Barzilai N. Benefits of metformin in attenuating the hallmarks of aging. Cell Metabolism . 2020;32(1):15–30. doi: 10.1016/j.cmet.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Xiong W., Sun K. Y., Zhu Y., Zhang X., Zhou Y. H., Zou X. Metformin alleviates inflammation through suppressing FASN-dependent palmitoylation of Akt. Cell Death & Disease . 2021;12(10):p. 934. doi: 10.1038/s41419-021-04235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ma R., Yi B., Riker A. I., Xi Y. Metformin and cancer immunity. Acta Pharmacologica Sinica . 2020;41(11):1403–1409. doi: 10.1038/s41401-020-00508-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Feng W., Liu J., Ao H., Yue S., Peng C. Targeting gut microbiota for precision medicine: focusing on the efficacy and toxicity of drugs. Theranostics . 2020;10(24):11278–11301. doi: 10.7150/thno.47289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Miao Z. F., Adkins-Threats M., Burclaff J. R., et al. A metformin-responsive metabolic pathway controls distinct steps in gastric progenitor fate decisions and maturation. Cell Stem Cell . 2020;26(6):910–925.e6. doi: 10.1016/j.stem.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Jiang L. L., Liu L. Effect of metformin on stem cells: molecular mechanism and clinical prospect. World Journal of Stem Cells . 2020;12(12):1455–1473. doi: 10.4252/wjsc.v12.i12.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chiang T. H., Chang W. J., Chen S. L., et al. Mass eradication of Helicobacter pylori to reduce gastric cancer incidence and mortality: a long-term cohort study on Matsu Islands. Gut . 2021;70(2):243–250. doi: 10.1136/gutjnl-2020-322200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included in the article.


