Highlights
-
•
Key anterior approaches differences in LSTV include vascular (aortic bifurcation/iliocaval confluence), muscular (psoas) and osseus anatomy (inter-crestal tangent/pubic symphysis), when compared to non-LSTV.
-
•
There are increased surgical deviations but not significantly greater complications for anterior approaches in LSTV.
-
•
Vascular awareness while accessing L45 will be in the presence of a more cephalad ABF and ICC with sacralized L5, and access to the deeper L56 level will be in the presence of a more caudal ABF and ICC in lumbarized S1.
Keywords: Lumbosacral transitional vertebrae, Bertolotti, Castelvi, ALIF, OLIF, ATP, LLIF, Retroperitoneal, ABF, Sacralized, Lumbarized
Abbreviations
- ABF
aortic bifurcation
- ALV
ascending lumbar vein
- A/O/LLIF
Anterior/Oblique/Lateral lumbar interbody fusion
- ATP
Anterior to Psoas (aka OLIF)
- AxialLIF
Axial lumbar interbody fusion
- CIV
Common Iliac Vein
- D/XLIF
Direct/extreme lateral lumbar interbody fusion (aka transpsoas)
- GRADE
Grades of Recommendation, Assessment Development, and Evaluation
- ICC
ilio-caval confluence
- ICT
intercrestal tangent; IS: isthmic spondylolisthesis
- IVC
inferior vena cava
- LSTV
Lumbosacral transitional vertebrae
- MeSH
Medical Subject Headings
- MRI
Magnetic Resonance Imaging
- PICO
Patient-Intervention-Comparison-Outcome
- PLIF
Posterior lumbar interbody fusion
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
1. Introduction
Lumbosacral transitional vertebrae (LSTV) are anatomical variants with adaptations to demands of sacral weight-bearing capacity, with a sacralized L5 better able to handle increased, and conversely, lumbarized S1 handling decreased burdens (Mahato, 2010). Mounting evidence supports a correlation between LSTV and low back pain, sacro-iliac dysfunction, and nerve root symptoms (Quinlan et al., 2006; Lian et al., 2018). The prevalence of LSTV is 30% of the normal population, including those with 6 lumbar vertebrae in 2–5.5% and 3–4 lumbar vertebrae in 1–7% (Bron et al., 2007; McCulloch and Waddell, 1980; Hsieh et al., 2000; Hanson et al., 2010). However, most surgical outcomes research exclude LSTV to optimise patient homogeneity.
Hypermobility of the suprajacent intervertebral space, contributes to increased torque and subsequent intervertebral disc degeneration which is higher than the non-LSTV population (Mallikarjunappa, 2019; Aihara et al., 2005; Farshad-Amacker et al., 2015). Fusion therefore demands enhanced stability measures to achieve a successful union. LSTV includes smaller and more asymmetrical facets and pedicles than non-LSTV anatomy, creating the potential for pedicle screw malposition and sub-optimal anchor strength (Fisher and Bordoni, 2019; Ono et al., 2018). With increasing degrees of sacralisation there is an increased incidence of non-union in these cases (Lee et al., 2018).
Anterior approaches are increasingly popular options for lumbar fusion, including direct or extreme lateral (DLIF/XLIF/LLIF respectively, also known as transpsoas), oblique (OLIF, also known as pre-psoas or ATP; anterior to psoas) and anterior (ALIF, usually considered midline anterior) interbody fusion. Reasons cited for this include a greater intradiscal implant footprint, increased restoration of segmental lordosis, and a lower rate of surgical morbidity, than traditional posterior approaches (Mobbs et al., 2013; Pradhan et al., 2002). Anterior approaches to the spine may be more challenging in the presence of atypical anatomy, which may accompany LSTV. Aside from complexities in identification of the correct level, there are additional anatomical, including vascular, muscular, neural, osseous and technique-based considerations. With a more recent surgical focus on restoration of segmental lordosis, anterior approaches are more commonly performed but without significant literature or collated data on their validity in the LSTV setting.
PICO: In patients with LSTV (P), who undergo an anterior approach for spinal fusion (I), compared to non-LSTV patients (C), the following outcomes were assessed (O).
-
•
What are the relevant anatomical aspects?
-
•
What further technique-based considerations are there?
-
•
What is the incidence of intra-operative surgical deviations and complications ?
2. Methods
2.1. Study design: systematic review
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed. A search was conducted using the MEDLINE and EMBASE databases of the English and French literature from January 1, 1970 to July 1, 2022. Inclusion criteria were studies that included lumbosacral transitional vertebrae (LSTV) and details relevant to anterior surgical approaches including lateral (transpsoas, LLIF), oblique (OLIF, ATP) and anterior (ALIF) surgery. The subject headings (MeSH [Medical Subject Headings]) in both databases were used in conjunction with key word variants to build gold-standard search strategies: “lumbosacral transitional vertebrae” “lumbarized” “sacralized” “Bertolotti” “Castelvi” AND any of “vasculature” “iliocaval confluence” “aortic bifurcation” “anterior lumbar interbody fusion” “oblique lumbar interbody fusion” “lateral lumbar interbody fusion”. The reference list of each relevant paper was cross checked for further relevant studies.
Each article from the primary search was evaluated for titles and abstracts for inclusion within the previously described parameters of the study. A secondary review was then taken of the full article where relevant to further discriminate primary findings. Study design, level of evidence and sample size were considered.
A qualitative analysis was performed for the answer to each research question with respect to the available literature across three domains including quantity, quality and consistency of support for the answer. Then, ratings of high, moderate, low, and very low were assigned to the outcome from each question, based on the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) guidelines (Phillips, 2021). Data was extracted by a single individual and verified independently by a second investigator.
3. Results
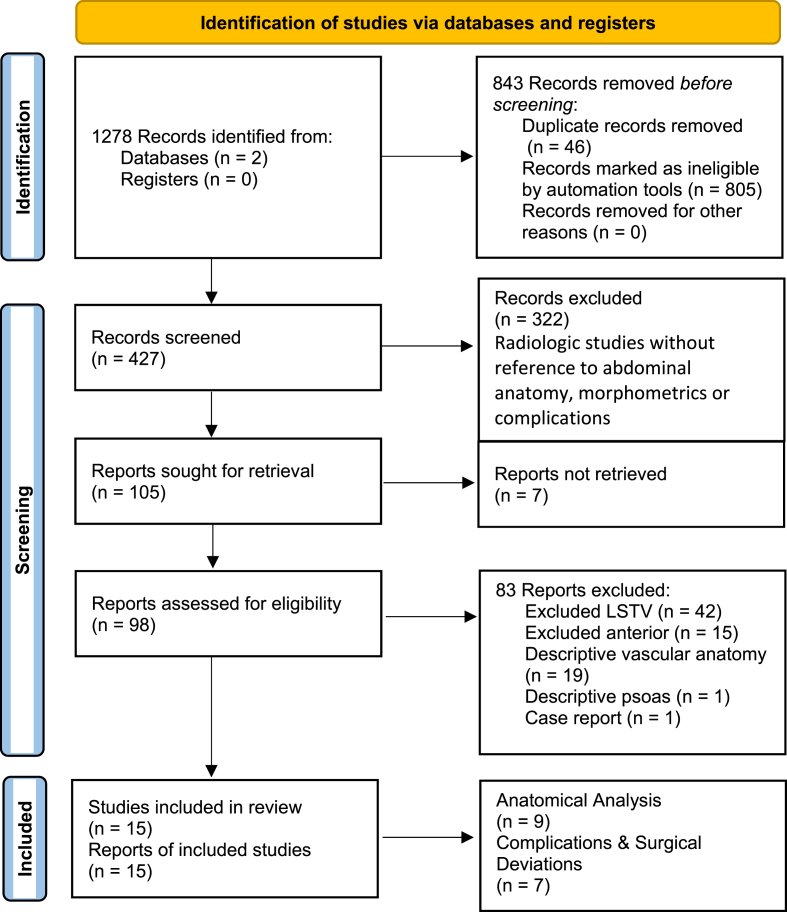
A total of 98 articles were identified (Fig. 1), with a final analysis of eight anatomical studies and seven studies on complications and surgical deviations. 83 papers were excluded from the final analysis but were considered for discussion. These excluded studies were relevant to anterior spine surgery (42- excluded LSTV to ensure homogeneity of their population), LSTV (15- LSTV morphometrics in the surgical context, but not anterior approaches), vascular anatomy (19- descriptive), psoas anatomy (1) and an unrelated case report (1), not both LSTV and ALIF/OLIF/LLIF surgery (Fig. 1). Nonetheless, these papers yielded valuable insight in terms of anatomical and approach considerations.
-
•
Sacralized L5 segment transfers motion and torque to the L4/L5 segment. Surgery is invariably required at the L4/L5 segment, also considered the “functional lumbosacral junction”, or “first mobile segment”. Most studies refer to this anatomical variant in association with the partial or complete ossification of the L5/S1 junction (Castellvi classification (Castellvi et al., 1984)).
-
•
Lumbarized S1 segment is otherwise considered as the L5/L6 segment. This is deeper in the pelvis, with a greater sacral slope and pelvic incidence. While of a similar prevalence, this variant is less commonly evaluated in the literature.
-
•
The anterior approach to either case will be very different, which will be discussed.
Fig. 1.
Flow diagram of systematic review. PRISMA (Page et al., 2021).
3.1. Anatomical considerations
Anatomical analysis yielded nine papers, all of which were based on radiological studies, with conflicting findings (Table 1).
Table 1.
Studies included in Anatomical Analysis n = 9. ICT: Iliac crest tangent, RR: retrospective review, PR: prospective review.
| Author | n (LSTV/Total) | Study Design | Level of Evidence | Findings | GRADE |
|---|---|---|---|---|---|
| Louie | 28/263 | MRI study | 3 | No association between teardrop psoas anatomy and LSTV | Mod |
| Gündüz | 39/55 | Control study, radiographic | 3 | ICT crossed the spine more often at L4 In LSTV than controls, ABF lower in LSTV 75.8% vs. 98.2%∗ | Mod |
| Josiah | 28 | CT & MRI | 4 | LSTV-iliac crest is more likely to be above the L4–5 disc space, association between LSTV and teardrop psoas anatomy, more cephalad ICC and AB | V Low |
| Molinares | 6/100 | RR MRI | 4 | LSTV does not negatively impact the oblique corridor to the L2–S1 | V Low |
| Chithriki | 37/442 | Pr MRI | 3 | Sacralisation L5- ABF more cephalad, more caudally in lumbarisation. ICC not measured (ABF at L3 in 59% in LSTV v L4 in 67% non-LSTV) | Mod |
| Lee | 127/534 | MRI | 4 | More cephalad ABF and ICC in sacralisation cases | Low |
| Jagannathan | 58/312 | MRI | 4 | More cephalad ABF and ICC in sacralisation cases | Low |
| Tureli | 505 | MRI | 4 | More cephalad ABF in sacralisation cases and more caudally in lumbarisation. ICC not measured | Low |
| Becker | 53/819 | CT | 4 | ICT, ICC & ABF was lower in 6L, higher in 4L, Psoas more anterior in LSTV | Mod |
Tear-drop psoas is considered where the posterior aspect of psoas major is anterior to the most posterior aspect of the disc or vertebral body, and the most anterior aspect of this muscle is no longer in contact with the vertebrae and detached anteriorly or laterally (Tanida et al., 2017). The psoas muscle was investigated on an MRI series by Louie et al. who differentiated between lumbarized and sacralized cases as part of their analysis, without demonstrating a higher incidence of tear-drop psoas in LSTV (contrasts with findings from complication/surgical deviation studies below) (Louie et al., 2017). It was noted on axial imaging at L4-5, that the psoas migrated anteriorly and laterally, with anterior migration of the lumbar plexus and posterolateral migration of the iliac vasculature. This may increase the risk of neurovascular injury during LLIF and OLIF procedures.
Gündüz and Josiah et al. found the LSJ to lie deeper in the pelvis in LSTV, where the intercrestal tangent (ICT) is more likely to cross at L4, a finding often consistent with a greater pelvic incidence and with a more horizontal sacrum (Gündüz et al., 2019; Josiah et al., 2017). Psoas muscles appeared to rise ventrally and laterally in patients with LSTV (Becker et al., 2022). These series were much smaller than that of Louie et al. The ascending lumbar vein (ALV) was found to course over the disc space into venous union and with the higher merger of the iliac veins. Access through the bifurcation of the inferior vena cava effectively reduced the annulotomy window and the size of the graft that can be used (Lee et al., 2018). It was noted in LSTV cases, that where the psoas was not to the side of the vertebrae, the iliac vessels occupied this space instead.
Molinares et al. analysed retroperitoneal trajectories to the lumbar spine on MRI, finding that although transitional anatomy has a greater incidence of altered vascular anatomy, it does not appear to negatively impact the oblique corridor to L2-S1 discs (Molinares et al., 2016). It was also found, while not restricted to LSTV, that with a lower ICC, direct anterior access to L5S1 was less probable. In 3 of 8 cases of ICC at mid-L5, oblique access was found instead. Chithriki, Lee, Jagannathan and Turelli et al. (Chithriki et al., 2002; Lee et al., 2007; Jagannathan et al., 2017; Tureli et al., 2014) found similar relationships between vasculature and functional lumbosacral junction, where either or both the ABF and ICC were positioned more cephalad with sacralized L5 cases and more caudal in lumbarized S1 cases. While Becker et al. enumerated as 4L 4L-LSTV, 6L and 6L-LSTV, results were similar to the above- ICT, ICC & ABF were more cephalad in sacralisation and lower in lumbarisation cases (Becker et al., 2022).
3.2. Technical consideration and complications
Intra-operative surgical deviations and complications analysis revealed seven studies (Table 2).
Table 2.
Studies included in Intra-operative Surgical Deviations and Complications Analysis n = 7. RR: retrospective review, CS: Case series, IS: isthmic spondylolisthesis.
| Author | n (LSTV/Total) | Study Design | Level of Evidence | Findings | GRADE |
|---|---|---|---|---|---|
| Weiner | 11/12 | RR | 3 | ALIF: Complications not reported. Approach lateral to ICC, similar to L4L5 approach. | Mod |
| Smith | 10/351 | RR | 4 | LLIF: L56 challenging, 8/10 displayed neuromonitoring findings preventing successful LLIF, teardrop-shaped psoas found. | Mod |
| Voyadzis | 3 | CS | 4 | 1/3 cases of abandoned LLIF had LSTV which was L5L6 instead of L4L5. | V low |
| Fantini | 1/345 | RR | 4 | ALIF: analysis of major vascular injury, total 10 of which 1 was LSTV | Mod |
| Moreau | 12/20 | RR | 4 | ALIF for L45 IS, No complications, L4-L5 was below the projection of the iliac crest in 92% of cases, left common iliac vein courses transversely across the left anterolateral aspect of the L4-L5 disc and L5 vertebral body favour a pure anterior approach (midline) or an exclusive posterior approach | Mod |
| Nourian | 49/204 | RR | 4 | Higher blood loss (347 v 262) compared to without LSTV | V Low |
| Chung | 8/127 | RR | 4 | OLIF, No intra-operative outcomes. Among the 127 cages inserted, 8 (6.2%) at L5-6 (lumbarisation) level. No intra-operative outcomes. | V Low |
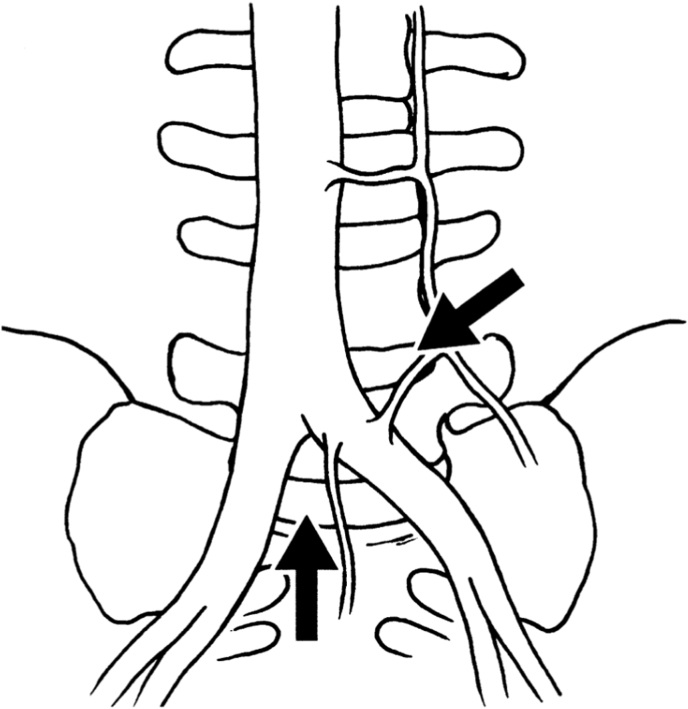
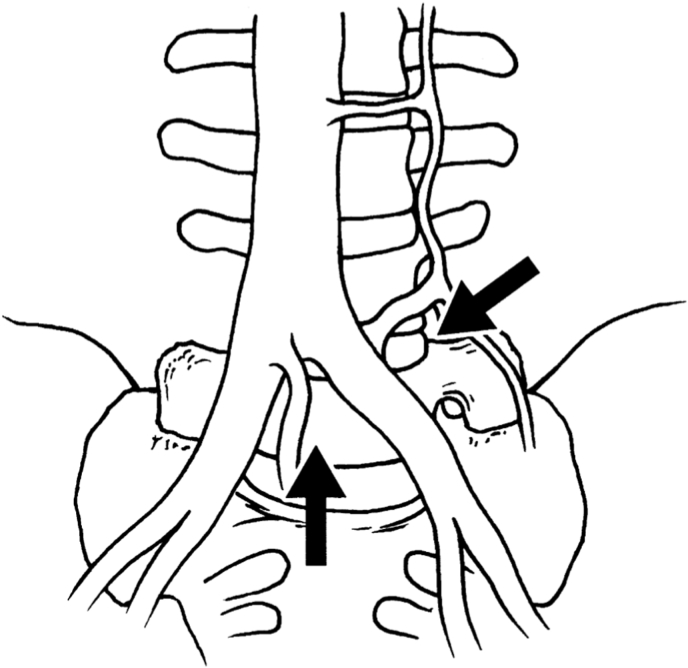
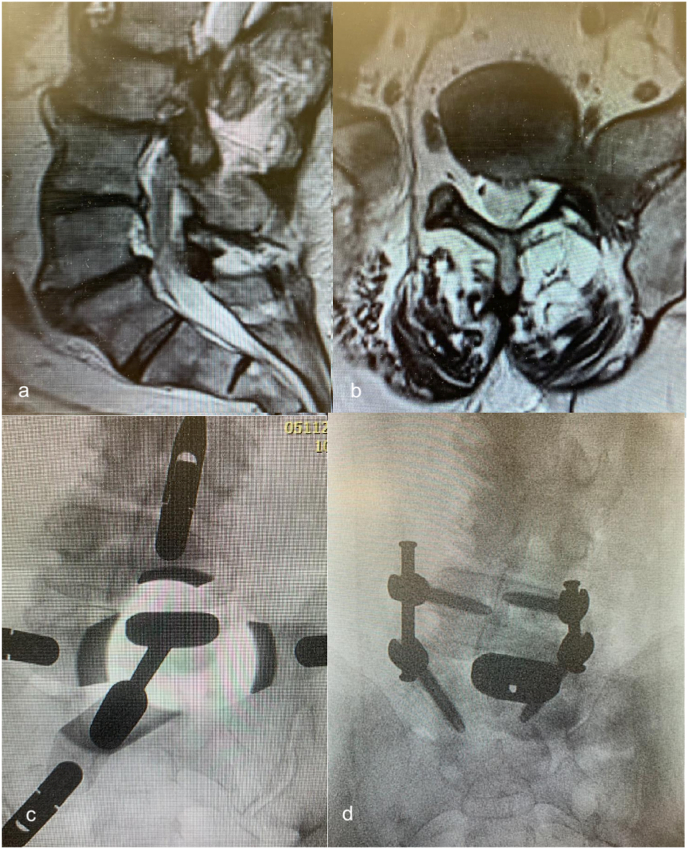
Weiner et al. noted in 11/12 sacralized L5 cases, that the surgical approach to the “functional lumbosacral junction” (L4L5 level) required access lateral to ABF/ICC [28, Fig. 2A 2B]. The ascending lumbar vein was more proximally located (closer to the junction with the IVC) and could form a common trunk with the iliolumbar vein. Both the median sacral and the ascending lumbar were found to be of increased diameter. Smith and Voyadzis et al. found difficulties with a lateral transpsoas approach, which had a high conversion to PLIF or AxialIF. Similar to Josiah et al., there was a higher prevalence of tear-drop psoas (Lian et al., 2018; Voyadzis et al., 2014), which prevented safe access to the disc space. Neurological injury to the femoral plexus was a risk factor in these cases, but there was no increased potential for vascular injury (Smith et al., 2012a). Fantini et al. reviewed a retrospective clinical series of all ALIF, with a 2.9% (10/345) rate of vascular injury, including one LSTV who suffered a left CIV injury with inadequate mobilisation of the iliac vessels, using a left paramedian approach (Fantini et al., 2007). The authors noted cephalization of the last mobile segment and/or caudalization of the iliac vein confluence, such that the last mobile segment is often located directly dorsal to the ICC, mandating an approach lateral to the ABF & ICC for the last mobile segment necessitating ligation and division of the ascending iliolumbar vein to obtain exposure.
Fig. 2.
A&2B: Adapted from Weiner et al. (Weiner et al., 2001). Access options on midline and lateral approaches for “Normal” and LSTV vascular anatomy. A: “Normal”: midline approach between ABF and ICC, lateral approach to L45. B: LSTV: midline approach is less likely whereas lateral approach may be considered, accepting anatomical variants including ABF, ICC and ALV.
Moreau et al. noted in a series of 20 anterior fusions in L4-L5 isthmic spondylolisthesis (IS) where LSTV (sacralized L5) was present in 12 (60%) (Moreau et al., 2016). In LSTV cases, L4-L5 was below the projection of the iliac crest in 92%. The CCI was more cephalad and there was a trend towards a steeper confluence angle of the common iliac veins in this group. No complication was noted, even if the approach was unusually difficult in 11 cases. The left CIV was noted to course transversely across the left anterolateral aspect of the L4-L5 disc and L5 vertebral body, increasing the risk of vascular injury. Contrary to other studies, the recommended approach for L4-L5 IS was a pure anterior approach (midline) or an exclusive posterior approach. Nourian et al. demonstrated increased blood loss in LSTV ((347 mls vs. 262 mls non-LSTV) (Nourian et al., 2016). Chung et al. demonstrated OLIF at L56 was technically feasible without reporting specific complications (Chung et al., 2018).
4. Discussion
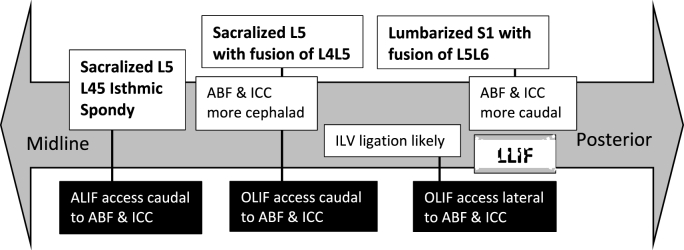
In the treatment of lumbar spine pathologies, employing anterior approaches for spinal fusion, including ALIF, OLIF and LLIF are increasingly prevalent lending to minimally invasive concepts, deformity corrections and high fusion rates. As the trajectory varies from direct anterior, to flank, to lateral, the obstacles in anterior approaches include the level and nature of the vasculature, the position of the psoas muscle and the iliac crest respectively (Fig. 7). Most common complications reported in over 11000 all-level ALIFs were venous injury (3.2%), retrograde ejaculation (2.7%), neurologic injury (2%), prosthesis related (2%), postoperative ileus (1.4%), superficial infection (1%) and others (1.3%) (Bateman et al., 2015). Arguably lower than this are early clinical results and complications associated with OLIF, which have shown incisional pain (2.2%), sympathetic chain injury (1.7%), neurological deficit (1.1%) and iliac vessel injury (1.1%) (Silvestre et al., 2012). Taking an accommodative approach in each case is critical to achieving surgical objectives, particularly where LSTV anatomy is expected to deviate from normal patterns (Fig. 2A & B). At the outset, osseus morphology with LSTV will demonstrate abnormal L5-Iliac crest enlarged and dysplastic transverse processes or enumeration variance in the case of lumbarisation. In this context, the evaluation of anatomic studies must be interpreted in terms of access to above the standard lumbosacral junction at the suprajacent L4/5, or below, at the L5/6 levels.
Fig. 7.
Algorithm for LSTV abdominal approach. ABF: Aortic Bifurcation; ICC: Ilio-Caval Confluence. Dashed line indicates contra-indication.
Vascular awareness while accessing L4/5 will be in the presence of a more cephalad ABF and ICC with sacralized L5 (far greater prevalence of pathology), but with safer experiences of approaching L45 lateral to the ABF/ICC. Conversely access to the deeper L5/6 level will be in the presence of a more caudal ABF and ICC in lumbarized S1 (Fig. 6). In non-LSTV, the laterality of vessels has greater implications with OLIF to L5S1, as the right side is considered to be more accessible (Song et al., 2019). The superior hypogastric (sympathetic) plexus should be considered because it courses on the left side of the aorta, anterior to the LCIV. The left-side approach to the L5S1 disc could damage the superior hypogastric plexus, resulting in excess vasodilation and warmth in the left foot and/or retrograde ejaculation (Paraskevas et al., 2008). Evaluation of vascular position and access corridor when evaluated on supine MRI scanning must recognise the change in position of these structures when operating from an oblique or lateral approach.
Fig. 6.
Lumbarisation L56, a: pre-existing L1-L4 spinal instrumentation, non-contiguous stenosis and instability L56 requiring revision with extension to the pelvis, b: MRI T2, distal segment degeneration L4L5 L5L6, c: MRI T2, L56 facet erosion, achievable midline vascular access noted (not used), d: post-revision radiograph, instrumentation L1-Pelvis with L56 bullet cage, e: SPECT scan, pseudarthrosis L56 with cage subsidence and cyst formation. An anterior approach at L56 would have allowed a greater implant footprint and height, thus optimising segmental lordosis and fusion.
LLIF should be considered a relative contra-indication in LSTV, largely due to tear-drop psoas, interposed iliac vessels in the lateral vertebral-psoas gutter and a higher intercrestal tangent (ICT). Evaluation of the position and size of the psoas should also be considered with an oblique approach (OLIF), for example, for L4L5 fusion with sacralized L5. While reported for LSTV, Louie et al. did not find a higher incidence of tear-drop psoas in LSTV, accepting that this analysis was done on a non-surgical cohort. Lumbarized S1 is a relative contraindication for lateral transpsoas interbody fusion (L5/L6), largely because of a psoas that resembles that of the normal L5/S1 level (Smith et al., 2012b). A sacralized L5 may contain variant psoas anatomy and an anteriorly displaced lumbar plexus (Xu et al., 2018). As the psoas travels caudally, the plexus generally migrates from a dorsal to ventral location on the lateral aspect of the disc. Given the recent trend for prone lateral interbody (LLIF) or single position 360° surgery (LLIF with percutaneous pedicle screw insertion), LSTV cases should not be considered for these procedures for the reasons outlined.
An L4/5 IS yielded a 60% incidence of LSTV, with smaller pars interarticularis, pre-disposing the patient to IS but also alternative vascular findings (Moreau et al., 2016). Contrary to the findings above, the authors found it better to access the L45 disc through a direct anterior route (ALIF), because of significantly more cephalad ABF/ICC. Ould-Slimane et al. (2020) previously noted that IS at L5S1 induces a geometric deformation of the lumbosacral hinge which modifies its anatomical relations with the ICC. The anterior approach to L45 in the presence of an L5S1 IS is possible between the iliac veins (Xu et al., 2018).
One must display a heightened awareness of appropriate level surgery with transitional anatomy. Sacralized L5 exhibits a spectrum of elongated transverse process(es) to complete sacral fusion (Castellvi et al., 1984). Intra-operative display of pre-operative radiographs may also help recognition of the morphology of the fusion level. The lumbosacral junction lies deeper in the pelvis than non-LSTV, where the ICT is more likely to cross at L4 (Louie et al., 2017).
Major haemorrhage is a known and feared complication of anterior access surgery. While instances were reported, this review did not find significantly higher incidences of major haemorrhage in LSTV cases. Intra-operative blood loss was reported as higher than non-LSTV cases (Fantini et al., 2007). While vascular complications were not significantly higher, surgical deviations were more common. However, lessons learned from difficult access cases can mitigate against this, particularly with recognised psoas, vascular and osseus factors on pre-operative evaluation (Fig. 2). Vascular injuries are under-reported and can be unforgiving. While standardising the anterior approach normally provides a “safe system” routine, varying the side or the angle of the approach must raise the awareness of the operator that the safe zone for dissection may therefore be the inverse of the normal routine. Marking the retractors to remind the operator would help mitigate against surgical injury. This is also relevant for pledget dissection which is best conducted in the direction of the relevant vessel as opposed to away from it.
While anatomic studies have identified a lower ABF and ICC in lumbarisation cases (L5/L6), surgical studies have not shown details of surgical deviations or complications. Series of fusions at L5/L6 (any approach) are not described in the literature. Described alternative fusion methods for L5/L6 include PLIF, AxialLIF (Weiner et al., 2001) or OLIF (Nourian et al., 2016) but complications have not been reported. L56 patients have up to 20° higher pelvic incidence and up to 11° greater sacral slope than standard measurements respectively (Price et al., 2016). The pubic bone may therefore inhibit access to L56 if using ALIF.
These techniques must also incorporate other known approach considerations. Correct positioning warrants supervision and communication, with co-ordination of intra-operative imaging. A perivascular fat-strip can determine retraction potential of the iliac veins, although it is less likely to exist at LS levels and its presence is not proven as a safety factor. Requirement for secondary insertion of pedicle screws or osteotomy is influenced by the pathology, including degenerative deformity, isthmic spondylolisthesis, high sacral slope and segmental hypermobility/instability or ability to safely apply a plate (Ahern et al., 2020). Exposure to the disc space will dictate annulotomy size, affecting intradiscal height correction. Additional factors influence the optimal approach including previous abdominal scarring or truncal obesity. Fusion of multiple levels may necessitate mobilisation of the iliac vein or IVC which has a higher vascular injury rate. Placing the patient in a semi-lateral position and using OLIF therefore allows versatility where it allows safe access to either medial or caudal to ABF/ICC (Molloy et al., 2016). Key to this utilitarian approach is using the appropriate obliquus internus approach window that facilitates optimal access to the disc space. This obviates the need for converting a failed OLIF procedure into a PLIF/TLIF/AxiaLIF. Recognising safe boundaries is of critical importance and therefore, aborting surgery to instead provide posterior fusion is safer than a significant vascular injury with its associated blood loss and risk of mortality.
4.1. Illustrative cases are described (Fig. 2, Fig. 3, Fig. 4, Fig. 5)
Fig. 3.
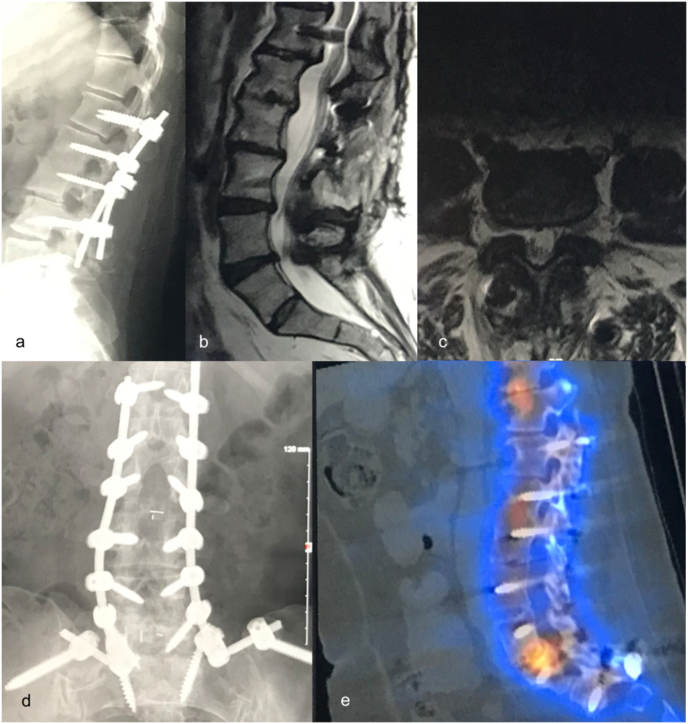
Sacralized L5 case. a: disc degeneration at the functional (L45) lumbosacral level; b: axial MRI view of pathology, fluid in facets indicating significant segmental mobility, anterior vessels demonstrate right side artery and vein (R CIA and CIV) which are not in the surgical corridor, left side (L CIA and CIV) are in close proximity, traversing the surgical corridor from medial to lateral. Optimal disc access was unclear. Left side OLIF approach anterolateral to the disc revealed inadequate access but safely allowed access medial to the left CIA and CIV, thus caudal to the ABF/ICC; c: intervertebral cage and pedicle screws in situ.
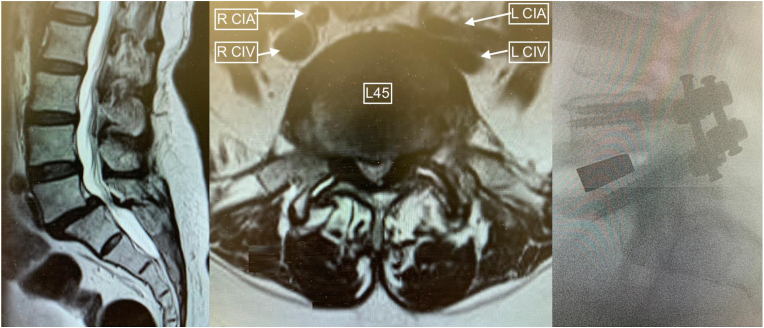
Fig. 4.
Sacralized L5 case. a: 10 years of mid-flexion instability, mild MRI evidence of disc degeneration at the functional lumbosacral level; b: high bifurcation revealed direct anterior access to the disc space, normally considered approachable from anterolateral access; c: intervertebral cage with facet joint wedges, note the high inter-crestal tangent (mid-L4).
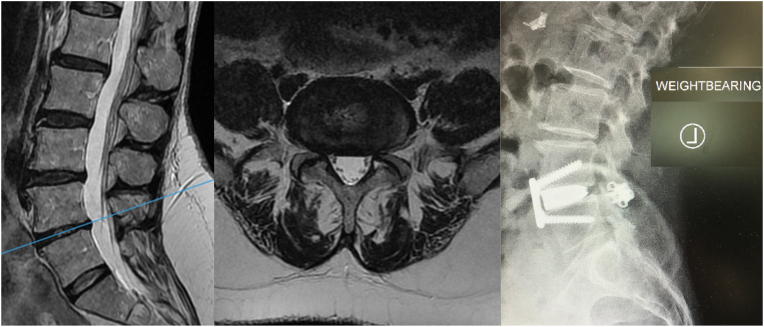
Fig. 5.
Sacralized L5 with coronal deformity. a: MRI T2 b: MRI Axial L4L5 (functional lumbosacral joint). The surgical approach to the bifurcation appeared accessible through a midline approach caudal to the bifurcation. The L45 transitional segment disc height approximates that of a L5S1 disc; c: an OLIF approach (semi-lateral position, flank incision) was taken as a utilitarian approach. The disc was difficult to access through the midline and a lateral trajectory was taken instead. This required a second IO window but ultimately was safely achievable with OLIF. d: final radiograph with partial coronal correction.
While a criticism of posterior approaches for LSTV is the increased risk of non-union, a limitation of this review was that rate of fusion was not reported in the analysed studies. Arterial thrombosis is another recognised complication of this approach, not reported in the literature on LSTV, and relevant where anterior or lateral osteophytes would dictate the approach to the disc space instead. Disc height restoration is a commonly quoted feat of ALIF, OLF and LLIF but these were not reported for LSTV. The studies were heterogenous, with findings from radiological, cadaveric and surgical research. However, surgical outcomes are contingent on these pre- and intra-operative factors.
In conclusion, this systematic review seeks to identify characteristics of anterior approaches to the LSTV spine and promote identification of safe surgery for LSTV using the anatomical and surgical papers reporting on the subject. While anatomy is usually altered in these cases, vascular, muscular and osseous anatomy is predictably altered, sufficient on pre-operative analysis to facilitate a surgical plan. Fluid versatility is required on behalf of the spine surgeon to optimise the approach and in particular, interchange between ALIF and OLIF, access the appropriate level lateral or caudal to the ABF/ICC and mitigate for potential posterior surgery if needed. The OLIF approach seems a more utilitarian option in this regard. This review also arms the surgeon with enhanced awareness and insight for anterior approaches in the non-LSTV population, providing a further layer of safety and optimisation of results.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author statement
Derek T Cawley Material preparation, analysis, methodology, approved the version to be published, primary author of manuscript. Roozbeh Shafafi Material preparation, methodology, commented on previous versions of the manuscript, approved current version of manuscript, agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Obiekezie Agu Editing, commented on previous versions of the manuscript, approved current version of manuscript. Sean Molloy Material preparation, analysis, methodology, approved the version to be published, agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved, supervisor.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Prof F Kandziora
Contributor Information
D.T. Cawley, Email: derek.cawley@materprivate.ie.
R. Shafafy, Email: roozbeh.shafafy@gmail.com.
O. Agu, Email: o.agu@nhs.net.
S. Molloy, Email: seanmolloy@nhs.net.
References
- Ahern D.P., Welch-Phillips A., Cawley D.T., Butler J.S. Is multilevel anterior lumbar interbody fusion (ALIF) superior to pedicle subtraction osteotomy (PSO) for degenerative lumbar deformity? Clinical Spine Surgery. 2020;33(1):1–4. doi: 10.1097/BSD.0000000000000817. Feb 1. [DOI] [PubMed] [Google Scholar]
- Aihara T., et al. Intervertebral disc degeneration associated with lumbosacral transitional vertebrae: a clinical and anatomical study. J. Bone Jt Surg. Br. 2005;87(5):687–691. doi: 10.1302/0301-620X.87B5.15727. [DOI] [PubMed] [Google Scholar]
- Bateman D.K., Millhouse P.W., Shahi N., Kadam A.B., Maltenfort M.G., Koerner J.D., Vaccaro A.R. Anterior lumbar spine surgery: a systematic review and meta-analysis of associated complications. Spine J. 2015;15(5):1118–1132. doi: 10.1016/j.spinee.2015.02.040. May 1. Epub 2015 Feb 26. PMID: 25728552. [DOI] [PubMed] [Google Scholar]
- Becker L., Adl Amini D., Ziegeler K., et al. Approach-related anatomical differences in patients with lumbo-sacral transitional vertebrae undergoing lumbar fusion surgery at level L4/5. Arch. Orthop. Trauma. Surg. 2022 doi: 10.1007/s00402-021-04303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bron J.L., van Royen B.J., Wuisman P.I. The clinical significance of lumbosacral transitional anomalies. Acta Orthop. Belg. 2007;73:687–695. [PubMed] [Google Scholar]
- Castellvi A.E., Goldstein L.A., Chan D.P. Lumbosacral transitional vertebrae and their relationship with lumbar extradural defects. Spine. 1984;9(5):493–495. doi: 10.1097/00007632-198407000-00014. [DOI] [PubMed] [Google Scholar]
- Chithriki M., Jaibaji M., Steele R. The anatomical relationship of the aortic bifurcation to the lumbar vertebrae: a MRI study. Surg. Radiol. Anat. 2002;24(5):308–312. doi: 10.1007/s00276-002-0036-3. Dec 1. [DOI] [PubMed] [Google Scholar]
- Chung N.S., Lee H.D., Jeon C.H. Accuracy of the lateral cage placement under intraoperative C-arm fluoroscopy in oblique lateral interbody fusion. J. Orthop. Sci. 2018;23(6):918–922. doi: 10.1016/j.jos.2018.07.010. Nov 1. [DOI] [PubMed] [Google Scholar]
- Fantini G.A., Pappou I.P., Girardi F.P., Sandhu H.S., Cammisa F.P., Jr. Major vascular injury during anterior lumbar spinal surgery: incidence, risk factors, and management. Spine. 2007;32(24):2751–2758. doi: 10.1097/BRS.0b013e31815a996e. Nov 15. [DOI] [PubMed] [Google Scholar]
- Farshad-Amacker N.A., Herzog R.J., Hughes A.P., Aichmair A., Farshad M. Associations between lumbosacral transitional anatomy types and degeneration at the transitional and adjacent segments. Spine J. 2015;15(6):1210–1216. doi: 10.1016/j.spinee.2013.10.029. [DOI] [PubMed] [Google Scholar]
- Fisher M., Bordoni B. StatPearls Publishing; Treasure Island (FL): 2019. Anatomy, Bony Pelvis and Lower Limb, Pelvic Joints. [PubMed] [Google Scholar]
- Gündüz N., Günaydin G.D., Eser M.B., Aslan A., Kabaalioğlu A. Role of iliac crest tangent in correct numbering of lumbosacral transitional vertebrae. Turk. J. Med. Sci. 2019;49(1):184–189. doi: 10.3906/sag-1807-258. Feb 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson E.H., Mishra R.K., Chang D.S., et al. Sagittal whole-spine magnetic resonance imaging in 750 consecutive outpatients: accurate determination of the number of lumbar vertebral bodies. J. Neurosurg. Spine. 2010;12:47–55. doi: 10.3171/2009.7.SPINE09326. [DOI] [PubMed] [Google Scholar]
- Hsieh C.Y., Vanderford J.D., Moreau S.R., et al. Lumbosacral transitional segments: classification, prevalence, and effect on disk height. J. Manip. Physiol. Ther. 2000;23:483–489. doi: 10.1067/mmt.2000.108817. [DOI] [PubMed] [Google Scholar]
- Jagannathan D., Indiran V., Hithaya F., Alamelu M., Padmanaban S. Role of anatomical landmarks in identifying normal and transitional vertebra in lumbar spine magnetic resonance imaging. Asian Spine J. 2017;11(3):365. doi: 10.4184/asj.2017.11.3.365. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josiah D.T., Boo S., Tarabishy A., Bhatia S. Anatomical differences in patients with lumbosacral transitional vertebrae and implications for minimally invasive spine surgery. J. Neurosurg. Spine. 2017;26(2):137–143. doi: 10.3171/2016.6.SPINE1691. Feb 1. [DOI] [PubMed] [Google Scholar]
- Lee C.H., Park C.M., Kim K.A., Hong S.J., Seol H.Y., Kim B.H., Kim J.H. Identification and prediction of transitional vertebrae on imaging studies: anatomical significance of paraspinal structures. Clin. Anat. 2007;20(8):905–914. doi: 10.1002/ca.20540. Nov. [DOI] [PubMed] [Google Scholar]
- Lee G.W., Shin J.H., Ryu S.M., Ahn M.W. The impact of L5 sacralization on fusion rates and clinical outcomes after single-level posterior lumbar interbody fusion (PLIF) at L4–L5 level. Clinical Spine Surgery. 2018;31(1):E62–E68. doi: 10.1097/BSD.0000000000000536. Feb 1. [DOI] [PubMed] [Google Scholar]
- Lian J., Levine N., Cho W. A review of lumbosacral transitional vertebrae and associated vertebral numeration. Eur. Spine J. 2018;27(5):995–1004. doi: 10.1007/s00586-018-5554-8. May 1. [DOI] [PubMed] [Google Scholar]
- Louie P.K., Narain A.S., Hijji F.Y., Yacob A., Yom K.H., Phillips F.M., Singh K. Radiographic analysis of psoas morphology and its association with neurovascular structures at L4-5 with reference to lateral approaches. Spine. 2017;42(24):E1386–E1392. doi: 10.1097/BRS.0000000000002303. Dec 15. [DOI] [PubMed] [Google Scholar]
- Mahato N.K. Morphological traits in sacra associated with complete and partial lumbarization of first sacral segment. Spine J. 2010;10(10):910–915. doi: 10.1016/j.spinee.2010.07.392. [DOI] [PubMed] [Google Scholar]
- Mallikarjunappa B. Lumbosacral transitional vertebra: prevalence and its impact on transitional and adjacent lumbar discs. IOSR J. Dental Med. Sci. e-ISSN. 2019;2279-0853 01-08. p-ISSN: 2279-0861. 18, 8. 9 (August. [Google Scholar]
- McCulloch J.A., Waddell G. Variation of the lumbosacral myotomes with bony segmental anomalies. J. Bone Joint Surg. Br. 1980;62- B:475–480. doi: 10.1302/0301-620X.62B4.7430228. [DOI] [PubMed] [Google Scholar]
- Mobbs R.J., Loganathan A., Yeung V., Rao P.J. Indications for anterior lumbar interbody fusion. Orthop. Surg. 2013;5(3):153–163. doi: 10.1111/os.12048. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinares D.M., Davis T.T., Fung D.A. Retroperitoneal oblique corridor to the L2–S1 intervertebral discs: an MRI study. J. Neurosurg. Spine. 2016;24(2):248–255. doi: 10.3171/2015.3.SPINE13976. Feb 1. [DOI] [PubMed] [Google Scholar]
- Molloy S., Butler J.S., Benton A., Malhotra K., Selvadurai S., Agu O. A new extensile anterolateral retroperitoneal approach for lumbar interbody fusion from L1 to S1: a prospective series with clinical outcomes. Spine J. 2016;16(6):786–791. doi: 10.1016/j.spinee.2016.03.044. Jun 1. [DOI] [PubMed] [Google Scholar]
- Moreau P.E., Flouzat-Lachaniette C.H., Lebhar J., Mirouse G., Poignard A., Allain J. Particularities of anterior fusion in L4-L5 isthmic spondylolisthesis. J. Orthop. Traumatol.: Surgery & Research. 2016;102(6):755–758. doi: 10.1016/j.otsr.2016.05.006. Oct 1. [DOI] [PubMed] [Google Scholar]
- Nourian A.A., Cunningham C.M., Bagheri A., Bruffey J.D., Eastlack R.K. Effect of anatomic variability and level of approach on perioperative vascular complications with anterior lumbar interbody fusion. Spine. 2016;41(2):E73–E77. doi: 10.1097/BRS.0000000000001160. Jan 1. [DOI] [PubMed] [Google Scholar]
- Ono T., Tarukado K., Tono O., Harimaya K., Morishita Y., Nakashima Y., Doi T. The morphological relationship between lumbosacral transitional vertebrae and lumbosacral pedicle asymmetry. Spine Surg. Related Res. 2018;2(1):77–81. doi: 10.22603/ssrr.2017-0019. Jan 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ould-Slimane M., Damade C., Gillibert A., Michelin P., Latrobe C., Guigui P., Ferrero E., Gauthé R. Iliocava junction to L4-L5 disc anatomical relationship in L5-S1 isthmic spondylolisthesis. J. Orthop. Traumatol.: Surgery & Research. 2020;106(6):1195–1201. doi: 10.1016/j.otsr.2020.02.013. Oct 1. [DOI] [PubMed] [Google Scholar]
- Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraskevas G., Tsitsopoulos P., Papaziogas B., et al. Variability in superior hypogastric plexus morphology and its clinical applications: a cadaveric study. Surg Radiol Anat SRA. 2008;30:481–488. doi: 10.1007/s00276-008-0352-3. [DOI] [PubMed] [Google Scholar]
- Phillips M. Healthcare recommendations: grades of recommendation, assessment, development, and evaluation (GRADE) approach. Evidence-Based Orthopedics. 2021:19–23. Aug 30. [Google Scholar]
- Pradhan B.B., Nassar J.A., Delamarter R.B., Wang J.C. Single-level lumbar spine fusion: a comparison of anterior and posterior approaches. J. Spinal Disord. Tech. 2002;15:355–361. doi: 10.1097/00024720-200210000-00003. [DOI] [PubMed] [Google Scholar]
- Price R., Okamoto M., Le Huec J.C., Hasegawa K. Normative spino-pelvic parameters in patients with the lumbarization of S1 compared to a normal asymptomatic population. Eur. Spine J. 2016;25(11):3694–3698. doi: 10.1007/s00586-016-4794-8. Nov. [DOI] [PubMed] [Google Scholar]
- Quinlan J.F., Duke D., Eustace S. Bertolotti's syndrome: a cause of back pain in young people. J Bone Joint. British volume. 2006;88(9):1183–1186. doi: 10.1302/0301-620X.88B9.17211. Sep. [DOI] [PubMed] [Google Scholar]
- Silvestre C., Mac-Thiong J.M., Hilmi R., et al. Complications and morbidities of mini-open anterior retroperitoneal lumbar interbody fusion: oblique lumbar interbody fusion in 179 patients. Asian Spine J. 2012;6:89–97. doi: 10.4184/asj.2012.6.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W.D., Youssef J.A., Christian G., Serrano S., Hyde J.A. Lumbarized sacrum as a relative contraindication for lateral transpsoas interbody fusion at L5-6. Clinical Spine Surgery. 2012;25(5):285–291. doi: 10.1097/BSD.0b013e31821e262f. Jul 1. [DOI] [PubMed] [Google Scholar]
- Smith W.D., Youssef J.A., Christian G., Serrano S., Hyde J.A. Lumbarized sacrum as a relative contraindication for lateral transpsoas interbody fusion at L5-6. Clinical Spine Surgery. 2012;25(5):285–291. doi: 10.1097/BSD.0b013e31821e262f. Jul 1. [DOI] [PubMed] [Google Scholar]
- Song S.J., Shin M.H., Kim J.T. Anatomical feasibility of right oblique approach for L5-S1 oblique lumbar interbody fusion. World Neurosurgery. 2019;132:e403–e408. doi: 10.1016/j.wneu.2019.08.135. Dec 1. [DOI] [PubMed] [Google Scholar]
- Tanida S., Fujibayashi S., Otsuki B., Masamoto K., Matsuda S. Influence of spinopelvic alignment and morphology on deviation in the course of the psoas major muscle. J. Orthop. Sci. 2017;22(6):1001–1008. doi: 10.1016/j.jos.2017.08.002. Nov 1. [DOI] [PubMed] [Google Scholar]
- Tureli D., Ekinci G., Baltacioglu F. Is any landmark reliable in vertebral enumeration? A study of 3.0-Tesla lumbar MRI comparing skeletal, neural, and vascular markers. Clin. Imag. 2014;38(6):792–796. doi: 10.1016/j.clinimag.2014.05.001. Nov 1. [DOI] [PubMed] [Google Scholar]
- Voyadzis J.M., Felbaum D., Rhee J. The rising psoas sign: an analysis of preoperative imaging characteristics of aborted minimally invasive lateral interbody fusions at L4–5: report of 3 cases. J. Neurosurg. Spine. 2014;20(5):531–537. doi: 10.3171/2014.1.SPINE13153. May 1. [DOI] [PubMed] [Google Scholar]
- Weiner B.K., Walker M., Fraser R.D. Vascular anatomy anterior to lumbosacral transitional vertebrae and implications for anterior lumbar interbody fusion. Spine J. 2001;1(6):442–444. doi: 10.1016/s1529-9430(01)00126-7. Nov 12. [DOI] [PubMed] [Google Scholar]
- Xu D.S., Walker C.T., Godzik J., Turner J.D., Smith W., Uribe J.S. Minimally invasive anterior, lateral, and oblique lumbar interbody fusion: a literature review. Ann. Transl. Med. 2018;6(6) doi: 10.21037/atm.2018.03.24. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]