Abstract
This Graphical Review provides an overview of amide bond activation achieved by selective oxidative addition of the N–C(O) acyl bond to transition metals and nucleophilic acyl addition, resulting in acyl and decarbonylative coupling together with key mechanistic details pertaining to amide bond distortion underlying this reactivity manifold.
Keywords: C–N activation, amide bond activation, acyl coupling, decarbonylative coupling, acyl addition, catalysis
Graphical Abstract

The importance of amide bond is undeniable. The amide bond is the fundamental linkage of life in peptides and proteins. Due to its special dipolar character, amides are indispensable in pharmaceuticals, pesticides, polymers. At present, more than 50% of drug candidates contain amide bonds. Remarkably, reactions of amides are the most common type of reactions used in current medicinal chemistry.
Typical planar amides feature strong amidic resonance, nN → π*C=O conjugation (15–20 kcal/mol), which renders amide bond cleavage extremely difficult. However, the amide bond can be sterically-twisted or electronically-activated by functionalizing the nitrogen atom of the amide bond. In this way, the amide bond resonance can be significantly decreased or diverted onto the activating group, thus enabling highly selective activation of N–C(O) amide bonds.
The recent years have seen an explosion of amide bond activation methods. Although the concept of amide bond twisting and the concurrent decrease of amidic resonance in bridged lactams was proposed as early as in 1930s, it was not until 2015 that generic acyclic twisted amides were used for the first time as cross-coupling partners in selective N–C(O) bond activation, thus effectively serving as surrogates for acyl and aryl halides and pseudohalides in transition-metal-catalysis.
Amide bond activation can be categorized as acyl coupling and decarbonylative coupling. This reactivity is triggered by selective oxidative addition of the N–C(O) acyl bond to transition metal, leading to either direct transmetalation or CO de-insertion. Furthermore, the successful use of amides as acyl halide equivalents in transition-metal-catalysis spearheaded the development of an array of highly selective methods for nucleophilic acyl addition to amide bonds, resulting in an alternative disconnection to acyl products. In many cases, the direct nucleophilic acyl addition shows advantages over transition-metal-catalyzed variants; however, it should be noted that these manifolds are broadly complementary.
This Graphical Review provides an overview of the key studies in amide bond activation covering the period of 2015 to 2022. The goal of this Graphical Review is to provide a summary of the reactions developed and manifolds established in the main areas of amide bond activation, including acyl and decarbonylative coupling as well as acyl nucleophilic addition, while highlighting the underlying mechanisms and amides that are critical to this reactivity manifold. Throughout the review we have attempted to cite the seminal reports and precedents. However, the Reader should note that due to the format of this review and the large number of contributions, the review is not comprehensive.
Throughout the review, the reactions are categorized by the type of mechanism. An important aspect that the Reader should pay special attention to is the role of specific amides that participate in each reaction of manifold. In general, sterically-twisted or electronically-activated amides can be prepared (1) from carboxylic acids or derivatives, or (2) from generic 1° or 2° amides. Both methods are valuable in terms of synthetic advantages of amide bonds in cross-coupling and acyl addition chemistry. However, for derivatization of biomolecules and late-stage functionalization of pharmaceuticals, only amides that can be generically prepared from 1° or 2° amides are useful. We hope that the review will stimulate further progress in this tremendously important field of chemistry.
Figure 1.

Amide bond activation: concept and discoveries.1
Figure 2.
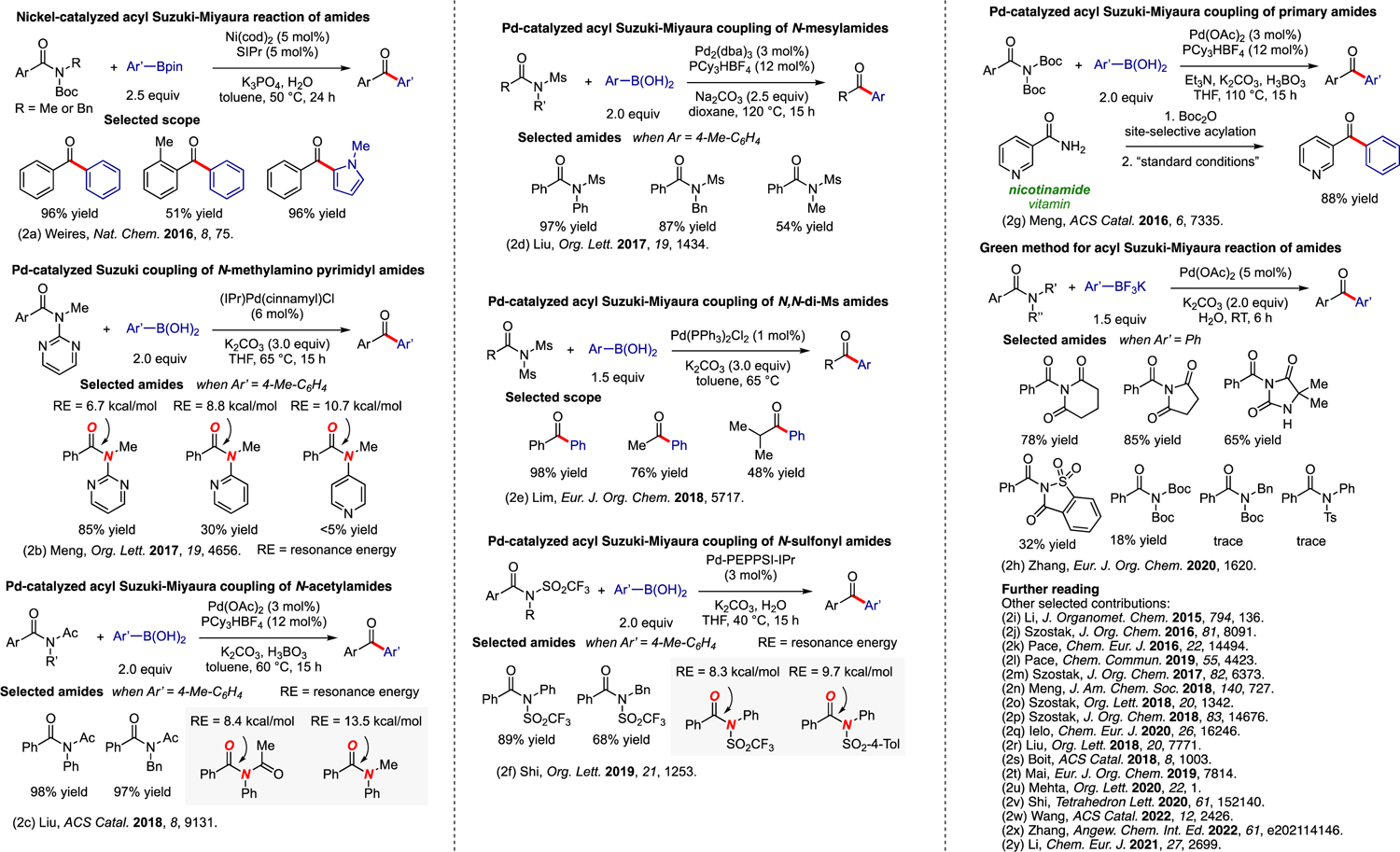
Transition-metal-catalyzed acyl Suzuki-Miyaura coupling of amides.2
Figure 3.

Synthesis of ketone via transition-metal-catalysis and metal-fee conditions.3
Figure 4.

Amides as acylating reagents in various synthetic methodologies.4
Figure 5.

Cross-coupling of amides with versatile partners.5
Figure 6.

Transamidation of amides under metal-catalysis and metal-free conditions.6
Figure 7.

Esterification of amides via metal-catalysis and metal-free conditions.7
Figure 8.

Decarbonylative cross-coupling of amides: discoveries and mechanism.8
Figure 9.

Decarbonylative cross-coupling of amides: construction of carbon-carbon and carbon-hydrogen bonds.9
Figure 10.

Decarbonylative cross-coupling of amides: construction of carbon-heteroatom bonds.10,11
Acknowledgment
We thank the current and former members of the Szostak group who have contributed to establishing the concept of amide bond activation.
Funding Information
We thank the NSF (CAREER CHE-1650766), the NIH (R35GM133326), Rutgers University, Overseas High-Level Talents Fund of Shanghai, and Shanghai University for generous support.
Biographies

Chengwei Liu received his PhD from Rutgers University with Prof. Michal Szostak in 2020. He conducted his postdoctoral research at University of Oxford with Prof. Stephen P. Fletcher from 2020 to 2021. Then he performed as an Assistant Professor at Nanjing University of Information Science and Technology from 2021 to 2022. In the summer of 2022, he joined the faculty at Shanghai University, where he started his independent career and was promoted to Full Professor in 2022. His research group is focused on amide bond activation, C–O bond activation, C–S bond activation, and lanthanide organometallic chemistry.

Michal Szostak received his Ph.D. from the University of Kansas in 2009. He carried out postdoctoral research at Princeton University and University of Manchester. In 2014, he joined the faculty at Rutgers University, where he is currently Professor of Chemistry. His research group developed the concept of acyclic twisted amide bond activation. In 2022, he edited the book “Amide Bond Activation: Concepts and Reactions”. His current research is focused on the development of new synthetic methodology based on transition-metal-catalysis, amide bond activation, NHC ligands, inert bond activation, and application to the synthesis of biologically active molecules. He is the author of over 240 publications.
Footnotes
Conflict of Interest
The authors declare no competing financial interest.
References
- (1).(a) Szostak M Amide Bond Activation: Concepts and Reactions, Wiley, 2022. [Google Scholar]; (b) Hie L; Fine Nathel NF; Shah TK; Baker EL; Hong X; Yang YF; Liu P; Houk KN; Garg NK Nature 2015, 524, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Meng G; Szostak M Org. Lett 2015, 17, 4364. [DOI] [PubMed] [Google Scholar]; (d) Li X; Zou G Chem. Commun 2015, 51, 5089. [DOI] [PubMed] [Google Scholar]; (e) Szostak M; Aube J Chem. Rev 2013, 113, 5701. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Meng G; Zhang J; Szostak M Chem. Rev 2021, 121, 12746. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Meng G; Shi S; Szostak M Synlett 2016, 27, 2530. [Google Scholar]; (h) Liu C; Szostak M Chem. Eur. J 2017, 23, 7157. [DOI] [PubMed] [Google Scholar]; (i) Meng G; Szostak M Eur. J. Org. Chem 2018, 2352. [Google Scholar]; (j) Takise R; Muto K; Yamaguchi J Chem. Soc. Rev 2017, 46, 5864. [DOI] [PubMed] [Google Scholar]; (k) Kaiser D; Bauer A; Lemmerer M; Maulide N Chem. Soc. Rev 2018, 47, 7899. [DOI] [PubMed] [Google Scholar]; (l) Dander JE; Garg NK ACS Catal. 2017, 7, 1413. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Adachi S; Kumagai N; Shibasaki M Tetrahedron Lett. 2018, 59, 1147. [Google Scholar]; (n) Guo L; Rueping M Chem. Eur. J 2018, 24, 7794. [DOI] [PubMed] [Google Scholar]; (o) Bourne-Branchu Y; Gosmini C; Danoun G Chem. Eur. J 2019, 25, 2663. [DOI] [PubMed] [Google Scholar]; (p) Chaudhari MB; Gnanaprakasam B Chem. Asian J 2019, 14, 76. [DOI] [PubMed] [Google Scholar]; (q) Li G; Ma S; Szostak M Trends Chem. 2020, 2, 914. [Google Scholar]; (r) Gao P; Rahman MM; Zamalloa A; Feliciano J; Szostak MJ Org. Chem 2023, 88, 10.1021/acs.joc.2c01094. [DOI] [PubMed] [Google Scholar]; (s) Feng M; Zhang H; Maulide N Angew. Chem. Int. Ed 2022, 61, e202212213. [DOI] [PMC free article] [PubMed] [Google Scholar]; (t) Liu C; Meng G; Liu Y; Liu R; Lalancette R; Szostak R; Szostak M Org. Lett 2016, 18, 4194. [DOI] [PubMed] [Google Scholar]; (u) Meng G; Szostak R; Szostak M Org. Lett 2017, 19, 3596. [DOI] [PubMed] [Google Scholar]; (v) Osumi Y; Liu C; Szostak M Org. Biomol. Chem 2017, 15, 8867. [DOI] [PubMed] [Google Scholar]; (w) Rahman MM; Buchspies J; Szostak M Catalysts 2019, 9, 129. [Google Scholar]; (x) Jian J; He Z; Zhang Y; Liu T; Liu L; Wang Z; Wang H; Wang S; Zeng Z Eur. J. Org. Chem 2020, 4176. [Google Scholar]; (y) Buchspies J; Rahman MM; Szostak R; Szostak M Org. Lett 2020, 22, 4703. [DOI] [PubMed] [Google Scholar]; (z) Meng G; Szostak M Org. Biomol. Chem 2016, 14, 5690. [DOI] [PubMed] [Google Scholar]; (aa) Wu H; Li Y; Cui M; Jian J; Zeng Z Adv. Synth. Catal 2016, 358, 3876. [Google Scholar]; (ab) Cui M; Chen Z; Liu T; Wang H; Zeng Z Tetrahedron Lett. 2017, 58, 3819. [Google Scholar]; (ac) Wang T; Guo J; Wang H; Guo H; Jia D; Zhang W; Liu LJ Organomet. Chem 2018, 877, 80. [Google Scholar]; (ad) Liu C; Lalancette R; Szostak R; Szostak M Org. Lett 2019, 21, 7976. [DOI] [PubMed] [Google Scholar]; (ae) Rahman MM; Liu C; Bisz E; Dziuk B; Lalancette R; Wang Q; Chen H; Szostak R; Szostak MJ Org. Chem 2020, 85, 5475. [DOI] [PubMed] [Google Scholar]; (af) Ma S; Zhou T; Li G; Szostak M Adv. Synth. Catal 2020, 362, 1887. [Google Scholar]; (ag) Luo Z; Liu T; Guo W; Wang Z; Huang J; Zhu Y; Zeng Z Org. Process Res. Dev 2018, 22, 1188. [Google Scholar]; (ah) Wang CA; Liu C; Szostak M Org. Process Res. Dev 2020, 24, 1043. [Google Scholar]; (ai) Rahman MM; Pyle DJ; Bisz E; Dziuk B; Ejsmont K; Lalancette R; Wang Q; Chen H; Szostak R; Szostak MJ Org. Chem 2021, 86, 10455. [DOI] [PubMed] [Google Scholar]
- (2).(a) Weires NA; Baker EL; Garg NK Nat. Chem 2016, 8, 75. [DOI] [PubMed] [Google Scholar]; (b) Meng G; Lalancette R; Szostak R; Szostak M Org. Lett 2017, 19, 4656. [DOI] [PubMed] [Google Scholar]; (c) Liu C; Li G; Shi S; Meng G; Lalancette R; Szostak R; Szostak M ACS Catal. 2018, 8, 9131. [Google Scholar]; (d) Liu C; Liu Y; Liu R; Lalancette R; Szostak R; Szostak M Org. Lett 2017, 19, 1434. [DOI] [PubMed] [Google Scholar]; (e) Lim M; Kim H; Ban J; Son J; Lee JK; Min SJ; Lee SU; Rhee H Eur. J. Org. Chem 2018, 5717. [Google Scholar]; (f) Shi S; Lalancette R; Szostak R; Szostak M Org. Lett 2019, 21, 1253. [DOI] [PubMed] [Google Scholar]; (g) Meng G; Shi S; Szostak M ACS Catal. 2016, 6, 7335. [Google Scholar]; (h) Zhang Y; Wang Z; Tang Z; Luo Z; Wu H; Liu T; Zhu Y; Zeng Z Eur. J. Org. Chem 2020, 1620. [Google Scholar]; (i) Li X; Zou GJ Organomet. Chem 2015, 794, 136. [Google Scholar]; (j) Szostak R; Shi S; Meng G; Lalancette R; Szostak MJ Org. Chem 2016, 81, 8091. [DOI] [PubMed] [Google Scholar]; (k) Pace V; Holzer W; Meng G; Shi S; Lalancette R; Szostak R; Szostak M Chem. Eur. J 2016, 22, 14494. [DOI] [PubMed] [Google Scholar]; (l) Pace V; Holzer W; Ielo L; Shi S; Meng G; Hanna M; Szostak R; Szostak M Chem. Commun 2019, 55, 4423. [DOI] [PubMed] [Google Scholar]; (m) Szostak R; Meng G; Szostak MJ Org. Chem 2017, 82, 6373. [DOI] [PubMed] [Google Scholar]; (n) Meng G; Shi S; Lalancette R; Szostak R; Szostak MJ Am. Chem. Soc 2018, 140, 727. [DOI] [PubMed] [Google Scholar]; (o) Szostak R; Szostak M Org. Lett 2018, 20, 1342. [DOI] [PubMed] [Google Scholar]; (p) Szostak R; Liu C; Lalancette R; Szostak MJ Org. Chem 2018, 83, 14676. [DOI] [PubMed] [Google Scholar]; (q) Ielo L; Pace V; Holzer W; Rahman MM; Meng G; Szostak R; Szostak M Chem. Eur. J 2020, 26, 16246. [DOI] [PubMed] [Google Scholar]; (r) Liu C; Shi S; Liu Y; Liu R; Lalancette R; Szostak R; Szostak M Org. Lett 2018, 20, 7771. [DOI] [PubMed] [Google Scholar]; (s) Boit TB; Weires NA; Kim J; Garg NK ACS Catal. 2018, 8, 1003. [DOI] [PMC free article] [PubMed] [Google Scholar]; (t) Mai WP; Liu Y; Sui HD; Xiao YM; Mao P; Lu K Eur. J. Org. Chem 2019, 7814 [Google Scholar]; (u) Mehta MM; Boit TB; Dander JE; Garg NK Org. Lett 2020, 22, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]; (v) Shi W; Sun G; Zou G Tetrahedron Lett. 2020, 61, 152140. [Google Scholar]; (w) Wang CA; Rahman MM; Bisz E; Dziuk B; Szostak R; Szostak M ACS Catal. 2022, 12, 2426. [Google Scholar]; (x) Zhang J; Zhang P; Shao L; Wang R; Ma Y; Szostak M Angew. Chem. Int. Ed 2022, 61, e202114146. [DOI] [PubMed] [Google Scholar]; (y) Li CX; Ning Q; Zhao W; Cao HJ; Wang YP; Yan H; Lu CS; Liang Y Chem. Eur. J 2021, 27, 2699. [DOI] [PubMed] [Google Scholar]
- (3).(a) Lei P; Meng G; Szostak M ACS Catal. 2017, 7, 1960. [Google Scholar]; (b) Lei P; Meng G; Ling Y; An J; Szostak MJ Org. Chem 2017, 82, 6638. [DOI] [PubMed] [Google Scholar]; (c) Lei P; Meng G; Shi S; Ling Y; An J; Szostak R; Szostak M Chem. Sci 2017, 8, 6525. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Shi S; Nolan SP; Szostak M Acc. Chem. Res 2018, 51, 2589. [DOI] [PubMed] [Google Scholar]; (e) Lei P; Meng G; Ling Y; An J; Nolan SP; Szostak M Org. Lett 2017, 19, 6510. [DOI] [PubMed] [Google Scholar]; (f) Lei P; Ling Y; An J; Nolan SP; Szostak M Adv. Synth. Catal 2019, 361, 5654. [Google Scholar]; (g) Li G; Lei P; Szostak M; Casals-Cruanas E; Poater A; Cavallo L; Nolan SP ChemCatChem 2018, 10, 3096. [Google Scholar]; (h) Shi S; Szostak M Chem. Commun 2017, 53, 10584. [DOI] [PubMed] [Google Scholar]; (i) Zhou T; Li G; Nolan SP; Szostak M Org. Lett 2019, 21, 3304. [DOI] [PubMed] [Google Scholar]; (j) Li G; Zhou T; Poater A; Cavallo L; Nolan SP; Szostak M Catal. Sci. Technol 2020, 10, 710. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Buchspies J; Rahman MM; Szostak M Catalysts 2020, 10, 372. [Google Scholar]; (l) Zhou T; Ma S; Nahra F; Obled AMC; Poater A; Cavallo L; Cazin CSJ; Nolan SP; Szostak M iScience 2020, 23, 101377. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Lei P; Li G; Ling Y; An J; Nolan SP; Szostak M Synthesis 2021, 53, 682. [Google Scholar]; (n) Lei P; Mu Y; Wang Y; Wang Y; Ma Z; Feng J; Liu X; Szostak M ACS Sustainable Chem. Eng 2021, 9, 552. [Google Scholar]; (o) Lei P; Wang Y; Mu Y; Wang Y; Ma Z; Feng J; Liu X; Szostak M ACS Sustainable Chem. Eng 2021, 9, 14937. [Google Scholar]; (p) Buchspies J; Rahman MM; Szostak M Molecules 2021, 26, 188. [DOI] [PMC free article] [PubMed] [Google Scholar]; (q) Shi S; Szostak M Chem. Eur. J 2016, 22, 10420. [DOI] [PubMed] [Google Scholar]; (r) Shi S; Szostak M Synthesis 2017, 49, 3602. [Google Scholar]; (s) Shi S; Szostak M Org. Lett 2016, 18, 5872. [DOI] [PubMed] [Google Scholar]; (t) Simmons BJ; Weires NA; Dander JE; Garg NK ACS Catal. 2016, 6, 3176. [DOI] [PMC free article] [PubMed] [Google Scholar]; (u) Dorval C; Stetsiuk O; Gaillard S; Dubois E; Gosmini C; Danoun G Org. Lett 2022, 24, 2778. [DOI] [PubMed] [Google Scholar]; (v) Dorval C; Dubois E; Bourne-Branchu Y; Gosmini C; Danoun G Adv. Synth. Catal 2019, 361, 1777. [Google Scholar]; (w) Bao CC; Du HZ; Luo YL; Guan BT Commun. Chem 2021, 4, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]; (x) Kim J; Park MS; Lee S; Song KH Tetrahedron Lett. 2022, 111, 154201. [Google Scholar]; (y) Liu C; Achtenhagen M; Szostak M Org. Lett 2016, 18, 2375. [DOI] [PubMed] [Google Scholar]; (z) Chen C; Liu P; Luo M; Zeng X ACS Catal. 2018, 8, 5864. [Google Scholar]; (aa) Sureshbabu P; Azeez S; Muniyappan N; Sabiah S; Kandasamy JJ Org. Chem 2019, 84, 11823. [DOI] [PubMed] [Google Scholar]; (ab) Li G; Szostak M Chem. Eur. J 2020, 26, 611. [DOI] [PubMed] [Google Scholar]; (ac) Li G; Szostak M Org. Biomol. Chem 2020, 18, 3827. [DOI] [PubMed] [Google Scholar]
- (4).(a) Ni S; Zhang W; Mei H; Han J; Pan Y Org. Lett 2017, 19, 2536. [DOI] [PubMed] [Google Scholar]; (b) Zhuo J; Zhang Y; Li Z; Li C ACS Catal. 2020, 10, 3895. [Google Scholar]; (c) Yu CG; Matsuo Y Org. Lett 2020, 22, 950. [DOI] [PubMed] [Google Scholar]; (d) Idris MA; Lee S Org. Lett 2020, 22, 9190. [DOI] [PubMed] [Google Scholar]; (e) He Z; Yan C; Zhang M; Irfan M; Wang Z; Zeng Z Synthesis 2022, 54, 705. [Google Scholar]; (f) Liu X; Hsiao CC; Guo L; Rueping M Org. Lett 2018, 20, 2976. [DOI] [PubMed] [Google Scholar]; (g) Meng G; Szostak M Org. Lett 2018, 20, 6789. [DOI] [PubMed] [Google Scholar]; (h) Chen J; Xu M; Yu S; Xia Y; Lee S Org. Lett 2020, 22, 2287. [DOI] [PubMed] [Google Scholar]; (i) Liu Y; Meng G; Liu R; Szostak M Chem. Commun 2016, 52, 6841. [DOI] [PubMed] [Google Scholar]; (j) Liu Y; Liu R; Szostak M Org. Biomol. Chem 2017, 15, 1780. [DOI] [PubMed] [Google Scholar]; (k) Li J; Yao J; Chen L; Zou D; Walsh PJ; Liang G Org. Chem. Front 2021, 8, 6344. [Google Scholar]; (l) Amani J; Alam R; Badir S; Molander GA Org. Lett 2017, 19, 2426. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Shi W; Zou G Molecules 2018, 23, 2412. [DOI] [PMC free article] [PubMed] [Google Scholar]; (n) Mahesh S; Tang KC; Raj M Molecules 2018, 23, 2615. [DOI] [PMC free article] [PubMed] [Google Scholar]; (o) Wang C; Huang L; Wang F; Zou G Tetrahedron Lett. 2018, 59, 2299. [Google Scholar]; (p) Kadam AA; Metz TL; Qian Y; Stanley LM ACS Catal. 2019, 9, 5651. [Google Scholar]; (q) Koeritz MT; Burgett RW; Kadam AA; Stanley LM Org. Lett 2020, 22, 5731. [DOI] [PubMed] [Google Scholar]; (r) Reina A; Krachko T; Onida K; Bouyssi D; Jeanneau E; Monteiro N; Amgoune A ACS Catal. 2020, 10, 2189. [Google Scholar]; (s) Kerackian T; Reina A; Bouyssi D; Monteiro N; Amgoune A Org. Lett 2020, 22, 2240. [DOI] [PubMed] [Google Scholar]; (t) Kerackian T; Bouyssi D; Pilet G; Medebielle M; Monteiro N; Vantourout JC; Amgoune A ACS Catal. 2022, 12, 12315. [Google Scholar]
- (5).(a) Cui M; Wu H; Jian J; Wang H; Liu C; Daniel S; Zeng Z Chem. Commun 2016, 52, 12076. [DOI] [PubMed] [Google Scholar]; (b) Karthik S; Gandhi T Org. Lett 2017, 19, 5486. [DOI] [PubMed] [Google Scholar]; (c) Li W; Zhang S; Feng X; Yu X; Yamamoto Y; Bao M Org. Lett 2021, 23, 2521. [DOI] [PubMed] [Google Scholar]; (d) Simmons BJ; Hoffmann M; Hwang J; Jackl MK; Garg NK Org. Lett 2017, 19, 1910. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Simmons BJ; Ramirez M; Garg NK Org. Synth 2019, 96, 436. [Google Scholar]; (f) Boit TB; Mehta MM; Kim J; Baker EL; Garg NK Angew. Chem. Int. Ed 2021, 60, 2472. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Dander JE; Giroud M; Racine S; Darzi ER; Alvizo O; Entwistle D; Garg NK Commun. Chem 2019, 2, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Knapp RR; Bulger AS; Garg NK Org. Lett 2020, 22, 2833. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Bulger AS; Witkowski DC; Garg NK Org. Synth 2022, 99, 305. [Google Scholar]; (j) Rahman MM; Szostak M Org. Lett 2021, 23, 4818. [DOI] [PubMed] [Google Scholar]; (k) Idris MA; Song KH; Lee S Adv. Synth. Catal 2022, 364, 2449. [Google Scholar]; (l) Medina JM; Moreno J; Racine S; Du S; Garg NK Angew. Chem. Int. Ed 2017, 56, 6567. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Walker JA; Vickerman KL; Humke JN; Stanley LM J. Am. Chem. Soc 2017, 139, 10228. [DOI] [PubMed] [Google Scholar]; (n) Luo Z; Wu H; Li Y; Chen Y; Nie J; Lu S; Zhu Y; Zeng Z Adv. Synth. Catal 2019, 361, 4117. [Google Scholar]; (o) Azeez S; Sureshbabu P; Sabiah S; Kandasamy J Org. Biomol. Chem 2022, 20, 2048. [DOI] [PubMed] [Google Scholar]; (p) Liu LL; Chen P; Sun Y; Wu Y; Chen S; Zhu J; Zhao YJ Org. Chem 2016, 81, 11686. [DOI] [PubMed] [Google Scholar]; (q) Chu CQ; Dang LJ Org. Chem 2018, 83, 5009. [DOI] [PubMed] [Google Scholar]; (r) Wang H; Zhang SQ; Hong X Chem. Commun 2019, 55, 11330. [DOI] [PubMed] [Google Scholar]
- (6).(a) Baker EL; Yamano MM; Zhou Y; Anthony SM; Garg NK Nat. Commun 2016, 7, 11554. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Meng G; Lei P; Szostak M Org. Lett 2017, 19, 2158. [DOI] [PubMed] [Google Scholar]; (c) Idris MA; Lee S Org. Chem. Front 2020, 7, 2737. [Google Scholar]; (d) Liu Y; Shi S; Achtenhagen M; Liu R; Szostak M Org. Lett 2017, 19, 1614. [DOI] [PubMed] [Google Scholar]; (e) Liu Y; Achtenhagen M; Liu R; Szostak M Org. Biomol. Chem 2018, 16, 1322. [DOI] [PubMed] [Google Scholar]; (f) Rahman MM; Li G; Szostak MJ Org. Chem 2019, 84, 12091. [DOI] [PubMed] [Google Scholar]; (g) Li G; Szostak M Nat. Commun 2018, 9, 4165. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Li G; Ji CL; Hong X; Szostak MJ Am. Chem. Soc 2019, 141, 11161. [DOI] [PubMed] [Google Scholar]; (i) Zuo D; Wang Q; Liu L; Huang T; Szostak M; Chen T Angew. Chem. Int. Ed 2022, 61, e202202794. [DOI] [PubMed] [Google Scholar]; (j) Li G; Xing Y; Zhao H; Zhang J; Hong X; Szostak M Angew. Chem. Int. Ed 2022, 61, e202200144. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Zhang J; Zhao H; Li G; Zhu X; Shang L; He Y; Liu X; Ma Y; Szostak M Org. Biomol. Chem 2022, 20, 5981. [DOI] [PubMed] [Google Scholar]; (l) Li G; Szostak M Chem. Rec 2020, 20, 649. [DOI] [PubMed] [Google Scholar]; (m) Li G; Szostak M Synthesis 2020, 52, 2579. [Google Scholar]; (n) Guo W; Huang J; Wu H; Liu T; Luo Z; Jian J; Zeng Z Org. Chem. Front 2018, 5, 2950. [Google Scholar]; (o) Zhang Y; Ye X; Liu S; Chen W; Majeed I; Liu T; Zhu Y; Zeng Z Org. Biomol. Chem 2021, 19, 8566. [DOI] [PubMed] [Google Scholar]; (p) Dander JE; Baker EL; Garg NK Chem. Sci 2017, 8, 6433. [DOI] [PMC free article] [PubMed] [Google Scholar]; (q) Singh S; Popuri S; Junaid QM; Sabiah S; Kandasamy J Org. Biomol. Chem 2021, 19, 7134. [DOI] [PubMed] [Google Scholar]; (r) Sureshbabu P; Azeez S; Chaudhary P; Kandasamy J Org. Biomol. Chem 2019, 17, 845. [DOI] [PubMed] [Google Scholar]; (s) Sureshbabu P; Azeez S; Pattanaik K; Sabiah S; Kandasamy J Asian J. Org. Chem 2022, 11, e202200076. [Google Scholar]; (t) Subramani M; Rajendran SK Eur. J. Org. Chem 2019, 3677. [Google Scholar]; (u) Yang D; Shin T; Kim H; Lee S Org. Biomol. Chem 2020, 18, 6053. [DOI] [PubMed] [Google Scholar]; (v) Joseph D; Park MS; Lee S Org. Biomol. Chem 2021, 19, 6227. [DOI] [PubMed] [Google Scholar]
- (7).(a) Hie L; Baker EL; Anthony SM; Desrosiers JN; Senanayake C; Garg NK Angew. Chem. Int. Ed 2016, 55, 15129. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Bourne-Branchu Y; Gosmini C; Danoun G Chem. Eur. J 2017, 23, 10043. [DOI] [PubMed] [Google Scholar]; (c) Li G; Lei P; Szostak M Org. Lett 2018, 20, 5622. [DOI] [PubMed] [Google Scholar]; (d) Wu H; Guo W; Daniel S; Li Y; Liu C; Zeng Z Chem. Eur. J 2018, 24, 3444. [DOI] [PubMed] [Google Scholar]; (e) Wybon CCD; Mensch C; Hollanders C; Gadais C; Herrebout WA; Ballet S; Maes BU W. ACS Catal 2018, 8, 203. [Google Scholar]; (f) Ye D; Liu Z; Chen H; Sessler JL; Lei C Org. Lett 2019, 21, 6888. [DOI] [PubMed] [Google Scholar]; (g) Wang Q; Liu L; Dong J; Tian Z; Chen T New J. Chem 2019, 43, 9384. [Google Scholar]; (h) Rahman MM; Li G; Szostak M Synthesis 2020, 52, 1060. [Google Scholar]; (i) Li Y; Wu H; Zeng Z Eur. J. Org. Chem 2019, 4357. [Google Scholar]; (j) Liu Y; Mo X; Majeed I; Zhang M; Wang H; Zeng Z Org. Biomol. Chem 2022, 20, 1532. [DOI] [PubMed] [Google Scholar]; (k) Weires NA; Caspi DD; Garg NK ACS Catal. 2017, 7, 4381. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Dander JE; Morrill LA; Nguyen MM; Chen S; Garg NK J. Chem. Educ 2019, 96, 776. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Nagae H; Hirai T; Kato D; Soma S; Akebi SY; Mashima K Chem. Sci 2019, 10, 2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).(a) Meng G; Szostak M Angew. Chem. Int. Ed 2015, 54, 14518. [DOI] [PubMed] [Google Scholar]; (b) Liu C; Meng G; Szostak MJ Org. Chem 2016, 81, 12023. [DOI] [PubMed] [Google Scholar]; (c) Liu C; Szostak M Org. Biomol. Chem 2018, 16, 7998. [DOI] [PubMed] [Google Scholar]; (d) Ji CL; Hong XJ Am. Chem. Soc 2017, 139, 15522. [DOI] [PubMed] [Google Scholar]; (e) Tong WY; Ly TD; Zhao TT; Wu YB; Wang X Chem. Commun 2020, 56, 113. [DOI] [PubMed] [Google Scholar]; (f) Li R; Xu H; Zhao N; Jin X; Dang YJ Org. Chem 2020, 85, 833. [DOI] [PubMed] [Google Scholar]; (g) Shi S; Meng G; Szostak M Angew. Chem. Int. Ed 2016, 55, 6959. [DOI] [PubMed] [Google Scholar]; (h) Zhou T; Ji CL; Hong X; Szostak M Chem. Sci 2019, 10, 9865. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Luo Z; Xiong L; Liu T; Zhang Y; Lu S; Chen Y; Guo W; Zhu Y; Zeng ZJ Org. Chem 2019, 84, 10559. [DOI] [PubMed] [Google Scholar]; (j) Srimontree W; Chatupheeraphat A; Liao HH; Rueping M Org. Lett 2017, 19, 3091. [DOI] [PubMed] [Google Scholar]; (k) Liu L; Zhou D; Liu M; Zhou Y; Chen T Org. Lett 2018, 20, 2741. [DOI] [PubMed] [Google Scholar]; (l) Chatupheeraphat A; Liao HH; Srimontree W; Guo L; Minenkov Y; Poater A; Cavallo L; Rueping MJ Am. Chem. Soc 2018, 140, 3724. [DOI] [PubMed] [Google Scholar]
- (9).(a) Meng G; Szostak M Org. Lett 2016, 18, 796. [DOI] [PubMed] [Google Scholar]; (b) Meng G; Szostak M ACS Catal. 2017, 7, 7251. [Google Scholar]; (c) Wu H; Liu T; Cui M; Li Y; Jian J; Wang H; Zeng Z Org. Biomol. Chem 2017, 15, 536. [DOI] [PubMed] [Google Scholar]; (d) Xiong L; Deng R; Liu T; Luo Z; Wang Z; Zhu XF; Wang H; Zeng Z Adv. Synth. Catal 2019, 361, 5383. [Google Scholar]; (e) Dey A; Sasmal S; Seth K; Lahiri GK; Maiti D ACS Catal. 2017, 7, 433. [Google Scholar]; (f) Yue H; Guo L; Lee SC; Liu X; Rueping M Angew. Chem. Int. Ed 2017, 56, 3972. [DOI] [PubMed] [Google Scholar]; (g) Mondal M; Bharali P New J. Chem 2017, 41, 13211. [Google Scholar]; (h) Hu J; Wang M; Pu X; Shi Z Nat. Commun 2017, 8, 14993. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Shi S; Szostak M Org. Lett 2017, 19, 3095. [DOI] [PubMed] [Google Scholar]; (j) Chatupheeraphat A; Liao HH; Lee SC; Rueping M Org. Lett 2017, 19, 4255. [DOI] [PubMed] [Google Scholar]; (k) Long Y; Zheng Y; Xia Y; Qu L; Yang Y; Xiang H; Zhou X ACS Catal. 2022, 12, 4688. [Google Scholar]; (l) Zhou PX; Shi S; Wang J; Zhang Y; Li C; Ge C Org. Chem. Front 2019, 6, 1942. [Google Scholar]
- (10).(a) Hu J; Zhao Y; Liu J; Zhang Y; Shi Z Angew. Chem. Int. Ed 2016, 55, 8718. [DOI] [PubMed] [Google Scholar]; (b) Shi S; Szostak M ACS Omega 2019, 4, 4901. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Bie F; Liu X; Shi Y; Cao H; Han Y; Szostak M; Liu CJ Org. Chem 2020, 85, 15676. [DOI] [PubMed] [Google Scholar]; (d) Liu C; Szostak M Angew. Chem. Int. Ed 2017, 56, 12718. [DOI] [PubMed] [Google Scholar]; (e) Liu X; Yue H; Jia J; Guo L; Rueping M Chem. Eur. J 2017, 23, 11771. [DOI] [PubMed] [Google Scholar]; (f) Lee SC; Guo L; Yue H; Liao HH; Rueping M Synlett 2017, 28, 2594. [Google Scholar]; (g) Lee SC; Liao HH; Chatupheeraphat A; Rueping M Chem. Eur. J 2018, 24, 3608. [DOI] [PubMed] [Google Scholar]; (h) Bie F; Liu X; Cao H; Shi Y; Zhou T; Szostak M; Liu C Org. Lett 2021, 23, 8098. [DOI] [PubMed] [Google Scholar]; (i) Yue H; Guo L; Liao HH; Cai Y; Zhu C; Rueping M Angew. Chem. Int. Ed 2017, 56, 4282. [DOI] [PubMed] [Google Scholar]
- (11).(a) For additional information, see:Long Y; Su Z; Zheng Y; He S; Zhong J; Xiang H; Zhou X ACS Catal. 2020, 10, 5, 3398. [Google Scholar]; (b) Ito Y; Nakatani S; Shiraki R; Kodama T; Tobisu MJ Am. Chem. Soc 2022, 144, 662. [DOI] [PubMed] [Google Scholar]; (c) Zhang ZB; Ji CL; Yang C; Chen J; Hong X; Xia JB Org. Lett 2019, 21, 1226. [DOI] [PubMed] [Google Scholar]; (d) Gao P; Szostak M Org. Lett 2020, 22, 6010. [DOI] [PubMed] [Google Scholar]; (e) Zhang ZB; Yang Y; Yu ZX; Xia JB ACS Catal. 2020, 10, 5419. [Google Scholar]; (f) Min KH; Iqbal N; Cho EJ Org. Lett 2022, 24, 989. [DOI] [PubMed] [Google Scholar]; (g) Hollanders C; Renders E; Gadais C; Masullo D; Van Raemdonck L; Wybon CCD; Martin C; Herrebout WA; Maes BUW; Ballet S ACS Catal. 2020, 10, 4280. [Google Scholar]; (h) Lee GS; Won J; Choi S; Baik M-H; Hong SH Angew. Chem., Int. Ed 2020, 59, 16933. [DOI] [PubMed] [Google Scholar]; (i) Sun W; Wang L; Hu Y; Wu X; Xia C; Liu C Nat. Commun 2020, 11, 3113. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Chen J; Joseph D; Xia Y; Lee SJ Org. Chem 2021, 86, 5943. [DOI] [PubMed] [Google Scholar]; (k) Joseph D; Lee S Org. Lett 2022, 24, 6186. [DOI] [PubMed] [Google Scholar]; (l) Xu Y; Wang B; Jiang J; Yu H; Fu YJ Org. Chem 2019, 84, 9474. [DOI] [PubMed] [Google Scholar]; (m) Xie PP; Qin ZX; Zhang SQ; Hong X ChemCatChem 2021, 13, 3536. [Google Scholar]; (n) Tomasini M; Zhang J; Zhao H; Besalu E; Falivene L; Caporaso L; Szostak M; Poater A Chem. Commun 2022, 58, 9950. [DOI] [PubMed] [Google Scholar]


