Abstract
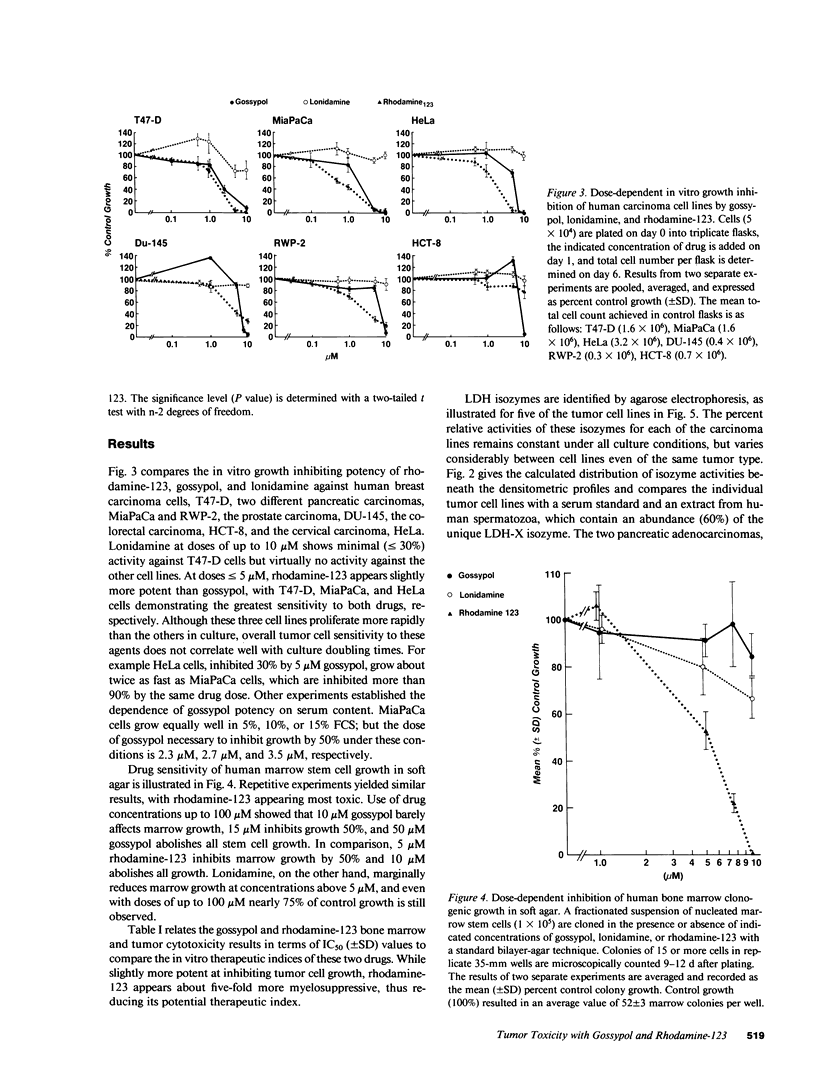
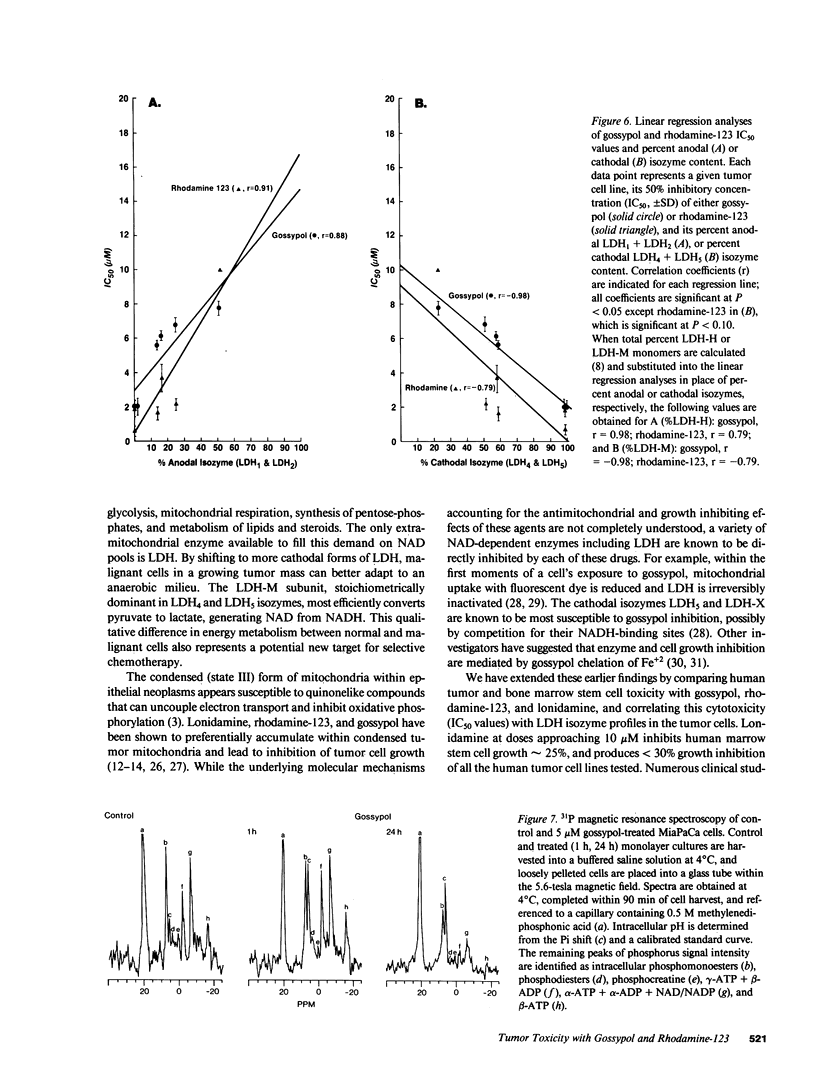
Three compounds that share specific antimitochondrial properties are gossypol, rhodamine-123, and lonidamine. We compare the antiproliferative activities of these drugs against six human cell lines derived from breast (T47-D), pancreas (MiaPaCa, RWP-2), prostate (DU-145), colon (HCT-8), and cervix (HeLa) carcinomas. Tumor cells enriched in cathodal LDH isozymes (LDH4 and LDH5) are significantly more sensitive to gossypol and rhodamine-123. When compared for ability to inhibit growth of human marrow in soft agar, 10 microM gossypol shows little effect on colony formation whereas 10 microM rhodamine-123 completely prevents stem cell growth, suggesting that gossypol may have the most favorable therapeutic index. Within 24 h of drug administration, there is a relative increase in intracellular inorganic phosphate pools and a marked decline in soluble high-energy phosphates in sensitive tumor cells, as measured by 31P magnetic resonance spectroscopy. These studies suggest that specific antimitochondrial agents might be selectively administered on the basis of tumor LDH isozyme content and noninvasively monitored for antiproliferative activity by 31P spectroscopy.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN J. M. Multiple forms of lactic dehydrogenase in tissues of the mouse: their specificity, cellular localization, and response to altered physiological conditions. Ann N Y Acad Sci. 1961 Nov 2;94:937–951. doi: 10.1111/j.1749-6632.1961.tb35586.x. [DOI] [PubMed] [Google Scholar]
- Abou-Donia M. B. Physiological effects and metabolism of gossypol. Residue Rev. 1976;61:125–160. doi: 10.1007/978-1-4613-9401-3_5. [DOI] [PubMed] [Google Scholar]
- Balinsky D., Greengard O., Cayanis E., Head J. F. Enzyme activities and isozyme patterns in human lung tumors. Cancer Res. 1984 Mar;44(3):1058–1062. [PubMed] [Google Scholar]
- Balinsky D., Platz C. E., Lewis J. W. Isozyme patterns of normal, benign, and malignant human breast tissues. Cancer Res. 1983 Dec;43(12 Pt 1):5895–5901. [PubMed] [Google Scholar]
- Benz C., Cadman E., Gwin J., Wu T., Amara J., Eisenfeld A., Dannies P. Tamoxifen and 5-fluorouracil in breast cancer: cytotoxic synergism in vitro. Cancer Res. 1983 Nov;43(11):5298–5303. [PubMed] [Google Scholar]
- Benz C., Cadman E. Modulation of 5-fluorouracil metabolism and cytotoxicity by antimetabolite pretreatment in human colorectal adenocarcinoma HCT-8. Cancer Res. 1981 Mar;41(3):994–999. [PubMed] [Google Scholar]
- Benz C., Hollander C., Miller B. Endocrine-responsive pancreatic carcinoma: steroid binding and cytotoxicity studies in human tumor cell lines. Cancer Res. 1986 May;46(5):2276–2281. [PubMed] [Google Scholar]
- Bernal S. D., Lampidis T. J., McIsaac R. M., Chen L. B. Anticarcinoma activity in vivo of rhodamine 123, a mitochondrial-specific dye. Science. 1983 Oct 14;222(4620):169–172. doi: 10.1126/science.6623064. [DOI] [PubMed] [Google Scholar]
- Burt C. T., Koutcher J. A. Multinuclear NMR studies of naturally occurring nuclei. J Nucl Med. 1984 Feb;25(2):237–248. [PubMed] [Google Scholar]
- Butrimovitz G. P., Farina F., Sharlip I. Micromethod for determination of lactate dehydrogenase isoenzyme C4 activity in human seminal plasma. Clin Chem. 1983 Aug;29(8):1518–1521. [PubMed] [Google Scholar]
- Cohen S. M., Ogawa S., Rottenberg H., Glynn P., Yamane T., Brown T. R., Shulman R. G. P nuclear magnetic resonance studies of isolated rat liver cells. Nature. 1978 Jun 15;273(5663):554–556. doi: 10.1038/273554a0. [DOI] [PubMed] [Google Scholar]
- De Martino C., Battelli T., Paggi M. G., Nista A., Marcante M. L., D'Atri S., Malorni W., Gallo M., Floridi A. Effects of Lonidamine on murine and human tumor cells in vitro. A morphological and biochemical study. Oncology. 1984;41 (Suppl 1):15–29. doi: 10.1159/000225881. [DOI] [PubMed] [Google Scholar]
- GOLDMAN R. D., KAPLAN N. O., HALL T. C. LACTIC DEHYDROGENASE IN HUMAN NEOPLASTIC TISSUES. Cancer Res. 1964 Apr;24:389–399. [PubMed] [Google Scholar]
- Lampidis T. J., Bernal S. D., Summerhayes I. C., Chen L. B. Selective toxicity of rhodamine 123 in carcinoma cells in vitro. Cancer Res. 1983 Feb;43(2):716–720. [PubMed] [Google Scholar]
- Lee C. Y., Moon Y. S., Yuan J. H., Chen A. F. Enzyme inactivation and inhibition by gossypol. Mol Cell Biochem. 1982 Sep 3;47(2):65–70. doi: 10.1007/BF00234406. [DOI] [PubMed] [Google Scholar]
- Nass M. M. Analysis of methylglyoxal bis(guanylhydrazone)-induced alterations of hamster tumor mitochondria by correlated studies of selective rhodamine binding, ultrastructural damage, DNA replication, and reversibility. Cancer Res. 1984 Jun;44(6):2677–2688. [PubMed] [Google Scholar]
- Pedersen P. L. Tumor mitochondria and the bioenergetics of cancer cells. Prog Exp Tumor Res. 1978;22:190–274. doi: 10.1159/000401202. [DOI] [PubMed] [Google Scholar]
- Roberts J. K., Wade-Jardetzky N., Jardetzky O. Intracellular pH measurements by 31P nuclear magnetic resonance. Influence of factors other than pH on 31P chemical shifts. Biochemistry. 1981 Sep 15;20(19):5389–5394. doi: 10.1021/bi00522a006. [DOI] [PubMed] [Google Scholar]
- Stefanini M. Enzymes, isozymes, and enzyme variants in the diagnosis of cancer. A short review. Cancer. 1985 May 1;55(9):1931–1936. doi: 10.1002/1097-0142(19850501)55:9<1931::aid-cncr2820550917>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Summerhayes I. C., Lampidis T. J., Bernal S. D., Nadakavukaren J. J., Nadakavukaren K. K., Shepherd E. L., Chen L. B. Unusual retention of rhodamine 123 by mitochondria in muscle and carcinoma cells. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5292–5296. doi: 10.1073/pnas.79.17.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso W. W., Lee C. S. Gossypol uncoupling of respiratory chain and oxidative phosphorylation in ejaculated boar spermatozoa. Contraception. 1982 Jun;25(6):649–655. doi: 10.1016/0010-7824(82)90066-x. [DOI] [PubMed] [Google Scholar]
- Tuszynski G. P., Cossu G. Differential cytotoxic effect of gossypol on human melanoma, colon carcinoma, and other tissue culture cell lines. Cancer Res. 1984 Feb;44(2):768–771. [PubMed] [Google Scholar]
- WARBURG O. On the origin of cancer cells. Science. 1956 Feb 24;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Wang Y., Rao P. N. Effect of gossypol on DNA synthesis and cell cycle progression of mammalian cells in vitro. Cancer Res. 1984 Jan;44(1):35–38. [PubMed] [Google Scholar]
- Wilkie D. Antimitochondrial drugs in cancer chemotherapy: preliminary communication. J R Soc Med. 1979 Aug;72(8):599–601. doi: 10.1177/014107687907200810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood D. C., Varela V., Palmquist M., Weber F. Serum lactic dehydrogenase and isoenzyme changes in clinical cancer. J Surg Oncol. 1973;5(3):251–257. doi: 10.1002/jso.2930050308. [DOI] [PubMed] [Google Scholar]







