Abstract
The reprogramming of lipid metabolism and its association with oncogenic signaling pathways within the tumor microenvironment (TME) have emerged as significant hallmarks of cancer. Lipid metabolism is defined as a complex set of molecular processes including lipid uptake, synthesis, transport, and degradation. The dysregulation of lipid metabolism is affected by enzymes and signaling molecules directly or indirectly involved in the lipid metabolic process. Regulation of lipid metabolizing enzymes has been shown to modulate cancer development and to avoid resistance to anticancer drugs in tumors and the TME. Because of this, understanding the metabolic reprogramming associated with oncogenic progression is important to develop strategies for cancer treatment. Recent advances provide insight into fundamental mechanisms and the connections between altered lipid metabolism and tumorigenesis. In this review, we explore alterations to lipid metabolism and the pivotal factors driving lipid metabolic reprogramming, which exacerbate cancer progression. We also shed light on the latest insights and current therapeutic approaches based on small molecular inhibitors and phytochemicals targeting lipid metabolism for cancer treatment. Further investigations are worthwhile to fully understand the underlying mechanisms and the correlation between altered lipid metabolism and carcinogenesis.
Keywords: Tumor microenvironment, Lipid uptake, Lipolysis, Lipogenesis, Cholesterol transport, Phytochemical
Introduction
The tumor microenvironment (TME) is a highly heterogeneous environment consisting of many distinct cell types and molecules released by tumor cells, cancer-associated fibroblasts (CAFs), and infiltrating immune cells. The representative cells that surround and support cancer cells in the TME include CAFs, tumor-endothelial cells (TECs), cancer-associated adipocytes (CAAs), tumor-associated neutrophils (TANs), and tumor-associated macrophages (TAMs) (Chen et al. 2015). In addition, non-cancer cells such as endothelial cells, adipocytes, immune/inflammatory cells, and myeloid-derived suppressor cells (MDSCs) can interact directly and/or indirectly with cancer cells in the TME. This collaborative interplay provides favorable conditions for tumor development, growth, and progression (Gonzalez et al. 2018; Petrova et al. 2018; Cao 2019; Cheng et al. 2019; Greten and Grivennikov 2019; Bui et al. 2021). Indeed, numerous studies have demonstrated that the communication between cancer cells and surrounding cells in the TME promotes cancer metastasis and confers chemoresistance through the establishment of metabolic reprogramming, which involves the alteration of tumor metabolism (DeBerardinis and Chandel 2016; Chen et al. 2022; Li et al. 2022; Liu et al. 2022a, b). Metabolic reprogramming and alteration of oncogenic signaling pathways related to metabolic processes have emerged as hallmarks of cancer (Zhu et al. 2022). Thus, it would be essential to understand the metabolic reprogramming related to oncogenic progression to develop strategies for cancer treatment. In this review, we focus on the critical factors in the TME driving lipid metabolic reprogramming to encourage the progression of cancer. In addition, we explore the latest insights and current therapeutic approaches based on small molecular inhibitors and phytochemicals targeting lipid metabolism for cancer treatment because of few reviews summarizing cancer lipid metabolism by phytochemicals.
Altered lipid metabolism in cancer cells of the TME
Tumor cells need to rewire their metabolic pathways to regulate nutrient intake and metabolism to maintain energy production. Lipids are not only essential energy sources but also membrane components necessary for tumor growth and metabolism. In addition, lipids mediate cell signaling pathways and control redox homeostasis. Numerous proteins and genes responsible for lipid metabolism are regulatory mediators of tumor survival and progression, as well as mutual communication between cancer cells and the TME (Ventura et al. 2015; Gupta et al. 2017). Lipid metabolism is defined as a complex set of molecular processes, including lipid uptake, synthesis, transport, and degradation (Harayama and Riezman 2018). The dysregulation of lipid metabolism is affected by enzymes and signaling molecules that are directly or indirectly involved in the lipid metabolic process. Such imbalance can alter membrane composition, gene expression, and downstream signaling pathway activity, which control cell proliferation, motility, inflammation, and survival (Zechner et al. 2012; Harayama and Riezman 2018). Thus, regulation of lipid-metabolizing enzymes has been shown to modulate cancer development and to avoid resistance to anticancer drugs in tumors and the TME (Ventura et al. 2015; Gupta et al. 2017). Exogenous lipids produced by the TME can also affect tumor malignancy and inflammation (Gupta et al. 2017). The following sections describe diverse signaling pathways responsible for lipid metabolic reprogramming-mediated cancer progression, focusing on the related regulatory proteins and genes.
Lipid uptake by CD36 and its alteration in cancer
Tumor cells require a variety of transporters to mediate the trafficking of lipids for oxidation or to activate oncogenic signaling pathways. Cancer cells tend to uptake fatty acids from the extracellular environment to meet their needs for growth (Zhang et al. 2022a, b). Cluster of differentiation 36 (CD36) as a scavenger receptor is a macrophage receptor for oxidized low-density lipoprotein (Endemann et al. 1993). CD36, also known as fatty acid translocase protein, functions in the binding or trans-membrane uptake of long-chain fatty acids (Abumrad et al. 1993). CD36 is a membrane glycoprotein that consists of two short intracellular domains, two transmembrane segments, and a large extracellular domain with a hydrophobic sequence where lipid ligands bind (Glatz et al. 2010; Pepino et al. 2014; Glatz and Luiken 2018). High expression of CD36 has been observed in diverse cancer types, including liver cancer (Zhao et al. 2018), breast cancer (Yang et al. 2020), colorectal cancer (Drury et al. 2020), and gastric cancer (Jiang et al. 2019). Gyamfi et al. recently reported that CD36 is a key player in the interaction between adipocytes and breast cancer cells, suggesting its potential as a therapeutic target in the TME. Human breast cancer cells co-cultured with adipocytes show upregulation of CD36 expression, with fatty acid import into the cytosol or mitochondria (Gyamfi et al. 2021). Increased CD36 expression activates the STAT3 signaling pathway mediating adipocyte-induced epithelial–mesenchymal transition (EMT) and stemness. In addition, increased CD36 expression occurs with increased fatty acid-binding protein 4 (FABP4) expression, and their direct interaction regulates the import, transport, and metabolism of fatty acid (Gyamfi et al. 2021). Inhibition of CD36 and FABP4 significantly reduces the proliferation, migration, invasiveness, and tumorsphere-forming capacity of breast cancer cells, which is associated with reduced tumorigenicity in a xenograft mouse model (Gyamfi et al. 2021).
The stability and function of CD36 are regulated by post-translational modifications, including palmitoylation, glycosylation, phosphorylation, and ubiquitination (Glatz et al. 2010; Pepino et al. 2014; Glatz and Luiken 2018). Intracellular lipid accumulation by dysregulated lipid metabolism is associated with non-alcoholic steatohepatitis (NASH), a subset of non-alcoholic fatty liver disease (NAFLD) (Chavez-Tapia et al. 2012). Expression of soluble CD36 is significantly increased in patients with advanced steatosis of NAFLD (Garcia-Monzon et al. 2014). Palmitoylation is a covalent attachment of palmitate to cysteine residues of the protein, and increased palmitoylation of CD36 in NASH facilitates fatty acid uptake and lipid accumulation (Zhao et al. 2018). Inhibition of CD36 palmitoylation interferes with the binding and uptake of fatty acids. It alleviates liver tissue inflammation by inactivating the JNK signaling pathway while interfering with CD36/Fyn/Lyn complex formation (Zhao et al. 2018). In addition, O-linked β-N-acetylglucosamine (O-GlcNAc) glycosylation called an O-GlcNAcylation is modulated by O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA), which are responsible for O-GlcNAc addition and removal (Zachara and Hart 2002). O-GlcNAcylation modulates protein functions by regulating subcellular localization, protein stability, transcriptional activity, and protein–protein interaction (Zachara and Hart 2002; Chatham et al. 2021). Dysregulation in O-GlcNAc cycling has been implicated in the progression of various chronic human diseases related to inflammation and tumor growth (Chatham et al. 2021; Ouyang et al. 2022). Interestingly, elevated fatty acid uptake activity promotes the metastatic potential of gastric cancer cells in a CD36-dependent manner via upregulation of O-GlcNAcylation (Jiang et al. 2019). Increased O-GlcNAcylation level accelerates the transcription of CD36 by activating the NF-κB pathway, and CD36 can be directly modified at Ser468 and Thr470 residues by O-GlcNAc (Jiang et al. 2019). Consequently, the induction of CD36 expression facilitates fatty acid uptake in gastric cancer cells, forming a vicious cycle between O-GlcNAcylation and CD36 transcription. This relationship may be correlated with poor survival in gastric cancer (Jiang et al. 2019).
Recently, the role of CD36 has been receiving attention not only in cancer cells but also in immune cells within TME. Intratumoral regulatory T (Treg) cells exhibit expression of metabolic genes associated with lipid metabolism compared to circulating Treg cells. Intratumoral Treg cells, in particular, show a substantial increase in the expression of CD36 (Wang et al. 2020a, b, c). CD36-mediated metabolic adaptation supports the survival and suppressive functions of intratumoral Treg cells, which is mediated by mitochondrial fitness and NAD production (Wang et al. 2020a, b, c). In contrast, it has been demonstrated that CD8+ tumor-infiltrating lymphocytes were shown to have reduced anti-tumor function, which is attributed to an increase in lipid peroxidation resulting from the uptake of fatty acids and oxidized lipids mediated by CD36 (Ma et al. 2021; Xu et al. 2021). In addition, tumor-associated macrophages (TAMs) residing in TME accumulate lipids through enhanced lipid uptake via CD36. This accumulation leads to the differentiation and functional alteration of pro-tumoral M2 type TAMs characterized by high levels of fatty acid oxidation (Su et al. 2020a, b; Yang et al. 2022). Therefore, further research and understanding are needed on the regulatory mechanism involving post-translational modifications of CD36 in controlling cell–cell communication within the TME since CD36 is a potential biomarker and therapeutic target for cancer.
Alteration of lipogenesis in cancer
Tumor cells obtain fatty acids from de novo lipogenesis and require activation of fatty acid synthesis to meet biosynthetic and bioenergetics requirements during carcinogenesis and tumorigenesis (Mounier et al. 2014). Lipogenesis encompasses the process of fatty acid triglyceride synthesis from acetyl-CoA synthesized by glucose or other substrates. Acetyl-CoA is carboxylated by acetyl-CoA carboxylase 1 (ACC1), converting to malonyl-CoA. ACC1 is located in the cytoplasm and acts as a rate-limiting enzyme in de novo fatty acid synthesis, producing malonyl-CoA as a substrate for forming palmitate catalyzed by fatty acid synthase (FASN) (Wakil and Abu-Elheiga 2009). ACC2 localizes in the mitochondria and regulates the activity of carnitine palmitoyltransferase 1 (CPT1), involved in the β-oxidation of fatty acids (Wakil and Abu-Elheiga 2009). Malonyl-CoA acts as an inhibitor of the CPT1, offering a pathway choice between fatty acid synthesis and β-oxidation. In addition, stearoyl-CoA desaturase 1 (SCD1) is a Δ9 desaturase, which catalyzes the insertion of a cis double bond at the Δ9 position of 12–19 carbon saturated fatty acids, converting them to monounsaturated fatty acids. SCD1 affects lipid composition, membrane fluidity, and functionality, which are essential for maintaining cellular integrity (Rodriguez-Cuenca et al. 2016). Sterol regulatory-element binding proteins (SREBPs) are membrane-bound transcription factors that induce the expression of genes related to lipogenesis and cholesterol biosynthesis (Schiliro and Firestein 2021). The expression of enzymes and genes involved in lipogenesis is associated with oncogenic signaling in tumors, facilitating immune system evasion, tumor growth, and chemoresistance (Flaveny et al. 2015).
Acetyl-CoA carboxylase 1 (ACC1)
ACC1 is abundantly expressed in various cancer cells, and its overexpression is associated with poor prognosis in cancer patients. ACC1 knockdown using siRNA inhibits the proliferation of highly lipogenic prostate cancer LNCaP, glioblastoma U87 EGFRvIII cell lines, colon cancer HCT-116, and liver cancer HepG2 cells (Brusselmans et al. 2005; Zhan et al. 2008; Jones et al. 2017; Ye et al. 2019a, b) but does not affect the proliferation of normal cells (Brusselmans et al. 2005). In addition, the expression level of ACC1 positively correlates with prolyl isomerase Pin1 expression in human prostate cancer specimens (Ueda et al. 2019). Pin1 recognizes the phosphorylated Ser/Thr-Pro motif and facilitates the cis–trans isomerization of proline (Stukenberg and Kirschner 2001). Direct binding of ACC1 to the WW domain of Pin1 improves the stability and activity of ACC1 protein by blocking lysosome-mediated degradation (Ueda et al. 2019). High expression of ACC1 protein in prostate cancer cells is caused by Pin1-mediated posttranscriptional level, contributing to increased FA contents that support cell proliferation (Ueda et al. 2019). Moreover, ACCs reconstruct lipogenesis-dependent cancer metabolism in head and neck squamous cell carcinoma (HNSCC), so HNSCC cells can survive inhibition of the Warburg effects by cetuximab treatment (Luo et al. 2017). HNSCC cells with acquired cetuximab resistance exhibit increased expression and activation of ACC1. Combination treatment with ACC allosteric inhibitor TOFA and cetuximab produces a more potent anti-tumor effect of cetuximab-resistant HNSCC xenograft (Luo et al. 2017).
Tumor-specific CD4+ T cells can support therapeutic effects by maintaining effector CD8+ T cells, which are important to reduce the exhaustion of CD8+ T cells mediated by PD-1 and TRAIL-mediated apoptosis during initial tumor elimination (Church et al. 2014). It has been reported that differentiation of Th17 cells depends on ACC1-mediated de novo fatty acid synthesis (Berod et al. 2014; Endo et al. 2015). The function of Th17 cells in TME remains unclear, but it can favor tumor growth by promoting angiogenesis or suppressing tumor immunity. Inhibition of fatty acid biosynthesis by T cell-specific deletion of ACC1 in mice attenuates Th17 cell-mediated inflammatory diseases (Berod et al. 2014). Furthermore, genetic deletion of ACC1 enhances the formation of memory CD4+ T cells, which exhibits the phenotype characterized by low fatty acid biosynthesis and high respiratory capacity (Endo et al. 2019).
Although ACC1 is recognized as an oncoprotein in various cancer cells including prostate cancer, glioblastoma, colon cancer, and liver cancer (Brusselmans et al. 2005; Zhan et al. 2008; Jones et al. 2017; Ueda et al. 2019; Ye et al. 2019a, b), there is evidence that ACC1 can act as a tumor suppressor by modulating the energy homeostasis or attenuating protein acetylation in tumor xenograft models (Jeon et al. 2012; Rios Garcia et al. 2017). Under metabolic energy stress conditions, AMPK can accelerate the survival of lung cancer by governing the NADPH level and maintaining the ATP level. Knockdown of ACC1 reduces the NADP+/NADPH ratio and ROS level, which promotes tumor formation in the lung cancer xenograft model using human A549 cells (Jeon et al. 2012). In addition, ACC1 inactivation by leptin or TGFβ treatment drives the invasion and metastasis of breast cancer cells, which is mediated by the elevation of acetyl-CoA production. Subsequently, protein acetylation, particularly transcription factor Smad2, leads to the upregulation of EMT markers such as vimentin and N-cadherin (Rios Garcia et al. 2017). In acute myeloid leukemia (AML), the Trib1-COP1 complex, an E3 ubiquitin ligase, is a substrate for ACC and induces proteasome-mediated degradation. Its degradation may initiate metabolic reprogramming to support the energy source for the progression of leukemia cells (Ito et al. 2021). Moreover, the stabilization of ACC1 suppresses the growth of human AML cells by inducing loss of self-renewal activity in leukemia-initiating cells, which is mediated by increased ROS levels and NADPH consumption (Ito et al. 2021). Based on previous reports, further detailed studies are needed to understand the physiology of ACC1, which can act as either a tumor suppressor or an oncogene depending on the complexity of the TME in terms of energy balance.
Fatty acid synthase (FASN)
High expression of FASN, a critical regulator of lipid metabolism, is associated with cancer progression in various types of cancer in the breast, prostate, ovary, and liver (Flavin et al. 2010; Fhu and Ali 2020). FASN acts as an oncogenic factor due to its role in regulating fatty acid synthesis or inducing aberrant lipogenesis in cancer cells (Che et al. 2020). FASN has been identified as a poor prognostic factor in patients with colon cancer, cervical cancer, cholangiocarcinoma, and clear cell renal cell carcinoma (Yuan et al. 2020; Tomacha et al. 2021; Drury et al. 2022; Du et al. 2022). FASN catalyzes the biosynthesis of the fatty acid palmitate, which accelerates RhoU palmitoylation, a critical step in regulating the turnover of focal adhesions (Baenke et al. 2013). Silencing FASN in prostate cancer leads to a reduction of RhoU palmitoylation and decreases the protein stability of Cdc42, which is connected to the suppression of migration and invasion (De Piano et al. 2020, 2021). In cholangiocarcinoma, the knockdown of FASN suppresses cell growth, migration, and invasion, which is associated with decreased cellular levels of palmitic amide, a fatty acid amide derived from palmitic acid (Tomacha et al. 2021). In addition, FASN promotes lymph node metastasis via cholesterol reprogramming and lymphangiogenesis in cervical cancer. FASN activates the c-Src-PI3K-Akt-FAK signaling pathway via cholesterol reprogramming, defined as lipid rafts and actin skeleton remodeling, leading to enhanced cell migration and invasion (Du et al. 2022). FASN also induces lymphangiogenesis by secreting PDGF-AA and IGFBP-3 (Du et al. 2022). Moreover, mutant K-Ras has been shown to stimulate lipogenesis by controlling lipogenic enzymes, including ACC1 and FASN (Kerk et al. 2021). Knockdown of K-Ras suppresses the levels of ACC1 and FASN expression, contributing to the suppression of spheroid formation through ROS production in K-Ras-activated pancreatic cancer cells (Terado et al. 2022).
Recently, Schroeder et al. reported that the network between fatty acid biogenesis catalyzed by FASN and the interactions within the Bcl-2 family controls the mitochondrial priming response to apoptosis (Schroeder et al. 2021). Inhibition of FASN promotes an increase in the NADPH/NADP+ ratio in breast cancer cells; the redox imbalance leads to the activation of stress-related proapoptotic kinases such as JNK and p38 MAPK as well as energy-sensing AMP-activated protein kinase (AMPK) (Schroeder et al. 2021). Interestingly, pharmacological inhibition of FASN activity results in an upregulation of mRNA and protein levels of the BH3-only Bcl-2 members such as Bim, NOXA, and PUMA (Schroeder et al. 2021). These findings suggest that FASN inhibitory cancer cells acquire increased mitochondrial apoptotic priming that can induce apoptotic hypersensitivity to Bcl-2-specific BH3-mimetics.
Post-translational modifications such as ubiquitination and sumoylation of FASN protein protect FASN from proteasomal degradation (Fhu and Ali 2020). Ubiquitin-specific proteases (USPs) are responsible for cellular functions through interactions with various target proteins, and their expressions are correlated with worse prognosis in several human cancers (Cruz et al. 2021). FASN can interact with USPs and prevent ubiquitin-mediated degradation among several target proteins. Androgen-stimulated USP2a upregulation in prostate cancer has been shown to stabilize FASN expression by blocking its polyubiquitination (Graner et al. 2004). Another study demonstrated that interaction between USP38 and FASN enhances the stability of FASN protein and increases triglyceride production in gastric cancer cells, which contributes to cell proliferation, migration, and tumorigenesis (Zheng et al. 2022).
Elevated FASN expression in ovarian cancer cells results in the accumulation of lipids and subsequent inhibition of tumor-infiltrating dendritic cells, which are required to sustain the capacity of anti-tumor T cells (Jiang et al. 2018). Hence, by targeting FASN, anti-tumor immunity can be enhanced by reducing lipid accumulation-induced dysfunction in dendritic cells (Jiang et al. 2018). In addition, intratumoral Treg cells display elevated SREBP activity, which results in FASN-dependent fatty acid synthesis (Lim et al. 2021). Deletion of FASN in Treg cells inhibits the growth of MC38 and B16 tumors (Lim et al. 2021). Interestingly, the heightened expression of PD-1 in Treg cells infiltrating tumors depends on SREBP activation, indicating a potential avenue for cancer immunotherapy targeting the lipid metabolism of Treg cells (Lim et al. 2021). Recent reports indicate that activated fatty acid metabolism by FASN is associated with reduced immune infiltration in male breast cancer, leading to the promotion of metastasis (Sun et al. 2023). These studies support FASN as a critical factor in abnormal lipid metabolism of cancer progression, and further detailed studies are needed to understand the function of FASN in reprogramming lipid metabolism.
ATP-citrate lyase (ACLY)
ACLY is a nuclear-cytosolic homotetrameric enzyme that catalyzes the production of cytosolic acetyl-CoA, generating a substrate for de novo biosynthesis of fatty acids and cholesterol (Chypre et al. 2012). Acetyl-CoA provides an acetyl donor for the acetylation of cytosolic and nuclear proteins, including transcription factors and histones, which control gene expression related to cancer metabolism (Wellen et al. 2009). ACLY is highly expressed or activated in several cancers, supporting tumor cell growth through lipogenesis (Bauer et al. 2005; Zaidi et al. 2012). ACLY depletion in various cancer cell lines exerts an anti-proliferative effect through the generation of mitochondrial ROS and AMPK activation accompanied by triglyceride accumulation and down-regulation of carnitine palmitoyltransferase 1A (Migita et al. 2013, 2014). In addition, glucose injection induces acetylation of lysine residues of ACLY in lung cancer A549 cells and mouse liver, blocking ubiquitination and increasing ACLY protein expression (Lin et al. 2013). Lung cancer tissues consistently exhibit enhanced ACLY acetylation that contributes to lipogenesis and promotes tumor cell proliferation (Lin et al. 2013).
As mentioned earlier, ACLY is a nucleocytosolic enzyme that produces acetyl-CoA from citrate and plays a critical role in determining the level of histone acetylation. ACLY phosphorylation activated by Akt promotes histone acetylation in both cancer and immune cells present in TME, inducing their proliferation (Lee et al. 2014; Covarrubias et al. 2016; Osinalde et al. 2016). Histone acetylation in human glioma and prostate tumors responds to acetyl-CoA production levels in the nucleus, which is accelerated upon increased phosphorylation of ACLY by Akt (Lee et al. 2014). In addition, M2 macrophage activation can be induced by IL-4-triggered acetyl-CoA production and histone acetylation as a consequence of Akt-mediated ACLY activation (Covarrubias et al. 2016). Recently, it has been increasingly emphasized that intracellular acetyl-CoA level in TME plays a vital role in regulating the fate of T cells (Vodnala et al. 2019). Phosphorylation of ACLY is induced upon IL-2-triggered Akt activation and generates acetyl-CoA that serves as a substrate for histone acetyltransferases. It stimulates the acetylation of histones in promoters of cell cycle-related genes, causing T-cell proliferation (Osinalde et al. 2016). IL-12-stimulated CD8+ T cells exhibit increased intracellular acetyl-CoA levels due to high expression of ACLY, resulting in the maintenance of IFNγ production and lipid biosynthesis for energy demand in nutrient-deprived TME (Chowdhury et al. 2022).
Acyl-CoA synthetase short-chain family member 2 (ACSS2)
ACSS2, one of the acyl-CoA synthetase short-chain family members, is a nucleocytosolic enzyme that catalyzes the conversion of acetate to acetyl-CoA, which is highly expressed in various tumors (Liu et al. 2022a, b). It has been proven through in vitro and in vivo experiments that ACSS2 plays an important role in tumor cell survival under hypoxia in lung cancer, breast cancer, melanoma, and colon cancer (Yoshii et al. 2009). ACSS2 is a critical enzyme that supplies a key source of acetyl-CoA for tumors using acetate as a carbon source to maintain lipid synthesis and histone acetylation (Comerford et al. 2014; Bulusu et al. 2017). SREBP transcriptionally upregulates ACSS, and this upregulation is highly expressed in tumor cells, as a response to the acidic TME (Kondo et al. 2017). In addition, under metabolic stress such as hypoxia or low serum, ACSS2 is required for the utilization and uptake of acetate and also supports the biosynthesis of membrane phospholipids for tumor cell growth (Kamphorst et al. 2014; Schug et al. 2015; Gao et al. 2016). Silencing ACSS2 caused significant inhibition of spheroid formation and markedly reduced xenograft tumor growth derived from breast and prostate cancer cells (Schug et al. 2015). In addition, ACSS2 induced histone acetylation at the FASN promoter region, which upregulated FASN expression to enhance lipid synthesis to promote the survival of hepatocellular carcinoma (Gao et al. 2016). ACSS2 is also overexpressed in malignant plasma cells derived from patients with myeloma. In particular, the expression of ACSS2 is significantly higher in obese myeloma patients (Li et al. 2021). Treatment of adipocyte-secreted angiotensin II enhanced the expression of ACSS2 in myeloma cells, which promotes tumorigenesis by maintaining the stability of interferon regulatory factor 4 (IRF4), an oncogenic protein (Li et al. 2021). ACSS2-mediated IRF4 acetylation results in the elevation of IRF4 protein level due to dysregulation of p62-mediated lysosomal degradation in myeloma (Li et al. 2021). Moreover, the acetylation of HIF-2α in the presence of ACSS2 significantly increased the expression level of HIF-2α target genes, potentially linking tumor cell growth and metastasis (Chen et al. 2017; Nagati et al. 2019).
Like ACLY, enforced expression of ACSS2 in T cells increased acetate-derived carbon incorporation in citrate and fatty acid, whereas reducing ACSS expression in T cells impairs IFN-γ production by tumor-infiltrating lymphocytes and tumor clearance (Qiu et al. 2019). Acetate promotes histone acetylation and restores chromatin accessibility in glucose-limited T cells, following enhanced IFN-γ production in an ACSS-dependent manner (Qiu et al. 2019).
Stearoyl-CoA desaturase 1 (SCD1)
Stearoyl-CoA desaturase-1 (SCD1) converts saturated fatty acids (SFA) into monounsaturated fatty acids (MUFA), which are used for the synthesis of phospholipids, triglycerides, and cholesterol esters, important components of membrane phospholipids. The MUFA/SFA ratio influences the plasma membrane fluidity and signal transduction (Kim and Ntambi 1999). Knockdown of SCD1 in human lung adenocarcinoma A549 cells reduced the MUFA/SFA ratio in cell membrane lipids, leading to the inactivation of Akt signaling, impairing lipogenesis (Scaglia and Igal 2008). Mice inoculated with SCD1-depleted A549 cells showed a reduction in tumor development compared with control cell-injected tumors (Scaglia and Igal 2008). In addition, inhibition of SCD1 activity suppressed migration and invasion by regulating EMT in colorectal cancer HCT116 cells harboring high expression levels of SCD1, which might be related to reduced level of MUFA and the MUFA/SFA ratio in SCD1 knockdown cancer cells (Ran et al. 2018). SCD1 is highly expressed in a variety of human cancer tissues including breast, prostate, lung, colon, kidney, and ovarian cancer (Roongta et al. 2011; von Roemeling et al. 2013; Ran et al. 2018), and reports have shown SCD1 overexpression to function as an oncogene in various cancers and to predict poor clinical outcome (Huang et al. 2016; Wang et al. 2016; Wang et al. 2020a, b, c).
Acidosis of TMEs contributes to tumor progression, invasion, and resistance to chemotherapy (Kato et al. 2013). Interestingly, melanoma (Mel501) cells exhibited a higher level of unsaturated species than saturated fatty acids under acidic conditions, which appears to be associated with the upregulation of SCD expression (Urbanelli et al. 2020). Acidification activates the PI3K/Akt signaling pathway to increase the expression of SCD1, which then binds to PPARα and promotes hepatic tumorigenesis through lipid metabolic reprogramming (Ding et al. 2022). In addition, the treatment of cobalt chloride, a hypoxia imitative agent, induced the expression of SCD-1 mRNA in human clear cell renal carcinoma Caki-2 cells, accompanied by increased mRNA levels of HIF1A and HIF2A (Melana et al. 2021). Moreover, the conversion of SFA (stearic acid; 18:0) into MUFA (oleic acid; 18:1n-9) is promoted under hypoxic conditions, which favors tumor cell proliferation (Melana et al. 2021). Furthermore, there is clinical significance of SCD1 and FABP4 expression in primary human tumors and metastatic tissues that relapse after first-line chemo- or hormone therapy in breast cancer patients. Notably, FABP4 was detected in blood vessels and adipocytes adjacent to metastatic relapse tissue of human breast cancer patients (Luis et al. 2021). In the syngeneic Lewis lung carcinoma (LLC) mouse tumor model and mouse xenograft model using MDA-MB-231 breast cancer cells, FABP4 expression was upregulated by hypoxia and re-oxygenation in adipocytes and tumor endothelial cells but not in cancer cells (Luis et al. 2021). Conversely, SCD1 expression was high in tumor sections, which leads to fatty acid desaturation, supporting cancer cell membrane fluidity and migration (Luis et al. 2021). FABP4-induced lipid droplet formation in cancer cells has been shown to provide a resource for survival under hypoxia and oxidative stress-induced ferroptosis, which might promote tumor recurrence (Luis et al. 2021).
In contrast to the previous reports, Ducheix et al. reported that intestinal-specific Scd1 (iScd1) knockout mice are prone to trigger inflammation and cancer in the gut compared with controls, in response to decreased hepatic MUFA proportion (Ducheix et al. 2018, 2022). In a diethylnitrosamine-induced model of HCC, disturbance of the hepatic fatty acid profile induced by iScd1 deletion favors tumor progression of extensive hepatic tumors and more frequent metastasis in the lungs compared with wild-type mice (Ducheix et al. 2022). Depending on the type of cancer, the physiology of SCD1 can differ. Further investigations into the functions of SCD1 in the lipid metabolism of individual cancer would contribute to the development of anticancer drugs.
Lipolysis and cancer progression
ATGL and HSL
Lipolysis is the metabolic process of hydrolyzing triglyceride (TG) into glycerol and free fatty acids. Several factors are related to lipolysis and cancer. Adipose triglyceride lipase (ATGL) and hormone-sensitive lipase (HSL) are key enzymes involved in the breakdown of intracellular TG, providing free fatty acids that can provide energy for cancer cell growth and migration (Zechner et al. 2012). ATGL is found in breast cancer cells, and its higher expression level is associated with more aggressive tumors (Wang et al. 2017). Its expression is amplified upon interaction with adipocytes to trigger the release of free fatty acids that are channeled into fatty acid β-oxidation. This metabolic process is prevalent in cancer cells but not in normal breast epithelial cells (Wang et al. 2017). In addition, increased expression of ATGL accelerates the growth of CRC cells, while its suppression enhances apoptosis in these cells. ATGL promotes the lipolytic process in CRC cells. Overexpression of ATGL in SW480 cells leads to increased levels of FFA and decreased levels of TAG compared to control cells. Conversely, HCT116 cells with ATGL knockdown exhibit decreased levels of FFA. ATGL-mediated lipolysis provides FFA for cholesterol metabolism and CoA biosynthesis (Yin et al. 2021). ATGL level is elevated in human colonic tumors, and their expression is further amplified by obesity. The increase in ATGL, influenced by the obesity-related compound oleic acid, enhances cancer cell migration. This migratory effect can be mitigated by inhibiting ATGL, which contributes to broad transcriptional changes in human colon cancer cells related to growth and metabolism (Iftikhar et al. 2021). However, the genetic ablation of neutrophil ATGL in the orthotopic 4T1 tumor model results in an increase in lung metastases of breast cancer cells (Li et al. 2020). The authors suggest that it would be intriguing to elucidate the roles played by bioactive lipid mediators originating from lung neutrophils within the lung metastatic niche, as well as to investigate the potential transfer of lipids from neutrophils to tumor cells in TME (Li et al. 2020).
The level of HSL mRNA in adipose tissue of cancer patients showed a notable increase compared to control patients. Additionally, cancer patients displayed a twofold elevation in both serum triacylglycerol and serum free fatty acid levels (Thompson et al. 1993). However, a study by Xu and colleagues recently revealed that HSL deficiency correlates with inflammation in both adipose tissue and the pancreas, accelerating pancreatic ductal adenocarcinoma (PDAC) in the KrasG12D mouse model (Xu et al. 2018). Administration of HSL inhibitors has been proposed for the management of cachexia (Das et al. 2011). Additionally, the precise effect of chronic HSL suppression on the progression of PDAC needs to be investigated.
FABP4
FABP4, known as adipocyte fatty acid binding protein, exhibits high expression in adipocytes. During the adipocyte differentiation process, FABP4 interacts with HSL to regulate lipolysis (Furuhashi 2019). Exogenous FABP4 interacts with adipocytes, promoting differentiation and facilitating p38/HSL-mediated lipolysis (Dou et al. 2020). Additionally, FABP4 plays a significant role in tumor transformation, proliferation, metastasis, and drug resistance by enhancing lipid transport (Guaita-Esteruelas et al. 2018). Co-culture of cancer cells with adipocytes triggers lipolysis in adipocytes, leading to subsequent β-oxidation through lipid transfer to cancer cells, which has been demonstrated in breast and ovarian cancers (Nieman et al. 2011; Attane et al. 2020). FABP4 can bind reversibly to long-chain fatty acids and is highly expressed in adipocytes (Furuhashi and Hotamisligil 2008). FABP4 expression is strongly exhibited at the interface between adipocyte and ovarian cancer cells, enhancing lipid availability in cancer cells and promoting rapid tumor growth and metastasis (Nieman et al. 2011). In a co-culture model involving ovarian cancer cells and adipocytes, treatment with an FABP4 inhibitor attenuated lipid accumulation in the cancer cells, reducing migration and invasion of cancer cells facilitated by adipocytes (Nieman et al. 2011). A link between FABP4 and lipolysis in the survival and growth of cancer cells through adipocytes has also been reported in breast cancer tissues (Kim et al. 2020).
MIC-1
Macrophage inhibitory cytokine-1 (MIC-1), a stress response cytokine, belongs to the transforming growth factor beta (TGF-β) superfamily as a divergent member (Bootcov et al. 1997). MIC-1 is recognized as a protumorigenic marker associated with progressive prostate cancers. Studies have consistently demonstrated its presence in prostate cancer tissues, influencing cell proliferation and invasion (Bauskin et al. 2006; Bruzzese et al. 2014; Jones et al. 2015). Notably, the role of a high-fat diet in prostate cancer progression has been studied (Huang et al. 2012, 2014; Wu et al. 2016). A high-fat diet markedly enhanced adipocyte infiltration and adipose lipolysis in a mouse intraperitoneal xenograft model of prostate cancer, which facilitates tumor progression through the expression and secretion of MIC-1 (Huang et al. 2021). Furthermore, the co-culture of prostate cancer PC-3 with surrounding adipocytes stimulates the secretion of MIC-1, while prostate stromal fibroblasts release IL-8, both influenced by the fatty acids secreted through activated lipolysis (Huang et al. 2021). Collectively, these findings underscore the intricate interplay between lipid metabolism, the TME, and the expression and effects of MIC-1 in prostate cancer progression.
ADM
Exosomal adrenomedullin (ADM), a multifactorial hypoxia-inducible peptide derived from pancreatic cancer cells, was shown to induce lipolysis in adipose tissues (Sagar et al. 2016). This effect extends to exosomal ADM derived from CAFs, which promotes lipolysis in both mouse 3T3-L1 and human adipocytes (Kong et al. 2018). Adding to this complex network, recent findings by Pare et al., reveal that MCF-7 mammospheres produce ADM, which modifies the phenotype of cancer-associated adipocytes via the ADM receptor in a paracrine manner. Stimulation of adipocytes by ADM promotes lipolysis through the phosphorylation of hormone-sensitive lipase (HSL) and uncoupling protein 1 (UCP1) expression in breast adipocytes, which may provide energy to cancer cells or remodel the TME (Pare et al. 2020). Therefore, the emerging understanding of the involvement of exosomal ADM in adipose tissue interactions and lipolysis within the TME offers a perspective on the intricate crosstalk among cancer cells, and adipocytes, and their mutual influence. In Fig. 1, we illustrate the potential signaling that may modulate tumor progression through the stimulation of lipolysis in the tumor microenvironment.
Fig. 1.

Possible regulatory mechanism of lipolysis in tumor microenvironment. Lipolysis is the metabolic process in which triglyceride (TG) is broken down into glycerol and free fatty acids (FA). Overexpression of ATGL and HSL in adipocyte and cancer cells leads to the release of FAs which are used in fatty acid β-oxidation. Adipocyte fatty acid binding protein (FABP4) can bind to long-chain fatty acids reversibly and triggers lipolysis in adipocytes, enabling lipid transfer to cancer cells and promoting β-oxidation. In addition, the interaction of cancer cells with surrounding adipocytes stimulates the secretion of macrophage inhibitory cytokine-1 (MIC-1) and the release of IL-8 by stromal normal fibroblasts, influenced by the fatty acids secreted through activated lipolysis. Exosomal adrenomedullin (ADM) is a multifactorial, hypoxia-inducible peptide facilitates lipolysis through interaction with adipocytes within the tumor microenvironment. The proteins associated with lipolysis highlight the complex interactions between lipid metabolism and the tumor microenvironment, playing a crucial role in the cancer progression, migration, and invasion
ABC transporter for cholesterol transport and chemoresistance
Biosynthesis of cholesterol begins with acetyl-CoA derived from mitochondria and transported to the cytosol through a series of chemical reactions known as the mevalonate pathway. Condensation of acetyl-CoA with acetoacetyl-CoA forms 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA), which is then converted by HMG-CoA reductase (HMG-CoAR) to mevalonate (Sharpe and Brown 2013) and then further converted to cholesterol (Sharpe and Brown 2013). Cholesterol is an essential constituent of the cellular membrane, which plays an important role in maintaining the stability of the cellular membrane, supporting cell proliferation, and serving as a precursor for the biosynthesis of steroid hormones (Ghayee and Auchus 2007; Hu et al. 2010; Arita et al. 2015). In addition, several studies have suggested that cholesterol accumulation promotes cancer development and resistance to anticancer drugs (Yue et al. 2014; Yan et al. 2020). Cholesterol enriched within the TME leads to increased endoplasmic reticulum stress in CD8+ T cells, resulting in the upregulation of immune checkpoint expression, particularly PD-1, at the transcriptional level. This cholesterol-induced response contributes to the functional exhaustion of CD8+ T cells (Ma et al. 2019). Statin, the inhibitor for cholesterol synthesis, has the capability to transcriptionally suppress PD-L1 expression. As a result, they can mitigate the aggressiveness of NSCLC through ferroptosis (Mao et al. 2022). Moreover, in the meta-analysis of NSCLC immunotherapy cohorts, stains enhance the drug-response effect of anti-PD-1 therapy and prolong the survival of NSCLC patients (Mao et al. 2022).
ABCA1
ATP-binding cassette (ABC) transporters, one of the most prominent families of integral plasma membrane proteins, are involved in cholesterol transport across the cell membrane. Notably, the relationship between ABC transporter A1 (ABCA1) and cholesterol is becoming prominent in cancer (Pasello et al. 2020). In response to elevated cellular cholesterol levels, ABCA1 expression is upregulated by blocking the ubiquitination and subsequent proteasomal degradation, leading to an increase in free cholesterol efflux (Hsieh et al. 2014). The expression of ABCA transporters is mainly regulated by the sterol regulatory element-binding protein 2 (SREBP2) and liver X receptor (LXR) (Goldstein et al. 2006; Gabbi et al. 2014). It has been reported that cholesterols are converted to oxysterols and prevent the translocation of SREBP2 to the nucleus, leading to ABCA1 expression. LXR acts as a sensor of cholesterol homeostasis and interacts with retinoid X receptor (RXR), which forms a heterodimeric complex under high-cholesterol conditions (Costet et al. 2000; Venkateswaran et al. 2000). The implications of ABC transporter expression in cancer are controversial. In prostate cancer cells, the reduced expression of ABCA1 with promoter hypermethylation maintains elevated intracellular cholesterol levels, contributing to aggressive prostate cancer progression (Lee et al. 2013). However, Gao et al. reported that ABCA1 suppression impairs malignant phenotypes of epithelial ovarian cancer (EOC) cells and reduces the formation of EOC spheroids, which may be associated with the induction of intracellular cholesterol (Gao et al. 2022). In triple-negative breast cancer, elevated cholesterol levels from ABCA1 knockdown in membranes of breast cancer cells diminished membrane fluidity, hindering cell migration (Zhao et al. 2016). This process might be associated with apoptosis triggering in cholesterol-rich environments (Gajate and Mollinedo 2001). Cholesterol efflux mediated by ABCA1 overexpression promoted EMT and increased invasive capacity by maintaining caveolin-1 stability in colorectal cancer, the expression level of which could be used as a prognostic biomarker in colon cancer patients (Aguirre-Portoles et al. 2018).
ABCB1
ABC transporter B1 (ABCB1), also known as multidrug resistance protein 1 (MDR1) or P-glycoprotein, facilitates cholesterol distribution in the plasma membrane using floppase (Le May et al. 2013). ABCB1 substrates preferentially accumulate in cholesterol-rich regions of the membrane, and their pumping activity is enhanced in the presence of cholesterol (Subramanian et al. 2016). In chemoresistant colon cancer HT29 (HT29-dx) cells, the reduction of cholesterol synthesis is regulated by attenuated activity and expression of HMG-CoAR through induction of the E3 ligase Trc8, reducing cell viability (Gelsomino et al. 2013). This change is accompanied by decreased ABCB1 surface level in HT29-dx cells, which may exert a pro-immunogenic effect in response to chemotherapy through the restoration of extracellular HMGB1 (high mobility group box 1) expression, an index of necrosis and immunogenic death (Gelsomino et al. 2013). These data suggest that ABCB1 not only participates in cholesterol transport within the plasma membrane but also contributes to the development of chemoresistance in response to anticancer drugs.
ABCG1
ABCG1, also known as ABC transporter G1, participates in the process of reverse cholesterol transport by expelling excess cholesterol from cells into high-density lipoprotein particles (Kennedy et al. 2005). A deficiency of ABCG1 in mice significantly inhibits the subcutaneous growth of B16-melanoma and MB49-bladder carcinoma cells (Sag et al. 2015). In addition, the absence of ABCG1 in macrophages has been demonstrated to enhance their inflammatory characteristics and diminish the growth of subcutaneous tumors in mice following a Western-like diet (Sag et al. 2015). In Abcg1−/− mice, tumor growth is linked to a change in the macrophage phenotype within the tumor, transitioning from a tumor-promoting M2 to a M1 tumor-fighting profile (Sag et al. 2015). In lung cancer HKULC4 cells, the overexpression of ABCG1 not only stimulates cell proliferation but also increases the expression of anti-apoptotic proteins, namely BCL2 and MCL1 (Tian et al. 2017a, b). Enhanced cholesterol efflux promotes IL-4-mediated reprogramming, which includes the suppression of gene expression induced by IFNγ (Goossens et al. 2019). Genetic deletion of ABCG1 reverses the tumor-promoting functions of TAMs and leads to a reduction in tumor burden (Goossens et al. 2019). Various ABC transporters, including ABCG1, are considered as therapeutic targets for improving cancer treatment.
Targeting lipid metabolism for cancer treatment
In the preceding section, we explored the fundamental factors that underlie the reprogramming of lipid metabolism, which propels the progression of cancer. Furthermore, we assessed the interplay between cancer progression and altered lipid metabolism, encompassing crucial processes such as lipid uptake, lipolysis, and lipogenesis within the TME. With a focus on these factors responsible for lipid metabolic reprogramming, driving cancer progression, there is considerable potential in devising novel approaches for cancer treatment. Notably, clinical trials are currently evaluating the efficacy of fluvastatin, simvastatin, and denifanstat, which target lipid metabolisms such as HMG-CoAR or FASN in several carcinomas, including NSCLC, breast cancer, ovarian cancer, and prostate cancer (Han et al. 2011; Longo et al. 2020; Falchook et al. 2021). In addition, it is well documented that a variety of phytochemicals suppress cancer development and progression through modulating lipid metabolism. While small molecules trigger a specific target to regulate lipid metabolism (Fig. 2), phytochemicals often have the advantage of simultaneously affecting multiple targets (Fig. 3). In this context, we introduce a range of small molecular inhibitors and a variety of phytochemicals that target lipid metabolism, expanding beyond those previously mentioned.
Fig. 2.
Modulation of lipid metabolism by small molecular inhibitors to treat cancer. Small molecules specifically inhibit their target enzymes involved in lipid metabolism through direct binding and/or suppression of activity (Fatostatin, SREBP inhibitor; Cerulenin, FASN inhibitor; ND-654, ACC inhibitor; SB-204990, ACLY inhibitor). As a consequence, altered lipid modulation remodels TME less favorable for cancer promotion and progression
Fig. 3.
Potential multi-target effects of dietary phytochemicals on lipid metabolism in cancer. Unlike small molecular inhibitors, dietary phytochemicals simultaneously modulate multiple targets involved in lipid metabolism, rather than triggering specific single enzyme. This multi-target approach can influence various aspects of TME, while preventing development of resistance to a single pathway
Small molecular inhibitors
Fatostatin
Fatostatin is a small molecule non-sterol diarylthiazole derivative that is a specific inhibitor of the SREBP cleavage-activating protein (SCAP) required for SREBP activation (Fig. 2, Table 1). It can bind to SCAP directly, inhibiting the activation of SREBP (Kamisuki et al. 2009). Targeting the SREBP-mediated lipogenic program by fatostatin led to a blockade of tumor growth as well as distant metastasis by decreasing the frequency of mitotic cancer cells in the Pml/Pten double-null mouse model of prostate cancer (Chen et al. 2018). Fatostatin decreases the expression of SREBP target genes such as FASN, SCD-1, HMGCR, HMGCS1, MVK, MVD, and LDLR in prostate cancer cells, followed by a reduction in intracellular fatty acid and total cholesterol levels (Li et al. 2014). Similar findings have been reported in human endometrial carcinoma cells, and an antitumor effect was also observed in a subcutaneous HEC-1A endometrial and androgen-insensitive C4-2B prostate xenograft model (Li et al. 2014; Yao et al. 2020). In addition, temozolomide-resistant glioblastoma multiforme U87 (U87R) cells exhibit a significant increase in cholesterol and fatty acid synthesis, alongside the reduction of lipid unsaturation, resulting in diminished membrane fluidity (Choo et al. 2023). Fatostatin shows more potent cell growth inhibitory effects in U87R cells compared to sensitive U87 cells (Choo et al. 2023). Moreover, fatostatin leads to a significant decrease in the level of the active form of SREBP1 in pancreatic cancer MIAPaCa-2 cells. This reduction is associated with cytotoxicity and reduction of proteins involved in lipid biosynthesis such as FASN, SCD-1, and HMG-CoAR (Siqingaowa et al. 2017). Similar outcomes have been observed in various cell lines such as glioma, prostate, and breast cancer, demonstrating the potential of fatostatin to attenuate tumor growth (Gholkar et al. 2016).
Table 1.
Effects of small molecule inhibitors of lipid metabolism on TME
| Categories | Small molecule inhibitors | Molecular targets | Chemical structure | Effects on TME | References |
|---|---|---|---|---|---|
| Non-sterol diarylthiazole derivative | Fatostatin |
• Directly bind to SCAP • Inhibit SREBP activation |
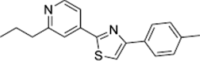 |
• Reduce tumor growth and metastasis in prostate cancer mouse model • Anti-cancer effects in human endometrial carcinoma and prostate cancer xenograft models • Inhibit tumor growth in glioma, prostate, and breast cancer cell lines |
Chen et al. (2018) Yao et al. (2020) and Li et al. (2014) Gholkar et al. (2016) |
| Antifungal antibiotic | Cerulenin | • Directly bind to FASN | 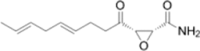 |
• Suppress proliferation of human prostate cancer cells • Reduce tumorsphere formation • Reduce invasiveness and stemness in patient-derived glioma stem cells • Inhibit migration and invasion of cervical cancer cells • Reduce lymph node metastasis in the metastatic mouse model of cervical cancer |
Nishi et al. (2016) Brandi et al. (2017) Yasumoto et al. (2016) Du et al. (2022) Du et al. (2022) |
| Liver-specific ACC inhibitor | ND-654 |
• Bind to ACC • Inhibit ACC phosphorylation by AMPK |
 |
• Suppress proliferation of human hepatic carcinoma cells | Lally et al. (2019) |
| γ-Lactone prodrug | SB-204990 | • Inhibit ACLY |  |
• Disrupt the mitochondrial membrane potential and inhibit cell growth in various human cancer cell lines • Inhibit growth of tumor xenografts |
Hatzivassiliou et al. (2005) Hatzivassiliou et al. (2005) |
Cerulenin
Cerulenin covalently binds to the ketoacyl synthase domain of FASN (Fig. 2, Table 1) (Kuhajda et al. 2000) to significantly suppress the proliferation of human pancreatic cancer MiaPaCa-2 and AsPC-1 cells (Nishi et al. 2016). In addition, treatment of tumorsphere Panc1 cells with cerulenin leads to reduced cell viability and significant changes in cell morphology. There is also a reduction in spheroids, suggesting cytoskeletal reorganization (Brandi et al. 2017). Similar to the findings of these studies, treatment with cerulenin, an inhibitor of FASN, reduced the invasiveness of patient-derived glioma stem cells as well as the expression of stemness markers such as CD133 and Sox2. These findings imply that FASN-driven de novo lipogenesis plays a crucial role in maintaining cancer stemness (Yasumoto et al. 2016). Consistent with this, impeding the activity of FASN by cerulenin in SKBR3 results in high impairment of cellular proliferation (Stoiber et al. 2018). Recently, Du et al. demonstrated that cerulenin impairs the migration and invasion of cervical cancer, specifically HeLa and CaSki cells, through cholesterol reprogramming (Du et al. 2022). Moreover, cerulenin effectively reduced the lymph node volumes and the percentage of popliteal lymph nodes in a metastatic mouse model of cervical cancer (Du et al. 2022).
ND-654
ND-646 and ND-654 compounds bind to the arginine residue within the biotin carboxylase domain of the ACC protein (Fig. 2, Table 1), effectively inhibiting both its dimerization and enzymatic activity. While ND-646 is broadly distributed throughout the body, ND-654 is designed to facilitate increased uptake in the liver (Svensson et al. 2016; Lally et al. 2019). Treating hepatic cellular carcinoma (HCC) HepG2 cells with liver-specific ND-654 leads to a reduction in the level of ACC phosphorylation by AMPK, which is followed by a decrease in cell proliferation. Interestingly, HepG2 cells expressing a mutation in ACC serine phosphorylation sites exhibit enhanced lipogenesis and proliferation (Lally et al. 2019). These studies provide valuable insights into the significance of de novo lipogenesis and dysregulation of AMPK-mediated ACC phosphorylation in the development of HCC. Furthermore, the findings highlight the potential of ACC inhibitors as a targeted approach for cancer treatment.
SB-204990
SB-204990, a γ-lactone prodrug of the potent ACLY inhibitor SB-201076 (Fig. 2, Table 1), effectively suppresses the synthesis of cholesterol and fatty acid in both HepG2 cells and rats (Pearce et al. 1998). In various human cancer cell lines, SB-204990 disrupts the mitochondrial membrane potential, impedes cell cycle progression, and inhibits cell growth. These effects are closely linked to the impairment of glycolytic metabolism and glucose-dependent lipid synthesis (Hatzivassiliou et al. 2005). Notably, intraperitoneal administration of SB-204990 significantly inhibits tumor growth in nude mice with xenografts from mouse pancreatic ductal cell lines carrying oncogenic K-rasG12D alleles (Hatzivassiliou et al. 2005). Furthermore, treatment with SB-204990 in K-Ras-driven cancer cells results in a significant loss of cell viability in the absence of serum and during glutamine deprivation (Hatzivassiliou et al. 2005; Hatipoglu et al. 2022; Sola-Garcia et al. 2023). From the studies, ACL is identified as a potential therapeutic target and provides the rationale for the development of ACL inhibitors for cancer treatment.
Phytochemicals
Apigenin
Apigenin is a flavonoid abundant in parsley, onions, oranges, and chamomile and is effective in treating asthma, shingles, and cancer (Fig. 3, Table 2) (Su et al. 2020a, b). In combination with other chemotherapeutics, such as doxorubicin, apigenin can sensitize inhibitory effects on tumor growth and proliferation (Gao et al. 2013, 2018; Nozhat et al. 2021). In addition to anti-cancer effects, apigenin plays a role in lipid metabolism and has been reported to attenuate lipid accumulation in human hepatic cancer cell lines and adipocytes (Ono and Fujimori 2011; Lu et al. 2019; Hsu et al. 2021). In a high-fat diet (HFD)-induced obese mouse model, apigenin reduced body weight and visceral adipose tissue through downregulation of FASN, SCD1, or CD36, suggesting an anti-adipogenic effect (Su et al. 2020a, b; Wu et al. 2021). Moreover, apigenin can activate lipolysis. In human hepatocarcinoma HepG2 cells, apigenin-induced autophagy-dependent lipid degradation (Lu et al. 2020). Similarly, apigenin treatment upregulated the expression of genes responsible for lipolysis, including ATGL, HSL, FOXO1, and ACC in adipose tissues of HFD-induced obese mice (Table 2) (Sun and Qu 2019). These data suggest that apigenin can exert an anti-cancer effect by regulating lipid metabolism, although further investigation is required to confirm the phenomenon in vitro and in vivo.
Table 2.
Effects of phytochemicals on lipid metabolism
| Categories | Phytochemicals | Origins | Chemical structure | Effects on lipid metabolism | References |
|---|---|---|---|---|---|
| Flavonoid | Apigenin | Parsley, Onion, Chamomile |  |
• Reduce lipid accumulation in cancer cells and adipocytes • Promote lipid degradation in HepG2 cells • Reduce visceral adipose tissues (↓FASN, SCD1, CD36) in HFD mice • Induce lipolysis-responsible genes (ATGL, HSL, FOXO1, and ACC) in HFD mice |
Ono and Fujimori (2011), Lu et al. (2019), and Hsu et al. (2021) Lu et al. (2020) Su et al. (2020a, b), and Wu et al. (2021) Sun and Qu (2019) |
| Polyphenol | Curcumin | Curcuma longa | 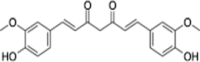 |
• Inhibit lipid accumulation and adipogenesis in adipocytes (↑ACC phosphorylation, fatty acid oxidation) • Reduce fatty acid uptake and biosynthesis (↓CD36, FASN) in NAFLD mice • Promote cholesterol efflux in macrophages |
Ejaz et al. (2009) Yan et al. (2018) |
| Polyphenolic flavonoid | EGCG | Green tea | 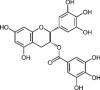 |
• Inhibit lipid accumulation, dipogenesis, and cell proliferation in adipocytes • Promote lipolysis (↑HSL, autophagy) • Reduce white adipose tissue weight in HFD mice • Inhibit lipogeneic gene expression (ACC1, FASN, SCD1) • Induce lipolysis gene expression (HSL, ATGL) • Decrease fatty acid synthesis in cancer cells and xenograft tumors (↓FASN, ACC, ACLY) • Reduce triglyceride level and inhibits lipolysis in Huh7 cells • Promote fat clearance in cancer cells (↑autophagy) |
Moon et al. (2007), and Kim and Sakamoto (2012) Lee et al. (2009), Zhou et al. (2014), and Kim et al. (2017) Choi et al. (2020) Li et al. (2018), Choi et al. (2020), and Lee et al. (2009) Khiewkamrop et al. (2022) Suihara et al. (2021) Zhou et al. (2014) |
| Polyphenol | Resveratrol | Grapes, Berries | 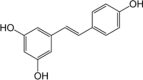 |
• Inhibit adipogenesis (↓FASN, PPARγ) in adipocytes • Reduce lipid accumulation in HFD mice • Increase fatty acid oxidation (↓ACC activation) in mice • Promote cholesterol efflux in macrophages |
Rayalam et al. (2008), Santos et al. (2014), and Chang et al. (2016) Kim et al. (2011), and Andrade et al. (2014) Gimeno-Mallench et al. (2019) |
| Flavonoid | Scutellarin | Scutellaria baicalensis | 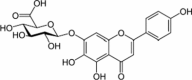 |
• Inhibit adipogenesis in adipocytes • Inhibit hepatic lipid accumulation in HepG2 and HFD mice (↓CD36, FASN ACC) |
Lu et al. (2013) |
| Flavonoid | Silibinin | Milk thistle | 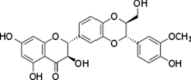 |
• Inhibit lipid accumulation and proliferation in adipocytes (↓ FASN) • Reduce white adipose tissue weight and triglyceride accumulation in HFD mice • Inhibit lipid accumulation in HepG2 cells and tumor xenografts (↓ FASN, ACC) |
Yang et al. (2021) Alsaggar et al. (2020) Deep et al. (2017) |
| Flavonoid | Quercetin | Red onion, Apple, Berries, Citrus fruits |  |
• Inhibit differentiation and lipid accumulation in adipocytes • Reduces white adipose tissues and hepatic lipid accumulation in HFD mice (↓ FASN, CD36) • Inhibits fatty acid accumulation in HepG2 cells (↓ FASN) |
Hong et al. (2021), and Seo et al. (2015) Jung et al. (2013) |
Curcumin
Curcumin is a well-known chemopreventive phytochemical extracted from the rhizome of Curcuma longa that is widely used as a spice in Asian countries (Table 2) (Tomeh et al. 2019). In addition to anti-inflammatory and anti-carcinogenic effects, curcumin is closely associated with lipid metabolism (Nosrati-Oskouie et al. 2021). Curcumin facilitates ACC phosphorylation and fatty acid oxidation in 3T3-L1 adipocytes, while inhibiting lipid accumulation and adipogenesis (Ejaz et al. 2009; Tian et al. 2017a, b; Wu et al. 2019). In HFD-induced obese mice, dietary curcumin reduced body weight gain, body fat, lipid levels, and hepatic lipid accumulation (Ejaz et al. 2009; Kobori et al. 2018). Yan et al. have demonstrated that oral administration of curcumin reduced CD36 and FASN expression in the liver of NAFLD mice (Table 2), implying its inhibitory effects on fatty acid uptake and biosynthesis (Yan et al. 2018). Moreover, curcumin promoted cholesterol efflux via ABCA1 in THP-1 macrophages to prevent atherosclerosis (Lin et al. 2015; Tan et al. 2021). In line with that, randomized controlled trial studies showed that curcumin supplementation improved lipid profiles in individuals at risk of cardiovascular diseases, polycystic ovary syndrome, and metabolic syndrome, further supporting the effects of curcumin on lipid metabolism (Tabrizi et al. 2018; Sohaei et al. 2019; Rafiee et al. 2021).
Curcumin functions in the lipid metabolism of various types of cancers, inducing apoptosis in human breast cancer MDA-MB-231 and liver cancer HepG2 cell lines, which is mediated by the downregulation of FASN (Fan et al. 2014, 2016). In addition to its inhibitory effect on lipogenesis, curcumin decreased cholesterol absorption into human colorectal cancer Caco-2 cells, suppressing cancer cell proliferation (Qin et al. 2023). Moreover, curcumin attenuated stem cell phenotypes of human breast cancer cells through the downregulation of fatty acid desaturases, such as SCD, FADS1, and FASD2 (Colacino et al. 2016). In tumor-bearing mice, curcumin combined with sorafenib enhanced serum lipid profiles and reduced FASN expression compared to the sorafenib-only-treated group (Man et al. 2020). Moreover, curcumin further inhibited tumor growth and EMT in sorafenib-treated mice, which suggests that curcumin sensitizes cells to the anti-cancer effects of sorafenib through alteration of lipid metabolism (Man et al. 2020). Taken together, these results suggest that curcumin exerts anti-tumorigenic effects by altering lipid metabolism (Fig. 3).
Epigallocatechin gallate (EGCG)
EGCG, a polyphenolic flavonoid, is a main bioactive ingredient in green tea that has been found to possess diverse biological functions and health benefits, particularly in cancers and metabolic syndrome (Legeay et al. 2015; Alam et al. 2022). EGCG has been reported to inhibit adipogenesis, lipid accumulation, and cell proliferation in 3T3-L1 adipocytes (Moon et al. 2007; Kim and Sakamoto 2012). In addition to the anti-lipogenic effect, EGCG promoted HSL-mediated lipolysis in 3T3-L1 cells (Lee et al. 2009). Moreover, EGCG led to autophagy-induced lipolysis in vivo and in vitro (Zhou et al. 2014; Kim et al. 2017). These lipid-modulating effects of EGCG have been demonstrated in diet-induced obese animal models as well. EGCG intake reduced body weight gain and white adipose tissue weight in HFD-induced obese mice (Choi et al. 2020). Moreover, EGCG significantly improved serum lipid profiles in HFD-fed mice and rats (Li et al. 2018; Li and Wu 2018). EGCG can stimulate lipid catabolism through inhibition of the expression of lipogenic genes such as ACC1, FASN, and SCD1 and through induction of lipolysis-involved genes, like HSL and ATGL (Table 2) (Lee et al. 2009; Li et al. 2018; Choi et al. 2020).
In cancer settings, EGCG has been reported to suppress tumorigenesis by regulating lipid metabolism. EGCG treatment resulted in increased apoptosis and decreased fatty acid synthesis through downregulation of FASN, ACC, and ACLY in human colorectal cancer cell lines as well as in a tumor xenograft mouse model (Khiewkamrop et al. 2022). Similarly, EGCG inhibited FASN and subsequent fatty acid synthesis, which mediated apoptosis in human prostate and liver cancer cells (Brusselmans et al. 2003; Khiewkamrop et al. 2022). Moreover, EGCG markedly reduced intracellular triglyceride levels and accelerated lipolysis in human hepatoma Huh7 cells (Suihara et al. 2021). In particular, EGCG can stimulate autophagy-dependent fat clearance in human liver cancer cells (Zhou et al. 2014). These data suggest a cross-link between the lipid-modulating and anti-carcinogenic effects of EGCG.
Resveratrol
Resveratrol is a potent anti-oxidative phytochemical mainly found in grapes and berries and can effectively prevent cardiovascular diseases, carcinogenesis, and metabolic syndrome (Table 2) (Ko et al. 2017; Hou et al. 2019). In particular, resveratrol activates SIRT1 but downregulates PPARγ, implying its role in lipid metabolism. In 3T3-L1 adipocytes, resveratrol suppresses adipogenesis through the downregulation of FASN and PPARγ and leads to apoptosis (Rayalam et al. 2008; Santos et al. 2014; Chang et al. 2016). In HFD-fed obese mice, resveratrol can reduce body weight and lipid accumulation, while enhancing lipid profiles, which seems to be mediated by the expression of key adipogenic genes, such as SREBP-1 and FASN (Kim et al. 2011; Andrade et al. 2014). Consistently, a meta-analysis showed that resveratrol intake can enhance lipid profiles in obese people (Zhou et al. 2022). Konings et al. have shown that resveratrol intake reduced abdominal subcutaneous adipocyte size in obese men (Konings et al. 2014). These data suggest that resveratrol possesses an anti-adipogenic effect in vitro and in vivo. Moreover, resveratrol affects lipolysis and cholesterol transport, facilitating basal and/or drug-induced triglyceride hydrolysis in fat cells (Szkudelska et al. 2009; Gomez-Zorita et al. 2013). Resveratrol upregulated ATGL expression at mRNA and protein levels in 3T3-L1 cells (Lasa et al. 2012). In old healthy mice, resveratrol inhibited the expression and activation of ACC1, resulting in increased fatty acid oxidation (Gimeno-Mallench et al. 2019). Of note, resveratrol accelerated ABCA1/ABCG1-mediated cholesterol efflux in human and murine-derived macrophages, implying its preventive role in atherosclerosis (Voloshyna et al. 2013; Ye et al. 2019a, b). Overall, resveratrol is involved in diverse processes of lipid metabolism, conferring protection against obesity and cardiovascular diseases.
Regarding its anti-carcinogenic and lipid-modulating effects, resveratrol has been thought to suppress tumor promotion and progression by altering lipid metabolism. Resveratrol significantly inhibited lipid synthesis, cell proliferation, and stemness by downregulating the expression of lipogenic genes, such as SREBP1 and its downstream ACLY1, ACC1, and FASN, in human breast and pancreatic cancer cells (Pandey et al. 2011; Khan et al. 2014; Zhou et al. 2019). Consistently, resveratrol inhibited adipocyte hypertrophy in the mammary fat pad of HFD-fed obese mice, leading to suppression of tumor growth (Rossi et al. 2018). In hepatoma-bearing rats, resveratrol intake reduced tumor size, tumor metastasis, and serum cholesterol level (Miura et al. 2003). Fukuda et al. have shown that resveratrol inhibited oral cancer cell growth by blocking epidermal FABP expression in vitro as well as in the xenograft model (Fukuda et al. 2022). Taken together, these findings indicate that resveratrol can inhibit lipogenesis and lipid uptake, leading to the suppression of tumor promotion and progression.
Scutellarin
Scutellarin is isolated from Scutellaria baicalensis, an herb widely used in Chinese medicine to treat inflammatory diseases and hyperlipidemia (Table 2) (Wang and Ma 2018). Scutellarin has been reported to exert chemopreventive and anti-cancer effects in various types of cancers (Li et al. 2010; Wang et al. 2010; Xu and Zhang 2013). In terms of lipid metabolism, scutellarin inhibited adipogenesis through PPARα in 3T3-L1 cells (Lu et al. 2013). Moreover, scutellarin attenuated hepatic lipid accumulation in both HepG2 cells and HFD-fed mice, which was mediated by the upregulation of CD36, FASN, and ACC (Luan et al. 2020; Zhang et al. 2022a, b). In line with that, scutellarin can protect against nonalcoholic fatty liver diseases and hyperlipidemia in animal models (Fan et al. 2017; Zhang et al. 2022a, b). Thus, scutellarin is a feasible candidate for suppressing tumor promotion and progression through the modulation of adipogenesis.
Silibinin
Silibinin is a flavonoid derived from the seeds of Silybum marianum (milk thistle) and is well-known for its hepatoprotective and chemopreventive activities (Table 2) (Abenavoli et al. 2010; Cheung et al. 2010). It has been reported that silibinin can improve lipid metabolism in obese mice, based on proteomics analysis (Wang et al. 2020a, b, c). In adipocytes, silibinin suppressed lipid accumulation and cell proliferation, possibly through the downregulation of FASN (Ka et al. 2009; Suh et al. 2015). In HFD-fed obese mice, silibinin intake reduced body weight gain, white adipose tissue weight, and adipocyte hypertrophy (Alsaggar et al. 2020). In the liver of HFD-fed mice, silibinin attenuated the expression of FASN and ACC and triglyceride accumulation, protecting against liver steatosis (Yang et al. 2021).
Silibinin can affect tumorigenesis through lipid modulation as well. Silibinin has been reported to attenuate adipogenesis in various types of cancers. In HepG2 cells, silibinin inhibited the expression of FASN and ACC and lipid deposition (Yang et al. 2021). In human endometrial and prostate cancer cell lines, silibinin effectively blocked the expression and/or activation of SREBP1, SCD-1, FASN, and ACLY, resulting in suppression of lipid accumulation and cancer cell proliferation (Nambiar et al. 2014; Shi et al. 2019). Interestingly, silibinin suppressed expression levels of genes responsible for lipogenesis and cell invasion in HepG2 cells exposed to sera from obese males, suggesting silibinin as a potential chemotherapeutic in obesity-induced cancer (Miethe et al. 2017). Similarly, silibinin inhibited hypoxia-induced lipogenesis and angiogenesis under hypoxic conditions in human prostate cancer cells (Deep et al. 2017). Furthermore, silibinin administration dramatically decreased tumor growth, intratumoral lipid accumulation, and expression levels of FASN and ACC in the tumor xenograft mouse model (Deep et al. 2017). Thus, silibinin may be useful for the treatment of cancer patients with highly lipogenic phenotypes.
Quercetin
Quercetin is a dietary flavonoid abundant in red onions, apples, berries, and citrus fruits that exhibits antioxidant, anti-obesity, and anti-cancer effects (Table 2) (Shabir et al. 2022). In adipocytes, quercetin inhibited differentiation and fat accumulation in 3T3-L1 cells (Hong et al. 2021). Quercetin provoked upregulation of ATGL and HSL expression and downregulation of FASN and LPL expression in OP9 cells that differentiate into adipocytes (Seo et al. 2015). In HFD-induced obese mice, dietary quercetin significantly reduced body weight gain, white adipose tissues, and hepatic lipid accumulation (Jung et al. 2013). Moreover, quercetin attenuated expression levels of CD36 and FASN in the liver of these obese mice (Jung et al. 2013). Thus, quercetin exerts its anti-obesity effect by preventing adipogenesis and promoting lipolysis.
In HepG2 liver cancer cells, quercetin downregulated FASN expression and accumulation of intracellular fatty acids, resulting in increased apoptosis (Li et al. 2013; Zhao et al. 2014). Similarly, quercetin blocked FASN expression and cell proliferation in human nasopharyngeal carcinoma cell lines (Daker et al. 2013). Quercetin intake suppressed tumor size and lipid accumulation in tumor-bearing mice, suggesting a correlation between the anti-cancer and lipid-modulating effects of quercetin (Ruidas et al. 2022). In Fig. 3, an illustration is presented depicting proteins related to lipid metabolism that can be influenced by phytochemicals.
Concluding remarks and perspectives
Metabolic reprogramming within the TME has been recognized as a crucial hallmark of cancer (Pavlova and Thompson 2016). Tumors require a huge supply of energy to expedite promotion and progression. To date, investigations into metabolic alteration in cancer have focused on glucose utilization, referred to as the Warburg effect. Lipid metabolism is emerging as another key player in TME-related metabolic reprogramming (Snaebjornsson et al. 2020). TME represents altered lipid metabolic pathways, which are closely associated with the activation of oncogenic signals (Fernandez et al. 2020). Thus, reprogrammed lipid-modulating genes in TME may be novel targets for the prevention and treatment of cancers. Targeting lipid metabolic reprogramming factors that promote cancer progression would support new strategies for cancer treatment. As numerous phytochemicals exert anti-obesity and anti-cancer effects concomitantly, it would be worth repurposing lipid-modulating drugs and/or phytochemicals as chemotherapeutics. Therefore, further investigations are needed to fully understand the underlying mechanisms and the correlation between altered lipid metabolism and tumorigenesis.
Acknowledgements
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2020R1A2C1103139 to D.-H.K.; NRF-2022M3A9F3016364 to N.-Y.S.; NRF-2020R1A2C2008672 and RS-2023-00217123 to H.Y).
Declarations
Conflict of interest
The authors declared no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Do-Hee Kim and Na-Young Song have contributed equally to this study.
References
- Abenavoli L, Capasso R, Milic N, Capasso F. Milk thistle in liver diseases: past, present, future. Phytother Res. 2010;24:1423–1432. doi: 10.1002/ptr.3207. [DOI] [PubMed] [Google Scholar]
- Abumrad NA, El-Maghrabi MR, Amri EZ, Lopez E, Grimaldi PA. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J Biol Chem. 1993;268:17665–17668. doi: 10.1016/S0021-9258(17)46753-6. [DOI] [PubMed] [Google Scholar]
- Aguirre-Portoles C, Feliu J, Reglero G, Ramirez de Molina A. ABCA1 overexpression worsens colorectal cancer prognosis by facilitating tumour growth and caveolin-1-dependent invasiveness, and these effects can be ameliorated using the BET inhibitor apabetalone. Mol Oncol. 2018;12:1735–1752. doi: 10.1002/1878-0261.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam M, Ali S, Ashraf GM, Bilgrami AL, Yadav DK, Hassan MI. Epigallocatechin 3-gallate: from green tea to cancer therapeutics. Food Chem. 2022;379:132135. doi: 10.1016/j.foodchem.2022.132135. [DOI] [PubMed] [Google Scholar]
- Alsaggar M, Bdour S, Ababneh Q, El-Elimat T, Qinna N, Alzoubi KH. Silibinin attenuates adipose tissue inflammation and reverses obesity and its complications in diet-induced obesity model in mice. BMC Pharmacol Toxicol. 2020;21:8. doi: 10.1186/s40360-020-0385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade JM, Paraiso AF, de Oliveira MV, Martins AM, Neto JF, Guimaraes AL, de Paula AM, Qureshi M, Santos SH. Resveratrol attenuates hepatic steatosis in high-fat fed mice by decreasing lipogenesis and inflammation. Nutrition. 2014;30:915–919. doi: 10.1016/j.nut.2013.11.016. [DOI] [PubMed] [Google Scholar]
- Arita Y, Nishimura S, Ishitsuka R, Kishimoto T, Ikenouchi J, Ishii K, Umeda M, Matsunaga S, Kobayashi T, Yoshida M. Targeting cholesterol in a liquid-disordered environment by theonellamides modulates cell membrane order and cell shape. Chem Biol. 2015;22:604–610. doi: 10.1016/j.chembiol.2015.04.011. [DOI] [PubMed] [Google Scholar]
- Attane C, Milhas D, Hoy AJ, Muller C. Metabolic remodeling induced by adipocytes: a new Achilles' Heel in invasive breast cancer? Curr Med Chem. 2020;27:3984–4001. doi: 10.2174/0929867325666180426165001. [DOI] [PubMed] [Google Scholar]
- Baenke F, Peck B, Miess H, Schulze A. Hooked on fat: the role of lipid synthesis in cancer metabolism and tumour development. Dis Model Mech. 2013;6:1353–1363. doi: 10.1242/dmm.011338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer DE, Hatzivassiliou G, Zhao F, Andreadis C, Thompson CB. ATP citrate lyase is an important component of cell growth and transformation. Oncogene. 2005;24:6314–6322. doi: 10.1038/sj.onc.1208773. [DOI] [PubMed] [Google Scholar]
- Bauskin AR, Brown DA, Kuffner T, Johnen H, Luo XW, Hunter M, Breit SN. Role of macrophage inhibitory cytokine-1 in tumorigenesis and diagnosis of cancer. Cancer Res. 2006;66:4983–4986. doi: 10.1158/0008-5472.CAN-05-4067. [DOI] [PubMed] [Google Scholar]
- Berod L, Friedrich C, Nandan A, Freitag J, Hagemann S, Harmrolfs K, Sandouk A, Hesse C, Castro CN, Bahre H, Tschirner SK, Gorinski N, Gohmert M, Mayer CT, Huehn J, Ponimaskin E, Abraham WR, Muller R, Lochner M, Sparwasser T. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat Med. 2014;20:1327–1333. doi: 10.1038/nm.3704. [DOI] [PubMed] [Google Scholar]
- Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, Zhang HP, Donnellan M, Mahler S, Pryor K, Walsh BJ, Nicholson RC, Fairlie WD, Por SB, Robbins JM, Breit SN. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci USA. 1997;94:11514–11519. doi: 10.1073/pnas.94.21.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandi J, Dando I, Pozza ED, Biondani G, Jenkins R, Elliott V, Park K, Fanelli G, Zolla L, Costello E, Scarpa A, Cecconi D, Palmieri M. Proteomic analysis of pancreatic cancer stem cells: functional role of fatty acid synthesis and mevalonate pathways. J Proteomics. 2017;150:310–322. doi: 10.1016/j.jprot.2016.10.002. [DOI] [PubMed] [Google Scholar]
- Brusselmans K, De Schrijver E, Heyns W, Verhoeven G, Swinnen JV. Epigallocatechin-3-gallate is a potent natural inhibitor of fatty acid synthase in intact cells and selectively induces apoptosis in prostate cancer cells. Int J Cancer. 2003;106:856–862. doi: 10.1002/ijc.11317. [DOI] [PubMed] [Google Scholar]
- Brusselmans K, De Schrijver E, Verhoeven G, Swinnen JV. RNA interference-mediated silencing of the acetyl-CoA-carboxylase-alpha gene induces growth inhibition and apoptosis of prostate cancer cells. Cancer Res. 2005;65:6719–6725. doi: 10.1158/0008-5472.CAN-05-0571. [DOI] [PubMed] [Google Scholar]
- Bruzzese F, Hagglof C, Leone A, Sjoberg E, Roca MS, Kiflemariam S, Sjoblom T, Hammarsten P, Egevad L, Bergh A, Ostman A, Budillon A, Augsten M. Local and systemic protumorigenic effects of cancer-associated fibroblast-derived GDF15. Cancer Res. 2014;74:3408–3417. doi: 10.1158/0008-5472.CAN-13-2259. [DOI] [PubMed] [Google Scholar]
- Bui TM, Yalom LK, Sumagin R. Tumor-associated neutrophils: orchestrating cancer pathobiology and therapeutic resistance. Expert Opin Ther Targets. 2021;25:573–583. doi: 10.1080/14728222.2021.1954162. [DOI] [PubMed] [Google Scholar]
- Bulusu V, Tumanov S, Michalopoulou E, van den Broek NJ, MacKay G, Nixon C, Dhayade S, Schug ZT, Vande Voorde J, Blyth K, Gottlieb E, Vazquez A, Kamphorst JJ. Acetate recapturing by nuclear acetyl-CoA synthetase 2 prevents loss of histone acetylation during oxygen and serum limitation. Cell Rep. 2017;18:647–658. doi: 10.1016/j.celrep.2016.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y. Adipocyte and lipid metabolism in cancer drug resistance. J Clin Investig. 2019;129:3006–3017. doi: 10.1172/JCI127201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Lin KY, Peng KY, Day YJ, Hung LM. Resveratrol exerts anti-obesity effects in high-fat diet obese mice and displays differential dosage effects on cytotoxicity, differentiation, and lipolysis in 3T3-L1 cells. Endocr J. 2016;63:169–178. doi: 10.1507/endocrj.EJ15-0545. [DOI] [PubMed] [Google Scholar]
- Chatham JC, Zhang J, Wende AR. Role of O-linked N-acetylglucosamine protein modification in cellular physiology. Physiol Rev. 2021;101:427–493. doi: 10.1152/physrev.00043.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez-Tapia NC, Rosso N, Tiribelli C. Effect of intracellular lipid accumulation in a new model of non-alcoholic fatty liver disease. BMC Gastroenterol. 2012;12:20. doi: 10.1186/1471-230X-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che L, Chi W, Qiao Y, Zhang J, Song X, Liu Y, Li L, Jia J, Pilo MG, Wang J, Cigliano A, Ma Z, Kuang W, Tang Z, Zhang Z, Shui G, Ribback S, Dombrowski F, Evert M, Pascale RM, Cossu C, Pes GM, Osborne TF, Calvisi DF, Chen X, Chen L. Cholesterol biosynthesis supports the growth of hepatocarcinoma lesions depleted of fatty acid synthase in mice and humans. Gut. 2020;69:177–186. doi: 10.1136/gutjnl-2018-317581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Zhuang X, Lin L, Yu P, Wang Y, Shi Y, Hu G, Sun Y. New horizons in tumor microenvironment biology: challenges and opportunities. BMC Med. 2015;13:45. doi: 10.1186/s12916-015-0278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Xu M, Nagati J, Garcia JA. Coordinate regulation of stress signaling and epigenetic events by Acss2 and HIF-2 in cancer cells. PLoS ONE. 2017;12:e0190241. doi: 10.1371/journal.pone.0190241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Zhang J, Sampieri K, Clohessy JG, Mendez L, Gonzalez-Billalabeitia E, Liu XS, Lee YR, Fung J, Katon JM, Menon AV, Webster KA, Ng C, Palumbieri MD, Diolombi MS, Breitkopf SB, Teruya-Feldstein J, Signoretti S, Bronson RT, Asara JM, Castillo-Martin M, Cordon-Cardo C, Pandolfi PP. An aberrant SREBP-dependent lipogenic program promotes metastatic prostate cancer. Nat Genet. 2018;50:206–218. doi: 10.1038/s41588-017-0027-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhu H, Yin Y, Jia S, Luo X. Colorectal cancer: metabolic interactions reshape the tumor microenvironment. Biochim Biophys Acta Rev Cancer. 2022;1877:188797. doi: 10.1016/j.bbcan.2022.188797. [DOI] [PubMed] [Google Scholar]
- Cheng HS, Lee JXT, Wahli W, Tan NS. Exploiting vulnerabilities of cancer by targeting nuclear receptors of stromal cells in tumor microenvironment. Mol Cancer. 2019;18:51. doi: 10.1186/s12943-019-0971-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung CW, Gibbons N, Johnson DW, Nicol DL. Silibinin—a promising new treatment for cancer. Anticancer Agents Med Chem. 2010;10:186–195. doi: 10.2174/1871520611009030186. [DOI] [PubMed] [Google Scholar]
- Choi C, Song HD, Son Y, Cho YK, Ahn SY, Jung YS, Yoon YC, Kwon SW, Lee YH. Epigallocatechin-3-gallate reduces visceral adiposity partly through the regulation of beclin1-dependent autophagy in white adipose tissues. Nutrients. 2020 doi: 10.3390/nu12103072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo M, Mai VH, Kim HS, Kim DH, Ku JL, Lee SK, Park CK, An YJ, Park S. Involvement of cell shape and lipid metabolism in glioblastoma resistance to temozolomide. Acta Pharmacol Sin. 2023;44:670–679. doi: 10.1038/s41401-022-00984-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S, Kar A, Bhowmik D, Gautam A, Basak D, Sarkar I, Ghosh P, Sarkar D, Deka A, Chakraborty P, Mukhopadhyay A, Mehrotra S, Basak S, Paul S, Chatterjee S. Intracellular acetyl CoA potentiates the therapeutic efficacy of antitumor CD8+ T cells. Cancer Res. 2022;82:2640–2655. doi: 10.1158/0008-5472.CAN-21-4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church SE, Jensen SM, Antony PA, Restifo NP, Fox BA. Tumor-specific CD4+ T cells maintain effector and memory tumor-specific CD8+ T cells. Eur J Immunol. 2014;44:69–79. doi: 10.1002/eji.201343718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chypre M, Zaidi N, Smans K. ATP-citrate lyase: a mini-review. Biochem Biophys Res Commun. 2012;422:1–4. doi: 10.1016/j.bbrc.2012.04.144. [DOI] [PubMed] [Google Scholar]
- Colacino JA, McDermott SP, Sartor MA, Wicha MS, Rozek LS. Transcriptomic profiling of curcumin-treated human breast stem cells identifies a role for stearoyl-CoA desaturase in breast cancer prevention. Breast Cancer Res Treat. 2016;158:29–41. doi: 10.1007/s10549-016-3854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comerford SA, Huang Z, Du X, Wang Y, Cai L, Witkiewicz AK, Walters H, Tantawy MN, Fu A, Manning HC, Horton JD, Hammer RE, McKnight SL, Tu BP. Acetate dependence of tumors. Cell. 2014;159:1591–1602. doi: 10.1016/j.cell.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costet P, Luo Y, Wang N, Tall AR. Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J Biol Chem. 2000;275:28240–28245. doi: 10.1074/jbc.M003337200. [DOI] [PubMed] [Google Scholar]
- Covarrubias AJ, Aksoylar HI, Yu J, Snyder NW, Worth AJ, Iyer SS, Wang J, Ben-Sahra I, Byles V, Polynne-Stapornkul T, Espinosa EC, Lamming D, Manning BD, Zhang Y, Blair IA, Horng T. Akt-mTORC1 signaling regulates Acly to integrate metabolic input to control of macrophage activation. Elife. 2016 doi: 10.7554/eLife.11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz L, Soares P, Correia M. Ubiquitin-specific proteases: players in cancer cellular processes. Pharmaceuticals (basel) 2021 doi: 10.3390/ph14090848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daker M, Bhuvanendran S, Ahmad M, Takada K, Khoo AS. Deregulation of lipid metabolism pathway genes in nasopharyngeal carcinoma cells. Mol Med Rep. 2013;7:731–741. doi: 10.3892/mmr.2012.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SK, Eder S, Schauer S, Diwoky C, Temmel H, Guertl B, Gorkiewicz G, Tamilarasan KP, Kumari P, Trauner M, Zimmermann R, Vesely P, Haemmerle G, Zechner R, Hoefler G. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science. 2011;333:233–238. doi: 10.1126/science.1198973. [DOI] [PubMed] [Google Scholar]
- De Piano M, Manuelli V, Zadra G, Otte J, Edqvist PD, Ponten F, Nowinski S, Niaouris A, Grigoriadis A, Loda M, Van Hemelrijck M, Wells CM. Lipogenic signalling modulates prostate cancer cell adhesion and migration via modification of Rho GTPases. Oncogene. 2020;39:3666–3679. doi: 10.1038/s41388-020-1243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Piano M, Manuelli V, Zadra G, Loda M, Muir G, Chandra A, Morris J, Van Hemelrijck M, Wells CM. Exploring a role for fatty acid synthase in prostate cancer cell migration. Small GTPases. 2021;12:265–272. doi: 10.1080/21541248.2020.1826781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis RJ, Chandel NS. Fundamentals of cancer metabolism. Sci Adv. 2016;2:e1600200. doi: 10.1126/sciadv.1600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deep G, Kumar R, Nambiar DK, Jain AK, Ramteke AM, Serkova NJ, Agarwal C, Agarwal R. Silibinin inhibits hypoxia-induced HIF-1alpha-mediated signaling, angiogenesis and lipogenesis in prostate cancer cells: in vitro evidence and in vivo functional imaging and metabolomics. Mol Carcinog. 2017;56:833–848. doi: 10.1002/mc.22537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding M, Zhang S, Guo Y, Yao J, Shen Q, Huang M, Chen W, Yu S, Zheng Y, Lin Y, Yan W, Liu Z, Su T, Lu L. Tumor microenvironment acidity triggers lipid accumulation in liver cancer via SCD1 activation. Mol Cancer Res. 2022;20:810–822. doi: 10.1158/1541-7786.MCR-21-0699. [DOI] [PubMed] [Google Scholar]
- Dou HX, Wang T, Su HX, Gao DD, Xu YC, Li YX, Wang HY. Exogenous FABP4 interferes with differentiation, promotes lipolysis and inflammation in adipocytes. Endocrine. 2020;67:587–596. doi: 10.1007/s12020-019-02157-8. [DOI] [PubMed] [Google Scholar]
- Drury J, Rychahou PG, He D, Jafari N, Wang C, Lee EY, Weiss HL, Evers BM, Zaytseva YY. Inhibition of fatty acid synthase upregulates expression of CD36 to sustain proliferation of colorectal cancer cells. Front Oncol. 2020;10:1185. doi: 10.3389/fonc.2020.01185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury J, Young LEA, Scott TL, Kelson CO, He D, Liu J, Wu Y, Wang C, Weiss HL, Fan T, Gentry MS, Sun R, Zaytseva YY. Tissue-specific downregulation of fatty acid synthase suppresses intestinal adenoma formation via coordinated reprograming of transcriptome and metabolism in the mouse model of Apc-driven colorectal cancer. Int J Mol Sci. 2022;23:6510. doi: 10.3390/ijms23126510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Q, Liu P, Zhang C, Liu T, Wang W, Shang C, Wu J, Liao Y, Chen Y, Huang J, Tan H, Zhao Y, Xia M, Liu J, Yao S. FASN promotes lymph node metastasis in cervical cancer via cholesterol reprogramming and lymphangiogenesis. Cell Death Dis. 2022;13:488. doi: 10.1038/s41419-022-04926-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducheix S, Peres C, Hardfeldt J, Frau C, Mocciaro G, Piccinin E, Lobaccaro JM, De Santis S, Chieppa M, Bertrand-Michel J, Plateroti M, Griffin JL, Sabba C, Ntambi JM, Moschetta A. Deletion of stearoyl-CoA desaturase-1 from the intestinal epithelium promotes inflammation and tumorigenesis, reversed by dietary oleate. Gastroenterology. 2018;155:1524–1538. doi: 10.1053/j.gastro.2018.07.032. [DOI] [PubMed] [Google Scholar]
- Ducheix S, Piccinin E, Peres C, Garcia-Irigoyen O, Bertrand-Michel J, Fouache A, Cariello M, Lobaccaro JM, Guillou H, Sabba C, Ntambi JM, Moschetta A. Reduction in gut-derived MUFAs via intestinal stearoyl-CoA desaturase 1 deletion drives susceptibility to NAFLD and hepatocarcinoma. Hepatol Commun. 2022;6:2937–2949. doi: 10.1002/hep4.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejaz A, Wu D, Kwan P, Meydani M. Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice. J Nutr. 2009;139:919–925. doi: 10.3945/jn.108.100966. [DOI] [PubMed] [Google Scholar]
- Endemann G, Stanton LW, Madden KS, Bryant CM, White RT, Protter AA. CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem. 1993;268:11811–11816. doi: 10.1016/S0021-9258(19)50272-1. [DOI] [PubMed] [Google Scholar]
- Endo Y, Asou HK, Matsugae N, Hirahara K, Shinoda K, Tumes DJ, Tokuyama H, Yokote K, Nakayama T. Obesity drives Th17 cell differentiation by inducing the lipid metabolic kinase, ACC1. Cell Rep. 2015;12:1042–1055. doi: 10.1016/j.celrep.2015.07.014. [DOI] [PubMed] [Google Scholar]
- Endo Y, Onodera A, Obata-Ninomiya K, Koyama-Nasu R, Asou HK, Ito T, Yamamoto T, Kanno T, Nakajima T, Ishiwata K, Kanuka H, Tumes DJ, Nakayama T. ACC1 determines memory potential of individual CD4(+) T cells by regulating de novo fatty acid biosynthesis. Nat Metab. 2019;1:261–275. doi: 10.1038/s42255-018-0025-4. [DOI] [PubMed] [Google Scholar]
- Falchook G, Infante J, Arkenau HT, Patel MR, Dean E, Borazanci E, Brenner A, Cook N, Lopez J, Pant S, Frankel A, Schmid P, Moore K, McCulloch W, Grimmer K, O'Farrell M, Kemble G, Burris H. First-in-human study of the safety, pharmacokinetics, and pharmacodynamics of first-in-class fatty acid synthase inhibitor TVB-2640 alone and with a taxane in advanced tumors. EClinicalMedicine. 2021;34:100797. doi: 10.1016/j.eclinm.2021.100797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H, Tian W, Ma X. Curcumin induces apoptosis of HepG2 cells via inhibiting fatty acid synthase. Target Oncol. 2014;9:279–286. doi: 10.1007/s11523-013-0286-5. [DOI] [PubMed] [Google Scholar]
- Fan H, Liang Y, Jiang B, Li X, Xun H, Sun J, He W, Lau HT, Ma X. Curcumin inhibits intracellular fatty acid synthase and induces apoptosis in human breast cancer MDA-MB-231 cells. Oncol Rep. 2016;35:2651–2656. doi: 10.3892/or.2016.4682. [DOI] [PubMed] [Google Scholar]
- Fan H, Ma X, Lin P, Kang Q, Zhao Z, Wang L, Sun D, Cheng J, Li Y. Scutellarin prevents nonalcoholic fatty liver disease (NAFLD) and hyperlipidemia via PI3K/AKT-dependent activation of nuclear factor (erythroid-derived 2)-like 2 (Nrf2) in rats. Med Sci Monit. 2017;23:5599–5612. doi: 10.12659/msm.907530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez LP, Gomez de Cedron M, Ramirez de Molina A. Alterations of lipid metabolism in cancer: Implications in prognosis and treatment. Front Oncol. 2020;10:577420. doi: 10.3389/fonc.2020.577420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fhu CW, Ali A. Fatty acid synthase: an emerging target in cancer. Molecules. 2020 doi: 10.3390/molecules25173935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaveny CA, Griffett K, El-Gendy Bel D, Kazantzis M, Sengupta M, Amelio AL, Chatterjee A, Walker J, Solt LA, Kamenecka TM, Burris TP. Broad anti-tumor activity of a small molecule that selectively targets the Warburg effect and lipogenesis. Cancer Cell. 2015;28:42–56. doi: 10.1016/j.ccell.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavin R, Peluso S, Nguyen PL, Loda M. Fatty acid synthase as a potential therapeutic target in cancer. Future Oncol. 2010;6:551–562. doi: 10.2217/fon.10.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Ogasawara Y, Hayashi H, Inoue K, Sakashita H. Resveratrol inhibits proliferation and induces autophagy by blocking SREBP1 expression in oral cancer cells. Molecules. 2022 doi: 10.3390/molecules27238250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuhashi M. Fatty acid-binding protein 4 in cardiovascular and metabolic diseases. J Atheroscler Thromb. 2019;26:216–232. doi: 10.5551/jat.48710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuhashi M, Hotamisligil GS. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov. 2008;7:489–503. doi: 10.1038/nrd2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbi C, Warner M, Gustafsson JA. Action mechanisms of Liver X Receptors. Biochem Biophys Res Commun. 2014;446:647–650. doi: 10.1016/j.bbrc.2013.11.077. [DOI] [PubMed] [Google Scholar]
- Gajate C, Mollinedo F. The antitumor ether lipid ET-18-OCH(3) induces apoptosis through translocation and capping of Fas/CD95 into membrane rafts in human leukemic cells. Blood. 2001;98:3860–3863. doi: 10.1182/blood.v98.13.3860. [DOI] [PubMed] [Google Scholar]
- Gao AM, Ke ZP, Wang JN, Yang JY, Chen SY, Chen H. Apigenin sensitizes doxorubicin-resistant hepatocellular carcinoma BEL-7402/ADM cells to doxorubicin via inhibiting PI3K/Akt/Nrf2 pathway. Carcinogenesis. 2013;34:1806–1814. doi: 10.1093/carcin/bgt108. [DOI] [PubMed] [Google Scholar]
- Gao X, Lin SH, Ren F, Li JT, Chen JJ, Yao CB, Yang HB, Jiang SX, Yan GQ, Wang D, Wang Y, Liu Y, Cai Z, Xu YY, Chen J, Yu W, Yang PY, Lei QY. Acetate functions as an epigenetic metabolite to promote lipid synthesis under hypoxia. Nat Commun. 2016;7:11960. doi: 10.1038/ncomms11960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao AM, Zhang XY, Hu JN, Ke ZP. Apigenin sensitizes hepatocellular carcinoma cells to doxorubic through regulating miR-520b/ATG7 axis. Chem Biol Interact. 2018;280:45–50. doi: 10.1016/j.cbi.2017.11.020. [DOI] [PubMed] [Google Scholar]
- Gao J, Jung M, Williams RT, Hui D, Russell AJ, Naim AJ, Kamili A, Clifton M, Bongers A, Mayoh C, Ho G, Scott CL, Jessup W, Haber M, Norris MD, Henderson MJ, On Behalf of Australian Ovarian Cancer S Suppression of the ABCA1 cholesterol transporter impairs the growth and migration of epithelial ovarian cancer. Cancers (basel) 2022 doi: 10.3390/cancers14081878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Monzon C, Lo Iacono O, Crespo J, Romero-Gomez M, Garcia-Samaniego J, Fernandez-Bermejo M, Dominguez-Diez A, Rodriguez de Cia J, Saez A, Porrero JL, Vargas-Castrillon J, Chavez-Jimenez E, Soto-Fernandez S, Diaz A, Gallego-Duran R, Madejon A, Miquilena-Colina ME. Increased soluble CD36 is linked to advanced steatosis in nonalcoholic fatty liver disease. Eur J Clin Investig. 2014;44:65–73. doi: 10.1111/eci.12192. [DOI] [PubMed] [Google Scholar]
- Gelsomino G, Corsetto PA, Campia I, Montorfano G, Kopecka J, Castella B, Gazzano E, Ghigo D, Rizzo AM, Riganti C. Omega 3 fatty acids chemosensitize multidrug resistant colon cancer cells by down-regulating cholesterol synthesis and altering detergent resistant membranes composition. Mol Cancer. 2013;12:137. doi: 10.1186/1476-4598-12-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghayee HK, Auchus RJ. Basic concepts and recent developments in human steroid hormone biosynthesis. Rev Endocr Metab Disord. 2007;8:289–300. doi: 10.1007/s11154-007-9052-2. [DOI] [PubMed] [Google Scholar]
- Gholkar AA, Cheung K, Williams KJ, Lo YC, Hamideh SA, Nnebe C, Khuu C, Bensinger SJ, Torres JZ. Fatostatin inhibits cancer cell proliferation by affecting mitotic microtubule spindle assembly and cell division. J Biol Chem. 2016;291:17001–17008. doi: 10.1074/jbc.C116.737346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno-Mallench L, Mas-Bargues C, Ingles M, Olaso G, Borras C, Gambini J, Vina J. Resveratrol shifts energy metabolism to increase lipid oxidation in healthy old mice. Biomed Pharmacother. 2019;118:109130. doi: 10.1016/j.biopha.2019.109130. [DOI] [PubMed] [Google Scholar]
- Glatz JFC, Luiken J. Dynamic role of the transmembrane glycoprotein CD36 (SR-B2) in cellular fatty acid uptake and utilization. J Lipid Res. 2018;59:1084–1093. doi: 10.1194/jlr.R082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatz JF, Luiken JJ, Bonen A. Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol Rev. 2010;90:367–417. doi: 10.1152/physrev.00003.2009. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell. 2006;124:35–46. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Gomez-Zorita S, Treguer K, Mercader J, Carpene C. Resveratrol directly affects in vitro lipolysis and glucose transport in human fat cells. J Physiol Biochem. 2013;69:585–593. doi: 10.1007/s13105-012-0229-0. [DOI] [PubMed] [Google Scholar]
- Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 2018;32:1267–1284. doi: 10.1101/gad.314617.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens P, Rodriguez-Vita J, Etzerodt A, Masse M, Rastoin O, Gouirand V, Ulas T, Papantonopoulou O, Van Eck M, Auphan-Anezin N, Bebien M, Verthuy C, Vu Manh TP, Turner M, Dalod M, Schultze JL, Lawrence T. Membrane cholesterol efflux drives tumor-associated macrophage reprogramming and tumor progression. Cell Metab. 2019;29:1376–1389.e1374. doi: 10.1016/j.cmet.2019.02.016. [DOI] [PubMed] [Google Scholar]
- Graner E, Tang D, Rossi S, Baron A, Migita T, Weinstein LJ, Lechpammer M, Huesken D, Zimmermann J, Signoretti S, Loda M. The isopeptidase USP2a regulates the stability of fatty acid synthase in prostate cancer. Cancer Cell. 2004;5:253–261. doi: 10.1016/s1535-6108(04)00055-8. [DOI] [PubMed] [Google Scholar]
- Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51:27–41. doi: 10.1016/j.immuni.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guaita-Esteruelas S, Guma J, Masana L, Borras J. The peritumoural adipose tissue microenvironment and cancer. The roles of fatty acid binding protein 4 and fatty acid binding protein 5. Mol Cell Endocrinol. 2018;462:107–118. doi: 10.1016/j.mce.2017.02.002. [DOI] [PubMed] [Google Scholar]
- Gupta S, Roy A, Dwarakanath BS. Metabolic cooperation and competition in the tumor microenvironment: implications for therapy. Front Oncol. 2017;7:68. doi: 10.3389/fonc.2017.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyamfi J, Yeo JH, Kwon D, Min BS, Cha YJ, Koo JS, Jeong J, Lee J, Choi J. Interaction between CD36 and FABP4 modulates adipocyte-induced fatty acid import and metabolism in breast cancer. NPJ Breast Cancer. 2021;7:129. doi: 10.1038/s41523-021-00324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JY, Lee SH, Yoo NJ, Hyung LS, Moon YJ, Yun T, Kim HT, Lee JS. A randomized phase II study of gefitinib plus simvastatin versus gefitinib alone in previously treated patients with advanced non-small cell lung cancer. Clin Cancer Res. 2011;17:1553–1560. doi: 10.1158/1078-0432.CCR-10-2525. [DOI] [PubMed] [Google Scholar]
- Harayama T, Riezman H. Understanding the diversity of membrane lipid composition. Nat Rev Mol Cell Biol. 2018;19:281–296. doi: 10.1038/nrm.2017.138. [DOI] [PubMed] [Google Scholar]
- Hatipoglu A, Menon D, Levy T, Frias MA, Foster DA. Inhibiting glutamine utilization creates a synthetic lethality for suppression of ATP citrate lyase in KRas-driven cancer cells. PLoS ONE. 2022;17:e0276579. doi: 10.1371/journal.pone.0276579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzivassiliou G, Zhao F, Bauer DE, Andreadis C, Shaw AN, Dhanak D, Hingorani SR, Tuveson DA, Thompson CB. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell. 2005;8:311–321. doi: 10.1016/j.ccr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Hong SY, Ha AW, Kim W. Effects of quercetin on cell differentiation and adipogenesis in 3T3-L1 adipocytes. Nutr Res Pract. 2021;15:444–455. doi: 10.4162/nrp.2021.15.4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou CY, Tain YL, Yu HR, Huang LT. The effects of resveratrol in the treatment of metabolic syndrome. Int J Mol Sci. 2019 doi: 10.3390/ijms20030535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh V, Kim MJ, Gelissen IC, Brown AJ, Sandoval C, Hallab JC, Kockx M, Traini M, Jessup W, Kritharides L. Cellular cholesterol regulates ubiquitination and degradation of the cholesterol export proteins ABCA1 and ABCG1. J Biol Chem. 2014;289:7524–7536. doi: 10.1074/jbc.M113.515890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu MC, Guo BC, Chen CH, Hu PA, Lee TS. Apigenin ameliorates hepatic lipid accumulation by activating the autophagy-mitochondria pathway. J Food Drug Anal. 2021;29:240–254. doi: 10.38212/2224-6614.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Zhang Z, Shen WJ, Azhar S. Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutr Metab (lond) 2010;7:47. doi: 10.1186/1743-7075-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, Narita S, Numakura K, Tsuruta H, Saito M, Inoue T, Horikawa Y, Tsuchiya N, Habuchi T. A high-fat diet enhances proliferation of prostate cancer cells and activates MCP-1/CCR2 signaling. Prostate. 2012;72:1779–1788. doi: 10.1002/pros.22531. [DOI] [PubMed] [Google Scholar]
- Huang M, Narita S, Inoue T, Tsuchiya N, Satoh S, Nanjo H, Sasaki T, Habuchi T. Diet-induced macrophage inhibitory cytokine 1 promotes prostate cancer progression. Endocr Relat Cancer. 2014;21:39–50. doi: 10.1530/ERC-13-0227. [DOI] [PubMed] [Google Scholar]
- Huang J, Fan XX, He J, Pan H, Li RZ, Huang L, Jiang Z, Yao XJ, Liu L, Leung EL, He JX. SCD1 is associated with tumor promotion, late stage and poor survival in lung adenocarcinoma. Oncotarget. 2016;7:39970–39979. doi: 10.18632/oncotarget.9461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, Narita S, Koizumi A, Nara T, Numakura K, Satoh S, Nanjo H, Habuchi T. Macrophage inhibitory cytokine-1 induced by a high-fat diet promotes prostate cancer progression by stimulating tumor-promoting cytokine production from tumor stromal cells. Cancer Commun (lond) 2021;41:389–403. doi: 10.1002/cac2.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iftikhar R, Penrose HM, King AN, Samudre JS, Collins ME, Hartono AB, Lee SB, Lau F, Baddoo M, Flemington EF, Crawford SE, Savkovic SD. Elevated ATGL in colon cancer cells and cancer stem cells promotes metabolic and tumorigenic reprogramming reinforced by obesity. Oncogenesis. 2021;10:82. doi: 10.1038/s41389-021-00373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Nakamae I, Kato JY, Yoneda-Kato N. Stabilization of fatty acid synthesis enzyme acetyl-CoA carboxylase 1 suppresses acute myeloid leukemia development. J Clin Investig. 2021 doi: 10.1172/JCI141529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon SM, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485:661–665. doi: 10.1038/nature11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Fang X, Wang H, Li D, Wang X. Ovarian cancer-intrinsic fatty acid synthase prevents anti-tumor immunity by disrupting tumor-infiltrating dendritic cells. Front Immunol. 2018;9:2927. doi: 10.3389/fimmu.2018.02927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Wu N, Xu B, Chu Y, Li X, Su S, Chen D, Li W, Shi Y, Gao X, Zhang H, Zhang Z, Du W, Nie Y, Liang J, Fan D. Fatty acid-induced CD36 expression via O-GlcNAcylation drives gastric cancer metastasis. Theranostics. 2019;9:5359–5373. doi: 10.7150/thno.34024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AC, Antillon KS, Jenkins SM, Janos SN, Overton HN, Shoshan DS, Fischer EG, Trujillo KA, Bisoffi M. Prostate field cancerization: deregulated expression of macrophage inhibitory cytokine 1 (MIC-1) and platelet derived growth factor A (PDGF-A) in tumor adjacent tissue. PLoS ONE. 2015;10:e0119314. doi: 10.1371/journal.pone.0119314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JE, Esler WP, Patel R, Lanba A, Vera NB, Pfefferkorn JA, Vernochet C. Inhibition of acetyl-CoA carboxylase 1 (ACC1) and 2 (ACC2) reduces proliferation and de novo lipogenesis of EGFRvIII human glioblastoma cells. PLoS ONE. 2017;12:e0169566. doi: 10.1371/journal.pone.0169566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CH, Cho I, Ahn J, Jeon TI, Ha TY. Quercetin reduces high-fat diet-induced fat accumulation in the liver by regulating lipid metabolism genes. Phytother Res. 2013;27:139–143. doi: 10.1002/ptr.4687. [DOI] [PubMed] [Google Scholar]
- Ka SO, Kim KA, Kwon KB, Park JW, Park BH. Silibinin attenuates adipogenesis in 3T3-L1 preadipocytes through a potential upregulation of the insig pathway. Int J Mol Med. 2009;23:633–637. doi: 10.3892/ijmm_00000174. [DOI] [PubMed] [Google Scholar]
- Kamisuki S, Mao Q, Abu-Elheiga L, Gu Z, Kugimiya A, Kwon Y, Shinohara T, Kawazoe Y, Sato S, Asakura K, Choo HY, Sakai J, Wakil SJ, Uesugi M. A small molecule that blocks fat synthesis by inhibiting the activation of SREBP. Chem Biol. 2009;16:882–892. doi: 10.1016/j.chembiol.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Kamphorst JJ, Chung MK, Fan J, Rabinowitz JD. Quantitative analysis of acetyl-CoA production in hypoxic cancer cells reveals substantial contribution from acetate. Cancer Metab. 2014;2:23. doi: 10.1186/2049-3002-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Ozawa S, Miyamoto C, Maehata Y, Suzuki A, Maeda T, Baba Y. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013;13:89. doi: 10.1186/1475-2867-13-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MA, Barrera GC, Nakamura K, Baldan A, Tarr P, Fishbein MC, Frank J, Francone OL, Edwards PA. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005;1:121–131. doi: 10.1016/j.cmet.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Kerk SA, Papagiannakopoulos T, Shah YM, Lyssiotis CA. Metabolic networks in mutant KRAS-driven tumours: tissue specificities and the microenvironment. Nat Rev Cancer. 2021;21:510–525. doi: 10.1038/s41568-021-00375-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A, Aljarbou AN, Aldebasi YH, Faisal SM, Khan MA. Resveratrol suppresses the proliferation of breast cancer cells by inhibiting fatty acid synthase signaling pathway. Cancer Epidemiol. 2014;38:765–772. doi: 10.1016/j.canep.2014.09.006. [DOI] [PubMed] [Google Scholar]
- Khiewkamrop P, Surangkul D, Srikummool M, Richert L, Pekthong D, Parhira S, Somran J, Srisawang P. Epigallocatechin gallate triggers apoptosis by suppressing de novo lipogenesis in colorectal carcinoma cells. FEBS Open Bio. 2022;12:937–958. doi: 10.1002/2211-5463.13391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Ntambi JM. Regulation of stearoyl-CoA desaturase genes: role in cellular metabolism and preadipocyte differentiation. Biochem Biophys Res Commun. 1999;266:1–4. doi: 10.1006/bbrc.1999.1704. [DOI] [PubMed] [Google Scholar]
- Kim H, Sakamoto K. (−)-Epigallocatechin gallate suppresses adipocyte differentiation through the MEK/ERK and PI3K/Akt pathways. Cell Biol Int. 2012;36:147–153. doi: 10.1042/CBI20110047. [DOI] [PubMed] [Google Scholar]
- Kim S, Jin Y, Choi Y, Park T. Resveratrol exerts anti-obesity effects via mechanisms involving down-regulation of adipogenic and inflammatory processes in mice. Biochem Pharmacol. 2011;81:1343–1351. doi: 10.1016/j.bcp.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Kim SN, Kwon HJ, Akindehin S, Jeong HW, Lee YH. Effects of epigallocatechin-3-gallate on autophagic lipolysis in adipocytes. Nutrients. 2017 doi: 10.3390/nu9070680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HM, Lee YK, Kim ES, Koo JS. Energy transfer from adipocytes to cancer cells in breast cancer. Neoplasma. 2020;67:992–1001. doi: 10.4149/neo_2020_191017N1050. [DOI] [PubMed] [Google Scholar]
- Ko JH, Sethi G, Um JY, Shanmugam MK, Arfuso F, Kumar AP, Bishayee A, Ahn KS. The role of resveratrol in cancer therapy. Int J Mol Sci. 2017 doi: 10.3390/ijms18122589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobori M, Takahashi Y, Takeda H, Takahashi M, Izumi Y, Akimoto Y, Sakurai M, Oike H, Nakagawa T, Itoh M, Bamba T, Kimura T. Dietary intake of curcumin improves eIF2 signaling and reduces lipid levels in the white adipose tissue of obese mice. Sci Rep. 2018;8:9081. doi: 10.1038/s41598-018-27105-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo A, Yamamoto S, Nakaki R, Shimamura T, Hamakubo T, Sakai J, Kodama T, Yoshida T, Aburatani H, Osawa T. Extracellular acidic pH activates the sterol regulatory element-binding protein 2 to promote tumor progression. Cell Rep. 2017;18:2228–2242. doi: 10.1016/j.celrep.2017.02.006. [DOI] [PubMed] [Google Scholar]
- Kong F, Li L, Du Y, Zhu H, Li Z, Kong X. Exosomal adrenomedullin derived from cancer-associated fibroblasts promotes lipolysis in adipose tissue. Gut. 2018;67:2226–2227. doi: 10.1136/gutjnl-2017-315778. [DOI] [PubMed] [Google Scholar]
- Konings E, Timmers S, Boekschoten MV, Goossens GH, Jocken JW, Afman LA, Muller M, Schrauwen P, Mariman EC, Blaak EE. The effects of 30 days resveratrol supplementation on adipose tissue morphology and gene expression patterns in obese men. Int J Obes (lond) 2014;38:470–473. doi: 10.1038/ijo.2013.155. [DOI] [PubMed] [Google Scholar]
- Kuhajda FP, Pizer ES, Li JN, Mani NS, Frehywot GL, Townsend CA. Synthesis and antitumor activity of an inhibitor of fatty acid synthase. Proc Natl Acad Sci USA. 2000;97:3450–3454. doi: 10.1073/pnas.97.7.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lally JSV, Ghoshal S, DePeralta DK, Moaven O, Wei L, Masia R, Erstad DJ, Fujiwara N, Leong V, Houde VP, Anagnostopoulos AE, Wang A, Broadfield LA, Ford RJ, Foster RA, Bates J, Sun H, Wang T, Liu H, Ray AS, Saha AK, Greenwood J, Bhat S, Harriman G, Miao W, Rocnik JL, Westlin WF, Muti P, Tsakiridis T, Harwood HJ, Jr, Kapeller R, Hoshida Y, Tanabe KK, Steinberg GR, Fuchs BC. Inhibition of acetyl-CoA carboxylase by phosphorylation or the inhibitor ND-654 suppresses lipogenesis and hepatocellular carcinoma. Cell Metab. 2019;29:174–182.e175. doi: 10.1016/j.cmet.2018.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasa A, Schweiger M, Kotzbeck P, Churruca I, Simon E, Zechner R, Portillo MP. Resveratrol regulates lipolysis via adipose triglyceride lipase. J Nutr Biochem. 2012;23:379–384. doi: 10.1016/j.jnutbio.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Le May C, Berger JM, Lespine A, Pillot B, Prieur X, Letessier E, Hussain MM, Collet X, Cariou B, Costet P. Transintestinal cholesterol excretion is an active metabolic process modulated by PCSK9 and statin involving ABCB1. Arterioscler Thromb Vasc Biol. 2013;33:1484–1493. doi: 10.1161/ATVBAHA.112.300263. [DOI] [PubMed] [Google Scholar]
- Lee MS, Kim CT, Kim IH, Kim Y. Inhibitory effects of green tea catechin on the lipid accumulation in 3T3-L1 adipocytes. Phytother Res. 2009;23:1088–1091. doi: 10.1002/ptr.2737. [DOI] [PubMed] [Google Scholar]
- Lee BH, Taylor MG, Robinet P, Smith JD, Schweitzer J, Sehayek E, Falzarano SM, Magi-Galluzzi C, Klein EA, Ting AH. Dysregulation of cholesterol homeostasis in human prostate cancer through loss of ABCA1. Cancer Res. 2013;73:1211–1218. doi: 10.1158/0008-5472.CAN-12-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JV, Carrer A, Shah S, Snyder NW, Wei S, Venneti S, Worth AJ, Yuan ZF, Lim HW, Liu S, Jackson E, Aiello NM, Haas NB, Rebbeck TR, Judkins A, Won KJ, Chodosh LA, Garcia BA, Stanger BZ, Feldman MD, Blair IA, Wellen KE. Akt-dependent metabolic reprogramming regulates tumor cell histone acetylation. Cell Metab. 2014;20:306–319. doi: 10.1016/j.cmet.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legeay S, Rodier M, Fillon L, Faure S, Clere N. Epigallocatechin Gallate: a review of its beneficial properties to prevent metabolic syndrome. Nutrients. 2015;7:5443–5468. doi: 10.3390/nu7075230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wu S. Epigallocatechin gallate suppresses hepatic cholesterol synthesis by targeting SREBP-2 through SIRT1/FOXO1 signaling pathway. Mol Cell Biochem. 2018;448:175–185. doi: 10.1007/s11010-018-3324-x. [DOI] [PubMed] [Google Scholar]
- Li H, Huang D, Gao Z, Lv Y, Zhang L, Cui H, Zheng J. Scutellarin inhibits cell migration by regulating production of αvβ6 integrin and E-cadherin in human tongue cancer cells. Oncol Rep. 2010;24:1153–1160. doi: 10.3892/or_00000967. [DOI] [PubMed] [Google Scholar]
- Li X, Wang R, Zhou N, Wang X, Liu Q, Bai Y, Bai Y, Liu Z, Yang H, Zou J, Wang H, Shi T. Quercetin improves insulin resistance and hepatic lipid accumulation in vitro in a NAFLD cell model. Biomed Rep. 2013;1:71–76. doi: 10.3892/br.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Chen YT, Hu P, Huang WC. Fatostatin displays high antitumor activity in prostate cancer by blocking SREBP-regulated metabolic pathways and androgen receptor signaling. Mol Cancer Ther. 2014;13:855–866. doi: 10.1158/1535-7163.MCT-13-0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Gao C, Yan P, Zhang M, Wang Y, Hu Y, Wu X, Wang X, Sheng J. EGCG reduces obesity and white adipose tissue gain partly through AMPK activation in mice. Front Pharmacol. 2018;9:1366. doi: 10.3389/fphar.2018.01366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Lu M, Shi J, Gong Z, Hua L, Li Q, Lim B, Zhang XH, Chen X, Li S, Shultz LD, Ren G. Lung mesenchymal cells elicit lipid storage in neutrophils that fuel breast cancer lung metastasis. Nat Immunol. 2020;21:1444–1455. doi: 10.1038/s41590-020-0783-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Liu H, He J, Wang Z, Yin Z, You G, Wang Z, Davis RE, Lin P, Bergsagel PL, Manasanch EE, Wong STC, Esnaola NF, Chang JC, Orlowski RZ, Yi Q, Yang J. Acetyl-CoA synthetase 2: a critical linkage in obesity-induced tumorigenesis in myeloma. Cell Metab. 2021;33:78–93.e77. doi: 10.1016/j.cmet.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Lv X, Han C, Kong Y, Dai Z, Huo D, Li T, Li D, Li W, Wang X, Zhao Q, Ming J, Yang W, Chen Y, Wu X. Enhancer reprogramming promotes the activation of cancer-associated fibroblasts and breast cancer metastasis. Theranostics. 2022;12:7491–7508. doi: 10.7150/thno.75853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SA, Wei J, Nguyen TM, Shi H, Su W, Palacios G, Dhungana Y, Chapman NM, Long L, Saravia J, Vogel P, Chi H. Lipid signalling enforces functional specialization of T(reg) cells in tumours. Nature. 2021;591:306–311. doi: 10.1038/s41586-021-03235-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R, Tao R, Gao X, Li T, Zhou X, Guan KL, Xiong Y, Lei QY. Acetylation stabilizes ATP-citrate lyase to promote lipid biosynthesis and tumor growth. Mol Cell. 2013;51:506–518. doi: 10.1016/j.molcel.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin XL, Liu MH, Hu HJ, Feng HR, Fan XJ, Zou WW, Pan YQ, Hu XM, Wang Z. Curcumin enhanced cholesterol efflux by upregulating ABCA1 expression through AMPK-SIRT1-LXRalpha signaling in THP-1 macrophage-derived foam cells. DNA Cell Biol. 2015;34:561–572. doi: 10.1089/dna.2015.2866. [DOI] [PubMed] [Google Scholar]
- Liu C, Li C, Liu Y. The role of metabolic reprogramming in pancreatic cancer chemoresistance. Front Pharmacol. 2022;13:1108776. doi: 10.3389/fphar.2022.1108776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Liu N, Wang J, Fu S, Wang X, Chen D. Acetyl-CoA synthetase 2 as a therapeutic target in tumor metabolism. Cancers (basel) 2022 doi: 10.3390/cancers14122896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo J, Hamilton RJ, Masoomian M, Khurram N, Branchard E, Mullen PJ, Elbaz M, Hersey K, Chadwick D, Ghai S, Andrews DW, Chen EX, van der Kwast TH, Fleshner NE, Penn LZ. A pilot window-of-opportunity study of preoperative fluvastatin in localized prostate cancer. Prostate Cancer Prostatic Dis. 2020;23:630–637. doi: 10.1038/s41391-020-0221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K, Han M, Ting HL, Liu Z, Zhang D. Scutellarin from Scutellaria baicalensis suppresses adipogenesis by upregulating PPARα in 3T3-L1 cells. J Nat Prod. 2013;76:672–678. doi: 10.1021/np300889y. [DOI] [PubMed] [Google Scholar]
- Lu J, Meng Z, Cheng B, Liu M, Tao S, Guan S. Apigenin reduces the excessive accumulation of lipids induced by palmitic acid via the AMPK signaling pathway in HepG2 cells. Exp Ther Med. 2019;18:2965–2971. doi: 10.3892/etm.2019.7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Meng Z, Chen Y, Yu L, Gao B, Zheng Y, Guan S. Apigenin induced autophagy and stimulated autophagic lipid degradation. Food Funct. 2020;11:9208–9215. doi: 10.1039/d0fo00949k. [DOI] [PubMed] [Google Scholar]
- Luan H, Huo Z, Zhao Z, Zhang S, Huang Y, Shen Y, Wang P, Xi J, Liang J, Wu F. Scutellarin, a modulator of mTOR, attenuates hepatic insulin resistance by regulating hepatocyte lipid metabolism via SREBP-1c suppression. Phytother Res. 2020;34:1455–1466. doi: 10.1002/ptr.6582. [DOI] [PubMed] [Google Scholar]
- Luis G, Godfroid A, Nishiumi S, Cimino J, Blacher S, Maquoi E, Wery C, Collignon A, Longuespee R, Montero-Ruiz L, Dassoul I, Maloujahmoum N, Pottier C, Mazzucchelli G, Depauw E, Bellahcene A, Yoshida M, Noel A, Sounni NE. Tumor resistance to ferroptosis driven by Stearoyl-CoA Desaturase-1 (SCD1) in cancer cells and Fatty Acid Biding Protein-4 (FABP4) in tumor microenvironment promote tumor recurrence. Redox Biol. 2021;43:102006. doi: 10.1016/j.redox.2021.102006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Hong Y, Lu Y, Qiu S, Chaganty BK, Zhang L, Wang X, Li Q, Fan Z. Acetyl-CoA carboxylase rewires cancer metabolism to allow cancer cells to survive inhibition of the Warburg effect by cetuximab. Cancer Lett. 2017;384:39–49. doi: 10.1016/j.canlet.2016.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Bi E, Lu Y, Su P, Huang C, Liu L, Wang Q, Yang M, Kalady MF, Qian J, Zhang A, Gupte AA, Hamilton DJ, Zheng C, Yi Q. Cholesterol induces CD8(+) T cell exhaustion in the tumor microenvironment. Cell Metab. 2019;30:143–156.e145. doi: 10.1016/j.cmet.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Xiao L, Liu L, Ye L, Su P, Bi E, Wang Q, Yang M, Qian J, Yi Q. CD36-mediated ferroptosis dampens intratumoral CD8(+) T cell effector function and impairs their antitumor ability. Cell Metab. 2021;33:1001–1012.e1005. doi: 10.1016/j.cmet.2021.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man S, Yao J, Lv P, Liu Y, Yang L, Ma L. Curcumin-enhanced antitumor effects of sorafenib via regulating the metabolism and tumor microenvironment. Food Funct. 2020;11:6422–6432. doi: 10.1039/c9fo01901d. [DOI] [PubMed] [Google Scholar]
- Mao W, Cai Y, Chen D, Jiang G, Xu Y, Chen R, Wang F, Wang X, Zheng M, Zhao X, Mei J. Statin shapes inflamed tumor microenvironment and enhances immune checkpoint blockade in non-small cell lung cancer. JCI Insight. 2022;7:e161940. doi: 10.1172/jci.insight.161940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melana JP, Mignolli F, Stoyanoff T, Aguirre MV, Balboa MA, Balsinde J, Rodriguez JP. The hypoxic microenvironment induces stearoyl-CoA desaturase-1 overexpression and lipidomic profile changes in clear cell renal cell carcinoma. Cancers (basel) 2021;13:2962. doi: 10.3390/cancers13122962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miethe C, Nix H, Martin R, Hernandez AR, Price RS. Silibinin reduces the impact of obesity on invasive liver cancer. Nutr Cancer. 2017;69:1272–1280. doi: 10.1080/01635581.2017.1367935. [DOI] [PubMed] [Google Scholar]
- Migita T, Okabe S, Ikeda K, Igarashi S, Sugawara S, Tomida A, Taguchi R, Soga T, Seimiya H. Inhibition of ATP citrate lyase induces an anticancer effect via reactive oxygen species: AMPK as a predictive biomarker for therapeutic impact. Am J Pathol. 2013;182:1800–1810. doi: 10.1016/j.ajpath.2013.01.048. [DOI] [PubMed] [Google Scholar]
- Migita T, Okabe S, Ikeda K, Igarashi S, Sugawara S, Tomida A, Soga T, Taguchi R, Seimiya H. Inhibition of ATP citrate lyase induces triglyceride accumulation with altered fatty acid composition in cancer cells. Int J Cancer. 2014;135:37–47. doi: 10.1002/ijc.28652. [DOI] [PubMed] [Google Scholar]
- Miura D, Miura Y, Yagasaki K. Hypolipidemic action of dietary resveratrol, a phytoalexin in grapes and red wine, in hepatoma-bearing rats. Life Sci. 2003;73:1393–1400. doi: 10.1016/s0024-3205(03)00469-7. [DOI] [PubMed] [Google Scholar]
- Moon HS, Chung CS, Lee HG, Kim TG, Choi YJ, Cho CS. Inhibitory effect of (−)-epigallocatechin-3-gallate on lipid accumulation of 3T3-L1 cells. Obesity (silver Spring) 2007;15:2571–2582. doi: 10.1038/oby.2007.309. [DOI] [PubMed] [Google Scholar]
- Mounier C, Bouraoui L, Rassart E. Lipogenesis in cancer progression (review) Int J Oncol. 2014;45:485–492. doi: 10.3892/ijo.2014.2441. [DOI] [PubMed] [Google Scholar]
- Nagati JS, Xu M, Garcia T, Comerford SA, Hammer RE, Garcia JA. A substitution mutation in a conserved domain of mammalian acetate-dependent acetyl CoA synthetase 2 results in destabilized protein and impaired HIF-2 signaling. PLoS ONE. 2019;14:e0225105. doi: 10.1371/journal.pone.0225105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambiar DK, Deep G, Singh RP, Agarwal C, Agarwal R. Silibinin inhibits aberrant lipid metabolism, proliferation and emergence of androgen-independence in prostate cancer cells via primarily targeting the sterol response element binding protein 1. Oncotarget. 2014;5:10017–10033. doi: 10.18632/oncotarget.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, Romero IL, Carey MS, Mills GB, Hotamisligil GS, Yamada SD, Peter ME, Gwin K, Lengyel E. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi K, Suzuki K, Sawamoto J, Tokizawa Y, Iwase Y, Yumita N, Ikeda T. Inhibition of fatty acid synthesis induces apoptosis of human pancreatic cancer cells. Anticancer Res. 2016;36:4655–4660. doi: 10.21873/anticanres.11016. [DOI] [PubMed] [Google Scholar]
- Nosrati-Oskouie M, Aghili-Moghaddam NS, Sathyapalan T, Sahebkar A. Impact of curcumin on fatty acid metabolism. Phytother Res. 2021;35:4748–4762. doi: 10.1002/ptr.7105. [DOI] [PubMed] [Google Scholar]
- Nozhat Z, Heydarzadeh S, Memariani Z, Ahmadi A. Chemoprotective and chemosensitizing effects of apigenin on cancer therapy. Cancer Cell Int. 2021;21:574. doi: 10.1186/s12935-021-02282-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M, Fujimori K. Antiadipogenic effect of dietary apigenin through activation of AMPK in 3T3-L1 cells. J Agric Food Chem. 2011;59:13346–13352. doi: 10.1021/jf203490a. [DOI] [PubMed] [Google Scholar]
- Osinalde N, Mitxelena J, Sanchez-Quiles V, Akimov V, Aloria K, Arizmendi JM, Zubiaga AM, Blagoev B, Kratchmarova I. Nuclear phosphoproteomic screen uncovers ACLY as mediator of IL-2-induced proliferation of CD4+ T lymphocytes. Mol Cell Proteomics. 2016;15:2076–2092. doi: 10.1074/mcp.M115.057158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang M, Yu C, Deng X, Zhang Y, Zhang X, Duan F. O-GlcNAcylation and its role in cancer-associated inflammation. Front Immunol. 2022;13:861559. doi: 10.3389/fimmu.2022.861559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey PR, Okuda H, Watabe M, Pai SK, Liu W, Kobayashi A, Xing F, Fukuda K, Hirota S, Sugai T, Wakabayashi G, Koeda K, Kashiwaba M, Suzuki K, Chiba T, Endo M, Fujioka T, Tanji S, Mo YY, Cao D, Wilber AC, Watabe K. Resveratrol suppresses growth of cancer stem-like cells by inhibiting fatty acid synthase. Breast Cancer Res Treat. 2011;130:387–398. doi: 10.1007/s10549-010-1300-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare M, Darini CY, Yao X, Chignon-Sicard B, Rekima S, Lachambre S, Virolle V, Aguilar-Mahecha A, Basik M, Dani C, Ladoux A. Breast cancer mammospheres secrete Adrenomedullin to induce lipolysis and browning of adjacent adipocytes. BMC Cancer. 2020;20:784. doi: 10.1186/s12885-020-07273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasello M, Giudice AM, Scotlandi K. The ABC subfamily A transporters: multifaceted players with incipient potentialities in cancer. Semin Cancer Biol. 2020;60:57–71. doi: 10.1016/j.semcancer.2019.10.004. [DOI] [PubMed] [Google Scholar]
- Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce NJ, Yates JW, Berkhout TA, Jackson B, Tew D, Boyd H, Camilleri P, Sweeney P, Gribble AD, Shaw A, Groot PH. The role of ATP citrate-lyase in the metabolic regulation of plasma lipids. Hypolipidaemic effects of SB-204990, a lactone prodrug of the potent ATP citrate-lyase inhibitor SB-201076. Biochem J. 1998;334:113–119. doi: 10.1042/bj3340113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepino MY, Kuda O, Samovski D, Abumrad NA. Structure-function of CD36 and importance of fatty acid signal transduction in fat metabolism. Annu Rev Nutr. 2014;34:281–303. doi: 10.1146/annurev-nutr-071812-161220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova V, Annicchiarico-Petruzzelli M, Melino G, Amelio I. The hypoxic tumour microenvironment. Oncogenesis. 2018;7:10. doi: 10.1038/s41389-017-0011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Su Q, Li X, Shao M, Zhang Y, Yu F, Ni Y, Zhong J. Curcumin suppresses cell proliferation and reduces cholesterol absorption in Caco-2 cells by activating the TRPA1 channel. Lipids Health Dis. 2023;22:6. doi: 10.1186/s12944-022-01750-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Villa M, Sanin DE, Buck MD, O'Sullivan D, Ching R, Matsushita M, Grzes KM, Winkler F, Chang CH, Curtis JD, Kyle RL, Van Teijlingen BN, Corrado M, Haessler F, Alfei F, Edwards-Hicks J, Maggi LB, Jr, Zehn D, Egawa T, Bengsch B, Klein Geltink RI, Jenuwein T, Pearce EJ, Pearce EL. Acetate promotes T Cell effector function during glucose restriction. Cell Rep. 2019;27:2063–2074.e2065. doi: 10.1016/j.celrep.2019.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiee S, Bagherniya M, Askari G, Sathyapalan T, Jamialahmadi T, Sahebkar A. The effect of curcumin in improving lipid profile in patients with cardiovascular risk factors: a systematic review of clinical trials. Adv Exp Med Biol. 2021;1291:165–177. doi: 10.1007/978-3-030-56153-6_10. [DOI] [PubMed] [Google Scholar]
- Ran H, Zhu Y, Deng R, Zhang Q, Liu X, Feng M, Zhong J, Lin S, Tong X, Su Q. Stearoyl-CoA desaturase-1 promotes colorectal cancer metastasis in response to glucose by suppressing PTEN. J Exp Clin Cancer Res. 2018;37:54. doi: 10.1186/s13046-018-0711-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayalam S, Yang JY, Ambati S, Della-Fera MA, Baile CA. Resveratrol induces apoptosis and inhibits adipogenesis in 3T3-L1 adipocytes. Phytother Res. 2008;22:1367–1371. doi: 10.1002/ptr.2503. [DOI] [PubMed] [Google Scholar]
- Rios Garcia M, Steinbauer B, Srivastava K, Singhal M, Mattijssen F, Maida A, Christian S, Hess-Stumpp H, Augustin HG, Muller-Decker K, Nawroth PP, Herzig S, Berriel Diaz M. Acetyl-CoA carboxylase 1-dependent protein acetylation controls breast cancer metastasis and recurrence. Cell Metab. 2017;26:842–855.e845. doi: 10.1016/j.cmet.2017.09.018. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Cuenca S, Whyte L, Hagen R, Vidal-Puig A, Fuller M. Stearoyl-CoA desaturase 1 is a key determinant of membrane lipid composition in 3T3-L1 adipocytes. PLoS ONE. 2016;11:e0162047. doi: 10.1371/journal.pone.0162047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roongta UV, Pabalan JG, Wang X, Ryseck RP, Fargnoli J, Henley BJ, Yang WP, Zhu J, Madireddi MT, Lawrence RM, Wong TW, Rupnow BA. Cancer cell dependence on unsaturated fatty acids implicates stearoyl-CoA desaturase as a target for cancer therapy. Mol Cancer Res. 2011;9:1551–1561. doi: 10.1158/1541-7786.MCR-11-0126. [DOI] [PubMed] [Google Scholar]
- Rossi EL, Khatib SA, Doerstling SS, Bowers LW, Pruski M, Ford NA, Glickman RD, Niu M, Yang P, Cui Z, DiGiovanni J, Hursting SD. Resveratrol inhibits obesity-associated adipose tissue dysfunction and tumor growth in a mouse model of postmenopausal claudin-low breast cancer. Mol Carcinog. 2018;57:393–407. doi: 10.1002/mc.22763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruidas B, Sur TK, Das Mukhopadhyay C, Sinha K, Som Chaudhury S, Sharma P, Bhowmick S, Majumder R, Saha A. Quercetin: a silent retarder of fatty acid oxidation in breast cancer metastasis through steering of mitochondrial CPT1. Breast Cancer. 2022;29:748–760. doi: 10.1007/s12282-022-01356-y. [DOI] [PubMed] [Google Scholar]
- Sag D, Cekic C, Wu R, Linden J, Hedrick CC. The cholesterol transporter ABCG1 links cholesterol homeostasis and tumour immunity. Nat Commun. 2015;6:6354. doi: 10.1038/ncomms7354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar G, Sah RP, Javeed N, Dutta SK, Smyrk TC, Lau JS, Giorgadze N, Tchkonia T, Kirkland JL, Chari ST, Mukhopadhyay D. Pathogenesis of pancreatic cancer exosome-induced lipolysis in adipose tissue. Gut. 2016;65:1165–1174. doi: 10.1136/gutjnl-2014-308350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos JC, Gotardo EM, Brianti MT, Piraee M, Gambero A, Ribeiro ML. Effects of yerba mate, a plant extract formulation (“YGD”) and resveratrol in 3T3-L1 adipogenesis. Molecules. 2014;19:16909–16924. doi: 10.3390/molecules191016909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaglia N, Igal RA. Inhibition of stearoyl-CoA desaturase 1 expression in human lung adenocarcinoma cells impairs tumorigenesis. Int J Oncol. 2008;33:839–850. [PubMed] [Google Scholar]
- Schiliro C, Firestein BL. Mechanisms of metabolic reprogramming in cancer cells supporting enhanced growth and proliferation. Cells. 2021 doi: 10.3390/cells10051056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder B, Vander Steen T, Espinoza I, Venkatapoorna CMK, Hu Z, Silva FM, Regan K, Cuyas E, Meng XW, Verdura S, Arbusa A, Schneider PA, Flatten KS, Kemble G, Montero J, Kaufmann SH, Menendez JA, Lupu R. Fatty acid synthase (FASN) regulates the mitochondrial priming of cancer cells. Cell Death Dis. 2021;12:977. doi: 10.1038/s41419-021-04262-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schug ZT, Peck B, Jones DT, Zhang Q, Grosskurth S, Alam IS, Goodwin LM, Smethurst E, Mason S, Blyth K, McGarry L, James D, Shanks E, Kalna G, Saunders RE, Jiang M, Howell M, Lassailly F, Thin MZ, Spencer-Dene B, Stamp G, van den Broek NJ, Mackay G, Bulusu V, Kamphorst JJ, Tardito S, Strachan D, Harris AL, Aboagye EO, Critchlow SE, Wakelam MJ, Schulze A, Gottlieb E. Acetyl-CoA synthetase 2 promotes acetate utilization and maintains cancer cell growth under metabolic stress. Cancer Cell. 2015;27:57–71. doi: 10.1016/j.ccell.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo YS, Kang OH, Kim SB, Mun SH, Kang DH, Yang DW, Choi JG, Lee YM, Kang DK, Lee HS, Kwon DY. Quercetin prevents adipogenesis by regulation of transcriptional factors and lipases in OP9 cells. Int J Mol Med. 2015;35:1779–1785. doi: 10.3892/ijmm.2015.2185. [DOI] [PubMed] [Google Scholar]
- Shabir I, Kumar Pandey V, Shams R, Dar AH, Dash KK, Khan SA, Bashir I, Jeevarathinam G, Rusu AV, Esatbeyoglu T, Pandiselvam R. Promising bioactive properties of quercetin for potential food applications and health benefits: a review. Front Nutr. 2022;9:999752. doi: 10.3389/fnut.2022.999752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe LJ, Brown AJ. Controlling cholesterol synthesis beyond 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) J Biol Chem. 2013;288:18707–18715. doi: 10.1074/jbc.R113.479808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Zhou Q, Gao S, Li W, Li X, Liu Z, Jin P, Jiang J. Silibinin inhibits endometrial carcinoma via blocking pathways of STAT3 activation and SREBP1-mediated lipid accumulation. Life Sci. 2019;217:70–80. doi: 10.1016/j.lfs.2018.11.037. [DOI] [PubMed] [Google Scholar]
- Siqingaowa SS, Gopalakrishnan V, Taghibiglou C. Sterol regulatory element-binding protein 1 inhibitors decrease pancreatic cancer cell viability and proliferation. Biochem Biophys Res Commun. 2017;488:136–140. doi: 10.1016/j.bbrc.2017.05.023. [DOI] [PubMed] [Google Scholar]
- Snaebjornsson MT, Janaki-Raman S, Schulze A. Greasing the wheels of the cancer machine: the role of lipid metabolism in cancer. Cell Metab. 2020;31:62–76. doi: 10.1016/j.cmet.2019.11.010. [DOI] [PubMed] [Google Scholar]
- Sohaei S, Amani R, Tarrahi MJ, Ghasemi-Tehrani H. The effects of curcumin supplementation on glycemic status, lipid profile and hs-CRP levels in overweight/obese women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled clinical trial. Complement Ther Med. 2019;47:102201. doi: 10.1016/j.ctim.2019.102201. [DOI] [PubMed] [Google Scholar]
- Sola-Garcia A, Caliz-Molina MA, Espadas I, Petr M, Panadero-Moron C, Gonzalez-Moran D, Martin-Vazquez ME, Narbona-Perez AJ, Lopez-Noriega L, Martinez-Corrales G, Lopez-Fernandez-Sobrino R, Carmona-Marin LM, Martinez-Force E, Yanes O, Vinaixa M, Lopez-Lopez D, Reyes JC, Dopazo J, Martin F, Gauthier BR, Scheibye-Knudsen M, Capilla-Gonzalez V, Martin-Montalvo A. Metabolic reprogramming by Acly inhibition using SB-204990 alters glucoregulation and modulates molecular mechanisms associated with aging. Commun Biol. 2023;6:250. doi: 10.1038/s42003-023-04625-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoiber K, Naglo O, Pernpeintner C, Zhang S, Koeberle A, Ulrich M, Werz O, Muller R, Zahler S, Lohmuller T, Feldmann J, Braig S. Targeting de novo lipogenesis as a novel approach in anti-cancer therapy. Br J Cancer. 2018;118:43–51. doi: 10.1038/bjc.2017.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stukenberg PT, Kirschner MW. Pin1 acts catalytically to promote a conformational change in Cdc25. Mol Cell. 2001;7:1071–1083. doi: 10.1016/s1097-2765(01)00245-3. [DOI] [PubMed] [Google Scholar]
- Su P, Wang Q, Bi E, Ma X, Liu L, Yang M, Qian J, Yi Q. Enhanced lipid accumulation and metabolism are required for the differentiation and activation of tumor-associated macrophages. Cancer Res. 2020;80:1438–1450. doi: 10.1158/0008-5472.CAN-19-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su T, Huang C, Yang C, Jiang T, Su J, Chen M, Fatima S, Gong R, Hu X, Bian Z, Liu Z, Kwan HY. Apigenin inhibits STAT3/CD36 signaling axis and reduces visceral obesity. Pharmacol Res. 2020;152:104586. doi: 10.1016/j.phrs.2019.104586. [DOI] [PubMed] [Google Scholar]
- Subramanian N, Schumann-Gillett A, Mark AE, O'Mara ML. Understanding the accumulation of P-glycoprotein substrates within cells: the effect of cholesterol on membrane partitioning. Biochim Biophys Acta. 2016;1858:776–782. doi: 10.1016/j.bbamem.2015.12.025. [DOI] [PubMed] [Google Scholar]
- Suh HJ, Cho SY, Kim EY, Choi HS. Blockade of lipid accumulation by silibinin in adipocytes and zebrafish. Chem Biol Interact. 2015;227:53–62. doi: 10.1016/j.cbi.2014.12.027. [DOI] [PubMed] [Google Scholar]
- Suihara S, Ishisaka A, Murakami A. (−)-Epigallocatechin-3-O-gallate at a high concentration may induce lipolysis via ATP consumption by activation of stress defense mechanisms. Biosci Biotechnol Biochem. 2021;85:411–420. doi: 10.1093/bbb/zbaa056. [DOI] [PubMed] [Google Scholar]
- Sun YS, Qu W. Dietary Apigenin promotes lipid catabolism, thermogenesis, and browning in adipose tissues of HFD-Fed mice. Food Chem Toxicol. 2019;133:110780. doi: 10.1016/j.fct.2019.110780. [DOI] [PubMed] [Google Scholar]
- Sun H, Zhang L, Wang Z, Gu D, Zhu M, Cai Y, Li L, Tang J, Huang B, Bosco B, Li N, Wu L, Wu W, Li L, Liang Y, Luo L, Liu Q, Zhu Y, Sun J, Shi L, Xia T, Yang C, Xu Q, Han X, Zhang W, Liu J, Meng D, Shao H, Zheng X, Li S, Pan H, Ke J, Jiang W, Zhang X, Han X, Chu J, An H, Ge J, Pan C, Wang X, Li K, Wang Q, Ding Q. Single-cell transcriptome analysis indicates fatty acid metabolism-mediated metastasis and immunosuppression in male breast cancer. Nat Commun. 2023;14:5590. doi: 10.1038/s41467-023-41318-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson RU, Parker SJ, Eichner LJ, Kolar MJ, Wallace M, Brun SN, Lombardo PS, Van Nostrand JL, Hutchins A, Vera L, Gerken L, Greenwood J, Bhat S, Harriman G, Westlin WF, Harwood HJ, Jr, Saghatelian A, Kapeller R, Metallo CM, Shaw RJ. Inhibition of acetyl-CoA carboxylase suppresses fatty acid synthesis and tumor growth of non-small-cell lung cancer in preclinical models. Nat Med. 2016;22:1108–1119. doi: 10.1038/nm.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szkudelska K, Nogowski L, Szkudelski T. Resveratrol, a naturally occurring diphenolic compound, affects lipogenesis, lipolysis and the antilipolytic action of insulin in isolated rat adipocytes. J Steroid Biochem Mol Biol. 2009;113:17–24. doi: 10.1016/j.jsbmb.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Tabrizi R, Vakili S, Lankarani KB, Akbari M, Mirhosseini N, Ghayour-Mobarhan M, Ferns G, Karamali F, Karamali M, Taghizadeh M, Kouchaki E, Asemi Z. The effects of curcumin on glycemic control and lipid profiles among patients with metabolic syndrome and related disorders: a systematic review and meta analysis of randomized controlled trials. Curr Pharm Des. 2018;24:3184–3199. doi: 10.2174/1381612824666180828162053. [DOI] [PubMed] [Google Scholar]
- Tan C, Zhou L, Wen W, Xiao N. Curcumin promotes cholesterol efflux by regulating ABCA1 expression through miR-125a-5p/SIRT6 axis in THP-1 macrophage to prevent atherosclerosis. J Toxicol Sci. 2021;46:209–222. doi: 10.2131/jts.46.209. [DOI] [PubMed] [Google Scholar]
- Terado T, Kim CJ, Ushio A, Minami K, Tambe Y, Kageyama S, Kawauchi A, Tsunoda T, Shirasawa S, Tanaka H, Inoue H. Cryptotanshinone suppresses tumorigenesis by inhibiting lipogenesis and promoting reactive oxygen species production in KRAS activated pancreatic cancer cells. Int J Oncol. 2022 doi: 10.3892/ijo.2022.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MP, Cooper ST, Parry BR, Tuckey JA. Increased expression of the mRNA for hormone-sensitive lipase in adipose tissue of cancer patients. Biochim Biophys Acta. 1993;1180:236–242. doi: 10.1016/0925-4439(93)90044-2. [DOI] [PubMed] [Google Scholar]
- Tian C, Huang D, Yu Y, Zhang J, Fang Q, Xie C. ABCG1 as a potential oncogene in lung cancer. Exp Ther Med. 2017;13:3189–3194. doi: 10.3892/etm.2017.4393. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Tian L, Song Z, Shao W, Du WW, Zhao LR, Zeng K, Yang BB, Jin T. Curcumin represses mouse 3T3-L1 cell adipogenic differentiation via inhibiting miR-17-5p and stimulating the Wnt signalling pathway effector Tcf7l2. Cell Death Dis. 2017;8:e2559. doi: 10.1038/cddis.2016.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomacha J, Dokduang H, Padthaisong S, Namwat N, Klanrit P, Phetcharaburanin J, Wangwiwatsin A, Khampitak T, Koonmee S, Titapun A, Jarearnrat A, Khuntikeo N, Loilome W. Targeting fatty acid synthase modulates metabolic pathways and inhibits cholangiocarcinoma cell progression. Front Pharmacol. 2021;12:696961. doi: 10.3389/fphar.2021.696961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomeh MA, Hadianamrei R, Zhao X. A review of curcumin and its derivatives as anticancer agents. Int J Mol Sci. 2019 doi: 10.3390/ijms20051033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K, Nakatsu Y, Yamamotoya T, Ono H, Inoue Y, Inoue MK, Mizuno Y, Matsunaga Y, Kushiyama A, Sakoda H, Fujishiro M, Takahashi SI, Matsubara A, Asano T. Prolyl isomerase Pin1 binds to and stabilizes acetyl CoA carboxylase 1 protein, thereby supporting cancer cell proliferation. Oncotarget. 2019;10:1637–1648. doi: 10.18632/oncotarget.26691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanelli L, Buratta S, Logozzi M, Mitro N, Sagini K, Raimo RD, Caruso D, Fais S, Emiliani C. Lipidomic analysis of cancer cells cultivated at acidic pH reveals phospholipid fatty acids remodelling associated with transcriptional reprogramming. J Enzyme Inhib Med Chem. 2020;35:963–973. doi: 10.1080/14756366.2020.1748025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkateswaran A, Laffitte BA, Joseph SB, Mak PA, Wilpitz DC, Edwards PA, Tontonoz P. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXR alpha. Proc Natl Acad Sci USA. 2000;97:12097–12102. doi: 10.1073/pnas.200367697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura R, Mordec K, Waszczuk J, Wang Z, Lai J, Fridlib M, Buckley D, Kemble G, Heuer TS. Inhibition of de novo palmitate synthesis by fatty acid synthase induces apoptosis in tumor cells by remodeling cell membranes, inhibiting signaling pathways, and reprogramming gene expression. EBioMedicine. 2015;2:808–824. doi: 10.1016/j.ebiom.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodnala SK, Eil R, Kishton RJ, Sukumar M, Yamamoto TN, Ha NH, Lee PH, Shin M, Patel SJ, Yu Z, Palmer DC, Kruhlak MJ, Liu X, Locasale JW, Huang J, Roychoudhuri R, Finkel T, Klebanoff CA, Restifo NP. T cell stemness and dysfunction in tumors are triggered by a common mechanism. Science. 2019 doi: 10.1126/science.aau0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voloshyna I, Hai O, Littlefield MJ, Carsons S, Reiss AB. Resveratrol mediates anti-atherogenic effects on cholesterol flux in human macrophages and endothelium via PPARgamma and adenosine. Eur J Pharmacol. 2013;698:299–309. doi: 10.1016/j.ejphar.2012.08.024. [DOI] [PubMed] [Google Scholar]
- von Roemeling CA, Marlow LA, Wei JJ, Cooper SJ, Caulfield TR, Wu K, Tan WW, Tun HW, Copland JA. Stearoyl-CoA desaturase 1 is a novel molecular therapeutic target for clear cell renal cell carcinoma. Clin Cancer Res. 2013;19:2368–2380. doi: 10.1158/1078-0432.CCR-12-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakil SJ, Abu-Elheiga LA. Fatty acid metabolism: target for metabolic syndrome. J Lipid Res. 2009;50(Suppl):S138–143. doi: 10.1194/jlr.R800079-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Ma Q. Clinical benefits and pharmacology of scutellarin: a comprehensive review. Pharmacol Ther. 2018;190:105–127. doi: 10.1016/j.pharmthera.2018.05.006. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Li XL, Wang QF, Mehendale SR, Yuan CS. Selective fraction of Scutellaria baicalensis and its chemopreventive effects on MCF-7 human breast cancer cells. Phytomedicine. 2010;17:63–68. doi: 10.1016/j.phymed.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Xu Y, Zhu L, Zou Y, Kong W, Dong B, Huang J, Chen Y, Xue W, Huang Y, Zhang J. High expression of stearoyl-CoA desaturase 1 predicts poor prognosis in patients with clear-cell renal cell carcinoma. PLoS ONE. 2016;11:e0166231. doi: 10.1371/journal.pone.0166231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YY, Attane C, Milhas D, Dirat B, Dauvillier S, Guerard A, Gilhodes J, Lazar I, Alet N, Laurent V, Le Gonidec S, Biard D, Herve C, Bost F, Ren GS, Bono F, Escourrou G, Prentki M, Nieto L, Valet P, Muller C. Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells. JCI Insight. 2017;2:e87489. doi: 10.1172/jci.insight.87489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Shi M, Ji J, Cai Q, Zhao Q, Jiang J, Liu J, Zhang H, Zhu Z, Zhang J. Stearoyl-CoA desaturase 1 (SCD1) facilitates the growth and anti-ferroptosis of gastric cancer cells and predicts poor prognosis of gastric cancer. Aging (albany NY) 2020;12:15374–15391. doi: 10.18632/aging.103598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Chen S, Ren L, Wang Y, Li Z, Song T, Zhang H, Yang Q. The effect of silibinin on protein expression profile in white adipose tissue of obese mice. Front Pharmacol. 2020;11:55. doi: 10.3389/fphar.2020.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Franco F, Tsui YC, Xie X, Trefny MP, Zappasodi R, Mohmood SR, Fernandez-Garcia J, Tsai CH, Schulze I, Picard F, Meylan E, Silverstein R, Goldberg I, Fendt SM, Wolchok JD, Merghoub T, Jandus C, Zippelius A, Ho PC. CD36-mediated metabolic adaptation supports regulatory T cell survival and function in tumors. Nat Immunol. 2020;21:298–308. doi: 10.1038/s41590-019-0589-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324:1076–1080. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Powers S, Zhu W, Hannun YA. Substantial contribution of extrinsic risk factors to cancer development. Nature. 2016;529:43–47. doi: 10.1038/nature16166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LY, Chen CW, Chen LK, Chou HY, Chang CL, Juan CC. Curcumin attenuates adipogenesis by inducing preadipocyte apoptosis and inhibiting adipocyte differentiation. Nutrients. 2019 doi: 10.3390/nu11102307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Guo T, Deng R, Liu L, Yu Y. Apigenin ameliorates insulin resistance and lipid accumulation by endoplasmic reticulum stress and SREBP-1c/SREBP-2 pathway in palmitate-induced HepG2 cells and high-fat diet-fed mice. J Pharmacol Exp Ther. 2021;377:146–156. doi: 10.1124/jpet.120.000162. [DOI] [PubMed] [Google Scholar]
- Xu H, Zhang S. Scutellarin-induced apoptosis in HepG2 hepatocellular carcinoma cells via a STAT3 pathway. Phytother Res. 2013;27:1524–1528. doi: 10.1002/ptr.4892. [DOI] [PubMed] [Google Scholar]
- Xu M, Chang HH, Jung X, Moro A, Chou CEN, King J, Hines OJ, Sinnett-Smith J, Rozengurt E, Eibl G. Deficiency in hormone-sensitive lipase accelerates the development of pancreatic cancer in conditional KrasG12D mice. BMC Cancer. 2018;18:797. doi: 10.1186/s12885-018-4713-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Chaudhary O, Rodriguez-Morales P, Sun X, Chen D, Zappasodi R, Xu Z, Pinto AFM, Williams A, Schulze I, Farsakoglu Y, Varanasi SK, Low JS, Tang W, Wang H, McDonald B, Tripple V, Downes M, Evans RM, Abumrad NA, Merghoub T, Wolchok JD, Shokhirev MN, Ho PC, Witztum JL, Emu B, Cui G, Kaech SM. Uptake of oxidized lipids by the scavenger receptor CD36 promotes lipid peroxidation and dysfunction in CD8(+) T cells in tumors. Immunity. 2021;54:1561–1577.e1567. doi: 10.1016/j.immuni.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Zhang Y, Zhang X, Aa J, Wang G, Xie Y. Curcumin regulates endogenous and exogenous metabolism via Nrf2-FXR-LXR pathway in NAFLD mice. Biomed Pharmacother. 2018;105:274–281. doi: 10.1016/j.biopha.2018.05.135. [DOI] [PubMed] [Google Scholar]
- Yan A, Jia Z, Qiao C, Wang M, Ding X. Cholesterol metabolism in drug resistant cancer (Review) Int J Oncol. 2020;57:1103–1115. doi: 10.3892/ijo.2020.5124. [DOI] [PubMed] [Google Scholar]
- Yang PB, Hou PP, Liu FY, Hong WB, Chen HZ, Sun XY, Li P, Zhang Y, Ju CY, Luo LJ, Wu SF, Zhou JX, Wang ZJ, He JP, Li L, Zhao TJ, Deng X, Lin T, Wu Q. Blocking PPARγ interaction facilitates Nur77 interdiction of fatty acid uptake and suppresses breast cancer progression. Proc Natl Acad Sci USA. 2020;117:27412–27422. doi: 10.1073/pnas.2002997117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Liu Q, Zhang H, Wang Y, Li Y, Chen S, Song G, Ren L. Silibinin improves nonalcoholic fatty liver by regulating the expression of miR-122: an in vitro and in vivo study. Mol Med Rep. 2021 doi: 10.3892/mmr.2021.11974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Qin H, Li Y, Xiao A, Zheng E, Zeng H, Su C, Luo X, Lu Q, Liao M, Zhao L, Wei L, Varghese Z, Moorhead JF, Chen Y, Ruan XZ. CD36-mediated metabolic crosstalk between tumor cells and macrophages affects liver metastasis. Nat Commun. 2022;13:5782. doi: 10.1038/s41467-022-33349-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Chen S, Li W. Fatostatin inhibits the development of endometrial carcinoma in endometrial carcinoma cells and a xenograft model by targeting lipid metabolism. Arch Biochem Biophys. 2020;684:108327. doi: 10.1016/j.abb.2020.108327. [DOI] [PubMed] [Google Scholar]
- Yasumoto Y, Miyazaki H, Vaidyan LK, Kagawa Y, Ebrahimi M, Yamamoto Y, Ogata M, Katsuyama Y, Sadahiro H, Suzuki M, Owada Y. Inhibition of fatty acid synthase decreases expression of stemness markers in glioma stem cells. PLoS ONE. 2016;11:e0147717. doi: 10.1371/journal.pone.0147717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye B, Yin L, Wang Q, Xu C. ACC1 is overexpressed in liver cancers and contributes to the proliferation of human hepatoma Hep G2 cells and the rat liver cell line BRL 3A. Mol Med Rep. 2019;19:3431–3440. doi: 10.3892/mmr.2019.9994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye G, Chen G, Gao H, Lin Y, Liao X, Zhang H, Liu X, Chi Y, Huang Q, Zhu H, Fan Y, Dong S. Resveratrol inhibits lipid accumulation in the intestine of atherosclerotic mice and macrophages. J Cell Mol Med. 2019;23:4313–4325. doi: 10.1111/jcmm.14323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Li W, Mo L, Deng S, Lin W, Ma C, Luo Z, Luo C, Hong H. Adipose triglyceride lipase promotes the proliferation of colorectal cancer cells via enhancing the lipolytic pathway. J Cell Mol Med. 2021;25:3963–3975. doi: 10.1111/jcmm.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii Y, Furukawa T, Yoshii H, Mori T, Kiyono Y, Waki A, Kobayashi M, Tsujikawa T, Kudo T, Okazawa H, Yonekura Y, Fujibayashi Y. Cytosolic acetyl-CoA synthetase affected tumor cell survival under hypoxia: the possible function in tumor acetyl-CoA/acetate metabolism. Cancer Sci. 2009;100:821–827. doi: 10.1111/j.1349-7006.2009.01099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Yang X, Li Y, Liu Q, Wu F, Qu H, Gao H, Ge J, Xu Y, Wang H, Wang Y, Zhao Z. Expression and prognostic significance of fatty acid synthase in clear cell renal cell carcinoma. Pathol Res Pract. 2020;216:153227. doi: 10.1016/j.prp.2020.153227. [DOI] [PubMed] [Google Scholar]
- Yue S, Li J, Lee SY, Lee HJ, Shao T, Song B, Cheng L, Masterson TA, Liu X, Ratliff TL, Cheng JX. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab. 2014;19:393–406. doi: 10.1016/j.cmet.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachara NE, Hart GW. The emerging significance of O-GlcNAc in cellular regulation. Chem Rev. 2002;102:431–438. doi: 10.1021/cr000406u. [DOI] [PubMed] [Google Scholar]
- Zaidi N, Swinnen JV, Smans K. ATP-citrate lyase: a key player in cancer metabolism. Cancer Res. 2012;72:3709–3714. doi: 10.1158/0008-5472.CAN-11-4112. [DOI] [PubMed] [Google Scholar]
- Zechner R, Zimmermann R, Eichmann TO, Kohlwein SD, Haemmerle G, Lass A, Madeo F. FAT SIGNALS—lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 2012;15:279–291. doi: 10.1016/j.cmet.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y, Ginanni N, Tota MR, Wu M, Bays NW, Richon VM, Kohl NE, Bachman ES, Strack PR, Krauss S. Control of cell growth and survival by enzymes of the fatty acid synthesis pathway in HCT-116 colon cancer cells. Clin Cancer Res. 2008;14:5735–5742. doi: 10.1158/1078-0432.CCR-07-5074. [DOI] [PubMed] [Google Scholar]
- Zhang C, Zhu N, Li H, Gong Y, Gu J, Shi Y, Liao D, Wang W, Dai A, Qin L. New dawn for cancer cell death: emerging role of lipid metabolism. Mol Metab. 2022;63:101529. doi: 10.1016/j.molmet.2022.101529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Huo Z, Luan H, Huang Y, Shen Y, Sheng L, Liang J, Wu F. Scutellarin ameliorates hepatic lipid accumulation by enhancing autophagy and suppressing IRE1alpha/XBP1 pathway. Phytother Res. 2022;36:433–447. doi: 10.1002/ptr.7344. [DOI] [PubMed] [Google Scholar]
- Zhao P, Mao JM, Zhang SY, Zhou ZQ, Tan Y, Zhang Y. Quercetin induces HepG2 cell apoptosis by inhibiting fatty acid biosynthesis. Oncol Lett. 2014;8:765–769. doi: 10.3892/ol.2014.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Prijic S, Urban BC, Tisza MJ, Zuo Y, Li L, Tan Z, Chen X, Mani SA, Chang JT. Candidate antimetastasis drugs suppress the metastatic capacity of breast cancer cells by reducing membrane fluidity. Cancer Res. 2016;76:2037–2049. doi: 10.1158/0008-5472.CAN-15-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Zhang C, Luo X, Wang P, Zhou W, Zhong S, Xie Y, Jiang Y, Yang P, Tang R, Pan Q, Hall AR, Luong TV, Fan J, Varghese Z, Moorhead JF, Pinzani M, Chen Y, Ruan XZ. CD36 palmitoylation disrupts free fatty acid metabolism and promotes tissue inflammation in non-alcoholic steatohepatitis. J Hepatol. 2018;69:705–717. doi: 10.1016/j.jhep.2018.04.006. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Shang Y, Xu R, Yan X, Wang X, Cai J, Bai Z, Liu X, Yin J, Zhang J, Zhang Z. Ubiquitin specific peptidase 38 promotes the progression of gastric cancer through upregulation of fatty acid synthase. Am J Cancer Res. 2022;12:2686–2696. [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Farah BL, Sinha RA, Wu Y, Singh BK, Bay BH, Yang CS, Yen PM. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, stimulates hepatic autophagy and lipid clearance. PLoS ONE. 2014;9:e87161. doi: 10.1371/journal.pone.0087161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Qian W, Ma J, Cheng L, Jiang Z, Yan B, Li J, Duan W, Sun L, Cao J, Wang F, Wu E, Wu Z, Ma Q, Li X. Resveratrol enhances the chemotherapeutic response and reverses the stemness induced by gemcitabine in pancreatic cancer cells via targeting SREBP1. Cell Prolif. 2019;52:e12514. doi: 10.1111/cpr.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Wang Y, Han X, Fu S, Zhu C, Chen Q. Efficacy of resveratrol supplementation on glucose and lipid metabolism: a meta-analysis and systematic review. Front Physiol. 2022;13:795980. doi: 10.3389/fphys.2022.795980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Zhu X, Wu Y. Effects of glucose metabolism, lipid metabolism, and glutamine metabolism on tumor microenvironment and clinical implications. Biomolecules. 2022;12:580. doi: 10.3390/biom12040580. [DOI] [PMC free article] [PubMed] [Google Scholar]



