Abstract
Introduction:
An international database was created by the International Association for the Study of Lung Cancer (IASLC) to inform the 9th edition of the Tumor – Node – Metastasis (TNM) classification of lung cancer. The present analyses concern its T component.
Methods:
Data on 124,581 patients diagnosed with lung cancer from January 1, 2011, to December 31, 2019, were submitted to the IASLC database. Of these, 33,982 met the inclusion criteria for the clinical T (cT) analysis, and 30,715 met the inclusion criteria for the pathologic post-surgical (pT) analysis. Survival was measured from date of diagnosis or operation for clinically and pathologically staged tumors, respectively. T descriptors were evaluated in univariate analysis and multivariable Cox regression analysis adjusted for age, sex, histological type, and geographic region.
Results:
Comprehensive survival analysis showed that the existing 8th edition T-component criteria performed adequately in the 9th edition dataset. Although pathological chest wall / parietal pleura involvement (PL 3) yielded a worse survival compared to the other T3 descriptors, with a similar survival as T4 tumors, this difference was not observed for clinical chest wall / PL 3 tumors. Due to these inconsistent findings, no reallocation of chest wall / PL 3 tumors is advised.
Conclusions:
The T-subcommittee members proposed not to implement any changes and keep the current 8th edition T-descriptors for the 9th edition.
Keywords: lung cancer, lung cancer staging, T component, T descriptors, TNM classification
Graphical Abstract

INTRODUCTION
In the 8th edition of the tumor, node and metastasis (TNM) classification of lung cancer published in 2015 profound changes were made concerning the T-descriptors .1 A diameter of 3 cm was kept to distinguish T1 from T2 tumors. Size was found to be an important prognostic factor. Increments of 1 cm were implemented until 5 cm resulting in the following subdivisions: T1a (≤ 1 cm), T1b (> 1 cm and ≤ 2 cm), T1c (>2 cm and ≤ 3 cm), T2a (>3 cm and ≤ 4 cm), T2b (>4 cm and ≤ 5 cm). Tumors > 5 cm and ≤ 7 cm were reclassified as T3, and tumors > 7 cm as T4. Involvement of the main bronchus was defined as a T2 descriptor regardless of the distance from the carina. Partial and total atelectasis or pneumonitis were also grouped as T2 descriptors. Diaphragmatic invasion was reclassified as T4 and mediastinal pleura invasion was deleted as a T-descriptor. Furthermore, for part-solid lesions only the solid or invasive part was recommended to determine the T-size.2 An overview of the 8th edition T-classification is provided in Supplemental Table 1.
After publication of the 8th edition of the TNM classification, the International Association for the Study of Lung Cancer (IASLC) launched the third phase of the International Staging Project in 2018.3 To update and refine the TNM classification a new database was created for cases diagnosed between 2011 and 2019 with follow-up data until the end of December 2021.4 This database was used to inform the 9th edition of the TNM classification.
The present article summarizes the results of the analyses of the new IASLC database performed by the members of the T-Subcommittee of the IASLC Staging and Prognostic Factors Committee (SPFC) and the statisticians of Cancer Research And Biostatistics (CRAB) concerning the T-component of the TNM classification and its descriptors. The analyses were conducted to achieve predefined objectives: to validate the descriptors of the 8th classification using the database for the 9th edition; to assess whether further adaptations to the T-descriptors were necessary; and to specifically assess the prognostic impact of chest wall / parietal pleura (PL 3) invasion to determine whether reallocation of such tumors is warranted on the basis of patient outcomes.5 To this end specific methods and guiding principles for the development of the 9th edition were developed.6
MATERIAL AND METHODS
Population
A total of 124,581 cases were submitted to the 9th edition staging project (Supplemental Fig. 1). Of those, 73,197 satisfied the general inclusion criteria including valid histological type, survival time, diagnosis date between 2011 and 2019, valid stage, and non-small cell lung cancer (NSCLC). One large registry dataset with patients diagnosed in 2010 (outside of the prespecified time window) was included in the analysis given the substantial sample size and high quality of the data, and one site was excluded from the final analysis due to concerns about aberrant patterns of correlation between clinical and pathologic N-category information. Of the included patients, 33,982 and 30,715 met the inclusion criteria for evaluation of the clinical T-component (cT) and pathologic T-component (pT) analyses, respectively. To further inform the T stage analysis, data on the solid (clinical) or invasive (pathologic) size was sought for all analyses. However, characteristics of the submitted data left some uncertainty to how size was determined in some cases, likely due to the fact that the focus on solid/invasive size for staging purposes was not published until 2016, and the time it takes for awareness and for local data collection systems to adapt.2 Despite attempts to be as specific as possible, it proved difficult to identify with certainty cases involving only solid/invasive size, or to precisely define the proportion of such cases among all submitted cases. However, it appears that the issue mainly pertains to the T1 category; for T2-T4 the proportion of cases in which an uncertain size measurement determined the T category is estimated to be <5%. Furthermore, approximately 5,000 cases involving uncertainty regarding the nature of the size measurement involved a 2010 cohort from Japan; analyses with and without these cases did not meaningfully change the presence of or magnitude of differences between T categories and subcategories. Finally, among T1 subcategories, in which the issue of solid/invasive versus total size is likely to play the largest role, a subset analysis involving only non-mucinous adenocarcinoma cases with invasive size recorded and diagnosed in 2018 or later demonstrated stepwise progressively worse overall survival (OS) with increasing T1 subcategories (although the sample size limited the feasibility of statistical comparisons). Therefore, a more inclusive approach was chosen for the analyses and figures in this paper, involving largely cases submitted with an invasive size measurement but with a proportion in whom the size measurement was uncertain, or likely represented total size. A valid N component as well M0 status were also required, in addition to passing general quality control screens.
When analyzing the data on visceral pleural invasion which is a T2 descriptor, for some centers a discrepancy was noted between visceral pleural invasion and pleural status (PL). To correct this issue, a cleaning step was introduced by CRAB to correct for visceral pleural invasion status for one site, which provided corrected data to support this cleaning step.
The number of cases and patients’ characteristics are shown in Table 1 and Supplemental Table 2). Of clinical stage cases, 31,329 were managed surgically and 2,350 were managed non-surgically (Supplemental Table 2). Among those with pathologic N0 tumors, 21,484 patients who did not receive neoadjuvant treatment were available for the main pT analysis, and 804 patients were treated with neoadjuvant therapy and further analyzed.
Table 1.
Patient characteristics. Upper panel shows clinical T (cT) cases, lower panel pathological T (pT) cases. Criteria for T-descriptor analysis: cases were M0 non-small cell lung cancer, had T-descriptors to support the assigned T-category (cases were excluded if the provided T-descriptors failed to confirm the reported T-category), had valid N-category provided, and passed general quality control screens.
| All cT | cT1 | cT2 | cT3 | cT4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |
| Grand Total | 33,982 | (100%) | 18,765 | (100%) | 8,941 | (100%) | 3,681 | (100%) | 2,595 | (100%) |
| Region, n (%) | ||||||||||
| Asia | 22,513 | (66%) | 13,385 | (71%) | 5,946 | (67%) | 1,938 | (53%) | 1,244 | (48%) |
| Europe | 4,175 | (12%) | 1,564 | (8%) | 1,168 | (13%) | 779 | (21%) | 664 | (26%) |
| North America | 6,324 | (19%) | 3,396 | (18%) | 1,576 | (18%) | 812 | (22%) | 540 | (21%) |
| Rest of World | 970 | (3%) | 420 | (2%) | 251 | (3%) | 152 | (4%) | 147 | (6%) |
| GDP, n (%) | ||||||||||
| Low | 11,271 | (33%) | 6,423 | (34%) | 2,955 | (33%) | 1,053 | (29%) | 840 | (32%) |
| Mid | 14,363 | (42%) | 8,307 | (44%) | 3,928 | (44%) | 1,347 | (37%) | 781 | (30%) |
| High | 8,348 | (25%) | 4,035 | (22%) | 2,058 | (23%) | 1,281 | (35%) | 974 | (38%) |
| Sex, n (%) | ||||||||||
| Male | 16,231 | (48%) | 8,288 | (44%) | 4,397 | (49%) | 1,972 | (54%) | 1,574 | (61%) |
| Female | 17,749 | (52%) | 10,475 | (56%) | 4,551 | (51%) | 1,709 | (46%) | 1,021 | (39%) |
| No Data | 2 | (0%) | 2 | (0%) | . | . | . | . | . | . |
| Age, n (%) | ||||||||||
| Less than 65 | 14,728 | (43%) | 8,311 | (44%) | 3,642 | (41%) | 1,511 | (41%) | 1,264 | (49%) |
| 65 or Older | 19,188 | (56%) | 10,413 | (55%) | 5,281 | (59%) | 2,166 | (59%) | 1,328 | (51%) |
| No Data | 66 | (0%) | 41 | (0%) | 18 | (0%) | 4 | (0%) | 3 | (0%) |
| NSCLC Histology, n (%) | ||||||||||
| AIS | 444 | (1%) | 377 | (2%) | 38 | (0%) | 15 | (0%) | 14 | (1%) |
| Adenocarcinoma | 23,954 | (70%) | 15,269 | (81%) | 5,599 | (63%) | 1,880 | (51%) | 1,206 | (46%) |
| Adenosquamous | 657 | (2%) | 245 | (1%) | 253 | (3%) | 105 | (3%) | 54 | (2%) |
| Large Cell | 425 | (1%) | 130 | (1%) | 126 | (1%) | 93 | (3%) | 76 | (3%) |
| NSCLC NOS | 347 | (1%) | 52 | (0%) | 99 | (1%) | 79 | (2%) | 117 | (5%) |
| Squamous | 8,155 | (24%) | 2,692 | (14%) | 2,826 | (32%) | 1,509 | (41%) | 1,128 | (43%) |
| Resection, n (%) | ||||||||||
| Nonsurgical | 2,350 | (7%) | 393 | (2%) | 525 | (6%) | 528 | (14%) | 904 | (35%) |
| Surgical R0 | 29,305 | (86%) | 17,525 | (93%) | 7,650 | (86%) | 2.,762 | (75%) | 1,368 | (53%) |
| Surgical R1 or R2 | 1,099 | (3%) | 379 | (2%) | 361 | (4%) | 214 | (6%) | 145 | (6%) |
| Surgical R Unknown | 925 | (3%) | 330 | (2%) | 333 | (4%) | 139 | (4%) | 123 | (5%) |
| Surgical Status Unknown | 303 | (1%) | 138 | (1%) | 72 | (1%) | 38 | (1%) | 55 | (2%) |
| N-component, n (%) | ||||||||||
| N0 | 27,971 | (82%) | 17,507 | (93%) | 6,936 | (78%) | 2,315 | (63%) | 1,213 | (47%) |
| N1 | 2,358 | (7%) | 593 | (3%) | 886 | (10%) | 543 | (15%) | 336 | (13%) |
| N2 | 2,838 | (8%) | 577 | (3%) | 923 | (10%) | 646 | (18%) | 692 | (27%) |
| N3 | 815 | (2%) | 88 | (0%) | 196 | (2%) | 177 | (5%) | 354 | (14%) |
| Grand Total | 28,934 | (100%) | 10,725 | (100%) | 12,406 | (100%) | 3,736 | (100%) | 2,076 | (100%) |
| Region, n (%) | ||||||||||
| Asia | 18,386 | (64%) | 5,904 | (55%) | 8,781 | (71%) | 2,409 | (64%) | 1,292 | (63%) |
| Europe | 3,345 | (12%) | 1,152 | (11%) | 1,279 | (10%) | 516 | (14%) | 398 | (19%) |
| North America | 5,764 | (20%) | 2,941 | (27%) | 1,877 | (15%) | 659 | (18%) | 287 | (14%) |
| Rest of World | 1,439 | (5%) | 728 | (7%) | 469 | (4%) | 152 | (4%) | 90 | (4%) |
| GDP, n (%) | ||||||||||
| Low | 9,583 | (33%) | 4,705 | (44%) | 3,394 | (27%) | 779 | (21%) | 705 | (34%) |
| Mid | 11,014 | (38%) | 2,143 | (20%) | 6,190 | (50%) | 1,921 | (51%) | 760 | (37%) |
| High | 8,337 | (29%) | 3,877 | (36%) | 2,822 | (23%) | 1,036 | (28%) | 602 | (29%) |
| Sex, n (%) | ||||||||||
| Male | 14,206 | (49%) | 5,000 | (47%) | 5,952 | (48%) | 2,000 | (54%) | 1,254 | (61%) |
| Female | 14,726 | (51%) | 5.723 | (53%) | 6,454 | (52%) | 1,736 | (46%) | 813 | (39%) |
| No Data | 2 | (0%) | 2 | (0%) | . | . | . | . | . | . |
| Age, n (%) | ||||||||||
| Less than 65 | 13,449 | (46%) | 5,785 | (54%) | 5,190 | (42%) | 1,453 | (39%) | 1,021 | (49%) |
| 65 or Older | 15,430 | (53%) | 4,907 | (46%) | 7,197 | (58%) | 2,281 | 61%) | 1,045 | (51%) |
| No Data | 55 | (0%) | 33 | (0%) | 19 | (0%) | 2 | (0%) | 1 | (0%) |
| NSCLC Histology, n (%) | ||||||||||
| AIS | 0 | (0%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 0 | (0%) |
| Adenocarcinoma | 20,937 | (72%) | 9,171 | (86%) | 8,752 | (71%) | 2,016 | (54%) | 998 | (48%) |
| Adenosquamous | 615 | (2%) | 89 | (1%) | 356 | (3%) | 120 | (3%) | 50 | (2%) |
| Large Cell | 420 | (1%) | 64 | (1%) | 180 | (1%) | 97 | (3%) | 79 | (4%) |
| NSCLC NOS | 105 | (0%) | 26 | (0%) | 31 | (0%) | 28 | (1%) | 20 | (1%) |
| Squamous | 6,857 | (24%) | 1,375 | (13%) | 3,087 | (25%) | 1,475 | (39%) | 920 | (45%) |
| Resection, n (%) | ||||||||||
| Nonsurgical | 0 | (0%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 0 | (0%) |
| Surgical R0 | 27,097 | (94%) | 10,203 | (95%) | 11,669 | (94%) | 3,419 | (92%) | 1,806 | (87%) |
| Surgical R1 or R2 | 858 | (3%) | 92 | (1%) | 367 | (3%) | 197 | (5%) | 202 | (10%) |
| Surgical R Unknown | 979 | (3%) | 430 | (4%) | 370 | (3%) | 120 | (3%) | 59 | (3%) |
| Surgical Status Unknown | 0 | (0%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 0 | (0%) |
| N-component, n (%) | ||||||||||
| N0 | 21,484 | (74%) | 9,441 | (88%) | 8,571 | (69%) | 2,381 | (64%) | 1,091 | (53%) |
| N1 | 3,563 | (12%) | 659 | (6%) | 1,774 | (14%) | 684 | (18%) | 446 | (22%) |
| N2 | 3,770 | (13%) | 606 | (6%) | 2,002 | (16%) | 656 | (18%) | 506 | (24%) |
| N3 | 117 | (0%) | 19 | (0%) | 59 | (0%) | 15 | (0%) | 24 | (1%) |
GDP, Gross Domestic Product categories were based on the 2017 World Bank GDP per capita, where <$30,000 was categorized as Low, $30,000-$40,000 as Middle, and >$40,000 as High; NSCLC, Non-Small Cell Lung Cancer; AIS, Adenocarcinoma in situ; NOS, Not Otherwise Specified
Forty-three percent of all clinical T patients were younger than 65 years, and 53% of all pathological T patients were 65 years or older (Table 1).
Regarding specific pathologic type, 76% of patients had NSCLC with non-squamous and non-neuroendocrine histology in the cT and pT subgroups (Supplemental Fig. 2, Table 1). Adenocarcinoma was the most frequent histologic type, found in 70% and 72% of cT and pT tumors, respectively.
Most of the included patients came from Asia (66% cT, 64% pT). The majority of patients had N0 tumors (82% cT, 74% pT). Surgical treatment was provided in 93% of all patients with clinically staged tumors.
For patients with pT tumors, complete R0 resection was achieved in 94%, R1 or R2 resection in 3%, and the R-descriptor was unknown in 3%.
Statistical Analysis
The primary endpoint was OS , which was measured from the date of diagnosis for patients with clinically staged tumors, and date of operation for those with pathologically staged cancers. After screening for eligibility and data completeness, survival between T-categories was explored graphically using the Kaplan-Meier method.7 Pairwise differences in survival between adjacent T-categories were tested using the log rank test.8 Reproducibility of findings was further assessed in subgroup analyses including N-component subgroups, histologic type, region, diagnosis year, and treatment with neoadjuvant therapy. Prognostic groups were assessed using multivariable Cox proportional hazards regression analysis stratified by data source with a distinction between electronic data capturing (EDC) and batch datasets.9
Adjustments were made for age (<65 versus ≥65), sex (male versus female), histologic type (squamous versus non-squamous), and geographic region (Europe, North America, or Rest of World [ROW] versus Asia). All survival and regression analyses were performed using SAS 9.4 (SAS Institute, Cary, NC) or R (R Foundation for Statistical Computing, Vienna, Austria).
T-descriptors were analyzed separately among T1, T2, T3, and T4, to verify that all T-descriptors within the same category had similar survival. Kaplan Meier curves were used to visualize clinical and pathologic T-descriptors, comparing survival patterns of T-descriptors within a category, and to the T-category one level higher and lower. Any T-descriptor with fewer than 50 cases was collapsed into “Other Single Descriptor” category based on the recommendation that it is questionable to include descriptors in the definition of stage classification that are reported in less than 50 cases as, generally, there are too few events to permit a robust survival analysis.6 If more than one T-descriptor was reported, the patient was included in a “Multiple Descriptors” category. Descriptors identified for potential reclassification, based on their respective survival outcome compared with other descriptors in the same or adjacent category, were then evaluated in a multivariate Cox regression analysis stratified by data source and adjusted for age, sex, histologic type, and geographic region. Specific comparisons were made between the survival of patients with a given descriptor against other cases within its category as defined by the 8th edition, and against those in the proposed category. If a given descriptor was significantly different from others in the same 8th edition category, and similar to those in an adjacent category, in both the clinical and pathologic analysis, it was considered to be evidence in support of potential re-categorization. Hazard ratios for individual T-descriptors were compared to the survival of patients in the T-category above and below.
Decisions on recommendations
Preliminary analyses of the new IASLC database were presented at several virtual meetings of the SPFC Core Committee including CRAB statisticians and at an in-person meeting on August 5, 2022 during the World Conference on Lung Cancer (WCLC) in Vienna, Austria. Final recommendations were agreed upon after discussion of the new analyses during a virtual meeting on October 4, 2022. Further detailed statistical analyses were conducted by CRAB and finalized on October 4, 2023.
RESULTS
T-component validation
The existing T-component categories of the 8th edition (Supplemental Table 1) were validated in the 9th edition dataset. These analyses generally demonstrated good, ordered discrimination between the various T categories and subcategories in various N and R-status cohorts. Furthermore, assessments of generalizability were undertaken.
Survival curves of patients with clinical and pathologic stage tumors by T categories demonstrated consistent ordering with increasing T in an R-any, N0 cohort (Fig 1 A,B) and in an N-any R0 cohort (Fig 2 A,B). The differences between adjacent T categories and subcategories in these graphs are generally clinically and statistically significant. The validation of the existing 8th edition T descriptors was further confirmed in clinical and pathologic stage tumors with multivariable regression analyses (Table 2), which adjusted for confounders of age, sex, histologic type, region and stratified by data source.
Figure 1A and 1B.

Survival curves for the different T-categories to validate the 8th edition criteria for M0,N0, any R cases (clinical staging, left panel; pathological staging, right panel).
Figure 2A and 2B.
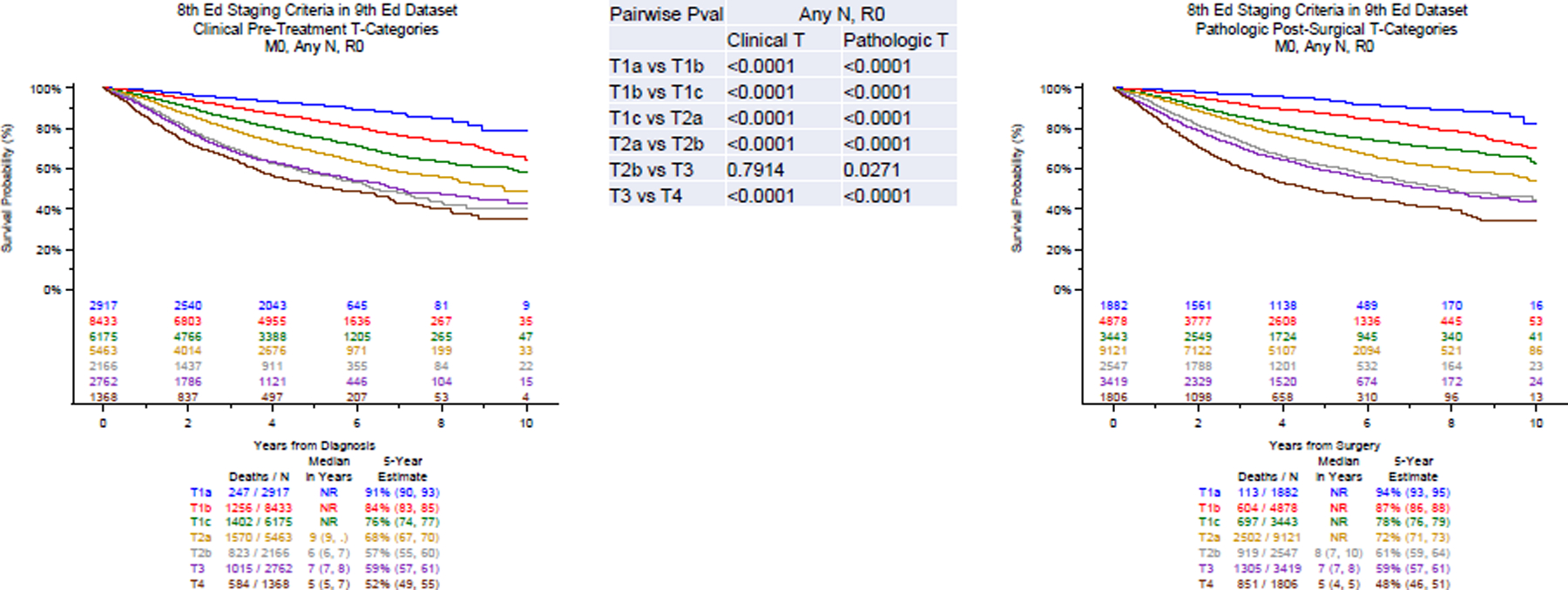
Survival curves for the different T-categories to validate the 8th edition criteria for R0 cases only ( clinical staging, left panel; pathological staging, right panel).
Table 2.
Multivariable survival analyses of T-component stratified by data source. Clinical T-component (upper panel), pathologic T-component (lower panel). Hazard Ratios reflect the risk associated among those with the trait, versus the reference category in parentheses. P-value from Wald χ2 test in adjusted Cox regression.
| Clinical T-component N=33,545; R2=36.3536 |
|||
|---|---|---|---|
| n/N (%) | HR (95% CI) | P-value | |
| T1b (vs T1a) | 8,937/33,545 (26.64%) | 1.81 (1.59–2.07) | <.0001 |
| T1c (vs T1b) | 6,664/33,545 (19.87%) | 1.54 (1.43–1.65) | <.0001 |
| T2a (vs T1c) | 6,290/33,545 (18.75%) | 1.36 (1.27–1.45) | <.0001 |
| T2b (vs T2a) | 2,512/33,545 (7.49%) | 1.35 (1.25–1.45) | <.0001 |
| T3 (vs T2b) | 3,598/33,545 (10.73%) | 1.10 (1.01–1.19) | 0.0239 |
| T4 (vs T3) | 2,475/33,545 (7.38%) | 1.52 (1.41–1.63) | <.0001 |
| Age 65 or Older (vs younger than 65) | 18,962/33,545 (56.53%) | 1.43 (1.37–1.50) | <.0001 |
| Female (vs Male) | 17,603/33,545 (52.48%) | 0.96 (0.92–1.00) | 0.0392 |
| Europe (vs Asia) | 4,002/33,545 (11.93%) | 1.55 (1.45–1.65) | <.0001 |
| North America (vs Asia) | 6,256/33,545 (18.65%) | 1.33 (1.26–1.41) | <.0001 |
| Rest of World (vs Asia) | 927/33,545 (2.76%) | 1.80 (1.59–2.04) | <.0001 |
| Squamous (vs Non-squamous) | 8,133/33,545 (24.25%) | 1.40 (1.34–1.47) | <.0001 |
|
Pathologic T-component
N=28,771; R 2 =34.5095 |
|||
| n/N (%) | HR (95% CI) | P-value | |
| T1b (vs T1a) | 5,105/28,771 (22.09%) | 1.97 (1.62–2.40) | <.0001 |
| T1c (vs T1b) | 3,604/28,771 (15.57%) | 1.64 (1.47–1.82) | <.0001 |
| T2a (vs T1c) | 9,648/28,771 (24.96%) | 1.36 (1.25–1.48) | <.0001 |
| T2b (vs T2a) | 2,707/28,771 (8.07%) | 1.32 (1.23–1.42) | <.0001 |
| T3 (vs T2b) | 3,706/28,771 (13.36%) | 1.11 (1.02–1.20) | 0.0115 |
| T4 (vs T3) | 2,046/28,771 (7.54%) | 1.40 (1.29–1.52) | <.0001 |
| Age 65 or Older (vs younger than 65) | 15,377/28,771 (49.29%) | 1.45 (1.38–1.52) | <.0001 |
| Female (vs Male) | 14,677/28,771 (46.91%) | 0.85 (0.81–0.89) | <.0001 |
| Europe (vs Asia) | 3,310/28,771 (15.61%) | 1.41 (1.31–1.52) | <.0001 |
| North America (vs Asia) | 5,741/28,771 (23.85%) | 1.34 (1.26–1.43) | <.0001 |
| Rest of World (vs Asia) | 1,424/28,771 (6.12%) | 1.38 (1.25–1.52) | <.0001 |
| Squamous (vs Non-squamous) | 6,848/28,771 (23.09%) | 1.31 (1.24–1.38) | <.0001 |
HR, Hazard Ratio; 95% CI, 95% confidence interval; n, number of patients with the trait; N, total number of patients evaluated; %, percent with the trait; vs, versus
Although appropriate ordering was maintained, the pairwise comparison of survival of patients with T2b and T3 tumors was small and not statistically significant. However, the adjusted multivariate analyses did confirm statistically different outcomes in both clinical and pathologic stage tumors (Table 2).
Generalizability of the T-category classification was further analyzed in multiple subsets. In general, ordering and discrimination were demonstrated, although the assessment is hampered in some subsets by limited sample size and likely confounders. Survival graphs were evaluated for patients with clinical and pathologic stage tumors by histologic subsets (squamous vs other NSCLC; Supplemental Fig. 3), subsets by region (Asia, Europe, North America, ROW; Supplemental Fig. 4 and 5), T categories in subsets by N status (N any, N1, N2 and N3 (Supplemental Fig. 6 and 7), subsets by treatment approach (surgical versus nonsurgical treatment, and primary resection versus neoadjuvant therapy and resection (Supplemental Fig. 8), diagnosis before 2018 or from 2018 on (Supplemental Fig. 9), and finally subsets by EDC and batch data only (Supplemental Fig. 10). As shown in these supplemental figures, significant differences are noted between various regions, histological categories, etc. Generally, a patient with cT1a NSCLC (any N, any R) has a 5-year survival rate of 91%, but for cT1a squamous cell carcinoma 5-year survival drops to 70% in contrast to cT1a non-squamous cell carcinoma with a 5-year survival rate of 92%. An Asian patient with cT1a NSCLC has a 5-year survival rate of 93%, in contrast to 79% for a North American patient, and 71% for an European patient. Some of these subset analyses are likely strongly influenced by confounding factors, e.g., patients receiving neoadjuvant therapy are very likely to have had nodal involvement, and likely to have had a reasonable response, thus undergoing subsequent resection.
The impact of the T category is potentially obscured by the impact of strong confounders. The granularity of the available data did not allow exploration, or adjustment for such potential confounders.
Separate analysis of the different T-descriptors were made regarding clinical and pathologic T1 and T2 size only (Supplemental Fig. 11), clinical T2-descriptors (Supplemental Fig. 12), pathologic T2-descriptors (Supplemental Fig. 13), clinical T3-descriptors (Supplemental Fig. 14), pathologic T3-descriptors (Supplemental Fig. 15), clinical T4-descriptors (Supplemental Fig. 16), and pathologic T4-descriptors (Supplemental Fig. 17). In general, a good discrimination was obtained between the various T-categories. Some T-descriptors may be considered for upgrade to a higher category but currently, there were no consistent findings between clinical and pathologic staging. In addition, the sample sizes in several subgroups were too small for meaningful analysis. These descriptors should be considered for reevaluation in the 10th edition.
Chest wall and PL 3 invasion
The T-descriptors subcommittee investigated the hypothesis that patients with tumors with obvious chest wall or parietal pleura (PL 3) invasion had a worse prognosis compared to patients with tumors involving the other single T3 descriptors, as was suggested by a detailed analysis of the 8th edition IASLC database.5
No clinically relevant or statistically significant OS differences were seen when comparing patients with clinical stage tumors involving chest wall / PL 3 invasion versus those with tumors involving other single T3 descriptors. This was seen in a cohort with any degree of nodal involvement (N-any; Fig. 3A, 5-year OS 47% versus 51% p=0.0721, respectively), as well as in a cohort without nodal involvement (N0; Fig.3B, 5-year OS 57% versus 56%, p=0.8093). The lack of a survival difference between patients with clinical stage tumors involving chest wall / PL 3 invasion versus those with tumors involving other single T3 descriptors remained in both N-any and N0 cohorts further subdivided into those undergoing surgical or non-surgical treatment (Supplemental Fig. 18 and 19).
Figure 3A and 3B.

Survival curves according to the 8th edition criteria for patients with clinically staged T3 tumors with any nodal involvement (left panel) and N0 only (right panel)
On the other hand, clinically relevant and statistically significant lower survival was apparent when comparing patients with pathologic stage tumors involving chest wall / PL 3 invasion versus those with tumors involving other single T3 descriptors. This was seen in an N-any cohort (Fig. 4A, 5-year OS 53% versus 60%, p<0.0001) as well as in a N0 cohort (Fig 4B, 5-year OS 55% versus 68%, p<0.0001.
Figure 4A and 4B.

Survival curves according to the 8th edition criteria for patients with pathologically staged T3 tumors with any nodal involvement (left panel) and N0 only (right panel)
Finally, the OS of patients with chest wall/PL3 tumors remained better than that of patients with T4 tumors in most analyses (Fig. 3A–3B and 4A), with the exception of a pathologic stage N0, R-any cohort (Fig 4B).
DISCUSSION
In the 8th edition of the TNM classification profound changes were made to the T-descriptors, especially related to specific tumor size.1 Increments of 1 cm were implemented for T1 and T2 tumors with 3 cm remaining the landmark separating T1 from T2 tumors (Supplemental Table 1). T1 tumors were subdivided into T1a, T1b, and T1c, which is especially relevant for smaller lesions that are screening- or incidentally-detected pulmonary nodules. Recently, these tumors have been extensively studied with the main focus on the extent of resection to determine whether sublobar resection, comprising wedge excision and anatomical segmentectomy, is an oncological valid treatment for tumors 2 cm or smaller. Results of two large randomized trials have recently become available.10–12 The Japanese JCOG 0802 randomized phase III trial showed a significant survival advantage for anatomical segmentectomy compared to lobectomy, although local recurrences were more frequently observed in the segmentectomy arm.10 In contrast, the US CALGB 140503 (Alliance) randomized phase III trial showed similar survival results for wedge resections, segmentectomies and lobectomies for early lung cancers up to 2 cm in size, but strict operative criteria were applied consisting of frozen section analysis of hilar lymph node station 10 and a minimum of 2 mediastinal lymph node stations.11–12 A peculiar finding was also that in general, survival was better for Japanese patients compared to North American patients, which is also observed in the IASLC database (Supplemental Fig. 4 and 5). As shown in the supplemental figures, also significant survival differences were observed regarding specific histology (squamous versus non-squamous), region (Asia versus Europe versus North America), treatment (neoadjuvant versus non-neoadjuvant), data collection (EDC versus batch data) and time of diagnosis,. The exact causes for these differences cannot be determined from the database, but are probably multifactorial including genetic background, life style and the fact that most cases came from Asia, which may skew the overall survival results. It should also be noted that we wanted to obtain a dataset that has sufficient sample size to evaluate the different T-descriptors, rather than getting a dataset that is a representative sample of lung cancers worldwide.After thorough analysis of the database to inform the 9th edition of the TNM classification, the descriptors put forward by the 8th edition were found to be valid with a good separation of the different survival curves as outlined in the results.
For the first time, post-induction cases (ypT) could be studied to a larger extent. OS was worse for all T-categories compared to non-induction cases demonstrating that biological behavior may be different with more aggressive tumors undergoing neoadjuvant therapy (Supplemental Fig. 8). However, sample sizes for some subgroups were small; so, the findings should be confirmed in larger patient populations.
As suggested by the T-subcommittee, chest wall and parietal pleural (PL 3) invasion were studied in more detail. Prevalence of these tumors is less than 10%. Even large series may suffer from a selection bias and specific treatment protocols have not been clearly established.13 Prognostic differences between different T3 descriptors were studied in an analysis of the Surveillance, Epidemiology, and End-Results (SEER) database.14 The authors concluded that different T-descriptors have different outcome and that the T3 category should be reconsidered in the upcoming edition of the TNM classification.14
An expert consensus statement on resection of chest wall tumors and reconstruction was recently published.15 The expert group concluded that for NSCLC invading the chest wall, wide excision with neoadjuvant and / or adjuvant therapy is recommended for cancers classified as T3-4N0-1M0.15
When analyzing survival data from the IASLC database used to determine the 8th edition of the TNM classification, a better OS was found in patients with node-negative pT3 tumors defined by size or a separate nodule, compared to pT3 tumors defined by chest wall invasion, parietal pleural invasion, or by multiple T3 features.5 This raised the question whether to subdivide pT3 tumors into pT3a consisting of those tumors defined by size or a separate nodule, and pT3b tumors defined by invasion of the parietal pleura or chest wall. However, to develop proposals for refinement in the 9th edition, a significant difference was only found for pathological staging, and not for clinically staged tumors. Given these inconsistent findings the consensus within the T-subcommittee was that there was insufficient evidence to change the chest wall / PL 3 classification as a T3 descriptor and reallocate it to the T4 category.
Most probably, the discrepancy between clinical and pathological staging of parietal pleura / chest wall invasion can be explained by the low sensitivity of chest CT scanning in this setting, which has been shown to be only 42% in contrast to the detailed pathological evaluation provided in the pT3 descriptor.16
Separate tumor nodules can be categorized as T3, T4 or M1a if they are present in the same lobe, an ipsilateral other lobe, or a contralateral lobe, respectively. A specific paper will address these multiple nodules as was done for the 8th edition by Detterbeck et al. 17
The data submitted to IASLC/CRAB was not sufficiently reliable to analyze the 8th edition TNM proposal to use invasive size rather than total size for the size T-descriptor for part-solid and part-lepidic nonmucinous lung adenocarcinomas, because this was officially proposed by the IASLC in 2016 2 , and adopted by the UICC and AJCC in 2017.2,18,19 The time period for diagnosis of the cases submitted spanned from 2011 to 2019, hence the majority of the cases submitted to IASLC/CRAB were diagnosed before this recommendation was made. Because most of the submitted cases failed to provide the total size in addition to the required solid/invasive size, this point could not be analyzed. It has been shown that application of this principle in pathologic stage I-IIA non-mucinous lung adenocarcinomas may result in downstaging 22% of tumors.20
A similar issue relates to the entities of adenocarcinoma in situ (AIS) and minimally invasive adenocarcinoma (MIA) which were first defined by the IASLC/ATS/ERS adenocarcinoma classification in 2011 and only recognized by the WHO Classification in 2015 21,22. Then AIS and MIA were formally added to the TNM Stage Classification in 2017 near the end of the data collection period for this database, making it difficult to have suitable data to analyze regarding these newly introduced entities.17–19 Also, spread through air spaces (STAS) is considered a specific histologic descriptor and is the subject of a separate paper 23. Efforts will be made to obtain higher quality data on these histologic issues in the collection of data to analyze for the 10th edition.
In conclusion, after analyzing the survival results of the current database established to inform the 9th edition of the TNM classification, the T-subcommittee members proposed no changes to the current 8th edition T-descriptors for the 9th edition. Specific recommendations will be made when preparing the 10th edition database, for which a specific working group has been created. Currently, proposals include further detailed analysis of neoadjuvant ypT cases, different T descriptors, and invasive versus total tumor size for part-solid and part-lepidic lesions in a larger, multicentric patient population. Moreover, analysis in specific subpopulations (e.g. specific regions, obesity, biomarkers) as well as time-based intervals will be considered for the 10th edition.
Supplementary Material
Funding:
This research is supported by a grant from AstraZeneca. This research was supported in part through the NIH/NCI Cancer Center Support Grant P30 CA008748, and R01CA172253. KN acknowledges support from the IASLC for employees of Cancer Research And Biostatistics (CRAB) to attend the IASLC World Conference on Lung Cancer for the Staging Project as statistical consultants.
APPENDIX 1.
IASLC Staging and Prognostic Factors Committee:
Hisao Asamura (chair), Keio University, Tokyo, Japan; Valerie Rusch (chair elect) Memorial Sloan Kettering Cancer Center, New York, New York, USA; Ramón Rami-Porta (past chair), Hospital Universitari Mutua Terrassa, Terrassa, Spain; Luiz Henrique Araujo, Brazilian National Cancer Institute, Rio de Janeiro, Brazil; David Beer, University of Michigan, Ann Arbor, Michigan, USA; Pietro Bertoglio, IRCCS Azienda Ospedaliero Universitaria di Bologna, Bologna, Italy; Ricardo Beyruti, University of São Paulo Medical School, Sao Paolo, Brazil; Andrea Bille, Guy’s Hospital, London, United Kingdom; Souheil Boubia, Department of Thoracic surgery, University Hospital Ibn Rochd, Laboratoire de Pathologie Cellulaire et Moléculaire Hassan II University of Casablanca, Casablanca, Morocco; Elisabeth Brambilla, Centre Hospitalier Universitaire, Grenoble, France, University of Grenoble Alpes, Grenoble, France; A. K. Cangir, Ankara University Faculty of Medicine, Ankara, Turkey; David Carbone, The Ohio State University, Columbus, Ohio, USA; Vanessa Cilento, Cancer Research And Biostatistics, Seattle, Washington, USA; Casey Connolly, IASLC, Denver, Colorado, USA; Gail Darling, University of Toronto, Toronto, Canada; Frank Detterbeck, Yale University School of Medicine, New Haven, Connecticut, USA; Daniel Dibaba, Cancer Research And Biostatistics, Seattle, Washington, USA; Xavier Benoit D’Journo, Aix-Marseille University, Marseille, France; Jessica Donington, University of Chicago, Chicago, Illinois, USA; Wilfried Eberhardt, West German Cancer Centre, University Hospital Essen, Essen, Germany; John Edwards, Northern General Hospital, Sheffield, United Kingdom; Megan Eisele, Cancer Research And Biostatistics, Seattle, Washington, USA; Jeremy Erasmus, M. D. Anderson Cancer Center, Houston, Texas, USA; Wentao Fang, Department of Thoracic Surgery, Shanghai Chest Hospital, Jiaotong University Medical School, Shanghai, People’s Republic of China; Dean Fennell, Leicester Cancer Research Centre, Department of Genetics and Genome Biology, University of Leicester and University Hospital of Leicester National Health Service Trust, Leicester, United Kingdom; Kwun Fong, University of Queensland Thoracic Research Centre, Brisbane, Australia; Françoise Galateau-Salle, Centre Hospitalier Universitaire, Caen, France; Oliver Gautschi, Cancer Center, Cantonal Hospital Lucerne, Lucerne, Switzerland; Ritu R. Gill, Beth Israel Lahey Health, Boston, Massachussetts, USA; Dorothy Giroux, Cancer Research And Biostatistics, Seattle, Washington, USA; Meredith Giuliani, The Princess Margaret Cancer Centre/University Health Network, Toronto, Ontario, Canada; Department of Otolaryngology - Head and Neck Surgery, The University of Toronto, Toronto, Ontario, Canada; Jin Mo Goo, Seoul National University, Seoul, Republic of Korea; Seiki Hasegawa, Hyogo College of Medicine, Nishinomiya, Japan; Emily Goren, Cancer Research And Biostatistics, Seattle, Washington, USA; Fred Hirsch, Center for Thoracic Oncology, Tisch Cancer Institute, Mount Sinai Health System, New York, New York, USA; Antje Hoering, Cancer Research And Biostatistics, Seattle, Washington, USA; Hans Hoffman, Technical University of Munich, Munich, Germany; Wayne Hofstetter, M. D. Anderson Cancer Center, Houston, Texas, USA; James Huang, Memorial Sloan Kettering Cancer Center, New York, New York, USA; Philippe Joubert, Quebec Heart and Lung Institute, Quebec, Canada; Kemp H. Kernstine, The University of Texas Southwestern Medical Center, Dallas, Texas, USA; Keith Kerr, University of Aberdeen, School of Medicine and Dentistry, Aberdeen, United Kingdom; Young Tae Kim, Seoul National University, Seoul, Republic of Korea; Hong Kwan Kim, Samsung Medical Center, Seoul, Republic of Korea; Hedy Kindler, The University of Chicago Medical Center, Chicago, Illinois, USA; Yolande Lievens, Radiation Oncology department, Ghent University Hospital and Ghent University, Ghent, Belgium; Hui Liu, Sun Yat-Sen University Cancer Center, Guangdong Sheng, People’s Republic of China; Donald E Low, Virginia Mason Medical Center, Seattle, Washington, USA; Gustavo Lyons, Buenos Aires British Hospital, Buenos Aires, Argentina; Heber MacMahon, University of Chicago, Chicago, Illinois, USA; Alyson Mahar, School of Nursing, Queen’s University, Ontario, Canada; Mirella Marino, IRCCS Regina Elena National Cancer Institute, Rome, Italy; Edith M. Marom, University of Tel Aviv, the Chaim Sheba Medical Center, Tel Aviv, Israel; José-María Matilla, Valladolid University Hospital, Valladolid, Spain; Jan van Meerbeeck, Antwerp University and Antwerp University Hospital, Antwerp, Belgium; Luis M. Montuenga, Center of Applied Medical Research, University of Navarra, Pamplona, Spain and Centro de Investigación Biomédica en Red de Cáncer, Spain; Andrew G.Nicholson, Royal Brompton and Harefield Hospitals, Guy’s and St Thomas’ NHS Foundation Trust and Imperial College, London, United Kingdom; Katie Nishimura, Cancer Research And Biostatistics, Seattle, Washington, USA; Anna Nowak, University of Western Australia, Perth, Australia; Isabelle Opitz, University Hospital Zurich, Zurich, Switzerland; Meinoshin Okumura, National Hospital Organization Osaka Toneyama Medical Center, Osaka, Japan; Raymond U. Osarogiagbon, Baptist Cancer Center, Memphis, Tennessee, USA; Harvey Pass, New York University, New York, New York, USA; Marc de Perrot, University of Toronto, Toronto, Canada; Helmut Prosch, Medical University of Vienna, Vienna, Austria; David Rice, M. D. Anderson Cancer Center, Houston, Texas, USA; Andreas Rimner, Memorial Sloan Kettering Cancer Center, New York, New York, USA; Robert T. Ripley, Baylor College of Medicine, Michael E. DeBakey Department of Surgery, Houston, Texas, USA; Adam Rosenthal, Cancer Research And Biostatistics, Seattle, Washington, USA; Enrico Ruffini, University of Torino, Torino, Italy; Shuji Sakai, Tokyo Women’s Medical University, Tokyo, Japan; Paul Van Schil, Antwerp University and Antwerp University Hospital, (Edegem) Antwerp, Belgium; Navneet Singh, Postgraduate Institute of Medical Education and Research, Chandigarh, India; Francisco Suárez, Clínica Santa María, Santiago, Chile; Ricardo M. Terra, University of Sao Paulo, Sao Paulo, Brazil; William D Travis, Memorial Sloan Kettering Cancer Center, New York, New York, USA; Ming S. Tsao, Princess Margaret Cancer Centre, Toronto, Canada; Paula Ugalde, Brigham & Women’s Hospital, Boston, Massachusetts, USA; Shun-ichi Watanabe, National Cancer Center Hospital, Tokyo, Japan; Ignacio Wistuba, The University of Texas M. D. Anderson Cancer Center, Houston, Texas, USA; Murry Wynes, IASLC, Denver, Colorado, USA; Yasushi Yatabe, National Cancer Center Hospital, Tokyo, Japan.
Advisory Board to the Lung Cancer Domain:
Samuel Armato, The University of Chicago, Chicago, USA; Lawek Berzenji, University of Antwerp, Antwerp, Belgium; Alex Brunelli, St. James’s University Hospital, Leeds, UK; Giuseppe Cardillo, Azienda Ospedaliera San Camilo Forlanini, Rome, Italy; Jason Chang, Memorial Sloan Kettering Cancer Center, New York, New York, USA; Keneng Chen, Peking University, Beijing Cancer Hospital, Beijing, China; Wendy Cooper, Royal Prince Alfred Hospital, NSW Health Pathology, Sydney, Australia; Pier Luigi Filosso, University of Torino, Torino, Italy; Liyan Jiang, Shanghai Chest Hospital, Shanghai, People’s Republic of China; Nagla Karim, Inova Cancer Institute-University of Virginia, Virginia, USA; Peter Kneuertz, The Ohio State University College of Medicine, Ohio, USA; Mark Krasnik, Gentofte University Hospital, Copenhagen, Denmark; Kaoru Kubota, Nippon Medical School Hospital, Tokyo, Japan; Catherine Labbe, Quebec Heart and Lung Institute, Quebec, Canada; Ho Yun Lee, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea; Eric Lim, Imperial College and the Royal Brompton Hospital, London, United Kingdom; Geoffrey Liu, Princess Margaret Cancer Centre, University of Toronto, Toronto, Canada; Hongxu Liu, Cancer Hospital of China Medical University, Liaoning Cancer Hospital & Institute, Liaoning, China; Philip Mack, Mount Sinai, New York, New York, USA; David Naidich, NYU-Langone Medical Center, New York, New York, USA; Mizuki Nishino, Brigham and Women’s Hospital and Dana-Farber Cancer Institute, Boston, Massachusetts, USA; Marcin Ostrowski, Medical University of Gdańsk, Gdańsk, Poland; Charles Powell, Mount Sinai School of Medicine, New York, New York, USA; Carolyn Presley, The Ohio State University, Ohio, USA; Paul Martin Putora, Kantonsspital St.Gallen, St. Gallen, Switzerland; Natasha Rekhtman, Memorial Sloan Kettering Cancer Center, New York, New York, USA; Harry Ren, Shanghai Pulmonary Hospital, Shanghai, China; M Patricia Rivera, University of North Carolina, Dept of Medicine, Chapel Hill, North Carolina, USA; Gaetano Rocco, Memorial Sloan Kettering Cancer Center, New York, New York, USA; Maria Teresa Ruiz Tzukazan, Pontifical Catholic University of Rio Grande do Sul, PUCRS, Porto Alegre, Brazil; Robert Samstein, Mount Sinai, New York, New York, USA; Yu Yang Soon, National University Hospital, Harvard University Hospital, Singapore; Kenichi Suda, Kindai University Faculty of Medicine, Osaka, Japan; Martin Tammemägi, Department of Community Health Sciences, Ontario, Canada; Lynn Tanoue ,Yale University, Dept of Medicine, New Haven, Connecticut, USA; Akif Turna, Istanbul University, Cerrahpasa Medical School, Istanbul, Turkey; Benny Weksler, University of Tennesse Health Science Center, Tennessee, USA; Terence Williams, City of Hope comprehensive cancer center, California, USA ; Dawei Yang Zhongshan Hospital Fudan University, Shanghai, China; Jeff Yang, Massachusetts General Hospital/Harvard Medical School, Massachusetts, USA; Masaya Yotsukura, Department of Thoracic Surgery, National Cancer Center Hospital, Tokyo, Japan.
Advisory Board to the Thymic Tumor Domain:
Usman Ahmad, Cleveland Clinic, Cleveland, Ohio, USA, Thoracic Surgery, Heart, Vascular and Thoracic Institute, Cleveland Clinic and Cleveland Clinic Abu Dhabi, United Arab Emirates; Sarit Appel, Sheba Medical Center, Ramat Gan, Israel; Cecilia Brambilla, Royal Brompton and Harefield hospital, Guy’s and St. Thomas NHS Foundation Trust, London, UK; Conrad B. Falkson, Queen’s University, Kingston, Ontario, Canada; Pier Luigi Filosso, University of Torino, Torino, Italy; Giuseppe Giaccone, Weill-Cornell Medicine, New York, New York, USA; Francesco Guerrera, University of Torino, Torino, Italy; Maurizio Infante, University and Hospital Trust Azienda Ospedaliera Universitaria Integrata, Verona, Italy; Dong Kwan Kim, Asan Medical Center, Seoul, and University of Ulsan College of Medicine, Seoul, Republic of Korea; Marco Lucchi, Division of Thoracic Surgery, Azienda Ospedaliero-Universitaria Pisana, Pisa, Italy; Anja Roden, Laboratory Medicine and Pathology, Mayo Clinic Rochester, Minnesota, USA; Charles B. Simone II, New York Proton Center and Memorial Sloan Kettering Cancer Center, New York, USA.
Advisory Board to the Esophageal Cancer Domain:
Mark Ferguson, The University of Chicago, Chicago, USA.
Advisory Board to the Mesothelioma Domain:
Jennifer Sauter, Memorial Sloan Kettering Cancer Center, New York, New York, USA; Andrea Wolf, Icahn School of Medicine at Mount Sinai, New York, New York, USA.
APPENDIX 2. Chairpersons and Members of the Subcommittees of the Lung Cancer, Thymic Epithelial Tumors, Pleural Mesothelioma and Esophageal Cancer Domains of the IASLC Staging and Prognostic Factors Committee
IASLC Staging and Prognostic Factors Committee Chair: Hisao Asamura.
Lung Cancer Domain
Lung Cancer Domain Chair: Paul Van Schil.
Lung Cancer Domain Vice Chair: Kemp H. Kernstine.
Lung Cancer Domain T Descriptors Subcommittee
Hisao Asamura (chair), Young Tae Kim (co-chair) Pietro Bertoglio, Ayten K. Cangir, Jessica Donington, Wentao Fang, Yolande Lievens, Hiu Liu, Gustavo Lyons, Shuji Sakai, William Travis, Paula Ugalde, Paul Van Schil, Jeff Yang, Masaya Yotsukura.
Lung Cancer Domain N Descriptors Subcommittee
James Huang (chair), Raymond U. Osarogiagbon (co-chair), Andrea Bille, Giuseppe Cardillo, Kemp H. Kernstine, Hong Kwan Kim, Kaoru Kubota, Yolande Lievens, Eric Lim, Edith M. Marom, Helmut Prosch, Paul Martin Putora, David Rice, Gaetano Rocco, Valerie Rusch, Paul Van Schil, Isabelle Opitz, Francisco Suárez, Jeff Yang, Shun-ichi Watanabe.
Lung Cancer Domain M Descriptors Subcommittee
Kwun Fong (chair), Wilfried Eberhardt (co-chair), Jeremy Erasmus, Yolande Lievens, Mirella Marino, Edith M. Marom, Paul Martin Putora, Navneet Singh, Francisco Suárez.
Lung Cancer Domain Lepidic & GGO Subcommittee
William Travis (chair), Philippe Joubert (co-chair), Hisao Asamura, Frank Detterbeck, Giuseppe Cardillo, Wendy Cooper, Ritu R. Gill, Jin Mo Goo, Young Tae Kim, Ho Yun Lee, Heber MacMahon, Edith M. Marom, David Naidich, Andrew G. Nicholson, Mizuki Nishino, Helmut Prosch, Ramon Rami-Porta, Valerie Rusch, Shuji Sakai, Yasushi Yatabe, Shun-ichi Watanabe.
Lung Cancer Domain Neuroendocrine Tumors Subcommittee
Ming Tsao (chair), Andrew G. Nicholson, (co-chair), Ricardo Beyruti, Frank Detterbeck, Wilfried Eberhardt, Pier Luigi Filosso, Yolande Lievens, Eric Lim, Geoffrey Liu, José-María Matilla, Natasha Rekhtman, William Travis, Jeff Yang, Yasushi Yatabe.
Lung Cancer Domain Stage Group Subcommittee
Hisao Asamura (chair), Giuseppe Cardillo, Frank Detterbeck, John Edwards, Kwun Fong, Meredith Giuliani, James Huang, Kemp H. Kernstine, Edith M. Marom, Andrew G. Nicholson, Ramón Rami-Porta, William Travis, Ming Tsao, Paul Van Schil, Shun-ichi Watanabe.
Lung Cancer Domain Lymph Node Chart Subcommittee
Shun-ichi Watanabe (chair), Jin Mo Goo (co-chair), Hisao Asamura, Hans Hoffman, James Huang, Kemp H. Kernstine, Yolanda Lievens, Raymond U. Osarogiagbon, Paul Martin Putora, Ramón Rami-Porta, Valerie Rusch, Paul Van Schil, Jeff Yang.
Lung Cancer Domain Validation and Methodology Subcommittee
Frank Detterbeck (chair), Alyson Mahar (co-chair), Hisao Asamura, Meredith Giuliani, Mirella Marino, Raymond U. Osarogiagbon, Valerie Rusch.
Lung Cancer Domain Prognostic Factors Subcommittee
Frank Detterbeck (chair), Raymond U. Osarogiagbon (co-chair), Alex Brunelli, Kwun Fong, Meredith Giuliani, James Huang, Young Tae Kim, Mark Krasnik, Hiu Liu, Jan van Meerbeeck, Luis M. Montuenga, Andrew G. Nicholson, Paul Martin Putora, Valerie Rusch, Robert Samstein, Navneet Singh, Martin Tammemägi, Ricardo Terra, Ming Tsao, Akif Turna, Terence Williams.
Lung Cancer Domain R Factor Subcommittee
John Edwards (chair), Marcin Ostrowski (co-chair), Souheil Boubia, Jessica Donnington, Hans Hoffman, Maurizio Infante, Mirella Marino, Edith M. Marom, Jun Nakajima, Andrew G. Nicholson, Paul Van Schil, William Travis, Ming Tsao, Yasushi Yatabe.
Lung Cancer Domain Imaging Subcomittee
Jim Mo Goo (chair), Ritu R. Gill (co-chair), Helmut Prosch (co-chair), Samuel Armato, Hui Liu, Heber MacMahon, Edith M. Marom, David Naidich, Charles Powell, Paul Van Schil, William Travis.
Lung Cancer Domain Multiple Pulmonary Nodules Subcommittee
Frank Detterbeck (chair), Edith Marom (co-chair), Sarit Appel, Jason Chang, Keneng Chen, Nicolas Girard, Jin Mo Goo, Young Tae Kim, Heber MacMahon, Andrew G. Nicholson, Paul Martin Putora, Natasha Rekhtman, M Patricia Rivera, Lynn Tanoue, Ricardo M. Terra, William Travis, Paula Ugalde, Yasushi Yatabe.
Lung Cancer Domain Molecular Subcommittee
David Carbone (co-chair), Fred Hirsch (co-chair), Luiz Henrique Araujo, Hisao Asamura, Elisabeth Brambilla, Jason Chang, Frank Detterbeck, Oliver Gautschi, Nagla Karim, Keith Kerr, Peter Kneuertz, Eric Lim, Philip Mack, José-María Matilla, Luis M. Montuenga, Andrew G. Nicholson, Raymond U. Osarogiagbon, Harvey Pass, Carolyn J Presley, Ramón Rami-Porta, Natasha Rekhtman, Harry Ren, Robert Samstein, Kenichi Suda, Ricardo M. Terra, William Travis, Ming Tsao, Terence Williams, Ignacio Wistuba, Dawei Yang, Yasushi Yatabe.
Lung Cancer Domain Database
Paula Ugalde (chair), Pietro Bertoglio (co-chair), Sarit Appel, Philippe Joubert, Catherine Labbe, Hongxu Liu, Gustavo Lyons, José-María Matilla, Robert Samstein, Ricardo Terra, Maria Teresa Ruiz Tzukazan, Benny Weksler.
Cancer Research And Biostatistics
Vanessa Cilento, Daniel Dibaba, Megan Eisele, Dorothy Giroux, Emily Goren, Antje Hoering, Katie Nishimura, Adam Rosenthal.
Thymic Epithelial Tumors Domain
Enrico Ruffini (chair), James Huang (co-chair), Usman Ahmad, Sarit Appel, Andrea Bille, Souheil Boubia, Cecilia Brambilla, Ayten K. Cangir, Frank Detterbeck, Conrad Falkson, Wentao Fang, Pier Luigi Filosso, Giuseppe Giaccone, Nicolas Girard, Francesco Guerrera, Maurizio Infante, Dong Kwan Kim, Marco Lucchi, Mirella Marino, Edith M. Marom, Andrew Nicholson, Meinoshin Okumura, Andreas Rimner, Anja Roden, Charles B. Simone II.
Thymic Domain T descriptor:
Andrew Nicholson (chair), Cecilia Brambilla, Ayten K. Cangir, Maurizio Infante, Mirella Marino, Edith M. Marom, Meinoshin Okumura.
Thymic Domain N descriptor:
Wentao Fang (chair), Frank Detterbeck, Pier Luigi Filosso, Marco Lucchi, Edith M. Marom, Charles B. Simone II.
Thymic Domain M descriptor:
Nicolas Girard (chair), Usman Ahmad, Sarit Appel, Conrad Falkson, Wentao Fang, Giuseppe Giaccone, Dong Kwan Kim, Edith M. Marom, Andreas Rimner.
Thymic Domain Database subcommittee:
Pier Luigi Filosso (chair), Usman Ahmad, Andrea Billè, Souheil Boubia, Frank Detterbeck, Wentao Fang, Nicolas Girard, Francesco Guerrera, James Huang, Dong Kwan Kim, Meinoshin Okumura, Enrico Ruffini.
Pleural Mesothelioma Domain
Valerie Rusch (chair), Anna Nowak (co-chair), Pietro Bertoglio, Andrea Billè, Ayten K. Cangir, Dean Fennell, Françoise Galateau, Ritu R. Gill, Seiki Hasegawa, Hong Kwan Kim, Hedy Kindler, Jan van Meerbeeck, Isabelle Opitz, Harvey Pass, Marc de Perrot, David Rice, Andreas Rimner, Robert T. Ripley, Jennifer Sauter, Ming Tsao, David Waller, Andrea Wolf.
Esophageal Cancer Domain
Wentao Fang (chair), Xavier D’Journo (co-chair), Gail Darling, Jeremy Erasmus, Mark Ferguson, Wayne Hofstetter, Hong Kwan Kim, Donald Low, Paula Ugalde.
APPENDIX 3. Participating Institutions in the third phase of the IASLC Lung Cancer Staging Project
Participating institutions ordered by number of eligible cases submitted
I. Yoshino, Japanese Joint Lung Cancer Registry, Chiba, Japan (23,663 cases); T. Muley, Thoraxklinik, University Hospital Heidelberg, Heidelberg, Germany (8887 cases); W. Li, CAALC: West China Hospital, Sichuan University, Chengdu, China (7345 cases); Y. Kim, Korean Association for Lung Cancer, Seoul, South Korea (4622 cases); H.K. Kim, Samsung Medical Center, Seoul, South Korea (4130 cases); F. Griesinger, CRISP, Berlin, Germany (5482 cases)*; J. Huang, Memorial Sloan Kettering Cancer Center, New York, USA (3146 cases); R. Osarogiagbon, Baptist Memorial Hospital, Memphis, USA (3021cases); S. Park, Seoul National University Hospital, Seoul, South Korea (2542 cases); G. Liu, Princess Margaret Cancer Center, Toronto, Canada (2280 cases); N. Singh, Postgraduate Institute of Medical Education & Research (PGIMER), Chandigarh, India (2060 cases); P. Ugalde Figueroa, IUCPQ - Université Laval, Quebec, Canada (2018 cases); P. Kneuertz, The Ohio State University, Columbus, USA (1819 cases); J. Shih, Taiwan Society of Pulmonary and Critical Care Medicine, Taipei, Taiwan (1481 cases); S. Jordan, The Royal Brompton Hospital & E. Beddow, Harefield Hospital, part of Guy’s & St. Thomas’ NHS Foundation Trust, London, UK (1434 cases); B. McCaughan, University of Sydney, Newtown, Australia (1368 cases); H. Liu, Liaoning Cancer Hospital, Shenyang, China (1161 cases); A. K. Cangir, Ankara University School of Medicine, Ankara-Sihhiye, Turkey (887 cases); A. Billè, Guy's Hospital, London, UK (882 cases); F. Leo, S Luigi Hospital, University of Turin, Orbassano, Torino, Italy (840 cases); H. Liu, Sun Yat-sen University Cancer Center, Guangzhou, China (825 cases); M. Redman, SWOG-0819, Seattle, USA (782 cases); H. Pass, NYU Langone Medical Center and Cancer Center, New York, USA (762 cases); J. Sun, CAALC: Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China (634 cases); K. Fong, The University of Queensland TPCH Thoracic Research Centre, Brisbane, Australia (577 cases); R. Terra, University of Sao Paulo Medical School, Sao Paulo, Brazil (555 cases); N. Wu, Second Department of Thoracic Surgery, Peking University Cancer, Beijing, China (455 cases); K. Chen, First Department of Thoracic Surgery, Peking University Cancer H, Beijing, China (451 cases); A. Mohan, All India Institute of Medical Sciences, New Delhi, India (448 cases); P. Van Schil, University Hospital Antwerp, Dept of Pneumology, Edegem, Belgium (304 cases); P. Bertoglio, IRCCS Sacro Cuore-Don Calabria Hospital, Negrar, Italy (298 cases); C. Yang, Massachusetts General Hospital, Boston, USA (295 cases); R. Moises, Hospital de Rehabilitación Respiratoria María Ferrer, Buenos Aires, Argentina (264 cases); A. Turna, Istanbul University-Cerrahpasa, Cerrahpasa Medical Faculty, Istanbul, Turkey (238 cases); A. Celik, Gazi University Faculty of Medicine, Ankara, Turkey (193 cases); M. Modesto Alapont, GCCB3: Consorcio Hospitalario Provincial de Castellón, Castellón, Spain (165 cases); L. Sánchez Moreno and M. Zabaleta Murguiondo, GCCB3: Hospital Universitario Marqués de Valdecilla, Santander, Spain (165 cases); C. Longo, Instituto COI, Rio de Janeiro, Brazil (150 cases); H. Zhou, Suining Central Hospital, Suining, China (147 cases); E. Pirondini, ASST San Gerardo, Monza, Italy (144 cases); G. Lyons, Hospital Británico de Buenos Aires, Buenos Aires, Argentina (143 cases); I. Gkiozos, Athens School of Medicine, Athens, Greece (133 cases); K. Kernstine, UT Southwestern Medical Center at Dallas, Dallas, USA (132 cases); M. Serra Mitjans and R. Costa, GCCB3: Hospital Mútua Terrassa. Barcelona (124 cases); M. Genovés Crespo and A. Nuñez Ares, GCCB3: Complejo Hospitalario Universitario of Albacete, Albacete, Spain (114 cases); C. Lee, Seoul National University Bundang Hospital, Seongnam, South Korea (104 cases); Y.K. Pang, Malaysian Thoracic Society, Kuala Lumpur, Malaysia (99 cases); N. Evans, Thomas Jefferson University Hospital, Philadelphia, USA (98 cases); F. Hirsch, Icahn School of Medicine at Mount Sinai, New York, USA (84 cases); M. Ridai, University Hospital of Casablanca, Casablanca, Morocco (83 cases); C. Martínez Barenys and J. Sanz Santos, GCCB3: Hospital Universitari Germans Trias i Pujol, Badalona, Spain (77 cases); J. Sauleda Roig, Hospital Universitari Son Espases, Palma de Mallorca, Spain (76 cases); H. Hoffmann, University of Munich - Division of Thoracic Surgery, Munich, Germany (75 cases); M.A. Iñiguez-García, National Institute of Respiratory Diseases, Mexico City, Mexico (74 cases); L.H. de Lima Araujo, Brazilian National Cancer Institute (INCA), Rio de Janeiro, Brazil (72 cases); C. Grohé, Evangelische Lungenklinik Berlin - NET Registry, Berlin, Germany (71 cases); D. Ball, Peter MacCallum Cancer Institute, Melbourne, Australia (70 cases); J.C. Peñalver Cuesta, GCCB3: Fundación Instituto Valenciano de Oncología, Valencia, Spain (65 cases); N. Tarek, Ain Shams University Hospitals, Cairo, Egypt (64 cases); D. Yang, CAALC: Zhongshan Hospital Fudan University, Shanghai, China (63 cases); D. Sánchez, GCCB3: Hospital Clínic, Barcelona, Spain (62 cases); J.A. Gullón Blanco, GCCB3: Hospital Universitario San Agustín, Avilés, Asturias, Spain (61 cases); L. Montuenga, CIMA/Clínica Universidad de Navarra, Pamplona, Spain (55 cases); G. Galán Gil and R. Guijarro Jorge, GCCB3: Hospital Clínico Universitario de Valencia, Valencia, Spain (52 cases); C. García Rico, J.M. Matilla and B. de Vega Sánchez, GCCB3: Hospital Clínico Universitario de Valladolid, Valladolid, Spain (50 cases); A. Rodríguez Fuster and V. Curall, GCCB3: Hospital del Mar, Barcelona, Spain (50 cases); L. Miravet, GCCB3: Hospital La Plana, Castellón, Spain (49 cases); J. Abal Arca and I. Parente Lamelas, GCCB3: Complexo Hospitalario Universitario Ourense, Ourense, Spain (48 cases); E. Melis, IRCCS Regina Elena National Cancer Institute, Rome, Italy (41 cases); S. García Fuika, GCCB3: Hospital UA Txagorritxu, Vitoria-Gasteiz, Spain (34 cases); K. Tournoy, University Hospital Ghent, Ghent, Belgium (33 cases); M. Zuil Martín, GCCB3: Hospital Royo Villanova, Zaragoza, Spain (31 cases); L. García Aranguena, GCCB3: Hospital Sierrallana, Torrelavega, Cantabria, Spain (28 cases); O. Arrieta, Instituto Nacional de Cancerología, Mexico City, Mexico (28 cases); M. G. Blum , Penrose Cancer Center, Colorado Springs, USA (28 cases); D. Mishra, BP Koirala Institute of Health Sciences, Dharan, Nepal (25 cases); J.M. García Prim, Hospital Clínico Universitario de Santiago, Santiago de Compostela, Spain (25 cases); M. Mariñán Gorospe, Hospital San Pedro de Logroño, Logroño, Spain (24 cases); R. Stirling, The Alfred Hospital, Melbourne, Australia (23 cases); B. Steen, GCCB3: Hospital de Alcorcón, Madrid, Spain (23 cases); D. Chimondeguy, Hospital Universitario Austral, Buenos Aires, Argentina (22 cases); F.J. Montoro Zulueta, GCCB3: Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain (22 cases); M. Paradela de la Morena and A. Souto Alonso, GCCB3: Complejo Hospitalario Universitario de A Coruña, La Coruña, Spain (21 cases); R. Cordovilla and T. Gómez Hernández, GCCB3: Hospital Universitario de Salamanca, Salamanca, Spain (21 cases); C. Thomas, Mayo Clinic Rochester, Rochester, Minessota, USA (20 cases); J. Hernández Hernández, and I. Lobato Astiárraga, GCCB3: Complejo Asistencial de Ávila, Ávila, Spain (19 cases); I. Macía Vidueira and S.Padrones, GCCB3: Hospital de Bellvitge, Barcelona, Spain (16 cases); J.R. Jarabo Salcedo and B. Morales Chacón, GCCB3: Hospital Clínico San Carlos, Madrid, Spain (16 cases); Y. L. Wu, Guangdong General Hospital, Guangzhou, China (15 cases); E. Martínez Tellez, J.C. Trujillo and V. Pajares Ruiz, GCCB3: Hospital de la Santa Creu i Sant Pau, Barcelona, Spain (14 cases); L. Bai, CAALC: Xinqiao Hospital, No. 3 Army Medical University, Chongqing, China (14 cases); R. Magaroles and L. de Esteban Júlvez, Hospital Universitari Joan XXIII, Tarragona, Spain (14 cases); R. Melchor Íñiguez, Fundación Jiménez Díaz, Madrid, Spain (14 cases); I.R. Embun Flor and P.Teller Justes, GCCB3: Hospital Clínico Universitario Lozano Blesa, Zaragoza, Spain (13 cases); C.M. Ariza Prota, GCCB3: Hospital Universitario Asturias, Oviedo, Spain (13 cases); M. J. Pavón Fernández, Hospital Severo Ochoa, Leganés, Spain (13 cases); J. Menéndez, Hospital General de Agudos José M. Penna, Buenos Aires, Argentina (11 cases); S. Defranchi, Hospital Universitario-Fundación Favaloro, Buenos Aires, Argentina (11 cases); E. Martínez Tellez, Hospital de Terrassa, Terrassa, Spain (11 cases).
The following institutions submitted ten eligible cases or less listed alphabetically.
M. Curado, A.C. Camargo Cancer Center, Sao Paulo, Brazil; A. Badawy, Alexandria University, Alexandria, Egypt; X. Zhang, CAALC: Henan Provincial People's Hospital, Zhengzhou, China; Q. Wang, CAALC: The Second Hospital of Dalian Medical University, Dalian, China; S. Han, CAALC: Zhongda Hospital Affiliated to Southeast University, Nanjing, China; D. Levy Faber, Carmel Medical Center, Haifa, Israel; P. García Herreros, Clínica Cardiovid, Medellín, Antioquia, Colombia; F. Suárez, Clínica Santa María, Santiago, Chile; D. Subotic, Clinical Center of Serbia, Belgrade, Serbia; T. Horvath, Czech Republic-Urazova nemocnice Brno, BRNO, Czech Republic; M. Velásquez, Fundación Clínica Valle del Lili, Cali, Colombia; T. Ruiz Albi, GCCB3: Hospital Río Hortega, Valladolid, Spain; M. Serraj, Hassan II University Hospital, Fez, Morocco; V. Baysungur, Health Science University Sureyyapasa Thoracic and Chest Disease, Istambul, Turkey; M. Raíces, Hospital Italiano de Buenos Aires, Argentina; M.J. Pavón Fernández, GCCB3: Hospital Severo Ochoa, Leganés, Madrid, Spain; V. Cvijanovic, Military Medical Academy, Belgrade, Serbia; M. Zereu, Pavilhao Pereira Filho, Santa Casa de Porto Alegre, Brazil; W. Aguiar, SECITOR - Servico de Cirurgia Toracica de Recife, Recife, Brazil.
* CRISP is an AIO study (project no. AIO TRK-0315) under the medical leadership of the Executive Committee (Prof. F. Griesinger (Oldenburg), Prof. M. Thomas (Heidelberg), Dr. M. Sebastian (Frankfurt) and Dr. W. Eberhardt (Essen)). CRISP is conducted by AIO-Studien-gGmbH (sponsor) in cooperation with iOMEDICO (conception, project management, analysis). CRISP is supported by AstraZeneca GmbH, Boehringer Ingelheim Pharma GmbH & Co. KG, Bristol-Myers Squibb GmbH & Co. KGaA, Celgene GmbH, Lilly Deutschland GmbH, MSD Sharp & Dohme GmbH, Novartis Pharma GmbH, Pfizer Pharma GmbH, Roche Pharma AG and Takeda Pharma Vertrieb GmbH & Co. KG. However, these companies have no input into or influence over data analysis, data interpretation, or writing of the manuscript.
Footnotes
CRediT Authorship Contribution Statement
Paul E. Van Schil: Conceptualization, Methodology, Investigation, Writing-original draft.
Hisao Asamura: Conceptualization, Methodology, Investigation, Writing-review and editing.
Katherine K. Nishimura, Dorothy Giroux: Methodology, Formal analysis, Investigation, Resources, Data curation, Writing-original draft, Writing-review and editing.
Ramon Rami-Porta, Frank Detterbeck: Conceptualization, Methodology, Investigation, Writing-original draft, Writing-review and editing.
Young Tae Kim, Pietro Bertoglio, Ayten K. Cangir, Jessica Donington, Wentao Fang, Yolande Lievens, Hui Liu, Gustavo Lyons, Shuji Sakai, William D. Travis, Paula Ugalde, Jeff Yang, Masaya Yotsukura: Conceptualization, Writing-review and editing.
Disclosures:
Dr. Van Schil reports consulting fees from AstraZeneca, BMS, MSD, Roche, Janssen; honoraria from AstraZeneca, BMS, MSD, Roche, and Janssen; leadership positions at the IASLC and BACTS. Dr. Asamura has no relevant disclosures. Dr. Nishimura has no relevant disclosures. Dr. Rami-Porta has no relevant disclosures. Dr. Kim reports honoraria from AstraZeneca and Roche. Dr. Bertoglio has no relevant disclosures. Dr. Cangir has no relevant disclosures. Dr. Donington Dr. Fang has no relevant disclosures Ms. Giroux has no relevant disclosures. Dr. Lievens reports grants and contracts for ImmunoSABR EU Project and HERO-VBHC; consulting fees from AstraZeneca. Dr. Liu Dr. Lyons Dr. Sakai Dr. Travis reports grants from the NCI (MSKCC Core Grant: p30 CA008748). Dr. Ugalde reports honoraria from AstraZeneca, MSD, BMS, Medtronic, and Johnson & Johnson. Dr. Yang reports honoraria from AstraZeneca. Dr. Yotsukura has no relevant disclosures. Dr. Detterbeck has no relevant disclosures
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Rami-Porta R, Bolejack V, Crowley J, Ball D, Kim J, Lyons G, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10(7):990–1003. [DOI] [PubMed] [Google Scholar]
- 2.Travis WD, Asamura H, Bankier AA, Beasley MB, Detterbeck F, Flieder DB, et al. The IASLC Lung Cancer Staging Project: Proposals for Coding T Categories for Subsolid Nodules and Assessment of Tumor Size in Part-Solid Tumors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2016;11(8):1204–23. [DOI] [PubMed] [Google Scholar]
- 3.Giroux DJ, Van Schil P, Asamura H, Rami-Porta R, Chansky K, Crowley JJ, et al. The IASLC Lung Cancer Staging Project: A Renewed Call to Participation. J Thorac Oncol 2018;13(6):801–9. [DOI] [PubMed] [Google Scholar]
- 4.Asamura H, Nishimura KK, Giroux DJ, Chansky K, Hoering A, Rusch V et al. Members of the IASLC Staging and Prognostic Factors Committee and of the Advisory Boards, and Participating Institutions. . IASLC lung cancer staging project: the new database to inform innovations in the ninth edition of the TNM classification of lung cancer. J Thor Oncol 2023; 18(5):564–575. [DOI] [PubMed] [Google Scholar]
- 5.Ugalde P, Marques E, Cilento VJ, Giroux D, Nishimura KK, Detterbeck FC et al. Completeness of resection and long-term survival of patients undergoing resection for T3 non-small-cell lung cancer: an International Association for the Study of Lung Cancer analysis. . J Thorac Oncol 2023; S1556-0864(23)01078 [DOI] [PubMed]
- 6.Detterbeck FC, Nishimura KK, Cilento VJ, Giuliani M, Marino M, Osarogiagbon RU, et al. The International Association for the Study of Lung Cancer Staging Project: Methods and Guiding Principles for the Development of the Ninth Edition TNM Classification. J Thorac Oncol 2022;17(6):806–15. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan EL MP. Nonparametric estimation from incomplete observations. . J Am Statist Assoc 1958;53:457–81. [Google Scholar]
- 8.Mantel N Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 1966;50(3):163–70. [PubMed] [Google Scholar]
- 9.Cox D Regression models and life‐tables. . J Royal Statist Assoc 1972;34:187–202. [Google Scholar]
- 10.Saji H, Okada M, Tsuboi M, Nakajima R, Suzuki K, Aokage K, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399(10335):1607–17. [DOI] [PubMed] [Google Scholar]
- 11.Altorki NK, Wang X, Kozono D, Watt C, Landreneau R, Wigle D et al. Lobar or sublobar resection for peripheral clinical stage IA ≤2 cm non-small cell lung cancer (NSCLC): results from an international randomized phase III trial (CALGB 140503 [Alliance]). J Thorac Oncol 2022;17(9):S1–S2 Meeting abstract PL03.6. [Google Scholar]
- 12.Altorki N, Wang X, Kozono D, Watt C, Landrenau R, Wigle D, et al. Lobar or sublobar resection for peripheral stage IA non-small-cell lung cancer. N Engl J Med 2023;388(6):489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanuti M Surgical Management of lung cancer involving the chest wall. Thorac Surg Clin 2017;27(2):195–9. [DOI] [PubMed] [Google Scholar]
- 14.Cai JS, Yang F, Wang X. Is there a prognostic difference among T3N0-3M0 NSCLC patients with different T3 descriptors? Eur J Cardiothorac Surg 2023; 63(3):ezac558. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Yan X, Zhao J, Chen C, Chen C, Chen J, et al. Expert consensus on resection of chest wall tumors and chest wall reconstruction. Transl Lung Cancer Res 2021;10(11):4057–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bandi V, Lunn W, Ernst A, Eberhardt R, Hoffmann H, Herth FJ. Ultrasound vs. CT in detecting chest wall invasion by tumor: a prospective study. Chest 2008;133(4):881–6. [DOI] [PubMed] [Google Scholar]
- 17.Detterbeck FC, Nicholson AG, Franklin WA, Marom EM, Travis WD, Girard N, et al. The IASLC Lung Cancer Staging Project: Summary of Proposals for Revisions of the Classification of Lung Cancers with Multiple Pulmonary Sites of Involvement in the Forthcoming Eighth Edition of the TNM Classification. J Thorac Oncol 2016;11(5):639–50. [DOI] [PubMed] [Google Scholar]
- 18.O'Sullivan B, Mason M, Asamura H. Lung, pleural and thymic tumors In: Brierley JD, Gospodarowicz MK, Wittekind C, editors. UICC TNM Classification of Malignant Tumors, West Sussex, United Kingdom: Wiley, Blackwell; 2017. p. 105–17. [Google Scholar]
- 19.Rami-Porta R, Asamura H, Travis WD, Rusch VW. Lung. In: Amin MB, editor. AJCC Cancer Staging Manual 8th edition. American Joint Committee on Cancer; Chicago, 2017. p. 431–56.20. [DOI] [PubMed] [Google Scholar]
- 20.Kameda K, Eguchi T, Lu S, Qu Y, Tan KS, Kadota K, et al. Implications of the eighth edition of the TNM proposal: invasive versus total tumor size for the T descriptor in pathologic stage I-IIA lung adenocarcinoma. J Thorac Oncol 2018;13(12):1919–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, et al. The New IASLC/ATS/ERS international multidisciplinary lung adenocarcinoma classification. J Thoracic Oncol 2011;6:244–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart Lyon: International Agency for Research on Cancer; 2015, pages 44–50. [DOI] [PubMed] [Google Scholar]
- 23.Travis WD, Eisele M, Nishimura KK, Aly R, Bertoglio P, Detterbeck F et al. The IASLC Lung Cancer Staging Project: Recommendation to introduce Spread Through Air Spaces (STAS) as a histologic descriptor in the 9th edition of the TNM classification of lung cancer. Analysis of 4,061 pathologic stageI non-small cell lung carcinomas. J Thorac Oncol submitted [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


