Abstract
This study aimed to explore the role and mechanism of umbilical cord mesenchymal stem cells (UCMSCs) in regulating inflammation of bronchial epithelial cells. Transforming growth factor beta-1 (TGF-β1) was used to induce inflammation in human bronchial epithelial cells. Cell proliferation was detected through CCK8 and cell apoptosis was detected by Annexin V and propidium iodide double staining. E-cadherin and α-smooth muscle actin (α-SMA) were detected by immunofluorescence, and tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6) in culture medium supernatant were detected by ELISA. The expression of E-cadherin, α-SMA, Sonic hedgehog (Shh), Gli1 and Snail was detected by Western blot analysis. Compared with the control group, bronchial epithelial cells treated with TGF-β1 showed significantly decreased proliferation, increased apoptosis, increased secretion of TNF-α and IL-6, increased expression of α-SMA, Shh, Gli1 and Snail and decreased E-cadherin expression. However, co-culture with UCMSCs inhibited TGF-β1-induced changes in human bronchial epithelial cell proliferation, apoptosis, secretion of TNF-α and IL-6 and activation of the Hedgehog pathway. In conclusion, UCMSCs have protective effects on TGF-β1-induced inflammation in human bronchial epithelial cells by regulating the Hedgehog pathway.
Key words: asthma, umbilical cord mesenchymal stem cells, Hedgehog pathway, inflammation
Introduction
Bronchial asthma is a disease associated with immune dysfunction, and its incidence is increasing worldwide.1 Asthma is characterized by allergy, airway hyperresponsiveness, inflammation, bronchial airway remodeling, and increased number of immune cells in the airways.2 The pathogenesis of asthma is complex, with a chronic inflammatory response involving T cells, monocytes, mast cells, eosinophils, and other cells and their secreted components. Asthma is characterized by the infiltration of epithelial cells with a large number of inflammatory cells, resulting in subepithelial fibrosis and airway remodeling due to the secretion of collagen, and accompanied by the hypertrophy and proliferation of airway smooth muscle cells.2
Mesenchymal stem cells (MSCs), also known as “mesenchymal stromal cells”, are multipotent stromal cells.3 Recent studies have revealed that in addition to repairing damaged tissues and replacing damaged functional cells, MSCs have strong inflammatory and immunomodulatory effects. MSCs play a central role in immunomodulation by secreting cytokines such as interleukin 6 (IL-6), transforming growth factor beta-1 (TGF-β1) and human leukocyte antigen G5 (HLA-G5), which inhibit immune cell responses and T cell activation while promoting the ratio of regulatory T cells (Tregs).4 Studies have shown that MSCs have tremendous regenerative and immunomodulatory potential and have been tested in numerous preclinical and clinical studies in various degenerative, hematological and inflammatory diseases including experimental models of asthma.5
Hedgehog (Hh) is a gene found in Drosophila, and three Hedgehog signaling proteins are expressed in mammals: Sonic hedgehog (Shh), Desert hedgehog (Dhh) and Indian hedgehog (Ihh), of which Ihh is only involved in cartilage development; Dhh is only involved in germ cell development; and Shh is most widely expressed in various tissues and organs.6,7 Hedgehog signaling plays a regulatory role in airway asthma remodeling.8 Therefore, this study aimed to explore whether umbilical cord MSCs (UCMSCs) could inhibit asthma by regulating Hedgehog pathway.
Materials and Methods
Human bronchial epithelial cells
Human bronchial epithelial cell line BEAS-2B was purchased from iCell Bioscience, Inc. (Shanghai, China) and stained for Pan Cytokeratin (PCK) for cell identification. Briefly, cells were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS, pH 7.4) for 10 min at room temperature. The cells were washed with PBS and then incubated with primary antibody for PCK (Cat# bs- 5352r, 1:100 dilution; Bioss, Woburn, MA, USA) overnight at 4°C, washed with PBS and incubated with secondary antibody cy3 (Cat# AS007, 1:200 dilution; ABclonal, Woburn, MA, USA) for 1 h at room temperature. For negative control, the cells were incubated with PBS instead of anti-PCK antibody. The nuclei were stained with 1 μg/mL 4’,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich, St. Louis, MA, USA) in the dark for 1 min at room temperature, and the slices were sealed with blocking solution containing anti-fluorescence quencher, and observed under fluorescence microscope (CKX53; Olympus, Tokyo, Japan) equipped with a Zyla 4.6 sCMOS camera to record the images. Immunolabeling was assessed by ImageJ software analysis program (NIH Image, Bethesda, MD, USA).
Identification of UcMscs
The protocols of isolating UCMSCs were approved by Ethics Committee of Fuzhou No.1 Hospital Affiliated with Fujian Medical University (approval No. 20210752) and volunteers signed informed consents. Umbilical cords were obtained from volunteer women with caesarean delivery, washed with PBS under sterile condition and cut into small segments. After removing blood vessels, the segments were transferred into 25 cm2 flasks with DMEM (Gibco, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS, Gibco) and incubated at 37°C in humidified atmosphere with 5% CO2. The medium was changed every 3 days. After 2 weeks, umbilical cord segments were removed and the adherent UCMSCs were collected, washed twice with PBS, and incubated with CD29 PE antibody (1:500 dilution, Cat# 303003; Biolegend, San Diego, CA, USA), CD90 FITC antibody (1:500 dilution, Cat# 328108; Biolegend) and CD44 PE/Cy7 antibody (1:500 dilution, Cat# 25-0441-82; Invitrogen, Waltham, MA, USA) for 20 min at room temperature in the dark. The cells were washed twice with PBS and resuspended in PBS for detection by NovoCyte™ flow cytometer (NovoCyte 2060R; Eisen Bio, Hangzhou, China). All experiments were performed in triplicate.
Cell treatment
BEAS-2B cells were cultured and divided into 4 groups: 1) control group (control); 2) asthma model group (model); 3) asthma model + inhibitor group (KAAD-Cyclopamine); 4) asthma model + mesenchymal stem cells group (UCMSCs). Asthma models were prepared by treating human bronchial epithelial cells with 10 ng/mL TGF-β1 (HY-P7118, MCE) for 7 h. The asthma model + inhibitor group was treated with 100 nM inhibitor, KAADCyclopamine (HY-100535, MCE). The asthma model + MSC group was a co-culture of UCMSCs and human bronchial epithelial cells in Transwell co-culture system.
CCK8 assay
BEAS-2B cells were seeded in 96-well plates and after treatment 10 ul CCK8 reagent (Dojindo, Mashiki, Japan) was added to each well and incubated for 2 h; the absorbance value of each well was detected at 450 nm by a microplate reader.
Flow cytometry analysis of apoptosis
Total 1.106 cells were collected and washed twice with PBS. Cells were then stained with Annexin V-FITC and propidium iodide (PI) (AP101-100-kit; Multi Sciences, Hangzhou, China) for 10 min at room temperature in the dark. The stained cells were detected by a flow cytometer (BD LSRII; BD Biosciences, Franklin Lakes, NJ, USA). For flow cytometry 1x105 events were recorded for each sample, and all experiments were made in triplicate.
Immunofluorescence staining
Cells were fixed in 4% paraformaldehyde in PBS for 10 min at room temperature, washed with PBS and then incubated with primary antibody for E-cadherin (Cat# af0131, 1:200 dilution; Affinity, Cincinnati, OH, USA) and α-smooth muscle actin (α- SMA) (Cat# 19245, 1:100 dilution; Cell Signaling Technology, Danvers, MA, USA) overnight at 4°C. The cells were washed with PBS and incubated with secondary antibody cy3 (AS007, 1:200 dilution; ABclonal) for 1 h at room temperature. For negative control, the cells were incubated with PBS instead of primary antibody. The nuclei were stained with 1 μg/mL DAPI in the dark for 1 min at room temperature, and the slices were sealed with blocking solution containing anti-fluorescence quencher, and observed under fluorescence microscope (CKX53, Olympus) equipped with a Zyla 4.6 sCMOS camera to record the images. The immunolabeling was assessed by ImageJ software analysis program.
ELISA
The cell culture supernatant was collected, and the contents of tumor necrosis factor alpha (TNF-α) and IL-6 were detected by using ELISA assay TNF-α kit (MM-0122H2, Jiangsu Enzyme Free Industry Co., Ltd., Nantong, China) and ELISA assay IL-6 kit (MM-0049H2, Jiangsu Enzyme Free Industry Co., Ltd.).
Western blot analysis
Cells were lysed, total proteins of the cells were collected and analyzed by the BCA protein quantification kit (E-BC-K318-M, Elabscience, Houston, TX, USA). Proteins were separated by electrophoresis and transferred to membranes. The membranes were blocked and incubated with primary antibody for E-cadherin (Cat# Sc-8426, 1:1000 dilution; Santa Cruz, Biotechnology, Dallas, TX, USA), α-SMA (Cat# ab124964, 1:1000 dilution; Abcam), Shh (Cat# 20697-1-AP, 1:500 dilution; Proteintech, Rosemont, IL, USA), Gli1 (Cat# A8387, 1:1000 dilution; ABclonal), Snail (Cat# AF6032, 1:500 dilution; Affinity) overnight at 4°C. After washing, the membranes were incubated with secondary antibodies (1:5000 dilution; Abcam) at room temperature for 1 h. The blots were developed and pictures were taken. The band’s intensity was measured by ImageJ software
Statistical analysis
GraphPad Prism 9.0.0 software was used for statistical analysis. All experiments were repeated three times, and data were expressed as mean ± standard deviation (X±SD). One-way ANOVA was used for comparison of multiple groups, with p<0.05 indicating significant differences.
Results
Identification of human bronchial epithelial cells and UcMscs
As shown in Figure 1A, we observed strong staining of PCK in human bronchial epithelial cells. On the other hand, positive expression of protein by flow cytometry analysis of UCMSCs was 97.79% for CD44, 99.42% for CD29 and 98.91% for CD90, indicating successful identification of UCMSCs (Figure 1B).
Effects of Hedgehog pathway inhibitor and Mscs on the proliferation and apoptosis of human bronchial epithelial cells
As shown in Figure 2A, cell proliferation in Model group decreased significantly compared with control group. Cell proliferation in KAAD-cyclopamine group increased slightly while cell proliferation in UCMSCs group increased significantly, compared with model group. As shown in Figure 2B, apoptosis significantly increased in model group compared with control group. Compared with model group, apoptosis significantly decreased in KAADcyclopamine group and UCMSCs group.
Effects of Hedgehog pathway inhibitor and Mscs on the secretion of inflammatory factors in human bronchial epithelial cells
As shown in Figure 3, TNF-α and IL-6 contents in the supernatants of cells in Model group increased significantly compared with control group. However, TNF-α and IL-6 contents in the supernatants of cells in KAAD-cyclopamine group and UCMSCs group decreased significantly compared with Model group.
Figure 1.
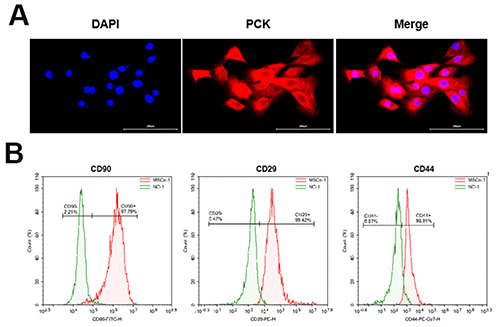
Identification of human bronchial epithelial cells and hUCMSCs. A) Identification of human bronchial epithelial cells; scale bars: 100 μm. B) Identification of hUCMSCs.
Effect of Hedgehog pathway inhibitor and Mscs on E-cadherin and α-sMa expression in human bronchial epithelial cells
Immunofluorescence staining showed that, compared with control group, the expression of E-cadherin in model group decreased significantly and the expression of α-SMA increased significantly. Compared with model group, the expression of Ecadherin in the KAAD-cyclopamine group and UCMSCs group increased significantly and the expression of α-SMA decreased significantly (Figure 4A). Western blot analysis confirmed that Ecadherin expression decreased significantly and α-SMA expression increased significantly in Model group compared with control group. Compared with model group, E-cadherin expression in KAAD-cyclopamine group and UCMSCs group increased significantly, and α-SMA expression decreased significantly (Figure 4B).
Effect of Hedgehog pathway inhibitor and Mscs on the expression of shh, Gli1, and snail in human bronchial epithelial cells
As shown in Figure 5, the expression of Shh, Gli1, and Snail increased in model group compared with control group. Compared with model group, the expression levels of α-SMA, Shh, Gli1 and Snail in KAAD-cyclopamine group and UCMSCs group decreased significantly.
Figure 2.

Effect ofH pathway inhibitor and MSCs on the proliferation and apoptosis of human bronchial epithelial cells. A) CCK8 assay of cell proliferation. B) Flow cytometry analysis of apoptosis. *p<0.05 compared with control group; #p<0.05 compared model group.
Figure 3.

Effect of Hedgehog pathway inhibitor and UCMSCs on the secretion of inflammatory factors in human bronchial epithelial cells. TNF-α and IL-6 in the supernatants were detected by ELISA. *p<0.05 compared with control group; #p<0.05 compared model group.
Discussion
Asthma is a chronic inflammatory disease of the airways characterized by airway inflammation and airway hyperresponsiveness. Inflammation occurs due to the infiltration of eosinophils, macrophages, lymphocytes and neutrophils into the bronchial lumen and lung tissue.9 It has been shown that Shh proteins are secreted by bronchial epithelial cells. Epithelial to mesenchymal transition of tracheal epithelial cells is one of the important factors leading to tracheal remodeling.10 Therefore, in this study asthma model was established in human bronchial epithelial cells with the induction of TGF-β1. Bone marrow-derived MSCs and adiposederived MSCs have been shown to be effective in the treatment of asthma.11 UCMSCs have been less studied in asthma treatment. Therefore, in this study we tested the effects of UCMSCs on cell model of asthma.
TGF-β1 can cause apoptosis in a variety of cells, such as spermatogenic cells, vascular smooth muscle cells and human bronchial epithelial cells.12 Shh signaling pathway is in a state of inhibition when Ptch binds to Smo under normal conditions and inhibits downstream pathway.6 In this study we confirmed that TGF-β1 caused apoptosis in human bronchial epithelial cells. In addition, the action of KAAD-cyclopamine, a suppressor of Shh pathway, and UCMSCs can inhibit TGF-β1-induced apoptosis in human bronchial epithelial cells.
Figure 4.

Effect of Hedgehog pathway inhibitor and UCMSCs on E-cadherin and α-SMA expression in human bronchial epithelial cells. A) Immunofluorescence staining; scale bars: 100 μm. B) Western blot analysis. *p<0.05 compared with control group; #p<0.05 compared model group.
Figure 5.

Effect of Hedgehog pathway inhibitor and UCMSCs on the expression of Shh, Gli1 and Snail in human bronchial epithelial cells. *p<0.05 compared with control group; #p<0.05 compared model group.
Bronchial asthma is a metabolic disease involving multiple inflammatory cells, inflammatory mediators and cytokines. IL-6 is a pleiotropic cytokine with biphasic anti-inflammatory and proinflammatory effects. It has been shown that IL-6 is involved in the pathogenesis of asthma. IL-6 is a pro-inflammatory cytokine, and it can promote the release of other inflammatory factors and aggravate the inflammatory response. IL-6 also promotes the proliferation and differentiation of B cells to produce excessive IgE, which causes mast cells and basophils to release large amounts of bioactive mediators to aggravate asthma attacks.13 TNF-α is mainly produced by activated macrophages, and is a pro-inflammatory cytokine that plays an important role in the regulation of immune response and inflammation. TNF-α can exacerbate inflammatory response by stimulating the release of inflammatory factors such as IL-6 from endothelial cells and tissue macrophages.14 The results of this study confirm that the levels of inflammatory factors IL-6 and TNF-α significantly increased in in vitro asthma cell model, and significantly decreased after treatment with an inhibitor of the Shh pathway or after co-culture with UCMSCs. These results suggest that both inhibition of the Hedgehog pathway and treatment with UCMSCs have therapeutic effects on asthma.
Shh signaling is involved in the development of the mammalian lung.15 The mode of action of Shh signaling in fetal lung development is the interaction between epithelial cells derived from the endoderm and mesenchymal cells from the mesoderm.16 When the Shh signaling is activated and delivered to the downstream transcription factor Gli family, its stabilizing transcription factor (cubitus interruptus, Ci) is translocated to the nucleus and activates downstream target gene transcription. The activation of Gli1 expression increases the expression of Snail, a cell migrationassociated transcription factor that promotes epithelial to mesenchymal transition.17
α-SMA is an important indicator of early airway remodeling in asthma, and co-culture of FIZZ1-expressing alveolar epithelial cells with lung fibroblasts revealed significantly higher expression of α-SMA and type I collagen, indicating that FIZZ1 has a role in stimulating lung myofibroblast differentiation.18,19 It has been shown that α-SMA expression is upregulated, thereby causing luminal constriction and wall thickening, and regulating early airway remodeling in asthma.20 The results of the present study show that α-SMA, Shh, Gli1 and Snail expression significantly elevated while E-cadherin expression decreased significantly in cell asthma model. The expression of α-SMA, Shh, Gli1 and Snail decreased significantly and the expression of E-cadherin increased after the action of the Shh pathway inhibitor or co-culture with UCMSCs. These results show that Shh signaling plays a role in the pathogenesis of asthma, and UCMSCs may regulate Shh signaling for asthma treatment.
A recent study reported the administration of UCMSCs to inhibit inflammation in mouse asthma model.21 However, the underlying signaling mechanism remains unclear. To our knowledge, this is the first study to reveal that the Hedgehog signaling pathway plays an important role in mediating the effects of UCMSCs on TGF-β induced inflammation of human bronchial epithelial cells. Our findings suggest the potential application of UCMSCs in asthma therapy.
Funding Statement
Funding: this study was supported by Natural Science Foundation of Fujian Province (no. 2021J011339) and key Clinical Specialty Discipline Construction Program of Fuzhou, Fujian, China (no. 20220102).
References
- 1.Holgate S, Wenzel S, Postma D, Weiss S, Renz H, Sly P. Asthma. Nature Rev Disease Primers 2015;1:15025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohn L, Elias J, Chupp G. Asthma: mechanisms of disease persistence and progression. Ann Rev Immunol 2004;22:789-815. [DOI] [PubMed] [Google Scholar]
- 3.Paim TC, Wink MR. The versatility of mesenchymal stem cells: From regenerative medicine to COVID, what is next? Biocell 2022;46:913-22. [Google Scholar]
- 4.Kim O-H, Kim E-R, Park J-H, Lee H-J. Applications of scaffolds: Tools for enhancing the immunomodulation of mesenchymal stromal cells. Biocell 2022;46:1439-43. [Google Scholar]
- 5.Tynecka M, Moniuszko M, Eljaszewicz A. Old Friends with unexploited perspectives: current advances in mesenchymal stem cell-based therapies in asthma. Stem Cell Rev Rep 2021;17:1323-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang J. Hedgehog signaling mechanism and role in cancer. Semin Cancer Biol 2021;85:107-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu X, Li Y, Liu G, Li K, Chen P, Gao Y, et al. MiR-378a-3p acts as a tumor suppressor in gastric cancer via directly targeting RAB31 and inhibiting the Hedgehog pathway proteins GLI1/2. Cancer Biol Med 2022;19:1662-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeng L, Barkat M, Syed S, Shah S, Abbas G, Xu C, et al. Hedgehog signaling: linking embryonic lung development and asthmatic airway remodeling. Cells 2022;11:1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Dong Y, Du X. Lung development: AT1 and AT2 property. Biocell 2020;44:1-5. [Google Scholar]
- 10.Ramachandran J, Zhou W, Bardenhagen A, Nasr T, Yates E, Zorn A, et al. Hedgehog regulation of epithelial cell state and morphogenesis in the larynx. eLife 2022;18:e77055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi JY, Hur J, Jeon S, Jung CK, Rhee CK. Effects of human adipose tissue- and bone marrow-derived mesenchymal stem cells on airway inflammation and remodeling in a murine model of chronic asthma. Sci Rep 2022;12:12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bai L, Yu Z, Wang C, Qian G, Wang G. Dual role of TGF-β1 on Fas-induced apoptosis in lung epithelial cells. Respir Physiol Neurobiol 2011;177:241-6. [DOI] [PubMed] [Google Scholar]
- 13.Permaul P, Peters MC, Petty CR, Cardet JC, Phipatanakul W. The association of plasma IL-6 with measures of asthma morbidity in a moderate-severe pediatric cohort aged 6-18 years. J Allergy Clin Immunol Pract 2021;9:2916-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H, Wang M, Xu Y. Understanding the mechanisms underlying obesity in remodeling the breast tumor immune microenvironment: from the perspective of inflammation. Cancer Biol Med 2023;20:268-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang C, Cassandras M, Peng T. The role of Hedgehog Signaling in adult lung regeneration and maintenance. J Dev Biol 2019;7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang L, Jin M, Gu R, Xiao K, Lu M, Huo X, et al. miR-199a- 5p Reduces chondrocyte hypertrophy and attenuates osteoarthritis progression via the Indian Hedgehog signal pathway. J Clin Med 2023;12:1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu H, Shi Y, Wan X, Liu Y, Shu H, Huang F, et al. Effect of peroxiredoxin 1 on the biological function of airway epithelial cells and epithelial-mesenchymal transition. Biocell 2022;46:2671-80. [Google Scholar]
- 18.Cigna N, Farrokhi Moshai E, Brayer S, Marchal-Somme JL, Wemeau-Stervinou L, Fabre A, et al. The hedgehog system machinery controls transforming growth factor-β-dependent myofibroblastic differentiation in humans: involvement in idiopathic pulmonary fibrosis. Am J Pathol 2012;181:2126-37. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Li F, Yang M, Wu J, Zhao J, Gong W, et al. FIZZ1 promotes airway remodeling through the PI3K/Akt signaling pathway in asthma. Exp Therap Med 2014;7:1265-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wieczfinska J, Pawliczak R. Anti-fibrotic effect of ciglitazone in HRV-induced airway remodelling cell model. J Cell Mol Med 2023;31:133-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Y Kang H, Bang JY, Shin JW, Kim HY, Cho SH, et al. Intratracheal administration of mesenchymal stem cells modulates lung macrophage polarization and exerts anti-asthmatic effects. Sci Rep 2022;12:11728. [DOI] [PMC free article] [PubMed] [Google Scholar]


