Abstract
Objectives
The aim of this study was to describe the abdominal ultrasonographic findings in cats with confirmed or presumed feline infectious peritonitis (FIP).
Methods
This was a retrospective study performed in an academic veterinary hospital. The diagnosis of FIP was reached on review of history, signalment, clinical presentation, complete blood count, biochemistry panel, peritoneal fluid analysis, cytology and/or histopathology results from abnormal organs, and/or molecular testing (immunohistochemical or FIP coronavirus [FCoV] RT-PCR). Cats with confirmed FIP by molecular testing or with a highly suspicious diagnosis of FIP were included. Abdominal ultrasound examination findings were reviewed.
Results
In total, 25 cats were included. Common clinical signs/pathology findings included hyperglobulinemia (96%), anorexia/hyporexia (80%) and lethargy (56%). Abdominal ultrasound findings included effusion in 88% and lymphadenopathy in 80%. Hepatic changes were noted in 80%, the most common being hepatomegaly (58%) and a hypoechoic liver (48%). Intestinal changes were noted in 68% of cats, characterized by asymmetric wall thickening and/or loss of wall layering, with 52% being ileocecocolic junction and/or colonic in location. Splenic changes were present in 36% of cats, including splenomegaly, mottled parenchyma and hypoechoic nodules. Renal changes were present in 32%, encompassing a hypoechoic subcapsular rim and/or cortical nodules. Mesenteric and peritoneal abnormalities were seen in 28% and 16% of cats, respectively. Most cats (92%) had two or more locations of abdominal abnormalities on ultrasound.
Conclusions and relevance
The present study documents a wider range and distribution of ultrasonographic lesions in cats with FIP than previously reported. The presence of effusion and lymph node, hepatic and/or gastrointestinal tract changes were the most common findings, and most of the cats had a combination of two or more abdominal abnormalities.
Keywords: Ultrasound, feline infectious peritonitis, abdomen, viral diseases
Introduction
Feline infectious peritonitis (FIP) has a high incidence in multi-cat populations and is most prevalent in young cats.1 –3 The pathogenesis is still incompletely understood. The FIP coronavirus (FCoV) is a monocyte/macrophage-tropic mutant of the feline enteric coronavirus (FECoV).4,5 Two clinical manifestations are described; the effusive and non-effusive forms. 6 FIP is a systemic disease with variable clinical and imaging presentations. Historically considered a fatal disease, recent research has revealed efficacy of new antivirals in FIP treatment, making an early diagnosis even more critical.7 –12
Immunohistochemistry (IHC) for the FCoV antigen in affected tissues is considered the gold standard for the diagnosis of FIP. 7 In the absence of a definitive diagnosis, background, clinical signs, routine laboratory tests and molecular analyses can be combined to establish a high index of suspicion of FIP.7,13
A previous study has described the abdominal ultrasonographic (US) findings in 16 cats with FIP. 14 Cats had abdominal effusion (75%) or abnormalities in the kidneys (69%), lymph nodes (LNs) (56%) and/or liver (37%). In 82% of the cats, the gastrointestinal (GI) tract was unremarkable. 14
Our hypothesis was that FIP has a higher prevalence of abdominal lesions, especially in the intestinal tract. The aim of our study was to describe abdominal US lesions in cats with FIP and evaluate features that might support its diagnosis.
Materials and methods
Inclusion criteria
The database of the Foster Animal Hospital for Small Animals between 2013 and 2022 was searched for cats with a confirmed or highly suspicious diagnosis of FIP and that had abdominal US performed within 7 days of admission. Historical and clinicopathologic information was recorded, including sex, age, breed, presenting clinical complaint, duration of clinical signs, complete blood count (CBC), serum biochemical findings, abdominal fluid analysis, molecular testing, cyto/histopathologic findings and outcome.
The diagnosis of FIP was established based on signalment, clinical presentation (fever, ascites, ocular or neurologic signs), clinical pathology (low serum albumin:globulin ratio <0.6, lymphopenia <0.9 K/µ, anemia [packed cell volume <30%]), highly proteinaceous effusion (>3.5 g/dl, 13 reference interval [RI] <2.5 g/dl; low to moderate cellularity, pyogranulomatous inflammation, low albumin:globulin ratio <0.8), pyogranulomatous inflammation and vasculitis on tissue, positive FCoV antigen on immunohistochemistry (IHC) on tissue or fluids or RT-PCR demonstrating FCoV messenger RNA (mRNA) in effusions. 15
Cats with a definitive diagnosis of FIP had supportive clinical presentation, and clinicopathologic and histopathologic findings, along with one of the following: (1) positive FCoV IHC on tissue or fluid; or (2) positive FCoV RT-PCR mRNA on effusion. Highly suspicious cats had a supportive clinical presentation, low albumin:globulin ratio in serum, characteristic fluid cytology (if effusion was present) and, if obtained, cyto/histopathology consistent with pyogranulomatous inflammation. Patients with known comorbidities were excluded.
Abdominal ultrasound
Cats were scanned using an Epic 7G or an iU22, with a curvilinear 8–5 MHz, or linear 12–5 MHz or 18–5 MHz transducer (Phillips). Images, video clips and reports were reviewed by a board-certified radiologist (DP) and a diagnostic imaging resident (TM).
The presence, echogenicity, amount of peritoneal/retroperitoneal effusion and mesenteric fat echogenicity were evaluated. Evaluation of abdominal LNs included thickness, echogenicity and margination. LNs >5 mm thick were considered enlarged. 16 Hepatobiliary assessment included subjective liver size, echotexture and echogenicity, focal lesions (number and size), common bile duct dilation (>4 mm), and gallbladder wall thickening (>1 mm) and contents. 17 GI tract evaluation included wall thickness, layering, and extent and distribution of lesion(s). Wall thickness was considered normal if it was 2–4 mm for the stomach, up to 2.5 mm for the duodenum and jejunum, 2.8 mm for the ileum and 1.5 mm for the colon. 18 The splenic size was considered normal if the mean thickness was ⩽1 cm, as previously described. 19 Splenic echogenicity and echotexture were subjectively assessed. Renal evaluation included size, shape, echogenicity, presence of focal lesions, subcapsular rim and pyelectasia. Urinary bladder wall thickness and contents were reported if abnormal.
Statistics
Descriptive statistics were performed and data were presented as median (range) for continuous variables and frequency counts for categorical variables.
Results
A total of 25 cats met the inclusion criteria. 7 Of them, 12 (48%) had a definitive diagnosis of FIP. These cats had supportive clinical presentation, clinical pathology, fluid analysis (8/12 cats), cytology (8/12 cats), histopathology (4/12 cats) and either positive FCoV IHC on tissue or fluid (7/12 cats) or a positive FCoV RT-PCR test (5/12 cats). In total, 13/25 cats were highly suspicious for FIP based on supportive clinical presentation, clinical pathology, fluid analysis (7/13 cats) and/or cytology (7/13 cats) and/or histology (8/13 cats) consistent with pyogranulomatous inflammation.
Signalment and clinical presentation
The median age was 3.5 years (range 0.3–12.2 years). Of the 25 cats, nine (36%) were aged <2 years and five (20%) were aged ⩾11 years. Most cats (n = 14) were domestic shorthair cats. Other breeds included domestic longhair (n = 3), Siberian (n = 2) and one each of the following breeds: Ragdoll, Bengal, domestic medium hair, Maine Coon, Egyptian Mau and Persian.
Of the 25 cats, 13 were spayed females, 11 were castrated males and one was an intact male. Of the 25 cats, seven were adopted from a shelter, seven were from a multi-cat household, five were indoor-only with no other pets in the household and two were obtained from breeders. Information about the environment was not available for four cats.
The most common clinical signs in the 25 cats were anorexia/hyporexia (n = 20), lethargy (n = 14), vomiting (n = 7), dehydration (n = 9), pyrexia (n = 9), diarrhea (n = 5) and weight loss (n = 5). Most cats (17/25) had a duration of clinical signs of over 1 week and 8/25 cats had clinical signs for less than 1 week. Neurologic signs were noted in 2/25 cats: one had seizures and the other had non-ambulatory tetraparesis. Ocular signs were reported in 2/25 cats: one cat had anisocoria and the other had uveitis.
Ultrasonographic findings
Individual US findings are presented in Table 1. All 25 cats had ultrasonographic abnormalities.
Table 1.
Summary of abdominal ultrasonographic findings in 25 cats with confirmed or presumed feline infectious peritonitis (FIP): cases 1–12 are confirmed; cases 13–25 are highly suspicious for FIP
| Case | Effusion | Mesentery | Lymphadenopathy | Liver | Intestinal tract | Spleen | Kidneys |
|---|---|---|---|---|---|---|---|
| 1 | Mild (retroperitoneal) | NA | Jejunal, colic | NA | NA | Two small hypoechoic nodules | LK: hyperechoic nodule and pyelectasia |
| 2 | Scant | NA | PD | Hypoechoic hepatomegaly | Thickening with lost wall layering of the ICJ and transverse and proximal descending colon | NA | NA |
| 3 | Severe | Hyperechoic, lobulated | Ileocecal | Hypoechoic | Diffuse SI muscularis thickening | NA | NA |
| 4 | Severe | Hyperechoic, lobulated* | NI | Hepatomegaly | NA | NA | NA |
| 5 | Mild | NA | Hepatic, PD, jejunal, ileocecal, colic | Hypoechoic hepatomegaly | ICJ thickening with altered layering and focal lost layering | Mottled | NA |
| 6 | Severe | Hyperechoic, lobulated* | NI | Hypoechoic hepatomegaly | NA | Splenomegaly | NA |
| 7 | Severe | Hypoechoic nodules | PD, jejunal | Hyperechoic hepatomegaly | NA | Splenomegaly | NA |
| 8 | Moderate | Hypoechoic nodules | Jejunal, ileocecal | Hepatomegaly | Thickened, layered ileum | NA | NA |
| 9 | Severe | Hyperechoic, hypoechoic nodules* | NI | Hyperechoic | NA | Splenomegaly, hyperechoic nodules | NA |
| 10 | No | NA | Ileocecal, colic | NA | ICJ mass. Descending colonic wall thickening | NA | NA |
| 11 | Mild | NA | Ileocecal | Hypoechoic | Descending colon thickening | NA | NA |
| 12 | Scant | NA | Hepatic, jejunal, ileocecal | Hypoechoic hepatomegaly | ICJ thickening with altered layering | NA | NA |
| 13 | Scant | NA | Hepatic, PD, medial iliac | Hyperechoic hepatomegaly | Focal colonic wall thickening, altered to lost layering | Mottled splenomegaly | RK: renomegaly, subcapsular rim |
| 14 | Moderate | Peritoneal nodule | Jejunal, colic | Hepatomegaly, hyperechoic nodules | Thickened descending colon with reactive lymphoid nodules | Splenomegaly | NA |
| 15 | Scant | NA | Hepatic, colic | NA | Eccentric thickening of descending colon, altered layering | NA | NA |
| 16 | Scant | NA | NA | Hyperechoic | NA | NA | NA |
| 17 | Moderate | NA | Jejunal | NA | Jejunal thickening, altered to lost layering | NA | Bilateral subcapsular rim and hyperechoic nodules |
| 18 | Scant (retroperitoneal) | NA | NA | NA | NA | NA | Bilateral renomegaly, subcapsular rim, pyelectasia, hyperechoic nodules |
| 19 | Severe | Hyperechoic and lobulated | PD | Hypoechoic | Thickened descending colon, altered to lost layering | Splenomegaly | RK: hyperechoic nodule and subcapsular rim |
| 20 | Mild | NA | Hepatic, ileocecal, colic | Hypoechoic | Jejunal thickening. ICJ thickening | NA | LK: renal nodule |
| 21 | Mild | NA | Jejunal | Hypoechoic | NA | NA | NA |
| 22 | No | NA | Jejunal, ileocecal | Hyperechoic hepatomegaly | ICJ thickening with altered to lost layering | NA | NA |
| 23 | Mild | NA | Hepatic, gastric | Hypoechoic hepatomegaly | Jejunal wall thickening, altered layering. Descending colon: eccentric thickening | Mottled splenomegaly | NA |
| 24 | Scant | NA | Jejunal | Hypoechoic hepatomegaly | Thickened ileum with altered to loss of layering | NA | Bilateral renomegaly, subcapsular rim, pyelectasia, cortical nodules |
| 25 | No | NA | Hepatic, jejunal, colic, medial iliac | Hypoechoic hepatomegaly | Thickened colonic wall, lost layering | NA | LK: cortical nodules |
Also showed a thickened parietal peritoneum
ICJ = ileocecocolic junction; LK = left kidney; NA = no abnormalities detected; NI = not identified; PD = pancreaticoduodenal lymph node; RK = right kidney; SI = small intestine
Abdominal effusion (peritoneal or retroperitoneal) was present in 22/25 cats (scant in seven cats, mild in six cats, moderate in three cats and severe in six cats). Effusion was anechoic in 13 cats and mildly echogenic in nine cats.
The mesentery was abnormal in 7/25 cats. A hyperechoic/lobular mesentery was seen in 4/7 cats, and 3/7 cats had hypoechoic nodules. The peritoneum was thickened in 3/25 cats (Figure 1) and 1/25 cats had a parietal peritoneal nodule.
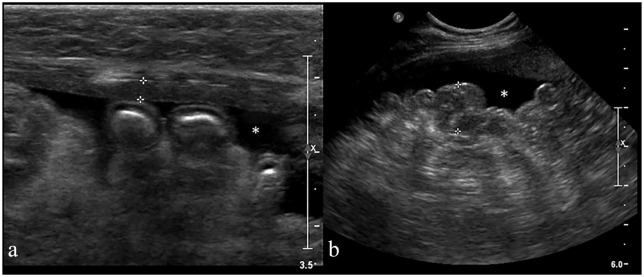
Figure 1.
Longitudinal ultrasonographic (US) image of the abdomen in a 12-year-old female cat with feline infectious peritoniris (case 9). (a) Part of the moderately thickened parietal peritoneum is seen between the calipers (2.6 mm). (b) The hyperechoic mesentery appears nodular (between the calipers: 1.2 cm) and irregular. Anechoic peritoneal effusion (asterisk) was also present.
A total of 20/25 cats had LN changes. Multifocal abdominal lymphadenopathy (two or more LNs) was identified in 13/20 cats and perinodal hyperechoic fat was noted in 4/20 cats. The jejunal LNs were enlarged and hypoechoic in 11/20 cats. Markedly enlarged (up to 20 mm), lobulated, hypoechoic jejunal LNs were noted in 1/11 cats (Figure 2). The ileocecal LNs were enlarged (thickness up to 8.4 mm) and hypoechoic in 8/20 cats. The colic LNs were enlarged and hypoechoic in 7/20 cats, measuring up to 18 mm. The hepatic LN was enlarged, measuring up to 14 mm, and hypoechoic in 7/20 cats; 6/7 cats had hepatic changes. The gastric LN was enlarged (6 mm) in one cat. The pancreaticoduodenal LN was enlarged and hypoechoic in 5/20 cats (thickness up to 10 mm). The medial iliac LNs were hypoechoic in 2/20 cats. LN images were not available in 3/25 cats.
Figure 2.
Jejunal suppurative lymphadenitis in a 7-month-old female cat (case 21). Longitudinal ultrasound image of the enlarged jejunal lymph nodes. Note the cranial mesenteric vein (asterisk). The most dorsally located jejunal lymph node is markedly enlarged, hypoechoic, with an ill-defined anechoic cranial pole (white arrow) and slightly lobulated. Regional perinodal fat is hyperechoic, supportive of steatitis (white arrowhead)
The liver was abnormal in 20/25 (80%) cats. Of them, hepatomegaly was noted in 13 cats. The liver was diffusely hypoechogenic in 12 cats and diffusely hyperechogenic in five cats. Of the 20 cats, one had multiple small (up to 2.4 mm) hyperechoic nodules, some distorting the capsule (Figure 3). One cat had gallbladder wall thickening (3 mm) and a mildly distended common bile duct (5 mm). The pancreas was unremarkable in all cats in which it was imaged (20/25 cats).
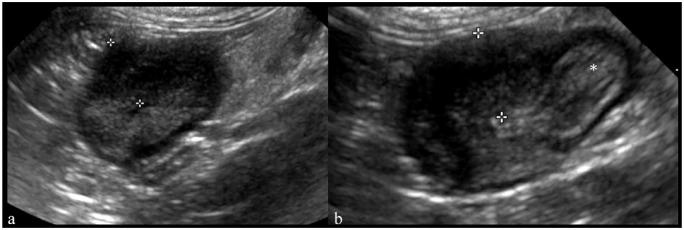
Figure 3.
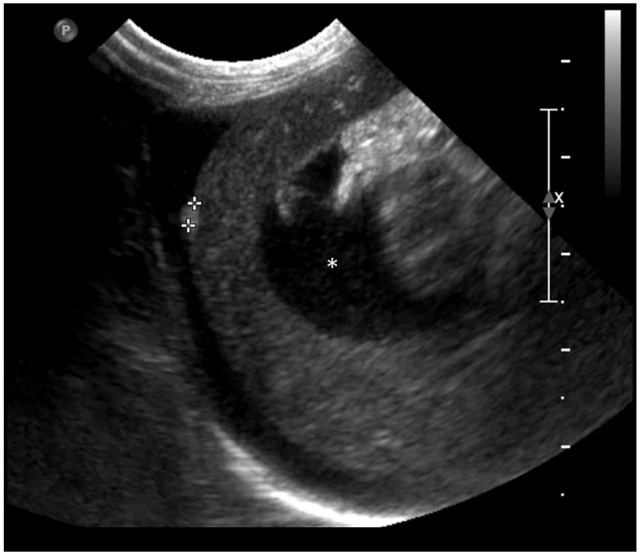
Longitudinal ultrasound image of the liver in an 8-year-old male neutered cat with feline infectious peritonitis (case 14). A 2.4 mm ellipsoid hyperechoic nodule (cursors) is noted within the periphery of the liver, bulging along the hepatic capsule. Serosal granuloma was diagnosed by histopathology. Slightly echogenic peritoneal effusion is also noted (asterisk)
Intestinal abnormalities were found in 17/25 cats, with 13/17 being ICJ and/or colonic changes. Of these 13 cats, in six, primarily ICJ lesions were noted, and in nine, colonic lesions were noted. Of the nine cats with colonic lesions, two had a mass-like lesion marked by eccentric, mixed echogenicity and colonic wall thickening, measuring up to 3 cm in width. The remaining 7/9 cats showed colonic wall thickening (up to 6 mm; range 2.6–6 mm).
Ileal wall thickening (up to 6 mm) with altered layering (thickened muscular layer) was noted in 2/17 cats. A total of 6/17 cats had ileocecocolic junction (ICJ) lesions. Wall thickening affecting the ileum, cecum and colonic walls was seen in 5/6 cats, and loss of wall layering in 2/5 cats. An ICJ marked thickening was seen in 1/6 cats (Figure 4). This lesion was heterogeneously hypoechoic, with faint, ill-defined and irregular hyperechoic areas, extending to the ascending and transverse colon with loss of wall layering, extending approximately over 3.5 cm in length (case 10).
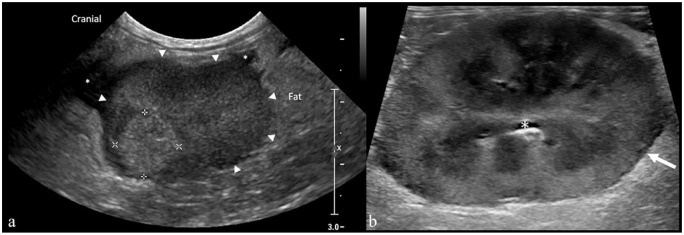
Figure 4.
Transverse ultrasound image of (a) the ascending colon and (b) ileocolic junction in a 3-year-old female cat with feline infectious peritonitis (case 10). (a) The ascending colon is thickened with lost wall layering (between the calipers: 8.5 mm). (b) An ileocecocolic junction-centered colonic wall thickening with loss of wall layering (between the calipers: 8 mm). The ileum is also seen (asterisk)
The jejunum had changes in 4/17 cats, with segmental muscular layer thickening in 3/4 cats. A long asymmetrical thickened segment of jejunum (>15 cm) with altered to lost wall layering (up to 3.8 mm) was seen in 1/4 cats. The stomach and duodenum were unremarkable.
The spleen was abnormal in 9/25 (36%) cats; splenomegaly was observed in 7/9 cats and a mottled parenchyma in 3/9 cats. Two small hypoechoic splenic nodules (up to 5 mm in diameter) were identified in another cat. One cat had multiple small (up to 4.3 mm) hyperechoic nodules, thought to represent myelolipomas.
Abnormal renal findings were seen in 8/25 cats: decreased corticomedullary distinction in five cats; a hypoechoic subcapsular rim in five cats (bilateral in three cats and unilateral in two cats) (Figure 5); and pyelectasia (up to 7 mm) in three cats. Of the eight cats, two had bilateral renomegaly and two had unilateral renomegaly (up to 4.9 cm in length). Hyperechoic cortical nodules were seen bilaterally in three cats and unilaterally in four cats (Figure 5).
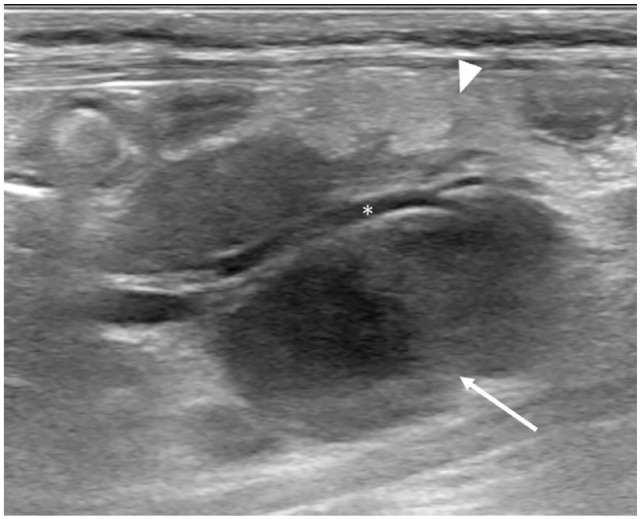
Figure 5.
(a) Parasagittal ultrasound image of the right kidney in an 8-month-old cat with feline infectious peritonitis (case 19) showing a 1 cm hyperechoic cortical nodule (cursors). There is concurrent retroperitoneal anechoic effusion (asterisks). (b) Pyogranulomatous nephritis in a 1-year-old cat with feline infectious peritonitis (case 18). Longitudinal ultrasound image of the enlarged (4.7 cm long) right kidney with heterogeneous parenchyma and slightly irregular margination. A thin hypoechoic rim is also noted at the outer cortical (white arrow). The perirenal fat is hyperechoic. The asterisk indicates a scant amount of urine in the renal pelvis. Pyogranulomatous nephritis was diagnosed on necropsy
The abdominal lesions had a predominantly multifocal distribution with involvement of two or more organ/structures in 92% of cats.
Clinical pathology and fluid analysis
The serum albumin:globulin ratio was <0.77 in 24/25 (96%) cases. Of the 25 cats, 15 had anemia (hematocrit <35); in 13/15 cats, this was non-regenerative. Lymphopenia was present in 12/25 cats.
Increased total serum bilirubin was seen in 10/25 cats (median 2 mg/dl; range 0.5–8.3 mg/dl; RI <0.3 mg/dl). Serum alkaline phosphatase (188 U/l, RI <79 U/l) or serum alanine aminotransferase (224 U/l; RI <145 U/l) was increased in only one cat. Of the 25 cats, nine had an increase in serum aspartate aminotransferase, which was typically very mild (median 78 U/l; range 61–232 U/l; RI <42 U/l). All of these cats also had increased serum bilirubin. In 4/9 cats with increased serum aspartate transaminase (AST), there was a concurrent increase in creatinine kinase activity, suggesting a muscle source for the serum AST. Two cats had an elevated serum creatinine concentration.
The results of the fluid analysis are summarized in Table 2.
Table 2.
Summary of clinical signs, histopathology, necropsy, cytology, abdominal fluid analysis and molecular testing in 25 cats with confirmed or presumed feline infectious peritonitis (FIP): cases 1–12 are confirmed; cases 13–25 are highly suspicious for FIP
| Case | Clinical signs | Histopathology and necropsy | Cytology | Abdominal fluid analysis | PCR or IHC positive |
|---|---|---|---|---|---|
| 1* | Fever, lethargy | Necropsy: lymphoplasmacytic portal hepatitis. Pyogranulomatous lymphadenitis, nephritis, splenitis, meningoencephalitis and pleuropneumonia | Kidney: possible neutrophilic inflammation | None | IHC |
| 2 | Diarrhea, lethargy | Ocular signs: uveitis and pyogranulomatous perineuritis | None | None | IHC |
| 3 | Fever, emesis, anorexia | None | None | High-protein transudate (mixed inflammation) | IHC |
| 4 | Fever, lethargy, emesis. | None | Mesentery: mixed neutrophilic/macrophagic inflammation | None | PCR |
| 5 | Fever, lethargy, weight loss | None | None | Exudate (neutrophilic inflammation) | PCR |
| 6 | Fever, lethargy, anorexia |
None | Possible mild neutrophilic hepatitis | Exudate (neutrophilic inflammation) | PCR |
| 7 | Emesis, anorexia | None | Liver: possible neutrophilic inflammation Jejunal LN: neutrophilic/macrophagic inflammation |
Exudate (mixed inflammation) | PCR |
| 8 | Chronic diarrhea, hyporexia, lethargy | None | Mesenteric nodule: necrosis with mixed inflammation | Exudate (neutrophilic inflammation) | IHC |
| 9 | Fever, hyporexia, lethargy | None | None | High-protein transudate (neutrophilic inflammation) | IHC |
| 10 | Fever, lethargy, emesis | Biopsy: ICJ: pyogranulomatous enterocolitis and lymphadenitis | Colon: neutrophilic inflammation Jejunal LN: reactive |
None | IHC |
| 11 | Chronic diarrhea, lethargy, anorexia | Biopsy: pyogranulomatous lymphoplasmacytic hepatitis, colitis and peritonitis Necropsy: diffuse severe peritonitis Pyogranulomatous vasculitis and enterocolitis |
Colon: mixed inflammation Colic LN: non-diagnostic |
High-protein transudate (mixed inflammation) | IHC |
| 12 | Weight loss, hyporexia | None | Jejunal lymph node: moderate neutrophilic/macrophagic inflammation Scant heterogeneous lymphoid population |
High-protein transudate (mixed inflammation) | PCR |
| 13 | Hyporexia | Biopsy: hepatic pyogranulomas | Spleen and liver: moderate neutrophilic/macrophagic inflammation | None | |
| 14 | Hyporexia | Necropsy: pyogranulomatous serositis and vasculitis (diffuse) | None | High-protein transudate (mixed inflammation) | |
| 15 | Constipation, hyporexia | None | Non-diagnostic | Exudate (mixed inflammation) | |
| 16* | Fever, hyporexia, weight loss | Necropsy: fibrinous peritonitis and pleuritis Hepatic lipidosis with lymphoplasmacytic portal infiltrate. Neutrophilic and lymphoplasmacytic interstitial nephritis. Meningoencephalitis | None | None | |
| 17 | Fever, anorexia | None | Kidney: mild neutrophilic inflammation Liver: neutrophilic inflammation; mild hepatocellular lipid vacuolation Spleen: no evidence of inflammation or neoplasia |
High-protein transudate (mixed inflammation) | |
| 18 | Weakness, hyporexia | Necropsy: pyogranulomatous and lymphoplasmacytic nephritis and vasculitis Multifocal telangiectasis (peliosis hepatis) | None | None | |
| 19 | Anorexia, weight loss | None | None | High-protein transudate (mixed inflammation) | |
| 20 | Fever, hyporexia weight loss | Necropsy: bicavitary serofibrinous effusion Pyogranulomatous to suppurative lymphadenitis and nephritis. Fibrino-necrotizing splenic capsulitis cholestasis; multifocal, acute hepatic necrosis | None | Exudate (mixed inflammation) | |
| 21 | Fever, hyporexia, lethargy | Necropsy: omentum, small intestine, lymph node: lymphohistiocytic vasculitis with fibrinoid necrosis. Suppurative lymphadenitis with fibrinous peritonitis. Acute pulmonary edema | Jejunal LN: neutrophilic inflammation | Exudate (mixed inflammation) | |
| 22 | Fever, hyporexia, lethargy | Necropsy: pyogranulomatous and necrotizing lymphadenitis. Hepatic lipidosis; neutrophilic cholangitis; lymphoplasmacytic periportal hepatitis. Small intestine (Peyer’s patches): pyogranulomatous lymphadenitis | Marked hepatocellular distinct vacuolation (lipid), possible neutrophilic inflammation. Lymph node: neutrophilic/macrophagic inflammation, possible necrosis | None | |
| 23 | Fever, hyporexia, lethargy | None | ICJ mass: neutrophilic inflammation | Exudate (mixed inflammation) | |
| 24 | Anorexia, lethargy | Necropsy: pyogranulomatous nephritis, enterocolitis, lymphadenitis, hepatitis and vasculitis | None | None | |
| 25 | Anorexia, lethargy | None | Hepatic and medial iliac LN: pyogranulomatous lymphadenitis | None |
Cases that had concurrent neurologic signs
ICJ = ileocecocolic junction; IHC = immunohistochemistry; LN = lymph node
An abdominal fluid analysis was performed in 15/25 cats and revealed high protein content most consistent with FIP. Results included exudates with mixed inflammation in five cats, exudates with neutrophilic inflammation in three cats, high-protein transudate with mixed inflammation in six cats and with neutrophilic inflammation in one cat. In all 15 cases, neutrophils were non-degenerate, and no infectious organisms were identified. Bacterial culture was not performed on any of the abdominal fluid samples.
Cytology and necropsy
Individual cytologic and histopathologic findings are presented in Table 2.
US-guided fine-needle aspiration of abdominal structures was performed in 15/25 cases. Histopathology was available in 12/25 cats: on post-mortem (n = 9); US-guided tissue biopsies of liver (n = 1 [case 11]); colonic wall/mass and LNs (n = 2 [cases 10 and 11]); and enucleated eye (n = 1). Case 11 had a liver biopsy and post-mortem evaluation. The post-mortem evaluation revealed lesions typical of FIP 13 in all nine cats.
Hepatic abnormalities were noted in 8/12 cats with histopathology, including granulomatous hepatitis (n = 3), moderate to severe lipidosis (n = 2), lymphoplasmacytic portal hepatitis (n = 1) and peliosis hepatis (n = 1).
Intestinal histopathology was abnormal in 5/12 cats. Multifocal pyogranulomatous or lymphohistiocytic lesions (vasculitis or enterocolitis) were seen in five cats (cases 10, 11, 21, 22 and 24). Cat 22 had inflammatory lesions confined to the intestinal lymphatic vessels.
Pyogranulomatous splenitis (in 2/12 cats) and acute fibrino-necrotizing capsulitis (in 1/12 cats) were diagnosed on splenic cytology or histopathology. The cats with pyogranulomatous splenitis had either hypoechoic nodules or mottled splenomegaly on US, while the cat with capsulitis had a normal spleen on US.
Abnormal renal histopathology was noted in 5/12 cats and revealed pyogranulomatous nephritis and vasculitis in four cats (cases 1, 18, 20 and 24), and neutrophilic and lymphoplasmacytic nephritis in one cat (case 16) with an unremarkable kidney on US.
A total of 11 cats had cytologic (n = 6) or histopathologic (n = 5) evidence of pyogranulomatous (n = 8) or suppurative inflammation (n = 3) in one or more abdominal LNs; all these LNs were abnormal on US.
Outcome
Of the 25 cats, 12 were euthanized during the first week of presentation. Three were euthanized after 1, 3 and 5 months of supportive care treatment, owing to worsening of clinical signs. Of the 25 cats, eight were discharged with supportive care and lost to follow-up. Two cats (cases 5 and 12) treated with a trial drug for FIP (GS-441524) 20 showed improvement of the clinical signs and resolution of abdominal effusion. Case 5 had an ultrasound 10 weeks after therapy, which showed resolution of the pretreatment hepatic and intestinal lesions. Case 12 received antiviral treatment for 5 weeks and had resolution of the previously seen ICJ-centered thickening at a 2-month US recheck, though hypoechoic hepatomegaly persisted.
Discussion
The present study supports our hypothesis that FIP has a high prevalence of abdominal US lesions, more than previously described. 14 Abdominal effusion was the most common finding on US (88%). Ultrasound lesions in the LNs (80%), liver (80%) and intestines (68%) were also common. In addition, all cats had two or more affected areas on ultrasound.
In the present study, US lesions affecting the intestines were seen in 68% (17/25) of cats, compared with 13% in a previous publication. 14 Ileocecocolic and ICJ/colonic changes were most common, with an incidence of 52% (13/25 cats). The most common abnormalities were wall thickening (11/17 cats) and altered to lost wall layering (11/17 cats). Three cats had marked thickening in the colon (n = 2) or at the ICJ (n = 1). Differential diagnoses for these lesions would include neoplasia (eg, lymphoma, mast cell tumor, carcinoma), fungal disease and feline gastrointestinal eosinophilic sclerosing fibroplasia (FGESF). Neoplastic lesions tend to be poorly echogenic with more extensive loss of wall layering than seen in this study.21 –23 The mixed echogenicity within the lesions in our study likely corresponded to fibrotic regions. This change can also be seen in sclerosing mast cell tumors or FGESF.24 –26 Therefore, cytologic or histopathologic evaluation of intestinal lesions remain necessary to confirm the diagnosis.
The effusive form is the most common presentation of FIP 27 and acquisition of fluid from these cats for analysis is important in establishing a diagnosis of this viral disease. Ultrasound was able to identify abdominal effusion in 22/25 (88%) cats, with seven (28%) cats having only a scant amount. Thus, US evaluation permits the identification and acquisition of fluid even in cats with no obvious effusion on physical examination, including those with neuro/ocular changes.
Of the 25 cats, 11 had US abnormalities of the mesentery or parietal peritoneum. These US findings had not been previously reported in cats with FIP and may be secondary to granulomatous-necrotizing processes. 28 Differential diagnoses for the mesenteric changes would include carcinomatosis, lymphomatosis, sarcomatosis, mesothelioma or chronic peritonitis.29,30 Thickened parietal peritoneum (3/25 cats) and parietal peritoneal nodule (1/3 cats) were not previously reported in FIP. The thickened peritoneum likely corresponds to the fibrinous serositis/peritonitis described in FIP. 31 Since all cats with these changes had moderate to severe abdominal effusion, we speculate that the presence of fluid facilitates the identification of these lesions on US.
Out of 25 cats, 20 had evidence of lymphadenopathy, which is often reported with FIP.28,32 Differential diagnoses for abdominal lymphadenopathy in cats include infectious diseases (eg, fungal, protozoal, bacterial), round cell neoplasia (RCN) and reactive lymphadenomegaly.33 –36
The liver is frequently affected in FIP.37 –40 In our study, 20/25 (80%) cats had diffuse or focal hepatic ultrasonographic changes. The most common US abnormalities in our study were hepatomegaly (13/25, 52%) and diffuse hypoechogenicity (12/25, 48%). Too few cats had histopathologic evaluation of the liver to draw any conclusions about typical US changes. In terms of clinical pathology, 9/10 cats with hyperbilirubinemia had ultrasound abnormalities of the liver, with the most common findings being hepatomegaly (n = 6) and a hypoechoic echotexture (n = 7). Likewise, a high percentage of cats with elevated serum AST (n = 8) had an abnormal liver on US, with the most common finding being a hypoechoic liver (n = 6). The one cat with an increased serum ALT had a normal US.
The spleen showed US abnormalities in 9/25 (36%) cats, while a previous study reported splenic changes in only 12% of cats. 14 Splenomegaly was the most common abnormality; however, it is difficult to assess the clinical relevance of this finding as only 3/9 cases had histopathological confirmation. Pyogranulomatous necrotizing splenitis and acute fibrino-necrotizing splenic capsulitis, seen in our study, have been reported in FIP. 28 A mottled spleen was seen in three cats in our study population. This is a non-specific US finding and has been reported with extramedullary hematopoiesis, lymphoid hyperplasia, passive congestion, round cell neoplasia, carcinoma, histiocytic sarcoma, as well as granulomatous splenitis.23,41 –43
Renal lesions in our population included renomegaly in 4/25 (16%) cats and the presence of a subcapsular hypoechoic rim in 5/25 (20%) cats. A higher prevalence of these lesions (31%) was previously reported. 14 The hypoechoic rim in FIP may be due to cellular infiltration in the outer renal cortex, 37 similar to renal lymphoma. 44 Hyperechoic cortical nodules were seen in 4/5 cats in our study and, histopathologically, pyogranulomatous inflammation typical of FIP lesions was documented. 14
Only one cat with renal ultrasound changes had elevations in serum urea nitrogen or creatinine, indicating the likely presence of subclinical disease in some cats with FIP.
Two cats in this study received a novel antiviral therapy8,9,12,13,20 and follow-up US showed partial to complete resolution of changes, suggesting that US may be a valuable tool in assessing response to treatment with this trial drug. Persistent US changes despite curative treatment with antiviral therapy have been documented in cats with FIP.9,12 Hence, a better understanding of FIP lesions that are detectable with ultrasound pretreatment is beneficial.
The present study has some limitations. These include a lack of the gold standard diagnosis in 13 cats, the collection of data from ultrasonographic examinations performed by different operators and ultrasound machines, which may have led to inconsistency in documenting findings, a lack of histopathologic or cytologic correlates to all the US changes detected, and a small sample size, especially when evaluating imaging findings for a multi-organ distributed disease. Larger prospective multi-institutional studies focused on in-depth correlation between imaging and histopathologic features are encouraged, especially in the light of promising antiviral treatment.
Conclusions
We report a high incidence of ultrasonographic abnormalities in the liver, LNs, GI tract, mesentery/peritoneum, spleen and kidney in cats with FIP. All cats had two or more abnormalities on abdominal US, likely reflecting the multisystemic nature of this infection, despite non-specific signs and neuro/ocular abnormalities.
Although the US imaging features were not specific, the combined presence of effusion and involvement of several organs, along with the signalment, history, clinical signs and clinicopathologic data, assist in prioritizing FIP as a diagnosis. In addition, US facilitates the sampling of abdominal fluid and/or affected organs for histopathology, immunohistochemistry or molecular testing, which are critical in making a definitive diagnosis of FIP.
Footnotes
Accepted: 4 November 2023
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: The work described in this manuscript involved the use of non-experimental (owned or unowned) animals. Established internationally recognized high standards (‘best practice’) of veterinary clinical care for the individual patient were always followed and/or this work involved the use of cadavers. Ethical approval from a committee was therefore not specifically required for publication in JFMS. Although not required, where ethical approval was still obtained, it is stated in the manuscript.
Informed consent: Informed consent (verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (experimental or non-experimental animals, including cadavers) for all procedure(s) undertaken (prospective or retrospective studies). No animals or people are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Thiago R Müller  https://orcid.org/0000-0002-7494-8588
https://orcid.org/0000-0002-7494-8588
Francisco O Conrado  https://orcid.org/0000-0001-5055-2637
https://orcid.org/0000-0001-5055-2637
References
- 1. Riemer F, Kuehner KA, Ritz S, et al. Clinical and laboratory features of cats with feline infectious peritonitis – a retrospective study of 231 confirmed cases (2000–2010). J Feline Med Surg 2016; 18: 348–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Felten S, Hartmann K. Diagnosis of feline infectious peritonitis: a review of the current literature. Viruses 2019; 11. DOI: 10.3390/v11111068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rohrbach BW, Legendre AM, Baldwin CA, et al. Epidemiology of feline infectious peritonitis among cats examined at veterinary medical teaching hospitals. J Am Vet Med Assoc 2001; 218: 1111–1115. [DOI] [PubMed] [Google Scholar]
- 4. Foley JE, Lapointe JM, Koblik P, et al. Diagnostic features of clinical neurologic feline infectious peritonitis. J Vet Intern Med 1998; 12: 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pedersen NC. An update on feline infectious peritonitis: virology and immunopathogenesis. Vet J 2014; 201: 123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pedersen NC. A review of feline infectious peritonitis virus infection: 1963–2008. J Feline Med Surg 2009; 11: 225–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thayer V, Gogolski S, Felten S, et al. 2022 AAFP/EveryCat feline infectious peritonitis diagnosis guidelines. J Feline Med Surg 2022; 24: 905–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Krentz D, Zenger K, Alberer M, et al. Curing cats with feline infectious peritonitis with an oral multi-component drug containing GS-441524. Viruses 2021; 13. DOI: 10.3390/v13112228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zwicklbauer K, Krentz D, Bergmann M, et al. Long-term follow-up of cats in complete remission after treatment of feline infectious peritonitis with oral GS-441524. J Feline Med Surg 2023; 25. DOI: 10.1177/1098612X231183250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Green J, Syme H, Tayler S. Thirty-two cats with effusive or non-effusive feline infectious peritonitis treated with a combination of remdesivir and GS-441524. J Vet Intern Med 2023; 37: 1784–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coggins SJ, Norris JM, Malik R, et al. Outcomes of treatment of cats with feline infectious peritonitis using parenterally administered remdesivir, with or without transition to orally administered GS-441524. J Vet Intern Med 2023; 37: 1772–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taylor SS, Coggins S, Barker EN, et al. Retrospective study and outcome of 307 cats with feline infectious peritonitis treated with legally sourced veterinary compounded preparations of remdesivir and GS-441524 (2020–2022). J Feline Med Surg 2023; 25. DOI: 10.1177/1098612X231194460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tasker S. Diagnosis of feline infectious peritonitis: update on evidence supporting available tests. J Feline Med Surg 2018; 20: 228–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lewis KM, O’Brien RT. Abdominal ultrasonographic findings associated with feline infectious peritonitis: a retrospective review of 16 cases. J Am Anim Hosp Assoc 2010; 46: 152–160. [DOI] [PubMed] [Google Scholar]
- 15. Simons FA, Vennema H, Rofina JE, et al. A mRNA PCR for the diagnosis of feline infectious peritonitis. J Virol Methods 2005; 124: 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. d’Anjou MA, Carmel EN. Abdominal cavity, lymph nodes, and great vessels. In: Penninck D, d’Anjou MA. (eds). Atlas of small animal ultrasonography. 2nd ed. Iowa: John Wiley & Sons, 2015, pp 455–480. [Google Scholar]
- 17. d’Anjou MA, Penninck D. Liver. In: Penninck D, d’Anjou MA. (eds). Atlas of small animal ultrasonography 2nd ed. Iowa: John Wiley & Sons, 2015, pp 183–238. [Google Scholar]
- 18. Di Donato P, Penninck D, Pietra M, et al. Ultrasonographic measurement of the relative thickness of intestinal wall layers in clinically healthy cats. J Feline Med Surg 2014; 16: 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sayre RS, Spaulding KA. Formulation of a standardized protocol and determination of the size and appearance of the spleen in healthy cats. J Feline Med Surg 2014; 16: 326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jones S, Novicoff W, Nadeau J, et al. Unlicensed GS-441524-like antiviral therapy can be effective for at-home treatment of feline infectious peritonitis. Animals 2021; 11. DOI: 10.3390/ani11082257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grooters AM, Biller DS, Ward H, et al. Ultrasonographic appearance of feline alimentary lymphoma. Vet Radiol Ultrasound 1994; 35: 468–472. [Google Scholar]
- 22. Rivers BJ, Walter PA, Feeney DA, et al. Ultrasonographic features of intestinal adenocarcinoma in five cats. Vet Radiol Ultrasound 1997; 38: 300–306. [DOI] [PubMed] [Google Scholar]
- 23. Sato AF, Solano M. Ultrasonographic findings in abdominal mast cell disease: a retrospective study of 19 patients. Vet Radiol Ultrasound 2004; 45: 51–57. [DOI] [PubMed] [Google Scholar]
- 24. Weissman A, Penninck D, Webster C, et al. Ultrasonographic and clinicopathological features of feline gastrointestinal eosinophilic sclerosing fibroplasia in four cats. J Feline Med Surg 2013; 15: 148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Munday JS, Martinez AW, Soo M. A case of feline gastrointestinal eosinophilic sclerosing fibroplasia mimicking metastatic neoplasia. N Z Vet J 2014; 62: 356–360. [DOI] [PubMed] [Google Scholar]
- 26. Linton M, Nimmo JS, Norris JM, et al. Feline gastrointestinal eosinophilic sclerosing fibroplasia: 13 cases and review of an emerging clinical entity. J Feline Med Surg 2015; 17: 392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pedersen NC. A review of feline infectious peritonitis virus infection: 1963–2008. J Feline Med Surg 2009; 11: 225–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kipar A, Köhler K, Leukert W, et al. A comparison of lymphatic tissues from cats with spontaneous feline infectious peritonitis (FIP), cats with FIP virus infection but no FIP, and cats with no infection. J Comp Pathol 2001; 125: 182–191. [DOI] [PubMed] [Google Scholar]
- 29. Monteiro CB, O’Brien RT. A retrospective study on the sonographic findings of abdominal carcinomatosis in 14 cats. Vet Radiol Ultrasound 2004; 45: 559–564. [DOI] [PubMed] [Google Scholar]
- 30. Morgan KRS, North CE, Thompson DJ. Sonographic features of peritoneal lymphomatosis in 4 cats. J Vet Intern Med 2018; 32: 1178–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Weiss RC, Scott FW. Pathogenesis of feline infectious peritonitis: pathologic changes and immunofluorescence. Am J Vet Res 1981; 42: 2036–2048. [PubMed] [Google Scholar]
- 32. Kipar A, Koehler K, Bellmann S, et al. Feline infectious peritonitis presenting as a tumour in the abdominal cavity. Vet Rec 1999; 144: 115–118. [DOI] [PubMed] [Google Scholar]
- 33. Gabor LJ, Malik R, Canfield PJ. Clinical and anatomical features of lymphosarcoma in 118 cats. Aust Vet J 1998; 76: 725–732. [DOI] [PubMed] [Google Scholar]
- 34. Hittmair K, Krebitz Gressl E, Kubber Heiss AK, et al. Feline alimentary lymphosarcoma: radiographical, ultrasonographical, histological and virological findings. Eur J Companion Anim Pract 2001; 11: 119–128. [Google Scholar]
- 35. Zwingenberger AL, Marks SL, Baker TW, et al. Ultrasonographic evaluation of the muscularis propria in cats with diffuse small intestinal lymphoma or inflammatory bowel disease. J Vet Intern Med 2010; 24: 289–292. [DOI] [PubMed] [Google Scholar]
- 36. Daniaux LA, Laurenson MP, Marks SL, et al. Ultrasonographic thickening of the muscularis propria in feline small intestinal small cell T-cell lymphoma and inflammatory bowel disease. J Feline Med Surg 2014; 16: 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kipar A, May H, Menger S, et al. Morphologic features and development of granulomatous vasculitis in feline infectious peritonitis. Vet Pathol 2005; 42: 321–330. [DOI] [PubMed] [Google Scholar]
- 38. Wang YT, Su BL, Hsieh LE, et al. An outbreak of feline infectious peritonitis in a Taiwanese shelter: epidemiologic and molecular evidence for horizontal transmission of a novel type II feline coronavirus. Vet Res 2013; 44. DOI: 10.1186/1297-9716-44-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hartmann K. Feline infectious peritonitis. Vet Clin North Am Small Anim Pract 2005; 35: 39–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Giordano A, Paltrinieri S, Bertazzolo W, et al. Sensitivity of Tru-cut and fine-needle aspiration biopsies of liver and kidney for diagnosis of feline infectious peritonitis. Vet Clin Pathol 2005; 34: 368–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hanson JA, Papageorges M, Girard E, et al. Ultrasonographic appearance of splenic disease in 101 cats. Vet Radiol Ultrasound 2001; 42: 441–445. [DOI] [PubMed] [Google Scholar]
- 42. Quinci M, Sabattini S, Agnoli C, et al. Ultrasonographic honeycomb pattern of the spleen in cats: correlation with pathological diagnosis in 33 cases. J Feline Med Surg 2020; 22: 800–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Allan R, Thompson KG, Halsey TR. Splenic mast cell tumour and mastocytaemia in a cat: case study and literature review. N Z Vet J 2000; 48: 117–121. [DOI] [PubMed] [Google Scholar]
- 44. Valdés-Martínez A, Cianciolo R, Mai W. Association between renal hypoechoic subcapsular thickening and lymphosarcoma in cats. Vet Radiol Ultrasound 2007; 48: 357–360. [DOI] [PubMed] [Google Scholar]