Abstract
Flat epithelium (FE) is a condition characterized by the loss of both hair cells (HCs) and supporting cells and the transformation of the organ of Corti into a simple flat or cuboidal epithelium, which can occur after severe cochlear insults. The transcription factors Gfi1, Atoh1, Pou4f3, and Six1 (GAPS) play key roles in HC differentiation and survival in normal ear. Previous work using a single transcription factor, Atoh1, to induce HC regeneration in mature ears in vivo usually produced very few cells and failed to produce HCs in severely damaged organs of Corti, especially those with FE. Studies in vitro suggested combinations of transcription factors may be more effective than any single factor, thus the current study aims to examine the effect of co-overexpressing GAPS genes in deafened mature guinea pig cochleae with FE. Deafening was achieved through the infusion of neomycin into the perilymph, leading to the formation of FE and substantial degeneration of nerve fibers. Seven days post neomycin treatment, adenovirus vectors carrying GAPS were injected into the scala media and successfully expressed in the FE. One or two months following GAPS inoculation, cells expressing Myosin VIIa were observed in regions under the FE (located at the scala tympani side of the basilar membrane), rather than within the FE. The number of cells, which we define as induced HCs (iHCs), was not significantly different between one and two months, but the larger N at two months made it more apparent that there was significantly more iHCs in GAPS treated animals than in controls. Additionally, qualitative observations indicated that ears with GAPS gene expression in the FE had more nerve fibers than FE without the treatment. In summary, our results showed that co-overexpression of GAPS enhances the potential for HC regeneration in a severe lesion model of FE.
Keywords: Cochlea, Deafness, Flat epithelium, Adenovirus, Gene transfer, Hair cell regeneration
1. Introduction
Hearing depends on the transduction of sound to neural signals, a process largely attributed to the sensory epithelium in the cochlea, the organ of Corti, which consists of mechanosensitive hair cells (HCs), surrounding supporting cells (SCs), and neural endings. Within this epithelium, the HCs, arrayed in a single row of inner HCs and 3 rows of outer HCs, fulfil the crucial function of converting sound waves into electrical signals. Because of the lack of regenerative capability for HCs in the mammalian cochlea, loss of HCs leads to lifelong sensorineural hearing loss (SNHL). Therefore, it is necessary to explore effective regenerative strategies for replacing lost HCs. Given that HCs in non-mammalian vertebrates can spontaneously regenerate (Corwin and Cotanche, 1988; Ryals and Rubel, 1988), researchers have tried to explore the possibility of inducing mammalian auditory HC regeneration.
Several research avenues have been used for testing means to induce HC regeneration in the mammalian cochlea. Based on the findings that avian auditory HCs can be replaced by transdifferentiation of SCs within the sensory epithelium (Raphael, 1992; Stone and Rubel, 2000), induced transdifferentiation has been studied using transgenesis or gene transfer approaches. Studies using transgenic mice have demonstrated that manipulation of gene expression of HC genes like Atoh1 can induce formation of new HCs (Iyer and Groves, 2021). Genes identified in the transgenic mouse studies and in developmental studies (Driver and Kelley, 2020) have become useful in gene transfer approaches, which are feasible for clinical use in humans. Gene transfer techniques employing viruses or other transfer vectors to deliver genes to target cells within the inner ear bear a clinical application value (Lee et al., 2020). Relevant to the work presented here, the target cells are the non-sensory cells in deaf ears which degrade to the state of a flat epithelium (FE).
Transcription factors (TFs) are essential in regulating gene expression patterns within tissues, controlling cell fate, and influencing their morphological and functional development (Mulvaney and Dabdoub, 2012; Schimmang, 2013). In the process of inner ear development, Atoh1, a basic helix-loop-helix TF, plays a crucial role in inducing the differentiation of HCs (Bermingham et al., 1999; Woods et al., 2004). Studies using transgenesis have demonstrated that Atoh1 alone is capable of inducing the formation of HCs in vitro (Jen et al., 2019), as well as in neonatal transgenic mice in vivo (Kelly et al., 2012; Liu et al., 2012). Studies using gene transfer methods showed that transdifferentiation of SCs into new HCs can be accomplished using forced expression of the Atoh1 gene in cultures (Shou et al., 2003) and in mature ears in vivo (Kawamoto et al., 2003). However, the work in mature ears in vivo has produced variable results that were partially dependent on the severity of the lesion, with failure to induce regeneration in the severely traumatized organ of Corti (Izumikawa et al., 2008).
A possible way to enhance the ability of TF gene transfer to form new HCs is to use a combination of 2 or more TFs. For instance, Gfi1 and Pou4f3 have garnered attention after being identified as crucial factors for HC differentiation and survival (Costa et al., 2015). Pou4f3 is a downstream target of Atoh1 activation in HCs, while Gfi1 is the target of Pou4f3 (Wallis et al., 2003; Xiang et al., 1997). Moreover, a combination of 4 genes, namely Gfi1, Atoh1, Pou4f3, and Six1 (GAPS), possesses the capacity to convert fibroblast cells into HCs (Menendez et al., 2020). These results motivated our experiments using GAPS expressing viral vectors in an in vivo guinea pig model of severe lesion in the organ of Corti.
We chose to design and develop our viral approach using adenovirus. One reason is the ability of these viruses to accommodate a relatively large set of transgenes, which is necessary for inserting all 4 GAPS genes. The other reason is the need to transduce the SCs in the auditory epithelium. These cells do not efficiently express transgenes after adeno-associated virus infection in mature ears (Kilpatrick et al., 2011) but they do express transgenes more efficiently after adenovirus infection (Excoffon et al., 2006; Ishimoto et al., 2002; Lee et al., 2020). The route of administration of the adenovirus is also critical. Inoculation into the perilymphatic space has demonstrated suboptimal HC transduction when utilizing adenovirus vectors (Dazert et al., 1997; Stover et al., 1999). In contrast, robust transduction was demonstrated after scala media infusion in both mouse and guinea pig models (Excoffon et al., 2006; Ishimoto et al., 2002; Lee et al., 2020). The explanation for the difference remains obscure; nevertheless, we chose the scala media route because of the success of previous studies that used it.
Both mice and guinea pigs commonly serve as animal models for SNHL. The loss of HCs can be induced by an ototoxic drug, by exposure to prolonged or extremely loud auditory signals, or by aging (Hawkins, 1973; Wagner and Shin, 2019). Some lesions involve the direct loss of only HCs, while the SCs are not initially affected and retain their differentiated state. In other cases, such as prolonged post-cochlear implantation periods (Nadol et al., 1994), exposure to ototoxic drugs at certain doses and concentrations (Kim and Raphael, 2007), and certain hereditary cochlear pathologies (Webster, 1992), both HCs and SCs incur damage. This more extensive damage results in the structural transformation of the organ of Corti into a flat or cuboidal simple epithelium, a condition referred to as FE (Wang et al., 2017). To date, no method has been established to induce cochlear FE in the mature mouse cochlea; therefore, the guinea pig emerged as the sole suitable model for inducing FE (Izumikawa et al., 2008; Kim and Raphael, 2007).
In this study, we introduced an adenovirus vector carrying the 4 GAPS genes into the deafened mature guinea pig cochlea through infusion into scala media in vivo. Deafening was achieved by administering neomycin into the scala tympani, leading to the loss of HCs and appearance of a FE. Contrary to our previous research findings, which reported no morphological changes in the FE following the overexpression of Atoh1 alone (Izumikawa et al., 2008), our current investigation demonstrates that the adenovirus-mediated introduction of this combination of 4 TFs can convert the cells within the FE into Myosin VIIa-positive cells which we define here as induced hair cells (iHCs). Our findings offer preliminary evidence suggesting that the delivery of GAPS could potentially constitute an effective strategy for managing hearing loss associated with the formation of a FE in the mature cochlea.
2. Materials and methods
2.1. Animals and groups
All animal experiments were conducted with the approval of the Institutional Animal Care & Use Committee (IACUC) at the University of Michigan (UM) and followed recognized veterinary protocols. UM is accredited by AAALAC International, and the Animal Care & Use Program conforms to the standards outlined in the Guide for the Care and Use of Laboratory Animals (NRC 2011). UM has an approved assurance statement on file with the Office of Laboratory Animal Welfare (OLAW).
Initially, a total of 59 young guinea pigs (Elm Hill Breeding Laboratory) weighing 285–447g were used in this study. Animals were group-housed, whenever possible, up to 6 animals per drawer. Singly housed animals continued to gain weight similar to their cohorts. Vivarium lights were on an automatic timer and were turned on at 6 am and off at 6 pm. Unilateral deafening was induced in all animals using 15% neomycin, followed by the administration of one of the following treatments seven days later: Ad-EF1a-Gfi1-Atoh1-Pou4f3-Six1-Venus (Ad.GAPS-Venus) and Ad.EF1a-Venus.
During the deafening surgery, 7 guinea pigs died immediately after the neomycin infusion. Additionally, 1 guinea pig was found to have an inner ear malformation, specifically a natural hole on the basal turn. After the adenovirus inoculation, 1 guinea pig was euthanized 11 days after surgery because of a fever and a marked weight reduction relative to its contemporaries. These animals were eliminated from study; the remaining 50 animals were distributed among groups as described below.
2.1.1. Scala media infusion groups
One animal group was employed for the specific purpose of immunocytochemical detection of the control vector, Ad.EF1a-Venus (N=6) at seven days post viral vector inoculation via the preferred scala media route. Two other groups were euthanized after longer periods, at either one month (N=9) or two months (N=25) following inoculation with Ad.GAPS-Venus to evaluate induction of HCs by this vector. As the control for these groups, another long-term group was euthanized at two months post inoculation with Ad.EF1a-Venus (N=4). A total of 44 animals were involved in experiments with scala media inoculation of the viral vector.
2.1.2. Scala tympani infusion group
The remaining 6 of the 50 experimental animals were used in scala tympani inoculation assays. In these groups, only Ad.GAPS-Venus was injected, to assess transduction efficiency by this route. Animals were euthanized at either one month (N=4) or two months (N=2) post inoculation.
2.2. Deafening and inoculation surgery
The animals were anesthetized with a combination of xylazine (intramuscularly (IM), 10 mg/kg, Covetrus, Inc. USA) and ketamine HCL (IM, 40 mg/kg, Dechra Veterinary Products, Inc. USA). The analgesic ketoprofen (1 mg/kg, Zoetis, Inc. USA) was given by subcutaneous injection. An eye lubricant was applied. Local anesthesia was administered subcutaneously along the postauricular region to be incised using 1% lidocaine hydrochloride (Fresenius Kabi, Inc. USA). Unilateral deafness in the animals was accomplished by infusing 10 μl of 15% neomycin (PCCA and Fagron, USA) diluted in sterile water into the left ear. The selected concentration of neomycin is designed to eliminate all HCs and induce significant morphological alterations in supporting cells from the base to the 3rd turn of the guinea pig cochleae. The animals were placed in a lateral recumbent position on a water-circulating heating blanket, and a surgical incision was made in the left postauricular region. The temporal bone was exposed and opened using scalpel and forceps, providing a clear view of the basal turn of the cochlea. The neomycin was delivered via a microcannula through a cochleostomy into scala tympani at the base of the cochlea using a syringe pump (1 μl/min). Following the infusion, the cochleostomy was covered with fascia, the temporal bone opening was closed using carboxylate cement (3M Durelon, Germany), and both the subcuticular and skin layers were sutured. This procedure has been shown to result in profound deafness with no measurable auditory brainstem responses (Yamagata et al., 2004), as expected with a complete absence of cochlear HCs.
Five μl of viral vectors Ad.GAPS-Venus or Ad.EF1a-Venus were introduced into the left ear seven days after the deafening surgery. These procedures were also conducted under anesthesia as previously described. For the scala media surgery, a comprehensive description of the method can be found in our previously published paper (Ishimoto et al., 2002), with the notable exception that the inoculation targeted the 2nd turn of the cochlea. The procedure induces a mechanical lesion to HCs when performed in a normal cochlea (Kawamoto et al., 2003) but in the current experiments, the epithelium was flat before the injection into the scala media. The surgical procedure for the scala tympani was consistent with that of the deafening surgery. Viral vectors were delivered through the same cochleostomy created on the basal turn during the deafening procedure.
Following surgical procedures, the xylazine reversal drug, atipamezole (0.5 mg/kg, Orion Corp, Espoo, Finland), was administered subcutaneously. Guinea pigs were given warm saline (2–3 ml/kg) subcutaneously. The analgesic ketoprofen was continued for two days following the procedure, along with subcutaneous saline. Critical care, an herbivore supplement, was given per os the day of scala media surgery and then as needed to support animals with weight loss.
2.3. Adenoviral vectors
A schematic of the viral vector construct is presented in Supplementary Fig. 1. Codon-optimized DNA sequences for human GFI1, ATOH1, POU4F3 and SIX1, together with an mVenus fluorescent protein DNA sequence were synthesized by GeneArt/Thermo. The stop codon was removed from each of the first 4 coding regions, and each DNA coding sequence was separated from its downstream neighbor with a picornavirus P2A sequence to allow expression of multiple proteins from the same transcript. Ad-EF1a-Venus, 1.3×1012 VP/ml. Ad.GAPS-Venus, 1.4×1012 VP/ml were generated by Vector Biolabs, USA.
2.4. Cochlear whole mount and immunostaining
The animals were deeply anesthetized as previously described, followed by decapitation, temporal bone removal, and collection of cochleae. The round and oval window membranes were opened, a local perfusion was performed through a hole in the apex with 4% paraformaldehyde in phosphate-buffered saline (PBS). The temporal bones were immersed in 4% paraformaldehyde for 3h for fixation, then rinsed with PBS. Additional dissection was carried out to remove the stria vascularis, Reissner’s membrane, and the tectorial membrane. Afterward, the tissue was permeabilized using 0.3% Triton X-100 in PBS for 30 min at room temperature. For staining with primary antibodies, samples were blocked using 5% normal goat serum (NGS) in PBS for 30 min at room temperature. Tissues were then incubated overnight at 4°C for 1 hour at room temperature in primary antibodies. Primary antibodies used in the current study were rabbit anti-Myosin VIIa (diluted 1:600 in 1% NGS, Proteus Biosciences, Inc. USA), mouse anti-GFP antibody (diluted 1:300 in 1% NGS, Invitrogen, USA), mouse anti-neurofilament 200 kD (diluted 1:200 in 1% NGS, Millipore Sigma, USA). After rinsing in PBS, samples were incubated in fluorescence-labeled secondary antibodies for 1h at room temperature. Secondary antibodies used in the current study were goat anti-rabbit Alexa Fluor 594 (diluted 1:1000 in PBS, Invitrogen, USA), goat anti-mouse Alexa Fluor 488 (diluted 1:600 in PBS, Invitrogen, USA), goat anti-mouse IgG1 Alexa Fluor 350 (diluted 1:200 in PBS, Invitrogen, USA). Alexa Fluor 350 phalloidin (diluted 1:200 in PBS, Invitrogen, USA) and Alexa Fluor 488 phalloidin (diluted 1:300 in PBS, Invitrogen, USA) were used to stain F-actin. After rinsing in PBS, samples were further dissected to separate individual cochlear turns and mounted on glass slides using ProLong Gold (Invitrogen, USA) and kept in a slide box in the dark at 4°C until they were viewed.
2.5. Microscopy
The samples were imaged using a Leica DMRB epifluorescence microscope and captured using a CCD Cooled SPOT-RT monochrome digital camera (Diagnostic Instruments, Sterling Heights, MI, USA). Digital images were processed using Adobe Photoshop. For confocal microscopy, we obtained optical sections on a Leica SP8 confocal microscope with Leica Application Suite (LAS) X software. 3D images were created using LAS X software with the 3D Viewer module.
2.6. Statistical analysis
iHC were identified by Myosin VIIa-positive staining and tabulated by turn as well as summed across all turns for each animal. Descriptive statistics were employed to characterize the distribution of Myosin VIIa-positive cells across the 4 cochlear turns. To test whether the proportions of animals with Myosin VIIa-positive cells differed as a function of time (one month vs. two months post inoculation), we constructed a 2 × 2 contingency table of the number of animals that had Myosin VIIa-positive cells at each time point and the number that did not, then performed a Chi-square test to determine whether there was a statistically significant difference between the distributions (Zar, 1999). We also used this method to test whether the observed distributions differed significantly from an expectation of no animals with Myosin VIIa-positive cells in the absence of the vector. To evaluate differences in the numbers of Myosin VIIa-positive cells, t-tests were employed to compare the mean number of iHCs across different time points and groups. The statistical tests described above were performed using the R functions chisq.test and t.test, respectively, in the stats package accompanying base R, version 4.3.1 (R Core Team, 2023).
3. Results
3.1. Morphological changes in the auditory epithelium following neomycin infusion
Morphological analysis indicated that in contrast to the normal structure seen in a whole-mount of the auditory epithelium (Fig. 1a), after neomycin infusion, the auditory epithelium transitioned to a FE state (Fig. 1b). In most cases (49 of 50 animals), the FE area extended from the base to at least the lower end of the 3rd turn, with the remainder of the organ of Corti showing HC loss, scar formation, and reorganization of apical cell-cell adherens junctions. In 40 of these animals, the FE state extended to at least the middle of the 3rd turn. Only 8 animals had FE that extended into the apical turn (Fig. 2). Thus, the predominant presentation in the apical cochlear turn was the absence of HCs and the emergence of scar tissue rather than FE. Because Myosin VIIa-positive cells in the scarred regions could represent newly generated or residual HCs, our analysis only focused on areas manifesting the FE state.
Fig. 1.

Whole mounts of the auditory epithelium stained with markers for actin (green) and Myosin VIIa (red) and photographed with epifluorescence. (a) In the normal auditory epithelium, one array of inner hair cells (I), 3 arrays of outer hair cells (1, 2, 3, indicating the 1st, 2nd, and 3rd rows), and pillar cells (P) are displayed. (b) Seven days after neomycin administration, the distribution of actin in adherens junctions between cells reveals a single layer FE of non-sensory cells with irregular apical outlines; whereas HCs and differentiated SCs are absent. All images at the same magnification; scale bar = 50 μm.
Fig. 2.

Whole-mounts of the neomycin-deafened auditory epithelium from the 4 cochlear turns of a guinea pig, from apex (a) to base (d), demonstrating FE without HCs or SCs throughout the cochlear duct. All images at the same magnification, scale bar = 50 μm.
The thinness of the FE can be appreciated by confocal analysis of the tissue as seen in Supplementary material, figure S1 and movie S2. Both movie and still image clearly demonstrate that the thickness of the FE is similar to that of the vas spirale, the blood vessel that resides in the basilar membrane on the scala tympani side of the epithelium. The organization and dimensions of the FE also can be appreciated in cross sections of plastic embedded cochleae, which were shown previously, along with corresponding whole-mounts (Shibata et al., 2010), especially in figures 1 c–d and 3a.
Fig. 3.

Whole mounts of the FE stained with markers for actin (blue) and GFP (green) and photographed with epifluorescence. Seven days after Ad.EF1a-Venus delivery through a scala media infusion, gene expression was detected at the level of the FE, as seen in lower magnification (a, scale bar = 100 μm) and at higher magnification (b, scale bar = 20 μm). The approximate location of the habenula perforata is marked with a dashed line and the medial aspect of the epithelium is at the bottom of the images.
3.2. Gene expression in FE after adenovirus inoculation
To verify gene expression in FE cells after adenoviral inoculation via the scala media and scala tympani, two groups of animals comprising of a total of 6 animals were euthanized seven days after delivery of Ad.EF1a-Venus or Ad.GAPS-Venus. Gene expression was identified through GFP specific antibodies (Fig. 3). The densest accumulation of GFP-positive cells was observed at the area close to the inoculation site in the 2nd turn, diminishing as the distance from the site increased.
3.3. Quantification of iHCs in FE after adenovirus inoculation
Control animals that were deafened and inoculated with Ad.EF1a-Venus and assessed 2 months later (N=4) had no Myosin VIIa-positive cells throughout the cochlea. Animals injected with Ad.GAPS-Venus via scala media and analyzed one month later, yielded 4 out of 9 animals with Myosin VIIa-positive cells (Fig. 4a). One of these animals had several hundred positive cells, which were mostly in the 2nd turn. No Myosin VIIa-positive cells were found in the apical turn of any animals in this group. The distribution of cells by turn in the Myosin VIIa-positive animals is shown in Table 1.
Fig. 4.
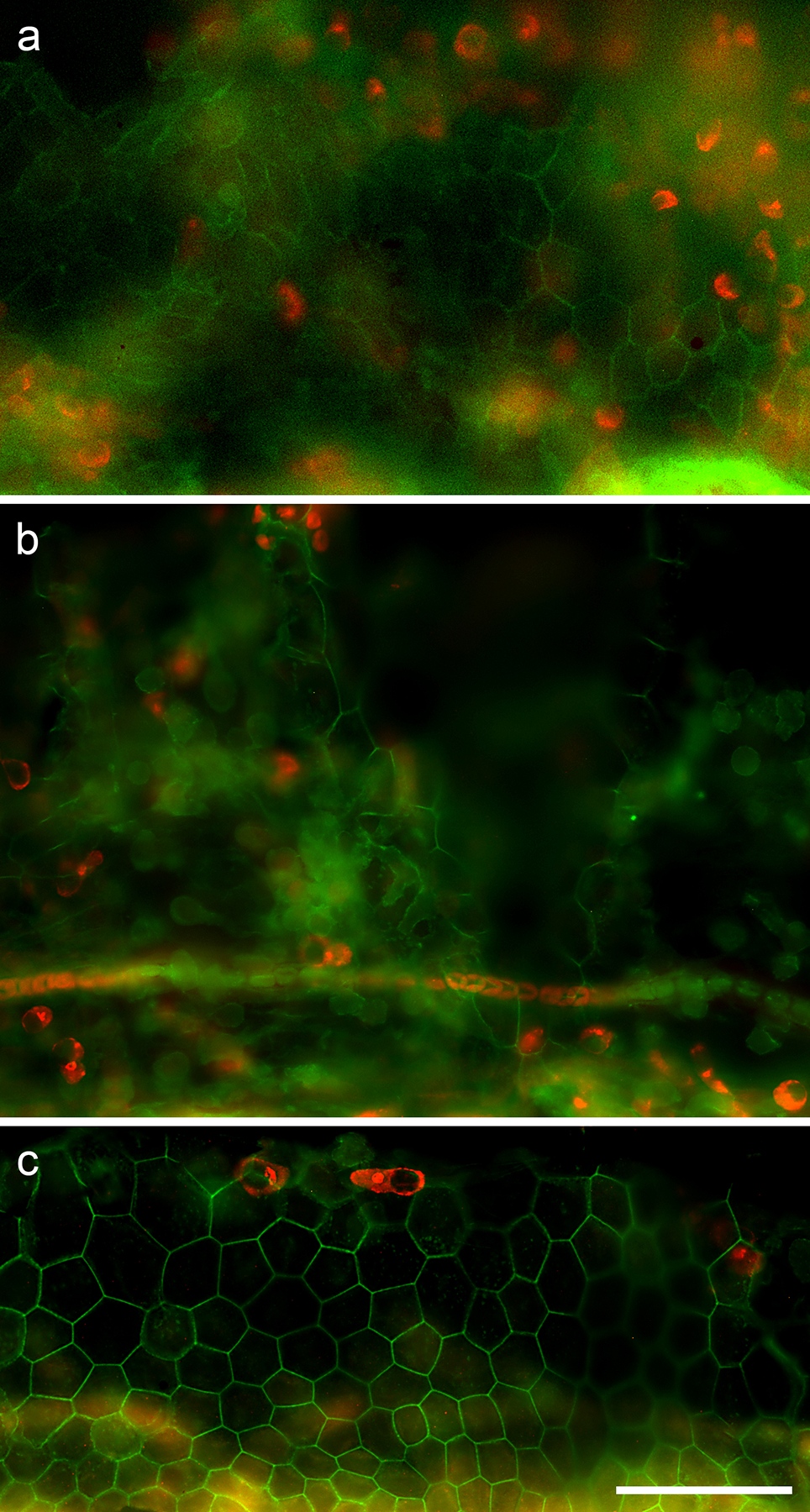
Whole mounts of the FE stained with markers for actin (green) and Myosin VIIa (red) and photographed with epifluorescence. (a) One month after Ad.GAPS-Venus delivery through scala media infusion, Myosin VIIa-positive iHCs are seen at a focal plane of the scala tympani side of the basilar membrane, under the FE. (b) Two months after Ad.GAPS-Venus delivery through scala media infusion, Myosin VIIa-positive cells are seen on the scala tympani side under the level of the FE. (c) Two months after Ad.GAPS-Venus delivery through scala media infusion, Myosin VIIa-positive iHCs are seen at the level of the FE. All images at the same magnification, scale bar = 50 μm.
Table 1.
The distribution of cells by turn in the Myosin VIIa-positive animals one month after Ad.GAPS-Venus injection.
| ID | Turn 1 (Basal turn) |
Turn 2 | Turn 3 | Turn 4 (Apical turn) |
Total |
|---|---|---|---|---|---|
| YJL-09 | 135 | 578 | 63 | 0 | 776 |
| YJL-13 | 0 | 7 | 0 | 0 | 7 |
| YJL-15 | 0 | 0 | 22 | 0 | 22 |
| YJL-17 | 0 | 0 | 2 | 0 | 2 |
Animals injected with Ad.GAPS-Venus via scala media and analyzed two months later yielded 8 out of 25 animals with at least 1 Myosin VIIa-positive cell (Fig. 4b). Although this is a slightly smaller proportion of animals with positive results (32% vs 44%), the difference in proportion was not statistically significant based on a 2 × 2 contingency table analysis (χ2=0.449, p=0.503). Taken together, 12 of 34 animals (35%) in the two groups of treated animals yielded evidence of Myosin VIIa-positive cells, which is significantly more than the expected number of zero (χ2=14.57, p=0.00013).
In contrast, none of the 6 animals injected with Ad.GAPS-Venus via scala tympani had Myosin VIIa-positive cells in any turn. There was no difference between data collected 1 or 2 months after virus injection, and the data were not different from the control animals that were only deafened.
An important difference among the animals that had Myosin VIIa-positive cells was that those cells were generally more numerous and more widely distributed in the animals allowed to survive for two months instead of one. After one month, only 1 of 4 animals had positive cells outside of the first turn and it still had 74% of the Myosin VIIa-positive cells in the 2nd turn (the site of injection). In contrast, 6 of 8 animals with Myosin VIIa-positive cells at two months after inoculation had Myosin VIIa-positive cells in at least 2 turns, and 4 of 8 had positive cells in 3 turns (Table 2).
Table 2.
The distribution of cells by turn in the Myosin VIIa-positive animals two months after Ad.GAPS-Venus injection
| ID | Turn 1 (Basal turn) |
Turn 2 | Turn 3 | Turn 4 (Apical turn) |
Total |
|---|---|---|---|---|---|
| YJL-12 | 2 | 11 | 19 | 0 | 32 |
| YJL-20 | 0 | 1 | 0 | 0 | 1 |
| YJL-22 | 154 | 144 | 9 | 0 | 307 |
| YJL-27 | 0 | 45 | 1 | 0 | 46 |
| YJL-36 | 37 | 15 | 37 | 4 | 93 |
| YJL-37 | 0 | 15 | 2 | 0 | 17 |
| YJL-39 | 16 | 143 | 4 | 0 | 164 |
| YJL-41 | 0 | 22 | 0 | 0 | 22 |
When all animals injected via scala media were included, the average number of Myosin VIIa-positive cells at one month was not significantly greater than zero (89.7 cells, t=0.985, p=0.353). At two months, the average number of cells was smaller but much closer to significant (27.3 cells, t=1.93, p=0.066) due to the larger N and smaller variance (4,796 vs. 66,294). However, analyses of only those animals with Myosin VIIa-positive cells indicated that the yield could be substantial (Table 2). Again, the average number of cells per Myosin VIIa-positive animal in the one-month group was greater than in the two-months group (201.75 vs 85.25), but the variance also was larger in the one month-group. Consequently, the average for the Myosin VIIa-positive animals at one month still was not significantly different from zero (t=1.05, p=0.37), but the average for Myosin VIIa-positive animals at two months was (t=2.32, p=0.05).
During the microscopic examination of the samples, it was possible to determine the focal plane of view such that areas beneath the basilar membrane (scala tympani side) could be distinguished from areas of the FE. We determined that in most cases, the iHCs were located on the scala tympani side of the basilar membrane (Fig. 4a–b) rather than in the FE itself. The cells were located in a focal plane that was clearly below the prominent blood vessels of the basilar membrane. Among all newly generated HCs, only 1 iHC was observed at the FE level in an animal examined two months post-operation (Fig. 4c).
3.4. Connection of nerve fiber and iHCs in the FE
To assess spatial relationships between neural fibers and iHCs, we stained for neurofilament in addition to Myosin VIIa and actin in deafened animals two months after adenoviral inoculation via scala media injection. In regions displaying iHCs under the FE, we noted nerve fibers extending towards the FE area; however, in most cases, no nerve fibers entered the FE (Fig. 5a). Where fibers were observed in the FE, they did not appear to contact iHCs (Fig. 5b). In one exceptional case (Fig. 5c), the nerve fiber appeared to terminate in the immediate vicinity of an iHC raising the possibility that iHCs can attract innervation.
Fig. 5.

Whole mounts of the FE stained with markers for Myosin VIIa (red), actin (green) and neurofilament (blue) and photographed with epifluorescence. (a) In a control cochlea that was only deafened, neural fibers were sparse and seldom extended far beyond the habenula perforata (dotted line). (b) Two months after Ad.GAPS-Venus delivery through scala media infusion, several fibers could be seen passing through regions with numerous Myosin VIIa-positive iHCs. (c) In rare cases, a nerve fiber appeared to terminate in the immediate vicinity of a Myosin VIIa-positive cell (arrow). All images at the same magnification, scale bar = 50 μm.
4. Discussion
In the current study, we confirmed that after infusing neomycin into the perilymph of the guinea pig cochlea, both HCs and SCs were absent and the auditory epithelium was replaced by flat cells, referred to as the FE. A combination of 4 TFs (GAPS) delivered into scala media via an adenovirus (Ad.GAPS-Venus) can successfully express those genes in the FE region and induce the regeneration of iHCs in the mature deafened cochlea. Notably, the position of iHCs was under the basilar membrane, even though the regeneration was achieved only through scala media infusion rather than scala tympani infusion.
Typically, HCs are more vulnerable than SCs to insults such as acoustic trauma, aging, and aminoglycosides ototoxicity. Consequently, in regions of the sensory epithelium that lose HCs, SCs usually remain intact. When SCs persist in areas of lost HCs, they retain their general structure, and participate in scarring in which their apical domain expands to fill the spaces of the lost HCs (Anttonen et al., 2014; Raphael and Altschuler, 1991; Sugawara et al., 2005). In contrast, extremely severe insults can lead to the disappearance of both HCs and SCs, transforming the sensory epithelium into a layer of non-specialized, flattened cells, termed FE (Kim and Raphael, 2007). The exact mechanism underlying the formation of FE in the mammalian inner ear remains unclear, but there are two possibilities to explain it. One possibility is that SCs, comprising Deiters cells, pillar cells, and phalangeal cells, undergo dedifferentiation post-injury, leading to the formation of FE. An alternative theory contends that following damage leading to the loss of both HCs and SCs, surrounding sensory epithelial cells migrate to the regions previously occupied by HCs and SCs. This was supported by a study from Taylor et al. (Taylor et al., 2012), who demonstrated elevated expression levels of Cx26 and Cx30 (the connexins characteristic of Hensen cells and Claudius cells) in areas flanking the organ of Corti. Moreover, Ladrech et al. (Ladrech et al., 2017) indicated that fibroblasts from the cochlear lateral wall contribute to the epithelial reorganization of the organ of Corti following hair cell depletion.
There are several reasons for studying HC regeneration in the FE. First, this condition appears in the clinical setting. A recent study assessing both HC and SC viability in 274 human inner ear samples with SNHL revealed that FE occur in the basal 20% of the cochlea (Kaur et al., 2023). Although less common than cases with surviving SCs, this clinical reality needs to be therapeutically addressed. Second, the FE presents an interesting biological model. Based on the morphology of the FE, it likely has a low differentiation state, and may therefore possess intrinsic stem cell characteristics. As such, FE cells may potentially transdifferentiate into new HCs or SCs. Therefore, an in-depth understanding of FE biology may advance this lesion model for transdifferentiation research.
Atoh1 is a key TF during HC differentiation (Bermingham et al., 1999; Woods et al., 2004) and is sufficient to drive the differentiation of non-sensory cells into HCs when overexpressed in embryonic, neonatal transgenic mice, or cultures (Ahmed et al., 2012; Jen et al., 2019; Kelly et al., 2012; Shou et al., 2003). However, the efficiency of HC reprogramming declines with age, and in severe lesion models, such as the FE (Iyer and Groves, 2021; Izumikawa et al., 2008). Given that human cochleae become functionally mature neonatally (Hepper and Shahidullah, 1994) and SNHL is predominantly observed in adults, it is imperative to seek strategies that augment the capacity of Atoh1 in promoting HC regeneration in the mature cochlea. Pou4f3 and Gfi1 are two additional HC-specific TFs that are expressed downstream of Atoh1 during HC maturation and play a crucial role in maintaining HC survival and function (Wallis et al., 2003; Xiang et al., 1997). Another TF, Six1, acts as a key inducer for Atoh1 activation and regulates inner ear morphogenesis by controlling the otic epithelium’s programmed cell death and proliferative growth (Ahmed et al., 2012; Zheng et al., 2003). Although our earlier research findings indicated no morphological changes of the FE upon overexpression of Atoh1 alone (Izumikawa et al., 2008), the present study reveals that the co-overexpression of GAPS can stimulate HC regeneration in cochleae with a FE. This suggests that the combination of other genes with Atoh1 offers a viable strategy for enhancing transdifferentiation efficiency and extends the results of previous studies employing a combinatorial approach in vivo (Lee et al., 2020). Costa et al. (Costa et al., 2015) applied the combined Atoh1, Gfi1, and Pou4f3 and demonstrated its potential to reprogram embryonic stem cells and chick otic epithelial cells into cells with HC characteristics. This strategy was also confirmed by an in vivo study, showing the superior efficiency of this combination of 3 genes in converting neonatal Lgr5+ SCs to HC-like cells when compared to Atoh1 alone (Chen et al., 2021). Moreover, Menendez et al. (Menendez et al., 2020) added Six1 and reported that this combination of 4 TF genes (GAPS) can effectively reprogram fibroblast cells into HCs. These studies offer insights into the use of combinations of TF genes, but their primary focus on embryonic or neonatal cells limits their relevance for extrapolating to deafness in the mature human cochlea. Our research builds upon these discoveries by pioneering its application in a realistic in vivo model of deafness in adult guinea pigs.
Surprisingly, our study revealed that after co-overexpression of GAPS in the FE region, the predominant HC regeneration occurred on the scala tympani side of the basilar membrane. We currently cannot ascertain the precise origin of the Myosin VIIa-positive cells identified on the scala tympani side of basilar membrane, but there are several possibilities that merit consideration. One possibility is that some transfected cells may have migrated from the FE through the basilar membrane. An alternative is that the Myosin VIIa-positive cells were transdifferentiated mesothelial cells (tympanic border cells) residing on the scala tympani side of the basilar membrane. The feasibility for this to occur was previously shown by Jan et al. who demonstrated in culture that tympanic border cells (mesothelial cells) can act as progenitors for hair cells (Jan et al., 2013). However, the Jan study used transgenesis of cells in immature ears and it remains unclear whether cells from mature ears can be transformed by the same technique. Another hypothesis involves the basilar membrane macrophages. In the organ of Corti, macrophages are predominantly located along the perilymphatic side of the basilar membrane (Liu et al., 2018; O’Malley et al., 2016), and exhibit the capacity to extend projections through the basilar membrane (Okayasu et al., 2020). Thus, we hypothesize that these macrophages, whose numbers are increased after a lesion in the organ of Corti (Kaur et al., 2015) are recruited to lesioned areas to engulf cells compromised by adenovirus vector infection and upon expression of the viral gene load they subsequently express the HC marker. To shed light on these hypotheses, we infused GAPS into the scala tympani. No iHCs were found after this effort; however, the volume of the scala tympani much exceeds that of the scala media and the concentration of the adenovirus would have been greatly diminished. Further research on this approach will be needed to determine an optimal concentration and volume of adenovirus to enhance potential efficacy.
Because regenerated HCs can only lead to restoration of hearing if they also attract innervation, we also examined the spatial relationship between the iHCs and the auditory neurons. Our qualitative observation indicated that ears with GAPS gene expression in the FE had more nerve fibers than FE without the treatment. There was an occasional proximity between a nerve ending and an iHC. Further work is needed to better characterize the presence of synapses and the connectivity between neurons and iHCs over time.
It is also important to assess other physiological parameters of the iHCs to better determine their functional potential. We postulate that if these cells form connections with the neural network and demonstrate mature traits linked to neurotransmitter synthesis and release, they might play a role in eliciting action potentials in auditory neurons even when located in the scala tympani side of the basilar membrane. We speculate that the sodium-rich environment of the perilymph can support depolarization, as seen in other systems with lower potassium concentration such as the vestibular system or the lateral line. It is also necessary to better integrate HC regeneration with neuronal survival, particularly by augmenting the concentration of neurotrophins in cochlear fluids, as previously shown (Shibata et al., 2010).
5. Conclusions
Our results indicate that co-overexpression of Gfi1, Pou4f3, and Six1 alongside Atoh1 enhances the potential for HC regeneration within a severe lesion model characterized by FE in the guinea pig, compared to using Atoh1 alone. Nonetheless, it remains uncertain whether these newly formed HCs were transdifferentiated from the FE cells, as they appear to be situated on the scala tympani side of the basilar membrane. Further investigation into the origin and function of these iHCs is required.
Supplementary Material
Fig. S2: A confocal microscope projection of optical sections showing the FE and the vas spirale (arrow). Scale bar = 25 μm.
Fig. S1. Schematics of the Ad5 vectors used for the experiments. The upper shows the control vector with the Venus insert. The lower shows the order of the transcription factor genes and spacers. Codon-optimized DNA sequences for human GFI1, ATOH1, POU4F3 and SIX1, together with an mVenus fluorescent protein DNA sequence were synthesized by GeneArt/Thermo. The stop codon was removed from each of the first 4 coding regions, and each DNA coding sequence was separated from its downstream neighbor with a picornavirus P2A sequence to allow expression of multiple proteins from the same transcript. Ad-EF1a-Venus, 1.3×1012 VP/ml. Ad.GAPS-Venus, 1.4×1012 VP/ml were generated by Vector Biolabs, USA.
Fig. S3: A movie render of the FE. The diameter of the blood vessel is similar to the depth of the FE cells adjacent to it.
Acknowledgements
This work was supported by the Hearing Restoration Project (HRP) and RO1 DC014832 both to Y.R. and A.K.G.
Citations
- Ahmed M, Wong EY, Sun J, Xu J, Wang F, and Xu PX (2012). Eya1-Six1 interaction is sufficient to induce hair cell fate in the cochlea by activating Atoh1 expression in cooperation with Sox2. Dev Cell 22, 377–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anttonen T, Belevich I, Kirjavainen A, Laos M, Brakebusch C, Jokitalo E, and Pirvola U (2014). How to bury the dead: elimination of apoptotic hair cells from the hearing organ of the mouse. J Assoc Res Otolaryngol 15, 975–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, and Zoghbi HY (1999). Math1: an essential gene for the generation of inner ear hair cells. Science 284, 1837–1841. [DOI] [PubMed] [Google Scholar]
- Chen Y, Gu Y, Li Y, Li GL, Chai R, Li W, and Li H (2021). Generation of mature and functional hair cells by co-expression of Gfi1, Pou4f3, and Atoh1 in the postnatal mouse cochlea. Cell Rep 35, 109016. [DOI] [PubMed] [Google Scholar]
- Corwin JT, and Cotanche DA (1988). Regeneration of sensory hair cells after acoustic trauma. Science 240, 1772–1774. [DOI] [PubMed] [Google Scholar]
- Costa A, Sanchez-Guardado L, Juniat S, Gale JE, Daudet N, and Henrique D (2015). Generation of sensory hair cells by genetic programming with a combination of transcription factors. Development 142, 1948–1959. [DOI] [PubMed] [Google Scholar]
- Dazert S, Battaglia A, and Ryan AF (1997). Transfection of neonatal rat cochlear cells in vitro with an adenovirus vector. International Journal of Developmental Neuroscience 15, 595–600. [DOI] [PubMed] [Google Scholar]
- Driver EC, and Kelley MW (2020). Development of the cochlea. Development 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Excoffon KJ, Avenarius MR, Hansen MR, Kimberling WJ, Najmabadi H, Smith RJ, and Zabner J (2006). The Coxsackievirus and Adenovirus Receptor: A new adhesion protein in cochlear development. Hear Res 215, 1–9. [DOI] [PubMed] [Google Scholar]
- Hawkins JE Jr. (1973). Comparative otopathology: aging, noise, and ototoxic drugs. Advances in Oto-Rhino-Laryngology 20, 125–141. [DOI] [PubMed] [Google Scholar]
- Hepper PG, and Shahidullah BS (1994). Development of fetal hearing. Arch Dis Child Fetal Neonatal Ed 71, F81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto S, Kawamoto K, Kanzaki S, and Raphael Y (2002). Gene transfer into supporting cells of the organ of Corti. Hear Res 173, 187–197. [DOI] [PubMed] [Google Scholar]
- Iyer AA, and Groves AK (2021). Transcription Factor Reprogramming in the Inner Ear: Turning on Cell Fate Switches to Regenerate Sensory Hair Cells. Frontiers in cellular neuroscience 15, 660748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumikawa M, Batts SA, Miyazawa T, Swiderski DL, and Raphael Y (2008). Response of the flat cochlear epithelium to forced expression of Atoh1. Hear Res 240, 52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan TA, Chai R, Sayyid ZN, van Amerongen R, Xia A, Wang T, Sinkkonen ST, Zeng YA, Levin JR, Heller S, et al. (2013). Tympanic border cells are Wnt-responsive and can act as progenitors for postnatal mouse cochlear cells. Development 140, 1196–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jen HI, Hill MC, Tao L, Sheng K, Cao W, Zhang H, Yu HV, Llamas J, Zong C, Martin JF, et al. (2019). Transcriptomic and epigenetic regulation of hair cell regeneration in the mouse utricle and its potentiation by Atoh1. eLife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur C, Van Orden M, O’Malley JT, Wu PZ, and Liberman MC (2023). Supporting-cell vs. hair-cell survival in the human cochlea: Implications for regenerative therapies. Hear Res 435, 108815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur T, Zamani D, Tong L, Rubel EW, Ohlemiller KK, Hirose K, and Warchol ME (2015). Fractalkine Signaling Regulates Macrophage Recruitment into the Cochlea and Promotes the Survival of Spiral Ganglion Neurons after Selective Hair Cell Lesion. J Neurosci 35, 15050–15061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto K, Ishimoto S, Minoda R, Brough DE, and Raphael Y (2003). Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci 23, 4395–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MC, Chang Q, Pan A, Lin X, and Chen P (2012). Atoh1 directs the formation of sensory mosaics and induces cell proliferation in the postnatal mammalian cochlea in vivo. J Neurosci 32, 6699–6710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick LA, Li Q, Yang J, Goddard JC, Fekete DM, and Lang H (2011). Adeno-associated virus-mediated gene delivery into the scala media of the normal and deafened adult mouse ear. Gene Therapy 18, 569–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, and Raphael Y (2007). Cell division and maintenance of epithelial integrity in the deafened auditory epithelium. Cell Cycle 6, 612–619. [DOI] [PubMed] [Google Scholar]
- Ladrech S, Eybalin M, Puel JL, and Lenoir M (2017). Epithelial-mesenchymal transition, and collective and individual cell migration regulate epithelial changes in the amikacin-damaged organ of Corti. Histochem Cell Biol 148, 129–142. [DOI] [PubMed] [Google Scholar]
- Lee S, Song JJ, Beyer LA, Swiderski DL, Prieskorn DM, Acar M, Jen HI, Groves AK, and Raphael Y (2020). Combinatorial Atoh1 and Gfi1 induction enhances hair cell regeneration in the adult cochlea. Scientific reports 10, 21397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Molnar M, Garnham C, Benav H, and Rask-Andersen H (2018). Macrophages in the Human Cochlea: Saviors or Predators-A Study Using Super-Resolution Immunohistochemistry. Front Immunol 9, 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Dearman JA, Cox BC, Walters BJ, Zhang L, Ayrault O, Zindy F, Gan L, Roussel MF, and Zuo J (2012). Age-dependent in vivo conversion of mouse cochlear pillar and Deiters’ cells to immature hair cells by Atoh1 ectopic expression. J Neurosci 32, 6600–6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez L, Trecek T, Gopalakrishnan S, Tao L, Markowitz AL, Yu HV, Wang X, Llamas J, Huang C, Lee J, et al. (2020). Generation of inner ear hair cells by direct lineage conversion of primary somatic cells. eLife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvaney J, and Dabdoub A (2012). Atoh1, an essential transcription factor in neurogenesis and intestinal and inner ear development: function, regulation, and context dependency. J Assoc Res Otolaryngol 13, 281–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadol JB Jr., Ketten DR, and Burgess BJ (1994). Otopathology in a case of multichannel cochlear implantation. Laryngoscope 104, 299–303. [DOI] [PubMed] [Google Scholar]
- O’Malley JT, Nadol JB Jr., and McKenna MJ (2016). Anti CD163+, Iba1+, and CD68+ Cells in the Adult Human Inner Ear: Normal Distribution of an Unappreciated Class of Macrophages/Microglia and Implications for Inflammatory Otopathology in Humans. Otol Neurotol 37, 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayasu T, Quesnel AM, O’Malley JT, Kamakura T, and Nadol JB Jr. (2020). The Distribution and Prevalence of Macrophages in the Cochlea Following Cochlear Implantation in the Human: An Immunohistochemical Study Using Anti-Iba1 Antibody. Otol Neurotol 41, e304–e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2023). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. [Google Scholar]
- Raphael Y (1992). Evidence for supporting cell mitosis in response to acoustic trauma in the avian inner ear. J Neurocytol 21, 663–671. [DOI] [PubMed] [Google Scholar]
- Raphael Y, and Altschuler RA (1991). Reorganization of cytoskeletal and junctional proteins during cochlear hair cell degeneration. Cell Motility & the Cytoskeleton 18, 215–227. [DOI] [PubMed] [Google Scholar]
- Ryals BM, and Rubel EW (1988). Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science 240, 1774–1776. [DOI] [PubMed] [Google Scholar]
- Schimmang T (2013). Transcription factors that control inner ear development and their potential for transdifferentiation and reprogramming. Hear Res 297, 84–90. [DOI] [PubMed] [Google Scholar]
- Shibata SB, Cortez SR, Beyer LA, Wiler JA, Di Polo A, Pfingst BE, and Raphael Y (2010). Transgenic BDNF induces nerve fiber regrowth into the auditory epithelium in deaf cochleae. Exp Neurol 223, 464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou J, Zheng JL, and Gao WQ (2003). Robust generation of new hair cells in the mature mammalian inner ear by adenoviral expression of Hath1. Mol Cell Neurosci 23, 169–179. [DOI] [PubMed] [Google Scholar]
- Stone JS, and Rubel EW (2000). Cellular studies of auditory hair cell regeneration in birds. Proc Natl Acad Sci U S A 97, 11714–11721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover T, Yagi M, and Raphael Y (1999). Cochlear gene transfer: round window versus cochleostomy inoculation. Hear Res 136, 124–130. [DOI] [PubMed] [Google Scholar]
- Sugawara M, Corfas G, and Liberman MC (2005). Influence of supporting cells on neuronal degeneration after hair cell loss. J Assoc Res Otolaryngol 6, 136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RR, Jagger DJ, and Forge A (2012). Defining the cellular environment in the organ of Corti following extensive hair cell loss: a basis for future sensory cell replacement in the Cochlea. PLoS One 7, e30577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner EL, and Shin JB (2019). Mechanisms of Hair Cell Damage and Repair. Trends Neurosci 42, 414–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis D, Hamblen M, Zhou Y, Venken KJ, Schumacher A, Grimes HL, Zoghbi HY, Orkin SH, and Bellen HJ (2003). The zinc finger transcription factor Gfi1, implicated in lymphomagenesis, is required for inner ear hair cell differentiation and survival. Development 130, 221–232. [DOI] [PubMed] [Google Scholar]
- Wang GP, Basu I, Beyer LA, Wong HT, Swiderski DL, Gong SS, and Raphael Y (2017). Severe streptomycin ototoxicity in the mouse utricle leads to a flat epithelium but the peripheral neural degeneration is delayed. Hear Res 355, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster DB (1992). Degeneration followed by partial regeneration of the organ of Corti in deafness (dn/dn) mice. Experimental Neurology 115, 27–31. [DOI] [PubMed] [Google Scholar]
- Woods C, Montcouquiol M, and Kelley MW (2004). Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nat Neurosci 7, 1310–1318. [DOI] [PubMed] [Google Scholar]
- Xiang M, Gan L, Li D, Chen ZY, Zhou L, O’Malley BW Jr., Klein W, and Nathans J (1997). Essential role of POU-domain factor Brn-3c in auditory and vestibular hair cell development. Proc Natl Acad Sci U S A 94, 9445–9450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata T, Miller JM, Ulfendahl M, Olivius NP, Altschuler RA, Pyykko I, and Bredberg G (2004). Delayed neurotrophic treatment preserves nerve survival and electrophysiological responsiveness in neomycin-deafened guinea pigs. J Neurosci Res 78, 75–86. [DOI] [PubMed] [Google Scholar]
- Zar JH (1999) Biostatistical Analysis, 4th edition. Prentice Hall, Inc., NJ. [Google Scholar]
- Zheng W, Huang L, Wei ZB, Silvius D, Tang B, and Xu PX (2003). The role of Six1 in mammalian auditory system development. Development 130, 3989–4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S2: A confocal microscope projection of optical sections showing the FE and the vas spirale (arrow). Scale bar = 25 μm.
Fig. S1. Schematics of the Ad5 vectors used for the experiments. The upper shows the control vector with the Venus insert. The lower shows the order of the transcription factor genes and spacers. Codon-optimized DNA sequences for human GFI1, ATOH1, POU4F3 and SIX1, together with an mVenus fluorescent protein DNA sequence were synthesized by GeneArt/Thermo. The stop codon was removed from each of the first 4 coding regions, and each DNA coding sequence was separated from its downstream neighbor with a picornavirus P2A sequence to allow expression of multiple proteins from the same transcript. Ad-EF1a-Venus, 1.3×1012 VP/ml. Ad.GAPS-Venus, 1.4×1012 VP/ml were generated by Vector Biolabs, USA.
Fig. S3: A movie render of the FE. The diameter of the blood vessel is similar to the depth of the FE cells adjacent to it.


