Abstract
Background and aim:
There are many epidemiological pieces of evidence that show combined pulmonary fibrosis and emphysema (CPFE) patients have an increased risk of lung cancer. We conducted a systematic review of all published data to define the characteristics and treatments of lung cancer that develops in CPFE by performing a meta-analysis.
Methods:
Databases including PubMed, Medline and Web of Science (updated to July, 2021) were searched to find original articles that related to lung cancer in CPFE(CPFE-LC) patients and a meta-analysis was used to analyze the included 15 articles. Stata17.0 software was performed for this meta-analysis.
Results:
Fifteen original studies that assessed 5933 patients were included in this meta-analysis. In the pooled data, people with CPFE-LC were elderly (70.58 years) and heavy smokers (0.959, 45.793 pack-years), with a male predominance (0.959). Most lung cancer in CPFE was located in the lower lobe (0.533) and obvious areas of pulmonary fibrosis (0.516). The highest prevalence of cellular subtypes of lung cancer in CPFE was squamous carcinoma (SqCC, 0.437) and chemotherapy was the primary treatment (0.387). The mortality rate was 0.720(95%CI: 0.657-0.783) and the 5-year survival rate was 0.250 (95%CI: 0.133-0.368). The main cause of death was infection (0.268) and respiratory failure was the main cause of death after surgery (0.392).
Conclusions:
Lung cancer in CPFE, most commonly SqCC, presents in elderly heavy smokers with a male, located in the lower lobe of the lung and the areas of fibrosis predominance. Chemotherapy is the primary treatment and the optimal treatment remains to be explored.
Keywords: CPFE, combined pulmonary fibrosis and emphysema, pulmonary fibrosis, lung cancer
Introduction
Pulmonary fibrosis and emphysema are both pathological diagnoses, which are considered to be independent and incompatible diseases. However, the coexistence of fibrosis and emphysema in individuals has been gradually recognized since 1990 (1). In 2005, Cotton et al. (2) defined CPFE as upper lobe emphysema and lower lobe fibrosis in high-resolution computed tomography (HRCT). Family history of disease, male, smoking, age, various types of environmental effects such as organic and inorganic dust and medical treatments are risk factors that increase the risk of CPFE (3-5). CPFE has been increasingly recognized as a separate clinical entity, characterized by progressively worse respiratory symptoms, a decline in lung function and high mortality(3, 6, 7). According to Mejia’s (8) research, patients with CPFE have poorer survival than patients with idiopathic pulmonary fibrosis (IPF) alone and pulmonary hypertension is an independent predictor of mortality (6).
Emphysema and pulmonary fibrosis are two important risk factors for lung cancer (9). CPFE, which has both characteristics of fibrosis and emphysema, is more likely to develop lung cancer than patients with chronic obstructive pulmonary disease (COPD) or IPF alone (10). Kitaguchi et al. (11) have suggested that 46.8% of CPFE patients are associated with lung cancer. Previous studies have shown that lung cancer in CPFE is mainly SqCC, followed by adenocarcinoma (9, 12-22). However, other studies indicated that adenocarcinoma was the most common type (23-26). Four studies suggested that most patients with CPFE-LC were in the advanced stage of lung cancer (16, 18, 19, 24, 25), while more studies showed that most patients were in the early stage (13-15, 17, 20-23, 26). The location of lung cancer on CT has also been reported. Some studies showed that most lung cancer in CPFE was located in the upper lobe (13, 21, 26), while others suggested that lung cancer was more common in the lower lobe (18, 19, 22).
The onset of CPFE-LC is insidious and the 5-year survival rate after diagnosis ranges from 18.7 to 36.9% (14, 20, 26). Several studies have indicated that the survival rate of CPFE-LC patients is significantly lower than that of IPF-LC, emphysema-LC and LC patients alone. However, the statistical results of the specific rate have varied (14, 20, 26). The treatment of patients with CPFE-LC is challenging because treatments, including chemotherapy, surgery and radiotherapy, may induce acute exacerbation or pneumonia even death.
Despite previously published studies about the prevalence of lung cancer in CPFE patients, the characteristics of lung cancer in CPFE have not been fully evaluated and the treatment has not yet reached a consensus. Therefore, we conducted a meta-analysis of relevant studies published in recent years on CPFE-LC to find disease predictors and provide evidence-based basis for its early diagnosis and treatment.
Material and methods
Literature retrieval strategy
We searched PubMed, Embase and Cochrane Library databases for CPFE studies with lung cancer. The following keywords were used to perform our research: ((“Lung Neoplasms”[Mesh]) OR ((Pulmonary Neoplasms) OR (Neoplasms, Lung) OR (Lung Neoplasm) OR (Neoplasm, Lung) OR (Neoplasms, Pulmonary) OR (Neoplasm, Pulmonary) OR (Lung Cancer) OR (Cancer, Lung) OR (Cancers, Lung) OR (Lung Cancers)) OR (Cancer of Lung) OR (Cancer of the Lung) OR (Pulmonary Cancers)) OR (Cancers, Pulmonary)) OR (Cancer, Pulmonary) OR (Pulmonary Cancer))) AND (“pulmonary fibrosis” AND “emphysema” AND [“fibrosis” OR “fibroses” OR “fibrosing” OR “alveolitis” OR “alveolitides”] AND [“combine*” OR “cryptogen*”]). The starting point of the search time was set to 2005 and the language was restricted to English.
Inclusion and exclusion criteria
Inclusion criteria based on PICO(related to Evidence-Based Medicine)(27). (1) Population: Cohort and retrospective studies that investigated LC in CPFE patients; (2) Intervention: Surgical resection or radiological and pathologically confirmed cancer; (3) Comparison: cancers develop in non-CPFE (fibrosis, emphysema and normal); (4) Outcome: Describe the clinical feathers of LC in CPFE patients and other risk factors; (5) Data was available for further meta-analysis.
Exclusion criteria were as follows: (1) Case reports, reviews, letters, Comments, conference results and meta-analysis; (2) Non-accessible full text; (3) The number of cases included was too small, less than 10; (4) Duplicated papers; (5) Non-Chinese or English literature.
Quality assessment
Two researchers independently reviewed all titles and abstracts and a full-text review was carried out using inclusion and exclusion criteria. We assessed the quality of the original studies by Newcastle-Ottawa Quality Assessment Scale (NOS). Each study received a score from 0 to 9, with scores above six considered high quality.
Statistical analysis
Stata 17.0 software was used for meta-analysis and sensitivity analysis was performed to analyze heterogeneity. Heterogeneity analysis was evaluated using the Cochran test (Q) and I2 index included in the study. P < 0.05 and I2 > 50% were deemed to indicate substantial heterogeneity and the random-effect model was used. On the contrary, P >0.05 and I2 <50% indicated no significant heterogeneity and the fixed-effect model was used. The odds ratio (OR) and weighted mean difference (WMD) were used to compare continuous and dichotomous variables, respectively. The Egger and Begg’s tests were evaluated to examine the publication bias. A funnel plot was used to analyze the occurrence bias of more than 10 articles.
Result
Literature retrieval results
Based on performed searches, 442 preliminary papers were obtained and 312 duplicates were removed; 105 were excluded after reading questions and abstracts, because the studies were animal experiments, literature reviews, meta-analyses and conference papers; 10 were excluded after reading the full papers. Finally, 15 papers(12-26) that met the requirements were included, all of which were in English. The literature quality scores were 8-9, indicating that all the included studies were of high quality. Finally, 15 studies were included in this meta-analysis. Most studies were carried out in Japan and Korea.
Characteristics of the studies
A total of 15 studies were entered into the final list. The characteristics of the studies were summarized in Table 1. The PRISMA flowchart (Figure 1) showed the literature selection and identification process. A total of 5933 patients were enrolled, including 746 CPFE patients with lung cancer. The studies included were published from 2014 to 2020.
Table 1.
Characteristics of studies included in the meta-analysis.
| Study/year | country | Na | Enrolled period | Group | NOS score | ||
|---|---|---|---|---|---|---|---|
| LC | CPFE | CPFE-LC | |||||
| Fujiwara/2012 | Japan | 274 | 36 | 36 | 2003-2011 | CPFE-LC/fibrosis-LC/ emphysema-LC/normal-LC |
9 |
| Girard/2014 | France | 47 | 47 | 47 | 2003-2012 | CPFE-LC | 8 |
| Hata A/2016 | Korea | 250 | 11 | 11 | 2008-2016 | CPFE-LC/IP-LC/emphysema-LC/normal-LC | 9 |
| Kim H/2019 | Korea | 234 | 16 | 16 | 2010-2017 | CPFE-LC/IPF-LC/emphysema-LC/ COPD-LC/controlb-LC |
9 |
| Kumagai S /2014 | Japan | 365 | 20 | 20 | 2007-2012 | CPFE-LC/fibrosis-LC/ emphysema-LC/normal-LC |
9 |
| Kwak N/2014 | Korea | 25 | 48 | 12 | 2000-2011 | CPFE-LC/IPF-LC/emphysema-LC | 8 |
| Mimae T/2016 | Japan | 2295 | 151 | 151 | 2008-2010 | CPFE-LC/non-CPFE-LC | 9 |
| Minegishi Y/ 2014 | Japan | 1536 | 88 | 88 | 1998-2011 | CPFE-LC/non-CPFE-LC | 9 |
| Moon SW/2019 | Korea | 283 | 107 | 107 | 2003-2018 | CPFE-NSCLC/IPF-NSCLC | 9 |
| Nasim F/2020 | Japan | 26 | 230 | 26 | 1995-2017 | CPFE-LC/only CPFE | 9 |
| Oh JY/2020 | Korea | 61 | 227 | 61 | 2004-2016 | CPFE-LC/only CPFE | 9 |
| Otsuka H/2016 | Japan | 831 | 23 | 23 | 2004-2014 | CPFE-LC/IPF-LC/emphysema-LC | 9 |
| Takenaka T/2018 | Japan | 274 | 17 | 17 | 2005-2011 | CPFE-NSCLC/fibrosis-NSCLC/emphysema-NSCLC/normal-NSCLC | 8 |
| Ueno F/2017 | Japan | 59 | 59 | 59 | 2001-2015 | CPFE-LC | 8 |
| Zhang M/2016 | Japan | 985 | 72 | 72 | 1995-2013 | CPFE-LC/non-CPFE-LC | 9 |
Abbreviations: COPD: chronic obstructive pulmonary disease; NSCLC: non-small cell lung carcinoma; LC: lung cancer; NOS: Newcastle-Ottawa Quality Assessment Scale. a number of included patients; b Non-COPD, non-CPFE, and non-IPF.
Figure 1.

A flow diagram of the study.
Clinical characters
In the pooled data, most of the patients with CPFE-LC were elderly (70.58 years) and heavy smokers (98.3%, 45.793 pack-years), with a male predominance (95.9%, Fig 2). Three papers mentioned clubbing fingers, with a combined incidence of 0.396 (95%CI: 0.221-0.571). Only Girard et al. (13) reported the crackles (38%). FIve studies reported the BMI and 3 papers (21, 25, 26) reported the KL-6, which were estimated to be 22.888 (95%CI: 22.773-23.002) and 612.452 (95%CI: 521.022-703.881). See Table 2.
Table 2.
The main clinical features of CPFE-LC in Meta-analysis.
| Variables | N (study)a | Pooled data | P | Heterogeneity testing model |
|---|---|---|---|---|
| Male | 15 | 0.959 (0.933,0.980) | 0.045 | Random |
| Smoking | 11 | 0.983 (0.916,1.000) | <0.001 | Random |
| Age, year | 15 | 70.580 (69.646,71.515) | <0.001 | Random |
| Smoking index, Pack-year | 6 | 45.793 (42.027,49.559) | 0.549 | Fixed |
| BMI | 5 | 22.888 (22.773,23.002) | 0.926 | Fixed |
| finger clubbing | 3 | 0.396 (0.221,0.571) | 0.011 | Random |
| KL-6, U/mL | 3 | 612.452 (521.022,703.881) | <0.001 | Random |
Abbreviations: BMI: Body Mass Index; KL-6: Krebs von den Lungen-6. anumber of studies included in the meta-analysis.
Figure 2.

Forest plot for male patients in CPFE-LC.
Pulmonary function test
In the pulmonary function tests, the pooled data of %VC, FVC, FEV1(Figure 3) and FEV1/FVC1 was estimated to be 101.543, 87.573, 81.604 and 69.627, respectively. However, the mean DLCO was 61.907. See Table 3.
Figure 3.

Forest plot for FEV1 in the patients of CPFE-LC.
Table 3.
The pulmonary function parameters of CPFE-LC in Meta-analysis.
| Variables | N (study)a | Pooled data | P | Heterogeneity testing model |
|---|---|---|---|---|
| VC,% | 3 | 101.543 (99.468,103.618) | 0.433 | fixed |
| FVC, %pred | 4 | 87.573 (82.306,92.841) | 0.072 | random |
| FEV1, %pred | 7 | 81.604 (74.964,88.244) | <0.001 | random |
| FEV1/FVC,% | 5 | 69.627 (66.359,72.894) | 0.071 | random |
| DLCO, %pred | 5 | 61.907 (54.251,69.564) | <0.001 | random |
Abbreviations: VC: vital capacity; FVC: forced vital capacity; FEV1: forced expiratory volume in one second; DLCO: diffusing capacity of the lung for carbon monoxide. anumber of studies included in the meta-analysis.
Pathological types and clinical stages of lung cancer
The most common histological type of lung cancer was SqCC (0.437, 95%CI: 0.374-0.500, Figure 4), followed by adenocarcinoma (0.340, 95%CI: 0.274-0.405) and the majority were in stage I (0.442, 95%CI: 0.312-0.573). See Table 4.
Figure 4.

Forest plot for squamous carcinoma in the patients of CPFE-LC.
Table 4.
Pathologic types and clinical stages of lung cancer of CPFE-LC in Meta-analysis.
| Variables | N (study)a | Pooled data (95%CI) | P | Heterogeneity testing model |
|---|---|---|---|---|
| Adenocarcinoma | 15 | 0.340 (0.274,0.405) | <0.001 | Random |
| Squamous carcinoma | 15 | 0.437 (0.374,0.500) | <0.001 | Random |
| Other types | 14 | 0.184 (0.119,0.259) | <0.001 | Random |
| Clinical stage | ||||
| Stage I | 14 | 0.442 (0.312,0.573) | <0.001 | Random |
| Stage II | 14 | 0.183 (0.134,0.232) | 0.001 | Random |
| Stage III | 13 | 0.176 (0.124,0.228) | <0.001 | Random |
| Stage IV | 8 | 0.162 (0.051,0.314) | <0.001 | Random |
| Unknown stage | 4 | 0.033 (0.000-0.105) | 0.001 | Random |
a number of studies included in the meta-analysis.
The features of patients with CPFE-LC on chest CT
CT results showed that most of the lung cancer in CPFE-LC was located in the lower lobe (0.533, 95%CI: 0.429-0.638, Fig 5), contacted the pleura (0.459, 95%CI: 0.117-0.801) and obvious areas of pulmonary fibrosis (0.516, 95%CI: 0.153-0.879). Centriacinar (0.541) and paraseptal emphysema (0.545) were common in CPFE-LC. However, for the type of fibrosis, honeycombing (0.537) and reticular opacity (0.667) were easier to find. See Table 5.
Table 5.
The features of patients with CPFE-LC on chest computed tomography scan in Meta-analysis.
| Variables | N (study)a | Pooled data (95%CI) | P | Heterogeneity testing model |
|---|---|---|---|---|
| Localization of cancer | ||||
| Upper lobe | 6 | 0.433(0.310,0.556) | <0.001 | Random |
| Middle lobe | 6 | 0.102(0.032,0.171) | <0.001 | Random |
| Lower lobe | 5 | 0.533(0.429,0.638) | 0.026 | Random |
| In the emphysema | 2 | 0.151(0.088,0.227) | <0.001 | Random |
| In the fibrosis | 3 | 0.516(0.153,0.879) | <0.001 | Random |
| Pleural contact | 4 | 0.459(0.117,0.801) | <0.001 | Random |
| Pleural un-contact | 2 | 0.276(0.139,0.435) | <0.001 | Random |
| Fibrosis | ||||
| Honeycombing | 3 | 0.537(0.209,0.864) | <0.001 | Random |
| Ground glass opacity | 2 | 0.297(0.199,0.395) | 0.366 | Fixed |
| Reticular opacity | 2 | 0.667(0.296,1.038) | <0.001 | Random |
| Traction bronchiectasis | 2 | 0.194(0.114,0.289) | <0.001 | Random |
| Emphysema | ||||
| Centrilobular | 2 | 0.541(0.171,0.910) | <0.001 | Random |
| Paraseptal | 2 | 0.545(0.190,0.900) | 0.003 | Random |
| Mixed | 2 | 0.382(0.262,0.502) | 0.265 | Fixed |
anumber of studies included in the meta-analysis.
Figure 5.
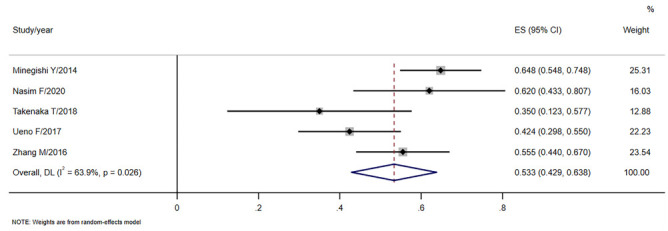
Forest plot for the tumor located in the lower lobe.
The clinical characters compared with IPF-LC
Compared with IPF-LC, CPFE-LC patients had a younger age at diagnosis (WMD=-0.228, P=0.767), a higher smoking index (WMD=12.624, P<0.001, Fig 6) and a smaller BMI (WMD=-0.515, P=0.155). In the pulmonary function tests, the FVC (WMD=1.868, P=0.319) and FEV1% (WMD=5.151, P-value=0.004) were higher than IPF-LC patients, while the FEV1 / FVC was lower (WMD=-4.238, P<0.001). Among them, the P values of smoking index, FVC and FEV1 / FVC were <0.05 and considered significant. See Table 6.
Table 6.
Comparison of characteristics between CPFE-LC and IPF-LC in Meta-analysis.
| Variables | N (study)a | N (CPFE-LC)b | N (IPF-LC)c | WMD (95%CI) | P-value | Heterogeneity | |
|---|---|---|---|---|---|---|---|
| I2 (%) | P(h) | ||||||
| age | 4 | 158 | 255 | -0.228 (-1.740,1.283) | 0.767 | 0 | 0.664 |
| pack-years | 4 | 158 | 255 | 12.624 (7.791,17.458) | <0.001* | 45.4 | 0.139 |
| BMI | 2 | 119 | 224 | -0.515 (-1.224,0.194) | 0.155 | 0 | 0.922 |
| FVC% | 3 | 135 | 246 | 1.868 (-1.809,5.544) | 0.319 | 60.2 | 0.081 |
| FEV1% | 4 | 158 | 255 | 5.151 (-8.65,-1.653) | 0.004* | 20.9 | 0.285 |
| FEV1/FVC% | 3 | 135 | 246 | -4.238 (-6.304,-2.172) | <0.001* | 0 | 0.513 |
anumber of studies included in the meta-analysis; bnumber of patients in CPFE-LC group; cnumber of patients in IPF-LC group. LC, lung cancer. * P-value<0.05 has been considered significant.
Figure 6.
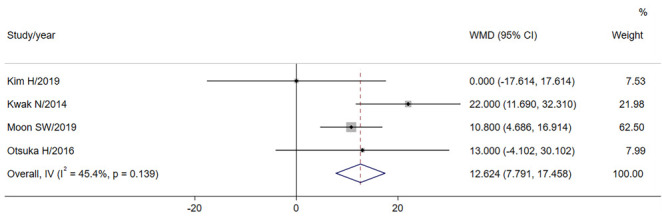
Forest plot for the comparison of pack-years.
Treatments
Nowadays, chemotherapy was the main treatment (0.387, 0.329-0.445, Fig 7), followed by surgery (0.318,0.259-0.377). The mortality rate was 0.720 and the 5-year survival rate was 0.250. The main cause of death was infection, with a combined incidence of 0.268. The main cause of death after lung cancer surgery was respiratory failure(0.392,0.245-0.638). See Table 7.
Table 7.
Treatment, modality and survival indicators of patients with CPFE-LC in the Meta-analysis.
| Variables | N (study)a | Pooled data, 95%CI | P | Heterogeneity testing model |
|---|---|---|---|---|
| Treatment | ||||
| Chemotherapy | 5 | 0.387 (0.329,0.445) | 0.052 | Fixed |
| Radiotherapy | 4 | 0.151 (0.028,0.337) | <0.001 | Random |
| Surgery | 4 | 0.318 (0.259,0.377) | 0.838 | Fixed |
| Other treatment | 3 | 0.272 (0.163,0.380) | 0.122 | Random |
| Survival and mortality indicators | ||||
| Mortality rate | 3 | 0.720 (0.657,0.783) | 0.365 | Fixed |
| Five-year survival rate | 3 | 0.250 (0.133,0.368) | 0.486 | Fixed |
| Cause of death | ||||
| Lung cancer | 8 | 0.238 (0.134,0.359) | <0.001 | Random |
| AEILD | 8 | 0.219 (0.145,0.302) | 0.020 | Random |
| Infection | 3 | 0.268 (0.065,0.471) | 0.010 | Random |
| Not related to CPFE and LC | 4 | 0.079 (0.046,0.111) | 0.386 | Fixed |
| Other causes | 6 | 0.306 (0.129,0.482) | <0.001 | Random |
| After surgery | ||||
| Mortality rate at 90 days | 3 | 0.074 (0.036,0.121) | 0.627 | Fixed |
| Acute exacerbation | 2 | 0.143 (0.068,0.218) | 0.158 | Fixed |
| Died of lung cancer | 5 | 0.220 (0.132,0.322) | 0.355 | Fixed |
| Died of respiratory failure | 3 | 0.392 (0.245,0.638) | 0.258 | Fixed |
| Died of other causes | 2 | 0.344 (0.179,0.508) | 0.923 | Fixed |
Abbreviations: AEILD: acute exacerbation of interstitial lung disease. anumbers of studies included in the meta-analysis.
Figure 7.

Forest plot for chemotherapy.
Publication bias and sensitivity analysis
Publication bias
The Egger test was used to analyze the publication bias of more than 10 articles included in the analysis. Taking the proportion of male patients as an example (p=0.016, <0.05), the funnel diagram was drawn (Figure 8) and the two sides of the funnel diagram were not completely symmetric, indicating that there was a certain publication bias.
Figure 8.

Publication bias in the proportion of male patients.
Sensitivity analysis
Random and fixed-effect model were used to estimate the combined rate and confidence interval of the research factors with P < 0.05, such as males, age, KL-6, FEV1 and so on. It was found that the results obtained by the two test models were roughly the same, indicating that the comprehensive analysis results of this study were reliable (Table 8).
Table 8.
Comparison of fixed and random effects.
| Variables | N (study)a | Random-effect model (Pooled data, 95%CI) | Fixed-effect model (Pooled data, 95%CI) |
|---|---|---|---|
| Male | 15 | 0.959 (0.933,0.980) | 0.958 (0.940,0.973) |
| Age, year | 15 | 70.580 (69.646,71.515) | 71.443 (71.232,71.655) |
| KL-6, U/mL | 3 | 612.452 (521.022,703.881) | 633.067 (619.612,646.521) |
| FEV1, %pred | 7 | 81.604 (74.964,88.244) | 73.700 (72.780,74.619) |
| DLCO, %pred | 5 | 61.907 (54.251,69.564) | 54.027 (533.364,54.589) |
anumbers of studies included in the meta-analysis.
Discussion
The total sample size of patients with CPFE was 5933, among whom 746 had lung cancer. This meta-analysis indicated that people with CPFE-LC were elderly heavy smokers with a male predominance. Most lung cancer was located in the lower lobe and obvious areas of fibrosis. SqCC was the most common type of lung cancer, followed by adenocarcinoma. Patients with CPFE-LC had poor survival and chemotherapy was the primary treatment.
Smoking, male and age are risk factors in developing lung cancer in CPFE (28). SqCC has been reported to be more closely associated with smoking than adenocarcinoma, which may be one reason for the higher incidence of this type of cancer in CPFE. The level of serum KL-6 at diagnosis is an independent prognostic determinant in patients with CPFE-LC(29, 30), suggesting that increased KL-6 is associated with disease progression and poor prognosis. Previous studies have suggested that the pathogenesis of CPFE-LC may be related to matrix metalloproteinase-9 (MMP-9) and Transforming growth factor-β1 (TGF-β1) genetic polymorphisms (31), DNA hypermethylation, epithelial-mesenchymal transition (EMT), miRNA dysregulation and other factors (32, 33) and the specific mechanism still needs to be further studied and confirmed.
We found that compared with emphysema, the peripheral fibrosis area of CPFE may be closely related to the development of lung cancer. Zhang et al. (22) suggested that lung cancer in CPFE may arise from dysplastic epithelium in the fibrotic area around the tumor. Evidence has represented that pulmonary fibrosis and lung cancer share several cellular and molecular processes that drive the progression of both pathologies, such as fibroblast transformation, proliferation and activation, endoplasmic reticulum stress, oxidative stress and many genetic and epigenetic marks that predispose patients with fibrosis to lung cancer development (34). Epithelial-mesenchymal transition (EMT), the key feature of epithelial fibrosis, also plays an important role in lung cancer (35, 36). Calio et al. (37) have speculated in a study of patients with IPF and lung cancer that cancer may arise from transformed small airways in honeycomb lung areas where abnormal bronchiolar proliferation takes place, suggesting a direct relationship between fibrosis and lung cancer (38).
At present, there is no optimal treatment for patients with CPFE-LC. The majority in this study were treated with chemotherapy (0.387), followed by surgery (0.318). Minegishi et al. (18) found that in the selection of first-line chemotherapy regimens, carboplatin plus paclitaxel was mainly used in CPFE-NSCLC patients, while platinum-agent plus etoposide in CPFE-SCLC. Acute exacerbation of disease caused by chemotherapy may cause death and the tumor recurrence rate after postoperative adjuvant chemoradiotherapy was high (23). With the rapid development of immuno-oncology, immune checkpoint inhibitors (ICIs) are often used as the standard of care for advanced NSCLC. Recent studies have shown that combined immunotherapy and radiotherapy can improve the prognosis of low-metastatic or advanced lung cancer with tolerable side effects (39, 40). Molecularly targeted drugs provide a new option for the treatment of CPFE-LC patients. Nintedanib, as an anti-fibrotic drug, has been used as a conventional treatment in CPFE patients. Studies have suggested that as a multi-targeted tyrosine kinase inhibitor, nintedanib also has anti-tumor effects because it can inhibit tumor angiogenesis, thereby inhibiting tumor growth and metastasis (41). Yamanaka et al. (42) found that therapeutic strategies combining conventional cytotoxic agents with nintedanib are promising for overcoming refractory intrahepatic cholangiocarcinoma. kato et al. (43) demonstrated that nintedanib not only anti-fibrosis but also exerted a combined anti-tumor effect by attenuating the immunosuppressive nature of the tumour microenvironment and promoting the intratumoural accumulation and activation of CD8+ T cells. Therefore, for all patients with CPFE-LC, it is recommended that nintedanib be actively used in clinical therapy. Patients with CPFE-LC often have severe dyspnea and poor cardiopulmonary reserve and many can’t tolerate it but often undergo invasive surgery and treatment (9), which can cause iatrogenic complications and lead to death. Therefore, procedures and other treatment modalities for patients with CPFE-LC should not be as aggressive as those for lung cancer without CPFE.
The prognosis of lung cancer patients with CPFE is poor and CPFE has been reported to be a worse prognostic factor for lung cancer compared to emphysema or fibrosis alone(23, 44). In our study, the mortality rate was 0.720 and the 5-year survival rate was 0.250, which were consistent with those of previous studies. Usui et al. (44) reported that the median survival duration of patients with CPFE-LC is 10.8 months. Kumagai, S et al. found that NSCLC recurred earlier and more frequently (50%) in patients and that patients with CPFE had shorter OS after recurrence than those without CPFE. The probability of acute exacerbation (AE) after treatment is closely related to the prognosis. Moon, S.W., et al suggested that CPFE may increase the risk of AE regardless of whether invasive or non-invasive treatment is used. Iwata et al. (45) showed that perioperative pirfenidone could significantly reduce the incidence of postoperative AE in lung cancer patients with IPF, which may also be true for CPFE patients and further research is needed.
Limitations
One of the main limitations of this meta-analysis to be mentioned is that we only used pooled data rather than individual data. The existence of selection bias is another limitation because most of the articles included in this study were from Japan and Korea, which may limit the generalization of these research results. Moreover, because few studies included in this study have investigated the survival time and detailed treatment methods, this meta-analysis could not focus on these factors.
Conclusion
In conclusion, the high prevalence of CPFE-LC is more observed in elderly men who smoke and is more evident in the progression of cancer, SqCC and mostly limited affects the fibrosis area and the lower part of the lung. At present, there are challenges in the treatment of patients with CPFE-LC. In addition to the treatment of lung cancer, the existence of emphysema-fibrosis should also be considered. Therefore, it is recommended to closely follow up with the high-risk population, regularly review lung function and chest CT and put forward new requirements for clinical research.
Funding:
Project of Chongqing Natural Science Foundation Committee, Grant/ Award Number: cstc2020jcyj-msxmX0359.
Conflict of Interest:
Each author declares that he or she has no commercial associations (e.g., consultancies, stock ownership, equity interest, patent/licensing arrangement, etc.) that might pose a conflict of interest in connection with the submitted article.
References
- Wiggins J, Strickland B, Turner-Warwick M. Combined cryptogenic fibrosing alveolitis and emphysema: the value of high resolution computed tomography in assessment. Respir Med. 1990;84(5):365–9. doi: 10.1016/s0954-6111(08)80070-4. doi: 10.1016/s0954-6111(08)80070-4. [DOI] [PubMed] [Google Scholar]
- Cottin V, Nunes H, Brillet PY, et al. Combined pulmonary fibrosis and emphysema: a distinct underrecognised entity. Eur Respir J. 2005;26(4):586–93. doi: 10.1183/09031936.05.00021005. doi: 10.1183/09031936.05.00021005. [DOI] [PubMed] [Google Scholar]
- Hirano ACG, Targueta EP, Ferraz de Campos FP, et al. Severe pulmonary hypertension due to combined pulmonary fibrosis and emphysema: another cause of death among smokers. Autops Case Rep. 2017;7(2):15–26. doi: 10.4322/acr.2017.022. doi: 10.4322/acr.2017.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottin V, Cordier JF. Combined pulmonary fibrosis and emphysema in connective tissue disease. Curr Opin Pulm Med. 2012;18(5):418–27. doi: 10.1097/MCP.0b013e328356803b. doi: 10.1097/MCP.0b013e328356803b. [DOI] [PubMed] [Google Scholar]
- Roshan R, Guptal M, Kulshrestha R, Menon B, Chhabra SK. Combined pulmonary fibrosis and emphysema in a welder. Monaldi Arch Chest Dis. 2012;77(1):26–8. doi: 10.4081/monaldi.2012.164. doi: 10.4081/monaldi.2012.164. [DOI] [PubMed] [Google Scholar]
- Sugino K, Ishida F, Kikuchi N, et al. Comparison of clinical characteristics and prognostic factors of combined pulmonary fibrosis and emphysema versus idiopathic pulmonary fibrosis alone. Respirology. 2014;19(2):239–245. doi: 10.1111/resp.12207. doi: 10.1111/resp.12207. [DOI] [PubMed] [Google Scholar]
- Tokgoz Akyıl F, Sevim T, Akman C, et al. The predictors of mortality in IPF - Does emphysema change the prognosis? Sarcoidosis Vasc Diffuse Lung Dis. 2016;33(3):267–274. [PubMed] [Google Scholar]
- Mejía M, Carrillo G, Rojas-Serrano J, et al. Idiopathic pulmonary fibrosis and emphysema: decreased survival associated with severe pulmonary arterial hypertension. Chest. 2009;136(1):10–15. doi: 10.1378/chest.08-2306. doi: 10.1378/chest.08-2306. [DOI] [PubMed] [Google Scholar]
- Lin H, Jiang S. Combined pulmonary fibrosis and emphysema (CPFE): an entity different from emphysema or pulmonary fibrosis alone. J Thorac Dis. 2015;7(4):767–79. doi: 10.3978/j.issn.2072-1439.2015.04.17. doi: 10.3978/j.issn.2072-1439.2015.04.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hage R, Gautschi F, Steinack C, Schuurmans MM. Combined Pulmonary Fibrosis and Emphysema (CPFE) Clinical Features and Management. Int J Chron Obstruct Pulmon Dis. 2021;16:167–177. doi: 10.2147/COPD.S286360. doi: 10.2147/copd.S286360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitaguchi Y, Fujimoto K, Hanaoka M, Kawakami S, Honda T, Kubo K. Clinical characteristics of combined pulmonary fibrosis and emphysema. Respirology. 2010;15(2):265–71. doi: 10.1111/j.1440-1843.2009.01676.x. doi: 10.1111/j.1440-1843.2009.01676.x. [DOI] [PubMed] [Google Scholar]
- Fujiwara A, Tsushima K, Sugiyama S, et al. Histological types and localizations of lung cancers in patients with combined pulmonary fibrosis and emphysema. Thorac Cancer. 2013;4(4):354–360. doi: 10.1111/1759-7714.12023. doi: 10.1111/1759-7714.12023. [DOI] [PubMed] [Google Scholar]
- Girard N, Marchand-Adam S, Naccache J-M, et al. Lung Cancer in Combined Pulmonary Fibrosis and Emphysema: A Series of 47 Western Patients. Journal of Thoracic Oncology. 2014;9(8):1162–1170. doi: 10.1097/JTO.0000000000000209. doi: https://doi.org/10.1097/JTO.0000000000000209. [DOI] [PubMed] [Google Scholar]
- Hata A, Sekine Y, Kota O, Koh E, Yoshino I. Impact of combined pulmonary fibrosis and emphysema on surgical complications and long-term survival in patients undergoing surgery for non-small-cell lung cancer. Int J Chron Obstruct Pulmon Dis. 2016;11:1261–8. doi: 10.2147/COPD.S94119. doi: 10.2147/copd.S94119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Yoo H, Pyo H, et al. Impact Of Underlying Pulmonary Diseases On Treatment Outcomes In Early-Stage Non-Small Cell Lung Cancer Treated With Definitive Radiotherapy. Int J Chron Obstruct Pulmon Dis. 2019;14:2273–2281. doi: 10.2147/COPD.S210759. doi: 10.2147/copd.S210759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak N, Park CM, Lee J, et al. Lung cancer risk among patients with combined pulmonary fibrosis and emphysema. Respir Med. 2014;108(3):524–30. doi: 10.1016/j.rmed.2013.11.013. doi: 10.1016/j.rmed.2013.11.013. [DOI] [PubMed] [Google Scholar]
- Mimae T, Suzuki K, Tsuboi M, et al. Severity of lung fibrosis affects early surgical outcomes of lung cancer among patients with combined pulmonary fibrosis and emphysema. Medicine (Baltimore) 2016;95(29):e4314. doi: 10.1097/MD.0000000000004314. doi: 10.1097/md.0000000000004314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minegishi Y, Kokuho N, Miura Y, et al. Clinical features, anti-cancer treatments and outcomes of lung cancer patients with combined pulmonary fibrosis and emphysema. Lung Cancer. 2014;85(2):258–63. doi: 10.1016/j.lungcan.2014.05.016. doi: 10.1016/j.lungcan.2014.05.016. [DOI] [PubMed] [Google Scholar]
- Nasim F, Moua T. Lung cancer in combined pulmonary fibrosis and emphysema: a large retrospective cohort analysis. ERJ Open Res. 2020;6(4) doi: 10.1183/23120541.00521-2020. doi: 10.1183/23120541.00521-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka H, Sugino K, Hata Y, et al. Clinical features and outcomes of patients with lung cancer as well as combined pulmonary fibrosis and emphysema. Mol Clin Oncol. 2016;5(3):273–278. doi: 10.3892/mco.2016.954. doi: 10.3892/mco.2016.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno F, Kitaguchi Y, Shiina T, et al. The Preoperative Composite Physiologic Index May Predict Mortality in Lung Cancer Patients with Combined Pulmonary Fibrosis and Emphysema. Respiration. 2017;94(2):198–206. doi: 10.1159/000477587. doi: 10.1159/000477587. [DOI] [PubMed] [Google Scholar]
- Zhang M, Yoshizawa A, Kawakami S, et al. The histological characteristics and clinical outcomes of lung cancer in patients with combined pulmonary fibrosis and emphysema. Cancer Med. 2016;5(10):2721–2730. doi: 10.1002/cam4.858. doi: 10.1002/cam4.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai S, Marumo S, Yamanashi K, et al. Prognostic significance of combined pulmonary fibrosis and emphysema in patients with resected non-small-cell lung cancer: a retrospective cohort study. Eur J Cardiothorac Surg. 2014;46(6):e113–9. doi: 10.1093/ejcts/ezu384. doi: 10.1093/ejcts/ezu384. [DOI] [PubMed] [Google Scholar]
- Moon SW, Park MS, Kim YS, et al. Combined pulmonary fibrosis and emphysema and idiopathic pulmonary fibrosis in non-small cell lung cancer: impact on survival and acute exacerbation. BMC Pulm Med. 2019;19(1):177. doi: 10.1186/s12890-019-0951-2. doi: 10.1186/s12890-019-0951-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JY, Lee YS, Min KH, et al. Impact and prognosis of lung cancer in patients with combined pulmonary fibrosis and emphysema. Sarcoidosis Vasc Diffuse Lung Dis. 2020;37(4):e2020020. doi: 10.36141/svdld.v37i4.7316. doi: 10.36141/svdld.v37i4.7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka T, Furuya K, Yamazaki K, Miura N, Tsutsui K, Takeo S. The prognostic impact of combined pulmonary fibrosis and emphysema in patients with clinical stage IA non-small cell lung cancer. Surg Today. 2018;48(2):229–235. doi: 10.1007/s00595-017-1577-8. doi: 10.1007/s00595-017-1577-8. [DOI] [PubMed] [Google Scholar]
- da Costa Santos CM, de Mattos Pimenta CA, Nobre MR. The PICO strategy for the research question construction and evidence search. Rev Lat Am Enfermagem. 2007;15(3):508–11. doi: 10.1590/s0104-11692007000300023. doi: 10.1590/s0104-11692007000300023. [DOI] [PubMed] [Google Scholar]
- Koo HJ, Do KH, Lee JB, Alblushi S, Lee SM. Lung Cancer in Combined Pulmonary Fibrosis and Emphysema: A Systematic Review and Meta-Analysis. PLoS One. 2016;11(9):e0161437. doi: 10.1371/journal.pone.0161437. doi: 10.1371/journal.pone.0161437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitaguchi Y, Fujimoto K, Hotta J, Horie S, Hirayama J, Hanaoka M. Clinical Characteristics and Predictors of Mortality in Patients with Combined Pulmonary Fibrosis and Emphysema Syndrome and Lung Cancer. J Pulm Respir Med. 2015;5(3):263. doi: 10.4172/2161-105X.1000263. [Google Scholar]
- Ohnishi H, Yokoyama A, Kondo K, et al. Comparative study of KL-6, surfactant protein-A, surfactant protein-D, and monocyte chemoattractant protein-1 as serum markers for interstitial lung diseases. Am J Respir Crit Care Med. 2002;165(3):378–81. doi: 10.1164/ajrccm.165.3.2107134. doi: 10.1164/ajrccm.165.3.2107134. [DOI] [PubMed] [Google Scholar]
- Xu L, Bian W, Gu XH, Shen C. Genetic polymorphism in matrix metalloproteinase-9 and transforming growth factor-β1 and susceptibility to combined pulmonary fibrosis and emphysema in a Chinese population. Kaohsiung J Med Sci. 2017;33(3):124–129. doi: 10.1016/j.kjms.2016.12.004. doi: 10.1016/j.kjms.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou W, Hu S, Li C, et al. Cigarette Smoke Induced Lung Barrier Dysfunction, EMT, and Tissue Remodeling: A Possible Link between COPD and Lung Cancer. Biomed Res Int. 2019;2019:2025636. doi: 10.1155/2019/2025636. doi: 10.1155/2019/2025636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A, Horie M, Micke P, Nagase T. The Role of TGF-β Signaling in Lung Cancer Associated with Idiopathic Pulmonary Fibrosis. Int J Mol Sci. 2018;19(11) doi: 10.3390/ijms19113611. doi: 10.3390/ijms19113611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarelli AV, Masciale V, Aramini B, et al. Molecular Mechanisms and Cellular Contribution from Lung Fibrosis to Lung Cancer Development. Int J Mol Sci. 2021;22(22) doi: 10.3390/ijms222212179. doi: 10.3390/ijms222212179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenburg G, Hay ED. Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J Cell Biol. 1982;95(1):333–9. doi: 10.1083/jcb.95.1.333. doi: 10.1083/jcb.95.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood M Q, Sohal S S, Shukla SD, et al. Epithelial mesenchymal transition in smokers: large versus small airways and relation to airflow obstruction. Int J Chron Obstruct Pulmon Dis. 2015;10:1515–24. doi: 10.2147/COPD.S81032. doi: 10.2147/copd.S81032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliò A, Lever V, Rossi A, et al. Increased frequency of bronchiolar histotypes in lung carcinomas associated with idiopathic pulmonary fibrosis. Histopathology. 2017;71(5):725–735. doi: 10.1111/his.13269. doi: 10.1111/his.13269. [DOI] [PubMed] [Google Scholar]
- Gao L, Xie S, Liu H, et al. Lung cancer in patients with combined pulmonary fibrosis and emphysema revisited with the 2015 World Health Organization classification of lung tumors. Clin Respir J. 2018;12(2):652–658. doi: 10.1111/crj.12575. doi: 10.1111/crj.12575. [DOI] [PubMed] [Google Scholar]
- Bauml JM, Mick R, Ciunci C, et al. Pembrolizumab After Completion of Locally Ablative Therapy for Oligometastatic Non-Small Cell Lung Cancer: A Phase 2 Trial. JAMA Oncol. 2019;5(9):1283–1290. doi: 10.1001/jamaoncol.2019.1449. doi: 10.1001/jamaoncol.2019.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theelen W, Peulen HMU, Lalezari F, et al. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients With Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019;5(9):1276–1282. doi: 10.1001/jamaoncol.2019.1478. doi: 10.1001/jamaoncol.2019.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid U, Liesenfeld KH, Fleury A, Dallinger C, Freiwald M. Population pharmacokinetics of nintedanib, an inhibitor of tyrosine kinases, in patients with non-small cell lung cancer or idiopathic pulmonary fibrosis. Cancer Chemother Pharmacol. 2018;81(1):89–101. doi: 10.1007/s00280-017-3452-0. doi: 10.1007/s00280-017-3452-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka T, Harimoto N, Yokobori T, et al. Nintedanib inhibits intrahepatic cholangiocarcinoma aggressiveness via suppression of cytokines extracted from activated cancer-associated fibroblasts. Br J Cancer. 2020;122(7):986–994. doi: 10.1038/s41416-020-0744-7. doi: 10.1038/s41416-020-0744-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato R, Haratani K, Hayashi H, et al. Nintedanib promotes antitumour immunity and shows antitumour activity in combination with PD-1 blockade in mice: potential role of cancer-associated fibroblasts. Br J Cancer. 2021;124(5):914–924. doi: 10.1038/s41416-020-01201-z. doi: 10.1038/s41416-020-01201-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui K, Tanai C, Tanaka Y, Noda H, Ishihara T. The prevalence of pulmonary fibrosis combined with emphysema in patients with lung cancer. Respirology. 2011;16(2):326–31. doi: 10.1111/j.1440-1843.2010.01907.x. doi: 10.1111/j.1440-1843.2010.01907.x. [DOI] [PubMed] [Google Scholar]
- Iwata T, Yoshida S, Nagato K, et al. Experience with perioperative pirfenidone for lung cancer surgery in patients with idiopathic pulmonary fibrosis. Surg Today. 2015;45(10):1263–70. doi: 10.1007/s00595-014-1071-5. doi: 10.1007/s00595-014-1071-5. [DOI] [PubMed] [Google Scholar]


