Abstract
Background and Aims
In many systems, postfire vegetation recovery is characterized by temporal changes in plant species composition and richness. We attribute this to changes in resource availability with time since fire, with the magnitude of species turnover determined by the degree of resource limitation. Here, we test the hypothesis that postfire species turnover in South African fynbos heathland is powered by fire-modulated changes in nutrient availability, with the magnitude of turnover in nutrient-constrained fynbos being greater than in fertile renosterveld shrubland. We also test the hypothesis that floristic overlaps between fynbos and renosterveld are attributable to nutritional augmentation of fynbos soils immediately after fire.
Methods
We use vegetation survey data from two sites on the Cape Peninsula to compare changes in species richness and composition with time since fire.
Key Results
Fynbos communities display a clear decline in species richness with time since fire, whereas no such decline is apparent in renosterveld. In fynbos, declining species richness is associated with declines in the richness of plant families having high foliar concentrations of nitrogen, phosphorus and potassium and possessing attributes that are nutritionally costly. In contrast, families that dominate late-succession fynbos possess adaptations for the acquisition and retention of sparse nutrients. At the family level, recently burnt fynbos is compositionally more similar to renosterveld than is mature fynbos.
Conclusions
Our data suggest that nutritionally driven species turnover contributes significantly to fynbos community richness. We propose that the extremely low baseline fertility of fynbos soils serves to lengthen the nutritional resource axis along which species can differentiate and coexist, thereby providing the opportunity for low-nutrient extremophiles to coexist spatially with species adapted to more fertile soil. This mechanism has the potential to operate in any resource-constrained system in which episodic disturbance affects resource availability.
Keywords: Alpha diversity, community richness, fire, temporal turnover, fynbos, resource availability, soil nutrients, species richness, postfire succession, vascular plants
INTRODUCTION
Fire influences landscape-scale species richness in many ecosystems through its effect on vegetation structure and composition. In the moist tropics, for example, the presence of fire can switch the vegetation from a fire-averse forest state to a fire-prone grassland or savanna state, with the exclusion of fire having the reverse effect (Murphy and Bowman, 2012; Pausas and Keeley, 2014a; Pausas and Bond, 2020). Given that the spread of fire is influenced by multiple factors that vary across the landscape, fires tend to be patchy, and many tropical landscapes constitute grassland/savanna–forest mosaics (Murphy and Bowman, 2012; Das et al., 2015; Staver et al., 2017; Beckett and Bond, 2019). Importantly, given that grassland/savanna and forest communities comprise different species (Bond and Parr, 2010; Aleman et al., 2020), this has implications for landscape-scale and regional species richness (Kelly and Brotons, 2017; Pausas and Ribeiro, 2017; Erdős et al., 2018). In a similar manner, fire promotes the emergence of forest–shrubland (fynbos) mosaics in the Cape Floristic Region of South Africa (Coetsee et al., 2015; Cramer et al., 2019a; Slingsby et al., 2020), whose cumulative species richness is enhanced by high floristic turnover between these vegetation types (Power et al., 2017).
Besides facilitating landscape-scale coexistence of fire-averse and fire-prone vegetation types, fire also promotes diversity within fire-prone vegetation types. Given that fire-adapted/exapted (see Bradshaw et al., 2011) species vary in their responses to fire and the time frames over which these responses manifest, fire-prone vegetation can display a pronounced postfire succession, usually characterized by a net decline in apparent species richness with time since fire and by temporal turnover in plant species composition and abundance (e.g. Kruger and Bigalke, 1984; Hoffman et al., 1987; Guo, 2001; Slingsby et al., 2017; He et al., 2019). This temporal dispersion of the prevalence of species following fire has the effect of alleviating interspecific competition and facilitating plot-scale coexistence, with positive consequences for alpha diversity (Cowling, 1987; Thuiller et al., 2007). Moreover, because most fire-prone environments show spatial variation in fire history and/or regime (i.e. pyrodiversity; Martin and Sapsis, 1992; Steel et al., 2021) and because plant species differ with respect to the fire regime characteristics that favour their performance and persistence (e.g. some species are favoured by high fire frequency, others not; Magadzire et al., 2019), fire also enhances beta diversity through the provision of diverse, spatially dispersed fire niches (Martin and Sapsis, 1992; He et al., 2019). These patterns are consistent with the generally positive effect of environmental heterogeneity on species richness (Ricklefs, 1977; Kerr and Packer, 1997; Stein et al., 2014; Udy et al., 2021).
Although postfire change in plant community composition and richness is well documented, its proximate drivers remain poorly explored. Consistent with a view that postfire species turnover reflects differential adaptation/exaptation of species to changing conditions along the postfire succession sequence (He et al., 2019), we propose that the magnitude of species turnover following fire is determined by the extent to which fire and subsequent regrowth transform plant resource availability. Fire potentially enhances the availabilities of light, soil moisture (Clemente et al., 2005) and soil nutrients (Kutiel and Naveh, 1987; Caon et al., 2014), with these resources commonly declining in availability with time since fire. Which resources are most influential in modulating the floristic impact of fire, however, i likely to vary from one system to another. In light-limited tall conifer forests, for example, the role of fire in opening canopy gaps and thus enhancing light availability might be of principal importance in driving species turnover (e.g. Spies and Franklin, 1989), whereas in open sclerophyll shrublands on low-nutrient soils, nutrient augmentation associated with ash deposition might be more influential (e.g. Lambers et al., 2022).
The Cape Floristic Region (CFR) of South Africa has some of the poorest soils globally, with mean soil nitrogen and phosphorus concentrations lower than in any other mediterranean climate region (Stock and Verboom, 2012). Unsurprisingly, vegetation distribution and characteristics in the CFR are strongly influenced by bedrock and soil properties (Specht and Moll, 1983; Campbell, 1986; Cowling et al., 1992; Bergh et al., 2014; but for a contrasting view, see Esler et al., 2015). For example, where the nutritionally impoverished sands derived from quartzitic and calcrete rocks predominantly support sclerophyllous fynbos shrubland, the heavier and more fertile soils derived from shales and granites support grassier, semi-deciduous renosterveld and karroid shrubland. Importantly, because many functional traits, including nutritional traits, of plants show phylogenetic signal, with family- and order-level lineages differing in their functional attributes (e.g. Stock and Verboom, 2012; Cornwell et al., 2014; Verboom et al., 2017), these vegetation types are differentiated floristically at the levels of genus, family and order (Bergh et al., 2014). Where fynbos is dominated by lineages having low-nutrient adaptations/exaptations, such as woodiness, sclerophylly, low tissue nutrient concentrations, low growth rate and specialized root systems (e.g. Ericaceae, Proteaceae and Restionaceae; reviewed by Cramer et al., 2014), succulent karoo and renosterveld lineages are characterized by adaptations/exaptations to seasonal aridity, including annualness, succulence, geophytism, leaf deciduousness, high tissue nutrient concentrations and high growth rate (e.g. Aizoaceae, Asteraceae and Poaceae; Verboom et al., 2004, 2017; Cramer et al., 2014). Despite a pronounced difference in the fertility of fynbos vs. renosterveld soils (Specht and Moll, 1983; Campbell, 1986; Cramer et al., 2019b), a surprisingly large number of taxa straddle the fynbos and renosterveld floras (Bergh et al., 2014; Verboom et al., 2014), resulting in the latter being termed a ‘transitional’ vegetation type (i.e. between fynbos and succulent karoo; Cowling, 1983). We propose that the floristic overlap between fynbos and renosterveld is explained by fluctuations in the availability of nutrients in fynbos (Brown and Mitchell, 1986; Stock and Lewis, 1986), with fire-mediated ash deposition producing a window of opportunity for high-nutrient-adapted taxa that are otherwise more typical of fertile renosterveld and succulent karoo systems.
Here, we test the hypothesis that fynbos communities display a change in species composition and a net decline in species richness with time since fire and that these effects are a consequence of the high-nutrient pulse that manifests immediately in the wake of fire. We discuss the consequences of this phenomenon for community richness (i.e. alpha diversity) in fynbos. We also test the hypothesis that the floristic affinity of fynbos to renosterveld is attributable to the postfire nutrient pulse observed in fynbos, this providing a transient niche for the entry of high-nutrient-adapted renosterveld lineages. To test these ideas, we compare postfire changes in species richness and composition between mountain fynbos shrubland growing on highly infertile, quartzite-derived sands and renosterveld shrubland growing on more fertile, shale-derived clays. We predict that the postfire decline in species richness is more pronounced in fynbos than in renosterveld, reflecting the disappearance of species belonging to high-nutrient-adapted families. We also predict that the floristic similarity (at the family level) of fynbos to renosterveld declines as a function of increasing vegetation age.
MATERIALS AND METHODS
Source data
Plant community composition data were obtained from two relevé-based vegetation surveys, one based in the former Cape of Good Hope Nature Reserve (Taylor 1969; hereafter referred to as ‘Cape Point’) and the second on Signal Hill (Joubert and Moll, 1992; hereafter referred to as ‘Signal Hill’). Both study areas are located on the Cape Peninsula, where they are situated <70 km apart and form part of the Table Mountain National Park. The vegetation of Cape Point comprises largely mesic fynbos (Peninsula Sandstone Fynbos and Hangklip Sand Fynbos; Rebelo et al., 2006) growing on acid sands derived from Table Mountain Group quartzites, whereas the vegetation of Signal Hill comprises principally renosterveld (Peninsula Shale Renosterveld; Rebelo et al., 2006) growing on clay-rich soils derived from Malmesbury Group shales. The coastal margin at Cape Point, however, supports two non-fynbos vegetation types, namely Cape Flats Dune Strandveld (Rebelo et al., 2006) and Cape Seashore Vegetation (Mucina et al., 2006). Although the fertility of soils at Cape Point and Signal Hill has not been quantified, the greater fertility of shale-derived, renosterveld soils relative to quartzite-derived, fynbos soils is well documented (Specht and Moll, 1983; Campbell, 1986; Cramer et al., 2014, 2019b). Although we would ideally have included additional fynbos and renosterveld sites in this study, this was not possible owing to the scarcity of surveys giving estimates of vegetation age, and of renosterveld surveys generally.
The studies by Taylor (1969) and Joubert and Moll (1992) used a 10 m × 5 m relevé or plot as a survey unit, with the vegetation in surveyed plots ranging in age from 1 to 40 years in the Cape Point data set and from 1 to 20 years in the Signal Hill data set. In the case of the Cape Point plots, vegetation age had been estimated using counts of annual growth nodes of proteaceous shrubs (Hall, 1959; Taylor 1969), whereas for the Signal Hill plots they had been inferred using City of Cape Town fire records (Joubert 1991: appendix 7). Neither study detailed the characteristics of the most recent fires. To facilitate comparison between the two data sets and because we were interested primarily in successional change over a typical fynbos inter-fire interval (10–20 years; Kraaij et al., 2014), we excluded 12 plots from the Cape Point data set whose postfire vegetation age exceeded 20 years. We also excluded 17 plots located <500 m from the coast, because they do not contain typical fynbos communities, presumably on account of their soils being enriched by marine deposits (Nyaga et al., 2015). Consequently, our Cape Point data set comprised 59 plots containing a total of 336 species, with the number of species per plot ranging from 6 to 79 and having a mean of 40.9 (Supplementary Data Fig. S1A). Although the Cape Point plots were resurveyed in 1996 (Privett et al., 2001), we elected to use the original survey data because only 81 of the original 100 plots were relocated in the 1996 survey and because the original survey captured a broader spread of vegetation ages, including several recently burnt plots. For Signal Hill, we used only plots associated with Subcommunity 1.1, as designated by Joubert and Moll (1992), because the area occupied by Subcommunity 1.2 had been planted with pines until 1976, after which it had been cleared and protected from fire. Consequently, our Signal Hill data set comprised 35 plots containing a total of 174 species, with the number of species per plot ranging from 29 to 73, with a mean of 46.4 (Supplementary Data Fig. S1B). Unfortunately, the vegetation in most plots was rather old (>12 years), with only two plots of age 1 year.
Both source studies recorded species data as ordinal, Braun-Blanquet cover–abundance classes, which are not conducive to sampling standardization using taxon sampling (rarefaction) curves. Therefore, we determined species richness as the number of species recorded in each plot, regardless of their abundance. We acknowledge that, in so doing, what we term species richness is more correctly species density (i.e. number of species per unit area) sensuGotelli and Colwell (2001). We also acknowledge that these are once-off surveys and that we rely on patterns of variation across spatially dispersed plots of unequal age as a substitute for time.
Postfire changes in species richness and composition
The relationship of species richness to postfire vegetation age in both fynbos (Cape Point) and renosterveld (Signal Hill) was assessed using ordinary least squares regression, as implemented in R v.4.2.1 (R Core Team, 2021), across the set of plots in each study area. To determine which plant families are responsible for any observed changes in overall species richness with time since fire, we assessed the species richness–vegetation age relationship separately for each plant family occurring in ≥20 plots in either vegetation type, with this prevalence threshold being applied to ensure sufficient statistical power to enable detection of a relationship. This was done separately for the Cape Point and Signal Hill plots.
To probe further which lineages dominate early vs. late postfire succession fynbos communities at Cape Point, we determined, for each species, the mean age of the vegetation in all sites in which it was recorded. Using functions in ape v.5.6-2 (Paradis and Schliep, 2019) and phytools v.1.0-3 (Revell, 2012), maximum likelihood ancestral character state estimation was then used to map this variable on a phylogenetic tree generated with V.PhyloMaker2 (Jin and Qian, 2019, 2022). We also used the phylosig function in phytools to assess whether this variable displays significant phylogenetic signal relative to λ = 0 (Pagel, 1999).
For each data set, we then used ordinary least squares regression and phylogenetically generalized least squares regression, as implemented in phylolm v.2.6.2 (Ho and Ané, 2014), to assess whether the sign and strength of the species richness–vegetation age relationship shown by different plant families is related to their foliar nutrient concentrations. In these analyses, the response variable was the species richness–vegetation age correlation (r) and the predictor the mean foliar concentration of nitrogen [N], phosphorus [P] or potassium [K]. Given that the latter variables were based on foliar nutrient concentration data reported by Verboom et al. (2017), these analyses were necessarily confined to the ten high-prevalence families for which Verboom et al. (2017) provided such data, i.e. Asteraceae, Cyperaceae, Ericaceae, Fabaceae, Iridaceae, Rosaceae, Proteaceae, Restionaceae, Rutaceae and Scrophulariaceae. Although the data provided by Verboom et al. (2017) are not based on samples from Cape Point or Signal Hill, or even from species occurring at these localities, the existence of strong phylogenetic signal in foliar nutrient traits, especially foliar [P] and [K] (Verboom et al., 2017), validates the use of trait means derived from these data as broad general proxies for family-specific foliar nutrient concentrations. Phylogenetic regression was done using a pruned version of the phylogeny presented by Verboom et al. (2017).
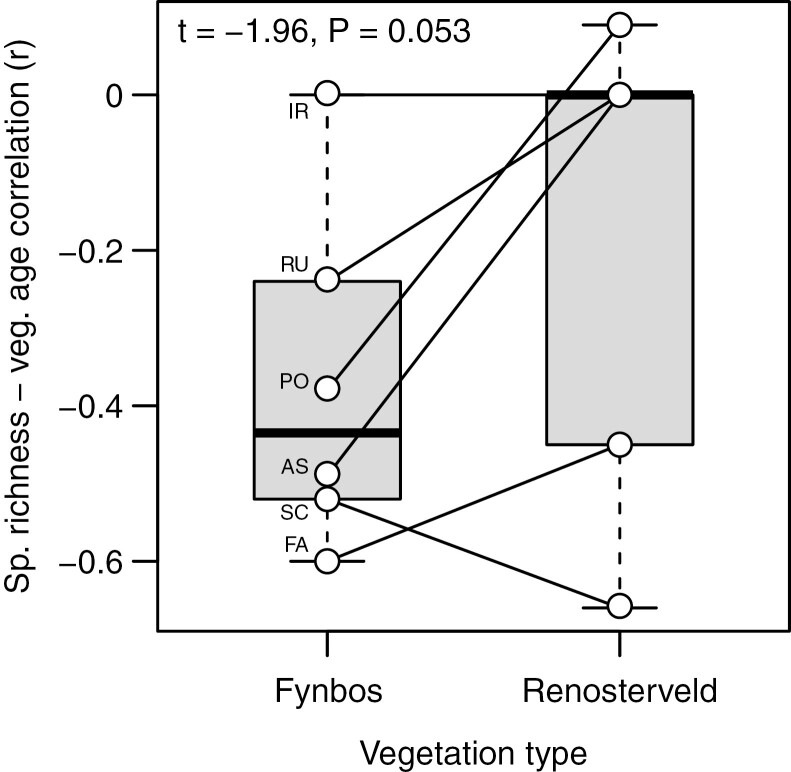
Finally, to test the prediction that postfire changes in species composition are more pronounced in nutrient-poor fynbos than in more fertile renosterveld, we used a paired-sample t test to compare the strength of the species richness–vegetation age correlation across the six families common to the Cape Point and Signal Hill data sets (i.e. Asteraceae, Fabaceae, Iridaceae, Poaceae, Rubiaceae and Scrophulariaceae).
Postfire vegetation age and the fynbos–renosterveld floristic relationship
To test the hypothesis that the floristic affinities of fynbos to renosterveld reflect the presence of high-nutrient-adapted elements (families) in the early postfire period, we used non-metric multidimensional scaling (NMDS) with Bray–Curtis (Sørenson) dissimilarity, as implemented in vegan v.2.6-2 (Oksanen et al., 2022), to quantify patterns of family-level compositional similarity across the full set of sites at both Cape Point and Signal Hill. NMDS was preferred over other ordination methods (e.g. principal components analysis) because it is comparatively assumption free and has been shown generally to outperform other methods in recovering community similarity patterns (Fasham et al., 1977). In our data set, a high frequency of zeros introduces a zero-bounding issue, resulting in violation of the principal components analysis assumption of linearity. We applied NMDS to a site × family community matrix, in which cell entries describe the number of species representing a particular family at a particular site. We predicted that recently burnt fynbos plots would occupy positions in ordination space closer to the renosterveld plots than older fynbos plots. To assess this statistically, we tested whether the fynbos (i.e. Cape Point) plots displayed a relationship between postfire vegetation age and their score on the first multidimensional scaling axis (MDS1), which describes the separation of fynbos and renosterveld plots.
RESULTS
Postfire changes in species richness and composition
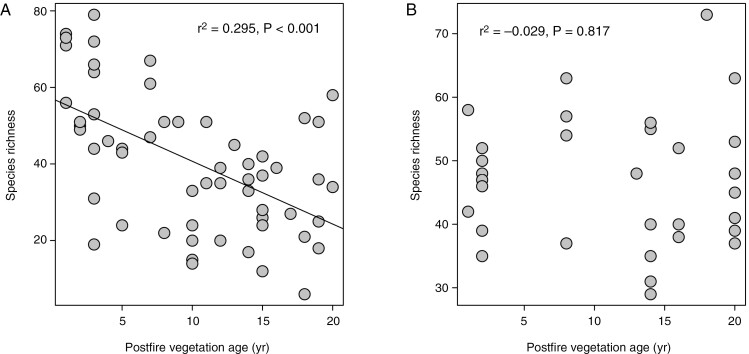
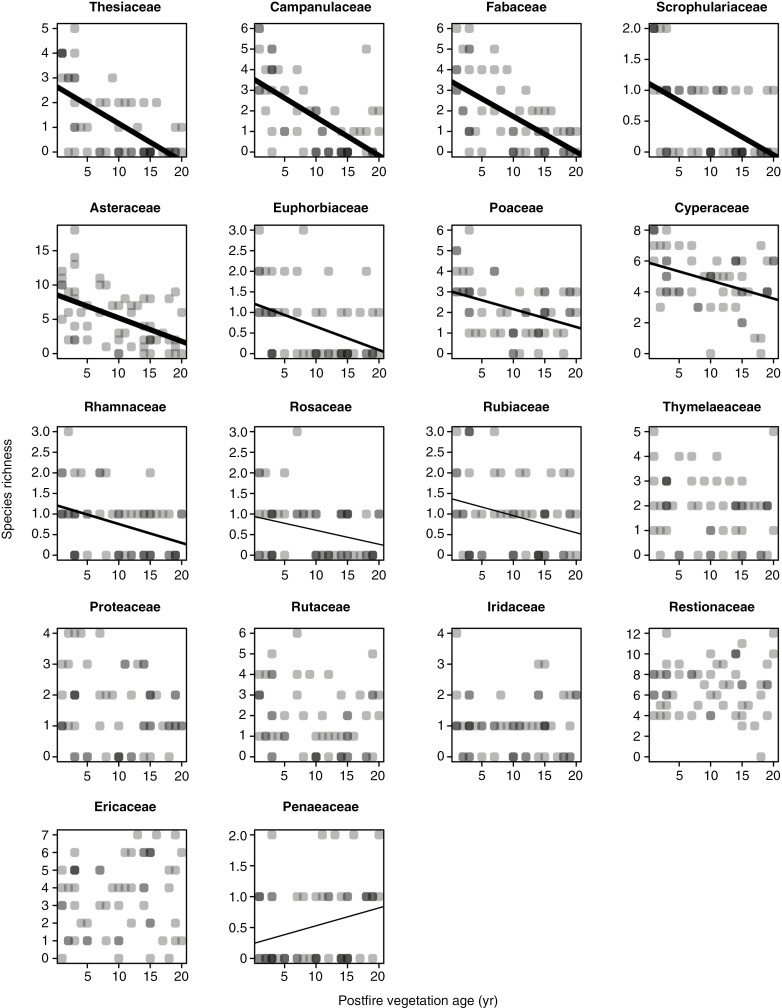
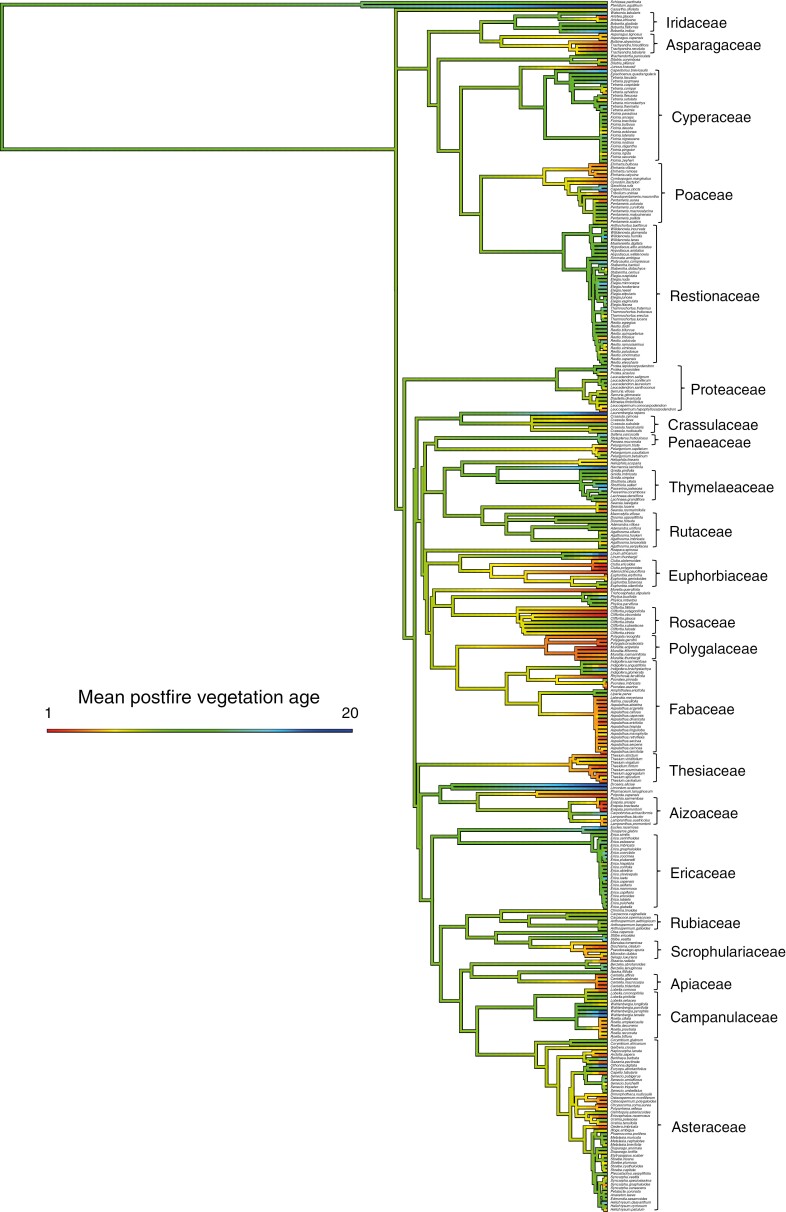
The Cape Point fynbos plots display a strong decline in overall species richness with time since fire (Fig. 1A), with this pattern reflecting declines in the species richness of 11 of 18 families meeting our prevalence threshold (Fig. 2), including several that are prominent in renosterveld vegetation, both at Signal Hill and elsewhere. Examples of the latter include Campanulaceae, Fabaceae, Scrophulariaceae, Asteraceae, Euphorbiaceae and Poaceae. In contrast, many of the more typical fynbos-specialist taxa, including Proteaceae, Diosmeae (Rutaceae), Restionaceae, Ericaceae and Penaeaceae, show no evidence of declining species richness, with Penaeaceae even showing an increase in species richness with time since fire. Consistent with these patterns, a reconstruction of the mean age of the vegetation occupied by each of the Cape Point species (Fig. 3) reveals significant phylogenetic signal (λ = 0.632, P < 0.001), with lineages showing strong postfire species richness declines (Fig. 2) also reflecting an association with younger vegetation. Although they are not represented in Fig. 2 because they fail to meet our prevalence threshold, Aizoaceae, Apiaceae and Crassulaceae also fall into this category (Fig. 3).
Fig. 1.
Relationships of species richness to postfire vegetation age across the Cape Point fynbos plots (A) and the Signal Hill renosterveld plots (B).
Fig. 2.
Relationships of species richness to postfire vegetation age across the Cape Point fynbos plots, by family. Only families present in ≥20 plots are included. Given that symbols have a transparent fill, dark points represent instances of multiple overlapping points. The significance of relationship is indicated by line thickness, with thin lines representing P < 0.05, medium lines P < 0.01 and thick lines P < 0.001.
Fig. 3.
Maximum likelihood phylogenetic reconstruction of the mean age of the vegetation occupied by the Cape Point species. Branch shading indicates vegetation age association, with red and blue, respectively, indicating an association with young (i.e. recently burnt) and old vegetation. Important family-level lineages are indicated on the right of the tree.
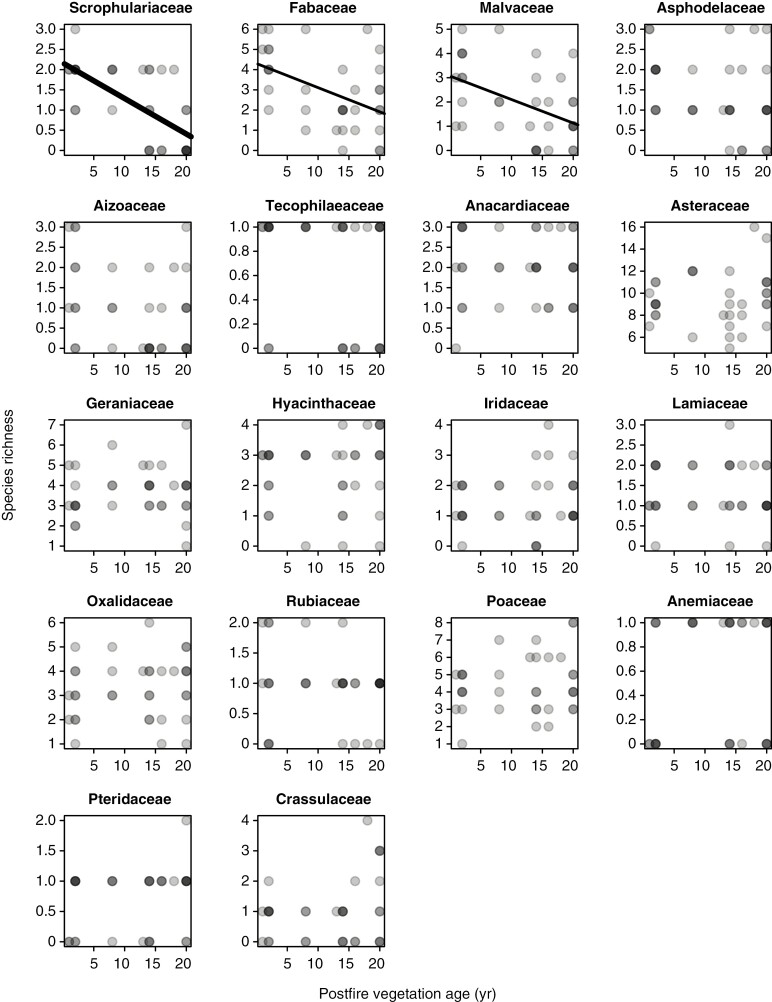
In contrast to the Cape Point plots, and despite declines being apparent in Scrophulariaceae, Fabaceae and Malvaceae (Fig. 4), the Signal Hill renosterveld plots show no decline in overall species richness with time since fire (Fig. 1B). This suggests that the impacts of fire on species richness and community composition are less pronounced in renosterveld than in fynbos, an inference which is further supported by the species richness–vegetation age correlation being generally weaker in renosterveld than in fynbos, when compared across the six families common to both data sets (Fig. 5).
Fig. 4.
Relationships of species richness to postfire vegetation age across the Signal Hill renosterveld plots, by family. Only families present in ≥20 plots are included. Given that symbols have a transparent fill, dark points represent instances of multiple overlapping points. The significance of relationship is indicated by line thickness, with thin lines representing P < 0.05, medium lines P < 0.01 and thick lines P < 0.001.
Fig. 5.
Box-and-whisker comparison, by family, of the species richness–vegetation age correlation, for the Cape Point fynbos (Fig. 2) and Signal Hill renosterveld (Fig. 4) plots. The six families included, by virtue of their presence in ≥20 plots at both Cape Point and Signal Hill, are as follows: AS, Asteraceae; FA, Fabaceae; IR, Iridaceae; PO, Poaceae; RU, Rubiaceae; SC, Scrophulariaceae. The whiskers depict the minimum–maximum ranges, the boxes the 0.25–0.75 interquartile ranges, and the dark bar the median, across the six families.
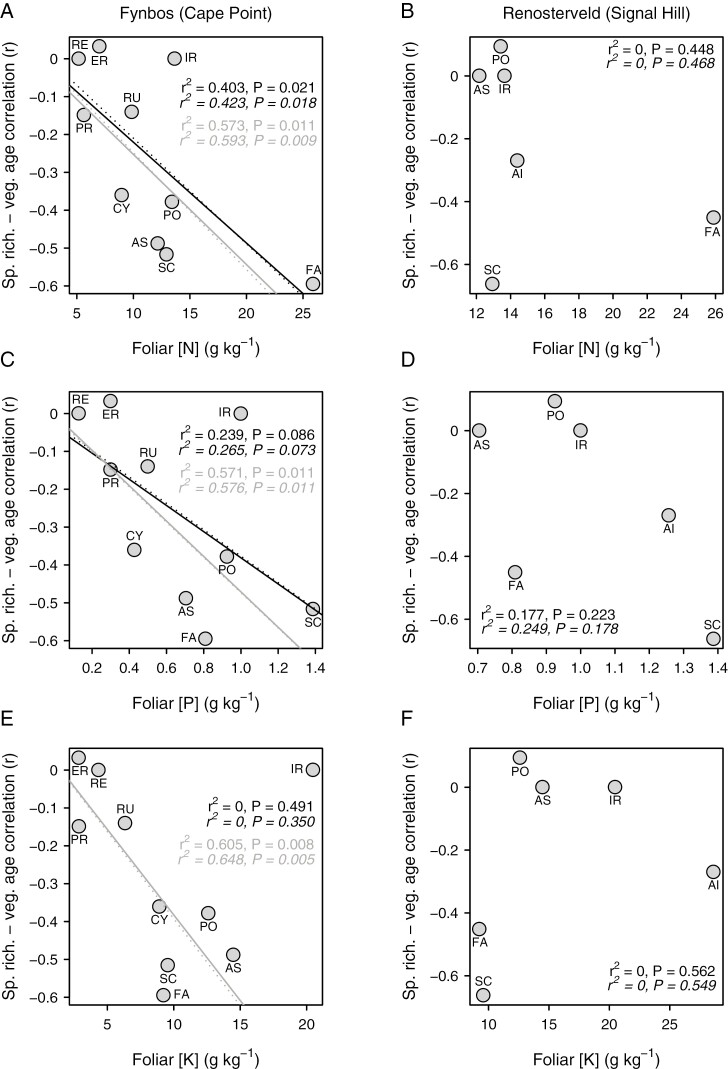
Besides differing between two vegetation types, the species richness–vegetation age correlation is negatively related to foliar [N], [P] and [K] across families in the Cape Point (fynbos) plots, at least with the exclusion of geophytic Iridaceae, which is a consistent outlier (Fig. 6A, C, E). Although this relationship is robust to the inclusion of Iridaceae in the case of [N] (Fig. 6A), in the case of [P] and [K] it results in a loss of significance, although only marginally so in the case of [P] (Fig. 6C). In the case of [N], the relationship is also robust to the exclusion of Fabaceae (ordinary least squares regression: r2 = 0.668, P = 0.008; phylogenetically generalized least squares regression: r2 = 0.686, P = 0.007). In contrast to the situation at Cape Point, the species richness–vegetation age correlation is unrelated to foliar nutrient concentration across families in the Signal Hill (renosterveld) plots (Fig. 6B, D, F).
Fig. 6.
Relationship of the species richness–vegetation age correlation to mean foliar nitrogen (A, B), phosphorus (C, D) and potassium (E, F) concentrations, across families present in ≥20 plots at Cape Point (left panels; n = 10) and Signal Hill (right panels; n = 6). Family codes are as follows: AS, Asteraceae; CY, Cyperaceae; ER, Ericaceae; FA, Fabaceae; IR, Iridaceae; PO, Poaceae; PR, Proteaceae; RE, Restionaceae; RU, Rutaceae; SC, Scrophulariaceae. Regression lines are fitted using both ordinary least squares (black lines) and phylogenetically generalized least squares (grey lines), with the outlying Iridaceae (IR) both included (solid lines) and excluded (dotted lines).
Postfire vegetation age and the fynbos–renosterveld floristic relationship
Application of NMDS to a matrix recording the numbers of species from each family observed in each plot across the combined data sets yields a two-dimensional biplot with a stress value of 0.093, and in which the fynbos and renosterveld plot clusters separate out along MDS1 (Fig. 7A). Consistent with our second hypothesis, younger plots in the fynbos cluster tend to be situated closer to the renosterveld cluster (Fig. 7A; higher MDS1 scores), with a significant negative relationship between MDS1 score and vegetation age, across the fynbos plots, identifying this pattern as significant (Fig. 7B).
Fig. 7.
(A) Non-metric multidimensional scaling biplot extracted from a site × family community matrix comprising both the Cape Point fynbos (triangles) and Signal Hill renosterveld (circles) plot data. Cell entries in the input matrix describe the number of species representing a particular family at a particular site, and between-plot distances are based on Bray–Curtis dissimilarity. Symbol size describes postfire vegetation age, with larger symbols representing recently burnt plots. The associated stress value is 0.093. (B) Relationship of the MDS1 score (from panel A), describing the family-level compositional similarity of the Cape Point fynbos plots to the Signal Hill renosterveld plots (high values indicate greater similarity), as a function of postfire fynbos vegetation age.
DISCUSSION
Our data demonstrate pronounced changes in fynbos plant community composition with time since fire, with some families showing marked declines in species richness and others not, and with overall species richness experiencing a net decline with time since fire. These patterns are more pronounced in fynbos than in renosterveld. Although we are conscious of our data being drawn from only a pair of sites (Cape Point and Signal Hill), the generality of these patterns is corroborated by our personal field observations. It is also supported by the literature, which reveals postfire species richness declines to be a feature of many fire-prone systems (e.g. Kruger and Bigalke, 1984; Hoffman et al., 1987; Guo 2001; He et al., 2019) and postfire changes in family-level composition to be a common feature of phosphorus-constrained, fire-prone vegetation (e.g. Lambers et al., 2022). Echoing patterns reported here, Kruger (1987: pp. 72–74) identified geophytes and postfire ephemerals as the most important contributors to elevated postfire species richness in fynbos, with these elements being drawn principally from Aizoaceae, Asteraceae, Campanulaceae, Crassulaceae, Fabaceae, Iridaceae, Scrophulariaceae and Thesiaceae.
We hypothesized that postfire changes in fynbos plant community composition and richness are powered principally by changes in soil nutrient availability with time following fire (Brown and Mitchell, 1986; Stock and Lewis, 1986), and three patterns support this idea. First, the postfire decline in overall species richness is apparent only in the fynbos vegetation at Cape Point, not in the renosterveld vegetation at Signal Hill. Although soil fertility at these sites has not been measured, the greater fertility of shale-derived, renosterveld soils relative to quartzite-derived, fynbos soils is well documented (Specht and Moll, 1983; Campbell, 1986; Cramer et al., 2014, 2019b), and we propose that the stronger postfire species richness spike observed in fynbos is attributable to the greater nutritional impact of ash deposition there. Second, the decline in overall species richness observed in fynbos is replicated across multiple plant families, with the species richness–vegetation age correlation being generally negative and stronger in fynbos than in renosterveld. Third, at Cape Point the species richness–vegetation age correlation is related to family-level variation in foliar nutrient concentrations (ionome sensuSalt et al., 2008), a trait showing strong phylogenetic signal and apparently having a strong genetic basis (Broadley et al., 2004; Kerkhoff et al., 2006; Verboom et al., 2017). Given the importance of foliar nutrient concentrations as determinants of plant growth potential in conditions of high nutrient availability (Elser et al., 2000; Wright et al., 2004), this pattern almost certainly reflects the ability of some lineages, notably Thesiaceae, Campanulaceae, Fabaceae, Scrophulariaceae, Asteraceae, Euphorbiaceae, Poaceae, Cyperaceae, Aizoaceae and Crassulaceae, to respond to the postfire nutrient pulse observed in fynbos, and their inability to cope with the low-nutrient conditions that characterize later postfire succession. Although Verboom et al. (2017) identified some of these families as nutritional generalists, in contrast to the low-nutrient specialists that dominate mature fynbos, they might, alternatively, be high-nutrient specialists. This is, perhaps, more consistent with the lack of a clear relationship between specialization and disturbance response in plants (Vázquez and Simberloff, 2002).
Although we argue for a principal role of nutrient augmentation in stimulating postfire changes in species richness and composition in fynbos, we do not exclude a role for other resources, such as light, whose availability is also influenced by fire. For example, mechanical defoliation, which increases light but not nutrient availability, has been shown to enhance the richness, density and flowering of geophytes in fynbos, albeit not to the same extent as fire (Verboom et al., 2002; Vlok 2020). Interestingly, in geophytic Ehrharta (Poaceae) postfire flowering levels are achieved only with a combination of fertilization and defoliation (Verboom et al., 2002), which suggests that the responsiveness of high-nutrient-adapted plants to nutrient augmentation is contingent on a simultaneous increase in light availability.
Beyond having higher foliar nutrient concentrations, the families that dominate early postfire succession fynbos possess other traits that reflect a high demand of soil nutrients, especially P and K. For example, the ability of Fabaceae to acquire N symbiotically offers significant benefits, but only in conditions of high P availability (Power et al., 2010, 2011). In the presence of low P, a trade-off between investment in N2 fixation and in traits enabling the acquisition of sparse P might compromise the ability of legumes to acquire P (Power et al., 2010; Cramer et al., 2014), thereby upsetting the N:P stoichiometry of these plants (Güsewell, 2004; Neugebauer et al., 2018). This might explain the general failure of Fabaceae species to persist beyond the earliest stages of postfire succession, as P becomes scarcer. Succulence, as exemplified by Aizoaceae, Crassulaceae and Euphorbiaceae, also appears to carry a high soil nutrient demand. In part, this might be attributable to the low and sporadic availability of soil moisture in environments favoured by succulents, this limiting opportunities for nutrient uptake and necessitating an association with more fertile soils (Ripley et al., 2013). In addition, the tendency of Aizoaceae and Crassulaceae to maintain high foliar concentrations of calcium, magnesium, potassium and sodium (White et al., 2016; Verboom et al., 2017), possibly as an anti-herbivore defence (Franceschi and Nakata 2005), might necessitate an association with conditions of high soil fertility. There is also evidence to show that high tissue concentrations of inorganic salts are a requirement for CO2 fixation in succulents using the crassulacean acid metabolic pathway (Bloom, 1979), although this might better explain the high postfire incidence of Crassulaceae than that of Aizoaceae (see Ripley et al., 2013). Although the strong association of hemiparasitic Thesiaceae with the postfire nutrient flush seems surprising, given the apparent ability of these plants to source both carbon and minerals from their hosts (Fer et al., 1993, 1994; Luo and Guo, 2010; Giesemann and Gebauer, 2022), the low nutrient concentrations in the tissues of potential host species might limit the efficacy of parasitism as a nutrient acquisition strategy in fynbos, especially later in the postfire succession sequence. Finally, although the nutrient dependence of Asteraceae, Campanulaceae, Cyperaceae, Poaceae and Scrophulariaceae has been little studied, a high incidence of herbaceousness and annuality in these families, paired with the production of dense inflorescences in Asteraceae, Cyperaceae, Poaceae and some Scrophulariaceae, suggests a general proclivity for fast growth and increased dependence on seed-mediated persistence (e.g. Garnier, 1992; Garnier and Vancaeyzeele, 1994; Verboom et al., 2004, 2012), with a need to bear the high nutrient costs that these incur (Bloom et al., 1985). Indeed, the nutrient cost associated with seed production is predicted generally to be high in short-lived plants whose persistence across fire cycles depends on the establishment of soil-based seed banks (e.g. many Fabaceae, Thesiaceae).
In contrast to the lineages discussed above, several of the families that dominate the later stages of postfire succession possess traits that facilitate the acquisition of sparse nutrients from soils that are critically infertile because nutrients, besides being scarce, are also bound and thus inaccessible (Lambers et al., 2011; Cramer et al., 2014; Raven et al., 2018). These traits include the formation in Proteaceae, Restionaceae and schoenoid Cyperaceae of short-lived cluster roots, which can mobilize and rapidly absorb insoluble inorganic P (Lambers et al., 2006), and the formation in Ericaceae of associations with ericoid ectomycorrhizas, which are capable of hydrolysing organic forms of P (Smith and Read, 2008; Raven et al., 2018). Significantly, although Cyperaceae display a decline in species richness with time since fire, this pattern reflects a decline in the species richness of non-schoenoid sedges, which lack root specializations. The schoenoid sedges, which commonly possess dauciform roots (Shane et al., 2006), show no such decline (Supplementary Data Fig. S2).
Beyond adaptations for enhanced nutrient acquisition, most of the lineages that dominate mature fynbos also possess traits that enhance nutrient retention. These include the production of small leaves with low specific leaf area (Ordoñez et al., 2009; Yates et al., 2010), as observed in fynbos Rhamnaceae (i.e. Phylica), Rutaceae (i.e. Diosmeae) and Penaeaceae (Onstein et al., 2014; Onstein and Linder, 2016), or leaf loss and the transfer of photosynthetic function to the high-shoot-mass-per-area culms, as observed in Restionaceae (Ehmig et al., 2019). In all these plants, the functional significance of producing low-specific leaf area/high-shoot-mass-per-area photosynthetic tissues relates to the greater longevity of these tissues (Wright et al., 2004), this facilitating nutrient retention and functioning as an adaptation to low soil fertility (Cramer et al., 2014). Underground storage structures, as observed in Iridaceae, represent an alternative mechanism for the resorption and retention of nutrients assimilated during the postfire nutrient flush and initially stored in the leaves. In Iridaceae and other fynbos geophytes, annual corm replacement is prioritized over other activities, including flowering, with the developing daughter corm being the dominant nutrient sink (Verboom et al., 2002; Vlok, 2021) and with resorption of nutrients from the senescing leaves into the corm (or bulb) being exceptionally efficient (Ruiters et al., 1993, 1994). Thus, although these species continue to produce a few leaves every year through the postfire succession sequence to replenish carbohydrate stocks, a high resorption efficiency ensures that nutrient loss is minimal and explains the ability of Iridaceae to produce unusually high-nutrient leaves in nutritionally impoverished, mature fynbos environments (Fig. 5). Significantly, sexual reproduction (flowering and fruiting) in fynbos geophytes typically takes place only in the first year or two after fire (Levyns, 1929; Linder, 1990; Le Maitre and Brown, 1992; Vlok, 2020), apparently as a plastic response to increased light and nutrient availability (Verboom et al., 2002; Vlok, 2020).
Given the greater fertility of renosterveld relative to fynbos, the observation that renosterveld-associated plant families dominate the early postfire window in fynbos and disappear as the vegetation matures provides further support for the idea that these changes are nutritionally driven. It also supports the hypothesis that the floristic similarities between fynbos and renosterveld owe their existence to the role of fire in providing a transient high-nutrient niche in fynbos. Given that renosterveld and fynbos represent floristically distinct, biome-like entities (e.g. Bergh et al., 2014), the dispersion of floristic elements representing each along a postfire succession sequence identifies fynbos as a composite biome, i.e. a biome comprising two biome-like elements that coexist spatially, but which are temporally dispersed. This perspective departs from the conventional view of biomes as purely spatial entities that are stable at least on shorter, ecological time scales, and which can be mapped (Higgins et al., 2016; Mucina, 2019; Conradi et al., 2020).
Whether or not categorical assignment of species to a biome is useful in this context, the recognition that fire enables the spatial coexistence of floristically distinct assemblages contributes to our understanding of fynbos species richness. Just as spatial heterogeneity promotes landscape-scale coexistence and species richness through the provision of diverse niches (Stein et al., 2014; Udy et al., 2021), so too the effect of fire in generating diverse nutritional niches must enhance species richness, although in this case the temporal rather than spatial dispersion of these niches allows for species richness to be enhanced even at the community scale (alpha diversity).
In this context, it is interesting that the alpha diversity of fynbos is commonly considered to be unexceptional (e.g. Cowling, 1983; Cowling et al., 1992, 1996; Simmons and Cowling, 1996), although Bond (1983), comparing community richness across a range of mediterranean-climate and temperate ecosystems, considered fynbos communities to ‘rank as rich in the world’s vegetation’. Bond also recognized the sensitivity of alpha diversity estimation to the time of sampling, including the inclusion or exclusion of ephemeral life forms, such as annuals and geophytes. Given that estimates of fynbos alpha diversity are typically based on vegetation surveys conducted at a particular point along the postfire succession sequence, and often several years following fire when community richness is in decline, they are likely to underestimate the true alpha diversity. Expanding the analogy of Pausas and Keeley (2014b), who compare the multi-year fire cycle in a shrubland ecosystem like fynbos with the annual moisture cycle in a seasonally arid ecosystem, sampling mature fynbos to determine species richness is akin to sampling a seasonally arid ecosystem towards the end of the dry season. This highlights the challenges in detecting and attributing the impacts of global change drivers on species richness and composition in this ecosystem (Slingsby et al., 2017).
Our data suggest that the postfire recovery cycle has a greater impact on apparent species composition and richness in fynbos than in renosterveld. We attribute this to the low nutritional status of fynbos relative to renosterveld soils, which ensures that the nutritional impact of ash deposition is greater in fynbos. Viewed through the lens of classical coexistence theory (MacArthur and Levins, 1967), we suggest that the exceptionally low baseline nutrient content of fynbos soils has the effect of lengthening the nutritional resource axis along which species can differentiate and coexist, thus providing space for low-nutrient extremophiles (mid to late postfire succession) in addition to species that favour more fertile conditions (early postfire succession). This effect might also explain variation in alpha richness between the five mediterranean-climate regions of the world, with the intercepts of regional log(species richness)–log(area) curves (data from Cowling et al., 2015: fig. 2) being positively related (Supplementary Data Fig. S3; r2 = 0.793, P = 0.027) to soil [P] (data from Stock and Verboom, 2012: table 1). In the context of mediterranean-climate vegetation, of course, the association of high species richness with infertile soils has long been appreciated (e.g. Cowling et al., 1996), with different mechanisms being invoked to account for it. Some authors have invoked the emergence of diverse adaptations to nutrient scarcity, particularly phosphate scarcity, in facilitating local species coexistence and thus contributing to species richness (e.g. Lambers et al., 2011, 2018; Laliberté et al., 2014; Zemunik et al., 2015; Hayes et al., 2021), whereas others have invoked the effect of edaphic extremity in accentuating the spatial variability of soil fertility, in addition to the role of fire in generating temporal variability (Cramer and Verboom, in press).
Although our work addresses the impact of fire-modulated nutrient dynamics on fynbos species richness, we note that the mechanism we invoke is applicable to any system in which episodic disturbance affects the availability of a key resource. In tropical rainforest systems, for example, extreme light limitation imposed by a continuous, tiered canopy is interrupted episodically by treefall events, which enable the establishment of light-loving species (Denslow, 1987) and enhance species richness (Strong, 1977; Phillips et al., 1994). Thus, the phenomenon is likely to be general, although the key resources that stimulate species turnover following disturbance might vary between systems.
Supplementary data
Supplementary data are available at Annals of Botany online and consist of the following.
Figure S1: histograms describing the age distribution of the Cape Point fynbos plots (A) and Signal Hill renosterveld plots (B) included in this study. Figure S2: relationships of species richness to postfire vegetation age across the Cape Point fynbos plots, for the non-schoenoid (A) and schoenoid (B) Cyperaceae. Figure S3: relationship of mean community richness (i.e. alpha diversity) to mean soil phosphorus concentration ([P]) across the five mediterranean-type ecosystems of the world.
ACKNOWLEDGEMENTS
This paper has benefitted from discussions with a range of colleagues over several years, notably David Ackerly, Nicola Bergh, William Bond, Richard Cowling, Peter Linder, Eugene Moll, Simon Power and William Stock.
Contributor Information
G Anthony Verboom, Bolus Herbarium, University of Cape Town, Private Bag X3, Rondebosch, 7701, South Africa; Department of Biological Sciences, University of Cape Town, Private Bag X3, Rondebosch, 7701, South Africa.
Jasper A Slingsby, Department of Biological Sciences, University of Cape Town, Private Bag X3, Rondebosch, 7701, South Africa; Centre for Statistics in Ecology, Environment and Conservation, University of Cape Town, Private Bag X3, Rondebosch, 7701, South Africa; Fynbos Node, South African Environmental Observation Network (SAEON), Cape Town, South Africa.
Michael D Cramer, Department of Biological Sciences, University of Cape Town, Private Bag X3, Rondebosch, 7701, South Africa.
FUNDING
Funding to support for this research was provided by the University of Cape Town.
LITERATURE CITED
- Aleman JC, Fayolle A, Favier C, et al. 2020. Floristic evidence for alternative biome states in tropical Africa. Proceedings of the National Academy of Sciences of the United States of America 117: 28183–28190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett H, Bond WJ.. 2019. Fire refugia facilitate forest and savanna co‐existence as alternative stable states. Journal of Biogeography 46: 2800–2810. [Google Scholar]
- Bergh NG, Verboom GA, Rouget M, Cowling RM.. 2014. Vegetation types of the greater cape floristic region. In: Allsopp NA, Colville JF, Verboom GA, eds. Fynbos: ecology, evolution, and conservation of a megadiverse region. Oxford: Oxford University Press, 1–25. [Google Scholar]
- Bloom AJ. 1979. Salt requirement for crassulacean acid metabolism in the annual succulent, Mesembryanthemum crystallinum. Plant Physiology 63: 749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom AJ, Chapin FS III, Mooney HA.. 1985. Resource limitation in plants—an economic analogy. Annual Review of Ecology and Systematics 16: 363–392. [Google Scholar]
- Bond W. 1983. On alpha diversity and the richness of the Cape flora: a study in southern Cape fynbos. In: Kruger FJ, Mitchell DT, Jarvis JUM, eds. Mediterranean-type ecosystems: the role of nutrients. Berlin and Heidelberg: Springer, 337–356. [Google Scholar]
- Bond WJ, Parr CL.. 2010. Beyond the forest edge: ecology, diversity and conservation of the grassy biomes. Biological Conservation 143: 2395–2404. [Google Scholar]
- Bradshaw SD, Dixon DW, Hopper SD, Lambers H, Turner SR.. 2011. Little evidence for fire-adapted plant traits in Mediterranean climate regions. Trends in Plant Science 16: 69–76. [DOI] [PubMed] [Google Scholar]
- Broadley MR, Bowen HC, Cotterill HL, et al. 2004. Phylogenetic variation in the shoot mineral concentration of angiosperms. Journal of Experimental Botany 55: 321–336. [DOI] [PubMed] [Google Scholar]
- Brown G, Mitchell DT.. 1986. Influence of fire on the soil phosphorus status in sand plain lowland fynbos, south-western Cape. South African Journal of Botany 52: 67–72. [Google Scholar]
- Campbell BM. 1986. Montane plant communities of Fynbos Biome. Vegetatio 66: 3–16. [Google Scholar]
- Caon L, Vallejo VR, Ritsema CJ, Geissen V.. 2014. Effects of wildfire on soil nutrients in Mediterranean ecosystems. Earth-Science Reviews 139: 47–58. [Google Scholar]
- Clemente AS, Rego FC, Correia OA.. 2005. Growth, water relations and photosynthesis of seedlings and resprouts after fire. Acta Oecologica 27: 233–243. [Google Scholar]
- Coetsee C, Bond WJ, Wigley BJ.. 2015. Forest and fynbos are alternative states on the same nutrient poor geological substrate. South African Journal of Botany 101: 57–65. [Google Scholar]
- Conradi T, Slingsby JA, Midgley GF, Nottebrock H, Schweiger AH, Higgins SI.. 2020. An operational definition of the biome for global change research. The New Phytologist 227: 1294–1306. [DOI] [PubMed] [Google Scholar]
- Cornwell WK, Westoby M, Falster DS, et al. 2014. Functional distinctiveness of major plant lineages. Journal of Ecology 102: 345–356. [Google Scholar]
- Cowling RM. 1983. Diversity relations in Cape shrublands and other vegetation in the southeastern Cape, South Africa. Vegetatio 54: 103–127. [Google Scholar]
- Cowling RM. 1987. Fire and its role in coexistence and speciation in Gondwanan shrublands. South African Journal of Science 83: 106–112. [Google Scholar]
- Cowling RM, Holmes PM, Rebelo AG.. 1992. Plant diversity and endemism. In: Cowling RM, ed. The ecology of fynbos: nutrients, fire and diversity. Cape Town: Oxford University Press, 62–112. [Google Scholar]
- Cowling RM, Rundel PW, Lamont BB, Arroyo MK, Arianoutsou M.. 1996. Plant diversity in Mediterranean-climate regions. Trends in Ecology & Evolution 11: 362–366. [DOI] [PubMed] [Google Scholar]
- Cowling RM, Potts AJ, Bradshaw PL, et al. 2015. Variation in plant diversity in Mediterranean-climate ecosystems: the role of climatic and topographical stability. Journal of Biogeography 42: 552–564. [Google Scholar]
- Cramer MD, Verboom GA.. 2024. Quantitative evaluation of the drivers of species richness in a Mediterranean ecosystem (Cape, South Africa). Annals of Botany 133: 801–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer MD, West AG, Power SC, Skelton S, Stock WD.. 2014. Plant ecophysiological diversity. In: Allsopp NA, Colville JF, Verboom GA, eds. Fynbos: ecology, evolution, and conservation of a megadiverse region. Oxford: Oxford University Press, 248–272. [Google Scholar]
- Cramer MD, Power SC, Belev A, et al. 2019a. Are forest-shrubland mosaics of the Cape Floristic Region an example of alternate stable states? Ecography 42: 717–729. [Google Scholar]
- Cramer MD, Wootton LM, van Mazijk R, Verboom GA.. 2019b. New regionally modelled soil layers improve prediction of vegetation type relative to that based on global soil models. Diversity and Distributions 25: 1736–1750. [Google Scholar]
- Das A, Nagendra H, Anand M, Bunyan M.. 2015. Topographic and bioclimatic determinants of the occurrence of forest and grassland in tropical montane forest-grassland mosaics of the Western Ghats, India. PLoS One 10: e0130566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denslow JS. 1987. Tropical rainforest gaps and tree species diversity. Annual Review of Ecology and Systematics 18: 431–451. [Google Scholar]
- Ehmig M, Coiro M, Linder HP.. 2019. Ecophysiological strategy switch through development in heteroblastic species of Mediterranean ecosystems – an example in the African Restionaceae. Annals of Botany 123: 611–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elser JJ, Sterner RW, Gorokhova EA, et al. 2000. Biological stoichiometry from genes to ecosystems. Ecology Letters 3: 540–550. [Google Scholar]
- Erdős L, Kröel-Dulay G, Bátori Z, et al. 2018. Habitat heterogeneity as a key to high conservation value in forest-grassland mosaics. Biological Conservation 226: 72–80. [Google Scholar]
- Esler KJ, Von Staden L, Midgley GF.. 2015. Determinants of the Fynbos/Succulent Karoo biome boundary: insights from a reciprocal transplant experiment. South African Journal of Botany 101: 120–128. [Google Scholar]
- Fasham MJR. 1977. A comparison of nonmetric multidimensional scaling, principal components and reciprocal averaging for the ordination of simulated coenoclines, and coenoplanes. Ecology 58: 551–561. [Google Scholar]
- Fer A, Simier P, Arnaud MC, Rey L, Renaudin S.. 1993. Carbon acquisition and metabolism in a root hemiparasitic angiosperm, Thesium humile (Santalaceae) growing on wheat (Triticum vulgare). Functional Plant Biology 20: 15–24. [Google Scholar]
- Fer A, Russo N, Simier P, Arnaud MC, Thalouarn P.. 1994. Physiological changes in a root hemiparasitic angiosperm, Thesium humile (Santalaceae), before and after attachment to the host plant (Triticum vulgare). Journal of Plant Physiology 143: 704–710. [Google Scholar]
- Franceschi VR, Nakata PA.. 2005. Calcium oxalate in plants: formation and function. Annual Review of Plant Biology 56: 41–71. [DOI] [PubMed] [Google Scholar]
- Garnier E. 1992. Growth analysis of congeneric annual and perennial grass species. Journal of Ecology 80: 665–675. [Google Scholar]
- Garnier E, Vancaeyzeele S.. 1994. Carbon and nitrogen content of congeneric annual and perennial grass species: relationships with growth. Plant, Cell & Environment 17: 399–407. [Google Scholar]
- Giesemann P, Gebauer G.. 2022. Distinguishing carbon gains from photosynthesis and heterotrophy in C3-hemiparasite–C3-host pairs. Annals of Botany 129: 647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotelli NJ, Colwell RK.. 2001. Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecology Letters 4: 379–391. [Google Scholar]
- Guo Q. 2001. Early postfire succession in California chaparral: changes in diversity, density, cover and biomass. Ecological Research 16: 471–485. [Google Scholar]
- Güsewell S. 2004. N:P ratios in terrestrial plants: variation and functional significance. The New Phytologist 164: 243–266. [DOI] [PubMed] [Google Scholar]
- Hall AV. 1959. Observations on the distribution and ecology of Orchidaceae in the Muizenberg Mountains, Cape Peninsula. Journal of South African Botany 25: 265–278. [Google Scholar]
- Hayes PE, Nge FJ, Cramer MD, et al. 2021. Traits related to efficient acquisition and use of phosphorus promote diversification in Proteaceae in phosphorus-impoverished landscapes. Plant and Soil 462: 67–88. [Google Scholar]
- He T, Lamont BB, Pausas JG.. 2019. Fire as a key driver of Earth’s biodiversity. Biological Reviews of the Cambridge Philosophical Society 94: 1983–2010. [DOI] [PubMed] [Google Scholar]
- Higgins SI, Buitenwerf R, Moncrieff GR.. 2016. Defining functional biomes and monitoring their change globally. Global Change Biology 22: 3583–3593. [DOI] [PubMed] [Google Scholar]
- Ho LST, Ané C.. 2014. A linear-time algorithm for Gaussian and non-Gaussian trait evolution models. Systematic Biology 63: 397–408. [DOI] [PubMed] [Google Scholar]
- Hoffman MT, Moll EJ, Boucher C.. 1987. Post-fire succession at Pella, a South African lowland fynbos site. South African Journal of Botany 53: 370–374. [Google Scholar]
- Jin Y, Qian H.. 2019. VPhyloMaker: an R package that can generate very large phylogenies for vascular plants. Ecography 42: 1353–1359. [Google Scholar]
- Jin Y, Qian H.. 2022. VPhyloMaker2: an updated and enlarged package that can generate very large phylogenies for vascular plants. Plant Diversity 44: 335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubert C. 1991. History and description of contemporary vegetation, Signal Hill, Cape Town. MSc Thesis, University of Cape Town, South Africa. [Google Scholar]
- Joubert C, Moll EJ.. 1992. A phytosociological study of Signal Hill, Cape Town, utilizing both perennial and ephemeral species. Bothalia 22: 255–282. [Google Scholar]
- Kelly LT, Brotons L.. 2017. Using fire to promote biodiversity. Science 355: 1264–1265. [DOI] [PubMed] [Google Scholar]
- Kerkhoff AJ, Fagan WF, Elser JJ, Enquist BJ.. 2006. Phylogenetic and growth form variation in the scaling of nitrogen and phosphorus in the seed plants. The American Naturalist 168: E103–E122. [DOI] [PubMed] [Google Scholar]
- Kerr JT, Packer L.. 1997. Habitat heterogeneity as a determinant of mammal species richness in high-energy regions. Nature 385: 252–254. [Google Scholar]
- Kraai T, van Wilgen BW.. 2014. Drivers, ecology, and management of fire in fynbos. In: Allsopp NA, Colville JF, Verboom GA, eds. Fynbos: ecology, evolution, and conservation of a megadiverse region. Oxford: Oxford University Press, 47–72. [Google Scholar]
- Kruger FJ. 1987. Succession after fire in selected fynbos communities of the south-western Cape. PhD Thesis, University of the Witwatersrand, South Africa. [Google Scholar]
- Kruger FJ, Bigalke RC.. 1984. Fire in fynbos. In: de Van Booysen P, Tainton NM, eds. Ecological effects of fire in South African ecosystems. Berlin and Heidelberg: Springer, 67–114. [Google Scholar]
- Kutiel P, Naveh Z.. 1987. The effect of fire on nutrients in a pine forest soil. Plant and Soil 104: 269–274. [Google Scholar]
- Laliberté E, Lambers H, Burgess TI, Wright SJ.. 2015. Phosphorus limitation, soil-borne pathogens and the coexistence of plant species in hyperdiverse forests and shrublands. New Phytologist 206: 507–521. [DOI] [PubMed] [Google Scholar]
- Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas E.. 2006. Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Annals of Botany 98: 693–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambers H, Brundrett MC, Raven JA, Hopper SD.. 2011. Plant mineral nutrition in ancient landscape: high plant species diversity on infertile soils is linked to functional diversity for nutritional strategies. Plant and Soil 348: 7–27. [Google Scholar]
- Lambers H, Albornoz F, Kotula L, et al. 2018. How belowground interactions contribute to the coexistence of mycorrhizal and non-mycorrhizal species in severely phosphorus-impoverished hyperdiverse ecosystems. Plant and Soil 424: 11–33. [Google Scholar]
- Lambers H, de Britto Costa P, Cawthray GR, et al. 2022. Strategies to acquire and use phosphorus in phosphorus-impoverished and fire-prone environments. Plant and Soil 476: 133–160. [Google Scholar]
- Le Maitre DC, Brown PJ.. 1992. Life cycles and fire-stimulated flowering in geophytes. In: van Wilgen BW, Richardson DM, Kruger FJ, Hensbergen HJ, eds. Fire in South African mountain fynbos: ecosystem, community and species response at Swartboskloof. Berlin and Heidelberg: Springer, 145–160. [Google Scholar]
- Levyns MR. 1929. Veld-burning experiments at Ida’s Valley, Stellenbosch. Transactions of the Royal Society of South Africa 17: 61–92. [Google Scholar]
- Linder HP. 1990. Vegetative morphology and interfire survival strategies in the Cape fynbos grasses. Bothalia 20: 91–103. [Google Scholar]
- Luo FL, Guo QS.. 2010. Influences of host species on transpiration, photosynthesis, chlorophyll and mineral contents of medicinal hemiparasite Thesium chinense Turcz. Acta Physiologiae Plantarum 32: 1093–1102. [Google Scholar]
- MacArthur R, Levins R.. 1967. The limiting similarity, convergence, and divergence of coexisting species. The American Naturalist 101: 377–385. [Google Scholar]
- Magadzire N, de Klerk HM, Esler KJ, Slingsby JA.. 2019. Fire and life history affect the distribution of plant species in a biodiversity hotspot. Diversity and Distributions 25: 1012–1023. [Google Scholar]
- Martin RE, Sapsis DB.. 1992. Fires as agents of biodiversity: pyrodiversity promotes biodiversity. In: Kerner HM, ed. Proceedings of the conference on biodiversity of northwest California ecosystems. Berkeley: University of California, 150–157. [Google Scholar]
- Mucina L. 2019. Biome: evolution of a crucial ecological and biogeographical concept. The New Phytologist 222: 97–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucina L, Adams JB, Knevel IC, et al. 2006. Coastal vegetation of South Africa. In: Mucina L, Rutherford MC, eds. The vegetation of South Africa, Lesotho and Swaziland. Pretoria: South African National Biodiversity Institute, 658–696. [Google Scholar]
- Murphy BP, Bowman DM.. 2012. What controls the distribution of tropical forest and savanna? Ecology Letters 15: 748–758. [DOI] [PubMed] [Google Scholar]
- Neugebauer K, Broadley MR, El-Serehy HA, et al. 2018. Variation in the angiosperm ionome. Physiologia Plantarum 163: 306–322. [DOI] [PubMed] [Google Scholar]
- Nyaga JM, Neff JC, Cramer MD.. 2015. The contribution of occult precipitation to nutrient deposition on the west coast of South Africa. PLoS One 10: e0126225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksanen J, Blanchet FG, Friendly M, et al. 2022. Vegan: community ecology package. Version 2.6-2. https://cran.r-project.org/web/packages/vegan/ [Google Scholar]
- Onstein RE, Linder HP.. 2016. Beyond climate: convergence in fast evolving sclerophylls in Cape and Australian Rhamnaceae predates the Mediterranean climate. Journal of Ecology 104: 665–677. [Google Scholar]
- Onstein RE, Carter RJ, Xing Y, Linder HP.. 2014. Diversification rate shifts in the Cape Floristic Region: the right traits in the right place at the right time. Perspectives in Plant Ecology, Evolution and Systematics 16: 331–340. [Google Scholar]
- Ordoñez JC, van Bodegom PM, Witte JPM, Wright IJ, Reich PB, Aerts R.. 2009. A global study of relationships between leaf traits, climate and soil measures of nutrient fertility. Global Ecology and Biogeography 18: 137–149. [Google Scholar]
- Pagel M. 1999. Inferring the historical patterns of biological evolution. Nature 401: 877–884. [DOI] [PubMed] [Google Scholar]
- Paradis E, Schliep K.. 2019. Ape 50: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35: 526–528. [DOI] [PubMed] [Google Scholar]
- Pausas JG, Bond WJ.. 2020. Alternative biome states in terrestrial ecosystems. Trends in Plant Science 25: 250–263. [DOI] [PubMed] [Google Scholar]
- Pausas JG, Keeley JE.. 2014a. Abrupt climate-independent fire regime changes. Ecosystems 17: 1109–1120. [Google Scholar]
- Pausas JG, Keeley JE.. 2014b. Evolutionary ecology of resprouting and seeding in fire-prone ecosystems. New Phytologist 204: 55–65. [DOI] [PubMed] [Google Scholar]
- Pausas JG, Ribeiro E.. 2017. Fire and plant diversity at the global scale. Global Ecology and Biogeography 26: 889–897. [Google Scholar]
- Phillips OL, Hall P, Gentry AH, Sawyer SA, Vasquez R.. 1994. Dynamics and species richness of tropical rain forests. Proceedings of the National Academy of Sciences of the United States of America 91: 2805–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power SC, Cramer MD, Verboom GA, Chimphango SB.. 2010. Does phosphate acquisition constrain legume persistence in the fynbos of the Cape Floristic Region? Plant and Soil 334: 33–46. [Google Scholar]
- Power SC, Cramer MD, Verboom GA, Chimphango SBM.. 2011. Legume seeders of the Cape Floristic Region inhabit more fertile soils than congeneric resprouters—sometimes. Plant Ecology 212: 1979–1989. [Google Scholar]
- Power SC, Verboom GA, Bond WJ, Cramer MD.. 2017. Environmental correlates of biome-level floristic turnover in South Africa. Journal of Biogeography 44: 1745–1757. [Google Scholar]
- Privett SDJ, Cowling RM, Taylor HC.. 2001. Thirty years of change in the fynbos vegetation of the Cape of Good Hope Nature Reserve, South Africa. Bothalia 31: 99–115. [Google Scholar]
- Raven JA, Lambers H, Smith SE, Westoby M.. 2018. Costs of acquiring phosphorus by vascular land plants: patterns and implications for plant coexistence. The New Phytologist 217: 1420–1427. [DOI] [PubMed] [Google Scholar]
- R Core Team. 2021. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. http://www.R-project.org/ [Google Scholar]
- Rebelo AG, Boucher C, Helme N, Mucina L, Rutherford MC.. 2006. Fynbos Biome. In: Mucina L, Rutherford MC, eds. The vegetation of South Africa, Lesotho and Swaziland. Pretoria: South African National Biodiversity Institute, 52–219. [Google Scholar]
- Revell LJ. 2012. Phytools: an R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution 3: 217–223. [Google Scholar]
- Ricklefs RE. 1977. Environmental heterogeneity and plant species diversity: a hypothesis. The American Naturalist 111: 376–381. [Google Scholar]
- Ripley BS, Abraham T, Klak C, Cramer MD.. 2013. How succulent leaves of Aizoaceae avoid mesophyll conductance limitations of photosynthesis and survive drought. Journal of Experimental Botany 64: 5485–5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiters C, McKenzie B.. 1994. Seasonal allocation and efficiency patterns of biomass and resources in the perennial geophyte Sparaxis grandiflora subspecies fimbriata (Iridaceae) in lowland coastal fynbos, South Africa. Annals of Botany 74: 633–646. [Google Scholar]
- Ruiters C, McKenzie B, Raitt LM.. 1993. Life-history studies of the perennial geophyte Haemanthus pubescens L. subspecies pubescens (Amaryllidaceae) in lowland coastal fynbos, South Africa. International Journal of Plant Sciences 154: 441–449. [Google Scholar]
- Salt DE, Baxter I, Lahner B.. 2008. Ionomics and the study of the plant ionome. Annual Review of Plant Biology 59: 709–733. [DOI] [PubMed] [Google Scholar]
- Shane MW, Cawthray GR, Cramer MD, Kuo J, Lambers H.. 2006. Specialized ‘dauciform’ roots of Cyperaceae are structurally distinct, but functionally analogous with ‘cluster’ roots. Plant, Cell & Environment 29: 1989–1999. [DOI] [PubMed] [Google Scholar]
- Simmons MT, Cowling RM.. 1996. Why is the Cape Peninsula so rich in plant species? An analysis of the independent diversity components. Biodiversity and Conservation 5: 551–573. [Google Scholar]
- Slingsby JA, Merow C, Aiello-Lammens M, Silander JA Jr. 2017. Intensifying postfire weather and biological invasion drive species loss in a Mediterranean-type biodiversity hotspot. Proceedings of the National Academy of Sciences of the United States of America 114: 4697–4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slingsby JA, Moncrieff GR, Rogers AJ, February EC.. 2020. Altered ignition threatens a hyperdiverse fire-dependent ecosystem. Global Change Biology 26: 616–628. [DOI] [PubMed] [Google Scholar]
- Smith SE, Read DJ.. 2008. Mycorrhizal symbiosis. London: Academic Press and Elsevier. [Google Scholar]
- Specht RL, Moll EJ.. 1983. Mediterranean-type heathlands and sclerophyllous shrublands of the world: an overview. In: Kruger FJ, Mitchell DT, Jarvis JUM, eds. Mediterranean-type ecosystems: the role of nutrients. Berlin and Heidelberg: Springer, 41–65. [Google Scholar]
- Spies TA, Franklin JF.. 1989. Gap characteristics and vegetation response in coniferous forests of the Pacific northwest. Ecology 70: 543–545. [Google Scholar]
- Staver AC, Botha J, Hedin L.. 2017. Soils and fire jointly determine vegetation structure in an African savanna. The New Phytologist 216: 1151–1160. [DOI] [PubMed] [Google Scholar]
- Steel ZL, Collins BM, Sapsis DB, Stephens SL.. 2021. Quantifying pyrodiversity and its drivers. Proceedings Biological Sciences 288: 20203202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein A, Gerstner K, Kreft H.. 2014. Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecology Letters 17: 866–880. [DOI] [PubMed] [Google Scholar]
- Stock WD, Lewis OA.. 1986. Soil nitrogen and the role of fire as a mineralizing agent in a South African coastal fynbos ecosystem. Journal of Ecology 74: 317–328. [Google Scholar]
- Stock WD, Verboom GA.. 2012. Phylogenetic ecology of foliar N and P concentrations and N:P ratios across mediterranean-type ecosystems. Global Ecology and Biogeography 21: 1147–1156. [Google Scholar]
- Strong DR Jr. 1977. Epiphyte loads, tree falls, and perennial forest disruption: a mechanism for maintaining higher tree species richness in the tropics without animals. Journal of Biogeography 4: 215–218. [Google Scholar]
- Taylor HC. 1969. A vegetation survey of the Cape of Good Hope Nature Reserve. MSc Thesis, University of Cape Town, South Africa. [Google Scholar]
- Thuiller W, Slingsby JA, Privett SDJ, Cowling RM.. 2007. Stochastic species turnover and stable coexistence in a species-rich, fire-prone plant community. PLoS One 2: e938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udy K, Fritsch M, Meyer KM, et al. 2021. Environmental heterogeneity predicts global species richness patterns better than area. Global Ecology and Biogeography 30: 842–851. [Google Scholar]
- Vázquez DP, Simberloff D.. 2002. Ecological specialization and susceptibility to disturbance: conjectures and refutations. The American Naturalist 159: 606–623. [DOI] [PubMed] [Google Scholar]
- Verboom GA, Stock WD, Linder HP.. 2002. Determinants of postfire flowering in the geophytic grass Ehrharta capensis. Functional Ecology 16: 705–713. [Google Scholar]
- Verboom GA, Linder HP, Stock WD.. 2004. Testing the adaptive nature of radiation: growth form and life history divergence in the African grass genus Ehrharta (Poaceae: Ehrhartoideae). American Journal of Botany 91: 1364–1370. [DOI] [PubMed] [Google Scholar]
- Verboom GA, Moore TE, Hoffmann V, Cramer MD.. 2012. The roles of climate and soil nutrients in shaping the life histories of grasses native to the Cape Floristic Region. Plant and Soil 355: 323–340. [Google Scholar]
- Verboom GA, Stock WD, Cramer MD.. 2017. Specialization to extremely low-nutrient soils limits the nutritional adaptability of plant lineages. The American Naturalist 189: 684–699. [DOI] [PubMed] [Google Scholar]
- Vlok JH. 2020. Responses of Watsonia fourcadei and other geophytes to burning and slashing treatments in South Outeniqua Sandstone Fynbos. South African Journal of Botany 135: 268–273. [Google Scholar]
- Vlok JH. 2021. Survival strategy of Watsonia fourcadei (Iridaceae); allocation of resources to sexual reproduction and vegetative survival of the parent plant. South African Journal of Botany 139: 192–196. [Google Scholar]
- White PJ, Bowen HC, Broadley MR, et al. 2004. Evolutionary origins of abnormally large shoot sodium accumulation in nonsaline environments within the Caryophyllales. The New Phytologist 214: 284–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Westoby M, et al. 2004. The worldwide leaf economics spectrum. Nature 428: 821–827. [DOI] [PubMed] [Google Scholar]
- Yates MJ, Verboom GA, Rebelo AG, Cramer MD.. 2010. Ecophysiological significance of leaf size variation in Proteaceae from the Cape Floristic Region. Functional Ecology 24: 485–492. [Google Scholar]
- Zemunik G, Turner BL, Lambers H, Laliberté E.. 2015. Diversity of plant nutrient-acquisition strategies increases during long-term ecosystem development. Nature Plants 1: 15050. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.