Abstract
Background
Older adults with asthma have the greatest burden and worst outcomes, and there is increasing evidence that chronic cough (CC) is associated with asthma severity and poor prognosis. However, the clinical characteristics of older adult patients with both asthma and CC remain largely unknown.
Methods
Participants with stable asthma underwent two cough assessments within 3 months to define the presence of CC. Patients were divided into four groups based on CC and age (cut-off ≥60 years). Multidimensional assessment was performed at baseline, followed by a 12-month follow-up to investigate asthma exacerbations. Logistic regression models were used to explore the interaction effect of CC and age on asthma control and exacerbations.
Results
In total, 310 adult patients were prospectively recruited and divided into four groups: older CC group (n=46), older non-CC group (n=20), younger CC group (n=112) and younger non-CC group (n=132). Compared with the younger non-CC group, the older CC group had worse asthma control and quality of life and increased airflow obstruction. The older CC group showed an increase in moderate-to-severe exacerbations during the 12-month follow-up. There was a significant interaction effect of CC and ageing on the increased moderate-to-severe exacerbations (adjusted risk ratio 2.36, 95% CI 1.47–3.30).
Conclusion
Older asthma patients with CC have worse clinical outcomes, including worse asthma control and quality of life, increased airway obstruction and more frequent moderate-to-severe exacerbations, which can be partly explained by the interaction between CC and ageing.
Tweetable abstract
Older patients with asthma and chronic cough have worse asthma control and more moderate-to-severe exacerbations, which could be partly explained by an interaction effect of chronic cough and ageing https://bit.ly/3PX74xF
Introduction
Asthma is a chronic inflammatory airway disease characterised by cough, dyspnoea, wheezing, chest tightness with variable airflow limitation and airway hyperresponsiveness [1]. The prevalence of asthma among patients older than 60 years is >10% [2]. This population is increasing with an ageing society, adding a heavy socioeconomic burden to the healthcare system [1]. Notably, older patients with asthma have the greatest burden of disease and worst outcomes owing to asthma exacerbations [3, 4], which may be partly explained by comorbidities and age-related changes, such as ageing lung function and distinct airway inflammation [5]. Given these complexities, there is a need to further study the features of asthma in this vulnerable population and identify the factors associated with asthma exacerbations to formulate more effective treatments.
Cough is the most troublesome symptom for patients with asthma [6, 7]. The worldwide prevalence of chronic cough (CC) is approximately 10%, with up to 6% in the Chinese population aged ≥50 years, resulting in impaired quality of life [8, 9]. Managing chronic airway diseases through “treatable traits” has been proposed as a way to apply personalised medicine to improve outcomes [10]. In recent years, more and more attention has been paid to cough as a crucial pulmonary treatable trait for asthma management. There is increasing evidence that CC is associated with asthma severity and poor prognosis [11, 12]. A recently published cross-sectional study found that CC in patients with asthma was associated with worse respiratory symptoms and greater healthcare utilisation [13]. Furthermore, we recently found that CC is associated with increased airway inflammation and worse clinical outcomes in asthma [14]. However, the clinical characteristics of asthma and CC in the older adult population remain largely unknown.
Therefore, this study aimed to explore the clinical characteristics of older adult patients with asthma and CC using a multidimensional assessment in a real-world setting, and to identify implications for asthma management in older individuals.
Methods
Study design
This was a prospective 12-month cohort study in a real-world setting. At baseline, all participants underwent a multidimensional assessment described previously [15]. During the 12-month follow-up period, patients underwent face-to-face visits (or by telephone if unable to attend) to collect detailed information about exacerbations. The Institutional Review Board at the West China Hospital approved all protocols (2014-30), and written informed consent was obtained from all participants.
Participants
Participants aged ≥18 years with stable asthma were consecutively recruited from September 2016 to January 2021, based on the Australasian Severe Asthma Network [16]. Asthma was diagnosed according to Global Initiative for Asthma (GINA) guidelines [17], based on current episodic respiratory symptoms and confirmed by evidence of variable expiratory airflow limitation with either airway hyperresponsiveness challenged by methacholine or bronchodilator responsiveness with improvement of forced expiratory volume in 1 s (FEV1) >12% and >200 mL after a short-acting β2-agonist. The diagnostic criteria of asthma in this study are described in the supplementary file. Stable asthma was defined as an exacerbation-free condition with no respiratory infection or no change in maintenance therapy in the preceding 4 weeks before study entry.
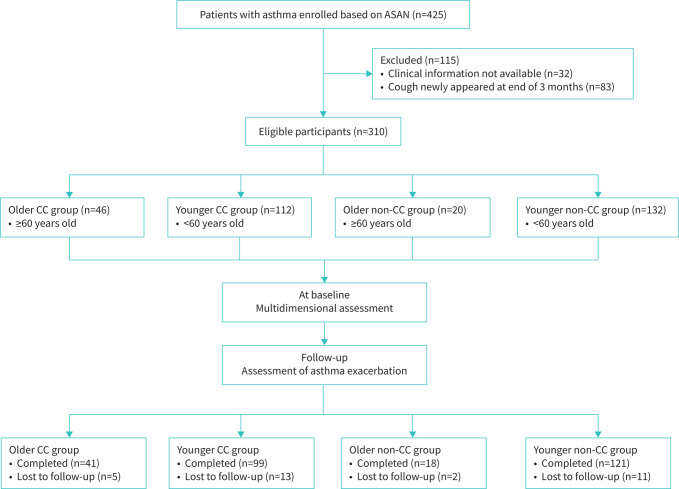
As a real-world study, indications for patient treatment were based on the GINA guidelines [17]. At screening stage, all participants received anti-asthma therapy for 3 months. If participants had poor asthma control or asthma exacerbations, step-up treatment was applied after 3 months of therapy. Otherwise, maintenance therapy was applied after 3 months. After the screening stage, patients were asked whether they had cough persisting for at least 3 months. Then those patients were classified into four conditions: a) cough absent at the beginning of screening but newly appeared at the end of 3 months; b) cough present at the beginning of screening persists despite 3-month anti-asthma therapy; c) cough present only at the beginning of screening but resolved by the end of 3 months; d) cough absent both at screening and during the 3 months. The participants with condition “a” were excluded as representative of a case of unstable asthma. The participants with condition “b” were defined as the CC group, and participants with conditions “c” or “d” were defined as the non-CC group (figure 1). In this study, CC was defined as cough that persisted for at least 3 months despite GINA-guided treatment. Moreover, patients were classified into the older group (≥60 years) or younger group (18–59 years) based on their age at study entry. Accordingly, patients with chronic asthma were classified into four groups: the older CC group, the younger CC group, the older non-CC group and the younger non-CC group.
FIGURE 1.
Flowchart of study. ASAN: Australasian Severe Asthma Network; CC: chronic cough.
Multidimensional assessment
All participants underwent multidimensional assessment, which has been described previously [15]. Comprehensive clinical data were collected at baseline (the end of the 3-month anti-asthma therapy), including demographic and clinical characteristics such as comorbidities, medication use, lung function, asthma control and asthma exacerbations in the previous year. Detailed definitions of comorbidities are described in supplementary table S1. Asthma control and quality of life were assessed using the six-item Asthma Control Questionnaire (ACQ-6) and Asthma Quality of Life Questionnaire (AQLQ) [18, 19], respectively. Dyspnoea, chest tightness and wheezing intensity were evaluated using a visual analogue scale (VAS). The VAS of dyspnoea, chest tightness and wheezing ranged from 0 (no symptoms) to 100 mm (the worst symptoms ever) [20]. Spirometry was measured based on the American Thoracic Society (ATS)/European Respiratory Society (ERS) recommendations [21]. Skin prick tests were performed for 11 common allergens, including dog hair, cat hair, cockroach, pollen (ragweed, birch, maize and London plane), mould (Alternaria tenuis and Aspergillus fumigatus) and house dust mites (Dermatophagoides pteronyssinus and Dermatophagoides farinae). Atopy was defined by the presence of at least one positive skin prick test [15]. In addition, all participants underwent blood sampling and sputum induction. More details are provided in the supplementary file.
In this study, cough severity and cough-related quality of life were assessed using a cough symptom score [14], cough VAS [22] and the Chinese version of the Leicester Cough Questionnaire (LCQ) [23]. The LCQ consists of 19 questions that cover three subdomains reflecting physical, social and psychological issues. A higher score indicates a better cough-related quality of life. The cough symptom score, ranging from 0 to 3, reflects cough frequency and severity for both the daytime and night-time (from 0=no cough to 3=serious impact on daily activity or sleep due to cough) (supplementary material). The cough VAS ranged from 0 (no cough) to 100 mm (the worst cough ever). Individuals were asked to place a mark perpendicular to the line at a position corresponding to their subjective assessment.
Definitions of asthma phenotypes
Asthma phenotypes were defined as per our previously published studies [15]. In terms of the inflammatory phenotypes [24], patients with a sputum proportion <61% neutrophils and ≥3% eosinophils were classified as having eosinophilic asthma, those with ≥61% neutrophils and <3% eosinophils as having neutrophilic asthma, those with ≥61% neutrophils and ≥3% eosinophils as having mixed granulocytic asthma and those with <61% neutrophils and <3% eosinophils as having paucigranulocytic asthma. Late-onset asthma was defined as age of asthma onset ≥12 years [25]. Allergic asthma was defined as positive skin prick tests and allergy symptoms [26]. Asthma with fixed airflow limitation was defined as an FEV1/forced vital capacity <70% after inhalation of bronchodilator [27].
Definitions of asthma exacerbations within a following 12-month period
The primary outcome was identified as moderate-to-severe exacerbations during the 12-month follow-up. Asthma exacerbations were defined based on the ATS/ERS statement [28]. Therefore, a moderate asthma exacerbation was defined as any increase in rescue bronchodilator use for at least 2 days or any temporary increase in inhaled corticosteroid (ICS)-formoterol as reliever, or an emergency department visit or an unscheduled visit while not requiring oral corticosteroids (OCSs) due to asthma symptoms worsening. A severe asthma exacerbation was defined as worsening of asthma symptoms that led to one of the following: ≥3 days of OCS treatment or a temporary increase in OCS maintenance dosage, an emergency department or intensive care unit visit requiring OCS or an asthma-specific hospitalisation. For consistency, courses of corticosteroids separated by one or more weeks were classified as separate severe exacerbations.
Statistical analysis
Continuous variables are presented as mean±sd or median (interquartile range (IQR)) based on their distributions. When possible, all continuous data were transformed into a normal distribution. Categorical variables are presented as n (%). For two-group comparisons, a t-test or Mann–Whitney test was performed for continuous variables based on their distributions. For four groups, a one-way ANOVA or Kruskal–Wallis test was performed. A Chi-square test or Fisher's exact test was used for categorical data, as appropriate. Bonferroni's correction was used for multiple comparisons.
Logistic regression models were used to explore the association of CC and age on asthma control at baseline and exacerbations in the 12-month follow-up, adjusted for sex, body mass index (BMI), smoking, prebronchodilator FEV1 % predicted, ICS dosage and VAS scores of dyspnoea, wheezing and chest tightness. The interaction effect of CC and age was assessed through the incorporation of an interaction term into regression models. Odds ratios (ORs) are used to present the association between CC and age with asthma control at baseline. Risk ratios are used to present the association between CC and age with asthma exacerbations in the 12-month follow-up. In addition, we performed a sensitivity analysis by excluding patients with COPD as one common risk factor for CC. Statistical analysis was performed using IBM SPSS software (version 26.0; IBM, Armonk, NY, USA). A two-sided p-value <0.05 was considered statistically significant.
Results
Participant characteristics at baseline
We identified 46 older asthma patients with CC (older CC group), 112 younger asthma patients with CC (younger CC group), 20 older asthma patients without CC (older non-CC group) and 132 younger asthma patients without CC (younger non-CC group) (figure 1). CC was significantly more frequent in the older than younger subjects (69.7% versus 45.9%; p<0.001). The baseline characteristics are summarised in table 1. A history of smoking was more common in the older CC group than younger CC group. The proportion of atopy in the older CC group was lower than that in the younger CC group and the older non-CC group. In addition, the younger CC group had greater airway obstruction than the younger non-CC group. The older CC group had a higher proportion of COPD than the younger CC group.
TABLE 1.
Demographic and clinical characteristics of the included patients with asthma grouped by chronic cough (CC) and age
| Variables | Older patients | Younger patients | Adjusted p-values # | p-values between four groups ¶ | |||||
| CC | Non-CC | CC | Non-CC | Older CC versus Younger CC | Older non-CC versus Younger non-CC | Older CC versus Older non-CC | Younger CC versus Younger non-CC | ||
| Subjects (n) | 46 | 20 | 112 | 132 | |||||
| Age (years) | 66.0 (62.8, 71.0) | 65.0 (62.3, 68.0) | 45.0 (32.5, 52.0) | 41.0 (33.3, 47.0) | <0.001 | <0.001 | 0.999 | 0.192 | <0.001 |
| Female | 22 (47.8) | 11 (55.0) | 80 (71.4) | 96 (72.7) | 0.020 | 0.424 | 0.999 | 0.999 | 0.008 |
| BMI (kg·m−2), mean±sd | 23.4±3.2 | 23.3±2.7 | 22.9±3.5 | 22.8±3.1 | 0.999 | 0.999 | 0.999 | 0.999 | 0.678 |
| Smoking | 0.040 + | 0.036 + | 0.999+ | 0.756 | <0.001 + | ||||
| Never | 26 (56.5) | 12 (60.0) | 88 (78.6) | 115 (87.1) | |||||
| Former | 15 (32.6) | 6 (30.0) | 14 (12.5) | 11 (8.3) | |||||
| Current | 5 (10.9) | 2 (10.0) | 10 (8.9) | 6 (4.5) | |||||
| Smoking (pack-years) | 23.5 (9.5, 39.0) | 34.8 (14.7, 44.5) | 17.5 (5.9, 29.5) | 5.3 (2.1, 6.8) | 0.016 | <0.001 | 0.999 | 0.156 | <0.001 |
| Asthma duration (years) | 5.5 (2.0, 10.3) | 6.0 (3.0, 21.0) | 7.0 (3.0, 23.0) | 8.0 (3.0, 25.8) | 0.999 | 0.999 | 0.999 | 0.999 | 0.721 |
| Asthma family history | 19 (41.3) | 7 (35.0) | 42 (37.8) | 46 (34.8) | 0.999 | 0.999 | 0.999 | 0.999 | 0.874 |
| Atopy | 7 (15.9) | 11 (55.0) | 62 (56.9) | 90 (68.2) | <0.001 | 0.980 | <0.001 | 0.280 | <0.001 |
| Pre-FEV1 (% predicted) | 71.5 (42.5, 88.0) | 82.0 (62.8, 95.8) | 75.5 (61.5, 91.0) | 85.0 (72.0, 94.0) | 0.260 | 0.999 | 0.328 | 0.020 | 0.001 |
| Pre-FEV1/FVC | 0.6 (0.5, 0.7) | 0.7 (0.6, 0.7) | 0.7 (0.6, 0.8) | 0.7 (0.7, 0.8) | 0.008 | 0.040 | 0.364 | 0.004 | <0.001 |
| Comorbidities | |||||||||
| Rhinitis | 23 (51.1) | 6 (30.0) | 76 (67.9) | 85 (64.4) | 0.196 | 0.012 | 0.456 | 0.999 | 0.005 |
| Eczema | 4 (8.9) | 4 (20.0) | 17 (15.2) | 29 (21.9) | 0.999 | 0.999+ | 0.952+ | 0.708 | 0.200+ |
| Nasal polyps | 3 (6.7) | 3 (15.0) | 22 (19.6) | 10 (7.6) | 0.180 | 0.999+ | 0.999+ | 0.020 | 0.020 + |
| GORD | 6 (13.3) | 3 (15.0) | 6 (5.4) | 5 (3.8) | 0.416+ | 0.284+ | 0.999+ | 0.999 | 0.042 + |
| COPD | 14 (30.4) | 6 (30.0) | 12 (10.7) | 3 (2.3) | 0.008 | <0.001 + | 0.999 | 0.024 | <0.001 + |
| Bronchiectasis | 5 (11.1) | 0 (0) | 12 (10.7) | 4 (3.0) | 0.999+ | 0.999+ | 0.999+ | 0.064 | 0.034 + |
| Anxiety | 0 (0) | 0 (0) | 8 (7.1) | 3 (2.3) | 0.424+ | 0.999+ | NA | 0.272 | 0.103+ |
| Depression | 0 (0) | 0 (0) | 9 (8.0) | 3 (2.3) | 0.240+ | 0.999+ | NA | 0.152 | 0.050 + |
| Osteoporosis | 6 (13.3) | 3 (15.0) | 3 (2.7) | 4 (3.0) | 0.068+ | 0.196+ | 0.999+ | 0.999+ | 0.007 + |
| Diabetes | 2 (4.4) | 2 (10.0) | 1 (0.9) | 1 (0.8) | 0.792+ | 0.184+ | 0.999+ | 0.999+ | 0.027 + |
| Medications | |||||||||
| ICS/LABA | 45 (97.8) | 20 (100.0) | 111 (99.1) | 131 (99.2) | 0.999+ | 0.999+ | 0.999+ | 0.999+ | 0.636+ |
| ICS dosage (BDP, μg·day−1) | 600 (400, 1000) | 400 (400, 1000) | 400 (400, 800) | 400 (400, 1000) | 0.164 | 0.999 | 0.999 | 0.999 | 0.254 |
| LTRA | 32 (69.6) | 14 (70.0) | 98 (87.5) | 107 (81.1) | 0.028 | 0.988+ | 0.999 | 0.684 | 0.032 + |
| LAMA | 3 (6.5) | 0 (0) | 1 (0.9) | 2 (1.5) | 0.300+ | 0.999+ | 0.999+ | 0.999+ | 0.168+ |
| Theophylline | 4 (8.7) | 1 (5.0) | 8 (7.1) | 5 (3.8) | 0.999+ | 0.999+ | 0.999+ | 0.980 | 0.452+ |
| GINA treatment steps 1 and 2/3/4/5 (n) | 0/23/12/11 | 0/12/6/2 | 2/76/16/18 | 2/83/24/23 | 0.408+ | 0.999+ | 0.999+ | 0.999+ | 0.419 |
| Exacerbation in past year | |||||||||
| Moderate-to-severe exacerbation | 22 (47.8) | 7 (35.0) | 60 (53.6) | 61 (46.2) | 0.999 | 0.999 | 0.999 | 0.999 | 0.408 |
| Severe exacerbation | 15 (32.6) | 5 (25.0) | 31 (27.7) | 27 (20.5) | 0.999 | 0.999+ | 0.999 | 0.748 | 0.350 |
| Unscheduled visit | 13 (28.3) | 2 (10.0) | 41 (36.6) | 47 (35.9) | 0.999 | 0.084 | 0.496+ | 0.999 | 0.096 |
| Emergency department visit | 6 (13.0) | 2 (10.0) | 11 (9.8) | 16 (12.2) | 0.999+ | 0.999+ | 0.999+ | 0.999 | 0.928+ |
| Hospitalisation | 1 (2.2) | 0 (0) | 6 (5.4) | 1 (0.8) | 0.999+ | 0.999+ | 0.999+ | 0.200+ | 0.143+ |
Data are presented as median (Q1, Q3) or n (%), unless otherwise indicated. Bold text indicates statistically significant differences. BMI: body mass index; pre: prebronchodilator; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; GORD: gastro-oesophageal reflux disease; ICS: inhaled corticosteroid; LABA: long-acting β2-agonist; BDP: beclomethasone dipropionate; LTRA: leukotriene receptor antagonist; LAMA: long-acting muscarinic antagonist; GINA: Global Initiative for Asthma; NA: not available. #: adjusted p-values from t-test, Mann–Whitney U test, Chi-square test and Fisher's exact test; ¶: p-values from Kruskal–Wallis test; +: p-values from Fisher's exact test.
Respiratory symptoms and asthma control
The younger CC group had a higher proportion of patients with dyspnoea, wheezing and chest tightness compared with the younger non-CC group (table 2). Wheezing was more common in the older CC group. The older CC group had a cough VAS median score of 30.00 mm (IQR 23.75–50.00 mm) and a LCQ median total score of 19.17 (IQR 15.31–20.35). The younger CC group had a cough VAS median score of 20.00 mm (IQR 10.00–35.00 mm) and a LCQ median total score of 18.57 (IQR 16.70–20.21). In addition, the older CC group had a lower proportion of controlled asthma and worse asthma quality of life. The younger CC group had worse asthma control and asthma quality of life than the younger non-CC group.
TABLE 2.
Respiratory symptoms and asthma control in patients grouped by chronic cough (CC) and age
| Variables | Older CC | Older non-CC | Younger CC | Younger non-CC | H /U/χ2 | p-value |
| Subjects (n) | 46 | 20 | 112 | 132 | ||
| Dyspnoea | 28 (60.9)# | 7 (35.0) | 59 (52.7)# | 32 (24.2) | 29.507 | <0.001 |
| Wheezing | 24 (52.2)#,+ | 3 (15.0) | 51 (45.5)# | 23 (17.4) | 33.216 | <0.001 |
| Chest tightness | 18 (39.1) | 2 (10.0)¶ | 62 (55.4)# | 36 (27.3) | 27.431 | <0.001 |
| Dyspnoea VAS score | 10 (0, 42.5)# | 0 (0, 17.5) | 5 (0, 25)# | 0 (0, 0) | 33.830 | <0.001§ |
| Wheezing VAS score | 7.5 (0, 40)#,+ | 0 (0, 0) | 0 (0, 13.75)# | 0 (0, 0) | 37.376 | <0.001§ |
| Chest tightness VAS score | 0 (0, 22.5) | 0 (0, 0)¶ | 7.5 (0, 20)# | 0 (0, 8.75) | 27.616 | <0.001§ |
| Cough VAS score | 30 (23.75, 50) | NA | 20 (10, 35) | NA | 1724 | 0.001 ƒ |
| Daytime cough symptom score | 1 (1, 1.25) | 0 (0, 0) | 1 (1, 1) | 0 (0, 0) | 2236.5 | 0.073ƒ |
| Night-time cough symptom score | 1 (1, 2) | 0 (0, 0) | 1 (0, 1) | 0 (0, 0) | 1953.5 | 0.008ƒ |
| LCQ | ||||||
| Physical domain | 5.9 (4.9, 6.6) | NA | 6.0 (5.4, 6.5) | NA | 2680.5 | 0.622ƒ |
| Psychological domain | 6.5 (5.4, 7.0) | NA | 6.3 (5.4, 7.0) | NA | 2524 | 0.910ƒ |
| Social domain | 6.6 (5.2, 7.0) | NA | 6.5 (5.3, 7.0) | NA | 2447.5 | 0.676ƒ |
| Total score | 19.2 (15.3, 20.4) | NA | 18.6 (16.7, 20.2) | NA | 2551 | 0.994ƒ |
| ACQ score | 0.8 (0.3, 1.7)#,¶,+ | 0.1 (0.0, 0.5) | 0.3 (0.0, 0.8)# | 0.0 (0.0, 0.2) | 60.493 | <0.001§ |
| Asthma control by ACQ score | 44.414 | <0.001 ## | ||||
| Controlled | 22 (47.8)#,¶,+ | 17 (85.0) | 82 (73.2)# | 122 (92.4) | ||
| Somewhat controlled | 12 (26.1)# | 2 (10.0) | 15 (13.4) | 8 (6.1) | ||
| Poorly controlled | 12 (26.1)# | 1 (5.0) | 15 (13.4)# | 2 (1.5) | ||
| AQLQ score | 6.0 (5.2, 6.5)#,+ | 6.7 (6.3, 7.0)¶ | 6.2 (5.6, 6.5)# | 6.6 (6.3, 6.9) | 62.200 | <0.001§ |
Data are presented as median (Q1, Q3) or n (%), unless otherwise indicated. Bold text indicates statistically significant differences. VAS: visual analogue scale; LCQ: Leicester Cough Questionnaire; ACQ: Asthma Control Questionnaire; AQLQ: Asthma Quality of Life Questionnaire; NA: not available. #: p<0.05 versus Younger non-CC group; ¶: p<0.05 versus Younger CC group; +: p<0.05 versus Older non-CC group; §: Kruskal–Wallis test; ƒ: Mann–Whitney U test; ##: Fisher's exact test.
Inflammatory biomarkers in induced sputum and blood
Inflammatory biomarkers in induced sputum and blood are summarised in table 3. The absolute number and percentage of sputum neutrophil counts were significantly elevated in the older CC group compared with the younger non-CC group. The level of IgE was significantly decreased in the older CC group compared with the younger non-CC group.
TABLE 3.
Airway and systemic inflammation in patients grouped by chronic cough (CC) and age
| Variables | Older CC | Older non-CC | Younger CC | Younger non-CC | H/F | p-value # |
| Induced sputum, n | 34 | 14 | 80 | 102 | ||
| Total cell count (106·mL−1) | 3.56 (2.33, 6.55)¶,+ | 2.49 (2.19, 4.71) | 2.43 (1.38, 4.07) | 2.19 (1.35, 3.2) | 12.656 | 0.005 |
| Eosinophils (106·mL−1) | 1.20 (0.14, 7.02) | 0.00 (0.00, 1.18) | 0.81 (0.00, 15.06) | 0.00 (0.00, 5.81) | 8.153 | 0.043 |
| Eosinophils (%) | 0.50 (0.19, 1.38) | 0.00 (0.00, 0.25) | 0.38 (0.00, 5.13) | 0.00 (0.00, 2.56) | 7.033 | 0.071 |
| Neutrophils (106·mL−1) | 182.07 (42.56, 361.42)¶ | 105.23 (28.52, 409.87) | 68.10 (24.46, 169.68) | 48.51 (17.01, 133.13) | 11.426 | 0.01 |
| Neutrophils (%) | 52.13 (22.38, 77.75)¶ | 43.25 (10.85, 82.94) | 36.25 (13.00, 64.16) | 26.42 (9.81, 50.07) | 11.704 | 0.008 |
| Macrophages (106·mL−1) | 119.12 (27.10, 180.83) | 116.49 (50.95, 210.31) | 92.01 (49.95, 164.35) | 124.38 (71.46, 195.91) | 3.948 | 0.267 |
| Macrophages (%) | 36.39 (10.88, 63.13)¶ | 55.50 (16.69, 81.96) | 48.59 (20.69, 78.54) | 70.50 (42.98, 87.31) | 18.382 | <0.001 |
| Lymphocytes (106·mL−1) | 1.44 (0.00, 3.13) | 1.42 (0.00, 3.28) | 1.29 (0.36, 4.66) | 1.05 (0.24, 2.76) | 1.276 | 0.735 |
| Lymphocytes (%) | 0.38 (0.00, 0.81) | 0.38 (0.00, 1.56) | 0.50 (0.25, 1.67) | 0.50 (0.25, 1.00) | 2.228 | 0.527 |
| Blood, n | 46 | 20 | 110 | 131 | ||
| IgE (IU·mL−1) | 54.70 (18.05, 159.75)¶ | 73.15 (27.71, 286.75) | 123.50 (49.95, 245.25) | 129.50 (46.00, 314.50) | 11.416 | 0.003 |
| White blood cells (109·L−1) | 5.70 (5.00, 7.01) | 5.46 (4.82, 6.46) | 5.65 (4.56, 6.66) | 5.68 (4.68, 6.75) | 1.525 | 0.676 |
| Eosinophils (109·L−1) | 0.16 (0.09, 0.30) | 0.17 (0.09, 0.31) | 0.21 (0.11, 0.31) | 0.17 (0.11, 0.30) | 3.289 | 0.349 |
| Eosinophils (%) | 2.71 (1.50, 4.90) | 3.27 (1.36, 5.69) | 3.49 (2.12, 5.62) | 3.13 (1.83, 4.93) | 3.748 | 0.290 |
| Neutrophils (109·L−1) | 3.60 (3.08, 4.35) | 3.26 (2.66, 3.91) | 3.42 (2.70, 4.37) | 3.37 (2.63, 4.46) | 2.128 | 0.546 |
| Neutrophils (%) | 63.90 (56.01, 68.31) | 59.77 (56.21, 65.06) | 61.29 (55.74, 68.29) | 60.44 (54.06, 66.52) | 2.954 | 0.399 |
| Basophils (109·L−1) | 0.03 (0.02, 0.05) | 0.03 (0.01, 0.04) | 0.03 (0.02, 0.05) | 0.03 (0.02, 0.05) | 3.915 | 0.271 |
| Basophils (%) | 0.57 (0.42, 0.87) | 0.52 (0.27, 0.67) | 0.59 (0.40, 0.78) | 0.60 (0.40, 0.97) | 3.208 | 0.361 |
| Monocytes (109·L−1) | 0.42 (0.32, 0.50)¶,+ | 0.39 (0.31, 0.47) | 0.32 (0.26, 0.41) | 0.33 (0.26, 0.43) | 16.019 | 0.001 |
| Monocytes (%) | 6.88 (5.70, 7.92)¶,+ | 7.01 (6.46, 8.09)¶,+ | 5.95 (4.78, 7.23) | 6.02 (5.04, 7.13) | 17.680 | 0.001 |
| Lymphocytes (109·L−1) | 1.46 (1.11, 1.86) | 1.48 (1.24, 1.68) | 1.56 (1.21, 1.82) | 1.58 (1.32, 1.94) | 3.528 | 0.317 |
| Lymphocytes (%) | 26.09±7.25 | 27.92±6.35 | 27.23±7.29 | 28.93±8.25 | 1.894 | 0.131 |
Data are presented as median (Q1, Q3) or n (%), unless otherwise indicated. Bold text indicates statistically significant differences. #: p-values comparing four groups are from Kruskal–Wallis test and one-way ANOVA; ¶: p<0.05 versus Younger non-CC group; +: p<0.05 versus Younger CC group.
Asthma phenotypes
A greater proportion of neutrophilic asthma was found in the older CC group compared with the younger non-CC group, whereas a lower proportion of allergic asthma phenotype occurred in the older CC group (supplementary table S3). Furthermore, a higher proportion of fixed airflow limitation asthma was observed in the older CC group compared with the younger CC group.
Association between CC and age with asthma control at baseline
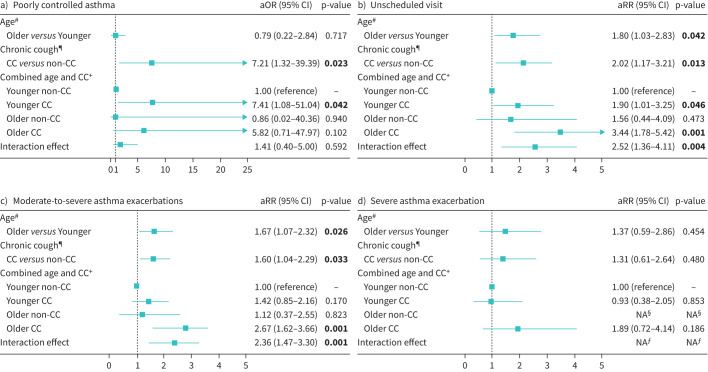
The logistic regression model indicated CC was independently associated with poor asthma control (adjusted OR 7.21, 95% CI 1.32–39.39, p=0.023) (figure 2). In addition, no significant interaction was observed between CC and age in relationship to poor asthma control at baseline.
FIGURE 2.
Associations of chronic cough (CC) and age with a) poorly controlled asthma at baseline, b) unscheduled visits in the 12-month follow-up, c) moderate-to-severe asthma in the 12-month follow-up and d) severe asthma exacerbation in the 12-month follow-up. Poorly controlled asthma defined as Asthma Control Questionnaire score ≥1.5. Bold text indicates statistically significant results. aOR: adjusted odds ratio; aRR: adjusted risk ratio; NA: not available. #: adjusted for CC, sex, body mass index (BMI), smoking, prebronchodilator forced expiratory volume in 1 s (pre-FEV1) % predicted, inhaled corticosteroid (ICS) dosage and visual analogue scale (VAS) scores of dyspnoea, wheezing and chest tightness; ¶: adjusted for age, sex, BMI, smoking, pre-FEV1 % predicted, ICS dosage and VAS scores of dyspnoea, wheezing and chest tightness; +: adjusted for sex, BMI, smoking, pre-FEV1 % predicted, ICS dosage and VAS scores of dyspnoea, wheezing and chest tightness; §: no patients experienced future severe exacerbation in the older non-CC group; ƒ: the interaction effect for future severe exacerbation was not calculated.
Exacerbations in the following 12 months
A total of 89.1% of the older CC patients (n=41), 90.0% of the older non-CC patients (n=18), 88.4% of the younger CC patients (n=99) and 91.7% of the younger non-CC patients (n=121) completed the 12-month follow-up (supplementary table S4). A greater proportion of moderate-to-severe asthma exacerbations was observed in the older CC group compared with the older non-CC group within the 12-month follow-up (60.98% versus 22.22%, p=0.037). Unadjusted logistic regression models indicated that older patients with asthma were at higher risk for moderate-to-severe exacerbations (risk ratio 1.86, 95% CI 1.32–2.41, p=0.001) and unscheduled visits (risk ratio 1.92, 95% CI 1.18–2.83, p=0.01) than younger patients with asthma. Patients with CC were at higher risk for moderate-to-severe exacerbations (risk ratio 2.09, 95% CI 1.48–2.75, p<0.001) and unscheduled visits (risk ratio 2.28, 95% CI 1.40–3.43, p=0.001) than patients without CC.
In the adjusted exacerbation analyses, older patients with asthma were at higher risk for moderate-to-severe exacerbations (adjusted risk ratio 1.67, 95% CI 1.07–2.32, p=0.026) than younger patients with asthma. CC was associated with higher risk for moderate-to-severe exacerbations (adjusted risk ratio 1.60, 95% CI 1.04–2.29, p=0.033) and unscheduled medical visits (adjusted risk ratio 2.02, 95% CI 1.17–3.21, p=0.013) (figure 2). The older CC group was at higher risk for moderate-to-severe exacerbations (adjusted risk ratio 2.67, 95% CI 1.62–3.66, p=0.001) and unscheduled medical visits (adjusted risk ratio 3.44, 95% CI 1.78–5.42, p=0.001) than the younger non-CC group in the adjusted exacerbation analyses. Furthermore, interaction effects of CC and age on risk of future moderate-to-severe exacerbations (adjusted risk ratio 2.36, 95% CI 1.47–3.30, p=0.001) and unscheduled visits (adjusted risk ratio 2.52, 95% CI 1.36–4.11, p=0.004) were observed.
In the sensitivity analysis excluding patients with COPD, the interaction effects of CC and age were associated with higher risk of future moderate-to-severe exacerbations (adjusted risk ratio 2.15, 95% CI 1.20–3.21, p=0.013) and unscheduled visits (adjusted risk ratio 2.16, 95% CI 1.01–3.95, p=0.048).
Discussion
To the best of our knowledge, this is the first prospective cohort study to explore the characteristics of older adult patients with asthma and CC. We found that older asthma patients with CC had worse clinical outcomes, as indicated by increased respiratory symptoms, worse asthma control, poorer asthma-related quality of life and increased airway obstruction, compared to younger asthma patients without CC. CC is an independent signature associated with poor asthma control and future asthma exacerbations. Furthermore, interaction effects of CC and age on the increased risk of future exacerbations were observed. These findings suggest that CC is associated with asthma severity and prognosis, and that more attention should be paid to cough in the management of older patients with asthma.
This interaction effect between CC and ageing on future asthma exacerbations could be explained by several factors involved in inflammation. First, the older participants with CC had significantly increased either type 2 (T2) or non-T2 inflammation (i.e. eosinophils and neutrophils in induced sputum), which resulted in more respiratory symptoms that required an elevated ICS dose, although this difference did not reach statistical significance when comparing ICS dosage equivalent between the four groups. Second, airway eosinophilia can increase neuronal sensitivity, which enhances the cough reflex, leading to increased symptom of cough [29]. Third, non-T2 inflammation, such as increased neutrophils, in the older CC group is associated with cough. Interferon-γ (IFN-γ), a non-T2 biomarker, has recently been found to enhance cough reflex sensitivity by inducing calcium influx and it is associated with increased cough in patients with chronic refractory cough [30, 31]. We found an increased level of sputum IFN-γ in older patients with asthma in our recently published study [27]. Increased non-T2 inflammation, i.e. neutrophils and IFN-γ, are associated with reduced response to ICS [32]. Accordingly, cough is a complex airway neuronal reflex in patients with asthma, yet little is known about the underlying neuronal and inflammation mechanisms, which need to be explored in future studies [33].
Asthma in the older population might represent a different phenotype of asthma, which is more complex owing to comorbidities and age-related changes [27]. In this study, older asthma patients with CC had worse asthma control and quality of life than those without CC, indicated that CC is associated with adverse clinical outcomes. Furthermore, CC has been recognised as a separate heterogeneous entity [34, 35]. Asthmatic cough is a common cough phenotype identified as a cough-related treatable trait [34]. This phenotype can respond to anti-inflammatory asthma therapy; therefore, therapy containing ICS is considered as first-line therapy for this condition. However, multidimensional assessment is critical for the management of CC in patients with asthma because of the overlapping cough-related traits such as COPD and gastro-oesophageal reflux disease indicated in this study, especially in the older patients. Additionally, a higher prevalence of chronic rhinosinusitis and hypertension associated with cough is also found in older asthma patients [2]. Patients with asthma and these comorbidities often have additional symptoms, such as heartburn, regurgitation and nasal congestion, and have poor health outcomes [3]. Early recognition of these cough-related traits is helpful for trait-specific therapy. Additionally, patients who have persistent cough despite optimal treatment of cough-related conditions can be classified as having chronic refractory cough, for which neuromodulatory therapies such as gabapentin and pregabalin can be useful [36].
In this observational study, increased sputum eosinophilic inflammation was found in the CC group compared with the non-CC group. Our recently published study found that asthma patients with CC had elevated airway inflammation [14]. But Marsden et al. [37] found no significant correlation between objective cough frequency and sputum eosinophils in a group of patients with asthma. These contradictory findings could be at least explained by a cross-sectional design. Recently, a crossover randomised controlled study by Satia et al. [29] directly investigated the effects of inducing airway inflammation on cough, and compared cough responses to inhaled capsaicin in patients with mild steroid-naive atopic asthma during and 24 h after inhaled allergen challenge compared with diluent control. They found that allergen-induced airway eosinophilia led to increased cough reflex sensitivity associated with an increase in 24-h spontaneous coughing. In addition, eosinophils were found to be associated with increased cough reflex sensitivity in an animal model in published studies [38]. These findings indicate that airway eosinophilic inflammation is important in the pathogenesis of cough. Anti-inflammatory therapy is crucial in the management of patients with asthmatic cough, because some patients improved after 3 months of anti-asthma therapy in this study.
Our study had several limitations that need to be considered. First, cough is an important symptom associated with asthma severity. However, we did not measure objective cough frequency. It remains unclear how cough frequency is associated with future asthma exacerbations. Second, the cut-off age of 60 years for defining an older population may be controversial because the cut-off ages vary across different countries, guidelines or laws [1, 39, 40]. However, we performed additional analyses using ages 65 or 70 years as cut-offs and splitting ages into decile groups, and these did not change the findings from the main analysis. Additionally, CC might be due to multiple reasons: we did not validate these potential components. Finally, we did not reassess the cough symptoms in the 1-year follow-up, which might be useful to understand the clinical prognosis.
Conclusion
Older adult asthma patients with CC exhibited poor asthma control and quality of life, increased airway obstruction and more frequent moderate-to-severe exacerbations. Furthermore, interaction effects between CC and ageing on the future risk of asthma exacerbations were identified, indicating that assessment of cough in older adult patients cannot be neglected.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00461-2023.SUPPLEMENT (287.3KB, pdf)
Acknowledgements
We are grateful to Michelle Gleeson (Hunter Medical Research Institute, the University of Newcastle, Australia) and Zhi Lin (West China Hospital, Sichuan University, China) for their sputum processing and thank all participants who volunteered in this study.
Provenance: Submitted article, peer reviewed.
This study is registered at www.chictr.org.cn with identifier number ChiCTR-OOC-16009529.
Ethics statement: This study was performed in accordance with the Declaration of Helsinki. This human study was approved by the institutional review board at West China Hospital of Sichuan University, approval number 2014-30. All adult participants provided written informed consent to participate in this study, before enrolment.
Author contributions: F.D. Jin, J. Wang and G. Wang contributed to the study conception and design. Material preparation, data collection and analysis were performed by all authors. The first draft of the manuscript was written by F.D. Jin and J. Wang, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Conflict of interest: W-J. Song is Deputy Chief Editor of this journal. The other authors declare that they have no relevant conflicts of interest.
Support statement: This study was supported by the National Natural Science Foundation of China (81920108002 and 82370044); the 1.3.5 Project for Disciplines of Excellence–Clinical Research Incubation Project, West China Hospital, Sichuan University (2018HXFH016); the Science and Technology Bureau of Chengdu City, China (No. 2021-GH03-00007-HZ); and the Sichuan Science and Technology Program, Sichuan, China (No. 2022YFS0263 and 2023NSFSC1455). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Skloot GS, Busse PJ, Braman SS, et al. An official American Thoracic Society workshop report: evaluation and management of asthma in the elderly. Ann Am Thorac Soc 2016; 13: 2064–2077. doi: 10.1513/AnnalsATS.201608-658ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boulet LP, Robitaille C, Deschesnes F, et al. Comparative clinical, physiological, and inflammatory characteristics of elderly subjects with or without asthma and young subjects with asthma. Chest 2017; 152: 1203–1213. doi: 10.1016/j.chest.2017.09.019 [DOI] [PubMed] [Google Scholar]
- 3.Sohn KH, Song WJ, Park JS, et al. Risk factors for acute exacerbations in elderly asthma: what makes asthma in older adults distinctive? Allergy Asthma Immunol Res 2020; 12: 443–453. doi: 10.4168/aair.2020.12.3.443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang AL, Lahousse L, Dahlin A, et al. Novel genetic variants associated with inhaled corticosteroid treatment response in older adults with asthma. Thorax 2023; 78: 432–441. doi: 10.1136/thoraxjnl-2021-217674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunn RM, Busse PJ, Wechsler ME. Asthma in the elderly and late-onset adult asthma. Allergy 2018; 73: 284–294. doi: 10.1111/all.13258 [DOI] [PubMed] [Google Scholar]
- 6.Song WJ, Won HK, Lee SY, et al. Patients’ experiences of asthma exacerbation and management: a qualitative study of severe asthma. ERJ Open Res 2021; 7: 00528-2020. doi: 10.1183/23120541.00528-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmes J, O'Neill V, McGarvey LP, et al. Adverse perception of cough in patients with severe asthma: a discrete choice experiment. ERJ Open Res 2023; 9: 00442-2022. doi: 10.1183/23120541.00442-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song WJ, Chang YS, Faruqi S, et al. The global epidemiology of chronic cough in adults: a systematic review and meta-analysis. Eur Respir J 2015; 45: 1479–1481. doi: 10.1183/09031936.00218714 [DOI] [PubMed] [Google Scholar]
- 9.Huang K, Gu X, Yang T, et al. Prevalence and burden of chronic cough in China: a national cross-sectional study. ERJ Open Res 2022; 8: 00075-2022. doi: 10.1183/23120541.00075-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDonald VM, Fingleton J, Agusti A, et al. Treatable traits: a new paradigm for 21st century management of chronic airway diseases: Treatable Traits Down Under International Workshop report. Eur Respir J 2019; 53: 1802058. doi: 10.1183/13993003.02058-2018 [DOI] [PubMed] [Google Scholar]
- 11.de Marco R, Marcon A, Jarvis D, et al. Prognostic factors of asthma severity: a 9-year international prospective cohort study. J Allergy Clin Immunol 2006; 117: 1249–1256. doi: 10.1016/j.jaci.2006.03.019 [DOI] [PubMed] [Google Scholar]
- 12.Thomson NC, Chaudhuri R, Messow CM, et al. Chronic cough and sputum production are associated with worse clinical outcomes in stable asthma. Respir Med 2013; 107: 1501–1508. doi: 10.1016/j.rmed.2013.07.017 [DOI] [PubMed] [Google Scholar]
- 13.Colak Y, Afzal S, Lange P, et al. Role and impact of chronic cough in individuals with asthma from the general population. J Allergy Clin Immunol Pract 2019; 7: 1783–1792. doi: 10.1016/j.jaip.2019.02.021 [DOI] [PubMed] [Google Scholar]
- 14.Deng SJ, Wang J, Liu L, et al. Chronic cough in asthma is associated with increased airway inflammation, more comorbidities, and worse clinical outcomes. Allergy Asthma Proc 2022; 43: 209–219. doi: 10.2500/aap.2022.43.220022 [DOI] [PubMed] [Google Scholar]
- 15.Wu WW, Zhang X, Li M, et al. Treatable traits in elderly asthmatics from the Australasian Severe Asthma Network: a prospective cohort study. J Allergy Clin Immunol Pract 2021; 9: 2770–2782. doi: 10.1016/j.jaip.2021.03.042 [DOI] [PubMed] [Google Scholar]
- 16.Wang G, Wang F, Gibson PG, et al. Severe and uncontrolled asthma in China: a cross-sectional survey from the Australasian Severe Asthma Network. J Thorac Dis 2017; 9: 1333–1344. doi: 10.21037/jtd.2017.04.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Global Initiative for Asthma (GINA) . Global Strategy for Asthma Management and Prevention. 2014. Available from: http://ginasthma.org/
- 18.Juniper EF, Norman GR, Cox FM, et al. Comparison of the standard gamble, rating scale, AQLQ and SF-36 for measuring quality of life in asthma. Eur Respir J 2001; 18: 38–44. doi: 10.1183/09031936.01.00088301 [DOI] [PubMed] [Google Scholar]
- 19.Juniper EF, Bousquet J, Abetz L, et al. Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the Asthma Control Questionnaire. Respir Med 2006; 100: 616–621. doi: 10.1016/j.rmed.2005.08.012 [DOI] [PubMed] [Google Scholar]
- 20.Mador MJ, Kufel TJ. Reproducibility of visual analog scale measurements of dyspnea in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1992; 146: 82–87. doi: 10.1164/ajrccm/146.1.82 [DOI] [PubMed] [Google Scholar]
- 21.Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med 2011; 184: 602–615. doi: 10.1164/rccm.9120-11ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pavord ID, Chung KF. Management of chronic cough. Lancet 2008; 371: 1375–1384. doi: 10.1016/S0140-6736(08)60596-6 [DOI] [PubMed] [Google Scholar]
- 23.Ma W, Yu L, Wang Y, et al. Changes in health-related quality of life and clinical implications in Chinese patients with chronic cough. Cough 2009; 5: 7. doi: 10.1186/1745-9974-5-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDonald VM, Hiles SA, Godbout K, et al. Treatable traits can be identified in a severe asthma registry and predict future exacerbations. Respirology 2019; 24: 37–47. doi: 10.1111/resp.13389 [DOI] [PubMed] [Google Scholar]
- 25.Holguin F, Bleecker ER, Busse WW, et al. Obesity and asthma: an association modified by age of asthma onset. J Allergy Clin Immunol 2011; 127: 1486–1493. doi: 10.1016/j.jaci.2011.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Kampen V, de Blay F, Folletti I, et al. EAACI position paper: skin prick testing in the diagnosis of occupational type I allergies. Allergy 2013; 68: 580–584. doi: 10.1111/all.12120 [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Zhang X, Zhang L, et al. Age-related clinical characteristics, inflammatory features, phenotypes, and treatment response in asthma. J Allergy Clin Immunol Pract 2023; 11: 210–219. doi: 10.1016/j.jaip.2022.09.029 [DOI] [PubMed] [Google Scholar]
- 28.Reddel HK, Taylor DR, Bateman ED, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med 2009; 180: 59–99. doi: 10.1164/rccm.200801-060ST [DOI] [PubMed] [Google Scholar]
- 29.Satia I, Watson R, Scime T, et al. Allergen challenge increases capsaicin-evoked cough responses in patients with allergic asthma. J Allergy Clin Immunol 2019; 144: 788–795. doi: 10.1016/j.jaci.2018.11.050 [DOI] [PubMed] [Google Scholar]
- 30.Deng Z, Zhou W, Sun J, et al. IFN-γ enhances the cough reflex sensitivity via calcium influx in vagal sensory neurons. Am J Respir Crit Care Med 2018; 198: 868–879. doi: 10.1164/rccm.201709-1813OC [DOI] [PubMed] [Google Scholar]
- 31.Sun J, Zhan C, Deng Z, et al. Expression of interferon-γ and its effect on cough hypersensitivity in chronic refractory cough patients. Thorax 2022; 77: 621–624. doi: 10.1136/thoraxjnl-2021-218403 [DOI] [PubMed] [Google Scholar]
- 32.Li JJ, Wang W, Baines KJ, et al. IL-27/IFN-γ induce MyD88-dependent steroid-resistant airway hyperresponsiveness by inhibiting glucocorticoid signaling in macrophages. J Immunol 2010; 185: 4401–4409. doi: 10.4049/jimmunol.1001039 [DOI] [PubMed] [Google Scholar]
- 33.Lai K, Satia I, Song WJ, et al. Cough and cough hypersensitivity as treatable traits of asthma. Lancet Respir Med 2023; 11: 650–662. doi: 10.1016/S2213-2600(23)00187-X [DOI] [PubMed] [Google Scholar]
- 34.Morice AH, Millqvist E, Bieksiene K, et al. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J 2020; 55: 1901136. doi: 10.1183/13993003.01136-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung KF, McGarvey L, Song WJ, et al. Cough hypersensitivity and chronic cough. Nat Rev Dis Primers 2022; 8: 45. doi: 10.1038/s41572-022-00370-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gibson P, Wang G, McGarvey L, et al. Treatment of unexplained chronic cough: CHEST guideline and expert panel report. Chest 2016; 149: 27–44. doi: 10.1378/chest.15-1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marsden PA, Satia I, Ibrahim B, et al. Objective cough frequency, airway inflammation, and disease control in asthma. Chest 2016; 149: 1460–1466. doi: 10.1016/j.chest.2016.02.676 [DOI] [PubMed] [Google Scholar]
- 38.Fang Z, Huang C, Zhang J, et al. Traffic‐related air pollution induces non‐allergic eosinophilic airway inflammation and cough hypersensitivity in guinea‐pigs. Clin Exp Allergy 2019; 49: 366–377. 10.1111/cea.2019.49.issue-3. [DOI] [PubMed] [Google Scholar]
- 39.Dyer CA, Hill SL, Stockley RA, et al. Quality of life in elderly subjects with a diagnostic label of asthma from general practice registers. Eur Respir J 1999; 14: 39–45. doi: 10.1034/j.1399-3003.1999.14a09.x [DOI] [PubMed] [Google Scholar]
- 40.Agondi RC, Andrade MC, Takejima P, et al. Atopy is associated with age at asthma onset in elderly patients. J Allergy Clin Immunol Pract 2018; 6: 865–871. doi: 10.1016/j.jaip.2017.10.028 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00461-2023.SUPPLEMENT (287.3KB, pdf)