Abstract
OBJECTIVES:
The objective of this study was to determine the association of the use of extracorporeal cardiopulmonary resuscitation (ECPR) with survival to hospital discharge in pediatric patients with a noncardiac illness category. A secondary objective was to report on trends in ECPR usage in this population for 20 years.
DESIGN:
Retrospective multicenter cohort study.
SETTING:
Hospitals contributing data to the American Heart Association’s Get With The Guidelines-Resuscitation registry between 2000 and 2021.
PATIENTS:
Children (<18 yr) with noncardiac illness category who received greater than or equal to 30 minutes of cardiopulmonary resuscitation (CPR) for in-hospital cardiac arrest.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
Propensity score weighting balanced ECPR and conventional CPR (CCPR) groups on hospital and patient characteristics. Multivariable logistic regression incorporating these scores tested the association of ECPR with survival to discharge. A Bayesian logistic regression model estimated the probability of a positive effect from ECPR. A secondary analysis explored temporal trends in ECPR utilization. Of 875 patients, 159 received ECPR and 716 received CCPR. The median age was 1.0 [interquartile range: 0.2–7.0] year. Most patients (597/875; 68%) had a primary diagnosis of respiratory insufficiency. Median CPR duration was 45 [35–63] minutes. ECPR use increased over time (p < 0.001). We did not identify differences in survival to discharge between the ECPR group (21.4%) and the CCPR group (16.2%) in univariable analysis (p = 0.13) or propensity-weighted multivariable logistic regression (adjusted odds ratio 1.42 [95% CI, 0.84–2.40; p = 0.19]). The Bayesian model estimated an 85.1% posterior probability of a positive effect of ECPR on survival to discharge.
CONCLUSIONS:
ECPR usage increased substantially for the last 20 years. We failed to identify a significant association between ECPR and survival to hospital discharge, although a post hoc Bayesian analysis suggested a survival benefit (85% posterior probability).
Keywords: cardiopulmonary resuscitation, extracorporeal cardiopulmonary resuscitation, extracorporeal membrane oxygenation, in-hospital cardiac arrest, pediatrics
Approximately 15,200 children require cardiopulmonary resuscitation (CPR) for in-hospital cardiac arrests (IHCAs) annually in the United States (1). Pediatric IHCA survival rates have improved over time from 18% in 2000 to 38% in 2018, but a substantial proportion of patients do not achieve return of spontaneous circulation (ROSC) (2, 3). Thus, extracorporeal CPR (ECPR), the implementation of venoarterial extracorporeal membrane oxygenation (VA-ECMO) during CPR, is used as a rescue therapy for refractory IHCA (2–4).
The association of ECPR with pediatric IHCA survival outcomes has been reported in several studies, but the majority have largely focused on children with underlying cardiac disease (5–12). In this patient population, ECPR is associated with higher rates of survival to hospital discharge when compared with conventional CPR (CCPR) (6). Thus, based on the currently available evidence, international CPR guidelines and International Liaison Committee on Resuscitation consensus statements advise consideration of ECPR for children with existing cardiac disease who have IHCA in settings with existing ECMO protocols (13, 14). Data regarding the association of ECPR with IHCA outcomes in children without primary cardiac diagnoses are lacking. Guidelines specifically acknowledge these lack of data and are unable to provide recommendations in the noncardiac population (10, 13–15). Thus, ECPR deployment in noncardiac patients is determined based on hospital-specific practice guidelines (16, 17).
To address this critical knowledge gap, the primary objective of this study was to determine the association between the use of ECPR and survival to hospital discharge in children with noncardiac illness category who received greater than or equal to 30 minutes of CPR for IHCA reported to the American Heart Association’s Get With The Guidelines-Resuscitation registry (GWTG-R). We hypothesized that rates of survival to hospital discharge would be higher in children who received ECPR than children who received CCPR. A secondary objective was to determine temporal trends in ECPR usage in the noncardiac population.
METHODS
Overview
This was a retrospective cohort study using data from the GWTG-R registry of in-hospital resuscitation events using Utstein-style data reporting (18). The design and reporting of GWTG-R have been described previously (www.heart.org/resuscitation). The Children’s Hospital of Philadelphia Institutional Review Board determined this study exempt as it did not meet criteria for human subject research (IRB#21018505).
Inclusion and Exclusion Criteria
Pediatric IHCA index events (first IHCA in a given hospitalization) with a total CPR duration of greater than or equal to 30 minutes between January 1, 2000, and May 4, 2021, were eligible for inclusion. Subjects were included if they had an illness category classified as “medical noncardiac” or “surgical noncardiac” and excluded if they had a cardiac illness category. Patients with a “trauma” illness category were also excluded due to the anticipation that ECPR would be rarely used in this patient population and that the overall low number of patients in this category would lead to difficulty with achieving balance in statistical models. This definition does not fully exclude patients with cardiac diagnoses, as the definition of illness category in the GWTG-R registry is based on the subject’s primary diagnosis at the time of the event. For example, a subject with a history of a structural cardiac defect admitted with pneumonia would be classified as “medical noncardiac” at the time of event, while a patient admitted with myocarditis would be classified as “medical cardiac.” A CPR duration threshold of at least 30 minutes was selected to avoid inclusion of: 1) patients who required CPR during cannulation for ECMO that preceded cardiac arrest and 2) CCPR patients for whom teams were not committed to similar resuscitation efforts as what would be required for ECPR (e.g., minimize inclusion of CCPR patients who received CPR without reasonable expectation of survival). The ECPR group was defined by patients who received “cardiopulmonary bypass/extracorporeal CPR” under the GWTG-R registry category for non-drug interventions and had sustained return of circulation (ROC) for at least 20 minutes. Thus, only events with successful cannulation were included as we theorized there could be interinstitutional discrepancies in recording ECPR as an intervention if the cannulation was not completed.
To be eligible, patients were required to be treated in a hospital with at least 3 years of registry data during the study period and at least three total GWTG-R entries in the calendar year of the event. Patients were only included if they were treated in a hospital with at least one ECPR case meeting our inclusion criteria in the same calendar year or any earlier year to compare patients with ECPR vs. CCPR, rather than patients treated at ECPR vs. non-ECPR centers. GWTG-R does not report ECMO volume per center, so this served as a surrogate for determining a center’s available ECPR system and resources, which has shown association with survival (19). However, the analysis of ECPR usage temporal trends did not exclude patients based on ECPR utilization of the center. Events were excluded if they occurred outside predefined inpatient locations (ICUs, diagnostic/intervention areas, and general inpatient wards) or the emergency department. Patients were excluded if data on non-drug interventions, survival to hospital discharge, sex, or pre-existing conditions were missing. All acute stroke patients were excluded based on the relative contraindication for ECMO. Only years with complete data were included in the analysis of ECPR usage trends; this was limited to events between January 1, 2000, and December 31, 2020.
Outcomes
The primary outcome was survival to hospital discharge. An exploratory outcome was survival with a favorable outcome (pediatric cerebral performance category [PCPC] score of 1, 2, or 3 or no change from admission PCPC score) in the subset of patients with available admission and discharge PCPC data.
Statistical Analyses
Categorical variables were summarized as counts and percentages and continuous variables as median, first, and third quartiles. Fisher exact test (with a Monte Carlo approximation when response had more than two categories) compared categorical variables and Wilcoxon rank-sum test compared continuous variables between ECPR and CCPR patients.
To assess the association of ECPR vs. CCPR with outcomes, propensity-weighted regression was used to reduce the effect of variables that confound the relationship between CPR type and outcomes (age, sex, race, illness category, pre-existing medical conditions, event location, first documented rhythm, time of event: night/weekend vs. weekday, hospital size, calendar year of event, and CPR duration) (20). This robust approach involved three main steps. First, a logistic regression model was created to estimate each patient’s probability (propensity) to receive ECPR and a butterfly plot of propensities was created by ECPR status (Supplemental Fig. 1, http://links.lww.com/CCM/H468). For patients missing race, “missing” was coded as a separate category that was used to achieve balance in the propensity-weighted regression. Second, patients were weighted using stabilized inverse probability of treatment weights. Using this method rather than propensity matching allowed a greater number of patients to be included in the final cohort. This method applied a weight to each patient based on the patient’s probability to receive treatment to create balance across the potential confounders between the two groups (21). Differences in proportions were compared between patients with ECPR and CCPR and summarized as counts and percentages in the original and weighted cohorts. Groups were considered well-balanced when the absolute standardized difference was <0.10 after propensity weighting (20). Third, a logistic regression model was created using the weighted cohort to assess the association of ECPR with survival to hospital discharge. This model was then adjusted for any covariate that did not meet the criteria for sufficient balance after propensity weighting. Because of our hypothesis that CPR duration would be strongly associated with both receiving ECPR as well as survival to hospital discharge, CPR duration was included as an a priori covariate in the logistic regression model despite achieving our goal balance through propensity weighting (22, 23). Site was included as a random effect to account for within-hospital correlation. Neurologic outcomes were evaluated for those patients included in the propensity-weighted regression, but due to the small number of patients with available PCPC scores, an adjusted odds ratio (OR) was not calculated.
Specific attention was taken to control for CPR duration in the final models. The odds of survival decrease sharply for each minute of CPR initially, but the slope quickly levels out. In order to appropriately capture the relationship, a piecewise linear spline of CPR duration was used as the covariate for CPR duration. This allowed the slope to change (become less steep) at 45 and 60 minutes after the start of CPR. CPR duration was also truncated after 120 minutes to address the lack of change in odds of survival after that time.
A post hoc Bayesian estimation of the logistic regression model was created to determine the probability of a positive effect of ECPR on survival to hospital discharge. For the purposes of this analysis, minimally informative priors (scaled Student t distributions with 3 degrees of freedom, centered at 0, with scale parameter resulting in priors with sd = 175) were chosen for all regression parameters without incorporation of subjective prior beliefs for estimation to be driven by the data while applying the same propensity weighting described earlier. The aim was to estimate the posterior probabilities that the treatment effect exceeded a range of potential values for the minimal clinically important treatment effect (OR > 0.9, OR > 0.95, OR > 1, OR > 1.05, OR > 1.10, OR > 1.15, OR > 1.20, OR > 1.25). This range was selected to include the potential for harm (OR < 1) in addition to the probability of treatment benefit. This method was included to provide a clinically relevant evaluation of benefit of a rare therapy that has been accepted by many into practice for rescue of refractory IHCA.
The usage of ECPR over time was assessed using the trends test. All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC) or R 4.0.1 (R Foundation for Statistical Computing, Vienna, Austria) with reported p values based on a two-sided alternative and deemed significant if less than 0.05.
RESULTS
Unweighted Cohort Characteristics
After sequential exclusions (Fig. 1), 875 patients were included in the primary analysis; 159 received ECPR and 716 received CCPR. The unweighted prearrest patient and event characteristics are described in Table 1, arrest characteristics in Table 2, and hospital characteristics of the patients in the final cohort are described in Supplemental Table 1 (http://links.lww.com/CCM/H468). The ECPR cohort patients were older, had lower frequency of baseline CNS depression, and higher frequency of pre-existing diagnosis of pneumonia. Patients receiving ECPR had a longer median duration of CPR 56 [interquartile range: 45–82] vs. 43 [34–58] minutes for CCPR (p < 0.001). Patients receiving ECPR were more likely to have an arterial catheter in place prior to arrest, received more epinephrine doses, had a shorter epinephrine dosing interval, and had higher frequency of receiving alternative pharmacologic interventions during arrest (calcium, bicarbonate, and alternative vasopressors). Information on the demographics of patients excluded for treatment in non-ECPR performing centers is provided in Supplemental Table 2 (http://links.lww.com/CCM/H468).
Figure 1.
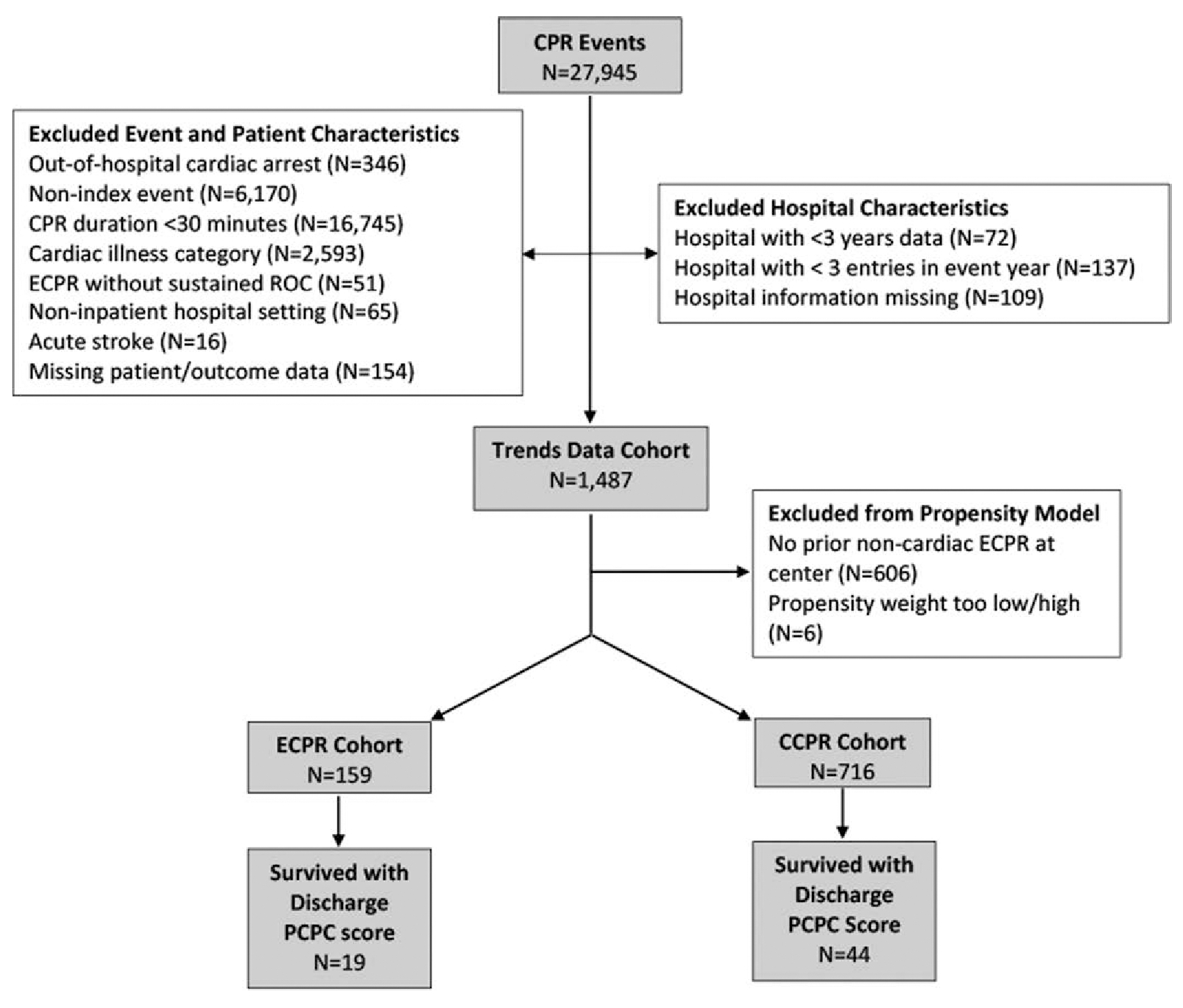
Patient selection flow diagram of sequential exclusions of patients included in the final cohort separated by extracorporeal cardiopulmonary resuscitation (ECPR) vs. conventional CPR (CCPR) status. PCPC = pediatric cerebral performance category, ROC = return of circulation.
TABLE 1.
Prearrest Patient and Event Characteristics by Extracorporeal Cardiopulmonary Resuscitation Status
| ECPR Status |
||||
|---|---|---|---|---|
| Characteristics | Conventional Cardiopulmonary Resuscitation (n = 716) | ECPR (n = 159) | Overall (n = 875) | p |
|
|
||||
| Patient and event characteristics | ||||
| Age at CPA event (yr) | 1.0 [0.1, 7.0] | 2.0 [0.6, 8.0] | 1.0 [0.2, 7.0] | 0.015a |
| Age at CPA event | 0.021a | |||
| <1 mo | 180 (25.1%) | 21 (13.2%) | 201 (23.0%) | |
| 1 mo–< 1 yr | 155 (21.6%) | 37 (23.3%) | 192 (21.9%) | |
| 1 yr–<8 yr | 208 (29.1%) | 61 (38.4%) | 269 (30.7%) | |
| 8–≤18 yr | 173 (24.2%) | 40 (25.2%) | 213 (24.3%) | |
| Sex | 0.218b | |||
| Male | 404 (56.4%) | 81 (50.9%) | 485 (55.4%) | |
| Female | 312 (43.6%) | 78 (49.1%) | 390 (44.6%) | |
| Race | ||||
| White | 358 (50.0%) | 74 (46.5%) | 432 (49.4%) | |
| Black/others | 229 (32.0%) | 48 (30.2%) | 277 (31.7%) | |
| Unknown | 129 (18.0%) | 37 (23.3%) | 166 (19.0%) | |
| Location of event | 0.099b | |||
| ICU | 558 (77.9%) | 126 (79.2%) | 684 (78.2%) | |
| Emergency department | 72 (10.1%) | 11 (6.9%) | 83 (9.5%) | |
| General inpatient | 57 (8.0%) | 9 (5.7%) | 66 (7.5%) | |
| OR | 29 (4.1%) | 13 (8.2%) | 42 (4.8%) | |
| Night, weekend, or holiday event | 384 (53.6%) | 76 (47.8%) | 460 (52.6%) | 0.189b |
| Pre-existing conditions | ||||
| Acute CNS nonstroke event | 59 (8.2%) | 8 (5.0%) | 67 (7.7%) | 0.190b |
| Baseline depression in CNS function | 149 (20.8%) | 16 (10.1%) | 165 (18.9%) | 0.002b |
| Cardiac malformation-acyanotic | 47 (6.6%) | 8 (5.0%) | 55 (6.3%) | 0.589b |
| Cardiac malformation-cyanotic | 28 (3.9%) | 7 (4.4%) | 35 (4.0%) | 0.823b |
| Congenital malformation (noncardiac) | 146 (20.4%) | 30 (18.9%) | 176 (20.1%) | 0.743b |
| Hepatic insufficiency | 45 (6.3%) | 10 (6.3%) | 55 (6.3%) | 1.000b |
| Hypotension/hypoperfusion | 195 (27.2%) | 51 (32.1%) | 246 (28.1%) | 0.242b |
| Major trauma | 25 (3.5%) | 2 (1.3%) | 27 (3.1%) | 0.203b |
| Metastatic or hematologic malignancy | 69 (9.6%) | 18 (11.3%) | 87 (9.9%) | 0.558b |
| Metabolic/electrolyte abnormality | 148 (20.7%) | 34 (21.4%) | 182 (20.8%) | 0.830b |
| Pneumonia | 68 (9.5%) | 25 (15.7%) | 93 (10.6%) | 0.032b |
| Renal insufficiency | 90 (12.6%) | 22 (13.8%) | 112 (12.8%) | 0.694b |
| Respiratory insufficiency | 493 (68.9%) | 104 (65.4%) | 597 (68.2%) | 0.398b |
| Sepsisc | 206 (28.8%) | 53 (33.3%) | 259 (29.6%) | 0.251b |
CPA = cardiopulmonary arrest, ECPR = extracorporeal cardiopulmonary resuscitation.
Wilcoxon rank-sum test.
Fisher exact test (Monte Carlo approximate for tables larger than 2 × 2).
In Get With The Guidelines-Resuscitation registry data until the year 2018 used the term septicemia.
TABLE 2.
Arrest Characteristics by Extracorporeal Cardiopulmonary Resuscitation Status
| ECPR Status |
||||
|---|---|---|---|---|
| Characteristics | Conventional Cardiopulmonary Resuscitation (n = 716) | ECPR (n = 159) | Overall (n = 875) | p |
|
|
||||
| Interventions in place at time of arrest | ||||
| Arterial cathetera | 161 (22.5%) | 57 (35.8%) | 218 (24.9%) | <0.001b |
| Dialysisa | 33 (4.6%) | 6 (3.8%) | 39 (4.5%) | 0.832b |
| Type of assisted ventilation | 0.823b | |||
| None | 151 (21.1%) | 35 (22.0%) | 186 (21.3%) | |
| Invasive assisted ventilation | 485 (677%) | 109 (68.6%) | 594 (67.9%) | |
| Noninvasive assisted ventilation | 80 (11.2%) | 15 (9.4%) | 95 (10.9%) | |
| Vasoactive agenta | 260 (36.4%) | 71 (44.7%) | 331 (37.9%) | 0.058b |
| Arrest characteristics | ||||
| First documented rhythm | 0.005b | |||
| Bradycardia with poor perfusion | 335 (50.4%) | 55 (36.7%) | 390 (47.9%) | |
| Asystole | 122 (18.3%) | 25 (16.7%) | 147 (18.0%) | |
| Pulseless electrical activity | 168 (25.3%) | 62 (41.3%) | 230 (28.2%) | |
| Ventricular tachycardia | 15 (2.3%) | 3 (2.0%) | 18 (2.2%) | |
| Ventricular fibrillation | 6 (0.9%) | 1 (0.7%) | 7 (0.9%) | |
| Other | 19 (2.9%) | 4 (2.7%) | 23 (2.8%) | |
| Unknown | 51 (7.1%) | 9 (5.7%) | 60 (6.9%) | |
| Cardiopulmonary resuscitation duration (min)c | 43.0 [34.0, 58.5] | 56.0 [45.0, 82.0] | 45.0 [35.0, 63.0] | <0.001d |
| Total number of epinephrine dosese | 7.0 [4.0, 10.0] | 11.0 [6.5, 15.0] | 7.0 [4.0, 11.0] | <0.001d |
| Time to first epinephrine (min)f | 1.0 [0.0, 3.0] | 1.0 [0.0, 3.0] | 1.0 [0.0, 3.0] | 0.895d |
| Epinephrine dosing interval (min)g | 7.2 [4.6, 12.5] | 5.1 [4.0, 8.5] | 6.6 [4.5, 11.7] | <0.001d |
| Vasopressor(s) other than epinephrine bolus | 284 (39.7%) | 94 (59.1%) | 378 (43.2%) | <0.001b |
| Epinephrine bolus | 680 (95.0%) | 155 (97.5%) | 835 (95.4%) | 0.210b |
| Sodium bicarbonate | 519 (72.5%) | 133 (83.6%) | 652 (74.5%) | 0.003b |
| Calcium chloride or gluconate | 450 (62.8%) | 131 (82.4%) | 581 (66.4%) | <0.001b |
| Atropine | 184 (25.7%) | 42 (26.4%) | 226 (25.8%) | 0.842b |
| Lidocaine | 52 (7.3%) | 12 (7.5%) | 64 (7.3%) | 0.867b |
| Amiodarone | 31 (4.3%) | 9 (5.7%) | 40 (4.6%) | 0.527b |
ECPR = extracorporeal cardiopulmonary resuscitation.
Missing data on one patient.
Fisher exact test (Monte Carlo approximate for tables larger than 2 × 2).
Truncated after 120 min.
Wilcoxon rank-sum test.
Missing data on 70 patients.
Truncated after 30 min. Missing data on 77 patients who received at least one dose of epinephrine.
Detined as total resuscitation duration after the first dose of epinephrine divided by the total number of administered epinephrine doses
Model Development and Weighting
Balance was achieved for the majority of propensity model covariates (Supplemental Table 3, http://links.lww.com/CCM/H468), with the exception of race, sex, hypotension/hypoperfusion, sepsis/pneumonia, and baseline depression in CNS function, which did not meet the goal of less than 0.10 for sufficient balance in the propensity model. Based on this residual imbalance, these covariates were included in the multivariable logistic regression model. Supplemental Data (http://links.lww.com/CCM/H468) include the characteristics of the six patients not included in the final cohort due to their propensity scores (Supplemental Table 4, http://links.lww.com/CCM/H468) and the primary drivers of the propensity model (Supplemental Table 5, http://links.lww.com/CCM/H468). Later event year, hospital size, and the absence of baseline CNS depression were the covariates with the greatest association with receiving ECPR.
Outcomes
Before propensity weighting, survival to hospital discharge was similar between groups (ECPR: 34/159 [21.4%] vs. CCPR: 116/716 [16.2%]; p = 0.13; Table 3). Data regarding PCPC are also included in Table 3 but was limited due to a degree of missingness (58% missing data among survivors). In the multivariable logistic regression model incorporating propensity weights, the adjusted OR of survival to hospital discharge with ECPR was 1.42 (95% CI, 0.84–2.40; p = 0.19). The Bayesian analysis estimated an 85.1% posterior probability of a population-level OR greater than 1 for survival to hospital discharge with ECPR vs. CCPR (Table 4).
TABLE 3.
Outcomes by Extracorporeal Cardiopulmonary Resuscitation Status: Univariate Outcomes
| ECPR Status |
||||
|---|---|---|---|---|
| Outcomes | Conventional Cardiopulmonary Resuscitation | ECPR | p | |
|
|
||||
| Sustained return of spontaneous circulation | 306/716 (42.7%) | 0/159 (0.0%) | <0.001a | |
| 24-hr survival | 204/716 (28.5%) | 117/159 (73.6%) | <0.001a | |
| Survival to hospital discharge | 116/716 (16.2%) | 34/159 (21.4%) | 0.130a | |
| PCPC at hospital dischargec | 0.211b | |||
| 1 | 17/44 (38.6%) | 11/19 (57.9%) | ||
| 2 | 9/44 (20.5%) | 3/19 (15.8%) | ||
| 3 | 8/44 (18.2%) | 2/19 (10.5%) | ||
| 4 | 9/44 (20.5%) | 2/19 (10.5%) | ||
| 5 | 1/44 (2.3%) | 1/19 (5.3%) | ||
| Change in PCPC from admit to hospital dischargec | 0.143b | |||
| 0 | 36/44 (81.8%) | 12/19 (63.2%) | ||
| 1 | 3/44 (6.8%) | 4/19 (21.1%) | ||
| 2 | 2/44 (4.5%) | 1/19 (5.3%) | ||
| 3 | 2/44 (4.5%) | 1/19 (5.3%) | ||
| 4 | 1/44 (2.3%) | 1/19 (5.3%) | ||
| Hospital discharge with PCPC of 1, 2, or no worse than baselinec | 39/44 (88.6%) | 15/19 (78.9%) | 0.434a | |
| Hospital discharge with PCPC of 1, 2, 3, or no worse than baselinec | 40/44 (90.9%) | 16/19 (84.2%) | 0.422a | |
ECPR = extracorporeal cardiopulmonary resuscitation; PCPC = pediatric cerebral performance category.
Fisher exact test.
Wilcoxon rank-sum test.
Summarized on those who survived to hospital discharge.
TABLE 4.
Probability of Treatment Effect With Extracorporeal Cardiopulmonary Resuscitation by Bayesian Analysis
| OR > 0.90 | OR > 0.95 | OR > 1 | OR > 1.05 | OR > 1.10 | OR > 1.15 | OR > 1.20 | OR > 1.25 | |
|---|---|---|---|---|---|---|---|---|
| P(OR) | 0.93 | 0.89 | 0.85 | 0.81 | 0.76 | 0.71 | 0.65 | 0.60 |
OR = odds ratio.
This model assumes minimally informative prior beliefs for all logistic regression parameters.
Supplemental Figure 2 (http://links.lww.com/CCM/H468) depicts frequency and survival outcomes of ECPR and CCPR relative to CPR duration. Duration was distributed relatively evenly across all 15-minute duration intervals for the ECPR group, while more than half of CCPR events had CPR durations of 30–45 minutes.
Figure 2 displays the use of ECPR over time. Compared with reference year of 2000, ECPR usage increased over time (test of trends p < 0.001).
Figure 2.
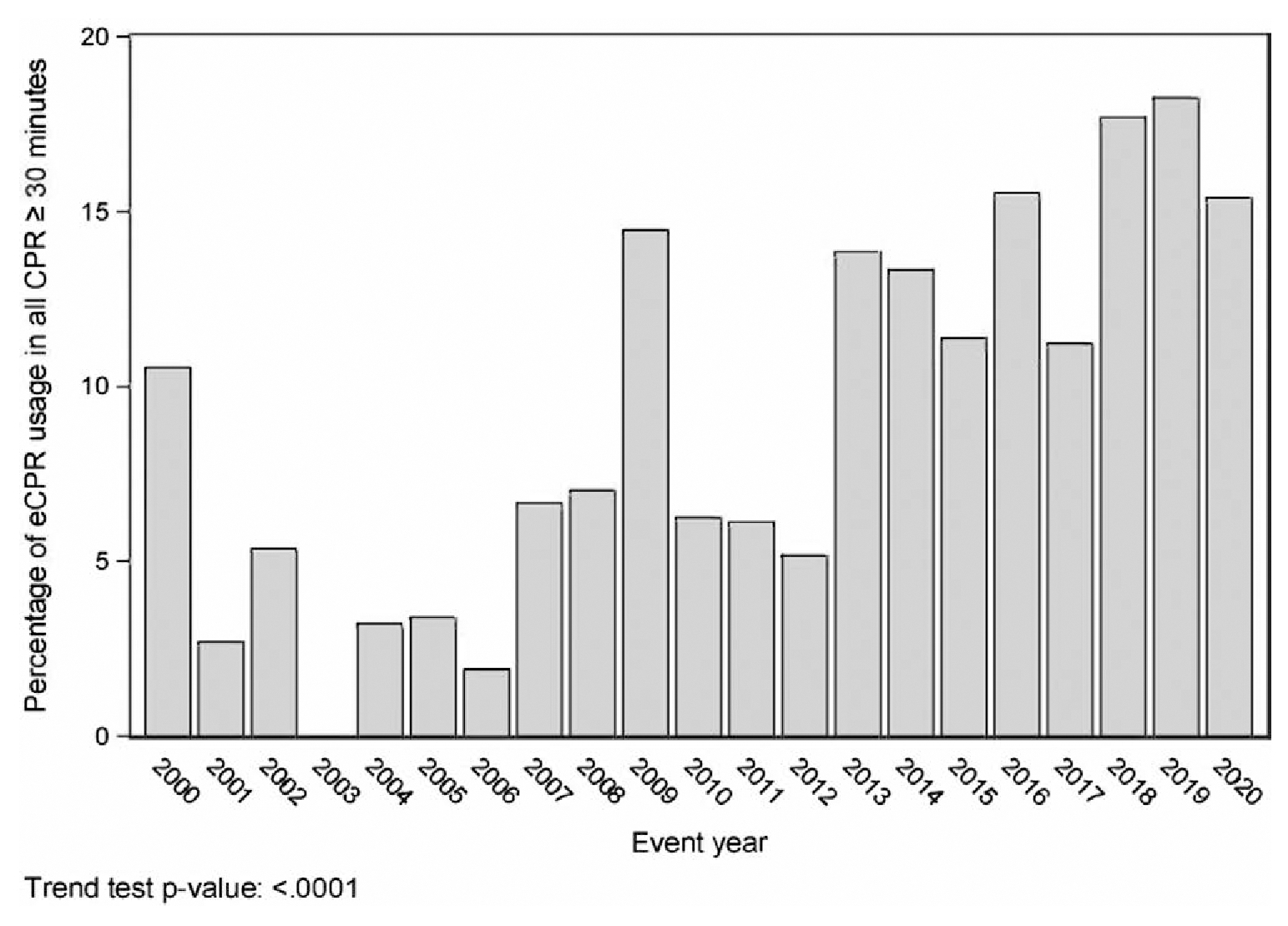
Percentage of pediatric patients with a noncardiac illness category receiving greater than or equal to 30min of cardiopulmonary resuscitation (CPR) for in-hospital cardiac arrests who received treatment with extracorporeal CPR (ECPR) out of all the CPR events in this cohort by event year.
DISCUSSION
Using a large, multicenter national IHCA database and robust methods of controlling for potential confounders, we did not identify a statistically significant difference in survival to hospital discharge between ECPR compared with CCPR in children with noncardiac illness categories who received at least 30 minutes of CPR. Using a Bayesian probabilistic approach, we were able to estimate an 85% probability of a positive effect on survival to hospital discharge with the usage of ECPR. Across a 20-year span of cardiac arrest data reported to the GWTG-R registry, we demonstrated an increase in the percentage of children treated with ECPR for prolonged IHCA greater than or equal to 30 minutes.
To the best of our knowledge, this study represents the largest study of pediatric ECPR in the noncardiac population and enhances our understanding of the epidemiology and outcomes of patients treated with this therapy. Contrary to our hypothesis, we were unable to identify an association between ECPR and survival to hospital discharge in this noncardiac illness category cohort. Prior studies have shown better outcomes among cardiac patients who receive ECPR but have not focused on the outcomes of this unique population (5, 7, 9). Despite including two decades of GWTG-R registry data, the number of ECPR patients included in this study who underwent greater than or equal to 30 minutes of CPR remained relatively low (n = 159) and the width of the 95% CI for ECPR’s adjusted OR (95% CI, 0.84–2.40) suggests that inadequate statistical power may have hampered our ability to detect a positive finding. This small sample size also limited the ability to analyze survival based on event year, which may have shown improvement in ECPR outcomes over the last two decades.
To that end, the Bayesian analysis suggests a potential positive treatment effect of ECPR on survival to discharge. This statistical approach calculates the probability of treatment benefit—in this case, 85%—and this probability can be used as a more intuitive measure of evidence for a population-level benefit of ECPR. This calculation of probability provides an additional method for interpreting data compared with the frequentist calculation of p value and 95% CI, which are not synonymous with probability of treatment effect (24, 25). In the analysis of a rare but clinically used treatment such as ECPR, this probabilistic estimate of treatment benefit can add to our interpretation of these data and potentially aid in clinical decision making regarding the use of ECPR. In addition to the 85% probability of survival benefit with the use of ECPR, the low (7%) probability of an OR for survival less than 0.9 suggests that ECPR is very unlikely to expose patients to a considerable risk of harm. However, we can only assess harm defined as increased odds of death with the data available. These findings support the ongoing usage and development of ECPR systems.
Compared with children with cardiac illness, for whom ECPR is associated with survival benefit, ECPR outcomes in this cohort may differ for both systems and physiologic reasons. From a systems standpoint, ECPR is a resource intensive therapy with success relying on a multidisciplinary response team. As the majority of these systems in pediatrics have been focused on the cardiac population (26, 27), cardiac center teams may be more efficient in the deployment of ECPR. The cardiac cohort comprised largely of surgical cardiac patients (6), who often have an established relationship with a cardiothoracic surgeon and at times the ability to cannulate an open chest. Conversely, in a recent survey of pediatric noncardiac ECPR programs in North America, most noncardiac cannulations are performed by general surgeons, who are less likely to be involved in the patient’s care before IHCA (16). System factors may increase time to cannulation and CPR duration before ROC in the noncardiac population. In our study, the median time to ROC for those who received ECPR was 56 minutes, whereas in recent studies of ECPR for cardiac patients the median time to ROC was 25 minutes (28). In this cohort of prolonged cardiac arrest, we observed high rates of calcium and bicarbonate administration, despite current recommendations to avoid the universal use of these therapies (29–31). This observation may provide insight into the physiologic derangements observed during prolonged cardiac arrest or may indicate common use of these medications regardless of indication in children requiring prolonged CPR. From the physiologic standpoint, IHCA etiology differs between these populations. Patients with noncardiac diagnoses and refractory IHCA may be more likely to have heterogenous physiologic derangements and multisystem organ dysfunction leading up to the IHCA than their cardiac counterparts (2, 32–35).
Despite the absence of formal recommendations for ECPR in noncardiac patients and the lack of survival benefit in limited case series and prior reports (8, 36), we observed a significant temporal increase in ECPR use among centers reporting to the GWTG-R registry. This increased utilization of ECPR may reflect a combination of more aggressive treatment of refractory IHCA and recognition that prolonged CPR is not inherently futile. Although prolonged IHCA is associated with lower rates of survival compared with briefer IHCA events, some children do survive even after more than an hour of CPR (23). Increasing ECPR usage may contribute to improved survival in cases of prolonged IHCA, providing a rescue therapy for IHCA when ROSC is not otherwise achieved (5, 34, 35). Such anecdotal observations may contribute to institutional decisions to pursue this therapy in the noncardiac population in the absence of robust epidemiologic support. During this study period there has likely been substantial evolution and refinement of ECMO and ECPR methods and technology; however, limited sample size precluded a temporal analysis of survival in this trends analysis. The ideal design and patient eligibility for ECPR remains unknown and likely involves patient, location, and team factors beyond the primary illness category (5, 37, 38). Future work should focus on determining subsets of patients with the greatest survival benefit as well as the development of robust ECPR systems for populations outside of surgical cardiac patients.
Our registry-based data and retrospective analysis present several limitations. First, this retrospective cohort study cannot account for all potential forms of selection bias. We elected to restrict patients to only those with prolonged IHCA treated at ECPR centers but recognize this may introduce unmeasured selection bias, despite the use of propensity weighting and multivariable regression to control for suspected confounders. Second, the analysis was limited by the relatively small sample size of ECPR subjects compared with prior studies of ECPR in cardiac and adult populations. Third, the use of a multicenter data registry introduces the possibility of recording errors despite rigorous efforts on the part of the GWTG-R to ensure data integrity. Fourth, limitations in data granularity may have resulted in misclassifications, such as cases of postarrest ECMO deployment used following ROSC being classified as ECPR. Fifth, neurologic outcome data were limited due to missing PCPC data for many survivors, creating a notable challenge when reporting on survival after prolonged cardiac arrest. We recommend future study to address this knowledge gap in neurologic outcomes following noncardiac ECPR. Sixth, ECMO complication data are not recorded in the GWTG-R database, limiting the details of our survival outcomes. Finally, given limitations of data availability, we could not incorporate CPR mechanics into our interpretation of outcomes (39, 40). Given these limitations introduced through the use of retrospective data, there continues to be a need for prospective studies of ECPR in this population; whether equipoise exists for randomized controlled trials of ECPR vs. CCPR should be explored.
CONCLUSIONS
ECPR use in children with noncardiac illness has significantly increased in the last 20 years. Compared with CCPR, ECPR was not associated with a statistically significant increase in survival to discharge among children who required greater than or equal to 30 minutes of CPR. However, this may have been limited by statistical power and a Bayesian model estimated a positive effect of ECPR usage as well as low odds of harm with ECPR.
Supplementary Material
KEY POINTS.
Question:
The purpose of this investigation was to determine the association between the use of extracorporeal cardiopulmonary resuscitation (ECPR) and survival to hospital discharge in pediatric patients with a noncardiac illness category.
Findings:
The primary results of this investigation showed no statistically significant survival benefit with the usage of ECPR in a frequentist statistical model but did show a high probability of treatment benefit (85%) with a Bayesian statistical model.
Meaning:
This study did not identify a clear survival advantage with ECPR for noncardiac pediatric patients but the findings support the ongoing development of ECPR systems and further study in this population.
ACKNOWLEDGMENTS
The Get With The Guidelines-Resuscitation (GWTG-R) program is provided by the American Heart Association. Hospitals participating in the registry submit clinical information regarding the medical history, hospital care, and outcomes of consecutive patients hospitalized for cardiac arrest using an online, interactive case report form and Patient Management Tool (IQVIA, Parsippany, NJ). All participating institutions were required to comply with local regulatory and privacy guidelines and, if required, to secure institutional review board approval. Because data were used primarily at the local site for quality improvement, sites were granted a waiver of informed consent under the common rule. IQVIA serves as the data collection (through their Patient Management Tool) and coordination center for the American Heart Association/American Stroke Association Get With The Guidelines programs. Additionally, we are thankful to Dr. Nadir Yehya and Dr. Robert A. Berg for their collaboration and guidance, and Kathyrn Graham for her collaboration and expertise with Get With The Guidelines.
The GWTG-R Pediatric Research Task Force members include: Anne-Marie Guerguerian, MD, PhD, FRCPC, FAAP, FAHA; Caitlin E. O’Brien, MD, MPH; Ericka L. Fink, MD, MS; Javier J. Lasa, MD, FAAP; Joan S. Roberts, MD; Lillian Su, MD; Linda L. Brown, MD, MSCE; Maya Dewan, MD, MPH; Melania M. Bembea, MD, MPH, PhD; Monica Kleinman, MD; Noorjahan Ali, MD, MS, FAAP; Punkaj Gupta, MBBS; Robert M. Sutton, MD, MSCE; Ron Reeder, MS, PhD; and Todd Sweberg, MD, MBA.
This study was supported by the Children’s Hospital of Philadelphia Resuscitation Science Center and the Department of Anesthesiology and Critical Care Medicine. Dr. Himebauch’s effort was supported by the National Institutes of Health (NIH) National Heart, Lung, and Blood Institute (NHLBI) (K23HL153759). Dr. Morgan’s effort was supported by the NIH NHLBI (K23HL148541).
Drs. Himebauch, Reeder, Kilbaugh, Barney, Topjian, Sutton, and Morgan report grant funding from the National Institutes of Health (NIH). Drs. Lasa, Raymond, Topjian, Sutton, and Morgan are volunteers with the American Heart Association Emergency Cardiovascular Care Committee and serve as writing group members for pediatric resuscitation guidelines. Dr. Raymond reports being a paid consultant of New England Research Institutes, as a member of the adjudication committee for the COMPASS trial. Drs. Himebauch and Morgan received support for article research from the NIH. Drs. Reeder, Alvey, and Race’s institution received funding from the Children’s Hospital of Philadelphia. Dr. Slovis received funding from GlaxoSmithKline. Dr. Topjian received funding for expert testimony; Their institution received funding from the NIH. Dr. Sutton’s institution received funding from the National Heart, Lung, and Blood Institute.
Footnotes
The remaining authors have disclosed that they do not have any potential conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
REFERENCES
- 1.Holmberg MJ, Ross CE, Fitzmaurice GM, et al. : Annual incidence of adult and pediatric in-hospital cardiac arrest in the United States. Circ Cardiovasc Qual Outcomes 2019; 12:1–8 [PMC free article] [PubMed] [Google Scholar]
- 2.Hamzah M, Othman HF, Almasri M, et al. : Survival outcomes of in-hospital cardiac arrest in pediatric patients in the USA. Eur J Pediatr 2021; 180:2513–2520 [DOI] [PubMed] [Google Scholar]
- 3.Holmberg MJ, Wiberg S, Ross C, et al. : Trends in survival after pediatric in-hospital cardiac arrest in the United States. Circulation 2019; 140:1398–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Girotra S, Spertus JA, Li Y, et al. ; American Heart Association Get With the Guidelines–Resuscitation Investigators: Survival trends in pediatric in-hospital cardiac arrests an analysis from get with the guidelines-resuscitation. Circ Cardiovasc Qual Outcomes 2013; 6:42–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bembea MM, Ng DK, Rizkalla N, et al. : Outcomes after extracorporeal cardiopulmonary resuscitation of pediatric in-hospital cardiac arrest: a report from the get with the guidelines-resuscitation and the extracorporeal life support organization registries. Crit Care Med 2019; 47:e278–e285 [DOI] [PubMed] [Google Scholar]
- 6.Lasa JJ, Rogers RS, Localio R, et al. : Extracorporeal cardiopulmonary resuscitation (E-CPR) during pediatric in-hospital cardiopulmonary arrest is associated with improved survival to discharge. Circulation 2016; 133:165–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esangbedo ID, Brunetti MA, Campbell FM, et al. : Pediatric extracorporeal cardiopulmonary resuscitation: a systematic review. Pediatr Crit Care Med 2020; 21:e934–e943 [DOI] [PubMed] [Google Scholar]
- 8.Morris MC, Wernovsky G, Nadkarni VM: Survival outcomes after extracorporeal cardiopulmonary resuscitation instituted during active chest compressions following refractory inhospital pediatric cardiac arrest*. Pediatr Crit Care Med 2004; 5:440–446 [DOI] [PubMed] [Google Scholar]
- 9.Thiagarajan RR, Laussen PC, Rycus PT, et al. : Extracorporeal membrane oxygenation to aid cardiopulmonary resuscitation in infants and children. Circulation 2007; 116:1693–1700 [DOI] [PubMed] [Google Scholar]
- 10.Guerguerian A-M, Sano M, Todd M, et al. : Pediatric extracorporeal cardiopulmonary resuscitation ELSO guidelines. ASAIO J 2021; 67:229–237 [DOI] [PubMed] [Google Scholar]
- 11.Holmberg MJ, Geri G, Wiberg S, et al. ; International Liaison Committee on Resuscitation’s (ILCOR) Advanced Life Support and Pediatric Task Forces: Extracorporeal cardiopulmonary resuscitation for cardiac arrest: A systematic review. Resuscitation 2018; 131:91–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sperotto F, Saengsin K, Danehy A, et al. : following ECPR in pediatric cardiac patients: Planning for an interventional trial. Resuscitation 2021; 167:12–21 [DOI] [PubMed] [Google Scholar]
- 13.Topjian AA, Raymond TT, Atkins D, et al. : 2020 American Heart Association Guidelines for cardiopulmonary resuscitation and emergency cardiovascular care, circulation part 4: Pediatric basic and advanced life support resuscitation and emergency cardiovascular care. Circulation 2020; 142:469–523 [DOI] [PubMed] [Google Scholar]
- 14.Soar J, MacOnochie I, Wyckoff MH, et al. : 2019 International consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations: Summary from the basic life support; advanced life support; pediatric life support; neonatal life support; education, implementation, and teams; and first aid task forces. Circulation 2019; 140:e826–e880 [DOI] [PubMed] [Google Scholar]
- 15.Soar J, Böttiger BW, Carli P, et al. : European Resuscitation Council Guidelines 2021: Adult advanced life support. Resuscitation 2021; 161:115–151 [DOI] [PubMed] [Google Scholar]
- 16.Rice-townsend SE, Brogan TV, Digeronimo RJ, et al. : Characteristics of pediatric non-cardiac eCPR programs in United States and Canadian hospitals: A cross-sectional survey. J Pediatr Surg 2022; 57:23. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen D-A, De Mul A, Hoskote AU, et al. ; PALISI, ESPNIC, ANZICS PSG: Factors associated with initiation of extracorporeal cardiopulmonary resuscitation in the pediatric population: An international survey. ASAIO J 2022; 68:413–418 [DOI] [PubMed] [Google Scholar]
- 18.Nolan JP, Berg RA, Andersen LW, et al. ; Utstein Collaborators: Cardiac arrest and cardiopulmonary resuscitation outcome reports: Update of the utstein resuscitation registry template for in-hospital cardiac arrest: A consensus report from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia). Resuscitation 2019; 144:166–177 [DOI] [PubMed] [Google Scholar]
- 19.Pollack BE, Barbaro RP, Gorga SM, et al. : Hospital ECMO capability is associated with survival in pediatric cardiac arrest. Resuscitation 2023; 188:109853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haukoos JS, Lewis RJ: The propensity score. JAMA 2015; 314:1637–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chesnaye NC, Stel VS, Tripepi G, et al. : An introduction to inverse probability of treatment weighting in observational research. Clin Kidney J 2022; 15:14–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wengenmayer T, Rombach S, Ramshorn F, et al. : Influence of low-flow time on survival after extracorporeal cardiopulmonary resuscitation (eCPR). Crit Care 2017; 21:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matos RI, Watson RS, Nadkarni VM, et al. ; American Heart Association’s Get With The Guidelines–Resuscitation (Formerly the National Registry of Cardiopulmonary Resuscitation) Investigators: Duration of cardiopulmonary resuscitation and illness category impact survival and neurologic outcomes for in-hospital pediatric cardiac arrests. Circulation 2013; 127:442–451 [DOI] [PubMed] [Google Scholar]
- 24.Bitti JA, Yulei H: Bayesian analysis: a practical approach to interpret clinical trials and create clinical practice guidelines. Circ Cardiovasc Qual Outcomes 2017; 10:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yarnell CJ, Abrams D, Baldwin MR, et al. : Personal view clinical trials in critical care: can a Bayesian approach enhance clinical and scientific decision making?. Lancet Respir 2022; 9:207–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laussen PC, Guerguerian AM: Establishing and sustaining an ECPR program. Front Pediatr 2018; 6:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sawyer T, Burke C, Mcmullan DM, et al. : Impacts of a pediatric extracorporeal cardiopulmonary resuscitation (ECPR) simulation training program. Acad Pediatr 2019; 19:566–571 [DOI] [PubMed] [Google Scholar]
- 28.Sperotto F, Saengsin K, Danehy A, et al. : Modeling severe functional impairment or death following ECPR in pediatric cardiac patients: Planning for an interventional trial. Resuscitation 2021; 167:12–21 [DOI] [PubMed] [Google Scholar]
- 29.Hsu CH, Couper K, Nix T, et al. ; Advanced Life Support and Paediatric Life Support Task Forces at the International Liaison Committee on Resuscitation (ILCOR): Calcium during cardiac arrest: A systematic review. Resusc Plus 2023; 14:100379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cashen K, Reeder RW, Ahmed T, et al. ; for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network (CPCCRN) and National Heart Lung and Blood Institute ICU-RESUScitation Project Investigators: Sodium bicarbonate use during pediatric cardiopulmonary resuscitation: A secondary analysis of the ICU-RESUScitation Project Trial*. Pediatr Crit Care Med 2022; 23:784–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Srinivasan V, Morris MC, Helfaer MA, et al. ; American Heart Association National Registry of CPR Investigators: Calcium use during in-hospital pediatric cardiopulmonary resuscitation: A report from the national registry of cardiopulmonary resuscitation. Pediatrics 2008; 121:e1144–e1151 [DOI] [PubMed] [Google Scholar]
- 32.Ortmann L, Prodhan P, Gossett J, et al. ; American Heart Association's Get With the Guidelines–Resuscitation Investigators: Outcomes after in-hospital cardiac arrest in children with cardiac disease a report from get with the guidelines-resuscitation. Circulation 2011; 124:2329–2337 [DOI] [PubMed] [Google Scholar]
- 33.Lasa JJ, Jain P, Raymond TT, et al. : Extracorporeal cardiopulmonary resuscitation in the pediatric cardiac population: In search of a standard of care. Pediatr Crit Care Med 2018; 19:125–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farhat A, Ling RR, Jenks CL, et al. : Outcomes of pediatric extracorporeal cardiopulmonary resuscitation: A systematic review and meta-analysis. Crit Care Med 2021; 49:682–692 [DOI] [PubMed] [Google Scholar]
- 35.Barbaro R, Paden M, Guner Y, et al. : Pediatric extracorporeal life support organization registry international report 2016. ASAIO J 2017; 63:456–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fiser RT, Morris MC: Extracorporeal cardiopulmonary resuscitation in refractory pediatric cardiac arrest. Pediatr Clin North Am 2008; 55:929–941 [DOI] [PubMed] [Google Scholar]
- 37.Kane DA, Thiagarajan RR, Wypij D, et al. : Rapid-response extracorporeal membrane oxygenation to support cardiopulmonary resuscitation in children with cardiac disease. Circulation 2010; 122:S241–S248 [DOI] [PubMed] [Google Scholar]
- 38.Sood N, Sangari A, Goyal A, et al. : Predictors of survival for pediatric extracorporeal cardiopulmonary resuscitation: A systematic review and meta-analysis. Med (United States) 2022; 101:e30860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taeb M, Levin AB, Spaeder MC, et al. : Comparison of pediatric cardiopulmonary resuscitation quality in classic cardiopulmonary resuscitation and extracorporeal cardiopulmonary resuscitation events using video review*. Pediatr Crit Care Med 2018; 19:831–838 [DOI] [PubMed] [Google Scholar]
- 40.Lauridsen KG, Lasa JJ, Raymond TT, et al. : Association of chest compression pause duration prior to E-CPR cannulation with cardiac arrest survival outcomes. Resuscitation 2022; 22:1–8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


