Abstract
As the incorporation of heterocycles increases the physical characteristics and biological activity of pharmacological molecules, heterocyclic scaffolds are commonly discovered as common cores in a wide spectrum of biologically active drugs. In the contemporary context, many heterocycles have arisen, playing vital roles in diverse pharmaceutical compounds that benefit humanity. Over 85 % of FDA-approved medication molecules contain heterocycles, and most importantly, numerous heterocyclic medicinal molecules indicate potential benefits against a range: of malignancies. The unique flexibility and dynamic core scaffold of these compounds have aided anticancer research. These medications are used to treat cancer patients by targeting particular genes, enzymes, and receptors. Aside from the drugs that are now on the market, numerous forms are being researched for their potential anti-cancer activity. Here in this review, we classified some molecules and biologically active heterocycles containing anticancer medicinal moieties approved by the FDA between 2019 and 2021 based on their use in various forms of cancer. We will focus on those that are suitable for cancer treatment, as well as the essential biochemical mechanisms of action, biological targets, synthetic methods, and inherent limiting considerations in their use.
List of Abbreviations
- ABCB1
ATP Binding Cassette Subfamily B Member 1
- ABCG2
ATP Binding Cassette Subfamily G Member 2
- ABL
Abelson
- ADC
Antibody-drug conjugate
- ADCC
Antibody-dependent cellular cytotoxicity
- ADCP
Antibody-dependent cellular phagocytosis
- AKT
Ak strain transforming
- ALT
Alanine aminotransferase
- AML
Acute myeloid leukaemia
- AR
Androgen receptor
- AST
Aspartate aminotransferase
- BCMA
B-cell maturation antigen
- BCR
B-cell antigen receptor
- BLK
B-lymphoid tyrosine kinase
- BRAF
v-raf murine sarcoma viral oncogene homolog B1
- BTK
Bruton tyrosine kinase
- CCDC6-RET
Coiled Coil Domain Containing 6- Rearrange:d during transfection
- CCL19
Chemokine ligand 19
- CD79b
Cluster of differentiation 79B
- CDA
Cytidine deaminase
- CDK
Cyclin-dependent kinase
- CK1ε
Casein kinase 1 epsilon
- CMML
Chronic myelomonocytic leukaemia
- CSF1R
Colony Stimulating Factor 1 Receptor
- CXCL12
C-X-C motif chemokine 12
- DDR1
Discoidin domain receptor 1
- DLBCL
Diffuse large B-cell lymphoma
- DXd
Derivative of exatecan or DX-8951 derivative
- ECOG
Eastern Cooperative Oncology Group
- EGFR
Epidermal growth factor receptor
- ERBB
Erythroblastic Oncogene B
- ERK
Extracellular signal-related kinase
- EZH2
enhancer of zeste homolog 2
- FDC
Fixed dose oral combination
- FGFR
Fibroblast growth factor receptor
- FLT
Fms Related Receptor Tyrosine Kinase
- FR
Folate receptor
- FSH
Follicle-stimulating hormone
- GnRH
Gonadotropin-releasing hormone
- HER
Human epidermal growth factor receptor
- HPMC
Hydroxypropyl methylcellulose
- HSPCs
Hematopoietic stem and progenitor cells
- IgG1
Immunoglobulin G1
- JAK2
Janus Associated Kinase 2
- KIF5B-RET
Kinesin family member 5B- Rearrange:d during transfection
- KRAS
Kirsten rat sarcoma viral oncogene homolog
- LH
Luteinizing hormone
- mAb
Monoclonal antibody
- MAPK
Mitogen-activated protein kinase
- mc-vc-PAB
-valine-citrulline-p-aminobenzyloxycarbonyl
- MCL
Mantle cell lymphoma
- MDS
Myelodysplastic syndromes
- MEK
Mitogen-activated protein kinase
- MET exon 14
Mesenchymal epithelial transition factorexon 14
- MF
Myelofibrosis
- MMAE
Monomethyl auristatin E
- MMAF
Monomethyl auristatin F
- MTC
Metastatic medullary thyroid cancer
- mTNBC
Metastatic triple-negative breast cancer
- MZL
Marginal zone lymphoma
- NETs
Neuro-endocrine tumors
- NF1
Neurofibromatosis type 1
- NSCLC
Non-small cell lung cancer
- NTRK
Neurotrophic tyrosine receptor kinase
- PD-1
Programmed Cell Death Protein 1
- PDGFR
Platelet-derived growth factor receptor
- PDGFRA D842V
platelet-derived growth factor alpha D842V
- PDL1
Programmed death-ligand 1
- PET
Positron emission tomography
- PI3K
Phosphatidylinositol 3-kinase
- PI3Kα
Phosphatidylinositol-3-kinase alpha
- PIK3CA
Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha
- PR
Progesterone receptor
- PSMA
Prostate-specific membrane antigen
- RAF
Rapidly Accelerated Fibrosarcoma
- RAS
Rat Sarcoma
- RCC
Renal cell carcinoma
- RET
Rearrange:d during transfection
- ROS1
c-ros oncogene 1
- RRMM
Relapsed or refractory multiple myeloma
- SSTR2
Somatostatin receptor 2
- STAT
Signal transducer and activator of transcription
- SN-38
7-ethyl-10-hydroxy-camptothecin
- TIE2
Tyrosine kinase with immunoglobulin and epidermal growth factor homology domains 2
- TRKA
Tropomyosin Receptor Kinase A
- Trop-2
Trophoblast cell-surface antigen-2
- TSP
Thrombospondin
- TYK
Tyrosine Kinase
- VEGFR
Vascular endothelial growth factor receptor
- XPO1
Exportin-1
Introduction
In organic chemistry, heterocyclic chemistry is considered a prominent area, and a large number of current publications in organic chemistry are reported in this field. Natural products, including carbohydrates, alkaloids (viz., reserpine, atropine and morphine), amino acids, proteins, nucleic acids, hemoglobin, hormones, enzyme co-factors, and, vitamins are mostly made up of heterocycles. The biologically significant polymeric molecules RNA (Ribonucleic acid) and DNA (Deoxyribonucleic acid) periodically contain heterocyclic scaffolds consisting of nitrogenous bases like adenine, guanine, cytosine, uracil, and thymine. The crucial roles of such heterocycles pretty much in all aspects of biochemical processes, such as the transmission of nerve impulses across the body, various chemical reactions for the provision of energy, transfer of genetic information, and vision, as well as in the metabolism of every single one of the living beings, which are essential to sustain lives, make the evolution of the biochemistry frequently revolve around imitating such structural characteristics [1,2]. Additionally, there are various other uses of heterocycles as chiral auxiliaries, synthetic intermediates, fertilizers, pesticides, organic catalysts, protecting groups, corrosion inhibitors, antioxidants, pigments, copolymers, metal ligands in asymmetric catalytic inorganic synthesis, disinfectants, and medicines [[3], [4], [5]]. When it comes to the pharmaceutical industry, heterocyclic molecules are of great significance. The core structures of a broad range: of drug categories are heterocyclic compounds with diverse biological activities, including anti-inflammatory, antiviral, antibacterial, antihistaminic, anti-Parkinson's disease, antiepileptic, analgesic, immunomodulatory agents, antimalarial, anti-obesity, local anaesthetic, antimicrobial, antianxiety, anti-diabetic, antidepressant, antioxidant, antineoplastic, anticonvulsant, antifungal, antihypertensive, antitubercular, antitumor, and anti-cancer [[4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16]]. All the top five small molecule drug brands by retail sales worldwide in 2022 contain active ingredients with heterocyclic moieties, and the total revenue obtained from these five drugs was around 66 billion U.S. dollars (Fig. 1) [17].
Fig. 1.
The active ingredients containing heterocyclic fragments of the top 5 small molecule drug brands by retail sales in 2022 worldwide.
Heterocyclic motifs often exist in several drug molecules because of the engagement of numerous intermolecular interactions, such as hydrogen bond donor/acceptor capability, metal co-ordination bonds, van der Waals, pi-stacking interactions, and hydrophobic forces, which interactions help the drug molecules to effectively bind target enzymes and receptors in a multitude of ways [2,18]. The type or size of the ring of heterocycles, along with the substituted groups, as well as structural permutations, enable them to interact with correspondingly different structural arrangements of enzyme binding pockets, which strongly influence the physicochemical properties and consequently, the pharmacological characteristics of the molecules.
Cancer, defined by abnormal cell growth that can attack the function of the organs, is one of the most fatal diseases with a high mortality rate—nearly 10 million deaths as reported in 2020 [[19], [20], [21]]. The generic term cancer is also referred to as benign and malignant tumors. It is a disease where a broad class of disorders can affect any region of the body with the quick development of aberrant cells that proliferate outside of their normal borders and have the potential to infiltrate nearby bodily components and eventually expand to other organs. The spreading of cancer to other organs is because of metastasis and widespread metastases are regarded as the primary reason for cancer deaths. There are various kinds of cancers according to the location of the cancer cells such as lung cancer, GIST (gastrointestinal stromal tumors), lymphoma, myeloma, ovarian cancer, prostate cancer, breast cancer, bladder cancer, renal cell carcinoma, epithelioid sarcoma and so on. According to the number of active pharmaceuticals in the globe as of 2022, among the top 10 diseases, cancers take up eight spots, and even the top two out of the top three diseases are cancers: breast cancer (888 medications) and non-small cell lung cancer (832 medications), respectively [22]. A multi-stage process that often moves from a pre-cancerous lesion to a malignant tumor, results in the change of normal cells into tumour cells, which is how cancer develops. These modifications come about as a result of a person's genetic makeup interacting with three different types of external factors [23].
-
(A)
chemical carcinogens, like aflatoxin (a family of toxins or fungal contaminants in food), components of tobacco smoke, arsenic (a drinking water contaminant), asbestos, and alcohol;
-
(B)
physical carcinogens, namely ionizing radiation and ultraviolet; and (C) biological carcinogens—for example, infections from certain bacteria, viruses, or parasites.
Taking into consideration all ages, both genders, and all types of cancers, the total number of new cancer patients in 2020, is estimated to be 19,292,789, among which 9,503,710 (49.3 %) people, i.e., most of the people, belong to Asia continent (Fig. 2) [24]. Among the new cases of cancer, breast cancer (2,261,419; Crude rate/100,000: 58.5), lung cancer (2,206,771; Crude rate/100,000: 28.3), colorectum cancer (1,931,590; Crude rate/100,000: 24.8), and prostate cancer(1,414,259; Crude rate/100,000: 36.0) are predominant (Fig. 3) [[24], [25], [26]]. The statistics of incidence and mortality rates in 2020 show that, in the case of males, lung cancer has a high incidence rate as well as a very high death rate (Fig. 4). However, though prostate and colorectum cancers have a high incidence rate, the mortality rates are comparatively low.
Fig. 2.
The worldwide estimated number of new incidents of all cancers, distributed by region, considering all ages and both genders in 2020.
Fig. 3.
Worldwide crude incidence rates by type of cancer in all ages and both genders in 2020 (Excluding Non-melanoma skin cancers).
Fig. 4.
Comparison of the crude incidence and mortality rates of males and females of all ages in the world in 2020 (Excluding Non-melanoma skin cancers).
The incidence rates of the stomach, liver, and esophagus cancers are not so high, but these cancers have high death rates compared to the incidence rates. Analysing the data on female cancer patients in 2020, it can be deduced that breast cancer is the most common cancer discovered in females and causes the highest number of deaths among females. Besides that, the colorectum, lung, and cervix uteri are also usually observed in females. However, the incident cases along with mortality cases of lung cancer are scarce among females with respect to males.
The treatments of cancer involve clinical trials, chemotherapy, hormone therapy, personalized and targeted therapies, surgery, maintenance therapy, bone marrow/stem cell transplantation, immunotherapy and vaccination, radiation therapy and integrative medication [27]. Several medications containing biotech and small molecules as the main ingredients were employed at various stages of therapy. Some medications can also be administered intravenously for diagnostic purposes. The main goal of these anticancer agents is to eliminate tumor cells and restrict metastasis via inhibiting phosphorylation, signalling and proliferation. These anticancer agents interact with various genes, enzymes, and receptors such as EGFR; Folate receptor; PSMA; KRAS; MET; VEGFR-1,2,3,4; PDGFR; CDK-4/6; PI3Kα; PI3Kδ; CK1ε; PDGFRA; BTK; XPO1; FGFR1,2,3,4 etc. and govern their activities to inhibit the signalling and tumor cell division. In cancer therapy and medications, heterocycle moieties play an important role in drug design due to their biological activity and versatility. Especially oxygen, nitrogen and sulphur-containing heterocyclic scaffolds were found in these types of anticancer drugs [28,29]. Development and manufacturing of effective anticancer medicines have always been the core of interest for numerous research organisations. Previously various review articles regarding prescribed heterocyclic drug molecules [30] and FDA-approved drug molecules [31] were published. In this review, we addressed various heterocycle-containing biotech and small molecule drugs with anti-cancer properties approved by the FDA from 2019 to 2021, as summarised in Table 1, and discussed about their synthetic procedures and mechanisms of action as well as their adverse effects.
Table 1.
An Illustrative List of Approved Anti-Cancer Drugs that are discussed in this review.
| No. | Drug Name | Active Ingredients | Company | Target | FDA Approval Date | Clinical Applications |
|---|---|---|---|---|---|---|
| 1 | Piqray | Alpelisib | Novartis | PI3K | May 24, 2019 | To treat breast cancer |
| 2 | Enhertu | Fam-trastuzumab deruxtecan-nxki | Daiichi Sankyo Company Ltd. and AstraZeneca | HER2 | December 20, 2019 | |
| 3 | Tukysa | Tucatinib | Array BioPharma | HER2 and HER3 | April 17, 2020 | |
| 4 | Trodelvy | Sacituzumab govitecan-hziy | Immunomedics | Topoisomerase I inhibitor | April 22, 2020 | |
| 5 | Rozlytrek | Entrectinib | Genentech, Inc. | NTRK | August 15, 2019 | To treat patients with NSCLC |
| 6 | Tabrecta | Capmatinib | Novartis Oncology | c-Met/HGFR | May 6, 2020 | |
| 7 | Gavreto | Pralsetinib | Blueprint Medicines Corporation | RET | September 04, 2020 | |
| 8 | Tepmetko | Tepotinib | Merck | MET exon 14 skipping mutations | March 25, 2020 | |
| 9 | Exkivity | Mobocertinib | Takeda Pharmaceutical Company Limited | EGFR | September 15, 2021 | |
| 10 | Zepzelca | Lurbinectedin | Pharma Mar, S.A. | Alkylating drug | June 15, 2020 | To treat patients with SCLC |
| 11 | Cosela | Trilaciclib | G1 Therapeutics | CDK4 and CDK6 | February 12, 2021 | |
| 12 | Nubeqa | Darolutamide | Orion and Bayer | Androgen receptor (AR) andprogesterone receptor (PR) | July 30, 2019 | To treat patients with prostate cancer |
| 13 | Orgovyx | Relugolix | Myovant Sciences | GnRH | December 18, 2020 | |
| 14 | Lumakras | Sotorasib | Amgen | KARSG12C | March 28, 2021 | To treat patients with Ovarian cancer |
| 15 | Cytalux | Pafolacianine | On Target Laboratories, Inc. | Folate receptors | November 29, 2021 | To detect ovarian cancer lesions |
| 16 | Retevmo | Selpercatinib | Loxo Oncology | VEGFR1 and VEGFR3 | May 08, 2020 | Use in the treatment of Lung and Thyroid Cancers |
| 17 | Ayvakit | Avapritinib | Blueprint Medicines Corporation | PDGFRA, PDGFRA D842 mutants, KIT exon 11, 11/17 and 17 mutants | January 09, 2020 under | To treat patients with GIST |
| 18 | Qinlock | Ripretinib | Deciphera Pharmaceuticals, Inc. | KIT and PDGFRA mutants | May 15, 2020 | |
| 19 | Fotivda | Tivozanib | AVEO Oncology | VEGFR-1, VEGFR-2 and VEGFR-3 | March 10, 2021 | To treat patients with renal cell carcinoma |
| 20 | Balversa | Erdafitinib | Janssen Pharmaceutical Companies | FGFR1, 2, 3 and 4 | April 12, 2019 | To treat patients with Bladder cancer |
| 21 | Padcev | Enfortumab vedotin-ejfv | Astellas Pharma Support SolutionsSM | Nectin-4-expressing cells | December 13, 2019 | |
| 22 | Polivy | Polatuzumab vedotin-piiq | Genentech | CD79b | June 10, 2019 | To treat patients with lymphoma |
| 23 | Brukinsa | Zanubrutinib | BeiGene | Cysteine residue in Bruton tyrosine kinase (BTK) active site | November 14, 2019 | |
| 24 | Ukoniq | Umbralisib | TG Therapeutics | PI3Kδ and CK1ε kinase | February 5, 2021 | |
| 25 | Xpovio | Selinexor | Karyopharm Therapeutics | XPO1 inhibitor | July 03, 2019 | To treat patients with Myeloma |
| 26 | Blenrep | Belantamab mafodotin-blmf | GlaxoSmithKline | BCMA | August 05, 2020 | |
| 27 | Pemazyre | Pemigatinib | Incyte Corporation | FGFR1, FGFR2, FGFR3 and FGFR4 | April 17, 2020 | To treat adult patients with cholangiocarcinoma |
| 28 | Tazverik | Tazemetostat | Epizyme, in collaboration with Eisai | EZH2 | January 23, 2020 | To treat patients with epithelioid sarcoma |
| 29 | Inrebic | Fedratinib | Celgene Corporation | JAK2 | August 16, 2019 | To treat patients with myelofibrosis |
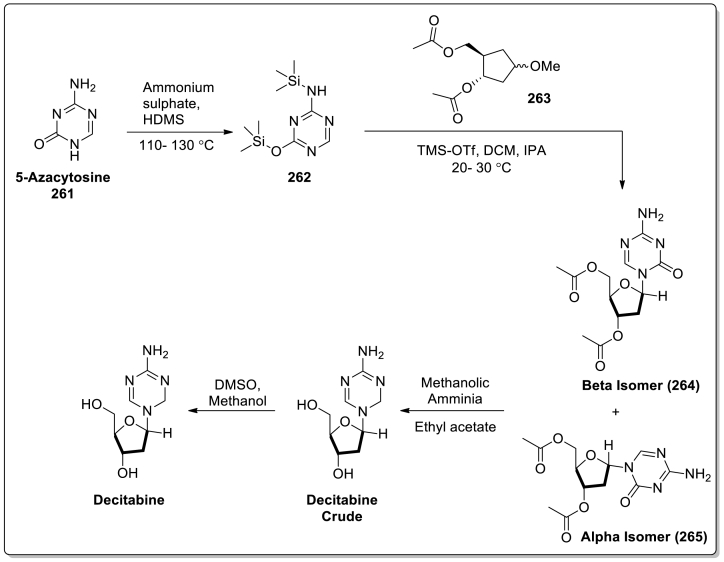
| 30 | Inqovi | Decitabine, andCedazuridine | Astex Pharmaceuticals | Nucleoside metabolic inhibitor | July 7, 2020 | To treat patients with MDS |
| 31 | Koselugo | Selumetinib | AstraZeneca | MEK1/2 | April 10, 2020 | To treat patients with NF1 |
| 32 | Pylarify | Piflufolastat F 18 | Progenics Pharmaceuticals Inc. | PSMA | May 27, 2021 | Diagnosis of prostate cancer |
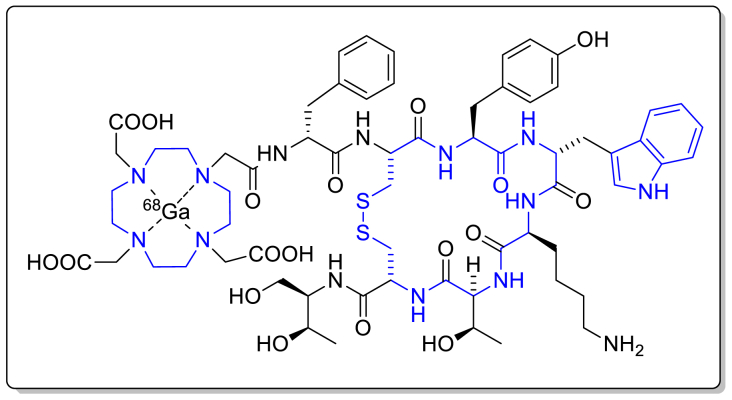
| 33 | Ga-68-DOTATOC | Ga-68-DOTATOC | Evergreen Theragnostics, Inc. | Somatostatin receptors | August 21, 2019 | Diagnosis of NETs |
| 34 | Detectnet | Copper Cu-64 Dotatate Injection | RadioMedix, and Curium | somatostatin receptors | September 03, 2020 |
1. Breast cancer
1.1. Active ingredient name: alpelisib
Drug Brand Name: Piqray.
Background and Mechanism of Action: Alpelisib, under the brand name PIQRAY, was developed by Novartis and was approved by the FDA on 24th May 2019 for the treatment of metastatic or advanced breast cancer [32,33]. It is an orally accessible phosphatidylinositol 3-kinase (PI3K) inhibitor that shows antitumor activity. It mainly targets phosphatidylinositol-3-kinase alpha (PI3Kα), a catalytic subunit of PI3K which is responsible for proliferation, survival, differentiation and metabolism. Alpelisib and Fulvestrant were combinedly used in the treatment of patients with HER2-negetive, HR-positive, PIK3CA-mutated metastatic or advanced breast cancer. Alpelisib primarily inhibits the activity of phosphatidylinositol-3 kinase alpha (PI3Kα) [33]. It is important to avoid coadministration of alpelisib with CYP3A4 inducers or BCRP inhibitors, because for 1st case, the concentration of alpelisib decreases which effects its activity and for the second case, the concentration of alpelisib increases which may increase its adverse effects. Alpelisib primarily inhibits the activity of phosphatidylinositol-3 kinase alpha (PI3Kα). In vitro and in vivo models, the critical lipid kinase PI3Kα was activated via the gene encoding of α-subunit of PI3K which led to the activation of Akt-signaling, cellular transformation and formation of tumors. Alpelisib restricts phosphorylation of PI3K downstream as well as Akt and shows activity in PIK3CA mutate cell lines. In xenograft models, it was found that, Alpelisib repressed the PI3K/Akt signalling and restricted tumor growth [33].
Structure.

Molecular Formula: C19H22F3N5O2S
IUPAC name: (2S)–N1-[4-Methyl-5-[2-(2,2,2-trifluoro-1,1-dimethylethyl)-4-pyridinyl]-2-thiazolyl]-1,2-pyrrolidinedicarboxamide.
Type: Small molecule.
Dosage: Patients have to take 300 mg of PIQRAY (two 150 mg tablets), orally without chewing, crushing or splitting the tablets, once every day with food until disease progression or unacceptable toxicity [33]. Patients have to take a missed dose within 9 h of its actual time otherwise it is better to skip that dose for that day. Patients were advised not to take an additional dose of PIQRAY after vomiting occurs.
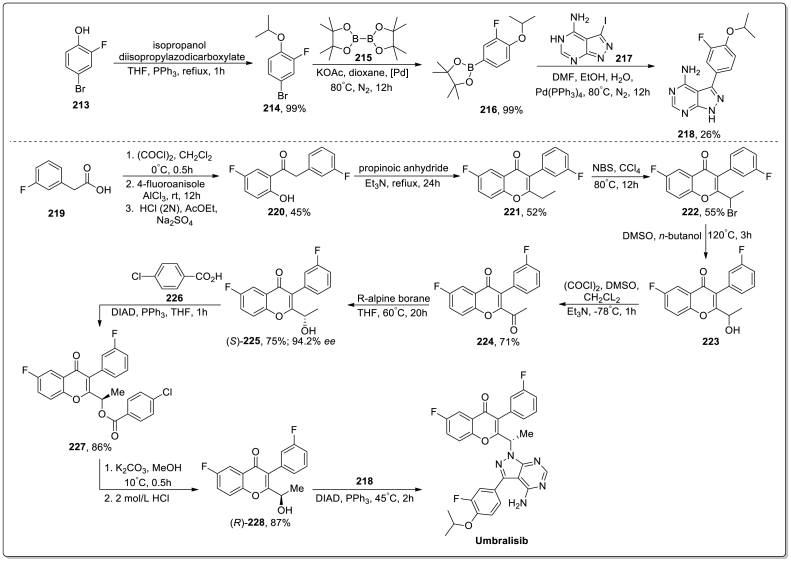
Synthesis: The synthesis of Alpelisib was discussed in Scheme 1 [34]. Initially, compound 1 was reacted with oxalyl chloride in reflux condition to give the acid chloride 2 which underwent cycloaddition reaction with (E)-4-methoxy-3-buten-2-one (3) in presence of LiHMDS/THF to obtain compound 4. Next, compound 4 reacted with aqueous ammonia at 65 °C to produce compound 5 which gave 4-bromopyridine derivative 6 via the reaction with Phosphoryl bromide in DCM. After that, a cross-coupling reaction occurred between 6 and 2-acetamido-4-methylthiazole (7) in the presence of palladium catalyst and gave the compound 8 which went through hydrolysis process to produce compound 9. At last, compound 9 was treated with carbonyldiimidazole (CDI) to get intermediate 10 which underwent substitution reaction with l-prolineamide to give the desired product Alpelisib in 87 % yield.
Scheme 1.
Synthesis of Alpelisib.
Adverse effects: The safety information of PIQRAY was elaborated after it was administered in 571 patients having HER2-negative, HER2-positive and advanced or metastatic breast cancer [33]. Patients were divided into two different group for the medication process. First group of 284 patients were treated with 300 mg of PIQRAY plus fulvestrant and another group of 287 patients were treated with placebo plus fulvestrant.
Two patients who received the dose of PIQRAY plus fulvestrant, died due to cardio-respiratory arrest (1) and second primary malignancy (1). 35 % of patients who received the dose of PIQRAY plus fulvestrant, experienced serious adverse effect included diarrhea (2.8 %), abdominal pain (2.1 %), rash (3.5 %), hyperglycemia (10 %), kidney injury (2.5 %) and anemia (2.1 %). Among patients receiving PIQRAY with fulvestrant, 4.6 % discontinued both PIQRAY and fulvestrant, whereas 21 % discontinued PIQRAY alone due to adverse effects. ARs caused dose reductions in 55 % of individuals taking PIQRAY with fulvestrant.
Some common adverse effects [33] (≥20 %) are creatinine increased; rash; diarrhea; glucose increased; lymphocyte count decreased; nausea; fatigue; GGT increased; ALT increased; aPTT prolonged; hemoglobin decreased; lipase increased; vomiting; decreased appetite; weight decreased; glucose decreased; calcium decreased, and alopecia.
1.2. Active ingredient name: fam-trastuzumab deruxtecan-nxki
Drug Brand Name: Enhertu.
Background and Mechanism of Action:
On December 20, 2019, Fam-trastuzumab deruxtecan-nxki (ENHERTU®), manufactured and marketed by Daiichi Sankyo, Inc., received accelerated approval from the FDA in the USA for the treatment in adults who are suffering from HER2-positive breast cancer that cannot be eliminated via surgery (unresectable) or has expanded to other bodily organs (metastatic) [35,36]. This drug should be administered to those patients who have already been gone through the treatment for metastatic HER2-derived breast cancer with anti-HER2-based regimens more than two times.
Fam-trastuzumab deruxtecan-nxki, a conjugate of DNA topoisomerase I inhibitor, as well as an antibody triggered by human epidermal growth factor receptor 2 (HER2), is being globally developed and commercialised as ENHERTU® by Daiichi Sankyo, Inc. in collaboration with AstraZeneca to treat HER2-induced solid tumors including gastric cancer, non-small cell lung cancer, colorectal cancer, and breast cancer [35,36]. As an antibody-drug conjugate (ADC), Fam-trastuzumab deruxtecan-nxki contains three components [36].
-
I.
a derivative of exatecan, DXd, a topoisomerase inhibitor;
-
II.
an anti-HER2, humanized Immunoglobulin G1 (IgG1) monoclonal antibody (mAb);
-
III.
a protease-cleavable and tetrapeptide-based maleimide linker, which covalently links the topoisomerase inhibitor with the anti-HER2 IgG1mAb.
The topoisomerase inhibitor, DXd and the tetrapeptide-based maleimide linker are collectively designated as Deruxtecan. Each antibody molecule contains approximately 8deruxtecan molecules. The linker and the topoisomerase inhibitor are obtained through chemical synthesis, while the generation of the antibody takes place in Chinese hamster ovary cells with the help of recombinant DNA technology.
ENHERTU® (fam-trastuzumab deruxtecan-nxki) is sterile and each single-dose vial of preservative-free lyophilized white to yellowish white-powdered ENHERTU contains 100 mg offam-trastuzumab deruxtecan-nxki.
Fam-trastuzumab deruxtecan-nxki attaches to HER2 on the tumor cells, which results in the internalization followed by intracellular linker cleavage of Fam-trastuzumab deruxtecan-nxki due to the effect of lysosomal enzymes [36]. In addition, the release of membrane-permeable DXd leads to DNA-damage and the death of apoptotic cells.
Structure.
Type: Biotech.
Dosage: ENHERTU® should only be used as an intravenous infusion and is forbidden to be applied as an intravenous bolus or push [36]. Also, it is advised to avoid using ENHERTU in combination with or in place of adotrastuzumab emtansine or trastuzumab. 5.4 mg/kg of ENHERTU is recommended to use once every three weeks of a 21-day cycle until unacceptable toxicity or disease progression.
Adverse effects:
The clinical study of ENHERTU was analyzed based on the reports of pooled 234 patients suffering from unresectable or metastatic HER2-positive breast cancer, who were given ENHERTU at the minimum dose of 5.4 mg/kg in DESTINY-Breast01 ((NCT03248492, Trial 1) and Study DS8201 A-J101 (NCT02564900, Trial 2) with a median treatment duration of 7 months (range: 0.7 to 31) [36]. The median age of the patients was 56 years (range: 28–96), among which female patients were 99.6 %, patients with age <65 years were 74 %, and 5 % of patients were 75 years of age or older. The ECOG (Eastern Cooperative Oncology Group) performance status of the patients at baseline was 0 (58 %) or 1 (42 %).
There were no discernible variations in overall efficacy between patients over 65 and younger ones. However, in the case of Grade 3-4 adverse reactions, patients 65 years of age or older (53 %) experienced more Grade 3–4 adverse responses in comparison to younger patients (42 %). 20 % of patients, who were provided ENHERTU, showed some severe adverse reactions, such as pneumonia, cellulitis, vomiting, intestinal obstruction, nausea, interstitial lung disease, and hypokalemia, and for 4.3 % of patients, the adverse reactions were fatal.
The adverse effects that are generally observed (≥20 %) [36] are as follows fatigue; cough; alopecia; thrombocytopenia; nausea; constipation; diarrhea; anemia; leukopenia; vomiting; decreased appetite; and neutropenia (Low count of white blood cells).
1.3. Active ingredient name: Tucatinib
Drug Brand Name: Tukysa.
Background and Mechanism of Action:
Tucatinib is a kinase inhibitor that was initially manufactured by Array BioPharma (a subsidiary of Pfizer) and later by Seattle Genetics, under the brand name TUKYSA™, available as 50 mg and 150 mg film-coated tablets for oral administration [37,38]. On April 17, 2020, Tucatinib received approval from the FDA in the USA and in May 2020 in Switzerland for the treatment of adults suffering from human epidermal growth factor receptor (HER)2-positive breast cancer, where it cannot be removed through surgery (advanced unresectable) or has already spread to the other body parts (metastatic), which includes brain metastases. Tucatinib should be used in those cases where the patients have already gone through the earlier treatment for metastatic disease with one or more anti-HER2-based regimens, in combination with two other medications, capecitabine and trastuzumab.
Tucatinib hampers the activation of ERBB-2 signal transduction pathways by reversibly binding to the ATP pocket of the HER2 receptor's internal domain [37,39]. Including HER2 or HER3 interactions, these signal transduction pathways are crucial for the evasion of apoptosis. In vivo, Tucatinib acts by hindering the growth of the tumors induced by HER2, whereas in vitro, by restricting the phosphorylation of HER2 and HER3, with the inhibition of downstream AKT and MAPK (mitogen-activated protein kinase) signaling and cell proliferation, Tucatinib performs its antitumor activity against HER2-induced tumor cells [38]. However, the combined use of trastuzumab and Tucatinib appears to be more fruitful, exhibiting greater anti-tumor activity in vivo and in vitro, in contrast to when either drug is applied alone.
Structure.
Molecular Formula:C26H24N8O2
Chemical Name: (N4-(4-([1,2,4]triazolo[1,5-a]pyridin-7-yloxy)-3-methylphenyl)-N6-(4,4-dimethyl-4,5-dihydrooxazol-2-yl)quinazoline-4,6-diamine.
Type: Small Molecule.
Dosage: 300 mg of TUKYSA tablet is recommended to be swallowed orally as a whole rather than crushing, chewing or splitting the tablet before swallowing, with or without food two times daily at the same time around 12 h apart, combined with capecitabine and trastuzumab until the toxicity becomes unacceptable or the illness worsens [38]. Also, the patients should be instructed not to ingest a tablet that is cracked, broken or otherwise not intact.
200 mg of TUKYSA is recommended to intake orally daily two times for the patients who are suffering from severe hepatic impairment [38].
Synthesis: There is a well-established and popular synthetic route for the preparation of Tucatinib developed by Joseph P. LYSSIKATOS and Co. from Array BioPharma Inc., United States (World Intellectual Property Organization, Patent Number-WO2007059257) [40]. However, in 2021, Yaodong Lyu et al. also proposed another different pathway for the synthesis of Tucatinib, as shown in Scheme 2 [41]. Here, the process was initiated with a two-step process. At first, the reaction between 4-chloropyridin-2-amine (11) and N,N-dimethylformamide dimethyl acetal (DMF-DMA) followed by hydroxylamine hydrochloride in the second step led to the formation of N-hydroxy-formimidamide 12 (89 % yield). Then the compound 12 converted to the triazolo[1,5-a]pyridine compound 13 (71 % yield), when reacted with trifluoroacetic acid anhydride (TFAA) in the presence of tetrahydrofuran (THF) at 60–70 °C, after reworking the reaction conditions reported by Rajaram Ayothiraman and team [42]. After that, the key intermediate 15 was obtained in 73 % yield via heating the compound 13 and commercially accessible 4-amino-2-methylphenol (14) in the presence of DMF/K2CO3 at 130–140 °C for 16 h. The heating of the compounds 15 and 16 in acetic acid resulted in the generation of the compound 17, which was then reduced via catalytic hydrogenation to provide the aniline compound 18. Next, in DMF, the compound 18 was treated with adenosine monophosphate (AMP) and 1,1′-ahiocarbonyldiimidazole (TCDI) at 5–25 °C for 4 h to afford the thiourea compound 19 in 81 % yield. In the last step, the desired product Tucatinib was obtained in 77 % yield when the compound 19 reacted with p-TsCl and NaOH in THF at the temperature of 50–60 °C for 3 h.
Scheme 2.
Synthesis of Tucatinib.
Adverse effects:
The randomized (2:1), double-blind, placebo-controlled clinical trial HER2CLIMB (NCT02614794) included 612 patients with a median age of 54 years (range: 22–82), to evaluate the potency of TUKYSA along with capecitabine and trastuzumab, among which TUKYSA plus capecitabine and trastuzumab was given to 404 patients, and the median treatment duration was 5.8 months (range: 3 days to 2.9 years) [38]. Among these patients, 116 patients (or 19 %) were 65 years of age or older. Female patients were 99 %, and ECOG performance status was 1 in 51 % cases. 26 % of patients, who were provided with TUKYSA experienced serious adverse effects, like abdominal pain, nausea, diarrhea, seizure, and vomiting, and two percent of patients experienced fatal adverse reactions. The patients ≥65 years (34 %) experienced more serious adverse reactions than the patients <65 years (24 %). Overall, there weren't any observable differences in how effective TUKYSA was in patients over 65 compared to younger ones. Unfortunately, the number of patients aged over 75 years was too low to evaluate the efficacy and safety of the treatment.
The typical adverse effects (≥20 %) [38] are abdominal pain; diarrhea; stomatitis (mouth sores); palmar-plantar erythrodysesthesia; anemia or decrease of red blood cell counts; hepatotoxicity; decreased appetite; rash; nausea; vomiting; headache; and fatigue.
1.4. Active ingredient name: sacituzumab govitecan-hziy
Drug Brand Name: Trodelvy.
Background and Mechanism of Action: On April 22, 2020, the FDA approved Sacituzumab govitecan-hziy, manufactured by Immunomedics, Inc., in the USA, to treat metastatic triple-negative breast cancer (mTNBC) in adult patients, who have already undergone at least two earlier treatments for the metastatic disease [43,44].
The active reagent of Trodelvy™, is Sacituzumab govitecan-hziy, an antibody bound to the trophoblast cell-surface antigen-2 (Trop-2) and conjugated to the drug SN-38, a topoisomerase I inhibitor [43,44]. Sacituzumab govitecan-hziy consists of three parts [44].
-
I.
a topoisomerase I inhibitor, the drug SN-38;
-
II.
the humanized monoclonal Trop-2-mediated antibody, hRS7 IgG1κ, also known as Sacituzumab;
-
III.
a hydrolysable linker, CL2A, which connects SN-38 with the humanized monoclonal antibody.
The small molecule units CL2AandSN-38 are chemically synthesized, whereas the recombinant monoclonal antibody is obtained from mammalian (murine myeloma) cells. Every antibody molecule of Sacituzumab govitecan-hziy incorporates 7–8 molecules of SN-38 on average.
Sacituzumab is considered a humanized antibody, which can perceive Trop-2 [44]. The topoisomerase I inhibitor, SN-38 is a small molecule that is connected covalently via a linker to the antibody. According to the pharmacology data [44], cancer cells triggered by Trop-2 are attached to sacituzumab govitecan-hziy, which is internalized with the liberation of SN-38 through the hydrolysis of the linker. Also, the interaction of SN-38 with topoisomerase I leads to the inhibition of the re-ligation of the single strand breaks activated by topoisomerase I and apoptosis and cell death after the damage to the resulting DNA.
Structure.
Type: Biotech.
Dosage: 10 mg/kg of TRODELVY™ is recommended to administer as an intravenous infusion only once on Day 1 and Day 8 every week of the 21-day treatment cycles, until disease progression or unacceptable toxicity [44]. Dosage more than 10 mg/kg is forbidden to administer.
Adverse effects:
Based on the information from the single arm, open-label clinical study (IMMU-132-01, NCT01631552) enrolling 408 patients who were suffering from metastatic triple-negative breast cancer (mTNBC) and other malignancies and had undergone a systemic treatment regimen for the advanced disease [44]. Among these patients, 108 patients with mTNBC who had undergone a minimum of two previous treatments were considered in a subset to study the efficacy of TRODELVY, and the median duration for the treatment was 5.1 months (range: 0–51 months). Among the patients receiving TRODELVY, 35 % (144/408) of all patients and 18 % (19/108) patients with mTNBC were ≥65 years old, the median being 55 years (range: 31–80 years). 99 % of the patients were female. There was no comprehensive difference in the effectiveness and safety of these patients compared to the younger ones.
31 % patients were affected by serious adverse reactions. The most common serious adverse reactions were diarrhea, vomiting, pleural effusion, dehydration, dyspnea, neutropenia, nausea, pneumonia, anemia and febrile neutropenia.
The commonly observed adverse effects [44] (>25 %) in patients suffering from mTNBC are vomiting; nausea; decreased appetite; diarrhea; anemia; abdominal pain; constipation; neutropenia; fatigue; rash; and alopecia.
2. Lung cancer
2. A. Non-small cell lung cancer
2. A.1. Active ingredient name: Entrectinib
Drug Brand Name: Rozlytrek.
Background and Mechanism of Action: ROZLYTREK® (Entrectinib) capsules are distributed as printed hard-shell capsules, which can be yellow opaque HPMC capsules with 100 mg Entrectinib or orange opaque HPMC capsules with 200 mg Entrectinib for oral use, and are developed by Roche to treat several solid tumors with ROS1 or NTRK1/2/3 gene fusions. Genentech, Inc., a member of the Roche group [45], is the manufacturer of Entrectinib (Rozlytrek®) in the USA, and on August 15, 2019, the FDA approves Entrectinib in the USA [46], for the treatment of.
-
I.
Adult patients who are suffering from metastatic non-small cell lung cancer (NSCLC) [47,48], which:
-
a)
is developed due to abnormal ROS1 genes; and
-
b)
has already spread to other body parts (metastatic).
-
II.
Adult and pediatric or adolescent patients (12–17 years of age) having solid tumors [47,49], which:
-
a)
are generated because of a certain neurotrophic tyrosine receptor kinase (NTRK) gene;
-
b)
are complicated to remove through surgery or are metastatic; and
-
c)
cannot be treated with other acceptable treatments, or the effects of the other treatments are counterproductive.
Entrectinib (Rozlytrek®), a white to pale pink-coloured powder, is an inhibitor of the tyrosine kinases tropomyosin receptor kinases (TRK)TRKA, TRKB, and TRKC (encoded by the neurotrophic tyrosine receptor kinase [NTRK] genes NTRK1, NTRK2, and NTRK3, respectively), anaplastic lymphoma kinase (ALK) with central nervous system (CNS) activity, and proto-oncogene tyrosine-protein c-ros oncogene 1 (ROS1) with IC50 values greater than 5 nM [50,47]. In vitro, similar activity was noticed for M5, the major active metabolite of Entrectinib, against ALK, ROS1, and TRK. Fusion proteins containing ALK, ROS1, or TRK kinase domains have the potential to direct tumorigenic potential by hyperactivating downstream signalling pathways, resulting in unrestrained cell growth. Entrectinib showed its inhibitory activity on downstream pathways, which include cell cycle arrest and apoptosis, inhibition of Trk phosphorylation, tumor growth inhibition, and inhibition of cell proliferation in vitro and in vivo.
Structure.
Molecular Formula: C31H34F2N6O2
Chemical Name:
N-[5-(3,5-difluorobenzyl)-1H-indazol-3-yl]-4-(4-methylpiperazin-1-yl)-2-(tetrahydro-2H-pyran-4-ylamino) benzamide.
Type: Small Molecule.
Dosage: (1) Dosage Recommended for ROS1-Positive Non-Small Cell Lung Cancer (NSCLC): 600 mg of ROZLYTREK® is recommended to be taken orally with or without food once daily until the condition progresses or the toxicity becomes intolerable [47].
-
(2)
Dosage Recommended for NTRK Gene Fusion-Positive Solid Tumors:
-
i.
For Adults:
600 mg of ROZLYTREK® is recommended to be taken orally with or without food once daily until the condition progresses or there is intolerable toxicity [47].
-
ii.
For Pediatric Patients aged 12–17 years (Adolescents):
In the case of pediatric patients 12–17 years of age, ROZLYTREK® is administered based on the body surface area (BSA) of the patients, as mentioned in the following table [47]. ROZLYTREK® is advised to be consumed orally with or without food, once daily until the condition progresses or the toxicity becomes intolerable.
| Body Surface Area (BSA) | Recommended Dosage (Once daily for Oral Use) |
|---|---|
| >1.50 m2 | 600 mg |
| 1.11–1.50 m2 | 500 mg |
| 0.91–1.10 m2 | 400 mg |
Synthesis:
In 2021, Morgan J. Cordell and team introduced an exceptional and amazing methodology for the synthesis of the Entrectinib using Cross-Coupling via Photo-Redox in flow (Scheme 3) [51]. The involvement of the flow chemistry results in fewer steps than the earlier reported synthetic procedure of Entrectinib. Initially, the SNAr reaction between 4-aminotetrahydropyran and the compound 20 in the presence of DABCO and DMSO at 100 °C for 16 h provided the intermediate 21 in 85 %. Then, the compound 21 was converted to the intermediate 22 with the protection of the secondary amine by TFAA (98 %). After that, the intermediate 22 underwent C–N cross-coupling photo-redox reaction with N-methylpiperazine in flow with a flow rate of 93.3 μL/min (2.8 mL plate volume) for 180 min in the presence of 10 mol% of NiBr2, 0.2 mol% of Ru(bpy)3(PF6)2, 2.0 equiv. of DABCO to produce the intermediate 23 in 32 %, which was then hydrolyzed using TFA to form the carboxylic acid 24 in >99 % yield. Next, the generation of the acid chloride of the intermediate 24 from the intermediate 23, followed by the slow addition of the aminoimidazole 25 and then purification afforded the compound 26 in 52 % [52]. Finally, the expected product Entrectinib was obtained in 66 % through the deprotection of the amine group using triethylamine (TEA) in methanol.
Scheme 3.
Synthesis of Entrectinib.
Adverse effects:
ROZLYTREKTM was approved by the FDA based on the information from four clinical trials on 355 patients, among whom 48 % (n = 172) of the patients were exposed to ROZLYTREK for at least 6 months and 24 % (n = 84) of the patients were exposed for at least 1 year.46 The study of ROZLYTREK was done in one dose-finding trial [ALKA (n = 57)] in adults, one dose-finding and activity-estimating trial in adults [STARTRK-1, or NCT02097810 (n = 76)], one dose-finding and activity-evaluating trial in pediatric and adult patients [STARTRK-NG (n = 16)], as well as one single arm and activity-evaluating trial in adults [STARTRK-2, or NCT02568267 (n = 206)]. The median age of the patients was 55 years (range: 4–86 years), with 5 % (n = 17) being <18 years old and 55 % being female. 20 % of the patients had NTRK gene fusions, while 42 % had ROS1 gene fusions.
39 % of patients experienced severe adverse effects. The most common serious adverse reactions (≥2 %) were respiratory failure, pleural effusion, pyrexia, sepsis, pneumonia, pulmonary embolism, and dyspnea. Fatal adverse effects were sepsis, large intestine perforation, tumor lysis syndrome, pneumonia, dyspnea, and completed suicide.
The general adverse effects (≥20 %) [47] are dyspnea; increased weight; dysgeusia; vision disorders; cognitive impairment; diarrhea; pyrexia; fatigue; dysesthesia; arthralgia; dizziness; constipation; vomiting; myalgia; cough; nausea, and edema.
2. A.2. Active ingredient name: Capmatinib
Drug Brand Name: Tabrecta.
Background and Mechanism of Action: On May 6, 2020, FDA approved Capmatinib, to treat metastatic non-small cell lung cancer (NSCLC) with mutated tumors leading to MET exon 14 skipping [53]. The mesenchymal-epithelial transition (MET) inhibitor (kinase inhibitor), Capmatinib can be obtained as the yellow powder Capmatinib hydrochloride and it is the active ingredient of TABRECTA™, an ovaloid, unscored tablet with bevelled edges coated with curved film, developed by Novartis Oncology, under license from Incyte Corporation to be used orally for the treatment of cancers [53,54]. The tablets of TABRECTA are provided with 150 mg of pale orange-brown Capmatinib (176.55 mg Capmatinib hydrochloride anhydrous) or 200 mg of yellow Capmatinib (235.40 mg Capmatinib hydrochloride anhydrous).
Capmatinib attacks MET, which includes exon 14 skipping-induced mutant variant and inhibits cancer cell growth produced by mutant METexon 14 skipping at clinically achievable concentrations [54]. In a protein, the lack of exon 14 in MET leads to the missing of the regulatory domain, which diminishes its negative regulation, resulting in the intensification of the downstream MET signalling. The anti-tumor activity of Capmatinib is observed in murine tumor xenograft models, which is derived from human lung tumors with either MET amplification or a mutation resulting in MET exon 14 skipping. Capmatinib shows its preventive effect by inhibiting the phosphorylation of mutant and wild-type MET, which are activated by MET amplification or by the hepatocyte growth factor, and it results in the hindering of MET-mediated phosphorylation of downstream signalling proteins, in addition to the proliferation and survival of MET-dependent tumor or cancer cells [54].
Structure.
Molecular Formula:C23H21Cl2FN6O2.
Chemical Name:
2-Fluoro-N-methyl-4-[7-(quinolin-6-ylmethyl)imidazo[1,2 b][1,2,4]triazin-2-yl]benzamide—hydrogen chloride—water (1/2/1)
Type: Small Molecule.
Dosage: 400 mg of TABRECTA is recommended to be swallowed orally as whole tablets without chewing, breaking, or crushing, with or without food twice daily.54 For the missing dose or the dose which is vomited, is advised to call it quits and wait for the dose scheduled at the next time.
Synthesis: Novartis Pharma AG unfolded a synthetic route for the synthesis of Capmatinib, as presented in Scheme 4 (World Intellectual Property Organization, Patent Number- WO2021165818) [55]. At first, 4-bromo-2-fluorobenzonitrile (27) reacted with isopropyl magnesium chloride lithium chloride (28) to form the complex 29. 2,2-dimethoxy-1-morpholinoethan-1-one (30) (60 %), generated from the nucleophilic substation reaction between morpholine (40) and methyl 2,2-dimethoxyacetate (39), underwent substation reaction with the complex 29 to provide the compound 31. After that, the reaction between the compound 31 with aminoguanidine hydrochloride (32) in the presence of propionic acid and 2-propanol led to the formation of the compound 34 in a two-step pathway through the formation and cyclization of the intermediate 33. Then, in presence of ethylene glycol, the compound 34 reacted with 1-(2-chloro-1-hydroxy-3-(quinolin-6-yl)propyl)pyrrolidine-2,5-dione (35) to give the compound 2-fluoro-4-(7-(quinolin-6-ylmethyl)imidazo[1,2-b][1,2,4]triazin-2-yl)benzonitrile (36), which then hydrolyzed to the compound 37 (86 %). Later, the compound 37 reacted with methylamine and carbonyldiimidazole (CDI) and afforded the amidation product 38 (94 %), which then provided the desired crystalline form of Capmatinib (92 %) in the presence of HCl.
Scheme 4.
Synthesis of Capmatinib.
Adverse effects:
Based on the open-label, non-randomized, multicenter, multi-cohort clinical study, named GEOMETRY mono-1 (NCT02414139), where the number of participating patients was 334, 57 % of them being 65 years of age or older, the FDA approved TABRECTA.54 Between the patients aged ≥65 years and younger patients, no overall variations in safety or efficacy were found. Among these patients, 97 patients (69 previously treated and 28 treatment-naïve patients) with median age of 71 years (range: 49–90 years) and 24 % and 75 % having ECOG Performance Status (PS) 0 and 1, respectively, were selected to study the benefits of the drug TABRECTA, and out of these patients, retests were performed on 78 patient samples using the FDA-approved FoundationOne® CDx (56 previously treated patients and 22 treatment-naive) to find out the mutations resulting in MET exon 14 skipping. Among these 78 patients, 73 samples could be assessed (53 previously treated patients and 20 treatment-naive), out of which 72 samples (52 previously treated patients and 20 treatment-naive) had a mutation triggering MET exon 14 skipping, and that showed an estimated positive percentage agreement of 99 % (72/73) between the clinical trial assay and the FDA-approved assay and were proven to have a mutation that results in MET exon 14 skipping.
51 % of the patients who were provided TABRECTA experienced serious adverse reactions, including pleural effusion, dyspnea, vomiting, pneumonia, nausea and general physical health deterioration. The fatality rate was 0.3 % (1 patient) because of pneumonitis.
The adverse effects caused by taking TABRECTA [54] are (≥20 %) fatigue: nausea: vomiting; decreased appetite; peripheral edema, and dyspnea.
2.A.3. Active ingredient name: Pralsetinib
Drug Brand Name: Gavreto.
Background and Mechanism of Action: Pralsetinib, developed by Blueprint Medicines Corporation under the trade name Gavreto, was approved by FDA on September 04, 2020, as a kinase inhibitor to treat adult patients suffering from metastatic non-small cell lung cancer (NSCLC), which is generated due to the anomalous rearranged during transfection (RET) genes [[56], [57], [58]].
As a kinase inhibitor, Pralsetinib is very effective against oncogenic RET fusions (CCDC6-RET) and wild-type RET, as well as mutations (RET V804 M, RET M918T, and RET V804L) with very low (less than 0.5 nM) half maximal inhibitory concentrations (IC50s) [57]. In purified enzyme assays, TRKC, JAK1-2, VEGFR2, FLT3, FGFR1, DDR1, TRKA, and PDGFRb at higher concentrations were restrained by Pralsetinib. Also, in cellular assays, RET was prevented by Pralsetinib at 12-, 14-, and 40-fold lower concentrations than JAK2, VEGFR2, and FGFR2, respectively.
Structure.

Molecular Formula: C27H32FN9O2.
Chemical Name:(cis)-N-((S)-1-(6-(4-fluoro-1H-pyrazol-1-yl)pyridin-3-yl)ethyl)-1-methoxy-4-(4-methyl-6-(5 methyl-1H-pyrazol-3-ylamino)pyrimidin-2-yl)cyclohexanecarboxamide.
Type: Small Molecule.
Dosage: It is recommended to take 400 mg (4 capsules) of GAVRETO orally on an empty stomach at one time daily.57 It is forbidden to consume food for at least 1 h after and 2 h before taking the drug.
Synthesis: Jason D. Brubaker and Co. (assigned to Blueprint Medicines Corporation) have invented a synthetic procedure for Pralsetinib (United States, Patent Number-US20170121312) (Scheme 5) [59,60]. Upon the treatment of sodium methanethiol, 2,4-dichloro-6-methylpyrimidine (41) converted to sulfide 42 in 42 % yield through nucleophilic replacement of 4-Cl. Then, the cross-coupling reaction between the sulfide 42 and methyl 4-iodo-1-methoxycyclohexane-1- carboxylate (43) resulted in the formation of the coupling product 44 in 70 % yield with the use of PdCl2(dppf). After that, the sulphone 45, generated in 89 % yield through oxidation of the compound 44 by m-CPBAin the solvent dichloromethane (DCM), was hydroxylated to form the compound 46 in 79 % yield. The chlorination of the compound 46 through substitution with the treatment of phosphorus oxychloride (POCl3) provided the chlorinated product 47 (85 %), which then afforded the compound 49 using 5-methyl-1H-pyrazol-3-amine (48). Later, the desired Pralsetinib was obtained in 46 % yield through a three-step reaction. The compound 49 was hydrolyzed by sodium hydroxide, then treated with the compound 50 in the presence of diisopropylethylamine (DIPEA or iPr2Net) and PyBOP in DMF, leading to the generation of a mixture of trans- and cis-isomers. Finally, the preferred cis-isomer was separated from the trans-isomer by supercritical fluid chromatography (SFC), and the desired chiral Pralsetinib was isolated.
Scheme 5.
Synthesis of Pralsetinib.
Adverse effects:
The non-randomized, multicenter, multi-cohort, open-label clinical study designated as ARROW (NCT03037385) involving 438 patients, with median age 60 years (range: 26–87 years) having RET altered solid tumors was arranged to investigate the benefits of the drug GAVRETO.57 94 % of the patients had the ECOG performance status 0–1, while 6 % of the patients had the performance status 2.
45 % of patients receiving GAVRETO suffered Serious adverse reactions, such as sepsis, pneumonia, pyrexia, urinary tract infection, and pneumonitis. 5 % of the patients experienced fatal adverse reactions, including sepsis (n = 2), and pneumonia (n = 3).
The commonly observed adverse effects are57 pneumonitis/interstitial lung disease; hemorrhagic events or bleeding problems; hypertension/high blood pressure; risk of impaired wound healing; hepatotoxicity or liver problem; tiredness; constipation, etc.
2.A.4. Active ingredient name: Tepotinib
Drug Brand Name: Tepmetko.
Background and Mechanism of Action: Tepotinib, under the brand name of TEPMETKO was developed by Merck and was approved by the FDA on 2nd February 2021 for the treatment of patients with non-small cell lung cancers (NSCLCs) caused by MET exon 14 skipping mutations [61,62]. In vivo, Tepotinib caused tumors to shrink in human cancer murine xenograft models in vivo, regardless of whether MET activation was reliant on hepatocyte growth factor. This kinase inhibitor targets MET exon 14 skipping mutations. Tepotinib restricts both HGF-dependent and independent MET phosphorylation (HGF = hepatocyte growth factor) [62]. It prevents downstream signalling. Tepotinib also restricted imidazoline 1 and melatonin 2 receptors at clinically acquirable concentrations. It constrains tumor cell multiplication and tumor cell migration.
Structure.

Molecular Formula: C29H28N6O2.
IUPAC name:3-[1-[[3-[5-[(1-methylpiperidin-4-yl)methoxy]pyrimidin-2-yl]phenyl]methyl]-6-oxopyridazin-3-yl]benzonitrile.
Type: Small Molecule.
Dosage: The patient should have to take 450 mg of TEPMETKO once a day at the same time with food without chewing or crushing the tablets [62]. If a dose was missed by more than 8 h, the patient needs to take the next dose on the next day as prescribed and the patient doesn't need to make up that missed dose.
Synthesis: Dieter Dorsch and co-workers developed some synthetic procedures for the synthesis of various kinase inhibitors in 2015, where we found the synthetic route to Tepotinib (Scheme 6) [63]. Initially, 5-Boron-2-iodpyrimidin (52) was coupled with 3-(Hydroxymethyl)phenylboronic acid (51) via the Suzuki coupling reaction, yielding compound 53, which was chlorinated to yield 5-Bromo-2-(3-(chloromethyl)phenyl)pyrimidine (54). Then, the compound 54 reacted with 3-(6-Oxo-1,6-dihydro-3-pyridazinyl)benzonitrile (55) to produce the compound 56, which underwent Miyaura borylation and gave the intermediate 58. The compound 59 was then formed as a result of the reaction between 58 and sodium perborate. Finally, compound 59 reacted with 1-methyl-4-piperidin-4-yl)methanol (60) via the Mitsunobu reaction, yielding the desired compound Tepotinib.
Scheme 6.
Synthesis of Tepotinib.
Adverse effects: 450 mg of TEPMETKO was administered once daily in 225 patients having NSCLC with MET exon 14 skipping mutation and data regarding its adverse effects were studied. 45 % of patients experienced serious adverse effects included pneumonia (5 %), general health deterioration (3.5 %), dyspnea (3.9 %), edema (3.9 %), musculoskeletal pain (2 %), pleural effusion (7 %), and pulmonary embolism (2 %). 3 of patients experienced fatal adverse reaction included pneumonitis (n = 1), hepatic failure (n = 1), and dyspnea from fluid overload (n = 1). TEPMETKO was discontinued permanently in 20 % of patients because of an adverse response. Dyspnea (1.6 %), pleural effusion (2 %), Edoema (5 %) and general health worsening were the most common side effects (>1 %) resulting to permanent withdrawal of TEPMETKO.
Dose reductions were required in 30 % of TEPMETKO individuals due to an adverse response.
Edoema (19 %), pleural effusion (2.7 %), and elevated blood creatinine (2.7 %) were among the adverse events that necessitated dosage reductions in more than 2 % of TEPMETKO patients. Tepmetko may cause some serious adverse effects [62] (≥20 %), like edema; diarrhea; nausea; fatigue; musculoskeletal pain; dyspnea; Other Grade 3 to 4 laboratory abnormalities (≥2 %) were decreased albumin; decreased lymphocytes; increased gamma-glutamyltransferase; increased amylase; decreased sodium; increased ALT; decreased haemoglobin and increased AST.
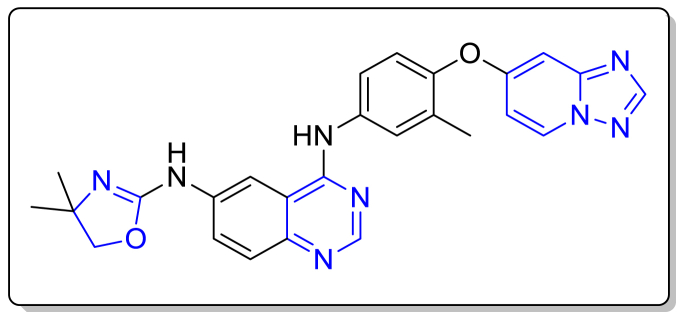
2.A.5. Active ingredient name: Mobocertinib
Drug Brand Name: Exkivity.
Background and Mechanism of Action: Mobocertinib, under the brand name of Exkivity, got the FDA approval on 15th September 2021 [64,65]. This is an orally available drug used to treat non-small cell lung cancer occurred by exon 20 insertion mutations. Mobocertinib is a kinase inhibitor which targets human epidermal growth factor receptor (EGFR). It is used to treat patients with non-small cell lung cancer with exon 20 insertion mutations [64]. This Epidermal growth factor receptor (EGFR) kinase inhibitor binds with EGFR exon 20 insertion mutation at a low concentration. In-vitro, it was found that Mobocertinib also restricts the activity of HER2, HER4, and BLK at clinically acceptable concentrations. Cultural cell models showed that Mobocertinib obstructed the proliferation of cells caused by EGFR exon 20 insertion mutation [65].
Structure.
Molecular Formula: C32H39N7O4.
IUPAC name: Propan-2-yl 2-[4-[2-(dimethylamino)ethyl-methylamino]-2-methoxy-5-(prop-2-enoylamino)anilino]-4-(1-methylindol-3-yl)pyrimidine-5-carboxylate.
Type: Small Molecule.
Dosage: Exkivity 160 mg capsules must be taken orally once daily by patients without opening, chewing, or dissolving the capsule. Patients must omit the missed dose if it has been more than 6 h since it was last taken and proceed with the next dose as directed [65].
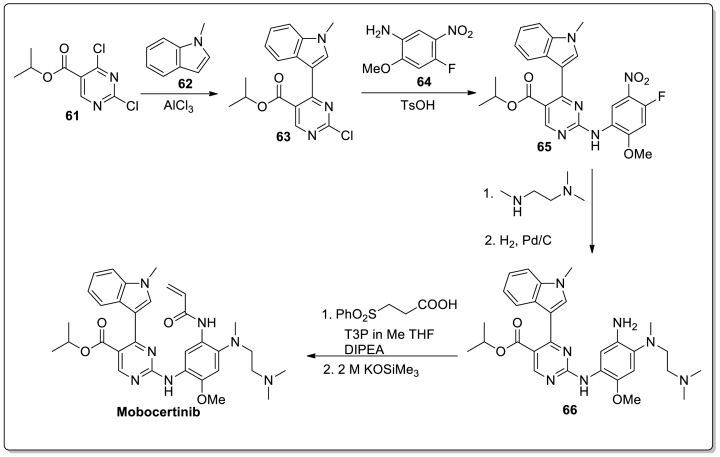
Synthesis: Jun Wang et al. developed a procedure for the synthesis of Mobocertinib in 2022 (Scheme 7) [66]. The synthetic procedure started with a cross-coupling reaction that occurred between isopropyl 2,4-dichloropyrimidine-5-carboxylate (61) and 1-methylindole (62), yielding a 5-(3-indolyl)pyrimidine intermediate (63). Then the intermediate 63 underwent a substitution reaction with 4-fluoro-2- methoxy-5-nitroaniline (64) to form the compound 65. The intermediate 65 is then coupled with N,N,N′-trimethylethylenediamine, and the nitro-group is reduced in the presence of Pd/C to give the intermediate 66. Finally, an 89 % yield was obtained by amide coupling of 66 with 3-(phenylsulfonyl) propanoic acid in the presence of propylphosphonic anhydride, followed by potassium trimethylsilanolate-mediated phenylsulfonyl group elimination. The overall yield of this six-step process is 61 %.
Scheme 7.
Synthesis of Mobocertinib.
Adverse effects: EXKIVITY's safety was assessed in a subgroup of patients in Study AP32788-15-101 who had EGFR exon 20 insertion mutation-positive locally progressed or metastatic NSCLC and had previously undergone platinum-based chemotherapy. EXKIVITY 160 mg once day was given to 114 individuals until illness progression or unacceptable toxicity occurred; 60 % were treated for 6 months or longer, and 14 % were exposed for more than a year [66].
Of the individuals who got EXKIVITY, 46 % experienced serious side effects. Diarrhea, dyspnea, vomiting, pyrexia, acute renal damage, nausea, pleural effusion, and heart failure were serious adverse events that occurred in 2 % of individuals. 1.8 % of individuals experienced fatal adverse effects including pneumonitis (0.9 %) and heart failure (0.9 %). 17 % of patients who got EXKIVITY experienced permanent discontinuance. Diarrhea and nausea were side effects that necessitated the permanent cessation of EXKIVITY in at least 2 % of patients. 51 % of patients had to stop the medication of EXKIVITY because of an adverse response. Diarrhea, nauseousness, and vomiting were adverse events that >5 % of patients had to stop taking their medication for. 25 % of patients had their EXKIVITY dosage reduced as a result of a negative response. Diarrhea was the adverse event that required a dosage decrease in >5 % of participants.
The most common adverse effects [66] (>20 %) are fatigue; diarrhea; dry skin; nausea; vomiting; stomatitis, and paronychia.
2.Bsmall cell lung cancer
2.B.1. Active ingredient name: Lurbinectedin
Drug Brand Name: Zepzelca.
Background and Mechanism of Action:
Lurbinectedin is an oncogenic transcription inhibitor 67, and an alkylating drug 68. Pharma Mar, S.A. is the developer of Lurbinectedin, which is marketed as ZepzelcaTM 67. On June 15, 2020, Lurbinectedin was first approved by FDA in the USA for the treatment of metastatic (spread to other body parts) small cell lung cancer (SCLC) with progression of the disease on or after platinum-based chemotherapy in adult patients [68].
Lurbinectedin acts as an alkylating agent, attaching to guanine residues present in the minor groove of DNA to form adducts, leading to the curving of the DNA helix nearing the major groove [68]. The formation of the adduct sets in motion a series of events, that can influence the successive activity of DNA to bind proteins, as well as DNA repair pathways and, some transcription factors. These incidents result in perturbation of the cell cycle and ultimately the death of the cell.
Structure.

Molecular Formula:C41H44N4O10S.
IUPAC Name:
(1′R,6R,6aR,7R,13S,14S,16R)-8,14-dihydroxy-6′,9-dimethoxy-4,10,23-trimethyl-19-oxo-2′,3′,4′,6,7,9′,12,13,14,16-decahydro-6aH-spiro[7,13-azano-6,16-(epithiopropanooxymethano)[1,3]dioxolo[7,8]isoquinolino[3,2-b][3]benzazocine-20,1′-pyrido[3,4-b]indol]-5-yl acetate.
Type: Small Molecule.
Dosage: Every 21 days, the drug Zepzelca (3.2 mg/m2) is proposed to be infused directly into the vein (intravenous infusion) over 60 min [68]. The treatment should be continued until unacceptable toxicity or disease progression.
Synthesis: In 2019, Weiming He et al. unfolded a procedure for the synthesis of Lurbinectedin, as shown in Scheme 8 [69]. The alcohol 74 was prepared from ˪-tyrosine through a 6-step procedure, provided by Ruijiao Chen and team [70]. At first, the compound 68, formed by the protection of the amino group of the starting compound 67 with benzyloxy-carbonyl chloride (CbzCl), reacted with HCHO to produce the compound 69 in 85 % yield. Methylation (etherification and esterification), followed by dihydroxylation of the compound 69 provided the compound 70 (92 % yield over two steps), which underwent formylation with α,α-dichloromethyl methyl ether (71) using TiCl4 in DCM to generate the formylation product 72 in 82 % yield. Then, applying 3-chloroperbenzoic acid (m-CPBA) followed by reduction using LiBH4, the compound 72 was converted to the amino alcohol 73 (80 % yield for the two steps), which produced the compound 74 in 98 % yield through Pd-catalyzed hydrogenolysis with the elimination of the amine protecting group Cbz-.
Scheme 8.
Synthesis of Lurbinectedin.
The cycloaddition reaction (Pictet–Spengler reaction) of the compound 74 with the subsequent protection by (Boc)2O led to the formation of the tetrahydroisoquinoline75 (82 % yield after the two steps), which was then oxidised by Salcomine O2 in acetonitrile to give the product 76 in 85 % yield. Then, the irradiation of the compound 76 with blue light in THF at rt afforded the alcohol 77. After the hydroxyl group in the phenyl ring of the compound 77was protected with a benzyl group, the alcoholic hydroxyl group underwent Swern oxidation to form the aldehyde 79 (95 % yield), which was then subjected to Intermolecular Pictet–Spengler reaction with the amino alcohol 74 to generate the cyclization product 80 in 67 % yield as a major isomer. The reductive amination for the introduction of the requisite methyl group and subsequent protection of the compound 80 by an allyl group led to the formation of the compound 81 in 88 % overall yield. Next, the compound 81 underwent Swern oxidation, followed by the deprotection and intramolecular Strecker reaction to provide the compound 82 in 86 % yield after the two steps. Then, the compound 82 was treated with boron trichloride for the deprotection with the elimination of benzyl-protecting groups, affording the phenol 83 (92 %), which was oxidised by benzeneselenic anhydride via a position-selective angular hydroxylation to generate dihydroxy dienone 84 in 86 % yield. The condensation reaction between the compound 84 and (R)-N-Alloc-S-Fm-Cys furnished the ester 85 in 84 % yield. After that, the ester 85 underwent macrocyclization as per Corey's one-pot process [71] to give the lactone 86 in 51 % yield. Next, in the presence of Pd(PPh3)4, the Pd0-catalyzed reduction of the compound 86 resulted in the removal of both the alloc- and allyl-protecting groups, providing the amine 87 (85 %), which was then oxidised using 4-formyl-1-methylpyridinium and benzenesulphonate, then DBU (1,8-Diazabicyclo [5.4.0]undec-7-ene) and later oxalic acid to deliver the keto ester 89 in 52 % yield. Finally, the desired product Lurbinectedin was obtained in overall 77 % yield, after Pictet–Spengler reaction of the keto ester 89 proceeded with 2-(5-methoxy-1H-indol-3-yl)ethanamine hydrochloride salt (90) as a condenser.
Adverse effects:
The drug ZEPZELCA got approval besed on the open-label, multicenter, multi-cohort clinical study PM1183-B-005-14 (Study B-005; NCT02454972), in which 554 patients with advanced solid tumors participated.68 Out of 554 patients, 105 patients having median age of 60 years (range: 40 to 83) with prior treatment of SCLC (small cell lung cancer) and progressing on or after platinum-based chemotherapy, who were enrolled to evaluate the efficacy of ZEPZELCA.
Among them, 35 % (37) of the patients were ≥65 years old. The cases of serious adverse reactions were higher in the patients ≥65 years (49 %) of age experienced than the younger ones (26 %).
34 % of the patients, who were provided with ZEPZELCA experienced serious adverse reactions, like neutropenia, anemia, pneumonia, dyspnea, respiratory tract infection, thrombocytopenia, and febrile neutropenia.
The most common adverse effects, as well as laboratory abnormalities (≥20 %)68 caused by ZEPZELCATM, are nausea; increased glucose; decreased albumin; decreased appetite; increased creatinine; fatigue; constipation; thrombocytopenia; leukopenia; vomiting; cough; anemia; dyspnea; increased alanine aminotransferase; musculoskeletal pain; decreased sodium; increased aspartate aminotransferase; lymphopenia; diarrhea; decreased magnesium, and neutropenia.
2.B.2. active ingredient name: Trilaciclib
Drug Brand Name: Cosela.
Background and Mechanism of Action: Trilaciclib, developed by G1 Therapeutics under the brand name of COSELA, was first approved by the FDA on February 12, 2021 for the treatment of patients having myelosuppression (a condition where a decrease of bone marrow activity was observed, resulting in low blood cell production), a side effect of chemotherapy used in the treatment of small cell lung cancer [[72], [73], [74]]. Trilaciclib is a water-soluble yellow coloured solid that acts as a cyclin-dependent kinase 4/6 (CDK4/6) inhibitor. When Trilaciclib was administered before chemotherapy, it reduced apoptosis in CDK4/6 cell lines. As a result, transient G1 cell cycle arrest decreased cytotoxic chemotherapy agents’ toxicity. Overall, it restricts HSPCs (hematopoietic stem and progenitor cells) augmentation, which increase the circulation of RBCs, neutrophils and platelets.74.
Structure.
Molecular Formula: C24H30N8O.
IUPAC name: 4-[[5-(4-methylpiperazin-1-yl)pyridin-2-yl]amino]spiro[1,3,5,11-tetrazatricyclo[7.4.0.02,7]trideca-2,4,6,8-tetraene-13,1′-cyclohexane]-10-one.
Type: Small Molecule.
Dosage: Normally, the patient's healthcare provider would recommend 240 mg/m2 of COSELA per dose [74]. On each scheduled day, patients must complete a 30-min intravenous infusion 4 h before chemotherapy. The interval between two consecutive doses should be at most 28 h. When a COSELA dose is missed, the patient should discontinue chemotherapy on that day and resume both on the next scheduled day.
Synthesis: In the year 2021, Smith and team proposed a synthetic route of Trilaciclib derivatives (Scheme 9) [75]. The synthesis procedure started with 5-bromo-2,4-dichloropyrimidine (92), that on nucleophilic substitution reaction with 91 gave the compound 93 which underwent Sonogashira coupling gave intermediate 95. Then, the intermediate 95 underwent cyclization followed by lactamization gave the key spirocycle 97 which further reacted with 2-aminopyridine derivative 98 in the presence of Pd-catalyst to form Trilaciclib.
Scheme 9.
Synthesis of Trilaciclib.
Adverse effects: COSELA's adverse effects were assessed in three studies. Study 1 for patients who newly diagnosed ES-SCLC and never went through any chemotherapy. 105 patients underwent this medication where 85 % of them were treated with COSELA and 91 % of the patients received placebo finished the induction treatment after four rounds. Study 2 for 75 patients who also are newly diagnosed ES-SCLC and never went through any chemotherapy. 76 % of these patients received COSELA and 87 % of patients received placebo finished the induction treatment after four rounds. For every group the average treatment was 6 cycled. Study 3 for patients having ES-SCLC who went through chemotherapy. 38 % patients received COSELA and 29 % patients received placebo finished ≥5 cycles of therapy [76].
Thirty percent of individuals taking COSELA experienced severe adverse effects. Respiratory failure, bleeding, and thrombosis were among the serious adverse responses that were observed in >3 % of patients who received COSELA. 9 % of patients who got COSELA experienced a permanent discontinuance as a result of an adverse response. Patients receiving COSELA experienced the following adverse reactions that resulted in the permanent discontinuation of any study treatment: pneumonia (2 %), asthenia (2 %), injection-site reaction, thrombocytopenia, cerebrovascular accident, ischemic stroke, infusion-related reaction, respiratory failure, and myositis (1 % each). 5 % of those taking COSELA experienced fatal adverse effects. Pneumonia (2 %), respiratory failure (2 %), acute respiratory failure (1 %), hemoptysis (1 %), and cerebrovascular accident (1 %), among other adverse effects, have been fatal for patients using COSELA. 4.1 % of patients receiving COSELA experienced infusion interruptions as a result of a negative reaction.
Some common adverse effects (≥10 % of patients with ≥2 % difference in incidence compared to placebo) [76] of Cosela are headache; abnormal liver function; breathing problem; low level of calcium or potassium and tiredness.
3. Prostate cancer
3.1. Active ingredient name: darolutamide
Drug Brand Name: Nubeqa.
Background and Mechanism of Action: Darolutamide, under the brand name of NUBEQA, was developed by Orion and Bayer and was approved by the FDA on 30th July 2019 for the treatment of patients having non-metastatic and castration-resistant prostate cancer [77,78]. This androgen receptor (AR) antagonist is an optically active, yellowish white or white to greyish crystalline powder with a pH value of around 11.75, soluble in tetrahydrofuran but insoluble in aqueous medium. Against the wild type androgen receptor, darolutamide, its (S,R)- and (S,S)-diastereomers, and keto-darolutamide displayed IC50 values of 60–100 nmol/L after 5670–7650 nmol/L following stimulation with the agonist R1881 at a dosage of 1 nmol/L, and 5670–7650 nmol/L after stimulation with R1881 at a dose of 10 nmol/L. Darolutamide showed IC50 values of less than 200 nmol/L against most other androgen receptor mutants tested following stimulation with R1881 0.1 nmol/L, while keto-darolutamide demonstrated considerable action against W742C and W742L androgen receptor mutants. Darolutamide restricts AR nuclear translocation, androgen binding, and AR-mediated transcription [78]. In vitro, various metabolites, ketodarolutamide showed similar activity to Darolutamide. In vitro, Darolutamide also performed as a progesterone receptor (PR) antagonist, but its activity is very low compared to AR. Darolutamide diminished tumour cell volume in mouse xenograft model, and in vitro, it restricted prostate cancer cell proliferation.
Structure.
Molecular Formula: C19H19ClN6O2.
IUPAC name: N-{(2S)-1-[3-(3-chloro-4-cyanophenyl)-1H-pyrazol-1-yl]propan-2-yl}-5-(1-hydroxyethyl)-1H-pyrazole-3-carboxamide.
Type: Small molecule.
Dosage: The suggested dose of NUBEQA is 600 mg (two 300 mg tablets) orally, twice, daily with food. Patients are recommended to swallow whole tablets without chewing them [78]. Patients have to take a gonadotropin-releasing hormone (GnRH) analogue along with NUBEQA or should go through a bilateral orchiectomy. In case of a missed dose, patients have to take that missed dose as early as possible before the next scheduled dose.
Synthesis: Orion Corporation developed an effective synthetic methodology for the synthesis of Darolutamide, as shown in Scheme 10 [79]. At first, 1-(oxan-2-yl)-1H-pyrazole (99) participated in a coupling reaction with compound 100 to produce 101 which underwent Suzuki-coupling with 4-bromo-2-chlorobenzonitrile (102) and afforded 2-Chloro-4-[1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazol-5-yl]benzonitrile (103) in 92 % yield. After that, hydrolysis of 103 in the presence of HCl gave the compound 104 which further reacted with tert-butyl (S)-(1-hydroxypropan-2-yl)carbamate (105) to form the chiral compound 106. Then, compound 106 went through an amide condensation reaction with compound 107 to produce compound 108 which on reduction with NaBH4 gave the desired drug Darolutamide in 76 % yield.
Scheme 10.
Synthesis of Darolutamide.
Adverse effects: Patients with castration-resistant non-metastatic prostate cancer were enrolled in the ARAMIS clinical investigation, a randomized (2:1), double-blind, placebo-controlled trial that involved many centers. Patients in this research got either a dose of placebo twice a day or NUBEQA daily at a dose of 600 mg. In patients who got NUBEQA, the median exposure time was 14.8 months (the range was 0–44.3 months) [78].
25 % of patients who received NUBEQA and 20 % of patients who received placebo experienced serious adverse reaction. Pneumonia, urinary retention, and hematuria were serious negative side effects that occurred in more than 1 % of individuals who got NUBEQA. In total, 3.9 % of patients using NUBEQA and 3.2 % of patients taking a placebo perished from adverse events. For NUBEQA, these reactions included death (0.4 %), cardiac arrest (0.2 %), heart failure (0.3 %), pulmonary embolism (0.2 %), and general physical health deterioration (0.2 %). Of patients using NUBEQA or a placebo, 9 % had to stop treatment permanently because of side effects. Cardiac failure (0.4 %) and mortality (0.4 %) were the most prevalent side effects necessitating permanent discontinuance in individuals receiving NUBEQA. In 6 % of patients receiving NUBEQA, dosage reductions were made as a result of adverse effects. Nausea (0.3 %), hypertension (0.3 %), and fatigue (0.7 %), were the side effects that required dosage decrease the most often in individuals using NUBEQA.
Some common adverse effects [78] (≥2 %) are pain in extremity; fatigue, and rash.
3.2. Active ingredient name: Relugolix
Drug Brand Name: Orgovyx.
Background and Mechanism of Action: In 2019, Orgovyx, developed by Takeda and ASKA Pharmaceutical in Japan and branded as Relumina, was approved for the treatment of uterine fibroids relatedsymptoms [80]. On December 18, 2020, FDA sanctioned the authorization of Relugolix to Myovant Sciences, designated as the drug name Orgovyx, to treat the advanced prostate cancer in adults [81,82]. Relugolix, a gonadotropin-releasing hormone (GnRH) antagonist, non-peptide active molecule, is used orally to treat several diseases caused by disorders associated with sex hormones.
The competitive binding of Relugolix with pituitary GnRH receptors leads to the depletion of the discharge of the follicle-stimulating hormone (FSH) and luteinizing hormone (LH).81 Thus Relugolix minimizes the release of testosterone.
Structure.
Molecular Formula: C29H27F2N7O5S.
Chemical Name:
N-(4-{1-[(2,6-difluorophenyl)methyl]-5-[(dimethylamino)methyl]-3-(6-methoxypyridazin-3-yl)-2,4-dioxo-1,2,3,4 tetrahydrothieno[2,3-d]pyrimidin-6-yl}phenyl)-N′-methoxyurea.
Type: Small Molecule.
Dosage: On the first day, the treatment has to begin with a dosage of 360 mg ORGOVYX and then orally 120 mg once per day [81]. The time for taking the drug each day with or without food has to be maintained at approximately the same time. The tablets are required to swallow as a whole without chewing or crushing the tablets as prescribed for effective results.
Synthesis: Kazuhiro Miwa and team provided a methodology for the preparation of Relugolix in 2011, as shown in Scheme 11 [83]. At first, the compound 109 was ethoxycarbonylated using ethyl chloroformate in toluene and then alkylated by 2-(chloromethyl)-1,3-difluorobenzene (111) to produce the N,N-disubstituted amine 112 (93 %), which reacted with N-bromosuccinimide (NBS)accompanied by azobisisobutyronitrile (AIBN) to generate the bromomethyl derivative 113. After that, the reaction between the derivative 113 and 2-methoxy-N-methylethan-1-amine (114) led to the formation of the compound 115. Next, the nitro group of the tertiary amine 115 was hydrogenated in the presence of Pd/C-catalyst, and the compound 116 (97 %) was obtained. Through a two-step reaction process, treating with 1,10-carbonyldiimidazole (CDI) followed by N-methoxyamine hydrochloride (MeONH2) resulted in the urea derivative 117 (89 %). Then the derivative 117 was converted to thiophene-3-carboxylic acid 118 (96 %) through selective hydrolysis in the presence of 2 N NaOH. After that the compound 118 proceeded through condensation reaction to provide the thieno[2,3-d]pyrimidine-2,4-dione derivative 120 (69 %) when treated with 6-methoxypyridazin-3-amine (119) and diethylphosphorocyanidate (DEPC) followed by sodium methoxide. In the final step, the reaction of compound 120 with 1-chloroethylchloroformate (121) generated an intermediary quaternary ammonium salt, which underwent nucleophilic substitution reaction with dimethylamine and the desired Relugolix was obtained in 44 % yield.
Scheme 11.
Synthesis of Relugolix.
Adverse effects:
In the open label and randomized clinical study HERO (NCT03085095), the efficacy and safety of the drug ORGOVYX were examined with a total of randomized 934 male patients with advanced prostate cancer.81 The 930 patients were to receive ORGOVYX (n = 622) or leuprolide acetate, for 48 weeks in a 2:1 ratio, and the median age was 71 years (range 47–97 years). Among these 622 patients taking ORGOVYX, the age of 35 % patients were ≥75 years and the age of 81 % patients were ≥65 years. Age did not play a clinically significant role in the pharmacokinetics or testosterone response of OGROVYX, as assessed by pharmacokinetics/pharmacodynamic assays and population pharmacokinetics in men aged 45–91 years.
A 12 % incidence rate of serious adverse reactions was observed in patients treated with OGROVYX, including arrhythmia, myocardial infarction, urinary tract infection, haemorrhage, and acute kidney injury. 0.8 % of patients taking ORGOVYX experienced fatal adverse reactions, such as myocardial infarction, injury, and acute kidney disease.
The frequent adverse effects [81] (≥10 %) are hot flushes; increased aspartate aminotransferase (AST); increased alanine aminotransferase (ALT); increased blood fat levels (increased triglycerides); fatigue; diarrhea; glucose increased; constipation; musculoskeletal pain (back, muscle, bone, or joint pain) and decreased blood hemoglobin levels.
4. Ovarian cancer
4.1. Active ingredient name: sotorasib
Drug Brand Name: Lumakras.
Background and Mechanism of Action: Sotorasib (also known as AMG-510), under the brand name of LUMAKRAS, was developed by Amgen and was approved by the FDA on 28th March 2021 for the treatment of KRAS G12C mutant non-small cell lung cancer [84,85]. This first-in-class KRAS G12C inhibitor readily binds with the cysteine of KARSG12C. It forms an irreversible, covalent bond which leads to deactivation of the protein, resulting the prevention of downstream signalling to restrict cell division and promotes apoptosis only in tumor cell lines [86]. In vitro, Sotorasib inhibited the recombinant mutant KRAS G12C/C118A's SOS1-catalyzed nucleotide exchange. Only the G12C-containing peptide of KRAS was covalently changed, according to a study of the cysteine proteome of cells that had been treated with sotorasib. Additionally, sotorasib specifically reduced the viability of KRAS G12C mutant strains [85].
Structure.

Molecular Formula: C30H30F2N6O3.
IUPAC name: 6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(4-methyl-2-propan-2-ylpyridin-3-yl)-4-[(2S)-2-methyl-4-prop-2-enoylpiperazin-1-yl]pyrido[2,3-d]pyrimidin-2-one.
Type: Small Molecule.
Dosage: The patients should have to take 960 mg (eight 120 mg tablets) of LUMAKRAS once at the same time every day until disease progression or toxicity.86 If a dose was missed by more than 6 h, the patient needs to take the next dose on the next day as prescribed. The tablets are required to swallow as a whole without chewing, crushing or splitting.
Synthesis: In the year 2021, a synthetic route was developed for the treatment of NSCLC caused by KRAS G12C Mutation as shown in Scheme 12 [87]. Initially, 2,6-Dichloro-5-fluoronicotinamide (122) was treated with oxalyl chloride at 40 °C to produce the intermediate 123, which further reacted with 2-isopropyl-4-methyl-3-pyridine amine 124 and gave the urea derivative 125. After that, the cyclization of the urea derivative 125 led to the formation of rac-dione 126. Then, rac-dione 126 underwent chiral separation in the presence of (+)-2,3-dibenzoyl-d-tartrate ((+)-DBTA) to obtain M-dione M-127in 37 % yield on two steps. Later, compound 128 was obtained from the reaction between M-128 and POCl3 which further reacted with 1.2 equiv. of 4-N-Boc-2-methyl-piperazine (129) to produce compound 130. After that, compound 130 underwent Suzuki coupling and Boc-deprotection reaction to obtain the compound 133. To conclude the synthetic procedure, compound 133 reacted with acryloyl chloride via 1,2-substitution process and the desired compound Sotorasib was obtained.
Scheme 12.
Synthesis of Sotorasib.
Adverse effects: The safety information of LUMAKRAS was collected after it was administered orally in 204 patients. Patients were treated with 960 mg of LUMAKRAS daily until disease progression or toxicity. 39 % of those exposed for at least six months, and 3 % were exposed for more than a year. Patients who underwent LUMAKRAS had a median age of 66 years (range: 37 to 86), with 55 % of them being female [86].
Of the individuals who received LUMAKRAS, 50 % experienced serious adverse effects. In more than 2 % of individuals, pneumonia (8 %), hepatotoxicity (3.4 %), and diarrhea (2 %), all caused serious adverse events. 3.4 % of patients who received LUMAKRAS experienced fatal adverse effects as a result of pneumonitis (0.4 %), respiratory failure (0.8 %), cardiac arrest (0.4 %), heart failure (0.4 %), pneumonia (0.4 %), and stomach ulcer (0.4 %). LUMAKRAS was discontinued permanently in 9 % of patients because to an adverse response. Hepatotoxicity (4.9 % of patients) was one of the adverse responses that led to the permanent cessation of LUMAKRAS. 34 % of patients interrupted their dosage due to some adverse effects. These adverse effects (≥2 %) included diarrhea (8 %), hepatotoxicity (11 %), nausea (2.9 %), musculoskeletal pain (3.9 %), and pneumonia (2.5 %).
The general adverse effects [86] (≥20 %) are given bellow nausea; liver problems; diarrhea; tiredness; cough and muscle or bone pain.
4.2. Active ingredient name: Pafolacianine
Drug Brand Name: Cytalux.
Background and Mechanism of Action: Pafolacianine was approved by the FDA on 29th November 2021 to identify malignant cancer cells in patients with ovarian cancer. Pafolacianine injection is a non-polar, sterile and dark bluish green coloured clear solution that recommended for use in adult ovarian cancer patients as a supplement for intraoperative detection of malignant tumors [88,89]. This fluorescent drug has a ∼1 nM affinity for the membrane-bound protein known as the folate receptor (FR), which is overexpressed in ovarian cancer.89 Pafolacianine absorbs near infrared light (760 nm–785 nm) with a peak absorption of 776 nm andproduces fluorescence in the range of 790 nm–815 nm with a peak emission of 796 nm.
Structure.
Molecular Formula: C61H67N9O17S4.
IUPAC name: 2-[(E)-2-[(3E)-2-[4-[(2S)-2-[[4-[(2-amino-4-oxo-3H-pteridin-6-yl)methylamino]benzoyl]amino]-2-carboxyethyl]phenoxy]-3-[(2E)-2-[3,3-dimethyl-5-sulfo-1-(4-sulfobutyl)indol-2-ylidene]ethylidene]cyclohexen-1-yl]ethenyl]-3,3-dimethyl-1-(4-sulfobutyl)indol-1-ium-5-sulfonate.
Type: Small Molecule.
Dosage: A single intravenous infusion of 0.025 mg/kg of CYTALUX, diluted in 250 mL of 5 % Dextrose Injection, should be given over the course of 60 min using a special infusion line, 1 h to 9 h before surgery [89].
Adverse effects: CYTALUX was given intravenously to 294 individuals at a dose of 0.025 mg/kg. The average age of the patients was 63.5 years, with 51 % being 65 or older. Females made up 89 % of the patients.
Most common adverse effects that occurred in ≥1 % of patients are flushing (1.7 %), chest discomfort (1 %), abdominal pain (2.7 %), hypersensitivity (1 %), nausea (15 %), dyspepsia (1 %), pruritus (1 %), and vomiting (5.8 %).
5. Lung and thyroid cancers
5.1. Active ingredient name: Selpercatinib
Drug Brand Name: Retevmo.
Background and Mechanism of Action:
Selpercatinib, a slightly hygroscopic, white to light yellow powder, is a receptor tyrosine kinase RET (rearranged during transfection) inhibitor [90,91]. RETEVMO™ (Selpercatinib), marketed as capsules of 40 mg or 80 mg hard gelatin is used orally to treat cancers possessing RET alterations, developed by Loxo Oncology (acquired by Eli Lilly and Company) [91,92]. Selpercatinib obtained its first approval from FDA in the USA on May 8, 2020, for the treatment of abnormal RET (rearranged during transfection) genes-induced cancers [90,91,93], including.
-
I.
advanced or metastatic medullary thyroid cancer (MTC) in adults and children ≥12 years of age;
-
II.
advanced or metastatic thyroid cancer in adults and children ≥12 years of age, when the radioactive iodine applied to the patients had no effect or lost its efficacy on them;
-
III.
metastatic non-small cell lung cancer (NSCLC) in adult patients.
In aqueous medium, Selpercatinib is slightly soluble at neutral pH and easily soluble at low pH, ensuring the pH-dependency in water solution.
Selpercatinib shows its inhibiting activity against wild-type and multiple mutated RET isoforms along with Vascular endothelial growth factor receptor 1 (VEGFR1) and VEGFR3 with IC50 values of 0.92–67.8 nM [91]. Also, in other enzyme assays, Fibroblast growth factor receptor(FGFR) 1, 2, and 3 were inhibited at higher concentrations, and in cellular assays, at approximately 60-fold lesser concentrations than FGR1 and 2 as well as 8-fold lesser concentration than VEGFR3, RET was inhibited by Selpercatinib. RET in-frame fusions with a variety of partners resulting in chromosomal rearrangements or specific point mutations in RET can lead to the constitutive activation of chimeric RET fusion proteins, functioning as carcinogenic drivers with the promotion of cell proliferation. Selpercatinib is effective against mutations and gene fusions, including RET V804 M, CCDC6-RET, RET M918T, and KIF5B-RET.
Structure.
Molecular Formula: C29H31N7O3.
Chemical Name:
6-(2-hydroxy-2-methylpropoxy)-4-(6-(6-((6-methoxypyridin-3-yl)methyl)-3,6-diazabicyclo[3.1.1]heptan-3-yl)pyridin-3-yl)pyrazolo[1,5-a]pyridine-3-carbonitrile.
Type: Small Molecule.
Dosage: The dosage of RETEVMO™ (Selpercatinib) is recommended on the basis of body weight [91].
-
❖
Less than 50 kg: 120 mg;
-
❖
50 kg or greater: 160 mg.
RETEVMO is advised to be consumed twice daily (every 12 h or so, roughly), by swallowing the whole capsules, without chewing or crushing, until disease progression or unacceptable toxicity. The patients are only allowed to take the missed dose if the duration of the next scheduled dose is 6 h.
Synthesis: The methodology of the synthesis of Selpercatinib disclosed by Array BioPharma Inc. is described in Scheme 13 (World Intellectual Property Organization, Patent Number-WO2019075108) [94]. The nucleophilic substation reaction of 2,4,6-trimethylbenzenesulfonyl chloride (134) with tert-butyl hydroxycarbamate (135) in the presence of methyl tertiary-butyl ether (MTBE) and triethylamine (TEA) at 0 °C furnished the compound 136 in 89 % yield, which was then treated with trifluoroacetic acid (TFA) at 0 °C to produce the intermediate 137. Then, the intermediate 137 reacted with the compound 138 at 0 °C to form the intermediate 139 in 83 % yield, which underwent [3 + 2] cycloaddition reaction in DMF and TEA providing the compound 141. The compound 141 was decarboxylated with the use of aqueous HBr at 80 °C to give the product 142. Next, the Vilsmeier-Haack reaction of the compound 142 using POCl3 and DMF at 0 °C afforded the corresponding aldehyde143 (86 %), which was then converted to the imine 144 through the condensation reaction of the aldehyde 143 and hydroxylamine hydrochloride. Further oxidation of the imine 144 by Ac2O at 140 °C provided the compound 145 in 81 % yield. The compound 145 was demethylated by AlCl3 in DCE at 76 °C generating the compound 146 in 98 % yield. Next, the reaction of the compound 146 with 2,2-dimethyloxirane (147) in basic solution produced the ring-opening product 148 in 84 % yield. After that, the coupling compound 150 was acquired in 95 % yield through the Suzuki-Miyaura reaction of the compound 148 with the compound 149. The compound 150 was then transformed to the compound 152 via substation reaction with the compound 151. Then, N-Boc deprotection in the presence of hydrochloric acid in dioxane led to the formation of the compound 153. Finally, the desired product Selpercatinib was obtained in 59 % yield through Borch reductive amination of the compound 153 with 6-methoxynicotinaldehyde (154) using NaBH(AcO)3 in DCM.
Scheme 13.
Synthesis of Selpercatinib.
Adverse effects:
The FDA approved RETEVMO on the basis of the open-label, multi-cohort, and multicentred clinical trial LIBRETTO-001 (NCT03157128), in which a total number of participating patients were 702, with a median age of 59 years (range 15–92 years) and 0.3 % being pediatric patients (12–16 years of age).91 For the analysis of the treatment-naïve RET fusion-positive NSCLC, 39 patients with a median age of 61 years (range 23–86)were taken into consideration, whereas 105 patients with a median age of 61 years (range 23–81), who had RET fusion-positive NSCLC and were also previously treated with platinum chemotherapy, were enrolled to explore the efficacy of RETEVMO. Among these 702 patients, the number of patients aged ≥75 years old was 67 (10 %), and the number of patients aged ≥65 years was 239 (34 %).
33 % of patients receiving RETEVMO had serious adverse reactions, the most common being pneumonia. 3 % of patients suffered fatal adverse reactions, including respiratory failure (n = 3), sepsis (n = 3), and cardiac arrest (n = 3).
The frequently observed adverse effects,91 taking into consideration the laboratory abnormalities (≥25 %) are increased glucose; increased aspartate aminotransferase (AST); increased alanine aminotransferase (ALT); increased alkaline phosphatase; decreased leukocytes; increased creatinine; decreased albumin (protein levels) in blood; decreased calcium; diarrhea; decreased sodium; hypertension; decreased platelets; dry mouth; constipation; increased total cholesterol; edema; rash, and fatigue.
6. Gastrointestinal stromal tumour (GIST)
6.1. Active ingredient name: avapritinib
Drug Brand Name: Ayvakit.
Background and Mechanism of Action: Avapritinib was approved by FDA on 9th January 2020 under the brand name of AYVAKIT for treating adults with gastrointestinal stromal tumors (GIST) that have spread to other body areas or that cannot be surgically removed [95], [96]Avapritinib is an orally available tyrosine kinase inhibitor of cell growth factor receptor KIT and platelet-derived growth factor receptor alpha (PDGFRA). The solubility of Avapritinib is pH-dependent. With the increasing pH of any solution, solubility of Avapritinib decreases. Avapritinib plasma concentrations were elevated when AYVAKIT and a strong or moderate CYP3A inhibitor are administered together, which may increase its adverse reactions. So, avoiding coadministration with strong or moderate CYP3A inhibitors is important. On the other hand avapritinib plasma concentrations were decreased when it coadministered with moderate or strong CYP3A inducers which decreases its effectuality. So, avoiding coadministration with moderate or strong CYP3A inducers is also important [97]. Avapritinib targets PDGFRA and PDGFRA D842 mutats and attacks various KIT exon 11, 11/17 and 17 mutants by less than 25 nM half-maximal inhibitory concentrations (IC50s). Mutations in PDGFRA and KIT can promote autophosphorylation and activation of those receptors, leading to tumor cell proliferation [95,97]. Avapritinib inhibits the transporters ABCB1 and ABCG2, which are involved in the multidrug resistance phenotype of certain malignancies. In vitro, it inhibits autophosphorylation of PDGFRA D842V and KIT D816V.
Structure.

Molecular Formula: C26H27FN10.
IUPAC name: (1S)-1-(4-fluorophenyl)-1-[2-[4-[6-(1-methylpyrazol-4-yl)pyrrolo[2,1-f][1,2,4]triazin-4-yl]piperazin-1-yl]pyrimidin-5-yl]ethanamine.
Type: Small Molecule.
Dosage: On an empty stomach, orally, 1 h before or 2 h after ingesting food, the recommended daily dose of AYVAKIT is 300 mg. Patients have to continue their treatment until progression. Making up a dose that was missed within 8 h of the next dose's scheduled time is prohibited.97.
Synthesis: In the year of 2015, Hodous et al. developed a methodology for the synthesis of Avapritinib (Scheme 14) [98]. According to this methodology, one of its key intermediate, (4-fluorophenyl)(2-(piperazin-1-yl)pyrimidin-5-yl)methanone (162) was synthesized through a five-step synthetic procedure. Initially, ethyl 2-chloropyrimi dine-5-carboxylate (155) reacted with Boc-protected 1,4-piperazine (156) in the presence of DIPEA to produce the compound 157, which underwent base hydrolysis to form 2-(4-(tert-butoxycarbonyl)piperazin-1-yl)pyrimidine-5-carboxylic acid (158). After that, the reaction between the compound 158 and N,O-dimethylhydroxylamine (159) gave the intermediate 160, which underwent Grignard reaction followed by Boc-deprotection reaction afforded one of the key intermediate 162. Next, another key intermediate, 4-chloro-6-(1-methyl-1H-pyrazol-4-yl)pyrrolo[1,2-f][1,2,4]triazine (172) was also synthesized through a five-step synthetic procedure. At first, diphenylphosphinic chloride (163) reacted with hydroxylamine hydrochloride and the intermediate 164 was formed which further reacted with 4-bromo-1H-pyrrole-2-carboxylic acid methyl ester (165) and gave the compound 166. The compound 166 then reacted with formamide (167) to obtain 6-bromo-3H-pyrrolo[2,1-f][1,2,4]triazin-4-one (168) which underwent Suzuki coupling gave the compound 6-(1-methyl-1H-pyrazol-4-yl)pyrrolo[1,2-f ][1,2,4]triazin 4(3H)-one (170). Then, compound 170was treated with phosphorus oxychloride to obtain the key intermediate 172. After that, the two intermediates 162 and 172 participated in a substitution reaction under acidic condition at room temperature and yielded compound 173 which reacted with (S)-2-methylpropane-2-sulfinamide (174) at 70 °C for 18 h and gave the intermediate 175. Later it was treated with methylmagnesium bromide to obtain 176 which on reaction with HCl in 1,4-dioxane/MeOH followed by purification via chiral SFC afforded the desired product Avapritinib in 68.3 % yield.
Scheme 14.
Synthesis of Avapritinib.
Adverse effects: The safety information of AYVAKIT was evaluated in 204 patients diagnosed with GIST. The median age of AYVAKIT patients was 62 years (range: 29–90 years), 60 % were 65 years old, and 62 % were male. Patients received 300 or 400 mg of AYVAKIT orally once every day [97].
In 52 % of individuals who received AYVAKIT, serious adverse events occurred. Anaemia (9 %), stomach discomfort (3 %), pleural effusion (3 %), sepsis (3 %), gastrointestinal haemorrhage (2 %), vomiting (2 %), acute renal damage (2 %), pneumonia (1 %), and tumour haemorrhage (1 %), all occurred in 1 % of patients who received AYVAKIT. 49 % of patients reduced their dosage of AYVAKIT due to some adverse effect like anemia, fatigue, memory impairment, hyperbilirubinemia, nausea and periorbital edema. 3.4 % of patients experienced fatal adverse effects. Sepsis and tumour haemorrhage (1 % each) were fatal adverse events that occurred in more than one patient.
The common adverse effects (incidence ≥20 %) are [97] nausea; vomiting; diarrhea; increased lacrimation; rash and dizziness; edema; hair colour changes; constipation; fatigue/asthenia, and abdominal pain.
6.2. Active ingredient name: Ripretinib
Drug Brand Name: Qinlock.
Background and Mechanism of Action: Ripretinib, developed and marketed by Deciphera Pharmaceuticals, Inc. under the name of QINLOCK™, is used to treat the cancers induced by platelet derived growth factor receptor A (PDGFRA) or/and KIT proto-oncogene receptor tyrosine kinase (KIT) together with gastrointestinal stromal tumour (GIST) [[99], (a)]. Ripretinib was first approved by FDA in the USA on May 15, 2020, for the treatment of adult patients suffering from GIST, a type of bowel, stomach, or esophagus tumor [100]. The drug is administered to the patients, whose disease [101].
-
a.
has permeated the entire body (referred to as metastatic GIST);
-
b.
is not surgically removable;
-
c.
has undergone prior treatments at least three times.
The oval, white to off-white looking, and orally used tablets of QINLOCK™, contain 50 mg of lipophilic, white to off-white solid crystalline Ripretinib, which is a type-II tyrosine switch control inhibitor (a), [100]. Ripretinib is a weak base and insoluble in water.
Ripretinib functions as a type II “switch-control” tyrosine kinase inhibitor that makes the activation “switch’’ (loop) assume an inactive conformation to inhibit KIT and PDGFRA kinase, along with wild type, primary, and secondary mutations (b), [100]. The switch control mechanism consists of two components: (A) As an antagonist, Ripretinib hinders switches from adopting the active state of type I; and (B) As an agonist, it sustains switches in the inactive state of type II. Ripretinib is also active in vitro against several other kinases, for example, TIE2, BRAF, VEGFR2, and PDGFRB.
Preventing KIT phosphorylation and proliferation, Ripretinib influences apoptosis in the GIST cell lines originated in treatment-resistant patients, cell lines of other cancers with PDGFRA or KIT mutations (for example, acute myeloid leukaemia and systemic mastocytosis), and the cell lines transfected with the mutations triggered by PDGFRA or KIT.99.
Structure.

Molecular Formula: C24H21BrFN5O2.
Chemical Name:
1-(4-bromo-5-[1-ethyl-7-(methylamino)-2-oxo-1,2-dihydro-1,6-naphthyridin-3-yl]-2-fluorophenyl)-3-phenylurea.
Type: Small Molecule.
Dosage: Once daily, with or without food, 150 mg of QINLOCK is recommended to be given to swallow orally as whole tablets, every day at the same time until the condition progresses, or the toxicity becomes intolerable [100]. It is also advised to consume the missed dose if the missed scheduled dose does not exceed 8 h.
Synthesis: Among several reported synthetic procedures, the recent synthetic approach for the synthesis of Ripretinib was depicted by Srinivas Oruganti and Co. as described in Scheme 15 (World Intellectual Property Organization, Patent Number-WO2022162690) [102]. Initially, the compound 178 was treated with 70 % ethylamine solution in water in the presence of acetonitrile at 25–35 °C for 22–24 h under nitrogen-atmosphere to produce the compound 179 in 90.6 %. The compound 179 was reduced by DIBAL-H (Diisobutylaluminum hydride) providing the alcohol 180 (85.8 % yield), which was oxidised by activated manganese dioxide to give the aldehyde 181 in 84.9 %. After that, the aldehyde 181 underwent [4 + 2] cycloaddition reaction with the compound 182 in the presence of DMA and 40 % KF/Al2O3 (N, N-dimethylacetamide) at 25–35 °C for 1–2 h in N2-atmosphere to furnish the compound 183 in 79.1 %. Then, the nucleophilic substitution reaction of the compound 183 with methylamine led to the formation of the compound 184. Lastly, the desired product Ripretinib was obtained in 75.3 % yield when the compound 184 reacted with phenyl phenylcarbamate (186) with the addition of tetrahydrofuran (THF) followed by 1-methylpyrrolidine (185).
Scheme 15.
Synthesis of Ripretinib.
Adverse effects:
Based upon the international, double-blind, randomized (2:1), multi-center, placebo-controlled clinical trial labelled as INVICTUS (NCT03353753), with a total of 351 participating patients.100 The efficacy of QINLOCK was evaluated based on the information of 129 randomized patients, 85 to QINLOCK and 44 to placebo, with a median age of 60 years (range: 29–83 years); the population of patients aged ≥65 years was 39 %. Among the patients who were provided with QINLOCK, 9 % were ≥75 years old, and the population of the patients aged between 65 and 74 years was 24 %. However, the comparison of the efficacy of QINLOCK between older and younger patients was not decided due to the inadequate number of patients.
Of the patients receiving QINLOCK, 31 % showed serious adverse reactions, including nausea, abdominal pain, vomiting, and anemia.
The reported adverse reactions (≥20 %) [100] commonly observed are nausea; myalgia (muscle pain); constipation; decreased appetite; vomiting; abdominal pain; diarrhea; fatigue; palmar-plantar erythrodysesthesia (skin problem in the palms and soles), and alopecia (hair loss).
7. Renal cell carcinoma (kidney)
7.1. Active ingredient name: tivozanib
Drug Brand Name: Fotivda.
Background and Mechanism of Action: 3 % of cancer cases found in adults are caused by renal cell carcinoma (RCC). Tivozanib, under the brand name of FOTIVDA, was first approved by the FDA on 10th march, 2021 for the treatment of patients having advanced renal cell carcinoma (RCC) [103,104]. From tumour xenograft models it was found that, tivozanib restricts angiogenesis, vascular permeability, and tumour cell proliferation in many tumour cell lines along with human RCC. Tivozanib is a VEGFR tyrosin kinase inhibitor that restricts phosphorylation. Normally two types of Tivozanib (FOTIVDA) capsule was prepared for treatment. (i) FOTIVDA 1.34 mg capsule contains 1.5 mg of tivozanib and (ii) FOTIVDA 0.89 mg capsule contains 1.0 mg of Tivozanib along with same amount of other components. This tyrosin kinase inhibitor FOTIVDA, also known as Tivozanib was developed for the treatment of adult patients having refractory or relapsed RCC [103]. After administration of tivozanib, VEGF-A's serum levels were increased and that of VEGFR-2 decreased. Tivozanib also inhibits phosphorylation of VEGFR-1, and VEGFR-3 [104]. In glioblastoma, tivozanib restricts pathway of polo-like kinase 1 resulting G2/M cell cycle arrest, and also it includes downregulation of cyclin B1, Aurora kinase A and B, and CDC25C.
Structure.

Molecular Formula: C22H19ClN4O5.
IUPAC name: 1-[2-chloro-4-(6,7-dimethoxyquinolin-4-yl)oxyphenyl]-3-(5-methyl-1,2-oxazol-3-yl)urea.
Type: Small Molecule.
Dosage: Patients have to take 1.34 mg of FOTIVDA once daily for 21 days, followed by one week off for a 28-day cycle.104 Patients have to take FOTIVDA capsules with water without chopping, chewing, or opening them. It is forbidden to take two doses at the same time after a missed dose.
Synthesis: In the year of 2016, Chunping Zhu and team developed a synthetic path of tivozanib (Scheme 16) [105]. Initially, nitration of 1-(3,4-dimethoxyphenyl)ethan-1-one (187) gave 1-(4,5-dimethoxy-2-nitrophenyl)ethan-1-one (188) in 86 % yield which further reacted with DMF-DMA in toluene solvent at 110 °C to give 3-(dimethylamino)-1-(2-nitrophenyl)prop-2-en-1-one (189). After that, 6,7-Dimethyloxyquinoline-4-ol (190) was obtained by treating the compound 189 with H2/Ni at room temperature. After that, chlorination of Compound 190 led to the formation of 4-chloroquinoline (191) in 78 % yield. Then, 4-amino-3-chlorophenol hydrochloride (192) reacted with compound 191 in the presence of tBuOK to obtain 2-chloro-4-((6,7-dimethoxyquinoline-4-yl)oxy)aniline (193) in 77 % yield. Finally, the reaction of compound 193 with methyl(5-methylisoxazol-3-yl)carbamate (194) gave the desired moiety Tivozanib in 98.9 % purity after recrystallization.
Scheme 16.
Synthesis of Tivozanib.
Adverse effects: The safety information of FOTIVDA was explained after it was administered in 350 patients having relapsed or refractory renal cell carcinoma (RCC) who previously went through some systemic treatments. Patients were divided into two groups randomly. One group received 1.34 mg of FOTIVDA every day for 21 days followed by 7 days off in a 28-day cycle and another group received 400 mg of sorafenib twice a day orally [104]. In the first group of patients, 53 % of them were exposed to FOTIVDA, for 6 months or more and 31 % were treated for more than one year. 45 % of patients who received FOTIVDA, experienced serious adverse effect and adverse reactionsin more than 2 % of patients included hepatobiliary disorders (2.3 %), bleeding (3.5 %), acute kidney injury (2.3 %), thromboembolism (3.5 %), and thromboembolism (2.9 %). 8 % of patients who received FOTIVDA, were observed fatal adverse reaction including subdural hematoma (0.6 %), hepatobiliary disorders (1.2 %), pneumonia (1.7 %), respiratory failure (1.2 %), myocardial infarction (0.6 %), and cerebrovascular accident (0.6 %). 21 % of patients who received FOTIVDA experienced an adverse effect and among them more than 2 patients were diagnosed with pneumonia, hepatobiliary disorders and fatigue resulting dosage discontinuation. Dosage interruptions occurred in 48 % patients who received FOTIVDA included hypertension, nausea, fatigue, and decreased appetite. 24 % of patients who received FOTIVDA reduced their dosage after experienced some adverse reaction included diarrhea, fatigue, and decreased appetite [104].
The typical adverse effects [104] (≥20 %) are nausea; tiredness; decreased appetite; diarrhea; cough; decreased levels of salt (sodium) and phosphate in the blood; mouth sores; low levels of thyroid hormones and increased levels of lipase in the blood.
8. Bladder cancer
8.1. Active ingredient name: Erdafitinib
Drug Brand Name: Balversa.
Background and Mechanism of Action: Erdafitinib, under the brand name of BALVERSA, was developed by Janssen Pharmaceutical Companies and was approved by the FDA on 12th April 2019 for the treatment of patients having metastatic or locally advanced urothelial carcinoma that has FGFR2 or 3 mutations and progressed one-line of prior platinum-containing chemotherapy with 12 months of adjuvant or neoadjuvant platinum-containing chemotherapy [106,107]. This drug was also being tested for the treatment of non-small cell lung cancer, liver cancer, cholangiocarcinoma, prostate cancer, oesophageal cancer and lymphoma. In vivo, Erdaftinib treatment in mice with xenograft tumors made from SNU-16 human gastric cancer cells amplifying FGFR2 decreased tumor growth in a manner dependent on dose. It also showed antitumor activity in mice with xenograft tumors made from LUX001 cells. In vitro, Balversa inhibits activity of FGFR1, 2, 3 and 4. It also attached to KIT, CSF1R, FLT4, PDGFRA, RET, VEGFR2 and PDGFRB. Erdafitinib restricts phosphorylation and signalling, decreasing cell viability in cell lines with FGFR mutations, point mutations, and amplification of cancer cells and gene fusions. Erdafitinib inhibited tumor growth in xenograft models and FGFR-expressing cell lines including bladder cancer [107].
Structure.

Molecular Formula: C25H30N6O2
IUPAC name: N'-(3,5-dimethoxyphenyl)-N'-[3-(1-methylpyrazol-4-yl)quinoxalin-6-yl]-N-propan-2-ylethane-1,2-diamine.
Type: Small Molecule.
Dosage: Initially, patients have to take 8 mg of BALVERSA orally once daily. Then, based on serum phosphate (PO4) levels patients have to increase their dosage up to 9 mg (three 3 mg tablets) once daily.107 Tablets have to be swollen whole without chewing it with or without food until disease progression or unacceptable toxicity. For a missed dose, patients have to take BALVERSA™ on the same day as soon as possible rather than taking an extra dose on the next scheduled day.
Synthesis: The synthetic route of Erdafitinib was shown in Scheme 17 [108]. The carbonyl group present on compound 195 reacted with POCl3 at 100 °C followed by the reaction with K2CO3 to give the intermediate 196 which underwent cross-coupling reaction with 197 to obtain compound 198. Then, compound 198 cross-coupled with 3,5-diethoxy aniline (199) in the presence of Pd(OAc)2 and BINAP to obtain intermediate 200 which further participated in substituted reaction with 201 to produce 202 in 94 % yield. After that, compound 203 was obtained from the reaction that occurred between MsCl and 202 which further underwent amine substitution reaction to produce the desired drug Erdafitinib in 87 % yield.
Scheme 17.
Synthesis of Erdafitinib.
Adverse effects: The adverse effects were elaborated after it was administered in 87 patients, diagnosed with locally advanced or metastatic urothelial carcinoma. Initially, 8 mg of BALVERSA was administered once daily, with an increased 9 mg dose of BALVERSA with phosphate levels [107].
Some patients were diagnosed with acute myocardial infarction, a fatal effect of BALVERSA. Serious adverse reaction like eye disorders (10 %) was found in 41 % of patients. Due to eye disorder (6 %), 13 % of patients discontinued their doses. 68 % of patients faced dose interruptions due to some most frequent adverse effects like stomatitis (17 %), palmar-plantar erythro-dysaesthesia syndrome (8 %), eye disorders (17 %), and hyperphosphatemia (24 %). 53 % patients faced dose reduction due to the adverse effects like nail dystrophy (6 %), stomatitis (15 %), eye disorders (23 %), palmar-plantar erythro-dysaesthesia syndrome (7 %), paronychia (7 %), and hyperphosphatemia (7 %).
Some of its common adverse effects are [107] stomatitis; diarrhea; albumin decreased; constipation; sodium decreased; dry skin; calcium increased; fatigue; decreased appetite; hemoglobin decreased; nausea, and onycholysis.
8.2. Active ingredient name: enfortumab vedotin-ejfv
Drug Brand Name: Padcev.
Background and Mechanism of Action:
For injection, PADCEV™ (enfortumab vedotin-ejfv), developed by Astellas Pharma, is administered for intravenous use as a white to off-white, preservative-free, sterile lyophilized powder, in a single-dose vial. On December 13, 2019, the US FDA approved Enfortumab vedotin-ejfv (PADCEV™) in the USA, to treat urothelial carcinoma, a type of urinary tract and bladder cancer in adult patients [109,110], where.
-
i.
the patients have already received immunotherapy drugs, like PD-1 and PDL1 inhibitor;
-
ii.
the cancer has spread to other parts of the body (metastatic or locally advanced urothelial carcinoma);
-
iii.
the patients have already undergone chemotherapy containing platinum.
Enfortumab vedotin-ejfv, an antibody-drug conjugate (ADC), is composed of [109,110].
-
I.
a fully human, anti-Nectin-4 IgG1 (Immunoglobulin G1) kappa monoclonal antibody (AGS-22C3), which is linked to;
-
II.
monomethyl auristatin E (MMAE), a small molecule microtubule disrupting agent, through;
-
III.
SGD-1006, a protease-cleavable linker composed of maleimidocaproyl valine-citrulline (vc).
Every single antibody molecule contains approximately four MMAE molecules. The generation of Enfortumab vedotin-ejfv takes place through the chemical conjugation of small molecule components and the antibody, where the conjugation occurs on the cysteine residues, consisting of the interchain disulfide bonds present in the antibody, to afford a product with a drug-to-antibody ratio of around 3.8:1. The small molecule components are chemically synthesized whereas the antibody is afforded by mammalian (Chinese hamster ovary) cells.
Through a protease-cleavable linker, the microtubule-disrupting agent MMAE is connected to the humanIgG1anti-Nectin-4 antibody, an adhesion protein that is detected on the cellsurface [109]. Based on the nonclinical data, the attachment of the enfortumab vedotin-ejfv with the Nectin-4-expressing cells leads to the internalization of the ADC-Nectin-4 complex, releasing MMAE through the proteolytic cleavage and the liberation of MMAE interrupts the microtubule network within the cell, which results in the cell cycle arrest and death of apoptotic cell.
Structure.
Type: Biotech.
Dosage:
1.25 mg/kg of PADCEVTM (for patients ≥100 kg, the dose should not exceed 125 mg) is recommended to apply as an intravenous infusion for over 30 min on Days 1, 8 and 15 of a 28-day cycle, until the condition worsens or there is intolerable toxicity.109.
Adverse effects:
The efficacy of PADCEV was investigated from the information of the clinical studies, named EV-301, EV-201, EV-101 (NCT02091999), and EV-102 (NCT03070990), using PADCEV as a single agent in 680 patients; among them, 65 % (n = 440) were ≥65 years old, and 25 % (n = 168) were ≥75 years old [109].
-
(a)
EV-301: In EV-301, 296 patients were enrolled, with a median age of 68 years (range: 30–88 years) and 77 % being male. The incidence rate of serious adverse reactions including acute kidney injury, pneumonia, and urinary tract infection was 47 %. were the frequently observed serious adverse reactions. In 3 % of cases, fatal adverse reactions were observed. The most common fatal adverse reactions were septic shock, pelvic abscess, hepatic dysfunction, pneumonitis, hyperglycemia, and multiorgan dysfunction.
-
(b)
EV-201, Cohort 1: In EV-201, Cohort 1, the total number of participants was 125, with a median age of 69 years (range: 40–84 years) and 70 % being male. 46 % of patients suffered serious adverse reactions, including sepsis, dyspnea, urinary tract infection, acute kidney injury, neutropenia, rash, cellulitis, diarrhea, and febrile. 3.2 % of patients suffered fatal adverse reactions, such as cardiac disorder, aspiration pneumonia, pneumonitis, sepsis, and acute respiratory failure.
-
(c)
EV-201, Cohort 2: In EV-201, Cohort 2, the total number of participating patients was 89. 39 % of patients had serious adverse reactions, including sepsis, pneumonia, and diarrhea. 8 % of patients had fatal adverse reactions The frequent fatal adverse reactions were metabolic acidosis, pneumonitis, pneumonia, multiorgan dysfunction, and sepsis.
The most common adverse effects along with laboratory abnormalities (≥20 %) [109] are diarrhea; decreased neutrophils; increased creatinine; rash; pruritus; decreased albumin; nausea; dry skin; decreased sodium; increased lipase; decreased lymphocytes; decreased appetite; decreased platelets; peripheral neuropathy; decreased phosphate; increased aspartate aminotransferase; increased alanine aminotransferase; increased urate; decreased hemoglobin; increased glucose; decreased weight; fatigue; anemia; dysgeusia, and alopecia.
9. Lymphoma
9.1. Active ingredient name: polatuzumab vedotin-piiq
Drug Brand Name: Polivy.
Background and Mechanism of Action: For injection, POLIVY™ (polatuzumab vedotin-piiq), developed by Genentech, Inc., a subsidiary of Roche, to treat haematological malignancies, is distributed as a preservative-free, white to greyish-white lyophilized sterile powder and, for intravenous infusion, it resembles a cake after reconstitution and dilution. On June 10, 2019, the US FDA approved Polatuzumab vedotin-piiq combined with bendamustine and rituximab, for the treatment of refractory (no improvement) or relapsed (the return of the disease) diffuse large B-cell lymphoma (DLBCL) in adults who are already treated with at least two earlier therapies [111,112]. Polatuzumab vedotin-piiq (Polivy™), an antibody-drug conjugate (ADC) contains a monoclonal antibody triggered by CD79b (Cluster of Differentiation 79B), a B-cell receptor unit [111,112]. Polatuzumab vedotin-piiq is made up of three components.
-
I.
the small molecule and anti-mitotic cytotoxic agent, Monomethyl auristatin E (MMAE);
-
II.
the humanizedIgG1 (immunoglobulin G1) monoclonal antibody directed against human CD79b, a B cell receptor component; and
-
III.
maleimidocaproyl-valine-citrulline-p-aminobenzyloxycarbonyl (mc-vc-PAB), a protease-cleavable linker, which connects MMAE to polatuzumab antibody covalently.
Each antibody molecule is linked to 3.5 molecules of MMAE on average. The chemical conjugation of the small molecule components and the antibody results in the formation of Polatuzumab vedotin-piiq. The small molecule components are synthesized chemically, whereas the mammalian (Chinese hamster ovary) cells provided the antibody [112].
Polatuzumab vedotin-piiq acts against dividing B-cells through the attachment of the monoclonal antibody to CD79b, a protein component of the B-cell receptor on the B-cell surface, followed by internalization, which leads to the breaking of the linker by lysosomal proteases, releasing MMAE intercellularly [112]. MMAE kills the dividing cells by binding with microtubules, thus inhibiting cell division and inducing apoptosis.
Structure.
Molecular Formula: C6670H10317N1745O2087S40.
Type: Biotech.
Dosage: 1.8 mg/kg of POLIVYTM is recommended as an intravenous infusion over 90 min each 21 days for 6 cycles in conjugation with bendamustine and a rituximab product in any sequence on the first day of each cycle.112 If the preceding infusion is tolerated, subsequent infusions may be given over 30 min. If not formerly premedicated, an antihistamine and antipyretic are suggested to be applied at least 30 min prior to POLIVYTM.
Adverse effects:
In the clinical trial GO29365 (NCT02257567), the patency of the drug POLIVY was studied with randomized 45 patients (a) receiving POLIVY, combined with a bendamustine and rituximab product (BR) (n = 6) in a single arm study, and (b) receiving POLIVY in combination with BR or BR alone (n = 39 treated per arm) in an open-label randomized study, every 21 days for 6 treatment cycles [112]. The median age of the patients was 67 years (range: 33–86).
Serious adverse reactions occurred in 64 % of the patients receiving POLIVY plus BR, which included pyrexia, pneumonia, sepsis, and febrile neutropenia. 7 % of patients treated with POLIVY plus BR suffered fatal adverse reactions within 90 days since the last treatment.
The most prevalent adverse effects (20 %) [112] are as follows decreased appetite; anemia; diarrhea; neutropenia; fatigue; pneumonia; peripheral neuropathy; pyrexia, and thrombocytopenia.
9.2. Active ingredient name: Zanubrutinib
Drug Brand Name: Brukinsa.
Background: Zanubrutinib, under the brand name of BRUKINSA, was developed by BeiGene and was approved by the FDA on November 14, 2019 for the treatment of adult patients having mantle cell lymphoma (MCL) who previously went through at least one treatment [113,114]. It is an orally available Bruton tyrosine kinase (BTK) inhibitor. Zanubrutinib binds with cysteine residue in Bruton tyrosine kinase (BTK) active site to inhibit its activity resulting inhibition of cytokine receptor pathways and B-cell antigen receptor (BCR) signaling. BTK signaling is responsible for activation of pathways that lead to B-cell proliferation, chemotaxis, trafficking and adhesion.114.
Structure.

Molecular Formula: C27H29N5O3.
IUPAC name: (7S)-2-(4-phenoxyphenyl)-7-(1-prop-2-enoylpiperidin-4-yl)-4,5,6,7-tetrahydropyrazolo[1,5-a]pyrimidine-3-carboxamide.
Type: Small Molecule.
Dosage: The prescribed dose of BRUKINSA is orally, 160 mg twice or 320 mg once daily, until unacceptable toxicity or disease progression.114 BRUKINSA should be swallowed with water without opening, chewing, or breaking it. In the event of a missed dose, patients must take Brukinsa immediately, followed by their regular prescribed schedule the following day.
Synthesis: Synthesis of Zanubrutinib initiated with the treatment of 4-Phenoxybenzoic acid (204) with thionyl chloride resulting in the formation of acyl chloride which further reacted with malononitrile to afford 205 in 93 % yield. Then, compound 205 went through a substitution reaction with Trimethyl orthoformate (TMOF) to produce intermediate 206 which underwent cycloaddition reaction and gave 5-Amino-3-(4-phenoxyphenyl)-1H-pyrazole-4-carbonitrile (207) in 69 % yield. Then, compound 207 and tert-butyl-4-[(2E)-3-(dimethylamino)prop-2-enoyl]piperidine-1-carboxylate (208) went through a cycloaddition reaction to give the intermediate 209. Then, the intermediate underwent reduction of six-membered ring and hydrolysis under the basic condition to give compound 210 which then went through Boc-deprotection reaction and gave compound 211. At last, the reaction of compound 211 with acryloyl chloride (212) at room temperature followed by chiral resolution gave the drug Zanubrutinib in good yield (Scheme 18) [115].
Scheme 18.
Synthesis of Zanubrutinib.
Adverse effects: Data regarding the adverse effects of BRUKINSA was analyzed after it was administered in 118 patients, diagnosed with Mantle Cell Lymphoma (MCL). The average age of patients was 62 years (ranges from 34 to 86 years) including 75 % male. 94 % of the patients underwent Eastern Cooperative Oncology Group (ECOG) performance with a status of 0–1. Two types of BRUKINSA doses were administered on different patients. Some patients received 160 mg of BRUKINSA twice daily and some patients received 320 mg of BRUKINSA once daily.114 79 % of the patients continued this medication for 6 months or more whereas 68 % continued for more than one year.
7 % of the patients experiences fatal events within one month of the last BRUKINSA dose including pneumonia and cerebral haemorrhage. 31 % patients experienced serious adverse effects including haemorrhage (5 %) and pneumonia (11 %).
Some common adverse effects (≥20 %) [114] of Zanubrutinib are cough; hemoglobin decreased; diarrhea; neutrophil count decreased; white blood cell count decreased; platelet count decreased; bruising; upper respiratory tract infection, and rash.
9.3. Active ingredient name: umbralisib
Drug Brand Name: Ukoniq.
Background and Mechanism of action: Umbralisib tosylate was developed by TG Therapeutics and approved by the FDA on 5th February 2021 for the treatment of patients with marginal zone lymphoma (MZL) who already went through an anti-CD20-based regimen and relapsed or refractory follicular lymphoma who previously went through ≥3 lines of systemic treatment [116]. It acts as a dual phosphatidylinositol 3-kinase delta (PI3Kδ) and casein kinase 1 epsilon (CK1ε) inhibitor. From Biochemical studies it was found that Umbralisib can also restricts some mutated form of ABL, which leads to the inhibition of cell proliferation, adhesion, and migration. In vitro research demonstrated that umbralisib's suppression of CK1 shields Tregs from the negative effects of PI3K inhibition. In addition, umbralisib synergized with carfilzomib to inhibit cap-dependent translation of tumor-promoting genes in diffuse large B-cell lymphoma (DLBCL) and mantle cell lymphoma (MCL) cell lines, which resulted in marked anti-mitotic and cytotoxic effects in Hodgkin-derived cell lines.116,117.
Structure.

Molecular Formula: C31H24F3N5O3.
IUPAC name: (S)-2-(1-(4-amino-3-(3-fluoro-4-isopropoxyphenyl)-1H-pyrazolo [3, 4-d] pyrimidin-1-yl)-ethyl)-6-fluoro-3-(3-fluorophenyl)-4H-chromen-4-one-4methylbenzenesulfonate.
Type: Small Molecule.
Dosage: UKONIQ's suggested oral dose is 800 mg once daily at the same time each day with food.117 Patients have to swallow the whole tablet without crushing, breaking, cutting or chewing it. Patients were advised not to take an additional dose after vomiting.
Synthesis: TG Therapeutics and Rhizen patented a procedure for the synthesis of Umbralisib via the reaction of two key intermediates 1H-pyrazolo[5,4-d]pyrimidine (218) and chiral alcohol 225 (Scheme 19) [117]. The intermediate 218 was synthesized via 3 step process, where initially, a mitsunobu type reaction occurred between 4-bromo-2-fluorophenol (213) between isopropanol and give the intermediate 214 which further on substitution reaction with diboronic ester 215 to afford 216. Later, compound 216 underwent Suzuki coupling reaction with 3-iodo-5H-pyrazolo[3,4-d]pyrimidin-4-amine resulting in one of the key intermediate 218. 3-fluorophenylacetic acid (219) underwent acid chloride formation followed by Friedel-Crafts acylation and quenching with HCl afforded the intermediate 220. After that, compound 221 was formed via a cyclization reaction which occurred between 220 and propionic anhydride. The compound 221 then underwent allylic bromination reaction to obtain 222 which was further converted into alcohol 223 and then ketone 224 via Swern oxidation and subsequently asymmetric reduction to give chiral (S)-alcohol 225. The (S)-alcohol is then converted into its R-isomer 228 via a two-step process. At last, 228 and 218 condensed with each other DIAD and PPh3 to generate the desired product Umbralisib.
Scheme 19.
Synthesis of Umbralisib.
Adverse effects: The safety information of UKONIQ was analyzed from a population of 221 adults, where 37 % of them were diagnosed with marginal zone lymphoma and 63 % of the population was diagnosed with follicular lymphoma. The average was 66 years which range:s from 29 to 88 years. 43 % of them were female and 97 % of the population underwent Eastern Cooperative Oncology Group (ECOG) performance with a status of 0–1. No patients were previously exposed to any kind of PI3K inhibitor. Patients underwent a treatment of UKONIQ with a dose of 800 mg per day [118].
18 % of the patients experienced serious adverse effects after administration of UKONIQ. More than 2 % of patients, observed with serious adverse effects were diagnosed with pneumonia (3 %), urinary tract infection (2 %), sepsis (2 %), and diarrhea-colitis (4 %). Permanent discontinuation took place for 14 % of patients due to adverse effects like transaminase elevation (5 %) and diarrhea-colitis (6 %). Dose reduction took place for 11 % of patients and more than 4 % of patients reduced their dose due to the adverse effect diarrhea-colitis (4 %). Dosage interruptions took place for 43 % of patients and ≥5 % of patients interrupted their dosage for some adverse effects like transaminase elevation (7 %), vomiting (5 %), neutropenia (5 %), upper respiratory tract infection (5 %), and diarrhea-colitis (18 %).
Typically (≥15 %) it shows following adverse effects [118] diarrhea-colitis; nausea; vomiting; upper respiratory tract infection; increased creatinine; transaminase elevation; neutropenia; decreased appetite; abdominal pain; musculoskeletal pain; fatigue, and anemia.
10. Myeloma
10.1. Active ingredient name: selinexor
Drug Brand Name: Xpovio.
Background and Mechanism of action: Selinexor, under the brand name XPOVIO, was developed by Karyopharm Therapeutics and was approved by the FDA on 3rd July 2019 for the treatment of adults having relapsed or refractory multiple myeloma (RRMM) [119,120]. It is an orally available tablet that restricts function of Exportin-1 (XPO1). XPOVIO is a blue coloured, film-coated, round, bi-convex tablet that contains colloidal silicon dioxide, magnesium stearate, Opadry 200 clear, povidone K30, croscarmellose sodium, Opadry II blue, sodium lauryl sulphate and microcrystalline cellulose. Selinexor is also undergoing some clinical development to study its applicability in various other hematological and solid cancers. Selinexor therapy led to the nuclear localization of tumor suppressor proteins in preclinical and clinical investigations as a result, apoptosis increased and proliferation decreased in cancer cell line. Inhibition of exportin 1 (XPO1) primarily shattered three-dimensional nuclear orientation of cancer cells. With IC50 values of less than 0.5 M, Selinexor demonstrated cytotoxicity in various myeloid leukaemia cell lines. In preclinical investigations, selinexor coupled with melphalan, dexamethasone, or carfilzomib to treat myeloma shown synergistic anticancer efficacy.119 From nonclinical studies, it was found that, Selinexor reversibly restricts nuclear export signalling of tumor suppressor proteins (TSPs), mRNAs of oncogenic proteins, growth regulators by blocking exportin 1 (XPO1). Due to the inhibition of XPO1, accumulation of TSPs increased in the nucleus, reduction of various oncoproteins occurred, apoptosis of malignant cells and cell cycle arrest took place.120.
Structure.
Molecular Formula: C17H11F6N7O.
IUPAC name: (2Z)‐3‐{3‐[3,5‐bis(trifluoromethyl)phenyl]‐1H‐1,2,4‐triazol‐1‐yl}‐N'‐(pyrazin‐2‐yl)prop‐2‐enehydrazide.
Type: Small Molecule.
Dosage: The prescribed starting dose for XPOVIO™ is 80 mg (four 20 mg tablets) on days 1 and 3 of every week. The recommended dosage of dexamethasone is 20 mg along with every XPOVIO dose [120]. Patients have to take XPOVIO™ tablets orally with water without breaking, crushing, chewing or dividing them. In case of missed dosages, patients have to continue their regular dose as per prescription. If vomiting occurs, patients have to take next scheduled dose without repeating that dose.
Synthesis: The synthesis route was shown in Scheme 20 [121]. The process for the synthesis of Selinexor was initiated with the reaction of 3,5-bis(trifluoromethyl)benzonitrile (229) with NaSH in the presence of magnesium chloride hexahydrate at low temperature which gave the compound 230. After that, compound 230 was treated with dimethylformamide dimethylacetal (231) at 0–5 °C and the intermediate 232 was formed which further reacted with hydrazine hydrate at same temperature for around 120 min and gave 3-(3,5-bis(trifluoromethyl)phenyl)-lH-l,2,4-triazole (233). Next, compound 233 underwent substitution reaction with the ester 234 to obtain compound 235 which underwent base catalyzed hydrolysis and gave (Z)-3-(3-(3,5-bis(trifluoromethyl)phenyl)-lH-l,2,4-triazol-l-yl)acrylic acid (236). At last, the reaction of compound 236 with 2-hydrazinopyrazine (237) followed by purification of the yield by methanol and Norit-CGP grade charcoal gave the pure yield of Selinexor in 91.15 % yield.
Scheme 20.
Synthesis of Selinexor.
Adverse effects: Data, regarding adverse reactions were described by administering XPOVIO in 202 patients with a median age of 64 years (range:s between 35 and 86 years) having RRMM. Patients were treated with 80 mg of XPOVIO along with 20 mg of dexamethasone on every alternative day of each week. The average duration of medication was 8 weeks with an average dose of 115.4 mg per week. The rate of treatment discontinuation raised up to 27 %, because of its adverse effect, 65.3 % of patients had their XPOVIO dosage halted, and 53.3 % had their dose reduced. The fatal adverse reaction rate was 8.9 % and ≥4 % of patients discontinued this medication due to frequent adverse effects like thrombocytopenia, nausea, and fatigue [120].
The typical adverse effects (≥20 %) of Selinexor are [120] thrombocytopenia; hyponatremia; decreased appetite; fatigue; nausea; decreased weight; anemia; hyponatremia; leukopenia; neutropenia; decreased appetite; upper respiratory tract infection; constipation and dyspnea.
10.2. Active ingredient name: belantamab mafodotin-blmf
Drug Brand Name: Blenrep.
Background and Mechanism of Action:
FDA first approved this drug on August 05, 2020, for the treatment of refractory or relapsed multiple myeloma in adults who have already at least gone through four earlier therapies, which involve a proteasome inhibitor, an anti-CD38 monoclonal antibody, and an immunomodulatory agent 122. The name of the developer of this drug, BlenrepTM is GlaxoSmithKline. Belantamab mafodotin-blmf, a monoclonal antibody-drug conjugate (ADC) and an antibody led by B-cell maturation antigen (BCMA), is employed in the treatment of a type of blood cancer, identified as multiple myeloma [122], [123]. It consists of three components 123.
-
I.
afucosylated, humanized immunoglobulin G1 (IgG1) monoclonal antibody;
-
II.
a protease-resistant maleimidocaproyl linker;
-
III.
the microtubule inhibitor MMAF (Monomethylauristatin F).
Belantamab mafodotin-blmf contains an afucosylated IgG1antibody portion that targets BCMA, a protein exhibited by multiple myeloma cells and normal B lymphocytes 123. The small unit MMAF acts as a microtubule inhibitor. After attaching to BCMA, the internalization of belantamab mafodotin-blmf leads to the liberation of MMAF through proteolytic cleavage, and the free MMAF obstructs the microtubule network, which results in apoptosis and cell cycle arrest. Belantamab mafodotin-blmf displays its antitumor activity via antibody-dependent cellular phagocytosis (ADCP) and antibody-dependent cellular cytotoxicity (ADCC)123.
Structure.
Molecular Formula: C6484H10008N1728O2030S44 (C49H66N6O11)4
Type: Biotech.
Dosage: It is suggested to consume 2.5 mg/kg of BLENREP as per actual body weight over around 30 min triweekly (once every 3 weeks) as intravenous infusion, as late as the disease condition reaches to the undesirable noxious state123.
Adverse effects:
The FDA approved BLENREP based on the evidence from the clinical trial DREAMM-2 (NCT 03525678), a multicenter, open-label study 123. In this trial, 218 patients participated; among them, the number of patients aged 65–75 years was 43 %, and those aged ≥75 years were 17 %. Of the patients, who suffered Keratopathy, 73 % were ≥65 years old and 80 % were <65 years old.
Among these patients, 95 were provided with 2.5 mg/kg BLENREP (recommended dosage) intravenously once every 3 weeks. 3.2 % of patients suffered fatal adverse reactions, including lung infection, cardiac arrest, and sepsis. 40 % of patients had serious adverse reactions, such as pyrexia, hypercalcemia, pneumonia, infusion-related reactions, sepsis, and renal impairment.
The grade 3 or 4 abnormalities 123 (incidents ≥5 %) most commonly observed in laboratories are thrombocytopenia (decrease in platelets); decreased neutrophils; decreased hemoglobin; decreased lymphocytes; increased gamma-glutamyl transferase, and creatinine increased. The typical adverse effects (≥20 %) [123] are decreased visual acuity; keratopathy (change of corneal epithelium on eye exam); blurred vision; infusion-related reactions; nausea; pyrexia, and fatigue.
11. Cholangiocarcinoma
11.1. Active ingredient name: Pemigatinib
Drug Brand Name: Pemazyre.
Background and Mechanism of Action: Pemigatinib, a white to off-white, non-hygroscopic solid, is the kinase inhibitor of fibroblast growth factor receptor (FGFR) 1, FGFR2 and FGFR3 and is being developed by Incyte Corporation under the brand name Pemazyre™ as a treatment of FGFR-induced malignancies, which also includes cholangiocarcinoma (bile duct cancer) [124,125]. On April 17, 2020, Pemigatinib (PEMAZYRE™) was approved by FDA in the USA, for the treatment of adults, who are previously treated with chemotherapy, have cancer due to FGFR2 fusion or other rearrange:ment, or cholangiocarcinoma cannot be removed by surgery (unresectable) and spread to the other body parts (metastatic cholangiocarcinoma) [125]. PEMAZYRE tablets are administrated for oral use and available as uncoated tablets. The tablets contain Pemigatinib of 4.5 mg, 9 mg, or 13.5 mg. In the case of solubility, Pemigatinib is found to be pH dependent. With the increase in pH, the solubility of Pemigatinib decreases.
As a kinase inhibitor, Pemigatinib attacks FGFR1, FGFR2 and FGFR3 with IC50 values below 2 nM, as well as FGFR4 nearly at100 times higher concentration in vitro than those that target FGFR1, 2, and 3 [125]. The constitutive FGFR signalling assists the proliferation and survival of cancerous (malignant) cells. Through hindering the phosphorylation and signalling of FGFR1, 2, and 3, Pemigatinib diminishes the viability of cells in the cancer cell lines by triggering the FGFR amplifications and fusions that activate the constitutive FGFR signalling.
Structure.
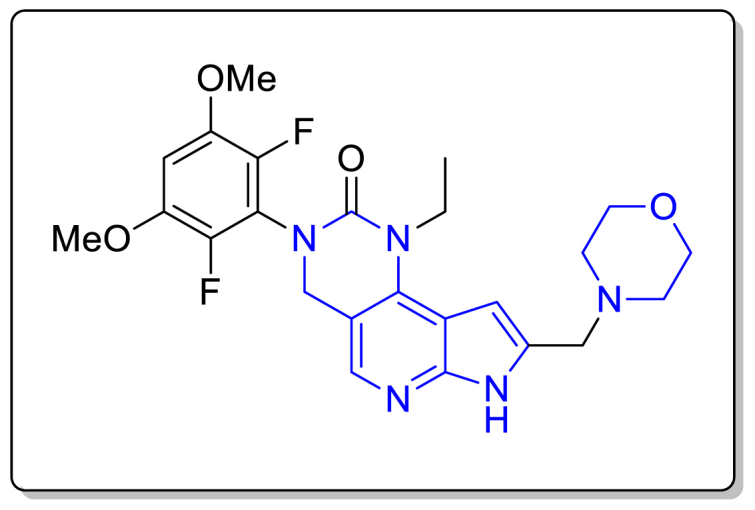
Molecular Formula:C24H27F2N5O4.
Chemical Name:
3-(2,6-difluoro-3,5-dimethoxyphenyl)-1-ethyl-8-(morpholin-4-ylmethyl)-1,3,4,7-tetrahydro-2H-pyrrolo[3′,2':5,6]pyrido[4,3 d]pyrimidin-2-one.
Type: Small Molecule.
Dosage: 13.5 mg of PEMAZYRE™ is recommended to be swallowed orally as a whole, instead of chewing, crushing, crushing, or dissolving the tablets, with or without food, more or less at the same time daily, for 14 successive days with a subsequent 7-days break from the treatment in a 21-day cycle, until disease progression or unacceptable toxicity [125]. If vomiting takes place or the patient is four or more hours late for a PEMAZYRE dosage, it is advised to continue the dose in the next schedule.
Synthesis: In 2021, Liangxing Wu et al. reported the synthetic route of Pemigatinib, as shown in Scheme 21 126. The starting material 238 underwent nucleophilic aromatic substitution (SNAr) reaction with ethylamine in the presence of 2-methoxyethanol at 130 °C to provide the compound 239 in 92 % yield. The condensation reaction between the compound 239 and 2,6-difluoro-3,5-dimethoxyaniline (240) led to the formation of an imine intermediate, which was reduced to form the diamine 241 in 82 % yield over the two steps. Then, the cyclization of the diamine 241 in the presence of triphosgene gave the tricyclic urea 242 (92 %). Next, after protecting the pyrrole nitrogen in 242 using benzene sulfonyl chloride, the aldehyde 244 was obtained in 91 % yield through selective deprotonation by LDA in the presence of DMF. After that, aldehyde 244 underwent reductive amination reaction with morpholine to produce the compound 245 (95 %). In the final step, deprotecting the sulfonyl group by applying tetra-n-butylammonium fluoride (TBAF) afforded the desired product Pemigatinib in good yield.
Scheme 21.
Synthesis of Pemigatinib.
Adverse effects:
The safety and efficacy of the drug PEMAZYRE were investigated in the open-label, multicenter, single-arm trial, labelled FIGHT-202 (NCT02924376), where the number of participating patients was 146 with a median age of 59 years (range: 26-78)[125]. The median treatment duration was 181 days (range: 7–730 days). 4.1 % of patients suffered fatal adverse reactions, such as bile duct obstruction, pleural effusion, failure to thrive, sepsis, and cholangitis. Serious adverse reactions were observed in ≥2 % of patients taking PEMAZYRE. The most common serious adverse effects were acute kidney injury, hyponatremia, urinary tract infection, hypercalcemia, thrive, and small intestinal obstruction.
The frequently observed adverse effects (≥20 %) [125] are:
dry eye; dry mouth; dry skin; nausea; vomiting; diarrhea; constipation; decreased appetite; abdominal pain; nail toxicity; hypophosphatemia; hyperphosphatemia; arthralgia; fatigue; back pain; alopecia; stomatitis, and dysgeusia.
12. Epithelioid Sarcoma
12.1. Active ingredient name: tazemetostat
Drug Brand Name: Tazverik.
Background and Mechanism of Action: Tazemetostat, under the brand name of TAZVERIK, was developed by Epizyme and Eisai and was approved by the FDA on 23rd January 2020 for the treatment of patients aged ≥16 years having metastatic or locally advanced epithelioid sarcoma 127. It is a first-in-class enhancer of zeste homolog 2 (EZH2) inhibitor, a histone-lysine N-methyltransferase enzyme [127], [128]. Tazemetostat is also being tested in clinical trials in numerous countries across the world for the treatment of different cancers, including diffuse large B-cell lymphoma and mesothelioma. Besides inhibition of methyltransferase, EZH2 and some EZH2 mutations like Y646X and A687V, also restrict EZH1 with IC50 of 392 nM which is 36 times more than IC50 for inhibition of EZH2 [128]. EZH2 acts as a catalytic subunit of polycomb repressive complex 2 (PRC2), trimethylation of histone H3 which is responsible for transcriptional repression. The PRC2 function can antagonized by SWItch/Sucrose Non-Fermentable (SWI/SNF) complexes in the control of gene expression. In preclinical investigations, the benefit of combining tazemetostat with other medications was evaluated, tazemetostat showed synergistic effects when it was combined with glucocorticoid receptor agonists, B-cell receptor pathway modulators, venetoclax in lymphoma cell lines or in prostate cancer cell line 127.
Structure.

Molecular Formula: C34H44N4O4.
IUPAC name: N-[(4,6-dimethyl-2-oxo-1H-pyridin-3-yl)methyl]-3-[ethyl(oxan-4-yl)amino]-2-methyl-5-[4-(morpholin-4-ylmethyl)phenyl]benzamide.
Type: Small Molecule.
Dosage: Normally, the patient's healthcare provider recommends 800 mg of TAZVERIK twice daily. Patients have to swallow whole tablets of TAZVERIK orally, without chewing or cutting them, until disease progression or unacceptable toxicity [128]. If a dosage is missed or vomiting occurs after TAZVERIK, do not take an extra dose; instead, proceed with the next regular dose.
Synthesis: The synthetic route was discussed in Scheme 22 [129]. The synthesis of Tazemetostat was initiated with bromination of 3-nitro-o-toluic acid (246) which gave the 5-Bromo-2-methyl-3-nitrobenzoic acid (248). After that, methylation of the carboxylic group followed by reduction of nitro group afforded compound 250 which underwent 2 times reductive amination to form 252 which gave the compound 253 via base hydrolysis. After that, compound 253 reacted with 254 at room temperature to afford 255 which finally underwent Suzuki-coupling reaction to give the desired drug Tazemetostat.
Scheme 22.
Synthesis of Tazemetostat.
Adverse effects: Patients (n = 62) with epithelioid sarcoma were enrolled in a Cohort of Study EZH-202, which examined the safety of TAZVERIK. Patients were received a dose of 800 mg of TAZVERIK orally twice daily, and among them 44 % were treated for 6 months and 24 % were treated more than one year [128]. 37 % of patients who received TAZVERIK experienced serious adverse effects and among them, more than 3 % were suffered from peural effusion, haemorrhage, skin infection, respiratory distress, pain and dyspnea.
Common adverse effects of Tazemetostat are [128] fatigue; decreased appetite; pain; constipation; vomiting, and nausea.
13. Myelofibrosis
13.1. Active ingredient name: Fedratinib
Drug Brand Name: Inrebic.
Background and Mechanism of Action:
The US-FDA approved Fedratinib in the USA on August 16, 2019, to treat intermediate-2 or high-risk primary or secondary (post-essential thrombocythemia or post-polycythemia vera) myelofibrosis (MF) in adult patents [130], [131], [132]. The JAK2-selective inhibitor, Fedratinib (INREBIC®) is used orally for the treatment of myelofibrosis, a rare bone marrow chronic disorder, in which scar tissue develops in the bone marrow and the generation of blood cells switches to the liver and spleen from the bone marrow, which results in organ enlargement 130.
As a kinase inhibitor, Fedratinib acts against mutationally activated and wild type Janus Associated Kinase 2 (JAK2) and FMS-like tyrosine kinase 3 (FLT3) 131. Fedratinib can be considered a JAK2-selective inhibitor and shows greater inhibitory activity for JAK2 over the other family members like JAK1, JAK3 and TYK2 (Tyrosine Kinase 2). Myeloproliferative neoplasms (MPNs), along with polycythemia vera and myelofibrosis cause the anomalous activation of JAK2.In cell models, where FLT3ITD or JAK2V617F was exhibiting mutational activity, the activator of transcription (STAT3/5) proteins and the phosphorylation of signal transducer were diminished by Fedratinib, which resulted in the prevention of cell proliferation, as well as the apoptotic cell death.
Structure.
Molecular Formula:C27H36N6O3S·2HCl·H2O.
Chemical Name:
N-tert-butyl-3-[(5-methyl-2-{[4-(2-pyrrolidin-1-ylethoxy)phenyl]amino}pyrimidin-4-yl)amino]benzenesulfonamide dihydrochloride monohydrate.
Type: Small Molecule.
Dosage: The patients, who have a baseline platelet count of at least 50 x 109/L, are recommended to take orally 400 mg of INREBIC® daily with or without food [131]. In the event of a missed INREBIC® dose, patients should take the next scheduled dose the following day.
Patients undergoing Ruxolitinib (Jakafi®) treatment for myelofibrosis (MF) must reduce and eventually stop according to the Ruxolitinib prescribing information prior to the introduction of INREBIC® [131,133,134].
Synthesis: A synthetic route of Fedratinib is provided by Arvind Jayan and Co.as described in Scheme 23 (World Intellectual Property Organization, Patent Number: WO2012061833) [135]. At first, the pyrimidine compound 257 and 3-bromo-N-tert-butyl-benzenesulfonamide (258) were refluxed in the presence of Pd2(dba)3, Xantphos and cesium carbonate in dioxane under Argon (Ar) atmosphere for 3 h to produce the intermediate 259 in 98 % yield. Then, the intermediate 259 was treated with phenylamine compound 260 in acetic acid with microwave (MW) irradiation at 150 °C for 20 min to generate the desired product Fedratinib in 27 % yield.
Scheme 23.
Synthesis of Fedratinib.
Adverse effects:
The safety of the drug INREBIC was examined in the JAKARTA trial, a randomized, double-blind, placebo-controlled clinical trial where 96 patients received placebo and another group of 96 patients 400 mg of INREBIC daily [131]. The median exposure duration to the drug INREBIC 400 mg daily was 15.5 months, while that duration to placebo was 6 months or until disease progression. 65 years (range: 27–86 years) was the median age of the patients getting INREBIC, with 92 % having a PS of ECOG of 0–1.
21 % of the patients receiving INREBIC 400 mg daily had serious adverse reactions, including anemia and cardiac failure, whereas 1 % of patients suffered cardiogenic shock as fatal adverse reaction.
The most frequently observed adverse effects (≥20 %) [131] are nausea; vomiting; diarrhea, and anemia.
14 Myelodysplastic syndromes
14.1. Active ingredients Names:(A) decitabine, and (B) cedazuridine
Drug Brand Name: Inqovi.
Background and Mechanism of Action:
Inqovi™ acquired its first approval from FDA in Canada and USA on July 7, 2020, as a treatment of chronic myelomonocytic leukaemia (CMML) and myelodysplastic syndromes (MDS) [136,137]. Taiho Pharmaceutical and Astex Pharmaceuticals, a wholly-owned subsidiary company of Otsuka Pharmaceutical Co., Ltd. (Tokyo, Japan), developed a fixed dose oral combination (FDC) of cedazuridine and decitabine and marketed as Inqovi® to treat several cancers, such as chronic myelomonocytic leukaemia (CMML), myelodysplastic syndromes (MDS), and acute myeloid leukaemia (AML) [136,138,139]. Taiho Oncology, Inc., and Taiho Pharma Canada, Inc. are responsible for the commercialization of Inqovi™ in the USA and Canada, respectively [136,139,140]. INQOVI™ contains decitabine and cedazuridine as active ingredients. Decitabine acts as a nucleoside metabolic inhibitor, and Cedazuridine serve as a cytidine deaminase inhibitor [136,137].
-
(A)
Mechanism of Action of Decitabine: As a nucleoside metabolic inhibitor, Decitabine leads to the cellular differentiation and/or apoptosis and DNA hypomethylation when it phosphorylates and directly incorporates into DNA, hindering DNA methyltransferase [137]. Hypomethylation caused by Decitabine in cancer cells, may bring back the usual function to genes, which are risky for the control of proliferation and cellular differentiation. Also, in vitro, methylation of DNA is inhibited by Decitabine. However, Decitabine is ineffective against non-proliferating cells.
-
(B)
Mechanism of Action of Cedazuridine: The enzyme cytidine deaminase (CDA) plays the role of catalysis for the breakdown of cytidine as well as the cytidine analogue Decitabine [137]. If the level of CDA in the liver and gastrointestinal tract is high, it can lead to the degradation of Decitabine, restricting its oral bioavailability. Since Cedazuridine acts as a CDA inhibitor, it is combined with Decitabine to increase the effectiveness of Decitabine, hence the drug.
Structure.
Molecular Formula.
-
(A)
Decitabine: C8H12N4O4
-
(B)
Cedazuridine: C9H14F2N2O5
Chemical Name.
-
(A)
Decitabine: 4-amino-1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,3,5-triazin-2(1H)-one
-
(B)
Cedazuridine: (4R)-1-[(2R,4R,5R)-3,3-difluoro-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-4-hydroxy-1,3-diazinan-2-one
Type.
-
(A)
Decitabine: Small Molecule
-
(B)Cedazuridine: Small Molecule
- Dosage: 1 tablet containing 100 mg cedazuridine and 35 mg decitabine is recommended to be consumed orally on an empty stomach once per day on Days 1 through 5 of each 28-day cycle [137]. The intake of the drug has to be continued for at least 4 cycles until unacceptable toxicity or disease progression occurs.
Synthesis.
-
(A)
Synthesis of Decitabine:
Shilpa Medicare Limited, India provided a synthetic procedure for the preparation of Decitabine (India, Patent Number: IN2015CH04265) (Scheme 24) [141]. Initially, in the presence of ammonium sulphate 5-Azacytosine (261) treated with hexamethyldisilazane (HDMS) at 110–130 °C to generate protected 5-Azacytosine 262, which reacted with l-methoxy-3,5-di-O-acetyl-2-deoxy ribose (263) at 20–30 °C to afford the mixture of compounds Beta isomer (264) and Alpha isomer (265). The separation of crude Decitabine from the Alpha isomer (265) was accomplished using methanolic ammonia, followed by washing with ethyl acetate. At last, the crude Decitabine was purified with the use of methanol and pure crystalline Decitabine was isolated.
-
(B)
Synthesis of Cedazuridine:
Scheme 24.
Synthesis of Decitabine using 5-azacytosine.
In 2014, Dana Ferraris and team suggested a methodology for the preparation of Cedazuridine from Gemcitabine (266) (Scheme 25) [142]. At first, Gemcitabine (266) was hydrolyzed in the presence of Rh/C catalyst to provide 2′-deoxy-2′,2′-difluorodihydrouridine 267. Then, the epimers 268 (29 %) and 269 (31 %) were obtained through the reduction of the compound 267 using sodium borohydride. The desired Cedazuridine (epimer 268) was isolated by preparative HPLC.
Scheme 25.
Synthesis of Cedazuridine from Gemcitabine.
Adverse effects:
The safety of the drug INQOVI was investigated in Clinical Study ASTX727-01-B (NCT02103478) and (NCT03306264) [137]. 213 patients in Study ASTX727-01-B and 80 patients in Study ASTX727-02, a total of 213 patients, participated. 24 % of patients taking INQOVI were exposed to the drug for >1 year, and 24 % were exposed for ≥6 months. 6 % of the patients had fatal adverse reactions, including respiratory failure, septic shock, sepsis, cerebral haemorrhage, pneumonia, and sudden death. Serious adverse reactions, such as sepsis, pneumonia, and neutropenia, occurred in >5 % of patients.
The usual adverse effects (≥20 %) [137] of the drug are fatigue; headache; dyspnea; upper respiratory tract infection; rash; increased transaminase; febrile neutropenia; pneumonia; decreased appetite; edema; dizziness; diarrhea, etc. The regular Grade 3 or 4 laboratory abnormalities (≥50 %) [137] are decreased neutrophil count; decreased platelet count; decreased leukocytes and decreased hemoglobin.
15. Neurofibromatosis type- 1
15.1. Active ingredient name: selumetinib
Drug Brand Name: Koselugo.
Background and Mechanism of Action: Selumetinib, under the brand name of KOSELUGO, was developed by AstraZeneca and was approved by FDA on 10th April 2020 for the treatment of patients having neurofibromatosis type 1 (NF1) 143. It is a monomorphic white to yellowish crystalline substance that shows anticancer activity by inhibiting mitogen-activated protein kinase 1 and 2 (MEK1/2) [144]. Selumetinib inhibits signalling of the abnormal protein which responsible for the tumour growth. The US Food and medication Administration has also designated selumetinib as an orphan medication for adjuvant therapy of differentiated thyroid carcinoma in stages III or IV. Mitogen-activated protein kinase kinases 1 and 2 (MEK1/2) and extracellular signal-related kinase (ERK) are important components for RAS-RAF-MEK-ERK signalling responsible for various types of cancers. In the study on the Neurofibromatosis type 1 (NF1) genetic model of mice, epitomize of human NF1 model shows inhibition of phosphorylation on the dosing of Selumetinib, resulting neurofibroma proliferation reduction 144.
Structure.

Molecular Formula: C17H15BrClFN4O3.
IUPAC name: 6-(4-bromo-2-chloroanilino)-7-fluoro-N-(2-hydroxyethoxy)-3-methylbenzimidazole-5-carboxamide.
Type: Small Molecule.
Dosage: The suggested KOSELUGO dose is 25 mg/m2 orally twice daily on an empty stomach, i.e., food should not be taken 1 h after or 2 h before taking the dose [144]. Patients have to swallow the whole capsule with water without dissolving or opening it. If vomiting occurs after taking KOSELUGO, do not take another dosage; instead, proceed with the next planned dose.
Synthesis: Wallace and co-workers developed a methodology for the synthesis of Selumetinib which discussed in Scheme 26 [145]. Initially, 2,3,4-trichlorobenzoic acid (270) went through a nitration process to afford 2,3,4-Trifluoro-5-nitrobenzoic acid (271) which further reacted with ammonium hydroxide at 0 °C to obtain 272. This intermediate 272 then went through an esterification process in presence of TMSCHN2 to afford methyl ester 273 which subsequently reacted with aniline and compound 274 was found. After that, on reduction of compound 274 using Pd(OH)2/C followed by reaction with formic acid in ethanol gave the intermediate 275 in 86 % yield. Then, the 4′-position and 2′-position of that free-phenyl ring was brominated and chlorinated sequentially by using NBS and NCS respectively to produce 277 which underwent N-methylation reaction and gave the substance 278. Then ester hydrolysis followed by substitution reaction with o-(2-vinyloxy-ethyl)-hydroxylamine (280) gave the compound 281 which underwent acid hydrolysis reaction to afford the desired drug Selumetinib.
Scheme 26.
Synthesis of Selumetinib.
Adverse effects: The information in the WARNINGS AND PRECAUTIONS section describes according to the administration of KOSELUGO to 74 pediatric patients at doses ranging from 20 to 30 mg/m2 orally twice daily in SPRINT. These patients' exposure to KOSELUGO lasted 12 months or more (91 %), more than 2 years (74 %), or more than 4 years (23 %), including dose interruptions.
24 % of those KOSELUGO-administered patients experienced serious adverse reactions including diarrhea, hypoxia and anemia. Dosage interruptions occurred in 80 % of patients and 24 % of patients were received dose reductions because of some serious adverse reaction.
Most common adverse effects [144] (≥40 %) are abdominal pain; nausea; vomiting; acneiform rash; dry skin; diarrhea; pyrexia; fatigue; pruritus and musculoskeletal pain.
16. Drugs for the detection of cancer cells
16.A. Detection of prostate cancer
16.A.1. Active ingredient name: Piflufolastat F 18
Drug Brand Name: Pylarify.
Background and Mechanism of Action: Piflufolastat F 18, under the brand name PYLARIFY, was developed by Progenics Pharmaceuticals Inc. and was approved by the FDA on 26th May 2021 [146,147]. This urea-based radioactive tracer used in positron emission tomography (PET) of prostate-specific membrane antigen (PSMA) positive lesions in males having prostate cancer with suspected metastasis or recurrence. Prostate-specific membrane antigen (PSMA), a transmembrane glycoprotein is usually found on normal prostate cell buit's expression level on prostate cancer cells is even higher than elsewhere in the body [147]. Piflufolastat F 18 binds with malignant prostate cancer cells having excessive amounts of PSMA and enables positron emission tomography. The action of androgen deprivation treatment and other medications targeting the androgen pathway may affect the absorption of pifufolastat F 18 in prostate cancer. Serum PSA levels can effect PET imagining using Piflufolastat F 18 and Risk variables such as Gleason score and tumour stage can affect imaging of metastatic pelvic lymph nodes prior to initial decisive treatment.146.
Structure.
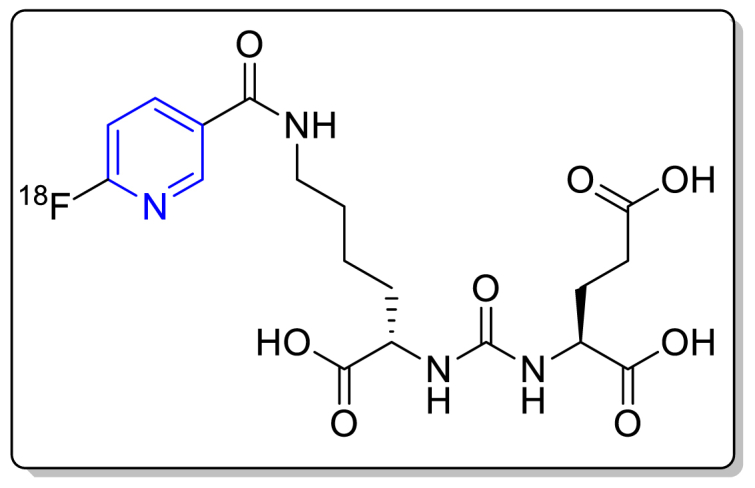
Molecular Formula: C18H23FN4O8.
IUPAC name: (2S)-2-[[(1S)-1-carboxy-5-[(6-(18F)fluoranylpyridine-3-carbonyl)amino]pentyl]carbamoylamino]pentanedioic acid.
Type: Small Molecule.
Dosage: The recommended amount of Pylarify is 9 mCi (333 MBq) with a suitable rang of 8 mCi–10 mCi (296 MBq–370 MBq) administered as a single dose of intravenous injection [147].
Synthesis: In the year of 2016, Vincent Bouvet et al. developed a methodology for the radiosynthesis of Piflufolastat F 18 (Scheme 27) [148]. For the synthesis of trimethylammonium salt 285, initially active ester N,N,N-tri-methyl-5-((2,3,5,6-tetrafluorophenoxy)carbonyl)-pyridin-2-aminium triflate 283 was prepared from 6-chloronicotinic acid 282 with small modifications based on the synthetic scheme developed by Dag E. Olberg and co-othurs [149]. Then, trimethylammonium salt 285 was prepared from the acylation reaction occurred between active ester 283 and compound 284. The nucleophilic heteroaromatic substitution reaction, occurred between activated [18F]fluoride, that was synthesized from [18O]H2O and the trimethylammonium salt 285 yielded compound 286. At last, removal of the protecting group (tert-butyl ester) by acidic cleavage gave the desired drug Piflufolastat F 18.
Scheme 27.
Synthesis of Piflufolastat F 18.
Adverse effects: PYLARIFY was tested for safety in 593 individuals, each of whom received one dosage with average injected activity 9.2 ± 0.7 mCi (340 ± 26 MBq) [147]. More than 0.5 % of patients showed adverse reactions and 0.2 % of patients having a history of allergic condition showed hypersensitivity reaction.
The most common adverse effects [147] are dysgeusia (2 % patients); headache (2 % patients), and fatigue (1 % patients).
16.B. Detection of neuroendocrine tumors (NETs)
16.B.1. Active ingredient name: Ga-68-DOTATOC
Drug Brand Name: Ga-68-DOTATOC.
Background and Mechanism of Action: Ga-68-DOTATOC was approved by FDA on August 21, 2019, and this intravenous injection used as a radioactive diagnostic agent in the treatment of adult and pediatric patients having somatostatin receptor-positive neuroendocrine tumors (NETs) [150,151]. Ga-68-DOTATOC is a cyclic 8 amino acid peptide which contains a covalently bound chelator. Its amino acid sequences are: H-D-Phe-Cys-Tyr-D-Trp-Lys-Thr-Cys-Thr-OH and it also contains one disulfide bond. One Ga-68-DOTATOC intravenous injection contains 0.5 mCi/mL to 4 mCi/mL (18.5 MBq/mL to 148 MBq/mL) of Ga 68 DOTATOC, octreotide (3.6 mcg/mL) and ethanol (10 % v/v) in NaCl (9 mg/mL) solution. This pyrogen-free, colourless clear buffer solution has a pH between 4 and 8. Ga 68 DOTATOC attaches to the somatostatin receptors and it shows the greatest affinity towards subtype 2 receptors (sstr2 151. Ga 68 DOTATOC attaches to somatostatin receptors that overexpress in malignant neuroendocrine cells. Gallium 68 is a radionuclide that emits β+ which correlates to 511 keV annihilation photons and permits positron emission tomography (PET) imaging.
Structure.
Molecular Formula: C65H89GaN14O18S2.
IUPAC name: Gallium-68 labelled 2-[4-[2-[[(2R)-1-[[(4R,7S,10S,13R,16S,19R)-10-(4-aminobutyl)-4-[[(2R,3R)-1,3 dihydroxybutan-2-yl]carbamoyl]-7-[(1R)-1-hydroxyethyl]-16-[(4-hydroxyphenyl)methyl]-13-(1H-indol3-ylmethyl)-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentazacycloicos-19-yl]amino]-1-oxo-3 phenylpropan-2-yl]amino]-2-oxoethyl]-7,10-bis(carboxymethyl)-1,4,7,10-tetrazacyclododec-1-yl]acetic acid.
Type: Small Molecule.
Dosage: The suggested amount of radioactivity to apply in adults for PET imaging is 148 MBq (4 mCi) with a range: lies between 111 MBq and 185 MBq (3 mCi–5 mCi). This imaging agent should be administered as intravenous injection with 10 s/mL injection rate [151]. For pediatric patients, the suggested amount of radioactivity for PET imaging is 1.59 MBq/kg of body weight within a range: between 11.1 MBq and 111 MBq (0.3 mCi–3 mCi) as intravenous injection with same injection rate.
Adverse effects: Ga-68 DOTATOC Injection's safety and effectiveness were demonstrated in two single-center, open-label trials (Study A and Study B), in which 282 patients (59 % females) with confirmed or suspected SSTR-positive NETs received a single dose of Ga-68 DOTATOC [151]. At the time of Ga-68 DOTATOC imaging, 84 % of patients had a history of malignancy. The average age was 54 years (with a range: of 4–82 years).
The reported adverse effect arises with a rate of <2 % is a Gastrointestinal Disorders: nausea and adverse effects arises with a rate of <1 % are flushing, a vascular disorder and pruritus, a skin and subcutaneous Tissue disorder [151].
16.B.2. Active ingredient name: copper Cu-64 Dotatate Injection
Drug Brand Name: Detectnet.
Background and Mechanism of Action: Copper Cu-64 Dotatate Injection (DetectnetTM) was developed by RadioMedix, Inc. as a radioactive diagnostic agent and on September 03, 2020, it was approved by FDA to spot somatostatin receptor-positive neuroendocrine tumors (NETs) when using positron emission tomography (PET) scan[152], [153].
Copper Cu-64 dotatate targets the cells exhibiting somatostatin receptors involving malignant neuroendocrine cells, which tend to overexpress SSTR2 receptors 153. It has the highest affinity for marking subtype 2 receptors (SSTR2). As a positron (β+) emitting radionuclide, Copper Cu-64 is convenient to use in positron emission tomography (PET) scanning.
Structure.
Molecular Formula: C65H88CuN14O19S2.
Chemical Name: Copper (Cu-64)-N-[(4,7,10-tricarboxymethyl-1,4,7,10 tetraazacyclododec-1-yl)acetyl]-D-phenylalanyl-L-cysteinyl-l-tyrosyl-d-tryptophanyl-L-lysyl-L-threoninyl-L-cysteinyl-l-threonine-cyclic (2–7) disulfide.
Type: Small Molecule.
Dosage: It is recommended to take 148 MBq (4 mCi) of Detectnet™ administered as intravenous bolus injection [153]. 45–90 min after injecting the drug, the images of PET scan can be initiated to obtain.
Adverse effects:
The population of the trail of efficacy and safety trials of the drug Detectnet was 71; among them, the number of healthy volunteers was 21 [153]. Because of the radioactivity, the use of copper Cu-64 dotatate may have the chance of exposing to radiation for lifetime [152,153]. The commonly observed adverse effects are: vascular disorders (flushing) and gastrointestinal disorders (vomiting, nausea).
Conclusions
Despite substantial efforts, cancer remains a significant concern in illness treatment, even with the introduction of new medicines. One of the key challenges in the developing world is the rising prevalence of cancer. Novel therapeutic agents are badly needed for the treatment of malignancies that have developed resistance to current medications. As a result, the synthesis of effective therapeutic molecules with anticancer activity has piqued the interest of many researchers, and mostly heterocycles were found to be the most important and predominant in those anticancer medications due to their versatility and inherent resourcefulness as well as their physicochemical potencies. Heterocyclic rings in those drug molecules can bind to receptors via a variety of intermolecular forces, including hydrogen bonding, p-p stacking interactions, van der Waals and hydrophobic interactions, dipole-induced dipole, and coordinated covalent bonding with metals at the target site. In terms of method of action, different types of anticancer medications function in different ways. For example, some treatments work by adding an alkyl group to DNA to make cell proliferation harder (alkylating agent). Some medications (antimetabolites) function by interfering with DNA synthesis. Some medications impede cell division. Heterocyclic anticancer medications are only one type of anticancer drug, and their method of action varies according to the drug. In this review, we have classified 34 heterocycle-containing drug moieties according to their application in various types of cancers that were approved by the FDA between 2019 and 2021. Also, their mechanism of action, synthetic route, and adverse effects were discussed. The majority of pharmaceuticals were synthesized for the treatment of lung cancer; however, for certain tumors, only one therapy was accessible at that time frame. We could expect more advancements, particularly in sequences, due to the multi-step nature required to build new heterocyclic drug molecules. Without a doubt, the present demand for pharmaceutical corporations to produce novel species more quickly and at a cheaper cost will promote research and discovery, enabling numerous new ways. This review will be of interest to many organic synthetic chemists, as well as pharmacists, biologists, and anticancer drug practitioners and specialists.
Expert Opinion
The heterocyclic drugs are gaining significance in cancer treatment because of their mode of action, many enzyme pocket sites, and target specificity. The researchers are developing more anticancer heterocyclic drugs for FDA approval, and demands are increasing in the market daily. In a short period, 34 medications, as given in this review, got FDA approval with a wide variety of covering cancer treatment. Heterocyclic chemotypes like imidazole, having a moiety anticancer compound, was patented by Dr Mossaraf Hossain and have tremendous potential of becoming an anticancer therapeutic agent in future. Because of the limitation of page number and focusing on FDA-approved heterocyclic compounds, we have not touched the biological aspect of each drug in detail.
We aim to give readers more information about FDA-approved heterocyclic anticancer compounds. Those interested in more details about particular combinations could get it from the FDA site. If we go into more detail, the review's focus will change, and it will be lengthy.
Data Availability Statement
Data will be made available on request.
Additional information
No additional information is available for this paper.
CRediT authorship contribution statement
Mossaraf Hossain: Writing - review & editing, Writing - original draft, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Imran Habib: Writing - review & editing, Writing - original draft, Data curation, Conceptualization. Koustav Singha: Writing - review & editing, Writing - original draft, Data curation, Conceptualization. Anoop Kumar: Writing - review & editing, Validation, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The author (MH) highly acknowledges UGC-SRG-India (No.F.30-597/2021-BSR) for financial support. The authors IH and KS thank West Bengal govt. for SVMCM fellowship.
References
- 1.Martins P., Jesus J., Santos S., Raposo L.R., Roma-Rodrigues C., Baptista P.V., Fernandes A.R. Heterocyclic anticancer compounds: recent advances and the paradigm shift towards the use of nanomedicine's tool box. Molecules. 2015;20(9):16852–16891. doi: 10.3390/molecules200916852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pearce Simon. The importance of heterocyclic compounds in anti-cancer drug design. Drug Discovery. 2017;67 [Google Scholar]
- 3.Whitesell J.K. C2 symmetry and asymmetric induction. Chem. Rev. 1989;89(7):1581–1590. [Google Scholar]
- 4.Peerzada M.N., Hamel E., Bai R., Supuran C.T., Azam A. Deciphering the key heterocyclic scaffolds in targeting microtubules, kinases and carbonic anhydrases for cancer drug development. Pharmacol. Ther. 2021;225 doi: 10.1016/j.pharmthera.2021.107860. [DOI] [PubMed] [Google Scholar]
- 5.Sachdeva H., Khaturia S., Saquib M., Khatik N., Khandelwal A.R., Meena R., Sharma K. Oxygen-and sulphur-containing heterocyclic compounds as potential anticancer agents. Appl. Biochem. Biotechnol. 2022;194(12):6438–6467. doi: 10.1007/s12010-022-04099-w. [DOI] [PubMed] [Google Scholar]
- 6.Akhtar J., Khan A.A., Ali Z., Haider R., Yar M.S. Structure-activity relationship (SAR) study and design strategies of nitrogen-containing heterocyclic moieties for their anticancer activities. Eur. J. Med. Chem. 2017;125:143–189. doi: 10.1016/j.ejmech.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 7.Saraswat P., Jeyabalan G., Hassan M.Z., Rahman M.U., Nyola N.K. Review of synthesis and various biological activities of spiro heterocyclic compounds comprising oxindole and pyrrolidine moieties. Synth. Commun. 2016;46(20):1643–1664. [Google Scholar]
- 8.Gao F., Wang T., Xiao J., Huang G. Antibacterial activity study of 1, 2, 4-triazole derivatives. Eur. J. Med. Chem. 2019;173:274–281. doi: 10.1016/j.ejmech.2019.04.043. [DOI] [PubMed] [Google Scholar]
- 9.Sharma V., Gupta M., Kumar P., Sharma A. A comprehensive review on fused heterocyclic as DNA Intercalators: promising anticancer agents. Curr. Pharm. Des. 2021;27(1):15–42. doi: 10.2174/1381612826666201118113311. [DOI] [PubMed] [Google Scholar]
- 10.Al-Mulla A. A review: biological importance of heterocyclic compounds. Der Pharma Chem. 2017;9(13):141–147. [Google Scholar]
- 11.Darque A., Dumètre A., Hutter S., Casano G., Robin M., Pannecouque C., Azas N. Synthesis and biological evaluation of new heterocyclic quinolinones as anti-parasite and anti-HIV drug candidates. Bioorg. Med. Chem. Lett. 2009;19(20):5962–5964. doi: 10.1016/j.bmcl.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 12.Metwally N.H., Abdelrazek F.M., Eldaly S.M. Synthesis and anticancer activity of some new heterocyclic compounds based on 1-cyanoacetyl-3, 5-dimethylpyrazole. Res. Chem. Intermed. 2016;42:1071–1089. [Google Scholar]
- 13.Belwal S., Saritha R., Sachdeva H., Kiran G. Synthesis, characterization and prediction of anticancer potentiality of some novel green nanoparticles by molecular docking and ADMET techniques. Bull. Chem. Soc. Ethiop. 2019;33(3):493–504. [Google Scholar]
- 14.Chernyshov V.V., Yarovaya O.I., Fadeev D.S., Gatilov Y.V., Esaulkova Y.L., Muryleva A.S., Sinegubova K.O., Zarubaev V.V., Salakhutdinov N.F. Single-stage synthesis of heterocyclic alkaloid-like compounds from (+)-camphoric acid and their antiviral activity. Mol. Divers. 2020;24:61–67. doi: 10.1007/s11030-019-09932-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saini M.S., Kumar A., Dwivedi J., Singh R. A review: biological significances of heterocyclic compounds. Int. J. Pharm. Sci. Res. 2013;4(3):66–77. [Google Scholar]
- 16.Alrooqi M., Khan S., Alhumaydhi F.A., Asiri S.A., Alshamrani M., Mashraqi M.M., Alzamami A., Alshahrani A.M., Aldahish A.A. A therapeutic journey of pyridine-based heterocyclic compounds as potent anticancer agents: a review (from 2017 to 2021) Anti Cancer Agents Med. Chem. 2022;22(15):2775–2787. doi: 10.2174/1871520622666220324102849. [DOI] [PubMed] [Google Scholar]
- 17.https://www.drugdiscoverytrends.com/50-of-2022s-best-selling-pharmaceuticals/ (Accessed on May 8, 2023)
- 18.Tandon R., Singh I., Luxami V., Tandon N., Paul K. Recent advances and developments of in vitro evaluation of heterocyclic moieties on cancer cell lines. Chem. Rec. 2019;19(2-3):362–393. doi: 10.1002/tcr.201800024. [DOI] [PubMed] [Google Scholar]
- 19.Kocarnik J.M., Compton K., Dean F.E., Fu W., Gaw B.L., Harvey J.D., Henrikson H.J., Lu D., Pennini A., Xu R., Ababneh E. Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: a systematic analysis for the global burden of disease study 2019. JAMA Oncol. 2022;8(3):420–444. doi: 10.1001/jamaoncol.2021.6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Begley C.G., Ellis L.M. Raise standards for preclinical cancer research. Nature. 2012;483(7391):531–533. doi: 10.1038/483531a. [DOI] [PubMed] [Google Scholar]
- 21.https://www.statista.com/topics/8292/cancer-worldwide/#topicOverview
- 22.https://www.statista.com/statistics/791347/top-diseases-worldwide-by-number-of-active-drugs/
- 23.https://www.who.int/news-room/fact-sheets/detail/cancer
- 24.https://gco.iarc.fr/
- 25.https://www.statista.com/statistics/288559/new-cancer-cases-worldwide-by-type/
- 26.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 27.https://www.cancer.net/navigating-cancer-care/how-cancer-treated
- 28.Sachdeva H., Khaturia S., Saquib M., Khatik N., Khandelwal A.R., Meena R., Sharma K. Oxygen-and sulphur-containing heterocyclic compounds as potential anticancer agents. Appl. Biochem. Biotechnol. 2022;194(12):6438–6467. doi: 10.1007/s12010-022-04099-w. [DOI] [PubMed] [Google Scholar]
- 29.Lang D.K., Kaur R., Arora R., Saini B., Arora S. Nitrogen-containing heterocycles as anticancer agents: an overview. Anti Cancer Agents Med. Chem. 2020;20(18):2150–2168. doi: 10.2174/1871520620666200705214917. [DOI] [PubMed] [Google Scholar]
- 30.Heravi M.M., Zadsirjan V. Prescribed drugs containing nitrogen heterocycles: an overview. RSC adv. 2020;10(72):44247–44311. doi: 10.1039/d0ra09198g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan S., Luo Y.Q., Zuo J.H., Liu H., Li F., Yu B. New drug approvals for 2020: synthesis and clinical applications. Eur. J. Med. Chem. 2021;215 doi: 10.1016/j.ejmech.2021.113284. [DOI] [PubMed] [Google Scholar]
- 32.Markham A. Alpelisib: first global approval. Drugs. 2019;79(11):1249–1253. doi: 10.1007/s40265-019-01161-6. [DOI] [PubMed] [Google Scholar]
- 33.https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212526s000lbl.pdf
- 34.Furet P., Guagnano V., Fairhurst R.A., Imbach-Weese P., Bruce I., Knapp M., Fritsch C., Blasco F., Blanz J., Aichholz R., Hamon J., Fabbro D., Caravatti G. Discovery of NVP-BYL719 a potent and selective phosphatidylinositol-3 kinase alpha inhibitor selected for clinical evaluation. Bioorg. Med. Chem. Lett. 2013;23(13):3741–3748. doi: 10.1016/j.bmcl.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Keam S.J. Trastuzumab deruxtecan: first approval. Drugs. 2020;80(5):501–508. doi: 10.1007/s40265-020-01281-4. [DOI] [PubMed] [Google Scholar]
- 36.https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761139s000lbl.pdf
- 37.Lee A. Tucatinib: first approval. Drugs. 2020;80(10):1033–1038. doi: 10.1007/s40265-020-01340-w. [DOI] [PubMed] [Google Scholar]
- 38.https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213411s000lbl.pdf
- 39.Kulukian A., Lee P., Taylor J., Rosler R., de Vries P., Watson D., Forero-Torres A., Peterson S. Preclinical activity of HER2-selective tyrosine kinase inhibitor tucatinib as a single agent or in combination with trastuzumab or docetaxel in solid tumor models. Mol. Cancer Ther. 2020;19(4):976–987. doi: 10.1158/1535-7163.MCT-19-0873. [DOI] [PubMed] [Google Scholar]
- 40.https://scifinder-n.cas.org/pdf/v1/AUTH_5060d187888f42fe97a1589aed05c6d6/full-patentpak-fulltext-45/TXA8rNu4EDSmnXGV3Y_esTb2awIAhFqyHsk9HFX9lp0.pdf?temp_url_sig=06502849a35068768ef74246d401a3a0d76b8ec7&temp_url_expires=1675799036&inline
- 41.Lyu Y., Huang L., Zhu X., Lu S., Mao Y. A practical alternate synthesis of tucatinib. Org. Prep. Proced. Int. 2021;53(6):554–561. [Google Scholar]
- 42.Ayothiraman R., Bandaru D., Paranthaman R., Fenster M., Eastgate M.D., Vaidyanathan R. T3P-Mediated N–N cyclization for the synthesis of 1, 2, 4-triazolo [1, 5-a] pyridines, org. Process Res. Dev. 2019;23(11):2510–2515. [Google Scholar]
- 43.Syed Y.Y. Sacituzumab govitecan: first approval. Drugs. 2020;80:1019–1025. doi: 10.1007/s40265-020-01337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761115s000lbl.pdf
- 45.https://www.gene.com/media/press-releases/14802/2019-08-15/fda-approves-genentechs-rozlytrek-entrec
- 46.https://www.fda.gov/news-events/press-announcements/fda-approves-third-oncology-drug-targets-key-genetic-driver-cancer-rather-specific-type-tumor
- 47.https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212726s000lbl.pdf
- 48.https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trials-snapshots-rozlytrek-0
- 49.https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trials-snapshots-rozlytrek
- 50.Al-Salama Z.T., Keam S.J. Entrectinib: first global approval. Drugs. 2019;79(13):1477–1483. doi: 10.1007/s40265-019-01177-y. [DOI] [PubMed] [Google Scholar]
- 51.Cordell M.J., Adams M.R., Vincent‐Rocan J.F., Riley J.G. Total synthesis of Entrectinib with key photo‐redox mediated cross‐coupling in flow. Eur. J. Org. Chem. 2022;2022(4) [Google Scholar]
- 52.Lombardi Borgia A., Menichincheri M., Orsini P., Panzeri A., Perone E., Vanotti E., Nesi M. 2009. Substituted Indazole Derivatives Active as Kinase Inhibitors. WO 2009/013126A1. [Google Scholar]
- 53.Dhillon S. Capmatinib: first approval. Drugs. 2020;80(11):1125–1131. doi: 10.1007/s40265-020-01347-3. [DOI] [PubMed] [Google Scholar]
- 54.https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213591s000lbl.pdf
- 55.https://scifinder-n.cas.org/patent-viewer?docuri=DDnnxYvOJOCikaCgcbParo9GppoJ9mqGXVnT-rPNjHU&markedFullTextKey=k2kR2ercOp3nWFzC6WN4wNZAwtpcN_fpOqt65T6BwDY.pdf&fullTextKey=vP1O8vYpv2TJoELGoghXxPOzbVPee99j4994xqo5PKY.pdf
- 56.Markham A. Naxitamab: first approval. Drugs. 2021;81(2):291–296. doi: 10.1007/s40265-021-01467-4. [DOI] [PubMed] [Google Scholar]
- 57.https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213721s000lbl.pdf
- 58.https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trial-snapshot-gavreto
- 59.Brubaker J.D., Kim J.L., Wilson K.J., Wilson D., DiPietro L.V. U.S. Patent No. US20170121312; 2017. Inhibitors of RET. [Google Scholar]
- 60.Yuan S., Luo Y.Q., Zuo J.H., Liu H., Li F., Yu B. New drug approvals for 2020: synthesis, and clinical applications. Eur. J. Med. Chem. 2021;215 doi: 10.1016/j.ejmech.2021.113284. [DOI] [PubMed] [Google Scholar]
- 61.Paik P.K., Felip E., Veillon R., Sakai H., Cortot A.B., Garassino M.C., Mazieres J., Viteri S., Senellart H., Van Meerbeeck J., Raskin J. Tepotinib in non–small-cell lung cancer with MET exon 14 skipping mutations. N. Engl. J. Med. 2020;383(10):931–943. doi: 10.1056/NEJMoa2004407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214096s000lbl.pdf
- 63.Dorsch D., Schadt O., Stieber F., Meyring M., Grädler U., Bladt F., Friese-Hamim M., Knühl C., Pehl U., Blaukat A. Identification and optimization of pyridazinones as potent and selective c-Met kinase inhibitors. Bioorg. Med. Chem. Lett. 2015;25(7):1597–1602. doi: 10.1016/j.bmcl.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 64.Markham A. Mobocertinib: first approval. Drugs. 2021;81(17):2069–2074. doi: 10.1007/s40265-021-01632-9. [DOI] [PubMed] [Google Scholar]
- 65.https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215310s000lbl.pdf
- 66.Wang J., Lam D., Yang J., Hu L. Discovery of mobocertinib, a new irreversible tyrosine kinase inhibitor indicated for the treatment of non-small-cell lung cancer harboring EGFR exon 20 insertion mutations. Med. Chem. Res. 2022;31(10):1647–1662. doi: 10.1007/s00044-022-02952-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Markham A. Lurbinectedin: first approval. Drugs. 2020;80(13):1345–1353. doi: 10.1007/s40265-020-01374-0. [DOI] [PubMed] [Google Scholar]
- 68.https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213702s000lbl.pdf
- 69.He W., Zhang Z., Ma D. A scalable total synthesis of the antitumor agents et‐743 and Lurbinectedin. Angew. Chem. Int. Ed. 2019;58(12):3972–3975. doi: 10.1002/anie.201900035. [DOI] [PubMed] [Google Scholar]
- 70.Chen R., Zhu D., Hu Z., Zheng Z., Chen X. A new approach to the synthesis of l-3-hydroxy-4-methoxy-5-methyl-phenylalanine derivatives from L-tyrosine. Tetrahedron: Asymmetry. 2010;21(1):39–42. [Google Scholar]
- 71.Corey E.J., Gin D.Y., Kania R.S. Enantioselective total synthesis of ecteinascidin 743. J. Am. Chem. Soc. 1996;118(38):9202–9203. [Google Scholar]
- 72.Bisi J.E., Sorrentino J.A., Roberts P.J., Tavares F.X., Strum J.C. Preclinical characterization of G1T28: a novel CDK4/6 inhibitor for reduction of chemotherapy-induced myelosuppression. Mol. Cancer Ther. 2016;15(5):783–793. doi: 10.1158/1535-7163.MCT-15-0775. [DOI] [PubMed] [Google Scholar]
- 73.https://www.fda.gov/news-events/press-announcements/fda-approves-drug-reduce-bone-marrow-suppression-caused-chemotherapy
- 74.https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214200s000lbl.pdf
- 75.https://patentimages.storage.googleapis.com/b1/75/b1/fa05793bcbe3e9/US20210122755A1.pdf
- 76.https://www.drugs.com/mtm/trilaciclib.html
- 77.Markham A., Duggan S. Darolutamide: first approval. Drugs. 2019;79(16):1813–1818. doi: 10.1007/s40265-019-01212-y. [DOI] [PubMed] [Google Scholar]
- 78.https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212099Orig1s000lbl.pdf
- 79.https://patentimages.storage.googleapis.com/d6/33/d3/ab10d1b99f87ef/WO2016162604A1.pdf
- 80.Markham A. Relugolix: first global approval. Drugs. 2019;79(6):675–679. doi: 10.1007/s40265-019-01105-0. [DOI] [PubMed] [Google Scholar]
- 81.https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/214621s000lbl.pdf
- 82.https://www.fda.gov/news-events/press-announcements/fda-approves-first-oral-hormone-therapy-treating-advanced-prostate-cancer
- 83.Miwa K., Hitaka T., Imada T., Sasaki S., Yoshimatsu M., Kusaka M., Tanaka A., Nakata D., Furuya S., Endo S., Hamamura K., Kitazaki T. Discovery of 1-{4-[1-(2, 6-difluorobenzyl)-5-[(dimethylamino) methyl]-3-(6-methoxypyridazin-3-yl)-2, 4-dioxo-1, 2, 3, 4-tetrahydrothieno [2, 3-d] pyrimidin-6-yl] phenyl}-3-methoxyurea (TAK-385) as a potent, orally active, non-peptide antagonist of the human gonadotropin-releasing hormone receptor. J. Med. Chem. 2011;54(14):4998–5012. doi: 10.1021/jm200216q. [DOI] [PubMed] [Google Scholar]
- 84.Canon J., Rex K., Saiki A.Y., Mohr C., Cooke K., Bagal D., Gaida K., Holt T., Knutson C.G., Koppada N., Lanman B.A. The clinical KRAS (G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019;575(7781):217–223. doi: 10.1038/s41586-019-1694-1. [DOI] [PubMed] [Google Scholar]
- 85.Blair H.A. Sotorasib: first approval. Drugs. 2021;81(13):1573–1579. doi: 10.1007/s40265-021-01574-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214665s000lbl.pdf
- 87.Kocienski P. Synthesis of sotorasib. Synfacts. 2021;17(10):1071. [Google Scholar]
- 88.Dindere M.E., Tanca A., Rusu M., Liehn E.A., Bucur O. Intraoperative tumor detection using pafolacianine. Int. J. Mol. Sci. 2022;23(21) doi: 10.3390/ijms232112842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/214907s000lbl.pdf
- 90.Markham A. Selpercatinib: first approval. Drugs. 2020;80(11):1119–1124. doi: 10.1007/s40265-020-01343-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213246s000lbl.pdf
- 92.https://investor.lilly.com/news-releases/news-release-details/lilly-completes-acquisition-loxo-oncology
- 93.https://www.fda.gov/drugs/development-approval-process-drugs/drug-trials-snapshots-retevmo
- 94.https://scifinder-n.cas.org/patent-viewer?docuri=yaMTCSpPI_VB6_zZs7_-XVPvRlO0fEk9CqqiSP3Bwtc&markedFullTextKey=sj43_1cDVManyV63LAtE_qGPtvi_SJrjycZVDMwYsqk.pdf&fullTextKey=65CLdYmnVN1FCfLaYxWWv5ffpUK1ktOaPp60fxuNiVw.pdf
- 95.Wu C.P., Lusvarghi S., Wang J.C., Hsiao S.H., Huang Y.H., Hung T.H., Ambudkar S.V. Avapritinib: a selective inhibitor of KIT and PDGFRα that reverses ABCB1 and ABCG2-mediated multidrug resistance in cancer cell lines. Mol. Pharm. 2019;16(7):3040–3052. doi: 10.1021/acs.molpharmaceut.9b00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mazzocca A., Napolitano A., Silletta M., Spalato Ceruso M., Santini D., Tonini G., Vincenzi B. New frontiers in the medical management of gastrointestinal stromal tumours. Ther. Adv. Med. Oncol. 2019;11 doi: 10.1177/1758835919841946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212608s000lbl.pdf
- 98.https://patentimages.storage.googleapis.com/03/05/e5/083c33225b302d/US9200002.pdf
- 99.(a) Dhillon S. Ripretinib: first approval. Drugs. 2020;80(11):1133–1138. doi: 10.1007/s40265-020-01348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Smith B.D., Kaufman M.D., Lu W.P., Gupta A., Leary C.B., Wise S.C., Rutkoski T.J., Ahn Y.M., Al-Ani G., Bulfer S.L., Caldwell T.M. Ripretinib (DCC-2618) is a switch control kinase inhibitor of a broad spectrum of oncogenic and drug-resistant KIT and PDGFRA variants. Cancer Cell. 2019;35(5):738–751. doi: 10.1016/j.ccell.2019.04.006. [DOI] [PubMed] [Google Scholar]; (c) Schneeweiss M., Peter B., Bibi S., Eisenwort G., Smiljkovic D., Blatt K., Jawhar M., Berger D., Stefanzl G., Herndlhofer S., Greiner G. The KIT and PDGFRA switch-control inhibitor DCC-2618 blocks growth and survival of multiple neoplastic cell types in advanced mastocytosis. Haematologica. 2018;103(5):799. doi: 10.3324/haematol.2017.179895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213973s000lbl.pdf
- 101.https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trial-snapshot-qinlock
- 102.https://scifinder-n.cas.org/patent-viewer?docuri=aZfbOFZJWwOlwnLZSMUQc_cPSjrJLbmGSw4drwCh7FU&markedFullTextKey=gC3ru_3TIB-2BKdLVdMBfZycA5rsAb1I_f4o_gQ1DCo.pdf&fullTextKey=U9VLL7hjkwSlsvgU5OMMa_Rv_-KjW5cVmOw_pTQpaqs.pdf
- 103.Santoni M., Massari F., Piva F., Carrozza F., Di Nunno V., Cimadamore A., Martignetti A., Montironi R., Battelli N. Tivozanib for the treatment of renal cell carcinoma. Expert Opin. Pharmacother. 2018;19(9):1021–1025. doi: 10.1080/14656566.2018.1480722. [DOI] [PubMed] [Google Scholar]
- 104.https://www.aveooncology.com/fotivdapi.pdf
- 105.Zhu C., Mao Y., Wang H., Xu J. A new and practical synthesis of tivozanib. Heterocycles: an international journal for reviews and communications in heterocyclic chemistry. 2016;92(10):1882–1887. [Google Scholar]
- 106.Markham A. Erdafitinib: first global approval. Drugs. 2019;79(9):1017–1021. doi: 10.1007/s40265-019-01142-9. [DOI] [PubMed] [Google Scholar]
- 107.https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/BALVERSA-pi.pdf
- 108.Vermeulen W., Hostyn S.A., Cuyckens F.A.C., Jones R.M., Broggini D.F.D. 2015. Quinoxaline Derivatives Useful as FGFR Kinase Modulators. WO 2015144803. [Google Scholar]
- 109.https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761137s006s008lbl.pdf
- 110.https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trials-snapshots-padcev
- 111.Deeks E.D. Polatuzumab vedotin: first global approval. Drugs. 2019;79(13):1467–1475. doi: 10.1007/s40265-019-01175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761121s000lbl.pdf
- 113.Syed Y.Y. Zanubrutinib: first approval. Drugs. 2020;80(1):91–97. doi: 10.1007/s40265-019-01252-4. [DOI] [PubMed] [Google Scholar]
- 114.https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/213217s000lbl.pdf
- 115.Guo Y., Liu Y.E., Hu N., Yu D., Zhou C., Shi G., Zhang B., Wei M., Liu J., Luo L., Tang Z. Discovery of zanubrutinib (BGB-3111), a novel, potent, and selective covalent inhibitor of Bruton's tyrosine kinase. J. Med. Chem. 2019;62(17):7923–7940. doi: 10.1021/acs.jmedchem.9b00687. [DOI] [PubMed] [Google Scholar]
- 116.Dhillon S., Keam S.J. Umbralisib: first approval. Drugs. 2021;81:857–866. doi: 10.1007/s40265-021-01504-2. [DOI] [PubMed] [Google Scholar]
- 117.https://scifinder-n.cas.org/pdf/v1/AUTH_5060d187888f42fe97a1589aed05c6d6/full-patentpak-fulltext-19/7ejBm8QuDQo5K2PvYGfc8vq7jErbJhWumi4ctP9Xua4.pdf?temp_url_sig=cef9396527e8b4988f94031cf8068be3c9579467&temp_url_expires=1675930073&inline
- 118.https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/213176s000lbl.pdf
- 119.Syed Y.Y. Selinexor: first global approval. Drugs. 2019;79(13):1485–1494. doi: 10.1007/s40265-019-01188-9. [DOI] [PubMed] [Google Scholar]
- 120.https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212306s000lbl.pdf
- 121.https://scifinder-n.cas.org/pdf/v1/AUTH_5060d187888f42fe97a1589aed05c6d6/full-patentpak-fulltext-33/hslhKLVpfjxVt5c_SNpFGR8beWfVqkjkYPPXjUm58VA.pdf?temp_url_sig=1ed947525e18d0d3607c33697634a5a91af73db9&temp_url_expires=1679639622&inline
- 122.Markham A. Belantamab mafodotin: first approval. Drugs. 2020;80(15):1607–1613. doi: 10.1007/s40265-020-01404-x. [DOI] [PubMed] [Google Scholar]
- 123.https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761158s000lbl.pdf
- 124.Hoy S.M. Pemigatinib: first approval. Drugs. 2020;80(9):923–929. doi: 10.1007/s40265-020-01330-y. [DOI] [PubMed] [Google Scholar]
- 125.https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213736s000lbl.pdf
- 126.Wu L., Zhang C., He C., Qian D., Lu L., Sun Y., Xu M., Zhuo J., Liu P.C.C., Klabe R., Wynn R., Covington M., Gallagher K., Leffet L., Bowman K., Diamond S., Koblish H., Zhang Y., Soloviev M., Hollis G., Burn T.C., Scherle P., Yeleswaram S., Huber R., Yao W. Discovery of pemigatinib: a potent and selective fibroblast growth factor receptor (FGFR) inhibitor. J. Med. Chem. 2021;64(15):10666–10679. doi: 10.1021/acs.jmedchem.1c00713. [DOI] [PubMed] [Google Scholar]
- 127.Hoy S.M. Tazemetostat: first approval. Drugs. 2020;80(5):513–521. doi: 10.1007/s40265-020-01288-x. [DOI] [PubMed] [Google Scholar]
- 128.https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/211723s000lbl.pdf
- 129.Kuntz K.W., Campbell J.E., Keilhack H., Pollock R.M., Knutson S.K., Porter-Scott M., Richon V.M., Sneeringer C.J., Wigle T.J., Allain C.J., Majer C.R. The importance of being me: magic methyls, methyltransferase inhibitors, and the discovery of tazemetostat. J. Med. Chem. 2016;59(4):1556–1564. doi: 10.1021/acs.jmedchem.5b01501. [DOI] [PubMed] [Google Scholar]
- 130.Blair H.A. Fedratinib: first approval. Drugs. 2019;79(15):1719–1725. doi: 10.1007/s40265-019-01205-x. [DOI] [PubMed] [Google Scholar]
- 131.https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212327s000lbl.pdf
- 132.https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trials-snapshots-inrebic
- 133.https://www.jakafi.com/myelofibrosis/mf-home
- 134.https://www.jakafi.com/pdf/prescribing-information.pdf
- 135.https://scifinder-n.cas.org/patent-viewer?docuri=oTXwAsCXAawSbkXZE5pf8Nap3OYUMP1qbLaneLz401M&markedFullTextKey=RqI9cAovlSeY5JwlKUiOlMQ8QDGG_ILDkoNeXXR1U9g.pdf&fullTextKey=MEuFoflGTYH-_I9wfYJKfDO-rFD-MpSKGluzaGVMVlo.pdf
- 136.Dhillon S. Decitabine/cedazuridine: first approval. Drugs. 2020;80(13):1373–1378. doi: 10.1007/s40265-020-01389-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212576s000lbl.pdf
- 138.https://www.otsuka.co.jp/en/company/newsreleas es/2014/20140331_1.html
- 139.https://www.otsuka.co.jp/en/company/newsreleases/2019/20190607_2.html
- 140.https://www.taihooncology.eu/news/astex-pharmaceuticals-taiho-oncology-and-otsuka-pharmaceutical-announce-fda-and-health-canada-approval-of-inqovi-decitabine-and-cedazuridine-tablets-oral-hypomethylating-agent-hma-therapy-for-intermediate-and-high-risk-mds-and-cmml/
- 141.https://scifinder-n.cas.org/searchDetail/reference/638f1f0a8643a84f11aa0b8c/referenceDetails
- 142.Ferraris D., Duvall B., Delahanty G., Mistry B., Alt J., Rojas C., Rowbottom C., Sanders K., Schuck E., Huang Kuan-Chun, Redkar S., Slusher B.B., Tsukamoto T. J. Med. Chem. 2014;57(6):2582–2588. doi: 10.1021/jm401856k. [DOI] [PubMed] [Google Scholar]
- 143.Markham A., Keam S.J. Selumetinib: first approval. Drugs. 2020;80:931–937. doi: 10.1007/s40265-020-01331-x. [DOI] [PubMed] [Google Scholar]
- 144.https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213756s000lbl.pdf
- 145.https://patentimages.storage.googleapis.com/96/e2/dc/cdbf17557dc962/US20030232869A1.pdf
- 146.Keam S.J. Piflufolastat F 18: diagnostic first approval. Mol. Diagn. Ther. 2021;25(5):647–656. doi: 10.1007/s40291-021-00548-0. [DOI] [PubMed] [Google Scholar]
- 147.https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214793s000lbl.pdf
- 148.Bouvet V., Wuest M., Jans H.S., Janzen N., Genady A.R., Valliant J.F., Benard F., Wuest F. Automated synthesis of [18F] DCFPyL via direct radiofluorination and validation in preclinical prostate cancer models. EJNMMI Res. 2016;6(1):1–15. doi: 10.1186/s13550-016-0195-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Olberg D.E., Arukwe J.M., Grace D., Hjelstuen O.K., Solbakken M., Kindberg G.M., Cuthbertson A. One step radiosynthesis of 6-[18F] fluoronicotinic acid 2, 3, 5, 6-tetrafluorophenyl ester ([18F] F-Py-TFP): a new prosthetic group for efficient labeling of biomolecules with fluorine-18. J. Med. Chem. 2010;53(4):1732–1740. doi: 10.1021/jm9015813. [DOI] [PubMed] [Google Scholar]
- 150.Hennrich U., Benešová M. [68Ga] Ga-DOTA-TOC: the first FDA-approved 68Ga-radiopharmaceutical for PET imaging. Pharmaceuticals. 2020;13(3):38. doi: 10.3390/ph13030038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/210828s000lbl.pdf
- 152.https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trials-snapshots-detectnet
- 153.https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213227s000lbl.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.