Abstract
To investigate the influencing factors of in-hospital acute heart failure (AHF) in patients with acute exacerbation of chronic obstructive pulmonary disease (AECOPD), and to construct and validate a risk prediction nomogram model. Three Hundred Thirty patients with AECOPD admitted to our hospital from June 2020 to June 2023 were retrospectively analyzed as a training set for the construction of the model. Three Hundred Twenty-five AECOPD patients admitted to the Second People’s Hospital of Hefei from 2006 to June 2023 were also collected as the validation set for the validation of the model. A nomogram model was constructed to predict the risk of nosocomial AHF in patients with AECOPD, and C-index and receiver operating characteristic curve were drawn to assess the predictive predictive efficacy of the model. Model fit was evaluated by Hosmer–Lemeshow test, calibration curve was drawn to evaluate the calibration of the model; decision curve was drawn to analyze the net benefit rate of this nomogram model. Multivariate logistic regression analysis indicated that body mass index, mmRC grade, neutrophils, lymphocytes, hemoglobin, creatinine, PO2, PCO2, and Homocysteine were independent risk factors for in-hospital AHF in patients with AECOPD. To construct a nomogram model for risk prediction of in-hospital AHF in patients with AECOPD. The C-index of the training set was 0.949 (95% CI: 0.91–0.961); the C-index of the validation set was 0.936 (95% CI: 0.911–0.961) suggesting good model discrimination. The receiver operating characteristic curve calculated area under curve for the training set was 0.949 (95% CI: 0.928–0.97); area under curve for the validation set was 0.936 (95% CI: 0.91–0.961) suggesting good model accuracy. The results of Hosmer-Lemeshoe goodness-of-fit test and calibration curve analysis showed that the calibration curve of this nomogram model was close to the ideal curve. The clinical decision curve also showed good clinical net benefit of the nomogram model. Body mass index, mmRC grade, neutrophils, lymphocytes, hemoglobin, creatinine, PO2, PCO2, and Homocysteine are risk factors for in-hospital AHF in AECOPD patients, and nomogram models constructed based on the above factors have some predictive value for in-hospital AHF in AECOPD patients. It is also vital for nursing staff to strengthen nursing care.
Keywords: acute exacerbation of chronic obstructive pulmonary disease, acute heart failure, AECOPD, AHF, multicenter, nomogram, nursing
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a respiratory disease characterized by persistent respiratory symptoms and incompletely reversible airflow limitation.[1] Common symptoms of COPD are cough, sputum, and dyspnea. If the symptoms worsen dramatically, requiring a change in treatment regimen, it is called acute exacerbation of chronic obstructive pulmonary disease (AECOPD).[2] AECOPD can lead to a sharp decline in pulmonary function, quickly complicated pneumonia, hypoxemia, pulmonary heart disease, and other diseases, and is the most common cause of increased hospitalization rate. Still, it it also leads to COPD patients death.[3–5] Heart failure is one of the most essential comorbidities in patients with AECOPD. COPD patients with systolic or diastolic heart failure have been reported to have a prevalence of 20% to 70%, with higher rates of hospitalization and mortality compared to patients without heart failure.[5,6] Therefore, early assessment of the condition of COPD patients, timely judgment of the severity of the disease, and intervention are essential to reduce patient mortality.
This study aimed to identify independent factors influencing in-hospital acute heart failure (AHF) in patients with AECOPD by Nomogram. The Nomogram is based on the analysis results of COX proportional hazards, or logistic regression model, which is graphical and visualized for the prediction of individual disease risk and is more intuitive and easy to be popularized and applied in clinical practice. Compared with traditional risk scoring systems, nomogram models integrate more risk factors, obtain numerical probabilities of target events, more accurately quantify risk, and are more flexible to apply.[7] The Nomogram method for diagnosing high-risk patients can be conveniently used in clinical practice and guide clinical decision-making. The prediction model of nosocomial AHF in AECOPD has not been established at home and abroad. Therefore, we intend to develop and externally validate a nomogram model to predict the risk of in-hospital AHF in AECOPD patients and provide vital information for clinical decision-making.
2. Materials and methods
2.1. Study population
Three hundred thirty patients with AECOPD admitted to our hospital from June 2020 to June 2023 were retrospectively analyzed as a training set for the construction of the model. Three Hundred Twenty-five AECOPD patients admitted to the Second People’s Hospital of Hefei from 2006 to June 2023 were also collected as the validation set for the validation of the model. AECOPD was defined based on the International Classification of Diseases, Ninth Revision (ICD-9) code 491.21. For patients with multiple hospitalizations, only the first hospitalization was registered. Diagnosis of acute heart failure: clinical manifestations such as shortness of breath, orthopnea, pulmonary rales, pink foamy sputum; NT-proBNP: > 450 ng/L in patients under 50 years old, > 900 ng/L in patients over 50 years old, > 1800 ng/L in patients over 75 years old, and > 1200 ng/L in patients with renal insufficiency (glomerular filtration rate < 60 mL/minutes). Exclusion criteria : Combined with other respiratory diseases such as bronchial asthma, tuberculosis, etc.; Accompanied by severe heart, liver, and kidney dysfunction or abnormalities; Combined with immunodeficiency and malignant tumors; other exclusion criteria include length of hospital stay < 48 hours, data loss > 10%. This study was approved by the Ethics Committee of Fuyang Cancer Hospital and the Second People’s Hospital of Hefei.
2.2. Study method
2.2.1. Data collection.
Demographic characteristics and clinical data at admission were collected from AMI patients through a hospital electronic case system, including age, gender, body mass index (BMI), smoking, hypertension, diabetes, coronary heart disease (CHD), pulmonary heart disease (PHD), mmRC grade, forced respiratory volume in 1 second (FEV1)% predicted, FEV1/forced vital capacity (FVC), neutrophils, lymphocytes, hemoglobin, platelets, albumin (ALB), C-reactive protein (CRP), blood urea nitrogen (BUN), creatinine (SCR), uric acid (UA), cystatin C Homocysteine (Hcy), NT-ProBNP, PO2, PCO2, alanine aminotransferase (ALT), and glutamic oxaloacetic transaminase (AST).
2.2.2. Nomogram establishment and verification.
Multivariate logistic regression was used to analyze independent predictors of in-hospital AHF in patients with AECOPD. A nomogram model was constructed to predict the risk of nosocomial AHF in patients with AECOPD using predictors, and C-index and receiver operating characteristic (ROC) curves were plotted to assess the predictive efficacy of the model for predicting the risk of nosocomial AHF in patients with AECOPD. The model fit was evaluated by the Hosmer–Lemeshow test, and draw the calibration curve and Brier score evaluation model calibration; the decision curve was drawn to analyze the net benefit rate of this nomogram model in predicting nosocomial AHF in AECOPD patients.
2.2.3. Statistical methods.
Statistical analysis and plotting were performed using SPSS 26.0, R4.2.1. Kolmogorov-Smirnov normality test was performed on the measurement data, which conformed to the normal distribution and was expressed as mean ± standard deviation and independent sample t-test was used for comparison between the 2 groups; the measurement data without normal distribution were expressed as median M (P25, P75), and Mann–Whitney U test was used for comparison between the 2 groups; the adoption rate of enumeration data was expressed, and chi-square test was used for comparison between the 2 groups; multivariate logistic regression was used to analyze the independent risk factors affecting nosocomial AHF in AECOPD patients; C-index, area under ROC curve, calibration curve and clinical decision curve were calculated. All statistics were performed using 2-sided tests, and P < .05 was considered statistically significant.
3. Results
3.1. Comparison of clinical data between the AECOPD group and AECOPD combined with the AHF group
Of 330 AECOPD patients in the training set, 130 developed AHF in-hospital. There was no significant difference in age, gender, the proportion of patients with diabetes, the proportion of patients with a smoking history, and AST between the 2 groups (P > .05); there were significant differences in BMI, combined hypertension, CHD, PHD, mmRC grade, FEV1% predicted, FEV1/FVC, SCR, UA, BUN, neutrophils, lymphocytes, hemoglobin, platelets, ALB, CRP, Hcy, NT-ProBNP, ALT, AST, PO2, and PCO2 between the 2 groups (P < .05). In the validation set of 325 AECOPD patients, 125 developed AHF in the hospital. There was no significant difference in gender, BMI, the proportion of patients with diabetes, and AST between the 2 groups (P > .05); there was a substantial age difference, the proportion of patients with a smoking history, the proportion of patients with hypertension, CHD, PHD, mmRC grade, FEV1% predicted, FEV1/FVC, SCR, UA, BUN, neutrophils, lymphocytes, hemoglobin, platelets, ALB, CRP, Hcy, NT-ProBNP, ALT, AST, PO2, and PCO2 between the 2 groups (P < .05). As shown in Table 1.
Table 1.
Comparison of general clinical data between training set and validation set.
| Variables | Training set | P value | Validation set | P value | ||
|---|---|---|---|---|---|---|
| AECOPD | AECOPD + AHF | AECOPD | AECOPD + AHF | |||
| Age, yr | 75.61 ± 7.31 | 77.15 ± 6.83 | .055 | 74.88 ± 7.12 | 77.88 ± 6.43 | <.001* |
| Gender, male, n (%) | 119 (59.5) | 75 (57.69) | .744 | 115 (57.5) | 70 (56) | .79 |
| BMI, kg/m2 | 23.09 ± 3.98 | 21.98 ± 2.13 | .001* | 23.15 ± 4.08 | 22.58 ± 3.22 | .165 |
| Hypertension,n (%) | 32 (16) | 38 (29.23) | .004* | 35 (17.5) | 39 (31.2) | .004* |
| Diabetes, n (%) | 15 (7.5) | 10 (7.69) | 0.949 | 19 (9.5) | 11 (8.8) | .179 |
| Smoking, n (%) | 133 (66.5) | 94 (72.31) | .266 | 115 (57.5) | 86 (68.8) | .041* |
| CHD, n (%) | 17 (8.5) | 33 (25.38) | <.001* | 20 (10) | 33 (26.4) | <.001* |
| PHD, n (%) | 5 (2.5) | 15 (11.54) | .001* | 8 (4) | 15 (12) | .006* |
| MMRC Grade, n (%) | 25 (12.5) | 42 (32.31) | <.001* | 21 (10.5) | 34 (27.2) | <.001* |
| FEV1% Predicted | 95.82 ± 7.51 | 46.52 ± 5.6 | <.001* | 93.25 ± 6.73 | 47.52 ± 5.26 | <.001* |
| FEV1/FVC | 81.02 ± 5.72 | 43.89 ± 4.18 | <.001* | 83.02 ± 5.45 | 41.89 ± 4.29 | <.001* |
| SCR, umol/L | 92.36 ± 14.04 | 101 ± 12.07 | <.001* | 95.36 ± 14.03 | 105 ± 12.09 | <.001* |
| UA, umol/L | 289.76 ± 55.12 | 320.44 ± 85.4 | <.001* | 300.76 ± 55.11 | 330.44 ± 85.69 | .001* |
| BUN, mmol/L | 7.91 ± 2.08 | 8.71 ± 2.88 | .007* | 6.24 ± 1.65 | 6.89 ± 2.05 | .003* |
| Neutrophils,×109/L | 4.28 ± 1.76 | 6.34 ± 1.93 | <.001 | 4.86 ± 1.78 | 6.98 ± 1.94 | <.001* |
| Lymphocytes | 1.32 ± 0.52 | 0.94 ± 0.3 | <.001* | 1.42 ± 0.52 | 1.04 ± 0.31 | <.001* |
| Hemoglobin,g/L | 132.12 ± 22.97 | 125.84 ± 27.26 | .031* | 130.12 ± 21.02 | 123.84 ± 25.39 | .022* |
| Platelets,×109/L | 193.51 ± 38.89 | 219.31 ± 51.56 | <.001* | 183.49 ± 29.06 | 209.3 ± 41.91 | <.001* |
| ALB, g/L | 34.91 ± 7.99 | 31.93 ± 9.48 | .003* | 36.91 ± 8 | 33.93 ± 9.51 | .004* |
| CRP | 8.79 ± 4.13 | 11.77 ± 4.99 | <.001* | 9.84 ± 5.03 | 12.79 ± 5.95 | <.001* |
| Hcy | 14.63 ± 2.3 | 17.18 ± 4.04 | <.001* | 16.63 ± 2.3 | 19.18 ± 4.05 | <.001* |
| NT-ProBNP | 127.64 ± 28.67 | 2787.4 ± 531.78 | <.001* | 147.64 ± 28.68 | 2787.4 ± 631.93 | <.001* |
| ALT | 24.44 ± 8.56 | 22.28 ± 6.03 | .007* | 27.44 ± 8.55 | 25.28 ± 6.04 | .008* |
| AST | 20.36 ± 7.39 | 21.08 ± 6.23 | .344 | 23.36 ± 7.4 | 24.08 ± 6.27 | .367 |
| PO2 | 77.56 ± 11.65 | 68.48 ± 9.76 | <.001* | 79.56 ± 11.65 | 70.48 ± 9.83 | <.001* |
| PCO2 | 36.96 ± 8.36 | 43.75 ± 9.46 | <.001* | 38.96 ± 8.36 | 45.76 ± 9.48 | <.001* |
AECOPD = acute exacerbation of chronic obstructive pulmonary disease, AHF = acute heart failure, ALB = albumin, ALT = alanine aminotransferase, AST = glutamic oxaloacetic transaminase, BMI = body mass index, BUN = blood urea nitrogen, CHD = coronary heart disease, CRP = C-reactive protein, FEV1 = forced respiratory volume in 1 second, FVC = forced vital capacity, Hcy = homocysteine, PHD = pulmonary heart disease, SCR = creatinine, UA = uric acid.
P < .05.
3.2. Development of a risk prediction model for nosocomial AHF in patients with AECOPD
3.2.1. Univariate and multivariate logistic regression analysis model construction.
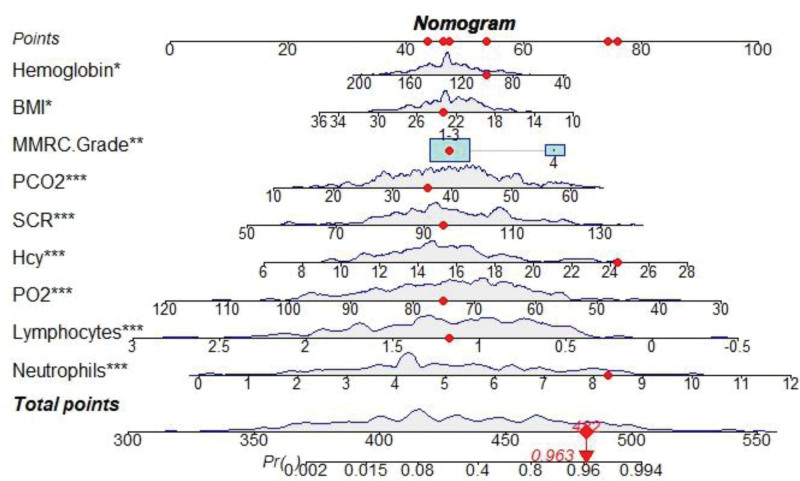
Statistically significant variables (P < .05) in Table 1 were included in univariate logistic regression analysis to screen out univariate risk factors for in-hospital AHF in AECOPD patients, as shown in Table 2. Meanwhile, significant variables in univariate logistic regression analysis were included in multivariate logistic regression analysis. The results showed that BMI, mmRC grade, neutrophils, lymphocytes, hemoglobin, creatinine, PO2, PCO2, and Hcy were independent risk factors for in-hospital AHF risk in patients with AECOPD (P < .05), as shown in Table 2. A nomogram of the predictive model for in-hospital development of AHF in AECOPD patients was drawn according to the predictive variables and is shown in Figure 1. Each predictor variable corresponds to a specific score on the horizontal axis of the nomogram score. The scores corresponding to the 4 predictor variables are summed to obtain a total score corresponding to the predicted AHF risk at the bottom of the Nomogram.
Table 2.
Univariate and multivariate logistic regression analysis.
| Univariate logistic regression analysis | Multivariate logistic regression analysis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | S.E | Wald | Odds ratio | 95% CI | P value | β | S.E | Wald |
Odds ratio | 95% CI | P value | |
| BMI | −0.098 | 0.035 | 8.09 | 0.906 | (0.847, 0.97) | .004 | -0.168 | 0.073 | 5.247 | 0.846 | (0.733, 0.976) | .022* |
| Hypertension | −0.774 | 0.273 | 8.054 | 0.461 | (0.27, 0.787) | .005 | −0.324 | 0.881 | 0.135 | 0.723 | (0.129, 4.068) | .713 |
| CHD | −1.298 | 0.324 | 16.063 | 0.273 | (0.145, 0.515) | <.001 | −2.08 | 1.084 | 3.683 | 0.125 | (0.015, 1.045) | .055 |
| PHD | −0.1627 | 0.53 | 9.434 | 0.197 | (0.07, 0.555) | .002 | 1.025 | 1.19 | 0.742 | 2.787 | (0.271, 28.704) | .389 |
| MMRC Grade | −1.206 | 0.284 | 17.988 | 0.299 | (0.171, 0.523) | <.001 | −1.855 | 0.566 | 10.733 | 0.156 | (0.052,0 .475) | .001* |
| Neutrophils | 0.602 | 0.077 | 61.075 | 1.826 | (1.57, 2.124) | <.001 | 0.678 | 0.135 | 25.355 | 1.97 | (1.513, 2.565) | <.001* |
| Hemoglobin | −0.01 | 0.005 | 4.946 | 0.99 | (0.981, 0.999) | .026 | −0.026 | 0.011 | 6.002 | 0.974 | (0.954, 0.995) | .014* |
| Platelets | 0.013 | 0.003 | 23.07 | 1.013 | (1.008, 1.018) | <.001 | 0.009 | 0.005 | 2.778 | 1.009 | (0.998, 1.02) | .096 |
| CRP | 0.146 | 0.027 | 28.51 | 1.157 | (1.097, 1.221) | <.001 | 0.188 | 0.055 | 11.565 | 1.207 | (1.083, 1.346) | .096 |
| ALB | −0.04 | 0.013 | 8.983 | 0.961 | (0.936, 0.986) | .003 | 0 | 0.028 | 0 | 1 | (0.946, 1.056) | .987 |
| SCR | 0.049 | 0.009 | 27.658 | 1.051 | (1.031, 1.07) | <.001 | 0.068 | 0.019 | 0.624 | 1.07 | (1.032, 1.111) | <.001* |
| BUN | 0.136 | 0.047 | 8.229 | 1.145 | (1.044, 1.257) | .004 | 0.072 | 0.091 | 0.624 | 1.075 | (0.899, 1.285) | .43 |
| UA | 0.006 | 0.002 | 14.347 | 1.006 | (1.003, 1.01) | <.001 | 0.005 | 0.003 | 2.539 | 1.006 | (0.999, 1.012) | .111 |
| PO2 | −0.078 | 0.012 | 40.202 | 0.925 | (0.903, 0.948) | <.001 | −0.117 | 0.026 | 19.997 | 0.89 | (0.845, 0.936) | <.001* |
| PCO2 | 0.087 | 0.014 | 36.484 | 1.091 | (1.061, 1.123) | <.001 | 0.087 | 0.029 | 9.109 | 1.091 | (1.031, 1.154) | .003* |
| Hcy | 0.26 | 0.042 | 38.649 | 1.297 | (1.195, 1.408) | <.001 | 0.307 | 0.083 | 13.781 | 1.359 | (1.156, 1.598) | <.001* |
| Lymphocytes | −1.987 | 0.305 | 42.384 | 0.137 | (0.075, 0.249) | <.001 | −2.894 | 0.573 | 25.536 | 0.055 | (0.018, 0.17) | <.001* |
| ALT | −0.037 | 0.015 | 6.08 | 0.964 | (0.935, 0.992) | .014 | −0.055 | 0.031 | 3.133 | 0.947 | (0.891, 1.006) | .077 |
ALB = albumin, ALT = alanine aminotransferase, BMI = body mass index, BUN = blood urea nitrogen, CHD = coronary heart disease, CRP = C-reactive protein, Hcy = homocysteine, PHD = pulmonary heart disease, SCR = creatinine, UA = uric acid.
P < .05.
Figure 1.
A nomogram of acute in-hospital heart failure in patients with AECOPD. AECOPD = acute exacerbation of chronic obstructive pulmonary disease.
3.2.2. Nomogram model validation.
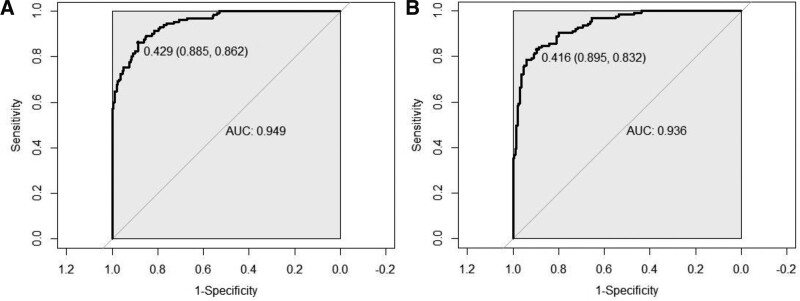
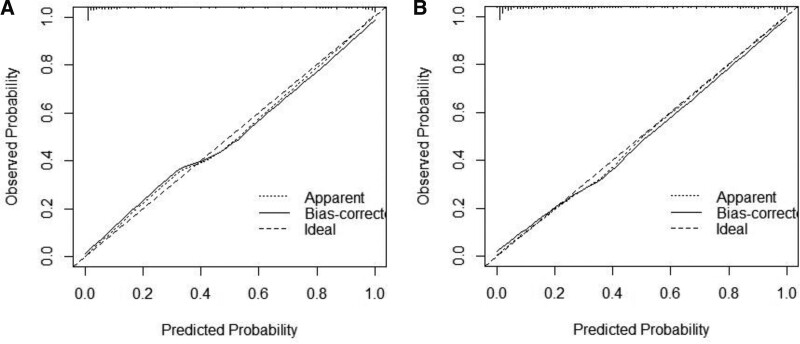
The C-index of the training set was 0.949 (95% CI: 0.91–0.961); the C-index of the validation set was 0.936 (95% CI: 0.911–0.961), suggesting good model discrimination. The ROC curve calculated area under curve (AUC) for the training set was 0.949 (95% CI: 0.928–0.97); AUC for the validation set was 0.936 (95% CI: 0.91–0.961), suggesting good model accuracy, as shown in Figure 2. The results of the Hosmer–Lemeshoe goodness-of-fit test showed that this nomogram model fitted well in the training set (χ2 = 2.8046, P = .246) and in the validation group (χ2 = 2.05, P = .3588). The calibration curve analysis results showed that the calibration curve of this nomogram model for predicting in-hospital AHF in AECOPD patients in the training set and validation set was close to the ideal curve, the Brier score was 0.137, suggesting that nomogram models predicted in-hospital AHF in AECOPD patients with good correlation and strong calibration with internal sampling.as shown in Figure 3.
Figure 2.
ROC curve was used to assess the predictive power of the model to predict the risk of in-hospital AHF in patients with AECOPD. AECOPD = acute exacerbation of chronic obstructive pulmonary disease, AHF = acute heart failure, ROC = receiver operating characteristic.
Figure 3.
Calibration curve; (A) training set calibration curve.
3.2.3. Clinical decision analysis for nomogram models.
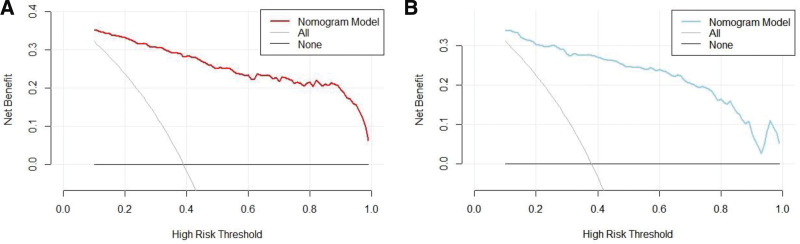
The clinical decision curve analysis (DCA) was plotted with the probability of high-risk threshold as the abscissa and the net benefit rate as the ordinate, where the probability of high-risk threshold was set to (0, 1), the solid black line represents the net benefit rate of in-hospital AHF in none of the patients, the solid gray line represents the net benefit rate of in-hospital AHF in all patients, the red curve represents the decision curve of the nomogram model of the training set, and the blue sky represents the decision curve of the validation set. Compared with all subjects who were positive or negative, the Nomogram predicted the net benefit of the model in the interval of 0.1 to 0.99 for the probability of DCA threshold in the training and validation sets, suggesting that the nomogram model could bring net clinical benefit to patients when the threshold probability was 0.1 to 0.99, as shown in Figure 4.
Figure 4.
Clinical decision curve analysis for nomogram models.
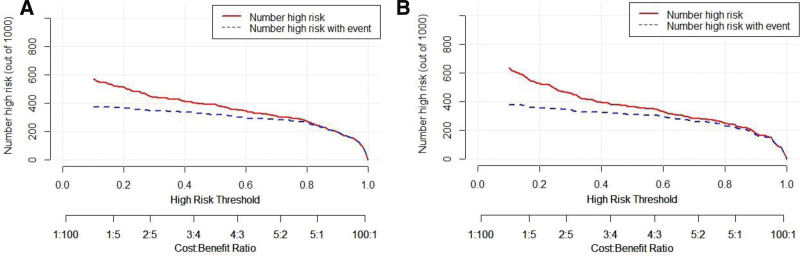
3.2.4. Clinical Impact Curve for nomogram models.
The clinical impact curve can further reflect the risk stratification of 1000 people predicted by the nomogram model, showing the “loss: benefit” axis, with 8 scales assigned, panel A represents the training set, panel B represents the validation set, and gray represents the actual occurrence of AHF in AECOPD patients in the hospital. It is obvious that the training set curve and validation set curve have small differences from the actual disease curve. These results suggest that nomogram model has a good application in the clinical occurrence of nosocomial AHF in patients with AECOPD, as shown in Figure 5.
Figure 5.
Clinical impact curve analysis for nomogram models.
4. Discussion
COPD is currently one of the diseases with a dramatic increase in morbidity and mortality worldwide, and its prevalence gradually increases with age. In elderly patients, AECOPD is more likely to induce cardiac insufficiency, which seriously affects the quality of life of elderly patients and even leads to death. Therefore, it is essential to early detect heart failure and perform early intervention in elderly patients. At present, brain natriuretic peptide or echocardiography is mainly used to diagnose heart failure in clinical practice, but the actual operation steps are cumbersome, the cost is high, and the patient compliance is poor, which limits its wide application in outpatient clinics and primary hospitals. Therefore, it is necessary to find a simple and economical prediction model in clinical practice to help clinicians early detect patients with AECOPD and acute heart failure.
To better personalized assess the risk prognosis of nosocomial AHF in patients with AECOPD, this study used multivariate logistic regression analysis to screen BMI, mmRC grade, neutrophils, lymphocytes, hemoglobin, creatinine, PO2, PCO2, and Hcy as independent risk factors for nosocomial AHF in patients with AECOPD. Studies have shown that low BMI is a risk factor for accelerated decline in lung function, while high BMI may have a protective effect.[8,9] Because obese COPD patients exhibit less pulmonary hyper congestion and higher inspiratory capacity and inspiratory capacity/total lung capacity ratio compared to COPD patients with lower BMI, these mechanical changes may positively impact the prognosis of obese COPD patients.[10] COPD exacerbations, in particular, result in increased resting energy expenditure, decreased peripheral oxygen supply, and increased pulmonary and systemic inflammation.[9] A foreign study confirmed that heart failure patients with higher BMI had a better prognosis[11]; however, some studies demonstrated that BMI showed a U-shaped curve relationship with the prognosis of heart failure, and studies confirmed that both too-high and too-low BMI levels were risk factors for poor prognosis of heart failure.[12] Our study also showed that BMI was an independent protective factor for the risk of in-hospital AHF in patients with AECOPD, with AECOPD patients with higher BMI having a more negligible risk of AHF.
The most common cause of AECOPD is respiratory tract infection, and neutrophils are its primary inflammatory cells; neutrophils adhere to respiratory endothelial cells in response to chemokines, release proteolytic enzymes and oxygen free radicals, degrade alveolar elastin and collagen, and lead to emphysema resulting in airway dysfunction.[13,14] Neutrophils can stimulate cytokine production and contribute to changes in the microbiota of patients with AECOPD.[15] Increased white blood cell count is associated with advanced heart failure hospitalization and mortality. In addition, neutrophilia is associated with an increased incidence in patients with acute heart failure and with acute myocardial infarction. At the same time, relative lymphopenia is an independent predictor of mortality in heart failure. Neutrophil numbers and lymphocyte numbers are markers of inflammation, and they vary with the severity of inflammation. Because increases in corticosteroid levels and lymphopenia are expected in response to stress.[16] Lymphopenia has also been reported to be caused by increased lymphocyte apoptosis during the acute phase of inflammation. Foreign studies have found that lower lymphocyte percentage can be used as an independent predictor of prognosis in patients with acute coronary syndrome. Relatively low lymphocyte percentages have also been associated with hospitalization and mortality in patients with acute heart failure.[17,18]In addition, some studies have shown that elevated neutrophil counts are also associated with this. Our study is similar to previous studies in that neutrophils are an independent risk factor for developing acute heart failure in AECOPD patients. Lymphocytes are an independent protective factor.
The traditional view is that chronic hypoxia is present in COPD, which leads to polycythemia, and anemia is rare. While an increasing number of studies have shown that anemia is not uncommon in COPD patients, the impact on patients may be underestimated. The relationship between anemia and COPD has been re-recognized in recent years and has received much attention from scholars.[19,20] Anemia may increase with duration and severity.[21] At the same time, low hemoglobin leads to poor blood oxygen-carrying ability, which will cause insufficient blood supply and oxygen supply to tissues and organs and affect tissue and organ function[22,23]; blood viscosity is reduced, which increases the return blood volume, thereby increasing cardiac load and quickly causing heart failure.[24] At the same time, we found that arterial PO2 and PCO2 are the influencing factors, which may be due to the poor pulmonary ventilation and ventilation function of AECOPD patients themselves, resulting in increased myocardial oxygen consumption due to decreased arterial PO2, acute myocardial output reduced due to diastolic and systolic dysfunction after the occurrence of acute heart failure, pulmonary and peripheral circulatory resistance increased, resulting in insufficient blood perfusion of tissues and organs, and then causing cardiac dysfunction leading to increased arterial PCO2.
Studies have shown that Hcy can be used as an inflammatory marker to predict acute exacerbations of COPD. Levels of Hcy influence inflammatory processes such as oxidative stress, adhesion molecules, leukocyte adhesion, reduced NO bioavailability, or endothelial dysfunction and injury.[25] Acute exacerbation of COPD leads to enhanced oxidative stress in the lungs and whole body of AECOPD patients, which reduces the activity and number of methionine synthase and cystathionine β synthase, critical enzymes of Hcy catabolism, and impaired metabolism of Hcy accumulates in the body to form Hcy.[26] Glutathione, as an essential antioxidant that neutralizes oxygen free radicals, Hcy interferes with glutathione synthesis in AECOPD patients, causing redox imbalance and oxidative stress to be further exacerbated in AECOPD patients. Therefore, Hcy is both a marker and cause of oxidative stress and an essential mediator in the inflammatory process of AECOPD, and Hcy may be involved in the pathogenesis of AECOPD by regulating inflammatory factors in vivo. Our study also showed that Hcy levels were elevated in COPD patients and higher in AECOPD patients with AHF than in AECOPD patients. Multivariate logistic regression analysis showed that Hcy was an independent risk factor for acute heart failure in AECOPD patients.
Renal function refers to the function that the kidney excretes metabolites in the body and maintains the stability of electrolytes such as potassium, sodium, and calcium and acid-base balance in the body. And renal function parameters include blood urea nitrogen, creatinine, blood uric acid, and urinary albumin. Blood urea nitrogen and creatinine are well-established renal markers, and both are associated with prognosis in patients with acute heart failure.[27,28] In COPD, when the body is stimulated by harmful gases and particles such as smoking, there is a continuous chronic inflammatory response and oxidative stress in the lungs, resulting in lung tissue injury and decreased lung function.[29] When lung function declines, oxygen intake in the body is significantly reduced, tissue hypoxia occurs, and is more prominent in patients with acute exacerbations. On this basis, further studies have proposed that creatinine urate, as an integrated indicator of uric acid and creatinine, can rule out the effect of renal metabolism on uric acid and may reflect the degree of hypoxia more than uric acid.[30] Our study showed that serum creatinine was higher in AECOPD patients with heart failure than in AECOPD patients alone. Multivariate logistic regression analysis showed that serum creatinine was an independent risk factor for acute heart failure in patients with AECOPD.
To better predict the risk of acute heart failure in patients with AECOPD. We developed and validated a nomogram model to predict risk. A nomogram is a visual graph composed of line segments of different lengths that are used to predict the probability of a clinical event, is based on a multivariate regression model, and is drawn after integrating multiple clinical indicators. In this study, we constructed a nomogram model for risk prediction of acute heart failure in AECOPD patients based on indicators that were statistically different in multivariate logistic regression analysis. The results showed that the C-index of the training set predicted by the nomogram model to develop AHF in AECOPD patients was 0.949 (95% CI: 0.91–0.961); the C-index of the validation set was 0.936 (95% CI: 0.911–0.961) suggesting that the model discriminated well. The ROC curve calculated AUC for the training set was 0.949 (95% CI: 0.928–0.97); AUC for the validation set was 0.936 (95% CI: 0.91–0.961), suggesting good model accuracy. The results of the Hosmer–Lemeshoe goodness-of-fit test showed that this nomogram model fitted well in the training set (χ2 = 2.8046, P = .246) and in the validation group (χ2 = 2.05, P = .3588). The results of the calibration curve analysis showed that the calibration curve of this nomogram model for predicting in-hospital AHF in AECOPD patients in the training set and validation set was close to the ideal curve. DCA analysis showed that the net benefit of the nomogram prediction model was higher in the interval of threshold probability between 0.1 and 0.99, suggesting that when the threshold probability was 0.1 to 0.99, the nomogram model could bring net clinical benefit to patients, confirming that the prediction model had a high clinical response rate.
There are still shortcomings in this study: first, this study is a 2-center study with a limited sample size and cannot avoid bias; second, in terms of model validation, further validation from a multicenter large-scale prospective study is required. Therefore, in terms of this model’s clinical application and promotion, large-sample, multicenter clinical data are still needed to provide more evidence to support further exploration of the influencing factors of acute heart failure in AECOPD patients and optimize the nomogram model.
5. Conclusion
In summary, the results of this study showed that BMI, mmRC grade, neutrophils, lymphocytes, hemoglobin, creatinine, PO2, PCO2, and Hcy were independent risk factors for in-hospital AHF risk in patients with AECOPD. The nomogram model for in-hospital acute heart failure risk prediction in patients with AECOPD constructed based on the above-influencing factors had good discrimination, calibration, and clinical effectiveness and could be used as an effective tool for early clinical prediction of in-hospital acute heart failure risk in patients with AECOPD. At the same time, nursing staff could focus on high-risk groups, achieve early detection, early reporting, and early treatment, and improve the prognosis of patients.
Author contributions
Conceptualization: Lina Yan.
Data curation: Hao-Ran Ma.
Formal analysis: Min Chen.
Methodology: Hui Wei.
Writing – original draft: Lina Yan.
Writing – review & editing: Lina Yan.
Abbreviations:
- AECOPD
- acute exacerbation of chronic obstructive pulmonary disease
- AHF
- acute heart failure
- ALB
- albumin
- ALT
- Alanine aminotransferase
- AST
- glutamic oxaloacetic transaminase
- AUC
- area under curve
- BMI
- body mass index
- BUN
- blood urea nitrogen
- CHD
- coronary heart disease
- COPD
- Chronic obstructive pulmonary disease
- CRP
- C-reactive protein
- DCA
- decision curve analysis
- FEV1
- forced respiratory volume in 1 second
- FVC
- forced vital capacity
- Hcy
- homocysteine
- PHD
- pulmonary heart disease
- ROC
- receiver operating characteristic
- SCR
- Creatinine
- UA
- Uric acid
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Studies involving human participants were reviewed and approved by the Ethics Committee of the Second People’s Hospital of Hefei and the Ethics Committee of Fuyang Cancer Hospital. According to national laws and institutional requirements, this study does not require written informed consent to participate.
The authors have no funding and conflicts of interest to disclose.
How to cite this article: Yan L-N, Chen M, Wei H, Ma H-R. Construction and validation of nomogram prediction model for risk of acute heart failure in patients with acute exacerbation of chronic obstructive pulmonary disease. Medicine 2024;103:1(e36840).
Contributor Information
Min Chen, Email: 531532545@qq.com.
Hui Wei, Email: 591314659@qq.com.
Hao-Ran Ma, Email: 244888866@qq.com.
References
- [1].Brennan M, McDonnell MJ, Walsh SM, et al. Review of the prevalence, pathogenesis and management of OSA-COPD overlap. Sleep Breath. 2022;26:1551–60. [DOI] [PubMed] [Google Scholar]
- [2].Feng Z, Zhang L, Yu H, et al. High-flow nasal cannula oxygen therapy versus non-invasive ventilation for AECOPD patients after extubation: a systematic review and meta-analysis of randomized controlled trials. Int J Chron Obstruct Pulmon Dis. 2022;17:1987–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Alparslan Bekir S, Tuncay E, Gungor S, et al. Can red blood cell distribution width (RDW) level predict the severity of acute exacerbation of chronic obstructive pulmonary disease (AECOPD)? Int J Clin Pract. 2021;75:e14730. [DOI] [PubMed] [Google Scholar]
- [4].Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report GOLD executive summary. Am J Respir Crit Care Med. 2017;195:557–82. [DOI] [PubMed] [Google Scholar]
- [5].Yao C, Liu X, Tang Z. Prognostic role of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio for hospital mortality in patients with AECOPD. Int J Chron Obstruct Pulmon Dis. 2017;12:2285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bhatt SP, Dransfield MT. Chronic obstructive pulmonary disease and cardiovascular disease. Transl Res. 2013;162:237–51. [DOI] [PubMed] [Google Scholar]
- [7].Iasonos A, Schrag D, Raj GV, et al. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26:1364–70. [DOI] [PubMed] [Google Scholar]
- [8].Grigsby MR, Siddharthan T, Pollard SL, et al. Low body mass index is associated with higher odds of COPD and lower lung function in low- and middle-income countries. COPD. 2019;16:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sun Y, Milne S, Jaw JE, et al. BMI is associated with FEV (1) decline in chronic obstructive pulmonary disease: a meta-analysis of clinical trials. Respir Res. 2019;20:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Spelta F, Fratta Pasini AM, Cazzoletti L, et al. Body weight and mortality in COPD: focus on the obesity paradox. Eat Weight Disord. 2018;23:15–22. [DOI] [PubMed] [Google Scholar]
- [11].Dong B, Yao Y, Xue R, et al. Distinct implications of body mass index in different subgroups of non-obese patients with heart failure with preserved ejection fraction: a latent class analysis of data from the TOPCAT trial. BMC Med. 2022;20:423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nochioka K, Shiba N, Kohno H, et al. Both high and low body mass indexes are prognostic risks in Japanese patients with chronic heart failure: implications from the CHART study. J Card Fail. 2010;16:880–7. [DOI] [PubMed] [Google Scholar]
- [13].Sakurai K, Chubachi S, Irie H, et al. Clinical utility of blood neutrophil-lymphocyte ratio in Japanese COPD patients. BMC Pulm Med. 2018;18:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].O’Donnell R, Peebles C, Ward J, et al. Relationship between peripheral airway dysfunction, airway obstruction, and neutrophilic inflammation in COPD. Thorax. 2004;59:837–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pascual-González Y, López-Sánchez M, Dorca J, et al. Defining the role of neutrophil-to-lymphocyte ratio in COPD: a systematic literature review. Int J Chron Obstruct Pulmon Dis. 2018;13:3651–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Thomson SP, Gibbons RJ, Smars PA, et al. Incremental value of the leukocyte differential and the rapid creatine kinase-MB isoenzyme for the early diagnosis of myocardial infarction. Ann Intern Med. 1995;122:335–41. [DOI] [PubMed] [Google Scholar]
- [17].Núñez J, Núñez E, Miñana G, et al. Effectiveness of the relative lymphocyte count to predict one-year mortality in patients with acute heart failure. Am J Cardiol. 2011;107:1034–9. [DOI] [PubMed] [Google Scholar]
- [18].Ali S, Shahbaz AU, Nelson MD, et al. Reduced relative lymphocyte count in African-Americans with decompensated heart failure. Am J Med Sci. 2009;337:156–60. [DOI] [PubMed] [Google Scholar]
- [19].Toft-Petersen AP, Torp-Pedersen C, Weinreich UM, et al. Association between hemoglobin and prognosis in patients admitted to hospital for COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:2813–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Vasquez A, Logomarsino JV. Anemia in chronic obstructive pulmonary disease and the potential role of iron deficiency. COPD. 2016;13:100–9. [DOI] [PubMed] [Google Scholar]
- [21].Boutou AK, Hopkinson NS, Polkey MI. Anaemia in chronic obstructive pulmonary disease: an insight into its prevalence and pathophysiology. Clin Sci (Lond). 2015;128:283–95. [DOI] [PubMed] [Google Scholar]
- [22].Beck-da-Silva L, Piardi D, Soder S, et al. IRON-HF study: a randomized trial to assess the effects of iron in heart failure patients with anemia. Int J Cardiol. 2013;168:3439–42. [DOI] [PubMed] [Google Scholar]
- [23].O’Meara E, Rouleau JL, White M, et al. Heart failure with anemia: novel findings on the roles of renal disease, interleukins, and specific left ventricular remodeling processes. Circ Heart Fail. 2014;7:773–81. [DOI] [PubMed] [Google Scholar]
- [24].Beavers CJ, Alburikan KA, Rodgers JE, et al. Distinguishing anemia and iron deficiency of heart failure: signal for severity of disease or unmet therapeutic need? Pharmacotherapy. 2014;34:719–32. [DOI] [PubMed] [Google Scholar]
- [25].Al Mutairi F. Hyperhomocysteinemia: clinical insights. J Cent Nerv Syst Dis. 2020;12:1179573520962230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fimognari FL, Loffredo L, Di Simone S, et al. Hyperhomocysteinaemia and poor vitamin B status in chronic obstructive pulmonary disease. Nutr Metab Cardiovasc Dis. 2009;19:654–9. [DOI] [PubMed] [Google Scholar]
- [27].Brisco MA, Zile MR, Ter Maaten JM, et al. The risk of death associated with proteinuria in heart failure is restricted to patients with an elevated blood urea nitrogen to creatinine ratio. Int J Cardiol. 2016;215:521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kajimoto K, Minami Y, Sato N, et al. Serum sodium concentration, blood urea nitrogen, and outcomes in patients hospitalized for acute decompensated heart failure. Int J Cardiol. 2016;222:195–201. [DOI] [PubMed] [Google Scholar]
- [29].Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–65. [DOI] [PubMed] [Google Scholar]
- [30].Rumora L, Hlapčić I, Popović-Grle S, et al. Uric acid and uric acid to creatinine ratio in the assessment of chronic obstructive pulmonary disease: Potential biomarkers in multicomponent models comprising IL-1beta. PLoS One. 2020;15:e0234363. [DOI] [PMC free article] [PubMed] [Google Scholar]