Abstract
Background:
Cancer and cardiovascular disease (CVD) are major causes of morbidity and mortality in the United States (US). Cancer survivors have increased risks for CVD and CVD-related mortality due to multiple factors including cancer treatment-related cardiotoxicity. Disparities are rooted in differential exposure to risk factors and social determinants of health (SDOH) including systemic racism. This review aimed to assess SDOH's role in disparities, document CVD-related disparities among US cancer survivors, and identify literature gaps for future research.
Methods:
Following the Peer Review of Electronic Search Strategies (PRESS) guidelines, MEDLINE, PsycINFO, and Scopus were searched on March 15, 2021, with an update conducted on September 26, 2023. Articles screening was performed using the PRISMA 2020, a pre-defined PICO framework, and the Rayyan platform. A modified version of the Newcastle Ottawa Scale was used to assess the risk of bias, and RAW Graphs for alluvial charts. This review is registered with PROSPERO under ID #CRD42021236460.
Results:
Out of 7,719 retrieved articles, 24 were included, and discussed diverse SDOH that contribute to CVD-related disparities among cancer survivors. The 24 included studies had a large combined total sample size (n=7,704,645; median=19,707). While various disparities have been investigated, including rural-urban, sex, socioeconomic status, and age, a notable observation is that non-Hispanic Black cancer survivors experience disproportionately adverse CVD outcomes when compared to non-Hispanic White survivors. This underscores historical racism and discrimination against non-Hispanic Black individuals as fundamental drivers of CVD-related disparities.
Conclusions:
Stakeholders should work to eliminate the root causes of disparities. Clinicians should increase screening for risk factors that exacerbate CVD-related disparities among cancer survivors. Researchers should prioritise the investigation of systemic factors driving disparities in cancer and CVD and develop innovative interventions to mitigate risk in cancer survivors.
Introduction
Cancer and Cardiovascular Disease
Cancer and cardiovascular disease (CVD) are leading causes of morbidity and mortality in the United States (US) [1]. Both pathologies are closely linked due to shared risk factors (e.g., low physical activity [PA], poor diet, obesity, etc.), and adverse effects of cancer treatment drugs through systemic inflammation, among other complex pathophysiological pathways [2-5]. Scholars have established a bidirectional causal relationship between cancer and CVD [6]. Having one disease increases the risk of having the other, and both pathologies often co-occur [6,7]. For example, myocardial infarction (MI) accelerates breast cancer (BC) metastasis and cancer-specific mortality [8]. Similarly, adverse outcomes of cancer treatments increase the likelihood of having CVD [9,10] and CVD mortality [11-13]. Advances in cancer care, including improved cancer screening and treatment options, have increased survivorship [3], and CVD is a major survivorship threat. Further, studies have shown that cancer survivors are more probable to die from CVD than cancer itself [13].
Introduction to Health Disparities
While every cancer survivor is at increased risk for CVD, the risk is not evenly distributed. Disparities exist, and some groups are at higher risk than others. Health disparities are “preventable differences in indicators of health of different population groups, often defined by race/ethnicity, sex/gender, educational level, socioeconomic status (SES), disability status, and geographic location of residence” [14,15] which are unfairly and unjustly distributed across different social groups [16]. Disparities have long been, and continue to be a significant public health concern. Disparities have detrimental effects on populations that have historically been targeted for marginalisation, leaving them disadvantaged and susceptible not only within the US but also in broader global contexts. Reducing health disparities was one of the main objectives of Healthy People 2010 and 2020 visions [17] with goals to improve health for all groups and achieve health equity [14]. Unfortunately, disparities persist across multiple measures of health outcomes. Disparities reduction remains a foundational framework for Healthy People 2030 [18]. Members of disadvantaged groups including those living with disabilities [14], those living in rural areas [19-21], minoritised groups by sexual and/or gender identities (SGM) [22] and race and ethnicity [23-25], and those of lower SES [26] continue to be disproportionately affected by health disparities.
Key factors that contribute to disparities include social determinants of health (SDOH) or non-medical factors that influence health outcomes. SDOH is an umbrella term encompassing the conditions in which people are born, grow, live, play, work, and age, as defined by the 2005–2008 World Health Organisation’s Commission on SDOH [27,28]. The National Institutes of Health Centers for Population Health and Health Disparities (CPHHD) Conceptual Framework relates SDOH (population risk) to individual risk and its link to adverse health outcomes [29]. The CPHHD framework has three key components, including distal factors, intermediate factors, and proximal factors, which interact in influencing population and individual health outcomes. Within the CPHHD framework, distal factors have an impact on intermediate factors, and these intermediate factors, in turn, influence proximal and/or individual-level factors that are intricately linked to individual health.
In the CPHHD framework, distal factors include institutional context (healthcare systems, legal systems, political systems, etc.), policies and the policymaking process, and their effects on social conditions (culture, norms, prejudice, etc.), and are collectively termed fundamental causes. Intermediate factors include social relationships, social contexts, and physical contexts through which individuals experience distal effects including neighbourhood physical environment, communities, and social networks. Proximal factors include individual demographic characteristics and individual risk factors that affect biological responses, and pathways to individual health outcomes.
Through intermediate factors, the CPHHD framework posits that the socioeconomic and political environment affects individual sociodemographic factors (i.e., health insurance coverage, health status, education, employment/income, housing insecurity and/or homelessness), individual risks factors (i.e., tobacco or alcohol use, use of greenspaces or other natural and healing environments, PA, loneliness, psychosocial stress, use of social services, trust/mistrust in the healthcare system and use of the system), individual biologic or genetic pathways (chronic stress and allostatic load, genetic ancestry and related mechanisms including intra/extra cellular signalling and mutations), and individual biological responses (i.e., alcoholism, obesity, depression, hypertension, high cholesterol, and other comorbid conditions) [29], all of which can culminate into unfavourable health outcomes for individuals who are disproportionately exposed to adverse life experiences; and this can lead to transgenerational transmission of health disparities [30].
Inequities in the distribution of resources that promote good health, and differential access to resources needed to manage individual health risks stemming from biology or other factors, lead to poorer health outcomes for disadvantaged populations [29]. Structural and systemic barriers, including racial and ethnic discrimination, along with reduced or poorer access to medical care and other salutogenic resources such as greenspaces experienced by disadvantaged population groups increase their vulnerability and exacerbate disparities in health outcomes [26,31-33].
Social scientists have been investigating sociopolitical processes that create conditions leading to health disparities [16,27], including structural, institutional, and historical racism [34,35] which are considered distal factors within the CPHHD framework. One of the most used frameworks, and closely related to the CPHHD framework, is the biopsychosocial model of health, which emphasises the interconnectedness among biological factors, psychological factors, and social factors at the individual level. The biopsychosocial model explains disease development through complex interaction among biological factors (such as genetics and biochemical), psychological factors (including mood, personality, and behaviour), and socioenvironmental factors (i.e., cultural, familial, socioeconomic, medical, neighbourhoods, etc.) in producing and reproducing health [36]. These factors are all nested within the CPHHD framework, illustrating their interconnectedness in understanding health disparities and outcomes. Health disparities are deeply rooted in structural determinants of health (distal factors), mainly through their influence on psychological and physiological stressors, such as threats within the neighbourhood environment, including neighbourhood disinvestment and/or area-level poverty (intermediate factors). For instance, social risk factors have been linked to poorer health outcomes including higher risk for mortality in the US [37]. Intermediate factors influence threats at the family or individual level such as household income, lack of insurance, inadequate access to health care, educational inequalities, and behavioural factors such as smoking, racial segregation, poor diet, and inadequate PA [27,38], and biological consequences such chronic stress and allostatic load [39-43].
Brief Overview of Potential Context-Specific Causes of Disparities
Different individual and community-specific contexts impact SES differently and contribute to health disparities in the general population. For example, health disparities between rural and urban areas in the US have been attributed to SDOH factors operating at both individual and community levels, as well as barriers within the healthcare system, all situated within the CPHHD framework [44]. Individual factors specific to rural areas that contribute to poor CVD outcomes for instance include higher risk of traditional CVD risk factors including diabetes, obesity, and hypertension, among others [45]. Health system-level factors that contribute to poor health outcomes in rural areas encompass reduced healthcare access, including longer distances to tertiary healthcare centres, lower number of practising physicians per resident, lower rates of insurance coverage, and limited public health and healthcare services infrastructure when compared to urban areas [45]. Rural Americans face additional challenges related to SDOH, including relatively lower income levels, lower education levels, more precarious employment opportunities, substandard housing conditions (including inadequate heating or plumbing systems, leaks, and pests), higher unmet transportation needs due to limited vehicle access and inadequate public transportation infrastructure, and higher rates of food insecurity [45]. Additionally, rural residents might not fully use government benefits intended to deal with SDOH such as the Supplemental Nutrition Assistance Program aimed at reducing food insecurity. Factors like misconceptions about eligibility, stigma in smaller communities, and the long distances to stores where they can redeem benefits, all contribute to reduced participation in these available government resources [45]. As a result, rural cancer survivors experience a disproportionate burden of disparities in healthcare and health outcomes [46].
SGM communities continue to face both societal and internalised homophobia, exacerbating the structural barriers that hinder their reach to healthcare services [22]. Furthermore, the medical education system (considered a distal factor within the CPHHD framework), along with the limited cultural competency education and training for healthcare providers to deal with specific needs of SGM individuals, contribute to health disparities within this population [47]. Additionally, different intersecting factors including increased risk of experiencing bullying, harassment, and substance use [48], and other factors that discourage SGM individuals from seeking healthcare services, contribute to lower utilisation levels for both mental health services [49]; and primary health care services [50]. Consequently, cancer survivors within SGM communities are disproportionately affected by disparities in healthcare and health outcomes [51]. For example, a recent systematic review found that sexual minority men have disproportionately higher CVD risk related to tobacco use, alcohol consumption, illicit drug use, poor mental health, and body mass index [52]. Additionally, scholars have linked social conditions such as stigma, prejudice, and discrimination to observed disparities through pathways of a hostile and stressful social environment that causes mental health problems [53].
Individuals with disabilities also have disability-related challenges that limit health service utilisation. Some of those challenges include increased health threats to which people with disabilities are more susceptible, which may include physical, medical, cognitive, emotional, or psychosocial stressors [54]. Moreover, historical disadvantages such as disability-based discrimination and the institutionalisation of individuals with significant disabilities, as well as more recent challenges where individuals with disabilities were not recognised as a population deserving of more public health attention, increase the disadvantages faced by individuals with disabilities [55]. Additionally, individuals with disabilities tend to have lower education levels, lower employment rates, fewer household resources, and poorer overall health than those without disabilities [55,56]. The convergence of these multiple threats intersects and exacerbates disadvantageous environments for individuals with disabilities, ultimately contributing to poorer health outcomes [57], including increased rates of CVD risk factors (i.e., diabetes) and CVD conditions (i.e., hypertension) [58], and cancer disparities [59].
American Indian and Alaska Native/Tribal communities have also historically had limited reach to quality health care, which is a contributor to disparities in poor health outcomes [31]; and cancer survivors within the American Indian and Alaska Native communities are disproportionately affected [60,61]. Although Indian Health Services (IHS) clinics have been created to allow federally recognised tribes to have rights to healthcare services, many tribes without federal recognition are not eligible for IHS services; and therefore, lack access to care [31,62]. In addition, the IHS program is chronically underfunded and is mainly limited to rural reservations [62-64]. The limited funding of the IHS significantly impairs its effectiveness, and its restriction to rural reservation lands restricts access for members of federally recognised tribes living in urban areas unless they travel longer distances to healthcare services to seek the care they need [31]. Further, additional factors exacerbate disparities within tribal communities, including language and communication barriers, higher rates of poverty, limited insurance coverage, a lack of diversity in the healthcare workforce, and experiences of discrimination [31].
Last, but not least, minoritised groups have a history of being targeted for marginalisation, resulting in substantial disadvantages. The US has a history marked by chattel slavery, which was succeeded by systemic laws that restricted reach to economic opportunities for formerly enslaved people following abolition of slavery [65-67]. Some of those colour-coded and discriminatory laws, such as redlining, led to systemic disinvestments in Black/African American neighbourhoods. This neighbourhood disinvestment has been linked to poor neighbourhood physical environments, including disproportionate vulnerability to extreme weather events related to climate change [68,69] as well as adverse health outcomes, including those related to CV health and cancer [68,70-73]. Redlining, neighbourhood disinvestment, and community disadvantage contribute to poor health outcomes [72,73], including increased CVD risk in a variety of ways. In disinvested neighbourhoods, the absence of grocery stores, often referred to as “food deserts,” creates food-insecure communities with limited reach to healthy food options [74-77]. Food-insecure neighbourhoods exhibit restricted availability, access, utilisation and stability of healthy and/or fresh food [75,76,78], all of which are key pillars of food security [79]. Food insecurity is linked to unfavourable health status [80,81] and CVD risk factors [82-85], including unhealthy dietary patterns [86-88], obesity [86,87,89-98], and diabetes [99-106], subsequently leading to poor CV health outcomes [107-111].
Another facet of neighbourhood disadvantage is the high prevalence of fast food restaurants, often referred to as “food swamps” [112]. Food swamps contribute to healthful food insecurity [113] by fostering an environment in which residents are encouraged to consume highly processed and unhealthy junk foods [114]. Consumption of junk foods increases CV risk factors [115,116]. In addition to critical links between food deserts, food swamps, and food insecurity in relationship to CV risk factors, disinvested lower SES neighbourhoods have relatively poorer quality greenspaces [117], poorer quality recreational facilities, poor neighbourhood walkability, limited or poor quality bike paths, and other neighbourhood risk factors such as higher concentration of smoke shops and tobacco or alcoholic products, increased risk of air pollution, and increase risk for CVD [118]. All these intersecting social and built neighbourhood environmental risk factors collectively amplify the risk for obesity, which is the main pathogen behind high rising rates of chronic diseases including CVD, and cancer [119,120]. This, in turn, contributes to disparities in CVD outcomes, particularly among racial and ethnic minority cancer survivors [121]. It is therefore not surprising that individuals who have been targeted for marginalisation, including African Americans, often exhibit earlier onset of multiple traditional CV risk factors. This, in turn, increases their risk for CVD conditions such as heart failure, stroke and peripheral vascular disease [67], and leads to disparities in cancer survivorship-related outcomes [122].
Objective of the Review
While existing reviews have examined CVD disparities in healthy populations with a focus on racial disparities [123,124], there is currently no systematic review focused on CVD disparities among cancer survivors in the US. This gap is substantial considering the higher risk for CVD among cancer survivors, a topic extensively explored in the Cardio-Oncology literature [1,5]. In this review, the aim was to identify various disparities in CVD outcomes among cancer survivors in the US, identify literature gaps, and offer insights to guide future research efforts. A comprehensive literature review was conducted, encompassing various forms of disparities, including racial and ethnic, urban versus rural, SGM versus heterosexual/cis-gender, SES, and any other measure of disparities that scholars have investigated thus far. The primary research question was as follow: “What are the established disparities in CVD outcomes among cancer survivors in the US?”
Methods
This review adhered to a predefined protocol that was developed in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) guidelines [125] and used the PRISMA 2020 checklist as a reporting guide [126].
Literature Search
A comprehensive literature search was developed with a medical librarian and peer-reviewed using the Peer Review of Electronic Search Strategies (PRESS) guideline [127]. Searches were originally conducted in MEDLINE (Ovid), APA PsycINFO, and Scopus on March 15, 2021, and results were previously reported in an abstract [128]. Updated searches were performed on September 26, 2023. This process ensured the inclusion of all relevant papers. Databases were considered because we needed to include citation databases of peer-reviewed literature with potential of having medical sciences articles from the National Library of Medicine's bibliographic database (Ovid), source-neutral abstracts and citation databases curated by independent subject matter experts (Scopus), and a pool of citations and other abstracts of literature in the field of psychology and sociology (PsycINFO). Searches were limited to articles written in English.
Search strategies were created using medical subject headings and keywords combined with database-specific advanced search techniques. Medical subject headings terms and keywords were identified to represent disparities, CVD outcomes and cancer. Some keywords used include injustice, stroke, and neoplasm to respectively represent disparities, CVD outcomes, and cancer survivorship. The full search strategy is further detailed in Appendix A. All articles retrieved from the literature searches were downloaded into EndNote and duplicate articles were removed. All unique publications were uploaded into Rayyan Artificial Intelligence, an online instrument for systematic review [129]. The web app facilitated the article selection process, eased the collaboration between two independent reviewers (JCB and IA or SR), and enabled blinding of the two reviewers from each other during article screening, a process that has widely been used in previous systematic reviews [33,130-132].
Articles Selection Process
The following Population, Exposure, Comparison, Comparison, Setting (PECOS) framework [133,134] was followed in the article inclusion and exclusion criteria:
P (Population): Cancer survivors, following the National Cancer Institute (NCI) definition of cancer survivorship, the period of time from clinical diagnosis until the patient’s end of life [135].
E (Exposure ): Any (no restrictions)
C (Comparison): All types of individuals belonging to least disadvantaged groups such as Non-Hispanic Whites (NHWs), heterosexuals, or Cis genders, those in higher SES, or those without disabilities.
O (Outcomes): All CVD-related outcomes including blood pressure and major adverse cardiovascular events as defined in previous studies [136-138] such as the occurrence of MI (fatal and nonfatal), heart failure (congestive heart failure, systolic heart failure, diastolic heart failure), cerebrovascular disease or CV accident or stroke (fatal and nonfatal), or coronary artery bypass grafting and cardiac arrest. Congenital heart diseases are excluded as outcomes.
S (Setting): Must be studying the US population-based sample; studies looking at any other country, other than the US were excluded.
Eligibility criteria were piloted using a random selection of articles from the initial article pool to ensure clarity in expectations between reviewers. Next, two blinded reviewers (JCB and IA or SR) independently screened titles of all results of the literature search against eligibility criteria. The goal was to exclude articles that did not clearly meet the PECOS criteria by reading articles’ titles. Discrepancies between reviewers were resolved through a mutual discussion or sought arbitration or mediation from third reviewer (KMMB) if needed. The same process was repeated for abstract screening for the remaining articles. Next, reference lists of all remaining articles were screened to identify relevant publications not retrieved by electronic database searches. The article selection process was repeated with additional articles from references list. Both reviewers independently read full methods sections of every remaining article to make sure that all PECOS criteria have strictly been adhered to.
Articles Eligibility Criteria
Inclusion criteria
In the original database search, inclusion criteria required presence of keywords related to health disparities, CVD outcomes, or cancer survivorship in article title, abstract, or full text. Any measure of health disparities was considered including age, sex, gender identity, sexual orientation, race/ethnicity, rurality or urbanicity, SES, disability status, or geographic location [139]. Consideration was given to standard outcome measures, such as rates, proportions, percentages, or means used to present differential outcomes between groups being compared, as these measures have been suggested throughout the health disparities literature [140].
Exclusion criteria
The exclusion criteria for articles were based on PECOS criteria, resulting in the exclusion of studies that did not investigate CVD-related disparities, did not measure at least one cardiovascular outcome, involved populations other than cancer survivors, included non-empirical or non-original research articles (e.g., reviews, letters to the editor, policy papers, abstracts, and protocols), were published in languages other than English due to translation logistics, or were conducted outside of the US. Table 1 summarises inclusion and exclusion criteria.
Table 1.
Article inclusion and exclusion criteria.
| Inclusion | Exclusion |
|---|---|
| Studies investigating CVD-related disparities | Studies that investigate disparities other than CVD-related disparities were excluded. |
| Measuring at least one CVD-related outcome in terms of disparities | Any other outcome studied other than CVD |
| Study population: Cancer survivors only; and the US study setting only. No age restriction. | Any population other than cancer survivors or study settings outside of the US |
| Empirical and original studies only with no temporality restriction on CVD outcome (including comorbid CVD with cancer regardless of the time of diagnosis) | All type of review articles, letters to editor, case reports, policy and position papers, abstracts only and protocols were excluded |
| Publication in English Language only | Articles published in any other language other than English |
| Existence of full text | Lack of full text |
| Peer reviewed articles only | Non peer reviewed articles were excluded |
Risk of Bias Assessment
Risk of bias was assessed using the Newcastle Ottawa Scale (NOS), which was modified to include case-control studies and cohort studies, a method that was used in previous systematic reviews [33]. Briefly, the NOS scale has eight items, and each can be scored with one point, except the compatibility item which can be assigned up to two points, resulting in a total of nine points. Studies with a score of five or more are considered low risk of bias while those with a score of less than five are considered moderate to high risk of bias. Two independent reviewers (JCB and IA or SR) assessed all included articles and resolved all discrepancies until a consensus was reached.
Data Extraction
From the studies, a comprehensive array of data was extracted, covering various dimensions: (1) study settings, spanning US regions (National, Northeast, Southeast, and West), (2) specific disparities explored, including racial and ethnic disparities, gender disparities, age disparities, rural-urban disparities, disparities associated with education, SES, disability, cancer stage at diagnosis, sexual orientation/identity, and others, (3) metrics for CVD outcomes, encompassing measures like CVD risk and mortality, (4) comparison groups, defined by populations belonging to less disadvantaged groups like NHWs or individuals with higher SES, (5) cancer type, classified as specific or any cancer type, along with cancer stage at diagnosis, (6) specifics regarding the statistical analyses conducted, (7) key findings from the included studies, and lastly, (8) conclusions as articulated by the respective authors of the included studies.
Extracted information is summarised in Table 2 [141-164]. In addition, the extracted information was used to create a small Excel dataset, which, in turn, was used to produce an alluvial chart for graphical illustration of the review findings. The Excel dataset is annexed in Appendix B. This review is registered with PROSPERO, the National Institute for Health Research, International Prospective Register of Systematic Reviews, with registration number: CRD42021236460.
Table 2.
Summary of characteristics of all articles include in the review.
| Author, Year, and title |
Setting/ Dataset/ Years |
Sample Size |
Study type and/or Dataset used |
Age: Mean (± SD)years or Range |
Cancer type and stage at diagnosis |
Disparity type and Description |
CVD- related Outcome |
Statistical Analyses |
Main findings |
Conclusions |
|---|---|---|---|---|---|---|---|---|---|---|
| Al-Kindi et al. (2015) [141] Trends and disparities in cardiovascular mortality among survivors of Hodgkin Lymphoma |
SEER National 1990 to 2011 | n=19,781 53% Male & 83% White | Surveillance, Epidemiology, and End Results (SEER) of the National Cancer Institute (NCI) | 33 (± 8.3) at cancer diagnosis Range: 20-49 years |
Hodgkin Lymphoma Stage I-IV |
Racial disparities Gender disparities Age disparities |
Trends of cardiovascular disease mortality (CVM) | Kaplan-Meier method with the log-rank test for analyses of cumulative incidence. Cox proportional hazard | Risk of CVM was higher in men, older and Black patients. Black people: HR: 1.97. Men: HR, 2.2 Older at diagnosis: HR, 1.073 per year. |
CV screening and risk modification should be intensified in HL patients at increased risk (men, older at diagnosis and Black patients) |
| Al-Sadawi et al. (2021) [142] Racial and socioeconomic disparities in cardiotoxicity among women with HER2-positive breast cancer |
New York Northeastern 2004 to 2013 | n=1,399 women with HER2-positive breast cancer (BC) Black (n=169) White (n=1,064) Other (n=166) |
Retrospective cohort study Memorial Sloan Kettering Cancer Center and New York BC databases SES data: US Census 2013-2017American Community Survey |
Median Age (Interquartile range) 51 (44-59) Black: 51.6 (44.6, 59.3) White: 51.0 (43.5, 59.2) Other 50.9 (42.7, 57.5) |
BC Cancer stage: I II III ER-positive. PR-positive |
Racial disparities socioeconomic status (SES) disparities | Cardiotoxicity: Heart failure Asymptomatic decline in left ventricular ejection fraction (LVEF, absolute decrease ≥ 10% to < 53%, or ≥ 16%) |
Kruskal-Wallis test for continuous data Pearson’s chi squared test for categorical data. Univariable and multivariable logistic regression |
Higher levels of hypertension among Black women. Lower SES among Black women than other races Adjusted risk for cardiotoxicity is higher among Black women that White women. |
There is high level of risk for cardiotoxicity among Black women during HER2-targetedBC therapy |
| Berkman et al. (2014) [143] Racial differences in breast cancer, cardiovascular disease, and all-cause mortality among women with ductal carcinoma in situ of the breast |
SEER National 1978 to 2010 | 67,514 women: 6,113 Black women 54,518 White women |
Cohort study National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) registry |
Age at diagnosis 40-49 years 50-59 years 60-69 years ≥70 years |
BC: Ductal carcinoma in situ of the breast | Racial disparities | Incidence of BC and CVD death | Kaplan-Meier product-limit Competing risks analysis Hazard ratios and 95% CI computed based on the log-rank test. |
Higher risk for death is more prevalent among Black women with DCIS than White women. Higher risk for all cause death, BC death and CVD death among Black women than White women. |
Higher risk of all cause death and CVD specific death than BC specific death among women with DCIS. Higher risk of death for Black women compared to White women. |
| Berkman et al. (2017) [144] Racial differences in 20-year cardiovascular mortality risk among childhood and young adult cancer survivors |
SEER National 1973 to 2011 | Total: 164,316 Black: 16,060 White: 16,060 |
The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) registries | Age of diagnosis diagnosed at age 0–14 years. diagnosed at age 15–34 years |
All cancers: Leukemias Hodgkin lymphoma Non-Hodgkin lymphoma Central nervous system (CNS) Germ cell Thyroid Melanoma | Racial disparities | Risk for any death and CVD death | Kaplan–Meier and Cumulative incidence curves Log-rank test Cox proportional hazards models with competing risk analysis |
Black survivors had a higher risk for CVD mortality (HR: 2.13) compared to White survivors. Persisted risk for CVD among Black survivors at 5 years (HR: 2.38), 10-years (HR: 2.59) and 20-years (HR: 2.31) post diagnosis. |
Higher risk of CVD mortality, differing by cancer type was prevalent among Black survivors of childhood or young adults than White survivors. |
| Gallicchio et al. (2017) [145] Cardiovascular health among Black and White breast cancer patients initiating aromatase inhibitor therapy |
Baltimore, MD Northeast region 2011 to April 2015 |
n=146 Black: n=45 White: n=101 |
Prospective Cohort study Data: Baseline data collection |
Mean age of the study sample was 63.2 years (SD=7.8) | BC Stage I–III |
Racial disparities | The 10-year Atherosclerotic cardiovascular disease (ASCVD) event risk score and the number of CVD risk factors | Chi-squared and Fisher’s exact tests Student’s t-tests Wilcoxon rank sum tests for continuous variables with a skewed distribution |
Higher median 10-year ASCVD event risk score among Black than White patients. Higher number of CVD risk factors among Black than White patients. Higher rates of hypertension more likely to be found among Black than White patients. |
Higher 10-year ASCVD event score and higher number of CVD risk factors are found in Black than White patients. Racial disparities are associated to treatment issues and increased risk for negative outcomes for Black patients |
| Keegan et al. (2018) [146] Sociodemographic disparities in the occurrence of medical conditions among adolescent and young adult Hodgkin lymphoma survivors |
West 1996 to 2012 | 5,085 patients | Population-based study California Cancer Registry linked to hospitalization data. |
Age at diagnosis: 15–39 years |
Hodgkin lymphoma (HL) | SES disparities Racial and ethnic disparities |
Incidence of circulatory system diseases | Gray’s K-sample test statistic Multivariable Cox proportional hazards regression Martingale residuals |
Higher probability of circulatory system diseases (HR=1.58, 95% CI=1.17–2.14) among Black survivors compared to NHW survivors Higher likelihood of circulatory system diseases among adolescents and young adults with public or no insurance compared to those with private or military insurance. |
Higher risk for circulatory system conditions and mortality among adolescent and young adult Black HL survivors of an those without insurance or with or public insurance |
| Litvak et al. (2018) [147] Racial disparities in the rate of cardiotoxicity of HER2-targeted therapies among women with early breast cancer |
Baltimore, MD Northeast January 2005 to March 2015 |
Entire Cohort: n=216 White: n=157 Black Patients n=59 |
Retrospective study Data collected from the Sidney Kimmel Comprehensive Cancer Centre at Johns Hopkins | Age: Entire Cohort 52 ± 12 White Patients 53 ± 12 Black patients 51 ± 11 |
Early Breast Cancer: Stage I, II, III |
Racial disparities | Cardiotoxicity Assessed with a decline in LVEF to<50% AND An absolute drop in LVEF of 10% from baseline. and incomplete therapy (<52 weeks of HER2-targeted therapy) |
Descriptive statistics Correlation Kaplan-Meier method Univariate and multivariable Cox regression analyses |
Higher prevalence of hypertension at diagnosis and a higher rate antihypertensive among Black compared to White women. Increased risk of cardiotoxicity among Black patients. Higher correlation between cardiotoxicity event and incomplete therapy |
Higher rate of cardiotoxicity among Black patients treated with HER2-targeted therapies than White patients. Incomplete therapy was higher among Black patients. |
| Polednak, 2004 [148] Racial differences in mortality from obesity-related chronic diseases in US women diagnosed with breast cancer. |
SEER National 1975 to 1995 | 233,329 women | US National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) |
57.0 years for African Americans 57.1 years for Asian Americans Whites (61.9 years) |
BC Stage: local, regional, distant, or unknown |
Racial disparities | Death from obesity-related causes: Coronary heart disease (CHD), hypertension, and cerebrovascular disease |
Cox proportional hazards regression | Higher risks for obesity-related causes (hypertension, coronary heart disease, and cerebrovascular disease) among African American women compared to White women | Risks for obesity-related causes (hypertension, coronary heart disease, and cerebrovascular disease) are higher for Black women compared to White women |
| Troeschel et al. (2019) [149] Race differences in cardiovascular disease and breast cancer mortality among US women diagnosed with invasive breast cancer |
SEER National 1990 to 2014 | 407,587 non-Hispanic Black (NHB) and White (NHW) women | Surveillance, Epidemiology, and End Results (SEER) registries | Age at diagnosis (<55,55–68, 69+ years) | BC Localized Regional Distant | Racial disparities | Cumulative incidence and Mortality due to CVD | Cox Proportional hazard with Fine-Gray methods for competing risks. Likelihood ratio tests (LRT) |
CVD-related mortality was higher among younger NHB than NHW White women. CVD mortality disparities reduced with increasing age |
CVD-related mortality risk is higher among NHB compared to NHW survivors; and higher racial differences are among younger women. |
| Williams et al. (2020) [150] The association between hypertension and race/ethnicity among breast cancer survivors |
NHANES National 1999 to 2014. | 524 BC survivors 107 African American 417 White |
National Health and Nutrition Examination Surveys 1999–2014 | Age 65.9 ± 0.8 NHB: 66.3 ± 0.8 NHW 61.8 ± 1.9 |
BC | Racial and ethnic disparities | Hypertension prevalence | Modified Poisson regression. Prevalence ratios (PR) |
Higher prevalence of hypertension among African American BC survivors than White BC survivors | Higher prevalence of hypertension among African American BC survivors than White survivors, and might experience higher risk of CVD-related death |
| Du and Song (2022) [151] Racial disparities in treatments and mortality among a large population-based cohort of older men and women with colorectal cancer |
SEER National 2007 to 2015 | n=101,250 men and women (65 years and older) | Surveillance, Epidemiology, and End Results (SEER) registries | Age at diagnosis (65-69, 70-74, 75-79, 80-84, and 85+ years) | Cancer stage: in-situ, local, regional, distant, and unknown or missing Colorectal cancer (CRC) |
Racial Disparities | Prevalence and treatment of hypertension | Chi-square statistics for categorical variables Multiple logistic regression models Cox proportional hazard regression models |
There are substantial racial and ethnic disparities in the prevalence of hypertension and in receiving antihypertensive treatment. | There were racial disparities in prevalence of and treatment for hypertension |
| Connor et al. (2022) [152] Racial disparities in mortality outcomes among women diagnosed with breast cancer in Maryland: Impact of cardiovascular disease and clinical characteristics | East Maryland Cancer Registry |
n=36,088 women (25,181 Whites and 10,907 Blacks) | Maryland Cancer Registry 2007 to 2017 | 18 years and older | Primary invasive BC | Racial disparities | CVD-related mortality | Cox proportional hazards regression Fine Gray regression models for CVD-related mortality sdHR. |
Black women had a higher hazard of CVD mortality (sdHR, 1.33; 95% confidence interval [CI], 1.17-1.51) compared to White women. | Black women have a higher hazard for CVD mortality in comparison with White women. |
| Datta et al. (2022) [153] Racial disparities in cancer-associated thrombosis |
West The California Cancer Registry |
n=942,109 | The California Cancer Registry linked to the California Patient Discharge Dataset and Emergency Department Utilization database (2005-2017). | Age not clarified | Any cancer type, excluding nonmalignant cases. | Race and ethnicity | Incidence of cancer-associated thrombosis (CAT) | Multivariable Cox proportional hazards regression models | Black/African Americans had the highest 12-month cumulative incidence of CAT across all cancer types except for myeloma, with hazard ratios ranging from 1.27 to 1.69 compared with Whites | Blacks/African Americans had a higher incidence of CAT for all tumour types except myeloma compared to NHW patients |
| Mukherjee et al. (2022) [154] Racial and rural–urban disparities in cardiovascular risk factors among patients with head and neck cancer in a clinical cohort |
University of Alabama at Birmingham Health System | n=2,262 | Electronic health records (EHR) data (2012 and 2018) | Median age at diagnosis: 61 | Head and neck squamous cell carcinoma (HNSCC) | Racial and ethnicity Rural and urban disparities |
Prevalence of CVD risk factors (hypertension, dyslipidaemia and diabetes) at baseline and incidence at 1-year post HNSCC diagnosis | Logistic and robust Poisson regression | Hypertension was less prevalent in rural HNSCC patients, compared to urban HNSCC patients. No racial differences in hypertension prevalence were reported |
Urban HNSCC had a higher risk of hypertension prevalence than rural patients. No racial differences in hypertension prevalence were reported. |
| Du and Song (2023) [155] Age and racial disparities in the utilization of anticancer, antihypertension, and anti-diabetes therapies, and in mortality in a large population-based cohort of older women with breast cancer |
National SEER 2007-2015 |
n=92,829 women | Surveillance, Epidemiology, and End Results | 65 and older | BC Stage: in situ, AJCC stages I–IV, or unknown |
Age and Racial Disparities | Hypertension prevalence Utilization of antihypertensive |
Chi-square statistics for difference in distribution at baseline characteristics. Multiple logistic regression. Cox proportional hazard regression model. |
NHB women or older women (75 years or older) had the highest prevalence for hypertension compared to NHW women or younger women (65-74 years old) Black women were significantly more likely to receive antihypertensive (1.26, 1.19-1.33) compared to White women |
NHB women or older women at highest risk for hypertension Black women were more likely to receive antihypertensive than White women. |
| Nielsen et al. (2023) [156] Determining the association of rurality and cardiovascular disease among prostate cancer survivors |
West | n=19,632 3,379 rural and 16,253 urban |
The Utah Population Database, linked to the Utah Cancer Registry, and electronic medical records from Utah hospitals | 18 years and older at diagnosis | Prostate | Urban-rural disparities | Prevalence for: Hypertension, diseases of arteries, diseases of veins, and heart failure. |
Cox proportional hazards | Rural survivors had a lower risk of hypertension (HR 0.90), diseases of arteries (HR 0.92), and veins (HR 0.92) but a higher risk of heart failure (HR 1.17) | Rural survivors were less likely to be diagnosed with screen-detected cardiovascular disease but more likely to have heart failure. |
| Johnson et al. (2022) [157] Disparities in comorbidities in lung cancer: findings from the behavioural risk factor surveillance system |
National BRSS 2014 to 2018 | n=594 individuals with lung cancer (217 men and 377 women) | Behavioural Risk Factor Surveillance System | 18 years and older | Lung | Sexual disparities Racial/ethnic disparities |
Incident heart attack History of Stroke |
Descriptive statistics Chi2 test of independence Multiple logistic regression |
Men were more likely to suffer from heart attack. NHB individuals are more likely to suffer from stroke than NHW. Black individuals were more likely to suffer from stroke and less likely to suffer from heart attack than White |
Men face higher vulnerability to heart attack than women. NHB individuals face higher vulnerability to stroke than NHW individuals. White men face higher vulnerability to heart attacks and angina. |
| Appiah et al. (2021) [158] The influence of individual and neighbourhood-level characteristics on rural-urban disparities in cardiovascular disease mortality among U.S. women diagnosed with breast and gynaecologic cancers | National SEER 2000 to 2016 |
n=1,139,767 | Surveillance, Epidemiology, and End Results linked with Area Health Resource Files for neighbourhood-level factors | 20 years and older | Breast and Gynaecologic cancer | Rural-urban disparities | CVD mortality | Standardized mortality ratios (SMRs) Multilevel Cox models |
Women with BGC in rural communities had higher CVD deaths than those in urban communities | Women with BGC in rural communities had higher CVD deaths than those in urban communities |
| Noyd et al. (2023) [159] Cardiovascular risk factor disparities in adult survivors of childhood cancer compared with the general population |
National CCSS 1970 to 1999 |
n=16,457 | The Childhood Cancer Survivor Study (CCSS) dataset | Age not clearly defined, other than survivors lived for at least 5 years after diagnosis. | Childhood cancer | Racial and ethnicity | CVD risk factors | Multivariable piecewise exponential regression | NHB (n=1,092) and Hispanic (n=1,405) survivors compared with NHW (n=13,960) survivors reported a higher cumulative incidence of multiple CVRFs (17.7%, 16.6%, and 12.3%, respectively) | NHBs and Hispanic survivors face higher vulnerability to CVD risk factors. |
| Shi et al. (2021) [160] Insurance disparity in cardiovascular mortality among non-elderly cancer survivors |
National SEER 2007 to 2016 |
n=768,055 | Surveillance, Epidemiology and End Results | 18 to 64 years old at the time of diagnosis | Any cancer types. SEER stage: in situ, Regional, Distant |
Insurance type, proxy for SES. Non-Medicaid versus Medicaid or no insurance. | CVD mortality | Standardized mortality ratios (SMRs) Fine-Grey Model |
CVD death in patients with Medicaid was higher than in those with non-Medicaid insurance. Older age, male gender, and Black race were all associated with increased CVD mortality. | Survivors with Medicaid or without insurance face higher vulnerability to CVD mortality compared to those with non-Medicaid insurance. Men, older age, and NHBs individuals also have higher vulnerability. |
| Nyrop et al. (2021) [161] Obesity, comorbidities, and treatment selection in Black and White women with early breast cancer |
UNC Southeast 1992 to 2004 and 2010 to 2014 |
n=548 women | University of North Carolina Linebarger Comprehensive Cancer Centre database | Mean age: 55 (range, 25-83) years for Black women 57 (range, 24-92) years for White women |
Early-stage breast cancer (stages I-III) | Race and ethnicity | Prevalence of hypertension at diagnosis | Fisher exact test for comparison of frequencies for Black and White subjects. Log binomial regression | Black women had higher frequencies of hypertension (PR, 1.45; 95% CI, 1.19-1.75; P=.0002). | Black women face higher vulnerability to hypertension |
| Appiah et al. (2022) [162] A prospective population-based study of Cardiovascular disease mortality following treatment for breast cancer among men in the United States, 2000-2019 |
National SEER 2000 to 2019 |
n=5,216 men with breast cancer treated with chemotherapy | Surveillance, Epidemiology, and End Results | Age: 40 years and older |
Breast cancer | Race and ethnicity | CVD mortality | Cox proportional hazards with competing risk models | Treatment with chemotherapy was associated with higher risk of CVD mortality in men with breast cancer; and Hispanic men had the highest risk (HR: 3.96, 95%CI: 1.31-12.02) than non-Hispanic Black and non-Hispanic White men | Hispanic men with BC face higher vulnerability to CVD mortality after chemotherapy treatment. |
| DeRemer et al. (2023) [164] Racial and ethnic differences in cardiac surveillance evaluation of patients treated with anthracycline-based chemotherapy |
Southeast January 1, 2012, to April 30, 2020 |
n=5,430 patients treated with anthracycline-based chemotherapy | OneFlorida Clinical Research Consortium database. | Median age=53 years | Any cancer types | Race and ethnicity | Undergoing cardiac surveillance evaluation before and 6 to 12 months after exposure to anthracycline treatment | Multivariable logistic regression | NHB patients had a lower likelihood of receiving a baseline echocardiogram than NHW patients or any baseline cardiac surveillance. Compared with NHW patients, Hispanic patients received significantly less cardiac surveillance at the 6-month and 12-month time points. |
NHB and Hispanic patients have a lower likelihood of receiving cardiac surveillance, and baseline (NHB) and at 6 and 12 months (Hispanic) compared to NHW. |
| Zhu et al. (2023) [163] Racial and ethnic disparities in all-cause and cardiovascular mortality among cancer patients in the US | National SEER 2000 to 2018 |
n=3,674,511 | Surveillance, Epidemiology, and End Results | 18 years and older | Any of the 10 most prevalent cancers (Any cancer type) |
Race and ethnicity | CVD mortality | Cox regression models with Fine and Gray's method for competing risks | NHB individuals had higher CVD mortality (HR: 1.25; 95% CI: 1.24-1.27) than NH White patients. | NHB individuals face higher vulnerability to CVD mortality than NHW. |
Results
PRISMA Chart Illustrating Articles Selection Process
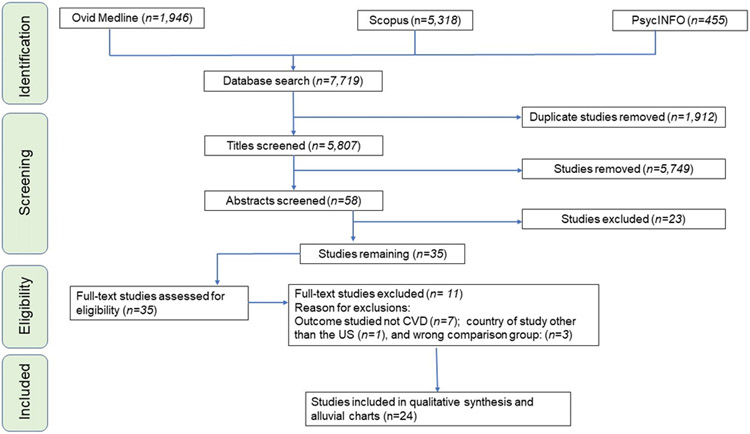
Following a systematic article inclusion and exclusion process, 24 articles meeting the pre-defined inclusion and exclusion criteria were identified from the initial pool of 7,719 articles retrieved from database searches, as illustrated graphically in the PRISMA 2020 chart (see Figure 1). Excluded studies include non-empirical studies, those without a CVD outcome, those investigating a different study population other than cancer survivors and other exclusion criteria (see Table 1).
Figure 1. Graphical illustration of the PRISMA flowchart detailing the article selection process based on the pre-defined PECOS framework that guided the inclusion and exclusion criteria.
Abbreviations: PECOS, Population, Exposure, Comparison, Comparison, Setting; PRISMA, Preferred Reporting Items for Systematic Review and Meta-Analysis.
Included Studies Risk of Bias
The risk of bias assessment table scores, along with item-scoring guidelines, are described in Table 3 [141-164]. The average score for the studies included is 8.9 out of 9, suggesting a low risk of bias.
Table 3.
Risk of bias Assessment: Modified NOS for cohort or case control or cross-sectional studies.
| Selection | Compatibility | Outcomes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study: Author (year) | Item 1 |
Item 2 |
Item 3 |
Item 4 |
Item 5 | Item 6 |
Item 7 |
Item 8 |
||
| 1 | Al-Kindi et al. (2015) | 9/9 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| 2 | Al-Sadawi et al. (2021) [142] | 9/9 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| 3 | Berkman et al. (2014) [143] | 9/9 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| 4 | Berkman et al. (2017) [144] | 9/9 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| 5 | Gallicchio et al. (2017) [145] | 9/9 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| 6 | Keegan et al. (2018) [146] | 9/9 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| 7 | Litvak et al. (2018) [147] | 9/9 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| 8 | Polednak et al. (2004) [148] | 9/9 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| 9 | Troeschel et al. (2019) [149] | 9/9 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| 10 | Williams et al. (2020) [150] | 9/9 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| 11 | Du and Song, (2022) [151] | 9/9 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| 12 | Connor et al. (2022) [152] | 9/9 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| 13 | Datta et al. (2022) [153] | 9/9 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| 14 | Mukherjee et al. (2022) [154] | 9/9 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| 15 | Du and Song (2023) [155] | 9/9 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| 16 | Nielsen et al. (2023) [156] | 9/9 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| 17 | Johnson et al. (2022) [157] | 8/9 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 1 |
| 18 | Appiah et al. (2021) [158] | 9/9 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| 19 | Noyd et al. (2023) [159] | 8/9 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 1 |
| 20 | Shi et al. (2021) [160] | 9/9 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| 21 | Nyrop et al. (2021) [161] | 9/9 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| 22 | Appiah et al. (2022) [162] | 9/9 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| 23 | Zhu et al. (2023) [163] | 9/9 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| 24 | DeRemer et al. (2023) [164] | 9/9 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| Final Average score | 8.9/9 | |||||||||
Summary Characteristics of the 24 Articles Included in the Review
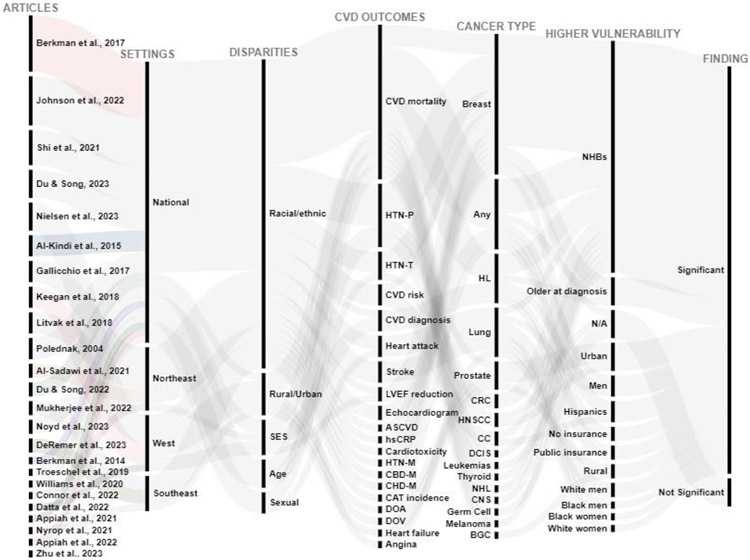
The 24 included studies had a large combined total sample size (n=7,704,645; median= 19,707). Those 24 articles meeting the pre-determined inclusion criteria are summarised in Table 2 by authors and year, study setting (National or other specific US regions), sample size, study type, follow-up/duration, age, type of disparity, disparity description with groups being compared, CV health-related outcomes, statistical analyses conducted, main findings and authors conclusion. Additionally, the alluvial chart summarises review’s results, incorporating key details including authors and year, study setting, disparity type, CVD outcomes, cancer type, disadvantaged groups, and findings' significance (Figure 2). This method has been employed in previous systematic reviews [33,132,165].
Figure 2. Disparities in cardiovascular diseases (CVD) outcomes among cancer survivors in the US.
The first column represents authors and year of each study, the second column represents the US geographical settings of studies, the third column represents different disparities investigated in each statistical test conducted, the fourth column represents CVD-related outcomes investigated, the fifth column represents types of cancer for different study populations, the sixth column represents the reported disadvantaged groups, while the seventh column represents statistical significance reported by studies. This graphical representation shows an overall trend in findings across studies for all statistical tests conducted for specific CVD outcomes investigated.
Abbreviations: NHB, non-Hispanic Black; CBD-M, cerebrovascular disease related mortality; HTN-T, hypertension treatment receipt; ASCVD, atherosclerotic cardiovascular disease risk score; CHD-M, coronary heart disease related mortality; HTN-M, hypertension related mortality; BC, breast cancer; HL, Hodgkin Lymphoma, DCIS, ductal carcinoma in situ of the breast; CNS, central nervous system; NHL, non-Hodgkin Lymphoma; hsCRP, high sensitivity C-reactive protein; CAT, cancer-associated thrombosis; HTN-P, hypertension prevalence; LVEF, left ventricular ejection fraction; HNSCC, head and neck squamous cell carcinoma; CRC, colorectal cancer; CC, childhood cancer; SES, socioeconomic status; BGC, breast and gynaecologic cancer; DOA, diseases of the arteries; DOV, diseases of the veins.
Significant Racial and Ethnic Disparities Were Identified in This Review
Twenty-three out of 24 included articles (96.8%) focused on racial and ethnic disparities, specifically comparing Non-Hispanic Black (NHB) to NHW individuals, all reporting substantial disadvantages for NHB individuals. A single study (4.1%) reported mixed findings, including a higher risk of stroke among NHB individuals diagnosed with lung cancer compared to NHW individuals, but also reported a higher risk of heart attack among NHW individuals compared to NHB individuals diagnosed with lung cancer [157]. Additionally, Johnson et al. (2022) found a higher risk of angina among Black women diagnosed with lung cancer compared to White women diagnosed with lung cancer [157]. Some studies reporting on racial disparities also considered other types, such as SES [141,146,160], sexual [141,157,160], age [141,155,160], and rural-urban disparities [154]. In contrast to Nielsen et al. [156], Mukherjee et al. [154] found a higher risk of hypertension among patients with head and neck squamous cell carcinoma (HNSCC) living in urban areas compared to those in rural areas [154]. A single study out of 24 (4.1%) by Nielsen et al. (2023) focused solely on rural-urban disparities, and reported mixed findings, including a lower risk of hypertension and diseases of arteries for rural cancer survivors but a higher risk of congestive heart failure compared to urban survivors [156].
In their 2015 study, Al-Kindi et al. investigated disparities related to gender, age, and differences in outcomes by cancer stage at diagnosis [141]. They found an increased risk of CVD mortality among men and older individuals at cancer diagnosis compared to women and younger individuals at diagnosis, respectively [141]. Similarly, Shi et al. [160] identified an higher risk of CVD mortality among men and older individuals at diagnosis compared to women or younger individuals. Du and Song [155] investigated age disparities in the utilisation of antihypertension medications in a large population-based cohort of older women with breast cancer. They reported that the oldest of these women have a higher prevalence of hypertension and received more hypertension treatment compared to younger women [155].
Keegan et al. [146] examined SES disparities and found a higher likelihood of circulatory system diseases diagnosis among adolescent and young adult HL survivors without insurance, those with public insurance, and those residing in low SES neighbourhoods compared to survivors with private insurance, military insurance, and those residing in higher SES neighbourhoods, respectively. In a similar vein, Shi et al. (2021) found an increased risk of CVD mortality among non-elderly cancer survivors without insurance or with public insurance compared to those with private insurance [160]. In contrast, Al-Sadawi et al. [142] reported no difference in the risk for cardiotoxicity between individuals living in low- and high-SES neighbourhoods.
Geographical Settings or Regions of Studies and Datasets Used
In total, 14 out of 24 included studies (58.3%) used national datasets without a specific focus on any particular region of the US [141,143,144,148-151,155,157-160,162,163] and were classified as ‘National.’ Eleven of those 14 studies (78.6%) used the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) cancer registries [141,143,144,148,149,151,155,158,160,162,163]. Three of the 14 (21.4%) used other national datasets [150,157,159]. A study by Williams et al. [150] used the National Health and Nutrition Examination Survey dataset, one of Centers for Disease Control’s National Center for Health Statistics, designed to assess health and nutritional status of adults and children in the US [166]. Johnson et al. [157] utilised data from the 2014, 2016, 2017, and 2018 Behavioural Risk Factor Surveillance System, a Centers for Disease Control-sponsored self-report telephone survey collecting various health-related risk behaviours, chronic health conditions, and preventive services data from noninstitutionalised adult citizens in the US. Noyd et al. [159] used data from the Childhood Cancer Survivor Study, an NCI-funded initiative aimed at better understanding the late effects of childhood cancer.
Ten out of the 24 included studies (41.7%) used data from different US regions. Four of these (40%) were conducted in the Northeast region of the US [142,145,147,152]. Among those four, one study (25%) used the Memorial Sloan Kettering Cancer Centre dataset in New York [142], while three studies (75%) were conducted in Baltimore, Maryland. One of these studies used data from the Sidney Kimmel Comprehensive Cancer Centre at Johns Hopkins [147], the second study used clinical data from the Prevention and Research Centre at Mercy Medical Centre [145], while the third one used data from the Maryland Cancer Registry [152]. Three of the 10 studies (30%) were conducted in the West region [146,153,156]. Of the three studies, two used data from the California Cancer registry [146,153] while one study used data from the Utah Population Database [156]. The remaining three of the 10 studies (30%) were conducted in the Southeast region [154,161,164]. These three studies used a variety of datasets, one with data from the Georgia Cancer registry [167], another one with the OneFlorida Consortium database [164], and the third with data from the University of Alabama at Birmingham Health System’s NCI designated cancer centre [154].
Cardiovascular Diseases and Related Outcomes Investigated
The most prevalent outcome investigated was CVD mortality, which was included in nine of 24 (37.5%) studies [141,143,144,149,152,158,160,162,163]. All these nine studies reported distinct racial and ethnic disparities, with NHB individuals being disproportionately affected by CVD mortality compared to their NHW counterparts [141,143,144,149,152,160]. Additionally, Appiah et al. [162] reported a higher risk of CVD mortality among Hispanic survivors compared to NHW survivors. Furthermore, sexual, age, SES, and rural-urban disparities in CVD mortality were identified. Men, older individuals at cancer diagnosis, those residing in rural areas, and those without insurance or with public insurance were reported to have a higher risk for CVD mortality compared to women, younger individuals, those residing in urban areas, and those with private insurance [141,158,160].
The second most reported outcome was hypertension prevalence, investigated in seven out of 24 studies (29.2%%) [147,150,151,154-156,161], closely related to the receipt of antihypertension treatment, which was the third most investigated outcome in three out of 24 studies (12.5%) [147,151,155]. All these studies reported an increased risk for hypertension prevalence and a higher likelihood of receiving hypertensive medications among NHB survivors compared to their NHW counterparts, except for two studies. The study by Mukherjee et al. [154] found no racial and ethnic differences in hypertension prevalence among patients with head and neck squamous cell carcinoma but reported a higher risk for survivors residing in urban areas. Nielsen et al. [156], who investigated only rural-urban disparities, reported higher hypertension prevalence among prostate cancer survivors residing in urban areas compared to their counterparts residing in rural areas . Additionally, Du and Song [155] reported both higher hypertension prevalence and a higher likelihood of receiving antihypertensive medications among older breast cancer survivors compared to younger survivors.
The fourth and fifth most prevalent outcomes investigated were CVD risk factors, including clinical risk factors (such as hypertension, high cholesterol, smoking, etc., when combined) and CVD diagnosis. These outcomes were investigated in three of 24 studies [145,146,159]. Gallicchio et al. [145] reported CVD risk factors by using the sum of the defined risk factors (obese, current smoker, physical inactivity, hypertension, diabetes, and high cholesterol). Gallicchio et al. [145] also reported the patient’s 10-year atherosclerotic CVD (ASCVD) event risk score which included nonfatal MI, fatal coronary heart disease (CHD), nonfatal stroke, and fatal stroke and obtained from the patient’s questionnaire, medical chart, and blood samples. Gallicchio et al. [145] found higher median ASCVD event risk scores and higher rates of hypertension, diabetes, and obesity among Black than White patients. Keegan et al. [146] reported the likelihood of occurrence of circulatory system diseases among adolescent and young adult Hodgkin Lymphoma survivors (≥2 years after diagnosis). Noyd et al. [159] used incidence rate ratios for two or more CVD risk factors and found higher cumulative incidence in NHB and Hispanic adult survivors of childhood cancer compared to their NHW counterparts [142].
One of the outcomes investigated is related to cancer therapy-related adverse cardiotoxicity, as explored by three studies [142,147,153], which is noteworthy due to the known adverse effects of cancer therapies on the vasculature [5]. Al-Sadawi et al. [142] defined cardiotoxicity as clinical heart failure or asymptomatic left ventricular ejection fraction (LVEF) decline (absolute decrease ≥10% to < 53%, or ≥16%) while Litvak et al. (147) defined cardiotoxicity as a decline in LVEF to <50% and absolute drop in LVEF of ≥10% from baseline after 10-year follow-up from January 2005 to March 2015 or incomplete treatment with human epidermal growth factor receptor 2 (HER2)-targeted therapy (<52 weeks). Datta et al. [153] reported racial disparities in a 12-month cumulative incidence of cancer-associated thrombosis. All three studies found an increased risk of cardiotoxicity in Black women BC survivors compared to White women, highlighting racial and ethnic disparities.
Additional CVD-related outcomes that were less frequently reported included incidence of heart attack, stroke or angina [157], the incidences of heart failure, diseases or arteries, or diseases of veins [156], cardiac screening with echocardiograms [164], CHD-related mortality [148], cerebrovascular disease-related mortality [148] and high-sensitivity C-reactive protein (hsCRP), a CVD pathological marker for systemic inflammation [145]. Polednak et al. [148] reported increased risk for CHD and cerebrovascular diseases at both the 5-year and the 10-year follow-up for Black compared to White individuals. Gallicchio et al. [145] found that hsCRP was significantly higher in Black BC patients compared to their White counterparts. DeRemer et al. [164] reported that NHB patients had a lower likelihood of receiving a baseline echocardiogram than NHW patients or any baseline cardiac surveillance. Furthermore, DeRemer et al. [164] reported that Hispanic patients received significantly less cardiac surveillance at the 6-month and 12-month time points, respectively, compared with NHW patients.
Different Cancer Types Investigated and Stages at Diagnosis
Breast cancer, including breast ductal carcinoma in situ, was the most common type of cancer investigated in 13 out the 24 studies [142,143,145,147-150,152,155,158,161,162]. Two of those 13 studies looked at specific types of BC [142,143]. Al-Sadawi et al. [142] focused on women with HER2 BC because of known risks associated with treatments including cardiotoxicity. Berkman et al. [143] focused on breast ductal carcinoma in situ which represents 15%–20% of new annual BC diagnoses in the US. Three out of the 24 studies looked at survivors of Hodgkin Lymphoma [141,144,146] while five out of the 24 focused on any cancer type [144,153,160,163,164]. One of the five studies focused on childhood or young adulthood cancers including leukemias, germ cell cancer, cancer of the central nervous system, Hodgkin Lymphoma, non-Hodgkin Lymphoma, melanoma, and thyroid cancer [144]. Another one looked at hepatocellular carcinoma, lung cancer, and pancreatic cancer [168]. Three studies looked at one specific type of cancer, one focused on patients with lung cancer [157], another on prostate cancer [156], and a third on colorectal cancer [151]. Lastly, Mukherjee et al. [154] focused on patients with head and neck squamous cell carcinoma.
Statistical Analyses Used in Included Studies
Statistical analysis approaches varied across studies depending on data type, study type and specific research questions. Most studies used Cox proportional hazards models in identification of disparities between groups by measures of hazard ratios [141,144,146-149,152,153,156,158,160]. Some studies used survival analysis with Kaplan-Meier curves and log-rank tests to determine CVD-related death cumulative incidences [141,143,146,147,149] or survival rates at different time points post diagnosis such as years 1, 5, 10, and 20 [143] or differences in time-to-event endpoints between groups [144]. Two studies used multivariable logistic regression modelling to assess associations between predictors and dichotomous outcomes [142,157]. One study used multivariable piecewise exponential regression to estimate incidence rate ratios [159].
Three studies used slightly different approaches than most common analyses in the studies above. Nyrop et al. [161] used Fisher’s exact test to compare frequencies for Black and White patients. Investigating differences between Black and White cancer patients, Gallicchio et al. [145] used chi-square and Fisher’s exact tests for categorical variables, Student’s t-tests for continuous variables with a normal distribution, and Wilcoxon rank sum tests for continuous variables with a skewed distribution. Williams et al. [150] used a modified Poisson regression to estimate the prevalence ratios with their respective 95% confidence interval (CI) in measuring racial disparities in hypertension prevalence.
Discussion
In this review, the objective was to document disparities in CVD-related outcomes among cancer survivors in the US. Using a pre-defined PICOS framework to guide articles' inclusion and exclusion criteria, the PRESS guidelines in database searches, and the PRISMA 2020 checklist as the reporting guideline, disparities related to race and ethnicity, sex, age, rural/urban, and SES were identified. Investigated CVD-related outcomes encompassed cardiac screening, CVD risk factors, CVD diagnosis (including specific measures like heart failure, heart attack, angina, diseases of arteries, diseases of veins, or stroke), CVD-related mortality, cancer treatment-related cardiotoxicity, and one CVD pathological biomarker of systemic inflammation (hsCRP). Prior reviews on CVD disparities do exist; however, there has not been a systematic review dedicated to cancer survivors in the US comprehensively exploring various causes of disparity beyond race and ethnicity. To the best of our knowledge, this systematic review aims to bridge this knowledge gap, representing the first of its kind.
The findings of this review suggest that non-Hispanic Black (NHB) cancer survivors are disproportionately impacted by adverse CVD-related outcomes. This includes a lower likelihood of receiving cardiac screening, a higher prevalence of hypertension, a higher likelihood of receiving antihypertensive medications (another proxy for higher hypertension prevalence), increased CVD risk factors, and an higher risk of CVD-related death compared to NHW individuals (see Figure 2). Additionally, individuals with lower SES among cancer survivors, including those without insurance or with public insurance, are disproportionately affected by adverse cardiovascular outcomes compared to those with military or private insurance [146,160]. Three studies investigating age and sex reported substantial adverse cardiovascular outcomes for individuals with a male sex assigned at birth and older individuals at the time of cancer diagnosis [141,155,157]. These findings suggest an urgent need for relevant stakeholders to design and implement specific, targeted interventions for individuals who are known to be at an increased disadvantage. Mixed findings on rural-urban disparities were reported in the reviewed studies [154,156,158].
While all disparities were considered in this review, the findings suggest that racial and ethnic disparities are most investigated in CVD-related outcomes among cancer survivors in the US. Rural-urban disparities, age, SES, and sexual disparities were explored to a lesser extent in this review. Other disparity types (such as disability, gender identity, etc.) were not identified in this review. The alluvial chart synthesis indicates that racial/ethnic minority cancer survivors are disproportionately affected by adverse CVD outcomes. This might be due to complex structural factors including the historical legacy of slavery and subsequent systemic racism and discrimination in access to opportunities. The US history was characterised by socioeconomic inequalities [169] and injustices that date back to the institution of American slavery in the 1500s. African American people were systematically exploited in chattel slavery and lived through post-slavery racially discriminatory policies and practices that targeted them for marginalisation, and put them at increased risk for disadvantages and a lifetime of social, neighbourhood, economic, and environmental stressors [170]. Health is influenced by numerous factors and is not produced or maintained in isolation. Complex biopsychosocial factors intersect in producing and reproducing health. The legacy of historical racially discriminatory policies or distal factors manifests through differences in access to wealth, leading to various disparities [171], including environmental exposures [172,173], health status, reach to healthcare [174,175], healthcare utilisation and quality of care received [175,176], cancer stage at diagnosis [177], and protective factors such as the quality and quantity of greenspaces [178] and PA [179]. These disparities ultimately increase the risk of CVD risk factors and lead to poor CVD outcomes [180]. The frequent investigation of BC is unsurprising given its higher burden.
The findings of this review complement previous work in CVD disparities among populations that have been historically targeted for marginalisation in the US [181-183], by adding a specific focus to cancer survivors to the published literature. In Communities of Colour, for instance, some of the known factors that exacerbate CVD disparities include a high overall prevalence of CVD risk factors that are unrecognised and not treated due social economic factors such as poverty and/or limited access to insurance [181,184,185], reduced access to healthcare services [186-188], a reduced likelihood of receiving potentially beneficial treatments [183], poor neighbourhood environments, and racial residential segregation [189], among other multiple complex and intersecting risk factors. It is noteworthy that one paper identified in this review, focusing on cardiac screening, reported racial disparities, with NHB individuals less likely to be screened compared to their NHW counterparts [164]. This observation suggests that disparities reported in cardiovascular outcomes might be underreported, and therefore, the actual disparities could be higher or more extensive than documented in this review. Race-based residential segregation and socioeconomic inequalities serve as fundamental (or distal) factors influencing both intermediate factors (social and physical environment risk factors) and proximate factors (stressors, psychosocial factors, social integration and support, and health behaviours), thereby increasing CVD risk factors [189]. For instance, racial residential segregation (or isolation) has been linked to poorer overall and breast cancer-specific survival among older NHB women diagnosed with breast cancer in the US [190]. Given the higher CVD risk among cancer survivors, addressing the fundamental or root causes of disparities becomes imperative to enhance CVD outcomes before and/or during cancer survivorship.
Structural and strategic interventions targeting socioeconomic inequalities, including addressing upstream social and environmental determinants of health at the neighbourhood level, are crucial for mitigating disparities and enhancing outcomes for all. Moreover, clinical interventions in primary care and Cardio-Oncology clinics that improve screening for CVD risk factors among cancer survivors at considerable risk could enhance early diagnosis, facilitate recommendations for specialised care plans, mitigate adverse cardiotoxic outcomes, and reduce disparities. Increased collaboration among primary care, oncology, cardiology, and Cardio-Oncology fields could enhance the management of CVD risks and mitigate cancer treatments related to cardiotoxicity among high-risk cancer survivors. Equity-focused frameworks should be considered in designing and deploying these innovative interventions to ensure that racial and ethnic minorities, including Black people, and individuals in low SES living conditions receive special attention, considering their higher risk compared to other groups.
In addition to clinical interventions, public health interventions addressing systemic disinvestments can help reduce equity gaps. Neighbourhood-level interventions aimed at improving the social and built environment, such as the equitable distribution of greenspaces and enhancing housing (cost, conditions, consistency, and context), are promising avenues to mitigate neighbourhood-level risk factors contributing to health disparities [191,192]. Greenspace plays a role in reducing existing income-related health inequalities by modifying pathways through which low socioeconomic position can lead to disease [193]. For instance, higher quality residential environment promotes park use, which positively contributes to residents’ weekly amounts of overall PA and recreational walking [194]. Individuals in low-income neighbourhoods, unlike those in high-income areas, use parks or greenspaces less frequently due to poor perceived accessibility and safety [195]. Reduced access to greenspace in low-income neighbourhoods predisposes residents to lower PA levels [196], increasing their risk of chronic diseases, including CVD and cancer [197]. Furthermore, improvements in four dimensions of housing—stability, quality and safety, affordability and accessibility, and neighbourhood environment—can enhance overall health and improve CVD outcomes [198]. Strategic investments in greenspace and housing could be key interventions to reduce cardiovascular disparities, particularly among cancer survivors in the US [32,33].
Implications for Future Research
The results from this review suggest overwhelming evidence of racial and ethnic disparities in cardiovascular health outcomes among cancer survivors. While systemic disinvestment, limited reach to salutogenic resources, and chronic exposure to pathogenic neighbourhood environments are known pathways to poor health outcomes for communities of colour, the role of historical and/or contemporary systemic racism in poor CVD outcomes could benefit from further investigation. In future research, incorporating measures of historical and/or contemporary racism and discrimination is essential to understand their direct effects on Cardio-Oncology disparities. This approach is particularly important as it enables stakeholders to take tangible actions to deal with broken systems by targeting distal actors or root causes of disparities. Additionally, understanding the role of salutogenic factors, such as neighbourhood greenspaces and quality, equitable housing, is critical. These factors play a crucial role in improving neighbourhood social and built environments, potentially contributing to the reduction of Cardio-Oncology disparities [1]. Moreover, understanding disparities related to different gender identities, which may differ from biologically assigned sex at birth, in CVD outcomes among cancer survivors is crucial. Identifying everyone at an increased risk for poorer outcomes is essential for designing public health and clinical interventions informed by critical gaps. Additionally, in this systematic review, a single study investigated a CVD pathological inflammatory biomarker, the high-sensitivity C-reactive protein, and found that NHB individuals are disproportionately affected [145]. Disparities in other CVD pathological biomarkers of inflammation remain under-investigated and poorly understood among high-risk cancer survivors; and yet, inflammation is a critical pathological risk factor for atherosclerosis [199,200]. More inflammatory biomarkers, particularly pro-inflammatory cytokines, such as interleukins including IL-6, IL-1α, and IL-1β, and tumour necrosis factor-alpha should be investigated in future disparity research due to their known implication in atherosclerosis [199] and their existing known associations with reduced overall cancer survival [201]. Investigating these biomarkers will contribute to understanding their role in disparities, informing future Cardio-Oncology interventions, and ensuring that the most susceptible individuals are targeted.
Limitations
Despite adhering to the rigour of PRISMA requirements, this study has a few limitations. There is a possibility that the review may have unintentionally omitted some relevant publications. Efforts were made to minimise such omissions by applying the PRESS guideline and examining the references of included articles for additional studies that might meet the inclusion criteria. All disparities identified in this review are limited to those reported and published in academic, peer-reviewed journals. Consequently, any unpublished or non-peer-reviewed work is not included in this report, and we may have underreported the extent of existing disparities. Despite these limitations, the review establishes a vital foundation for interventions emphasising CVD risk screening, monitoring, prevention, and management tailored for specific susceptible groups including cancer survivors. The goal is to reduce adverse outcomes in Cardio-Oncology disparities and enhance health equity. Equity and justice-informed research and interventions are essential in dismantling the root causes of disparities, and structural barriers to equitable and just health systems.
Conclusion
Racial and ethnic minority cancer survivors face an higher risk of adverse CVD outcomes in the US compared to NHWs. Disparities pertaining to other SES characteristics (income, gender, education, insurance, age, etc.) or rural-urban distinctions have received less attention. Notably, no study exploring tribal-related disparities was identified in this review. Implementing a systems-thinking approach is crucial for policymakers and stakeholders to deal with the root causes of disparities and various forms of injustice. This includes removing systemic barriers that hinder individuals' access to essential resources necessary for maintaining good health [202]. Clinical practitioners should enhance screening for risk factors contributing to adverse CVD outcomes, particularly among individuals historically targeted for marginalisation, such as racial and ethnic minorities, and other disadvantaged groups. As future studies identify additional susceptible groups, like tribal communities or those with a specific gender identity that might differ from their biologically assigned sex at birth, similar attention to screening practices is warranted. Researchers, especially those in the cardio-oncology field, should broaden their focus and explore innovative approaches to reduce health disparities and advance health equity. These approaches necessitate targeted and sustainable actions to establish an equitable social system that is fair to everyone, without any discriminatory bias, to advance intergenerational health equity and justice.
Supplementary Material
Item assessment description/guidelines:
Representativeness of exposed group/Case definition/Sample: one star was given if the exposed cohort is representative of the community average and well described or if the cases are well defined or if the sample is representative of the average target population (random sampling). No star was given if no case is defined or described or if the cohort is not described or no description of sampling strategy.
Non-exposed group/Cases/sample size: One star was given if non-exposed cohort was drawn from the same community as the exposed cohort or if cases are similar or if the sample is well justified. No star was given if there is potential for selection bias or if not stated or if exposed cohort is drawn from a different source than exposed cohort or if no description is given or if the sample size is not justified.
Ascertainment of exposure/control selection: one star was given if community controls were used or if study used secure records (e.g., database records) or used a structured interview. No star was given if self-reports were used or not described or hospital controls or no description of control sources.
Demonstration of outcome/control definition: One star was given if there is a valid measure for outcome or if there is a demonstration that outcome of interest was not present at start of study or if controls developed no disease at the end of the study. No star was given if no adequate description is given for controls at the end of the study.
Compatibility and controlling factors between groups: One star was given if the study controls for the most important covariates. A second star was given if the study controls for additional factors. If no covariate is controlled for, no star was given.
Outcome assessment: One star was given if the study clearly defines outcomes and how they were assessed. No star was given if the outcome is not described.
Exposure duration: One star was given if raters perceive that exposure duration was long enough to observe differences in outcomes. No star was given if raters perceived duration as inadequate.
Cohorts follow-up/statistical test: One star was given if all subjects were followed up until completion or if the raters perceive the number of subjects lost to follow as small enough to not introduce any bias or if the statistical test used to analyse the data is clearly described and appropriate. No star was given if raters perceived that too many subjects were lost to follow-up or if the statistical test is not described.
Funding Sources
The work is supported by an American Heart Association Scientific focused research network on disparities in Cardio-oncology grants (K.M.M.B: grant ID # 863108; and A.M.B.: grant ID #:863107), the American Heart Association Research supplement to promote diversity in Science (J.C.B.; grant ID # 960133), NIH (National Institutes of Health) grants: R01HL133029 (A.M.B.), R01CA214805 (K.M.M.B), the Medical College of Wisconsin Cancer Center grants (KM.M.B), and by the Medical College of Wisconsin Cardiovascular center grant 'We Care Fund" (A.M.B.; grant ID # 3308140).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
There are no conflicts of interest to disclose.
Appendices. Supplementary Data
The full database search strategy is included in Appendix A, alluvial charts data files are in Appendix B, and the PRISMA 2020 reporting checklist is provided in Appendix C.
References
- [1].Bikomeye JC, Beyer AM, Kwarteng JL, Beyer KMM. Greenspace, inflammation, cardiovascular health, and cancer: a review and conceptual framework for greenspace in cardio-oncology research. Int J Environ Res Public Health 2022;19:2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation 2016;133:1104–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dent SF, Kikuchi R, Kondapalli L, Ismail-Khan R, Brezden-Masley C, Barac A, et al. Optimizing cardiovascular health in patients with cancer: a practical review of risk assessment, monitoring, and prevention of cancer treatment-related cardiovascular toxicity. Am Soc Clin Oncol Educ B 2020;40:501–15. [DOI] [PubMed] [Google Scholar]
- [4].Thotamgari SR, Sheth AR, Grewal US. Racial disparities in cardiovascular disease among patients with cancer in the United States: the elephant in the room. EClinicalmedicine 2022;44:101297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bikomeye JC, Terwoord ID, Santos JH, Beyer AM. Emerging mitochondrial signaling mechanisms in cardio-oncology: beyond oxidative stress. Am J Physiol Heart Circ Physiol 2022;323:H702–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bertero E, Ameri P, Maack C. Bidirectional relationship between cancer and heart failure: old and new issues in cardio-oncology. Card Fail Rev 2019;5:106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Weaver KE, Foraker RE, Alfano CM, Rowland JH, Arora NK, Bellizzi KM, et al. Cardiovascular risk factors among long-term survivors of breast, prostate, colorectal, and gynecologic cancers: a gap in survivorship care? J Cancer Surviv 2013;7:253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Koelwyn GJ, Newman AAC, Afonso MS, van Solingen C, Corr EM, Brown EJ, et al. Myocardial infarction accelerates breast cancer via innate immune reprogramming. Nat Med 2020;26:1452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Aleman BMP, Moser EC, Nuver J, Suter TM, Maraldo MV, Specht L, et al. Cardiovascular disease after cancer therapy. EJC Suppl 2014;12:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Barac A, Murtagh G, Carver JR, Chen MH, Freeman AM, Herrmann J, et al. Cardiovascular health of patients with cancer and cancer survivors: A roadmap to the next level. J Am Coll Cardiol 2015;65:2739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bradshaw PT, Stevens J, Khankari N, Teitelbaum SL, Neugut AI, Gammon MD. Cardiovascular disease mortality among breast cancer survivors. Epidemiology 2016;27:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gernaat SAM, Ho PJ, Rijnberg N, Emaus MJ, Baak LM, Hartman M, et al. Risk of death from cardiovascular disease following breast cancer: a systematic review. Breast Cancer Res Treat 2017;164:537–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res 2011;13:R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Office of Disease Prevention and Health Promotion. Disparities ∣ Healthy people; 2020. Available at: https://www.healthypeople.gov/2020/about/foundation-health-measures/Disparities. [accessed January 18, 2021].
- [15].Chan WC, Wright C, Riddell T, Wells S, Kerr AJ, Gala G, et al. Ethnic and socioeconomic disparities in the prevalence of cardiovascular disease in New Zealand. New Zeal. N Z Med J 2008;121:11–20. [PubMed] [Google Scholar]
- [16].Adler NE, Stewart J. Health disparities across the lifespan: meaning, methods, and mechanisms. Ann N Y Acad Sci 2010;1186:5–23. [DOI] [PubMed] [Google Scholar]
- [17].United States Department of Human Services Healthy people; 2010. Available at: https://www.cdc.gov/nchs/data/hpdata2010/hp2010_final_review.pdf. [accessed March 20, 2021].
- [18].United States Department of Health and Human Services. Office of Disease Prevention and Health Promotion healthy people, vol. 2030. Framework. Available at: https://health.gov/healthypeople/about/healthy-people-2030-framework. [accessed March 20, 2021]. [Google Scholar]
- [19].Hartley D. Rural health disparities, population health, and rural culture. Am J Public Health 2004;94:1675–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sparks PJ. Rural health disparities. In: Kulcsár LJ, Curtis KJ, eds. International Handbook of Rural Demography: Berlin: Springer; 2012. p. 255–71. [Google Scholar]
- [21].Florence J, Pack RP, Southerland JL, Wykoff RF. The depth of rural health disparities in America: ABCDE’s. Rural Popul Heal Determ Disparities Solut 2012:51–72. [Google Scholar]
- [22].Mayer KH, Bradford JB, Makadon HJ, Stall R, Goldhammer H, Landers S. Sexual and gender minority health: what we know and what needs to be done. Am J Public Health 2008;98:989–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Williams DR, Sternthal M. Understanding racial-ethnic disparities in health: sociological contributions. J Health Soc Behav 2010;51(Suppl):S15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nelson A. Unequal treatment: confronting racial and ethnic disparities in health care. J Natl Med Assoc 2002;94:666–8. [PMC free article] [PubMed] [Google Scholar]
- [25].Fiscella K, Franks P, Gold MR, Clancy CM. Inequality in quality: addressing socioeconomic, racial, and ethnic disparities in health care. JAMA 2000;283:2579–84. [DOI] [PubMed] [Google Scholar]
- [26].Ren XS, Amick BC, Williams DR. Racial/ethnic disparities in health: the interplay between discrimination and socioeconomic status. Ethn Dis 1999;9:151–65. [PubMed] [Google Scholar]
- [27].Braveman P, Gottlieb L. The social determinants of health: it’s time to consider the causes of the causes. Public Health Rep 2014;129(Suppl 2):19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].WHO Commission on Social Determinants of Health Closing the gap in a generation: health equity through action on the social determinants of health. Available at: https://www.who.int/publications/i/item/9789241563703 [accessed October 20, 2022]. [DOI] [PubMed]
- [29].Warnecke RB, Oh A, Breen N, Gehlert S, Paskett E, Tucker KL, et al. Approaching health disparities from a population perspective: the National Institutes of Health centers for population health and health disparities. Am J Public Health 2008;98:1608–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jones NL, Gilman SE, Cheng TL, Drury SS, Hill CV, Geronimus AT. Life course approaches to the causes of health disparities. Am J Public Health 2019;109:S48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].National Indian Council on Aging (NICOA). American Indian health disparities. Available at: https://www.nicoa.org/elder-resources/health-disparities/. [accessed April 18, 2022].
- [32].Bikomeye JC, Namin S, Anyanwu C, Rublee CS, Ferschinger J, Leinbach K, et al. Resilience and equity in a time of crises: investing in public urban greenspace is now more essential than ever in the US and beyond. Int J Environ Res Public Health 2021;18:8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bikomeye JC, Balza JS, Kwarteng JL, Beyer AM, Beyer KMM. The impact of greenspace or nature-based interventions on cardiovascular health or cancer-related outcomes: A systematic review of experimental studies. PLoS One 2022;17:e0276517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zambrana RE, Williams DR. The intellectual roots of current knowledge on racism and health: relevance to policy and the national equity discourse. Health Aff (Millwood) 2022;41:163–70. [DOI] [PubMed] [Google Scholar]
- [35].Hahn RA, Truman BI, Williams DR. Civil rights as determinants of public health and racial and ethnic health equity: health care, education, employment, and housing in the United States. SSM Popul Health 2018;4:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Engel GL. The need for a new medical model: a challenge for biomedicine. Science 1977;196:129–36. [DOI] [PubMed] [Google Scholar]
- [37].Galea S, Tracy M, Hoggatt KJ, DiMaggio C, Karpati A. Estimated deaths attributable to social factors in the United States. Am J Public Health 2011;101:1456–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].The Center for Disease Control and Prevention health disparities among youth ∣ Adolescent and school health: Centers for Disease Control and Prevention (U.S.). Available at: https://www.cdc.gov/healthyyouth/disparities/index.htm [accessed January 18, 2021].
- [39].Carlson ED, Chamberlain RM. Allostatic load and health disparities: a theoretical orientation. Res Nurs Health 2005;28:306–15. [DOI] [PubMed] [Google Scholar]
- [40].McEwen BS, Seeman T. Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Ann N Y Acad Sci 1999;896:30–47. [DOI] [PubMed] [Google Scholar]
- [41].Guidi J, Lucente M, Sonino N, Fava GA. Allostatic load and its impact on health: a systematic review. Psychother Psychosom 2021;90:11–27. [DOI] [PubMed] [Google Scholar]
- [42].AI Ribeiro, Amaro J, Lisi C, Fraga S. Neighborhood socioeconomic deprivation and allostatic load: a scoping review. Int J Environ Res Public Health 2018;15:1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tan M, Mamun A, Kitzman H, Mandapati SR, Dodgen L. Neighborhood disadvantage and allostatic load in African American women at risk for obesity-related diseases. Prev Chronic Dis 2017;14:E119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Schopfer DW. Rural health disparities in chronic heart disease. Prev Med (Baltimore) 2021;152:106782. [DOI] [PubMed] [Google Scholar]
- [45].Harrington RA, Califf RM, Balamurugan A, Brown N, Benjamin RM, Braund WE, et al. Call to action: rural health: A presidential advisory from the American Heart Association and American Stroke Association. Circulation 2020;141:e615–44. [DOI] [PubMed] [Google Scholar]
- [46].Weaver KE, Geiger AM, Lu L, Case LD. Rural-urban disparities in health status among US cancer survivors. Cancer 2013;119:1050–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bonvicini KA. LGBT healthcare disparities: what progress have we made? Patient Educ Couns. patient ed. 2017;100:2357–61. [DOI] [PubMed] [Google Scholar]
- [48].Reisner SL, Greytak EA, Parsons JT, Ybarra ML. Gender minority social stress in adolescence: disparities in adolescent bullying and substance use by gender identity. J Sex Res 2015;52:243–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Burgess D, Tran A, Lee R, van Ryn M. Effects of perceived discrimination on mental health and mental health services utilization among gay, lesbian, bisexual and transgender persons. J LGBT Health Res 2007;3:1–14. [DOI] [PubMed] [Google Scholar]
- [50].Whitehead J, Shaver J, Stephenson R. Outness, stigma, and primary health care utilization among rural LGBT populations. PLoS One 2016;ll:e0146139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Cheung CK, Lee H, Levin NJ, Choi E, Ross VA, Geng Y, et al. Disparities in cancer care among sexual and gender minority adolescent and young adult patients: A scoping review. Cancer Med 2023;12:14674–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Caceres BA, Brody A, Luscombe RE, Primiano JE, Marusca P, Sitts EM, et al. A systematic review of cardiovascular disease in sexual minorities. Am J Public Health 2017;107:e13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Meyer IH. Prejudice, social stress, and mental health in lesbian, gay, and bisexual populations: conceptual issues and research evidence. Psychol Bull 2003;129:674–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Wisdom JP, McGee MG, Horner-Johnson W, Michael YL, Adams E, Berlin M. Health disparities between women with and without disabilities: a review of the research. Soc Work Public Health 2010;25:368–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Krahn GL, Walker DK, Correa-De-Araujo R. Persons with disabilities as an unrecognized health disparity population. Am J Public Health 2015;105(Suppl 2):S198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Disability and health in the United States; 2001–2005. Available at: https://stacks.cdc.gov/view/cdc/6983 [accessed May 9, 2022].
- [57].Shandra CL. Disability as inequality: social disparities, health disparities, and participation in daily activities. Soc Forces 2018;97:157–92. [Google Scholar]
- [58].Anderson LL, MapelLentz S, Larson SA. Physical disability and health disparities. In Hollar D, ed. Advances in Exercise and Health for People With Mobility Limitations. Cham: Springer International Publishing; 2019. p. 41–59. [Google Scholar]
- [59].Hughes RB, Robinson-Whelen S, Knudson C. Cancer disparities experienced by people with disabilities. Int J Environ Res Public Health 2022;19:9187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Cavanagh BM, Wakefield CE, McLoone JK, Garvey G, Cohn RJ. Cancer survivorship services for indigenous peoples: where we stand, where to improve? A systematic review. J Cancer Surviv 2016;10:330–41. [DOI] [PubMed] [Google Scholar]
- [61].Guadagnolo BA, Petereit DG, Coleman CN. Cancer care access and outcomes for American Indian populations in the United States: challenges and models for progress. Semin Radiat Oncol 2017;27:143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Weinstein JN, Geller A, Negussie Y, Baciu A. Native American health: historical and legal context. In: National Academies of Sciences, Engineering, and Medicine. Communities in Action: Pathways to Health Equity: Washington: National Academies Press; 2017. p. 1–558. [PubMed] [Google Scholar]
- [63].Sequist TD, Cullen T, Bernard K, Shaykevich S, Orav EJ, Ayanian JZ. Trends in quality of care and barriers to improvement in the Indian Health Service. J Gen Intern Med 2011;26:480–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Warne D, Frizzell LB. American Indian health policy: historical trends and contemporary issues. Am J Public Health 2014;104(Suppl 3):S263–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].U.S. National Park Service slaves of Andrew Johnson - Andrew Johnson national historic site. Available at: https://www.nps.gov/anjo/learn/historyculture/slaves.htm. [accessed February 14, 2021].
- [66].Hummel J. Emancipating slaves, enslaving free men: a history of the American Civil War. Open Court 2013; ISBN 0812698444. [Google Scholar]
- [67].Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, et al. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation 2017;136:e393–423. [DOI] [PubMed] [Google Scholar]
- [68].Bikomeye JC, Rublee CS, Beyer KMM. Positive externalities of climate change mitigation and adaptation for human health: a review and conceptual framework for public health research. Int J Environ Res Public Health 2021;18:2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Rublee C, Bikomeye J, Rao S, Husain M, Beyer K. Climate mitigation and adaptation is cancer prevention and control. J Clim Chang Heal 2023;10:100209. [Google Scholar]
- [70].Lee EK, Donley G, Ciesielski TH, Gill I, Yamoah O, Roche A, et al. Health outcomes in redlined versus non-redlined neighborhoods: A systematic review and meta-analysis. Soc Sci Med 2022;294:114696. [DOI] [PubMed] [Google Scholar]
- [71].Mujahid MS, Gao X, Tabb LP, Morris C, Lewis TT. Historical redlining and cardiovascular health: the Multi-Ethnic Study of Atherosclerosis. Proc Natl Acad Sci U S A 2021;118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Bikomeye JC, Zhou YM, McGinley EL, Canales B, Yen TWF, Beyer KMM. Historical redlining and breast cancer survival in the United States: evidence from the 2010–2017 SEER Medicare linked dataset. J Clin Oncol 2022;40:1095. [Google Scholar]
- [73].Bikomeye JC, Zhou Y, McGinley EL, Canales B, Yen TWF, Tarima S, et al. Historical redlining and breast cancer treatment and survival among older women in the United States. J Natl Cancer Inst 2023;115:652–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Fitzpatrick K, Greenhalgh-Stanley N, Ver Ploeg M. The impact of food deserts on food insufficiency and SNAP participation among the elderly. Am J Agric Econ 2016;98:19–40. [Google Scholar]
- [75].Camp NL. Food insecurity and food deserts. Nurse Pract 2015;40:32–6. [DOI] [PubMed] [Google Scholar]
- [76].Ver Ploeg M. Access to affordable, nutritious food is limited in “food deserts”. Available at: https://www.ers.usda.gov/amber-waves/2010/march/access-to-affordable-nutritious-food-is-limited-in-food-deserts/, [accessed March 20, 2021]. [Google Scholar]
- [77].Morton LW, Bitto EA, Oakland MJ, Sand M. Solving the problems of Iowa food deserts: food insecurity and civic structure. Rural Sociol 2005;70:94–112. [Google Scholar]
- [78].Walker RE, Keane CR, Burke JG. Disparities and access to healthy food in the United States: a review of food deserts literature. Health Place 2010;16:876–84. [DOI] [PubMed] [Google Scholar]
- [79].Committee on World Food Security (CFS) Global Strategic Framework for Food Security & Nutrition (GSF). Available at: http://www.fao.org/fileadmin/templates/cfs/Docs1314/GSF/GSF_Version_3_EN.pdf. [accessed April 16, 2021].
- [80].Stuff JE, Casey PH, Szeto KL, Gossett JM, Robbins JM, Simpson PM, et al. Household food insecurity is associated with adult health status. J Nutr 2004;134:2330–5. [DOI] [PubMed] [Google Scholar]
- [81].Vozoris NT, Tarasuk VS. Household food insufficiency is associated with poorer health. J Nutr 2003;133:120–6. [DOI] [PubMed] [Google Scholar]
- [82].Mendy VL, Vargas R, Cannon-Smith G, Payton M, Enkhmaa B, Zhang L. Food insecurity and cardiovascular disease risk factors among Mississippi adults. Int J Environ Res Public Health 2018;15:2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Vercammen KA, Moran AJ, McClain AC, Thorndike AN, Fulay AP, Rimm EB. Food security and 10-year cardiovascular disease risk among U.S. adults. Am J Prev Med 2019;56:689–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Mahmoodi MR, Najafipour H, Mohsenpour MA, Amiri M. The relationship between food insecurity with cardiovascular risk markers and metabolic syndrome components in patients with diabetes: a population-based study from Kerman coronary artery disease risk study. J Res Med Sci 2017;22:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Liu Y, Eicher-Miller HA. Food insecurity and cardiovascular disease risk. Curr Atheroscler Rep 2021;23:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Dharod JM, Croom JE, Sady CG. Food insecurity: its relationship to dietary intake and body weight among Somali refugee women in the United States. J Nutr Educ Behav 2013;45:47–53. [DOI] [PubMed] [Google Scholar]
- [87].Morales ME, Berkowitz SA. The relationship between food insecurity, dietary patterns, and obesity. Curr Nutr Rep 2016;5:54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Mello JA, Gans KM, Risica PM, Kirtania U, Strolla LO, Fournier L. How is food insecurity associated with dietary behaviors? An analysis with low-income, ethnically diverse participants in a nutrition intervention study. J Am Diet Assoc 2010;110:1906–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Crawford PB, Webb KL. Unraveling the paradox of concurrent food insecurity and obesity. Am J Prev Med 2011;40:274–5. [DOI] [PubMed] [Google Scholar]
- [90].Franklin B, Jones A, Love D, Puckett S, Macklin J, White-Means S. Exploring mediators of food insecurity and obesity: a review of recent literature. J Community Health 2012;37:253–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Metallinos-Katsaras E, Must A, Gorman K. A longitudinal study of food insecurity on obesity in preschool children. J Acad Nutr Diet 2012;112:1949–58. [DOI] [PubMed] [Google Scholar]
- [92].Adams EJ, Grummer-Strawn L, Chavez G. Food insecurity is associated with increased risk of obesity in California women. J Nutr 2003;133:1070–4. [DOI] [PubMed] [Google Scholar]
- [93].Dinour LM, Bergen D, Yeh MC. The food insecurity–obesity paradox: a review of the literature and the role food stamps may play. J Am Diet Assoc 2007;107:1952–61. [DOI] [PubMed] [Google Scholar]
- [94].Kaur J, Lamb MM, Ogden CL. The association between food insecurity and obesity in children-the National Health and Nutrition Examination Survey. J Acad Nutr Diet 2015;115:751–8. [DOI] [PubMed] [Google Scholar]
- [95].Kaiser LL, Townsend MS, Melgar-Quiñonez HR, Fujii ML, Crawford PB. Choice of instrument influences relations between food insecurity and obesity in Latino women. Am J Clin Nutr 2004;80:1372–8. [DOI] [PubMed] [Google Scholar]
- [96].Olson CM, Strawderman MS. The relationship between food insecurity and obesity in rural childbearing women. J Rural Health 2008;24:60–6. [DOI] [PubMed] [Google Scholar]
- [97].Pan L, Sherry B, Njai R, Blanck HM. Food insecurity is associated with obesity among US adults in 12 states. J Acad Nutr Diet 2012;112:1403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Martin MA, Lippert AM. Feeding her children, but risking her health: the intersection of gender, household food insecurity and obesity. Soc Sci Med 2012;74:1754–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Walker RJ, Williams JS, Egede LE. Pathways between food insecurity and glycaemic control in individuals with type 2 diabetes. Public Health Nutr 2018;21:3237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Gucciardi E, Vahabi M, Norris N, Del Monte JP, Farnum C. The intersection between food insecurity and diabetes: a review. Curr Nutr Rep 2014;3:324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Gucciardi E, Vogt JA, DeMelo M, Stewart DE. Exploration of the relationship between household food insecurity and diabetes in Canada. Diabetes Care 2009;32:2218–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Seligman HK, Bindman AB, Vittinghoff E, Kanaya AM, Kushel MB. Food insecurity is associated with diabetes mellitus: results from the National Health Examination and Nutrition Examination Survey (Nhanes) 1999–2002. J Gen Intern Med 2007;22:1018–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Vijayaraghavan M, Jacobs EA, Seligman H, Fernandez A. The association between housing instability, food insecurity, and diabetes self-efficacy in low-income adults. J Health Care Poor Underserved 2011;22:1279–91 [DOI] [PubMed] [Google Scholar]
- [104].Moreno G, Morales LS, Isiordia M, de Jaimes FN, Tseng CH, Noguera C, et al. Latinos with diabetes and food insecurity in an agricultural community. Med Care 2015;53:423–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Heerman WJ, Wallston KA, Osborn CY, Bian A, Schlundt DG, Barto SD, et al. Food insecurity is associated with diabetes self-care behaviours and glycaemic control. Diabet Med 2016;33:844–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Ippolito MM, Lyles CR, Prendergast K, Marshall MB, Waxman E, Seligman HK. Food insecurity and diabetes self-management among food pantry clients. Public Health Nutr 2017;20:183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Morales ME, Epstein MH, Marable DE, Oo SA, Berkowitz SA. Food insecurity and cardiovascular health in pregnant women: results from the Food for Families Program, Chelsea, Massachusetts, 2013–2015. Prev Chronic Dis 2016;13:E152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Saiz AM, Aul AM, Malecki KM, Bersch AJ, Bergmans RS, LeCaire TJ, et al. Food insecurity and cardiovascular health: findings from a statewide population health survey in Wisconsin. Prev Med 2016;93:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Sun Y, Liu B, Rong S, Du Y, Xu G, Snetselaar LG, et al. Food insecurity is associated with cardiovascular and all-cause mortality among adults in the United States. J Am Heart Assoc 2020;9:e014629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Hamedi-Shahraki S, Mir F, Amirkhizi F. Food insecurity and cardiovascular risk factors among Iranian women. Ecol Food Nutr 2021;60:163–81. [DOI] [PubMed] [Google Scholar]
- [111].Castillo DC, Ramsey NLM, Yu SS, Ricks M, Courville AB, Sumner AE. Inconsistent access to food and cardiometabolic disease: the effect of food insecurity. Curr Cardiovasc Risk Rep 2012;6:245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Hager ER, Cockerham A, O’Reilly N, Harrington D, Harding J, Hurley KM, et al. Food swamps and food deserts in Baltimore City, MD, USA: associations with dietary behaviours among urban adolescent girls. Public Health Nutr 2017;20:2598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Jin H, Lu Y. Evaluating consumer nutrition environment in food deserts and food swamps. Int J Environ Res Public Health 2021;18:2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Fielding JE, Simon PA. Food deserts or food swamps?: comment on “fast food restaurants and food stores”. Arch Intern Med 2011;171:1171–2. [DOI] [PubMed] [Google Scholar]
- [115].Azemati B, Kelishadi R, Ahadi Z, Shafiee G, Taheri M, Ziaodini H, et al. Association between junk food consumption and cardiometabolic risk factors in a national sample of Iranian children and adolescents population: the Caspian-V study. Eat Weight Disord 2020;25:329–35. [DOI] [PubMed] [Google Scholar]
- [116].Bains A, Rashid MA. Junk food and heart disease: the missing tooth. J R Soc Med 2013;106:472–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Hughey SM, Walsemann KM, Child S, Powers A, Reed JA, Kaczynski AT. Using an environmental justice approach to examine the relationships between park availability and quality indicators, neighborhood disadvantage, and racial/ethnic composition. Landsc Urban Plan 2016;148:159–69. [Google Scholar]
- [118].Malambo P, Kengne AP, De Villiers A, Lambert EV, Puoane T. Built environment, selected risk factors and major cardiovascular disease outcomes: a systematic review. PLoS One 2016;11:e0166846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Beilin L, Huang RC. Childhood obesity, hypertension, the metabolic syndrome and adult cardiovascular disease. Clin Exp Pharmacol Physiol 2008;35:409–11. [DOI] [PubMed] [Google Scholar]
- [120].van Emmerik NMA, Renders CM, van de Veer M, van Buuren S, van der Baan-Slootweg OH, Kistvan Holthe JE, et al. High cardiovascular risk in severely obese young children and adolescents. Arch Dis Child 2012;97:818–21 LP – 821. [DOI] [PubMed] [Google Scholar]
- [121].Coughlin SS, Datta B, Guha A, Wang X, Weintraub NL. Cardiovascular conditions and obesity among gynecologic cancer survivors: results from the 2020 behavioral risk factor surveillance system survey. Gynecol Oncol 2022;165:405–9. [DOI] [PubMed] [Google Scholar]
- [122].Woods LM, Rachet B, Coleman MP. Origins of socio-economic inequalities in cancer survival: a review. Ann Oncol 2006;17:5–19. [DOI] [PubMed] [Google Scholar]
- [123].Balarajan R. Ethnic differences in mortality from ischaemic heart disease and cerebrovascular disease in England and Wales. BMJ 1991;302:560–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Cruickshank JK, Mbanya JC, Wilks R, Balkau B, Forrester T, Anderson SG, et al. Hypertension in four African-origin populations: current ‘Rule of Halves’, quality of blood pressure control and attributable risk of cardiovascular disease. J Hypertens 2001;19:41–6. [DOI] [PubMed] [Google Scholar]
- [125].Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol 2016;75:40–6. [DOI] [PubMed] [Google Scholar]
- [128].Bikomeye JC, Awoyinka I, Kwarteng JL, Beyer AM, Beyer KM. Disparities in cardiovascular disease outcomes among cancer survivors in the United States: a systematic review. Circulation 2023;147(Suppl 1):AP576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan---a web and mobile app for systematic reviews. Syst Rev 2016;5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Berkman LF, Melchior M, Chastang JF, Niedhammer I, Leclerc A, Goldberg M. Social integration and mortality: a prospective study of French employees of Electricity of France-Gas of France: the GAZEL Cohort. Am J Epidemiol 2004;159:167–74. [DOI] [PubMed] [Google Scholar]
- [131].Balza JS, Bikomeye JC, Beyer KMM, Rublee C, Flynn KE. Elevated blood lead levels of refugee children in the United States: a systematic review of recent literature (2011–2021). Rev Environ Health 2023;38:361–83. [DOI] [PubMed] [Google Scholar]
- [132].Bikomeye JC, Balza J, Beyer KM. The impact of schoolyard greening on children’s physical activity and socioemotional health: a systematic review of experimental studies. Int J Environ Res Public Health 2021;18:535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Morgan RL, Whaley P, Thayer KA, Schünemann HJ. Identifying the PECO: A framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ Int 2018;121:1027–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Nang C, Piano B, Lewis A, Lycett K, Woodhouse M. Using the PICOS model to design and conduct a systematic search: a speech pathology case study. Available at: https://ro.ecu.edu.au/cgi/viewcontent.cgi?referer=&httpsredir=1&article=1010&context=ecupres. [accessed December 3, 2020]. [Google Scholar]
- [135].National Cancer Institute Definition of survivorship - NCI Dictionary of Cancer Terms. Available at: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/survivorship. [accessed January 14, 2021].
- [136].Kokkinos PF, Faselis C, Myers J, Narayan P, Sui X, Zhang J, et al. Cardiorespiratory fitness and incidence of major adverse cardiovascular events in US veterans: a cohort study. Mayo Clin Proc 2017;92:39–48. [DOI] [PubMed] [Google Scholar]
- [137].Blood Pressure Lowering Treatment Trialists' Collaboration, Turnbull F, Neal B, Ninomiya T, Algert C, Arima H, et al. Effects of different regimens to lower blood pressure on major cardiovascular events in older and younger adults: meta-analysis of randomised trials. BMJ 2008;336:1121–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med 2006;355:2631–9. [DOI] [PubMed] [Google Scholar]
- [139].Francis DK, Bennett NR, Ferguson TS, Hennis AJ, Wilks RJ, Harris EN, et al. Disparities in cardiovascular disease among Caribbean populations: a systematic literature review. BMC Public Health 2015;15:828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Nkimbeng M, Cudjoe J, Turkson-Ocran RA, Commodore-Mensah Y, Thorpe RJ, Szanton SL. Disparities in the prevalence and correlates of disability in older immigrants in the USA: a systematic review of the literature. J Racial Ethn Health Disparities 2019;6:552–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Al-Kindi SG, Abu-Zeinah GF, Kim CH, Hejjaji V, William BM, Caimi PF, et al. Trends and disparities in cardiovascular mortality among survivors of Hodgkin lymphoma. Clin Lymphoma Myeloma Leuk 2015;15:748–52. [DOI] [PubMed] [Google Scholar]
- [142].Al-Sadawi M, Hussain Y, Copeland-Halperin RS, Tobin JN, Moskowitz CS, Dang CT, et al. Racial and socioeconomic disparities in cardiotoxicity among women with HER2-positive breast cancer. Am J Cardiol 2021;147:116–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Berkman A, F Cole B, Ades PA, Dickey S, Higgins ST, Trentham-Dietz A, et al. Racial differences in breast cancer, cardiovascular disease, and all-cause mortality among women with ductal carcinoma in situ of the breast. Breast Cancer Res Treat 2014;148:407–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Berkman AM, Brewster AM, Jones LW, Yu J, Lee JJ, Peng SA, et al. Racial differences in 20-year cardiovascular mortality risk among childhood and young adult cancer survivors. J Adolesc Young Adult Oncol 2017;6:414–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Gallicchio L, Calhoun C, Riseberg D, Helzlsouer K. Cardiovascular health among black and White breast cancer patients initiating aromatase inhibitor therapy. Breast J 2017;23:206–9. [DOI] [PubMed] [Google Scholar]
- [146].Keegan THM, Li Q, Steele A, Alvarez EM, Brunson A, Flowers CR, et al. Sociodemographic disparities in the occurrence of medical conditions among adolescent and young adult Hodgkin lymphoma survivors. Cancer Causes Control 2018;29:551–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Litvak A, Batukbhai B, Russell SD, Tsai HL, Rosner GL, Jeter SC, et al. Racial disparities in the rate of cardiotoxicity of HER2-targeted therapies among women with early breast cancer. Cancer 2018;124:1904–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Polednak AP. Racial differences in mortality from obesity-related chronic diseases in US women diagnosed with breast cancer. Ethn Dis 2004;14:463–8. [PubMed] [Google Scholar]
- [149].Troeschel AN, Liu Y, Collin LJ, Bradshaw PT, Ward KC, Gogineni K, et al. Race differences in cardiovascular disease and breast cancer mortality among US women diagnosed with invasive breast cancer. Int J Epidemiol 2019;48:1897–905. [DOI] [PubMed] [Google Scholar]
- [150].Williams MS, Beech BM, Griffith DM Jr. Thorpe, R.J. the association between hypertension and race/ethnicity among breast cancer survivors. J Racial Ethn Heal Disparities 2020;7:1172–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Du XL, Song L. Racial disparities in treatments and mortality among a large population-based cohort of older men and women with colorectal cancer. Cancer Treat Res Commun 2022;32:100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Connor AE, Kaur M, Sheng JY, Hayes JH. Racial disparities in mortality outcomes among women diagnosed with breast cancer in Maryland: impact of cardiovascular disease and clinical characteristics. Cancer 2022;128:727–36. [DOI] [PubMed] [Google Scholar]
- [153].Datta T, Brunson A, Mahajan A, Keegan T, Wun T. Racial disparities in cancer-associated thrombosis. Blood Adv 2022;6:3167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Mukherjee A, Wiener HW, Griffin RL, Lenneman C, Chatterjee A, Nabell LM, et al. Racial and rural-urban disparities in cardiovascular risk factors among patients with head and neck cancer in a clinical cohort. Head Neck 2022;44:1563–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Du XL, Song L. Age and racial disparities in the utilization of anticancer, antihypertension, and anti-diabetes therapies, and in mortality in a large population-based cohort of older women with breast cancer. J Racial Ethn Health Disparities 2023;10:446–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Nielsen S, O’Neil B, Chang CP, Mark B, Snyder J, Deshmukh V, et al. Determining the association of rurality and cardiovascular disease among prostate cancer survivors. Urol Oncol 2023;41:429.e15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Johnson LA, Briley P, Bolin LP, Kitko L, Ellis C. Disparities in comorbidities in lung cancer: findings from the behavioral risk factor surveillance system. Cancer Nurs 2022;45:E883–9. [DOI] [PubMed] [Google Scholar]
- [158].Appiah D, Farias RM, Olokede OA, Nwabuo CC, Bhende KM, Ebong IA, et al. The influence of individual and neighborhood-level characteristics on rural-urban disparities in cardiovascular disease mortality among U.S. women diagnosed with breast and gynecologic cancers. Gynecol Oncol 2021;161:483–90. [DOI] [PubMed] [Google Scholar]
- [159].Noyd DH, Liu Q, Yasui Y, Chow EJ, Bhatia S, Nathan PC, et al. Cardiovascular Risk Factor Disparities in Adult Survivors of Childhood Cancer Compared With the General Population. JACC CardioOncology 2023;5:489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Shi T, Jiang C, Zhu C, Wu F, Fotjhadi I, Zarich S. Insurance disparity in cardiovascular mortality among non-elderly cancer survivors. Cardiooncology 2021;7:11. doi: 10.1186/s40959-021-00098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Nyrop KA, Damone EM, Deal AM, Carey LA, Lorentsen M, Shachar SS, et al. Obesity, comorbidities, and treatment selection in Black and White women with early breast cancer. Cancer 2021;127:922–30. doi: 10.1002/cncr.33288. [DOI] [PubMed] [Google Scholar]
- [162].Appiah D, Mai M, Parmar K. A Prospective Population-Based Study of cardiovascular Disease Mortality following Treatment for Breast Cancer among Men in the United States, 2000–2019. Curr Oncol 2022;30:284–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Zhu C, Shi T, Jiang C, Liu B, Baldassarre LA, Zarich S. Racial and ethnic disparities in all-cause and cardiovascular mortality among cancer patients in the U.S. JACC CardioOncol 2023;5:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].DeRemer DL, Nguyen NK, Guha A, Ahmad FS, Cooper-DeHoff RM, Pepine CJ, et al. Racial and ethnic differences in cardiac surveillance evaluation of patients treated with anthracycline-based chemotherapy. J Am Heart Assoc 2023;12:e027981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Beltrán Ponce SE, Abunike SA, Bikomeye JC, Sieracki R, Niyonzima N, Mulamira P, et al. Access to radiation therapy and related clinical outcomes in patients with cervical and breast cancer across sub-Saharan Africa: a systematic review. JCO Glob Oncol 2023;9:e2200218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].The Center for Disease Control and Prevention. National Center for Health Statistics NHANES - about the national health and nutrition examination survey. Available at: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm. [accessed July 14, 2021].
- [167].Collin LJ, Troeschel AN, Liu Y, Gogineni K, Borger K, Ward KC, et al. A balancing act: racial disparities in cardiovascular disease mortality among women diagnosed with breast cancer. Ann Cancer Epidemiol 2020;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Gad MM, Saad AM, Al-Husseini MJ, Abushouk AI, Salahia S, Rehman KA, et al. Temporal trends, ethnic determinants, and short-term and long-term risk of cardiac death in cancer patients: a cohort study. Cardiovasc Pathol 2019;43:107147. [DOI] [PubMed] [Google Scholar]
- [169].Ski CF, King-Shier KM, Thompson DR. Gender, socioeconomic and ethnic/racial disparities in cardiovascular disease: A time for change. Int J Cardiol 2014;170:255–7. [DOI] [PubMed] [Google Scholar]
- [170].Council NR Population, C. on Understanding Racial and Ethnic Differences in Health in Late Life: A Research Agenda: Washington: National Academies Press; 2004. [PubMed] [Google Scholar]
- [171].Crossney KB, Bartelt DW. The legacy of the home owners’ loan corporation. Hous Policy Debate 2005;16:547–74. [Google Scholar]
- [172].Namin S, Xu W, Zhou Y, Beyer K. The legacy of the Home Owners’ Loan Corporation and the political ecology of urban trees and air pollution in the United States. Soc Sci Med 2020;246:112758. [DOI] [PubMed] [Google Scholar]
- [173].Grove M, Ogden L, Pickett S, Boone C, Buckley G, Locke DH, et al. The legacy effect: understanding how segregation and environmental injustice unfold over time in Baltimore. Ann Am Assoc Geogr 2018;108:524–37. [Google Scholar]
- [174].Yearby R. Racial disparities in health status and access to healthcare: the continuation of inequality in the United States due to structural racism. American J Econ Sociol 2018;77:1113–52. [Google Scholar]
- [175].Copeland VC. African Americans: disparities in health care access and utilization. Health Soc Work 2005;30:265–70. [DOI] [PubMed] [Google Scholar]
- [176].Mateo CM, Williams DR. Racism: a fundamental driver of racial disparities in health-care quality. Nat Rev Dis Primers 2021;7:20. [DOI] [PubMed] [Google Scholar]
- [177].Williams F, Thompson E. Disparities in breast cancer stage at diagnosis: importance of race, poverty, and age. J Health Dispar Res Pract 2017;10:34–45. [PMC free article] [PubMed] [Google Scholar]
- [178].Nardone A, Rudolph KE, Morello-Frosch R, Casey JA. Redlines and greenspace: the relationship between historical redlining and 2010 greenspace across the United States. Environ Health Perspect 2021;129:17006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Gordon-Larsen P, Nelson MC, Page P, Popkin BM. Inequality in the built environment underlies key health disparities in physical activity and obesity. Pediatrics 2006;117:417–24. [DOI] [PubMed] [Google Scholar]
- [180].Brondolo E, Love EE, Pencille M, Schoenthaler A, Ogedegbe G. Racism and hypertension: a review of the empirical evidence and implications for clinical practice. Am J Hypertens 2011;24:518–29. [DOI] [PubMed] [Google Scholar]
- [181].Kurian AK, Cardarelli KM. Racial and ethnic differences in cardiovascular disease risk factors: a systematic review. Ethn Dis 2007;17:143–52. [PubMed] [Google Scholar]
- [182].Ferdinand KC, Yadav K, Nasser SA, Clayton-Jeter HD, Lewin J, Cryer DR, et al. Disparities in hypertension and cardiovascular disease in blacks: the critical role of medication adherence. J Clin Hypertens (Greenwich) 2017;19:1015–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Graham G. Disparities in cardiovascular disease risk in the United States. Curr Cardiol Rev 2015;11:238–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Bonow RO, Grant AO, Jacobs AK. The cardiovascular state of the union: confronting healthcare disparities. Circulation 2005;111:1205–7. [DOI] [PubMed] [Google Scholar]
- [185].Graham G. Population-based approaches to understanding disparities in cardiovascular disease risk in the United States. Int J Gen Med 2014;7:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Brown ER, Ojeda VD W R, Levan R. Racial and ethnic disparities in access to health insurance and health care. Available at: https://www.kff.org/wp-content/uploads/2013/01/racial-and-ethnic-disparities-in-access-to-health-insurance-and-health-care-report.pdf. [accessed December 5, 2020]. [Google Scholar]
- [187].Price JH, Khubchandani J, McKinney M, Braun R. Racial/ethnic disparities in chronic diseases of youths and access to health care in the United States. BioMed Res Int 2013;2013:787616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Weinick RM, Zuvekas SH, Cohen JW. Racial and ethnic differences in access to and use of health care services, 1977 to 1996. Med Care Res Rev 2000;57(Suppl 1):36–54. [DOI] [PubMed] [Google Scholar]
- [189].Schulz AJ, Kannan S, Dvonch JT, Israel BA, Allen III A, James SA, et al. Social and physical environments and disparities in risk for cardiovascular disease: the healthy environments partnership conceptual model. Environ Health Perspect 2005;113:1817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Canales B, Laud PW, Tarima S, Zhou Y, Bikomeye JC, McGinley EL, et al. Isolation and survival: the impact of local and MSA isolation on survival among non-Hispanic Black women diagnosed with breast cancer in the United States using a SEER-Medicare cohort. Health Place 2023;83:103090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Rigolon A, Browning MHEM, McAnirlin O, Yoon HV. Green space and health equity: A systematic review on the potential of green space to reduce health disparities. Int J Environ Res Public Health 2021;18:2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Swope CB, Hernández D. Housing as a determinant of health equity: a conceptual model. Soc Sci Med 2019;243:112571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Mitchell R, Popham F. Effect of exposure to natural environment on health inequalities: an observational population study. Lancet 2008;372:1655–60. [DOI] [PubMed] [Google Scholar]
- [194].Leslie E, Cerin E, Kremer P. Perceived neighborhood environment and park use as mediators of the effect of area socio-economic status on walking behaviors. J Phys Act Health 2010;7:802–10. [DOI] [PubMed] [Google Scholar]
- [195].Jones A, Hillsdon M, Coombes E. Greenspace access, use, and physical activity: understanding the effects of area deprivation. Prev Med 2009;49:500–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Kavanagh AM, Goller JL, King T, Jolley D, Crawford D, Turrell G. Urban area disadvantage and physical activity: a multilevel study in Melbourne, Australia. J Epidemiol Community Health 2005;59:934–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Kristensen PL, Wedderkopp N, Møller NC, Andersen LB, Bai CN, Froberg K. Tracking and prevalence of cardiovascular disease risk factors across socio-economic classes: a longitudinal substudy of the European Youth Heart Study. BMC Public Health 2006;6:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Sims M, Kershaw KN, Breathett K, Jackson EA, Lewis LM, Mujahid MS, et al. Importance of housing and cardiovascular health and well-being: a scientific statement from the American Heart Association. Circ Cardiovasc Qual Outcomes 2020;13:E000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Goyal A, Belur AD, Dey AK, Mehta NN Chapter 7. Blood inflammatory biomarkers of cardiovascular disease. In: Elsevier, edr. Biomarkers in Cardiovascular Disease; 2019. p. 71–9. [Google Scholar]
- [200].Held C, White HD, Stewart RAH, Budaj A, Cannon CP, Hochman JS, et al. Inflammatory biomarkers interleukin-6 and C-reactive protein and outcomes in stable coronary heart disease: experiences from the STABILITY (stabilization of atherosclerotic plaque by initiation of darapladib therapy) trial. J Am Heart Assoc 2017;6:e005077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Pierce BL, Ballard-Barbash R, Bernstein L, Baumgartner RN, Neuhouser ML, Wener MH, et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol 2009;27:3437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].House J, Williams DR, Smedley BD, Syme SL. Understanding and reducing socioeconomic and racial/ethnic disparities in health. In: Promoting Health: Intervention Strategies from Social and Behavioral Research: Washington, DC: National Academy Press; 2000. p. 81–124. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.