Abstract
Introduction/Objective:
The KidneyIntelX is a multiplex, bioprognostic, immunoassay consisting of 3 plasma biomarkers and clinical variables that uses machine learning to predict a patient’s risk for a progressive decline in kidney function over 5 years. We report the 1-year pre- and post-test clinical impact on care management, eGFR slope, and A1C along with engagement of population health clinical pharmacists and patient coordinators to promote a program of sustainable kidney, metabolic, and cardiac health.
Methods:
The KidneyIntelX in vitro prognostic test was previously validated for patients with type 2 diabetes and diabetic kidney disease (DKD) to predict kidney function decline within 5 years was introduced into the RWE study (NCT04802395) across the Health System as part of a population health chronic disease management program from [November 2020 to April 2023]. Pre- and post-test patients with a minimum of 12 months of follow-up post KidneyIntelX were assessed across all aspects of the program.
Results:
A total of 5348 patients with DKD had a KidneyIntelX assay. The median age was 68 years old, 52% were female, 27% self-identified as Black, and 89% had hypertension. The median baseline eGFR was 62 ml/min/1.73 m2, urine albumin-creatinine ratio was 54 mg/g, and A1C was 7.3%. The KidneyIntelX risk level was low in 49%, intermediate in 40%, and high in 11% of cases. New prescriptions for SGLT2i, GLP-1 RA, or referral to a specialist were noted in 19%, 33%, and 43% among low-, intermediate-, and high-risk patients, respectively. The median A1C decreased from 8.2% pre-test to 7.5% post-test in the high-risk group (P < .001). UACR levels in the intermediate-risk patients with albuminuria were reduced by 20%, and in a subgroup treated with new scripts for SGLT2i, UACR levels were lowered by approximately 50%. The median eGFR slope improved from −7.08 ml/min/1.73 m2/year to −4.27 ml/min/1.73 m2/year in high-risk patients (P = .0003), −2.65 to −1.04 in intermediate risk, and −3.26 ml/min/1.73 m2/year to +0.45 ml/min/1.73 m2/year in patients with low-risk (P < .001).
Conclusions:
Deployment and risk stratification by KidneyIntelX was associated with an escalation in action taken to optimize cardio-kidney-metabolic health including medications and specialist referrals. Glycemic control and kidney function trajectories improved post-KidneyIntelX testing, with the greatest improvements observed in those scored as high-risk.
Keywords: diabetic kidney disease, early-stage, KidneyIntelX, Real World Evidence, precision medicine, treatment management
Introduction
Chronic kidney disease (CKD) is a major cause of morbidity and mortality in the United States and affects approximately 38 million adults, 1 with type 2 diabetes (T2D) being the leading precursor for disease onset. 2 Early-stage diabetic kidney disease (DKD) is defined by the presence of elevated urinary albumin creatinine ratio (UACR, ≥30 mg/g) or estimated glomerular filtration rate (eGFR, 30-59 ml/min/1.73 m2) in a person with T2D. 1 The clinical course of DKD, depending on the phenotype, is heterogeneous and includes fluctuating levels of albuminuria and eGFR, with varied temporal relationships between the 2.2,3 Some individuals experience a decline in kidney function without albuminuria, which is still associated with significantly higher hospitalization rates, especially when compared with an eGFR of >90 ml/min/1.73 m2. 4 Without therapeutic intervention, DKD can progress to kidney failure, cardiovascular events, and even death.4 -7 Unfortunately, despite available kidney protective medications, only a small proportion of eligible patients are currently on these therapies.6,8 -10 The reasons for this are multifactorial, but a lack of early and accurate risk stratification to identify those most at risk for DKD progression is a key problem.8,11 Therapeutic inertia is common in kidney disease, due to inadequate ability to determine who will derive the most (absolute) benefit from new treatments for a silent condition that takes years to realize the gains of therapy on a patient-level basis.
KidneyIntelX is a plasma multiplex immunoassay that combines measured values of 3 proteins (ie, soluble tumor necrosis factor receptors 1 and 2 [sTNFR1, sTNFR2], and kidney injury molecule [KIM-1] with clinical variables to produce a level of risk predicting progressive decline in kidney function over 5-years. The test was introduced by the Population Health team and Ambulatory leadership at the MS Health System (MSHS) as part of an institutional effort to improve outcomes of patients with T2D and existing early-stage CKD. We have previously reported on the 6-month interim results of a real-world evidence (RWE) study using the KidneyIntelX test, which demonstrated changes in clinical management of patients with T2D and early-stage (Grade (G)1-G3) DKD. 11 Here, we sought to ascertain the 1-year data from the RWE KidneyIntelX program at MSHS. Our focus was on sustainability of the KidneyIntelX testing platform as a program of personalized risk assessment with an emphasis on treatment with cardio-renal protective agents, utilization of consult services, advanced practice provider driven protocols post-testing, and impact on A1C, UACR, and the eGFR slope as drivers of patient-provider compliance and improved outcomes.
Methods
The Population Health team and Ambulatory leadership at MSHS introduced the KidneyIntelX test, as part of an RWE study (NCT04802395) throughout primary care/internal medicine and selected endocrinology, nephrology, and cardiology clinics. 11 Specifically, the KidneyIntelX test combines the blood-based biomarkers sTNFR1, sTNFR2, and Kim-1 with standard clinical features including eGFR, UACR, serum Calcium, A1C, systolic blood pressure, platelet count, and AST (aspartate aminotransferase) to predict a progressive decline in kidney function within 5 years. Of note, the KidneyIntelX test was clinically validated 12 and an interim risk level correlation on clinical decisions and measured outcomes from an interim 6-month assessment has been published. 11 The inclusion criteria was that all patients have T2D and early stage (concurrent) DKD (with documented eGFR of 30 to 59 ml/min/1.73 m2 [G3a, G3b] or eGFR ≥ 60 ml/min/1.73 m2 with albuminuria, [UACR] ≥30 mg/g [A2, A3]) between the ages of 23 to 79. We excluded patients with eGFR < 30 or ≥60 ml/min/1.73 m2 without albuminuria, patients with end-stage kidney disease or on renal recovery treatments at time of enrollment, pregnant, currently hospitalized, on Embrel or with active malignancies. Patients with a scheduled appointment at a participating practice were identified through an automated process and providers were alerted of eligibility. Both the eGFR and UACR values used to identify intended use patients was based on a minimum of 2 measurements taken at least 90 days apart. All enrolled patients had a KidneyIntelX test between November 2020 and April 2023 (Supplemental Figure S1). Participating providers received a 1-time, in-service training by the study principal investigator team or population health staff member. The training included an explanation of the KidneyIntelX test and generated risk level, integration into routine clinical workflow, and recommended clinical management based on MSHS ambulatory care standard for CDK and KDIGO/American Diabetes Association (ADA) guidelines.2,13,14 The study was approved by the MS Institutional Review Board with individual patient consent waived due to minimal risk, patient volume, and number of participating providers.
This is a longitudinal collection of participant clinical data, provider clinical decisions, and actions/outcomes captured via electronic medical record (EMR) data stored in EPIC on all enrolled RWE patients. Enrolled patients were included in this analysis if there was minimum of 12-month post-test data available. Demographics, encounters, consultation referrals, medication history, changes in A1C, UACR, eGFR slope, and laboratory values were extracted and deidentified on all participants with a KidneyIntelX test result at pre-test baseline, 0 to 3 months, and designated minimum 12-month post-test period for all subsequent analyses. Ambulatory care decisions were assessed for patients at each risk level by frequency of encounters with providers, and referrals to specialty services that included condition management programs, a registered dietician, nephrology, and endocrinology. Medical management was captured through EMR abstraction at specified time points and informal surveys. Only patients with complete clinical data files were included in the outcome studies.
Dissemination of KidneyIntelX test results varied based on level of risk, which were available after 5 days of sample collection. Patients with a low-risk result received an automated message/letter explaining the results, as did their provider. All patients with intermediate- or high-risk results received a phone call from the care navigation team (CNT) member explaining the result. The CNT also contacted the ordering provider regarding their patient’s results and recommended care pathway actions. These recommendations included opportunities for renal protective medication management and the patient’s eligibility for referrals, which includes remote chronic condition management programs, a registered dietician, clinical pharmacist, or sub-specialist. Upon referral to chronic condition management programs, clinical pharmacists become a central role in patient-provider education and co-management of patients with uncontrolled hypertension or diabetes. Co-management focused on promoting cardiac-kidney-metabolic health and included initiation and optimization of anti-hypertensive agents, the addition of sodium glucose transporter 2 inhibitors (SGLT2i), and or glucagon-like peptide-1 receptor agonists (GLP-1RA) therapy with appropriate monitoring. Patients were also enrolled into Remote Patient Monitoring (RPM) programs for blood pressure control and provided OMRON blood pressure systems for at home use, monitoring of patient compliance, and adherence to treatment plans. There was a specific focus on socioeconomic barriers to care to promote patient engagement with established programs within the health system leveraged to improve medication access.
The primary objective was the demonstration of a sustained impact of the KidneyIntelX risk assessment platform on clinical management and outcomes for patients with T2D and early-stage CKD. Targeted measures included a 20% proportional change in clinic visit frequency, use of consult services or novel medications (eg, SGLT2i), and clinically significant improvement in A1C, UACR, and eGFR slope. Every provider received a KidneyIntelX test report that contained low-, intermediate-, and high-risk levels and a guideline-based clinical care pathway developed and approved by MSHS Population Health. Each report contained the recommended care pathway based on the respective risk level that included visit frequency (1-3x/year), specialist referral, and dose/class of medication (example report provided in Supplemental Figure S2). Baseline pre- and 12-month post-test assessment of physician type/encounter/timing, new referrals to specialty consult), and new prescriptions (SGLT2i, GLP-1RA) were recorded. New prescription activity was documented based on an electronic chart evaluation of KidneyIntelX patients using their 12-month pre-test existing SGLT2i prescriptions versus 12-month post-test documentation of new prescriptions in collaboration with population health pharmacy support. The focus on SGLT2i and GLP-1 RA reflects the recommendations from both the American Diabetes Association (ADA) and Kidney Disease Improving Global Outcomes (KDIGO) guidelines highlighting their important role in cardiac and kidney health. The intent was to assess impact of KidneyIntelX risk on provider use of these agents for the most appropriate patients. Patient compliance regarding medication use with evidence of glucose and blood pressure control were supervised through RPM as part of MSHS population health chronic disease management and clinical pharmacy program.
Statistical Analyses
This is a 12-month update of a decision impact study to evaluate the results of the KidneyIntelX test on clinical management and outcomes. The primary measures were the percent change (target was minimum 20%) using descriptive statistics. This includes odds ratio (OR) with 95% confidence intervals (CI) and P-values generated via a paired t-test between pre-and post-test values, with a focus on high- versus low-risk patients. The pre- and post-test eGFR slopes were calculated using 2 eGFR values prior to a baseline test, 1 eGFR at the time of testing, and then 2 additional eGFR values post testing. There had to be a minimum of 365 days between all measurements, which required a 2-year pre- and post-test assessment window. If patients did not match these criteria, they were not included in the slope analysis. Adherence to a 12 month pre (baseline) post test time window was utilized for all clinical measured outcomes including A1C and uACR.
Results
Of the total 5348 patients enrolled with DKD, 2569 patients had been followed for a minimum 12-month post-KidneyIntelX test and were included in the current clinical management and outcome assessment study. All patients had a confirmed diagnosis of early-stage DKD (eGFR 1-3; A2, A3, and HbA1C ≥6.5%). The patients were seen at 25 primary care and specialty practice locations by 80 providers across the MSHS. Complete demographic information of the cohort is provided in Table 1. Briefly, the median baseline age was 68 years old, median BMI of 30 kg/m2, 52% female, 27% Black, 88% with hypertension, and 9% with stage A to D American Heart Association functional classified heart failure. The baseline median systolic blood pressure (SBP) was 132 mmHg, with eGFR of 61.9 ml/min/1.73 m2, UACR of 54 mg/g, and 7.3% A1C. Only patients with complete clinical outcome measures were included in subsequent analyses with <5% of the entire cohort missing any clinical data.
Table 1.
All Patient RWE Cohort Demographics Including Individual Risk Levels.
| All patients | Low-risk | Intermediate-risk | High-risk | |
|---|---|---|---|---|
| N = 2569 | N = 1261 | N = 1017 | N = 291 | |
| Age, years median (IQR) | 68 (60, 74) | 69 (62, 74) | 67 (59, 74) | 66 (57, 71) |
| % Female | 52 | 53 | 53 | 49 |
| Race, n (%) | ||||
| Black | 706 (27) | 343 (27) | 276 (27) | 87 (30) |
| Non-Black (White/other) | 1863 (73) | 918 (53) | 741 (53) | 204 (49) |
| BMI, kg/m2 (median) | 30 | 30 | 31 | 30 |
| Comorbidities | ||||
| Hypertension, n (%) | 2279 (89) | 1109 (88) | 907 (89) | 263 (90) |
| CAD, n (%) | 540 (21) | 248 (20) | 225 (22) | 67 (23) |
| Heart failure, n (%) | 234 (9) | 86 (7) | 114 (11) | 34 (12) |
Abbreviations: BMI, body mass index; CAD, coronary artery disease; IQR, interquartile range.
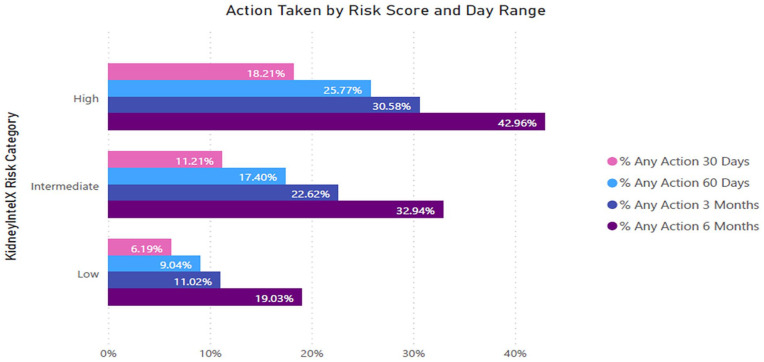
At 12 months, the RWE KidneyintelX risk stratification was comparable with the original clinical validation study for intermediate- (40% vs 37%) and low-risk (49% vs 46%), with percentage of high-risk patients slightly higher in the original validation study than at the 1-year mark (17% vs 11%, respectively). 12 Self-identified race was also similar across risk stratifications. During the minimum 12-month period, 85% (2178/2569) of enrolled patients were seen by their PCP within the first 3-months post-test. As such, post-test actions were taken in 19% of low-risk, 33% of intermediate-risk, and 43% of high-risk cases (OR = 3.20, 95% CI: 2.41-4.23 for high- vs low-risk; Figure 1).
Figure 1.
Action taken post-KidneyIntelX result stratified by level of risk. The amount of time until a medical action was initiated (ie, referral to specialty clinics, new prescriptions) post-KidneyIntelX is presented according to level of patient risk. Time to action was divided into the following periods: 30-day (pink), 60-day (teal), 3-month (dark blue), and 6-month (purple) post-test result.
Investigation into new referrals was observed to be 6% in low-risk, 10% in intermediate-risk, and 20% in high-risk groups (OR = 3.82, 95% CI: 2.59-6.61 for high- vs low-risk, Figure 2a). Interestingly, the increase in high-risk patients for a new referral was almost 4x that of low-risk patients. Patients were referred mostly to endocrinology, followed by nephrology and a small percentage to a nutrition service (Figure 2b).
Figure 2.
Occurrence of specialty referrals post-KidneyIntelX result according to risk. (a) referral to a specialist based on the KidneyIntelX risk category. Time to referral was divided into the following periods: 30-day (pink), 60-day (teal), 3-month (dark blue), and 6-month (purple) post-test result. (b) Sankey Flow diagram demonstrating proportion of referrals based on provider.
Next, we evaluated prescription usage at 12 months. Among anti-hypertensive medications, there was an 8% increase in new angiotensin-converting enzyme inhibitors (ACEI) prescriptions in high-risk patients. A similar 8% dose adjustment of angiotensin receptor blockers (ARBs) in KidneyIntelX high- and intermediate-risk groups was also observed. Even more pronounced was the impact on new prescriptions for SGLT2i at 1-year. Nineteen percent of high-risk cases had a new prescription for SGLT2i vs only 4% of low-risk cases (OR = 4.98, 95% CI: 3.27-7.60 for high- vs low-risk, Figure 3a). This is nearly a 5x rate increase in initiation among high-risk patients compared to low-risk patients. Primary care represented 22% of the ordering physicians for SGLT2i in the high-risk group, followed by nephrologists (17%) and endocrinologists (11%, Figure 3b). As a cohort, usage of SGLT2i among patients increased from 30% at pre-test to 40% post-test, with a relative 61% increase in patients that scored as high-risk (Figure 4). By comparison, there was a modest, but still risk-appropriate usage of GLP-1RAs with a higher pre-test use than SGLT2i (OR = 2.14, 95% CI: 1.19-3.76 for high- vs low-risk, Figure 4). A detailed comparison of absolute versus relative % change between risk groups for each treatment is provided in Supplemental Table S1.
Figure 3.
New SGLT2i prescriptions post-KidneyIntelX result stratified by risk and referring physician. (a) Post-test result with all patients followed for 12 months in the high- (19%) versus low-risk (4%) KidneyIntelX groups. Time to new SGLT2i Rx was divided into the following periods: 30-day (pink), 60-day (teal), 3-month (dark blue), and 6-month (purple) post-test result. (b) The physician type most likely to order SGLT2i. Referral physicians were categorized as follows: Endocrinology (dark blue), Nephrologist (orange), and Primary Care Physician (light blue).
Abbreviations: Rx, prescription; SGLT2i, sodium glucose transporter 2 inhibitors.
Figure 4.

Percent change in prescription usage pre- and post-test, stratified by level of risk. The increase in usage post-KidneyIntelX test result is primarily observed in the high- and intermediate-risk groups (red and orange, respectively).
Abbreviations: GLP-1RA, glucagon-like peptide 1 receptor agonist; SGLT2i, sodium glucose transporter 2 inhibitor.
The 12-month results corroborate the original interim 6-month observations that KidneyIntelX testing and the combined increased use of the SGLT2i and GLP-1RA lowered A1C levels in the high-risk group (8.2% vs 7.5%, P < .001) but not significant in the intermediate risk (7.4% vs 7.3%) and low-risk groups (7.1% vs 7.0%), both P = .9. Significant reductions in the median UACR levels were also recorded, especially for patients of intermediate-risk which increased by 20% (Table 2). As such, a subgroup analysis of intermediate risk patients that received new prescriptions for SGLT2i revealed this group of patients also had a 50% reduction in UACR levels. The improvement in eGFR slope was observed across all risk categories (P < .001 for all), with the change between pre- and post-testing for eGFR slopes recorded as +3.9,+1.6, and +3.8 ml/min/1.73 m2/year for low-, intermediate-, and high-risk, respectively (Figure 5).
Table 2.
Pre- and Post-Test Clinical Variables.
| All patients | Low-risk | Intermediate-risk | High-risk | |
|---|---|---|---|---|
| N = 2569 | N = 1261 | N = 1017 | N = 291 | |
| Pre-test (median) | ||||
| SBP, mmHg | 132 | 130 | 135 | 138 |
| DBP, mmHg | 77 | 77 | 78 | 78 |
| eGFR, ml/min/1.73 m2 | 61 | 61 | 66 | 48 |
| UACR, mg/g | 54 | 31 | 97 | 635 |
| A1C, % | 7.3 | 7.1 | 7.4 | 8.2 |
| BMI, kg/m2 | 30.2 | 30.1 | 30.4 | 30.0 |
| Post-test (median) | ||||
| SBP, mmHg | 131 | 130 | 133 | 137 |
| DBP, mmHg | 76 | 76 | 76 | 76 |
| eGFR, ml/min/1.73 m2 | 61 | 61 | 65 | 43 |
| UACR, mg/g | 48 | 29 | 76 | 572 |
| A1C, % | 7.2 | 7.0 | 7.3 | 7.5 |
| BMI, kg/m2 | 29.6 | 29.5 | 29.7 | 29.5 |
Abbreviations: A1c, hemoglobin A1C; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure; UACR, urine albumin-to-creatinine ratio.
Figure 5.

Change in eGFR slope over time. The eGFR slope improved between pre- and post-test result across all risk categories (P < .001 for all). Data presented as change in eGFR slope. Low-risk (green), intermediate-risk (orange), and high-risk (red).
Abbreviation: eGFR, estimated glomerular filtration rate.
The MSHS Population Health DKD Care Navigation Team (CNT) had completed a brief post-test informal survey on a subgroup of the entire enrolled population. Of the 1379 patients enrolled from 1 January 2022 – 30 September 2023, 165 were high-risk, 960 intermediate-risk, and 254 were low-risk. With an emphasis on intermediate- and high-risk patients, all were successfully contacted by nurse practitioners from CNT and completed a brief post-test informal survey. Fifty-two percent of patients were knowledgeable of the KidneyIntelX test and 75% were aware that diabetes was a major risk factor. Importantly, 98% were appreciative of the call and 97% were motivated to act. Overall, 30% of patients were interested in receiving dietary education. This was slightly higher among high-risk patients with 44% (n = 73) expressing interest in nutritional intervention and 59% (n = 43) of these patients actually met with a dietician. Thus, this data supports that that disease awareness, risk understanding, and education has a positive impact on patient motivation to change behavior, which will likely influence compliance.
During the same study period, the clinical pharmacist group engaged with 489 KidneyIntelX high- and intermediate-risk patients. In this capacity they supported the post-test management of SGLT2i, GLP-1RA, and ACE/ARBs either as new scripts or optimization of the pre-test dose. The most significant impact was on the use of SGLT2i, with a pre-post-test increase of 31% to 53% for high-risk patients and 29% to 54% in patients with intermediate-risk. As part of triaging patients for active monitoring, 312/489 patients were referred to the hypertension (HTN) MSHS RPM program, which includes daily at-home blood pressure monitoring and a monthly clinical pharmacist telehealth visit with a target of <140/90 mmHg as per HEDIS HTN quality measure. 15 Briefly, blood pressure was obtained monthly and analyzed every 90 days with the last 14 days of each 90-day period used to calculate % of patients meeting their target blood pressure of <140/90 mmHg. Of the 312 patients, there are currently 200 patients enrolled and managed in this activity with a mean decrease in SBP of 11.8 mmHg and diastolic blood pressure by 6.5 mmHg over 12 months. The percentage of high- and intermediate-risk patients successfully controlling their blood pressure increased from 33% at baseline to 61% by 12 months.
Discussion
Clinical Relevance of Key Findings
This study suggests that 12 months after the initial KidneyIntelX risk assessment, the combined change in management of care and the positive adjustment in select clinical variables in patients with early-stage DKD is a sustainable approach for improving long-term outcomes. The evolution of this “predictive-personalized-preventive and participatory” program emanated from a proposal of systems medicine originally identified by Hood 16 and is aligned with the newly directed Cardiac-Kidney-Metabolic Health initiative put forward by the American Heart Association. 17 The intention was to utilize the KidneyIntelX risk level report as an educational implementation platform. As such, this would advance both the American Diabetes Association and KDIGO guidelines to facilitate the deployment of a precision medicine patient-centric interdisciplinary plan for chronic disease management. The ability to guide clinical decisions based on an individual patient’s level of risk for kidney function decline is a significant step toward the actualization of biomarker-based precision medicine in chronic disease. 18 This is particularly important given DKD is present in 1/3 of adults with T2D, yet most people are unaware they have the condition. 19 This lack of awareness is due to the asymptomatic presentation of DKD coupled with the intra-individual variability of eGFR and UACR lab values used to diagnose and risk assess DKD. 20 Moreover, UACR is often not performed at the frequency recommended by guidelines or HEDIS measure for this population.15,21,22 The current diagnostic approach, especially when used to predict progression of early-stage DKD, is further flawed with the use of staging systems such as KDIGO that rely on small changes within these highly variable markers to stratify patients’ risk of disease progression on an individual level. 23
Findings in Context of the Current Literature
In the current update, the level of high- versus low-risk continues to play an important role in promoting increased patient engagement, with 43% of high-risk patients having some action taken within the first 3 months such as seeing their PCP, receiving a new specialist referral, or new prescriptions for SGLT2i compared to 19% of low-risk patients. For this cohort, most disease management decisions were driven primarily by the PCP’s. Of relevance, the increase in new prescriptions for SGLT2i extended to the intermediate-risk group (pre-test 29% versus post-test 54%) when the population health pharmacists were engaged with these patients. This contrasts with the reported modest increase in SGLT2i and GLP-1RA use in a Beyond Diabetes comprehensive (provider-centric) educational initiative (that included CME educational surveys and point-of-care, EMR patient-level decision support tools), and highlights the challenges when relying solely on education, especially in a complex chronic disease, to motivate the use of new agents. As such, of the 1630 providers (59% internal medicine), only 32% initiated new scripts post-education. 24 While we observed an increase in both SGLT2i and GLP1-RA, the observed change in use or modification of ACE/ARBs continues to be low across all risk groups, likely due to the high penetrance at both pre- and post-test time points within this population.
Implications of Findings
A key element for a successful decision impact study is a comprehensive multi-faceted and multi-disciplinary approach that can address patient adherence, lifestyle modifications, and pharmacologic treatments.25,26 The resources required to achieve this are often costly and limited in comparison to the population’s need. Utilizing the KidneyIntelX test to identify patients at intermediate- and high-risk for DKD progression, the program was able to focus its efforts. The CNT has interacted with over 1300 patients, assessing patient awareness regarding risk factors for DKD, and emphasizing the importance of diet, as well as trying to gain an understanding of the patient’s general motivation for action. Through these efforts, the CNT identified 73 high-risk patients who expressed interest in meeting with a dietician, and 59% of whom met with a dietician. Furthermore, attaining blood pressure control is a critical component in preventing the progression of DKD and is a core effort of the current RWE program. 27 As such, the RPM identified a decrease in SBP of 11.8 mmHg over 12 months with 33% of patients from baseline (pre-test) successfully meeting the <140/90 target and controlling their blood pressure 61% of the time by 12 months.
This 12-month update provided evidence of consistent changes in several clinical outcomes, including the lowering of A1C levels by 0.7%, which was clinically significant in high-risk patients.28,29 Also noted was a decrease in UACR, specifically for intermediate-risk patients with microalbuminuria that is hypothesized to be the result of optimized ACEi/ARBs per guidelines as well as the use of SGLT2i recommendations. Finally, improvement in kidney function was observed across all risk categories, as shown by a clinical and statistically significant improvement (positive change) in eGFR slope between time points. Noteworthy is the recommendation by the National Kidney Foundation, the US Food and Drug Administration, and the European Medicines Agency that eGFR slope reduction of 0.75 ml/min/1.73 m2/year was protective for end-stage kidney disease.30,31 Collectively, achieving blood pressure targets, reduction in A1C and attenuation of eGFR slope decline demonstrate the potential for implementing change and improving the overall cardiac-kidney and metabolic health in this population. Long-term outcomes such as emergency room visits, frequency of dialysis, and kidney transplant will be reported as the data matures.
Limitations
The current study is not without limitations. First, there was insufficient data available on cardiovascular events including heart failure and myocardial infarction. Second, the number of individual patients contacted by the CNT or enrolled in the RPM program represents a small percentage of the overall enrolled population. Efforts are underway to increase out-reach across individual clinics, patients, and providers. Third, we did not actively collect per-patient health insurance coverage information, which could potentially impact access to care and novel therapies. Moreover, patient smoking history and socioeconomic status, as well as an in-depth assessment of the impact of self-identified race on risk level association and access to care will be reported in future studies. Comprehensive efforts are underway to perform such analyses including insurance coverage subgroup to assess impact on care and cost. Finally, we did not isolate the impact of KidneyIntelX availability on outcomes without a multi-disciplinary support team pre- and post-test. The intent of the RWE study was to evaluate the post-test impact of KidneyIntelX on provider management decisions, patient behavior, and clinical outcomes. As such, our pre-test, pre-multidisciplinary baseline results serves as an acceptable alternative to a randomized control group, which is difficult and ethically challenging to effectively administer across an integrated health care system.
Conclusion
The 1-year successful deployment and risk stratification by KidneyIntelX in the RWE precision medicine program was associated with escalation in actions taken to optimize cardio-metabolic-kidney health. Glycemic control, albuminuria, and eGFR slopes all improved post-KidneyIntelX testing with the greatest improvement observed in high-risk patients. Continued engagement and education by care navigators and clinical pharmacists served to further provide patient-level sustainability required for improved long-term DKD outcomes.
Supplemental Material
Supplemental material, sj-docx-1-jpc-10.1177_21501319231223437 for A Real-World Precision Medicine Program Including the KidneyIntelX Test Effectively Changes Management Decisions and Outcomes for Patients With Early-Stage Diabetic Kidney Disease by Joji Tokita, David Lam, Aida Vega, Stephanie Wang, Leonard Amoruso, Tamara Muller, Nidhi Naik, Shivani Rathi, Sharlene Martin, Azadeh Zabetian, Catherine Liu, Catherine Sinfield, Tony McNicholas, Fergus Fleming, Steven G. Coca, Girish N Nadkarni, Roger Tun, Mike Kattan, Michael J. Donovan and Arshad K. Rahim in Journal of Primary Care & Community Health
Acknowledgments
The authors would like to acknowledge all patients and providers participating in this important Population Health program with special mention to the clinical pharmacy team including Angela Knight, PharmD, BCACP, Amanda Vanic, PharmD, BCACP, Christina Lim, PharmD, BCPS, Opeyemi Ogedenbe, PharmD, BCGP, Rachel Lumish, PharmD, BCACP, CDCES, Jacquline Mullakary, PharmD, BCACP, CDE, AE-C, Ruchi Tiwari, PharmD, MS, Susan Mashni, PharmD, BCPS. Also to be acknowledged is JetPub Scientific Communications LLC, which assisted the authors in the preparation of this manuscript.
Footnotes
Author Contributions: JT, DL, AV, SW, LA, TM, NN, SR, SM, AZ. CL, CS, SC, GN, RT, MJD, and AR provided overall content for the general theme, results, and discussion for the publication. TM, MK, GN, and SC provided data analytics and statistical support. SC, DL, AR, and FF provided editorial oversight. TM, NN, SR, SM, AZ, and CL provided all content associated with CNT and Pharmacy patient evidence.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MJD, TM, FF, and RT are employees of Renalytix; SC, GN, MK, and AZ are consultants for Renalytix, All remaining authors have nothing to disclose.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by Renalytix.
Ethical Standards: All authors assert that all procedures contributing to this work comply with the ethical standards of the Mount Sinai Institutional Review Board (IRB).
Ethics and Consent to Participate: In accordance with the Declaration of Helsinki, this study was approved by the Mount Sinai Institutional Review Board (IRB). Individual patient consent waived due to patient volume and number of providers.
IRB Approval: Mount Sinai Institutional Review Board.
Study Enrollment Dates: November 2020 to March 2023.
Trial Registration Number: Real World Evidence (RWE) study, NCT04802395.
ORCID iD: Michael J. Donovan  https://orcid.org/0000-0003-0772-598X
https://orcid.org/0000-0003-0772-598X
Supplemental Material: Supplemental material for this article is available online.
References
- 1. USRDS. Anon: Annual Data Report. 2023. Accessed November 1, 2023. https://usrds-adr.niddk.nih.gov/2023
- 2. Kasiske BL, Wheeler DC, et al. Official JOurnal Of the internatiOnal SOciety Of nephrOlOgy KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney International Supplements (2013) 3m vii. Accessed August 22, 2022. https://www.publicationethics.org
- 3. Oshima M, Shimizu M, Yamanouchi M, et al. Trajectories of kidney function in diabetes: a clinicopathological update. Nat Rev Nephrol. 2021;17:740-750. Accessed August 22, 2022. https://www.nature.com/articles/s41581-021-00462-y [DOI] [PubMed] [Google Scholar]
- 4. Grams ME, Coresh J, Matsushita K, et al. Estimated glomerular filtration rate, albuminuria, and adverse outcomes: an individual-participant data meta-analysis. JAMA. 2023;330: 1266-1277. Accessed November 1, 2023. https://pubmed.ncbi.nlm.nih.gov/37787795/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dunkler D, Gao P, Lee SF, et al. Risk prediction for early CKD in type 2 diabetes. Clin J Am Soc Nephrol. 2015;10:1371-1379. Accessed August 22, 2022. https://pubmed.ncbi.nlm.nih.gov/26175542/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kagoma YK, Weir MA, Iansavichus AV, et al. Impact of estimated GFR reporting on patients, clinicians, and health-care systems: a systematic review. Am J Kidney Dis. 2011;57: 592-601. Accessed August 22, 2022. https://pubmed.ncbi.nlm.nih.gov/21146269/ [DOI] [PubMed] [Google Scholar]
- 7. Sprangers B, Evenepoel P, Vanrenterghem Y. Late referral of patients with chronic kidney disease: no time to waste. Mayo Clin Proc. 2006;81:1487-1494. Accessed August 22, 2022. https://pubmed.ncbi.nlm.nih.gov/17120405/ [DOI] [PubMed] [Google Scholar]
- 8. Hingwala J, Wojciechowski P, Hiebert B, et al. Risk-based triage for nephrology referrals using the kidney failure risk equation. Can J Kidney Heal Dis. 2017;4:2054358117722782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boulware LE, Troll MU, Jaar BG, et al. Identification and referral of patients with progressive CKD: a national study. Am J Kidney Dis. 2006;48:192-204. Accessed August 22, 2022. https://pubmed.ncbi.nlm.nih.gov/16860184/ [DOI] [PubMed] [Google Scholar]
- 10. Agrawal V, Ghosh AK, Barnes MA, et al. Perception of indications for nephrology referral among internal medicine residents: a national online survey. Clin J Am Soc Nephrol. 2009;4:323-328. Accessed August 22, 2022. https://pubmed.ncbi.nlm.nih.gov/19218472/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tokita J, Vega A, Sinfield C, et al. Real world evidence and clinical utility of KidneyIntelX on patients with early-stage diabetic kidney disease: interim results on decision impact and outcomes. J Prim Care Community Health. 2022; 13:21501319221138196. Accessed November 1, 2023. https://pubmed.ncbi.nlm.nih.gov/36404761/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chan L, Nadkarni GN, Fleming F, et al. Derivation and validation of a machine learning risk score using biomarker and electronic patient data to predict progression of diabetic kidney disease. Diabetologia. 2021;64:1504-1515. Accessed August 22, 2022. https://pubmed.ncbi.nlm.nih.gov/33797560/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. American Diabetes Association. Anon: Volume 43 Issue Supplement_1 | Diabetes Care | American Diabetes Association. Accessed August 22, 2022. https://diabetesjournals.org/care/issue/43/Supplement_1
- 14. de Boer IH, Caramori ML, Chan JCN, et al. Executive summary of the 2020 KDIGO diabetes management in CKD guideline: evidence-based advances in monitoring and treatment. Kidney Int. 2020;98:839-848. Accessed August 22, 2022. https://pubmed.ncbi.nlm.nih.gov/32653403/ [DOI] [PubMed] [Google Scholar]
- 15. NCQA. Anon: 2023. HEDIS measures and technical resources - NCQA. Accessed November 2, 2023. https://www.ncqa.org/hedis/measures/
- 16. Hood L. Systems biology and p4 medicine: past, present, and future. Rambam Maimonides Med J. 2013;4:e0012. Accessed November 3, 2023. https://pubmed.ncbi.nlm.nih.gov/23908862/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ndumele CE, Rangaswami J, Chow SL, et al. Cardiovascular-kidney-metabolic health: a presidential advisory from the American Heart Association. Circulation. 2023;148:1606-1635. Accessed November 3, 2023. https://pubmed.ncbi.nlm.nih.gov/37807924/ [DOI] [PubMed] [Google Scholar]
- 18. Nageeta F, Waqar F, Allahi I, et al. Medicine and surgery. Cureus. 2023;15:e45575. doi: 10.7759/cureus.45575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chu CD, McCulloch CE, Banerjee T, et al. CKD awareness among US adults by future risk of kidney failure. Am J Kidney Dis. 2020;76:174-183. Accessed August 22, 2022. https://pubmed.ncbi.nlm.nih.gov/32305206/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rodriguez F, Lee DJ, Gad SS, et al. Real-world diagnosis and treatment of diabetic kidney disease. Adv Ther. 2021;38:4425-4441. Accessed November 3, 2023. https://pubmed.ncbi.nlm.nih.gov/34254257/ [DOI] [PubMed] [Google Scholar]
- 21. Stempniewicz N, Vassalotti JA, Cuddeback JK, et al. Chronic kidney disease testing among primary care patients with type 2 diabetes across 24 U.S. health care organizations. Diabetes Care. 2021;44:2000-2009. Accessed August 22, 2022. https://pubmed.ncbi.nlm.nih.gov/34233925/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Waikar SS, Rebholz CM, Zheng Z, et al. Biological variability of estimated GFR and albuminuria in CKD. Am J Kidney Dis. 2018;72:538-546. Accessed August 22, 2022. https://pubmed.ncbi.nlm.nih.gov/30031564/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Major RW, Cockwell P, Nitsch D, et al. The next step in chronic kidney disease staging: individualized risk prediction. Kidney Int. 2022;102:456-459. Accessed August 22, 2022. https://pubmed.ncbi.nlm.nih.gov/35842063/ [DOI] [PubMed] [Google Scholar]
- 24. Hirsh BJ, Hirsch JS, Hmoud H, et al. A system approach to improving guideline-directed therapy for cardio-renal-metabolic conditions: the “beyond diabetes” initiative. Am J Prev Cardiol. 2023;16:100608. Accessed November 8, 2023. https://pubmed.ncbi.nlm.nih.gov/37822579/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Blonde L, Umpierrez GE, Reddy SS, et al. American Association of Clinical Endocrinology Clinical practice guideline: developing a diabetes mellitus comprehensive care plan-2022 update. Endocr Pract. 2022;28:923-1049. Accessed November 3, 2023. https://pubmed.ncbi.nlm.nih.gov/35963508/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kumar M, Dev S, Khalid MU, et al. The bidirectional link between diabetes and kidney disease: mechanisms and management. Cureus. 2023;15:e45615. Accessed November 3, 2023. https://pubmed.ncbi.nlm.nih.gov/37868469/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Elsayed NA, Aleppo G, Aroda VR, et al. Cardiovascular disease and risk management: standards of care in diabetes-2023. Diabetes Care. 2023;46:S158-S190. Accessed November 3, 2023. https://pubmed.ncbi.nlm.nih.gov/36507632/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qaseem A, Wilt TJ, Kansagara D, et al. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med. 2018;168: 569-576. Accessed November 3, 2023. https://pubmed.ncbi.nlm.nih.gov/29507945/ [DOI] [PubMed] [Google Scholar]
- 29. Eldib AH, Dhaver S, Al-Badri M, et al. Magnitude of A1C improvement in relation to baseline A1C and amount of weight loss in response to intensive lifestyle intervention in real-world diabetes practice: 13 years of observation. J Diabetes. 2023;15: 532-538. Accessed November 3, 2023. https://pubmed.ncbi.nlm.nih.gov/37194402/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grams ME, Sang Y, Ballew SH, et al. Evaluating glomerular filtration rate slope as a surrogate end point for ESKD in clinical trials: an individual participant meta-analysis of observational data. J Am Soc Nephrol. 2019;30:1746-1755. Accessed November 3, 2023. https://pubmed.ncbi.nlm.nih.gov/31292199/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Levey AS, Gansevoort RT, Coresh J, et al. Change in albuminuria and GFR as end points for clinical trials in early stages of CKD: a scientific workshop sponsored by the national kidney foundation in collaboration with the US food and drug administration and European medicines agency. Am J Kidney Dis. 2020;75:84-104. Accessed November 3, 2023. https://pubmed.ncbi.nlm.nih.gov/31473020/ [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jpc-10.1177_21501319231223437 for A Real-World Precision Medicine Program Including the KidneyIntelX Test Effectively Changes Management Decisions and Outcomes for Patients With Early-Stage Diabetic Kidney Disease by Joji Tokita, David Lam, Aida Vega, Stephanie Wang, Leonard Amoruso, Tamara Muller, Nidhi Naik, Shivani Rathi, Sharlene Martin, Azadeh Zabetian, Catherine Liu, Catherine Sinfield, Tony McNicholas, Fergus Fleming, Steven G. Coca, Girish N Nadkarni, Roger Tun, Mike Kattan, Michael J. Donovan and Arshad K. Rahim in Journal of Primary Care & Community Health