Abstract
Purpose:
This study aimed to document the spectrum of ocular manifestations of hematological malignancies presenting to a tertiary health center in Eastern India and their association with blood parameters.
Methods:
This hospital-based cross-sectional study was conducted from August 2021 to July 2022. Patients diagnosed with leukemia, lymphoma, and multiple myeloma were enrolled in the study. A comprehensive ophthalmic evaluation was done in each case.
Results:
A total of 97 patients with a confirmed diagnosis of hematological malignancies and meeting the inclusion and exclusion criteria were included in the study. Ocular manifestations were noted in 48 (49.48%) patients. Acute lymphocytic leukemia accounted for 35.4% of cases, followed by acute myeloid leukemia (31.25%), lymphoma (4.2%), and minimum manifestation in multiple myeloma (2.1%) patients. Among 48 patients with ocular manifestations, anterior segment involvement was found in 6.2% of cases, with subconjunctival hemorrhage being the most common, and the posterior segment was involved in 100% of patients, with intraretinal hemorrhages being the most common manifestation. A statistically significant association was noted between hemoglobin, total red blood cell count, and total platelet count with posterior segment manifestations (p < 0.001). On multivariable logistic regression, only total leucocyte count and total platelet count were significant predictors for ocular manifestation.
Conclusion:
Indirect involvement of the retina is the most common ocular pathology in hematological malignancies, with intraretinal hemorrhages being the most common finding. Ophthalmic examination is highly recommended as a part of the routine evaluation at the time of diagnosis of hematological malignancies and periodically thereafter to diagnose any ocular involvement.
Keywords: Hematological, leukemia, lymphoma, malignancy, ophthalmic
Hematological malignancies include disorders of erythrocytes, leukocytes, platelets, and diseases of coagulation and plasma proteins. They are called leukemia, lymphoma, and myeloma, according to the types of cells affected. Thomas Hodgkin, in 1832, was the first to describe hematological malignancy, which was named Hodgkin disease in his honor after almost 30 years.[1] The ophthalmic manifestations of hematological malignancies could result from direct neoplastic infiltration of ocular tissues or indirect ocular involvement as associated with hematological abnormalities and treatment complications.[2] The involvement can be intraocular, adnexal, orbital tissue, and the neuro-ophthalmic manifestation of central nervous system (CNS) involvement.
Among all ocular manifestations, leukemic retinopathy is the most clinically apparent manifestation, and Liebreich was the first to describe this in the 1860s.[3] Primary leukemic infiltrates manifest as choroidal infiltration, orbital hemorrhages, proptosis, optic nerve infiltration, and cranial nerve palsy. Ocular manifestations in lymphoma are relatively rare, and most of these occur due to direct infiltration of the adnexa, orbit, and intraocular tissue, which present as proptosis or conjunctival mass, uveitis, retinitis, and vasculitis.[3] Multiple myeloma affects the orbital and ocular tissue by direct infiltration or extramedullary plasmacytoma and manifests as proptosis, compressive mass lesions, corneal deposits, and ciliary body cysts. Another mechanism of ocular involvement is hyperviscosity syndrome due to elevated monoclonal immunoglobulins, which leads to hyperviscosity retinopathy, retinal microaneurysm, choroidal infiltrate, and retinal venous occlusion.[4]
Ophthalmic manifestations are usually proportional to the disease severity but sometimes may be the presenting manifestation of an underlying hematological disorder and may occur before the presentation of systemic disease.[5] Ophthalmic manifestations may also signal an isolated focal relapse after complete recovery from systemic disease.[6] Therefore, all patients with hematological malignancy will need ophthalmological evaluation for the disease’s diagnosis, relapse, and prognosis. We aimed to study the spectrum of ocular manifestations of hematological malignancies presenting to a tertiary health center in Eastern India and their association with various blood parameters.
Methods
The present study is a hospital-based cross-sectional study conducted in the Department of Ophthalmology of a tertiary care hospital in Eastern India from August 2021 to July 2022. Institutional ethical committee clearance was obtained before the study (DRI/IMS.SH/SOA/2021/161 dated August 31, 2021). The study adhered to the basic tenets of the Helsinki Declaration. Written informed consent was obtained from each patient before enrolling them in the study. Consent for patients aged less than 18 years was obtained from their parents/guardians.
All new and previously (on treatment) diagnosed cases of leukemia, lymphoma, multiple myeloma, and plasma cell dyscrasia attending the Department of Ophthalmology from August 2021 to July 2022 were included in the study. In all patients, diagnosis of hematological malignancy was based on complete blood count (CBC), microscopic examination (blood film), bone marrow biopsy/aspirate, cytochemistry, histology, immunohistochemistry, and flow cytometry (immuno-phenotyping).[7] Ocular manifestations due to direct tumor infiltration were considered primary manifestations of hematological malignancies. Manifestations due to abnormal blood parameters were considered secondary ocular manifestations. Patients with systemic disorders such as diabetes, hypertension, autoimmune disease, vascular occlusions, positive serological testing for Human Immunodeficiency Virus, significant cataract obstructing the fundus view, and history of any intraocular surgery within the past 3 months were excluded from the study. Severely ill patients who could not provide consent for examination were excluded from the study. Rao soft sample size calculator was used to determine the sample size. Using a confidence interval of 95%, a sample size of 92 was deemed adequate for the study. The study ultimately included 97 patients for statistical analysis.
A brief history and demographic details were obtained from each patient. Best-corrected visual acuity (BCVA) was determined using Snellen’s chart. Extraocular movements were evaluated by checking the eyeball movement in nine cardinal gazes. Detailed anterior segment examination was performed using a slit lamp. Intraocular pressure measurement was done using Goldmann’s applanation tonometer. Both the eyes of the patient were dilated using a combination of 1% tropicamide and 10% phenylephrine. Funduscopy was done on a slit lamp with a +78-D lens. The peripheral retinal examination was done using an indirect ophthalmoscope with a +20-D lens. The presence of proptosis was evaluated using Hertel’s exophthalmometer and plain ruler. Anterior and posterior segment photographs were obtained for records except in patients unable to cooperate for clinical pictures. In debilitated patients, the initial examination was done at the bedside, and retinal examination was done using a direct ophthalmoscope and an indirect ophthalmoscope. Patients were then examined in the Ophthalmology outpatient department if necessary. Neuroimaging was done in patients with primary ocular manifestations to rule out CNS metastasis and ocular/orbital tumors.
Statistical analysis
Appropriate statistical analysis was done at the end of the study period by using SPSS software version 21. Descriptive statistics were presented as frequency and proportion for categorical variables. Continuous variables were presented as mean (± standard deviation) or median depending upon the normality of the data. Independent t-test, or its nonparametric equivalent, Mann–Whitney U test, was used to compare continuous variables between two groups.
One-way analysis of variance (ANOVA), or its nonparametric equivalent, Kruskal–Wallis H test, was used to compare continuous variables between more than two groups. p < 0.05 was considered statistically significant.
Results
This study included 97 patients diagnosed with hematological malignancy, either newly diagnosed or under treatment. The study included 44 (45.4%) newly diagnosed and 53 (54.6%) previously diagnosed patients on treatment for hematological malignancy. The mean age of the patients was 36.20 ± 15.51 years (range: 9–70 years). There were 67 (69.1%) males and 30 (30.9%) females. Maximum patients were aged 20–40 years [Table 1]. Types of hematological malignancies noted were leukemia (77 patients, 79.38%), multiple myeloma (11 patients, 11.34%), and lymphoma (9 patients, 9.27%). The majority of the patients were referred from the Department of Hematology for an ophthalmic assessment irrespective of the presence or absence of ocular symptoms. Only three patients (3.09%) presented directly to the Ophthalmology department with vision complaints. These three patients, on the basis of their ophthalmic findings, were later referred to the Hematology department and then diagnosed with a hematological malignancy.
Table 1.
Age distribution of patients according to hematological malignancy
| Age group | Lymphoma (n=9) | MM (n=11) | AML (n=23) | ALL (n=33) | CML (n=21) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| <20 years | 2 (22.22%) | 0 | 5 (21.73%) | 15 (45.45%) | 0 | |||||
| 21–40 years | 2 (22.22%) | 2 (18.18%) | 10 (43.47%) | 12 (36.36%) | 12 (57.14%) | |||||
| 41–60 years | 3 (33.33%) | 6 (54.54%) | 8 (34.78%) | 6 (18.18%) | 7 (33.33%) | |||||
| >60 years | 2 (22.22%) | 3 (27.27%) | 0 | 0 | 2 (9.52%) |
MM: Multiple myeloma; AML: Acute myeloid leukemia; ALL: Acute lymphocytic leukemia; CML: cChronic myeloid leukemia
Loss of vision was the most common complaint seen in 21 (21.65%) cases. Headache and redness of the eyes were noted in five (5.15%) and four (4.12%) cases, respectively. BCVA <3/60 was noted in 17 eyes. Good vision (BCVA >6/18) was seen in multiple myeloma, and poor vision (BCVA <3/60) in acute myeloid leukemia (AML). Ocular manifestations were noted in 48 cases (49.48%), among which 45 patients (46.39%) were of leukemia, two cases (2.06%) were of lymphoma, and one case (1.03%) was of multiple myeloma. Forty-nine patients (50.51%) did not have ocular manifestations during the examination. Unilateral involvement was noted in six (12.5%) cases, and bilateral involvement was noted in 42 (87.5%) cases. Vision-threatening complications were noted in 14 patients, presenting as subhyaloid hemorrhage in 10 (10.3%) cases, exudative retinal detachment in two cases, and macular edema and optic nerve head infiltration in one case each.
Anterior segment manifestations were noted in six cases (12.5%), while posterior segment manifestations were seen in all 48 patients (100%) with ocular manifestations. The most common anterior segment manifestation was subconjunctival hemorrhage (4.1%), followed by a relative afferent pupillary defect (2.1%). The most common posterior segment manifestation was intraretinal hemorrhage (42.3%), followed by preretinal hemorrhage (14.14%) [Figs. 1 and 2a and b]. Ocular manifestations have been highlighted in Table 2.
Figure 1.

(a and b) Color fundus photograph of a patient with acute lymphoblastic leukemia showing bilateral intraretinal and preretinal hemorrhages. (c) Color fundus photograph of the right eye of a patient with acute myeloid leukemia showing retinal hemorrhages and cotton wool spots (d) Color fundus photograph of the left eye of a patient with acute myeloid leukemia with good vision showing intraretinal hemorrhages
Table 2.
Ocular manifestation according to the type of hematological malignancy in the study population
| Ocular manifestation | Hematological malignancies |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ALL | AML | CML | Lymphoma | MM | ||||||
| Anterior segment | ||||||||||
| SCH | 1 | 3 | - | - | - | |||||
| RAPD | 2 | - | - | - | - | |||||
| WNL | 30 | 20 | 21 | 9 | 11 | |||||
| Posterior segment | ||||||||||
| IRH | 12 | 13 | 13 | 2 | 1 | |||||
| PRH | 8 | 3 | 2 | 1 | - | |||||
| CWS | 5 | 2 | 2 | - | 1 | |||||
| DE | 2 | 3 | 2 | 1 | - | |||||
| OA | 1 | - | - | - | - | |||||
| ME | - | - | - | 1 | - | |||||
| CRVO | - | 1 | - | 1 | - | |||||
| PPE | 1 | - | - | - | - | |||||
| ONHI | 1 | - | - | - | - | |||||
| ERD | 2 | - | - | - | - | |||||
| WNL | 16 | 8 | 8 | 7 | 10 | |||||
ALL: Acute lymphocytic leukemia; AML: Acute myeloid leukemia; CML: Chronic myeloid leukemia; MM: Multiple myeloma; SCH: Subconjunctival hemorrhage; RAPD: Relative afferent pupillary defect; WNL: Within normal limits; IRH: Intraretinal hemorrhage; PRH: Pre-retinal hemorrhage; CWS: Cotton wool spots; DE: Disc edema; OA: Optic atrophy; ME: Macular edema; CRVO: Central retinal vein occlusion; PPE: Peripapillary edema; ONHI: Optic nerve head infiltration; ERD: Exudative retinal detachment
Low hemoglobin was statistically significant among patients with a normal anterior segment compared to patients with anterior segment manifestations (p = 0.041) and those with a normal posterior segment compared to patients with posterior segment manifestations (p = 0.008). A statistically significant association was found between total red blood cell count (TRBC) with posterior segment manifestations (p = 0.001). Similarly, a statistically significant association was noted between low total platelet count (TPC) and posterior segment manifestations (p < 0.001). On multivariable logistic regression analysis to assess the blood parameters associated with the presence of ocular manifestation, only total leucocyte count (TLC) (p = 0.028) and TPC (p = 0.024) were found to be significant predictors of the presence of ocular findings [Table 3].
Table 3.
Multivariable logistic regression to assess the blood parameters associated with the presence of ocular manifestation
| Variables | Adjusted odds ratio (95%CI) | P | ||
|---|---|---|---|---|
| Hemoglobin | 1.07 (0.75–1.53) | 0.694 | ||
| TRBC | 0.49 (0.19–1.27) | 0.141 | ||
| TLC | 1.007 (1.001–1.012) | 0.028 | ||
| TPC | 0.996 (0.992–0.999) | 0.024 |
TRBC: Total red blood cell count; TLC: Total leucocyte count; TPC: Total platelet count
Discussion
Ophthalmic manifestations in hematological malignancies have been reported in the literature, ranging from 24% to 70%.[8] This wide variation in prevalence may imply the transient nature of ocular findings associated with hematological malignancies, which may wax and wane with time and treatment. The wide variance reported can also be due to the setting in which previous studies have been conducted. Results are expected to be different if the study is conducted in a primary oncology referral center as compared to an eye hospital. Hematological malignancies may present with or be associated with ocular disorders. Therefore, recognizing the various ocular manifestations of hematological malignancy is essential to assess the course and prognosis of the disease. During the study period, 97 patients diagnosed with hematological malignancies were examined for the presence or absence of ocular manifestations and their association with blood parameters. We found this sample size adequate for evaluating the study at 95% confidence intervals. The sample size of this study is comparable with the 102 patients in a study conducted by Dhasmana R et al.,[9] 111 patients in a study conducted by Omoti AE et al.,[10] and 100 patients studied by Menon LM et al.[11]
The age in the study population ranged from 9 to 70 years with a mean (± SD) age of 36.20 ± 15.51 years which is comparable with the mean age of 39.27 ± 19.76 years reported by Dhasmana R et al.,[9] the mean age of 39.73 ± 22.1 years reported by Koshy J et al.,[12] and the mean age of 32.7 ± 9.8 years reported by Eze et al.[13] in leukemia patients. In our study, acute lymphocytic leukemia (ALL) was seen more commonly in younger age groups (<20 years of age) as compared to AML and chronic myeloid leukemia (CML) patients, which were primarily seen in 20–40 years of age. Lymphoma and multiple myeloma were more commonly seen in the age group of 41–60 years. Similar findings were reported by Dhasmana R et al.[9] Of 97 patients, 69.1% (67) were males and 30.9% (30) were females. The male preponderance may be due to better access to healthcare facilities than females or to estrogen’s protective effect against retinopathy in women.[14] It is comparable to the study by Dhasmana R et al.,[9] which reported 64.7% males and 35.3% females. Other studies have also reported male preponderance: 63.5% males in a study by Koshy J et al.,[12] 62.5% males in a study by Eze et al.,[13] and 63.5% males in a study by Bouazza M et al.[15]
Leukemia was the most common hematological malignancy seen in our study, followed by multiple myeloma and lymphoma, similar to the study by Eze et al.,[13] who reported the findings in 72 subjects of leukemia, and Bukhari Z et al.,[16] who also reported ALL predominance. In our study population, 13.4% of patients had BCVA <6/18, associated with severe posterior segment involvement and vision-threatening complications. Similar visual loss in 13.7% of patients was reported by Dhasmana R et al.,[9] which differed from Eze et al.,[13] in which visual loss was reported in 32.1% of cases. This variation is because of the transient nature of ocular findings associated with hematological malignancies.
The prevalence of ocular manifestations in the present study is 49.48%. This is comparable to the 43.8% prevalence reported in the study by Koshy J et al.[12] from Northern India and 35.4% reported in a study by Reddy SC et al.[2] from Malaysia. However, our results differ from the study by Eze B et al.[13] from Nigeria, which reported a prevalence of 77.8%. This difference may be because they have exclusively included only leukemia patients in their study. In our study, among 48 patients with ocular manifestations, one patient (2.1%) of ALL was proven to have primary leukemic infiltration as optic nerve infiltration [Fig. 2c]. Subtle enhancement of the left optic nerve was observed in the above case on contrast study with magnetic resonance imaging [Fig. 2d]. In contrast, the remaining (97.9%) cases were due to rheological abnormalities such as anemia, thrombocytopenia, and hyperviscosity. Leukemic optic nerve infiltration indicates an advanced stage of disease and poor prognosis because it is primarily associated with meningeal leukemia and CNS involvement.
Figure 2.
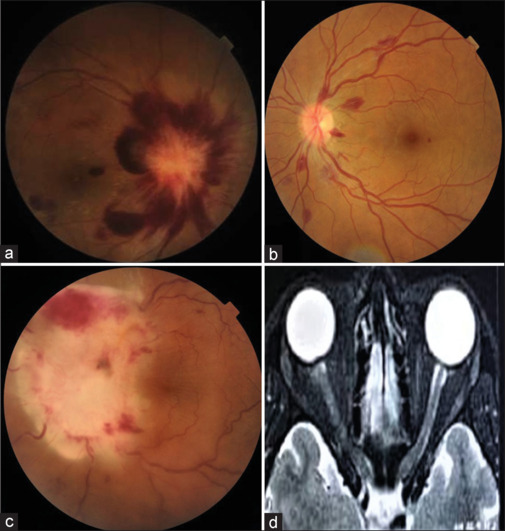
(a) Color fundus photograph of the right eye of a patient with acute lymphoblastic leukemia showing disc edema and retinal hemorrhages (b) Color fundus photograph of the left eye of a patient who primarily presented to the ophthalmology department showing multiple white-centered retinal hemorrhages. The patient was later diagnosed with chronic myeloid leukemia. (c) Color fundus photograph of the left eye of a patient with acute lymphoblastic leukemia showing leukemic optic nerve infiltration (d) Magnetic resonance imaging of the same patient showing subtle enhancement of the left optic nerve with indistinct margins
Anterior segment manifestations were noted in six cases (12.5%), while posterior segment manifestations were seen in all 48 patients (100%) with ocular manifestations. Some subjects had more than one manifestation in one or both eyes. Anterior segment manifestations were noted in acute leukemia only. Anterior segment manifestation in subconjunctival hemorrhage was noted in 4.1% of patients. Bukhari et al.[16] reported subconjunctival hemorrhage in 11.1% of cases. This difference is because they considered only leukemia patients in their study. Posterior segment manifestations were most prominent in our study, particularly in leukemia. We noted different manifestations, with retinal hemorrhages being the most common finding, followed by cotton wool spots. Neuro-ophthalmic manifestations in disc edema, optic atrophy, and optic nerve head infiltration were noted in nine cases. Central retinal vein occlusion was seen in one patient each with AML and lymphoma. Similar findings were seen in various other studies as well.[11,16,17,18]
Ocular lesions are proportional to the severity of hematological malignancy and sometimes can be the presenting sign of the illness or an isolated focal relapse after complete recovery. Similarly, our study revealed one refractory ALL revealed by optic nerve head infiltration and exudative retinal detachment. Patients with multiple myeloma may present with infiltration of orbit, conjunctiva, uvea, lacrimal sac, lacrimal gland, chorioretinopathy, neuro-ophthalmic abnormalities, corneal deposits, and opportunistic infections.[19] However, none of these findings were noted in our study, possibly due to the small sample size. There is sufficient literature on ocular manifestations in hematological malignancy but minimal studies on the association of blood parameters with ocular manifestations in hematological malignancy. Some studies have shown a direct relationship between blood parameters and ocular manifestation.[9,16,20] Lower values of TPC, hemoglobin, TRBC, and high TLC have been associated with a higher incidence of ocular manifestations. The present study found a significant association of anterior segment manifestation with lower mean hemoglobin (p = 0.041), whereas mean TRBC, TLC, and TPC were statistically insignificant (p > 0.05). The reduced mean value of hemoglobin (p = 0.008), TRBC (p = 0.001), and mean TPC (p < 0.001) had a significant association with posterior segment involvement, whereas mean TLC showed a statistically insignificant difference (p = 0.689).
Retinal hemorrhages and cotton wool spots are hematological malignancy’s most striking posterior segment features. Retinal hemorrhages are associated with anemia and thrombocytopenia, and venous tortuosity or occlusions are related to hyperviscosity syndrome. Development of cotton wool spots occurs due to occlusion of precapillary arterioles, which results in retinal ischemia. In this study, reduced hemoglobin (p < 0.001) and TRBC (p < 0.05) had a significant association with retinal hemorrhages and cotton wool spots while showing no association with disc edema (p > 0.05). Similar results were noted in the studies by Suresh K et al.,[14] Dhasmana R et al.,[9] Soman S et al.,[20] and Bukhari Z et al.[16] However, in these studies, the ocular manifestations were compared irrespective of the involved segment of the eye, TRBC values were not correlated, and only leukemia cases were considered. Low platelet count (<0.05) and higher TLC were associated with retinal hemorrhages. However, cotton wool spots and disc edema had no significant association, which is comparable with Dhasmana R et al.[9] (p = 0.004) and Reddy SC et al.[2] study (p = 0.03) but differ from Bukhari Z et al. (p = 0.06).[16]
On multivariable logistic regression analysis to assess the blood parameters associated with the presence of ocular manifestations, only TLC (p = 0.028) and TPC (p = 0.024) were significant predictors of ocular manifestation. With one unit (i.e. thousands per μL) increase in TLC, the probability of the presence of ocular manifestation increased by 1.007 times (aOR: 1.007; 95% CI: 1.001-1.012), and with one unit (i.e. thousands per μL) increase in TPC, the probability of the presence of ocular manifestation decreased by 0.4% (aOR: 0.996; 95% CI: 0.992-0.999). Soman S et al.[20] showed that hemoglobin level was the only predictor of developing subhyaloid hemorrhage. They interpreted that every 1 g/L increment in hemoglobin level led to a 30% reduction in the likelihood of developing subhyaloid hemorrhage. They also found that increased platelet count reduced the probability of overall ophthalmic manifestations.
The present study has a few limitations. This is a single-center hospital-based cross-sectional study. We considered a small number of patients with hematological malignancy. Hence, the results may not correctly reflect the prevalence of ophthalmic manifestations in the general population. The observations in our study require more investigations in the setting of a prospective study. The present study did not study the stage or severity of the systemic disease. The correlation of the stage of the disease with the blood parameters could have increased the clinical significance of the study. Dry eye disease has been reported to be the most common ocular manifestation in the study by Bouazza M et al.[15] This might reflect more a side effect associated with the treatment of leukemia rather than the disease itself. The present study is not designed to evaluate for the presence of dry eye disease, and this is a shortcoming of the study.
Conclusion
We conclude that indirect involvement of the retina is the most common ocular pathology in hematological malignancies, with intraretinal hemorrhages being the most common finding. Furthermore, according to our study, TLC and TPC were significant predictors of ocular manifestations in hematological malignancies. Many patients with ocular manifestations do not complain about eye symptoms; thus, ophthalmic examination is highly recommended as a part of the routine evaluation at the time of diagnosis of hematological malignancies and periodically after that to diagnose any ocular manifestation. This highlights the significance of an ophthalmologist’s role in preventing severe ocular damage in patients with hematological malignancies and monitoring the course of the disease.
Financial support and sponsorship:
Nil.
Conflicts of interest:
There are no conflicts of interest.
References
- 1.Aisenberg AC. Historical review of lymphomas. Br J Haematol. 2000;109:466–76. doi: 10.1046/j.1365-2141.2000.01988.x. [DOI] [PubMed] [Google Scholar]
- 2.Reddy SC, Jackson N, Menon BS. Ocular involvement in leukemia--A study of 288 cases. Ophthalmologica. 2003;217:441–5. doi: 10.1159/000073077. [DOI] [PubMed] [Google Scholar]
- 3.Orhan B, Malbora B, Akça Bayar S, Avcı Z, Alioğlu B, Özbek N. Ophthalmologic findings in children with leukemia: A single-center study. Turk J Ophthalmol. 2016;46:62–7. doi: 10.4274/tjo.03880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Omoti AE, Omoti CE. Ophthalmic manifestations of multiple myeloma. West Afr J Med. 2007;26:265–8. doi: 10.4314/wajm.v26i4.28324. [DOI] [PubMed] [Google Scholar]
- 5.Singh AD. The prevalence of ocular disease in chronic lymphocytic leukemia. Eye (Lond) 2003;17:3–4. doi: 10.1038/sj.eye.6700278. [DOI] [PubMed] [Google Scholar]
- 6.Sharma T, Grewal J, Gupta S, Murray PI. Ophthalmic manifestations of acute leukaemias: The ophthalmologist’s role. Eye (Lond) 2004;18:663–72. doi: 10.1038/sj.eye.6701308. [DOI] [PubMed] [Google Scholar]
- 7.Bahakeem E, Qadah T. Current diagnostic methods for hematological malignancies: A mini-review. Pharmacophore. 2020;11:63–8. [Google Scholar]
- 8.Ilo OT, Adenekan AO, Alabi AS, Onakoya AO, Aribaba OT, Kehinde MO, et al. Ocular manifestations of leukaemia: A teaching hospital experience. Niger Postgrad Med J. 2019;26:205–10. doi: 10.4103/npmj.npmj_50_19. [DOI] [PubMed] [Google Scholar]
- 9.Dhasmana R, Prakash A, Gupta N, Verma SK. Ocular manifestations in leukemia and myeloproliferative disorders and their association with hematological parameters. Ann Afr Med. 2016;15:97–103. doi: 10.4103/1596-3519.188887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Omoti AE, Omoti CE, Momoh RO. Ophthalmic disorders in adult lymphoma patients in Africa. Middle East Afr J Ophthalmol. 2009;16:252–5. doi: 10.4103/0974-9233.58420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menon LM, John E, Deepthi R, Shaji A. Prevalence of ocular manifestation in haematological malignancies-A clinical study. IOSR J Dent Med Sci. 2018;17:22–8. [Google Scholar]
- 12.Koshy J, John MJ, Thomas S, Kaur G, Batra N, Xavier WJ. Ophthalmic manifestations of acute and chronic leukemias presenting to a tertiary care center in India. Indian J Ophthalmol. 2015;63:659–64. doi: 10.4103/0301-4738.169789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eze BI, Ibegbulam GO, Ocheni S. Ophthalmic manifestations of leukemia in a tertiary hospital population of adult nigerianafricans. Middle East Afr J Ophthalmol. 2010;17:325–9. doi: 10.4103/0974-9233.71599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suresh K, Sampath R, Tanvi Ocular manifestations in hematological disorders. SRMC. 2011;4:1–4. [Google Scholar]
- 15.Bouazza M, Youssefi H, Bouanani N. Ocular manifestations in hematological disorders. Cureus. 2022;14:e27941. doi: 10.7759/cureus.27941. doi: 10.7759/cureus. 27941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bukhari ZM, Alzahrani A, Alqarni MS, Alajmi RS, Alzahrani A, Almarzouki H, et al. Ophthalmic manifestations in acute leukemia patients and their relation with hematological parameters in a tertiary care center. Cureus. 2021;13:e19384. doi: 10.7759/cureus.19384. doi: 10.7759/cureus. 19384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nuzzi R, Scalabrin S, Becco A, Panzica G. Gonadal hormones and retinal disorders: A review. Front Endocrinol (Lausanne) 2018;9:66. doi: 10.3389/fendo.2018.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pandharinath JT, Chandrakant SS, Pandharinath JD. Ophthalmic manifestations of common haematological disorders. J Evol Med Dent Sci. 2014;3:10510–6. [Google Scholar]
- 19.Chin KJ, Kempin S, Milman T, Finger PT. Ocular manifestations of multiple myeloma: Three cases and a review of the literature. Optometry. 2011;82:224–30. doi: 10.1016/j.optm.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Soman S, Kasturi N, Srinivasan R, Vinod KV. Ocular manifestations in leukemias and their correlation with hematologic parameters at a tertiary care setting in South India. Ophthalmol Retina. 2018;2:17–23. doi: 10.1016/j.oret.2017.05.009. [DOI] [PubMed] [Google Scholar]


