Abstract
Over the last two decades, there has been a concerted effort by researchers to mass propagate Eurycoma longifolia and improve the yield of its very important and sought-after anti-cancer and aphrodisiac bioactive compounds. To achieve this, various techniques have been used to mass propagate and improve the yield of these bioactive compounds in tissue cultures. These techniques include the optimization of media conditions and application of various types and combinations of plant growth regulators (PGRs). In addition, some elicitation techniques have been used to improve the synthesis of these bioactive compounds. However, in comparison with other herbal species with similar economic importance, many techniques have not been applied to E. longifolia. Adopting the most recent methodologies would ensure efficiency and sustainability in the in vitro production of bioactive compounds in E. longifolia. Therefore, in this review, we present an up-to-date record on the success stories in the tissue culture techniques and synthesis of bioactive compounds. In addition, we attempted to identify some of the missing links on the road to the effective and sustainable biotechnological utilization of this super important biological resource.
Keywords: Eurycoma longifolia, plant tissue culture, elicitation, biosynthesis, bioactive compounds
1. Introduction
Eurycoma longifolia Jack is a versatile tree of the Simaroubaceae family, popularly known as the Quassia family. It belongs to the genus Eurycoma together with two other two species, namely, Eurycoma apiculata A. W. Benn., which occurs in Malaysia and Indonesia, and E. harmandiana Pierre, which grows along the axis between Thailand and Laos. Although these species share some similarities in terms of chromosome number [1] and basic phytochemicals [2], E. longifolia is more widespread and more popular, thanks to its numerous bioactive compounds [2] and uses. It is native to southeast Asia, occurring mainly in Indonesia, Malaysia, Singapore, and Thailand. It is also found in Brunei Darussalam, Cambodia, Laos, Vietnam, southern Myanmar and the Philippines [3,4,5]. E. longifolia has various local names such as Tongkat Ali in Malaysia, Pasak Bumi in Indonesia, Ian-don in Thailand, Cây bách bệnh in Vietnam, and Tho nan in Laos [6].
E. longifolia is a medium-sized tree that typically reaches a height of about 15 to 18 m as undergrowth in forests [7]. This wild natural resource served as the sole source of tongkat ali products until recently, when growing demand exerted pressure on the wild resource, leading to the establishment of commercial plantations [8].
E. longifolia is regarded as one of the most valuable medicinal plants. In Vietnam, it is listed in the pharmacopoeia, and it is locally known as “chy ba binh”, which literally means tree that cures hundreds of diseases [9]. It is considered a national treasure in Malaysia [10] and a popular medicinal plant in Indonesia [11]. E. longifolia is utilized in both traditional herbal medicine and modern pharmaceutical applications. Additionally, it is employed in food supplements and the cosmetic industry. The multitude of applications has contributed to elevating E. longifolia’s status in both the commercial and scientific realms.
Traditionally, various parts of the E. longifolia tree are used to treat many diseases, which include skin itching, dysentery, stomach worms, diarrhea, and fever [12]. The root extract of E. longifolia is highly valued for its aphrodisiac properties and its efficacy in treating chronic diseases such as cancer and diabetes [13]. It is also employed for conditions such as leukemia, syphilis, fever, and osteoporosis. Additionally, it serves as an antibiotic, aids in slowing the aging process, and helps reduce stress and anxiety. Moreover, it is used in addressing gynecological disorders [14].
Scientific experiments using in vitro systems, animal models, and clinical trials have established the antimalarial [15], cytotoxic, anticancer [16,17,18,19], antidiabetic, aphrodisiac, proandrogenic, and antimicrobial effects [20,21,22] of E. longifolia. In addition, its efficacy in treating male sexual dysfunctions [23] and osteoporosis [24] has been confirmed. Furthermore, clinical studies have shown that eurycomanone, the major bioactive molecule in E. longifolia, is effective against lung, breast, gastric, and colon cancer [25]. It also exhibits wound healing properties [26] and effectiveness against bacteria, fungi, protozoa [27,28], dengue [29], and coronavirus [30].
E. longifolia has been extensively researched for its phytochemicals and bioactive compounds isolated from its root, leaf, and stem [31]. These compounds form a group of quassinoids/degraded triterpenoids [32], including eurycomalactone, eurycomanone, eurycomanol, and others [33,34,35,36]. Abubakar et al. [37] reported over 70 bioactive compounds from various parts of E. longifolia in their review. In the last decade, numerous new bioactive compounds have been reported [29,32,38,39,40,41,42,43,44]. This trend is expected to continue.
The products of E. longifolia are gaining public acceptance across the globe, with hundreds of products being registered by the relevant authorities. The Malaysian Ministry of Health estimated the total value of E. longifolia at USD 1.7 billion, projecting an annual increase of 15% [45]. This lucrative market has attracted significant interest, raising concerns about product adulteration [46] and emphasizing the need for product authentication [47,48,49].
The approved products are already in international markets [50], leading to increased pressure on resources and prompting the implementation of government laws to ensure sustainability [45]. Ensuring sustainability in the utilization of plant resources can be achieved through effective propagation techniques. In the case of E. longifolia, numerous research reports focus on in vitro propagation and various strategies for enhancing the synthesis of bioactive compounds.
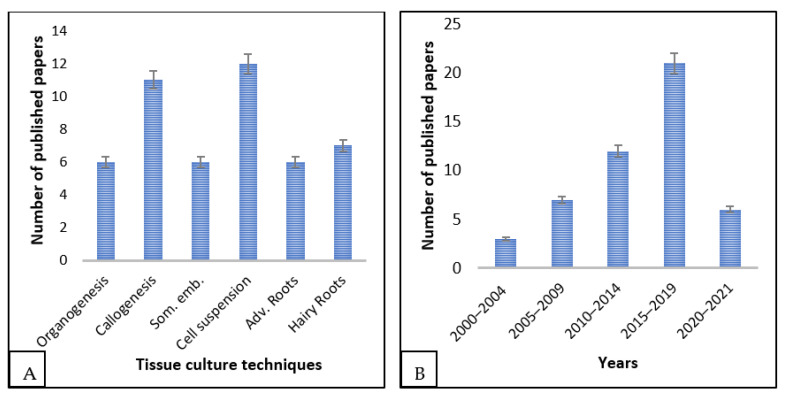
The first report on E. longifolia tissue culture surfaced in 2000. Since then, numerous papers, covering various tissue culture techniques, have been published (Figure 1A). Notably, from 2015 to 2019, there has been a surge in interest in the tissue culture of this medicinal tree (Figure 1B), which is expected to intensify in the coming years.
Figure 1.
Current trend in E. longifolia tissue culture; (A) various tissue culture techniques employed in E. longifolia, (B) occurrence of publications of E. longifolia over the years. The last bar represents only two years.
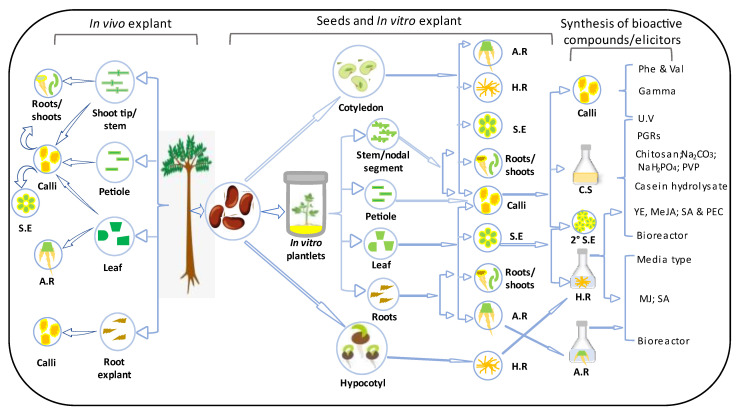
Therefore, there is a need to compile information for convenient access, facilitating the comparison of techniques and aiding decision-making. This report serves as a comprehensive guide for the development and utilization of E. longifolia resources, offering updates on successful tissue culture techniques and in vitro synthesis of bioactive compounds (Figure 2). Recommendations are also provided for future possibilities to ensure effective and sustainable biotechnological exploitation.
Figure 2.
Summary of the successes in the tissue culture and in vitro synthesis of bioactive compounds in E. longifolia. S.E = somatic embryo; A.R = adventitious roots; H.R = hairy roots; C.S = cell suspension; Phe = phenylalanine; Val = valine; PEC = pectin; YE = yeast extract; MeJa = methyl jasmonate; JA = jasmonic acids; SA = salicylic acid; 2° = secondary; Na2CO3 = sodium carbonate; NaH2PO4 = monosodium phosphate; PVP = polyvinylpyrrolidone.
2. Tissue Culture Techniques for Mass Propagation in E. longifolia
2.1. Techniques for Direct Organogenesis
The induction of organs, such as roots and shoots, directly on the explant is a micropropagation technique that allows for the swift clonal mass propagation of plants. In E. longifolia, various plant growth regulators (PGRs) have been utilized to stimulate direct organogenesis (Table 1). Hussein et al. [51], using shoot tips as explants, induced shoots on Murashige and Skoog (MS) medium with 5.0 mg/L kinetin and roots on 0.5 mg/L indole-3-butyric acid (IBA). In a similar vein, Hussein et al. [52], utilizing roots as explants, generated shoots on Driver and Kuniyuki Walnut (DKW) media with 1.0 mg/L zeatin, and induced roots on MS medium with 0.5 mg/L IBA. Similarly, stems were used to induce shoots on woody plant medium (WPM) supplemented with 2.0 mg/L each of 6-benzylaminopurine (BAP) and zeatin, and roots were induced on MS medium with 0.5 mg/L IBA. Using nodal segments, cotyledons, and in vitro leaves as explants, shoots were induced on both half-strength and full-strength MS supplemented with 0.5 mg/L BAP and 1.0 mg/L BAP, respectively. Additionally, roots were induced on half-strength MS supplemented with 10 mg/L and 0.5 mg/L IBA and full-strength MS supplemented with 0.5 mg/L IBA [53,54,55]. The light conditions used in the above studies were 16L: 8D with an intensity of 35–150 µmol/m2/s.
Table 1.
Summary of the techniques for organogenesis in E. longifolia showing the explant and the media conditions used with the corresponding morphogenic response.
| Explants | Media + PGR | Other Culture Conditions | Morphogenic Response/Outcome | Refs. |
|---|---|---|---|---|
| Shoot tips | BAP, KIN, and Zeatin | 16L: 8D lighting of 150 µmol/m2/s | 5.0 mg/L KIN induced shoots while 0.5 mg/L IBA induced roots | [51] |
| In vitro roots and stems | Shooting: MS, DKW or WPM + BAP, KIN, and Zeatin Rooting: MS + IBA |
16L: 8D lighting of 150 µmol/m2/s | DKW + 1.0 mg/L kinetin + 1.0 mg/L zeatin formed shoots on root explants while WPM + 2.0 mg/L BAP + 2 mg/L zeatin formed shoots on stem explants MS + 0.5 mg/L IBA formed roots on shoot explants |
[52] |
| Nodal segments | Shooting: BAP + KIN Rooting: IBA |
Continuous light | ½ MS + 0.5 mg/L BAP produced shoots while ½ MS + 10 mg/L IBA produced roots | [53] |
| Cotyledons | Shooting: BAP + KIN and TDZ Rooting: IBA + NAA |
16L: 8D lighting of 35 µmol/m2/s |
1.0 mg/L BAP and 0.5 mg/L IBA produced better shoots and roots, respectively | [54] |
| In vitro leaves | Shooting: MS + BAP; KIN and TDZ Rooting: ½ MS + IBA and NAA |
16L: 8D lighting of 35 µmol/m2/s | 1.0 mg/L BAP produced shoots directly; and ½ MS + 0.5 mg/L IBA produced roots on the shoots | [55] |
Key: PGRs = plant growth regulators; BAP = 6-benzylaminopurine; KIN = kinetin; IBA = indole-3-butyric acid; TDZ = thidiazuron; NAA = 1-naphthaleneacetic acid; MS = Murashige and Skoog medium; DKW = Driver and Kuniyuki Walnut medium; WPM = woody plant medium.
From the above results, it can be deduced that BAP (0.5–2.0 mg/L) resulted in a higher percentage and larger shoot formation regardless of the explants used. Similarly, IBA at 0.5 mg/L was found to be best for root induction compared to other auxins. This shows that these PGRs are superior to others in the organogenesis of E. longifolia.
2.2. Techniques for Callogenesis and Callus Elicitation
Callus induction is a crucial event in various tissue and cell culture systems, as it can function as a transient tissue for organogenesis, somatic embryogenesis, cell culture [56], and more. Additionally, it can serve as a latent tissue for the synthesis of bioactive compounds. In Table 2, the main techniques for the induction, proliferation, and elicitation of callus in E. longifolia are summarized. Siregar et al. [57] tested the effects of different genotypes, media, and naphthaleneacetic acid (NAA) concentrations on callus induction using leaves as explants. The result showed that MS modification and the genotype have effects on callus induction.
Siregar et al. [58] investigated the effects of BAP and NAA on callus formation using various explants. They found that MS supplemented with 8.0 mg/L NAA and 2.0 mg/L BAP yielded the highest callus biomass on petioles. In contrast, Mahmood et al. [59], using various plant parts as explants, discovered that different concentrations of 2,4-dichlorophenoxyacetic acid (2,4-D) and picloram were effective in inducing callus. Similar results have shown that 1.0 mg/L 2,4-D is effective in callus induction [60].
Studies on the effects of different concentrations of 2,4-D and NAA revealed that 1.0 mg/L NAA plus 1.0 mg/L BAP induced callus on leaves, while 1.0 mg/L 2,4-D plus 1.0 mg/L BAP induced callus on petioles [61]. Furthermore, the subculture of callus on 1.5 mg/L NAA and 1.0 mg/L kinetin resulted in improved biomass [62].
The production of bioactive compounds in E. longifolia callus has been documented. According to Rosli et al. [63], higher levels of methoxycanthin-6-one were reported on quarter-strength MS (¼ MS) with 2% fructose and 2 mg/L dicamba, along with the addition of 1.65 × 10−2 mg/L phenylalanine. Furthermore, gamma irradiation [64] has been shown to reduce callus biomass, total phenolics, and flavonoids. Interestingly, increasing the dose to around 60 Gy enhanced the synthesis of soluble protein. This suggests that gamma irradiation at a specific dose can stimulate changes in certain metabolic pathways. However, the available data are insufficient to fully explain the mechanisms involved.
Table 2.
Summary of the techniques for callogenesis and callus elicitation in E. longifolia, indicating the explant, the media, and the culture conditions used with the results.
| Explants | Media + PGR + Additives | Other Culture Conditions | Morphogenic Response/Outcome | Refs. |
|---|---|---|---|---|
| Leaves | MS + NAA and various macro nutrients | Various plant sources | Eu 9 plant, pH 5.75, and modified MS formed more callus | [57] |
| Leaves, stems, and petioles | MS + BAP, NAA | 24L: 0D lighting of 30 µmol/m2/s | 8.0 mg/L (43.01 µM) NAA + 2.0 mg/L (8.88 µM) BAP formed higher callus on petioles, while 10 mg/L NAA formed callus on leaves | [58] |
| All plant parts | MS, SH, WH, and B5 + auxins, sugars, and amino acids | ¼ MS + 2% fructose + 2 mg/L dicamba and 1.65 × 10−2 mg/L phenylalanine produced higher 9-MCO | [63] | |
| All plant parts | MS + 2,4-D, dicamba, picloram, NAA, and IAA | Continuous dark | 1.0–4.0 mg/L 2,4-D produced callus on leaf, petioles, rachis, stem, root, and cotyledon explants, etc. | [65] |
| Callus | MS + 1 mg/L 2,4-D | 16L: 8D lighting of 15 µmol/m2/s and gamma | Gamma radiation decreased biomass, total phenol, and flavonoids but improved soluble protein at 60 Gy | [64] |
| Leaves and petioles | MS + 2,4-D, NAA, BAP, and KIN | 1.0 mg/L (1.0 ppm) NAA + 1 mg/L BAP induced callus on leaves, and 1.0 mg/L (1.0 ppm) 2,4-D + 1 mg/L BAP induced callus on petioles | [61] | |
| Root segments | MS + 1 mg/L 2,4-D | 16L: 8D lighting of 40 µmol/m2/s | Treatment produced callus | [60] |
| Callus | MS + 2,4-D, NAA, and KIN | 8L: 16D lighting | 1.5 mg/L NAA and 1.0 mg/L KIN produced better biomass | [62] |
Key: PGRs = plant growth regulators; BAP = 6-benzylaminopurine; KIN = kinetin; NAA = 1-naphthaleneacetic acid; 2,4-D = 2,4-dichlorophenoxyacetic acid; IAA = indole-3-acetic acid; MS = Murashige and Skoog medium; SH = Schenk and Hildebrandt medium; WH = White’s medium; B5 = Gamborg (B5) medium; Gy = Gray; 9-MCO = 9-methoxycanthin-6-one; Eu 9 = code given by the author.
Different photoperiods, including continuous dark and different light intensities ranging from 15 to 50 µmol/m2/s [58,65], were applied. The multiplicity in induction conditions highlights that callus in E. longifolia can be induced from different explants using various types, combinations, and concentrations of PGRs and under different light conditions. However, leaves seem to be the predominant explants used, with 2,4-D and NAA emerging as the most frequently employed PGRs.
2.3. Techniques for Induction and Multiplication of Somatic Embryos (SEs)
Somatic embryogenesis is a significant event that can enhance the success of clonal and mass propagation, cryopreservation, synthetic seed production, and/or genetic improvement of important plant species [66,67,68,69,70]. In E. longifolia, the successful induction of somatic embryos has been reported (Table 3).
Table 3.
Techniques for the induction of somatic embryos (SEs) in E. longifolia showing the explant, media and culture conditions and morphogenic response.
| Explants | Culture Media + Additives | Other Culture Conditions | Morphogenic Changes/Results | Refs. |
|---|---|---|---|---|
| Immature cotyledons | MS + 2,4-D, NAA, KIN, and BAP | Both light and darkness | Direct SEs produced on NAA while indirect on 2,4-D | [71] |
| All plant parts | MS + 2,4-D, IAA, IBA, dicamba, and NAA | 16L: 8D lighting of 150 µmol/m2/s | Cotyledons on 1 mg/L 2,4-D produced embryonic callus (EC) and subculture of EC on 0.5 mg/L KIN, and 1 mg/L 2,4-D produced higher yield of SEs | [72] |
| Secondary callus | MS + 2,4-D and BAP or KIN | 12L: 12D lighting and agitated at 120 rpm | 1–2.5 mg/L 2,4-D, 2 mg/L BAP, and KIN produced SEs | [73] |
| Cotyledons | Modified MS + IBA, Zeatin, and TDZ | Activated charcoal and in dark | 0.1 zeatin + 0.2 IBA + 0.12 TDZ produced highest SEs, and 2 mg/L IBA + 0.075 mg/L TDZ produced secondary SEs | [74] |
| Primary SEs | MS + IBA + Zeatin + TDZ and 0.1 g/L AC | RITA ® bioreactors | Immersion rate of 5 min every 4 h produced highest number of SEs | [75] |
| Cotyledons | MS + 0.2 mg/L IBA + 0.1 mg/L Zeatinand 0.12 mg/L TDZ | Complete darkness | Globular SEs were produced | [75] |
Key: SE = somatic embryo; EC = embryonic calli; BAP = 6-benzylaminopurine; KIN = kinetin; IBA = indole-3-butyric acid; TDZ = thidiazuron; NAA = 1-naphthaleneacetic acid; 2,4-D = 2,4-dichlorophenoxyacetic acid; MS = Murashige and Skoog medium.
Aziz et al. [71] induced direct somatic embryos (SEs) and indirect ones (via embryonic calli (EC)) from immature cotyledons on NAA and 2,4-D, respectively. Similarly, Hussein et al. [72], using different explants, concluded that cotyledonary tissues produced EC on 1.0 mg/L 2,4-D, and EC subculture on 0.5 mg/L kinetin plus 1 mg/L 2,4-D resulted in a higher yield. Furthermore, EC were used to induce SEs on liquid media containing 1–2.5 mg/L 2,4-D, 2.0 mg/L BAP, and kinetin [73]. Additionally, Dalila et al. [74] found that the highest percentage of SE was produced using modified MS containing 0.1 mg/L zeatin, 0.2 mg/L IBA, and 0.12 mg/L TDZ. Mohd et al. [75] also utilized the mentioned liquid media and varied the immersion frequencies in RITA® bioreactors. They found that an immersion frequency of 5 min every 4 h produced the highest SE.
From all the studies above, it is evident that cotyledons are the only explants to successfully produce SEs on MS supplemented with NAA, 2,4-D, and zeatin. This may result from specific cells in cotyledons that are more responsive to these hormones, leading to the initiation of embryonic cells. Furthermore, SEs can be induced in E. longifolia both in total darkness and different photoperiods, demonstrating the light independency of the process that leads to somatic embryo formation.
3. Techniques for Improving the Synthesis of Bioactive Compounds in E. longifolia
3.1. Techniques for the Establishment of Cell Suspension Culture and Synthesis of Bioactive Compounds
Plant cell suspension cultures are increasingly being utilized for synthesizing bioactive compounds. This approach, applied to E. longifolia, offers a convenient method for producing compounds for agricultural, pharmaceutical, and industrial applications. Various culture conditions and elicitation techniques have been studied (as summarized in Table 4). It should be noted that the initial steps of callus formation have already been presented in the earlier sections of this review. Therefore, only the steps involved in the establishment of cell suspensions from the callus are highlighted in this section.
One of the earliest reports is that of Siregar et al. [57], in which the effects of adjusting MS nutrients and pH level on growth were tested. The best results were recorded at pH 5.75 in the modified MS. Similarly, under these conditions, variations between different cell sources were tested [76]. The results indicated that different cell lines react differently, with Eu9 producing the highest biomass and Eu8 producing the highest level of alkaloids. Other studies revealed that MS supplemented with 0.5 mg/L (2.69 µM) NAA and 0.25 mg/L (1.13 µM) 2,4-D produced more 9-methoxycanthin-6-one and 9-hydroxycanthin-6-one alkaloids [58], and MS with modified nutrients yielded even better levels of biomass and alkaloids [77].
Studies on the effects of carbon and nitrogen sources have shown that glucose and potassium nitrate (KNO3) produced the highest cell growth and soluble protein in CS cultures [78]. In a similar study, Shim et al. [60] revealed that full-strength MS supplemented with 3.0 mg/L NAA, 3% sucrose, and 0:60 ratio of NH4+:NO3− produced better biomass. Additionally, the content of eurycomanone in the CS improved on MS plus 1.2 mg/L NAA and 1.0 mg/L kinetin [79].
Table 4.
Summary of various techniques for establishment and elicitation of cell suspension culture in E. longifolia including the media and culture conditions and the corresponding outcome.
| Medium + PGR | Suspension Culture Conditions | Objective | Outcome | Refs. |
|---|---|---|---|---|
| MS + NAA | 1.0 g cells shaken in 20 mL MS at 130 rpm under 24L: 0D lighting | Effects of pH and Macro-nutrients | Cells grew better in 5.75 pH and modified MS | [57] |
| MS + NAA | 1.0 g cells shaken in 20 mL MS at 130 rpm under 24L: 0D lighting | Effects of cell source and pH on canthin-6-one | Eu9 produced highest biomass while Eu8 produced more canthin-6-one at pH 5.75 | [76] |
| MS + NAA and 2,4-D | 0.5 g cells shaken in 20 mL MS at 130 rpm | Effects of PGR on growth and synthesis | MS + 0.5 mg/L (2.69 µM) NAA and 0.25 mg/L (1.13 µM) 2,4-D gave better 9-MCO and 9-HCO alkaloid | [58] |
| MS + 0.5 mg/L NAA and 0.25 mg/L 2,4-D | 1.0 g cells shaken in 20 mL MS at 130 rpm under 18 μE/m2/s | Effects of modified MS | Modified MS formed more biomass and biosynthesis | [77] |
| Modified MS | Callus in MS + Chitosan, Na2CO3, NaH2PO4, and PVP and 24L: 0D lighting of 32.5 µmol/m2/s | Elicitation | 100 g/L chitosan produced more biomass; 150 produced the highest 9-MCO and 9-HCO; 2.0 mg/L and 20 mg/L NaH2PO4 produced the highest biomass and alkaloid, respectively | [80] |
| MS + 0.5 mg/L NAA and 0.25 mg/L 2,4-D | 1.0 g cells in 25 mL MS + casein hydrolysate and shaking at 130 rpm under 1525 lux of light | Effects of casein hydrolysate and light on biomass and 9-MCO | 0.1–2.0% casein hydrolysate and 1525 lux of light improved the synthesis of 9-MCO | [81] |
| MS + 1.0 mg/L 2,4-D; sugar and nitrogen sources | Callus shaken at 100 rpm under 16L: 8D lighting | Optimization for growth | Glucose and KNO3 produced the highest cell growth, soluble protein, and activity of peroxidase | [78] |
| MS + 2,4-D and KIN | Cells suspended in ½ MS and shake at 100 rpm | Elicitation with UV | UV + 1.1 mg/L (1.1 ppm) 2,4-D and 1.0 mg/L (1.0 ppm) KIN improved alkaloids synthesis | [82] |
| MS + IBA, NAA, sugar, and nitrogen sources | 5.0 g cells shaken in 100 mL MS at 110 rpm under 40 µmol/m2/s, 16L: 8D lighting | Optimization for growth | Full-strength MS, 3.0 mg/L NAA, 3% sucrose, and 0:60 NH4+:NO3− produced better biomass | [60] |
| MS + NAA and KIN; sugar sources | 3.0 g callus shaken in 50 mL MS at 120 rpm under 500 lux | Optimization for eurycomanone synthesis | 1.2 mg/L NAA and 1.0 mg/L KIN produced more eurycomanone | [79] |
| MS + NAA and KIN; YE, MeJa, and SA | 3.0 g callus shaken in 50 mL MS at 120 rpm under 500 lux | Elicitation | 200 mg/L YE, 20 µM each of MeJa and SA produced more eurycomanone | [83] |
| MS + NAA and 2,4-D; YE, PEC, and VAL | 1.0 g callus shaken in 20 mL MS at 120 rpm under 32.5 µmol/m2/s | Biotic elicitation | Elicitation improved biosynthesis | [84] |
Key: PGRs = plant growth regulators; KIN = kinetin; IBA = indole-3-butyric acid; NAA = 1-naphthaleneacetic acid; 2,4-D = 2,4-dichlorophenoxyacetic acid; MS = Murashige and Skoog medium; YE = Yeast extract; MeJa = methyl jasmonate; SA = salicylic acid; PEC = pectin; VAL = valine; PVP = polyvinylpyrrolidone; 9-MCO = 9-methoxycanthin-6-one; 9-HCO = 9-hydroxycanthin-6-one; Eu 8 and Eu 9 = code given by the author.
Different elicitation techniques using both biotic and abiotic factors on the CS of E. longifolia have also been reported. Keng et al. [80], using modified MS and different elicitation agents, reported that 100–150 g/L chitosan produced higher biomass and 9-methoxycanthin-6-one and 9-hydroxycanthin-6-one. However, NaCO3 and PVP inhibited growth but had no effect on alkaloid synthesis. Similarly, Siregar et al. [81] tested the elicitation effects of casein hydrolysate and found that 0.1–2% casein hydrolysate, along with 1525 lux of light, improved the synthesis of 9-methoxycanthin-6-one. Additionally, the effect of UV irradiation on the CS resulted in improved the alkaloids content [82].
In addition to physical and chemical elicitation, biotic elicitors such as yeast extract (YE), methyl jasmonate (MeJa), salicylic acid (SA), pectin (PEC), and valine (VAL) were tested in E. longifolia. Nhan and Loc [83] found that 200 mg/L YE and 20 µM each of MeJa and SA were ideal for enhancing the synthesis of eurycomanone. Similarly, Kwan et al. [84] reported that YE, PEC, and VAL have positive effects on the synthesis of bioactive compounds in E. longifolia.
From Table 4, it can be inferred that the most appropriate PGR combination for establishing cell suspension cultures in E. longifolia is either a combination of 5.0 mg/L NAA and 2.5 mg/L 2,4-D or 1.2 mg/L NAA and 1.0 mg/L kinetin. This shows the cells can respond positively to a variety of PGRs. Additionally, the optimal culture density and shaking frequency were determined to be 1.0 g/20 mL and 120 rpm, respectively. Biotic elicitation techniques have been shown to be efficient in enhancing the cell biomass and synthesis of bioactive compounds. This could help positively towards the mass production of phytochemicals in E. longifolia.
3.2. Induction, Proliferation, and Elicitation of Adventitious Roots (ARs)
Adventitious roots (ARs) are induced to propagate true-to-type plants and/or to produce bioactive compounds from medicinal plants. In E. longifolia, there are only a few reports on the induction and/or elicitation of ARs (Table 5).
Ali et al. [85] observed that ARs could be produced on leaf explants by adjusting the MS medium to contain half the normal quantity of nitrate plus 5.0 mg/L IBA. Similarly, Lulu et al. [86] achieved successful ARs production on leaf explants using ¾ MS strength and 3.0 mg/L IBA. Consistent results were reported by Cui et al. [87], where ¾ MS with 3.0 mg/L IBA led to improved ARs and metabolite production. Giap et al. [88], using cotyledons as explants and testing various PGR combinations, established that MS with 1.5 mg/L NAA and 0.1 mg/L BA is optimal for ARs induction. Furthermore, 3.0 mg/L NAA with 50 g/L sucrose was found to induce better ARs, while elicitation with MeJa showed a reduction in alkaloid contents [89].
Table 5.
Summary of the techniques for induction, growth, and elicitation of adventitious roots (ARs) showing the explants, media, and culture conditions and the corresponding outcome.
| Explants | Media + PGR and Other Culture Conditions | Morphogenic Response/Outcome | Refs. |
|---|---|---|---|
| Leaves | MS + IBA, IAA, NAA, and sucrose | MS (½ nitrate) + 5.0 mg/L IBA produced better ARs | [85] |
| Leaves | MS + IBA, NAA, and IAA; different carbon source in continuous dark | MS + 3.0 mg/L NAA and 50 g/L sucrose produced better biomass | [89] |
| In vitro roots | MS + 5.0 KIN and MeJa under 16L: 8D lighting of 150 µmol/m2/s | MeJa reduced alkaloids concentration | [90] |
| In vitro leaves | ¾ MS + 3.0 mg/L IBA + 30 g/L sucrose in dark condition | Treatment produced ARs | [86] |
| In vitro cotyledons | MS + BA and NAA under 16L: 8D lighting of 2500–3000 lux | 1.5 mg/L NAA and 0.1 mg/L BA produced ARs on cotyledons | [88] |
| Leaves | Diff. media type, and IBA and sucrose in dark condition | ¾ MS + 3.0 mg/L IBA and 30 g/L sucrose produced better ARs and metabolites | [87] |
Key: PGRs= plant growth regulators; BA = benzyl adenine; KIN = kinetin; IBA = indole-3-butyric acid; NAA = 1-naphthaleneacetic acid; IAA = indole-3-acetic acid; MS = Murashige and Skoog medium; MeJa = methyl jasmonate.
From these results, it can be deduced that ARs are mostly induced using leaves as explants with 3.0–5.0 mg/L IBA under dark conditions. However, other PGR combinations, such as NAA and BA, are suitable for cotyledonary explants. This explains the facts that different tissues response differently to different PGRs depending on their cell types, endogenous hormones, and total physiology.
3.3. Transformation with A. rhizogene and Induction of Hairy Roots (HRs)
HRs are engineered to serve as living factories for producing valuable phytochemicals for pharmaceutical, cosmetic, and agricultural applications. Inducing HRs marks a milestone in plant biotechnology, enabling the large-scale production of bioactive compounds without the need for PGRs [91,92,93,94].
In E. longifolia, limited reports document the successful generation of HRs (Table 6). Balakrishnan et al. [95] achieved the successful generation of HRs using somatic embryos (SEs) with two strains of A. rhizogenes (AR12 and AR14). The infected tissues were cultured in the dark for three days, and ultimately, a transient expression of the β-glucuronidase (GUS) gene was detected.
Table 6.
Techniques for A. rhizogenes transformation and/or induction of hairy roots (HRs) in E. longifolia showing different co-culture techniques and their outcome.
| Starting Material | A. rhizogenes Strain and Media | Inoculation/Transformation | Initial Co-Culture Conditions | Subculture Media | Outcome | Refs. |
|---|---|---|---|---|---|---|
| Somatic embryos (SEs) | AR12 and AR14 strain grown in LB medium | SEs immersed in bacterial suspension for 20 min | Infected tissue inoculated in MS + 0.5 mg/L IBA and 1% PVP in dark | MS + 500 mg/L cefotaxime | Transient GUS expression observed in the explants | [95] |
| In vivo and in vitro plants, seedlings, embryos, and SEs | MAFF106590, 106591, 201265, 301726, and 720002 strains in LB medium | A. rhizogene suspension injected into explants | Infected tissues inoculated in MS in dark | MS + 300 mg/L cefotaxime | Only MAF201265, 301726 and 720002 induced HRs in hypocotyls region | [96] |
| Cotyledons and hypocotyl | ATTC 15834 strain in YMB medium | Explants incubated on bacterial plates for 30 min | Infected tissues inoculated on WPM under low light | WPM + 500 mg/L cefotaxime | HR tips appeared from the explants | [39] |
Key: LB = Luria–Bertani media; YMB = yeast mannitol broth medium; WPM = woody plant medium; MS = Murashige and Skoog medium IBA = indole-3-butyric acid; PVP = polyvinylpyrrolidone; GUS = β-glucuronidase.
In research conducted by Danial et al. [96], various explants and strains of A. rhizogenes were tested, revealing that two strains induced HRs on the plants’ hypocotyl region. Similarly, Ngoc et al. [39] successfully produced HRs from in vitro cotyledons and hypocotyls using A. rhizogenes strain ATTC 15834.
The generation of hairy roots from E. longifolia may not have received significant attention or could have presented challenges. Nevertheless, the current results suggest that hairy roots can be induced from hypocotyl and cotyledon tissues, which are somewhat related in plants, explaining their ability to differentiate into HRs after successful transformation. Furthermore, successful transfection can be achieved through co-culturing tissues and bacteria using various media compositions.
3.4. Improving the Synthesis of Bioactive Compounds in Hairy Roots (HRs) Culture
Following the successful induction of HRs, the focus shifts to optimizing the growth conditions and synthesizing target bioactive compounds. HRs exhibit rapid growth even without PGRs, prompting the exploration of factors such as media type, culture density, and external elicitors. The effects of different media and the elicitation on E. longifolia HRs are summarized in Table 7.
Studies investigating the effects of media types and elicitation techniques on the growth and synthesis of hairy roots revealed that Gamborg B5, Schenk and Hildebrandt media (SH), and woody plant medium (WPM) supported the maximum growth and synthesis of alkaloids [97,98]. Furthermore, elicitation with jasmonic acid (JA), YE, MeJa, and SA enhanced the synthesis of canthin-6-one alkaloids [98,99].
Table 7.
Techniques of maintenance and elicitation of HR cultures in E. longifolia indicating various media types and their effects.
| Medium + Additives | Culture Conditions | Objective | Outcome | Refs. |
|---|---|---|---|---|
| B5, ½ B5, SH, ½ SH, N6, and ½ N6 | 0.2 g HRs agitated at 110 rpm | Effects of media type | B5 supported maximum growth and production of alkaloid | [97] |
| MS + MeJa + SA | 0.2 g HRs in 50 mL MS at 110 rpm | Elicitation for 9-MCO production | MeJa and SA at 0.1 mM each produced high amounts of 9-MCO | [99] |
| WPM, MS and SH + JA and YE | 0.3 g HRs in 100 mL media and agitated at 80 rpm | Elicitation for improved biomass and 9-MCO | SH and WPM produced the best biomass and 9-MCO alkaloids, and JA and YE elicitation improved only 9-MCO synthesis | [98] |
Key: B5 = Gamborg B5 medium; SH = Schenk and Hildebrandt media; N6 = Chu (N6) medium; MS = Murashige and Skoog medium; WPM = woody plant medium; MeJa = methyl jasmonate; JA = jasmonic acid; YE = yeast extract; SA = salicylic acid; 9-MCO = 9-methoxycanthin-6-one.
Based on the results presented in Table 7, it can be concluded that elicitation techniques have positive effects on the synthesis of bioactive compounds in the HRs of E. longifolia. This can therefore add to the inherent abilities of HRs. The findings also indicate that HRs can grow well on a variety of media. Furthermore, agitation at 110 rpm in dark conditions is the most frequently employed technique.
3.5. Upscale Production of Bioactive Compounds in Bioreactors
Bioreactors are utilized for the scaled-up production or synthesis of bioactive compounds in living cells or tissues. Bioreactors offer a superior alternative to other culture systems [100] because of their efficient contact between cells or tissues and the medium, as well as their improved aeration and growth.
There are only a few reports on the use of bioreactors for the large-scale production of bioactive compounds in E. longifolia (Table 8). Natanael et al. [82] tested the effects of bioreactors and UV irradiation on CS. The results showed that UV has a positive effect on the synthesis of canthin-6-one and β-carboline. In a similar study, Shim et al. [60] found out that a CS density of 50 g/L at an aeration rate of 0.3 vvm was optimal for growth and the synthesis of phenolics.
In other studies, adventitious roots (ARs) were fed to a bioreactor at a density of 5.0 g/L and an aeration rate of 0.1 vvm to test the effects of nitrogen sources [86]. The results showed that a ratio of 1:2 NH4+ and NO3− was optimal for growth and synthesis. In Fan et al. [101], ARs were fed to bioreactors containing ¾ MS to optimize the growth conditions. The results showed that 40 g/L sucrose, a 5.0 g/L cell density, and a 0.05 vvm aeration rate were optimum for the growth synthesis of eurycomanone. Additionally, the effects of bioreactors on the HRs resulted in improved biomass and synthesis of canthin-6-one alkaloids compared to shake flasks [102].
In summary, three types of tissues (CS, HRs, and ARs) have been reported for use in bioreactors. These tissues exhibit little variation in their culture conditions. Generally, different types and strengths of media are applicable. ARs and HRs were cultured in the dark, while CS requires light. The aeration rate ranged between 0.05 and 0.3 vvm. Therefore, the large-scale production of E. longifolia products is possible using bioreactors.
Table 8.
Techniques for the large-scale production of bioactive metabolites in E. longifolia using bioreactor including the explant used, the culture condition, and the results.
| Explants | Bioreactor Type | Culture Media/Condition | Inoculation Condition | Outcome/Opt. Condition | Refs. |
|---|---|---|---|---|---|
| CS | Bubble column | ½ MS with 25 g/L sucrose + 1.1 and 1.0 mg/L 2,4-D and KIN | 5.0 g/L cells at 0.3 vvm aeration and 18L: 6D UV | UV improved canthin-6-one and β-carboline | [82] |
| ARs | 5 L balloon- type bubble |
¾ MS + IBA and NAA and varying ratios of NH4+:NO3− | 5.0 g/L ARs at 0.1 vvm aeration in dark | IBA, NAA, and 1:2 NH4+:NO3− are optimum | [86] |
| CS | 5 L balloon-type bubble | MS + 3.0 mg/L NAA, 3% sucrose and 0:60 NH4+:NO3− | 40–80 g/L cell at 0.05–0.3 vvm and 16L: 8D lighting of 40 µmol/m2/s | 50 g/L and 0.3 vvm improved biomass and phenols | [60] |
| HRs | 20 L spherical bubble | Liq. WPM with 30 g/L sucrose and 40 mg/L YE | 3.0 g/L HRs inoculated at 1.5 vvm in dark | Bioreactor improved biomass and synthesis of canthin-6-one alkaloids | [102] |
| ARs | 5 L bubble column | ¾ MS with diff. sucrose cons. + 3 mg/L IBA | 2.5–5.0 g/L ARs inoculated at 0.05–0.1 vvm in dark | 40 g/L sucrose, 5.0 g/L density, and 0.05 vvm were optimum for biomass and eurycomanone synthesis | [101] |
| HRs | 5 L bioreactor | MS basal medium | Dark conditions | Biomass improved by 20-fold | [103] |
Key: CS = cell suspension; ARs = adventitious roots; HRs = hairy roots; KIN = kinetin; IBA = indole-3-butyric acid; NAA = 1-naphthaleneacetic acid; MS = Murashige and Skoog medium; WPM = woody plant medium; YE = yeast extract.
4. Future Perspectives
Despite considerable efforts to enhance the yield of bioactive metabolites in E. longifolia, there is still a need for improvement to ensure sustainability. Adopting recent techniques already applied to other valuable medicinal plants would further enhance the synthesis of metabolites. The following sections briefly outline promising areas in tissue culture and biotechnological advancements that have not yet been applied to E. longifolia.
4.1. Elicitation Techniques
Elicitation induces stress in cells and tissues, enhancing secondary metabolite production. Biotic elicitation agents (bacteria, fungi, etc.) and abiotic stressors (heavy metal ions, UV radiation, nanoparticles, etc.) are utilized in various plants. Despite the proven effectiveness of elicitation techniques [104,105,106,107,108,109,110], research in this area on E. longifolia is insufficient.
4.1.1. Biotic Elicitation
E. longifolia has been tested with only a few biotic elicitors, such as YE, JA, and SA. Many other biotic elicitors, including aspergillus and fusarium [111], remain unexplored. Additionally, effective elicitation agents like algae [112,113], proteins, and plant-growth-promoting microorganisms such as rhizobacteria and trichoderma [114,115], etc., have not been investigated. This represents a gap in academic research.
4.1.2. Abiotic Elicitation
Although physical elicitation methods are highly effective [115], limited research has been conducted on E. longifolia. While UV and gamma radiation have been tested, the details of their mode of action remain unclear. Furthermore, essential abiotic elicitation techniques such as LED [116] and nanoparticles [117] have not been explored.
Different LED monochromatic lights have been employed individually or in combinations to enhance the synthesis of crucial metabolites. LEDs have the ability to improve metabolite production in both callus [118,119,120,121,122] and cell suspension [123,124]. Moreover, several studies highlight the effectiveness of LEDs in enhancing the synthesis of bioactive compounds in various in vitro cultures [116]. Additionally, numerous research papers document the efficacy of nanoparticle elicitation in enhancing metabolite production [125,126,127,128]. Therefore, there is a need to explore these aspects in E. longifolia.
4.2. Modern Breeding Strategies for Improved Biosynthesis
Modern molecular breeding techniques have been employed to enhance the synthesis of various plant substances. Initially, molecular markers [129] were used to identify existing variations in the population. Subsequently, omics technologies [130,131,132] can be utilized to identify the specific biosynthetic pathways and their underlying genes. In ginkgo, for instance, transcriptomics and metabolomics have been instrumental in improving bioactive metabolites [133,134,135,136]. Similar techniques have been applied to enhance the biosynthesis of taxol and other metabolites in yew trees [137,138,139,140,141]. Additional recent and efficient techniques include metabolic engineering and synthetic biology [142,143,144,145,146,147] for directly enhancing the synthesis of target metabolites.
The application of modern breeding technologies in E. longifolia is still in its early stages. Currently, only a limited number of studies have explored genetic variation in E. longifolia. These studies have utilized molecular markers such as inter-retrotransposon amplified polymorphism (IRAP), single-nucleotide polymorphism (SNP), random amplified polymorphic DNA (RAPD), simple sequence repeat (SSR), and microsatellites [148,149,150,151]. Additionally, DNA barcoding has been employed for the characterization of E. longifolia [152]. There is a clear need to expand research in this field to enhance the synthesis of bioactive metabolites, highlighting a substantial gap in the overall biotechnological exploration of E. longifolia.
Author Contributions
Conceptualization, S.S. (Sani Sale), S.S. (Sreeramanan Subramaniam) and M.F.M.A.; writing—original draft, S.S. (Sani Sale); writing—review and editing, S.S. (Sani Sale), S.S. (Sreeramanan Subramaniam) and M.F.M.A.; visualization, S.S. (Sani Sale); methodology, S.S. (Sani Sale), S.S. (Sreeramanan Subramaniam) and M.F.M.A.; resources, M.F.M.A.; validation, S.S. (Sreeramanan Subramaniam) and M.F.M.A.; project administration, S.S. (Sreeramanan Subramaniam) and M.F.M.A.; funding acquisition, M.F.M.A. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The authors wish to acknowledge Universiti Sains Malaysia for providing the necessary facilities. The authors would like to thank the financial support from the Ministry of Higher Education Malaysia for the Fundamental Research Grant Scheme with project code: FRGS/1/2020/STG03/USM/02/21. The authors also express gratitude to Gombe State University for support through the Tertiary Education Trust Fund (TETFund).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Zulfahmi A.R., Irfan M., Rosmaina M.N. Chromosome Numbers and Karyotypes of Eurycoma longifolia Jack and Eurycoma Apiculata a.w Benn (Simaroubaceae) Pak. J. Biotechnol. 2018;15:969–973. [Google Scholar]
- 2.Chaingam J., Choonong R., Juengwatanatrakul T., Kanchanapoom T., Putalun W., Yusakul G. Evaluation of anti-inflammatory properties of Eurycoma longifolia Jack and Eurycoma harmandiana Pierre in vitro cultures and their constituents. Food Agric. Immunol. 2022;33:530–545. doi: 10.1080/09540105.2022.2100324. [DOI] [Google Scholar]
- 3.Bhat R., Karim A. Tongkat Ali (Eurycoma longifolia Jack): A review on its ethnobotany and pharmacological importance. Fitoterapia. 2010;81:669–679. doi: 10.1016/j.fitote.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Chua LS L., Kamarudin S., Markandan M., Hamidah M. A preliminary checklist of vascular plants from the Machinchang Range, Pulau Langkawi, Peninsular Malaysia. Malay. Nat. J. 2005;57:155–172. [Google Scholar]
- 5.Wizneh F.M., Asmawi M.Z. Eurycoma longifolia jack (Simarubaceae); Advances in its medicinal potentials. Pharmacogn. J. 2014;6:1e9. doi: 10.5530/pj.2014.4.1. [DOI] [Google Scholar]
- 6.Hidayati S., Zuhud E.A., Adiyaksa I.K., Al Manar P. Review: Etnotaksonomi dan bioekologi tumbuhan pasak bumi (Eurycoma longifolia Jack.) J. Pengelolaan Sumberd. Alam Dan Lingkung. (J. Nat. Resour. Environ. Manag.) 2021;11:177–178. doi: 10.29244/jpsl.11.2.177-178. [DOI] [Google Scholar]
- 7.Nordin M.S. Distribution of the Population of Tongkat Ali (Eurycoma Spp.) in Malaysia Based on Data Taken from Herbarium Records. Med. Aromat. Plants. 2014;3:2167-0412. doi: 10.4172/2167-0412.1000155. [DOI] [Google Scholar]
- 8.Wan-Muhammad-Azrul W.A., Mohd-Farid A., Lee S.Y., Sajap A.S., Omar D., Mohamed R. A survey on the occurrence of pests and diseases in tongkat ali (Eurycoma longifolia) plantations in Peninsular Malaysia. J. Trop. For. Sci. 2018;30:362–375. doi: 10.26525/jtfs2018.30.3.362375. [DOI] [Google Scholar]
- 9.Thoi L.V., Suong N.N. Constituents of Eurycoma longifolia Jack. J. Org. Chem. 1970;35:1104–1109. doi: 10.1021/jo00829a053. [DOI] [PubMed] [Google Scholar]
- 10.Mohamed A.N., Vejayan J., Yusoff M.M. Review on Eurycoma longifolia Pharmacological and Phytochemical Properties. J. Appl. Sci. 2015;15:831–844. doi: 10.3923/jas.2015.831.844. [DOI] [Google Scholar]
- 11.Susilowati A., Rachmat H.H., Elfiati D., Hasibuan M.H. The composition and diversity of plant species in pasak bumi’s (Eurycoma longifolia) habitat in Batang Lubu Sutam forest, North Sumatra, Indonesia. Biodiversitas J. Biol. Divers. 2019;20:413–418. doi: 10.13057/biodiv/d200215. [DOI] [Google Scholar]
- 12.Kuo P.-C., Damu A.G., Lee K.-H., Wu T.-S. Cytotoxic and antimalarial constituents from the roots of Eurycoma longifolia. Bioorganic Med. Chem. 2004;12:537–544. doi: 10.1016/j.bmc.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 13.Tsai C.-H., Fang T.-C., Liao P.-L., Liao J.-W., Chan Y.-J., Cheng Y.-W., Li C.-H. The powdered root of Eurycoma longifolia Jack improves beta-cell number and pancreatic islet performance through pdx1 induction and shows antihyperglycemic activity in db/db mice. Nutrients. 2020;12:2111. doi: 10.3390/nu12072111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdul Rahman S., Ahamad A.N., Kit-Lam C. Effects of Eurycoma longifolia Standardized Extract on Estradiol Valerate-Induced Teratogenicity in Female Rats. Int. J. Pure Appl. Math. 2018;118:1–23. [Google Scholar]
- 15.Chan K.-L., Choo C.-Y., Abdullah N.R. Semisynthetic 15-O-Acyl- and 1,15-Di-O-acyleurycomanones from Eurycoma longifolia as potential antimalarials. Planta Med. 2005;71:967–969. doi: 10.1055/s-2005-864188. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen-Pouplin J., Tran H., Tran H., Phan T.A., Dolecek C., Farrar J., Tran T.H., Caron P., Bodo B., Grellier P. Antimalarial and cytotoxic activities of ethnopharmacologically selected medicinal plants from South Vietnam. J. Ethnopharmacol. 2007;109:417–427. doi: 10.1016/j.jep.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Nurhanan M.Y., Hawariah L.P.A., Ilham A.M., Shukri M.A.M. Cytotoxic effects of the root extracts of Eurycoma longifolia Jack. Phytother. Res. 2005;19:994–996. doi: 10.1002/ptr.1759. [DOI] [PubMed] [Google Scholar]
- 18.Ye G., Xu M., Shu Y., Sun X., Mai Y., Hong Y., Zhang J., Tian J. A Quassinoid Diterpenoid Eurycomanone from Eurycoma longifolia Jack Exerts Anti-Cancer Effect through Autophagy Inhibition. Molecules. 2022;27:4398. doi: 10.3390/molecules27144398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okba M.M., Ezzat M.I., Shehabeldine A.M., Ezzat S.M. Eurycomanol and eurycomanone as potent inducers for cell-cycle arrest and apoptosis in small and large human lung cancer cell lines. Nat. Prod. Res. 2022;37:1856–1862. doi: 10.1080/14786419.2022.2119387. [DOI] [PubMed] [Google Scholar]
- 20.Ang H.H., Ikeda S., Gan E.K. Evaluation of the potency activity of aphrodisiac in Eurycoma longifolia Jack. Phytother. Res. 2001;15:435–436. doi: 10.1002/ptr.968. [DOI] [PubMed] [Google Scholar]
- 21.Farouk A.-E., Benafri A. Antibacterial activity of Eurycoma longifolia Jack. A Malaysian medicinal plant. Saudi Med. J. 2007;28:1422–1424. [PubMed] [Google Scholar]
- 22.Khanam Z., Wen C.S., Bhat I.U.H. Phytochemical screening and antimicrobial activity of root and stem extracts of wild Eurycoma longifolia Jack (Tongkat Ali) J. King Saud Univ.-Sci. 2015;27:23–30. doi: 10.1016/j.jksus.2014.04.006. [DOI] [Google Scholar]
- 23.Thu H.E., Mohamed I.N., Hussain Z., Jayusman P.A., Shuid A.N. Eurycoma longifolia as a potential adaptogen of male sexual health: A systematic review on clinical studies. Chin. J. Nat. Med. 2017;15:71–80. doi: 10.1016/s1875-5364(17)30010-9. [DOI] [PubMed] [Google Scholar]
- 24.Effendy N.M., Mohamed N., Muhammad N., Mohamad I.N., Shuid A.N. Eurycoma longifolia: Medicinal plant in the prevention and treatment of male osteoporosis due to androgen deficiency. Evid.-Based Complement. Altern. Med. 2012;2012:125761. doi: 10.1155/2012/125761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thu H.E., Hussain Z., Mohamed I.N., Shuid A.N. Eurycoma longifolia, A Potential Phytomedicine for the Treatment of Cancer: Evidence of p53-mediated Apoptosis in Cancerous Cells. Curr. Drug Targets. 2017;19:1109–1126. doi: 10.2174/1389450118666170718151913. [DOI] [PubMed] [Google Scholar]
- 26.Al-Bayati M.R.Y., Hussein Y.F., Faisal G.G., Fuaat A.A., Affandi K.A., Abidin M.A.Z. The Effect of Eurycoma longifolia Jack Tongkat Ali Hydrogel on Wound Contraction and Re-Epithelialization in In Vivo Excisional Wound Model. Open Access Maced. J. Med. Sci. 2022;10:634–643. doi: 10.3889/oamjms.2022.9140. [DOI] [Google Scholar]
- 27.Thu H.E., Hussain Z., Mohamed I.N., Shuid A.N. Recent Advances in Antibacterial, Antiprotozoal and Antifungal Trends of Eurycoma longifolia: A Review of Therapeutic Implications and Future Prospects. Curr. Drug Targets. 2018;19:1657–1671. doi: 10.2174/1389450119666180219123815. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y.-X., An X.-L., Xu Y.-N., Hao Y.-J., Piao X.-C., Jin M.-Y., Lian M.-L. Antibacterial and antibiofilm properties of dichloromethane fraction of extracts from adventitious roots of Eurycoma longifolia against Staphylococcus aureus. LWT. 2022;162:113438. doi: 10.1016/j.lwt.2022.113438. [DOI] [Google Scholar]
- 29.He X., Zheng Y., Tian C., Wen T., Yang T., Yu J., Fang X., Fan C., Liu J., Yu L. Quassinoids from Eurycoma longifolia with antiviral activities by inhibiting dengue virus replication. Phytomedicine. 2023;110:154650. doi: 10.1016/j.phymed.2023.154650. [DOI] [PubMed] [Google Scholar]
- 30.Choonong R., Ruangdachsuwan S., Churod T., Palabodeewat S., Punyahathaikul S., Juntarapornchai S., Ketsuwan K., Komaikul J., Masrinoul P., Kitisripanya T., et al. Evaluating the in vitro Efficacy of Quassinoids from Eurycoma longifolia and Eurycoma harmandiana against Common Cold Human Coronavirus OC43 and SARS-CoV-2 Using In-Cell Enzyme-Linked Immunosorbent Assay. J. Nat. Prod. 2022;85:2779–2788. doi: 10.1021/acs.jnatprod.2c00736. [DOI] [PubMed] [Google Scholar]
- 31.Rehman S.U., Choe K., Yoo H.H. Review on a traditional herbal medicine, Eurycoma longifolia Jack (Tongkat Ali): Its traditional uses, chemistry, evidence-based pharmacology and toxicology. Molecules. 2016;21:331. doi: 10.3390/molecules21030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ismail N.I.M., Chua L.S. Solvent Partition for Terpenoid Rich Fraction from Crude Extract of Eurycoma longifolia; Proceedings of the Third International Conference on Separation Technology 2020 (ICoST 2020); Johor, Malaysia. 15–16 August 2020; pp. 62–67. [DOI] [Google Scholar]
- 33.Chan K.L., O’Neill M.J., Phillipson J.D., Warhurst D.C. Plants as sources of antimalarial drugs. Part 3. Eurycoma longifolia. Planta Med. 1986;52:105–107. doi: 10.1055/s-2007-969091. [DOI] [PubMed] [Google Scholar]
- 34.Darise M., Kohda H., Mizutani K., Tanaka O. Eurycomanone and eurycomanol, quassinoids from the roots of Eurycoma longifolia. Phytochemistry. 1982;21:2091–2093. doi: 10.1016/0031-9422(82)83050-1. [DOI] [Google Scholar]
- 35.Miyake K., Tezuka Y., Awale S., Li F., Kadota S. Quassinoids from Eurycoma longifolia. J. Nat. Prod. 2009;72:2135–2140. doi: 10.1021/np900486f. [DOI] [PubMed] [Google Scholar]
- 36.Chua L.S., Segaran A., Wong H.J. LC-PDA-MS/MS-based characterization of key phytochemicals in Eurycoma longifolia roots. J. Chromatogr. Sci. 2021;59:659–669. doi: 10.1093/chromsci/bmab041. [DOI] [PubMed] [Google Scholar]
- 37.Abubakar B.M., Salleh F.M., Wagiran A. Chemical Composition of Eurycoma longifolia (Tongkat Ali) and the Quality Control of its Herbal Medicinal Products. J. Appl. Sci. 2017;17:324–338. doi: 10.3923/jas.2017.324.338. [DOI] [Google Scholar]
- 38.Meng D., Li X., Han L., Zhang L., An W. Four new quassinoids from the roots of Eurycoma longifolia Jack. Fitoterapia. 2014;92:105–110. doi: 10.1016/j.fitote.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 39.Ngoc P.B., Pham T.B., Nguyen H.D., Tran T.T., Chu H.H., Chau V.M., Lee J.-H., Nguyen T.D. A new anti-inflammatory β-carboline alkaloid from the hairy-root cultures of Eurycoma longifolia. Nat. Prod. Res. 2016;30:1360–1365. doi: 10.1080/14786419.2015.1056187. [DOI] [PubMed] [Google Scholar]
- 40.Park S., Nhiem N.X., Van Kiem P., Van Minh C., Tai B.H., Kim N., Yoo H.H., Song J.-H., Ko H.-J., Kim S.H. Five new quassinoids and cytotoxic constituents from the roots of Eurycoma longifolia. Bioorganic Med. Chem. Lett. 2014;24:3835–3840. doi: 10.1016/j.bmcl.2014.06.058. [DOI] [PubMed] [Google Scholar]
- 41.Ruan J., Li Z., Zhang Y., Chen Y., Liu M., Han L., Zhang Y., Wang T. Bioactive Constituents from the Roots of Eurycoma longifolia. Molecules. 2019;24:3157. doi: 10.3390/molecules24173157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang W.-Q., Shao X.-H., Deng F., Hu L.-J., Xiong Y., Huang X.-J., Fan C.-L., Jiang R.-W., Ye W.-C., Wang Y. Unprecedented Quassinoids from Eurycoma longifolia: Biogenetic Evidence and Antifeedant Effects. J. Nat. Prod. 2020;83:1674–1683. doi: 10.1021/acs.jnatprod.0c00244. [DOI] [PubMed] [Google Scholar]
- 43.Yang W.-Q., Tang W., Huang X.-J., Song J.-G., Li Y.-Y., Xiong Y., Fan C.-L., Wu Z.-L., Wang Y., Ye W.-C. Quassinoids from the roots of Eurycoma longifolia and their anti-proliferation activities. Molecules. 2021;26:5939. doi: 10.3390/molecules26195939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y., Zhao W., Ruan J., Wichai N., Li Z., Han L., Zhang Y., Wang T. Anti-inflammatory canthin-6-one alkaloids from the roots of Thailand Eurycoma longifolia Jack. J. Nat. Med. 2020;74:804–810. doi: 10.1007/s11418-020-01433-6. [DOI] [PubMed] [Google Scholar]
- 45.Brinckmann J., Brendler T. Tongkat ali Eurycoma longifolia Family: Simaroubaceae. American Botanical Council; Austin, TX, USA: 2019. pp. 6–16. [Google Scholar]
- 46.Abubakar B.M., Salleh F.M., Omar M.S.S., Wagiran A. Assessing product adulteration of Eurycoma longifolia (Tongkat Ali) herbal medicinal product using DNA barcoding and HPLC analysis. Pharm. Biol. 2018;56:368–377. doi: 10.1080/13880209.2018.1479869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fadzil N.F., Wagiran A., Salleh F.M., Abdullah S., Izham N.H.M. Authenticity testing and detection of Eurycoma longifolia in commercial herbal products using bar-high resolution melting analysis. Genes. 2018;9:408. doi: 10.3390/genes9080408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mutschlechner B., Schwaiger S., Tran T.V.A., Stuppner H. Development of a selective HPLC-DAD/ELSD method for the qualitative and quantitative assessment of commercially available Eurycoma longifolia products and plant extracts. Fitoterapia. 2018;124:188–192. doi: 10.1016/j.fitote.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 49.Serag A., Zayed A., Mediani A., Farag M.A. Integrated comparative metabolite profiling via NMR and GC–MS analyses for tongkat ali (Eurycoma longifolia) fingerprinting and quality control analysis. Sci. Rep. 2023;13:2533. doi: 10.1038/s41598-023-28551-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sambandan T., Rha C., Kadir A.A., Aminudim N., Saad J.M. Bioactive Fraction of Eurycoma longifolia. United State Pat. 2006;7:1–9. [Google Scholar]
- 51.Hussein S., Ibrahim R., Kiong A.L.P., Fadzillah N.M., Daud S.K. Multiple shoots formation of an important tropical medicinal plant, Eurycoma Iongifolia Jack. Plant Biotechnol. 2005;22:349–351. doi: 10.5511/plantbiotechnology.22.349. [DOI] [Google Scholar]
- 52.Hussein S., Ibrahim R., Kiong A.L.P. Adventitious Shoots Regeneration from Root and Stem Explants of Eurycoma longifolia Jack—An Important Tropical Medicinal Plants. Int. J. Agric. Res. 2006;1:183–193. doi: 10.3923/ijar.2010.543.553. [DOI] [Google Scholar]
- 53.Hassan N.H., Abdullah R., Kiong L.S., Ahmad A.R., Abdullah N., Zainudin F., Ismail H., Rahman A.S.S. Micropropagation and production of eurycomanone, 9-methoxycanthin-6-one and canthin-6-one in roots of Eurycoma longifolia plantlets. Afr. J. Biotechnol. 2012;11:6818–6825. doi: 10.5897/ajb11.3414. [DOI] [Google Scholar]
- 54.Alttaher A., Yusof Z., Mahmood M., Shaharuddin N. High-frequency induction of multi-ple shoots and plant regeneration from cotyledonary node explants of tongkat ali (Eurycoma longifolia jack) Appl. Ecol. Environ. Res. 2020;18:6321–6333. doi: 10.15666/aeer/1805_63216333. [DOI] [Google Scholar]
- 55.Alttaher A.G.A., Yusof Z.N.B., Shaharuddin N.A. Direct Shoot Organogenesis and Clonal Fidelity Confirmation of Tongkat Ali (Eurycoma longifolia) using Molecular Markers. Int. J. Agric. Biol. 2021;26:9–16. doi: 10.17957/IJAB/15.1802. [DOI] [Google Scholar]
- 56.Fehér A. Callus, dedifferentiation, totipotency, somatic embryogenesis: What these terms mean in the era of molecular plant biology? Front. Plant Sci. 2019;10:536. doi: 10.3389/fpls.2019.00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Siregar L.A.M., Lai-Keng C., Peng-Lim B. Selection of Cell Source and the Effect of pH and MS Macronutrients on Biomass Production in Cell Cultures of Tongkat Ali (Eurycoma longifolia Jack) J. Plant Biotechnol. 2003;5:131–135. [Google Scholar]
- 58.Siregar L.A.M., Keng C.L., Lim B.P. Pertumbuhan dan Akumulasi Alkaloid dalam Kalus dan Suspensi Sel Eurycoma longifolia Jack. J. Ilm. Pertan. Kult. 2006;41:19–27. [Google Scholar]
- 59.Mahmood M., Normi R., Subramaniam S. Distribution of 9-methoxycanthin-6-one from the intact plant parts and callus cultures of Eurycoma longifolia (Tongkat Ali) Aust. J. Crop Sci. 2011;5:1565–1569. [Google Scholar]
- 60.Shim K.-M., Murthy H.N., Park S.-Y., Rusli I., Paek K.-Y. Production of biomass and bioactive compounds from cell suspension cultures of Eurycoma longifolia in balloon type bubble bioreactors. Korean J. Hortic. Sci. Technol. 2015;33:251–258. doi: 10.7235/hort.2015.14102. [DOI] [Google Scholar]
- 61.Rosmaina R., Zulfahmi Z., Sutejo P., Ulfiatun U., Maisupratina M. Induksi Kalus Pasak Bumi (Eurycoma longifolia Jack) Melalui Eksplan Daun Dan Petiol. J. Agroteknologi. 2015;6:33. doi: 10.24014/ja.v6i1.1567. [DOI] [Google Scholar]
- 62.Nhân N.H., Quảng H.T., Lộc N.H. Ảnh Hưởng Của Môi Trường Nuôi Cấy Lên Khả Năng Sinh Trưởng Của Callus Cây Bách Bệnh (Eurycoma longifolia Jack) Hue Univ. J. Sci. Nat. Sci. 2019;128:69–76. doi: 10.26459/hueuni-jns.v128i1E.5451. [DOI] [Google Scholar]
- 63.Rosli N., Maziah M., Chan K.L., Sreeramanan S. Factors affecting the accumulation of 9-methoxycanthin-6-one in callus cultures of Eurycoma longifolia. J. For. Res. 2009;20:54–58. doi: 10.1007/s11676-009-0010-6. [DOI] [Google Scholar]
- 64.Hussein S., Ling A.P.K., Lau C.Y., Ong S.L., Harun A.R. Morphological and Biochemical Responses of Eurycoma longifolia Callus to Gamma Irradiation. Bioremediat. Biodivers. Bioavailab. 2012;6:3–7. [Google Scholar]
- 65.Mahmood M., Normi R., Subramaniam S. Optimization of suitable auxin application in a recalcitrant woody forest plant of Eurycoma longifolia (Tongkat Ali) for callus induction. Afr. J. Biotechnol. 2010;9:8417–8428. [Google Scholar]
- 66.Kumar V., Van Staden J. New insights into plant somatic embryogenesis: An epigenetic view. Acta Physiol. Plant. 2017;39:194. doi: 10.1007/s11738-017-2487-5. [DOI] [Google Scholar]
- 67.Isah T. Induction of somatic embryogenesis in woody plants. Acta Physiol. Plant. 2016;38:118. doi: 10.1007/s11738-016-2134-6. [DOI] [Google Scholar]
- 68.Gray D.J., Purohit A., Triglano R.N. Somatic embryogenesis and development of synthetic seed technology. CRC Crit. Rev. Plant Sci. 1991;10:33–61. doi: 10.1080/07352689109382306. [DOI] [Google Scholar]
- 69.O’brien C., Hiti-Bandaralage J., Folgado R., Hayward A., Lahmeyer S., Folsom J., Mitter N. Cryopreservation of woody crops: The avocado case. Plants. 2021;10:934. doi: 10.3390/plants10050934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guan Y., Li S.-G., Fan X.-F., Su Z.-H. Application of somatic embryogenesis in woody plants. Front. Plant Sci. 2016;7:938. doi: 10.3389/fpls.2016.00938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aziz S., Akeng G., Kandasamy K.I. Induction of somatic embryos from cotyledonary tissue of Tongkat Ali (Eurycoma longifolia) J. Trop. Med. Plants. 2000;1:53–59. [Google Scholar]
- 72.Hussein S., Ibrahim R., Kiong A.L.P., Fadzillah N.M., Daud S.K. Micropropagation of Eurycoma longifolia Jack via Formation of Somatic Embryogenesis. Asian J. Plant Sci. 2005;4:472–485. doi: 10.3923/ajps.2005.472.485. [DOI] [Google Scholar]
- 73.Rahmawati A., Esyanti R.R. Analysis of Secondary Metabolite Production in Somatic Embryo of Pasak Bumi (Eurycoma longifolia Jack.) Procedia Chem. 2014;13:112–118. doi: 10.1016/j.proche.2014.12.014. [DOI] [Google Scholar]
- 74.Dalila Z.D., Hafsah J., Rokiah Z., Rodziah K., Madihah M.N. Thidiazuron induces high frequency direct somatic embryogenesis growth from cotyledon culture of Eurycoma longifolia. Sains Malays. 2015;44:913–920. doi: 10.17576/jsm-2015-4407-01. [DOI] [Google Scholar]
- 75.Mohd N.M., Ja’Afar H., Zawawi D.D., Alias N. In vitro somatic embryos multiplication of Eurycoma longifolia Jack using temporary immersion system RITA®. Sains Malays. 2017;46:897–902. doi: 10.17576/jsm-2017-4606-08. [DOI] [Google Scholar]
- 76.Siregar L.A.M., Chan L.K., Boey P.L. Effect of cell source and pH of culture medium on the production of canthin-6-one alkaloids from the cell cultures of Tongkat Ali (Eurycoma longifolia Jack) Plant Biotechnol. 2004;6:125–130. [Google Scholar]
- 77.Siregar L.A.M., Keng C.L., Lim B.P. Effects of Medium Constituents on Growth and Canthinone Accumulation in Cell Suspension Cultures of Eurycoma longifolia Jack. HAYATI J. Biosci. 2009;16:69–77. doi: 10.4308/hjb.16.2.69. [DOI] [Google Scholar]
- 78.Lim F.C.P., Ling A.P.K., Hii S.L., Hussein S. Towards understanding of physiological changes in cell culture of recalcitrant woody plant, Eurycoma longifolia, in response to carbon and nitrogen sources. J. Med. Plant Res. 2011;5:3200–3209. [Google Scholar]
- 79.Nhan N.H., Loc N.H. Production of eurycomanone from cell suspension culture of Eurycoma longifolia. Pharm. Biol. 2017;55:2234–2239. doi: 10.1080/13880209.2017.1400077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Keng C.L., Wei A.S., Bhatt A. Elicitation effect on cell biomass and production of alkaloids in cell suspension culture of the tropical tree Eurycoma longifolia. UNED Res. J. 2010;2:239–244. doi: 10.22458/urj.v2i2.160. [DOI] [Google Scholar]
- 81.Siregar L.A.M., Lai-Keng C., Peng-Lim B. Effects of Casein Hydrolisate and Light Intensity on Production of Biomass and Canthinone Alkaloid in Cell Suspension Cultures of Pasak Bumi (Eurycoma longifolia Jack) Makara J. Sci. 2010;14:15–21. [Google Scholar]
- 82.Natanael J., Esyanti R.R., Manurung R. Growth Kinetics and Secondary Metabolite Production of Eurycoma longifolia Jack Cell Culture Elicitated by Uv in Flask Scale and Bubble Column Bioreactor Scale. Int. J. Tech. Res. 2014;5:29–32. [Google Scholar]
- 83.Nhan N.H., Loc N.H. Enhancement of eurycomanone biosynthesis in cell culture of long jack (Eurycoma longifolia) by elicitor treatment. J. Plant Biotechnol. 2018;45:340–346. doi: 10.5010/JPB.2018.45.4.340. [DOI] [Google Scholar]
- 84.Kwan L.S., Tan S.Y., Hirata Y., Chan L.-K., Nagaoka Y., Uesato S., Boey P.L. Biotic elicitation at different feeding time in cell suspension cultures of Eurycoma longifolia Jack, a valuable medicinal plant, for enhancement of cytotoxic activity of bioactive compounds against human colon cancer cell line. Vitr. Cell. Dev. Biol.-Plant. 2021;58:15–27. doi: 10.1007/s11627-021-10189-x. [DOI] [Google Scholar]
- 85.Ali W.N.W., Wahab S.S.A., Seman Z., Wahab M.R.A., Yasin M.R.M., Hussein S., Harun A.R., Mohamad A., Ibrahim R. Nuclear Malaysia Technical Convention 2009. Malaysian Nuclear Agency Document Delivery Center; Bangi, Malaysia: 2009. Production of adventitious root of Eurycoma longifolia jack using air- lift bioreactor system; p. 6. [Google Scholar]
- 86.Lulu T., Park S.-Y., Ibrahim R., Paek K.-Y. Production of biomass and bioactive compounds from adventitious roots by optimization of culturing conditions of Eurycoma longifolia in balloon-type bubble bioreactor system. J. Biosci. Bioeng. 2015;119:712–717. doi: 10.1016/j.jbiosc.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 87.Cui X.-H., Murthy H.N., Zhang J.-D., Song H.-L., Jiang Y.-J., Qi W.-W., Li Y.Y., Paek K.-Y., Park S.-Y. Effect of nutritional factors on the accretion of secondary metabolites in Malaysian ginseng adventitious root cultures. Plant Biotechnol. Rep. 2020;14:381–386. doi: 10.1007/s11816-019-00592-7. [DOI] [Google Scholar]
- 88.Giap T.D., Minh N.N., Vinh B.T., Van Loc P. Study on Adventitious Root Formation of Tongkat Ali Tree (Eurycoma longifolia Jack.) by In Vitro Culture. Tạp Chí Khoa Học Công Nghệ Và Thực Phẩm 13. 2017;13:84–88. [Google Scholar]
- 89.Hussein S., Pick Kiong Ling A., Hann Ng T., Ibrahim R., Kee Yoeup P. “Adventitious roots induction of recalcitrant tropical woody plant, Eurycoma longifolia Materials and methods Surface Sterilization of Explants. Rom. Biotechnol. Lett. 2012;17:7026–7035. [Google Scholar]
- 90.Chee F.M., Rathinam X., Danial M., Lam C.K., Qui M.H., Subramaniam S. Effects of methyl-jasmonate on 9-methoxycanthin-6-one content in Eurycoma longifolia (Tongkat ali) root culture. Pak. J. Bot. 2015;47:897–904. [Google Scholar]
- 91.Morey K.J., Peebles C.A.M. Hairy roots: An untapped potential for production of plant products. Front. Plant Sci. 2022;13:2808. doi: 10.3389/fpls.2022.937095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zárate R. Plant Secondary Metabolism Engineering: Methods, Strategies, Advances, and Omics. Chem. Biol. 2010;3:629–668. doi: 10.1016/b978-008045382-8.00068-x. [DOI] [Google Scholar]
- 93.Gutierrez-Valdes N., Häkkinen S.T., Lemasson C., Guillet M., Oksman-Caldentey K.-M., Ritala A., Cardon F. Hairy Root Cultures—A Versatile Tool with Multiple Applications. Front. Plant Sci. 2020;11:33. doi: 10.3389/fpls.2020.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hussain M.J., Abbas Y., Nazli N., Fatima S., Drouet S., Hano C., Abbasi B.H. Root Cultures, a Boon for the Production of Valuable Compounds: A Comparative Review. Plants. 2022;11:439. doi: 10.3390/plants11030439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Balakrishnan B., Julkifle A.L., Alwee S.S.R.S., Keng C.L., Subramaniam S. The Effect of Strain Virulence on Agrobacterium Rhizogenes Transformation Efficiency in Eurycoma longifolia; Proceedings of the 2nd Annual International Conference, Syiah Kuala University & The 8th IMT-GT Uninet Biosciences Conference; Banda Aceh, Indonesia. 22–24 November 2012; pp. 431–433. [Google Scholar]
- 96.Danial M., Keng C.L., Alwee S.S.R.S., Subramaniam S. Hairy roots induction from difficult-to-transform pharmacologically important plant Eurycoma longifolia using wild strains of Agrobacterium rhizogenes. J. Med. Plants Res. 2012;6:479–487. doi: 10.5897/jmpr11.1217. [DOI] [Google Scholar]
- 97.Nazirah A., Nor Hasnida H., Abdul Rashih A., Siti Suhaila A.R., Haliza I., Muhammad Fuad Y. Effects of Different Culture Media on The Growth and Production of Secondary Metabolites in Eurycoma longifolia Hairy−Root Culture. In: Rahim S., Lim H.F., Farhana M.M.H., Mahmudin S., editors. Proceedings of the Conference on Forestry and Forest Products Research 2013; Kuala Lumpur, Malaysia. 12–13 November 2013; Kuala Lumpur, Malaysia: Forest Research Institute Malaysia; 2013. pp. 372–375. [Google Scholar]
- 98.Tran T.T., Nguyen N.T., Pham N.B., Chu H.N., Nguyen T.D., Kishimoto T., Van Chau M., Chu H.H. Hairy root cultures of Eurycoma longifolia and production of anti-inflammatory 9-methoxycanthin-6-one. Nat. Prod. Commun. 2018;13:1934578X1801300507. doi: 10.1177/1934578X1801300507. [DOI] [Google Scholar]
- 99.Nazirah A., Nor-Hasnida H., Ismanizan I., Norlia B., Abdul-Rashih A., Muhammad-Fuad Y., Mohd-Saifuldullah A. Production Of 9-Methoxycanthin-6-One in Elicited Eurycoma longifolia Hairy Root. J. Trop. For. Sci. 2018;30:606–614. doi: 10.26525/jtfs2018.30.4.606614. [DOI] [Google Scholar]
- 100.Valdiani A., Hansen O.K., Nielsen U.B., Johannsen V.K., Shariat M., Georgiev M.I., Omidvar V., Ebrahimi M., Dinanai E.T., Abiri R. Bioreactor-based advances in plant tissue and cell culture: Challenges and prospects. Crit. Rev. Biotechnol. 2018;39:20–34. doi: 10.1080/07388551.2018.1489778. [DOI] [PubMed] [Google Scholar]
- 101.Fan M., An X., Cui X., Jiang X., Piao X., Jin M., Lian M. Production of eurycomanone and polysaccharides through adventitious root culture of Eurycoma longifolia in a bioreactor. Biochem. Eng. J. 2021;171:108013. doi: 10.1016/j.bej.2021.108013. [DOI] [Google Scholar]
- 102.Trang T.T. Nghiên Cứu Tăng Cường Tích Luỹ Một Số Alkaloid Có Hoạt Tính Sinh Học Từ Sinh Khối Rễ Tơ Cây Bá Bệnh (Eurycoma longifolia Jack) Nuôi Cấy Trên Hệ Thống Bioreactor 20 Lít. Bộ Giáo Dục Và Đào Tạo Viện Hàn Lâm Khoa Học Và Công Nghệ Việt Nam; Hanoi, Vietnam: 2020. [Google Scholar]
- 103.Yunos N.M., Amin N.D.M., Jauri M.H., Ling S.K., Hassan N.H., Sallehudin N.J. The In Vitro Anti-Cancer Activities and Mechanisms of Action of 9-Methoxycanthin-6-one from Eurycoma longifolia in Selected Cancer Cell Lines. Molecules. 2022;27:585. doi: 10.3390/molecules27030585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fazili M.A., Bashir I., Ahmad M., Yaqoob U., Geelani S.N. In vitro strategies for the enhancement of secondary metabolite production in plants: A review. Bull. Natl. Res. Cent. 2022;46:35. doi: 10.1186/s42269-022-00717-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Halder M., Sarkar S., Jha S. Elicitation: A biotechnological tool for enhanced production of secondary metabolites in hairy root cultures. Eng. Life Sci. 2019;19:880–895. doi: 10.1002/elsc.201900058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Khan A.K., Kousar S., Tungmunnithum D., Hano C., Abbasi B.H., Anjum S. Nano-elicitation as an effective and emerging strategy for in vitro production of industrially important flavonoids. Appl. Sci. 2021;11:1694. doi: 10.3390/app11041694. [DOI] [Google Scholar]
- 107.Largia M.J.V., Shilpha J., Satish L., Swamy M.K., Ramesh M. Elicitation: An Efficient Strategy for Enriched Production of Plant Secondary Metabolites. In: Swamy M.K., Kumar A., editors. Phytochemical Genomics. Springer; Singapore: 2022. pp. 477–497. [DOI] [Google Scholar]
- 108.Miladinova-Georgieva K., Geneva M., Stancheva I., Petrova M., Sichanova M., Kirova E. Effects of Different Elicitors on Micropropagation, Biomass and Secondary Metabolite Production of Stevia rebaudiana Bertoni—A Review. Plants. 2023;12:153. doi: 10.3390/plants12010153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ramirez-Estrada K., Vidal-Limon H., Hidalgo D., Moyano E., Golenioswki M., Cusidó R.M., Palazon J. Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules. 2016;21:182. doi: 10.3390/molecules21020182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shakya P., Marslin G., Siram K., Beerhues L., Franklin G. Elicitation as a tool to improve the profiles of high-value secondary metabolites and pharmacological properties of Hypericum perforatum. J. Pharm. Pharmacol. 2019;71:70–82. doi: 10.1111/jphp.12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Elshahawy O.A.M., Zeawail M.E.-F., Hamza M.A., Elateeq A.A., Omar M.A. Improving the Production of Total Phenolics and Flavonoids and the Antioxidant Capacity of Echinacea purpurea Callus through Biotic Elicitation. Egypt. J. Chem. 2022;65:137–149. doi: 10.21608/ejchem.2022.145210.6328. [DOI] [Google Scholar]
- 112.Rakesh B., Praveen N. Biotic elicitation mediated in vitro production of L-DOPA from Mucuna pruriens (L.) DC. Cell Cultures. Vitr. Cell. Dev. Biol.-Plant. 2022;58:1077–1089. doi: 10.1007/s11627-022-10303-7. [DOI] [Google Scholar]
- 113.Bavi K., Khavari-Nejad R.A., Najafi F., Ghanati F. Phenolics and terpenoids change in response to yeast extract and chitosan elicitation in Zataria multiflora cell suspension culture. 3 Biotech. 2022;12:163. doi: 10.1007/s13205-022-03235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bhaskar R., Xavier L.S.E., Udayakumaran G., Kumar D.S., Venkatesh R., Nagella P. Biotic elicitors: A boon for the in-vitro production of plant secondary metabolites. Plant Cell Tiss. Organ. Cult. 2022;149:7–24. doi: 10.1007/s11240-021-02131-1. [DOI] [Google Scholar]
- 115.Guru A., Dwivedi P., Kaur P., Pandey D.K. Exploring the role of elicitors in enhancing medicinal values of plants under in vitro condition. S. Afr. J. Bot. 2022;149:1029–1043. doi: 10.1016/j.sajb.2021.10.014. [DOI] [Google Scholar]
- 116.Hashim M., Ahmad B., Drouet S., Hano C., Abbasi B.H., Anjum S. Comparative effects of different light sources on the production of key secondary metabolites in plants in vitro cultures. Plants. 2021;10:1521. doi: 10.3390/plants10081521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Selivanov N.Y., Selivanova O.G., Sokolov O.I., Sokolova M.K., Sokolov A.O., Bogatyrev V.A., Dykman L.A. Effect of gold and silver nanoparticles on the growth of the Arabidopsis thaliana cell suspension culture. Nanotechnol. Russ. 2017;12:116–124. doi: 10.1134/S1995078017010104. [DOI] [Google Scholar]
- 118.Khurshid R., Ullah M.A., Tungmunnithum D., Drouet S., Shah M., Zaeem A., Hameed S., Hano C., Abbasi B.H. Lights triggered differential accumulation of antioxidant and antidiabetic secondary metabolites in callus culture of Eclipta alba L. PLoS ONE. 2020;15:e0233963. doi: 10.1371/journal.pone.0233963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lian T.T., Moe M.M., Kim Y.J., Bang K.S. Effects of different colored leds on the enhancement of biologically active ingredients in callus cultures of Gynura procumbens (lour.) merr. Molecules. 2019;24:4336. doi: 10.3390/molecules24234336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nadeem M., Abbasi B.H., Younas M., Ahmad W., Zahir A., Hano C. LED-enhanced biosynthesis of biologically active ingredients in callus cultures of Ocimum basilicum. J. Photochem. Photobiol. B Biol. 2019;190:172–178. doi: 10.1016/j.jphotobiol.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 121.Tariq U., Ali M., Abbasi B.H. Morphogenic and biochemical variations under different spectral lights in callus cultures of Artemisia absinthium L. J. Photochem. Photobiol. B Biol. 2014;130:264–271. doi: 10.1016/j.jphotobiol.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 122.Younas M., Drouet S., Nadeem M., Giglioli-Guivarc’H N., Hano C., Abbasi B.H. Differential accumulation of silymarin induced by exposure of Silybum marianum L. callus cultures to several spectres of monochromatic lights. J. Photochem. Photobiol. B Biol. 2018;184:61–70. doi: 10.1016/j.jphotobiol.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 123.Ali M., Abbasi B.H. Light-induced fluctuations in biomass accumulation, secondary metabolites production and antioxidant activity in cell suspension cultures of Artemisia absinthium L. J. Photochem. Photobiol. B Biol. 2014;140:223–227. doi: 10.1016/j.jphotobiol.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 124.Beuel A.-K., Jablonka N., Heesel J., Severin K., Spiegel H., Rasche S. LEDitSHAKE: A lighting system to optimize the secondary metabolite content of plant cell suspension cultures. Sci. Rep. 2021;11:23353. doi: 10.1038/s41598-021-02762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Al-Qudah T., Mahmood S.H., Abu-Zurayk R., Shibli R., Khalaf A., Lambat T.L., Chaudhary R.G. Nanotechnology Applications in Plant Tissue Culture and Molecular Genetics: A Holistic Approach. Curr. Nanosci. 2022;18:442–464. doi: 10.2174/1573413717666211118111333. [DOI] [Google Scholar]
- 126.Anjum S., Anjum I., Hano C., Kousar S. Advances in nanomaterials as novel elicitors of pharmacologically active plant specialized metabolites: Current status and future outlooks. RSC Adv. 2019;9:40404–40423. doi: 10.1039/C9RA08457F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mahajan S., Kadam J., Dhawal P., Barve S., Kakodkar S. Application of silver nanoparticles in in-vitro plant growth and metabolite production: Revisiting its scope and feasibility. Plant Cell Tissue Organ Cult. 2022;150:15–39. doi: 10.1007/s11240-022-02249-w. [DOI] [Google Scholar]
- 128.Iqbal Z., Javad S., Naz S., Shah A.A., Shah A.N., Paray B.A., Gulnaz A., Abdelsalam N.R. Elicitation of the in vitro Cultures of Selected Varieties of Vigna radiata L. with Zinc Oxide and Copper Oxide Nanoparticles for Enhanced Phytochemicals Production. Front. Plant Sci. 2022;13:908532. doi: 10.3389/fpls.2022.908532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Song Z., Xingfeng L. Recent Advances in Molecular Marker-Assisted Breeding for Quality Improvement of Traditional Chinese Medicine. Curr. Pharm. Biotechnol. 2021;22:867–875. doi: 10.2174/1389201021666200430121013. [DOI] [PubMed] [Google Scholar]
- 130.Joshi D.C., Zhang K., Wang C., Chandora R., Khurshid M., Li J., He M., Georgiev M., Zhou M. Strategic enhancement of genetic gain for nutraceutical development in buckwheat: A genomics-driven perspective. Biotechnol. Adv. 2020;39:107479. doi: 10.1016/j.biotechadv.2019.107479. [DOI] [PubMed] [Google Scholar]
- 131.Wang C., Chen L., Cai Z.C., Chen C., Liu Z., Liu X., Zou L., Chen J., Tan M., Wei L., et al. Comparative Proteomic Analysis Reveals the Molecular Mechanisms Underlying the Accumulation Difference of Bioactive Constituents in Glycyrrhiza uralensis Fisch under Salt Stress. J. Agric. Food Chem. 2020;68:1480–1493. doi: 10.1021/acs.jafc.9b04887. [DOI] [PubMed] [Google Scholar]
- 132.Wang Z., Zhao M., Cui H., Li J., Wang M. Transcriptomic Landscape of Medicinal Dendrobium Reveals Genes Associated with the Biosynthesis of Bioactive Components. Front. Plant Sci. 2020;11:391. doi: 10.3389/fpls.2020.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mohanta T.K. Advances in Ginkgo biloba research: Genomics and metabolomics perspectives. Afr. J. Biotechnol. 2012;11:15936–15944. doi: 10.5897/ajb12.627. [DOI] [Google Scholar]
- 134.Guo J., Wu Y., Wang G., Wang T., Cao F. Integrated analysis of the transcriptome and metabolome in young and mature leaves of Ginkgo biloba L. Ind. Crop. Prod. 2020;143:111906. doi: 10.1016/j.indcrop.2019.111906. [DOI] [Google Scholar]
- 135.Xu F., Li L., Zhang W., Cheng H., Sun N., Cheng S., Wang Y. Isolation, characterization, and function analysis of a flavonol synthase gene from Ginkgo biloba. Mol. Biol. Rep. 2012;39:2285–2296. doi: 10.1007/s11033-011-0978-9. [DOI] [PubMed] [Google Scholar]
- 136.Chen Q., Yan J., Meng X., Xu F., Zhang W., Liao Y., Qu J. Molecular Cloning, Characterization, and Functional Analysis of Acetyl-CoA C-Acetyltransferase and Mevalonate Kinase Genes Involved in Terpene Trilactone Biosynthesis from Ginkgo biloba. Molecules. 2017;22:74. doi: 10.3390/molecules22010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang K., Jiang L., Wang X., Han H., Chen D., Qiu D., Yang Y. Transcriptome-wide analysis of AP2/ERF transcription factors involved in regulating Taxol biosynthesis in Taxus × media. Ind. Crop. Prod. 2021;171:113972. doi: 10.1016/j.indcrop.2021.113972. [DOI] [Google Scholar]
- 138.Jiang L., Zhang K., Lü X., Yang L., Wang S., Chen D., Yang Y., Qiu D. Characterization and expression analysis of genes encoding Taxol biosynthetic enzymes in Taxus spp. J. For. Res. 2021;32:2507–2515. doi: 10.1007/s11676-021-01290-3. [DOI] [Google Scholar]
- 139.Yanfang Y., Kaikai Z., Liying Y., Xing L., Ying W., Hongwei L., Qiang L., Duanfen C., Deyou Q. Identification and characterization of MYC transcription factors in Taxus sp. Gene. 2018;675:1–8. doi: 10.1016/j.gene.2018.06.065. [DOI] [PubMed] [Google Scholar]
- 140.Li T., Li B., Liao C., Zhang H., Wang L., Fu T., Xue S., Sun T., Xu X., Fan X., et al. Transcriptome analysis provides insights into light condition effect on paclitaxel biosynthesis in yew saplings. BMC Plant Biol. 2022;22:577. doi: 10.1186/s12870-022-03958-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cheng J., Wang X., Liu X., Zhu X., Li Z., Chu H., Wang Q., Lou Q., Cai B., Yang Y., et al. Chromosome-level genome of Himalayan yew provides insights into the origin and evolution of the paclitaxel biosynthetic pathway. Mol. Plant. 2021;14:1199–1209. doi: 10.1016/j.molp.2021.04.015. [DOI] [PubMed] [Google Scholar]
- 142.Chandran H., Meena M., Barupal T., Sharma K. Plant tissue culture as a perpetual source for production of industrially important bioactive compounds. Biotechnol. Rep. 2020;26:e00450. doi: 10.1016/j.btre.2020.e00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jan R., Asaf S., Numan M., Lubna, Kim K.M. Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy. 2021;11:968. doi: 10.3390/agronomy11050968. [DOI] [Google Scholar]
- 144.Liu Q., Yang F., Zhang J., Liu H., Rahman S., Islam S., Ma W., She M. Application of CRISPR/Cas9 in crop quality improvement. Int. J. Mol. Sci. 2021;22:4206. doi: 10.3390/ijms22084206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Marchev A.S., Yordanova Z.P., Georgiev M.I. Green (cell) factories for advanced production of plant secondary metabolites. Crit. Rev. Biotechnol. 2020;40:443–458. doi: 10.1080/07388551.2020.1731414. [DOI] [PubMed] [Google Scholar]
- 146.Rizzo P., Altschmied L., Ravindran B.M., Rutten T., D’auria J.C. The biochemical and genetic basis for the biosynthesis of bioactive compounds in Hypericum perforatum L., one of the largest medicinal crops in Europe. Genes. 2020;11:1210. doi: 10.3390/genes11101210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhu Q., Wang B., Tan J., Liu T., Li L., Liu Y.-G. Plant Synthetic Metabolic Engineering for Enhancing Crop Nutritional Quality. Plant Commun. 2020;1:100017. doi: 10.1016/j.xplc.2019.100017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fadilah W.N., Mohamad O., Zaki A.M., Shamsiah A. Assessment of IRAP Markers to evaluate the genetic diversity of Eurycoma longifolia. Pertanika J. Trop Agric. Sci. 2019;42:921–937. [Google Scholar]
- 149.Lee C.T., Norlia B., Tnah L.H., Lee S.L., Ng C.H., Ng K.K., Nor-Hasnida H., Nurul-Farhanah Z., Suryani C.S., Nur-Nabilah A. Isolation and characterisation of SSR markers in tongkat ali (Eurycoma longifolia) using next-generation sequencing approach. J. Trop. For. Sci. 2018;30:279–291. doi: 10.26525/jtfs2018.30.3.279291. [DOI] [Google Scholar]
- 150.Osman A., Jordan B., Lessard P.A., Muhammad N., Haron M.R., Riffin N.M., Sinskey A.J., Rha C., Housman D.E. Genetic Diversity of Eurycoma longifolia Inferred from Single Nucleotide Polymorphisms. Plant Physiol. 2003;131:1294–1301. doi: 10.1104/pp.012492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tnah L.H., Lee C.T., Lee S.L., Ng K.K.S., Ng C.H., Hwang S.S. Microsatellite markers of an important medicinal plant, Eurycoma longifolia (Simaroubaceae), for DNA profiling. Am. J. Bot. 2011;98:e130–e132. doi: 10.3732/ajb.1000469. [DOI] [PubMed] [Google Scholar]
- 152.Yulita K., Susilowati A., Rachmat H., Hidayat A. DNA barcode of Eurycoma longifolia Jack (Simaroubaceae) from Sumatra, Indonesia based on trnL-F plastid sequence; Proceedings of the Presented at the 1st International Electronic Conference on Forests; Online. 15–30 November 2020; 8074. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.