Abstract
Objective
Cervical squamous intraepithelial lesion (SIL) and cervical cancer are major threats to females’ health and life in China, and we aimed to estimate the economic burden associated with their diagnosis and treatment.
Methods
A nationwide multicenter, cross-sectional, hospital-based survey was conducted in 26 qualified hospitals across seven administrative regions of China. We investigated females who had been pathologically diagnosed with SIL and cervical cancer, and included five disease courses (“diagnosis”, “initial treatment”, “chemoradiotherapy”, “follow-up” and “recurrence/progression/metastasis”) to estimate the total costs. The median and interquartile range (IQR) of total costs (including direct medical, direct non-medical, and indirect costs), reimbursement rate by medical insurance, and catastrophic health expenditures in every clinical stage were calculated.
Results
A total of 3,471 patients in different clinical stages were analyzed, including low-grade SIL (LSIL) (n=549), high-grade SIL (HSIL) (n=803), cervical cancer stage IA (n=226), IB (n=610), IIA (n=487), IIB (n=282), III (n=452) and IV (n=62). In urban areas, the estimated total costs of LSIL and HSIL were  1,637.7 (IQR:
1,637.7 (IQR: 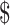 956.4−
956.4−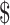 2,669.2) and
2,669.2) and  2,467.1 (IQR:
2,467.1 (IQR: 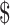 1,579.1−
1,579.1−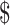 3,762.3), while in rural areas the costs were
3,762.3), while in rural areas the costs were 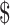 459.0 (IQR:
459.0 (IQR: 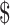 167.7−
167.7−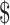 1,330.3) and
1,330.3) and  1,230.5 (IQR:
1,230.5 (IQR: 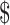 560.6−
560.6− 2,104.5), respectively. For patients with cervical cancer stage IA, IB, IIA, IIB, and III−IV, the total costs were
2,104.5), respectively. For patients with cervical cancer stage IA, IB, IIA, IIB, and III−IV, the total costs were  15,034.9 (IQR:
15,034.9 (IQR:  11,083.4−
11,083.4−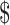 21,632.4),
21,632.4),  19,438.6 (IQR:
19,438.6 (IQR: 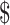 14,060.0−
14,060.0−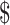 26,505.9),
26,505.9), 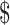 22,968.8 (IQR:
22,968.8 (IQR: 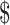 16,068.8−
16,068.8−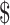 34,615.9),
34,615.9), 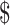 26,936.0 (IQR:
26,936.0 (IQR: 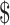 18,176.6−
18,176.6− 41,386.0) and
41,386.0) and  27,332.6 (IQR:
27,332.6 (IQR: 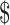 17,538.7−
17,538.7−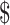 44,897.0), respectively. Medical insurance covered 43%−55% of direct medical costs for cervical cancer patients, while the coverage for SIL patients was 19%−43%. For most cervical cancer patients, the expense was catastrophic, and the extent of catastrophic health expenditure was about twice large for rural patients than that for urban patients in each stage.
44,897.0), respectively. Medical insurance covered 43%−55% of direct medical costs for cervical cancer patients, while the coverage for SIL patients was 19%−43%. For most cervical cancer patients, the expense was catastrophic, and the extent of catastrophic health expenditure was about twice large for rural patients than that for urban patients in each stage.
Conclusions
The economic burden of SIL and cervical cancer in China is substantial, with a significant proportion of the costs being avoidable for patients with LSIL. Even for those with medical insurance, catastrophic health expenditures are also a major concern for patients with cervical cancer, particularly for those living in rural areas.
Keywords: Squamous intraepithelial lesion, cervical cancer, economic burden, medicare, catastrophic health expenditures
Introduction
Cervical cancer is the fifth most commonly diagnosed cancer and the seventh leading cause of mortality in Chinese females (1). China accounts for one-fifth of the global burden of cervical cancer, with an estimated 109,741 new cases and 59,060 deaths in 2020 (2). According to the research on natural history of cervical cancer, it takes at least 10 years for human papillomavirus (HPV) infection to progress to malignancy. Effective interventions for cervical squamous intraepithelial lesion (SIL) can help reduce the disease burden of cervical cancer. However, despite these interventions, the age-standardized incidence and mortality of cervical cancer in China are increasing rapidly at an average annual percentage change (AAPC) of 8.5% and 5.4%, respectively (1). Furthermore, the mean age at cervical cancer diagnosis was 5−10 years younger than that reported before 2000 in China, particularly for the vulnerable population in urban areas (3), seriously threatening their health and quality of life (3,4).
The increasing disease burden of cervical cancer cause considerable economic losses to patients, families, and society. Studies from the United States (5), Canada (6), India (7), and Norway (8) have estimated the economic burden of cervical cancer by considering direct costs, as well as indirect costs associated with individuals who temporarily or permanently leave their employment due to illness. These costs they estimated were used to inform decisions on intervention priorities, assess the effectiveness of existing prevention and treatment programs, and predict expenditures and potential economic impacts on new interventions (6). However, despite several regional studies on treatment-related costs of cervical cancer in China, it is challenging to evaluate the representativeness of the population and the quality of data (9). Also, although diagnosis and treatment guidelines for SIL and cervical cancer have been available in China (10-12), it is unclear about the compliance with the guidelines and what proportion of non-normative treatments were performed. A series of insurance programs aimed to mitigate healthcare inequalities, and the insurance reimbursements policy hugely alleviated the economic burden on patients (13). However, there is little evidence-based research on the impact of cervical cancer insurance reimbursement and whether the out-of-pocket costs are financially devastating for people living in rural and urban areas.
In the present study, we aim to estimate the costs associated with various treatment patterns and payment methods for health care to provide scientific evidence of the economic burden of cervical disease. The results of this study will not only encourage clinicians to adopt standardized clinical treatment patterns, but also facilitate more rational allocation of the health insurance funds for cervical lesions treatment (14). Additionally, detailed and comprehensive costs of SIL and cervical cancer are crucial for policy-makers to assess the cost-effectiveness of cervical cancer prevention and control strategies, as well as to optimize cancer-related policies in China.
Materials and methods
Study design
We conducted a nationwide multicenter cross-sectional, hospital-based survey in China from August 2020 to June 2021. To ensure the nationally representative of the study, a total of 26 qualified hospitals in seven administrative regions of China (namely North, Northeast, East, Central, South, Southwest, and Northwest China) were selected. In each region, three to five hospitals mainly responsible for the diagnosis and treatment of cervical lesions were selected. The number of the study population recruited in each region was allocated depending on the overall sample requirement as well as the proportion of the population in each region to the national population. The number of patients recruited by each hospital was determined by its admitting capacity. Considering the capability of the hospitals in providing diagnosis and treatment services, females with cervical precancerous, including low-grade squamous intraepithelial lesion (LSIL) and high-grade squamous intraepithelial lesion (HSIL), were recruited in the hospitals located in both rural and urban areas. And females with cervical cancer were recruited in the hospitals located in the urban areas. Written informed consent was obtained from all participants. The study has been approved by the Ethics Committees of the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences (NCC/CHCAMS) (No. 22/364-3566).
Study population
The inclusion criteria for our study were as follows: 1) patients diagnosed pathologically with LSIL, HSIL, and invasive cervical cancer stages IA, IB, IIA, IIB, III, and IV; 2) outpatient and inpatient who had a complete diagnosis and treatment information from the surveyed hospitals during their disease course, and the follow-up had been lasted for ≥1 year(s) and ≤5 years; and 3) patients with common chronic diseases could be included in this survey as long as the treatment for these diseases did not have a significant impact on the overall treatment cost for SIL and cervical cancer. The exclusion criteria were as follows: 1) patients who had other primary neoplasms or serious complications like coronary heart disease and organ transplantation; or 2) patients who could not provide valid informed consent.
Disease course
To comprehensively assess the economic burden of the whole disease, we have considered five courses of the disease, including “diagnosis”, “initial treatment”, “chemoradiotherapy”, “follow-up”, and “recurrence/progression/metastasis” (“R/P/M”). The “diagnosis” included the period before the pathology report confirms a diagnosis. The “initial treatment” consisted of surgery or pharmacotherapy for LSIL and HSIL, surgery and preoperative neoadjuvant therapy for stages I and II, and chemoradiotherapy (including patients undergoing surgery) for stages III and IV. It should be noted that patients in “diagnosis” and “initial treatment” were the same person. The “chemoradiotherapy” incorporated radiotherapy and chemotherapy (including patients undergoing chemoradiotherapy alone without surgery) for stages I and II. The “follow-up” was set for all the patients (from LSIL to stage IV) treated or those without treatment. The “R/P/M” was only for patients with HSIL and above.
Relevant definition
Treatment recommendations of Chinese guidelines for SIL and cervical cancer are summarized in Supplementary Table S1. Treatment disparities and non-normativity were defined as treatments inconsistent with the guideline recommendations. Overtreatment occurs when patients receive unnecessary treatment or when simple procedures become complicated. The management of LSIL is complex. According to the Chinese guideline for comprehensive prevention and control of cervical cancer (11), females with LSIL do not usually require treatment. However, for females with pathologically diagnosed LSIL, further management is suggested according to the cytological results to avoid misdiagnosis. In the present study, we defined 86.1% of females with LSIL and who have been treated were overtreatment, considering the misdiagnosis possibilities and its future progression to the HSIL (15,16). And hysterectomy and chemoradiotherapy conducted for HSIL were also deemed excessive. Additionally, loop electrosurgical excision procedure (LEEP) or cold knife conization (CKC) for cervical cancer was non-normative in principle.
Table S1. Summary of treatment recommendations for cervical SIL and cervical cancer in China (10-12).
| Stage | Recommendations |
| SIL, squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; AIS, adenocarcinoma in situ. | |
| LSIL | In principle, no treatment is advised, clinical observation and follow-up instead. |
| HSIL | Adequate colposcopy: cervical conization could be performed, or cervical ablation could be carefully selected. Insufficient colposcopy: cervical conization should be selected. Hysterectomy is not recommended. |
| AIS | For patients who wish not to preserve fertility, hysterectomy is advised. For those who wish to preserve fertility, cervical conization could be performed. |
| IA1 | Patients in stage IA1 who have no fertility requirements are advised to receive extrafascial hysterectomy. If patients wish to preserve fertility, cervical conization could be performed. Patients with negative margins should be followed up regularly. The lymph node metastasis rate of stage IA1 is <1%, hence there is no need for lymph node resection for stage IA1 patients. However, if lymphovascular space is invaded, cervical conization (negative incision margins) or modified radical hysterectomy with pelvic lymphadenectomy should be performed. |
| IA2 | The lymph node metastasis rate for stage IA2 cervical cancer is 3%−5%. Subradical hysterectomy (type II modified radical hysterectomy) and pelvic lymphadenectomy may be required. If patients have fertility requirements, cervical conization (negative incision margins) or radical trachelectomy or pelvic lymphadenectomy may be selected. For patients who wish to preserve fertility, radical trachelectomy may be advised. |
| IB1, IB2, IIA1 | The surgical procedures are type III radical hysterectomy and pelvic lymphadenectomy ± para-aortic lymph node sampling. For patients who wish to preserve fertility and the cervical tumor diameter is ≤2 cm, radical trachelectomy and pelvic lymphadenectomy ± para-aortic lymph node sampling could be performed. |
| IB3, IIA2 | 1) concurrent chemoradiotherapy; 2) radical hysterectomy, pelvic lymph node dissection, para-aortic lymph node sampling, and postoperative individualized adjuvant therapy; 3) hysterectomy after neoadjuvant chemotherapy; 4) adjuvant hysterectomy after concurrent chemoradiation. |
| IIB− IVA |
Concurrent chemoradiation |
| IVB | Systematic treatment is the principal therapy complemented by supportive treatment, with certain patients having palliative surgery or individualized radiotherapy |
Data collection
The main data in this study included basic characteristics (age, ethnicity, home address, occupation, education, marital status, etc.), clinical information (diagnosis and pathology classification, disease courses, treatment patterns, etc.), and costs (direct medical costs, direct non-medical costs, and indirect costs). All costs incurred during the “diagnosis”, “initial treatment”, “chemoradiotherapy”, “follow-up”, and “R/P/M” of each stage of cervical lesions were estimated. For the “follow-up” of the disease, we have collected both the costs that have been spent so far and the follow-up schedule that has been completed to further calculate the total costs of the entire follow-up process. Data collection was conducted through the hospital information systems (HIS) and face-to-face questionnaires. Treatment-related costs of the whole process of cervical disease were collected from a social perspective.
Statistical analysis
We used mean and standard deviation (SD) to describe continuous normally distributed variables such as patients’ age. For categorical variables like home address, occupation, education, marital status, pathology classification, etc., we provided percentages. For skewed distributed costs, we calculated the median and interquartile range [M (IQR)], and Q1−3 IQR and Q3+3 IQR were used to delete outliers of the costs.
The total economic burden of SIL and cervical cancer in China was estimated using a bottom-up approach. The median total costs per patient in every disease course were calculated by summing up the direct medical cost, direct non-medical cost, and indirect cost. Direct medical costs included all medical care expenses associated with the treatment process in the surveyed hospitals such as fees for bunk, examination, assay, drug, treatment, surgery, radiotherapy, chemotherapy, and others. It also covered the expenses of outpatient, inpatient, and drug purchase out of the surveyed hospitals. Direct non-medical costs included fees for transportation, accommodation, nutrition, and caregiver wages due to the disease. Indirect costs were the lost income due to absence from work, which were calculated using the human capital approach. For each patient and family member, we multiplied the days of absence from work due to the illness by the average daily income converted from the annual disposable income per capita.
Our study relied on the probability of a patient undergoing a specific disease course to calculate the median total costs of the corresponding disease course. We obtained these probabilities through the literature review and more details are in Supplementary Table S2. Since the disease course was similar between stages III and IV of cervical cancer, we estimated the mean total costs of the two stages to represent the total cost of stage III−IV.
Table S2. Estimated costs of each stage of cervical lesions throughout disease courses ( ).
).
| Stages | Courses of disease | Median total costs (IQR) | Weight (%) | Reference | Estimated total costs (IQR) |
| IQR, interquartile range; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion. | |||||
| Rural | |||||
| LSIL | Diagnosis | 48.3 (21.4−127.1) | 100 | Assumed | 459.0 (167.7−1,330.3) |
| Initial treatment | 171.4 (56.6−555.8) | 100 | Assumed | ||
| Follow-up | 246.7 (92.4−667.5) | 97.0 | Assumed | ||
| HSIL | Diagnosis | 88.2 (77.1−215.3) | 100 | Assumed | 1,230.5 (560.6−2,104.5) |
| Initial treatment | 717.1 (374.3−1,097.8) | 100 | Assumed | ||
| Follow-up | 408.3 (98.5−763.1) | 97.0 | Assumed | ||
| Recurrence/progress | 1,814.6 (847.0−3,201.3) | 1.6 | (18) | ||
| Urban | |||||
| LSIL | Diagnosis | 285.7 (170.1−481.5) | 100 | Assumed | 1,637.7 (956.4−2,669.2) |
| Initial treatment | 684.4 (379.1−1,067.5) | 100 | Assumed | ||
| Follow-up | 688.2 (419.7−1,154.9) | 97.0 | Assumed | ||
| HSIL | Diagnosis | 293.6 (172.1−497.1) | 100 | Assumed | 2,467.1 (1,579.1−3,762.3) |
| Initial treatment | 1,250.7 (862.7−1,698.2) | 100 | Assumed | ||
| Follow-up | 921.4 (547.1−1,562.7) | 97.0 | Assumed | ||
| Recurrence/progress | 1,814.6 (847.0−3,201.3) | 1.6 | (18) | ||
| National | |||||
| IA | Diagnosis | 354.6 (170.9−749.8) | 100 | (19) | 15,034.9 (11,083.4−21,632.4) |
| Initial treatment | 3,863.1 (3,229.9−5,490.8) | 100 | (19) | ||
| Chemoradiotherapy | 14,313.8 (10,535.9−19,690.5) | 63.5 | (19) | ||
| Follow-up | 1,657.7 (927.4−2,837.8) | 97.0 | (20) | ||
| Recurrence/metastasis | 4,288.0 (3,313.4−4,840.4) | 2.8 | (21) | ||
| IB | Diagnosis | 371.4 (179.3−815.1) | 100 | (19) | 19,438.6 (14,060.0−26,505.9) |
| Initial treatment | 6,554.2 (5,243.3−8,002.1) | 100 | (19) | ||
| Chemoradiotherapy | 12,739.1 (10,214.8−16,915.1) | 63.5 | (19) | ||
| Follow-up | 3,418.4 (1,621.5−6,186.1) | 97.0 | (20) | ||
| Recurrence/metastasis | 10,861.5 (5,668.7−9,284.8) | 10.2 | (21) | ||
| IIA | Diagnosis | 328.6 (185.7−580.0) | 100 | (19) | 22,968.8 (16,068.8−34,615.9) |
| Initial treatment | 6,605.8 (4,974.9−9,751.4) | 100 | (19) | ||
| Chemoradiotherapy | 14,701.9 (10,333.8−19,216.9) | 79.6 | (19) | ||
| Follow-up | 2,448.4 (1,256.7−5,360.0) | 97.0 | (20) | ||
| Recurrence/metastasis | 11,787.7 (8,816.4−22,823.8) | 16.6 | (21) | ||
| IIB | Diagnosis | 487.9 (213.3−1,449.6) | 100 | (19) | 26,936.0 (18,176.6−41,386.0) |
| Initial treatment | 7,481.9 (5,023.7−11,424.8) | 100 | (19) | ||
| Chemoradiotherapy | 13,698.6 (10,579.8−17,820.3) | 79.6 | (19) | ||
| Follow-up | 4,877.0 (1,997.2−9,014.8) | 95.6 | (22) | ||
| Recurrence/metastasis | 15,739.3 (12,077.2−26,428.0) | 21.6 | (21) | ||
| III | Diagnosis | 343.0 (228.5−717.0) | 100 | (19) | 26,243.6 (17,208.5−39,075.6) |
| Initial treatment | 15,016.7 (11,269.3−20,222.1) | 100 | (19) | ||
| Follow-up | 4,989.8 (2,054.0−10,158.9) | 95.6 | (22) | ||
| Recurrence/metastasis | 17,772.6 (10,892.9−24,490.1) | 34.4 | (21) | ||
| IV | Diagnosis | 792.7 (214.6−1,339.0) | 100 | (19) | 28,421.5 (17,869.0−50,718.4) |
| Initial treatment | 12,882.5 (9,083.9−20,620.9) | 100 | (19) | ||
| Follow-up | 9,239.6 (4,391.1−19,633.8) | 95.6 | (22) | ||
| Recurrence/metastasis | 11,263.3 (8,328.8−19,025.9) | 52.5 | (21) | ||
To calculate the reimbursement rate of health insurance, we subtracted the proportion of out-of-pocket medical expenses from the direct medical costs. Additionally, we also calculated the catastrophic health expenditure, which occurs when the out-of-pocket medical expenses exceed 40% of the annual disposable income per capita (17). Wilcoxon rank-sum test was used to compare the difference between subordinate categories of the costs. Statistical analyses were performed using the software R (Version 4.1.1; R Foundation for Statistical Computing, Vienna, Austria). Results were considered statistically significant at P<0.05.
Results
Basic characteristics
A total of 3,471 patients were included in our final analysis, which included LSIL (n=549), HSIL (n=803), stage IA (n=226), IB (n=610), IIA (n=487), IIB (n=282), III (n=452), and IV (n=62). The average age of patients was 49.3±10.9 years old, 32.7% (1,136/3,471) of them were younger than 45 years old. The sample distribution across the seven administrative regions in China was as follows: North (n=310), Northeast (n=290), East (n=915), Central (n=507), South (n=315), Southwest (n=644), and Northwest (n=490). More details are available in Supplementary Table S3.
Table S3. General characteristics of patients with cervical SIL and cervical cancer.
| Variables | n (%) | ||||||||
| LSIL (N=549) | HSIL (N=803) | IA (N=226) | IB (N=610) | IIA (N=487) | IIB (N=282) | III (N=452) | IV (N=62) | Total (N=3,471) | |
| SIL, squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; NA, not available. *, the age of one patient with LSIL and one patient with stage IB was unknown. | |||||||||
| Age (year)* | |||||||||
| <30 | 28 (5.1) | 46 (5.7) | 4 (1.8) | 14 (2.3) | 5 (1.0) | 4 (1.4) | 2 (0.4) | 0 (0) | 103 (3.0) |
| 30−44 | 236 (43.0) | 348 (43.3) | 85 (37.6) | 163 (26.7) | 87 (17.9) | 30 (10.6) | 74 (16.4) | 10 (16.1) | 1,033 (29.8) |
| 45−59 | 253 (46.1) | 331 (41.2) | 104 (46.0) | 343 (56.2) | 280 (57.5) | 153 (54.3) | 253 (56.0) | 38 (61.3) | 1,755 (50.6) |
| ≥60 | 31 (5.6) | 78 (9.7) | 33 (14.6) | 89 (14.6) | 115 (23.6) | 95 (33.7) | 123 (27.2) | 14 (22.6) | 578 (16.7) |
| Area | |||||||||
| North | 60 (10.9) | 74 (9.2) | 20 (8.8) | 43 (7.0) | 31 (6.4) | 42 (14.9) | 34 (7.5) | 6 (9.7) | 310 (8.9) |
| Northeast | 71 (12.9) | 87 (10.8) | 10 (4.4) | 36 (5.9) | 26 (5.3) | 23 (8.2) | 31 (6.9) | 6 (9.7) | 290 (8.4) |
| East | 121 (22.0) | 229 (28.5) | 75 (33.2) | 167 (27.4) | 116 (23.8) | 73 (25.9) | 118 (26.1) | 16 (25.8) | 915 (26.4) |
| Central | 83 (15.1) | 97 (12.1) | 12 (5.3) | 106 (17.4) | 100 (20.5) | 32 (11.3) | 68 (15.0) | 9 (14.5) | 507 (14.6) |
| South | 55 (10.0) | 79 (9.8) | 25 (11.1) | 53 (8.7) | 44 (9.0) | 15 (5.3) | 38 (8.4) | 6 (9.7) | 315 (9.1) |
| Southwest | 102 (18.6) | 127 (15.8) | 52 (23.0) | 111 (18.2) | 87 (17.9) | 52 (18.4) | 99 (21.9) | 14 (22.6) | 644 (18.6) |
| Northwest | 57 (10.4) | 110 (13.7) | 32 (14.2) | 94 (15.4) | 83 (17.0) | 45 (16.0) | 64 (14.2) | 5 (8.1) | 490 (14.1) |
| Residence | |||||||||
| Urban | 252 (45.9) | 390 (48.6) | 86 (38.1) | 207 (33.9) | 148 (30.4) | 99 (35.1) | 112 (24.8) | 17 (27.4) | 1,311 (37.8) |
| Rural | 297 (54.1) | 413 (51.4) | 140 (61.9) | 403 (66.1) | 339 (69.6) | 183 (64.9) | 340 (75.2) | 45 (72.6) | 2,160 (62.2) |
| Occupation | |||||||||
| Famer | 119 (21.7) | 179 (22.3) | 45 (19.9) | 149 (24.4) | 152 (31.2) | 105 (37.2) | 168 (37.2) | 24 (38.7) | 941 (27.1) |
| Worker/staff | 240 (43.7) | 303 (37.7) | 77 (34.1) | 184 (30.2) | 108 (22.2) | 53 (18.8) | 96 (21.2) | 14 (22.6) | 1,075 (31.0) |
| Self-employed | 98 (17.9) | 145 (18.1) | 34 (15.0) | 93 (15.2) | 49 (10.1) | 27 (9.6) | 36 (8.0) | 5 (8.1) | 487 (14.0) |
| Retired | 33 (6.0) | 65 (8.1) | 31 (13.7) | 66 (10.8) | 74 (15.2) | 48 (17.0) | 60 (13.3) | 10 (16.1) | 387 (11.1) |
| Student/unemployed/unknown | 59 (10.7) | 111 (13.8) | 39 (17.3) | 118 (19.3) | 104 (21.4) | 49 (17.4) | 92 (20.4) | 9 (14.5) | 581 (16.7) |
| Education | |||||||||
| Elementary school and below | 96 (17.5) | 186 (23.2) | 50 (22.1) | 169 (27.7) | 193 (39.6) | 121 (42.9) | 185 (40.9) | 27 (43.5) | 1,027 (29.6) |
| Junior high school | 154 (28.1) | 214 (26.7) | 87 (38.5) | 229 (37.5) | 156 (32.0) | 90 (31.9) | 145 (32.1) | 19 (30.6) | 1,094 (31.5) |
| High school/technical secondary school |
108 (19.7) | 154 (19.2) | 49 (21.7) | 125 (20.5) | 89 (18.3) | 52 (18.4) | 101 (22.3) | 13 (21.0) | 691 (19.9) |
| University/college and above | 191 (34.8) | 249 (31.0) | 40 (17.7) | 87 (14.3) | 49 (10.1) | 19 (6.7) | 21 (4.6) | 3 (4.8) | 659 (19.0) |
| Marital status | |||||||||
| Married | 492 (89.6) | 730 (90.9) | 209 (92.5) | 555 (91.0) | 450 (92.4) | 256 (90.8) | 412 (91.2) | 54 (87.1) | 3,158 (91.0) |
| Divorce/cohabitation/widowed | 36 (6.6) | 34 (4.2) | 15 (6.6) | 46 (7.5) | 37 (7.6) | 24 (8.5) | 38 (8.4) | 8 (12.9) | 238 (6.9) |
| Unmarried | 21 (3.8) | 39 (4.9) | 2 (0.9) | 9 (1.5) | 0 (0) | 2 (0.7) | 2 (0.4) | 0 (0) | 75 (2.2) |
| Histological type | |||||||||
| Squamous cell carcinoma | NA | NA | 206 (91.2) | 509 (83.4) | 446 (91.6) | 263 (93.3) | 409 (90.5) | 53 (85.5) | 1,886 (89.0) |
| Adenocarcinoma | NA | NA | 17 (7.5) | 84 (13.8) | 36 (7.4) | 17 (6.0) | 36 (8.0) | 7 (11.3) | 197 (9.3) |
| Adenosquamous carcinoma or others |
NA | NA | 3 (1.3) | 17 (2.8) | 5 (1.0) | 2 (0.7) | 7 (1.5) | 2 (3.2) | 36 (1.7) |
Treatment patterns
The treatment patterns varied among different disease courses in our study. For “initial treatment”, LEEP was selected mostly for LSIL, accounting for 50.7% (116/229) in urban areas and 39.0% (23/59) in rural areas. And 51.3% (153/298) of CKC, 40.6% (121/298) of LEEP, and 2.3% (7/298) of thermal ablation (TA) or modified radical hysterectomy (Type B) were chosen for HSIL in urban areas, while 47.5% (29/61) of LEEP, 37.7% (23/61) of CKC, and 18.0% (11/61) of simple hysterectomy (Type A) were performed in rural areas. The overtreatment rates of LSIL and HSIL were 57.1% and 7.5%, respectively. The main treatment modality for the other disease courses is available in Table 1. For stages I−II and III−IV in “initial treatment”, radical hysterectomy (Type C) (range: 32.7%−54.2%) and concurrent chemoradiotherapy (CCRT) (range: 74.0%−75.0%) were the dominant treatment methods, respectively. Nevertheless, the proportion of non-normative treatment was 16.4% (48/292), 2.5% (6/237), and 0.9% (2/219) for stage I, II, and III, respectively. The main treatment modality for the other disease courses is available in Table 2.
Table 1. Top two or three treatment modalities in each disease course of SIL.
| Stage | Rural areas | Urban areas | |||||||
| Initial treatment | % (n/N) | Follow-up | % (n/N) | Initial treatment | % (n/N) | Follow-up | % (n/N) | ||
| SIL, squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesions; LEEP, loop electrosurgical excision procedure; TCM, Traditional Chinese Medicine; CKC, cold knife conization; Type A, simple hysterectomy; FWT, follow-up without treatment; TA, thermal ablation; Type B, modified radical hysterectomy. | |||||||||
| LSIL | LEEP | 39.0 (23/59) | FWT | 56.9 (33/58) | LEEP | 50.7 (116/229) | FWT | 68.0 (138/203) | |
| TCM | 23.7 (14/59) | TCM | 20.7 (12/58) | CKC | 8.7 (20/229) | TCM | 12.8 (26/203) | ||
| CKC | 3.4 (2/59) | − | − | TCM | 8.7 (20/229) | − | − | ||
| HSIL | LEEP | 47.5 (29/61) | FWT | 67.2 (39/58) | CKC | 51.3 (153/298) | FWT | 81.1 (184/227) | |
| CKC | 37.7 (23/61) | TCM | 20.7 (12/58) | LEEP | 40.6 (121/298) | TCM | 8.4 (19/227) | ||
| Type A | 18.0 (11/61) | − | − | TA/Type B | 2.3 (7/298) | − | − | ||
Table 2. Top two or three treatment modalities in each disease course of cervical cancer.
| Stage | Treatment | |||||||
| Initial treatment | % (n/N) | Chemoradiotherapy | % (n/N) | Follow-up | % (n/N) | R/P/M | % (n/N) | |
| R/P/M, recurrence/progression/metastasis; Type C, radical hysterectomy; CKC, cold knife conization; Type B, modified radical hysterectomy; ET, extensive trachelectomy; UEH, ultra-extensive hysterectomy; PNC, preoperative neoadjuvant chemotherapy; CCRT, concurrent chemoradiotherapy; SC, simple chemotherapy; PC, palliative chemotherapy; PAR, postoperative adjuvant radiotherapy; SR, simple radiotherapy; FWT, follow-up without treatment; TCM, Traditional Chinese Medicine; Type A, simple hysterectomy. | ||||||||
| IA | Type C | 32.7 (35/107) | CCRT | 50.0 (9/18) | FWT | 77.5 (62/80) | Type A | 33.3 (7/21) |
| CKC | 26.2 (28/107) | SC | 33.3 (6/18) | TCM | 6.3 (5/80) | CKC | 28.6 (6/21) | |
| Type B | 15.9 (17/107) | PAR | 16.7 (3/18) | − | − | ET/Type C/CCRT | 9.5 (2/21) | |
| IB | Type C | 42.7 (79/185) | CCRT | 52.2 (109/209) | FWT | 86.3 (151/175) | CCRT | 43.9 (18/41) |
| ET | 18.4 (34/185) | SC | 21.5 (45/209) | TCM | 6.3 (11/175) | SC | 12.2 (5/41) | |
| UEH | 16.8 (31/185) | PAR | 14.4 (30/209) | − | − | UEH | 7.3 (3/41) | |
| IIA | Type C | 54.2 (117/216) | CCRT | 61.0 (75/123) | FWT | 77.2 (98/127) | CCRT | 38.1 (8/21) |
| UEH | 15.7 (34/216) | PAR | 21.1 (26/123) | TCM | 8.7 (11/127) | SC | 33.3 (7/21) | |
| PNC | 15.7 (34/216) | SC | 13.0 (16/123) | − | − | ET/PC | 14.3 (3/21) | |
| IIB | Type C | 52.4 (11/21) | CCRT | 84.7 (127/150) | FWT | 82.0 (73/89) | CCRT | 50.0 (11/22) |
| UEH | 23.8 (5/21) | SR | 10.0 (15/150) | TCM | 10.1 (9/89) | SC | 22.7 (5/22) | |
| CCRT | 23.8 (5/21) | SC | 7.3 (11/150) | − | − | PC | 9.1 (2/22) | |
| III | CCRT | 74.0 (162/219) | CCRT | 71.6 (154/215) | FWT | 69.9 (116/166) | CCRT | 55.2 (37/67) |
| Type C | 10.0 (22/219) | Type C | 10.2 (22/215) | TCM | 12.7 (21/166) | SC | 20.9 (14/67) | |
| SC | 8.7 (19/219) | ET/SC | 8.8 (19/215) | − | − | PC | 7.5 (5/67) | |
| IV | CCRT | 75.0 (21/28) | CCRT | 73.1 (19/26) | FWT | 81.8 (9/11) | CCRT | 39.1 (9/23) |
| SC | 17.9 (5/28) | SC | 19.2 (5/26) | TCM | 18.2 (2/11) | SC | 34.8 (8/23) | |
| UEH/PC | 7.1 (2/28) | UEH/PC | 7.7 (2/26) | − | − | PC | 13.0 (3/23) | |
Estimated total cost per patient and its composition in each clinical stage
For patients with SIL, the estimated total costs of LSIL and HSIL in urban areas were 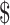 1,637.7 (IQR:
1,637.7 (IQR: 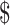 956.4−
956.4− 2,669.2) and
2,669.2) and  2,467.1 (IQR:
2,467.1 (IQR: 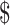 1,579.1−
1,579.1− 3,762.3), whereas
3,762.3), whereas 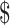 459.0 (IQR:
459.0 (IQR: 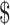 167.7−
167.7−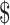 1,330.3) and
1,330.3) and 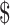 1,230.5 (IQR:
1,230.5 (IQR: 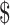 560.6−
560.6−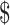 2,104.5) in rural areas. The median costs of the same disease course for SIL were lower in rural areas than those in urban areas (P<0.001). It was higher for HSIL compared to LSIL both in the urban (“diagnosis”: P=0.812, “initial treatment”: P<0.001, “follow-up”: P<0.001) and rural areas (“diagnosis”: P=0.116, “initial treatment”: P<0.001, “follow-up”: P=0.333), respectively. The total costs of invasive cervical cancer were exactly $15,034.9 (IQR:
2,104.5) in rural areas. The median costs of the same disease course for SIL were lower in rural areas than those in urban areas (P<0.001). It was higher for HSIL compared to LSIL both in the urban (“diagnosis”: P=0.812, “initial treatment”: P<0.001, “follow-up”: P<0.001) and rural areas (“diagnosis”: P=0.116, “initial treatment”: P<0.001, “follow-up”: P=0.333), respectively. The total costs of invasive cervical cancer were exactly $15,034.9 (IQR: 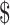 11,083.4−
11,083.4− 21,632.4),
21,632.4),  19,438.6 (IQR:
19,438.6 (IQR: 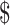 14,060.0−
14,060.0− 26,505.9),
26,505.9), 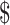 22,968.8 (IQR:
22,968.8 (IQR: 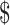 16,068.8−
16,068.8−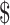 34,615.9),
34,615.9), 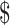 26,936.0 (IQR:
26,936.0 (IQR:  18,176.6−
18,176.6−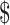 41,386.0), and
41,386.0), and  27,332.6 (IQR:
27,332.6 (IQR: 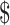 17,538.7−
17,538.7− 44,897.0) for stage IA, IB, IIA, IIB, and III−IV, respectively. As expected, the costs for patients with SIL and cervical cancer generally increased with the severity of the disease. While the costs differences of various clinical stages of cervical cancer in “diagnosis” (P=0.059) and “chemoradiotherapy” (P=0.164) were not statistically significant, but the differences in “initial treatment”, “follow-up”, and “R/P/M” all approached statistical significance with P<0.001. When analyzing differences in disease courses at the same clinical stage, we found marked differences between distinct disease courses (P<0.001). Specifically, for clinical stages I−II, the costs of “chemoradiotherapy” were higher than that of “diagnosis”, “initial treatment”, and “follow-up” (P<0.05), while the differences between “R/P/M” and “chemoradiotherapy” were not statistically significant. For stage III, there were no statistical differences in costs between “initial treatment” and “R/P/M”, both of which were higher than “diagnosis” and “follow-up” (P<0.05). For stage IV, no statistical differences in costs were found in “initial treatment”, “follow-up”, and “R/P/M”, all of those were higher than “diagnosis” (Supplementary Table S2).
44,897.0) for stage IA, IB, IIA, IIB, and III−IV, respectively. As expected, the costs for patients with SIL and cervical cancer generally increased with the severity of the disease. While the costs differences of various clinical stages of cervical cancer in “diagnosis” (P=0.059) and “chemoradiotherapy” (P=0.164) were not statistically significant, but the differences in “initial treatment”, “follow-up”, and “R/P/M” all approached statistical significance with P<0.001. When analyzing differences in disease courses at the same clinical stage, we found marked differences between distinct disease courses (P<0.001). Specifically, for clinical stages I−II, the costs of “chemoradiotherapy” were higher than that of “diagnosis”, “initial treatment”, and “follow-up” (P<0.05), while the differences between “R/P/M” and “chemoradiotherapy” were not statistically significant. For stage III, there were no statistical differences in costs between “initial treatment” and “R/P/M”, both of which were higher than “diagnosis” and “follow-up” (P<0.05). For stage IV, no statistical differences in costs were found in “initial treatment”, “follow-up”, and “R/P/M”, all of those were higher than “diagnosis” (Supplementary Table S2).
The estimated total costs per patient in each clinical stage included direct medical costs ( 440.5−
440.5−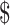 25,304.0 for LSIL to stage III−IV), direct non-medical costs (
25,304.0 for LSIL to stage III−IV), direct non-medical costs ( 18.5−
18.5−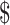 1,040.9), and indirect costs (
1,040.9), and indirect costs (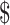 0−
0−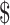 1,267.0). Direct medical costs accounted for the majority (88%−96%) of the total costs, followed by indirect costs (0−10%) and direct non-medical costs (1%−4%). Furthermore, the expenses distribution of different disease courses in direct medical costs varied among clinical stages, which incorporated “diagnosis” (
1,267.0). Direct medical costs accounted for the majority (88%−96%) of the total costs, followed by indirect costs (0−10%) and direct non-medical costs (1%−4%). Furthermore, the expenses distribution of different disease courses in direct medical costs varied among clinical stages, which incorporated “diagnosis” (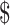 44.6−
44.6−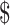 431.2), “initial treatment” (
431.2), “initial treatment” (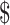 173.2−
173.2−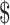 13,605.9), “chemoradiotherapy” (
13,605.9), “chemoradiotherapy” (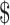 8,927.0−
8,927.0−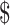 13,605.9), “follow-up” (
13,605.9), “follow-up” ( 222.6−
222.6−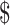 5,249.0), and “R/P/M” (
5,249.0), and “R/P/M” ( 27.1−
27.1−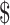 6,017.9) (Figure 1, Supplementary Table S4).
6,017.9) (Figure 1, Supplementary Table S4).
Figure 1.
Estimated total costs and its composition in each stage of cervical lesions. R/P/M, recurrence/progression/metastasis; LSIL-R, low-grade squamous intraepithelial lesion in rural areas; HSIL-R, high-grade squamous intraepithelial lesions in rural areas; LSIL-U, low-grade squamous intraepithelial lesion in urban areas; HSIL-U, high-grade squamous intraepithelial lesions in urban areas.
Table S4. Estimated total cost and its composition in each stage of cervical lesions ($).
| Cost types | Rural areas | Urban areas | IA | IB | IIA | IIB | III−IV | ||||
| LSIL | HSIL | LSIL | HSIL | ||||||||
| LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; R/P/M, recurrence/progression/metastasis. †, all percentages are the proportions of the direct medical cost, direct non-medical cost, and indirect cost in total cost; ‡, all percentages are the proportions of five disease courses in direct medical cost; *, patients with cervical cancer stage III and IV in the “initial treatment” mainly receive chemoradiotherapy. | |||||||||||
| Total cost | 459.0 | 1,230.5 | 1,637.7 | 2,467.1 | 15,034.9 | 19,438.6 | 22,968.8 | 26,936.0 | 27,332.6 | ||
| Direct medical cost† | 440.5 (0.96) |
1,085.8 (0.88) |
1,513.7 (0.92) |
2,224.5 (0.90) |
14,229.4 (0.95) |
18,290.9 (0.94) |
21,691.0 (0.94) |
25,067.3 (0.93) |
25,304.0 (0.93) |
||
| Diagnosis‡ | 44.6 (0.10) |
82.3 (0.08) |
268.7 (0.18) |
271.3 (0.12) |
297.2 (0.02) |
301.4 (0.02) |
279.6 (0.01) |
421.0 (0.02) |
431.2 (0.02) |
||
| Initial treatment‡ | 173.2 (0.39) |
631.7 (0.58) |
632.2 (0.42) |
1,196.6 (0.54) |
3,715.2 (0.26) |
6,292.8 (0.34) |
6,039.6 (0.28) |
7,104.7 (0.28) |
13,605.9 (0.54)* |
||
| Chemoradiotherapy‡ | NA | NA | NA | NA | 8,927.0 (0.63) |
7,943.8 (0.43) |
11,314.6 (0.52) |
10,555.4 (0.42) |
|||
| Follow up‡ | 222.6 (0.51) |
344.7 (0.32) |
612.9 (0.40) |
728.2 (0.33) |
1,181.4 (0.08) |
2,600.1 (0.14) |
2,277.5 (0.10) |
3,613.3 (0.14) |
5,249.0 (0.21) |
||
| R/P/M‡ | NA | 27.1 (0.02) |
NA | 28.3 (0.01) |
108.6 (0.01) |
1,152.8 (0.06) |
1,779.7 (0.08) |
3,373.0 (0.13) |
6,017.9 (0.24) |
||
| Direct non-medical cost† | 18.5 (0.04) |
21.5 (0.02) |
68.5 (0.04) |
94.9 (0.04) |
210.5 (0.01) |
419.8 (0.02) |
443.6 (0.02) |
601.7 (0.02) |
1,040.9 (0.04) |
||
| Indirect cost† | 0 (0) |
123.2 (0.10) |
55.5 (0.03) |
147.6 (0.06) |
595.0 (0.04) |
727.8 (0.04) |
834.2 (0.04) |
1,267.0 (0.05) |
987.7 (0.04) |
||
Reimbursement rate of medical insurance for direct medical costs
For people with medical insurance, the reimbursement rates were 19% and 35% for rural residents with LSIL and HSIL, and the proportions were 34% and 43% for urban residents. Specifically, there was no difference between rural and urban residents with HSIL in “diagnosis”, whereas the reimbursement rates of LSIL in “diagnosis” (P=0.004) and HSIL in “initial treatment” (P=0.01) were higher for urban residents than rural populations. Meanwhile, none of the other differences in disease courses for SIL were statistically significant. Moreover, the reimbursement rate for rural residents with cervical cancer was 46%−55%, and 43%−52% for urban populations. There was no statistically significant difference between rural and urban cancer patients among each disease course in every clinical stage, with exception of the higher reimbursement rate for urban residents with stage IB in the “follow-up” (P=0.003) than rural (Figure 2).
Figure 2.
Reimbursement rate of medical insurance for direct medical costs among rural and urban insured patients in each stage of cervical lesions. LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesions.
Catastrophic health expenditure
The study found that patients with LSIL and HSIL did not experience catastrophic health expenses, whether they lived in urban or rural areas. Out-of-pocket spending accounted for 0.07−0.09 times the annual disposable income per capita separately for insured urban residents and 0.18−0.26 times for insured rural residents. For those without medical insurance, the figures were 0.09−0.11 times for urban residents and 0.23−0.34 times for rural residents. However, patients with stage IB and above faced catastrophic health expenses regardless of their location or insurance status. The out-of-pocket spending was the highest for rural residents without medical insurance, accounting for 1.78−3.59 times the annual disposable income per capita (Figure 3B), while it was the lowest for the urban insured population accounting for 0.50−0.83 times the annual disposable income per capita (Figure 3A). For urban residents with stage IA, the out-of-pocket expenditure was not catastrophic, accounting for 0.22−0.25 times the annual disposable income per capita. However, for rural populations at the same stage, it was catastrophic, accounting for 0.84−0.96 times the annual disposable income per capita. The details are described in Figure 3. For patients with SIL, the proportion of out-of-pocket spending to annual disposable income per capita was higher in those without medical insurance than in those insured (P<0.05), except for urban residents with HSIL, where the difference was not statistically significant. There was no significant difference of the proportion between insured and uninsured cervical cancer patients. Additionally, the severity of catastrophic health expenditure was greater for rural patients than urban patients (P<0.05), except in stage III−IV, where the difference between rural and urban residents without medical insurance was not statistically significant.
Figure 3.
Proportion of out-of-pocket medical expenditures to annual disposable income per capita in each stage of cervical lesions. (A) Urban residents; (B) Rural residents. LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesions.
Discussion
In our study, we mainly found that LSIL was often overtreated, and different degrees of non-normative treatments were conducted for the other clinical stages. The economic burden of SIL and cervical cancer was substantial, with the direct medical costs accounting for the largest proportion of the total costs, and the costs of chemoradiotherapy accounting for about half of the direct medical costs. Additionally, we found that cancer patients often faced catastrophic health expenditures, despite having medical insurance. And such expenditures were not observed among SIL patients. The interventions recommended by global guidelines vary among countries and regions regarding health care accessibility. For patients with SIL, most guidelines suggest follow-up and surveillance for cervical intraepithelial neoplasia (CIN) 1, with treatment for sustained CIN1 lasting two years (23-25), and resection being applicable if colposcopy is unsatisfactory (26). Chinese guidelines recommended follow-up in such cases, and diagnostic conization if necessary (11,27,28). A meta-analysis showed (29) that the annual natural probability of progression from CIN1 to CIN2 and above was 5.7%, much lower than keeping CIN1 (40.0%) or reversing to normal (57.8%). Hence, it was sufficient to develop an individualized follow-up management strategy primarily for LSIL. However, our study found that LSIL was overtreated, and partial treatment patterns for HSIL and cervical cancer were non-normative in China. As a vast territory country, Chinese availability of medical resources and clinicians’ professional capabilities vary greatly among regions at different levels of development. Therefore, it is crucial to strengthen skills training for healthcare providers and increase the assistance of medical resources to enable clinicians to choose optimized individualized treatment strategies according to local medical resources and standard treatment guidelines.
Our study aimed to evaluate the total costs per patient of SIL and cervical cancer with regards to the entire disease course and the ways of payment for healthcare in China. We conducted a national multicenter cross-sectional hospital-based survey to comprehensively analyze the costs. The total costs of LSIL and HSIL in rural areas were lower than those in urban areas, which probably due to the availability of healthcare, frequency of visits, and charging standards. Direct medical costs accounted for the majority of the total costs, and were the dominant treatment-related expenditures for patients. To reduce unnecessary treatment costs fundamentally, it is essential to standardize and transparentize charges items, avoid bundled and repeated fee-for-service, and adjust the charges standards for medical services appropriately.
Furthermore, the composition of costs in direct medical expenses varied among different courses of disease. Specifically, the costs of “chemoradiotherapy” for cancer patients were tremendous. Chemotherapy drugs are extremely expensive, especially imported ones, and advanced medical equipment and high frequency are demanded for radiotherapy, nevertheless, both of which produce side effects. During “chemoradiotherapy”, treatment of complications to maintain physical functions adds an additional financial burden to the disease course. Given the constraints above, the health authorities are commonly faced with the challenge of implementing more effective cancer treatment programs with lower costs, not only in cervical cancer (30). Incorporating more chemotherapy drugs into the medical insurance list and optimizing the radiotherapy regimen would be effective strategies to alleviate the financial burden among cancer patients.
Medical insurance is an effective tool to prevent poverty caused by illness through a risk-sharing mechanism (31). The benefits package, reimbursement rates, and caps of social health insurance programs can affect patients’ behavior when seeking care (32). For patients with SIL, the reimbursement rate was lower for rural residents (19%−35%) than for urban residents (34%−43%). For patients with invasive cancers, the reimbursement rate was generally consistent for both rural and urban residents. However, the annual disposable income per capita was much lower for rural residents (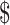 2,447.3) than for urban residents (
2,447.3) than for urban residents ( 6,262.0) (33), leading to greater catastrophic health spending for the former. The situation was even worse for uninsured populations. Out-of-pocket spending reached 3.59 times and 1.97 times the annual disposable income per capita for rural and urban residents without medical insurance, respectively. Although the accessibility and affordability of primary healthcare have significantly improved through increased government funding and universal health insurance coverage since the healthcare reform in 2009, there are still significant challenges that vary greatly across regions. Differences exist not only in financing levels but also in reimbursement ratios and coverage among different medical insurance systems. Optimizing the financing mechanism of medical insurance, increasing the coverage rate, and investment in medicare for rural residents, especially in critical diseases, are significant measures to prevent catastrophic medical expenditure and ensure health equity. Moreover, further promotion of cervical cancer screening is expected to fundamentally prevent the progression of precancerous lesions to invasive cancer, thereby reducing the economic burden associated with cancer treatment.
6,262.0) (33), leading to greater catastrophic health spending for the former. The situation was even worse for uninsured populations. Out-of-pocket spending reached 3.59 times and 1.97 times the annual disposable income per capita for rural and urban residents without medical insurance, respectively. Although the accessibility and affordability of primary healthcare have significantly improved through increased government funding and universal health insurance coverage since the healthcare reform in 2009, there are still significant challenges that vary greatly across regions. Differences exist not only in financing levels but also in reimbursement ratios and coverage among different medical insurance systems. Optimizing the financing mechanism of medical insurance, increasing the coverage rate, and investment in medicare for rural residents, especially in critical diseases, are significant measures to prevent catastrophic medical expenditure and ensure health equity. Moreover, further promotion of cervical cancer screening is expected to fundamentally prevent the progression of precancerous lesions to invasive cancer, thereby reducing the economic burden associated with cancer treatment.
Studies on the economic burden of cervical cancer across China were limited. The treatment costs of cervical cancer were found to be consistent with a modeling study that simulated the lifetime cost of HPV-related diseases (34). Previous field studies only incorporate women with CIN2 and above (35,36), which ignored the additional costs of non-normative treatment of LSIL (CIN1). Meanwhile, our findings were not comparable with the previous studies due to factors such as measurement methods, physician treatment preferences, charges standard and inflation. Previous research primarily focused on direct economic burden alone, localized areas of mainland China, and indirect costs without considering the impact on the patients’ families (35,36) Our study collected nationwide representative data from the perspective of society and considered full courses of the disease. Additionally, the present study included the lost wages of patients along with their families being unable to go to work due to illness, both of which have been ignored by most previous studies.
This study has some notable strengths. First, we comprehensively assessed the cost of diagnosis and treatment for all SIL and cervical cancer stages (from stage LSIL to stage IV) and whole courses of the disease in China. Second, the survey was conducted across seven administrative regions of China, ensuring that the data we collected are truly representative of the economic burden of the disease in the country. However, our study is subject to several limitations. First, direct non-medical and indirect costs were supplementarily collected through questionnaires, which may have led to recalling bias. Second, due to limited available data in China, there were limited possibilities for follow-up on SIL cases, which might underestimate or overestimate the costs of SIL using a substitution rate of 97% of early cervical cancer. Third, the study population was primarily hospital-based, which might have resulted in selection bias in the analysis of LSIL treatment modalities. Further studies are necessary to assess the risk of over-treatment and should be conducted longitudinally on a population basis. Fourth, the psychosocial repercussions for the patient as well as the life-year lost prematurely due to the disease had not been evaluated, that may lead to an underestimation of the total cost.
Conclusions
Our study found that LSIL was sometimes treated too aggressively in China, resulting in unnecessary costs. And non-normative treatment patterns for HSIL and cervical cancer also existed in hospitals located in different regions of China. More attention should be paid to strengthening and improving clinicians’ professional capabilities. The total costs of SIL and cervical cancer were substantial in China. For patients with cervical cancer, even with medical insurance, the cost of treatment could be catastrophic, especially for those living in rural areas. Therefore, rapidly scaling up HPV vaccination and enhancing cervical screening are urgently needed to reduce the incidence of cervical cancer and prevent early precancerous lesions from developing into invasive cancer. These preventive measures could significantly reduce the economic burden of cancer treatment at advanced stages. Furthermore, it is necessary to standardize the current treatment patterns of the disease and increase the medicare payment to effectively reduce the relevant economic burden for the patients. Our study also provides important parameters for the health economic evaluation of health technology assessment as well as strategies for cervical cancer prevention. All above findings could help to inform policy making in the comprehensive tertiary prevention of cervical cancer.
Acknowledgements
This work was supported by the Bill and Melinda Gates Foundation (No. OPP1216421) and CAMS Innovation Fund for Medical Sciences (CIFMS) (No. 2021-I2M-1-004).
Acknowledgements
This work was supported by the Bill and Melinda Gates Foundation (No. OPP1216421) and CAMS Innovation Fund for Medical Sciences (CIFMS) (No. 2021-I2M-1-004).
References
- 1.Zheng R, Zhang S, Zeng H, et al Cancer incidence and mortality in China, 2016. J Natl Cancer Cent. 2022;2:1–9. doi: 10.1016/j.jncc.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, et al Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Li X, Zheng R, Li X, et al Trends of incidence rate and age at diagnosis for cervical cancer in China, from 2000 to 2014. Chin J Cancer Res. 2017;29:477–86. doi: 10.21147/j.issn.1000-9604.2017.06.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Recommendations to assure the quality, safety and efficacy of recombinant human papillomavirus virus-like particle vaccines, Annex 4, TRS No 999. Available online: https://www.who.int/publications/m/item/recombinant-hpv-like-particle-vaccines-annex-4-trs-no-999
- 5.Shah R, Nwankwo C, Kwon Y, et al Economic and humanistic burden of cervical cancer in the United States: Results from a nationally representative survey. J Womens Health (Larchmt) 2020;29:799–805. doi: 10.1089/jwh.2019.7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Righolt CH, Pabla G, Mahmud SM The direct medical costs of diseases associated with human papillomavirus infection in Manitoba, Canada. Appl Health Econ Health Policy. 2018;16:195–205. doi: 10.1007/s40258-017-0367-1. [DOI] [PubMed] [Google Scholar]
- 7.Singh MP, Chauhan AS, Rai B, et al Cost of treatment for cervical cancer in India. Asian Pac J Cancer Prev. 2020;21:2639–46. doi: 10.31557/APJCP.2020.21.9.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hylin H, Thrane H, Pedersen K, et al The healthcare costs of treating human papillomavirus-related cancers in Norway. BMC Cancer. 2019;19:426. doi: 10.1186/s12885-019-5596-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma L, Wang Y, Gao X, et al Economic evaluation of cervical cancer screening strategies in urban China. Chin J Cancer Res. 2019;31:974–83. doi: 10.21147/j.issn.1000-9604.2019.06.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Health Commission of People’s Republic of China Chinese guidelines for diagnosis and treatment of cervical cancer 2018 (English version) Chin J Cancer Res. 2019;31:295–305. doi: 10.21147/j.issn.1000-9604.2019.02.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chinese Society for Colposcopy and Cervical Pathology of China Healthy Birth Science Association Expert consensus on cervical cancer screening and abnormal management in China (second) Zhongguo Fu Chan Ke Lin Chuang Za Zhi (in Chinese) 2017;18:286–8. doi: 10.13390/j.issn.1672-1861.2017.03.041. [DOI] [Google Scholar]
- 12.National Health Commission of the People’s Republic of China National guidelines for diagnosis and treatment of cervical cancer 2022 in China (English version) Chin J Cancer Res. 2022;34:256–69. doi: 10.21147/j.issn.1000-9604.2022.03.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H, Jiang L Catastrophic medical insurance in China. Lancet. 2017;390:1724–5. doi: 10.1016/S0140-6736(17)32603-X. [DOI] [PubMed] [Google Scholar]
- 14.Luengo-Fernandez R, Leal J, Gray A, et al Economic burden of cancer across the European Union: a population-based cost analysis. Lancet Oncol. 2013;14:1165–74. doi: 10.1016/S1470-2045(13)70442-X. [DOI] [PubMed] [Google Scholar]
- 15.Duesing N, Schwarz J, Choschzick M, et al Assessment of cervical intraepithelial neoplasia (CIN) with colposcopic biopsy and efficacy of loop electrosurgical excision procedure (LEEP) Arch Gynecol Obstet. 2012;286:1549–54. doi: 10.1007/s00404-012-2493-1. [DOI] [PubMed] [Google Scholar]
- 16.Egemen D, Cheung LC, Chen X, et al Risk estimates supporting the 2019 ASCCP risk-based management consensus guidelines. J Low Genit Tract Dis. 2020;24:132–43. doi: 10.1097/LGT.0000000000000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cylus J, Thomson S, Evetovits T Catastrophic health spending in Europe: equity and policy implications of different calculation methods. Bull World Health Organ. 2018;96:599–609. doi: 10.2471/BLT.18.209031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L, Liu L, Tao X, et al Analysis of recurrence and its influencing factors in patients with cervical HSIL within 24 months after LEEP. Zhonghua Fu Chan Ke Za Zhi (in Chinese) 2019;54:534–40. doi: 10.3760/cma.j.issn.0529-567x.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Li S, Hu T, Lv W, et al Changes in prevalence and clinical characteristics of cervical cancer in the People’s Republic of China: A study of 10, 012 cases from a nationwide working group. Oncologist. 2013;18:1101–7. doi: 10.1634/theoncologist.2013-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu G, Ren L, Ji W, et al, Analysis of prognostic factors for recurrence of vaginal stump in 493 cases of stage I−IIA cervical cancer after radical hysterectomy. Zhonghua Fang She Zhong Liu Xue Za Zhi (in Chinese) 2019;28:353-7.
- 21.Cao Y. Investigation and analysis of cervical cancer recurrence based on bid data of clinical diagnosis and treatment of cervical cancer in China. Southern Medical University, Guangzhou, 2022.
- 22.Li Z. Analysis between NLR and SII and recurrence for stage IIB−IVA cervical cancer. Hebei University of Engineering, Handan, 2021.
- 23.Perkins RB, Guido RS, Castle PE, et al 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102–31. doi: 10.1097/LGT.0000000000000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bentley J, Bertrand M, Brydon L, et al Colposcopic management of abnormal cervical cytology and histology. J Obstet Gynaecol Can. 2012;34:1188–202. doi: 10.1016/S1701-2163(16)35468-8. [DOI] [PubMed] [Google Scholar]
- 25.Bhatla N, Singhal S, Saraiya U, et al Screening and management of preinvasive lesions of the cervix: Good clinical practice recommendations from the Federation of Obstetrics and Gynaecologic Societies of India (FOGSI) J Obstet Gynaecol Res. 2020;46:201–14. doi: 10.1111/jog.14168. [DOI] [PubMed] [Google Scholar]
- 26.Jordan J, Martin-Hirsch P, Arbyn M, et al European guidelines for clinical management of abnormal cervical cytology, part 2. Cytopathology. 2009;20:5–16. doi: 10.1111/j.1365-2303.2008.00636.x. [DOI] [PubMed] [Google Scholar]
- 27.National Center for Women and Children’s Health. Guidelines for Comprehensive Prevention and Control of Cervical Cancer. Beijing: People’s Medical Publishing House, 2017.
- 28.Committee of Gynecological Oncology, China Anti-Cancer Association Guidelines for the diagnosis and treatment of cervical cancer (2021 edition) Zhongguo Ai Zheng Za Zhi (in Chinese) 2021;31:474–89. doi: 10.19401/j.cnki.1007-3639.2021.06.06. [DOI] [Google Scholar]
- 29.You T, Xia C, Chen M, et al Meta-analysis of annual probability of outcome for different cervical disease states. Zhonghua Yi Xue Za Zhi (in Chinese) 2021;101:1899–907. doi: 10.3760/cma.j.cn112137-20201216-03372. [DOI] [PubMed] [Google Scholar]
- 30.Tang CH, Pwu RF, Tsai IC, et al Costs of cervical cancer and precancerous lesions treatment in a publicly financed health care system. Arch Gynecol Obstet. 2010;281:683–95. doi: 10.1007/s00404-009-1218-6. [DOI] [PubMed] [Google Scholar]
- 31.Dickman SL, Himmelstein DU, Woolhandler S Inequality and the health-care system in the USA. Lancet. 2017;389:1431–41. doi: 10.1016/S0140-6736(17)30398-7. [DOI] [PubMed] [Google Scholar]
- 32.Li X, Lu J, Hu S, et al The primary health-care system in China. Lancet. 2017;390:2584–94. doi: 10.1016/S0140-6736(17)33109-4. [DOI] [PubMed] [Google Scholar]
- 33.National Bureau of Statistics. Resident Income and Consumption in 2020. Available online: https://www.stats.gov.cn/xxgk/sjfb/zxfb2020/202101/t20210118_1812464.html
- 34.Ding W, Ma Y, Ma C, et al The lifetime cost estimation of human papillomavirus-related diseases in China: A modeling study. J Transl Int Med. 2021;9:200–11. doi: 10.2478/jtim-2021-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tao S, Peng J, Wang Y, et al Study on direct economic burden and influencing factors in patients with cervical cancer and precancerous lesions. Zhonghua Yu Fang Yi Xue Za Zhi (in Chinese) 2018;52:1281–6. doi: 10.3760/cma.j.issn.0253-9624.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 36.Peng J, Tao S, Wen Y, et al Cost-effectiveness analysis of cervical cancer screening strategies in urban China. Zhonghua Zhong Liu Za Zhi (in Chinese) 2019;41:154–60. doi: 10.3760/cma.j.issn.0253-3766.2019.02.015. [DOI] [PubMed] [Google Scholar]





