Abstract
Background
Lumenless leads (LLLs) are widely used for left bundle branch area pacing (LBBAP). Recently, stylet-driven leads (SDLs) have also been used for LBBAP.
Objective
The purpose of this study was to evaluate the acute performance of SDLs during LBBAP in comparison with LLLs.
Methods
Consecutive patients undergoing LBBAP for bradycardia or cardiac resynchronization therapy indications at 2 high-volume, early conduction system pacing adopters, tertiary centers were included from January 2019 to July 2023. Patients received either SDLs or LLLs at the discretion of the implanting physician. Acute performance and follow-up data of both lead types were evaluated.
Results
A total of 925 LBBAP implants were included, 655 using LLLs and 270 using SDLs. Overall, LBBAP acute success was significantly higher with LLLs than SDLs (95.3% vs 85.1%, respectively; P <.001) even after the learning curve (97% vs 86%; P = .013). LLLs were implanted in more mid-basal septal positions in comparison with SDLs, which tended to be implanted in more inferior and mid-apical septal positions. Acute lead-related complications were higher with SDLs than LLLs (15.9% vs 6.1%, respectively; P <.001) with 15 cases of lead damage during implant (4.4% vs 0.5%; P <.001) but decreased with acquired experience and were comparable in the last 100 patients included in each group. Lead implant and fluoroscopy times were shorter for SDLs, with lead dislodgment occurring in 0.9% with LLLs and 1.5% with SDLs (P = .489).
Conclusion
Acute lead performance proved to be different between LLLs and SDLs. A specific learning curve should be considered for SDLs even for implanters with extensive previous experience with LLLs.
Keywords: Lumenless leads, Stylet-driven leads, Left bundle branch area pacing, Conduction system pacing, Physiological pacing
Graphical abstract
Key Findings.
-
▪
Acute performance of stylet-driven leads (SDLs) is different compared with lumenless leads (LLLs) during left bundle branch area pacing.
-
▪
Implant success is significantly higher with LLLs.
-
▪
Lead implant and fluoroscopy times are significantly shorter when SDLs are used.
-
▪
SDLs usually are implanted in more inferior and mid-apical septal positions than are LLLs.
-
▪
A specific learning curve should be considered for SDLs even for experienced implanters of LLLs.
Introduction
Conduction system pacing (CSP) has recently evolved as a novel physiological pacing modality.1 Lumenless leads (LLLs) with a fixed, extended helix have been widely used for both His-bundle pacing and left bundle branch area pacing (LBBAP), and most of the worldwide CSP experience has been reported with use of the model 3830 SelectSecureR lead from Medtronic (Minneapolis, MN).2, 3, 4, 5, 6, 7 More recently, stylet-driven leads (SDLs) from different manufacturers have been incorporated into LBBAP.8,9 The design and structural characteristics of both lead types are significantly different and may have an impact on LBBAP outcomes. Detailed data regarding the acute and short-term performance of SDLs in this setting are scarce. The aim of this study was to evaluate the acute performance of SDLs during LBBAP in comparison with LLLs in 2 high-volume CSP centers.
Methods
We conducted an observational retrospective study including consecutive patients undergoing LBBAP for bradycardia or cardiac resynchronization therapy (CRT) indications at 2 institutions (Hospital Universitari i Politècnic La Fe, Valencia, Spain, and Geisinger Heart Institute, Wilkes Barre, Pennsylvania) from January 2019 to July 2023. SDLs were available for CSP at both centers from January 2021, and since then patients have received either SDLs or LLLs at the discretion of the implanting physician. During the implant procedure, all patients were connected to a 12 lead-electrocardiogram (ECG) of an electrophysiological recording system, and unipolar and unfiltered electrograms from the lead tip were recorded and analyzed offline. All ECG measurements were performed offline by an electrophysiological recording system at a sweep speed of 100 mm/s. The institutional review board committee of both institutions approved the study protocol, and all patients gave written informed consent for the implant before the procedure. The research in this study was conducted according to the Helsinki Declaration guidelines on human research.
LLL LBBAP implant technique
The LBBAP implant technique using the 3830 LLL has been previously described.10,11 In brief, after vascular access was obtained, a fixed curve delivery sheath (C315His) with the LLL inside was advanced to the tricuspid annulus. The His-bundle area was located either anatomically using electrogram references or with use of contrast. The sheath then was advanced 15–20 mm toward the right ventricular apex using gentle counterclockwise torque. Unipolar pacing was used to evaluate the pacing morphology from the right side of the interventricular septum looking for a “W” pattern in lead V1 and discordant paced QRS in leads II and III. At this point, the lead was penetrated in the septum using rapid turns under fluoroscopic guidance. Optimal penetration of the lead was monitored using lead tip unfiltered electrogram, unipolar pacing morphology, lead impedance, fixation beats, and/or contrast delivered through the sheath. LBBAP criteria were evaluated, then the sheath was slit and the lead secured to the muscular plane.
SDL LBBAP implant technique
The principles of the implant technique with SDLs were the same as those for LLLs in terms of location of the lead insertion site. SDLs from 3 different manufacturers were used in the study (Solia S60 from Biotronik, Berlin, Germany; 4197-59 Ingevity or Ingevity plus from Boston Scientific, Marlborough, MA; and Tendril 2088TC from Abbott, Sylmar, CA) and implanted using available sheaths (Selectra 42-55, SPSCC 2/3, or His-Pro, respectively). The helix was extended before the lead was introduced into the sheath, and additional rotations on the lead pin were applied to pretension the lead using the stylet insertion tool in order to avoid helix retraction during lead penetration for the Solia lead. For Boston and Abbott SDLs, the helix extraction/retraction tool was connected to the lead pin while the lead was turned, and careful attention was given to helix behavior during lead penetration using fluoroscopy to confirm complete helix exposure. Once the sheath was in an adequate right ventricular septal position, the lead with the helix extended and the stylet fully inserted to the tip was advanced and rapidly rotated. Continuous unipolar pacing via the stylet during lead penetration was used to monitor lead impedance and pacing morphology. Once the electrical parameters and LBBAP criteria were considered adequate, the stylet and the sheath were partially removed from the lead tip, and the sheath was slit.
Definitions and outcomes
Implant success was defined as the presence of left bundle branch pacing (LBBP) or left ventricular septal pacing (LVSP) criteria at the end of the procedure.11 LBBP was considered in the presence of a paced QRS with right bundle branch block morphology and at least 1 of the following criteria: (1) QRS transition (nonselective [NS-LBBP to selective (S)-LBBP or NS-LBBP to LVSP) during threshold testing or programmed stimulation; (2) presence of LBB potential with current of injury; (3) retrograde His-bundle potential with stim-His <35 ms; or (4) left bundle potential–V6 R-wave peak time (RWPT) = stim–V6 RWPT (±10 ms). In the absence of “r” prime wave in lead V1, LBBP was considered if QRS transition during pacing threshold or during programmed ventricular stimulation was present. Patients with evidence of a deep septal position of the lead and paced QRS with right bundle branch block morphology but not fulfilling LBBP criteria were classified as LVSP.
Acute performance of both lead types was evaluated comparing LBBAP implant success rate (defined by the presence of LBBP or LVSP criteria), electrical parameters, ECG characteristics, and lead-related complications. Acute LBBAP lead-related complications included acute LBBAP lead microdislodgment, septal perforation, septal hematoma, and lead damage during implant. Acute lead microdislodgment during implant was defined as the loss of previously achieved LBBAP criteria after sheath slitting without significant change in fluoroscopic lead position occurring before the end of the procedure. Septal perforation was defined as an overt perforation with complete loss of capture identified by contrast injection through the sheath but also included those cases with a significant reduction in the current of injury amplitude (<4 mV) or presence of a QS pattern in the unfiltered channel associated with a sudden increase of the pacing threshold and resulting in a change of the lead deployment position.
Additionally, conduction system capture criteria were assessed at the end of the procedure and before patient discharge by 12-lead ECG performed during asynchronous ventricular pacing (or synchronous pacing in case of absence of intrinsic rhythm) looking for V1 “r” prime wave loss and significant paced QRS widening and change in V6 RWPT >10 ms at working output in comparison with paced QRS morphology obtained during lead implant. Special care was taken to procure comparable precordial lead positions in the electrophysiology laboratory and during predischarge 12 lead-ECG.
All patients underwent pre- and postprocedural transthoracic echocardiography (TTE) to evaluate potential complications and the final ventricular lead position. The final ventricular lead position within the septum was estimated using paced QRS axis (classified as inferior [leads II and III positive], superior [leads II and III predominantly negative], or intermediate [lead II predominantly positive and negative component in lead III]), fluoroscopic orthogonal views (left anterior oblique 30° and right anterior oblique 30°), and postprocedural TTE; and classified as basal, mid-basal, or mid-apical septum by dividing the interventricular septum into 3 parts from the annular region to the apex and the mid-septal area in 2 equivalent portions (mid-basal and mid-apical).
Because the introduction of SDLs for CSP in the 2 participating centers occurred after extensive experience with LLLs, a potential bias toward preferential utilization of LLLs for the a priori most challenging cases was considered. In order to avoid this potential bias, acute lead performance was evaluated in patients without structural heart disease and in the last 100 patients for each lead type to elucidate the influence of the learning curve associated with the introduction of SDLs.
Statistical analysis
Data are given as mean ± SD or median [interquartile range] for continuous variables. For categorical variables, absolute frequencies and percentages are used. Discrete variables were compared using χ2 test. Continuous variables were compared using the Student t test or the analysis of variance as appropriate. Two-tailed P <.05 was considered significant. All analyses were performed using SPSS Version 28.0.1.1 (IBM SPSS Statistics, Armonk, NY).
Results
A total of 925 patients undergoing LBBAP at both participant institutions were included in the study. Baseline characteristics of the patients are given in Table 1. LLLs were used in 655 patients, and SDLs were used in 270 patients (213 Solia S60 leads, 32 Ingevity leads, and 25 Tendril 2088TC leads). Patients in the LLL group more frequently had structural heart disease as well as a CRT indication for pacing, and baseline QRS was significantly wider in comparison with patients in the SDL group. Systematic use of contrast through the sheath after complete lead deployment was used in 610 of 925 patients.
Table 1.
Baseline patient characteristics
| Overall (N = 925) | LLL (n = 655) | SDL (n = 270) | P value | |
|---|---|---|---|---|
| Age (y) | 75 ± 13 | 74 ± 14 | 76 ± 10 | .013 |
| Hypertension | 685 (74) | 480 (73) | 205 (76) | .107 |
| Diabetes | 332 (36) | 239 (37) | 93 (34) | .667 |
| Chronic kidney disease | 339 (37) | 247 (38) | 92 (34) | .495 |
| Structural heart disease | 481 (52) | 372 (57) | 109 (40) | <.001 |
| NYHA functional class | .005 | |||
| I | 350 (38) | 233 (36) | 117 (43) | |
| II | 433 (47) | 311 (47) | 122 (45) | |
| III | 124 (13) | 104 (16) | 20 (7) | |
| IV | 2 (0.2) | 1 (0.2) | 1 (0.4) | |
| Baseline LVEF (%) | 54 ± 15 | 53 ± 15 | 57 ± 12 | <.001 |
| LVEF ≤50% | 259 (28) | 210 (32) | 49 (18) | <.001 |
| Ischemic heart disease | 194 (21) | 158 (24) | 36 (13) | .003 |
| Valvular heart disease | 203 (22) | 141 (22) | 62 (23) | .662 |
| Interventricular septum width (mm) | 12.4 ± 5.1 | 12.2 ± 2.5 | 12.9 ± 8.6 | .042 |
| LA diameter (mm) | 41 ± 8 | 41 ± 8 | 41 ± 7 | .372 |
| Pacing indication | <.001 | |||
| SND | 171 (18) | 97 (15) | 74 (27) | |
| AVB | 554 (60) | 382 (58) | 172 (64) | |
| CRT | 165 (18) | 145 (22) | 20 (7) | |
| Ablate and pace | 35 (4) | 31 (5) | 4 (2) | |
| Baseline QRS duration (ms) | 138 ± 37 | 141 ± 37 | 132 ± 36 | .002 |
| Baseline QRS morphology | <.001 | |||
| Normal | 301 (33) | 198 (30) | 103 (38) | |
| RBBB | 239 (26) | 166 (25) | 73 (27) | |
| LBBB | 201 (22) | 155 (24) | 46 (17) | |
| IVCD | 41 (5) | 25 (4) | 16 (6) | |
| Paced | 128 (14) | 106 (16) | 22 (8) |
Values are given as mean ± SD or n (%) unless other indicated.
AVB = atrioventricular block; CRT = cardiac resynchronization therapy; IVCD = intraventricular conduction disturbance; LA = left atrium; LBBB = left bundle branch block; LLL = lumenless lead; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association; RBBB = right bundle branch block; SDL = stylet-driven lead; SND = sinus node disease.
Implant success, paced QRS characteristics, and electrical parameters
Overall, implant success defined as LBBAP criteria at the end of the procedure was achieved in 92.3% of patients (95.3% of patients with LLLs and 85.1% with SDLs; P <.001) (Table 2). Implant success was significantly higher among patients receiving a pacemaker for bradycardia indications (97.3% for LLLs and 85.9% for SDLs) than those with CRT indications (88.3% for LLLs and 75% for SDLs; P <.001) for the comparison between pacing indications.
Table 2.
Procedure details
| Overall (N = 925) | LLL (n = 655) | SDL (n = 270) | P value | |
|---|---|---|---|---|
| Implanted device type | <.001 | |||
| Single-chamber PM | 75 (8) | 53 (8) | 22 (8) | |
| Dual-chamber PM | 709 (77) | 479 (73) | 230 (85) | |
| CRT device | 141 (15) | 123 (19) | 18 (7) | |
| LBBAP lead implant time (min) | 23 ± 11 | 24 ± 23 | 21 ± 15 | .006 |
| LBBAP implant fluoroscopy (min) | 11 ± 9 | 12 ± 9 | 10 ± 8 | .010 |
| No. of screw attempts | 2.6 ± 1.8 | 2.6 ± 1.8 | 2.5 ± 1.8 | .644 |
| No. of lead turns >15∗ | 308 (51)a | 283 (63)a | 25 (16)a | <.001 |
| LBBAP success | 852 (92) | 624 (95) | 228 (84) | <.001 |
| LBBAP criteria | <.0001 | |||
| LBBP criteria | 575 (62) | 427 (65) | 148 (55) | |
| LVSP criteria | 277 (30) | 197 (30) | 80 (30) | |
| "r" prime in lead V1 | 810 (88) | 588 (90) | 222 (82) | .009 |
| LB potential | 401 (43) | 311 (47) | 90 (33) | <.0001 |
| QRS transition during pacing threshold | 385 (42) | 278 (42) | 107 (40) | .508 |
| V6 RWPT (ms) | 82 ± 16 | 82 ± 16 | 81 ± 15 | .365 |
| V1–V6 interpeak (ms) | 40±13 | 40±13 | 39 ±13 | .379 |
| Paced QRS axis | <.001 | |||
| Inferior | 311 (34) | 246 (38) | 65 (24) | |
| Intermediate | 322 (35) | 227 (35) | 95 (35) | |
| Superior | 264 (29) | 171 (26) | 93 (34) | |
| Septal lead position | <.001 | |||
| Basal | 108 (12) | 83 (13) | 25 (9) | |
| Mid-basal | 699 (76) | 511 (78) | 188 (69) | |
| Mid-apical | 70 (8) | 32 (5) | 38 (14) | |
| R-wave sensing (mV) | 10.8 ± 5.7 | 10.9 ± 5.8 | 10.3 ± 5.3 | .112 |
| Pacing threshold (V) | 0.86 ± 0.39 | 0.80 ± 0.34 | 1.0 ± 0.46 | <.001 |
| Impedance (Ω) | 912 ± 34 | 948 ± 235 | 822 ± 208 | <.001 |
| Final paced QRS duration (from pacing spike) (ms) | 155 ± 19 | 156 ± 20 | 153 ± 17 | .016 |
| Final paced QRS duration (only QRS) (ms) | 115 ± 15 | 115 ± 15 | 114 ± 15 | .193 |
Values are given as n (%) or mean ± SD unless other indicated.
LB = left bundle; LBBAP = left bundle branch area pacing; LBBP = left bundle branch pacing; LVSP = left ventricular septal pacing; PM = pacemaker; RWPT = R-wave peak time; other abbreviations as in Table 1.
Number of lead turns was evaluated in 610 patients (450 with LLLs and 160 with SDLs).
Among patients with successful LBBAP, LBB capture criteria were achieved in 65.2% of cases with LLLs vs 55.2% for SDLs (P <.001,) whereas LVSP was achieved in 30.1% and 29.9%, respectively. Mean LBBAP lead implant time was significantly shorter for patients with SDLs than in those with LLLs (20.9 ± 14.5 minutes vs 24.5 ± 23.2 minutes, respectively; P = .006) as was fluoroscopy time (10.6 ± 8.6 minutes vs 11.8 ± 9.5 minutes, respectively; P = .010). The number of lead turns needed to penetrate the septum was significantly higher for LLLs (63% of LLL patients needed >15 turns vs 16% for SDLs; P <.001). However, the number of attempts to implant the lead was comparable between the 2 lead types (2.5 ± 1.8 for SDLs vs 2.6 ± 1.8 for LLLs; P = .640).
In terms of acute electrical parameters, LBBAP lead pacing threshold was significantly higher and lead impedance significantly lower for SDLs than for LLLs at implant, whereas R-wave sensing was comparable between the 2 groups (Table 3). Up to 27.7% of patients with SDLs had an acute pacing threshold >1.5 V at 0.5 ms in comparison with 11.5% of patients with LLLs (P <.001).
Table 3.
Electrical parameters in the different subgroups
| Overall |
Patients without structural heart disease |
Last 100 patients |
||||
|---|---|---|---|---|---|---|
| LLL (n = 655) | SDL (n = 270) | LLL (n = 280) | SDL (n = 151) | LLL (n = 100) | SDL (n = 100) | |
| Implant electrical parameters | ||||||
| Sensed R-wave amplitude (mV) | 10.9 ± 5.8 | 10.3 ± 5.3 | 11.2 ± 5.5 | 10.4 ± 5.4 | 9.9 ± 5.4 | 10.5 ± 5.4 |
| Unipolar pacing threshold (V) | 0.80 ± 0.34 | 1.0 ± 0.46∗ | 0.79 ± 0.34 | 0.96 ± 0.41∗ | 0.96 ± 0.42 | 1.0 ± 0.55 |
| Unipolar lead impedance (Ω) | 948 ± 235 | 822 ± 208∗ | 940 ± 239 | 788 ± 201∗ | 1041 ± 202 | 838 ± 211∗ |
| Paced QRS duration (from pacing spike) (ms) | 156 ± 20 | 153 ± 17∗ | 149 ± 17 | 149 ± 15 | 164 ± 20 | 154 ± 15∗ |
| Paced QRS duration (only QRS) (ms) | 115 ± 15 | 114 ± 15 | 111 ± 13 | 111 ± 14 | 121 ± 14 | 115 ± 14∗ |
| Last follow-up electrical parameters† | ||||||
| Sensed R-wave amplitude (mV) | 15.4 ± 7.2 | 13.4 ± 5.4∗ | 15.9 ± 7.5 | 12.9 ± 5.1∗ | 17.1 ± 8.5 | 13.1 ± 5.3∗ |
| Unipolar pacing threshold (V) | 0.84 ± 0.94 | 0.88 ± 0.34 | 0.88 ± 0.87 | 0.95 ± 0.37 | 0.85 ± 0.36 | 1.01 ± 0.36∗ |
| Unipolar lead impedance (Ω) | 481 ± 102 | 508 ± 123∗ | 505 ± 101 | 534 ± 102∗ | 512 ± 74 | 556 ± 89∗ |
Values are given as mean ± SD.
Abbreviations as in Table 1.
Indicates statistical significance (P <.05) in comparison of LLLs vs SDLs.
Median follow-up 16.4 [9.6–24.6] months.
Left bundle potentials were more frequently seen with LLLs (47.6% vs 34%; P <.001), whereas the presence of QRS transitions during pacing threshold was comparable between the 2 groups (42.4% for LLLs vs 39.9% for SDLs; P = .508) (Table 2). Paced QRS showed an inferior axis more frequently with LLLs (38.2% for LLLs vs 25.7% for SDLs; P <.001), whereas a superior paced QRS axis was more commonly seen with SDLs (26.6% for LLLs vs 36.8% for SDLs; P = .013) (Supplemental Figures 1 and 2). Postprocedure echocardiographic LBBAP lead position evaluation revealed a basal, mid-basal, and mid-apical septal lead position in 13.2%, 81.7%, and 5.1%, respectively, for LLLs; and in 10%, 74.9%, and 15.1%, respectively, for SDLs (P <.001).
Acute complications
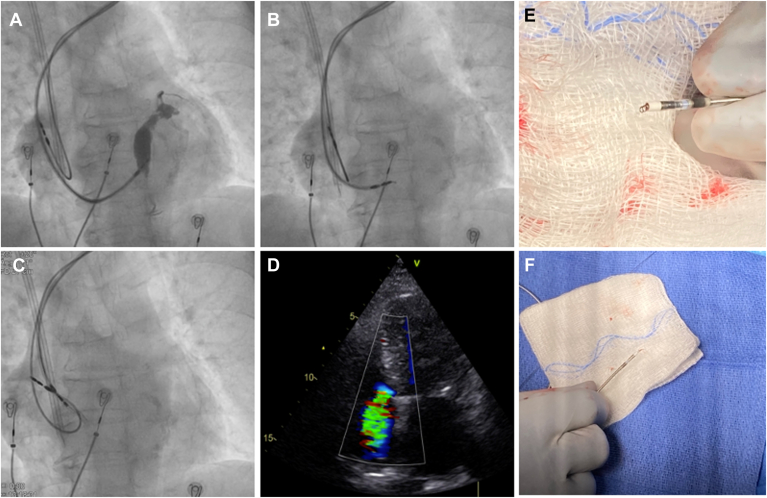
Septal perforation (9.2% for SDLs vs 4.9% for LLLs; P = .021) was significantly more frequent with SDLs but was not associated with any clinical consequence, and all the procedures could be successfully completed without the need for additional interventions (Table 4). Septal hematoma was observed in 2 SDLs cases, with spontaneous resolution in otherwise asymptomatic patients (Figure 1). Lead damage during implant requiring lead replacement due to helix entrapment or distortion occurred in 12 SDLs cases (4.4%) and in 3 LLLs cases (0.5%) (P <.001) (Table 5): 11 with the Solia S60 lead (5.2%); 3 with the 3830 lead (0.5%); and 1 with the 7742-59 Ingevity lead (3%). In 14 of the 15 cases, the lead could be completely removed using counterclockwise lead rotations and simple traction. In the remaining case (Solia S60 lead), the helix was entrapped within the interventricular septum, and counterclockwise rotations of the lead and simple traction could not remove the lead (Figure 2 and Supplemental Videos 1 to 4). Countertraction with the sheath also was unsuccessful, so a locking stylet (LLD, Spectranetics, Colorado Springs, CO) was used to extract the lead. TTE performed in this patient at the end of the procedure revealed severe tricuspid regurgitation that was not present at the beginning. In the remaining 14 cases, no clinical or echocardiographic consequences were noted. Significant distortion of the helix was noted upon lead extraction in all 15 cases (Figure 2).
Table 4.
Complications
| Overall (N = 925) | LLL (n = 655) | SDL (n = 270) | P value | |
|---|---|---|---|---|
| Implant LBBAP lead-related complications | 83 (9.0) | 40 (6.1) | 43 (15.9) | <.001 |
| Septal perforation | 56 (6.1) | 32 (4.9) | 24 (8.9) | .023 |
| Septal hematoma | 2 (0.2) | 0 | 2 (0.7) | .085 |
| Acute lead microdislodgment | 16 (2.1) | 6 (1.1) | 10 (4.7) | .003 |
| Lead damage during implant | 15 (1.6) | 3 (0.5) | 12 (4.4) | <.001 |
| Significant paced QRS changes before discharge | 46 (5.5) | 21 (3.5) | 25 (10.6) | <.001 |
| LBBAP lead dislodgment during follow-up | 10 (1.1) | 6 (0.9) | 4 (1.5) | .489 |
| Non-LBBAP lead-related complications | 13 (2.6) | 11 (3) | 2 (1.5) | .529 |
| Pocket hematoma | 8 (1.3) | 6 (1.3) | 2 (1.3) | 1 |
| Pneumothorax | 4 (0.7) | 4 (0.7) | 0 | .577 |
| Right atrial lead dislodgment | 5 (0.8) | 3 (0.7) | 2 (1.3) | .610 |
| Other | 2 (0.2) | 2 (0.3) | 0 | .549 |
Figure 1.
Acute septal hematoma in a patient who underwent left bundle branch area pacing (LBBAP) using a stylet-driven lead (Solia S60). A: Four-chamber view of transthoracic echocardiography (TTE) performed just before the procedure. B: Same TTE view performed just after the procedure shows significant thickening of the interventricular septum in the region of final LBBAP lead placement, suggesting the presence of a septal hematoma. The patient was completely asymptomatic, and the septal thickening resolved spontaneously after 2 weeks.
Table 5.
Clinical characteristics, tools, and outcomes of patients with a damaged lead during LBBAP lead implant
| Age (y) | Sex | Pacing indication | Structural heart disease | IVS (mm) | Lead type | Sheath | Lead model | Outcome | Clinical consequences | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 85 | M | AV block | Valvular, TAVR | 15.7 | SDL | Selectra 3D 55-42 | Solia S60 | LBBAP with second Solia lead | No |
| 2 | 71 | F | AV block | No | 12 | SDL | Selectra 3D 55-42 | Solia S60 | LBBAP with 3830 | Severe TR |
| 3 | 86 | M | AV block | No | 12 | SDL | SPCC 3 | Ingevity 7742-59 | LBBAP with 3830 | No |
| 4 | 79 | M | AV block | Valvular, TAVR | 10.5 | SDL | Selectra 3D 55-42 | Solia S60 | LBBAP with 3830 | No |
| 5 | 81 | M | AV block | Valvular, TAVR | 14 | SDL | Selectra 3D 55-42 | Solia S60 | Conventional myocardial pacing with second Solia S60 lead | No |
| 6 | 83 | F | AV block | Valvular, TAVR | 14.4 | SDL | Selectra 3D 55-42 | Solia S60 | Conventional myocardial pacing with second Solia S60 lead | No |
| 7 | 73 | M | SSS | No | 10 | LLL | C315His | 3830-69 | LBBAP with second 3830 | No |
| 8 | 81 | M | AV block | No | 14 | LLL | C315His | 3830-69 | LBBAP with second 3830 | No |
| 9 | 75 | F | SSS | No | 8.9 | LLL | C315His | 3830-69 | LBBAP with second 3830 | No |
| 10 | 87 | F | AV block | CAD | 11 | SDL | Selectra 3D 55-42 | Solia S60 | LBBAP with 3830 | No |
| 11 | 52 | M | AV block | NICM | 12 | SDL | Selectra 3D 55-42 | Solia S60 | LBBAP with 3830 | No |
| 12 | 74 | M | AV block | CAD | 9.4 | SDL | Selectra 3D 55-42 | Solia S60 | LBBAP with second Solia lead | No |
| 13 | 81 | F | AV block | CAD | 8.8 | SDL | Selectra 3D 55-42 | Solia S60 | LBBAP with second Solia lead | No |
| 14 | 75 | F | SSS | No | 12 | SDL | Selectra 3D 55-42 | Solia S60 | LBBAP with second Solia lead | No |
| 15 | 81 | M | SSS | CAD | 11 | SDL | Selectra 3D 55-42 | Solia S60 | LBBAP with second Solia lead | No |
Figure 2.
A: Septal contrast staining and visualization of the septal perforation veins during contrast injection through the sheath after penetration of a stylet-driven lead (SDL) (Solia S60) (fluoroscopic left anterior oblique [LAO] 30° view). B: Same patient during lead penetration in a different position in the LAO view. Significant helix distortion was noted, and multiple attempts to unscrew the lead were unsuccessful. C: The lead was extracted with a lead locking device (LLD, Spectranetics) with complete helix elongation. D: TTE performed after the procedure revealed the presence of a severe tricuspid regurgitation that was not present before the implant. E, F: Other examples of helix distortion after screwing attempts in SDLs. Abbreviations as in Figure 1.
LBBAP lead acute microdislodgment after sheath slitting, defined as significant paced QRS morphology changes (loss of previously present “r” prime wave in V1, loss of previously present LBB capture criteria, and/or paced QRS widening), occurred more frequently with SDLs than with LLLs (4.7% vs 1.1%, respectively; P = .003) (Figure 3). None of these cases underwent lead reposition following the implant physician criteria because either myocardial capture or LVSP still was present in all cases. Overall, acute implant LBBAP lead-related complications (including septal perforation, septal hematoma, lead damage, and acute LBBAP lead microdislodgment) were significantly higher for SDLs than LLLs (15.9% vs 6.1%, respectively; P <.001) (Table 4). Contrast injection through the sheath after lead deployment was used in 610 patients (450 LLLs and 160 SDLs) and resulted in visualization of septal perforator veins or septal contrast staining in 5.6% (9/160 cases) of cases with SDLs and 2% (9/450 cases) with LLLs (P = .028).
Figure 3.
Examples of left bundle branch area pacing lead microdislodgment after implant (24 hours later) before discharge in a patient with stylet-driven lead (SDL) (left) and a patient with lumenless lead (LLL) (right). In both cases, significant paced QRS changes can be observed with loss of the “r” prime wave in lead V1 and QRS duration prolongation. Paper speed = 25 mm/s.
Significant paced QRS morphology changes occurring after the implant but before hospital discharge were documented more frequently in SDLs than LLLs (10.6% vs 3.5% LLLs, respectively; P <.001).
Patients without structural heart disease
When exclusively considering patients without structural heart disease (280 LLLs and 151 SDLs), implant success rate still was significantly higher in the LLL than the SDL group (98.6% vs 91.3%, respectively; P = .001), with LBB capture criteria present in 77.5% of LLLs and 70.7% of SDLs (P = .001). Lead location based on TTE was more frequently basal or mid-basal in the LLL group, with more mid-apical septal lead locations in the SDL group (6.7% LLLs vs 17% SDLs, respectively; P = .004). An inferiorly directed paced QRS axis was significantly more frequent for LLLs than SDLs (33.9% vs 21.9%, respectively; P = .011), whereas a superiorly directed paced QRS axis was more frequently seen with SDLs than LLLs (34.4% vs 24.5%; P = .033). Lead implant time (20.6 ± 16 minutes for SDLs vs 24.4 ± 22 minutes for LLLs; P = .023) and fluoroscopy time (9.7 ± 8.2 minutes vs 11.3 ± 8.3 minutes, respectively, P = .032) were significantly lower for SDLs. Acute lead-related complications were also more frequent with SDLs than LLLs (11.3% vs 3.9%, respectively; P = .007) in this subgroup, including a higher rate of lead damage during implant requiring lead replacement (4% for SDLs vs 1.1% for LLLs; P = .072) and lead perforations (6.5% for SDLs vs 2.5% for LLLs; P = .106), which did not reach statistical significance.
Learning curve
Considering the potential influence of the learning curve on lead implant outcomes, the last 100 patients of both groups were evaluated. Acute implant success rate was still significantly better for LLLs in this subgroup (97% vs 86%; P = .013). However, the percentage of patients showing LBB capture criteria was comparable (60% for LLLs vs 59% for SDLs; P = 1). Lead position within the septum evaluated by TTE showed a basal position in 21.6% of LLLs vs 12.2% for SDLs; mid-basal septal position in 72.2% vs 69.4%, respectively; and mid-apical position in 6.2% vs 18.4%, respectively (P = .014). The paced QRS axis was inferior in 57% of LLLs vs 31% of SDLs (P <.001) and superior in 21% vs 35%, respectively (P = .027).
Although numerically higher with SDLs, acute LBBAP lead-related complications were comparable between the last 100 patients of both groups (9% for LLLs vs 14% for SDLs, respectively; P = .376), with a similar rate of septal perforation (9% for LLLs vs 8% for SDLs). There were 3 cases of acute lead microdislodgment, 2 cases of lead damage during implant, and 2 septal hematomas in the SDL group, whereas none of these events occurred in the LLL group.
Lead performance during follow-up
With median follow-up of 16.4 [9.6–24.6] month, electrical parameters remained stable with comparable pacing threshold between the 2 groups but with higher R-wave sensing values and lower unipolar pacing impedance values for LLLs than for SDLs (Table 3). Unipolar pacing threshold >2.5 V at last follow-up was seen in 2 patients in the LLL group and in 1 patient in the SDL group. Lead dislodgment occurred in 10 patients (1.5%): 6 (0.9%) in the LLL group and 4 (1.5%) in the SDLs group (P = .489). Five of the 10 patients underwent lead revision (3 in the LLLs group and 2 in the SDLs group). In the remaining 5 patients, myocardial capture with adequate electrical parameters was still present, and no further interventions were performed.
Discussion
The principal findings of this study are as follows. (1) LLLs were associated with a significantly higher implant success rate (either LBBP capture or LVSP) even after the learning curve. (2) SDLs usually were implanted in more inferior and less basal positions in the septum than were LLLs. (3) Acute complications were significantly higher with SDLs, especially lead damage, septal perforation, and acute lead microdislodgment, after sheath slitting. (4) Significant QRS morphology changes after implant but before hospital discharge were more frequent in SDLs. (5) SDLs were associated with faster lead implant and a reduction in fluoroscopy time. (6) Acquired experience with SDLs significantly decreased acute lead-related complications, which were comparable in the last 100 patients included in each group.
Different sheath delivery and lead design may play a significant role during LBBAP lead implantation. Most of the previously published experience with LBBAP has been achieved with the SelectSecure 3830 lead, which is a 4.1F LLL with a 1.8-mm electrically active fixed helix delivered through the C315His or C304His sheath. In contrast, SDLs have a larger lead diameter (5.6F for the Solia S lead, 5.7F for the 2088TC Tendril lead, and 6F for the Ingevity lead) and an extendable/retractable helix with a variable length (1.8–2 mm). Limited experience with SDLs in the setting of LBBAP has been reported.7, 8, 9,12, 13, 14, 15 In a multicenter experience, de Pooter et al9 reported the results of 353 patients who underwent LBBAP using SDLs (Biotronik Solia S60 lead), with a 94% implant success rate defined as either LBBP (73%) or LVSP (27%). Interestingly, these results were obtained in centers with a wide range of experience (1 of the 8 participating centers contributed 162 patients and 5 centers contributed <40 patients). A low rate of complications was reported with 1.4% lead revision at mean follow-up of 9 ± 5 months, 2% septal perforations, and 1.4% septal coronary artery fistulae. However, detailed descriptions of acute lead performance and complications during implant and follow-up are lacking. More recently, Sritharan et al12 prospectively compared a group of 153 patients receiving SDLs from 4 different manufacturers with 153 patients receiving LLLs and showed comparable implant success rates as well as comparable rates of macro- and microdislodgment. However, and in line with our observations, helix damage was significantly higher with SDLs, and, although not statistically significant, macro- and microdislodgment rates were numerically higher with SDLs and loss of conduction system capture during follow-up doubled the rates observed with LLLs.
In our experience, using strict criteria for LBBAP, SDLs were associated with a significantly lower implant success rate than LLLs (84% vs 95%, respectively), with LBB capture criteria achieved in 55% of SDLs and 65% of LLLs. SDLs were implanted in more inferior and mid-apical positions in the interventricular septum as revealed by both the paced QRS axis and the lead position evaluated by TTE after the procedure. Larger lead body diameter and stiffness may play a key role in directing the lead more inferiorly and more apically during implantation. For this reason and despite the higher stiffness of the SDL delivery sheath, the posterior curve of the sheath tends to lose shape, and this is especially relevant after multiple delivery attempts.
A higher rate of acute lead instability early after the implant and before hospital discharge was also noted with SDLs in our series, as expressed by the rate of acute microdislodgment after sheath slitting and the rate of significant paced QRS morphology changes before hospital discharge (Figure 3). Importantly, acute lead instability did not result in significantly different lead performance during follow-up, with lead dislodgment rates comparable between the 2 groups (1.5% for SDLs vs 0.9% for LLLs; P = .489). Lead septal perforation also was significantly more frequent with SDLs, although none of these events had clinical consequences for the patients in our series. Up to 12 cases (4.4%) of lead damage due to helix entrapment resulting in helix elongation/distortion were recorded in the SDL group, resulting in lead replacement without further clinical consequences in 11 of the 12 patients. However, 1 patient experienced tricuspid valve damage related to helix entrapment during lead removal attempts that required additional lead extraction tools and developed severe tricuspid regurgitation that was not present before the procedure. Tan et al13 reported SDL damage during lead implant and repositioning, including helix damage and helix fracture, in up to 31% of patients; in 2 of the patients, helix fragments could not be removed from the interventricular septum. In contradistinction, only 3 cases of lead damage occurred in the LLL group (0.5%), all of them easily resolved with counterclockwise lead rotation without any clinical consequences. Of note, our results highlight the different acute performance of both lead types probably related to different lead design and behavior during LBBAP implantation. Whether the greater acute lead instability can be minimized by changes in lead implant technique or is intrinsically related to lead design remains to be proven, but the incidence of these events, although diminished, still occurred in SDLs after the learning curve despite special care being taken in lead management during the implant procedure. Coaxial disposition of the lead and the delivery sheath has been suggested as a requirement for avoiding SDLs deformation during implant according to an animal model.15 In the same manner, if lead repositioning is needed during the implantation, counterclockwise lead rotation and gentle traction should be applied to remove the lead before any manipulation of the sheath. Of note, in our series, LBBAP could be achieved on the first lead position only in 41.3% without differences between lead types (40% for LLLs vs 44.4% for SDLs; P = .213), which indicates that most patients will need lead repositioning during LBBAP implants.
The study was performed in 2 high-volume CSP implanting centers that were early adopters of the technique, and the introduction of SDLs in their practice occurred after a significant time period using LLLs. As a result, a new learning curve with SDLs occurred, but the use of LLLs for CSP was the standard of care in the 2 centers. This fact inevitably contributed to a selection bias of more complex patients toward the use of LLLs, as expressed by the significantly higher percentage of patients with structural heart disease, lower baseline ejection fraction, and more CRT indications in the LLL group. For this reason, we compared the performance of the 2 lead types depending on the presence of structural heart disease in an attempt to balance the clinical patient’s profile and case complexity between the 2 groups. However, the same findings as in the overall population were observed: higher implant success rate, higher LBB capture criteria, fewer acute lead-related complications, and a more basal and less inferior lead position with LLLs.
In addition, in an attempt to reduce selection bias and the influence of the learning curve on the results, the last 100 patients of each group were compared. This comparison also showed a higher implant success rate for LLLs and more inferior and less basal positions for SDLs. However, acute lead-related complications were comparable in this subgroup of patients, thus highlighting the importance of a specific learning curve with SDLs but still demonstrating 2% lead damage within the SDL group and 3% acute lead dislodgment after sheath slitting.
Of interest, SDLs were associated with significantly lower lead implant time, fluoroscopy time, and number of lead turns to penetrate the septum. The fact that continuous pacing while screwing the lead is easily possible with SDLs as well as the higher torque and push associated with the larger lead diameter and the use of an inserted stylet may explain the reduction in implant time. These findings were also previously described by Braunstein et al16 and may reflect some of the most important features regarding the use of SDLs for CSP, which are the higher penetration power within the septum related to a higher sheath stiffness, the larger lead body diameter, and the presence of an inner lumen with an stylet, all of them providing more power during lead penetration. However, in our series, this did not translate into a lower number of lead penetration attempts for SDLs and probably reflects that these same features make more difficult the appropriate sheath and lead alignment toward the septum and also lead stability during penetration. Also remarkable is the higher incidence of acute lead microdislodgment with SDLs, which could also be explained by the larger lead tip size, more abrupt transition between lead tip and helix diameters, and overall higher lead stiffness resulting in higher tension forces within the septum during beating of the heart.17 The higher proportion of SDL cases with acute pacing threshold >1.5 V but with complete normalization during follow-up would also reflect more extensive local trauma/edema induced by a larger lead tip diameter. In the same manner, contrast staining or visualization of perforator veins was more frequent with SDLs, which also reinforces this hypothesis.
Specific recommendations during SDL implant
Given these previous considerations, special care should be taken when using SDLs for LBBAP both for achieving successful LBBAP as well as preventing potential complications. Based on our experience, specific recommendations should be incorporated to the SDL implant workflow. In contradistinction to LLLs, forward lead pushing should be avoided during SDL lead turning if the lead does not advance smoothly through the septum. The stylet should be fully loaded to the lead tip during penetration to ensure appropriate lead tip orientation because the stylet typically tends to retract during this process. Lead screwing should be immediately stopped if bending of the lead tip is observed. If helix entrapment is suspected, lead counterclockwise turns should be applied without pulling back the lead and before any sheath manipulation to avoid lead entanglement. A specific SDL learning curve should be considered even in the presence of a large previous experience with LLLs for CSP because lead design and behavior during implant are considerably different. Lead and sheath design are rapidly evolving, and the development of new tools should facilitate LBBAP implantation and probably will overcome the current limitations of the technique.
Study limitations
This was an observational and retrospective study, so inherent bias associated with this study design should be taken into consideration. The study was performed at 2 tertiary centers with high CSP volume and experience, so the results should not be extrapolated to a different environment. As discussed in the Methods, a potential bias toward the utilization of LLLs in patients with a more complex clinical profile could be present. In order to avoid this limitation, a subgroup analysis of patients without structural heart disease was included. In the same manner, the influence of the learning curve associated with the introduction of SDLs was also analyzed by comparing the last 100 patients treated with each lead type. Finally, the previous experience using LLLs for His-bundle pacing may have facilitated the transition toward LBBAP with this specific lead type and may have introduced further bias into the results.
Conclusion
The use of LLLs for LBBAP resulted in a significantly higher implant success rate in comparison with SDLs even after the learning curve. SDLs were frequently implanted in a more inferior and mid-apical septal lead position than LLLs, with significantly shorter lead implant and fluoroscopy times. Electrical parameters were stable in both groups, and lead dislodgment rates were comparable during follow-up. Greater experience with SDLs significantly decreased the number of complications. A specific learning curve should be considered for SDLs even for implanters with large previous experience with LLLs.
Acknowledgments
Funding Sources
The authors have no funding sources to disclose.
Disclosures
Óscar Cano reports being s consultant for and receiving research support from Medtronic; and being a consultant for Abbott, Biotronik, Boston Scientific, and MicroPort. Faiz A. Subzposh reports honoraria from Medtronic. Pugazhendi Vijayaraman reports honoraria, consultant, research, and fellowship support from Medtronic; being a consultant for Abbott and Eaglepoint LLC; honoraria from Boston Scientific and Biotronik; and patent for a His-bundle pacing delivery tool. Syeda Atiqa Batul reports honoraria from Medtronic. All other authors have no conflicts of of interest to disclose.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
All patients gave written informed consent for the implant before the procedure.
Ethics Statement
The institutional review board committee of both institutions approved the study protocol. The research in this study was conducted according to the Helsinki Declaration guidelines on human research.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hroo.2023.11.014.
Appendix. Supplementary Data
References
- 1.Vijayaraman P., Chelu M.G., Curila K., et al. Cardiac conduction system pacing: a comprehensive update. JACC Clin Electrophysiol. 2023 doi: 10.1016/j.jacep.2023.06.005. S2405-500X(23)00391-2. [DOI] [PubMed] [Google Scholar]
- 2.Kircanski B., Boveda S., Prinzen F., et al. Conduction system pacing in everyday clinical practice: EHRA physician survey. Europace. 2023;25:682–687. doi: 10.1093/europace/euac201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keene D., Anselme F., Burri H., et al. Conduction system pacing, a European survey: insights from clinical practice. Europace. 2023;25 doi: 10.1093/europace/euad019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdelrahman M., Subzposh F.A., Beer D., et al. Clinical outcomes of His bundle pacing compared to right ventricular pacing. J Am Coll Cardiol. 2018;71:2319–2330. doi: 10.1016/j.jacc.2018.02.048. [DOI] [PubMed] [Google Scholar]
- 5.Sharma P.S., Patel N.R., Ravi V., et al. Clinical outcomes of left bundle branch area pacing compared to right ventricular pacing: results from the Geisinger-Rush Conduction System Pacing Registry. Heart Rhythm. 2022;19:3–11. doi: 10.1016/j.hrthm.2021.08.033. [DOI] [PubMed] [Google Scholar]
- 6.Vijayaraman P., Sharma P.S., Cano Ó., et al. Comparison of left bundle branch area pacing and biventricular pacing in candidates for resynchronization therapy. J Am Coll Cardiol. 2023;82:228–241. doi: 10.1016/j.jacc.2023.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Jastrzębski M., Kiełbasa G., Cano O., et al. Left bundle branch area pacing outcomes: the multicentre European MELOS study. Eur Heart J. 2022;43:4161–4173. doi: 10.1093/eurheartj/ehac445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Pooter J., Calle S., Timmermans F., Van Heuverswyn F. Left bundle branch area pacing using stylet-driven pacing leads with a new delivery sheath: a comparison with lumen-less leads. J Cardiovasc Electrophysiol. 2021;32:439–448. doi: 10.1111/jce.14851. [DOI] [PubMed] [Google Scholar]
- 9.De Pooter J., Ozpak E., Calle S., et al. Initial experience of left bundle branch area pacing using stylet-driven pacing leads: a multicenter study. J Cardiovasc Electrophysiol. 2022;33:1540–1549. doi: 10.1111/jce.15558. [DOI] [PubMed] [Google Scholar]
- 10.Huang W., Chen X., Su L., Wu S., Xia X., Vijayaraman P. A beginner's guide to permanent left bundle branch pacing. Heart Rhythm. 2019;16:1791–1796. doi: 10.1016/j.hrthm.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 11.Burri H., Jastrzebski M., Cano Ó., et al. EHRA clinical consensus statement on conduction system pacing implantation: endorsed by the Asia Pacific Heart Rhythm Society (APHRS), Canadian Heart Rhythm Society (CHRS), and Latin American Heart Rhythm Society (LAHRS) Europace. 2023;25:1208–1236. doi: 10.1093/europace/euad043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sritharan A., Kozhuharov N., Masson N., Bakelants E., Valiton V., Burri H. Procedural outcome and follow-up of stylet-driven leads compared with lumenless leads for left bundle branch area pacing. Europace. 2023;25 doi: 10.1093/europace/euad295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan E.S.J., Lee J.Y., Boey E., et al. Use of extendable helix leads for conduction system pacing: differences in lead handling and performance lead design impacts conduction system pacing. J Cardiovasc Electrophysiol. 2022;33:1550–1557. doi: 10.1111/jce.15528. [DOI] [PubMed] [Google Scholar]
- 14.le Polain de Waroux J.B., Wielandts J.Y., Gillis K., et al. Repositioning and extraction of stylet-driven pacing leads with extendable helix used for left bundle branch area pacing. J Cardiovasc Electrophysiol. 2021;32:1464–1466. doi: 10.1111/jce.15030. [DOI] [PubMed] [Google Scholar]
- 15.Okubo Y., Miyamoto S., Oguri N., et al. Lead deformation of the stylet-driven lead in left bundle branch area pacing. Heart Rhythm. 2023;20:781–782. doi: 10.1016/j.hrthm.2023.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Braunstein E.D., Kagan R.D., Olshan D.S., et al. Initial experience with stylet-driven versus lumenless lead delivery systems for left bundle branch area pacing. J Cardiovasc Electrophysiol. 2023;34:710–717. doi: 10.1111/jce.15789. [DOI] [PubMed] [Google Scholar]
- 17.Jastrzębski M., Moskal P., Hołda M.K., et al. Deep septal deployment of a thin, lumenless pacing lead: a translational cadaver simulation study. Europace. 2020;22:156–161. doi: 10.1093/europace/euz270. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.