Abstract
Background
Previous studies on the distribution of mycetoma globally have failed to identify Ecuador as an endemic country.
Methods
We present data on 35 cases of mycetoma in Ecuador between 1955 and 2021: 5 cases from our experience and 30 cases from the literature.
Results
Eight cases of eumycetoma (23%) and 27 cases of actinomycetoma (77%) were diagnosed. Most cases originated from the coastal region of Ecuador.
Conclusions
For the first time in an English-language publication, this communication confirms the presence of mycetoma in Ecuador, securing Ecuador's position on the global mycetoma map.
Keywords: mycetoma, neglected tropical disease, skin infections
Introduction
Mycetoma is a neglected tropical disease. It is a chronic destructive infection of subcutaneous tissue and deeper structures, including bone, caused by bacteria (actinomycetoma) or fungi (eumycetoma). Cases occur predominantly throughout the tropical and subtropical regions of the world, although the epidemiology of the disease is poorly understood. This has been identified as a research priority. Recent reviews on the distribution of mycetoma globally1,2 have failed to identify Ecuador as a country where mycetoma occurs, and maps of mycetoma distribution, including those produced by the World Health Organization, show Ecuador as a country for which there are ‘no data available’.3
Methods
We present data on 35 cases of mycetoma in Ecuador: 5 cases from our own experience and an additional 30 cases4,5 from the literature. The details of these cases are summarised in Table 1.
Table 1.
Clinical and epidemiological characteristics
| Characteristics | Existing literature (n=30)3,4 | Present study (n=5) | Total (n=35) |
|---|---|---|---|
| Age (years), n (%) | |||
| <10 | 0 (0) | 0 (0) | 0 (0) |
| 11–20 | 4 (13.3) | 0 (0) | 4 (11.4) |
| 21–30 | 10 (33.3) | 1 (20) | 11 (31.4) |
| 31–40 | 5 (16.7) | 1 (20) | 6 (17.1) |
| 41–50 | 5 (16.7) | 2 (40) | 7 (20) |
| 51–60 | 0 (0) | 1 (20) | 1 (2.9) |
| 61–70 | 3 (10) | 0 (0) | 3 (8.6) |
| 71–80 | 1 (3.3) | 0 (0) | 1 (2.9) |
| Data not available | 2 (6.7) | 0 (0) | 2 (5.7) |
| Sex, n (%) | |||
| Male | 20 (66.7) | 3 (60) | 23 (65.7) |
| Female | 10 (33.3) | 2 (40) | 12 (34.3) |
| Occupation, n (%) | |||
| Farmer | 20 (66.7) | 3 (60) | 23 (65.7) |
| Housekeeper | 9 (30) | 1 (20) | 10 (28.6) |
| Factory worker | 1 (3.3) | 0 (0) | 1 (2.9) |
| Teacher | 0 (0) | 1 (20) | 1 (2.9) |
| Origin (province), n (%) | |||
| Coastal region | 24 (80) | 3 (60) | 27 (77.1) |
| Guayas | 10 (33.3) | 2 (40) | 12 (34.3) |
| Los Rios | 5 (16.7) | 1 (20) | 6 (17.1) |
| Manabi | 6 (20) | 0 (0) | 6 (17.1) |
| El Oro | 3 (10) | 0 (0) | 3 (8.6) |
| Andes (mountain) region | 4 (13.3%) | 1 (20) | 5 (14.3) |
| Azuay | 2 (6.7) | 0 (0) | 2 (5.7) |
| Bolivar | 1 (3.3) | 0 (0) | 1 (2.9) |
| Cotopaxi | 1 (3.3) | 0 (0) | 1 (2.9) |
| Santo Domingo | 0 (0) | 1 (20) | 1 (2.9) |
| Amazon region | 0 (0) | 1 (20) | 1 (2.9) |
| Napo | 0 (0) | 1 (20) | 1 (2.9) |
| Data not available | 2 (6.7) | 0 (0) | 2 (5.7) |
| Type of mycetoma, n (%) | |||
| Actinomycetoma | 27 (90) | 0 (0) | 27 (77.1) |
| Eumycetoma | 3 (10) | 5 (100) | 8 (22.9) |
| Anatomical location, n (%) | |||
| Foot | 22 (73.3) | 2 (40) | 24 (68.6) |
| Ankle | 1 (3.3) | 1 (20) | 2 (5.7) |
| Leg | 3 (10) | 1 (20) | 4 (11.4) |
| Buttock | 1 (3.3) | 0 (0) | 1 (2.9) |
| Back | 3 (10) | 0 (0) | 3 (8.6) |
| Arm | 0 (0) | 1 (20) | 1 (2.9) |
| Duration of symptoms (years), n (%) | |||
| <1 | 2 (6.7) | 0 (0) | 2 (5.7) |
| 1–5 | 16 (53.3) | 5 (100) | 21 (60) |
| 6–10 | 5 (16.7) | 0 (0) | 5 (14.3) |
| 11–20 | 3 (10) | 0 (0) | 3 (8.6) |
| ≥21 | 1 (3.3) | 0 (0) | 1 (2.9) |
| Data not available | 3 (10) | 0 (0) | 3 (8.6) |
| Causative agent, n (%) | |||
| Bacterial | |||
| Nocardia brasiliensis | 9 (30) | 0 (0) | 9 (25.7) |
| Nocardia asteroides | 6 (20) | 0 (0) | 6 (17.1) |
| Nocardia sp. | 3 (10) | 0 (0) | 3 (8.6) |
| Actinomadura madurae | 2 (6.7) | 0 (0) | 2 (5.7) |
| Unidentified actinomycete | 7 (23.3) | 0 (0) | 7 (20) |
| Fungal | |||
| Fusarium sp. | 0 (0) | 3 (60) | 3 (8.6) |
| Madurella sp. | 1 (3.3) | 1 (20) | 2 (5.7) |
| Cylindrocarpon sp. | 0 (0) | 1 (20) | 1 (2.9) |
| Pyrenochaeta sp. | 1 (3.3) | 0 (0) | 1 (2.9) |
| Pseudallescheria boydii | 1 (3.3) | 0 (0) | 1 (2.9) |
The five cases from our experience were all eumycetomas, diagnosed between 2017 and 2021, at two institutions in Ecuador. Of the 30 cases from the existing literature, 29 were collected at the Mycology Department of the National Institute of Hygiene and Tropical Medicine ‘Leopoldo Izquieta Pérez’ between 1955 and 1978,4 work that was presented at the 1st International Symposium on Mycetomas in Barquisimeto, Venezuela in 1978. All cases were diagnosed using routine methods, including microscopy, culture and histopathology; causative organisms were identified based on their microscopic and macroscopic morphological features. Molecular diagnostic tools were not available.
Results
Of the 35 cases presented here, young adults were most commonly affected, with the majority of cases (68.6%) occurring across the third, fourth and fifth decades of life. Two-thirds of patients were male, and the most common occupations were farmers and agricultural workers (65.7%) followed by housekeepers (28.6%).
The majority of cases (77.1%) were actinomycetomas. Nocardia species were the most common causative agents, with Nocardia brasiliensis most frequently isolated. In one-quarter of the actinomycetoma cases, an actinomycete was detected but the species could not be identified. Fusarium species were the most common causative agents of eumycetoma. Among the other fungal isolates, two were identified as Madurella species, one recorded as ‘Madurella mycetomi’ (Madurella mycetomatis) and the other as Madurella sp., and one case was caused by Pseudallescheria boydii (Scedosporium apiospermum).
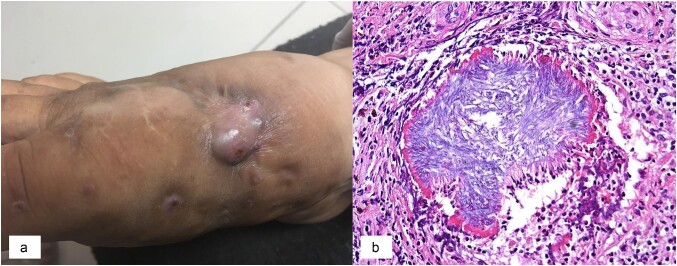
The foot was the site of infection in 68.6% of cases (Figure 1), the back was affected in 8.6% of cases and the upper arm in 2.9%. The duration of symptoms prior to diagnosis was 1–5 y for the majority (60.0%) of patients, but ranged from 5 months to 50 y.
Figure 1.
(a) Eumycetoma of the foot in a female patient from a rural area of Guayas province in coastal Ecuador. (b) Haematoxylin and eosin–stained tissue section showing pale eumycotic grains caused by Fusarium sp. (×20).
On the distribution of mycetoma within Ecuador, most of the cases (77.1%) originated from provinces in the Pacific coastal region—Guayas, Los Rios, Manabí and El Oro. Only one patient originated from Amazonian Ecuador. Guayas province contained the greatest number of cases, many of which were located close to the city of Guayaquil, from towns situated along the Babahoyo and Daule Rivers and their tributaries. These rivers join to form the Guayas River before entering the Pacific Ocean.
Discussion
For the first time in an English-language publication, this communication confirms the presence of mycetoma in Ecuador and secures Ecuador's position as an endemic country on the global mycetoma map.
Most cases of mycetoma occur between latitudes 30°N and 15°S, referred to as the mycetoma belt.1,6 Sudan reports the highest number of cases, followed by Mexico and India. Many endemic areas have a tropical climate, with high temperatures, annual rainfall of 50–1000 mm and well-defined dry and rainy seasons.7,8 Ecuador is situated on the equator and is composed of three regions, each with distinct geography and climate: the Pacific coast, the Andes and the Amazon.
The geographic distribution of mycetoma and the variation in causative agents, observed both between and within countries, is poorly understood.
In Mexico, actinomycetoma accounts for 96.5% of all cases, the majority caused by N. brasiliensis, and only 3.5% of cases are eumycetomas. However, there are regions within Mexico where eumycetoma accounts for up to 20% of cases.9 The relative frequency of eumycetoma is higher in Argentina (42% of cases) and even higher in parts of India and Sudan (62% and 79% of cases, respectively). Data from modelling studies in Sudan have shown that aridity and proximity to water predict the occurrence of both eumycetoma and actinomycetoma, while a greater diversity of thorny trees is a predictor of eumycetoma occurrence.8
The limited data that are available suggest that mycetoma in Ecuador is predominantly coastal in distribution.
The climate of coastal Ecuador shares similarities with many mycetoma-endemic regions, being classified as ‘dry semi-hot tropical’ with rainfall of <1000 mm/y and a dry season from June to December. The vegetation in this region broadly consists of light xerophytic forest interspersed with savanna. In the vicinity of Guayaquil, where most mycetoma cases occur, pellic vertisols are the predominant soil type. These soils can be extremely dry and hard during the summer (dry season) and wet and swampy during the winter. For agricultural workers in these areas, this can involve working with their feet submerged in water or soil. Pellic vertisols are also found in parts of the Pacific coastal lowlands of Mexico, which are highly endemic for mycetoma.
The distribution of cases in Ecuador could be explained in part by geographic factors, but may also reflect limited access to healthcare and sociodemographic barriers in the Andes and Amazon regions. This highlights the importance of reporting and publishing even small numbers of cases where data are scarce, to allow a more accurate estimation of disease burden and permit analyses of mycetoma distribution to better understand potential environmental risk factors.
Contributor Information
David J Chandler, Department of Global Health and Infection, Brighton and Sussex Medical School, Brighton, BN1 9PX, UK; Dermatology Department, Brighton General Hospital, University Hospitals Sussex NHS Foundation Trust, Brighton, BN2 3EW, UK.
Luis Escalante, Universidad Espíritu Santo, Guayaquil, Ecuador; Universidad de Guayaquil, Guayaquil, Ecuador.
Astrid Maldonado, Universidad de Guayaquil, Guayaquil, Ecuador; EPHORA Research Group, Guayaquil, Ecuador.
Sonia Tello, Department of Pathology, Hospital Axxis, Quito, Ecuador.
Shirley Orellana, Hospital General del Norte de Guayaquil Los Ceibos, Guayaquil, Ecuador.
Edgar Escalante, Hospital Carlos Andrade Marin, Quito, Ecuador.
Authors’ contributions
DC and LE conceived the study. LE, AM, ST, SO and EE were responsible for data collection. DC was responsible for analysis and interpretation of the data and drafted the manuscript. All authors critically revised the manuscript for intellectual content and read and approved the final manuscript. DC and LE are guarantors of the paper.
Acknowledgements
None.
Funding
None.
Competing interests
None declared.
Ethical approval
Not required.
Data Availability
All data are incorporated into the article and its online supplementary material.
References
- 1. van de Sande WWJ. Global burden of human mycetoma: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2013;7(11):e2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Emery D, Denning DW. The global distribution of actinomycetoma and eumycetoma. PLoS Negl Trop Dis. 2020;14(9):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . Mycetoma reported cases, latest year available. 2019. Available from: https://cdn.who.int/media/docs/default-source/ntds/mycetoma-chromoblastomycosis-and-other-deep-mycoses/mycetoma-cases-reported-latest-year-available.pdf?sfvrsn=f91e3125_2
- 4. Lazo SRF. Micetomas en el Ecuador. Rev Ecuat Hig Med Trop. 1979;32(1):39–44. [Google Scholar]
- 5. Torres M, Medina F. Micetoma en gluteo. Gac Dermatolog Ecuator. 1998;1. [Google Scholar]
- 6. Zijlstra EE, van de Sande WWJ, Welsh O et al. Mycetoma: a unique neglected tropical disease. Lancet Infect Dis. 2016;16(1):100–12. [DOI] [PubMed] [Google Scholar]
- 7. Develoux M. Epidemiologic aspects of mycetoma in Africa. J Fungi (Basel, Switzerland). 2022;8:1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hassan R, Simpson H, Cano J et al. Modelling the spatial distribution of mycetoma in Sudan. Trans R Soc Trop Med Hyg. 2021;115(10):1144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Estrada R, Chávez-López G, Estrada-Chávez G et al. Mycetoma and the Community Dermatology Program, Mexico. Trans R Soc Trop Med Hyg. 2021;115(4):383–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are incorporated into the article and its online supplementary material.