Summary
The evolutionary trajectory of glioblastoma is a multifaceted biological process that extends beyond genetic alterations alone. Here, we perform an integrative proteogenomic analysis of 123 longitudinal glioblastoma pairs and identify a highly proliferative cellular state at diagnosis and replacement by activation of neuronal transition and synaptogenic pathways in recurrent tumors. Proteomic and phosphoproteomic analyses reveal that the molecular transition to neuronal state at recurrence is marked by post-translational activation of the WNT/PCP signaling pathway and BRAF protein kinase. Consistently, multi-omic analysis of Patient-Derived Xenograft (PDX) models mirror similar patterns of evolutionary trajectory. Inhibition of BRAF kinase impair both neuronal transition and migration capability of recurrent tumor cells, phenotypic hallmarks of post-therapy progression. Combinatorial treatment of temozolomide (TMZ) with BRAF inhibitor, vemurafenib, significantly extends the survival of PDX models. This study provides comprehensive insights into the biological mechanisms of glioblastoma evolution and treatment resistance, highlighting promising therapeutic strategies for clinical intervention.
Keywords: Longitudinal glioblastoma, Proteogenomics, Recurrence, BRAF, Neuronal, Synapse
eTOC Blurb
Kim et al., reveal that glioblastoma develops therapeutic resistance through a neuronal transition caused by changes in the WNT/PCP pathway and BRAF kinase, as shown by extensive proteogenomic analysis. Notably, inhibiting BRAF kinase disrupts this lethal transformation, offering potential therapeutic avenues.
Graphical Abstract

Introduction
Glioblastoma (GBM) is the most common and lethal primary brain tumor in adults with a median survival of less than 15 months, despite continuous efforts in treatment innovations1–4. A major roadblock that contributes to such a dismal prognosis is tumor relapse, which is observed in over 90% of patients5. While the standard of care for primary treatment has been largely established, recurrent GBM remains therapeutically unresolved due to the complexity of tumor heterogeneity and limited understanding of the evolutionary dynamics after therapy.
To address these challenges, several studies generated genomic data from two or more longitudinal GBM samples and characterized the evolution of the molecular profiles in response to treatment6–12. These studies showed that recurrent GBMs undergo a complex clonal evolution under therapeutic pressure driving genetic drifts that follow both neutral and selective evolutionary models13,14. Whereas emerging knowledge of GBM evolution is covered through the profiling of their genomes and transcriptomes, these platforms have failed to identify consistent trajectories of evolution that could explain the therapeutic resistance invariably associated with recurrent disease.
When combined with genomics and transcriptomics, proteomics provides another paradigm for investigating a hidden dimension of cancer biology that has gone largely undetected. The Clinical Proteomic Tumor Analysis Consortium (CPTAC) has implemented a significant effort to profile a wide spectrum of malignant tumor types using multiple proteogenomic platforms. This approach has generated tumor classifications and subgroups that are associated with distinct patient prognoses and potential therapeutic vulnerabilities15–26. Here, we performed comprehensive proteogenomic analyses of 123 matched primary and recurrent GBMs and adopted the integration of genomics and deep proteomics characterization to extract the significant changes that drive GBM evolution under therapy. The proteogenomic inspection of the longitudinal GBM cohort identified the activation of neuronal programs as the primary mechanism of evolution driving tumor recurrence after therapy. Integrated multi-omics analyses uncovered the activation of the WNT/PCP pathway and the B-Raf Proto-Oncogene (BRAF) kinase as molecular determinants of neuronal transition in recurrent GBM, thus offering diagnostic and therapeutic tools to manage tumor recurrence in patients with GBM.
Results
The molecular trajectory of longitudinal glioblastoma
To investigate the underlying mechanisms that drive the complex molecular trajectory of GBM evolution, we collected 123 matched longitudinal GBM specimens from 5 different institutions. Among them, 122 were characterized by Whole-Exome Sequencing (WES) with 91 having matched blood-derived normal DNA, 86 by Whole-Transcriptome Sequencing (WTS), and 105 by mass spectrometry-based proteomics to provide robust quantification of proteins and phospho-proteins (Figure 1A and Table S1).
Figure 1. The molecular trajectory of longitudinal glioblastoma.
(A) Summary of profiling platforms and number of matched longitudinal GBM patient samples analyzed by each molecular platform. The color white indicates matched samples not profiled by the indicated platform. SNU: Seoul National University, PS: Hôpital de la Pitié-Salpêtrière, SMC: Samsung Medical Center, CNU: Chonnam National University, and NCC: National Cancer Center. (B) Comparison of tumor mutational burden and aneuploidy fraction between IDH mutant and IDH wild-type primary and matched recurrent GBMs. Box plots span from the first to third quartiles, middle line represents median, and whiskers represent the 1.5x interquartile range. The P-values were calculated by the Wilcoxon rank-sum test. (C) Somatic genomic landscape of GBM driver genes grouped by oncogenic pathways. IDH mutant samples are grouped on the right side of the panel. Patients were ordered according to progression-free survival (PFS, months). Genomic alterations shared between primary and recurrent tumors are in green, private alterations are in yellow (primary specific) or purple (recurrent specific); in red are shared alterations with mutation replacement). The bar graph on the right represents the overall frequency rate of each shared or private genomic alteration. (D) Bubble plot illustrating the frequency of fCNVs and non-synonymous mutations of GBM driver genes in exclusively primary (yellow, left axis), exclusively recurrent (purple, right axis), and shared (green, upper axis) between primary and recurrent GBM. GBM driver genes with genetic alterations in at least 4 primary recurrent pairs are represented. Red and blue gene labels denote oncogenes and tumor suppressor genes, respectively. The size of each node represents the number of patients harboring the corresponding genetic alterations. (E) Bubble plot illustrating the frequency of fCNVs and non-synonymous mutations of all the genes in exclusively primary (yellow, left axis), exclusively recurrent (purple, right axis), and shared between primary and recurrent GBM (green, upper axis) GBM tumors (Fisher’s exact test, p < 0.10). Representative genes involved in neuronal differentiation, cell cycle, mitosis, and DNA repair are indicated.
Among 122 patients with available WES data, 8 patients had acquired the hypermutator phenotype at recurrence10,27–29 (Figure S1A). These recurrent tumors were characterized by genetic alterations in mismatch repair encoding genes, including MSH3, MSH6, MLH1, and PMS2 and temozolomide-induced mutation signatures (Figure S1A). Hypermutated recurrent tumors had a high mutation load and increased number of somatic mutations-derived neoantigens30,31 (Figure S1B-C). When we excluded hypermutant tumors, we observed a significant increase in the tumor mutational burden upon recurrence in both Isocitrate Dehydrogenase 1 (IDH1)-mutant and IDH1-wild-type GBM, whereas the aneuploidy fraction did not exhibit a significant difference (Figure 1B).
Next, we investigated the occurrence of major driver mutations and copy number alterations during GBM evolution. Initially, we focused on the well-recognized drivers that constitute the core oncogenic pathways of GBM, including receptor tyrosine kinase (RTK)-RAS signaling, phosphoinositide-3-kinase (PI3K), p53, chromatin modification, and cell cycle pathways. Mutations in IDH1 exhibited coherent stability during temporal evolution in agreement with the suggestion that IDH1 mutation is one of the early glioma-initiating events10 (Figure 1C). Similarly, chromosomal amplification of EGFR, deletion of CDKN2A, and somatic mutations of PTEN, ATRX, and TP53 were highly conserved across untreated and recurrent tumors. However, we discovered several genetic alterations that were frequently lost in recurrent tumors, including chromosomal amplification of CDK6, MDM4, and CCND2 (Figure 1D). As these drivers are primarily involved in cell cycle and proliferation kinetics, we hypothesized that recurrent GBMs are not sustained by conventional mechanisms enhancing cell cycle progression and proliferation.
To assess the functional impacts of copy number variations on gene expression in cis (functional Copy Number Variation, fCNV) at different stages of tumor progression, we performed an integrative analysis of mRNA expression and CNVs32,33. Overall, we discovered that major GBM driver alterations, including CDKN2A, TP53, EGFR, and PTEN were maintained from primary to recurrent tumors. Conversely, several genes were differentially altered in primary and recurrent GBM. Among the genes more frequently altered at diagnosis were CDK6 and MET oncogenes (amplified) and the NF1 tumor suppressor (mutated/deleted) (Figure 1D). Conversely, albeit small in number, somatic mutations or deletions of PI3KR1 were found at higher frequency in recurrent tumors. When we expanded the integrative analysis to non-driver genes, we found that functional amplification of genes that are involved in cell cycle, mitosis, and DNA repair activities (ERCC2, HUS1, PDGFA, RCF2, and BRAT1) was significantly enriched in GBM at diagnosis and depleted at recurrence. In contrast, recurrent GBMs were marked by functional amplification of neuronal differentiation-promoting genes, including SGK2, LIN7B, STX1A, and CDK5 (Figure 1E), suggesting that cell proliferation may represent a driving force behind GBM tumor expansion before treatment. However, following standard-of-care therapy, recurrent GBM acquires capabilities distinct from cell cycle and proliferation, possibly involving co-option of neuronal activities.
Collectively, our results highlight that genetic alterations remain highly conserved throughout GBM progression and integrative proteogenomic characterization could potentially provide unprecedented insights into the complex molecular structure of GBM evolution that were largely hidden from genomics or transcriptomics alone.
Impact of EGFR genomic alteration on downstream signaling in recurrent GBM
Previous studies have identified recurrent genomic aberrations in the core oncogenic pathways of GBM that constitute complex canonical signal transduction28. Among them, chromosomal amplifications in genes encoding receptor tyrosine kinases (RTKs) have been frequently identified34–37. When we investigated these key oncogenic signaling pathways at the protein level during GBM evolution, we discovered that FGFR2/3, NTRK2/3, and BRAF proteins were predominantly enriched in recurrent tumors, while the abundance of EGFR, CDK4, and CDK6 proteins was increased in primary tumors (Figure 2A). The accumulation of these oncogenic proteins was not associated with corresponding changes in gene copy number and/or mRNA levels, which points towards post-transcriptional mechanisms of regulation of these protein kinases during GBM evolution. Genomic alterations in EGFR are among the major contributors to GBM oncogenesis38–40. As such, many clinical trials have been initiated to assess the therapeutic efficacy of EGFR-mediated therapy. While the majority of the studies have demonstrated the significance of the functional role of EGFR in primary GBM, its role in driving GBM recurrence remains elusive. Thus, we analyzed the dynamics of EGFR genomic alterations in matched primary-recurrent GBM samples. We identified three distinct groups of patients; one group exhibited EGFR alteration in both primary and recurrent tumors, while in the other two groups, EGFR alteration was either lost or gained in the recurrent tumor (Figure 2B). As expected, loss of EGFR alterations at recurrence was associated with the decrease of EGFR protein (Figure 2C). Strikingly, reduction of EGFR abundance and phosphorylation was also detected in those tumors that maintained EGFR alterations at recurrence, suggesting that post-translational mechanisms operate during evolution to abate EGFR signaling (Figures 2D and S2).
Figure 2. Impact of EGFR genomic alteration on downstream signaling upon recurrence.
(A) Analysis of up-regulated protein in key oncogenic GBM pathways including RTK-RAS, p53, cell cycle, and chromatin modification. The pie charts show the percentage of primary (yellow) and recurrent (purple) samples showing up-regulated proteins. Selected phosphosites are indicated and color-coded according to elevation in primary or recurrent tumors. (B) Somatic genomic landscape of EGFR alterations, including amplification (Amp), mutation (Mut), EGFRvIII, and Fusion (Fus). Patients have been categorized as “Shared” if EGFR alterations are shared between primary and recurrent tumors (green), “Loss” (blue) if EGFR alterations are lost at recurrence, or “Gain” (Red) if it was gained in the recurrent tumor. (C) Comparison of EGFR protein abundance between matched primary (P) and recurrent (R) tumors in the corresponding patient groups. Box plots span from the first to third quartiles and middle line represents median. The P-values were calculated by the Wilcoxon rank-sum test. (D) Violin plots of the phosphorylation abundance in EGFR sites between primary and recurrent GBM patients in the “Shared” group. The P-values were calculated by the Wilcoxon rank-sum test. (E) Correlation plot of EGFR protein abundance with key regulators of PI3K-AKT-PTEN-mTOR and the RAS-RAF-MEK-ERK downstream pathways. Pearson Correlation Coefficient (PCC) is indicated once each protein abundance was normalized across samples. (F) Dots plot showing the changes of phosphorylation abundance in EGFR substrates when comparing recurrent and primary GBMs. The P-values were calculated by the Wilcoxon rank-sum test.
See also Figure S2.
EGFR executes oncogenic activities via two primary downstream signaling, the PI3K-AKT-PTEN-mTOR and the RAS-RAF-MEK-ERK pathways41–43. To investigate the impact of EGFR genomic alterations on these signals, we interrogated proteomics data to determine the correlation between EGFR and signaling effector molecules. We found a statistically significant positive correlation between EGFR and GAB1 with AKT1 and AKT2 proteins but a negative correlation with BRAF and MAPK1/3 proteins (Figure 2E). When we investigated the phosphorylation status of EGFR and GAB1, the docking factor for EGFR-mediated PI3K-AKT activation42, we found that phospho-EGFR (Y1197, S1166) was significantly reduced in recurrent tumors that maintained EGFR genetic alterations as well as in those in which lesions were lost at recurrence (Figure 2D). Concordantly, phospho-GAB1 (S402, T503) was significantly decreased at recurrence (Figures 2F and S2). Together, our results suggest that EGFR signaling is largely deactivated in recurrent GBM, likely as a consequence of EGFR protein reduction during evolution.
Proteogenomic characterization of longitudinal GBM reveals increased neuronal activities at recurrence
To identify biological features active in recurrent GBM, we interrogated the differential proteome and phospho-proteome of matched GBM pairs. We uncovered 377 proteins and 1,820 phospho-proteins that were increased in recurrent GBMs, including SNAP25, TUBB4A, NELF, DAAM1/2, STMN1, and MBP (Figure 3A). Pathway enrichment analysis revealed that recurrent tumors were characterized by the elevation of proteins and phospho-proteins functionally involved in neuron structure and function and synapse formation (both pre- and post-synaptic activities). Examples of biological pathways significantly overrepresented in recurrent GBM were “Ion channel activity”, “Action potential”, “Synaptic plasticity”, “Neurotransmitter transport”, “Neuron projection”, “Neuron differentiation”, and “Axon”. These pathways include either key structural neuronal proteins or proteins involved in neuron activities, namely the transmission of electrical and chemical signals. On the other hand, GBM at diagnosis was primarily enriched with activation of DNA replication, cellular proliferation, and MAPK regulation as well as extracellular matrix functions and EGFR signaling (Figures 3B-C, S3A, and Table S2). Next, to investigate the relevance of protein regulation in comparison with transcriptional control for GBM progression, we integrated transcriptomic, proteomic, and phospho-proteomic data to identify proteins and phosphoproteins differentially abundant in recurrent versus primary tumors showing concordant/discordant changes in mRNA. While most proteins in recurrent GBM had a concordant change of corresponding mRNAs (n=8,207, PLP1, GFAP, FBXO2, STMN4), numerous molecules showed mRNA-protein dissociation (SYT4/11, DAAM1/2, BRAF, EGFR, and its substrate, GAB1) (Figure 3D). The biological pathway enrichment analysis revealed that proteins and phospho-proteins increased at recurrence were significantly associated with synapse formation, GTPase activity, and oxidative phosphorylation. Conversely, proteins and phosphoproteins implicated in cell division, mRNA processing, and transcription were more abundant in primary GBM (Figure 3E).
Figure 3. Proteogenomic characterization of longitudinal GBM reveals increased neuronal activities in recurrent GBM.
(A) Volcano plot representation of proteins (upper panel) and phosphoproteins (bottom panel) with differential abundance. x-axis indicates the difference of median expression between primary and recurrent GBMs; y-axis indicates the statistical significance derived from Wilcoxon rank-sum test. The P-values for each protein and phospho-protein were calculated by the Wilcoxon rank-sum test. (B) single sample Gene Set Enrichment Analysis (ssGSEA) analysis of proteins and phosphoproteins between primary and recurrent GBMs using all genesets available in the MSigDB. The P-values were calculated by the Wilcoxon rank-sum test for each geneset between primary and recurrent tumors. (C) Comparison of major synaptic pathway activities between matched primary and recurrent tumors. The lines within each violin plot represent the 25th, 50th, and 75th quantiles. The P-value was calculated by the Wilcoxon rank-sum test. (D) Scatter plot showing the ratio of mRNAs, proteins, and phosphoproteins expression/abundance significantly changed between recurrent and primary GBMs. Red and blue dotted-line quadrants indicate upregulated and downregulated proteins, and phosphoproteins, respectively without any mRNA changes. The P-values were calculated by the Wilcoxon rank-sum test. (E) GO enrichment analysis of proteins and phosphoproteins from the red and blue quadrants in (D). The q values were calculated using the Benjamini-Hochberge method. (F-G) Cis and trans effects of major genomic alterations including mutations and CNVs on protein (F) and phosphoprotein (G) levels. Cis and trans effects of each genomic alteration were categorized into primary-specific (yellow), recurrent-specific (purple), or shared (green); see the track on the left of the panel. The P-values were calculated by the Wilcoxon rank-sum test.
We then explored the functional impact of genetic alterations on global and phosphoprotein abundance, both cis-acting (cognate gene product) and trans-acting (other gene products) at recurrence. We found a strong effect in cis for EGFR, with significant decreases at recurrence in total protein and phosphorylation abundance at multiple sites (S1071, S1166, T693) (Figure 3F-G). These results suggest that EGFR alterations in GBM contribute to tumor initiation but are no longer required for tumor evolution. At the trans-acting level, recurrent tumors harboring multiple GBM driver genetic alterations (e.g., EGFR, PDGFRA, PTEN, TP53) exhibited elevated protein and phosphoprotein levels of members of the RAS pathway (NTRK2/3, BRAF, and MAP2K1/2), WNT pathway (DAAM1/2) and proteins involved in synapse formation (PRKCZ, SNAP25, SYN1, and GRIA2) (Figure 3F-G). Collectively, our results demonstrate that recurrent GBMs are uniquely characterized by neuronal signaling programs, which may be driven by activation of RAS and WNT signaling and contribute to disabling the cell proliferation mechanisms essential for the expansion of primary GBM.
Integrative multi-omics subtyping reveals enrichments of the neuronal GBM subtype at recurrence
We explored genomics, transcriptomics, proteomics, and phosphoproteomics and clustered matched primary and recurrent GBM pairs using the recently described pathway based GBM classifier32,33. The classifier distinguishes four tumor subtypes grouped into two functional branches, neurodevelopment (proliferative/progenitor, PPR and neuronal, NEU) and metabolism (glycolytic/plurimetabolic, GPM and mitochondrial, MTC). We found that each platform provided an enrichment of the molecular elements defining each functional subgroup and recapitulated the predominant biological activities of each GBM subtype (Figure 4A). Then, we quantified the frequency of subtype transition in matched primary and recurrent tumors and discovered that recurrent GBM exhibited a significantly lower frequency of PPR and a higher frequency of NEU subtypes (Figure 4B). To determine the potential impact of genetic changes, we compared driver and non-driver genetic alterations between primary non-NEU and recurrent NEU GBM pairs. Major GBM drivers such as CDKN2A/B, EGFR, TP53, and PTEN occurred similarly in both primary and recurrent tumors. Recurrent tumors acquired fCNV amplification of genes involved in neuronal differentiation and synaptic activity such as BRAF, STX1A, KCNN1, SCN1B, and ACTL6B and fCNV deletion of genes involved in cell cycle, mitosis, and DNA repair, including CHEK2, ASPM, CENPW, CDK1, and MDC1. Conversely, primary tumors mainly harbored preferential fCNV gain of genes involved in cell cycle, mitosis, and DNA repair functions and fCNV loss of genes controlling neuronal differentiation and specialized neuronal functions (Figure 4C). We then integrated the global proteome and phospho-proteome to identify phosphorylation sites that are differentially modified in the absence or presence of corresponding protein changes. NEU recurrent GBM showed elevated phosphorylation of neuronal proteins (STX4-S36, DLGAP4-S405/S156/T915, CAMK2D-S338/S276) without significant change in the corresponding protein (neutral). Proteins whose phosphorylation and global abundance were significantly increased in recurrent NEU tumors were involved in neuronal structure/functions and the BRAF/MAPK pathway (Figure S3B). Conversely, non-NEU primary GBM of the matched NEU tumors exhibited elevated levels of phosphorylation in a variety of proteins involved in chromatin remodeling and DNA damage/repair, without changes in global abundance. Consistent with global proteome analyses, EGFR phosphorylation at Y1197, S1071, and T693 residues were among the top differentially increased phosphosites in non-NEU primary tumors. Together, these results indicate that the evolutionary transition towards the neuronal state in recurrent GBM is regulated by both genetic and post-genetic molecular events that include de-activation of EGFR and activation of BRAF-MAPK signaling pathways.
Figure 4. Integrative multi-omics clustering reveals the transition of GBM at diagnosis toward neuronal state at recurrence.
(A) Heatmap of multi-omic features significantly associated with each subtype according to the proteomic-based functional classification of primary and recurrent GBM. Upper track, functional classification; bottom track, tumor type. Columns are individual tumors and rows are features. First panel, mutation calls are in green (p < 0.10, Fisher’s exact test). Second panel, fCNV gain or amplification calls are in red and orange (p < 0.05, Fisher’s exact test), respectively. Third panel, fCNV heterozygous or homozygous deletion calls (p < 0.05, Fisher’s exact test) are in blue and cyan, respectively. Fourth and fifth panels, 150 highest scoring genes/proteins in the gene/protein expression/abundance ranked list of each of the four GBM subtypes (MWW-GST test). Sixth panel, significant outlier phosphorylated proteins in each functional GBM subtype (p < 0.05, BlackSheep). Biological pathways significantly enriched (Fisher exact test, p < 0.05) and representative genetic alterations specific to each GBM subtype are indicated on the right. (B) Proteomic-based functional subtyping of primary and recurrent GBM. The transition plot of functional subtypes in primary and recurrent GBM shows an increased frequency of the NEU subtype at recurrence (p = 0.0099, χ2 test). (C) Bubble plot frequency of primary non-neuronal to recurrent neuronal matched pairs of GBM harboring fCNV in genes significantly associated with primary or recurrent tumors (Fisher’s exact test, p < 0.05). (D) Comparison of MGMT, MSH2, MLH1, and PMS2 protein abundance between matched primary and recurrent tumors that transition into C2-type. Box plots span from the first to third quartiles and middle line represents median. The P-values were calculated by the Wilcoxon rank-sum test. (E) Graphical illustration of the down-regulation of mismatch repair (MMR) encoding proteins leading to TMZ resistance. (PPR : proliferative/progenitor, NEU : neuronal, GPM : glycolytic/plurimetabolic, MTC : mitochondrial, CL : classical, MES : mesenchymal, PN : proneural)
See also Figures S3-5.
We sought to orthogonally confirm the pattern of GBM evolution through the unsupervised clustering of matched primary and recurrent GBM pairs using our multi-omics data cohort. The analysis returned three distinct tumor clusters. Cluster 1 (C1) was associated with epithelial-to-mesenchymal transition, inflammation, and response to interferon-γ and was enriched with tumors classified as GPM and mesenchymal by the pathway-based and transcriptional GBM classifiers, respectively32,44 (Figure S3C and S4A). Cluster 2 (C2) samples were characterized by enrichments of neuronal features, aligned with coherent activation of proteins and phospho-proteins that were associated with neuron projection, synaptic plasticity, and neurotransmitter activity. Accordingly, C2 exhibited enrichment with tumors classified within the NEU subtype (Figure S3C and S4A). Cluster 3 (C3) tumors demonstrated increased cell cycle and chromosomal remodeling activities, were largely composed of Ki-67-positive tumors and were mostly classified as PPR by the pathway-based and proneural by the transcriptional GBM classifiers (Figure S3C and S4A). To determine the effects of the treatment on the identified clusters during GBM progression, we quantified the extent of subtype transition in matched primary and recurrent tumors. While primary GBMs were mainly enriched for C1- and C3-type tumors, recurrent GBMs were largely composed of C2-type tumors (16% at diagnosis to 63% at recurrent) (Figure S4B-D), thus independently validating the finding that recurrent GBMs exhibit increased neuronal functions.
In an effort to further investigate the biological components that were associated with subtype transition and treatment resistance at recurrence, we analyzed the protein abundance of key molecules that regulate DNA repair mechanisms. Chemoresistance in glioblastoma is largely attributed to the inhibition of demethylating enzyme O6-methylguanine-DNA methyltransferase (MGMT) expression by the methylation of MGMT promoter45,46. Expression and abundance of MGMT gene and protein, respectively, were decreased in MGMT-methylated primary GBM (Figure S5A). In agreement with previous reports, DNA methylation of MGMT was highly associated with prolonged survival47,48 (Figure S5B). Moreover, when patients were classified according to MGMT protein abundance, patients harboring tumors with high MGMT expression exhibited decreased survival, despite having MGMT methylation (Figure S5C). Conversely, patients with unmethylated MGMT showed no significant difference in survival.
When we asked whether MGMT-methylated or MGMT-unmethylated primary GBM exhibited preferential transition toward a specific functional subtype at recurrence, we found that 48.6% of MGMT-unmethylated primary GBM recurred as neuronal tumors (Figure S5D). Consistently, C2-subtype tumors at recurrence demonstrated increased MGMT protein and down-regulation of mismatch repair (MMR) proteins, including MSH2, ML1, and PMS2 (Figure 4D). Conversely, tumors that relapse as either C1- or C3- subtype, demonstrated no significant difference (Figure S5E-F). While previous studies indicate that mismatch repair deficiency, primarily caused by loss-of-function mutations49, leads to TMZ resistance during GBM progression, our findings suggest that protein inactivation, possibly through protein degradation, may also be a significant mechanism contributing to GBM recurrence (Figure 4E).
Single-cell analysis and functional experimental models reveal enrichments of neuronal activity in recurrent tumor cells
To assess whether the enrichments of neuronal and synaptic activities at recurrence were tumor cell-intrinsic and not derived from the microenvironment, we conducted single-cell RNA sequencing on four paired primary and recurrent tumor tissue specimens. We first distinguished 4,228 malignant cells and 12,258 non-malignant cells based on large‐scale chromosomal copy number variations (CNVs) in each cell inferred by inferCNV (Figure 5A-B)50,51. Dimensionality reduction (Uniform Manifold Approximation and Projection, UMAP) revealed eight major non-malignant cell types with high expression of distinct marker genes including oligodendrocytes, myeloid, T cells, astrocytes, oligodendrocyte progenitor cells (OPCs), endothelial, pericytes, and neurons (Figure 5C). The comparative analysis of the tumor microenvironment (TME) between primary and recurrent GBM revealed that oligodendrocytes and neurons increased in recurrent tumors at the expense of myeloid and T cells (Figure 5D-E). As far as tumor cell states were concerned, we analyzed the evolutionary trajectory of recurrent GBM based on pathway-based functional states32 and found that GBM evolution was marked by a reduction in PPR (from 14.2% to 10.5%) and MTC (from 23.8% to 16.8%) and gain of NEU states (from 36.2% to 49.0%; Figure 5F, upper panel). On the contrary, the other glioma cell state classifier, which includes NPC-like, OPC-like, AC-like, and MES-like states52 did not uncover significant changes between primary and recurrent GBMs (Figure 5F, lower panel). Computation of the PPR and NEU cell state activity confirmed the global increase of neuronal functions at the expense of stem/progenitor-like activities (Figure 5G). Finally, we calculated a ‘simplicity score’ for each single cell as a continuous measure of the strength of the state within a four-quadrant plot corresponding to the four transcriptomic states32. The quadrant/state clustering of tumors indicated that neuronal activity is the dominant biological feature of recurrent tumors (Figure 5H).
Figure 5. Single-cell analysis reveals increase of tumor-intrinsic neuronal state and neurons and oligodendrocytes in the TME of recurrent GBM.
(A) UMAP plot of single cells from matched primary and recurrent tumors (4 patients, 8 tumors), colored according to malignant or non-malignant cell subpopulations (left panel) and primary or recurrent status (right panel). (B) UMAP plot of single cells colored according to the median of the copy number of genes in chromosome 7 (left panel) and chromosome 10 (right panel) predicted by inferCNV as hallmarks of glioblastoma. (C) UMAP plot of single cells colored according to the malignant and non-malignant cell types (left panel) and averaged gene expression of corresponding marker genes (right panel). OPC, Oligodendrocyte progenitor cells. (D) Bar plots showing the cell type composition in each tumor analyzed. (E) Enrichment of non-malignant cell types between primary and recurrent GBM. Asterisks indicate chi-squared test derived standardized residuals above 2.5 as index of the statistical significance and strength of the association. (F) Tumor cell type distribution based on functional pathway (upper panel) and glioma cell state (lower panel). Asterisks indicate chi-squared test derived standardized residuals above 2.5 as index of the statistical significance and strength of the association. AC, Astrocyte. (G) Box plots showing neuronal and proliferative-progenitor state enrichment scores (NES) in each individual tumor cell from primary and recurrent GBM. Cells are colored according to the neuronal (blue) or proliferative-progenitor (cyan) state enrichment score. Box plots span from the first to third quartiles and whiskers show 1.5× interquartile range (Wilcoxon test). (H) Two-dimensional plot of functional tumor cell state enrichment scores. Each quadrant corresponds to one GBM subtype, and the position of dots (tumor cells) reflects the relative subtype-specific NES of each cell as indicated on the x and y axes; cells are colored according to primary and recurrent status.
To experimentally validate our findings regarding the essential components of recurrent GBMs, namely neurogenesis and synapse formation, we developed in vitro models using a co-culture system. In this system, we co-cultured matched primary and recurrent GBM patient-derived tumor cells (PDCs) labeled with GFP alongside mouse cortical neurons. Our results demonstrated that recurrent GBM cells, compared to primary tumors, are characterized by an increased number of pre-synapses when evaluated through quantitative immunofluorescence and immunoblot analysis for synapsin-1 (a pre-synapse marker) (Figure 6A). Moreover, recurrent tumor cells generated a large number of synapses per cell and displayed longer neurites compared to cells derived from primary tumors (Figure 6B). Furthermore, we performed double immunofluorescence analysis of matched primary and recurrent GBM samples, using markers against SOX2 (GBM tumor marker) and DAMM1 (neuronal development). Based on the previously reported neuronal transformation at the periphery of primary GBM32, we selected areas from both the core and infiltrative periphery of primary tumors. Interestingly, the expression of DAMM1 was almost undetectable in both areas of the primary tumor, regardless of the regions analyzed. In contrast, recurrent tumors exhibited higher levels of DAMM1 expression in the tumor core, with a marked increase observed at the infiltrative edge (Figures 6C-D and S6). These results provide additional evidence supporting the role of neurogenesis and synapse formation as key hallmarks of recurrent GBM.
Figure 6. Functional validation of enhanced synaptic activity in recurrent GBM.

(A) Co-culture of paired patient-derived GBM cells (primary and recurrent) and mouse cortical neuron cells. Expression of MAP2, synapsin-1 in paired GBM cells was measured by immunoblot analysis. The number of presynapses was analyzed. Data are represented as mean ± SD from triplicate wells. Statistical significance was assessed using Student’s t-test.Scale bars = 20 μm. (B) Immunofluorescence stains with MAP2 (red), synapsin-1 (green), and human-specific nuclei (white) antibodies in the co-culture condition of (A). The neurite length of GBM cells and the number of the synapse was analyzed by counting colocalized punta of synapsin-1 and MAP2. Data are represented as mean ± SD from triplicate wells. Statistical significance was assessed using Student’s t-test. Scale bars = 20 μm. (C-D) Representative images (C) and quantification (D) of immunostaining with DAAM1 (red) and SOX2 (green) antibodies in patient GBM tissue specimens. The ratio of DAAM1+ cells among SOX2+ cells is shown in primary-recurrent paired GBM tissues whole sections from 4 patients. Scale bars = 40 μm.
See also Figure S6.
Modeling of neuronal transition at recurrence using in vivo model system and preclinical testing of BRAF as a therapeutic target
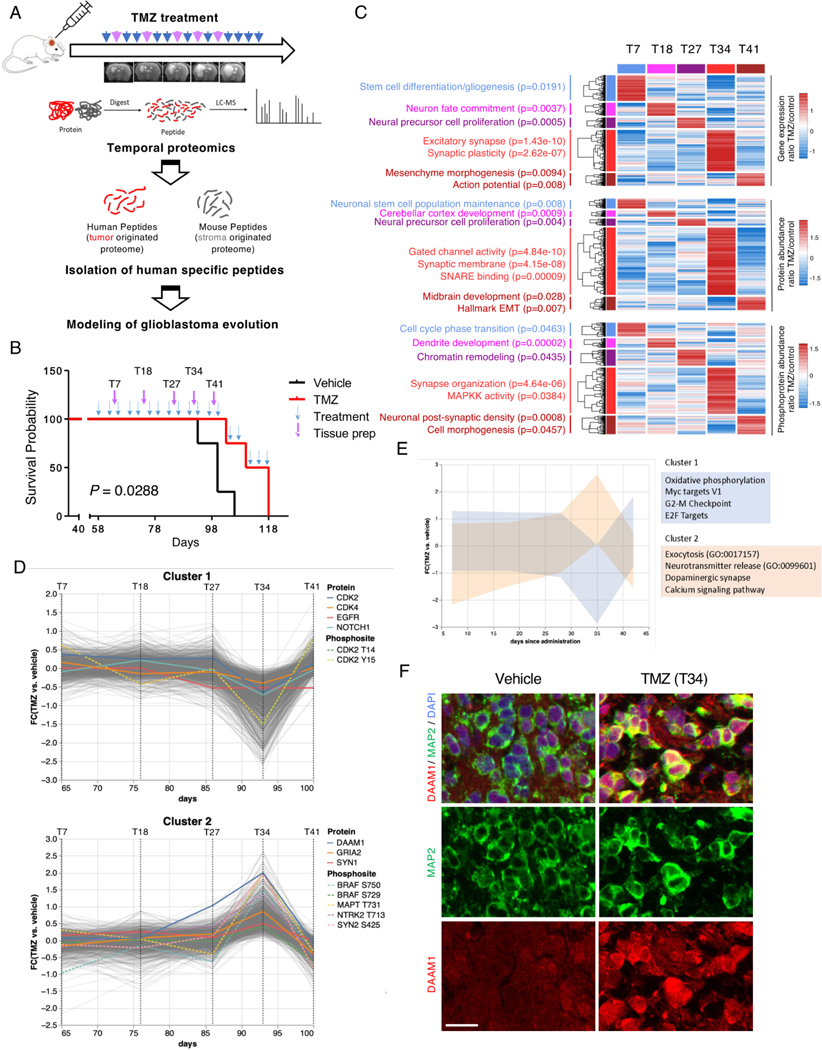
To investigate the dynamic regulation of the transcriptome, proteome, and phosphoproteome during the evolutionary trajectory of neuronal recurrent GBM, we generated a PDX model using tumor cells from a patient who underwent neuronal transition after therapy. Figure 7A outlines the experimental procedure, which mimicked the patient’s clinical course, from tumor assessment by magnetic resonance imaging (MRI) after intracranial injection of PDCs in mice. Mice were then treated with temozolomide (TMZ) or vehicle and GBM tissue specimens were collected at multiple time points (from day 7 to 41) for proteogenomic analysis. Tissue collection was based on MRI results that represent the recurrence status of the GBM model. Consistent with previous reports3,53–55, TMZ treatment significantly prolonged the overall mouse survival (Figure 7B). To characterize each time point, we interrogated transcriptomics, proteomics, and phospho-proteomics data to identify unique multi-omics features. The initial time points (T7, T18, and T27) exhibited increased stemness activities, cell proliferation, and chromatin remodeling (Figure 7C). At T34, (34 days after the start of TMZ treatment) we observed the strongest enrichment of neuronal features, particularly related to synaptic structure and organization. Similar patterns were also observed in a separate PDX model that was treated with TMZ and collected and analyzed at serial time points (Figure S7A). The phospho-proteome at this stage also showed activation of MAPK kinases. An independent analysis using unsupervised co-regulation clustering revealed two distinct clusters of proteins and phosphosites that diverged significantly at T34 (Figure 7D-E). Cluster 1, reduced at T34, was significantly enriched in cell cycle proteins, oxidative phosphorylation, MYC, and E2F targets. Conversely, cluster 2, which became abundant at T34, showed enrichment in proteins and pathways involved in neuronal functions, such as neurotransmitter release, dopaminergic synapse, and calcium signaling (Figure 7D-E). Immunofluorescence analysis confirmed increased expression of DAMM1 at T34 in TMZ-treated PDXs compared with vehicle-treated tumors (Figure 7F).
Figure 7. In vivo modeling of neuronal transition at recurrence.
(A) Overview of experimental design for proteogenomics analysis and modeling for therapeutic resistance by TMZ treatment in PDX models. (B) Survival curves of mice treated with 10 mg/kg TMZ (n=4) or vehicle (n=4) (p = 0.0288, log-rank Mantel-Cox test). T indicates the time point (in days) of tissue preparation for proteogenomic analysis. (C) Heat map of the transcriptome (upper panel), proteome (middle panel), and phospho-proteome (bottom panel) of PDX-GBM treated multiple times with TMZ. Differential expression/abundance at each time point was compared to the other times (log2(FC) TMZ/control > 0.58). Biological pathways significantly enriched for each platform analyzed are presented on the left (Fisher exact test, p < 0.05). (D) Co-regulation clustering of temporal proteomics data from PDX contrasting TMZ and vehicle treatment reveals two distinct clusters of proteins having the opposite expression pattern by day 35 after administration. (E) Pathway enrichment analysis of the two co-expressed clusters of proteins from Figure 7C. (F) Representative multiplex immunohistochemistry for MAP2 and DAAM1in TMZ- and vehicle-treated PDX model. Scale bars = 20 μm.
See also Figure S7.
To identify potential therapeutical targets driving the neuronal transition in recurrent GBM, we reconstructed a primary-recurrent GBM-specific kinase–phosphosite interaction network using SPHINKS, a recently developed tool that integrates proteomics and phospho-proteomics profiles to build an interactome of kinase–phospho-substrate pairs that are scored according to the strength of their interaction across all samples33. We implemented single-sample Master Kinase analysis33 to explore the dynamic interplay between kinase activity and its phospho-substrates. Serine/threonine-protein kinase BRAF emerged as among the most active kinases in both multi-omics-based cluster C2, mainly composed of neuronal recurrent GBM, and functional classifier-based NEU subtype (Figure 8A-B). Considering the availability of BRAF inhibitors that have shown treatment efficacy in patients with various cancer types harboring BRAF alterations56–58, we evaluated the therapeutic effect of two BRAF inhibitors, vemurafenib and dabrafenib, on neurite growth in GBM cells. Treatment with BRAF inhibitors at a concentration of 1μM resulted in collapsed neurites in recurrent GBM cells (Figure 8C). A spreading assay further confirmed that vemurafenib reduced neuronal cell migration, with the effect being most prominent in recurrent GBM cells (Figure S7B-D). Moreover, vemurafenib markedly reduced MAPK1/3 phosphorylation in recurrent GBM cells but had no effect in primary tumor cells (Figure 8D). Additionally, the combination of vemurafenib with TMZ significantly extended the overall survival and reduced the tumor size of GBM PDX models (Figure 8E-F). These findings collectively suggest that BRAF drives neuronal attributes during GBM evolution toward the neuronal state and that BRAF inhibitors can serve as useful therapeutic tools to prevent neuronal transformation associated with drug resistance in recurrent GBM.
Figure 8. Identification of BRAF as a key therapeutic target of GBM evolution.

(A) Heat map of master kinases with differential activity in each unsupervised multi-omics-based (left) and functional (right) GBM subtypes. (B) Box plots showing BRAF activity in each individual tumor stratified according unsupervised multi-omics based (left) or functional GBM subtype (rigth panel). Tumors are colored according to primary or recurrent status. Box plots span from the first to third quartiles, middle line represents median, and whiskers show 1.5× interquartile range. (left panel, C2 versus all other subtypes: p = 1.71e-08; right panel, NEU versus all other subtypes: p = 2.21e-05; Wilcoxon test) (C) Representative images (left) and quantification (right) of spreading assays in paired GBM cells treated with vehicle, 100 nM vemurafenib, or 100 nM dabrafenib (PMZ). Scale bars = 10 μm. Data are represented as mean ± SD from triplicate wells. Statistical significance was assessed using Student’s t-test (**p < 0.01; ***p < 0.001). (D) Immunoblot analysis of MAPK signaling-related proteins in paired GBM cells treated with vemurafenib. (E) Survival analysis of in vivo PDX models that were treated with either vehicle (n=6), TMZ (n=6), vemurafenib (n=6), or the combination of TMZ with vemurafenib (n=6) (p = 7.2X10−3, log-rank Mantel-Cox test). (F) Representative MRI and H&E sections of the mouse brains from (E). Scale bars = 2 mm.
See also Figures S7 and S8.
In conclusion, our analysis of matched primary-recurrent human GBM, PDX mouse models, and in vitro experiments demonstrate that GBM evolution under therapy primarily involves a transition toward the neuronal cellular state with the acquisition of synapse hallmarks. This transition is associated with multi-level activation of WNT/PCP signaling, a significant contribution of DAMM1, and dominance of BRAF activity (Figure S8).
Discussion
In this study, we present a large dataset of matched primary and recurrent GBM profiled by multi-omics platforms. The primary innovation of the study is the application of proteomics and phospho-proteomics to investigate the evolutionary trajectory of GBM. Recent work by the GLASS consortium defined the transcriptional changes sustained by recurrent glioma59. The limited correlation between mRNA and protein levels with more than 60% variance between protein levels and corresponding mRNA left unanswered key questions related to the protein effectors of GBM evolution under therapy. Our work uncovers the proteomic and phosphoproteomic changes leading to GBM recurrence and provides insights that can be used for diagnostics and precision therapeutics to prevent and/or delay drug resistance.
Consistent with previous reports, we found that the main chromosomal arm-level alterations (gain of chromosome 7 and loss of 10), which are known to drive GBM initiation, are highly conserved from diagnosis to recurrence, further supporting that genomics is not an adequate platform to inform on molecular targets driving the evolution of GBM after therapy. The paradigm for this conclusion is that de-activation of EGFR signaling at recurrence is consequent to the loss of EGFR protein and phosphorylation and independent of the status of the EGFR gene. Besides reinforcing the notion that protein level changes are better predictors of the evolutionary trajectory of GBM than genetic alterations, our observation suggests that EGFR amplification may be required for glioma initiation but is no longer necessary for tumor maintenance under therapeutic pressure. It is tempting to speculate that failure of EGFR targeting in recurrent GBM is caused by loss of EGFR dependency of GBM cells after exposure to standard-of-care treatment. We found that GBM evolution under therapy is marked by the collapse of the cell cycle, mitosis, and DNA damage/repair activities sustained by the loss of the genetic alterations and reduced abundance of the proteins involved in these functions. Conversely, recurrent tumors acquire neuronal and synaptic functions correlating with the gain of neuronal transition-promoting genes, thus qualifying them as drivers of recurrence with the potential to be included as novel biomarkers for targeted therapy.
Genomics, proteomics, and phosphoproteomics all independently captured significant upregulation of specialized neuronal and synaptic signaling programs in recurrent GBM. The enrichment of neuronal programs in a specific subtype of GBM and in recurrent tumors has been recently highlighted by a novel GBM classification that was guided by single-cell transcriptomic analysis and encapsulated biological activities as fundamental traits for the classifier32. Using the large longitudinal multi-omics cohort of GBM originally presented in this study, we determined that the most frequent functional subtype at recurrence was the C2 subtype that closely resembled the previous neuronal cellular states. Although the activation of a similar neuronal program has been recapitulated at the T34 stage in GBM xenograft models treated with TMZ, we also observed a shift toward a more mesenchymal phenotype at the T41 time-point, in agreement with previous studies indicating that gain of mesenchymal features may be associated with glioma progression, possibly steered by the tumor’s microenvironment13,14,60–62. However, the basis for the MES shift in recurrent GBM is not completely understood.
The proteomics approach and longitudinal analysis allowed us to trace mechanistically how evolving GBM executes the transition toward the neuronal state. Activation of neuronal programs coincides with activation at multiple levels of the RAS-MAPK pathway. Specifically, activation of neuronal attributes/functions coincided with elevation and hyperphosphorylation of RAS, BRAF, and MAPK proteins. Thus, we propose that in a large fraction of GBM acquiring resistance to standard-of-care and transiting towards the neuronal state, a pro-proliferation EGFR mediated signaling is converted to “pro-neuronal transition” activities via elevation of neurotrophic receptors (NTRK2/3) and the RAS-BRAF-MAPK pathway. We experimentally showed that the BRAF kinase is required to maintain axon projection and tumor cell migration in GBM cells as inhibition of BRAF led to the collapse of both attributes specifically in cells from recurrent tumors. The BRAF-MAPK signaling cascade operates in parallel with the non-canonical WNT pathway, which is similarly mediated by an increase in protein and phosphorylation of two main regulators, DAAM1 and DAAM2 that promote cytoskeletal rearrangement via RHOA promoting axonal outgrowth and cellular motility63–66. Indeed, the DAAM1 protein is up-regulated at recurrence in the GBM neuronal subtype and could be used as a marker for patient classification.
The link between axonal extension by neuronal tumor cells, the establishment of synaptic connectivity, and enhanced invasiveness are in line with recent reports showing that the acquisition of specialized neuronal functions is associated with the ability of GBM cells to invade the normal brain7,32,67. We experimentally demonstrated that increased neurite length and activation of synapse formation are both specific hallmarks of recurrent GBM.
The identification of increased phosphorylation and protein level of the BRAF kinase underscores MAPK signaling as the main driver pathway of the non-neuronal to neuronal subtype switch during GBM evolution. It is a longstanding knowledge that BRAF kinase plays a crucial role in neuronal differentiation68,69. BRAF controls pre-natal and postnatal neuronal morphogenesis and axon regeneration in injured mature CNS via the classical cell autonomous RAF-MEK signaling68–70, but also regulates synaptic transmission via the same MEK–ERK pathway71. Moreover, mutation or dysregulation of BRAF signaling impacts neuronal development and is associated with various neurodevelopmental disorders71,72. We speculate that the physiological functions of BRAF are hijacked by glioma cells promoting axon growth and synaptogenesis, therefore driving glioma invasion and connectivity with normal brain cells and affecting patient outcome73,74. Our results support the notion that BRAF blockade may reverse these changes, thus introducing BRAF inhibitors as a potential therapeutic option for recurrent GBM. Phase 2 studies and VE-BASKET trials suggested that the use of the second generation of selective BRAF inhibitors vemurafenib and dabrafenib, targeting the downstream MAPK signaling pathway, may be effective for the treatment of GBM as well as other cancer types75. The proteogenomic characterization, at multi-omics levels, of longitudinal GBM presented here has allowed us to address biological questions on this lethal disease that remained unexplored. The biological mechanisms underlying the subtype transition from non-neuronal at diagnosis to the neuronal subtype at recurrence have been uncovered through the exceptional profiling of multi-omics data of a large number of matched GBM pairs. The identification of DAAM1 as a potential biomarker of GBM evolution and BRAF as a therapeutically actionable target offers unexpected opportunities to manage recurrence in GBM patients.
STAR Methods
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Jong Bae Park (jbp@ncc.re.kr).
Material availability
All material generated in this study is available from the lead contact upon request with appropriate material transfer agreements.
Date and code availability
Data generated for this study are available through the Sequence Read Archive (SRA : PRJNA1051047) and Proteomic Data Commoms (PDC : PDC000514, PDC000515). The source codes used for all customized computational analyses we performed in this manuscript are available at GitHub at https://github.com/ablancsong/Longitudinal_GBM, https://github.com/lucgar/MAKINA and https://github.com/lucgar/GBMstates.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental model and study participant details
Clinical specimens of GBM
Tumor and blood samples from 123 qualified cases were collected from 5 tissue source sites in strict accordance with the CPTAC-3 protocol with informed consent from the patients. No adjacent tissue was collected as part of this study. This study contained both males (n = 79) and females (n = 44) from 2 different countries. Histopathologically defined adult glioblastoma tumors were only considered for analysis, with an age range of 1–84. Clinical data were obtained from the tissue source sites and reviewed for correctness and completeness of data.
Experimental Animal Ethics Statement
The mice used in this study were female BALB/c nude mice and ICR mice (for primary Mouse cortical neuron culture). All mice were handled and treated according to the animal care and handling protocols approved by the Institutional Animal Care and Use Committee of the National Cancer Center or Seoul National University.
Method Details
Genomic and Transcriptomic Analysis
Cryopulverization of Tissue Samples
Frozen tissue samples were weighed and washed with cold Phosphate-Buffered Saline (PBS) to remove blood contamination, put into tissueTUBE (Covaris, Woburn, MA), snap-frozen in liquid nitrogen, and pulverized using cryoPREP Tissue Disruption system (CP02, Covaris). The pulverized tissue powder was aliquoted from 10–20mg for DNA, RNA, and protein extraction.
Genomic DNA and Total RNA Extraction and Quality Check
Genomic DNA was extracted from cryopulverized tissue powders using a MagNA Pure 24 Total NA Isolation kit with a MagNAPure 24 automated instrument (Roche, Switzerland). Genomic DNA was extracted from the buffy coat using Maxwell® 16 Blood DNA Purification Kit with Maxwell® 16 automated instrument (Promega, USA). Purified gDNA was analyzed for concentration and purity with Nanodrop 8000 (Thermofisher, USA). The integrity of gDNA was analyzed with 1% gel electrophoresis. DNA quantitation was analyzed using Qubit dsDNA BR Assay Kit Thermofisher). Total RNA was extracted from pulverized tissue powders using RNeasy Mini Kit with a QIAcube automated instrument (Qiagen, Germany). Purified RNA was analyzed concentration and purity with Nanodrop 8000 (Thermofisher). For RNA quality analysis, RNA was analyzed using RNA Nano 6000 Kit with BioAnalyzer 2100 (Agilent, USA).
Whole-Exome Sequencing
WES of paired-end reads were generated using Illumina NovaSeq for genome sequencing. Genomic DNA was fragmentation using a Covaris LE220 instrument (Covaris), and the library kit used Twist Human Core Exome (Twist Bioscience, South San Francisco, USA).
Whole-Transcriptome Resequencing
Total RNA concentration was calculated by Quant-IT RiboGreen. To assess the integrity of the total RNA, samples are run on the TapeStation RNA screen tape. Only high-quality RNA preparations, with RIN greater than 7.0, were used for RNA library construction. A library was independently prepared with 1ug of total RNA for each sample by Illumina TruSeq Stranded mRNA Sample Prep Kit. The first step in the workflow involves purifying the poly-A-containing mRNA molecules using poly‐T‐attached magnetic beads. Following purification, the mRNA is fragmented into small pieces using divalent cations under elevated temperatures. The cleaved RNA fragments are copied into first-strand cDNA using SuperScript II reverse transcriptase and random primers. This is followed by second-strand cDNA synthesis using DNA Polymerase I, RNase H, and dUTP. These cDNA fragments then go through an end repair process, the addition of a single ‘A’ base, and then ligation of the adapters. The products are then purified and enriched with PCR to create the final cDNA library. The libraries were quantified using KAPA Library Quantification kits for Illumina Sequencing platforms according to the qPCR Quantification Protocol Guide (KAPA BIOSYSTEMS, #KK4854) and qualified using the TapeStation D1000 Screen Tape (Agilent Technologies, # 5067–5582). Indexed libraries were then submitted to an Illumina NovaSeq (Illumina, Inc., San Diego, CA, USA), and the paired-end (2×100 bp) sequencing was performed by Macrogen Incorporated.
Data Preprocessing
Mutation analysis
The paired-end sequenced reads from the FASTQ files were aligned on the human genome reference (hg19) and aligned using Burrows–Wheeler Aligner94. SAMtools81, Picard (https://broadinstitute.github.io/picard/), Genome Analysis Toolkit (GATK)82, and dbSNP95 were used BAM files were preprocessed for handling, sorting, removing duplicate reads, and realigning reads around potential small indels, respectively. Single nucleotide variants (SNVs) were generated by VarScan284, Freebayes85, Strelka86, VarDict87, MuTect96, and SomaticIndelDetector97 were used to predict the confidence for somatic mutations from tumor and matched normal pairs. Variant Effect Predictor (VEP)98 was used to annotate somatic mutations with potential functional effects and other significant information83. The functional effect of missense SNVs and in-frame indels was determined using multiple prediction algorithms. MutationTaster288, Polyphen289, Provean90, and SIFT91 were applied to predict the pathogenicity of missense SNVs99. A total of 122 longitudinal pair samples were analyzed.
Copy number alteration analysis
Copy number alterations were generated and calculated by ngCGH (https://github.com/seandavi/ngCGH) python package aCGH-like data using the WES data. Tumors and matched normal blood were used to generate a curated amplification/deletion profile of the corresponding gene-based read counts. Copy-number thresholds for CN variations (CNV) calls were assessed using GISTIC scores (−2 homozygous deletion, −1 heterozygous deletion, 0 no change, 1 gain, 2 amplification). Non-protein-coding genes were removed from the analysis. To select CNV calls that impact gene expression in cis, functional copy number variation (fCNV) analysis was performed as described32. A total of 122 longitudinal pair samples were analyzed.
Gene expression analysis
RNA sequencing data was aligned and processed by in-house custom pipelines. Data processing and analysis were performed using the R/Bioconductor libraries. Normalized gene expression values were calculated using TPM76. A total of 86 longitudinal pair samples were analyzed.
Genetic alteration frequency analysis in driver genes during evolution
The association of fCNVs in driver genes with primary and recurrent tumor subgroups was determined by comparing the number of samples harboring a specific CNV in each group using the two-tailed Fisher’s exact test. Only genes with fCNV calls in at least five pairs (either primary or recurrent or both specimens) in the dataset were examined. We selected CNVs that scored as statistically significant according to p < 0.10. A total of 122 longitudinal pair samples were analyzed.
Single-nuclei data processing
Gene expression was derived as counts-per-million (CPM). We divided the CPM values by 10 as the size of single-cell libraries is estimated to be in the order of 100,000 transcripts. Low-quality cells were filtered out based on the low number of detected genes (n = 1000) or high expression of mitochondrially-encoded genes (a proxy for dying cells; cut-off = 1%). Gene expression was log2-transformed and lowly-expressed genes were filtered out.
Cells were clustered using graph-based clustering (Louvain’s algorithm) of a 2-dimensional UMAP space. Malignant and non-malignant cells were distinguished by inferring the copy number of chromosome 7 and chromosome 10 (hallmarks of glioblastoma) from gene expression using inferCNV tool51. Myeloid, T cells, OPC, oligodendrocytes, neurons, astrocytes, endothelial, and pericytes cells were characterized based on the expression of known marker genes.
Malignant cells were assigned to either pathway-based or previously described transcriptomic states based on the highest significant score using single sample MWW-GST100 (ssMWW-GST) and the subtype-specific gene signatures reported in original publications32,52. The simplicity score for each cell was derived by computing the difference between the normalized enriched score (NES) of the assigned state and any other state as indicated in the axis labels in Figure 5H. For example, the upper right quadrant is composed of NEU cells with x-axis values computed as NES NEU - NES PPR and y-axis values as NES NEU – NES GPM. Association between normal cell types or tumor cell states and primary and recurrent status was assessed by chi-squared test.
Proteomic and Phosphoproteomic Analysis
Protein Extraction and Tryptic Digestion
For TMT11-plex proteomic and phosphoproteomic experiments, cryopulverized tissue powder samples were solubilized in SDS solubilization buffer (5% SDS, 50mM TEAB pH 8.5) using S220 Focused-ultrasonicator (Covaris). Proteins were tryptic digested using S-Trap™ spin columns (Protifi, Huntington, NY) with the manufacturer’s instructions. The samples were reduced by DTT and alkylated by iodoacetamide (IAA). After quenching the alkylation reaction, additional SDS and phosphoric acid were added so that the final concentration was 5% SDS and 1.2% phosphoric acid. Acidified samples were mixed with 90% methanol in 100mM TEAB, loaded into the S-Trap micro columns, and incubated with mass spec grade trypsin/LysC (Promega) for 3 h at 47°C. Eluted peptides were evaporated using a vacuum concentrator and cleaned up using C18 spin columns (Thermofisher).
TMT 11-plex Labeling
Desalted peptide samples were reconstituted in 100mM TEAB, and labeled using TMT11plex reagents. Each prepared TMT reagent was transferred to the peptide sample, the mixture was incubated for 1 h, quenched by the addition of 8 mL of 5% hydroxylamine, and incubated for 15 min at room temperature. Differently labeled 11plex peptides were pooled and dried using a vacuum concentrator.
Peptide Fractionation by Mid-pH Reverse Phase Liquid Chromatography
The pooled 11plex TMT-labeled sample was separated using the Agilent 1260 Infinity HPLC system (Agilent, Palo Alto, CA)101. Solvents A and B were 10 mM triethylammonium bicarbonate (TEAB) in water (pH 7.5) and, 10 mM TEAB in 90% acetonitrile (ACN, pH 7.5) respectively. The pooled sample was fractionated by Xbridge C18 analytical column (4.6 mm × 250 mm, 130 Å, 5 um) with a gradient from 0% solvent B for 15 min, 0 to 5% solvent B for 10 min, 5 to 35% solvent B for 60 min, from 35 to 70% solvent B for 15 min, 70% solvent B for 10 min, 70 to 0% solvent B for 10 min at a flow rate of 500 μL/min. A total of 96 fractions were collected every minute from 15 to 110 min and were pooled into 24 non-continuous peptide fractions with a stepwise concentration strategy102. Following desalting with C18 desalting columns (Thermofisher), 5% of each fraction was aliquoted and dried by Speed-vac concentrator for global proteome analysis. The remaining 95% concatenated fractions were further combined into 12 fractions, and dried using a vacuum concentrator for phosphopeptide enrichment.
Phosphopeptide Enrichment
Phosphopeptide enrichment was performed using Fe-NTA immobilized metal affinity chromatography method101. Brifely, Ni-NTA magnetic agarose beads (Qiagen, Valencia, CA) were washed with deionized water and treated with 100mM EDTA, pH 8.0 for 30 minutes with end-over-end rotation. The EDTA solution was removed, and the beads were washed with deionized water and treated with 10mM aqueous FeCl3 metal ion solution for 30min with end-over-end rotation. After the removal of excess metal ions, the beads were washed with deionized water, resuspended in 1:1:1 acetonitrile/methanol/0.01% acetic acid solution, and aliquoted into microcentrifuge tubes. Fractionated peptide samples were resuspended in resuspension buffer (80% acetonitrile, 0.1% TFA). After washing the aliquoted Fe3+-NTA beads with resuspension buffer, the resuspended peptide sample was added and treated with end-over-end rotation for 30 minutes. The supernatant was collected and dried for future reference, the beads were washed with resuspension buffer and the remaining solution was discarded. The enriched phosphopeptide was eluted with 1:1:1 acetonitrile/2.5% Ammonia water/2mM phosphate buffer, acidified with 10% TFA solution, and dried using a vacuum concentrator.
LC-MS/MS Analysis
TMT-labeled peptides prepared for global proteome and phosphoproteome analysis were resuspended with 0.1% formic acid in water, separated, and analyzed using a Thermo Scientific Ultimate 3000 RSLCnano system (Thermo Scientific) coupled to a Q Exactive HF-X hybrid quadrupole-orbitrap mass spectrometer (Thermo Scientific). Solvents A and B were 0.1% FA in water and 0.1% FA in acetonitrile, respectively. The peptides were loaded onto the trap column (Acclaum PepMap™ 100, 75μm x 2cm), separated by the analytical column (EASY-Spray column, 75μm x 50cm, Thermofisher) with a gradient from 5 to 24% solvent B for 150min, 24 to 36% solvent B for 30 min (for global proteome); 5 to 24% solvent B for 170min, 24 to 36% solvent B for 10 min (for phosphoproteome) at a flow rate 300 nL/min. The Q Exactive HF-X Orbitrap mass analyzer was operated in a top 10 data-dependent method. Full MS scans were acquired over the range m/z 350–2000 with a mass resolution of 120,000 (at m/z 200). The AGC target value was 3.00E+06. The ten most intense peaks with charge state 2–6 were fragmented in the higher-energy collisional dissociation (HCD) collision cell with a normalized collision energy of 32 and tandem mass spectra were acquired in the Orbitrap mass analyzer with a mass resolution of 45,000 at m/z 200.
Mass Spectrometry Data Analysis
Database Search and Processing
Raw mass spectrometry files were converted to open mzML format using the msconvert utility of the ProteoWizard software suite. The mzML spectral data were mapped to the human UniProt database (UP000005640.fasta.gz). FragPipe (ver.17.0), including MSFragger, Philosopher and TMT-integrator program92. All identified proteins had an FDR of ≤ 1%, which was calculated at the peptide level. For the global proteome, the search parameters allowed for tryptic specificity of up to two missed cleavages, with carbamidomethyl-modifications of cysteine as a fixed modification and oxidation of methionine as a dynamic modification. The mass search parameters for −1, 0, +1, +2, and +3 ions included mass error tolerances of 20 ppm for precursor ions. For the phosphoproteome, the search parameters allowed for tryptic specificity of up to two missed cleavages, with modified serine, tyrosine, and threonine as variable modifications. The mass search parameters for 0, +1, +2, and +3 ions included mass error tolerances of 20 ppm for precursor ions. The protein expression was quantified with the isobaric TMT 11 option and normalized to the relative expression ratio against the global reference pool of each TMT 11 set. Batch corrections were applied to global proteome and phosphoproteome abundance data to remove the technical differences between different TMT runs before performing advanced analysis. To remove batch effects, ComBat, an R library with tumor/normal status adjustment, was applied78. To impute missing values, the DreamAI package was used to perform an imputation of the proteins and phosphopeptides with a missing rate of less than 50%. The resulting global proteome numbering 10,533 and phosphoproteome numbering 13,328 were used for downstream analysis. A total of 105 longitudinal pair samples were analyzed for global proteome and a total of 89 pair samples were analyzed for phosphor-proteome.
Multi-omics clustering using unsupervised classification
We selected the following proteogenomic features for multi-omics clustering: RNA, global protein, phosphoprotein expression. All data tables were then concatenated and all data with no missing values were used. We used unsupervised clustering of tumor samples and to identify proteogenomic features that show characteristic expression patterns for each cluster. The median absolute deviation (MAD) is used for the measurement of variance corresponding to the absolute deviation from the median, and genes based on MAD10 were used for clustering. We selected k = 3 to confirm the specific difference between the clustering results and the biological pathways of multi-omics. A total of 86 longitudinal pair samples with all available datasets were analyzed.
Co-expressed gene clustering
To analyze co-regulated global protein and phosphorylation clusters across temporal and treatment conditions in the NCC-772 PDX model, we used Abu-Jamous and Kelly’s clust algorithm103 on the normalized and imputed global proteome and phosphoproteome ratio data. This unsupervised clustering algorithm initializes multiple k-means clusters of genes with varying values of k and filters significant seed clusters using M-N scatter plot selection104 and outlier removal. Clust was run with z-score normalization (parameter -n 4) and tightness 0.25 (-t 0.25) to generate two clusters of sizes 839 and 540. The differentially expressed genes and biological pathways across these clusters were computed with GSEApy (https://github.com/zqfang/GSEApy) against the “KEGG_2021_Human” and “GO_Biological_Process_2021” databases.
Pearson Correlation Coefficient in EGFR-altered GBM
We employed the Pearson correlation coefficients method to measure the relationships between proteins that are associated with key regulators such as PI3K, AKT, PTEN, and mTOR, with the downstream pathways of RAS, RAF, MEK, and ERK. These correlations were assessed based on the protein abundance of EGFR. A total of 36 longitudinal pair samples with shared EGFR alterations were analyzed.
Proteomics-based functional classification of primary and recurrent GBM
Classification of CPTAC-GBM tumors in functional subtypes has been recently reported with the identification of subtype-specific protein signatures33. The 50 highest-scoring proteins from these signatures were used to calculate the enrichment of each functional GBM subtype (normalized enrichment score of subtype activity, NES) for each tumor using single-sample MWW-GST (ssMWW-GST). Each tumor was assigned to a subtype only if NES was higher than 0.58 and the enrichment significance (FDR) < 0.05, otherwise the tumor was classified as undefined. If a tumor has shown statistically significant enrichment of multiple subtypes, the subtype with the highest score has been assigned. Matched pairs where at least one between primary or recurrent specimens was classified as undefined have been removed. A total of 105 longitudinal pair samples were analyzed.
Identification of functional subtypes-specific multi-omics features
To define the functional pathways enriched in each GBM subtype, we aggregated the MSigDB c5.bp, c5.mf, c5.cc, Hallmark and KEGG collections of gene sets, retaining only pathways composed of at least 15 genes, resulting in 5,032 gene sets. Having classified both primary and recurrent GBM tumors, we then generated the ranked lists of genes and proteins with differential expression/abundance in each GBM subtype compared to the others using the transcriptomic and proteomics data, respectively, and the Mann Whitney Wilcoxon (MWW) test. We defined as subtype-specific gene expression and protein abundance signatures the 150 highest scoring genes/proteins in the ranked lists. These proteins/genes were used as input features of the pathway enrichment analysis to define the biological functions significantly active in NEU recurrent when compared to primary tumors (Fisher’s exact test, p < 0.05). The association of fCNVs with distinct GBM subtypes was determined by comparing the number of samples harboring a specific CNV in each subtype with the other groups using the two-tailed Fisher’s exact test (for one subtype compared to all others combined). Only genes with fCNV calls in at least three samples in the dataset were examined. We selected CNVs that scored as statistically significant according to p < 0.10. Driver genes or genes included in specific biological pathways as representative of each subtype were listed in the integrative heat map. We applied BlackSheep’s differential extreme value analysis module to define the list of outlier phosphorylated proteins in functional GBM subtypes (p < 0.05). To identify only sites whose phosphorylation is not explained by protein abundance, global protein and phosphorylation abundance was compared between primary non-neuronal and recurrent neuronal tumor subgroups using Wilcoxon test. Phosphorylation or protein changes were considered significantly different if they had p < 0.01 and log2(FC) > 0.3.
Identification of subtype-specific master kinases
Using the entire cohort of primary-recurrent GBM, we reconstructed an unbiased interactome of kinases-substrates using SPHINKS, a recently developed machine-learning method that uses a semi-supervised approach to integrate proteomics and phospho-proteomics data and infer active master kinases responsible of the phospho-program in each GBM subtype. The activity of each master kinase in each sample has been derived as previously described33. We selected master kinases that showed a significant difference in activity in one subtype compared to the others using MWW test (effect size >0.3 and p < 0.01).
Cells and cell culture conditions
Human primary GBM cells were maintained in DMEM/F-12 50/50 supplemented with B27, EGF (10 ng/ml), bFGF (5 ng/ml). All cells were repeatedly screened for mycoplasma.
Primary Culture of Cortical Neurons
Primary cortical neurons dissected from E15 mice as previously reported105 with minor modifications. Cerebral cortices of E15 mice were dissected out and digested papain diluted in HBSS containing DNase for 40 min at 37°C. Enzyme-digested cortices were washed three times with MEM and dissociated in MEM. The dissociated neurons were centrifuged to remove the supernatant and cells were resuspended with a suitable volume of prewarmed Neurobasal Medium supplemented with GlutaMAX and B27 and the neurons were plated on poly-L-lysine-coated plastic dishes. Then the cells were cultured for 10 days. Half-media changes were performed at DIV 3, 7, and 10 with the respective media types.
Cell spreading assay
Plates (96 wells) were coated with poly-d-lysine followed by OMgp (25 ng). GBM cells (1×104) were seeded on each 96-well plate. After 18 h immunofFuorescence staining of phalloidin was performed essentially as described106. For the counting of normally spread cells, images were taken from triplicate wells independently (Zeiss) and the length of the cell exceeding 20 μm were considered as normally spread cells.
Cell migration assay on OMgp coated plate
Plates (96 wells) were coated with nitrocellulose membrane, poly-d-lysine or OMgp (25 μg/ml). After OMgp coating, those well were coated with fibronectin. Then, primary GBM cells (1×104) were seeded on OMgp + fibronectin coated well and applied real time imaging for 24h with or without vemurafenib or dabrafenib in every 15 min..
Western blot
Proteins were extracted with RIPA buffer with complete protease inhibitors, separated by electrophoresis, transferred to PVDF membranes (Millipore), and blocked with 5% skim milk. Primary antibodies against phospho-p44/42 MAPK (1:1000, 42–44 kDa), p38 MAPK (1:1000, 40 kDa), and GAPDH (1:1000, 37 kDa) were incubated overnight at 4°C. Immunoreactive bands were visualized using peroxidase-labelled affinity purified secondary antibodies and the detection reagent Amersham ECL prime western blotting detection reagent (GE Healthcare). In every western blot panel, more than three independent experiments were performed, and one representative immunoblot was shown.
In vivo study
We anesthetized mice using Zoletil 50 to perform brain orthotopic injection. For the orthotopic mouse model, cells were first suspended in DMEM and DMEM/F-12 supplemented with B27, EGF (10 ng/mL) and bFGF (5 ng/mL), and then transplanted into the left striatum of 5-week-old female BALB/c nude mice by stereotactic injection. The injection coordinates were 2.2 mm to the left of the midline and 0.2 mm posterior to the bregma at a depth of 3.5 mm. The tumors were extracted, pooled for each experimental group, and mechanically disaggregated using stainless steel operating scissors. The brain of each mouse was harvested and fixed in 4% PFA for H&E and IHC staining and PBS for proteomics analysis. For therapeutic resistance model by Temozolomide (TMZ) treatment. TMZ (10 mg/kg) treatment for twice a week and vemurafenib(75 mg/kg) treatment for daily b.i.d was orally administered. Survival was analyzed using GraphPad PRISM software (version 7; GraphPad PRISM, La Jolla, CA, USA).
Immunofluorescence staining
For observation of histological features, mice were anaesthetized with isoflurane and killed by transcardial perfusion with 10 ml of PBS, followed by 10 ml of 4% paraformaldehyde solution. Brains were removed, fixed with 4% paraformaldehyde for 24 h at 4℃. Antigen retrieval was performed by pressure cooking for 5 min in citrate buffer (pH 6.0). The tissue slides were immunostained for the cancer stem cell markers, SOX2 (1:200) and HuNu (1:500), as MAP2 (1:500), synapsin-1 (1:200), and DAAM1 (1:100) by incubating overnight at 4 °C in a humidified chamber with primary antibody, diluted for the working concentration with antibody diluent buffer (IHC World). Immunoreactive proteins were visualized with the appropriate Alexa Fluor 488- or Alexa Fluor 568- or Alexa Fluor 647-conjugated secondary antibody (1:500). Nuclei were stained with 4’,6-diamidino-2-phenylindole (DAPI, 1:50000). Fluorescent slides were scanned using the Vectra Polaris 3.0 (Akoya Biosciences, Marlborough, MA, USA) using 20×magnifcation and auto-estimated exposure times. Whole slide scan was imaged using 4 epi-fluorescent filters (DAPI, Opal 520, Opal 620, Opal 690). High resolution fluorescence images were obtained using an LSM 780 confocal laser-scanning microscope (Carl Zeiss).
Identification of multi-omics features in distinct time points after TMZ treatment in PDX model of GBM
Genes/proteins/phosphoproteins with fold-change, derived as difference between the expression of each time point and the average expression of the others, above 0.58 have been defined as differential features. Those genes/proteins/phosphoproteins have been used to perform enrichment analysis (Fisher’s exact test, p < 0.05).
Quantification and statistical analysis
All data were analyzed and processed using R statistical computing environment (version 4.2.2). Details of specific functions and libraries are provided in the methods sections. Significance was determined by Mann-Whitney U test (Wilcoxon rank-sum test) and Fisher’s exact test, Student’s t-test, Paired t-test, Chi-squared test, Pearson Correlation Coefficient. P values <0.05 is considered statistically significant. Survival comparisons were quantified using log-rank Mantel-Cox test.
Supplementary Material
Table S1. GBM patient clinical characteristics, genomic, and proteomic information, related to Figure 1
Table S2. GO Analysis between primary and recurrent GBM, related to Figure 3
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| GAPDH(14C10) | Cell signaling | 2118S |
| P38 MAPK | Cell signaling | 9212 |
| MAP2 | R&D systems | MAB8304 |
| MAP2 | Synaptic Systems | 188 004 |
| Synapsin-1 | Synaptic Systems | 106 011 |
| HuNu | Sigma-aldrich | MAB1281 |
| DAAM1 | proteintech | 14876-1-AP |
| Phospho-p44/42 MAP kinase(Thr202/Tyr204) | Cell signaling | 4370 |
| SOX2 | R&D systems | AF2018 |
| KPL Affinity purified antibody Peroxidase labeled Goat anti-Mouse IgG | Seracare | 5450-0011 |
| Alexa Fluor 488 conjugated secondary antibody | Thermo-Fisher Scientific | A21206 |
| Alexa Fluor 568-conjugated secondary antibody | Thermo-Fisher Scientific | A10037 |
| Alexa Fluor 647-conjugated secondary antibody | Thermo-Fisher Scientific | A21447 |
| Biological samples | ||
| Human GBM tumor tissue samples and blood samples | National Cancer Center, Seoul National University College of Medicine, Samsung Medical Center, Chonnam National University Hwasun Hospital, Hôpital de la Pitié-Salpêtrière | See Table S1 for details |
| Mouse tumors and samples for the PDX | National Cancer Center | |
| Chemicals, peptides, and recombinant proteins | ||
| Quant-IT RiboGreen | Invitrogen | R11490 |
| Dithiothreitol | Thermo Scientific | R0861 |
| Iodoacetamide | Sigma-Aldrich | I6125 |
| Phosphoric acid | Sigma-Aldrich | 695017 |
| Trypsin/Lys-C Mix, Mass Spec Grage | Promega | V5072 |
| UltraPure 0.5M EDTA, pH8.0 | Invitrogen | 15575020 |
| Iron (III) chloride | Sigma Aldrich | 451649 |
| Ammonium hydroxide solution | Fluka | 17837 |
| Sodium phosphate monobasic | Sigma-Aldrich | S0751 |
| Sodium phosphate dibasic | Sigma-Aldrich | S9763 |
| Complete Protease Inhibitor Cocktail | Roche | 11697498001 |
| PhosSTOP | Roche | 4906845001 |
| Ni-NTA agarose beads | Qiagen | 36113 |
| 50% Hydroxylamine | Thermo Scientific | 90115 |
| Phosphatase Inhibitor Cocktail (100x) | Gendepot | P3200-001 |
| Protease inhibitor cocktail | Roche | 11836153001 |
| Sodium Dodecylsulfate | Thermo Scientific | J75819 |
| Water, with 0.1% (v/v) Formic acid, Optima® LC/MS grade | Fisher chemical | LS118 |
| 1M Triethylammonium bicarbonate (TEAB) | Thermo Scientific | 90114 |
| 10X Phosphate Buffered Saline | Corning | 46-013-CM |
| Phosphate-Buffered Saline | Welgene | LB-001-02 |
| Methanol | Honeywell | AH230-4 |
| Acetonitrile | J.T Baker | 9017-03 |
| Water | Millipore | 1.15333.2500 |
| 2-Propanol | J.T Baker | 9095-03 |
| Acetic acid | Merk | 1.00063 |
| Formic acid | Sigma-Aldrich | 5.33002.0050 |
| Trifluoroacetic acid | Sigma Aldrich | 302031 |
| RIPA Buffer | Biochemicals | R3001 |
| DMEM/F-12 | Welgene | LM002-04 |
| B27 | Invitrogen | 17504-044 |
| EGF | R&D Systems | 236-EG-01M |
| bFGF | R&D Systems | 4114-TC-01M |
| Papain | Worthington | LS003120 |
| DNase | Sigma | D5026-376KU |
| Zoletil 50 | Virbac | |
| MEM | Cellgro | 10-010-CV |
| poly-d-lysine | Sigma | 27964-99-4 |
| skim milk | BD | 232100 |
| Neurobasal Medium | Gibco | 10888-022 |
| Recombinant Human OMgp Protein, CF | R&D system | 1673-OM |
| Temozolomide (TMZ) | Sigma | T2577 |
| Vemurafenib | Medchemexpress | 918504-65-1 |
| Dabrafenib | Medchemexpress | HY-14660A |
| Alexa Fluor™ 568 Phalloidin | invitrogen | A12380 |
| GlutaMAX | Gibco | 35050061 |
| DAPI | Sigma | D9542 |
| IHC-Tek Antibody Diluent pH7.4 | IHCWORLD | IW-1000 |
| Critical commercial assays | ||
| MagNA Pure 24 Total NA Isolation kit | Roche | 07658036001 |
| Maxwell® 16 Blood DNA Purification Kit | Promega | AS1010 |
| TapeStation RNA screen tape | Agilent | 5067-5576 |
| SuperScript II reverse transcriptase | Invitrogen | 18064014 |
| TruSeq Stranded mRNA Sample Prep Kit | Illumina | RS-122-2101 |
| TMT10plex Isobaric Label Reagent Set plus TMT11-131C | Thermo Scientific | A37725 |
| Deposited data | ||
| WES and Bulk-RNA seq data for GBM samples | This paper | SRA: PRJNA1051047 (https://www.ncbi.nlm.nih.gov/sra) |
| Proteome and Phosphoproteome data for GBM evolution | This paper | PDC: PDC000514, PDC000515 (https://proteomic.datacommons.cancer.gov/pdc) |
| Experimental models: Organisms/strains | ||
| Balb/c-nu | orientbio | 01142040 |
| CrlOri : CD1(ICR)_TP | orientbio | |
| ICR_TP15 | DBL | |
| Software and algorithms | ||
| RSEM (v1.55.0) | Li et al.76 | https://github.com/deweylab/RSEM |
| HISAT (v2.0.522) | Pertea et al.77 | https://github.com/DaehwanKimLab/hisat2 |
| ComBat (v3.20.0) | Johnson et al.78 | https://bioconductor.org/packages/SVA |
| DreamAI | DreamAI | https://github.com/WangLab-MSSM/DreamAI |
| ssGSEA (v1.48.3) | Hänzelmann et al.79 | https://bioconductor.org/packages/GSVA |
| BWA (v0.7.17) | Li et al.80 | https://github.com/lh3/bwa |
| Samtools (v1.18) | Li et al.81 | http://www.htslib.org |
| GATK (v4.2.4.0) | DePristo et al.82 | https://gatk.broadinstitute.org |
| Picard | Picard | https://broadinstitute.github.io/picard |
| ngCGH | ngCGH | https://github.com/seandavi/ngCGH |
| VEP (v106) | McLaren et al.83 | https://asia.ensembl.org/info/docs/tools/vep |
| VarScan2 (v2.4.0) | Koboldt et al.84 | https://varscan.sourceforge.net/ |
| Freebayes | Garrison et al.85 | https://github.com/freebayes/freebayes |
| Strelka | Saunders et al.86 | https://github.com/Illumina/strelka |
| VarDict | Lai et al.87 | https://github.com/AstraZeneca-NGS/VarDict |
| MutationTaster2 | Schwarz et al.88 | https://www.mutationtaster.org/ |
| Polyphen2 | Adzhubei et al.89 | http://genetics.bwh.harvard.edu/pph2/ |
| Provean (v1.1.3) | Choi et al.90 | http://provean.jcvi.org/index.php |
| SIFT | Sim et al.91 | https://sift.bii.a-star.edu.sg/ |
| inferCNV (v1.1.0) | inferCNV | https://github.com/broadinstitute/infercnv |
| MSFragger (v3.4) | Kong et al.92 | https://github.com/Nesvilab/MSFragger/ |
| Philosopher (v4.1.0) | da Veiga Leprevost et al.93 | https://github.com/nesvilab/philosopher/ |
| R 4.2.2 | R Core Team | https://www.R-project.org/ |
Highlights.
Longitudinal proteogenomic analysis reveals increased neuronal state at recurrence.
Neuronal transition associates with activation of WNT/PCP pathway and BRAF kinase.
Resistance to therapy via neuronal transition is recapitulated in GBM PDX models.
BRAF kinase inhibition impairs the neuronal transition of GBM at recurrence.
Acknowledgments
This work was supported by the grant NCC-1810860 from National Cancer Center of Korea to J.B.P., by grants NRF-2021R1A2C3013315, NRF-2021M3F7A1083230 from National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT to J.B.P. This research was also supported by NCC-1810863 from National Cancer Center of Korea to H.Y. and by the Korea Evaluation Institute of Industrial Technology grant funded by the Korean government (MSIT) (20009125) to J.K.S.
This work was also supported by NIH grant R35CA253183 to A.I., by INCa, the French Ministry of Solidarity and Health and Inserm (INCA-DGOS-Inserm_12560) to M.S. and under the auspices of a Memorandum of Understanding between proteogenomic Research Project of NCC, Korea and the U.S. National Cancer Institute’s International Cancer Proteogenome Consortium (ICPC). ICPC encourages international cooperation among institutions and nations in proteogenomic cancer research in which proteogenomic datasets are made available to the public. We thank Dr. Henry Rodriguez and Dr. Ana I. Robles from the U.S. National Cancer Institute’s Clinical Proteomic Tumor Analysis Consortium (CPTAC) for helpful discussions.
Footnotes
Declaration of interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lapointe S, Perry A, and Butowski NA (2018). Primary brain tumours in adults. Lancet 392, 432–446. 10.1016/S0140-6736(18)30990-5. [DOI] [PubMed] [Google Scholar]
- 2.Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, et al. (2021). The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol 23, 1231–1251. 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. (2005). Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352, 987–996. 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Fougner V, Hasselbalch B, Lassen U, Weischenfeldt J, Poulsen HS, and Urup T. (2022). Implementing targeted therapies in the treatment of glioblastoma: Previous shortcomings, future promises, and a multimodal strategy recommendation. Neurooncol Adv 4, vdac157. 10.1093/noajnl/vdac157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weller M, Cloughesy T, Perry JR, and Wick W. (2013). Standards of care for treatment of recurrent glioblastoma--are we there yet? Neuro Oncol 15, 4–27. 10.1093/neuonc/nos273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barthel FP, Johnson KC, Varn FS, Moskalik AD, Tanner G, Kocakavuk E, Anderson KJ, Abiola O, Aldape K, Alfaro KD, et al. (2019). Longitudinal molecular trajectories of diffuse glioma in adults. Nature 576, 112–120. 10.1038/s41586-019-1775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varn FS, Johnson KC, Martinek J, Huse JT, Nasrallah MP, Wesseling P, Cooper LAD, Malta TM, Wade TE, Sabedot TS, et al. (2022). Glioma progression is shaped by genetic evolution and microenvironment interactions. Cell 185, 2184-2199 e2116. 10.1016/j.cell.2022.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim J, Lee IH, Cho HJ, Park CK, Jung YS, Kim Y, Nam SH, Kim BS, Johnson MD, Kong DS, et al. (2015). Spatiotemporal Evolution of the Primary Glioblastoma Genome. Cancer Cell 28, 318–328. 10.1016/j.ccell.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Kim H, Zheng S, Amini SS, Virk SM, Mikkelsen T, Brat DJ, Grimsby J, Sougnez C, Muller F, Hu J, et al. (2015). Whole-genome and multisector exome sequencing of primary and post-treatment glioblastoma reveals patterns of tumor evolution. Genome Res 25, 316–327. 10.1101/gr.180612.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Cazzato E, Ladewig E, Frattini V, Rosenbloom DI, Zairis S, Abate F, Liu Z, Elliott O, Shin YJ, et al. (2016). Clonal evolution of glioblastoma under therapy. Nat Genet 48, 768–776. 10.1038/ng.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JK, Wang J, Sa JK, Ladewig E, Lee HO, Lee IH, Kang HJ, Rosenbloom DS, Camara PG, Liu Z, et al. (2017). Spatiotemporal genomic architecture informs precision oncology in glioblastoma. Nat Genet 49, 594–599. 10.1038/ng.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korber V, Yang J, Barah P, Wu Y, Stichel D, Gu Z, Fletcher MNC, Jones D, Hentschel B, Lamszus K, et al. (2019). Evolutionary Trajectories of IDH(WT) Glioblastomas Reveal a Common Path of Early Tumorigenesis Instigated Years ahead of Initial Diagnosis. Cancer Cell 35, 692–704 e612. 10.1016/j.ccell.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Hoogstrate Y, Draaisma K, Ghisai SA, van Hijfte L, Barin N, de Heer I, Coppieters W, van den Bosch TPP, Bolleboom A, Gao Z, et al. (2023). Transcriptome analysis reveals tumor microenvironment changes in glioblastoma. Cancer Cell 41, 678–692 e677. 10.1016/j.ccell.2023.02.019. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Jung J, Babikir H, Shamardani K, Jain S, Feng X, Gupta N, Rosi S, Chang S, Raleigh D, et al. (2022). A single-cell atlas of glioblastoma evolution under therapy reveals cell-intrinsic and cell-extrinsic therapeutic targets. Nat Cancer 3, 1534–1552. 10.1038/s43018-022-00475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao L, Huang C, Cui Zhou D, Hu Y, Lih TM, Savage SR, Krug K, Clark DJ, Schnaubelt M, Chen L, et al. (2021). Proteogenomic characterization of pancreatic ductal adenocarcinoma. Cell 184, 5031–5052 e5026. 10.1016/j.cell.2021.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark DJ, Dhanasekaran SM, Petralia F, Pan J, Song X, Hu Y, da Veiga Leprevost F, Reva B, Lih TM, Chang HY, et al. (2019). Integrated Proteogenomic Characterization of Clear Cell Renal Cell Carcinoma. Cell 179, 964–983 e931. 10.1016/j.cell.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dou Y, Kawaler EA, Cui Zhou D, Gritsenko MA, Huang C, Blumenberg L, Karpova A, Petyuk VA, Savage SR, Satpathy S, et al. (2020). Proteogenomic Characterization of Endometrial Carcinoma. Cell 180, 729–748 e726. 10.1016/j.cell.2020.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gillette MA, Satpathy S, Cao S, Dhanasekaran SM, Vasaikar SV, Krug K, Petralia F, Li Y, Liang WW, Reva B, et al. (2020). Proteogenomic Characterization Reveals Therapeutic Vulnerabilities in Lung Adenocarcinoma. Cell 182, 200–225 e235. 10.1016/j.cell.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krug K, Jaehnig EJ, Satpathy S, Blumenberg L, Karpova A, Anurag M, Miles G, Mertins P, Geffen Y, Tang LC, et al. (2020). Proteogenomic Landscape of Breast Cancer Tumorigenesis and Targeted Therapy. Cell 183, 1436–1456 e1431. 10.1016/j.cell.2020.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petralia F, Tignor N, Reva B, Koptyra M, Chowdhury S, Rykunov D, Krek A, Ma W, Zhu Y, Ji J, et al. (2020). Integrated Proteogenomic Characterization across Major Histological Types of Pediatric Brain Cancer. Cell 183, 1962–1985 e1931. 10.1016/j.cell.2020.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Satpathy S, Krug K, Jean Beltran PM, Savage SR, Petralia F, Kumar-Sinha C, Dou Y, Reva B, Kane MH, Avanessian SC, et al. (2021). A proteogenomic portrait of lung squamous cell carcinoma. Cell 184, 4348–4371 e4340. 10.1016/j.cell.2021.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vasaikar S, Huang C, Wang X, Petyuk VA, Savage SR, Wen B, Dou Y, Zhang Y, Shi Z, Arshad OA, et al. (2019). Proteogenomic Analysis of Human Colon Cancer Reveals New Therapeutic Opportunities. Cell 177, 1035–1049 e1019. 10.1016/j.cell.2019.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang LB, Karpova A, Gritsenko MA, Kyle JE, Cao S, Li Y, Rykunov D, Colaprico A, Rothstein JH, Hong R, et al. (2021). Proteogenomic and metabolomic characterization of human glioblastoma. Cancer Cell 39, 509–528 e520. 10.1016/j.ccell.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang B, Wang J, Wang X, Zhu J, Liu Q, Shi Z, Chambers MC, Zimmerman LJ, Shaddox KF, Kim S, et al. (2014). Proteogenomic characterization of human colon and rectal cancer. Nature 513, 382–387. 10.1038/nature13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H, Liu T, Zhang Z, Payne SH, Zhang B, McDermott JE, Zhou JY, Petyuk VA, Chen L, Ray D, et al. (2016). Integrated Proteogenomic Characterization of Human High-Grade Serous Ovarian Cancer. Cell 166, 755–765. 10.1016/j.cell.2016.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mertins P, Mani DR, Ruggles KV, Gillette MA, Clauser KR, Wang P, Wang X, Qiao JW, Cao S, Petralia F, et al. (2016). Proteogenomics connects somatic mutations to signalling in breast cancer. Nature 534, 55–62. 10.1038/nature18003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson BE, Mazor T, Hong C, Barnes M, Aihara K, McLean CY, Fouse SD, Yamamoto S, Ueda H, Tatsuno K, et al. (2014). Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science 343, 189–193. 10.1126/science.1239947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cancer Genome Atlas Research, N. (2008). Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455, 1061–1068. 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunter C, Smith R, Cahill DP, Stephens P, Stevens C, Teague J, Greenman C, Edkins S, Bignell G, Davies H, et al. (2006). A hypermutation phenotype and somatic MSH6 mutations in recurrent human malignant gliomas after alkylator chemotherapy. Cancer Res 66, 3987–3991. 10.1158/0008-5472.CAN-06-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Touat M, Li YY, Boynton AN, Spurr LF, Iorgulescu JB, Bohrson CL, Cortes-Ciriano I, Birzu C, Geduldig JE, Pelton K, et al. (2020). Mechanisms and therapeutic implications of hypermutation in gliomas. Nature 580, 517–523. 10.1038/s41586-020-2209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bouffet E, Larouche V, Campbell BB, Merico D, de Borja R, Aronson M, Durno C, Krueger J, Cabric V, Ramaswamy V, et al. (2016). Immune Checkpoint Inhibition for Hypermutant Glioblastoma Multiforme Resulting From Germline Biallelic Mismatch Repair Deficiency. J Clin Oncol 34, 2206–2211. 10.1200/JCO.2016.66.6552. [DOI] [PubMed] [Google Scholar]
- 32.Garofano L, Migliozzi S, Oh YT, D’Angelo F, Najac RD, Ko A, Frangaj B, Caruso FP, Yu K, Yuan J, et al. (2021). Pathway-based classification of glioblastoma uncovers a mitochondrial subtype with therapeutic vulnerabilities. Nat Cancer 2, 141–156. 10.1038/s43018-020-00159-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Migliozzi S, Oh YT, Hasanain M, Garofano L, D’Angelo F, Najac RD, Picca A, Bielle F, Di Stefano AL, Lerond J, et al. (2023). Integrative multi-omics networks identify PKCdelta and DNA-PK as master kinases of glioblastoma subtypes and guide targeted cancer therapy. Nat Cancer 4, 181–202. 10.1038/s43018-022-00510-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh D, Chan JM, Zoppoli P, Niola F, Sullivan R, Castano A, Liu EM, Reichel J, Porrati P, Pellegatta S, et al. (2012). Transforming fusions of FGFR and TACC genes in human glioblastoma. Science 337, 1231–1235. 10.1126/science.1220834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu KV, Chang JP, Parachoniak CA, Pandika MM, Aghi MK, Meyronet D, Isachenko N, Fouse SD, Phillips JJ, Cheresh DA, et al. (2012). VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell 22, 21–35. 10.1016/j.ccr.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snuderl M, Fazlollahi L, Le LP, Nitta M, Zhelyazkova BH, Davidson CJ, Akhavanfard S, Cahill DP, Aldape KD, Betensky RA, et al. (2011). Mosaic amplification of multiple receptor tyrosine kinase genes in glioblastoma. Cancer Cell 20, 810–817. 10.1016/j.ccr.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 37.Puputti M, Tynninen O, Sihto H, Blom T, Maenpaa H, Isola J, Paetau A, Joensuu H, and Nupponen NN (2006). Amplification of KIT, PDGFRA, VEGFR2, and EGFR in gliomas. Mol Cancer Res 4, 927–934. 10.1158/1541-7786.MCR-06-0085. [DOI] [PubMed] [Google Scholar]
- 38.Network TC (2013). Corrigendum: Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 494, 506. 10.1038/nature11903. [DOI] [PubMed] [Google Scholar]
- 39.Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, et al. (2010). Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17, 98–110. 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, Misra A, Nigro JM, Colman H, Soroceanu L, et al. (2006). Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 9, 157–173. 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 41.Kolch W, Halasz M, Granovskaya M, and Kholodenko BN (2015). The dynamic control of signal transduction networks in cancer cells. Nat Rev Cancer 15, 515–527. 10.1038/nrc3983. [DOI] [PubMed] [Google Scholar]
- 42.Mattoon DR, Lamothe B, Lax I, and Schlessinger J. (2004). The docking protein Gab1 is the primary mediator of EGF-stimulated activation of the PI-3K/Akt cell survival pathway. BMC Biol 2, 24. 10.1186/1741-7007-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holland EC, Celestino J, Dai C, Schaefer L, Sawaya RE, and Fuller GN (2000). Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nat Genet 25, 55–57. 10.1038/75596. [DOI] [PubMed] [Google Scholar]
- 44.Wang Q, Hu B, Hu X, Kim H, Squatrito M, Scarpace L, deCarvalho AC, Lyu S, Li P, Li Y, et al. (2017). Tumor Evolution of Glioma-Intrinsic Gene Expression Subtypes Associates with Immunological Changes in the Microenvironment. Cancer Cell 32, 42–56 e46. 10.1016/j.ccell.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Molenaar RJ, Verbaan D, Lamba S, Zanon C, Jeuken JW, Boots-Sprenger SH, Wesseling P, Hulsebos TJ, Troost D, van Tilborg AA, et al. (2014). The combination of IDH1 mutations and MGMT methylation status predicts survival in glioblastoma better than either IDH1 or MGMT alone. Neuro Oncol 16, 1263–1273. 10.1093/neuonc/nou005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mansouri A, Hachem LD, Mansouri S, Nassiri F, Laperriere NJ, Xia D, Lindeman NI, Wen PY, Chakravarti A, Mehta MP, et al. (2019). MGMT promoter methylation status testing to guide therapy for glioblastoma: refining the approach based on emerging evidence and current challenges. Neuro Oncol 21, 167–178. 10.1093/neuonc/noy132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, et al. (2005). MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352, 997–1003. 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 48.Ceccarelli M, Barthel FP, Malta TM, Sabedot TS, Salama SR, Murray BA, Morozova O, Newton Y, Radenbaugh A, Pagnotta SM, et al. (2016). Molecular Profiling Reveals Biologically Discrete Subsets and Pathways of Progression in Diffuse Glioma. Cell 164, 550–563. 10.1016/j.cell.2015.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weller M, Stupp R, Reifenberger G, Brandes AA, van den Bent MJ, Wick W, and Hegi ME (2010). MGMT promoter methylation in malignant gliomas: ready for personalized medicine? Nat Rev Neurol 6, 39–51. 10.1038/nrneurol.2009.197. [DOI] [PubMed] [Google Scholar]
- 50.Gavish A, Tyler M, Greenwald AC, Hoefflin R, Simkin D, Tschernichovsky R, Galili Darnell N, Somech E, Barbolin C, Antman T, et al. (2023). Hallmarks of transcriptional intratumour heterogeneity across a thousand tumours. Nature 618, 598–606. 10.1038/s41586-023-06130-4. [DOI] [PubMed] [Google Scholar]
- 51.Patel AP, Tirosh I, Trombetta JJ, Shalek AK, Gillespie SM, Wakimoto H, Cahill DP, Nahed BV, Curry WT, Martuza RL, et al. (2014). Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 344, 1396–1401. 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neftel C, Laffy J, Filbin MG, Hara T, Shore ME, Rahme GJ, Richman AR, Silverbush D, Shaw ML, Hebert CM, et al. (2019). An Integrative Model of Cellular States, Plasticity, and Genetics for Glioblastoma. Cell 178, 835–849 e821. 10.1016/j.cell.2019.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang J, Stevens MF, and Bradshaw TD (2012). Temozolomide: mechanisms of action, repair and resistance. Curr Mol Pharmacol 5, 102–114. 10.2174/1874467211205010102. [DOI] [PubMed] [Google Scholar]
- 54.Wick W, Platten M, and Weller M. (2009). New (alternative) temozolomide regimens for the treatment of glioma. Neuro Oncol 11, 69–79. 10.1215/15228517-2008-078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang B, Yu Z, and Liang R. (2021). Effect of long-term adjuvant temozolomide chemotherapy on primary glioblastoma patient survival. BMC Neurol 21, 424. 10.1186/s12883-021-02461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nobre L, Zapotocky M, Ramaswamy V, Ryall S, Bennett J, Alderete D, Balaguer Guill J, Baroni L, Bartels U, Bavle A, et al. (2020). Outcomes of BRAF V600E Pediatric Gliomas Treated With Targeted BRAF Inhibition. JCO Precis Oncol 4. 10.1200/PO.19.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gentilcore G, Madonna G, Mozzillo N, Ribas A, Cossu A, Palmieri G, and Ascierto PA (2013). Effect of dabrafenib on melanoma cell lines harbouring the BRAF(V600D/R) mutations. BMC Cancer 13, 17. 10.1186/1471-2407-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang H, Higgins B, Kolinsky K, Packman K, Go Z, Iyer R, Kolis S, Zhao S, Lee R, Grippo JF, et al. (2010). RG7204 (PLX4032), a selective BRAFV600E inhibitor, displays potent antitumor activity in preclinical melanoma models. Cancer Res 70, 5518–5527. 10.1158/0008-5472.CAN-10-0646. [DOI] [PubMed] [Google Scholar]
- 59.Consortium G. (2018). Glioma through the looking GLASS: molecular evolution of diffuse gliomas and the Glioma Longitudinal Analysis Consortium. Neuro Oncol 20, 873–884. 10.1093/neuonc/noy020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bhat KPL, Balasubramaniyan V, Vaillant B, Ezhilarasan R, Hummelink K, Hollingsworth F, Wani K, Heathcock L, James JD, Goodman LD, et al. (2013). Mesenchymal differentiation mediated by NF-kappaB promotes radiation resistance in glioblastoma. Cancer Cell 24, 331–346. 10.1016/j.ccr.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schmitt MJ, Company C, Dramaretska Y, Barozzi I, Gohrig A, Kertalli S, Grossmann M, Naumann H, Sanchez-Bailon MP, Hulsman D, et al. (2021). Phenotypic Mapping of Pathologic Cross-Talk between Glioblastoma and Innate Immune Cells by Synthetic Genetic Tracing. Cancer Discov 11, 754–777. 10.1158/2159-8290.CD-20-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sa JK, Chang N, Lee HW, Cho HJ, Ceccarelli M, Cerulo L, Yin J, Kim SS, Caruso FP, Lee M, et al. (2020). Transcriptional regulatory networks of tumor-associated macrophages that drive malignancy in mesenchymal glioblastoma. Genome Biol 21, 216. 10.1186/s13059-020-02140-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Habas R, Kato Y, and He X. (2001). Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell 107, 843–854. 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- 64.Yan T, Zhang A, Shi F, Chang F, Mei J, Liu Y, and Zhu Y. (2018). Integrin alphavbeta3-associated DAAM1 is essential for collagen-induced invadopodia extension and cell haptotaxis in breast cancer cells. J Biol Chem 293, 10172–10185. 10.1074/jbc.RA117.000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alkailani MI, Aittaleb M, and Tissir F. (2022). WNT signaling at the intersection between neurogenesis and brain tumorigenesis. Front Mol Neurosci 15, 1017568. 10.3389/fnmol.2022.1017568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu G, Yan T, Li X, Sun J, Zhang B, Wang H, and Zhu Y. (2018). Daam1 activates RhoA to regulate Wnt5ainduced glioblastoma cell invasion. Oncol Rep 39, 465–472. 10.3892/or.2017.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Venkataramani V, Yang Y, Schubert MC, Reyhan E, Tetzlaff SK, Wissmann N, Botz M, Soyka SJ, Beretta CA, Pramatarov RL, et al. (2022). Glioblastoma hijacks neuronal mechanisms for brain invasion. Cell 185, 2899–2917 e2831. 10.1016/j.cell.2022.06.054. [DOI] [PubMed] [Google Scholar]
- 68.York RD, Yao H, Dillon T, Ellig CL, Eckert SP, McCleskey EW, and Stork PJ (1998). Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature 392, 622–626. 10.1038/33451. [DOI] [PubMed] [Google Scholar]
- 69.Vossler MR, Yao H, York RD, Pan MG, Rim CS, and Stork PJ (1997). cAMP activates MAP kinase and Elk-1 through a B-Raf- and Rap1-dependent pathway. Cell 89, 73–82. 10.1016/s0092-8674(00)80184-1. [DOI] [PubMed] [Google Scholar]
- 70.Vithayathil J, Pucilowska J, Goodnough LH, Atit RP, and Landreth GE (2015). Dentate Gyrus Development Requires ERK Activity to Maintain Progenitor Population and MAPK Pathway Feedback Regulation. J Neurosci 35, 6836–6848. 10.1523/JNEUROSCI.4196-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lim SY, Menzies AM, and Rizos H. (2017). Mechanisms and strategies to overcome resistance to molecularly targeted therapy for melanoma. Cancer 123, 2118–2129. 10.1002/cncr.30435. [DOI] [PubMed] [Google Scholar]
- 72.Zhong J, Li X, McNamee C, Chen AP, Baccarini M, and Snider WD (2007). Raf kinase signaling functions in sensory neuron differentiation and axon growth in vivo. Nat Neurosci 10, 598–607. 10.1038/nn1898. [DOI] [PubMed] [Google Scholar]
- 73.Krishna S, Choudhury A, Keough MB, Seo K, Ni L, Kakaizada S, Lee A, Aabedi A, Popova G, Lipkin B, et al. (2023). Glioblastoma remodelling of human neural circuits decreases survival. Nature 617, 599–607. 10.1038/s41586-023-06036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Venkatesh HS, Morishita W, Geraghty AC, Silverbush D, Gillespie SM, Arzt M, Tam LT, Espenel C, Ponnuswami A, Ni L, et al. (2019). Electrical and synaptic integration of glioma into neural circuits. Nature 573, 539–545. 10.1038/s41586-019-1563-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bouche V, Aldegheri G, Donofrio CA, Fioravanti A, Roberts-Thomson S, Fox SB, Schettini F, and Generali D. (2021). BRAF Signaling Inhibition in Glioblastoma: Which Clinical Perspectives? Front Oncol 11, 772052. 10.3389/fonc.2021.772052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li B, and Dewey CN (2011). RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12, 323. 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pertea M, Kim D, Pertea GM, Leek JT, and Salzberg SL (2016). Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat Protoc 11, 1650–1667. 10.1038/nprot.2016.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Johnson WE, Li C, and Rabinovic A. (2007). Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 8, 118–127. 10.1093/biostatistics/kxj037. [DOI] [PubMed] [Google Scholar]
- 79.Hanzelmann S, Castelo R, and Guinney J. (2013). GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinformatics 14, 7. 10.1186/1471-2105-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li H, and Durbin R. (2010). Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26, 589–595. 10.1093/bioinformatics/btp698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, and Genome Project Data Processing, S. (2009). The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079. 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, Philippakis AA, del Angel G, Rivas MA, Hanna M, et al. (2011). A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet 43, 491–498. 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McLaren W, Gil L, Hunt SE, Riat HS, Ritchie GR, Thormann A, Flicek P, and Cunningham F. (2016). The Ensembl Variant Effect Predictor. Genome Biol 17, 122. 10.1186/s13059-016-0974-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Koboldt DC, Zhang Q, Larson DE, Shen D, McLellan MD, Lin L, Miller CA, Mardis ER, Ding L, and Wilson RK (2012). VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res 22, 568–576. 10.1101/gr.129684.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Erik Garrison GM (2012). Haplotype-based variant detection from short-read sequencing. arXiv 1207.3907.
- 86.Saunders CT, Wong WS, Swamy S, Becq J, Murray LJ, and Cheetham RK (2012). Strelka: accurate somatic small-variant calling from sequenced tumor-normal sample pairs. Bioinformatics 28, 1811–1817. 10.1093/bioinformatics/bts271. [DOI] [PubMed] [Google Scholar]
- 87.Lai Z, Markovets A, Ahdesmaki M, Chapman B, Hofmann O, McEwen R, Johnson J, Dougherty B, Barrett JC, and Dry JR (2016). VarDict: a novel and versatile variant caller for next-generation sequencing in cancer research. Nucleic Acids Res 44, e108. 10.1093/nar/gkw227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schwarz JM, Cooper DN, Schuelke M, and Seelow D. (2014). MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods 11, 361–362. 10.1038/nmeth.2890. [DOI] [PubMed] [Google Scholar]
- 89.Adzhubei I, Jordan DM, and Sunyaev SR (2013). Predicting functional effect of human missense mutations using PolyPhen-2. Curr Protoc Hum Genet Chapter 7, Unit7 20. 10.1002/0471142905.hg0720s76. [DOI] [PMC free article] [PubMed]
- 90.Choi Y, and Chan AP (2015). PROVEAN web server: a tool to predict the functional effect of amino acid substitutions and indels. Bioinformatics 31, 2745–2747. 10.1093/bioinformatics/btv195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sim NL, Kumar P, Hu J, Henikoff S, Schneider G, and Ng PC (2012). SIFT web server: predicting effects of amino acid substitutions on proteins. Nucleic Acids Res 40, W452–457. 10.1093/nar/gks539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kong AT, Leprevost FV, Avtonomov DM, Mellacheruvu D, and Nesvizhskii AI (2017). MSFragger: ultrafast and comprehensive peptide identification in mass spectrometry-based proteomics. Nat Methods 14, 513–520. 10.1038/nmeth.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.da Veiga Leprevost F, Haynes SE, Avtonomov DM, Chang HY, Shanmugam AK, Mellacheruvu D, Kong AT, and Nesvizhskii AI (2020). Philosopher: a versatile toolkit for shotgun proteomics data analysis. Nat Methods 17, 869–870. 10.1038/s41592-020-0912-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li H, and Durbin R. (2009). Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760. 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Smigielski EM, Sirotkin K, Ward M, and Sherry ST (2000). dbSNP: a database of single nucleotide polymorphisms. Nucleic Acids Res 28, 352–355. 10.1093/nar/28.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cibulskis K, Lawrence MS, Carter SL, Sivachenko A, Jaffe D, Sougnez C, Gabriel S, Meyerson M, Lander ES, and Getz G. (2013). Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol 31, 213–219. 10.1038/nbt.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Banerji S, Cibulskis K, Rangel-Escareno C, Brown KK, Carter SL, Frederick AM, Lawrence MS, Sivachenko AY, Sougnez C, Zou L, et al. (2012). Sequence analysis of mutations and translocations across breast cancer subtypes. Nature 486, 405–409. 10.1038/nature11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McLaren W, Pritchard B, Rios D, Chen Y, Flicek P, and Cunningham F. (2010). Deriving the consequences of genomic variants with the Ensembl API and SNP Effect Predictor. Bioinformatics 26, 2069–2070. 10.1093/bioinformatics/btq330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.D’Angelo F, Ceccarelli M, Tala Garofano L, Zhang J, Frattini V, Caruso FP, Lewis G, Alfaro KD, Bauchet L, et al. (2019). The molecular landscape of glioma in patients with Neurofibromatosis 1. Nat Med 25, 176–187. 10.1038/s41591-018-0263-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Frattini V, Pagnotta SM, Tala Fan JJ, Russo MV, Lee SB, Garofano L, Zhang J, Shi P, Lewis G, et al. (2018). A metabolic function of FGFR3-TACC3 gene fusions in cancer. Nature 553, 222–227. 10.1038/nature25171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mun DG, Bhin J, Kim S, Kim H, Jung JH, Jung Y, Jang YE, Park JM, Kim H, Jung Y, et al. (2019). Proteogenomic Characterization of Human Early-Onset Gastric Cancer. Cancer Cell 35, 111–124 e110. 10.1016/j.ccell.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 102.Wang Y, Yang F, Gritsenko MA, Wang Y, Clauss T, Liu T, Shen Y, Monroe ME, Lopez-Ferrer D, Reno T, et al. (2011). Reversed-phase chromatography with multiple fraction concatenation strategy for proteome profiling of human MCF10A cells. Proteomics 11, 2019–2026. 10.1002/pmic.201000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Abu-Jamous B, and Kelly S. (2018). Clust: automatic extraction of optimal co-expressed gene clusters from gene expression data. Genome Biol 19, 172. 10.1186/s13059-018-1536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Abu-Jamous B, Fa R, Roberts DJ, and Nandi AK (2015). UNCLES: method for the identification of genes differentially consistently co-expressed in a specific subset of datasets. BMC Bioinformatics 16, 184. 10.1186/s12859-015-0614-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jang EH, Sim A, Im SK, and Hur EM (2016). Effects of Microtubule Stabilization by Epothilone B Depend on the Type and Age of Neurons. Neural Plast 2016, 5056418. 10.1155/2016/5056418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fournier AE, GrandPre T, and Strittmatter SM (2001). Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature 409, 341–346. 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. GBM patient clinical characteristics, genomic, and proteomic information, related to Figure 1
Table S2. GO Analysis between primary and recurrent GBM, related to Figure 3