Abstract
Objective
We previously reported the successful outcomes in severe acute pancreatitis (SAP) after continuous hemodialysis using a polymethylmethacrylate hemofilter (PMMA-CHD). The present study makes informative suggestions regarding the initiation and termination of PMMA-CHD.
Methods
We retrospectively studied 63 patients with SAP admitted to the intensive care unit between January 1, 2011, and December 31, 2022, including 30 who received PMMA-CHD therapy for renal dysfunction. Statistical significance was evaluated using a multiple logistic regression analysis for severity scores, prognostic factor scores in the Japanese severity criteria, the Kidney Disease: Improving Global Outcomes (KDIGO) stage, and the lung injury score (LIS).
Results
At the onset of blood purification therapy using PMMA-CHD, a significant increase in the KDIGO stage was shown, with a cutoff value of 2.0. The prognostic factor score and LIS at the start of blood purification therapy were significantly high, with a cutoff value of 3.0. Analyses of severity scores, the KDIGO stage, and the LIS before the start of PMMA-CHD were also increased significantly, with cutoff values of +2.0, +1.0, and +3.0, respectively. Furthermore, on analyses of improvements in values after starting PMMA-CHD, the value of KDIGO staging significantly decreased, and the cutoff value was -2.0. The prognostic factor score was also significantly decreased, with a cutoff value of -2.0.
Conclusion
Prognostic factor scores of the Japanese severity criteria and LIS, as well as the KDIGO stage, are valuable indicators for determining the start and end of PMMA-CHD therapy.
Keywords: continuous hemodialysis, severe acute pancreatitis, acute kidney injury, Japanese prognostic factor, lung injury, polymethylmethacrylate hemofilter
Introduction
We previously reported the usefulness of continuous hemodialysis (CHD) using a polymethylmethacrylate (PMMA) hemofilter (PMMA-CHD) for treating severe acute pancreatitis (SAP) (1). In a previous study, patients with SAP requiring PMMA-CHD had a significantly higher Acute Physiology and Chronic Health Evaluation (APACHE) II score, Sequential Organ Failure Assessment (SOFA) score, Kidney Disease: Improving Global Outcomes (KDIGO) stage, and lung injury score (LIS) than in those not requiring PMMA-CHD. PMMA-CHD improved these outcomes in critically ill patients, and the actual mortality ratios were lower than those predicted by the APACHE II scores.
However, the advantages of blood purification therapy for SAP in improving mortality, reducing the length of intensive care unit (ICU) stay, and reducing total hospital costs remain uncertain (2). Patients with SAP experience platelet and endothelial dysfunctions induced by hypercytokinemia, which can lead to a hypercoagulable state due to the activation of tissue factors, deterioration of antithrombotic reactions by AT-III and the protein C system, and inhibition of the fibrinolytic system (3). These hypercoagulable changes in critically ill patients may be associated with a decreased filter life during blood purification therapy (4).
Theoretically, continuous hemofiltration (CHF) is expected to be more effective than CHD for cytokines (5), although its filter life is shorter than that of CHD (6). Furthermore, coagulable changes in hemofilters during blood purification due to chemo-structural changes on the PMMA membrane surface are encountered as complications (7). Therefore, a high dose of anticoagulants is required, which can cause hemorrhagic complications in critically ill patients, and nafamostat mesilate can be safely used for blood purification therapy in such patients (8). However, CHF is significantly more expensive than CHD (9). Consequently, we routinely use CHD to extend the use of PMMA hemofilters with nafamostat mesilate as an anticoagulant in our medical practice for the treatment of SAP to achieve favorable clinical results, as described previously (1).
Although many studies on blood purification therapy for SAP have been reported, the appropriate timing of its start and end, even for acute kidney injury (AKI) in critically ill patients, remains unclear (10). Xie et al. demonstrated that an intra-abdominal pressure >20 mmHg measured by the intravesical pressure was a useful indicator for CHF onset in patients with SAP using an AN69 hemofilter (11). However, this index is not suitable for general use.
Therefore, in the present study, we reviewed our clinical experience with SAP to provide informative suggestions regarding the initiation and termination of PMMA-CHD.
Materials and Methods
Study design, patients, and treatments
This study was approved by the Ethics Committee on Human Research of our hospital (Permission No. 2211).
We retrospectively reviewed 63 patients who had been diagnosed with SAP according to the Japanese guidelines for the management of acute pancreatitis (12) for patients admitted to the ICU between January 1, 2011, and December 31, 2022. According to the Japanese criteria, SAP is defined as grade 2 or 3 pancreatic tissue involvement, as evaluated by contrast-enhanced computed tomography (CT), with an optional evaluation based on prognostic factors (12). Initially, patients were treated using conventional therapy (1). Nevertheless, when patients had anuria/oliguria with a urine output of ≦200 mL/8-12 h after ICU admission, they were treated with PMMA-CHD. Among them, 30 developed AKI with or without respiratory failure during their ICU stay and were treated with PMMA-CHD. We divided the patients into two groups (those with and without PMMA-CHD) and analyzed their data.
Blood purification therapy
We routinely use CHD instead of CHF or CHDF to extend hemofilter lifetime (1). ACH-ΣⓇ (Asahi-Kasei, Tokyo, Japan) was used as the CHD equipment, and 1.3- or 1.8-m2 PMMA hemofilters (Hemofeel CH-1.3W/1.8 WⓇ; Toray, Tokyo, Japan) were used for their cytokine adsorption capability (13). The dialysate flow rate was set at 600 mL/h because of national health insurance requirements in Japan. Nafamostat mesilate (Torii Pharmaceutical, Tokyo, Japan), a synthetic serine protease inhibitor, was administered as an anticoagulant at 30-40 mg/h. The detailed procedure for PMMA-CHD was described in our previous study (1).
The evaluation of the patients' condition severity
The systemic conditions of the patients were evaluated using the severity of organ dysfunction based on APACHE II (14) and SOFA scores (15). Prognostic factors were scored according to severity criteria in Japanese guidelines (12). The kidney function was evaluated by the KDIGO stage (16), and lung injury was evaluated by the LIS (17). The status of the pancreas and/or surrounding tissues was classified based on the CT grading of the Japanese severity criteria (12) and Balthazar CT severity index (18).
Statistical analyses
Values are expressed as the mean ± standard deviation. Categorical variables were analyzed using Pearson's chi-squared or Fisher's exact test. Wilcoxon's rank-sum test was used for two-group comparisons. Multiple comparisons were performed using the Bonferroni-Dunn test. A multiple logistic regression analysis was used to determine the association of PMMA-CHD therapy with any three parameters among the APACHE II score, SOFA score, prognostic factor score, KDIGO stage, and LIS; among logistic regression models, those showing p<0.01 in the whole model test, p>0.05 in lack of fit, and an area under the curve (AUC) of ≧0.7 in a receiver operating characteristic (ROC) analysis were selected and used in this study. These statistical models dismissed the probability of chance correlation due to the small sample size using the Y-scrambling test (19). The cutoff values were calculated using an ROC analysis. The JMP ver. 17 statistical software program (SAS Institute, Cary, USA) was used for all analyses.
Results
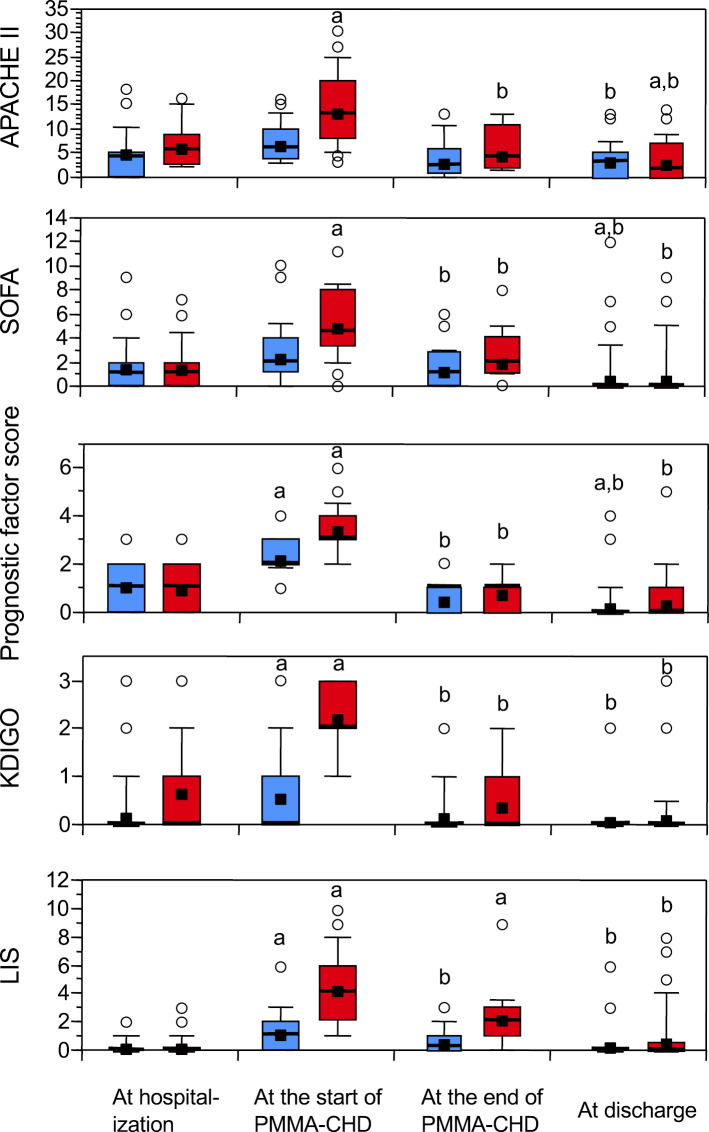
We retrospectively studied 63 patients with SAP admitted to the ICU between January 1, 2011 and December 31, 2022. Of these patients, 30 underwent PMMA-CHD because of renal and/or pulmonary dysfunction. The baseline characteristics of the patients are summarized in Table 1. No significant differences were observed in the two-group analyses of age, sex, or etiology between patients requiring PMMA-CHD and those who did not. The average duration from hospitalization to ICU admission in all patients was 16.9±17.9 (2-92) h without any marked difference between the groups. There were also no significant differences in the levels of amylase, C-reactive protein, or lactate dehydrogenase or white blood cell counts between the two groups for cases with the poorest values until patient admission to the ICU (Table 1). At the onset of PMMA-CHD, the APACHE II and SOFA scores, prognostic factor scores of the Japanese severity criteria, KDIGO stage, and LIS were significantly higher in patients requiring PMMA-CHD than in those not requiring PMMA-CHD (Table 1). These severity scores (Figure) significantly increased at the start of PMMA-CHD compared with those at hospitalization and then significantly declined after PMMA-CHD therapy (Figure).
Table 1.
Profiles and Data of Patients Requiring or Not Requiring PMMA-CHD.
| Patients not requiring PMMA-CHD | Patients requiring PMMA-CHD | p value | |
|---|---|---|---|
| Numbers of patients | 33 | 30 | |
| Age | 53.8±18.6 | 51.9±17.0 | 0.635 |
| Sex (male:female) | 23:10 | 19:11 | 0.593 |
| Etiology | 0.926 | ||
| Alcohol abuse | 20 | 20 | |
| Cholelithiasis | 8 | 3 | |
| Others | 4 | 7 | |
| Radiological severity of most worsen score after hospitalization | |||
| CT grade of Japanese criteria | 2.6±0.5 | 2.5±0.5 | 0.182 |
| Balthazar CTSI score | 5.5±1.0 | 6.5±1.6 | 0.253 |
| Laboratory data (the most worsen value before ICU admission) | |||
| Amylase (IU/L) | 1,455.1±1,632.0 | 1,425.1±1,398.4 | 0.810 |
| CRP (mg/dL) | 10.01±10.54 | 12.97±11.78 | 0.186 |
| Creatinine (mg/dL) | 1.16±0.80 | 1.47±1.37 | 0.842 |
| LDH (U/L) | 373.5±169.1 | 657.3±1096.9 | 0.120 |
| WBC (counts/µL) | 12,724.2±4,338.9 | 12,353.3±5,810.0 | 0.531 |
| Hours from hospitalization to ICU admission | 13.2±18.6 | 16.9±17.9 | 0.564 |
| Hours to start of PMMA-CHD from hospitalization | 27.6±22.7 (4-89) | ||
| Physical conditions at the start of PMMA-CHDa | |||
| APACHE II score | 7.2±3.8 | 13.8±7.3 | <0.001 |
| SOFA score | 2.7±2.4 | 5.2±2.9 | <0.001 |
| Prognostic factor scoreb | 2.30±0.68 | 3.47±1.04 | <0.001 |
| KDIGO stage | 0.61±0.79 | 2.27±0.74 | <0.001 |
| LIS | 1.2±1.3 | 4.4±2.8 | <0.001 |
| Days of blood purification | 4.8±3.8 (1-16) | ||
| Days of ICU care | 4.6±3.7 (1-15) | 9.0±6.0 (2-25) | <0.001 |
| Mechanical ventilation | 2/30 (0.7%) | 18/30 (60.0%) | <0.001 |
| Physical conditions at the end of PMMA-CHDc | |||
| APACHE II score | 3.5±3.3 | 4.6±3.0 | 0.066 |
| SOFA score | 1.4±1.4 | 2.1±1.4 | 0.009 |
| Prognostic factor scoreb | 0.64±0.65 | 1.37±0.76 | <0.001 |
| KDIGO stage | 0.03±0.17 | 0.20±0.48 | 0.068 |
| LIS | 0.5±0.8 | 2.0±1.8 | <0.001 |
| Days of hospital stay | 16.9±11.7 | 29.2±21.3 | 0.002 |
| 28-day survive | 31/33 (93.9%) | 30/30 (100%) | 0.493 |
| Survive at discharge | 31/33 (93.9%) | 28/30 (93.3%) | 1.000 |
Values are expressed as the mean±standard deviation.
aValues of patients not requiring blood purification denote those on the day after ICU admission.
bTotal points of prognostic factors in the Japanese severity criteria.
cValues of patients not requiring PMMA-CHD denote those at the end of ICU care.
PMMA-CHD: continuous hemodialysis with a polymethylmethacrylate membrane hemofilter, CRP: C-reactive protein, LDH: lactate dehydrogenase, WBC: white blood cell, APACHE: Acute Physiology and Chronic Health Evaluation, SOFA: Sequential Organ Failure Assessment, KDIGO: Kidney Disease: Improving Global Outcome, LIS: lung injury score, CTSI: computed tomography severity index, ICU: intensive care unit
Figure.
Changes in the APACHE II score, SOFA score, prognostic factor score of the Japanese severity criteria, KDIGO stage, and LIS during ICU care. For patients who did not require PMMA-CHD therapy, the values at the start of PMMA-CHD were denoted by the values on the day after ICU admission, and the values at the end of PMMA-CHD were denoted by the values at the end of ICU care. Red boxes: PMMA-CHD group (n=30); blue boxes: non-CHD group (n=33). Boxes: 25-75th percentiles. Error bars indicate 10th and 90th percentiles. Bold horizontal lines indicate medians, and closed squares indicate mean values. Open circles represent outliers. Statistical significance within each group was evaluated using multiple comparisons (Bonferroni-Dunn test): (a) p<0.05, compared with the initial value at hospitalization; and (b) p<0.05, compared with the second value at the start of PMMA-CHD. APACHE: Acute Physiology and Chronic Health Evaluation, SOFA: Sequential Organ Failure Assessment, KDIGO: Kidney Disease: Improving Global Outcome, LIS: lung injury score
To estimate the ability to indicate the start or end of PMMA-CHD, we used multiple logistic regression analyses to assess the correlations between the APACHE II score, SOFA score, prognostic factor scores, KDIGO stage, and LIS. In the analyses of hospitalization data among prognostic factor scores, KDIGO stage, and LIS at hospitalization, the KDIGO stage showed a relatively high odds ratio and good 95% confidence interval (CI) (p=0.044) (Table 2A). The cutoff value of the KDIGO stage at hospitalization was calculated as 1.0 (Table 2B). For the other regression models, we could not evaluate any associations because the statistical models were insignificant (p>0.05, for whole-model tests and/or p<0.05, for lack of fit; data not shown).
Table 2.
Association between Severity Scores (Prognostic Factor Score, KDIGO, and LIS) and the Initiation of PMMA-CHD Therapy at Hospitalization.
| (A) Analysis of the values at the hospitalization | ||||||
|---|---|---|---|---|---|---|
| Association among prognostic factor scorea score, KDIGO stage, and LIS | ||||||
| Coefficient | SE | Wald χ2 | p value | OR | 95% CI of OR | |
| Intercept | -0.267 | 0.406 | ||||
| Prognostic factor scorea | -0.197 | 0.301 | 0.429 | 0.512 | 0.821 | 0.444-1.467 |
| KDIGO stage | 0.750 | 0.372 | 4.071 | 0.044 | 2.117 | 1.072-4.752 |
| LIS | 0.167 | 0.438 | 0.112 | 0.737 | 1.158 | 0.468-2.889 |
| (B) Cut-off value at the start of PMMA-CHD | ||||||
| Cut-off value | Sensitivity | Specificity | AUC | |||
| KDIGO stage | 1.0 | 47.7% | 81.8% | 0.643 | ||
aTotal points of prognostic factors listed in the Japanese severity criteria.
AUC: area under the curve (C-statistics), ROC analysis: receiver operating characteristic, SE: standard error, OR: odds ratio, CI: confidence interval, Other abbreviations are listed in Table 1.
In the analysis of the data at the start of PMMA-CHD, the KDIGO stage was the most significant (p<0.001), with a markedly high odds ratio and 95% CI (Table 3A, 2-4). The cutoff value of the KDIGO stage at PMMA-CHD initiation was calculated as 2.0 (Table 3B). In addition, the p values of the prognostic factor score and LIS (Table 3A, 1-4) were significant in these models because of the relatively high odds ratios and 95% CI. The cutoff value was 3.0 for both the prognostic factor score and LIS (Table 3B). To analyze the association with progression of systemic deterioration, we analyzed increases in the SOFA score, KDIGO stage, prognostic factor score, and LIS in the ICU using differences obtained by subtracting the values at the start of PMMA-CHD from those at hospitalization (Δ1 values). A multiple logistic regression analysis of these Δ1 values revealed significant increases in the odds ratios and 95% CIs (Table 4A), and the cutoff values of the prognostic factor score, KDIGO stage, and LIS were +2.0, +1.0, and +3.0, respectively (Table 4B).
Table 3.
Association between Severity Scores (APACHE II, SOFA, Prognostic Factor Score, KDIGO, and LIS) and Initiation of PMMA-CHD Therapy.
| (A) Analysis of the values at the start of PMMA-CHDa | ||||||
|---|---|---|---|---|---|---|
| (1) Association among APACHE II score, KDIPO stage, and prognostic factor scoreb | ||||||
| Coefficient | SE | Wald χ2 | p value | OR | 95% CI of OR | |
| Intercept | -5.057 | 1.331 | ||||
| APACHE II score | 0.123 | 0.081 | 2.293 | 0.130 | 1.130 | 0.971-1.341 |
| SOFA score | 0.002 | 0.170 | <0.001 | 0.988 | 1.002 | 0.715-1.410 |
| Prognostic factor scoreb | 1.331 | 0.501 | 10.123 | 0.008 | 3.787 | 1.573-11.312 |
| (2) Association among APACHE II score, KDIGO stage, and LIS | ||||||
| Coefficient | SE | Wald χ2 | p value | OR | 95% CI of OR | |
| Intercept | -4.703 | 1.219 | ||||
| APACHE II score | 0.064 | 0.084 | 0.586 | 0.444 | 1.066 | 0.912-1.282 |
| KDIGO stage | 1.941 | 0.601 | 10.423 | 0.001 | 6.969 | 2.525-28.565 |
| LIS | 0.465 | 0.231 | 4.058 | 0.044 | 1.592 | 1.081-2.785 |
| (3) Association among SOFA score, KDIGO stage, and LIS | ||||||
| Coefficient | SE | Wald χ2 | p value | >OR | 95% CI of OR | |
| Intercept | -4.164 | 1.108 | ||||
| SOFA score | -0.145 | 0.203 | 0.510 | 0.475 | 0.865 | 0.556-1.265 |
| KDIGO stage | 2.166 | 0.614 | 12.447 | <0.001 | 8.723 | 3.102-36.870 |
| LIS | 0.661 | 0.293 | 5.102 | 0.024 | 1.937 | 1.203-3.906 |
| (4) Association among prognostic factor scoreb, KDIGO stage, and LIS | ||||||
| Coefficient | SE | Wald χ2 | p value | OR | 95% CI of OR | |
| Intercept | -7.335 | 2.398 | ||||
| Prognostic factor scoreb | 1.157 | 0.688 | 3.266 | 0.071 | 3.179 | 0.912-14.495 |
| KDIGO stage | 2.106 | 0.667 | 9.967 | 0.002 | 8.218 | 2.790-42.544 |
| LIS | 0.363 | 0.229 | 2.507 | 0.113 | 1.438 | 0.967-2.479 |
| (B) Cut-off value at the start of PMMA-CHD | ||||||
| Cut-off value | Sensitivity | Specificity | AUC | |||
| Prognostic factor scoreb | 3.0 | 83.3% | 63.6% | 0.816 | ||
| KDIGO stage | 2.0 | 83.3% | 87.9% | 0.915 | ||
| LIS | 3.0 | 66.7% | 87.9% | 0.846 | ||
aValues of patients not requiring blood purification denote those on the day after admission to the ICU.
bTotal points of prognostic factors listed in the Japanese severity criteria.
Abbreviations listed in Tables 1 and 2.
Table 4.
Association between the Start of PMMA-CHD and Increased Scores before Blood Purification Therapy.
| (A) Multiple logistic regression analysis of increasing values (Δ1: increasing difference of the start of PMMA-CHDa from that at hospitalization) | ||||||
|---|---|---|---|---|---|---|
| (1) Association among Δ1-APACHE II score, Δ1-KDIGO stage, and Δ1-prognostic factor scoreb | ||||||
| Coefficient | SE | Wald χ2 | p value | OR | 95% CI of OR | |
| Intercept | -2.686 | 0.720 | ||||
| Δ1-APACHE II score | 0.162 | 0.097 | 2.747 | 0.096 | 1.176 | 0.984-1.454 |
| Δ1-SOFA score | 0.298 | 0.240 | 1.574 | 0.216 | 1.347 | 0.862-2.242 |
| Δ1-Prognostic factor scoreb | 0.764 | 0.368 | 4.322 | 0.038 | 2.147 | 1.109-4.779 |
| (2) Association among Δ1-APACHE II score, Δ1-KDIGO stage, and Δ1-LIS | ||||||
| Coefficient | SE | Wald χ2 | p value | OR | 95% CI of OR | |
| Intercept | -3.477 | |||||
| Δ1-APACHE II score | -0.204 | 0.098 | 4.287 | 0.038 | 0.816 | 0.662-0.982 |
| Δ1-KDIGO stage | -1.441 | 0.491 | 8.608 | 0.003 | 0.237 | 0.078-0.559 |
| Δ1-LIS | -0.633 | 0.286 | 4.910 | 0.027 | 0.531 | 0.273-0.855 |
| (3) Association among Δ1-SOFA score, Δ1-KDIGO stage, and Δ1-LIS | ||||||
| Coefficient | SE | Wald χ2 | p value | OR | 95% CI of OR | |
| Intercept | -3.087 | 0.777 | ||||
| Δ1-SOFA score | -0.228 | 0.219 | 1.084 | 0.298 | 0.796 | 0.483-1.181 |
| Δ1-KDIGO stage | -1.300 | 0.458 | 8.056 | 0.005 | 0.273 | 0.098-0.618 |
| Δ1-LIS | -0.655 | 0.277 | 5.579 | 0.018 | 0.519 | 0.273-0.831 |
| (4) Association among Δ1-prognostic factor scoreb, Δ1-KDIGO stage, and Δ1-LIS | ||||||
| Coefficient | SE | Wald χ2 | p value | OR | 95% CI of OR | |
| Intercept | -3.383 | 0.903 | ||||
| Δ1-Prognostic factor scoreb | 0.516 | 0.442 | 1.363 | 0.243 | 1.676 | 0.723-4.253 |
| Δ1-KDIGO stage | 1.297 | 0.458 | 8.017 | 0.005 | 3.659 | 1.615-10.154 |
| Δ1-LIS | 0.637 | 0.285 | 4.988 | 0.026 | 1.891 | 1.157-3.633 |
| (B) Cut-off value at the start of PMMA-CHD | ||||||
| Cut-off value | Sensitivity | Specificity | AUC | |||
| Δ1-Prognostic factor scoreb | +2.0 | 76.7% | 66.7% | 0.788 | ||
| Δ1-KDIGO stage | +1.0 | 90.0% | 43.3% | 0.845 | ||
| Δ1-LIS | +3.0 | 66.7% | 90.9% | 0.859 | ||
For the multivariate analysis of values at the end of PMMA-CHD, we analyzed the difference from the start of PMMA-CHD to the end of blood purification (Δ2 values). We were unable to evaluate the association between the APACHE II, SOFA, and prognostic factor scores because the statistical model was insignificant (data not shown). However, the declining values in the prognostic factor score (Δ2-prognosutic factor score) and KDIGO stage (Δ2-KDIGO) were significant, and the odds ratios and 95% CI were relatively high (Table 5A). The cutoff value was -2.0 for both the Δ2-prognostic factor score and Δ2-KDIGO (Table 5B).
Table 5.
Association between the End of PMMA-CHD and Decreased Scores after Blood Purification Therapy.
| (A) Multiple logistic regression analysis of declining values (Δ2: decreasing difference of the enda from the startb of PMMA-CHD) | ||||||
|---|---|---|---|---|---|---|
| (1) Association among Δ2-APACHE II score, Δ2-KDIGO stage, and Δ2-LIS | ||||||
| Coefficient | SE | Wald χ2 | p value | OR | 95% CI of OR | |
| Intercept | -4.071 | 1.018 | ||||
| Δ2-APACHE II score | 0.193 | 0.109 | 3.104 | 0.078 | 1.213 | 1.009-1.549 |
| Δ2-KDIGO stage | 1.826 | 0.497 | 13.526 | <0.001 | 6.211 | 2.647-19.371 |
| Δ2-LIS | 0.401 | 0.291 | 1.889 | 0.169 | 1.493 | 0.894-2.868 |
| (2) Association among Δ2-SOFA score, Δ2-KDIGO stage, and Δ2-LIS | ||||||
| Coefficient | SE | Wald χ2 | p value | OR | 95% CI of OR | |
| Intercept | -3.300 | 0.838 | ||||
| Δ2-SOFA score | -0.045 | 0.156 | 0.084 | 0.711 | 0.956 | 0.696-1.302 |
| Δ2-KDIGO stage | 2.012 | 0.528 | 14.548 | <0.001 | 7.481 | 3.045-25.219 |
| Δ2-LIS | 0.551 | 0.275 | 4.023 | 0.045 | 1.735 | 1.069-3.193 |
| (3) Association among Δ2-prognostic factor scorec, Δ2-KDIGO stage, and Δ2-LIS | ||||||
| Coefficient | SE | Wald χ2 | p value | OR | 95% CI of OR | |
| Intercept | -3.149 | 1.161 | ||||
| Δ2-Prognostic factor scorec | -0.895 | 0.471 | 3.610 | 0.057 | 2.448 | 1.035-6.752 |
| Δ2-KDIGO stage | -1.549 | 0.435 | 12.671 | <0.001 | 4.708 | 2.199-12.492 |
| Δ2-LIS | 0.625 | 0.272 | 5.292 | 0.021 | 0.535 | 0.295-0.873 |
| (A) Cut-off of the difference Δ2 for the end of PMMA-CHD | ||||||
| Cut-off value | Sensitivity | Specificity | AUC | |||
| Δ2-Prognostic factor scorec | -2.0 | 70.0% | 45.5% | 0.597 | ||
| Δ2-KDIGO stage | -2.0 | 76.7% | 90.9% | 0.890 | ||
aValues of patients not requiring blood purification denote those at the end of ICU care.
bValues of patients not requiring blood purification denote those on the day after ICU admission.
cTotal points of prognostic factors listed in the Japanese severity criteria.
Abbreviations are listed in Tables 1 and 2.
During CHD treatment, several sessions of PMMA-CHD therapy required unanticipated exchange with a new circuit because of filter/circuit clotting due to the hypercoagulable state in patients with SAP. However, none of the patients experienced massive blood loss or required blood transfusion. Furthermore, none of the PMMA-CHD sessions were interrupted by adverse events, such as hemorrhagic complications. After treatment, all 30 patients requiring PMMA-CHD survived until day 28 (Table 1), and 28 patients recovered fully and were discharged from the hospital. However, two patients died: one of pancreatic cancer on day 59, and the other of ICU-acquired weakness with severe pneumothorax from unidentified causes on day 58. Of the 33 patients who did not require PMMA-CHD, 31 survived and were discharged from the hospital; however, 2 patients in this group died, both from severe alcoholic liver failure, after recovering from SAP on days 26 and 29.
Discussion
In this study, the deterioration of AKI at stage 2 in the KDIGO analysis indicated the start of PMMA-CHD therapy. General deterioration indicated by a prognostic factor score ≧3.0 and pulmonary deterioration with an LIS ≧3.0 could function as supplementary information for the start of PMMA-CHD. On comparison with data at hospitalization, systemic exacerbations, indicating by increases of +2.0 in the prognostic factor score, +1.0 in the KDIGO stage, and +3.0 in the LIS, were deemed useful indicators for the start of PMMA-CHD.
PMMA-CHD was started 4 to 89 h after ICU admission in the present study. The KDIGO stage at hospitalization was significantly correlated with blood purification therapy (Table 2). Therefore, we suggest that kidney injury is the principal cause of organ failure in patients requiring blood purification therapy. Serum creatinine levels did not differ significantly between patients with and without PMMA-CHD (Table 1). Conversely, the KDIGO stage showed a significant correlation with the initiation of PMMA-CHD in logistic analyses. Therefore, we suggest that a decrease in urine volume within a few days of SAP onset is important for determining the deterioration of kidney injury and initiation of blood purification. In addition, SAP progresses to multiple organ dysfunction a few days after the onset of acute pancreatitis (20). Systemic deterioration accompanied by kidney injury was assessed using the prognostic factor score and LIS in the present study, which provided useful information for the initiation of PMMA-CHD.
Regarding continuous renal replacement therapy (CRRT), many guidelines for AKI, including the Japanese guideline for sepsis, imply that kidney injury of KDIGO stage 3 should be an indicator for its initiation, except in cases of life-threatening complications, such as hyperkalemia and pulmonary edema (21). As such, the guidelines do not recommend our KDIGO stage 2 cutoff value at the start of PMMA-CHD. Xu et al. showed that the onset of CHF in patients with SAP at KDIGO stage 2 could effectively reduce intra-abdominal pressure, which correlated with blood interleukin (IL)-8 levels, and the start of stage 2 KDIGO decreased the mortality ratio to 8.1% compared to 36.4% mortality in the non-CRRT group (22,23). However, randomized clinical trials and meta-analyses of CRRT for AKI in critically ill patients have failed to clarify the advantages of starting therapy at lower KDIGO stages (24,25). The initiation of PMMA-CHD therapy in patients with stage 2 KDIGO thus remains to be addressed in future studies.
A meta-analysis showed that the urine volume after starting renal replacement therapy (RRT) for AKI in critically ill patients had a modest predictive ability for discontinuation (26). A prospective multicenter study of 92 critically ill patients revealed that creatinine clearance and the creatinine ratio on day 2/day 0 were associated with successful completion of RRT (27). In addition, a recovered urine volume was associated with the 60-day survival after discontinuation (28). Our findings suggested an improvement in AKI as the KDIGO stage declined to over -2.0, thus indicating the end of PMMA-CHD for SAP.
The aim of CRRT in patients with SAP is to reduce excess inflammatory responses, as immune dysregulation can cause the development of AKI with systemic exacerbation (29). CRRT using cytokine-adsorbing hemofilters has been widely found to show an improvement in patients with SAP, with a significant elimination of IL-6 and other inflammatory cytokines (13,25). Lower levels of IL-6 and osteopontin upon discontinuation of RRT are associated with a better prognosis in critically ill patients (28). Therefore, declining levels of serum IL-6 or other biomarkers may be useful indicators for the end of blood purification therapy for SAP in future studies.
The prognostic factor score of the Japanese severity criteria was a better predictor of hospital mortality than the APACHE II score (12). The Japanese prognostic factor score also showed a better predictive ability than the APACHE II score for the development of severe complications during the course of SAP (30). In this study, a noteworthy connection was established between the prognostic factor score and initiation and completion of PMMA-CHD. Nevertheless, no associations were detected between the APACHE II and SOFA scores. Thus, the prognostic factor score showed more utility for indicating the start and end of blood purification therapy than the APACHE II and SOFA scores.
Several limitations associated with the present study warrant mention. This was a single-center retrospective study, and the sample size was small, producing limited statistical predictions. Further studies with determinable statistical analyses are required to confirm the appropriate timing of the start and end of PMMA-CHD.
Conclusion
After systemic deterioration due to progressive inflammation in patients with SAP, AKI of KDIGO stage ≧2 is a suggestive indicator of the initiation of PMMA-CHD. Multiple organ failure, as denoted by a prognostic factor score ≧3.0 and LIS ≧3.0, was also useful for deciding on initiation. An increasing KDIGO stage (+1.0), prognostic factor score (+2.0), and LIS (+3.0) after hospitalization provided supplementary information regarding when to start blood purification therapy. After blood purification therapy, decreases in the prognostic factor score (-2.0) and KDIGO stage (-2.0) indicated the end of PMMA-CHD therapy.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors thank Mr. Kei Sakugawa, who operated the computer software program and provided advice on the statistical analyses in this study.
References
- 1.Kinjoh K, Nagamura R, Sakuda Y, et al. Clinical efficacy of blood purification using a polymethylmethacrylate hemofilter for the treatment of severe acute pancreatitis. Acute Crit Care 37: 398-406, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin Y, He S, Gong J, et al. Continuous veno-venous hemofiltration for severe acute pancreatitis. Cochrane Database Syst Rev 10: CD012959, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gui M, Zhao B, Huang J, Chen E, Qu H, Mao E. Pathogenesis and therapy of coagulation disorders in severe acute pancreatitis. J Inflamm Res 16: 57-67, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brain M, Winson E, Roodenburg O, McNeil J. Non anti-coagulant factors associated with filter life in continuous renal replacement therapy (CRRT): a systematic review and meta-analysis. BMC Nephrol 18: 69, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hattori N, Oda S. Cytokine-adsorbing hemofilter: old but new modality for septic acute kidney injury. Ren Replace Ther 2: 41, 2016. [Google Scholar]
- 6.Mann L, Ten Eyck P, Wu C, et al. CVVHD results in longer filter life than pre-filter CVVH: results of a quasi-randomized clinical trial. PLoS One 18: e0278550, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oshihara W, Fujieda H, Ueno Y. A new poly(methyl methacrylate) membrane dialyzer, NF, with adsorptive and antithrombotic properties. Contrib Nephrol 189: 230-236, 2017. [DOI] [PubMed] [Google Scholar]
- 8.Shinoda T. Anticoagulation in acute blood purification for acute renal failure in critical care. Contrib Nephrol 166: 119-125, 2010. [DOI] [PubMed] [Google Scholar]
- 9.Duriseti P, Idrees N, Aldairem A, Jaber BL, Balakrishnan VS. Cost analysis of two modalities of continuous renal replacement therapy. Hemodial Int 25: 173-179, 2021. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed AR, Obilana A, Lappin D. Renal replacement therapy in the critical care setting. Crit Care Res Pract 2019: 6948710, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie Y, Yuan Y, Su W, et al. Effect of continuous hemofiltration on severe acute pancreatitis with different intra-abdominal pressure: a cohort study. Medicine (Baltimore) 100: e27641, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takada T, Isaji S, Mayumi T, et al. JPN clinical practice guidelines 2021 with easy-to-understand explanations for the management of acute pancreatitis. J Hepatobiliary Pancreat 29: 1057-1083, 2022. [DOI] [PubMed] [Google Scholar]
- 13.Kishikawa T, Fujieda H, Sakaguchi H. Comprehensive analysis of cytokine adsorption properties of polymethyl methacrylate (PMMA) membrane material. J Artif Organs 25: 343-349, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 13: 818-829, 1985. [PubMed] [Google Scholar]
- 15.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22: 707-710, 1996. [DOI] [PubMed] [Google Scholar]
- 16.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 120: c179-c184, 2012. [DOI] [PubMed] [Google Scholar]
- 17.Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis 138: 720-723, 1988. [DOI] [PubMed] [Google Scholar]
- 18.Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology 174: 331-336, 1990. [DOI] [PubMed] [Google Scholar]
- 19.Livingstone DL. Multiple regression: robustness, chance effects, the comparison of models and selection bias. In: A Practical Guide to Scientific Data Analysis. 1st ed. John Wiley & Sons, Hoboken, 2009: 174-183. [Google Scholar]
- 20.Heckler M, Hackert T, Hu K, Halloran CM, Büchler MW, Neoptolemos JP. Severe acute pancreatitis: surgical indications and treatment. Langenbecks Arch Surg 406: 521-535, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egi M, Ogura H, Yatabe T, et al. The Japanese Clinical Practice Guidelines for Management of Sepsis and Septic Shock 2020 (J-SSCG 2020). J Intensive Care 9: 53, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu JM, Yang HD, Tian XP. Effects of early hemofiltration on organ function and intra-abdominal pressure in severe acute pancreatitis patients with abdominal compartment syndrome. Clin Nephrol 92: 243-249, 2019. [DOI] [PubMed] [Google Scholar]
- 23.Xu J, Cui Y, Tian X. Early continuous veno-venous hemofiltration is effective in decreasing intra-abdominal pressure and serum interleukin-8 level in severe acute pancreatitis patients with abdominal compartment syndrome. Blood Purif 44: 276-282, 2017. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Liu C, Mao Z, Li Q, Zhou F. Timing of renal replacement therapy initiation for acute kidney injury in critically ill patients: a systematic review of randomized clinical trials with meta-analysis and trial sequential analysis. Crit Care 25: 15, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.An JN, Kim SG, Song YR. When and why to start continuous renal replacement therapy in critically ill patients with acute kidney injury. Kidney Res Clin Pract 40: 566-577, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katulka RJ, Al Saadon A, Sebastianski M, et al. Determining the optimal time for liberation from renal replacement therapy in critically ill patients: a systematic review and meta-analysis (DOnE RRT). Crit Care 24: 50, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stads S, Kant KM, de Jong MFC, et al. Predictors of short-term successful discontinuation of continuous renal replacement therapy: results from a prospective multicentre study. BMC Nephrol 20: 129, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang T, Sun S, Zhao Y, et al. Biomarkers upon discontinuation of renal replacement therapy predict 60-day survival and renal recovery in critically ill patients with acute kidney injury. Hemodial Int 22: 56-65, 2018. [DOI] [PubMed] [Google Scholar]
- 29.Dambrauskas Z, Giese N, Gulbinas A, et al. Different profiles of cytokine expression during mild and severe acute pancreatitis. World J Gastroenterol 16: 1845-1853, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ikeura T, Horibe M, Sanui M, et al. Validation of the efficacy of the prognostic factor score in the Japanese severity criteria for severe acute pancreatitis: a large multicenter study. United European Gastroenterol Journal 5: 389-397, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]