Abstract
Microbial biosurfactants surpass synthetic alternatives due to their biodegradability, minimal toxicity, selective properties, and efficacy across a wide range of environmental conditions. Owing to their remarkable advantages, biosurfactants employability as effective emulsifiers and stabilizers, antimicrobial and antioxidant attributes, rendering them for integration into food preservation, processing, formulations, and packaging. The biosurfactants can also be derived from various types of food wastes. Biosurfactants are harnessed across multiple sectors within the food industry, ranging from condiments (mayonnaise) to baked goods (bread, muffins, loaves, cookies, and dough), and extending into the dairy industry (cheese, yogurt, and fermented milk). Additionally, their impact reaches the beverage industry, poultry feed, seafood products like tuna, as well as meat processing and instant foods, collectively redefining each sector’s landscape. This review thoroughly explores the multifaceted utilization of biosurfactants within the food industry as emulsifiers, antimicrobial, antiadhesive, antibiofilm agents, shelf-life enhancers, texture modifiers, and foaming agents.
Keywords: Biosurfactant, Low-cost substrate, Food sector, Sustainability, Antimicrobial
Introduction
Surfactants are chemical compounds consists of amphipathic molecules comprising both hydrophilic and hydrophobic moieties. Regarding the attributes of surfactants, they are defined by essential characteristics, including the critical micelle concentration, hydrophilic-lipophilic balance, chemical composition, and the charge present in the hydrophilic head group (Shoeb et al., 2013). The surfactants market is poised to witness a growth in size from 18.82 million tons in 2023 to 21.35 million tons by 2028, with a projected compound annual growth rate (CAGR) of 2.55%. Surfactants have played a pivotal role in the food industry. Surfactants perform diverse roles, including lubrication, emulsification, structure-building, aeration, quality enhancement, shelf-life extension, anti-adhesive, and moisture retention in various food products (Sharma 2014a). Utilizing compounds like sorbitol esters, their ethoxylates, and sucrose esters for emulsion, the industry has crafted a diverse array of food products, including mayonnaise, salad creams, dressings, and desserts. Moreover, sulfonyl stands as a prevalent surfactant extensively employed in coatings, inks, and adhesives utilized across numerous food-packaging applications (Nerin et al., 2018).
In this context, notwithstanding their benefits, biosurfactants hold an edge over synthetic counterparts due to factors like biodegradability, reduced toxicity, potential for sustainable manufacturing, and versatile functionalities, rendering them a more eco-conscious and adaptable option across a spectrum of industrial uses (Johnson et al., 2021). These particular biosurfactants are produced by microorganisms, specifically fungi, yeast, bacteria, and actinomycetes. The biosurfactant realm prominently features rhamnolipids from Pseudomonas aeruginosa, surfactin by Bacillus subtilis, sophorolipids from Salmonella bombicola, and alasan of Acinetobacter radioresistens, all serving as remarkable microbial-driven examples (Naughton et al., 2019). Biosurfactants offer distinct advantages over their synthetic counterparts, including biodegradability, minimal toxicity, heightened selectivity, and effectiveness across wide-ranging conditions of pH, temperature, and salinity (Vijayakumar and Saravanan, 2015). The global market for biosurfactants increased at a CAGR of 7.8% from $4.07 billion in 2022 to $4.39 billion in 2023 and reached $6 billion in 2027 at a CAGR of 8.1%. Prominent contributors to the biosurfactants market, spanning various countries, include Evonik Industries AG (Germany), BASF SE (Germany), Jeneil (USA), Givaudan (Switzerland), and Ecover (Belgium), among several others.
Within the realm of the food industry, biosurfactants exhibit the capability to create stable emulsions, rendering them valuable as wetting agents, food additives, and preservatives. The potential application in the food sector is centered on their adeptness as emulsifiers, improving attributes such as rheology, water retention, ingredient homogenization, and ease of handling (Bjerk et al., 2021). Moreover, biosurfactants showcase the capability to enhance not only product safety but also its inherent quality by mitigating contamination and microbial adhesion. These biomolecules bestow heightened resistance against oxidative degradation, when incorporated into formulations, consequently elongating the product's shelf life (Nalini et al., 2020). As a result, there has been a discernible shift towards harnessing these natural compounds as robust substitutes for synthetic counterparts, a trend characterized by a burgeoning scholarly interest (Mouafo et al., 2020b). While holding promising advantages, the complete integration of biosurfactants within the food industry remains an area that requires further exploration. In the scope of this review, a thorough investigation is undertaken to explore the diverse biosurfactant applications in the context of food. Additionally, a comprehensive analysis is presented concerning the classification and production methodologies of biosurfactants obtained from economically viable substrates.
Various types of biosurfactant
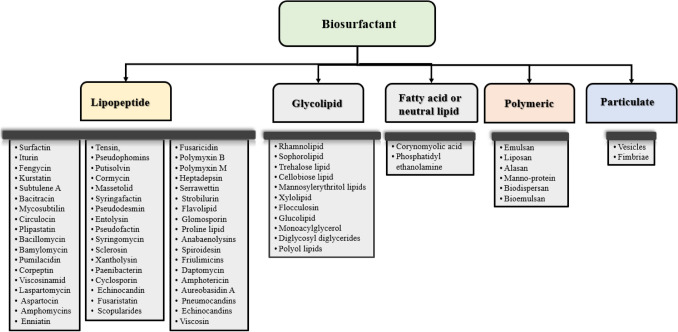
Biosurfactants can be categorized as glycolipids, lipopeptides, phospholipids, fatty acids, neutral lipids, polymeric and particulate surfactants based on their chemical composition and structure. The various classes of biosurfactants have been shown in Fig. 1.
Fig. 1.
Various types of microbial biosurfactants and its characteristics
Glycolipids
Glycolipids consist of a hydrophobic lipid tail and a carbohydrate portion, which are joined either through covalent bonding or connected by a glycosidic bond. Rhamnolipids, sophorolipids, trehalose lipids, cellobiose lipids, manno sylerythritol lipids, succinoyl trehalolipids, and xylolipids stand as significant and pivotal sub-classifications within the domain of glycolipids (Venkataraman et al., 2022). Microbial glycolipids exhibit a diverse range of intriguing functional characteristics. These encompass the capacity to lower surface and interfacial tension, adeptness in both emulsification and de-emulsification processes, notable foaming capabilities, effective solubilization and mobilization capacities, as well as the ability to create pores. Additionally, their noteworthy physicochemical attributes include resilience under demanding conditions such as extreme pH levels, high salinity, and elevated temperatures. Glycolipids differ in terms of the composition of carbohydrate and lipid moiety (Mnif and Ghribi, 2016).
Lipopeptides
Lipopeptides stand out as one of the most widely recognized categories of biosurfactants. In terms of structure, they are composed of a fusion between a fatty acid and a peptide segment (Wang et al., 2018). This amalgamation represents a collection of variations distinguished by the makeup of the peptide section, the extension of the fatty acid chain, and the linkage connecting these two components. Notably, surfactin, iturin, and fengycin stand out as renowned examples, finding applications in the food industry. With their diverse structural variations, lipopeptides excel at lowering surface and interfacial tension (Lin et al., 2020). They undertake a multitude of roles, including emulsification, dispersion, foaming, moisturization, and even hydrocarbon solubilization and metal binding. These compounds have an array of qualities, such as antibacterial, antiviral, anticancer, and insecticidal activities.
Fatty acids or neutral lipids
Various strains of bacteria and yeast have the capability to generate substantial amounts of fatty acids and phospholipid surfactants as they undergo growth while being nurtured on n-alkanes (Vijayakumar and Saravanan, 2015). On adjacent carbon atoms, corynomycolic acid has two hydrophilic heads (COOH and OH) and two hydrophobic tails (Kawase et al., 2015). Corynebacterinae has lipid-rich cell walls with 87 outer membrane layers controlled by long-chain alkyl hydroxy fatty acid (called corynomycolic acids in Corynebacterium). These membrane layers are covalently conjugated to arabinogalactan and trehalose as trehalose monomycolate and trehalose dimycolate (Schick et al., 2017). In regard to the food industry, neutral lipids and phospholipids fulfill diverse roles in the food industry: emulsification, texture enhancement, and flavor encapsulation. Phospholipids stabilize oil–water mixtures in dressings mayonnaise and enhance texture in spreads, baked goods, and confectionery. They also improve dough consistency, bread volume, shelf life, and nutritional content (Rahaman et al., 2023). In dairy products, they enhance stability and texture, while neutral lipids are suitable for high-temperature cooking due to their high smoke points and oxidative stability (Fookao et al., 2022).
Polymeric
Numerous bacteria create high molecular weight polymeric biosurfactants, termed bioemulsifiers, to differentiate them from low molecular weight metabolites (Bagheri et al., 2022). Lipomanan, liposan, emulsan, and alasan are the most well-known polymeric biosurfactants. Nevertheless, at low concentrations, emulsan is considered effective. Emulsan, an extracellular mixture of acylated polysaccharides generated primarily by Acinetobacter sp., is a widely used polymeric bioemulsifier. Acinetobacter radioresistens produces alasan, a compound of anionic polysaccharides consisting of covalently bonded alanine-rich proteins with three primary proteins. The water-soluble liposan bioemulsifier is composed of an organic solvent fermented by Yarrowia lipolytica yeast. This protein-carbohydrate complex constituting 83% carbohydrate and 17% protein is synthesized by the yeast Yarrowia lipolytica (Alizadeh-Sani et al., 2018). In the food business, polymeric surfactants are used for emulsification, foam stabilization, texture improvement, and raising the solubility of hydrophobic components in food products (Anal et al., 2019).
Particulate
Particulate biosurfactants generate microemulsion by partitioning external membrane vesicles, influencing alkane absorption in microorganisms. Vesicles with a diameter of 20–50 nm and buoyancy density of 1.158 cubic cm are found in Acinetobacter sp. They are made up of phospholipids, proteins, and lipopolysaccharides. Particulate biosurfactants, including extracellular membrane vesicles, partition oil–water mixture and generate micro-emulsion (at the interface) to allow microbes to absorb alkane (Adetunji and Olaniran, 2021). Particulate surfactants are used in the food industry to alter texture, stabilize emulsions, augment flavor delivery, and enhance the quality and appearance of food items. In order to seamlessly and effectively incorporate them into a wide range of food applications, it is imperative to conduct further research to comprehensively uncover their potential (Nitschke and Silva, 2018).
Furthermore, biosurfactants have been categorized into several classes based on their microbial origin. Among these, the most notable and recognized classifications are elaborated below. The biosurfactant classified based on microbial origin with their characteristics and the application in the food industry has been jotted down (Table 1).
Table 1.
An overview of the types and characteristics of microbial biosurfactants
| Biosurfactant | Microorganism | Yield | Time (h) | Characteristics | Food application | References |
|---|---|---|---|---|---|---|
| Rhamnolipid | Pseudomonas aeruginosa MTCC 2297 | 9.18 g/L | 24 |
ST: 31.33 mN/m CMC: 92.4 mg/L |
Antiadhension, Antibiofilm | George and Jayachandran (2013) |
| Sophorolipid | Candida albicans SC5314 | 1600 mg/L |
E24: 51% ST: 42 N/m |
Anti-bacterial, Emulsifier | Gaur et al. (2019) | |
| Trehalose lipid | Rhodococcus fascians BD8 | 20 g/L | 120 |
ST: 34 mN/m CMC: 0.140 mg/mL |
Antiadhesive agent | Janek et al. (2018) |
| Cellobiose lipid | Cryptococcus humicola JCM 1461 | – | – | CMC: 3.3 mg/L | – | Morita et al. (2011) |
| Emulsan | Acinetobacter calcoaceticusPTCC131 | 0.9 g/L | 48 |
E24: 98% ST: 24 mN/m |
Bioemulsifier | Amani and Kariminezhad (2016) |
| Biodispersan | Bacillus subtilis N3-1P | 216 |
ST: 27.9 mN/m CMC: 0.18 g/L |
Oil Dispersion | Zhu et al. (2020) | |
| Corynomyolic acid | P aeruginosa 2Bf | 12.46 mg/mL | 40 | – | Bioemulsifier | Ghilamicael, 2003) |
| Phosphatidyl ethanolamine | Consortium of five bacterial isolates | 74.49% | – | ST: 36 mN/m | Emulsifier | Deshmukh et al. (2012) |
| Liposan | Saccharomyces cerevisiae 2031 | – | 48 | E24: 58% | Bioemulsifier | Alcantara et al. (2012) |
| Alasan | A. radioresistens KA53 | 500 µg/mL | – | – | Bioemulsifier | Barkay et al. (1999) |
| Surfactin | Bacillus subtilis | – | 36 | ST: 26.9 mN/m CMC: 33 mg/L | Reduce the adhesion | Zezzi do Valle Gomes and Nitschke (2012) |
| Iturin | Bacillus amyloliquefaciens | 179.22 mg/l | 72 | CMC: 1.2 × 10−4 M | Antifungal and antibacterial in food packaging | Shi et al. (2018) |
| Fengycin | B. mojavensis B0621A | 247.3 mg/L | 72 | CMC: 1.2 ± 0.1 μM | Antifungal properties | Eeman et al. (2014) |
| Kurstatin | B. thuringiensis CIP 110220 | 24 | Antifungal | Abderrahmani et al. (2011) | ||
| Subtulene A | Bacillus subtilis SSE4 | 18 | Antifungal | Thasana et al. (2010) | ||
| Bacitracin | Bacillus cereus NK1 | 48 | CMC: 45 mg/L | Antimicrobial activity | Sriram et al. (2011) | |
| Mycosubtilin | Bacillus sp. MTCC 5877 | – | 72 |
E24: 50% ST: 32 mN/m |
Antibiofilm property | Anjum et al. (2016) |
| Circulocin | Bacillus circulans, J2154 | 210 mg/L | 168 | – | Antibacterial | He et al. (2001) |
| Plipastatin | B. subtilis 168 B. subtilis 3NA | 10 mg/L | 72 | – | Antifungal | Vahidinasab et al. (2020) |
| Bacillomycin | Bacillus 35 amyloliquefaciens FZB42 | 25 μg/mL | Inhibition of ochratoxin A production in food samples | Qian et al. (2015) | ||
| Pumilacidin | Bacillus pumilus | 70 µg/mL | 96 | Controls food poisoning | From et al. (2007) | |
| Entolysin | Pseudomonas entomophila | – | 16 | – | Heamolytic activity | Vallet-Gely et al. (2010) |
| Pseudofactin | Pseudomonas fluorescens BD5 | 72 mg/L | – | ST: 31.5 mN/m |
Antifungal, biosurfactant |
Janek et al. (2010) |
| Syringomycin | Pseudomonas syringae | – | 40 | – | Phytotoxic, antifungal, heamolytic activity | Anselmi et al. (2011) |
| Sclerosin | Pseudomonas sp. strain DF41 | – | 96 | – |
Antifungal, Antibacterial activity |
Berry et al. (2012) |
| Xantholysin | Pseudomonas putida BW11M1 | – | 48 | – |
Antifungal, Inhibits biofilm formation |
Li et al. (2013) |
| Paenibacterin | Paenibacillus thiaminolyticus | 6.8 µg/mL | 72 | – | Inhibiting food-born pathogens | Li et al. (2018) |
| Fusaricidin | Paenibacillus bovis sp. nov BD3526 | 96 | Antimicrobial | Hua et al. (2020) | ||
| Heptadepsin | Paenibacillus sp. BML771-113F9 | Antimicrobial activity: 30 μg/mL inhibition | Inhibit the adhesion molecules | Ohno et al. (2004) | ||
| Serrawettin | Serratia marcescens | 120 | ST: 30 mN/m | Antimicrobial, antitumor and plant protecting properties | Zhang et al. (2021) | |
| Flavolipid | Flavobacterium sp. strain MTN11 | 0.1 g/L | 192 | ST: 26.0 mN/m | Emulsifier | Bodour et al. (2004) |
| Glomosporin | Glomospora sp. | Antifungal | Sato et al. (2000) | |||
| Anabaenolysins | Anabaena sp. | 72 | Antimicrobial | Shishido et al. (2015) | ||
| Spiroidesin | Cyanobacterium anabaena | 96 | Inhibit toxic cyanobacterium Microcystis aeruginosa | Kaya et al. (2002) | ||
| Friulimicins | Actinoplanes utahensis | 120 | – | Emulsifier | Aretz et al. (2000) | |
| Daptomycin | Streptomyces roseosporus | 474 mg/L | 140 | – | Antimicrobial | Yuan et al. (2016) |
| Amphotericin | Streptomyces nodosus | 15.6 g/L | 144 | Antifungal drug | Huang et al. (2021) | |
| Brevibacillin | Brevibacillus laterosporus | – | 48 | – | Antibacterial | Xu et al. (2016) |
ST surface tension, E24 emulsification index 24, CMC critical micellar concentration
Rhamnolipid
Rhamnolipids are predominantly composed of crystalline acids characterized by the presence of one or two rhamnose units connected by β-hydroxylated fatty acid chains. Microorganisms such as Pseudomonas sp., Burkholderia sp., Acinetobacter sp., and Sphingomonas sp. are the primary producers of rhamnolipid (Haba et al., 2014). They exhibit several advantageous characteristics, encompassing antimicrobial, foam-forming (Campos et al., 2015), solubilization and biodegradability, biocompatibility, and metal-chelating properties. Upon examination through various studies, it was noted that rhamnolipids exhibited surface tension values spanning from 25 to 41 mN/m, with corresponding critical micelle concentration (CMC) values in the range of 20–230 mg/L. Moreover, these biosurfactants demonstrated robust stability over wide pH levels and temperatures. Rhamnolipids exhibit both lipophilic and hydrophilic characteristics, which are attributed to their structural composition and the fermentation process involved in their production. In the context of the food industry, they function as stabilizers, antimicrobials (Malakar et al., 2021), emulsifiers (Abbasi et al., 2020), antibiofilm and anti-adhesive agents that help prominently in food preservation (Araujo et al., 2016).
Sophorolipid
Sophorolipids are disaccharide sophoroses, intricately linked through β-glycosidic bonds to the hydroxyl group situated at the penultimate carbon of fatty acids. The terminal carboxyl group has the potential to adopt a lactonic conformation or be hydrolyzed, resulting in the formation of an anionic surfactant. Sophorolipid production is sourced from Candida sp., Rhodotorula sp., Starmerella sp., and Wickerhamomyces sp. (Punrat et al., 2020). Based on previous studies, the approximate measurements of surface tension yielded values spanning from 31.4 to 52.5 mN/m, accompanied by CMC values ranging between 40.1 and 110 mg/L. Sophorolipids play a prominent role as emulsifiers in the food industry, offering inherent antimicrobial properties that contribute to improved food preservation and safety (Zhu et al., 2023). Additionally, they possess algicidal properties and can enhance the water-holding capacity of food (Solaiman et al., 2015). These attributes make sophorolipids predominantly valuable in applications such as food packaging and ensuring food safety (Gaur et al., 2019).
Trehalose lipid
Trehalolipids encompass a diverse category of glycolipids composed of disaccharide trehalose linked to mycolic acids with long-chain fatty acids through an ester bond. They are predominantly produced by various actinobacterial genera, including Mycobacterium sp., Nocardia sp., Corynebacterium sp., Arthrobacter sp., and Rhodococcus sp. (Franzetti et al., 2010). Trehaloselipids are a family of biosurfactants with an amphiphilic structure allows them to successfully reduce surface tension (~ 24–58 mN/m) and stabilize interfaces between various substances, such as oil and water. They have outstanding emulsification abilities and have the ability to produce stable foams at a key micelle concentration (~ 25–166.16 mg/L). Their suitability for applications is further boosted by their biodegradability, pH adaptability, and heat stability. Trehalose lipids have applications in the food domain, attributed to their potential to reduce interfacial tension and enhance the apparent solubility of hydrophobic substances (Patil and Pratap, 2018).
Surfactin
Surfactin represents a cyclic lipopeptide characterized by a solitary hydroxy fatty acid chain and the linkage of seven amino acid residues through a lactone bond, yielding a compact cyclic lactone ring structure. Surfactin, primarily produced by Bacillus sp., stands out for its notable surface-active properties (lowest surface tension attained ~ 28.98 mN/m), and it showcases a spectrum of significant activities, including potent antiviral, antibacterial, and antitumoral (Qin et al., 2023). Within the food industry, surfactin proves its versatility by serving as an emulsifier, enhancing antioxidant stability (Giri et al., 2019), preventing adhesion (Zezzi do Valle Gomes and Nitschke, 2012), inhibiting spore formation, and its natural antimicrobial properties (Jiang et al., 2016), especially anti-listeria activity (Sabaté and Audisio, 2013). These diverse functionalities play a pivotal role in maintaining and ensuring the safety and preservation of food items (Hoffmann et al., 2021).
Fengycin
Fengycin, derived from various Bacillus subtilis strains, constitutes cyclic decapeptides characterized by a peptide chain of ten amino acids (Villegas-Escobar et al., 2013). This chain is intricately linked through a fatty acid chain of lactone bonds, culminating in synthesizing a closed cyclic peptide ring structure. Fengycin showcases robust antifungal efficacy, effectively restraining the growth of diverse food pathogens, with a notable emphasis on filamentous fungi (Lin et al., 2020). With its robust antifungal capabilities, fengycin can play a pivotal role in bolstering food safety and extending the shelf life of the products.
Iturin
Iturin stands as a family of cyclic lipopeptides distinguished by a peptide segment intricately bound to a β-amino fatty acid. This linkage occurs through amide bonds connecting with the individual amino acid residues (Zhao et al., 2021). Functioning as a distinctive category of pore-forming lipopeptides, they are notably acknowledged for their potent antifungal effects against a diverse spectrum of pathogenic yeasts and fungi. Through its potent antifungal capabilities, iturin can effectively thwart the growth of detrimental fungi responsible for food deterioration, thereby aiding in the preservation of food quality thereby extending the shelf life of perishable products (Kourmentza et al., 2021).
Food waste-derived biosurfactant production
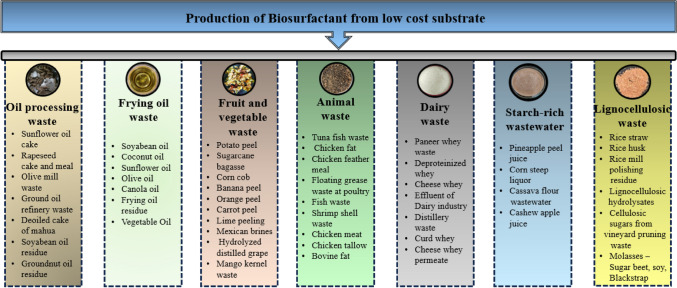
Over the past decade, the demand for affordable biosurfactant production materials has surged, driving research into renewable options. The low-cost food waste utilized for the production of biosurfactants is shown in Fig. 2. Food and agricultural waste are being investigated as potential substrates for biosurfactant production (Rivera et al., 2019). Utilizing waste materials as substrates offers a dual advantage by enhancing process profitability and addressing the challenge of managing rapidly accumulating waste (Rene et al., 2020). Nevertheless, in addition to cost-effectiveness, factors such as substrate stability, composition, form, and required quantity are pivotal considerations when selecting a suitable substrate for biosurfactant production (Varjani et al., 2020).
Fig. 2.
Production of microbial biosurfactants by employing low-cost food processing industry waste as substrate
Fruits and vegetable waste
Every year, a staggering amount of over 2.5 million tonnes of fruit and vegetable waste is generated globally. The extraction of juices and derivatives from fruits and vegetables yields a substantial volume of waste, encompassing peels from fruits like apples, bananas, oranges, and carrots. These discarded peels harbor the potential to function as invaluable substrates for the biosurfactant production process (Sharma and Vashist, 2015). For instance, isolated from hydrocarbon-contaminated soil, Pseudomonas aeruginosa SR17 demonstrated its remarkable capability for biosurfactant production, yielding 2.7 g/L of biosurfactant by utilizing waste whey as its primary substrate. This output was further augmented to 4.8 g/L through the introduction of mineral salts and glucose supplementation (Patowary et al., 2016). The biosurfactant rhamnolipid was produced via submerged fermentation of Pseudomonas aeruginosa MTCC 2297 using orange peel. This yielded 9.18 g/L of biosurfactant, which lowered surface tension to 31.3 mN/m (George and Jayachandran, 2013). Utilizing Bacillus subtilis SASCBT01 and cassava peel waste as a substrate, the biosurfactant production yielded 3 g/L within a 96 h timeframe with an emulsifying activity of 65% (Selvam et al., 2021). In another study, Pseudomonas aeruginosa ATCC 10145 produced 11.7 g/L of glycolipid biosurfactant, utilizing 120 g of soy molasses for fermentation (Rodríguez-Fernández et al., 2010). Rhamnolipids are derived from jackfruit waste through Pseudomonas aeruginosa SR17, yielding 2.3 g/L. The resulting biosurfactant demonstrates notable emulsification capacity in addition to its antifungal activity (Patowary et al., 2022).
Starch-rich wastewater
Furthermore, the starch extraction process from crops such as rice, wheat, cassava, and potatoes results in significant wastewater production (Bjerk et al., 2021). This starch-rich wastewater, containing husks, can potentially serve as a valuable resource for the manufacturing of biosurfactants. For instance, utilizing cassava wastewater, a byproduct abundant in carbohydrates resulting from cassava flour production shows considerable potential as a valuable resource for the synthesis of surfactin through the enzymatic actions of B. subtilis (Nitschke and Pastore, 2006). In another instance, cassava wastewater, combined with frying oil, was employed as a substrate for the production of rhamnolipid by utilizing Pseudomonas aeruginosa. This process resulted in a yield of 660 mg/L of rhamnolipid, effectively reducing the surface tension to 30 mN/m (Costa et al., 2009). In another case, Pseudomonas aeruginosa utilized rice water as a substrate to yield a substantial 9.35 g/L of rhamnolipid production (Poonguzhali et al., 2022). In a study, Bacillus subtilis utilized cassava wastewater along with whey and activated carbon as substrates for surfactin production, resulting in a yield of 27.07 mg/L and achieving a surface tension reduction to 29 mN/m (de Andrade et al., 2016).
Oil processing waste
Annually, around the world, approximately 200 million gallons of oil waste are being disposed of. Substantial oil processing on a notable level results in the generation of notable waste streams, encompassing soap stock, marine oils, lard, tallow, and liberated fatty acids acquired during the extraction of seed oils (Rufino et al., 2007). Effectively managing the disposal of this waste remains a persistent problem, prompting researchers to delve into the potential utilization of these waste materials as substrates for biosurfactants (Gaur et al., 2019). Within this framework, Bacillus subtilis is employed to generate surfactin utilizing sunflower cake and rapeseed cake as substrates, resulting in yields of 1.19 g/L and 1.45 g/L, respectively (Ciurko et al., 2022). In a study evaluated by Partovi et al. (2013), it was discovered that waste generated from refining of soybean oil could be effectively repurposed to produce biosurfactants. The biosurfactant was synthesized using Pseudomonas aeruginosa MR01 and exhibited a noteworthy yield of 14.55 g/L. This finding underscores the potential of utilizing soybean oil refining waste as a valuable resource in the creation of biosurfactants, highlighting both the environmental and economic benefits of this approach (Partovi et al., 2013). Within this specific context, lipopeptides have been synthesized using waste derived from the groundnut oil processing. Bacillus pseudomycoides was harnessed for the production of lipopeptides, which exhibited noteworthy antibacterial efficacy against food-borne pathogens, displaying a minimum inhibitory concentration (MIC) value of 50 μg/mL and also effectively reducing the surface tension to a remarkable 31.6 mN/m with their emulsification activity measuring at 81.2% (Chittepu, 2019). Harnessing oil processing waste for biosurfactant production not only optimizes resource utilization but also exemplifies a commitment to the principles of a circular economy, thereby presenting a dual advantage.
Frying oil waste
A substantial amount of waste frying oils is generated globally, especially in densely populated nations. For instance, in China, the quantity reaches 5.6 million tons, while the USA accounts for 1.2 million tons, India accounts for 1.1 million tons, and the EU accounts for approximately 0.9 million tons (Teixeira et al., 2018). Apart from processing oil waste, frying oil can also be used as the substrate for the economical production of biosurfactants. In a fed-batch process utilizing Pseudomonas aeruginosa ATCC 9027 as the microorganism and waste frying oil as the exclusive carbon source, the optimal production of rhamnolipids occurred after 72 h, resulting in a peak yield of 8.5 g/L (Luo et al., 2013). In this context, rhamnolipids derived from Pseudomonas aeruginosa, in conjunction with waste frying oil, demonstrated a production yield of 6.3 g/L. This rhamnolipid formulation exhibited a surface tension of 30 mN/m, coupled with an emulsification index of 84% with kerosene (Pathania and Jana, 2020). In another study, with Pseudomonas aeruginosa, the highest biosurfactant production of 11 g/L was attained using waste cooking oil as substrate. This biosurfactant exhibited a surface tension of 26.2 mN/m and an emulsification index of 62.5 ± 0.3% (Sharma et al., 2019). Furthermore, the frying oil waste has also found its application in the producing sophorolipid. Frying oil, along with palm oil, has been utilized to produce sophorolipid by Starmerella bombicola, yielding 142.8 g/L (Hirata et al., 2021). Beyond glycolipids, frying oil can also serve as a substrate for the production of lipopeptides. Bacillus subtilis employs waste frying oil as a substrate to generate surfactin, showcasing its potential for such endeavors (Valenzuela‐Ávila et al., 2020). Within the context, Bacillus pumilus was harnessed to employ waste frying oil as a substrate for the purpose of biosurfactant production, yielding a notable 5.7 g/L (Oliveira and Garcia-Cruz, 2013). The utilization of waste frying oil as a substrate for value-added products has gained popularity, potentially contributing to the establishment of large-scale economic processes and fostering a circular economy.
Lignocellulosic waste
Recent statistics reveal that the annual production of lignocellulose biomass on a global scale stands at roughly 181.5 billion tons. However, only 8.2 billion tons of lignocellulose biomass are utilized out of this vast amount. Besides, a wide array of lignocellulosic waste materials, including sugarcane bagasse (Marcelino et al., 2019), rice straw (Panjiar et al., 2020), wheat bran (Liu et al., 2017), coconut oil (Murugan and Rengaswamy, 2011), and rice bran (Bhardwaj et al., 2015), have been extensively examined for their potential in biosurfactant production. For example, lignocellulosic waste derived from rice straw and corn cobs has been employed for the purpose of biosurfactant production by Achromobacter sp. The sequential hydrolysis of rice straw and corn cobs yielded sugars at rates of 2.2 g/L and 7.8 g/L, respectively (Sari et al., 2020). The effective use of agro-wastes, specifically corn oil and cassava waste flour by Pseudomonas aeruginosa, to produce biosurfactants with yields of 0.60 mg/mL and 0.61 mg/mL, respectively (Varadharajan and Subramaniyan, 2014). Antarctic yeast strain, identified as Naganishia adellienses L95, was employed in the production of a biosurfactant using detoxified hemicellulosic hydrolysate from sugarcane straw. The process resulted in a substantial yield of 4.27 g/L of the biosurfactant, exhibiting an impressive emulsification activity of 52% (Chaves et al., 2021). These research findings collectively suggest that lignocellulosic residues offer a promising and economical alternative as carbon sources for biosurfactant production.
Dairy waste
With a daily milk processing capacity of around 500,000 l, a fully scaled dairy production unit can produce an estimated 200 to 350 kg of waste. In addition to the aforementioned points, dairy waste can also be utilized for the purpose of biosurfactant production. The dairy sector generates a substantial volume of waste in the form of by-products, including whey, buttermilk, and related derivatives (Usmani et al., 2022; Park et al., 2021). Evidently, only about half of the overall waste produced by the dairy industry is repurposed for valuable applications like animal feed, while the remaining portion is treated as disposable waste (Adesra et al., 2021). A yield of 4.8 g/L of rhamnolipid was achieved through the utilization of paneer whey by Pseudomonas aeruginosa strain SR17. This biosurfactant exhibited notable surface-active characteristics, as evidenced by a surface tension of 26.5 mN/m, a CMC of 11 mg/L, and demonstrated potential emulsifying properties (Patowary et al., 2016). In this context, by utilizing whey, Bacillus licheniformis strain M104 has produced a lipopeptide with substantial potential for antimicrobial activity (Gomaa, 2013). Bacillus sp. strain BioSol021 has successfully synthesized surfactin by employing cheese whey and winery flotation wastewater as substrates, yielding 1.54 g/L and 2.65 g/L, respectively. The resultant surfactin exhibits notable antifungal properties against aflatoxigenic Aspergillus flavus (Dmitrović et al., 2022). In another study, Bacillus subtilis ATCC 6633 employed rehydrated whey powder as the substrate to synthesize surfactin, achieving a yield of 0.24 g/L (Cagri-Mehmetoglu et al., 2012). As demonstrated in these studies, the utilization of dairy waste for biosurfactant production not only aligns with the principles of a circular economy but also proves to be a cost-effective approach.
Animal waste
The meat processing sector produces substantial quantities of waste materials like animal fat, tallow, and lard, which have been harnessed for the synthesis of biosurfactants. For instance, Bacillus subtilis ATCC 21332 effectively generated biosurfactants by utilizing fish peptone as a comprehensive substrate, leading to the noteworthy outcome of achieving the highest surfactin productivity of 274 mg/L during pilot-scale trials. This underscores the promising feasibility of utilizing fish waste as an economical substrate for large-scale surfactin production (Hu et al., 2021). Using chicken tallow as the carbon substrate, Yarrowia lipolytica produced biosurfactant with a yield of 1.41 g/L along lipase enzyme and microbial lipid (Radha et al., 2020a). In another study by the same group, Yarrowia lipolytica MTCC9520 demonstrated the ability to produce lipopeptides using chicken tallow as the substrate, resulting in a substantial maximum yield of 4.4 g/L. This process also exhibited noteworthy emulsification activity, reaching a peak of 55%, and concurrently lowering the surface tension to 37 mN/m after 96 h of cultivation (Radha et al., 2020b). The utilization of animal waste for biosurfactant production is an ongoing exploration, with its potential utilization showing promise; however, further studies are necessary to realize its applications. This approach not only addresses waste reduction but also aligns with the principles of a circular economy.
The utilization of affordable substrates presents the prospect of advantageous outcomes in terms of waste management and cost minimization for the production of biosurfactants. The resulting decrease in expenses is pivotal for extending the environmental applications of biosurfactants on a larger scale. Moreover, the purification aspect of biosurfactants has been extensively detailed in the research conducted by (Venkataraman et al., 2022).
Various applications of biosurfactants in food sectors
Biosurfactants play a crucial role in the realms of food processing, medical intervention, and therapeutic applications, serving as invaluable additives and refining agents (Sharma and Saharan, 2014). They exert their influence by not only heightening the palatability and safety of consumables but also contributing significantly to facets like nutritional content (Jemil et al., 2020), textural uniformity (Ravindran et al., 2023), flavour profile (Zhang et al., 2014), and the comprehensive security of the end product (De Giani et al., 2021). Furthermore, biosurfactants undertake the tasks of emulsification facilitators, viscosity enhancers, and promoters of optimal moisture retention. Various application of biosurfactant in the food sector has been shown in Fig. 3. Biosurfactants find applications in various sectors within the food industry, including food processing, food formulation, food packaging, and food preservation (Table 2).
Fig. 3.

Application of microbial biosurfactants in food industry
Table 2.
Food applications of various microbial biosurfactants
| Biosurfactant concentration | Origin | Food sector | Target | Additive | Remarks | References |
|---|---|---|---|---|---|---|
| Food preservation | ||||||
| Bacillomycin D (50 mg/L) | Bacillus sp | Cherry | Antifungal activity against Botrytis cinerea and Rhizopus stolonifer | Chitosan |
Enhance the cherry tomato shelf life MIC value: R. stolonifer (100 mg/L) and B. cinerea (50 mg/L) |
Lin et al. (2021) |
| Biosurfactant (10 mg/mL) | Lactobacillus paracasei subsp. tolerans N2 | Meat industry (Beef) | Antiadhesive property against S. aureus STP1, Bacillus sp. BC1 and S. xylosus STP2 | – |
Inhibit growth leading to meat spoilage Inhibition rate: 100% MIC value: 12.5 mg/mL |
Mouafo et al. (2020a) |
| Biosurfactants (0.4 g/100 g) | Lactobacillus paracasei subsp. tolerans N2 and Lactobacillus casei subsp. casei TM1B | Goat meat | Antibacterial activity against Pseudomonas and Escherichia | – |
Increased the shelf life upto 15 days Growth reduced from 4.76 Log CFU/g to 1.29 Log CFU/g |
Mouafo et al. (2020b) |
| Brevibacillin 3,200 μg/mL | Brevibacillus laterosporus | – | Antibacterial activity against Listeria monocytogenes, Bacillus cereus, and Alicyclobacillus acidoterrestris | – |
Eradicate the multidrug resistant organism Can be utilized as food additive MIC range: 1–8 μg/mL |
Xu et al. (2016) |
| Cyclolipopeptides (3%) | Bacillus subtilis | Berries | Antifungal activity against Aspergillus niger | Sodium alginate |
Multifunctional coating film for berries-Stoma of peel (10 μm) Inhibiting respiration of the berries Zone of inhibition range: 33.2–55.8 mm |
Xu et al. (2020) |
| Fengycin and surfactin (1 mg/mL) | Bacillus sp. YJ17 | Dairy products | Antibacterial activities against Pseudomonas aeruginosa and Staphylococcus aureus | – |
Established study in deep-sea water environment Zone of inhibition range: 6.5 -24.17 mm |
Gu et al. (2022) |
| Fengycins | Bacillus amyloliquefaciens JFL21 | Sea-food | Antimicrobial efficacy against L. monocytogenes, A. hydrophila, and C. gloeosporioides | – |
Evaluvation of food production chain MIC value: 25–50 ug/mL |
Lin et al. (2020) |
| Iturin A (0.13–0.76 mg/mL) | Bacillus amyloliquefaciens LZ-5 | Orange juice | Antimicrobial activity against Saccharomyces cerevisiae | – |
Juice product stabilization MIC value: 0.76 mg/mL |
Shi et al. (2018) |
| Iturin A (36 μg/ mL) | B. amyloliquefaciens BUZ-14 | Fruits | Antifungal activity against Monilinia species and P. expansum, | – |
Eradicate postharvest diseases Induce antagonistic activity MIC range: 16.9 and 33.9 μg/mL |
Calvo et al. (2019) |
| Iturin V (125 µg/mL) | Lactobacillus sp. M31 | Aquatic food | Antibacterial activity against Pseudomonas and Vibrio | Lactic aid |
Control pathogens during the processing of food items MIC value: 25 µg/mL |
Singh et al. (2021) |
| Iturin, fengycin and surfactin | B. amyloliquefaciens ssp. plantarum | Fermented food | Antibacterial activity against Bacillus cereus |
Inhibit the pathogenic organism in food Safeguarding the plant contamination Inhibition activity reduced to 75% while increasing temperature |
Compaoré et al. (2013) | |
| Lipopeptide | Bacillus amyloliquefaciens | Soya bean | Antibacterial activity against, Listeria monocytogenes, Penicillium sp. and Bacillus cereus | – | Inhibit foodborne pathogens | Lee et al. (2016b) |
| Lipopeptide (1 mg/mL) | Bacillus subtilis | Shrimp meat | Antibacterial against Staphylococcus aureus | – |
Enhances the food safety Suppress the enterotoxin production MIC value: 1.25 mg/mL |
Zhang et al. (2017b) |
| Lipopeptide (1 mg/mL) | Marine bacterium | Shrimp | Antimicrobial activity against pathogenic Vibrio parahaemolyticus SF14 | – |
Adjust the gut microbiome to improve shrimp health Enhance the immunity of aquaculture Mortality rate: 50% |
Prathiviraj et al. (2021) |
| Lipopeptide (250 µg/mL) | Bacillus methylotrophicus DCS1 | Ground beef patties | Antioxidant activity determined for lipid oxidation by emulsification | – |
Enhance the nutritional properties Extension of shelf life Antioxidant concentration: 250 µg/mL |
Jemil et al. (2020) |
| Mannosylerythritol lipids (10 mg/mL) | Pseudozyma aphidis DSM 70725 | Dairy, and fishery products | Antibacterial activity against Staphylococcus aureus | – |
Inhibit planktonic cells Inhibit the biofilm formation Prevent contamination in food contact surfaces MIC value: 0.625 mg/mL |
Shu et al. (2020 |
|
Mannosylerythritol lipids-A (128 μg/mL) |
Pseudozyma aphidis | Milk | Antibacterial effects against Listeria monocytogenes | – |
MIC value: 32 μg/mL Potent antimicrobial agent |
Liu et al. (2020) |
| Mycosubtilin and surfactin mixtures (1–16 mg/L) | Bacillus sp. | Meat, cheese and beverages | Antimicrobial activity against Paecilomyces variotti, Candida krusei and Byssochlamys fulva | – |
Extension of shelf life Maximum rate of inhibition: 73.2% Potential food additives |
Kourmentza et al. (2021) |
| Rhamnolipid (1 mg/mL) | Pseudomonas sp., | Vegetables and fruits (potato, tomato and lemon) | Antifungal activity against A. oryzae, F. solani, and Curvularia sp | – |
Extending shelf life and quality Also exhibit antiadhesive properties |
Sharma et al. (2018) |
| Rhamnolipid (100 µg/mL) | Pseudomonas aeruginosa UKMP14T | – | Antibacterial activity against Escherichia coli and Staphylococcus aureus | – |
Recovery of high value product MIC value: 10 µg/mL |
Firdose et al. (2021) |
| Rhamnolipid (16.5–500 µg/mL) | Pseudomonas aeruginosa JS29 | – | Antimicrobial activity against Staphylococcus aureus | Zinc oxide nanoparticles |
Inhibit the formation of biofilm on food material MIC value at 500 µg/mL |
Malakar et al. (2021) |
| Sophorolipid | Candida bombicola | Meat or cheese products | Antibacterial activity against. Listeria monocytogenes and Salmonella sp | Palmitic acid, oleic acid and stearic acid |
Enhance food safety Microbial population reduction: 0.2–0.8 log CFU/mL |
Zhang et al. (2016) |
| Sophorolipid (1800 μg/mL) | Starmerella bombicola | – | Antifungal activity against the food borne fungi Aspergillus sp. | – |
Controlling the microbial spoilage of foods Enhancing food safety MIC range: 225–729 µg/mL |
Hipólito et al. (2020) |
| Surfactin (1 mg/mL) | Bacillus subtilis | Honey | Effectiveness against various strains of L. monocytogenes in terms of its anti-Listeria activity | – |
New tool as food preservative Sustain antagonistic activity under extreme temperatures and pH MIC value: 0.125 mg/mL |
Sabaté and Audisio, 2013) |
| Surfactin (1 mg/mL) | Bacillus subtilis | Broilers | Antimicrobial activity against Staphylococcus aureus, Escherichia coli, C. perfringens and Salmonella typhimurium | – |
Potential feed additives Substitutes for antibiotics in poultry industry MIC value: 100 µg/mL |
Cheng et al. (2018) |
| Surfactin (3 mg/mL) | Bacillus velezensis SK | Aquatic food | Antimicrobial agent against Bacillus cereus | – |
Prevent food borne pathogen Utilized as probiotics in aquacultures Exhibit antimicrobial activity: 1600 AU/mL |
Barale et al. (2022) |
| Surfactin A (1 mg/mL) | Bacillus amyloliquefaciens | Kiwifruit | Antifungal activity against Botryosphaeria dothidea | – |
Delay fruit senescence Enhance kiwifruit disease resitance Inhibition rate: 73.12% |
Pang et al. (2021) |
| Surfactin, iturin and fengycin (2 mg/mL) | Bacillus subtilis | Honey | Antibacterial activity against and S. aureus, B. cereus and Listeria monocytogenes | – |
Inhibitory activity against food borne pathogens MIC range: 0.1–2 mg/mL |
Torres et al. (2015) |
| Surfactin, iturin, and fengycin (4096 μg/mL) | Bacillus velezensis | Camellia assamica leaves | Antimicrobial activity against Aspergillus sp, Staphylococcus aureus, Listeria monocytogenes, and Salmonella typhimurium | – |
Inhibit the toxic fungi in food industry MIC value of bacteria: 512 − 2048 μg/mL MIC value of fungi: 128 − 256 μg/mL |
Li et al. (2020) |
| Food packaging | ||||||
| Brevibacillin V (64 μg/mL) | Brevibacillus laterosporus fmb70 | Skim milk preservation | Antibacterial activity against S. aureus and L. monocytogenes | – |
Inhibit the food borne pathogen Food preservative for skim milk MIC range: 4–64 μg/mL |
Wu et al. (2019) |
| Fengycin and iturin (1 mg/mL) | Bacillus sp | Fermented food products | Antibacterial activity against Bacillus cereus, Enterococcus faecalis and Listeria monocytogenes | – |
Enhance the Cheonggukjang fermentation against pathogen Maximum antifungal unit: 160 AU/mL |
Lee et al. (2016a) |
| Iturin A (100 μg/mL) | Bacillus subtilis | Chicken | Antimicrobila activity against E. coli, S. aureus and Salmonella | Silver naoparticles |
Antimicrobial designed to safeguard food items Prevent spoilage during storage Zone of inhibition range: 8–18.2 mm |
Zhao et al. (2021) |
| Lipopeptides (10 mg/mL) | Bacillus | – | Antibacterial activity of L. monocytogenes | Biopolymer (pectin) |
Antimicrobial food packaging Water vapour permeability Zone of inhibition: 2–4 mm |
Verónica et al. (2023) |
| Lipopeptide | Bacillus sp | – | Antibacterial activity against Salmonella typhimurium and Pythium myriotylum | Nanocomposite |
Useful in food packaging wraps Water adsorbtion capacity: 94.6% |
Jayakumar et al. (2021) |
| Sophorolipid | Starmerella bombicola | – | Antifungal properties against Botrytis cinerea | Cassava starch |
Food packaging to manage and mitigate foodborne pathogens Inhibition rate: 100% |
Hipólito et al. (2021) |
| Sophorolipid | Starmerella bombicola | Chicken | Antimicrobial efficacy against Clostridium perfringens and Campylobacter jejuni | Lactic acid |
Reduces chicken pathogens Natural sanitizer MIC range: 0.003–0.5% |
Silveira et al. (2019) |
| Sophorolipid (4 mg/mL) | Starmerella bombicola | Poultry feed | Antibacterial activity against Listeria monocytogenes, Staphylococcus aureus and Salmonella sp. | Polylactic acid |
Plasticizer agent Reduced tensile strength Enhanced thermomechanical properties Inhibition rate:50% |
Silveira et al. (2020) |
| Sophorolipid | Candida bombicola | Feed | Antimicrobial activity against Propionibacterium acnes | Biopolymer |
Utilized in food packaging Algicidal property Zone of inhibition: 5–8 mm |
Solaiman et al. (2015) |
| Sensory and texture evaluation | ||||||
| Lichenysin (111.74 μg/L) | Bacillus licheniformis | Beverage | Flavour enhancer | – |
Add flavour to beverages Enhance the aroma of the product MIC value: 160 μg/L |
Zhang et al. (2014) |
| Lipopeptide | Bacillus licheniformis | Yoghurt | Flavour and texture enhancer | – |
Enhanced flavor and stability Probiotic supplementation |
Ravindran et al. (2022) |
| Lipopeptide | Bacillus licheniformis MS48 | Cookie | Antioxidant activity indicates the conformation changes in gluten | – |
Improved emulsification:70% Enhance the textural of cookie Improve sensory properties |
Ravindran et al. (2023) |
| Mannosylerythritol Lipid-A | Moesziomyces aphidis DM34 | Dough and bread | Reduced the moisture migration capacity and increased water holding capacity | – |
Maximum springiness: 77.47% Highest specific volume: 2.981 mL/g |
Liu et al. (2024) |
| Mannosylerythritol Lipid-A (0.5%, 1% and 1.5%) | Pseudozyma aphidis | Dough and bread | Antibacterial activity against Bacillus cereus |
Antibacterial rate: 75.54% spore and 99.97% for vegetative spores Increased the gas retention ability and loaf volume Enhance the bread textural property |
Shu et al. (2022) | |
| Mannosylerythritol Lipid-A (128 μg/mL) | Pseudozyma aphidis | Antibacterial activity against L. monocytogenes | Lactic acid |
Antibacterial rate: 81.8% Incorporate significant changes in amino acid and carbohydrate metabolism |
Liu et al. (2022) | |
| Rhamnolipid (1 mg/mL) | Pseudomonas aeruginosa | Ice cream, loaf and mayonnaise | Antimicrobial activity against Candida albicans, Rhodotorula sp, and Bacillus subtilis | – |
Zone of inhibition: 17–30 mm Enhances the taste, flavour and texture |
AK et al. (2016) |
| Emulsification | ||||||
| Biosurfactant | Lactobacillus pentosus Tw226 | Essential oils | Biosurfactants that are useful to form and stabilize oil in water emulsions | – |
Stabilization of oil in water emulsions Emulsification range: 82 and 98% |
Lara et al. (2022) |
| Biosurfactant (0.7%) | Candida utilis | Food emulsion | Formulations of mayonnaise | – |
Enhance the emulsification, Improve foaming and wetting Total amount of lipid: 65.88% |
Campos et al. (2015) |
| Biosurfactant (1%) | Candida utilis | – | Bio-emulsifier in food products | – |
Enhances emulsification property Emulsification index: 73% |
Campos et al. (2014) |
| Lipopeptide (10 mg/mL) | Bacillus cereus UCP 1615 | Cookie emulsion | Anti-oxidant activity against Bacillus cereus | – |
Total antioxidant capacity:476.43 ± 12.34% Green additive in the food industry Economically viable |
Durval et al. (2021a) |
| Lipopeptide (125 μg/mL) | Nesterenkonia sp | Emulisification (muffin processing) | Emulsification activity observed was 75% | – |
Improved the quality of food Potential emulsifier utilized as food additives MIC value: 125 μg/mL Antibacterial and antibiofilm activity against Staphylococcus aureus |
Kiran et al. (2017) |
| Rhamnolipid | Pseudomonas aeruginosa | Essential oils | Emulsification index range from 10–42.8% | – |
Increase their availability Potential antimicrobial against of C. albicans and S. aureus MIC value: 0.125% |
Haba et al. (2014) |
| Rhamnolipid and Sophorolipid | Pseudomonas aeruginosa | Beverage industry emulsion |
Water emulsion formulation with 2.75% sophorolipids and 0.662% rhamnolipids |
– |
Rhamnolipids improve the stability of emulsion system (92.64%) Viscosity ratio: 0.338 |
Abbasi et al. (2020) |
| Sophorolipid (300 mg/l) | Wickerhamomyces anomalus MUE24 | Food retrogradation (Rice flour) | Holds the emulsifying capacity for the vegetables oil | – |
Potential to enhance rice flour's retrogradation Increase the swelling power and water-holding capacity Water holding capacity improved from 1.3 to 1.6 g/g |
Punrat et al. (2020) |
| Sophorolipid (60 mg/L) | Candida albicans SC5314 | Vegetable oils | Higher emulsification was observed at pH 7.0 in the range of 51–53% | – |
Thermotolerant upto 120 °C Inhibition rate: 65.8% Bacillus subtilis |
Gaur et al. (2019) |
| Surfactin (0.01%) | Bacillus subtilis | – | Reducing the interfacial tension by oil in water emulsion | – |
Enhancing the emulsion formation: 83.26% Stabilization effect of emulsion |
Hoffmann et al. (2021) |
| Surfactin (0.2 mmol/L) | Bacillus subtilis | Docosahexaenoic acid | Improved the antioxidation stability of the oil-in-water microemulsion | – |
Minimized the oxidation of DHA within the microemulsion Emulsion diameter reduced from 140 to 15 nm |
He et al. (2017) |
| Food processing | ||||||
| Biosurfactant | Levilactobacillus brevis | Dairy industry | Emulsification of product enhanced production of biosurfactant | – |
Also applicable in medical industry Emulsification indices of 17.14% and 37.5% were achieved with hexadecane and crude oil, respectively |
Soleiman Meiguni et al. (2022) |
| Biosurfactant (5 mg/mL) | Bacillus subtilis VSG4 and Bacillus licheniformis VS16 | Antibiofilm and antioxidant activity against Staphylococcus aureus, Salmonella typhimurium, and Bacillus cereus | – |
Antioxidant range: 69.1–73.5% by DPPH Antibiofilm range: 61.1–68.4% Enhanced antiadhesive property |
Giri et al. (2019) | |
| Brevilaterin B (10 mmol/L) | B. laterosporus S62-9 | Instant food | Antibacterial activity against L. monocytogenes | – |
Induced the membrane related pathways Stress response activation at the gene level Fluorescence intensity reduces to 59.13% |
Liu et al. (2023) |
| Iturin A | Bacillus velezensis | Honey production | Antifungal activity against Aspergillus fumigatus | – |
Ecofriendly Antifungal activity unit:20–1600 AU/mL |
Xiong et al. (2022) |
| Lipopeptides (64 μg/L) | Bacillus subtilis | Fermented products | Antagonistic activity against Bacillus cereus and Listeria monocytogenes | – |
Exhibit the stability over board pH and temperature Shows antibacterial activity without inhibiting the useful organism for fermentation MIC range: 64—128 μg/mL |
Wu et al. (2013) |
| Mannosylerythritol Lipid-A (16 μg/mL-32 μg/mL) | Pseudozyma aphidis | Food processing | Antibacterial activity against L. monocytogenes | – |
MICvalue: 32 μg/mL Enhances food safety |
Liu et al. (2022) |
| Mannosylerythritol Lipid-A (0.5%) | Acinetobacter calcoaceticus RAG-1 (ATCC 31012) | Bread | Bioemulsifier and anti-staling agent | – |
Enhances the shelf-life of bread Texture analysis: 425.7 to 210.25 N |
Sadeghi et al. (2023) |
| Paenibacterin (1.7 μg/mL) | Paenibacillus thiaminolyticus | Food processing | Antibiofilm activity against Listeria monocytogenes | Chitosan |
Gene regulation in biofilm formation Inhibition of biofilm in surfaces MIC value: 6.8 µg/mL |
Li et al. (2018) |
| Rhamnolipid | P. aeruginosa PA1 | – | Prevent the biofilm formation against L. monocytogenes and Pseudomonas fluorescens on food contact servies | – |
Antiadhesion rate:79% Antibiofilm rate:83% |
Araujo et al. (2016) |
| Rhamnolipid | Pseudomonas aeruginosa | Cat fish | Antimicrobial activity against Flavobacterium columnare | – | Survival rate of cat fish:50% | Zhang et al. (2017a) |
| Rhamnolipid (1 mg/mL) | P. aeruginosa | Poultry feed | Antibiofilm property against S. enteritidis, E. coli, and C. jejuni | – |
Natural disinfectant Maximum antibiofilm activity: 87.67% |
Carvalho et al. (2022) |
| Surfactin | Bacillus subtilis | – | Antiadhesion activity against L. monocytogenes and S. enteritidis |
Adhesion rate: 63.8% Potential food additive Prevent adhesion in stainless steel and polypropylene surfaces |
Nitschke et al. (2009) | |
| Surfactin and rhamnolipid | Bacillus subtilis and Pseudomonas aeruginosa | Food contact surface | Antiadhesion activity against Staphylococcus aureus, Listeria monocytogenes and Salmonella enteritidis | Polystyrene (model surface) |
Adhesion rate of surfactin:58.5% Adhesion rate of rhamnolipid:24% |
Zezzi do Valle Gomes and Nitschke (2012) |
| Food security | ||||||
| Fengycin (1 mg/L) | Bacillus subtilis | Rice | Antifungal activity against Magnaporthe grisea | – |
Induce bursts of reactive oxygen species Utilized as biocontrol agents Zone of inhibition: 2.25 cm |
Linlin and Chaomin (2018) |
| Fengycin A (10–25 μg/mL) | Bacillus megaterium WL-3 | Potato | Anti-oomycete activity against Phytophthora infestans | – |
Control potato late blight promote potato plant growth Inhibition rate: 73.3–84.6% |
Wang et al., (2020) |
| Fengycin H (1 mg/mL) | Bacillus cabrialesii BH5 | Tomato | Inhibition against Botrytis cinerea | – |
Prevent gray mold disease Act as biological control for crop protection Inhibition rate:61–87% |
Zhou et al. (2021) |
| Lipopeptide (50 μg/mL) | Bacillus subtilis strain 109GGC020 | Wheat | Antifungal activity against Magnaporthe oryzae | – |
Biocontrol agents Decrease wheat blast disease Inhibition percentage:51.4–60.9% |
Chakraborty et al. (2020) |
| Surfactin (50 μg/mL) | Bacillus subtilis | Food security | Antimicrobial activity against B. pumilus and Micrococcus luteus | – |
Inhibit the fungal contamination Enhance the food safety MIC value: 50 μg/mL |
Jiang et al. (2016) |
MIC minimum inhibitory concentration
Food processing and formulation
Biosurfactants find application across sectors, notably in food processing, as eco-friendly replacements for traditional emulsifiers. These biologically sourced compounds effectively stabilize oil–water mixtures, enhancing product quality, lifespan and aligning with consumer preferences for sustainability (Caporgno et al., 2019). In food formulations, biosurfactants serve as environmentally conscious alternatives to conventional emulsifiers (Campos et al., 2014), excelling in stabilizing oil–water blends (He et al., 2017), extending product shelf life (Sharma et al., 2015) and satisfying customer preferences for eco-friendly ingredients (Gaur et al., 2019).
Emulsifier and texture enhancer
Emulsifiers stabilize oil–water mixtures, yet the trend leans towards natural, eco-friendly choices. Bio-based surfactants replace synthetic ones in food, ensuring both consumer satisfaction and environmental responsibility. Emulsifiers play a vital role in food formulation by mitigating interfacial tension and averting phase clustering (Hoffmann et al., 2021). Low-molecular-weight surfactants are key to preserving emulsion stability, while microbial surfactants enhance not only consistency and rheological properties but also prolong the product's shelf life (Nalini et al., 2020).
Industries such as dairy production (Gu et al., 2022), fermented goods (Compaoré et al., 2013), bread production (Shu et al., 2022), and distilleries utilize biosurfactants to realize intended product outcomes (Sałek and Euston, 2019). In dairy processing, emulsions are extensively employed across a range of consumable products, encompassing milk, butter, margarine, mayonnaise, and ice cream. Incorporating 0.7% (w/v) of a high molecular weight biosurfactant sourced from C. utilis, combined with guar gum, into the mayonnaise formulation results in a product that maintains excellent shelf-life stability. Notably, no visible phase separation occurs even after a duration of 4 weeks under refrigeration (Campos et al., 2014).
In the baked goods sector, Mnif et al. (2012) discovered that lipopeptide biosurfactant from the B. subtilis BS1 strain incorporated into bread dough at a ratio of 0.075 wt% resulted in improved characteristics of the bread products. The research conducted by (Zouari et al., 2016) demonstrated that integrating B. subtilis SPB1 biosurfactant into cookie dough at a concentration of 0.1% relative to the flour weight led to notable improvements in the textural attributes of the dough. Utilizing lipopeptides from B. subtilis as emulsifying agents resulted in decreased dough firmness and improved batter consistency. Consequently, the inclusion of these biosurfactants led to the production of cookies with a considerably softer texture (Zouari et al., 2016). Upon the utilization of the biosurfactant extracted from Nesterenkonia sp. to enhance muffin texture, a plethora of supplementary benefits ensue. These encompass a notable reduction in attributes such as hardness, chewiness, and gumminess, particularly when juxtaposed with control samples. This effect is particularly conspicuous when the formulation incorporates 0.75% lipopeptide into the preparation mixture (Kiran et al., 2017). Incorporating a biosurfactant derived from the actinobacteria Nesterenkonia alba into muffin dough resulted in a softer texture, enhanced uniformity, and decreased chewiness (Kiran et al., 2017). Utilizing the biosurfactant from Candida bombicola URM 3718 as a dietary addition took the form of cupcakes. Within the cupcake dessert formulation, the biosurfactant was introduced as a replacement for 50%, 75%, and 100% of the initial vegetable fat content. Remarkably, the cupcakes' textural characteristics remained largely unaffected when portions or the entirety of the vegetable fat were substituted with biosurfactants (Silva et al., 2020).
In cereals and grains, a biosurfactant derived from Wickerhamomyces anomalus, specifically a sophorolipid, successfully lowered the surface tension from 52.5 to 36.0 mN/m. This particular biosurfactant has been noted for its ability to enhance several properties of rice flour, including retrodegradation, swelling power, and water-holding capacity (Punrat et al., 2020). In beverages, biosurfactants play a pivotal role, especially in formulating, producing, and stabilizing various beverage types. A key aspect of beverage production involves preparing stable emulsions, where biosurfactants demonstrate their prowess in establishing enduring emulsions, serving as emulsifying or de-emulsifying agents (DeGroff, 2010). Numerous established products employ aeration, wherein the inclusion of air, manifested as bubbles, imparts diverse textures and sensory experiences to these items. Incorporating air into the matrix of food significantly influences its texture, resulting in enhanced firmness and smoother and lighter characteristics (Zúñiga and Aguilera, 2008).
In edible oils, lipoprotein biosurfactants synthesized by specific Bacillus sp. LB5a, along with rhamnolipids generated by Pseudomonas aeruginosa 47T2, contribute to the creation of stable emulsions using coconut fat and soybean oil. These characteristics render them valuable for their application as emulsifying agents within the food sector.
Food preservation and packaging
Beyond their emulsification capacity, biosurfactants also offer other advantageous traits that contribute to the preservation and enhancement of shelf life in food products.
Antimicrobials
Biological surface-active compounds have demonstrated antimicrobial properties against different bacterial such as Salmonella typhimurium, Listeria monocytogenes, Staphylococcus aureus, Bacillus subtilis, Botrytis cinerea, Escherichia coli, Clostridium perfringens and more (Leong et al., 2017). The antimicrobial capability of microbiological surfactants is due to their adherence capability to cell interfaces, causing the structural integrity of cell membranes to deteriorate and contributing to the subsequent breakdown of the feeding chain (Overney et al., 2017). The degradation of membrane-associated phospholipids causes an increase in cellular sponginess. Because of the disruption in cellular membranes caused by biosurfactant contact, the fat concentration in the membranes varies (Zhang et al., 2020). The biosurfactant engages effectively with the fatty acids, resulting in a blockade of the membrane-bound enzymes and releasing the internal cytoplasm constituents (Magalhães and Nitschke, 2013).
The surge in listeriosis incidence over the past decade can be primarily ascribed to shifts in dietary patterns, notably the widespread adoption of prepared foods as a regular component of daily menus (Zainith et al., 2020). Rhamnolipids were efficient at regulating Listeria monocytogenes from adhering to surfaces and have shown the capacity to reduce the adhesion of bacteria (Kumar and Chandra, 2020). These substances demonstrate a remarkable inhibition of Listeria monocytogenes adherence, with a reduction of approximately 82% compared to sodium dodecyl sulphate, leading to a significant 23% decrease in adherence.
Surfactin's antibacterial ability was assessed towards seven different Listeria monocytogenes, which have been identified as resistant to the bacteriocins in a dose of 0.125 mg/mL. Through meticulous investigation, surfactin exhibited a remarkable preservation of its anti-listerial capability across a broad pH spectrum ranging from 2 to 10. Moreover, even after exposure to harsh conditions, such as 15 min at 121 °C, it remained effective in combatting Listeria. Furthermore, it demonstrated an impressive resistance to enzyme-mediated proteolytic treatment, highlighting its robustness as an anti-listerial agent (Jia et al., 2020). The results of the present investigation suggest that surfactin is capable of being used as a unique technique for inhibiting or controlling Listeria monocytogenes during culinary processing (Sadh et al., 2018).
In juices, a study by Joe et al. (2012), a nanoemulsion derived from surfactin and sunflower oil exhibited strong antibacterial effects against pathogens like Salmonella typhi, Listeria monocytogenes, and Staphylococcus aureus. This nanoemulsion also demonstrated antifungal properties against Rhizopus nigricans, Penicillium sp., and Aspergillus niger. Additionally, it exhibited sporicidal effects against Bacillus circulans and Bacillus cereus. Practical assessments extended to mixed vegetables, apple juice, milk, and raw chicken indicated a significant reduction in bacterial and fungal growth, emphasizing its microbial control effectiveness.
Antibiofilm
Biofilms pose a significant challenge to the food sector, given their potential as enduring sources of contamination, culminating in both food spoilage and the potential spread of diseases (Giri et al., 2019). A proactive strategy to counter bacterial adherence and consequent biofilm development involves the preliminary application of biosurfactants on surfaces. These agents adeptly alter surface physicochemical attributes, thereby influencing bacterial interactions and impeding adhesion mechanisms (Kim et al., 2023). Pseudofactin II significantly deters bacterial biofilm adhesion, with 36% to 90% of inhibition rates. Targeting species like E. coli, Proteus mirabilis, Staphylococcus epidermidis, Enterococcus faecalis, and Enterococcus hirae, this effect spans substrates such as silicone, polystyrene, and glass. Similarly, against C. albicans yeast biofilms, Pseudofactin II notably achieves 92% to 99% inhibition at 0.5 mg/mL (Janek et al., 2012). Quinn et al. (2013) demonstrated the efficacy of rhamnolipid in suppressing single-species biofilms of S. aureus, B. subtilis, and M. luteus. Lipopeptides akin to fengycin demonstrate the capacity to proficiently curtail biofilm formation in Gram-positive bacteria like S. aureus, achieving dispersion rates reaching 90%. Similarly, their efficacy extends to Gram-negative bacteria such as E. coli, eliciting dispersion rates of up to 97% (Rivardo et al., 2010). MEL-A showed significant antibiofilm efficacy against S. aureus, with a biofilm eradication concentration of 32 μg/mL. MELs also demonstrated antibiofilm activity against Methicillin-resistant S. aureus (MRSA), a drug-resistant bacterium recognized for its biofilm-forming ability, which is especially significant due to the high morbidity and mortality linked with MRSA infections (Shu et al., 2020). Derived from Candida sphaerica, Lunasan exhibits notable efficacy in deterring bacterial adhesion. Notably, it curtails adhesion rates among various bacteria, encompassing P. aeruginosa, Streptococcus sanguinis, and S. agalactiae, with inhibition reaching levels as high as 92% (Luna et al., 2011). In a parallel vein, Candida lipolytica's rufisan showcases the capacity to impede biofilm formation by bacteria, including S. agalactiae, S. aureus, and S. mutans. This inhibitory prowess becomes apparent at concentrations equal to or exceeding 0.75 μg/mL (Rufino et al., 2011). Pseudomonas aeruginosa PA1 and B. subtilis ATCC 21332 exhibit notable antiadhesive characteristics, inhibiting biofilm formation on various surfaces, particularly with P. fluorescens ATCC 13525 and L. monocytogenes ATCC 19112/7644 (Araujo et al., 2016).
Antioxidant
Antioxidants are essential to extend food shelf life by preventing lipid oxidation, which leads to rancidity and undesirable changes in food products (Durval et al., 2021a). Biosurfactants offer a promising approach as antioxidants, potentially increasing the longevity of food items. Biosurfactants manifest substantial potential as antioxidants, garnering recognition for their multifaceted interfacial and biochemical characteristics. This recognition further underscores their prominence within this particular context (Singh et al., 2019). Recent investigations into the antioxidative prowess of biosurfactants have unveiled the potential for these compounds to serve as substitutes, and potentially even comprehensive replacements, for synthetic antioxidants (Silva et al., 2020).
The antioxidative properties of bioactive surface-active agents from Lactobacillus casei MRTL3 strain have been empirically demonstrated. These findings highlight the biosurfactant's potential for application in food processing (Sharma and Singh Saharan, 2014). Assessment of the antioxidant potential of rhamnolipids and surfactin revealed their capacity to effectively counteract free radicals and curtail peroxidative reactions within lipids, attributed to the presence of unsaturated fatty acids. Notably, Bacillus amyloliquefaciens NS6 exhibited excellent intrinsic surface activity, contributing to its exceptional antioxidative characteristics (Ciurko et al., 2022). Biosurfactants from B. subtilis VSG4 and Bacillus licheniformis VS16 were evaluated for their potential to counteract oxidants. Results showed that these biosurfactants exhibited a range of 69.1% to 73.5% scavenging capacity for free radicals in the case of B. subtilis VSG4, and a range of 63.3% to 69.8% for Bacillus licheniformis VS16 biosurfactants (Giri et al., 2019). The research conducted by (Gargouri et al., 2017)., discloses that the biosurfactant originating from the Stenotrophomonas B-2 strain showcases notable antioxidant properties and demonstrates the potential for various industrial applications. Furthermore, the researchers emphasized the aptness of this biosurfactant for utilization across the realms of food, cosmetics, and detergent industries. The antioxidative attribute plays a pivotal role in safeguarding product quality, flavor, color, and aroma while simultaneously prolonging the product's shelf life. Furthermore, incorporating antioxidants in consumables promises to mitigate the risk of cardiovascular and degenerative disorders (Durval et al., 2021b).
Future prospective and conclusion
Biosurfactants exhibit numerous characteristics that hold potential across various domains within the food industry. Nonetheless, the synthesis of biosurfactants remains constrained by intricacies embedded within the fine-tuning of the production process, the concomitant outlay of purification procedures, the subdued yield quantum, and the conformational disparities characterizing the end product. These manifold aspects collectively cast a shadow upon the cost-efficiency and market competitiveness vis-à-vis their chemical surfactant counterparts. As a corollary, there exists an exigency to orchestrate concerted endeavors toward unearthing steadfast and economically viable methodologies conducive to the large-scale procurement of biosurfactants. In the context of cost-effective production and applications within the food industry, the emergence of biosurfactant is a promising indicator of future developments. In the foreseeable years, these biosurfactants are poised to become essential components in food processing, serving as both integral constituents in formulations and additives.
Acknowledgements
The authors would like to thank the financial support from the fundamental research funds for SRMIST, Chennai, India.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abbasi H, Karimi S, Gharibzahedi SMT. Rhamnolipid as a unique emulsifier to stabilize sesame oil-in-water beverage emulsions formed by ultrasound-induced cavitation: optimizing the formulation and physical properties. Journal of Food Processing and Preservation. 2020;44:e14810. [Google Scholar]
- Abderrahmani A, Tapi A, Nateche F, Chollet M, Leclère V, Wathelet B, Hacene H, Jacques P. Bioinformatics and molecular approaches to detect NRPS genes involved in the biosynthesis of kurstakin from Bacillus thuringiensis. Applied Microbiology and Biotechnology. 2011;92:571–581. doi: 10.1007/s00253-011-3453-6. [DOI] [PubMed] [Google Scholar]
- Adesra A, Srivastava VK, Varjani S. Valorization of dairy wastes: integrative approaches for value added products. Indian Journal of Microbiology. 2021;61:270–278. doi: 10.1007/s12088-021-00943-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adetunji AI, Olaniran AO. Production and potential biotechnological applications of microbial surfactants: An overview. Saudi Journal of Biological Sciences. 2021;28:669–679. doi: 10.1016/j.sjbs.2020.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Asady AK, Majeed GH,,Al-Waely WA. Study of rhamnolipids cytotoxicity, inhibitory effect on some microorganisms and applying in food products. International Journal of Bio-Technology and Research. 6: 1-8 (2016)
- Alcantara VA, Pajares IG, Simbahan JF, Rubio MD. Substrate dependent production and isolation of an extracellular biosurfactant from Saccharomyces cerevisiae 2031. Philippine Journal of Science. 2012;141:13–24. [Google Scholar]
- Alizadeh-Sani M, Hamishehkar H, Khezerlou A, Azizi-Lalabadi M, Azadi Y, Nattagh-Eshtivani E, Fasihi M, Ghavami A, Aynehchi A, Ehsani A. Bioemulsifiers derived from microorganisms: Applications in the drug and food industry. Advanced Pharmaceutical Bulletin. 2018;8:191. doi: 10.15171/apb.2018.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amani H, Kariminezhad H. Study on emulsification of crude oil in water using emulsan biosurfactant for pipeline transportation. Petroleum Science and Technology. 2016;34:216–222. [Google Scholar]
- Anal AK, Shrestha S, Sadiq MB. Biopolymeric-based emulsions and their effects during processing, digestibility and bioaccessibility of bioactive compounds in food systems. Food Hydrocolloids. 2019;87:691–702. [Google Scholar]
- Anjum F, Gautam G, Edgard G, Negi S. Biosurfactant production through Bacillus sp. MTCC 5877 and its multifarious applications in food industry. Bioresource Technology. 213: 262-269 (2016) [DOI] [PubMed]
- Anselmi M, Eliseo T, Zanetti-Polzi L, Fullone MR, Fogliano V, Di Nola A, Paci M, Grgurina I. Structure of the lipodepsipeptide syringomycin E in phospholipids and sodium dodecylsulphate micelle studied by circular dichroism, NMR spectroscopy and molecular dynamics. Biochimica et Biophysica Acta. 1808: 2102-2110 (2011) [DOI] [PubMed]
- Anti-adhesion/antibiofilm and antimicrobial effects Araujo LV de, Guimarães CR, Marquita RL da S, Santiago VMJ, de Souza MP, Nitschke M and Freire DMG. Rhamnolipid and surfactin. Food Control. 2016;63:171–178. [Google Scholar]
- Aretz W, Meiwes J, Seibert G, Vobis G, Wink J. Friulimicins: novel lipopeptide antibiotics with peptidoglycan synthesis inhibiting activity from Actinoplanes friuliensis sp. nov. I. Taxonomic studies of the producing microorganism and fermentation. The Journal of Antibiotics. 53: 807-815 (2000) [DOI] [PubMed]
- Bagheri H, Mohebbi A, Amani FS, Naderi M. Application of low molecular weight and high molecular weight biosurfactant in medicine/biomedical/pharmaceutical industries. In: Green Sustainable Process for Chemical and Environmental Engineering and Science. pp. 1-60 (2022)
- Barale SS, Ghane SG, Sonawane KD. Purification and characterization of antibacterial surfactin isoforms produced by Bacillus velezensis SK. Amb Express. 2022;12:1–20. doi: 10.1186/s13568-022-01348-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkay T, Navon-Venezia S, Ron EZ, Rosenberg E. Enhancement of solubilization and biodegradation of polyaromatic hydrocarbons by the bioemulsifier alasan. Applied and Environmental Microbiology. 1999;65:2697–2702. doi: 10.1128/aem.65.6.2697-2702.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry CL, Brassinga AKC, Donald LJ, Fernando WGD, Loewen PC, de Kievit TR. Chemical and biological characterization of sclerosin, an antifungal lipopeptide. Canadian Journal of Microbiology. 2012;58:1027–1034. doi: 10.1139/w2012-079. [DOI] [PubMed] [Google Scholar]
- Bhardwaj G, Cameotra SS, Chopra HK. Isolation and purification of a new enamide biosurfactant from Fusarium proliferatum using rice-bran. RSC Advances. 2015;5:54783–54792. [Google Scholar]
- Bjerk TR, Severino P, Jain S, Marques C, Silva AM, Pashirova T, Souto EB. Biosurfactants: properties and applications in drug delivery, biotechnology and ecotoxicology. Bioengineering. 2021;8:115. doi: 10.3390/bioengineering8080115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodour AA, Guerrero-Barajas C, Jiorle B V, Malcomson ME, Paull AK, Somogyi A, Trinh LN, Bates RB, Maier RM. Structure and characterization of flavolipids, a novel class of biosurfactants produced by Flavobacterium sp. strain MTN11. Applied and Environmental Microbiology. 70: 114-120 (2004) [DOI] [PMC free article] [PubMed]
- Cagri-Mehmetoglu A, Kusakli S, van de Venter M. Production of polysaccharide and surfactin by Bacillus subtilis ATCC 6633 using rehydrated whey powder as the fermentation medium. Journal of Dairy Science. 2012;95:3643–3649. doi: 10.3168/jds.2012-5385. [DOI] [PubMed] [Google Scholar]
- Calvo H, Mendiara I, Arias E, Blanco D, Venturini ME. The role of iturin A from B. Amyloliquefaciens BUZ-14 in the inhibition of the most common postharvest fruit rots. Food Microbiology. 82: 62-69 (2019) [DOI] [PubMed]
- Campos JM, Stamford TLM, Rufino RD, Luna JM, Stamford TCM, Sarubbo LA. Formulation of mayonnaise with the addition of a bioemulsifier isolated from Candida utilis. Toxicology Reports. 2015;2:1164–1170. doi: 10.1016/j.toxrep.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos JM, Stamford TLM, Sarubbo LA. Production of a Bioemulsifier with Potential Application in the Food Industry. Applied Biochemistry and Biotechnology. 2014;172:3234–3252. doi: 10.1007/s12010-014-0761-1. [DOI] [PubMed] [Google Scholar]
- Caporgno MP, Haberkorn I, Böcker L, Mathys A. Cultivation of Chlorella protothecoides under different growth modes and its utilisation in oil/water emulsions. Bioresource Technology. 2019;288:121476. doi: 10.1016/j.biortech.2019.121476. [DOI] [PubMed] [Google Scholar]
- Carvalho D, Menezes R, Chitolina GZ, Kunert-Filho HC, Wilsmann DE, Borges KA, Furian TQ, Salle CTP, Moraes HL de S, do Nascimento VP. Antibiofilm activity of the biosurfactant and organic acids against foodborne pathogens at different temperatures, times of contact, and concentrations. Brazilian Journal of Microbiology. 53: 1051-1064 (2022) [DOI] [PMC free article] [PubMed]
- Chakraborty M, Mahmud NU, Gupta DR, Tareq FS, Shin HJ, Islam T. Inhibitory effects of linear lipopeptides from a marine Bacillus subtilis on the wheat blast fungus Magnaporthe oryzae Triticum. Frontiers in Microbiology. 11: 655(2020) [DOI] [PMC free article] [PubMed]
- Chaves FS, Brumano LP, Franco Marcelino PR, da Silva SS, Sette LD, Felipe M. Biosurfactant production by Antarctic-derived yeasts in sugarcane straw hemicellulosic hydrolysate. Biomass Conversion and Biorefinery. 13: 1-11 (2021)
- Cheng Y-H, Zhang N, Han J-C, Chang C-W, Hsiao FS-H, Yu Y-H. Optimization of surfactin production from Bacillus subtilis in fermentation and its effects on Clostridium perfringens-induced necrotic enteritis and growth performance in broilers. Journal of Animal Physiology and Animal Nutrition. 102: 1232-1244 (2018) [DOI] [PubMed]
- Chittepu OR. Isolation and characterization of biosurfactant producing bacteria from groundnut oil cake dumping site for the control of foodborne pathogens. Grain & Oil Science and Technology. 2019;2:15–20. [Google Scholar]
- Ciurko D, Czyżnikowska Ż, Kancelista A, Łaba W, Janek T. Sustainable production of biosurfactant from agro-industrial oil wastes by Bacillus subtilis and its potential application as antioxidant and ACE inhibitor. International Journal of Molecular Sciences. 2022;23:10824. doi: 10.3390/ijms231810824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compaoré CS, Nielsen DS, Sawadogo‐Lingani H, Berner TS, Nielsen KF, Adimpong DB, Diawara B, Ouédraogo GA, Jakobsen M, Thorsen L. Bacillus amyloliquefaciens ssp. plantarum strains as potential protective starter cultures for the production of Bikalga, an alkaline fermented food. Journal of Applied Microbiology. 115: 133-146 (2013) [DOI] [PubMed]
- Costa SG, Lépine F, Milot S, Déziel E, Nitschke M, Contiero J. Cassava wastewater as a substrate for the simultaneous production of rhamnolipids and polyhydroxyalkanoates by Pseudomonas aeruginosa. Journal of Industrial Microbiology and Biotechnology. 2009;36:1063–1072. doi: 10.1007/s10295-009-0590-3. [DOI] [PubMed] [Google Scholar]
- de Andrade CJ, de Andrade LM, Bution ML, Dolder MAH, Barros FFC, Pastore GM. Optimizing alternative substrate for simultaneous production of surfactin and 2, 3-butanediol by Bacillus subtilis LB5a. Biocatalysis and Agricultural Biotechnology. 2016;6:209–218. [Google Scholar]
- DeGroff D. The Craft of the Cocktail: Everything You Need to Know to Be a Master Bartender, with 500 Recipes. Clarkson Potter (2010)
- Deshmukh C, Jagtap CB, Titus S, Kumar P. Isolation and characterization of fatty acid esters and phosphatidylethanolamine surfactants from a consortium of marine bacteria. 41: 398-404 (2012)
- Dmitrović S, Pajčin I, Vlajkov V, Grahovac M, Jokić A, Grahovac J. Dairy and wine industry effluents as alternative media for the production of Bacillus-based biocontrol agents. Bioengineering. 2022;9:663. doi: 10.3390/bioengineering9110663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durval IJB, Ribeiro BG, Aguiar JS, Rufino RD, Converti A, Sarubbo LA. Application of a biosurfactant produced by Bacillus cereus UCP 1615 from waste frying oil as an emulsifier in a cookie formulation. Fermentation. 2021;7:189. [Google Scholar]
- Durval IJB, da Silva IA, Sarubbo LA. Application of microbial biosurfactants in the food industry. In: Microbial Biosurfactants. 1–10 (2021b)
- Eeman M, Olofsson G, Sparr E, Nasir MN, Nylander T, Deleu M. Interaction of fengycin with stratum corneum mimicking model membranes: A calorimetry study. Colloids and Surfaces b: Biointerfaces. 2014;121:27–35. doi: 10.1016/j.colsurfb.2014.05.019. [DOI] [PubMed] [Google Scholar]
- Firdose A, Msarah MJ, Chong NHH, Aqma WS. Extraction and antimicrobial activity of rhamnolipid biosurfactant produced by Pseudomonas aeruginosa UKMP14T. Malaysian Journal of Microbiology. 17: (2021)
- Fookao AN, Mbawala A, Nganou ND, Nguimbou RM, Mouafo HT. Improvement of the texture and dough stability of milk bread using bioemulsifiers/biosurfactants produced by lactobacilli isolated from an indigenous fermented milk (pendidam) LWT. 2022;163:113609. [Google Scholar]
- Franzetti A, Gandolfi I, Bestetti G, Smyth TJP, Banat IM. Production and applications of trehalose lipid biosurfactants. European Journal of Lipid Science and Technology. 2010;112:617–627. [Google Scholar]
- From C, Hormazabal V, Granum PE. Food poisoning associated with pumilacidin-producing Bacillus pumilus in rice. International Journal of Food Microbiology. 2007;115:319–324. doi: 10.1016/j.ijfoodmicro.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Gargouri B, Contreras M del M, Ammar S, Segura-Carretero A, Bouaziz M. Biosurfactant production by the crude oil degrading Stenotrophomonas sp. B-2: chemical characterization, biological activities and environmental applications. Environmental Science and Pollution Research. 24: 3769-3779 (2017) [DOI] [PubMed]
- Gaur VK, Regar RK, Dhiman N, Gautam K, Srivastava JK, Patnaik S, Kamthan M, Manickam N. Biosynthesis and characterization of sophorolipid biosurfactant by Candida spp.: application as food emulsifier and antibacterial agent. Bioresource Technology. 285: 121314 (2019) [DOI] [PubMed]
- George S, Jayachandran K. Production and characterization of rhamnolipid biosurfactant from waste frying coconut oil using a novel Pseudomonas aeruginosa D. Journal of Applied Microbiology. 2013;114:373–383. doi: 10.1111/jam.12069. [DOI] [PubMed] [Google Scholar]
- De Giani A, Zampolli J, Di Gennaro P. Recent trends on biosurfactants with antimicrobial activity produced by bacteria associated with human health: different perspectives on their properties, challenges, and potential applications. Frontiers in Microbiology. 2021;12:655150. doi: 10.3389/fmicb.2021.655150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri SS, Ryu EC, Sukumaran V, Park SC. Antioxidant, antibacterial, and anti-adhesive activities of biosurfactants isolated from Bacillus strains. Microbial Pathogenesis. 2019;132:66–72. doi: 10.1016/j.micpath.2019.04.035. [DOI] [PubMed] [Google Scholar]
- Gomaa EZ. Antimicrobial activity of a biosurfactant produced by Bacillus licheniformis strain M104 grown on whey. Brazilian Archives of Biology and Technology. 2013;56:259–268. [Google Scholar]
- Gu Y, Zheng R, Sun C, Wu S. Isolation, identification and characterization of two kinds of deep-sea bacterial lipopeptides against foodborne pathogens. Frontiers in Microbiology. 13: (2022) [DOI] [PMC free article] [PubMed]
- Haba E, Bouhdid S, Torrego-Solana N, Marqués AM, Espuny MJ, García-Celma MJ, Manresa A. Rhamnolipids as emulsifying agents for essential oil formulations: Antimicrobial effect against Candida albicans and methicillin-resistant Staphylococcus aureus. International Journal of Pharmaceutics. 2014;476:134–141. doi: 10.1016/j.ijpharm.2014.09.039. [DOI] [PubMed] [Google Scholar]
- He H, Shen B, Korshalla J, Carter GT. Circulocins, new antibacterial lipopeptides from Bacillus circulans, J2154. Tetrahedron. 2001;57:1189–1195. [Google Scholar]
- He Z, Zeng W, Zhu X, Zhao H, Lu Y, Lu Z. Influence of surfactin on physical and oxidative stability of microemulsions with docosahexaenoic acid. Colloids and Surfaces b: Biointerfaces. 2017;151:232–239. doi: 10.1016/j.colsurfb.2016.12.026. [DOI] [PubMed] [Google Scholar]
- Hipólito A, Caretta T de O, Silveira VAI, Bersaneti GT, Mali S, Celligoi MAPC. Active biodegradable cassava starch films containing sophorolipids produced by Starmerella bombicola ATCC® 22214TM. Journal of Polymers and the Environment. 29: 3199-3209 (2021)
- Hipólito A, da Silva RAA, de Oliveira Caretta T, Silveira VAI, Amador IR, Panagio LA, Borsato D, Celligoi MAPC. Evaluation of the antifungal activity of sophorolipids from Starmerella bombicola against food spoilage fungi. Biocatalysis and Agricultural Biotechnology. 2020;29:101797. [Google Scholar]
- Hirata Y, Igarashi K, Ueda A, Quan GL. Enhanced sophorolipid production and effective conversion of waste frying oil using dual lipophilic substrates. Bioscience, Biotechnology, and Biochemistry. 2021;85:1763–1771. doi: 10.1093/bbb/zbab075. [DOI] [PubMed] [Google Scholar]
- Hoffmann M, Mück D, Grossmann L, Greiner L, Klausmann P, Henkel M, Lilge L, Weiss J, Hausmann R. Surfactin from Bacillus subtilis displays promising characteristics as O/W-emulsifier for food formulations. Colloids and Surfaces b: Biointerfaces. 2021;203:111749. doi: 10.1016/j.colsurfb.2021.111749. [DOI] [PubMed] [Google Scholar]
- Hu J, Luo J, Zhu Z, Chen B, Ye X, Zhu P, Zhang B. Multi-scale biosurfactant production by Bacillus subtilis using tuna fish waste as substrate. Catalysts. 2021;11:456. [Google Scholar]
- Hua B, Feng H, Han J, Qiao Z, Wang X, Zhang Q, Liu Z, Wu Z. Isolation and characterization of a new fusaricidin-type antibiotic produced by Paenibacillus bovis sp. nov BD3526. Current Microbiology. 77: 3990-3999 (2020) [DOI] [PubMed]
- Huang K, Zhang B, Shen Z-Y, Cai X, Liu Z-Q, Zheng Y-G. Enhanced amphotericin B production by genetically engineered Streptomyces nodosus. Microbiological Research. 2021;242:126623. doi: 10.1016/j.micres.2020.126623. [DOI] [PubMed] [Google Scholar]
- Janek T, Krasowska A, Czyżnikowska Ż, Łukaszewicz M. Trehalose lipid biosurfactant reduces adhesion of microbial pathogens to polystyrene and silicone surfaces: an experimental and computational approach. Frontiers in Microbiology. 2018;9:2441. doi: 10.3389/fmicb.2018.02441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janek T, Łukaszewicz M, Krasowska A. Antiadhesive activity of the biosurfactant pseudofactin II secreted by the Arctic bacterium Pseudomonas fluorescens BD5. BMC Microbiology. 2012;12:1–9. doi: 10.1186/1471-2180-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janek T, Łukaszewicz M, Rezanka T, Krasowska A. Isolation and characterization of two new lipopeptide biosurfactants produced by Pseudomonas fluorescens BD5 isolated from water from the Arctic Archipelago of Svalbard. Bioresource Technology. 2010;101:6118–6123. doi: 10.1016/j.biortech.2010.02.109. [DOI] [PubMed] [Google Scholar]
- Jayakumar A, Radoor S, Nair IC, Siengchin S, Parameswaranpillai J, Radhakrishnan EK. Polyvinyl alcohol-nanocomposite films incorporated with clay nanoparticles and lipopeptides as active food wraps against food spoilage microbes. Food Packaging and Shelf Life. 2021;30:100727. [Google Scholar]
- Jemil N, Ouerfelli M, Almajano MP, Elloumi-Mseddi J, Nasri M, Hmidet N. The conservative effects of lipopeptides from Bacillus methylotrophicus DCS1 on sunflower oil-in-water emulsion and raw beef patties quality. Food Chemistry. 2020;303:125364. doi: 10.1016/j.foodchem.2019.125364. [DOI] [PubMed] [Google Scholar]
- Jia Z, Gwynne L, Sedgwick AC, Müller M, Williams GT, Jenkins ATA, James TD, Schönherr H. Enhanced Colorimetric Differentiation between Staphylococcus aureus and Pseudomonas aeruginosa Using a Shape-Encoded Sensor Hydrogel. ACS Applied Bio Materials. 2020;3:4398–4407. doi: 10.1021/acsabm.0c00403. [DOI] [PubMed] [Google Scholar]
- Jiang J, Gao L, Bie X, Lu Z, Liu H, Zhang C, Lu F, Zhao H. Identification of novel surfactin derivatives from NRPS modification of Bacillus subtilis and its antifungal activity against Fusarium moniliforme. BMC Microbiology. 2016;16:31. doi: 10.1186/s12866-016-0645-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe MM, Bradeeba K, Parthasarathi R, Sivakumaar PK, Chauhan PS, Tipayno S, Benson A, Sa T. Development of surfactin based nanoemulsion formulation from selected cooking oils: Evaluation for antimicrobial activity against selected food associated microorganisms. Journal of the Taiwan Institute of Chemical Engineers. 2012;43:172–180. [Google Scholar]
- Johnson P, Trybala A, Starov V, Pinfield VJ. Effect of synthetic surfactants on the environment and the potential for substitution by biosurfactants. Advances in Colloid and Interface Science. 2021;288:102340. doi: 10.1016/j.cis.2020.102340. [DOI] [PubMed] [Google Scholar]
- Kawase T, Sumida S, Oida T. Hybrid biosurfactant: Syntheses of hybrid corynomycolic acid and its monolayer formation. Tenside, Surfactants, Detergents. 2015;52:219–229. [Google Scholar]
- Kaya K, Mahakhant A, Keovara L, Sano T, Kubo T, Takagi H. Spiroidesin, a Novel Lipopeptide from the Cyanobacterium Anabaena spiroides That Inhibits Cell Growth of the Cyanobacterium Microcystis aeruginosa. Journal of Natural Products. 2002;65:920–921. doi: 10.1021/np010660x. [DOI] [PubMed] [Google Scholar]
- Kiran GS, Priyadharsini S, Sajayan A, Priyadharsini GB, Poulose N, Selvin J. Production of lipopeptide biosurfactant by a marine Nesterenkonia sp. and its application in food industry. Frontiers in Microbiology. 8: 1138 (2017) [DOI] [PMC free article] [PubMed]
- Kim JY, Moon EC, Kim J-Y, Kim HJ, Heo K, Shim J-J, Lee J-L. Lactobacillus helveticus HY7801 ameliorates bacterial vaginosis by inhibiting biofilm formation and epithelial cell adhesion of Gardnerella vaginalis. Food Science and Biotechnology. 2023;32:507–515. doi: 10.1007/s10068-022-01208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourmentza K, Gromada X, Michael N, Degraeve C, Vanier G, Ravallec R, Coutte F, Karatzas KA, Jauregi P. Antimicrobial activity of lipopeptide biosurfactants against foodborne pathogen and food spoilage microorganisms and their cytotoxicity. Frontiers in Microbiology. 2021;11:561060. doi: 10.3389/fmicb.2020.561060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Chandra R. Ligninolytic enzymes and its mechanisms for degradation of lignocellulosic waste in environment. Heliyon. 6: 2 (2020) [DOI] [PMC free article] [PubMed]
- Lara VM, Vallejo M, Parada R, Henao Ossa JS, Gliemmo MF, Campos CA. Characterization of the emulsifying activity of biosurfactants produced by lactic acid bacteria isolated from the Argentinian Patagonia. Journal of Dispersion Science and Technology. 2022;43:902–909. [Google Scholar]
- Lee M-H, Lee J, Nam Y-D, Lee JS, Seo M-J, Yi S-H. Characterization of antimicrobial lipopeptides produced by Bacillus sp. LM7 isolated from chungkookjang, a Korean traditional fermented soybean food. International Journal of Food Microbiology. 221: 12-18 (2016a) [DOI] [PubMed]
- Lee JY, Shim JM, Yao Z, Liu X, Lee KW, Kim H-J, Ham K-S, Kim JH. Antimicrobial activity of Bacillus amyloliquefaciens EMD17 isolated from Cheonggukjang and potential use as a starter for fermented soy foods. Food Science and Biotechnology. 2016;25:525–532. doi: 10.1007/s10068-016-0073-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong D, NicAogáin K, Luque-Sastre L, McManamon O, Hunt K, Alvarez-Ordóñez A, Scollard J, Schmalenberger A, Fanning S, O’Byrne C, Jordan K. A 3-year multi-food study of the presence and persistence of Listeria monocytogenes in 54 small food businesses in Ireland. International Journal of Food Microbiology. 2017;249:18–26. doi: 10.1016/j.ijfoodmicro.2017.02.015. [DOI] [PubMed] [Google Scholar]
- Li R, Du W, Yang J, Liu Z, Yousef AE. Control of Listeria monocytogenes biofilm by paenibacterin, a natural antimicrobial lipopeptide. Food Control. 2018;84:529–535. [Google Scholar]
- Li W, Rokni-Zadeh H, De Vleeschouwer M, Ghequire MGK, Sinnaeve D, Xie G-L, Rozenski J, Madder A, Martins JC, De Mot R. The antimicrobial compound xantholysin defines a new group of Pseudomonas cyclic lipopeptides. PloS One. 2013;8:e62946. doi: 10.1371/journal.pone.0062946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F-Z, Zeng Y-J, Zong M-H, Yang J-G, Lou W-Y. Bioprospecting of a novel endophytic Bacillus velezensis FZ06 from leaves of Camellia assamica: Production of three groups of lipopeptides and the inhibition against food spoilage microorganisms. Journal of Biotechnology. 2020;323:42–53. doi: 10.1016/j.jbiotec.2020.07.021. [DOI] [PubMed] [Google Scholar]
- Lin F, Huang Z, Chen Y, Zhou L, Chen M, Sun J, Lu Z, Lu Y. Effect of combined Bacillomycin D and chitosan on growth of Rhizopus stolonifer and Botrytis cinerea and cherry tomato preservation. Journal of the Science of Food and Agriculture. 2021;101:229–239. doi: 10.1002/jsfa.10635. [DOI] [PubMed] [Google Scholar]
- Lin L-Z, Zheng Q-W, Wei T, Zhang Z-Q, Zhao C-F, Zhong H, Xu Q-Y, Lin J-F, Guo L-Q. Isolation and characterization of fengycins produced by Bacillus amyloliquefaciens JFL21 and its broad-spectrum antimicrobial potential against multidrug-resistant foodborne pathogens. Frontiers in Microbiology. 2020;11:579621. doi: 10.3389/fmicb.2020.579621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linlin Z, Chaomin S. Fengycins, Cyclic Lipopeptides from Marine Bacillus subtilis Strains, Kill the Plant-Pathogenic Fungus Magnaporthe grisea by Inducing Reactive Oxygen Species Production and Chromatin Condensation. Applied and Environmental Microbiology. 2018;84:e00445–e518. doi: 10.1128/AEM.00445-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Gu S, Shi Y, Chen Q. Alleviative effects of mannosylerythritol lipid-A on the deterioration of internal structure and quality in frozen dough and corresponding steamed bread. Food Chemistry. 2024;431:137122. doi: 10.1016/j.foodchem.2023.137122. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ning Y, Chen Z, Han P, Zhi T, Li S, Ma A, Jia Y. Transcriptomics reveals substance biosynthesis and transport on membranes of Listeria monocytogenes affected by antimicrobial lipopeptide brevilaterin B. Food Science and Human Wellness. 2023;12:1359–1368. [Google Scholar]
- Liu X, Shu Q, Chen Q, Pang X, Wu Y, Zhou W, Wu Y, Niu J, Zhang X. Antibacterial efficacy and mechanism of mannosylerythritol lipids-A on Listeria monocytogenes. Molecules. 2020;25:4857. doi: 10.3390/molecules25204857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhang L, Pang X, Wu Y, Wu Y, Shu Q, Chen Q, Zhang X. Synergistic antibacterial effect and mechanism of high hydrostatic pressure and mannosylerythritol Lipid-A on Listeria monocytogenes. Food Control. 2022;135:108797. [Google Scholar]
- Liu J, Zhu N, Yang J, Yang Y, Wang R, Liu L, Yuan H. Lipopeptide produced from Bacillus sp. W112 improves the hydrolysis of lignocellulose by specifically reducing non-productive binding of cellulases with and without CBMs. Biotechnology for Biofuels. 10: 1-12 (2017) [DOI] [PMC free article] [PubMed]
- Luna JM, Rufino RD, Sarubbo LA, Rodrigues LRM, Teixeira JAC, de Campos-Takaki GM. Evaluation antimicrobial and antiadhesive properties of the biosurfactant Lunasan produced by Candida sphaerica UCP 0995. Current Microbiology. 2011;62:1527–1534. doi: 10.1007/s00284-011-9889-1. [DOI] [PubMed] [Google Scholar]
- Luo Z, Yuan X, Zhong H, Zeng G, Liu Z, Ma X, Zhu Y. Optimizing rhamnolipid production by Pseudomonas aeruginosa ATCC 9027 grown on waste frying oil using response surface method and batch-fed fermentation. Journal of Central South University. 2013;20:1015–1021. [Google Scholar]
- Magalhães L, Nitschke M. Antimicrobial activity of rhamnolipids against Listeria monocytogenes and their synergistic interaction with nisin. Food Control. 2013;29:138–142. [Google Scholar]
- Malakar C, Patowary K, Deka S, Kalita MC. Synthesis, characterization, and evaluation of antibacterial efficacy of rhamnolipid-coated zinc oxide nanoparticles against Staphylococcus aureus. World Journal of Microbiology and Biotechnology. 2021;37:1–14. doi: 10.1007/s11274-021-03160-w. [DOI] [PubMed] [Google Scholar]
- Marcelino PRF, Peres GFD, Terán-Hilares R, Pagnocca FC, Rosa CA, Lacerda TM, Dos Santos JC, Da Silva SS. Biosurfactants production by yeasts using sugarcane bagasse hemicellulosic hydrolysate as new sustainable alternative for lignocellulosic biorefineries. Industrial Crops and Products. 2019;129:212–223. [Google Scholar]
- Mnif I, Besbes S, Ellouze R, Ellouze-Chaabouni S, Ghribi D. Improvement of bread quality and bread shelf-life by Bacillus subtilis biosurfactant addition. Food Science and Biotechnology. 2012;21:1105–1112. [Google Scholar]
- Mnif I, Ghribi D. Glycolipid biosurfactants: main properties and potential applications in agriculture and food industry. Journal of the Science of Food and Agriculture. 2016;96:4310–4320. doi: 10.1002/jsfa.7759. [DOI] [PubMed] [Google Scholar]
- Morita T, Ishibashi Y, Fukuoka T, Imura T, Sakai H, Abe M, Kitamoto D. Production of glycolipid biosurfactants, cellobiose lipids, by Cryptococcus humicola JCM 1461 and their interfacial properties. Bioscience, Biotechnology and Biochemistry. 2011;75:1597–1599. doi: 10.1271/bbb.110036. [DOI] [PubMed] [Google Scholar]
- Mouafo HT, Baomog AMB, Adjele JJB, Sokamte AT, Mbawala A, Ndjouenkeu R. Microbial Profile of Fresh Beef Sold in the Markets of Ngaoundéré, Cameroon, and Antiadhesive Activity of a Biosurfactant against Selected Bacterial Pathogens. Journal of Food Quality. 2020;2020:5989428. [Google Scholar]
- Mouafo HT, Mbawala A, Tanaji K, Somashekar D, Ndjouenkeu R. Improvement of the shelf life of raw ground goat meat by using biosurfactants produced by lactobacilli strains as biopreservatives. LWT. 2020;133:110071. [Google Scholar]
- Murugan G, Rengaswamy P. Isolation, screening and production of biosurfactant by Pseudomonas sp. isolated from mangrove forest soil using coconut oil cake as substrate. Journal of Basic and Applied Biology. 5: 251-257 (2011)
- Nalini S, Parthasarathi R, Inbakanadan D. Biosurfactant in food and agricultural application. Environmental Biotechnology. 2020;2:75–94. [Google Scholar]
- Naughton PJ, Marchant R, Naughton V, Banat IM. Microbial biosurfactants: current trends and applications in agricultural and biomedical industries. Journal of Applied Microbiology. 2019;127:12–28. doi: 10.1111/jam.14243. [DOI] [PubMed] [Google Scholar]
- Nerin C, Canellas E, Vera P, Garcia-Calvo E, Luque-Garcia JL, Cámara C, Ausejo R, Miguel J, Mendoza N. A common surfactant used in food packaging found to be toxic for reproduction in mammals. Food and Chemical Toxicology. 2018;113:115–124. doi: 10.1016/j.fct.2018.01.044. [DOI] [PubMed] [Google Scholar]
- Nitschke M, Silva SS e. Recent food applications of microbial surfactants. Critical Reviews in Food Science and Nutrition. 58: 631-638 (2018) [DOI] [PubMed]
- Nitschke M, Araújo L V, Costa SGVAO, Pires RC, Zeraik AE, Fernandes ACLB, Freire DMG, Contiero J. Surfactin reduces the adhesion of food‐borne pathogenic bacteria to solid surfaces. Letters in Applied Microbiology. 49: 241-247 (2009) [DOI] [PubMed]
- Nitschke M, Pastore GM. Production and properties of a surfactant obtained from Bacillus subtilis grown on cassava wastewater. Bioresource Technology. 2006;97:336–341. doi: 10.1016/j.biortech.2005.02.044. [DOI] [PubMed] [Google Scholar]
- Ohno O, Ikeda Y, Sawa R, Igarashi M, Kinoshita N, Suzuki Y, Miyake K, Umezawa K. Isolation of Heptadepsin, a Novel Bacterial Cyclic Depsipeptide that Inhibits Lipopolysaccharide Activity. Chemistry and Biology. 2004;11:1059–1070. doi: 10.1016/j.chembiol.2004.05.016. [DOI] [PubMed] [Google Scholar]
- de Oliveira JG, Garcia-Cruz CH. Properties of a biosurfactant produced by Bacillus pumilus using vinasse and waste frying oil as alternative carbon sources. Brazilian Archives of Biology and Technology. 2013;56:155–160. [Google Scholar]
- Overney A, Jacques-André-Coquin J, Ng P, Carpentier B, Guillier L, Firmesse O. Impact of environmental factors on the culturability and viability of Listeria monocytogenes under conditions encountered in food processing plants. International Journal of Food Microbiology. 2017;244:74–81. doi: 10.1016/j.ijfoodmicro.2016.12.012. [DOI] [PubMed] [Google Scholar]
- Pang L, Xia B, Liu X, Yi Y, Jiang L, Chen C, Li P, Zhang M, Deng X, Wang R. Improvement of antifungal activity of a culture filtrate of endophytic Bacillus amyloliquefaciens isolated from kiwifruit and its effect on postharvest quality of kiwifruit. Journal of Food Biochemistry. 2021;45:e13551. doi: 10.1111/jfbc.13551. [DOI] [PubMed] [Google Scholar]
- Panjiar N, Mattam AJ, Jose S, Gandham S, Velankar HR. Valorization of xylose-rich hydrolysate from rice straw, an agroresidue, through biosurfactant production by the soil bacterium Serratia nematodiphila. Science of the Total Environment. 2020;729:138933. doi: 10.1016/j.scitotenv.2020.138933. [DOI] [PubMed] [Google Scholar]
- Park H-R, Kim G-H, Na Y, Oh J-E, Cho M-S. Physicochemical and sensory properties of protein-fortified cookies according to the ratio of isolated soy protein to whey protein. Food Science and Biotechnology. 2021;30:653–661. doi: 10.1007/s10068-021-00909-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partovi M, Lotfabad TB, Roostaazad R, Bahmaei M, Tayyebi S. Management of soybean oil refinery wastes through recycling them for producing biosurfactant using Pseudomonas aeruginosa MR01. World Journal of Microbiology and Biotechnology. 2013;29:1039–1047. doi: 10.1007/s11274-013-1267-7. [DOI] [PubMed] [Google Scholar]
- Pathania AS, Jana AK. Utilization of waste frying oil for rhamnolipid production by indigenous Pseudomonas aeruginosa: Improvement through co-substrate optimization. Journal of Environmental Chemical Engineering. 2020;8:104304. [Google Scholar]
- Patil HI, Pratap AP. Production and quantitative analysis of trehalose lipid biosurfactants using high-performance liquid chromatography. Journal of Surfactants and Detergents. 2018;21:553–564. [Google Scholar]
- Patowary R, Patowary K, Kalita MC, Deka S. Utilization of paneer whey waste for cost-effective production of rhamnolipid biosurfactant. Applied Biochemistry and Biotechnology. 2016;180:383–399. doi: 10.1007/s12010-016-2105-9. [DOI] [PubMed] [Google Scholar]
- Patowary R, Patowary K, Kalita MC, Deka S, Lam SS, Sarma H. Green production of noncytotoxic rhamnolipids from jackfruit waste: process and prospects. Biomass Conversion and Biorefinery. 2022;12:4375–4388. [Google Scholar]
- Poonguzhali P, Rajan S, Parthasarathi R, Srinivasan R, Kannappan A. Optimization of biosurfactant production by Pseudomonas aeruginosa using rice water and its competence in controlling Fusarium wilt of Abelmoschus esculentus. South African Journal of Botany. 2022;151:144–157. [Google Scholar]
- Prathiviraj R, Rajeev R, Fernandes H, Rathna K, Lipton AN, Selvin J, Kiran GS. A gelatinized lipopeptide diet effectively modulates immune response, disease resistance and gut microbiome in Penaeus vannamei challenged with Vibrio parahaemolyticus. Fish and Shellfish Immunology. 2021;112:92–107. doi: 10.1016/j.fsi.2021.02.018. [DOI] [PubMed] [Google Scholar]
- Punrat T, Thaniyavarn J, Napathorn SC, Anuntagoolb J, Thaniyavarn S. Production of a sophorolipid biosurfactant by Wickerhamomyces anomalus MUE24 and its use for modification of rice flour properties. ScienceAsia. 46: 1 (2020)
- Qian S, Lu H, Meng P, Zhang C, Lv F, Bie X, Lu Z. Effect of inulin on efficient production and regulatory biosynthesis of bacillomycin D in Bacillus subtilis fmbJ. Bioresource Technology. 2015;179:260–267. doi: 10.1016/j.biortech.2014.11.086. [DOI] [PubMed] [Google Scholar]
- Qin W-Q, Fei D, Zhou L, Guo Y-J, An S, Gong O-H, Wu Y-Y, Liu J-F, Yang S-Z, Mu B-Z. A new surfactin-C 17 produced by Bacillus subtilis TD7 with a low critical micelle concentration and high biological activity. New Journal of Chemistry. 2023;47:7604–7612. [Google Scholar]
- Quinn GA, Maloy AP, Banat MM, Banat IM. A comparison of effects of broad-spectrum antibiotics and biosurfactants on established bacterial biofilms. Current Microbiology. 2013;67:614–623. doi: 10.1007/s00284-013-0412-8. [DOI] [PubMed] [Google Scholar]
- Radha P, Prabhu K, Jayakumar A, AbilashKarthik S, Ramani K. Biochemical and kinetic evaluation of lipase and biosurfactant assisted ex novo synthesis of microbial oil for biodiesel production by Yarrowia lipolytica utilizing chicken tallow. Process Biochemistry. 2020;95:17–29. [Google Scholar]
- Radha P, Suhazsini P, Prabhu K, Jayakumar A, Kandasamy R. Chicken tallow, a renewable source for the production of biosurfactant by Yarrowia lipolytica MTCC9520, and its application in silver nanoparticle synthesis. Journal of Surfactants and Detergents. 2020;23:119–135. [Google Scholar]
- Rahaman SM, Bhattarai A, Kumar D, Singh B, Saha B. Application of biosurfactants as emulsifiers in the processing of food products with diverse utilization in the baked goods. In: Applications of Next Generation Biosurfactants in the Food Sector. 203–237 (2023)
- Ravindran A, Kiran GS, Selvin J. Revealing the effect of lipopeptide on improving the probiotics characteristics: Flavor and texture enhancer in the formulated yogurt. Food Chemistry. 2022;375:131718. doi: 10.1016/j.foodchem.2021.131718. [DOI] [PubMed] [Google Scholar]
- Ravindran A, Selvin J, Seghal Kiran G. Effective utilization of wheat gluten by lipopeptide biosurfactant in the formulation of cookies. Journal of the Science of Food and Agriculture. 2023;103:4685–4691. doi: 10.1002/jsfa.12557. [DOI] [PubMed] [Google Scholar]
- Rene ER, Ge J, Kumar G, Singh RP, Varjani S. Resource recovery from wastewater, solid waste, and waste gas: engineering and management aspects. Environmental Science and Pollution Research. 2020;27:17435–17437. doi: 10.1007/s11356-020-08802-4. [DOI] [PubMed] [Google Scholar]
- Rivardo F, Martinotti MG, Turner RJ, Ceri H. The activity of silver against Escherichia coli biofilm is increased by a lipopeptide biosurfactant. Canadian Journal of Microbiology. 2010;56:272–278. doi: 10.1139/w10-007. [DOI] [PubMed] [Google Scholar]
- Rivera ÁD, Urbina MÁM, López y López VE. Advances on research in the use of agro-industrial waste in biosurfactant production. World Journal of Microbiology and Biotechnology. 35: 155 (2019) [DOI] [PubMed]
- Rodríguez-Fernández DE, Rodríguez-León JA, de Carvalho JC, Thomaz-Soccol V, Parada JL, Soccol CR. Recovery of phytase produced by solid-state fermentation on citrus peel. Brazilian Archives of Biology and Technology. 2010;53:1487–1496. [Google Scholar]
- Rufino RD, Luna JM, Sarubbo LA, Rodrigues LRM, Teixeira JAC, Campos-Takaki GM. Antimicrobial and anti-adhesive potential of a biosurfactant Rufisan produced by Candida lipolytica UCP 0988. Colloids and Surfaces b: Biointerfaces. 2011;84:1–5. doi: 10.1016/j.colsurfb.2010.10.045. [DOI] [PubMed] [Google Scholar]
- Rufino RD, Sarubbo LA, Campos-Takaki GM. Enhancement of stability of biosurfactant produced by Candida lipolytica using industrial residue as substrate. World Journal of Microbiology and Biotechnology. 2007;23:729–734. [Google Scholar]
- Sabaté DC, Audisio MC. Inhibitory activity of surfactin, produced by different Bacillus subtilis subsp. subtilis strains, against Listeria monocytogenes sensitive and bacteriocin-resistant strains. Microbiological Research. 168: 125-129 (2013) [DOI] [PubMed]
- Sadeghi H, Rashedi H, Mazaheri Assadi M, Seyedin Ardebili M. Potential application of bioemulsifier RAG-1 as an anti-staling agent in flat bread quality. Journal of Food Science and Technology. 2023;60:2619–2627. doi: 10.1007/s13197-023-05784-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadh PK, Duhan S, Duhan JS. Agro-industrial wastes and their utilization using solid state fermentation: a review. Bioresources and Bioprocessing. 2018;5:1. [Google Scholar]
- Sałek K, Euston SR. Sustainable microbial biosurfactants and bioemulsifiers for commercial exploitation. Process Biochemistry. 2019;85:143–155. [Google Scholar]
- Sari SK, Trikurniadewi N, Ibrahim SNMM, Khiftiyah AM, Abidin AZ, Nurhariyati T. Bioconversion of agricultural waste hydrolysate from lignocellulolytic mold into biosurfactant by Achromobacter sp. BP (1) 5. Biocatalysis and Agricultural Biotechnology. 24: 101534 (2020)
- Sato T, Ishiyama D, Honda R, Senda H, Konno H, Tokumasu S, Kanazawa S. Glomosporin, a novel antifungal cyclic depsipeptide from Glomospora sp. I. Production, isolation, physico-chemical properties and biological activities. The Journal of Antibiotics. 53: 597-602 (2000) [DOI] [PubMed]
- Schick J, Etschel P, Bailo R, Ott L, Bhatt A, Lepenies B, Kirschning C, Burkovski A, Lang R. Toll-like receptor 2 and Mincle cooperatively sense corynebacterial cell wall glycolipids. Infection and Immunity. 85: 10-1128 (2017) [DOI] [PMC free article] [PubMed]
- Selvam K, Senthilkumar B, Selvankumar T. Optimization of low‐cost biosurfactant produced by Bacillus subtilis SASCBT01 and their environmental remediation potential. Letters in Applied Microbiology. 72: 74-81 (2021) [DOI] [PubMed]
- Sharma D, Ansari MJ, Gupta S, Al Ghamdi A, Pruthi P, Pruthi V. Structural characterization and antimicrobial activity of a biosurfactant obtained from Bacillus pumilus DSVP18 grown on potato peels. Jundishapur Journal of Microbiology. 8(9): e21257 (2015) [DOI] [PMC free article] [PubMed]
- Sharma S, Datta P, Kumar B, Tiwari P, Pandey LM. Production of novel rhamnolipids via biodegradation of waste cooking oil using Pseudomonas aeruginosa MTCC7815. Biodegradation. 2019;30:301–312. doi: 10.1007/s10532-019-09874-x. [DOI] [PubMed] [Google Scholar]
- Sharma V, Garg M, Devismita T, Thakur P, Henkel M, Kumar G. Preservation of microbial spoilage of food by biosurfactantbased coating. Asian Journal of Pharmaceutical and Clinical Research. 11: 98 (2018)
- Sharma R. Surfactants: basics and versatility in food industries. PharmaTutor. 2014;2:17–29. [Google Scholar]
- Sharma D, Singh Saharan B. Simultaneous production of biosurfactants and bacteriocins by probiotic Lactobacillus casei MRTL3. International Journal of Microbiology. (2014b) [DOI] [PMC free article] [PubMed]
- Sharma D, Vashist H. Hydrodistillation and Comparative Report of Percentage Yield on Leaves and Fruit Peels from Different Citrus Plants of Rutaceae Family. Journal of Plant Sciences. 2015;10:75–78. [Google Scholar]
- Shi J, Zhu X, Lu Y, Zhao H, Lu F, Lu Z. Improving iturin A production of Bacillus amyloliquefaciens by genome shuffling and its inhibition against Saccharomyces cerevisiae in orange juice. Frontiers in Microbiology. 2018;9:2683. doi: 10.3389/fmicb.2018.02683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishido TK, Jokela J, Kolehmainen C-T, Fewer DP, Wahlsten M, Wang H, Rouhiainen L, Rizzi E, De Bellis G, Permi P. Antifungal activity improved by coproduction of cyclodextrins and anabaenolysins in Cyanobacteria. Proceedings of the National Academy of Sciences. 2015;112:13669–13674. doi: 10.1073/pnas.1510432112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoeb E, Akhlaq F, Badar U, Akhter J, Imtiaz S. Classification and industrial applications of biosurfactants. Academic Research International. 2013;4:243. [Google Scholar]
- Shu Q, Wei T, Liu X, Liu S, Chen Q. The dough-strengthening and spore-sterilizing effects of mannosylerythritol lipid-A in frozen dough and its application in bread making. Food Chemistry. 2022;369:131011. doi: 10.1016/j.foodchem.2021.131011. [DOI] [PubMed] [Google Scholar]
- Shu Q, Wei T, Lu H, Niu Y, Chen Q. Mannosylerythritol lipids: dual inhibitory modes against Staphylococcus aureus through membrane-mediated apoptosis and biofilm disruption. Applied Microbiology and Biotechnology. 2020;104:5053–5064. doi: 10.1007/s00253-020-10561-8. [DOI] [PubMed] [Google Scholar]
- Silva IA, Veras BO, Ribeiro BG, Aguiar JS, Guerra JMC, Luna JM, Sarubbo LA. Production of cupcake-like dessert containing microbial biosurfactant as an emulsifier. PeerJ. 2020;8:e9064. doi: 10.7717/peerj.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira VAI, Marim BM, Hipólito A, Gonçalves MC, Mali S, Kobayashi RKT, Celligoi MAPC. Characterization and antimicrobial properties of bioactive packaging films based on polylactic acid-sophorolipid for the control of foodborne pathogens. Food Packaging and Shelf Life. 2020;26:100591. [Google Scholar]
- Silveira VAI, Nishio EK, Freitas CAUQ, Amador IR, Kobayashi RKT, Caretta T, Macedo F, Celligoi MAPC. Production and antimicrobial activity of sophorolipid against Clostridium perfringens and Campylobacter jejuni and their additive interaction with lactic acid. Biocatalysis and Agricultural Biotechnology. 2019;21:101287. [Google Scholar]
- Singh SS, Akhtar MdN, Sharma D, Mandal SM, Korpole S. Characterization of Iturin V, a novel antimicrobial lipopeptide from a potential probiotic strain Lactobacillus sp. M31. Probiotics and Antimicrobial Proteins. 13: 1766-1779 (2021) [DOI] [PubMed]
- Singh P, Patil Y, Rale V. Biosurfactant production: emerging trends and promising strategies. Journal of Applied Microbiology. 2019;126:2–13. doi: 10.1111/jam.14057. [DOI] [PubMed] [Google Scholar]
- Solaiman DKY, Ashby RD, Zerkowski JA, Krishnama A, Vasanthan N. Control-release of antimicrobial sophorolipid employing different biopolymer matrices. Biocatalysis and Agricultural Biotechnology. 2015;4:342–348. [Google Scholar]
- Soleiman Meiguni F, Imanparast S, Salimi F, Nemati F. The Probiotic Biosurfactant From Levilactobacillus brevis Strain F20 Isolated from a Diary Product with Potential Food Applications. Food Biotechnology. 2022;36:394–411. [Google Scholar]
- Sriram MI, Kalishwaralal K, Deepak V, Gracerosepat R, Srisakthi K, Gurunathan S. Biofilm inhibition and antimicrobial action of lipopeptide biosurfactant produced by heavy metal tolerant strain Bacillus cereus NK1. Colloids and Surfaces b: Biointerfaces. 2011;85:174–181. doi: 10.1016/j.colsurfb.2011.02.026. [DOI] [PubMed] [Google Scholar]
- Teixeira MR, Nogueira R, Nunes LM. Quantitative assessment of the valorisation of used cooking oils in 23 countries. Waste Management. 2018;78:611–620. doi: 10.1016/j.wasman.2018.06.039. [DOI] [PubMed] [Google Scholar]
- Thasana N, Prapagdee B, Rangkadilok N, Sallabhan R, Aye SL, Ruchirawat S, Loprasert S. Bacillus subtilis SSE4 produces subtulene A, a new lipopeptide antibiotic possessing an unusual C15 unsaturated β-amino acid. FEBS Letters. 2010;584:3209–3214. doi: 10.1016/j.febslet.2010.06.005. [DOI] [PubMed] [Google Scholar]
- UV-MALDI-TOF MS analysis of its bioactive compounds. Torres MJ, Petroselli G, Daz M, Erra-Balsells R and Audisio MC. Bacillus subtilis subsp. subtilis CBMDC3f with antimicrobial activity against Gram-positive foodborne pathogenic bacteria. World Journal of Microbiology and Biotechnology. 2015;31:929–940. doi: 10.1007/s11274-015-1847-9. [DOI] [PubMed] [Google Scholar]
- Usmani Z, Sharma M, Gaffey J, Sharma M, Dewhurst RJ, Moreau B, Newbold J, Clark W, Thakur VK, Gupta VK. Valorization of dairy waste and by-products through microbial bioprocesses. Bioresource Technology. 2022;346:126444. doi: 10.1016/j.biortech.2021.126444. [DOI] [PubMed] [Google Scholar]
- Vahidinasab M, Lilge L, Reinfurt A, Pfannstiel J, Henkel M, Morabbi Heravi K, Hausmann R. Construction and description of a constitutive plipastatin mono-producing Bacillus subtilis. Microbial Cell Factories. 2020;19:1–12. doi: 10.1186/s12934-020-01468-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela-Ávila L, Miliar Y, Moya-Ramírez I, Chyhyrynets O, García-Román M, Altmajer-Vaz D. Effect of emulsification and hydrolysis pretreatments of waste frying oil on surfactin production. Journal of Chemical Technology & Biotechnology. 2020;95:223–231. [Google Scholar]
- Vallet-Gely I, Novikov A, Augusto L, Liehl P, Bolbach G, Péchy-Tarr M, Cosson P, Keel C, Caroff M, Lemaitre B. Association of hemolytic activity of Pseudomonas entomophila, a versatile soil bacterium, with cyclic lipopeptide production. Applied and Environmental Microbiology. 2010;76:910–921. doi: 10.1128/AEM.02112-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadharajan S, Subramaniyan V. Production of biosurfactant by Pseudomonas aeruginosa PB3A using agroindustrial wastes as a carbon source. Malaysian Journal of Microbiology. 2014;10:57–62. [Google Scholar]
- Varjani S, Lee D-J, Zhang Q. Valorizing agricultural biomass for sustainable development: biological engineering aspects. Bioengineered. 2020;11:522–523. doi: 10.1080/21655979.2020.1759185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataraman S, Rajendran DS, Kumar PS, Vo D-VN, Vaidyanathan VK. Extraction, purification and applications of biosurfactants based on microbial-derived glycolipids and lipopeptides: a review. Environmental Chemistry Letters. 1-22. (2022)
- Verónica CM, Julia TM, M. SA, Carina AM, Alejandra BM. Antibacterial activity of Bacillus lipopeptides vehiculized and delivered by biopolymeric films. Food and Bioprocess Technology. (2023)
- Vijayakumar S, Saravanan V. Biosurfactants-types, sources and applications. Research Journal of Microbiology. 2015;10:181–192. [Google Scholar]
- Villegas-Escobar V, Ceballos I, Mira JJ, Argel LE, Orduz Peralta S, Romero-Tabarez M. Fengycin C produced by Bacillus subtilis EA-CB0015. Journal of Natural Products. 2013;76:503–509. doi: 10.1021/np300574v. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liang J, Zhang C, Wang L, Gao W, Jiang J. Bacillus megaterium WL-3 Lipopeptides Collaborate Against Phytophthora infestans to Control Potato Late Blight and Promote Potato Plant Growth. Frontiers in Microbiology. 11: (2020) [DOI] [PMC free article] [PubMed]
- Wang C, Zhao L, Xia S, Zhang T, Cao R, Liang G, Li Y, Meng G, Wang W, Shi W. De novo design of α-helical lipopeptides targeting viral fusion proteins: a promising strategy for relatively broad-spectrum antiviral drug discovery. Journal of Medicinal Chemistry. 2018;61:8734–8745. doi: 10.1021/acs.jmedchem.8b00890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W-J, whey S-M, Ahn B-Y. Isolation and characterization of an antimicrobial substance from Bacillus subtilis BY08 antagonistic to Bacillus cereus and Listeria monocytogenes. Food Science and Biotechnology. 22: 433-440 (2013)
- Wu Y, Zhou L, Lu F, Bie X, Zhao H, Zhang C, Lu Z, Lu Y. Discovery of a Novel Antimicrobial Lipopeptide, Brevibacillin V, from Brevibacillus laterosporus fmb70 and Its Application on the Preservation of Skim Milk. Journal of Agricultural and Food Chemistry. 2019;67:12452–12460. doi: 10.1021/acs.jafc.9b04113. [DOI] [PubMed] [Google Scholar]
- Xiong ZR, Cobo M, Whittal RM, Snyder AB, Worobo RW. Purification and characterization of antifungal lipopeptide produced by Bacillus velezensis isolated from raw honey. Plos One. 2022;17:e0266470. doi: 10.1371/journal.pone.0266470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, En H, Chunhua Y, Liwen Z, E YA. Isolation and structural elucidation of Brevibacillin, an antimicrobial lipopeptide from Brevibacillus laterosporus that combats drug-resistant gram-positive bacteria. Applied and Environmental Microbiology. 82: 2763-2772 (2016) [DOI] [PMC free article] [PubMed]
- Xu L, Zhang B, Qin Y, Li F, Yang S, Lu P, Wang L, Fan J. Preparation and characterization of antifungal coating films composed of sodium alginate and cyclolipopeptides produced by Bacillus subtilis. International Journal of Biological Macromolecules. 2020;143:602–609. doi: 10.1016/j.ijbiomac.2019.12.051. [DOI] [PubMed] [Google Scholar]
- Yuan P-H, Zhou R-C, Chen X, Luo S, Wang F, Mao X-M, Li Y-Q. DepR1, a TetR family transcriptional regulator, positively regulates daptomycin production in an industrial producer, Streptomyces roseosporus SW0702. Applied and Environmental Microbiology. 2016;82:1898–1905. doi: 10.1128/AEM.03002-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zainith S, Chowdhary P, Mani S, Mishra S. Microbial ligninolytic enzymes and their role in bioremediation. In: Microorganisms for Sustainable Environment and Health. 179–203 (2020)
- Zezzi do Valle Gomes M, Nitschke M. Evaluation of rhamnolipid and surfactin to reduce the adhesion and remove biofilms of individual and mixed cultures of food pathogenic bacteria. Food Control. 25: 441-447 (2012)
- Zhang X, Ashby R, Solaiman DKY, Uknalis J, Fan X. Inactivation of Salmonella spp. and Listeria spp. by palmitic, stearic, and oleic acid sophorolipids and thiamine dilauryl sulfate. Frontiers in Microbiology. 7: 2076 (2016) [DOI] [PMC free article] [PubMed]
- Zhang D, Beck BH, Lange M, Zhao H, Thongda W, Ye Z, Li C, Peatman E. Impact of oral and waterborne administration of rhamnolipids on the susceptibility of channel catfish (Ictalurus punctatus) to Flavobacterium columnare infection. Fish & Shellfish Immunology. 2017;60:44–49. doi: 10.1016/j.fsi.2016.10.053. [DOI] [PubMed] [Google Scholar]
- Zhang J, Bilal M, Liu S, Zhang J, Lu H, Luo H, Luo C, Shi H, Iqbal HMN, Zhao Y. Isolation, Identification and Antimicrobial Evaluation of Bactericides Secreting Bacillus subtilis Natto as a Biocontrol Agent. Processes. 2020;8:259. [Google Scholar]
- Zhang N, Pu Y, Sun L, Wang Y, Deng Q, Xu D, Liu Y, Hussain M, Gooneratne R. Modeling the effects of different conditions on the inhibitory activity of antimicrobial lipopeptide (AMPNT-6) against Staphylococcus aureus growth and enterotoxin production in shrimp meat. Aquaculture International. 2017;25:57–70. [Google Scholar]
- Zhang K, Tao W, Lin J, Wang W, Li S. Production of the biosurfactant serrawettin W1 by Serratia marcescens S-1 improves hydrocarbon degradation. Bioprocess and Biosystems Engineering. 2021;44:2541–2552. doi: 10.1007/s00449-021-02625-4. [DOI] [PubMed] [Google Scholar]
- Zhang R, Wu Q, Xu Y, Qian MC. Isolation, Identification, and Quantification of Lichenysin, a Novel Nonvolatile Compound in Chinese Distilled Spirits. Journal of Food Science. 2014;79:C1907–C1915. doi: 10.1111/1750-3841.12650. [DOI] [PubMed] [Google Scholar]
- Zhao X, Wang K, Ai C, Yan L, Jiang C, Shi J. Improvement of antifungal and antibacterial activities of food packages using silver nanoparticles synthesized by iturin A. Food Packaging and Shelf Life. 2021;28:100669. [Google Scholar]
- Zhou L, Song C, Muñoz CY, Kuipers OP. Bacillus cabrialesii BH5 protects tomato plants against botrytis cinerea by production of specific antifungal compounds. Frontiers in Microbiology. 12: 707609 (2021) [DOI] [PMC free article] [PubMed]
- Zhu J, Huang T, Chen X, Tian D, Wang L, Gao R. Preparation and characterization of vanillin-conjugated chitosan-stabilized emulsions via a Schiff-base reaction. Food Science and Biotechnology. 32: 1-11 (2023) [DOI] [PMC free article] [PubMed]
- Zhu Z, Zhang B, Cai Q, Ling J, Lee K, Chen B. Fish waste based lipopeptide production and the potential spplication as a bio-dispersant for oil spill control. Frontiers in Bioengineering and Biotechnology. 8: 734 (2020) [DOI] [PMC free article] [PubMed]
- Zouari R, Besbes S, Ellouze-Chaabouni S, Ghribi-Aydi D. Cookies from composite wheat-sesame peels flours: Dough quality and effect of Bacillus subtilis SPB1 biosurfactant addition. Food Chemistry. 2016;194:758–769. doi: 10.1016/j.foodchem.2015.08.064. [DOI] [PubMed] [Google Scholar]
- Zúñiga RN, Aguilera JM. Aerated food gels: fabrication and potential applications. Trends in Food Science & Technology. 2008;19:176–187. [Google Scholar]