Abstract
Metabolic diseases have overtaken infectious diseases as the most serious public health issue and economic burden in most countries. Moreover, metabolic diseases increase the risk of having infectious diseases. The treatment of metabolic disease may require a long-term strategy of taking multiple medications, which can be costly and have side effects. Attempts to expand the therapeutic use of vaccination to prevent or treat metabolic diseases have attracted significant interest. A growing body of evidence indicates that Bacillus Calmette-Guérin (BCG) offers protection against non-infectious diseases. The non-specific effects of BCG occur likely due to the induction of trained immunity. In this regard, understanding how BCG influences the development of chronic metabolic health including liver diseases would be important. This review focuses on research on BCG, the constellation of disorders associated with metabolic health issues including liver diseases and diabetes as well as how BCG affects the gut microbiome, immunity, and metabolism.
Keywords: Bacillus Calmette-Guérin (BCG), Liver disease, Vaccination, Gut microbiota, Metabolic diseases, Trained immunity, Diabetes
1. Introduction
Metabolic syndrome is characterized by abdominal obesity, insulin and glucose resistance, hyperglycemia, and dyslipidemia; these health issues include dysfunctional hepatic metabolism, diabetes, and cardiovascular disorders.1,2 Metabolic syndrome affects people over age 60; however, the incidence varies by gender, age, and ethnicity.3 Early prevention is crucial to reduce the financial burden on the health care system and the medical expenditures associated with it.
Obesity and metabolic disorders are not only intertwined with each other but also closely linked with immunological responses via pathogen sensing systems, nutrition, and inflammation-related pathways. Therefore, metabolic control and immunological response are firmly linked, and the proper operation relies on the appropriate function of the other.4 This interaction may be considered a central homeostatic mechanism.
Bacillus Calmette-Guérin (BCG) vaccine was produced over 100 years ago to provide immunization against tuberculosis (TB); it was later found that it has off-target effects. Because of its non-specific effects, BCG was first used for bladder cancer treatment over 40 years ago.5 In recent years, it has been shown that BCG has the potential to manage and treat autoimmune and even neurological disorders, including type 1 diabetes mellitus (T1DM), Alzheimer's disease, multiple sclerosis, and Parkinson's disease.6
BCG works by inducing immunological responses that influence inflammatory indicators, implying that immunization may affect inflammation-associated metabolic phenotypes and disease processes.7, 8, 9 This review focuses on research on the effects of BCG on metabolism and its possible underlying mechanisms.
2. BCG, trained immunity, and epigenetics
The innate immune system recognizes the external stimulation promptly and mounts a defensive reaction against the foreign pathogen. The adaptive immune system creates a long-term memory of the infectious agent to protect the host from re-infection.10,11 Additionally, the innate immune system possesses adaptive features, a trait now known as trained immunity.12 The onset of heterologous vaccination effects, as well as infection-prevention effects, all rely on innate immunity memory, or trained immunity regulated by monocytes.12,13 However, trained immunity can also be maladaptive in the context of chronic inflammatory disorders. Thus, trained immunity can explain the epidemiological correlations between infections and the development of atherosclerotic cardiovascular disease.14,15
“Immunometabolism” is a fast-emerging subject that has biomedical applications because it explains the interactions between the immune system and the metabolic processes.16,17 These two important research areas are connected in many ways. On the one hand, immune cells in the liver and adipose tissue control metabolic processes, including insulin action and lipolysis.18, 19, 20, 21 On the other hand, the metabolism of protein, lipid, and carbohydrate can influence immune response.20
2.1. Epigenetic effects of BCG
When trained immunity occurs, monocytes undergo epigenetic rewiring. Trimethylated histone H3 lysine 4 (H3K4me3), an activating histone modification, is prevalent in the proximal regulatory areas of pro-inflammatory cytokine genes including tumor necrosis factor-alpha (TNF-α) and interleukin (IL)-6 in trained monocytes.22 Another sign of BCG-induced trained immunity is reduced trimethylated histone H3 lysine 9 (H3K9me3) at the promoters of TNF-α and IL-6.22 Moreover, trained immunity has been proposed to have anti-cancer effect. These anti-tumor benefits derive from the epigenetic rewiring of multipotent progenitors in the bone marrow, which overrides the immunosuppressive tumor microenvironment.23 In this context, it has been demonstrated that systemic treatment of BCG or beta-glucan rewires hematopoietic stem cells in the bone marrow through a type II interferon or IL-1 response, which confers trained protective immunity against TB.24
Recent studies have demonstrated that a Western diet intake also establishes a trained immune system. At molecular level, trained immunity affects metabolic pathways that are dictated by epigenetic processes involving H3K4me3 and acetylation of histone 3 at lysine 27 (H3K27ac) in monocytes and macrophages.25,26 A proof-of-concept study in patients with familial hypercholesterolemia reported that monocytes exhibit promoter enrichment of H3K4me3 and cytokine production capacity. In addition, those epigenetic effects lasted months post-statin treatment.27 Animal studies showed that atherosclerosis-prone low-density lipoprotein receptor-deficient (Ldlr−/−) mice fed a Western diet for four weeks had transcriptional and pro-inflammatory epigenetic modifications in their circulatory monocytes and myeloid progenitor cells providing evidence for trained immunity development. In addition, when the innate immune system is stimulated, a Western diet furthers inflammation.28 Moreover, the trained immunological phenotype was sustained despite the mice being fed a regular diet and having their cholesterol and systemic inflammatory indicators restored to normal.28
2.2. Other immune cells
In addition to monocyte-mediated trained immunity, T cells and B cells have been implicated in the protective roles of BCG against TB. T cell responses in particular the induction of Th1 responses to BCG have been described comprehensively.29 Furthermore, B cell responses have recently been linked to BCG-mediated protection against TB which has also been reviewed.30,31
Although trained immunity has been considered as the primary mechanism for combating bladder cancer, direct robust evidence is lacking.22 Additionally, it has been shown that BCG immunotherapy inhibits bladder tumors by inducing tumor-specific IFN-γ+CD4+ T cell-dependent responses.32 Collectively, BCG can induce several different immune cells to combat TB or bladder cancer.
3. Metabolic pathways and trained immunity
It has been shown that increased glycolysis, glutaminolysis, and cholesterol synthesis are essential for trained immunity development in the monocytes based on integrated transcriptomics and metabolomics data.33
3.1. Glycolysis
Glycolysis is a fundamental process in trained immunity and is often attributed to immune cell activation. Increased glycolysis observed in activated T cells leads to induced glucose metabolism in pro-inflammatory macrophages, lactate production, as well as adenosine triphosphate (ATP) synthesis.34, 35, 36
In addition to BCG, beta-glucans also have trained immunity property. Beta-glucans are soluble fibers from different sources such as fungi, bacteria, yeasts, and certain plants. Saccharomyces cerevisiae, a yeast has abundant beta-glucans, which has been shown to reduce the risk of cardiovascular diseases.37 It is reported that beta-glucans can induce trained immunity by shifting cellular metabolism from oxidative phosphorylation toward glucose fermentation regulated by the protein kinase B (AKT)/mammalian target of rapamycin (mTOR) pathway. This metabolic switch is vital in inducing trained immunity. Moreover, a long-term increase in glycolysis, as well as pro-inflammatory phenotypes, were reported in mice with beta-glucan-induced trained immunity. Together, beta-glucan-induced trained immunity is accompanied by an increased high glycolytic rate and reduced oxidative phosphorylation.37,38 In contrast to beta-glucans, BCG-induced trained immunity enhances both glycolysis and oxygen consumption rate. Although different training stimuli establishes distinct metabolic pathways, it is important to note that glycolysis and lactate production remain more significant than oxidative phosphorylation in both beta-glucan- and BCG-induced trained immunity.39
3.2. Glutaminolysis
Glutamine is the most prevalent free amino acid in the body. It is important in proliferation, cell survival, and plays a key role in immune activation.40 Trained immunity boosts glutaminolysis, which converts glutamine to glutamate and further into alpha-ketoglutarate, succinate, fumarate, and malate. Inhibition of glycolysis or glutaminolysis can prohibit beta-glucan-induced trained immunity and reduce fumarate concentration in trained monocytes.40
Previous research has linked epigenetic remodeling of chromatin to trained immunity.26 It has been shown that fumarate by inhibiting lysine demethylase (5BKDM5), a histone demethylase, can increase H3K4me3 in the promoters of pro-inflammatory cytokine genes.33 Moreover, in a clinical study, BCG upregulates the expression of genes involved in glutaminolysis (solute carrier family 1 member 5 (SLC1A5), glutaminase (GLS), and glutamate dehydrogenase (GLUD)) and glycolysis (phosphofructokinase (PFK), hexokinase 2 (HK2), and lactate dehydrogenase A (LDHA)) up to 56 weeks, contributing to long-term epigenetic reprogramming of trained monocytes.39
3.3. The cholesterol biosynthesis pathway (mevalonate pathway)
The enzyme 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase (HMGCR) catalyzes the conversion of HMG-CoA to mevalonate, which is a rate-limiting step in the biosynthesis of cholesterol. The mevalonate pathway involves a variety of cell activities including establishing trained innate immunity. Incubating monocytes with mevalonate promote a trained immunity phenotype. Moreover, monocytes obtained from patients with mevalonate accumulation showed trained immunity phenotype featured by increased cytokines as well as elevated expression of genes in the glycolysis pathway.40 Statins are HMGCR inhibitors and are used to treat hypercholesterolemia. Statins impaired the memory of monocytes as well as the effects of BCG in bladder cancer treatment.33,41 These findings further strengthen the relevance of mevalonate metabolism in BCG-induced trained immunity.
Together, there are substantial changes in metabolic pathways associated with trained immunity. The relationship between BCG and metabolic syndrome remains to be explored. Recent clinical studies revealed that BCG treatment improves T1DM and atherosclerosis.42 In addition, metabolic diseases have been improved in bladder cancer patients who had intravesical BCG therapy. Those findings suggested a strong relationship between BCG and metabolism.43 The effects of BCG-induced trained immunity on metabolic activity and epigenetic rewiring are depicted in Fig. 1.
Fig. 1.
BCG induces trained immunity which has effects on metabolic activity and epigenetic rewiring. Increased glycolysis, glutaminolysis, and cholesterol synthesis are essential for BCG-induced trained immunity development in the monocytes. In addition, when trained immunity occurs, monocytes undergo epigenetic rewiring. H3K4me3, an activating histone modification, is prevalent in the proximal regulatory areas of pro-inflammatory cytokine genes including TNF-α and IL-6 in trained monocytes. Another sign of BCG-induced trained immunity is reduced H3K9me3 at the promoters of TNF-α and IL-6. Importantly, metabolites such as acetyl-CoA in trained monocytes serve as cofactors for acetyltransferases. Collectively, the integration of metabolism and epigenetic rewiring occurs during BCG-induced trained immunity. Abbreviations: α-KG, alpha-ketoglutarate; ATP, adenosine triphosphate; BCG, Bacillus Calmette-Guérin; H3K4me3, trimethylated histone H3 lysine 4; H3K9me3, trimethylated histone H3 lysine 9; IL-6, interleukin-6; TCA, tricarboxylic acid; TNF-α, tumor necrosis factor-alpha.
4. BCG and systemic inflammation
Increased metabolic rate is linked with inflammation, which can occur during tissue-destructive processes via blood-derived compounds. The purpose of inflammation is to kill or isolate the source of disturbance, remove injured tissue, and restore homeostasis. Inflammation and its associated multi-organ abnormalities are strongly linked. Therefore, inflammation influences the severity of non-alcoholic fatty liver disease (NAFLD), type 2 diabetes mellitus (T2DM), and cardiovascular disease.
Additional studies in rodents reported the effects of alcohol on the effectiveness of BCG vaccination. When mice consumed ethanol prior to BCG vaccination, they were not protected against Mycobacterium tuberculosis (M. tuberculosis). In contrast, mice that consumed ethanol after BCG vaccination had protection against M. tuberculosis challenge.44,45 Therefore, ethanol interferes with trained immunity, but ethanol does not interfere with already established trained immunity induced by BCG.45
In 60–80 years old people, one dose of BCG could reduce pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β), chemokines (C-C motif chemokine ligand 2 (CCL2) and C-X-C motif chemokine ligand 10 (CXCL10)), acute phase proteins such as C-reactive protein, and matrix metalloproteinases. This implies that BCG reverses both immunological senescence and inflammation, resulting in significant implications for the prevention of immune-related illnesses associated with inflammation, such as infectious diseases associated with immune senescence and cardiovascular disorders.46 BCG can reduce systemic inflammation in a gender-specific manner.47 BCG-Bulgaria vaccination was done in a cohort of 303 healthy volunteers followed by monitoring systemic inflammation and assessing trained immunity. Results showed that BCG reduced systemic inflammation and the effects were stronger in men than women.47 However, estradiol or dihydrotestosterone was not able to induce training or tolerance in monocytes.48 Other mechanisms that account for the gender difference remain to be uncovered.
Taken together, trained immunity contributes to the development of autoimmune diseases as well as atherosclerosis by increasing the circulation of pro-inflammatory cytokines.49 However, BCG can reduce the risk of developing asthma, eczema, and atopic allergy.50,51 How BCG vaccine lowers inflammation while enhancing trained immunity is still unknown.
5. BCG and liver diseases
Trained immunity is linked with increased pro-inflammatory mediators like IL-1β, TNF-α, and IL-6, as well as increased expression of markers on innate immune cells associated with antigen presentation to T cells. An animal study demonstrated that BCG lowered plasma non-high-density lipoprotein (HDL) cholesterol in mice by enhancing hepatic uptake of cholesterol-enriched lipoprotein remnants and decreasing gastrointestinal cholesterol absorption.52
In NAFLD models, intravenous administration of BCG in 6 weeks old ob/ob mice prevented NAFLD development by altering the physiological condition of both adipocytes and hepatocytes. BCG reduced fatty acid-induced endoplasmic reticulum (ER) stress in the livers of ob/ob mice.53 BCG treatment also considerably elevated adiponectin serum levels as well as reduced hepatic triglyceride storage, liver macrovesicular steatosis, and lipogenic-related gene expression. Furthermore, BCG administration significantly decreased serum insulin levels and epididymal white adipose tissue weight revealing extensive metabolic benefits.53
M. tuberculosis can also influence hepatic lipid metabolism in humans. For example, compared to healthy controls, Nigerian TB patients showed reduced cholesterol levels compared to the control population.54 Additionally, individuals with pulmonary TB had lower serum cholesterol, low-density lipoprotein (LDL), HDL, and total cholesterol compared with those without TB.55 Moreover, TB patients had reduced serum medium-chain fatty acids.56 Reduced lipids may be due to triglyceride uptake by M. tuberculosis.57
However, in immunocompromised situations, BCG vaccination has been shown to increase the liver size, fibrosis, cirrhosis, and development of hepatic granulomas, which are localized collections of inflammatory cells.58 When this occurs, cytokines and other pro-inflammatory chemicals, such as inducible nitric oxide synthase (iNOS)-derived nitric oxide, are released into the circulation; they damage the liver and cause inflammation in mice.59 Moreover, soluble TNF-α, a critical cytokine in liver damage, is required in hepatic granuloma development.59
BCG administration in immunocompromised individuals carries the potential of causing BCGitis and liver cirrhosis.60 A case study reported a 62-year-old bladder cancer patient treated with BCG developed hepatic cirrhosis and increased serum markers associated with liver injury.61 Similarly, another case study showed a 47-year-old bladder cancer patient developed acute hepatitis after BCG intravesical therapy.62 Together, BCG may provide preventive effects in liver disease development in the absence of pre-existing pathological condition.
6. BCG and diabetes
T1DM affects the insulin-secreting islets of Langerhans, causing pancreatic dysfunction in children. T1DM requires lifetime insulin therapy and carries a high mortality risk.63 Latent autoimmune diabetes in adults (LADA) has an average onset age of 30 years. T2DM is the most common type of diabetes connected to obesity and insulin resistance. High blood sugar is a common feature for all three types of diabetes.64
6.1. T1DM
Insulin administration and peripheral insulin resistance reduction are the two most common methods used for blood sugar management.2 In T1DM patients, aerobic glycolysis is diminished, and oxidative phosphorylation becomes the dominant metabolic pathway, characterized by low glucose consumption, high ketone generation, and the use of tricarboxylic acid cycle.2,65 BCG induces TNF-α releasing, which reduces the levels of suppressor T cells that are responsible for pancreatic islet cell destruction in T1DM. Another mechanism by which BCG can combat T1DM is shifting glucose metabolism from overactive oxidative phosphorylation to aerobic glycolysis.65 Aerobic glycolysis is a metabolic state characterized by rapid ATP production and high glucose consumption by cells.66 BCG induces a dramatic shift in immunometabolism by shifting the dependence from oxidative phosphorylation to aerobic glycolysis, leading in a reduction in blood glucose levels.2,66,67
Given that T1DM patients have a significantly elevated level of oxidative phosphorylation prior to BCG therapy, BCG normalizes immunological metabolism by boosting aerobic glycolysis, a scenario in which glucose use is swiftly regulated. M. tuberculosis also has the capability to alter aerobic glycolysis for trained immunological actions.37
Several studies have reported the beneficial metabolic changes caused by BCG using various methodologies, including transcriptome profiling, epigenetics, metabolomics, and clinical monitoring both in vivo and in vitro settings. BCG increased early glycolytic enzymes including hexokinase and phosphofructokinase, reduced the activity of late glycolytic enzymes such as pyruvate kinase, increased lactate generation, and lowered overactive oxidative phosphorylation in immune cells.2 The second dose of BCG appears to cure these immunological inadequacies by resetting the metabolism of immune system through shifting the dependence from oxidative phosphorylation to aerobic glycolysis.68
Clinical studies also support the benefits of BCG against T1DM. Patients with T1DM who received two doses of BCG can achieve normal blood sugar levels about 3 years later. Once the normal blood sugar level was restored, the therapeutic impact persisted longer than 5 years.2 Furthermore, immune cells may also be involved in the switch to aerobic glycolysis. Together, BCG leads to a long-term and sustained reduction in blood sugar levels.
Regarding the impact of BCG on insulin, animal models showed that BCG vaccination inhibits T cells from destroying insulin-secreting cells, allowing the pancreas to regenerate and begin producing insulin.69 A birth cohort analysis reported that BCG vaccination or the age at which it was administered did not link with the prevalence of T1DM in children aged 10–18 years. Although BCG vaccination has been demonstrated to have a promising effect on T1DM, it did not prevent adolescents from developing the disease.70
It has been shown that BCG vaccination promoted glycolysis and restored normoglycemia in people with acute autoimmune diabetes.2,65 Moreover, BCG can prevent T1DM and LADA via other immunological mechanisms. BCG may favor immunological tolerance through the enhancement of regulatory mechanisms.71 Moreover, BCG leads to epigenetic modifications in innate immune cells to induce pro-inflammatory pathways and counteract autoimmune processes.72,73 These mechanisms may also limit obesity-related chronic inflammation and protect against insulin resistance in different forms of diabetes. Further, the long-lasting effects of BCG by one dose neonatal injection have been re-evaluated and extended up to 50 years.71,74
6.2. T2DM
T2DM is mainly linked to lifestyle.75 In a 60-year follow-up analysis of the BCG vaccine, a statistically significant reduction in T2DM incidence was found in American Indians and Alaska Natives.76,77 A BCG vaccination registry data from Canada showed that the protective effects of BCG were seen in both T1DM and T2DM who received vaccination between the ages of 22 and 44. Additionally, the beneficial effects against T2DM were more profound in the cohort that received T2DM medications.78 The same benefits were demonstrated in animal models. BCG was administered to T2DM mice infected with M. tuberculosis, and serum cholesterol levels were decreased 4–6 months later.79
7. BCG and cardiovascular diseases
Hypercholesterolemia is the common source of atherosclerosis since it increases the production of foam cells in the arterial wall. Innate and adaptive immune cell recruitment, as well as other inflammatory processes, contribute to plaque formation in the future, and may lead to arterial constriction and plaque rupture.78 Repeated injections of non-viable BCG reduced atherosclerosis in apolipoprotein E-deficient (Apoe−/−) and Ldlr−/− mice but had no significant effects on plasma cholesterol levels. In addition, enhanced circulating IL-10 levels and reduced serum levels of pro-inflammatory cytokines were found in these mouse models.80
In hyperlipidemic APOE∗3-Leiden cholesteryl ester transfer protein (E3L.CETP) mice, BCG reduced plasma non-HDL cholesterol levels by improving hepatic cholesterol clearance.52 BCG also reduced foam cell formation by activating circulating T cells. Additionally, BCG significantly slowed down atherosclerosis progression and lowered lesion severity.52 Another mouse study reported that the administration of BCG combined with Toll-like receptor 4 (TLR4) agonist prevented cardiovascular hypertrophy and fibrosis by regulating immune microenvironment and skewing the Th1 immune responses.81 Conversely, another study reported that intranasal administration of BCG increased the extent of atherosclerosis formation in the aortas of Western diet-fed hyperlipidemic Ldlr−/− mice after 16 weeks.82
Lipids are required for both immune cells and M. tuberculosis. Cholesterols are required for immune cells to grow and maintain cell membrane integrity.83 Additionally, M. tuberculosis needs cholesterol to enter macrophages.84 In consistency, it has been shown that BCG administration can lower cholesterol and lipid content in hyperlipidemic mouse models.52 However, BCG injection with a dose similar to human use increased lymphocyte and monocyte activation and facilitated atherosclerosis development in rabbits.85 The effects of BCG on metabolic diseases are depicted in Fig. 2 and summarized in Table 1.44, 45, 46, 47,52,53,58,77,79,80,85
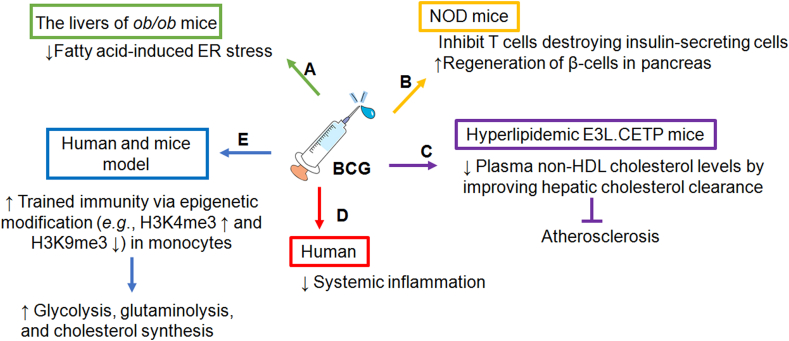
Fig. 2.
Effects of BCG on metabolic diseases. (A) Intravenous administration of BCG in ob/ob mice prevents NAFLD development by altering the physiological condition of both adipocytes and hepatocytes. BCG reduces fatty acid-induced ER stress in the livers of ob/ob mouse model. (B) BCG vaccination inhibits T cells from destroying insulin-secreting cells, allowing the pancreas to regenerate and begin producing insulin in animal models. (C) In hyperlipidemic E3L.CETP mice, BCG reduces plasma non-HDL cholesterol levels by improving hepatic cholesterol clearance. BCG can delay atherosclerosis progression by decreasing foam cells and plaque formations in mice. (D) BCG reduces systemic inflammation in humans and the effects are stronger in men than in women. How BCG vaccine lowers inflammation while enhancing trained immunity is still unknown. (E) BCG induces trained immunity in monocytes via epigenetic rewiring. Abbreviations: BCG, Bacillus Calmette-Guérin; E3L.CETP, APOE∗3-Leiden cholesteryl ester transfer protein; ER, endoplasmic reticulum; HDL, high-density lipoprotein; NAFLD, non-alcoholic fatty liver disease; NOD, nonobese diabetic.
Table 1.
Effects of BCG on metabolic diseases.
| Author and reference | Models or participants | Study details (time of BCG vaccination) | Dosage and route | Main findings |
|---|---|---|---|---|
| Porretta et al.44 | Male C57BL/6J mice were fed an alcohol-containing diet. BCG was administered either as a preventative or therapeutic drug. |
Three weeks prior or subsequent to the initiation of the alcohol diet. | 4 × 105 CFU (SC) | Mice consumed ethanol after BCG vaccination were protected against a subsequent M. tuberculosis challenge. When mice consumed ethanol prior to BCG vaccination, they were not protected against a subsequent pulmonary challenge with M. tuberculosis. |
| Mendenhall et al.45 | BCG-vaccinated BALB/c mice were exposed to alcohol via Lieber Decarli alcohol-containing diet or a liquid control diet. | Two weeks prior to the cytokine evaluation. | 2 × 106 CFU (IV) | Alcohol consumption down-regulated IFN-γ and immune responses in the mice vaccinated with BCG compared to mice on the liquid control diet. |
| Pavan et al.46 | A population-based study on 82 elderly individuals, between the ages of 60 and 80. | One month prior to cytokine/chemokine evaluation. | Freeze-dried 0.1 mL BCG adult dose (Intradermal) | BCG reduced pro-inflammatory cytokine levels (including TNF-α, IL-6, and IL-1β) and chemokines (including CCL2 and CXCL10) in elderly individuals. |
| Koeken et al.47 | A population-based study including 307 healthy volunteers. | Three months prior to protein measurements. | 0.1 mL BCG-Bulgaria (Intradermal) | BCG immunization lowered systemic inflammation in a gender-dependent manner as immunization reduced the pro-inflammatory proteins in the male participants. |
| van Dam et al.52 | Hyperlipidemic E3L.CETP mice. | Three weeks subsequent to the initiation of the Western diet. | 5 × 106 CFU (IV) | BCG reduced plasma non-HDL-cholesterol and atherosclerotic lesion formation. |
| Inafuku et al.53 | C57BL/6 mice/leptin-deficient ob/ob mice. | Four weeks prior to the measurement of biochemical parameters. | 1 × 108 CFU (IV) | BCG decreased white adipose tissue, serum insulin, hepatic triglyceride, and alleviated hepatic steatosis. |
| Wangoo et al.58 | Murine fibrosis model-BALB/c mice. | Three weeks prior to the measurement of granuloma formation. | 4 × 106 and 1 × 106 CFU (IV) | BCG induced hepatic granuloma formation at 4 × 106. Fewer or no granulomas were found at 1 × 106 CFU. |
| Aronson et al.77 | A placebo-controlled BCG trail among American Indians and Alaska Natives. | 60-year follow-up study. | Strain 317 in a dose of 0.15 mg, and strain 575 in a dose of 0.1 mg. | BCG-vaccinated individuals had a lower risk of type 2 diabetes than unvaccinated subjects. |
| Radhakrishnan et al.79 | A combination of streptozotocin and nicotinamide was used to induce hyperglycemic C57BL/6J mice (T2DM mice). | 4–6 months prior to cholesterol measurement. | 1 × 106 CFU (SC) | Cholesterol levels were significantly decreased at 4–6 months after BCG administration in T2DM mice. |
| Ovchinnikova et al.80 | Hypercholesterolemia Ldlr−/− and Apoe−/− mice. | 24 weeks prior to flow cytometric analysis. | Freeze-dried BCG (100 μg) (SC) | BCG exerted anti-atherogenic effects by elevation of IL-10 levels and expansion of Foxp3+ Tregs. |
| Lamb et al.85 | Cholesterol-fed rabbit. | Eight weeks prior to the initiation of the cholesterol diet. | 4–13 × 106 CFU (SC) | Immunization with human equivalent dose of BCG showed pro-atherogenic effects in the cholesterol-fed rabbit model. |
Abbreviations: Apoe−/−, apolipoprotein E-deficient; BCG, Bacillus Calmette-Guérin; CCL2, C-C motif chemokine ligand 2; CFU, colony-forming unit; CXCL10, C-X-C motif chemokine ligand 10; E3L.CETP, APOE∗3-Leiden cholesteryl ester transfer protein; HDL, high-density lipoprotein; IFN-γ, interferon-gamma; IL, interleukin; IV, intravenous; Ldlr−/−, low-density lipoprotein receptor-deficient; M. tuberculosis, Mycobacterium tuberculosis; NAFLD, non-alcoholic fatty liver disease; SC, subcutaneously; TNF-α, tumor necrosis factor-alpha.
8. BCG and microbiota
The gut microbiome can influence the effectiveness of vaccination via their metabolites, microbial ligands, or both. Short-chain fatty acids (SCFAs) produced by bacteria directly affect myeloid cells. For example, butyric acid, an SCFA, produced by bacterial fermentation of fiber, can alter the function of intestinal macrophages and hepatic inflammation.86,87 Moreover, microbiome may alter vaccination effectiveness.88,89
8.1. Human studies
A prospective observational study on infants reported that Bifidobacterium dominance in the gut may increase thymic development and responses to both oral and parenteral vaccines including BCG, tetanus, and polio.90 Moreover, the abundance of Clostridiales, Pseudomonadales, and Enterobacteriales was associated with decreased responsiveness to vaccine and systemic inflammation.90 Another human study showed BCG immunization in healthy individuals effectively increased the abundance of 43 immunomodulatory taxa. Trained immunity was associated with 27 specific species. Specific immunity induced after ex vivo restimulation with Staphylococcus aureus showed a correlation with three species, i.e., Streptococcus thermophilus, Eggerthella lenta, and Ruminococcus.91 Further, ex vivo blood restimulation with Staphylococcus aureus resulted in increased cytokine IL-6, IL-1β, and TNF-α production, this response was negatively linked with the abundance of Roseburia. Conversely, specific immunity against M. tuberculosis stimulation, as indicated by IFN-γ production, was favorably linked with Eggerthella lenta and Ruminococcus.91 The immunomodulatory taxa also have significant influence on circulating metabolites, with Roseburia having the most significant effects not only on trained immune responses but also on phenylalanine metabolism.91
8.2. Animal studies
Mice born and breastfed by mothers exposed to antibiotics had reduced responses to influenza vaccine, pneumococcal conjugate vaccine, and BCG vaccination. Notably, in those mice, antibiotics caused dysbiosis was marked by loss of Bacteroides and decreased Akkermansia. These findings show that early life colonization with specific bacteria is essential for efficient antigen-specific antibody release. Thus, gut dysbiosis is one of the most significant factors affecting vaccine efficacy. Gut dysbiosis induced by antibiotics significantly decreased the activation of CD4+ as well as CD8+ T cells and reduced the memory T cells in the lung of BCG-vaccinated mice.92 Additional evidence generated from animal trials demonstrated the significance of the gut microbiota on BCG-associated trained immunity. Parenteral BCG vaccination can alter the structure of the gut microbiome and metabolites, leading to the induction of trained immunity in the lung.93 Another study in mice reported that BCG immunization had a similar Firmicutes to Bacteroides ratio, compared with mice infected with M. tuberculosis.94 BCG increased the abundances of Cyanobacteria, Proteobacteria, Bacteroidetes, and Firmicutes, but reduced Tenericutes at the phylum level. At the genus level, BCG increased the abundance of Romboutsia, Bacteroides, Intestinimonas, Turicibacter, and Clostridium, but decreased Alistipes, Bacillus, and Rhizobium.94 Further, high fat diets have been shown to limit microbiota diversity and diminish the anti-TB effects of the BCG vaccine. High fat diet-associated dysbiosis, marked by an increase in the genera Alistipes, Parasuterella, Mucispirillum, and Akkermansia can be contributing factors to the diminished effects of BCG.95 Together, gut microbiome has a significant role in BCG-induced immunity. Due to controversial data, the role of specific microbiota such as Akkermansia on BCG efficacy needs more investigation.
9. Future directions
Because of the off-target effects, the utilization of BCG to prevent TB has been expanded to combat metabolic diseases. When considering the effects of BCG, the route of administration and adequate dosage as well as frequency can be important. The pre-existing health condition is also critically important. Moreover, utilization of BCG as an adjuvant therapy with other drugs may balance or mediate the trained immunity effects of BCG in metabolic diseases. The benefits of BCG in preventing or treatment of metabolic disease warrants further investigation and validation. This is particularly important for those diseases such as non-alcoholic steatohepatitis that currently still lack of treatment option. Furthermore, whether the beneficial effects of BCG are exclusively due to trained immunity remains to be investigated. Additional research is also needed to study the effects of attenuated strain of BCG or combined effects of BCG and probiotics or prebiotics on metabolism and immunity disorders to provide new therapeutic or preventive options.
Authors' contributions
M. U. Ijaz, F. Vaziri, and Y.-J. Y. Wan conducted literature search and wrote the manuscript. All authors read and approved the final manuscript.
Declarations of competing interest
The authors declare that they have no conflict of interest.
Acknowledgements
This study is supported by grants funded by the USA National Institutes of Health (R01CA222490).
Footnotes
Edited by Yuxia Jiang and Peiling Zhu.
References
- 1.Grundy S.M., Cleeman J.I., Daniels S.R., et al. Diagnosis and management of the metabolic syndrome: an American heart association/national heart, lung, and blood institute scientific statement: executive summary. Crit Pathw Cardiol. 2005;4:198–203. doi: 10.1097/00132577-200512000-00018. [DOI] [PubMed] [Google Scholar]
- 2.Kühtreiber W.M., Tran L., Kim T., et al. Long-term reduction in hyperglycemia in advanced type 1 diabetes: the value of induced aerobic glycolysis with BCG vaccinations. NPJ Vaccines. 2018;3:23. doi: 10.1038/s41541-018-0062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engin A. The definition and prevalence of obesity and metabolic syndrome. Adv Exp Med Biol. 2017;960:1–17. doi: 10.1007/978-3-319-48382-5_1. [DOI] [PubMed] [Google Scholar]
- 4.Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 5.Lamm D.L. Bacillus Calmette-Guerin immunotherapy of superficial bladder cancer. Nihon Hinyokika Gakkai Zasshi. 1995;86:9–10. doi: 10.5980/jpnjurol.86.9. [DOI] [PubMed] [Google Scholar]
- 6.Angelidou A., Pittet L.F., Faustman D., Curtis N., Levy O. BCG vaccine's off-target effects on allergic, inflammatory, and autoimmune diseases: worth another shot? J Allergy Clin Immunol. 2022;149:51–54. doi: 10.1016/j.jaci.2021.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moliva J.I., Turner J., Torrelles J.B. Immune responses to bacillus Calmette-Guérin vaccination: why do they fail to protect against mycobacterium tuberculosis? Front Immunol. 2017;8:407. doi: 10.3389/fimmu.2017.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Classen J.B. Review of evidence that epidemics of type 1 diabetes and type 2 diabetes/metabolic syndrome are polar opposite responses to iatrogenic inflammation. Curr Diabetes Rev. 2012;8:413–418. doi: 10.2174/157339912803529869. [DOI] [PubMed] [Google Scholar]
- 9.Paragh G., Seres I., Harangi M., Fulop P. Dynamic interplay between metabolic syndrome and immunity. Adv Exp Med Biol. 2014;824:171–190. doi: 10.1007/978-3-319-07320-0_13. [DOI] [PubMed] [Google Scholar]
- 10.Janeway C.A., Jr., Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 11.Bonilla F.A., Oettgen H.C. Adaptive immunity. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S33–S40. doi: 10.1016/j.jaci.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 12.Netea M.G., Domínguez-Andrés J., Barreiro L.B., et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20:375–388. doi: 10.1038/s41577-020-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Netea M.G., Giamarellos-Bourboulis E.J., Domínguez-Andrés J., et al. Trained immunity: a tool for reducing susceptibility to and the severity of SARS-CoV-2 infection. Cell. 2020;181:969–977. doi: 10.1016/j.cell.2020.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Musher D.M., Abers M.S., Corrales-Medina V.F. Acute infection and myocardial infarction. N Engl J Med. 2019;380:171–176. doi: 10.1056/NEJMra1808137. [DOI] [PubMed] [Google Scholar]
- 15.Leentjens J., Bekkering S., Joosten L.A.B., Netea M.G., Burgner D.P., Riksen N.P. Trained innate immunity as a novel mechanism linking infection and the development of atherosclerosis. Circ Res. 2018;122:664–669. doi: 10.1161/CIRCRESAHA.117.312465. [DOI] [PubMed] [Google Scholar]
- 16.Lee Y.S., Olefsky J. Chronic tissue inflammation and metabolic disease. Genes Dev. 2021;35:307–328. doi: 10.1101/gad.346312.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pålsson-McDermott E.M., O'Neill L.A.J. Targeting immunometabolism as an anti-inflammatory strategy. Cell Res. 2020;30:300–314. doi: 10.1038/s41422-020-0291-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daemen S., Schilling J.D. The interplay between tissue niche and macrophage cellular metabolism in obesity. Front Immunol. 2020;10:3133. doi: 10.3389/fimmu.2019.03133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee A.H., Dixit V.D. Dietary regulation of immunity. Immunity. 2020;53:510–523. doi: 10.1016/j.immuni.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Remmerie A., Martens L., Scott C.L. Macrophage subsets in obesity, aligning the liver and adipose tissue. Front Endocrinol (Lausanne) 2020;11:259. doi: 10.3389/fendo.2020.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trim W.V., Lynch L. Immune and non-immune functions of adipose tissue leukocytes. Nat Rev Immunol. 2022;22:371–386. doi: 10.1038/s41577-021-00635-7. [DOI] [PubMed] [Google Scholar]
- 22.van Puffelen J.H., Keating S.T., Oosterwijk E., et al. Trained immunity as a molecular mechanism for BCG immunotherapy in bladder cancer. Nat Rev Urol. 2020;17:513–525. doi: 10.1038/s41585-020-0346-4. [DOI] [PubMed] [Google Scholar]
- 23.Priem B., van Leent M.M.T., Teunissen A.J.P., et al. Trained immunity-promoting nanobiologic therapy suppresses tumor growth and potentiates checkpoint inhibition. Cell. 2020;183:786–801. doi: 10.1016/j.cell.2020.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan N., Downey J., Sanz J., et al. M. tuberculosis reprograms hematopoietic stem cells to limit myelopoiesis and impair trained immunity. Cell. 2020;183:752–770.e22. doi: 10.1016/j.cell.2020.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuhtreiber W.M., Takahashi H., Keefe R.C., et al. BCG vaccinations upregulate Myc, a central switch for improved glucose metabolism in diabetes. iScience. 2020;23 doi: 10.1016/j.isci.2020.101085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kleinnijenhuis J., Quintin J., Preijers F., et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A. 2012;109:17537–17542. doi: 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bekkering S., Stiekema L.C.A., Bernelot Moens S., et al. Treatment with statins does not revert trained immunity in patients with familial hypercholesterolemia. Cell Metab. 2019;30:1–2. doi: 10.1016/j.cmet.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 28.Christ A., Gunther P., Lauterbach M.A.R., et al. Western diet triggers NLRP3-dependent innate immune reprogramming. Cell. 2018;172:162–175.e14. doi: 10.1016/j.cell.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dockrell H.M., Smith S.G. What have we learnt about BCG vaccination in the last 20 years? Front Immunol. 2017;8:1134. doi: 10.3389/fimmu.2017.01134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Achkar J.M., Chan J., Casadevall A. B cells and antibodies in the defense against Mycobacterium tuberculosis infection. Immunol Rev. 2015;264:167–181. doi: 10.1111/imr.12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferluga J., Yasmin H., Al-Ahdal M.N., Bhakta S., Kishore U. Natural and trained innate immunity against Mycobacterium tuberculosis. Immunobiology. 2020;225 doi: 10.1016/j.imbio.2020.151951. [DOI] [PubMed] [Google Scholar]
- 32.Antonelli A.C., Binyamin A., Hohl T.M., Glickman M.S., Redelman-Sidi G. Bacterial immunotherapy for cancer induces CD4-dependent tumor-specific immunity through tumor-intrinsic interferon-gamma signaling. Proc Natl Acad Sci U S A. 2020;117:18627–18637. doi: 10.1073/pnas.2004421117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arts R.J., Novakovic B., Ter Horst R., et al. Glutaminolysis and fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity. Cell Metab. 2016;24:807–819. doi: 10.1016/j.cmet.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang R., Dillon C.P., Shi L.Z., et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35:871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donnelly R.P., Finlay D.K. Glucose, glycolysis and lymphocyte responses. Mol Immunol. 2015;68(2 Pt C):513–519. doi: 10.1016/j.molimm.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 36.Jha A.K., Huang S.C., Sergushichev A., et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015;42:419–430. doi: 10.1016/j.immuni.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Cheng S.C., Quintin J., Cramer R.A., et al. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345 doi: 10.1126/science.1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riksen N.P., Netea M.G. Immunometabolic control of trained immunity. Mol Aspect Med. 2021;77 doi: 10.1016/j.mam.2020.100897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arts R.J.W., Carvalho A., La Rocca C., et al. Immunometabolic pathways in BCG-induced trained immunity. Cell Rep. 2016;17:2562–2571. doi: 10.1016/j.celrep.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sikalidis A.K. Amino acids and immune response: a role for cysteine, glutamine, phenylalanine, tryptophan and arginine in T-cell function and cancer? Pathol Oncol Res. 2015;21:9–17. doi: 10.1007/s12253-014-9860-0. [DOI] [PubMed] [Google Scholar]
- 41.Hoffmann P., Roumeguere T., Schulman C., van Velthoven R. Use of statins and outcome of BCG treatment for bladder cancer. N Engl J Med. 2006;355:2705–2707. doi: 10.1056/NEJMc062714. [DOI] [PubMed] [Google Scholar]
- 42.Lu K., Su B., Meng X. Recent advances in the development of vaccines for diabetes, hypertension, and atherosclerosis. J Diabetes Res. 2018;2018 doi: 10.1155/2018/1638462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lenis A.T., Asanad K., Blaibel M., Donin N.M., Chamie K. Association between metabolic syndrome and recurrence of nonmuscle invasive bladder cancer following bacillus Calmette-Guerin treatment. Urol Pract. 2018;5:132–138. doi: 10.1016/j.urpr.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Porretta E., Happel K.I., Teng X.S., Ramsay A., Mason C.M. The impact of alcohol on BCG-induced immunity against Mycobacterium tuberculosis. Alcohol Clin Exp Res. 2012;36:310–317. doi: 10.1111/j.1530-0277.2011.01624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mendenhall C.L., Finkelman F., Means R.T., Jr., et al. Cytokine response to BCG infection in alcohol-fed mice. Alcohol. 1999;19:57–63. doi: 10.1016/s0741-8329(99)00018-x. [DOI] [PubMed] [Google Scholar]
- 46.Pavan Kumar N., Padmapriyadarsini C., Rajamanickam A., et al. Effect of BCG vaccination on proinflammatory responses in elderly individuals. Sci Adv. 2021;7 doi: 10.1126/sciadv.abg7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koeken V.A., de Bree L.C.J., Mourits V.P., et al. BCG vaccination in humans inhibits systemic inflammation in a sex-dependent manner. J Clin Invest. 2020;130:5591–5602. doi: 10.1172/JCI133935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Bree L.C.J., Janssen R., Aaby P., et al. The impact of sex hormones on BCG-induced trained immunity. J Leukoc Biol. 2018;104:573–578. doi: 10.1002/JLB.5MA0118-027R. [DOI] [PubMed] [Google Scholar]
- 49.Bekkering S., Joosten L.A., van der Meer J.W., Netea M.G., Riksen N.P. Trained innate immunity and atherosclerosis. Curr Opin Lipidol. 2013;24:487–492. doi: 10.1097/MOL.0000000000000023. [DOI] [PubMed] [Google Scholar]
- 50.Marks G.B., Ng K., Zhou J., et al. The effect of neonatal BCG vaccination on atopy and asthma at age 7 to 14 years: an historical cohort study in a community with a very low prevalence of tuberculosis infection and a high prevalence of atopic disease. J Allergy Clin Immunol. 2003;111:541–549. doi: 10.1067/mai.2003.171. [DOI] [PubMed] [Google Scholar]
- 51.Kowalewicz-Kulbat M., Locht C. BCG and protection against inflammatory and auto-immune diseases. Expert Rev Vaccines. 2017;16:1–10. doi: 10.1080/14760584.2017.1333906. [DOI] [PubMed] [Google Scholar]
- 52.van Dam A.D., Bekkering S., Crasborn M., et al. BCG lowers plasma cholesterol levels and delays atherosclerotic lesion progression in mice. Atherosclerosis. 2016;251:6–14. doi: 10.1016/j.atherosclerosis.2016.05.031. [DOI] [PubMed] [Google Scholar]
- 53.Inafuku M., Matsuzaki G., Oku H. Intravenous Mycobacterium bovis Bacillus Calmette-Guerin ameliorates nonalcoholic fatty liver disease in obese, diabetic ob/ob mice. PLoS One. 2015;10 doi: 10.1371/journal.pone.0128676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taylor G.O., Bamgboye A.E. Serum cholesterol and diseases in Nigerians. Am J Clin Nutr. 1979;32:2540–2545. doi: 10.1093/ajcn/32.12.2540. [DOI] [PubMed] [Google Scholar]
- 55.Deniz O., Gumus S., Yaman H., et al. Serum total cholesterol, HDL-C and LDL-C concentrations significantly correlate with the radiological extent of disease and the degree of smear positivity in patients with pulmonary tuberculosis. Clin Biochem. 2007;40:162–166. doi: 10.1016/j.clinbiochem.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 56.Daniel J., Maamar H., Deb C., Sirakova T.D., Kolattukudy P.E. Mycobacterium tuberculosis uses host triacylglycerol to accumulate lipid droplets and acquires a dormancy-like phenotype in lipid-loaded macrophages. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weiner J., 3rd, Parida S.K., Maertzdorf J., et al. Biomarkers of inflammation, immunosuppression and stress with active disease are revealed by metabolomic profiling of tuberculosis patients. PLoS One. 2012;7 doi: 10.1371/journal.pone.0040221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wangoo A., Brown I.N., Marshall B.G., Cook H.T., Young D.B., Shaw R.J. Bacille Calmette-Guerin (BCG)-associated inflammation and fibrosis: modulation by recombinant BCG expressing interferon-gamma (IFN-gamma) Clin Exp Immunol. 2000;119:92–98. doi: 10.1046/j.1365-2249.2000.01100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Olleros M.L., Vesin D., Fotio A.L., et al. Soluble TNF, but not membrane TNF, is critical in LPS-induced hepatitis. J Hepatol. 2010;53:1059–1068. doi: 10.1016/j.jhep.2010.05.029. [DOI] [PubMed] [Google Scholar]
- 60.Hanna L., Ubee S.S., Boddy J., Cooke P.W. Efficacy and complications of intravesical BCG in immunocompromised patients. BJU Int. 2014;113:691–693. doi: 10.1111/bju.12562. [DOI] [PubMed] [Google Scholar]
- 61.Vallilas C., Zachou M., Dolkiras P., et al. Difficulties in diagnosing and treating disseminated Bacillus Calmette-Guerin (BCG) infection after intravesical BCG therapy in a patient with liver cirrhosis: a case report. Am J Case Rep. 2021;22 doi: 10.12659/AJCR.933006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abid H., Figuigui M., Adil Ibrahimi S., et al. Acute hepatitis induced by intravesical BCG therapy: a rare but serious complication. Case Reports Hepatol. 2021;2021 doi: 10.1155/2021/4574879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Katsarou A., Gudbjornsdottir S., Rawshani A., et al. Type 1 diabetes mellitus. Nat Rev Dis Primers. 2017;3 doi: 10.1038/nrdp.2017.16. [DOI] [PubMed] [Google Scholar]
- 64.Pieralice S., Pozzilli P. Latent autoimmune diabetes in adults: a review on clinical implications and management. Diabetes Metab J. 2018;42:451–464. doi: 10.4093/dmj.2018.0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kuhtreiber W.M., Faustman D.L. BCG therapy for type 1 diabetes: restoration of balanced immunity and metabolism. Trends Endocrinol Metab. 2019;30:80–92. doi: 10.1016/j.tem.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 66.Shi L., Eugenin E.A., Subbian S. Immunometabolism in tuberculosis. Front Immunol. 2016;7:150. doi: 10.3389/fimmu.2016.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gleeson L.E., Sheedy F.J., Palsson-McDermott E.M., et al. Cutting edge: Mycobacterium tuberculosis induces aerobic glycolysis in human alveolar macrophages that is required for control of intracellular bacillary replication. J Immunol. 2016;196:2444–2449. doi: 10.4049/jimmunol.1501612. [DOI] [PubMed] [Google Scholar]
- 68.Faustman D.L., Wang L., Okubo Y., et al. Proof-of-concept, randomized, controlled clinical trial of Bacillus-Calmette-Guerin for treatment of long-term type 1 diabetes. PLoS One. 2012;7 doi: 10.1371/journal.pone.0041756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kodama S., Kuhtreiber W., Fujimura S., Dale E.A., Faustman D.L. Islet regeneration during the reversal of autoimmune diabetes in NOD mice. Science. 2003;302:1223–1227. doi: 10.1126/science.1088949. [DOI] [PubMed] [Google Scholar]
- 70.Corsenac P., Parent M.E., Benedetti A., Richard H., Stager S., Rousseau M.C. Association between Bacillus Calmette-Guerin vaccination and type 1 diabetes in adolescence: a population-based birth cohort study in Quebec, Canada. Prev Med. 2022;154 doi: 10.1016/j.ypmed.2021.106893. [DOI] [PubMed] [Google Scholar]
- 71.Ahmed A., Rakshit S., Adiga V., et al. A century of BCG: impact on tuberculosis control and beyond. Immunol Rev. 2021;301:98–121. doi: 10.1111/imr.12968. [DOI] [PubMed] [Google Scholar]
- 72.Ristori G., Faustman D., Matarese G., Romano S., Salvetti M. Bridging the gap between vaccination with Bacille Calmette-Guerin (BCG) and immunological tolerance: the cases of type 1 diabetes and multiple sclerosis. Curr Opin Immunol. 2018;55:89–96. doi: 10.1016/j.coi.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 73.Leong I. BCG vaccination for type 1 diabetes mellitus. Nat Rev Endocrinol. 2018;14:503. doi: 10.1038/s41574-018-0064-7. [DOI] [PubMed] [Google Scholar]
- 74.Lobo N., Brooks N.A., Zlotta A.R., et al. 100 years of Bacillus Calmette-Guérin immunotherapy: from cattle to COVID-19. Nat Rev Urol. 2021;18:611–622. doi: 10.1038/s41585-021-00481-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ashcroft F.M., Rorsman P. Diabetes mellitus and the beta cell: the last ten years. Cell. 2012;148:1160–1171. doi: 10.1016/j.cell.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Usher N.T., Chang S., Howard R.S., et al. Association of BCG vaccination in childhood with subsequent cancer diagnoses: a 60-year follow-up of a clinical trial. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aronson N.E., Santosham M., Comstock G.W., et al. Long-term efficacy of BCG vaccine in American Indians and Alaska Natives: a 60-year follow-up study. JAMA. 2004;291:2086–2091. doi: 10.1001/jama.291.17.2086. [DOI] [PubMed] [Google Scholar]
- 78.Corsenac P., Parent M.E., Mansaray H., et al. Early life Bacillus Calmette-Guerin vaccination and incidence of type 1, type 2, and latent autoimmune diabetes in adulthood. Diabetes Metab. 2022;48 doi: 10.1016/j.diabet.2022.101337. [DOI] [PubMed] [Google Scholar]
- 79.Radhakrishnan R.K., Thandi R.S., Tripathi D., et al. BCG vaccination reduces the mortality of Mycobacterium tuberculosis-infected type 2 diabetes mellitus mice. JCI Insight. 2020;5 doi: 10.1172/jci.insight.133788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ovchinnikova O.A., Berge N., Kang C., et al. Mycobacterium bovis BCG killed by extended freeze-drying induces an immunoregulatory profile and protects against atherosclerosis. J Intern Med. 2014;275:49–58. doi: 10.1111/joim.12127. [DOI] [PubMed] [Google Scholar]
- 81.Liu Y.Y., Cai W.F., Yang H.Z., et al. Bacillus Calmette-Guerin and TLR4 agonist prevent cardiovascular hypertrophy and fibrosis by regulating immune microenvironment. J Immunol. 2008;180:7349–7357. doi: 10.4049/jimmunol.180.11.7349. [DOI] [PubMed] [Google Scholar]
- 82.Huaman M.A., Qualls J.E., Jose S., et al. Mycobacterium bovis Bacille-Calmette-Guerin infection aggravates atherosclerosis. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.607957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Getz G.S., Reardon C.A. The mutual interplay of lipid metabolism and the cells of the immune system in relation to atherosclerosis. Clin Lipidol. 2014;9:657–671. doi: 10.2217/clp.14.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ouellet H., Johnston J.B., de Montellano P.R. Cholesterol catabolism as a therapeutic target in Mycobacterium tuberculosis. Trends Microbiol. 2011;19:530–539. doi: 10.1016/j.tim.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lamb D.J., Eales L.J., Ferns G.A. Immunization with bacillus Calmette-Guerin vaccine increases aortic atherosclerosis in the cholesterol-fed rabbit. Atherosclerosis. 1999;143:105–113. doi: 10.1016/s0021-9150(98)00284-6. [DOI] [PubMed] [Google Scholar]
- 86.Chang P.V., Hao L., Offermanns S., Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci U S A. 2014;111:2247–2252. doi: 10.1073/pnas.1322269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sheng L., Jena P.K., Hu Y., et al. Hepatic inflammation caused by dysregulated bile acid synthesis is reversible by butyrate supplementation. J Pathol. 2017;243:431–441. doi: 10.1002/path.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ciabattini A., Olivieri R., Lazzeri E., Medaglini D. Role of the microbiota in the modulation of vaccine immune responses. Front Microbiol. 2019;10:1305. doi: 10.3389/fmicb.2019.01305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zimmermann P., Curtis N. The influence of the intestinal microbiome on vaccine responses. Vaccine. 2018;36:4433–4439. doi: 10.1016/j.vaccine.2018.04.066. [DOI] [PubMed] [Google Scholar]
- 90.Huda M.N., Ahmad S.M., Alam M.J., et al. Bifidobacterium abundance in early infancy and vaccine response at 2 years of age. Pediatrics. 2019;143 doi: 10.1542/peds.2018-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Strazar M., Mourits V.P., Koeken V., et al. The influence of the gut microbiome on BCG-induced trained immunity. Genome Biol. 2021;22:275. doi: 10.1186/s13059-021-02482-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nadeem S., Maurya S.K., Das D.K., Khan N., Agrewala J.N. Gut dysbiosis thwarts the efficacy of vaccine against Mycobacterium tuberculosis. Front Immunol. 2020;11:726. doi: 10.3389/fimmu.2020.00726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jeyanathan M., Vaseghi-Shanjani M., Afkhami S., et al. Parenteral BCG vaccine induces lung-resident memory macrophages and trained immunity via the gut-lung axis. Nat Immunol. 2022;23:1687–1702. doi: 10.1038/s41590-022-01354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Silva F., Enaud R., Creissen E., Henao-Tamayo M., Delhaes L., Izzo A. Mouse subcutaneous BCG vaccination and Mycobacterium tuberculosis infection alter the lung and gut microbiota. Microbiol Spectr. 2022;10 doi: 10.1128/spectrum.01693-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Arias L., Goig G.A., Cardona P., et al. Influence of gut microbiota on progression to tuberculosis generated by high fat diet-induced obesity in C3HeB/FeJ mice. Front Immunol. 2019;10:2464. doi: 10.3389/fimmu.2019.02464. [DOI] [PMC free article] [PubMed] [Google Scholar]