Abstract
Introduction
Longitudinal effect of diet-induced obesity on bone is uncertain. Prior work showed both no effect and a decrement in bone density or quality when obesity begins prior to skeletal maturity. We aimed to quantify long-term effects of obesity on bone and bone marrow adipose tissue (BMAT) in adulthood.
Methods
Skeletally mature, female C57BL/6 mice (n = 70) aged 12 weeks were randomly allocated to low-fat diet (LFD; 10% kcal fat; n = 30) or high-fat diet (HFD; 60% kcal fat; n = 30), with analyses at 12, 15, 18, and 24 weeks (n = 10/group). Tibial microarchitecture was analyzed by µCT, and volumetric BMAT was quantified via 9.4T MRI/advanced image analysis. Histomorphometry of adipocytes and osteoclasts, and qPCR were performed.
Results
Body weight and visceral white adipose tissue accumulated in response to HFD started in adulthood. Trabecular bone parameters declined with advancing experimental age. BV/TV declined 22% in LFD (p = 0.0001) and 17% in HFD (p = 0.0022) by 24 weeks. HFD failed to appreciably alter BV/TV and had negligible impact on other microarchitecture parameters. Both dietary intervention and age accounted for variance in BMAT, with regional differences: distal femoral BMAT was more responsive to diet, while proximal femoral BMAT was more attenuated by age. BMAT increased 60% in the distal metaphysis in HFD at 18 and 24 weeks (p = 0.0011). BMAT in the proximal femoral diaphysis, unchanged by diet, decreased 45% due to age (p = 0.0002). Marrow adipocyte size via histomorphometry supported MRI quantification. Osteoclast number did not differ between groups. Tibial qPCR showed attenuation of some adipose, metabolism, and bone genes. A regulator of fatty acid β-oxidation, cytochrome C (CYCS), was 500% more abundant in HFD bone (p < 0.0001; diet effect). CYCS also increased due to age, but to a lesser extent. HFD mildly increased OCN, TRAP, and SOST.
Conclusions
Long-term high fat feeding after skeletal maturity, despite upregulation of visceral adiposity, body weight, and BMAT, failed to attenuate bone microarchitecture. In adulthood, we found aging to be a more potent regulator of microarchitecture than diet-induced obesity.
Keywords: Obesity, Aging, Osteoporosis, Translational research, Advanced image analysis (MRI-CT)
Plain Language Summary
The long-term effects of obesity on the adult skeleton remain unknown. Our long-term study of obesity in adult mice showed that despite increases in fat stores, including fat housed within bone, obesity failed to harm bone. Early aging – in contrast to obesity – emerged as the principal negative regulator of bone.
Introduction
Despite known harmful metabolic effects of obesity in human and animal studies, obesity is known to promote accrual of bone mass [1–3] with an unclear impact on osteoporotic fractures according to a recent meta-analysis [4]. Studies point to adverse bone outcomes in sarcopenic obesity, as opposed to obesity alone, which implicates additional factors such as exercise/physical activity as potential mediators [5]. Obesity associates with more distal limb fractures and less hip fractures [6–8]; however, clinical impact of this and mechanistic underpinnings are unclear.
In terms of functional musculoskeletal decline with age, mice show reduced grip strength by 24 weeks [9], not unlike humans, where grip strength drops in the third decade of life [10]. Bone loss follows a similar trajectory, particularly affecting trabecular bone which begins to decline after peak bone mass, between 12 and 24 weeks in mice. As such, loss of bone mass begins well before old age [11, 12], a trait that tracks with the human timeline [13, 14]. Both rodents and humans accrue adiposity at multiple sites concomitant with an age-related decline in bone density, and yet despite this association, the precise role of increased adiposity in mediating bone density diminution remains undetermined.
Here, we chose to study early age-related bone loss in mice, comparable to humans aged 20–35 years [15, 16] and asked whether HFD-induced obesity attenuates this as well as the marrow adipose depot, a site known to expand both with aging and obesity [17, 18]. We compared the long-term impact of high fat feeding on bone and bone marrow adipose tissue (BMAT) quantified volumetrically via 9.4T magnetic resonance imaging (MRI) in skeletally mature mice. Despite robust increases in weight, visceral adiposity, and BMAT in groups fed HFD until age 18 and 24 weeks, we discovered bone quantity to be mostly unaffected by this dietary intervention. Our analysis shows that bone loss due to aging is measurable by 15 weeks of age, which strengthens a body of work showing early aging as a powerful mediator of bone loss. This loss is not exacerbated by obesity and higher BMAT in young adulthood. In contrast to prior work, we report here that in skeletally mature female C57BL/6 mice, long-term HFD did not alter age-related trabecular bone, despite a significant impact on visceral adiposity, lipid, and metabolism gene expression, and bone marrow adiposity.
Materials and Methods
Animals and Dietary Experimental Intervention
Procedures and ethical guidelines of the University of North Carolina’s Institutional Animal Care and Use Committee (IACUC) were adhered throughout the study. Female, 12-week-old C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME, USA) were housed in controlled light (12-h light/12-h dark), temperature (21–22°C), and humidity (range 30–70%) conditions, with ad libitum access to food and water, and acclimated for 5 days prior to randomization. Females were chosen due to clinical relevance for osteoporosis. Animals were randomized to one of two dietary groups: a low-fat diet (LFD, 10% kcal fat; #12450B, Research Diets, New Brunswick, NJ, USA; n = 30) or a high-fat diet (HFD, 60% kcal fat; #D12492, Research Diets; n = 30) with a control group (n = 10), sacrificed at 12 weeks of age, after acclimatization. Mice exposed to long-term dietary intervention were sacrificed at 15, 18, and 24 weeks of age (n = 10/group). Body weight was measured weekly through study termination.
3D Quantification and Imaging of Marrow Adipose Tissue via MRI
Quantification of BMAT was performed via high-resolution 9.4T MRI with advanced image analysis for 3D volumetric BMAT analysis, a method previously validated against both osmium-stained µCT with advanced image analysis, as well as histomorphometry [18, 19]. Water and fat maps of murine femora were obtained with a 2-dimensional RARE imaging sequence with the following parameters: RARE factor = 4, TE = 28 ms, TR = 4,000 ms, number of averages = 4, number of slices = 24, slice thickness = 0.5 mm, in-plane resolution = 100 × 100 μm2, matrix size = 130 × 130 (pixels). Utilizing the fact that the fat and water protons have an NMR frequency separation of 3.5 ppm, a Gaussian-shaped 90-degree saturation pulse with a width of 2 ms was applied preceding the RARE sequence to suppress the fat or water signal while leaving the other signal unaffected. Fat and water images were acquired by setting the saturation pulse frequency to be the same as the water and fat frequencies, respectively.
In our processing workflow, we manually subdivided the full images holding samples into individual images for each bone. Then, we employed water images to manually outline femoral cortical bone masks using Insight ITK-SNAP [20] (open-access http://www.itksnap.org). Using these bone masks, interior bone regions were masked from other image parts in both the water and fat maps. Next, we established a common, study-specific reference space by computing an unbiased average MRI image [21] from the masked water maps using the ANTS registration software [22]. All individual water and fat maps were then propagated into the common space, where voxel-wise correspondence allows direct comparison of intensities. Average fat maps for each experimental group were computed in the common space and superimposed on the common, average water image for visualization of group fat maps. Fat map intensities were represented with a colored heat map in 3D Slicer (software development info) for visualization (open access www.slicer.org) [20, 23]. 3D Slicer is a platform distributed under a BSD-style open-source license that is broadly compatible with the OpenSource Definition by the Open Source Initiative and holds no restrictions on legal uses of the software. For BMAT quantification, we created a regional label map of the femur, excluding cortical bone but focusing on regions for the epiphysis, metaphysis, and diaphysis. Intensity-weighted volume of BMAT was then quantified via regional fat histograms.
Osteoclast Quantification via Histology
Femurs were fixed in 10% formalin, decalcified in formic acid (UN3412 Immunocal, StatLab, McKinney, TX, USA), paraffin-embedded and sectioned longitudinally at 5 μm, and mounted on glass slides. Xylene-deparaffinized sections were rehydrated with graded ethanol, rinsed with deionized water, and stained for tartrate-resistant acid phosphatase (TRAP) by a buffer containing naphthol-AS-BI-phosphate (70485; Sigma-Aldrich, St. Louis, MO, USA) followed by a buffer containing sodium nitrite (237213; Sigma-Aldrich) and pararosaniline dye (215600; Sigma-Aldrich). Fast Green stain was applied (F7252; Sigma-Aldrich), dehydrated, then exposed to xylene before mounting with Cytoseal (8312-4; Thermo Fisher Scientific, Waltham, MA, USA). Images were obtained via an Olympus IX81, and TRAP was quantified using open-source software [24].
Marrow Adipocyte Quantification via Histology
Fixed and decalcified femurs were embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin (MHS16; Sigma-Aldrich, St. Louis, MO, USA) [18, 25]. Imaging on an Olympus X81 (microscope) at ×4 and ×40 magnification. The ×40 images were obtained at the distal femoral metaphysis, where lipid content is maximal. ImageJ (NIH, Bethesda, MD) [26] was used to isolate adipocytes within ×40 images and to quantify adipocyte size as previously described [18, 27]. Briefly, globular maxima were removed manually to isolate defined adipocytes. The “Analyze Particles” function was used to outline cells and calculate area in pixels. A lower limit of 1,000 pixels was applied to n = 2–3/group with a minimum of 5 distinct histologic regions per section, as well as a minimum of 3 sections per animal. A minimum of 20 adipocytes were analyzed for adipocyte area measurement per mouse in each section. To assess adipocyte number via ImageJ, the marrow cavity at the distal metaphysis was imaged at a magnification of ×4 and adipocytes were manually counted. Adipocytes were additionally probed n = 2–3/group via immunohistochemistry staining for perilipin 1 (PLIN1) (online suppl. Table 1; for all online suppl. material, see https://doi.org/10.1159/000536159).
Bone Microarchitecture, Geometry, and Biomechanical Assessment
Bone microarchitecture parameters of the proximal tibial metaphysis and mid-diaphysis were quantified ex vivo via high-resolution µCT (Scanco Medical, vivaCT40, Brüttisellen, Switzerland) at the UNC Chapel Hill Biomedical Imaging Research Center (resolution 12 mm; E 55 kVa; I 145 mA) [19, 28]. The region-of-interest along the tibial metaphysis was defined as a region 1,000 µm beneath the metaphyseal growth plate. Briefly, the interface of trabecular and cortical surfaces was manually contoured. To resolve the compartments, natural contouring was preserved along the endosteal surface, where the cortical surface was delineated from trabecular struts based on variations in discrete density values (mgHA/cm3). Subsequently, an automated algorithm was applied to quantify bone in each compartment. Parameters analyzed in trabecular bone include the following: ratio of the segmented bone volume to the total volume of region-of-interest (bone volume fraction; BV/TV); measure of average number of trabeculae per unit length (trabecular number); mean thickness of trabeculae, assessed using direct 3 D methods (trabecular thickness [Tb.Th]); mean distance between trabeculae, assessed using direct 3 D methods (trabecular separation). Parameters in cortical bone include the following: total cross-sectional area inside the periosteal envelope (Tt.Ar); cortical area (Ct.Ar) calculated from cortical volume divided by the number of slices x slice thickness; Ct.Ar fraction (Ct.Ar/Tt.Ar); average cortical thickness (Ct.Th) normalized to Tt.Ar of the region-of-interest. Additional variables for trabecular regions include structure model index, connectivity density (Conn.D), degree of anisotropy, and apparent trabecular bone mineral density. In the cortical compartment or the tibial mid-diaphysis, the following are reported: apparent cortical bone mineral density, Ct.Th, and polar moment of inertia of inertia around the X- or Y-axes, a biomechanical measure of resistance to bending.
Bone mineral density in trabecular and cortical compartments is shown in the Results section (quantified by 3D μCT in mg of HA/cm3). In preclinical rodent models, 3D μCT remains the method of choice for quantification of trabecular and cortical bone [29, 30] and has been shown to correlate with DEXA BMD, as well as histomorphometry [31] while also providing bone qualitative biomechanical measures. This differs from humans, where clinical DEXA is used as the gold standard to predict fracture in the entire skeleton. Quantitative CT methods in humans are reserved for research studies, due to high radiation exposure and lack of sufficient clinical reference databases.
Real-Time Quantitative PCR
Whole tibial mRNA was reverse transcribed and analyzed via real-time qPCR as previously described [32, 33]. Briefly, 10 μL of cDNA from each experimental condition was pooled and diluted 1:10 to 1:10,000 to generate a 5-point standard curve. A non-template control was added to each PCR reaction. Standards and samples were run in duplicate. PCR products were normalized to GAPDH, quantified via the 2((−) (delta) (delta CT)) method. PCR primer sequences are supplied (online suppl. Tables 1, 2).
Statistical Analysis
Analyses were performed using GraphPad Prism Version 9.1.0 (GraphPad, San Diego, CA, USA). We applied the two-way analysis of the variance or ANOVA (age x diet) with correction for multiple comparisons via Tukey’s post hoc test, a mixed model, or nested [34] ANOVA, as appropriate. Details of analyses are noted in figure legends. Significance was defined at an alpha of less than 0.05.
Results
HFD started after skeletal maturity increases body weight and visceral adiposity. Previously, we investigated the effect of HFD feeding, initiated at the early age of 4 weeks, to investigate diet-induced obesity and running exercise on BMAT and bone [18]. The post-weaning dietary intervention is a valuable tool; however, implementation concomitant with rapid skeletal growth may lower physiologic relevance for studying age-related osteoporosis. For increased relevance to the adult human state, unaffected by growth, we wished to study the effect of HFD as well as LFD after skeletal maturity had been attained.
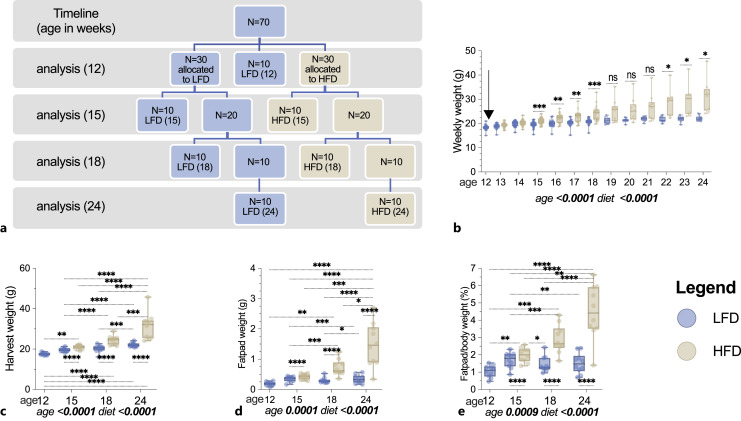
Herein, we present a longitudinal study of n = 70 C57BL/6 female mice where dietary interventions were started at age 12 and maintained until 24 weeks of age. Analyses were made at 12, 15, 18, and 24 weeks (Fig. 1a). Dietary comparisons include LFD where 10% of the calories were derived from fat, i.e., lower than typical standard chow diets for mice, while experimental HFD group received 60% of calories from fat.
Fig. 1.
Body weight and white adipose tissue accumulate in response to HFD, initiated in adulthood. Female 12-week-old B6 mice were allocated to LFD (n = 40 LFD) or HFD (n = 30 HFD) interventions for a 12-week experimental period. A schematic demonstrating the experimental timeline (a) is provided. The weekly experimental (b) as well as harvest (c) weights are plotted. Arrow in (b) denotes start of experimental intervention. Harvest fat pad weight (d) and fat pad as a % of body weight (e) are shown. Data in (b) were analyzed via mixed effects with multiplicity corrected p values via Bonferroni. Data (c–e) analyzed via two-way ANOVA with Turkey’s post hoc; p values for main effects (age, diet) shown below plots; data as individual animal data points. Box plot horizontal lines represent medians ± IQR. Group differences shown if p < 0.05 as follows: *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****p < 0.0001.
Weight between groups diverged by 3 weeks post-intervention at 15 weeks of age (Fig. 1b). Body weights rose throughout the study; final weights at 24 weeks were significantly higher than the 12-week-old baseline (+20% LFD; +70% HFD; p < 0.0001 for both; Fig. 1b). Perigonadal fat pad weights reflected a significant increase in white, visceral adipose stores by 15 weeks, comparing HFD versus LFD (Fig. 1c, d). Body weight and gonadal fat pad weight differed significantly between LFD and HFD groups at each analysis (15, 18, and 24 weeks; Fig. 1c–e). Perigonadal fat pad weight, as a percent of body weight, increased 324% (Fig. 1e, p < 0.0001) in HFD at 24 weeks as compared to baseline, while LFD fat pads were not significantly changed at 24 weeks (Fig. 1e).
Thus, in addition to an effect of HFD intervention, we also found that LFD tempered age-related weight gain known to occur on chow diets, comparable to prior studies in male mice [35]. This stands in contrast to the continuous weight gain in both LFD- and HFD-fed animals – when fed post-weaning for similar duration [35].
HFD, despite Increases in Weight and Adiposity, Did Not Worsen Age-Related Declines in Microarchitecture
To quantify the effect of long-term HFD on the adult skeleton, after peak bone mass has been attained, µCT scans were performed at the tibial diaphysis and proximal metaphysis, analyzed for trabecular and cortical microarchitecture. At the end of the experimental period, or age 24 weeks, BV/TV (−21% LFD vs. −17% HFD) and trabecular number (−26% LFD vs. −28% HFD) were significantly lower compared to 12 weeks and notably not offset by diet (Fig. 2a). Apparent trabecular bone mineral density declined ∼15% at 24 weeks in both HFD and LFD (vs. 12 weeks, p < 0.05; Fig. 2a). Although diet accounted for some of the variance in Tb.Th and Conn.D, a significant HFD-dose-response did not emerge (Fig. 2a). Conn.D was minimally attenuated at 15 weeks and clearly was lower at 24 weeks (−34 in HFD and – 28% in LFD) (Fig. 2a). Expected Tb.Th response to early aging (Fig. 2a) is thought to represent thickening of existing trabeculae, as thinner ones are resorbed [12]. It is important to note that age accounted for most variance in trabecular microarchitecture parameters (Fig. 2a). For example, diet accounted for 3% of the variance in Tb. Th and Conn.D, while age accounted for >50% of the variance (Fig. 2a). The cortical compartment is known to be stable up to 1 year of age in female C57BL/6 mice; therefore, our finding of mostly stable microarchitecture and biomechanical parameters with age (Fig. 2b, c) in this cohort is supported by others [12].
Fig. 2.
Trabecular bone parameters decline with experimental age, with lesser attenuation of the cortical compartment. Female 12-week-old skeletally mature B6 mice were allocated to LFD or HFD intervention for 12 weeks (n = 10/group). a Trabecular bone microarchitecture was assessed at the proximal tibial metaphysis via micro-CT. b Quantitative measures of cortical bone at the mid-tibial diaphysis via μCT. c Biomechanical measures of the cortical mid-diaphysis. Data as individual animal data points. Box plot horizontal lines represent medians ± IQR; analysis via two-way ANOVA with Tukey’s post hoc; p values for main effects (age, diet) are noted below plots, and between-group comparisons added if p < 0.05 as follows: *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****p < 0.0001. Measures of resistance to bending pMOI of Ixx- or Iyy-axes. Tb.BMDa, apparent trabecular bone mineral density; Tb.BV/TV, trabecular bone volume fraction; Tb.N, trabecular number per unit length; Tb.Th, trabecular thickness via direct 3D methods; Tb.Sp, trabecular separation, or the mean distance between trabecular using direct 3D methods; Tb.Conn.D, trabecular connectivity density; Tb.SMI, trabecular structural model index; Conn.D, trabecular connectivity density; Ct.Ar/Tt.Ar, cortical bone volume fraction; Tt.Ar, total area; Ct.Ar, cortical area; Ct.BMDa, apparent cortical bone mineral density; Ct.Th, cortical thickness; pMOI, polar moment of inertia; Ixx, inertia around the X-axis; Iyy, inertia around the Y-axis.
BMAT Rose in Response to HFD and Age in the Distal Femur
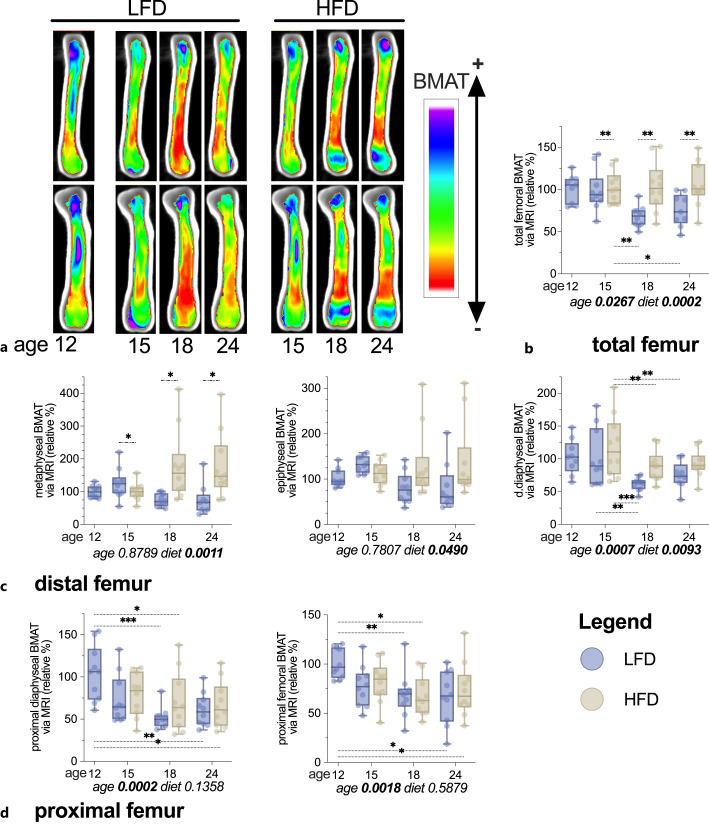
We next asked if HFD and early aging associate with a rise in BMAT. Volumetric 3D MRI via 9.4T scanner [18, 19, 28] to quantify BMAT relative to bone volume, using advanced image analysis techniques (Fig. 3a–d), shows reproducible visualization and quantification of BMAT. BMAT distribution via color heat map was acquired (Fig. 3a) as individual animal’s fat maps, superimposed for an average image per experimental group. Both diet and age accounted for variance in total femoral BMAT; however, we found regional differences in BMAT response.
Fig. 3.
Dietary intervention and aging associated with increased quantity of BMAT via 9.4 Tesla MRI during the experimental period, with the largest effect of diet localized to the distal femoral compartment. Skeletally mature female 12-week-old B6 mice allocated to LFD or HFD for a 12-week period. a Visualization of BMAT by MRI. Each image is an average of each of the individual images superimposed on each other per experimental group; top panel, sagittal cut; bottom panel, coronal cut. Data as individual animal data points. Box plot horizontal lines represent medians ± IQR expressed as % relative to 12-week LFD. Volumetric analyses on the whole femur (b) as well as pre-specified anatomical regions in the distal femur (c) and proximal femur (d) were analyzed via two-way ANOVA with Tukey’s post hoc; p values for main effects (age, diet) noted below plots; between-group differences shown if *, p < 0.05; **, p < 0.01; ***, p < 0.001.
BMAT is known to be maximal in proximity to physes, and a volumetric assessment was performed of proximal and distal regions without the femur. Total BMAT was ∼75% higher in HFD than in LFD at 18 and 24 weeks of age with an effect of age as well (Fig. 3a; p = 0.0238). Anticipated rise in total femoral BMAT with early aging was offset by LFD (Fig. 3b). Distal femoral BMAT was more responsive to dietary intervention, while proximal femoral BMAT was more attenuated by age. In the distal metaphysis, BMAT was ∼60% higher in HFD versus LFD at 18 and 24 weeks (p = 0.0011; diet effect; Fig. 3c). BMAT in the proximal diaphysis, unchanged by diet, sank ∼45% due to aging (p = 0.0002; age effect; Fig. 3d). Thus, proximal femoral BMAT at a younger age transferred in distribution toward the distal femur with early aging and HFD intervention (Fig. 3c, d). In sum, BMAT rose in response to high fat dietary intervention in the femur, particularly distally.
High Fat Feeding Prompted Bone Marrow Adipocyte Hypertrophy in Adulthood
Histomorphometric analysis of BMAT was performed using hematoxylin staining of paraffin-embedded femurs. Staining for the lipid droplet PLIN1 provided excellent identification of adipocyte cell edges rather than simply white space, allowing precise measures of fat cell size (Fig. 4a–d). Bone marrow adipocyte size rose through 18 and 24 weeks (Fig. 4a–d) in HFD. Compared to LFD, HFD average adipocyte area (µm2) was higher (p = 0.0067 diet effect) and aging also contributed to the increase in adipocyte size (p = 0.0017 age effect) (Fig. 4d). Interestingly, adipocyte number was not meaningfully altered (Fig. 4d). These histologic measures confirm age and age-induced increases in adipocyte size of BMAT depot, limited by LFD, consistent with MRI data.
Fig. 4.
Bone marrow adipocyte size is increased in HFD initiated in adulthood. Female 12-week-old C57BL/6 mice allocated to baseline 12-week-old LFD or HFD interventions for a 12-week experimental period. a, b Bone marrow adipocyte area quantified via ImageJ. b The Gaussian distribution using a non-linear least squares regression to visualize 95% CI (horizontal lines) of adipocyte size per group, including the means. c Representative distal femoral sections stained via methyl green and probed via immunohistochemistry for PLIN1 via brightfield microscopy at indicated magnification. d Adipocyte number per mm2 via ImageJ. e Osteoclast number per bone surface. d and e are shown as individual animal data with bars indicating median. a shows individual measurements per animal (M1, mouse 1; M2, mouse 2; M3, mouse 3). Two-way ANOVA p values for main effects (age, diet) below (d and e) in italics; between-group differences shown as follows: *, p < 0.05; **, p < 0.01.
Diet and Age Attenuate Lipid, Inflammatory, and Bone Marker Expression in the Tibia
Lipid and metabolism (AP2, CD36, PLIN1, PLIN5, FCYCS, ASN, LEPR), bone (OCN, TRAP SOST, RANKL), and inflammation (TNFα, GCSF, CD68) markers were queried via RT-qPCR in whole tibia (Fig. 5). PLIN1, a negative regulator of lipid droplet lipolysis via ATGL, was decreased by both aging (p = 0.0311) and HFD (p = 0.0068) (Fig. 5). That mitochondrial β-oxidation marker cytochrome C (CYCS) rises with age (p = 0. 0.0193) and HFD (p < 0.0001) reinforces that lipid substrates are used as fuel within bone [18, 36]. Indeed, CYCS expression was ∼500% higher in HFD-fed bone versus LFD (p < 0.0001; diet effect; Fig. 5). Age also increased CYCS but to a lesser extent. In terms of bone turnover markers, HFD raised the expression of some (OCN, TRAP, SOST) though the micro-CT data did not demonstrate a strong dietary effect on bone microarchitecture in these analyses.
Fig. 5.
Diet and experimental age effect on adipose, metabolism, bone, and inflammatory markers in the tibia. Female 12-week-old C57BL/6 mice allocated to baseline 12-week-old control group (CTL) as well as LFD or HFD interventions for a 12-week experimental period. Target gene mRNA % relative to GAPDH was quantified via qPCR (n = 3–4) in murine tibia for adipose/lipid droplet, metabolism, bone turnover, and inflammatory markers. Data as individual animal data points with bars indicating median ± range. Analysis via 2-way ANOVA with Tukey’s post hoc; p values for main effects (age, diet) shown above each plot.
Discussion
In this study, we asked whether HFD, and the resultant increased BMAT, might worsen age-related bone density decline in skeletally mature, female mice. As predicted [12, 37], even during this early period of adulthood, 3–6-month mice significantly reduced trabecular bone quantity and quality, with a lesser impact on the cortical compartment. We found HFD to associate with higher BMAT in the whole femur and particularly in the distal femur. The diet-induced increase in BMAT was mediated by an increase in adipocyte size, rather than number, which also responded to increased age, indicating that lipid intake resulted in augmented storage in this depot. Consistent with increased marrow adipocyte size, AP2/FABP4 (regulator of lipolysis), CYCS (role in β-oxidation), as well as CD36 (regulator of fatty acid uptake) increased in HFD bone, supporting that bone can rely on fatty acids as a fuel source [36, 38, 39]. Our prior work in skeletally immature mice showed HFD associated with lower cortical bone volume fraction, whereas in the present analysis with intervention begun in adults, HFD failed to attenuate similar cortical parameters. Hence, prior effect in younger mice may have been related to growth, hormonal milieu during growth, or interaction between obesity and such factors [40]. Osteoclast numbers were similar between HFD and LFD with comparable microarchitectural data. The relevance of raised expression of turnover markers in HFD including TRAP, OCN, and SOST is uncertain. Our study adds to prior investigations that suggest HFD-induced obesity, initiated after skeletal maturity – in and of itself – does not alter bone fragility, despite augmenting BMAT. Importantly, bone microarchitecture significantly declines with early aging, which is unaffected by high fat feeding/obesity. The initiation of HFD after skeletal maturity in this study sets it apart from prior investigations in the bone field.
The impact of HFD-induced obesity on bone health and marrow adipose tissue in adulthood remains uncertain. Despite a reciprocal relationship between the osteoblast and the adipocyte [41], both arising from mesenchymal stem cell (MSC) precursors, body mass, and BMD –at the level of the whole organism – do not inversely correlate [42]. Clinically, obesity associates with lower rates of hip fractures [43] and higher rates of humerus or tibial fractures [7, 8, 44], proposed to be linked to soft tissue padding at these sites, though this still is undecided. Recent analyses show a negative relationship between visceral adiposity and BMD, as opposed to subcutaneous adiposity, which had little association with BMD [45–49]. And yet, in our present translational investigation of obesity in adult mice, increased visceral adiposity did not alter BMD. This points to other factors, e.g., steroid hormones, physical activity [50], insulin sensitivity [51], and lean mass [52, 53] which may further explain clinical differences in fracture risk in the setting of obesity. Despite significant upregulation of BMAT and changes in its distribution, in tandem with white adipose accumulation, bone quantity and quality remain similar in obese and non-obese mice. As such, our experiments confirm no direct effects of BMAT induced by HFD on bone, at least at this level.
Evolutionarily conserved, age-related bone losses in early adulthood represent a knowledge gap in our understanding of osteoporosis [54, 55]. Shortly after mammals stop growing, musculoskeletal aging begins [11, 12, 56–58], a period critical for optimization of bone health before life-long declines ensue [15, 59, 60]. On the cellular level, bone-forming MSCs lose proliferative capacity as early as 7–12 weeks of age [61] like other cells [62]. Broadly, aging prompts processes including senescence, stem cell exhaustion, oxidative stress, epigenetic alterations, altered autophagy, and altered intercellular communication [63]. Senescence markers such as the cyclin-dependent kinase inhibitor P16INK4a rise in rodents, already at 12 weeks of age [64], a rise that continues into old age [61]. In the murine femur, senescence-associated β-galactosidase increases as early as 8 weeks, highlighting the fact that these pathways rise well before old age [65, 66]. While adequate calcium intake and physical activity are understood as major determinants of peak bone mass, neither can counteract age-related declines [67]. Our study compliments clinical observational data showing an association between obesity and improved bone density in adulthood [68]; however, there are no comparable interventional studies in humans since this intervention, particularly for extended periods of time, is harmful to metabolic health and thus not viable. The converse effect of weight loss via medical or surgical interventions in humans carries known, adverse effect on the skeleton [69].
In sum, our data support that obesity and higher BMAT do little to attenuate early age-related losses. Our emphasis was on female mice due to clinical relevance to post-menopausal osteoporosis. That we did not test male animals is a limitation of our work, but less of a clinical objective. Additionally, the few available bone analyses of HFD provided after skeletal maturity were in males and lend support to ones presented herein [3, 35, 70]. In terms of molecular/qPCR analyses, these are performed on a heterogenous population of cells in the bone and therefore cannot be fully attributed to MSC, adipocytes, osteoblasts, osteocytes, or hematopoietic cells. For example, AP2/FABP4 expression is a feature not only of adipocytes, but also in immune cells of the macrophage lineage [71] which may indicate that the sources of FABP4, or other transcripts in our analyses, reflect other cells than the marrow adipocyte.
This longitudinal analysis from 3 to 6 months spans from peak bone mass to the start of early, age-related bone loss, supplying data on HFD/obesity effect in adult animals. We noted significant impacts of HFD on BMAT and fat metabolism, as well as concurrent expansion of peripheral and marrow adipose stores and marrow adipocyte size. Our data confirm prior work showing that obesity – to a degree enough to change BMAT – does not meaningfully exacerbate the significant bone loss accompanying early aging, while also uniquely addressing the outcome in skeletally mature animals. While non-pharmacologic measures such as exercise [72–75] have relevance to bone health in aging, it is less likely that dietary manipulation of lipid contributes. In sum, long-term HFD augmented BMAT and fat metabolism, with no apparent effect on bone quantity or quality in skeletally mature female mice.
Statement of Ethics
Procedures and ethical guidelines of the University of North Carolina’s Institutional Animal Care and Use Committee (IACUC) were adhered throughout the study. The following body approved the use of vertebrate animals for this study: University of North Carolina’s Institutional Animal Care and Use Committee. This study protocol was reviewed and approved by UNC IACUC, approval number(s) 14-291.0, 17-270.0, and 20-208.0. The UNC Division of Comparative Medicine (DCM) also known as the Division of Laboratory Animal Medicine (DLAM) is responsible for the care of all vertebrate animals on the UNC-CH campus. The University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee (IACUC) oversees the university’s animal care and use program and is responsible for reviewing all animal care applications using vertebrate animals, ensuring compliance with federal animal welfare regulations, inspecting animal facilities and investigator laboratories, investigating animal concerns, and overseeing training and educational programs. The division’s program is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International (AAALAC) since 1973 and meets US Department of Agriculture and Public Health Service Standards and Regulations.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The following research project grant awards from the National Institutes of Health (NIH) supported the work conducted in this proposal: (PI: Styner) R01AR073264 (institute: NIAMS or National Institute for Arthritis and Musculoskeletal and Skin Diseases) and KL2TR002490 (institute: NCATS or National Center for Advancing Translational Sciences), and (PI: Rubin) R01AR075803 (institute: NIAMS or National Institute for Arthritis and Musculoskeletal and Skin Diseases).
Author Contributions
Authors approved the final manuscript. Specific author contributions in addition to manuscript approval are as follows: Cody McGrath, Sarah E. Little-Letsinger, Buer Sen, Zhihui Xie, Gunes Uzer, Guniz B. Uzer, Xiaopeng Zong, and Martin A. Styner (acquisition, analysis, or interpretation of data), Gabriel M. Pagnotti (interpretation of data, drafting the work, and providing review that is critically important for intellectual content), Janet Rubin (conception as well as interpretation of the data), and Maya Styner (conception or design of work, acquisition, analysis, or interpretation of data). Affiliations are the location where most of the work was performed if an author has more than one affiliation.
Funding Statement
The following research project grant awards from the National Institutes of Health (NIH) supported the work conducted in this proposal: (PI: Styner) R01AR073264 (institute: NIAMS or National Institute for Arthritis and Musculoskeletal and Skin Diseases) and KL2TR002490 (institute: NCATS or National Center for Advancing Translational Sciences), and (PI: Rubin) R01AR075803 (institute: NIAMS or National Institute for Arthritis and Musculoskeletal and Skin Diseases).
Data Availability Statement
All data generated or analyzed during this study are included in this article and its supplementary material files. Further inquiries can be directed to the corresponding author.
Supplementary Material
References
- 1. Reid IR. Fat and bone. Arch Biochem Biophys. 2010;503(1):20–7. [DOI] [PubMed] [Google Scholar]
- 2. Shapses SA, Pop LC, Wang Y. Obesity is a concern for bone health with aging. Nutr Res. 2017;39:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lecka-Czernik B, Stechschulte LA, Czernik PJ, Dowling AR. High bone mass in adult mice with diet-induced obesity results from a combination of initial increase in bone mass followed by attenuation in bone formation; implications for high bone mass and decreased bone quality in obesity. Mol Cell Endocrinol. 2015;410:35–41. [DOI] [PubMed] [Google Scholar]
- 4. Turcotte A-F, O’Connor S, Morin SN, Gibbs JC, Willie BM, Jean S, et al. Association between obesity and risk of fracture, bone mineral density and bone quality in adults: a systematic review and meta-analysis. PLoS One. 2021;16(6):e0252487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gandham A, Mesinovic J, Jansons P, Zengin A, Bonham MP, Ebeling PR, et al. Falls, fractures, and areal bone mineral density in older adults with sarcopenic obesity: a systematic review and meta‐analysis. Obes Rev. 2021;22(5):e13187. [DOI] [PubMed] [Google Scholar]
- 6. Turcotte A, Jean S, Morin SN, Mac‐Way F, Gagnon C. Relationships between obesity and incidence of fractures in a middle-aged population: a study from the CARTaGENE cohort. Jbmr Plus. 2023;7(5):e10730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Compston JE, Watts NB, Chapurlat R, Cooper C, Boonen S, Greenspan S, et al. Obesity is not protective against fracture in postmenopausal women: glow. Am J Med. 2011;124(11):1043–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Prieto-Alhambra D, Premaor MO, Fina Avilés F, Hermosilla E, Martinez-Laguna D, Carbonell-Abella C, et al. The association between fracture and obesity is site-dependent: a population-based study in postmenopausal women. J Bone Miner Res. 2012;27(2):294–300. [DOI] [PubMed] [Google Scholar]
- 9. Yanai S, Endo S. Functional aging in male C57bl/6J mice across the life-span: a systematic behavioral analysis of motor, emotional, and memory function to define an aging phenotype. Front Aging Neurosci. 2021;13:697621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim CR, Jeon Y-J, Kim MC, Jeong T, Koo WR. Reference values for hand grip strength in the South Korean population. PLoS One. 2018;13(4):e0195485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, et al. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem. 2007;282(37):27285–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Glatt V, Canalis E, Stadmeyer L, Bouxsein ML. Age-related changes in trabecular architecture differ in female and male C57bl/6J mice. J Bone Miner Res. 2007;22(8):1197–207. [DOI] [PubMed] [Google Scholar]
- 13. Slemenda C, Longcope C, Peacock M, Hui S, Johnston CC. Sex steroids, bone mass, and bone loss. A prospective study of pre-peri-and postmenopausal women. J Clin Invest. 1996;97(1):14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Raisz LG, Seeman E. Causes of age-related bone loss and bone fragility: an alternative view. J Bone Miner Res. 2001;16(11):1948–52. [DOI] [PubMed] [Google Scholar]
- 15. Hill C, James RS, Cox VM, Seebacher F, Tallis J. Age-related changes in isolated mouse skeletal muscle function are dependent on sex, muscle, and contractility mode. Am J Physiol Regul Integr Comp Physiol. 2020;319(3):R296–314. [DOI] [PubMed] [Google Scholar]
- 16. Kilborn SH, Trudel G, Uhthoff H. Review of growth plate closure compared with age at sexual maturity and lifespan in laboratory animals. Contemp Top Lab Anim. 2002;41(5):21–6. [PubMed] [Google Scholar]
- 17. Styner M, Thompson WR, Galior K, Uzer G, Wu X, Kadari S, et al. Bone marrow fat accumulation accelerated by high fat diet is suppressed by exercise. Bone. 2014;64:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Styner M, Pagnotti GM, McGrath C, Wu X, Sen B, Uzer G, et al. Exercise decreases marrow adipose tissue through β-oxidation in obese running mice. J Bone Miner Res. 2017;32(8):1692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McGrath C, Sankaran JS, Misaghian‐Xanthos N, Sen B, Xie Z, Styner MA, et al. Exercise degrades bone in caloric restriction, despite suppression of Marrow Adipose Tissue (MAT). J Bone Miner Res. 2020;35(1):106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116–28. [DOI] [PubMed] [Google Scholar]
- 21. Joshi S, Davis B, Jomier M, Gerig G. Unbiased diffeomorphic atlas construction for computational anatomy. Neuroimage. 2004;23(Suppl 1):S151–60. [DOI] [PubMed] [Google Scholar]
- 22. Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54(3):2033–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin J-C, Pujol S, et al. 3D slicer as an image computing platform for the quantitative imaging network. Magn Reson Imaging. 2012;30(9):1323–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van't Hof RJ, Rose L, Bassonga E, Daroszewska A. Open source software for semi-automated histomorphometry of bone resorption and formation parameters. Bone. 2017;99:69–79. [DOI] [PubMed] [Google Scholar]
- 25. Longobardi L, Li T, Myers TJ, O’Rear L, Ozkan H, Li Y, et al. TGF-β type II receptor/MCP-5 axis: at the crossroad between joint and growth plate development. Dev Cell. 2012;23(1):71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Parlee SD, Lentz SI, Mori H, MacDougald OA. Quantifying size and number of adipocytes in adipose tissue. Methods Enzymol. 2014;537:93–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McGrath C, Little-Letsinger SE, Sankaran JS, Sen B, Xie Z, Styner MA, et al. Exercise increases bone in SEIPIN deficient lipodystrophy, despite low marrow adiposity. Front Endocrinol. 2021;12:782194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R. Guidelines for assessment of bone microstructure in rodents using micro–computed tomography. J Bone Miner Res. 2010;25(7):1468–86. [DOI] [PubMed] [Google Scholar]
- 30. Kim Y, Brodt MD, Tang SY, Silva MJ. Skeletal development and repair, methods and protocols. Methods Mol Biol. 2020;2230:169–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barou O, Valentin D, Vico L, Tirode C, Barbier A, Alexandre C, et al. High-resolution three-dimensional micro-computed tomography detects bone loss and changes in trabecular architecture early: comparison with DEXA and bone histomorphometry in a rat model of disuse osteoporosis. Invest Radiol. 2002;37(1):40–6. [DOI] [PubMed] [Google Scholar]
- 32. Morton TL, Galior K, McGrath C, Wu X, Uzer G, Uzer GB, et al. Exercise increases and Browns muscle lipid in high-fat diet-fed mice. Front Endocrinol. 2016;7:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Styner M, Meyer MB, Galior K, Case N, Xie Z, Sen B, et al. Mechanical strain downregulates C/EBPβ in MSC and decreases endoplasmic reticulum stress. PLoS One. 2012;7(12):e51613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhao J, Wang C, Totton SC, Cullen JN, O’Connor AM. Reporting and analysis of repeated measurements in preclinical animals experiments. PLoS One. 2019;14(8):e0220879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ionova-Martin SS, Wade JM, Tang S, Shahnazari M, Ager JW, Lane NE, et al. Changes in cortical bone response to high-fat diet from adolescence to adulthood in mice. Osteoporos Int. 2011;22(8):2283–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Alekos N, Kushwaha P, Kim S, Li Z, Abood A, Dirckx N, et al. Mitochondrial β-oxidation of adipose-derived fatty acids by osteoblasts fuels parathyroid hormone-induced bone formation. Jci Insight. 2023;8(6):e165604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Matkovic V, Jelic T, Wardlaw GM, Ilich JZ, Goel PK, Wright JK, et al. Timing of peak bone mass in Caucasian females and its implication for the prevention of osteoporosis. Inference from a cross-sectional model. J Clin Invest. 1994;93(2):799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Flaherty SE 3rd, Grijalva A, Xu X, Ables E, Nomani A, Ferrante AW Jr. A lipase-independent pathway of lipid release and immune modulation by adipocytes. Science. 2019;363(6430):989–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alekos NS, Moorer MC, Riddle RC. Dual effects of lipid metabolism on osteoblast function. Front Endocrinol. 2020;11:578194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Clark EM, Ness AR, Tobias JH. Adipose tissue stimulates bone growth in prepubertal children. J Clin Endocrinol Metab. 2006;91(7):2534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung U, Kubota N, et al. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004;113(6):846–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Johansson H, Kanis JA, Odén A, McCloskey E, Chapurlat RD, Christiansen C, et al. A meta-analysis of the association of fracture risk and body mass index in women. J Bone Miner Res. 2014;29(1):223–33. [DOI] [PubMed] [Google Scholar]
- 43. Leslie WD, Orwoll ES, Nielson CM, Morin SN, Majumdar SR, Johansson H, et al. Estimated lean mass and fat mass differentially affect femoral bone density and strength index but are not FRAX independent risk factors for fracture. J Bone Miner Res. 2014;29(11):2511–9. [DOI] [PubMed] [Google Scholar]
- 44. Beck TJ, Petit MA, Wu G, LeBoff MS, Cauley JA, Chen Z. Does obesity really make the femur stronger? BMD, geometry, and fracture incidence in the women’s health initiative-observational study. J Bone Miner Res. 2009;24(8):1369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jain RK, Vokes T. Visceral adipose tissue is negatively associated with bone mineral density in NHANES 2011-2018. J Endocr Soc. 2023;7(4):bvad008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gilsanz V, Chalfant J, Mo AO, Lee DC, Dorey FJ, Mittelman SD. Reciprocal relations of subcutaneous and visceral fat to bone structure and strength. J Clin Endocrinol Metab. 2009;94(9):3387–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ng AC, Melton LJ, Atkinson EJ, Achenbach SJ, Holets MF, Peterson JM, et al. Relationship of adiposity to bone volumetric density and microstructure in men and women across the adult lifespan. Bone. 2013;55(1):119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhu K, Hunter M, James A, Lim EM, Cooke BR, Walsh JP. Relationship between visceral adipose tissue and bone mineral density in Australian baby boomers. Osteoporos Int. 2020;31(12):2439–48. [DOI] [PubMed] [Google Scholar]
- 49. Compston JE, Flahive J, Hosmer DW, Watts NB, Siris ES, Silverman S, et al. Relationship of weight, height, and body mass index with fracture risk at different sites in postmenopausal women: the Global Longitudinal study of Osteoporosis in Women (GLOW). J Bone Miner Res. 2014;29(2):487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Armstrong ME, Spencer EA, Cairns BJ, Banks E, Pirie K, Green J, et al. Body mass index and physical activity in relation to the incidence of hip fracture in postmenopausal women. J Bone Miner Res. 2011;26(6):1330–8. [DOI] [PubMed] [Google Scholar]
- 51. Yamaguchi T, Kanazawa I, Yamamoto M, Kurioka S, Yamauchi M, Yano S, et al. Associations between components of the metabolic syndrome versus bone mineral density and vertebral fractures in patients with type 2 diabetes. Bone. 2009;45(2):174–9. [DOI] [PubMed] [Google Scholar]
- 52. Christensen JD, Lungu AO, Cochran E, Collins MT, Gafni RI, Reynolds JC, et al. Bone mineral content in patients with congenital generalized lipodystrophy is unaffected by metreleptin replacement therapy. J Clin Endocrinol Metab. 2014;99(8):E1493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sornay-Rendu E, Boutroy S, Vilayphiou N, Claustrat B, Chapurlat RD. In obese postmenopausal women, bone microarchitecture and strength are not commensurate to greater body weight: the Os des Femmes de Lyon (OFELY) study. J Bone Miner Res. 2013;28(7):1679–87. [DOI] [PubMed] [Google Scholar]
- 54. Cameron N, Demerath EW. Critical periods in human growth and their relationship to diseases of aging. Am J Phys Anthropol. 2002;119(S35):159–84. [DOI] [PubMed] [Google Scholar]
- 55. Kralick AE, Zemel BS. Evolutionary perspectives on the developing skeleton and implications for lifelong health. Front Endocrinol. 2020;11:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jilka RL. The relevance of mouse models for investigating age-related bone loss in humans. Journals Gerontology Ser. 2013;68(10):1209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Halloran BP, Ferguson VL, Simske SJ, Burghardt A, Venton LL, Majumdar S. Changes in bone structure and mass with advancing age in the male C57BL/6J mouse. J Bone Miner Res. 2002;17(6):1044–50. [DOI] [PubMed] [Google Scholar]
- 58. Kelly A, Shults J, Mostoufi-Moab S, McCormack SE, Stallings VA, Schall JI, et al. Pediatric bone mineral accrual Z-score calculation equations and their application in childhood disease. J Bone Miner Res. 2019;34(1):195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sayer AA, Syddall H, Martin H, Patel H, Baylis D, Cooper C. The developmental origins of sarcopenia. J Nutr Health Aging. 2008;12(7):427–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cooper C, Melton LJ. Epidemiology of osteoporosis. Trends Endocrinol Metab. 1992;3(6):224–9. [DOI] [PubMed] [Google Scholar]
- 61. Stolzing A, Scutt A. Age-related impairment of mesenchymal progenitor cell function. Aging Cell. 2006;5(3):213–24. [DOI] [PubMed] [Google Scholar]
- 62. Xie K, Fuchs H, Scifo E, Liu D, Aziz A, Aguilar-Pimentel JA, et al. Deep phenotyping and lifetime trajectories reveal limited effects of longevity regulators on the aging process in C57BL/6J mice. Nat Commun. 2022;13(1):6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Little-Letsinger SE, Rubin J, Diekman B, Rubin CT, McGrath C, Pagnotti GM, et al. Exercise to mend aged-tissue crosstalk in bone targeting osteoporosis & osteoarthritis. Semin Cell Dev Biol. 2022;123:22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Safwan-Zaiter H, Wagner N, Michiels J-F, Wagner K-D. Dynamic spatiotemporal expression pattern of the senescence-associated factor p16Ink4a in development and aging. Cells. 2022;11(3):541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Li C, Chai Y, Wang L, Gao B, Chen H, Gao P, et al. Programmed cell senescence in skeleton during late puberty. Nat Commun. 2017;8(1):1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Storer M, Mas A, Robert-Moreno A, Pecoraro M, Ortells MC, Di Giacomo V, et al. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell. 2013;155(5):1119–30. [DOI] [PubMed] [Google Scholar]
- 67. Westerterp KR, Yamada Y, Sagayama H, Ainslie PN, Andersen LF, Anderson LJ, et al. Physical activity and fat-free mass during growth and in later life. Am J Clin Nutr. 2021;114(5):1583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Maïmoun L, Mura T, Leprieur E, Avignon A, Mariano-Goulart D, Sultan A. Impact of obesity on bone mass throughout adult life: influence of gender and severity of obesity. Bone. 2016;90:23–30. [DOI] [PubMed] [Google Scholar]
- 69. Gagnon C, Schafer AL. Bone health after bariatric surgery. Jbmr Plus. 2018;2(3):121–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Doucette CR, Horowitz MC, Berry R, MacDougald OA, Anunciado‐Koza R, Koza RA, et al. A high fat diet increases bone Marrow Adipose Tissue (MAT) but does not alter trabecular or cortical bone mass in C57bl/6J mice. J Cell Physiol. 2015;230(9):2032–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Liang X, Gupta K, Quintero JR, Cernadas M, Kobzik L, Christou H, et al. Macrophage FABP4 is required for neutrophil recruitment and bacterial clearance in Pseudomonas aeruginosa pneumonia. Faseb J. 2019;33(3):3562–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pagnotti GM, Adler BJ, Green DE, Chan ME, Frechette DM, Shroyer KR, et al. Low magnitude mechanical signals mitigate osteopenia without compromising longevity in an aged murine model of spontaneous granulosa cell ovarian cancer. Bone. 2012;51(3):570–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pagnotti GM, Styner M. Exercise regulation of marrow adipose tissue. Front Endocrinol. 2016;7:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pagnotti GM, Styner M, Uzer G, Patel VS, Wright LE, Ness KK, et al. Combating osteoporosis and obesity with exercise: leveraging cell mechanosensitivity. Nat Rev Endocrinol. 2019;15(6):339–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Watson SL, Weeks BK, Weis LJ, Harding AT, Horan SA, Beck BR. High-intensity resistance and impact training improves bone mineral density and physical function in postmenopausal women with osteopenia and osteoporosis: the LIFTMOR randomized controlled trial. J Bone Miner Res. 2018;33(2):211–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article and its supplementary material files. Further inquiries can be directed to the corresponding author.