Abstract
Metal-organic frameworks (MOFs) are porous, crystalline materials constructed from organic linkers and inorganic nodes with potential utility in gas separations, drug delivery, sensing, and catalysis. Small variations in MOF synthesis conditions can lead to a range of accessible frameworks with divergent chemical or photophysical properties. New methods to controllably access phases with tailored properties would broaden the scope of MOFs that can be reliably prepared for specific applications. Herein, we demonstrate that simply increasing the reaction concentration during the solvothermal synthesis of M2(dobdc) (M = Mg, Mn, Ni; dobdc4− = 2,5-dioxido-1,4-benzenedicarboxylate) MOFs unexpectedly leads to trapping of a new framework termed CORN-MOF-1 (CORN = Cornell University) instead. In-depth spectroscopic, crystallographic, and computational studies support that CORN-MOF-1 has a similar structure to M2(dobdc) but with partially protonated linkers and charge-balancing or coordinated formate groups in the pores. The resultant variation in linker spacings causes CORN-MOF-1 (Mg) to be strongly photoluminescent in the solid state, whereas H4dobdc and Mg2(dobdc) are weakly emissive due to excimer formation. In-depth photophysical studies suggest that CORN-MOF-1 (Mg) is the first MOF based on the H2dobdc2− linker that likely does not emit via an excited state intramolecular proton transfer (ESIPT) pathway. In addition, CORN-MOF-1 variants can be converted into high-quality samples of the thermodynamic M2(dobdc) phases by heating in N,N-dimethylformamide (DMF). Overall, our findings support that high-concentration synthesis provides a straightforward method to identify new MOFs with properties distinct from known materials and to produce highly porous samples of MOFs, paving the way for the discovery and gram-scale synthesis of framework materials.
Graphical Abstract

Introduction.
Selectively isolating a single phase with tailored properties from a reaction mixture in which multiple phases can form is a fundamental challenge in materials synthesis. This challenge is illustrated by metal-organic frameworks (MOFs), which are porous crystalline materials constructed from polytopic organic linkers and inorganic nodes with potential utility for chemical separations, catalysis, drug delivery, sensing, and beyond.1–4 Because metals can form multiple nodes and linkers can access distinct protonation states or coordination modes, many frameworks can potentially form from the combination of a given metal salt and linker.5–8 As such, small variations in synthesis conditions can lead to drastically different reaction outcomes. Controlling the topology of a framework is critical, as small changes in framework architecture can have a profound influence on physical properties. Due to these limitations, MOF syntheses are often optimized on a trial-and-error basis, contributing to the perception that their synthesis remains a “black box.”8–10
To ensure phase purity, MOFs are typically prepared under thermodynamic control, which involves synthesis under dilute solvothermal conditions at high reaction temperatures to maximize the reversibility of framework self-assembly.8 Unfortunately, the large solvent volumes required for traditional solvothermal synthesis necessitate the use of liters of solvent to produce grams of MOF, contributing greatly to the cost of MOFs on scale and generating significant waste.11 High-concentration solvothermal synthesis would enable MOF production with reduced solvent use, but mixtures of phases12 or low-quality materials13–16 are generally obtained under these conditions; successful syntheses of porous, phase-pure MOFs under high-concentration conditions (>0.25 M in linker) without seeding remain remarkably rare.17–20 Concentration-dependent selection between distinct MOF phases has also not been reported to date.
The MOF-74, CPO-27, or M2(dobdc) (M = Mg, Mn, Fe, Co, Ni, Cu, Zn, Cd; dobdc4− = 2,5-dioxido-1,4-benzenedicarboxylate) family of frameworks embodies the aforementioned synthetic challenges (Figure 1).21–24 These materials represent a canonical family of frameworks due to their promise for chemical separations,25,26 gas storage,23 and catalysis,27,28 yet they are typically prepared under dilute solvothermal conditions (~0.01 M). In addition, variations in solvent, temperature, metal precursor, and other synthesis parameters have been shown to produce a range of different Mg,29–35 Mn,36,37 Ni,38 Co,38–41 Cu,25 and Zn41–47 phases. Notably, some of these frameworks exhibit detectable photoluminescence (PL) in the solid state,29–31,35,36,48 demonstrating that small changes in linker protonation state or spatial orientation can have a profound effect on framework photophysical properties. A critical barrier to predicting the preferred phase(s) is a lack of chemical insight into the processes underpinning M2(dobdc) self-assembly.9,49–51 For example, while step-wise linker deprotonation (H4dobdc → H2dobdc2− → dobdc4−) through distinct phases has been established under mechanochemical conditions with basic metal precursors,44,52 its general role in M2(dobdc) formation under solvothermal conditions remains unclear.12,39
Figure 1.
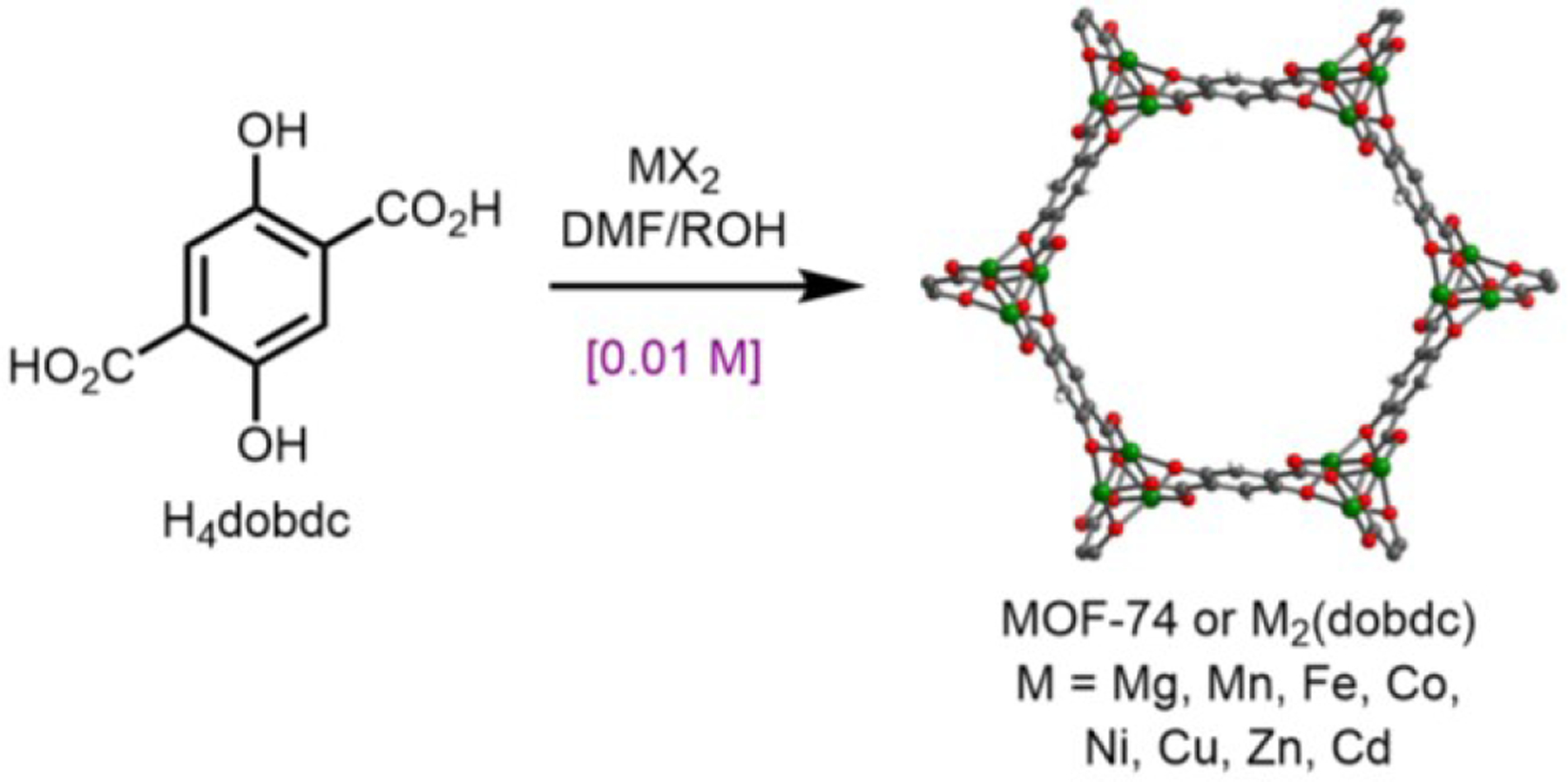
Standard synthesis of M2(dobdc) under dilute solvothermal conditions (concentration of linker shown). R = alkyl, H.
Herein, we demonstrate that increasing the linker concentration in M2(dobdc) (M = Mg, Mn, Ni) syntheses induces the clean formation of a new microporous phase, M2(HCO2)2(H2dobdc) or CORN-MOF-1 (CORN = Cornell University), with partially protonated linkers. To the best of our knowledge, this represents the first example of a phase-to-phase transition induced by changing only the concentration of a MOF synthesis. Moreover, CORN-MOF-1 (Mg) is intensely photoluminescent in the solid state, while H4dobdc and Mg2(dobdc) are nearly non-emissive. In-depth photophysical studies suggest that this is due to non-radiative excimer formation in H4dobdc and Mg2(dobdc) that is absent in CORN-MOF-1 (Mg). As such, our work demonstrates that high-concentration solvothermal synthesis provides a straightforward avenue to reduce the amount of solvent required to prepare MOFs, isolate new phases that provide mechanistic insight into MOF formation, and discover new materials with distinct (photo)physical properties compared to known thermodynamic phases.
Results and Discussion.
High concentration synthesis of CORN-MOF-1 (Mg).
Owing to its high density of coordinatively unsaturated Mg2+ sites and low molecular weight, Mg2(dobdc) exhibits high adsorption capacities for many gases.22,25,53 As such, it is a promising material for applications in chemical separations and gas storage.26,54,55 Decreasing the volume of solvent required to prepare high-quality Mg2(dobdc) would facilitate its gram-scalable synthesis, especially by non-specialists.13 However, previous reports suggest that even a slight increase in the Mg2(dobdc) synthesis concentration (up to 0.05 M in linker) without stirring leads to a mixture of non-porous phases.12 Therefore, we asked: can Mg2(dobdc) actually be synthesized at high reaction concentrations?
Solvothermal syntheses of Mg2(dobdc) in an 18:1:1 mixture of N,N-dimethylformamide (DMF), ethanol (EtOH), and H2O at 120 °C for 24 h22 were carried out at linker concentrations ranging from 0.01 M to 1.50 M with 2.50 equiv. of Mg(NO3)2·6H2O at every concentration (Figure 2a–b, Supporting Information or SI Figure S1, see SI Section 3 for details). Notably, vigorous stirring (700 rpm) was required to produce samples free from inorganic impurities such as Mg(HCO2)2.
Figure 2.

a) Synthesis of Mg2(dobdc) and/or CORN-MOF-1 (Mg) at varying linker concentration. b) PXRD patterns at increasing reaction concentrations. Transition from Mg2(dobdc) (dashed gray lines) to CORN-MOF-1 (Mg) (dashed pink lines) is indicated. The pattern corresponding to the previously reported SCXRD structure of Zn2(dobdc) is included for reference (black). c) SEM image of CORN-MOF-1 (Mg) synthesized at 0.15 M with stirring. d) SEM image of Mg2(dobdc) synthesized at 0.01 M without stirring.
At H4dobdc concentrations between 0.01 and 0.05 M, phase-pure Mg2(dobdc) was obtained, as confirmed by comparison of the PXRD pattern to that simulated from the single-crystal X-ray diffraction (SCXRD) structure of Zn2(dobdc).56 Intriguingly, simply increasing the linker concentration to 0.08 M led to contamination of Mg2(dobdc) with a new phase, which we term CORN-MOF-1 (Mg). We hypothesize that CORN-MOF-1 (Mg) is a kinetic phase closely related in structure to Mg2(dobdc).12,44 Indeed, higher reaction temperatures (140–160 °C) were found to favor Mg2(dobdc) over CORN-MOF-1 (Mg) at an intermediate linker concentration of 0.08 M (SI Figure S31). The PXRD pattern of this material does not match any known crystalline phase constructed from Mg2+ ions and H2dobdc2− or dobdc4− linkers (SI Figure S2). At reaction concentrations between 0.10 and 1.50 M, only phase-pure CORN-MOF-1 (Mg) is obtained. The transition from Mg2(dobdc) to CORN-MOF-1 (Mg) was also evident by scanning electron microscopy (SEM) (Figure 2c–d). Hexagonal needles/rods characteristic of M2(dobdc) materials24 were obtained at low concentrations (Figure 2d), whereas hexagonal plates corresponding to CORN-MOF-1 (Mg), along with other less well-defined crystallites, were obtained at higher reaction concentrations (Figure 2c, SI Figure S13). This synthesis could be reproducibly carried out on 15 mmol scale using only 10 mL of organic solvent to yield >5 g of CORN-MOF-1 (Mg) in a single batch (SI Figure S3). Notably, the same phase change was observed in 1:1 DMF:methanol (MeOH), another solvent system commonly used to prepare Mg2(dobdc) analogues,57 as confirmed by PXRD and SEM (SI Section 6). These results indicate that a clean phase change from Mg2(dobdc) to CORN-MOF-1 (Mg) is generally favored at high reaction concentrations.
Due to the unusual conditions under which CORN-MOF-1 (Mg) forms—high reaction concentrations with vigorous stirring—we have been unable to grow sufficiently large crystals of this material to enable structural elucidation by SCXRD. Attempts to determine the structure of CORN-MOF-1 (Mg) via electron diffraction were only partially successful (SI Section 16). The collected reflections match well to those observed by PXRD, but only a partial data set could be collected due to the anisotropic nature of the plate-like crystals of CORN-MOF-1 (Mg) (Figure 2c). Therefore, the structure of this MOF was interrogated using PXRD (Figure 3) and a host of spectroscopic and analytical methods (Figure 4).
Figure 3.

Pawley refinement of the PXRD pattern (λ = 1.5406 Å) of CORN-MOF-1 (Mg). The shown fit corresponds to the space group with a = 27.612(2) Å and c = 7.2853(3) Å. Rwp = 3.85%. The black ticks indicate calculated Bragg peak positions.
Figure 4.

a) ATR-IR spectra of Mg2(dobdc) and CORN-MOF-1 (Mg). b) 77 K N2 adsorption (closed circles) and desorption (open circles) isotherms of activated CORN-MOF-1 (Mg). Inset: DFT-calculated pore size distribution of CORN-MOF-1 (Mg), assuming a cylindrical pore shape with a metal oxide surface. c) 1H and d) CP 13C MAS SSNMR spectra of Mg2(dobdc) and CORN-MOF-1 (Mg). All SSNMR data were collected at a field strength of 9.4 T and a MAS rate of 25 kHz.
Pawley refinement of the PXRD pattern of CORN-MOF-1 (Mg) prepared at a reaction concentration of 0.1 M revealed that it could be fit well (Rwp = 3.85%) as a single phase in the space group (the same space group as Mg2(dobdc)) with a = 27.612(2) Å and c = 7.2853(3) Å (Figure 3, SI Section 18). We note that the R3, , R32, R3m, and space groups cannot be differentiated using PXRD alone. These unit cell parameters are similar to those previously reported for Mg2(dobdc) (, a = 25.865(4) Å, c = 6.911(1) Å)58 but slightly expanded in a and c, which accounts for the lower-angle first reflection of CORN-MOF-1 (Mg) compared to Mg2(dobdc) (Figure 2b). Similar parameters were determined for samples prepared at reaction concentrations of 0.2 and 1.5 M (SI Section 18).
We have consistently observed that the lowest angle reflection in the PXRD pattern of well-activated M2(dobdc) frameworks (2θ ≈ 6.8°), which corresponds to the plane, is the most intense reflection (see SI Figure S28 for an example). Accordingly, this plane contains a large portion of the linker molecules. In contrast, the most or second-most intense reflection in the PXRD pattern of CORN-MOF-1 (Mg) is generally observed at 2θ ≈ 12.8 °, which corresponds to the (101) plane. The reflection is found on the right shoulder of this reflection and may contribute to its overall intensity, but its contribution is likely minimal because their combined peak shape is that of a well-defined single reflection originating from the (101) plane. In the space group, the (101) plane is roughly perpendicular to the c axis and the plane is parallel to it, meaning that in Mg2(dobdc) the (101) plane includes the pore volume and the plane draws a narrow line through it. As such, this qualitative argument indicates that CORN-MOF-1 (Mg) likely contains significant electron density within the framework pores (i.e., more atoms in the (101) plane) that is absent in Mg2(dobdc). Unfortunately, the disordered nature of this electron density precludes exact structure solution by PXRD.
In order to gain further insight into the chemical species occupying the pores of CORN-MOF-1 (Mg), this material was characterized by attenuated total reflectance infrared (ATR-IR) spectroscopy after extensive washing with MeOH to remove residual DMF from the pores (Figure 4a, SI Figure S4 and Table S2). CORN-MOF-1 (Mg) contains an additional carbonyl C=O stretch (1655 cm–1) that is absent in Mg2(dobdc). The same C=O stretch (1644 cm−1) was observed by transmission IR after activation of CORN-MOF-1 (Mg) under vacuum (SI Figure S5), suggesting that it does not correspond to trapped solvent. Based on its frequency,59 we hypothesize that this additional carbonyl stretch is due to formate (HCO2−). Indeed, acidic digestion of CORN-MOF-1 (Mg) reproducibly yielded a 1:2 mixture of H4dobdc and formic acid, as confirmed by solution-state 1H and 13C NMR (SI Figures S6–8). Residual DMF was not detected, nor was a significant amount of HCO2H observed in an acid-digested sample of Mg2(dobdc) prepared under traditional solvothermal conditions (SI Figure S8). Accounting for charge-balancing and the observed formate:linker ratio, these findings are consistent with a potential (desolvated) molecular formula of Mg2(HCO2)2(H2dobdc) for CORN-MOF-1 (Mg). Combustion elemental analysis of this material yielded C (32.09%) and H (3.49%) values that are in reasonable agreement with the molecular formula of Mg2(HCO2)2(H2dobdc)(H2O)2 (32.39% C, 2.72% H), assuming partial hydration under ambient conditions (SI Table S3). This molecular formula would indicate that the phenols of the linker are protonated in CORN-MOF-1 (Mg), with charge-balancing or coordinated formate groups in the pores. The observed formates likely result from DMF decomposition during MOF formation.60 Consistently, CORN-MOF-1 (Mg) could be prepared in N,N-diethylformamide but not in N,N-dimethylacetamide at any concentration (SI Figures S36–37).
The presence of additional species within the pores of CORN-MOF-1 (Mg) is further supported by 77 K N2 adsorption measurements (Figure 4b). Soaking as-synthesized CORN-MOF-1 (Mg) in MeOH and dichloromethane enabled its desolvation under high vacuum at 30 °C without significant loss in crystallinity (SI Figure S12). The 77 K N2 adsorption/desorption isotherms of CORN-MOF-1 (Mg) confirm that it is microporous, with Brunauer–Emmett–Teller (BET) and Langmuir surface areas of 403 m2/g and 479 ± 1 m2/g, respectively (Figure 4b, SI Figures S9–10). CORN-MOF-1 (Mg) is the highest surface area Mg-based MOF containing H2dobdc2− linkers that has been reported to date (SI Table S11). The BET surface area of this material is smaller than that reported for Mg2(dobdc) (1743 m2/g).54 Likewise, the density functional theory (DFT)-calculated pore size distribution of CORN-MOF-1 (Mg) assuming a cylindrical pore shape with a metal oxide surface is ~9.9 Å (Figure 4b inset, SI Figure S11), which is smaller than the crystallographically determined pore aperture of Mg2(dobdc) (11 Å).58 Together, these findings support that CORN-MOF-1 (Mg) possesses smaller guest-accessible pores than Mg2(dobdc), likely due to partial pore-filling by formate groups.
The presence of additional charge-balancing formate groups in CORN-MOF-1 (Mg) was corroborated by magic angle spinning (MAS) 1H and cross-polarized (CP) 13C solid-state NMR (SSNMR) spectroscopies (Figure 4c–d, SI Section 14). As expected, the 1H and 13C MAS SSNMR spectra of Mg2(dobdc) and CORN-MOF-1 (Mg) possess several resonances that can be assigned to the aromatic linker. Additionally, the 1H and 13C MAS SSNMR spectra of CORN-MOF-1 (Mg) contain resonances at 9 ppm and 164 ppm, respectively, that are absent from the corresponding spectra of Mg2(dobdc). These resonances can be assigned to the extra formate groups in CORN-MOF-1 (Mg).61 The 1H MAS SSNMR spectrum of CORN-MOF-1 (Mg) contains another additional resonance at 11.6 ppm, which likely corresponds to phenol groups on the linker.62 Together with the IR and PXRD data, the SSNMR spectra suggest that CORN-MOF-1 (Mg) is structurally similar to Mg2(dobdc) but with additional formate groups in the pores along with partially protonated linker molecules.
Further mechanistic studies support the presence of protonated phenols and charge-balancing formate groups in CORN-MOF-1 (Mg) (SI Section 6). The addition of acid (benzoic, acetic, or pivalic acid) to the standard Mg2(dobdc) synthesis conditions was found to induce the formation of CORN-MOF-1 (Mg) at a relatively low concentration of 0.05 M (SI Figure S38). This is consistent with one of the expected consequences of increasing the reaction concentration, which is to decrease the amount of basic N,N-dimethylamine generated in situ from DMF decomposition (relative to the linker), and with previous studies suggesting that H4dobdc is deprotonated step-wise during the self-assembly of M2(dobdc).12,44 The addition of acid likely consumes some of the generated N,N-dimethylamine, favoring partially deprotonated H2dobdc2− (as in CORN-MOF-1) over fully deprotonated dobdc4− (as in MOF-74).
First-principles DFT calculations were carried out to evaluate potential structures for CORN-MOF-1 (Mg) (SI Section 17). A structural model in which each Mg2+ center is bound by one bidentate and one bridging formate group (Structure A, SI Figure S97) shows the best match to the experimental SSNMR and IR data but a poor match to the experimental PXRD data (SI Figures S99–S101), suggesting that this model accurately reflects the chemical environment of the formates but not their spatial arrangement in the MOF. The mismatch between the experimental and predicted PXRD data may also originate from disordered solvent and/or ligand molecules present in the pores. The disordered pore environment of CORN-MOF-1 (Mg) hinders structural modelling and elucidation by PXRD and remains an active area of investigation in our groups.
Photophysical analysis of H4dobdc, CORN-MOF-1 (Mg), and Mg2(dobdc).
The presence of partially protonated linkers in CORN-MOF-1 (Mg) should make this material photoluminescent in the solid state. The H2dobdc2− linker has been used to prepare a range of photoluminescent MOFs, which are generally proposed to emit via an excited state intramolecular proton transfer (ESIPT) pathway (Figure S10).29–31,35,36,48,63–66 Consistently, CORN-MOF-1 (Mg) fluoresces intensely upon irradiation with UV light in the solid state, whereas Mg2(dobdc) and H4dobdc emit only weakly (Figure 5a). This initial observation demonstrates how subtle changes in MOF structure—stemming from a simple increase in the synthesis concentration—can significantly modulate MOF photophysical properties. Notably, CORN-MOF-1 (Mg) possesses a higher BET surface area (403 m2/g) than all reported photoluminescent MOFs constructed from the H2dobdc2− linker (Table S10). In addition, these alternative photoluminescent phases are typically identified by laboriously changing the metal salt, solvent, reaction temperature, and synthesis time (Table S10), whereas CORN-MOF-1 can be obtained by a simple change in reaction concentration. To understand why CORN-MOF-1 (Mg) fluoresces strongly in the solid state but Mg2(dobdc) and H4dobdc do not, detailed PL measurements were carried out (Figures 6–7, SI Section 15).
Figure 5.

a) Visual comparison of H4dobdc (left), CORN-MOF-1 (Mg) (center), and Mg2(dobdc) (right) upon irradiation with UV light (230 nm), confirming the uniquely strong solid-state photoluminescence of CORN-MOF-1 (Mg). b) Relative PL intensities of CORN-MOF-1 (Mg), Mg2(dobdc), and H4dobdc powder.
Figure 6.

a) Normalized, solvent-dependent PL spectra for H4dobdc (0.1 mM). Normalized, solvent-dependent PL spectra for suspensions (1 mg/mL) of b) Mg2(dobdc) and c) CORN-MOF-1 (Mg). d) Spectral slices of the H4dobdc powder time-resolved PL capturing prompt emission (0 ns) and delayed excimer emission (5 ns) as a function of temperature. e) Equivalent spectral slices of the Mg2(dobdc) time-resolved PL capturing prompt (0 ns) and delayed excimer (3 ns) emission. Temperature-dependent spectra for H4dobdc and Mg2(dobdc) are normalized to the prompt PL signal to demonstrate how the excimer PL intensity increases at lower temperature due to the suppressed nonradiative decay. Time zeroes were set at the point of maximum PL intensity, and gate widths of 1 ns were used. f) Normalized, temperature-dependent steady state PL of CORN-MOF-1 (Mg). THF = tetrahydrofuran, iPrOH = isopropanol, rt = room temperature (295 K).
Figure 7.

a) PL decay curves for 0.1 mM H4dobdc in THF (dark green triangles), CORN-MOF-1 (Mg) powder (blue circles), H4dobdc powder (light green triangles), and Mg2(dobdc) powder (red squares). Decay curves were extracted by integrating over the entire PL spectrum. b) Spectral evolution of H4dobdc powder within the first 3 ns. c) Spectral evolution of Mg2(dobdc) within the first 1.5 ns. The excimer formation is near the 1 ns resolution of the ICCD. d) Spectral evolution of CORN-MOF-1 (Mg) within the first 25 ns.
We acquired steady-state PL spectra of H4dobdc and the two MOFs dissolved or suspended in a range of solvents to evaluate the potential role of the ESIPT pathway (Figure 6a–c). As expected, the PL line-shape of H4dobdc is highly solvent dependent, consistent with the ESIPT mechanism (Figure 6a, see SI Section 15 for further discussion). Crucially, there are no such changes in the UV-Vis absorption line-shape across this series, supporting that solvent polarity and hydrogen-bonding ability predominantly affect the excited state (i.e., not the ground state) of this molecule (SI Figure S80–81). For Mg2(dobdc) suspended in various solvents, the weak PL is essentially independent of solvent polarity—except for MeOH or H2O, in which the MOF fully or partially degrades (Figure 6b, SI Figure S79). This result is expected, as Mg2(dobdc) does not have free phenols and thus cannot emit via an ESIPT pathway. Surprisingly, the emission of CORN-MOF-1 (Mg) is similarly solvent-independent (Figure 6c). This suggests that the strong PL either does not occur via ESIPT or that the phenol groups are not accessible to solvent molecules, potentially because they are blocked by formates (see SI Section 15 for further discussion). Notably, CORN-MOF-1 (Mg) also (partially) degrades in MeOH and H2O (SI Figures S79, S104), precluding the collection of reliable PL data in these solvents.
We evaluated the temperature-dependent PL using a gated intensified CCD camera with 480 ps resolution (Figure 6d–f). Unlike common single-wavelength techniques such as time-correlated single photon counting, this approach permits sensitive, direct detection of the full PL spectral evolution over the decay lifetime.67 The strong emission of H4dobdc in solution is quenched in the solid state (Figure 5). Intermolecular interactions evidently prevent the ESIPT mechanism, and rapid non-radiative decay dominates in solid H4dobdc. This effect manifests spectrally as a rapid transition into a weak, red-shifted state. The relative strength of this emission is significantly increased upon cooling (Figure 6d), indicating a suppression of a major non-radiative decay channel. This behavior is typical of excimers.68 The same basic behavior—fast conversion into a red-shifted excimer state—is observed in Mg2(dobdc) powder as well (Figure 6e), indicating that the non-emissive nature of this MOF stems from rapid quenching into a dark excimer. The degree of red-shift in the excimer is smaller in Mg2(dobdc) than in H4dobdc powder, indicating that intermolecular interactions are weaker in Mg2(dobdc). This is likely due to the larger spacing between linkers in the porous MOF compared to the dense H4dobdc solid (SI Figure S85). The excimer in Mg2(dobdc) also progressively blue-shifts on cooling. This effect has previously been observed in molecular films and is linked to the charge-transfer character of the excimer,68 although its detailed origin remains unclear. No change in the prompt PL is observed at lower temperatures in either case. In contrast to both of these materials, the steady-state PL of CORN-MOF-1 (Mg) is essentially temperature-independent (Figure 6f), indicating that excimers do not play a major role in the photophysics of this material.
These observations are supported by analysis of the decay lifetimes and detailed spectral evolutions (Figure 7). In THF (0.1 mM), H4dobdc exhibits single-component exponential decay, consistent with the ESIPT mechanism (Figure 7a, see SI Figure S83 for other solvents). Both H4dobdc powder and Mg2(dobdc) demonstrate two-component exponential decay, with a faster and a slower time constant relative to the free linker (Figure 7a, Table S9). Consistent with our assignment above and with previous reports,69–73 we assign the faster time constant to quenching of the S1→S0 emission, while the longer lifetime corresponds to emission from an excimer. The significantly shortened τ1 in Mg2(dobdc) and H4dobdc powder explains why these samples do not visually appear bright under UV irradiation. This rapid decay is accompanied by a clear red-shift and peak broadening, hallmarks of excimer formation in Mg2(dobdc) and H4dobdc powder (Figure 7b–c). This transition is better resolved in H4dobdc powder than in Mg2(dobdc) because the excimer formation process is slower in H4dobdc than in Mg2(dobdc) (Table S9). Excimer formation is common in pure organic films/powders, and the close packing of dobdc4− linkers in Mg2(dobdc) likely accounts for excimer formation in this material (SI Figure S85).24,74 Together, these photophysical data support that excimer formation quenches the PL of Mg2(dobdc) and H4dobdc powder, and that dissolving H4dobdc prevents excimer formation and allows for bright emission via an ESIPT mechanism.
Importantly, these features of excimer formation are entirely absent in the time-resolved PL spectra of CORN-MOF-1 (Mg) (Figure 7d). Instead, we observe the same spectral shape from initial excitation out to longer timescales. In this sense, the spectral features of CORN-MOF-1 (Mg) most closely resemble those of dissolved H4dobdc (Figure S82). Because CORN-MOF-1 (Mg) does not show any evidence of excimer formation, the spacing of linker molecules must be far enough apart on average for it to behave like a network of non-interacting linker molecules.30 Thus, the emission mechanism of CORN-MOF-1 (Mg) is fundamentally different than all other reported H2dobdc2−-based MOFs (Table S10). The non-single component decay kinetics reflect disorder in the environment of the emitting molecules, for instance due to defect or surface sites or variable coupling of the protonated linker molecules to formates within the pores. The PL decay curve for CORN-MOF-1 (Mg) can be equally fit to a stretched exponential function, indicating only a small degree of energetic disorder (Figure S86). However, anisotropy measurements show no resolvable energy transfer, in line with lack of spectral diffusion during PL decay (SI Figure S87).
In addition to the rapid dynamics observed in these MOFs, blue-shifted emission was also detected on much longer timescales (SI Figures S86–89). This emission is extremely weak and could possibly arise from upconversion in the MOFs. This phenomenon remains an active area of investigation in our groups.
Overall, these findings support that high-concentration synthesis provides a straightforward means to “turn on” the photoluminescence of M2(dobdc) derivatives.29–31,35,36 The rare combination of porosity and strong solid-state fluorescence in a Mg-based MOF (Table S10) makes CORN-MOF-1 (Mg) well-suited for applications in sensing and solid-state lighting that are not available to the parent Mg2(dobdc).75–77
Conversion of CORN-MOF-1 to M2(dobdc).
Our structural and photophysical studies thus far indicate that CORN-MOF-1 (Mg) is akin to partially formed Mg2(dobdc), with the linker phenol groups protonated and charge-balancing and/or coordinating formate groups in the pores. Previous studies have detected phases with H2dobdc2− linkers during MOF self-assembly, but it remains unclear if they can generally be converted to the corresponding M2(dobdc) frameworks under synthetically relevant conditions.12,34,39 We hypothesized that CORN-MOF-1 could potentially be converted into Mg2(dobdc) by treatment with an appropriate base to fully deprotonate the linkers, such as N,N-dimethylamine generated from the decomposition of DMF (SI Section 4).
Consistent with this hypothesis, heating CORN-MOF-1 (Mg) in DMF at 150 °C for 5 days allowed for its conversion into phase-pure Mg2(dobdc), as confirmed by PXRD and a distinct color change from tan to yellow (Figure 8a–b). Conversion of large hexagonal plates of CORN-MOF-1 (Mg) into rods of Mg2(dobdc) could also be monitored over time by SEM (Figure 8c–f), confirming that this process occurs as a solid-to-solid transformation (SI Figures S18−19, S22). After soaking in MeOH to remove soluble impurities, the formate groups originally present in CORN-MOF-1 (Mg) were also absent in converted Mg2(dobdc) by ATR-IR (SI Figure S24). The overall process of synthesizing CORN-MOF-1 (Mg) and converting it into Mg2(dobdc) could be carried out on at least 10 mmol scale in good yield (4.23 g, 87% yield), in contrast to the traditional solvothermal synthesis of Mg2(dobdc), which failed on >1 g scale in our hands (SI Section 5).22
Figure 8.

a) Synthesis of Mg, Mn and Ni analogues of CORN-MOF-1, and M2(dobdc) converted from the corresponding CORN-MOF-1 analogues. b) PXRD patterns of CORN-MOF-1 (Mg, Mn and Ni) and the converted M2(dobdc) (M = Mg, Mn and Ni) analogues. SEM images of the conversion of CORN-MOF-1 (Mg) hexagonal plates to Mg2(dobdc) rods by heating in DMF at 150 °C after c) 0 h, d) 24 h, e) 72 h, f) 120 h. R = alkyl, H.
After desolvation of the converted Mg2(dobdc), its porosity was assessed using 77 K N2 adsorption/desorption isotherms (Table 1, SI Figures S25–26). Intriguingly, its BET (1829 ± 3 m2/g) and Langmuir (2060 ± 2 m2/g) surface areas were found to be slightly higher than the BET (1743 m2/g)54 and Langmuir (1957 ± 2 m2/g)25 surface areas reported for this material. Overall, these results suggest that the two-step synthesis of Mg2(dobdc) has improved scalability and leads to higher surface area MOF compared to the traditional solvothermal synthesis under dilute conditions.
Table 1.
BET surface areas of M2(dobdc) samples prepared from the corresponding CORN-MOF-1 analogues.
Generalization to other M2(dobdc) variants.
One of the most intriguing features of the M2(dobdc) family is the breadth of cations that can be incorporated into this framework.21–24 As such, we examined the generality of CORN-MOF-1 formation at high reaction concentrations with different metals (Figure 8). CORN-MOF-1 (Mn) also forms during attempted high-concentration syntheses of Mn2(dobdc) (Figure 8b, SI Figure S39, SI Section 7). This material could be converted into Mn2(dobdc) by heating in DMF (Figure 8b, SI Section 8). Consistent with the results observed for the Mg analogue, the BET surface area of Mn2(dobdc) prepared from CORN-MOF-1 (Mn) was higher (1165 ± 9 m2/g, Table 1, SI Figure S56–57) than that reported previously for this MOF (1102 m2/g).22 As previous studies into the mechanism of M2(dobdc) formation have focused on the Mg, Co, Ni, and Zn congeners,12,39,44,52 this represents the first experimental evidence that Mn2(dobdc) likely also forms via step-wise deprotonation of the linker.
Carrying out the synthesis of Ni2(dobdc) at high reaction concentrations consistently produced a mixture of CORN-MOF-1 (Ni) and Ni2(dobdc) (Figure 8b, SI Figure S63, SI Section 10). The observation of Ni2(dobdc) even at concentrations as high as 1.5 M is likely due to the poor reversibility of Ni2(dobdc) formation, which arises from the strong Ni–O bonds comprising this MOF. Once again, phase-pure Ni2(dobdc) could be prepared on multi-gram scale from the mixture containing CORN-MOF-1 (Ni) (Figure 8b, SI Section 11) with a higher BET surface area (1270 ± 1, Table 1, SI Figures S71–72) than material prepared by the traditional solvothermal route (1218 m2/g).54 Ni2(dobdc) prepared via CORN-MOF-1 (Ni) also exhibits better-defined crystallites by SEM compared to material prepared under traditional conditions (SI Figure S68). Overall, these findings suggest that the phase change to CORN-MOF-1 at higher reaction concentrations is general among M2(dobdc) frameworks. The large scales at which these two-step syntheses furnish high-quality M2(dobdc) without the requirement for specialized equipment gives this method an edge over state-of-the-art alternatives, such as mechanochemical syntheses that require ball mills.38,44,78,79
A break in this trend was observed for the Zn congener (SI Section 13). Although CORN-MOF-1 (Zn) was not observed, its attempted synthesis at a linker concentration of 1.0 M yielded the known one-dimensional polymer Zn2(H2dobdc) instead (SI Figure S77).44,46 Previous studies suggest that this material is a kinetic intermediate formed en route to Zn2(dobdc) under mechanochemical conditions.44 Thus, its formation under similar conditions as CORN-MOF-1 (Mg, Mn, Ni) further supports that the CORN-MOF-1 congeners are formed during the solvothermal syntheses of the corresponding M2(dobdc) analogues. CORN-MOF-1 (Zn) is likely not formed due to the lability of Zn–O bonds compared to Mg–O, Mn–O, and Ni–O bonds, and the flexibility of Zn coordination complexes. The observed 1D polymer Zn2(H2dobdc), which has an octahedral geometry with two weakly bound solvent molecules, is likely more stable than the analogous CORN-MOF-1 phase.
CONCLUSION
Herein, we have demonstrated that simply increasing the concentration of the solvothermal synthesis of M2(dobdc) analogues (M = Mg, Mn, Ni, Zn) up to 150-fold leads to a new phase, CORN-MOF-1, with protonated phenol groups and charge-balancing and/or coordinated formate groups in the pores. This finding supports previous findings that step-wise linker deprotonation occurs during the self-assembly of M2(dobdc) materials12,39,44,52 and implicates formate as a non-innocent species during MOF formation in formamide solvents.60 Critically, CORN-MOF-1 (Mg) is highly photoluminescent in the solid-state, whereas closely-related H4dobdc and Mg2(dobdc) are only weakly emissive, which photophysical studies confirm is due to the suppression of dark excimer formation in CORN-MOF-1 (Mg). Notably, CORN-MOF-1 (Mg) is the first framework based on the H2dobdc2− linker that is not proposed to emit via an ESIPT pathway, broadening the scope of photophysical properties that can be unlocked using these materials. Together, these findings support that high-concentration synthesis represents an underutilized method to gain mechanistic insight into the pathways of MOF self-assembly and to identify new phases with distinct (photo)physical properties from known materials.
In addition, we have shown that kinetic phases formed at high concentrations can be converted into the known thermodynamic M2(dobdc) materials. The produced frameworks are highly crystalline and possess BET surface areas that are consistently higher than those obtained by traditional solvothermal synthesis. This novel two-step synthetic approach is amenable to the multigram-scale synthesis of M2(dobdc) (M = Mg, Mn, Ni) materials without the need for specialized equipment.
We anticipate that these findings will guide the development of high-concentration syntheses of other MOFs. Two desirable outcomes can be envisaged: the formation of the same phase as the reaction concentration is increased, which allows for MOFs to be prepared with significantly reduced solvent use, or the formation of new phases with distinct properties from known combinations of linkers and metal salts. In the latter case, changing the concentration of a MOF synthesis is much simpler than evaluating dozens of conditions to produce a new MOF phase from a given linker-metal combination. As such, high-concentration solvothermal synthesis should be added to the growing lexicon of methods available for the synthesis of MOFs and other metal-organic materials. Future work will focus on growing high-quality single-crystals of CORN-MOF-1 variants to enable their structure determination and investigating the high-concentration solvothermal syntheses of other MOFs.
Experimental
Characterization Details.
Full details of all measurements are provided in the SI. ATR-IR spectra were collected on a Bruker Tensor II spectrometer equipped with a diamond ATR attachment. Surface area data were collected on either a Micromeritics ASAP 2020 or a Micromeritics 3-Flex gas sorption analyzer using ultrapure N2 (99.999%) and a liquid N2 bath. Laboratory PXRD data were collected on a Rigaku Ultima IV diffractometer equipped with a Cu Kα source (λ = 1.5406 Å). 1H NMR data were collected on a Bruker INOVA 500 MHZ spectrometer and are referenced to residual solvent. Thermogravimetric analysis (TGA) experiments were conducted using a TA Instruments TGA Q500 under a flow of dry N2 (60 mL/min). Masses are uncorrected for buoyancy effects. Differential scanning calorimetry (DSC) measurements were conducted using a TA Instruments Q1000 modulated differential scanning calorimeter (MDSC) under an atmosphere of dry He.
SSNMR experiments were performed at a field strength of 9.4 T (400 MHz for 1H) using a Bruker Avance I console with a 2.5 mm magic angle spinning (MAS) probe. All SSNMR experiments were performed at a MAS rate of 25 kHz. 1H NMR spectra were recorded via a simple pulse-acquire sequence, with the recycle delay adjusted to yield quantitative data. A 1H probe background spectrum was also recorded and subtracted from the data to give the final spectra. 13C NMR spectra were obtained by cross polarization (CP) from 1H with a contact time of 1 ms (unless otherwise specified) and with continuous wave 1H decoupling. NMR spectra were referenced to adamantane at 1.8 ppm for 1H and at 38.5 ppm for 13C (left-hand resonance).
SEM images were collected at 1.0 kV using a Zeiss Gemini 500 Scanning Electron Microscope. The powder samples were immobilized on a carbon tape mounted on an aluminum stub. The samples were blown using compressed air to remove excess material not stuck to the tape and then were coated with a carbon layer on samples dried at 30 °C prior to analysis.
Detailed procedures for PL and TEM measurements, as well as DFT calculations, are provided in the SI.
Representative synthesis of CORN-MOF-1 (Mg).
A 75 mL screw-cap high-pressure flask equipped with a stir bar was charged with Mg(NO3)2•6H2O (9.62 g, 37.5 mmol, 2.50 equiv.), H4dobdc (2.97 g, 15.0 mmol, 1.00 equiv.), DMF (9.0 mL), ethanol (0.5 mL), and water (0.5 mL). The flask was sealed and placed in a silicone oil bath, and the reaction mixture was heated to 120 °C while stirring vigorously (1000 rpm). The reaction mixture was allowed to stir at 120 °C for 24 h. At this time, the reaction mixture was allowed to cool to room temperature and filtered. The resulting solid was washed thoroughly with DMF (100 mL) and MeOH (100 mL). Drying under vacuum yielded CORN-MOF-1 (Mg) as a tan solid (~6.6 g).
Supplementary Material
ACKNOWLEDGMENT
We thank Drs. Craig Brown and Benjamin Trump (NIST) for assistance with analyzing PXRD data and helpful discussions. We thank Kaitlyn Keasler (Cornell University) for helpful discussions and for reproducing the synthesis of CORN-MOF-1 (Mg).
Funding Sources
The development of scalable routes to prepare M2(dobdc) analogues was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number R35GM138165 (A.H., T.A.P., M.N., J.J.F.R., P.J.M.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We acknowledge the support of a Camille Dreyfus Teacher-Scholar Award to P.J.M. (TC-23–048). Further support was provided by a Cornell University College of Arts and Sciences New Frontiers Grant awarded to P.J.M. and A.J.M. A.J.M. acknowledges the donors of the American Chemical Society Petroleum Research Fund for partial support of this research. J.O. was supported by the National Institute of General Medical Sciences under award F32GM143925. J.-H.L. was supported by the KIST Institutional Program (Project No. 2E32531) and the National Center for Materials Research Data (NCMRD) (Project No. 2021M3A7C2089739) and the program of Future Hydrogen Original Technology Development (Project No. 2021M3I3A1083946), through the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT. Computational resources provided by KISTI Supercomputing Center (Project No. KSC-2020-CRE-0361). T.R. acknowledges the support of the Welch Foundation (Grant No.: N-2012–20220331). We acknowledge support from a UKRI Future Leaders Fellowship to A.C.F. (MR/T043024/1). Z. S. and D.A.M. acknowledge support from the Center for Alkaline-Based Energy Solutions (CABES), an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Basic Energy Sciences, under Award DE-SC0019445. This work made use of the Cornell Center for Materials Research Shared Facilities, which are supported through the NSF MRSEC program (DMR-1719875). 1H NMR data were collected on a Bruker INOVA 500 MHz spectrometer that was purchased with support from the NSF (CHE-1531632).
Footnotes
ASSOCIATED CONTENT
Supporting Information. Details of synthesis and characterization of all MOFs, PL measurements, and DFT calculations. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- (1).Bavykina A; Kolobov N; Khan IS; Bau JA; Ramirez A; Gascon J Metal–Organic Frameworks in Heterogeneous Catalysis: Recent Progress, New Trends, and Future Perspectives. Chem. Rev 2020, 120 (16), 8468–8535. 10.1021/acs.chemrev.9b00685. [DOI] [PubMed] [Google Scholar]
- (2).Sun Y; Zheng L; Yang Y; Qian X; Fu T; Li X; Yang Z; Yan H; Cui C; Tan W Metal–Organic Framework Nanocarriers for Drug Delivery in Biomedical Applications. Nano-Micro Lett 2020, 12 (1), 103. 10.1007/s40820-020-00423-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Furukawa H; Cordova KE; O’Keeffe M; Yaghi OM The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341 (6149), 1230444–1230444. 10.1126/science.1230444. [DOI] [PubMed] [Google Scholar]
- (4).Li J-R; Sculley J; Zhou H-C Metal–Organic Frameworks for Separations. Chem. Rev 2012, 112 (2), 869–932. 10.1021/cr200190s. [DOI] [PubMed] [Google Scholar]
- (5).Gong X; Noh H; Gianneschi NC; Farha OK Interrogating Kinetic versus Thermodynamic Topologies of Metal–Organic Frameworks via Combined Transmission Electron Microscopy and X-Ray Diffraction Analysis. J. Am. Chem. Soc 2019, 141 (15), 6146–6151. 10.1021/jacs.9b01789. [DOI] [PubMed] [Google Scholar]
- (6).Pang J; Yuan S; Qin J; Liu C; Lollar C; Wu M; Yuan D; Zhou H-C; Hong M Control the Structure of Zr-Tetracarboxylate Frameworks through Steric Tuning. J. Am. Chem. Soc 2017, 139 (46), 16939–16945. 10.1021/jacs.7b09973. [DOI] [PubMed] [Google Scholar]
- (7).Ma J; Tran LD; Matzger AJ Toward Topology Prediction in Zr-Based Microporous Coordination Polymers: The Role of Linker Geometry and Flexibility. Cryst. Growth Des 2016, 16 (7), 4148–4153. 10.1021/acs.cgd.6b00698. [DOI] [Google Scholar]
- (8).Stock N; Biswas S Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites. Chem. Rev 2012, 112 (2), 933–969. 10.1021/cr200304e. [DOI] [PubMed] [Google Scholar]
- (9).Van Vleet MJ; Weng T; Li X; Schmidt JR In Situ, Time-Resolved, and Mechanistic Studies of Metal–Organic Framework Nucleation and Growth. Chem. Rev 2018, 118 (7), 3681–3721. 10.1021/acs.chemrev.7b00582. [DOI] [PubMed] [Google Scholar]
- (10).Yuan S; Qin J-S; Li J; Huang L; Feng L; Fang Y; Lollar C; Pang J; Zhang L; Sun D; Alsalme A; Cagin T; Zhou H-C Retrosynthesis of Multi-Component Metal−organic Frameworks. Nat. Commun 2018, 9 (1), 808. 10.1038/s41467-018-03102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Ryu U; Jee S; Rao PC; Shin J; Ko C; Yoon M; Park KS; Choi KM Recent Advances in Process Engineering and Upcoming Applications of Metal–Organic Frameworks. Coord. Chem. Rev 2021, 426, 213544. 10.1016/j.ccr.2020.213544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Du Bois DR; Wright KR; Bellas MK; Wiesner N; Matzger AJ Linker Deprotonation and Structural Evolution on the Pathway to MOF-74. Inorg. Chem 2022, 61, 11, 4550–4554. 10.1021/acs.inorgchem.1c03988. [DOI] [PubMed] [Google Scholar]
- (13).Du Bois DR; Matzger AJ Metal–Organic Framework Seeding to Drive Phase Selection and Overcome Synthesis Limitations. Cryst. Growth Des 2022, 22 (11), 6379–6383. 10.1021/acs.cgd.2c00762. [DOI] [Google Scholar]
- (14).Li Q; Gies J; Yu X; Gu Y; Terfort A; Kind M Concentration‐Dependent Seeding as a Strategy for Fabrication of Densely Packed Surface‐Mounted Metal–Organic Frameworks (SURMOF) Layers. Chem. Eur. J 2020, 26 (23), 5185–5189. 10.1002/chem.202000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).McKinstry C; Cussen EJ; Fletcher AJ; Patwardhan SV; Sefcik J Effect of Synthesis Conditions on Formation Pathways of Metal Organic Framework (MOF-5) Crystals. Cryst. Growth Des 2013, 13 (12), 5481–5486. 10.1021/cg4014619. [DOI] [Google Scholar]
- (16).Forster PM; Stock N; Cheetham AK A High-Throughput Investigation of the Role of PH, Temperature, Concentration, and Time on the Synthesis of Hybrid Inorganic-Organic Materials. Angew. Chem. Int. Ed 2005, 44 (46), 7608–7611. 10.1002/anie.200501766. [DOI] [PubMed] [Google Scholar]
- (17).Jerozal R; Pitt T; MacMillan S; Milner P High-Concentration Self-Assembly of Zirconium- and Hafnium-Based Metal-Organic Materials. J. Am. Chem. Soc 2023, 145 (24), 13273–13283. 10.1021/jacs.3c02787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Siegelman RL; McDonald TM; Gonzalez MI; Martell JD; Milner PJ; Mason JA; Berger AH; Bhown AS; Long JR Controlling Cooperative CO2 Adsorption in Diamine-Appended Mg2(dobpdc) Metal–Organic Frameworks. J. Am. Chem. Soc 2017, 139 (30), 10526–10538. 10.1021/jacs.7b05858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Furukawa H; Gándara F; Zhang Y-B; Jiang J; Queen WL; Hudson MR; Yaghi OM Water Adsorption in Porous Metal–Organic Frameworks and Related Materials. J. Am. Chem. Soc 2014, 136 (11), 4369–4381. 10.1021/ja500330a. [DOI] [PubMed] [Google Scholar]
- (20).Morelli Venturi D; Campana F; Marmottini F; Costantino F; Vaccaro L Extensive Screening of Green Solvents for Safe and Sustainable UiO-66 Synthesis. ACS Sustainable Chem. Eng 2020, 8 (46), 17154–17164. 10.1021/acssuschemeng.0c05587. [DOI] [Google Scholar]
- (21).Xiao T; Liu D The Most Advanced Synthesis and a Wide Range of Applications of MOF-74 and Its Derivatives. Microporous Mesoporous Mater 2019, 283, 88–103. 10.1016/j.micromeso.2019.03.002. [DOI] [Google Scholar]
- (22).Caskey SR; Wong-Foy AG; Matzger AJ Dramatic Tuning of Carbon Dioxide Uptake via Metal Substitution in a Coordination Polymer with Cylindrical Pores. J. Am. Chem. Soc 2008, 130 (33), 10870–10871. 10.1021/ja8036096. [DOI] [PubMed] [Google Scholar]
- (23).Dietzel PDC; Panella B; Hirscher M; Blom R; Fjellvåg H Hydrogen Adsorption in a Nickel Based Coordination Polymer with Open Metal Sites in the Cylindrical Cavities of the Desolvated Framework. Chem. Commun 2006, No. 9, 959. 10.1039/b515434k. [DOI] [PubMed] [Google Scholar]
- (24).Rosi NL; Kim J; Eddaoudi M; Chen B; O’Keeffe M; Yaghi OM Rod Packings and Metal−Organic Frameworks Constructed from Rod-Shaped Secondary Building Units. J. Am. Chem. Soc 2005, 127 (5), 1504–1518. 10.1021/ja045123o. [DOI] [PubMed] [Google Scholar]
- (25).Queen WL; Hudson MR; Bloch ED; Mason JA; Gonzalez MI; Lee JS; Gygi D; Howe JD; Lee K; Darwish TA Comprehensive Study of Carbon Dioxide Adsorption in the Metal–Organic Frameworks M2(dobdc) (M= Mg, Mn, Fe, Co, Ni, Cu, Zn). Chem. Sci 2014, 5 (12), 4569–4581. 10.1039/C4SC02064B. [DOI] [Google Scholar]
- (26).Zick ME; Lee J-H; Gonzalez MI; Velasquez EO; Uliana AA; Kim J; Long JR; Milner PJ Fluoroarene Separations in Metal–Organic Frameworks with Two Proximal Mg2+ Coordination Sites. J. Am. Chem. Soc 2021, 143, 1948–1958. 10.1021/jacs.0c11530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Xiao DJ; Bloch ED; Mason JA; Queen WL; Hudson MR; Planas N; Borycz J; Dzubak AL; Verma P; Lee K; Bonino F; Crocellà V; Yano J; Bordiga S; Truhlar DG; Gagliardi L; Brown CM; Long JR Oxidation of Ethane to Ethanol by N2O in a Metal–Organic Framework with Coordinatively Unsaturated Iron(II) Sites. Nat. Chem 2014, 6 (7), 590–595. 10.1038/nchem.1956. [DOI] [PubMed] [Google Scholar]
- (28).Calleja G; Sanz R; Orcajo G; Briones D; Leo P; Martínez F Copper-Based MOF-74 Material as Effective Acid Catalyst in Friedel–Crafts Acylation of Anisole. Catalysis Today 2014, 227, 130–137. 10.1016/j.cattod.2013.11.062. [DOI] [Google Scholar]
- (29).Huang P; Liu Y; Karmakar A; Yang Q; Li J; Wu F-Y; Deng K-Y Tuning the Excited-State Intramolecular Proton Transfer (ESIPT)-Based Luminescence of Metal–Organic Frameworks by Metal Nodes toward Versatile Photoluminescent Applications. Dalton Trans 2021, 50 (20), 6901–6912. 10.1039/D1DT00728A. [DOI] [PubMed] [Google Scholar]
- (30).Douvali A; Tsipis AC; Eliseeva SV; Petoud S; Papaefstathiou GS; Malliakas CD; Papadas I; Armatas GS; Margiolaki I; Kanatzidis MG; Lazarides T; Manos MJ Turn-On Luminescence Sensing and Real-Time Detection of Traces of Water in Organic Solvents by a Flexible Metal-Organic Framework. Angew. Chem 2015, 127 (5), 1671–1676. 10.1002/ange.201410612. [DOI] [PubMed] [Google Scholar]
- (31).Jayaramulu K; Kanoo P; George SJ; Maji TK Tunable Emission from a Porous Metal–Organic Framework by Employing an Excited-State Intramolecular Proton Transfer Responsive Ligand. Chem. Commun 2010, 46 (42), 7906. 10.1039/c0cc02069a. [DOI] [PubMed] [Google Scholar]
- (32).Henkelis SE; McCormick LJ; Cordes DB; Slawin AMZ; Morris RE Synthesis and Crystallographic Characterisation of Mg(H2Dhtp)(H2O)5·H2O. Inorg. Chem. Commun 2016, 65, 21–23. 10.1016/j.inoche.2016.01.007. [DOI] [Google Scholar]
- (33).Cheansirisomboon A; Salinas-Uber J; Massera C; Roubeau O; Youngme S; Gamez P One-Pot Multiple Metal-Organic Framework Formation: Concomitant Generation of Structural Isomers or of Drastically Distinct Materials: One-Pot Multiple Metal-Organic Framework Formation. Eur. J. Inorg. Chem 2014, 2014 (26), 4385–4393. 10.1002/ejic.201402475. [DOI] [Google Scholar]
- (34).Dietzel PDC; Blom R; Fjellvåg H Base-Induced Formation of Two Magnesium Metal-Organic Framework Compounds with a Bifunctional Tetratopic Ligand. Eur. J. Inorg. Chem 2008, 2008 (23), 3624–3632. 10.1002/ejic.200701284. [DOI] [Google Scholar]
- (35).Douvali A; Papaefstathiou GS; Gullo MP; Barbieri A; Tsipis AC; Malliakas CD; Kanatzidis MG; Papadas I; Armatas GS; Hatzidimitriou AG; Lazarides T; Manos MJ Alkaline Earth Metal Ion/Dihydroxy–Terephthalate MOFs: Structural Diversity and Unusual Luminescent Properties. Inorg. Chem 2015, 54 (12), 5813–5826. 10.1021/acs.inorgchem.5b00539. [DOI] [PubMed] [Google Scholar]
- (36).Henkelis SE; Rademacher D; Vogel DJ; Valdez NR; Rodriguez MA; Shea-Rohwer LE; Nenoff TM Luminescent Properties of DOBDC Containing MOFs: The Role of Free Hydroxyls. ACS Appl. Mater. Interfaces 2020, 12 (20), 22845–22852. 10.1021/acsami.0c02829. [DOI] [PubMed] [Google Scholar]
- (37).Gao Q; Jiang F-L; Wu M-Y; Huang Y-G; Wei W; Zhang Q-F; Hong M-C Crystal Structures, Topological Analyses, and Magnetic Properties of Manganese-Dihydroxyterephthalate Complexes. Aust. J. Chem 2010, 63 (2), 286–262. 10.1071/CH09378. [DOI] [Google Scholar]
- (38).Ayoub G; Karadeniz B; Howarth AJ; Farha OK; Đilović I; Germann LS; Dinnebier RE; Užarević K; Friščić T Rational Synthesis of Mixed-Metal Microporous Metal–Organic Frameworks with Controlled Composition Using Mechanochemistry. Chem. Mater 2019, 31 (15), 5494–5501. 10.1021/acs.chemmater.9b01068. [DOI] [Google Scholar]
- (39).Rosnes MH; Mathieson JS; Törnroos KW; Johnsen RE; Cronin L; Dietzel PDC Electrospray Mass Spectrometry Investigation into the Formation of CPO-27. Cryst. Growth Des 2019, 19 (4), 2089–2096. 10.1021/acs.cgd.8b01657. [DOI] [Google Scholar]
- (40).Flores LS; Alcântara SP; de Lima GCG; Yoshida MI; Corrêa CC Vibrational Analysis and Crystal Structure of Two New 1D CuII and CoII Coordination Polymers, Involving the Ligands 2,5-Dihydroxyterephthalate and Glutarate. Vibrational Spectroscopy 2016, 86, 302–310. 10.1016/j.vibspec.2016.08.010. [DOI] [Google Scholar]
- (41).Rosa IML; Costa MCS; Vitto BS; Amorim L; Correa CC; Pinheiro CB; Doriguetto AC Influence of Synthetic Methods in the Structure and Dimensionality of Coordination Polymers. Cryst. Growth Des 2016, 16 (3), 1606–1616. 10.1021/acs.cgd.5b01716. [DOI] [Google Scholar]
- (42).Gheorghe A; Imaz I; van der Vlugt JI; Maspoch D; Tanase S Tuning the Supramolecular Isomerism of MOF-74 by Controlling the Synthesis Conditions. Dalton Trans 2019, 48 (27), 10043–10050. 10.1039/C9DT01572H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Kim D; Coskun A Template-Directed Approach Towards the Realization of Ordered Heterogeneity in Bimetallic Metal-Organic Frameworks. Angew. Chem. Int. Ed 2017, 56 (18), 5071–5076. 10.1002/anie.201702501. [DOI] [PubMed] [Google Scholar]
- (44).Julien PA; Užarević K; Katsenis AD; Kimber SAJ; Wang T; Farha OK; Zhang Y; Casaban J; Germann LS; Etter M; Dinnebier RE; James SL; Halasz I; Friščić T In Situ Monitoring and Mechanism of the Mechanochemical Formation of a Microporous MOF-74 Framework. J. Am. Chem. Soc 2016, 138 (9), 2929–2932. 10.1021/jacs.5b13038. [DOI] [PubMed] [Google Scholar]
- (45).Luo F; Yan C; Dang L; Krishna R; Zhou W; Wu H; Dong X; Han Y; Hu T-L; O’Keeffe M; Wang L; Luo M; Lin R-B; Chen B UTSA-74: A MOF-74 Isomer with Two Accessible Binding Sites per Metal Center for Highly Selective Gas Separation. J. Am. Chem. Soc 2016, 138 (17), 5678–5684. 10.1021/jacs.6b02030. [DOI] [PubMed] [Google Scholar]
- (46).Ghermani NE; Morgant G; d’Angelo J; Desmaële D; Fraisse B; Bonhomme F; Dichi E; Sgahier M Covalently Bonded Infinite Zigzag Chain Structure in a Novel Zn(II) Complex of 2,5-Dihydroxy-1,6-Benzenedicarboxylic Acid. Polyhedron 2007, 26 (12), 2880–2884. 10.1016/j.poly.2007.01.025. [DOI] [Google Scholar]
- (47).Zhao Y; Shao L; Li L; Wang S; Song G; Gao Z; Zhang X; Wang T; Li Y; Zhang L; Li W; Meng F; Fu Y Novel Zinc-Based Infinite Coordination Polymer for Highly Selective Ammonia Gas Sensing at Room Temperature. BCSJ 2020, 93 (9), 1070–1073. 10.1246/bcsj.20200132. [DOI] [Google Scholar]
- (48).Lefton JB; Pekar KB; Haris U; Zick ME; Milner PJ; Lippert AR; Pejov L; Runčevski T Defect Formation and Amorphization of Zn-MOF-74 Crystals by Post-Synthetic Interactions with Bidentate Adsorbates. J. Mater. Chem. A 2021, 9, 19698–19704. 10.1039/D0TA10613E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Albuquerque GH; Fitzmorris RC; Ahmadi M; Wannenmacher N; Thallapally PK; McGrail BP; Herman GS Gas–Liquid Segmented Flow Microwave-Assisted Synthesis of MOF-74(Ni) under Moderate Pressures. CrystEngComm 2015, 17 (29), 5502–5510. 10.1039/C5CE00848D. [DOI] [Google Scholar]
- (50).El Osta R; Feyand M; Stock N; Millange F; Walton RI Crystallisation Kinetics of Metal Organic Frameworks From in Situ Time-Resolved X-Ray Diffraction. Powder Diffr 2013, 28 (S2), S256–S275. 10.1017/S0885715613000997. [DOI] [Google Scholar]
- (51).Haque E; Jhung SH Synthesis of Isostructural Metal–Organic Frameworks, CPO-27s, with Ultrasound, Microwave, and Conventional Heating: Effect of Synthesis Methods and Metal Ions. Chem. Eng. J 2011, 173 (3), 866–872. 10.1016/j.cej.2011.08.037. [DOI] [Google Scholar]
- (52).Beamish-Cook J; Shankland K; Murray CA; Vaqueiro P Insights into the Mechanochemical Synthesis of MOF-74. Cryst. Growth Des 2021, 21 (5), 3047–3055. 10.1021/acs.cgd.1c00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Mason JA; Veenstra M; Long JR Evaluating Metal–Organic Frameworks for Natural Gas Storage. Chem. Sci 2014, 5 (1), 32–51. 10.1039/C3SC52633J. [DOI] [Google Scholar]
- (54).Dietzel PDC; Georgiev PA; Eckert J; Blom R; Strässle T; Unruh T Interaction of Hydrogen with Accessible Metal Sites in the Metal–Organic Frameworks M2(dhtp) (CPO-27-M; M = Ni, Co, Mg). Chem. Commun 2010, 46 (27), 4962. 10.1039/c0cc00091d. [DOI] [PubMed] [Google Scholar]
- (55).Remy T; Peter SA; Denayer JFM Selective Dynamic CO2 Separations on Mg-MOF-74 at Low Pressures: A Detailed Comparison with 13X. J. Phys. Chem. C 2013, 117 (18), 9301–9310. 10.1021/jp401923v. [DOI] [Google Scholar]
- (56).Dietzel PDC; Johnsen RE; Blom R; Fjellvåg H Structural Changes and Coordinatively Unsaturated Metal Atoms on Dehydration of Honeycomb Analogous Microporous Metal–Organic Frameworks. Chem. Eur. J 2008, 14 (8), 2389–2397. 10.1002/chem.200701370. [DOI] [PubMed] [Google Scholar]
- (57).Milner PJ; Martell JD; Siegelman RL; Gygi D; Weston SC; Long JR Overcoming Double-Step CO2 Adsorption and Minimizing Water Co-Adsorption in Bulky Diamine-Appended Variants of Mg 2 (Dobpdc). Chem. Sci 2018, 9 (1), 160–174. 10.1039/C7SC04266C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Moon H-S; Moon J-H; Chun DH; Park YC; Yun YN; Sohail M; Baek K; Kim H Synthesis of [Mg2 (DOBDC)(DMF)2]@polystyrene Composite and Its Carbon Dioxide Adsorption. Microporous Mesoporous Mater 2016, 232, 161–166. 10.1016/j.micromeso.2016.06.014. [DOI] [Google Scholar]
- (59).Moreno MA; Gálvez O; Maté B; Herrero VJ; Escribano R Formate Ion: Structure and Spectroscopic Properties. J. Phys. Chem. A 2011, 115 (1), 70–75. 10.1021/jp108326x. [DOI] [PubMed] [Google Scholar]
- (60).Choi JI; Chun H; Lah MS Zirconium-Formate Macrocycles and Supercage: Molecular Packing versus MOF-like Network for Water Vapor Sorption. J. Am. Chem. Soc 2018, 140 (34), 10915–10920. 10.1021/jacs.8b06757. [DOI] [PubMed] [Google Scholar]
- (61).Lucier BEG; Zhang Y; Huang Y Complete Multinuclear Solid-State NMR of Metal-Organic Frameworks: The Case of α-Mg-Formate. Concepts Magn. Reson. Part A 2016, 45A (6), e21410. 10.1002/cmr.a.21410. [DOI] [Google Scholar]
- (62).Hanrahan MP; Venkatesh A; Carnahan SL; Calahan JL; Lubach JW; Munson EJ; Rossini AJ Enhancing the Resolution of 1H and 13C Solid-State NMR Spectra by Reduction of Anisotropic Bulk Magnetic Susceptibility Broadening. Phys. Chem. Chem. Phys 2017, 19 (41), 28153–28162. 10.1039/C7CP04223J. [DOI] [PubMed] [Google Scholar]
- (63).Cho E; Lee TS Manipulation of Intramolecular Hydrogen Bonds in Single-Benzene Derivatives: Esterase Sensing, Fluorescence Patterning, and Inkless Writing. Sens. Actuators B: Chem 2020, 319, 128307. 10.1016/j.snb.2020.128307. [DOI] [Google Scholar]
- (64).Ren X; Wang J; Peng Z; Lu L Direct Monitoring of Trace Water in Li-Ion Batteries Using Operando Fluorescence Spectroscopy. Chem. Sci 2018, 9 (1), 231–237. 10.1039/C7SC03191B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Della Pia A; Luo D; Blackwell R; Costantini G; Martsinovich N Molecular Self-Assembly of Substituted Terephthalic Acids at the Liquid/Solid Interface: Investigating the Effect of Solvent. Faraday Discuss 2017, 204, 191–213. 10.1039/C7FD00112F. [DOI] [PubMed] [Google Scholar]
- (66).Denisov GS; Golubev NS; Schreiber VM; Shajakhmedov Sh. S.; Shurukhina AV Excited State Intramolecular Proton Transfer and Dual Emission of the Cyclic Homo- and Heterodimers of 2-Hydroxy and 2,6-Dihydroxy Benzoic Acids. J. Mol. Struct 1996, 381 (1–3), 73–81. 10.1016/0022-2860(96)09315-5. [DOI] [Google Scholar]
- (67).Halder A; Bain DC; Oktawiec J; Addicoat MA; Tsangari S; Fuentes-Rivera JJ; Pitt TA; Musser AJ; Milner PJ Enhancing Dynamic Spectral Diffusion in Metal–Organic Frameworks through Defect Engineering. J. Am. Chem. Soc 2023, 145 (2), 1072–1082. 10.1021/jacs.2c10672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Musser AJ; Rajendran SK; Georgiou K; Gai L; Grant RT; Shen Z; Cavazzini M; Ruseckas A; Turnbull GA; Samuel IDW; Clark J; Lidzey DG Intermolecular States in Organic Dye Dispersions: Excimers vs. Aggregates. J. Mater. Chem. C 2017, 5 (33), 8380–8389. 10.1039/C7TC02655B. [DOI] [Google Scholar]
- (69).Yu J; Park J; Van Wyk A; Rumbles G; Deria P Excited-State Electronic Properties in Zr-Based Metal–Organic Frameworks as a Function of a Topological Network. J. Am. Chem. Soc 2018, 140 (33), 10488–10496. 10.1021/jacs.8b04980. [DOI] [PubMed] [Google Scholar]
- (70).Deria P; Yu J; Smith T; Balaraman RP Ground-State versus Excited-State Interchromophoric Interaction: Topology Dependent Excimer Contribution in Metal–Organic Framework Photophysics. J. Am. Chem. Soc 2017, 139 (16), 5973–5983. 10.1021/jacs.7b02188. [DOI] [PubMed] [Google Scholar]
- (71).Tang B; Liu H; Li F; Wang Y; Zhang H Single-Benzene Solid Emitters with Lasing Properties Based on Aggregation-Induced Emissions. Chem. Commun 2016, 52 (39), 6577–6580. 10.1039/C6CC02616H. [DOI] [PubMed] [Google Scholar]
- (72).Cheng X; Wang K; Huang S; Zhang H; Zhang H; Wang Y Organic Crystals with Near-Infrared Amplified Spontaneous Emissions Based on 2′-Hydroxychalcone Derivatives: Subtle Structure Modification but Great Property Change. Angew. Chem. Int. Ed 2015, 54 (29), 8369–8373. 10.1002/anie.201503914. [DOI] [PubMed] [Google Scholar]
- (73).Cheng X; Li F; Han S; Zhang Y; Jiao C; Wei J; Ye K; Wang Y; Zhang H Emission Behaviors of Unsymmetrical 1,3-Diaryl-β-Diketones: A Model Perfectly Disclosing the Effect of Molecular Conformation on Luminescence of Organic Solids. Sci. Rep 2015, 5 (1), 9140. 10.1038/srep09140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).de Oliveira A; de Lima GF; De Abreu HA Structural and Electronic Properties of M-MOF-74 (M = Mg, Co or Mn). Chem. Phys. Lett 2018, 691, 283–290. 10.1016/j.cplett.2017.11.027. [DOI] [Google Scholar]
- (75).Leith GA; Martin CR; Mayers JM; Kittikhunnatham P; Larsen RW; Shustova NB Confinement-Guided Photophysics in MOFs, COFs, and Cages. Chem. Soc. Rev 2021, 50 (7), 4382–4410. 10.1039/D0CS01519A. [DOI] [PubMed] [Google Scholar]
- (76).Rice AM; Martin CR; Galitskiy VA; Berseneva AA; Leith GA; Shustova NB Photophysics Modulation in Photoswitchable Metal–Organic Frameworks. Chem. Rev 2020, 120 (16), 8790–8813. 10.1021/acs.chemrev.9b00350. [DOI] [PubMed] [Google Scholar]
- (77).Dolgopolova EA; Rice AM; Martin CR; Shustova NB Photochemistry and Photophysics of MOFs: Steps towards MOF-Based Sensing Enhancements. Chem. Soc. Rev 2018, 47 (13), 4710–4728. 10.1039/C7CS00861A. [DOI] [PubMed] [Google Scholar]
- (78).Wang Z; Li Z; Ng M; Milner PJ Rapid Mechanochemical Synthesis of Metal–Organic Frameworks Using Exogenous Organic Base. Dalton Trans 2020, 49 (45), 16238–16244. 10.1039/D0DT01240H. [DOI] [PubMed] [Google Scholar]
- (79).Chen EY; Mandel RM; Milner PJ Evaluating Solvothermal and Mechanochemical Routes towards the Metal–Organic Framework Mg2(m-dobdc). CrystEngComm 2022, 24 (41), 7292–7297. 10.1039/D2CE00739H. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


