Abstract
Introduction
Cognitive dysfunction or deficits are common in patients with major depressive disorder (MDD). The current study systematically reviews and meta-analyzes multiple domains of cognitive impairment in patients with MDD.
Methods
PubMed/MEDLINE, PsycINFO, Cochrane Library, Embase, Web of Science, and Google Scholar were searched from inception through May 17, 2023, with no language limits. Studies with the following inclusion criteria were included: (1) patients with a diagnosis of MDD using standardized diagnostic criteria; (2) healthy controls (i.e., those without MDD); (3) neuropsychological assessments of cognitive impairment using Cambridge Neuropsychological Test Automated Battery (CANTAB); and (4) reports of sufficient data to quantify standardized effect sizes. Hedges’ g standardized mean differences (SMDs) with corresponding 95% confidence intervals (CIs) were used to quantify effect sizes of cognitive impairments in MDD. SMDs were estimated using a fixed- or random-effects models.
Results
Overall, 33 studies consisting of 2,596 subjects (n = 1,337 for patients with MDD and n = 1,259 for healthy controls) were included. Patients with MDD, when compared to healthy controls, had moderate cognitive deficits (SMD, −0.39 [95% CI, −0.47 to −0.31]). In our subgroup analyses, patients with treatment-resistant depression (SMD, −0.56 [95% CI, −0.78 to −0.34]) and older adults with MDD (SMD, −0.51 [95% CI, −0.66 to −0.36]) had greater cognitive deficits than healthy controls. The effect size was small among unmedicated patients with MDD (SMD, −0.19 [95% CI, −0.37 to −0.00]), and we did not find any statistical difference among children. Cognitive deficits were consistently found in all domains, except the reaction time. No publication bias was reported.
Conclusion
Because cognitive impairment in MDD can persist in remission or increase the risk of major neurodegenerative disorders, remediation of cognitive impairment in addition to alleviation of depressive symptoms should be an important goal when treating patients with MDD.
Keywords: Cognition, Cognitive impairment, Depression, Major depressive disorder, Cambridge Neuropsychological Test Automated Battery
Introduction
Major depressive disorder (MDD) is one of the most common and disabling psychiatric disorders, affecting about 280 million individuals (or 5% of the adult population) globally [1, 2]. Cognitive dysfunction or deficits are well established in patients with MDD [3–6]. For example, about two-thirds of patients with MDD reported cognitive impairment [7], and two meta-analytic studies [8, 9] reported that patients with MDD, when compared to healthy controls, had small to moderate effect sizes of cognitive deficits. Furthermore, such cognitive deficits in MDD play a principal mediating role of psychosocial impairment, including workforce performance [3, 4, 10], and may elevate the risk of developing other chronic conditions, such as Alzheimer’s disease and related dementias (ADRD) [11, 12].
To date, four meta-analytic studies [8, 9, 13, 14] have compared the cognitive deficits (or impairment) between currently depressed patients with a clinical diagnosis of MDD and healthy subjects. One study [8] published in 2009 examined (1) episodic memory (e.g., immediate and delayed recall, learning and recognition), (2) executive function (e.g., working memory, selective attention, cognitive flexibility, set shifting, planning/problem solving), (3) processing speed, (4) semantic memory, and (5) visuospatial memory using multiple types of neuropsychological tests. This study included 14 studies for its final systematic review and meta-analysis and concluded that patients with MDD had significant cognitive deficits in episodic memory, executive function, and processing speed, but not for sematic or visuospatial memory. Another study conducted in 2013 [9] included 24 studies and found moderate cognitive deficits in executive function, memory, and attention among patients with MDD when compared to healthy controls. Other older studies [13, 14] produced similar findings.
There are several limitations in previous studies, which our study aims to address. First, as stated in the previous study [9], one of methodological drawbacks is related to use of different, multiple neuropsychological tests that were used across the studies and lack of implementation of standardized effect sizes to reflect the magnitude of cognitive impairment [8, 13, 14]. Furthermore, earlier studies may not reflect the results as more contemporary studies have improved methodological approaches. Second, earlier studies did not distinguish effect sizes of cognitive impairment by medication status [8, 14], treatment-resistant status (e.g., those who are eligible for electroconvulsive therapy) [8, 9, 13, 14], or among children or older adult populations [8, 9, 13]. Third, these studies [8, 9, 13, 14] did not assess (1) potential moderators (e.g., age, sex, sample sizes, and geographic regions) using meta-regression analyses and/or (2) quality assessment of individual studies, including publication bias. Our study aimed to explicitly address these gaps.
While there are multiple neuropsychological tests to assess cognitive impairment in MDD, there is no gold standard [3]. Consistent with an earlier study [9], we focused on studies that employed the Cambridge Neuropsychological Test Automated Battery (CANTAB) [15] that was originally developed to assess multiple domains of cognitive function in the 1980s. It provides objective, culturally sensitive, and validated measures of cognitive function that are highly correlated to neural networks [15]. Multidimensional domains of cognitive function (e.g., executive function, memory, attention, and reaction) using multiple measures are described in Table 1. Furthermore, CANTAB is a widely used neuropsychological assessment across diverse clinical conditions (e.g., mood disorders, traumatic brain injury, and ADRD) and study settings globally, resulting in over 3,000 peer-reviewed publications. In the era of digital health and telehealth, CANTAB is also widely known for its accessibility and validity of digital cognitive assessments [15].
Table 1.
Neuropsychological assessments of cognitive impairment according to Cambridge Neuropsychological Test Automated Battery (CANTAB)
| Domain | Measure | Description and outcome of interest | Reference |
|---|---|---|---|
| Executive function | (One Touch) stockings of Cambridge (OTS/SOC) | • This task was derived from the Tower of London test and assesses visual planning, reasoning, and impulsivity | Owen et al. [16] |
| • Outcome measures analyzed were the number/percentage correct or number of moves above the minimum (for all problems or difficult [four/five-move] problems) | |||
| Spatial working memory (SWM) | • This self-ordered search task is based on foraging behavior and assesses working memory and strategy use. Participants search for tokens without returning to previous token locations | Owen et al. [17] | |
| • Outcome measure analyzed was between-search errors | |||
| Intra-extra dimensional set shift (IED) | • This test of cognitive flexibility, analogous to the Wisconsin Card Sorting Test (WCST), has multiple stages segregating cognitive processes that assess rule learning, rule reversal, and attentional set shifting | Rogers et al. [18] | |
| • Outcome measures analyzed were total errors, extradimensional shift errors (adjusted), or stages completed | |||
| Spatial span (SSP) | • This is a task of spatial short-term memory based on the Corsi block-tapping task | Kempton et al. [19] | |
| • Outcome measure analyzed was spatial span | |||
| Memory | Delayed matching to sample (DMS) | • In this test, participants remember the visual features of a complex, abstract target stimulus, and select it from a choice of four target patterns after a variable delay | Robbins et al. [20] |
| • Outcome measures analyzed were total/percentage correct (for all trials or 12-s delay trials) | |||
| Paired associates learning (PAL) | • In this test, participants learn the locations of a progressively increasing number of abstract stimuli | Sahakian et al. [21] | |
| • Outcome measures analyzed were total errors (adjusted) or first trials correct | |||
| Pattern recognition memory (PRM) | • This is a two-forced-choice test of abstract visual pattern recognition memory | Owen et al. [17] | |
| • Outcome measures analyzed were total/percentage correct | |||
| Spatial recognition memory (SRM) | • This two-forced-choice discrimination paradigm tests spatial recognition memory | Owen et al. [17] | |
| • Outcome measures analyzed were total/percentage correct | |||
| Attention | Rapid visual information processing (RVP) | • This is a continuous performance test that assesses sustained attention, signal detection, and impulsivity. Participants monitor a stream of single digits for three-digit target sequences | Sahakian et al. [22] |
| • Outcome measures analyzed were target sensitivity or total hits/omissions | |||
| Reaction | Reaction time (RTI) | • This is a test of simple and five-choice reaction time | Sahakian et al. [23] |
| • Outcome measure analyzed was five-choice reaction time |
This table is adapted from the CANTAB manual and previous studies [9].
The objective of this study was to conduct and update a systematic review and meta-analysis with meta-regression to synthesize the evidence of cognitive impairment in patients with MDD relative to healthy controls, using a single neuropsychological test battery, CANTAB. Our rationale for using CANTAB measures alone was to enable assessment of a wide range of cognitive domains while maintaining inter-study homogeneity of measurements. Consistent with earlier studies [8, 9], we hypothesized that cognitive deficits would be found in patients with MDD when compared to healthy subjects.
Methods
Search Strategy
The protocol pertaining to this study was registered on PROSPERO (CRD42023433574). A systematic search was conducted from inception to May 23, 2023. The following databases were systematically searched: PubMed/MEDLINE, PsycINFO, Web of Science, the Cochrane Library, and Embase using Medical Subject Headings (MeSH) terms and text keywords. We also manually searched all relevant studies on Google Scholar (https://scholar.google.com/). No language restrictions were imposed. Search strategies are provided in eTable 1 (for all online suppl. material, see https://doi.org/10.1159/000535665) in the Supplement, which also covers the search terms used in the previous study [9]. This study followed the preferred reporting items for meta-analysis of observational studies in epidemiology (MOOSE) reporting guidelines [24] (online suppl. eTable 2). Our study used publicly available data and did not include human participant research. As per 45 CFR §46.102(f), this study was not submitted for institutional review board approval and did not require informed consent procedures.
Eligibility Criteria and Study Selection
Inclusion criteria were established prior to article reviews and were as follows: (1) patients with a diagnosis of MDD using standardized diagnostic criteria (e.g., Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition [DSM-5] or International Statistical Classification of Diseases and Related Health Problems, Tenth Revision [ICD-10]); (2) healthy controls (i.e., those [a] without MDD or other psychiatric conditions and [b] are similar to patients with MDD otherwise in terms of patient characteristics); (3) neuropsychological assessments of cognitive impairment using CANTAB (Table 1) in both patients with MDD and healthy controls; and (4) reports of sufficient data (e.g., mean differences and standard deviations) to quantify standardized effect sizes. For patients with MDD, severity of depressive symptoms using validated measures (e.g., Montgomery-Asberg Depression Rating Scale [MADRS] and Hamilton Depression Rating Scale [HDRS or HAMD]) should also have been reported. Exclusion criteria were (1) nonhuman studies and (2) no use of validated measures for MDD or outcomes of interest. We only considered currently depressed patients and not remitted depressed patients because a study focusing on the latter group has been recently reported [25].
Study Identification and Data Extraction
Titles and abstracts were independently screened by two reviewers (T.G.R. and S.R.S.), and articles identified as potentially relevant by at least one reviewer were retrieved and duplicates were removed. Full-text articles were independently screened by the same reviewers, and discrepancies were resolved through discussion. Data from included articles were independently extracted by the two reviewers using a pilot-tested data extraction form and then corroborated, with discrepancies resolved through discussion. Information to be extracted was established a priori and included: study characteristics (e.g., PICOTS framework), participant characteristics and subgroups, sample source and collection period, modes of ascertainment, methods of data analysis, selection of cases and controls, and quantitative data pertaining to any primary and secondary outcomes along with covariates. For longitudinal or interventional studies, only baseline data were used. To ensure the absence of overlapping data and to maintain the meta-integrity, data and references for each included study were carefully cross-checked.
Assessment Methodological Quality
The risk of bias and methodological quality were evaluated using the Newcastle-Ottawa scale for the assessment of the quality of non-randomized studies in meta-analyses [26]. We assessed eight items across three domains: selection (adequate definition of case; representativeness of case; definition of control; selection of control); comparability of cases and controls based on the design or analysis; and exposure/outcome (ascertainment of exposure or outcome; same method of ascertainment for cases and controls; and nonresponse rate). The overall quality was classified as being indicative of a “good quality,” “fair quality,” or “poor quality” [26].
Publication Bias
Publication bias is always a concern for evidence synthesis. We used funnel plots to visually explore for evidence of association between the effect sizes and statistical precision [27]. We supplemented visual assessments with statistical testing of funnel plot asymmetry using Egger’s test (i.e., a weighted linear regression of effect size vs. precision) and Begg and Mazumdar’s test (i.e., rank correlation test). Evidence of associations between effect sizes and precision across studies may indicate design heterogeneity, chance, or selection biases that operate cross the evidence base (e.g., publication and outcome reporting bias).
Statistical Analysis
We used a Hedges’ g standardized mean difference (SMD) for differences in severity of cognitive impairment between patients with MDD and healthy controls. The weight of each study was determined using an inverse-variance method [27], with a restricted maximum likelihood estimator technique [28]. We used both Cochran’s Q-statistic and I2 statistic to quantify the proportions of heterogeneity due to within- and between-study variations [27]. To adequately estimate the overall effect sizes, SMDs with their corresponding 95% confidence intervals (CIs) were calculated using a fixed- or random-effects models, depending on the model assumptions [27]. More specifically, a random-effects model was used when I2 statistic was >50%, and a fixed-effects model was used when I2 statistic was <50%. We reported both random- and fixed-effects models when I2 statistics was 50%. When describing heterogeneity using the I2 statistic, we followed the guidance from the Cochrane handbook (i.e., 0–40% as minimal; 30–60% as moderate; 50–90% as substantial; and 75–100% as considerable heterogeneity) [27].
When some studies reported multiple effect sizes for a single domain (e.g., reporting effect sizes of two or more measures for executive function), we used a two-stage meta-analysis. In the first stage, we obtained the overall effect size estimate of multiple measures within the study using a three-level meta-analysis [29]. In the second stage, we pooled and obtained the overall effect size estimate using one effect size from each study. This approach avoids potential duplicates of the samples included. In addition, we performed four subgroup analyses: (1) unmedicated MDD versus healthy controls; (2) treatment-resistant depression (TRD) (defined by individual studies) versus healthy controls; (3) MDD versus healthy controls among children aged ≤18; and (4) MDD versus healthy controls among older adults ≥50.
We also conducted moderator analyses using meta-regression analyses in each of 10 outcome measures by study sample size; age; female sex (%); children, older adults; medication status; TRD status; and region. Medication status (yes or no) refers to any antidepressants (with or without other psychotropic medications) prescribed for managing MDD. TRD was defined as a failure to respond to two or more trials of antidepressant therapies. In particular, those with electroconvulsive therapy referral [30] or deep brain stimulation recipients [31, 32] were considered to have TRD. When identifying potential moderators, we used the variance of the true effects using a restricted maximum likelihood estimator [27]. We used a statistical software, R 4.2.1. (R Foundation for Statistical Computing) for all analyses using the “meta,” “rmeta,” and “metafor” packages [33]. A two-sided p value <0.05 was considered for statistical significance. Finally, as mentioned earlier, we assessed publication bias (or small-study effects) using funnel plots [27]. Egger’s test and Begg and Mazumdar’s test were also performed when assessing the publication bias [27]. Unless otherwise noted, a two-sided p value <0.05 was considered statistically significant.
Results
Characteristics of Included Studies
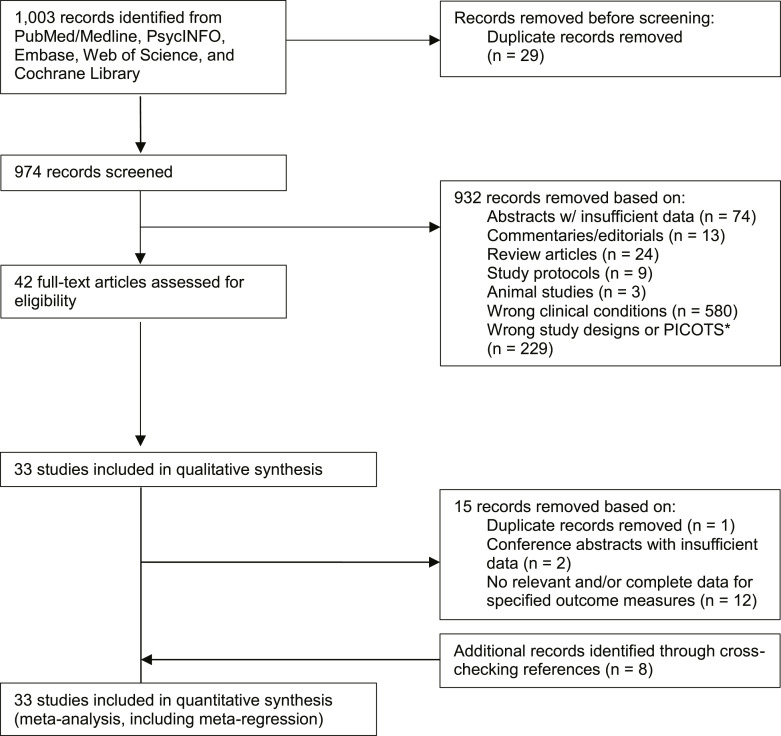
The literature search yielded 982 articles, of which 42 were eligible after screening titles and abstracts and removing duplicates. Of these eligible studies, 15 were further excluded after full-text screening. Two independent investigators (T.G.R. and S.R.S.) discovered 8 additional studies by manually searching reference lists and Google Scholar (Fig. 1). Overall, 33 studies [30–32, 34–63] consisting of 2,596 subjects (n = 1,337 for patients with MDD and n = 1,259 for healthy controls) were included in the review (Table 2). All studies were conducted in hospital settings, except one study [49] that did not specify the study setting. Four studies [34, 40, 43] focused on older adult populations; two studies [55, 60] focused on child populations; and three studies [30–32] focused on patients with TRD. Of 33 studies, 10 (30.3%) were conducted in the UK, 7 (23.3%) were conducted in the USA, and 16 (48.5%) were conducted in other regions. Sample sizes ranged from 30 to 265. Table 2 provides details of characteristics, including severity of depressive symptoms in patients with MDD, for all included studies.
Fig. 1.
Study selection flowchart. *PICOTS indicates population, intervention, comparator, outcome, timing, and setting.
Table 2.
Selected characteristics of included studies for both qualitative and quantitative analyses (n = 33)
| Author (year) | Country (setting) | Major depressive disorder (MDD) | Healthy control | Note | |||||
|---|---|---|---|---|---|---|---|---|---|
| sample size (% female) | age, years±SD | diagnostic criteria | severity of MDD (mean ± SD) | medication status | sample size (% female) | age, years±SD | |||
| Beats et al. [34] (1996) | UK (inpatient) | 24 (60) | 72±5.9 | DSM-III-R | HAMD-x: 29.6±5.1; MADRS: 40.3±7.2 | 21 medicated | 15 (50) | 69.3±6.6 | Aged ≥60 only |
| Elliott et al. [35] (1996) | UK (inpatient) | 28 (68) | 49.9±1.7 | DSM-III-R | HAMD-x: 22.4±0.8; MADRS: 34.0±1.1 | All medicated | 22 (68.2) | 48.1±1.2 | Aged 40–70 |
| Elliott et al. [36] (1997) | UK (inpatient) | 6 (16.7) | 34.7±n/a | DSM-IV | HAMD-x: 23.8; MADRS: 35.3 | 5 medicated | 6 (16.7) | 31±n/a | |
| Purcell et al. [37] (1997) | Australia (hospital) | 20 (60) | 31.2±n/a | DSM-IV | HAMD-24: 22.6±5.6 | 12 medicated | 20 (60) | 37±n/a | |
| Sweeney et al. [38] (2000) | USA (hospital) | 58 (66.7) | 32.3±9.1 | DSM-IV | HAMD-17: 21.6±4.3 | Medicated patients | 51 (76.5) | 36.3±9.7 | |
| Grant et al. [39] (2001) | USA (hospital) | 48 (61.0) | 39±10.4 | DSM-IV | HAMD-17: 16.7±5.4 | All unmedicated patients for 28 days prior to testing | 31 (63.9) | 40.2±9.7 | |
| Swainson et al. [40] (2001) | UK (hospital) | 37 (n/a) | 60.8±8.6 | DSM-IV | HAMD-x: 21.4±6.2 | n/a | 39 (n/a) | 64.6±8.5 | |
| Murphy et al. [41] (2003) | UK (hospital) | 27 (51.9) | 38.9±9.7 | DSM-IV | HAMD-x: 23.6±4.2; MADRS: 34.3±5.4 | 26 medicated | 23 (52.2) | 39.1±10.8 | |
| Porter et al. [42] (2003) | UK (outpatient) | 44 (65.9) | 32.9±10.6 | DSM-IV | HAMD-17: 21.1±4.4; MADRS: 28.9±5.5; BDI: 27.9±10.2 | All unmedicated | 44 (65.9) | 32.3±11.4 | |
| O’Brien et al. [43] (2004) | UK (hospital) | 61 (78.7) | 73.9±6.7 | DSM-IV | MADRS: 30.7±7.1 | Mostly medicated | 40 (75%) | 73.3±6.7 | Aged ≥60 only |
| Erickson et al. [44] (2005) | UK (hospital) | 20 (50.0) | 37.2±11.9 | DSM-IV | MADRS: 25.4±7.1 | All unmedicated for 3 weeks prior to testing | 20 (50.0) | 36.5±11.1 | All had illness onset before age 40 |
| Kyte et al. [45] (2005) | UK (hospital) | 30 (60.0) | 15.3±2.5 | K-SADS-PL | HAMD-x: 10.9±6.8 | Medicated and unmedicated | 49 (59.2) | 15.2±2.1 | |
| Taylor Tavares et al. [46] (2007) | USA (hospital) | 22 (70.6%) | 32.6±2.7 | DSM-IV | MADRS: 25.5±7.5 | All unmedicated | 25 (72.0) | 34.8±1.8 | |
| Matthews et al. [47] (2008) | UK (hospital) | 14 (100) | 14.5±1.2 | ICD-10; CAPA-C | MFQ: 41.3±10.4 | All unmedicated | 14 (100) | 14.4±1.0 | |
| Michopoulos et al. [48] (2008) | Greece (inpatient) | 40 (100) | 52.7±10.8 | DSM-IV-TR | HAMD-17: 20.0±4.0 | All medicated | 20 (100) | 49.8±12.7 | |
| Cannon et al. [49] (2009) | USA (n/a) | 18 (61.1) | 31±11 | DSM-IV | MADRS: 22±5.3 | All unmedicated | 19 (57.9) | 31±8.5 | |
| Reppermund et al. [50] (2009) | Germany (inpatient) | 53 (52.8) | 43.5±8.0 | DSM-IV | HAMD-x: 25.1±5.1 | 50 medicated | 13 (53.8) | 46.4±9.5 | |
| Heinzel et al. [51] (2010) | Germany (inpatient) | 20 (55.0) | 40.0±9.9 | DSM-IV | HAMD-21: 33.1±7.1; BDI: 29.9±4.9 | All unmedicated for 1 week prior to testing | 29 (72.4) | 35.3±7.3 | |
| Lyche et al. [52] (2010) | Norway (hospital) | 37 (62.2) | 44.2±12.3 | DSM-IV | BDI: 21.4±11.1 | 13 medicated | 91 (69.2) | 35.8±12.0 | |
| Maalouf et al. [53] (2010) | USA (outpatient) | 20 (80.0) | 34.2±9.4 | DSM-IV | HAMD-25: 24.8±5.8 | All medicated | 28 (67.9) | 31.9±9.4 | |
| Braw et al. [54] (2011) – young | Israel (outpatient) | 30 (66.7) | 17.1±0.5 | DSM-IV | CDRS-R: 67.5±2.0; BDI: 32.6±1.3 | All unmedicated for 1 month prior to testing | 30 (60.0) | 17.5±0.6 | Aged <25 |
| Braw et al. [54] (2011) – middle-aged | Israel (outpatient) | 30 (53.3) | 35.0±1.0 | DSM-IV | HAMD-17: 32.5±1.1; BDI: 33.5±1.4 | All unmedicated for 1 month prior to testing | 30 (53.3) | 34.5±1.1 | Aged 25–45 |
| Braw et al. [54] (2011) – older adults | Israel (outpatient) | 25 (56.0) | 54.0±0.9 | DSM-IV | HAMD-17: 31.3±1.3; BDI: 30.8±1.4 | All unmedicated for 1 month prior to testing | 25 (68.0) | 54.2±0.9 | Aged 46–65 |
| Maalouf et al. [55] (2011) | USA (hospital) | 20 (85.0) | 15.3±1.6 | DSM-IV | CDRS: 58.6±10.9 | 13 medicated | 17 (52.9) | 15.2±1.8 | Aged ≤18 |
| Tsaltas et al. [30] (2011) – non-TRD | Greece (hospital) | 15 (100) | 47.8±11.7 | DSM-IV-TR | HAMD-24: 27.6±5.6 | All medicated | 15 (100) | 49.3±11.6 | |
| Tsaltas et al. [30] (2011) – TRD | Greece (hospital) | 15 (100) | 48.5±11.2 | DSM-IV-TR | HAMD-24: 31.9±6.5 | All medicated | 15 (100) | 49.3±11.6 | |
| Boeker et al. [56] (2012) | Switzerland (inpatient) | 28 (46.4) | 39.7±11.4 | DSM-x; HAMD-21 ≥24; BDI ≥24 | HAMD-21: 28.5±7.0; BDI: 25.9±8.2 | 19 were medicated | 28 (46.4) | 35.0±7.4 | |
| Bourke et al. [57] (2012) | New Zealand (outpatient) | 101 (69.3) | 38.1±11.4 | DSM-IV | HAMD-17: 16.7±5.7; MADRS: 24.2±7.0 | All unmedicated | 76 (65.8) | 38.1±13 | |
| Hermens et al. [58] (2013) | Australia (hospital) | 48 (66.7) | 21.7±3.2 | DSM-IV-TR | HAMD-17: 13.7±6.2 | Some medicated | 21 (66.7) | 22.9±3.1 | |
| Moreines et al. [31] (2014) | USA (hospital) | 17 (58.8) | 42±8.9 | DSM-IV | HAMD-17: 23.8±3.2; BDI-II: 37.7±11.3 | Mostly medicated | 15 (40.0) | 36±10.7 | |
| Yang et al. [59] (2015) | China (hospital) | 51 (60.8) | 31±9.5 | DSM-IV | HAMD-17: 22.9±4.3 | All unmedicated | 51 (60.8) | 31.1±9.3 | |
| Shehab et al. [60] (2016) | Lebanon (hospital) | 24 (66.7) | 14.8±1.6 | DSM-IV-TR | CDRS: 51±8; BDI: 28±8 | n/a | 24 (58.3) | 14.3±1.6 | Aged 12–18 |
| Bergfeld et al. [32] (2017) | The Netherlands (outpatient) | 25 (68.0) | 53.1±8.4 | n/a | HAMD-17: 13.6±7.8 | All medicated | 21 (61.9) | 53.5±8 | Patients with TRD eligible for deep brain stimulation |
| Sanchez-Carro et al. [61] (2021) | Spain (outpatient) | 74 (70.3) | 49.7±10.3 | DSM-IV-TR | HAMD-17: 20.0±5.4 | Mostly medicated | 68 (70.6) | 48.3±10.3 | |
| Yang et al. [62] (2021) | China (hospital) | 100 (61.0) | 27.1±8.4 | DSM-IV | HAMD-17: 23.3±5.3 | All unmedicated | 165 (66.1) | 26.0±7.1 | |
| Luo et al. [63] (2022) | China (hospital) | 107 (62.6) | 34.6±8.9 | DSM-5 | HAMD-x: 20.7±5.9 | All medicated | 74 (56.8) | 35.1±4.2 | |
BDI, Beck Depression Inventory; CAPA, Child and Adolescent Psychiatric Assessment; CDRS, Child Depression Rating Scale; DSM, Diagnostic and Statistical Manual of Mental Disorders; HDRS or HAMD, Hamilton Depression Rating Scale; ICD, International Classification of Diseases; K-SADS, Kiddie Schedule for Affective Disorders and Schizophrenia; MADRS, Montgomery-Åsberg Depression Rating Scale; MFQ, Mood and Feelings Questionnaire; SD, standard deviation; TRD, treatment-resistant depression.
Neuropsychological Assessments of Cognitive Impairment
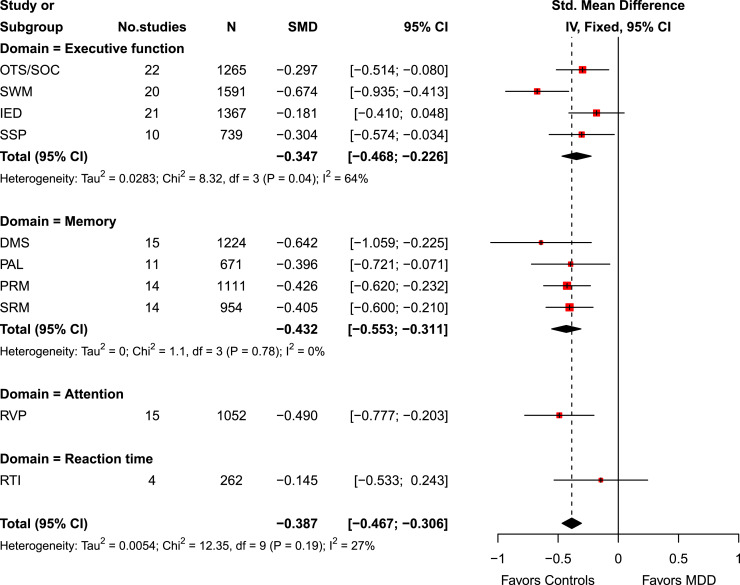
Overall, patients with MDD, when compared to healthy controls, had significant moderate cognitive deficits (SMD, −0.39 [95% CI, −0.47 to −0.31]; Cochran’s Q-statistic, p = 0.19) with minimal heterogeneity due to within- and between-study variations (I2 statistic = 27%) (Fig. 2). While there was no significant difference in reaction time between patients with MDD and healthy controls (SMD, −0.15 [95% CI, −0.53 to 0.24]), patients with MDD had significant moderate cognitive deficits in executive function (SMD, −0.35 [95% CI, −0.47 to −0.23]), memory (SMD, −0.43 [95% CI, −0.55 to −0.31]), and attention (SMD, −0.49 [95% CI, −0.78 to −0.20]) (Fig. 2). Neuropsychological assessments by individual outcome measures were also reported (online suppl. eFig. 1).
Fig. 2.
Forest plot of neuropsychological assessments in patients with major depressive disorder (MDD) versus healthy controls. The random-effects model was used based on the overall I2 statistic, which was 27%. In other words, the heterogeneity due to within- and between-study variations was moderate.
Subgroup Analyses of Cognitive Impairment between MDD and Healthy Controls
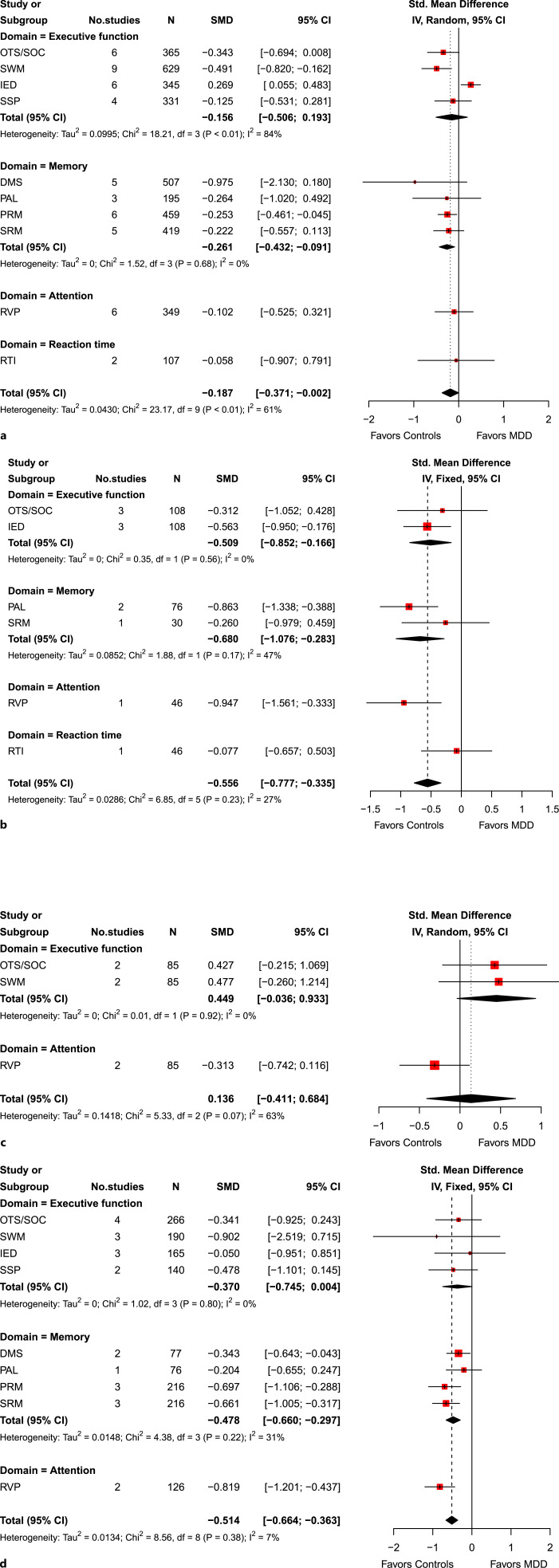
We further performed four subgroup analyses (Fig. 3). First, we compared patients with MDD who were not prescribed medications (e.g., antidepressants) versus healthy controls (Fig. 3a). Patients with unmedicated MDD had relatively small but significant cognitive deficits when compared to healthy controls (SMD, −0.19 [95% CI, −0.37 to −0.00]; Cochran’s Q-statistic, p < 0.01). Heterogeneity due to within- and between-study variations was substantial, however (I2 statistic = 61%). Memory was the only domain that had a significant difference between the two groups (SMD, −0.26 [95% CI, −0.43 to −0.09]).
Fig. 3.
Subgroup analyses of neuropsychological assessments in patients with major depressive disorder (MDD) versus healthy controls. a Unmedicated MDD versus healthy controls. b Treatment-resistant depression (TRD) versus healthy controls. c MDD versus healthy controls among children. d MDD versus healthy controls among older adults.
Second, we compared patients with TRD versus healthy controls (Fig. 3b), and the effect size was moderate (SMD, −0.56 [95% CI, −0.78 to −0.34]; Cochran’s Q-statistic, p = 0.23), indicating that patients with TRD had moderate cognitive deficits. The heterogeneity due to within- and between-study variations was minimal in this case (I2 statistic = 27%). Patients with TRD had significant cognitive deficits in terms of executive function, memory, and attention, but not in reaction time.
Third, we compared patients with MDD versus healthy controls among children (Fig. 3c). We did not find any statistically significant difference in cognitive impairment among children. Last, we compared patients with MDD versus health controls among older adults (Fig. 3d). Among older adults, patients with MDD had moderate cognitive deficits when compared to healthy controls (SMD, −0.51 [95% CI, −0.66 to −0.36]; Cochran’s Q-statistic, p = 0.38). Heterogeneity due to within- and between-study variations was minimal in this case (I2 statistic = 7%). In particular, patients with MDD had significant moderate cognitive deficits in memory (SMD, −0.48 [95% CI, −0.66 to −0.30]) and attention (SMD, −0.82 [95% CI, −1.20 to −0.44]).
Moderator Analysis
We have also fully explored potential moderating roles of the following variables in each of 10 outcome measures using meta-regression models: study sample size; age; female sex (%); children; older adults; medication status; TRD status; and region by individual outcome measures (online suppl. eTable 3). The study sample size was a potential moderator for delayed matching to sample (p < 0.001) and paired associates learning (p = 0.02). Age was a potential moderator for spatial recognition memory (p = 0.04). Being a child was a potential moderator for (one touch) stockings of Cambridge (OTS/SOC) (p = 0.03). Medication status was a potential moderator for intra-extra dimensional set shift (p = 0.003) and rapid visual information processing (RVP) (p = 0.01). Finally, geographic region was a potential moderator for pattern recognition memory (p = 0.006).
Methodological Quality and Publication Bias
Methodological quality of the included studies was considered “good,” and we provided our justification on online suppl. eTable 4 in the supplement. In our main analysis, we did not find any potential publication bias (online suppl. eFig. 2) using the Egger’s test (p = 0.74) and Begg and Mazumdar’s test (p = 0.93). In our subgroup analyses, we did not find any evidence for publication bias (online suppl. eFig. 3). We also reported the tests of publication bias by individual outcome measures (online suppl. eFig. 4), in which we did not find any evidence for publication bias.
Discussion
The current study updates previous systematic review and meta-analytic works [8, 9]. Our findings are consistent with these earlier studies [8, 9], indicating that those who are currently depressed, when compared to healthy subjects, had significant moderate cognitive deficits across the domains of executive function, memory, and attention, but not in the reaction time domain. Furthermore, our study extends findings of previous studies [8, 9] by conducting subgroup analyses by medication status, treatment-resistant status, and among children and older adult populations. In our subgroup analyses, currently depressed patients who are unmedicated had a small effect size of cognitive deficits, and no significant effect sizes were found among children, but those with treatment resistance and among older adults had moderate to large effect sizes of cognitive deficits across multiple cognitive domains. In summary, our systematic review and meta-analysis demonstrate that moderate cognitive deficits are found in currently depressed patients when compared to healthy controls.
Our study only included studies that had used CANTAB tasks to assess cognitive function in currently depressed patients relative to healthy controls. While the magnitudes of cognitive deficits recorded in our findings are broadly in line with previous studies, our non-significant finding of cognitive deficit in reaction time in MDD when compared to healthy controls contrasted with the literature [9]. This may be due in part to variability of patient characteristics and/or study designs and settings. Meta-analytic research across findings from different cognition tests is needed to further assess the generalizability of our findings in the future.
This study highlights the moderate to large deficits in cognition among those with TRD and in the older adult population. In particular, moderate to large deficits were found (1) in the domains of executive function, memory, and attention among patients with TRD and (2) in the domains of memory and attention in older patients with MDD. These findings extend to the earlier study reported by Rock and his colleagues [9]. Because impaired cognitive functioning in MDD is associated with poor response to antidepressant treatment [64, 65] and deteriorated psychosocial functioning [3], pharmacological and psychosocial interventions should be tailored to address cognitive impairment in these subgroup populations.
To date, relatively few interventions have been shown to improve cognitive functioning in MDD [66, 67]. Pharmacological interventions have mixed findings. While one meta-analysis suggested that antidepressants, particularly SSRIs, may improve multiple domains of cognition in adults with MDD [68], some studies [69, 70] suggested differential effects of antidepressants on cognition, requiring further research. Furthermore, use of antidepressants and/or other psychotropic medications should be carefully considered since the medications themselves can have detrimental effects on cognitive performance due in part to withdrawal symptoms and/or other iatrogenic factors (e.g., extrapyramidal side effects affecting cognitive disturbances) [71]. Cognition-oriented non-pharmacological interventions (e.g., cognitive-behavioral therapy and cognitive remediation therapy) are also found to show promising results for improving cognitive impairment in MDD [69, 72]. Because cognitive deficits may even persist in remission from MDD [25] or result in other neurodegenerative disorders (e.g., ADRD [11, 12]), both alleviation of depressive symptoms and remediation of cognitive impairment should be prioritized when treating individuals with MDD.
There are several limitations in our study. First, included studies had relatively small sample sizes, which may have undermined the internal or external validity of studies. However, we did not detect any publication bias in our meta-analysis, and methodological quality assessment of individual studies was considered “good.” Second, while we attempted to control for and/or identify potential moderators in our meta-regression analyses (online suppl. eTable 3), it was not possible to control for other possible confounders (e.g., early or late onset of illness, duration of illness, number of episodes, and psychotic features) due to insufficient data reported in individual studies. Third, we only had two studies focusing on children [55, 60], and in particular, children’s executive function was different from findings from other subgroups. It is clinically important to note potential heterogeneity across subpopulations, and more studies are needed using the CANTAB measures to assess cognitive functioning among children with MDD in order to compare or contrast with findings from a previous study [14]. Furthermore, the number of effect sizes was not always sufficient in our subgroup analyses (e.g., 2 studies in reaction time in Fig. 3a), and interpretations should account for this potential limitation.
In conclusion, this study demonstrates that patients with MDD are likely to have significant, moderate cognitive deficits when compared to healthy subjects. Further, some subpopulation groups (e.g., those with TRD or older adults with MDD) are likely to have greater effect sizes of cognitive deficits. Because cognitive impairment can persist in remission or lead to other neurodegenerative disorders (e.g., ADRD), remediation of cognitive impairment in addition to alleviation of depressive symptoms should be an important goal when treating patients with MDD.
Statement of Ethics
An ethics statement is not applicable because this study is based exclusively on published literature. Written informed consent was not required as this study is based exclusively on published literature.
Conflict of Interest Statement
Each author completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and had none directly related to this manuscript. Rhee was supported in part by the National Institute on Aging (NIA) (#R21AG070666; R21AG078972), the National Institute of Mental Health (#R21MH117438), the National Institute on Drug Abuse (#R21DA057540), and the Institute for Collaboration on Health, Intervention, and Policy (InCHIP) of the University of Connecticut. Dr. Rhee serves as a review committee member for Patient-Centered Outcomes Research Institute (PCORI) and Substance Abuse and Mental Health Services Administration (SAMHSA) and has received honoraria payments from PCORI and SAMHSA. Dr. Rhee has also served as a stakeholder/consultant for PCORI and received consulting fees from PCORI. Dr. Rhee serves as an advisory committee member for International Alliance of Mental Health Research Funders (IAMHRF). Dr. Rhee is currently a co-editor-in-chief of Mental Health Science and has received honorarium payments annually from the publisher, John Wiley & Sons, Inc. Shim, Kaster, D’Andrea, and Steffens reported none. Manning has received research grant funding from the National Institute of Mental Health (#K21MH118420) and the Alzheimer’s Association. He serves as a consultant for Brandywine Assisted Living. Tennen was supported in part by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) (#P50AA027055 and #T32AA00729) and by the National Institute of Dental and Craniofacial Research (NIDCR) (#U01DE028520). He serves as a consultant to Yale University School of Medicine (NIAAA #R01AA026844) for which he receives a consulting fee. Dr. Tennen is the editor of the Journal of Personality and receives honorarium payments annually from the publisher, John Wiley & Sons, Inc. Forester has received research grant funding from Biogen, Eisai, Rogers Family Foundation, Spier Family Foundation, and the NIH. He also serves in the Pharmacy and Therapeutics Committee for CVS Health and is a consultant for Patina Health and Rippl Care. Nierenberg is supported by grants from the Patient-Centered Outcomes Research Institute (PaCR-2017C2-8169, XPPRN-1512-33786, XPPRN-1512-33786), the Thomas P. Hackett, MD Endowed Chair in Psychiatry at MGH, and the Dauten Family Center for Bipolar Treatment Innovation. Nierenberg is a consultant for Abbott Laboratories, Alkermes, American Psychiatric Association, Appliance Computing Inc., Mindsite, Basilea, BrainCells Inc., Brandeis University, Bristol-Myers Squibb, Clintara, Corcept, Dey Pharmaceuticals, Dainippon Sumitomo (now Sunovion), Eli Lilly, Epiq, Mylan, Forest, Genaissance, Genentech, GlaxoSmithKline, Hoffmann-La Roche, Infomedics, Intra-Cellular Therapies, Lundbeck, Janssen Pharmaceutical, Jazz Pharmaceuticals, MedAvante, Merck, Methylation Sciences, Naurex, NeuroRx, Novartis, Otsuka, Pamlabs, Parexel, Pfizer, PGx Health, Ridge Diagnostics Shire, Schering-Plough, Somerset, Sunovion, Takeda Pharmaceuticals, Targacept, and Teva; consulted through the Massachusetts General Hospital Clinical Trials Network and Institute for AstraZeneca, BrainCells Inc., Dainippon Sumitomo/Sepracor, Johnson and Johnson, LaboPharm, Merck, Methylation Science, Novartis, PGx Health, Shire, Schering-Plough, Targacept, and Takeda/Lundbeck Pharmaceuticals; receives grant or research support from American Foundation for Suicide Prevention, Agency for Healthcare Research and Quality, Brain and Behavior Research Foundation, Bristol-Myers Squibb, Cederroth, Cephalon, Cyberonics, Elan, Eli Lilly, Forest, GlaxoSmithKline, Janssen Pharmaceutical, Intra-Cellular Therapies, Lichtwer Pharma, Marriott Foundation, Mylan, National Institute of Mental Health, Pamlabs, Patient-Centered Outcomes Research Institute, Pfizer, Shire, Stanley Foundation, Takeda, and Wyeth-Ayerst; honoraria include Belvoir Publishing, University of Texas Southwestern Dallas, Brandeis University, Bristol-Myers Squibb, Hillside Hospital, American Drug Utilization Review, American Society for Clinical Psychopharmacology, Baystate Medical Center, Columbia University, Controlled Risk Insurance Company, Dartmouth Medical School, Health New England, Harold Grinspoon Charitable Foundation, Imedex, Israel Society for Biological Psychiatry, Johns Hopkins University, MJ Consulting, New York State, Medscape, MBL Communications Inc., Massachusetts General Hospital Psychiatry Academy, National Association of Continuing Education, Physicians Postgraduate Press, State University of New York Buffalo, University of Wisconsin, University of Pisa, University of Michigan, University of Miami, University of Wisconsin at Madison, World Congress of Brain Behavior and Emotion, American Professional Society of ADHD and Related Disorders, International Society for Bipolar Disorder, SciMed, Slack Publishing, Wolters Kluwer Publishing, the American Society for Clinical Psychopharmacology (formerly NCDEU), Rush Medical College, Yale University School of Medicine, National Nuclear Data Center, Nova Southeastern University, National Alliance on Mental Illness, Institute of Medicine, Continued Medical Education Institute, and the International Society for CNS Clinical Trials and Methodology; is currently or was formerly on the advisory boards of Appliance Computing Inc., BrainCells Inc., Eli Lilly, Genentech, Johnson and Johnson, Takeda/Lundbeck, Targacept, and Infomedics; owns stock options in Appliance Computing Inc., BrainCells Inc., and MedAvante; has copyrights to the Clinical Positive Affect Scale and the Massachusetts General Hospital Structured Clinical Interview for the Montgomery Asberg Depression Scale exclusively licensed to the Massachusetts General Hospital Clinical Trials Network and Institute.McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China (NSFC); speaker/consultation fees from Lundbeck, Janssen, Alkermes, Mitsubishi, Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, AbbVie, Atai Life Sciences. Dr. Roger McIntyre is a CEO of Braxia Scientific Corp.
Funding Sources
This study received no funding.
Author Contributions
Study concept, design, and supervision: Rhee; data acquisition, statistical analyses, and drafting of the manuscript: Rhee and Shim; and interpretation of data and critical revision of the manuscript for important intellectual content: Rhee, Shim, Manning, Tennen, Kaster, d’Andrea, Forester, Nierenberg, McIntyre, and Steffens. Rhee had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding Statement
This study received no funding.
Data Availability Statement
Data are publicly available. All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Supplementary Material
References
- 1. Rhee TG, Wilkinson ST, Steffens DC, Rosenheck RA, Olfson M. Prevalence of treatment for depression among US adults who screen positive for depression, 2007-2016. JAMA Psychiatry. 2020;77(11):1193–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . Depressive disorder (depression). 2023.
- 3. McIntyre RS, Cha DS, Soczynska JK, Woldeyohannes HO, Gallaugher LA, Kudlow P, et al. Cognitive deficits and functional outcomes in major depressive disorder: determinants, substrates, and treatment interventions. Depress Anxiety. 2013;30(6):515–27. [DOI] [PubMed] [Google Scholar]
- 4. Maj M, Stein DJ, Parker G, Zimmerman M, Fava GA, De Hert M, et al. The clinical characterization of the adult patient with depression aimed at personalization of management. World Psychiatr. 2020;19(3):269–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rhee TG, Steffens DC. Major depressive disorder and impaired health-related quality of life among US older adults. Int J Geriatr Psychiatry. 2020;35(10):1189–97. [DOI] [PubMed] [Google Scholar]
- 6. Rhee TG, Mohamed S, Rosenheck RA. Stages of major depressive disorder and behavioral multi-morbidities: findings from nationally representative epidemiologic study. J Affect Disord. 2021;278:443–52. [DOI] [PubMed] [Google Scholar]
- 7. Butters MA, Whyte EM, Nebes RD, Begley AE, Dew MA, Mulsant BH, et al. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry. 2004;61(6):587–95. [DOI] [PubMed] [Google Scholar]
- 8. McDermott LM, Ebmeier KP. A meta-analysis of depression severity and cognitive function. J Affect Disord. 2009;119(1–3):1–8. [DOI] [PubMed] [Google Scholar]
- 9. Rock PL, Roiser JP, Riedel WJ, Blackwell AD. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med. 2014;44(10):2029–40. [DOI] [PubMed] [Google Scholar]
- 10. McIntyre RS, Best MW, Bowie CR, Carmona NE, Cha DS, Lee Y, et al. The THINC-integrated tool (THINC-it) screening assessment for cognitive dysfunction: validation in patients with major depressive disorder. J Clin Psychiatry. 2017;78(7):873–81. [DOI] [PubMed] [Google Scholar]
- 11. Potter GG, Steffens DC. Contribution of depression to cognitive impairment and dementia in older adults. Neurologist. 2007;13(3):105–17. [DOI] [PubMed] [Google Scholar]
- 12. Steffens DC, Potter GG. Geriatric depression and cognitive impairment. Psychol Med. 2008;38(2):163–75. [DOI] [PubMed] [Google Scholar]
- 13. Bora E, Harrison BJ, Yucel M, Pantelis C. Cognitive impairment in euthymic major depressive disorder: a meta-analysis. Psychol Med. 2013;43(10):2017–26. [DOI] [PubMed] [Google Scholar]
- 14. Wagner S, Muller C, Helmreich I, Huss M, Tadic A. A meta-analysis of cognitive functions in children and adolescents with major depressive disorder. Eur Child Adolesc Psychiatry. 2015;24(1):5–19. [DOI] [PubMed] [Google Scholar]
- 15. Cognition Cambridge . Digital cognitive assessments. 2023. [Google Scholar]
- 16. Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW. Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia. 1990;28(10):1021–34. [DOI] [PubMed] [Google Scholar]
- 17. Owen AM, Sahakian BJ, Semple J, Polkey CE, Robbins TW. Visuo-spatial short-term recognition memory and learning after temporal lobe excisions, frontal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1995;33(1):1–24. [DOI] [PubMed] [Google Scholar]
- 18. Rogers RD, Blackshaw AJ, Middleton HC, Matthews K, Hawtin K, Crowley C, et al. Tryptophan depletion impairs stimulus-reward learning while methylphenidate disrupts attentional control in healthy young adults: implications for the monoaminergic basis of impulsive behaviour. Psychopharmacology. 1999;146(4):482–91. [DOI] [PubMed] [Google Scholar]
- 19. Kempton S, Vance A, Maruff P, Luk E, Costin J, Pantelis C. Executive function and attention deficit hyperactivity disorder: stimulant medication and better executive function performance in children. Psychol Med. 1999;29(3):527–38. [DOI] [PubMed] [Google Scholar]
- 20. Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbitt P. Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5(5):266–81. [DOI] [PubMed] [Google Scholar]
- 21. Sahakian BJ, Morris RG, Evenden JL, Heald A, Levy R, Philpot M, et al. A comparative study of visuospatial memory and learning in Alzheimer-type dementia and Parkinson’s disease. Brain. 1988;111(Pt 3)(Pt 3):695–718. [DOI] [PubMed] [Google Scholar]
- 22. Sahakian B, Jones G, Levy R, Gray J, Warburton D. The effects of nicotine on attention, information processing, and short-term memory in patients with dementia of the Alzheimer type. Br J Psychiatry. 1989;154:797–800. [DOI] [PubMed] [Google Scholar]
- 23. Sahakian BJ, Owen AM, Morant NJ, Eagger SA, Boddington S, Crayton L, et al. Further analysis of the cognitive effects of tetrahydroaminoacridine (THA) in Alzheimer’s disease: assessment of attentional and mnemonic function using CANTAB. Psychopharmacology. 1993;110(4):395–401. [DOI] [PubMed] [Google Scholar]
- 24. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12. [DOI] [PubMed] [Google Scholar]
- 25. Semkovska M, Quinlivan L, O'Grady T, Johnson R, Collins A, O'Connor J, et al. Cognitive function following a major depressive episode: a systematic review and meta-analysis. Lancet Psychiatry. 2019;6(10):851–61. [DOI] [PubMed] [Google Scholar]
- 26. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. [DOI] [PubMed] [Google Scholar]
- 27. Cooper H, Hedges LV, Valentine JC. The handbook of research synthesis and meta-analysis. Russell Sage Foundation; 2019. [Google Scholar]
- 28. Veroniki AA, Jackson D, Viechtbauer W, Bender R, Bowden J, Knapp G, et al. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods. 2016;7(1):55–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Van den Noortgate W, Lopez-Lopez JA, Marin-Martinez F, Sanchez-Meca J. Three-level meta-analysis of dependent effect sizes. Behav Res Methods. 2013;45(2):576–94. [DOI] [PubMed] [Google Scholar]
- 30. Tsaltas E, Kalogerakou S, Papakosta VM, Kontis D, Theochari E, Koutroumpi M, et al. Contrasting patterns of deficits in visuospatial memory and executive function in patients with major depression with and without ECT referral. Psychol Med. 2011;41(5):983–95. [DOI] [PubMed] [Google Scholar]
- 31. Moreines JL, McClintock SM, Kelley ME, Holtzheimer PE, Mayberg HS. Neuropsychological function before and after subcallosal cingulate deep brain stimulation in patients with treatment-resistant depression. Depress Anxiety. 2014;31(8):690–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bergfeld IO, Mantione M, Hoogendoorn MLC, Ruhe HG, Horst F, Notten P, et al. Impact of deep brain stimulation of the ventral anterior limb of the internal capsule on cognition in depression. Psychol Med. 2017;47(9):1647–58. [DOI] [PubMed] [Google Scholar]
- 33. Shim SR, Kim SJ. Intervention meta-analysis: application and practice using R software. Epidemiol Health. 2019;41:e2019008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Beats BC, Sahakian BJ, Levy R. Cognitive performance in tests sensitive to frontal lobe dysfunction in the elderly depressed. Psychol Med. 1996;26(3):591–603. [DOI] [PubMed] [Google Scholar]
- 35. Elliott R, Sahakian BJ, McKay AP, Herrod JJ, Robbins TW, Paykel ES. Neuropsychological impairments in unipolar depression: the influence of perceived failure on subsequent performance. Psychol Med. 1996;26(5):975–89. [DOI] [PubMed] [Google Scholar]
- 36. Elliott R, Baker SC, Rogers RD, O'Leary DA, Paykel ES, Frith CD, et al. Prefrontal dysfunction in depressed patients performing a complex planning task: a study using positron emission tomography. Psychol Med. 1997;27(4):931–42. [DOI] [PubMed] [Google Scholar]
- 37. Purcell R, Maruff P, Kyrios M, Pantelis C. Neuropsychological function in young patients with unipolar major depression. Psychol Med. 1997;27(6):1277–85. [DOI] [PubMed] [Google Scholar]
- 38. Sweeney JA, Kmiec JA, Kupfer DJ. Neuropsychologic impairments in bipolar and unipolar mood disorders on the CANTAB neurocognitive battery. Biol Psychiatry. 2000;48(7):674–84. [DOI] [PubMed] [Google Scholar]
- 39. Grant MM, Thase ME, Sweeney JA. Cognitive disturbance in outpatient depressed younger adults: evidence of modest impairment. Biol Psychiatry. 2001;50(1):35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Swainson R, Hodges JR, Galton CJ, Semple J, Michael A, Dunn BD, et al. Early detection and differential diagnosis of Alzheimer’s disease and depression with neuropsychological tasks. Dement Geriatr Cogn Disord. 2001;12(4):265–80. [DOI] [PubMed] [Google Scholar]
- 41. Murphy FC, Michael A, Robbins TW, Sahakian BJ. Neuropsychological impairment in patients with major depressive disorder: the effects of feedback on task performance. Psychol Med. 2003;33(3):455–67. [DOI] [PubMed] [Google Scholar]
- 42. Porter RJ, Gallagher P, Thompson JM, Young AH. Neurocognitive impairment in drug-free patients with major depressive disorder. Br J Psychiatry. 2003;182:214–20. [DOI] [PubMed] [Google Scholar]
- 43. O’Brien JT, Lloyd A, McKeith I, Gholkar A, Ferrier N. A longitudinal study of hippocampal volume, cortisol levels, and cognition in older depressed subjects. Am J Psychiatry. 2004;161(11):2081–90. [DOI] [PubMed] [Google Scholar]
- 44. Erickson K, Drevets WC, Clark L, Cannon DM, Bain EE, Zarate CA Jr., et al. Mood-congruent bias in affective go/no-go performance of unmedicated patients with major depressive disorder. Am J Psychiatry. 2005;162(11):2171–3. [DOI] [PubMed] [Google Scholar]
- 45. Kyte ZA, Goodyer IM, Sahakian BJ. Selected executive skills in adolescents with recent first episode major depression. J Child Psychol Psychiatry. 2005;46(9):995–1005. [DOI] [PubMed] [Google Scholar]
- 46. Taylor Tavares JV, Clark L, Cannon DM, Erickson K, Drevets WC, Sahakian BJ. Distinct profiles of neurocognitive function in unmedicated unipolar depression and bipolar II depression. Biol Psychiatry. 2007;62(8):917–24. [DOI] [PubMed] [Google Scholar]
- 47. Matthews K, Coghill D, Rhodes S. Neuropsychological functioning in depressed adolescent girls. J Affect Disord. 2008;111(1):113–8. [DOI] [PubMed] [Google Scholar]
- 48. Michopoulos I, Zervas IM, Pantelis C, Tsaltas E, Papakosta VM, Boufidou F, et al. Neuropsychological and hypothalamic-pituitary-axis function in female patients with melancholic and non-melancholic depression. Eur Arch Psychiatry Clin Neurosci. 2008;258(4):217–25. [DOI] [PubMed] [Google Scholar]
- 49. Cannon DM, Klaver JM, Peck SA, Rallis-Voak D, Erickson K, Drevets WC. Dopamine type-1 receptor binding in major depressive disorder assessed using positron emission tomography and [11C]NNC-112. Neuropsychopharmacology. 2009;34(5):1277–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Reppermund S, Ising M, Lucae S, Zihl J. Cognitive impairment in unipolar depression is persistent and non-specific: further evidence for the final common pathway disorder hypothesis. Psychol Med. 2009;39(4):603–14. [DOI] [PubMed] [Google Scholar]
- 51. Heinzel A, Northoff G, Boeker H, Boesiger P, Grimm S. Emotional processing and executive functions in major depressive disorder: dorsal prefrontal activity correlates with performance in the intra-extra dimensional set shift. Acta Neuropsychiatry. 2010;22(6):269–79. [DOI] [PubMed] [Google Scholar]
- 52. Lyche P, Jonassen R, Stiles TC, Ulleberg P, Landrø NI. Cognitive control functions in unipolar major depression with and without Co-morbid anxiety disorder. Front Psychiatr. 2010;1:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Maalouf FT, Klein C, Clark L, Sahakian BJ, Labarbara EJ, Versace A, et al. Impaired sustained attention and executive dysfunction: bipolar disorder versus depression-specific markers of affective disorders. Neuropsychologia. 2010;48(6):1862–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Braw Y, Aviram S, Bloch Y, Levkovitz Y. The effect of age on frontal lobe related cognitive functions of unmedicated depressed patients. J Affect Disord. 2011;129(1–3):342–7. [DOI] [PubMed] [Google Scholar]
- 55. Maalouf FT, Brent D, Clark L, Tavitian L, McHugh RM, Sahakian BJ, et al. Neurocognitive impairment in adolescent major depressive disorder: State vs. Trait illness markers. J Affect Disord. 2011;133(3):625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Boeker H, Schulze J, Richter A, Nikisch G, Schuepbach D, Grimm S. Sustained cognitive impairments after clinical recovery of severe depression. J Nerv Ment Dis. 2012;200(9):773–6. [DOI] [PubMed] [Google Scholar]
- 57. Bourke C, Porter RJ, Carter JD, McIntosh VV, Jordan J, Bell C, et al. Comparison of neuropsychological functioning and emotional processing in major depression and social anxiety disorder subjects, and matched healthy controls. Aust N Z J Psychiatry. 2012;46(10):972–81. [DOI] [PubMed] [Google Scholar]
- 58. Hermens DF, Lee RS, De Regt T, Lagopoulos J, Naismith SL, Scott EM, et al. Neuropsychological functioning is compromised in binge drinking young adults with depression. Psychiatry Res. 2013;210(1):256–62. [DOI] [PubMed] [Google Scholar]
- 59. Yang X, Ma X, Huang B, Sun G, Zhao L, Lin D, et al. Gray matter volume abnormalities were associated with sustained attention in unmedicated major depression. Compr Psychiatry. 2015;63:71–9. [DOI] [PubMed] [Google Scholar]
- 60. Shehab AAS, Brent D, Maalouf FT. Neurocognitive changes in selective serotonin reuptake inhibitors - treated adolescents with depression. J Child Adolesc Psychopharmacol. 2016;26(8):713–20. [DOI] [PubMed] [Google Scholar]
- 61. Sánchez-Carro Y, Portella MJ, Leal-Leturia I, Salvat-Pujol N, Etxandi M, de Arriba-Arnau A, et al. Age at illness onset and physical activity are associated with cognitive impairment in patients with current diagnosis of major depressive disorder. J Affect Disord. 2021;279:343–52. [DOI] [PubMed] [Google Scholar]
- 62. Yang X, Qi S, Wang M, Calhoun VD, Sui J, Li T, et al. Subtypes of depression characterized by different cognitive decline and brain activity alterations. J Psychiatr Res. 2021;138:413–9. [DOI] [PubMed] [Google Scholar]
- 63. Luo W, Luo L, Wang Q, Li Y, Zhang Y, Hu Y, et al. Disorder-specific impaired neurocognitive function in major depression and generalized anxiety disorder. J Affect Disord. 2022;318:123–9. [DOI] [PubMed] [Google Scholar]
- 64. Potter GG, Blackwell AD, McQuoid DR, Payne ME, Steffens DC, Sahakian BJ, et al. Prefrontal white matter lesions and prefrontal task impersistence in depressed and nondepressed elders. Neuropsychopharmacology. 2007;32(10):2135–42. [DOI] [PubMed] [Google Scholar]
- 65. Story TJ, Potter GG, Attix DK, Welsh-Bohmer KA, Steffens DC. Neurocognitive correlates of response to treatment in late-life depression. Am J Geriatr Psychiatry. 2008;16(9):752–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rosenblat JD, Kakar R, McIntyre RS. The cognitive effects of antidepressants in major depressive disorder: a systematic review and meta-analysis of randomized clinical trials. Int J Neuropsychopharmacol. 2015;19(2):pyv082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Baune BT, Brignone M, Larsen KG. A network meta-analysis comparing effects of various antidepressant classes on the digit symbol substitution test (DSST) as a measure of cognitive dysfunction in patients with major depressive disorder. Int J Neuropsychopharmacol. 2018;21(2):97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Prado CE, Watt S, Crowe SF. A meta-analysis of the effects of antidepressants on cognitive functioning in depressed and non-depressed samples. Neuropsychol Rev. 2018;28(1):32–72. [DOI] [PubMed] [Google Scholar]
- 69. Baune BT, Renger L. Pharmacological and non-pharmacological interventions to improve cognitive dysfunction and functional ability in clinical depression: a systematic review. Psychiatry Res. 2014;219(1):25–50. [DOI] [PubMed] [Google Scholar]
- 70. Huang IC, Chang TS, Chen C, Sung JY. Effect of vortioxetine on cognitive impairment in patients with major depressive disorder: a systematic review and meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2022;25(12):969–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fava GA, Rafanelli C. Iatrogenic factors in psychopathology. Psychother Psychosom. 2019;88(3):129–40. [DOI] [PubMed] [Google Scholar]
- 72. Sociali A, Borgi M, Pettorruso M, Di Carlo F, Di Natale C, Tambelli A, et al. What role for cognitive remediation in the treatment of depressive symptoms? A superiority and noninferiority meta-analysis for clinicians. Depress Anxiety. 2022;39(7):586–606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are publicly available. All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.