Acclimating broad whitefish (Coregonus nasus) and saffron cod (Eleginus gracilis) to 5#x00B0;C and 15#x00B0;C resulted in a shift in temperature tolerances through changes in CTmax and HSP70 protein and mRNA expression. These responses indicate that these two species have the potential to acclimate to temperature changes associated with climate change.
Keywords: Arctic teleosts, critical thermal maximum, HSP70 expression, thermal plasticity
Abstract
The thermally dynamic nearshore Beaufort Sea, Alaska, is experiencing climate change-driven temperature increases. Measuring thermal tolerance of broad whitefish (Coregonus nasus) and saffron cod (Eleginus gracilis), both important species in the Arctic ecosystem, will enhance understanding of species-specific thermal tolerances. The objectives of this study were to determine the extent that acclimating broad whitefish and saffron cod to 5°C and 15°C changed their critical thermal maximum (CTmax) and HSP70 protein and mRNA expression in brain, muscle and liver tissues. After acclimation to 5°C and 15°C, the species were exposed to a thermal ramping rate of 3.4°C · h−1 before quantifying the CTmax and HSP70 protein and transcript concentrations. Broad whitefish and saffron cod acclimated to 15°C had a significantly higher mean CTmax (27.3°C and 25.9°C, respectively) than 5°C-acclimated fish (23.7°C and 23.2°C, respectively), which is consistent with trends in CTmax between higher and lower acclimation temperatures. There were species-specific differences in thermal tolerance with 15°C-acclimated broad whitefish having higher CTmax and HSP70 protein concentrations in liver and muscle tissues than saffron cod at both acclimation temperatures. Tissue-specific differences were quantified, with brain and muscle tissues having the highest and lowest HSP70 protein concentrations, respectively, for both species and acclimation temperatures. The differences in broad whitefish CTmax between the two acclimation temperatures could be explained with brain and liver tissues from 15°C acclimation having higher HSP70a-201 and HSP70b-201 transcript concentrations than control fish that remained in lab-acclimation conditions of 8°C. The shift in CTmax and HSP70 protein and paralogous transcripts demonstrate the physiological plasticity that both species possess in responding to two different acclimation temperatures. This response is imperative to understand as aquatic temperatures continue to elevate.
Introduction
Broad whitefish (Iñupiaq name—Aanaakłiq) and saffron cod (Iñupiaq name—uugaq) are two species of Arctic fishes that co-occur in the nearshore Beaufort Sea, Alaska, during the ice-free period (Wolotira, 1985; Fechhelm et al., 1992; Griffiths et al., 1992; Tallman and Reist, 1997; Mueter et al., 2016). In this region, both species occupy an important intermediate trophic level connecting primary producers and higher trophic consumers (Frost and Lowry, 1981; Tallman and Reist, 1997; Reusser et al., 2016). Additionally, broad whitefish is an important subsistence resource for Indigenous coastal Alaskan communities (Fechhelm et al., 1992; Tallman and Reist, 1997). Anthropogenic-driven climate change is impacting the nearshore area and has been linked to an increase in aquatic temperatures, with some reports indicating an increase four times faster than any other region (Rantanen et al., 2022). Additionally, this area is inherently thermally dynamic and can experience unstable temperatures due to wind-driven currents and river discharge (Mccain et al., 2014; Hamman et al., 2021). Environmental thermal variability is one of the most important abiotic factors regulating fish abundance and distribution as poikilothermic organisms (Reist et al., 2006; Somero, 2010; Zhang and Kieffer, 2014; Khalsa et al., 2021). Some aspects of these species’ thermal tolerances have been studied (Bilyk and Sformo, 2021), but understanding how broad whitefish and saffron cod may respond to increasing environmental temperatures changes at an organismal and molecular level will aid in predicting the impact and response of these species as climate change continues in the nearshore Beaufort Sea.
There are a variety of biological processes that work in concert to produce an organism’s thermal tolerance (Yamashita et al., 2010). One important biological process is the induced expression of the molecular chaperones known as inducible heat shock proteins (HSP), which are upregulated due to a stressor, such as elevated temperature, to maintain cellular homeostasis (Dietz and Somero, 1993; White et al., 1994; Iwama et al., 1998; Somero, 2010). The 70-kDa HSP (HSP70) is the most highly conserved induced HSP across teleost species, and there are several HSP70 transcripts that can have unique species and tissue-specific expression patterns (Lindquist, 1986; White et al., 1994; Iwama et al., 1998; Feder and Hofmann, 1999; Santoro, 1999; Basu et al., 2002; Yamashita et al., 2010). Additionally, the production of HSP can change depending on the thermal environment (Hofmann, 1999).
Organisms can shift their HSP production in different thermal regimes, which can result in a phenotypically plastic upper thermal tolerance threshold, allowing organisms to persist across a range of temperatures (Dietz and Somero, 1992; Basu et al., 2002; Somero, 2010). For example, seasonal temperature changes have resulted in summer-acclimated fish having higher levels of constitutive HSP and higher induction temperatures for the inducible cognate versus winter-acclimated populations (Dietz and Somero, 1992; Dietz, 1994; Hofmann, 1999; Currie et al., 2000). The critical thermal maximum (CTmax) is one such plastic physiological trait commonly measured as an indicator of an organism’s upper thermal tolerance threshold, and it is measured as the highest sustained temperature resulting in physiological impairment, such as loss of equilibrium, but preceding mortality (Beitinger et al., 2000; Zhang and Kieffer, 2014). Measuring CTmax, the underlying HSP70 gene expression and protein synthesis, and how these parameters change in differing thermal conditions will improve understanding of overall upper thermal threshold and the extent that it can shift in response to environmental changes (Basu et al., 2002).
The objectives of this study were to: (1) determine the CTmax in broad whitefish and saffron cod at two acclimation temperatures; (2) quantify HSP70 protein concentration in brain, liver and muscle tissues to determine if there are differences among tissue types, acclimation temperatures and species; and (3) measure HSP70 transcript abundance in broad whitefish liver and muscle tissues to test for significant differences between tissue types and acclimation temperatures. This information could aid future predictions in the responses and distribution of these two fishes in a warming climate regime (Reist et al., 2006; Somero, 2010).
Materials and Methods
Sample collection
All fish sampling, transport and laboratory experiments were conducted in accordance with the Alaska Department of Fish and Game Aquatic Resource Permit (ADF&G; numbers CF-20-021 and CF-21-009) and the University of Alaska Fairbanks Institutional Animal Care and Use Committee (IACUC) protocol (protocol numbers 1054743, 1615559 and 197441). Paired fyke nets (1.8 × 1.7 m, with 12.77-mm stretch mesh netting) were deployed 60 m from shore with a lead net and two blocking nets (60 × 1.8 m and 15 × 1.8 m, 25-mm stretch mesh; Supplementary Fig. S1). Salinity and temperature were measured daily using a handheld probe (YSI® Pro20i; YSI Inc., Yellow Springs, Ohio). Broad whitefish (n = 17) and saffron cod (n = 35) were sampled in 2020 and 2021, respectively (Supplementary Table S2). Fish were placed in polyethylene bags (15–20 individuals per bag) with Beaufort Sea water before being shipped by air to the University of Alaska Fairbanks (UAF). At UAF, fish were placed in a recirculating rearing tank system maintained at 8°C to lab-acclimate for 6 weeks. Broad whitefish were kept at a salinity of 3.5 ppt while saffron cod were held at a salinity of 9.0 ppt throughout the duration of acclimation and experimentation periods. These salinities were chosen based on the conditions these species have been found in (Reusser et al., 2016). Both species were fed 0.5 g per fish of frozen blood worms (Glycera spp.) daily.
Thermal ramping experiment and critical thermal maximum determination
Broad whitefish were divided in to two experimental tanks set to 5°C or 15°C while saffron cod were divided in to two experimental tanks per acclimation temperature (four tanks total) (76.2 × 45.72 × 30.48 cm, 110 l), and both species were allowed to acclimate for a week (Dyer et al., 1991; Lutterschmidt and Hutchison, 1997). These temperatures were the mean minimum and maximum temperatures experienced from 30 June to 22 August 2019 in the nearshore Beaufort Sea (Gatt et al., 2019). Water temperature in the experimental tanks was monitored daily and maintained within 1.0°C of the target temperature. Each tank was fitted with an aquarium air pump (Tetra, Blacksburg, Virginia) and two air stones to maintain oxygen levels. Daily feeding was carried out as described previously, and water changes were conducted daily in the 5°C-acclimation tanks and weekly in the 15°C-acclimation tanks to maintain balanced water chemistry.
At the end of the 1-week acclimation period, fish were placed in individual plastic containers fitted with an air stone and aerator that were placed in a water bath matching the respective acclimation temperature. Thermal ramping was carried out using an 800-W titanium heater fitted in each water bath (Finnex, Chicago, Illinois). The ramping rate was set to 3.4°C · h−1, which was the most rapid water temperature rate of change observed in the Beaufort Sea nearshore area during the 2019 ice-free period (Gatt et al., 2019). Temperature was monitored and adjusted to ensure there was a linear increase in water temperature matching the ramping rate (Supplementary Fig. S2). Individual fish were continually monitored until they demonstrated a loss of equilibrium (LOE), which occurred once the fish turned over and could not right itself after 5 seconds. The temperature at which this occurred was recorded as the CTmax end-point (Becker and Genoway, 1979; Saravia et al., 2021). Fish were then immediately euthanized with an overdose (100 mg/l) of MS-222 before fork length (in millimeters) and wet weight (in grams) were measured, and a 1-cm2 section of pectoral muscle and liver tissue and the entirety of brain tissue were removed and placed into individual cryovials, flash-frozen in liquid nitrogen and stored at −80°C for future analysis. All dissecting instruments were sterilized with 70% molecular-grade ethanol and wiped clean between each tissue collection.
HSP70 protein concentration quantification
Total protein was extracted from each tissue sample using a homogenization buffer (Supplementary Table S1), and the protein concentration was quantified using the Pierce™ Coomassie (Bradford) Protein Assay Kit (ThermoFisher Scientific, Waltham, Massachusetts). A western blot assay was carried out following the methods in Kelley et al. (2013). Briefly, 10 μg of total protein from each sample was separated by electrophoresis on 10% polyacrylamide gels. Proteins were transferred to nitrocellulose membranes and incubated in monoclonal mouse anti-HSP70 antibody (1:1000) and a donkey anti-Mouse IgG (H + L) secondary antibody (1:10000; Supplementary Information 1). Nitrocellulose membranes were exposed to chemiluminescence (SuperSignal reagent; Pierce, Rockford, Illinois) and imaged using the Amerhsam Imager chemiluminescent setting (GE Healthcare, Chicago, Illinois; Supplementary Fig. S3). HSP70 protein concentrations were quantified using ImageJ (v. 1.8.0_172) following the protocol outlined by ImageJ (Abràmoff et al., 2004) and Gassmann et al. (2009) to calculate the optical density (OD) unit. An internal standard (broad whitefish fish #5 brain tissue) was used to normalize measurements between blots.
RNA-seq and HSP70 transcript quantification
Broad whitefish that remained in lab-acclimation conditions at 8°C were used as control samples for mRNA quantification. The transcriptomes from liver (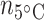 = 6;
= 6; 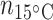 = 6;
= 6; 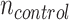 = 4) and muscle (
= 4) and muscle (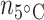 = 5;
= 5; 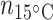 = 6;
= 6; 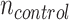 = 4) were obtained from Poly(A) selected sequencing libraries using 2x150 chemistry on a Illumina instrument. Transcript abundance was determined using the functions implemented in Salmon (v. 1.6.0, github.com/COMBINE-lab/salmon). Rainbow trout (Oncorhynchus mykiss) gene annotation dataset (downloaded from Ensembl v. 105) was used as the reference transcriptome. The quantification values were normalized using transcript per million (TPM) method (Li and Dewey, 2011). The TPM values in two protein-coding paralogous HSP70 transcripts (HSP70a-201 and HSP70b-201) were analysed for differential gene expression between the control and temperature-treated samples (Ojima et al., 2005a).
= 4) were obtained from Poly(A) selected sequencing libraries using 2x150 chemistry on a Illumina instrument. Transcript abundance was determined using the functions implemented in Salmon (v. 1.6.0, github.com/COMBINE-lab/salmon). Rainbow trout (Oncorhynchus mykiss) gene annotation dataset (downloaded from Ensembl v. 105) was used as the reference transcriptome. The quantification values were normalized using transcript per million (TPM) method (Li and Dewey, 2011). The TPM values in two protein-coding paralogous HSP70 transcripts (HSP70a-201 and HSP70b-201) were analysed for differential gene expression between the control and temperature-treated samples (Ojima et al., 2005a).
Data analysis
All statistical analyses were conducted in R (v. 4.2.1; R core team, 2022; tidyr, tidyselect, dplyr, lubridate, ggplot2, here, MASS, ggsignif, rstatix, lmtest, ggpubr, FSA, outliers; Ahlmann-Eltze and Patil, 2021; Grolemund and Wickham, 2011; Henry and Wickham, 2022; Kassambara, 2023a; Kassambara, 2023b; Komsta, 2022; Müller, 2020; Ogle et al., 2023; Venables and Ripley, 2002; Wickham et al., 2019; Zeileis and Hothorn, 2002). A Grubb’s test was used to identify outliers in the CTmax and HSP70 protein concentrations; there were too few data points in the mRNA expression dataset to remove outliers. If a high or low outlier was identified with a P-value of ≤0.05, the data value was removed. Data from both species and acclimation temperatures was kept together and a linear regression model was used to determine if fish weight, length and the interaction of these two variables correlated with CTmax. The datasets were then separated by species and treatment group, but the CTmax, HSP70 protein concentration and HSP70 mRNA expression datasets failed to meet the normality assumption for parametric tests. As a result, non-parametric statistical analyses were used with an  = 0.05 and Bonferroni-corrected P-values. A Wilcoxon rank-sum test was used to compare the CTmax values between each acclimation temperature and between species at the same acclimation temperature. The acclimation response ratio (ARR), a measurement used to compare the change in thermal tolerance threshold between species, was calculated as:
= 0.05 and Bonferroni-corrected P-values. A Wilcoxon rank-sum test was used to compare the CTmax values between each acclimation temperature and between species at the same acclimation temperature. The acclimation response ratio (ARR), a measurement used to compare the change in thermal tolerance threshold between species, was calculated as: 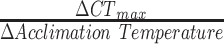 (Kelley, 2014). For the HSP70 protein expression, a Wilcoxon rank-sum test was used to compare means of the HSP70 protein concentrations between the acclimation temperatures in the three tissue samples and between species at the same acclimation temperature. A Kruskal–Wallis test was used to determine any difference in protein expression based on tissue type for each species, which was followed by a post hoc Dunn’s test to determine which pairwise comparisons were significantly different. A Wilcoxon rank-sum test was used to compare the TPM values between muscle and liver tissue samples at the same acclimation temperature. A Kruskal–Wallis test was used to determine if acclimation temperature influenced mRNA expression, and a post hoc Dunn’s test was used to determine which pairwise acclimation temperatures were different.
(Kelley, 2014). For the HSP70 protein expression, a Wilcoxon rank-sum test was used to compare means of the HSP70 protein concentrations between the acclimation temperatures in the three tissue samples and between species at the same acclimation temperature. A Kruskal–Wallis test was used to determine any difference in protein expression based on tissue type for each species, which was followed by a post hoc Dunn’s test to determine which pairwise comparisons were significantly different. A Wilcoxon rank-sum test was used to compare the TPM values between muscle and liver tissue samples at the same acclimation temperature. A Kruskal–Wallis test was used to determine if acclimation temperature influenced mRNA expression, and a post hoc Dunn’s test was used to determine which pairwise acclimation temperatures were different.
Results
Measurement of critical thermal maximum
There was no effect of fish weight (P = 0.672), length (P = 0.908) or the interaction of weight × length (P = 0.633) on the mean CTmax. For the linear model between acclimation temperature (Ta) and CTmax, there was a positive relationship for broad whitefish and saffron cod in addition to broad whitefish having a higher slope than saffron cod (Table 1). The acclimation response ratio for broad whitefish was 0.3895 and 0.2890 for saffron cod. Mean CTmax in 15°C-acclimated fish was higher than the group acclimated to 5°C by 3.6°C in broad whitefish (W = 72; P < 0.001) and by 2.7°C in saffron cod (W = 224; P < 0.0001; Fig. 1; Table 1; Supplementary Table S3). The mean CTmax in 15°C-acclimated fish was 1.4°C higher for broad whitefish than saffron cod (W = 110; P < 0.01; Fig. 1; Table 1; Supplementary Table S3), but there was no difference between the two species when acclimated to 5°C (W = 81; P > 0.05).
Table 1.
The mean CTmax and HSP70 protein concentrations for broad whitefish and saffron cod at both acclimation temperatures. Additionally, the HSP70a-201 and HSP70b-201 abundances for broad whitefish are provided
| Acclimation temperature | CT max ( o C) | Tissue | HSP70 concentration (OD unit) | mRNA expression (TPM) |
|---|---|---|---|---|
| Broad whitefish (93–148 mm, 8.6–32.0 g) | ||||
| 5oC (n = 8) | 23.7 ± 1.36 | Brain | 0.95 ± 0.06 | NA |
| Liver | 0.80 ± 0.26 | 135 ± 116 (HSP70a-201) 238 ± 197 (HSP70b-201) | ||
| Muscle | 0.47 ± 0.14 | 106 ± 29.2 (HSP70a-201) 210 ± 57.9 (HSP70b-201) | ||
| 15oC (n = 9) | 27.3 ± 1.06 | Brain | 0.87 ± 0.10 | NA |
| Liver | 0.61 ± 0.07 | 443 ± 389 (HSP70a-201) 844 ± 734 (HSP70b-201) | ||
| Muscle | 0.32 ± 0.19 | 760 ± 388 (HSP70a-201) 1657 ± 953 (HSP70b-201) | ||
| Control (n = 4) | NA | Liver | NA | 3.97 ± 2.39 (HSP70a-201) 8.34 ± 2.68 (HSP70b-201) |
| Muscle | NA | 42 ± 62.7 (HSP70a-201) 92.2 ± 136 (HSP70b-201) | ||
| Saffron cod (121–170 mm, 8.0–26.1 g) | ||||
| 5oC (n = 15) | 23.2 ± 0.79 | Brain | 0.91 ± 0.10 | NA |
| Liver | 0.21 ± 0.09 | |||
| Muscle | 0.04 ± 0.04 | |||
| 15oC (n = 16) | 25.9 ± 0.66 | Brain | 0.93 ± 0.16 | |
| Liver | 0.07 ± 0.06 | |||
| Muscle | 0.03 ± 0.02 | |||
Figure 1.

The CTmax (°C) for broad whitefish and saffron cod at the 5°C and 15°C acclimation temperatures. The median (line) and mean (dot) values are reported. Significant differences are denoted by *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 1 × 10−4 from a Wilcoxon rank-sum test.
HSP70 protein synthesis during CTmax assay
The antibody used in this study recognizes both the constitutive and inducible HSP70 protein, and thus produces a measure of total HSP 70-kDa protein abundance (Dietz and Somero, 1993; Yoo and Janz, 2003). Under the experimental conditions, any measured changes in HSP70 abundance are expected to be a result of changes in the inducible HSP70 levels (Li et al., 2007). There were no differences in the mean HSP70 protein concentration (hereafter referred to as just protein) between the two acclimation temperatures for the same tissue type (Wliver = 12.5; Wmuscle = 16.0; Wbrain = 17.0; P > 0.05; Table 1; Fig. 2a; Supplementary Table S4). However, tissue type did influence the mean protein concentration (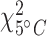 = 14.6;
= 14.6; 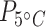 < 0.0001;
< 0.0001;  = 21.0,
= 21.0,  < 0.0001; Supplementary Table S5). Brain tissue at the 15°C acclimation temperature had a higher mean protein concentration by 43% compared to liver and by almost 3 times compared to muscle (tbrain*liver = −2.5; Pbrain*liver < 0.05; tbrain*muscle = −4.6, Pbrain*muscle < 0.0001; Table 1; Fig. 2b). However, 5°C-acclimated brain tissue only had a higher mean protein concentration compared to the muscle samples by 102% (tbrain*muscle = −3.8; Pbrain*muscle < 0.001; Table 1; Fig. 2b).
< 0.0001; Supplementary Table S5). Brain tissue at the 15°C acclimation temperature had a higher mean protein concentration by 43% compared to liver and by almost 3 times compared to muscle (tbrain*liver = −2.5; Pbrain*liver < 0.05; tbrain*muscle = −4.6, Pbrain*muscle < 0.0001; Table 1; Fig. 2b). However, 5°C-acclimated brain tissue only had a higher mean protein concentration compared to the muscle samples by 102% (tbrain*muscle = −3.8; Pbrain*muscle < 0.001; Table 1; Fig. 2b).
Figure 2.

a. Comparisons of 70-kDa heat shock protein (HSP70) concentrations (OD unit) between acclimation temperatures 5°C and 15°C and between broad whitefish and saffron cod. The median (line) and mean (dot) values are reported. Significant differences are denoted by *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 1 × 10−4 from a Wilcoxon rank-sum test. b. Comparisons of HSP70 protein concentrations (OD unit) between brain, liver and muscle samples in broad whitefish and saffron cod and between acclimation temperatures 5°C and 15°C. The median (line) and mean (dot) values are reported. Significant differences are denoted by *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 1 × 10−4 from a Kruskal-Wallis test followed by a Dunn’s post hoc test.
The 5°C-acclimated saffron cod liver tissue had three times the protein concentration than the 15°C-acclimated samples (Wliver = 8.5; P < 0.05; Table 1; Supplementary
Table S4), but there was no difference in mean protein concentration in the other two tissues (Wmuscle = 118.5; Wbrain = 148; P > 0.05; Table 1; Fig. 2a; Supplementary Table S4). Tissue type had an effect on mean protein concentration values ( = 38.5;
= 38.5; 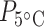 < 0.0001;
< 0.0001;  = 26.9,
= 26.9, 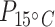 < 0.0001; Table 1; Supplementary Table S5), with brain tissues having 13.3 and 31 times higher mean protein concentration than liver and muscle at acclimation temperature 15°C (tbrain*liver = −3.1, Pbrain*liver < 0.01; tbrain*muscle = −5.1, Pbrain*muscle < 0.0001; Table 1; Fig. 2b) and 4.3 and 22.8 times higher protein concentration in liver and muscle at acclimation temperature 5°C (tbrain*liver = −3.2, Pbrain*liver < 0.01; tbrain*muscle = −6.2, Pbrain*muscle < 0.0001; Table 1). The mean protein concentration in 5°C-acclimated saffron cod liver tissue was five times higher than in muscle (tliver*muscle = −2.8, Pliver*muscle < 0.05; Table 1; Fig. 2b). Broad whitefish had a higher mean protein concentration than saffron cod by 10.7 times at 15°C and 11.8 times at 5°C in the muscle tissue (
< 0.0001; Table 1; Supplementary Table S5), with brain tissues having 13.3 and 31 times higher mean protein concentration than liver and muscle at acclimation temperature 15°C (tbrain*liver = −3.1, Pbrain*liver < 0.01; tbrain*muscle = −5.1, Pbrain*muscle < 0.0001; Table 1; Fig. 2b) and 4.3 and 22.8 times higher protein concentration in liver and muscle at acclimation temperature 5°C (tbrain*liver = −3.2, Pbrain*liver < 0.01; tbrain*muscle = −6.2, Pbrain*muscle < 0.0001; Table 1). The mean protein concentration in 5°C-acclimated saffron cod liver tissue was five times higher than in muscle (tliver*muscle = −2.8, Pliver*muscle < 0.05; Table 1; Fig. 2b). Broad whitefish had a higher mean protein concentration than saffron cod by 10.7 times at 15°C and 11.8 times at 5°C in the muscle tissue (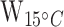 = 133;
= 133; 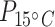 < 0.001;
< 0.001; 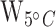 = 128,
= 128, 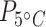 < 0.001; Table 1; Supplementary Table S4), and by 8.7 times at 15°C and 3.8 times at 5°C in liver tissue (
< 0.001; Table 1; Supplementary Table S4), and by 8.7 times at 15°C and 3.8 times at 5°C in liver tissue ( = 63;
= 63; 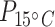 < 0.01;
< 0.01; 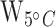 = 112,
= 112, 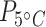 < 0.0001; Table 1; Fig. 2a; Supplementary Table S4).
< 0.0001; Table 1; Fig. 2a; Supplementary Table S4).
HSP70 mRNA abundance
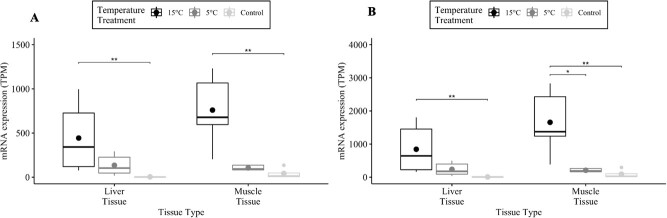
Acclimation temperature had an effect on mean TPM values in liver ( = 9.9; Pa < 0.01;
= 9.9; Pa < 0.01; 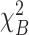 = 9.9; PB < 0.01; Table 1) and muscle (
= 9.9; PB < 0.01; Table 1) and muscle (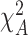 = 11.2; Pa < 0.01;
= 11.2; Pa < 0.01;  = 10.7; PB < 0.01; Table 1; Supplementary Table S7) tissues. Mean liver and muscle TPM values were 111.6 times higher and 18.1 times higher, respectively, at the 15°C acclimation temperature versus the control group in HSP70a-201 (tliver = −3.2, Pliver < 0.01; tmuscle = −3.2; Pmuscle < 0.01; Table 1). Mean liver and muscle TPM values were 101.2 times higher and 17.8 times higher, respectively, at the 15°C acclimation temperature versus the control group in HSP70b-201 (tliver = −3.2; Pliver < 0.01; tmuscle = −3.0; Pmuscle < 0.01; Table 1; Fig. 3). The HSP70b-201 transcript had 7.9 times the mean TPM when acclimated to 15°C versus 5°C in muscle (T = −2.4; P < 0.05; Table 1; Fig. 3) tissue. There were no significant differences in mean TPM values between the two tissue types at the same acclimation temperature in HSP70a-201 (
= 10.7; PB < 0.01; Table 1; Supplementary Table S7) tissues. Mean liver and muscle TPM values were 111.6 times higher and 18.1 times higher, respectively, at the 15°C acclimation temperature versus the control group in HSP70a-201 (tliver = −3.2, Pliver < 0.01; tmuscle = −3.2; Pmuscle < 0.01; Table 1). Mean liver and muscle TPM values were 101.2 times higher and 17.8 times higher, respectively, at the 15°C acclimation temperature versus the control group in HSP70b-201 (tliver = −3.2; Pliver < 0.01; tmuscle = −3.0; Pmuscle < 0.01; Table 1; Fig. 3). The HSP70b-201 transcript had 7.9 times the mean TPM when acclimated to 15°C versus 5°C in muscle (T = −2.4; P < 0.05; Table 1; Fig. 3) tissue. There were no significant differences in mean TPM values between the two tissue types at the same acclimation temperature in HSP70a-201 ( = 9;
= 9; 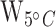 = 13;
= 13; 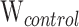 = 2; P > 0.05; Table 1; Supplementary
Table S6) and HSP70b-201 (
= 2; P > 0.05; Table 1; Supplementary
Table S6) and HSP70b-201 ( = 9;
= 9; 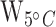 = 13;
= 13; 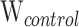 = 0.0; P > 0.05; Table 1; Supplementary
Table S6).
= 0.0; P > 0.05; Table 1; Supplementary
Table S6).
Figure 3.
The mRNA HSP70 TPM between broad whitefish liver and muscle tissue samples in addition to between the acclimation temperatures 5°C and 15°C and the control group. The control group were broad whitefish samples that were left in lab-acclimation conditions at 8°C. The image on the left is a result from transcript A, and on the right is transcript B. The median (line) and mean (dot) values are reported. Significant differences are denoted by *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 1 × 10−4 from a Kruskal-Wallis test followed by a Dunn’s post hoc test.
Discussion
Comparison of mean CTmax temperatures
The increase in mean CTmax as acclimation temperatures increased indicated that both species were successful in shifting their upper thermal tolerance. Between the two species, broad whitefish had a higher acclimation response ratio and CTmax at 15°C acclimation than saffron cod, indicating that it exhibits a greater degree of thermal plasticity (Kelley, 2014; Semsar-kazerouni and Verberk, 2018). We expected that broad whitefish would have a lower thermal tolerance and smaller acclimation response ratio than saffron cod since saffron cod has a broader geographic range and experiences higher environmental temperatures than broad whitefish (Fechhelm et al., 1992; Reusser et al., 2016). The CTmax and thermal ranges of these species were also compared to data of other fishes from different studies.
It should be noted that the ramping rates used in each study differed with this study, which could impact the CTmax data. However, the relevant thermal tolerance information is still important to compare to better understand species-specific differences. The CTmax for 5°C- and 15°C-acclimated broad whitefish was lower than reported for most like-acclimated salmonids, and the 5°C-acclimated CTmax was within 0.4°C of a CTmax previously reported for 9°C-acclimated broad whitefish (Table 2; Bilyk and Sformo, 2021). The 5°C-acclimated saffron cod had a CTmax that was higher than reported for 6.5°C-acclimated Arctic cod (Boreogadus saida), but the CTmax at both acclimation temperatures was lower than other fishes (Table 2; Bilyk and Sformo, 2021). In addition to the CTmax, there were differences in the thermal ranges (the slope of the CTmax equation; CTmax = M*TemperatureAcclimation + B) between these and other species (Table 2). Broad whitefish had a broader thermal range than other salmonids, which may be necessary to tolerate the thermally dynamic nearshore environment of the Beaufort Sea (Table 2; Nati et al., 2021). Conversely, saffron cod had a narrower thermal range than most other comparable fishes, which suggests that this species tolerates a smaller range of temperatures (Table 2). The lower CTmax and differences in thermal ranges for broad whitefish and saffron cod compared to the other fishes indicated species-specific thermal tolerances (Table 2; Nati et al., 2021).
Table 2.
A comparison of different mean CTmax and the equations between CTmax and acclimation temperature (Ta) from other teleost studies
| Species name | Acclimation temperature (°C) | Thermal ramping rate (°C · h −1 ) | CT max temperature ± SD (°C) | CT max equation | Reference |
|---|---|---|---|---|---|
| C. nasus (broad whitefish) | 5 | 3.4 | 23.7 ± 1.36 | 3 CTmax = 0.390*Ta + 21.18393, R2 = 0.6916, P < 0.0001 | This study |
| 15 | 3.4 | 27.3 ± 1.06 | |||
| E. gracilis (saffron cod) | 5 | 3.4 | 23.2 ± 0.786 | 3 CTmax = 0.289*Ta + 21.6273, R2 = 0.7928, P < 0.0001 | This study |
| 15 | 3.4 | 25.9 ± 0.655 | |||
| C. nasus (broad whitefish) | 9 | 18.0 | 23.3 ± 0.84 | NA | (Bilyk and Sformo, 2021) |
| B. saida (Arctic cod) | 0.5 | 3.0 | 14.94 | CTmax = 0.43*Ta + 14.24, R2 = 0.74, P < 0.0001 | (Drost et al., 2016) |
| 3.5 | 3.0 | 15.54 | |||
| 6.5 | 3.0 | 17.14 | |||
| Coregonus clupeaformis (lake whitefish) | 6 | 12.0 | 23.9 ± 0.15 | 1 CTmax = 0.175*Ta + 22.867 | (Manzon et al., 2022) |
| 12 | 12.0 | 25.0 ± 0.33 | |||
| 18 | 12.0 | 26.0 ± 0.58 | |||
| Acipenser brevirostrum (shortnose sturgeon) | 10 | 6.0 | 27.6 ± 0.35 | CTmax = 0.52*Ta + 22.87, R2 = 0.803, P < 0.001 | (Zhang and Kieffer, 2014) |
| 15 | 6.0 | 31.5 ± 0.91 | |||
| 20 | 6.0 | 32.8 ± 1.17 | |||
| O. mykiss (rainbow trout) | 10 | 18.0 | 28.0 ± 0.36 | CTmax = 0.18*Ta + 26.23, R2 = 0.975, P < 0.0001 | (Currie et al., 1998) |
| 15 | 18.0 | 29.1 ± 0.27 | |||
| 20 | 18.0 | 29.8 ± 0.36 | |||
| Oncorhynchus kisutch (coho salmon) | 5 | 6.0 | 24.844 | NA | (Becker and Genoway, 1979) |
| 5 | 18.0 | 25.324 | |||
| 15 | 6.0 | 28.134 | |||
| 15 | 18.0 | 28.704 | |||
| Oncorhynchus clarkii (cutthroat trout) | 10 | 24.0 | 27.63 ± 0.08 | 2 CTmax = 0.255*Ta + 25.5 | (Heath, 1963; Beitinger et al., 2000) |
| 15 | 24.0 | 29.06 ± 0.05 | |||
| 20 | 24.0 | 29.88 ± 0.09 |
1= calculated by this researcher based on the paper’s data.
2 = no R2 or P-value was reported for the equation.
3= only two acclimation temperatures were used to make the equation.
4 = no standard deviation values were reported.
HSP70 protein synthesis during CTmax assay
The few significant differences between HSP70 protein concentration and acclimation temperatures were contrary to other studies that have reported enhanced in vivo and in vitro protein synthesis at higher acclimation temperatures (Dietz, 1994; Iwama et al., 1999; Dalvi et al., 2012). The observed tissue-specific HSP70 protein concentrations could be explained by underlying differences in the heat shock response between the tissue types, including differences in the constitutive HSP levels, different induction temperatures for the inducible HSP and variations in protein denaturation and proteolysis of damaged proteins (Dietz and Somero, 1993; Smith et al., 1999). Additionally, tissue-specific protein expression patterns have been reported in other teleosts, such as Atlantic salmon (Salmo salar), but the tissue type with the highest or lowest concentration tends to vary by species (Smith et al., 1999; Dalvi et al., 2012).
The differences in protein concentration between broad whitefish and saffron cod has been reported in other species. Atlantic salmon, a mesothermic salmonid, showed a unique HSP expression profile relative to the eurythermal fathead minnow (Pimephales promelas) and mummichog (Fundulus heteroclitus) (Smith et al., 1999). Species have unique thermal histories which, in addition to environmental factors, can impact the stress response, and these factors could have resulted in the species-specific protein concentrations observed in the current study (Iwama et al., 1999). The significantly higher HSP70 protein concentration in broad whitefish supports the higher CTmax at the 15°C acclimation temperature relative to saffron cod. Previous research has reported higher levels of HSP70 correlates with higher acclimation temperatures, which results in an overall increase in upper thermal tolerance (Dalvi et al., 2012).
HSP70 mRNA abundance
The upregulation of HSP70a-201 and HSP70b-201 as acclimation temperature increased has been reported before in rainbow trout erythrocytes and cell lines where elevated HSP70 mRNA correlated with rising exposure temperatures (Currie et al., 2000; Ojima et al., 2005a; Yamashita et al., 2010). The upregulated expression for 15°C-acclimated broad whitefish could explain the significantly higher CTmax at the same temperature as higher HSP70 transcript levels have been correlated with warmer acclimated organisms, including the longjaw mudsucker (Gillichthys mirabilis) (Dietz, 1994; Hofmann, 1999). Higher mRNA transcript concentration in 15°C-acclimated broad whitefish suggested that there is a temperature-dependent induction profile for HSP70 mRNA, which could explain the observed CTmax for this species.
The genes encoding these transcripts are paralogous and have demonstrated unique expression profiles in rainbow trout cell lines before, with HSP70b-201 having higher relative concentrations than HSP70a-201 (Ojima et al., 2005b). Additionally, the heat shock transcription factor, HSF1, are encoded by two paralogous genes, which could result in unique HSP70 transcript concentrations due to differential binding rates to the heat shock element (Ojima et al., 2005b). The paralogous genes encoding both the HSP70 and HSF1 transcripts could account for the unique concentrations observed in the two transcripts. Even with this differential expression, both HSP70 transcripts are vital in the heat stress response for fish, which is likely why both transcripts were significantly upregulated at the 15°C acclimation temperature (Ojima et al., 2005a). Further research is needed to confirm the exact genetic mechanisms producing HSP70 transcripts in broad whitefish.
There were no significant differences in transcript concentrations between tissue types, contrasting the differences observed in HSP70 protein concentration between tissue type. The lack of tissue-specific differences could be due to differences in transcription and translation rates. Smith et al. (1999) found that the maximum rate of protein synthesis occurred after 2 hours of thermal shock in Atlantic salmon, but mRNA transcript concentrations continued to increase past this point. This paper suggested that existing HSP70 mRNA are translated into protein before more are transcribed. It is possible that, within the time frame of this experiment, the mRNA transcript levels had not yet increased to match the protein concentrations. There could potentially be other post-transcriptional regulations that are occurring during the heat shock response, and it is something that has been observed in Arctic charr (Salvelinus alpinus) and Atlantic salmon (Lewis et al., 2016). Further experimentation is needed to determine what, if any, transcriptional or translational modifications are occurring in broad whitefish.
Implications of thermal tolerance variation under ongoing climate change
There has been little to no data on the physiological and molecular parameters driving the upper thermal tolerance of broad whitefish and saffron cod. Given the accelerated rate of climate change, ecological importance of both species and the subsistence use of broad whitefish, understanding these parameters for both species will provide insight into the potential both species have in responding to elevated temperatures (Frost and Lowry, 1981; Fechhelm et al., 1992; Tallman and Reist, 1997; Reusser et al., 2016). The results of this study suggest that broad whitefish and saffron cod in the nearshore Beaufort Sea can shift their upper thermal tolerance due to phenotypic plasticity driven by underlying molecular mechanisms. Both species demonstrated a broad range of HSP70 protein expression, which is common for species living in a variable environment and could explain the higher CTmax at this temperature for 15°C-acclimated broad whitefish (White et al., 1994; Currie et al., 2000). Further, the higher CTmax, acclimation response ratio and HSP70 protein concentration for broad whitefish suggests they are more physiologically capable of responding to heat stress than saffron cod. The shifts in HSP70 protein and mRNA concentrations with higher in situ temperature changes could result in an increased tolerance to future temperature changes (Iwama et al., 1999), but it should be noted that the observed upper thermal tolerance in these lab conditions does not necessarily correlate to survivability in the nearshore Beaufort Sea if temperatures reach or exceed 15°C.
While successfully shifting an organism’s upper thermal tolerance threshold is critical to returning to organismal homeostasis, this response could also come at a cost. Studies have shown that increasing acclimation temperatures results in the convergence of the CTmax and incipient lethal temperatures, which limits continued acclimation (Somero, 2010). It would be beneficial to determine the upper temperature limit for broad whitefish and saffron cod to gauge their acclimation potential at temperatures warmer than 15°C. Additionally, the inducible heat shock proteins interfere with ongoing cellular processes, and the increased transcription of HSP mRNA is energetically costly and not sustainable (Feder and Hofmann, 1999; Hofmann, 1999; Lewis et al., 2016). The acclimation limit and energetic expenditure of continually producing HPS70 emphasizes the potential cost of inducible thermal tolerance for these two species.
The evidence of phenotypic plasticity in broad whitefish and saffron cod suggests that they have the potential to respond to future increases in environmental temperature in the nearshore Beaufort Sea. The thermal tolerance data from this study paired with the CTmax measurements in other Arctic teleost species in this region shows a variety of species-specific thermal tolerance responses (Drost et al., 2016; Bilyk and Sformo, 2021). Given these species-specific responses, changing thermal parameters may influence the distribution and abundance of fishes in the nearshore Beaufort Sea (Reist et al., 2006; Somero, 2010; Priest et al., 2022). There has already been a recent notable increase in broad whitefish and saffron cod abundance (Hamman et al., 2021; Priest et al., 2022). Overall, the current study provided detailed insights into the physiological and molecular parameters driving the upper thermal tolerance shifts demonstrated in broad whitefish and saffron cod, and this phenotypic plasticity may be used in response to future changes in thermal conditions in the nearshore Beaufort Sea.
Acknowledgments
Thank you to Hilcorp Alaska LLC for providing the funding for this work and the Endicott camp for providing the infrastructure we used and logistical support. We appreciate the support of graduate students Kyle Gatt and Jonah Bacon and technicians Feyne Elmore, Sarah Barnes, Alex Page and Amanda Frantz for the assistance in fish collection and sample processing. Finally, thank you to Dr Kristen O’Brien for providing materials that were integral to completion of this project.
Author Contributions
C.R.W. conceived the presented ideas, was the lead in all experiments, wrote the proposal to secure the URSA grant and wrote the original draft of this manuscript with input from all authors. T.M.S., A.L.K. and J.A.L. assisted in experiment conceptualization, implementation and investigation in addition to formal analysis. A.L.K. provided all materials for the HSP70 protein concentration experiment and assisted in western blot experimentation. J.A.L. wrote the proposal to secure the grant for broad whitefish RNA-seq analysis and helped submit all broad whitefish tissue samples. T.M.S. wrote the proposal to secure the Hilcorp grant that provided funding for field work in Prudhoe Bay, AK, in addition to assisting in sample collection and implementation of the infrastructure to hold all live fish samples. All authors provided critical feedback over the course of the experiment and reviewed and edited the agreed-upon final manuscript.
Conflict of Interest
These authors declare no conflict of interest.
Funding
This work was supported by Hillcorp Alaska, LLC [grant number 2255–00659.14.11.60], Alaska Established Program to Stimulate Competitive Research (EPSCoR) National Science Foundation (NSF) award [grant number OIA-1757348] and the Undergraduate Research and Scholarly Activity (URSA) Mentor award [grant number M21–6]. Additionally, research reported in this publication was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number 2P20GM103395. The content is solely the authors’ responsibility and does not necessarily reflect the official views of the NIH. Funding to help with the publication of this article was provided by the University of Alaska Fairbanks Vice Chancellor for Research.
Supplementary Material
Data Availability
The data used for this article is available as a supplementary .CSV file.
Contributor Information
Carolyn R Waterbury, Department of Fisheries, University of Alaska Fairbanks, Fairbanks AK 99705, USA.
Trent M Sutton, Department of Fisheries, University of Alaska Fairbanks, Fairbanks AK 99705, USA.
Amanda L Kelley, Department of Marine Biology, University of Alaska Fairbanks, Fairbanks AK 99705, USA.
J Andrés López, Department of Fisheries, University of Alaska Fairbanks, Fairbanks AK 99705, USA.
Supplementary Material
Supplementary material is available at Conservation Physiology online.
References
- Abràmoff MD, Magalhães PJ, Ram SJ (2004) Image processing with ImageJ. Biophotonics Int 11: 36–42. [Google Scholar]
- Ahlmann-Eltze C, Patil I (2021) Ggsignif: R package for displaying significance brackets for 'ggplot2'. PsyArxiv . 10.31234/osf.io/7awm6. [DOI] [Google Scholar]
- Basu N, Todgham AE, Ackerman PA, Bibeau MR, Nakano K, Schulte PM, Iwama GK (2002) Heat shock protein genes and their functional significance in fish. Gene 295: 173–183. 10.1016/s0378-1119(02)00687-x. [DOI] [PubMed] [Google Scholar]
- Becker CD, Genoway RG (1979) Evaluation of the critical thermal maximum for determining thermal tolerance of freshwater fish. Environ Biol Fishes 4: 245–256. 10.1007/BF00005481. [DOI] [Google Scholar]
- Beitinger TL, Bennett WA, Mccauley RW (2000) Temperature tolerances of North American freshwater fishes exposed to dynamic changes in temperature. Environ Biol Fishes 58: 237–275. 10.1023/A:1007676325825. [DOI] [Google Scholar]
- Bilyk KT, Sformo TL (2021) Varying heat tolerance among Arctic nearshore fishes. Polar Biology 44: 607–612. 10.1007/s00300-021-02815-6. [DOI] [Google Scholar]
- Currie RJ, Bennett WA, Beitinger TL (1998) Critical thermal minima and maxima of three freshwater game-fish species acclimated to constant temperatures. Environ Biol Fishes 51: 187–200. 10.1023/A:1007447417546. [DOI] [Google Scholar]
- Currie S, Moyes CD, Tufts BL (2000) The effects of heat shock and acclimation temperature on hsp70 and hsp30 mRNA expression in rainbow trout: in vivo and in vitro comparisons. J Fish Biol 56: 398–408. 10.1111/j.1095-8649.2000.tb02114.x. [DOI] [Google Scholar]
- Dalvi RS, Pal AK, Tiwari LR, Baruah K (2012) Influence of acclimation temperature on the induction of heat-shock protein 70 in the catfish Horabagrus brachysoma (Günther). Fish Physiol Biochem 38: 919–927. 10.1007/s10695-011-9578-9. [DOI] [PubMed] [Google Scholar]
- Dietz TJ (1994) Acclimation of the threshold induction temperatures for 70-kDa and 90-kDa heat shock proteins in the fish Gillichthys mirabilis. J Exp Biol 188: 333–338. 10.1242/jeb.188.1.333. [DOI] [PubMed] [Google Scholar]
- Dietz TJ, Somero GN (1992) The threshold induction temperature of the 90-kDa heat shock protein is subject to acclimatization in eurythermal goby fishes (genus Gillichthys). Proceedings of the National Academy of Science 89: 3389–3393. 10.1073/pnas.89.8.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz TJ, Somero GN (1993) Species-and tissue-specific synthesis patterns for heat-shock proteins HSP70 and HSP90 in several marine teleost fishes. Physiol Zool 66: 863–880. 10.1086/physzool.66.6.30163744. [DOI] [Google Scholar]
- Drost HE, Lo M, Carmack EC, Farrell AP (2016) Acclimation potential of Arctic cod (Boreogadus saida) from rapidly warming Arctic Ocean. J Exp Biol 219: 3114–3125. 10.1242/jeb.140194. [DOI] [PubMed] [Google Scholar]
- Dyer SD, Dickson KL, Zimmerman EG, Sanders BM (1991) Tissue specific patterns of synthesis of heat-shock proteins and thermal tolerance of the fathead minnow (Pimephales promelas). Can J Zool 69: 2021–2027. 10.1139/z91-282. [DOI] [Google Scholar]
- Fechhelm RG, Dillinger RE, Gallaway BJ, Griffiths WB (1992) Modeling of in situ temperature and growth relationships for yearling broad whitefish in Prudhoe Bay, Alaska. Trans Am Fish Soc 121: 1–12. . [DOI] [Google Scholar]
- Feder ME, Hofmann GE (1999) Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol 61: 243–282. 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- Frost KJ, Lowry LF (1981) Trophic importance of some marine gadids in northern Alaska and their body-otolith size relationship. Fish Bull 79: 187–192. [Google Scholar]
- Gassmann M, Grenacher B, Rohde B, Vogel J (2009) Quantifying Western blots: pitfalls of densitometry. Electrophoresis 30: 1845–1855. 10.1002/elps.200800720. [DOI] [PubMed] [Google Scholar]
- Gatt KP, Hamman CR, Priest JT, Green DG, Sutton TM (2019) Beaufort Sea long-term nearshore fish monitoring program: 2019 annual report. In Report for Hilcorp Alaska, LLC by the University of Alaska Fairbanks. College of Fisheries and Ocean Sciences, Department of Fisheries, Fairbanks, AK. [Google Scholar]
- Griffiths WB, Gallaway BJ, Gazey WJ, Dillinger RE (1992) Growth and condition of Arctic cisco and broad whitefish as indicators of causeway-induced effects in the Prudhoe Bay region, Alaska. Trans Am Fish Soc 121: 557–577. . [DOI] [Google Scholar]
- Grolemund G, Wickham H (2011) Dates and times made easy with lubridate. J Stat Softw 40: 1–25. 10.18637/jss.v040.i03. https://www.jstatsoft.org/v40/i03/. [DOI] [Google Scholar]
- Hamman CR, Bacon JA, Sutton TM (2021) Beaufort Sea long-term nearshore fish monitoring program: 2021 annual report. In Report for Hilcorp Alaska, LLC by the University of Alaska Fairbanks. College of Fisheries and Ocean Sciences, Department of Fisheries, Fairbanks, AK. [Google Scholar]
- Heath WG (1963) Thermoperiodism in sea-run cutthroat trout (Salmo clarki clarki). Science 142: 486–488. 10.1126/science.142.3591.486. [DOI] [PubMed] [Google Scholar]
- Henry L, Wickham H (2022) Tidyselect: select from a set of strings. R package version 1.2.0. https://CRAN.R-project.org/package=tidyselect.
- Hofmann GE (1999) Ecologically relevant variation in induction and function of heat shock proteins in marine organisms. Am Zool 39: 889–900. 10.1093/icb/39.6.889. [DOI] [Google Scholar]
- Iwama GK, Thomas PT, Forsyth RB, Vijayan MM (1998) Heat shock protein expression in fish. Reviews in Fish Biology and Fisheries 8: 35–56. 10.1023/A:1008812500650. [DOI] [Google Scholar]
- Iwama GK, Vijayan MM, Forsyth RB, Ackerman PA (1999) Heat shock proteins and physiological stress in fish. Am Zool 39: 901–909. 10.1093/icb/39.6.901. [DOI] [Google Scholar]
- Kassambara A (2023a) Ggpubr: 'ggplot2' based publication ready plots. R package version 0.6.0. https://CRAN.R-project.org/package=ggpubr.
- Kassambara A (2023b) Rstatix: pipe-friendly framework for basic statistical tests. R package version 0.7.2. https://CRAN.R-project.org/package=rstatix.
- Kelley AL (2014) The role thermal physiology plays in species invasion. Conservation Physiology 2: 1–14. 10.1093/conphys/cou045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AL, Rivera CE, Buckley BA (2013) Cold tolerance of the invasive Carcinus maenas in the East Pacific: molecular mechanisms and implications for range expansion in a changing climate. Biol Invasions 15: 2299–2309. 10.1007/s10530-013-0454-7. [DOI] [Google Scholar]
- Khalsa NS, Gatt KP, Sutton TM, Kelley AL (2021) Characterization of the abiotic drivers of abundance of nearshore Arctic fishes. Ecol Evol 11: 11491–11506. 10.1002/ece3.7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komsta L (2022) Outliers: tests for outliers. R package version 0.15. https://CRAN.R-project.org/package=outliers.
- Lewis M, Götting M, Anttila K, Kanerva M, Prokkola JM, Seppänen E, Kolari I, Nikinmaa M (2016) Different relationship between hsp70 mRNA and hsp70 levels in the heat shock response of two salmonids with dissimilar temperature preference. Front Physiol 7: 1–12. 10.3389/fphys.2016.00511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Dewey CN (2011) RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12: 1–16. 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Qin JG, Abbott CA, Li X, Benkendorff K (2007) Synergistic impacts of heat shock and spawning on the physiology and immune health of Crassostrea gigas: an explanation for summer mortality in Pacific oysters. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology 293: R2353–R2362. 10.1152/ajpregu.00463.2007. [DOI] [PubMed] [Google Scholar]
- Lindquist S (1986) The heat shock response. Annu Rev Biochem 55: 1151–1191. 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Lutterschmidt WI, Hutchison VH (1997) The critical thermal maximum: history and critique. Can J Zool 75: 1561–1574. 10.1139/z97-783. [DOI] [Google Scholar]
- Manzon LA, Zak MA, Agee M, Boreham DR, Wilson JY, Somers CM, Manzon RG (2022) Thermal acclimation alters both basal heat shock protein gene expression and the heat shock response in juvenile lake whitefish (Coregonus clupeaformis). J Therm Biol 104: 103185–103111. 10.1016/j.jtherbio.2021.103185. [DOI] [PubMed] [Google Scholar]
- Mccain KA, Raborn Scott W, Fechhelm RG, Raborn SW (2014) Year 31 of the Long-Term Monitoring of Nearshore Beaufort Sea Fishes in the Prudhoe Bay Region: 2013 Annual Report. Report for BP Exploration (Alaska) Inc. by LGL Alaska Research Associates, Inc, Anchorage, Alaska, p. 72. [Google Scholar]
- Mueter FJ, Nahrgang J, John Nelson R, Berge J (2016) The ecology of gadid fishes in the circumpolar Arctic with a special emphasis on the polar cod (Boreogadus saida). Polar Biology 39: 961–967. 10.1007/s00300-016-1965-3. [DOI] [Google Scholar]
- Müller K (2020). Here: a simpler way to find your files. R package version 1.0.1. https://CRAN.R-project.org/package=here.
- Nati JJH, Svendsen MBS, Marras S, Killen SS, Steffensen JF, McKenzie DJ, Domenici P (2021) Intraspecific variation in thermal tolerance differs between tropical and temperate fishes. Sci Rep 11: 21272–21278. 10.1038/s41598-021-00695-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogle DH, Doll JC, Wheeler AP, Dinno A (2023) FSA: simple fisheries stock assessment methods. R package version 0.9.4. https://CRAN.R-project.org/package=FSA.
- Ojima N, Yamashita M, Watabe S (2005a) Comparative expression analysis of two paralogous Hsp70s in rainbow trout cell exposed to heat stress. Biochim Biophys Acta Rev Cancer 1681: 99–106. 10.1016/j.bbaexp.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Ojima N, Yamashita M, Watabe S (2005b) Quantitative mRNA expression profiling of heat-shock protein families in rainbow trout cells. Biochim Biophys Acta Rev Cancer 1681: 99–106. 10.1016/j.bbaexp.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Priest JT, Mueter FJ, Raborn SW, Sutton TM (2022) Effects of environmental variables on a nearshore arctic fish community, 2001–2018. Polar Biology 45: 585–599. 10.1007/s00300-022-03013-8. [DOI] [Google Scholar]
- R Core Team (2022) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/ [Google Scholar]
- Rantanen M, Karpechko AY, Lipponen A, Nordling K, Hyvärinen O, Ruosteenoja K, Vihma T, Laaksonen A (2022) The Arctic has warmed nearly four times faster than the globe since 1979. Communications Earth & Environment 3: 1–10. 10.1038/s43247-022-00498-3. [DOI] [Google Scholar]
- Reist JD, Wrona FJ, Prowse TD, Power M, Dempson JB, Beamish RJ, King JR, Carmichael TJ, Sawatzky CD (2006) General effects of climate change on Arctic fishes and fish populations. Ambio 35: 370–380. 10.1579/0044-7447(2006)35[370:GEOCCO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Reusser DA, Frazier ML, Loiselle RA, Lee H, Thorsteinson LK (2016) Arctic climate change – a tale of two cods. In Thorsteinson LK, Love MS, eds, Alaska Arctic Marine Fish Ecology Catalog: U.S. Geological Survey Scientific Investigations Report 2016–5038 (OCS Study, BOEM 2016–048). U.S. Geological Survey, Virginia, pp. 659–679. [Google Scholar]
- Santoro GM (2000) Heat shock factors and the control of the stress response. Biochem Pharmacol 59: 55–63. 10.1016/s0006-2952(99)00299-3. [DOI] [PubMed] [Google Scholar]
- Saravia J, Paschke K, Oyarzún-Salazar R, Christina Cheng C-H, Navarro JM, Vargas-Chacoff JM (2021) Effects of warming rates on physiological and molecular components of response to CTMax heat stress in the Antarctic fish Harpagifer antarcticus. Journal of Thermal Biology 99: 1–11. 10.1016/j.jtherbio.2021.103021. [DOI] [PubMed] [Google Scholar]
- Semsar-kazerouni M, Verberk WC (2018) It’s about time: linkages between heat tolerance, thermal acclimation and metabolic rate at different temporal scales in the freshwater amphipod Gammarus fossarum Koch, 1836. J Therm Biol 75: 31–37. 10.1016/j.jtherbio.2018.04.016. [DOI] [PubMed] [Google Scholar]
- Smith TR, Tremblay GC, Bradley TM (1999) Characterization of the heat shock protein response of Atlantic salmon (Salmo salar). Fish Physiol Biochem 20: 279–292. 10.1023/A:1007743329892. [DOI] [Google Scholar]
- Somero GN (2010) The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J Exp Biol 213: 912–920. 10.1242/jeb.037473. [DOI] [PubMed] [Google Scholar]
- Tallman RF, Reist JD (1997) The proceedings of the broad whitefish workshop: the biology, traditional knowledge and scientific management of broad whitefish (Coregonus nasus (Pallas)) in the lower Mackenzie River. Canadian Technical Report of Fisheries and Aquatic Sciences 2193: 1–227. [Google Scholar]
- Venables WN, Ripley BD (2002) Modern Applied Statistics with S, EdFourth. Springer, New York, ISBN 0-387-95457-0. [Google Scholar]
- White CN, Hightower LE, Jack R (1994) Variation in heat-shock proteins among species of desert fishes (Poeciliidae, Poeciliopsis). Mol Biol Evol 11: 106–119. 10.1093/oxfordjournals.molbev.a040085. [DOI] [PubMed] [Google Scholar]
- Wickham H, Averick M, Bryan J, Chang W, McGowan LD, François R, Grolemund G, Hayes A, Henry L, Hester Jet al. (2019) Welcome to the tidyverse. Journal of Open Source Software 4: 1686. 10.21105/joss.01686. [DOI] [Google Scholar]
- Wolotira RJ (1985) Saffron cod (Eleginus gracilis) in western Alaska: the resource and its potential. NOAA Technical Memorandum NMFS F/NWC-79: 1–127. [Google Scholar]
- Yamashita M, Yabu T, Ojima N (2010) Stress protein HSP70 in fish. Aqua-BioSci Monogr (ABSM) 3: 111–141. 10.5047/absm.2010.00304.0111. [DOI] [Google Scholar]
- Yoo JL, Janz DM (2003) Tissue-specific HSP70 levels and reproductive physiological responses in fishes inhabiting a metal-contaminated creek. Arch Environ Contam Toxicol 45: 110–120. 10.1007/s00244-002-0109-7. [DOI] [PubMed] [Google Scholar]
- Zeileis A, Hothorn T (2002) Diagnostic checking in regression relationships. R News 2: 7–10. https://CRAN.R-project.org/doc/Rnews/. [Google Scholar]
- Zhang Y, Kieffer JD (2014) Critical thermal maximum (CTmax) and hematology of shortnose sturgeons (Acipenser brevirostrum) acclimated to three temperatures. Can J Zool 92: 215–221. 10.1139/cjz-2013-0223. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.