Highlights
-
•
Aberrant brain dynamics across schizophrenia and mood disorder were investigated.
-
•
Energy landscape analysis was used in this study.
-
•
Brain dynamics was more disrupted in schizophrenia than in mood disorder.
-
•
Aberrant brain dynamics and cognition in schizophrenia are associated with each other.
Keywords: Energy-landscape analysis, Resting-state fMRI, Default mode network, Psychiatric disorder
Abstract
Introduction
The dynamics of large-scale networks, which are known as distributed sets of functionally synchronized brain regions and include the visual network (VIN), somatomotor network (SMN), dorsal attention network (DAN), salience network (SAN), limbic network (LIN), frontoparietal network (FPN), and default mode network (DMN), play important roles in emotional and cognitive processes in humans. Although disruptions in these large-scale networks are considered critical for the pathophysiological mechanisms of psychiatric disorders, their role in psychiatric disorders remains unknown. We aimed to elucidate the aberrant dynamics across large-scale networks in patients with schizophrenia (SZ) and mood disorders.
Methods
We performed energy-landscape analysis to investigate the aberrant brain dynamics of seven large-scale networks across 50 healthy controls (HCs), 36 patients with SZ, and 42 patients with major depressive disorder (MDD) recruited at Wakayama Medical University. We identified major patterns of brain activity using energy-landscape analysis and estimated their duration, occurrence, and ease of transition.
Results
We identified four major brain activity patterns that were characterized by the activation patterns of the DMN and VIN (state 1, DMN (-) VIN (-); state 2, DMN (+) VIN (+); state 3, DMN (-) VIN (+); and state 4, DMN (+) VIN (-)). The duration of state 1 and the occurrence of states 1 and 2 were shorter in the SZ group than in HCs and the MDD group, and the duration of state 3 was longer in the SZ group. The ease of transition between states 3 and 4 was larger in the SZ group than in the HCs and the MDD group. The ease of transition from state 3 to state 4 was negatively associated with verbal fluency in patients with SZ. The current study showed that the brain dynamics was more disrupted in SZ than in MDD.
Conclusions
Energy-landscape analysis revealed aberrant brain dynamics across large-scale networks between SZ and MDD and their associations with cognitive abilities in SZ, which cannot be captured by conventional functional connectivity analyses. These results provide new insights into the pathophysiological mechanisms underlying SZ and mood disorders.
1. Introduction
Schizophrenia (SZ) and mood disorders are diagnosed and treated as distinct psychiatric disorders (Fischer, 2012, Malhi and Mann, 2018). Delusions and hallucinations are the two key symptoms that characterize SZ (Tandon et al., 2013), while depressed mood and loss of interest are the core symptoms of depressive episodes of mood disorders (Uher et al., 2014). Furthermore, while antipsychotics are used as medications for SZ, antidepressants are used for the treatment of major depressive disorder (MDD), which is categorized as a mood disorder. However, recent studies have shown that SZ and mood disorders share overlapping commonalities from multiple perspectives. SZ and mood disorders share overlapping genetic factors (Gandal et al., 2018, Lee et al., 2013, Pettersson et al., 2016, Purcell et al., 2009), gray matter loss (Goodkind et al., 2015), functional brain network dysfunction (Ishida et al., 2023, Lee et al., 2013, Wei et al., 2017, Xia et al., 2018), and clinical symptoms such as depression and anxiety (Chen et al., 2019). Thus, unraveling the extent of commonalities and distinctions between SZ and mood disorders from a pathophysiological perspective is indispensable for providing deeper insights into the biological underpinnings of these disorders.
Cognitive processes or mental states are organized by the systematic dynamics of the activation patterns of distributed sets of brain regions known as large-scale functional networks, including the visual network (VIN), somatomotor network (SMN), dorsal attention network (DAN), salience network (SAN), limbic network (LIN), frontoparietal network (FPN), and default mode network (DMN) (Allen et al., 2014, Bassett et al., 2011, Braun et al., 2015, Breakspear, 2017, Kucyi et al., 2017, Watanabe and Rees, 2017, Zalesky et al., 2014). Disruptions in these network dynamics are considered to underlie the psychopathological mechanisms of psychiatric disorders, including SZ and mood disorders (Dong et al., 2018; Dosenbach et al.; Gong et al., 2021, Goya-Maldonado et al., 2016, Kaiser et al., 2015, Menon, 2011, Moran et al., 2013, Mulders et al., 2015, Palaniyappan et al., 2011, Seeley et al., 2007, Sha et al., 2019). Recent studies have attempted to reveal such aberrant network connections between SZ and mood disorders using resting-state functional magnetic resonance imaging (rs-fMRI) (Goya-Maldonado et al., 2016, Menon, 2011, Moran et al., 2013, Mulders et al., 2015, Palaniyappan et al., 2011). However, only a limited number of studies directly compared HC, SZ and mood disorders, and their results were inconsistent. For example, while Baker et al. showed that graded disruption of the FPN was commonly associated with affective and psychotic illnesses (Baker et al., 2019), Sha et al. found that DMN-SN and SN-FPN hypoconnectivity and DMN-FPN and DMN-SN hyperconnectivity were common alterations across psychiatric disorders, including SZ and mood disorders (Sha et al., 2019). Thus, further direct comparisons between HC, SZ and mood disorders are required for establishing consistent evidence for the similarities and distinctions between SZ and mood disorders. Furthermore, considering the importance of the whole-brain synchronous brain dynamics for the emotional state and cognitive processes (Bassett et al., 2011, Braun et al., 2015, Kucyi et al., 2017), elucidating the aberrant brain dynamics of SZ and mood disorders was also essential for revealing the underlying pathophysiological mechanisms.
Energy-landscape analysis, which is based on statistical physics, has recently emerged as a technique to directly capture the characteristics of complex dynamic brain systems with multiple coexisting stable states and transitions from one stable state to another owing to random fluctuations considering second-order interactions between the regions of interest (Watanabe et al., 2013, Watanabe et al., 2014a, Watanabe et al., 2014b). It can identify multiple stable brain-activity patterns (local minimum states) that appear more frequently than other activity patterns in a data-driven manner. In this model, stable and unstable brain activity patterns can be considered states with lower and higher energies, respectively. Thus, we constructed the energy landscape of stable brain-activity patterns based on a pairwise maximum entropy model (MEM) and characterized the brain dynamics by quantifying the ease of transitions between these multiple stable brain-activity patterns (Ezaki et al., 2018, Ezaki et al., 2017, Watanabe et al., 2014a). Several studies have used energy-landscape analysis to investigate the relationship between the dynamics of brain activity, cortical structure, and behavior underpinning bistable perception in healthy controls (HC) (Watanabe et al., 2014b), the changes in brain dynamics associated with the age-related cognitive decline in HC (Ezaki et al., 2018), and the brain dynamics related to auditory attentional fluctuations in HC (Kondo et al., 2022). Furthermore, energy-landscape analysis has also been applied to investigate the aberrant brain dynamics of patients with stroke (Fan et al., 2022) and psychiatric disorders (Jun et al., 2022, Jun et al., 2023, Regonia et al., 2021, Watanabe and Watanabe, 2023, Watanabe and Rees, 2017), providing novel insights into whole-brain neural dynamics and the pathophysiological mechanisms of various neuropsychiatric disorders.
According to previous studies, SZ exhibits a more pronounced disruption in brain volumes (Okada et al., 2023), white matter microabnormalities (Koshiyama et al., 2020), abnormal FC (Baker et al., 2019), and brain activity patterns during working memory tasks (Wang et al., 2021) when compared to MDD. These data suggest that the common aberrant brain dynamics might be more profoundly compromised in SZ than in MDD. However, no previous studies, particularly those utilizing energy-landscape analysis, have simultaneously explored these aberrant brain dynamics across extensive networks in patients with both SZ and MDD. To this end, we applied energy-landscape analysis to explore the presence of unique brain dynamic patterns in SZ and MDD, and to determine if the degree of dynamic impairment in each disorder is associated with the severity of its symptoms, using a resting-state functional MRI dataset that encompassed 50 HCs, 36 patients with SZ, and 42 patients with MDD. This approach may help to gain a deeper insight into the complex neurofunctional variances that underpin these psychiatric disorders.
2. Materials and methods
2.1. Study participants and psychopathology assessments
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Wakayama Medical University. Written informed consent was obtained from all participants. We recruited 50 HCs, 36 patients with SZ, and 42 patients with MDD from the Wakayama Medical University Hospital. The diagnoses of SZ and MDD were independently made by two well-experienced psychiatrists in accordance with the criteria set forth in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). One psychiatrist examined the patients face-to-face and the other examined the validity of the diagnosis by reviewing medical records. The inclusion criteria for the HC group were age ≤60 years and no previous or current history of psychiatric disorders. For the patient groups, we included individuals aged ≤60 years who had received accurate clinical diagnoses of SZ or MDD based on the criteria delineated in the DSM-5. The exclusion criteria for all participants were the presence or history of neurovascular diseases, traumatic brain injury, pregnancy, or any contraindication for undergoing an MRI scan.
Table 1 provides an overview of the demographic data, including neuropsychological scale scores and clinical characteristics of the participants. To maintain data integrity, participants whose head movements exceeded 2 mm were excluded from the analysis. Consequently, 8 participants from the HC group, 10 from the SZ group, and 6 from the MDD group were excluded. Therefore, our analysis included data from 104 participants.
Table 1.
Demographic characteristics of the participants.
| HC | SZ | MDD | |
|---|---|---|---|
| Number of participants | 42 | 26 | 36 |
| Male/Female | 25/17 | 10/16 | 22/14 |
| Age | 38.3 ± 9.9 | 42.2 ± 9.7 | 43.8 ± 9.7 |
| PANSS-positive | 13.9 ± 5.1 | ||
| PANSS-negative | 17.8 ± 5.9 | ||
| PANSS general | 31.5 ± 8.2 | ||
| PANSS total | 63.2 ± 17.7 | ||
| HAMD | 9.0 ± 6.5 | ||
| BACS | |||
| Verbal memory | 35.0 ± 11.5 | 43.0 ± 8.1 | |
| Working memory | 16.6 ± 4.8 | 20.6 ± 4.0 | |
| Motor function | 63.3 ± 19.8 | 74.8 ± 15.8 | |
| Verbal fluency | 37.9 ± 11.0 | 49.4 ± 9.5 | |
| Attention | 49.5 ± 12.2 | 59.8 ± 9.2 | |
| Executive function | 13.1 ± 6.8 | 17.3 ± 2.9 | |
| CPZ-equivalent dose | 518.8 ± 294.2 (n = 25) |
Abbreviations: HC, healthy controls; SZ, patients with schizophrenia; MDD, patients with major depressive disorder; PANSS, Positive and Negative Symptom Scale; HAMD, Hamilton Rating Scale for Depression; BACS, Brief Assessment of Cognition in Schizophrenia.
The CPZ-equivalent dose for one patient with SZ was missing.
The Positive and Negative Symptom Scale (PANSS) was used to assess symptom severity in patients with SZ (Kay et al., 1987). The Hamilton Rating Scale for Depression (HAMD) was used to assess symptom severity in patients with MDD (Hamilton, 1960). The Japanese version of the Brief Assessment of Cognition in Schizophrenia (BACS-J) was used to assess SZ and MDD (Kaneda et al., 2007, Keefe et al., 2004). Medication-equivalent doses of SZ were estimated using the chlorpromazine (CPZ)-equivalent dose. CPZ-equivalent dose data for one patient with SZ were missing.
2.2. MRI acquisition and preprocessing for functional MR images
All MRI data were acquired using a 3 T Philips Achieva scanner (Philips, Amsterdam, Netherlands) equipped with a 32-channel head coil. Each participant underwent 10-min rs-fMRI and high-resolution anatomical MRI. Details of the MR sequences are provided in Supplementary Material 1.
We used SPM12 (Wellcome Department of Cognitive Neurology, https://www.fil.ion.ucl.ac.uk/spm/) to pre-process the MRI data. We applied slice-timing correction and functional images were realigned to the first image to correct for head movements in the scanner. The corrected functional scans were co-registered to the anatomical T1 image in the native space and normalized to the Montral Neurological Institute (MNI) space using a unified segment and Diffeomorphic Anatomical Registration through Exponential Lie Algebra (DARTEL) in SPM12. Spatial smoothing was applied to the normalized images (2 mm isotropic voxel) with a Gaussian kernel of full-width half-maximum at 6 mm. We also normalized the segmented gray matter (GM) image to individually specify the voxels in the gray matter for extraction of the BOLD time series. Furthermore, we normalized the segmented cerebrospinal fluid (CSF) and white matter (WM) images to extract non-neural signals of the CSF and WM regions. Patients with head motion of >2 mm were excluded from the study.
The 400 regions of interest (ROIs) were defined as 4-mm spheres around the center coordinates determined in the previous studies (Schaefer et al., 2018). These ROIs were classified into seven functionally independent networks based on their study (Schaefer et al., 2018), namely, the VIN, SMN, DAN, SAN, LIN, FPN, and DMN. The BOLD time course of each network was calculated by averaging the BOLD signal time courses in the 400 spheres of ROIs corresponding to each network. We removed non-neural signal sources from the BOLD time-courses of the seven networks using linear regression with nine parameters, including six motion parameters and signals averaged over the CSF, WM, and whole brain. Finally, bandpass temporal filtering (0.01–0.1 Hz) was applied.
2.3. Energy-landscape analysis
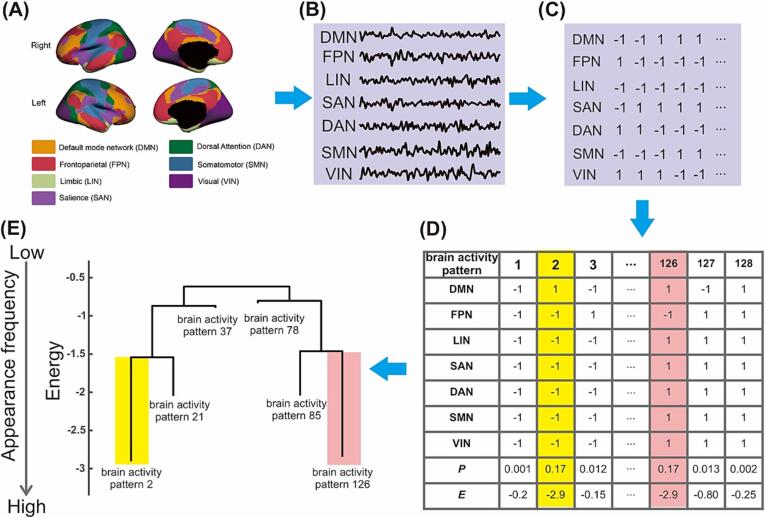
Our analysis pipeline is schematically shown in Fig. 1. We fitted the pairwise MEM to the preprocessed fMRI data in accordance with previous studies (Ezaki et al., 2018, Ezaki et al., 2017, Watanabe et al., 2014b, Watanabe and Rees, 2017) by using the Energy Landscape Analysis Toolkit (https://sites.google.com/site/ezakitakahiro/software) (Ezaki et al., 2017). For each network BOLD time-course of each participant, we binarized the BOLD time-course with the threshold set at its average value. We regarded BOLD activity that was larger/smaller than the time-averaged threshold as active/inactive (+1/-1), and then concatenated the binarized BOLD signals from all the participants in the same group for each network activity. In this method, the activity pattern at time t in each network i was specified by a N-dimensional binary vector where was the binarized activity (either active [+1] or inactive [-1]) and N (=7) was the number of the networks. The number of possible activity patterns was and the kth brain activity pattern was described by (k = 1, 2, …, ). In the MEM, the frequency of the brain activity pattern follows the Boltzmann distribution,
where ) represents the energy for activity pattern defined by
here, we calculated and by maximum-likelihood estimation to adjust the model-based activation of network i, and the model-based pairwise interaction between networks i and j, toward the empirical activation of network i and j, , respectively. Here, and
Fig. 1.
Procedures performed in energy-landscape analysis. (A) Regions of interest from the Schafer atlas. (B) Blood oxygen level-dependent signals of seven functionally different brain networks were extracted, and (C) they were binarized by the mean of each time series. (D) The distribution of the frequency of activity patterns was fitted by a pairwise MEM model, and (E) the energy landscape was constructed from the MEM model. Abbreviation; MEM, maximum entropy.
. This energy value does not indicate any biological energy. It is a statistical index that indicates the probability of the occurrence of each brain activity pattern. In other words, the activity patterns with lower energy values tended to occur more frequently. Next, we confirmed whether the pairwise MEM accurately fit the data by assessing the accuracy measures in the same manner as in previous studies (Ezaki et al., 2018, Ezaki et al., 2017, Watanabe and Rees, 2017). Details are described in the Supplementary Materials.
The energy landscape (disconnectivity graph) was defined as a graph of the brain-activity pattern with the corresponding energy fitted in a pairwise MEM (Ezaki et al., 2018, Ezaki et al., 2017, Watanabe and Rees, 2017). Brain-activity patterns were regarded as neighbors if they differed in only one active or inactive brain region. Local minima were then identified as activity patterns whose energy values were smaller than those of all neighboring patterns. Thus, local minimum patterns are more likely to appear than all neighboring patterns. We searched for local energy minima, and then summarized all brain-activity patterns into local minimum basins, a family of brain activity patterns consisting of patterns neighboring a local minimum pattern, in a data-driven manner. These basins were computed as follows: First, we selected one network-activity pattern and evaluated whether it had any neighboring patterns with smaller energy values. If there was no neighboring pattern with a smaller energy value, we defined the pattern as a local minimum pattern; otherwise, we searched for a neighboring pattern with a smaller energy. The search process was iterated until a local minimum pattern was obtained. We assigned the initial state to the local minimum basin. This procedure was iterated for all patterns in the pairwise MEM. Thus, we assigned all patterns to a single local minimum basin.
We focused on the following parameters that characterize brain dynamics in the energy-landscape analysis: mean duration, occurrence, and transition probability. The mean duration of each state represents the duration for which each state continues. The occurrence of a brain state represents the frequency at which each brain state occurs per second. The transition probability from state i to state j (i j) indicates the ratio of the number of the transitions from state i to state j to the total numbers of transitions from state i to the other states, which reflects the ease of the transitions from state i to state j. These three parameters were calculated for each participant on the basis of the group-level energy landscape. To validate the characteristics of brain dynamics acquired from the empirical data, we performed a random-walk simulation of the energy landscape, as in previous studies (Ezaki et al., 2018, Ezaki et al., 2017, Watanabe et al., 2014b, Watanabe and Rees, 2017). The details are provided in the Supplementary Materials.
2.4. Group comparison of the brain dynamics and associations between brain dynamics and symptoms
We compared the mean duration, occurrence, and transition probabilities across the HC, SZ, and MDD groups by using analysis of covariance (ANCOVA), with age and sex as nuisance covariates (Bonferroni-corrected p < 0.05). A post-hoc Tukey’s multiple-comparison test was performed for parameters showing significant differences in ANCOVA (corrected p < 0.05). To investigate how the aberrant brain dynamics of each psychiatric disorder were associated with its symptoms, we calculated Pearson’s correlation coefficients between symptom indices (PANSS and BACS scores for SZ and HAMD and BACS scores for MDD) and the brain dynamics parameters of each psychiatric disorder that significantly differed from those of HCs (Bonferroni-corrected p < 0.05). If we found significant associations between brain dynamics characteristics and symptom and cognitive scores, we also confirmed whether these correlations remained significant after controlling for age and medications (Bonferroni-corrected p < 0.05).
3. Results
3.1. Identification of local minimum basins and comparison of energy-landscape structures
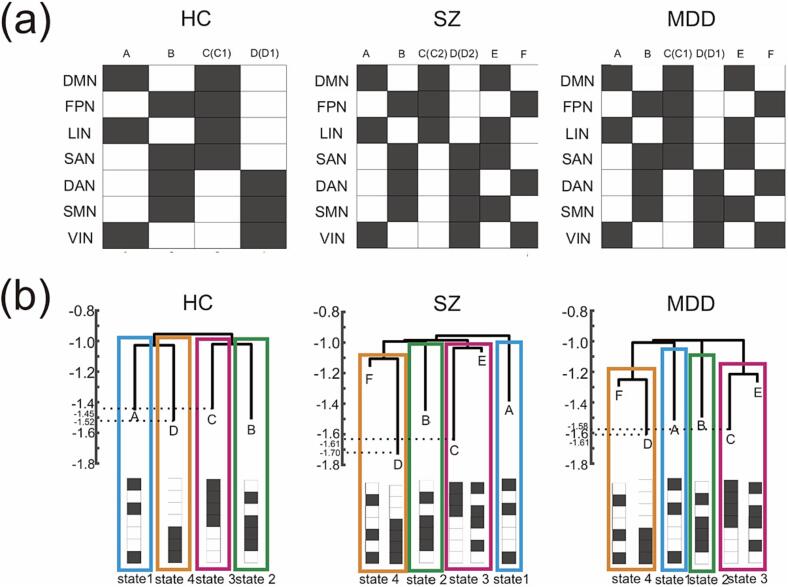
Four local minimum basins (basins A–D) were identified in the HCs and six local minimum basins (basins A–F) were identified in the SZ and MDD group. In the SZ group, while four basins (A, B, E and F) were common in MDD and SZ and two basins (C(C1) and D (D1)) were common in HC and MDD, basins C(C2) and D (D2) were SZ-specific basins (Fig. 2).
Fig. 2.
Identification of the local minimum and comparison of the energy-landscape structures. (Upper) The activity patterns of local minima identified in the energy landscape analysis. (Bottom) Disconnectivity graphs of the energy-landscape structure. Lower energy reflects more stable and more frequent occurrence of a local minimum pattern, while higher energy reflects less stable and less frequent occurrence. HC, healthy controls; SZ, patients with schizophrenia; MDD, patients with major depressive disorder.
Specifically, state 1 was characterized as the state where FPN, SAN, DAN, and SMN were active and DMN, LIN, and VIN were inactive, state 2 as the state where FPN, SAN, DAN, and SMN were inactive, state 3 as the state where DAN and VIN were active and DMN and LIN were inactive, state 4 as the state where DAN and VIN were inactive and DMN and LIN were active.
Each graph consists of four types of sub-branches (Fig. 2). One consisted of basin A, the second consisted of basin B, the third consisted of basin C (C1) in HC (basins C (C1) and E in MDD, or basins C (C2) and E in SZ), and the fourth consisted of basin D (D1) in HC (basins D (D1) and F in MDD, or basins D (D2) and F in SZ). On the basis of the hierarchical characteristics and similarity of the energy-landscape structures, we classified basin A as state 1, basin B as state 2, basin C (C1) in HC (basins C (C1) and E in MDD or basins C (C2) and E in SZ) as state 3, and basin D (D1) in HC (basins D (D1) and F in MDD or basins D (D2) and F in SZ) as state 4. Although the activity patterns of basins C (C2) and D (D2) in the SZ group were not exactly the same as those of basins C (C1) and D (D1) in the other groups, we assigned basins C (C2) and D (D2) to states 3 and 4, respectively, for the following reasons. First, the activity patterns of basins C1 (D1) and C2 (D2) were spatially similar, and they differed depending on whether the SAN was active or inactive. Second, the basin that belonged to the same sub-branch as basin C2 (D2) in the SZ group was basin E (F), which was the same basin belonging to the same sub-branch as basin C1 (D1) belonging to the MDD groups. Thus, the hierarchical structure of the disconnectivity graphs was similar across the groups if we assigned basins C (C2) and D (D2) to states 3 and 4, respectively, in the SZ group. This simplification made it possible to directly compare the characteristics of the brain dynamics among the three groups.
3.2. Characterization of brain dynamics
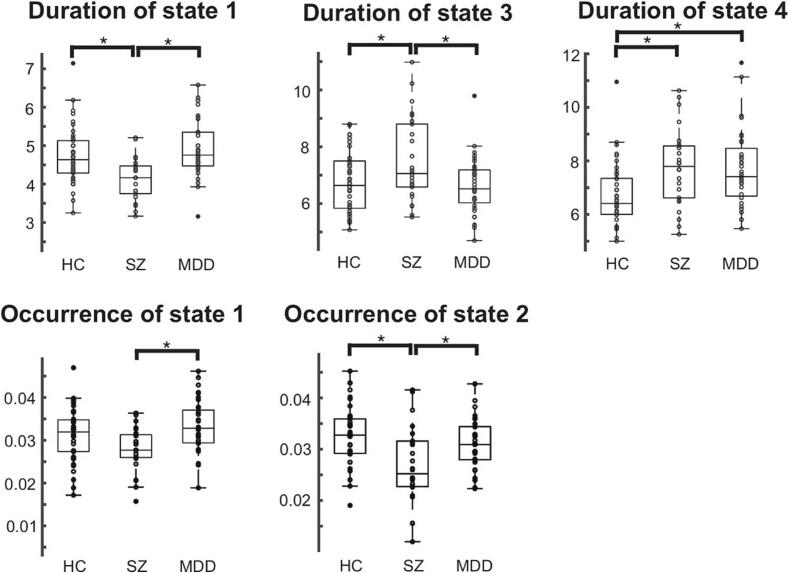
The duration of states 1, 3, and 4, and the occurrence of states 1 and 2 differed significantly across the HC, SZ, and MDD groups (ANCOVA with age and sex as nuisance covariates: Bonferroni-corrected p < 0.05). As shown in Fig. 3, the duration of state 1 and the occurrence of states 1 and 2 were significantly longer in the HC and MDD groups than in the SZ group. The duration of state 3 was significantly longer in the SZ group than in the HC and MDD groups. The duration of state 4 was longer in the SZ and MDD groups than that in the HC group (Fig. 3).
Fig. 3.
Group comparison of the duration and occurrence across brain states. The durations of states 1, 3, and 4, and the occurrence of states 1 and 2 were significantly different among the HC, SZ, and MDD groups (ANCOVA including age and sex as nuisance covariates: p < 0.001). Tukey’s multiple-comparison test was performed to investigate which pairs of groups showed significant differences: * corrected p < 0.05. HC, healthy controls; SZ, schizophrenia; MDD, major depressive disorder.
The random-walk simulation confirmed that the duration and occurrence of states 1 and the occurrence of state 2 were longer in the HC and MDD groups than in the SZ group (Figure S2). Furthermore, the random-walk simulation confirmed that the duration of state 3 was longer in patients with SZ than in HCs and patients with MDD, and that the duration of state 4 was longer in patients with SZ and MDD than in HCs (Figure S2).
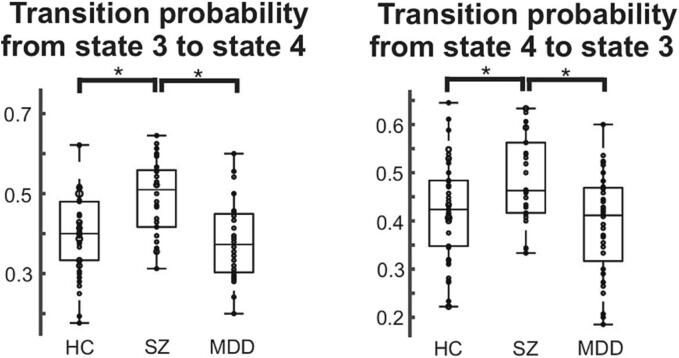
We found that the transition probabilities from state 3 to state 4 and from state 4 to state 3 were significantly different across the three groups (ANCOVA with age and sex as nuisance covariates: Bonferroni-corrected p < 0.05; Fig. 4). The transition probabilities from state 3 to state 4 and from state 4 to state 3 were higher in patients with SZ than in HCs and patients with MDD. Furthermore, the random-walk simulation revealed that the transition probabilities from state 3 to state 4 and from state 4 to state 3 were higher in patients with SZ than in HCs and patients with MDD, thus validating the results of our empirical data (Figure S4).
Fig. 4.
Group comparison of the direct transition probabilities across the brain states. The direct transition probabilities from state 3 to state 4 and state 4 to state 3 were significantly different among the HC, SZ, and MDD groups, including age and sex as nuisance covariates (p < 0.001). Tukey’s multiple-comparison test was performed to investigate which pairs of groups showed significant differences: *corrected p < 0.05. HC, healthy controls; SZ, schizophrenia; MDD, major depressive disorder.
3.3. Associations between brain dynamics and symptoms
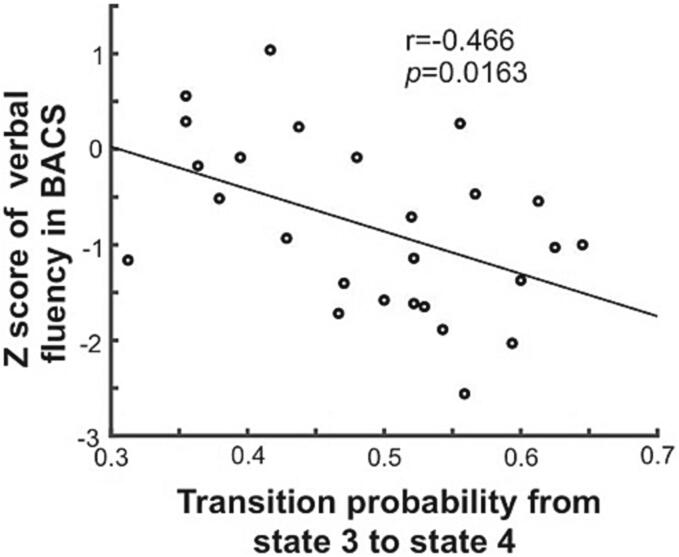
In the SZ group, the transition probability from state 3 to state 4 was negatively correlated with the scores for verbal fluency in the BACS (r = -0.466, p = 0.0163, Bonferroni-corrected p < 0.05; Fig. 5). This correlation remained significant after controlling for age (uncorrected p = 0.0221) and the chlorpromazine-equivalent dose (uncorrected p < 0.0001). One patient with SZ was eliminated from the partial correlation analysis with the effect of the CPZ-equivalent dose as a nuisance covariate, since the CPZ-equivalent dose data for this patient were missing. In patients with MDD, no significant association was found between aberrant brain dynamics and symptom severity after Bonferroni correction.
Fig. 5.
Association between brain dynamics and the scores of BACS for SZ. The duration of state 4 was positively correlated with the verbal memory scores in the BACS, while the occurrence of state 1 and the direct transition frequency from state 1 to state 4 were negatively correlated with the verbal memory scores in the BACS. BACS, Brief Assessment of Cognition in Schizophrenia; SZ, schizophrenia.
4. Discussion
In this study, we applied energy-landscape analysis to elucidate the aberrant brain dynamics of large-scale functional networks across SZ and MDD. We identified similar energy-landscape structures consisting of four states (state 1–4) in the HC, SZ, and MDD groups. The duration of state 1 and the occurrence of states 1 and 2 were longer in the HC and MDD groups than in the SZ group, and the duration of state 3 was longer in the SZ group than in the HC and MDD groups. The duration of state 4 was longer in patients with SZ and MDD than in HCs. The transition probabilities between states 3 and 4 were significantly higher in the SZ group than in the HC and MDD groups. Finally, the transition probability from state 3 to state 4 was associated with verbal fluency in the SZ group.
All groups showed similar energy-landscape structures consisting of states 1, 2, 3, and 4 (Fig. 2). However, state 3 (state 4) consisted of local minimum activity patterns C (C2) and E (D (D2) and F) rather than C (C1) and E (D (D1) and F) in the SZ group, whereas state 3 (state 4) consisted of local minimum activity patterns C (C1) and E (D (D1) and F) in the other disorder groups. The difference between brain-activity patterns C1 and C2 (D1 and D2) depended on whether the SAN was active or inactive. This might be related to the imbalance in the switch between the DMN (related to inner cognitive processing) and the FPN (related to external goal-directed regulation) modulated by the SAN (related to salience processing) in patients with SZ, which leads to aberrant assignment of saliency to internal mental events and goal-directed external stimuli (Manoliu et al., 2014, Palaniyappan et al., 2013).
Most properties of brain dynamics were the most disrupted in SZ. Specifically, the duration of state 1 and the occurrence of states 1 and 2 were shorter in the SZ group than in the HC and MDD groups, while the duration of state 3 was longer in the SZ group than in the HC and MDD groups, and that of state 4 was longer in the SZ group than in the HC group (Fig. 3). Since states 1 and 2 (states 3 and 4) were characterized by the coactivation/co-deactivation (anti-activation) of DMN and VIN (Fig. 2), the brain dynamics of SZ were characterized by shorter (longer) stays and less (more) frequent appearances of states in which the DMN and VIN were coactivated/co-deactivated (anti-activated). Thus, the DMN-VIN FC was lower in the SZ group than in the HC and MDD groups. Furthermore, the bidirectional transition probabilities between states 3 and 4 were significantly higher in the SZ group than in the HC and MDD groups (Fig. 3). Therefore, the bidirectional switches between state 3 (deactivated DMN and activated VIN) and state 4 (activated DMN and deactivated VIN) were more likely to occur in patients with SZ than in HCs and patients with MDD. Patients with SZ have reduced VIN-DMN connectivity (Peng et al., 2021) and reduced VIN-to-DMN causal connections than HCs (Ishida et al., 2023), suggesting that our results confirmed these findings from the perspective of brain dynamics. The dynamic pattern between the VIN and DMN is a potential indicator for diagnosing psychiatric disorders.
The duration of state 4 was significantly longer in patients with MDD than in the HCs. FPN-DAN hypoconnectivity and FPN-DMN hyperconnectivity have been consistently reported in previous studies on MDD (Hasler and Northoff, 2011, Kaiser et al., 2015, Liston et al., 2014); These findings are consistent with our results since a longer duration of the local minimum state D (belonging to state 4) implied a longer duration of anti-activation of the FPN and DAN and coactivation of the FPN and DMN. Thus, FPN-DAN hypoconnectivity and FPN-DMN hyperconnectivity partly originated from the longer stay in state D, where the FPN and DAN were anti-activated and the FPN and DMN were coactivated (activated DMN, activated FPN, and deactivated DAN), which could not be captured by conventional FC analysis. Therefore, energy-landscape analysis could reveal how aberrant brain dynamics in psychiatric disorders lead to disrupted static FC properties. Furthermore, a longer stay in state 4 corresponded to a longer duration of DMN activation, which is consistent with the previous studies showing that hyperactivity within the DMN is related to negative rumination in patients with MDD (Hamilton et al., 2015, Kaiser et al., 2015, Koster et al., 2011, Sheline et al., 2009).
Furthermore, the ease of transition from state 3 to state 4 was negatively correlated with the verbal-fluency score on the BACS in patients with SZ. This indicated that patients with SZ were more likely to transit from the brain state where the DMN was inactive and the DAN was active (state 3, which is related to external directed attention and working memory) to the state where the DMN was active and the DAN was inactive (state 4, which is related to internal self-referential mental activities), and that this ease of transition from the external attention mode to the internal self-referential mode was negatively associated with verbal fluency in patients with SZ. This may suggest that patients with SZ experience difficulties in paying attention to external events and that their attention to external events soon switches to their internal activities, potentially causing disruption of executive functions in patients with SZ. Verbal fluency is based on the complex nature of psychological mechanisms (Ross et al., 2007). It requires mental flexibility, multitasking, efficient retrieval, and recall of words (Henry and Crawford, 2004) and can be partly attributed to executive function (Aita et al., 2019). The anti-correlation between the DMN and DAN is related to cognitive processes, including executive function (Boord et al., 2017, Chai et al., 2014, Spreng et al., 2016) and disrupted anti-correlation is associated with poor cognitive performance in SZ (Hasenkamp et al., 2011, Jiang et al., 2013, Williamson, 2007). Our findings may capture the dynamic aspects of the relationship between disrupted DMN-DAN connections and cognitive dysfunction in patients with SZ.
The duration of state 1 and occurrence of state 2 were shorter in SZ than in HC and MDD, the duration of state 3 was longer in SZ than in HC and MDD, and the bidirectional transition probabilities between state 3 and 4 were higher in SZ than in HC and MDD, suggesting that the brain dynamics was more disrupted in SZ than in MDD and HC. Shorter duration of state 1 and occurrence of state 2 in SZ (shorter duration and occurrence of the states where DMN/FPN was inactive/active or active/inactive) meant that the DMN-FPN FC was greater in SZ than in MDD. This was in line with a previous study (Gong et al., 2017) showing a greater DMN-FPN FC in SZ than in MDD. Wang et al., reported that patients with SZ showed stronger fMRI response in regions of the DMN during performance of working memory task than patients with MDD (Wang et al., 2021). Our findings showed that the more disrupted brain dynamics in SZ was related to the more impaired coordination of self-monitoring and task-performance in SZ than in MDD, which might lead to the increased cognitive dysfunction and clinical symptoms in SZ compared to MDD (Reichenberg et al., 2009, Velthorst et al., 2017).
Recently, a growing body of evidence suggests that SZ and mood disorders, including bipolar disorder (BD), shared a spectrum of symptoms, genetics and neuroanatomical aberrations albeit with varying degrees of severity (Buckholtz and Meyer-Lindenberg, 2012, Clementz et al., 2022, Romero et al., 2022). Thus, we extended our analysis to a limited dataset of patients with BD (n = 12), as detailed in the supplementary materials. We found that the duration of state 1 and the occurrence of states 1 and 2 seemed to be smaller in patients with BD than in HCs and patients with MDD (Supplementary Figs. 2–5), and that the transition probabilities between states 3 and 4 appeared to be larger in patients with BD than in HCs and patients with MDD. These observations suggest that the characteristics of aberrant brain dynamics in BD may represent an intermediate spectrum between SZ and MDD. However, it's important to note that these trends should warrant further validation through future studies with larger sample sizes of BD patients.
The current study had some limitations. First, sample size employed was relatively modest. To enhance the robustness of our findings, future investigations should encompass larger and more diverse cohorts, not restricted solely to SZ and MDD, but also encompassing BD and various other psychiatric disorders. Second, we performed global signal regression as a preprocessing step, similar to previous studies that applied energy-landscape analysis to resting-state fMRI data (Ezaki et al., 2018, Watanabe and Rees, 2017). However, the justification for performing global signal regression remains a topic of debate. Third, we considered only the seven functional networks of interest because our method was limited to a relatively small number of ROIs (approximately 10 ROIs), which may have resulted in the loss of more detailed information acquired at a finer 3-mm3 voxel level (Sporns et al., 2005). Fourth, although we integrated the two local minima (the local minimum C and E/ the local minimum D and F) into one state (state 3/state 4) for the direct comparison, it is still controversial whether two local minima could be integrated as one macro state.
5. Conclusion
Energy-landscape analysis revealed both the commonalities and distinctions of brain dynamics across SZ and mood disorders. In addition, the aberrant dynamics pattern in SZ was associated with cognitive function in this disorder. Thus, energy-landscape analysis could help elucidating aberrant brain dynamics in psychiatric disorders and provide new insights into the pathophysiological mechanisms underlying psychiatric disorders.
CRediT authorship contribution statement
Takuya Ishida: Writing – original draft, Software, Methodology, Conceptualization. Shinichi Yamada: Writing – review & editing. Kasumi Yasuda: Investigation. Shinya Uenishi: Investigation. Atsushi Tamaki: Investigation. Michiyo Tabata: Investigation. Natsuko Ikeda: Investigation. Shun Takahashi: Writing – review & editing. Sohei Kimoto: Writing – review & editing, Supervision, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by JSPS KAKENHI (JP18K07570, JP23K06992, and JP23H02841), the Naito Foundation and Takeda Science Foundation.
Ethics approval statement
This study was approved by the ethics committee of the Wakayama Medical University (No. 2223).
Patient consent statement
Informed consent has been obtained from all individuals included in this study.
Permission to reproduce material from other sources
Not applicable. All materials were originally produced for the current study.
Clinical trial registration
This study is registered to UMIN000036498.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2024.103574.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
Information Theory and Statistical Mechanics
- Aita S.L., Beach J.D., Taylor S.E., Borgogna N.C., Harrell M.N., Hill B.D. Executive, language, or both? An examination of the construct validity of verbal fluency measures. Appl. Neuropsychol. Adult. 2019;26:441–451. doi: 10.1080/23279095.2018.1439830. [DOI] [PubMed] [Google Scholar]
- Allen E.A., Damaraju E., Plis S.M., Erhardt E.B., Eichele T., Calhoun V.D. Tracking whole-brain connectivity dynamics in the resting state. Cereb. Cortex. 2014;24:663–676. doi: 10.1093/cercor/bhs352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J.T., Dillon D.G., Patrick L.M., Roffman J.L., Brady R.O., Jr., Pizzagalli D.A., Öngür D., Holmes A.J. Functional connectomics of affective and psychotic pathology. PNAS. 2019;116:9050–9059. doi: 10.1073/pnas.1820780116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett D.S., Wymbs N.F., Porter M.A., Mucha P.J., Carlson J.M., Grafton S.T. Dynamic reconfiguration of human brain networks during learning. PNAS. 2011;108:7641–7646. doi: 10.1073/pnas.1018985108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boord P., Madhyastha T.M., Askren M.K., Grabowski T.J. Executive attention networks show altered relationship with default mode network in PD. Neuroimage Clin. 2017;13:1–8. doi: 10.1016/j.nicl.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U., Schäfer A., Walter H., Erk S., Romanczuk-Seiferth N., Haddad L., Schweiger J.I., Grimm O., Heinz A., Tost H., Meyer-Lindenberg A., Bassett D.S. Dynamic reconfiguration of frontal brain networks during executive cognition in humans. PNAS. 2015;112:11678–11683. doi: 10.1073/pnas.1422487112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breakspear M. Dynamic models of large-scale brain activity. Nat. Neurosci. 2017;20:340–352. doi: 10.1038/nn.4497. [DOI] [PubMed] [Google Scholar]
- Buckholtz, J.W., Meyer-Lindenberg, A., 2012. Psychopathology and the human connectome: toward a transdiagnostic model of risk for mental illness. Neuron. © 2012 Elsevier Inc, United States, pp. 990-1004. [DOI] [PubMed]
- Chai X.J., Ofen N., Gabrieli J.D., Whitfield-Gabrieli S. Selective development of anticorrelated networks in the intrinsic functional organization of the human brain. J. Cogn. Neurosci. 2014;26:501–513. doi: 10.1162/jocn_a_00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R.A., Lee C.Y., Lee Y., Hung C.F., Huang Y.C., Lin P.Y., Lee S.Y., Wang L.J. Defining cognitive profiles of depressive patients using the Brief Assessment of Cognition in Affective Disorders. PeerJ. 2019;7:e7432. doi: 10.7717/peerj.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementz B.A., Parker D.A., Trotti R.L., McDowell J.E., Keedy S.K., Keshavan M.S., Pearlson G.D., Gershon E.S., Ivleva E.I., Huang L.Y., Hill S.K., Sweeney J.A., Thomas O., Hudgens-Haney M., Gibbons R.D., Tamminga C.A. Psychosis biotypes: replication and validation from the B-SNIP consortium. Schizophr. Bull. 2022;48:56–68. doi: 10.1093/schbul/sbab090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong D., Wang Y., Chang X., Luo C., Yao D. Dysfunction of large-scale brain networks in schizophrenia: a meta-analysis of resting-state functional connectivity. Schizophr. Bull. 2018;44:168–181. doi: 10.1093/schbul/sbx034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezaki T., Watanabe T., Ohzeki M., Masuda N. Energy landscape analysis of neuroimaging data. Philos Trans A Math Phys Eng. Sci. 2017:375. doi: 10.1098/rsta.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezaki T., Sakaki M., Watanabe T., Masuda N. Age-related changes in the ease of dynamical transitions in human brain activity. Hum. Brain Mapp. 2018;39:2673–2688. doi: 10.1002/hbm.24033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L., Li C., Huang Z.G., Zhao J., Wu X., Liu T., Li Y., Wang J. The longitudinal neural dynamics changes of whole brain connectome during natural recovery from poststroke aphasia. Neuroimage Clin. 2022;36 doi: 10.1016/j.nicl.2022.103190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B.A. A review of American psychiatry through its diagnoses: the history and development of the Diagnostic and Statistical Manual of Mental Disorders. J. Nerv. Ment. Dis. 2012;200:1022–1030. doi: 10.1097/NMD.0b013e318275cf19. [DOI] [PubMed] [Google Scholar]
- Gandal M.J., Haney J.R., Parikshak N.N., Leppa V., Ramaswami G., Hartl C., Schork A.J., Appadurai V., Buil A., Werge T.M., Liu C., White K.P., Horvath S., Geschwind D.H. Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science. 2018;359:693–697. doi: 10.1176/appi.focus.17103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Q., Hu X., Pettersson-Yeo W., Xu X., Lui S., Crossley N., Wu M., Zhu H., Mechelli A. Network-level dysconnectivity in drug-naïve first-episode psychosis: dissociating transdiagnostic and diagnosis-specific alterations. Neuropsychopharmacology. 2017;42:933–940. doi: 10.1038/npp.2016.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J., Wang J., Chen P., Qi Z., Luo Z., Huang L., Wang Y. Large-scale network abnormality in bipolar disorder: A multimodal meta-analysis of resting-state functional and structural magnetic resonance imaging studies. J. Affect. Disord. 2021;292:9–20. doi: 10.1016/j.jad.2021.05.052. [DOI] [PubMed] [Google Scholar]
- Goodkind M., Eickhoff S.B., Oathes D.J., Jiang Y., Chang A., Jones-Hagata L.B., Ortega B.N., Zaiko Y.V., Roach E.L., Korgaonkar M.S., Grieve S.M., Galatzer-Levy I., Fox P.T., Etkin A. Identification of a common neurobiological substrate for mental illness. JAMA Psychiat. 2015;72:305–315. doi: 10.1001/jamapsychiatry.2014.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goya-Maldonado R., Brodmann K., Keil M., Trost S., Dechent P., Gruber O. Differentiating unipolar and bipolar depression by alterations in large-scale brain networks. Hum. Brain Mapp. 2016;37:808–818. doi: 10.1002/hbm.23070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.P., Farmer M., Fogelman P., Gotlib I.H. Depressive rumination, the default-mode network, and the Dark Matter of Clinical Neuroscience. Biol. Psychiatry. 2015;78:224–230. doi: 10.1016/j.biopsych.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenkamp W., James G.A., Boshoven W., Duncan E. Altered engagement of attention and default networks during target detection in schizophrenia. Schizophr. Res. 2011;125:169–173. doi: 10.1016/j.schres.2010.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G., Northoff G. Discovering imaging endophenotypes for major depression. Mol. Psychiatry. 2011;16:604–619. doi: 10.1038/mp.2011.23. [DOI] [PubMed] [Google Scholar]
- Henry J.D., Crawford J.R. A meta-analytic review of verbal fluency performance following focal cortical lesions. Neuropsychology. 2004;18:284–295. doi: 10.1037/0894-4105.18.2.284. [DOI] [PubMed] [Google Scholar]
- Ishida T., Nakamura Y., Tanaka S.C., Mitsuyama Y., Yokoyama S., Shinzato H., Itai E., Okada G., Kobayashi Y., Kawashima T., Miyata J., Yoshihara Y., Takahashi H., Morita S., Kawakami S., Abe O., Okada N., Kunimatsu A., Yamashita A., Yamashita O., Imamizu H., Morimoto J., Okamoto Y., Murai T., Kasai K., Kawato M., Koike S. Aberrant large-scale network interactions across psychiatric disorders revealed by large-sample multi-site resting-state functional magnetic resonance imaging datasets. Schizophr. Bull. 2023;49:933–943. doi: 10.1093/schbul/sbad022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T., Zhou Y., Liu B., Liu Y., Song M. Brainnetome-wide association studies in schizophrenia: the advances and future. Neurosci. Biobehav. Rev. 2013;37:2818–2835. doi: 10.1016/j.neubiorev.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Jun, M., Akihiko, S., Takahiro, E., Masanori, I., Takanori, K., Naoki, M., Yasuo, M., Yuki, S., Nobukatsu, S., Shisei, T., Shiho, U., Toshihiko, A., Toshiya, M., Hidehiko, T., 2022. Associations of conservatism/jumping to conclusions biases with aberrant salience and default mode network. medRxiv, 2022.2009.2029.22280497.
- Jun, M., Toby, W.-B., Thomas, S., Toshihiko, A., Nicola, C., Jennifer, C., Nicolas, A.C., Emrah, D., Takahiro, E., Masaki, F., Carolyn, H., Masanori, I., Kouhei, K., Kiyoto, K., Takanori, K., Shinsuke, K., Akira, K., Naoki, M., Susumu, M., Yasuo, M., Toshiya, M., Kiyotaka, N., Frederick, N., Kazutaka, O., Naohiro, O., Yuki, S., Nobukatsu, S., Tsutomu, T., Shinichi, U., Yoshiyuki, W., Crystal, C.W., Hidenaga, Y., Yuka, Y., Ryota, H., Hidehiko, T., Akira, S., Philip, M., 2023. Functional alterations of two salience-related systems jointly and independently contribute to psychosis. medRxiv, 2021.2010.2002.21264326.
- Kaiser R.H., Andrews-Hanna J.R., Wager T.D., Pizzagalli D.A. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiat. 2015;72:603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda Y., Sumiyoshi T., Keefe R., Ishimoto Y., Numata S., Ohmori T. Brief assessment of cognition in schizophrenia: validation of the Japanese version. Psychiatry Clin Neurosci Australia. 2007:602–609. doi: 10.1111/j.1440-1819.2007.01725.x. [DOI] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Keefe R.S., Goldberg T.E., Harvey P.D., Gold J.M., Poe M.P., Coughenour L. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res Netherlands. 2004:283–297. doi: 10.1016/j.schres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Kondo H.M., Terashima H., Ezaki T., Kochiyama T., Kihara K., Kawahara J.I. Dynamic transitions between brain states predict auditory attentional fluctuations. Front. Neurosci. 2022;16 doi: 10.3389/fnins.2022.816735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiyama D., Fukunaga M., Okada N., Morita K., Nemoto K., Usui K., Yamamori H., Yasuda Y., Fujimoto M., Kudo N., Azechi H., Watanabe Y., Hashimoto N., Narita H., Kusumi I., Ohi K., Shimada T., Kataoka Y., Yamamoto M., Ozaki N., Okada G., Okamoto Y., Harada K., Matsuo K., Yamasue H., Abe O., Hashimoto R., Takahashi T., Hori T., Nakataki M., Onitsuka T., Holleran L., Jahanshad N., van Erp T.G.M., Turner J., Donohoe G., Thompson P.M., Kasai K. White matter microstructural alterations across four major psychiatric disorders: mega-analysis study in 2937 individuals. Mol. Psychiatry. 2020;25:883–895. doi: 10.1038/s41380-019-0553-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster E.H., De Lissnyder E., Derakshan N., De Raedt R. Understanding depressive rumination from a cognitive science perspective: the impaired disengagement hypothesis. Clin. Psychol. Rev. 2011;31:138–145. doi: 10.1016/j.cpr.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Kucyi A., Hove M.J., Esterman M., Hutchison R.M., Valera E.M. Dynamic brain network correlates of spontaneous fluctuations in attention. Cereb. Cortex. 2017;27:1831–1840. doi: 10.1093/cercor/bhw029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S.H., Ripke, S., Neale, B.M., Faraone, S.V., Purcell, S.M., Perlis, R.H., Mowry, B.J., Thapar, A., Goddard, M.E., Witte, J.S., Absher, D., Agartz, I., Akil, H., Amin, F., Andreassen, O.A., Anjorin, A., Anney, R., Anttila, V., Arking, D.E., Asherson, P., Azevedo, M.H., Backlund, L., Badner, J.A., Bailey, A.J., Banaschewski, T., Barchas, J.D., Barnes, M.R., Barrett, T.B., Bass, N., Battaglia, A., Bauer, M., Bayés, M., Bellivier, F., Bergen, S.E., Berrettini, W., Betancur, C., Bettecken, T., Biederman, J., Binder, E.B., Black, D.W., Blackwood, D.H., Bloss, C.S., Boehnke, M., Boomsma, D.I., Breen, G., Breuer, R., Bruggeman, R., Cormican, P., Buccola, N.G., Buitelaar, J.K., Bunney, W.E., Buxbaum, J.D., Byerley, W.F., Byrne, E.M., Caesar, S., Cahn, W., Cantor, R.M., Casas, M., Chakravarti, A., Chambert, K., Choudhury, K., Cichon, S., Cloninger, C.R., Collier, D.A., Cook, E.H., Coon, H., Cormand, B., Corvin, A., Coryell, W.H., Craig, D.W., Craig, I.W., Crosbie, J., Cuccaro, M.L., Curtis, D., Czamara, D., Datta, S., Dawson, G., Day, R., De Geus, E.J., Degenhardt, F., Djurovic, S., Donohoe, G.J., Doyle, A.E., Duan, J., Dudbridge, F., Duketis, E., Ebstein, R.P., Edenberg, H.J., Elia, J., Ennis, S., Etain, B., Fanous, A., Farmer, A.E., Ferrier, I.N., Flickinger, M., Fombonne, E., Foroud, T., Frank, J., Franke, B., Fraser, C., Freedman, R., Freimer, N.B., Freitag, C.M., Friedl, M., Frisén, L., Gallagher, L., Gejman, P.V., Georgieva, L., Gershon, E.S., Geschwind, D.H., Giegling, I., Gill, M., Gordon, S.D., Gordon-Smith, K., Green, E.K., Greenwood, T.A., Grice, D.E., Gross, M., Grozeva, D., Guan, W., Gurling, H., De Haan, L., Haines, J.L., Hakonarson, H., Hallmayer, J., Hamilton, S.P., Hamshere, M.L., Hansen, T.F., Hartmann, A.M., Hautzinger, M., Heath, A.C., Henders, A.K., Herms, S., Hickie, I.B., Hipolito, M., Hoefels, S., Holmans, P.A., Holsboer, F., Hoogendijk, W.J., Hottenga, J.J., Hultman, C.M., Hus, V., Ingason, A., Ising, M., Jamain, S., Jones, E.G., Jones, I., Jones, L., Tzeng, J.Y., Kähler, A.K., Kahn, R.S., Kandaswamy, R., Keller, M.C., Kennedy, J.L., Kenny, E., Kent, L., Kim, Y., Kirov, G.K., Klauck, S.M., Klei, L., Knowles, J.A., Kohli, M.A., Koller, D.L., Konte, B., Korszun, A., Krabbendam, L., Krasucki, R., Kuntsi, J., Kwan, P., Landén, M., Långström, N., Lathrop, M., Lawrence, J., Lawson, W.B., Leboyer, M., Ledbetter, D.H., Lee, P.H., Lencz, T., Lesch, K.P., Levinson, D.F., Lewis, C.M., Li, J., Lichtenstein, P., Lieberman, J.A., Lin, D.Y., Linszen, D.H., Liu, C., Lohoff, F.W., Loo, S.K., Lord, C., Lowe, J.K., Lucae, S., MacIntyre, D.J., Madden, P.A., Maestrini, E., Magnusson, P.K., Mahon, P.B., Maier, W., Malhotra, A.K., Mane, S.M., Martin, C.L., Martin, N.G., Mattheisen, M., Matthews, K., Mattingsdal, M., McCarroll, S.A., McGhee, K.A., McGough, J.J., McGrath, P.J., McGuffin, P., McInnis, M.G., McIntosh, A., McKinney, R., McLean, A.W., McMahon, F.J., McMahon, W.M., McQuillin, A., Medeiros, H., Medland, S.E., Meier, S., Melle, I., Meng, F., Meyer, J., Middeldorp, C.M., Middleton, L., Milanova, V., Miranda, A., Monaco, A.P., Montgomery, G.W., Moran, J.L., Moreno-De-Luca, D., Morken, G., Morris, D.W., Morrow, E.M., Moskvina, V., Muglia, P., Mühleisen, T.W., Muir, W.J., Müller-Myhsok, B., Murtha, M., Myers, R.M., Myin-Germeys, I., Neale, M.C., Nelson, S.F., Nievergelt, C.M., Nikolov, I., Nimgaonkar, V., Nolen, W.A., Nöthen, M.M., Nurnberger, J.I., Nwulia, E.A., Nyholt, D.R., O'Dushlaine, C., Oades, R.D., Olincy, A., Oliveira, G., Olsen, L., Ophoff, R.A., Osby, U., Owen, M.J., Palotie, A., Parr, J.R., Paterson, A.D., Pato, C.N., Pato, M.T., Penninx, B.W., Pergadia, M.L., Pericak-Vance, M.A., Pickard, B.S., Pimm, J., Piven, J., Posthuma, D., Potash, J.B., Poustka, F., Propping, P., Puri, V., Quested, D.J., Quinn, E.M., Ramos-Quiroga, J.A., Rasmussen, H.B., Raychaudhuri, S., Rehnström, K., Reif, A., Ribasés, M., Rice, J.P., Rietschel, M., Roeder, K., Roeyers, H., Rossin, L., Rothenberger, A., Rouleau, G., Ruderfer, D., Rujescu, D., Sanders, A.R., Sanders, S.J., Santangelo, S.L., Sergeant, J.A., Schachar, R., Schalling, M., Schatzberg, A.F., Scheftner, W.A., Schellenberg, G.D., Scherer, S.W., Schork, N.J., Schulze, T.G., Schumacher, J., Schwarz, M., Scolnick, E., Scott, L.J., Shi, J., Shilling, P.D., Shyn, S.I., Silverman, J.M., Slager, S.L., Smalley, S.L., Smit, J.H., Smith, E.N., Sonuga-Barke, E.J., St Clair, D., State, M., Steffens, M., Steinhausen, H.C., Strauss, J.S., Strohmaier, J., Stroup, T.S., Sutcliffe, J.S., Szatmari, P., Szelinger, S., Thirumalai, S., Thompson, R.C., Todorov, A.A., Tozzi, F., Treutlein, J., Uhr, M., van den Oord, E.J., Van Grootheest, G., Van Os, J., Vicente, A.M., Vieland, V.J., Vincent, J.B., Visscher, P.M., Walsh, C.A., Wassink, T.H., Watson, S.J., Weissman, M.M., Werge, T., Wienker, T.F., Wijsman, E.M., Willemsen, G., Williams, N., Willsey, A.J., Witt, S.H., Xu, W., Young, A.H., Yu, T.W., Zammit, S., Zandi, P.P., Zhang, P., Zitman, F.G., Zöllner, S., Devlin, B., Kelsoe, J.R., Sklar, P., Daly, M.J., O'Donovan, M.C., Craddock, N., Sullivan, P.F., Smoller, J.W., Kendler, K.S., Wray, N.R., 2013. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet 45, 984-994. [DOI] [PMC free article] [PubMed]
- Liston C., Chen A.C., Zebley B.D., Drysdale A.T., Gordon R., Leuchter B., Voss H.U., Casey B.J., Etkin A., Dubin M.J. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol. Psychiatry. 2014;76:517–526. doi: 10.1016/j.biopsych.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi G.S., Mann J.J. Depression. Lancet. 2018;392:2299–2312. doi: 10.1016/S0140-6736(18)31948-2. [DOI] [PubMed] [Google Scholar]
- Manoliu A., Riedl V., Zherdin A., Mühlau M., Schwerthöffer D., Scherr M., Peters H., Zimmer C., Förstl H., Bäuml J., Wohlschläger A.M., Sorg C. Aberrant dependence of default mode/central executive network interactions on anterior insular salience network activity in schizophrenia. Schizophr. Bull. 2014;40:428–437. doi: 10.1093/schbul/sbt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Moran L.V., Tagamets M.A., Sampath H., O'Donnell A., Stein E.A., Kochunov P., Hong L.E. Disruption of anterior insula modulation of large-scale brain networks in schizophrenia. Biol. Psychiatry. 2013;74:467–474. doi: 10.1016/j.biopsych.2013.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulders P.C., van Eijndhoven P.F., Schene A.H., Beckmann C.F., Tendolkar I. Resting-state functional connectivity in major depressive disorder: A review. Neurosci. Biobehav. Rev. 2015;56:330–344. doi: 10.1016/j.neubiorev.2015.07.014. [DOI] [PubMed] [Google Scholar]
- Okada N., Fukunaga M., Miura K., Nemoto K., Matsumoto J., Hashimoto N., Kiyota M., Morita K., Koshiyama D., Ohi K., Takahashi T., Koeda M., Yamamori H., Fujimoto M., Yasuda Y., Hasegawa N., Narita H., Yokoyama S., Mishima R., Kawashima T., Kobayashi Y., Sasabayashi D., Harada K., Yamamoto M., Hirano Y., Itahashi T., Nakataki M., Hashimoto R.I., Tha K.K., Koike S., Matsubara T., Okada G., van Erp T.G.M., Jahanshad N., Yoshimura R., Abe O., Onitsuka T., Watanabe Y., Matsuo K., Yamasue H., Okamoto Y., Suzuki M., Turner J.A., Thompson P.M., Ozaki N., Kasai K., Hashimoto R. Subcortical volumetric alterations in four major psychiatric disorders: a mega-analysis study of 5604 subjects and a volumetric data-driven approach for classification. Mol. Psychiatry. 2023 doi: 10.1038/s41380-023-02141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniyappan L., Mallikarjun P., Joseph V., White T.P., Liddle P.F. Regional contraction of brain surface area involves three large-scale networks in schizophrenia. Schizophr. Res. 2011;129:163–168. doi: 10.1016/j.schres.2011.03.020. [DOI] [PubMed] [Google Scholar]
- Palaniyappan L., Simmonite M., White T.P., Liddle E.B., Liddle P.F. Neural primacy of the salience processing system in schizophrenia. Neuron. 2013;79:814–828. doi: 10.1016/j.neuron.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y., Zhang S., Zhou Y., Song Y., Yang G., Hao K., Yang Y., Li W., Lv L., Zhang Y. Abnormal functional connectivity based on nodes of the default mode network in first-episode drug-naive early-onset schizophrenia. Psychiatry Res. 2021;295 doi: 10.1016/j.psychres.2020.113578. [DOI] [PubMed] [Google Scholar]
- Pettersson E., Larsson H., Lichtenstein P. Common psychiatric disorders share the same genetic origin: a multivariate sibling study of the Swedish population. Mol. Psychiatry England. 2016:717–721. doi: 10.1038/mp.2015.116. [DOI] [PubMed] [Google Scholar]
- Purcell S.M., Wray N.R., Stone J.L., Visscher P.M., O'Donovan M.C., Sullivan P.F., Sklar P. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regonia P.R., Takamura M., Nakano T., Ichikawa N., Fermin A., Okada G., Okamoto Y., Yamawaki S., Ikeda K., Yoshimoto J. Modeling heterogeneous brain dynamics of depression and melancholia using energy landscape analysis. Front. Psych. 2021;12 doi: 10.3389/fpsyt.2021.780997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A., Harvey P.D., Bowie C.R., Mojtabai R., Rabinowitz J., Heaton R.K., Bromet E. Neuropsychological function and dysfunction in schizophrenia and psychotic affective disorders. Schizophr. Bull. 2009;35:1022–1029. doi: 10.1093/schbul/sbn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero, C., Werme, J., Jansen, P.R., Gelernter, J., Stein, M.B., Levey, D., Polimanti, R., de Leeuw, C., Posthuma, D., Nagel, M., van der Sluis, S., 2022. Exploring the genetic overlap between twelve psychiatric disorders. Nat Genet. © 2022. The Author(s), under exclusive licence to Springer Nature America, Inc., United States, pp. 1795-1802. [DOI] [PubMed]
- Ross T.P., Calhoun E., Cox T., Wenner C., Kono W., Pleasant M. The reliability and validity of qualitative scores for the Controlled Oral Word Association Test. Arch. Clin. Neuropsychol. 2007;22:475–488. doi: 10.1016/j.acn.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Schaefer A., Kong R., Gordon E.M., Laumann T.O., Zuo X.N., Holmes A.J., Eickhoff S.B., Yeo B.T.T. Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb. Cortex. 2018;28:3095–3114. doi: 10.1093/cercor/bhx179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha Z., Wager T.D., Mechelli A., He Y. Common dysfunction of large-scale neurocognitive networks across psychiatric disorders. Biol. Psychiatry. 2019;85:379–388. doi: 10.1016/j.biopsych.2018.11.011. [DOI] [PubMed] [Google Scholar]
- Sheline Y.I., Barch D.M., Price J.L., Rundle M.M., Vaishnavi S.N., Snyder A.Z., Mintun M.A., Wang S., Coalson R.S., Raichle M.E. The default mode network and self-referential processes in depression. PNAS. 2009;106:1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O., Tononi G., Kötter R. The human connectome: A structural description of the human brain. PLoS Comput. Biol. 2005;1:e42. doi: 10.1371/journal.pcbi.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng R.N., Stevens W.D., Viviano J.D., Schacter D.L. Attenuated anticorrelation between the default and dorsal attention networks with aging: evidence from task and rest. Neurobiol. Aging. 2016;45:149–160. doi: 10.1016/j.neurobiolaging.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon R., Gaebel W., Barch D.M., Bustillo J., Gur R.E., Heckers S., Malaspina D., Owen M.J., Schultz S., Tsuang M., Van Os J., Carpenter W. Definition and description of schizophrenia in the DSM-5. Schizophr. Res. 2013;150:3–10. doi: 10.1016/j.schres.2013.05.028. [DOI] [PubMed] [Google Scholar]
- Uher R., Payne J.L., Pavlova B., Perlis R.H. Major depressive disorder in DSM-5: implications for clinical practice and research of changes from DSM-IV. Depress. Anxiety. 2014;31:459–471. doi: 10.1002/da.22217. [DOI] [PubMed] [Google Scholar]
- Velthorst E., Fett A.J., Reichenberg A., Perlman G., van Os J., Bromet E.J., Kotov R. The 20-year longitudinal trajectories of social functioning in individuals with psychotic disorders. Am. J. Psychiatry. 2017;174:1075–1085. doi: 10.1176/appi.ajp.2016.15111419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Cheng B., Roberts N., Wang S., Luo Y., Tian F., Yue S. Shared and distinct brain fMRI response during performance of working memory tasks in adult patients with schizophrenia and major depressive disorder. Hum. Brain Mapp. 2021;42:5458–5476. doi: 10.1002/hbm.25618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, D., Watanabe, T., 2023. Distinct Frontoparietal Brain Dynamics Underlying the Co-Occurrence of Autism and ADHD. eNeuro 10. [DOI] [PMC free article] [PubMed]
- Watanabe T., Hirose S., Wada H., Imai Y., Machida T., Shirouzu I., Konishi S., Miyashita Y., Masuda N. A pairwise maximum entropy model accurately describes resting-state human brain networks. Nat. Commun. 2013;4:1370. doi: 10.1038/ncomms2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Hirose S., Wada H., Imai Y., Machida T., Shirouzu I., Konishi S., Miyashita Y., Masuda N. Energy landscapes of resting-state brain networks. Front. Neuroinf. 2014;8:12. doi: 10.3389/fninf.2014.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Masuda N., Megumi F., Kanai R., Rees G. Energy landscape and dynamics of brain activity during human bistable perception. Nat. Commun. 2014;5:4765. doi: 10.1038/ncomms5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Rees G. Brain network dynamics in high-functioning individuals with autism. Nat. Commun. 2017;8:16048. doi: 10.1038/ncomms16048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S., Womer F., Geng H., Jiang X., Zhou Q., Chang M., Zhou Y., Tang Y., Wang F. Similarities and differences of functional connectivity in drug-naïve, first-episode adolescent and young adult with major depressive disorder and schizophrenia. Sci. Rep. 2017;7:44316. doi: 10.1038/srep44316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson P. Are anticorrelated networks in the brain relevant to schizophrenia? Schizophr. Bull. 2007;33:994–1003. doi: 10.1093/schbul/sbm043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia C.H., Ma Z., Ciric R., Gu S., Betzel R.F., Kaczkurkin A.N., Calkins M.E., Cook P.A., García de la Garza A., Vandekar S.N., Cui Z., Moore T.M., Roalf D.R., Ruparel K., Wolf D.H., Davatzikos C., Gur R.C., Gur R.E., Shinohara R.T., Bassett D.S., Satterthwaite T.D. Linked dimensions of psychopathology and connectivity in functional brain networks. Nat. Commun. 2018;9:3003. doi: 10.1038/s41467-018-05317-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A., Fornito A., Cocchi L., Gollo L.L., Breakspear M. Time-resolved resting-state brain networks. PNAS. 2014;111:10341–10346. doi: 10.1073/pnas.1400181111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.