Abstract
Inguinal hernia repair is one of the most commonly performed surgical activities worldwide. Given the circumstances, understanding and identifying the risk and the protective factors is an essential step in order to prevent, diagnose and treat such a common condition. For a long time, obesity was generally considered to be a risk factor in the occurrence of an inguinal hernia. Studies have provided some unexpected data, suggesting that it might actually be a protective factor. This review aims to provide an overview on this topic, taking into account systemic aspects such as collagen distribution and metabolism. In inguinal hernia patients, the ratio between type I collagen and type III collagen is decreased, with type III collagen being responsible for the weakness of the abdominal wall. In obese patients, the extracellular matrix becomes richer in collagen, especially type I collagen, which will generate strength and stiffness. Obesity seems to be a protective factor indeed, but in order to understand the underlying mechanism and to choose the optimal surgical approach, further research is needed.
Keywords:inguinal hernia, obesity, risk factor.
INTRODUCTION
Inguinal hernia is one of the most common surgical conditions worldwide, affecting males more than females (1). It implies a herniation of the intra-abdominal organs, as a result of the weakness of the abdominal wall. There are many categories of inguinal hernia, based on multiple criteria. Therefore, inguinal hernia can be direct or indirect, bilateral or unilateral, initial or recurrent. The etiology is unclear, with multiple factors being correlated with the occurrence or recurrence of this pathology. Genetics, for example, is considered to be involved into the pathophysiology of inguinal hernias (2). An increased intra-abdominal pressure was traditionally correlated with the occurrence of inguinal hernia. As a consequence, activities that increased intra-abdominal pressure, such as lifting weights or even long hours of standing/walking increased the risk of requiring surgical assistance for lateral inguinal hernia (3, 4). However, this statement did not apply to medial inguinal hernias (3, 4). This could also explain why rural residence might be a risk factor for developing inguinal hernias (5).
Smoking seems to be involved into the recurrence of inguinal hernia, as it affects the extracellular matrix and consecutively the process of wound repair (6). This can explain the fact that smoking cessation was recommended before a surgery (7). However, the involvement of tobacco use into the occurrence of primary inguinal hernias is uncertain and some studies state that heavy smoking actually decreases the risk of developing an inguinal hernia (8). This unexpected protective role might be explained by the correlation between smoking and a low body mass index (BMI), as we will further see in this review (9). There are studies that even deny the influence of tobacco consumption on the outcomes of surgery (10).
Obesity is considered to be a wide-spreading disease of the developed world (11). Obesity refers to excess body weight and it implies a BMI greater than 30 kg/m^2. It is correlated with numerous health risks. Obesity leads to insulin resistance, which can also lead to hyperinsulinemia and diabetes, hence the higher incidence of diabetes mellitus among obese individuals (12). Then, obesity is undeniably correlated with cardiovascular disease, but we must remember that obesity involves not only an increase in weight, but also changes in the metabolic status. Therefore, BMI is not an absolute predictor of cardiovascular disease. Visceral abdominal adipose fat (VAT) is related to health risks, including the cardiovascular risk, more than subcutaneous adipose fat (SAT), the ratio between VAT and SAT also being correlated with mortality and cardiac events (13). Therefore, because of the distribution of VAT and/or SAT, two individuals with the same BMI could have a different metabolic and cardiovascular profile (14). In obese patients, fat cell hypertrophy and hyperplasia were documented (15). As a consequence, the extracellular matrix undergoes a remodeling process (16).
Once diagnosed, the only kind of treatment for inguinal hernia is the surgical one. There are two types of surgery: open surgery and laparoscopic surgery, the former being associated with a better recovery, a shorter hospitalization time and less chronic pain (17, 18). In spite of it, laparoscopic surgery was also associated with a higher recurrence rate (19). The open surgery can also be divided into tissue repair and mesh repair. The former has been proven to be a better alternative, as it reduces the late recurrence rates (20). The guidelines state that mesh repair is mandatory when approaching an inguinal hernia case, unless there are specifical contraindications (21).
For many years, obesity has been considered a key factor in the development of an inguinal hernia because it was associated with an elevated intra-abdominal pressure. Nonetheless, more and more studies are revealing that obesity, as counterintuitive as it may sound, might actually be a protective factor and not a risk one.
The purpose of this paper is to evaluate the results of studies that discuss the involvement of obesity in etiology of inguinal hernia and to provide an overall perspective on the actual knowledge on this topic.
MATERIAL AND METHODS
An extensive search was performed, using the following medical databases: Scopus, Web of Science, PubMed and SpringerLink. The search formula contained terms such as “obesity”, “inguinal hernia”, “risk”, “BMI”, ”body mass index”, ”intra-abdominal pressure”, ”waist circumference”, ”MMPs”, ”collagen”, ”TIMPs”. The articles eligible for this review were original studies available on the databases mentioned above up to 2007 and were all written in English. To further refine the selection process, only articles that were research articles, case studies, systematic reviews and meta-analyses were considered. Opinion pieces, commentaries and editorials were explicitly excluded to ensure the gathered data was empirical and rooted in evidence- based research. It is important to note that this article is structured as a narrative review, offering a descriptive and interpretative synthesis of the relevant literature on the topic.
DISCUSSION
Intra-abdominal pressure was considered to be involved into the development of inguinal hernias (22). Also, a positive correlation between the intra-abdominal pressure and body mass index was found (23). Therefore, we would expect Obesity and Inguinal Hernias obesity to also be involved into the physiopathology of inguinal hernias. Multiple studies have shown that, however, an increased BMI actually involved a decreased risk of developing inguinal hernia. The occurrence of inguinal hernia was associated with normal rather than low or high values of the BMI (24-31).
Obese and overweight patients were less likely to develop such a condition. There were several possible explanations for this phenomenon. First, the excessive fat might act as a plug, as a barrier, herniation of the abdominal organs becoming more difficult. Then, it is more difficult to diagnose an inguinal hernia if the patient is obese (25, 32). Also, they are more likely to have a sedentary lifestyle, with physical activity being an important risk factor for inguinal hernias. It is important to mention that the patients taken into consideration were usually the ones that actually underwent the surgery, while obese patients suffer from multiple comorbidities which make them non-eligible for the surgery (25).
Because BMI does not reflect the abdominal fat exclusively, other variables were taken into account, such as waist circumference. However, more recent studies denied most of the explanations above, by considering only diagnosed hernias and including the patients who were diagnosed, but did not undergo surgery. Waist circumference, which is considered to be related to the intra-abdominal visceral fat, was measured and the results remained unchanged: the higher the waist circumference, the lower the risk of developing an inguinal hernia. A strong hypothesis might be that the abdominal fat provides support, counterbalancing the weakness of some abdominal areas (26).
In an attempt to evaluate all the implications of the obesity in the pathophysiology of inguinal hernias, there have been found significantly proved relations between obesity and age, respectively between obesity and the size of the defect. However, the type and content of the inguinal hernia were not in a significant relation with the patients’ BMI (27).
Even though higher BMI implies a lower risk for developing inguinal hernias, obesity plays a role in this pathology, which proves the importance of this topic. Surgeons must know the implications and particularities of the obese patients, especially because obesity becomes more and more common, while inguinal hernias affect an important part of the population. Studies provide data which cannot lead to a universally accepted truth. For example, some studies have shown that overweight and obese patients are more likely to develop recurrent hernias, hence the surgeons must be aware of their risk and these patients need a proper follow-up and a careful monitoring (28). However, other studies deny this correlation, stating that the outcomes of the surgery are not significantly different among obese patients: neither the recurrence risk, nor the complications increase with the BMI (29, 24, 33). Nonetheless, some difficulties, such as the increased risk of surgical site infections (24, 34) or the increased operative time (30) might arise when performing surgery on obese patients. These difficulties might plead in favor of performing a laparoscopic intervention instead of an open one. The open one might be more economic, but the laparoscopic one diminishes the risk of the surgical site infections in obese patients (34). There is a significant correlation between body mass index and the age of the occurrence (27, 31). However, most articles, in spite of some debatable particularities of the obese patients, conclude that there is no higher risk when it comes to these patients, the surgery being safe and efficient.
Some studies suggested that inguinal hernias are not just the result of a primary defect, but they could also arise as the consequence of a systemic disease regarding collagen distribution (35). This hypothesis could be also suggested if we take into account the genetic aspects of inguinal hernia disease (36), as well as the higher recurrence of inguinal hernia amongst patients diagnosed with congenital collagenopathy (37). This is why, in the following part, we will discuss some particularities of the extracellular matrix in inguinal hernia patients, as well as in obese patients.
Extracellular matrix in patients with inguinal hernia
Collagen is one of the most important compounds of the extracellular matrix and it might be as well involved in the development of inguinal hernias. There are multiple types of collagen, with each of them having its own particularities and being found in certain structures and organs. Therefore, we need to take a brief look at two of these types before explaining the mechanisms behind the development of inguinal hernia.
Type I collagen represents the prevalent form of protein in the human body (38). It is a fibril-forming type of collagen, which is known to be remarkably resistant to the activity of proteases and is considered to be responsible for the mechanical strength of various structures such as skin, blood vessels, fascial tissue, cornea or tendons. It also plays a role in wound healing, being found in old mature scars. Type III collagen, on the other hand, is much less stable, less cros- sed-linked and it is usually found in hollow organs. It can be identified as well in other structures, associated with type I collagen. It also forms fibers, known as reticular fibers, which are organized in a rather loose network. It provides cellular support, confers elasticity to the tissues and it is found in the early stages of wound repair. In fascia transversalis, type I collagen is prevalent (39).
Multiple studies have shown that, in patients with inguinal hernia, the ratio between type I and type III collagen is decreased (40). Collagen type I is not necessarily decreased, but there is a significant increase in type III collagen synthesis (41). Other studies state that type I collagen in fascia transversalis might be decreased, but this also proves the lower type I/type III collagen ratio (40). Type III collagen, being much less resistant, might be responsible for the weakness of the abdominal wall (42). The ratio mentioned before was shown to be decreased both in the skin as well as in the abdominal wall fascia (43). The fact that no significant difference could be found between the skin and fascia transversalis suggests that in patients with collagen-related conditions which can lead to inguinal hernias, a skin biopsy should be enough (40). Moreover, in patients with inguinal hernia, an increased turnover of type IV collagen and a decreased turnover of type V collagen have been discovered (44-46).
The extracellular matrix undergoes dynamic changes and MMPs (matrix metalloproteinases) play a fundamental role in the degradation of EMC components. TIMPs are inhibitors of matrix metalloproteinases and they can be divided into four categories: TIMP-1, TIMP-2, TIMP-3 and TIMP-4. The quantity of collagen itself is not the only variable that might interfere with the development of inguinal hernia. The levels of MMPs and TIMPs were also measured and studied. The values of multiple metalloproteinases were increased in patients with inguinal hernia, while those of TIMPs were decreased (47). Other papers state that only TIMP-2 was decreased, while TIMP-1 was significantly increased or even not significantly different (48) (49). MMP-1 and MMP-13 are enzymes involved in the degradation of types I, II and III collagen and they have been shown to play a role in the pathophysiology of inguinal hernia (49, 50). However, MMP-2 was found to be the most important metalloproteinase involved in the development of inguinal hernias. Its level was elevated both in the serum and tissues, which proved there was a systemic increase of MMP-2 (51). MMP-2 and MMP-9 are also called gelatinases and they play a role in the degradation of type IV collagen, which should explain the high turnover rate mentioned above. However, MMP-2 can also degrade types I, II and III collagen. While some authors proved that MMP-2 and MMP-9 are elevated in both plasma and tissue, others discovered low blood levels of both gelatinases (52, 48). The value of TIMP-2 was decreased in patients with inguinal hernia; also, the levels of MMP-2 m-RNA and TIMP-2 m-RNA were significantly lower (53). This would explain the intense proteolyitic activity of matrix metalloproteinases, which would further lead to dysregulations in collagen distribution and metabolism. As we could see, there are still many debatable aspects regarding the way MMPs and TIMPs increase or decrease in inguinal hernia patients, but most studies stated that there was a significant change and an imbalance between the activity of MMPs and TIMPs, which could lead to the development of this disease.
Extracellular matrix in patients with obesity and/or metabolic disease
Obesity has become a major struggle around the world, as its prevalence, associated risks and obesity-related deaths have significantly increased over the last decades (54). The structural, biochemical and molecular changes related to this condition are various and complex. In the current section of our paper, changes occurred in the structure of the extracellular matrix as a result of the obesity are discussed.
In obese patients, the extracellular matrix (ECM) goes through a remodeling process in order to allow the expansion, respectively the shrinking of the adipose tissue associated with weight gain and loss. In the adipose tissue, the main types of collagen found in ECM were type I and type III (discussed above) as well as type V or type VI (55). Adipocytes are the principal collagen- secreting cells in the adipose tissue. In obese individuals, the accumulation of collagen finally leads to adipose tissue fibrosis. Therefore, the ECM gains more strength and stiffness, type I collagen being the main protein (56). A flexible ECM allows the expansion of the adipose tissue, while a rigid and stiff ECM inhibits this expansion, leading to several conditions related to obesity. Therefore, while taking into account that the main type of collagen involved is type I, the strength of the ECM could explain the lower rates of inguinal hernia amongst obese people.
With regard to the modulators of the extracellular matrix, MMPs and TIMPs, there were significant changes in patients with metabolic syndrome or type 2 diabetes, pathologies which can be correlated with obesity. Therefore, MMP-2 and MMP-9 (the gelatinases that have a higher value and might also be involved in the development of inguinal hernia) are increased in this type of patients (57). This statement alone could justify the positive correlation between obesity and inguinal hernia. However, the values of TIMP-1 and TIMP-2 were also significantly increased (57), as opposed to what happens in IH patients.
CONCLUSION
Studies have tried to provide a better understanding on this unknown mechanism: although increased intra-abdominal pressure seems to be correlated with a higher risk for developing an inguinal hernia and obesity implies an elevated intra-abdominal pressure, it seems that a higher BMI is correlated with a lower chance of developing this condition. There was proven that significant changes in collagen metabolism and distribution were involved in both obesity/metabolic disease and inguinal hernia, which could explain this correlation. Also, in both cases there is an imbalance between the activity of matrix metalloproteinases and the activity of TIMPs. The mechanism behind this correlation is not fully understood and further research is needed.
Conflict of interests: none declared.
Financial support: none declared.
TABLE 1.
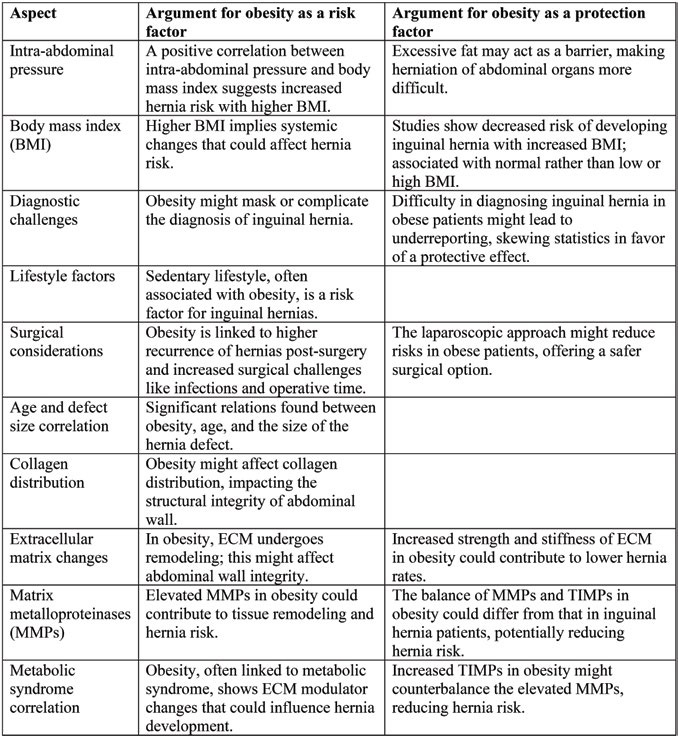
Synthesis of data regarding the complex interplay of risk and protection factors regarding obesity and inguinal hernia
Contributor Information
Ruxandra ROSCA, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania.
Dan Nicolae PADURARU, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; University Emergency Hospital Bucharest, Romania.
Alexandra BOLOCAN, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; University Emergency Hospital Bucharest, Romania.
Florentina MUSAT, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; University Emergency Hospital Bucharest, Romania.
Daniel ION, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; University Emergency Hospital Bucharest, Romania.
Octavian ANDRONIC, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; University Emergency Hospital Bucharest, Romania.
References
- 1.Fitzgibbons RJ, Forse RA. Groin Hernias in Adults. New Engl J Med. 2015;372:756–763. doi: 10.1056/NEJMcp1404068. [DOI] [PubMed] [Google Scholar]
- 2.Yen HC, et al. Sex-specific genetic variants associated with adult-onset inguinal hernia in a Taiwanese population. Int J Med Sci. 2023;20:607–615. doi: 10.7150/ijms.82331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vad MV, Frost P, Bay-Nielsen M, Svendsen SW. Impact of occupational mechanical exposures on risk of lateral and medial inguinal hernia requiring surgical repair. Occup Environ Med. 2012;69:802. doi: 10.1136/oemed-2012-100787. [DOI] [PubMed] [Google Scholar]
- 4.Vad MV, Frost P, Rosenberg J, et al. Inguinal hernia repair among men in relation to occupational mechanical exposures and lifestyle factors: a longitudinal study. Occup Environ Med. 2017;74:769. doi: 10.1136/oemed-2016-104160. [DOI] [PubMed] [Google Scholar]
- 5.Ruhl CE, Everhart JE. Risk Factors for Inguinal Hernia among Adults in the US Population. Am J Epidemiol. 2007;165:1154–1161. doi: 10.1093/aje/kwm011. [DOI] [PubMed] [Google Scholar]
- 6.Sørensen LT, Zillmer R, Ågren M, et al. Effect of smoking, abstention, and nicotine patch on epidermal healing and collagenase in skin transudate. Wound Repair and Regeneration. 2009;17:347–353. doi: 10.1111/j.1524-475X.2009.00479.x. [DOI] [PubMed] [Google Scholar]
- 7.Landin M, et al. The effect of tobacco use on outcomes of laparoscopic and open inguinal hernia repairs: a review of the NSQIP dataset. Surg Endosc. 2017;31:917–921. doi: 10.1007/s00464-016-5055-y. [DOI] [PubMed] [Google Scholar]
- 8.Hemberg A, Holmberg H, Norberg M, Nordin P. Tobacco use is not associated with groin hernia repair, a population-based study. Hernia. 2017;21:517–523. doi: 10.1007/s10029-017-1617-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piirtola M, et al. Association of current and former smoking with body mass index: A study of smoking discordant twin pairs from 21 twin cohorts. PLoS One. 2018;13:e0200140. doi: 10.1371/journal.pone.0200140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yergin CG, Ding DD, Phillips S, et al. The effect of smoking status on inguinal hernia repair outcomes: An ACHQC analysis. Surg Endosc. 2023;37:5464–5471. doi: 10.1007/s00464-023-10055-4. [DOI] [PubMed] [Google Scholar]
- 12.Al Amiri E, et al. The prevalence, risk factors, and screening measure for prediabetes and diabetes among Emirati overweight/obese children and adolescents. BMC Public Health. 2015;15:1298. doi: 10.1186/s12889-015-2649-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ladeiras-Lopes R, et al. The Ratio Between Visceral and Subcutaneous Abdominal Fat Assessed by Computed Tomography Is an Independent Predictor of Mortality and Cardiac Events. Revista Española de Cardiología (English Edition) 2017. [DOI] [PubMed]
- 14.Liu J. et al. Impact of Abdominal Visceral and Subcutaneous Adipose Tissue on Cardiometabolic Risk Factors: The Jackson Heart Study. J Clin Endocrinol Metab. 2020;95:5419–5426. doi: 10.1210/jc.2010-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drolet R, et al. Hypertrophy and hyperplasia of abdominal adipose tissues in women. Int J Obes. 2008;32:283–291. doi: 10.1038/sj.ijo.0803708. [DOI] [PubMed] [Google Scholar]
- 16.Khan T, et al. Metabolic Dysregulation and Adipose Tissue Fibrosis: Role of Collagen VI,”. Mol Cell Biol. [DOI] [PMC free article] [PubMed]
- 17.Eklund A, Montgomery A, Bergkvist L, et al. Chronic pain 5 years after randomized comparison of laparoscopic and Lichtenstein inguinal hernia repair. Br J Surg. 2010;97:600–608. doi: 10.1002/bjs.6904. [DOI] [PubMed] [Google Scholar]
- 18.Payiziwula J, et al. Laparoscopy Versus Open Incarcerated Inguinal Hernia Repair in Octogenarians: Single-Center Experience With World Review. Surg Laparosc Endosc Percutan Tech. 2019;29:138–140. doi: 10.1097/SLE.0000000000000629. [DOI] [PubMed] [Google Scholar]
- 19.Ramsay G, Scott NW, Jansen JO. A 19 year population-based cohort study analysing reoperation for recurrence following laparoscopic and open inguinal hernia repairs. Hernia. 2020;24:793–800. doi: 10.1007/s10029-019-02073-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee CS, et al. Retrospective study on prevalence of recurrent inguinal hernia: A large-scale multi-institutional study. Ann Surg Treat Res. 2020;98:51–55. doi: 10.4174/astr.2020.98.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miserez M, et al. Update with level 1 studies of the European Hernia Society guidelines on the treatment of inguinal hernia in adult patients. Hernia. 2014;18:151–163. doi: 10.1007/s10029-014-1236-6. [DOI] [PubMed] [Google Scholar]
- 22.Soucasse A, Jourdan A, Edin L, et al. A better understanding of daily life abdominal wall mechanical solicitation: Investigation of intra-abdominal pressure variations by intragastric wireless sensor in humans. Med Eng Phys. 2022;104:103813. doi: 10.1016/j.medengphy.2022.103813. [DOI] [PubMed] [Google Scholar]
- 23.Smit M, Werner MJ, Lansink-Hartgring AO, et al. How central obesity influences intra-abdominal pressure: a prospective, observational study in cardiothoracic surgical patients. Ann Intensive Care. 2016;6:99. doi: 10.1186/s13613-016-0195-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shrestha S, Upadhyay PK. Prevalence of obesity in inguinal hernia repair patients in a tertiary care center. Journal of the Nepal Medical Association. 2021;59:156–159. doi: 10.31729/jnma.5636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zendejas B, Hernandez-Irizarry R, Ramirez T, et al. Relationship between body mass index and the incidence of inguinal hernia repairs: A population-based study in Olmsted County, MN. Hernia. 2014;18:283–288. doi: 10.1007/s10029-013-1185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hemberg A, Montgomery A, Holmberg H, Nordin P. Waist Circumference is not Superior to Body Mass Index in Predicting Groin Hernia Repair in Either Men or Women. World J Surg. 2022;46:401–408. doi: 10.1007/s00268-021-06359-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zade M, Sridharan S. Comparison of Incidence of Inguinal Hernia among Obese and Normal Individuals: A Retrospective Analysis. J Pharm Res Int. 2021;33:711–714. [Google Scholar]
- 28.Lee SS, Jung HJ, Park BS, et al. Surgical Aspects of Recurrent Inguinal Hernia in Adults. Am Surg. 2016;82:1063–1067. [PubMed] [Google Scholar]
- 29.Huerta S, Tran N, Yi B, Pham T. Outcomes of obese compared to non-obese veterans undergoing open inguinal hernia repair: a case–control study. Hernia. 2021;25:1289–1294. doi: 10.1007/s10029-021-02382-z. [DOI] [PubMed] [Google Scholar]
- 30.Chinn J, et al. Comparison of BMI on operative time and complications of robotic inguinal hernia repair at a VA medical center. Surg Endosc. 2022;36:9398–9402. doi: 10.1007/s00464-022-09259-x. [DOI] [PubMed] [Google Scholar]
- 31.Park CY, Kim JC, Kim DY, Kim SK. Inguinal hernia repair in overweight and obese patients. J Korean Surg Soc. 2011;81:205–210. doi: 10.4174/jkss.2011.81.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Goede B, et al. Risk factors for inguinal hernia in middle-aged and elderly men: Results from the Rotterdam Study. Surgery (United States) 2015. [DOI] [PubMed]
- 33.Kudsi OY, Bou-Ayash N, Gokcal F. Comparison of perioperative outcomes between non-obese and obese patients undergoing robotic inguinal hernia repair: a propensity score matching analysis. Hernia. 2022;26:1033–1039. doi: 10.1007/s10029-021-02433-5. [DOI] [PubMed] [Google Scholar]
- 34.Willoughby AD, Lim RB, Lustik MB. Open versus laparoscopic unilateral inguinal hernia repairs: defining the ideal BMI to reduce complications. Surg Endosc. 2017;31:206–214. doi: 10.1007/s00464-016-4958-y. [DOI] [PubMed] [Google Scholar]
- 35.Koruth S, Narayanaswamy Chetty YV. Hernias – Is it a primary defect or a systemic disorder? Role of collagen III in all hernias – A case control study. Ann Med Surg. 2017;19:37–40. doi: 10.1016/j.amsu.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jansen PL, Klinge U, Jansen M, Junge K. Risk factors for early recurrence after inguinal hernia repair. BMC Surg. 2009;9:18. doi: 10.1186/1471-2482-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang HH, Juan YS, Li CC, et al. Congenital collagenopathies increased the risk of inguinal hernia developing and repair: analysis from a nationwide population-based cohort study. Sci Rep 2022. [DOI] [PMC free article] [PubMed]
- 38.Stefanovic B. RNA protein interactions governing expression of the most abundant protein in human body, type I collagen. Wiley Interdisciplinary Reviews: RNA. 2013;4:535–545. doi: 10.1002/wrna.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henriksen NA, Yadete DH, Sorensen LT, et al. Connective tissue alteration in abdominal wall hernia. Br J Surg. 2011;98:210–219. doi: 10.1002/bjs.7339. [DOI] [PubMed] [Google Scholar]
- 40.Prasanna S, Sekaran PG, Sivakumar A, Govindan VK. Role of Collagen in the Etiology of Inguinal Hernia Patients: A Case-Control Study. Cureus. 2023;15:e43479. doi: 10.7759/cureus.43479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyer ALM, Berger E, Monteiro O Jr, et al. Quantitative and qualitative analysis of collagen types in the fascia transversalis of inguinal hernia patients. Arq Gastroenterol. 2007;44:230–234. doi: 10.1590/s0004-28032007000300010. [DOI] [PubMed] [Google Scholar]
- 42.Henriksen NA, Yadete DH, Sorensen LT, et al. Connective tissue alteration in abdominal wall hernia,”. British Journal of Surgery. 2011;98:210–219. doi: 10.1002/bjs.7339. [DOI] [PubMed] [Google Scholar]
- 43.Peeters E, De Hertogh G, Junge K, et al. Skin as marker for collagen type I/III ratio in abdominal wall fascia. Hernia. 2014;18:519–525. doi: 10.1007/s10029-013-1128-1. [DOI] [PubMed] [Google Scholar]
- 44.Henriksen NA, et al. The collagen turnover profile is altered in patients with inguinal and incisional hernia. Surgery (United States) 2015. [DOI] [PubMed]
- 45.Medical D, Rosenberg J, Jeekel J, Montgomery A. PhD Thesis Danish Medical Journal 2014.
- 46.Lorentzen L, et al. Type V Collagen is Persistently Altered After Inguinal Hernia Repair. Scand J Surg. 2018;107:212–217. doi: 10.1177/1457496918766694. [DOI] [PubMed] [Google Scholar]
- 47.Isik A, Gursul C, Peker K, et al. Metalloproteinases and Their Inhibitors in Patients with Inguinal Hernia. World J Surg. 2017;41:1259–1266. doi: 10.1007/s00268-016-3858-6. [DOI] [PubMed] [Google Scholar]
- 48.Strohalmová S, et al. The Effect of Surgery on the Levels of Matrix Metalloproteinases in Patients with Inguinal Hernia. Physiol Res. 2021;70:627–634. doi: 10.33549/physiolres.934625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Durukan U, et al. The role of tissue inhibitor of metalloproteinases in the aetiology of inguinal and incisional hernias,”. Int Wound J. 2022;19:1502–1508. doi: 10.1111/iwj.13746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aren A, Gökçe AH, Gökçe FS, Dursun N. Roles of matrix metalloproteinases in the etiology of inguinal hernia. Hernia. 2011;15:667–671. doi: 10.1007/s10029-011-0846-5. [DOI] [PubMed] [Google Scholar]
- 51.Śmigielski J, Kołomecki K, Ziemniak P, et al. Degradation of Collagen by Metalloproteinase 2 in Patients with Abdominal Hernias. Eur Surg Res. 2008;42:118–121. doi: 10.1159/000187643. [DOI] [PubMed] [Google Scholar]
- 52.Antoniou GA, Tentes IK, Antoniou SA, et al. Matrix Metalloproteinase Imbalance in Inguinal Hernia Formation. J Invest Surg. 2011;24:145–150. doi: 10.3109/08941939.2011.558610. [DOI] [PubMed] [Google Scholar]
- 53.Wang D, Han Y, Xu X, et al. Matrix Metalloproteinases (MMP-2) and Tissue Inhibitors of Metalloproteinases (TIMP-2) in Patients with Inguinal Hernias. World J Surg. 2020;44:3679–3686. doi: 10.1007/s00268-020-05674-0. [DOI] [PubMed] [Google Scholar]
- 54.Boutari C, Mantzoros CS. A 2022 update on the epidemiology of obesity and a call to action: as its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism. 2022;133:155217. doi: 10.1016/j.metabol.2022.155217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mori S, Kiuchi S, Ouchi A, et al. Characteristic expression of extracellular matrix in subcutaneous adipose tissue development and adipogenesis; Comparison with visceral adipose tissue. Int J Biol Sci. 2014;10:825–833. doi: 10.7150/ijbs.8672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu X, et al. Enhanced migration of murine fibroblast-like 3T3-L1 preadipocytes on type I collagen-coated dish is reversed by silibinin treatment. Mol Cell Biochem. 2018;441:35–62. doi: 10.1007/s11010-017-3173-z. [DOI] [PubMed] [Google Scholar]
- 57.Hopps E, Lo Presti R, Montana M, et al. Gelatinases and Their Tissue Inhibitors in a Group of Subjects with Metabolic Syndrome. J Invest Med. 2013;61:978–983. doi: 10.2310/JIM.0b013e318294e9da. [DOI] [PubMed] [Google Scholar]


