Abstract
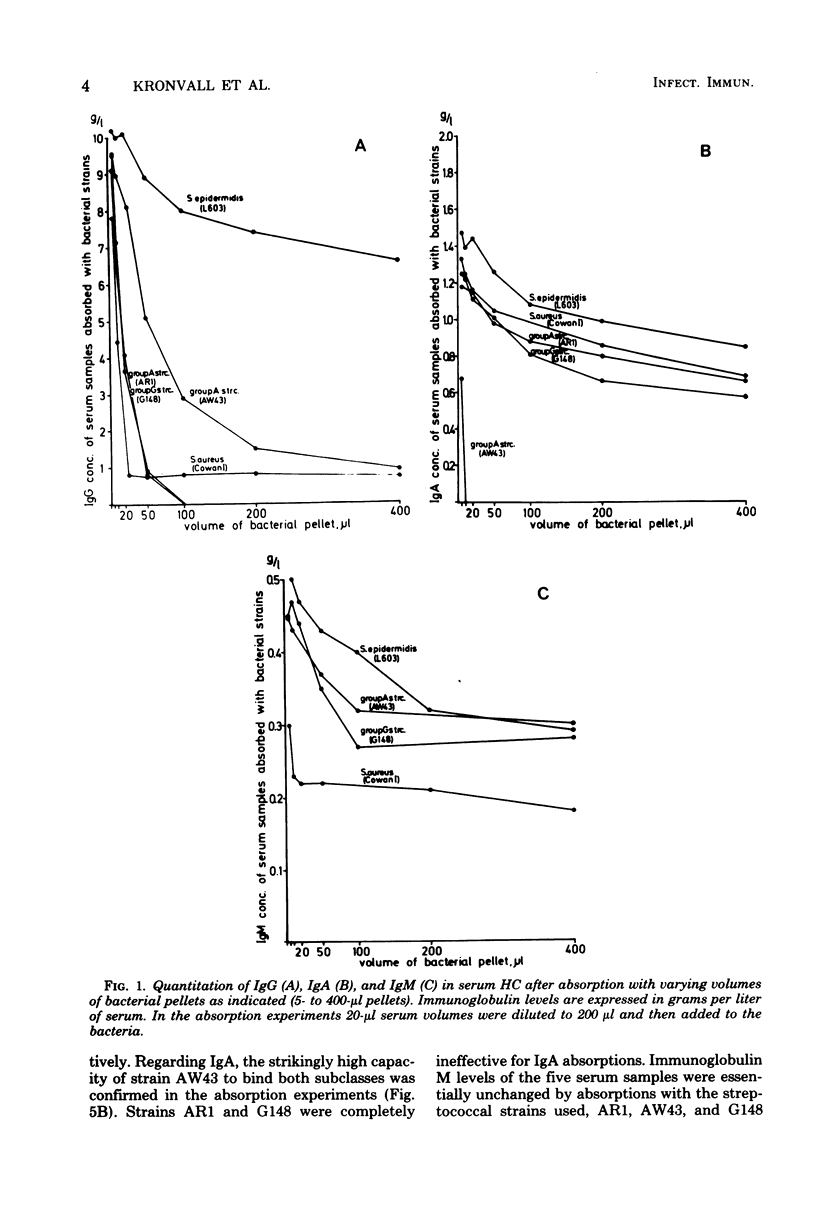
Five gram-positive bacterial strains were selected for absorption studies of human serum samples. Strain AR1 (group A, M-type 1) and G148 (group G), with strong immunoglobulin G (IgG) binding capacities, and strain AW43 (group A, M-type 60), binding both IgA1 and IgA2, were compared with Staphylococcus aureus Cowan I and with Staphylococcus epidermidis L603. Both AR1 and G148 were capable of completely absorbing out serum IgG. In contrast, S. aureus Cowan I left a fraction unabsorbed, as expected from its known lack of IgG3 binding. Strain AW43 absorbed out all serum IgA, using a 10-microliter bacterial pellet for 20 microliter of serum. Serum IgM levels were slightly reduced by S. aureus Cowan I absorption. On the basis of the experiments, a bacterial mixture was designed consisting of S. aureus Cowan I and group A streptococcus strains AR1 and AW43, with absorption characteristics suitable for use in discriminating between early IgM and late IgG and IgA immune responses in routine serological work. A new type of bacteria-mammalian protein binding was discovered. Human serum albumin was completely absorbed out by strain G148 and to a lesser extent by strain AR1 and AW43. S. aureus Cowan I and S. epidermidis were negative. The binding capacity of G148 for albumin equalled that of Cowan I for IgG. The binding pattern of albumin to the strains was different from those of IgG, IgA, IgM, fibrinogen, haptoglobin, or aggregated beta 2-microglobulin and therefore seems to represent another type of bacterial-mammalian interaction with a specific albumin receptor on the surface of streptococci.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ankerst J., Christensen P., Kjellén L., Kronvall G. A rountine diagnostic test for IgA and IgM antibodies to rubella virus: absorption of IgG with Staphylococcus aureus. J Infect Dis. 1974 Sep;130(3):268–273. doi: 10.1093/infdis/130.3.268. [DOI] [PubMed] [Google Scholar]
- Arvidson S., Holme T., Wadström T. Influence of cultivation conditions on the production of extracellular proteins by Staphylococcus aureus. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(3):399–405. doi: 10.1111/j.1699-0463.1971.tb00079.x. [DOI] [PubMed] [Google Scholar]
- Banck G., Forsgren A. Many bacterial species are mitogenic for human blood B lymphocytes. Scand J Immunol. 1978;8(4):347–354. doi: 10.1111/j.1365-3083.1978.tb00528.x. [DOI] [PubMed] [Google Scholar]
- Brown J. P., Klitzman J. M., Hellström I. Radioimmunoassay of murine leukemia virus p30 using Staphylococcus aureus as immunoadsorbent. J Immunol Methods. 1978;21(1-2):23–33. doi: 10.1016/0022-1759(78)90220-x. [DOI] [PubMed] [Google Scholar]
- Brunda M. J., Minden P., Sharpton T. R., McClatchy J. K., Farr R. S. Precipitation of radiolabeled antigen-antibody complexes with protein A-containing Staphylococcus aureus. J Immunol. 1977 Jul;119(1):193–198. [PubMed] [Google Scholar]
- Brunner H., Schaeg W., Brück U., Schummer U., Schiefer H. G. A staphylococcal radioimmunoassay for detection of antibodies to Mycoplasma pneumoniae. Med Microbiol Immunol. 1977 May 18;163(1):25–35. doi: 10.1007/BF02126706. [DOI] [PubMed] [Google Scholar]
- Cullen S. E., Schwartz B. D. An improved method for isolation of H-2 and Ia alloantigens with immunoprecipitation induced by protein A-bearing staphylococci. J Immunol. 1976 Jul;117(1):136–142. [PubMed] [Google Scholar]
- DUTHIE E. S. The action of fibrinogen on certain pathogenic cocci. J Gen Microbiol. 1955 Oct;13(2):383–393. doi: 10.1099/00221287-13-2-383. [DOI] [PubMed] [Google Scholar]
- Figenschau K. J., Ulstrup J. C. Staphylococcal radioimmunoassay for hepatitis B antigen and antibody. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Jun;82(3):422–428. doi: 10.1111/j.1699-0463.1974.tb02346.x. [DOI] [PubMed] [Google Scholar]
- Forsgren A., Sjöquist J. "Protein A" from S. aureus. I. Pseudo-immune reaction with human gamma-globulin. J Immunol. 1966 Dec;97(6):822–827. [PubMed] [Google Scholar]
- Ginsberg M. H., Morrison D. C. The selective binding of aggregated IgG to lipid A-rich bacterial lipopolysaccharides. J Immunol. 1978 Jan;120(1):317–319. [PubMed] [Google Scholar]
- Goding J. W. Use of staphylococcal protein A as an immunological reagent. J Immunol Methods. 1978;20:241–253. doi: 10.1016/0022-1759(78)90259-4. [DOI] [PubMed] [Google Scholar]
- Handsher R., Fogel A. Modified staphylococcal absorption method used for detecting rubella-specific immunoglobin M antibodies during a rubella epidemic. J Clin Microbiol. 1977 Jun;5(6):588–592. doi: 10.1128/jcm.5.6.588-592.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe M., Closs O., Bjune G., Kronvall G., Axelsen N. H. Mycobacterium leprae specific antibodies detected by radioimmunoassay. Scand J Immunol. 1978;7(2):111–120. doi: 10.1111/j.1365-3083.1978.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Harboe M., Fölling I. Recognition of two distinct groups of human IgM and IgA based on different binding to staphylococci. Scand J Immunol. 1974;3(4):471–482. doi: 10.1111/j.1365-3083.1974.tb01280.x. [DOI] [PubMed] [Google Scholar]
- Jahrling P. B., Hesse R. A., Metzger J. F. Radioimmunoassay for quantitation of antibodies to alphaviruses with staphylococcal protein A. J Clin Microbiol. 1978 Jul;8(1):54–60. doi: 10.1128/jcm.8.1.54-60.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson S., Kronvall G. The use of protein A-containing Staphylococcus aureus as a solid phase anti-IgG reagent in radioimmunoassays as exemplified in the quantitation of alpha-fetoprotein in normal human adult serum. Eur J Immunol. 1974 Jan;4(1):29–33. doi: 10.1002/eji.1830040108. [DOI] [PubMed] [Google Scholar]
- KANTOR F. S. FIBRINOGEN PRECIPITATION BY STREPTOCOCCAL M PROTEIN. I. IDENTITY OF THE REACTANTS, AND STOICHIOMETRY OF THE REACTION. J Exp Med. 1965 May 1;121:849–859. doi: 10.1084/jem.121.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Klareskog L., Banck G., Forsgren A., Peterson P. A. Binding of HLA antigen-containing liposomes to bacteria. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6197–6201. doi: 10.1073/pnas.75.12.6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronvall G. A surface component in group A, C, and G streptococci with non-immune reactivity for immunoglobulin G. J Immunol. 1973 Nov;111(5):1401–1406. [PubMed] [Google Scholar]
- Kronvall G., Bjune G., Stanford J., Menzel S., Samuel D. Mycobacterial antigens in antibody responses of leprosy patients. Int J Lepr Other Mycobact Dis. 1975 Oct-Dec;43(4):306–306. [PubMed] [Google Scholar]
- Kronvall G., Myhre E. B., Björck L., Berggård I. Binding of aggregated human beta2-microglobulin to surface protein structure in group A, C, and G streptococci. Infect Immun. 1978 Oct;22(1):136–142. doi: 10.1128/iai.22.1.136-142.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronvall G., Williams R. C., Jr Differences in anti-protein A activity among IgG subgroups. J Immunol. 1969 Oct;103(4):828–833. [PubMed] [Google Scholar]
- Köhler W., Prokop O. Relationship between haptoglobin and Streptococcus pyogenes T4 antigens. Nature. 1978 Jan 26;271(5643):373–373. doi: 10.1038/271373a0. [DOI] [PubMed] [Google Scholar]
- LAURELL C. B. ANTIGEN-ANTIBODY CROSSED ELECTROPHORESIS. Anal Biochem. 1965 Feb;10:358–361. doi: 10.1016/0003-2697(65)90278-2. [DOI] [PubMed] [Google Scholar]
- Laurell C. B. Electroimmuno assay. Scand J Clin Lab Invest Suppl. 1972;124:21–37. doi: 10.3109/00365517209102748. [DOI] [PubMed] [Google Scholar]
- Lind I., Harboe M., Folling I. Protein A reactivity of two distinct groups of human monoclonal IgM. Scand J Immunol. 1975;4(8):843–848. doi: 10.1111/j.1365-3083.1975.tb03726.x. [DOI] [PubMed] [Google Scholar]
- Loos M., Wellek B., Thesen R., Opferkuch W. Antibody-independent interaction of the first component of complement with Gram-negative bacteria. Infect Immun. 1978 Oct;22(1):5–9. doi: 10.1128/iai.22.1.5-9.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallinson H., Roberts C., Bruce White G. B. Staphylococcal protein A; its preparation and an application to rubella serology. J Clin Pathol. 1976 Nov;29(11):999–1002. doi: 10.1136/jcp.29.11.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Myhre E. B., Holmberg O., Kronvall G. Immunoglobulin-binding structure on bovine group G streptococci different from type III Fc receptors on human group G streptococci. Infect Immun. 1979 Jan;23(1):1–7. doi: 10.1128/iai.23.1.1-7.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myhre E. B., Kronvall G. Heterogeneity of nonimmune immunoglobulin Fc reactivity among gram-positive cocci: description of three major types of receptors for human immunoglobulin G. Infect Immun. 1977 Sep;17(3):475–482. doi: 10.1128/iai.17.3.475-482.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick C. C., Virella G., Koistinen J., Fudenberg H. H. Differential binding of IgA proteins of different subclasses and allotypes to Staphylococcal protein A. Z Immunitatsforsch Immunobiol. 1977 Dec;153(5):466–469. [PubMed] [Google Scholar]
- Saltvedt E., Harboe M. Binding of IgA to protein-A-containing staphylococci: relationship to subclasses. Scand J Immunol. 1976;5(10):1103–1108. doi: 10.1111/j.1365-3083.1976.tb00250.x. [DOI] [PubMed] [Google Scholar]
- Skaug K., Gaarder P. I. An indirect immunofluorescent antibody test for determination of Rubella virus specific IgM antibodies. Elimination of secondary IgM rheumatoid factor staining after absorption of serum IgG with Staphylococcal protein A. Acta Pathol Microbiol Scand C. 1978 Feb;86(1):33–35. doi: 10.1111/j.1699-0463.1978.tb02554.x. [DOI] [PubMed] [Google Scholar]
- Skaug K., Tjotta E. Diagnosis of recent Herpes simplex infections. A modified immunofluorescent test for the detection of specific Herpes simplex IgM antibodies after staphylococcal adsorption of IgG. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Jun;82(3):323–328. [PubMed] [Google Scholar]