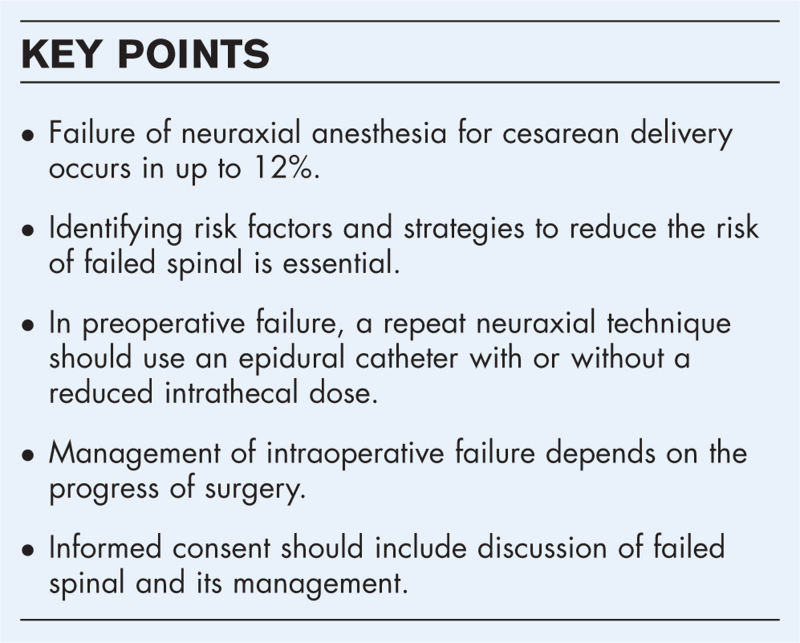
Abstract
Purpose of review
There is an increasing awareness of the significance of intraoperative pain during cesarean delivery. Failure of spinal anesthesia for cesarean delivery can occur preoperatively or intraoperatively. Testing of the neuraxial block can identify preoperative failure. Recognition of the risk of high neuraxial block in repeat spinal in case of preoperative failure is important.
Recent finding
Knowledge of risk factors for block failure facilitates prevention by selecting the most appropriate neuraxial procedure, adequate intrathecal doses and choice of technique. Intraoperative pain is not uncommon, and neither obstetricians nor anesthesiologists can adequately identify intraoperative pain. Early intraoperative pain should be treated differently from pain towards the end of surgery.
Summary
Block testing is crucial to identify preoperative failure of spinal anesthesia. Repeat neuraxial is possible but care must be taken with dosing. In this situation, switching to a combined spinal epidural or an epidural technique can be useful. Intraoperative pain must be acknowledged and adequately treated, including offering general anesthesia. Preoperative informed consent should include block failure and its management.
Keywords: combined spinal epidural, failed spinal, high spinal, intraoperative pain, repeat spinal
INTRODUCTION
‘The mother is awake and typically not sedated while undergoing major abdominal surgery for a seismic life event: the arrival of her baby. This is a rare – or unique – experience, the memory of which will be greatly influenced by the quality of care she receives’ [1▪▪].
A retrospective review of more than 5300 spinal anesthesia (SA) for cesarean delivery (CD) found a SA failure rate of 2.1% [95% confidence interval (CI) 1.7–2.5] [2▪▪]. The authors defined failure as need for repeat anesthesia [general anesthesia (GA) or neuraxial procedure] within one hour of injection of intrathecal medication. Another 2.0% (95% CI 1.6–2.4) received supplementary analgesia and/or sedation [2▪▪]. A total of 4.1% (95% CI 3.6–4.7) needed GA, repeat neuraxial procedure or analgesic supplementation. In another retrospective study in 5015 women, 5.5% (5.5%, 95% CI 4.9–6.2) received GA, repeat neuraxial procedure or analgesic supplementation [3]. A systematic review of elective CD by Patel et al.[4▪] found a failure rate of 10.2% (95% CI 9.0–11.4) with a prevalence of only 2 GA per 3497 patients, a very low rate of GA. In this systematic review, epidural supplementation of combined spinal epidural (CSE) anesthesia was included in the definition of failure.
According to the Royal College of Anaesthetists (RCoA) improvement compendium, the quality criteria of best practice the rate of conversion of regional to GA should be below 1% in elective CD and less than 5% in category 2–3 [5]. Real-life data shows that these standards of care seem difficult to achieve. For example, in the study by Adesope et al.[3] the overall incidence of SA failure was 5.5% and failure rate did not differ between scheduled and unscheduled CD. We believe that conversion rates are indicators to be used with great caution since they do not reflect intraoperative pain. Furthermore, aiming for a lower rate carries the risk of more women in pain.
Box 1.
no caption available
What is a failed spinal?
Although failure rates of SA for CD have been discussed for more than 30 years, the definition of failure has only recently been questioned [1▪▪], especially in consideration of the patient's experience [6]. In fact, analgesic supplementation or intraoperative conversion to GA certainly underestimates the incidence of intraoperative pain. Although awareness under GA has received a lot of attention, this is not the case for ‘awareness’ under regional anesthesia. On the other hand, in medico-legal claims of obstetric anesthesia, pain during CD is more frequent than accidental awareness under GA [7▪▪,8].
Although the RCoA improvement compendium suggests that the rate of pain during CD carried out under regional anesthesia could be an outcome to monitor, no acceptable rate is specified [5].
What are reasons for failure?
Previous narrative reviews have detailed the possible causes of SA failure [9,10]. The vast majorities of failures are likely due to technical problems or operator related, whereas ineffective drug action and local anesthetic resistance are probably extremely rare. Common technical problems include: failed/difficult lumbar puncture; patient positioning; needle type; needle insertion; loss of injectate; inadequate intrathecal spread; or errors in injectate preparation such as inadequate dose, type of drugs or density. A broad spectrum of local anesthetic concentrations in CSF were measured in case of failed SA [11]. Other known issues are pseudo-successful lumbar punctures. They occur when a reflux of clear fluid is mistaken for CSF when it is in fact due to a previous injection (local anesthetic or epidural saline). Pseudo-successful lumbar punctures can also occur in case of Tarlov cyst [12] or when accidental needle movement or needle position results in partial injection into the epidural space [9,10]. The frequency of occurrence of these various etiologies and their relative contributions to SA failures in clinical practice are largely unknown.
Prevention
SA is one of the most reliable regional block methods [9]. The best way to handle failed SA is similar to management of the difficult airway in obstetrics: anticipate the risk and avoid it – but have a good plan if you do have to manage it. The first step to minimize the risk of failed SA is to recognize the importance of a meticulous approach when performing the block [9]. This includes positioning of the patient, identification of subarachnoid space, injection of the solution and patient management throughout the procedure [9].
Knowledge of risk factors for SA failures is obviously critical in order to handle modifiable risk factors.
Risk factors for spinal failure
There are two recent retrospective studies, which analyzed factors associated with SA failure and are summarized in Table 1. Stav et al.[13▪▪] included 4305 patients and calculated odds ratios for conversion to GA, while Jin et al.[2▪▪] included 5361 patients and calculated odds ratios for repeat neuraxial technique or conversion to GA.
Table 1.
Factors associated with spinal anesthesia failure
| Parameter | Odds ratio | 95% Confidence interval | Reference |
| Previous CD | 11.33 | 7.09–18.20 | [2▪▪] |
| Tubal ligation | 8.23 | 3.12–19.20 | [2▪▪] |
| Peripartum hemorrhage | 5.96 | 1.09–25.18 | [13▪▪] |
| Size of spinal needle 27 G vs. 25 G | 5.08 | 1.91–13.27 | [2▪▪] |
| Height of lumbar puncture (L4/5 vs. L3/4 or L2/3) | 1.81 | 1.06–3.10 | [2▪▪] |
| Emergency CD | 1.68 | 0.99–2.80 | [2▪▪] |
| Surgical duration (per minute) | 1.03 | 1.02–1.04 | [13▪▪] |
| Body mass index (per kg/m2) | 0.94 | 0.90–0.98 | [2▪▪] |
| Gestational age (per week) | 0.91 | 0.84–0.99 | [2▪▪] |
| Dose of bupivacaine | 0.54 | 0.38–0.75 | [13▪▪] |
CD, cesarean delivery.
Jin et al.[2▪▪] also ranked risk factors for requirement of alternate anesthetic technique or analgesic supplementation when 12 mg hyperbaric bupivacaine with fentanyl and morphine was used. The rank of these risk factors were:
-
(1)
Previous CD (independent of the number of CD)
-
(2)
Longer surgery duration
-
(3)
Lower birth weight
-
(4)
Lower gestational age
-
(5)
Lower body mass index
-
(6)
Tubal ligation
Lower birth weight and lower gestational weight are obviously related. Adescope et al.[3] found increased SA failure rates in neonates below 28 weeks of gestation or birth weight below 2500 g. In preterm CD, less women reached a sensory level of T4, and it took longer to get there when compared to term CD [14]. The hypothesis is that in pregnant women lower dose of intrathecal drugs are required because there is a decrease in subarachnoid and epidural volumes due to uterine enlargement, caval obstruction and epidural vein distension. However, in preterm pregnancies and in lower birth weight the volume of intrathecal/epidural volume might be less affected, and the usual reduced intrathecal doses may prove insufficient [3]. The same might be true for patients with low body mass index [2▪▪].
Interestingly, the lumbar level of spinal puncture can also influence the risk of SA failure, as the failure rate seems higher with punctures at L4/5 or L5/S1 when compared to higher levels [2▪▪]. The local anesthetic solution might be entrapped below lumbar lordosis, especially if performed in the sitting position [9]. On the other hand, a higher lumbar level cannot be recommended without a word of caution: clinical determination of lumbar level is alarmingly inaccurate [15] and direct needle trauma to the conus has been described [16]. It seems cautious to select a lower lumbar level unless the correct lumbar level is verified by ultrasound.
The use of intravenous prophylactic phenylephrine to prevent hypotension appears to influence the spinal block. In a randomized trial, Xiao et al.[17] found a 20% higher ED95 for hyperbaric bupivacaine when combined with a prophylactic phenylephrine infusion, as compared to a placebo infusion. Of note, for methodological reasons, SA were performed in this study without the use of intrathecal adjuvants.
Patient positioning can influence intrathecal spread of local anesthetics. When comparing sitting vs. lateral position for placement of SA, the lateral position leads to a significantly faster onset of sensory block by 3 min, while motor block was only slightly faster [18]. The lateral position also led to less hypotension and higher maternal satisfaction scores. The final height of the block was not different.
Lack of expertise also is likely to influence the failure rate. In a recent retrospective matched case–control study, the rate of SA failure was higher when performed by third year residents compared to registrars [19].
Table 2 summarizes strategies to minimize the risk of SA failure.
Table 2.
Suggested strategies to reduce the risk of failed spinal anesthesia
| Adequate dosing of local anesthetic | Increase with low birth weight, <29 weeks of gestation, prophylactic vasopressor infusion |
| Add intrathecal lipophilic opioid | Fentanyl or Sufentanil |
| Consider CSE in expected longer surgical duration | Particularly in repeat CD, tubal ligation |
| Appropriate motor and sensory testing before start of surgery | Consider repeat spinal if no effect at all. Otherwise, consider reduced dose spinal, CSE or change to epidural anesthesia |
CD, cesarean delivery; CSE, combined spinal epidural technique.
PREOPERATIVE FAILURE
Testing the block
Testing of the block should be performed with the intention of excluding an inadequate block, and not to confirm that the block is working. In a case series of obstetric litigation, 42% of surgeries started despite evidence of inadequate neuraxial anesthesia [8].
There is no reason to test for the upper sensory level of SA until some extent of motor block has been established in the lower extremities. The easiest method to test for motor block is the straight leg test, where the patient is asked to lift the legs against gravity. The narration of Susanna Stanford is a frightening example that asking the same question over and over again (i.e. testing for cold sensation) eventually leads to a (false) positive answer [6]. Recently, a working group by the Obstetric Anaesthetist's Association (OAA) in the UK has published guidelines on prevention and management of intraoperative pain during CD [7▪▪]. These guidelines also cover informed consent and management of intraoperative pain and give a detailed recommendation on preoperative assessment of neuraxial block [7▪▪].
A level of T5 to loss of touch sensation is required for CD [7▪▪]. With a sensory level of T10 a forceps test is probably already negative. For psychological reasons, we recommend that anesthesiologists do not allow surgeons to perform a forceps test at the site of incision before the sensory level required for surgery, i.e. loss of touch sensation at T5 or above, has been reached.
Repeat neuraxial or general anesthesia?
In case of preoperative failure of SA, the question arises whether to repeat a neuraxial procedure or to switch to GA. There are concerns about repeating a neuraxial procedures with the two most important being, firstly an excessive spread of the second dose leading to high or total spinal block, and secondly a theoretical increased risk of direct nerve trauma, as adjacent nerve tissue might already be anesthetized [9]. In case of a total failure of the first intrathecal injection, that is, absence of any measurable effect, including sacral dermatomes, there is probably no additional risk in repeating the SA with the usual dosage [9]. In case of partial effect of SA or in the situation of unsuccessful extension of epidural labor analgesia for CD, there must be a word of caution about a second SA. In a randomized trial of repeat spinal after failed SA for CD, the use of 12 mg of hyperbaric bupivacaine significantly increased the incidence of high spinal block, hypotension, bradycardia and respiratory compromise when compared to 10 mg [20]. In case of a repeat injection, additives such as opioids should be omitted. Reassuringly, there was no report of cardiac arrest in repeat SA and only one event in a patient with SA following failed epidural augmentation in the 7th national audit project (NAP7) in the UK [21▪]. Nevertheless, the authors advocate to use of strategies to reduce the risk of high block when using a second neuraxial technique [21▪]. A reasonable approach in case of partial failure is to use CSE with a reduced intrathecal dose or to switch to a pure epidural technique.
INTRAOPERATIVE FAILURE
Definition and perception of intraoperative pain
Conversion of regional anesthesia to GA or use of intravenous opioids has been used as a definition of intraoperative pain, but as already mentioned these are inadequate measures of intraoperative pain [1▪▪]. Despite evidence of intraoperative pain, 33% of anesthesiologists did not accept failure of the block and did not act accordingly [8]. A recent prospective observational study found intraoperative pain to be reported by the patient in 11.9% (95% CI 7.9–17.5) of the patients undergoing CD under SA [22▪▪]. This number is of even higher significance, as women with a sensory block below T4 to pinprick and T2 to cold were excluded, as were prolonged surgical procedures lasting more than 90 min. Of note, the presence or absence of intraoperative pain was reported by the patients using a questionnaire administered in post anesthesia care unit. One third of patients reported pain before delivery, two thirds after delivery. Most importantly, obstetricians and anesthesiologists were unable to accurately identify intraoperative pain of parturients. The false negative rate of pain perception was 82.6% and 52.2% for obstetricians and anesthesiologists, respectively [22▪▪].
Management of intraoperative pain
Management of intraoperative pain is crucial, as patients can experience psychological trauma not only by the pain itself, but as well as by how it is managed [1▪▪]. There are different options to react to insufficient intraoperative SA, and these depend on the urgency of the situation and on the stage of the cesarean delivery. The surgeon should be asked to stop the surgery [7▪▪]. Except for the moment between uterine incision and delivery, this should always be possible. In the statements of the Committee on Obstetric Anesthesia of the American Society of Anesthesiologists several systemic and inhaled medications are proposed for supplementation of inadequate regional blockade during CD [23] Unless contra-indicated, first line treatment should consist of repeated intravenous boluses of fast acting opioids, such as remifentanil (20 μg), alfentanil (250–500 μg) or fentanyl (25–50 μg) since they alleviate intraoperative pain. Some do also recommend small doses of ketamine (10 mg), alpha-2 adrenergic agonists or nitrous oxide. Pure hypnotic agents such as midazolam and propofol should not be used without an analgesic. The time of intraoperative pain is important. In the case of early intraoperative pain, i.e. before delivery, intravenous opioids are unlikely to be effective. In this situation, the patient should be offered GA. If pain is perceived towards the end of surgery – such as due to prolonged surgical time, then wound infiltration with local aesthetics by the surgeon can be an effective method. Intraabdominal dissemination of 2-Chloroprocaine 3% has been reported to reduce intraoperative pain after delivery [24]. Experience with this method is, however, limited to one published case series of 32 patients. Most importantly, believe the patient if she says she is in pain. There should be a low threshold to offer GA. Recommendations for the management of intraoperative pain are summarized in Table 3.
Table 3.
Management of intraoperative pain
| If the patient says she is in pain, believe her |
| Ask the surgeon to stop the surgery |
| If pain occurs before delivery, general anesthesia should be offered |
| Repeat boluses of fast acting opioids might relieve the pain |
| Hypnotic agents should be used cautiously and in conjunction with fast acting opioids |
| Offer general anesthesia |
| Surgical wound infiltration if pain is towards end of surgery |
| Document patient perceptions and all measures taken in the patient chart |
Documentation
Meticulous documentation is essential. This includes assessment of the block with all tested modalities. Intraoperative pain and all measures taken to improve the situation should also be documented, as well as the patient's response. If the patient is offered general anesthesia, this should be visible from the notes.
Follow-up of patients who experienced intraoperative pain is important [7▪▪]. Postoperative visits prior to hospital discharge should be systematic, documented and repeated if necessary. Sometimes questions arise only weeks or months following a traumatic event. We do offer interdisciplinary meetings with the patient and her partner to discuss possible reasons, room for improvement and anticipate care during a potential future pregnancy and delivery.
Informed consent
Given the knowledge on failing spinal anesthesia for CD summarized in this article, it would seem obvious that the process of informed consent includes information on expected and unexpected sensations during CD under regional anesthesia [7▪▪]. This information should include the frequency of failure of the technique and the different options to improve the situation, including the possibility of conversion to GA. Following the OAA guideline, general anesthesia should not only be discussed as a rescue technique, but as the primary alternative to neuraxial anesthesia [7▪▪].
Only 23% of survey respondents had formal training of intraoperative pain under neuraxial anesthesia and only 30% had written guidelines for follow up of such patients [25▪].
CONCLUSION
Pre- or intraoperative failure of SA for CD occurs in up to 12%. Meticulous testing of neuraxial block allows for identification of failure before the start of surgery. Knowledge of risk factors for failed spinal allow for selection and adjustment of the anesthetic technique, such as adequate intrathecal dosing or use of CSE. In the case of repeat neuraxial technique, a strategy to reduce the risk of high block should be chosen. Management of intraoperative pain starts by trusting the patient when she expresses pain and appropriate measures will depend on the progress of surgery. The process of preoperative informed consent should include information on expected sensations during CD, frequency of failure and options for treatment.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1▪▪.Stanford SER. What is ‘genuine’ failure of neuraxial anaesthesia? Anaesthesia 2022; 77:523–526. [DOI] [PubMed] [Google Scholar]; The view of the patient is invaluable in the discussion on intraoperative pain. The author arguments against conversion rate to general anesthesia being a useful measure of intraoperative pain.
- 2▪▪.Jin SY, Munro A, Aidemouni M, et al. The incidence and predictors of failed spinal anesthesia after intrathecal injection of local anesthetic for cesarean delivery: a single-center, 9-year retrospective review. Anesth Analg 2024; 138:430–437. [DOI] [PubMed] [Google Scholar]; This large retrospective study identified the most important predictors of spinal anesthesia failure in a multivariable analysis. A dominance analysis ranks the predictors according to their importance.
- 3.Adesope OA, Einhorn LM, Olufolabi AJ, et al. The impact of gestational age and fetal weight on the risk of failure of spinal anesthesia for cesarean delivery. Int J Obstet Anesth 2016; 26:8–14. [DOI] [PubMed] [Google Scholar]
- 4▪.Patel R, Kua J, Sharawi N, et al. Inadequate neuraxial anaesthesia in patients undergoing elective caesarean section: a systematic review. Anaesthesia 2022; 77:598–604. [DOI] [PubMed] [Google Scholar]; This systematic review was focused on elective surgery only.
- 5.Purva M, Kinsella S. Chereshneva M, Johnston C, Colvin J, Peden C. Caesarean section anaesthesia: technique and failure rate. Royal College of Anaesthetists, Raising the standard: RCoA quality improvement compendium. London:2020. [Google Scholar]
- 6.Stanford SE, Bogod DG. Failure of communication: a patient's story. Int J Obstet Anesth 2016; 28:70–75. [DOI] [PubMed] [Google Scholar]
- 7▪▪.Plaat F, Stanford SER, Lucas DN, et al. Prevention and management of intra-operative pain during caesarean section under neuraxial anaesthesia: a technical and interpersonal approach. Anaesthesia 2022; 77:588–597. [DOI] [PMC free article] [PubMed] [Google Scholar]; These recommendations on prevention and management of failed spinal are exceptionally practical.
- 8.McCombe K, Bogod DG. Learning from the law. A review of 21 years of litigation for pain during caesarean section. Anaesthesia 2018; 73:223–230. [DOI] [PubMed] [Google Scholar]
- 9.Fettes PD, Jansson JR, Wildsmith JA. Failed spinal anaesthesia: mechanisms, management, and prevention. Br J Anaesth 2009; 102:739–748. [DOI] [PubMed] [Google Scholar]
- 10.Parikh KS, Seetharamaiah S. Approach to failed spinal anaesthesia for caesarean section. Indian J Anaesth 2018; 62:691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steiner LA, Hauenstein L, Ruppen W, et al. Bupivacaine concentrations in lumbar cerebrospinal fluid in patients with failed spinal anaesthesia. Br J Anaesth 2009; 102:839–844. [DOI] [PubMed] [Google Scholar]
- 12.Hoppe J, Popham P. Complete failure of spinal anaesthesia in obstetrics. Int J Obstet Anesth 2007; 16:250–255. [DOI] [PubMed] [Google Scholar]
- 13▪▪.Stav M, Matatov Y, Hoffmann D, et al. Incidence of conversion to general anaesthesia and need for intravenous supplementation in parturients undergoing caesarean section under spinal anaesthesia: a retrospective observational study. Acta Anaesthesiol Scand 2023; 67:29–35. [DOI] [PubMed] [Google Scholar]; A large retrospective analysis of factors contributing to spinal failure with focus on need for general anesthesia or analgesic supplementation.
- 14.James KS, McGrady E, Patrick A. Combined spinal-extradural anaesthesia for preterm and term caesarean section: is there a difference in local anaesthetic requirements? Br J Anaesth 1997; 78:498–501. [DOI] [PubMed] [Google Scholar]
- 15.Chin A, Crooke B, Heywood L, et al. A randomised controlled trial comparing needle movements during combined spinal-epidural anaesthesia with and without ultrasound assistance. Anaesthesia 2018; 73:466–473. [DOI] [PubMed] [Google Scholar]
- 16.Reynolds F. Damage to the conus medullaris following spinal anaesthesia. Anaesthesia 2001; 56:238–247. [DOI] [PubMed] [Google Scholar]
- 17.Xiao F, Drzymalski D, Liu L, et al. Comparison of the ED50 and ED95 of intrathecal bupivacaine in parturients undergoing cesarean delivery with or without prophylactic phenylephrine infusion: a prospective, double-blind study. Reg Anesth Pain Med 2018; 43:885–889. [DOI] [PubMed] [Google Scholar]
- 18.Manouchehrian N, Moradi A, Torkashvand L. Comparative study of effect of spinal anesthesia in sitting and lateral positions on the onset time of sensory block and hemodynamic condition in cesarean section: a randomized clinical trial. Anesth Pain Med 2021; 11:e111483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Punchuklang W, Nivatpumin P, Jintadawong T. Total failure of spinal anesthesia for cesarean delivery, associated factors, and outcomes: a retrospective case–control study. Medicine 2022; 101:e29813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhar D, RoyBasunia S, Das A, et al. Repeat spinal anesthesia in cesarean section: a comparison between 10 mg and 12 mg doses of intrathecal hyperbaric (0.05%) bupivacaine repeated after failed spinal anesthesia: a prospective, parallel group study. Anesth Essays Res 2016; 10:362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21▪.Lucas DN, Kursumovic E, Cook TM, et al. Cardiac arrest in obstetric patients receiving anaesthetic care: results from the 7th National Audit Project of the Royal College of Anaesthetists. Anaesthesia 2024; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]; A summary of perioperative cardiac arrest in obstetric anaesthesia as identified during the national audit program (NAP7).
- 22▪▪.Keltz A, Heesen P, Katz D, et al. Intraoperative pain during caesarean delivery: Incidence, risk factors and physician perception. Eur J Pain 2022; 26:219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]; At last a study asking the parturients about intraoperative pain. They also compared experienced intraoperative pain with assessment by obstetricians and anesthesiologists.
- 23. American Society of Anesthesiologists. Statement on pain during cesarean delivery. Available at: https://www.asahq.org/standards-and-practice-parameters/statement-on-pain-during-cesarean-delivery [Accessed: 19 January 2024]. [Google Scholar]
- 24.Werntz M, Burwick R, Togioka B. Intraperitoneal chloroprocaine is a useful adjunct to neuraxial block during cesarean delivery: a case series. Int J Obstet Anesth 2018; 35:33–41. [DOI] [PubMed] [Google Scholar]
- 25▪.Patel R, Russell R, Plaat F, et al. Inadequate neuraxial anaesthesia during caesarean delivery: a survey of practitioners. Int J Obstet Anesth 2023; 56:103905. [DOI] [PubMed] [Google Scholar]; This survey highlights common practice in the United Kingdom in case of failed spinal for cesarean delivery.