Summary
The site specific recombination pathway of bacteriophage λ encompasses iso-energetic but highly directional and tightly regulated integrative and excisive reactions that integrate and excise the vial chromosome into and out of the bacterial chromosome. The reactions require 240 bp of phage DNA and 21 bp of bacterial DNA comprising 16 protein binding sites that are differentially used in each pathway by the phage-encoded Int and Xis proteins and the host-encoded IHF and FIS proteins. Structures of higher-order protein-DNA complexes of the four-way Holliday junction recombination intermediates provided clarifying insights into the mechanisms, directionality, and regulation of these two pathways, which are tightly linked to the physiology of the bacterial host cell. Here we review our current understanding of the mechanisms responsible for regulating and executing λ site-specific recombination, with an emphasis on key studies completed over the last decade.
Keywords: bacteriophage lambda, integration, site-specific recombination, viral integration, viral excision, DNA-bending, Holliday junction, tyrosine recombinase
Graphical Abstract
Bacteriophage λ uses site-specific recombination to integrate and excise the vial chromosome into and out of the bacterial chromosome. Structures of higher-order protein-DNA complexes of the four-way Holliday junction recombination intermediates provided clarifying insights into the mechanisms, directionality, and regulation of these two pathways.

1. Introduction
The Lambda (λ) virus is one of the first well-characterized examples of viruses with alternative lifestyles. In addition to the infective, or lytic, lifestyle -- during which the injected viral genome is replicated and decoded into those proteins necessary to manufacture more virus that will be released from the cell (with or without killing the host cell) -- these viruses also have a dormant or latent lifestyle. During the latter, which is also called lysogeny when it occurs in bacterial cells, the viral genome is largely shut down and remains quiescent within the host cell for many generations until some changes in the external environment or the physiology of the host cell reawakens the viral genome to transition into a productive infectious cycle (Figure 1). Nature has evolved several different strategies for how the quiescent lifestyle is maintained, including insertion of the viral genome into the chromosome of the cellular host where it is replicated at each cell division. The locus of viral insertion can be rather promiscuous, as in the case of Herpes and Adenoviruses and many tumor viruses, such as HIV and Epstein Barr virus, or it can be very precise as in the paradigmatic case of the bacteriophage λ virus. The λ paradigm also showcases a temporal specificity tightly linking recombination to the physiology of the bacterial host, a strategy that seems to have been highly conserved among lysogenic viruses utilizing a tyrosine recombinase (Smyshlyaev, Bateman and Barabas, 2021). The most recent review of the λ site-specific recombination pathway (Landy, 2015) appears in a comprehensive compendium of reviews on the larger topic of “Mobile DNA” (Craig, N. L. et al., 2015).
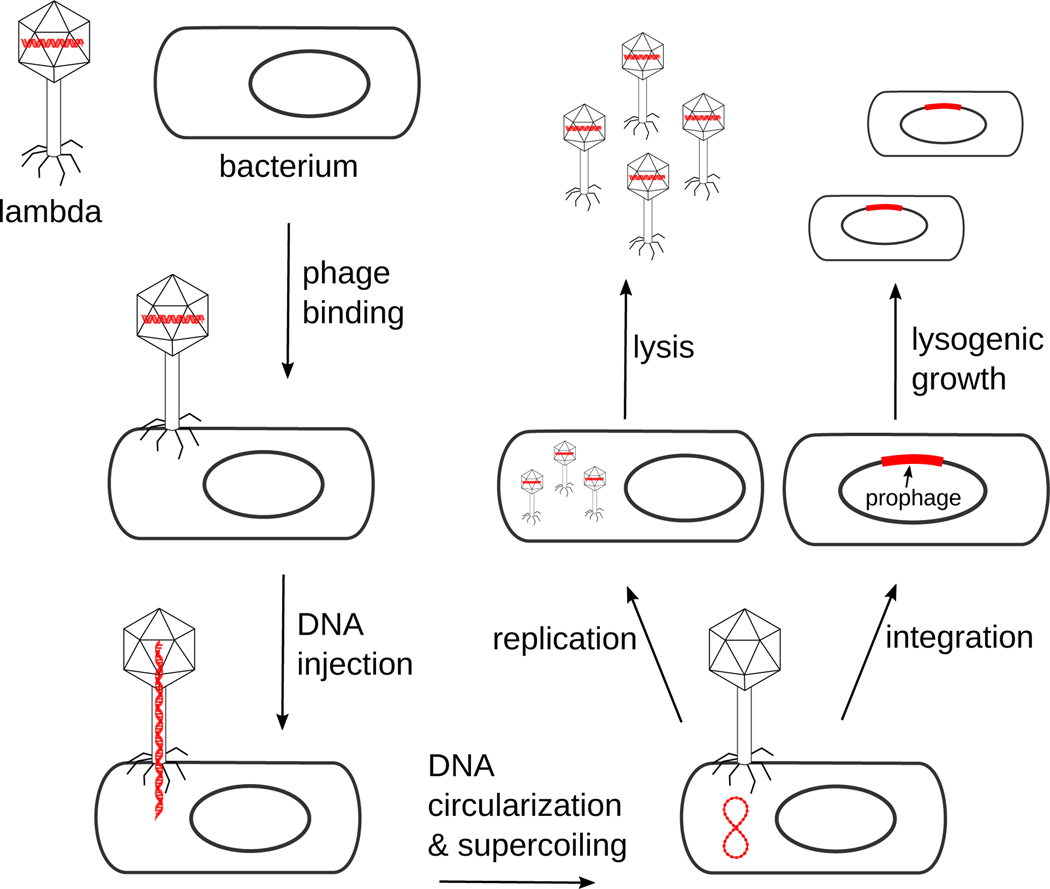
Figure 1.
Life styles of bacteriophage lambda. During infection, the phage particle binds to a bacterial host cell and injects its linear DNA genome into the cell. Host factors circularize and supercoil the lambda DNA. Depending upon regulatory signals from the phage and the bacterial cell the phage proceeds down the lytic or lysogenic path. In the former the phage DNA is transcribed, replicated, and packaged into new phage particles that are released from the lysed cell. In the lysogenic pathway the phage DNA is integrated into the host chromosome at a specific site using the phage-encoded integrase (Int) enzyme and the host integration host factor (IHF) protein. Lambda can then undergo lysogenic growth, where almost all of the viral genes are repressed and the prophage is replicated along with the host chromosome. During lysogenic growth signals from the environment and/or the host cause induction of the prophage and excision from the host chromosome (not shown in this figure). The excision reaction is catalyzed by Int, IHF, and the phage-encoded Xis protein. Transcription of lambda genes to produce new phage particles and to lyse the cell represent the lytic phase of growth. The phage DNA is drawn in red in this figure.
In addition to their important roles in biology, the site-specific recombination reactions from phage λ and related systems have been exploited for over thirty years in diverse applications involving in vivo and in vitro manipulations of DNA (Hirano et al., 2011; Wang et al., 2011; Fogg et al., 2014; Nafissi and Slavcev, 2014; Olorunniji, Rosser and Stark, 2016). The highly specific and directional integration and excision reactions of phage λ, for example, have been instrumental in generating genome-scale libraries that allow fascile in vitro manipulation of the cloned inserts (Hartley, Temple and Brasch, 2000). These “Gateway®” reactions (Katzen, 2007) have become ubiquitous in molecular biology, with the “BP” (integrative) reaction used to establish clones and the “LR” (excisive) reaction used to move inserts between vectors. Until recently, the mechanistic and structural bases for these efficient, but complex DNA rearrangements remained poorly understood. Here we review the history and most recent high resolution structures and models for the site-specific recombination pathway of bacteriophage λ and summarize our current understanding of the mechanisms of integrative and excisive site-specific recombination.
2. The Campbell Model
The first outlines of the λ paradigm emerged from Allan Campbell’s insightful proposal in 1962 that following viral infection the λ chromosome was circularized and could be integrated into the Escherichia coli host chromosome by means of a site-specific recombination between specific DNA loci or attachment (att) sites on the viral and bacterial chromosomes (Campbell, 1962). Based upon genetic analyses of these loci they were (conceptually) designated attP, or POP’, and attB, or BOB’, on the viral and bacterial chromosomes respectively. The resulting integrated “prophage” is flanked by junctions between the viral and host DNA sequences called attL or BOP’ and attR or POB’. During excision of the viral chromosome the attL and attR sites recombine with each other to regenerate attP and attB in preparation for a cycle of lytic growth (see Figure 2). Fifteen years later, the Campbell model was confirmed biochemically when the DNA sequences for attP, attL, and attR were determined (Landy and Ross, 1977).
Figure 2.
Site-specific integration of λ DNA into and excision out of the bacterial chromosome. Integration occurs between the phage attP site (C’-O-C) and the bacterial attB site (B-O-B’). The hybrid sites C’-O-B and C-O-B’ that are generated are called attL and attR, respectively. During excision, recombination between attL and attR sites results in free phage DNA containing attP and the unaltered bacterial chromosome. Additional binding sites for Int, IHF, and Xis located on the attP “arms” that flank C and C’ are not shown here. The full attP site is 240 bp in length, vs. the minimal 21 bp core attB site.
2.1. Secondary att Sites and Transducing Phage
In a cell with a deletion of the attB target site the λ chromosome can integrate with greatly reduced efficiency at other “secondary” att sites on the E. coli chromosome. A hierarchy of secondary att sites, each with its own characteristic efficiency, reflects the extent of deviation from the canonical features of attB, which are described further below. Powerful genetic selections enable the identification of extremely poor mimics whose recombination efficiencies can be reduced as much as nine orders of magnitude relative to attB. Aberrant excision from these sites, or from the attB locus, which is located between the gal and bio genes, leads to the creation of “specialized transducing phage” that have incorporated E.coli genes adjacent to the locus of λ insertion. The aberrant excision occurs via a rare illegitimate recombination, involving sequences with very little homology, between a site on the bacterial chromosome and a site in a nonessential region of the viral chromosome. It results in the formation of a viral chromosome lacking the pseudo prophage att site and some nonessential virus sequences linked to an adjacent segment of bacterial DNA, as illustrated in Figure 3 (Kumagai and Ikeda, 1991; Weisberg, R.A., 1996).
Figure 3.
Generation of transducing phage λ. Inefficient and rare integrations at a degenerate mimic of the canonical attB site (called a secondary att sites; attA in this figure) positions the λ prophage adjacent to E. coli genes X and Y. A rare illegitimate recombination, involving sequences with very little homology, between a site on the bacterial chromosome and a site in a nonessential region of the viral chromosome results in the formation of a viral chromosome linked to an adjacent segment of bacterial DNA, in this case gene X. This new transuding phage lacks the A’OC site and some adjacent nonessential viral sequences. The resulting phage can be used to transfer gene X into new host cells.
3. The Proteins
Following from the Campbell model, the ensuing years of genetic, biochemical, kinetic, and structural studies were designed around the goal of understanding how the λ integrase (Int) protein carries out a recombination reaction that is highly directional and tightly regulated with an ensemble of three accessory DNA bending proteins acting on 240 bp of DNA encoding 16 protein binding sites (see Figure 4). This complexity, and its tight coupling at the mechanistic level with the physiology of the host cell, distinguishes λ recombination (and other regulated pathways) from many other bacteriophage site-specific pathways, such as the well-studied Cre-lox pathway of bacteriophage P1.
Figure 4.
The protein binding sites that comprise complete att DNA sites. Integrative recombination between supercoiled attP and linear attB requires Int and the host-encoded IHF and gives rise to an integrated phage chromosome bounded by attL and attR. Excisive recombination between attL and attR to regenerate attP and attB additionally requires the phage-encoded Xis protein (which inhibits integrative recombination) and is stimulated by the host-encoded Fis protein. Both reactions proceed through the stepwise cleavage and strand exchange pathway shown in Figure 8. Integration and excision use different subsets of protein binding sites in the P and P′ arms, as indicated by the filled boxes: Int arm-type P1, P2, P′1, P′2, and P′3 (light green); IHF, H1, H2, and H′ (yellow); Xis, X1, X1.5, and X2 (gold); and Fis (pink). The four core-type Int binding sites, C, C′, B, and B′ (dark green boxes) are each bound by the core-binding and catalytic domains. The integration reaction requires four Int proteins bridging between core and arm binding sites, as shown in Figures 9 & 10. The excision reaction requires four Int proteins binding to core sites, but only three bridges to arm sites are required.
Genetic analyses also established that in the λ pathway both the integration and excision reactions required the virally encoded Integrase protein (Int) while the excision reaction additionally required the virally encoded excision protein (Xis) (Gingery and Echols, 1967; Gottesman and Yarmolinsky, 1968; Enquist and Weisberg, 1976). They further established that a host-encoded protein IHF (Integration Host Factor) was required for both reactions (Miller and Friedman, 1980). An additional host-encoded protein, FIS (Factor for Inversion Stimulation) stimulates the excision reaction under conditions when Xis is limiting but is not required when the concentration of Xis is sufficiently high (Thompson et al., 1987).
3.1. Int Protein
The founding breakthrough for in vitro studies of λ recombination was Howard Nash’s purification of the virally-encoded integrase protein (Int) (Nash, 1974). Int is generally regarded as the founding member of the tyrosine recombinase family, even though many family members are not strictly recombinases and carry out such diverse functions as chromosome segregation, chromosome copy number control, gene expression, conjugative transposition, gene dissemination and viral integration and excision. Family membership is defined by an active site tyrosine, which cleaves and reseals DNA through the formation of a covalent 3’-phospho-tyrosine high-energy intermediate without the requirement for any high energy cofactors (Nash, 1981; Craig and Nash, 1983; Nunes-Düby, Matsumoto and Landy, 1987; Kitts and Nash, 1988a)and several conserved amino acids comprising a common active site core (Nunes-Düby et al., 1998). All of the family members use this same chemistry to carry out DNA rearrangements via the formation and resolution of a four-way DNA junction, or Holliday junction (HJ) intermediate, as described further below (Nunes-Düby, Matsumoto and Landy, 1987). Although these four-way junctions are common intermediates in many different pathways responsible for rearranging DNA in evolution, heredity, and gene expression, they have been particularly well-studied in the tyrosine recombinase family (Craig, N. L. et al., 2015).
Biochemical and crystallographic studies have shown that the 356 amino acid λ Int consists of three domains, all of which make contact with DNA: a small amino-terminal domain (NTD) that binds with high affinity to a family of DNA sequences called “arm-type” binding sites (Sarkar et al., 2002; Wojciak et al., 2002; Fadeev, Sam and Clubb, 2009); a central domain that binds with low affinity to a family of DNA sequences called “core-type” sites, and a C-terminal catalytic domain that contains the active site tyrosine nucleophile along with several other highly conserved residues comprising the catalytic pocket which defines the tyrosine recombinase family (Figure 5). The central and catalytic domains form a C-clamp-like structure around the core-type binding sites where DNA cleavage, strand exchange, and ligation reactions are carried out (Aihara et al., 2003; Biswas et al., 2005). In addition to serving as the initiator of DNA binding the NTD also functions to inhibit DNA binding by the CTD -- and this inhibition is relieved when the NTD binds DNA. Thus, Int is a hetero-bivalent topoisomerase that is also self-inhibited in the absence of an appropriate NTD target (Sarkar, Radman-Livaja and Landy, 2001; Warren, Laxmikanthan and Landy, 2008).
Figure 5.
Structure of heterobivalent Int bound to arm- and core-type DNA sites. The conserved catalytic tyrosine responsible for cleavage of the core sites is shown in red and is poised to cleave at the edges of the overlap region of the core site. Based on pdb entry 1Z1G (Biswas et al., 2005). The N-terminal arm-binding domain of Int binds to one of the five arm-type DNA sites located outside of the core DNA region while the C-terminal core-binding and catalytic domains form a clamp around each core half-site sequence. The Int protein thus forms intra- and inter-molecular bridges between the core- and arm-type sites facilitated by the accessory DNA bending proteins (as shown in Figures 8, 9, & 10).
3.2. Integration Host Factor
All of the accessory proteins required for the λ recombination reactions are small, site-specific, DNA bending proteins. The first to be purified and characterized was the 21-kDa host-encoded Integration Host Factor (IHF) (Craig and Nash, 1984) which participates in many diverse cellular reactions such as regulation of gene transcription, initiation of DNA replication, transposition, and phage packaging; for reviews see (Rice, 1997; Swinger and Rice, 2004; Johnson, 2005), but it was first discovered and characterized by virtue of its required role in λ recombination (Miller et al., 1979; Miller, Kirk and Echols, 1981). IHF consists of two subunits that share approximately 30% identity to each other and also to the family of type II DNA-binding proteins that includes major histone-like proteins of E.coli such as HU. Each of the intertwined subunits contributes a long β ribbon arm that curls around the DNA and interacts exclusively with a minor groove to induce a DNA bend of more than 160⁰ primarily at two large kinks 9 bp apart (Rice et al., 1996) (see Figure 6). IHF binding sites share little overall sequence identity, with most of the conservation observed at 10 positions in the right arm. The left arms are often A/T-rich and contain A-tract sequences (Goodrich, Schwartz and McClure, 1990)
Figure 6.
Structure of integration host factor (IHF) bound to H’ DNA site. IHF is a tightly associated heterodimer composed of α and β subunits. β-hairpins from each subunit create sharp kinks in the DNA separated by 9-bp. The result is an overall bend in the DNA of ~160°. The H’ sequence (one of the three IHF binding sites, as shown in Figure 8) provides both specific IHF interactions and non-specific conformational flexibility in the DNA. Modified from pdb entry 1IHF (Rice et al., 1996).
3.3. Xis
The virally-encoded 72 amino acid Xis protein is also a DNA bending protein and functions in the excision reaction by binding and bending DNA at three adjacent sequences in the att site P arm (see Figure 7A) (Sam et al., 2002). In addition to stimulating excisive recombination in vivo more than 106 –fold it is also inhibitory for the integrative reaction (Nash, 1975; Abremski and Gottesman, 1982). As a result of flexible recognition surfaces Xis is a relatively promiscuous binder of DNA which means that it is easily distracted from its intended att site target in vivo, where there is a huge excess of nonspecific DNA, thus explaining the large differences observed between excisive recombination in vivo and in vitro with respect to the FIS stimulation described below.
Figure 7.
Structures of Xis and Fis bound to DNA. A) Three Xis molecules bind cooperatively to successive sites (named X1, X1.5, and X2) to form a mini-filament on DNA. Xis binding results in a smooth bend in the DNA. Based on pdb entry 2IEF (Abbani et al., 2007). B) Fis binds as a homodimer to its DNA binding site, resulting in a bend similar in magnitude to that seen with Xis. Based on pdb entry 3IV5 (Stella, Cascio and Johnson, 2010). In the λ P-arm, the Fis and Xis binding sites are adjacent, allowing for synergy between Fis binding and recruitment of the first Xis subunit at X2 (see Fig 8).
3.4. FIS
The host-encoded FIS protein is the only one of the four proteins that was known and studied prior to the discovery of its role in the λ recombination reaction (Kostrewa et al., 1991; Yuan et al., 1991). This 196 amino acid protein was initially identified as a factor in promoting site-specific recombination by DNA invertases (Johnson, Bruist and Simon, 1986; Koch and Kahmann, 1986). It is a nucleoid associated protein of global, structural, and regulatory importance whose role in determining overall chromosome structure figures significantly in regulating genes encoding topoisomerases, in the initiation of DNA replication, in the initiation of mutagenic double strand break repair as part of the SOS response, in several DNA transposition reactions, and in the regulation of transcription at many different genes by several different mechanisms (Finkel and Johnson, 1992; Schneider, Ross and Gourse, 2003; Johnson, 2005; Browning, Grainger and Busby, 2010; Moore et al., 2015). This large number of critical sites of action is undoubtedly related to the dramatic fluctuation of intracellular FIS levels as a function of cellular physiology (Thompson et al., 1987; Ball et al., 1992; Ninnemann, Koch and Kahmann, 1992; Mallik et al., 2006).
The role of FIS protein in λ site-specific recombination was first discovered in in vitro reactions where it was found that when Xis is limiting, FIS stimulates excisive recombination up to 20-fold and it binds cooperatively with Xis in the P arm of attR (Thompson et al., 1987). However, FIS stimulates excision in vivo by 100 to 1,000-fold, suggesting that, at least under some conditions, the level of available Xis is limiting, possibly due to the distraction of the promiscuous Xis by the large number of potential DNA binding sites, which of course are not present in the purified in vitro reactions (Ball and Johnson, 1991).
Based upon their biochemical and crystallographic studies the Johnson lab proposed that FIS initially searches for DNA with an intrinsically narrow minor groove, where AT composition and not sequence is the critical determinant (Stella, Cascio and Johnson, 2010)(see Figure 7B). While intrinsic DNA bends are not very important for FIS binding, the bends it induces in cooperation with its binding partners are crucial for its many functions and are likely facilitated by mutually compatible changes in DNA shape. They proposed that in vivo binding of FIS to its target site on the P arm attracts the otherwise peripatetic Xis to bind to its overlapping (X2) site, which then recruits Xis to the adjacent two Xis binding sites (X1.5 and X1) and they built a model in which their observed protein induced DNA distortions are proposed to favor cooperative binding of FIS and Xis (Papagiannis et al., 2007).
4. Attachment Site DNA (att)
Determination of the 240 bp DNA sequence necessary to comprise the minimum phage att site (POP’) was a surprising harbinger of the reaction complexity (Landy and Ross, 1977). Indeed, it is this complexity that confers both directionality and regulation upon the λ recombination reaction and distinguishes it from its Cre and Flp tyrosine recombinase family siblings. However, it shares with these siblings and most family members a common organization of DNA sequences and Int binding sites in the region where the DNAs are cut, rearranged, and resealed (see articles in (Craig, N. L. et al., 2015) for details).
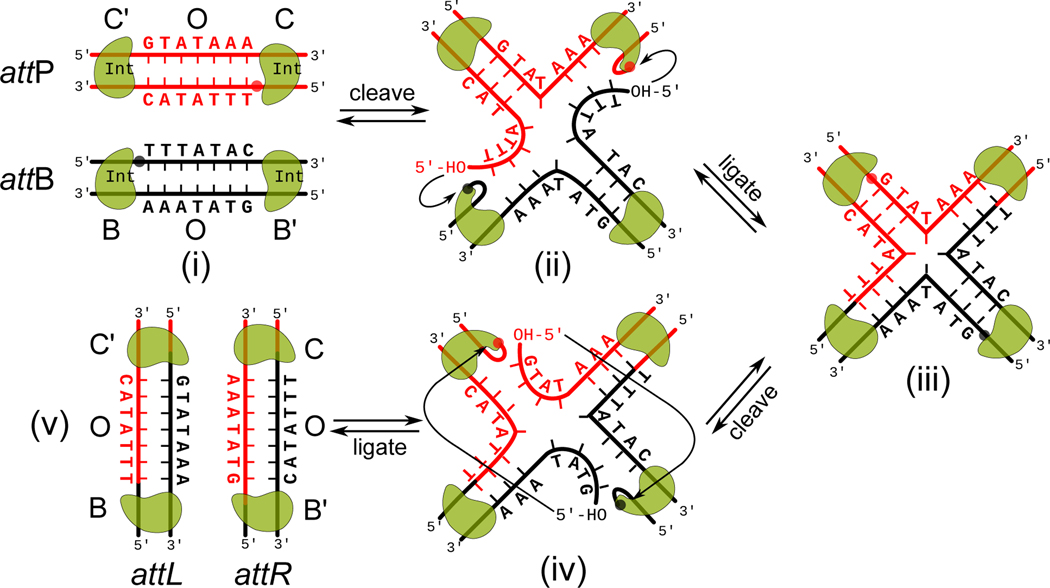
Each λ att site recombining partner DNA contains a seven bp “overlap” region (O) (6 to 8 bp in other systems), so called because it is identical in both recombining partners and in both of the recombination products. The overlap regions, which are defined by staggered nicks made by integrase during strand exchange are (chemically) heteroduplex in recombination products, with one strand from each recombination partner. The overlap regions are flanked by a pair of inverted repeat recombinase binding sites called core-type sites (nine bp each). Following the synapsis of two recombining partners recombination is initiated by Int cleavage of the “top” strands which are then swapped between partners to form a four-way DNA junction, or Holliday junction (HJ) (Figure 8 (i–iv) (Hsu and Landy, 1984; Nunes-Düby, Matsumoto and Landy, 1987; Kitts and Nash, 1988a, 1988b). Because the strand exchange involves the “swapping” of three/four base pairs only a slight rearrangement of the HJ-recombinase-tetramer is required to execute the second three/four base pair swap, following cleavage and prior to religation of the bottom strands, thus resolving the HJ to recombinant products (Nunes-Duby, Azaro and Landy, 1995). These features of HJ formation and resolution suggested a mechanism-based method for trapping HJ complexes that would prove critical in the structural studies described later. This was accomplished by recombining two att sites whose seven bp overlap sequences each has an unpaired 2 bp heteroduplex bubble adjacent to the first strand cleavage sites such that the first strand swap creates a fully base paired HJ. Adjacent to the second strand cleavage site the two overlap regions are homoduplex but of different sequences, such that strand swapping would create a heteroduplex bubble. The resulting fully base paired HJ is “trapped” because because resolution in either direction creates energetically unfavored heteroduplex products (going back would recreate the original unpaired bubbles and going forward would create two new unpaired bubbles) (Sun, 1990; Seah et al., 2014; Tong et al., 2014). A striking and significant feature of the λ Int reaction is that HJ formation is always initiated by cleavage and exchange of the same (top) strands in both integrative and excisive recombination, as expected for two distinct reactions that are not the reverse of one another.
Figure 8.
Biochemistry of recombination. Isoenergetic cleaving, recombining, and resealing DNA during recombination proceeds by two pairs of sequential single-strand DNA exchanges shown here as steps i->iii and steps iii->v. The phosphodiester linkages to be cleaved (indicated by solid circles) are staggered by seven base pairs that are identical in the substrate and product sites; they are referred to as the ‘overlap’ region (O). Viral and bacterial DNAs are colored as in Figures 1–3. (i) The attP (C’OC) and attB (BOB’) sites are aligned anti-parallel with respect to their identical overlap sequences. (ii) The Int subunits bound to the C and B core sites cleave the DNA to form covalent 3’-phosphotyrosine linkages, exposing free 5’-hydroxyl groups. The liberated DNA strands can melt away from their complementary strands and migrate to the identical complementary strand of the partner substrate. (iii) The 5’-OH groups attack the phosphotyrosine linkages at C’ and B to generate new DNA strands and form the four-way DNA (HJ) intermediate. (iv) A similar pair of single strand cleavage and exchange steps is executed by the Int subunits at C’ and B’ on the other sides of the overlap regions. (v) After resealing the nicked DNA strands following strand exchange, the HJ is resolved to recombinant products, attL (C’OB) and attR (COB’), and all four DNA strands have new junction sequences. In both integrative and excisive recombination λ Int always initiates cleavage at the C and B sites, which means that excision is not simply the reverse of integration, i.e., they are two distinct reactions.
All of the evidence suggests there are only small differences between the pathways of λ Int, Cre, and Flp at the level of HJ formation, structure, and resolution, and relatively minor differences in their respective protein chemistries of DNA cleavage and ligation. However, what does distinguish the λ Int reaction from the others are the DNA sequences and protein binding that lay outside of the core region.
In the λ system the nine bp “core-type” sites correspond directly to the bacterial DNA sequences originally designated as B and B’ sites, but in the much larger phage DNA sequences they are indicated as C and C’ to distinguish them from the other class of Int binding sites in the P and P’ arms that encode distant binding sites for the second, N-terminal DNA binding domain (NTD) of Int. Therefore the full phage DNA sequence is denoted as PCOC’P’, which means that the junction sites attL and attR have the arrangement BOC’P’ and PCOB’, respectively (see Figure. 4).
The initially puzzling observation that Int binds with higher affinity via its NTD to the distant arm-type sites than it does to the core-type sites, where the enzymology takes place via its core-catalytic CTD domain, was resolved by the finding that binding sites for the accessory DNA bending factors are positioned between the two classes of Int binding sites, as shown schematically in Figure 9. DNA binding and bending by the appropriate accessory protein(s) at their respective sites is thus necessary to “deliver” Int bound at the distal arm-type sites to the core region where the reactions are executed (Moitoso et al., 1989).
Figure 9.
Model for how DNA-bending proteins facilitate an Int bridge. A protein such as IHF can bring arm-binding and core-binding sites into proximity so that a molecule of Int can interact with both sites using its N-terminal arm-binding domain and its C-terminal core-binding and catalytic domains.
It should be noted that λ Int is also capable of carrying out an IHF-dependent reaction between two identical attL sites lacking a P’1 arm-type site (Segall and Nash, 1996). The existence of such pathways with unusual features and lacking the usual components raises interesting questions about the variety of recombinogenic complexes λ Int is capable of forming (Segall and Nash, 1993; Segall, 1998) and underscores the caveat of off-pathway reactions in the study of complex multi-component systems.
It is this requirement for the accessory DNA bending proteins that makes the directionality of λ recombination dependent on the expression of the phage-encoded Xis protein and additionally dependent upon the intracellular levels of IHF and FIS protein, both of which fluctuate dramatically with changes in the host physiology.
5. Influence of host physiology
The intracellular concentration of IHF increases approximately five- to seven-fold over a six hour span after entry into stationary phase and decreases when stationary phase cells are diluted into fresh medium and cell mass begins to double (Bushman et al., 1985; Ditto, Roberts and Weisberg, 1994). It is notable that although IHF is required for λ recombination, at high concentrations it tends to inhibit excisive recombination in vitro, the mechanism for which is suggested by structural models described below (Thompson et al., 1987). Analogously, but in the opposite direction, the intracellular levels of FIS drop dramatically when cells enter the stationary phase, as does occupation of the FIS binding site on att site DNA. The FIS levels increase 500-fold when stationary phase cells are diluted into fresh medium and reach a peak of 50,000 to 100,000 copies per cell as the culture enters exponential phase (Thompson et al., 1987; Ball et al., 1992; Ninnemann, Koch and Kahmann, 1992).
The effect of the changing intracellular levels of the host-encoded accessory proteins can be summarized as follows: integrative recombination is favored by higher concentrations of IHF and lower concentrations of FIS, when cells are entering stationary phase; while excisive recombination is favored by lower concentrations of IHF and higher concentrations of FIS, when cells are entering exponential phase. A logic for these regulatory modes would be consistent with the notion that viral excision and progression to the lytic cycle would be more favorable under conditions when a healthy cell can support robust viral development and also suggests the likelihood of finding in the near neighborhood healthy new host cells to infect. Accordingly, viral integration would be more favorable when the host cell is less able to support viral development and the near-neighboring cells would likely be less inviting for successful infection. However, there are other physiological and environmental factors that also play an important role in these reactions.
6. Kinetics
In order to determine how binding energy from the multiple protein-DNA interactions is used to achieve directionality and efficiency in the overall recombination pathways, single molecule experiments on a purified and minimized in vitro excisive recombination were used to distinguish kinetically relevant intermediates from off-pathway species (Mumm, Landy and Gelles, 2006). It was found that stable bent-DNA complexes containing Int, IHF, and Xis form rapidly and independently on attL and attR, and synapsis under these conditions is extremely rapid. Int mediated DNA cleavage is required before or immediately after synapsis to stabilize the synaptic complexes which are formed with high yield and go on to yield recombinant products with an impressive efficiency of nearly 100%. The rate-limiting step of excisive recombination occurs after synapsis but closely precedes, or is concomitant with, the appearance of a stable HJ (Mumm, Landy and Gelles, 2006), consistent with the observation that in solution the rates of stable HJ formation are similar to the rates of excisive recombination (Bankhead et al., 2003).
Given the intrinsic reversibility of the isoenergetic DNA cleavage and ligation chemistry of tyrosine family recombinases, the apparent irreversibility observed at each step in the λ recombination pathway (except for synapsis) indicates that the overall directionality of excisive recombination is a direct consequence of the sequence of protein-protein and protein-DNA interactions that efficiently drive the reaction forward through nearly every step. The proposed slow step in the reaction is a conformational change that stabilizes the HJ and there are several candidates for this rate-limiting step. The scissoring movement of the HJ arms or the reorientation of active and inactive pairs of Int protomers between the first and second pair of strand swaps are both suggested by comparison of the different X-ray crystal structures of tyrosine family recombinases complexed with their respective four-armed DNAs (Guo, Gopaul and Van Duyne, 1997; Van Duyne, 2002, 2008; Chen and Rice, 2003; Biswas et al., 2005).
A totally different aspect of kinetics in vivo concerns the process by which λ DNA finds it cognate attB in cells committed to lysogeny. Surprisingly, rather than actively searching, λ DNA remains confined to the point where it entered the cell and it is directed motion of the bacterial chromosome during replication that delivers attB to a waiting, relatively stationary, attP (Tal et al., 2014). Movement of attB in cells entering the lytic pathway has also been observed (Shao et al., 2017). In some cases, the λ genome may move to an alternative location in the cell prior to integration, perhaps establishing a new anchoring point for finding attB (Shao, Hawkins and Zeng, 2015). Analyses of λ-infected cells has also revealed that coinfecting phages establish their own spatially distinct cellular compartments where lytic and lysogenic programs can initiate independently, leading to both phage integration and lysis in the same cell (Shao et al., 2017; Trinh et al., 2020).
7. Bridging Patterns of Int
As noted above, λ Int is a heterobivalent DNA binding protein that forms higher-order complexes with an ensemble of accessory DNA bending proteins that function to deliver Ints bound at distal high-affinity arm-type sites to a core region where the DNA chemistry and strand exchanges are carried out by the catalytic/core binding domains (jointly referred to as the CTD). A summary of the Int bridges formed during excisive and integrative recombination is shown schematically in Figure 10 and the experiments used to map the interactions are discussed below.
Figure 10.
Schematic mapping of the bridges Int makes between core half-sites and arm-binding sites during integrative and excisive recombination. The P’1 and P’2 arm sites are used in both pathways. The P1 and P’3 sites are used only during integration and the P2 site is used only during excision.
Two different approaches were used to help decipher which core-type and arm-type Int binding sites are joined to one another by Int-mediated bridges. The chemical approach employed a disulfide trapping technology to introduce disulfide crosslinks at the protein-DNA interfaces between an Int NTD and its cognate arm-type site and between an Int CTD and its cognate core-type site. Using recombination intermediates consisting of trapped HJ-protein complexes, doubly cross-linked INTs were only observed with those att sites in which a cystamine-labeled arm site and a cystamine-labeled core site are bridged by the same INT molecule. In the integrative HJ recombination intermediate the bridges are: P’1 – C’; P’2 – C; P’3 – B’ and P1 – B while the excisive intermediate had the bridges: P’1 – C’; P’2 – B; P2 – B’, leaving the C core site as the one that does not form a bridge with any of the three arm-type sites required for excisive recombination (Tong et al., 2014).
The chemically determined Int bridges were confirmed by a genetic approach with full recombination reactions. Two chimeric recombinases engineered to recognize and bind different arm- and core-type binding sites were used in conjunction with “hybrid” att sites containing pairs of arm- and core-type sequences corresponding to either one or the other chimeric recombinase. The pairs of arm-core specificities that were functional confirmed the chemical cross-linking experiments and also provided complementary information as described below (Warren, Laxmikanthan and Landy, 2008).
Taken together, the results of these experiments argue strongly that each arm-core bridged pair enjoys a monogamous relationship throughout the course of the recombination reaction. The bridging results show that for excisive recombination the presynaptic partners have only intramolecular bridges, indicating that Int bridging is not a driving force in synapsis of attL and attR. This is also likely to be the case for integrative recombination, even though a fully assembled (supercoiled) attP complex is required for the capture of a naked attB and therefore involves two intermolecular bridges (Richet, Abcarian and Nash, 1988). It was postulated that the reason attB cannot bind INT protomers unless they are part of a higher-order complex stems from the need to overcome the NTD inhibition of the CTD function, as described above (Sarkar, Radman-Livaja and Landy, 2001). Models consistent with this interpretation, and additional insights regarding integrative synapsis come from the three-dimensional structure of an intact functional assembly of a trapped excisive recombination intermediate, as described below.
8. Structure of the Excisive HJ Complex
The molecular machinery that carries out λ site specific recombination was difficult to visualize and characterize because of its complexity and the difficulty of isolating intact functionally relevant complexes. The latter problem was solved by isolating stable HJ complexes trapped by in vitro recombination between attL and attR partners whose respective seven bp overlap sequences were designed to generate a fully paired HJ overlap sequence that was stable because either reversal or progression of the recombination reaction would generate mismatched (energetically disfavored) base pairs as discussed in section 4 (Sun, 1990; Seah et al., 2014; Tong et al., 2014). The resulting 240-bp complex bound specifically by 11 protein subunits (4 Int, 4 IHF, 3 Xis) was lightly cross-linked, purified, and analyzed by electron cryo-microscopy (cryo-EM) (Laxmikanthan et al., 2016). These results were then combined with data from X-ray crystal structures of relevant substructures involving Int (Biswas et al., 2005), IHF (Rice et al., 1996), Xis (Abbani et al., 2007), and FIS (Papagiannis et al., 2007) and studies with Fluorescence Resonance Energy Transfer (FRET) (Seah et al., 2014) to generate a model of the excisive recombination complex and the inferred complex for integrative recombination (Laxmikanthan et al., 2016).
The structure of the excisive HJ complex is shown in two orientations in Figure 11 and a video of the complex is available (https://elifesciences.org/articles/14313/figures#videos). Many of the protein-protein and protein-DNA interfaces are buried within the complex, making it difficult to visualize the individual Int bridges. To aid in visualization of the three-dimensional assembly, schematic representations of the complex are shown below the structural models. At the center of the excisive complex, an integrase tetramer is bound to a core-site HJ where strand exchange has taken place between the B half-site of attL and the C half-site of attR (see Figures 8 and 10). The most striking features of the complex are the tight bends of the P and P’ arms as they emerge from the C and C’ core half-sites and the overall compactness of the assembly.
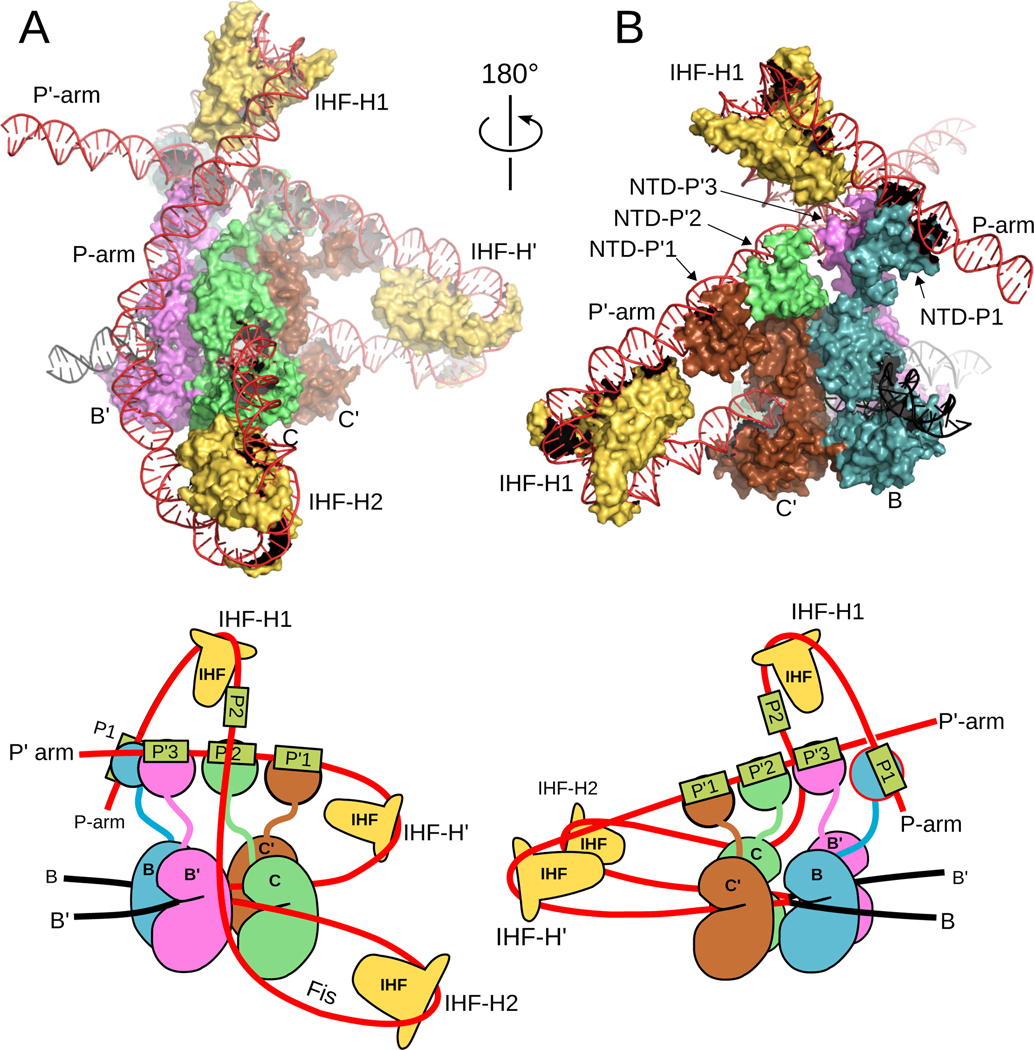
Figure 11.
Structure of the excisive Holliday junction complex. Coloring in the structures and in the schematic diagrams below follow the coloring used in Figure 10. Int CTDs are labeled according to the core half-site to which they are bound (B, B’, C, C’). A) View down the ‘top’ of the complex, towards the arm-binding domains (NTDs) of Int. The Int NTDs binding to the P’1, P’2, and P2 sites are indicated. The three Xis molecules bound to the P-arm are also indicated. This view highlights the sharp IHF-mediated DNA bends at H’ that delivers the P’1 and P’2 sites into bridging proximity of the core sites. The center of the DNA bend at H’ is approximately 100 Å from the center of the core sites. This view also illustrates the close association between the Int NTDs bound at P’1 and P’2 with Xis bound at the X1 and X1.5 sites. The close contact between Xis1 and the NTD bound at P2 can also be seen. B) Side view showing the bend of the P-arm in the excisive complex. The IHF-mediated bend at H2 turns the P-arm upwards and Xis provides the bend across the top of the complex to position P2 within bridging distance of the core site. The center of the DNA bend at H2 is approximately 120 Å from the center of the core binding sites. This view also provides an unobscured example of an Int bridging interaction, where the protomer bound at the B’ core site bridges to bind the P2 site.
IHF heterodimers bend the P arm at the H2 site and the P’ arm at the H’ site, in each case redirecting the DNA to cross over the N-terminal face of the Int tetramer. For the P’ arm, the IHF bend is sufficient to position the P’1 and P’2 arm-binding sites for bridging to the Int subunits bound at the C’ and B core sites, respectively (Figures 10 & 11). For the P arm, the IHF-mediated bend at H2 redirects the DNA to run upwards alongside the Int tetramer. Three Xis subunits provide a second change in direction of the P arm, directing it across the top of the Int tetramer roughly parallel to the P’-arm. As a result of the combined actions of IHF and Xis, the P2 arm-binding site is now positioned where it can be engaged by the Int subunit bound to the B’ core site. The P arm trajectory is also aided by an intrinsic A tract sequence found in the Fis binding site. Fis can be readily accommodated by the structure, as indicated in Figure 11.
The only component of the excisive HJ complex that is not accounted for in the cryo-EM complex is the NTD of Int bound at the C core site (Figure 10). This domain has no known function in excisive recombination, since only three arm-binding sites are used in the reaction (Numrych, Gumport and Gardner, 1990; Tong et al., 2014). The arm-binding domain of the Int molecule bound at C is not positioned directly above its connected CB domain; as indicated in Figure 11b, the Xis molecule bound at the X1 site instead occupies this space.
9. Two Roles for Xis During Excision
Xis bends the P arm to stabilize an attR complex configuration that is competent to synapse with an Int/IHF/attL complex to initiate excision. The cryo-EM structure revealed that Xis also mediates a substantial protein-protein interface between the P and P’ arms. The Xis molecules bound at the X1 and X1.5 sites of the P arm are packed tightly against the Int NTDs bound at the P’2 and P’1 sites, respectively (see Figure 11a). This interface is likely important, given how closely the P and P’ arms approach one another in the excisive complex.
The identification of a Xis-mediated interface between the P and P’ arms provides an explanation for how synapsis is facilitated in the excisive pathway. Int binds only weakly to the core sites of attL and attR and the Int-bound core sites do not form stable synaptic complexes that undergo recombination (Bushman et al., 1985). The three Int NTDs in the excisive complex are arranged in a nearly linear manner, where the NTD bound at P2 makes no contact with the NTDs bound at P’1 or P’2 (see Figure 11a). Thus, interactions beween NTDs are unlikely to contribute to synapsis during excision. Instead, Xis-NTD, and possibly Xis contacts to the NTD-CBD linker provide the interactions required to hold attL and attR together and allow the initial strand exchange to take place.
There are 18 residues at the C-terminus of Xis that were missing in the Xis•DNA crystal structures (Sam et al., 2004; Abbani et al., 2007) and are not included in the excisive complex model shown in Figure 11. Six of these residues are arginine or lysine, suggesting that the basic C-terminal tails of Xis could interact with P and/or P’ arm DNA, further stabilizing a synaptic interface. Thus, the cryo-EM structure revealed and explained two functional roles for Xis in excisive recombination: i) bending the P arm to place P2 in position for cooperative Int binding and formation of a synapsis-competent attR conformation; ii) mediating an Xis•Int interface between the P and P’ arms that stabilizes the attL x attR synaptic complex.
10. A Model for the Integrative HJ Complex
The integrative HJ intermediate is formed when a complex containing attP DNA, an Int tetramer, and three IHF heterodimers captures the attB site in the host genome and carries out top strand exchange between the core DNA sequences (see Figure 10 (A–B). A model for this complex, constructed based on the excisive complex structure shown in Figure 11, FRET-based studies (Seah et al., 2014), Int bridging data (Tong et al., 2014), and crystal structures of the components, is shown in Figure 12.
Figure 12.
A model for the integrative complex. Coloring and labels in the structures and in the schematic diagrams below follow the coloring used in Figures 10 & 11. A) View along the P-arm showing the upward trajectory of the P-arm following the IHF bend at H2. The DNA reverses course at the H1 site where IHF redirects the P-arm downwards towards the Int tetramer. The P-arm crosses over the P’-arm as a result of the bend at H1. B) View from the opposite side of the complex. The P’-arm engages three Int NTDs at the P’1, P’2, and P’3 sites which bridge to the C’, C, and B’ core sites, respectively. The P-arm can be seen crossing over the P’-arm in this orientation, where the P1 binding site is positioned to bind an Int NTD that bridges to the B core site. During the intitial steps of integration, Int will be tightly bound at P1 and P’3 and the respective CTDs of Int will test for the correct attB sequence in the E. coli chromosome. Labels and coloring schemes follow that shown in Figure 11.
In both the integrative and excisive complexes, IHF bending at the H’ site directs the P’ arm over the N-terminal face of the Int tetramer. In the integrative complex, Int bound at B’ bridges to the P’3 arm site, since Xis is not present to position the P2 site for binding. A striking aspect of this model is that the P’ arm trajectory requires no positional changes in order to readily accommodate binding of the Int NTDs at P’1, P’2, and P’3 that bridge to the C’, C, and B’ core sites, respectively. Thus, the P’ arm position and trajectory appear to be the same in excisive and integrative recombination.
In the absence of Xis, the P arm is not directed towards the Int NTDs. Instead, the H2 bend causes it to follow an upward trajectory beyond the P’ arm, where IHF binding at the H1 site reverses its course and directs it back towards the Int tetramer. This P arm trajectory places the high affinity P1 site within reach of the Int NTD bridging to the B core site. Thus, the P arm crosses over the P’ arm to position the P1 arm site during integration, but runs parallel to the P’ arm and promotes Int bridging between B’ and P2 during excision.
The P-P’ arm crossing shown in Figure 12 generates a negative DNA-crossing node, a feature that provides an explanation for the long-standing puzzle of why the topologies of integrative and excisive recombination are different and why supercoiling is required for efficient integration (Nash, 1975; Richet, Abcarian and Nash, 1988). The integrative model shown in Figure 12 cannot be easily constructed based on IHF bends at H2 and H1 alone; additional bending of the P-arm is required, which would occur naturally in a negatively supercoiled attP, but would be energetically unfavorable in linear substrates.
11. Regulated Directionality of λ site-specific recombination
The biochemical feature that defines excisive and integrative recombination as two distinct pathways (as opposed to a single bidirectional pathway) is the fact that both reactions are initiated by exchange at the B and C core sites to form their respective HJ intermediates (Nunes-Düby, Matsumoto and Landy, 1987; Kitts and Nash, 1988b). The structural models shown in Figures 11 and 12 indicate that the order of strand exchange in both pathways is determined prior to synapsis by the patterns of specific Int bridges between arm and core sites and by the IHF- and Xis-induced bends that enable those bridges. For example, the C’ and B bridges to the P’1 and P’2 sites of attL require a specific bend of the B-C’ core site, which in turn commits attL to cleave at the B core site in the synaptic complex with attR. Similarly, formation of an integration-competent attP assembly requires a specific bend direction of the C-C’ core site in order to form the correct bridging interactions.
The strong asymmetry provided by distinct attL and attR sites (as well as very different attP and attB sites used during integration) therefore ensures directionality in the reaction pathway and at the same time provides a mechanism to respond to the physiology of the host cell. This level of physiological control is lacking in simpler systems that use identical or nearly identical att-sites, such as the Cre-loxP system (Sternberg et al., 1981).
The excisive HJ complex structure explains how Xis is able to stimulate excision while inhibiting integration. During excision, Xis enhances formation of the Int bridge between the P2 arm site and the B’ core site by pronounced bending of the P arm. Xis interactions with the Ints bound to attL also facilitate synapsis of the attL and attR complexes. The same Xis-induced bending of the P-arm that is important for excision would prevent its upward trajectory in attP. Integration would therefore be inhibited, since the P-arm would not be able to cross over the P’-arm and position the P1 site for attB capture (Figure 12).
A long-standing conundrum has been to understand how the large attP complex captures a naked attB site (Richet, Abcarian and Nash, 1988). The integrative complex model shown in Figure 12 has an inherently flexible P arm that could allow for the dynamic binding required to embrace and test potential attB sites on the bacterial chromosome, a structural feature that is consistent with genetic and biochemical results (Tong et al., 2014).
12. Conclusions and outlook
The site specific recombination pathway of bacteriophage λ is responsible for the insertion and excision of the viral chromosome into and out of the chromosome of its E. coli bacterial host and it is executed by the virally encoded recombinase Int (integrase). The most distinguishing and interesting aspects of λ site specific recombination are the exquisite directionality and regulation used by the integration and excision pathways. The λ-encoded Int and Xis proteins, together with the host-encoded IHF and FIS proteins, interact with an extensive array of protein binding sites arranged on λ attP to run these two distinct reactions. Recent progress in mapping the Int bridging interactions between core and arm binding sites, combined with structural approaches using FRET and cryo-electron microscopy have revealed the molecular architectures responsible for catalyzing the integration and excision reactions. The structural complexity of these pathways mirrors the genetic and biochemical complexity that has been established over the past four decades. Sharp bends in the λ attachment sites, tightly packed multi-protein/DNA assemblies, and flexible Int bridging interactions all play important functional roles in orchestrating the steps involved in bringing the correct att sites together, cleaving and exchanging the DNA strands between the sites, and ensuring that the products are stable. This structural and mechanistic complexity of the two λ reactions is what enables their sophisticated tight linkage to the physiology of the host cell, an obvious advantage that explains why the majority of lysogenic phage dependent upon tyrosine recombinases seem to share this complexity (Smyshlyaev, Bateman and Barabas, 2021).
As noted in the introduction, site-specific recombinases such as Int have served as powerful tools in genome engineering and in vitro cloning applications. Bacterial viruses and their site-specific recombinases continue to receive attention from investigators interested in digitally and biochemically mining their diversity for new insights and for their potential usefulness for bioengineering (Srivastava and Thomson, 2016; Vijaya Chandra et al., 2016; Bessen et al., 2019; Durrant et al., 2020, 2020; Schmitt et al., 2022; Zhang, Azarin and Sarkar, 2022; Hackl et al., 2023). Highly detailed views of the molecular mechanisms involved in the well- studied model systems such as the λ system discussed here provide important frameworks and reference points for expanding these new directions.
Acknowledgements
This review is dedicated to the memory of Allan Campbell and Howard Nash, fathers of the genetics and biochemistry, respectively, of the λ site-specific recombination pathway. In our attempt to briefly summarize more than 60 years of work by a community of geneticists, biochemists, and structural biologists we regret not being able to cite all of the individuals contributing to the sophisticated understanding of this pathway. The authors’ studies on the mechanisms of viral integration and excision were supported by National Institutes of Health grants GM108751 (GV), GM062723 (AL) and GM033928 (AL). The authors declare no conflict of interests.
References
- Abbani M. et al. (2007) Structure of the cooperative Xis-DNA complex reveals a micronucleoprotein filament that regulates phage lambda intasome assembly, Proc Natl Acad Sci U S A, 104, 2109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abremski K. and Gottesman S. (1982) Purification of the bacteriophage lambda xis gene product required for lambda excisive recombination, The Journal of Biological Chemistry, 257, 9658–9662. [PubMed] [Google Scholar]
- Aihara H. et al. (2003) A conformational switch controls the DNA cleavage activity of lambda integrase, Mol Cell, 12, 187–98. [DOI] [PubMed] [Google Scholar]
- Ball CA et al. (1992) Dramatic changes in Fis levels upon nutrient upshift in Escherichia coli, Journal of Bacteriology, 174, 8043–8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball CA and Johnson RC (1991) Efficient excision of phage lambda from the Escherichia coli chromosome requires the Fis protein, Journal of Bacteriology, 173, 4027–4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankhead T. et al. (2003) Mutations at residues 282, 286, and 293 of phage lambda integrase exert pathway-specific effects on synapsis and catalysis in recombination, J Bacteriol, 185, 2653–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessen JL et al. (2019) High-resolution specificity profiling and off-target prediction for site-specific DNA recombinases, Nature Communications, 10, 1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas T. et al. (2005) A structural basis for allosteric control of DNA recombination by lambda integrase, Nature, 435, 1059–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning DF, Grainger DC and Busby SJ (2010) Effects of nucleoid-associated proteins on bacterial chromosome structure and gene expression, Current Opinion in Microbiology, 13, 773–780. [DOI] [PubMed] [Google Scholar]
- Bushman W. et al. (1985) Control of directionality in lambda site specific recombination, Science (80- ), 230, 906–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A. (1962) Episomes, in Caspari EW and Thoday JM (eds) Advances in Genetics. New York: Academic Press, 101–45. [Google Scholar]
- Chen Y. and Rice PA (2003) New insight into site-specific recombination from Flp recombinase-DNA structures, Annu Rev Biophys Biomol Struct, 32, 135–59. [DOI] [PubMed] [Google Scholar]
- Craig NL et al. (eds) (2015) Mobile DNA III. 3rd edn. Washington DC: ASM Press. [Google Scholar]
- Craig N. and Nash H. (1984) E. coli integration host factor binds to specific sites in DNA, Cell, 39, 707–16. [DOI] [PubMed] [Google Scholar]
- Craig NL and Nash HA (1983) The mechanism of phage λ site-specific recombination: Site-specific breakage of DNA by Int topoisomerase, Cell, 35, 795–803. [DOI] [PubMed] [Google Scholar]
- Ditto MD, Roberts D. and Weisberg RA (1994) Growth phase variation of integration host factor level in Escherichia coli, Journal of Bacteriology, 176, 3738–3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant MG et al. (2020) A Bioinformatic Analysis of Integrative Mobile Genetic Elements Highlights Their Role in Bacterial Adaptation, Cell Host & Microbe, 27, 140–153.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enquist LW and Weisberg RA (1976) The red plaque test: a rapid method for identification of excision defective variants of bacteriophage lambda, Virology, 72, 147–153. [DOI] [PubMed] [Google Scholar]
- Fadeev EA, Sam MD and Clubb RT (2009) NMR structure of the amino-terminal domain of the lambda integrase protein in complex with DNA: immobilization of a flexible tail facilitates beta-sheet recognition of the major groove, Journal of Molecular Biology, 388, 682–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel SE and Johnson RC (1992) The Fis protein: it’s not just for DNA inversion anymore, Molecular Microbiology, 6, 3257–3265. [DOI] [PubMed] [Google Scholar]
- Fogg PCM et al. (2014) New Applications for Phage Integrases, Journal of Molecular Biology, 426, 2703–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingery R. and Echols H. (1967) Mutants of bacteriophage lambda unable to integrate into the host chromosome., Proceedings of the National Academy of Sciences, 58, 1507–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich JA, Schwartz ML and McClure WR (1990) Searching for and predicting the activity of sites for DNA binding proteins: compilation and analysis of the binding sites for Escherichia coli integration host factor (IHF), Nucleic Acids Research, 18, 4993–5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman ME and Yarmolinsky MB (1968) Integration-negative mutants of bacteriophage lambda, Journal of Molecular Biology, 31, 487–505. [DOI] [PubMed] [Google Scholar]
- Guo F, Gopaul D. and Van Duyne G. (1997) Structure of Cre recombinase complexed with DNA in a site-specific recombination synapse, Nature, 389, 40–46. [DOI] [PubMed] [Google Scholar]
- Hackl T. et al. (2023) Novel integrative elements and genomic plasticity in ocean ecosystems, Cell, 186, 47–62.e16. [DOI] [PubMed] [Google Scholar]
- Hartley JL, Temple GF and Brasch MA (2000) DNA Cloning Using In Vitro Site-Specific Recombination, Genome Research, 10, 1788–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano N. et al. (2011) Site-specific recombinases as tools for heterologous gene integration, Applied Microbiology and Biotechnology, 92, 227–239. [DOI] [PubMed] [Google Scholar]
- Hsu PL and Landy A. (1984) Resolution of synthetic att-site Holliday structures by the integrase protein of bacteriophage lambda, Nature, 311, 721–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RC (2005) Major nucleoid proteins in the structure and function of the Escherichia coli chromosome, in Higgins NP (ed.) The Bacterial Chromosome. ASM Press, Washington D.C, 65–132. [Google Scholar]
- Johnson RC, Bruist MF and Simon MI (1986) Host protein requirements for in vitro site-specific DNA inversion, Cell, 46, 531–539. [DOI] [PubMed] [Google Scholar]
- Katzen F. (2007) Gateway® recombinational cloning: a biological operating system, Expert Opinion on Drug Discovery, 2, 571–589. [DOI] [PubMed] [Google Scholar]
- Kitts P. and Nash H. (1988a) An intermediate in the phage lambda site-specific recombination reaction is revealed by phosphorothioate substitution in DNA, Nucleic Acids Res, 16, 6839–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitts P. and Nash H. (1988b) Bacteriophage lambda site-specific recombination proceeds with a defined order of strand exchanges, J Mol Biol, 204, 95–107. [DOI] [PubMed] [Google Scholar]
- Koch C. and Kahmann R. (1986) Purification and properties of the Escherichia coli host factor required for inversion of the G segment in bacteriophage Mu, The Journal of Biological Chemistry, 261, 15673–15678. [PubMed] [Google Scholar]
- Kostrewa D. et al. (1991) Three-dimensional structure of the E. coli DNA-binding protein FIS, Nature, 349, 178–180. [DOI] [PubMed] [Google Scholar]
- Kumagai M. and Ikeda H. (1991) Molecular analysis of the recombination junctions of λ bio transducing phases, Molecular and General Genetics MGG, 230, 60–64. [DOI] [PubMed] [Google Scholar]
- Landy A. and Ross W. (1977) Viral Integration and Excision: Structure of the Lambda att Sites, Science, 197, 1147–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxmikanthan G. et al. (2016) Structure of a Holliday junction complex reveals mechanisms governing a highly regulated DNA transaction, eLife. Edited by Scheres SH, 5, e14313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallik P. et al. (2006) DksA is required for growth phase-dependent regulation, growth rate-dependent control, and stringent control of fis expression in Escherichia coli, Journal of Bacteriology, 188, 5775–5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller H. and Friedman D. (1980) An E. coli gene product required for lambda site-specific recombination, Cell, 20, 711–719. [DOI] [PubMed] [Google Scholar]
- Miller HI et al. (1979) Site-specific recombination of bacteriophage lambda: the role of host gene products, Cold Spring Harbor Symposia on Quantitative Biology, 43 Pt 2, 1121–1126. [DOI] [PubMed] [Google Scholar]
- Miller HI, Kirk M. and Echols H. (1981) SOS induction and autoregulation of the himA gene for site-specific recombination in Escherichia coli, Proceedings of the National Academy of Sciences of the United States of America, 78, 6754–6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moitoso et al. (1989) DNA looping generated by DNA bending protein IHF and the two domains of lambda integrase, Science (80- ), 244, 1457–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JM et al. (2015) Roles of Nucleoid-Associated Proteins in Stress-Induced Mutagenic Break Repair in Starving Escherichia coli, Genetics, 201, 1349–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumm JP, Landy A. and Gelles J. (2006) Viewing single lambda site-specific recombination events from start to finish, The EMBO journal, 25, 4586–4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nafissi N. and Slavcev R. (2014) Bacteriophage recombination systems and biotechnical applications, Applied Microbiology and Biotechnology, 98, 2841–2851. [DOI] [PubMed] [Google Scholar]
- Nash HA (1974) Purification of bacteriophage lambda Int protein, Nature, 247, 543–545. [DOI] [PubMed] [Google Scholar]
- Nash HA (1975) Integrative recombination of bacteriophage lambda DNA in vitro, Proceedings of the National Academy of Sciences of the United States of America, 72, 1072–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash HA (1981) Integration and excision of bacteriophage lambda: the mechanism of conservation site specific recombination, Annual Review of Genetics, 15, 143–167. [DOI] [PubMed] [Google Scholar]
- Ninnemann O, Koch C. and Kahmann R. (1992) The E.coli fis promoter is subject to stringent control and autoregulation., The EMBO Journal, 11, 1075–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numrych TE, Gumport RI and Gardner JF (1990) A comparison of the effects of single-base and triple-base changes in the integrase arm-type binding sites on the site-specific recombination of bacteriophage lambda, Nucleic Acids Research, 18, 3953–3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes-Düby S. et al. (1998) Similarities and differences among 105 members of the Int family of site-specific recombinases, Nucleic Acids Res, 26, 391–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes-Duby S, Azaro M. and Landy A. (1995) Swapping DNA strands and sensing homology without branch migration in lambda site-specific recombination, Curr Biol, 5, 139–48. [DOI] [PubMed] [Google Scholar]
- Nunes-Düby SE, Matsumoto L. and Landy A. (1987) Site-specific recombination intermediates trapped with suicide substrates, Cell, 50, 779–788. [DOI] [PubMed] [Google Scholar]
- Olorunniji FJ, Rosser SJ and Stark WM (2016) Site-specific recombinases: molecular machines for the Genetic Revolution, Biochemical Journal, 473, 673–684. [DOI] [PubMed] [Google Scholar]
- Papagiannis C. et al. (2007) Fis targets assembly of the Xis nucleoprotein filament to promote excisive recombination by phage lambda, J Mol Biol, 367, 328–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice P. et al. (1996) Crystal structure of an IHF-DNA complex: a protein-induced DNA U-turn, Cell, 876, 1295–306. [DOI] [PubMed] [Google Scholar]
- Rice PA (1997) Making DNA do a U-turn: IHF and related proteins, Current Opinion in Structural Biology, 7, 86–93. [DOI] [PubMed] [Google Scholar]
- Richet E, Abcarian P. and Nash H. (1988) Synapsis of attachment sites during lambda integrative recombination involves capture of a naked DNA by a protein-DNA complex, Cell, 52, 9–17. [DOI] [PubMed] [Google Scholar]
- Sam MD et al. (2002) Regulation of directionality in bacteriophage lambda site-specific recombination: structure of the Xis protein, Journal of Molecular Biology, 324, 791–805. [DOI] [PubMed] [Google Scholar]
- Sam MD et al. (2004) Crystal structure of the excisionase-DNA complex from bacteriophage lambda, Journal of Molecular Biology, 338, 229–240. [DOI] [PubMed] [Google Scholar]
- Sarkar D. et al. (2002) Differential affinity and cooperativity functions of the amino-terminal 70 residues of lambda integrase, J Mol Biol, 324, 775–89. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Radman-Livaja M. and Landy A. (2001) The small DNA binding domain of lambda integrase is a context-sensitive modulator of recombinase functions, EMBO J, 20, 1203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt LT et al. (2022) Prediction of designer-recombinases for DNA editing with generative deep learning, Nature Communications, 13, 7966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider DA, Ross W. and Gourse RL (2003) Control of rRNA expression in Escherichia coli, Current Opinion in Microbiology, 6, 151–156. [DOI] [PubMed] [Google Scholar]
- Seah NE et al. (2014) Nucleoprotein architectures regulating the directionality of viral integration and excision, Proceedings of the National Academy of Sciences of the United States of America, 111, 12372–12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segall A. (1998) Analysis of higher order intermediates and synapsis in the bent-L pathway of bacteriophage lambda site-specific recombination, J Biol Chem, 273, 24258–65. [DOI] [PubMed] [Google Scholar]
- Segall A. and Nash H. (1993) Synaptic intermediates in bacteriophage lambda site-specific recombination: integrase can align pairs of attachment sites, Embo Journal, 12, 4567–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segall A. and Nash H. (1996) Architectural flexibility in lambda site-specific recombination: three alternate conformations channel the attL site into three distinct pathways, Genes Cells, 1, 453–63. [DOI] [PubMed] [Google Scholar]
- Shao Q. et al. (2017) Lysis-lysogeny coexistence: prophage integration during lytic development, MicrobiologyOpen, 6, e00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Q, Hawkins A. and Zeng L. (2015) Phage DNA Dynamics in Cells with Different Fates, Biophysical Journal, 108, 2048–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyshlyaev G, Bateman A. and Barabas O. (2021) Sequence analysis of tyrosine recombinases allows annotation of mobile genetic elements in prokaryotic genomes, Molecular Systems Biology, 17, e9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava V. and Thomson J. (2016) Gene stacking by recombinases, Plant Biotechnology Journal, 14, 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella S, Cascio D. and Johnson RC (2010) The shape of the DNA minor groove directs binding by the DNA-bending protein Fis, Genes & Development, 24, 814–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg N. et al. (1981) Site-specific recombination and its role in the life cycle of bacteriophage P1, Cold Spring Harb Symp Quant Biol, 1, 297–309. [DOI] [PubMed] [Google Scholar]
- Sun KQ (1990) A study of DNA-DNA interactions during bacteriophage lambda integrative recombination. University of Illinois at Urbana-Champaign. [Google Scholar]
- Swinger KK and Rice PA (2004) IHF and HU: flexible architects of bent DNA, Current Opinion in Structural Biology, 14, 28–35. [DOI] [PubMed] [Google Scholar]
- Tal A. et al. (2014) Location of the unique integration site on an Escherichia coli chromosome by bacteriophage lambda DNA in vivo, Proceedings of the National Academy of Sciences of the United States of America, 111, 7308–7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. et al. (1987) Cellular factors couple recombination with growth phase: characterization of a new component in the lambda site-specific recombination pathway, Cell, 50, 901–8. [DOI] [PubMed] [Google Scholar]
- Tong W. et al. (2014) Mapping the λ Integrase bridges in the nucleoprotein Holliday junction intermediates of viral integrative and excisive recombination, Proceedings of the National Academy of Sciences of the United States of America, 111, 12366–12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh JT et al. (2020) Emerging heterogeneous compartments by viruses in single bacterial cells, Nature Communications, 11, 3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Duyne G. (2002) A structural view of tyrosine recombinase site-specific recombination, in Craig NL et al. (eds) Mobile DNA II. ASM Press, Washington DC, 93–117. [Google Scholar]
- Van Duyne G. (2008) Site-specific recombinases, in Protein-Nucleic Acid Interactions. Royal Society of Chemistry, 303–332. [Google Scholar]
- Vijaya Chandra SH et al. (2016) Conservative site-specific and single-copy transgenesis in human LINE-1 elements, Nucleic Acids Research, 44, e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. et al. (2011) Recombinase technology: applications and possibilities, Plant Cell Reports, 30, 267–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren D, Laxmikanthan G. and Landy A. (2008) A chimeric Cre recombinase with regulated directionality, Proceedings of the National Academy of Sciences of the United States of America, 105, 18278–18283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg RA (1996) Specialized transduction, in Neidhardt C. et al. (ed.) Escherichia coli and Salmonella. Cellular and Molecular Biology. ASM Press, Washington D.C, 2442–2448. [Google Scholar]
- Wojciak J. et al. (2002) Arm-site binding by lambda -integrase: solution structure and functional characterization of its amino-terminal domain, Proc Natl Acad Sci U S A, 99, 3434–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan HS et al. (1991) The molecular structure of wild-type and a mutant Fis protein: relationship between mutational changes and recombinational enhancer function or DNA binding, Proceedings of the National Academy of Sciences of the United States of America, 88, 9558–9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Azarin SM and Sarkar CA (2022) Model-guided engineering of DNA sequences with predictable site-specific recombination rates, Nature Communications, 13, 4152. [DOI] [PMC free article] [PubMed] [Google Scholar]