Abstract
Intratumoral bacteria have been implicated in driving tumor progression, yet effective treatments to modulate the tumor microbiome remain limited. In this study, we investigate the use of electroporation in combination with metronidazole to enhance the clearance of intracellular Fusobacterium nucleatum within pancreatic cancer cells. We explore various parameters, including electric field strength, pulse width, and pulse number to assess the permeability of pancreatic cancer cells infected with F. nucleatum, compared to non-infected cells of the same type. We subsequently quantify the clearance of intracellular bacteria when these pulsing schemes are applied to a suspension of infected pancreatic cancer cells in the presence of metronidazole. Our results reveal distinct differences in cell permeability between infected and non-infected cells, identifying a unique biophysical marker for host cells infected with F. nucleatum. We demonstrate that the combinatorial use of electroporation and metronidazole significantly enhances the delivery of metronidazole into host cells, leading to more effective clearance of intracellular F. nucleatum compared to independent treatments; we term this novel approach Electro-Antibacterial Therapy (EAT). EAT holds promise as an innovative strategy for addressing intratumoral bacteria in pancreatic cancer, other malignancies, and potentially treatment-resistant infections, offering new avenues for therapeutic intervention.
Keywords: Pulsed Electric Fields, Bacteria, Tumor Microbiome, Antibiotic, Electroporation, Fusobacterium
1. Introduction
Cancer, an evolving disease responsible for the loss of 10 million lives globally each year, has been revealed to not function in isolation but rather thrives due to its immune-suppressing and tumor-promoting environment [1]. The tumor microenvironment (TME) is a complex ecosystem made up of malignant cells alongside supporting immune cells, fibroblasts, and a modified extracellular matrix whose interactions promote tumor progression [2]. Apart from various eukaryotic cell types contributing to tumorigenesis, the presence of microbes and the role they may play in the disease have become new ventures of scholarly investigation. Recently, Hanahan has defined polymorphic microbiomes with emphasis on the role of bacteria to enhance metastases or modulate the immune response as an enabling hallmark of cancer [3]. Extra- and intratumoral microbiota have been observed to play a role in tumor formation, disease progression, treatment response, and patient prognosis [4].
Recently, we showed that an anaerobic bacterium, Fusobacterium nucleatum (F. nucleatum), commonly found in the oral cavity, can promote proliferation and migration across multiple cancer cell types including colorectal and pancreatic cancer cells [5,6]. The invasion of the bacteria into the cell induces these more aggressive phenotypes of the host cell by exploiting its autocrine and paracrine signaling pathways [6]. Further, Hayashi et al. showed that the intratumoral presence of F. nucleatum in human pancreatic ductal adenocarcinoma tissue samples correlated with decreased overall survival when compared to patients with no intratumoral colonization of F. nucleatum, suggesting that its presence in the TME promotes metastasis and disease progression of pancreatic cancer [7]. To address this particular cancer-promoting microbe, Bullman et al. observed treatment of F. nucleatum within the TME using an antibiotic, metronidazole (MTZ), reduced tumor burden in a mouse model though complete F. nucleatum clearance was not achieved [8]. Oral administration of MTZ, an antibiotic shown to be effective against Fusobacterium and the standard of care in the clinic, resulted in decreased bacterial load and overall tumor volume in patient-derived xenografts of colon cancer [8]. The observed effect of antibiotics on bacterial load and tumor volume could suggest that the aggressive phenotype of the host cell resulting from bacterial invasion is reversible once the bacteria is non-viable. While the presence of tumor-promoting bacteria decreased, the systemic antibiotic delivery failed to clear the entirety of tumor-associated bacteria. Effectively administering an antibiotic to target intracellular bacteria, however, remains a challenge as passive drug transport across the membrane is limited. [9]. We hypothesize electroporation, an electric field-based technique, can be used to permeabilize the cell membrane to induce malignant cell death and enhance antibiotic uptake by infected host cells for significant clearance of tumor-promoting intracellular bacteria. Electroporation uses pulsed electric fields (PEFs) to permeabilize the cell by creating hydrophilic pores in the membrane. The electropermeabilization effect of electroporation on a cell may be reversible, allowing for resealing and cell recovery after PEF exposure, or irreversible depending on the interplay among number of pulses, pulse width, and electric field strength. In oncology, a reversible electroporation technique, known as electrochemotherapy (ECT), is used clinically to enhance transport across the cell membrane for effective delivery of chemotherapeutics [10,11,12]. Similarly, because of its ability to preserve critical structures and recruit an immune response, irreversible electroporation (IRE) has been used as an ablation technique to induce malignant cell death as a direct result of the electric field to treat prostate, liver, and pancreatic cancers [13,14,15,16,17].
While electroporation has been widely adapted for oncology applications, it has also been proven as an effective method for inactivating bacterial growth for food and beverage industries [18,19]. Recent biomedical studies, such as those from Vadlamani et al., have shown that combinatorial application of nanosecond pulses and antibiotics yield higher inactivation of antibiotic-resistant strains when compared to antibiotics or electroporation alone [20,21]. While electroporation can irreversibly damage the membrane of microbes for food and biomedical applications on its own, this disruption requires extremely high-voltage pulses on the order of 5–20 kV/cm and does not translate easily to in vivo applications [22]. Ma et al. used an electroporation-based microdevice to increase the bioavailability of cell-permeating peptides for antisense inhibition of an intracellular Salmonella strain with a macrophage host. This study used field strengths of 1200 V/cm and millisecond pulses to yield a 9-fold decrease in intracellular bacteria [23]. Aside from this work, the effect of electroporation on bacteria has been primarily studied extracellularly leaving its potential advantages in treating intracellular bacteria largely unexplored.
The evolving knowledge of the TME and its role in disease progression has led to consideration of treatments that target its microenvironmental contributors [24,25]. The advantages of these approaches, however, are difficult to harness because of tumor complexity and conflicting roles of the targeted cell type to either promote or suppress tumor advancement [26]. In this study, we target tumor-promoting intracellular bacteria as a treatment modality that considers the contributions of bacteria to the TME and overall disease progression. Here, we propose biophysical disruption of infected host cells to enhance antibiotic uptake for treating intracellular cancer-promoting bacteria. This approach provides an alternative cancer treatment that overcomes challenges associated with hyper-specificity and complexity. This method, introduced here as Electro-Antibacterial Therapy (EAT), resulted in significant clearance of intracellular bacteria via both reversible and irreversible thresholds of the host cell. We demonstrate feasibility of EAT as an alternative approach for treating intracellular cancer-associated bacteria in vitro. We show evidence that expansion of this work could potentially yield a therapeutic technique not only useful for bulk tumor ablation in vivo, but also for targeting a recent hallmark of the disease that is progression-driving microbes present in the TME.
2. Methods
2.1. Bacteria culture
Fusobacterium nucleatum nucleatum ATCC 23726 (Manassas, VA) was cultured on solid agar plates made from Colombia broth supplemented with hemin and menadione (CBHK) under anaerobic conditions. Liquid cultures were initiated in CBHK media for 18 hr then back diluted and allowed to grow for 5 hr before being used for infection during early-mid log phase growth (O.D.600 = 0.25) (Fig. 1a). Cultures were then centrifuged at 1800×g for 5 mins and resuspended in unsupplemented Dulbecco’s Modified Eagle Medium (DMEM).
Fig. 1.
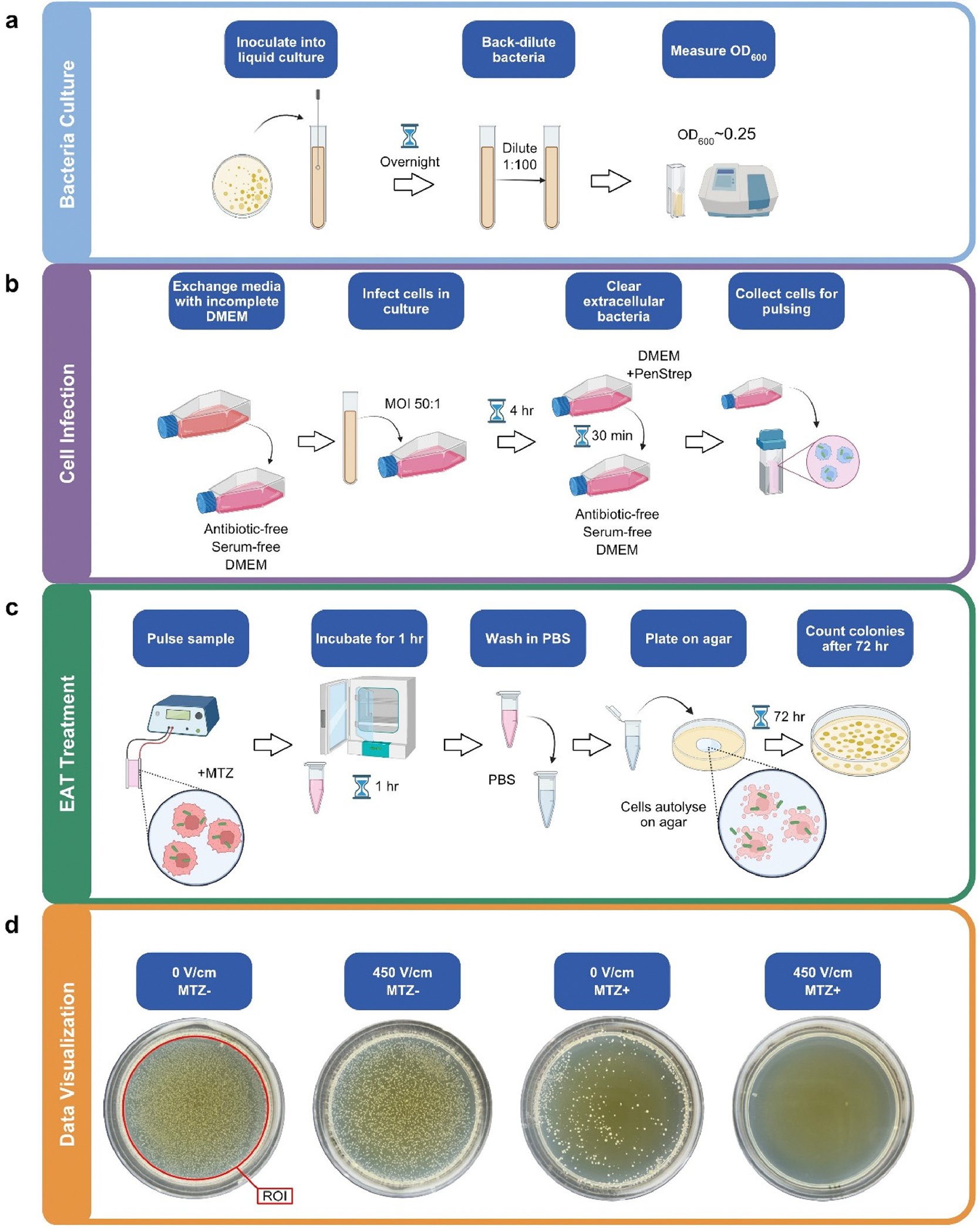
An overview of a) bacterial culture, b) cell infection, and c) pulsing treatment protocols. d) Representative images of agar plates 72 hr after treatment provide qualitative evidence for the efficacy of the control, independent, and combinatorial treatments. The ROI used in ImageJ is indicated here to quantify colony forming units.
2.2. Cell culture
Pancreatic epithelioid carcinoma PANC-1 cells were purchased from ATCC (CRL-1469) and cultured as recommended in DMEM supplemented with 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin. Prior to infection, confluent flasks were washed twice with PBS, and media replaced with serum- and antibiotic-free DMEM. PANC-1 cells were infected at a multiplicity of infection (MOI) of 50:1 (bacteria:host) under anaerobic conditions for 4 hr in serum- and antibiotic-free medium. Following infection, the cells were washed twice with phosphate-buffered saline (PBS), media replaced with supplemented DMEM, incubated for an additional 30 min, then trypsinized (Fig. 1b).
2.3. Determining electric field threshold of infected and non-infected cells
Infected and non-infected cells were suspended in serum- and antibiotic-free DMEM at 106 cells/mL. To determine the immediate permeability of cells while pulsing, propidium iodide (PI), a nuclear and chromosome counterstain, was added to the solution for a final concentration of 3 μg/mL. A volume of 337 μL of the solution was pipetted into a cuvette with 4 mm spacing. The cuvette was placed in a BTX Safety Stand 630B with Adjustable Gap (Holliston, MA). EAT pulsing schemes of eight 10 ms pulses were delivered via a BTX ECM830 Electroporation System (Holliston, MA) at 0, 150, 300, or 450 V/cm. The ECT pulsing scheme was delivered similarly with eight 100 μs pulses at 1200 V/cm. The IRE pulsing scheme was delivered as one hundred 100 μs pulses at 1200 V/cm. The experiments were performed in triplicates. The applied electric field strength of 1200 V/cm for IRE and ECT pulsing parameters was chosen after preliminary experiments indicated ~99% permeability of non-infected cells, data not shown. The samples were then placed in a 24-well plate and immediately processed for flow cytometry. To determine the long-term cell survival, samples were pulsed exactly as the samples investigating immediate permeability but did not contain PI in the solution when pulsing. These samples were placed in a 24-well plate and incubated at 5% CO2 and 37 °C for 24 hr. After 24 hr, adhered cells were trypsinized, PI was added to each well at 3 μL/mL and incubated with the stain for 15 min at 5% CO2 and 37 °C. The samples were then processed for flow cytometry.
2.3.1. Flow cytometry and cell viability quantification
Uptake of PI for each condition was quantified using a Bio-Rad S3e Cell Sorter (Hercules, CA). Flow cytometry data was processed using FlowJo (BD Life Sciences, Franklin Lakes, NJ) with gating for the main cell population (FSC Area vs. SSC Area) followed by singlet population (FSC Area vs. FSC height) before gating for fluorescence (FL4 Area) based on control populations of infected and non-infected cells. The percentages of PI+ cells for each applied field were gathered.
2.4. Electro-Antibacterial Therapy
2.4.1. Antibiotic solution preparation
Stock solutions of MTZ were made by dissolving powdered metronidazole (Research Products International M8100025.0, Mount Prospect, IL) in sterile dimethyl sulfoxide (DMSO) at a concentration of 30 mg/mL. The stock solution was added to unsupplemented DMEM to achieve 0 and 5 μg/mL MTZ solutions. The antibiotic concentration of 5μg/mL was determined by exposing infected cells to 0, 1, 5, and 10 μg/mL concentrations of antibiotic. When determining the efficacy of the concentrations on intracellular bacteria, 1 μg/mL showed no difference from the control and 10 μg/mL resulted in complete clearance of intracellular bacteria after 1 hr. A concentration of 5 μg/mL resulted in 60% inhibition of the bacteria and was chosen as the exploratory dose; data not shown. DMSO concentrations for all solutions were fixed at 0.3% and made within 1 hr of the experiment. Infected cells were suspended in antibiotic solutions at 106 cells/mL immediately before use. Extracellular bacteria viability in serum- and antibiotic-free DMEM with 0.3% DMSO after 1 hr was unaffected, data not shown.
2.4.2. Electro-Antibacterial Therapy treatment
PEF treatments were delivered by a BTX ECM830 Electroporation System to a cuvette with 4 mm spacing containing the sample. EAT pulsing schemes consisted of eight 10 ms pulses delivered at field strengths of 0, 300 (N = 3, n = 9), and 450 V/cm (N = 2, n = 6). For IRE, one hundred 100 μs pulses at a field strength of 1200 V/cm and a pulse repetition rate of one pulse per second were delivered (N = 2, n = 6). Pulsing parameters are summarized in Table 1. The temperature rise of the medium was measured using a fiber optic temperature sensor (TS3 Series, Optocon by Weidmann, Dresden, Germany) during and immediately after each treatment (n = 4). Measured temperature of the medium did not exceed an average of 37 °C after pulsing for experiments performed at room temperature (~ 20 °C) for all pulsing schemes except 450 V/cm. At this high energy pulsing scheme, the peak temperature stayed above 37 °C for an average of 14 s and above the threshold for thermal tissue damage (43 °C) for an average of 6 s. The measured average temperature rise can be found in Table 1. After pulsing, the cells were incubated at 5% CO2 and 37 °C for 1 hr. After 1 hr, the cells were centrifuged at 300×g for 5 min, supernatant was aspirated, and an equivalent volume of PBS was added. A volume of 250 μL of each washed sample was plated on individual agar plates and spread evenly by agitation. The cells auto-lysed on agar allowing any surviving intracellular bacteria to grow. The agar plates are allowed to dry and placed in an anaerobic chamber for 72 hr (Fig. 1c).
Table 1.
Pulsing parameters to assess cell permeability and Electro-Antibacterial Therapy.
| Label | Electric Field | Pulse Width | Number of Pulses | Specific Treatment Energy | Average Temperature Rise |
|---|---|---|---|---|---|
|
| |||||
| 0 V/cm | 0 V/cm | 0 s | 0 | 0 J/m3 | 0 °C |
| 150 V/cm | 150 V/cm | 10 ms | 8 | 3.02E7 J/m3 | 3.5 ± 1.1 °C |
| 300 V/cm | 300 V/cm | 10 ms | 8 | 12.1E7 J/m3 | 14.6 ± 1.8 °C |
| 450 V/cm | 450 V/cm | 10 ms | 8 | 27.2E7 J/m3 | 31.1 ± 4.3 °C |
| ECT | 1200 V/cm | 100 μs | 8 | 1.94E7 J/m3 | 1.6 ± 0.2 °C |
| IRE | 1200 V/cm | 100 μs | 100 | 24.2E7 J/m3 | 9.0 ± 0.6 °C |
2.4.3. Quantification of bacteria viability and data analysis
The measure of colony forming units (CFU) for each treatment group was used to quantify the delivery of MTZ intracellularly. After 72 hr, the plates were imaged using a cell phone camera and visible colony forming units were counted using an ImageJ macro (Fig. 1d). Statistical analysis of the data was performed using an ANOVA and Tukey tests for multiple comparisons in Graphpad Prism 9.5.1 software (Dotmatics, Boston, MA). The asterisks throughout this article are representative of statistical significance and indicate the following p-values: * for p < 0.05, ** for p < 0.01, *** for p < 0.001, **** for p < 0.0001.
3. Results and discussion
3.1. Determining electric field threshold of infected and non-infected cells
Immediate PI uptake and PI uptake after 24 hr were used to quantify cell permeability and cell survival, respectively. This characterization provides insight into the electric field threshold (EFT) needed to adequately deliver antibiotics into the cell and induce cell death. Immediate PI uptake served as a quantifiable analog for antibiotic uptake at each applied field, PI having a molecular weight of 668 Da compared to MTZ whose molecular weight is 171 Da. PI uptake after 24 hr provides a basis for cell death-inducing EFTs.
To determine the immediate permeabilization and the long-term survival of the cell due to pulsing, the percentage of cells uptaking PI at two different time points (0 and 24 hr) for both infected and non-infected cells were quantified and compared. PI uptake at 0 hr indicates immediate permeabilization of the cell, while PI uptake 24 hr later indicates that the membrane of the cell is still compromised and results in cell death. For both populations, the percentage of PI+ cells increases with applied electric field strength at 0 and 24 hr. For lower field strengths, it is anticipated that for some portion of the population, the permeability of the cell population is reversible and some level of recovery will be observed; that is, the pores generated in pulsing may reseal and the cell may remain viable. For higher field strengths, the reversibility in the cell population is lost as the high field induces cell death. At 450 V/cm, approximately 99% of infected and non-infected cells are uptaking PI at 0 and 24 hr, suggesting that the electric field at 450 V/cm is inducing cell death without any observable portion of the population recovering from the field (Fig. 2a). The applied field of 450 V/cm provides maximum permeability and cell death of the 10 ms pulse conditions explored (Fig. 2b).
Fig. 2.
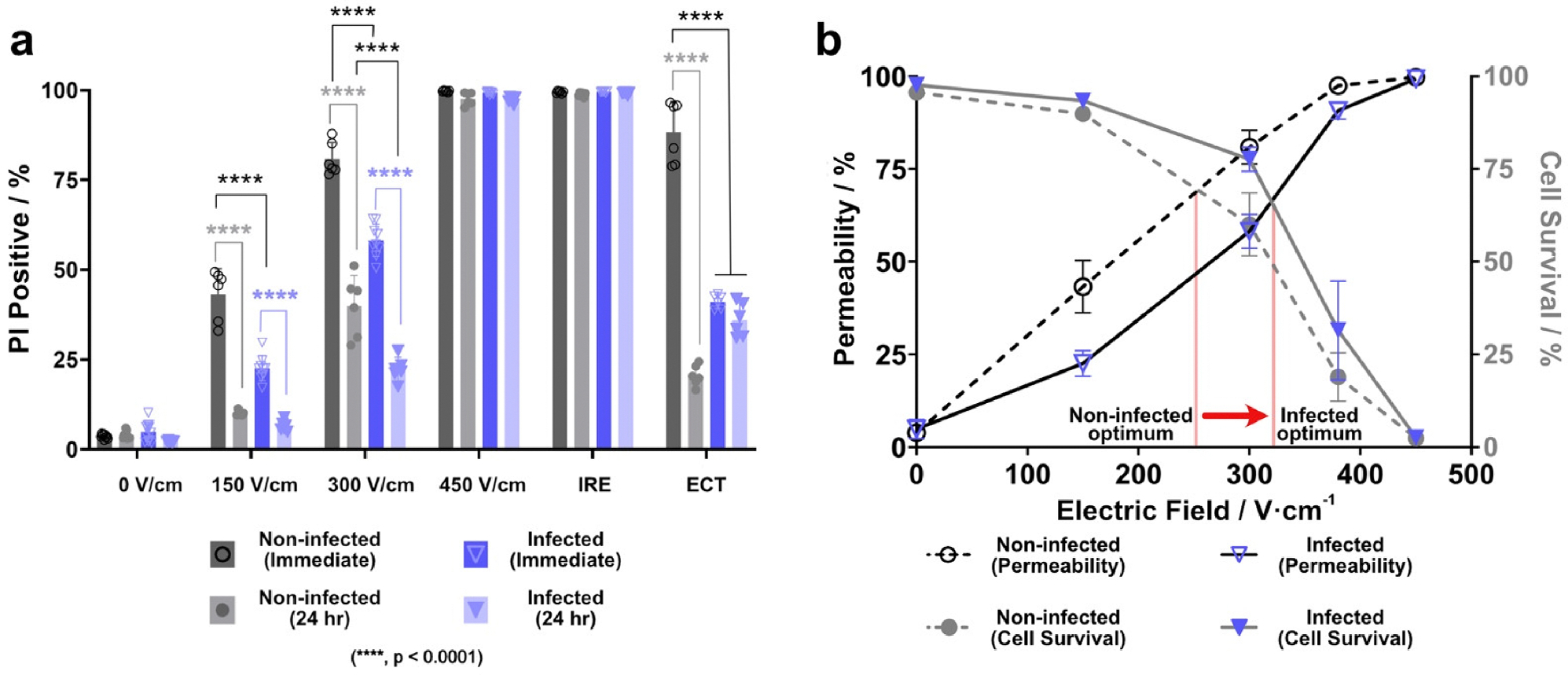
a) Percentage of the sample uptaking propidium iodide for applied field strength with 10 ms pulses, ECT, and IRE. Non-infected cells appear to be more susceptible to the field than infected cells. b) Maximum permeability and cell death occur for both infected and non-infected cells at 450 V/cm.
An irreversible regime is also observed for both cell populations experiencing an IRE pulsing scheme. IRE pulses yield a comparable 0 and 24 hr permeability to EAT pulses delivered at 450 V/cm. The permeability and cell death indicated by PI uptake is above 98% for both lower-field longer pulses and higher-field shorter pulses. Comparing applied energies, eight 10 ms pulses delivered at ~422 V/cm is equivalent to one hundred 100 μs pulses delivered at 1200 V/cm for IRE.
Reversibility can be seen for both infected and non-infected populations at lower fields such as 150 V/cm and 300 V/cm (Fig. 2a). Immediate permeabilization increased with field strength and, for a portion of the population, this permeability of the cell is not sustained at the 24 hr time point. Electro-Antibacterial Therapy regimes can be used for both intracellular microbe treatment and induction of host cell-death if applied at 450 V/cm. If optimizing drug delivery and cell viability is of interest, then delivering EAT at 300 V/cm would be more applicable for an infected cell population (Fig. 2b).
Interestingly, infected and non-infected cells yield a pronounced difference in permeability when exposed to the same field. A lower percentage of infected cells are permeabilized at applied field strengths of 150 and 300 V/cm. This suggests that infected cells might have a higher EFT to achieve the same permeability as non-infected cells and are, in general, less susceptible to the field. Changes in cell size due to infection can contribute to this effect. However, we saw no statistically significant differences between infected and noninfected cells when comparing median FSC areas, used here as a relative measurement of size (data not shown). Further, according to Fig. 2b, the point at which their permeability and cell survival curves intersect also differs between infected and non-infected cell populations. The point at which non-infected cells may have optimal cell viability and permeability occurs at ~254 V/cm, while infected cells may experience their optimal permeability and viability at a higher field of ~316 V/cm.
Another notable observation between infected and non-infected cells, as seen in Fig. 2a, is infected cells do not exhibit the anticipated recovery after pulsing as seen with non-infected cells. Non-infected cells are electropermeabilized in response to the ECT pulsing scheme and a lower PI+ population 24 hr later suggests that only a small portion of the noninfected cells are permanently damaged. Infected cells, however, have a low PI+ population in response to ECT pulses and a similar amount of PI+ cells 24 hr later. This indicates that infected cells appear to be irreversibly affected, albeit to a lesser effect than the non-infected cells, and show no obvious recovery 24 hr later. This is significant because it suggests that commonplace pulsed electric field treatments such as ECT might be significantly less effective on infected cells and subsequently leave behind cells that are likely to proliferate and migrate as a result of the microbes they are hosting. Similarly, the discrepancy between the two populations provides additional evidence that the invasion of F. nucleatum may alter the structure of the cell potentially making it resistant to certain treatments or even incognito to the immune system.
3.2. Electro-Antibacterial Therapy for intracellular bacteria
Viability of intracellular bacteria was investigated in response to antibiotics alone, electroporation alone, and a combinatorial approach of electroporation and antibiotics delivered simultaneously. Clinically, MTZ concentrations are dependent on dosage and have been found to be ~20 μg/mL in plasma when delivered intravenously and ~10 μg/mL when the same dose is given orally. Here, we choose a lower concentration (5 μg/mL) of MTZ to illustrate the effectiveness of enhanced treatment via pulsed electric fields and lower concentrations of locally-delivered antibiotics. For electroporation conditions, EAT pulses delivered at 300 V/cm (reversible EFT), and 450 V/cm and IRE at 1200 V/cm (irreversible EFTs) were evaluated for their ability to decrease intracellular bacterial viability in the presence and absence of MTZ.
In the absence of antibiotics, the bacteria viability is reduced due to the application of pulsed electric fields alone. Given that pulsed electric fields for the inactivation of bacteria are on the order of ~10 kV/cm, it is unlikely that the electric field delivered here is directly inducing death or inhibition of the bacteria. Rather, the decrease in intracellular bacteria viability is more likely to be a result of disrupted homeostasis of the host cell leading to various cell death mechanisms thought to be capable of clearing intracellular bacteria.
Likewise, MTZ alone yields a decrease in intracellular bacteria viability, even more so than pulsed electric fields alone. MTZ is typically thought to diffuse passively into bacteria, but studies have shown limited passive diffusion into mammalian cells prohibiting the drug from clearing intracellular bacteria [9]. Fig. 3 indicates that the drug is able to pass through the membrane and subsequently result in inactivation of intracellular F. nucleatum, albeit incomplete. In order to approach full clearance of intracellular bacteria, pulsed electric fields could be used to enhance transport of the drug through the cell membrane.
Fig. 3.
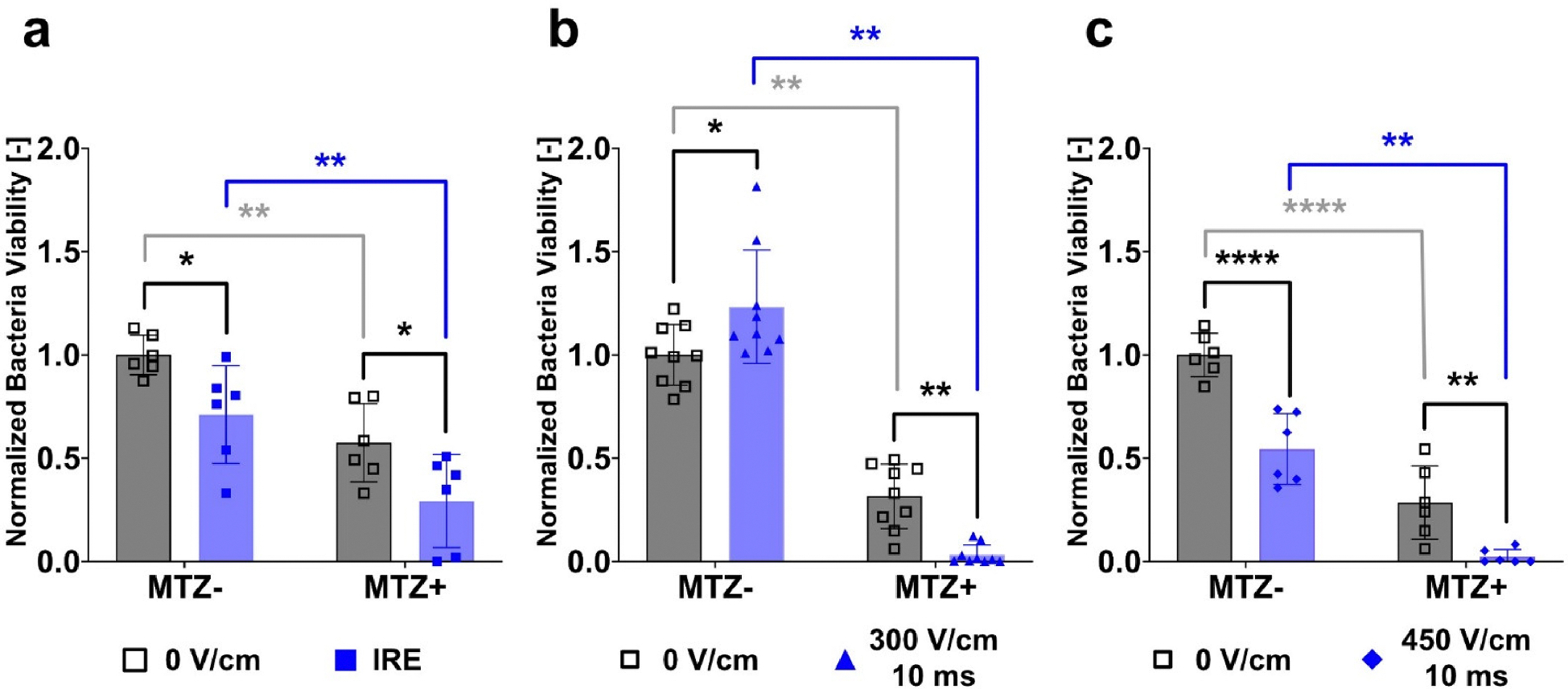
Electro-Antibacterial Therapy is an effective method for inactivating intracellular tumor-associated F. nucleatum. The quantified normalized intracellular bacteria viability due to a) IRE with or without MTZ (5 μg/mL) (N = 2, n = 6) is moderately effective at reducing bacteria viability. EAT 10 ms pulses delivered at b) 300 V/cm (N = 3, n = 9) and c) 450 V/cm (N = 2, n = 6) result in a decrease in intracellular bacteria viability with applied field or antibiotic alone, but result in maximum clearance of intracellular bacteria when used in synergy. The colony forming units quantified here are bacteria that were intracellular at the time of treatment.
As seen in Fig. 3, combinatorial therapy of EAT pulses delivered at 300 V/cm and 450 V/cm in the presence of MTZ yields the lowest levels of bacteria viability and results in significant clearance when compared to either antibiotics or pulsed electric fields alone. While MTZ and electroporation individually impacted and significantly reduced intracellular bacteria presence, the combination of the two treatments result in an efficacious clearance of ~99% of intracellular cancer-promoting microbes. Both the reversible (300 V/cm) and irreversible (450 V/cm) electric field thresholds result in effectively the same inactivation of bacteria (Fig. 3). This suggests that it is not required to permanently damage the host cell to enhance the delivery of MTZ into the cell. It is unclear whether the inactivation of F. nucleatum inside the cell at both field strengths is by the same mechanism; one could be uptaking the drug leading to inhibition of replication while the other could be disrupting the cell and releasing the intracellular bacteria into antibiotic medium. Of course, it remains possible that both treatments are effectively delivering the antibiotic into the cell and one simply also induces cell death.
While EAT pulses delivered at 450 V/cm yield the same permeability and viability as IRE pulses, the effect on intracellular bacteria differs. Irreversibly permeabilizing the host cell using standard IRE pulsing schemes does not equate to the bacterial inactivation seen using EAT pulses at 450 V/cm. While the cell is irreversibly damaged by both pulsing schemes, the data indicates that the way the cell is permeabilized will affect the delivery of MTZ into the cell and subsequent efficacy. This discrepancy between IRE and EAT at 450 V/cm indicates that lower-field longer pulses are more effective at bacterial clearance than higher-field shorter pulses. These findings suggest that standard non-thermal ablation techniques for treating solid tumors are not effective at clearing intracellular tumor-associated microbes.
The 450 V/cm pulsing scheme resulted in significant and observable electrolysis with visible bubbling in the cuvette. The use of electroporation that results in electrolysis has been shown to enhance cell death when compared to electroporation alone [27]. In this experiment, it is expected that electrolysis might play a role in the observed host cell and even intracellular bacteria death. Electrolysis, however, is not the only mechanism resulting in cell or bacteria death in this system. While cell death and bacterial clearance at 450 V/cm might be aided by electrolysis, the same level of clearance was quantified at 300 V/cm where less cell death and no electrolysis were observed. In addition, electrolysis inherently results in a current-dependent pH change at the electrodes. Rubinsky et al. also noted saline solutions within cuvettes became alkaline following all electroporation parameters tested [27]. Typical pH buffer systems found in electroporation buffers and DMEM do not have the buffering capacity to mitigate this pH change [28]. Antibiotic efficacy, degradation, and distribution can be affected by surrounding pH though MTZ is stable at a wide range of physiological pH levels [29]. More importantly, MTZ-membrane interactions can change as a result of surrounding pH, with MTZ demonstrating hydrophilic behavior at acidic pH levels and lipophilic behavior at a neutral pH [30]. Still, it remains difficult to predict how these changes to extracellular pH influence the efficacy of MTZ once intracellular.
Clinically, both intravenous and oral administration of MTZ lead to high bioavalability and peak plasma concentrations of 20 μg/mL. Systemic delivery of antibiotics, however, is also known to decimate tumor-suppressive bacteria and leads to poor gut health which has been linked to declining patient overall health and poor prognosis [31,32,33,34]. EAT resulted in significant clearance of intracellular tumor-associated Fusobacterium at a quarter of the clinical dose. By modulating the efficacy of antibiotics, EAT proposes a viable local treatment of the entire TME using fractional doses than those currently used, potentially curbing the microbiome disruptions associated with poor prognosis.
As seen in Fig. 3b, some trials indicated that the application of the pulsed electric fields alone might stimulate the growth of bacteria. In approximately 30% of the trials, the number of colony forming units increased when exposed to pulsed electric fields compared to the control. This could be attributed to a similar phenomenon seen in PEFs for bacteria growth whereby the intracellular bacteria are experiencing very low but stimulating electric fields inducing growth of the bacteria. Another possibility is the increase of available amino acids and other nutrients diffused into the host cells after permeabilization. Regardless, even when electric fields alone might have yielded growth of bacterial colonies upon culturing, the combinatorial treatment of EAT pulses in the presence of antibiotics still yielded significant clearance of the intracellular bacteria.
While the in vitro results are promising using electroporation to target cancer-promoting microbes, it is important to consider challenges that might arise when extending this work in vivo. One point of interest is the dense extracellular matrix characteristic of the pancreatic adenocarcinoma that might limit the diffusion of large molecules from reaching the core of the tumor. Similar efforts of electrochemotherapy or IRE with chemotherapeutics have been shown to have favorable outcomes for patients with locally advanced pancreatic cancer, increasing overall survival [35,36,37]. Further, antibiotics are magnitudes smaller than therapeutic agents such as bleomycin, that has been previously shown to be effective when used in conjunction with electroporation in pancreatic tumors in vivo. The antibiotic in this study, metronidazole, increases its efficacy in anaerobic conditions, which may suggest that when delivered locally, its effectiveness will increase as it penetrates the tumor.
Another important consideration regarding this treatment and its extension in vivo might be the impact of electroporation on the patient during and after treatment. The experiments performed in this work were performed at room temperature and the average temperature rise for each pulsing scheme are reported. Further work will need to be done to account for temperature rise at anatomically-relevant temperatures and tissue conductivities in order to avoid thermal damage. The pulsing schemes presented here are on par with pulsing schemes used in ongoing and completed gene electrotransfer clinical trials [38]. Also, the release of cancer-promoting microbes into the tumor microenvironment post-treatment should also be considered when expanding this work in vivo. The results presented here suggest that some intracellular bacteria can withstand electroporation treatment. If synergistic treatment is used, however, the results suggest that significant clearance can be achieved. In general, while these in vitro results are promising, further work must be done to investigate its usability, effectiveness, and challenges in vivo.
3.3. Future directions
Towards expanding this work in vivo, considerations of these pulsing schemes on the immune system will be necessary. Previous studies have shown that pulse length can dictate the cell death mechanism, damage-associated molecular pattern release, and both local and systemic immune cell recruitment. Within this study, we are maximizing the clearance of intracellular bacteria with fields that are reversible, or likely lead to a necrotic cell death mechanism. Future work will focus on translating these findings in vivo with a more holistic representation of the tumor microbiome and microenvironment.
With the identification of tumor-promoting bacteria comes the desire to control these microbiomes in vivo. Engineered bacteria, systemic antibiotics, and targeted nanoparticles have been used to this effect with varying degrees of success [39,40]. Here, we expand the toolbox to include Electro-Antibacterial Therapy, an adaptable technology that can be optimized for various antibiotics and bacterial species. While we show this technology in the context of F. nucleatum infected pancreatic cancer cells that might be found within the TME, we hypothesize that this approach can be applied to other scenarios. Intratumoral bacteria are capable of affecting tumor metabolism through bacterial-derived metabolites as well as modifying chemotherapeutics [41]. Geller et al. identified intratumoral Gammaproteobacteria capable of modifying gemcitabine, a chemotherapy used against pancreatic adenocarcinoma, thus inhibiting its cytotoxic effects on cancer cells in vitro and in vivo [42]. Recently, LaCourse et al. showed the chemotherapeutic 5-fluorouracil was a potent inhibitor of F. nucleatum and could be modified by E. coli into a nonactive form ineffective against both F. nucleatum and colorectal cancer cells [43]. The ability to control this E. coli population in vivo can impact the efficacy of 5-fluorouracil. In these cases, EAT applied at higher energies could be crucial in modulating the tumor microbiome with the additional benefit of causing irreversible electroporation to the tumor cells.
We anticipate this technology can also be applied to wound infections with deeply-seated bacterial populations which are difficult to target through traditional systemic and topical deliveries of antibiotics. EAT applied at 300 V/cm is capable of sustaining host viability, a desirable outcome in the wound environment, while reducing bacterial load. Caution is warranted when implementing this approach as further tissue damage and disruption of wound healing processes can occur. Electric fields have been investigated in the context of wound healing with the majority of work focusing on field strengths two to three orders of magnitude lower than typically used for electroporation applications [44,45]. While there is substantial evidence that low-level electric fields can produce a pro-regenerative wound environment, the same cannot be said about the PEFs investigated in this study [44,46]. Previous work by Wu et al. showed PEF’s can be used to clear Pseudomonas aeruginosa from a wound model [47]. Furthermore, the work presented here alludes to the potential of this therapy to be effective against drug-resistant bacterial species as well as other intracellular pathogenic bacteria. Small colony variants of Staphylococcus aureus can invade and persist within cells, replicating within intracellular reservoirs [48]. This phenotype can result in protection from antibiotics, similar to F. nucleatum. The use of a biophysical approach like EAT can reduce the need for tailored drug formulations and mechanisms due to its relatively indiscriminate nature of permeabilizing the entire cell population. Though molecule properties like size, charge, and stability still need to be considered, this makes it more amenable to broader use.
4. Conclusion
Intratumoral and intracellular microbes can be driving factors in the formation and progression of cancer. Until recently, these microbes have not been considered as targets for treatment. Here, we introduce Electro-Antibacterial Therapy as a modality to enhance antibiotic uptake by a malignant cancer cell hosting Fusobacterium nucleatum while also irreversibly damaging the cell. Using EAT pulsing schemes, intracellular bacteria viability decreased with independent treatment of metronidazole or pulsing. The combinatorial therapy of metronidazole and pulsing led to ~99% bacteria inhibition. We show the feasibility of EAT as a new paradigm for cancer treatment that considers the entire TME by inducing localized death of malignant cells and the microbes that drive disease progression.
Acknowledgements
The authors would like to thank Barath Udayasuryan for his contributions to the preliminary stages of this work. The authors are also grateful for the contributions of Tam Nguyen towards training bacteria handling and preparation.
Funding sources
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers P30CA012197, PO1CA207206-01, and RO1CA274439. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: The authors have a pending patent on the concept described in this article. Rafael V. Davalos has ownership interest for startup companies within the field of bioelectrics. In addition, Davalos also receives royalty income from technologies he has invented.
CRediT authorship contribution statement
Josie L. Duncan: Writing – review & editing, Writing – original draft, Visualization, Validation, Project administration, Methodology, Investigation, Formal analysis, Data curation. Raffae N. Ahmad: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. Hunter Danesi: Investigation, Formal analysis, Data curation. Daniel J. Slade: Writing – review & editing, Resources, Methodology, Investigation, Funding acquisition. Rafael V. Davalos: Writing – review & editing, Supervision, Resources, Project administration, Investigation, Funding acquisition, Conceptualization. Scott S. Verbridge: Writing – review & editing, Supervision, Resources, Project administration, Investigation, Funding acquisition, Conceptualization.
Data availability
Data will be made available on request.
References
- [1].Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, and Bray F, “Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries,” CA: A Cancer Journal for Clinicians, vol. 71, pp. 209–249, 5 2021. [DOI] [PubMed] [Google Scholar]
- [2].Baghban R, Roshangar L, Jahanban-Esfahlan R, Seidi K, Ebrahimi-Kalan A, Jaymand M, Kolahian S, Javaheri T, and Zare P, “Tumor microenvironment complexity and therapeutic implications at a glance,” Cell Communication and Signaling, vol. 18, p. 59, 12 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hanahan D, “Hallmarks of Cancer: New Dimensions,” Cancer Discovery, vol. 12, pp. 31–46, 1 2022. [DOI] [PubMed] [Google Scholar]
- [4].Yang L, Li A, Wang Y, and Zhang Y, “Intratumoral microbiota: roles in cancer initiation, development and therapeutic efficacy,” Signal Transduction and Targeted Therapy, vol. 8, p. 35, 1 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Casasanta MA, Yoo CC, Udayasuryan B, Sanders BE, Umaña A, Zhang Y, Peng H, Duncan AJ, Wang Y, Li L, Verbridge SS, and Slade DJ, “Fusobacterium nucleatum host-cell binding and invasion induces IL-8 and CXCL1 secretion that drives colorectal cancer cell migration,” Science Signaling, vol. 13, p. 9157, 7 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Udayasuryan B, Ahmad RN, Nguyen TT, Umaña A, Monét Roberts L, Sobol P, Jones SD, Munson JM, Slade DJ, Verbridge SS, Fusobacterium nucleatum induces proliferation and migration in pancreatic cancer cells through host autocrine and paracrine signaling, Sci. Signal. 15 (2022) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hayashi M, Ikenaga N, Nakata K, Luo H, Zhong P, Date S, Oyama K, Higashijima N, Kubo A, Iwamoto C, Torata N, Abe T, Yamada Y, Ohuchida K, Oda Y, and Nakamura M, “Intratumor Fusobacterium nucleatum promotes the progression of pancreatic cancer via the CXCL1-CXCR2 axis.,” Cancer science, vol. 114, pp. 3666–3678, 9 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bullman S, Pedamallu CS, Sicinska E, Clancy TE, Zhang X, Cai D, Neuberg D, Huang K, Guevara F, Nelson T, Chipashvili O, Hagan T, Walker M, Ramachandran A, Diosdado B, Serna G, Mulet N, Landolfi S, Ramon y Cajal S, Fasani R, Aguirre AJ, Ng K, Elez E, Ogino S, Tabernero J, Fuchs CS, Hahn WC, Nuciforo P, and Meyerson M, “Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer,” Science, vol. 358, pp. 1443–1448, 12 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ye P, Chang J, Foo LF, and Yap BC-M, “An early report: a modified porphyrin-linked metronidazole targeting intracellular Porphyromonas gingivalis in cultured oral epithelial cells,” International Journal of Oral Science, vol. 9, pp. 167–173, 9 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bastrup FA, Vissing M, and Gehl J, “Electrochemotherapy with intravenous bleomycin for patients with cutaneous malignancies, across tumour histology: a systematic review,” Acta Oncologica, vol. 61, pp. 1093–1104, 9 2022. [DOI] [PubMed] [Google Scholar]
- [11].Campana LG, Peric B, Mascherini M, Spina R, Kunte C, Kis E, Rozsa P, Quaglino P, Jones RP, Clover AJP, Curatolo P, Giorgione R, Cemazar M, Terlizzi F. d., Bosnjak M, and Sersa G, “Combination of Pembrolizumab with Electrochemotherapy in Cutaneous Metastases from Melanoma: A Comparative Retrospective Study from the InspECT and Slovenian Cancer Registry,” Cancers, vol. 13, p. 4289, 8 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Izzo F, Granata V, Fusco R, D’Alessio V, Petrillo A, Lastoria S, Piccirillo M, Albino V, Belli A, Tafuto S, Avallone A, Patrone R, and Palaia R, “Clinical Phase I/II Study: Local Disease Control and Survival in Locally Advanced Pancreatic Cancer Treated with Electrochemotherapy,” Journal of Clinical Medicine, vol. 10, p. 1305, 3 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Davalos RV, Mir LM, and Rubinsky B, “Tissue Ablation with Irreversible Electroporation,” Annals of Biomedical Engineering, vol. 33, pp. 223–231, 2 2005. [DOI] [PubMed] [Google Scholar]
- [14].Al-Sakere B, André F, Bernat C, Connault E, Opolon P, Davalos RV, Rubinsky B, and Mir LM, “Tumor Ablation with Irreversible Electroporation,” PLoS ONE, vol. 2, p. e1135, 11 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Aycock KN, Vadlamani RA, Jacobs EJ, Imran KM, Verbridge SS, Allen IC, Manuchehrabadi N, Davalos RV, Experimental and numerical investigation of parameters affecting high-frequency irreversible electroporation for prostate cancer ablation, J. Biomech. Eng. 144 (2022) 6. [DOI] [PubMed] [Google Scholar]
- [16].Arena CB, Sano MB, Rossmeisl JH, Caldwell JL, Garcia PA, Rylander MN, and Davalos RV, “High-frequency irreversible electroporation (H-FIRE) for non-thermal ablation without muscle contraction,” BioMedical Engineering OnLine, vol. 10, p. 102, 12 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Aycock KN and Davalos RV, “Irreversible Electroporation: Background, Theory, and Review of Recent Developments in Clinical Oncology,” Bioelectricity, vol. 1, pp. 214–234, 12 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mahnič-Kalamiza S, Vorobiev E, and Miklavčič D, “Electroporation in Food Processing and Biorefinery,” The Journal of Membrane Biology, vol. 247, pp. 1279–1304, 12 2014. [DOI] [PubMed] [Google Scholar]
- [19].Arshad RN, Abdul-Malek Z, Roobab U, Munir MA, Naderipour A, Qureshi MI, El-Din Bekhit A, Liu Z-W, and Aadil RM, “Pulsed electric field: A potential alternative towards a sustainable food processing,” Trends in Food Science & Technology, vol. 111, pp. 43–54, 5 2021. [Google Scholar]
- [20].Vadlamani RA, Dhanabal A, Detwiler DA, Pal R, McCarthy J, Seleem MN, and Garner AL, “Nanosecond electric pulses rapidly enhance the inactivation of Gram-negative bacteria using Gram-positive antibiotics.,” Applied microbiology and biotechnology, vol. 104, pp. 2217–2227, 3 2020. [DOI] [PubMed] [Google Scholar]
- [21].Vadlamani A, Detwiler DA, Dhanabal A, and Garner AL, “Synergistic bacterial inactivation by combining antibiotics with nanosecond electric pulses,” Applied Microbiology and Biotechnology, vol. 102, pp. 7589–7596, 9 2018. [DOI] [PubMed] [Google Scholar]
- [22].Lovšin Ž, Klančnik A, Kotnik T, Electroporation as an efficacy potentiator for antibiotics with different target sites, Front. Microbiol. 12 (2021) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ma S, Schroeder B, Sun C, Loufakis DN, Cao Z, Sriranganathan N, Lu C, Electroporation-based delivery of cell-penetrating peptide conjugates of peptide nucleic acids for antisense inhibition of intracellular bacteria, Integr. Biol. 6 (10) (2014) 973–978. [DOI] [PubMed] [Google Scholar]
- [24].Pitt JM, Marabelle A, Eggermont A, Soria J-C, Kroemer G, and Zitvogel L, “Targeting the tumor microenvironment: removing obstruction to anticancer immune responses and immunotherapy.,” Annals of oncology : official journal of the European Society for Medical Oncology, vol. 27, pp. 1482–92, 8 2016. [DOI] [PubMed] [Google Scholar]
- [25].Nejman D, Livyatan I, Fuks G, Gavert N, Zwang Y, Geller LT, Rotter-Maskowitz A, Weiser R, Mallel G, Gigi E, Meltser A, Douglas GM, Kamer I, Gopalakrishnan V, Dadosh T, Levin-Zaidman S, Avnet S, Atlan T, Cooper ZA, Arora R, Cogdill AP, Khan MAW, Ologun G, Bussi Y, Weinberger A, Lotan-Pompan M, Golani O, Perry G, Rokah M, Bahar-Shany K, Rozeman EA, Blank CU, Ronai A, Shaoul R, Amit A, Dorfman T, Kremer R, Cohen ZR, Harnof S, Siegal T, Yehuda-Shnaidman E, Gal-Yam EN, Shapira H, Baldini N, Langille MGI, Ben-Nun A, Kaufman B, Nissan A, Golan T, Dadiani M, Levanon K, Bar J, Yust-Katz S, Barshack I, Peeper DS, Raz DJ, Segal E, Wargo JA, Sandbank J, Shental N, and Straussman R, “The human tumor microbiome is composed of tumor type–specific intracellular bacteria,” Science, vol. 368, pp. 973–980, 5 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Xiao Y and Yu D, “Tumor microenvironment as a therapeutic target in cancer,” Pharmacology & Therapeutics, vol. 221, p. 107753, 5 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rubinsky L, Guenther E, Mikus P, Stehling M, and Rubinsky B, “Electrolytic Effects During Tissue Ablation by Electroporation,” Technology in Cancer Research & Treatment, vol. 15, pp. NP95–NP103, 10 2016. [DOI] [PubMed] [Google Scholar]
- [28].Li Y, Wu M, Zhao D, Wei Z, Zhong W, Wang X, Liang Z, and Li Z, “Electroporation on microchips: the harmful effects of pH changes and scaling down,” Scientific Reports, vol. 5, p. 17817, 12 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Erah P, “The stability of amoxycillin, clarithromycin and metronidazole in gastric juice: relevance to the treatment of Helicobacter pylori infection,” Journal of Antimicrobial Chemotherapy, vol. 39, pp. 5–12, 1 1997. [DOI] [PubMed] [Google Scholar]
- [30].Lopes-de Campos D, Nunes C, Sarmento B, Jakobtorweihen S, and Reis S, “Metronidazole within phosphatidylcholine lipid membranes: New insights to improve the design of imidazole derivatives,” European Journal of Pharmaceutics and Biopharmaceutics, vol. 129, pp. 204–214, 8 2018. [DOI] [PubMed] [Google Scholar]
- [31].McKee AM, Kirkup BM, Madgwick M, Fowler WJ, Price CA, Dreger SA, Ansorge R, Makin KA, Caim S,Le Gall G, Paveley J, Leclaire C, Dalby M, Alcon-Giner C, Andrusaite A, Feng T-Y, Di Modica M, Triulzi T, Tagliabue E, Milling SW, Weilbaecher KN, Rutkowski MR, Korcsḿ aros T, Hall LJ, and Robinson SD, “Antibiotic-induced disturbances of the gut microbiota result in accelerated breast tumor growth,” iScience, vol. 24, p. 103012, 9 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rooks MG and Garrett WS, “Gut microbiota, metabolites and host immunity,” Nature Reviews Immunology, vol. 16, pp. 341–352, 6 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zitvogel L, Galluzzi L, Viaud S, Vétizou M, Daillère R, Merad M, Kroemer G, Cancer and the gut microbiota: An unexpected link, Sci. Transl. Med. 7 (2015) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wu N, Yang X, Zhang R, Li J, Xiao X, Hu Y, Chen Y, Yang F, Lu N, Wang Z, Luan C, Liu Y, Wang B, Xiang C, Wang Y, Zhao F, Gao GF, Wang S, Li L, Zhang H, and Zhu B, “Dysbiosis Signature of Fecal Microbiota in Colorectal Cancer Patients,” Microbial Ecology, vol. 66, pp. 462–470, 8 2013. [DOI] [PubMed] [Google Scholar]
- [35].Granata V, Fusco R, Setola SV, Piccirillo M, Leongito M, Palaia R, Granata F, Lastoria S, Izzo F, Petrillo A, Early radiological assessment of locally advanced pancreatic cancer treated with electrochemotherapy, World J. Gastroenterol. 23 (26) (2017) 4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Tafuto S, von Arx C, De Divitiis C, Tracey Maura C, Palaia R, Albino V, Fusco R, Membrini M, Petrillo A, Granata V, and Izzo F, “Electrochemotherapy as a new approach on pancreatic cancer and on liver metastases,” International Journal of Surgery, vol. 21, pp. S78–S82, 9 2015. [DOI] [PubMed] [Google Scholar]
- [37].Probst U, Fuhrmann I, Beyer L, and Wiggermann P, “Electrochemotherapy as a New Modality in Interventional Oncology: A Review,” Technology in Cancer Research & Treatment, vol. 17, p. 153303381878532, 1 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Heller R, Heller LC, Gene electrotransfer clinical trials, Adv. Genet. 89 (2015) 235–262. [DOI] [PubMed] [Google Scholar]
- [39].Gao C, Wang X, Yang B, Yuan W, Huang W, Wu G, and Ma J, “Synergistic Target of Intratumoral Microbiome and Tumor by Metronidazole–Fluorouridine Nanoparticles,” ACS Nano, vol. 17, pp. 7335–7351, 4 2023. [DOI] [PubMed] [Google Scholar]
- [40].Gurbatri CR, Arpaia N, and Danino T, “Engineering bacteria as interactive cancer therapies,” Science, vol. 378, pp. 858–864, 11 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhou X, Kandalai S, Hossain F, Zheng Q, Tumor microbiome metabolism: A game changer in cancer development and therapy, Front. Oncol. 12 (2022) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Geller LT, Barzily-Rokni M, Danino T, Jonas OH, Shental N, Nejman D, Gavert N, Zwang Y, Cooper ZA, Shee K, Thaiss CA, Reuben A, Livny J, Avraham R, Frederick DT, Ligorio M, Chatman K, Johnston SE, Mosher CM, Brandis A, Fuks G, Gurbatri C, Gopalakrishnan V, Kim M, Hurd MW, Katz M, Fleming J, Maitra A, Smith DA, Skalak M, Bu J, Michaud M, Trauger SA, Barshack I, Golan T, Sandbank J, Flaherty KT, Mandinova A, Garrett WS, Thayer SP, Ferrone CR, Huttenhower C, Bhatia SN, Gevers D, Wargo JA, Golub TR, and Straussman R, “Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine,” Science, vol. 357, pp. 1156–1160, 9 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].LaCourse KD, Zepeda-Rivera M, Kempchinsky AG, Baryiames A, Minot SS, Johnston CD, and Bullman S, “The cancer chemotherapeutic 5-fluorouracil is a potent Fusobacterium nucleatum inhibitor and its activity is modified by intratumoral microbiota,” Cell Reports, vol. 41, p. 111625, 11 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Rajendran SB, Challen K, Wright KL, and Hardy JG, “Electrical Stimulation to Enhance Wound Healing,” Journal of Functional Biomaterials, vol. 12, p. 40, 6 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Xu J, Jia Y, Huang W, Shi Q, Sun X, Zheng L, Wang M, Li P, and Fan Y, “Non-contact electrical stimulation as an effective means to promote wound healing,” Bioelectrochemistry, vol. 146, p. 108108, 8 2022. [DOI] [PubMed] [Google Scholar]
- [46].Hlavac N, Bousalis D, Ahmad RN, Pallack E, Vela A, Li Y, Mobini S, Patrick E, and Schmidt CE, “Effects of Varied Stimulation Parameters on Adipose-Derived Stem Cell Response to Low-Level Electrical Fields,” Annals of Biomedical Engineering, vol. 49, pp. 3401–3411, 12 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wu M, Rubin AE, Dai T, Schloss R, Usta OB, Golberg A, and Yarmush M, “High-Voltage, Pulsed Electric Fields Eliminate Pseudomonas aeruginosa Stable Infection in a Mouse Burn Model,” Advances in Wound Care, vol. 10, pp. 477–489, 9 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Fraunholz M and Sinha B, “Intracellular staphylococcus aureus: Live-in and let die,” Frontiers in Cellular and Infection Microbiology, vol. 2, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.


