SUMMARY
Aging is underpinned by pronounced metabolic decline; however, the drivers remain obscure. Here we report that IgG accumulates during aging, particularly in white adipose tissue (WAT), to impair adipose tissue function and metabolic health. Caloric restriction (CR) decreases IgG accumulation in WAT, while replenishing IgG counteracts CR’s metabolic benefits. IgG activates macrophages via Ras signaling and consequently induces fibrosis in WAT through the TGF-β/SMAD pathway. Consistently, B cell null mice are protected from aging-associated WAT fibrosis, inflammation, and insulin resistance, unless exposed to IgG. Conditional ablation of the IgG recycling receptor, neonatal Fc receptor (FcRn), in macrophages prevents IgG accumulation in aging, resulting in prolonged healthspan and lifespan. Further, targeting FcRn by antisense oligonucleotide restores WAT integrity and metabolic health in aged mice. These findings pinpoint IgG as a hidden culprit in aging and enlighten a novel strategy to rejuvenate metabolic health.
Keywords: Aging, IgG, adipose tissue, fibrosis, metabolic dysfunction
eTOC blurb
In this study, Yu et al. demonstrate that IgG accumulates during aging, inducing adipose tissue fibrosis and metabolic dysfunction. Preventing IgG build-up by targeting the recycling receptor FcRn increases lifespan and improves the metabolic health of aged mice.
Graphical abstract

INTRODUCTION
Advances in science and technology have significantly extended human lifespan, but simultaneously aggravated age-associated complications. Age is a prominent risk factor for obesity, insulin resistance, type 2 diabetes, dyslipidemia, liver steatosis, etc.1,2 These metabolic conditions, in turn, dramatically impair human health and eventually lifespan.3 Multiple signaling pathways have been linked with the molecular signature of aging under the rubric of insulin/insulin-like growth factor-1 (IGF-1)/forkhead-box O transcription factor (FoxO; DAF-16 in C. elegans),4–6 mammalian target of rapamycin (mTOR),7,8 Sirtuins,9,10 and AMP-activated protein kinase (AMPK).11,12 These classic longevity pathways are all crucial for metabolic regulation, implying a metabolic basis of aging. However, The mechanistic details connecting metabolic dysregulation and aging remain poorly understood.
Adipose tissue is the primary site of energy storage, and maintaining its integrity is vital to energy homeostasis and metabolic health – processes often perturbed in aging. Indeed, adipose tissue is one of the few organs where organ-restricted intervention is sufficient to impact both lifespan and healthspan.13 Most longevity interventions in rodents are associated with alterations of adipose tissue function; examples include calorie restriction (CR),14 rapamycin treatment,15,16 surgical removal of visceral fat,17 and elimination of senescent cells.18 Age-associated transcriptomic changes occur earlier and with a greater magnitude in adipose tissue compared to other major metabolic organs,19 implying that adipose tissue could be driving at least a subset of the metabolic derangements in aging. Concurrent with aging, adipose tissue develops phenotypic hallmarks, including adipocyte hypertrophy, chronic inflammation, fibrosis, insulin resistance, and compromised adipogenesis, all of which are closely correlated with metabolic dysfunction.20–22 Nevertheless, the pathological remodeling of adipose tissue in aging remains an enigma, and a driving factor that could underlie multiple hallmarks is desired.
Antibodies, in the form of immunoglobulins (Igs), are the preeminent effectors for adaptive immune defense against pathogens. Among the five primary classes of antibodies, IgG accounts for ~80% of total serum antibodies. IgG consists of an antigen-binding fragment (Fab) and an Fc fragment. In the canonical adaptive immune response, B cells are activated in the presence of an antigen and produce specific antibodies to neutralize it through Fab, while simultaneously interacting with Fc gamma receptors (FcγRs) on innate immune cells to trigger antibody-dependent cytotoxicity and destroy antigen-containing pathogens through endosomal internalization.23,24 Unlike IgA or IgM, IgG has an exceptionally long half-life because it has a unique recycling mechanism. IgG is internalized into endosomes upon binding to its receptors (FcγRs). As the endosomes acidify during maturation, IgG is transferred from FcγRs to the neonatal Fc receptor (FcRn), the sole IgG recycling receptor. FcRn then recycles and releases IgG out of the plasma membrane at a neutral pH, while the unbound IgG is sorted for lysosomal degradation.25 Beyond the classic immune function, the role of IgG in metabolism is not appreciated. Of note, IgG is primarily considered a plasma protein, and its tissue presence, regulation, and function in the context of aging, if any, are unknown.
Here, we delved into the pathogenic remodeling of adipose tissue in aging to understand metabolic decline with age. We unexpectedly discovered a remarkable age-associated accumulation of IgG in white adipose tissue (WAT). Using in vitro and complementary in vivo models, we demonstrate that IgG is a driving factor of adipose tissue degeneration and metabolic decline in aging, and, therefore, an emerging therapeutic target to extend healthspan.
RESULTS
IgG is an age-associated factor
The most significant age-related transcriptomic changes in mice occur in the epididymal white adipose tissue (eWAT).19 However, there are possible discrepancies in gene expression caused by loss of proteostasis in aging.26 To better understand the age-associated pathologic remodeling of adipose tissue, we performed a quantitative proteomic comparison of eWAT from young (3-month-old) and aged (33-month-old) mice. Of the 1110 proteins identified in the 50 kD gel slice region that displayed the most noticeable difference according to Coomassie blue staining, there were 93 proteins within the size range between 35–60 kDa showing over 2-fold enrichments in aging, and 45 of them were immunoglobulin proteins (Figures 1A, 1B and Table S1). We first confirmed this accumulation by immunohistochemistry staining of the dominant Ig component IgG in the aged eWAT (Figure 1C). The accumulation of IgG in eWAT is age-associated, becoming noticeable at 6 months old and increasing further at 18 months old (Figures 1D, and S1A). Plasma IgG increased in parallel during aging in both male and female mice (Figures 1D, and S1A-C). Indeed, IgG accumulated across all examined tissues in aging, including subcutaneous inguinal WAT (iWAT), interscapular brown adipose tissue (BAT), liver, and kidney (Figure S1D), but the highest accumulation was in WAT (Figures 1E and S1E-F). The accumulated IgG proteins in aged eWAT consisted of all major subtypes, including IgG1, IgG2a, and IgG3, together with Ig isotypes IgM and IgA, suggesting polyclonal responses (Figure S1G). Although IgM and IgA increased in aging, unlike IgG, IgM is too large to diffuse into intercellular tissue fluid efficiently, and IgA is predominantly found in external secretions such as breast milk. Therefore, we focused on IgG in the context of adipose tissue. To learn the timing of IgG accumulation, we examined tissues at earlier ages and found that IgG started to accumulate in WAT at 4 months old, whereas only minimal levels were detected in the liver and muscle at even 9 months old (Figure S1H). Furthermore, this aging-associated accumulation of IgG was recapitulated in humans by western blotting analysis of human epicardial fat biopsies (Figure 1F). The IgG levels correlate with age but inversely correlate with the expression of adipocyte functional markers Pparg1, Pparg2, and Leptin (Figures 1G and S1I), implicating a detrimental effect.
Figure 1. IgG is preferentially accumulated in adipose tissue during aging.
(A) Coomassie blue staining of SDS-PAGE of total protein extracted from young (3 mon) and aged (33 mon) male mouse eWAT. Red bracket indicates the ~1 cm slice (showing the most significant changes at this region) for mass spectrometry analysis.
(B) Flow graph summary of mass spectrometric identification of immunoglobulin (Ig) proteins enriched in aged eWAT. The full list of significant differentially expressed peptides is provided in Table S1.
(C) Immunohistochemical (IHC) staining and quantification of IgG in eWAT from young and aged male mice. Scale bar, 100 μm, (n=5, 5).
(D) Western blot (WB) analysis of IgG heavy (H) and light (L) chains in eWAT protein extracts and plasma samples from C57BL/6J mice during aging. Ponceau red staining band was used as the loading control for the plasma samples. Mice were perfused to clear blood in the circulation before harvesting tissues.
(E) WB of IgG across different tissues from 33-month-old male mice. HSP90 was used as a loading control. Mice were perfused to clear blood in the circulation before harvesting tissues.
(F) WB analysis of IgG in human epicardial fat at the indicated ages.
(G) The correlations of human IgG (heavy chain) levels to age (quantifications of WB in 1F).
(H) Transcriptomic analyses reveal the top progressively increased (top) and decreased (bottom) biological processes (BPs) in eWAT during aging of C57BL/6J male mice. The complete BP list is provided in Figure S2.
See also Figures S1, S2, and Table S1.
Transcriptomic analyses of eWAT during aging indicate that the primary biological processes (BPs) that increase with age are immune pathways, particularly those involved in B cell development and activation, immunoglobulin production, and inflammatory responses (Figures 1H and S2A). On the other hand, BPs that progressively decrease with age are tightly associated with energy metabolism and adipocyte differentiation (Figures 1H and S2B). These results collectively indicate an inverse association of IgG levels with adipose tissue function in aging.
The metabolic improvement by caloric restriction is mediated through reducing IgG
Caloric Restriction (CR) is a common and effective anti-aging intervention.27 In 12-month-old C57BL/6J male mice, 4 weeks of CR improved insulin sensitivity (Figure S3A) and selectively decreased circulating IgG levels (by 60%) without affecting IgA or IgM levels (Figures 2A and 2B). CR specifically decreased IgG accumulation in eWAT, iWAT, and liver (Figures 2C, 2D, and S3B). This reduction in IgG in eWAT was associated with the upregulation of SirT1 and adipogenic genes (Pparg, Cebpa, and Srebf1), as well as repression of inflammation (Mcp1, Tnfa, and Il-6) and senescence markers (p16, p21, p53) (Figures 2E and S3C). These results reinforce IgG’s inverse correlation with adipose tissue function and further suggest that IgG accumulation can be interdicted.
Figure 2. The metabolic improvements by calorie restriction in aging are mediated through reducing IgG.
(A-E) 12-month-old male C57BL/6J mice were subjected to 30% caloric restriction (CR) for 4 weeks. (A-B) WB of plasma IgG, IgA, and IgM levels in ad libitum (ad lib) and CR mice (A) and quantifications (B). ***p<0.001 for CR vs ad lib control by 2-tailed student t-test (n=6, 6).
(C-D) WB analysis (C) and quantification (D) of IgG and SirT1 in protein lysates of eWAT and iWAT from ad lib and CR mice. HSP90 was used as the loading control, (n=6, 6).
(E) qPCR analysis of gene expression (arbitrary units, AU) in eWAT of a different cohort of 4-week CR and ad lib mice (n=8, 8).
(F-H) 5-month-old male C57BL/6J mice were intraperitoneally (i.p.) administered 3 mg mouse total IgG or vehicle (Veh) per week for 4 weeks since the beginning of CR. The regularly fed mice were included as the ad libitum control group.
(F) WB analysis of IgG in eWAT from mice treated with ad lib, CR-Veh and CR-IgG.
(G) qPCR analysis of eWAT gene expression from all three groups of mice (n=5, 8, 7).
(H) Insulin tolerance test (ITT) and the area under curve (AUC) after 3-wk treatment (n=5, 8, 7).
Data are presented as mean ± SEM; *p<0.05, **p<0.01 vs CR-Veh group by 2-tailed student t-test or one-way ANOVA.
See also Figure S3.
To assess any role of IgG in CR’s metabolic effects, we administered mouse total IgG via intraperitoneal (i.p.) injection to CR mice (5-month-old) to replenish IgG. Interestingly, the exogenous IgG predominantly accumulated in adipose tissues (eWAT and iWAT), compared with plasma and liver (Figures 2F and S3D-E). This elevated IgG restored the CR-suppressed inflammatory markers F4/80, Mcp1, and Tnfa and senescence genes p19, p21, and p53 in eWAT (Figure 2G). Moreover, IgG treatment attenuated the CR-induced expression of adipogenic genes Pparg and Srebf1, accompanied by repression of Adipoq (Figure 2G). Importantly, the insulin tolerance test revealed that IgG treatment counteracted the potent insulin-sensitizing function of CR without affecting body weight (Figures 2H and S3F). These data provide direct evidence of a pathogenic role of IgG in adipose tissue function and metabolic health.
IgG promotes adipose tissue fibrosis in aging
We further asked how IgG triggers adipose tissue degeneration in aging. Fibrosis is increasingly appreciated as a driving force for compromised adipocyte regeneration, insulin resistance, inflammation, and metabolic dysfunction in obesity.28 In aged mice, adipose tissue develops significant fibrosis (Figure 3A) that coincides with the accumulation of IgG and infiltration of macrophages (Figure 3B). The most prominent signaling pathway that triggers fibrosis is TGF-β-mediated phosphorylation of SMAD2/3 (Figure 3C).29 Supportively, SMAD2/3 phosphorylation was markedly induced in the eWAT of aged mice (Figure 3D). Conversely, CR prevalently suppressed fibrogenic genes and Tgfb in eWAT (Figure S4A), accompanied by inhibited SMAD2/3 phosphorylation (Figure S4B).
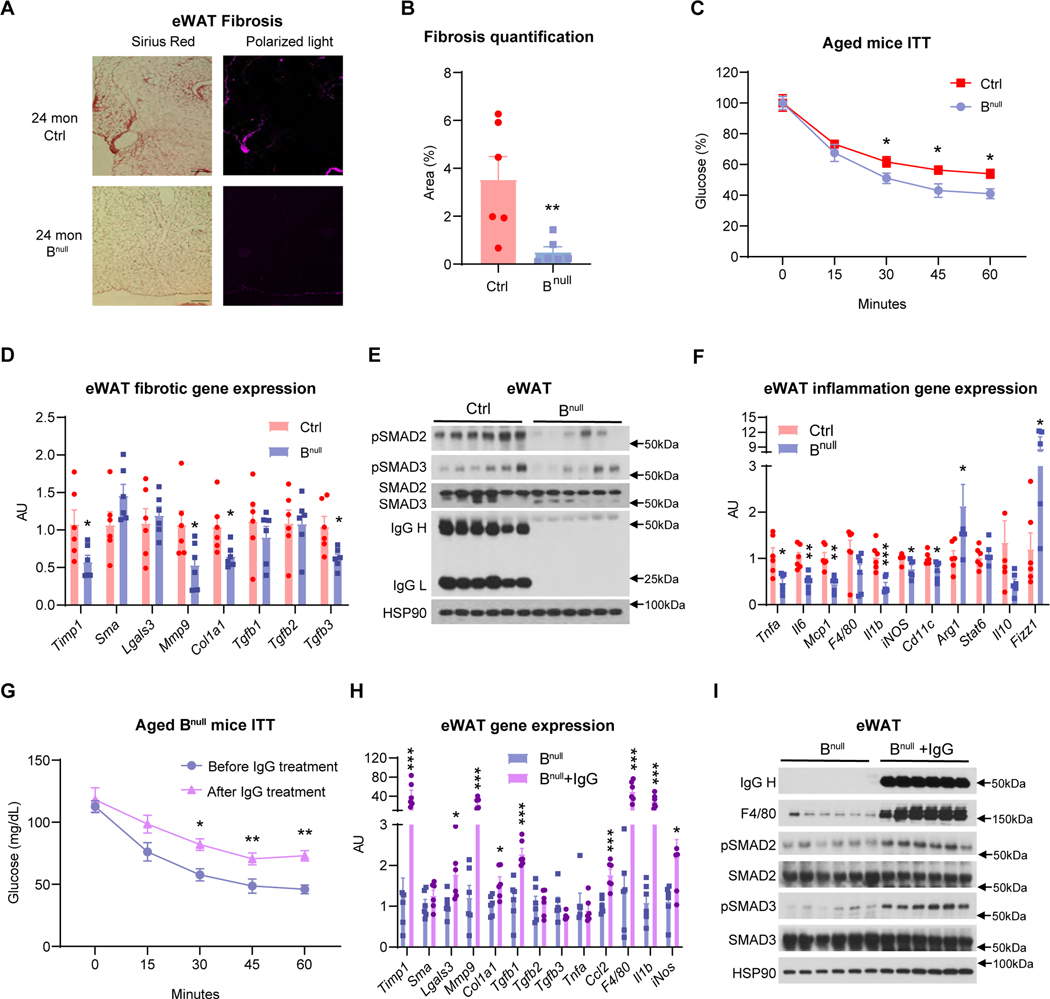
Figure 3. IgG activates macrophages to promote adipose tissue fibrosis.
(A) Picrosirius red staining of eWAT fibrosis in young (3-mon) and aged (33-mon) mice imaged by bright field and polarized-light microscopy and fibrosis area quantification (n=10, 10). Scale bar, 50 μm.
(B) Immunostaining of IgG, SMA, CD68, and DAPI of eWAT sections from young and aged mice. Scale bar, 50 μm.
(C) Schematic diagram of the TGF-β/SMAD signaling pathway to activate fibrotic response.
(D) WB analysis of phospho-SMAD2 and phospho-SMAD3 in the eWAT of young and aged mice. HSP90 was used as the loading control.
(E-G) Young C57BL/6J mice were i.p. injected with mouse total IgG (3 mg weekly) for 4 weeks.
(E) WB analysis of SMADs phosphorylation in the eWAT.
(F) qPCR analysis of fibrotic gene expression in the eWAT from Veh- or IgG-treated mice (n=5, 5).
(G) Enrichment of biological processes by IgG treatment in eWAT. The biological processes influenced by IgG treatment in eWAT were analyzed by DEG (Differentially Expressed Genes). The significant changed signal pathways were plotted for IgG treat vs. Vehicle. The top 15 significant biological processes (BPs) were ranked according to their FDR, and redundant terms were removed u REVIGO.
(H-I) Mouse BMDMs were treated with 200 μg/mL IgG for 24 hours, and RNA was extracted for RNA-seq and qPCR analysis
(H) Enriched BPs by IgG treatment in BMDMs. The BPs affected by IgG treatment in BMDM were analyzed by DEG. The significant changed signal pathways were plotted for IgG treat vs. Vehicle. The top 15 significant biological processes were ranked according to their FDR, and redundant terms were removed by REVIGO.
(I) qPCR analysis of the expression of inflammatory markers and Tgfb isoforms in BMDMs (n=3, 3).
(J) qPCR analysis of fibrotic genes’ expression in 3T3-L1 preadipocytes treated with conditioned media (CM) collected from vehicle- (Veh-CM) or IgG-treated (IgG-CM) BMDMs. 5 μM TGF-βR inhibitor LY2109761 or 10 ng/mL TGF-β was added as control treatments (n=3/group). *p<0.05, **p<0.01, ***P<0.001 vs Veh-CM; $ p<0.05, $ $ p<0.01, $ $ $ p<0.001 for IgG-CM + LY2109761 vs IgG-CM.
(K) WB analysis of TGF-β signaling in the CM-treated 3T3-L1 preadipocytes.
(L) WB analysis of ERK and MEK phosphorylation in BMDMs treated with 200 μg/mL IgG, 50 ng/mL LPS (proinflammatory activation), or 50 ng/mL IL-4 (alternative activation) for 24 hours.
(M) Ras activity was measured in the BMDMs treated with 200 μg/mL IgG or 50 ng/mL LPS for 24 hours. 50 ng/mL EGF was used as the positive control for Ras activation (n=3/group).
(N) ELISA determination of TGF-β production in the CM of Vehicle (Veh), IgG-, boiled IgG-, and IgG-ERK inhibitor- (1Um) treated BMDMs (n=6/group).
Data are presented as mean ± SEM, *p<0.05, **p<0.01, ***p<0.001 for the treatment group vs Vehicle group by 2-tailed student t-test or one-way ANOVA analysis.
See also Figure S4.
The correlations of adipose IgG and fibrosis in both aging and CR prompted us to test a fibrotic role of IgG in adipose tissue. To this end, we administered 3 mg mouse total IgG to young mice weekly to mimic its accumulation in aging. Four weeks of IgG treatment was sufficient to increase phosphorylation of SMAD2 and SMAD3 (Figure 3E) and stimulate fibrotic gene expression in eWAT, including Timp1, Mmp9, Col1a1, and Lgals3 together with Tgfb genes (Figure 3F). As such, IgG is uncovered as a causal factor of adipose tissue fibrosis in aging.
IgG activates macrophages to stimulate the fibrotic response
To understand this surprising fibrotic function of IgG, we first tested its direct effect on adipocyte progenitor cells but failed to activate the SMAD2/3 pathway (Figure S4C) or induce fibrotic genes in 3T3-L1 preadipocytes (Figure S4D), implying an indirect mechanism. RNA-seq analysis of eWAT from vehicle- or IgG-treated young mice revealed the top-upregulated BP in IgG-treated eWAT was the immune response (Figure 3G), as demonstrated by a 4.5-fold enrichment of differentially expressed genes (DEG) within inflammatory pathways compared to the background (Figure S4E). IgG is known to neutralize pathogens and induce inflammatory responses through targeting immune cells. Given the correlations of IgG and macrophages in WAT, we reasoned that macrophages might be the mediator of IgG. Indeed, IgG treatment directly activated bone marrow-derived monocytes (BMDMs) as demonstrated by the enriched BPs in inflammatory and metabolic responses from transcriptomic analyses (Figure 3H). IgG treatment potently activated inflammatory markers and simultaneously induced Tgfb genes (Figures 3I and S4F). Macrophages are appreciated as a major source of TGF-β.30 For functional test, we treated 3T3-L1 preadipocytes with conditioned media (CM) from Vehicle- or IgG-treated BMDMs. The representative fibrotic genes Sma, Timp1, Mmp9, Col3a1, and Lgals3 in 3T3-L1 cells were substantially induced by CM from IgG-treated BMDMs, largely mimicking TGF-β treatment; such effects are TGF-β-dependent as they were abrogated by TGF-β receptor (TGFβR) inhibitor LY2109761 (Figure 3J). Supportively, CM collected from IgG-treated BMDMs increased SMAD2 and SMAD3 phosphorylation in 3T3-L1 cells, which was blunted by the addition of LY2109761 (Figure 3K).
Next, we sought to understand the activation of macrophages by IgG. The MEK/ERK pathway is crucial for the pro-inflammatory activation of macrophages.31 The Toll-Like Receptor 4 (TLR4) ligand lipopolysaccharide (LPS) activated phosphorylation of MEK/ERK, while the anti-inflammatory activation by IL-4 blunted this effect (Figure 3L). Here we found that IgG treatment functioned similarly as LPS to induce MEK/ERK phosphorylation (Figure 3L). Consistently, ERK1/ERK2 pathway was among the top-induced BPs by IgG treatment in eWAT and BMDMs (Figures 3G, 3H). The classic MEK/ERK activation is mediated through the upstream Ras signaling and eventually converges with the TLR4 pathway (Figure S4G).32 Interestingly, IgG induced 4-fold Ras activation in BMDMs, in contrast to the more immediate effect of LPS (Figure 3M). Ras activation by IgG is likely mediated by its receptors FcγRs. Upon binding with IgG, FcγR clustering induces the activation of Src/Syk kinases, which triggers Ras activity (Figure S4G).33,34 Lastly, IgG treatment directly increased the amount of secreted TGF-β in the CM from BMDMs, while boiled IgG lost this effect (Figure 3N), indicating that native IgG is required to activate TGF-β production. Moreover, inhibition of ERK also abolished IgG’s effect (Figure 3N). Collectively, these data suggest that IgG activates macrophages through the Ras/MEK/ERK signaling pathway, stimulating TGF-β production to induce fibrogenesis in the adipose tissue microenvironment.
B cell null mice are protected from aging-associated adipose tissue fibrosis
To determine whether IgG is required for the aging-associated development of adipose tissue fibrosis, we employed B cell null (Bnull) mice, which are deprived of IgG together with IgA and IgM.35 24-month-old Bnull mice developed much less fibrosis in eWAT than the control mice (Figures 4A, 4B), and shown improved insulin sensitivity (Figure 4C). The reduced fibrosis was further confirmed by their decreased expression of fibrogenic genes, including Timp1, Col1a1, Mmp9, and Tgfb3 (Figure 4D). As expected, with the depletion of IgG, phosphorylation of SMAD2/3 was diminished in the Bnull eWAT (Figure 4E). In line with the stimulation of adipose tissue inflammation by IgG administration, Bnull mice showed alleviated immune cell infiltration (F4/80 and Cd11c) and inflammation (Tnfa, Il6, Mcp1, and Il1b) in eWAT (Figure 4F). On the other hand, macrophage anti-inflammatory genes Arg1 and Fizz1 were upregulated (Figure 4F). To directly assess any causal role of IgG in metabolic dysregulation in aged conditions, we administered mouse total IgG for 4 weeks to aged Bnull mice to replenish the circulating IgG. The 4-week IgG treatment offset the insulin-sensitizing effect in aged Bnull mice (Figure. 4G). Consistently, the fibrotic genes (Timp1, Lgals3, Mmp9, Col1a1, and Tgfb1) and inflammatory markers (Ccl2, F4/80, Il1b, and iNos) were upregulated in the eWAT (Figure. 4H). With the elevation of IgG in the eWAT of aged Bnull mice, phosphorylation of SMAD2 and SMAD3 was also elevated, accompanied by increased macrophage infiltration as indicated by the strong induction of F4/80 (Figure. 4I). Hence, B cells are involved in the development of adipose tissue fibrosis along with inflammation in aging, likely mediated through its main product – IgG.
Figure 4. Bnull mice are protected from aging-associated adipose tissue fibrosis.
(A-F) 24-month-old male control (Ctrl) and Bnull mice on the C57BL/6J background were used in the following studies (n=6, 6).
(A) Picrosirius red staining of eWAT sections imaged by brightfield and polarized-light microscopy. Scale bar, 100 μm.
(B) Quantification of eWAT fibrosis area of Ctrl and Bnull mice in (A), (n=6, 6).
(C) Insulin tolerance test (ITT) in aged Ctrl and Bnull mice.
(D) qPCR analysis of eWAT fibrotic gene expression.
(E) WB analysis of IgG and SMADs signaling in the eWAT tissue extracts. HSP90 was used as the loading control.
(F) qPCR analysis of pro- and anti-inflammatory genes in eWAT.
(G-I) 22–24-month-old Bnull mice were i.p. administered 3 mg mouse total IgG or vehicle (Veh) per week for 4 weeks (n=6).
(G) Insulin tolerance test (ITT) were compared before and after IgG treatment (n=6, 6).
(H) qPCR analysis of eWAT fibrotic and inflammatory gene expression after 4 weeks’ treatment. Aged Bnull mice were used as the control group (n=6, 6).
(I) WB analysis of eWAT proteins, with aged Bnull mice as the control group. HSP90 was used as the loading control.
Data are presented as mean ± SEM, *p<0.05, **p<0.01, and ***p<0.001 by 2-tailed student t-test.
Abolishing IgG recycling in macrophages prevents adipose tissue fibrosis in aging
The lack of mature B cells in Bnull mice leads to immunodeficiency in producing Ig isotypes (IgG, IgA, IgM) and B cell-derived cytokines. To isolate the role of IgG, we devised a strategy to specifically manipulate IgG levels without compromising its production. Previous studies show that IgG recycling has a greater impact on determining IgG levels than production by B cells.36 As the sole recycling receptor of IgG (Figure 5A), FcRn is abundantly expressed in myeloid cells and is reported to be critical for maintaining circulating IgG levels.37,38 We observed efficient internalization of IgG in macrophages (Figure S5A). Together with the stimulation of macrophage activation and infiltration into WAT by IgG, we hypothesized that FcRn-mediated IgG recycling in macrophages is involved in IgG accumulation during aging. To test this, we generated Fcgrt floxed mice (Figures S5B, S5C) and crossed them with a LyzM-Cre line to specifically knockout Fcgrt (encoding FcRn) in myeloid cells (FcRn mKO) (Figure 5B). Though FcRn is still expressed in other cell types in adipose tissue (Figure S5D), The half-life of circulating IgG was reduced by 80% in FcRn mKO mice (Figures 5C and S5E). As they aged, FcRn mKO mice modestly decreased IgG levels in the circulation, while the levels of IgA and IgM were unaffected (Figure S5F). FcRn also recycles albumin, but we observed a marginal decrease in the plasma albumin levels in the knockout animals (Figure S5F). Importantly, age-associated tissue IgG accumulation was prevented in mKO mice, especially adipose tissue (Figures 5D and S5G). Therefore, we conclude that IgG recycling by FcRn in macrophages is required for its age-associated accumulation.
Figure 5. Abolishing IgG recycling in macrophages prevents IgG accumulation and adipose tissue fibrosis in aged mice.
(A) Schematic diagram of FcRn-dependent IgG recycling.
(B) Fcgrt mRNA expression in peritoneal macrophages (MØ) isolated from 6-wk-old Fcgrtflox/flox;LysMcre (mKO) and Fcgrtflox/flox control (Ctrl) mice (n=3, 3).
(C) The half-life of circulating biotin-labelled mouse IgG in 15-mon-old Ctrl and mKO mice (n=5, 6).
(D) WB analysis of FcRn and IgG in eWAT from 15-mon-old Ctrl and mKO mice.
(E) Picrosirius red staining of eWAT fibrosis in 15-mon-old Ctrl and mKO mice imaged by brightfield and polarized-light microscopy. Scale bar, 200 μm.
(F) Quantification of fibrosis area shown in E (n=10, 10).
(G) WB analysis of SMADs phosphorylation in the eWAT of 15-mon-old Ctrl and mKO mice.
(H) qPCR analysis of eWAT fibrotic gene expression in 15-mon-old Ctrl and mKO mice (n=9, 10).
(I) qPCR analysis of eWAT inflammatory gene expression in 15-mon-old Ctrl and mKO mice (n=9, 10).
(J) H&E staining of eWAT and iWAT from 15-mon-old Ctrl and mKO mice. Scale bar, 200 μm.
(K) eWAT adipocyte size frequency distribution (n=10, 10).
Data are presented as mean ± SEM, *p<0.05, **p<0.01, and ***p<0.001 for mKO vs Ctrl by 2-tailed student t-test.
See also Figure S5.
Given the specific inhibition of IgG accumulation, this model allows us to dissect the effects of IgG on adipose tissue function. FcRn mKO mice were strongly protected from adipose tissue fibrosis at 15 months of age (Figures 5E, 5F), and the phosphorylation of SMAD2 and SMAD3 in their eWAT was potently reduced (Figure 5G). Consistently, the expression of fibrotic genes showed a broad decrease in the eWAT of aged mKO mice, in line with reduced expression of Tgfb2 and Tgfb3 (Figure 5H). Another hallmark of adipose tissue in aging is chronic inflammation. Preventing IgG accumulation resulted in the overall downregulation of inflammation and immune cell markers in aged mKO eWAT (Figure 5I). Adipocyte hypertrophy signifies adipose tissue inflammation and insulin resistance and is closely related to metabolic dysfunction.22 In line with their reduced fibrosis and inflammation, mKO mice showed alleviated adipocyte hypertrophy (Figures 5J, 5K). Therefore, preventing IgG accumulation retains adipose tissue health in aging.
mKO mice extend the healthspan and lifespan
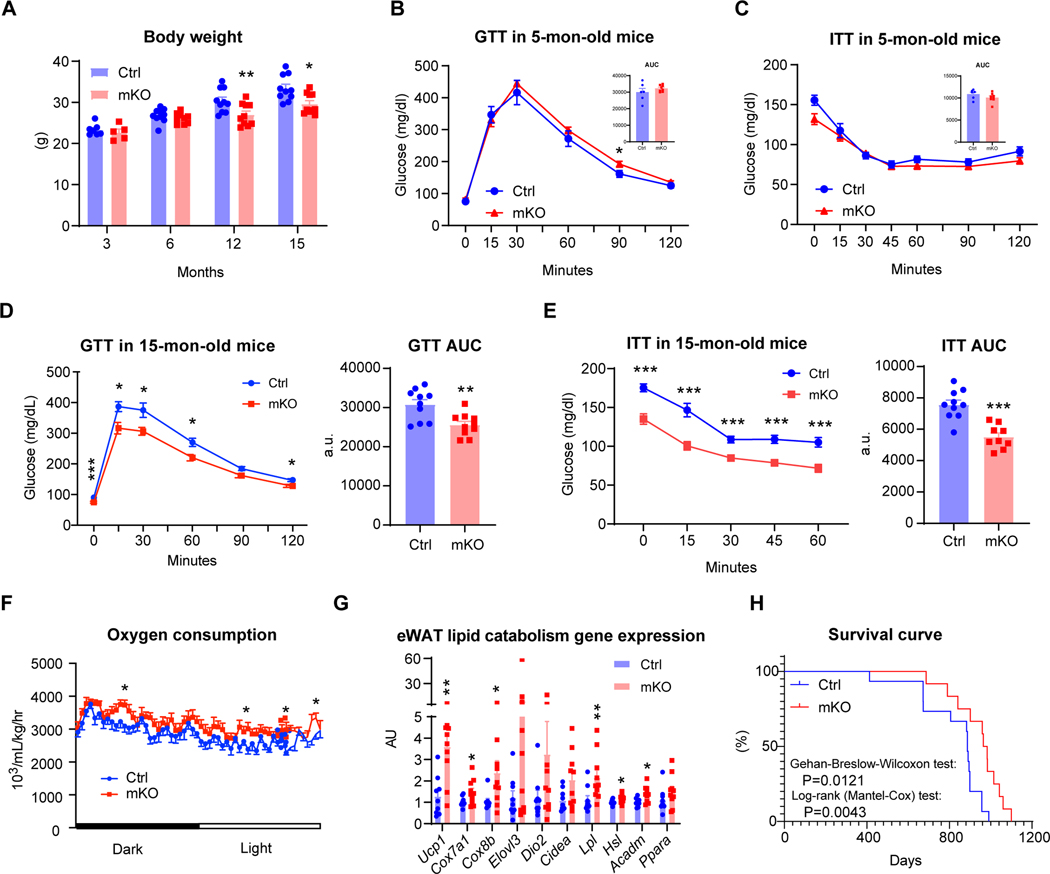
Adipose tissue IgG accumulation is not significant until approximately 6 months of age. We hereby detected negligible effects on body weight, glucose tolerance, and insulin sensitivity in 5-month-old mKO mice (Figures 6A-C). However, at 12 months of age, mKO mice started to weigh less than controls (Figure 6A). The age-associated declines in glucose homeostasis and insulin sensitivity were prevented in mKO mice (Figures 6D, 6E), accompanied by alleviated dyslipidemia (Figures S6A, S6B). Aging mKO mice better preserved their energy expenditure, as indicated by their increased oxygen consumption (Figure 6F) and higher energy expenditure (EE) despite higher food intake compared to their age-matched controls (Figures S6C, S6D). Their respiration exchange ratio (RER) was also higher, reinforcing higher carbohydrate consumption (Figure S6E). This increased EE was not explained by locomotor activity (Figure S6F). Instead, their eWAT showed a phenotype of brown remodeling with the increased expression of lipolytic genes (Lpl and Hsl) and lipid utilization genes (Ucp1, Cox7a1, Cox8b, and Acadm) (Figure 6G). Moreover, aging is associated with the decline in BAT function, characterized by lipid filling,39,40 whereas this whitening phenotypic conversion was inhibited in mKO mice (Figure S6G), as indicated by their upregulation of catabolic genes and glucose uptake gene Glut4 but downregulation of Fabp4, which encodes lipid droplet-binding protein aP2 (Figure S6H). As such, at the age of 15 months old, when metabolic decline is normally well underway, mKO mice are resistant to these changes, pointing to prolonged healthspan.
Figure 6. Prevention of IgG accumulation extends the healthspan and lifespan of mKO mice.
(A) Body weight of Ctrl and mKO mice at indicated ages; 3 mon (n= 7, 5), 6 mon (n=10, 9), 12 &15 mon (n=10, 10).
(B-C) Glucose tolerance test (GTT) and its area under curve (AUC) (B), ITT and its AUC (C) in 5-mon-old male Ctrl and mKO mice (n=6, 8).
(D-E) GTT (n=10, 10) (D) and ITT (n=10, 9) (E) with their AUC in 15-mon-old Ctrl and mKO mice.
(F) Oxygen consumption over a 24-hr dark/light cycle from indirect calorimetry in 15-mon-old Ctrl and mKO mice (n=10, 10).
(G) qPCR analysis of eWAT gene expression involved in lipid metabolism in 15-mon-old Ctrl and mKO mice (n=9, 10).
(H) Survival curve of Ctrl and mKO mice with Gehan-Breslow-Wilcoxon test: p=0.0121, and Log-rank (Mantel-Cox) test: p=0.0043.
Data are presented as mean ± SEM, *p<0.05, **p<0.01, and ***p<0.001 for mKO vs Ctrl by 2-tailed student t-test except H.
See also Figure S6.
Metabolic dysfunction will contribute to the development of complications in aging and ultimately shorten lifespan. We, therefore, investigated whether the delayed metabolic decline in mKO mice will benefit lifespan. After tracing these mice for over 3 years, we observed a ~12% increase in median survival time in the mKO mice compared to the control group (Figure 6H). Therefore, inhibiting IgG accumulation in aging prolongs both healthspan and lifespan in mice.
Targeting IgG recycling rejuvenates metabolic health in aging
From a therapeutic perspective, we designed an antisense oligonucleotide (ASO) against Fcgrt to rectify IgG accumulation in aging. We treated 70-week-old C57BL/6J male mice with control or FcRn ASO by i.p. injection twice weekly for up to 8 weeks (Figure 7A). FcRn ASO treatment efficiently knocked down FcRn in eWAT and other peripheral tissues (Figures 7B and S7A), resulting in decreased IgG levels in the plasma, WATs, BAT, and liver, and all major subtypes of IgG contributed to the decrease (Figures 7C, 7D and S7B, S7C). The FcRn knockdown (KD) mice did not gain weight like their controls over the treated period, owing to their higher oxygen consumption and EE revealed by indirect calorimetry (Figures 7E, 7F, and S7D). Similar to the mKO mice, FcRn KD increased food intake and RER without affecting locomotor activity (Figures S7E-S7G). Consistently, FcRn KD mice improved glucose tolerance and insulin sensitivity (Figures 7G, 7H). In contrast, KD FcRn in young (7 weeks old) mice, which have basal levels of IgG, had minimal effects on reducing IgG and, consequently, body weight, glucose tolerance, and insulin sensitivity (Figures S7I-S7L), supporting an IgG-dependent improvement. Therefore, targeting IgG recycling receptor FcRn rejuvenates metabolic functions in aging.
Figure 7. Targeting IgG recycling improves metabolism and adipose remodeling in aged mice.
(A) Experimental design for treating 70-week-old male mice with control (Ctrl) or FcRn antisense oligonucleotides (ASO) for 8 weeks. The following parameters were measured:
(B) WB analysis of FcRn in eWAT. GAPDH was used as the loading control.
(C) Plasma IgG levels determined by ELISA after 8 weeks of ASO treatment (n=7, 7).
(D) WB analysis of IgGs in eWAT with GAPDH as the loading control.
(E) Body weight curve during the treatment (n=8, 8).
(F) O2 consumption by indirect calorimetry.
(G) ITT after 5 weeks of ASO treatment (n=8, 8).
(H) GTT after 6 weeks of ASO treatment (n=8, 8).
(I) WAT and liver weights at the end of the 8-wk treatment (n=8, 8).
(J) H&E staining of eWAT, Scale bar, 100 μm.
(K) qPCR analysis of gene expression in eWAT (n=6, 6).
(L) qPCR analysis of fibrotic genes in eWAT (n=8, 8).
(M) WB analysis of TGF-β signaling in eWAT. HSP90 was used as the loading control.
Data are presented as mean ± SEM; *p<0.05, **p<0.01 for Ctrl vs FcRn ASO group by 2-tailed student t-test.
See also Figure S7.
FcRn KD reduced the weight of eWAT (64% reduction) and iWAT (33%) depots in aged mice (Figure 7I) and mitigated their adipocyte hypertrophy (Figures 7J, S7H), accompanied by the restored adipogenic gene expression (Pparg2, Fasn, Scd1, Adipoq, and Adipsin) (Figure 7K). In line with their increased EE, the KD mice showed increased expression of brown remodeling markers, e.g., Ucp1, Cidea, Cox8b, and SirT141 and downregulation of the white adipocyte-enriched gene Lep in eWAT (Figure 7K). Moreover, BAT whitening was alleviated (Figure S7H). Importantly, FcRn KD repressed the expression of fibrogenic and Tgfb genes (Figure 7L), supported by inhibited phosphorylation of SMAD2 and SMAD3 in the eWAT (Figure 7M). Collectively, targeting IgG recycling to inhibit IgG accumulation improves adipose tissue remodeling and rejuvenates metabolism in aging.
DISCUSSION
The present study unveils for the first time that IgG is an aging factor and its abnormal accumulation impairs metabolic health. Unlike its canonical immune-defensive function, IgG activates macrophage inflammatory response and prompts the TGF-β/SMAD pathway to induce adipose tissue fibrosis. This hitherto unrecognized pathogenic function of IgG connects multiple hallmarks of adipose tissue dysfunction, including chronic inflammation, fibrosis, impaired adipogenesis, adipocyte hypertrophy, and decreased catabolic activity, eventually leading to the loss of physiological integrity of adipose tissue in aging. Our data further reveal that FcRn-mediated IgG recycling in macrophages is required for IgG accretion in aging; thereby, intervening in this recycling process conveys therapeutic potential in rejuvenating metabolic health.
IgG is primarily considered a circulating factor, while its intra-tissue homeostasis is rarely appreciated. Though we observed correlations of IgG levels between tissues and circulation, adipose tissue IgG is more responsive to aging than plasma IgG. At the same level of circulating IgG, IgG accumulates faster and to a greater degree in adipose tissue compared to other tissues in aging, highlighting a specific regulation of IgG in adipose tissue. This could be related to efficient macrophage recycling and infiltration into adipose tissue under metabolic stress. Recent studies have also demonstrated age-related B cell infiltration into adipose tissue.42 These resident B cells likely contribute to the robust accumulation of IgG in adipose tissue during aging; the accumulated IgG will then worsen inflammation, fibrosis, and macrophage infiltration to self-accelerate this process. Moreover, FcRn also recycles albumin; however, we did not observe significant changes in albumin levels in either the mKO or FcRn ASO mouse model. Even if there is any possible detriment of marginal albumin decrease, it unlikely offsets the dominant metabolic improvements from abrogating IgG accumulation.
The function of IgG in adipose tissue pathological remodeling is actually supported by clues from previous studies. Bnull mice are protected from insulin resistance in diet-induced obesity.43–45 IgG2c, a pro-inflammatory isotype, was taken as a marker of adipose inflammation in diet-induced obesity. 43 The infiltrated B cells in obese adipose tissue are believed to interact with macrophages and T cells to induce adipose inflammation and cause insulin resistance and glucose intolerance through IgG-independent mechanisms.43–45 Interestingly, antibody production, the housekeeping function of B cells, was not counted, probably due to the traditional view of Igs as primarily plasma-relevant. In fact, the increased IgG in the circulation was noticed in older humans46 as well as in aging mice47 for decades, but the tissue accumulation remained not examined. Our finding of IgG as a pathogenic factor in adipose remodeling reveals a highly abundant, direct mediator of adipose-infiltrated B cells to impair metabolic function in aging. While future investigations are warranted to determine whether the accumulated IgG is accounted for by autoantibodies or not, IgG stands out as a unique factor to connect macrophages, adipocyte progenitor cells, adipocytes, and B cells – the major cell types that determine adipose health. The classic view of IgG as a circulating immune-defensive factor might be oversimplified in the context of adipose degeneration and the consequent metabolic dysfunction.
Here we primarily focused on fibrosis to delineate the pathogenic role of IgG in adipose tissue aging. Interestingly, the metabolic improvements in aged mKO mice are quite similar to those of Smad3−/− mice undergoing a high fat diet, including anti-obesity, improved insulin sensitivity and glucose tolerance, increased energy expenditure, and browning remodeling of WAT.48 Given the pronounced fibrogenic function of IgG and the driving role of fibrosis in adipose tissue degeneration, it is conceivable that this fibrotic pathway is among the major contributors to IgG’s detriments in adipose tissue. In addition, IgG is able to directly activate the inflammatory response of macrophages. These two parallel pathways will mutually aggravate each other to worsen adipose tissue derangement. However, it is not exclusive of other possible pathways that work in synchronization to execute IgG’s pathogenic function in aging, such as dampening BAT’s function.
By identifying IgG as a causal factor of metabolic dysfunction in aging, this work highlights the potential of inhibiting IgG accumulation as a novel therapeutic strategy to tackle age-associated metabolic decline. Various FcRn inhibitors have been developed for IgG engineering or for treating autoimmune diseases in the forms of antibodies,49,50 IgG mutants,38 and small peptides.51 These FcRn inhibitors could be repurposed to improve metabolic conditions and aging; however, the treatment should be carefully optimized as it may increase the risk of infection with uncontrolled IgG depletion.
Limitation of Study
We are cognizant of several limitations of the present study. First, we demonstrate preferential accumulation of IgG in adipose tissue during aging; however, we do not yet fully understand this preference. It may involve multiple processes in IgG homeostasis besides macrophage recycling, such as antigen presence, B cell infiltration and activation in adipose tissue, decreased clearance of IgG immune complexes, etc. Second, adipose tissue is unlikely the only effector tissue of IgG. Given the prevalent accumulation of IgG across tissues during aging, though at lower levels, IgG may target multiple cell types in different tissues to execute its pro-aging effects. Third, despite the prolonged lifespan of FcRn mKO mice, here we mainly investigate the physiological development of metabolic dysfunction during aging, which starts in middle age. It is envisioned that the further accretion of IgG in the advanced aging stage will contribute to aging-associated complications, such as cancer, dementia, and fragility. A systemic characterization of FcRn mKO mice in old age (e.g., >2 years old) will help to establish the full spectrum pro-aging effects of IgG. Last but not least, our discovery of IgG as an aging factor will invite caution of antibody immunotherapy. However, current studies on potential long-term effects of IgG on metabolism, particularly in human tissues and cells, are very limited.
STAR METHODS
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to the Lead Contact, Li Qiang (lq2123@cumc.columbia.edu)
Materials Availability
The Fcgrtflox/flox mouse line is available from the lead contact upon request.
Data and Code Availability
The RNA-seq data has been deposited to the NGDC (https://ngdc.cncb.ac.cn/) (PRJCA002140 & PRJCA020637) and are publicly available at the date of publication.
This paper does not report original code.
All the western blots raw data and excel spreadsheets containing the values for all graphs were provided in Data S1-Source data. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND STUDY PARTICIPANT DETAILS
Mice
All mice were on C57BL/6J background and maintained under standard laboratory conditions with ad libitum access to water and standard chow diet (except CR mice, PicoLab rodent diet 20, 5053). Mice were housed on a 12-hr light/12-hr dark cycle with lights on at 07:00 and off at 19:00. All animal experiments were performed in accordance with NIH guidelines for Animal Care and Use, approved and overseen by Columbia University Institutional Animal Care and Use Committee (IACUC).
Body composition was determined by EchoMRI. For glucose tolerance tests (GTT), mice were fasted overnight for 16 hours (17:00–09:00) and intraperitoneally injected with glucose at a dose of 2 g/kg·BW. Blood glucose levels were measured at basal state (0 min) and subsequently at 15, 30, 60, 90, and 120 min after injection. Insulin tolerance tests (ITT) were performed after a 4-hr (09:00–13:00) fast with intraperitoneal injection of human insulin (0.75 U/kg.BW). Blood glucose levels were measured via tail vein bleeding using a OneTouch glucometer. Plasma insulin concentrations were determined with Mouse Insulin ELISA Kit (Mercodia, USA). Infinity Triglyceride Reagent (Thermo Scientific) and NEFA-HR (Fujifilm Wako) were used to measure plasma triglyceride and NEFA levels, respectively.
Indirect calorimetry analyses were performed using the Comprehensive Laboratory Animal Monitoring System (CLAMS) equipped with an Oxymax Open Circuit Calorimeter System (Columbus Instruments). The following parameters of individual mice were monitored: food intake, O2 consumption (VO2), CO2 production (VCO2), heat, and locomotion. Mouse energy metabolism was analyzed according to the ANCOVA flowchart.52
For aging studies with ASO treatment, 70-wk-old mice were used. Fcgrt (5’-GATGATACATCAGTGG-3’) and control (5’-GGCCAATACGCCGTCA-3’) ASOs were injected intraperitoneally twice a week at a dose of 50 mg/kg·body weight (BW) into aged (70-week-old) and young (6-week-old) mice. Prior to sacrifice, mice were fasted overnight for 16 h and then refed for 4 h.
Mice were perfused with phosphate-buffered saline (PBS) for 5 min to clear the blood trace in tissues at sacrifice. Tissues were fixed in 10% formalin (in PBS) at 4 °C overnight for histological analyses or frozen for molecular analyses.
Calorie Restriction treatment
Wildtype C57BL/6J male mice at indicated ages were randomly assigned into ad libitum fed and CR groups. All mice were single-housed, and CR mice were fed 70% of the ad libitum food intake, consisting of one 2.6–2.8 g meal/day. Mice were monitored, including but not limited to body weight, GTT, ITT, etc.
IgG treatment of mice
Commercially purified mouse total IgG (>95%, ProteinMods and Cusabio) was dissolved in PBS. Male mice were administered with 3 mg IgG or vehicle (Veh) intraperitoneally per week. The selection of a 3 mg IgG dosage was based on its ability to elicit notable IgG accumulation in adipose tissue, akin to the levels observed in aging mice. This dosage resembles the cumulative endogenous IgG quantity present in the plasma of young adult mice (approximately 1.7 mg/mL in a plasma volume of around 2 mL), enabling us to mimic the amount of IgG in the plasma under physiological conditions.
Human tissues
Frozen heart tissues were obtained from the National Disease Research Interchange (NDRI). 5 cm × 5 cm left ventricle tissues were collected from normal human donors within 12 h post-death and kept frozen at −80 °C. The detailed age (sex) information is as follows: 24 (m), 37 (m), 41 (m), 47 (m), 71 (f), 80 (m), 82 (f), 100 (f).
METHOD DETAILS
Generation of Fcgrt conditional knockout mice
Fcgrt floxed mice were generated at the Columbia University Transgenic Mouse Shared Resource Core by flanking exons 2 and 3 of the Fcgrt gene (which contain the ATG start codon and first 325 nt of coding sequence). The strategy allows the deletion of the entire FcRn protein. A Loxp-Neo-Loxp (LNL) cassette was inserted in the intron upstream of exon 2 of the Fcgrt gene in a Bacterial Artificial Chromosome (BAC clone ID: RP23–476N22). The Neo cassette was removed by Cre recombinase, leaving behind one loxp site (L83). An Frt-Neo-Frt-Loxp (FNFL) cassette was inserted in the intron downstream of the Fcgrt exon 3. A gene targeting vector was constructed by retrieving the 2 kb short-homology arm (5’ to L83), the L83-FNFL cassette, and the 5 kb long-homology arm (end of FNFL cassette to 3’) into the pMCS-DTA vector carrying a DTA (Diphtheria Toxin Alpha chain) negative selection marker. The FNFL cassette confers G418 resistance during gene targeting in KV1 (129B6 hybrid) ES cells, and the DTA cassette provides an autonomous negative selection to reduce random integration events during gene targeting. Several positive targeted ES cell clones were identified and karyotyped. One of the targeted ES cell clones (2F2) was injected into C57BL/6J blastocysts to generate chimeric mice. Male chimeras were bred to homozygous ACTB (Flpe/Flpe) females on C57BL/6J background (Jackson Laboratory 005703) to transmit the floxed Fcgrt allele. The Fcgrtflox/+ mice were backcrossed to C57BL/6J mice for 6 generations and then bred with LyzM-Cre mice (Jackson Laboratory 004781 on C57BL/6J background) to conditionally knock out Fcgrt in macrophages. We genotyped the Fcgrt floxed allele using the following primer pair: forward: 5’-GGTGTCTTGGTATTGGGAGAAG-3’ and reverse: 5’-AGTCCAATTGCCGATTCTTG-3’. The amplicon from the floxed allele band is 598 bp, and the wildtype allele is 452 bp.
IgG half-life determination
200 µg of biotin-labeled mouse IgG (ProteinMods) was injected intraperitoneally into 15-mon-old FcRn mKO and age-matched control mice. Plasma was collected via tail vein bleeding at 3 h (as baseline) and at 1, 3, 7, 14, and 21 days after injection. Diluted plasma was resolved by SDS-PAGE and transferred to a PVDF membrane. The biotin signal was determined with goat anti-biotin HRP (Vector Laboratories Cat. No. SP-3010–1). Blots were quantified and normalized to the 3 h signal.
RNA extraction and qPCR analysis
RNA was extracted from tissues or cells using the IBI Tri-isolate total RNA kit (IBI Scientific). 1 μg total RNA was used to synthesize cDNA using the High-capacity cDNA Reverse Transcription kit (Applied Biosystems). A Bio-Rad CFX96 Real-Time PCR system with GoTaq qPCR Master Mix (Promega and Azura) was used to perform quantitative real-time PCR (qPCR). We calculated relative gene expression levels using the ΔΔCt method, and Rpl23 or cyclophilin A was used as the reference gene. QPCR primer sequences are available upon request.
RNA sequencing
RNA libraries for sequencing were prepared and sequenced by the professional services offered at Novogene, incorporating thorough examination of RNA quality and libraries through the Agilent 2100 platform. Subsequently, RNA libraries were multiplexed and sequenced utilizing the Illumina NovaSeq 6000 platform. Quality assurance was ensured by utilizing FastQC (v0.11.9) for the RNA-seq data.
Gene set enrichment analysis
To assess functional changes of eWAT during aging, we analyzed the RNA-seq dataset (NGDC: PRJCA002140) of wildtype C57BL/6J mouse eWAT at the ages of 8, 26, 60, 78, and 104 weeks to identify gene expression changes.19 We performed GSEA of BPs based on the gene rank of expression change for each pairwise comparison. We retained only BP with FDR < 0.05 in at least one comparison for downstream analysis. To identify upregulated or downregulated BPs during aging, we calculated the correlation between normalized enrichment score (NES) and age for each BP. We defined the BP with aging correlation > 0.95 in addition to NES of 104 weeks vs 8 weeks > 0 and NES of 78 weeks vs. 8 weeks > 0 as upregulated BP during aging. The downregulated BPs during aging were defined as BP with aging correlation < −0.7 in addition to NES of 104 weeks vs. 8 weeks < 0 and NES of 78 weeks vs. 8 weeks < 0.
RNA sequencing analyses were performed in the eWAT and BMDMs with and without IgG treatment. The RNA-seq data were deposited at the Genome Sequence Archive (GSA, https://ngdc.cncb.ac.cn/gsa/) database with the accession number PRJCA020637. The RNA-seq data was processed and analyzed as previously described.53 Briefly, the sequencing data was mapped to the mouse genome (GRCm39) using STAR (v2.7.7a) and calculated the read counts for each gene across the samples using feature Counts (v2.0.1). Gene expression was analyzed using edgeR. Normalization: Read counts were normalized across the samples using the Trimmed Mean of M-values (TMM) method. The normalized values were then converted to gene expression levels, represented as FPKM (fragments per kilo base per million). To prevent infinite values during log2FoldChange calculation, we added 0.5 to each FPKM value. Differentially Expressed Genes (DEG) were identified based on the criteria of log2FoldChange > 0.75 and an adjusted p-value of < 0.1. We showed the top 15 biological processes (BPs) in the results.
Cell culture
3T3-L1 cells were grown in high glucose DMEM (Corning Cat. No: 10–017) supplemented with 10% calf serum (CS, heat-inactivated, Gemini Bio-Products, Cat. No: 100506) and 1x Pen/Strep (Thermo Fisher). In the CM-treated assay, 3T3-L1 cells at >90% confluence were treated with the CM from BMDMs for 24 hours and then harvested for RNA and protein analyses.
Bone marrow-derived macrophages (BMDMs)
Bone marrow cells were collected from the femur and tibia of 8–12 weeks old mice, and erythrocytes were removed by resuspending in 1× red blood cell lysis buffer on ice. The remaining monocytes were cultured for 5–6 days in a petri dish in low glucose DMEM within 10% FBS and 1% penicillin-streptomycin supplemented with 10 ng/mL M-CSF. The cells were then seeded for macrophage activation in 12-well or 6-well plates for 24hr in the serum-free medium before stimulation by vehicle, indicated doses of IgG, 50 ng/mL LPS, or 50 ng/mL IL-4. IgG treatment was for 2 days. Conditioned media (CM) was collected from the 2nd day’s media to treat 3T3-L1 preadipocytes for 24 hours. Cells were then harvested for RNA and WB analyses. For assessing IgG uptake, BMDMs were treated with 50 µg/mL IgG-Cy5.5 overnight in the serum-free medium.
Western blotting
Tissues or cultured cells were homogenized in protein lysis buffer (50 mM Tris-HCl pH 7.4, 180 mM NaCl, 1% Triton X-100, 10% glycerol, and 1 mM EDTA) supplemented with 0.5 mM PMSF, and protease and phosphatase inhibitor cocktails (Sigma-Aldrich) or using the IntactProtein Lysis kit (GenulN Biotech, USA). Protein concentration was determined using the Pierce BCA Protein Assay kit (Thermo Fisher Scientific). Protein extracts (25–50 µg) were resolved by SDS-PAGE, transferred onto PVDF membranes, blocked for 1 hour with 5% skim milk in PBST, and incubated with primary antibodies overnight. For serum proteins, 1 µL plasma was diluted to 100 µL in protein lysis buffer, and 7–10 µL diluted plasma was subjected to SDS-PAGE. The antibodies used were anti-Hsp90 (Proteintech 131711-AP), anti-Sirt1 (Proteintech 13161–1-AP), anti-FcRn (Abcam 193148, Thermo Fisher Scientific PA5–79246, R&D AF6775), anti-IgG (Abcam 46540), anti-IgG1 (Bethyl A90–205P), anti-IgG2a (Bethyl A90–107P), anti-IgG3 (Bethyl A90–211P), anti-IgA (Bethyl A90–103P), anti-IgM (Jackson ImmunoResearch Laboratories 115–035-075), anti-F4/80 (Cell Signaling 70076), p-Smad2 (Cell Signaling 18338), p-Smad3 (Cell Signaling 9520), and p-Smad3 (Abcam ab52903), Smad2 (Cell Signaling 5339), and Smad2/3 (Cell Signaling 8685). Membranes were incubated with HRP-conjugated secondary antibodies and visualized by enhanced chemiluminescence (Thermo Fisher Scientific). Equal loading was confirmed using anti-GAPDH or anti-HSP90 (Proteintech) and/or membrane staining. Mouse plasma and tissue total IgG were also detected directly using secondary mouse antibodies conjugated with HRP (Sigma-Aldrich A9044, GE NA931), both of which gave results consistent with rabbit anti-IgG (Abcam 46540) followed by anti-rabbit secondary antibody. Human IgG was detected using goat-anti-human IgG HRP (Thermo Fisher Scientific 62–8420). Densitometric analyses of WB were performed using ImageJ and normalized to loading control.
Antibody and TGF-β ELISA
Plasma IgG levels were measured using the total IgG ELISA kit (Bethyl Laboratories E99–131). TGF-β in BMDM conditioned media was measured using the TGF-β1 ELISA kits (Proteintech KE10005).
Ras Activation Assay
BMDMs were treated with 100 ng/mL LPS or 200 µg/mL IgG for 1 hour, and EGF was used as the positive control for activating Ras. Ras activity was determined using the Ras G-LISA Activation Assay Kit (Cytoskeleton, BK131).
Proteomic analysis of adipose tissue proteins
Adipose tissue was lysed in protein lysis buffer, and 30 µg of total protein was separated on 8% SDS-PAGE and stained with SimplyBlue (Thermo Fisher Scientific). Protein gel slices were excised, and ingel digestion was performed. Mass spectrometry using a Thermo Scientific™ Orbitrap Fusion™ Tribrid™ mass spectrometer was performed at Columbia Herbert Irving Comprehensive Cancer Center core facility.
Histology and immunostaining
Tissues were stained with hematoxylin and eosin (H&E). For the immunohistochemical staining of IgG, sections were incubated with anti-IgG (Abcam 46540) at a 1:500 dilution overnight. For the immunofluorescent staining of F4/80, FcRn, and SMA, sections were incubated with anti-IgG (Invitrogen A31570), anti-F4/80 (Invitrogen MA5–16624), anti-FcRn (abcam ab193148), and anti-SMA (Cell signaling D4K9N) at 1:100–200 dilution. Quantification of fluorescence signal co-localization based on Colorc 2 plugin in Fiji software.
Statistical analysis
Statistical analyses were performed using Prism 8.0 software (Graphpad). We used the two-tailed Student’s t-test for comparison between two groups and one-way ANOVA for comparisons among three or more groups. p < 0.05 was adopted to declare statistical significance. All data were presented as means ± SEM (standard error). Sample sizes are included in the figure legends.
Supplementary Material
Table S1. Protein list from Mass Spectrometry, related to Figure 1.
KEY RESOURCE TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Experimental Models: Organisms | ||
| Mouse: C57BL/6J | The Jackson Laboratory | 000664 |
| Mouse: LyzM-Cre mice | The Jackson Laboratory | 004781 |
| Mouse: Fcgrt floxed mice | Generated in house | N/A |
| Mouse: B6.129S2-Ighmtm1Cgn/J | The Jackson Laboratory | 002288 |
| Cell line: 3T3-L1 cells | ATCC | CL-173 |
| Antibodies | ||
| anti-Sirt1 | Proteintech | PA1-054 |
| anti-biotin HRP | Vector Laboratories | SP-3010-1 |
| anti-pSmad2 | Cell Signaling | 18338 |
| anti-pSmad3 | Abcam | Ab52903 |
| anti-pSmad3 | Cell Signaling | 9520 |
| anti-Smad2 | Cell Signaling | 5339 |
| anti-Smad2/3 | Cell Signaling | 8685 |
| anti-Smad3 | Cell Signaling | 9513 |
| anti-FcRn | Thermo Fisher Scientific | PA5–79246 |
| anti-FcRn | Abcam | 193148 |
| anti-FcRn | R&D | AF6775 |
| anti-GAPDH HRP | Proteintech | HRP-60004 |
| anti-HSP90 | Proteintech | 13171-1-AP |
| anti-IgA | Bethyl Laboratories | A90-103P |
| anti-IgG | Abcam | 46540 |
| anti-IgG1 | Bethyl Laboratories | A90-205P |
| anti-IgG2a | Bethyl Laboratories | A90-107P |
| anti-IgG3 | Bethyl Laboratories | A90-211P |
| anti-IgM | Jackson ImmunoResearch Laboratories | 115-035-075 |
| anti-pERK | Cell Signaling | 9102S |
| anti-pMEK | Cell Signaling | 9121S |
| anti-human IgG HRP | Thermo Fisher Scientific | 62-8420 |
| Goat Anti-Human IgG | Abcam | ab6858 |
| anti-PPARγ | Cell Signaling | 2443 |
| anti-mouse IgG HRP 2nd antibody | GE | NA931 |
| anti-mouse IgG HRP 2nd antibody | Sigma-Aldrich | A9044 |
| anti-rabbit IgG HRP 2nd antibody | Sigma-Aldrich | A9169 |
| anti-F4/80 | Cell Signaling | D4C8V |
| anti-F4/80 | Invitrogen | MA5-16624 |
| anti-SMA | Cell Signaling | D4K9N |
| anti-CD68 | Bio Rad | MCA1957GA |
| anti-FcRn | Abcam | ab193148 |
| Alexa Fluor 594 donkey anti-Rat | Invitrogen | A21209 |
| Alexa Fluor 568 goat anti-Rabbit | Invitrogen | A11011 |
| Alexa Fluor 594 donkey anti-mouse | Invitrogen | A31570 |
| Alexa Fluor 647 goat anti-rat | Invitrogen | A21247 |
| Alexa Fluor 555 goat anti-rat | Invitrogen | A21434 |
| Alexa Fluor 488 donkey anti-rabbit | Invitrogen | A21206 |
| DAPI | Thermo Fisher Scientific | 62248 |
| Chemicals, materials | ||
| Liberase | Sigma-Aldrich | 5401127001 |
| biotin-labeled mouse IgG | ProteinMods | IMB |
| mouse IgG | ProteinMods | IMN |
| mouse IgG | Sigma-Aldrich | I5381 |
| mouse IgG | Cusabio | NP001601m |
| IgG-Cy5.5 | ProteinMods | IMY |
| Oil Red O Stain | Electron Microscopy Sciences | 26079 |
| M-CSF | Biolegend | 50-403-701 |
| TGF-β | Biolegend | 763102 |
| TGFβR inhibitor | MCE | LY2109761 |
| ERK inhibitor | Selleck | S7101 |
| Pierce ECL Western Blotting Substrate | Thermo Fisher Scientific | 32109 |
| Complete Protease Inhibitor Cocktail | Roche | 11697498001 |
| GoTaq Master Mix | Promega | A600A |
| Fetal Bovine Serum, heat inactivated | Corning | 35-011-CV |
| Calf Serum, heat inactivated | Gemini Bio-Products | 100506 |
| Critical Commercial Assays | ||
| NucleoSpin RNA Set for NucleoZOL | Macherey-Nagel | 740406 |
| High-Capacity cDNA Reverse Transcription Kit | Applied Biosystems (Fisher Scientific) | 43-688-14 |
| Quanti-blue™ medium | Invivogen | rep-qbs |
| AzuraView GreenFast qPCR Blue Mix LR | Azura | GreenFast qPCR Blue Mix LR |
| IntactProtein Lysis kit | GenulN Biotech | #415 |
| Pierce™ Protein A IgG Purification Kit | Thermo Fisher Scientific | 44667 |
| Melon Gel IgG Spin Purification Kit | Thermo Fisher Scientific | 45206 |
| Mouse insulin ELISA kit | Mercodia | 10-1247-01 |
| Mouse IgG ELISA kit | Bethyl Laboratories | E99-131 |
| Mouse TGF-β ELISA kit | Proteintech | KE10005 |
| NEFA-HR(2)-A assay | Wako | 434-91795 |
| NEFA-HR(2)-B assay | Wako | 434-91995 |
| Infinity triglycerides assay | Thermo | TR22421 |
| Ras G-LISA Activation Assay Kit | Cytoskeleton | BK131 |
| Oligonucleotides | ||
| Mouse Fcgrt ASO (Gen 2.5) | Ionis Pharmaceuticals Inc | ION 1089931 |
| Control ASO (Gen 2.5) | Ionis Pharmaceuticals Inc | ION 549144 |
| Deposited Data | ||
| Data S1 | Source Data underlying all graphs and figures. Related to Figures 1–7, S1-7. | |
| Software and Algorithms | ||
| ImageJ | https://imagej.nih.gov/ij/ | N/A |
| Prism 8 | GraphPad | N/A |
| R system: R v4.0.2 | R Core Team | https://cran.r-project.org/ |
| Programming environment of R: RStudio v1.4.1103 | RStudio Team | https://www.rstudio.com/ |
Highlights.
IgG accumulates in adipose tissue during aging and is reduced by caloric restriction.
IgG activates macrophages to induce adipose tissue fibrosis.
Preventing IgG accumulation in aging prolongs healthspan and lifespan.
Targeting IgG recycling receptor FcRn rejuvenates metabolic health in aged mice.
ACKNOWLEDGMENTS
We thank M. Graham at Ionis Pharmaceuticals for providing FcRn ASO and control ASO, K. Liu, J. Yang, and Q. Xu for technical support. This work was supported by NIH R01DK112943 (L.Q.), R01DK134471(L.Q.), and the Russell Berrie Foundation (L.Q.). We also thank the support of the NIDDK DRC grant P30DK063608 and the TBAC core.
Footnotes
DECLARATION OF INTERESTS
A patent application is pending by Columbia University.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barzilai N, Huffman DM, Muzumdar RH, and Bartke A. (2012). The critical role of metabolic pathways in aging. Diabetes 61, 1315–1322. 10.2337/db11-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hildrum B, Mykletun A, Hole T, Midthjell K, and Dahl AA. (2007). Age-specific prevalence of the metabolic syndrome defined by the International Diabetes Federation and the National Cholesterol Education Program: the Norwegian HUNT 2 study. BMC public health 7, 220. 10.1186/1471-2458-7-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grover SA, Kaouache M, Rempel P, Joseph L, Dawes M, Lau DC, and Lowensteyn I. (2015). Years of life lost and healthy life-years lost from diabetes and cardiovascular disease in overweight and obese people: a modelling study. Lancet Diabetes Endocrinol 3, 114–122. 10.1016/S2213-8587(14)70229-3. [DOI] [PubMed] [Google Scholar]
- 4.Kenyon CJ. (2010). The genetics of ageing. Nature 464, 504–512. 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 5.Junnila RK, List EO, Berryman DE, Murrey JW, and Kopchick JJ. (2013). The GH/IGF-1 axis in ageing and longevity. Nat Rev Endocrinol 9, 366–376. 10.1038/nrendo.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Heemst D. (2010). Insulin, IGF-1 and longevity. Aging Dis 1, 147–157. [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson SC, Rabinovitch PS, and Kaeberlein M. (2013). mTOR is a key modulator of ageing and age-related disease. Nature 493, 338–345. 10.1038/nature11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamming DW, Ye L, Sabatini DM, and Baur JA. (2013). Rapalogs and mTOR inhibitors as anti-aging therapeutics. J Clin Invest 123, 980–989. 10.1172/JCI64099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall JA, Dominy JE, Lee Y, and Puigserver P. (2013). The sirtuin family’s role in aging and age-associated pathologies. J Clin Invest 123, 973–979. 10.1172/JCI64094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merksamer PI, Liu Y, He W, Hirschey MD, Chen D, and Verdin E. (2013). The sirtuins, oxidative stress and aging: an emerging link. Aging (Albany NY) 5, 144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burkewitz K, Zhang Y, and Mair WB. (2014). AMPK at the nexus of energetics and aging. Cell Metab 20, 10–25. 10.1016/j.cmet.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salminen A, and Kaarniranta K. (2012). AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev 11, 230–241. 10.1016/j.arr.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Palmer AK, and Kirkland JL. (2016). Aging and adipose tissue: potential interventions for diabetes and regenerative medicine. Exp Gerontol 86, 97–105. 10.1016/j.exger.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barzilai N, and Gupta G. (1999). Revisiting the role of fat mass in the life extension induced by caloric restriction. J Gerontol A Biol Sci Med Sci 54, B89–96; discussion B97–88. [DOI] [PubMed] [Google Scholar]
- 15.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, et al. (2009). Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395. 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang GR, Chiu YS, Wu YY, Chen WY, Liao JW, Chao TH, and Mao FC. (2009). Rapamycin protects against high fat diet-induced obesity in C57BL/6J mice. J Pharmacol Sci 109, 496–503. [DOI] [PubMed] [Google Scholar]
- 17.Muzumdar R, Allison DB, Huffman DM, Ma X, Atzmon G, Einstein FH, Fishman S, Poduval AD, McVei T, Keith SW, and Barzilai N. (2008). Visceral adipose tissue modulates mammalian longevity. Aging Cell 7, 438–440. 10.1111/j.1474-9726.2008.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirkland JL, and Tchkonia T. (2017). Cellular Senescence: A Translational Perspective. EBioMedicine 21, 21–28. 10.1016/j.ebiom.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Q, Wan Q, Jiang Y, Liu J, Qiang L, and Sun L. (2020). A Landscape of Murine Long Non-Coding RNAs Reveals the Leading Transcriptome Alterations in Adipose Tissue during Aging. Cell Rep 31, 107694. 10.1016/j.celrep.2020.107694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, Khosla S, Jensen MD, and Kirkland JL. (2010). Fat tissue, aging, and cellular senescence. Aging Cell 9, 667–684. 10.1111/j.1474-9726.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karagiannides I, Tchkonia T, Dobson DE, Steppan CM, Cummins P, Chan G, Salvatori K, Hadzopoulou-Cladaras M, and Kirkland JL. (2001). Altered expression of C/EBP family members results in decreased adipogenesis with aging. American journal of physiology. Regulatory, integrative and comparative physiology 280, R1772–1780. 10.1152/ajpregu.2001.280.6.R1772. [DOI] [PubMed] [Google Scholar]
- 22.Gustafson B, Hammarstedt A, Hedjazifar S, and Smith U. (2013). Restricted adipogenesis in hypertrophic obesity: the role of WISP2, WNT, and BMP4. Diabetes 62, 2997–3004. 10.2337/db13-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McHeyzer-Williams LJ, Cool M, and McHeyzer-Williams MG. (2000). Antigen-specific B cell memory: expression and replenishment of a novel b220(−) memory b cell compartment. J Exp Med 191, 1149–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nimmerjahn F, Gordan S, and Lux A. (2015). FcgammaR dependent mechanisms of cytotoxic, agonistic, and neutralizing antibody activities. Trends Immunol 36, 325–336. 10.1016/j.it.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Roopenian DC, and Akilesh S. (2007). FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol 7, 715–725. 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 26.Hipp MS, Kasturi P, and Hartl FU. (2019). The proteostasis network and its decline in ageing. Nat Rev Mol Cell Biol 20, 421–435. 10.1038/s41580-019-0101-y. [DOI] [PubMed] [Google Scholar]
- 27.Anderson RM, Shanmuganayagam D, and Weindruch R. (2009). Caloric restriction and aging: studies in mice and monkeys. Toxicol Pathol 37, 47–51. 10.1177/0192623308329476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun K, Tordjman J, Clement K, and Scherer PE. (2013). Fibrosis and adipose tissue dysfunction. Cell Metab 18, 470–477. 10.1016/j.cmet.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Derynck R, and Zhang YE. (2003). Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425, 577–584. 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 30.Meng X. m., Nikolic-Paterson DJ, and Lan HY. (2016). TGF-β: the master regulator of fibrosis. Nature Reviews Nephrology 12, 325–338. 10.1038/nrneph.2016.48. [DOI] [PubMed] [Google Scholar]
- 31.Rao KM. (2001). MAP kinase activation in macrophages. J Leukoc Biol 69, 3–10. [PubMed] [Google Scholar]
- 32.McKay MM, and Morrison DK. (2007). Integrating signals from RTKs to ERK/MAPK. Oncogene 26, 3113–3121. 10.1038/sj.onc.1210394. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J, Guo J, Dzhagalov I, and He Y-W. (2005). An essential function for the calcium-promoted Ras inactivator in Fcγ receptor–mediated phagocytosis. Nature immunology 6, 911–919. 10.1038/ni1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Botelho RJ, Harrison RE, Stone JC, Hancock JF, Philips MR, Jongstra-Bilen J, Mason D, Plumb J, Gold MR, and Grinstein S. (2009). Localized diacylglycerol-dependent stimulation of Ras and Rap1 during phagocytosis. The Journal of biological chemistry 284, 28522–28532. 10.1074/jbc.M109.009514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, Tsui H, Wu P, Davidson MG, Alonso MN, et al. (2011). B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nature medicine 17, 610–617. 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim J, Hayton WL, Robinson JM, and Anderson CL. (2007). Kinetics of FcRn-mediated recycling of IgG and albumin in human: pathophysiology and therapeutic implications using a simplified mechanism-based model. Clin Immunol 122, 146–155. 10.1016/j.clim.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akilesh S, Christianson GJ, Roopenian DC, and Shaw AS. (2007). Neonatal FcR expression in bone marrow-derived cells functions to protect serum IgG from catabolism. J Immunol 179, 4580–4588. 10.4049/jimmunol.179.7.4580. [DOI] [PubMed] [Google Scholar]
- 38.Challa DK, Wang X, Montoyo HP, Velmurugan R, Ober RJ, and Ward ES. (2019). Neonatal Fc receptor expression in macrophages is indispensable for IgG homeostasis. mAbs 11, 848–860. 10.1080/19420862.2019.1602459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mancuso P, and Bouchard B. (2019). The Impact of Aging on Adipose Function and Adipokine Synthesis. Frontiers in endocrinology 10, 137. 10.3389/fendo.2019.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, et al. (2009). Identification and importance of brown adipose tissue in adult humans. The New England journal of medicine 360, 1509–1517. 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qiang L, Wang L, Kon N, Zhao W, Lee S, Zhang Y, Rosenbaum M, Zhao Y, Gu W, Farmer SR, and Accili D. (2012). Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Ppargamma. Cell 150, 620–632. 10.1016/j.cell.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Camell CD, Gunther P, Lee A, Goldberg EL, Spadaro O, Youm YH, Bartke A, Hubbard GB, Ikeno Y, Ruddle NH, et al. (2019). Aging Induces an Nlrp3 Inflammasome-Dependent Expansion of Adipose B Cells That Impairs Metabolic Homeostasis. Cell Metab. 10.1016/j.cmet.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, Tsui H, Wu P, Davidson MG, Alonso MN, et al. (2011). B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nature medicine 17, 610–617. 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ying W, Wollam J, Ofrecio JM, Bandyopadhyay G, El Ouarrat D, Lee YS, Oh DY, Li P, Osborn O, and Olefsky JM. (2017). Adipose tissue B2 cells promote insulin resistance through leukotriene LTB4/LTB4R1 signaling. J Clin Invest 127, 1019–1030. 10.1172/JCI90350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DeFuria J, Belkina AC, Jagannathan-Bogdan M, Snyder-Cappione J, Carr JD, Nersesova YR, Markham D, Strissel KJ, Watkins AA, Zhu M, et al. (2013). B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc Natl Acad Sci U S A 110, 5133–5138. 10.1073/pnas.1215840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Radl J, Sepers JM, Skvaril F, Morell A, and Hijmans W. (1975). Immunoglobulin patterns in humans over 95 years of age. Clin Exp Immunol 22, 84–90. [PMC free article] [PubMed] [Google Scholar]
- 47.Natsuume-Sakai S, Motonishi K, and Migita S. (1977). Quantitative estimations of five classes of immunoglobulin in inbred mouse strains. Immunology 32, 861–866. [PMC free article] [PubMed] [Google Scholar]
- 48.Yadav H, Quijano C, Kamaraju AK, Gavrilova O, Malek R, Chen W, Zerfas P, Zhigang D, Wright EC, Stuelten C, et al. (2011). Protection from obesity and diabetes by blockade of TGF-beta/Smad3 signaling. Cell Metab 14, 67–79. 10.1016/j.cmet.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuo TT, Baker K, Yoshida M, Qiao SW, Aveson VG, Lencer WI, and Blumberg RS. (2010). Neonatal Fc receptor: from immunity to therapeutics. J Clin Immunol 30, 777–789. 10.1007/s10875-010-9468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zuercher AW, Spirig R, Baz Morelli A, Rowe T, and Kasermann F. (2019). Next-generation Fc receptor-targeting biologics for autoimmune diseases. Autoimmun Rev 18, 102366. 10.1016/j.autrev.2019.102366. [DOI] [PubMed] [Google Scholar]
- 51.Mezo AR, McDonnell KA, Hehir CA, Low SC, Palombella VJ, Stattel JM, Kamphaus GD, Fraley C, Zhang Y, Dumont JA, and Bitonti AJ. (2008). Reduction of IgG in nonhuman primates by a peptide antagonist of the neonatal Fc receptor FcRn. Proc Natl Acad Sci U S A 105, 2337–2342. 10.1073/pnas.0708960105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tschop MH, Speakman JR, Arch JR, Auwerx J, Bruning JC, Chan L, Eckel RH, Farese RV Jr., Galgani JE, Hambly C, et al. (2011). A guide to analysis of mouse energy metabolism. Nat Methods 9, 57–63. 10.1038/nmeth.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou Q, Yu L, Cook JR, Qiang L, and Sun L. (2023). Deciphering the decline of metabolic elasticity in aging and obesity. Cell Metab 35, 1661–1671.e1666. 10.1016/j.cmet.2023.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Protein list from Mass Spectrometry, related to Figure 1.
Data Availability Statement
The RNA-seq data has been deposited to the NGDC (https://ngdc.cncb.ac.cn/) (PRJCA002140 & PRJCA020637) and are publicly available at the date of publication.
This paper does not report original code.
All the western blots raw data and excel spreadsheets containing the values for all graphs were provided in Data S1-Source data. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.