Abstract
OBJECTIVE
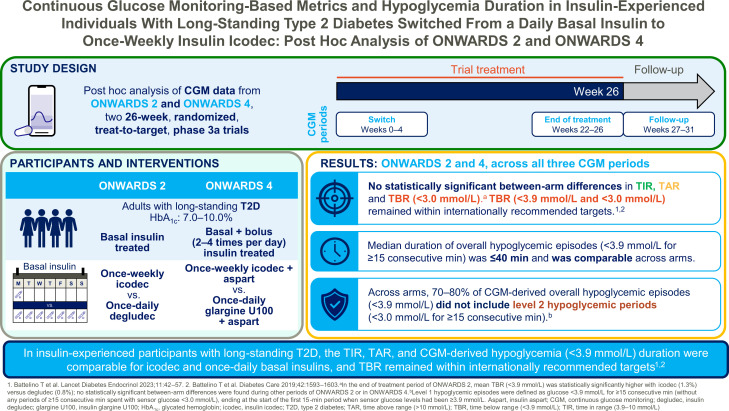
This post hoc analysis assessed continuous glucose monitoring (CGM)–based metrics and hypoglycemia duration with once-weekly insulin icodec versus once-daily basal insulin analogs in insulin-experienced individuals with long-standing type 2 diabetes from two 26-week phase 3a trials (ONWARDS 2 and ONWARDS 4).
RESEARCH DESIGN AND METHODS
Time in range (TIR) (3.9–10.0 mmol/L), time above range (TAR) (>10.0 mmol/L), and time below range (TBR) (<3.9 mmol/L and <3.0 mmol/L) were assessed during three CGM time periods (switch [weeks 0–4], end of treatment [weeks 22–26], and follow-up [weeks 27–31]) for icodec versus comparators (ONWARDS 2, insulin degludec [basal regimen]; ONWARDS 4, insulin glargine U100 [basal-bolus regimen]) using double-blind CGM data. CGM-derived hypoglycemic episode duration (<3.9 mmol/L) was assessed.
RESULTS
In both trials, there were no statistically significant differences in TIR, TAR, or TBR (<3.0 mmol/L) for icodec versus comparators across all time periods. In the end-of-treatment period, mean TIR was 63.1% (icodec) vs. 59.5% (degludec) in ONWARDS 2 and 66.9% (icodec) vs. 66.4% (glargine U100) in ONWARDS 4. Mean TBR <3.9 mmol/L and <3.0 mmol/L remained within recommended targets (<4% and <1%, respectively) across time periods and treatment arms. Hypoglycemic episode duration (<3.9 mmol/L) was comparable across time periods and treatment arms (median duration ≤40 min).
CONCLUSIONS
In insulin-experienced participants with long-standing type 2 diabetes, CGM-based TIR, TAR, and CGM-derived hypoglycemia duration (<3.9 mmol/L) were comparable for icodec and once-daily basal insulin analogs during all time periods. TBR remained within recommended targets.
Graphical Abstract
Introduction
By providing a comprehensive glucose profile, continuous glucose monitoring (CGM) data can offer a deeper understanding of variability in glucose levels, hyperglycemia, and hypoglycemia than self-measured blood glucose or HbA1c measurements alone (1). International consensus guidelines have been developed to ensure consistent and accurate CGM assessments and to provide definitions for key glycemic targets and guidance for the use of CGM assessments in clinical trials (2–4).
Insulin icodec is a basal insulin analog in clinical development with a mean half-life of ∼1 week, allowing for once-weekly dosing (5,6). In phase 2 trials in individuals with type 2 diabetes, once-weekly icodec treatment was associated with increased time in target glycemic range (TIR) (3.9–10.0 mmol/L) versus insulin glargine U100, without an increase in clinically significant hypoglycemia (<3.0 mmol/L) (7,8). The efficacy and safety of icodec have been further evaluated in six phase 3a trials forming the ONWARDS clinical program (6,9–14), including two trials in insulin-experienced individuals with long-standing type 2 diabetes: ONWARDS 2 and ONWARDS 4 (6,10,12).
In ONWARDS 2, icodec demonstrated noninferiority and superiority to insulin degludec for HbA1c reduction in basal insulin-treated individuals. Moreover, clinically significant or severe hypoglycemia rates were low, with numerically, but not statistically significantly higher event rates for icodec versus degludec (10). In ONWARDS 4, icodec demonstrated noninferiority to glargine U100 for HbA1c reduction in individuals with type 2 diabetes using a basal-bolus insulin regimen, with similar hypoglycemia rates in both treatment arms (12). In ONWARDS 2 and 4, during the end-of-treatment period (weeks 22–26), no statistically significant between-arm differences were found for CGM-based TIR (3.9–10.0 mmol/L) or time below range (TBR) (<3.0 mmol/L) (10,12).
Blinded CGM data, collected during three time periods of ONWARDS 2 and ONWARDS 4, can provide detailed clinically relevant information regarding glycemic metrics and hypoglycemia duration among individuals with long-standing type 2 diabetes with a higher risk of hypoglycemia who were switched from daily basal insulin to once-weekly icodec or a once-daily basal insulin comparator. These data can be used to further evaluate the respective effects and safety of the switching protocol, ongoing icodec treatment, and ending trial treatment. A post hoc analysis was conducted to explore these end points further.
Research Design and Methods
Trial Design and Participants
ONWARDS 2 and ONWARDS 4 were conducted in compliance with the principles of the Declaration of Helsinki and in accordance with the International Conference for Harmonization Good Clinical Practice guidelines (15). The designs, methods, and statistical analyses of the ONWARDS 2 and ONWARDS 4 trials have been described previously (6,10,12). Briefly, both ONWARDS 2 and ONWARDS 4 were randomized, open-label, treat-to-target, multicenter, phase 3a trials designed to evaluate the efficacy and safety of icodec in adults with type 2 diabetes with HbA1c 7.0–10.0% (53–86 mmol/mol). Each trial comprised a 2-week screening period, a 26-week treatment period, and a 5-week follow-up period (Supplementary Fig. 1).
ONWARDS 2 investigated treatment with once-weekly icodec versus once-daily degludec (with or without noninsulin glucose-lowering agents) in adults with type 2 diabetes previously treated with once- or twice-daily basal insulin (10). In ONWARDS 4, adults with type 2 diabetes treated with a basal-bolus insulin regimen (with or without noninsulin glucose-lowering agents) were randomized to receive once-weekly icodec or once-daily glargine U100, both in combination with two to four daily injections of insulin aspart.
Treatments
In both trials, for individuals switching to icodec, the weekly icodec dose was calculated by multiplying the pretrial daily basal insulin dose by 7. For the first injection only, a one-time additional 50% dose of icodec was also administered. Icodec and the once-daily basal insulin comparators were titrated weekly based on prebreakfast self-measured blood glucose (SMBG) values (target 4.4–7.2 mmol/L) (6,10,12).
In ONWARDS 4, the switch from the participant’s previous bolus insulin to aspart was done unit-to-unit per meal for both trial arms. The aspart dose could be altered during the first 8 weeks for safety reasons only. Thereafter, dose adjustments were made every 3–4 days based on preprandial and bedtime SMBG values. In both trials, sulfonylureas and glinides were discontinued at randomization.
CGM Assessments
In both trials, a blinded CGM device (Dexcom G6; Dexcom, San Diego, CA) was worn during the following trial periods: the switch period, defined as 0–4 weeks after initiation of randomized treatment; the end-of-treatment period, defined as 22–26 weeks after randomization (i.e., before the end of randomized treatment); and the follow-up period, defined as 27–31 weeks after randomization (i.e., after the end of randomized treatment) (6,10,12) (Supplementary Fig. 1). Per consensus guidelines, for a participant to be included in the summary of CGM glycemic control end points, ≥70% of the planned CGM measurements had to be available for the time period assessed (3).
Post Hoc Analysis: Outcomes
CGM-Based Glycemic Management
The CGM-based percentage of TIR (defined as sensor glucose 3.9–10.0 mmol/L), time above range (TAR) (sensor glucose >10.0 mmol/L), and TBR (sensor glucose <3.9 mmol/L and <3.0 mmol/L) were assessed during the switch, end-of-treatment, and follow-up periods. The proportions of participants attaining the three recommended CGM targets of >70% TIR, <25% TAR, and <4% TBR (<3.9 mmol/L) were reported during the end-of-treatment period (4). Glycemic variability was assessed based on the coefficient of variation percent (CV%) during the switch, end-of-treatment, and follow-up periods.
CGM-Derived Hypoglycemia Duration
In accordance with international consensus guidelines, CGM-derived overall hypoglycemic episodes were defined as ≥15 consecutive minutes of sensor glucose <3.9 mmol/L and were considered to have ended at the start of the first 15-min period with a sensor glucose ≥3.9 mmol/L (3). A level 1 hypoglycemic episode was defined as ≥15 consecutive minutes of sensor glucose <3.9 mmol/L (without any time periods of ≥15 consecutive minutes spent with levels <3.0 mmol/L), ending at the start of the first 15-min period with a sensor glucose ≥3.9 mmol/L (3). A level 2 hypoglycemic period was defined as sensor glucose <3.0 mmol/L for ≥15 consecutive minutes, ending at the start of the first 15-min period with a sensor glucose ≥3.0 mmol/L (3,4).
The median duration of CGM-derived overall hypoglycemic episodes (sensor glucose <3.9 mmol/L) and level 2 hypoglycemic periods (sensor glucose <3.0 mmol/L) was assessed during the switch, end-of-treatment, and follow-up periods. Overall hypoglycemic episodes (sensor glucose <3.9 mmol/L) were divided into clinically relevant subtypes based on the time spent with sensor glucose <3.0 mmol/L (no time, >0 to <15 consecutive minutes, or ≥15 consecutive minutes).
Basal Insulin Use After Trial Treatment
After trial treatment was ended, during the follow-up period, protocols for ONWARDS 2 and ONWARDS 4 recommended that participants be transferred to any available basal insulin at the discretion of the investigator. For participants who received icodec, the protocols recommended to initiate daily basal insulin 2 weeks after the last icodec dose or earlier if prebreakfast SMBG was >10.0 mmol/L. The proportion of participants who transitioned from the trial product to a daily basal insulin was assessed during the follow-up period. The timing of this transition was also captured.
Statistical Analysis
Statistical analyses were performed using SAS 9.4 software (SAS Institute Inc., Cary, NC). Descriptive summaries were performed using either SAS 9.4 or R 4.0.4.
CGM-based TIR and TAR were analyzed using an ANOVA model, with geographic region, personal CGM device use, and treatment assignment as fixed factors. The estimated treatment difference was calculated for icodec versus degludec (ONWARDS 2) and icodec versus glargine U100 (ONWARDS 4).
CGM-based TBR was analyzed using negative binomial regression with geographic region, personal CGM device use, and treatment assignment as fixed factors. The estimated rate ratio was calculated for icodec versus degludec (ONWARDS 2) and icodec versus glargine U100 (ONWARDS 4).
The binary response for meeting all three CGM targets (>70% TIR, <25% TAR, and <4% TBR) was analyzed using logistic regression with geographic region, personal CGM device use, and treatment assignment as fixed factors. The estimated odds ratio was calculated for icodec versus degludec (ONWARDS 2) and icodec versus glargine U100 (ONWARDS 4).
The statistical significance level was set to 0.05 (two-tailed hypothesis test) for all analyses, and there was no correction for multiplicity. Missing values from the end-of-treatment and follow-up periods were imputed using multiple imputation based on data from participants in the comparator arm who had completed randomized treatment. For ONWARDS 2, imputation used data for only those participants who had not initiated bolus insulin for >2 weeks at any time before the week 26 visit. No imputation was performed for the data assessed during the switch period; no imputation was performed for analyses of TBR.
Results
Participants
The post hoc analysis included the full analysis sets from each trial: 526 participants from ONWARDS 2 (icodec, n = 263; degludec, n = 263) and 582 from ONWARDS 4 (icodec, n = 291; glargine U100, n = 291). Baseline demographics and clinical characteristics of participants have been described previously (10,12). Mean (SD) diabetes duration was 16.7 (8.1) years in ONWARDS 2 and 17.1 (8.4) years in ONWARDS 4. Mean (SD) HbA1c was 8.13% (0.77%) (65 [8] mmol/mol) in ONWARDS 2 and 8.30% (0.88%) (67 [10] mmol/mol) in ONWARDS 4.
Switch Period (Weeks 0–4)
CGM-Based Glycemic Management
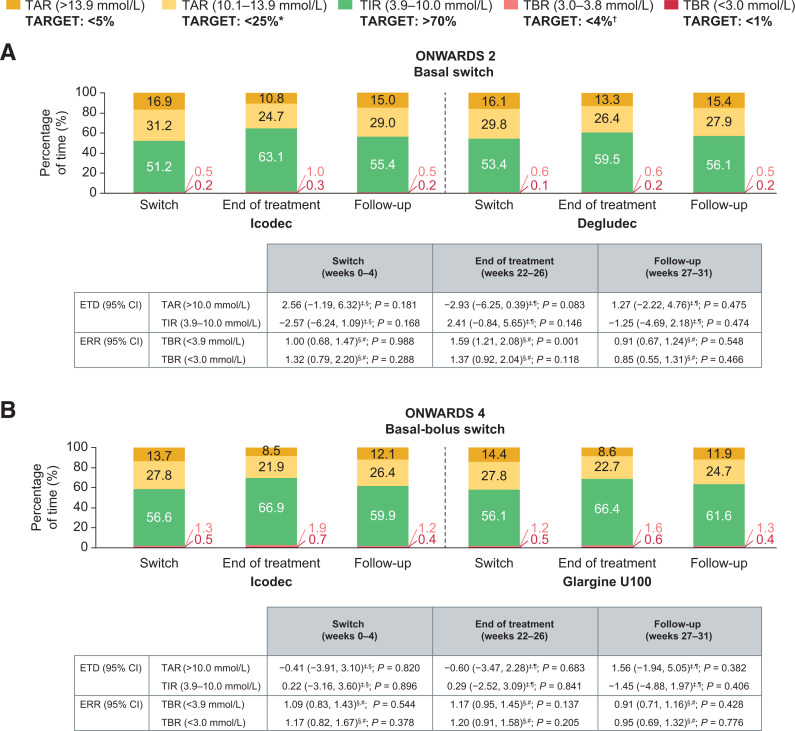
During the switch period, there were no statistically significant differences in mean percentage of CGM-based TIR, TAR, and TBR with icodec versus degludec in ONWARDS 2 (Fig. 1A) or versus glargine U100 in ONWARDS 4 (Fig. 1B). The mean percentage of TIR in ONWARDS 2 was 51.2% (equivalent to ∼12 h 17 min per day) in the icodec arm and 53.4% (equivalent to ∼12 h 49 min per day) in the degludec arm, whereas in ONWARDS 4, the percentage of TIR was 56.6% (equivalent to ∼13 h 35 min per day) in the icodec arm and 56.1% (equivalent to ∼13 h 28 min per day) in the glargine U100 arm (Supplementary Table 1).
Figure 1.
Mean CGM metrics during the switch periods (weeks 0–4), end-of-treatment periods (weeks 22–26), and follow-up periods (weeks 27–31) of ONWARDS 2 (A) and ONWARDS 4 (B). *Target includes TAR >13.9 mmol/L. †Target includes TBR <3.0 mmol/L. ‡Value is the estimated treatment difference (ETD) (percentage points) between the groups (icodec − degludec [ONWARDS 2]; icodec − glargine U100 [ONWARDS 4]). §Statistical analysis based on observed data. ¶Statistical analysis based on multiple imputed data. #Value is the estimated rate ratio (ERR) (icodec/degludec [ONWARDS 2]; icodec/glargine U100 [ONWARDS 4]). Statistical models include linear regression and negative binomial regression to obtain ETD and ERR, respectively. All models are adjusted for geographic region and use of personal CGM or intermittently scanned CGM device. CGM-recommended targets are based on international guidelines reported in Battelino et al. (4).
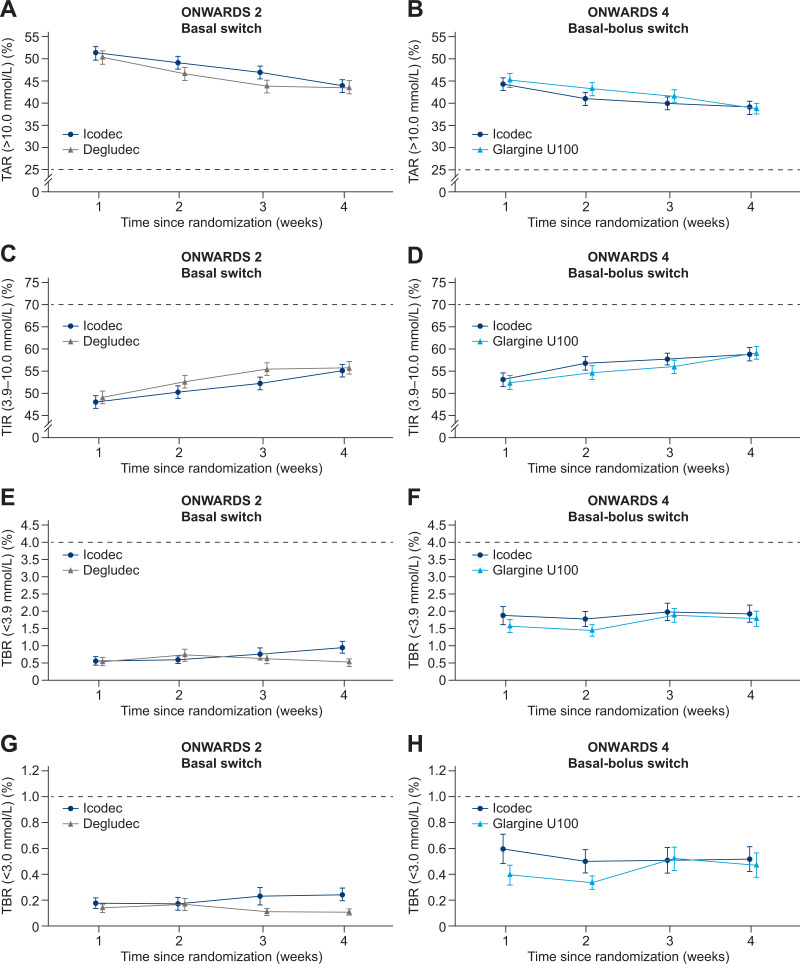
In both trials, there was no increase in mean percentage of TAR or decrease in mean percentage of TIR across weeks 0–4 in any treatment arm (Fig. 2A–D); the mean percentage of TIR increased during the switch period in both treatment arms (Fig. 2C and D). The mean percentage of TBR with sensor glucose <3.9 mmol/L (Fig. 2E and F) and TBR with sensor glucose <3.0 mmol/L (Fig. 2G and H) remained within the recommended targets (<4% and <1%, respectively) (2,4) in all treatment arms. There was no apparent clustering of overall hypoglycemic episodes (Supplementary Fig. 2A and B). Within-participant glycemic variability (geometric mean of CV% [CV%]) was comparable for icodec (28.84% [19.95%]) and degludec (29.50% [19.17%]) in ONWARDS 2, and for icodec (30.53% [23.44%]) and glargine U100 (31.27% [20.46%]) in ONWARDS 4 (Supplementary Fig. 3A and B).
Figure 2.
Percentage of CGM-based TAR in ONWARDS 2 (A) and ONWARDS 4 (B), TIR in ONWARDS 2 (C) and ONWARDS 4 (D), TBR (<3.9 mmol/L) in ONWARDS 2 (E) and ONWARDS 4 (F), and TBR (<3.0 mmol/L) in ONWARDS 2 (G) and ONWARDS 4 (H) by week during the switch period. Plots show mean (SE) (error bars). Percentage of time spent is defined as (number of recorded measurements in a given range/divided by the number of recorded measurements) × 100. Dashed lines indicate recommended glycemic targets (4).
CGM-Derived Hypoglycemia Duration
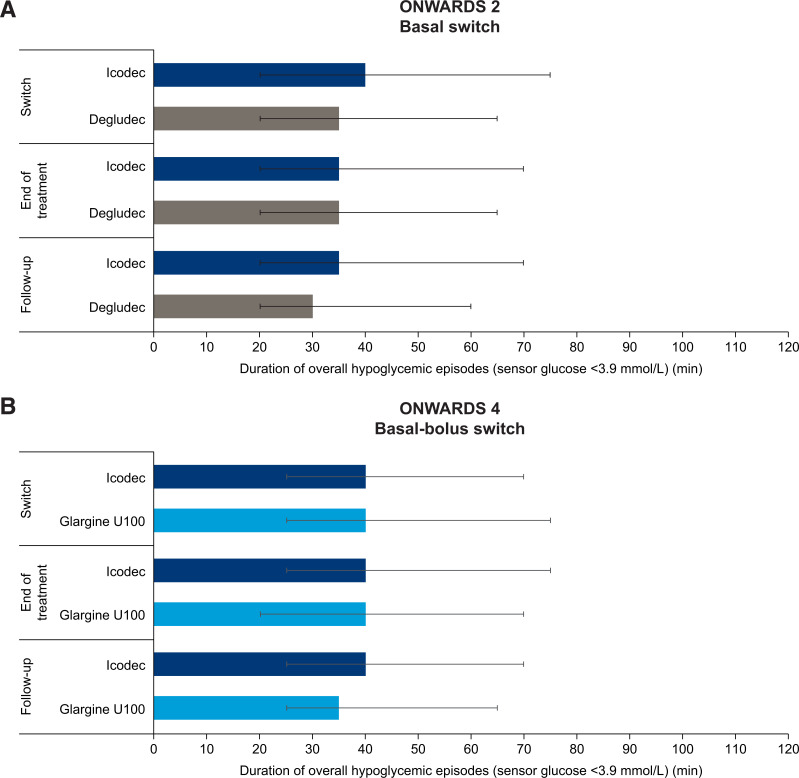
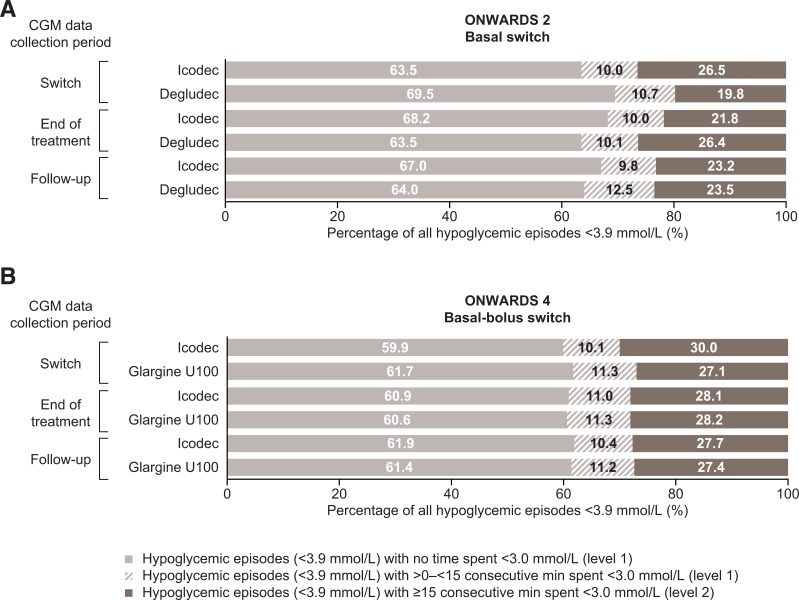
In both trials, the median duration of CGM-derived overall hypoglycemic episodes (sensor glucose <3.9 mmol/L) was comparable for icodec and the comparators. Median (interquartile range [IQR]) durations were 40 (20–75) min (icodec) and 35 (20–65) min (degludec) in ONWARDS 2 and 40 (25–70) min (icodec) and 40 (25–75) min (glargine U100) in ONWARDS 4 (Fig. 3A and B). Across the overall hypoglycemic episodes that occurred during the switch period, the majority included no time with sensor glucose <3.0 mmol/L: 63.5% (icodec) and 69.5% (degludec) in ONWARDS 2 and 59.9% (icodec) and 61.7% (glargine U100) in ONWARDS 4. Across trials, in all treatment arms, ∼10–11% of hypoglycemic episodes included >0 to <15 consecutive minutes with sensor glucose <3.0 mmol/L. The remaining episodes included ≥15 consecutive minutes with sensor glucose <3.0 mmol/L (Fig. 4A and B).
Figure 3.
Duration of overall CGM-derived hypoglycemic episodes (sensor glucose <3.9 mmol/L) during the switch, end-of-treatment, and follow-up periods of ONWARDS 2 (A) and ONWARDS 4 (B). Plots show median (IQR) (error bars). During the follow-up period, most participants started treatment with daily basal insulin; therefore, hypoglycemia duration is shown according to the treatment arm assigned during the trial.
Figure 4.
Classification of CGM-derived overall hypoglycemic episodes (sensor glucose <3.9 mmol/L) by time spent with sensor glucose <3.0 mmol/L during the switch, end-of-treatment, and follow-up periods of ONWARDS 2 (A) and ONWARDS 4 (B).
The median (IQR) duration of CGM-derived level 2 hypoglycemic periods (sensor glucose <3.0 mmol/L) was 40 (20–65) min (icodec) and 30 (20–55) min (degludec) in ONWARDS 2 and 30 (20–60) min (icodec) and 35 (20–60) min (glargine U100) in ONWARDS 4. Durations of CGM-derived overall hypoglycemic episodes (sensor glucose <3.9 mmol/L) by day during the switch period for both trials are shown in Supplementary Fig. 4A and B. In both trials, duration of hypoglycemic episodes remained generally stable in the icodec and comparator arms.
End-of-Treatment Period (Weeks 22–26)
CGM-Based Glycemic Management
During the end-of-treatment period, there was no statistically significant difference in mean percentage of CGM-based TIR or TAR with icodec versus degludec in ONWARDS 2 (Fig. 1A) or glargine U100 in ONWARDS 4 (Fig. 1B and Supplementary Table 1).
In ONWARDS 2, mean percentage of TBR (sensor glucose <3.9 mmol/L) was low in both arms (icodec, 1.3% [equivalent to ∼19 min/day]; degludec, 0.8% [equivalent to ∼11 min/day]) and within the recommended target of <4% (2); however, it was statistically significantly greater with icodec than with degludec (estimated rate ratio 1.59; 95% CI 1.21, 2.08; P = 0.001) (Fig. 1A). There were no statistically significant differences between icodec and degludec in mean percentage of TBR (sensor glucose <3.0 mmol/L) in ONWARDS 2. In ONWARDS 4, there was no statistically significant difference between treatment arms in mean percentage of TBR (sensor glucose <3.9 mmol/L and sensor glucose <3.0 mmol/L) (Fig. 1B).
No statistically significant differences between treatment arms were observed in ONWARDS 2 and ONWARDS 4 in the proportion of participants who achieved all three CGM targets (>70% TIR, <25% TAR, <4% TBR <3.9 mmol/L) (Supplementary Fig. 5A and B). Within-participant glycemic variability (geometric mean of CV% [CV%]) was similar for icodec (31.81% [17.04%]) and degludec (31.02% [16.02%]) in ONWARDS 2, and for icodec (33.26% [18.52%]) and glargine U100 (32.61% [16.27%]) in ONWARDS 4 (Supplementary Fig. 3C and D).
CGM-Derived Hypoglycemia Duration
In the end-of-treatment period, median duration of CGM-derived overall hypoglycemic episodes (sensor glucose <3.9 mmol/L) was comparable for icodec and the once-daily comparators. The median (IQR) duration of these hypoglycemic episodes was 35 (20–70) min (icodec) and 35 (20–65) min (degludec) in ONWARDS 2 and 40 (25–75) min (icodec) and 40 (20–70) min (glargine U100) in ONWARDS 4 (Fig. 3A and B).
Across the CGM-derived overall hypoglycemic episodes (sensor glucose <3.9 mmol/L) that occurred in the end-of-treatment period, the majority included no time with sensor glucose <3.0 mmol/L: 68.2% (icodec) and 63.5% (degludec) in ONWARDS 2 and 60.9% (icodec) and 60.6% (glargine U100) in ONWARDS 4. The percentage of episodes that included periods of ≥15 consecutive minutes with sensor glucose <3.0 mmol/L was 21.8% (icodec) and 26.4% (degludec) in ONWARDS 2 and 28.1% (icodec) and 28.2% (glargine U100) in ONWARDS 4 (Fig. 4A and B).
Median (IQR) duration of CGM-derived level 2 hypoglycemic periods (sensor glucose <3.0 mmol/L) was 30 (20–65) min (icodec) and 35 (20–65) min (degludec) in ONWARDS 2 and 30 (20–60) min (icodec) and 30 (20–55) min (glargine U100) in ONWARDS 4. Median duration of CGM-derived overall hypoglycemic episodes (sensor glucose <3.9 mmol/L) by day during the end-of-treatment periods of both trials is shown in Supplementary Fig. 4C and D. In both trials, duration of hypoglycemic episodes remained generally stable in the icodec arm across the week after injection. The glycemic metrics by day during the end-of-treatment period are summarized in Supplementary Fig. 6A and B. In both trials, mean percentage of TIR remained fairly stable throughout the week across arms; minor fluctuations were observed in TBR and TAR in the icodec arms, with the mean percentage of TBR (sensor glucose <3.9 mmol/L) tending to be slightly higher on days 2–4 versus other days of the week. A similar trend for slightly higher mean percentage of TBR (sensor glucose <3.9 mmol/L) on days 1–2 compared with other days of the week was observed for the degludec and glargine U100 arms in ONWARDS 2 and ONWARDS 4, respectively.
Follow-Up Period (Weeks 27–31)
Basal Insulin Use After Trial Treatment
In both trials, the majority of participants in the comparator arms (degludec in ONWARDS 2 and glargine U100 in ONWARDS 4) had begun alternative daily basal insulin by 1 day after ending trial treatment (Supplementary Fig. 7). Per the trial protocols, >70% and >60% of participants in the icodec arms of ONWARDS 2 and ONWARDS 4, respectively, had started daily basal insulin 2 weeks after the last icodec injection (i.e., 7 days after ending the treatment period).
CGM-Based Glycemic Management
There were no statistically significant differences in the mean percentage of CGM-based TIR, TAR, or TBR between arms in ONWARDS 2 (Fig. 1A) or ONWARDS 4 (Fig. 1B). For both trials, in participants who had previously received icodec or comparator during the trial, the mean percentage of TIR ranged from 55.4% to 61.6% in the follow-up period (Fig. 1A and B and Supplementary Table 1). Percentages of TAR, TIR, and TBR by week during weeks 27–31 are illustrated in Supplementary Fig. 8.
Glycemic variability (CV%) appeared to be lower in participants who had been treated with icodec than with degludec (ONWARDS 2) or glargine U100 (ONWARDS 4) during week 27. During weeks 28–31, variability was comparable between treatment arms in both trials (Supplementary Fig. 3E and F).
CGM-Derived Hypoglycemia Duration
During the follow-up period, median (IQR) duration of CGM-derived overall hypoglycemic episodes (sensor glucose <3.9 mmol/L) for participants who had received icodec during the trial was 35 (20–70) min in ONWARDS 2 and 40 (25–70) min in ONWARDS 4. Comparable durations were reported for hypoglycemic episodes in comparator arms (Fig. 3).
Across the CGM-derived overall hypoglycemic episodes (sensor glucose <3.9 mmol/L) that occurred in the follow-up period, the majority included no time with sensor glucose <3.0 mmol/L: 67.0% (icodec) and 64.0% (degludec) in ONWARDS 2 and 61.9% (icodec) and 61.4% (glargine U100) in ONWARDS 4. For both arms in both trials, some episodes included >0 to <15 consecutive minutes or ≥15 consecutive minutes with sensor glucose <3.0 mmol/L (Fig. 4). During the follow-up period, median (IQR) duration of CGM-derived level 2 hypoglycemic periods (sensor glucose <3.0 mmol/L) was 35 (20–60) min (icodec) and 30 (20–55) min (degludec) in ONWARDS 2 and 30 (20–55) min (icodec) and 30 (20–50) min (glargine U100) in ONWARDS 4.
Conclusions
In insulin-experienced participants with long-standing type 2 diabetes, compared with once-daily basal insulin analogs, switching from daily basal insulin to once-weekly icodec was associated with similar mean percentages of CGM-based TIR and TAR when assessed across three separate time periods in ONWARDS 2 and ONWARDS 4. Moreover, mean CGM-based TBR remained within the recommended targets in both arms in both trials (2). The duration of CGM-derived overall hypoglycemic episodes (sensor glucose <3.9 mmol/L) was comparable for icodec versus once-daily basal insulins, with median duration ≤40 min.
CGM data collection during the switch period allowed for detailed assessments of glycemic effects during the transition to icodec in insulin-experienced trial populations. Switching from a daily basal insulin regimen to once-weekly icodec, including a one-time additional 50% icodec dose at initiation, did not compromise glycemic control (no statistically significant difference in mean TAR or TIR) versus comparators, and mean TBR remained within the recommended targets (2). Furthermore, in both trials, the geometric mean of CV% was <36% throughout the switch period, indicating stable glucose levels for icodec by international consensus definitions (3). These results may reassure physicians that individuals who are treated with existing basal or basal-bolus insulin regimens can be switched to icodec with continued glucose control during the switch period.
During the end-of-treatment period, as previously reported, CGM-based glycemic control was similar with icodec versus once-daily insulin comparators, with mean percentage of TIR close to the recommended CGM target of >70% (2,10,12). In this post hoc analysis, although a statistically significantly higher mean percentage of TBR (sensor glucose <3.9 mmol/L) was reported for icodec versus degludec in ONWARDS 2, it was well within the recommended target (<4%), and no statistically significant difference was reported between arms for the mean percentage of time with sensor glucose <3.0 mmol/L (considered clinically significant according to the recent consensus statement [3]). Moreover, in ONWARDS 4, the mean percentage of TBR (sensor glucose <3.9 mmol/L and <3.0 mmol/L) was similar for the icodec and glargine U100 arms. Furthermore, as shown in the analysis of glycemic metrics by day in the end-of-treatment period, the mean percentage of TBR (sensor glucose <3.9 mmol/L) remained generally low across the days following day 1 (day of icodec injection) in ONWARDS 2 and 4 but appeared to be slightly higher on days 2–4 than on other days. This finding is potentially in alignment with previously reported pharmacodynamic data for icodec, which showed a close to even distribution of glucose-lowering effects over the week, with marginally higher effects on days 2–4 (16). Of note, a similar trend for a slightly higher mean percentage of TBR (<3.9 mmol/L) on days 1–2 was found in the comparator arms; this may reflect the initial glycemic changes in the days immediately following titration (which occurred once weekly on day 1), variability in carbohydrate intake across the week, or insulin dose downtitrations by the investigator during the latter part of the week (which were permitted for safety reasons). In both trials, the proportions of participants who attained all three recommended CGM targets during the end-of-treatment period were similar for the icodec and comparator arms, and although they were not very high, this may be expected in trial populations comprising participants with long-standing type 2 diabetes.
CGM data collected during the follow-up period are also clinically relevant for individuals who may need to stop treatment owing to various circumstances (e.g., hospitalization, pregnancy). A switch to daily insulin may be recommended 2 weeks after the last dose of icodec, or earlier, if prebreakfast SMBG is >10.0 mmol/L. Participants randomized to icodec received their last injection at the start of week 26, and most had switched to a daily basal insulin ∼2 weeks after their last injection. During the follow-up period, the finding of similar mean percentages of CGM-based TAR, TIR, and TBR between treatment arms in both trials may provide physicians with evidence to support the transition from icodec with appropriate timing of initiation of daily basal insulin treatments, if necessary.
In both trials, across all periods, the median duration of CGM-derived overall hypoglycemic episodes and level 2 hypoglycemic periods was ≤40 min and was comparable for icodec versus once-daily comparators. The median duration of hypoglycemia was not considered prolonged based on the consensus definition of prolonged events (120 min) (3); hence, the duration of hypoglycemia was not influenced by the long duration of action for icodec. Reassuringly, the majority of CGM-derived overall hypoglycemic episodes did not include level 2 hypoglycemic periods (sensor glucose <3.0 mmol/L for ≥15 min) during any of the CGM data collection periods in any treatment arm (4).
A strength of this analysis was the inclusion of data from two large, randomized, phase 3a trials, enabling comprehensive evaluation of CGM metrics in insulin-experienced participants with long-standing type 2 diabetes. The observed similarities in CGM-based metrics, particularly for TBR <3.0 mmol/L and CGM-derived hypoglycemia duration, between icodec and once-daily insulin analogs in these populations are reassuring. These findings are especially relevant when taken together with the positive HbA1c efficacy and self-reported hypoglycemia safety results of ONWARDS 2 and ONWARDS 4. Additionally, in both trials, CGM data were double-blind, so they could not influence changes to treatment or behavior, minimizing the potential confounding effect of CGM use. Finally, data obtained by assessing glycemic profiles across key periods during the clinical trials can provide useful information to assist health care providers with monitoring and prescribing decisions when switching individuals to and from once-weekly icodec.
The limitations of this study should also be considered. The ONWARDS 2 and 4 trials, and this post hoc analysis, were not designed to assess statistical differences between treatments for all CGM end points evaluated. Consequently, statistical testing was only performed for the mean CGM metrics reported in Fig. 1 and for the triple composite end point of meeting the international TIR, TAR, and TBR targets. Additionally, because CGM data were not captured at baseline and only during specific time periods, it was not possible to evaluate continuous changes from baseline during the trials. The treat-to-target trial design, with weekly assessments, should also be considered when interpreting these results. In clinical practice, titration of all insulins is highly individualized and may vary based on physician recommendations and individualized treatment needs, such as risk factors for hypoglycemia. Finally, although adults with long-standing type 2 diabetes were enrolled in ONWARDS 2 and ONWARDS 4, the generalizability of the results may be limited by the study criteria, particularly the exclusion of individuals with recent, recurrent severe hypoglycemic episodes or known hypoglycemic unawareness.
In conclusion, in this post hoc analysis of two large phase 3a trials in insulin-experienced adults with long-standing type 2 diabetes, treatment with once-weekly icodec versus once-daily basal insulin analogs was associated with comparable percentages of CGM-based TIR and TAR and CGM-derived median duration of hypoglycemic episodes. Furthermore, the percentage of CGM-based TBR remained within recommended targets across all arms. When considered alongside the results of the primary ONWARDS 2 and 4 studies, in which once-weekly icodec was associated with improved or similar glycemic control and no statistically significant increases in rates of clinically significant or severe hypoglycemic episodes versus once-daily basal insulin comparators, these results provide further reassurance for switching to icodec, a basal insulin analog with a long duration of action. Notably, treatment with once-weekly icodec could reduce injection burden from 365 to 52 basal insulin injections per year. Moreover, based on participant-reported outcomes in ONWARDS 2, treatment with once-weekly icodec was associated with improvements from baseline in treatment satisfaction compared with once-daily degludec (10). Taken together, these findings are relevant to clinical practice, where reduced injection burden and greater treatment satisfaction could contribute to improved adherence to basal insulin treatment.
This article contains supplementary material online at https://doi.org/10.2337/figshare.25104206.
Article Information
Acknowledgments. Medical writing support was provided by Samuel Bestall and Kate Ward of Oxford PharmaGenesis, Oxford, U.K., and funded by Novo Nordisk A/S.
Funding. This study was funded by Novo Nordisk A/S.
Duality of Interest. H.S.B. reported trial fees paid to his institution by Novo Nordisk during ONWARDS 3 and ONWARDS 5; he also reported trial fees paid to his institution by Amgen, AstraZeneca, Boehringer Ingelheim, Ceapro, Eli Lilly and Company, Gilead Sciences, Janssen Pharmaceuticals, Kowa Pharmaceuticals Co. Ltd., Ionis, Madrigal Pharmaceuticals, Merck, Novartis, Novo Nordisk, Pfizer, Sanofi, and Tricida outside the submitted work. B.Á., L.C., and C.L. are employees of Novo Nordisk A/S and may hold stock options. C.M. serves or has served on advisory boards for ActoBio Therapeutics, Avotres, AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Imcyse, Insulet, MannKind, Medtronic, MSD, Novartis, Novo Nordisk, Pfizer, Roche, Sandoz, Sanofi, Vertex Pharmaceuticals, and Zealand Pharma; financial compensation for this activity has been received by KU Leuven. KU Leuven has received research support for C.M. from ActoBio Therapeutics, Imcyse, Medtronic, Novo Nordisk, and Sanofi. C.M. participates or has participated in speaker’s bureaus for Boehringer Ingelheim, AstraZeneca, Eli Lilly and Company, Novartis, Novo Nordisk, and Sanofi; financial compensation for this activity has been received by KU Leuven. A.P.-T. performs research and serves as an advisor on behalf of her employer for Abbott, Dexcom, Eli Lilly and Company, Medtronic, Novo Nordisk, and Sanofi; there have been no direct or indirect transfers of funds. T.B. has served on advisory boards for Boehringer Ingelheim, Eli Lilly and Company, Indigo Diabetes, Medtronic, Novo Nordisk, and Sanofi and has received fees for participating in speaker’s bureaus for Abbott, AstraZeneca, Aventis, Eli Lilly and Company, Medtronic, Novo Nordisk, Roche, and Sanofi. T.B.’s institution has received research grant support from Abbott, the European Union, Medtronic, the National Institutes of Health, Novartis, Novo Nordisk, Sandoz, Sanofi, Zealand Pharma, and the Slovenian Research and Innovation Agency (grant P3-0343). No other potential conflicts of interest relevant to this article were reported.
Author Contributions. H.S.B., B.Á., L.C., C.L., C.M., A.P.-T., and T.B. provided substantial contributions to the conception and design of the study or the interpretation of data. All authors contributed to the review and revision of the manuscript and approved the final version. H.S.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented as posters or presentations at the 16th International Conference on Advanced Technologies & Treatments for Diabetes, Berlin, Germany, 22–25 February, 2023; the 83rd Scientific Sessions of the American Diabetes Association, San Diego, CA, 23–26 June 2023; and the European Association for the Study of Diabetes 59th Annual Meeting, Hamburg, Germany, and virtually, 2–6 October 2023.
Footnotes
Clinical trial reg. nos. NCT04770532 and NCT04880850, clinicaltrials.gov
References
- 1. Beyond A1C Writing Group . Need for regulatory change to incorporate beyond A1C glycemic metrics. Diabetes Care 2018;41:e92–e94 [DOI] [PubMed] [Google Scholar]
- 2. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 2019;42:1593–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care 2017;40:1631–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Battelino T, Alexander CM, Amiel SA, et al. Continuous glucose monitoring and metrics for clinical trials: an international consensus statement. Lancet Diabetes Endocrinol 2023;11:42–57 [DOI] [PubMed] [Google Scholar]
- 5. Nishimura E, Pridal L, Glendorf T, et al. Molecular and pharmacological characterization of insulin icodec: a new basal insulin analog designed for once-weekly dosing. BMJ Open Diabetes Res Care 2021;9:e002301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Philis-Tsimikas A, Bajaj HS, Begtrup K, et al. Rationale and design of the phase 3a development programme (ONWARDS 1-6 trials) investigating once-weekly insulin icodec in diabetes. Diabetes Obes Metab 2023;25:331–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bajaj HS, Bergenstal RM, Christoffersen A, et al. Switching to once-weekly insulin icodec versus once-daily insulin glargine U100 in type 2 diabetes inadequately controlled on daily basal insulin: a phase 2 randomized controlled trial. Diabetes Care 2021;44:1586–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lingvay I, Buse JB, Franek E, et al. A randomized, open-label comparison of once-weekly insulin icodec titration strategies versus once-daily insulin glargine U100. Diabetes Care 2021;44:1595–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosenstock J, Bain SC, Gowda A, et al. ; ONWARDS 1 Trial Investigators . Weekly icodec versus daily glargine U100 in type 2 diabetes without previous insulin. N Engl J Med 2023;389:297–308 [DOI] [PubMed] [Google Scholar]
- 10. Philis-Tsimikas A, Asong M, Franek E, et al. Switching to once-weekly insulin icodec versus once-daily insulin degludec in individuals with basal insulin-treated type 2 diabetes (ONWARDS 2): a phase 3a, randomised, open label, multicentre, treat-to-target trial. Lancet Diabetes Endocrinol 2023;11:414–425 [DOI] [PubMed] [Google Scholar]
- 11. Lingvay I, Asong M, Desouza C, et al. Once-weekly insulin icodec vs once-daily insulin degludec in adults with insulin-naive type 2 diabetes: the ONWARDS 3 randomized clinical trial. JAMA 2023;330:228–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mathieu C, Ásbjörnsdóttir B, Bajaj HS, et al. Switching to once-weekly insulin icodec versus once-daily insulin glargine U100 in individuals with basal-bolus insulin-treated type 2 diabetes (ONWARDS 4): a phase 3a, randomised, open-label, multicentre, treat-to-target, non-inferiority trial. Lancet 2023;401:1929–1940 [DOI] [PubMed] [Google Scholar]
- 13. Bajaj HS, Aberle J, Davies M, et al. Once-weekly insulin icodec with dosing guide app versus once-daily basal insulin analogues in insulin-naive type 2 diabetes (ONWARDS 5): a randomized trial. Ann Intern Med 2023;176:1476–1485 [DOI] [PubMed] [Google Scholar]
- 14. Russell-Jones D, Babazono T, Cailleteau R, et al. Once-weekly insulin icodec versus once-daily insulin degludec as part of a basal-bolus regimen in individuals with type 1 diabetes (ONWARDS 6): a phase 3a, randomised, open-label, treat-to-target trial. Lancet 2023;402:1636–1647 [DOI] [PubMed] [Google Scholar]
- 15. Dixon JR Jr. The International Conference on Harmonization Good Clinical Practice guideline. Qual Assur 1998;6:65–74 [DOI] [PubMed] [Google Scholar]
- 16. Pieber TR, Asong M, Fluhr G, et al. Pharmacokinetic and pharmacodynamic properties of once-weekly insulin icodec in individuals with type 2 diabetes. Diabetes Obes Metab 2023;25:3716–3723 [DOI] [PubMed] [Google Scholar]