Abstract
Introduction
Primary aldosteronism is characterized by the autonomous excretion of aldosterone, which may induce bone mineral disorders.
Methods
A total of 96 patients with primary aldosteronism were analyzed to identify differences in the regulation of serum calcium/phosphate balance between patients with unilateral and bilateral aldosterone hypersecretion and to determine whether or not adrenalectomy or mineralocorticoid receptor blockers affected such differences.
Results
Serum phosphate concentrations were significantly lower in patients with unilateral aldosterone hypersecretion than in patients with bilateral aldosterone hypersecretion (2.96±0.45 vs. 3.36±0.55 mg/dL, p<0.05), and recovered after adrenalectomy (2.96±0.45 vs. 3.49±0.32 mg/dL, p<0.01). In patients with bilateral aldosterone hypersecretion, the baseline serum phosphate levels were significantly lower in responders to mineralocorticoid receptor blocker treatment, defined as post-treatment plasma renin activity ≥1 ng/mL/h, than in non-responders. In responders, these levels tended to recover after treatment. A weak negative correlation between the plasma aldosterone concentration (PAC) and serum phosphate was observed, but there were no associations between the PAC and serum calcium concentration or between the aldosterone renin ratio and serum calcium and phosphate concentrations.
Conclusion
The effects on calcium/phosphate homeostasis may differ according to the primary aldosteronism subtype.
Keywords: primary aldosteronism, bone mineral disorders, mineralocorticoid receptor blocker, aldosterone-producing adenoma, bilateral adrenal hypersecretion
Introduction
Primary aldosteronism (PA), which is characterized by autonomous, excessive aldosterone secretion, is the most common cause of secondary hypertension (1). A recent meta-analysis showed that its prevalence is 3.2-12.7% in primary care facilities and 1-29% in specialized hospitals, which is higher than previously thought (2). Patients with PA are more likely to have various cardiovascular diseases, such as stroke, cardiomegaly, atrial fibrillation, coronary artery disease, and heart failure, than patients with essential hypertension (EH) (2-5), which makes it crucial to diagnose PA in its early phase, as its prognosis can be improved with appropriate treatment (6).
PA is mainly classified as unilateral hypersecretion, typically caused by an aldosterone-producing adenoma (APA), or bilateral hypersecretion, generally called idiopathic hyperaldosteronism (IHA). APA is characterized by excessive aldosterone secretion from an adrenal adenoma, usually presenting as a severe type of PA. In contrast, IHA is characterized by autonomous secretion of aldosterone resulting from bilateral adrenocortical hyperplasia, usually presenting as a milder form of PA (7). Therefore, it is often difficult to control blood pressure in patients with APA, who also more frequently have hypokalemia and cardiovascular diseases (8).
Recent reports have shown that hyperparathyroidism is prevalent in PA patients (9) and can also contribute to an increased risk of cardiovascular diseases (10,11). However, the extent of differences in hyperparathyroidism and bone mineral metabolism disorders between unilateral and bilateral PA, which have different clinical phenotypes, has not been fully investigated. Specific treatments, such as adrenalectomy and mineralocorticoid receptor blockers (MRBs), are also effective for alleviating bone mineral disorders in PA patients (12), but whether there is a difference in the effect of each treatment for bone mineral disorders in each type of PA patient has not been examined. In addition, patients with a sufficient elevation in their plasma renin activity (PRA) after treatment with MRBs have a reduced risk of cardiovascular diseases compared to patients without PRA elevation (6,13). The MRB-induced increase in PRA is thought to be associated with sufficient suppression of hyperaldosteronism, which may alleviate the risk of cardiovascular diseases in patients with PA. However, the association between the effects of MRB treatment and bone metabolic factors has not been clarified.
Therefore, in the present study, the impact of laterality, treatment methods, and treatment efficacy on calcium/phosphate regulation in patients with PA was investigated.
Materials and Methods
Study population
A total of 95 patients who presented to our center between April 2011 and March 2022 with a final diagnosis of PA and who met the criteria of plasma aldosterone concentration (PAC) >120 pg/mL, aldosterone renin ratio (ARR) >200, and positivity on at least one confirmatory test [captopril challenge test (CCT)], upright furosemide loading test, or saline loading test)] based on the JSH2019 guideline were included, as in our previous studies (14-16). Patients treated with vitamin D3 were excluded. Adrenal vein sampling (AVS) was performed for all patients included in this study. All included patients met the criteria of adrenal PAC ≥14,000 pg/mL in the unilateral or bilateral adrenal veins after adrenocorticotropic hormone stimulation, as in our previous studies (15,16). Unilateral aldosterone hypersecretion was diagnosed if the lateralized ratio, calculated as the adrenal vein A/C ratio on the high-value side/low-value side, was ≥4 (n=15). Otherwise, the patient was diagnosed with bilateral aldosterone hypersecretion (n=81).
All patients with unilateral aldosterone hypersecretion underwent adrenalectomy, and all patients with bilateral aldosterone hypersecretion received MRB treatment with either eplerenone or esaxerenone. For patients with unilateral aldosterone hypersecretion, blood samples collected during the first postoperative outpatient visit were regarded as “post-treatment.” For patients with bilateral aldosterone hypersecretion, blood samples collected at the outpatient visit one year after the start of MRB treatment were regarded as “post-treatment.” PRA ≥1 ng/mL/h at the post-treatment visit was considered sufficient aldosterone suppression, as previously described (6,13), and patients who met this criterion were considered “responders,” while those who did not were considered “non-responders.” All blood and urine samples were examined by SRL (Tokyo, Japan). Serum calcium concentrations were normalized to serum albumin (Alb) concentrations by “4-Alb (g/dL)”. The estimated daily sodium excretion in urine was calculated as described previously (17). The PAC was measured by a radioimmunoassay (RIA) until December 2020, after which it was measured using a chemiluminescent enzyme immunoassay and converted to estimated values using the RIA method (n=28) (18).
Opt-out consent was obtained for this study. The study protocol was approved by the Ethics Committee of The Jikei University School of Medicine (No. 32-029).
Statistical analyses
The SPSS software program, version 28 (IBM, Armonk, USA), was used for the data analysis. Data are presented as the mean±standard deviation. Statistical significance was set at p<0.05.
Differences in variables between unilateral and bilateral aldosterone hypersecretion and responders or non-responders were analyzed using a one-way analysis of variance. Comparisons between pre- and post-treatment values for patients with unilateral or bilateral aldosterone hypersecretion were analyzed using paired t-tests. The associations between PRA/PAC and serum calcium and phosphate concentrations were analyzed using a linear regression model.
Results
The comparison between unilateral and bilateral aldosterone hypersecretion
First, the pre-treatment characteristics of patients with unilateral and bilateral aldosterone hypersecretion were compared (Table 1). As previously reported (19), the PAC was higher and the PRA lower in patients with unilateral aldosterone hypersecretion than in those with bilateral aldosterone hypersecretion. In addition, the plasma sodium concentration was higher and potassium levels lower in patients with unilateral aldosterone hypersecretion than in those with bilateral aldosterone hypersecretion. Although the plasma calcium and alkaline phosphatase (ALP) concentrations were equivalent between the patient groups, the serum phosphate concentrations were significantly lower in patients with unilateral aldosterone hypersecretion than in those with bilateral aldosterone hypersecretion.
Table 1.
Comparison between Unilateral and Bilateral Aldosterone Hypersecretion.
| Unilateral (n=15) | Bilateral (n=80) | p value | ||||
|---|---|---|---|---|---|---|
| Age | 50.3±12.3 | 51.3±8.3 | 0.70 | |||
| Sex (male) | 67% | 61% | 0.70 | |||
| BMI (kg/m2) | 24.4±5.5 | 25.3±4.8 | 0.52 | |||
| Diabetes mellitus | 7% | 6% | 0.95 | |||
| Chronic kidney disease | 27% | 11% | 0.11 | |||
| Antihypertensive agents | ||||||
| Calcium channel blockers | 67% | 56% | 0.46 | |||
| α blockers | 20% | 1% | <0.01 | |||
| RAS inhibitors | 0% | 0% | N.A. | |||
| β blockers | 0% | 0% | N.A. | |||
| Diuretics | 0% | 1% | 0.67 | |||
| Other drugs | ||||||
| Potassium supplement | 67% | 0% | <0.01 | |||
| PRA (ng/mL/h) | 0.27±0.18 | 0.50±0.32 | <0.01 | |||
| PAC (pg/mL) | 381.1±371.8 | 184.1±71.5 | <0.01 | |||
| ARR | 2,571.2±4,139.8 | 470.7±305.6 | <0.01 | |||
| Cr (mg/dL) | 0.87±0.31 | 0.80±0.19 | 0.24 | |||
| eGFR (mL/min) | 73.9±20.3 | 74.5±14.2 | 0.85 | |||
| Na (mmol/L) | 143.1±2.0 | 141.6±1.3 | <0.01 | |||
| K (mmol/L) | 3.27±0.47 | 4.08±0.33 | <0.01 | |||
| Estimated sodium intake (mmol/day) | 127.1±36.3 | 139.9±40.4 | 0.19 | |||
| Ca (mg/dL) | 8.95±0.32 | 9.08±0.30 | 0.14 | |||
| iP (mg/dL) | 2.96±0.45 | 3.35±0.56 | <0.05 | |||
| ALP (IU/L) | 66.6±19.6 | 66.6±18.1 | 0.99 |
PAC: plasma aldosterone concentration, PRA: plasma renin activity, ARR: aldosterone renin ratio, N.A.: not analyzed
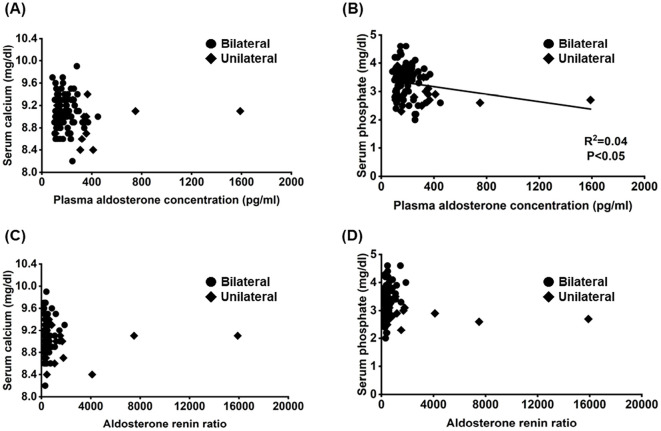
We hypothesized that high aldosterone secretion was responsible for hypophosphatemia in unilateral cases. Thus, the associations among the PAC, ARR, and serum calcium and phosphate concentrations were analyzed. There was a weak negative correlation between the PAC and serum phosphate levels, but no association was observed between the PAC and serum calcium concentration or between the ARR and serum calcium and phosphate concentrations (Figure). A correlation between the PAC and serum phosphate levels was not observed when patients with unilateral or bilateral aldosterone hypersecretion were analyzed separately.
Figure.
Associations between the plasma aldosterone concentration/aldosterone renin ratio (ARR) and serum calcium and phosphate concentrations.
Changes in serum calcium/phosphate and ALP values pre- and post-treatment
Next, the changes in serum calcium, phosphate, and ALP levels after the specific treatment were assessed (Table 2). Although the ALP concentration was slightly reduced in patients with bilateral aldosterone hypersecretion, serum calcium and phosphate concentrations were not altered after MRB treatment. In contrast, the serum phosphate concentration was significantly increased following adrenalectomy in patients with unilateral aldosterone hypersecretion.
Table 2.
Changes in Serum Calcium/phosphate and ALP Values Pre- and Post-treatment.
| Unilateral (n=15) | Pre-treatment | Post-treatment | p value | |||
|---|---|---|---|---|---|---|
| ALP (IU/L) | 66.6±19.6 | 72.9±20.2 | 0.24 | |||
| Ca (mg/dL) | 8.95±0.32 | 9.15±0.26 | 0.06 | |||
| iP (mg/dL) | 2.96±0.45 | 3.49±0.32 | <0.01 | |||
| Bilateral (n=80) | Pre-treatment | Post-treatment | p value | |||
| ALP (IU/L) | 66.6±18.1 | 62.3±16.1 | <0.01 | |||
| Ca (mg/dL) | 9.08±0.30 | 9.13±0.28 | 0.16 | |||
| iP (mg/dL) | 3.35±0.56 | 3.28±0.52 | 0.30 | |||
The comparison between responders and non-responders to MRB treatment
The baseline characteristics of patients with and without sufficient suppression of aldosterone secretion after MRB treatment were compared (Table 3). The PRA and PAC levels were higher in responders than in non-responders, whereas the ARR was equivalent between responders and non-responders. Although serum calcium and ALP concentrations were equivalent between responders and non-responders, serum phosphate concentrations were significantly lower in responders than in non-responders.
Table 3.
Comparison between Responders and Non-responders.
| Responders (n=25) | Non-responders (n=55) | p value | ||||
|---|---|---|---|---|---|---|
| Age | 51.6±8.4 | 51.1±8.4 | 0.80 | |||
| Sex (male) | 56% | 64% | 0.52 | |||
| BMI (kg/m2) | 26.5±5.3 | 24.7±4.6 | 0.13 | |||
| Diabetes mellitus | 4% | 7% | 0.58 | |||
| Chronic kidney disease | 20% | 7% | 0.10 | |||
| Antihypertensive agents | ||||||
| Calcium channel blockers | 68% | 51% | 0.16 | |||
| α-blockers | 0% | 2% | 0.50 | |||
| RAS inhibitors | 0% | 0% | N.A. | |||
| β-blockers | 0% | 0% | N.A. | |||
| Diuretics | 4% | 0% | 0.14 | |||
| Other drugs | ||||||
| Potassium supplement | 0% | 0% | N.A. | |||
| PRA (ng/mL/h) | 0.72±0.43 | 0.40±0.18 | <0.01 | |||
| PAC (pg/mL) | 239.8±85.8 | 158.8±46.0 | <0.01 | |||
| ARR | 415.7±257.2 | 495.8±324.3 | 0.28 | |||
| Cr (mg/dL) | 0.83±0.23 | 0.79±0.17 | 0.30 | |||
| eGFR (mL/min) | 71.2±15.2 | 76.4±13.5 | 0.13 | |||
| Na (mmol/L) | 141.5±1.3 | 141.6±1.3 | 0.71 | |||
| K (mmol/L) | 4.09±0.38 | 4.08±0.31 | 0.86 | |||
| Estimated sodium intake (mmol/day) | 135.3±45.4 | 144.2±36.5 | 0.35 | |||
| Ca (mg/dL) | 9.06±0.33 | 9.09±0.30 | 0.69 | |||
| iP (mg/dL) | 3.11±0.55 | 3.46±0.53 | <0.01 | |||
| ALP (IU/L) | 63.6±16.7 | 67.9±18.7 | 0.33 |
PAC: plasma aldosterone concentration, PRA: plasma renin activity, ARR: aldosterone renin ratio, N.A.: not analyzed
Changes in serum calcium/phosphate and ALP values with MRB treatment in responders and non-responders
Finally, differences between responder and non-responder patients in terms of changes in serum calcium, phosphate, and ALP levels after treatment were examined (Table 4). Serum calcium, phosphate, and ALP levels did not change markedly in responders. In contrast, although the serum calcium concentration was not altered, serum phosphate and ALP values were significantly decreased in non-responders after MRB treatment. In 17 patients, we were able to evaluate intact parathyroid hormone (iPTH) levels before and after treatment with MRBs, but no significant differences in changes were observed between responders and non-responders (Supplementary material). In addition, changes in iPTH levels were not correlated with changes in plasma calcium and phosphate concentrations.
Table 4.
Changes in Serum Calcium/phosphate and ALP Values with MRB Treatment in Responders and Non-responders.
| Responders (n=25) | Pre-treatment | Post-treatment | p value | |||
|---|---|---|---|---|---|---|
| ALP (IU/L) | 63.6±17.0 | 61.6±14.4 | 0.32 | |||
| Ca (mg/dL) | 9.06±0.33 | 9.20±0.28 | 0.06 | |||
| iP (mg/dL) | 3.11±0.55 | 3.27±0.40 | 0.23 | |||
| Non-responders (n=55) | Pre-treatment | Post-treatment | p value | |||
| ALP (IU/L) | 67.9±18.7 | 62.6±16.9 | <0.01 | |||
| Ca (mg/dL) | 9.09±0.29 | 9.10±0.27 | 0.78 | |||
| iP (mg/dL) | 3.46±0.53 | 3.29±0.57 | <0.05 | |||
Discussion
The risk of cardiovascular diseases is known to be increased in PA patients (5). Recently, several reports have also shown that the bone mineral density is lower and the prevalence of osteoporosis and vertebral fractures higher in patients with PA than in patients with essential hypertension or healthy individuals (20-23). These findings therefore suggest that bone mineral disorders, which are associated with aldosterone hypersecretion, may also increase the risk of various cardiovascular diseases (24).
In the present study, differences in baseline characteristics, including parameters associated with bone mineral metabolism, between patients with unilateral and bilateral aldosterone hypersecretion were examined. Plasma phosphate levels were significantly lower in patients with unilateral aldosterone hypersecretion than in those with bilateral aldosterone hypersecretion, which was corrected with adrenalectomy. In addition, baseline plasma phosphate levels were significantly lower in responders, defined as those who achieved sufficient aldosterone suppression with MRB, than in non-responders.
Law et al. (11) reported that the simultaneous administration of salt and aldosterone in healthy individuals increased urinary calcium excretion and plasma parathyroid hormone (PTH) levels, which were inhibited by spironolactone treatment, suggesting that aldosterone hypersecretion may lead to secondary hyperparathyroidism via hypercalciuria-induced hypocalcemia. The expression of angiotensin II and mineralocorticoid receptors in the parathyroid gland raises the possibility that activation of the renin-angiotensin aldosterone system may directly stimulate PTH secretion (25). Lenzini et al. (26) also showed that PTH secretion is markedly augmented by aldosterone stimulation, which is suppressed by concurrent treatment with canrenoic acid.
Conversely, calcium-sensing receptor (CaSR), vitamin D receptor, and PTH receptor are expressed in APAs (27), suggesting that there is a close relationship between aldosterone and PTH secretion (28). Indeed, recent meta-analyses have shown that the plasma calcium concentration is lower and the PTH level higher in patients with PA than in patients with essential hypertension (29). In addition, the PAC was positively correlated with PTH levels in patients with PA, and direct renin concentration was positively correlated with PTH levels in patients with hyperparathyroidism (30). Wang et al. (31) showed that the plasma PTH level was significantly higher in patients with APAs than in those with bilateral aldosterone hypersecretion. Zhang et al. (32) reported that PTH levels were significantly higher in patients with APA than in patients with nonfunctional adrenal adenomas, suggesting that PTH levels may be useful for distinguishing APAs from nonfunctional adrenal adenomas. PTH inhibits phosphate reabsorption via the proximal tubules, thereby decreasing serum phosphate (33), which may explain the significant difference in serum phosphate levels between patients with unilateral and bilateral aldosterone hypersecretion. In patients with APA, the amount of aldosterone secretion is higher (34) and the PTH level significantly lower after adrenalectomy than before the procedure (9), suggesting that elevated aldosterone secretion stimulates PTH secretion. This may occur either directly or through increased urinary calcium excretion, thereby decreasing the serum phosphate levels in patients with unilateral aldosterone hypersecretion.
Given the above, the correlation between PAC or ARR and serum calcium or phosphate levels was analyzed (Figure). Although there was a very weak correlation between the PAC and serum phosphate levels, there was no association between the PAC and serum calcium levels or between the ARR and serum calcium or phosphate levels. Thus, the lower plasma phosphate level observed in patients with unilateral aldosterone hypersecretion than in those with bilateral aldosterone hypersecretion may be partially independent of aldosterone secretion itself. Indeed, the association between the PAC and serum phosphate levels disappeared when unilateral and bilateral cases were analyzed separately, suggesting that the differences in serum phosphate concentrations in patients with unilateral and bilateral aldosterone hypersecretion may be related to the type of disease (e.g., APA or IHA) rather than the amount of aldosterone secretion. However, whether or not hypersecretion of PTH affects plasma phosphate levels in patients with unilateral aldosterone hypersecretion could not be determined, as the levels of iPTH pre- and post-MRB treatment could only be obtained from 17 patients. Furthermore, because we did not evaluate urinary calcium and phosphorus concentrations, we could not determine whether or not hyperphosphaturia resulted in reduced plasma phosphate levels in patients with unilateral aldosterone hypersecretion.
Of note, as many as 70% of PA patients have vitamin D deficiency (5,35), and the serum 25-(OH) vitamin D level is significantly lower in patients with unilateral aldosterone hypersecretion than in those with bilateral aldosterone hypersecretion (31), which might affect the plasma phosphate level in these patients. However, plasma vitamin D concentration was not measured in the present study. Although no correlation between PAC and 25-(OH) vitamin D concentration has been shown (36), further studies are needed to evaluate the effect of vitamin D metabolism in patients with PA. In summary, the present study suggests that calcium and phosphorus metabolism may differ between unilateral and bilateral PA. However, because the number of unilateral cases was relatively small, the characteristics of unilateral cases may not be accurately represented.
In patients with bilateral aldosterone excretion, whether or not the administration of MRBs improves bone metabolism is unclear. Rossi et al. (37) reported in a small number of PA cases that administration of spironolactone increased ionized calcium and decreased PTH concentrations. Similarly, specific treatments, such as adrenal surgery or spironolactone administration, were able to improve PTH levels in PA patients (12,37); however, Pilz et al. (38) reported that although plasma PTH levels tended to be decreased after spironolactone treatment (from 58.9±15.5 pg/mL to 47.1±18.6 pg/mL), the difference between values pre- and post-treatment with spironolactone was not significant. Similarly, Verheyen et al. (39) reported that eplerenone treatment for eight weeks did not alter the expression of markers associated with bone metabolism. In contrast, Adolf et al. (40) demonstrated that spironolactone treatment improved bone alkaline phosphatase, osteocalcin, and intact procollagen I N-terminal propeptide levels in postmenopausal patients with PA, whereas adrenalectomy did not. However, these studies did not consider whether or not sufficient suppression of mineralocorticoid activity was achieved with MRB administration.
Recent studies have shown that the cardioprotective effect of MRB treatment is mainly observed in patients with sufficient aldosterone suppression (6,13). Chen et al. (41) also reported that treatment with MRB improved arterial stiffness only in patients who achieved substantial suppression of aldosterone secretion. These results suggest that it is essential to evaluate whether or not the mineralocorticoid receptor activity is sufficiently suppressed with adequate MRB treatment. To this end, differences in parameters associated with bone mineral disorders between responders and non-responders, determined by post-treatment PRA ≥1 ng/mL/h as an indicator of sufficient aldosterone suppression, have been examined (6,13).
In the present study, we found that the PAC was higher and the serum phosphate concentration lower in responders than in non-responders. As the PAC was significantly higher in responders than in non-responders, aldosterone-induced hyperparathyroidism may be one reason for the reduced serum phosphate levels observed in responders. Although we did not measure iPTH levels in this study, the PAC and ARR were not correlated with serum phosphate levels in patients with bilateral aldosterone hypersecretion. Further studies will thus be necessary to clarify why serum phosphate levels were lower in responders.
In contrast, serum phosphate and ALP levels were significantly decreased after MRB treatment in non-responders. ALP is a hydrolytic enzyme that releases phosphate from its substrates. Half of the total ALP concentration originates from the liver, with the rest originating from the skeleton (42). The changes in serum phosphate and ALP levels were not correlated with each other, suggesting that the changes in ALP values may not be fully derived from changes in bone metabolism. In addition, we were unable to determine why the serum phosphate levels significantly decreased after MRB treatment in non-responders using the markers evaluated in our study. Further studies evaluating additional markers involved in bone metabolism, such as iPTH, vitamin D, and FGF23 levels, will be necessary to determine whether or not there is an association between the PA disease type or responsiveness to MRB treatment and the effects on PA-related bone mineral disorders.
Conclusion
In the present study, the serum phosphate level was lower in patients with unilateral aldosterone hypersecretion and MRB responders with bilateral aldosterone hypersecretion than in others. These results might reflect differences in bone metabolism disorders depending on the disease type and drug sensitivity.
The authors state that they have no Conflict of Interest (COI).
Miki Yarita-Kawana and Satoshi Kidoguchi contributed equally to this work.
Supplementary Material
The changes of intact parathyroid hormone (iPTH) in responders and non-responders pre- and post-treatment.
Acknowledgement
We thank Shelby King, PhD for editing this manuscript.
References
- 1.Reincke M, Bancos I, Mulatero P, Scholl UI, Stowasser M, Williams TA. Diagnosis and treatment of primary aldosteronism. Lancet Diabetes Endocrinol 9: 876-892, 2021. [DOI] [PubMed] [Google Scholar]
- 2.Käyser SC, Dekkers T, Groenewoud HJ, et al. Study heterogeneity and estimation of prevalence of primary aldosteronism: a systematic review and meta-regression analysis. J Clin Endocrinol Metab 101: 2826-2835, 2016. [DOI] [PubMed] [Google Scholar]
- 3.Savard S, Amar L, Plouin PF, Steichen O. Cardiovascular complications associated with primary aldosteronism: a controlled cross-sectional study. Hypertension 62: 331-336, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Ohno Y, Sone M, Inagaki N, et al. Prevalence of cardiovascular disease and its risk factors in primary aldosteronism: a multicenter study in Japan. Hypertension 71: 530-537, 2018. [DOI] [PubMed] [Google Scholar]
- 5.Monticone S, D'Ascenzo F, Moretti C, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 6: 41-50, 2018. [DOI] [PubMed] [Google Scholar]
- 6.Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol 6: 51-59, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young WF. Minireview: primary aldosteronism - changing concepts in diagnosis and treatment. Endocrinology 144: 2208-2213, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Heinrich DA, Adolf C, Rump LC, et al. Primary aldosteronism: key characteristics at diagnosis: a trend toward milder forms. Eur J Endocrinol 178: 605-611, 2018. [DOI] [PubMed] [Google Scholar]
- 9.Kometani M, Yoneda T, Aono D, et al. Primary aldosteronism with parathyroid hormone elevation: a single-center retrospective Study. Intern Med 60: 993-998, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hagström E, Hellman P, Larsson TE, et al. Plasma parathyroid hormone and the risk of cardiovascular mortality in the community. Circulation 119: 2765-2771, 2009. [DOI] [PubMed] [Google Scholar]
- 11.Law PH, Sun Y, Bhattacharya SK, Chhokar VS, Weber KT. Diuretics and bone loss in rats with aldosteronism. J Am Coll Cardiol 46: 142-146, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Ceccoli L, Ronconi V, Giovannini L, et al. Bone health and aldosterone excess. Osteoporos Int 24: 2801-2807, 2013. [DOI] [PubMed] [Google Scholar]
- 13.Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Incidence of atrial fibrillation and mineralocorticoid receptor activity in patients with medically and surgically treated primary aldosteronism. JAMA Cardiol 3: 768-774, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Umemura S, Arima H, Arima S, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens Res 42: 1235-1481, 2019. [DOI] [PubMed] [Google Scholar]
- 15.Kidoguchi S, Sugano N, Hayashi-Ishikawa N, Morisawa N, Tokudome G, Yokoo T. The characteristics of captopril challenge test-positive patients using various criteria. J Renin Angiotensin Aldosterone Syst 20: 1470320319870891, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kidoguchi S, Sugano N, Kawauchi R, et al. Evaluation of various confirmatory tests for the diagnosis of aldosterone-producing adenoma. J Renin Angiotensin Aldosterone Syst 21: 1470320320919610, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka T, Okamura T, Miura K, et al. A simple method to estimate populational 24-h urinary sodium and potassium excretion using a casual urine specimen. J Hum Hypertens 16: 97-103, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Naruse M, Katabami T, Shibata H, et al. Japan Endocrine Society clinical practice guideline for the diagnosis and management of primary aldosteronism 2021. Endocr J 69: 327-359, 2022. [DOI] [PubMed] [Google Scholar]
- 19.Ducher M, Mounier-Véhier C, Baguet JP, et al. Aldosterone-to-renin ratio for diagnosing aldosterone-producing adenoma: a multicentre study. Arch Cardiovasc Dis 105: 623-630, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Lv X, Hu H, Shen C, et al. Risk factors associated with lower bone mineral density in primary aldosteronism patients. Front Endocrinol (Lausanne) 13: 884302, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petramala L, Zinnamosca L, Settevendemmie A, et al. Bone and mineral metabolism in patients with primary aldosteronism. Int J Endocrinol 2014: 836529, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Notsu M, Yamauchi M, Yamamoto M, Nawata K, Sugimoto T. Primary aldosteronism as a risk factor for vertebral fracture. J Clin Endocrinol Metab 102: 1237-1243, 2017. [DOI] [PubMed] [Google Scholar]
- 23.Yokomoto-Umakoshi M, Sakamoto R, Umakoshi H, et al.; the Q-AND-A study group. Unilateral primary aldosteronism as an independent risk factor for vertebral fracture. Clin Endocrinol (Oxf) 92: 206-213, 2020. [DOI] [PubMed] [Google Scholar]
- 24.Tomaschitz A, Ritz E, Pieske B, et al. Aldosterone and parathyroid hormone: a precarious couple for cardiovascular disease. Cardiovasc Res 94: 10-19, 2012. [DOI] [PubMed] [Google Scholar]
- 25.Brown JM, Williams JS, Luther JM, et al. Human interventions to characterize novel relationships between the renin-angiotensin-aldosterone system and parathyroid hormone. Hypertension 63: 273-280, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lenzini L, Prisco S, Vanderriele PE, et al. PTH modulation by aldosterone and angiotensin II is blunted in hyperaldosteronism and rescued by adrenalectomy. J Clin Endocrinol Metab 104: 3726-3734, 2019. [DOI] [PubMed] [Google Scholar]
- 27.Gao X, Yamazaki Y, Tezuka Y, et al. The crosstalk between aldosterone and calcium metabolism in primary aldosteronism: a possible calcium metabolism-associated aberrant “neoplastic” steroidogenesis in adrenals. J Steroid Biochem Mol Biol 193: 105434, 2019. [DOI] [PubMed] [Google Scholar]
- 28.Chau K, Holmes D, Melck A, Chan-Yan C. Secondary hypertension due to concomitant aldosterone-producing adenoma and parathyroid adenoma. Am J Hypertens 28: 280-282, 2015. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Feng B. Association of serum parathyrine and calcium levels with primary aldosteronism: a meta-analysis. Int J Clin Exp Med 8: 14625-14633, 2015. [PMC free article] [PubMed] [Google Scholar]
- 30.Milicic Stanic B, Ilincic B, Zeravica R, Milicic Ivanovski D, Cabarkapa V, Mijovic R. The importance of correlation between aldosterone and parathyroid hormone in patients with primary hyperparathyroidism. Int J Endocrinol 2022: 3804899, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang A, Wang Y, Liu H, et al. Bone and mineral metabolism in patients with primary aldosteronism: a systematic review and meta-analysis. Front Endocrinol (Lausanne) 13: 1027841, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang LX, Gu WJ, Li YJ, et al. PTH is a promising auxiliary index for the clinical diagnosis of aldosterone-producing adenoma. Am J Hypertens 29: 575-581, 2016. [DOI] [PubMed] [Google Scholar]
- 33.Jacquillet G, Unwin RJ. Physiological regulation of phosphate by vitamin D, parathyroid hormone (PTH) and phosphate (Pi). Pflugers Arch 471: 83-98, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burrello J, Burrello A, Pieroni J, et al. Development and validation of prediction models for subtype diagnosis of patients with primary aldosteronism. J Clin Endocrinol Metab 105: dgaa379, 2020. [DOI] [PubMed] [Google Scholar]
- 35.Rossi GP, Lenzini L. Vitamin D supplementation: a novel therapy for aldosteronism? Nat Rev Endocrinol 16: 303-304, 2020. [DOI] [PubMed] [Google Scholar]
- 36.Zavatta G, Di Dalmazi G, Altieri P, et al. Association between aldosterone and parathyroid hormone levels in patients with adrenocortical tumors. Endocr Pract 28: 90-95, 2022. [DOI] [PubMed] [Google Scholar]
- 37.Rossi E, Sani C, Perazzoli F, Casoli MC, Negro A, Dotti C. Alterations of calcium metabolism and of parathyroid function in primary aldosteronism, and their reversal by spironolactone or by surgical removal of aldosterone-producing adenomas. Am J Hypertens 8: 884-893, 1995. [DOI] [PubMed] [Google Scholar]
- 38.Pilz S, Kienreich K, Drechsler C, et al. Hyperparathyroidism in patients with primary aldosteronism: cross-sectional and interventional data from the GECOH study. J Clin Endocrinol Metab 97: E75-E79, 2012. [DOI] [PubMed] [Google Scholar]
- 39.Verheyen N, Grübler MR, Meinitzer A, et al. Effect of eplerenone on markers of bone turnover in patients with primary hyperparathyroidism - the randomized, placebo-controlled EPATH trial. Bone 105: 212-217, 2017. [DOI] [PubMed] [Google Scholar]
- 40.Adolf C, Braun LT, Fuss CT, et al. Spironolactone reduces biochemical markers of bone turnover in postmenopausal women with primary aldosteronism. Endocrine 69: 625-633, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen ZW, Pan CT, Liao CW, et al. Implication of MR activity in posttreatment arterial stiffness reversal in patients with primary aldosteronism. J Clin Endocrinol Metab 108: 624-632, 2023. [DOI] [PubMed] [Google Scholar]
- 42.Riancho JA. Diagnostic approach to patients with low serum alkaline phosphatase. Calcif Tissue Int 112: 289-296, 2023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The changes of intact parathyroid hormone (iPTH) in responders and non-responders pre- and post-treatment.