Abstract
Homologous blood transfusion (HBT) is used for doping in endurance sports since the 1960s. The blood comes from a compatible donor, that is, someone with a compatible ABO and rhesus blood group. Despite been prohibited by the IOC in 1985, no detection method was available until 2003. Then came the idea to use red blood cells (RBC) minor blood groups antigens that constitute an “identity” card of someone's RBC to detect the presence of a second RBC population. The method validated for doping control samples uses flow cytometry after incubation of isolated RBC with eight to 12 primary antibodies against specific minor blood groups antigens. The presence of double populations of RBC is revealed by a major and a minor peak in a fluorescence histogram. The sensitivity was estimated sufficient to detect HBT for a few weeks. Despite the complexity and cost of the method, right after its application in 2004, several cases of HBT were identified but the number of cases dropped rapidly over the years. In the 2010s, other ways to detect HBT were developed and evaluated: indirect detection using the Athlete Biological Passport approach, and a few years later forensic DNA analysis to establish the presence of two different DNA in a blood sample after HBT. Despite the high specificity of the latter, the sensitivity was recently questioned in vivo. Nowadays, the flow cytometry method remains the method of choice for HBT detection and recent investigations helped to simplify the method and increase its specificity and sensitivity.
Keywords: DNA analysis, doping detection, flow cytometry, homologous blood transfusion, minor blood group antigens
This review is an overview of the methods to detect homologous blood transfusion (HBT) used for doping: flow cytometry and DNA analysis for direct detection and hematological module of the Athlete Biological Passport for indirect detection. Despite high specificity, detection of HBT by DNA analysis was recently challenged in vivo for its sensitivity. With recent technical progress, the flow cytometry method remains the method of choice with an improved sensitivity.

1. HOMOLOGOUS BLOOD TRANSFUSION DEVELOPMENT FOR MEDECINE
Homologous (or allogenic) blood transfusion (HBT) correspond to the infusion of someone's blood (whole blood or blood components in particular red blood cells [RBC]) into someone else sharing blood compatibility. The compatibility between the donor and the recipient is essential to avoid a reaction from the recipient's immune system against the foreign RBC introduced. This can lead to strong hemolysis associated to a series of serious side effects including renal failure and sometimes death. 1
The development of modern transfusion for medical application started after the discovery in 1901 by Dr. Karl Landsteiner of the ABO group system, opening the way to establish blood compatibility between donor and recipient. 2 This major blood group comprises the four main blood types (A, B, AB, and O) according to the expression or absence of expression of specific antigens (combinations of proteins and sugars) at the surface of RBC. The rhesus factor (expressed (Rh+) or not (Rh−)) was later discovered in 1940 leading to the blood compatibility chart still in use today (Figure 1). 3 At the same time, transfusion also benefited from the development of additives for blood preservation. 4 Blood bags containing appropriate buffers allow good conservation of blood stored refrigerated and the time between withdrawal and transfusion of a blood of good quality increased from a few days to several weeks (42 days in clinical medicine nowadays). While a bit more complex, the addition of glycerol to centrifuged RBC allows for freezing and conservation at −80°C for longer periods of time before reinfusion but requires several washes to eliminate the glycerol before transfusion can be performed. 5
FIGURE 1.

Major blood type compatibility chart for homologous transfusion (ABO system and rhesus status [RhD antigen]). Compatibility (white box) and uncompatibility (black box) are shown. O‐ blood type is a universal donor, AB+ blood type is universal recipient.
Large‐scale HBT started to be performed during the Second World War (WWII) and after the conflict transfusion numbers increased exponentially and are still used worldwide to treat a large number of chronic blood disease and medical emergencies and surgeries involving severe blood loss. 6
2. HBT AND EFFECTS ON PERFORMANCE
Following the increasing knowledge on transfusion after WWII, in 1947, research proved that allogenic blood transfusion increased tolerance to hypoxia in healthy young men, a first step in the demonstration of the ergogenic effect of HBT. 7 Direct scientific demonstration that blood transfusion has a significant effect on exercise and endurance was further performed in the 1970s–early 1980s. 8 , 9 It is now clearly established that transfusion of whole blood or RBC increase aerobic capacity (maximal oxygen consumption, VO2 max) and the time to exhaustion, and that this effect is also dependent of the volume of blood transfused (up 20%, 1 L of blood). 10 In the meantime, HBT has been used for doping (allegedly) during the 1960s in endurance sports like long‐distance athletics, cycling, or cross‐country skiing and HBT became a well‐used technique to boost performances during high‐level competitions including the Olympic Games in the 1970s and early 1980s. 11 After public revelations of use of HBT by Olympic champions like Karlo Maaninka who won medals in the 5 and 10 km track races after transfusion of 1 L of blood at 1980 Olympic Games and the US Olympic cycling team that collected nine medals in the 1984 Olympic games, HBT was unanimously condemned by both the public and scientific communities. 12 Soon after, now clearly considered as doping, blood transfusions in sports were banned by the International Olympic Committee (IOC) in 1986. However despite the dissuasion effect of this interdiction still in effect today, no detection method was available to control the athletes. At the same time the rise of recombinant erythropoietin (rEPO) as the new doping agent for endurance probably slowed down the use of transfusion for doping. Things changed again at the turn of year 2000 with the development of a globalized and more systematic antidoping system of survey with the creation of the World Anti‐Doping Agency (WADA) in 1999 and the introduction of a detection method for rEPO in 2000. 13 With EPO now detectable, concerns on a return to transfusion for doping emerged and the need of a detection method for HBT.
3. TOWARDS THE FIRST DETECTION METHOD: THE USE OF MINOR BLOOD GROUPS ANTIGENS
The idea leading to the first HBT detection method was to use some RBC characteristics that are almost unique to one individual to be able to identify the presence of a subgroup of RBC coming from another person. Progress in medical transfusion research conducted to the identification of hundreds of surface markers on the RBC membrane implicated in various functions (membrane structural integrity, transport of molecules through the membrane, receptors of extracellular ligands, adhesion molecules, etc.). 14 They are regrouped in human blood group systems (45 are recognized by the International Society of Blood Transfusion in 2023). 15 Each person expresses a specific pattern of these antigens. To limit the risks of hemolytic transfusion reaction (strong hemolysis of the blood transfused) or alloimmunization (antibodies produced against newly introduced RBC) for medical transfusion, the blood compatibility between the recipient and the donor bloods has to be monitored not only for the major blood group ABO but also for seven secondary blood group systems (Rh, Kell, Kidd, Duffy, MNS, P, Lewis, and Lutheran), each one regrouping several antigens. 16 To determine the status of expression of these antigens, an agglutination test is performed using antibodies specific to each antigen. Agglutination happens when antibodies find their corresponding antigen present at the membrane of the RBC thus indicating expression of this antigen by the person. With this knowledge 12 of the main common minor blood group antigens (Figure 2) known to have variable expression in the general population were chosen to characterize one person’ RBC for antidoping purpose. It was C, c, E, e from the Rh blood group, K, k from the Kell blood group, Jka, Jkb from the Kidd blood group, S, s from the MNS blood group and Fya, Fyb from the Duffy blood group. The group of Nelson M developed and validated a flow cytometry method to evaluate the status of expression (presence/absence) of each of these antigens in a blood sample and detect if a unique RBC population is present or two populations after an HBT. 17 , 18 , 19 Briefly starting from a whole blood diluted, blood was incubated in a series of tubes with antisera directed against one antigen. A secondary antibody conjugated with fluorescein is then added to detect RBC on which primary antibodies were fixed. Then cells were analyzed by flow cytometry, sorted (fluorescent/non fluorescent) and counted. Results appeared in a histogram of fluorescence showing a peak of fluorescence in case of expression of the antigen by the RBC or a peak of non‐fluorescence if the antigen was not expressed (Figure 3). In a normal blood sample for a single person, only one peak should be visible for each antigen. After an HBT, it was possible to detect the presence of a second peak in the histogram of fluorescence reflecting presence of a second population of RBC with a different expression status for some antigens (Figure 3). The criteria defined for an HBT positive result was to find double population (DP) for at least two antigens. This method was considered able to detect the introduction of 5% of stranger blood (one blood bag) for a few weeks and was applied for the first time during 2004 Olympic Games in Athens. Tyler Hamilton, Athens Gold medal champion in time‐trial cycling was the first athlete ever caught directly with this method. Its defense argued that the second RBC population could be linked to a “vanishing twin” disappeared at the embryonic state but with remaining cells producing different RBC but this was considered very unlikely and the athlete later confirmed having received a transfusion. 20 Of note, apart from transfusion, presence of mixed RBC populations can result from a bone marrow transplantation (such a major procedure is recorded and easy to document if needed) or chimerism an extremely rare event detectable by DNA analysis. 21
FIGURE 2.

Secondary (minor) blood group systems and their main antigens. The complete phenotype of RBC is performed as a precautionary measure before transfusion to provide best matched transfusion and prevent risk of alloimmunization. The antigens tested for each secondary blood group systems are listed. In red are presented the antigens selected as best targets for the detection of HBT by flow cytometry.
FIGURE 3.

Schematic representation of the flow cytometry method used for HBT detection.
The method was standardized to be applied in several antidoping laboratories: eight antigens were selected as the main target of interest (C, c, E, Jka, Jkb, Fya, Fyb and alternatively S or e), monoclonal antibodies IgM or IgG were recommended for use instead of polyclonal antibodies (antisera) to increase specificity and work were performed on fluorescence detection to increase signal detection for example by using an additional fluorescent tag phycoerythrin (PE). Detection of HBT was possible with only 0.3% to 2% of a second blood depending on the antigen considered. 21 , 22 Several high‐ranked athletes were caught like cyclist Alexander Vinokourov during the 2007 cycling competition Tour de France. 23
4. HBT DETECTION METHOD LIMITATIONS AND NEW STRATEGIES
By the 2010s, there were no more HBT cases detected by antidoping laboratories. It can be attributed to a change of method for blood doping with a rise in autologous transfusion (reinfusion of one's own blood after a certain amount of time) a practice performed at high‐scale by multiple athletes of various sports as revealed by Operation Puerto in 2006 24 and the persistence of doping with rEPO and new generations of analogs.
However, at the same time, the method was also less performed in antidoping laboratories: with less demand from the antidoping organizations, the method complex and costly was difficult to maintain. The evolution of doping toward lower volume of blood transfused and the need to perform the analysis fast to allow a potential counter‐analysis on a blood sample of good quality were also factors that could have impacted the performance of the test in detecting HBT. In addition some research focused on the risk of false negative results with the flow cytometry method: it can happen if the blood donor and the blood recipient share a similar pattern of expression for the antigens tested in the HBT detection method. Initially estimated below 0.2% when considering ABO, Rh status and the 12 selected minor blood groups antigens selected originally, 17 further studies of cohorts of athletes competing in the same sport in Russia and Italy showed that some of them shared the exact same phenotype for eight to 10 antigens or had only one different antigen (insufficient to declare an HBT case). 25 , 26 This reflected higher frequencies than theoretically expected for similar RBC phenotypes and consequently a higher risk of false negative samples with this method.
5. OTHER STRATEGIES EVALUATED TO DETECT HBT
Other strategies to detect HBT were evaluated at the same time. First to answer to the global problem of blood doping by transfusion (homologous or autologous) and/or erythropoiesis stimulation (rEPO and analogs, an indirect method of detection was implemented: the Athlete Biological Passport (ABP). 27 It is based on the longitudinal study of each athlete's hematological parameters in particular hemoglobin (HGB) and the percentage of young RBC (RET%), to detect atypical variations potentially linked to blood doping. The hematological ABP module was officially implemented by WADA in 2009 and studies demonstrated that it was indeed sensitive to detect use of rEPO or blood transfused in sufficient amount (one to two blood bags). HBT will conduct to an increase in HGB and slight decrease in RET% and will mainly be performed just before the competition. 28 The ABP had a real impact and lead to hundreds of athletes convicted for doping after evaluation of the ABP by independent hematological experts or after further testing initiated after observation of atypical variations. 29 Cheating athletes nevertheless can adapt their practice to the ABP system and it is thought that in recent years, lower doses of rEPO and lower volume of blood or other masking techniques were used to limit the amplitude of the hematological variations.
Another direct technique of detection of HBT with high specificity to avoid potential risk of false negative samples due to similar RBC phenotypes was also investigated. The idea was to use forensic DNA analysis to identify the presence of two DNA in a blood sample to reveal HBT. 30 , 31 DNA profiling has been developed with success and is a technique recognized for medical and legal application. Between 16 to 24 short tandem repeats (STR), repeated small sequences of nucleotides present at different position in the non‐coding region of human DNA are amplified and sequenced: the number of repetition is determined and visualized as a peak. Every individual possesses two DNA sequence, one from the father and one from the mother, regrouped in chromosomes pairs: a normal non‐mixed blood will contain only one DNA, showing for each STR either one peak (if the same number of repeats were present in the mother allele and the father allele) or two peaks (if the mother allele and the father allele had different number of repetitions). In case of the presence of another DNA, additional smaller peaks can be present for one or more STR if the number of repeats from the second DNA differs from the main DNA (Figure 4). Along with one or two major peaks, smaller third and four peaks are thus indicators of a second DNA in particular if they can be found in several STRs. The chance of sharing the same STR profile by this DNA profiling method is one over billions (except in case of homozygous twins).
FIGURE 4.

Schematic representation of the forensic DNA analysis method evaluated for HBT detection.
The limit of detection (LOD) of the method was estimated around 2% stranger blood. Interestingly, DNA analysis also does not require much volume (The genetic profile can be obtained even with a few microliters of whole blood).
Additional in vitro work in 2016 confirmed that mixes of bloods that would give false negative result for HBT if they were analyzed by flow cytometry (because the RBC from both subjects present the same phenotype of expression of the minor blood group antigens tested) were efficiently identified by forensic DNA analysis. 32 Despite this very high specificity, one potential limitation is the need to get enough DNA from the donor in the tested blood: RBC are enucleated cells that do not contain DNA, and the source of DNA in the blood will be the nucleated white cells. Removal of white blood cells using leukodepletion filter is an easy step performed to prevent some immune reactions post‐transfusion and can be done during the preparation of RBC concentrate (RBCC). This step will drastically decrease the number of DNA‐containing cells (>99.95%). 33 Interestingly, Stampella et al. also showed that despite a lower sensitivity, identification of the second DNA was still possible after adding 10% leukodepleted blood to another blood sample. 32 After these very encouraging results, the possibility to work from dried blood spots (DBS) instead of fresh blood was demonstrated without loss of sensitivity, which could simplify testing of athletes and allow reanalysis if needed after suspicious results from ABP or other sources of intelligence. As an alternative to STR profiling, another genotyping strategy was also recently evaluated to detect HBT from DBS with good sensitivity: by focusing on the detection of several single‐nucleotide polymorphisms (SNP), it allowed detection of blood mixes down to 1% stranger blood in ex vivo experiments. 34 The first in vivo evaluation of the detection of transfusion with a RBCC (without leukodepletion) was evaluated in 2022. 35 However, unexpected results were found: while DNA identification was successful in the RBCC showing an STR profile specific of the donor and distinct from the STR profile from the recipient, no trace of the donor DNA was found post‐transfusion of 150 ml RBCC (≈half a blood bag). The explanation might be that the white cells from the donor were recognized and eliminated very fast by the immune system of the recipient as previously suggested; 36 thus, the remains of the donor DNA were undetectable even just after 1‐day post‐transfusion. Further studies should clarify if forensic DNA analysis is really inefficient for HBT detection in vivo or could be effective in some cases (for example, transfusion of whole blood instead of a RBCC). In any case, the flow cytometry method de facto remains the method of choice to detect HBT.
6. LATEST EVOLUTION FOR THE FLOW CYTOMETRY METHOD
After years without cases, HBT returned to the spotlight in 2019 with the first adverse analytical findings in years, and two new cases occurred during 2020 Tokyo Olympic Games. 37 This confirmed that the survey of potential doping by HBT is still relevant and necessary. Progress was made in recent years to the flow cytometry method to simplify the analytical work, limit the risk of false negative and improve sensitivity.
A first step for the simplification and easier high‐throughput for the method was to perform all preparation steps in wells from 96‐wells microplate instead of working with single tubes. The technical improvement of the flow cytometer over the years allowed gain in sensitivity with a better separation of single cells by the fluidics system (hydrodynamic or acoustophoretic focusing) and the possibility to use brighter fluorescent tags for the antibodies. 38 In addition, the selection of a good combination of lasers (between violet, blue, red, and yellow, or green mostly) and fluorescent dyes allow multi‐color analysis with no or very little spectral overlap. 39 At the moment the primary antibodies used to detect the minor blood group antigens are not directly labelled with fluorescent tag but the use of secondary antibodies of different nature (IgG and IgM) allow detection of combination of two different antigens by well.
To increase specificity and avoid the risk of false negative, several studies were performed to evaluate the expression of minor blood groups antigens in populations of athletes of different origins. The most interesting antigens should be expressed a little above 50% to have a good distribution of expressing and non‐expressing persons increasing the chances to find differences after HBT. Huge differences in the frequency of expression of some antigens were found according to the ethnicity (Fyb and S were almost not expressed in athletes from Chinese origin but present in 50 to 80% of European). 26 Looking at athletes from the five continents and involved in various sports confirmed the interest of testing 11 different antigens for screening for HBT (C, c, E, e, Fya, Fyb, Jka, Jkb, S, s and K). 40
The detection of HBT with 150 ml RBCC that was not detected by forensic DNA analysis was efficiently performed by applying the latest development of the flow cytometry method and a window of detection of 50 days post‐transfusion was found. 35 The confirmation was performed by testing the presence of double population for each antigen presenting a DP in screening in a new experiment where RBC are incubated with three different dilutions of the primary antibodies (same as for the screening and two‐fold diluted and two‐fold concentrated) and the DP shall be confirmed with at least two of the three different concentrations of antibodies. DP for two different antigens are needed to consider a sample positive for HBT, while the sample will be considered atypical if only one DP is confirmed. Results showed that confirmation of HBT was possible on blood stored for 30 days at 4°C and was thus applicable also in case of a counter‐expertise (B analysis) ( 35 and Figure 5). This demonstrated that the flow cytometry is a powerful tool that should continue to be deployed in antidoping laboratories.
FIGURE 5.
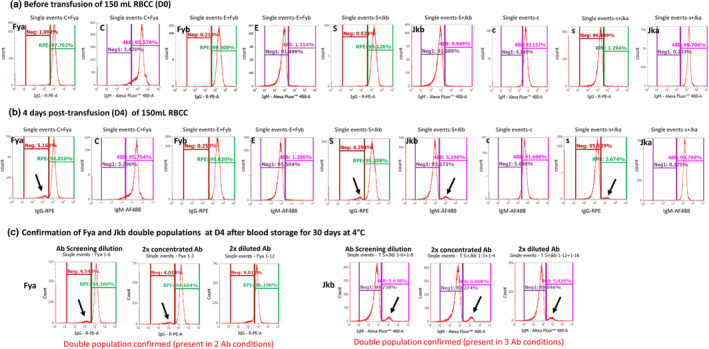
Screening of blood pre and post‐HBT and confirmation result of antigens showing double populations. An updated flow cytometry method validated by Marchand et al. was applied to blood samples collected in vivo pre and post‐HBT (see details in 34 ). Results of the screening are presented for one subject before (A) and 4 days after HBT (B) for 9 different antigens. Confirmation of antigens with double population was performed with the same flow cytometry method using 3 dilutions of the primary antibody (ab) on the same blood stored for 30 days at 4°C (C). Presence of a second peak separated from the main peak is indicated by a black arrow.
7. CONCLUSION
Twenty years after the introduction of the first direct method of detection of HBT by flow cytometry, this technique is still the most trustworthy to evaluate potential doping especially with the latest improvements. Despite a real potential and very high specificity, forensic DNA analysis for HBT detection needs more evaluation in vivo as recent concerns on the sensitivity have arisen. The indirect detection of HBT by evaluating changes detected using the ABP may also be affected by the lack of sensitivity in absence of other evidence. The search for good biomarkers of autologous transfusion continues and they might also be applicable to the detection of HBT in the future, but none have been fully validated yet. The recent cases in major competitions indicate that more testing for HBT is needed with blood samples collected in‐competition or post‐competition. More guidance on HBT detection by flow cytometry would benefit the antidoping laboratories as no guidelines or technical document have been published by WADA.
Marchand A, Ericsson M. Homologous blood transfusion and doping: Where are we now? Drug Test Anal. 2024;16(12):1479‐1486. doi: 10.1002/dta.3666
REFERENCES
- 1. Delaney M, Wendel S, Bercovitz RS, et al. Biomedical excellence for safer transfusion (BEST) collaborative. Transfusion reactions: prevention, diagnosis, and treatment. Lancet. 2016;388(10061):2825‐2836. doi: 10.1016/S0140-6736(15)01313-6 [DOI] [PubMed] [Google Scholar]
- 2. Boulton FE. Blood transfusion; additional historical aspects. Part 1. The birth of transfusion immunology. Transfus Med. 2013;23(6):375‐381. doi: 10.1111/tme.12075 [DOI] [PubMed] [Google Scholar]
- 3. Avent ND, Reid ME. The Rh blood group system: a review. Blood. 2000;95(2):375‐387. Erratum in: Blood 2000 Apr 1;95(7):2197.0/2016.0083–16 [PubMed] [Google Scholar]
- 4. Boulton FE. Blood transfusion; additional historical aspects. Part 2. The introduction of chemical anticoagulants; trials of 'Phosphate of soda'. Transfus Med. 2013;23(6):382‐388. doi: 10.1111/tme.12074 [DOI] [PubMed] [Google Scholar]
- 5. Chang A, Kim Y, Hoehn R, Jernigan P, Pritts T. Cryopreserved packed red blood cells in surgical patients: past, present, and future. Blood Transfus. 2017;15(4):341‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Giangrande PL. The history of blood transfusion. Br J Haematol. 2000;110(4):758‐767. doi: 10.1046/j.1365-2141.2000.02139.x [DOI] [PubMed] [Google Scholar]
- 7. Pace N, Lozner EL, Consolazio WV, Pitts GC, Pecora LJ. The increase in hypoxia tolerance of normal men accompanying the polycythemia induced by transfusion of erythrocytes. Am J Physiol. 1947;148(1):152‐163. doi: 10.1152/ajplegacy.1947.148.1.152 [DOI] [PubMed] [Google Scholar]
- 8. Ekblom B, Goldbarg AN, Gullbring B. Response to exercise after blood loss and reinfusion. J Appl Physiol. 1972;33(2):175‐180. doi: 10.1152/jappl.1972.33.2.175 [DOI] [PubMed] [Google Scholar]
- 9. Robertson RJ, Gilcher R, Metz KF, et al. Effect of induced erythrocythemia on hypoxia tolerance during physical exercise. J Appl Physiol Respir Environ Exerc Physiol. 1982;53(2):490‐495. doi: 10.1152/jappl.1982.53.2.490 [DOI] [PubMed] [Google Scholar]
- 10. Gledhill N. Blood doping and related issues: a brief review. Med Sci Sports Exerc. 1982;14(3):183‐189. doi: 10.1249/00005768-198203000-00005 [DOI] [PubMed] [Google Scholar]
- 11. Fitch KD. Blood doping at the Olympic games. J Sports Med Phys Fitness. 2017;57(11):1526‐1532. doi: 10.23736/S0022-4707.17.06948-1 [DOI] [PubMed] [Google Scholar]
- 12. Klein HG. Blood transfusion and athletics. Games people play. N Engl J Med. 1985;312(13):854‐856. doi: 10.1056/NEJM198503283121311 [DOI] [PubMed] [Google Scholar]
- 13. Catlin DH, Fitch KD, Ljungqvist A. Medicine and science in the fight against doping in sport. J Intern Med. 2008;264(2):99‐114. doi: 10.1111/j.1365-2796.2008.01993.x [DOI] [PubMed] [Google Scholar]
- 14. Reid ME, Yahalom V. Blood groups and their function. Baillieres Best Pract Res Clin Haematol. 2000;13(4):485‐509. doi: 10.1053/beha.2000.0096 [DOI] [PubMed] [Google Scholar]
- 15. Red Cell Immunogenetics and Blood Group Terminology https://www.isbtweb.org/isbt-working-parties/rcibgt.html) accessed 27 November 2023.
- 16. Subramaniyan R. Phenotyping of clinically significant blood group antigens among the south Indian donor population. Hematol Transfus Cell Ther. 2023;45(Suppl 2):S30‐S35. doi: 10.1016/j.htct.2021.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nelson M, Ashenden M, Langshaw M, Popp H. Detection of homologous blood transfusion by flow cytometry: a deterrent against blood doping. Haematologica. 2002;87(8):881‐882. [PubMed] [Google Scholar]
- 18. Nelson M, Popp H, Sharpe K, Ashenden M. Proof of homologous blood transfusion through quantification of blood group antigens. Haematologica. 2003;88(11):1284‐1295. [PubMed] [Google Scholar]
- 19. Nelson M, Cooper S, Nakhla S, Smith S, King M, Ashenden M. Validation of a test designed to detect blood‐doping of elite athletes by homologous transfusion. Aust J Med Sci. 2004;25(1):27‐33. doi: 10.3316/informit.260078420035121 [DOI] [Google Scholar]
- 20. Doping excuses: 13 of the most brazen and bizarre, https://www.cyclingnews.com/features/cycling-doping-excuses/ accessed 27 November 2023.
- 21. Giraud S, Robinson N, Mangin P, Saugy M. Scientific and forensic standards for homologous blood transfusion anti‐doping analyses. Forensic Sci Int. 2008;179(1):23‐33. doi: 10.1016/j.forsciint.2008.04.007 [DOI] [PubMed] [Google Scholar]
- 22. Voss SC, Thevis M, Schinkothe T, Schänzer W. Detection of homologous blood transfusion. Int J Sports Med. 2007;28(8):633‐637. doi: 10.1055/s-2007-965076 [DOI] [PubMed] [Google Scholar]
- 23. Vinokourov ban confirmed by CAS, https://www.cyclingnews.com/news/vinokourov-ban-confirmed-by-cas/ accessed 27 November 2023.
- 24. Mørkeberg J. Blood manipulation: current challenges from an anti‐doping perspective. Hematol am Soc Hematol Educ Program. 2013;2013(1):627‐631. doi: 10.1182/asheducation-2013.1.627 [DOI] [PubMed] [Google Scholar]
- 25. Krotov G, Nikitina M, Rodchenkov G. Possible cause of lack of positive samples on homologous blood transfusion. Drug Test Anal. 2014;6(11‐12):1160‐1162. doi: 10.1002/dta.1736 [DOI] [PubMed] [Google Scholar]
- 26. Donati F, de la Torre X, Pagliarosi S, Pirri D, Prevete G, Botrè F. Detection of homologous blood transfusion in sport doping by flow Cytofluorimetry: state of the art and new approaches to reduce the risk of false‐negative results. Front Sports Act Living. 2022;4:808449. doi: 10.3389/fspor.2022.808449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sottas PE, Robinson N, Rabin O, Saugy M. The athlete biological passport. Clin Chem. 2011;57(7):969‐976. doi: 10.1373/clinchem.2011.162271 [DOI] [PubMed] [Google Scholar]
- 28. Schumacher YO, Saugy M, Pottgiesser T, Robinson N. Detection of EPO doping and blood doping: the haematological module of the athlete biological passport. Drug Test Anal. 2012;4(11):846‐853. doi: 10.1002/dta.406 [DOI] [PubMed] [Google Scholar]
- 29. Faiss R, Saugy J, Saugy M. Fighting doping in elite sports: blood for all tests! Front Sports Act Living. 2019;1:30. doi: 10.3389/fspor.2019.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Donati F, Stampella A, de la Torre X, Botrè F. Investigation on the application of DNA forensic human identification techniques to detect homologous blood transfusions in doping control. Talanta. 2013;110:28‐31. doi: 10.1016/j.talanta.2013.02.042 [DOI] [PubMed] [Google Scholar]
- 31. Manokhina I, Rupert JL. A DNA‐based method for detecting homologous blood doping. Anal Bioanal Chem. 2013;405(30):9693‐9701. doi: 10.1007/s00216-013-7122-8 [DOI] [PubMed] [Google Scholar]
- 32. Stampella A, Di Marco S, Pirri D, de la Torre X, Botrè F, Donati F. Application of DNA‐based forensic analysis for the detection of homologous transfusion of whole blood and of red blood cell concentrates in doping control. Forensic Sci Int. 2016;265:204‐210. doi: 10.1016/j.forsciint.2016.04.021 [DOI] [PubMed] [Google Scholar]
- 33. Farrugia A, Tan Y, Romeo A, et al. Relative efficiency of leucocyte removal procedures for the production of leucocyte‐poor red cell concentrates assessed by flow cytometry. Vox Sang. 1994;66(3):153‐160. doi: 10.1111/j.1423-0410.1994.tb00302.x [DOI] [PubMed] [Google Scholar]
- 34. Donati F, Natalucci A, Concetti L, de la Torre X, Botrè F. A SNP‐based genotyping strategy to detect the abuse of homologous blood transfusion from dried blood spots. Drug Test Anal. 2024. doi: 10.1002/dta.3650. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 35. Marchand A, Roulland I, Semence F, et al. Evaluation of the detection of the homologous transfusion of a red blood cell concentrate in vivo for antidoping. Drug Test Anal. 2023;15(11‐12):1417‐1429. doi: 10.1002/dta.3448 [DOI] [PubMed] [Google Scholar]
- 36. Adams PT, Davenport RD, Reardon DA, Roth MS. Detection of circulating donor white blood cells in patients receiving multiple transfusions. Blood. 1992;80(2):551‐555. doi: 10.1182/blood.V80.2.551.551 [DOI] [PubMed] [Google Scholar]
- 37. Antidoping Rule Violations Olympic Games Tokyo 2020 https://ita.sport/sanction/olympic-games-tokyo-2020/ accessed 28 November 2023.
- 38. McKinnon KM. Flow cytometry: an overview. Curr Protoc Immunol. 2018;120:5.1.1‐5.1.11. doi: 10.1002/cpim.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maecker H, Trotter J. Selecting Reagents for Multicolor Flow cytometry. Application Note BD Biosciences 2012. https://web.mit.edu/flowcytometry/www/Becton%20Dickinson%20Multicolor_AppNote.pdf accessed 28 November 2023
- 40. Cristina Mirotti L, Renovato‐Martins M, du Rocher‐Silva B, et al. Minor red blood cell antigen phenotyping of athletes sampled in international competitions. Drug Test Anal. 2023;15(3):292‐298. doi: 10.1002/dta.3402 [DOI] [PubMed] [Google Scholar]


