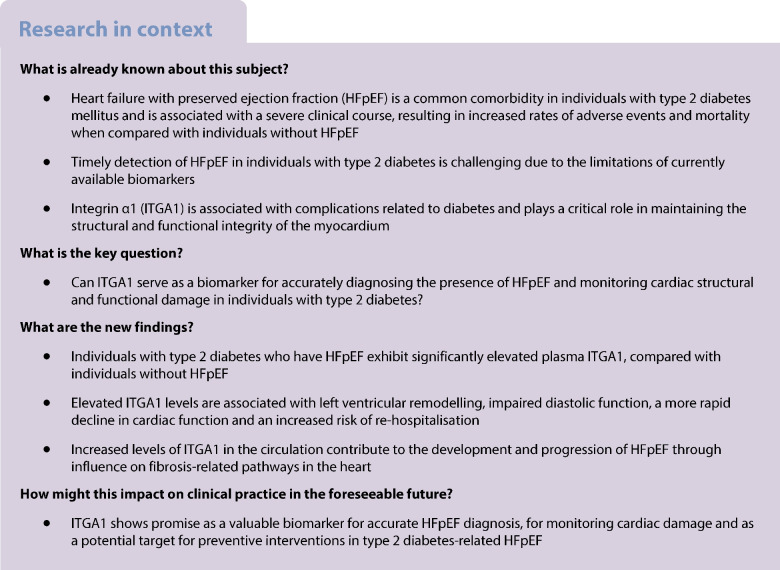
Abstract
Aims/hypothesis
Type 2 diabetes mellitus is known to contribute to the development of heart failure with preserved ejection fraction (HFpEF). However, identifying HFpEF in individuals with type 2 diabetes early on is often challenging due to a limited array of biomarkers. This study aims to investigate specific biomarkers associated with the progression of HFpEF in individuals with type 2 diabetes, for the purpose of enabling early detection and more effective management strategies.
Methods
Blood samples were collected from individuals with type 2 diabetes, both with and without HFpEF, for proteomic analysis. Plasma integrin α1 (ITGA1) levels were measured and compared between the two groups. Participants were further categorised based on ITGA1 levels and underwent detailed transthoracic echocardiography at baseline and during a median follow-up period of 30 months. Multivariable linear and Cox regression analyses were conducted separately to assess the associations between plasma ITGA1 levels and changes in echocardiography indicators and re-hospitalisation risk. Additionally, proteomic data for the individuals’ left ventricles, from ProteomeXchange database, were analysed to uncover mechanisms underlying the change in ITGA1 levels in HFpEF.
Results
Individuals with type 2 diabetes and HFpEF showed significantly higher plasma ITGA1 levels than the individuals with type 2 diabetes without HFpEF. These elevated ITGA1 levels were associated with left ventricular remodelling and impaired diastolic function. Furthermore, during a median follow-up of 30 months, multivariable analysis revealed that elevated ITGA1 levels independently correlated with deterioration of both diastolic and systolic cardiac functions. Additionally, higher baseline plasma ITGA1 levels independently predicted re-hospitalisation risk (HR 2.331 [95% CI 1.387, 3.917], p=0.001). Proteomic analysis of left ventricular myocardial tissue provided insights into the impact of increased ITGA1 levels on cardiac fibrosis-related pathways and the contribution made by these changes to the development and progression of HFpEF.
Conclusions/interpretation
ITGA1 serves as a biomarker for monitoring cardiac structural and functional damage, can be used to accurately diagnose the presence of HFpEF, and can be used to predict potential deterioration in cardiac structure and function as well as re-hospitalisation for individuals with type 2 diabetes. Its measurement holds promise for facilitating risk stratification and early intervention to mitigate the adverse cardiovascular effects associated with diabetes.
Data availability
The proteomic data of left ventricular myocardial tissue from individuals with type 2 diabetes, encompassing both those with and without HFpEF, is available from the ProteomeXchange database at http://proteomecentral.proteomexchange.org.
Graphical Abstract
Supplementary Information
The online version contains peer-reviewed but unedited supplementary material available at 10.1007/s00125-024-06109-4.
Keywords: Cardiac remodelling, Echocardiography, Heart failure with preserved ejection fraction, HFpEF, ITGA1, Plasma integrin α1, Type 2 diabetes
Introduction
Heart failure is a chronic and complex disease that poses a significant public health concern. Heart failure with preserved ejection fraction (HFpEF) constitutes approximately 50–55% of heart failure and its prevalence is increasing at a rate of around 1% per year [1]. Unlike heart failure with reduced ejection fraction, effective strategies for HFpEF management remain elusive [2]. Therefore, there is a pressing need to better understand the pathophysiology of HFpEF and screen potential risk factors to improve its prevention and management.
Type 2 diabetes mellitus is a common comorbidity in HFpEF; the presence of type 2 diabetes in individuals with HFpEF is associated with a higher rate of hospitalisation and mortality when compared with the absence of diabetes [3]. Approximately 45% of individuals with HFpEF have type 2 diabetes and the occurrence of comorbid type 2 diabetes is rising particularly among those with newly diagnosed HFpEF [4]. Unfortunately, the lack of obvious symptoms and limited biomarkers contribute to the delayed detection and treatment of HFpEF in individuals with type 2 diabetes [5]. Recent advances in peripheral blood analytical techniques and proteomics analysis have provided valuable insights into potential peripheral blood biomarkers for identifying HFpEF [6–8]. However, specific biomarkers for type 2 diabetes are limited and their relationship with heart structure and function, as well as future trends, has not been thoroughly analysed for most biomarkers [9]. To offset this deficiency, we conducted a plasma proteomics study on a well-matched subset of individuals with type 2 diabetes with and without HFpEF. Our analysis revealed a significant elevation in integrin α1 (ITGA1) levels among individuals with type 2 diabetes and HFpEF when compared with individuals with type 2 diabetes without HFpEF.
ITGA1 belongs to the integrin family, which consists of 18 α and 8 β subunits [10]. Traditionally, integrins are considered to be membrane-bound proteins that regulate cell-to-cell and cell-to-extracellular-matrix interactions [11]. However, it has been recently discovered that integrin subunits can exist in soluble forms in the circulation and have potential utility as diagnostic or prognostic markers for certain diseases [12]. For example, integrin β8 has been identified as a promising serum marker for the diagnosis, prognosis and surveillance of advanced colorectal cancer [13]. Additionally, the circulating levels of integrins β1, β2 and β3 can serve as diagnostic markers for venous thromboembolism [14]. ITGA1 is involved in cardiomyocyte adhesion and collagen secretion of myocardial fibroblasts, playing a critical role in cardiac remodelling [15, 16]. However, the relationship between ITGA1 plasma levels and the progression of left ventricular remodelling remains poorly understood. Therefore, this study aimed to validate ITGA1 as a diagnostic biomarker for identifying individuals with type 2 diabetes who are at a higher risk of developing HFpEF. Additionally, we aimed to establish a correlation between ITGA1 levels and abnormal cardiac structure and function, as well as explore its potential for predicting future cardiac deterioration and re-hospitalisation.
Methods
Study design
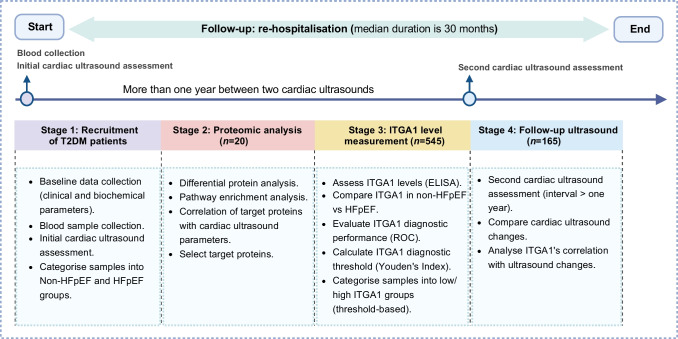
In our study on type 2 diabetes and HFpEF, we first recruited individuals with type 2 diabetes, collecting baseline data including demographics, clinical measurements and laboratory tests. These individuals were then categorised into non-HFpEF and HFpEF groups based on diagnostic criteria. In the proteomic analysis phase, we identified differentially expressed proteins related to HFpEF. Notably, the ‘hypertrophic cardiomyopathy’ pathway, which included proteins such as ITGA1, myosin heavy chain 7 (MYH7), desmin (DES), actin β (ACTB), IGF1 and calcium voltage-gated channel auxiliary subunit α2δ1 (CACNA2D1), was of particular interest due to its strong link to HFpEF. We correlated these proteins with echocardiographic data, finding ITGA1 to have significant correlations with cardiac structure and function. Subsequently, we focused on ITGA1, measuring its levels in both non-HFpEF and HFpEF groups and assessing its diagnostic utility. The final stage involved follow-up cardiac ultrasound assessments and monitoring of re-hospitalisation rates. The follow-up period, commencing from participant recruitment and initial blood sample collection, extended over a median duration of 30 months (Fig. 1).
Fig. 1.
Flow diagram of the study process. We first recruited individuals with type 2 diabetes and collected baseline data. The participants were then categorised into non-HFpEF and HFpEF groups based on diagnostic criteria. In the proteomic analysis phase, we identified differentially expressed proteins related to HFpEF and correlated these proteins with echocardiographic data, finding that ITGA1 correlated significantly with cardiac structure and function. Subsequently, we focused on ITGA1, measuring its levels in both non-HFpEF and HFpEF groups and assessing its diagnostic utility. The final stage involved follow-up cardiac ultrasound assessments and monitoring re-hospitalisation rates. The follow-up period, commencing from participant recruitment and initial blood sample collection, extended over a median duration of 30 months. T2DM, type 2 diabetes
Study population
All participants were recruited from the Cardiovascular Medical Centers of the University of Hong Kong - Shenzhen Hospital from June 2019 to June 2021. HFpEF was diagnosed according to the European Society of Cardiology guideline [17]: (1) presence of symptoms and/or signs of heart failure; (2) left ventricular ejection fraction (LVEF) ≥50%; and (3) N-terminal pro-B-type natriuretic peptide (NT-proBNp) >125 pg/ml. Type 2 diabetes was diagnosed using ADA guidelines [18]. Individuals meeting any of the following criteria were excluded: (1) LVEF <50% at any time; (2) isolated right heart failure due to pulmonary disease; (3) dyspnoea due to non-cardiac causes such as pulmonary disease, anaemia or severe obesity; or (4) severe valvular disease, infiltrative cardiomyopathy, congenital heart disease, chronic obstructive pulmonary disease (Global Initiative for Chronic Obstructive Lung Disease [GOLD] stage 3 or 4) or pericardial disease. The study was approved by the local ethics committee and written informed consent was obtained from all participants.
Clinical and biochemical data
Clinical measurements and blood sampling were conducted after participants had fasted overnight for at least 8 h. Age, sex (determined based on self-reported information) and detailed medical histories, including smoking status and the presence of hypertension, atrial fibrillation or coronary artery disease were recorded. Anthropometric measurements (body weight and height) were recorded and BMI was calculated. BP was measured at the end of the echocardiography examination after a 5 min rest. Fasting blood samples were collected for measuring HbA1c, glucose, lipid profile and serum creatinine levels and stored at −80°C for additional assays [19]. ITGA1 levels were measured using an ELISA kit (FineTest, China). The kit had an intra- and inter-assay coefficient of variation <8% and <10%, respectively, and a sensitivity limit of 0.188 ng/ml.
Sample preparation and proteomic analysis
Serum samples were thawed, centrifuged to remove debris, and high-abundance proteins were removed. Protein concentration was determined, and the proteins were reduced, alkylated and digested using the filter-aided sample preparation (FASP) method. Peptides were recovered by centrifugation and desalted using a Strata X SPE column (Phenomenex, CA, USA). Tryptic peptides were separated on a reverse-phase column with a solvent gradient. Orbitrap Exploris 480 mass spectrometer (Thermo Fisher Scientific, MA, USA) was used for peptide analysis, adjusting scan resolutions. Abundant precursors were selected for MS/MS analysis with high-energy collision dissociation (HCD) fragmentation. Proteome Discoverer search engine (v.2.4) was used for data analysis against a human protein database with specified criteria [20]. Mass error tolerances and false discovery rate (FDR) thresholds were adjusted. The detailed steps were followed as described in previous studies [21].
To further investigate the potential mechanisms linking elevated plasma ITGA1 levels to cardiac dysfunction and the development of HFpEF, we retrieved proteomic data for left ventricular myocardial tissue from individuals with type 2 diabetes with and without HFpEF from the ProteomeXchange database (http://proteomecentral.proteomexchange.org) and conducted a comparative proteomic analysis.
Echocardiography measurement
Standard two-dimensional echocardiography and tissue Doppler imaging was performed on recruited participants with a commercially available echocardiography system (VingmedE9; General Electric Vingmed Ultrasound, Horten, Norway) by skilled operators who were blinded to the participants’ clinical and biochemical characteristics. Participants were in the lateral decubitus position, and a 3.5 MHz transducer (General Electric Healthcare, IL, USA) was used to capture images and digitally store them in cine-loop format. Left ventricular end diastolic dimension (LVDD), left ventricular end systolic dimension (LVDS) and interventricular septal dimension at end-diastole (IVSD) were measured by the leading-edge-to-leading-edge method from two-dimensional guided M-mode tracings recorded at the parasternal long-axis view. Left ventricular mass was calculated according to the Devereux formula. Left ventricular volume and LVEF were measured using modified biplane Simpson’s method in apical four- and two-chamber views. Doppler imaging was applied to assess left ventricular diastolic function in apical four-chamber view. The transmitral early diastolic peak velocity (E) wave and transmitral late diastolic peak velocity (A) wave were measured, and the E/A ratio was calculated. The early diastolic peak velocity of mitral valve at septal or lateral annulus (e′) was measured by tissue Doppler imaging, and the average E/e′ was calculated [22].
Statistical analysis
Continuous variables were presented as mean ± SD, as well as frequencies and percentages for categorical variables. Normal distribution was assessed through the Kolmogorov–Smirnov test. For normally distributed variables, independent samples t tests were used to determine differences. Non-normally distributed or heterogeneous data were analysed using non-parametric tests. Categorical variables were compared using the χ2 test. In the small cohorts, variables including age, sex, duration of diabetes, BMI, smoking status, comorbidities and laboratory findings (glucose, HbA1c, albumin, triacyglycerol, total cholesterol, HDL-cholesterol, LDL-cholesterol, uric acid, and creatinine) were matched (p>0.05 for difference between groups).
The predictive ability of serum ITGA1 for HFpEF was assessed using the AUC in the receiver operating characteristic (ROC) curve. ITGA1’s Youden’s index was derived from the ROC curves. Changes in clinical characteristics and echocardiography indicators between baseline and follow-up were analysed using paired t tests or McNemar tests, as appropriate. Multivariable linear regression was used to examine the association between serum ITGA1 levels and changes in echocardiography indicators, taking into account the matching variables in the small cohorts. Kaplan–Meier survival curves with the logrank test were used to compare re-hospitalisation rates based on ITGA1 levels. The association between ITGA1 and re-hospitalisation risk was assessed using multivariable Cox regression, with significant variables from univariate analyses or biologically relevant factors included in the multivariable regression models.
IBM SPSS 26.0 (https://spss.en.softonic.com/) was used for all statistical analyses, with a two-sided p value <0.05 considered statistically significant.
Results
Baseline characteristics of the study population
Given the complex relationship between type 2 diabetes and HFpEF, identifying at-risk individuals is crucial for early intervention. To identify biomarkers that could better distinguish individuals with type 2 diabetes at higher risk of HFpEF development, a subset of 20 well-matched participants with type 2 diabetes was initially selected for a plasma proteomics study. This subset consisted of ten individuals with HFpEF and ten individuals without the condition (non-HFpEF). Electronic supplementary material (ESM) Table 1 provides detailed information on this initial study population.
Subsequently, an additional 545 participants with type 2 diabetes were enrolled for further evaluation, including blood tests and echocardiography. These participants were divided into non-HFpEF vs HFpEF groups and low-ITGA1 vs high-ITGA1 groups. Comparing the non-HFpEF and HFpEF groups, participants in the HFpEF group were found to be older, had a higher proportion of female participants and atrial fibrillation, and had a longer duration of diabetes (Table 1). Additionally, participants with HFpEF exhibited higher levels of creatinine, NT-proBNP and ITGA1, while having lower levels of albumin, compared with participants in the non-HFpEF group. In the comparison between the low-ITGA1 and high-ITGA1 groups, participants in the high-ITGA1 group were found to be older, had longer durations of diabetes, and had a higher proportion of atrial fibrillation, as well as lower proportion of arterial hypertension (Table 1). Furthermore, they presented elevated levels of uric acid, creatinine and NT-proBNP, along with lower levels of albumin.
Table 1.
Clinical characteristics of participants categorised by HFpEF and ITGA1
| Clinical characteristic | HFpEF | ITGA1 | ||||
|---|---|---|---|---|---|---|
| No (n=301) | Yes (n=244) | p value | Low (n=317) | High (n=228) | p value | |
| Age, years | 62.0±9.9 | 68.8±11.1 | <0.001 | 62.1±9.8 | 69.0±11.3 | <0.001 |
| Female sex, n (%) | 96 (31.9) | 101 (41.4) | 0.022 | 105 (33.1) | 92 (40.4) | 0.083 |
| Duration of diabetes, years | 7.9±6.9 | 10.9±8.3 | <0.001 | 7.7±6.7 | 11.4±8.5 | <0.001 |
| BMI, kg/m2 | 25.0±3.1 | 24.7±3.3 | 0.230 | 25.0±3.0 | 24.8±3.5 | 0.404 |
| Smoking, n (%) | 90 (29.9) | 73 (29.9) | 0.983 | 104 (32.8) | 59 (25.9) | 0.077 |
| Comorbidities, n (%) | ||||||
| Arterial hypertension | 253 (84.1) | 195 (79.9) | 0.209 | 271 (85.5) | 177 (77.6) | 0.018 |
| Atrial fibrillation history | 12 (4.0) | 56 (23.0) | <0.001 | 22 (6.9) | 46 (20.1) | <0.001 |
| Coronary artery disease | 254 (84.4) | 216 (88.5) | 0.163 | 270 (85.2) | 200 (87.7) | 0.395 |
| Laboratory findings | ||||||
| Glucose, mmol/l | 9.26±3.81 | 9.89±3.92 | 0.059 | 9.40±4.11 | 10.11±4.59 | 0.063 |
| HbA1c, mmol/mol | 58.36±15.28 | 60.09±18.36 | 0.058 | 58.43±16.74 | 59.69±16.95 | 0.390 |
| HbA1c, % | 7.49±1.40 | 7.74±1.68 | 0.058 | 7.50±1.53 | 7.61±1.55 | 0.390 |
| Albumin, g/l | 44.18±4.45 | 41.21±4.66 | <0.001 | 44.10±4.47 | 41.12±4.65 | <0.001 |
| Triacylglycerol, mmol/l | 2.39±1.71 | 2.03±1.43 | 0.010 | 2.28±1.72 | 2.15±1.41 | 0.340 |
| Total cholesterol, mmol/l | 3.99±1.28 | 3.76±1.19 | 0.031 | 3.93±1.31 | 3.82±1.14 | 0.350 |
| HDL-cholesterol, mmol/l | 1.09±0.30 | 1.09±0.30 | 0.934 | 1.09±0.30 | 1.08±0.30 | 0.697 |
| LDL-cholesterol, mmol/l | 2.32±1.04 | 2.16±1.01 | 0.069 | 2.28±1.07 | 2.20±0.97 | 0.374 |
| Uric acid, μmol/l | 343.99±103.12 | 360.98±120.20 | 0.076 | 342.34±104.39 | 364.47±119.32 | 0.022 |
| Creatinine, μmol/l | 77.11±17.83 | 149.51±76.74 | <0.001 | 77.29±17.45 | 154.33±81.88 | <0.001 |
| NT-proBNP, pg/ml | 58.12±47.04 | 813.21±779.30 | <0.001 | 75.56±50.47 | 843.25±912.01 | <0.001 |
| ITGA1, ng/ml | 3.21±0.97 | 9.65±13.57 | <0.001 | 2.93±0.67 | 10.41±13.68 | <0.001 |
Data are shown as mean ± SD or n (%)
Among the 545 participants with type 2 diabetes included in the study, 165 agreed to participate in follow-up and returned for echocardiography examinations over a median duration of 30 months (at least 12 months after the baseline examination). These participants were then categorised into low-ITGA1 and high-ITGA1 groups. The individuals with high-ITGA1 levels were found to be older (ESM Table 2). Additionally, compared with the low-ITGA1 group, the high-ITGA1 group had a longer duration of diabetes and displayed higher levels of creatinine and NT-proBNP, along with lower levels of albumin.
Plasma proteomic outcomes
The plasma proteins from participants in the non-HFpEF vs HFpEF groups were found to be distinguishable based on the results of principal component analysis (Fig. 2a). A total of 235 proteins with altered expression were identified between the two groups (81 downregulated proteins and 154 upregulated proteins in the HFpEF group compared with the non-HFpEF group) (Fig. 2b). Subsequent analysis using Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment revealed that the differentially expressed proteins were primarily involved in ten specific pathways. Among the analysed pathways, ‘hypertrophic cardiomyopathy’ stands out as a recognised contributor to HFpEF development due to its association with diastolic dysfunction and impaired heart filling (Fig. 2c). Within this pathway, ITGA1 demonstrated a stronger correlation with echocardiography indicators related to cardiac structure and function compared with the other five biomarkers (Fig. 2d and ESM Table 3). This suggests that ITGA1 could potentially serve as a valuable biomarker for assessing cardiac function in individuals with type 2 diabetes and for distinguishing those at a higher risk of developing HFpEF.
Fig. 2.
Plasma proteomic analysis comparing participants with type 2 diabetes with or without HFpEF. (a) Principal component analysis demonstrates distinguishable separation between the two groups. (b) Volcano plot showing differential proteins between the two groups. A total of 235 proteins exhibited significant alterations, including 81 downregulated and 154 upregulated proteins in the HFpEF group compared with the non-HFpEF group. (c) KEGG enrichment analysis revealed that the differentially expressed proteins were primarily involved in ten specific pathways. Among these pathways, ‘hypertrophic cardiomyopathy’ was closely linked to HFpEF. (d) Correlation analysis examining the relationship between proteins enriched in the ‘hypertrophic cardiomyopathy’ pathway and echocardiography indicators. ITGA1 demonstrated a stronger correlation with echocardiography indicators related to cardiac structure (left atrial diameter to left ventricular end systolic volume) and function (ejection fraction to e′ septal) compared with the other seven biomarkers. ACTB, actin β; CACNA2D1, calcium voltage-gated channel auxiliary subunit α2δ1; DES, desmin; Dim1, dimension 1; Dim2, dimension 2; e' lat, e' lateral; e' sep, e' septal; LAD, left atrial diameter; LVESV, left ventricular end systolic volume; MYH7, myosin heavy chain 7; PCA, principal component analysis; PPAR, peroxisome proliferator-activated receptor
ITGA1 levels and echocardiography indicators in individuals with type 2 diabetes with and without HFpEF
To further investigate ITGA1 as a diagnostic marker for HFpEF in individuals with type 2 diabetes, an additional cohort of 545 participants with type 2 diabetes was enrolled in the study (244 with HFpEF and 301 without HFpEF). Cardiac ultrasound assessment revealed that the participants with HFpEF exhibited several significant differences when compared with those without HFpEF, as shown in Table 2. Specifically, those with HFpEF demonstrated increased left ventricular wall thickness (p<0.05 for IVSD and p<0.01 for left ventricular posterior wall dimension [LVPWD]), mass (p<0.001) and volume (p<0.001). They also showed reduced systolic function (lower LVEF, p<0.001) and compromised diastolic function (lower e′ septal, e′ lateral, and E/A ratio, as well as higher average E/e′, all p<0.001) compared with individuals without HFpEF.
Table 2.
Echocardiographic characteristics of participants categorised by HFpEF and ITGA1
| Echocardiographic characteristic | HFpEF | ITGA1 | ||||
|---|---|---|---|---|---|---|
| No (n=301) | Yes (n=244) | p value | Low (n=317) | High (n=228) | p value | |
| IVSD, mm | 10.02±1.55 | 10.43±1.75 | 0.015 | 10.09±1.60 | 10.37±1.71 | 0.048 |
| LVPWD, mm | 9.85±1.61 | 10.33±1.98 | 0.003 | 9.93±1.61 | 10.25±2.02 | 0.038 |
| LVDD, mm | 45.95±3.90 | 48.39±5.86 | <0.001 | 46.61±4.08 | 47.64±6.05 | 0.017 |
| LVDS, mm | 29.19±4.18 | 32.19±5.55 | <0.001 | 29.80±4.21 | 31.55±5.91 | <0.001 |
| LV mass, g | 159.77±41.52 | 185.59±58.36 | <0.001 | 165.02±42.76 | 180.10±60.35 | 0.001 |
| LVEDV, ml | 97.94±19.14 | 112.89±30.13 | <0.001 | 101.34±20.32 | 109.21±31.26 | <0.001 |
| LVESV, ml | 33.90±10.92 | 43.79±18.14 | <0.001 | 35.87±12.20 | 41.75±18.45 | <0.001 |
| LVEF, % | 65.93±5.19 | 62.45±6.86 | <0.001 | 65.11±5.98 | 63.35±6.46 | 0.001 |
| E/A | 1.11±0.26 | 0.93±0.56 | 0.002 | 1.04±0.29 | 0.89±0.56 | 0.013 |
| e′ lateral, cm/s | 9.07±2.57 | 7.66±2.41 | <0.001 | 8.88±2.49 | 7.83±2.61 | <0.001 |
| e′ septal, cm/s | 6.58±1.96 | 5.79±1.72 | <0.001 | 6.54±2.01 | 5.79±1.63 | <0.001 |
| Average E/e′ | 10.72±3.14 | 14.41±6.06 | <0.001 | 11.30±3.88 | 13.86±5.97 | <0.001 |
Data are shown as mean ± SD
LV, left ventricular; LVEDV, left ventricular end diastolic volume; LVESV, left ventricular end systolic volume
The analysis of ITGA1 expression revealed its upregulation in the participants with type 2 diabetes who had HFpEF when compared with those who did not (Fig. 3a). Furthermore, ROC analysis demonstrated its strong predictive capacity for diagnosing HFpEF, as supported by an AUC value of 0.82, along with relatively higher positive (86.84%) and negative (85.49%) predictive values (Fig. 3b). Dividing the participants into low- and high-ITGA1 groups based on the Youden’s index of ITGA1, the high-ITGA1 group demonstrated increased left ventricular wall thickness (p<0.05 for IVSD and LVPWD), mass (p<0.01) and volume (p<0.001) (Table 2). The participants in the high-ITGA1 group also demonstrated reduced systolic function (lower LVEF, p<0.01) and impaired diastolic function (reduced e’ septal [p<0.001], e’ lateral [p<0.001] and E/A ratio [p<0.05], as well as elevated average E/e’ ratio [p<0.001]) compared with the low ITGA1 group.
Fig. 3.
Plasma ITGA1 as a diagnostic marker for HFpEF in individuals with type 2 diabetes. (a) Comparison of plasma ITGA1 levels between participants with type 2 diabetes with and without HFpEF. It indicated a notable increase in individuals with type 2 diabetes who also exhibited HFpEF, compared with those without HFpEF. Box plots show median (central line), 25th and 75th percentiles (box edges), and min/max values (whiskers), ***p<0.001. (b) ROC curves evaluating the diagnostic ability of plasma ITGA1 for HFpEF in individuals with type 2 diabetes. The ROC analysis underscored the dependable diagnostic capability of ITGA1 for HFpEF, demonstrated by an AUC value of 0.82
To further explore the impact of ITGA1 on the differences in echocardiographic indices between non-HFpEF and HFpEF groups, we divided the participants into low- and high-ITGA1 groups within each HFpEF/non-HFpEF group and assessed the variations in echocardiographic indices. For the non-HFpEF participants, the high-ITGA1 group exhibited worse left ventricular diastolic function, characterised by lower e′ septal (p<0.05), e′ lateral (p<0.05) and E/A ratio (p<0.001) (Table 3). For participants with HFpEF, the high-ITGA1 group was associated with higher left ventricular mass (p<0.01), reduced systolic function (reflected by lower LVEF, p<0.05), as well as impaired diastolic function indicated by lower E/A ratio (p<0.01) and higher average E/e′ ratio (p<0.001). These findings suggest that high ITGA1 levels may serve as a useful predictor of cardiac diastolic dysfunction in individuals with type 2 diabetes, regardless of the presence of HFpEF. Moreover, in individuals with HFpEF, high ITGA1 levels could indicate more severe cardiac structural and functional damage.
Table 3.
Echocardiographic variables of participants in the HFpEF and non-HFpEF groups according to the serum level of ITGA1
| Echocardiographic characteristic | Non-HFpEF | HFpEF | ||||
|---|---|---|---|---|---|---|
| Low ITGA1 (n=234) | High ITGA1 (n=67) | p value | Low ITGA1 (n=83) | High ITGA1 (n=161) | p value | |
| IVSD, mm | 9.93±1.81 | 9.94±1.53 | 0.193 | 10.29±1.82 | 10.48±1.74 | 0.390 |
| LVPWD, mm | 9.84±1.68 | 10.04±1.30 | 0.333 | 10.02±1.49 | 10.36±2.15 | 0.154 |
| LVDD, mm | 46.08±4.93 | 45.88±4.30 | 0.680 | 47.37±4.61 | 49.70±7.14 | 0.001 |
| LVDS, mm | 29.03±3.63 | 29.59±5.04 | 0.348 | 31.32±4.60 | 33.99±7.28 | <0.001 |
| LV mass, g | 160.42±40.89 | 157.48±43.86 | 0.610 | 172.64±48.60 | 193.96±57.36 | 0.004 |
| LVEDV, ml | 98.45±18.27 | 96.52±22.08 | 0.471 | 105.66±23.19 | 121.68±28.20 | <0.001 |
| LVESV, ml | 33.46±9.82 | 34.79±14.47 | 0.433 | 40.21±14.45 | 50.77±25.92 | <0.001 |
| LVEF, % | 66.18±4.91 | 65.93±5.27 | 0.688 | 62.52±7.41 | 60.16±8.99 | 0.018 |
| E/A | 0.94±0.28 | 0.82±0.21 | <0.001 | 0.89±0.63 | 0.72±0.31 | 0.004 |
| e′ lateral, cm/s | 6.80±1.98 | 6.17±1.98 | 0.012 | 5.94±1.53 | 5.65±1.60 | 0.175 |
| e′ septal, cm/s | 9.19±2.50 | 8.48±2.91 | 0.033 | 7.84±2.42 | 7.38±2.34 | 0.117 |
| Average E/e′ | 10.27±3.03 | 10.98±3.39 | 0.101 | 13.20±5.07 | 15.93±7.25 | <0.001 |
Data are shown as mean ± SD
LV, left ventricular; LVEDV, left ventricular end diastolic volume; LVESV, left ventricular end systolic volume
Association between ITGA1 levels and changes in echocardiography indicators
Among the 165 participants who underwent follow-up echocardiography examinations, 90 had low ITGA1 levels and 75 had high ITGA1 levels. Comparing the follow-up assessments with the baseline measurements, participants in the low-ITGA1 group did not show significant differences in most echocardiography indices, except for a minor decrease in the E/A ratio (Table 4 and ESM Fig. 1). In contrast, participants in the high-ITGA1 group exhibited noteworthy changes in several echocardiography indicators. Specifically, the high-ITGA1 group showed a significant increase in left ventricular wall thickness (p<0.01 for LVPWD), mass (p<0.001) and LV volume (p<0.01 for left ventricular end diastolic volume [LVEDV] and p=0.01 for left ventricular end systolic volume [LVESV]) during the follow-up period. Additionally, the high-ITGA1 group displayed reduced systolic function, as indicated by a decreased LVEF (p<0.01), and compromised diastolic function, characterised by reduced e′ septal (p<0.05), e′ lateral (p<0.05) and E/A ratio (p<0.05), as well as increased average E/e′ (p<0.05) at follow-up.
Table 4.
Changes in echocardiographic measures from baseline to follow-up
| Echocardiographic characteristic | Low ITGA1 (n=90) | High ITGA1 (n=75) | ||||
|---|---|---|---|---|---|---|
| Baseline | Follow-up | p value | Baseline | Follow-up | p value | |
| IVSD, mm | 9.93±1.81 | 9.94±1.53 | 0.956 | 10.09±1.77 | 10.46±1.95 | 0.102 |
| LVPWD, mm | 9.65±1.47 | 10.01±2.90 | 0.259 | 9.50±1.35 | 10.17±1.56 | 0.004 |
| LVDD, mm | 46.65±4.93 | 46.68±4.03 | 0.956 | 47.72±5.53 | 50.03±7.51 | 0.015 |
| LVDS, mm | 30.31±3.96 | 30.61±6.01 | 0.638 | 30.85±6.07 | 34.37±8.64 | 0.004 |
| LV mass, g | 179.45±56.54 | 182.44±67.23 | 0.134 | 182.39±64.24 | 199.60±62.19 | <0.001 |
| LVEDV, ml | 101.81±21.39 | 102.53±26.38 | 0.775 | 107.48±31.08 | 121.22±24.15 | 0.008 |
| LVESV, ml | 35.42±15.81 | 39.37±19.26 | 0.087 | 41.44±22.14 | 52.05±21.85 | 0.010 |
| LVEF, % | 64.71±6.10 | 62.57±9.23 | 0.051 | 63.31±7.63 | 59.50±8.72 | 0.007 |
| E/A | 1.04±0.23 | 0.87±0.21 | 0.034 | 0.92±0.12 | 0.78±0.20 | 0.012 |
| e' lateral, cm/s | 6.47±2.20 | 6.62±2.15 | 0.574 | 6.00±1.60 | 5.58±1.47 | 0.040 |
| e′ septal, cm/s | 8.50±2.82 | 8.36±2.61 | 0.657 | 7.88±3.08 | 7.08±2.83 | 0.045 |
| Average E/e′ | 10.62±3.87 | 10.76±4.70 | 0.767 | 12.09±3.74 | 14.24±3.30 | 0.015 |
Data are shown as mean ± SD
LV, left ventricular; LVEDV, left ventricular end diastolic volume; LVESV, left ventricular end systolic volume
To further investigate the relationship between plasma ITGA1 levels and changes in echocardiography indicators, linear regression analysis was conducted. In univariate linear regression, high ITGA1 levels at baseline were significantly associated with a decrease in LVEF and E/A ratio, and an increase in E/e′ ratio (all with p<0.05) (ESM Table 4). In the multivariable linear regression model, even after adjusting for baseline echocardiography indicators, sex, smoking status, duration of diabetes, total cholesterol, LDL-cholesterol and NT-proBNP, high baseline levels of ITGA1 remained independently associated with a decrease in LVEF and an increase in both the E/A ratio (p=0.001) and the E/e′ ratio (p=0.001) (Table 5). This suggests that an elevated circulating level of ITGA1 is an independent predictor of heart dysfunction, encompassing impaired diastolic and systolic functions, in type 2 diabetes patients.
Table 5.
Multiple linear regression showing the association between change in LVEF, E/A and average E/e′, and plasma ITGA1 levels
| Variable | △LVEF (%) | △E/A | △E/e′ | |||
|---|---|---|---|---|---|---|
| Standardised β | p value | Standardised β | p value | Standardised β | p value | |
| Baseline cardiac variables | −0.40 | 0.001 | −0.46 | 0.001 | −0.32 | 0.001 |
| ITGA1 | −0.26 | 0.007 | −0.15 | 0.030 | 0.29 | 0.002 |
| Sex | −0.17 | 0.056 | 0.01 | 0.116 | −0.19 | 0.032 |
| Smoking | 0.17 | 0.057 | −0.20 | 0.840 | −0.04 | 0.605 |
| Duration of diabetes, years | 0.01 | 0.917 | −0.01 | 0.991 | −0.01 | 0.975 |
| Total cholesterol, mmol/l | 0.07 | 0.509 | 0.06 | 0.732 | 0.02 | 0.943 |
| LDL-cholesterol, mmol/l | 0.34 | 0.072 | 0.28 | 0.779 | −0.03 | 0.875 |
| NT-proBNP, pg/ml | −0.23 | 0.005 | −4.04 | 0.001 | −0.02 | 0.883 |
Baseline cardiac variables indicate baseline LVEF (for change in LVEF), baseline E/A (for change in E/A) and baseline average E/e′ (for change in average E/e′), respectively
Association between ITGA1 levels and re-hospitalisation
During a median duration of 30 months, re-hospitalisation occurred in 33 participants (36.7%) in the low-ITGA1 group and in 51 participants (68.0%) in the high-ITGA1 group. The Kaplan–Meier survival curve illustrated that those participants in the high-ITGA1 group had a significantly increased risk of re-hospitalisation compared with those in the low ITGA1 group (Fig. 4, p<0.01). Moreover, a multivariate Cox regression analysis revealed that the baseline ITGA1 level was independently associated with re-hospitalisation (HR 2.331 [95% CI 1.387, 3.917], p=0.001) after adjusting for age, duration of diabetes, smoking, atrial fibrillation history and baseline creatinine levels (Table 6). This indicates that a higher plasma ITGA1 level is indicative of a significantly elevated risk of re-hospitalisation.
Fig. 4.
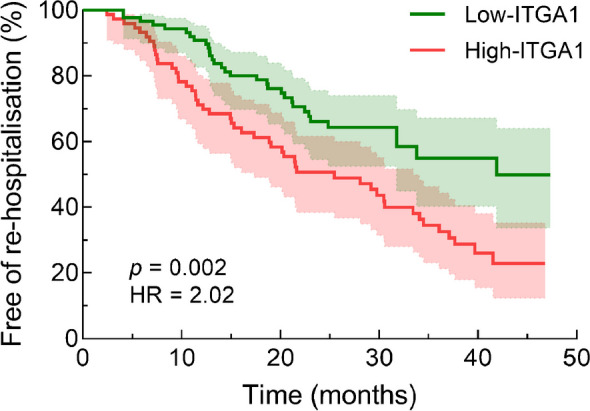
Kaplan–Meier survival curve for re-hospitalisation according to plasma ITGA1 levels. Participants in the high-ITGA1 group (n=75) had a significantly increased risk of re-hospitalisation compared with those in the low-ITGA1 group (n=90)
Table 6.
Cox regression analysis for re-hospitalisation in participants with type 2 diabetes
| Clinical characteristic | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Age | 1.020 | 1.002, 1.039 | 0.031 | 1.003 | 0.981, 1.025 | 0.814 |
| Sex (male vs female) | 0.725 | 0.466, 1.127 | 0.153 | |||
| Duration of diabetes | 1.034 | 1.008, 1.061 | 0.009 | 1.024 | 0.994, 1.055 | 0.123 |
| BMI | 1.005 | 0.963, 1.049 | 0.807 | |||
| Smoking (yes vs no) | 1.824 | 1.055, 3.154 | 0.031 | 1.561 | 0.851, 2.863 | 0.150 |
| Arterial hypertension (yes vs no) | 1.541 | 0.890, 2.669 | 0.122 | |||
| Atrial fibrillation history (yes vs no) | 1.907 | 1.116, 3.258 | 0.018 | 1.584 | 0.878, 2.860 | 0.127 |
| Coronary artery disease (yes vs no) | 0.790 | 0.344, 1.816 | 0.579 | |||
| Glucose | 0.989 | 0.952, 1.027 | 0.568 | |||
| HbA1c | 1.049 | 0.970, 1.134 | 0.233 | |||
| Albumin | 0.992 | 0.977, 1.008 | 0.323 | |||
| Triacylglycerol | 0.996 | 0.864, 1.148 | 0.956 | |||
| Total cholesterol | 0.885 | 0.771, 1.016 | 0.082 | |||
| HDL-cholesterol | 0.841 | 0.483, 1.467 | 0.543 | |||
| LDL-cholesterol | 1.010 | 0.975, 1.052 | 0.405 | |||
| Uric acid | 1.000 | 0.999, 1.002 | 0.736 | |||
| Creatinine | 1.001 | 1.000, 1.002 | 0.026 | 1.001 | 0.999, 1.002 | 0.349 |
| NT-proBNP | 1.000 | 0.999, 1.001 | 0.509 | |||
| ITGA1 level (high vs low) | 2.017 | 1.299, 3.131 | 0.002 | 2.331 | 1.387, 3.917 | 0.001 |
Mechanisms of elevated ITGA1 levels in HFpEF
Circulating proteins play a crucial role in heart biology, and changes in their levels contribute to various CVDs, such as heart failure, hypertension and ischaemic heart disease [23, 24]. To further investigate the potential mechanisms linking elevated plasma ITGA1 levels to cardiac dysfunction and the development of HFpEF, we retrieved proteomic data for left ventricular myocardial tissue from individuals with type 2 diabetes with and without HFpEF from the ProteomeXchange database (http://proteomecentral.proteomexchange.org, accessed on 10 July 2023) and conducted a comparative proteomic analysis. We observed significant differences in protein expression between the two groups (ESM Fig. 2a). Subsequent analysis using a protein–protein interaction (PPI) network identified 15 proteins as key targets of ITGA1 among the differentially expressed proteins, including seven that were downregulated and eight that were upregulated, in the HFpEF group (ESM Fig. 2b, c; ESM Table 5). Both Gene Ontology (GO) and KEGG enrichment analyses revealed that these proteins were mainly associated with myocardial fibrosis-related pathways (ESM Fig. 2d, e). These findings suggest that elevated ITGA1 levels may play a role in the promotion of myocardial fibrosis, thereby contributing to the development of HFpEF in individuals with type 2 diabetes.
Discussion
Type 2 diabetes is a known trigger for HFpEF, affecting ventricular relaxation/stiffness and coronary microvascular function. Individuals with type 2 diabetes who have HFpEF often experience a severe clinical course, resulting in increased rates of adverse events and mortality compared with individuals who do not have HFpEF [25]. However, the timely detection of HFpEF in individuals with type 2 diabetes is often overlooked due to the limitations of currently available biomarkers. To address this challenge, we conducted proteomics analysis using blood samples collected from individuals with type 2 diabetes with and without HFpEF. The analysis revealed significantly higher levels of plasma ITGA1 in those with HFpEF compared with those without HFpEF. Moreover, the participants with elevated ITGA1 levels demonstrated left ventricular remodelling, impaired diastolic function and a more rapid decline in cardiac function, along with an increased risk of re-hospitalisation over the follow-up period of 30 months.
ITGA1 is a member of the integrin family, which consist of α and β receptor subunits and act as transmembrane cell-adhesion molecules [10]. Integrins, including ITGA1, play a critical role in maintaining the structural and functional integrity of the myocardium in a healthy heart. However, in the presence of heart disease, integrin expression and function can be altered in response to abnormal stress signals, leading to cardiac remodelling [26, 27]. Suppressing ITGA1 expression has been shown to alleviate aggregation in the pathogenesis of cardiomyopathies, highlighting the strong association between abnormal ITGA1 expression and cardiac dysfunction [16]. Integrins, in general, have also been associated with cardiac dysfunction in diabetes. For instance, in diabetic cardiomyopathy, increased levels of integrin α11 expression can stimulate and activate TGF-β2, leading to collagen synthesis and myofibroblast differentiation and contributing to the development of fibrotic tissue [28]. Integrin α5 has been implicated in the regulation of vascular complications in type 1 diabetes [29]. In mouse models of diabetes, the combination of sKL and integrin β1 triggers the activation of the ERK1/2 pathway, leading to selective insulin resistance and myocardial fibrosis. Furthermore, ITGA1 has been implicated in diabetes-related complications. In mice with high-fat-induced insulin resistance, increased ITGA1 expression in hepatocytes is associated with impaired hepatic glucose metabolism, while the deletion of ITGA1 improves fatty liver conditions [30]. These findings suggest the potential involvement of ITGA1 in the cardiac remodelling associated with diabetes.
While integrin is primarily known as a transmembrane receptor, previous studies have demonstrated the existence of soluble forms of integrin in the bloodstream. These soluble forms are generated through proteolytic cleavage of the extracellular domain of integrin and have emerged as potential biomarkers for various diseases [31, 32]. For instance, high levels of circulating integrin have been implicated as diagnostic markers for venous thromboembolism [14] and enrichment of integrin αvβ1 has been observed in the circulation of individuals with advanced stages of breast cancer [33]. In individuals with colorectal cancer, the serum concentration of ITGA1 was also found to be significantly higher compared with that in healthy individuals and showed a significant association with metastatic (tumour, node, metastasis [TNM]) stage [34]. However, there is currently no reported research on the relationship between serum levels of ITGA1 and cardiac remodelling associated with type 2 diabetes.
In our study, we investigated the potential of ITGA1 as a diagnostic marker for HFpEF in individuals with type 2 diabetes. Additionally, we compared the echocardiographic profiles of participants categorised by ITGA1 levels at baseline and follow-up. Echocardiography, a widely accessible imaging technique, enables the identification of adverse left ventricular remodelling and diastolic dysfunction in individuals with type 2 diabetes [35]. Prior cross-sectional studies using echocardiography have highlighted various factors contributing to left ventricular hypertrophy and diastolic dysfunction in individuals with type 2 diabetes, including oxidative stress [36], autonomic dysfunction [37], microvascular disease [38], obesity [39] and poor glycaemic control [40]. Prospective echocardiography studies have also shown that longitudinal changes in left ventricular remodelling and myocardial dysfunction in individuals with type 2 diabetes are associated with factors such as retinopathy [41], B-type natriuretic peptide (BNP) [42], obesity [43] and female sex [44].
Our baseline assessments revealed that non-HFpEF participants with elevated ITGA1 levels exhibited marked left ventricular diastolic dysfunction, while in participants with HFpEF, higher ITGA1 levels were associated with increased left ventricular mass and deteriorations in both systolic and diastolic functions. This suggests that elevated ITGA1 levels could serve as an early indicator of cardiac dysfunction in individuals with type 2 diabetes without HFpEF, despite absence of overt heart failure symptoms. In contrast, in individuals with type 2 diabetes who display HFpEF, high ITGA1 levels indicate a more severe cardiac condition, encompassing both systolic and diastolic impairments, which may lead to a greater risk of adverse cardiovascular events and necessitate more intensive management. Additionally, our longitudinal analysis highlighted a significant correlation between baseline ITGA1 levels and the progression of cardiac dysfunction in individuals with type 2 diabetes. This progression, observed over the follow-up period, manifested as a decline in both diastolic and systolic functions. Furthermore, we observed a notable link between higher ITGA1 levels at baseline and an increased likelihood of re-hospitalisation during follow-up. This finding emphasises the strong relationship between ITGA1 levels and the risk of adverse clinical outcomes in these individuals.
While BNP and NT-proBNP are commonly used biomarkers for HFpEF, they primarily indicate significant cardiac damage or functional decline, reflecting notable pathological processes in the heart [45, 46]. In contrast, ITGA1 shows promise in assessing early-stage cardiac diastolic dysfunction. Our study revealed that even in individuals without HFpEF and with normal NT-proBNP levels, elevated levels of ITGA1 were consistently associated with reduced cardiac diastolic function compared with lower ITGA1 levels. These findings underscore the potential of ITGA1 as a marker for detecting early cardiac diastolic dysfunction and providing valuable insights into subclinical pathological changes that may precede the development of overt cardiac damage or functional impairment.
However, the underlying pathogenic mechanism linking elevated circulating levels of ITGA1 to adverse left ventricular remodelling and functional impairment in individuals with type 2 diabetes remains unclear. To investigate this, we utilised proteomic data from the left ventricle of individuals with type 2 diabetes with or without HFpEF. Intriguingly, we found that the proteins displaying strong associations with ITGA1 were significantly enriched in pathways related to myocardial fibrosis. Myocardial fibrosis plays a critical role in the development of HFpEF by contributing to cardiac dysfunction [47]. This process involves the excessive deposition of collagen and other extracellular matrix components in the myocardium, leading to increased myocardial stiffness and impaired relaxation during diastole. Furthermore, such fibrotic remodelling disrupts the normal architecture of the myocardium, compromising coordinated contraction and further exacerbating cardiac dysfunction [48]. Thus, our findings provide valuable insights into the molecular mechanisms by which ITGA1 influences myocardial fibrosis and contributes to the pathogenesis of HFpEF in individuals with type 2 diabetes.
Clinical implications
The study findings have important clinical implications for people with type 2 diabetes. ITGA1 may serve as a valuable biomarker for monitoring cardiac damage, diagnosing HFpEF accurately, predicting further deterioration in cardiac structure and function, and identifying individuals at higher risk of re-hospitalisation. Integrating ITGA1 into routine diagnostic protocols could improve the identification and management of HFpEF in individuals with type 2 diabetes. Additionally, ITGA1’s role in the pathophysiology of HFpEF highlights potential avenues for targeted therapeutic interventions. Modulating the expression or function of ITGA1, through the development of new drugs or repurposing existing medications, may help prevent or mitigate the onset of HFpEF in individuals with type 2 diabetes. These targeted interventions could potentially slow disease progression, enhance cardiac function and improve overall clinical outcomes.
Limitations
Our study had limitations, including a small sample size and a single-centre design. These may have affected the statistical power and introduced biases. Larger studies involving multiple centres are needed to confirm and extend our findings. Additionally, depending solely on proteomic data and bioinformatics analysis to demonstrate the impact of ITGA1 on HFpEF through influence on fibrotic pathways may introduce potential inaccuracies. Incorporating other approaches such as animal models or in vitro studies would provide a more comprehensive understanding of the underlying mechanisms. Future research should address these limitations to enhance the diagnostic and predictive value of ITGA1 for HFpEF.
Conclusion
Our study provides evidence supporting the involvement of ITGA1 in HFpEF among individuals with type 2 diabetes. We found that ITGA1 levels were significantly elevated in individuals with type 2 diabetes with HFpEF. Individuals with elevated ITGA1 levels exhibited left ventricular remodelling, impaired diastolic function and a faster decline in cardiac function, correlating with an increased risk of re-hospitalisation during the follow-up period. Further investigations using proteomic data from the left ventricle revealed that increased circulating levels of ITGA1 contribute to the development and progression of HFpEF by influencing fibrosis-related pathways in the heart. Based on these findings, ITGA1 holds potential as a prognostic marker for identifying high-risk individuals with type 2 diabetes who are prone to cardiovascular complications. Its measurement could facilitate risk stratification and early intervention to mitigate the adverse effects of HFpEF.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- A
Transmitral late diastolic peak velocity
- BNP
B-type natriuretic peptide
- E
Transmitral early diastolic peak velocity
- e′
Early diastolic peak velocity of mitral valve at septal or lateral annulus
- HFpEF
Heart failure with preserved ejection fraction
- ITGA1
Integrin α1
- IVSD
Interventricular septal dimension at end-diastole
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- LVDD
Left ventricular end diastolic dimension
- LVDS
Left ventricular end systolic dimension
- LVEF
Left ventricular ejection fraction
- LVPWD
Left ventricular posterior wall dimension
- NT-proBNP
N-terminal pro-B-type natriuretic peptide
- ROC
Receiver operating characteristic
Acknowledgements
We extend our gratitude to Q. Lai, Assistant Researcher in the Department of Cardiology at the University of Hong Kong-Shenzhen Hospital, for her diligent contribution in participant data entry. We also thank H. Sun from the Department of Microbiology, Li Ka Shing Faculty of Medicine, The University of Hong Kong for his valuable insights into experimental design and manuscript preparation.
Data availability
The proteomic data of left ventricular myocardial tissue from individuals with type 2 diabetes, encompassing both those with and without HFpEF, is available from the ProteomeXchange database at http://proteomecentral.proteomexchange.org. For individual participant data related to this study, availability will commence 12 to 36 months after publication. Researchers interested in these data are invited to submit their research proposals to the corresponding author, Cong Chen, at chenc6@hku-szh.org.
Funding
This project was supported by grants from National Nature Scientific Foundation of China (no. 82270400, no. 82300457), Guangdong Province Nature Scientific Foundation (no. 2022A1515110881, no. 2023A1515010731), Shenzhen City Nature Scientific Foundation (no. YCYJ20220530142410023) and Shenzhen City Technology Innovation Talents Development Program (no. RCBS20221008093335085, no. RCBS20221008093102010).
Authors’ relationships and activities
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Contribution statement
MQS, CC and KHY were responsible for designing the study and writing the initial draft of the manuscript. RW, MW and MYL played a key role in recruiting participants. SDC, WPL and YXW carried out the experiments and collected data. YLH and KLEY were in charge of performing the statistical analyses. JLC supervised the laboratory analyses. All authors actively participated in discussing the results and implications, and provided comments at all stages of the manuscript development. They all approved the final version of the manuscript. CC and KHY, as the guarantors of the work, had full access to all data in the study and take responsibility for the integrity and accuracy of the data analysis.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kaihang Yiu, Email: khkyiu@hku.hk.
Cong Chen, Email: chenc6@hku-szh.org.
References
- 1.Redfield MM, Borlaug BA. Heart failure with preserved ejection fraction: a review. JAMA. 2023;329(10):827–838. doi: 10.1001/jama.2023.2020. [DOI] [PubMed] [Google Scholar]
- 2.Borlaug BA. Evaluation and management of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2020;17(9):559–573. doi: 10.1038/s41569-020-0363-2. [DOI] [PubMed] [Google Scholar]
- 3.McHugh K, DeVore AD, Wu J, et al. Heart failure with preserved ejection fraction and diabetes: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(5):602–611. doi: 10.1016/j.jacc.2018.11.033. [DOI] [PubMed] [Google Scholar]
- 4.Echouffo-Tcheugui JB, Xu H, DeVore AD, et al. Temporal trends and factors associated with diabetes mellitus among patients hospitalized with heart failure: findings from Get With The Guidelines-Heart Failure registry. Am Heart J. 2016;182:9–20. doi: 10.1016/j.ahj.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 5.O'Keefe JH, Nassif ME, Magwire ML, O'Keefe EL, Lavie CJ. The elephant in the room: why cardiologists should stop ignoring type 2 diabetes. Prog Cardiovasc Dis. 2019;62(4):364–369. doi: 10.1016/j.pcad.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Sanders-van Wijk S, Tromp J, Beussink-Nelson L, et al. Proteomic evaluation of the comorbidity-inflammation paradigm in heart failure with preserved ejection fraction: results from the PROMIS-HFpEF Study. Circulation. 2020;142(21):2029–2044. doi: 10.1161/CIRCULATIONAHA.120.045810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kresoja KP, Rommel KP, Wachter R, et al. Proteomics to improve phenotyping in obese patients with heart failure with preserved ejection fraction. Eur J Heart Fail. 2021;23(10):1633–1644. doi: 10.1002/ejhf.2291. [DOI] [PubMed] [Google Scholar]
- 8.Michaëlsson E, Lund LH, Hage C, et al. Myeloperoxidase inhibition reverses biomarker profiles associated with clinical outcomes in HFpEF. JACC Heart Fail. 2023;11(7):775–787. doi: 10.1016/j.jchf.2023.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Laakso M. Biomarkers for type 2 diabetes. Mol Metab. 2019;27S:S139–S146. doi: 10.1016/j.molmet.2019.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moreno-Layseca P, Icha J, Hamidi H, Ivaska J. Integrin trafficking in cells and tissues. Nat Cell Biol. 2019;21(2):122–132. doi: 10.1038/s41556-018-0223-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li R, Frangogiannis NG. Integrins in cardiac fibrosis. J Mol Cell Cardiol. 2022;172:1–13. doi: 10.1016/j.yjmcc.2022.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Siekacz K, Kumor-Kisielewska A, Miłkowska-Dymanowska J, et al. Soluble ITGaM and ITGb2 integrin subunits are involved in long-term pulmonary complications after COVID-19 infection. J Clin Med. 2023;12(1):342. doi: 10.3390/jcm12010342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bengs S, Becker E, Busenhart P, et al. β(6) -integrin serves as a novel serum tumor marker for colorectal carcinoma. Int J Cancer. 2019;145(3):678–685. doi: 10.1002/ijc.32137. [DOI] [PubMed] [Google Scholar]
- 14.Yang F, Song Y, Song H, Zheng W, Wang L. The high expression of adhering and circulating integrin serves as a diagnostic marker in venous thromboembolism. Comput Math Methods Med. 2022;2022:9610071. doi: 10.1155/2022/9610071. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Chen C, Li R, Ross RS, Manso AM. Integrins and integrin-related proteins in cardiac fibrosis. J Mol Cell Cardiol. 2016;93:162–174. doi: 10.1016/j.yjmcc.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puzzi L, Borin D, Gurha P, et al. Knock down of plakophillin 2 dysregulates adhesion pathway through upregulation of miR200b and alters the mechanical properties in cardiac cells. Cells. 2019;8(12):1639. doi: 10.3390/cells8121639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pieske B, Tschöpe C, de Boer RA, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC) Eur Heart J. 2019;40(40):3297–3317. doi: 10.1093/eurheartj/ehz641. [DOI] [PubMed] [Google Scholar]
- 18.ElSayed NA, Aleppo G, Aroda VR, et al. 4. Comprehensive medical evaluation and assessment of comorbidities: standards of care in Diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S49–S67. doi: 10.2337/dc23-S004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu JH, Chen Y, Zhen Z, et al. Relationship of biomarkers of extracellular matrix with myocardial function in type 2 diabetes mellitus. Biomark Med. 2017;11(7):569–578. doi: 10.2217/bmm-2017-0044. [DOI] [PubMed] [Google Scholar]
- 20.MacCoss MJ, Wu CC, Yates JR., 3rd Probability-based validation of protein identifications using a modified SEQUEST algorithm. Anal Chem. 2002;74:5593–5599. doi: 10.1021/ac025826t. [DOI] [PubMed] [Google Scholar]
- 21.Wu D, Zhang S, Xie Z, et al. Plasminogen as a prognostic biomarker for HBV-related acute-on-chronic liver failure. J Clin Invest. 2020;130(4):2069–2080. doi: 10.1172/JCI130197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu MZ, Lee CH, Chen Y, et al. Association between adipocyte fatty acid-binding protein with left ventricular remodelling and diastolic function in type 2 diabetes: a prospective echocardiography study. Cardiovasc Diabetol. 2020;19(1):197. doi: 10.1186/s12933-020-01167-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng J, Haberland V, Baird D, et al. Phenome-wide Mendelian randomization mapping the influence of the plasma proteome on complex diseases. Nat Genet. 2020;52(10):1122–1131. doi: 10.1038/s41588-020-0682-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyngbakken MN, Myhre PL, Røsjø H, Omland T. Novel biomarkers of cardiovascular disease: applications in clinical practice. Crit Rev Clin Lab Sci. 2019;56(1):33–60. doi: 10.1080/10408363.2018.1525335. [DOI] [PubMed] [Google Scholar]
- 25.Gamrat A, Surdacki MA, Chyrchel B, Surdacki A. Endothelial dysfunction: a contributor to adverse cardiovascular remodeling and heart failure development in type 2 diabetes beyond accelerated atherogenesis. J Clin Med. 2020;9(7):2090. doi: 10.3390/jcm9072090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meagher PB, Lee XA, Lee J, Visram A, Friedberg MK, Connelly KA. Cardiac fibrosis: key role of integrins in cardiac homeostasis and remodeling. Cells. 2021;10(4):770. doi: 10.3390/cells10040770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lozano J, Rai A, Lees JG, et al. Scalable generation of nanovesicles from human-induced pluripotent stem cells for cardiac repair. Int J Mol Sci. 2022;23(22):14334. doi: 10.3390/ijms232214334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Talior-Volodarsky I, Connelly KA, Arora PD, Gullberg D, McCulloch CA. α11 integrin stimulates myofibroblast differentiation in diabetic cardiomyopathy. Cardiovasc Res. 2012;96(2):265–275. doi: 10.1093/cvr/cvs259. [DOI] [PubMed] [Google Scholar]
- 29.Chen M, Hu R, Cavinato C, et al. Fibronectin-integrin α5 signaling in vascular complications of type 1 diabetes. Diabetes. 2022;71(9):2020–2033. doi: 10.2337/db21-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams AS, Kang L, Zheng J, et al. Integrin α1-null mice exhibit improved fatty liver when fed a high fat diet despite severe hepatic insulin resistance. J Biol Chem. 2015;290(10):6546–6557. doi: 10.1074/jbc.M114.615716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stephens PE, Ortlepp S, Perkins VC, Robinson MK, Kirby H. Expression of a soluble functional form of the integrin alpha4beta1 in mammalian cells. Cell Adhes Commun. 2000;7(5):377–390. doi: 10.3109/15419060009109020. [DOI] [PubMed] [Google Scholar]
- 32.Alon R, Feigelson SW. Chemokine-triggered leukocyte arrest: force-regulated bi-directional integrin activation in quantal adhesive contacts. Curr Opin Cell Biol. 2012;24(5):670–676. doi: 10.1016/j.ceb.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 33.Zhang DX, Dang X, Vu LT, et al. αvβ1 integrin is enriched in extracellular vesicles of metastatic breast cancer cells: a mechanism mediated by galectin-3. J Extracell Vesicles. 2022;11(8):e12234. doi: 10.1002/jev2.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li H, Wang Y, Rong SK, et al. Integrin α1 promotes tumorigenicity and progressive capacity of colorectal cancer. Int J Biol Sci. 2020;16(5):815–826. doi: 10.7150/ijbs.37275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marwick TH, Ritchie R, Shaw JE, Kaye D. Implications of underlying mechanisms for the recognition and management of diabetic cardiomyopathy. J Am Coll Cardiol. 2018;71(3):339–351. doi: 10.1016/j.jacc.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 36.Zhao CT, Wang M, Siu CW, et al. Myocardial dysfunction in patients with type 2 diabetes mellitus: role of endothelial progenitor cells and oxidative stress. Cardiovasc Diabetol. 2012;11:147. doi: 10.1186/1475-2840-11-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sacre JW, Franjic B, Jellis CL, Jenkins C, Coombes JS, Marwick TH. Association of cardiac autonomic neuropathy with subclinical myocardial dysfunction in type 2 diabetes. JACC Cardiovasc Imaging. 2010;3(12):1207–1215. doi: 10.1016/j.jcmg.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 38.Moir S, Hanekom L, Fang ZY, et al. Relationship between myocardial perfusion and dysfunction in diabetic cardiomyopathy: a study of quantitative contrast echocardiography and strain rate imaging. Heart. 2006;92(10):1414–1419. doi: 10.1136/hrt.2005.079350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Jong KA, Czeczor JK, Sithara S, et al. Obesity and type 2 diabetes have additive effects on left ventricular remodelling in normotensive patients-a cross sectional study. Cardiovasc Diabetol. 2017;16(1):21. doi: 10.1186/s12933-017-0504-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zoppini G, Bergamini C, Bonapace S, et al. Association between subclinical left ventricular systolic dysfunction and glycemic control in asymptomatic type 2 diabetic patients with preserved left ventricular function. J Diabetes Complications. 2017;31(6):1035–1040. doi: 10.1016/j.jdiacomp.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 41.Walraven I, van den Hurk K, van 't Riet E, et al. Low-grade inflammation and endothelial dysfunction explain the association between retinopathy and left ventricular ejection fraction in men: an 8-year follow-up of the Hoorn Study. J Diabetes Complicat. 2014;28(6):819–823. doi: 10.1016/j.jdiacomp.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 42.Kroon MH, van den Hurk K, Alssema M, et al. Prospective associations of B-type natriuretic peptide with markers of left ventricular function in individuals with and without type 2 diabetes: an 8-year follow-up of the Hoorn Study. Diabetes Care. 2012;35(12):2510–2514. doi: 10.2337/dc11-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu MZ, Chen Y, Zou Y, et al. Impact of obesity on longitudinal changes to cardiac structure and function in patients with type 2 diabetes mellitus. Eur Heart J Cardiovasc Imaging. 2019;20(7):816–827. doi: 10.1093/ehjci/jey217. [DOI] [PubMed] [Google Scholar]
- 44.Wu MZ, Chen Y, Yu YJ, et al. Sex-specific pattern of left ventricular hypertrophy and diastolic function in patients with type 2 diabetes mellitus. Eur Heart J Cardiovasc Imaging. 2021;22(8):930–940. doi: 10.1093/ehjci/jeaa079. [DOI] [PubMed] [Google Scholar]
- 45.Meijers WC, Hoekstra T, Jaarsma T, van Veldhuisen DJ, de Boer RA. Patients with heart failure with preserved ejection fraction and low levels of natriuretic peptides. Neth Heart J. 2016;24(4):287–295. doi: 10.1007/s12471-016-0816-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anjan VY, Loftus TM, Burke MA, et al. Prevalence, clinical phenotype, and outcomes associated with normal B-type natriuretic peptide levels in heart failure with preserved ejection fraction. Am J Cardiol. 2012;110(6):870–876. doi: 10.1016/j.amjcard.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mishra S, Kass DA. Cellular and molecular pathobiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2021;18(6):400–423. doi: 10.1038/s41569-020-00480-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lam C, Voors AA, de Boer RA, Solomon SD, van Veldhuisen DJ. Heart failure with preserved ejection fraction: from mechanisms to therapies. Eur Heart J. 2018;39(30):2780–2792. doi: 10.1093/eurheartj/ehy301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The proteomic data of left ventricular myocardial tissue from individuals with type 2 diabetes, encompassing both those with and without HFpEF, is available from the ProteomeXchange database at http://proteomecentral.proteomexchange.org.
The proteomic data of left ventricular myocardial tissue from individuals with type 2 diabetes, encompassing both those with and without HFpEF, is available from the ProteomeXchange database at http://proteomecentral.proteomexchange.org. For individual participant data related to this study, availability will commence 12 to 36 months after publication. Researchers interested in these data are invited to submit their research proposals to the corresponding author, Cong Chen, at chenc6@hku-szh.org.