ABSTRACT
Microbial reduction of organic disulfides affects the macromolecular structure and chemical reactivity of natural organic matter. Currently, the enzymatic pathways that mediate disulfide bond reduction in soil and sedimentary organic matter are poorly understood. In this study, we examined the extracellular reduction of 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) by Shewanella oneidensis strain MR-1. A transposon mutagenesis screen performed with S. oneidensis resulted in the isolation of a mutant that lost ~90% of its DTNB reduction activity. Genome sequencing of the mutant strain revealed that the transposon was inserted into the dsbD gene, which encodes for an oxidoreductase involved in cytochrome c maturation. Complementation of the mutant strain with the wild-type dsbD partially restored DTNB reduction activity. Because DsbD catalyzes a critical step in the assembly of multi-heme c-type cytochromes, we further investigated the role of extracellular electron transfer cytochromes in organic disulfide reduction. The results indicated that mutants lacking proteins in the Mtr system were severely impaired in their ability to reduce DTNB. These findings provide new insights into extracellular organic disulfide reduction and the enzymatic pathways of organic sulfur redox cycling.
IMPORTANCE
Organic sulfur compounds in soils and sediments are held together by disulfide bonds. This study investigates how Shewanella oneidensis breaks apart extracellular organic sulfur compounds. The results show that an enzyme involved in the assembly of c-type cytochromes as well as proteins in the Mtr respiratory pathway is needed for S. oneidensis to transfer electrons from the cell surface to extracellular organic disulfides. These findings have important implications for understanding how organic sulfur decomposes in terrestrial ecosystems.
KEYWORDS: DTNB, TNB, outer membrane, organic sulfur, thiol, Shewanella, cysteine, cystine, glutathione, geomicrobiology
INTRODUCTION
Sulfur (S) redox cycling influences carbon respiration, nutrient availability, and contaminant sequestration in soils and sediments (1–3). Organic S is the primary form of sulfur in many terrestrial ecosystems (4, 5), and the oxidation of thiols in organic S produces disulfide bonds (R-S-S-R) which strongly affect the macromolecular structure and reactivity of natural organic matter (6, 7). The reduction of disulfide bonds in organic matter is a key environmental process that breaks up disulfide cross-linkages and liberates high-affinity sulfhydryl functional groups that are involved in trace nutrient binding and metal complexation (8, 9). Currently, the microbial processes that mediate disulfide bond reduction in soil and sedimentary organic matter are poorly understood.
Shewanella oneidensis strain MR-1 is a facultative anaerobe capable of catalyzing a diverse array of reduction reactions (10). This Gram-negative bacterium harbors a large number of multi-heme c-type cytochromes that facilitate the extracellular reduction of inorganic and organic substrates (11), including dissolved organic compounds such as flavins, quinones, and humic acids (12–14). Previous studies have shown that S. oneidensis can transfer electrons extracellularly to nitrogen atoms in riboflavin and riboflavin-5′-phosphate (15) and carbon atoms in the humic acid analog anthraquinone-2,6-disulfonate (16). Humic acids also contain disulfide bonds (7, 17), but very little is known about extracellular electron transfer to organic S in humic substances.
In this study, we conducted experiments to identify the genes involved in extracellular organic disulfide reduction by S. oneidensis. We employed transposon mutagenesis to isolate a S. oneidensis mutant impaired in organic disulfide reduction. The location of the transposon insertion was identified, and genetic complementation was carried out to restore the loss of disulfide reduction activity. S. oneidensis mutants that lacked outer-membrane cytochromes were also screened to further elucidate the mechanism of extracellular disulfide bond reduction. The results provide new insights into the enzymatic pathways that catalyze the breakup of organic disulfide bonds.
RESULTS AND DISCUSSION
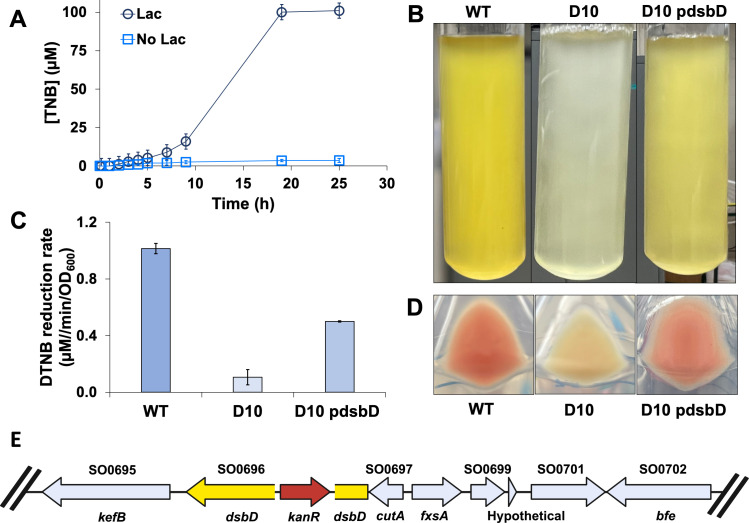
S. oneidensis reduced the organic disulfide 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) in an anaerobic M1 medium with lactate to form yellow-colored 2-nitro-5-thiobenzoate (TNB) (Fig. 1A). Complete DTNB reduction was observed in less than 20 hours of incubation. Cell suspensions in the M1 medium without lactate did not reduce DTNB. After screening 9,348 transposon mutants, a single mutant, designated as strain D10, was isolated, which was impaired in DTNB reduction. D10 did not produce a visible yellow-colored product (Fig. 1B) and lost approximately 90% of its DTNB reduction activity (Fig. 1C). D10 cells also lost the pink coloration characteristic of S. oneidensis (Fig. 1D). Genome sequencing of D10 revealed a transposon insertion within the dsbD gene (SO0696) (Fig. 1E), which encodes for an oxidoreductase involved in cytochrome c maturation (18). Complementation of the mutation using the wild-type (WT) dsbD sequence partially restored the abolished phenotype (Fig. 1C and D). The complemented mutant exhibited ~65% of the DTNB activity of MR-1, and pink coloration returned to the cells.
Fig 1.
DTNB reduction by the wild-type and mutant strains. (A) Reduction of DTNB to TNB by the wild-type strain in M1 medium with and without lactate. Each data point represents the average of three replicate experiments, and error bars indicate the standard deviation. (B) Overnight cultures grown in M1 lactate medium supplemented with 100 µM DTNB; wild-type (left), D10 mutant (middle), and complemented mutant D10 pdsbD (right). (C) Initial velocity of DTNB reduction for the wild-type, D10, and D10 pdsbD strains. (D) Cell pellets centrifuged; wild-type (left), D10 (middle), and D10 pdsbD (right). (E) Location of transposon insertion. The dsbD gene in yellow and the transposon in red.
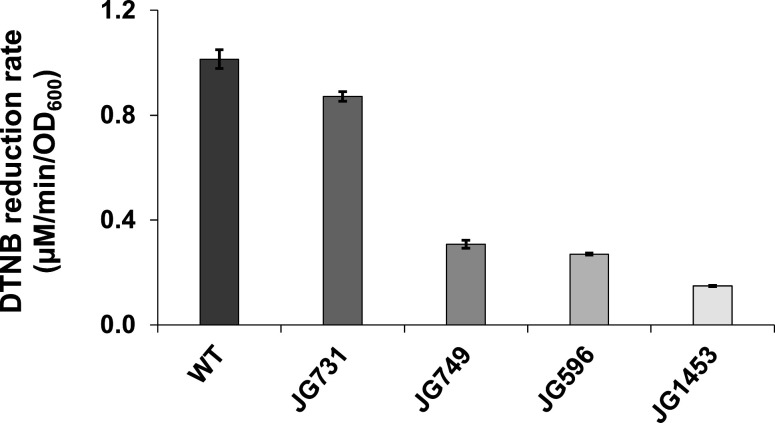
Because the dsbD gene is involved in cytochrome c maturation, we sought to investigate the role of the S. oneidensis Mtr system in extracellular disulfide bond reduction. Mutant strains JG731 (ΔmtrC), JG749 (ΔmtrC ΔomcA), JG596 (ΔmtrC ΔomcA ΔmtrF), and JG1453 (ΔmtrB ΔmtrE ΔmtrC ΔomcA ΔmtrF ΔmtrA ΔmtrD ΔdmsE ΔSO4360 ΔcctA) were tested for DTNB reduction activity. Mutants lacking the outer-membrane decaheme c-type cytochrome MtrC reduced DTNB at approximately 90% of the wild-type strain. Mutants lacking both the decaheme outer-membrane c-type cytochromes MtrC and OmcA lost more than half of their DTNB reduction activity. Additional deletion of the decaheme outer-membrane cytochrome MtrF had little effect on DTNB reduction. Mutant strain JG1453, which harbors two outer-membrane beta barrel deletions (MtrB and MtrF) and eight cytochrome deletions, reduced DTNB at less than 15% of the rate of MR-1 (Fig. 2).
Fig 2.
DTNB reduction by Mtr mutant strains JG731 (ΔmtrC), JG749 (ΔmtrC/ΔomcA), JG596 (ΔmtrC/ΔomcA/ΔmtrF), and JG1453 (ΔmtrB/ΔmtrE/ΔmtrC/ΔomcA/ΔmtrF/ΔmtrA/ΔmtrD/ΔdmsE/ΔSO4360/ΔcctA). Experiments were performed in triplicate, and error bars represent the standard deviation. Statistically significant differences were observed between the WT and all mutant strains (Student’s t-test, P < 0.01).
Enzymatic pathway of DTNB reduction
The results indicate that the mutation of the dsbD gene in S. oneidensis impairs DTNB reduction. DsbD is an inner-membrane protein that plays a critical role in the assembly of multi-heme c-type cytochromes in the periplasm. When apocytochromes are secreted into the periplasm, the cysteine residues associated with the heme-binding motifs (CxxC) are immediately oxidized. For heme ligation to occur, DsbD must convert the oxidized cysteines back to the reduced state (19). This enzymatic step is carried out by DsbD transferring electrons from the cytoplasmic thioredoxin system across the inner membrane to periplasmic apocytochromes through thiol-disulfide exchange reactions (20). Loss of DsbD disables this pivotal step in cytochrome maturation resulting in the inability of S. oneidensis to respire certain substrates anaerobically. Previous studies have shown that dsbD mutants are unable to respire cobalt (21) or transfer electrons extracellularly to electrodes (22). Beyond these phenotypes, our results demonstrate that a functional DsbD enzyme in S. oneidensis is also needed for the extracellular reduction of DTNB.
We observed that dsbD mutant cells lacked the distinctive pink coloration exhibited by the wild-type strain (Fig. 1D), which suggested that the absence of red-colored cytochromes was related to the loss of DTNB reduction activity (23, 24). The data presented in Fig. 2 show that mutants with a functional DsbD oxidoreductase but deletions in the Mtr system are greatly subdued in DTNB reduction. Anaerobic cultures of the wild-type strain incubated with lactate reduced DTNB (Fig. 1A) indicating that electrons are transferred from the electron transport chain via Mtr proteins to break apart extracellular organic disulfides. The Mtr system receives electrons from periplasmic electron carriers that regulate extracellular electron transfer efficiency (25). We observed progressive loss of DTNB reduction activity with successive cytochrome gene deletions, similar to other substrates that are dependent on the Mtr pathway (26–28).
Together, these data suggest that DTNB reduction occurs on the cell surface, most likely via direct electron transfer reactions with heme groups in outer-membrane cytochromes. While almost all of the DTNB reduction activity can be attributed to electron transfer reactions with Mtr proteins, we noted that DTNB reduction was not completely abolished in the Mtr mutant strains (Fig. 2). The low levels of residual activity in the mutants indicate that mechanisms other than the Mtr pathway might be involved in DTNB reduction. Besides Mtr cytochromes, the genome of S. oneidensis contains genes for additional outer-membrane multi-heme proteins (e.g., SO1659 and SO2931) that could potentially reduce DTNB. Furthermore, because of its low molecular weight, DTNB could also pass through outer-membrane porins and interact with periplasmic cytochromes. In S. oneidensis, the periplasmic octaheme cytochromes SirA and OTR catalyze the reduction of inorganic sulfur compounds sulfite and tetrathionate, respectively (29, 30). Whether or not periplasmic cytochromes can also reduce organic sulfur compounds is currently unknown. Cytoplasmic DTNB reduction is less plausible. Shewanella species employ dedicated transporters to import naturally occurring sulfur compounds across the inner membrane, and it is unlikely that synthetic DTNB is transported into the cytoplasm for intracellular reduction.
Environmental implications
Our results suggest that organic disulfides in soils and sediments can be biologically reduced by the Mtr system. Recent phylogenomic studies have shown that mtr genes are widespread in the Shewanella genus (10) and the Bacteria domain (31). If the Mtr system in these microorganisms can transfer electrons to disulfide bonds in naturally occurring organic matter, then our proposed enzymatic pathway has significant implications for organic sulfur redox cycling. Humic acids that participate in extracellular electron transfer reactions (12) are known to be heterogeneously cross-linked with disulfide bonds (17). The reduction of these disulfide bonds by the Mtr system would break apart humic acids, thus affecting the macromolecular structure and chemical reactivity. The extent to which this occurs in the environment would largely be dependent on the redox potential of the humic acid disulfide bonds. DTNB has a highly oxidized disulfide bond that is likely much more reactive than many naturally occurring organic disulfides. In complex molecules such as proteins, the redox potentials that have been measured for disulfide bonds range from −95 to −470 mV (32). These variations in redox potential are attributed to several factors, including sulfhydryl group pKa, entropy, and bond-strain energy. Similarly, humic acids are expected to harbor a large range of disulfide redox potentials, with the highest redox potential disulfide bonds being the most thermodynamically favorable for enzymatic reduction by the Mtr pathway.
Finally, the reduction of organic disulfides to thiols by S. oneidensis might also play a role in extracellular electron shuttling to Fe(III) minerals (33). Extracellular thiols can chemically reduce Fe(III) minerals such as ferrihydrite and smectite clays (34–37). Cysteine/cystine redox recycling by S. oneidensis has been shown to facilitate the reduction of ferruginous smectites (38). Cysteine-mediated Fe(III) reduction is thought to be driven by the production of reactive thiols by microbial cystine reduction, whereby extracellular cysteine acts as a conduit for electron transfer to mineral surfaces. The enzymatic reduction of naturally occurring disulfides and the coupled biotic–abiotic thiol/disulfide electron shuttling to Fe(III) minerals in soils and sediments merit further investigation.
MATERIALS AND METHODS
Bacterial strains and growth conditions
All strains and plasmids used in this study are listed in Table 1. Mutant strains containing gene deletions in the Mtr system were previously constructed by Coursolle and Gralnick (39). S. oneidensis MR-1 and its derivatives were maintained on a Luria–Bertani (LB) medium with the appropriate antibiotics. When required, the antibiotic concentrations were kanamycin at 50 µg/mL and chloramphenicol at 20 µg/mL. Experiments were conducted with cultures in an M1 lactate medium adapted from Myers and Nealson (40). This medium contained (per liter) 1.19 g (NH4)2SO4, 0.99 g K2HPO4, 0.45 g KH2PO4, 0.25 g MgSO4·7H2O, 0.07 g CaCl2·2H2O, 0.17 g NaHCO3, 10 mL of a trace metal solution (100×), 1 mL of a sodium selenite solution (6 mM), 14.8 mL of a sodium lactate solution (30% vol/vol), and 20 mL of an amino acid solution. The trace metal solution (100×) contained (per liter) 2.5 g Na2EDTA, 0.35 g H3BO3, 0.06 g NaCl, 0.15 g FeSO4·7 H2O, 0.12 g CoCl2·6 H2O, 0.13 g NiSO4·6 H2O, 0.08 g Na2MoO4, 0.02 g MnSO4·7H2O, 0.03 g ZnSO4·7 H2O, and 0.005 g CuSO4·7H2O. The amino acid solution contained (per 100 mL) 0.2 g each of serine, arginine, and glutamate.
TABLE 1.
Strains, plasmids, and primers
| Strains, plasmids, or primers | Characteristics | References | |
|---|---|---|---|
| S. oneidensis strains | |||
| JG274 | Shewanella oneidensis MR1; wild type | (40) | |
| D10 | Tn insertion in dsbD; kmR; dsbD::[oriR6K KmR] (SO0696); KmR | This study | |
| D10 pdsbD | D10 with pdsbD; KmR; CmR | This study | |
| JG596 | ΔmtrC/ΔomcA/ΔmtrF | (28) | |
| JG731 | ΔmtrC | (28) | |
| JG749 | ΔmtrC/ΔomcA | (28) | |
| JG1453 | ΔmtrB/ΔmtrE/ΔmtrC/ΔomcA/ΔmtrF/ΔmtrA/ΔmtrD/ΔdmsE/ΔSO4360/ΔcctA | (39) | |
| Escherichia coli strains | |||
| UQ950 | E. coli host for cloning | (41) | |
| UQ950 pdsbD | E. coli cloning host with pdsbD, CmR | This study | |
| WM3064 | Dap auxotroph; donor strain for conjugation | (41) | |
| WM3064 pdsbD | E. coli donor strain with pdsbD, CmR | This study | |
| Plasmids | |||
| pBBr-MCS1 | 4.7 kb broad-host range cloning vector plasmid: CmR, lacZ; 4,707 bp | (42) | |
| pdsbD | pBBr-MCS1 with dsbD and 21 bp upstream and 19 bp downstream; 6,580 bp (1873 dsbD PCR product) | This study | |
| Primers | |||
| dsbD-F | 5′-gtgagcgcgcgtaatacgactattggcgattaaactgcg; 19 bp downstream dsbD and includes 21 bp of T7 promoter pBBr-MCS1 | This study | |
| dsbD-R | 5′-ctccaattcgccctatagtggataaaagataacactcagc; 21 bp upstream dsbD start codon and includes 20 bp of T7 promoter pBBr-MCS1 | This study | |
| pBBr-MCS1-F | 5′-agtcgtattacgcgcgctcac; inside T7 promoter | This study | |
| pBBr-MCS1-R | 5′-cactatagggcgaattggag; inside T7 promoter | This study | |
DTNB reduction experiment
Organic disulfide reduction experiments were performed using the cell-impermeable compound DTNB, which is a synthetic colorogenic chemical also known as Ellman’s reagent (43). The reduction of the disulfide bond in DTNB produces TNB, which is a yellow-colored product that can be quantified spectrophotometrically. DTNB reduction experiments were carried out with S. oneidensis MR-1 and its derivatives grown in either LB broth or M1 medium supplemented with appropriate antibiotics. After cultures were incubated aerobically at 30°C for 20 hours, the cells were harvested by centrifugation at 8,000 × g for 10 minutes, washed once with 0.1 M NaCl, and resuspended with deoxygenated M1 lactate medium in N2-purged serum bottles sealed with rubber stoppers. DTNB was then added to the cell suspensions (OD600 = 0.3) at a final concentration of 100 µM and incubated anaerobically at 30°C. The cell suspensions were periodically sampled to monitor cell densities (OD600) and the formation of TNB. The concentrations of TNB were determined by centrifuging the samples and analyzing the supernatants spectrophotometrically at 412 nm (44). TNB calibration curves were constructed by reacting DTNB with known amounts of L-cysteine. All DTNB reduction experiments were conducted in triplicate with results reported as the mean and standard deviation of the triplicate experiments. Control experiments were performed with an M1 medium without lactate. Student’s t-test was used to determine if differences between the wild-type and mutant strains were statistically significant.
Transposon mutagenesis
S. oneidensis transposon mutants were generated previously by Brutinel and Gralnick (45). The transposon mutant library was cultured in LB broth with 50 µg/mL kanamycin, then plated on M1 lactate agar. Single colonies were picked and transferred to 96-well plates containing 250 µL of M1 lactate medium with 100 µM DTNB. Plates were then sealed in a plastic bag and incubated at 30°C. After 24 hours, cell densities and TNB concentrations were measured using a BioTek microplate reader at 600 and 412 nm, respectively. Nine thousand three hundred forty-eight transposon mutants were screened. Although genome coverage was not fully saturated, mutants that produced less than 70% of the TNB compared to the wild-type strain were isolated. These mutants were selected for further investigation.
After isolating a Tn mutant deficient in DTNB reduction, genomic DNA from the mutant strain was isolated using a Wizard HMW DNA extraction kit, and whole genome shotgun sequencing was performed by SeqCenter (Pittsburg, PA). Illumina reads were quality-checked with fastQC (46). Reads were assembled into contigs with SPAdes (47). Contigs were annotated using RAST with transposon components oriT and kanR genes used to locate the transposable element (48).
Complementation
The wild-type dsbD gene was PCR-amplified with primer set dsbD-F and dsbD-R (Table 1). The PCR product was cloned into a linearized pBBr-MCS1 plasmid using the Gibson assembly method and replicated in E. coli UQ950. The plasmid, designated as pdsbD, was then isolated and transferred into E. coli WM3064. Finally, the plasmid was transferred from E. coli WM3064 to the S. oneidensis dsbD mutant by conjugal mating, and transconjugants were selected using the appropriate antibiotics. The complemented mutant was designated as D10 pdsbD.
ACKNOWLEDGMENTS
We thank J.L. Goff for the helpful discussions on the DTNB transposon mutant screen and members of the Gralnick lab for their laboratory assistance.
This manuscript was greatly improved by the insightful comments provided by three anonymous reviewers.
Contributor Information
Jeffrey A. Gralnick, Email: gralnick@umn.edu.
Nathan Yee, Email: nyee@envsci.rutgers.edu.
Angela Re, Politecnico di Torino, Torino, Piemonte, Italy.
REFERENCES
- 1. Holmer M, Storkholm P. 2001. Sulphate reduction and sulphur cycling in lake sediments: a review. Freshw Biol 46:431–451. doi: 10.1046/j.1365-2427.2001.00687.x [DOI] [Google Scholar]
- 2. Santana MM, Dias T, Gonzalez JM, Cruz C. 2021. Transformation of organic and inorganic sulfur– adding perspectives to new players in soil and rhizosphere. Soil Biol Biochem 160:108306. doi: 10.1016/j.soilbio.2021.108306 [DOI] [Google Scholar]
- 3. Wang J, Dai J, Chen G, Jiang F. 2022. Role of sulfur biogeochemical cycle in mercury methylation in estuarine sediments: a review. J Hazard Mater 423:126964. doi: 10.1016/j.jhazmat.2021.126964 [DOI] [PubMed] [Google Scholar]
- 4. Ghani A, McLarren RG, Swift RS. 1993. Mobilization of recently-formed soil organic sulphur. Soil Biol Biochem 25:1739–1744. doi: 10.1016/0038-0717(93)90178-E [DOI] [Google Scholar]
- 5. Solomon D, Lehmann J, Tekalign M, Fritzsche F, Zech W. 2001. Sulfur fractions in particle-size separates of the sub-humid Ethiopian highlands as influenced by land use changes. Geoderma 102:41–59. doi: 10.1016/S0016-7061(00)00103-8 [DOI] [Google Scholar]
- 6. Vairavamurthy MA, Manowitz B, Maletic D, Wolfe H. 1997. Interactions of thiols with sedimentary particulate phase: studies of 3-mercaptopropionate in salt marsh sediments from Shelter Island, New York. Org Geochem 26:577–585. doi: 10.1016/S0146-6380(97)00019-3 [DOI] [Google Scholar]
- 7. Prietzel J, Thieme J, Salome M, Knicker H. 2007. Sulfur K-edge XANES spectroscopy reveals differences in sulfur speciation of bulk soils, humic acid, fulvic acid, and particle size separates. Soil Biol Biochem 39:877–890. doi: 10.1016/j.soilbio.2006.10.007 [DOI] [Google Scholar]
- 8. Hesterberg D, Chou JW, Hutchison KJ, Sayers DE. 2001. Bonding of Hg(II) to reduced organic sulfur in humic acid as affected by S/Hg ratio. Environ Sci Technol 35:2741–2745. doi: 10.1021/es001960o [DOI] [PubMed] [Google Scholar]
- 9. Martínez CE, McBride MB, Kandianis MT, Duxbury JM, Yoon S-J, Bleam WF. 2002. Zinc−sulfur and cadmium−sulfur association in metalliferous peats: evidence from spectroscopy, distribution coefficients, and phytoavailability. Environ Sci Technol 36:3683–3689. doi: 10.1021/es011333e [DOI] [PubMed] [Google Scholar]
- 10. Fredrickson JK, Romine MF, Beliaev AS, Auchtung JM, Driscoll ME, Gardner TS, Nealson KH, Osterman AL, Pinchuk G, Reed JL, Rodionov DA, Rodrigues JLM, Saffarini DA, Serres MH, Spormann AM, Zhulin IB, Tiedje JM. 2008. Towards environmental systems biology of Shewanella. Nat Rev Microbiol 6:592–603. doi: 10.1038/nrmicro1947 [DOI] [PubMed] [Google Scholar]
- 11. Hau HH, Gralnick JA. 2007. Ecology and biotechnology of the genus Shewanella. Annu Rev Microbiol 61:237–258. doi: 10.1146/annurev.micro.61.080706.093257 [DOI] [PubMed] [Google Scholar]
- 12. Hernandez ME, Newman DK. 2001. Extracellular electron transfer. Cell Mol Life Sci 58:1562–1571. doi: 10.1007/PL00000796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gralnick JA, Newman DK. 2007. Extracellular respiration. Mol Microbiol 65:1–11. doi: 10.1111/j.1365-2958.2007.05778.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Breuer M, Rosso KM, Blumberger J, Butt JN. 2015. Multi-haem cytochromes in Shewanella oneidensis MR-1: structures, functions and opportunities. J R Soc Interface 12:20141117. doi: 10.1098/rsif.2014.1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marsili E, Baron DB, Shikhare ID, Coursolle D, Gralnick JA, Bond DR. 2008. Shewanella secretes flavins that mediate extracellular electron transfer. Proc Natl Acad Sci U S A 105:3968–3973. doi: 10.1073/pnas.0710525105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Newman DK, Kolter R. 2000. A role for excreted quinones in extracellular electron transfer. Nature 405:94–97. doi: 10.1038/35011098 [DOI] [PubMed] [Google Scholar]
- 17. Vairavamurthy MA, Maletic D, Wang S, Manowitz B, Eglinton T, Lyons T. 1997. Characterization of sulfur-containing functional groups in sedimentary humic substances by X-ray absorption near-edge structure spectroscopy. Energy Fuels 11:546–553. doi: 10.1021/ef960212a [DOI] [Google Scholar]
- 18. Sanders C, Turkarslan S, Lee D-W, Daldal F. 2010. Cytochrome c biogenesis: the Ccm system. Trends Microbiol. 18:266–274. doi: 10.1016/j.tim.2010.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ito K, Inaba K. 2008. The disulfide bond formation (Dsb) system. Curr Opin Struct Biol 18:450–458. doi: 10.1016/j.sbi.2008.02.002 [DOI] [PubMed] [Google Scholar]
- 20. Feng X, Sun W, Kong L, Gao H. 2019. Distinct roles of Shewanella oneidensis thioredoxin in regulation of cellular responses to hydrogen and organic peroxides. Appl Environ Microbiol 85:e01700-19. doi: 10.1128/AEM.01700-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hau HH, Gilbert A, Coursolle D, Gralnick JA. 2008. Mechanism and consequences of anaerobic respiration of cobalt by Shewanella oneidensis strain MR-1. Appl Environ Microbiol 74:6880–6886. doi: 10.1128/AEM.00840-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Koga R, Matsumoto A, Kouzuma A, Watanabe K. 2020. Identification of an extracytoplasmic function sigma factor that facilitates c ‐type cytochrome maturation and current generation under electrolyte‐flow conditions in Shewanella oneidensis MR ‐1. Environ Microbiol 22:3671–3684. doi: 10.1111/1462-2920.15131 [DOI] [PubMed] [Google Scholar]
- 23. Guo K, Wang W, Wang H, Lu Z, Gao H. 2019. Complex oxidation of apocytochromes c during bacterial cytochrome c maturation. Appl Environ Microbiol 85:e01989-19. doi: 10.1128/AEM.01989-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guo K, Feng X, Sun W, Han S, Wu S, Gao H. 2022. NapB restores cytochrome c biosynthesis in bacterial dsbD-deficient mutants. Commun Biol 5:87. doi: 10.1038/s42003-022-03034-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sun W, Lin Z, Yu Q, Cheng S, Gao H. 2021. Promoting extracellular electron transfer of Shewanella oneidensis MR-1 by optimizing the periplasmic cytochrome c network. Front Microbiol 12:727709. doi: 10.3389/fmicb.2021.727709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baron D, LaBelle E, Coursolle D, Gralnick JA, Bond DR. 2009. Electrochemical measurement of electron transfer kinetics by Shewanella oneidensis MR-1. J Biol Chem 284:28865–28873. doi: 10.1074/jbc.M109.043455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Coursolle D, Baron DB, Bond DR, Gralnick JA. 2010. The Mtr respiratory pathway is essential for reducing flavins and electrodes in Shewanella oneidensis. J Bacteriol 192:467–474. doi: 10.1128/JB.00925-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Coursolle D, Gralnick JA. 2010. Modularity of the Mtr respiratory pathway of Shewanella oneidensis strain MR‐1. Mol Microbiol 77:995–1008. doi: 10.1111/j.1365-2958.2010.07266.x [DOI] [PubMed] [Google Scholar]
- 29. Mowat CG, Rothery E, Miles CS, McIver L, Doherty MK, Drewette K, Taylor P, Walkinshaw MD, Chapman SK, Reid GA. 2004. Octaheme tetrathionate reductase is a respiratory enzyme with novel heme ligation. Nat Struct Mol Biol 11:1023–1024. doi: 10.1038/nsmb827 [DOI] [PubMed] [Google Scholar]
- 30. Shirodkar S, Reed S, Romine M, Saffarini D. 2011. The octahaem SirA catalyses dissimilatory sulfite reduction in Shewanella oneidensis MR‐1. Environ Microbiol 13:108–115. doi: 10.1111/j.1462-2920.2010.02313.x [DOI] [PubMed] [Google Scholar]
- 31. Baker IR, Conley BE, Gralnick JA, Girguis PR. 2021. Evidence for horizontal and vertical transmission of Mtr-mediated extracellular electron transfer among the bacteria. mBio 13:e0290421. doi: 10.1128/mbio.02904-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wouters MA, Fan SW, Haworth NL. 2010. Disulfides as redox switches: from molecular mechanisms to functional significance. Antioxid Redox Signal 12:53–91. doi: 10.1089/ars.2009.2510 [DOI] [PubMed] [Google Scholar]
- 33. Wee SK. 2014. Novel pathway for microbial Fe(III) reduction: electron shuttling through naturally occurring thiols. PhD thesis. Georgia Institute of Technology. [Google Scholar]
- 34. Amirbahman A, Sigg L, Gunten UV. 1997. Reductive dissolution of Fe(III) (Hydr)oxides by cysteine: kinetics and mechanism. J Colloid Interface Sci 194:194–206. doi: 10.1006/jcis.1997.5116 [DOI] [PubMed] [Google Scholar]
- 35. Doong R-A, Schink B. 2002. Cysteine-mediated reductive dissolution of poorly crystalline iron(III) oxides by Geobacter sulfurreducens. Environ Sci Technol 36:2939–2945. doi: 10.1021/es0102235 [DOI] [PubMed] [Google Scholar]
- 36. Morrison KD, Bristow TF, Kennedy MJ. 2013. The reduction of structural iron in ferruginous smectite via the amino acid cysteine: implications for an electron shuttling compound. Geochimica et Cosmochimica Acta 106:152–163. doi: 10.1016/j.gca.2012.12.006 [DOI] [Google Scholar]
- 37. Eitel EM, Taillefert M. 2017. Mechanistic investigation of Fe(III) oxide reduction by low molecular weight organic sulfur species. Geochimica et Cosmochimica Acta 215:173–188. doi: 10.1016/j.gca.2017.07.016 [DOI] [Google Scholar]
- 38. Liu D, Dong H, Zhao L, Wang H. 2014. Smectite reduction by Shewanella species as facilitated by cystine and cysteine. Geomicrobiol J 31:53–63. doi: 10.1080/01490451.2013.806609 [DOI] [Google Scholar]
- 39. Coursolle D, Gralnick JA. 2012. Reconstruction of extracellular respiratory pathways for Iron(III) reduction in Shewanella oneidensis strain MR-1. Front Microbiol 3:56. doi: 10.3389/fmicb.2012.00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Myers CR, Nealson KH. 1988. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science 240:1319–1321. doi: 10.1126/science.240.4857.1319 [DOI] [PubMed] [Google Scholar]
- 41. Saltikov CW, Newman DK. 2003. Genetic identification of a respiratory arsenate reductase. Proc Natl Acad Sci U S A 100:10983–10988. doi: 10.1073/pnas.1834303100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM 2nd, Peterson KM. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176. doi: 10.1016/0378-1119(95)00584-1 [DOI] [PubMed] [Google Scholar]
- 43. Ellman GL. 1959. Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77. doi: 10.1016/0003-9861(59)90090-6 [DOI] [PubMed] [Google Scholar]
- 44. Eyer P, Worek F, Kiderlen D, Sinko G, Stuglin A, Simeon-Rudolf V, Reiner E. 2003. Molar absorption coefficients for the reduced Ellman reagent: reassessment. Anal Biochem 312:224–227. doi: 10.1016/s0003-2697(02)00506-7 [DOI] [PubMed] [Google Scholar]
- 45. Brutinel ED, Gralnick JA. 2012. Anomalies of the anaerobic tricarboxylic acid cycle in Shewanella oneidensis revealed by Tn‐seq. Mol Microbiol 86:273–283. doi: 10.1111/j.1365-2958.2012.08196.x [DOI] [PubMed] [Google Scholar]
- 46. Andrews S. 2010. FastQC: a quality control tool for high throughput sequence data. Available from: http://www.bioinformatics.babraham.ac.uk/projects/fastqc
- 47. Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477. doi: 10.1089/cmb.2012.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, Formsma K, Gerdes S, Glass EM, Kubal M, et al. 2008. The RAST server: rapid annotations using subsystems technology. BMC Genomics 9:75. doi: 10.1186/1471-2164-9-75 [DOI] [PMC free article] [PubMed] [Google Scholar]