Abstract
Background
The etiology for Attention Deficit Hyperactivity Disorder (ADHD) is generally unknown, but both genetics, biology and environment have been shown to increase the risk. The purpose of this study was to explore the prenatal risk factors, especially maternal antibiotics consumed before and during pregnancy, for the offspring for later being diagnosed with ADHD, and to find associations with neonatal biomarkers.
Methods
We included new-borns from the CODIBINE study, 465 children were ADHD cases and 10 954 children were controls. Ten biomarkers reflecting inflammation, neonatal stress, and/or neurologic development or damage were measured in dried blood spot samples drawn 2–3 days after birth. Maternal and child prescriptions of medication, birth data, and disorder codes were included in the statistical analyses.
Results
We found that maternal penicillin prescriptions until 2 years before birth increased the risk for offspring ADHD. The risk was higher with multiple prescriptions, both before and during pregnancy. Cases with maternal penicillin prescriptions had lower neonatal levels of epidermal growth factor (EGF) and soluble Tumor Necrosis Factor Receptor I (sTNF RI). Maternal prescriptions for psychotropic medication have, as expected, the highest correlation to offspring ADHD, but we found no differences in biomarkers in this group.
Conclusion
The fact that the offspring risk for ADHD was increased also with pre-pregnancy prescriptions of penicillin, indicates that it is not the penicillin that is the direct cause of the adverse effects. The significant differences in biomarkers strengthens the findings, as these could not be associated to other factors than maternal penicillin and offspring ADHD.
Keywords: ADHD, Penicillin, CODIBINE, Prenatal, Dried blood spot samples, Inflammation
Highlights
-
•
Maternal prescriptions of penicillins increases risk for offspring ADHD.
-
•
The offspring risk increases with multiple maternal prescriptions of penicillin.
-
•
Lower neonatal levels of EGF and sTNF RI in neonatal samples supports the finding.
-
•
Maternal psychotropic prescriptions had no association to neonatal biomarkers.
1. Introduction
Attention Deficit Hyperactivity Disorder (ADHD) is a childhood-onset neurodevelopmental disorder with multiple symptoms and an unknown and probably multifactorial etiology. The core symptoms are inattention, hyperactivity and impulsivity. There are several co-morbidities with other neurodevelopmental disorders, like autism spectrum disorders (ASD), communication and specific learning or motor disorders, intellectual disability, and tic disorders (Thapar and Cooper, 2016). People diagnosed with ADHD have a higher risk of anxiety, depression and schizophrenia later in life (Kanarik et al., 2022). Having an ADHD diagnosis is associated with lower levels of education, criminal records, risk behavior, and being unemployed (DuPaul et al., 2018; Mohr-Jensen et al., 2019). The risk of being diagnosed with ADHD in Denmark before the age of 18 is 3.04% in girls and 5.9% in boys (Dalsgaard et al., 2020). There are no diagnostic biomarkers for ADHD; a diagnosis is based on reported symptoms alone. Consequently, the symptoms may be both over and underestimated, resulting in both overdiagnosis and misdiagnosis (Yadav et al., 2021).
There is a strong genetic effect for the risk for ADHD (Demontis et al., 2019; Pingault et al., 2022). Other risk factors described for being diagnosed with ADHD are being born preterm, high or low maternal or paternal age (Min et al., 2021), low socioeconomic status, and maternal risk factors during pregnancy: pre-eclampsia, smoking, uterine infections, stress, obesity, asthma, and autoimmune disorders (Han et al., 2021b; Hegvik et al., 2021). As several of the above-mentioned risk factors may increase the maternal inflammatory state, systemic chronic inflammation and epigenetic changes has been suggested as the cause of the neurodevelopmental changes in the fetal brain (Furman et al., 2019; Han et al., 2021b).
Some studies indicate that prenatal exposure to antibiotics increases the risk for ADHD, where the number of prescriptions in pregnancy are proportional to the risk (Ai et al., 2021; Fan et al., 2020; Lavebratt et al., 2019), and early life exposure to antibiotics has been found to increase the risk in some (Slykerman et al., 2019), but not other studies (Axelsson et al., 2019; Hamad et al., 2019). Studies that have found an association between early life or prenatal maternal antibiotics use and ADHD in the offspring, have a high risk of comparability bias (Ai et al., 2021), and no biomarkers to support the findings.
The current study aimed to explore whether maternal antibiotic prescriptions were associated to offspring ADHD, and if so, to find neonatal biomarkers to support the association between maternal antibiotic prescriptions and the development of offspring ADHD. This is a part of the larger research project CODIBINE, Correlations and Diagnoses for Biomarkers in New-borns, aiming to explore biomarkers in new-borns correlated to birth, complications and diagnoses later in life.
2. Methods and materials
2.1. Sample selection and analysis
The CODIBINE cohort comprises all new-borns born with gestational age (GA) < 37 weeks (n = 7946) in Denmark between March 2009–March 2011, and maturely born controls with GA≥37 weeks (n = 7946) matched by birth hospital and day (Kiilerich et al., 2020). In the current study, we included all new-borns with GA≥32 weeks from the CODIBINE cohort, in total 11 419 individuals. In Denmark, most women get an early ultrasound screening as part of the first trimester combined screening for trisomy 13, 18 and 21, and here the GA is set (Steffensen et al., 2023). Thus, for most births, the registered GA is based on ultrasound and not the last menstrual period. The biomarkers were measured in dried blood spot samples (DBSS) from the Danish National Biobank originally collected for neonatal screening (Lund et al., 2020).
Cases were defined as all children with a registered ADHD diagnosis (ICD-10, International Classification of Diseases and Related Health Problems, code DF900) or children registered with prescriptions for ADHD medication (ATC (Anatomical Therapeutic Chemical Classification System) codes CO02AC02, N06BA04, N06BA09, and N06BA12) in the Medication register (Sundhedsstyrelsen), in total n = 465 ADHD cases, including 64 with ASD. In Denmark, only specialists in child psychiatry are allowed to prescribe ADHD medication for children, and only after a diagnosis has been set. Thus, one prescription was considered enough to confirm the diagnosis. All other samples were defined as controls (n = 10954), and were thus not matched with cases anymore from the original case-control cohort selection. Children with ICD-10 code for ASD (DF840, DF841) were excluded from the control group, and all multiple pregnancies were excluded from both case and control groups. There were 23 pairs of siblings other than twins/triplets in the study, but no siblings in the case group. The demographics for the cases and controls are shown in Table 1.
Table 1.
Demographic data grouped by ADHD or no ADHD in the offspring.
| ADHD (n = 465) | No ADHD (n = 10954) | |
|---|---|---|
| Age (in days) at sampling, number (% of groups) | ||
| 2 | 214 (46.0%) | 5328 (48.6%) |
| 3 | 239 (51.4%) | 5329 (48.6%) |
| 4 | 12 (2.6%) | 297 (2.7%) |
| Birth type, number (% of group) | ||
| In-labor C-section | 63 (13.5%) | 1150 (10.5%) |
| Pre-labor C-section | 81 (17.4%) | 1776 (16.2%) |
| Vaginal birth | 321 (69.0%) | 8028 (73.3%) |
| Maternal Body Mass Index class, number (% of groups) | ||
| Unknown | 14 (3.0%) | 502 (4.6%) |
| Underweight (<18.5) | 22 (4.7%) | 445 (4.1%) |
| Normal weight (18.5 to <25) | 240 (51.6%) | 6429 (58.7%) |
| Overweight (25 to <30) | 105 (22.6%) | 2213 (20.2%) |
| Obesity Class 1 (30 to <35) | 48(10.3%) | 865 (7.9%) |
| Obesity Class 2 (35 to <40) | 24 (5.2%) | 337 (3.1%) |
| Extreme Obesity (40 or higher) | 12 (2.6%) | 163 (1.5%) |
| Gestational age in weeks, number (% of groups) | ||
| 32 | 12 (2.6%) | 223 (2.1%) |
| 33 | 18 (3.9 %) | 345 (3.1%) |
| 34 | 23 (4.9%) | 644 (5.9%) |
| 35 | 56 (11.8%) | 1007 (9.2%) |
| 36 | 91 (19.6%) | 1877 (17.1%) |
| 37 | 23 (4.9%) | 347 (3.2%) |
| 38 | 42 (9.0%) | 968 (8.8%) |
| 39 | 58 (12.5%) | 1615 (14.7%) |
| 40 | 75 (16.1%) | 2075 (18.9%) |
| 41 | 51 (11.0%) | 1421 (13.0%) |
| 42+ | 17 (3.7%) | 422 (3.9%) |
| Gender, number (% of groups) | ||
| Female | 112 (24.1%) | 5163 (47.1%) |
| Male | 353 (75.9%) | 5791 (52.9%) |
| Maternal age | ||
| Mean (SD) | 28.6 (5.2) | 30.4 (5.0) |
| Range | 16–44 | 15–47 |
| Birth weight in grams | ||
| Mean (SD) | 3148 (687) | 3208 (681) |
| Range | 1165–5180 | 950–5670 |
The study was approved by the Danish Ethical Committee (VEK), Project-ID H-6-2014-078 and H-6-2014-079.
Analyses of the biomarkers were made on extractions from the dried blood spot samples using a Meso-Scale Discovery based in-house developed multiplex assay targeting 3 biomarkers for inflammation (Interleukin-18 (IL-18), Monocyte Chemotactic Protein (MCP-1), C-Reactive Protein (CRP)), 1 anti-inflammatory marker (soluble Tumor Necrosis Factor Receptor-1 (sTNF RI)), all as an indication for infection in the pregnancy. The other biomarkers have all been associated with ADHD or ASD in other studies, and may indicate problems with the neuronal development: 1 biomarker for stress (Heat Shock Protein-70 (HSP70)) (Özaslan et al., 2022), 2 growth factors (Vascular Endothelial Growth Factor A (VEGF-A) (Yurteri et al., 2019), Epidermal Growth Factor (EGF) (Tonhajzerova et al., 2021)), 3 neurotrophic biomarkers important for brain development and/or damage (Brain-Derived Neurotrophic Factor (BDNF) (Gumus et al., 2022), Neurotrophin-3 (NT-3) (Tostes et al., 2012), and S100 calcium binding protein B (S100B) (Ouadih-Moran et al., 2023)), as described previously (Kiilerich et al., 2021).
2.2. Data preparation
Maternal antibiotic prescriptions were the main focus, but to rule out other medication's effects, we evaluated all maternal prescriptions. Maternal prescription medicine in the last two years before birth were registered and put into 35 groups, based on the type of medication, defined by the ATC-codes, or ICD-10-codes as treatment code in the hospital, shown in Table 2 and Table S1. The rationale for the groups was the registered indication for each ATC-code as disorder groups, and not the drug as such. Antibiotics were divided into several groups depending on types and specific microbiologic effect, which was possible due to the high number of prescriptions (Table S1). Each medication group was further defined for each individual either as a “yes” or a “no” for any prescriptions for the specific medication at all, or conditional to the time relative to the pregnancy as “before”, “during”, “both before and during”, or “no”. The rationale for including prescriptions also before pregnancy, was that women tend to abrupt, if possible, medical treatments during pregnancy, and thus, we could overlook important disorders. The theory was that both indication and medicine could be factors of importance. Further, women with re-current infections before pregnancy were considered relevant to include, as infections may be on-going, also without any antibiotic prescriptions during pregnancy. We defined any registered antibiotics prescriptions received more than 14 days apart as separate prescriptions, and prescriptions received less than 14 days apart as part of the same prescription for the same infection. This assumption was made due to the fact that prescriptions often are made immediately in medical consultations with antibiotics as narrow spectrum as possible, and after no respond and/or microbiologic tests, are adjusted to other more broad spectrum antibiotics(Antibiotics Prescriptions, 2019). Further, prescriptions may be given repeatedly due to non adherence and thus failure in treatment (Jimmy and Jose, 2011).
Table 2.
Medication groups that were used as fixed factors in statistical calculations.
| Medication group – yes or no | Number | ATC-codes |
|---|---|---|
| Mental disorders (p-value<0.001) | 198 | N05AB03, N05AD01, N05AD03, N05AE04, N05AF01, N05AF03, N05AH03, N05AH04, N05AN01, N05AX08, N05AX12, N05BA01, N05BA02, N05BA04, N05BA08, N05BA09, N05BA12, N05BB01, N05AA02, N06AB03, N06AB04, N06AB05, N06AB06, N06AB10, N06AX03, N06AX11, N06AX16, N06AX21, N06AX22, N06BA04, N06AA09, N06AA10 |
| Vitamin B12 deficiency (p-value = 0.165) | 232 | B03BA01, B03BA02, B03BA03 |
| NSAIDs (p-value = 0.237) | 2229 | M01AB05, M01AB08, M01AB55, M01AC05, M01AE01, M01AE02, M01AE14, M01AG02, M01AX05, M02AA07, M02AA15 |
| Medication group – before and/or during pregnancy | Number before | Number during | Total number | ATC-codes |
|---|---|---|---|---|
| Penicillin (p-value = 0.011) | 3157 | 2149 | 4466 | J01CA01, J01CA02, J01CA04, J01CE02 |
| Analgesics (p-value = 0.122) | 630 | 219 | 739 | N02AG02, N02AJ06, N02AX02, N02BE01, N02AA01, N02AA05 |
2.3. Statistics
An overview of all statistical analyses performed are presented in Fig. 1.
Fig. 1.
An overview of all statistical analyses.
2.3.1. Finding maternal medication and demographic factors to use for analyses and adjustment
Associations between the maternal medication groups and ADHD in the offspring were examined using each medication group in a single logistic model as independent factors, with no medication from that group as the reference factor (Fig. 1 step A). ADHD in the offspring was used as the dependent variable, and the levels of the independent groups were defined as described earlier, calculating p-values and adjusting for multiple testing using false discovery rate (FDR)(Benjamini and Hochberg, 1995b) (Fig. 1 step A2). A type 3 analysis of covariance (ANCOVA) using the R-package car (Fox and Sanford, 2019) was performed on the model as well to test for the overall difference for each medication group (Fig. 1 step A1). Maternal penicillin was selected for further analyses (Fig. 1 step D).
To find other maternal medication groups that had a correlation with ADHD in the offspring and thereby could be confounders to control for in further analyses, a feature selection of all medication groups was performed using a backwards stepwise Akaike Information Criterion (AIC) from the R-package lmerTest (Kuznetsova and Rune, 2017) (Fig. 1 step B, Table S1). This was done on the above described logistic model, and the five maternal medication groups that were most associated with the risk for ADHD in the offspring, maternal penicillin included, were chosen performing type 3 ANCOVA (Fox and Sanford, 2019) on the model found by the feature selection (Fig. 1 step B1). The following maternal medication groups were included in subsequent models as control variable data (fixed factors); medication for mental disorders (schizophrenia and/or other psychotic conditions, bipolar disorders, anxiety, depression, and ADHD), B12 deficiency, analgesics, and non-steroid anti-inflammatory drugs (NSAID) (Fig. 1 step E, Table 2). Univariate analyses of all the medication groups were performed as well, showing similar results with high associations between ADHD in the offspring and the five medication groups.
Selected demographic data were tested for associations with ADHD using logistic regression with GA, maternal age, birth type, maternal Body Mass Index (BMI) class, birth weight, child gender, and the maternal medication groups found in the feature selection (Fig. 1 step B) as independent factors (Fig. 1 step C). An ANCOVA (Fox and Sanford, 2019) was used to determine which of the demographic factors in the model were significantly associated with ADHD in the offspring (Fig. 1 step C1). The gender of the child, BMI class of the mother, and the maternal age were found significantly associated to ADHD in the offspring, and were thus used as control factors in all further analyses (Fig. 1 step E).
Accordingly, the initial analysis demonstrating significant associations among the maternal medication groups (Fig. 1 step A) was repeated controlling for the age and the BMI class of the mother, and the gender of the child, giving similar results.
2.3.2. Analyses of the association between maternal penicillin and ADHD in the offspring
The association between maternal penicillin prescriptions and the risk of ADHD in the offspring was studied further using logistic regression.
ADHD in the offspring was set as the dependent variable and penicillin as an independent factor in four categories relative to the pregnancy; penicillin prescribed before, during, both before and during pregnancy, or not at all (Fig. 1 step F). The overall difference was tested with a type 3 ANCOVA (Fox and Sanford, 2019) (Fig. 1 step F1). We used contrasts to establish pairwise comparisons between the mother not having received any prescriptions for penicillin and having received prescriptions at the specific times relative to the pregnancy (treatments vs. control), adjusting the p-values for multiple testing within the comparison using FDR (Benjamini and Hochberg, 1995b) (Fig. 1 step F3). The contrasts were obtained using estimated marginal means (Lenth et al., 2021) (adjusted means), which were calculated using the chosen control variables to adjust for as nuisance factors with proportional weights (Fig. 1 step F2). The pairwise comparisons were performed using the scale of the log-odds ratio, while the adjusted means are back-transformed from the logit scale and are in response form (probability of ADHD in the child).
The time of maternal penicillin prescriptions for the offspring with only one registered maternal prescription have been analyzed further, with the same methods as described earlier, grouped into time slots (Fig. 1 step H). Similarly, the number of maternal penicillin prescriptions were analyzed to study effects of multiple prescriptions of maternal penicillin on ADHD in the offspring (Fig. 1 step G). More details of these analyses can be found in Table S2.
2.3.3. Analysis of biomarkers, maternal penicillin, and ADHD in the offspring
To find associations between each biomarker, maternal penicillin prescriptions, and ADHD in the offspring, linear models were build using the biomarkers as the dependent factors and the interaction between ADHD in the offspring and maternal penicillin prescriptions as the independent factors (Fig. 1 step J). Each biomarker was log-transformed using the natural logarithm to approach a normal distribution of the residuals, and the penicillin variable was grouped into none, one, or multiple prescriptions in one model and none or any prescriptions (yes or no) in another model per biomarker. The models included all the controlling factors described earlier and type 3 ANCOVA (Fox and Sanford, 2019) was used to detect the overall differences (Fig. 1 step J1). Adjusted biomarker means were calculated using the controlling factors as nuisance factors to proportionally adjust for them (Fig. 1 step J2), as described earlier. The adjusted means were used to find the pairwise contrasts and p-values for the difference between offspring with ADHD and without ADHD for each of the different maternal penicillin groups, adjusting the p-values for multiple comparisons within a model for a biomarker using FDR (Benjamini and Hochberg, 1995a) within each model for each biomarker (Fig. 1 step J3).
All analyses were performed in the statistical software R, version 4.1.1 (Team, 2021) using the packages lmerTest (Kuznetsova and Rune, 2017), emmeans (Lenth et al., 2021), car (Fox and Sanford, 2019), and tidyverse (Wickham et al., 2019). All plots were made with ggplot2 (Hadley, 2016).
3. Results
Maternal medication for mental disorders was the medicine group found to be most significantly associated with ADHD in the offspring (overall p-value<0.001). Maternal prescriptions of penicillin before and/or during pregnancy have the second highest association with ADHD in the offspring (overall adjusted p-value = 0.011). Calculating the adjusted mean risk of ADHD in the child and comparing treatments and controls for the different penicillin-groups, we found prescriptions before the pregnancy to be the most significant (adjusted p-value<0.001, OR = 1.53 [1.34–1.71]), and prescriptions both before and during pregnancy to be the second most significant (adjusted p-value = 0.049, OR = 1.44 [1.20–1.59]). The adjusted means with their standard error (SE) can be seen in Fig. 2A, and adjusted p-values and odds-ratios can be found in Table 3.
Fig. 2.
The mean probability of ADHD in the offspring for different maternal penicillin prescription groups
The error-bars indicate the SE of the mean, the stars indicate significant (p-value<0.05) difference between the group and no maternal penicillin prescribed. The overall p-value describes the overall association between the grouped maternal penicillin prescriptions and ADHD in the child found by the ANCOVA.
A: The adjusted mean probability of ADHD in the offspring compared to no maternal penicillin prescriptions, maternal penicillin prescriptions grouped by time relative to pregnancy: Before, during, or both before and during pregnancy.
B: The adjusted mean probability of ADHD in the offspring compared to no maternal penicillin prescriptions, maternal penicillin prescriptions grouped by time relative to pregnancy.
C: The adjusted mean probability of ADHD in the offspring compared to no maternal penicillin prescriptions.
Table 3.
Overall p-values for the association between ADHD in the offspring and maternal penicillin grouped in 4 different ways.
| Maternal penicillin | Odds ratio of ADHD risk (SE) | P-value | Overall p-value |
|---|---|---|---|
| Time relative to pregnancy | 0.002 | ||
| Before | 1.53 (1.35–1.71) | <0.001 | |
| Both | 1.44 (1.20–1.69) | 0.049 | |
| During | 1.20 (1.01–1.38) | 0.250 | |
| Amount of treatments | 0.001 | ||
| 1 | 1.29 (1.14–1.14) | 0.027 | |
| 2 | 1.66 (1.37–1.87) | 0.005 | |
| >2 | 1.69 (1.38–1.20) | 0.006 | |
| Amount of treatments | <0.001 | ||
| 1 | 1.29 (1.14–1.43) | 0.027 | |
| Multiple | 1.65 (1.44–1.86) | <0.001 | |
| Time relative to pregnancy, only one treatment/mother | 0.298 | ||
| During | 1.26 (1.05–1.47) | 0.318 | |
| 0–6m before | 1.39 (1.12–1.66) | 0.318 | |
| 6–12m before | 1.25 (1.00–1.50) | 0.345 | |
| 12m before-up to 2y before birth | 1.23 (0.91–1.56) | 0.423 | |
| Received at all (yes/no) | <0.001 |
Children whose mothers had more than one treatment of penicillin were excluded in analyses of the effect of penicillin prescriptions over time. We found no significant difference between the periods the prescriptions were received and the risk of ADHD in the offspring (Fig. 2B). Dividing the times for penicillin prescriptions further into groups with multiple prescriptions made each group too small, thus further calculations could not be performed, as shown in Table S2.
We found a significant overall difference in ADHD probability in the offspring given the amount of maternal penicillin prescriptions (overall p-value <0.001), as shown in Fig. 2C and Table 3.
The five most associated maternal medication groups found were, in ranked order, medication for mental disorders, penicillin, Vitamin B12 deficiency, NSAIDs, and analgesics. Their distributions relative to ADHD in the child are shown in Table 4. The distributions of other types of maternal antibiotics and the types of maternal penicillin relative to ADHD in the offspring can be found in Table S2 and Table 4, respectively.
Table 4.
The distribution of the maternal medication groups chosen by the AIC grouped by ADHD or no ADHD in the offspring.
| Maternal medication prescribed relative to pregnancy | ADHD | No ADHD |
|---|---|---|
| Penicillin | ||
| No | 233 (50.1%) | 6720 (61.3%) |
| Before | 130 (28.0%) | 2187 (20.0%) |
| Both before and during | 46 (9.9%) | 794 (7.2%) |
| During | 56 (12.0%) | 1253 (11.4%) |
| One treatment | 131 (28.2%) | 2713 (24.8%) |
| Multiple treatments | 101 (21.7%) | 1521 (13.9%) |
| Mental disorders | ||
| No | 374 (80.4%) | 9947 (90.8%) |
| Yes | 91 (19.6%) | 1007 (9.2%) |
| NSAIDs | ||
| No | 345 (74.2%) | 8845 (80.7%) |
| Yes | 120 (25.8%) | 2109 (19.3%) |
| Analgesic | ||
| No | 424 (91.2%) | 10256 (93.6%) |
| Before | 23 (4.9%) | 497 (4.5%) |
| Both before and during | 7 (1.5%) | 103 (0.9%) |
| During | 11 (2.4%) | 98 (0.9%) |
| B12 deficiency medication | ||
| No | 448 (96.3%) | 10739 (98.0%) |
| Yes | 17 (3.7%) | 215 (2.0%) |
|
Types of maternal penicillin prescribed | ||
| Ampicillin | ||
| No | 457 (98.3%) | 10834 (98.9%) |
| Yes | 8 (1.7%) | 120 (1.1%) |
| Pivampicillin | ||
| No | 424 (91.2%) | 10327 (94.3%) |
| Yes | 41 (8.8%) | 627 (5.7%) |
| Amoxicillin | ||
| No | 436 (93.8%) | 10382 (94.8%) |
| Yes | 29 (6.2%) | 572 (5.2%) |
| Phenoxymethylpenicillin | ||
| No | 267 (57.4%) | 7381 (67.4%) |
| Yes | 198 (42.6%) | 3573 (32.6%) |
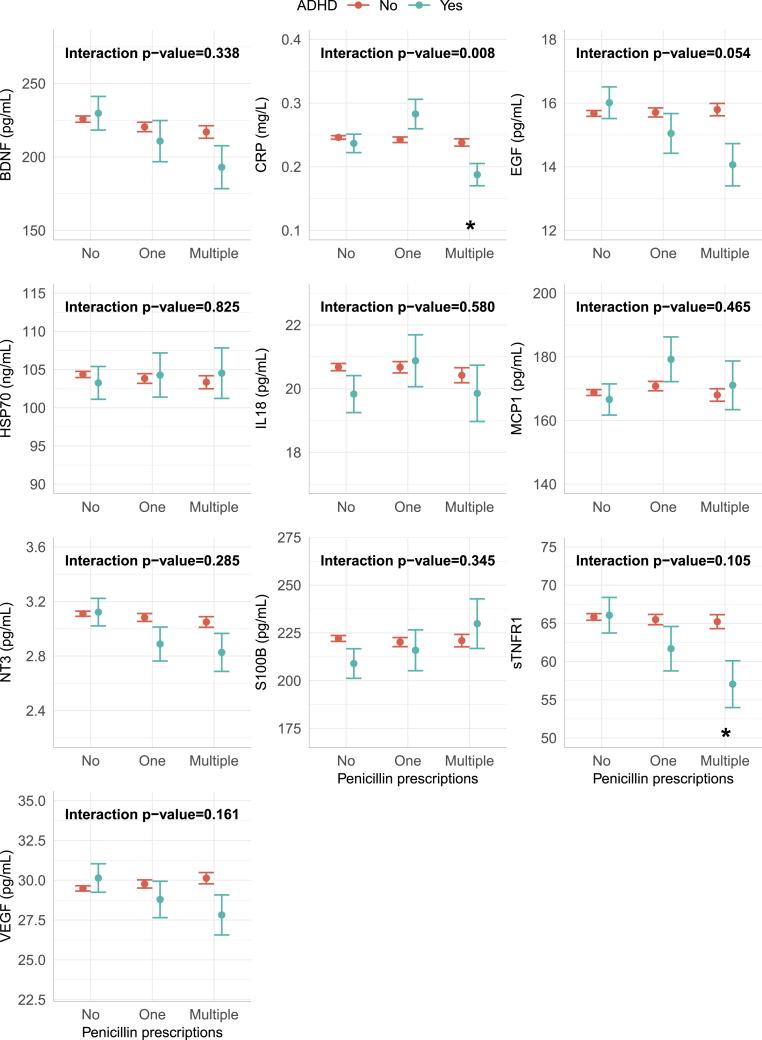
We found significant lower neonatal levels of EGF and sTNF RI in offspring later diagnosed with ADHD and maternal prescriptions of penicillin, compared to children without ADHD (Fig. 3) and an overall significant effect from the interaction between offspring ADHD and maternal penicillin prescriptions on EGF. Dividing into zero, one, or multiple descriptions of penicillin, neonatal levels of sTNF RI and CRP were significantly lower after multiple penicillin prescriptions in offspring later diagnosed with ADHD than without ADHD (Fig. 4). There was a significant overall effect on the inflammatory marker CRP. Further, there was a tendency of decreased levels of EGF for children later diagnosed with ADHD with multiple maternal penicillin prescriptions, though not significant. Similar tendencies were found for BDNF, NT3, and VEGF (Fig. 3, Fig. 4). All p-values and odds-ratios are shown in Tables S5 and S6.
Fig. 3.
Biomarker levels divided into maternal penicillin prescriptions
Adjusted means of the biomarkers for the interaction between ADHD in the offspring and maternal penicillin prescriptions.
The error-bars indicate the SE of the mean, the stars indicate significant (p-value<0.05) difference between the ADHD group and the control group within the indicated maternal penicillin prescription group. The interaction p-values describe the overall association between the specific biomarker and the interaction between maternal penicillin prescriptions and ADHD in the offspring.
Fig. 4.
Biomarker levels divided into the number of maternal penicillin prescriptions
Adjusted means of the biomarkers for the interaction between ADHD in the child and maternal penicillin prescriptions, prescriptions grouped by the amount of treatments. The error-bars indicate the SE of the mean, the stars indicate significant (p-value<0.05) difference between the ADHD group and the control group within the indicated maternal penicillin prescription group. The interaction p-values describe the overall association between the specific biomarker and the interaction between maternal penicillin prescriptions and ADHD in the offspring.
Maternal medication for mental disorders did not have any significant correlation to neonatal biomarkers (data not shown), and neither did ADHD diagnosis alone (Fig. 3).
The gender of the child, age of the mother, and BMI-class of the mother were significantly associated with ADHD, the distribution of these are shown in Table 1.
GA, birth weight, and birth type was not significantly associated with the risk of ADHD in the child in this study. Therefore, we do not expect these to influence any results in relation to ADHD, and analyses were thus not adjusted for these factors.
The demographic of the interaction between ADHD in the child and maternal penicillin prescriptions, and birth type can be found in Table S4.
We further tested if penicillin, the size of the placenta, and the risk of ADHD in the child had a combined effect on the biomarkers, but we did not find any significance (data not shown).
We tested the association between ADHD in the child and interactions between the five maternal medication groups, but found no significant interactions. Further, we studied associations between offspring ADHD and specifically maternal antibiotics for urinary tract infection, macrolides, psoriasis medication, and prescriptions for paracetamol and found no significance. We did not study other antibiotics groups, since the ADHD cases in these groups were too few.
4. Discussion
We found an association between offspring ADHD and maternal prescriptions for the penicillins ampicillin, pivampicillin, amoxicillin, and phenoxymethylpenicillin up to two years before birth and/or during pregnancy. More than one prescription of penicillin increased the risk (Fig. 2). Neonatal levels of the growth factor EGF and the anti-inflammatory marker sTNF RI were significantly lower in children with maternal prescriptions of penicillin that were later diagnosed with ADHD compared to children with maternal prescriptions of penicillin that did not have an ADHD diagnosis (Fig. 3). Neonatal levels of CRP and sTNF RI in the prior group were significantly lower after multiple maternal penicillin prescriptions (Fig. 4).
An association between prenatal antibiotics (both penicillins, β-lactams, and macrolides) exposure and ADHD in offspring has been described before (Ai et al., 2021; Fan et al., 2020), while the association of pre-pregnancy use of penicillins and later ADHD in offspring has, as far as we know, not before been reported. The observed differences in biomarkers and the risk for offspring ADHD were high er with more than one maternal penicillin prescription in the two years before birth. The indications for use for this group of penicillins are a wide range of infections, primarily upper and lower tract airway infections, ear and gastro-intestinal infections (Danish Medicine Information, 2022). Maternal genital tract infection with group B streptococcus, an infection treated with penicillin during pregnancy according to Danish guidelines (Danish Society for Obstetrics and Gynecology. Gruppe B Streptokokker - Early Onset Disease: Profylakse Inklusiv GBS Screening Intrapartum. https://www.dsog.dk/obstetrik.), has previously been associated with adverse neonatal outcomes (Huang et al., 2021). We do not know the specific indication for each penicillin prescription, and neither do we know which non-prescription drugs that also might have been consumed by the mothers during the infections. Another study indicate a connection between maternal urinary tract infections and ADHD in the offspring (Mann and McDermott, 2011), but we did not find this correlation (Table S1). The population in our study was quite different, as Mann and McDermott only included Medicaid-receivers (low income), and the study had a much higher ratio of black women included compared to Denmark. Thus, these two studies cannot be directly compared.
The fact that the risk was independent of the time, before or during the pregnancy, indicates that the penicillins itself are not the direct cause of the adverse effects. An explanation for the association between maternal penicillin consumption before pregnancy and ADHD in the offspring might be changes in the microbiota, where the induced changes in barrier microbiota opens up for opportunistic infections, or through changes in the microbiota and the gut-brain axis signaling (Lavebratt et al., 2019). The fact that repeated prescriptions before pregnancy increased the risk for offspring ADHD supports the theory of changes in the microbiota as the cause. Different types of antibiotics affects the microbiota in different ways (Ferrer et al., 2017), and this can explain the finding that women treated with other types of antibiotics did not have an association with offspring ADHD. Another possibility is that antibiotic treatments not always eradicate all bacteria of the infection, thus, the bacteria might proliferate again (Teimouri and Kolomeisky, 2019), and there might still be an ongoing infection during pregnancy.
Maternal immune activation, oxidative stress, and neuroinflammation has been described as possible causes for psychiatric disorders in the offspring (Corona, 2020; Han et al., 2021a), and are supported by the measured neonatal biomarkers in the current study, where we found significantly decreased neonatal levels of the anti-inflammatory marker sTNF RI in ADHD cases after maternal penicillin prescriptions. Elevated serum levels of inflammatory markers in children and youths with ADHD have been reported in previous studies (Chang et al., 2020; Darwish et al., 2019). In contrast, we found that CRP was significantly decreased after multiple maternal penicillin prescriptions in neonates later developing ADHD (Fig. 4), but was not significantly different between the cases and controls altogether (Fig. 3). The fact that CRP was lower in ADHD cases than controls could indicate an abnormal immune reaction in these individuals, or that the inflammation after multiple penicillin prescriptions is transient or delayed. We did not have enough individuals to look closer into the combination of actual time for penicillin prescriptions and the different levels of inflammatory markers.
The neonatal levels of the neurotrophic factor EGF was significantly decreased in the ADHD cases with maternal prescriptions of penicillin. EGF stimulates cell and neuronal proliferation, growth, and differentiation, and regeneration of the central nervous system cell, among them dopamine neurons and GABAergic cells (Futamura et al., 2003). EGF binds to EGF receptor, which plays a fundamental role during embryogenesis (Romano and Bucci, 2020). Decreased levels of EGF in schizophrenia patients has been described, where serum EGF concentrations were negatively correlated with cognitive performance (Zhang et al., 2020). Rat models have shown that EGF-treated rats can be used as animal models for psychiatric disorders due to changed behavior and/or cognitive dysfunctions (Futamura et al., 2003). Studies has described increased serum levels of EGF in youth with depressive or hypomanic/manic episodes in bipolar disorders (Skibinska et al., 2021). Thus, EGF's effect on cognitive functions is well described. We were not able to find any descriptions of EGF and ADHD. Animal models have shown that EGF do pass through the blood-brain barrier (Futamura et al., 2003), thus capillary levels are probably proportional to concentrations in the brain. In an earlier CODIBINE study, we showed that neonatal EGF levels depend on birth mode, where neonates born by vaginal delivery had significantly lower levels than neonates born by pre-labor cesarean section (Kiilerich et al., 2020). There were no significant differences in birth mode between the groups (Table 1), thus this cannot explain the observed differences in EGF levels.
We found a tendency, although not statistically significant after adjusting for multiple tests, for decreased neonatal levels of the neurotrophic factors BDNF and NT-3 in the ADHD-cases with maternal penicillin descriptions. Decreased plasma levels of BDNF in children and youth with ADHD has also been described by others (Chang et al., 2020). The neurotrophic factors support neuronal survival and differentiation, and improves the plasticity in the brain (Park and Poo, 2013). The psychostimulant drug Methylphenidate, and the non-stimulant drug Atomoxetin, both drugs used for ADHD treatment, have partly unknown mechanisms of action, but upregulates BDNF mRNA levels and gene expression, respectively, in rat brains (Fumagalli et al., 2010; Ribasés et al., 2008). This indicates that lack of neurotrophins are part of the etiology of the disorder. Further, polymorphisms in the BDNF and NT3 genes have earlier been found associated with ADHD (Ribasés et al., 2008). We have previously found decreased neonatal levels of BDNF in newborns later diagnosed with autism, while we did not see any associations with BDNF-levels in neonates later diagnosed with ADHD. In that study, we did not have access to information regarding maternal penicillin prescriptions (Skogstrand et al., 2019). Why the tendency for an association between the neurotrophic factors and ADHD only are seen in neonates after maternal pre-pregnancy or prenatal consumption of penicillin, is unclear.
Paracetamol is the recommended non-prescription drug in Denmark as an analgesic and antipyretic drug if pregnant (Rebordosa et al., 2009). Earlier studies have found that paternal or maternal prenatal consumption of paracetamol increases the risk for ADHD (Liew et al., 2014), but not pre-conceptional use (Ystrom et al., 2017). Thus, the pre-conceptional association of penicillins and offspring ADHD can probably not be explained by maternal pre-pregnancy consumption of paracetamol, but non-prescription drugs should not be forgotten as possible risk factors for ADHD before and/or during pregnancies. As we did not have any information regarding non-prescription drugs, this could not be adjusted for in the statistics.
The highest association of offspring ADHD and maternal medications was maternal consumption of medications for mental disorders. This was expected, as ADHD has a high genetic component (Demontis et al., 2019; Pingault et al., 2022), and ADHD is genetically associated to several other psychiatric disorders (Akingbuwa et al., 2023; Mattheisen et al., 2022). Maternal pre-pregnancy use of medications for mental disorders has previously been associated to ADHD in offspring (Yeh et al., 2021). The association was thus used as a sanity check of the study. Neonatal biomarkers in this case-group was not significantly different compared to controls despite maternal consumptions of penicillins. This strengthen the theory that ADHD is a multifactorial disorder that can be caused by either genetics and/or environmental factors.
Cesarean delivery has been associated to an increased risk for ADHD in the offspring (Lin et al., 2022). We have previously, in an earlier CODIBINE study comparing biomarkers after different delivery modes, found that it is not the cesarean delivery in itself, but the labor process (pre- or in-labor), that changes the levels of biomarkers (Kiilerich et al., 2020). In the current study, there was no difference in the number of cases of ADHD and birth mode, as shown in Table 1. Thus, the observed differences in biomarkers cannot be explained by the labor.
The strengths of the current study are the non-biased selection of cases and controls, as the coverage of neonatal screening in Denmark is >99% (Lund et al., 2012), thus we randomly selected newborn samples from almost all children born in Denmark in the time-period 2009–2011.
We do not know if the antibiotics were prescribed to a specific infection, or which one and the severity, or if the prescription was made as a precaution. Neither do we know the adherence of the different medications, that is, we do not know if the medication was taken as prescribed or used at all. Further, we do not know the maternal health status, other than the medications prescribed to the mothers 2 years before and until birth.
The medications were grouped after ATC-indications, although different medications in the same group may have very different pharmacological mechanisms. This may lead to confounding by indication, where some drugs may increase the risk for offspring ADHD even though sorted out in the statistics, as ruled out by other medications in the same group. To sort this out, we need more individuals using each medication.
We did not have information about other co-variables such as sociodemographic characteristics, smoking and alcohol consumption during pregnancy, and diagnosis in parents other than what maternal medications indicate. Further, we did not have information about paternal psychiatric disorders.
In conclusion, the current study indicates that maternal penicillin consumption, or the underlying infection, increases the risk for offspring ADHD. The significant differences in biomarkers strengthens the findings, as these could not be associated to other factors. Antibiotics should not be avoided if there are a treatable infection, but should not be given as a precaution. Further, there should be made a bacterial typing initially to find the most accurate antibiotic to avoid multiple antibiotics prescriptions.
Funding
This work was supported by ”Læge Sofus Carl Emil Friis og hustru Olga Doris Friis’ legat” and ”Fonden til Lægevidenskabens Fremme”. The sponsors were not involved in any part of the study.
CRediT authorship contribution statement
Solveig Holmgaard: Data curation, Formal analysis, Writing – original draft. Pia Kiilerich: Conceptualization, Writing – review & editing. Nis Borbye-Lorenzen: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. Kristin Skogstrand: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Declaration of competing interest
Solveig Holmgaard: Declarations of interest: none.
Pia Kiilerich: Declarations of interest: none.
Nis Borbye-Lorenzen: Declarations of interest: none.
Kristin Skogstrand: Declarations of interest: none.
Acknowledgements
Thanks to Malene Billsten Zent, Lisette Jensen, Karin Skaarup, and Karina Liebmann Madsen for skilful laboratory analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2024.100739.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Data availability
The data that has been used is confidential.
References
- Ai Y., Zhao J., Shi J., Zhu T.T. Antibiotic exposure and childhood attention-deficit/hyperactivity disorder: systematic review and meta-analysis. Psychopharmacology (Berl) 2021 Nov;238(11):3055–3062. doi: 10.1007/s00213-021-05989-3. [DOI] [PubMed] [Google Scholar]
- Akingbuwa W.A., Hammerschlag A.R., Allegrini A.G., Sallis H., Kuja-Halkola R., Rimfeld K., Lichtenstein P., Lundstrom S., Munafò M.R., Plomin R., Nivard M.G., Bartels M., Middeldorp C.M. Multivariate analyses of molecular genetic associations between childhood psychopathology and adult mood disorders and related traits. Am J Med Genet B Neuropsychiatr Genet. 2023;192:3–12. doi: 10.1002/ajmg.b.32922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antibiotics Prescriptions Guidlines. Danish Health and Medicines. Authority; n.d: 2019. [Google Scholar]
- Axelsson P.B., Clausen T.D., Petersen A.H., Hageman I., Pinborg A., Kessing L.V., Bergholt T., Rasmussen S.C., Keiding N., Løkkegaard E.C.L. Investigating the effects of cesarean delivery and antibiotic use in early childhood on risk of later attention deficit hyperactivity disorder. J Child Psychol Psychiatry. 2019;60:151–159. doi: 10.1111/jcpp.12961. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the royal statistical society series b-methodological. 1995;57:289–300. [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Roy. Stat. Soc. B. 1995;57:289–300. [Google Scholar]
- Chang J.P., Mondelli V., Satyanarayanan S.K., Chiang Y.J., Chen H.T., Su K.P., Pariante C.M. Cortisol, inflammatory biomarkers and neurotrophins in children and adolescents with attention deficit hyperactivity disorder (ADHD) in Taiwan. Brain Behav. Immun. 2020;88:105–113. doi: 10.1016/j.bbi.2020.05.017. [DOI] [PubMed] [Google Scholar]
- Corona J.C. Role of oxidative stress and neuroinflammation in attention-deficit/hyperactivity disorder. Antioxidants. 2020;9 doi: 10.3390/antiox9111039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard S., Thorsteinsson E., Trabjerg B.B., Schullehner J., Plana-Ripoll O., Brikell I., Wimberley T., Thygesen M., Madsen K.B., Timmerman A., Schendel D., McGrath J.J., Mortensen P.B., Pedersen C.B. Incidence rates and cumulative incidences of the full spectrum of diagnosed mental disorders in childhood and adolescence. JAMA Psychiatr. 2020;77:155–164. doi: 10.1001/jamapsychiatry.2019.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danish Medicine Information https://pro.medicin.dk
- Danish Society for Obstetrics and Gynecology Gruppe B Streptokokker - Early Onset Disease: Profylakse Inklusiv GBS Screening Intrapartum. https://www.dsog.dk/obstetrik
- Darwish A.H., Elgohary T.M., Nosair N.A. Serum interleukin-6 level in children with attention-deficit hyperactivity disorder (ADHD) J. Child Neurol. 2019;34:61–67. doi: 10.1177/0883073818809831. [DOI] [PubMed] [Google Scholar]
- Demontis D., Walters R.K., Martin J., Mattheisen M., Als T.D., Agerbo E., Baldursson G., Belliveau R., Bybjerg-Grauholm J., Bækvad-Hansen M., Cerrato F., Chambert K., Churchhouse C., Dumont A., Eriksson N., Gandal M., Goldstein J.I., Grasby K.L., Grove J., Gudmundsson O.O., Hansen C.S., Hauberg M.E., Hollegaard M.V., Howrigan D.P., Huang H., Maller J.B., Martin A.R., Martin N.G., Moran J., Pallesen J., Palmer D.S., Pedersen C.B., Pedersen M.G., Poterba T., Poulsen J.B., Ripke S., Robinson E.B., Satterstrom F.K., Stefansson H., Stevens C., Turley P., Walters G.B., Won H., Wright M.J., Andreassen O.A., Asherson P., Burton C.L., Boomsma D.I., Cormand B., Dalsgaard S., Franke B., Gelernter J., Geschwind D., Hakonarson H., Haavik J., Kranzler H.R., Kuntsi J., Langley K., Lesch K.P., Middeldorp C., Reif A., Rohde L.A., Roussos P., Schachar R., Sklar P., Sonuga-Barke E.J.S., Sullivan P.F., Thapar A., Tung J.Y., Waldman I.D., Medland S.E., Stefansson K., Nordentoft M., Hougaard D.M., Werge T., Mors O., Mortensen P.B., Daly M.J., Faraone S.V., Børglum A.D., Neale B.M. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat. Genet. 2019;51:63–75. doi: 10.1038/s41588-018-0269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPaul G.J., Morgan P.L., Farkas G., Hillemeier M.M., Maczuga S. Eight-year latent class trajectories of academic and social functioning in children with attention-deficit/hyperactivity disorder. J. Abnorm. Child Psychol. 2018;46:979–992. doi: 10.1007/s10802-017-0344-z. [DOI] [PubMed] [Google Scholar]
- Fan H., Gilbert R., O'Callaghan F., Li L. Associations between macrolide antibiotics prescribing during pregnancy and adverse child outcomes in the UK: population based cohort study. Bmj. 2020;368:m331. doi: 10.1136/bmj.m331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer M., Méndez-García C., Rojo D., Barbas C., Moya A. Antibiotic use and microbiome function. Biochem. Pharmacol. 2017;134:114–126. doi: 10.1016/j.bcp.2016.09.007. [DOI] [PubMed] [Google Scholar]
- Fox J.W., Sanford . third ed. 2019. An R Companion to Applied Regression'. [Google Scholar]
- Fumagalli F., Cattaneo A., Caffino L., Ibba M., Racagni G., Carboni E., Gennarelli M., Riva M.A. Sub-chronic exposure to atomoxetine up-regulates BDNF expression and signalling in the brain of adolescent spontaneously hypertensive rats: comparison with methylphenidate. Pharmacol. Res. 2010;62:523–529. doi: 10.1016/j.phrs.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Furman D., Campisi J., Verdin E., Carrera-Bastos P., Targ S., Franceschi C., Ferrucci L., Gilroy D.W., Fasano A., Miller G.W., Miller A.H., Mantovani A., Weyand C.M., Barzilai N., Goronzy J.J., Rando T.A., Effros R.B., Lucia A., Kleinstreuer N., Slavich G.M. Chronic inflammation in the etiology of disease across the life span. Nature medicine. 2019;25:1822–1832. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futamura T., Kakita A., Tohmi M., Sotoyama H., Takahashi H., Nawa H. Neonatal perturbation of neurotrophic signaling results in abnormal sensorimotor gating and social interaction in adults: implication for epidermal growth factor in cognitive development. Mol. Psychiatr. 2003;8:19–29. doi: 10.1038/sj.mp.4001138. [DOI] [PubMed] [Google Scholar]
- Gumus C., Yazici I.P., Yazici K.U., Ustundag B. Increased serum brain-derived neurotrophic factor, nerve growth factor, glial-derived neurotrophic factor and galanin levels in children with attention deficit hyperactivity disorder, and the effect of 10 Weeks methylphenidate treatment. Clin Psychopharmacol Neurosci. 2022;20:635–648. doi: 10.9758/cpn.2022.20.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley W. 2016. ggplot2: Elegant Graphics for Data Analysis. [Google Scholar]
- Hamad A.F., Alessi-Severini S., Mahmud S.M., Brownell M., Kuo I.F. Antibiotic exposure in the first year of life and the risk of attention-deficit/hyperactivity disorder: a population-based cohort study. Am. J. Epidemiol. 2019;188:1923–1931. doi: 10.1093/aje/kwz178. [DOI] [PubMed] [Google Scholar]
- Han V.X., Patel S., Jones H.F., Dale R.C. Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat. Rev. Neurol. 2021;17:564–579. doi: 10.1038/s41582-021-00530-8. [DOI] [PubMed] [Google Scholar]
- Han V.X., Patel S., Jones H.F., Nielsen T.C., Mohammad S.S., Hofer M.J., Gold W., Brilot F., Lain S.J., Nassar N., Dale R.C. Maternal acute and chronic inflammation in pregnancy is associated with common neurodevelopmental disorders: a systematic review. Transl. Psychiatry. 2021;11 doi: 10.1038/s41398-021-01198-w. 71-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegvik, T.A., Chen, Q., Kuja-Halkola, R., Klungsøyr, K., Butwicka, A., Lichtenstein, P., Almqvist, C., Faraone, S.V., Haavik, J., Larsson, H., 2021. Familial co-aggregation of attention-deficit/hyperactivity disorder and autoimmune diseases: a cohort study based on Swedish population-wide registers. Int. J. Epidemiol.2022 Jun 13;51(3):898-909. [DOI] [PMC free article] [PubMed]
- Huang J., Zheng L., Su Y., Wang F., Kong H., Chang Y., Xin H. Effects of group B streptococcus infection on vaginal micro-ecology and pregnancy outcomes of pregnant women in late pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021;267:274–279. doi: 10.1016/j.ejogrb.2021.11.419. [DOI] [PubMed] [Google Scholar]
- Jimmy B., Jose J. Patient medication adherence: measures in daily practice. Oman Med. J. 2011;26:155–159. doi: 10.5001/omj.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanarik M., Grimm O., Mota N.R., Reif A., Harro J. ADHD co-morbidities: a review of implication of gene × environment effects with dopamine-related genes. Neurosci. Biobehav. Rev. 2022;139 doi: 10.1016/j.neubiorev.2022.104757. [DOI] [PubMed] [Google Scholar]
- Kiilerich P., Cortes R., Lausten-Thomsen U., Borbye-Lorenzen N.S.K. Authorea.com; 2020. Neonatal Inflammation, Stress and Growth Factors after Vaginal Delivery, Pre-labour, and In-Labour Caesarean Section: a Retrospective Cohort Study. [Google Scholar]
- Kiilerich P., Cortes R., Lausten-Thomsen U., Borbye-Lorenzen N., Holmgaard S., Skogstrand K. Delivery modality affect neonatal levels of inflammation, stress, and growth factors. Front Pediatr. 2021;9 doi: 10.3389/fped.2021.709765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova A.C., Rune H.B. lmerTest package: tests in linear mixed effects models. J. Stat. Software. 2017;82(13):1–26. [Google Scholar]
- Lavebratt C., Yang L.L., Giacobini M., Forsell Y., Schalling M., Partonen T., Gissler M. Early exposure to antibiotic drugs and risk for psychiatric disorders: a population-based study. Transl. Psychiatry. 2019;9:317. doi: 10.1038/s41398-019-0653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth R.V.B., Paul, Herve Maxime, Love Jonathon, Riebl Hannes, Singmann Henrik. 2021. Emmeans: Estimated Marginam Means', Aka Least-Squares Means. R Package Version 1.6.3. [Google Scholar]
- Liew Z., Ritz B., Rebordosa C., Lee P.-C., Olsen J. Acetaminophen use during pregnancy, behavioral problems, and hyperkinetic disorders. JAMA Pediatr. 2014;168:313–320. doi: 10.1001/jamapediatrics.2013.4914. [DOI] [PubMed] [Google Scholar]
- Lin P.Y., Chen Y.L., Hsiao R.C., Chen H.L., Yen C.F. Risks of attention-deficit/hyperactivity disorder, autism spectrum disorder, and intellectual disability in children delivered by caesarean section: a population-based cohort study. Asian J Psychiatr. 2022;80 doi: 10.1016/j.ajp.2022.103334. [DOI] [PubMed] [Google Scholar]
- Lund A.M., Hougaard D.M., Simonsen H., Andresen B.S., Christensen M., Duno M., Skogstrand K., Olsen R.K., Jensen U.G., Cohen A., Larsen N., Saugmann-Jensen P., Gregersen N., Brandt N.J., Christensen E., Skovby F., Norgaard-Pedersen B. Biochemical screening of 504,049 newborns in Denmark, the Faroe Islands and Greenland--experience and development of a routine program for expanded newborn screening. Mol. Genet. Metabol. 2012;107:281–293. doi: 10.1016/j.ymgme.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Lund A., Wibrand F., Skogstrand K., Cohen A., Christensen M., Jäpelt R.B., Dunø M., Skovby F., Nørgaard-Pedersen B., Gregersen N., Andresen B.S., Olsen R.K.J., Hougaard D. Danish expanded newborn screening is a successful preventive public health programme. Dan Med J. 2020;67 [PubMed] [Google Scholar]
- Mann J.R., McDermott S. Are maternal genitourinary infection and pre-eclampsia associated with ADHD in school-aged children? J. Atten. Disord. 2011;15:667–673. doi: 10.1177/1087054710370566. [DOI] [PubMed] [Google Scholar]
- Mattheisen M., Grove J., Als T.D., Martin J., Voloudakis G., Meier S., Demontis D., Bendl J., Walters R., Carey C.E., Rosengren A., Strom N.I., Hauberg M.E., Zeng B., Hoffman G., Zhang W., Bybjerg-Grauholm J., Bækvad-Hansen M., Agerbo E., Cormand B., Nordentoft M., Werge T., Mors O., Hougaard D.M., Buxbaum J.D., Faraone S.V., Franke B., Dalsgaard S., Mortensen P.B., Robinson E.B., Roussos P., Neale B.M., Daly M.J., Børglum A.D. Identification of shared and differentiating genetic architecture for autism spectrum disorder, attention-deficit hyperactivity disorder and case subgroups. Nat. Genet. 2022;54:1470–1478. doi: 10.1038/s41588-022-01171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min X., Li C., Yan Y. Parental age and the risk of ADHD in offspring: a systematic review and meta-analysis. Int. J. Environ. Res. Publ. Health. 2021;18:4939. doi: 10.3390/ijerph18094939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr-Jensen C., Müller Bisgaard C., Boldsen S.K., Steinhausen H.C. Attention-Deficit/hyperactivity disorder in childhood and adolescence and the risk of crime in young adulthood in a Danish nationwide study. J. Am. Acad. Child Adolesc. Psychiatry. 2019;58:443–452. doi: 10.1016/j.jaac.2018.11.016. [DOI] [PubMed] [Google Scholar]
- Ouadih-Moran M., Muñoz-Hoyos A., D'Marco L., Molina-Carballo A., Seiquer I., Checa-Ros A. Is S100B involved in attention-deficit/hyperactivity disorder (ADHD)? Comparisons with controls and changes following a triple therapy containing methylphenidate, melatonin and ω-3 PUFAs. Nutrients. 2023;15 doi: 10.3390/nu15030712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özaslan A., Güney E., Gülbahar Ö., Büyüktaşkin Tunçtürk D., Arslan B., Güveli Bozkurt G.M. Serum heat Shock protein 70 level in children with attention deficiency hyperactivity disorder. Noro Psikiyatr Ars. 2022;59:63–67. doi: 10.29399/npa.27652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H., Poo M.M. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 2013;14:7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- Pingault, J.B., Barkhuizen, W., Wang, B., Hannigan, L.J., Eilertsen, E.M., Corfield, E., Andreassen, O.A., Ask, H., Tesli, M., Askeland, R.B., Davey Smith, G., Stoltenberg, C., Davies, N.M., Reichborn-Kjennerud, T., Ystrom, E., Havdahl, A., 2022. Genetic nurture versus genetic transmission of risk for ADHD traits in the Norwegian mother, father and child cohort study. Mol. Psychiatr.2023 Apr;28(4):1731-1738. [DOI] [PMC free article] [PubMed]
- Rebordosa C., Kogevinas M., Bech B.H., Sørensen H.T., Olsen J. Use of acetaminophen during pregnancy and risk of adverse pregnancy outcomes. Int. J. Epidemiol. 2009;38:706–714. doi: 10.1093/ije/dyp151. [DOI] [PubMed] [Google Scholar]
- Ribasés M., Hervás A., Ramos-Quiroga J.A., Bosch R., Bielsa A., Gastaminza X., Fernández-Anguiano M., Nogueira M., Gómez-Barros N., Valero S., Gratacòs M., Estivill X., Casas M., Cormand B., Bayés M. Association study of 10 genes encoding neurotrophic factors and their receptors in adult and child attention-deficit/hyperactivity disorder. Biol. Psychiatr. 2008;63:935–945. doi: 10.1016/j.biopsych.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Romano R., Bucci C. Role of EGFR in the nervous system. Cells. 2020;9 doi: 10.3390/cells9081887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibinska M., Kapelski P., Dmitrzak-Weglarz M., Lepczynska N., Pawlak J., Twarowska-Hauser J., Szczepankiewicz A., Rajewska-Rager A. Elevated epidermal growth factor (EGF) as candidate biomarker of mood disorders-longitudinal study in adolescent and young adult patients. J. Clin. Med. 2021;10 doi: 10.3390/jcm10184064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skogstrand K., Hagen C.M., Borbye-Lorenzen N., Christiansen M., Bybjerg-Grauholm J., Bækvad-Hansen M., Werge T., Børglum A., Mors O., Nordentoft M., Mortensen P.B., Hougaard D.M. Reduced neonatal brain-derived neurotrophic factor is associated with autism spectrum disorders. Transl. Psychiatry. 2019;9:252. doi: 10.1038/s41398-019-0587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slykerman R.F., Coomarasamy C., Wickens K., Thompson J.M.D., Stanley T.V., Barthow C., Kang J., Crane J., Mitchell E.A. Exposure to antibiotics in the first 24 months of life and neurocognitive outcomes at 11 years of age. Psychopharmacology (Berl) 2019;236:1573–1582. doi: 10.1007/s00213-019-05216-0. [DOI] [PubMed] [Google Scholar]
- Steffensen E.H., Pedersen L.H., Lou S., Vogel I. Impact of a prenatal screening program on the Down syndrome phenotype: an interrupted time series analysis. Acta Obstet. Gynecol. Scand. 2023;102:751–759. doi: 10.1111/aogs.14573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundhedsstyrelsen, Lægemiddelstatistikregisteret (LSR). https://www.esundhed.dk/Registre/Laegemiddelstatistikregisteret.
- Team R.C. R Foundation for Statistical Computing; Vienna: 2021. R: A Language and Environment for Statistical Computing.https://www.R-project.org/ [Google Scholar]
- Teimouri H., Kolomeisky A.B. Theoretical investigation of stochastic clearance of bacteria: first-passage analysis. J R Soc Interface. 2019;16 doi: 10.1098/rsif.2018.0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar A., Cooper M. Attention deficit hyperactivity disorder. Lancet. 2016;387:1240–1250. doi: 10.1016/S0140-6736(15)00238-X. [DOI] [PubMed] [Google Scholar]
- Tonhajzerova I., Ondrejka I., Ferencova N., Bujnakova I., Grendar M., Olexova L.B., Hrtanek I., Visnovcova Z. Alternations in the cardiovascular autonomic regulation and growth factors in autism. Physiol. Res. 2021;70:551–561. doi: 10.33549/physiolres.934662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tostes M.H., Teixeira H.C., Gattaz W.F., Brandão M.A., Raposo N.R. Altered neurotrophin, neuropeptide, cytokines and nitric oxide levels in autism. Pharmacopsychiatry. 2012;45:241–243. doi: 10.1055/s-0032-1301914. [DOI] [PubMed] [Google Scholar]
- Wickham H., Averick M., Bryan J., Chang W., McGowan L., François R., Grolemund G., Hayes A., Henry L., Hester J., Kuhn M., Pedersen T., Miller E., Bache S., Müller K., Ooms J., Robinson D.G., Seidel D.P., Spinu V., Takahashi K., Vaughan D., Wilke C., Woo K.H., Yutani H. Welcome to the tidyverse. J. Open Source Softw. 2019;4:1686. [Google Scholar]
- Yadav S.K., Bhat A.A., Hashem S., Nisar S., Kamal M., Syed N., Temanni M.R., Gupta R.K., Kamran S., Azeem M.W., Srivastava A.K., Bagga P., Chawla S., Reddy R., Frenneaux M.P., Fakhro K., Haris M. Genetic variations influence brain changes in patients with attention-deficit hyperactivity disorder. Transl. Psychiatry. 2021;11:349. doi: 10.1038/s41398-021-01473-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh T.C., Bai Y.M., Hsu J.W., Huang K.L., Tsai S.J., Chu H.T., Liang C.S., Chen M.H. Bipolar women's antepartum psychotropic exposure and offspring risk of attention-deficit/hyperactivity disorder and autism spectrum disorder. J. Affect. Disord. 2021;295:1407–1414. doi: 10.1016/j.jad.2021.09.016. [DOI] [PubMed] [Google Scholar]
- Ystrom E., Gustavson K., Brandlistuen R.E., Knudsen G.P., Magnus P., Susser E., Davey Smith G., Stoltenberg C., Surén P., Håberg S.E., Hornig M., Lipkin W.I., Nordeng H., Reichborn-Kjennerud T. Prenatal exposure to acetaminophen and risk of ADHD. Pediatrics. 2017;140 doi: 10.1542/peds.2016-3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurteri N., Şahin İ E., Tufan A.E. Altered serum levels of vascular endothelial growth factor and glial-derived neurotrophic factor but not fibroblast growth factor-2 in treatment-naive children with attention deficit/hyperactivity disorder. Nord. J. Psychiatr. 2019;73:302–307. doi: 10.1080/08039488.2019.1625437. [DOI] [PubMed] [Google Scholar]
- Zhang X., Xiao W., Chen K., Zhao Y., Ye F., Tang X., Du X. Decreased serum EGF in first-episode and chronic schizophrenia patients: negative correlation with psychopathology. Sci. Rep. 2020;10:6506. doi: 10.1038/s41598-020-63544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that has been used is confidential.