Abstract
Objective
In recent years, the prevalence of diabetic nephropathy (DN) has increased significantly. An increasing number of studies have shown that lymphocyte-associated inflammatory responses play a role in DN. This study aims to investigate the relationship between lymphocytes and DN in patients with autoimmune diabetes.
Methods
The clinical data of 226 patients with Type 1 diabetes (T1D) and 79 patients with latent autoimmune diabetes in adults (LADA) were retrospectively studied and stratified according to the urinary albumin to creatinine ratio (ACR). Risk factors associated with DN were analyzed using correlation analysis and logistic regression.
Results
In T1D and LADA patients, systolic blood pressure (SBP), uric acid duration, and diabetes duration in patients with normoalbuminuria were lower or shorter than those in patients with macroalbuminuria (P<0.05). The lymphocyte count of T1D patients was significantly higher than that in LADA patients (P<0.05), while the neutrophil to lymphocyte ratio (NLR) of T1D patients was significantly lower than that in LADA patients (P<0.05). The lymphocyte count in the T1D patients with normoalbuminuria was lower than that those with macroalbuminuria (P<0.05). The NLR was lower in the T1D patients with macroalbuminuria than those with microalbuminuria and normoproteinuria (all P<0.01). Based on logistic regression analysis, lymphocytes were independently associated with DN in T1D after adjusting for various known risk factors such as course of disease, age, gender, dyslipidemia, hypertension, and smoking status. Analysis of the receiver operating characteristic curve of subjects predicting lymphocytes in normoalbuminuria showed that the area under the curve was 0.601 (95% CI 0.510 to 0.693, P=0.039), and when the cutoff value of lymphocytes was 2.332, the sensitivity was 37.0%, and the specificity was 82.5%.
Conclusion
Lymphocyte counts in autoimmune diabetic patients are closely associated with DN, suggesting that lymphocyte-mediated inflammation may be involved in the pathogenesis of DN in autoimmune diabetic patients. This study provides a possible perspective for using lymphocytes as a potential biomarker for the early identification of individuals at risk for DN and potential therapeutic targets for DN.
Keywords: autoimmune diabetes mellitus, diabetic nephropathy, lymphocyte, biomarkers
Abstract
目的
近年来,随着糖尿病患病率的逐年增加,糖尿病肾病(diabetic nephropathy,DN)的患病率也在显著增加。糖尿病的发生机制复杂,淋巴细胞相关的炎症反应在DN中起作用。本研究旨在探讨自身免疫性糖尿病患者中淋巴细胞与DN的关系,为DN的预防和治疗探索新的方向。
方法
回顾性研究226例1型糖尿病(Type 1 diabetes,T1D)患者和79例成人隐匿性自身免疫性糖尿病(latent autoimmune diabetes in adults,LADA)患者的临床资料,并根据尿白蛋白与肌酐比值(albumin to creatinine ratio,ACR)进行分层分析。采用相关性分析、logistic回归等方法分析与DN相关的危险因素。
结果
在T1D和LADA患者中,与大量白蛋白尿患者比较,正常白蛋白尿患者的收缩压低、尿酸和糖尿病持续时间短(均P<0.05)。T1D患者的淋巴细胞计数较LADA患者明显增多(P<0.05),而T1D患者的淋巴细胞比值(neutrophil to lymphocyte ratio,NLR)显著低于LADA患者(P<0.05)。T1D患者中正常白蛋白尿组的淋巴细胞计数低于大量白蛋白尿组(P<0.05),而TID患者中大量白蛋白尿组的NLR显著低于微量白蛋白尿组和正常白蛋白尿组 (P<0.01)。运用斯谛回归分析,在调整了病程、年龄、性别、血脂异常、高血压、吸烟状况等各种已知的危险因素后,淋巴细胞与T1D中的DN独立相关。预测正常白蛋白尿的淋巴细胞的受试者操作特征曲线分析发现,曲线下面积为0.601(95% CI 0.510~0.693,P=0.039),且淋巴细胞截断值为2.332时,敏感度为37.0%,特异度为82.5%。
结论
自身免疫性糖尿病患者的淋巴细胞计数与DN密切相关,提示淋巴细胞介导的炎症可能参与了自身免疫性糖尿病患者DN的发病机制,并为使用淋巴细胞作为一种潜在的生物标志物来早期识别DN高危个体和DN的潜在治疗靶点提供了一个视角。
Keywords: 自身免疫性糖尿病, 糖尿病肾病, 淋巴细胞, 标志物
Diabetes mellitus (DM) is an endocrine and metabolic disease that has serious implications for human health. In recent years, the morbidity and mortality rates of diabetes have continued to rise at an alarming rate, and it is expected that by 2030, the number of people with diabetes will reach approximately 439 million worldwide[1]. The global prevalence of diabetes continues to rise. Diabetes and diabetes-related complications significantly affect patients’ quality of life and shorten their life expectancy compared to non-diabetic populations[2]. Latent autoimmune diabetes in adults (LADA) is a subtype of diabetes that belongs to autoimmune Type 1 diabetes (T1D). LADA refers to a type of diabetes that is characterized by slowly progressing autoimmune damage to islet β cells in the early clinical stages without the need for insulin therapy[3]. Diabetic nephropathy (DN) is a major chronic microvascular complication of diabetes and a major cause of end-stage renal disease (ESRD). DN is a global challenge and a major social and economic burden[4]. While the pathogenesis of DN is complex, poor glycemic control, hyperlipidemia, smoking, oxidative stress, accumulation of late glycation end products, and environmental, genetic and epigenetic factors play an important role in it[5].
DN is the leading cause of end-stage renal disease, accounting for approximately 50% of morbidity cases[6]. The main pathological features of DN include glomerulosclerosis, tubulointerstitial fibrosis, and renal vasculopathy. Its causative factors and pathogenesis are complex, mainly including metabolic disorders, genetic factors, oxidative stress, and inflammatory mechanisms[7].Diabetes mellitus (DM) is a chronic systemic disease. A large body of literature suggests that chronic inflammation is associated with insulin resistance and the development of DM. In addition, accumulating evidence suggests that the inflammatory response also plays a key role in the development and progression of DN[8].
Inflammation certainly plays an important role in DN progression, and the measurement of inflammatory markers is of great value in the diagnosis and treatment of DN[9].Lymphocytes are the main immune cell type associated with the inflammatory response, and an increasing number of studies suggest that their mediated inflammation plays a role in DN. During the inflammatory response in the kidney, leukocytes join the kidney under the action of chemokines and immune cell infiltration, especially cytotoxic CD8 lymphocyte infiltration, which plays an important role in the development of DN[10]. The results of available studies[11] have shown that there is a significant correlation between neutrophil to lymphocyte ratio (NLR) and DN.
In contrast, the application of many inflammatory markers is limited in daily clinical practice due to their cost and technical difficulties in measurement. Therefore, this study aims to evaluate the relationship between lymphocytes and DN and whether lymphocyte can be used as a predictor and prognostic risk marker for DN to reduce the occurrence of complications in patients with uncontrolled diabetes and to help physicians focus on patients early, and to help patients obtain the early intervention and then reduce the rate of progression to end-stage renal disease.
1. Subjects and methods
1.1. Subjects
The clinical data of 305 patients with autoimmune diabetes mellitus, including 226 patients with T1D and 79 patients with LADA, who attended the Department of Endocrinology and Metabolism at the Second Xiangya Hospital of Central South University between December 2012 and May 2021 were retrospectively studied. Demographic, clinical and laboratory data were recorded from the patient file records, including age, sex, smoking, weight, height, diabetes duration, glycosylated hemoglobin (HbA1c), blood pressure, serum urea, serum creatinine, serum albumin, urinary albumin to creatinine ratio (ACR), glomerular filtration rate (GFR), C-reactive protein (CRP), and complete blood count (CBC) [including total white blood cell count (WBC), lymphocyte count, NLR, monocyte count, total red blood cell count (RBC), hemoglobin (Hb), and platelet count (PLT)]. Body mass index (BMI) was calculated using the following formula: BMI=weight/height2. GFR was calculated according to the Modification of Diet in Renal Disease (MDRD) formula. The NLR was assessed by dividing the absolute neutrophil count by the absolute lymphocyte count. Smoking was defined as current smokers or former smokers. All abnormal or atypical WBCs and lymphocyte specimens were excluded.
Exclusion criteria: Type 2 diabetes, acute or chronic infection or inflammation, cancer, drugs (e.g. steroids) affecting WBC, hypertension, heart disease, systemic disease, any other known kidney disease, blood disorders, diseases affecting urinary protein excretion, dehydration, low GFR without microalbuminuria, and alcohol.
1.2. Methods
DN is typically characterized by the presence of proteinuria followed by a progressive decline in renal function. Therefore testing with fresh morning urine specimens obtained, the urinary ACR was calculated by dividing microalbumin by creatinine. According to the ACR, it was classified as no proteinuria or normoalbuminuria (ACR<30 mg/g), microalbuminuria or early DN (ACR: 30 to 300 mg/g), and macroalbuminuria or overt DN (ACR >300 mg/g).
The study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the Human Ethics Committee of the Second Xiangya Hospital of Central South University (NO: 2019-Research-40). Since this was a retrospective study, the requirement to obtain informed consent was waived.
1.3. Statistical analysis
All statistical analyses were performed using IBM Statistical Products, Services Solutions (SPSS) 26 and GraphPad 9.0 for statistical data (Armonk, N.Y., USA).The Kolmogorov-Smirnov test was first used to test the normality of the data. Normally distributed data are expressed as the mean±standard deviation, and one-way analysis of variance (ANOVA) tests were performed for comparisons between groups. Data with non-normal distributions were expressed as the the median (P 25, P 75) and log-transformed prior to analysis. For continuous data with non-normal distribution, values between groups were compared using the Kruskal-Wallis test. Correlations were assessed using the Pearson test. Binary logistic regression analysis was used to identify the risk factors for DN. The results were expressed in terms of significance (P), odds ratios (OR) and 95% confidence intervals (CI). In additions, lymphocyte prediction without albuminuria was performed in subjects with receiver operating characteristic curve analysis. P<0.05 was considered statistically significant.
2. Results
2.1. Clinical data analysis
A total of 226 patients with T1D and 79 patients with LADA were recruited for this study. Patients were assigned into 3 groups based on the ACR: normo-albuminuria, microalbuminuria, and macroalbuminuria. Table 1 summarizes the results of the one-way ANOVA regarding the clinical and laboratory characteristics of the study groups.
Table 1.
Characteristics of the study group of diabetic patients
| Variables | LADA | P | ||
|---|---|---|---|---|
| Normoalbuminuria | Microalbuminuria | Macroalbuminuria | ||
| Number | 61 | 10 | 8 | |
| Gender/No. | 35/26 | 7/3 | 4/4 | 0.667 |
| Age/year | 52.46±10.48 | 58.90±14.14 | 57.00±9.26 | 0.156 |
| Diabetes duration/year | 5.42±5.45 | 10.40±5.99* | 12.56±6.54* | 0.001 |
| Smoker/No. | 15 | 5 | 3 | 0.231 |
| WHR | 0.89±0.06 | 0.91±0.05 | 0.88±0.06 | 0.530 |
| BMI/(kg·m-2) | 21.59±2.79 | 22.86±3.62 | 21.96±2.42 | 0.429 |
| HbA1c/% | 9.45±2.35 | 9.69±2.53 | 9.89±2.17 | 0.864 |
| SBP/mmHg | 122.72±17.23 | 133.90±22.83 | 137.75±24.26* | 0.039 |
| DBP/mmHg | 77.00±9.82 | 79.90±15.50 | 85.13±10.74 | 0.121 |
| FCP/(pmol·L-1) | 90.00(30.85, 163.75) | 136.75(37.53, 224.88) | 70.55(9.48, 188.48) | 0.771 |
| PCP/(pmol·L-1) | 179.90(56.50, 312.78) | 251.65(76.80, 593.80) | 202.30(13.80, 255.23) | 0.778 |
| FBG/(mmol·L-1) | 8.31±4.31 | 8.07±3.55 | 6.56±3.73 | 0.579 |
| PBG/(mmol·L-1) | 13.50±6.18 | 12.66±4.92 | 11.81±5.81 | 0.746 |
| TBIL/(μmol·L-1) | 12.14±4.40 | 10.78±5.84 | 7.92±2.74 | 0.081 |
| DBIL/(μmol·L-1) | 3.88±1.44 | 3.28±1.65 | 2.64±1.18 | 0.073 |
| LDL-c/(mmol·L-1) | 2.43±0.64 | 2.38±0.78 | 2.37±0.94 | 0.965 |
| HDL-c/(mmol·L-1) | 1.29 | 1.34 | 1.18 | 0.241 |
| TC/(mmol·L-1) | 4.19±0.72 | 4.07±0.82 | 3.94±0.98 | 0.680 |
| TG/(mmol·L-1) | 0.93(0.68, 1.17) | 1.31(0.86, 1.70) | 1.3(0.91, 1.54) | 0.068 |
| Albumin/(g·L-1) | 37.44±2.96 | 36.38±2.91 | 33.21±3.92* | 0.003 |
| eGFR/[mL·(min-1·1.73 m-2)] | 102.23(80.56, 124.21) | 72.7(47.20, 144.44) | 59.29(42.45, 96.85)* | 0.022 |
| UA/(μmol·L-1) | 230.89±53.26 | 303.57±63.54* | 351.35±92.66* | <0.001 |
Table 1.
| Variables | T1D | P | ||
|---|---|---|---|---|
| Normoalbuminuria | Microalbuminuria | Macroalbuminuria | ||
| Number | 204 | 10 | 12 | |
| Gender/No. | 100/104 | 3/7 | 5/7 | 0.456 |
| Age/year | 21.67±14.92 | 28.50±28.66 | 33.50±17.06* | 0.017 |
| Diabetes duration/year | 5.13±5.05 | 7.27±7.59 | 11.03±5.12* | <0.001 |
| Smoker/No. | 72 | 4 | 5 | 0.870 |
| WHR | 0.84±0.07 | 0.83±0.08 | 0.87±0.07 | 0.296 |
| BMI/(kg·m-2) | 18.95±3.20 | 19.00±2.73 | 21.01±2.31* | 0.110 |
| HbA1c/% | 8.37±2.19 | 7.63±1.85 | 7.48±1.40 | 0.228 |
| SBP/mmHg | 112.94±15.53 | 114.20±20.91 | 139.42±34.21 | <0.001 |
| DBP/mmHg | 70.56±12.69 | 75.00±16.21 | 81.67±16.46* | 0.012 |
| FCP/(pmol·L-1) | 25.05(5.50, 89.58) | 10.05(5.50, 44.18) | 5.5(5.50, 157.50) | 0.405 |
| PCP/(pmol·L-1) | 41.00(5.70, 215.08) | 30.60(5.85, 86.50) | 17.10(5.50, 126.30) | 0.512 |
| FBG/(mmol·L-1) | 9.17±4.56 | 7.60±3.08 | 10.63±4.78 | 0.315 |
| PBG/(mmol·L-1) | 16.01±7.17 | 14.88±6.84 | 13.27±6.56 | 0.409 |
| TBIL/(μmol·L-1) | 12.26±5.35 | 11.68±3.62 | 9.46±4.00 | 0.194 |
| DBIL/(μmol·L-1) | 3.92±1.94 | 3.87±1.08 | 3.13±1.66 | 0.382 |
| LDL-c/(mmol·L-1) | 2.42±0.78 | 2.24±0.83 | 2.72±1.04 | 0.354 |
| HDL-c/(mmol·L-1) | 1.45 | 1.55 | 1.48 | 0.574 |
| TC/(mmol·L-1) | 4.28±0.91 | 4.15±0.83 | 4.63±1.26 | 0.416 |
| TG/(mmol·L-1) | 0.74(0.58, 1.11) | 0.86(0.49, 1.23) | 0.84(0.65, 1.25) | 0.422 |
| Albumin/(g·L-1) | 42.37±4.08 | 41.72±3.65 | 36.02±7.87* | <0.001 |
| eGFR/[mL·(min-1·1.73 m-2)] | 139.15(111.45, 168.47) | 110.36(72.18, 135.87)† | 57.24(50.42, 116.46)* | 0.001 |
| UA/(μmol·L-1) | 255.92±69.79 | 289.92±78.39 | 312.02±95.63* | 0.018 |
Data are expressed as the mean±standard deviation and median (P 25, P 75) for normally distributed data and non-normally distributed data, respectively. *P<0.05 vs the normoalbuminuria group; †P<0.05 vs the macroalbuminuria group. 1 mmHg=0.133 kPa. LADA: Latent autoimmune diabetes in adults; T1D: Type 1 diabetes; WHR: Waist to hip ratio; BMI: Body mass index; HbA1c: Glycosylated hemoglobin; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; FCP: Fasting C-peptide; PCP: 2-h Postprandial C-peptide; FBG: Fasting blood glucose; PBG: 2-h Postprandial blood glucose; TBIL: Total bilirubin; DBIL: Direct bilirubin; LDL-c: Low-density lipoprotein cholesterol; HDL-c: High-density lipoprotein cholesterol; TC: Total cholesterol; TG: Triglycerides; eGFR: Estimated glomerular filtration rate; UA: Uric acid.
continued
As shown in Table 1, in patients with T1D or LADA, systolic blood pressure (SBP), duration of uric acid, and diabetes duration were lower in patients with normoalbuminuria than those in patients with macroalbuminuria (all P<0.05). There was an increasing trend in age and diastolic blood pressure (DBP) in the 3 proteinuria groups in the T1D patients (P<0.05), but no significant differences were observed in LADA patients (P>0.05). Age, SBP, DBP, triglyceride (TG), glycated hemoglobin, fasting C-peptide (FCP), 2-h postprandial C-peptide (PCP), and BMI were lower in the T1D patients than those in the LADA patients (P<0.05). However, eGFR and high-density lipoprotein cholesterol (HDL-c) levels were higher in T1D patients than in LADA patients (all P<0.05).
2.2. Changes of lymphocyte count in autoimmune diabetes
As shown in Figure 1, WBC count, lymphocyte count, and NLR were compared between the T1D patients and the LADA patients. For the WBC count in all patients, there was no statistically significant difference between T1D and LADA (P>0.05). In all patients, the median lymphocyte count was higher in the T1D patients than in the LADA patients (P<0.01). In all patients, NLR was significantly lower in the T1D patients than in the LADA patients (P<0.01).
Figure 1. NLR (A), lymphocyte count (B), and white blood cell count (C) in patients with autoimmune diabetes mellitus.
Horizontal lines indicate median and quartiles, respectively; **P<0.01. NLR: Neutrophil to lymphocyte ratio; T1D: Type 1 diabetes; LADA: Latent autoimmune diabetes in adults.
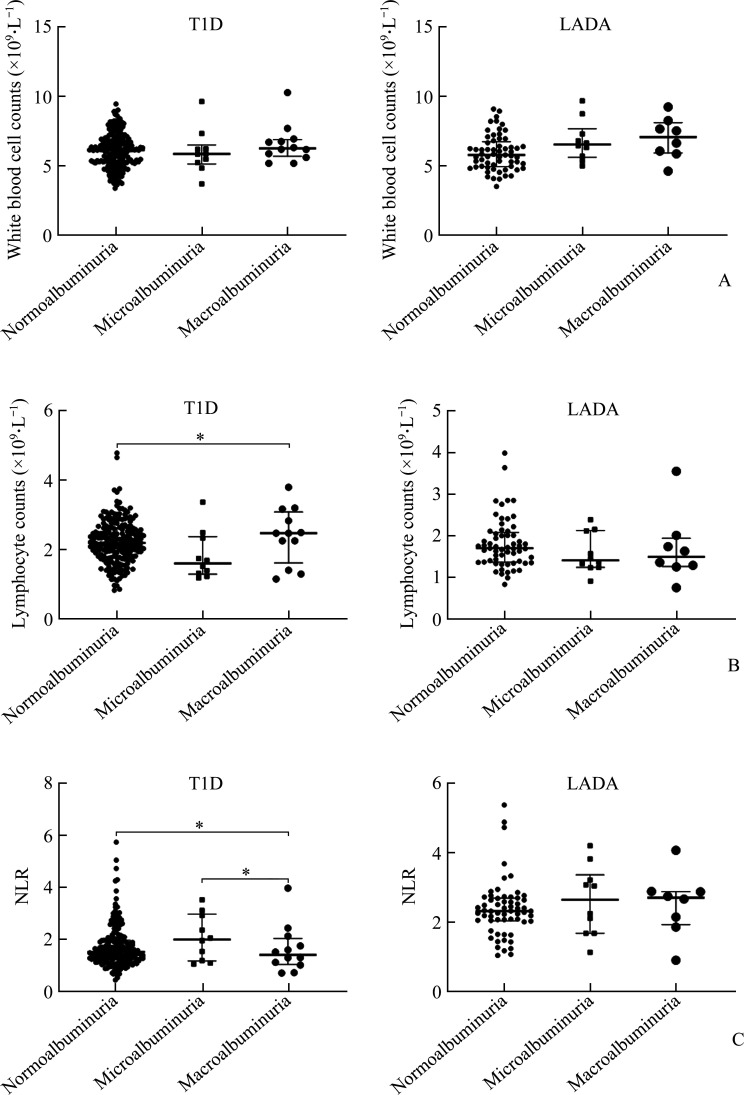
As shown in Figure 2, WBC, lymphocyte count, and NLR were analyzed and compared among T1D and LADA patients with normoalbuminuria, micro-albuminuria, and macroalbuminuria. The WBC increased gradually in the LADA patients with normoalbuminuria, microalbuminuria, and macro-albuminuria. The lymphocyte count in the T1D patients with normoalbuminuria was lower than those with the macroalbuminuria (P=0.023). The NLR was lower in the T1D patients with macroalbuminuria than those with microalbuminuria and normoproteinuria (all P<0.01), there was no significant difference in lymphocyte and NLR among the LADA patients with normoalbuminuria, microalbuminuria, and macroalbuminuria (all P>0.05).
Figure 2. Comparative plots of white blood cell count (A), lymphocyte count (B), and NLR (C) in different proteinuria groups of autoimmune diabetes mellitus.
*P<0.05. LADA: Latent autoimmune diabetes in adults; T1D: Type 1 diabetes; NLR: Neutrophil to lymphocyte ratio.
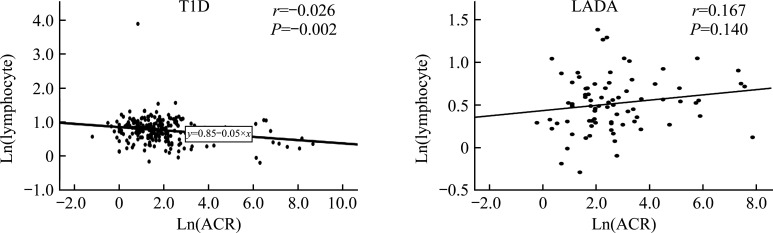
As shown in Figure 3, ACR was negatively correlated with lymphocytes in T1D (P<0.01), while there was no significant correlation in LADA patients.
Figure 3. Correlation between ACR and lymphocytes.
Ln: Natural logarithm; ACR: Albumin to creatinine ratio; LADA: Latent autoimmune diabetes in adults; T1D: Type 1 diabetes.
As shown in Table 2, in T1D, WBC was positively correlated with low-density lipoprotein cholesterol (LDL-c) (P<0.01) and negatively correlated with high-density lipoprotein cholesterol (HDL-c) (P<0.05). Lymphocyte count was positively correlated with total bilirubin (TBIL) and direct bilirubin (DBIL) (all P<0.05). The NLR was negatively correlated with eGFR (P<0.01) and positively correlated with diabetes duration, BMI, age, creatinine, SBP, and DBP (all P<0.01). In LADA, WBC were positively correlated with BMI, DBP, SBP, smoking, eGFR, and uric acid (all P<0.05). Lymphocyte count was positively correlated with SBP, DBP, and eGFR (all P<0.05), but negatively correlated with age (P<0.05). The NLR was positively correlated with age, diabetes duration, and creatinine (all P<0.05), and negatively correlated with eGFR (P<0.05).
Table 2.
Correlation of WBC, lymphocyte count, NLR with metabolic variables in different diabetes groups
| Variables | r T1D | r LADA | ||||
|---|---|---|---|---|---|---|
| WBC | Lymphocytes | NLR | WBC | Lymphocytes | NLR | |
| Diabetes duration | 0.097 | -0.081 | 0.322** | 0.153 | -0.034 | 0.235* |
| BMI | 0.097 | -0.057 | 0.332** | 0.227* | 0.147 | -0.023 |
| HbA1C | 0.104 | -0.032 | -0.030 | -0.100 | -0.054 | 0.028 |
| Age | 0.023 | -0.125 | 0.482** | -0.060 | -0.257* | 0.362** |
| WHR | 0.091 | -0.095 | 0.064 | 0.021 | -0.052 | 0.100 |
| Cr | 0.001 | -0.02 | 0.392** | 0.112 | -0.094 | 0.234* |
| TBIL | 0.003 | 0.161* | -0.056 | -0.216 | -0.125 | -0.047 |
| DBIL | -0.017 | 0.156* | -0.042 | -0.185 | -0.103 | -0.070 |
| FBG | 0.058 | 0.102 | 0.078 | 0.057 | 0.056 | 0.031 |
| PBG | -0.011 | 0.025 | -0.119 | 0.010 | -0.007 | 0.056 |
| FCP | 0.053 | 0.049 | 0.07 | 0.168 | 0.032 | 0.093 |
| PCP | 0.060 | 0.054 | -0.077 | 0.149 | 0.031 | 0.066 |
| SBP | 0.007 | -0.055 | 0.406** | 0.241* | 0.229* | -0.041 |
| DBP | -0.002 | -0.058 | 0.294** | 0.227* | 0.332** | -0.213 |
| TG | 0.114 | 0.023 | 0.052 | 0.187 | 0.084 | 0.081 |
| LDL-c | 0.190** | -0.052 | 0.076 | 0.207 | 0.081 | 0.079 |
| HDL-c | -0.143* | -0.040 | -0.145 | 0.048 | -0.174 | 0.024 |
| Gender | 0.012 | -0.087 | 0.111 | -0.126 | -0.009 | -0.139 |
| Smoking | -0.001 | -0.057 | 0.018 | 0.245* | 0.197 | -0.013 |
| eGFR | 0.046 | -0.011 | -0.183** | 0.395* | 0.225* | -0.268* |
| Albumin | -0.055 | 0.124 | -0.424 | -0.075 | 0.067 | -0.208 |
| UA | 0.020 | -0.056 | 0.051 | 0.337** | 0.091 | 0.141 |
*P<0.05, **P<0.01. LADA: Latent autoimmune diabetes in adults; T1D: Type 1 diabetes; WBC: White blood cell count; NLR: Neutrophil to lymphocyte ratio; BMI: Body mass index; HbA1c: Glycosylated hemoglobin; WHR: Waist to hip ratio; Cr: Creatinine; TBIL: Total bilirubin; DBIL: Direct bilirubin; FBG: Fasting blood glucose; PBG: 2-h Postprandial glucose; FCP: Fasting C-peptide; PCP: 2-h Postprandial C-peptide; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; TG: Triglycerides; LDL-c: Low-density lipoprotein cholesterol; HDL-c: High-density lipoprotein cholesterol; eGFR: Estimated glomerular filtration rate; UA: Uric acid.
As shown in Table 3, we first performed a univariate analysis with logistic regression analysis using DN staging with age, gender, diabetes duration, smoking, BMI, hypertension, dyslipidemia, FBG, HbA1c, FCP, and lymphocyte count as independent variables. The results showed that age, BMI, HbA1c, FCP, hpertension, and lymphocyte count were significantly associated with DN (all P<0.001) and were independent risk factors for DN. A further multiviriate analysis showed that age, BMI, HbA1c, FCP, hpertension, and lymphocyte count were all risk factors for DN (OR>1).
Table 3.
Logistic regression analysis of risk factors for diabetic nephropathy
| Variables | Univariate | Multivariate | 95% CI | ||
|---|---|---|---|---|---|
| P | OR | P | LCI | UCI | |
| Age | <0.001 | 1.124 | <0.001 | 1.088 | 1.162 |
| Gender | 0.111 | ||||
| Diabetes duration | 0.098 | ||||
| BMI | <0.001 | 1.148 | 0.066 | 0.991 | 1.330 |
| HbA1c | <0.001 | 1.138 | 0.191 | 0.937 | 1.381 |
| FBG | 0.077 | ||||
| FCP | <0.001 | 1.006 | 0.003 | 1.002 | 1.010 |
| Hypertension | 0.005 | 2.648 | 0.087 | 0.870 | 8.063 |
| Dyslipidemia | 0.391 | ||||
| Lymphocytes | <0.001 | 1.002 | 0.991 | 0.684 | 1.469 |
| Smoking | 0.279 | ||||
BMI: Body mass index; HbA1c: Glycosylated hemoglobin; FBG: Fasting blood glucose; FCP: Fasting C-peptide; LCI: Lower confidence limit; UCI: Upper confidence limit.
As shown in Figure 4, the analysis of the receiver operating characteristic curve of lymphocytes predicting subjects without albuminuria revealed that the area under the curve for lymphocytes was 0.601 (95% CI 0.510 to 0.693, P=0.039). The lymphocyte cutoff value of 2.332 had 37.0% sensitivity and 82.5% specificity, which indicates sufficient accuracy.
Figure 4. Receiver operating characteristic (ROC) curve analysis for predicting lymphocytes in normal proteinuria.
3. Discussion
LADA, a disease with a phenotype similar to type 2 diabetes (T2D), but with slow destruction of pancreatic β cells, has been recognized by the American Diabetes Association as a form of T1D in the 2022 classification. LADA accounts for 2% to 12% of all diabetic patients, affecting more than 10 million individuals in China. Multicenter studies[12-13] reported that 4% to 14% of patients initially diagnosed with T2D are further diagnosed as LADA based on autoantibody tests. As an autoimmune diabetes, LADA patients exhibit a mild autoimmune process,and β cell function declines more slowly. After onset, it tends not to require insulin therapy for at least more than 6 months in LADA patients. Therefore, there is a relatively long period before the patients develop pancreatic β cell failure[14].
The main objective of this study was to investigate and evaluate the predictive value of lymphocyte count in DN. The results showed that lymphocyte count were strongly and negatively associated with DN under normal conditions and when conventional risk factors were controlled.
The pathogenesis of DN is currently complex and is a serious complication in patients with autoimmune diabetes mellitus. The diagnosis of DN is traditionally based on microalbuminuria. Poor glycemic control, dyslipidemia, smoking, advanced glycosylation end products, and environmental and genetic cues play important role in the development of DN. Several studies[15-17] have linked DN to chronic inflammation, as various inflammatory molecules (e.g. adipokines, chemokines, adhesion molecules, and cytokines) may contribute to the development of DN. The application of many current inflammatory markers is limited in daily clinical practice due to their cost and technical difficulties of measurement. Notably, the NLR with lymphocytes is an easily measurable and inexpensive laboratory indicator calculated by routine analysis of leukocyte characteristics[18]. Lymphocytes, as one of the most important immune cell types associated with inflammation in the circulatory system, should also be involved in the pathogenesis of DN. In addition, hypertension also contributes to the onset and development of DN. Our result also revealed the elevated blood pressure in DN patients. Hyperglycemia is an important pathogenic mechanism leading to DN. Inflammatory factors can stimulate the release of vascular endothelial factor, damage glomerular endothelial cells, and enhance the adhesion and infiltration of monocytes to the vascular endothelium, resulting in proliferation of glomerular thylakoid cells and increased permeability of glomerular endothelial cells[19].
T1D is thought to be caused by autoimmune mechanisms mediating the destruction of insulin-producing β cells in the pancreatic islets. The classical view is that autoreactive T cells mistakenly destroy healthy (“innocent”) β cells[20]. T1D is thought to have a multifactorial pathogenesis in which both genetic and environmental factors play an important role. From a pathogenic perspective, the disease is the result of a breakdown in immune regulation, and dysregulated thymic and peripheral events subsequently leading to islet inflammation, usually characterized by adaptive and innate infiltrative effects. T and B lymphocytes are present and cytotoxic CD8+ T cells are the main population of β cells that can be targeted to express high levels of HLA class I molecules. In the autoimmune process, T helper (Th) cells contribute to autoreactive B cells that produce autoantibodies. Several studies[21-23] have demonstrated the cytolytic activity of natural killer cells on islet β cells and their role in disease development. T1D is orchestrated by islet-specific CD4+ and CD8+ T cells as well as B cells and involves the precise targeting and elimination of insulin-producing pancreatic β cells. CD8+ T cells are thought to directly target and kill β cells, while CD4+ T cells are thought to promote β cell death via cytokine secretion and help CD8+ T and B cells[24-25]. It has been found that T lymphocyte exosomes trigger apoptosis and the expression of genes involved in chemokine signaling, including Ccl2, Ccl7, and Cxcl10, only in β cells[25]. Induction of these genes may promote recruitment of immune cells and exacerbate β cell death during autoimmune attacks[24]. There are many pathways and mediators involved in the development and progression of DN, including oxidative stress, angiotensin II (Ang II), and inflammatory processes that are thought to play an important role[26]. Among these, inflammation, accompanied by the expression of inflammatory factors such as cytokines and chemokines that attract and activate immune cells, including potentially self-reactive T cells, such as CXCL10, appears to play a key role in the pathogenesis of T1D[27]. In addition, renal infiltration of lymphocytes has been observed in renal biopsies of patients with DN[28].
This study provides clinical evidence on the relationship between lymphocyte count and DN in patients with T1D and LADA. We found that lymphocyte count in T1D were higher than those in patients with LADA. Lymphocyte counts were higher in the T1D patients with macroproteinuria than those with normoproteinuria, suggesting that lymphocyte count is more accurate as an early predictive marker for autoimmune DN. Lymphocyte count in T1D patients were negatively correlated with ACR, consistent with the findings that low lymphocyte count in serum have been shown to be associated with DN[29]. A negative correlation with lymphocyte count was not found in LADA. This result may be related to the influence of the small specimens from the LADA patients, and may also be due to the fact that the degree of insulin resistance in LADA is similar to that of T2D and is often accompanied by metabolic syndrome, which is influenced by factors of long-term aging and chronic kidney injury, and the influence of inflammation on LADA is easily confused[30]. Both correlation and logistic regression analyses showed that lymphocyte count was an independent risk factor for DN in T1D and LADA with a negative correlation. The analysis of the receiver operating characteristic curve for predicting lymphocytes without albuminuria revealed that the area under the curve for lymphocytes was 0.601 and the cutoff value of 2.332 for lymphocytes had a sensitivity of 37.0% and a specificity of 82.5%, which indicates sufficient accuracy.
In addition, lymphocytes and NLR are influenced by genetic and non-genetic factors (gender, age, seasonal conditions, lifestyle and disease). Therefore, there are some limitations of this study, namely the small number of patients, the retrospective design, the fact that changes in other inflammatory markers were not assessed in this study and certain data were not available, such as chemokines, cytokines, lymphocyte taxa.
These findings support the role of lymphocytes in pathogenesis as a predictor of DN and as a prognostic risk marker. Lymphocytes are easy to calculate in the laboratory. Lymphocyte count tests are simple, can be performed routinely, and are cost effective.
Contributions: XIANG Zhongyuan Collected data, reviewed and revised the manuscript; LIU Wen’en Conceptualized and designed the study, critically reviewed and revised the manuscript; FU Sanya Collected data, conducted the statistical analysis, drafted the manuscript; HU Jinyi Conceptualized and designed the study, collected clinical information, critically reviewed and revised the manuscript. YANG Yanyi Reviewed and revised the manuscript, edited and submitted the manuscript. All authors have approved the final version of this manuscript.
Funding Statement
This work was supported by the National Natural Science Foundation of China (81870577).
Conflict of Interest
The authors declare that they have no conflicts of interest to disclose.
Footnotes
http://dx.chinadoi.cn/10.11817/j.issn.1672-7347.2023.230110
Note
http://xbyxb.csu.edu.cn/xbwk/fileup/PDF/2023111639.pdf
References
- 1. Zhang J, Liu JH, Qin XS. Advances in early biomarkers of diabetic nephropathy[J]. Rev Assoc Med Bras (1992), 2018, 64(1): 85-92. 10.1590/1806-9282.64.01.85. [DOI] [PubMed] [Google Scholar]
- 2. Li TT, Shen KY, Li JW, et al. Glomerular endothelial cells are the coordinator in the development of diabetic nephropathy[J]. Front Med, 2021, 8: 655639. 10.3389/fmed.2021.655639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hu JY, Zhang R, Zou HL, et al. Latent autoimmune diabetes in adults (LADA): from immunopathogenesis to immunotherapy[J]. Front Endocrinol, 2022, 13: 917169. 10.3389/fendo.2022.917169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sagoo MK, Gnudi L. Diabetic nephropathy: an overview[J]. Methods Mol Biol, 2020, 2067: 3-7. 10.1007/978-1-4939-9841-8_1. [DOI] [PubMed] [Google Scholar]
- 5. Papadopoulou-Marketou N, Paschou SA, Marketos N, et al. Diabetic nephropathy in type 1 diabetes[J]. Minerva Med, 2018, 109(3): 218-228. 10.23736/s0026-4806.17.05496-9. [DOI] [PubMed] [Google Scholar]
- 6. Kim H, Kim M, Lee HY, et al. Role of dendritic cell in diabetic nephropathy[J]. Int J Mol Sci, 2021, 22(14): 7554. 10.3390/ijms22147554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Magee C, Grieve DJ, Watson CJ, et al. Diabetic nephropathy: a tangled web to unweave[J]. Cardiovasc Drugs Ther, 2017, 31(5/6): 579-592. 10.1007/s10557-017-6755-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu JX, Liu XG, Li YP, et al. The association of neutrophil to lymphocyte ratio, mean platelet volume, and platelet distribution width with diabetic retinopathy and nephropathy: a meta-analysis[J]. Biosci Rep, 2018, 38(3): BSR20180172. 10.1042/BSR20180172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang Q, Wu H, Wo M, et al. Monocyte-lymphocyte ratio is a valuable predictor for diabetic nephropathy in patients with type 2 diabetes[J]. Medicine (Baltimore), 2020, 99(19): e20190. 10.1097/md.0000000000020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang MZ, Harris RC. Immunohistochemical staining of CD8α in diabetic mouse kidney[J]. Bio-protocol, 2019, 9(18): e3364[2023-03-10]. 10.21769/BioProtoc.3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khandare SA, Chittawar S, Nahar N, et al. Study of neutrophil-lymphocyte ratio as novel marker for diabetic nephropathy in type 2 diabetes[J]. Indian J Endocrinol Metab, 2017, 21(3): 387-392. 10.4103/ijem.IJEM_476_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Manisha AM, Shangali AR, Mfinanga SG, et al. Prevalence and factors associated with latent autoimmune diabetes in adults (LADA): a cross-sectional study[J]. BMC Endocr Disord, 2022, 22(1): 175. 10.1186/s12902-022-01089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thekra AZ, Molham AH, Riyadh SA. Latent autoimmune diabetes in adults (LADA) and its metabolic characteristics among yemeni type 2 diabetes mellitus patients[J]. Diabetes Metab Syndr Obes, 2021, 14: 4223-4232. 10.2147/DMSO.S332416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qiu J, Xiao Z, Zhang Z, et al. Latent autoimmune diabetes in adults in China[J]. Front Immunol, 2022, 13: 977413.10. 3389/fimmu.2022. 977413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jaaban M, Zetoune AB, Hesenow S, et al. Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as novel risk markers for diabetic nephropathy in patients with type 2 diabetes[J]. Heliyon, 2021, 7(7): e07564[2023-03-10]. 10.1016/j.heliyon.2021.e07564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tanase DM, Gosav EM, Anton MI, et al. Oxidative stress and NRF2/KEAP1/ARE pathway in diabetic kidney disease (DKD): New perspectives[J]. Biomolecules, 2022, 12(9): 1227. 10.3390/biom12091227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pichler R, Afkarian M, Dieter BP, et al. Immunity and inflammation in diabetic kidney disease: translating mechanisms to biomarkers and treatment targets[J]. Am J Physiol Renal Physiol, 2017, 312(4): F716-F731. 10.1152/ajprenal.00314.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wan H, Wang YY, Fang SJ, et al. Associations between the neutrophil-to-lymphocyte ratio and diabetic complications in adults with diabetes: a cross-sectional study[J]. J Diabetes Res, 2020, 2020: 6219545. 10.1155/2020/6219545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eid S, Sas KM, Abcouwer SF, et al. New insights into the mechanisms of diabetic complications: role of lipids and lipid metabolism[J]. Diabetologia, 2019, 62(9): 1539-1549. 10.1007/s00125-019-4959-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roep BO, Thomaidou S, van Tienhoven R, et al. Type 1 diabetes mellitus as a disease of the β-cell (do not blame the immune system?)[J]. Nat Rev Endocrinol, 2021, 17(3): 150-161. 10.1038/s41574-020-00443-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marca V, Gianchecchi E, Fierabracci A. Type 1 diabetes and its multi-factorial pathogenesis: the putative role of NK cells[J]. Int J Mol Sci, 2018, 19(3): 794. 10.3390/ijms19030794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gardner G, Fraker CA. Natural killer cells as key mediators in type i diabetes immunopathology[J]. Front Immunol, 2021, 12: 722979. 10.3389/fimmu.2021.722979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lang PA, Crome SQ, Xu HC, et al. NK cells regulate CD8 T cell mediated autoimmunity[J]. Front Cell Infect Microbiol, 2020, 10: 36. 10.3389/fcimb.2020.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martinov T, Fife BT. Type 1 diabetes pathogenesis and the role of inhibitory receptors in islet tolerance[J]. Ann NY Acad Sci, 2020, 1461(1): 73-103. 10.1111/nyas.14106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guay C, Kruit JK, Rome S, et al. Lymphocyte-derived exosomal microRNAs promote pancreatic β cell death and may contribute to type 1 diabetes development[J]. Cell Metab, 2019, 29(2): 348-361.e6. 10.1016/j.cmet.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 26. Samsu N. Diabetic nephropathy: challenges in pathogenesis, diagnosis, and treatment[J]. Biomed Res Int, 2021, 2021: 1497449. 10.1155/2021/1497449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Christen U, Kimmel R. Chemokines as drivers of the autoimmune destruction in type 1 diabetes: opportunity for therapeutic intervention in consideration of an optimal treatment schedule[J]. Front Endocrinol, 2020, 11: 591083. 10.3389/fendo.2020.591083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Winter L, Wong LA, Jerums G, et al. Use of readily accessible inflammatory markers to predict diabetic kidney disease[J]. Front Endocrinol (Lausanne), 2018, 9: 225. 10.3389/fendo.2018. 00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chittawar S, Dutta D, Qureshi Z, et al. Neutrophil-lymphocyte ratio is a novel reliable predictor of nephropathy, retinopathy, and coronary artery disease in indians with type-2 diabetes[J]. Indian J Endocrinol Metab, 2017, 21(6): 864-870. 10.4103/ijem.IJEM_197_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yu Y, Lin QQ, Ye DW, et al. Neutrophil count as a reliable marker for diabetic kidney disease in autoimmune diabetes[J]. BMC Endocr Disord, 2020, 20(1): 158. 10.1186/s12902-020-00597-2. [DOI] [PMC free article] [PubMed] [Google Scholar]