Abstract:
PURPOSE:
The aim was to study the characteristics of recurrence patterns in the form of scar recurrence, new lesions, and vitreous seeds which is necessary in anticipating future events for the management of retinoblastoma (RB).
METHODS:
This retrospective analytical observational study was conducted in a tertiary care hospital in South India; we included 64 eyes of 45 patients having RB from January 2019 to July 2020. The inclusion criterion was treatment-naïve patients with > 12 months of follow-up period. Recurrence patterns were defined as Pattern 1a and Pattern 1b: local and diffuse dissemination of vitreous seeds, respectively. Pattern 2: Scar recurrences: these are new tumor growths over chemoreduced lesions. Pattern 3: New lesions: local dissemination of subretinal seeds leading to new lesions elsewhere in the retina.
RESULTS:
A noncomparative analysis of 64 eyes of 45 patients having 108 lesions was studied; of which 28/45 (62.22%) were male and 17/45 (37.78%) were female. The mean time of presentation since the first clinical sign was 40 days (range: 10–180). The most common sign at presentation was leukocoria 42/64 (65.6%), followed by squint 4/64 (6.34%). Nineteen patients (42.22%) had bilateral RB, while 26 patients (57.78%) had unilateral RB. Primary enucleation was done for 19/26 eyes with advanced unilateral disease. Out of the total 32 eyes with subretinal tumor seeds at presentation, 17/32 eyes had a recurrence in the form of new lesions (Pattern 3) and 22/32 eyes had scar recurrence (Pattern 2). All of these 32 eyes were salvaged by local tumor consolidation methods. Recurrence due to vitreous seed dissemination was found in 18/64 eyes, in which diffuse dissemination (Pattern 1b) was present in 8/18 eyes (44.4%); all required enucleation even after local and systemic chemotherapeutic measures. Rest 10/18 eyes with local vitreous seeds (Pattern 1a) were cured at the end of the follow-up. Globe salvage was more with Pattern 1a rather than Pattern 1b even after additional intravitreal chemotherapy.
CONCLUSION:
All eyes with Patterns 2 and 3 were salvaged at the end of follow-up with local tumor consolidation methods, while the globe salvage rate with Pattern 1 was poor even with multiple doses of intravitreal chemotherapy. The rate of successful treatment for managing these recurrence patterns depends on early identification by regular follow-ups with detailed retina examination.
Keywords: Recurrence patterns, retinoblastoma, subretinal seeds, vitreous seeds
Introduction
Retinoblastoma (RB) tumor recurrence following all types of treatment has ranged from 6% to 45%.[1,2,3,4] Monitoring for active, recurrent disease remains crucial to ensure a favorable long-term prognosis.[1,2,3,4] Due to the discohesive nature of RB, there is intraocular dissemination of tumor seeds in the subretinal or vitreous space.[5] In this article, the cause of tumor recurrence can be postulated as the dissemination of two types of tumor cells; and they are (A) subretinal seeds which are responsible for scar recurrence and new lesions, and (B) vitreous seeds which are described in the form of localized and diffuse dissemination in vitreous. The subretinal seeds can either be fixed which will give rise to scar recurrences or due to their adherence-independent properties will migrate to the ora serrata[6] and will be responsible for a new lesion [Figures 1 and 2]. Scar recurrences can be explained by the inability of the present routes of antimitotic administration to achieve tumoricidal concentrations to the relatively avascular tumor areas[6,7] [Figures 1, 3, and 4]. In this article, vitreous seed recurrence is identified as prehyaloid, retrohyaloid, and free-floating seeds. In contrast to the free-floating vitreous seeds, the prehyaloid and retrohyaloid seeds are attached to the internal face of the hyaloid and internal limiting membrane, respectively, and tend to coalesce.[6] Free-floating vitreous seeds are morphologically classified as dust, sphere, i.e. local seeds [Figure 5], and cloud type, i.e. diffuse seeds [Figures 2 and 5]. The emergence of recurrent vitreous seeds signifies worsening tumor control.[5] Chemo reduction with focal consolidation is an important therapeutic approach for RB.[8,9] A major cause of treatment failure remains the persistence or recurrence of resistant vitreous and subretinal RB seeding.[1,2,3,4,5,6,7,8,9,10] Tumor control on chemoreduction alone, without considering the unseen presence of vitreous or subretinal seed, can lead to various types of recurrences during or after completing six cycles of standard chemotherapy. In this analysis, we classify a pattern of recurrence based on the source of tumor cells with its treatment protocol [Figures 6 and 7].
Figure 1.

Fundus picture of the eye with asterisk showing multiple scar recurrences on regressed tumor sites and arrowhead showing new lesion away from primary site disseminating due to their adherence-independent properties and Patterns 2 and 3
Figure 2.

Fundus picture of the eye on systemic chemotherapy with arrowheads showing diffuse vitreous seeds recurrence (Pattern 1b) due to widespread dissemination of tumor cells and asterisks showing new lesions on sites away from the primary lesion (Pattern 3)
Figure 3.
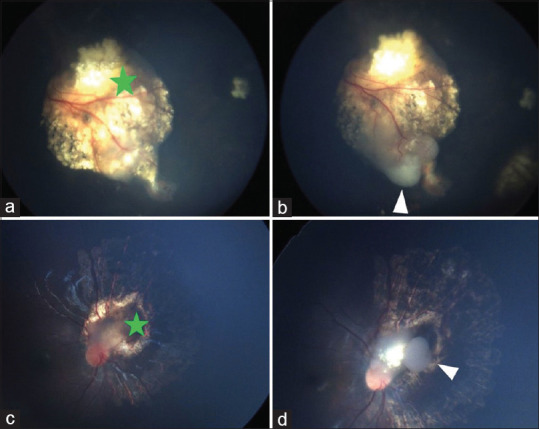
Fundus pictures of scar recurrences (Pattern 2). (a and c) Images with green asterisk showing regression after systemic chemotherapy, (b and d) showing white arrowheads are recurrences few months after the completion of systemic chemotherapy because of the persistence of subretinal tumor seeds due to failure of chemotherapy drugs to reach adequate mitotoxic levels (later on managed by local thermotherapy)
Figure 4.

Image (a) showing three tumor lesions, image (b) showing respective scar sites after systemic chemotherapy while image (c) with arrowhead shows tumor recurrence on the margin of the scar seen 5 months after the completion of systemic chemotherapy, which was later on managed by transpupillary thermotherapy (Pattern 2)
Figure 5.

Images (a and b) showing diffuse vitreous seed recurrence (Pattern 1b), while image (c) showing focal vitreous seed recurrence (Pattern 1a) around the localized area of the tumor
Figure 6.
Flowchart of the classification system of recurrence patterns of retinoblastoma based on the source of tumor seeds with their outcomes and management. TTT: Transpupillary thermotherapy, ICG: Indocyanine green, EUA: Examination under anesthesia
Figure 7.

Classification system of recurrence patterns of retinoblastoma based on the source of tumor seeds
Methods
This retrospective analytical observational study was conducted in a tertiary care hospital in South India; we included 64 eyes of 45 patients having RB from January 2019 to July 2020. All the eyes were classified as per the International Classification for Intraocular Retinoblastoma (ICRB)[11] system, in which Group A: RB ≤3 mm (in basal dimension or thickness); Group B: RB >3 mm (in basal dimension or thickness) or macular location (≤3 mm to foveola), juxtapapillary location (≤1.5 mm to disc), and subretinal fluid (≤3 mm from margin); Group C: RB with subretinal seeds ≤3 mm from tumor, vitreous seeds ≤3 mm from tumor, and both subretinal and vitreous seeds ≤3 mm from tumor; Group D: RB with subretinal seeds >3 mm from tumor, vitreous seeds >3 mm from tumor, and both subretinal and vitreous seeds >3 mm from RB; Group E: extensive RB occupying >50% globe or with neovascular glaucoma, opaque media from hemorrhage in the anterior chamber, vitreous or subretinal space, invasion of postlaminar optic nerve, choroid (>2 mm), sclera, orbit, and anterior chamber. Inclusion criteria were (1) treatment-naïve patients with >12 months of follow-up from the day of examination under anesthesia (EUA) and diagnosis of RB, (2) eyes with unilateral Group E RB who underwent primary enucleation were also included to study long-term prognosis and metastasis, and (3) eyes not showing any recurrences till 12 months were also included as these eyes had either subretinal or vitreous seeds at presentation. Patients who were not on regular follow-up were excluded. EUA was performed at every visit in which detailed anterior and posterior segment evaluation was done. An indirect ophthalmoscopic examination was done with indentation to check any lesions at the ora serrata for both eyes at every visit. The ocular status at presentation and follow-up was objectively monitored under EUA with fundus photography using RetCam and B-scan ultrasonography. First magnetic resonance imaging was performed just after the diagnosis of RB. Ocular oncologic follow-up with EUA was done every 3 weeks just before commencing the next chemo cycle. Recurrence of tumor was defined as the regrowth of retinal tumors on a new location or on tumor scars as well as the reappearance of vitreous seeds after an initial favorable response, or regression. Unresponsive disease was defined as the persistence of retinal tumors, vitreous or subretinal seeds after the six intravenous chemotherapy (IVC) cycles, with no appreciable sign of regression. Recurrences due to vitreous seeds were classified into Patterns 1a and 1b. Pattern 1a: localized dissemination of tumor seeds into vitreous (dust and spheres) and pre- or retrohyaloid positions. Pattern 1b: diffuse clouds of vitreous seeds or intracameral seeds. Pattern 2: scar recurrences, these are new tumor growths over chemoreduced lesions or scars. Pattern 3: new lesions away from primary tumors. Recurrent cases were treated with focal treatments such as transpupillary thermotherapy (TTT) with diode laser 810 nm (TTT), indocyanine green (ICG)-enhanced thermotherapy (ICG-TTT), and intravitreal injection (InVit) of topotecan hydrochloride 30 μg/0.03 mL. InVit topotecan was given with safety-enhanced technique. Enucleation was done as the last resort when additional chemoreduction treatments such as external beam radiation therapy (EBRT) and plaque brachytherapy failed. Consolidation of the regressed tumor was done with TTT just before chemotherapy to achieve tumor cell apoptosis and for the breakdown of blood-retinal barrier for increasing the availability of chemotherapeutic drugs. Most of the time, TTT consisted of power 250 mW over 5-min duration. ICG dye was administered with 0.5 mg/kg 1 min before applying TTT. The standard IVC protocol included six cycles of intravenous vincristine 0.05 mg/kg, etoposide 0.5 mg/kg, and carboplatin 18.6 mg/kg at every 3-week interval. Infants <1 month of age at diagnosis received a modified dosing regimen with a 50% decrease in all agents. Before and after each chemocycle, a detailed blood examination was done. Lesions at presentation were also counted and classified into anatomic locations into three regions as posterior pole, equator, and periphery. At the most final visit, the recorded data included the age of the last visit, status (cured/uncured), recurrence type, and treatment of recurrence (chemotherapy, thermotherapy, and intravitreal injection). The final ocular and systemic status was noted. The log-rank test and Kaplan–Meier survival were used to analyze the data, and P < 5% (0.05) was considered statistically significant.
Results
Patient characteristics and clinical presentation
A retrospective noncomparative analysis of 64 eyes of 45 patients having 108 lesions was studied; of which 28/45 (62.22%) were male and 17/45 (37.78%) were female. The mean time of presentation since the first clinical sign was 40 days (range: 10–180). The most common sign at presentation was leukocoria 42/64 (65.6%), followed by squint 4/64 (6.34%) and proptosis 4/64 (6.34%), and other signs were redness and discharge, while two of the patients were diagnosed on routine retinopathy of prematurity screening. The mean age of diagnosis was 22.15 ± 18.94 months (range: 1–84) [Table 1]. Bilateral cases were encountered in 19 (42.22%) patients, while 26 (57.78%) patients harbored unilateral RB. Primary enucleation was done for 19/26 eyes with advanced unilateral disease. As per the anatomical classification at the time of presentation of the total of 108 lesions, there were 68 lesions at the posterior pole, 21 lesions at the mid-periphery, and the rest 19 lesions at the periphery. Using the International RB Classification, one eye was Group A, 17 eyes were Group B, three eyes were Group C, nine eyes were Group D, and 34 eyes were presented with Group E RB. The mean number of follow-ups was 15.44. None of our patients underwent bilateral enucleation. The globe salvage rate at 12 months was 59.4%.
Table 1.
Description of demographics
| n (%) | |
|---|---|
| Total patients (n) | 45 |
| Gender | |
| Male | 62.2 |
| Female | 37.8 |
| Mean time of | 40 days |
| Presentation | (10–180) |
| Laterality | |
| Bilateral | 19 (42.22) |
| Unilateral | 26 (57.78) |
| Signs at presentation | |
| Leukocoria | 65.5 |
| Squint | 6.34 |
| Proptosis (rest were discharge and redness) | 6.34 |
| Mean age of diagnosis (months) | 22.15±18.94 (1–84) |
| Mean time of recurrence after initiation of systemic chemotherapy (months) | |
| Vitreous seeds | 5 (2–8) |
| Scar recurrence | 3 (2–6) |
| New lesions | 4 (2–8) |
Management and outcomes
At initial presentation, four eyes had seeds in the anterior chamber, and two eyes had visible pseudohypopyon. Out of the total of 32 eyes with subretinal tumor seeds at presentation, 17/32 eyes had a recurrence in the form of new lesions (Pattern 3), and scar recurrence (Pattern 2) was seen in 22/32 eyes; hence, 12/22 + 17 eyes showed a combination of both types of recurrences, while 5/32 eyes with subretinal seeds did not show any type of recurrences. There were a total of 8/64 unresponsive eyes. Secondary enucleation was done in 7/34 Group E eyes after the completion of six cycles of chemotherapy, of which two eyes had unilateral RB and five eyes had bilateral RB. Out of the total 45 patients, 31 (68.88%) patients received systemic chemotherapy. Central nervous system metastasis developed in 1/31 patient even after completing six cycles of chemotherapy and died thereafter. 1/14 patient with unilateral Group E underwent enucleation but did not show any risk factors on histopathology and hence was not given systemic chemotherapy, still developed metastasis and died. One patient had presented with trilateral RB.
Vitreous seed recurrences
On univariate analysis, the recurrence of vitreous seeds was significant with the size of RB, P < 0.006, such that maximum recurrent episodes were present in eyes with Group D (77.7%) RB. Recurrence due to vitreous seed dissemination was found even after mean three cycles of systemic chemotherapy in 18/64 eyes, in which 8/18 eyes (44.4%) had diffuse vitreous seed dissemination and required enucleation instead of additional systemic and intravitreal treatment, while the rest 10/18 eyes with local vitreous seeds were cured and developed calcified seeds at the end of the follow-up with successful globe salvage (55.5%). On average, two intravitreal topotecan 30 mcg/0.03 mL injections were required. Two eyes with diffuse vitreous seed dissemination required five doses of intravitreal topotecan 30 mcg/0.03 mL but were still not salvaged. The mean interval from the initiation of chemotherapy to the first vitreous seed recurrence was 5 months (range: 2–8 months).
Scar recurrence
With univariate analysis, scar recurrence had a statistically significant association (P < 0.001) with variables such as group, posterior pole lesion, number of cycles of chemotherapy, and TTT. Of 22 eyes having scar recurrence, 18 (81.8%) eyes had recurrence at the posterior pole, while four eyes had in the mid-periphery. Forty percentage of eyes with Group B RB and 28.6% of eyes with Group C RB had scar recurrences. On average, four sessions of TTT were required. ICG TTT was given to five eyes. Additional systemic chemotherapy was required only for 1/22 eyes, and the rest were managed only by TTT and ICG-TTT. All eyes were salvaged at the end of the follow-up. The mean interval of the initiation of systemic chemotherapy to scar recurrence (per eye) was 3 months (range: 2–6 months). The mean number of scar recurrence sites was 2 per eye.
New lesions
Univariate analysis showed a significant association of new lesions with the number of TTT sessions (P = 0.008). Of the total of 17 eyes that developed new lesions, on average, four sessions of TTT were required, and three eyes required additional ICG-TTT. Of the total of 17 eyes that developed new lesions, two eyes required additional systemic chemotherapy. None of these eyes had to undergo enucleation. All the eyes were salvaged at the end of the follow-up. The mean interval of the initiation of systemic chemotherapy to the new lesion (per eye) was 4 months (range: 2–8 months). The mean number of new lesions was 2 per each eye. 14/17 eyes (82.3%) had new lesions at the periphery, and 3/17 eyes had new lesions at the posterior pole [Table 2].
Table 2.
Description of management of different patterns of recurrence
| Pattern of recurrence | Management |
|---|---|
| Vitreous seed (18/64) | |
| Focal (10) | Systemic and intravitreal chemotherapy |
| Diffuse (8) | Enucleation |
| Scar recurrence | |
| Posterior pole (18) | TTT was given in all eyes, five eyes needed ICG-TTT (22/64) |
| Mid-periphery (4) | One eye was given additional systemic chemotherapy |
| New lesion | |
| Peripheral retina (14) | 15 eyes were cured with TTT alone while 17/64 |
| Posterior pole (3) | Two eyes needed additional chemotherapy |
TTT: Transpupillary thermotherapy, ICG: Indocyanine green
In this study, new lesions and scar recurrences were first treated with focal consolidation and only on being unresponsive were further treated with additional systemic chemotherapy. There was a 100% globe salvage rate for both these lesions. At the end of the follow-up of the total of 45 patients, two patients had died due to metastasis. The Kaplan–Meier survival estimate of different recurrence patterns was used to explain the relation of globe salvage rate over a given time. With reference to Pattern 1A, the globe salvage was 53.4% at 18 months, 52.3% at 2 years, and 50.5% at 5 years. EBRT was not given to any patient while intra-arterial chemotherapy was given to a patient who was then lost on follow-up.
Discussion
The subretinal seeds become independent due to their discohesive nature either floating under the retina as residual tumor cells known as subretinal seeds and into vitreous as vitreous seeds.[12,13,14]
Shields et al. in 2002 had classified three anatomic sites of tumor recurrence in retinal, subretinal and vitreous and stated that the only factor predictive of retinal tumor recurrence was the presence of tumor-associated subretinal seeds at the initial examination and both recurrence due to subretinal seeds and retinal tumor cannot be differentiated clinically.[15] Hence retinal tumor recurrence is considered under subretinal seeds in our study.
Recurrences due to vitreous seeds are classified into Patterns 1a and 1b.
Pattern 1a: Focal seeds: localized dissemination of tumor seeds into vitreous (dust and spheres), and into pre- or retrohyaloid positions. Their anatomical classification was done with the help of ultrasonography and clinical analysis.
Pattern 1b: Diffuse seeds: diffuse clouds of vitreous seeds or intracameral seeds due to widespread dissemination of tumor cells.
Pattern 1a is salvageable by intravitreal chemotherapy and additional cycles of chemotherapy. While for Pattern 1b, timely enucleation is very likely.
Pattern 2: Scar recurrences. These are new tumor growths over chemoreduced lesions. Suggesting sub retinal persistence of tumor seeds due to failure of chemo therapy drugs to reach adequate cytotoxic levels.[8]
Pattern 3: New lesions. Even after the commencement of systemic chemotherapy, there is local dissemination of subretinal seeds due to their adherence-independent properties which will result in new lesions elsewhere in the retina.[7,8]
For Patterns 2 and 3, consolidation of the regressed tumor was done with TTT to achieve tumor cell apoptosis and to break down the blood-retinal barrier to increase the availability of chemotherapeutic drugs to tumor cells. For resistant cases, the effect of thermotherapy may be enhanced by ICG-TTT;[16] in our study, Patterns 2 and 3 have almost always shown good response to local treatment and has resulted in a better globe salvage and visual prognosis on statistical comparison with Pattern 1. By multivariate analysis, the most important predictors of tumor recurrence in all three patterns were the size of the tumor and previous chemoreduction treatments (P < 0.001).
As seen in the current study and in studies of Shields et al.,[7,8] those at the greatest risk for retinal tumor recurrence were eyes with tumor-associated subretinal seeds at presentation, and also the eyes with subretinal seeds will develop recurrences in the form of Pattern 2 and 3 which were successfully treated with focal consolidations as soon as the recurrence was seen, and hence, all were salvaged.
Pattern 3 was present most commonly in the periphery up to the ora serrata (82.3%) probably due to the hypothesis that drugs might not travel into the end arterioles in the watershed zone due to uneven distribution of chemotherapeutic drugs and its deposition in more proximal vasculature[13] could possibly result in decreased therapeutic levels of the drug in this area. Hence, regular follow up every 3 weeks with EUA and fundus examination till the ora serrata was done so that recurrence patterns are not missed. There can be sudden vitreous dispersion of large tumor cells shortly after the initiation of chemotherapy due to a necrotic disruption of the internal limiting membrane.[13,14,15] Small size and diffuse distribution of Pattern 1A have resulted in the need for intravitreal chemotherapy to avoid enucleation. Instead of aggressive local chemotherapy, the globe salvage rate for resistant Pattern 1A is very poor, and is also seen in our study[10] that there was a statistically significant positive correlation (P < 0.005) between secondary enucleations and Pattern 1. As vitreous is an avascular tissue and systemic or arterial chemotherapy has limited transition to vitreous, vitreous seeding has been recognized as the strongest predictor of RB treatment failure.[12,13,14] Most of the secondary enucleations occurred due to refractory vitreous seeds[13,14] which is also seen in our study. Recurrence of Pattern 1a was given additional secondary systemic chemotherapies and intravitreal injections, while Pattern 1b was most commonly enucleated. Shields et al., in 2002, in a prospective study found recurrence of at least one retinal tumor per eye; recurrence with vitreous seeds and subretinal seeds is found in 50%, and 62% of eyes, respectively, at 5 years’ follow-up. The recurrence of even a group of subretinal seeds causing recurrent Patterns 2 and 3 during ongoing primary chemotherapy does not indicate failure of treatment modality compared to the thousands of seeds that would have been present initially.[3] In 2013, a study by Gündüz et al., including 171 RB eyes, reported that 17.5% had persistent disease and 25.7% had tumor recurrence. Of these 171 eyes that initially underwent chemoreduction, 33.3% underwent secondary enucleation. In that study, tumor group (Groups D and E), presence of vitreous and subretinal seeds, and number of chemotherapy and local treatments administered were determined as risk factors for treatment failure (i.e. enucleation).[17] As was found in our study, the presence of tumor size (tumor group) was found to be statistically associated with the recurrence of vitreous seeds. The most common occurrence of recurrence in form of pattern 1A was found in Group D tumors. Statistically increased number of intravitreal and systemic chemotherapy was associated with treatment failure of Pattern 1a, while an increased number of TTT sessions were associated with the number of recurrent episodes for Patterns 2 and 3. Shields’ et al., in a prospective study, found that the mean interval of recurrence after completion of chemotherapy for vitreous seeds was 2 months, and for subretinal seeds was 2 months,[15,18] which are consistent with our results of mean interval of recurrence after completion of chemotherapy for vitreous seeds was 5 months and subretinal seeds was 4 months after initiation of chemotherapy. Thus, EUA with detailed fundus examination and retinal indentation after initiation and completion of chemotherapy is necessary along with long-term monitoring for anticipating recurrence.
Conclusion
The purpose of this classification system is to anticipate future events if a particular recurrence pattern is seen and will be useful for the RB specialist to understand pathophysiology relative to the recurrence patterns and provide a window of time to expect each event. To our knowledge no previous study has proposed a classification system on recurrence patterns in retinoblastoma based on its source with treatment and their outcomes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Abramson DH, Greenfield DS, Ellsworth RM. Bilateral retinoblastoma. Correlations between age at diagnosis and time course for new intraocular tumors. Ophthalmic Paediatr Genet. 1992;13:1–7. doi: 10.3109/13816819209070046. [DOI] [PubMed] [Google Scholar]
- 2.Abramson DH, Gamell LS, Ellsworth RM, Kruger EF, Servodidio CA, Turner L, et al. Unilateral retinoblastoma: New intraocular tumours after treatment. Br J Ophthalmol. 1994;78:698–701. doi: 10.1136/bjo.78.9.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shields CL, Shelil A, Cater J, Meadows AT, Shields JA. Development of new retinoblastomas after 6 cycles of chemoreduction for retinoblastoma in 162 eyes of 106 consecutive patients. Arch Ophthalmol. 2003;121:1571–6. doi: 10.1001/archopht.121.11.1571. [DOI] [PubMed] [Google Scholar]
- 4.Shields CL, Mashayekhi A, Cater J, Shelil A, Meadows AT, Shields JA. Chemoreduction for retinoblastoma. Analysis of tumor control and risks for recurrence in 457 tumors. Am J Ophthalmol. 2004;138:329–37. doi: 10.1016/j.ajo.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 5.Munier FL. Classification and management of seeds in retinoblastoma. Ellsworth lecture ghent August 24th 2013 Ophthalmic Genet. 2014;35:193–207. doi: 10.3109/13816810.2014.973045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fabian ID, Stacey AW, Johnson KP, Onadim Z, Chowdhury T, Duncan C, et al. Primary intravenous chemotherapy for group D retinoblastoma: A 13-year retrospective analysis. Br J Ophthalmol. 2017;101:82–8. doi: 10.1136/bjophthalmol-2016-309710. [DOI] [PubMed] [Google Scholar]
- 7.Shields CL, Honavar SG, Shields JA, Demirci H, Meadows AT, Naduvilath TJ. Factors predictive of recurrence of retinal tumors, vitreous seeds, and subretinal seeds following chemoreduction for retinoblastoma. Arch Ophthalmol. 2002;120:460–4. [PubMed] [Google Scholar]
- 8.Shields CL, Shields JA. Editorial: Chemotherapy for retinoblastoma. Med Pediatr Oncol. 2002;38:377–8. doi: 10.1002/mpo.1353. [DOI] [PubMed] [Google Scholar]
- 9.Shields CL, Shields JA. Recent developments in the management of retinoblastoma. J Pediatr Ophthalmol Strabismus. 1999;36:8–18. doi: 10.3928/0191-3913-19990101-04. [DOI] [PubMed] [Google Scholar]
- 10.Francis JH, Marr BP, Abramson DH. Classification of vitreous seeds in retinoblastoma: Correlations with patient, tumor, and treatment characteristics. Ophthalmology. 2016;123:1601–5. doi: 10.1016/j.ophtha.2016.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphree AL, Chantada G. Staging and grouping of retinoblastoma. In: Singh AD, Damato BE, Peer J, Murphree AL, Perry JD, editors. Clinical Ophthalmic Oncology. Philadelphia: Elseiver; 2007. pp. 422–27. [Google Scholar]
- 12.Parness-Yossifon R, Bryar PJ, Weinstein JL, Srikumaran D, Mets MB. Sudden dispersion of retinoblastoma shortly after initial chemotherapy treatment. Am J Ophthalmol. 2009;147:903–6. doi: 10.1016/j.ajo.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Gündüz K, Günalp I, Yalçindağ N, Unal E, Taçyildiz N, Erden E, et al. Causes of chemoreduction failure in retinoblastoma and analysis of associated factors leading to eventual treatment with external beam radiotherapy and enucleation. Ophthalmology. 2004;111:1917–24. doi: 10.1016/j.ophtha.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 14.Manjandavida FP, Honavar SG, Reddy VA, Khanna R. Management and outcome of retinoblastoma with vitreous seeds. Ophthalmology. 2014;121:517–24. doi: 10.1016/j.ophtha.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Shields CL, Mashayekhi A, Au AK, Czyz C, Leahey A, Meadows AT, et al. The international classification of retinoblastoma predicts chemoreduction success. Ophthalmology. 2006;113:2276–80. doi: 10.1016/j.ophtha.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 16.Al-Haddad CE, Abdulaal M, Saab RH, Bashshur ZF. Indocyanine green-enhanced thermotherapy for retinoblastoma. Ocul Oncol Pathol. 2015;1:77–82. doi: 10.1159/000368558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gündüz K, Köse K, Kurt RA, Süren E, Taçyildiz N, Dinçaslan H, et al. Retinoblastoma in Turkey: Results from a tertiary care center in Ankara. J Pediatr Ophthalmol Strabismus. 2013;50:296–303. doi: 10.3928/01913913-20130730-02. [DOI] [PubMed] [Google Scholar]
- 18.Ancona-Lezama DA, Dalvin LA, Lucio-Alvarez JA, Jabbour P, Shields CL. Choroidal ischemia sparing the watershed zone following intra-arterial chemotherapy for retinoblastoma. Ocul Oncol Pathol. 2019;5:190–4. doi: 10.1159/000490856. [DOI] [PMC free article] [PubMed] [Google Scholar]



