Abstract
While the cardiovascular effects of Kolaviron (KV) and Garcinia kola (GK) are documented in the literature, a thorough search through literature revealed a fragmentation of information on the effect of KV and GK on cardiovascular diseases (CVDs). This systematic review aims to evaluate and summarize preclinical or clinical evidence on the effect of KV and GK on CVDs. Using the PRISMA guidelines, a systematic literature search was conducted in five medical databases (PubMed, Cochrane, EMBASE, CINAHL, and Web of Science). Inclusion criteria included both in vivo and in vitro studies related to CVDs. Eligible studies included those in which specific clinical parameters, CVD biomarkers, or voltage-gated channel effects were reported. The quality of the included studies was assessed using a modified Collaborative Approach to Meta-Analysis and Review of Animal Data from the Experimental Studies (CAMARADE) checklist. A total of 22 studies were included in this systematic review. The median and mean values of the included studies' quality scores were 6 and 5.864 ± 0.296, respectively. The results from the quality assessment of included studies validate their suitability, usefulness, and fit. Based on this systematic review, the effect of KV and GK on CVDs can be divided into eight emerging trends: (1) Anti-hypertensive/Blood pressure lowering effect; (2) Lipid profile improvement effect (3) Anti-atherosclerotic effect; (4) Anti-thrombotic effect; (5) Cardioprotection; (6) Vasodilatory effect; (7) Antioxidant effects; and (8) Genetic expression and therapeutic target for cardiovascular dysfunction. From this systematic review, it can be concluded that KV is helpful in managing CVD risk factors such as hypertension and high lipids/cholesterol. Several included studies in this review demonstrated the antihypertensive, lipid improvement, antioxidant, and signaling pathway modulation effects of KV. This potentially makes KV a good therapeutic target for the management of CVDs.
Keywords: Flavonoid, Kolaviron, Garcinia kola, Hypertension, Cardiovascular diseases, Antioxidants
Graphical abstract
1. Introduction
Cardiovascular diseases (CVDs) remain one of the leading causes of death in the global public health domain [1]. This is mainly driven by the substantial contribution it makes to the world's total disease burden, the excessive healthcare cost burden it leads to, and the immense conversations it generates in global public health circles [[2], [3], [4]]. An estimated 17.9 million lives are lost annually due to CVD; this constitutes 32% of all global deaths. Additionally, 85% of these deaths are due to heart attack and stroke, 38% of these deaths occur prematurely in people under 70 years of age, and 75% of these deaths occur in the low- and middle-income countries [1]. Indeed, cardiovascular disease is known to be the number one cause of years of life lost due to premature mortality [5,6].
High blood pressure, high blood cholesterol, and smoking are often a precursor to CVDs, and these factors are known to be the key modifiable risk factors for CVDs [1,3,4]. This is because they are associated with the restriction of blood flow to the heart, brain, or body. High blood pressure or Hypertension (HT) has been identified to be the most prevalent, potent, and leading preventable risk factor for CVDs [[7], [8], [9]]. Based on all of this, it can be postulated that by effectively controlling these key risk factors, the risk of mortality or morbidity associated with most CVDs can be reduced with a corresponding increase in the quality of life of affected CVD patients.
Indeed, the risk of CVDs can be prevented or managed by focussing on and modifying the most important behavioral (and modifiable) risk factors such as unhealthy diet, obesity, tobacco use, physical inactivity, high salt intake, and excessive consumption of alcohol [1,5]. Additionally, most CVDs can be treated via medical interventions to prevent death and improve the quality of life of CVD patients. Literature is replete with several medical interventions to manage CVD risk factors, including standard-of-care medications such as antihypertensives, hypolipidemic drugs, etc. [[10], [11], [12], [13], [14], [15], [16], [17]].
The use of herbal medicinal plants and flavonoids (obtained from natural sources) in managing CVDs and their risk factors is well documented [[18], [19], [20], [21], [22]]. Several studies have provided direct evidence of the relationship between consuming herbal medicinal plants (and their flavonoid content) and preventing CVD risk factors [23,24]. Furthermore, consuming flavonoids has been associated with antihypertensive effects, cardiovasculoprotective effects, and reduction of CVD disease progression [19,20,24]. As such, the importance of herbal medicinal plants and flavonoids obtained from these sources cannot be over-exaggerated in the management of CVDs.
Kolaviron (and its precursor, Garcinia kola), is an established source of flavonoids such as Kolaflavanone, Kolaflavone and Garcinia biflavonoids I & II and this constitutes its active ingredient [[25], [26], [27]]. Kolaviron (KV) is obtained from the defatted ethanolic extract of Garcinia kola Heckel (Family: Guttiferae) seeds, a widely available herbal medicinal plant in West African countries such as Nigeria, Ghana, and Cameroon [28,29]. Traditionally in Nigeria, Garcinia kola (GK) is locally regarded as “bitter kola, orogbo (in Southern Nigeria), aku-ilu (in Eastern Nigeria) and cida goro (in norhern Nigeria). GK is heavily used in folk traditional medicine and is sometimes referred to as a wonder plant because all parts of the plant are credited with medicinal uses [30]. Traditionally, extracts of GK have been used to relieve minor ailments such as chest colds, coughs, and colic and treat liver diseases and laryngitis [28].
KV and GK are familiar in the scientific research circles as several research and ethnopharmacological investigations have been conducted to report their numerous pharmacological activities or effects. These activities or effects include and may not be limited to their antimalarial [31], antiplasmodial [32], antiviral [27], antifungal and bactericidal [33], anti-ulcer [34], analgesic [35], antioxidant [[36], [37], [38], [39]], anti-inflammatory and hepatoprotective [35,40], hypoglycaemic [25,41], aromatase and alpha-glucosidase inhibitory [32] effects. Additionally, the hypolipidaemic, anti-atherogenic, lipid improvement, and cardioprotective effects of KV and GK have been documented in the literature [[41], [42], [43], [44], [45], [46], [47]]. Our previous studies reported the antihypertensive, hypolipidaemic, lipid improvement, and long-term safety of KV and GK [21,48,49].
While the cardiovascular effects of KV and GK are documented in the literature, a thorough search revealed a fragmentation of information on the impact of KV and GK on CVDs. It appears that no study or paper has attempted to critically review and synthesize the available information/research on the effect of KV and GK in CVDs. Additionally, a paper has yet to attempt to review and synthesize the various proposed and possible mechanisms through which KV and GK exert their pharmacological effects on CVDs. Therefore, the objective of this study is to evaluate and summarize existing preclinical or clinical evidence on the effect of KV and GK on CVDs. Additionally, this study will attempt to assess and summarize the possible underlying mechanisms proposed on how KV and GK exert their effects on CVDs.
2. Material and methods
2.1. Sources of information and search strategy
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used to perform a systematic literature search for this study [50]. PubMed, Cochrane, EMBASE, CINAHL, and Web of Science (WOS, previously known as Web of Knowledge) databases were searched in December 2022 and updated in August 2023 to identify articles and papers investigating the use of KV or GK in the management of CVDs. These databases were searched from their inception to date to capture all potentially relevant articles on the subject of this study.
The search strategy for this study consisted of a Boolean combination of keyword terms and controlled vocabulary as presented in Table 1 and represented as ((Kolaviron) OR (Garcinia kola)) AND (xxx). Here, xxx denotes the keyword (s) related to cardiovascular disease. This search strategy was used for PubMed with minor changes for other databases as needed. No restriction was enforced for language or date of publication for the search strategy across all five databases. However, duplicate research papers from the same or different databases were removed.
Table 1.
Search strategy.
| 1 | (Kolaviron or Garcinia kola) and cardiovascular disease |
|---|---|
| 2 | (Kolaviron or Garcinia kola) and cardiovascular |
| 3 | (Kolaviron or Garcinia kola) and vascular |
| 4 | (Kolaviron or Garcinia kola) and Antihypertensive |
| 5 | (Kolaviron or Garcinia kola) and Hypertension |
| 6 | (Kolaviron or Garcinia kola) and Blood Pressure |
| 7 | (Kolaviron or Garcinia kola) and Antioxidant |
| 8 | (Kolaviron or Garcinia kola) and (Antioxidant and antihypertensive) |
| 9 | (Kolaviron or Garcinia kola) and (Antioxidant and hypertension) |
| 10 | (Kolaviron or Garcinia kola) and Cholesterol |
| 11 | (Kolaviron or Garcinia kola) and Triglycerides |
| 12 | (Kolaviron or Garcinia kola) and Lipoprotein |
| 13 | (Kolaviron or Garcinia kola) and Ischemia |
| 14 | (Kolaviron or Garcinia kola) and Ischemia |
| 15 | (Kolaviron or Garcinia kola) and Angiotensin |
| 16 | (Kolaviron or Garcinia kola) and Nitric oxide |
| 17 | (Kolaviron or Garcinia kola) and (Nitric oxide and cardiovascular) |
| 18 | (Kolaviron or Garcinia kola) and (Nitric oxide and hypertension) |
| 19 | (Kolaviron or Garcinia kola) and Lipid lowering |
| 20 | (Kolaviron or Garcinia kola) and Vasodilation |
| 21 | (Kolaviron or Garcinia kola) and (Vasodilation and hypertension) |
| 22 | (Kolaviron or Garcinia kola) and (Vasodilation and cardiovascular) |
| 23 | (Kolaviron or Garcinia kola) and reactive oxygen species |
| 24 | (Kolaviron or Garcinia kola) and (reactive oxygen species and hypertension) |
| 25 | (Kolaviron or Garcinia kola) and (reactive oxygen species and cardiovascular) |
| 26 | (Kolaviron or Garcinia kola) and Oxidative stress |
| 27 | (Kolaviron or Garcinia kola) and (Oxidative stress and hypertension) |
| 28 | (Kolaviron or Garcinia kola) and (Oxidative stress and cardiovascular) |
| 29 | (Kolaviron or Garcinia kola) and Anti-proliferative |
| 30 | (Kolaviron or Garcinia kola) and Antiproliferative |
| 31 | (Kolaviron or Garcinia kola) and Hypocholesterolemia |
| 32 | (Kolaviron or Garcinia kola) and Hypocholesterolaemia |
| 33 | (Kolaviron or Garcinia kola) and Hypolipidemia |
| 34 | (Kolaviron or Garcinia kola) and Hypolipidaemia |
| 35 | (Kolaviron or Garcinia kola) and Atherogenic |
| 36 | (Kolaviron or Garcinia kola) and Atherosclerosis |
| 37 | (Kolaviron or Garcinia kola) and Arteriosclerosis |
| 38 | (Kolaviron or Garcinia kola) and Vascular smooth muscle cells |
| 39 | (Kolaviron or Garcinia kola) and Cardioprotective |
| 40 | (Kolaviron or Garcinia kola) and Cardioprotection |
| 41 | (Kolaviron or Garcinia kola) and Coronary artery disease |
| 42 | (Kolaviron or Garcinia kola) and Coronary heart disease |
| 43 | (Kolaviron or Garcinia kola) and Angina Pectoris |
| 44 | (Kolaviron or Garcinia kola) and Venous insufficiency |
| 45 | (Kolaviron or Garcinia kola) and Arrythmia |
| 46 | (Kolaviron or Garcinia kola) and Dysrhythmia |
| 47 | (Kolaviron or Garcinia kola) and Ischaemic heart disease |
| 48 | (Kolaviron or Garcinia kola) and Ischemic heart disease |
| 49 | (Kolaviron or Garcinia kola) and Myocardial infarction |
| 50 | (Kolaviron or Garcinia kola) and Congestive heart failure |
| 51 | (Kolaviron or Garcinia kola) and Thrombosis |
2.2. Eligibility criteria and selection process
Inclusion criteria: Includes in vivo (animal), in vitro, and clinical studies related to cardiovascular diseases or cardiometabolic diseases such as Diabetes mellitus. Eligible studies included those that had one or more clinical parameters, CVD biomarkers, or voltage-gated channel effects measured and reported in the study [[51], [52], [53], [54], [55], [56], [57], [58]].
The clinical parameters screened for include measurement of blood pressure, lipid levels, cholesterol levels, triglyceride levels, lipoprotein levels (High-density lipoprotein, Low-density lipoprotein, and Very Low-density lipoprotein), antioxidant levels, and atherogenic index. The CVD biomarkers screened for include C-reactive protein, Myeloperoxidase (MPO), Lactate dehydrogenase, troponin, myoglobin, creatine kinase (CK), B-type natriuretic peptide (BNP), N-terminal prohormone BNP (NT-proBNP), Lipoprotein-associated phospholipase A2, Fibrinogen, Trimethylamine N-oxide (TMAO), Cystatin C, Uric acid, Growth-differentiation factor-15, Pregnancy-associated plasma protein-A (PAPP-A), Matrix metalloproteinases (MMPs), Natriuretic peptides, Nitric oxide (NO), Angiotensin-converting enzyme (ACE), Protein kinases, and platelet aggregation. Voltage-gated channel effects screened for include potassium (K+), Calcium (Ca2+), or Sodium (Na2+) voltage-gated channel-mediated contraction or relaxation of tissues.
Exclusion criteria: There was no restriction for this study in terms of number of animals, dosage, administration method, or duration of treatment for either the animal or in vitro studies. There was no restriction on whether KV, GK, or both were used in the studies. However, non-CVD-related studies, non-KV or GK-related studies, review studies (literature review or systematic review papers), non-research papers (e.g., chemical information, chemical isolation, or patency papers), conference proceedings or abstract papers were excluded from this study.
Screening and evaluation of eligibility criteria of retrieved papers was conducted by two independent researchers (by reading the title, abstract, objective, results, and conclusion of each study). The inclusion of a study was confirmed based on the researchers' consensus. A process was put in place such that a third independent researcher settled any disagreement. All eligible papers were then read in full by the researchers. Any further exclusion as needed was carried out after all papers were read in full.
2.3. Data items, data collection, data validation and quality assessment
The following items were extracted from each study included in this systematic review: author-(s), year of publication, study design (animal study or in vitro study; If animal study, number of test animals as well as the strain of the animal is included. If in vitro, type and source of organ/cells used is included), treatment (whether KV or GK or both), dosage and duration of treatment, primary outcome measure, main results, and main conclusion from each study. The primary outcome measure should be one or more clinical parameters, CVD biomarkers, or voltage-gated channel effects measured and reported in the study. The first author conducted data extraction, and this was independently reviewed and validated by the secondary authors.
The quality of the included studies was assessed using a modified Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies (CAMARADE) checklist [59,60]. The checklist included the following criteria: (1) publication in peer-reviewed journals, (2) statement of control of temperature, (3) random allocation of treatment or control, (4) allocation concealment, (5) blinded assessment of outcome, (6) animal experimental model, (7) use of animals with induced CVD risk factors (e.g., hypertension, high lipid profile, diabetes mellitus), (8) sample size calculation, (9) statement of compliance with animal welfare regulation, and (10) statement of potential conflict of interest. Each study was assigned a quality score out of 10 maximum possible points by two reviewers, minimizing variance in the process. Each study was assessed on the CAMARADE checklist as either fulfilling (Yes) or not fulfilling (No) each of the CAMARADE checklist criteria. The number of “Yes” was then counted and reported over ten which is the maximum score possible. Please see Table 4 for details. The group median quality score for all eligible studies was calculated, and the mean quality score was expressed as mean ± SEM (standard error of the mean). This was followed by a One-way ANOVA with the Tukey multiple comparison test to evaluate for statistical differences between individual quality scores of included studies. All data analysis was done using GraphPad Prism 5 software (GraphPad Software Inc., La Jolla, CA, USA).
Table 4.
Quality assessment of included studies based on modified CAMARADES checklist.
| Author (s), Year | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Quality Score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adaramoye and Adeyemi, 2006 | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7 |
| Adaramoye and Lawal 2015 | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7 |
| Adaramoye and Medeiros 2009 | Y | Y | N | N | N | N | N | N | Y | Y | 4 |
| Adaramoye et al., 2005 | Y | Y | Y | N | N | Y | Y | N | N | N | 5 |
| Adaramoye et al., 2012 | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7 |
| Adejor et al., 2016 | Y | N | Y | N | N | Y | Y | N | Y | Y | 6 |
| Adoga et al., 2021 | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7 |
| Adoga et al., 2022 | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7 |
| Ajani et al., 2008 | Y | Y | Y | N | N | Y | Y | N | Y | N | 6 |
| Akinrinde et al., 2016 | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7 |
| Chen et al., 2022 | Y | Y | N | N | N | N | N | N | N | Y | 3 |
| Djétouan et al., 2022 | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7 |
| Oladapo et al., 2017 | Y | N | Y | N | N | Y | Y | N | Y | Y | 6 |
| Olajide 1999 | Y | Y | N | N | N | Y | Y | N | N | N | 4 |
| Olatoye et al., 2021 | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7 |
| Omole et al., 2018 | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7 |
| Oyagbemi et al., 2016a | Y | Y | N | N | N | N | N | N | N | Y | 3 |
| Oyagbemi et al., 2016b | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7 |
| Oyagbemi et al., 2018a | Y | N | Y | N | N | Y | Y | N | Y | Y | 6 |
| Oyagbemi et al., 2018b | Y | N | Y | N | N | Y | Y | N | Y | Y | 6 |
| Oyagbemi et al., 2018c | Y | Y | N | N | N | N | N | N | Y | Y | 4 |
| Uche and Osakpolo 2018 | Y | N | Y | N | N | Y | Y | N | Y | Y | 6 |
(1) Publication in peer reviewed journals, (2) Statement of control of temperature, (3) Random allocation of treatment or control, (4) Allocation concealment, (5) Blinded assessment of outcome, (6) Animal experimental model, (7) Use of animals with induced CVD risk factors (e.g. Hypertension, high lipid profile, diabetes mellitus), (8) Sample size calculation, (9) Statement of compliance with animal welfare regulation, and (10) Statement of potential conflict of interest. Y = Yes. N No.
Lastly, a risk of bias assessment of the eligible studies was conducted using the SYRCLE risk of bias tool [61,62] to evaluate the potential selection, performance, detection, attrition, and reporting biases in the eligible studies. Eligible studies were assessed based on the following criteria: (1) Random sequence generation (selection bias), (2) Allocation concealment (selection bias), (3) Blinding of participants and researchers (performance bias), (4) Blinding of outcome assessment (detection bias), (5) Incomplete outcome data (attrition bias), (6) Selective outcome reporting (reporting bias), (7) Other bias due to potential data contamination or analysis problems. The “Green” colour was used to indicate if the study met the criteria or if the study declared the criteria. The “Green” colour implied a low risk of bias. “Yellow” colour was used to indicate an unclear statement or declaration of the criteria and implied a high risk of bias. “Red” colour was used to suggest that the criteria was not declared or absent and implied a high risk of bias.
3. Results
3.1. Study selection
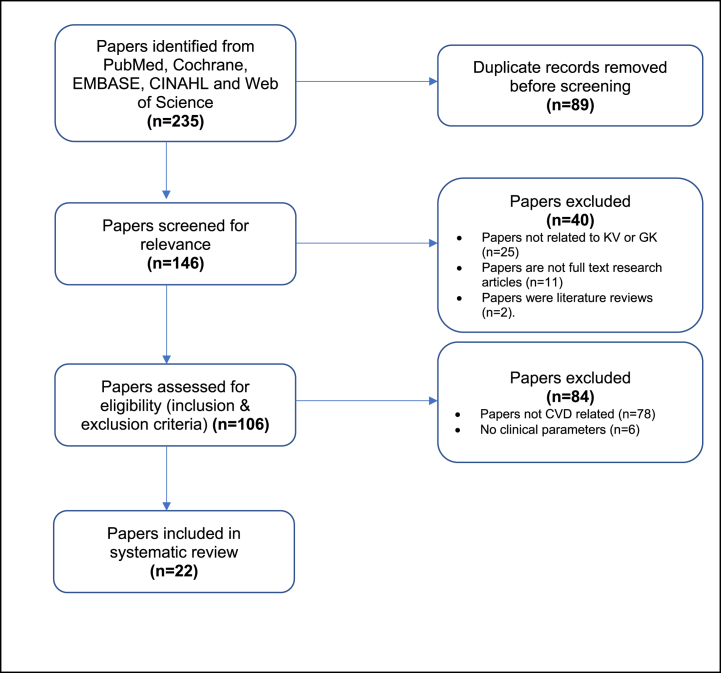
235 research papers were identified from the database search from PubMed, Cochrane, EMBASE, CINAHL, and Web of Science (WOS). Of these, 89 duplicates were removed before screening the papers for relevance. Through initial screening for relevance (by screening/reading the titles, abstracts, and conclusions of the research papers), 40 papers were excluded due to at least one of the following reasons: (1) Paper was not related to Kolaviron (KV) or Garcinia kola (GK); (2) Papers were not original full-text research articles (i.e. conference proceedings, conference abstracts, book chapters, papers on chemical information, chemical isolation, or patency papers); (3) Papers were focussed on reviews (literature review or systematic review papers) not related to KV, GK or cardiovascular diseases (CVDs). No paper was found to be solely focused on a literature review or systematic review of KV, GK, and CVDs.
106 papers were assessed for eligibility using our stated inclusion and exclusion criteria, and 84 papers were excluded as they did not meet the eligibility criteria. Papers excluded here were either not CVD-related (n = 78) or did not evaluate any clinical parameters or biomarkers stated in the inclusion criteria (n = 6). This resulted in 22 full-text papers that met this systematic review's eligibility criteria. Fig. 1 shows a flow chart of the literature search and selection process used in this systematic review.
Fig. 1.
Flow diagram of literature search and selection of papers included in systematic review.
3.2. Characteristics of included studies
The characteristics of the 22 studies included in this systematic review are summarized in Table 2 (for in vivo studies) and Table 3 (for in vitro studies). Upon analysis, 86% of the studies included were animal in vivo studies (n = 19) conducted in rats, while three papers (14%) were in vitro studies using different tissue cells. While clinical studies were not excluded from our database search strategy, our search did not return any clinical study conducted on the subject area yet. This is an important area for future research.
Table 2.
Characteristics (study design, protocol, and outcomes) of included in vivo animal studies.
| AUTHOR (S), YEAR | STUDY DESIGN | DOSE AND DURATION | PRIMARY OUTCOME MEASURE | MAIN RESULTS | STUDY'S MAIN CONCLUSION |
|---|---|---|---|---|---|
| ADARAMOYE AND ADEYEMI 2006 | 70 Male Wistar rats; n = 10/group; Simple randomization* | KV (100 mg/kg) daily; 2 weeks | CHOL, Lipid level, Triglycerides, LPO | Significant reduction in CHOL, Lipid levels and LPO | KV possesses significant hypolipidaemic effect |
| ADARAMOYE AND LAWAL 2015 | 42 Male Wistar rats; n = 6/group; Simple randomization* | KV (100 & 200 mg/kg) daily; 4 weeks | CK-MB, LDH, CHOL, Lipid level, Antioxidants, LPO, Atherogenic index | Significant reduction in CK-MB, LDH; Significant reduction in CHOL, Lipid levels and LPO, Significant increase in antioxidant levels | KV possesses significant cardioprotection |
| ADARAMOYE ET AL., 2005 | 28 Male Wistar rats; n = 4/group; Simple randomization* | KV (100 & 200 mg/kg) daily; 8 weeks | CHOL, Lipid level, Triglycerides, LPO | Significant reduction in CHOL, Lipid levels, Triglycerides, LPO | KV exhibits hypocholesterolaemic effect and possible anti-atherogenic effect |
| ADARAMOYE ET AL., 2012 | 24 Male Wistar rats; n = 6/group; Simple randomization* | KV (200 mg/kg) daily; 3 weeks | CK, Antioxidants, LPO | Significant reduction in CK, LPO; Significant increase in antioxidant levels | KV exhibits significant protective effect against l-Name, an hypertensive agent |
| ADEJOR ET AL., 2016 | 60 Male and female Wistar rats; n = 6/group; Simple randomization* | GK (200 mg/kg) daily; 3 weeks | CHOL, Lipid level, Atherogenic risk predictor indices | Significant reduction in CHOL, LDL; Significant increase in HDL, atherogenic risk predictor index | GK bioflavonoid has potential therapeutic efficacy in the management of hyperlipidaemia |
| ADOGA ET AL., 2021 | 48 Male Sprague Dawley rats; n = 6/group; Simple randomization* | KV (100 & 200 mg/kg) daily; 4 weeks | BP, CRP, Cardiac Troponin, CK-MB, CK, Lipid level, eNOS, ACE, antioxidant | Significant reduction in elevated BP, CRP, CK-MB, CK, Cardiac Troponin, ACE; increase in antioxidant levels, eNOS | KV attenuated oxidative cardiovascular injury and could serve a protective effect against cardiovascular injury |
| ADOGA ET AL., 2022 | 48 Male Sprague Dawley rats; n = 6/group; Simple randomization* | KV (100 & 200 mg/kg) daily; 4 weeks | P38 MAPK, ACE, eNOS, PK | Significant increase in eNOS; Significant decrease in P38 MAPK, ACE, PK | KV modulated the gene expression of P38 MAPK, ACE, eNOS, and PK suggesting a therapeutic effect and target for cardiovascular dysfunction |
| AJANI ET AL., 2008 | 30 Male Wistar rats; n = 5/group; Simple randomization* | KV (100 & 200 mg/kg) once to twice daily; 4 weeks | CHOL, Lipid level, Atherogenic index | No significant impact on CHOL, Lipid levels and Atherogenic index. | KV does not offer any cardioprotection |
| AKINRINDE ET AL., 2016 | 40 Male Wistar rats; n = 8/group; Simple randomization* | KV (100 & 200 mg/kg) daily; 2 weeks | BP, CRP, CK-MB, LDH, NO, Antioxidant, QRS, QT, Pwave | Significant reduction in BP, CRP, CK-MB, LDH: increase in antioxidant levels | KV is protective against cardiac damage, with possible BP lowering effect |
| DJÉTOUAN ET AL., 2022 | 30 Male Wistar rats; n = 6/group; Simple randomization* | GK (600 and 1000 mg/kg) daily; 4 weeks | CHOL, Lipid level | Significant reduction in Triglycerides, CHOL, LDL; significant Increase in HDL | GK could be a potential target for preventing dyslipidaemia and hyperglycaemia as well as management of cardiometabolic diseases |
| OLADAPO ET AL., 2017 | 48 Male Wistar rats; n = 8/group; Simple randomization* | KV (100 mg/kg) five times a week; 12 weeks | CHOL, Lipid level, Atherogenic index | Significant improvement in cholesterol, lipid profile and atherogenic index | KV could be beneficial in the management of atherosclerosis |
| OLAJIDE 1999 | Swiss Albino mice; n/group not stated; Simple randomization* | KV (100 mg/kg) daily; Duration Not mentioned | Platelet aggregation | KV displayed significant protection against thrombosis | KV possesses marked antithrombotic activity |
| OLATOYE ET AL., 2022 | 72 Male and female Wistar rats; n = 6/group; Simple randomization* | KV (50, 100 & 200 mg/kg) daily; 8 weeks | BP, CHOL, Lipid level, Antioxidants | Significant reduction in SBP, DBP & MAP; Significant reduction in CHOL and lipid levels; Increase in antioxidant levels. | KV possesses significant antihypertensive effect |
| OMOLE ET AL., 2018 | 30 Male Wistar rats; n = 5/group; Simple randomization* | KV (200 & 400 mg/kg) daily; 2 weeks | CK, LDH, Cardiac Troponin, Antioxidants, MPO | Significant reduction in CK, LDH, MPO, Cardiac Troponin; Increase in antioxidant levels | KV exhibits cardioprotective effect and maybe useful in management of cardiovascular injury |
| OYAGBEMI ET AL., 2016B | 60 Male Wistar rats; n = 10/group; Simple randomization* | KV and GK (100 & 200 mg/kg) daily; 14 days | HR, QRS, QT, Pwave, CK-MB, Lipid level, LDH, MPO, Antioxidant, | Significant amelioration of HR, QRS, QT, P-wave; Significant reduction in CK-MB, MPO, LDH; Increase in antioxidant level, Lipid profile improvement | KV and GK showed cardioprotective and anti-atherosclerotic effect |
| OYAGBEMI ET AL., 2018A | 60 Male rats; n = 10/group; Simple randomization* | KV (100 & 200 mg/kg) daily; 1 week | HR, QRS, QT, Pwave, Cardiac Troponin, CRP, CK-MB, LDH, NOS, Antioxidant, | Significant amelioration of HR, QRS, QT, P-wave; Significant reduction on CK-MB, LDH, CRP; Increase in antioxidant level, NOS | KV is cardioprotective causing reversal of cardiac damage |
| OYAGBEMI ET AL., 2018B | 40 Male Wistar rats; n = 10/group; Simple randomization* | KV (100 & 200 mg/kg) daily; 7 days | HR, QRS, QT, Pwave, Cardiac Troponin, CRP, NO, Antioxidant, | Significant amelioration of HR, QRS, QT, P-wave; Significant reduction on Cardiac Troponin, CRP; Increase in antioxidant level, NO Levels | KV may offer a new therapeutic regimen for treatment of cardo-renal dysfunction associated with arteriosclerosis, hypertension, and other CVDs |
| OYAGBEMI ET AL., 2018C | 15 Male Wistar rats (Isolated rat heart); n = 5/group; Simple randomization* | KV and GK. Dose and Duration not stated. | P38 MAPK, JNK | Significant reduction in P38 MAPK and p-JNK1 | KV and GK offer cardioprotection in ischaemic/reperfusion rat heart model |
| UCHE AND OSAKPOLO 2018 | 32 Male Sprague Dawley rats; n = 8/group; Simple randomization* | KV and GK (200 mg/kg) daily; 6 weeks | BP, CHOL, Lipid level | Significant reduction in SBP, DBP, MAP, PP; Lipid profile improvement | KV attenuates elevation in BP and ameliorates dyslipidaemia |
KV: Kolaviron; GK: Garcinia kola; GB1: Garcinia bioflavonoid I; BP: Blood Pressure; CHOL: Cholesterol; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure; MAP: Mean Arterial Pressure; Lipid level (Includes LDL, HDL); HDL: High density Lipoprotein; LDL: Low density Lipoprotein; LPO: Lipid Peroxidation; MPO: Myeloperoxidase; CK: Creatine Kinase; CK-MB: Creatine Kinase – Myoglobin binding; LDH: Lactate Dehydrogenase; CRP: C-reactive protein; NO: Nitric oxide; eNOS: Endothelial Nitric Oxide Synthase; ACE: Angiotensin converting enzyme; VSMC: Vascular smooth muscle cells; P38 MAPK: Mitogen activated protein kinases; PK: Protein Kinases; JNK: June N-Terminal Kinase; PP: Pulse Pressure; CVDs: Cardiovascular diseases; HR: Heart rate; QRS: Quantum Resonance System; *: Simple randomization into control and treatment groups.
Table 3.
Characteristics (study design, protocol, and outcomes) of included in vitro studies.
| AUTHOR (S), YEAR | STUDY DESIGN; CELL/TISSUES USED | DOSE AND DURATION | PRIMARY OUTCOME MEASURE | MAIN RESULTS | STUDY'S MAIN CONCLUSION |
|---|---|---|---|---|---|
| ADARAMOYE AND MEDEIROS 2009 | In Vitro; Isolated male superior mesenteric arteries obtained from male wistar rats. | KV (1, 10, 30, 100, 300, 500 & 1000 μg/ml); Duration not mentioned | Ca2+ or K+ mediated relaxation | Vasorelexant effect of KV demonstrated | KV possesses significant vasodilatory effect on the endothelium |
| CHEN ET AL., 2022 | In vitro; HepG2 cells obtained from Shangai Cell bank (Shangai, China) | GB1; dose not stated; 24 h duration. | Lipid levels, antioxidant | GBI ameliorated lipid deposition and reduced ROS production | GBI has significant bioactivity in improving lipid metabolism and profile. |
| OYAGBEMI ET AL., 2016A | In Vitro; VSMC obtained from RCMI Core Lab, TSU, Houston (Houston, USA). | KV (25–100 μg/ml); 4 days | NO, VSMC proliferation | Significant attenuation of increased NO levels, Significant reduction in VSMC growth | KV maybe able to mitigate against cardiovascular conditions that involve cell proliferation, free radical generation and inflammatory processes (eg Hypertension, diabetes and stroke) |
KV: Kolaviron; GK: Garcinia kola; GB1: Garcinia bioflavonoid I; NO: Nitric oxide; VSMC: Vascular smooth muscle cells; P38 MAPK: Mitogen activated protein kinases; PK: Protein Kinases; JNK: June N-Terminal Kinase.
For the animal studies, male test animals were the most prevalent (n = 14) with few studies using both male and female test animals (n = 2) or failing to specify the sex of the test animals (n = 2). Wistar rats were the most prevalent, followed by Sprague-Dawley rats. The number of test animals used in these studies varied from 24 to 72, with a total of 686 rats used in all studies. In all studies, the test animals were allowed to acclimatize to the environment before the start of experimentation. For the in vitro studies, the tissue cells used include isolated male superior mesenteric arteries (n = 1), HepG2 cells (n = 1), and Vascular smooth muscle cells (n = 1). No study used more than one tissue cell in its research.
While this systematic review did not enforce any restriction on the language in which the papers were written, all studies (n = 22) in this review were written in English. Most of the included studies were conducted in Nigeria, West Africa (n = 18), followed by South Africa (n = 2), Cote d’Ivoire, and China (n = 1). The duration of the animal studies in the eligible studies for this research varied from 1 week to 16 weeks. However, most studies (n = 11) were conducted for a minimum of 3 weeks (9 studies had a duration of 4 weeks and above, while two studies had a duration of at least three weeks). The duration of experimentation for the in vitro studies was mainly unstated, with only one study indicating a duration of 4 days.
Most of the included studies in this systematic review administered KV (n = 16). In contrast, others administered only GK (n = 2), a combination of KV and GK (n = 3), or Garcinia bioflavonoid 1 (GB1) as the drug of choice. GB1 is one of the main active chemical compositions of and isolated from KV or GK [63]. The most widely used route of administration was oral using the oral gavage, and administration was done daily. The dose of administration of KV to test animals (animal study) in the included eligible studies varied from 50 mg/kg to 400 mg/kg, with the most widely used dose being 100 mg/kg, which is the therapeutic dose [49,64]. The dose of GK administered in the included studies varied from 100 mg/kg to 600 mg/kg. For the in vitro study, KV was administered at a dose range of 1 μg/ml to 1000 μg/ml.
3.3. Methodological quality assessment of included studies
The quality of the included studies was evaluated using the 10-item modified CAMARADE checklist [59,60], as represented in Table 4. All studies included in this review were published in peer-reviewed journals (n = 22) but did not include a statement denoting allocation concealment, blinded assessment of outcome, or sample size calculation (N = 0). Of these studies, 77% (n = 17) included a statement of control of temperature and random allocation of treatment or control groups. Additionally, 82% of included studies (n = 18) demonstrated the use of animal experimental models, use of animals with induced CVD risk factors, and included a statement of compliance with animal welfare regulation while 86% of included studies (n = 19) included a statement of potential conflict of interest.
The median value of the quality score of included studies in this review was 6 (with a range of 3–7). The mean value of the quality score was 5.864 ± 0.296. Following a one-way ANOVA analysis with Tukey's multiple comparison test, there was no statistically significant (P>0.05) difference between the individual quality scores of the studies included in this systematic review.
3.4. Risk of bias assessment of included studies
The assessment of the risk of bias of included studies in this review is presented in Fig. 2 using the SYRCLE risk of bias tool [61,62]. All included studies had a low risk of attrition and reporting bias in terms of incomplete outcome data and selective outcome reporting. Additionally, almost all included studies had a low risk of performance and detection bias in terms of blinding of participants and blinding of outcome assessment. In terms of selection bias, most of the studies demonstrated a low risk related to random sequence generation, as almost all of them used a simple randomization process to assign test animals to different test groups randomly. However, these studies demonstrated an unclear risk related to allocation concealment. This is because it was unclear from the study protocol of these studies if the investigator allocating the test animals to groups could foresee such an assignment. Even though, a simple randomization method was used for most studies in this review. This is insufficient to suggest that the allocation to different groups was concealed from the investigator. Other sources of bias, such as data contamination problems, data analysis problems, or study design problems, were captured under “other bias.” The general lack of these problems denoted a low risk, and all included studies generally had a low risk in this regard.
Fig. 2.
Risk of bias assessment of eligible (selected) studies.
3.5. Effect of interventions (summary of outcomes from included studies)
Of all 22 eligible studies included in this review, 21 studies demonstrated significant positive results on the management of CVD or mitigation of CVD-associated risk factors (clinical parameters or biomarkers) evaluated in the study. Only one study showed no form of significant CVD benefit associated with the administration of KV or GK [44]. In this study, KV demonstrated no significant change in cholesterol, lipid, and atherogenic index levels. This study concluded that KV does not offer any form of cardioprotection.
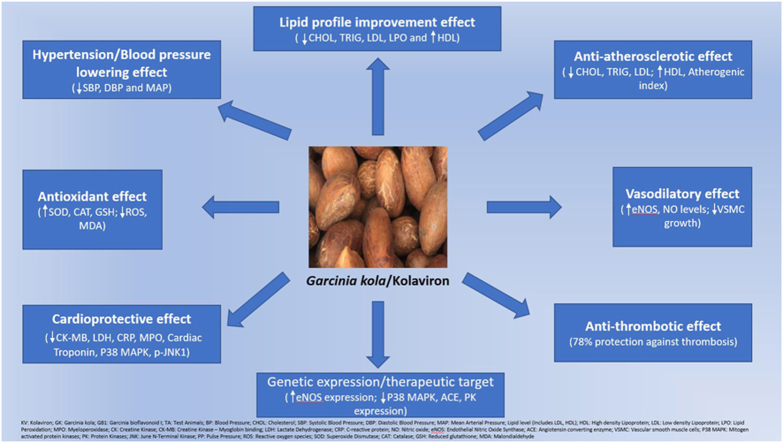
Based on the 21 studies that demonstrated significant mitigation of CVD risk factors, the impact of KV or GK on CVDs can be divided into eight emerging trends: (1) Hypertension/Blood pressure lowering effect; (2) Lipid profile improvement (Hypolipidaemic/hypocholesterolaemic effect); (3) Anti-atherosclerotic/Arteriosclerotic effect; (4) Anti-thrombotic effect; (5) Cardioprotection against cardiovascular injury; (6) Vasodilatory effect; (7) Antioxidant effects; and (8) Genetic expression and therapeutic target for cardiovascular dysfunction.
Of the 21 studies included in this review, six (n = 6) reported and demonstrated significant lipid profile improvement with the administration of KV or GK. KV or GK demonstrated marked hypolipidaemic, hypocholesterolaemic or anti-atherogenic effects in these studies. These studies confirm that KV or GK can be helpful in the management of lipid metabolic diseases such as hyperlipidaemia, hypercholesterolaemia, and dyslipidaemia, with the overall result of significantly reducing the levels of cholesterol, triglycerides, Low-density lipoprotein (LDL), Lipid peroxidation (LPO) and increasing the levels of High-density lipoprotein (HDL).
Furthermore, eight studies (n = 8) reported and demonstrated significant cardioprotection of KV or GK against cardiovascular injury. These studies demonstrated the ability of KV or GK to significantly lower Creatine Kinase (CK), Creatine Kinase – Myoglobin binding (CK-MB), LPO, Angiotensin-converting enzyme (ACE), Cardiac Troponin I, Lactate Dehydrogenase (LDH) as well as significantly increase antioxidant levels. Some of these studies significantly ameliorated Heart rate (HR), QRS, QT, and P-wave parameters.
Additionally, six studies (n = 6) reported and demonstrated significant blood pressure lowering and antihypertensive effects of KV or GK. In these studies, KV or GK demonstrated a marked reduction in Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP), Mean Arterial Pressure (MAP), Pulse Pressure (PP), and HR. In addition, these studies demonstrated the lipid profile and antioxidant improvement effect of KV or GK. Furthermore, two studies (n = 2) reported and demonstrated significant anti-atherosclerotic or anti-arteriosclerotic effects of KV or GK. One study each from the included studies demonstrated vasodilatory effect of KV on the endothelium, anti-thrombotic effect of KV and gene level therapeutic target for cardiovascular dysfunction. These effects are associated with the ability of KV or GK to vasodilate tissues, protect against thrombosis (78% protection), and significantly increase endothelial Nitric Oxide Synthase (eNOS) or significantly decrease Mitogen-activated protein kinases (P38 MAPK), ACE, and Protein Kinases (PK) respectively. Lastly, ten studies (n = 10) reported and demonstrated the antioxidant effect of KV and GK, the most reported pharmacological activity of KV and GK.
4. Discussion
Cardiovascular diseases (CVDs) are generally considered chronic diseases and, therefore, often require long-term treatment or management in affected patients. However, conventionally available standard-of-care medications have been associated with substantial side effects, huge economic costs that may lead to adherence problems, and potential worsening of CVD conditions in patients, especially in low- and middle-income countries [10,22,[66], [67], [68], [69]]. Therefore, the investigation into, development, and use of effective, affordable, and safe herbal medicines for the long-term management of CVDs is a research imperative, especially in low- and middle-income countries.
This systematic review of 22 eligible studies obtained from 5 different medical databases evaluated existing research evidence on the role of Kolaviron (KV) and Garcinia kola (GK) in the management of CVDs. This review found potential and promising evidence for the use of KV and GK in the management of several risk factors associated with CVDs, including hypertension and high blood cholesterol. Our methodological assessment of the quality of the studies included using the modified CAMARADE checklist suggests that the studies included in this systematic review are of relatively high quality, with no statistically significant difference in the quality of individual studies included in this review. Additionally, our assessment of the risk of bias of the included studies in this review using the SYRCLE tool suggests a low to moderate risk of bias. The results from the quality and risk of bias assessment of included studies validate the suitability, usefulness, and fit of included studies in this systematic review.
The effect of KV and GK on CVD and CVD risk factors from the included studies in this review can be divided into eight emerging trends: (1) Antihypertensive/Blood pressure lowering effect; (2) Lipid profile improvement (Hypolipidaemic/hypocholesterolaemic effect); (3) Anti-atherosclerotic/Arteriosclerotic effect; (4) Anti-thrombotic effect; (5) Cardioprotection against cardiovascular injury; (6) Vasodilatory effect; (7) Antioxidant effects; and (8) Genetic expression and therapeutic target for cardiovascular dysfunction.
4.1. Antihypertensive/blood pressure lowering effects of KV and GK
Hypertension (HT) is the leading preventable risk factor for CVDs and is strongly, independently, and linearly correlated with the risk of CVD and all-cause mortality [66]. In this review, we found promising evidence for the use of KV or GK in the management of HT. Of the 22 included papers, six (n = 6; 27%) demonstrated significant blood pressure lowering or anti-hypertensive effect of KV and GK, evidenced by a substantial reduction of Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP), Mean Arterial Pressure (MAP), Pulse Pressure (PP) and Heart rate (HR) in these studies.
While Uche and Osakpolo [43] demonstrated notable blood pressure (SBP, DBP, and MAP) lowering effect of KV in rats fed with the high salt diet for six weeks, Akinrinde et al. [57] demonstrated that rats treated with cobalt chloride and KV exhibited a dose-dependent reduction in SBP, DBP, and MAP when compared to animals treated with cobalt chloride only. High salt diet and Cobalt chloride are known to induce hypertension and lead to cardiac damage, respectively [43,57]. Olatoye et al. [21] demonstrated up to 32%, 23%, and 22% reduction in SBP, DBP, and MAP, respectively, in rats treated with KV in sucrose and ethanol experimental model of hypertension, while Adoga et al. [42] demonstrated that KV notably decreased elevated blood pressure levels in rats treated with KV and fructose/streptozotocin combination when compared to the control group. Oyagbemi et al., in several studies, demonstrated notable amelioration of heart rate elevated by different toxicants in each study [46,70,71]. Additionally, Oyagbemi and his team demonstrated notable amelioration of electrocardiogram parameters such as QRS, QT interval, and P-wave elevated by toxicants in these studies. QRS, QT interval, and P-wave are associated with the electrocardiogram measurement of how well the heart is performing its duty of pumping and distributing blood to the body system at a normal rate [72]. Finally, Adaramoye et al. [73] demonstrated the ability of KV to ameliorate the biochemical alterations caused by the administration of l-Name (N(G)-nitro-l-arginine methyl ester), a well-documented hypertensive agent [74]. All of these studies confirm the potential antihypertensive effect of KV, consistent with findings in literature in which herbal medicinal plants with flavonoid contents possess antihypertensive benefits, exert cardiovasculoprotective effects, and reduce the progression of CVDs [23,24].
4.2. Antioxidant effects of KV and GK
Apart from the antihypertensive effect observed in these studies, there are two more interesting general findings, which include the ability of KV to improve antioxidant and lipid profiles in test animals. Adaramoye et al., Akinrinde et al., Oyagbemi et al., Adoga et al., and Olatoye et al. [21,42,57,71,73,75] all demonstrated notable antioxidant effect of KV by improving the activities of antioxidant indices like Reduced glutathione (GSH), Superoxide Dismutase (SOD) and Catalase (CAT) in the blood and tissues (especially the heart, aorta, kidney, and liver) relative to the control groups. It is important to mention that the confirmed pharmacological effects of KV and GK are generally based on their known blood and tissue antioxidant activity [21,37,38,49]. A 90-day toxicological profiling of KV followed by a 30-day reversibility study suggested the long and sustained duration of antioxidant action of KV in blood and tissues long after the cessation of administration of KV [49]. This has huge implications for potential drug development and administration of KV in disease states.
4.3. Lipid profile improvement/anti-atherosclerotic/anti-thrombotic effects of KV and GK
Oyagbemi et al. [70], Uche and Osakpolo [43], Adoga et al. [42], and Olatoye et al. [21] demonstrated the possible lipid profile improvement ability of KV, which is associated with its ability to significantly reduce cholesterol, triglycerides, low-density lipoprotein (LDL) and increase high-density lipoprotein (HDL). This effect has a significant implication for the management of most cardiovascular diseases associated with lipid profile dysfunction, as a high lipid profile may lead to the accumulation of cholesterol in the blood vessels. This may inevitably lead to the clogging of the blood vessels, vasoconstriction of the blood vessels, and eventually an increase in blood pressure [45,65,[76], [77], [78]]. Hence, the ability of KV to improve lipid profile may have an impact on its ability to reduce blood pressure, ameliorate hypertension, and act as an antihypertensive agent.
Furthermore, the hypolipidaemic, hypocholesterolaemic, anti-atherosclerotic/arteriosclerotic effect of KV associated with its lipid profile improvement effect was demonstrated by Adaramoye et al., Adaramoye and Adeyemi, Adejor et al., Oladapo et al., and Djétouan et al. [41,45,[79], [80], [81]]. All these studies confirm the possible usefulness of KV in the management of CVDs associated with atherosclerosis, arteriosclerosis, hyperlipidaemia, hypercholesterolaemia, and dyslipidaemia. Indeed, the blood pressure lowering and lipid profile improvement potential of KV may be of high importance in the management of CVDs as hypertension and high lipid/cholesterol levels are the two key modifiable risk factors associated with CVDs [1,4].
In addition to the lipid profile improvement, hypolipidaemic, hypocholesterolaemic, and anti-atherosclerotic/arteriosclerotic effect of KV and GK, the possible antithrombotic effect of GK was demonstrated by Olajide [82]. Here, GK elicited a 78% protection against thrombosis. This is consistent with the literature in which flavonoids have been reported to have antithrombotic effects owing to their anti-inflammatory effects, platelet anti-aggregatory effect, prostaglandin synthesis inhibition, and ability to scavenge reactive oxygen species [[83], [84], [85]]. Indeed, the anti-inflammatory and antioxidant effects of GK have been confirmed in the literature [35,37].
4.4. Vasodilatory effect of KV and GK
In furtherance of the ability of KV to lower blood pressure, Adaramoye and Medeiros [86] provided evidence that KV may be able to induce vasodilation (or vasorelaxation) in isolated rat blood vessels. The findings from this study indicate that KV produces a concentration-dependent relaxation of rat superior mesenteric arteries, which involves blockade of extracellular Ca2+, inhibition of intracellular Ca2+ release, and the opening of potassium channels sensitive to 4-aminopyridine and charybdotoxin [86]. All of these effects provide some evidence that KV may be able to act as a Calcium channel blocker by blocking off Ca2+ influx through the Ca2+ channels present in the vascular smooth muscle cells [86]. This effect is similar to how Calcium channel blockers like Amlodipine are known to reduce blood pressure [87,88]. Indeed, the blood pressure-lowering effect of KV was comparable to that of Amlodipine in ethanol and sucrose experimental models of hypertension [21]. We should mention that calcium channel blockers and potassium channel openers are clinically used to manage hypertension due to their ability to induce vascular smooth muscle (VSMC) relaxation [86].
To bolster and validate the research on the ability of KV to induce VSMC relaxation, Oyagbemi et al. [89] demonstrated the possible effect of KV on VSMC relaxation, growth, and proliferation. The findings from this study confirmed that the treatment of VSMC with KV resulted in reduced VSMC growth and proliferation. The growth and proliferation of VSMC are associated with vasoconstriction, leading to an increase in blood pressure [86,89,90]. Furthermore, Oyagbemi et al. found that KV attenuated Angiotensin II-induced VSMC proliferation in a concentration- and time-dependent manner [89,91]. The role of Angiotensin II in the development of hypertension by promoting sodium and water retention and enhancing VSMC's vasoconstriction is well documented in the literature [92]. Adoga et al. [42] provided evidence for the ability of KV to inhibit the function of the enzyme, Angiotensin-converting enzyme (ACE), which is responsible for the conversion of Angiotensin I to Angiotensin II. By doing this, KV is possibly able to act as an Angiotensin-converting enzyme inhibitor (ACEI) and thereby possibly prevent vasoconstriction caused by Angiotensin II and prevent VSMC proliferation (leading to vasodilation). All of these, therefore, lead to a lowering of blood pressure. Indeed, the possible blood-lowering effect of KV has been demonstrated to be relatively similar to that of Lisinopril, an ACEI, in an experimental model of hypertension [48].
From our discussions so far, it is clear that the antihypertensive and cardiovascular effect of KV is one that involves several effects of KV which include (1) its blood pressure lowering effect, (2) its lipid profile improvement effect, (3) its antithrombotic effect, (4) its ability to induce vasodilation in VSMC via its ability to potentially block calcium channels and inhibit Angiotensin II led VSMC proliferation. In addition, the cardioprotective effect of KV against cardiotoxicity has been demonstrated in several experimental settings, alluding to its ability to protect the heart's functional integrity and its usefulness against cardiotoxic drugs [46,58].
4.5. Cardioprotective effect against cardiovascular injury
Adaramoye and Lawal, Omole et al., and Oyagbemi et al. [56,58,75] demonstrated the cardioprotective effect of KV in isoproterenol-induced cardiac injury, cyclophosphamide-induced cardiac toxicity, and ischaemic/reperfusion injury rat heart model respectively. Furthermore, Akinrinde et al. and Oyagbemi et al. [46,57,71] demonstrated the possible cardioprotective effect of KV in cobalt chloride-induced cardiorenal dysfunction, arsenic acid-induced cardiorenal dysfunction, and doxorubicin-induced cardiotoxicity respectively. The administration of KV in these cardiotoxic experimental settings ameliorated the induced alteration in heart tissue activities of lactate dehydrogenase (LDH), Creatine kinase (CK), Cardiac Troponin I (CTI), and C-reactive proteins (CRP), all of which can be indicative of cardiac damage or heart attack [51]. These results suggest that KV may be able to preserve the structural integrity of the cardiac cells and prevent the overproduction of LDH, CK, CTI, and CRP. These findings are consistent with the literature in which the cardioprotective effect of KV has been reported in cholesterol-fed rats [93]. The cardioprotective effect of KV may possibly be associated with its antioxidant activities as KV is able to significantly increase the antioxidant parameters, which were reduced by cardiotoxic drugs. An increase in antioxidant parameters in the aorta and heart tissues following the administration of KV has been reported by Olatoye et al. [21] and Olatoye and Akindele [49]. This is consistent with the findings of Mohamadin et al. and Ojha et al. [94,95], in which Andrographis paniculata extract and Lycopene increased cardiac antioxidant parameters in cardiotoxic experimental settings.
4.6. Genetic expression and therapeutic target for cardiovascular dysfunction
Findings from several studies included in this systematic review suggest that KV may mediate its CVD effects by regulating molecular signaling pathways that modulate various cellular functions [89]. Indeed, cardiovascular pathologies are characterized by vascular cell proliferation, inflammation, and increased oxidative stress that are associated with molecular signaling pathways and genetic expressions [57,96,97]. Adoga et al. [96] demonstrated the possible ability of KV to modulate (up-regulate) superoxide dismutase-2 (SOD-2), endothelial nitric oxide synthase (eNOS), and angiotensin-converting enzyme (ACE) mRNA expression compared to control in rats. By modulating the expression of these genes, it is possible that KV is able to exert its therapeutic effect and be a target for vascular dysfunction associated with CVD through the activation of the SOD-2, eNOS, and ACE signaling pathways [96]. For perspective, SOD-2 is one of the essential antioxidant enzymes that protect against elevated reactive oxygen species (ROS) in diverse organs and the vascular system, while eNOS plays a fundamental role in the vasorelaxation and modulation of involved in atherogenesis [21,98]. ACE, on the other hand, and as discussed earlier, plays a very important role in vasoconstriction by catalyzing the conversion of angiotensin I to angiotensin II [[99], [100], [101]].
Furthermore, Chen et al. [47] demonstrated that GBI, a significant constituent of KV, is able to reduce lipid deposition by upregulating the expression of Peroxisome proliferator-activated receptor-α (PPARα) and Sirtuin6 (SIRT6). In addition to this, Chen et al. demonstrated that KV is possibly able to suppress cell apoptosis by reducing oxidative stress and inflammatory factors of ROS, Interleukin-10 (IL10), and Tumour necrosis factor-α (TNFα), which are associated with lipid deposition. PPARα plays a vital role in lipid metabolism, and an upregulation of its expression will lead to the regulation and breakdown of lipids by facilitating the uptake and oxidation of fatty acids [102]. SIRT6 is also a key regulator of lipid metabolism, and it promotes liver oxidation of lipids by activating PPARα.
5. Limitation of study and future direction
The results and discussion from this systematic review should be considered within the context of some of its limitations. One of these limitations is that sex-specific differences were not evaluated in this systematic review. This cannot be overlooked, especially within the context of CVDs, in which studies have shown that there are indeed differences between the male and female sexes in terms of control and management of CVD risk factors [103,104]. Most of the test animals (approx. 80%) used in the included studies in this review were male animals, with very few studies using both male and female animals. Future reviews should consider evaluating sex-specific differences in terms of response to KV in the management of CVDs. Additionally, no clinical study in the literature has evaluated the effect of KV in CVDs in humans. It is recommended that future research should consider clinical evaluation of the effect of KV in the management of CVDs. Finally, a thorough quantitative synthesis of included studies in the form of a meta-analysis is recommended to further validate some of the findings in this paper.
6. Conclusion
From this systematic review, it can be concluded that KV is possibly helpful in the management of CVD risk factors such as hypertension and high lipids/cholesterol. Several included studies in this review demonstrated the possible antihypertensive, lipid improvement, antioxidant, and signaling pathway modulation effects of KV. This potentially makes KV an excellent therapeutic target for the management of CVDs and potentially a source of an investigational new drug for the management of CVDs.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure of interest
The authors report no conflict of interest.
Consent to participate
Not applicable.
Consent for publication
Available on request.
Availability of data and material
The data associated with this study has been deposited into a publicly available repository (Mendeley data) and is also available as an attachment to this journal article. Mendeley Data Reserved https://doi.org/10.17632/k7s3dsf7y9.1.
Code availability
Not applicable.
Author declaration
All listed authors have read and approved the submitted manuscript.
Ethics declaration
Review and approval by an ethics committee was not needed for this study because this is a systematic review study and not an animal study. Informed consent was not required for this study because this is a systematic review study, not a human one.
CRediT authorship contribution statement
Francis J. Olatoye: Writing – review & editing, Writing – original draft, Validation, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Abidemi.J. Akindele: Writing – review & editing, Supervision, Methodology, Conceptualization. Olufunsho Awodele: Writing – review & editing, Supervision, Methodology, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e27333.
Contributor Information
Francis J. Olatoye, Email: olatoyef@mcmaster.ca, foye57@yahoo.com.
Abidemi.J. Akindele, Email: jakindele@unilag.edu.ng.
Olufunsho Awodele, Email: oawodele@unilag.edu.ng.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.World Health Organization . WHO Fact Sheets; 2021. World Health Organisation Key Facts on Cardiovascular Diseases (CVDs)https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds [Google Scholar]
- 2.Forouzanfar M.H., Liu P., Roth G.A., Ng M., Biryukov S., Marczak L., et al. Global burden of hypertension and Systolic blood pressure of at least 110 to 115 mm Hg. JAMA, J. Am. Med. Assoc. 2017;317(6):648. doi: 10.1001/jama.2016.19043. 1990-2015.[Erratum appears in JAMA. 2017 Feb 14. PMID: 28114659] [DOI] [PubMed] [Google Scholar]
- 3.Vaduganathan M., Mensah G.A., Turco J.V., Fuster V., Roth G.A. The global burden of cardiovascular diseases and risk: a Compass for future health. J. Am. Coll. Cardiol. 2022 doi: 10.1016/j.jacc.2022.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Tsao C.W., Aday A.W., Almarzooq Z.I., Alonso A., Beaton A.Z., Bittencourt M.S., et al. Heart disease and stroke Statistics-2022 update: a report from the American heart association. Circulation. 2022;145 doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 5.Ruel M. Tackling the other Pandemic: the Rise in cardiovascular diseases. Can. J. Cardiol. 2022;38:849–851. doi: 10.1016/j.cjca.2022.06.001. [DOI] [Google Scholar]
- 6.Statistics Canada . 2022. Table 13-10-0801-01 Leading Causes of Death, Total Population (Age Standardization Using 2011 Population) [DOI] [Google Scholar]
- 7.Stanaway J.D., Afshin A., Gakidou E., Lim S.S., Abate D., Abate K.H., et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018:392. doi: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roth G.A., Abate D., Abate K.H., Abay S.M., Abbafati C., Abbasi N., et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maraboto C., Ferdinand K.C. Update on hypertension in African-Americans. Prog. Cardiovasc. Dis. 2020;63 doi: 10.1016/j.pcad.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Al Disi S.S., Anwar M.A., Eid A.H. Anti-hypertensive herbs and their mechanisms of action: Part I. Front. Pharmacol. 2016;6:1–24. doi: 10.3389/fphar.2015.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amsterdam E.A., Venugopal S., Bui J., Thevakumar B., Thinda A., Virk S., et al. Management of hypertension: JNC 8 and beyond. Cardiovasc Innov Appl. 2016;1 doi: 10.15212/cvia.2016.0030. [DOI] [Google Scholar]
- 12.Chiong J.R., Aronow W.S., Khan I.A., Nair C.K., Vijayaraghavan K., Dart R.A., et al. Secondary hypertension: current diagnosis and treatment. Int. J. Cardiol. 2008;124 doi: 10.1016/j.ijcard.2007.01.119. [DOI] [PubMed] [Google Scholar]
- 13.Taler S.J. Initial treatment of hypertension. N. Engl. J. Med. 2018;378 doi: 10.1056/nejmcp1613481. [DOI] [PubMed] [Google Scholar]
- 14.Sinha A., Bagga A. Evaluation and management of hypertension. Indian Journal of Practical Pediatrics. 2009;11 doi: 10.1016/b978-1-4160-5185-5.00065-1. [DOI] [Google Scholar]
- 15.Archer J.S. Evaluation and treatment of hypertension. Prim. Care Update OB/GYNS. 2000;7:1–6. doi: 10.1016/S1068-607X(99)00032-3. [DOI] [PubMed] [Google Scholar]
- 16.Gupta R., Guptha S. Strategies for initial management of hypertension. Indian J. Med. Res. 2010:132. [PMC free article] [PubMed] [Google Scholar]
- 17.Oliva R.V., Bakris G.L. Management of hypertension in the elderly population. Journals of Gerontology - Series A Biological Sciences and Medical Sciences. 2012;67 doi: 10.1093/gerona/gls148. [DOI] [PubMed] [Google Scholar]
- 18.Mashour N.H., Lin G.I., Frishman W.H. Herbal medicine for the treatment of cardiovascular disease: clinical considerations. Arch. Intern. Med. 1998;158 doi: 10.1001/archinte.158.20.2225. [DOI] [PubMed] [Google Scholar]
- 19.Rastogi S., Pandey M.M., Rawat A.K.S. Traditional herbs: a remedy for cardiovascular disorders. Phytomedicine. 2016;23 doi: 10.1016/j.phymed.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Shaito A., Thuan D.T.B., Phu H.T., Nguyen T.H.D., Hasan H., Halabi S., et al. Herbal medicine for cardiovascular diseases: Efficacy, mechanisms, and safety. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olatoye F.J., Akindele A.J., Onwe S. Ameliorative effect of Kolaviron, an extract of Garcinia kola seeds, on induced hypertension. J. Compl. Integr. Med. 2022;19:37–46. doi: 10.1515/jcim-2020-0354. [DOI] [PubMed] [Google Scholar]
- 22.Akindele A.J., Iyamu E.A., Dutt P., Satti N.K., Adeyemi O.O. Ameliorative effect of hydroethanolic leaf extract of Byrsocarpus coccineus in alcohol- and sucrose-induced hypertension in rats. J Tradit Complement Med. 2014;4:177–188. doi: 10.4103/2225-4110.129562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark J.L., Zahradka P., Taylor C.G. Efficacy of flavonoids in the management of high blood pressure. Nutr. Rev. 2015 doi: 10.1093/nutrit/nuv048. [DOI] [PubMed] [Google Scholar]
- 24.Maaliki D., Shaito A.A., Pintus G., El-Yazbi A., Eid A.H. Flavonoids in hypertension: a brief review of the underlying mechanisms. Curr. Opin. Pharmacol. 2019;45 doi: 10.1016/j.coph.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 25.Iwu M.M., Igboko O.A., Okunji C.O., Tempesta M.S. Antidiabetic and aldose reductase activities of biflavanones of Garcinia kola. J. Pharm. Pharmacol. 1990;42:290–292. doi: 10.1111/j.2042-7158.1990.tb05412.x. [DOI] [PubMed] [Google Scholar]
- 26.Iwu M., Igboko O. Flavonoids of Garcinia kola seeds. J. Nat. Prod. 1982;45:650–651. doi: 10.1021/np50023a026. [DOI] [Google Scholar]
- 27.Iwu M.M. Biflavanones of Garcinia: pharmacological and biological activities. Prog. Clin. Biol. Res. 1986;213:485–488. [PubMed] [Google Scholar]
- 28.Iwu M.M. second ed. 2014. Handbook of African Medicinal Plants. [DOI] [Google Scholar]
- 29.Iwu M.M. Antihepatoxic constituents of Garcinia kola seeds. Experientia. 1985;41:699–700. doi: 10.1007/BF02007729. [DOI] [PubMed] [Google Scholar]
- 30.Wickens G.E., Burkill H.M. The useful plants of west Tropical Africa. Kew Bull. 1986;41:471. doi: 10.2307/4102963. [DOI] [Google Scholar]
- 31.Konziase B. Protective activity of biflavanones from Garcinia kola against Plasmodium infection. J. Ethnopharmacol. 2015;172:214–218. doi: 10.1016/j.jep.2015.06.038. [DOI] [PubMed] [Google Scholar]
- 32.Antia B.S., Pansanit A., Ekpa O.D., Ekpe U.J., Mahidol C., Kittakoop P. α-Glucosidase inhibitory, aromatase inhibitory, and antiplasmodial activities of a biflavonoid gb1 from garcinia kola stem bark. Planta Med. 2010;76 doi: 10.1055/s-0029-1186081. [DOI] [PubMed] [Google Scholar]
- 33.Durand D.N., Farid B.M., Adolphe A., Haziz S., Pacome A.N., Hubert A.S., et al. Phytochemical screening and biological activities of Garcinia kola (bark, leaves, and seeds) collected in Benin. Afr. J. Microbiol. Res. 2015;9 doi: 10.5897/ajmr2015.7611. [DOI] [Google Scholar]
- 34.Ibironke G.F., Olaleye S.B., Balogun O., Aremu A. Effects of diets containing seeds of Garcinia kola (Heckel) on gastric acidity and experimental ulceration in rats. Phytother Res. 1997;11:312–313. doi: 10.1002/(SICI)1099-1573(199706)11:4<312::AID-PTR88>3.0.CO;2-7. [DOI] [Google Scholar]
- 35.Farombi E., Adewoye E., Owoyele B., Onasanwo S. Analgesic and anti-inflammatory effects of kolaviron (A Garcinia kola seed extract) Afr. J. Biomed. Res. 2000;3:171–174. [Google Scholar]
- 36.Adaramoye O.A., Farombi E.O., Adeyemi E.O., Emerole G.O. Comparative study on the antioxidant properties of flavonoids of Garcinia kola seeds. Pakistan J. Med. Sci. 2005;21:331–339. [Google Scholar]
- 37.Farombi E.O., Nwaokeafor I.A. Anti-oxidant mechanisms of kolaviron: studies on serum lipoprotein oxidation, metal chelation and oxidative membrane damage in rats. Clin. Exp. Pharmacol. Physiol. 2005;32:667–674. doi: 10.1111/j.0305-1870.2005.04248.x. [DOI] [PubMed] [Google Scholar]
- 38.Farombi E.O., Akanni O.O., Emerole G.O. Antioxidant and scavenging activities of flavonoid extract (Kolaviron) of Garcinia kola seeds. Pharm. Biol. 2002;40:107–116. doi: 10.1076/phbi.40.2.107.5838. [DOI] [Google Scholar]
- 39.Farombi E.O., Alabi M.C., Akuru T.O. Kolaviron modulates cellular redox status and impairment of membrane protein activities induced by potassium bromate (KBrO3) in rats. Pharmacol. Res. 2002;45:63–68. doi: 10.1006/phrs.2001.0907. [DOI] [PubMed] [Google Scholar]
- 40.Akintonwa A., Essien A.R. Protective effects of garcinia kola seed extract against paracetamol-induced hepatotoxicity in rats. J. Ethnopharmacol. 1990;29:207–211. doi: 10.1016/0378-8741(90)90057-Z. [DOI] [PubMed] [Google Scholar]
- 41.Adaramoye O.A., Adeyemi E.O. Hypoglycaemic and hypolipidaemic effects of fractions from kolaviron, a biflavonoid complex from Garcinia Kola in streptozotocin-induced diabetes mellitus rats. J. Pharm. Pharmacol. 2006;58:121–128. doi: 10.1211/jpp.58.1.0015. [DOI] [PubMed] [Google Scholar]
- 42.Adoga J.O., Channa M.L., Nadar A. Kolaviron attenuates cardiovascular injury in fructose-streptozotocin induced type-2 diabetic male rats by reducing oxidative stress, inflammation, and improving cardiovascular risk markers. Biomed. Pharmacother. 2021;144 doi: 10.1016/j.biopha.2021.112323. [DOI] [PubMed] [Google Scholar]
- 43.Uche O.K., Osakpolo R.F.A. Kolaviron attenuates elevation in blood pressure and ameliorates dyslipidemia in salt-induced hypertensive Sprague-Dawley rats. Afr. J. Biomed. Res. 2018;21:219–224. [Google Scholar]
- 44.Ajani E.O., Shallie P.D., Adegbesan B.O., Salau B.A., Adesanya M. Protective effect of Garcinia Kola (Kolaviron) extract on predisposition of rats to cardiovascular diseases following separate administration of amodiaquine and artesunate. Afr. J. Tradit., Complementary Altern. Med. 2008;5:180–186. doi: 10.4314/ajtcam.v5i2.31271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adaramoye O.A., Nwaneri V.O., Anyanwo K.C., Farombi E.O., Emerole G.O. Possible anti-atherogenic effect of kolaviron (a Garcinia kola seed extract) in hypercholesterolaemic rats. Clin. Exp. Pharmacol. Physiol. 2005;32:40–46. doi: 10.1111/j.1440-1681.2005.04146.x. [DOI] [PubMed] [Google Scholar]
- 46.Oyagbemi A.A., Omobowale T.O., Olopade J.O., Farombi E.O. Kolaviron and Garcinia kola attenuate doxorubicin-induced cardiotoxicity in Wistar rats. J. Compl. Integr. Med. 2018;15 doi: 10.1515/jcim-2016-0168. [DOI] [PubMed] [Google Scholar]
- 47.Chen H.X., Yang F., He X.Q., Li T., Sun Y.Z., Song J.P., et al. Garcinia biflavonoid 1 improves lipid metabolism in HepG2 cells via regulating PPARα. Molecules. 2022;27 doi: 10.3390/molecules27061978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olatoye F.J., Akindele A.J., Balogun O.E., Awodele O., Adejare A.A. Antihypertensive effect of Kolaviron, a bioflavonoid from Garcinia kola, in L-NAME induced hypertension in rats. Nat. Prod. Commun. 2023;18:1–10. [Google Scholar]
- 49.Olatoye F.J., Akindele A.J. Ninety-day oral toxicological profiling of Kolaviron (an extract of Garcinia kola) in male and female rats. Drug Chem. Toxicol. 2023;46:1–14. doi: 10.1080/01480545.2021.1997543. [DOI] [PubMed] [Google Scholar]
- 50.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kemp M., Donovan J., Higham H., Hooper J. Biochemical markers of myocardial injury. Br. J. Anaesth. 2004;93 doi: 10.1093/bja/aeh148. [DOI] [PubMed] [Google Scholar]
- 52.Viswanatha Swamy A.H.M., Patel U.M., Koti B.C., Gadad P.C., Patel N.L., Thippeswamy A.H.M. Cardioprotective effect of Saraca indica against cyclophosphamide induced cardiotoxicity in rats: a biochemical, electrocardiographic and histopathological study. Indian J. Pharmacol. 2013;45 doi: 10.4103/0253-7613.106434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zakynthinos E., Pappa N. Inflammatory biomarkers in coronary artery disease. J. Cardiol. 2009;53 doi: 10.1016/j.jjcc.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 54.Phosat C., Panprathip P., Chumpathat N., Prangthip P., Chantratita N., Soonthornworasiri N., et al. Elevated C-reactive protein, interleukin 6, tumor necrosis factor alpha and glycemic load associated with type 2 diabetes mellitus in rural Thais: a cross-sectional study. BMC Endocr. Disord. 2017;17 doi: 10.1186/s12902-017-0189-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patel V., Upaganlawar A., Zalawadia R., Balaraman R. Cardioprotective effect of melatonin against isoproterenol induced myocardial infarction in rats: a biochemical, electrocardiographic and histoarchitectural evaluation. Eur. J. Pharmacol. 2010;644 doi: 10.1016/j.ejphar.2010.06.065. [DOI] [PubMed] [Google Scholar]
- 56.Adaramoye O.A., Lawal S.O. Kolaviron, a biflavonoid fraction from Garcinia kola, protects against isoproterenol-induced injury by mitigating cardiac dysfunction and oxidative stress in rats. J. Basic Clin. Physiol. Pharmacol. 2015;26:65–72. doi: 10.1515/jbcpp-2013-0139. [DOI] [PubMed] [Google Scholar]
- 57.Akinrinde A.S., Omobowale O., Oyagbemi A., Asenuga E., Ajibade T. Protective effects of kolaviron and gallic acid against cobalt-chloride-induced cardiorenal dysfunction via suppression of oxidative stress and activation of the ERK signaling pathway. Can. J. Physiol. Pharmacol. 2016;94 doi: 10.1139/cjpp-2016-0197. [DOI] [PubMed] [Google Scholar]
- 58.Omole J.G., Ayoka O.A., Alabi Q.K., Adefisayo M.A., Asafa M.A., Olubunmi B.O., et al. Protective effect of kolaviron on cyclophosphamide-induced cardiac toxicity in rats. J Evid Based Integr Med. 2018;23 doi: 10.1177/2156587218757649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Macleod M.R., O'Collins T., Howells D.W., Donnan G.A. Pooling of animal experimental data reveals influence of study design and publication bias. Stroke. 2004;35 doi: 10.1161/01.STR.0000125719.25853.20. [DOI] [PubMed] [Google Scholar]
- 60.Bolan F., Louca I., Heal C., Cunningham C.J. The potential of biomaterial-based approaches as therapies for ischemic stroke: a systematic review and meta-analysis of pre-clinical studies. Front. Neurol. 2019;10 doi: 10.3389/fneur.2019.00924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arida R.M., Passos A.A., Graciani A.L., Brogin J.A.F., Ribeiro M. de AL., Faber J., et al. The potential role of previous physical Exercise Program to reduce Seizure Susceptibility: a systematic review and meta-analysis of animal studies. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.771123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hooijmans C.R., Rovers M.M., De Vries R.B.M., Leenaars M., Ritskes-Hoitinga M., Langendam M.W. SYRCLE's risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014;14 doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iwu M.M. Biflavonoids and glycosides of Garcinia kola stem bark. Planta Med. 1982;45 doi: 10.1055/s-2007-971307. [DOI] [PubMed] [Google Scholar]
- 64.Ayepola O.R., Chegou N.N., Brooks N.L., Oguntibeju O.O. Kolaviron, a Garcinia biflavonoid complex, ameliorates hyperglycemia-mediated hepatic injury in rats via suppression of inflammatory responses. BMC Compl. Alternative Med. 2013 doi: 10.1186/1472-6882-13-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lloyd-Jones D.M., Morris P.B., Ballantyne C.M., Birtcher K.K., Daly D.D., DePalma S.M., et al. 2017 focused update of the 2016 ACC Expert consensus Decision pathway on the role of non-Statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American college of cardiology task fo. J. Am. Coll. Cardiol. 2017;70:1785–1822. doi: 10.1016/j.jacc.2017.07.745. [DOI] [PubMed] [Google Scholar]
- 66.Mills K.T., Stefanescu A., He J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020;16 doi: 10.1038/s41581-019-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.World Health Organization . global health crises; Geneva: 2013. A Global Brief on Hypertension: Silent Killer. [Google Scholar]
- 68.Susalit E., Agus N., Effendi I., Tjandrawinata R.R., Nofiarny D., Perrinjaquet-Moccetti T., et al. Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: comparison with Captopril. Phytomedicine. 2011;18:251–258. doi: 10.1016/j.phymed.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 69.Wang J., Xiong X. Outcome measures of Chinese herbal medicine for hypertension: an overview of systematic reviews. Evid. base Compl. Alternative Med. 2012;2012 doi: 10.1155/2012/697237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oyagbemi A., Omobowale T., Farombi E. Kolaviron and Garcinia kola attenuate homocysteine-induced arteriosclerosis and cardiotoxicity in Wistar rats. Toxicol. Int. 2016;23:246–253. doi: 10.22506/ti/2016/v23/i3/146718. [DOI] [PubMed] [Google Scholar]
- 71.Oyagbemi A.A., Omobowale T.O., Asenuga E.R., Abiola J.O., Adedapo A.A., Yakubu M.A. Kolaviron attenuated arsenic acid induced-cardiorenal dysfunction via regulation of ROS, C-reactive proteins (CRP), cardiac troponin I (CTnI), and BCL2. J Tradit Complement Med. 2018;8:396–409. doi: 10.1016/j.jtcme.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen X., Shan H., Zhao J., Hong Y., Bai Y., Sun I., et al. L-type calcium current (I Ca,L) and inward rectifier potassium current (I K1) are involved in QT prolongation induced by arsenic trioxide in rat. Cell. Physiol. Biochem. 2010;26 doi: 10.1159/000324005. [DOI] [PubMed] [Google Scholar]
- 73.Adaramoye O.A., Nwosu I.O., Farombi E.O. Sub-acute effect of N G-nitro-l-arginine methyl ester (L-NAME) on biochemical indices in rats: protective effects of Kolaviron and extract of Curcuma longa L. Pharmacogn. Res. 2012;4:127–133. doi: 10.4103/0974-8490.99071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Seth M.K., Hussain M.E., Pasha S., Fahim M. Effects of a novel ACE inhibitor, 3-(3-thienyl)-l-alanyl-ornithyl-proline, on endothelial vasodilation and hepatotoxicity in L-NAME-induced hypertensive rats. Drug Des. Dev. Ther. 2016;10 doi: 10.2147/DDDT.S77761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oyagbemi A.A., Bester D., Esterhuyse J., Farombi E.O. Kolaviron and Garcinia kola seed extract protect against ischaemia/reperfusion injury on isolated rat hearts. Drug Res. 2018;68:286–295. doi: 10.1055/s-0043-123686. 10.1055/s-0043–123686. [DOI] [PubMed] [Google Scholar]
- 76.Koshy A.S., Anila L., Vijayalakshmi N.R. Flavonoids from Garcinia cambogia lower lipid levels in hypercholesterolemic rats. Food Chem. 2001;72:289–294. doi: 10.1016/S0308-8146(00)00225-9. [DOI] [Google Scholar]
- 77.Oyetayo F.L., Akomolafe S.F., Ogundumi O.A. Anti-hypercholesterolemic potential of diet supplemented with Anchomanes difformis and Pleurotus tuberregium tubers in high cholesterol fed rats. J. Diabetes Metab. Disord. 2020;19 doi: 10.1007/s40200-020-00615-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oparil S., Zaman M.A., Calhoun D.A. Pathogenesis of hypertension. Ann. Intern. Med. 2003 doi: 10.7326/0003-4819-139-9-200311040-00011. [DOI] [PubMed] [Google Scholar]
- 79.Adejor E.B., Ameh D.A., James D.B., Owolabi O.A., Ndidi U.S. Effects of Garcinia kola biflavonoid fractions on serum lipid profile and kidney function parameters in hyperlipidemic rats. Clinical Phytoscience. 2017;2 doi: 10.1186/s40816-016-0033-4. [DOI] [Google Scholar]
- 80.Djétouan K.J.M., Amonkan K.A., Yao A.R., Kanga A.J., Koko K.B., Kati-Coulibaly S. The effect of Ivory coast Garcinia kola Heckel (Guttiferae) seeds on hyperlipidemia, hyperglycemia and obesity in wistar rats Garcinia kola hyperlipidemia hyperglycemia obesity rat. Int J Nutr Sci. 2022;7:110–119. doi: 10.30476/IJNS.2022.95550.1189. [DOI] [Google Scholar]
- 81.Oladapo O., Quadri O., Ojora K., Ajani R. The effects of kolaviron on the atherogenic propensity of Nigeria local edible Oils in male wistar rats. J Pharm Res Int. 2017;18 doi: 10.9734/jpri/2017/35360. [DOI] [Google Scholar]
- 82.Olajide O.A. Investigation of the effects of selected medicinal plants on experimental thrombosis. Phytother Res. 1999;13 doi: 10.1002/(SICI)1099-1573(199905)13:3<231::AID-PTR414>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 83.Marzocchella L., Fantini M., Benvenuto M., Masuelli L., Tresoldi I., Modesti A., et al. Dietary flavonoids: molecular mechanisms of action as anti- inflammatory agents. Recent Pat. Inflamm. Allergy Drug Discov. 2011;5 doi: 10.2174/187221311797264937. [DOI] [PubMed] [Google Scholar]
- 84.Rathee P., Chaudhary H., Rathee S., Rathee D., Kumar V., Kohli K. Mechanism of action of flavonoids as anti-inflammatory agents: a review. Inflamm. Allergy - Drug Targets. 2009;8 doi: 10.2174/187152809788681029. [DOI] [PubMed] [Google Scholar]
- 85.Olajide O.A., Awe S.O., Makinde J.M. Pharmacological studies on Newbouldia laevis stem bark. Fitoterapia. 1997;68 [Google Scholar]
- 86.Adaramoye O.A., Medeiros I.A. Endothelium-independent vasodilation induced by kolaviron, a biflavonoid complex from Garcinia kola seeds, in rat superior mesenteric arteries. J. Smooth Muscle Res. 2009;45 doi: 10.1540/jsmr.45.39. [DOI] [PubMed] [Google Scholar]
- 87.Mehta J.L., Lopez L.M., Vlachakis N.D., Gradman A.H., Nash D.T., O'Connell M.T., et al. Double-blind evaluation of the dose-response relationship of amlodipine in essential hypertension. Am. Heart J. 1993 doi: 10.1016/0002-8703(93)90762-X. [DOI] [PubMed] [Google Scholar]
- 88.Jeffers B.W., Bhambri R., Robbins J. Uptitrating amlodipine significantly reduces blood pressure in diabetic patients with hypertension: a retrospective, pooled analysis. Vasc. Health Risk Manag. 2014;10:651–659. doi: 10.2147/VHRM.S64511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Oyagbemi A.A., Omobowale T.O., Adedapo A.A., Yakubu M.A. Kolaviron, biflavonoid complex from the seed of Garcinia kola attenuated angiotensin II- and lypopolysaccharide-induced vascular smooth muscle cell proliferation and nitric oxide production. Pharmacogn. Res. 2016;8:S50–S55. doi: 10.4103/0974-8490.178647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lacolley P., Regnault V., Nicoletti A., Li Z., Michel J.B. The vascular smooth muscle cell in arterial pathology: a cell that can take on multiple roles. Cardiovasc. Res. 2012;95:194–204. doi: 10.1093/cvr/cvs135. [DOI] [PubMed] [Google Scholar]
- 91.Oyagbemi A., Omobowale T., Adedapo A., Oyekan A., Yakubu M. Antiproliferative effect of kolaviron, a biflavonoid complex from the seed of Garcinia kola on vascular smooth muscle cells (VSMs) and A549 cancer cell line. Faseb. J. 2015;29 945–17. [Google Scholar]
- 92.Laursen J.B., Rajagopalan S., Galis Z., Tarpey M., Freeman B.A., Harrison D.G. Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation. 1997;95:588–593. doi: 10.1161/01.CIR.95.3.588. [DOI] [PubMed] [Google Scholar]
- 93.Nwaneri-chidozie V.O., Anyanwu K.C., Adaramoye O., Emerole E.O. Cardioprotective effect of Kolaviron (Garcinia kola seed extract) in cholesterol-fed rats. Int. J. Pharma Sci. Res. 2014;5:96–99. [Google Scholar]
- 94.Mohamadin A.M., Elberry A.A., Mariee A.D., Morsy G.M., Al-Abbasi F.A. Lycopene attenuates oxidative stress and heart lysosomal damage in isoproterenol induced cardiotoxicity in rats: a biochemical study. Pathophysiology. 2012;19 doi: 10.1016/j.pathophys.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 95.Ojha S., Bharti S., Golechha M., Sharma A.K., Rani N., Kumari S., et al. Andrographis paniculata extract protects against isoproterenol-induced myocardial injury by mitigating cardiac dysfunction and oxidative injury in rats. Acta Poloniae Pharmaceutica - Drug Research. 2012;69 [PubMed] [Google Scholar]
- 96.Adoga J.O., Channa M.L., Nadar A. Type-2 diabetic rat heart: the effect of kolaviron on mTOR-1, P70S60K, PKC-α, NF-kB, SOD-2, NRF-2, eNOS, AKT-1, ACE, and P38 MAPK gene expression profile. Biomed. Pharmacother. 2022;148 doi: 10.1016/j.biopha.2022.112736. [DOI] [PubMed] [Google Scholar]
- 97.Oyagbemi A.A., Bester D., Esterhuyse J., Farombi E.O. Kolaviron offered a novel cardioprotection against ischemic/reperfusion injury by modulation of Akt/protein kinase B (PKB)/p38MAPK/Caspase 3/poly‐ADP‐ribose polymerase signaling pathway. Faseb. J. 2020;34 doi: 10.1096/fasebj.2020.34.s1.00691. 1–1. [DOI] [Google Scholar]
- 98.Schiffrin E.L. Oxidative stress, nitric oxide synthase, and superoxide dismutase. Hypertension. 2008;51 doi: 10.1161/hypertensionaha.107.103226. [DOI] [PubMed] [Google Scholar]
- 99.Morgan T. Renin, angiotensin, sodium, and organ damage. Hypertens. Res. 2003;26:349–354. doi: 10.1291/hypres.26.349. [DOI] [PubMed] [Google Scholar]
- 100.Virdis A., Duranti E., Taddei S. Oxidative stress and vascular damage in hypertension: role of angiotensin ii. Int. J. Hypertens. 2011;2011 doi: 10.4061/2011/916310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ma T.K.W., Kam K.K.H., Yan B.P., Lam Y.Y. Renin-angiotensin-aldosterone system blockade for cardiovascular diseases: current status. Br. J. Pharmacol. 2010;160:1273–1292. doi: 10.1111/j.1476-5381.2010.00750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Montagner A., Polizzi A., Fouché E., Ducheix S., Lippi Y., Lasserre F., et al. Liver PPARα is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut. 2016;65 doi: 10.1136/gutjnl-2015-310798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reckelhoff J.F. Gender differences in hypertension. Curr. Opin. Nephrol. Hypertens. 2018;27 doi: 10.1097/MNH.0000000000000404. [DOI] [PubMed] [Google Scholar]
- 104.Song J.J., Ma Z., Wang J., Chen L.X., Zhong J.C. Gender differences in hypertension. J Cardiovasc Transl Res. 2020;13 doi: 10.1007/s12265-019-09888-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data associated with this study has been deposited into a publicly available repository (Mendeley data) and is also available as an attachment to this journal article. Mendeley Data Reserved https://doi.org/10.17632/k7s3dsf7y9.1.