Abstract
Purpose: To describe the first use of high-dose-rate yttrium-90 disc brachytherapy for choroidal melanoma. Methods: A 72-year-old patient had a cT1-category choroidal melanoma characterized by the presence of orange pigment, increasing subretinal fluid (SRF), and enlarging tumor thickness. It was treated with single-session, light-guided, light-defined yttrium-90-disc brachytherapy. Results: A specialized handheld applicator provided with 4 encircling lights was used to guide plaque placement and localize treatment. Unlike low-dose-rate plaques, high-dose-rate yttrium-90 required only 3 minutes 39 seconds. In this case, treatment did not require episcleral sutures, muscle relocation, outpatient dwell time, or a second surgery. High-dose-rate treatment improved radiation safety by eliminating perioperative exposure to health care personnel, the community, and the family. At the 13-month follow-up, the SRF and tumor thickness were diminished. There was no secondary cataract, radiation retinopathy, maculopathy, or optic neuropathy, and the visual acuity was 20/20. Conclusions: Yttrium-90 brachytherapy allowed for single-surgery, minimally invasive, outpatient irradiation of a choroidal melanoma.
Keywords: yttrium-90, high-dose rate, brachytherapy, melanoma, choroid, plaque
Introduction
Choroidal melanoma is currently widely treated with episcleral ophthalmic plaque radiation therapy. Stallard first used low-dose-rate solid cobalt-60, which evolved into Packer’s low-dose-rate iodine-125 seeds in gold-eye plaques.1–3 The latter was unidirectional into the eye and also comprised a lower level of energy. This improved the safety of the procedure by reducing radiation exposure to patients, clinicians, and health care personnel.
In 1991, an even lower energy seed, palladium-103, became commercially available for the treatment of cancer. 4 Palladium-103 seeds were the same size as iodine-125 seeds and, therefore, easy to use in gold-eye plaques. In addition, comparative dosimetry and clinical studies both showed that the lower energy palladium-103 reduced radiation exposure to most critical intraocular structures.1–5
In Europe and Russia, the dual beta-radiation plaque sources ruthenium-106/rhodium-106 and strontium-90/yttrium-90 were commercialized.6–12 The ruthenium-106/rhodium-106 plaques were multiday low dose rate, while the British strontium-90/yttrium-90 high-dose-rate sources were radiosimilar to the singular isotope yttrium-90 disc source described in the current case study.13–15 A key difference, however, was that the previous dual-isotope high-dose-rate strontium-90/yttrium-90 source was embedded at the distal end of a steel shaft, in contrast to the Liberty Vision yttrium-90 disc (Liberty Vision Corp), which is more like a seed and can be inserted into a clinical applicator. This allows additional capabilities for radiation localization and protection.
Monte-Carlo medical physics calculations showed that with the yttrium-90 disc located on the sclera posterior to the globe, only 12.0 mm of ocular tissue (sclera, choroid, retina, and vitreous) absorbed the yttrium-90 beta irradiation.14,15 Similarly, posterior to the source, there was a mean 0.75 mm of plastic, and a similar depth of orbital fat absorbed radiation in that direction. The dose lateral to the yttrium-90 disc exhibited a more acute drop-off as a result of yttrium-90 disc anisotropy and its surrounding radiation-absorbing plastic (Figure 1). 15
Figure 1.

Radiation fields anterior, posterior, and lateral to the yttrium-90 disc are shown. The inset values were calculated from Rivard et al. 15 (Illustration by Toby S. Welles.)
Radiation safety was one of the most important aspects of this treatment. A formal study was therefore performed using the radiation principle of “as low as reasonably achievable,” and the methods of treatment used in the study were found to be safe for the patient, surgeon, and operating room personnel. 14
A review of the literature found that beta radiation has long been widely used in the treatment of ophthalmic tumors and benign growths, including choroidal melanoma, retinoblastoma, choroidal metastasis, conjunctival squamous carcinoma, and conjunctival melanoma. Beta radiation has also been used in the treatment of benign tumors, such as choroidal hemangioma and retinal angioma, neovascular macular degeneration, eccentric disciform degeneration, and polypoidal choroidal vasculopathy.16–29
The most common and extensively cited ophthalmic use was high-dose-rate strontium-90/yttrium-90 to prevent pterygium-related episcleral fibrovascular growths. 30 However, clinical studies have described the use of high-dose-rate strontium-90/yttrium-90 to reduce the risk for trabeculectomy failure compared with either 5-fluorouracil or mitomycin, particularly for high-risk congenital and darkly pigmented eyes.31–37 For use in other organs, systemic yttrium-90 has been incorporated into microspheres that are widely used for transarterial radioembolization of primary and metastatic cancers.38–40 This paper presents a case of an American Joint Committee on Cancer (AJCC)–classified cT1a-category choroidal melanoma treated with high-dose-rate yttrium-90 brachytherapy.
Case Report
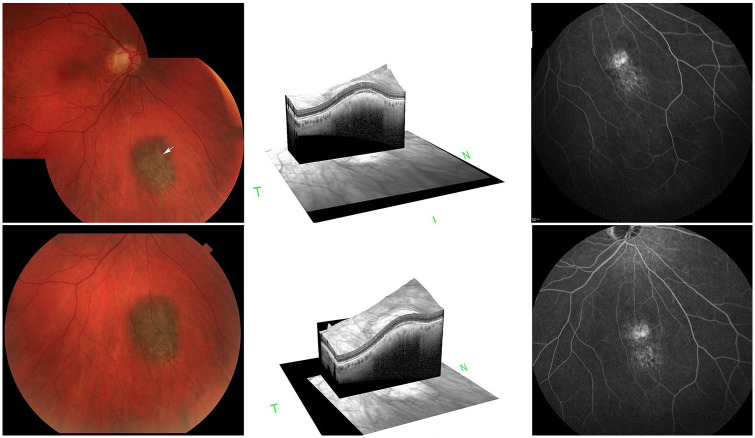
A 72-year-old patient was clinically diagnosed with an AJCC 8th edition cT1a-category posterior choroidal melanoma based on the presence of orange pigment, overlying exudative subretinal fluid (SRF), and enlarging tumor thickness (Figure 2). Ultrasound imaging showed a maximum tumor thickness of 1.3 mm and basal diameters of 4.1 mm and 2.9 mm. The underlying scleral thickness was 0.3 mm. After a discussion of the potential risks and benefits of continued observation or plaque brachytherapy (eg, iodine-125, palladium-103, yttrium-90), the patient requested high-dose-rate yttrium-90 plaque brachytherapy. Specifically, the patient noted that in consideration of his heart, lung, and kidney disease, he preferred a single-session radiation surgery. Once total-body positron emission tomography/computed tomography proved negative for metastatic melanoma or second nonocular cancer, the surgery was performed. 41
Figure 2.
(Top left) Fundus photograph (before yttrium-90 high-dose-rate brachytherapy) shows a small inferior choroidal melanoma with orange pigment lipofuscin (arrow). (Top middle) A 3-dimensional optical coherence tomography cross-section of the tumor shows subretinal fluid (SRF) over its apex. (Top right) A midphase fluorescein angiogram shows focal hyperfluorescence corresponding to the previously noted SRF. (Bottom) Early resolution of the orange pigment (left), SRF (middle), and minimally decreased fluorescein hyperfluorescence 13 months after yttrium-90 high-dose-rate brachytherapy.
The assembly of the Liberty Vision yttrium-90 disc–iWand P device, radiation safety testing, and low-dose-rate to high-dose-rate dose conversion have been described. 14 In this case, low-dose-rate to high-dose-rate dose conversion and US National Institute of Standards and Technology–traceable yttrium-90 disc calibration were performed. For treatment, this patient’s yttrium-90 disc activity was 15.48 mCi, and its diameter was 6.0 mm. The tumor’s apex (1.6 mm from episclera) was treated to a high-dose-rate 30 Gy (85 Gy low-dose-rate equivalent biologic effective dose). To achieve that dose, the duration of episcleral application was 3 minutes 39 seconds. 14
Access to the orbit, posterior to the globe and thus the episcleral target for iWand P application, required a minor conjunctival incision and opening of Tenon fascia. A dummy (nonradioactive) iWand P applicator was inserted so that its 4 lights were seen surrounding the tumor (Figure 3), showing an unobstructed pathway to normal high-dose-rate yttrium-90 disc plaque positioning. Specifically, in this case, no rectus or oblique muscle insertion, episcleral scarring, or fascia obstructed passage of the thin, loaded applicator tip. Once the dummy applicator was removed, the yttrium-90 disc-loaded iWand P applicator was inserted in the same location. The 4 applicator lights were again used to guide the 6.0 mm diameter source into place and maintain it in its therapeutic position (subjacent to the tumor) during treatment (Figure 3). Immediately after irradiation, the device was removed from the eye and placed into a shielded container. The conjunctiva was closed with a running 7-0 polyglactin suture.
Figure 3.
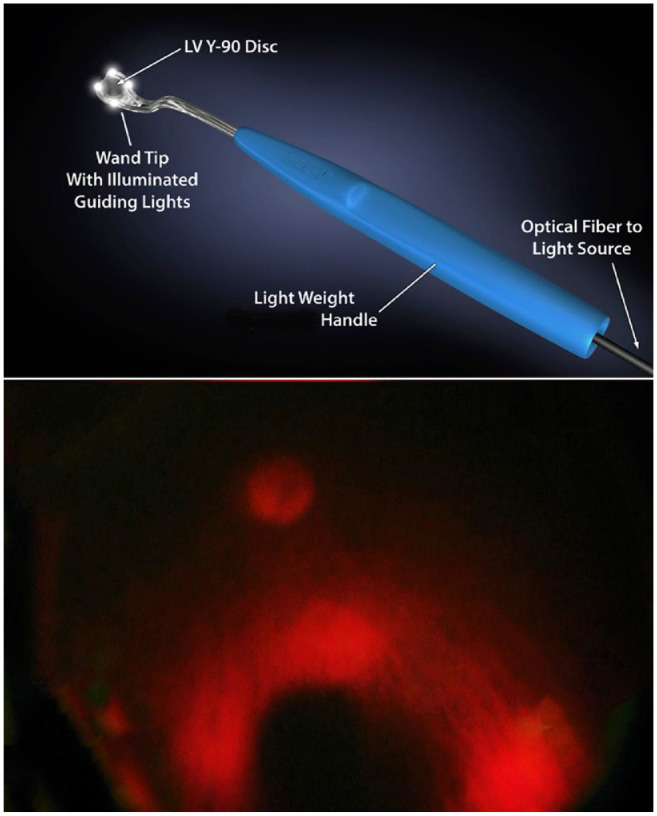
(Top) Lighted nodes on the anterior surface of the iWand P. (Bottom) Lighted nodes in place around the choroidal melanoma. Placement position of the yttrium-90 disc was ensured. (Illustration by Toby S. Welles.)
There were no acute surgical complications and, according to protocol, the patient was monitored on postoperative day 1 and then 1, 3, 6, and 13 months after treatment. At the last follow-up, there was slightly decreased orange pigment and apical SRF, while the tumor height had decreased to 1.0 mm. In addition, there were no signs of radiation cataract, retinopathy, or optic neuropathy. The patient’s visual acuity remained 20/20 (Figure 2), with no long-term surgical or radiation complications related to the application of this high-dose-rate yttrium-90 system.
Conclusions
The US Food and Drug Administration approved the Liberty Vision yttrium-90 disc beta-radiation source for clinical use as a strontium-90/yttrium-90 radiosimilar. 13 For use in treatment, it was affixed within an iWand P handheld applicator, whose function also includes 4 disc-surrounding lights used to guide the disc into position and ensure tumor targeting (Figure 3). In contrast to low-dose-rate plaque therapy, yttrium-90 high-dose-rate treatment allowed for a single, minimally invasive surgery that did not require indirect transillumination or ultrasonographic confirmation of the plaque position. The yttrium-90 disc was removed after minutes of treatment and thus did not require leaving the hospital with a radioactive device sewn to the eye. This eliminated the chance of postsurgical hospital and community radiation exposure. Having insertion and removal in a single session eliminated the need for a second surgery and its anesthesia-related risk (Figure 4). In the current case, with 13 months of follow-up, there have been no radiation complications.
Figure 4.
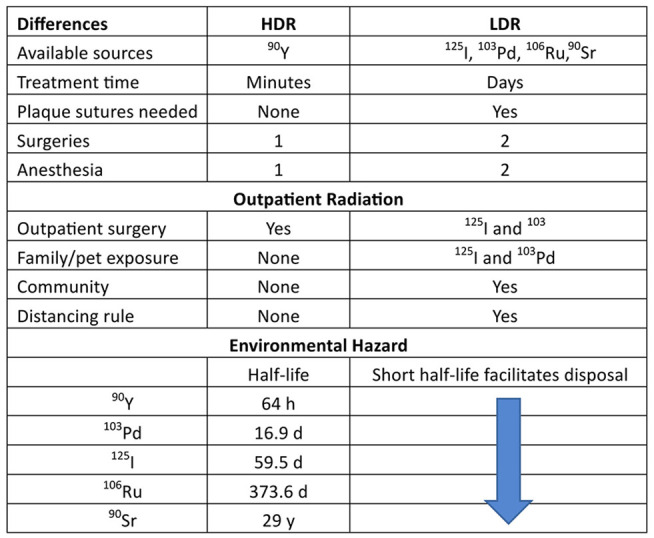
High-dose-rate vs low-dose rate brachytherapy for choroidal melanoma.
Abbreviations: 103Pd, palladium-103; 106Ru, ruthenium-106; 125I, iodine-125; 90Sr, strontium-90; 90Y, yttrium-90. Community, outpatient community radiation exposure; Distance rule, the distance a patient is required to stay away from others as an outpatient; Distancing rule, >3 feet away from others required in New York State for 125I and 103Pd, in-patient hospitalization for 106Ru; HDR, high-dose rate; LDR, low-dose rate.
Beta irradiation has been used in ophthalmology for more than 70 years. The previous beta sources have specific disadvantages compared with the high-dose-rate yttrium-90 system described in this case report. The previous sources are neither light-guided to the target nor light-defined during treatment, and although both are unshielded, several seconds were required to place the high-dose-rate yttrium-90 device vs the minutes required for placement and suturing of a low-dose-rate plaque (Figure 4).
Missotten et al 11 used a custom-made high-dose-rate strontium-90/yttrium-90 plaque to treat 46 choroidal melanomas. To do so, they modified an epibulbar SIAQ 7321 device applicator (Amersham Corp) containing 370 MBq (10 mCi) of strontium-90/yttrium-90 on the concave side shielded by 0.1 mm of platinum. The 16.0 mm radioactive disc element consisted of a 12.0 mm diameter central active surface surrounded by a 2.0 mm rim of inactive casing. The liberated strontium-90/yttrium-90 disc was then attached within an 18.5 mm ring to allow episcleral suturing. Thirteen of the 46 melanomas were larger than 10.0 mm in diameter, and 14 were larger than 4.0 mm in apical height. Missotten et al’s 11 high-dose-rate strontium-90/yttrium-90 treatments lasted 2 to 4 hours; there were 11 multiple radiation applications, and 7 tumors received additional xenon-arc photocoagulation. Despite using an 18.5 mm diameter plaque with a 12.0 mm central zone of activity, they were able to achieve a 93% local control rate. In contrast to the strontium-90/yttrium-90 high-dose-rate plaque, the yttrium-90 high-dose-rate disc–iWand P system used in this case was surrounded by guiding and radiation-defining lights and required minutes compared with hours of treatment. Exposure to radiation associated with plaque suturing during placement was also eliminated. Both methods comprised single-session, high-dose-rate brachytherapy treatments.
In conclusion, this case shows that single-session, high-dose-rate yttrium-90 surgical irradiation is feasible to treat choroidal melanoma.
Footnotes
Ethical Approval: The author(s) confirmed that any aspects of the work covered in this manuscript that involved human patients was conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged in the manuscript.
Statement of Informed Consent: The patient consented to publication of the case in writing. This report does not contain personal information that could lead to the identification of the patient. Therefore, this study conformed to the Declaration of Helsinki and the US Health Insurance Portability and Accountability Act of 1996. All participants gave informed consent for treatment with high-dose-rate yttrium-90 brachytherapy after a discussion of the relative risks and benefits of observation, resection, and all available forms of radiation sources. Patients also signed approval to participate in an independent safety and tolerability study (ethics and institutional review board approval by Advarra, Inc), sponsored by Liberty Vision with The New York Eye Cancer Center as its first participating site. The data collected in this study were approved by the institutional review board of Advarra, Inc.
Intellectual Property: The author(s) confirmed that due consideration was given to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication with respect to intellectual property. In doing so, the author(s) confirmed the regulations of the institutions concerning intellectual property were followed.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Finger is the chief executive officer of and owns private stock in LV Liberty Vision Corp and IP Liberty Vision Corp.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Paul T. Finger  https://orcid.org/0000-0002-8111-3896
https://orcid.org/0000-0002-8111-3896
References
- 1. American Brachytherapy Society - Ophthalmic Oncology Task Force. Electronic address: paulfinger@eyecancer.com; ABS – OOTF Committee. The American Brachytherapy Society consensus guidelines for plaque brachytherapy of uveal melanoma and retinoblastoma. Brachytherapy. 2014;13(1):1-14. doi: 10.1016/j.brachy.2013.11.008 [DOI] [PubMed] [Google Scholar]
- 2. Chiu-Tsao ST, Astrahan MA, Finger PT, et al. Dosimetry of 125I and 103Pd COMS eye plaques for intraocular tumors: report of Task Group 129 by the AAPM and ABS. Med Phys. 2012;39(10): 6161-6184. doi: 10.1118/1.4749933 [DOI] [PubMed] [Google Scholar]
- 3. Finger PT, Rivard MJ, Chaugule SS, et al. Ophthalmic radiotherapy: plaques and implants. In: Chaugule SS, Honavar SG, Finger PT, eds. Surgical Ophthalmic Oncology, A Collaborative Open-Access Reference. Vol. 1. 1st ed. Springer; 2019:147-158. [Google Scholar]
- 4. Finger PT, Moshfeghi DM, Ho TK. Palladium-103 ophthalmic plaque radiotherapy. Arch Ophthalmol. 1991;109(11):1610-1613. doi: 10.1001/archopht.1991.01080110148053 [DOI] [PubMed] [Google Scholar]
- 5. Finger PT, Chin KJ, Duvall G; Palladium-103 for Choroidal Melanoma Study Group. Palladium-103 ophthalmic plaque radiation therapy for choroidal melanoma: 400 treated patients. Ophthalmology. 2009;116(4):790-796.e1. doi: 10.1016/j.ophtha.2008.12.027 [DOI] [PubMed] [Google Scholar]
- 6. Lommatzsch PK. Results after beta-irradiation (106Ru/106Rh) of choroidal melanomas: 20 years’ experience. Br J Ophthalmol. 1986;70(11):844-851. doi: 10.1136/bjo.70.11.844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ballin R, Lommatzsch PK, Drost H, Ratajek B. A beta ray applicator (106Ru/106Rh) in the treatment of ciliary body melanomas. Klin Monatsbl Augenheilkd. 1985;187(2):144-146. doi: 10.1055/s-2008-1051007. German. [DOI] [PubMed] [Google Scholar]
- 8. Karimi S, Arabi A, Siavashpour Z, Shahraki T, Ansari I. Efficacy and complications of ruthenium-106 brachytherapy for uveal melanoma: a systematic review and meta-analysis. J Contemp Brachytherapy. 2021;13(3):358-364. doi: 10.5114/jcb.2021.106191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Ginderdeuren R, van Limbergen E, Spileers W. 18-years’ experience with high dose rate strontium-90 brachytherapy of small to medium sized posterior uveal melanoma. Br J Ophthalmol. 2005;89(10):1306-1310. doi: 10.1136/bjo.2005.068460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saakyan SV, Amiryan AG, Valskiy VV, Mironova IS. Plaque radiotherapy for anterior uveal melanomas. Vestn Oftalmol. 2015; 131(2):5-12. doi: 10.17116/oftalma201513125-11 [DOI] [PubMed] [Google Scholar]
- 11. Missotten L, Dirven W, Van der Schueren A, Leys A, De Meester G, Van Limbergen E. Results of treatment of choroidal malignant melanoma with high-dose-rate strontium-90 brachytherapy. A retrospective study of 46 patients treated between 1983 and 1995. Graefes Arch Clin Exp Ophthalmol. 1998;236(3):164-173. doi: 10.1007/s004170050059 [DOI] [PubMed] [Google Scholar]
- 12. Hughes WF., Jr. Beta radiation therapy in ophthalmology. Trans Am Ophthalmol Soc. 1952;50:469-549. [PMC free article] [PubMed] [Google Scholar]
- 13. U.S. Food & Drug Administration. FDA Clearance statement. May 29, 2020. Accessed 12 January 2024. https://www.accessdata.fda.gov/cdrh_docs/pdf19/K193602.pdf
- 14. Finger PT, Stewart R, Rivard MJ, et al. First clinical implementation of yttrium-90-disc brachytherapy after FDA clearance. Brachytherapy. 2023;22(3):416-427. doi: 10.1016/j.brachy.2023.02.004 [DOI] [PubMed] [Google Scholar]
- 15. Rivard MJ, Mohney K, Welles T, Finger P. Dosimetry of beta-emitting brachytherapy sources for age-related macular degeneration. Med Phys. 2018;45:e172. [Google Scholar]
- 16. Schueler AO, Flühs D, Anastassiou G, et al. Beta-ray brachytherapy with 106Ru plaques for retinoblastoma. Int J Radiat Oncol Biol Phys. 2006;65(4):1212-1221. doi: 10.1016/j.ijrobp.2006.02.002 [DOI] [PubMed] [Google Scholar]
- 17. Lommatzsch PK. Beta-ray treatment of malignant epibulbar melanoma. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1978;209(2):111-124. doi: 10.1007/BF00407844 [DOI] [PubMed] [Google Scholar]
- 18. Cohen VML, Papastefanou VP, Liu S, Stoker I, Hungerford JL. The use of strontium-90 Beta radiotherapy as adjuvant treatment for conjunctival melanoma. J Oncol. 2013;2013:349162. doi: 10.1155/2013/349162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Laskar S, Gurram L, Laskar SG, Chaudhari S, Khanna N, Upreti R. Superficial ocular malignancies treated with strontium-90 brachytherapy: long term outcomes. J Contemp Brachytherapy. 2015;7(5):369-373. doi: 10.5114/jcb.2014.55003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cerezo L, Otero J, Aragón G, et al. Conjunctival intraepithelial and invasive squamous cell carcinomas treated with strontium-90. Radiother Oncol. 1990;17(3):191-197. doi: 10.1016/0167-8140(90)90203-9 [DOI] [PubMed] [Google Scholar]
- 21. Elkon D, Constable WC. The use of strontium-90 in the treatment of carcinoma in situ of the conjunctiva. Am J Ophthalmol. 1979; 87(1):84-86. doi: 10.1016/0002-9394(79)90196-x [DOI] [PubMed] [Google Scholar]
- 22. Jaakkola A, Heikkonen J, Tommila P, Laatikainen L, Immonen I. Strontium plaque irradiation of subfoveal neovascular membranes in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 1998;236(1):24-30. doi: 10.1007/s004170050038 [DOI] [PubMed] [Google Scholar]
- 23. Chhablani J, Jager R, Ong J, et al. Two-year outcomes of episcleral brachytherapy adjunct to anti-VEGF therapy for treatment-resistant nAMD. Graefes Arch Clin Exp Ophthalmol. 2022;260(12): 3791-3798. doi: 10.1007/s00417-022-05736-0 [DOI] [PubMed] [Google Scholar]
- 24. Kishan AU, Modjtahedi BS, Morse LS, Lee P. Radiation therapy for neovascular age-related macular degeneration. Int J Radiat Oncol Biol Phys. 2013;85(3):583-597. doi: 10.1016/j.ijrobp.2012.07.2352 [DOI] [PubMed] [Google Scholar]
- 25. Naseripour M, Maleki A, Astaraki A, et al. Ruthenium-106 brachytherapy in the treatment of circumscribed choroidal hemangioma. Retina. 2018;38(5):1024-1030. doi: 10.1097/IAE.0000000000001616 [DOI] [PubMed] [Google Scholar]
- 26. Finger PT. Radiation therapy for exudative choroidal hemangioma. Indian J Ophthalmol. 2019;67(5):579-581. doi: 10.4103/ijo.IJO70719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dalbah S, Bechrakis NE, Thomasen H, et al. Brachytherapy for peripheral retinal capillary haemangioblastoma in von Hippel-Lindau disease. Klin Monbl Augenheilkd. 2021;238(7):781-787. doi: 10.1055/a-1391-9110 [DOI] [PubMed] [Google Scholar]
- 28. Petrarca R, Nau J, Dugel PU, Jackson TL. Epimacular brachytherapy for the treatment of retinal angiomatous proliferation. Retin Cases Brief Rep. 2012;6(4):353-357. doi: 10.1097/ICB.0b013e31823c12a3 [DOI] [PubMed] [Google Scholar]
- 29. Bornfeld N, Kreusel KM. Capillary hemangioma of the retina in cases of von Hippel-Lindau syndrome. New therapeutic directions. Ophthalmologe. 2007;104(2):114-118. doi: 10.1007/s00347-007-1485-1. German. [DOI] [PubMed] [Google Scholar]
- 30. Ali AM, Thariat J, Bensadoun RJ, et al. The role of radiotherapy in the treatment of pterygium: a review of the literature including more than 6000 treated lesions. Cancer Radiother. 2011;15(2): 140-147. doi: 10.1016/j.canrad.2010.03.020 [DOI] [PubMed] [Google Scholar]
- 31. Dhalla K, Cousens S, Bowman R, Wood M, Murdoch I. Is beta radiation better than 5 flurouracil as an adjunct for trabeculectomy surgery when combined with cataract surgery? A Randomised Controlled Trial. PLoS One. 2016;11(9):e0161674. doi: 10.1371/journal.pone.0161674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kirwan JF, Rennie C, Evans JR. Beta radiation for glaucoma surgery. Cochrane Database Syst Rev. 2012;2012(6):CD003433. doi: 10.1002/14651858.CD003433.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miller MH, Rice NS. Trabeculectomy combined with beta irradiation for congenital glaucoma. Br J Ophthalmol. 1991;75(10):584-590. doi: 10.1136/bjo.75.10.584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kirwan JF, Cousens S, Venter L, et al. Effect of beta radiation on success of glaucoma drainage surgery in South Africa: randomised controlled trial. BMJ. 2006;333(7575):942. doi: 10.1136/bmj.38971.395301.7C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. de Fendi LI, Arruda GV, Costa VP, Paula JS. Meta-analysis of beta radiation augmentation for trabeculectomy - results in distinct ethnic groups. Arq Bras Oftalmol. 2011;74(4):300-305. doi: 10.1590/s0004-27492011000400016 [DOI] [PubMed] [Google Scholar]
- 36. Murdoch I, Puertas R, Hamedani M, Khaw PT. Long-term safety and outcomes of β-radiation for trabeculectomy. J Glaucoma. 2023;32(3):171-177. doi: 10.1097/IJG.0000000000002144. [DOI] [PubMed] [Google Scholar]
- 37. Cook C, Perrott A, Mustak H, et al. Randomised clinical trial of trabeculectomy with mitomycin-C versus trabeculectomy with beta radiation. SA Ophthalmol J Spring. 2018;13(4):11-14. [Google Scholar]
- 38. Salem R, Johnson GE, Kim E, et al. Yttrium-90 radioembolization for the treatment of solitary, unresectable HCC: the LEGACY Study. Hepatology. 2021;74(5):2342-2352. doi: 10.1002/hep.31819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang EA, Broadwell SR, Bellavia RJ, Stein JP. Selective internal radiation therapy with SIR-Spheres in hepatocellular carcinoma and cholangiocarcinoma. J Gastrointest Oncol. 2017;8(2):266-278. doi: 10.21037/jgo.2016.11.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Levey AO, Elsayed M, Lawson DH, et al. Predictors of overall and progression-free survival in patients with ocular melanoma metastatic to the liver undergoing Y-90 radioembolization. Cardiovasc Intervent Radiol. 2020;43(2):254-263. doi: 10.1007/s00270-019-02366-8 [DOI] [PubMed] [Google Scholar]
- 41. Freton A, Chin KJ, Raut R, Tena LB, Kivelä T, Finger PT. Initial PET/CT staging for choroidal melanoma: AJCC correlation and second nonocular primaries in 333 patients. Eur J Ophthalmol. 2012;22(2):236-243. doi: 10.5301/ejo.5000049 [DOI] [PubMed] [Google Scholar]